User login
Defying Gravity
Last week, I fell in the driveway. I was taking the trash to the curb when I tripped and fell into the large blue recycling bin. Although it was 6 AM, I jumped up and looked around, horrified that someone may have seen my episode of gracelessness. I did bump my head and spent the rest of the day feeling stupid while rubbing the sore spot.
This incident, though more ego-bruising than anything, really conveyed to me the concerns of falling—not only for the elderly, but for all of us. So, not to be confused with the signature song from the musical Wicked sung by Elphaba (the Wicked Witch of the West) who has a desire to live without limits, my editorial this month discusses sobering limits in our everyday lives. And it does implicate gravity!
According to the National Council on Aging, about 1 in 3 adults ages 65 and older fall each year.1 Unintentional falls are the leading cause of nonfatal and fatal injuries (eg, hip fractures, head trauma) for older adults and result in about 57 fall-related deaths per 100,000 people per year.1,2 In 2013, more than 25,000 elderly individuals in the United States died from unintentional fall injuries.3
Who falls? Who doesn’t? But data indicate that among community-dwelling individuals older than 65, women fall more frequently than their male counterparts.4 But while injury and mortality rates rise dramatically for both sexes, regardless of race, after age 85, men in this age-group are more likely to die from a fall than are women.5
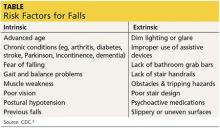
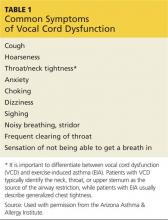
There are many risk factors that contribute to falling (see the table6), with gait and balance problems reported as the most significant contributor to falls among older adults.7 In addition, researchers report an increase in diseases linked to falls: diabetes, heart disease, stroke, arthritis, and Parkinson disease. In many cases, the medication used to treat the disease increases the risk for falling.8 It behooves every clinician to assess for and address any modifiable risk factors at each patient visit; one valuable resource is the CDC’s Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults.9
The issue of falling must be addressed on a larger scale, though. In response to the sobering statistics about falls, retirement communities, assisted living facilities, and nursing homes are trying to balance safety with their residents’ desire to live as they choose. They are hiring architects, interior designers, and engineers to find better ways to create safe living spaces, including installing floor lighting (similar to that on airplanes) that automatically illuminates a pathway to the bathroom when a resident gets out of bed.10
Continue for fall prevention in the community >>
But what about fall prevention in the community? A cost-benefit analysis revealed that community-based fall interventions are feasible and effective and provide a positive return on investment—no small consideration, given our current circumstances: Every 13 seconds, an older adult is treated in the emergency department for a fall, and every 29 minutes, an older adult dies from a fall-related injury.4,5 These estimates will likely rise as the population ages. The financial repercussions of falls and resultant morbidity and mortality may exceed $59 billion by 2020, according to the National Council on Aging.1 However, a 2013 report to Congress by the Centers for Medicare & Medicaid Services indicated that older adults’ participation in a falls prevention program has resulted in reduced health care costs.11
Over the decades, many different approaches have been used to enhance older adults’ participation in such programs. I am proud to report that my university is among the many organizations to address this issue. A.T. Still University of Health Sciences (ATSU) was recently awarded a $95,000 grant by the Baptist Hospitals and Health Systems (BHHS) to support the university’s Fall Prevention Outreach program—the largest university-based fall-prevention initiative in the country.12
Since the program began in 2008, more than 2,000 Arizonans have completed the eight-week curriculum, which gives older adults the tools they need to prevent falls and manage the often-paralyzing fear of falling that comes with growing older. Since injuries sustained from falls are the leading cause of accidental injury deaths in Arizonans ages 65 and older, according to the state’s Department of Health Services, this program gives us an opportunity to have a direct impact on our community.
ATSU uses A Matter of Balance, a nationally recognized fall-prevention curriculum developed by Boston University.13 After receiving special training, teams of ATSU physician assistant, physical therapy, occupational therapy, athletic training, and audiology students offer the curriculum, at no cost, to older citizens at 41 community sites in the Greater Phoenix area. Collaborations with partners ranging from local municipalities to assisted-living communities make the program possible.
Part of the BHHS grant funded the certification of 15 master trainers who teach the two-day A Matter of Balance curriculum to the ATSU students and the community volunteers who will, in turn, lead the sessions. The grant also funded the expansion of the program to an additional 24 sites, for a total of 65.12
For those who wish to identify appropriate evidence-based fall prevention programs in their community, the CDC developed a new guide, Preventing Falls: A Guide to Implementing Effective Community-Based Fall Prevention Programs.14 This “how-to” outlines how community-based organizations initiate and maintain effective programs. It focuses on implementation of fall prevention programs and offers strategies on program planning, development, implementation, and evaluation. This resource provides a solid starting point for those seeking to address this increasingly prevalent issue.
How do you investigate the risk for falls with your patients? I would be interested in hearing from you what resources are available in your community. You can contact me at [email protected].
REFERENCES
1. Cameron K, Schneider E, Childress D, Gilchrist C. (2015). National Council on Aging Falls Free® National Action Plan (2015). www.ncoa.org/FallsFreeNAP. Accessed November 5, 2015.
2. CDC. Important Facts about Falls. www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html. Accessed November 5, 2015.
3. CDC National Center for Injury Prevention and Control. Injury Prevention & Control: Data & Statistics (WISQARSTM). www.cdc.gov/injury/wisqars/. Accessed August 15, 2013.
4. Carande-Kulis I, Stevens JA, Florence CS, et al. Special Report from the CDC: a cost-benefit analysis of three older adult fall prevention interventions. J Safety Res. 2015;52:65-70.
5. CDC. WISQARS leading causes of nonfatal injury reports: 2006. Accessed November 13, 2006.
6. CDC. Risk factors for falls. http://www.cdc.gov/steadi/pdf/risk_factors_for_falls-a.pdf. Accessed November 4, 2015.
7. Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050-1056.
8. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75(1):51-61.
9. CDC. Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults. 3rd ed. www.cdc.gov/homeandrecreationalsafety/Falls/compendium.html. Accessed November 4, 2015.
10. Hafner K. Bracing for the falls of an aging nation. New York Times. November 2, 2014. www.nytimes.com/interactive/2014/11/03/health/bracing-for-the-falls-of-an-aging-nation.html?emc=edit_na_20141102&_r=0. Accessed November 4, 2015.
11. Houry D. The White House Conference on Aging and keeping older adults STEADI and free from falls. www.whitehouseconferenceonaging.gov/blog/post/the-white-house-conference-on-aging-and-keeping-older-adults-steadi-and-free-from-falls1.aspx. Accessed November 4, 2015.
12. Scott K. ATSU receives $95,000 grant to expand Fall Prevention Outreach program [news release]. October 1, 2015. http://news.atsu.edu/index.php/archives/category/arizona-campus. Accessed November 4, 2015.
13. MaineHealth Partnership for Healthy Aging. A Matter of Balance: Managing Concerns About Falls. www.mainehealth.org/AMatterofBalanceFrequentlyAskedQuestions#mob. Accessed November 4, 2015.
14. CDC. Preventing Falls: A Guide to Implementing Effective Community-Based Fall Prevention Programs. www.cdc.gov/HomeandRecreationalSafetyFalls/community_preventfalls.html. Accessed November 4, 2015.
Last week, I fell in the driveway. I was taking the trash to the curb when I tripped and fell into the large blue recycling bin. Although it was 6 AM, I jumped up and looked around, horrified that someone may have seen my episode of gracelessness. I did bump my head and spent the rest of the day feeling stupid while rubbing the sore spot.
This incident, though more ego-bruising than anything, really conveyed to me the concerns of falling—not only for the elderly, but for all of us. So, not to be confused with the signature song from the musical Wicked sung by Elphaba (the Wicked Witch of the West) who has a desire to live without limits, my editorial this month discusses sobering limits in our everyday lives. And it does implicate gravity!
According to the National Council on Aging, about 1 in 3 adults ages 65 and older fall each year.1 Unintentional falls are the leading cause of nonfatal and fatal injuries (eg, hip fractures, head trauma) for older adults and result in about 57 fall-related deaths per 100,000 people per year.1,2 In 2013, more than 25,000 elderly individuals in the United States died from unintentional fall injuries.3
Who falls? Who doesn’t? But data indicate that among community-dwelling individuals older than 65, women fall more frequently than their male counterparts.4 But while injury and mortality rates rise dramatically for both sexes, regardless of race, after age 85, men in this age-group are more likely to die from a fall than are women.5
There are many risk factors that contribute to falling (see the table6), with gait and balance problems reported as the most significant contributor to falls among older adults.7 In addition, researchers report an increase in diseases linked to falls: diabetes, heart disease, stroke, arthritis, and Parkinson disease. In many cases, the medication used to treat the disease increases the risk for falling.8 It behooves every clinician to assess for and address any modifiable risk factors at each patient visit; one valuable resource is the CDC’s Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults.9
The issue of falling must be addressed on a larger scale, though. In response to the sobering statistics about falls, retirement communities, assisted living facilities, and nursing homes are trying to balance safety with their residents’ desire to live as they choose. They are hiring architects, interior designers, and engineers to find better ways to create safe living spaces, including installing floor lighting (similar to that on airplanes) that automatically illuminates a pathway to the bathroom when a resident gets out of bed.10
Continue for fall prevention in the community >>
But what about fall prevention in the community? A cost-benefit analysis revealed that community-based fall interventions are feasible and effective and provide a positive return on investment—no small consideration, given our current circumstances: Every 13 seconds, an older adult is treated in the emergency department for a fall, and every 29 minutes, an older adult dies from a fall-related injury.4,5 These estimates will likely rise as the population ages. The financial repercussions of falls and resultant morbidity and mortality may exceed $59 billion by 2020, according to the National Council on Aging.1 However, a 2013 report to Congress by the Centers for Medicare & Medicaid Services indicated that older adults’ participation in a falls prevention program has resulted in reduced health care costs.11
Over the decades, many different approaches have been used to enhance older adults’ participation in such programs. I am proud to report that my university is among the many organizations to address this issue. A.T. Still University of Health Sciences (ATSU) was recently awarded a $95,000 grant by the Baptist Hospitals and Health Systems (BHHS) to support the university’s Fall Prevention Outreach program—the largest university-based fall-prevention initiative in the country.12
Since the program began in 2008, more than 2,000 Arizonans have completed the eight-week curriculum, which gives older adults the tools they need to prevent falls and manage the often-paralyzing fear of falling that comes with growing older. Since injuries sustained from falls are the leading cause of accidental injury deaths in Arizonans ages 65 and older, according to the state’s Department of Health Services, this program gives us an opportunity to have a direct impact on our community.
ATSU uses A Matter of Balance, a nationally recognized fall-prevention curriculum developed by Boston University.13 After receiving special training, teams of ATSU physician assistant, physical therapy, occupational therapy, athletic training, and audiology students offer the curriculum, at no cost, to older citizens at 41 community sites in the Greater Phoenix area. Collaborations with partners ranging from local municipalities to assisted-living communities make the program possible.
Part of the BHHS grant funded the certification of 15 master trainers who teach the two-day A Matter of Balance curriculum to the ATSU students and the community volunteers who will, in turn, lead the sessions. The grant also funded the expansion of the program to an additional 24 sites, for a total of 65.12
For those who wish to identify appropriate evidence-based fall prevention programs in their community, the CDC developed a new guide, Preventing Falls: A Guide to Implementing Effective Community-Based Fall Prevention Programs.14 This “how-to” outlines how community-based organizations initiate and maintain effective programs. It focuses on implementation of fall prevention programs and offers strategies on program planning, development, implementation, and evaluation. This resource provides a solid starting point for those seeking to address this increasingly prevalent issue.
How do you investigate the risk for falls with your patients? I would be interested in hearing from you what resources are available in your community. You can contact me at [email protected].
REFERENCES
1. Cameron K, Schneider E, Childress D, Gilchrist C. (2015). National Council on Aging Falls Free® National Action Plan (2015). www.ncoa.org/FallsFreeNAP. Accessed November 5, 2015.
2. CDC. Important Facts about Falls. www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html. Accessed November 5, 2015.
3. CDC National Center for Injury Prevention and Control. Injury Prevention & Control: Data & Statistics (WISQARSTM). www.cdc.gov/injury/wisqars/. Accessed August 15, 2013.
4. Carande-Kulis I, Stevens JA, Florence CS, et al. Special Report from the CDC: a cost-benefit analysis of three older adult fall prevention interventions. J Safety Res. 2015;52:65-70.
5. CDC. WISQARS leading causes of nonfatal injury reports: 2006. Accessed November 13, 2006.
6. CDC. Risk factors for falls. http://www.cdc.gov/steadi/pdf/risk_factors_for_falls-a.pdf. Accessed November 4, 2015.
7. Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050-1056.
8. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75(1):51-61.
9. CDC. Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults. 3rd ed. www.cdc.gov/homeandrecreationalsafety/Falls/compendium.html. Accessed November 4, 2015.
10. Hafner K. Bracing for the falls of an aging nation. New York Times. November 2, 2014. www.nytimes.com/interactive/2014/11/03/health/bracing-for-the-falls-of-an-aging-nation.html?emc=edit_na_20141102&_r=0. Accessed November 4, 2015.
11. Houry D. The White House Conference on Aging and keeping older adults STEADI and free from falls. www.whitehouseconferenceonaging.gov/blog/post/the-white-house-conference-on-aging-and-keeping-older-adults-steadi-and-free-from-falls1.aspx. Accessed November 4, 2015.
12. Scott K. ATSU receives $95,000 grant to expand Fall Prevention Outreach program [news release]. October 1, 2015. http://news.atsu.edu/index.php/archives/category/arizona-campus. Accessed November 4, 2015.
13. MaineHealth Partnership for Healthy Aging. A Matter of Balance: Managing Concerns About Falls. www.mainehealth.org/AMatterofBalanceFrequentlyAskedQuestions#mob. Accessed November 4, 2015.
14. CDC. Preventing Falls: A Guide to Implementing Effective Community-Based Fall Prevention Programs. www.cdc.gov/HomeandRecreationalSafetyFalls/community_preventfalls.html. Accessed November 4, 2015.
Last week, I fell in the driveway. I was taking the trash to the curb when I tripped and fell into the large blue recycling bin. Although it was 6 AM, I jumped up and looked around, horrified that someone may have seen my episode of gracelessness. I did bump my head and spent the rest of the day feeling stupid while rubbing the sore spot.
This incident, though more ego-bruising than anything, really conveyed to me the concerns of falling—not only for the elderly, but for all of us. So, not to be confused with the signature song from the musical Wicked sung by Elphaba (the Wicked Witch of the West) who has a desire to live without limits, my editorial this month discusses sobering limits in our everyday lives. And it does implicate gravity!
According to the National Council on Aging, about 1 in 3 adults ages 65 and older fall each year.1 Unintentional falls are the leading cause of nonfatal and fatal injuries (eg, hip fractures, head trauma) for older adults and result in about 57 fall-related deaths per 100,000 people per year.1,2 In 2013, more than 25,000 elderly individuals in the United States died from unintentional fall injuries.3
Who falls? Who doesn’t? But data indicate that among community-dwelling individuals older than 65, women fall more frequently than their male counterparts.4 But while injury and mortality rates rise dramatically for both sexes, regardless of race, after age 85, men in this age-group are more likely to die from a fall than are women.5
There are many risk factors that contribute to falling (see the table6), with gait and balance problems reported as the most significant contributor to falls among older adults.7 In addition, researchers report an increase in diseases linked to falls: diabetes, heart disease, stroke, arthritis, and Parkinson disease. In many cases, the medication used to treat the disease increases the risk for falling.8 It behooves every clinician to assess for and address any modifiable risk factors at each patient visit; one valuable resource is the CDC’s Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults.9
The issue of falling must be addressed on a larger scale, though. In response to the sobering statistics about falls, retirement communities, assisted living facilities, and nursing homes are trying to balance safety with their residents’ desire to live as they choose. They are hiring architects, interior designers, and engineers to find better ways to create safe living spaces, including installing floor lighting (similar to that on airplanes) that automatically illuminates a pathway to the bathroom when a resident gets out of bed.10
Continue for fall prevention in the community >>
But what about fall prevention in the community? A cost-benefit analysis revealed that community-based fall interventions are feasible and effective and provide a positive return on investment—no small consideration, given our current circumstances: Every 13 seconds, an older adult is treated in the emergency department for a fall, and every 29 minutes, an older adult dies from a fall-related injury.4,5 These estimates will likely rise as the population ages. The financial repercussions of falls and resultant morbidity and mortality may exceed $59 billion by 2020, according to the National Council on Aging.1 However, a 2013 report to Congress by the Centers for Medicare & Medicaid Services indicated that older adults’ participation in a falls prevention program has resulted in reduced health care costs.11
Over the decades, many different approaches have been used to enhance older adults’ participation in such programs. I am proud to report that my university is among the many organizations to address this issue. A.T. Still University of Health Sciences (ATSU) was recently awarded a $95,000 grant by the Baptist Hospitals and Health Systems (BHHS) to support the university’s Fall Prevention Outreach program—the largest university-based fall-prevention initiative in the country.12
Since the program began in 2008, more than 2,000 Arizonans have completed the eight-week curriculum, which gives older adults the tools they need to prevent falls and manage the often-paralyzing fear of falling that comes with growing older. Since injuries sustained from falls are the leading cause of accidental injury deaths in Arizonans ages 65 and older, according to the state’s Department of Health Services, this program gives us an opportunity to have a direct impact on our community.
ATSU uses A Matter of Balance, a nationally recognized fall-prevention curriculum developed by Boston University.13 After receiving special training, teams of ATSU physician assistant, physical therapy, occupational therapy, athletic training, and audiology students offer the curriculum, at no cost, to older citizens at 41 community sites in the Greater Phoenix area. Collaborations with partners ranging from local municipalities to assisted-living communities make the program possible.
Part of the BHHS grant funded the certification of 15 master trainers who teach the two-day A Matter of Balance curriculum to the ATSU students and the community volunteers who will, in turn, lead the sessions. The grant also funded the expansion of the program to an additional 24 sites, for a total of 65.12
For those who wish to identify appropriate evidence-based fall prevention programs in their community, the CDC developed a new guide, Preventing Falls: A Guide to Implementing Effective Community-Based Fall Prevention Programs.14 This “how-to” outlines how community-based organizations initiate and maintain effective programs. It focuses on implementation of fall prevention programs and offers strategies on program planning, development, implementation, and evaluation. This resource provides a solid starting point for those seeking to address this increasingly prevalent issue.
How do you investigate the risk for falls with your patients? I would be interested in hearing from you what resources are available in your community. You can contact me at [email protected].
REFERENCES
1. Cameron K, Schneider E, Childress D, Gilchrist C. (2015). National Council on Aging Falls Free® National Action Plan (2015). www.ncoa.org/FallsFreeNAP. Accessed November 5, 2015.
2. CDC. Important Facts about Falls. www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html. Accessed November 5, 2015.
3. CDC National Center for Injury Prevention and Control. Injury Prevention & Control: Data & Statistics (WISQARSTM). www.cdc.gov/injury/wisqars/. Accessed August 15, 2013.
4. Carande-Kulis I, Stevens JA, Florence CS, et al. Special Report from the CDC: a cost-benefit analysis of three older adult fall prevention interventions. J Safety Res. 2015;52:65-70.
5. CDC. WISQARS leading causes of nonfatal injury reports: 2006. Accessed November 13, 2006.
6. CDC. Risk factors for falls. http://www.cdc.gov/steadi/pdf/risk_factors_for_falls-a.pdf. Accessed November 4, 2015.
7. Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050-1056.
8. Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75(1):51-61.
9. CDC. Compendium of Effective Fall Interventions: What Works for Community-Dwelling Older Adults. 3rd ed. www.cdc.gov/homeandrecreationalsafety/Falls/compendium.html. Accessed November 4, 2015.
10. Hafner K. Bracing for the falls of an aging nation. New York Times. November 2, 2014. www.nytimes.com/interactive/2014/11/03/health/bracing-for-the-falls-of-an-aging-nation.html?emc=edit_na_20141102&_r=0. Accessed November 4, 2015.
11. Houry D. The White House Conference on Aging and keeping older adults STEADI and free from falls. www.whitehouseconferenceonaging.gov/blog/post/the-white-house-conference-on-aging-and-keeping-older-adults-steadi-and-free-from-falls1.aspx. Accessed November 4, 2015.
12. Scott K. ATSU receives $95,000 grant to expand Fall Prevention Outreach program [news release]. October 1, 2015. http://news.atsu.edu/index.php/archives/category/arizona-campus. Accessed November 4, 2015.
13. MaineHealth Partnership for Healthy Aging. A Matter of Balance: Managing Concerns About Falls. www.mainehealth.org/AMatterofBalanceFrequentlyAskedQuestions#mob. Accessed November 4, 2015.
14. CDC. Preventing Falls: A Guide to Implementing Effective Community-Based Fall Prevention Programs. www.cdc.gov/HomeandRecreationalSafetyFalls/community_preventfalls.html. Accessed November 4, 2015.
To Thine Own Health Be True
After 40 years as a PA (including 21 in academia), tucking myself safely into my seventh decade of life, I was feeling fairly invincible. Yes, I was experiencing increased fatigue and intermittent dizziness, but I attributed those to normal aging and my hectic schedule. Purely to get my colleagues and loved ones off my back, I agreed to see my primary care physician—but, feeling that I had little time for this and knowing that my health was just fine, thank you, I scheduled my appointment for a month later.
That appointment resulted in a referral to a cardiologist (which I didn’t think I needed) and a carotid ultrasound (which yielded negative results). I was still begrudging the time spent on these appointments, when my treadmill test revealed some ST changes in the lateral leads. OK, yes, I do have a positive family history for heart disease! But still, I was surprised.
This finding led to a nuclear treadmill test, which showed some ischemia. The cardiologist suggested an angiogram to rule out a probable false-positive result. Now, I really didn’t have time for this, either—but having gone this far, I felt it was important just to get it over with. Thank goodness I did!
During my angiogram (by the way, they give you great LaLa Land drugs), the interventional cardiologist discovered a 95% blockage of the circumflex, a 95% blockage of the right coronary artery, and a 60% blockage of the LAD. The big decision at that point, amidst the obvious shock of my mortality, was whether to opt for an open-heart bypass or stents. I chose stents (which, by the way, have had a significant impact on my energy level). My cardiologist tells me that, without the stents, if I had experienced a total occlusion event, I would not have survived. Quite a sobering thought!
My purpose in sharing this story is not to call attention to myself but rather to offer a wake-up call to those of us who think we are invincible. Many colleagues who have heard my story have decided to get their own long-delayed treadmill or other health-related tests. So here is my call to action to all of you: Take care of your health. The irony is that, for health care providers, this can be difficult.
