User login
NSCLC success story: Mortality down, survival improved
“This analysis shows for the first time that nationwide mortality rates for the most common category of lung cancer, NSCLC, are declining faster than its incidence, an advance that correlates with the [FDA] approval of several targeted therapies for this cancer in recent years,” coauthor Douglas Lowy, MD, deputy director, National Cancer Institute, Bethesda, Md., said in a statement.
“Major improvements have been made in NSCLC treatment with the advent of targeted therapies and immunotherapies,” lead author Nadia Howlander, PhD, National Cancer Institute, and colleagues observed.
“The survival benefit for patients with NSCLC treated with targeted therapy has been shown in clinical trials, but our study highlights their possible effect at the population level,” they added.
In contrast, mortality from SCLC has dropped only in tandem with a decline in the incidence of SCLC, and survival has remained largely unchanged, the same analysis showed.
NSCLC is by far the most common type of lung cancer, accounting for more than 75% of all lung cancer cases in the United States. SCLC accounts for about 13%.
The study was published online Aug. 12 in the New England Journal of Medicine.
“Although overall mortality from lung cancer has been declining in the United States, little is known about mortality trends according to cancer subtype at the population level because death certificates do not record subtype information,” the authors commented.
“To address this data limitation, the U.S. Surveillance, Epidemiology and End Results (SEER) program has linked mortality records to incidence cancer cases,” the authors explained. This allowed them to calculate incidence-based mortality among men and women in the United States.
The incidence-based mortality method that the researchers used was applied to the SEER data to describe population-level mortality trends in the United States that were attributable to each subtype of lung cancer as well as gender from 2001 to 2016.
Among men, the incidence of NSCLC decreased gradually by 1.9% a year from 2001 to 2008, then more dramatically by 3.1% a year from 2008 to 2016.
Corresponding incidence-based mortality rates among men dropped by 3.2% a year from 2006 to 2013, then again more dramatically by 6.3% a year from 2013 to 2016.
“The 2-year relative survival among patients with lung cancer improved substantially from 26% among men with NSCLC diagnosed in 2001 to 35% among those with NSCLC diagnosed in 2014,” the researchers added.
Among women, the incidence of NSCLC remained unchanged between 2001 and 2006, after which it began to drop by 1.5% a year from 2006 to 2016.
“In contrast, incidence-based mortality decreased slowly [among women] by 2.3% annually ... from 2006 through 2014 and then at a faster rate of 5.9% annually ... from 2014 through 2016,” the authors noted.
The 2-year relative survival rate for patients with NSCLC was higher among women than among men, improving from 35% in 2001 to 44% in 2014.
Improvements in survival were also observed for all races and ethnicities, despite concerns that new cancer treatments might increase treatment disparities between races, because they are all so expensive, the authors commented.
Mortality from SCLC declined by 4.3% a year among men, but that decline was entirely due to a similar decrease in the incidence of SCLC. Survival at 2 years for patients with this subtype of lung cancer remained largely unchanged over the same interval.
Genetic testing
The accelerating decline in NSCLC mortality starting in 2013 corresponds to the period in which clinicians began to routinely test for molecular alterations in epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), the authors pointed out.
In 2012, the National Comprehensive Cancer Network recommended that all patients with nonsquamous NSCLC undergo genetic testing for EGFR mutations and ALK rearrangements.
At about the same time, the FDA approved a number of targeted therapies for tumors that are sensitive to targeted tyrosine kinase inhibition.
More recently, immunotherapies that act as programmed cell death inhibitors have substantially improved NSCLC outcomes, the authors noted.
The first of these was approved for NSCLC in 2015 (pembrolizumab). It was followed by a number of similar agents. It is unlikely that their approval contributed to the observed decline in NSCLC mortality, which started to accelerate in 2013 in the United States, the authors commented.
Nevertheless, the effect that the immune checkpoint inhibitors has had on the survival of patients with NSCLC can be expected to continue and to extend improvement in survival beyond the current study endpoint in 2016, they suggest.
Another contributing factor is the decline in smoking that has occurred in the United States since the 1960s. This has led to the decrease in the incidence of lung cancer. The faster decrease in the incidence of SCLC, compared with NSCLC can be explained by the higher relative risk of smoking with regard to SCLC compared to NSCLC, they commented.
Similarly, the faster decrease in lung cancer incidence in men compared to women can be explained by the relative difference in the prevalence of smoking between men and women, they added.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“This analysis shows for the first time that nationwide mortality rates for the most common category of lung cancer, NSCLC, are declining faster than its incidence, an advance that correlates with the [FDA] approval of several targeted therapies for this cancer in recent years,” coauthor Douglas Lowy, MD, deputy director, National Cancer Institute, Bethesda, Md., said in a statement.
“Major improvements have been made in NSCLC treatment with the advent of targeted therapies and immunotherapies,” lead author Nadia Howlander, PhD, National Cancer Institute, and colleagues observed.
“The survival benefit for patients with NSCLC treated with targeted therapy has been shown in clinical trials, but our study highlights their possible effect at the population level,” they added.
In contrast, mortality from SCLC has dropped only in tandem with a decline in the incidence of SCLC, and survival has remained largely unchanged, the same analysis showed.
NSCLC is by far the most common type of lung cancer, accounting for more than 75% of all lung cancer cases in the United States. SCLC accounts for about 13%.
The study was published online Aug. 12 in the New England Journal of Medicine.
“Although overall mortality from lung cancer has been declining in the United States, little is known about mortality trends according to cancer subtype at the population level because death certificates do not record subtype information,” the authors commented.
“To address this data limitation, the U.S. Surveillance, Epidemiology and End Results (SEER) program has linked mortality records to incidence cancer cases,” the authors explained. This allowed them to calculate incidence-based mortality among men and women in the United States.
The incidence-based mortality method that the researchers used was applied to the SEER data to describe population-level mortality trends in the United States that were attributable to each subtype of lung cancer as well as gender from 2001 to 2016.
Among men, the incidence of NSCLC decreased gradually by 1.9% a year from 2001 to 2008, then more dramatically by 3.1% a year from 2008 to 2016.
Corresponding incidence-based mortality rates among men dropped by 3.2% a year from 2006 to 2013, then again more dramatically by 6.3% a year from 2013 to 2016.
“The 2-year relative survival among patients with lung cancer improved substantially from 26% among men with NSCLC diagnosed in 2001 to 35% among those with NSCLC diagnosed in 2014,” the researchers added.
Among women, the incidence of NSCLC remained unchanged between 2001 and 2006, after which it began to drop by 1.5% a year from 2006 to 2016.
“In contrast, incidence-based mortality decreased slowly [among women] by 2.3% annually ... from 2006 through 2014 and then at a faster rate of 5.9% annually ... from 2014 through 2016,” the authors noted.
The 2-year relative survival rate for patients with NSCLC was higher among women than among men, improving from 35% in 2001 to 44% in 2014.
Improvements in survival were also observed for all races and ethnicities, despite concerns that new cancer treatments might increase treatment disparities between races, because they are all so expensive, the authors commented.
Mortality from SCLC declined by 4.3% a year among men, but that decline was entirely due to a similar decrease in the incidence of SCLC. Survival at 2 years for patients with this subtype of lung cancer remained largely unchanged over the same interval.
Genetic testing
The accelerating decline in NSCLC mortality starting in 2013 corresponds to the period in which clinicians began to routinely test for molecular alterations in epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), the authors pointed out.
In 2012, the National Comprehensive Cancer Network recommended that all patients with nonsquamous NSCLC undergo genetic testing for EGFR mutations and ALK rearrangements.
At about the same time, the FDA approved a number of targeted therapies for tumors that are sensitive to targeted tyrosine kinase inhibition.
More recently, immunotherapies that act as programmed cell death inhibitors have substantially improved NSCLC outcomes, the authors noted.
The first of these was approved for NSCLC in 2015 (pembrolizumab). It was followed by a number of similar agents. It is unlikely that their approval contributed to the observed decline in NSCLC mortality, which started to accelerate in 2013 in the United States, the authors commented.
Nevertheless, the effect that the immune checkpoint inhibitors has had on the survival of patients with NSCLC can be expected to continue and to extend improvement in survival beyond the current study endpoint in 2016, they suggest.
Another contributing factor is the decline in smoking that has occurred in the United States since the 1960s. This has led to the decrease in the incidence of lung cancer. The faster decrease in the incidence of SCLC, compared with NSCLC can be explained by the higher relative risk of smoking with regard to SCLC compared to NSCLC, they commented.
Similarly, the faster decrease in lung cancer incidence in men compared to women can be explained by the relative difference in the prevalence of smoking between men and women, they added.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“This analysis shows for the first time that nationwide mortality rates for the most common category of lung cancer, NSCLC, are declining faster than its incidence, an advance that correlates with the [FDA] approval of several targeted therapies for this cancer in recent years,” coauthor Douglas Lowy, MD, deputy director, National Cancer Institute, Bethesda, Md., said in a statement.
“Major improvements have been made in NSCLC treatment with the advent of targeted therapies and immunotherapies,” lead author Nadia Howlander, PhD, National Cancer Institute, and colleagues observed.
“The survival benefit for patients with NSCLC treated with targeted therapy has been shown in clinical trials, but our study highlights their possible effect at the population level,” they added.
In contrast, mortality from SCLC has dropped only in tandem with a decline in the incidence of SCLC, and survival has remained largely unchanged, the same analysis showed.
NSCLC is by far the most common type of lung cancer, accounting for more than 75% of all lung cancer cases in the United States. SCLC accounts for about 13%.
The study was published online Aug. 12 in the New England Journal of Medicine.
“Although overall mortality from lung cancer has been declining in the United States, little is known about mortality trends according to cancer subtype at the population level because death certificates do not record subtype information,” the authors commented.
“To address this data limitation, the U.S. Surveillance, Epidemiology and End Results (SEER) program has linked mortality records to incidence cancer cases,” the authors explained. This allowed them to calculate incidence-based mortality among men and women in the United States.
The incidence-based mortality method that the researchers used was applied to the SEER data to describe population-level mortality trends in the United States that were attributable to each subtype of lung cancer as well as gender from 2001 to 2016.
Among men, the incidence of NSCLC decreased gradually by 1.9% a year from 2001 to 2008, then more dramatically by 3.1% a year from 2008 to 2016.
Corresponding incidence-based mortality rates among men dropped by 3.2% a year from 2006 to 2013, then again more dramatically by 6.3% a year from 2013 to 2016.
“The 2-year relative survival among patients with lung cancer improved substantially from 26% among men with NSCLC diagnosed in 2001 to 35% among those with NSCLC diagnosed in 2014,” the researchers added.
Among women, the incidence of NSCLC remained unchanged between 2001 and 2006, after which it began to drop by 1.5% a year from 2006 to 2016.
“In contrast, incidence-based mortality decreased slowly [among women] by 2.3% annually ... from 2006 through 2014 and then at a faster rate of 5.9% annually ... from 2014 through 2016,” the authors noted.
The 2-year relative survival rate for patients with NSCLC was higher among women than among men, improving from 35% in 2001 to 44% in 2014.
Improvements in survival were also observed for all races and ethnicities, despite concerns that new cancer treatments might increase treatment disparities between races, because they are all so expensive, the authors commented.
Mortality from SCLC declined by 4.3% a year among men, but that decline was entirely due to a similar decrease in the incidence of SCLC. Survival at 2 years for patients with this subtype of lung cancer remained largely unchanged over the same interval.
Genetic testing
The accelerating decline in NSCLC mortality starting in 2013 corresponds to the period in which clinicians began to routinely test for molecular alterations in epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), the authors pointed out.
In 2012, the National Comprehensive Cancer Network recommended that all patients with nonsquamous NSCLC undergo genetic testing for EGFR mutations and ALK rearrangements.
At about the same time, the FDA approved a number of targeted therapies for tumors that are sensitive to targeted tyrosine kinase inhibition.
More recently, immunotherapies that act as programmed cell death inhibitors have substantially improved NSCLC outcomes, the authors noted.
The first of these was approved for NSCLC in 2015 (pembrolizumab). It was followed by a number of similar agents. It is unlikely that their approval contributed to the observed decline in NSCLC mortality, which started to accelerate in 2013 in the United States, the authors commented.
Nevertheless, the effect that the immune checkpoint inhibitors has had on the survival of patients with NSCLC can be expected to continue and to extend improvement in survival beyond the current study endpoint in 2016, they suggest.
Another contributing factor is the decline in smoking that has occurred in the United States since the 1960s. This has led to the decrease in the incidence of lung cancer. The faster decrease in the incidence of SCLC, compared with NSCLC can be explained by the higher relative risk of smoking with regard to SCLC compared to NSCLC, they commented.
Similarly, the faster decrease in lung cancer incidence in men compared to women can be explained by the relative difference in the prevalence of smoking between men and women, they added.
The authors disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
HPV test is preferred method for cervical cancer screening: ACS
The American Cancer Society (ACS) has released updated guidelines for cervical cancer screening. The key recommendation is that primary human papillomavirus (HPV) testing is the preferred screening method, starting at the age of 25 and repeated every 5 years.
In the past, guidelines for cervical cancer screening recommended cytology (the Pap test) starting at 21 years of age and repeated every 3 years. In more recent years, cotesting (with both Pap and HPV tests) has been recommended.
Since the last ACS guidelines on cervical cancer screening were published in 2012, two HPV tests have been approved by the Food and Drug Administration (FDA) for use in primary HPV screening.
The new “streamlined recommendations can improve compliance and reduce potential harms,” commented Debbie Saslow, PhD, managing director, HPV/GYN Cancers, American Cancer Society.
The updated guidelines were published online July 30 in CA: A Cancer Journal for Clinicians.
“We now have stronger evidence to support starting cervical cancer screening at a later age and to recommend screening with the HPV test as the preferred test,” Saslow told Medscape Medical News. This also reflects the phasing out of cytology and cotesting, she added.
“This update is based on decades of studies comparing the effectiveness of HPV testing to cytology and is bolstered by evidence of the impact of HPV vaccination, including a dramatic decline in cervical precancers and, more recently, cervical cancers among young women,” she said.
