User login
Hibernoma
Hibernomas are rare benign soft-tissue tumors originally described as pseudolipomas by Merkel1 in 1906. Gery coined the term hibernoma in 1914, after noting the multivacuolated cytoplasm of the tumor cells and its resemblance to normal brown fat found in hibernating animals.2
Hibernomas represent 2% of all benign fat-containing tumors and are composed of brown adipocytes, which are histologically different from the white fat of lipomas. Hibernomas usually develop between ages 20 and 40 years, and their incidence is slightly higher in males.
Diffusely present in human newborns, brown fat usually regresses by 8 weeks of age.3 Residual brown fat deposits may remain in the neck, axilla, shoulder, thorax, thigh, retroperitoneum, and periscapular/interscapular regions.4 All these vestigial areas are therefore common locations of hibernomas, with the thigh accounting for up to 30% of cases.5 These tumors are seldom identified in the abdomen, popliteal fossa, or even intracranially. Injury to brown fat cells in these locations, either by infection, inflammation, or trauma, is considered a predisposing risk factor for development of hibernomas.6
Clinical Presentation
Clinically, hibernomas present as slow-growing, painless soft-tissue masses. Physical examination usually reveals a palpable, solitary, soft, and rubbery mass within the subcutaneous fat, which is freely mobile and not attached to deep layers. These tumors may rarely produce steroid hormones and result in a paraneoplastic syndrome. Even though these tumors are usually large at presentation, compression of adjacent structures seldom occurs.
Histology and Differential Diagnosis
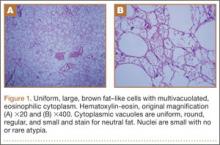
The characteristic hibernoma cell is a multivacuolated adipocyte with centrally located nucleus, indistinct nucleolus, and coarsely granular eosinophilic (or pale) cytoplasm (Figure 1). Cytoplasmic vacuoles are uniform, round, regular, and small and stain for neutral fat. Nuclei are usually small with no or rare atypia. These multivacuolated brown fat–like tumor cells usually stain positive for S100 and CD31, usually stain negative for CD34 and p53, and can show 11q13-21 rearrangements, also seen in lipomas and liposarcomas. Hibernomas have 4 histologic variants: typical (classic), myxoid, lipoma-like, and spindle-cell.5 The typical hibernoma, the most common, contains a varying mixture of brown and white fat cells. The myxoid type, second most common, is composed of hibernoma cells floating in a loose acellular myxoid stroma. The lipoma-like variant consists of a few scattered hibernoma cells in a predominance of white fat cells. The spindle-cell variant, the rarest, has features of typical hibernoma and spindle-cell lipoma.7
Grossly, hibernomas are well encapsulated, soft, and lobular with prominent feeding vessels.8 They typically are tan or brown because of their hypervascularity and abundant mitochondria. Tumor size ranges from 1 to 24 cm (mean, 9.4 cm).9 These tumors are well-defined intermuscular/intramuscular, subcutaneous, or retroperitoneal lesions that tend to grow along fascial planes and displace surrounding structures rather than invade them. Delicate branching capillaries are usually seen within the tumor.
Although rare, hibernoma should be included in the differential diagnosis of lipomatous soft-tissue tumors.10 Imaging findings of hibernoma are not specific; other differential diagnostic considerations for a mass with a signal similar to that of fat or containing large intratumoral vessels include angiolipoma, intramuscular hemangioma with fat, spindle-cell lipoma, pleomorphic lipoma, lipoblastoma, hemangiopericytoma, and hemangioblastoma,11-15 as well as malignant processes, including lipoma-like well-differentiated liposarcoma and myxoid liposarcoma.16 Other entities that should be considered include residual brown fat and rhabdomyoma.
Hibernomas are histologically distinguished from well-differentiated liposarcomas by location (liposarcomas tend to be deep), atypia, presence of a prominent “plexiform” capillary pattern, and specific molecular translocations, including t (12;16). Lipomas have lipocytes that are not multivacuolated, and residual brown fat does not present as a distinct mass. Rhabdomyomas are distinguished by an absence of cytoplasmic lipid vacuoles.