Those who care for others often have a tough time caring for themselves. We know that physicians are notoriously bad patients, and I think PAs/NPs are no different. We deal with life-and-death issues all the time, and outward displays of distress are, at a minimum, discouraged. In general, our training and accumulated experience help us to develop good coping skills: We are taught to ignore basic human needs (like hunger and fatigue) and to remain capable, competent, and compassionate clinicians under highly stressful conditions.
Nonetheless, we experience these high levels of stress and seldom act to relieve them. As long ago as 1886, Dr William Ogle exposed clinicians’ vulnerability to high mortality risks, but to this day, the subject remains fairly neglected.1 Perhaps as a result of stigma—we worry about confidentiality, or that our colleagues will consider us inadequate or incompetent clinicians, or that a display of “weakness” means we have failed in some way—we often wait too long to seek treatment. Often, it takes a crisis before we stop to care for ourselves.
Continue for suggestions on how to take care of yourself >>
We promote the health of our patients, but we often forget that if health is to be sustained, those who provide the help must be capable of caring for both themselves and others.2 Without being overly prescriptive, I would like to share with you some suggestions for how you can take care of yourself:
1. Maintain a positive attitude. Yes, we often set ourselves up for failure, but we must have patience with ourselves. There are countless ways to practice reflection and find some time to think; it could entail an artistic outlet such as painting, sculpting, sewing, or singing. A colleague of mine listens to affirmations (now available by smartphone app) that she says help her succeed in her daily endeavors.
2. Identify your support systems. You know who they are: family, friends, and colleagues who bring positive support to your life. Spend more time with them. Clinicians with strong family or social connections are generally healthier than those who lack a support network. Make plans with supportive family members and friends, or seek out activities where you can meet new people.
3. Choose healthy, well-balanced meals. This is probably one of the most difficult tasks, due to our hectic and busy lifestyles. We should strive for low-fat, low-sugar, low-salt, high-fiber, and reasonably low-calorie meals. And drink more water—at least 6 to 8 glasses per day.
4. Get an appropriate amount of sleep. Restorative rest is one of the things you can control and should—no, must—give priority to. It is known that sleep is key to competent delivery of care. The National Sleep Foundation suggests seven to nine hours per night for those ages 26 to 64 and seven to eight hours per night for those older than 65.3 (In fact, some studies have suggested an increase in stroke linked to sleeping longer in older age.4)
5. Exercise. Schedule time for a sustainable exercise program. Put it on your calendar, and make that the one meeting that is essential. (This is another area in which technology can help, by providing reminders that you need to get up and move.) You don’t have to go to the gym twice a day or run a marathon. Walking for an hour three or four times a week will make a difference; even a 5-minute walk after a stressful meeting can help. Just do it!
6. Schedule time for R&R. Over the past decade, a staggering number of studies have demonstrated that our work performance plummets when we work prolonged periods without a break. Use your vacation time! A recent Harvard Business Review article reported that employees who take vacation are actually more productive.5 Make time for yourself—no one else will!
7. Take care of your mental health. Stress is a fact of life. Do what you can to relieve it; develop good coping skills and use them. You may find that keeping a journal in which you can vent your frustrations and fears or taking your dog for a long walk helps to relieve tension. Find what works for you. And make sure to laugh and find the humor in life. Laughter has been shown to boost the immune system and ease pain. It’s a great way to relax!
8. Take care of your physical health. Caring for your body is one of the best things you can do for yourself. Reduce or eliminate risk factors. Please get your routine health promotion procedures done (eg, colonoscopy, Pap smear, mammogram). And when you experience signs of illness, don’t ignore them! Seek advice early for anything out of the ordinary, be it intermittent dizziness, recurrent fatigue, unintended weight loss, feelings of despair—I could go on.
If even a handful of you heed these suggestions, and doing so makes a difference in your health and longevity, then I have completed my mission for this editorial.
We should be role models for our patients. The societal focus on healthy lifestyles is a challenge for all of us, but the benefits are enormous. I would love to hear from you regarding ways to enhance our physical and mental health and avoid early morbidity or mortality. You can reach me at [email protected].
REFERENCES
1. Woods R. Physician, heal thyself: the health and mortality of Victorian doctors. Soc Hist Med. 1966;9(1):1-30.
2. Borchardt GL. Role models for health promotion: the challenge for nurses. Nurs Forum. 2000;35(3):29-32.
3. Hershkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40-43.
4. Leng Y, Cappuccio FP, Wainwright NWJ, et al. Sleep duration and risk of fatal and nonfatal stroke: a prospective study and meta-analysis. Neurology. 2015;84(11):1072-1079.
5. Friedman R. Dear boss: your team wants you to go on vacation. Harvard Bus Rev. June 15, 2015.
After 40 years as a PA (including 21 in academia), tucking myself safely into my seventh decade of life, I was feeling fairly invincible. Yes, I was experiencing increased fatigue and intermittent dizziness, but I attributed those to normal aging and my hectic schedule. Purely to get my colleagues and loved ones off my back, I agreed to see my primary care physician—but, feeling that I had little time for this and knowing that my health was just fine, thank you, I scheduled my appointment for a month later.
That appointment resulted in a referral to a cardiologist (which I didn’t think I needed) and a carotid ultrasound (which yielded negative results). I was still begrudging the time spent on these appointments, when my treadmill test revealed some ST changes in the lateral leads. OK, yes, I do have a positive family history for heart disease! But still, I was surprised.
This finding led to a nuclear treadmill test, which showed some ischemia. The cardiologist suggested an angiogram to rule out a probable false-positive result. Now, I really didn’t have time for this, either—but having gone this far, I felt it was important just to get it over with. Thank goodness I did!
During my angiogram (by the way, they give you great LaLa Land drugs), the interventional cardiologist discovered a 95% blockage of the circumflex, a 95% blockage of the right coronary artery, and a 60% blockage of the LAD. The big decision at that point, amidst the obvious shock of my mortality, was whether to opt for an open-heart bypass or stents. I chose stents (which, by the way, have had a significant impact on my energy level). My cardiologist tells me that, without the stents, if I had experienced a total occlusion event, I would not have survived. Quite a sobering thought!
My purpose in sharing this story is not to call attention to myself but rather to offer a wake-up call to those of us who think we are invincible. Many colleagues who have heard my story have decided to get their own long-delayed treadmill or other health-related tests. So here is my call to action to all of you: Take care of your health. The irony is that, for health care providers, this can be difficult.
Those who care for others often have a tough time caring for themselves. We know that physicians are notoriously bad patients, and I think PAs/NPs are no different. We deal with life-and-death issues all the time, and outward displays of distress are, at a minimum, discouraged. In general, our training and accumulated experience help us to develop good coping skills: We are taught to ignore basic human needs (like hunger and fatigue) and to remain capable, competent, and compassionate clinicians under highly stressful conditions.
Nonetheless, we experience these high levels of stress and seldom act to relieve them. As long ago as 1886, Dr William Ogle exposed clinicians’ vulnerability to high mortality risks, but to this day, the subject remains fairly neglected.1 Perhaps as a result of stigma—we worry about confidentiality, or that our colleagues will consider us inadequate or incompetent clinicians, or that a display of “weakness” means we have failed in some way—we often wait too long to seek treatment. Often, it takes a crisis before we stop to care for ourselves.
Continue for suggestions on how to take care of yourself >>
We promote the health of our patients, but we often forget that if health is to be sustained, those who provide the help must be capable of caring for both themselves and others.2 Without being overly prescriptive, I would like to share with you some suggestions for how you can take care of yourself:
1. Maintain a positive attitude. Yes, we often set ourselves up for failure, but we must have patience with ourselves. There are countless ways to practice reflection and find some time to think; it could entail an artistic outlet such as painting, sculpting, sewing, or singing. A colleague of mine listens to affirmations (now available by smartphone app) that she says help her succeed in her daily endeavors.
2. Identify your support systems. You know who they are: family, friends, and colleagues who bring positive support to your life. Spend more time with them. Clinicians with strong family or social connections are generally healthier than those who lack a support network. Make plans with supportive family members and friends, or seek out activities where you can meet new people.
3. Choose healthy, well-balanced meals. This is probably one of the most difficult tasks, due to our hectic and busy lifestyles. We should strive for low-fat, low-sugar, low-salt, high-fiber, and reasonably low-calorie meals. And drink more water—at least 6 to 8 glasses per day.
4. Get an appropriate amount of sleep. Restorative rest is one of the things you can control and should—no, must—give priority to. It is known that sleep is key to competent delivery of care. The National Sleep Foundation suggests seven to nine hours per night for those ages 26 to 64 and seven to eight hours per night for those older than 65.3 (In fact, some studies have suggested an increase in stroke linked to sleeping longer in older age.4)
5. Exercise. Schedule time for a sustainable exercise program. Put it on your calendar, and make that the one meeting that is essential. (This is another area in which technology can help, by providing reminders that you need to get up and move.) You don’t have to go to the gym twice a day or run a marathon. Walking for an hour three or four times a week will make a difference; even a 5-minute walk after a stressful meeting can help. Just do it!
6. Schedule time for R&R. Over the past decade, a staggering number of studies have demonstrated that our work performance plummets when we work prolonged periods without a break. Use your vacation time! A recent Harvard Business Review article reported that employees who take vacation are actually more productive.5 Make time for yourself—no one else will!
7. Take care of your mental health. Stress is a fact of life. Do what you can to relieve it; develop good coping skills and use them. You may find that keeping a journal in which you can vent your frustrations and fears or taking your dog for a long walk helps to relieve tension. Find what works for you. And make sure to laugh and find the humor in life. Laughter has been shown to boost the immune system and ease pain. It’s a great way to relax!
8. Take care of your physical health. Caring for your body is one of the best things you can do for yourself. Reduce or eliminate risk factors. Please get your routine health promotion procedures done (eg, colonoscopy, Pap smear, mammogram). And when you experience signs of illness, don’t ignore them! Seek advice early for anything out of the ordinary, be it intermittent dizziness, recurrent fatigue, unintended weight loss, feelings of despair—I could go on.
If even a handful of you heed these suggestions, and doing so makes a difference in your health and longevity, then I have completed my mission for this editorial.
We should be role models for our patients. The societal focus on healthy lifestyles is a challenge for all of us, but the benefits are enormous. I would love to hear from you regarding ways to enhance our physical and mental health and avoid early morbidity or mortality. You can reach me at [email protected].
REFERENCES
1. Woods R. Physician, heal thyself: the health and mortality of Victorian doctors. Soc Hist Med. 1966;9(1):1-30.
2. Borchardt GL. Role models for health promotion: the challenge for nurses. Nurs Forum. 2000;35(3):29-32.
3. Hershkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40-43.
4. Leng Y, Cappuccio FP, Wainwright NWJ, et al. Sleep duration and risk of fatal and nonfatal stroke: a prospective study and meta-analysis. Neurology. 2015;84(11):1072-1079.
5. Friedman R. Dear boss: your team wants you to go on vacation. Harvard Bus Rev. June 15, 2015.
After 40 years as a PA (including 21 in academia), tucking myself safely into my seventh decade of life, I was feeling fairly invincible. Yes, I was experiencing increased fatigue and intermittent dizziness, but I attributed those to normal aging and my hectic schedule. Purely to get my colleagues and loved ones off my back, I agreed to see my primary care physician—but, feeling that I had little time for this and knowing that my health was just fine, thank you, I scheduled my appointment for a month later.
That appointment resulted in a referral to a cardiologist (which I didn’t think I needed) and a carotid ultrasound (which yielded negative results). I was still begrudging the time spent on these appointments, when my treadmill test revealed some ST changes in the lateral leads. OK, yes, I do have a positive family history for heart disease! But still, I was surprised.
This finding led to a nuclear treadmill test, which showed some ischemia. The cardiologist suggested an angiogram to rule out a probable false-positive result. Now, I really didn’t have time for this, either—but having gone this far, I felt it was important just to get it over with. Thank goodness I did!
During my angiogram (by the way, they give you great LaLa Land drugs), the interventional cardiologist discovered a 95% blockage of the circumflex, a 95% blockage of the right coronary artery, and a 60% blockage of the LAD. The big decision at that point, amidst the obvious shock of my mortality, was whether to opt for an open-heart bypass or stents. I chose stents (which, by the way, have had a significant impact on my energy level). My cardiologist tells me that, without the stents, if I had experienced a total occlusion event, I would not have survived. Quite a sobering thought!
My purpose in sharing this story is not to call attention to myself but rather to offer a wake-up call to those of us who think we are invincible. Many colleagues who have heard my story have decided to get their own long-delayed treadmill or other health-related tests. So here is my call to action to all of you: Take care of your health. The irony is that, for health care providers, this can be difficult.
Those who care for others often have a tough time caring for themselves. We know that physicians are notoriously bad patients, and I think PAs/NPs are no different. We deal with life-and-death issues all the time, and outward displays of distress are, at a minimum, discouraged. In general, our training and accumulated experience help us to develop good coping skills: We are taught to ignore basic human needs (like hunger and fatigue) and to remain capable, competent, and compassionate clinicians under highly stressful conditions.
Nonetheless, we experience these high levels of stress and seldom act to relieve them. As long ago as 1886, Dr William Ogle exposed clinicians’ vulnerability to high mortality risks, but to this day, the subject remains fairly neglected.1 Perhaps as a result of stigma—we worry about confidentiality, or that our colleagues will consider us inadequate or incompetent clinicians, or that a display of “weakness” means we have failed in some way—we often wait too long to seek treatment. Often, it takes a crisis before we stop to care for ourselves.
Continue for suggestions on how to take care of yourself >>
We promote the health of our patients, but we often forget that if health is to be sustained, those who provide the help must be capable of caring for both themselves and others.2 Without being overly prescriptive, I would like to share with you some suggestions for how you can take care of yourself:
1. Maintain a positive attitude. Yes, we often set ourselves up for failure, but we must have patience with ourselves. There are countless ways to practice reflection and find some time to think; it could entail an artistic outlet such as painting, sculpting, sewing, or singing. A colleague of mine listens to affirmations (now available by smartphone app) that she says help her succeed in her daily endeavors.
2. Identify your support systems. You know who they are: family, friends, and colleagues who bring positive support to your life. Spend more time with them. Clinicians with strong family or social connections are generally healthier than those who lack a support network. Make plans with supportive family members and friends, or seek out activities where you can meet new people.
3. Choose healthy, well-balanced meals. This is probably one of the most difficult tasks, due to our hectic and busy lifestyles. We should strive for low-fat, low-sugar, low-salt, high-fiber, and reasonably low-calorie meals. And drink more water—at least 6 to 8 glasses per day.
4. Get an appropriate amount of sleep. Restorative rest is one of the things you can control and should—no, must—give priority to. It is known that sleep is key to competent delivery of care. The National Sleep Foundation suggests seven to nine hours per night for those ages 26 to 64 and seven to eight hours per night for those older than 65.3 (In fact, some studies have suggested an increase in stroke linked to sleeping longer in older age.4)
5. Exercise. Schedule time for a sustainable exercise program. Put it on your calendar, and make that the one meeting that is essential. (This is another area in which technology can help, by providing reminders that you need to get up and move.) You don’t have to go to the gym twice a day or run a marathon. Walking for an hour three or four times a week will make a difference; even a 5-minute walk after a stressful meeting can help. Just do it!
6. Schedule time for R&R. Over the past decade, a staggering number of studies have demonstrated that our work performance plummets when we work prolonged periods without a break. Use your vacation time! A recent Harvard Business Review article reported that employees who take vacation are actually more productive.5 Make time for yourself—no one else will!
7. Take care of your mental health. Stress is a fact of life. Do what you can to relieve it; develop good coping skills and use them. You may find that keeping a journal in which you can vent your frustrations and fears or taking your dog for a long walk helps to relieve tension. Find what works for you. And make sure to laugh and find the humor in life. Laughter has been shown to boost the immune system and ease pain. It’s a great way to relax!
8. Take care of your physical health. Caring for your body is one of the best things you can do for yourself. Reduce or eliminate risk factors. Please get your routine health promotion procedures done (eg, colonoscopy, Pap smear, mammogram). And when you experience signs of illness, don’t ignore them! Seek advice early for anything out of the ordinary, be it intermittent dizziness, recurrent fatigue, unintended weight loss, feelings of despair—I could go on.
If even a handful of you heed these suggestions, and doing so makes a difference in your health and longevity, then I have completed my mission for this editorial.
We should be role models for our patients. The societal focus on healthy lifestyles is a challenge for all of us, but the benefits are enormous. I would love to hear from you regarding ways to enhance our physical and mental health and avoid early morbidity or mortality. You can reach me at [email protected].
REFERENCES
1. Woods R. Physician, heal thyself: the health and mortality of Victorian doctors. Soc Hist Med. 1966;9(1):1-30.
2. Borchardt GL. Role models for health promotion: the challenge for nurses. Nurs Forum. 2000;35(3):29-32.
3. Hershkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40-43.
4. Leng Y, Cappuccio FP, Wainwright NWJ, et al. Sleep duration and risk of fatal and nonfatal stroke: a prospective study and meta-analysis. Neurology. 2015;84(11):1072-1079.
5. Friedman R. Dear boss: your team wants you to go on vacation. Harvard Bus Rev. June 15, 2015.
Going for the Gold
As we enter 2015 and approach the golden (50th) anniversary of both the NP and PA professions, dating back to Dr. Loretta Ford1 at the University of Colorado and Dr. Eugene Stead2 at Duke University (circa 1965), it is important to reflect on not only where we have been but also where we want our professions to go in the next half-century.
Since I am a PA, some may suggest that it is dangerous and ambitious for me to speak/project for both professions. They may also note that the professions are distinct and my suggestions and reflections do not apply to NPs. Well, I guess I am willing to take that risk. As a colleague, friend, and co-Editor-in-Chief of Marie-Eileen Onieal, I have found that we agree much more often on issues (both political and practical) than we disagree.
NPs and PAs have worked diligently over the past half-century to develop our individual professions, in terms of reimbursement, prescribing privileges, state licensure, commissioning in the uniformed services, and expansion of scope of practice. Studies continue to show that we provide quality health care with similar outcomes as physicians, thus making us cost-effective members of the health care team.3-7
So, here we are in 2015. While it is difficult to get accurate data, Table 1 shows the number of clinicians reported in the most recent US Health Workforce Chartbook.8 As we well know, our professions have grown exponentially from our humble beginnings in the mid-1960s. (The first class of PAs comprised four students, if you recall.)
At the risk of sounding like a fossil (Oh never mind—I am a fossil!), let me be presumptuous and share with you six areas in which I think both professions can “do more” through the second half-century of our existence. In my opinion, we should focus on:
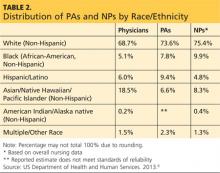
1. Diversity. As presented in Table 2, the majority of PAs and NPs are (non-Hispanic) white. Our efforts, should you agree with me, must start at the educational program level, through recruitment and retention of students from unrepresented and underrepresented groups. Another focal point should be the recruitment and retention of faculty and staff in these programs who reflect the diversity of our nation.
When I consider the origins of my profession, the key point is opportunity. Dr. Stead and other pioneers sought to fill a serious need for health care providers while also creating opportunities for military medics who were highly skilled but who did not meet requirements for many jobs within the medical community. Now it is time for us to ensure that other groups have similar opportunities to advance themselves and also fill gaps in the health care system.
Continue for more areas to focus on >>
2. Workforce planning and policy. This requires better data collection and an improved information infrastructure. In a 2014 editorial, Rod Hooker suggested that this is the golden age for PAs (about whom he was writing, although the same holds true for NPs) because US health care reform has identified our contributions to society and new policies place us upfront and center stage in producing labor solutions.9
It is time for our professions to do a better job in studying and reporting the important contributions we make in health care delivery. We need to have a place at the table—be it within a facility or at the state or federal level—whenever policy, utilization, and roles are discussed. Even if we as employees are subordinate to management in a particular setting, we should have a voice in how we are utilized in the workplace; we should ensure that those who make decisions—if we are not them—have the fullest picture of who we are and what we can do. It is not enough to use anecdotal evidence; we need to have data readily at hand that unequivocally support our assertions of value to patient care.
Hooker went so far as to call for the creation of a “physician assistant institute” to assist in the development and dissemination of reliable information about the profession. I think this is an idea whose time has come, but who will step up to the plate? If you are an NP, you may say, “What about us?” I would suggest this issue pertains to both of our professions—and in fact, this is an area in which I would encourage a collaborative effort. What a concept!
3. Ethics. In the past decade, there has been a renewed focus on maintaining an ethical culture in health care. As clinicians, we practice in an environment that affects the lives of everyone. Our patients and their family members expect high-quality care, patient safety, and use of the latest and most appropriate technology.
In the middle of this climate, we find a health care system that is probably the most entrepreneurial, corporatized, and profit-driven health system in the world. Most of our practice settings (outside the federal government) are for-profit entities. It is easy in this environment to fall victim to conflicts of interest and to acquire an economically oriented practice attitude.
If our first priority is the best possible health care for our patients, then integrity and ethical behavior naturally follow. Professionalism at its very heart places the patient’s interests ahead of our own. We must keep this in mind as we navigate the ever-changing health system.
Next page: Key area number four >>
4. Humanitarianism. I would encourage us to be actively engaged in promoting human welfare and social reforms with no prejudice on grounds of gender, sexual orientation, or religious or ethnic differences. Our goal should be to save lives, relieve suffering, and maintain human dignity.
How do we do this? Many of us have at some point in our careers volunteered our professional services at free clinics or other venues that provide health care to those who otherwise have difficulty accessing it. Some of us take that spirit a step further, traveling abroad on medical missions or responding to natural disasters or civil disturbances.
While using our medical and nursing skills for the good of society is ideal, we can also represent ourselves and our professions well by being good members of the community—not just the “medical” community. Perhaps we provide food and shelter to the homeless in our area or mentor young people.
Anything we do to help not only benefits us and the recipient; it also shows the community that our concept of “care” extends beyond the confines of the clinic or emergency department or operating theater in which we work. We have a very special calling, and I’m always pleased when our professional journals highlight the efforts of our colleagues to “help out”; their stories should inspire us all.
5. Appreciation. As NPs and PAs, we provide health care (whether from a medical or nursing standpoint), a revered calling since the days of Hippocrates. This privilege allows us to enter into a bond with our patients and assist them in a personal and fundamental way. It is no small thing to be involved in curing illness and promoting well-being. We should always remember that our role carries responsibility as well as provides rewards.
Despite the greater availability of diagnostic technologies and advancements in therapeutic abilities, we continue to see increasing disparities in the delivery of health care. Our professions must work diligently to find ways to address the issues of quality, accessibility, and cost of health care in the US. What kind of “stewards” will we be? That is our heritage!
Continue for the last area PAs and NPs need to grow in >>
6. Action. At the risk of sounding like a cheerleader, being part of the NP or PA profession is something special; it always has been and always will be. Our professions have grown impressively in numbers, utilization, and stature. We need to cultivate and support our professions and their representative organizations (ie, AANP and AAPA).