The American Society for Colposcopy and Cervical Pathology (ASCCP) said that it was preparing a response to these new guidelines, as is the American College of Obstetricians and Gynecologists (ACOG).
Cotesting or cytology alone
The updated guidelines recommend primary HPV testing as the preferred screening method for all women with a cervix. If primary HPV testing is not available, women should be screened with cotesting, which should also be performed every 5 years.
If only cytology is available, then women should be screened every 3 years.
The ACS authors point out that cotesting or cytology testing alone is still an acceptable option for cervical cancer screening, insofar as primary HPV testing using FDA-approved tests may not be available in some settings.
As more laboratories in the United States transition to FDA-approved tests for primary HPV testing, it is expected that the use of cotesting or cytology alone will be phased out.
The new guidelines also emphasize that women may discontinue screening at the age of 65 if they have not had cervical intraepitheal neoplasia of grade 2 or higher within the past 25 years and if they have tested negative over the past 10 years on all past screens.
The authors caution that past screens should only be considered negative if the patient has had two consecutive negative HPV tests or two consecutive negative cotests or three consecutive negative cytology tests within the past 10 years.
“These criteria do not apply to individuals who are currently under surveillance for abnormal screening results,” the authors state.
Women older than 65 for whom adequate documentation of prior screening is not available should continue to be screened until criteria for screening discontinuation are met, they add.
Screening may be discontinued among women with a limited life expectancy.
HPV vaccination
The authors note that HPV vaccination is expected to substantially change cervical cancer screening strategies.
In 2018, the National Immunization Survey–Teen, involving adolescents aged 13 to 17 years, showed that 68.1% of female patients were up to date on HPV vaccine recommendations, as were 51.1% of male patients.
“Cytology-based screening is much less efficient in vaccinated populations, as abnormal cytology disproportionately identifies minor abnormalities resulting from HPV types that are associated with lower cancer risk,” the reports’ authors point out.
As the prevalence of high-grade cervical abnormalities and the incidence of cervical cancer continue to decline, “the proportion of false-positive findings [on cytology alone] is expected to increase significantly,” they caution.
As a result, the ACS suggests that physicians will likely have to consider a patient’s vaccination status in tandem with cervical cancer screening results to arrive at an accurate assessment.
Raising starting age to 25 years
Saslow also noted that there were several reasons why it is now recommended that screening begin at the age of 25 instead of the age of 21, as in earlier guidelines.
“Firstly, less than 1% of cervical cancers are diagnosed before the age of 25 – so this is about 130 cases per year,” she explained.
Thanks to HPV vaccination, this percentage is further declining, “so screening is just not beneficial at this age,” Saslow emphasized.
Furthermore, the rate of false positives is much higher in younger patients, and a false-positive result can have a negative impact on pregnancy outcomes, she added.
Saslow also dismissed an article in favor of cotesting instead of HPV testing alone. That study, carried out by researchers at Quest Diagnostics and the University of Pittsburgh Medical Center, recommended cotesting, claiming that primary HPV testing is significantly less likely to detect cervical precancers or cervical cancer than cotesting.
“These data come from parties with a vested interest in preserving cytology as a screening test,” Saslow told Medscape Medical News. She noted that “these findings are not at all credible as judged by the scientific community.”
On the basis of their own modeling, ACS researchers estimate that “starting with primary HPV testing at age 25 will prevent 13% more cervical cancers and 7% more cervical cancer deaths” in comparison with cytology (Pap testing alone) beginning at the age of 21, then cotesting at the age of 30, Saslow said in a statement.
“Our model showed we could do that with a 9% increase in follow-up procedures but with 45% fewer tests required overall,” she added.
The new recommendations are not expected to create any change in the type or amount of care required by providers, and patients will not notice any difference, inasmuch as cotesting and primary HPV testing are performed the same way in the examination room, she added.
“Resistance [to the changes] is expected – and is already occurring – from laboratories and manufacturers of tests that will no longer be used once we transition from cotesting and, less commonly, Pap testing to primary HPV testing,” Saslow said.
However, providers need to be aware that HPV infection, as with any sexually transmitted disease, is associated with a certain stigma, and they need to take care in discussing potential HPV infection with their patients.
Good method
Medscape Medical News approached Mark Einstein, MD, president of the ASCCP and professor and chair of obstetrics, gynecology, and reproductive health at Rutgers Biomedical and Health Sciences, Newark, New Jersey, to comment on the new guidelines.
“First and foremost,” he said, “everything we want to do when it comes to screening is to maximize the identification of picking up a cancer and minimize the risk or potential harm of not only screening itself but of missing cancers, so any strategy that improves on the sensitivity of picking up a cancer is a good method.”
Nevertheless, inasmuch as the ASCCP is one of the foremost organizations involved in cervical cancer screening and management, its members need more time to take a closer look at the updated ACS guidelines before they, together with sister organizations, such as the ACOG, release an official statement as to whether or not they fully endorse the new guidelines.
The United States Preventive Services Task Force recently endorsed primary HPV testing (starting at age 30), but it also said that an alternative strategy is cotesting for women between 30 and 65 years of age, Einstein observed.
Asked to comment on the article from Quest Diagnostics and the University of Pittsburgh that recommended cotesting instead of primary HPV testing, Einstein said that suggestion should not be dismissed out of hand.
The ASCCP has asked the authors of that study for their data in order conduct an independent assessment of it, largely because the study was retrospective in nature. Because of that, “there may have been a few pieces of information that were missing in true real-time fashion,” he said. “Not having [both the primary HPV testing and the cytology results] in front of me might change the next thing I might recommend to the patient,” Einstein explained.
The bottom line is that, when comparing primary HPV testing alone, cytology alone, and cotesting and rates of cervical cancer at 5 years, “the biggest driver for true performance of positive predictive value is HPV,” Einstein said.
Nevertheless, cotesting does bring more information into the equation compared with primary HPV testing alone, although it also increases the potential for harm, including the harm of overtesting and conducting needless colposcopies, he added.
That said, starting primary HPV testing at the age of 25 rather than the age of 30, as was previously recommended, is very likely to lead to detection of spurious HPV infections because HPV infections are very common among women in their 20s, Einstein pointed out.
“This, too, could potentially lead to more colposcopies, which may cause harm from the procedure itself but also create a certain amount of anxiety and concern, so there is some harm in testing for HPV at an earlier age as well,” Einstein said.
Saslow and Einstein have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The American Cancer Society (ACS) has released updated guidelines for cervical cancer screening. The key recommendation is that primary human papillomavirus (HPV) testing is the preferred screening method, starting at the age of 25 and repeated every 5 years.
In the past, guidelines for cervical cancer screening recommended cytology (the Pap test) starting at 21 years of age and repeated every 3 years. In more recent years, cotesting (with both Pap and HPV tests) has been recommended.
Since the last ACS guidelines on cervical cancer screening were published in 2012, two HPV tests have been approved by the Food and Drug Administration (FDA) for use in primary HPV screening.
The new “streamlined recommendations can improve compliance and reduce potential harms,” commented Debbie Saslow, PhD, managing director, HPV/GYN Cancers, American Cancer Society.
The updated guidelines were published online July 30 in CA: A Cancer Journal for Clinicians.
“We now have stronger evidence to support starting cervical cancer screening at a later age and to recommend screening with the HPV test as the preferred test,” Saslow told Medscape Medical News. This also reflects the phasing out of cytology and cotesting, she added.
“This update is based on decades of studies comparing the effectiveness of HPV testing to cytology and is bolstered by evidence of the impact of HPV vaccination, including a dramatic decline in cervical precancers and, more recently, cervical cancers among young women,” she said.
The American Society for Colposcopy and Cervical Pathology (ASCCP) said that it was preparing a response to these new guidelines, as is the American College of Obstetricians and Gynecologists (ACOG).
Cotesting or cytology alone
The updated guidelines recommend primary HPV testing as the preferred screening method for all women with a cervix. If primary HPV testing is not available, women should be screened with cotesting, which should also be performed every 5 years.
If only cytology is available, then women should be screened every 3 years.
The ACS authors point out that cotesting or cytology testing alone is still an acceptable option for cervical cancer screening, insofar as primary HPV testing using FDA-approved tests may not be available in some settings.
As more laboratories in the United States transition to FDA-approved tests for primary HPV testing, it is expected that the use of cotesting or cytology alone will be phased out.
The new guidelines also emphasize that women may discontinue screening at the age of 65 if they have not had cervical intraepitheal neoplasia of grade 2 or higher within the past 25 years and if they have tested negative over the past 10 years on all past screens.
The authors caution that past screens should only be considered negative if the patient has had two consecutive negative HPV tests or two consecutive negative cotests or three consecutive negative cytology tests within the past 10 years.
“These criteria do not apply to individuals who are currently under surveillance for abnormal screening results,” the authors state.
Women older than 65 for whom adequate documentation of prior screening is not available should continue to be screened until criteria for screening discontinuation are met, they add.
Screening may be discontinued among women with a limited life expectancy.
HPV vaccination
The authors note that HPV vaccination is expected to substantially change cervical cancer screening strategies.
In 2018, the National Immunization Survey–Teen, involving adolescents aged 13 to 17 years, showed that 68.1% of female patients were up to date on HPV vaccine recommendations, as were 51.1% of male patients.
“Cytology-based screening is much less efficient in vaccinated populations, as abnormal cytology disproportionately identifies minor abnormalities resulting from HPV types that are associated with lower cancer risk,” the reports’ authors point out.
As the prevalence of high-grade cervical abnormalities and the incidence of cervical cancer continue to decline, “the proportion of false-positive findings [on cytology alone] is expected to increase significantly,” they caution.
As a result, the ACS suggests that physicians will likely have to consider a patient’s vaccination status in tandem with cervical cancer screening results to arrive at an accurate assessment.
Raising starting age to 25 years
Saslow also noted that there were several reasons why it is now recommended that screening begin at the age of 25 instead of the age of 21, as in earlier guidelines.
“Firstly, less than 1% of cervical cancers are diagnosed before the age of 25 – so this is about 130 cases per year,” she explained.
Thanks to HPV vaccination, this percentage is further declining, “so screening is just not beneficial at this age,” Saslow emphasized.
Furthermore, the rate of false positives is much higher in younger patients, and a false-positive result can have a negative impact on pregnancy outcomes, she added.
Saslow also dismissed an article in favor of cotesting instead of HPV testing alone. That study, carried out by researchers at Quest Diagnostics and the University of Pittsburgh Medical Center, recommended cotesting, claiming that primary HPV testing is significantly less likely to detect cervical precancers or cervical cancer than cotesting.
“These data come from parties with a vested interest in preserving cytology as a screening test,” Saslow told Medscape Medical News. She noted that “these findings are not at all credible as judged by the scientific community.”
On the basis of their own modeling, ACS researchers estimate that “starting with primary HPV testing at age 25 will prevent 13% more cervical cancers and 7% more cervical cancer deaths” in comparison with cytology (Pap testing alone) beginning at the age of 21, then cotesting at the age of 30, Saslow said in a statement.
“Our model showed we could do that with a 9% increase in follow-up procedures but with 45% fewer tests required overall,” she added.
The new recommendations are not expected to create any change in the type or amount of care required by providers, and patients will not notice any difference, inasmuch as cotesting and primary HPV testing are performed the same way in the examination room, she added.
“Resistance [to the changes] is expected – and is already occurring – from laboratories and manufacturers of tests that will no longer be used once we transition from cotesting and, less commonly, Pap testing to primary HPV testing,” Saslow said.
However, providers need to be aware that HPV infection, as with any sexually transmitted disease, is associated with a certain stigma, and they need to take care in discussing potential HPV infection with their patients.
Good method
Medscape Medical News approached Mark Einstein, MD, president of the ASCCP and professor and chair of obstetrics, gynecology, and reproductive health at Rutgers Biomedical and Health Sciences, Newark, New Jersey, to comment on the new guidelines.
“First and foremost,” he said, “everything we want to do when it comes to screening is to maximize the identification of picking up a cancer and minimize the risk or potential harm of not only screening itself but of missing cancers, so any strategy that improves on the sensitivity of picking up a cancer is a good method.”
Nevertheless, inasmuch as the ASCCP is one of the foremost organizations involved in cervical cancer screening and management, its members need more time to take a closer look at the updated ACS guidelines before they, together with sister organizations, such as the ACOG, release an official statement as to whether or not they fully endorse the new guidelines.
The United States Preventive Services Task Force recently endorsed primary HPV testing (starting at age 30), but it also said that an alternative strategy is cotesting for women between 30 and 65 years of age, Einstein observed.
Asked to comment on the article from Quest Diagnostics and the University of Pittsburgh that recommended cotesting instead of primary HPV testing, Einstein said that suggestion should not be dismissed out of hand.
The ASCCP has asked the authors of that study for their data in order conduct an independent assessment of it, largely because the study was retrospective in nature. Because of that, “there may have been a few pieces of information that were missing in true real-time fashion,” he said. “Not having [both the primary HPV testing and the cytology results] in front of me might change the next thing I might recommend to the patient,” Einstein explained.
The bottom line is that, when comparing primary HPV testing alone, cytology alone, and cotesting and rates of cervical cancer at 5 years, “the biggest driver for true performance of positive predictive value is HPV,” Einstein said.
Nevertheless, cotesting does bring more information into the equation compared with primary HPV testing alone, although it also increases the potential for harm, including the harm of overtesting and conducting needless colposcopies, he added.
That said, starting primary HPV testing at the age of 25 rather than the age of 30, as was previously recommended, is very likely to lead to detection of spurious HPV infections because HPV infections are very common among women in their 20s, Einstein pointed out.
“This, too, could potentially lead to more colposcopies, which may cause harm from the procedure itself but also create a certain amount of anxiety and concern, so there is some harm in testing for HPV at an earlier age as well,” Einstein said.
Saslow and Einstein have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The American Cancer Society (ACS) has released updated guidelines for cervical cancer screening. The key recommendation is that primary human papillomavirus (HPV) testing is the preferred screening method, starting at the age of 25 and repeated every 5 years.