Imaging
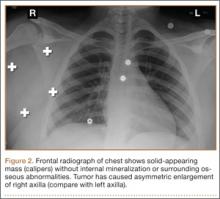
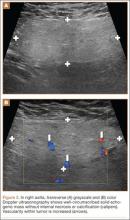
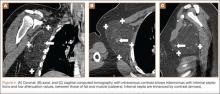
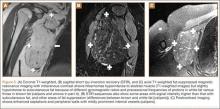
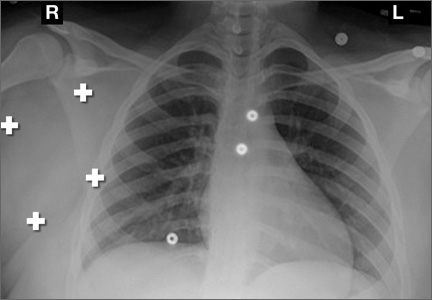
Conventional radiography may show a radiolucent mass without internal mineralization or associated osseous abnormalities4 (Figure 2). Calcifications are notably absent.17 Sonographically, hibernomas are well-circumscribed, solid, hyperechoic masses with increased internal vascular flow on both grayscale and color Doppler sampling; however their appearance is not pathognomonic (Figure 3). Angiography reveals a hypervascular tumor that may have internal arteriovenous shunting.18 Hibernomas have a heterogeneous appearance on computed tomography (CT) and magnetic resonance imaging (MRI) because of the variable distribution of brown fat cells, white fat cells, myxoid material, and spindle cells within the individual tumor subtypes.5 CT of these tumors shows internal septations and low attenuation values, between those of fat and muscle19 (Figure 4). Intravenous contrast enhances internal septa, but enhancement varies from none to intense, and from generalized to focal, depending on internal tumor composition.3,17,20-22
Hibernomas are usually hyperintense to skeletal muscle on T1-weighted MRI but slightly hypointense to subcutaneous fat because of the different gyromagnetic ratios and precessional frequencies of protons in white fat versus those in brown fat17 (Figure 5). Rarely, lesions are isointense to skeletal muscle on T1-weighted images.23 On T2-weighted images, high signal intensity similar to that of subcutaneous fat is typical.24 Flow voids can be readily identified.25 Short tau inversion recovery (STIR) MRI shows some areas with signal intensity higher than that of subcutaneous fat, and other areas of fat suppression.9 Ritchie and colleagues21 reported that hibernomas histologically composed of more than 70% multivacuolated adipocytes tended to have MRI signal characteristics different from those of subcutaneous fat, and those with less than 70% multivacuolated adipocytes tended to have signal characteristics paralleling those of subcutaneous fat. Myxoid hibernomas have higher signal intensity on T2-weighted and STIR MRI because of high water content.17,21,26,27
Hibernomas demonstrate moderate uptake on bone scintigraphy blood pool images and mild uptake on delayed images.4 Positron emission tomography (PET) is useful in differentiating hibernomas from other fat-containing lesions.9 Hibernomas demonstrate intense fluorine-18 fluorodeoxyglucose uptake because, unlike other adipogenic tumors, hibernomas contain abundant mitochondria and are highly metabolically active.28
Treatment and Prognosis
Complete surgical excision is the treatment of choice; given the behavior of the benign tumor, marginal complete excision is considered curative.5 Intralesional excision may be the only option for large tumors that are near nerves or vessels. However, intralesional excision may result in continued growth and local recurrence.
At surgery, these tumors usually are encapsulated and/or adherent to skeletal muscle or bone, without invasion, and easily separated from surrounding soft tissues.29 No specific surgical considerations are required beyond standard oncological principles, including careful dissection of adjacent nerves and vessels, and hemostasis. Hibernomas have the potential for significant bleeding during surgical excision. In this setting, embolization becomes a consideration, given the identification of large intratumoral vessels and the benign course of these lesions.
1. Merkel H. On a pseudolipoma of the breast. Beitr Pathol Anat. 1906;39:152-157.
2. Enzinger FM, Weiss SW. Benign lipomatous tumors. In: Enzinger FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis, MO: Mosby-Yearbook; 1994:420-423.
3. Alvine G, Rosenthal H, Murphey M, Huntrakoon M. Hibernoma. Skeletal Radiol. 1996;25(5):493-496.
4. Kumazoe H, Nagamatsu Y, Nishi T, Kimura YN, Nakazono T, Kudo S. Dumbbell-shaped thoracic hibernoma: computed tomography and magnetic resonance imaging findings. Jpn J Radiol. 2009;27(1):37-40.
5. Furlong MA, Fanburg-Smith JC, Miettinen M. The morphologic spectrum of hibernoma: a clinicopathologic study of 170 cases. Am J Surg Pathol. 2001;25(6):809-814.
6. Ucak A, Inan K, Onan B, Yilmaz AT. Resection of intrapericardial hibernoma associated with constrictive pericarditis. Interact Cardiovasc Thorac Surg. 2009;9(4):717-719.
7. Tomihama RT, Lindskog DM, Ahrens W, Haims AH. Hibernoma: a case report demonstrating usefulness of MR angiography in characterizing the tumor. Skeletal Radiol. 2007;36(6):541-545.
8. Choi J, Heiner J, Agni R, Hafez GR. Case of the season. Hibernoma. Semin Roentgenol. 2002;37(2):99-101.
9. Craig WD, Fanburg-Smith JC, Henry LR, Guerrero R, Barton JH. Fat-containing lesions of the retroperitoneum: radiologic-pathologic correlation. Radiographics. 2009;29(1):261-290.