In the early decades of each profession, individuals with a great deal of passion and dedication created, advanced, and led these organizations on both state and national levels—and even an international level. You know who they are! Without them, we would not have made the strides that we have as professionals—the attainment of licensure, authority, and reimbursement.
Now that we are being truly recognized for our role in the health care team and our contributions to patient care, some of us may become complacent with regard to our professional organizations. But there will always be legislative and regulatory gains to make, and strong representation (and “strength in numbers”) is the best way to achieve our professional goals. Please, let’s continue to support our organizations not only via membership (which provides funding for initiatives) but also by participating in whatever ways we can to further our professions.
If we are going to fully cement our place in America’s health care system, NPs and PAs must strive to keep up the national dialogue about our patients, their needs, and how our contributions address those needs. I hope you agree. Please share your thoughts with me at PAEditor@frontline medcom.com.
REFERENCES
1. Ridgway S. Loretta Ford, founded nurse practitioner movement. Working Nurse. www.workingnurse.com/articles/loretta-ford-founded-nurse-practitioner-movement. Accessed January 16, 2015.
2. Physician Assistant History Society. Eugene A. Stead Jr, MD. http://pahx.org/stead-jr-eugene. Accessed January 16, 2015.
3. Kartha A, Restuccia J, Burgess J, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014:9(10); 615-620.
4. Naylor MD, Kurtzman ET. The role of nurse practitioners in reinventing primary care. Health Affairs. 2010;29(5):893-899.
5. Horrocks S, Anderson E, Salisbury C. Systematic review of whether nurse practitioners working in primary care can provide equivalent care to doctors. BMJ. 2002;324:819-823.
6. Halter M, Drennan V, Chattopadhyay K, et al. The contribution of physician assistants in primary care: a systematic review. BMC Health Services Research. 2013;13:223-236.
7. Hooker RS, Everett CM. The contributions of physician assistants in primary care systems. Health Social Care Commun. 2012;20(1):20-31.
8. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. The US Health Workforce Chartbook. Part 1: Clinicians. Rockville, MD; 2013.
9. Hooker R. A physician assistant institute [editorial]. J Phys Assist Ed. 2014;25(3):5-6.
As we enter 2015 and approach the golden (50th) anniversary of both the NP and PA professions, dating back to Dr. Loretta Ford1 at the University of Colorado and Dr. Eugene Stead2 at Duke University (circa 1965), it is important to reflect on not only where we have been but also where we want our professions to go in the next half-century.
Since I am a PA, some may suggest that it is dangerous and ambitious for me to speak/project for both professions. They may also note that the professions are distinct and my suggestions and reflections do not apply to NPs. Well, I guess I am willing to take that risk. As a colleague, friend, and co-Editor-in-Chief of Marie-Eileen Onieal, I have found that we agree much more often on issues (both political and practical) than we disagree.
NPs and PAs have worked diligently over the past half-century to develop our individual professions, in terms of reimbursement, prescribing privileges, state licensure, commissioning in the uniformed services, and expansion of scope of practice. Studies continue to show that we provide quality health care with similar outcomes as physicians, thus making us cost-effective members of the health care team.3-7
So, here we are in 2015. While it is difficult to get accurate data, Table 1 shows the number of clinicians reported in the most recent US Health Workforce Chartbook.8 As we well know, our professions have grown exponentially from our humble beginnings in the mid-1960s. (The first class of PAs comprised four students, if you recall.)
At the risk of sounding like a fossil (Oh never mind—I am a fossil!), let me be presumptuous and share with you six areas in which I think both professions can “do more” through the second half-century of our existence. In my opinion, we should focus on:
1. Diversity. As presented in Table 2, the majority of PAs and NPs are (non-Hispanic) white. Our efforts, should you agree with me, must start at the educational program level, through recruitment and retention of students from unrepresented and underrepresented groups. Another focal point should be the recruitment and retention of faculty and staff in these programs who reflect the diversity of our nation.
When I consider the origins of my profession, the key point is opportunity. Dr. Stead and other pioneers sought to fill a serious need for health care providers while also creating opportunities for military medics who were highly skilled but who did not meet requirements for many jobs within the medical community. Now it is time for us to ensure that other groups have similar opportunities to advance themselves and also fill gaps in the health care system.
Continue for more areas to focus on >>
2. Workforce planning and policy. This requires better data collection and an improved information infrastructure. In a 2014 editorial, Rod Hooker suggested that this is the golden age for PAs (about whom he was writing, although the same holds true for NPs) because US health care reform has identified our contributions to society and new policies place us upfront and center stage in producing labor solutions.9
It is time for our professions to do a better job in studying and reporting the important contributions we make in health care delivery. We need to have a place at the table—be it within a facility or at the state or federal level—whenever policy, utilization, and roles are discussed. Even if we as employees are subordinate to management in a particular setting, we should have a voice in how we are utilized in the workplace; we should ensure that those who make decisions—if we are not them—have the fullest picture of who we are and what we can do. It is not enough to use anecdotal evidence; we need to have data readily at hand that unequivocally support our assertions of value to patient care.
Hooker went so far as to call for the creation of a “physician assistant institute” to assist in the development and dissemination of reliable information about the profession. I think this is an idea whose time has come, but who will step up to the plate? If you are an NP, you may say, “What about us?” I would suggest this issue pertains to both of our professions—and in fact, this is an area in which I would encourage a collaborative effort. What a concept!
3. Ethics. In the past decade, there has been a renewed focus on maintaining an ethical culture in health care. As clinicians, we practice in an environment that affects the lives of everyone. Our patients and their family members expect high-quality care, patient safety, and use of the latest and most appropriate technology.
In the middle of this climate, we find a health care system that is probably the most entrepreneurial, corporatized, and profit-driven health system in the world. Most of our practice settings (outside the federal government) are for-profit entities. It is easy in this environment to fall victim to conflicts of interest and to acquire an economically oriented practice attitude.
If our first priority is the best possible health care for our patients, then integrity and ethical behavior naturally follow. Professionalism at its very heart places the patient’s interests ahead of our own. We must keep this in mind as we navigate the ever-changing health system.
Next page: Key area number four >>
4. Humanitarianism. I would encourage us to be actively engaged in promoting human welfare and social reforms with no prejudice on grounds of gender, sexual orientation, or religious or ethnic differences. Our goal should be to save lives, relieve suffering, and maintain human dignity.
How do we do this? Many of us have at some point in our careers volunteered our professional services at free clinics or other venues that provide health care to those who otherwise have difficulty accessing it. Some of us take that spirit a step further, traveling abroad on medical missions or responding to natural disasters or civil disturbances.
While using our medical and nursing skills for the good of society is ideal, we can also represent ourselves and our professions well by being good members of the community—not just the “medical” community. Perhaps we provide food and shelter to the homeless in our area or mentor young people.
Anything we do to help not only benefits us and the recipient; it also shows the community that our concept of “care” extends beyond the confines of the clinic or emergency department or operating theater in which we work. We have a very special calling, and I’m always pleased when our professional journals highlight the efforts of our colleagues to “help out”; their stories should inspire us all.
5. Appreciation. As NPs and PAs, we provide health care (whether from a medical or nursing standpoint), a revered calling since the days of Hippocrates. This privilege allows us to enter into a bond with our patients and assist them in a personal and fundamental way. It is no small thing to be involved in curing illness and promoting well-being. We should always remember that our role carries responsibility as well as provides rewards.
Despite the greater availability of diagnostic technologies and advancements in therapeutic abilities, we continue to see increasing disparities in the delivery of health care. Our professions must work diligently to find ways to address the issues of quality, accessibility, and cost of health care in the US. What kind of “stewards” will we be? That is our heritage!
Continue for the last area PAs and NPs need to grow in >>
6. Action. At the risk of sounding like a cheerleader, being part of the NP or PA profession is something special; it always has been and always will be. Our professions have grown impressively in numbers, utilization, and stature. We need to cultivate and support our professions and their representative organizations (ie, AANP and AAPA).
In the early decades of each profession, individuals with a great deal of passion and dedication created, advanced, and led these organizations on both state and national levels—and even an international level. You know who they are! Without them, we would not have made the strides that we have as professionals—the attainment of licensure, authority, and reimbursement.
Now that we are being truly recognized for our role in the health care team and our contributions to patient care, some of us may become complacent with regard to our professional organizations. But there will always be legislative and regulatory gains to make, and strong representation (and “strength in numbers”) is the best way to achieve our professional goals. Please, let’s continue to support our organizations not only via membership (which provides funding for initiatives) but also by participating in whatever ways we can to further our professions.
If we are going to fully cement our place in America’s health care system, NPs and PAs must strive to keep up the national dialogue about our patients, their needs, and how our contributions address those needs. I hope you agree. Please share your thoughts with me at PAEditor@frontline medcom.com.
REFERENCES
1. Ridgway S. Loretta Ford, founded nurse practitioner movement. Working Nurse. www.workingnurse.com/articles/loretta-ford-founded-nurse-practitioner-movement. Accessed January 16, 2015.
2. Physician Assistant History Society. Eugene A. Stead Jr, MD. http://pahx.org/stead-jr-eugene. Accessed January 16, 2015.
3. Kartha A, Restuccia J, Burgess J, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014:9(10); 615-620.
4. Naylor MD, Kurtzman ET. The role of nurse practitioners in reinventing primary care. Health Affairs. 2010;29(5):893-899.
5. Horrocks S, Anderson E, Salisbury C. Systematic review of whether nurse practitioners working in primary care can provide equivalent care to doctors. BMJ. 2002;324:819-823.
6. Halter M, Drennan V, Chattopadhyay K, et al. The contribution of physician assistants in primary care: a systematic review. BMC Health Services Research. 2013;13:223-236.
7. Hooker RS, Everett CM. The contributions of physician assistants in primary care systems. Health Social Care Commun. 2012;20(1):20-31.
8. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. The US Health Workforce Chartbook. Part 1: Clinicians. Rockville, MD; 2013.
9. Hooker R. A physician assistant institute [editorial]. J Phys Assist Ed. 2014;25(3):5-6.
As we enter 2015 and approach the golden (50th) anniversary of both the NP and PA professions, dating back to Dr. Loretta Ford1 at the University of Colorado and Dr. Eugene Stead2 at Duke University (circa 1965), it is important to reflect on not only where we have been but also where we want our professions to go in the next half-century.
Since I am a PA, some may suggest that it is dangerous and ambitious for me to speak/project for both professions. They may also note that the professions are distinct and my suggestions and reflections do not apply to NPs. Well, I guess I am willing to take that risk. As a colleague, friend, and co-Editor-in-Chief of Marie-Eileen Onieal, I have found that we agree much more often on issues (both political and practical) than we disagree.
NPs and PAs have worked diligently over the past half-century to develop our individual professions, in terms of reimbursement, prescribing privileges, state licensure, commissioning in the uniformed services, and expansion of scope of practice. Studies continue to show that we provide quality health care with similar outcomes as physicians, thus making us cost-effective members of the health care team.3-7
So, here we are in 2015. While it is difficult to get accurate data, Table 1 shows the number of clinicians reported in the most recent US Health Workforce Chartbook.8 As we well know, our professions have grown exponentially from our humble beginnings in the mid-1960s. (The first class of PAs comprised four students, if you recall.)
At the risk of sounding like a fossil (Oh never mind—I am a fossil!), let me be presumptuous and share with you six areas in which I think both professions can “do more” through the second half-century of our existence. In my opinion, we should focus on:
1. Diversity. As presented in Table 2, the majority of PAs and NPs are (non-Hispanic) white. Our efforts, should you agree with me, must start at the educational program level, through recruitment and retention of students from unrepresented and underrepresented groups. Another focal point should be the recruitment and retention of faculty and staff in these programs who reflect the diversity of our nation.
When I consider the origins of my profession, the key point is opportunity. Dr. Stead and other pioneers sought to fill a serious need for health care providers while also creating opportunities for military medics who were highly skilled but who did not meet requirements for many jobs within the medical community. Now it is time for us to ensure that other groups have similar opportunities to advance themselves and also fill gaps in the health care system.
Continue for more areas to focus on >>
2. Workforce planning and policy. This requires better data collection and an improved information infrastructure. In a 2014 editorial, Rod Hooker suggested that this is the golden age for PAs (about whom he was writing, although the same holds true for NPs) because US health care reform has identified our contributions to society and new policies place us upfront and center stage in producing labor solutions.9
It is time for our professions to do a better job in studying and reporting the important contributions we make in health care delivery. We need to have a place at the table—be it within a facility or at the state or federal level—whenever policy, utilization, and roles are discussed. Even if we as employees are subordinate to management in a particular setting, we should have a voice in how we are utilized in the workplace; we should ensure that those who make decisions—if we are not them—have the fullest picture of who we are and what we can do. It is not enough to use anecdotal evidence; we need to have data readily at hand that unequivocally support our assertions of value to patient care.
Hooker went so far as to call for the creation of a “physician assistant institute” to assist in the development and dissemination of reliable information about the profession. I think this is an idea whose time has come, but who will step up to the plate? If you are an NP, you may say, “What about us?” I would suggest this issue pertains to both of our professions—and in fact, this is an area in which I would encourage a collaborative effort. What a concept!
3. Ethics. In the past decade, there has been a renewed focus on maintaining an ethical culture in health care. As clinicians, we practice in an environment that affects the lives of everyone. Our patients and their family members expect high-quality care, patient safety, and use of the latest and most appropriate technology.
In the middle of this climate, we find a health care system that is probably the most entrepreneurial, corporatized, and profit-driven health system in the world. Most of our practice settings (outside the federal government) are for-profit entities. It is easy in this environment to fall victim to conflicts of interest and to acquire an economically oriented practice attitude.
If our first priority is the best possible health care for our patients, then integrity and ethical behavior naturally follow. Professionalism at its very heart places the patient’s interests ahead of our own. We must keep this in mind as we navigate the ever-changing health system.
Next page: Key area number four >>
4. Humanitarianism. I would encourage us to be actively engaged in promoting human welfare and social reforms with no prejudice on grounds of gender, sexual orientation, or religious or ethnic differences. Our goal should be to save lives, relieve suffering, and maintain human dignity.
How do we do this? Many of us have at some point in our careers volunteered our professional services at free clinics or other venues that provide health care to those who otherwise have difficulty accessing it. Some of us take that spirit a step further, traveling abroad on medical missions or responding to natural disasters or civil disturbances.
While using our medical and nursing skills for the good of society is ideal, we can also represent ourselves and our professions well by being good members of the community—not just the “medical” community. Perhaps we provide food and shelter to the homeless in our area or mentor young people.
Anything we do to help not only benefits us and the recipient; it also shows the community that our concept of “care” extends beyond the confines of the clinic or emergency department or operating theater in which we work. We have a very special calling, and I’m always pleased when our professional journals highlight the efforts of our colleagues to “help out”; their stories should inspire us all.
5. Appreciation. As NPs and PAs, we provide health care (whether from a medical or nursing standpoint), a revered calling since the days of Hippocrates. This privilege allows us to enter into a bond with our patients and assist them in a personal and fundamental way. It is no small thing to be involved in curing illness and promoting well-being. We should always remember that our role carries responsibility as well as provides rewards.
Despite the greater availability of diagnostic technologies and advancements in therapeutic abilities, we continue to see increasing disparities in the delivery of health care. Our professions must work diligently to find ways to address the issues of quality, accessibility, and cost of health care in the US. What kind of “stewards” will we be? That is our heritage!
Continue for the last area PAs and NPs need to grow in >>
6. Action. At the risk of sounding like a cheerleader, being part of the NP or PA profession is something special; it always has been and always will be. Our professions have grown impressively in numbers, utilization, and stature. We need to cultivate and support our professions and their representative organizations (ie, AANP and AAPA).
In the early decades of each profession, individuals with a great deal of passion and dedication created, advanced, and led these organizations on both state and national levels—and even an international level. You know who they are! Without them, we would not have made the strides that we have as professionals—the attainment of licensure, authority, and reimbursement.
Now that we are being truly recognized for our role in the health care team and our contributions to patient care, some of us may become complacent with regard to our professional organizations. But there will always be legislative and regulatory gains to make, and strong representation (and “strength in numbers”) is the best way to achieve our professional goals. Please, let’s continue to support our organizations not only via membership (which provides funding for initiatives) but also by participating in whatever ways we can to further our professions.
If we are going to fully cement our place in America’s health care system, NPs and PAs must strive to keep up the national dialogue about our patients, their needs, and how our contributions address those needs. I hope you agree. Please share your thoughts with me at PAEditor@frontline medcom.com.
REFERENCES
1. Ridgway S. Loretta Ford, founded nurse practitioner movement. Working Nurse. www.workingnurse.com/articles/loretta-ford-founded-nurse-practitioner-movement. Accessed January 16, 2015.
2. Physician Assistant History Society. Eugene A. Stead Jr, MD. http://pahx.org/stead-jr-eugene. Accessed January 16, 2015.
3. Kartha A, Restuccia J, Burgess J, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014:9(10); 615-620.
4. Naylor MD, Kurtzman ET. The role of nurse practitioners in reinventing primary care. Health Affairs. 2010;29(5):893-899.
5. Horrocks S, Anderson E, Salisbury C. Systematic review of whether nurse practitioners working in primary care can provide equivalent care to doctors. BMJ. 2002;324:819-823.
6. Halter M, Drennan V, Chattopadhyay K, et al. The contribution of physician assistants in primary care: a systematic review. BMC Health Services Research. 2013;13:223-236.
7. Hooker RS, Everett CM. The contributions of physician assistants in primary care systems. Health Social Care Commun. 2012;20(1):20-31.
8. US Department of Health and Human Services, Health Resources and Services Administration, National Center for Health Workforce Analysis. The US Health Workforce Chartbook. Part 1: Clinicians. Rockville, MD; 2013.
9. Hooker R. A physician assistant institute [editorial]. J Phys Assist Ed. 2014;25(3):5-6.
Join Us in Celebrating
Clinician Reviews is celebrating its 25th year of publication in 2015. As the journal’s PA and NP Editors-in-Chief, we would like to be among the first to congratulate the publication on this milestone anniversary.
Paging through the inaugural issue (February 1991) today is an indication of how much has changed in 25 years (and, at the same time, how much hasn’t). In that issue, the editors acknowledged the service of military PAs and NPs who were deployed during Operation Desert Storm (at the time, a current conflict) and introduced an AIDS Update department because “the spread of human immunodeficiency virus (HIV) shows little sign of abating.”
Despite the passage of time, CR remains faithful to the editorial purpose established more than two decades ago. It was “designed to provide you with the information you need to keep abreast of the changes in medicine that occur on a daily basis.” And to the present day, the editorial staff and the editorial board have maintained their commitment to providing peer-reviewed, clinician-authored content that helps NPs and PAs provide the best possible patient care in an increasingly challenging environment. The journal has never lost its focus on primary care—the heart of health care in the United States—while acknowledging that primary care clinicians manage a wide variety of disease states and conditions.
The true strength of the journal has always been, and continues to be, the people affiliated with it. Special thanks to the dedicated editorial staff: Karen J. Clemments (Editor), Ann M. Hoppel (Managing Editor), Fran Hopkins (Senior Editor), and Kristen Garafano (Web Editor). This noteworthy anniversary would not be complete without extending special thanks to past Editors Robert E. De Donato, Jean Paternoster, and Maura Griffin, and longtime Senior Editor Christine Mooney Lukesh, who retired last year.
In addition, we acknowledge that no journal can be successful without the support and guidance of its editorial board. We have been especially blessed with colleagues from both the PA and NP professions—many of whom have been on the board for a decade or longer. Their names are listed here.
We, as Editors-in-Chief, would particularly like to thank you—our readers and colleagues—for your thoughtful feedback on our monthly editorials. It is rewarding to think that we have fostered discussion and fruitful debate on issues relevant to our professions—and we gain just as much perspective as we hope to impart. To those of you who have contributed manuscripts over the years, know that we are grateful for your professionalism, generosity with your time and expertise, and commitment to enhancing the knowledge of your fellow practitioners.
So, as CR marks its 25th anniversary, we invite our authors, peer reviewers, and readers to join us in celebrating. Thank you for your support for the past 25 years. Here’s to many more.
Clinician Reviews is celebrating its 25th year of publication in 2015. As the journal’s PA and NP Editors-in-Chief, we would like to be among the first to congratulate the publication on this milestone anniversary.
Paging through the inaugural issue (February 1991) today is an indication of how much has changed in 25 years (and, at the same time, how much hasn’t). In that issue, the editors acknowledged the service of military PAs and NPs who were deployed during Operation Desert Storm (at the time, a current conflict) and introduced an AIDS Update department because “the spread of human immunodeficiency virus (HIV) shows little sign of abating.”
Despite the passage of time, CR remains faithful to the editorial purpose established more than two decades ago. It was “designed to provide you with the information you need to keep abreast of the changes in medicine that occur on a daily basis.” And to the present day, the editorial staff and the editorial board have maintained their commitment to providing peer-reviewed, clinician-authored content that helps NPs and PAs provide the best possible patient care in an increasingly challenging environment. The journal has never lost its focus on primary care—the heart of health care in the United States—while acknowledging that primary care clinicians manage a wide variety of disease states and conditions.
The true strength of the journal has always been, and continues to be, the people affiliated with it. Special thanks to the dedicated editorial staff: Karen J. Clemments (Editor), Ann M. Hoppel (Managing Editor), Fran Hopkins (Senior Editor), and Kristen Garafano (Web Editor). This noteworthy anniversary would not be complete without extending special thanks to past Editors Robert E. De Donato, Jean Paternoster, and Maura Griffin, and longtime Senior Editor Christine Mooney Lukesh, who retired last year.
In addition, we acknowledge that no journal can be successful without the support and guidance of its editorial board. We have been especially blessed with colleagues from both the PA and NP professions—many of whom have been on the board for a decade or longer. Their names are listed here.
We, as Editors-in-Chief, would particularly like to thank you—our readers and colleagues—for your thoughtful feedback on our monthly editorials. It is rewarding to think that we have fostered discussion and fruitful debate on issues relevant to our professions—and we gain just as much perspective as we hope to impart. To those of you who have contributed manuscripts over the years, know that we are grateful for your professionalism, generosity with your time and expertise, and commitment to enhancing the knowledge of your fellow practitioners.
So, as CR marks its 25th anniversary, we invite our authors, peer reviewers, and readers to join us in celebrating. Thank you for your support for the past 25 years. Here’s to many more.
Clinician Reviews is celebrating its 25th year of publication in 2015. As the journal’s PA and NP Editors-in-Chief, we would like to be among the first to congratulate the publication on this milestone anniversary.