In the past, guidelines for cervical cancer screening recommended cytology (the Pap test) starting at 21 years of age and repeated every 3 years. In more recent years, cotesting (with both Pap and HPV tests) has been recommended.
Since the last ACS guidelines on cervical cancer screening were published in 2012, two HPV tests have been approved by the Food and Drug Administration (FDA) for use in primary HPV screening.
The new “streamlined recommendations can improve compliance and reduce potential harms,” commented Debbie Saslow, PhD, managing director, HPV/GYN Cancers, American Cancer Society.
The updated guidelines were published online July 30 in CA: A Cancer Journal for Clinicians.
“We now have stronger evidence to support starting cervical cancer screening at a later age and to recommend screening with the HPV test as the preferred test,” Saslow told Medscape Medical News. This also reflects the phasing out of cytology and cotesting, she added.
“This update is based on decades of studies comparing the effectiveness of HPV testing to cytology and is bolstered by evidence of the impact of HPV vaccination, including a dramatic decline in cervical precancers and, more recently, cervical cancers among young women,” she said.
The American Society for Colposcopy and Cervical Pathology (ASCCP) said that it was preparing a response to these new guidelines, as is the American College of Obstetricians and Gynecologists (ACOG).
Cotesting or cytology alone
The updated guidelines recommend primary HPV testing as the preferred screening method for all women with a cervix. If primary HPV testing is not available, women should be screened with cotesting, which should also be performed every 5 years.
If only cytology is available, then women should be screened every 3 years.
The ACS authors point out that cotesting or cytology testing alone is still an acceptable option for cervical cancer screening, insofar as primary HPV testing using FDA-approved tests may not be available in some settings.
As more laboratories in the United States transition to FDA-approved tests for primary HPV testing, it is expected that the use of cotesting or cytology alone will be phased out.
The new guidelines also emphasize that women may discontinue screening at the age of 65 if they have not had cervical intraepitheal neoplasia of grade 2 or higher within the past 25 years and if they have tested negative over the past 10 years on all past screens.
The authors caution that past screens should only be considered negative if the patient has had two consecutive negative HPV tests or two consecutive negative cotests or three consecutive negative cytology tests within the past 10 years.
“These criteria do not apply to individuals who are currently under surveillance for abnormal screening results,” the authors state.
Women older than 65 for whom adequate documentation of prior screening is not available should continue to be screened until criteria for screening discontinuation are met, they add.
Screening may be discontinued among women with a limited life expectancy.
HPV vaccination
The authors note that HPV vaccination is expected to substantially change cervical cancer screening strategies.
In 2018, the National Immunization Survey–Teen, involving adolescents aged 13 to 17 years, showed that 68.1% of female patients were up to date on HPV vaccine recommendations, as were 51.1% of male patients.
“Cytology-based screening is much less efficient in vaccinated populations, as abnormal cytology disproportionately identifies minor abnormalities resulting from HPV types that are associated with lower cancer risk,” the reports’ authors point out.
As the prevalence of high-grade cervical abnormalities and the incidence of cervical cancer continue to decline, “the proportion of false-positive findings [on cytology alone] is expected to increase significantly,” they caution.
As a result, the ACS suggests that physicians will likely have to consider a patient’s vaccination status in tandem with cervical cancer screening results to arrive at an accurate assessment.
Raising starting age to 25 years
Saslow also noted that there were several reasons why it is now recommended that screening begin at the age of 25 instead of the age of 21, as in earlier guidelines.
“Firstly, less than 1% of cervical cancers are diagnosed before the age of 25 – so this is about 130 cases per year,” she explained.
Thanks to HPV vaccination, this percentage is further declining, “so screening is just not beneficial at this age,” Saslow emphasized.
Furthermore, the rate of false positives is much higher in younger patients, and a false-positive result can have a negative impact on pregnancy outcomes, she added.
Saslow also dismissed an article in favor of cotesting instead of HPV testing alone. That study, carried out by researchers at Quest Diagnostics and the University of Pittsburgh Medical Center, recommended cotesting, claiming that primary HPV testing is significantly less likely to detect cervical precancers or cervical cancer than cotesting.
“These data come from parties with a vested interest in preserving cytology as a screening test,” Saslow told Medscape Medical News. She noted that “these findings are not at all credible as judged by the scientific community.”
On the basis of their own modeling, ACS researchers estimate that “starting with primary HPV testing at age 25 will prevent 13% more cervical cancers and 7% more cervical cancer deaths” in comparison with cytology (Pap testing alone) beginning at the age of 21, then cotesting at the age of 30, Saslow said in a statement.
“Our model showed we could do that with a 9% increase in follow-up procedures but with 45% fewer tests required overall,” she added.
The new recommendations are not expected to create any change in the type or amount of care required by providers, and patients will not notice any difference, inasmuch as cotesting and primary HPV testing are performed the same way in the examination room, she added.
“Resistance [to the changes] is expected – and is already occurring – from laboratories and manufacturers of tests that will no longer be used once we transition from cotesting and, less commonly, Pap testing to primary HPV testing,” Saslow said.
However, providers need to be aware that HPV infection, as with any sexually transmitted disease, is associated with a certain stigma, and they need to take care in discussing potential HPV infection with their patients.
Good method
Medscape Medical News approached Mark Einstein, MD, president of the ASCCP and professor and chair of obstetrics, gynecology, and reproductive health at Rutgers Biomedical and Health Sciences, Newark, New Jersey, to comment on the new guidelines.
“First and foremost,” he said, “everything we want to do when it comes to screening is to maximize the identification of picking up a cancer and minimize the risk or potential harm of not only screening itself but of missing cancers, so any strategy that improves on the sensitivity of picking up a cancer is a good method.”
Nevertheless, inasmuch as the ASCCP is one of the foremost organizations involved in cervical cancer screening and management, its members need more time to take a closer look at the updated ACS guidelines before they, together with sister organizations, such as the ACOG, release an official statement as to whether or not they fully endorse the new guidelines.
The United States Preventive Services Task Force recently endorsed primary HPV testing (starting at age 30), but it also said that an alternative strategy is cotesting for women between 30 and 65 years of age, Einstein observed.
Asked to comment on the article from Quest Diagnostics and the University of Pittsburgh that recommended cotesting instead of primary HPV testing, Einstein said that suggestion should not be dismissed out of hand.
The ASCCP has asked the authors of that study for their data in order conduct an independent assessment of it, largely because the study was retrospective in nature. Because of that, “there may have been a few pieces of information that were missing in true real-time fashion,” he said. “Not having [both the primary HPV testing and the cytology results] in front of me might change the next thing I might recommend to the patient,” Einstein explained.
The bottom line is that, when comparing primary HPV testing alone, cytology alone, and cotesting and rates of cervical cancer at 5 years, “the biggest driver for true performance of positive predictive value is HPV,” Einstein said.
Nevertheless, cotesting does bring more information into the equation compared with primary HPV testing alone, although it also increases the potential for harm, including the harm of overtesting and conducting needless colposcopies, he added.
That said, starting primary HPV testing at the age of 25 rather than the age of 30, as was previously recommended, is very likely to lead to detection of spurious HPV infections because HPV infections are very common among women in their 20s, Einstein pointed out.
“This, too, could potentially lead to more colposcopies, which may cause harm from the procedure itself but also create a certain amount of anxiety and concern, so there is some harm in testing for HPV at an earlier age as well,” Einstein said.
Saslow and Einstein have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Cancer patient organizations critically affected by pandemic
The COVID-19 pandemic has disrupted every aspect of cancer care, from diagnosis, treatment, and follow-up to participation in clinical trials, according to a new report that collected responses from cancer patient organizations around the world.
The report includes responses from 157 organizations in 56 countries, representing some 350,000 patients with cancer.
“The COVID-19 global pandemic has quite literally wreaked havoc with all of our lives but especially for cancer patients,” said the report’s author, Frances Reid, MBA, program director, World Ovarian Cancer Coalition.
“To those who have the power or influence to ensure that cancer treatment and services are not set back several years, please listen to those organizations who can articulate clearly the impact on patients, work with them, and act on it as soon as you can,” she added.
The new report, entitled “The Impact of COVID-19 on Cancer Patient Organisations,” was released on June 12. The organizations were surveyed from May 11 to May 25.
Cancer diagnosis
Two-thirds of the organizations surveyed said cancer screening programs had been canceled in their country, and 59% indicated they had seen a drop in urgent referrals for suspected cancer.
Some 44% said that access to pathology services had been reduced. One group in Australia reported that “results of pathology tests are taking longer to be returned. Generally a result would be returned within 48 hours. Since COVID-19, results are taking up to 7 days to be returned.”
As for treatment, 68% of organizations reported delays or cancellations of surgery or other treatments; 58% reported there had been a need to modify treatment protocols; and 48% indicated there had been a drop in participation in clinical trials.
Respondents were also concerned about reported increases in stress, anxiety, and isolation among many cancer patients. “Often at increased risk of infection and serious illness themselves ... many have been required to ‘shield’ from others, totally withdrawing from life outside their homes, thus increasing the already high levels of isolation they feel because of their life-limiting conditions,” the report notes.
In addition, some 60% of the organizations said that the pandemic had increased financial hardship among cancer patients. One US group commented: “Unemployment levels in the States similar to depression era. This has been a real challenge as many have lost insurance as well as jobs.”
Only a minority of respondents reported that cancer care was being offered in hospitals with no special arrangements in place to treat concomitant COVID-19 patients.
On the other hand, only 15% of respondents indicated that patients were being treated in a hospital that was not also caring for COVID-19 patients.
“Cancer will not wait for COVID-19 to pass, if it ever will, and the patient organizations are the key to minimizing the devastating impact [COVID-19 is having] on people with cancer,” Reid emphasized.
“More than ever, the patient/support services should be strengthened,” commented a group from France.
Patient services affected
“Almost all organisations (89%) have had to alter their services for people with cancer,” the report notes.
Two thirds of organizations involved in professional educational activities have had to change their services in some way, either by moving them online or stopping programs altogether, at least temporarily. “Some found that doctors and nurses are too busy with the pandemic to participate, and that their appetite for such activity is also diminished,” the report notes.
The volume of phone calls and emails increased in almost 6 of 10 organizations that provide support services for patients. Compared to prepandemic levels, volume increased by an average of 44%.
The most common queries raised by people with cancer (accounting for 85% of all queries) were questions about the risks of contracting COVID-19 and cancer treatments during the pandemic.
Some of the organizations also commented about how they had been affected. One group from Uganda said: “We had a sudden lockdown and we could not access office to give face to face counselling. We stopped research due to national guidelines on research. We continued giving information via phone and social media especially WhatsApp. We created groups for patients and counsellors to continue interacting.”
A group in Costa Rica reported: “We developed a new program of transfers from their homes to the hospital for cancer patients in chemotherapy and radiotherapy. 200 monthly transfers. We created a virtual community instead of our face-to-face support group, we started in April and we have 108 members, virtual sessions are held every two weeks.”
An organization based in the United States reported that it was “totally revamping our educational programs to be delivered in new ways in an online format ― not just replicating the in-person formats, but reaching out to our community and asking them what they would find the most valuable.”
Impact on fundraising
Almost 9 in 10 organizations raise funds to support their activities, the report notes. “A shocking 79% of organisations say they predict a fall in income over the next 12 months, with a further 16% not sure, leaving only 5% confident of their financial stability.”
Every type of fund-raising has been affected by COVID-19, from grants and major donors to community fund-raising events. Sixty percent of organisations said they were trying to find new ways to raise funds.
However, as one organization in Japan noted: “At the moment we can survive and feel it is unethical to ask the public for money when many are facing dire financial personal circumstances.”
A group from Australia commented: “Fundraising has been extremely difficult due to COVID-19 with distancing laws and no group gatherings as well as the economic downturn. Crisis appeals have been unsuccessful and all outdoor events and major events have been cancelled. In Australia we have had to contend with also the fires earlier in the year where a lot of money was donated to leaving other foundations struggling to get donor support.”
A little more than half (55%) of the organizations surveyed have had to cut costs.
Staffing cuts have been made in 1 in 10 of the organizations surveyed. A similar proportion of organizations have furloughed staff. Many if not all staff from numerous organizations are working from home.
A little more than half of those surveyed either provide funding for research or conduct research themselves, but only one quarter of them indicated there had been no change in their research projects. The others have indicated that they had to either reduce the scope of their research, put it on pause, or stop it altogether.
Three quarters of survey respondents noted that they had engaged in advocacy activities prior to the pandemic, and almost two thirds of them said they had to delay these activities.
Several of the organizations expressed thanks to the survey authors.
“COVID-19 is a global pandemic and cancer patients all around the world have similar worries, concerns and questions ― we are a small/medium organisation working in one country but believe in the power of community and coalitions and so this survey is a very welcome part of looking at this from a greater perspective,” commented one British group.
Reid has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The COVID-19 pandemic has disrupted every aspect of cancer care, from diagnosis, treatment, and follow-up to participation in clinical trials, according to a new report that collected responses from cancer patient organizations around the world.
The report includes responses from 157 organizations in 56 countries, representing some 350,000 patients with cancer.
“The COVID-19 global pandemic has quite literally wreaked havoc with all of our lives but especially for cancer patients,” said the report’s author, Frances Reid, MBA, program director, World Ovarian Cancer Coalition.
“To those who have the power or influence to ensure that cancer treatment and services are not set back several years, please listen to those organizations who can articulate clearly the impact on patients, work with them, and act on it as soon as you can,” she added.
The new report, entitled “The Impact of COVID-19 on Cancer Patient Organisations,” was released on June 12. The organizations were surveyed from May 11 to May 25.
Cancer diagnosis
Two-thirds of the organizations surveyed said cancer screening programs had been canceled in their country, and 59% indicated they had seen a drop in urgent referrals for suspected cancer.
Some 44% said that access to pathology services had been reduced. One group in Australia reported that “results of pathology tests are taking longer to be returned. Generally a result would be returned within 48 hours. Since COVID-19, results are taking up to 7 days to be returned.”