10. Vassos N, Lell M, Hohenberger W, Croner RS, Agaimy A. Deep-seated huge hibernoma of soft tissue: a rare differential diagnosis of atypical lipomatous tumor/well differentiated liposarcoma. Int J Clin Exp Pathol. 2013;6(10):2178-2184.
11. Mugel T, Ghossain MA, Guinet C, et al. MR and CT findings in a case of hibernoma of the thigh extending into the pelvis. Eur Radiol. 1998;8(3):476-478.
12. Kallas KM, Vaughan L, Haghighi P, Resnick D. Hibernoma of the left axilla; a case report and review of MR imaging. Skeletal Radiol. 2003;32(5):290-294.
13. Suh JS, Cho J, Lee SH, et al. Alveolar soft part sarcoma: MR and angiographic findings. Skeletal Radiol. 2000;29(12):680-689.
14. De Beuckeleer LH, De Schepper AM, Vandevenne JE, et al. MR imaging of clear cell sarcoma (malignant melanoma of the soft parts): a multicenter correlative MRI-pathology study of 21 cases and literature review. Skeletal Radiol. 2000;29(4):187-195.
15. Chu BC, Terae S, Hida K, Furukawa M, Abe S, Miyasaka K. MR findings in spinal hemangioblastoma: correlation with symptoms and with angiographic and surgical findings. AJNR Am J Neuroradiol. 2001;22(1):206-217.
16. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509-1517.
17. Anderson SE, Schwab C, Stauffer E, Banic A, Steinbach LS. Hibernoma: imaging characteristics of a rare benign soft tissue tumor. Skeletal Radiol. 2001;30(10):590-595.
18. Angervall L, Nilsson L, Stener B. Microangiographic and histological studies in 2 cases of hibernoma. Cancer. 1964;17:685-692.
19. Sansom HE, Blunt DM, Moskovic EC. Large retroperitoneal hibernoma—CT findings with pathological correlation. Clin Radiol. 1999;54(9):625-627.
20. Dursun M, Agayev A, Bakir B, et al. CT and MR characteristics of hibernoma: six cases. Clin Imaging. 2008;32(1):42-47.
21. Ritchie DA, Aniq H, Davies AM, Mangham DC, Helliwell TR. Hibernoma—correlation of histopathology and magnetic-resonance-imaging features in 10 cases. Skeletal Radiol. 2006;35(8):579-589.
22. Lee JC, Gupta A, Saifuddin A, et al. Hibernoma: MRI features in eight consecutive cases. Clin Radiol. 2006;61(12):1029-1034.
23. Chitoku S, Kawai S, Watabe Y, et al. Intradural spinal hibernoma: case report. Surg Neurol. 1998;49(5):509-513.
24. Baskurt E, Padgett DM, Matsumoto JA. Multiple hibernomas in a 1-month-old female infant. AJNR Am J Neuroradiol. 2004;25(8):1443-1445.
25. da Motta AC, Tunkel DE, Westra WH, Yousem DM. Imaging findings of a hibernoma of the neck. AJNR Am J Neuroradiol. 2006;27(8):1658-1659.
26. Cook MA, Stern M, de Siva RD. MRI of a hibernoma. J Comput Assist Tomogr. 1996;20(2):333-335.
27. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
28. Robison S, Rapmund A, Hemmings C, Fulham M, Barry P. False-positive diagnosis of metastasis on positron emission tomography–computed tomography imaging due to hibernoma. J Clin Oncol. 2009;27(6):994-995.
29. Kallas KM, Vaughan L, Haghighi P, Resnick D. Hibernoma of the left axilla; a case report and review of MR imaging. Skeletal Radiol. 2003;32(5):290-294.
Hibernomas are rare benign soft-tissue tumors originally described as pseudolipomas by Merkel1 in 1906. Gery coined the term hibernoma in 1914, after noting the multivacuolated cytoplasm of the tumor cells and its resemblance to normal brown fat found in hibernating animals.2
Hibernomas represent 2% of all benign fat-containing tumors and are composed of brown adipocytes, which are histologically different from the white fat of lipomas. Hibernomas usually develop between ages 20 and 40 years, and their incidence is slightly higher in males.