Paging through the inaugural issue (February 1991) today is an indication of how much has changed in 25 years (and, at the same time, how much hasn’t). In that issue, the editors acknowledged the service of military PAs and NPs who were deployed during Operation Desert Storm (at the time, a current conflict) and introduced an AIDS Update department because “the spread of human immunodeficiency virus (HIV) shows little sign of abating.”
Despite the passage of time, CR remains faithful to the editorial purpose established more than two decades ago. It was “designed to provide you with the information you need to keep abreast of the changes in medicine that occur on a daily basis.” And to the present day, the editorial staff and the editorial board have maintained their commitment to providing peer-reviewed, clinician-authored content that helps NPs and PAs provide the best possible patient care in an increasingly challenging environment. The journal has never lost its focus on primary care—the heart of health care in the United States—while acknowledging that primary care clinicians manage a wide variety of disease states and conditions.
The true strength of the journal has always been, and continues to be, the people affiliated with it. Special thanks to the dedicated editorial staff: Karen J. Clemments (Editor), Ann M. Hoppel (Managing Editor), Fran Hopkins (Senior Editor), and Kristen Garafano (Web Editor). This noteworthy anniversary would not be complete without extending special thanks to past Editors Robert E. De Donato, Jean Paternoster, and Maura Griffin, and longtime Senior Editor Christine Mooney Lukesh, who retired last year.
In addition, we acknowledge that no journal can be successful without the support and guidance of its editorial board. We have been especially blessed with colleagues from both the PA and NP professions—many of whom have been on the board for a decade or longer. Their names are listed here.
We, as Editors-in-Chief, would particularly like to thank you—our readers and colleagues—for your thoughtful feedback on our monthly editorials. It is rewarding to think that we have fostered discussion and fruitful debate on issues relevant to our professions—and we gain just as much perspective as we hope to impart. To those of you who have contributed manuscripts over the years, know that we are grateful for your professionalism, generosity with your time and expertise, and commitment to enhancing the knowledge of your fellow practitioners.
So, as CR marks its 25th anniversary, we invite our authors, peer reviewers, and readers to join us in celebrating. Thank you for your support for the past 25 years. Here’s to many more.
Normal and Abnormal Vocal Cord Movement
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[Courtesy of Arizona Asthma & Allergy Institute]
Click Here for Free CE/CME on Vocal Cord Dysfunction: Unmasking the Asthma Pretender
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[Courtesy of Arizona Asthma & Allergy Institute]
Click Here for Free CE/CME on Vocal Cord Dysfunction: Unmasking the Asthma Pretender
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[Courtesy of Arizona Asthma & Allergy Institute]
Click Here for Free CE/CME on Vocal Cord Dysfunction: Unmasking the Asthma Pretender
Vocal Cord Dysfunction: Unmasking the Asthma Pretender
CE/CME No: CR-1412
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the evolution in thinking about the pathogenesis of and treatment for vocal cord dysfunction (VCD).
• Describe the three primary functions of the healthy vocal cords.
• List the conditions or factors that may trigger VCD.
• Explain how to differentiate VCD from asthma.
• Develop a treatment plan for VCD that addresses both patient-specific VCD triggers and management of symptomatic episodes.
FACULTY
Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies and Randy D. Danielsen is a Professor and Dean at the Arizona School of Health Sciences, AT Still University, Mesa. Ms. MacConnell is also a clinical PA affiliated with Enticare, an otolaryngology practice in Chandler, Arizona. Susan Symington is a clinical PA with the Arizona Asthma & Allergy Institute, with which Dr. Danielsen is also affiliated.
Linda MacConnell and Randy Danielsen have no significant financial relationships to disclose. Susan Symington is a member of the speaker’s bureau for Teva Respiratory and Thermo Fisher Scientific, Inc.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2014.
Article begins on next page >>
The symptoms of vocal cord dysfunction (VCD) can be mistaken for those
of asthma or other respiratory illnesses. As a result, VCD is often misdiagnosed,
leading to unnecessary, ineffective, costly, or even dangerous treatment. Here are
the facts that will enable you to avoid making an erroneous diagnosis, choosing
potentially harmful treatment, and delaying effective treatment.
A 33-year-old oncology nurse, JD, had moved from Seattle to Phoenix about six months earlier for a job opportunity. Shortly after starting her new job, she had developed intermittent dyspnea on exertion, with a cough lasting several minutes at a time, along with a sensation of heaviness over the larynx and a choking sensation. These symptoms were precipitated by gastroesophageal reflux disease (GERD), postnasal drainage, stress, and significant environmental change (ie, Seattle to Phoenix). She noticed that, since moving to Phoenix, she frequently cleared her throat but denied any hoarseness, dysphagia, chest tightness, chest pain, or wheezing. She noted nasal congestion and clear nasal discharge on exposure to inhaled irritants (eg, woodstove smoke) and strong fragrances (eg, perfume or cologne).
On physical examination, the patient was alert, oriented, and in no acute distress. She was coughing intermittently but was able to speak in complete sentences. No stridor or dyspnea was noted, either on exertion (jogging in place) or at rest.
HEENT examination was normal, with no scalp lesions or tenderness; face, symmetric; light reflex, symmetric; conjunctivae, clear; sclera white, without lesions or redness; pupils, equal, reactive to light and accommodation; tympanic membranes and canals, clear with intact landmarks; no nasal deformities; nasal mucosa, mildly erythematous with mild engorgement of the turbinates; no nasal polyps seen; nasal septum midline without perforation; no sinus tenderness on percussion; pharynx, clear without exudate; uvula rises on phonation; and oral mucosa and gingivae, pink without lesions. Neck was supple without masses or thyromegaly, and trachea was midline. Lungs were clear to auscultation with normal respiratory movement and no accessory muscle use, with normal anteroposterior diameter. Heart examination revealed regular rate and rhythm, without murmur, clicks, or gallops.
Examination of the skin was normal, without rashes, hives, swelling, petechiae, or significant ecchymosis. There was no palpable cervical, supraclavicular, or axillary adenopathy.
Results of laboratory studies included a normal complete blood count with differential and a normal IgE level of 46.3 IU/mL. Spirometry testing revealed normal values without obstruction; however, there was a flattening of the inspiratory flow loop, with no reversibility after bronchodilator, which was highly suggestive of vocal cord dysfunction (VCD). Perennial nonallergic rhinitis (formerly called vasomotor rhinitis) was confirmed because the patient experienced fewer symptoms to perfume after nasal corticosteroid use. The patient’s GERD was generally well controlled with esomeprazole but was likely a contributing factor to her vocal cord symptoms.
On laryngoscopy, abnormal vocal cord movement toward the midline during both inspiration and expiration was visualized, confirming the diagnosis of VCD.
BACKGROUND
VCD is a partial upper airway obstruction caused by paradoxical adduction (medial movement) of the vocal cords.1 Although it is primarily associated with inspiration, it sometimes manifests during expiration as well.
The true incidence of VCD is uncertain; different studies have found incidence rates varying from 2% to 27%, with higher rates in patients with asthma.1,2 However, highlighting the risk for misdiagnosis, some 10% of patients evaluated for asthma unresponsive to aggressive treatment were found, in fact, to have VCD alone.2
Similarly, although VCD is generally more common in women than in men, the reported female-to-male ratio has varied from 2:1 to 4:1.1,2,4 Some reports suggest that VCD is seen more frequently in younger women, with average ages at diagnosis of 14.5 in adolescents and 33 in adults.2,3 Others identify a broader age range, with most patients older than 50.4
Historically, VCD has been known by a variety of names and has been observed clinically since 1842. In that year, Dunglison referred to it as hysteric croup, describing a disorder of the laryngeal muscles brought on by “hysteria.”5 Later, Mackenzie was able to visualize adduction of the vocal cords during inspiration in patients with stridor by using a laryngoscope.6 Osler demonstrated his understanding of the condition in 1902, stating, “Spasm of muscles may occur with violent inspiratory effort and great distress, and may even lead to cyanosis. Extraordinary cries may be produced either inspiratory or expiratory.”7
More recently, in 1974, Patterson et al reported finding laryngoscopic evidence of VCD, which they termed Munchausen’s stridor.8 They used this descriptor to report on the case of a young woman with 15 hospital admissions for this condition. At the time, the etiology of the condition was believed to be largely psychologic, and its evaluation was consigned to psychiatrists and other mental health practitioners.
As laryngoscopy became more widely available in the 1970s and 1980s, diagnosis of VCD increased, although the condition remains underrecognized.9 Ibrahim et al suggest that primary care clinicians may not be as aware of VCD as they should be and may not consider laryngoscopy for possible VCD in patients whose asthma is poorly controlled.2
Disagreement persists with regard to the preferred name for the condition. Because numerous disorders involve abnormal vocal cord function, Christopher proposed moving away from the broad term VCD and toward a more descriptive term: paradoxical vocal fold motion (PVFM) disorder.10 Interestingly, use of the two terms seems to be divided along specialty lines: VCD is preferred by allergy, pulmonology, and mental health specialists, while PVFM is favored by otolaryngology specialists and speech-language pathologists.11
Further complicating awareness and recognition of VCD is its longstanding reputation as a psychologic disorder. In fact, the paradigm has shifted away from defining VCD as a purely psychopathologic entity to the identification of numerous functional etiologies for the disorder. This, however, has resulted in many new terms to describe the condition, including nonorganic upper airway obstruction, pseudoasthma, irritable larynx syndrome, factitious asthma, spasmodic croup, functional upper airway obstruction, episodic laryngeal dyskinesia, functional laryngeal obstruction, functional laryngeal stridor, and episodic paroxysmal laryngospasm.1
Regardless of its name, an understanding of VCD is essential for both primary care and specialty clinicians because of its frequent misdiagnosis as asthma, allergies, or severe upper airway obstruction. When it is misdiagnosed as asthma, aggressive asthma treatments—to which VCD does not respond—may be prescribed, including high-dose inhaled and systemic corticosteroids and bronchodilators. Patients may experience multiple emergency department (ED) visits and hospitalizations and, in some cases, may be subjected to tracheostomies and intubation.
Continue for vocal cord physiology and functions >>
VOCAL CORD PHYSIOLOGY AND FUNCTIONS
The vocal cords are located within the larynx. Abduction, or opening, of the cords is controlled by the posterior cricoarytenoid muscle; adduction, or closing, occurs via contraction of the lateral cricoarytenoid muscle. These muscles are innervated by the recurrent laryngeal nerve to control the width of the space—the rima glottidis—between the cords. During inspiration, the glottis opens; during expiration, it narrows but remains open.12
The vocal cords are involved in three main functions: protection of the airway, respiration, and phonation (vocal production). These functions are at least partially controlled involuntarily by brain stem reflexes; however, only airway protection—the most important of these functions—is reflexive and involuntary.12 Respiration may be controlled voluntarily, and phonation is primarily voluntary. Closure of the vocal cords is under the control of the laryngeal nerve branches of the vagal nerve.12,13
The vocal cords normally abduct during inspiration to allow air to pass through them into the trachea and the lungs. Sniffing, puffing, snuffling, and panting also cause the vocal cords to abduct. The vocal cords adduct with phonation (talking, singing), coughing, clearing the throat, performing the Valsalva maneuver, and swallowing. During expiration, 10% to 40% adduction is considered normal.14
VOCAL CORD DYSFUNCTION
Pathogenesis and etiology
VCD is a nonspecific term, and a number of factors may be involved in its development.15 Although the precise cause of VCD is unknown, it is believed to result from laryngeal hyperresponsiveness. This exaggerated responsiveness may be prompted by irritant and nonirritant triggers of the sensory receptors in the larynx, trachea, and large airways that mediate cough and glottis closure reflexes.16
VCD may be among a group of airway disorders triggered by occupational exposures, including irritants and psychologic stressors. For example, occupationally triggered VCD was diagnosed in rescue, recovery, and cleanup workers at the World Trade Center disaster site.4
A history of childhood sexual abuse has also been associated by some researchers with the development of VCD. For example, Freedman et al reported that, of 47 patients with VCD, 14 identified such a history and five were suspected of having been sexually abused as children.17
Paradoxical movement of the vocal cords causes them to close when they should open. (Click here for a video on normal and abnormal vocal cord movement.) VCD generally occurs during inspiration, causing obstruction of the incoming air through the larynx. Symptoms of VCD frequently include dyspnea, coughing, wheezing, hoarseness, and tightness or pain in the throat.
Examination of the flow-volume loops recorded when a patient experiences “wheezing” during spirometry testing reveals a flattened inspiratory loop, indicating a decrease of airflow into the lungs (see Figure 1).13,16 “Wheezing” is actually a misnomer in this situation because the term typically refers to sounds that occur during expiration.
Triggers
Physiologic, psychologic, and neurologic factors may all contribute to VCD.1,15 Conditions that can trigger VCD include
• Asthma
• Postnasal drip
• Recent upper respiratory illness (URI)
• Talking, singing
• Exercise
• Cough
• Voice strain
• Stress, anxiety, tension, elevated emotions
• Common irritants (eg, strong smells)
• Airborne irritants
• Rhinosinusitis
• GERD
• Use of certain medications
Identification of a particular patient’s triggers is key to successful management of VCD.
PATIENT PRESENTATION
Although there is no “typical” patient with VCD, the condition occurs more frequently in women, with the most common age at onset between 20 and 40 years. However, VCD has been seen in very young children and in adults as old as 83, and its diagnosis in the pediatric population is increasing.18
The patient may present with complaints of atypical chest pain, throat tightness, stridor, choking, difficult vocalization, cough and sometimes dysphagia, GERD, or rhinosinusitis (see Table 1). These signs and symptoms may occur without provocation, or patients may relate a history of triggers such as anxiety, irritant exposure, or exercise. In fact, about 14% of VCD is associated with exercise, particularly in young female athletes who experience shortness of breath and even stridor with exercise.19
A characteristic finding on physical examination is inspiratory stridor, along with respiratory distress.20 The stridor is best auscultated not over the anterior chest wall but over the tracheal area of the anterior neck.
Continue for differential diagnosis >>
DIFFERENTIAL DIAGNOSIS
Distinguishing VCD from other disorders can be challenging. Differential diagnosis should include
• Non–vocal cord adduction disorders, such as thyroid goiter, upper airway hemorrhage, caustic ingestion, neoplastic disorders, rheumatoid cricoarytenoid arthritis, pharyngeal abscess, angioedema, pulmonary embolus21
• Anatomic defects (eg, laryngomalacia, subglottic stenosis)
• Tracheal masses (eg, enlarged thyroid gland)
• Vocal cord polyps
• Laryngospasm
• Vocal cord paresis
• Neurologic causes (eg, brain stem compression, severe cortical injury, nuclear or lower motor neuron injury, movement disorders)
• Nonorganic causes (eg, factitious symptoms or malingering; conversion disorder)22
• Reactive airway disease.
Some disorders are easier to distinguish from VCD than others. For example, although laryngospasm may produce similar symptoms, episodes are brief, lasting seconds to minutes; VCD episodes may last hours to days.
Asthma
Even the most astute clinician will be unable to obtain adequate information from the patient history to differentiate VCD from asthma. There is a significant overlap of symptoms—shortness of breath, cough, wheezing—and frequently, the diseases coexist. History is often negative for chest pain, but it is common for patients with VCD, when asked to describe their symptoms, to report chest tightness. The clinician therefore needs to ask the patient to point to where the tightness is felt—in the chest or in the neck over the laryngeal area—to distinguish the source.
Asthma symptoms usually increase over a few hours, days, or weeks but respond to medications that open the airway and reduce inflammation (inhaled β-agonists and corticosteroids). VCD symptoms usually occur or decrease suddenly and do not respond well to traditional asthma treatments.
Other differences between asthma and VCD symptoms include voice changes and time of day when symptoms occur. The person with VCD will experience voice changes, such as hoarseness, as well as prolonged coughing episodes. Patients with asthma may awaken at night because of breathlessness, while most patients with VCD experience symptoms only during the day.
The diagnosis is generally confirmed if VCD is seen on direct laryngoscopic visualization during a symptomatic episode. In terms of adduction, the anterior cords will appear normal, but the posterior portion of the cords will display the classic “glottis chink” (see Figure 2).9
If the diagnosis is in question, videostroboscopy, a technique that provides a magnified slow-motion view of vocal cord vibration, can help identify or exclude pathologic conditions of the vocal cords.23
Convincing the patient of the validity of the diagnosis may be problematic if the patient has been previously diagnosed with and treated for another condition. The diagnosis should be explained and the patient counseled what appropriate care for VCD entails (see discussion under “Patient education and self-care”).
TREATMENT
Acute episode
During an acute VCD episode, offering the patient calm reassurance can be effective in resolving the episode. Simple breathing guidance may also be beneficial; instructing the patient to breathe rapidly and shallowly (ie, pant) can result in immediate resolution of symptoms.24 The patient can be advised to utilize other techniques, such as diaphragmatic breathing, breathing through the nose, breathing through a straw, pursed-lip breathing, and exhaling with a hissing sound.25
Long-term management
Although various strategies are employed in the management of VCD, well-designed studies on which to base treatment decisions have not been performed. Of course, control and management of possible underlying triggers or disorders should be implemented. Because etiology is rarely known, treatment for VCD is generally empiric.
Evidence does exist, however, to suggest that voice therapy, the treatment of choice for muscle tension dysphonia, is also effective for VCD. Speech therapy with specific voice and breathing exercises can enable the patient to manage the condition, thereby reducing ED visits, hospitalizations, and treatment costs.26
Patient education and self-care
Patient education is a critical component of VCD management. The clinician should explain the functions of the larynx to the patient, including the normal functioning of the vocal cords during respiration, speaking, swallowing, coughing, throat clearing, and breath holding. It may also enhance patients’ understanding of VCD to view their diagnostic laryngoscopy or videostroboscopy films.21
The patient should be advised to rest the voice, hydrate, utilize sialagogues (lozenges, gum) to stimulate salivation, reduce exposure to triggers when possible, and decrease stress. She should be encouraged to track VCD triggers by documenting what she is doing, where, and when, at the time of a VCD episode.
Two exercises—“paused breathing” and “belly breathing”—can be used by patients to learn how to relax the vocal cords (see “Patient Handout”). Patients should practice these exercises three times a day so that they can be easily recalled and performed during VCD episodes.
Continue for outcomes >>
OUTCOMES
Little is known about long-term outcomes for patients with VCD. The current literature consists of poorly described and conflicting case reports and results of small trials. Although documentation is lacking, the authors agree that, by educating the patient about the diagnosis, teaching effective VCD management strategies, and referring patients for voice therapy, clinicians can help patients achieve signicant improvement. Further investigation is needed to enhance our knowledge of the causes of VCD and to research additional diagnostic modalities and treatments.2
CASE PATIENT
After diagnosing VCD, the clinician explained the normal functioning of the vocal cords and how certain factors may cause them to close during inspiration. The patient then understood why bronchodilator therapy had failed to relieve her symptoms. She was counseled to continue her inhaled nasal steroid and proton pump inhibitor for her perennial nonallergic rhinitis and GERD, respectively, because these conditions may trigger her VCD, and to take steps to manage her stress. She learned breathing techniques to alleviate acute episodes of VCD and was informed of the option of voice therapy with a speech therapist if needed.
At six-week follow-up, the patient reported that she was complying with her medication regimen, had made an effort to relax more, and had experienced no acute attacks of VCD since her last visit.
CONCLUSION
Patients with symptoms suggestive of VCD require a thorough evaluation, including laryngoscopic examination, to ensure accurate diagnosis and avoid a too-common misdiagnosis. Primary care clinicians should know about VCD and, if not trained in the performance of flexible laryngoscopy, should refer the symptomatic patient to a specialist for appropriate work-up.
1. Hoyte FCL. Vocal cord dysfunction. Immunol Allergy Clin N Am. 2013;33:1-22.
2. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83:164-172.
3. Powell DM, Karanfilov BI, Beechler KB, et al. Paradoxical vocal cord dysfunction in juveniles. Arch Otolaryngol Head Neck Surg. 2000;126(1):29-34.
4. Husein OF, Husein TN, Gardner R, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope. 2008; 118(4):740-747.
5. Dunglison RD. The Practice of Medicine. Philadelphia, PA: Lea and Blanchard; 1842:257-258.
6. MacKenzie M. Use of Laryngoscopy in Diseases of the Throat. Philadelphia, PA: Lindsey and Blackeston; 1869:246-250.
7. Osler W. Hysteria. In: The Principles and Practice of Medicine. 4th ed. New York, NY: Appleton; 1902:1111-1112.
8. Patterson R, Schatz M, Horton M. Munchausen’s stridor: non-organic laryngeal obstruction. Clin Allergy. 1974;4:307-310.
9. Christopher KL, Wood RP 2nd, Eckert RC, et al. Vocal cord dysfunction presenting as asthma. N Engl J Med. 1983;308(26):1566-1570.
10. Christopher KL. Understanding vocal cord dysfunction: a step in the right direction with a long road ahead. Chest. 2006;129(4):842-843.
11. Christopher KL, Morris MJ. Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol Clin N Am. 2010;43:43-66.
12. Sasaki CT, Weaver EM. Physiology of the larynx. Am J Med. 1997;103:9S-18S.
13. Balkissoon R. Occupational upper airway disease. Clin Chest Med. 2002;23:717-725.
14. Murakami Y, Kirschner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59-71.
15. Forrest LA, Husein T, Husein O. Paradoxical vocal cord motion disorder: classification and treatment. Laryngoscope. 2012;122:844-853.
16. Altman KW, Simpson CB, Amin MR, et al. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511.
17. Freedman MR, Rosenberg SJ, Schmaling KB. Childhood sexual abuse in patients with paradoxical vocal cord dysfunction. J Nerv Ment Dis. 1991;179(5):295-298.
18. Buddiga P. Vocal cord dysfunction. Medscape. http://emedicine.medscape.com/article/137782-overview. Accessed November 12, 2014.
19. Chiang T, Marcinow AM, deSilva BW, et al. Exercise-induced paradoxical vocal fold motion disorder: diagnosis and management. Laryngoscope. 2013;123:727-731.
20. Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest. 1999;116(6):1676-1682.
21. Hicks M, Brugman SM, Katial R. Vocal cord dysfunction/paradoxical vocal fold motion. Prim Care. 2008;35(1):81-103.
22. Maschka DA, Bauman NM, McCray PB, et al. A classification scheme for paradoxical vocal fold motion. Laryngoscope. 1997;107(11):1429-1435.
23. Uloza V, Vegiene A, Pribuisiene R, Saferis V. Quantitative evaluation of video laryngostroboscopy: reliability of the basic parameters. J Voice. 2013;27(3):361-368.
24. Pitchenik AF. Functional laryngeal obstruction relieved by panting. Chest. 1991;100(5):1465-1467.
25. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-160.
26. Carding PN, Horsley IA, Docherty GJ. A study of the effectiveness of voice therapy in the treatment of 45 patients with nonorganic dysphonia. J Voice. 1999;13(1):72-104.