As for treatment, 68% of organizations reported delays or cancellations of surgery or other treatments; 58% reported there had been a need to modify treatment protocols; and 48% indicated there had been a drop in participation in clinical trials.
Respondents were also concerned about reported increases in stress, anxiety, and isolation among many cancer patients. “Often at increased risk of infection and serious illness themselves ... many have been required to ‘shield’ from others, totally withdrawing from life outside their homes, thus increasing the already high levels of isolation they feel because of their life-limiting conditions,” the report notes.
In addition, some 60% of the organizations said that the pandemic had increased financial hardship among cancer patients. One US group commented: “Unemployment levels in the States similar to depression era. This has been a real challenge as many have lost insurance as well as jobs.”
Only a minority of respondents reported that cancer care was being offered in hospitals with no special arrangements in place to treat concomitant COVID-19 patients.
On the other hand, only 15% of respondents indicated that patients were being treated in a hospital that was not also caring for COVID-19 patients.
“Cancer will not wait for COVID-19 to pass, if it ever will, and the patient organizations are the key to minimizing the devastating impact [COVID-19 is having] on people with cancer,” Reid emphasized.
“More than ever, the patient/support services should be strengthened,” commented a group from France.
Patient services affected
“Almost all organisations (89%) have had to alter their services for people with cancer,” the report notes.
Two thirds of organizations involved in professional educational activities have had to change their services in some way, either by moving them online or stopping programs altogether, at least temporarily. “Some found that doctors and nurses are too busy with the pandemic to participate, and that their appetite for such activity is also diminished,” the report notes.
The volume of phone calls and emails increased in almost 6 of 10 organizations that provide support services for patients. Compared to prepandemic levels, volume increased by an average of 44%.
The most common queries raised by people with cancer (accounting for 85% of all queries) were questions about the risks of contracting COVID-19 and cancer treatments during the pandemic.
Some of the organizations also commented about how they had been affected. One group from Uganda said: “We had a sudden lockdown and we could not access office to give face to face counselling. We stopped research due to national guidelines on research. We continued giving information via phone and social media especially WhatsApp. We created groups for patients and counsellors to continue interacting.”
A group in Costa Rica reported: “We developed a new program of transfers from their homes to the hospital for cancer patients in chemotherapy and radiotherapy. 200 monthly transfers. We created a virtual community instead of our face-to-face support group, we started in April and we have 108 members, virtual sessions are held every two weeks.”
An organization based in the United States reported that it was “totally revamping our educational programs to be delivered in new ways in an online format ― not just replicating the in-person formats, but reaching out to our community and asking them what they would find the most valuable.”
Impact on fundraising
Almost 9 in 10 organizations raise funds to support their activities, the report notes. “A shocking 79% of organisations say they predict a fall in income over the next 12 months, with a further 16% not sure, leaving only 5% confident of their financial stability.”
Every type of fund-raising has been affected by COVID-19, from grants and major donors to community fund-raising events. Sixty percent of organisations said they were trying to find new ways to raise funds.
However, as one organization in Japan noted: “At the moment we can survive and feel it is unethical to ask the public for money when many are facing dire financial personal circumstances.”
A group from Australia commented: “Fundraising has been extremely difficult due to COVID-19 with distancing laws and no group gatherings as well as the economic downturn. Crisis appeals have been unsuccessful and all outdoor events and major events have been cancelled. In Australia we have had to contend with also the fires earlier in the year where a lot of money was donated to leaving other foundations struggling to get donor support.”
A little more than half (55%) of the organizations surveyed have had to cut costs.
Staffing cuts have been made in 1 in 10 of the organizations surveyed. A similar proportion of organizations have furloughed staff. Many if not all staff from numerous organizations are working from home.
A little more than half of those surveyed either provide funding for research or conduct research themselves, but only one quarter of them indicated there had been no change in their research projects. The others have indicated that they had to either reduce the scope of their research, put it on pause, or stop it altogether.
Three quarters of survey respondents noted that they had engaged in advocacy activities prior to the pandemic, and almost two thirds of them said they had to delay these activities.
Several of the organizations expressed thanks to the survey authors.
“COVID-19 is a global pandemic and cancer patients all around the world have similar worries, concerns and questions ― we are a small/medium organisation working in one country but believe in the power of community and coalitions and so this survey is a very welcome part of looking at this from a greater perspective,” commented one British group.
Reid has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The COVID-19 pandemic has disrupted every aspect of cancer care, from diagnosis, treatment, and follow-up to participation in clinical trials, according to a new report that collected responses from cancer patient organizations around the world.
The report includes responses from 157 organizations in 56 countries, representing some 350,000 patients with cancer.
“The COVID-19 global pandemic has quite literally wreaked havoc with all of our lives but especially for cancer patients,” said the report’s author, Frances Reid, MBA, program director, World Ovarian Cancer Coalition.
“To those who have the power or influence to ensure that cancer treatment and services are not set back several years, please listen to those organizations who can articulate clearly the impact on patients, work with them, and act on it as soon as you can,” she added.
The new report, entitled “The Impact of COVID-19 on Cancer Patient Organisations,” was released on June 12. The organizations were surveyed from May 11 to May 25.
Cancer diagnosis
Two-thirds of the organizations surveyed said cancer screening programs had been canceled in their country, and 59% indicated they had seen a drop in urgent referrals for suspected cancer.
Some 44% said that access to pathology services had been reduced. One group in Australia reported that “results of pathology tests are taking longer to be returned. Generally a result would be returned within 48 hours. Since COVID-19, results are taking up to 7 days to be returned.”
As for treatment, 68% of organizations reported delays or cancellations of surgery or other treatments; 58% reported there had been a need to modify treatment protocols; and 48% indicated there had been a drop in participation in clinical trials.
Respondents were also concerned about reported increases in stress, anxiety, and isolation among many cancer patients. “Often at increased risk of infection and serious illness themselves ... many have been required to ‘shield’ from others, totally withdrawing from life outside their homes, thus increasing the already high levels of isolation they feel because of their life-limiting conditions,” the report notes.
In addition, some 60% of the organizations said that the pandemic had increased financial hardship among cancer patients. One US group commented: “Unemployment levels in the States similar to depression era. This has been a real challenge as many have lost insurance as well as jobs.”
Only a minority of respondents reported that cancer care was being offered in hospitals with no special arrangements in place to treat concomitant COVID-19 patients.
On the other hand, only 15% of respondents indicated that patients were being treated in a hospital that was not also caring for COVID-19 patients.
“Cancer will not wait for COVID-19 to pass, if it ever will, and the patient organizations are the key to minimizing the devastating impact [COVID-19 is having] on people with cancer,” Reid emphasized.
“More than ever, the patient/support services should be strengthened,” commented a group from France.
Patient services affected
“Almost all organisations (89%) have had to alter their services for people with cancer,” the report notes.
Two thirds of organizations involved in professional educational activities have had to change their services in some way, either by moving them online or stopping programs altogether, at least temporarily. “Some found that doctors and nurses are too busy with the pandemic to participate, and that their appetite for such activity is also diminished,” the report notes.
The volume of phone calls and emails increased in almost 6 of 10 organizations that provide support services for patients. Compared to prepandemic levels, volume increased by an average of 44%.
The most common queries raised by people with cancer (accounting for 85% of all queries) were questions about the risks of contracting COVID-19 and cancer treatments during the pandemic.
Some of the organizations also commented about how they had been affected. One group from Uganda said: “We had a sudden lockdown and we could not access office to give face to face counselling. We stopped research due to national guidelines on research. We continued giving information via phone and social media especially WhatsApp. We created groups for patients and counsellors to continue interacting.”
A group in Costa Rica reported: “We developed a new program of transfers from their homes to the hospital for cancer patients in chemotherapy and radiotherapy. 200 monthly transfers. We created a virtual community instead of our face-to-face support group, we started in April and we have 108 members, virtual sessions are held every two weeks.”
An organization based in the United States reported that it was “totally revamping our educational programs to be delivered in new ways in an online format ― not just replicating the in-person formats, but reaching out to our community and asking them what they would find the most valuable.”
Impact on fundraising
Almost 9 in 10 organizations raise funds to support their activities, the report notes. “A shocking 79% of organisations say they predict a fall in income over the next 12 months, with a further 16% not sure, leaving only 5% confident of their financial stability.”
Every type of fund-raising has been affected by COVID-19, from grants and major donors to community fund-raising events. Sixty percent of organisations said they were trying to find new ways to raise funds.
However, as one organization in Japan noted: “At the moment we can survive and feel it is unethical to ask the public for money when many are facing dire financial personal circumstances.”
A group from Australia commented: “Fundraising has been extremely difficult due to COVID-19 with distancing laws and no group gatherings as well as the economic downturn. Crisis appeals have been unsuccessful and all outdoor events and major events have been cancelled. In Australia we have had to contend with also the fires earlier in the year where a lot of money was donated to leaving other foundations struggling to get donor support.”
A little more than half (55%) of the organizations surveyed have had to cut costs.
Staffing cuts have been made in 1 in 10 of the organizations surveyed. A similar proportion of organizations have furloughed staff. Many if not all staff from numerous organizations are working from home.
A little more than half of those surveyed either provide funding for research or conduct research themselves, but only one quarter of them indicated there had been no change in their research projects. The others have indicated that they had to either reduce the scope of their research, put it on pause, or stop it altogether.
Three quarters of survey respondents noted that they had engaged in advocacy activities prior to the pandemic, and almost two thirds of them said they had to delay these activities.
Several of the organizations expressed thanks to the survey authors.
“COVID-19 is a global pandemic and cancer patients all around the world have similar worries, concerns and questions ― we are a small/medium organisation working in one country but believe in the power of community and coalitions and so this survey is a very welcome part of looking at this from a greater perspective,” commented one British group.
Reid has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Dairy doesn’t do a body good in midlife women
Dairy consumption does not improve bone mineral density (BMD) or reduce the risk of osteoporotic fracture in women starting menopause, a new analysis of the Study of Women’s Health Across the Nation (SWAN) indicates.
And this was regardless of baseline menopausal status, say Taylor Wallace, PhD, of George Mason University, Fairfax, Va., and colleagues in their article published online in Menopause.
“Our previous work indicated a potential premenopausal critical window in regard to the effectiveness of calcium supplements,” they noted.
Clifford Rosen, MD, professor of medicine, Tufts University, Boston, said in an interview that he believes the study reinforces earlier work that dairy intake in women aged 45-55 years does not affect the rate of bone loss or fractures.
“The SWAN study is longitudinal and with sufficient numbers to support their conclusion,” Dr. Rosen said.
SWAN study: White women consume the most dairy
As dairy is known to be one of the foremost sources of calcium, along with other bone beneficial nutrients, Dr. Wallace and colleagues decided to examine intake of this food type with long-term bone health using the SWAN data.
The SWAN bone substudy started in 1996 and involved 3,302 pre- or early perimenopausal women aged 42-53 years. The sample size for the annualized rate of BMD loss and fracture analysis involved 1955 women.
A modified food frequency questionnaire was used at baseline, at visit 5, and again at visit 9 to record daily dairy consumption, among many other food items.
“Women were classified into four dairy groups based on this cumulative average dairy intake,” Wallace and colleagues note. Intake was grouped into < 0.5 servings/day; 0.5 to < 1.5 servings/day; 1.5 to < 2.5 servings/day, and ≥ 2.5 servings/day.
“Non-Hispanic white individuals were more likely to consume higher amounts of dairy compared to African American, Chinese, and Japanese individuals,” the authors noted.
They found no significant differences for baseline age, body mass index, femoral neck and lumbar spine BMD, calcium supplement use, or fracture history by dairy intake group.
There were also no differences in the hazard ratios or relative risk of nontraumatic fractures by frequency of daily dairy intake.
Findings on dairy and bone are inconsistent
The authors caution that several factors should be taken into account when considering these new findings.
“First, dairy intake was low [overall] among SWAN participants, with 65% reporting consumption of < 1.5 servings/day,” they point out.
Dairy intake was also “particularly low” among racial groups other than whites, which may be due to higher rates of lactose intolerance among ethnic minorities, they speculate.
They previously reported that the use of calcium dietary supplements in SWAN was associated with a lower annualized rate of femoral neck BMD loss as well as BMD loss at the lumbar spine over 10 years of follow-up, mainly in women who were premenopausal at baseline.
But no associations were observed in the risk of bone fracture in any women in that analysis, regardless of menopausal status.
In this new analysis, there were no significant differences in calcium supplement use across the dairy intake groups.
Dr. Wallace and colleagues also noted that the relevance of dairy product intake for bone health has been in question as some observational studies have even “suggested consumption to be associated with an increased risk of fractures.”
The lead author of one of these studies, Karl Michaelsson, MD, PhD, of Uppsala (Sweden) University, said in an interview that his study had looked only at milk intake, and the lack of benefit on bone health from high milk consumption may not apply to all dairy products.
We “may need to look at different types of dairy products,” he said.
Summing up, Stephanie Faubion, MD, MBA, medical director of the North American Menopause Society, said the new SWAN findings do add to the evidence base, “albeit inconsistent ... suggesting a lack of benefit from dairy intake on BMD and fracture risk.”
Vitamin D data were not available; dairy may help in this respect
Dr. Rosen also noted that no information was available on vitamin D levels in patients involved in SWAN, which he believes is a limitation of the study.
Nevertheless, “it is important to recognize that elderly individuals who increase their dairy intake may have health benefits as recognized in the Nurses’ Health Study, possibly due to increased protein intake, higher vitamin D levels, or greater calcium intake,” he observed.
A randomized trial of enhanced dairy intake in long-term care residents is currently underway, which should provide answers for a much more vulnerable population than those in the SWAN cohort, Dr. Rosen concluded.
Dr. Wallace has reported serving on the scientific advisory board of the Vitamin Shoppe and has received research support from the National Dairy Council and scientific consulting fees from several food companies. Dr. Rosen has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Dairy consumption does not improve bone mineral density (BMD) or reduce the risk of osteoporotic fracture in women starting menopause, a new analysis of the Study of Women’s Health Across the Nation (SWAN) indicates.