Diffusely present in human newborns, brown fat usually regresses by 8 weeks of age.3 Residual brown fat deposits may remain in the neck, axilla, shoulder, thorax, thigh, retroperitoneum, and periscapular/interscapular regions.4 All these vestigial areas are therefore common locations of hibernomas, with the thigh accounting for up to 30% of cases.5 These tumors are seldom identified in the abdomen, popliteal fossa, or even intracranially. Injury to brown fat cells in these locations, either by infection, inflammation, or trauma, is considered a predisposing risk factor for development of hibernomas.6
Clinical Presentation
Clinically, hibernomas present as slow-growing, painless soft-tissue masses. Physical examination usually reveals a palpable, solitary, soft, and rubbery mass within the subcutaneous fat, which is freely mobile and not attached to deep layers. These tumors may rarely produce steroid hormones and result in a paraneoplastic syndrome. Even though these tumors are usually large at presentation, compression of adjacent structures seldom occurs.
Histology and Differential Diagnosis
The characteristic hibernoma cell is a multivacuolated adipocyte with centrally located nucleus, indistinct nucleolus, and coarsely granular eosinophilic (or pale) cytoplasm (Figure 1). Cytoplasmic vacuoles are uniform, round, regular, and small and stain for neutral fat. Nuclei are usually small with no or rare atypia. These multivacuolated brown fat–like tumor cells usually stain positive for S100 and CD31, usually stain negative for CD34 and p53, and can show 11q13-21 rearrangements, also seen in lipomas and liposarcomas. Hibernomas have 4 histologic variants: typical (classic), myxoid, lipoma-like, and spindle-cell.5 The typical hibernoma, the most common, contains a varying mixture of brown and white fat cells. The myxoid type, second most common, is composed of hibernoma cells floating in a loose acellular myxoid stroma. The lipoma-like variant consists of a few scattered hibernoma cells in a predominance of white fat cells. The spindle-cell variant, the rarest, has features of typical hibernoma and spindle-cell lipoma.7
Grossly, hibernomas are well encapsulated, soft, and lobular with prominent feeding vessels.8 They typically are tan or brown because of their hypervascularity and abundant mitochondria. Tumor size ranges from 1 to 24 cm (mean, 9.4 cm).9 These tumors are well-defined intermuscular/intramuscular, subcutaneous, or retroperitoneal lesions that tend to grow along fascial planes and displace surrounding structures rather than invade them. Delicate branching capillaries are usually seen within the tumor.
Although rare, hibernoma should be included in the differential diagnosis of lipomatous soft-tissue tumors.10 Imaging findings of hibernoma are not specific; other differential diagnostic considerations for a mass with a signal similar to that of fat or containing large intratumoral vessels include angiolipoma, intramuscular hemangioma with fat, spindle-cell lipoma, pleomorphic lipoma, lipoblastoma, hemangiopericytoma, and hemangioblastoma,11-15 as well as malignant processes, including lipoma-like well-differentiated liposarcoma and myxoid liposarcoma.16 Other entities that should be considered include residual brown fat and rhabdomyoma.
Hibernomas are histologically distinguished from well-differentiated liposarcomas by location (liposarcomas tend to be deep), atypia, presence of a prominent “plexiform” capillary pattern, and specific molecular translocations, including t (12;16). Lipomas have lipocytes that are not multivacuolated, and residual brown fat does not present as a distinct mass. Rhabdomyomas are distinguished by an absence of cytoplasmic lipid vacuoles.
Imaging
Conventional radiography may show a radiolucent mass without internal mineralization or associated osseous abnormalities4 (Figure 2). Calcifications are notably absent.17 Sonographically, hibernomas are well-circumscribed, solid, hyperechoic masses with increased internal vascular flow on both grayscale and color Doppler sampling; however their appearance is not pathognomonic (Figure 3). Angiography reveals a hypervascular tumor that may have internal arteriovenous shunting.18 Hibernomas have a heterogeneous appearance on computed tomography (CT) and magnetic resonance imaging (MRI) because of the variable distribution of brown fat cells, white fat cells, myxoid material, and spindle cells within the individual tumor subtypes.5 CT of these tumors shows internal septations and low attenuation values, between those of fat and muscle19 (Figure 4). Intravenous contrast enhances internal septa, but enhancement varies from none to intense, and from generalized to focal, depending on internal tumor composition.3,17,20-22
Hibernomas are usually hyperintense to skeletal muscle on T1-weighted MRI but slightly hypointense to subcutaneous fat because of the different gyromagnetic ratios and precessional frequencies of protons in white fat versus those in brown fat17 (Figure 5). Rarely, lesions are isointense to skeletal muscle on T1-weighted images.23 On T2-weighted images, high signal intensity similar to that of subcutaneous fat is typical.24 Flow voids can be readily identified.25 Short tau inversion recovery (STIR) MRI shows some areas with signal intensity higher than that of subcutaneous fat, and other areas of fat suppression.9 Ritchie and colleagues21 reported that hibernomas histologically composed of more than 70% multivacuolated adipocytes tended to have MRI signal characteristics different from those of subcutaneous fat, and those with less than 70% multivacuolated adipocytes tended to have signal characteristics paralleling those of subcutaneous fat. Myxoid hibernomas have higher signal intensity on T2-weighted and STIR MRI because of high water content.17,21,26,27
Hibernomas demonstrate moderate uptake on bone scintigraphy blood pool images and mild uptake on delayed images.4 Positron emission tomography (PET) is useful in differentiating hibernomas from other fat-containing lesions.9 Hibernomas demonstrate intense fluorine-18 fluorodeoxyglucose uptake because, unlike other adipogenic tumors, hibernomas contain abundant mitochondria and are highly metabolically active.28
Treatment and Prognosis
Complete surgical excision is the treatment of choice; given the behavior of the benign tumor, marginal complete excision is considered curative.5 Intralesional excision may be the only option for large tumors that are near nerves or vessels. However, intralesional excision may result in continued growth and local recurrence.