CE/CME No: CR-1412
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the evolution in thinking about the pathogenesis of and treatment for vocal cord dysfunction (VCD).
• Describe the three primary functions of the healthy vocal cords.
• List the conditions or factors that may trigger VCD.
• Explain how to differentiate VCD from asthma.
• Develop a treatment plan for VCD that addresses both patient-specific VCD triggers and management of symptomatic episodes.
FACULTY
Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies and Randy D. Danielsen is a Professor and Dean at the Arizona School of Health Sciences, AT Still University, Mesa. Ms. MacConnell is also a clinical PA affiliated with Enticare, an otolaryngology practice in Chandler, Arizona. Susan Symington is a clinical PA with the Arizona Asthma & Allergy Institute, with which Dr. Danielsen is also affiliated.
Linda MacConnell and Randy Danielsen have no significant financial relationships to disclose. Susan Symington is a member of the speaker’s bureau for Teva Respiratory and Thermo Fisher Scientific, Inc.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2014.
Article begins on next page >>
The symptoms of vocal cord dysfunction (VCD) can be mistaken for those
of asthma or other respiratory illnesses. As a result, VCD is often misdiagnosed,
leading to unnecessary, ineffective, costly, or even dangerous treatment. Here are
the facts that will enable you to avoid making an erroneous diagnosis, choosing
potentially harmful treatment, and delaying effective treatment.
A 33-year-old oncology nurse, JD, had moved from Seattle to Phoenix about six months earlier for a job opportunity. Shortly after starting her new job, she had developed intermittent dyspnea on exertion, with a cough lasting several minutes at a time, along with a sensation of heaviness over the larynx and a choking sensation. These symptoms were precipitated by gastroesophageal reflux disease (GERD), postnasal drainage, stress, and significant environmental change (ie, Seattle to Phoenix). She noticed that, since moving to Phoenix, she frequently cleared her throat but denied any hoarseness, dysphagia, chest tightness, chest pain, or wheezing. She noted nasal congestion and clear nasal discharge on exposure to inhaled irritants (eg, woodstove smoke) and strong fragrances (eg, perfume or cologne).
On physical examination, the patient was alert, oriented, and in no acute distress. She was coughing intermittently but was able to speak in complete sentences. No stridor or dyspnea was noted, either on exertion (jogging in place) or at rest.
HEENT examination was normal, with no scalp lesions or tenderness; face, symmetric; light reflex, symmetric; conjunctivae, clear; sclera white, without lesions or redness; pupils, equal, reactive to light and accommodation; tympanic membranes and canals, clear with intact landmarks; no nasal deformities; nasal mucosa, mildly erythematous with mild engorgement of the turbinates; no nasal polyps seen; nasal septum midline without perforation; no sinus tenderness on percussion; pharynx, clear without exudate; uvula rises on phonation; and oral mucosa and gingivae, pink without lesions. Neck was supple without masses or thyromegaly, and trachea was midline. Lungs were clear to auscultation with normal respiratory movement and no accessory muscle use, with normal anteroposterior diameter. Heart examination revealed regular rate and rhythm, without murmur, clicks, or gallops.
Examination of the skin was normal, without rashes, hives, swelling, petechiae, or significant ecchymosis. There was no palpable cervical, supraclavicular, or axillary adenopathy.
Results of laboratory studies included a normal complete blood count with differential and a normal IgE level of 46.3 IU/mL. Spirometry testing revealed normal values without obstruction; however, there was a flattening of the inspiratory flow loop, with no reversibility after bronchodilator, which was highly suggestive of vocal cord dysfunction (VCD). Perennial nonallergic rhinitis (formerly called vasomotor rhinitis) was confirmed because the patient experienced fewer symptoms to perfume after nasal corticosteroid use. The patient’s GERD was generally well controlled with esomeprazole but was likely a contributing factor to her vocal cord symptoms.
On laryngoscopy, abnormal vocal cord movement toward the midline during both inspiration and expiration was visualized, confirming the diagnosis of VCD.
BACKGROUND
VCD is a partial upper airway obstruction caused by paradoxical adduction (medial movement) of the vocal cords.1 Although it is primarily associated with inspiration, it sometimes manifests during expiration as well.
The true incidence of VCD is uncertain; different studies have found incidence rates varying from 2% to 27%, with higher rates in patients with asthma.1,2 However, highlighting the risk for misdiagnosis, some 10% of patients evaluated for asthma unresponsive to aggressive treatment were found, in fact, to have VCD alone.2
Similarly, although VCD is generally more common in women than in men, the reported female-to-male ratio has varied from 2:1 to 4:1.1,2,4 Some reports suggest that VCD is seen more frequently in younger women, with average ages at diagnosis of 14.5 in adolescents and 33 in adults.2,3 Others identify a broader age range, with most patients older than 50.4
Historically, VCD has been known by a variety of names and has been observed clinically since 1842. In that year, Dunglison referred to it as hysteric croup, describing a disorder of the laryngeal muscles brought on by “hysteria.”5 Later, Mackenzie was able to visualize adduction of the vocal cords during inspiration in patients with stridor by using a laryngoscope.6 Osler demonstrated his understanding of the condition in 1902, stating, “Spasm of muscles may occur with violent inspiratory effort and great distress, and may even lead to cyanosis. Extraordinary cries may be produced either inspiratory or expiratory.”7
More recently, in 1974, Patterson et al reported finding laryngoscopic evidence of VCD, which they termed Munchausen’s stridor.8 They used this descriptor to report on the case of a young woman with 15 hospital admissions for this condition. At the time, the etiology of the condition was believed to be largely psychologic, and its evaluation was consigned to psychiatrists and other mental health practitioners.
As laryngoscopy became more widely available in the 1970s and 1980s, diagnosis of VCD increased, although the condition remains underrecognized.9 Ibrahim et al suggest that primary care clinicians may not be as aware of VCD as they should be and may not consider laryngoscopy for possible VCD in patients whose asthma is poorly controlled.2
Disagreement persists with regard to the preferred name for the condition. Because numerous disorders involve abnormal vocal cord function, Christopher proposed moving away from the broad term VCD and toward a more descriptive term: paradoxical vocal fold motion (PVFM) disorder.10 Interestingly, use of the two terms seems to be divided along specialty lines: VCD is preferred by allergy, pulmonology, and mental health specialists, while PVFM is favored by otolaryngology specialists and speech-language pathologists.11
Further complicating awareness and recognition of VCD is its longstanding reputation as a psychologic disorder. In fact, the paradigm has shifted away from defining VCD as a purely psychopathologic entity to the identification of numerous functional etiologies for the disorder. This, however, has resulted in many new terms to describe the condition, including nonorganic upper airway obstruction, pseudoasthma, irritable larynx syndrome, factitious asthma, spasmodic croup, functional upper airway obstruction, episodic laryngeal dyskinesia, functional laryngeal obstruction, functional laryngeal stridor, and episodic paroxysmal laryngospasm.1
Regardless of its name, an understanding of VCD is essential for both primary care and specialty clinicians because of its frequent misdiagnosis as asthma, allergies, or severe upper airway obstruction. When it is misdiagnosed as asthma, aggressive asthma treatments—to which VCD does not respond—may be prescribed, including high-dose inhaled and systemic corticosteroids and bronchodilators. Patients may experience multiple emergency department (ED) visits and hospitalizations and, in some cases, may be subjected to tracheostomies and intubation.
Continue for vocal cord physiology and functions >>
VOCAL CORD PHYSIOLOGY AND FUNCTIONS
The vocal cords are located within the larynx. Abduction, or opening, of the cords is controlled by the posterior cricoarytenoid muscle; adduction, or closing, occurs via contraction of the lateral cricoarytenoid muscle. These muscles are innervated by the recurrent laryngeal nerve to control the width of the space—the rima glottidis—between the cords. During inspiration, the glottis opens; during expiration, it narrows but remains open.12
The vocal cords are involved in three main functions: protection of the airway, respiration, and phonation (vocal production). These functions are at least partially controlled involuntarily by brain stem reflexes; however, only airway protection—the most important of these functions—is reflexive and involuntary.12 Respiration may be controlled voluntarily, and phonation is primarily voluntary. Closure of the vocal cords is under the control of the laryngeal nerve branches of the vagal nerve.12,13
The vocal cords normally abduct during inspiration to allow air to pass through them into the trachea and the lungs. Sniffing, puffing, snuffling, and panting also cause the vocal cords to abduct. The vocal cords adduct with phonation (talking, singing), coughing, clearing the throat, performing the Valsalva maneuver, and swallowing. During expiration, 10% to 40% adduction is considered normal.14
VOCAL CORD DYSFUNCTION
Pathogenesis and etiology
VCD is a nonspecific term, and a number of factors may be involved in its development.15 Although the precise cause of VCD is unknown, it is believed to result from laryngeal hyperresponsiveness. This exaggerated responsiveness may be prompted by irritant and nonirritant triggers of the sensory receptors in the larynx, trachea, and large airways that mediate cough and glottis closure reflexes.16
VCD may be among a group of airway disorders triggered by occupational exposures, including irritants and psychologic stressors. For example, occupationally triggered VCD was diagnosed in rescue, recovery, and cleanup workers at the World Trade Center disaster site.4
A history of childhood sexual abuse has also been associated by some researchers with the development of VCD. For example, Freedman et al reported that, of 47 patients with VCD, 14 identified such a history and five were suspected of having been sexually abused as children.17
Paradoxical movement of the vocal cords causes them to close when they should open. (Click here for a video on normal and abnormal vocal cord movement.) VCD generally occurs during inspiration, causing obstruction of the incoming air through the larynx. Symptoms of VCD frequently include dyspnea, coughing, wheezing, hoarseness, and tightness or pain in the throat.
Examination of the flow-volume loops recorded when a patient experiences “wheezing” during spirometry testing reveals a flattened inspiratory loop, indicating a decrease of airflow into the lungs (see Figure 1).13,16 “Wheezing” is actually a misnomer in this situation because the term typically refers to sounds that occur during expiration.
Triggers
Physiologic, psychologic, and neurologic factors may all contribute to VCD.1,15 Conditions that can trigger VCD include
• Asthma
• Postnasal drip
• Recent upper respiratory illness (URI)
• Talking, singing
• Exercise
• Cough
• Voice strain
• Stress, anxiety, tension, elevated emotions
• Common irritants (eg, strong smells)
• Airborne irritants
• Rhinosinusitis
• GERD
• Use of certain medications
Identification of a particular patient’s triggers is key to successful management of VCD.
PATIENT PRESENTATION
Although there is no “typical” patient with VCD, the condition occurs more frequently in women, with the most common age at onset between 20 and 40 years. However, VCD has been seen in very young children and in adults as old as 83, and its diagnosis in the pediatric population is increasing.18
The patient may present with complaints of atypical chest pain, throat tightness, stridor, choking, difficult vocalization, cough and sometimes dysphagia, GERD, or rhinosinusitis (see Table 1). These signs and symptoms may occur without provocation, or patients may relate a history of triggers such as anxiety, irritant exposure, or exercise. In fact, about 14% of VCD is associated with exercise, particularly in young female athletes who experience shortness of breath and even stridor with exercise.19
A characteristic finding on physical examination is inspiratory stridor, along with respiratory distress.20 The stridor is best auscultated not over the anterior chest wall but over the tracheal area of the anterior neck.
Continue for differential diagnosis >>
DIFFERENTIAL DIAGNOSIS
Distinguishing VCD from other disorders can be challenging. Differential diagnosis should include
• Non–vocal cord adduction disorders, such as thyroid goiter, upper airway hemorrhage, caustic ingestion, neoplastic disorders, rheumatoid cricoarytenoid arthritis, pharyngeal abscess, angioedema, pulmonary embolus21
• Anatomic defects (eg, laryngomalacia, subglottic stenosis)
• Tracheal masses (eg, enlarged thyroid gland)
• Vocal cord polyps
• Laryngospasm
• Vocal cord paresis
• Neurologic causes (eg, brain stem compression, severe cortical injury, nuclear or lower motor neuron injury, movement disorders)
• Nonorganic causes (eg, factitious symptoms or malingering; conversion disorder)22
• Reactive airway disease.
Some disorders are easier to distinguish from VCD than others. For example, although laryngospasm may produce similar symptoms, episodes are brief, lasting seconds to minutes; VCD episodes may last hours to days.
Asthma
Even the most astute clinician will be unable to obtain adequate information from the patient history to differentiate VCD from asthma. There is a significant overlap of symptoms—shortness of breath, cough, wheezing—and frequently, the diseases coexist. History is often negative for chest pain, but it is common for patients with VCD, when asked to describe their symptoms, to report chest tightness. The clinician therefore needs to ask the patient to point to where the tightness is felt—in the chest or in the neck over the laryngeal area—to distinguish the source.
Asthma symptoms usually increase over a few hours, days, or weeks but respond to medications that open the airway and reduce inflammation (inhaled β-agonists and corticosteroids). VCD symptoms usually occur or decrease suddenly and do not respond well to traditional asthma treatments.
Other differences between asthma and VCD symptoms include voice changes and time of day when symptoms occur. The person with VCD will experience voice changes, such as hoarseness, as well as prolonged coughing episodes. Patients with asthma may awaken at night because of breathlessness, while most patients with VCD experience symptoms only during the day.
The diagnosis is generally confirmed if VCD is seen on direct laryngoscopic visualization during a symptomatic episode. In terms of adduction, the anterior cords will appear normal, but the posterior portion of the cords will display the classic “glottis chink” (see Figure 2).9
If the diagnosis is in question, videostroboscopy, a technique that provides a magnified slow-motion view of vocal cord vibration, can help identify or exclude pathologic conditions of the vocal cords.23
Convincing the patient of the validity of the diagnosis may be problematic if the patient has been previously diagnosed with and treated for another condition. The diagnosis should be explained and the patient counseled what appropriate care for VCD entails (see discussion under “Patient education and self-care”).
TREATMENT
Acute episode
During an acute VCD episode, offering the patient calm reassurance can be effective in resolving the episode. Simple breathing guidance may also be beneficial; instructing the patient to breathe rapidly and shallowly (ie, pant) can result in immediate resolution of symptoms.24 The patient can be advised to utilize other techniques, such as diaphragmatic breathing, breathing through the nose, breathing through a straw, pursed-lip breathing, and exhaling with a hissing sound.25
Long-term management
Although various strategies are employed in the management of VCD, well-designed studies on which to base treatment decisions have not been performed. Of course, control and management of possible underlying triggers or disorders should be implemented. Because etiology is rarely known, treatment for VCD is generally empiric.
Evidence does exist, however, to suggest that voice therapy, the treatment of choice for muscle tension dysphonia, is also effective for VCD. Speech therapy with specific voice and breathing exercises can enable the patient to manage the condition, thereby reducing ED visits, hospitalizations, and treatment costs.26
Patient education and self-care
Patient education is a critical component of VCD management. The clinician should explain the functions of the larynx to the patient, including the normal functioning of the vocal cords during respiration, speaking, swallowing, coughing, throat clearing, and breath holding. It may also enhance patients’ understanding of VCD to view their diagnostic laryngoscopy or videostroboscopy films.21
The patient should be advised to rest the voice, hydrate, utilize sialagogues (lozenges, gum) to stimulate salivation, reduce exposure to triggers when possible, and decrease stress. She should be encouraged to track VCD triggers by documenting what she is doing, where, and when, at the time of a VCD episode.
Two exercises—“paused breathing” and “belly breathing”—can be used by patients to learn how to relax the vocal cords (see “Patient Handout”). Patients should practice these exercises three times a day so that they can be easily recalled and performed during VCD episodes.
Continue for outcomes >>
OUTCOMES
Little is known about long-term outcomes for patients with VCD. The current literature consists of poorly described and conflicting case reports and results of small trials. Although documentation is lacking, the authors agree that, by educating the patient about the diagnosis, teaching effective VCD management strategies, and referring patients for voice therapy, clinicians can help patients achieve signicant improvement. Further investigation is needed to enhance our knowledge of the causes of VCD and to research additional diagnostic modalities and treatments.2
CASE PATIENT
After diagnosing VCD, the clinician explained the normal functioning of the vocal cords and how certain factors may cause them to close during inspiration. The patient then understood why bronchodilator therapy had failed to relieve her symptoms. She was counseled to continue her inhaled nasal steroid and proton pump inhibitor for her perennial nonallergic rhinitis and GERD, respectively, because these conditions may trigger her VCD, and to take steps to manage her stress. She learned breathing techniques to alleviate acute episodes of VCD and was informed of the option of voice therapy with a speech therapist if needed.
At six-week follow-up, the patient reported that she was complying with her medication regimen, had made an effort to relax more, and had experienced no acute attacks of VCD since her last visit.
CONCLUSION
Patients with symptoms suggestive of VCD require a thorough evaluation, including laryngoscopic examination, to ensure accurate diagnosis and avoid a too-common misdiagnosis. Primary care clinicians should know about VCD and, if not trained in the performance of flexible laryngoscopy, should refer the symptomatic patient to a specialist for appropriate work-up.
CE/CME No: CR-1412
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the evolution in thinking about the pathogenesis of and treatment for vocal cord dysfunction (VCD).
• Describe the three primary functions of the healthy vocal cords.
• List the conditions or factors that may trigger VCD.
• Explain how to differentiate VCD from asthma.
• Develop a treatment plan for VCD that addresses both patient-specific VCD triggers and management of symptomatic episodes.
FACULTY
Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies and Randy D. Danielsen is a Professor and Dean at the Arizona School of Health Sciences, AT Still University, Mesa. Ms. MacConnell is also a clinical PA affiliated with Enticare, an otolaryngology practice in Chandler, Arizona. Susan Symington is a clinical PA with the Arizona Asthma & Allergy Institute, with which Dr. Danielsen is also affiliated.
Linda MacConnell and Randy Danielsen have no significant financial relationships to disclose. Susan Symington is a member of the speaker’s bureau for Teva Respiratory and Thermo Fisher Scientific, Inc.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2014.
Article begins on next page >>
The symptoms of vocal cord dysfunction (VCD) can be mistaken for those
of asthma or other respiratory illnesses. As a result, VCD is often misdiagnosed,
leading to unnecessary, ineffective, costly, or even dangerous treatment. Here are
the facts that will enable you to avoid making an erroneous diagnosis, choosing
potentially harmful treatment, and delaying effective treatment.
A 33-year-old oncology nurse, JD, had moved from Seattle to Phoenix about six months earlier for a job opportunity. Shortly after starting her new job, she had developed intermittent dyspnea on exertion, with a cough lasting several minutes at a time, along with a sensation of heaviness over the larynx and a choking sensation. These symptoms were precipitated by gastroesophageal reflux disease (GERD), postnasal drainage, stress, and significant environmental change (ie, Seattle to Phoenix). She noticed that, since moving to Phoenix, she frequently cleared her throat but denied any hoarseness, dysphagia, chest tightness, chest pain, or wheezing. She noted nasal congestion and clear nasal discharge on exposure to inhaled irritants (eg, woodstove smoke) and strong fragrances (eg, perfume or cologne).
On physical examination, the patient was alert, oriented, and in no acute distress. She was coughing intermittently but was able to speak in complete sentences. No stridor or dyspnea was noted, either on exertion (jogging in place) or at rest.
HEENT examination was normal, with no scalp lesions or tenderness; face, symmetric; light reflex, symmetric; conjunctivae, clear; sclera white, without lesions or redness; pupils, equal, reactive to light and accommodation; tympanic membranes and canals, clear with intact landmarks; no nasal deformities; nasal mucosa, mildly erythematous with mild engorgement of the turbinates; no nasal polyps seen; nasal septum midline without perforation; no sinus tenderness on percussion; pharynx, clear without exudate; uvula rises on phonation; and oral mucosa and gingivae, pink without lesions. Neck was supple without masses or thyromegaly, and trachea was midline. Lungs were clear to auscultation with normal respiratory movement and no accessory muscle use, with normal anteroposterior diameter. Heart examination revealed regular rate and rhythm, without murmur, clicks, or gallops.
Examination of the skin was normal, without rashes, hives, swelling, petechiae, or significant ecchymosis. There was no palpable cervical, supraclavicular, or axillary adenopathy.
Results of laboratory studies included a normal complete blood count with differential and a normal IgE level of 46.3 IU/mL. Spirometry testing revealed normal values without obstruction; however, there was a flattening of the inspiratory flow loop, with no reversibility after bronchodilator, which was highly suggestive of vocal cord dysfunction (VCD). Perennial nonallergic rhinitis (formerly called vasomotor rhinitis) was confirmed because the patient experienced fewer symptoms to perfume after nasal corticosteroid use. The patient’s GERD was generally well controlled with esomeprazole but was likely a contributing factor to her vocal cord symptoms.
On laryngoscopy, abnormal vocal cord movement toward the midline during both inspiration and expiration was visualized, confirming the diagnosis of VCD.
BACKGROUND
VCD is a partial upper airway obstruction caused by paradoxical adduction (medial movement) of the vocal cords.1 Although it is primarily associated with inspiration, it sometimes manifests during expiration as well.
The true incidence of VCD is uncertain; different studies have found incidence rates varying from 2% to 27%, with higher rates in patients with asthma.1,2 However, highlighting the risk for misdiagnosis, some 10% of patients evaluated for asthma unresponsive to aggressive treatment were found, in fact, to have VCD alone.2
Similarly, although VCD is generally more common in women than in men, the reported female-to-male ratio has varied from 2:1 to 4:1.1,2,4 Some reports suggest that VCD is seen more frequently in younger women, with average ages at diagnosis of 14.5 in adolescents and 33 in adults.2,3 Others identify a broader age range, with most patients older than 50.4
Historically, VCD has been known by a variety of names and has been observed clinically since 1842. In that year, Dunglison referred to it as hysteric croup, describing a disorder of the laryngeal muscles brought on by “hysteria.”5 Later, Mackenzie was able to visualize adduction of the vocal cords during inspiration in patients with stridor by using a laryngoscope.6 Osler demonstrated his understanding of the condition in 1902, stating, “Spasm of muscles may occur with violent inspiratory effort and great distress, and may even lead to cyanosis. Extraordinary cries may be produced either inspiratory or expiratory.”7
More recently, in 1974, Patterson et al reported finding laryngoscopic evidence of VCD, which they termed Munchausen’s stridor.8 They used this descriptor to report on the case of a young woman with 15 hospital admissions for this condition. At the time, the etiology of the condition was believed to be largely psychologic, and its evaluation was consigned to psychiatrists and other mental health practitioners.