And this was regardless of baseline menopausal status, say Taylor Wallace, PhD, of George Mason University, Fairfax, Va., and colleagues in their article published online in Menopause.
“Our previous work indicated a potential premenopausal critical window in regard to the effectiveness of calcium supplements,” they noted.
Clifford Rosen, MD, professor of medicine, Tufts University, Boston, said in an interview that he believes the study reinforces earlier work that dairy intake in women aged 45-55 years does not affect the rate of bone loss or fractures.
“The SWAN study is longitudinal and with sufficient numbers to support their conclusion,” Dr. Rosen said.
SWAN study: White women consume the most dairy
As dairy is known to be one of the foremost sources of calcium, along with other bone beneficial nutrients, Dr. Wallace and colleagues decided to examine intake of this food type with long-term bone health using the SWAN data.
The SWAN bone substudy started in 1996 and involved 3,302 pre- or early perimenopausal women aged 42-53 years. The sample size for the annualized rate of BMD loss and fracture analysis involved 1955 women.
A modified food frequency questionnaire was used at baseline, at visit 5, and again at visit 9 to record daily dairy consumption, among many other food items.
“Women were classified into four dairy groups based on this cumulative average dairy intake,” Wallace and colleagues note. Intake was grouped into < 0.5 servings/day; 0.5 to < 1.5 servings/day; 1.5 to < 2.5 servings/day, and ≥ 2.5 servings/day.
“Non-Hispanic white individuals were more likely to consume higher amounts of dairy compared to African American, Chinese, and Japanese individuals,” the authors noted.
They found no significant differences for baseline age, body mass index, femoral neck and lumbar spine BMD, calcium supplement use, or fracture history by dairy intake group.
There were also no differences in the hazard ratios or relative risk of nontraumatic fractures by frequency of daily dairy intake.
Findings on dairy and bone are inconsistent
The authors caution that several factors should be taken into account when considering these new findings.
“First, dairy intake was low [overall] among SWAN participants, with 65% reporting consumption of < 1.5 servings/day,” they point out.
Dairy intake was also “particularly low” among racial groups other than whites, which may be due to higher rates of lactose intolerance among ethnic minorities, they speculate.
They previously reported that the use of calcium dietary supplements in SWAN was associated with a lower annualized rate of femoral neck BMD loss as well as BMD loss at the lumbar spine over 10 years of follow-up, mainly in women who were premenopausal at baseline.
But no associations were observed in the risk of bone fracture in any women in that analysis, regardless of menopausal status.
In this new analysis, there were no significant differences in calcium supplement use across the dairy intake groups.
Dr. Wallace and colleagues also noted that the relevance of dairy product intake for bone health has been in question as some observational studies have even “suggested consumption to be associated with an increased risk of fractures.”
The lead author of one of these studies, Karl Michaelsson, MD, PhD, of Uppsala (Sweden) University, said in an interview that his study had looked only at milk intake, and the lack of benefit on bone health from high milk consumption may not apply to all dairy products.
We “may need to look at different types of dairy products,” he said.
Summing up, Stephanie Faubion, MD, MBA, medical director of the North American Menopause Society, said the new SWAN findings do add to the evidence base, “albeit inconsistent ... suggesting a lack of benefit from dairy intake on BMD and fracture risk.”
Vitamin D data were not available; dairy may help in this respect
Dr. Rosen also noted that no information was available on vitamin D levels in patients involved in SWAN, which he believes is a limitation of the study.
Nevertheless, “it is important to recognize that elderly individuals who increase their dairy intake may have health benefits as recognized in the Nurses’ Health Study, possibly due to increased protein intake, higher vitamin D levels, or greater calcium intake,” he observed.
A randomized trial of enhanced dairy intake in long-term care residents is currently underway, which should provide answers for a much more vulnerable population than those in the SWAN cohort, Dr. Rosen concluded.
Dr. Wallace has reported serving on the scientific advisory board of the Vitamin Shoppe and has received research support from the National Dairy Council and scientific consulting fees from several food companies. Dr. Rosen has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Dairy consumption does not improve bone mineral density (BMD) or reduce the risk of osteoporotic fracture in women starting menopause, a new analysis of the Study of Women’s Health Across the Nation (SWAN) indicates.
And this was regardless of baseline menopausal status, say Taylor Wallace, PhD, of George Mason University, Fairfax, Va., and colleagues in their article published online in Menopause.
“Our previous work indicated a potential premenopausal critical window in regard to the effectiveness of calcium supplements,” they noted.
Clifford Rosen, MD, professor of medicine, Tufts University, Boston, said in an interview that he believes the study reinforces earlier work that dairy intake in women aged 45-55 years does not affect the rate of bone loss or fractures.
“The SWAN study is longitudinal and with sufficient numbers to support their conclusion,” Dr. Rosen said.
SWAN study: White women consume the most dairy
As dairy is known to be one of the foremost sources of calcium, along with other bone beneficial nutrients, Dr. Wallace and colleagues decided to examine intake of this food type with long-term bone health using the SWAN data.
The SWAN bone substudy started in 1996 and involved 3,302 pre- or early perimenopausal women aged 42-53 years. The sample size for the annualized rate of BMD loss and fracture analysis involved 1955 women.
A modified food frequency questionnaire was used at baseline, at visit 5, and again at visit 9 to record daily dairy consumption, among many other food items.
“Women were classified into four dairy groups based on this cumulative average dairy intake,” Wallace and colleagues note. Intake was grouped into < 0.5 servings/day; 0.5 to < 1.5 servings/day; 1.5 to < 2.5 servings/day, and ≥ 2.5 servings/day.
“Non-Hispanic white individuals were more likely to consume higher amounts of dairy compared to African American, Chinese, and Japanese individuals,” the authors noted.
They found no significant differences for baseline age, body mass index, femoral neck and lumbar spine BMD, calcium supplement use, or fracture history by dairy intake group.
There were also no differences in the hazard ratios or relative risk of nontraumatic fractures by frequency of daily dairy intake.
Findings on dairy and bone are inconsistent
The authors caution that several factors should be taken into account when considering these new findings.
“First, dairy intake was low [overall] among SWAN participants, with 65% reporting consumption of < 1.5 servings/day,” they point out.
Dairy intake was also “particularly low” among racial groups other than whites, which may be due to higher rates of lactose intolerance among ethnic minorities, they speculate.
They previously reported that the use of calcium dietary supplements in SWAN was associated with a lower annualized rate of femoral neck BMD loss as well as BMD loss at the lumbar spine over 10 years of follow-up, mainly in women who were premenopausal at baseline.
But no associations were observed in the risk of bone fracture in any women in that analysis, regardless of menopausal status.
In this new analysis, there were no significant differences in calcium supplement use across the dairy intake groups.
Dr. Wallace and colleagues also noted that the relevance of dairy product intake for bone health has been in question as some observational studies have even “suggested consumption to be associated with an increased risk of fractures.”
The lead author of one of these studies, Karl Michaelsson, MD, PhD, of Uppsala (Sweden) University, said in an interview that his study had looked only at milk intake, and the lack of benefit on bone health from high milk consumption may not apply to all dairy products.
We “may need to look at different types of dairy products,” he said.
Summing up, Stephanie Faubion, MD, MBA, medical director of the North American Menopause Society, said the new SWAN findings do add to the evidence base, “albeit inconsistent ... suggesting a lack of benefit from dairy intake on BMD and fracture risk.”
Vitamin D data were not available; dairy may help in this respect
Dr. Rosen also noted that no information was available on vitamin D levels in patients involved in SWAN, which he believes is a limitation of the study.
Nevertheless, “it is important to recognize that elderly individuals who increase their dairy intake may have health benefits as recognized in the Nurses’ Health Study, possibly due to increased protein intake, higher vitamin D levels, or greater calcium intake,” he observed.
A randomized trial of enhanced dairy intake in long-term care residents is currently underway, which should provide answers for a much more vulnerable population than those in the SWAN cohort, Dr. Rosen concluded.
Dr. Wallace has reported serving on the scientific advisory board of the Vitamin Shoppe and has received research support from the National Dairy Council and scientific consulting fees from several food companies. Dr. Rosen has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Clinicians still unaware of need for genetic testing in NSCLC
Moreover, the majority of these clinicians believe that fewer than 50% of patients in their country undergo molecular testing, the same survey showed.
The survey was conducted by the International Association for the Study of Lung Cancer (IASLC); 2537 questionnaires from 102 countries were returned and analyzed.
It was published online May 20 in the Journal of Thoracic Oncology.
The results are concerning because “the risk of death for patients with NSCLC is substantially reduced when a gene alteration is identified and the available targeted therapy is administered,” the authors emphasize.
“Specific protocols to initiate reflex testing for guideline-recommended molecular markers would help providers consider molecular testing earlier and optimize tissue,” they suggest.
Surprised that clinicians were unaware of guidelines
“I was not surprised that we found suboptimal testing rates based on other research that has demonstrated the need to improve the quality of lung cancer in some areas,” corresponding author Matthew Smeltzer, PhD, University of Memphis, Tennessee, told Medscape Medical News in an email.
“However, I was surprised that so many respondents were unaware of guidelines,” he said.
The College of American Pathologists, IASLC, and Association for Molecular Pathology established evidence-based standards for the selection of NSCLC patients for molecular testing in 2013, and these guidelines were subsequently endorsed by the American Society of Clinical Oncology.
“We suspect that the level of access a provider has to targeted therapies does affect molecular testing rates,” Smeltzer acknowledged.
Molecular testing survey
“The survey included a seven-question introduction for all respondents and then divided respondents into one of three tracks,” the authors explain.
These tracks included respondents who requested tests and who treated patients (medical oncologists), those who analyzed and interpreted assays (pathologists), and those who acquired tissue samples (surgeons, pulmonologists, radiologists).
Countries were also grouped into five geographic regions — Asia, Europe, Latin America, United States, and Canada — and the rest of the world (ROW).
“Overall, respondents reported that molecular testing rates were lower than we would like but they were not satisfied with the current state of testing, and they reported higher testing rates in their own clinics,” Smeltzer noted.
However, when tests were ordered, “we found 99% of respondents in the requesting/treating track ordered tests for EGFR, 95% for ALK, 79% for ROS1, and < 50% ordered other tests,” the authors observe.
Indeed, EGFR, ALK, and ROS1 were the top three tests ordered across all regions, though less frequently so in the ROW, they add.
More than half of requesting/treating track respondents also order multiplex assays, although Latin America and the ROW did this less frequently than other regions.
Over 90% of respondents who perform or interpret assays indicated that they perform EGFR testing, while 83% of the same group do ALK testing; 69% tested for KRAS; 68% for BRAF, 64% for ROS1, and 56% for HER2. Fewer than half of them performed other tests.
Survey results also showed that EGFR, ALK, and KRAS are the top three tests performed across all regions, with no regional differences.
“Respondents also reported on the acquisition and testing of liquid biopsies,” survey authors point out.
Here, 87% of requesting/treating track respondents indicated that they “sometimes” request molecular testing on liquid biopsies, but the proportions of those who sometimes use liquid biopsy varied by region and were lowest in Latin America and the ROW.
A lower proportion of those who perform and interpret assays, at 69%, also offer tests on liquid biopsies, but this percentage, too, varied significantly by region, being the least frequently done in the United States and Canada, as well as in the ROW.
All the above tests are for genetic mutations or alterations that guide clinicians on use of targeted therapy directed at particular mutations, for example, drugs like erlotinib for EGFR and crizotinib for ALK.
However, immunotherapy with checkpoint inhibitors has also made a big impact on the treatment of NSCLC, and the use of these agents is sometimes guided by testing for programmed cell-death ligand (PD-L1).
PD-L1 is not a molecular marker per se, the authors note.
Nevertheless, “we found that 84% of respondents in the requesting/treating track ordered PD-L1 and 68% of respondents who perform or interpret assays report PD-L1 is offered in their own lab,” the authors observe.
Smeltzer commented that both approaches — targeted therapies and immunotherapy — have made inroads into the treatment of NSCLC, in some cases replacing chemotherapy.
He emphasized that “it is important to know if a specific oncogene driver is present before initiating immunotherapy treatment,” and noted that when tissue is sent out for both types of testing, the results for PD-L1 are usually available before the results for the full molecular testing panel are back.
Barriers to testing
“The most frequent barrier to molecular testing in every region was cost,” the survey authors note.
Insufficient amount of tumor cells was the main reason for molecular testing failures along with inadequate tissue quality.
The majority of respondents who order tests and treat patients were sure that the laboratories they use perform appropriate validation of molecular tests, while almost all of those who perform or interpret assays said they perform validation tests in their labs.
Only 30% of respondents who request tests and treat patients have access to molecular testing labs within their own institutions; the remaining respondents have to outsource testing completely or partially.
Most respondents who test and treat patients also have multidisciplinary tumor boards to discuss patients with NSCLC, but almost one quarter of the same group indicated their board met less than once a month.
“Turnaround time is a barrier to molecular testing across the world,” the authors continue, with 29% of those who request tests and treat patients reporting that it typically takes 10 days or more to receive molecular testing results.
Interestingly, the highest percentage of respondents who reported this long turnaround time were in North America.
Perhaps encouragingly, 41% of respondents who perform or interpret assays indicated they were dissatisfied with the condition of molecular testing in their country, although in this regard, the United States and Canada had the lowest rates of dissatisfaction.
In fact, 39% of those who request tests and treat patients ranked the conditions of molecular testing in their country as “average or below,” while 42% of respondents in the tissue acquisition track ranked the conditions of molecular testing as average or below, the worst rankings coming from Latin America and the ROW.
Low quality of tissue samples was another reason respondents expressed dissatisfaction with the current state of molecular testing in their country.
Smeltzer is a research consultant for the Association of Community Cancer Centers.
This article first appeared on Medscape.com.
Moreover, the majority of these clinicians believe that fewer than 50% of patients in their country undergo molecular testing, the same survey showed.
The survey was conducted by the International Association for the Study of Lung Cancer (IASLC); 2537 questionnaires from 102 countries were returned and analyzed.