At surgery, these tumors usually are encapsulated and/or adherent to skeletal muscle or bone, without invasion, and easily separated from surrounding soft tissues.29 No specific surgical considerations are required beyond standard oncological principles, including careful dissection of adjacent nerves and vessels, and hemostasis. Hibernomas have the potential for significant bleeding during surgical excision. In this setting, embolization becomes a consideration, given the identification of large intratumoral vessels and the benign course of these lesions.
Hibernomas are rare benign soft-tissue tumors originally described as pseudolipomas by Merkel1 in 1906. Gery coined the term hibernoma in 1914, after noting the multivacuolated cytoplasm of the tumor cells and its resemblance to normal brown fat found in hibernating animals.2
Hibernomas represent 2% of all benign fat-containing tumors and are composed of brown adipocytes, which are histologically different from the white fat of lipomas. Hibernomas usually develop between ages 20 and 40 years, and their incidence is slightly higher in males.
Diffusely present in human newborns, brown fat usually regresses by 8 weeks of age.3 Residual brown fat deposits may remain in the neck, axilla, shoulder, thorax, thigh, retroperitoneum, and periscapular/interscapular regions.4 All these vestigial areas are therefore common locations of hibernomas, with the thigh accounting for up to 30% of cases.5 These tumors are seldom identified in the abdomen, popliteal fossa, or even intracranially. Injury to brown fat cells in these locations, either by infection, inflammation, or trauma, is considered a predisposing risk factor for development of hibernomas.6
Clinical Presentation
Clinically, hibernomas present as slow-growing, painless soft-tissue masses. Physical examination usually reveals a palpable, solitary, soft, and rubbery mass within the subcutaneous fat, which is freely mobile and not attached to deep layers. These tumors may rarely produce steroid hormones and result in a paraneoplastic syndrome. Even though these tumors are usually large at presentation, compression of adjacent structures seldom occurs.
Histology and Differential Diagnosis
The characteristic hibernoma cell is a multivacuolated adipocyte with centrally located nucleus, indistinct nucleolus, and coarsely granular eosinophilic (or pale) cytoplasm (Figure 1). Cytoplasmic vacuoles are uniform, round, regular, and small and stain for neutral fat. Nuclei are usually small with no or rare atypia. These multivacuolated brown fat–like tumor cells usually stain positive for S100 and CD31, usually stain negative for CD34 and p53, and can show 11q13-21 rearrangements, also seen in lipomas and liposarcomas. Hibernomas have 4 histologic variants: typical (classic), myxoid, lipoma-like, and spindle-cell.5 The typical hibernoma, the most common, contains a varying mixture of brown and white fat cells. The myxoid type, second most common, is composed of hibernoma cells floating in a loose acellular myxoid stroma. The lipoma-like variant consists of a few scattered hibernoma cells in a predominance of white fat cells. The spindle-cell variant, the rarest, has features of typical hibernoma and spindle-cell lipoma.7
Grossly, hibernomas are well encapsulated, soft, and lobular with prominent feeding vessels.8 They typically are tan or brown because of their hypervascularity and abundant mitochondria. Tumor size ranges from 1 to 24 cm (mean, 9.4 cm).9 These tumors are well-defined intermuscular/intramuscular, subcutaneous, or retroperitoneal lesions that tend to grow along fascial planes and displace surrounding structures rather than invade them. Delicate branching capillaries are usually seen within the tumor.
Although rare, hibernoma should be included in the differential diagnosis of lipomatous soft-tissue tumors.10 Imaging findings of hibernoma are not specific; other differential diagnostic considerations for a mass with a signal similar to that of fat or containing large intratumoral vessels include angiolipoma, intramuscular hemangioma with fat, spindle-cell lipoma, pleomorphic lipoma, lipoblastoma, hemangiopericytoma, and hemangioblastoma,11-15 as well as malignant processes, including lipoma-like well-differentiated liposarcoma and myxoid liposarcoma.16 Other entities that should be considered include residual brown fat and rhabdomyoma.