As laryngoscopy became more widely available in the 1970s and 1980s, diagnosis of VCD increased, although the condition remains underrecognized.9 Ibrahim et al suggest that primary care clinicians may not be as aware of VCD as they should be and may not consider laryngoscopy for possible VCD in patients whose asthma is poorly controlled.2
Disagreement persists with regard to the preferred name for the condition. Because numerous disorders involve abnormal vocal cord function, Christopher proposed moving away from the broad term VCD and toward a more descriptive term: paradoxical vocal fold motion (PVFM) disorder.10 Interestingly, use of the two terms seems to be divided along specialty lines: VCD is preferred by allergy, pulmonology, and mental health specialists, while PVFM is favored by otolaryngology specialists and speech-language pathologists.11
Further complicating awareness and recognition of VCD is its longstanding reputation as a psychologic disorder. In fact, the paradigm has shifted away from defining VCD as a purely psychopathologic entity to the identification of numerous functional etiologies for the disorder. This, however, has resulted in many new terms to describe the condition, including nonorganic upper airway obstruction, pseudoasthma, irritable larynx syndrome, factitious asthma, spasmodic croup, functional upper airway obstruction, episodic laryngeal dyskinesia, functional laryngeal obstruction, functional laryngeal stridor, and episodic paroxysmal laryngospasm.1
Regardless of its name, an understanding of VCD is essential for both primary care and specialty clinicians because of its frequent misdiagnosis as asthma, allergies, or severe upper airway obstruction. When it is misdiagnosed as asthma, aggressive asthma treatments—to which VCD does not respond—may be prescribed, including high-dose inhaled and systemic corticosteroids and bronchodilators. Patients may experience multiple emergency department (ED) visits and hospitalizations and, in some cases, may be subjected to tracheostomies and intubation.
Continue for vocal cord physiology and functions >>
VOCAL CORD PHYSIOLOGY AND FUNCTIONS
The vocal cords are located within the larynx. Abduction, or opening, of the cords is controlled by the posterior cricoarytenoid muscle; adduction, or closing, occurs via contraction of the lateral cricoarytenoid muscle. These muscles are innervated by the recurrent laryngeal nerve to control the width of the space—the rima glottidis—between the cords. During inspiration, the glottis opens; during expiration, it narrows but remains open.12
The vocal cords are involved in three main functions: protection of the airway, respiration, and phonation (vocal production). These functions are at least partially controlled involuntarily by brain stem reflexes; however, only airway protection—the most important of these functions—is reflexive and involuntary.12 Respiration may be controlled voluntarily, and phonation is primarily voluntary. Closure of the vocal cords is under the control of the laryngeal nerve branches of the vagal nerve.12,13
The vocal cords normally abduct during inspiration to allow air to pass through them into the trachea and the lungs. Sniffing, puffing, snuffling, and panting also cause the vocal cords to abduct. The vocal cords adduct with phonation (talking, singing), coughing, clearing the throat, performing the Valsalva maneuver, and swallowing. During expiration, 10% to 40% adduction is considered normal.14
VOCAL CORD DYSFUNCTION
Pathogenesis and etiology
VCD is a nonspecific term, and a number of factors may be involved in its development.15 Although the precise cause of VCD is unknown, it is believed to result from laryngeal hyperresponsiveness. This exaggerated responsiveness may be prompted by irritant and nonirritant triggers of the sensory receptors in the larynx, trachea, and large airways that mediate cough and glottis closure reflexes.16
VCD may be among a group of airway disorders triggered by occupational exposures, including irritants and psychologic stressors. For example, occupationally triggered VCD was diagnosed in rescue, recovery, and cleanup workers at the World Trade Center disaster site.4
A history of childhood sexual abuse has also been associated by some researchers with the development of VCD. For example, Freedman et al reported that, of 47 patients with VCD, 14 identified such a history and five were suspected of having been sexually abused as children.17
Paradoxical movement of the vocal cords causes them to close when they should open. (Click here for a video on normal and abnormal vocal cord movement.) VCD generally occurs during inspiration, causing obstruction of the incoming air through the larynx. Symptoms of VCD frequently include dyspnea, coughing, wheezing, hoarseness, and tightness or pain in the throat.
Examination of the flow-volume loops recorded when a patient experiences “wheezing” during spirometry testing reveals a flattened inspiratory loop, indicating a decrease of airflow into the lungs (see Figure 1).13,16 “Wheezing” is actually a misnomer in this situation because the term typically refers to sounds that occur during expiration.
Triggers
Physiologic, psychologic, and neurologic factors may all contribute to VCD.1,15 Conditions that can trigger VCD include
• Asthma
• Postnasal drip
• Recent upper respiratory illness (URI)
• Talking, singing
• Exercise
• Cough
• Voice strain
• Stress, anxiety, tension, elevated emotions
• Common irritants (eg, strong smells)
• Airborne irritants
• Rhinosinusitis
• GERD
• Use of certain medications
Identification of a particular patient’s triggers is key to successful management of VCD.
PATIENT PRESENTATION
Although there is no “typical” patient with VCD, the condition occurs more frequently in women, with the most common age at onset between 20 and 40 years. However, VCD has been seen in very young children and in adults as old as 83, and its diagnosis in the pediatric population is increasing.18
The patient may present with complaints of atypical chest pain, throat tightness, stridor, choking, difficult vocalization, cough and sometimes dysphagia, GERD, or rhinosinusitis (see Table 1). These signs and symptoms may occur without provocation, or patients may relate a history of triggers such as anxiety, irritant exposure, or exercise. In fact, about 14% of VCD is associated with exercise, particularly in young female athletes who experience shortness of breath and even stridor with exercise.19
A characteristic finding on physical examination is inspiratory stridor, along with respiratory distress.20 The stridor is best auscultated not over the anterior chest wall but over the tracheal area of the anterior neck.
Continue for differential diagnosis >>
DIFFERENTIAL DIAGNOSIS
Distinguishing VCD from other disorders can be challenging. Differential diagnosis should include
• Non–vocal cord adduction disorders, such as thyroid goiter, upper airway hemorrhage, caustic ingestion, neoplastic disorders, rheumatoid cricoarytenoid arthritis, pharyngeal abscess, angioedema, pulmonary embolus21
• Anatomic defects (eg, laryngomalacia, subglottic stenosis)
• Tracheal masses (eg, enlarged thyroid gland)
• Vocal cord polyps
• Laryngospasm
• Vocal cord paresis
• Neurologic causes (eg, brain stem compression, severe cortical injury, nuclear or lower motor neuron injury, movement disorders)
• Nonorganic causes (eg, factitious symptoms or malingering; conversion disorder)22
• Reactive airway disease.
Some disorders are easier to distinguish from VCD than others. For example, although laryngospasm may produce similar symptoms, episodes are brief, lasting seconds to minutes; VCD episodes may last hours to days.
Asthma
Even the most astute clinician will be unable to obtain adequate information from the patient history to differentiate VCD from asthma. There is a significant overlap of symptoms—shortness of breath, cough, wheezing—and frequently, the diseases coexist. History is often negative for chest pain, but it is common for patients with VCD, when asked to describe their symptoms, to report chest tightness. The clinician therefore needs to ask the patient to point to where the tightness is felt—in the chest or in the neck over the laryngeal area—to distinguish the source.
Asthma symptoms usually increase over a few hours, days, or weeks but respond to medications that open the airway and reduce inflammation (inhaled β-agonists and corticosteroids). VCD symptoms usually occur or decrease suddenly and do not respond well to traditional asthma treatments.
Other differences between asthma and VCD symptoms include voice changes and time of day when symptoms occur. The person with VCD will experience voice changes, such as hoarseness, as well as prolonged coughing episodes. Patients with asthma may awaken at night because of breathlessness, while most patients with VCD experience symptoms only during the day.
The diagnosis is generally confirmed if VCD is seen on direct laryngoscopic visualization during a symptomatic episode. In terms of adduction, the anterior cords will appear normal, but the posterior portion of the cords will display the classic “glottis chink” (see Figure 2).9
If the diagnosis is in question, videostroboscopy, a technique that provides a magnified slow-motion view of vocal cord vibration, can help identify or exclude pathologic conditions of the vocal cords.23
Convincing the patient of the validity of the diagnosis may be problematic if the patient has been previously diagnosed with and treated for another condition. The diagnosis should be explained and the patient counseled what appropriate care for VCD entails (see discussion under “Patient education and self-care”).
TREATMENT
Acute episode
During an acute VCD episode, offering the patient calm reassurance can be effective in resolving the episode. Simple breathing guidance may also be beneficial; instructing the patient to breathe rapidly and shallowly (ie, pant) can result in immediate resolution of symptoms.24 The patient can be advised to utilize other techniques, such as diaphragmatic breathing, breathing through the nose, breathing through a straw, pursed-lip breathing, and exhaling with a hissing sound.25
Long-term management
Although various strategies are employed in the management of VCD, well-designed studies on which to base treatment decisions have not been performed. Of course, control and management of possible underlying triggers or disorders should be implemented. Because etiology is rarely known, treatment for VCD is generally empiric.
Evidence does exist, however, to suggest that voice therapy, the treatment of choice for muscle tension dysphonia, is also effective for VCD. Speech therapy with specific voice and breathing exercises can enable the patient to manage the condition, thereby reducing ED visits, hospitalizations, and treatment costs.26
Patient education and self-care
Patient education is a critical component of VCD management. The clinician should explain the functions of the larynx to the patient, including the normal functioning of the vocal cords during respiration, speaking, swallowing, coughing, throat clearing, and breath holding. It may also enhance patients’ understanding of VCD to view their diagnostic laryngoscopy or videostroboscopy films.21
The patient should be advised to rest the voice, hydrate, utilize sialagogues (lozenges, gum) to stimulate salivation, reduce exposure to triggers when possible, and decrease stress. She should be encouraged to track VCD triggers by documenting what she is doing, where, and when, at the time of a VCD episode.
Two exercises—“paused breathing” and “belly breathing”—can be used by patients to learn how to relax the vocal cords (see “Patient Handout”). Patients should practice these exercises three times a day so that they can be easily recalled and performed during VCD episodes.
Continue for outcomes >>
OUTCOMES
Little is known about long-term outcomes for patients with VCD. The current literature consists of poorly described and conflicting case reports and results of small trials. Although documentation is lacking, the authors agree that, by educating the patient about the diagnosis, teaching effective VCD management strategies, and referring patients for voice therapy, clinicians can help patients achieve signicant improvement. Further investigation is needed to enhance our knowledge of the causes of VCD and to research additional diagnostic modalities and treatments.2
CASE PATIENT
After diagnosing VCD, the clinician explained the normal functioning of the vocal cords and how certain factors may cause them to close during inspiration. The patient then understood why bronchodilator therapy had failed to relieve her symptoms. She was counseled to continue her inhaled nasal steroid and proton pump inhibitor for her perennial nonallergic rhinitis and GERD, respectively, because these conditions may trigger her VCD, and to take steps to manage her stress. She learned breathing techniques to alleviate acute episodes of VCD and was informed of the option of voice therapy with a speech therapist if needed.
At six-week follow-up, the patient reported that she was complying with her medication regimen, had made an effort to relax more, and had experienced no acute attacks of VCD since her last visit.
CONCLUSION
Patients with symptoms suggestive of VCD require a thorough evaluation, including laryngoscopic examination, to ensure accurate diagnosis and avoid a too-common misdiagnosis. Primary care clinicians should know about VCD and, if not trained in the performance of flexible laryngoscopy, should refer the symptomatic patient to a specialist for appropriate work-up.
1. Hoyte FCL. Vocal cord dysfunction. Immunol Allergy Clin N Am. 2013;33:1-22.
2. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83:164-172.
3. Powell DM, Karanfilov BI, Beechler KB, et al. Paradoxical vocal cord dysfunction in juveniles. Arch Otolaryngol Head Neck Surg. 2000;126(1):29-34.
4. Husein OF, Husein TN, Gardner R, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope. 2008; 118(4):740-747.
5. Dunglison RD. The Practice of Medicine. Philadelphia, PA: Lea and Blanchard; 1842:257-258.
6. MacKenzie M. Use of Laryngoscopy in Diseases of the Throat. Philadelphia, PA: Lindsey and Blackeston; 1869:246-250.
7. Osler W. Hysteria. In: The Principles and Practice of Medicine. 4th ed. New York, NY: Appleton; 1902:1111-1112.
8. Patterson R, Schatz M, Horton M. Munchausen’s stridor: non-organic laryngeal obstruction. Clin Allergy. 1974;4:307-310.
9. Christopher KL, Wood RP 2nd, Eckert RC, et al. Vocal cord dysfunction presenting as asthma. N Engl J Med. 1983;308(26):1566-1570.
10. Christopher KL. Understanding vocal cord dysfunction: a step in the right direction with a long road ahead. Chest. 2006;129(4):842-843.
11. Christopher KL, Morris MJ. Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol Clin N Am. 2010;43:43-66.
12. Sasaki CT, Weaver EM. Physiology of the larynx. Am J Med. 1997;103:9S-18S.
13. Balkissoon R. Occupational upper airway disease. Clin Chest Med. 2002;23:717-725.
14. Murakami Y, Kirschner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59-71.
15. Forrest LA, Husein T, Husein O. Paradoxical vocal cord motion disorder: classification and treatment. Laryngoscope. 2012;122:844-853.
16. Altman KW, Simpson CB, Amin MR, et al. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511.
17. Freedman MR, Rosenberg SJ, Schmaling KB. Childhood sexual abuse in patients with paradoxical vocal cord dysfunction. J Nerv Ment Dis. 1991;179(5):295-298.
18. Buddiga P. Vocal cord dysfunction. Medscape. http://emedicine.medscape.com/article/137782-overview. Accessed November 12, 2014.
19. Chiang T, Marcinow AM, deSilva BW, et al. Exercise-induced paradoxical vocal fold motion disorder: diagnosis and management. Laryngoscope. 2013;123:727-731.
20. Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest. 1999;116(6):1676-1682.
21. Hicks M, Brugman SM, Katial R. Vocal cord dysfunction/paradoxical vocal fold motion. Prim Care. 2008;35(1):81-103.
22. Maschka DA, Bauman NM, McCray PB, et al. A classification scheme for paradoxical vocal fold motion. Laryngoscope. 1997;107(11):1429-1435.
23. Uloza V, Vegiene A, Pribuisiene R, Saferis V. Quantitative evaluation of video laryngostroboscopy: reliability of the basic parameters. J Voice. 2013;27(3):361-368.
24. Pitchenik AF. Functional laryngeal obstruction relieved by panting. Chest. 1991;100(5):1465-1467.
25. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-160.
26. Carding PN, Horsley IA, Docherty GJ. A study of the effectiveness of voice therapy in the treatment of 45 patients with nonorganic dysphonia. J Voice. 1999;13(1):72-104.
1. Hoyte FCL. Vocal cord dysfunction. Immunol Allergy Clin N Am. 2013;33:1-22.
2. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83:164-172.
3. Powell DM, Karanfilov BI, Beechler KB, et al. Paradoxical vocal cord dysfunction in juveniles. Arch Otolaryngol Head Neck Surg. 2000;126(1):29-34.
4. Husein OF, Husein TN, Gardner R, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope. 2008; 118(4):740-747.
5. Dunglison RD. The Practice of Medicine. Philadelphia, PA: Lea and Blanchard; 1842:257-258.
6. MacKenzie M. Use of Laryngoscopy in Diseases of the Throat. Philadelphia, PA: Lindsey and Blackeston; 1869:246-250.
7. Osler W. Hysteria. In: The Principles and Practice of Medicine. 4th ed. New York, NY: Appleton; 1902:1111-1112.
8. Patterson R, Schatz M, Horton M. Munchausen’s stridor: non-organic laryngeal obstruction. Clin Allergy. 1974;4:307-310.
9. Christopher KL, Wood RP 2nd, Eckert RC, et al. Vocal cord dysfunction presenting as asthma. N Engl J Med. 1983;308(26):1566-1570.
10. Christopher KL. Understanding vocal cord dysfunction: a step in the right direction with a long road ahead. Chest. 2006;129(4):842-843.
11. Christopher KL, Morris MJ. Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol Clin N Am. 2010;43:43-66.
12. Sasaki CT, Weaver EM. Physiology of the larynx. Am J Med. 1997;103:9S-18S.
13. Balkissoon R. Occupational upper airway disease. Clin Chest Med. 2002;23:717-725.
14. Murakami Y, Kirschner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59-71.
15. Forrest LA, Husein T, Husein O. Paradoxical vocal cord motion disorder: classification and treatment. Laryngoscope. 2012;122:844-853.
16. Altman KW, Simpson CB, Amin MR, et al. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511.
17. Freedman MR, Rosenberg SJ, Schmaling KB. Childhood sexual abuse in patients with paradoxical vocal cord dysfunction. J Nerv Ment Dis. 1991;179(5):295-298.
18. Buddiga P. Vocal cord dysfunction. Medscape. http://emedicine.medscape.com/article/137782-overview. Accessed November 12, 2014.
19. Chiang T, Marcinow AM, deSilva BW, et al. Exercise-induced paradoxical vocal fold motion disorder: diagnosis and management. Laryngoscope. 2013;123:727-731.
20. Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest. 1999;116(6):1676-1682.
21. Hicks M, Brugman SM, Katial R. Vocal cord dysfunction/paradoxical vocal fold motion. Prim Care. 2008;35(1):81-103.
22. Maschka DA, Bauman NM, McCray PB, et al. A classification scheme for paradoxical vocal fold motion. Laryngoscope. 1997;107(11):1429-1435.
23. Uloza V, Vegiene A, Pribuisiene R, Saferis V. Quantitative evaluation of video laryngostroboscopy: reliability of the basic parameters. J Voice. 2013;27(3):361-368.
24. Pitchenik AF. Functional laryngeal obstruction relieved by panting. Chest. 1991;100(5):1465-1467.
25. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-160.
26. Carding PN, Horsley IA, Docherty GJ. A study of the effectiveness of voice therapy in the treatment of 45 patients with nonorganic dysphonia. J Voice. 1999;13(1):72-104.
Cruising With Disaster
I recently returned from a pleasure cruise to celebrate a milestone birthday. (I’m not telling you which one—but it did make me eligible for government health care!) Prior to embarking on this adventure, I had dreams of calm blue-green waters, endless plates of delectable food, sitting on the stateroom veranda drinking wine while watching the sunset, and exciting land and sea excursions.
However, I must admit that I also felt apprehensive. Just a year ago, one of my best friends lost his wife to severe influenza and necrotizing pneumonia while on a cruise. This disastrous event occurred very rapidly (what I call “from cough to death in less than 48 hours”) and in the same locale as my cruise. So, you can imagine my concern.
To prepare myself, I did a bit of research on the types of infectious diseases occurring aboard ship. The leading cause of human acute viral gastroenteritis on cruise ships is norovirus, outbreaks of which have increased significantly in recent years1 and always garner significant media attention.
However, the most frequent consultation with a ship’s medical team on most cruises is for respiratory illness.2 Outbreaks are not uncommon, particularly within flu season (October through May in the Northern hemisphere and April through September in the Southern hemisphere). Unfortunately, flu season is year round in the tropics and subtropics.3 There have also been occurrences of invasive meningococcal disease (four cases in the Mediterranean in October 2012, one of which was ultimately fatal)4 and cyclosporiasis (on two successive voyages embarking from Australia in 2010).5
Considering the detrimental consequences for passengers and the subsequent high costs for cruise companies, disease outbreaks on cruise ships represent a serious public health issue.6 Unfortunately, contagious disease on commercial ships tends to be poorly managed, as there is little capacity to confirm a case or isolate to curb transmission.7 (Personally, I was pleased—and relieved—to see that crew members stood outside every restaurant on the ship offering passengers hand disinfectant gel.)
I’m not trying to be alarmist. After all, I had a great cruise free of concerns. But I think anyone who sets sail should be aware of the circumstances should illness or injury occur. Here is some of what I learned through research and experience:
The cruise ship medical facility is not equivalent to your local hospital. Each ship in the fleet of a major cruise line contains a sick bay (to use a nautical term) with staff available 24 hours a day, but they are typically equipped to treat only minor, nonemergent conditions. I was told by the ship’s physician that he mostly sees upper respiratory infections, seasickness, and back pain. All ships have a physician on board who is trained in emergency medicine,8 and usually there are at least two RNs as well.
The medical facilities tend to resemble an infirmary or walk-in clinic rather than a “floating hospital.” There may be some simple equipment—a ventilator or a small x-ray machine—and the medical staff may be able to perform simple lab tests (eg, to check for infection or monitor glucose). But there is no capacity for advanced imaging, no ICU, and no store of blood in the event a transfusion is needed. Limited medications are available on board, but the inventories vary and are usually related to common conditions.
You should also know that there is no international body to regulate medical care on a passenger ship.9 The closest guidance comes from the American College of Emergency Physicians (ACEP), whose “Guidelines of Care for Cruise Ship Medical Facilities” was first published in 1996 (most recent revision, 2013).10,11 They stipulate that shipboard infirmaries must have equipment to handle a range of diagnoses and treatments: wheelchairs, a stretcher and backboard for spine immobilization, lab capabilities, oxygen, ECG, two defibrillators, cardiac monitors, and vital sign monitoring. Cruise RNs are required to have a minimum of two years of recent hospital experience, particularly in cardiac care, trauma, and internal medicine.
ACEP’s guidelines have become an international reference for the practice of cruise medicine.12 The proviso to their use is that they are based on member consensus rather than documented facts.
If there is an emergency on board, you are most likely on your own! You will be referred to a facility on land and disembarked to receive care—and the ship’s medical staff makes that call, not you. The “local” hospital may be at some distance from the port, necessitating transportation (which can be expensive), and in remote areas, the standards may not be what you are accustomed to in the United States. (There is the added difficulty that once you have recovered, you will have to make your own arrangements to get home.)
In the event of a serious emergency—a heart attack or stroke—the Coast Guard may step in to airlift you from the ship. But they aren’t obligated to risk a dangerous helicopter flight in bad weather, or if the ship is more than 250 miles from shore. Cruise ships do not commonly deviate course for evacuation purposes unless doing so will likely result in a markedly improved outcome.
On the next page: Will health insurance cover you?
See also: Dr. Bukata's comment on this editorial
What about health insurance? Will it cover you? Most plans do not cover medical services on board, which means you will pay out-of-pocket for any costs incurred. The bill can range from a few hundred to several thousand dollars. If you receive treatment or medicine from the ship’s medical team, the cost will be charged to your personal account. You could then file a claim with your personal insurance carrier to recoup whatever is covered by your health policy.
Travel insurance, which usually costs between 5% and 8% of the total cost of the trip, could cover the rest. In addition to medical costs, you can buy insurance policies that cover a wide range of trip interruption and cancellation situations, as well as medical evacuation.
Travel health insurance is your best protection. Consumer Reports recommends avoiding commission-driven policies sold by tour operators, cruise-line representatives, and travel agents. Instead, check out an online broker (such as insuremytrip.com) that sells coverage from multiple companies and allows you to tailor a plan to your needs.8
Many employers purchase travel insurance for their employees, which covers both business and pleasure trips. This type of policy is particularly convenient if an expensive emergency procedure, such as evacuation from the ship and/or a hospital stay in a foreign port, is necessary.