It was published online May 20 in the Journal of Thoracic Oncology.
The results are concerning because “the risk of death for patients with NSCLC is substantially reduced when a gene alteration is identified and the available targeted therapy is administered,” the authors emphasize.
“Specific protocols to initiate reflex testing for guideline-recommended molecular markers would help providers consider molecular testing earlier and optimize tissue,” they suggest.
Surprised that clinicians were unaware of guidelines
“I was not surprised that we found suboptimal testing rates based on other research that has demonstrated the need to improve the quality of lung cancer in some areas,” corresponding author Matthew Smeltzer, PhD, University of Memphis, Tennessee, told Medscape Medical News in an email.
“However, I was surprised that so many respondents were unaware of guidelines,” he said.
The College of American Pathologists, IASLC, and Association for Molecular Pathology established evidence-based standards for the selection of NSCLC patients for molecular testing in 2013, and these guidelines were subsequently endorsed by the American Society of Clinical Oncology.
“We suspect that the level of access a provider has to targeted therapies does affect molecular testing rates,” Smeltzer acknowledged.
Molecular testing survey
“The survey included a seven-question introduction for all respondents and then divided respondents into one of three tracks,” the authors explain.
These tracks included respondents who requested tests and who treated patients (medical oncologists), those who analyzed and interpreted assays (pathologists), and those who acquired tissue samples (surgeons, pulmonologists, radiologists).
Countries were also grouped into five geographic regions — Asia, Europe, Latin America, United States, and Canada — and the rest of the world (ROW).
“Overall, respondents reported that molecular testing rates were lower than we would like but they were not satisfied with the current state of testing, and they reported higher testing rates in their own clinics,” Smeltzer noted.
However, when tests were ordered, “we found 99% of respondents in the requesting/treating track ordered tests for EGFR, 95% for ALK, 79% for ROS1, and < 50% ordered other tests,” the authors observe.
Indeed, EGFR, ALK, and ROS1 were the top three tests ordered across all regions, though less frequently so in the ROW, they add.
More than half of requesting/treating track respondents also order multiplex assays, although Latin America and the ROW did this less frequently than other regions.
Over 90% of respondents who perform or interpret assays indicated that they perform EGFR testing, while 83% of the same group do ALK testing; 69% tested for KRAS; 68% for BRAF, 64% for ROS1, and 56% for HER2. Fewer than half of them performed other tests.
Survey results also showed that EGFR, ALK, and KRAS are the top three tests performed across all regions, with no regional differences.
“Respondents also reported on the acquisition and testing of liquid biopsies,” survey authors point out.
Here, 87% of requesting/treating track respondents indicated that they “sometimes” request molecular testing on liquid biopsies, but the proportions of those who sometimes use liquid biopsy varied by region and were lowest in Latin America and the ROW.
A lower proportion of those who perform and interpret assays, at 69%, also offer tests on liquid biopsies, but this percentage, too, varied significantly by region, being the least frequently done in the United States and Canada, as well as in the ROW.
All the above tests are for genetic mutations or alterations that guide clinicians on use of targeted therapy directed at particular mutations, for example, drugs like erlotinib for EGFR and crizotinib for ALK.
However, immunotherapy with checkpoint inhibitors has also made a big impact on the treatment of NSCLC, and the use of these agents is sometimes guided by testing for programmed cell-death ligand (PD-L1).
PD-L1 is not a molecular marker per se, the authors note.
Nevertheless, “we found that 84% of respondents in the requesting/treating track ordered PD-L1 and 68% of respondents who perform or interpret assays report PD-L1 is offered in their own lab,” the authors observe.
Smeltzer commented that both approaches — targeted therapies and immunotherapy — have made inroads into the treatment of NSCLC, in some cases replacing chemotherapy.
He emphasized that “it is important to know if a specific oncogene driver is present before initiating immunotherapy treatment,” and noted that when tissue is sent out for both types of testing, the results for PD-L1 are usually available before the results for the full molecular testing panel are back.
Barriers to testing
“The most frequent barrier to molecular testing in every region was cost,” the survey authors note.
Insufficient amount of tumor cells was the main reason for molecular testing failures along with inadequate tissue quality.
The majority of respondents who order tests and treat patients were sure that the laboratories they use perform appropriate validation of molecular tests, while almost all of those who perform or interpret assays said they perform validation tests in their labs.
Only 30% of respondents who request tests and treat patients have access to molecular testing labs within their own institutions; the remaining respondents have to outsource testing completely or partially.
Most respondents who test and treat patients also have multidisciplinary tumor boards to discuss patients with NSCLC, but almost one quarter of the same group indicated their board met less than once a month.
“Turnaround time is a barrier to molecular testing across the world,” the authors continue, with 29% of those who request tests and treat patients reporting that it typically takes 10 days or more to receive molecular testing results.
Interestingly, the highest percentage of respondents who reported this long turnaround time were in North America.
Perhaps encouragingly, 41% of respondents who perform or interpret assays indicated they were dissatisfied with the condition of molecular testing in their country, although in this regard, the United States and Canada had the lowest rates of dissatisfaction.
In fact, 39% of those who request tests and treat patients ranked the conditions of molecular testing in their country as “average or below,” while 42% of respondents in the tissue acquisition track ranked the conditions of molecular testing as average or below, the worst rankings coming from Latin America and the ROW.
Low quality of tissue samples was another reason respondents expressed dissatisfaction with the current state of molecular testing in their country.
Smeltzer is a research consultant for the Association of Community Cancer Centers.
This article first appeared on Medscape.com.
Moreover, the majority of these clinicians believe that fewer than 50% of patients in their country undergo molecular testing, the same survey showed.
The survey was conducted by the International Association for the Study of Lung Cancer (IASLC); 2537 questionnaires from 102 countries were returned and analyzed.
It was published online May 20 in the Journal of Thoracic Oncology.
The results are concerning because “the risk of death for patients with NSCLC is substantially reduced when a gene alteration is identified and the available targeted therapy is administered,” the authors emphasize.
“Specific protocols to initiate reflex testing for guideline-recommended molecular markers would help providers consider molecular testing earlier and optimize tissue,” they suggest.
Surprised that clinicians were unaware of guidelines
“I was not surprised that we found suboptimal testing rates based on other research that has demonstrated the need to improve the quality of lung cancer in some areas,” corresponding author Matthew Smeltzer, PhD, University of Memphis, Tennessee, told Medscape Medical News in an email.
“However, I was surprised that so many respondents were unaware of guidelines,” he said.
The College of American Pathologists, IASLC, and Association for Molecular Pathology established evidence-based standards for the selection of NSCLC patients for molecular testing in 2013, and these guidelines were subsequently endorsed by the American Society of Clinical Oncology.
“We suspect that the level of access a provider has to targeted therapies does affect molecular testing rates,” Smeltzer acknowledged.
Molecular testing survey
“The survey included a seven-question introduction for all respondents and then divided respondents into one of three tracks,” the authors explain.
These tracks included respondents who requested tests and who treated patients (medical oncologists), those who analyzed and interpreted assays (pathologists), and those who acquired tissue samples (surgeons, pulmonologists, radiologists).
Countries were also grouped into five geographic regions — Asia, Europe, Latin America, United States, and Canada — and the rest of the world (ROW).
“Overall, respondents reported that molecular testing rates were lower than we would like but they were not satisfied with the current state of testing, and they reported higher testing rates in their own clinics,” Smeltzer noted.
However, when tests were ordered, “we found 99% of respondents in the requesting/treating track ordered tests for EGFR, 95% for ALK, 79% for ROS1, and < 50% ordered other tests,” the authors observe.
Indeed, EGFR, ALK, and ROS1 were the top three tests ordered across all regions, though less frequently so in the ROW, they add.
More than half of requesting/treating track respondents also order multiplex assays, although Latin America and the ROW did this less frequently than other regions.
Over 90% of respondents who perform or interpret assays indicated that they perform EGFR testing, while 83% of the same group do ALK testing; 69% tested for KRAS; 68% for BRAF, 64% for ROS1, and 56% for HER2. Fewer than half of them performed other tests.
Survey results also showed that EGFR, ALK, and KRAS are the top three tests performed across all regions, with no regional differences.
“Respondents also reported on the acquisition and testing of liquid biopsies,” survey authors point out.
Here, 87% of requesting/treating track respondents indicated that they “sometimes” request molecular testing on liquid biopsies, but the proportions of those who sometimes use liquid biopsy varied by region and were lowest in Latin America and the ROW.
A lower proportion of those who perform and interpret assays, at 69%, also offer tests on liquid biopsies, but this percentage, too, varied significantly by region, being the least frequently done in the United States and Canada, as well as in the ROW.
All the above tests are for genetic mutations or alterations that guide clinicians on use of targeted therapy directed at particular mutations, for example, drugs like erlotinib for EGFR and crizotinib for ALK.
However, immunotherapy with checkpoint inhibitors has also made a big impact on the treatment of NSCLC, and the use of these agents is sometimes guided by testing for programmed cell-death ligand (PD-L1).
PD-L1 is not a molecular marker per se, the authors note.
Nevertheless, “we found that 84% of respondents in the requesting/treating track ordered PD-L1 and 68% of respondents who perform or interpret assays report PD-L1 is offered in their own lab,” the authors observe.
Smeltzer commented that both approaches — targeted therapies and immunotherapy — have made inroads into the treatment of NSCLC, in some cases replacing chemotherapy.
He emphasized that “it is important to know if a specific oncogene driver is present before initiating immunotherapy treatment,” and noted that when tissue is sent out for both types of testing, the results for PD-L1 are usually available before the results for the full molecular testing panel are back.
Barriers to testing
“The most frequent barrier to molecular testing in every region was cost,” the survey authors note.
Insufficient amount of tumor cells was the main reason for molecular testing failures along with inadequate tissue quality.
The majority of respondents who order tests and treat patients were sure that the laboratories they use perform appropriate validation of molecular tests, while almost all of those who perform or interpret assays said they perform validation tests in their labs.
Only 30% of respondents who request tests and treat patients have access to molecular testing labs within their own institutions; the remaining respondents have to outsource testing completely or partially.
Most respondents who test and treat patients also have multidisciplinary tumor boards to discuss patients with NSCLC, but almost one quarter of the same group indicated their board met less than once a month.
“Turnaround time is a barrier to molecular testing across the world,” the authors continue, with 29% of those who request tests and treat patients reporting that it typically takes 10 days or more to receive molecular testing results.
Interestingly, the highest percentage of respondents who reported this long turnaround time were in North America.
Perhaps encouragingly, 41% of respondents who perform or interpret assays indicated they were dissatisfied with the condition of molecular testing in their country, although in this regard, the United States and Canada had the lowest rates of dissatisfaction.
In fact, 39% of those who request tests and treat patients ranked the conditions of molecular testing in their country as “average or below,” while 42% of respondents in the tissue acquisition track ranked the conditions of molecular testing as average or below, the worst rankings coming from Latin America and the ROW.
Low quality of tissue samples was another reason respondents expressed dissatisfaction with the current state of molecular testing in their country.
Smeltzer is a research consultant for the Association of Community Cancer Centers.
This article first appeared on Medscape.com.
Single negative colonoscopy predicts low colorectal cancer risk
a new study concludes.
The population-based study showed a durable reduction in CRC risk over 17.4 years of follow-up.
“Our findings confirm that a 10-year interval between high-quality screening colonoscopies [as is currently recommended] is safe and that there is no benefit from more frequent screening,” lead author Nastazja Pilonis, MD, from the Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw, Poland, told Medscape Medical News.
“Furthermore, our findings suggest that this interval could even be prolonged, provided the baseline colonoscopy is of high quality,” she added.
However, she emphasized that “only high-quality colonoscopy provided a durable reduction in mortality risk,” and noted that “low-quality colonoscopy was associated with a significantly increased risk of CRC death after the first 5 years following the examination.”
The study was published online May 25 in the Annals of Internal Medicine.
Polish Colonoscopy Screening Program
The study included 165,887 average-risk patients enrolled in the Polish Colonoscopy Screening Program who had a single negative screening colonoscopy between October 2000 and December 2011.
Negative colonoscopy was defined as an examination where no evidence of any neoplastic lesion was found.
A high-quality screening colonoscopy was defined by three key properties: cecal intubation, adequate bowel preparation, and an endoscopist’s adenoma detection rate (ADR) of 20% or greater calculated on a yearly basis.
A total of 505 different endoscopists performed the colonoscopies over a median follow-up of 10.1 years.
Compared with the general population, among individuals with a negative colonoscopy, the incidence of CRC was 72% lower and CRC mortality was 81% lower over a period of 5.1 to 10 years, Pilonis and colleagues report.
“This was mainly driven by long-lasting reductions in CRC incidence and mortality (by 84% and 90%, respectively) after high-quality screening colonoscopies,” the investigators emphasize.
Beyond 10 years of follow-up, reductions in CRC incidence and mortality were similar to those observed for the earlier period of 5.1 to 10 years but only for participants who had had a high-quality screening colonoscopy, they emphasize.
Subgroup analyses
In addition, subgroup analyses showed that high-quality colonoscopy – although not those of low-quality – effectively reduced the incidence of, and mortality from, CRC in women and in the proximal colon.
As Pilonis pointed out, previous studies have suggested that women may not benefit from screening colonoscopy to the same extent as men. Plus previous research suggests a reduced CRC risk in the proximal colon relative to that in the distal colon.
Overall, standardized incidence ratios (SIRs) and standardized mortality ratios (SMRs) significantly differed between men and women in the current study, but this difference was not observed after high-quality examinations, the investigators report.
“This is an extremely important finding because, for the first time, we showed that when you have high-quality colonoscopy, women benefit from screening colonoscopy as much as men,” Pilonis emphasized.
Similarly, high-quality screening colonoscopy was associated with a 50% reduction in mortality in the proximal colon throughout the 17.4-year follow-up, whereas there was no decrease in mortality from CRC in the proximal colon with low-quality colonoscopies.