Hibernomas are histologically distinguished from well-differentiated liposarcomas by location (liposarcomas tend to be deep), atypia, presence of a prominent “plexiform” capillary pattern, and specific molecular translocations, including t (12;16). Lipomas have lipocytes that are not multivacuolated, and residual brown fat does not present as a distinct mass. Rhabdomyomas are distinguished by an absence of cytoplasmic lipid vacuoles.
Imaging
Conventional radiography may show a radiolucent mass without internal mineralization or associated osseous abnormalities4 (Figure 2). Calcifications are notably absent.17 Sonographically, hibernomas are well-circumscribed, solid, hyperechoic masses with increased internal vascular flow on both grayscale and color Doppler sampling; however their appearance is not pathognomonic (Figure 3). Angiography reveals a hypervascular tumor that may have internal arteriovenous shunting.18 Hibernomas have a heterogeneous appearance on computed tomography (CT) and magnetic resonance imaging (MRI) because of the variable distribution of brown fat cells, white fat cells, myxoid material, and spindle cells within the individual tumor subtypes.5 CT of these tumors shows internal septations and low attenuation values, between those of fat and muscle19 (Figure 4). Intravenous contrast enhances internal septa, but enhancement varies from none to intense, and from generalized to focal, depending on internal tumor composition.3,17,20-22
Hibernomas are usually hyperintense to skeletal muscle on T1-weighted MRI but slightly hypointense to subcutaneous fat because of the different gyromagnetic ratios and precessional frequencies of protons in white fat versus those in brown fat17 (Figure 5). Rarely, lesions are isointense to skeletal muscle on T1-weighted images.23 On T2-weighted images, high signal intensity similar to that of subcutaneous fat is typical.24 Flow voids can be readily identified.25 Short tau inversion recovery (STIR) MRI shows some areas with signal intensity higher than that of subcutaneous fat, and other areas of fat suppression.9 Ritchie and colleagues21 reported that hibernomas histologically composed of more than 70% multivacuolated adipocytes tended to have MRI signal characteristics different from those of subcutaneous fat, and those with less than 70% multivacuolated adipocytes tended to have signal characteristics paralleling those of subcutaneous fat. Myxoid hibernomas have higher signal intensity on T2-weighted and STIR MRI because of high water content.17,21,26,27
Hibernomas demonstrate moderate uptake on bone scintigraphy blood pool images and mild uptake on delayed images.4 Positron emission tomography (PET) is useful in differentiating hibernomas from other fat-containing lesions.9 Hibernomas demonstrate intense fluorine-18 fluorodeoxyglucose uptake because, unlike other adipogenic tumors, hibernomas contain abundant mitochondria and are highly metabolically active.28
Treatment and Prognosis
Complete surgical excision is the treatment of choice; given the behavior of the benign tumor, marginal complete excision is considered curative.5 Intralesional excision may be the only option for large tumors that are near nerves or vessels. However, intralesional excision may result in continued growth and local recurrence.
At surgery, these tumors usually are encapsulated and/or adherent to skeletal muscle or bone, without invasion, and easily separated from surrounding soft tissues.29 No specific surgical considerations are required beyond standard oncological principles, including careful dissection of adjacent nerves and vessels, and hemostasis. Hibernomas have the potential for significant bleeding during surgical excision. In this setting, embolization becomes a consideration, given the identification of large intratumoral vessels and the benign course of these lesions.
1. Merkel H. On a pseudolipoma of the breast. Beitr Pathol Anat. 1906;39:152-157.
2. Enzinger FM, Weiss SW. Benign lipomatous tumors. In: Enzinger FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis, MO: Mosby-Yearbook; 1994:420-423.
3. Alvine G, Rosenthal H, Murphey M, Huntrakoon M. Hibernoma. Skeletal Radiol. 1996;25(5):493-496.
4. Kumazoe H, Nagamatsu Y, Nishi T, Kimura YN, Nakazono T, Kudo S. Dumbbell-shaped thoracic hibernoma: computed tomography and magnetic resonance imaging findings. Jpn J Radiol. 2009;27(1):37-40.
5. Furlong MA, Fanburg-Smith JC, Miettinen M. The morphologic spectrum of hibernoma: a clinicopathologic study of 170 cases. Am J Surg Pathol. 2001;25(6):809-814.
6. Ucak A, Inan K, Onan B, Yilmaz AT. Resection of intrapericardial hibernoma associated with constrictive pericarditis. Interact Cardiovasc Thorac Surg. 2009;9(4):717-719.
7. Tomihama RT, Lindskog DM, Ahrens W, Haims AH. Hibernoma: a case report demonstrating usefulness of MR angiography in characterizing the tumor. Skeletal Radiol. 2007;36(6):541-545.