Not all perils at sea are contagious. Norovirus and motion sickness aren’t the only health concerns onboard. Falls and cardiac problems are also quite common. The CDC estimates that injuries, typically from slips, trips, or falls, account for 12% to 18% of shipboard medical visits. Obviously, anyone can fall, but those with limited mobility or who require assistance (eg, cane, walker, or wheelchair) are particularly at risk from wet decks and rough waters. (Factor in a cocktail or two, and even the normally ambulatory will have trouble navigating!)
If you have a bad outcome, don’t expect to win a lawsuit against the cruise line. Be sure to actually read the lengthy form you sign before boarding the ship. Courts have ruled that a cruise line may not be held vicariously liable for the negligence of a ship’s physician. There is no medical malpractice for care rendered on board. Traditionally, the physician and nurses are private contractors.
Ultimately, I think pleasure cruises are worth the time and effort for the prepared cruiser. I intend to go again. And this editorial is not an indictment of the physicians and nurses who work hard and do a wonderful job on cruises to maintain the health of passengers and crew. I just don’t want anyone caught unaware if they set sail for an adventure and find more than they wanted.
I would love to hear about any experience, positive or negative, that you have had with cruise medicine. Contact me at [email protected].
REFERENCES
1. Diskin AL, Caro GM, Dahl E. Acute gastroenteritis and video camera surveillance: a cruise ship case report. Int Marit Health. 2014; 65(1):20-22.
2. Williams S, Dahl E. Briefing notes on emergency medical disembarks by helicopter at sea in North America. Int Marit Health. 2014;65(1):7-12.
3. Brown CM, Cetron MS. Crossing borders: one world, global health. Clin Infect Dis. 2012; 54(11):v-vi.
4. Stefanelli P, Fazio C, Neri A, et al. Cluster of invasive Neisseria meningitidis infections on a cruise ship, Italy, October 2012. Euro Surveill. 2012;17(50):pii20336.
5. Gibbs RA, Nanyonjo R, Pingault NM, et al. An outbreak of Cyclospora infection on a cruise ship. Epidemiol Infect. 2013;141(3):508-516.
6. Bert F, Scaioli G, Gualano MR, et al. Norovirus outbreaks on commercial cruise ships: a systematic review and new targets for the public health agenda. Food Environ Virol. 2014; May 17. [Epub ahead of print.]
7. Bouricha M, Samad MA, Levy PY, et al. Point-of-care syndrome, rapid diagnosis of infections on commercial ships. J Travel Med. 2014;21(1):12-16.
8. Consumer Reports. 7 things you need to know about medical care on cruise ships. www.consumerreports.org/cro/news/ 2014/04/7-things-you-need-to-know-about-medical-care-on-cruise-ships/index.htm. Accessed July 17, 2014.
9. Dahl E. Cruise medicine: call for an international standard. Int Marit Health. 2001;52:24-26.
10. American College of Emergency Physicians. Guidelines of care for cruise ship medical facilities. Ann Emerg Med. 1996;27:846.
11. American College of Emergency Physicians. Health Care Guidelines for Cruise Ship Medical Facilities. www.acep.org/content.aspx?id=29500. Accessed July 16, 2014.
12. Cruise Lines International Association, Inc. Medical facilities. www.cruising.org/regula tory/policies/medical-facilities. Accessed July 16, 2014.
See also: Dr. Bukata's comment on this editorial
[Rick Bukata is the editor and founder of Emergency Medical Abstracts and has been studying the literature of emergency medicine in depth since 1977. Rick was awarded the Education Award of the American College of Emergency Physicians in 1993 and the College’s “Outstanding Speaker of the Year” award in 2000. He is a Clinical Professor of Emergency Medicine at the University of Southern California.]
I recently returned from a pleasure cruise to celebrate a milestone birthday. (I’m not telling you which one—but it did make me eligible for government health care!) Prior to embarking on this adventure, I had dreams of calm blue-green waters, endless plates of delectable food, sitting on the stateroom veranda drinking wine while watching the sunset, and exciting land and sea excursions.
However, I must admit that I also felt apprehensive. Just a year ago, one of my best friends lost his wife to severe influenza and necrotizing pneumonia while on a cruise. This disastrous event occurred very rapidly (what I call “from cough to death in less than 48 hours”) and in the same locale as my cruise. So, you can imagine my concern.
To prepare myself, I did a bit of research on the types of infectious diseases occurring aboard ship. The leading cause of human acute viral gastroenteritis on cruise ships is norovirus, outbreaks of which have increased significantly in recent years1 and always garner significant media attention.
However, the most frequent consultation with a ship’s medical team on most cruises is for respiratory illness.2 Outbreaks are not uncommon, particularly within flu season (October through May in the Northern hemisphere and April through September in the Southern hemisphere). Unfortunately, flu season is year round in the tropics and subtropics.3 There have also been occurrences of invasive meningococcal disease (four cases in the Mediterranean in October 2012, one of which was ultimately fatal)4 and cyclosporiasis (on two successive voyages embarking from Australia in 2010).5
Considering the detrimental consequences for passengers and the subsequent high costs for cruise companies, disease outbreaks on cruise ships represent a serious public health issue.6 Unfortunately, contagious disease on commercial ships tends to be poorly managed, as there is little capacity to confirm a case or isolate to curb transmission.7 (Personally, I was pleased—and relieved—to see that crew members stood outside every restaurant on the ship offering passengers hand disinfectant gel.)
I’m not trying to be alarmist. After all, I had a great cruise free of concerns. But I think anyone who sets sail should be aware of the circumstances should illness or injury occur. Here is some of what I learned through research and experience:
The cruise ship medical facility is not equivalent to your local hospital. Each ship in the fleet of a major cruise line contains a sick bay (to use a nautical term) with staff available 24 hours a day, but they are typically equipped to treat only minor, nonemergent conditions. I was told by the ship’s physician that he mostly sees upper respiratory infections, seasickness, and back pain. All ships have a physician on board who is trained in emergency medicine,8 and usually there are at least two RNs as well.
The medical facilities tend to resemble an infirmary or walk-in clinic rather than a “floating hospital.” There may be some simple equipment—a ventilator or a small x-ray machine—and the medical staff may be able to perform simple lab tests (eg, to check for infection or monitor glucose). But there is no capacity for advanced imaging, no ICU, and no store of blood in the event a transfusion is needed. Limited medications are available on board, but the inventories vary and are usually related to common conditions.
You should also know that there is no international body to regulate medical care on a passenger ship.9 The closest guidance comes from the American College of Emergency Physicians (ACEP), whose “Guidelines of Care for Cruise Ship Medical Facilities” was first published in 1996 (most recent revision, 2013).10,11 They stipulate that shipboard infirmaries must have equipment to handle a range of diagnoses and treatments: wheelchairs, a stretcher and backboard for spine immobilization, lab capabilities, oxygen, ECG, two defibrillators, cardiac monitors, and vital sign monitoring. Cruise RNs are required to have a minimum of two years of recent hospital experience, particularly in cardiac care, trauma, and internal medicine.
ACEP’s guidelines have become an international reference for the practice of cruise medicine.12 The proviso to their use is that they are based on member consensus rather than documented facts.
If there is an emergency on board, you are most likely on your own! You will be referred to a facility on land and disembarked to receive care—and the ship’s medical staff makes that call, not you. The “local” hospital may be at some distance from the port, necessitating transportation (which can be expensive), and in remote areas, the standards may not be what you are accustomed to in the United States. (There is the added difficulty that once you have recovered, you will have to make your own arrangements to get home.)
In the event of a serious emergency—a heart attack or stroke—the Coast Guard may step in to airlift you from the ship. But they aren’t obligated to risk a dangerous helicopter flight in bad weather, or if the ship is more than 250 miles from shore. Cruise ships do not commonly deviate course for evacuation purposes unless doing so will likely result in a markedly improved outcome.
On the next page: Will health insurance cover you?
See also: Dr. Bukata's comment on this editorial
What about health insurance? Will it cover you? Most plans do not cover medical services on board, which means you will pay out-of-pocket for any costs incurred. The bill can range from a few hundred to several thousand dollars. If you receive treatment or medicine from the ship’s medical team, the cost will be charged to your personal account. You could then file a claim with your personal insurance carrier to recoup whatever is covered by your health policy.
Travel insurance, which usually costs between 5% and 8% of the total cost of the trip, could cover the rest. In addition to medical costs, you can buy insurance policies that cover a wide range of trip interruption and cancellation situations, as well as medical evacuation.
Travel health insurance is your best protection. Consumer Reports recommends avoiding commission-driven policies sold by tour operators, cruise-line representatives, and travel agents. Instead, check out an online broker (such as insuremytrip.com) that sells coverage from multiple companies and allows you to tailor a plan to your needs.8
Many employers purchase travel insurance for their employees, which covers both business and pleasure trips. This type of policy is particularly convenient if an expensive emergency procedure, such as evacuation from the ship and/or a hospital stay in a foreign port, is necessary.
Not all perils at sea are contagious. Norovirus and motion sickness aren’t the only health concerns onboard. Falls and cardiac problems are also quite common. The CDC estimates that injuries, typically from slips, trips, or falls, account for 12% to 18% of shipboard medical visits. Obviously, anyone can fall, but those with limited mobility or who require assistance (eg, cane, walker, or wheelchair) are particularly at risk from wet decks and rough waters. (Factor in a cocktail or two, and even the normally ambulatory will have trouble navigating!)
If you have a bad outcome, don’t expect to win a lawsuit against the cruise line. Be sure to actually read the lengthy form you sign before boarding the ship. Courts have ruled that a cruise line may not be held vicariously liable for the negligence of a ship’s physician. There is no medical malpractice for care rendered on board. Traditionally, the physician and nurses are private contractors.
Ultimately, I think pleasure cruises are worth the time and effort for the prepared cruiser. I intend to go again. And this editorial is not an indictment of the physicians and nurses who work hard and do a wonderful job on cruises to maintain the health of passengers and crew. I just don’t want anyone caught unaware if they set sail for an adventure and find more than they wanted.
I would love to hear about any experience, positive or negative, that you have had with cruise medicine. Contact me at [email protected].
REFERENCES
1. Diskin AL, Caro GM, Dahl E. Acute gastroenteritis and video camera surveillance: a cruise ship case report. Int Marit Health. 2014; 65(1):20-22.
2. Williams S, Dahl E. Briefing notes on emergency medical disembarks by helicopter at sea in North America. Int Marit Health. 2014;65(1):7-12.
3. Brown CM, Cetron MS. Crossing borders: one world, global health. Clin Infect Dis. 2012; 54(11):v-vi.
4. Stefanelli P, Fazio C, Neri A, et al. Cluster of invasive Neisseria meningitidis infections on a cruise ship, Italy, October 2012. Euro Surveill. 2012;17(50):pii20336.
5. Gibbs RA, Nanyonjo R, Pingault NM, et al. An outbreak of Cyclospora infection on a cruise ship. Epidemiol Infect. 2013;141(3):508-516.
6. Bert F, Scaioli G, Gualano MR, et al. Norovirus outbreaks on commercial cruise ships: a systematic review and new targets for the public health agenda. Food Environ Virol. 2014; May 17. [Epub ahead of print.]
7. Bouricha M, Samad MA, Levy PY, et al. Point-of-care syndrome, rapid diagnosis of infections on commercial ships. J Travel Med. 2014;21(1):12-16.
8. Consumer Reports. 7 things you need to know about medical care on cruise ships. www.consumerreports.org/cro/news/ 2014/04/7-things-you-need-to-know-about-medical-care-on-cruise-ships/index.htm. Accessed July 17, 2014.
9. Dahl E. Cruise medicine: call for an international standard. Int Marit Health. 2001;52:24-26.
10. American College of Emergency Physicians. Guidelines of care for cruise ship medical facilities. Ann Emerg Med. 1996;27:846.
11. American College of Emergency Physicians. Health Care Guidelines for Cruise Ship Medical Facilities. www.acep.org/content.aspx?id=29500. Accessed July 16, 2014.
12. Cruise Lines International Association, Inc. Medical facilities. www.cruising.org/regula tory/policies/medical-facilities. Accessed July 16, 2014.
See also: Dr. Bukata's comment on this editorial
[Rick Bukata is the editor and founder of Emergency Medical Abstracts and has been studying the literature of emergency medicine in depth since 1977. Rick was awarded the Education Award of the American College of Emergency Physicians in 1993 and the College’s “Outstanding Speaker of the Year” award in 2000. He is a Clinical Professor of Emergency Medicine at the University of Southern California.]
I recently returned from a pleasure cruise to celebrate a milestone birthday. (I’m not telling you which one—but it did make me eligible for government health care!) Prior to embarking on this adventure, I had dreams of calm blue-green waters, endless plates of delectable food, sitting on the stateroom veranda drinking wine while watching the sunset, and exciting land and sea excursions.
However, I must admit that I also felt apprehensive. Just a year ago, one of my best friends lost his wife to severe influenza and necrotizing pneumonia while on a cruise. This disastrous event occurred very rapidly (what I call “from cough to death in less than 48 hours”) and in the same locale as my cruise. So, you can imagine my concern.
To prepare myself, I did a bit of research on the types of infectious diseases occurring aboard ship. The leading cause of human acute viral gastroenteritis on cruise ships is norovirus, outbreaks of which have increased significantly in recent years1 and always garner significant media attention.
However, the most frequent consultation with a ship’s medical team on most cruises is for respiratory illness.2 Outbreaks are not uncommon, particularly within flu season (October through May in the Northern hemisphere and April through September in the Southern hemisphere). Unfortunately, flu season is year round in the tropics and subtropics.3 There have also been occurrences of invasive meningococcal disease (four cases in the Mediterranean in October 2012, one of which was ultimately fatal)4 and cyclosporiasis (on two successive voyages embarking from Australia in 2010).5
Considering the detrimental consequences for passengers and the subsequent high costs for cruise companies, disease outbreaks on cruise ships represent a serious public health issue.6 Unfortunately, contagious disease on commercial ships tends to be poorly managed, as there is little capacity to confirm a case or isolate to curb transmission.7 (Personally, I was pleased—and relieved—to see that crew members stood outside every restaurant on the ship offering passengers hand disinfectant gel.)
I’m not trying to be alarmist. After all, I had a great cruise free of concerns. But I think anyone who sets sail should be aware of the circumstances should illness or injury occur. Here is some of what I learned through research and experience:
The cruise ship medical facility is not equivalent to your local hospital. Each ship in the fleet of a major cruise line contains a sick bay (to use a nautical term) with staff available 24 hours a day, but they are typically equipped to treat only minor, nonemergent conditions. I was told by the ship’s physician that he mostly sees upper respiratory infections, seasickness, and back pain. All ships have a physician on board who is trained in emergency medicine,8 and usually there are at least two RNs as well.
The medical facilities tend to resemble an infirmary or walk-in clinic rather than a “floating hospital.” There may be some simple equipment—a ventilator or a small x-ray machine—and the medical staff may be able to perform simple lab tests (eg, to check for infection or monitor glucose). But there is no capacity for advanced imaging, no ICU, and no store of blood in the event a transfusion is needed. Limited medications are available on board, but the inventories vary and are usually related to common conditions.
You should also know that there is no international body to regulate medical care on a passenger ship.9 The closest guidance comes from the American College of Emergency Physicians (ACEP), whose “Guidelines of Care for Cruise Ship Medical Facilities” was first published in 1996 (most recent revision, 2013).10,11 They stipulate that shipboard infirmaries must have equipment to handle a range of diagnoses and treatments: wheelchairs, a stretcher and backboard for spine immobilization, lab capabilities, oxygen, ECG, two defibrillators, cardiac monitors, and vital sign monitoring. Cruise RNs are required to have a minimum of two years of recent hospital experience, particularly in cardiac care, trauma, and internal medicine.
ACEP’s guidelines have become an international reference for the practice of cruise medicine.12 The proviso to their use is that they are based on member consensus rather than documented facts.
If there is an emergency on board, you are most likely on your own! You will be referred to a facility on land and disembarked to receive care—and the ship’s medical staff makes that call, not you. The “local” hospital may be at some distance from the port, necessitating transportation (which can be expensive), and in remote areas, the standards may not be what you are accustomed to in the United States. (There is the added difficulty that once you have recovered, you will have to make your own arrangements to get home.)
In the event of a serious emergency—a heart attack or stroke—the Coast Guard may step in to airlift you from the ship. But they aren’t obligated to risk a dangerous helicopter flight in bad weather, or if the ship is more than 250 miles from shore. Cruise ships do not commonly deviate course for evacuation purposes unless doing so will likely result in a markedly improved outcome.
On the next page: Will health insurance cover you?
See also: Dr. Bukata's comment on this editorial
What about health insurance? Will it cover you? Most plans do not cover medical services on board, which means you will pay out-of-pocket for any costs incurred. The bill can range from a few hundred to several thousand dollars. If you receive treatment or medicine from the ship’s medical team, the cost will be charged to your personal account. You could then file a claim with your personal insurance carrier to recoup whatever is covered by your health policy.
Travel insurance, which usually costs between 5% and 8% of the total cost of the trip, could cover the rest. In addition to medical costs, you can buy insurance policies that cover a wide range of trip interruption and cancellation situations, as well as medical evacuation.
Travel health insurance is your best protection. Consumer Reports recommends avoiding commission-driven policies sold by tour operators, cruise-line representatives, and travel agents. Instead, check out an online broker (such as insuremytrip.com) that sells coverage from multiple companies and allows you to tailor a plan to your needs.8
Many employers purchase travel insurance for their employees, which covers both business and pleasure trips. This type of policy is particularly convenient if an expensive emergency procedure, such as evacuation from the ship and/or a hospital stay in a foreign port, is necessary.
Not all perils at sea are contagious. Norovirus and motion sickness aren’t the only health concerns onboard. Falls and cardiac problems are also quite common. The CDC estimates that injuries, typically from slips, trips, or falls, account for 12% to 18% of shipboard medical visits. Obviously, anyone can fall, but those with limited mobility or who require assistance (eg, cane, walker, or wheelchair) are particularly at risk from wet decks and rough waters. (Factor in a cocktail or two, and even the normally ambulatory will have trouble navigating!)
If you have a bad outcome, don’t expect to win a lawsuit against the cruise line. Be sure to actually read the lengthy form you sign before boarding the ship. Courts have ruled that a cruise line may not be held vicariously liable for the negligence of a ship’s physician. There is no medical malpractice for care rendered on board. Traditionally, the physician and nurses are private contractors.
Ultimately, I think pleasure cruises are worth the time and effort for the prepared cruiser. I intend to go again. And this editorial is not an indictment of the physicians and nurses who work hard and do a wonderful job on cruises to maintain the health of passengers and crew. I just don’t want anyone caught unaware if they set sail for an adventure and find more than they wanted.
I would love to hear about any experience, positive or negative, that you have had with cruise medicine. Contact me at [email protected].
REFERENCES
1. Diskin AL, Caro GM, Dahl E. Acute gastroenteritis and video camera surveillance: a cruise ship case report. Int Marit Health. 2014; 65(1):20-22.
2. Williams S, Dahl E. Briefing notes on emergency medical disembarks by helicopter at sea in North America. Int Marit Health. 2014;65(1):7-12.
3. Brown CM, Cetron MS. Crossing borders: one world, global health. Clin Infect Dis. 2012; 54(11):v-vi.
4. Stefanelli P, Fazio C, Neri A, et al. Cluster of invasive Neisseria meningitidis infections on a cruise ship, Italy, October 2012. Euro Surveill. 2012;17(50):pii20336.
5. Gibbs RA, Nanyonjo R, Pingault NM, et al. An outbreak of Cyclospora infection on a cruise ship. Epidemiol Infect. 2013;141(3):508-516.
6. Bert F, Scaioli G, Gualano MR, et al. Norovirus outbreaks on commercial cruise ships: a systematic review and new targets for the public health agenda. Food Environ Virol. 2014; May 17. [Epub ahead of print.]
7. Bouricha M, Samad MA, Levy PY, et al. Point-of-care syndrome, rapid diagnosis of infections on commercial ships. J Travel Med. 2014;21(1):12-16.
8. Consumer Reports. 7 things you need to know about medical care on cruise ships. www.consumerreports.org/cro/news/ 2014/04/7-things-you-need-to-know-about-medical-care-on-cruise-ships/index.htm. Accessed July 17, 2014.
9. Dahl E. Cruise medicine: call for an international standard. Int Marit Health. 2001;52:24-26.
10. American College of Emergency Physicians. Guidelines of care for cruise ship medical facilities. Ann Emerg Med. 1996;27:846.
11. American College of Emergency Physicians. Health Care Guidelines for Cruise Ship Medical Facilities. www.acep.org/content.aspx?id=29500. Accessed July 16, 2014.
12. Cruise Lines International Association, Inc. Medical facilities. www.cruising.org/regula tory/policies/medical-facilities. Accessed July 16, 2014.
See also: Dr. Bukata's comment on this editorial
[Rick Bukata is the editor and founder of Emergency Medical Abstracts and has been studying the literature of emergency medicine in depth since 1977. Rick was awarded the Education Award of the American College of Emergency Physicians in 1993 and the College’s “Outstanding Speaker of the Year” award in 2000. He is a Clinical Professor of Emergency Medicine at the University of Southern California.]
Allergic Rhinitis & Immunotherapy: Hope or Hype
CE/CME No: CR-1403
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the pathophysiology and etiology of allergic rhinitis (AR).
• Describe the prevalence and types of AR.
• List the differential diagnoses for AR.
• Describe the historical and physical examination findings that are typical
of AR.
• Explain the indications for and types of allergy testing.
• Discuss the types of allergy desensitization therapies/immunotherapies.
FACULTY
Randy D. Danielsen is a Professor and Dean of the Arizona School of Health Sciences, A.T. Still University in Mesa, Arizona, and a long-time PA with the Arizona Asthma & Allergy Institute. Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies at the Arizona School of Health Sciences, A.T. Still University, and a formally trained otolaryngology PA.
The authors have no financial disclosures to report.
ACCREDITATION STATEMENT
Article begins on next page >>
Allergic rhinitis (AR), one of the most familiar complaints seen in primary care, is a common immunologic condition that occurs in genetically predisposed patients. AR is routinely treated through allergen avoidance and pharmacologic therapy. When these measures fail, however, immunologic treatment may be indicated. This review of AR and its treatment focuses on injection and oral immunotherapy.
Congestion; sneezing (particularly paroxysms); itchy nose, palate, and eyes; and runny nose are symptoms characteristic of allergic rhinitis (AR) seen every day in virtually all primary care offices. Patients are plagued not only by their symptoms, but also by AR-related sleep disturbances, resulting in fatigue and daytime sleepiness, irritability, and memory deficits. In children, these sleep disruptions may even cause behavioral disturbances. Depending on the cause, some patients may experience allergic symptoms only with outdoor environmental exposure and subsequent immunoglobulin E (IgE)–mediated responses to otherwise innocuous allergens, while others find that their symptoms are constant, occurring both indoors and out.