As Pilonis noted, lesions in the proximal colon are more subtle and are harder to detect.”It’s also easier to achieve good bowel preparation in the distal colon than in the proximal colon,” she added.
Women are also more prone to develop lesions in the right (proximal) side of the colon and appear to have more pain with colonoscopy than men, all of which could have contributed to previous reports of colonoscopy not being very effective in women or for the detection of lesions in the proximal colon, as Pilonis suggested.
As the authors explain, current guidelines recommend a 10-year screening interval for the average-risk patient when colonoscopy results are negative.
This interval was partially based on the estimated time it was thought to take an adenoma to progress to a carcinoma and partially on the estimated sensitivity of screening colonoscopy.
“We showed that high-quality is a prerequisite for safe intervals between colonoscopies, Pilonis said. “So I would say that if, at a certain age, a patient has a negative colonoscopy of high-quality, a negative colonoscopy is highly predictive of a very low future risk of CRC,” she added.
The study was funded by the Polish Ministry of Health.
This article first appeared on Medscape.com.
a new study concludes.
The population-based study showed a durable reduction in CRC risk over 17.4 years of follow-up.
“Our findings confirm that a 10-year interval between high-quality screening colonoscopies [as is currently recommended] is safe and that there is no benefit from more frequent screening,” lead author Nastazja Pilonis, MD, from the Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw, Poland, told Medscape Medical News.
“Furthermore, our findings suggest that this interval could even be prolonged, provided the baseline colonoscopy is of high quality,” she added.
However, she emphasized that “only high-quality colonoscopy provided a durable reduction in mortality risk,” and noted that “low-quality colonoscopy was associated with a significantly increased risk of CRC death after the first 5 years following the examination.”
The study was published online May 25 in the Annals of Internal Medicine.
Polish Colonoscopy Screening Program
The study included 165,887 average-risk patients enrolled in the Polish Colonoscopy Screening Program who had a single negative screening colonoscopy between October 2000 and December 2011.
Negative colonoscopy was defined as an examination where no evidence of any neoplastic lesion was found.
A high-quality screening colonoscopy was defined by three key properties: cecal intubation, adequate bowel preparation, and an endoscopist’s adenoma detection rate (ADR) of 20% or greater calculated on a yearly basis.
A total of 505 different endoscopists performed the colonoscopies over a median follow-up of 10.1 years.
Compared with the general population, among individuals with a negative colonoscopy, the incidence of CRC was 72% lower and CRC mortality was 81% lower over a period of 5.1 to 10 years, Pilonis and colleagues report.
“This was mainly driven by long-lasting reductions in CRC incidence and mortality (by 84% and 90%, respectively) after high-quality screening colonoscopies,” the investigators emphasize.
Beyond 10 years of follow-up, reductions in CRC incidence and mortality were similar to those observed for the earlier period of 5.1 to 10 years but only for participants who had had a high-quality screening colonoscopy, they emphasize.
Subgroup analyses
In addition, subgroup analyses showed that high-quality colonoscopy – although not those of low-quality – effectively reduced the incidence of, and mortality from, CRC in women and in the proximal colon.
As Pilonis pointed out, previous studies have suggested that women may not benefit from screening colonoscopy to the same extent as men. Plus previous research suggests a reduced CRC risk in the proximal colon relative to that in the distal colon.
Overall, standardized incidence ratios (SIRs) and standardized mortality ratios (SMRs) significantly differed between men and women in the current study, but this difference was not observed after high-quality examinations, the investigators report.
“This is an extremely important finding because, for the first time, we showed that when you have high-quality colonoscopy, women benefit from screening colonoscopy as much as men,” Pilonis emphasized.
Similarly, high-quality screening colonoscopy was associated with a 50% reduction in mortality in the proximal colon throughout the 17.4-year follow-up, whereas there was no decrease in mortality from CRC in the proximal colon with low-quality colonoscopies.
As Pilonis noted, lesions in the proximal colon are more subtle and are harder to detect.”It’s also easier to achieve good bowel preparation in the distal colon than in the proximal colon,” she added.
Women are also more prone to develop lesions in the right (proximal) side of the colon and appear to have more pain with colonoscopy than men, all of which could have contributed to previous reports of colonoscopy not being very effective in women or for the detection of lesions in the proximal colon, as Pilonis suggested.
As the authors explain, current guidelines recommend a 10-year screening interval for the average-risk patient when colonoscopy results are negative.
This interval was partially based on the estimated time it was thought to take an adenoma to progress to a carcinoma and partially on the estimated sensitivity of screening colonoscopy.
“We showed that high-quality is a prerequisite for safe intervals between colonoscopies, Pilonis said. “So I would say that if, at a certain age, a patient has a negative colonoscopy of high-quality, a negative colonoscopy is highly predictive of a very low future risk of CRC,” she added.
The study was funded by the Polish Ministry of Health.
This article first appeared on Medscape.com.
a new study concludes.
The population-based study showed a durable reduction in CRC risk over 17.4 years of follow-up.
“Our findings confirm that a 10-year interval between high-quality screening colonoscopies [as is currently recommended] is safe and that there is no benefit from more frequent screening,” lead author Nastazja Pilonis, MD, from the Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw, Poland, told Medscape Medical News.
“Furthermore, our findings suggest that this interval could even be prolonged, provided the baseline colonoscopy is of high quality,” she added.
However, she emphasized that “only high-quality colonoscopy provided a durable reduction in mortality risk,” and noted that “low-quality colonoscopy was associated with a significantly increased risk of CRC death after the first 5 years following the examination.”
The study was published online May 25 in the Annals of Internal Medicine.
Polish Colonoscopy Screening Program
The study included 165,887 average-risk patients enrolled in the Polish Colonoscopy Screening Program who had a single negative screening colonoscopy between October 2000 and December 2011.
Negative colonoscopy was defined as an examination where no evidence of any neoplastic lesion was found.
A high-quality screening colonoscopy was defined by three key properties: cecal intubation, adequate bowel preparation, and an endoscopist’s adenoma detection rate (ADR) of 20% or greater calculated on a yearly basis.
A total of 505 different endoscopists performed the colonoscopies over a median follow-up of 10.1 years.
Compared with the general population, among individuals with a negative colonoscopy, the incidence of CRC was 72% lower and CRC mortality was 81% lower over a period of 5.1 to 10 years, Pilonis and colleagues report.
“This was mainly driven by long-lasting reductions in CRC incidence and mortality (by 84% and 90%, respectively) after high-quality screening colonoscopies,” the investigators emphasize.
Beyond 10 years of follow-up, reductions in CRC incidence and mortality were similar to those observed for the earlier period of 5.1 to 10 years but only for participants who had had a high-quality screening colonoscopy, they emphasize.
Subgroup analyses
In addition, subgroup analyses showed that high-quality colonoscopy – although not those of low-quality – effectively reduced the incidence of, and mortality from, CRC in women and in the proximal colon.
As Pilonis pointed out, previous studies have suggested that women may not benefit from screening colonoscopy to the same extent as men. Plus previous research suggests a reduced CRC risk in the proximal colon relative to that in the distal colon.
Overall, standardized incidence ratios (SIRs) and standardized mortality ratios (SMRs) significantly differed between men and women in the current study, but this difference was not observed after high-quality examinations, the investigators report.
“This is an extremely important finding because, for the first time, we showed that when you have high-quality colonoscopy, women benefit from screening colonoscopy as much as men,” Pilonis emphasized.
Similarly, high-quality screening colonoscopy was associated with a 50% reduction in mortality in the proximal colon throughout the 17.4-year follow-up, whereas there was no decrease in mortality from CRC in the proximal colon with low-quality colonoscopies.
As Pilonis noted, lesions in the proximal colon are more subtle and are harder to detect.”It’s also easier to achieve good bowel preparation in the distal colon than in the proximal colon,” she added.
Women are also more prone to develop lesions in the right (proximal) side of the colon and appear to have more pain with colonoscopy than men, all of which could have contributed to previous reports of colonoscopy not being very effective in women or for the detection of lesions in the proximal colon, as Pilonis suggested.
As the authors explain, current guidelines recommend a 10-year screening interval for the average-risk patient when colonoscopy results are negative.
This interval was partially based on the estimated time it was thought to take an adenoma to progress to a carcinoma and partially on the estimated sensitivity of screening colonoscopy.
“We showed that high-quality is a prerequisite for safe intervals between colonoscopies, Pilonis said. “So I would say that if, at a certain age, a patient has a negative colonoscopy of high-quality, a negative colonoscopy is highly predictive of a very low future risk of CRC,” she added.
The study was funded by the Polish Ministry of Health.
This article first appeared on Medscape.com.
World first: Saliva test detects occult HPV-driven oropharyngeal cancer
A saliva test for detecting oropharyngeal cancer caused by human papillomavirus–16 (HPV-16) has scored a world first: It detected such a cancer in an asymptomatic adult.
If the finding can be replicated in a larger cohort of healthy asymptomatic individuals, widespread screening for HPV-16 – the main driver behind skyrocketing rates of oropharyngeal cancer – could be one step closer, the researchers suggested.
“Oropharyngeal squamous cell carcinomas often presents at a late stage with patients suffering huge morbidity as a result of treatment, [so] we must find strategies to detect these cancers earlier,” senior author Chamindie Punyadeera, PhD, Queensland University of Technology in Brisbane, Australia, told Medscape Medical News in an email.
“This study, for the first time, provides a solid scientific foundation to initiate a screening trial in high-risk individuals to detect HPV-driven oropharyngeal cancer, she added. “Saliva testing could be broadly implemented and [used] in a screening trial in the future,” she said.
The case report was published online March 31 in Frontiers in Oncology.
The saliva test was developed by Dr. Punyadeera and first author Kai Dun Tang, PhD, also from Queensland University of Technology. It is administered as an oral rinse: the individual swishes a saline solution around in his or her mouth for a minute or 2, and then spits the sample into a tube.
Prevalence Study
The saliva test was being scrutinized in an ongoing HPV-16 DNA prevalence study, which involved 650 healthy participants being tested for oral HPV-16 DNA.
“Of these, 3 have been identified to have persistent oral HPV-16 DNA infection,” the investigators reported.
After having approached these three participants, one middle-aged male who had been consistently HPV-16 DNA positive for a period of 36 months – and whose HPV-16 viral load had been steadily rising over time – was invited to attend an ear, nose, and throat clinic for assessment.
“Initial clinical examination of the oropharynx including palpation and white light revealed no significant abnormalities,” the researchers emphasized.
As Dr. Punyadeera explained, standard clinical assessment for oropharyngeal malignancy includes white light examination for masses, detection of irregularities or asymmetry of the underlying structures and palpation of the tonsil and tongue base.
Cross-sectional imaging with CT or MRI can be helpful as well, she said, but these imaging studies are unable to detect lesions smaller than a few millimeters in size.
In the case of this individual, salivary oral rinse samples had been collected at baseline, and again at 6, 12, and 36 months after study enrollment as well as 2 weeks after the patient decided to undergo a bilateral tonsillectomy.
DNA was extracted from the salivary oral rinse samples, as well as from the tonsillar tissue obtained after resection. HPV-16 DNA genotyping and viral loads were analyzed with a PCR assay.
Results from the salivary samples indicated that the patient’s HPV-16 DNA viral load had increased exponentially across the 36 months of follow-up, from 3.43 copies/50 ng at baseline to 1281.69 copies/50 ng at 36 months.
On surgery, the patient was found to have a 2 mm squamous cell carcinoma in the left tonsil, but all other oropharyngeal tissues were normal and HPV-16 DNA negative.
Two weeks after undergoing the tonsillectomy, the patient’s HPV-16 DNA viral load in the saliva samples became undetectable.
This case report demonstrates that salivary HPV can both detect smaller lesions than either clinical examination or even radiological investigation, and that the same salivary test can likely also be used to monitor treatment response, Dr. Punyadeera commented.
Long-term persistence
As researchers explained in their paper, long-term persistence of HPV-16 infection is most likely a prerequisite for the development of subsequent malignancy.
Unlike cervical cancer caused by HPV-16 infection, the natural history of HPV infection in the oropharyngeal cavity is not known.
However, clinical assessment of patients with either persistent HPV infection or microscopic carcinoma has failed to detect any identifiable abnormalities.
Thus, this is the first report of a histologically confirmed diagnosis of an asymptomatic occult oropharyngeal cancer detected by a screening test through serial measurements of HPV-16 DNA, the investigators emphasized.
The report also demonstrated that very early lesions can be eradicated with minimal morbidity. Unfortunately, most oropharyngeal cancer is currently diagnosed at much later stages, and surgical removal of these is often associated with significant disabilities including difficulties with swallowing and even communicating.
“It’s amazing to think that this man was cured of his disease with a 15-minute procedure which left him with no lasting issues at all,” Dr. Punyadeera commented. “We need to try and make this the norm, not the exception.
“So we must have a well-designed screening study using all the insights we have gained from this case. We owe it to patients to explore these findings to their fullest,” Dr. Punyadeera emphasized.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A saliva test for detecting oropharyngeal cancer caused by human papillomavirus–16 (HPV-16) has scored a world first: It detected such a cancer in an asymptomatic adult.
If the finding can be replicated in a larger cohort of healthy asymptomatic individuals, widespread screening for HPV-16 – the main driver behind skyrocketing rates of oropharyngeal cancer – could be one step closer, the researchers suggested.
“Oropharyngeal squamous cell carcinomas often presents at a late stage with patients suffering huge morbidity as a result of treatment, [so] we must find strategies to detect these cancers earlier,” senior author Chamindie Punyadeera, PhD, Queensland University of Technology in Brisbane, Australia, told Medscape Medical News in an email.
“This study, for the first time, provides a solid scientific foundation to initiate a screening trial in high-risk individuals to detect HPV-driven oropharyngeal cancer, she added. “Saliva testing could be broadly implemented and [used] in a screening trial in the future,” she said.
The case report was published online March 31 in Frontiers in Oncology.
The saliva test was developed by Dr. Punyadeera and first author Kai Dun Tang, PhD, also from Queensland University of Technology. It is administered as an oral rinse: the individual swishes a saline solution around in his or her mouth for a minute or 2, and then spits the sample into a tube.