8. Choi J, Heiner J, Agni R, Hafez GR. Case of the season. Hibernoma. Semin Roentgenol. 2002;37(2):99-101.
9. Craig WD, Fanburg-Smith JC, Henry LR, Guerrero R, Barton JH. Fat-containing lesions of the retroperitoneum: radiologic-pathologic correlation. Radiographics. 2009;29(1):261-290.
10. Vassos N, Lell M, Hohenberger W, Croner RS, Agaimy A. Deep-seated huge hibernoma of soft tissue: a rare differential diagnosis of atypical lipomatous tumor/well differentiated liposarcoma. Int J Clin Exp Pathol. 2013;6(10):2178-2184.
11. Mugel T, Ghossain MA, Guinet C, et al. MR and CT findings in a case of hibernoma of the thigh extending into the pelvis. Eur Radiol. 1998;8(3):476-478.
12. Kallas KM, Vaughan L, Haghighi P, Resnick D. Hibernoma of the left axilla; a case report and review of MR imaging. Skeletal Radiol. 2003;32(5):290-294.
13. Suh JS, Cho J, Lee SH, et al. Alveolar soft part sarcoma: MR and angiographic findings. Skeletal Radiol. 2000;29(12):680-689.
14. De Beuckeleer LH, De Schepper AM, Vandevenne JE, et al. MR imaging of clear cell sarcoma (malignant melanoma of the soft parts): a multicenter correlative MRI-pathology study of 21 cases and literature review. Skeletal Radiol. 2000;29(4):187-195.
15. Chu BC, Terae S, Hida K, Furukawa M, Abe S, Miyasaka K. MR findings in spinal hemangioblastoma: correlation with symptoms and with angiographic and surgical findings. AJNR Am J Neuroradiol. 2001;22(1):206-217.
16. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509-1517.
17. Anderson SE, Schwab C, Stauffer E, Banic A, Steinbach LS. Hibernoma: imaging characteristics of a rare benign soft tissue tumor. Skeletal Radiol. 2001;30(10):590-595.
18. Angervall L, Nilsson L, Stener B. Microangiographic and histological studies in 2 cases of hibernoma. Cancer. 1964;17:685-692.
19. Sansom HE, Blunt DM, Moskovic EC. Large retroperitoneal hibernoma—CT findings with pathological correlation. Clin Radiol. 1999;54(9):625-627.
20. Dursun M, Agayev A, Bakir B, et al. CT and MR characteristics of hibernoma: six cases. Clin Imaging. 2008;32(1):42-47.
21. Ritchie DA, Aniq H, Davies AM, Mangham DC, Helliwell TR. Hibernoma—correlation of histopathology and magnetic-resonance-imaging features in 10 cases. Skeletal Radiol. 2006;35(8):579-589.
22. Lee JC, Gupta A, Saifuddin A, et al. Hibernoma: MRI features in eight consecutive cases. Clin Radiol. 2006;61(12):1029-1034.
23. Chitoku S, Kawai S, Watabe Y, et al. Intradural spinal hibernoma: case report. Surg Neurol. 1998;49(5):509-513.
24. Baskurt E, Padgett DM, Matsumoto JA. Multiple hibernomas in a 1-month-old female infant. AJNR Am J Neuroradiol. 2004;25(8):1443-1445.
25. da Motta AC, Tunkel DE, Westra WH, Yousem DM. Imaging findings of a hibernoma of the neck. AJNR Am J Neuroradiol. 2006;27(8):1658-1659.
26. Cook MA, Stern M, de Siva RD. MRI of a hibernoma. J Comput Assist Tomogr. 1996;20(2):333-335.
27. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
28. Robison S, Rapmund A, Hemmings C, Fulham M, Barry P. False-positive diagnosis of metastasis on positron emission tomography–computed tomography imaging due to hibernoma. J Clin Oncol. 2009;27(6):994-995.
29. Kallas KM, Vaughan L, Haghighi P, Resnick D. Hibernoma of the left axilla; a case report and review of MR imaging. Skeletal Radiol. 2003;32(5):290-294.
1. Merkel H. On a pseudolipoma of the breast. Beitr Pathol Anat. 1906;39:152-157.
2. Enzinger FM, Weiss SW. Benign lipomatous tumors. In: Enzinger FM, Weiss SW, eds. Soft Tissue Tumors. 3rd ed. St. Louis, MO: Mosby-Yearbook; 1994:420-423.
3. Alvine G, Rosenthal H, Murphey M, Huntrakoon M. Hibernoma. Skeletal Radiol. 1996;25(5):493-496.
4. Kumazoe H, Nagamatsu Y, Nishi T, Kimura YN, Nakazono T, Kudo S. Dumbbell-shaped thoracic hibernoma: computed tomography and magnetic resonance imaging findings. Jpn J Radiol. 2009;27(1):37-40.