Prevalence Study
The saliva test was being scrutinized in an ongoing HPV-16 DNA prevalence study, which involved 650 healthy participants being tested for oral HPV-16 DNA.
“Of these, 3 have been identified to have persistent oral HPV-16 DNA infection,” the investigators reported.
After having approached these three participants, one middle-aged male who had been consistently HPV-16 DNA positive for a period of 36 months – and whose HPV-16 viral load had been steadily rising over time – was invited to attend an ear, nose, and throat clinic for assessment.
“Initial clinical examination of the oropharynx including palpation and white light revealed no significant abnormalities,” the researchers emphasized.
As Dr. Punyadeera explained, standard clinical assessment for oropharyngeal malignancy includes white light examination for masses, detection of irregularities or asymmetry of the underlying structures and palpation of the tonsil and tongue base.
Cross-sectional imaging with CT or MRI can be helpful as well, she said, but these imaging studies are unable to detect lesions smaller than a few millimeters in size.
In the case of this individual, salivary oral rinse samples had been collected at baseline, and again at 6, 12, and 36 months after study enrollment as well as 2 weeks after the patient decided to undergo a bilateral tonsillectomy.
DNA was extracted from the salivary oral rinse samples, as well as from the tonsillar tissue obtained after resection. HPV-16 DNA genotyping and viral loads were analyzed with a PCR assay.
Results from the salivary samples indicated that the patient’s HPV-16 DNA viral load had increased exponentially across the 36 months of follow-up, from 3.43 copies/50 ng at baseline to 1281.69 copies/50 ng at 36 months.
On surgery, the patient was found to have a 2 mm squamous cell carcinoma in the left tonsil, but all other oropharyngeal tissues were normal and HPV-16 DNA negative.
Two weeks after undergoing the tonsillectomy, the patient’s HPV-16 DNA viral load in the saliva samples became undetectable.
This case report demonstrates that salivary HPV can both detect smaller lesions than either clinical examination or even radiological investigation, and that the same salivary test can likely also be used to monitor treatment response, Dr. Punyadeera commented.
Long-term persistence
As researchers explained in their paper, long-term persistence of HPV-16 infection is most likely a prerequisite for the development of subsequent malignancy.
Unlike cervical cancer caused by HPV-16 infection, the natural history of HPV infection in the oropharyngeal cavity is not known.
However, clinical assessment of patients with either persistent HPV infection or microscopic carcinoma has failed to detect any identifiable abnormalities.
Thus, this is the first report of a histologically confirmed diagnosis of an asymptomatic occult oropharyngeal cancer detected by a screening test through serial measurements of HPV-16 DNA, the investigators emphasized.
The report also demonstrated that very early lesions can be eradicated with minimal morbidity. Unfortunately, most oropharyngeal cancer is currently diagnosed at much later stages, and surgical removal of these is often associated with significant disabilities including difficulties with swallowing and even communicating.
“It’s amazing to think that this man was cured of his disease with a 15-minute procedure which left him with no lasting issues at all,” Dr. Punyadeera commented. “We need to try and make this the norm, not the exception.
“So we must have a well-designed screening study using all the insights we have gained from this case. We owe it to patients to explore these findings to their fullest,” Dr. Punyadeera emphasized.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A saliva test for detecting oropharyngeal cancer caused by human papillomavirus–16 (HPV-16) has scored a world first: It detected such a cancer in an asymptomatic adult.
If the finding can be replicated in a larger cohort of healthy asymptomatic individuals, widespread screening for HPV-16 – the main driver behind skyrocketing rates of oropharyngeal cancer – could be one step closer, the researchers suggested.
“Oropharyngeal squamous cell carcinomas often presents at a late stage with patients suffering huge morbidity as a result of treatment, [so] we must find strategies to detect these cancers earlier,” senior author Chamindie Punyadeera, PhD, Queensland University of Technology in Brisbane, Australia, told Medscape Medical News in an email.
“This study, for the first time, provides a solid scientific foundation to initiate a screening trial in high-risk individuals to detect HPV-driven oropharyngeal cancer, she added. “Saliva testing could be broadly implemented and [used] in a screening trial in the future,” she said.
The case report was published online March 31 in Frontiers in Oncology.
The saliva test was developed by Dr. Punyadeera and first author Kai Dun Tang, PhD, also from Queensland University of Technology. It is administered as an oral rinse: the individual swishes a saline solution around in his or her mouth for a minute or 2, and then spits the sample into a tube.
Prevalence Study
The saliva test was being scrutinized in an ongoing HPV-16 DNA prevalence study, which involved 650 healthy participants being tested for oral HPV-16 DNA.
“Of these, 3 have been identified to have persistent oral HPV-16 DNA infection,” the investigators reported.
After having approached these three participants, one middle-aged male who had been consistently HPV-16 DNA positive for a period of 36 months – and whose HPV-16 viral load had been steadily rising over time – was invited to attend an ear, nose, and throat clinic for assessment.
“Initial clinical examination of the oropharynx including palpation and white light revealed no significant abnormalities,” the researchers emphasized.
As Dr. Punyadeera explained, standard clinical assessment for oropharyngeal malignancy includes white light examination for masses, detection of irregularities or asymmetry of the underlying structures and palpation of the tonsil and tongue base.
Cross-sectional imaging with CT or MRI can be helpful as well, she said, but these imaging studies are unable to detect lesions smaller than a few millimeters in size.
In the case of this individual, salivary oral rinse samples had been collected at baseline, and again at 6, 12, and 36 months after study enrollment as well as 2 weeks after the patient decided to undergo a bilateral tonsillectomy.
DNA was extracted from the salivary oral rinse samples, as well as from the tonsillar tissue obtained after resection. HPV-16 DNA genotyping and viral loads were analyzed with a PCR assay.
Results from the salivary samples indicated that the patient’s HPV-16 DNA viral load had increased exponentially across the 36 months of follow-up, from 3.43 copies/50 ng at baseline to 1281.69 copies/50 ng at 36 months.
On surgery, the patient was found to have a 2 mm squamous cell carcinoma in the left tonsil, but all other oropharyngeal tissues were normal and HPV-16 DNA negative.
Two weeks after undergoing the tonsillectomy, the patient’s HPV-16 DNA viral load in the saliva samples became undetectable.
This case report demonstrates that salivary HPV can both detect smaller lesions than either clinical examination or even radiological investigation, and that the same salivary test can likely also be used to monitor treatment response, Dr. Punyadeera commented.
Long-term persistence
As researchers explained in their paper, long-term persistence of HPV-16 infection is most likely a prerequisite for the development of subsequent malignancy.
Unlike cervical cancer caused by HPV-16 infection, the natural history of HPV infection in the oropharyngeal cavity is not known.
However, clinical assessment of patients with either persistent HPV infection or microscopic carcinoma has failed to detect any identifiable abnormalities.
Thus, this is the first report of a histologically confirmed diagnosis of an asymptomatic occult oropharyngeal cancer detected by a screening test through serial measurements of HPV-16 DNA, the investigators emphasized.
The report also demonstrated that very early lesions can be eradicated with minimal morbidity. Unfortunately, most oropharyngeal cancer is currently diagnosed at much later stages, and surgical removal of these is often associated with significant disabilities including difficulties with swallowing and even communicating.
“It’s amazing to think that this man was cured of his disease with a 15-minute procedure which left him with no lasting issues at all,” Dr. Punyadeera commented. “We need to try and make this the norm, not the exception.
“So we must have a well-designed screening study using all the insights we have gained from this case. We owe it to patients to explore these findings to their fullest,” Dr. Punyadeera emphasized.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Androgens may explain male vulnerability to COVID-19
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
Excess cancer deaths predicted as care is disrupted by COVID-19
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The majority of patients who have cancer or are suspected of having cancer are not accessing healthcare services in the United Kingdom or the United States because of the COVID-19 pandemic, the first report of its kind estimates.
As a result, there will be an excess of deaths among patients who have cancer and multiple comorbidities in both countries during the current coronavirus emergency, the report warns.
The authors calculate that there will be 6,270 excess deaths among cancer patients 1 year from now in England and 33,890 excess deaths among cancer patients in the United States. (In the United States, the estimated excess number of deaths applies only to patients older than 40 years, they note.)
“The recorded underlying cause of these excess deaths may be cancer, COVID-19, or comorbidity (such as myocardial infarction),” Alvina Lai, PhD, University College London, United Kingdom, and colleagues observe.
“Our data have highlighted how cancer patients with multimorbidity are a particularly at-risk group during the current pandemic,” they emphasize.
The study was published on ResearchGate as a preprint and has not undergone peer review.
Commenting on the study on the UK Science Media Center, several experts emphasized the lack of peer review, noting that interpretation of these data needs to be further refined on the basis of that input. One expert suggested that there are “substantial uncertainties that this paper does not adequately communicate.” But others argued that this topic was important enough to warrant early release of the data.
Chris Bunce, PhD, University of Birmingham, United Kingdom, said this study represents “a highly valuable contribution.”
“It is universally accepted that early diagnosis and treatment and adherence to treatment regimens saves lives,” he pointed out.
“Therefore, these COVID-19-related impacts will cost lives,” Bunce said.
“And if this information is to influence cancer care and guide policy during the COVID-19 crisis, then it is important that the findings are disseminated and discussed immediately, warranting their release ahead of peer view,” he added.
In a Medscape UK commentary, oncologist Karol Sikora, MD, PhD, argues that “restarting cancer services can’t come soon enough.”
“Resonably Argued Numerical Estimate”
“It’s well known that there have been considerable changes in the provision of health care for many conditions, including cancers, as a result of all the measures to deal with the COVID-19 crisis,” said Kevin McConway, PhD, professor emeritus of applied statistics, the Open University, Milton Keynes, United Kingdom.
“It seems inevitable that there will be increased deaths in cancer patients if they are infected with the virus or because of changes in the health services available to them, and quite possibly also from socio-economic effects of the responses to the crisis,” he continued.
“This study is the first that I have seen that produces a reasonably argued numerical estimate of the number of excess deaths of people with cancer arising from these factors in the UK and the USA,” he added.
Declines in Urgent Referrals and Chemo Attendance
For the study, the team used DATA-CAN, the UK National Health Data Research Hub for Cancer, to assess weekly returns for urgent cancer referrals for early diagnosis and also chemotherapy attendances for hospitals in Leeds, London, and Northern Ireland going back to 2018.
The data revealed that there have been major declines in chemotherapy attendances. There has been, on average, a 60% decrease from prepandemic levels in eight hospitals in the three regions that were assessed.
Urgent cancer referrals have dropped by an average of 76% compared to prepandemic levels in the three regions.
On the conservative assumption that the COVID-19 pandemic will only affect patients with newly diagnosed cancer (incident cases), the researchers estimate that the proportion of the population affected by the emergency (PAE) is 40% and that the relative impact of the emergency (RIE) is 1.5.
PAE is a summary measure of exposure to the adverse health consequences of the emergency; RIE is a summary measure of the combined impact on mortality of infection, health service change, physical distancing, and economic downturn, the authors explain.
Comorbidities Common
“Comorbidities were common in people with cancer,” the study authors note. For example, more than one quarter of the study population had at least one comorbidity; more than 14% had two.
For incident cancers, the number of excess deaths steadily increased in conjunction with an increase in the number of comorbidities, such that more than 80% of deaths occurred in patients with one or more comorbidities.
“When considering both prevalent and incident cancers together with a COVID-19 PAE of 40%, we estimated 17,991 excess deaths at a RIE of 1.5; 78.1% of these deaths occur in patients with ≥1 comorbidities,” the authors report.
“The excess risk of death in people living with cancer during the COVID-19 emergency may be due not only to COVID-19 infection, but also to the unintended health consequences of changes in health service provision, the physical or psychological effects of social distancing, and economic upheaval,” they state.
“This is the first study demonstrating profound recent changes in cancer care delivery in multiple centers,” the authors observe.
Lai has disclosed no relevant financial relationships. Several coauthors have various relationships with industry, as listed in their article. The commentators have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Increased risk of lung cancer with COPD, even in never smokers
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.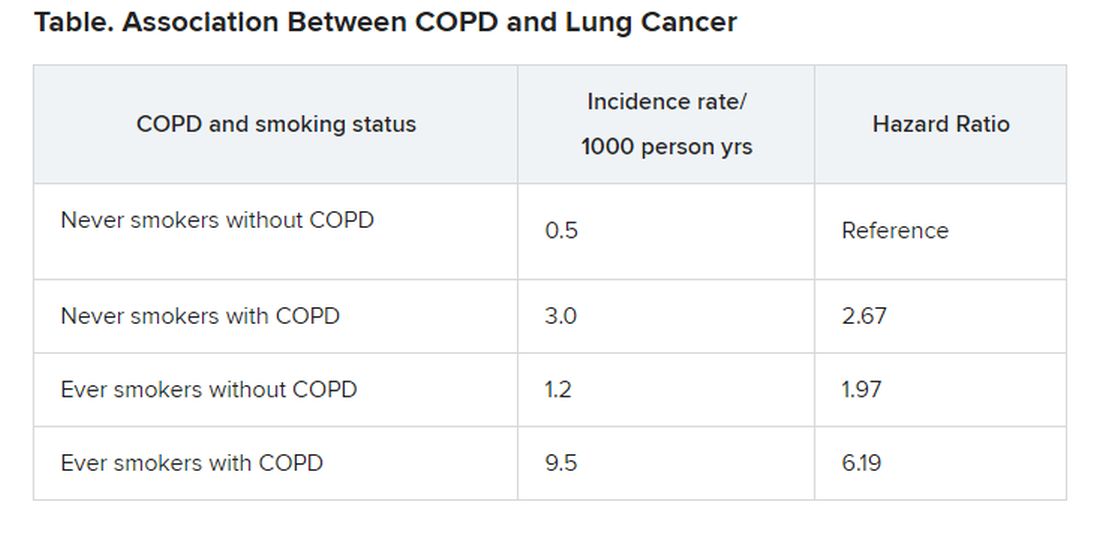
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.