5. Furlong MA, Fanburg-Smith JC, Miettinen M. The morphologic spectrum of hibernoma: a clinicopathologic study of 170 cases. Am J Surg Pathol. 2001;25(6):809-814.
6. Ucak A, Inan K, Onan B, Yilmaz AT. Resection of intrapericardial hibernoma associated with constrictive pericarditis. Interact Cardiovasc Thorac Surg. 2009;9(4):717-719.
7. Tomihama RT, Lindskog DM, Ahrens W, Haims AH. Hibernoma: a case report demonstrating usefulness of MR angiography in characterizing the tumor. Skeletal Radiol. 2007;36(6):541-545.
8. Choi J, Heiner J, Agni R, Hafez GR. Case of the season. Hibernoma. Semin Roentgenol. 2002;37(2):99-101.
9. Craig WD, Fanburg-Smith JC, Henry LR, Guerrero R, Barton JH. Fat-containing lesions of the retroperitoneum: radiologic-pathologic correlation. Radiographics. 2009;29(1):261-290.
10. Vassos N, Lell M, Hohenberger W, Croner RS, Agaimy A. Deep-seated huge hibernoma of soft tissue: a rare differential diagnosis of atypical lipomatous tumor/well differentiated liposarcoma. Int J Clin Exp Pathol. 2013;6(10):2178-2184.
11. Mugel T, Ghossain MA, Guinet C, et al. MR and CT findings in a case of hibernoma of the thigh extending into the pelvis. Eur Radiol. 1998;8(3):476-478.
12. Kallas KM, Vaughan L, Haghighi P, Resnick D. Hibernoma of the left axilla; a case report and review of MR imaging. Skeletal Radiol. 2003;32(5):290-294.
13. Suh JS, Cho J, Lee SH, et al. Alveolar soft part sarcoma: MR and angiographic findings. Skeletal Radiol. 2000;29(12):680-689.
14. De Beuckeleer LH, De Schepper AM, Vandevenne JE, et al. MR imaging of clear cell sarcoma (malignant melanoma of the soft parts): a multicenter correlative MRI-pathology study of 21 cases and literature review. Skeletal Radiol. 2000;29(4):187-195.
15. Chu BC, Terae S, Hida K, Furukawa M, Abe S, Miyasaka K. MR findings in spinal hemangioblastoma: correlation with symptoms and with angiographic and surgical findings. AJNR Am J Neuroradiol. 2001;22(1):206-217.
16. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509-1517.
17. Anderson SE, Schwab C, Stauffer E, Banic A, Steinbach LS. Hibernoma: imaging characteristics of a rare benign soft tissue tumor. Skeletal Radiol. 2001;30(10):590-595.
18. Angervall L, Nilsson L, Stener B. Microangiographic and histological studies in 2 cases of hibernoma. Cancer. 1964;17:685-692.
19. Sansom HE, Blunt DM, Moskovic EC. Large retroperitoneal hibernoma—CT findings with pathological correlation. Clin Radiol. 1999;54(9):625-627.
20. Dursun M, Agayev A, Bakir B, et al. CT and MR characteristics of hibernoma: six cases. Clin Imaging. 2008;32(1):42-47.
21. Ritchie DA, Aniq H, Davies AM, Mangham DC, Helliwell TR. Hibernoma—correlation of histopathology and magnetic-resonance-imaging features in 10 cases. Skeletal Radiol. 2006;35(8):579-589.
22. Lee JC, Gupta A, Saifuddin A, et al. Hibernoma: MRI features in eight consecutive cases. Clin Radiol. 2006;61(12):1029-1034.
23. Chitoku S, Kawai S, Watabe Y, et al. Intradural spinal hibernoma: case report. Surg Neurol. 1998;49(5):509-513.
24. Baskurt E, Padgett DM, Matsumoto JA. Multiple hibernomas in a 1-month-old female infant. AJNR Am J Neuroradiol. 2004;25(8):1443-1445.
25. da Motta AC, Tunkel DE, Westra WH, Yousem DM. Imaging findings of a hibernoma of the neck. AJNR Am J Neuroradiol. 2006;27(8):1658-1659.
26. Cook MA, Stern M, de Siva RD. MRI of a hibernoma. J Comput Assist Tomogr. 1996;20(2):333-335.
27. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
28. Robison S, Rapmund A, Hemmings C, Fulham M, Barry P. False-positive diagnosis of metastasis on positron emission tomography–computed tomography imaging due to hibernoma. J Clin Oncol. 2009;27(6):994-995.
29. Kallas KM, Vaughan L, Haghighi P, Resnick D. Hibernoma of the left axilla; a case report and review of MR imaging. Skeletal Radiol. 2003;32(5):290-294.