Although the prevalence of AR is increasing,1 allergies are certainly nothing new to humankind. In fact, hieroglyphics and Egyptian wall paintings have been discovered depicting a pharaoh dying from anaphylactic shock after receiving a wasp sting.2 In 1565, Leonardo Botallo described AR, calling it “rose catarrh” (mucous or phlegm) or “rose fever,” based on the mistaken idea that the symptoms were caused by rose pollen.2 John Babcock, an English physician, first diagnosed an upper respiratory disease that he called “hay fever” in 1819.
Seventy years later, Charles Blackley identified pollen as a cause of hay fever, documenting his findings in his 1873 book Experimental Researches on the Causes and Nature of Catarrhus Aestivus.2 Dr. Blackley performed the initial documented attempts at allergy desensitization treatments on himself—a willing patient, as he suffered from AR. He placed rye grass pollen onto his nasal mucosa, finding that after 30 minutes the nostril was completely occluded. He continued his experimentation by repeatedly exposing himself to pollen grains via abraded skin. Alas, he never noted any decrease in his symptoms.3
Presently, AR affects more than 55 million people in the United States4—approximately 10% to 30% of the adult population5 and more than 40% of children.6 The rising prevalence of AR is of concern in older adults, who tend to have related comorbidities (eg, chronic sinusitis, asthma, and otitis media). In fact, AR is the fifth most common chronic disease in the US.7
AR and its treatment impose a great economic burden on the health care system, critical in these days of affordable health care. In fact, in 2005 in the US, the overall (direct medical and indirect) cost of AR was $11.2 billion.8 Direct costs derive from office visits, diagnostic testing, and therapeutics. Costs are considerably higher when indirect expenses, including decreased productivity, missed school and missed workdays to care for children, and costs of travel to medical appointments, are included. In the US, approximately 3.5 million workdays and 2 million school days are lost each year due to AR.9 Decreases in productivity cost an estimated $600 per affected employee per year, all of which results in AR being the fifth costliest chronic disease.10,11
On the next page: Pathophysiology and examination >>
PATHOPHYSIOLOGY
AR is an immunologic disorder that occurs in genetically susceptible individuals who produce allergen-specific IgE antibody responses after environmental exposures. The IgE-mediated response causes inflammation of the nasal mucosa. Compared to controls, individuals with AR demonstrate increased amounts of IgE antibodies in the nasal mucosa.11 IgE binds to basophils in the bloodstream and mast cells in tissue. Allergens then attach to IgE on basophils and mast cells, which release histamines, prostaglandins, cytokines, and leukotrienes, with histamine being the most significant mediator in the inflammatory response.
In response to allergy-provoking substances, patients experience immediate- and late-phase symptoms. Symptoms of each stage are similar, but congestion is the hallmark of the late phase. While both phases are clinically important because of their contribution to the patient’s symptoms, most patients experience continued exposure to allergens, resulting in constant, overlapping symptoms.
HISTORY AND PHYSICAL EXAMINATION
Patients with AR relate a history of congestion, excessive mucous production, itchy, watery eyes, bouts of sneezing, and more systemic symptoms, such as headache, malaise, and excessive fatigue. It is important to evaluate the degree and duration of the symptoms, noting patterns and triggers, in an effort to confirm the diagnosis and to help the patient evaluate treatment options.
When taking the patient history, always review the family history, which is often notable for allergies and other atopic diseases. Be sure to ask about medications and recreational drug use; a number of substances have been implicated in the development of rhinitis, including anticholinergic medications, oxymetazoline (when overused), and cocaine. Also, question the patient about self-medication and treatment to determine what may or may not have provided relief. Further questioning may also reveal a history of comorbidities, including contact dermatitis, asthma, eczema, and chronic sinusitis.
The physical examination of the patient with rhinitis begins with observation of the patient’s outward appearance, which may reveal allergic shiners (dark to purplish areas under the eyes), conjunctivitis, an allergic salute (a transverse crease of the nose caused by upward rubbing of an itchy nose), mouth breathing, and a generally tired appearance. The nasal turbinates are swollen and often pale. Mucous secretions are usually thin and clear. Enlarged tonsils and posterior nasal drainage may be visualized. The types of AR are listed in Table 1.
On the next page: Diagnosis and treatment >>
DIAGNOSIS
Most of the clues needed to arrive at a diagnosis are discovered by taking a careful history and completing a physical examination. AR frequently underlies and/or coexists with acute upper respiratory infection (URI) and acute and chronic sinusitis. Differentiating acute URI and acute sinusitis from AR is usually relatively straightforward, based on the symptoms of the illness. The diagnosis of chronic sinusitis is made by radiologic imaging with CT scan.
Distinguishing nonallergic rhinitis (NAR) from AR can be far more difficult, because the symptoms of these conditions are similar and chronic in nature (see Table 2). Empiric treatment for AR may be attempted; however, further testing is often needed to differentiate the two. At this point, clinicians may choose to proceed with specific IgE blood tests. Alternatively, many medical practices are prepared to perform or refer for allergy skin testing.
TREATMENT
Avoidance of known triggers is the cornerstone of allergy treatment. Currently, the most effective pharmaceutical treatment for the majority of AR symptoms is inhaled nasal corticosteroids. Although less effective than corticosteroids, antihistamines—both nasal and oral—are a recommended addition to the regimen if the adverse effects and costs to the patient are tolerable. Other treatments include the leukotriene receptor antagonists, intranasal formulations of cromolyn, and the anticholinergic ipratropium bromide nasal spray, which is effective primarily on watery rhinorrhea. If symptoms are not controlled with medication, allergy immunotherapy (AI), the only known disease-modifying therapy for AR, may be indicated.
On the next page: Allergy testing >>
ALLERGY TESTING
In order to distinguish between AR and NAR and to direct treatment toward specific allergen avoidance and immunotherapy, providers have the choice of ordering in vitro blood IgE testing (to measure the antibodies that mediate an allergic response) or in vivo allergy skin testing (to measure the immune response to allergens that induces an allergic atopic reaction). Allergy testing is not a contemporary concept; the first allergy testing was documented in 1656 when Pierre Borel applied egg to a patient’s skin, which exhibited an allergic reaction.2
Allergy skin testing consists of applying multiple allergens to the skin of the patient’s forearms via tiny pinpricks while watching for immediate hypersensitivity reactions. The test begins with the placement of a drop of histamine to serve as a control. If after 10 minutes of watchful waiting the patient develops a reaction to the histamine (a positive test result), it is appropriate to test for antigens by placing drops of suspected allergen extracts on the skin.
After a period of time (usually 20 to 30 minutes), the area is inspected for allergic reaction. An immediate (early phase) wheal and flare (surrounding erythema) reaction may develop. This positive reaction indicates the presence of a mast cell–bound IgE antibody specific to the tested allergen. The size of the reactions is measured in millimeters, allowing for comparison to the histamine control.
A list of the commonly tested antigens in Arizona, as an example, is shown in Table 3. Antigens vary geographically and even from practice to practice. For up-to-date information on pollen counts by region, visit the American Academy of Allergy Asthma & Immunology Web site (see “Pollen Counts”).
On the next page: Patient selection and allergy immunotherapy >>
Patient Selection
After the clinician has determined that there is a high likelihood that the diagnosis is AR, allergy testing, needed to guide AI, is appropriate. Although not true of specific IgE testing, the accuracy of allergy skin testing results can be adversely affected by several medications. For example, some practitioners may choose to stop first-generation antihistamines two to three days before testing. It is generally accepted that the newer, second-generation antihistamines, which can affect skin-testing results longer, be stopped a week prior to testing.
Patients should be reminded that OTC sleep aids frequently contain antihistamines (particularly diphenhydramine) and that they must be discontinued prior to testing as well. Histamine H2-receptor antagonists such as cimetidine and ranitidine may be stopped a day or two before testing. Although β-blockers are only relatively contraindicated in both allergy testing and AI, many health care providers avoid testing and AI in patients taking oral and/or topical (eye drops) β-blocker therapy. Ultimately, the decision is made by individual health care practices.
In vivo allergy skin testing should not be performed on patients taking tricyclic antidepressants and monoamine oxidase inhibitors. Patients with significant cardiovascular disease should not undergo testing or treatment. Pregnancy is a relative contraindication, and allergy skin testing and AI are done only with obstetrician approval. Most allergists avoid allergy skin testing in pregnant women, however, because use of epinephrine, if required, introduces the risk for preterm labor.12,13 Special consideration should also be given to patients with immune deficiencies.
Setting for Allergy Evaluation and Treatment
Recently, many primary care practices have added allergy evaluation and management to the procedures and treatments they offer; however, evaluations and testing traditionally have been performed by allergy and immunology specialists, many of whom include PAs and NPs on their staff. PAs and NPs frequently manage the practices’ allergy programs. Allergists who are listed as American Board of Allergy and Immunology (ABAI)–certified have successfully passed the ABAI’s certifying examination. Other medical specialists, including otolaryngologists and the primary care specialties, are also well placed to evaluate patients with common allergy symptoms and provide appropriate treatment.
Allergy Immunotherapy
AI has now become more efficacious, safer, and more tolerable for the patient than when it was first introduced in 1911. At that time, Leonard Noon authored a brief article claiming that allergen-specific injections could modify AR. Unfortunately, Noon died at age 36 from tuberculosis, but his work was carried on by his associate, John Freeman. Together, they established the guidelines upon which contemporary AI is based, including the protocol to gradually increase the dose of allergen serum, starting with initial weekly to biweekly injections. They also voiced warnings about the potential for anaphylaxis.14 To this day, immunotherapy is still accomplished by the gradual administration of increasing amounts of the allergen to which the patient is sensitive. This tempers the abnormal immune response to that allergen, easing allergic symptoms.
In patients with IgE-mediated hypersensitivity reactions, confirmed by history, physical examination, and allergy skin testing, immunotherapy can be very effective. The results of immunotherapy may last for years and may even prevent the allergic march, the progression of allergic disease experienced by many patients that frequently begins early in life.15 This includes other allergy-related conditions, such as asthma and eczema (atopic dermatitis), and the acquisition of additional new allergies, including those to foods. AI also has been shown to decrease the frequency of comorbidities such as asthma.5 In addition, AI is used in carefully screened patients who desire to reduce the dosages of medication required to control their symptoms.
In a study published in 1999, Durham and colleagues clarified the questions surrounding the amount of time required for ongoing immunotherapy. They found that the desensitization and tolerance to allergens achieved by AI can last up to three years after a three- to four-year course of therapy. They also found that treatment should not start until an allergic component is identified by allergy skin testing or serum tests for allergen-specific IgE.16
As effective as immunotherapy has been documented to be, it is underused as a therapeutic modality. There are only 2 to 3 million patients receiving subcutaneous immunotherapy out of more than 55 million patients with AR.17
On the next page: Types of allergy immunotherapy >>
Types of AI
There are two categories of allergen desensitization therapy. The most common method is subcutaneous immunotherapy (SCIT), the so-called allergy shots. Less common and still somewhat controversial is sublingual immunotherapy (SLIT). Immunotherapy should be considered for patients who have secondary complications (eg, sinusitis, otitis) or asthma with allergies, or those in whom avoidance measures and medications fail. SCIT or SLIT may be desirable for patients with AR who have difficulty taking regular medications.
Subcutaneous immunotherapy. Currently, SCIT is the most recognized immunotherapy and the only one currently reimbursed by insurance. The procedure involves the subcutaneous injection of increasing doses of therapeutic solutions to which a patient has demonstrated sensitivity. The indications for this treatment are usually inhaled allergens, such as pollens and animal dander. SCIT may also be valuable in patients with asthma and atopic dermatitis.
The most common adverse reaction to allergy injections is a large localized reaction—primarily erythema, pruritus, discomfort, and possibly edema. Severe systemic reactions are extremely rare, with near-fatal to fatal reactions occurring at the rate of only 5.4 per million injections.11 The majority of these rare, albeit serious, complications are caused by higher-than-normal levels of pollen in the environment and dosing errors.11 Because of the uncommon but significant complications, patients undergoing immunotherapy should always receive injections in a medical office equipped with appropriate equipment and staff trained in handling anaphylaxis. It is standard protocol for patients to remain in the office for 30 minutes after administration for observation. Some clinicians prescribe epinephrine injectors (Epi-Pens) for patients to bring to every appointment as a condition for receiving their shot. Because of continuing controversy on this point, others only employ this requirement if the patient has a history of an adverse reaction.
Various protocols exist for the up-dosing of immunotherapy, most of which recommend weekly to twice-weekly injections prior to initiating maintenance therapy. Costs, risks, and benefits must be carefully considered and discussed with the patient prior to initiating immunotherapy.
Many insurance companies reimburse for immunotherapy, with varying copayments. Additionally, the time commitment may be taxing on the patient’s busy schedule. Weekly or biweekly appointments are required initially, and the patient must remain on site for half an hour. Although direct costs of SCIT are relatively easily measured and perhaps compensated, the indirect costs of time spent commuting and at the clinic are less tangible.
Success rates with SCIT may be more than 70% for certain allergens,18 but it is a long-term process with initial improvement often not seen until after six to 12 months of therapy. The benefits of therapy lead not only to reduction and suppression in symptoms (and medication), but also to reduction in comorbidity and lost school or workdays and improvement in quality of life.
Sublingual immunotherapy. Because of the possible safety concerns surrounding SCIT, along with problems relating to patient adherence to weekly office visits, alternative means of achieving allergen desensitization have been implemented. One of these methods, SLIT, has been increasingly supported by clinical evidence, especially in Europe. This therapy consists of applying aqueous allergen extract to the sublingual or oral mucosa, allowing it to be absorbed into the body. Subsequent swallowing of the extract allows the gut to respond with an increase in tolerance. The changes that result from this type of administration appear to be similar to those observed with SCIT.
SLIT has been in use for almost 30 years; the first published controlled study of this therapy was done in 1986 by Scadding and Brostoff.19 The World Allergy Organization recognized the safety and clinical efficacy of SLIT in 2009 after reviewing more than 60 controlled studies.20
A 2010 meta-analysis, reviewing documents from the prior 20 years of research, showed that SLIT decreased medication use and improved symptoms.21 Generally, SLIT was found to be more effective in adults than children. It was not as effective in patients with asthma as in those who were asthma-free. The timing of initiation of SLIT was also an important finding; when SLIT is used for grass pollen allergy, it should be started at least three months before the beginning of grass season.21 For other allergens, SLIT can be started at any time.
Because it is a home-based treatment, SLIT is far more convenient for the patient and therefore has become more popular in the US in the past decade. Its increased safety also contributes to its popularity. Although SLIT is commonly used in many parts of the world, at this writing, medications used in SLIT have not yet been approved by the US FDA. The FDA is currently reviewing two oral tablets for SLIT, and it is expected that its use in the US will increase once an approved product becomes available.22
It should be noted that no studies have directly compared SCIT and SLIT. Careful consultation between clinician and patient can help the patient arrive at the most appropriate modality for his or her condition based on symptomatology and lifestyle needs.
On the next page: Conclusion >>
CONCLUSION
For most patients, AR has little morbidity; however, for some whose rhinitis is moderate to severe, the complications can be a concern. If symptoms are not controlled with avoidance and/or medication, AI may be indicated. It comprises the building up of tolerance to the specific allergens as identified by allergy skin testing or in vitro specific IgE testing. AI, whether SCIT or SLIT, is the only means of altering the abnormal immune system response that underlies AR. Treatment may last as long as three to four years, which provides long-term efficacy of at least three years after cessation of therapy. The long-term prognosis for AR is excellent.
Appreciation is extended to Roxy Irestone, RN, Arizona Asthma & Allergy Institute, for her assistance is gathering factual information for this article.
1. Sibbald B, Rink E, D’Souza M. Is the prevalence of atopy increasing? Br J Gen Pract. 1990;40(337):338-340.
2. History of allergy. Allergy and Asthma Specialists Web site. www.allergyasthmaspecialist.com/allergy-and-asthma-clinic-of-kenosha-sc-education.htm. Accessed February 14, 2014.
3. Blackley CH. Hay Fever: Its Causes, Treatment, and Effective Prevention. London, England: Balliere, Tindall, & Cox; 1880.
4. Settipane RA. Rhinitis: a dose of epidemiological reality. Allergy Asthma Proc. 2003;24(3):147-154.
5. Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(2 suppl):S1-S84.
6. Wright AL, Holberg CJ, Halonen M, et al. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94(6):895 -901.
7. Bernstein JA. Allergic and mixed rhinitis: epidemiology and natural history. Allergy Asthma Proc. 2010;31:365-369.
8. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011; 106(suppl 2):S12-S16.
9. American Academy of Allergy, Asthma & Immunology. Task force on allergic disorders: promoting best practice: raising the standard of care for patients with allergic disorders. Executive summary report. 1998.
10. Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007; 28:3-9.
11. Tran NP, Vickery J, Blaiss MS. Management of rhinitis: allergic and non-allergic. Allergy Asthma Immunol Res. 2011;3:148-156.
12. Disease Summaries: Allergic Diseases and Asthma in Pregnancy. World Allergy Organization. www.worldallergy.org/professional/allergic_
diseases_center/allergy_in_pregnancy/. Accessed February 14, 2014.
13. Simons FE, Ardusso LR, Dimov V, et al. World Allergy Organization Anaphylaxis Guidelines: 2013 update of the evidence base. Int Arch Allergy Immunol. 2013;162:193-204.
14. Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;177:1572-1573.
15. Purello-D’Ambrosio F, Gangemi S, Merendino RA, et al. Prevention of new sensitizations in monosensitized subjects submitted to specific immunotherapy or not: a retrospective study. Clin & Exper Allergy. 2001;31:1295-1302.
16. Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468-475.
17. Mohapatra SS, Qazi M, Hellermann G. Immunotherapy for allergies and asthma: present and future. Curr Opin Pharmacol. 2010;10:276-288.
18. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007; Iss 1, Art No: CD001936.
19. Scadding GK, Brostoff J. Low dose sublingual therapy in patients with allergic rhinitis due to house dust mite.Clin Allergy. 1986;16:483-491.
20. Canonica GW, Bousquet J, Casale T, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009;64 suppl 91:1-59.
21. Di Bona D, Plaia A, Scafidi V, et al. Efficacy of sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2010;126:558-566.
22. Sikora JM, Tankersley MS; ACAAI Immunotherapy and Diagnostics Committee. Perception and practice of sublingual immunotherapy among practicing allergists in the United States: a follow-up survey. Ann Allergy Asthma Immunol. 2013;110:194-197.
CE/CME No: CR-1403
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the pathophysiology and etiology of allergic rhinitis (AR).
• Describe the prevalence and types of AR.
• List the differential diagnoses for AR.
• Describe the historical and physical examination findings that are typical
of AR.
• Explain the indications for and types of allergy testing.
• Discuss the types of allergy desensitization therapies/immunotherapies.
FACULTY
Randy D. Danielsen is a Professor and Dean of the Arizona School of Health Sciences, A.T. Still University in Mesa, Arizona, and a long-time PA with the Arizona Asthma & Allergy Institute. Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies at the Arizona School of Health Sciences, A.T. Still University, and a formally trained otolaryngology PA.
The authors have no financial disclosures to report.
ACCREDITATION STATEMENT
Article begins on next page >>
Allergic rhinitis (AR), one of the most familiar complaints seen in primary care, is a common immunologic condition that occurs in genetically predisposed patients. AR is routinely treated through allergen avoidance and pharmacologic therapy. When these measures fail, however, immunologic treatment may be indicated. This review of AR and its treatment focuses on injection and oral immunotherapy.
Congestion; sneezing (particularly paroxysms); itchy nose, palate, and eyes; and runny nose are symptoms characteristic of allergic rhinitis (AR) seen every day in virtually all primary care offices. Patients are plagued not only by their symptoms, but also by AR-related sleep disturbances, resulting in fatigue and daytime sleepiness, irritability, and memory deficits. In children, these sleep disruptions may even cause behavioral disturbances. Depending on the cause, some patients may experience allergic symptoms only with outdoor environmental exposure and subsequent immunoglobulin E (IgE)–mediated responses to otherwise innocuous allergens, while others find that their symptoms are constant, occurring both indoors and out.
Although the prevalence of AR is increasing,1 allergies are certainly nothing new to humankind. In fact, hieroglyphics and Egyptian wall paintings have been discovered depicting a pharaoh dying from anaphylactic shock after receiving a wasp sting.2 In 1565, Leonardo Botallo described AR, calling it “rose catarrh” (mucous or phlegm) or “rose fever,” based on the mistaken idea that the symptoms were caused by rose pollen.2 John Babcock, an English physician, first diagnosed an upper respiratory disease that he called “hay fever” in 1819.
Seventy years later, Charles Blackley identified pollen as a cause of hay fever, documenting his findings in his 1873 book Experimental Researches on the Causes and Nature of Catarrhus Aestivus.2 Dr. Blackley performed the initial documented attempts at allergy desensitization treatments on himself—a willing patient, as he suffered from AR. He placed rye grass pollen onto his nasal mucosa, finding that after 30 minutes the nostril was completely occluded. He continued his experimentation by repeatedly exposing himself to pollen grains via abraded skin. Alas, he never noted any decrease in his symptoms.3
Presently, AR affects more than 55 million people in the United States4—approximately 10% to 30% of the adult population5 and more than 40% of children.6 The rising prevalence of AR is of concern in older adults, who tend to have related comorbidities (eg, chronic sinusitis, asthma, and otitis media). In fact, AR is the fifth most common chronic disease in the US.7
AR and its treatment impose a great economic burden on the health care system, critical in these days of affordable health care. In fact, in 2005 in the US, the overall (direct medical and indirect) cost of AR was $11.2 billion.8 Direct costs derive from office visits, diagnostic testing, and therapeutics. Costs are considerably higher when indirect expenses, including decreased productivity, missed school and missed workdays to care for children, and costs of travel to medical appointments, are included. In the US, approximately 3.5 million workdays and 2 million school days are lost each year due to AR.9 Decreases in productivity cost an estimated $600 per affected employee per year, all of which results in AR being the fifth costliest chronic disease.10,11
On the next page: Pathophysiology and examination >>
PATHOPHYSIOLOGY
AR is an immunologic disorder that occurs in genetically susceptible individuals who produce allergen-specific IgE antibody responses after environmental exposures. The IgE-mediated response causes inflammation of the nasal mucosa. Compared to controls, individuals with AR demonstrate increased amounts of IgE antibodies in the nasal mucosa.11 IgE binds to basophils in the bloodstream and mast cells in tissue. Allergens then attach to IgE on basophils and mast cells, which release histamines, prostaglandins, cytokines, and leukotrienes, with histamine being the most significant mediator in the inflammatory response.
In response to allergy-provoking substances, patients experience immediate- and late-phase symptoms. Symptoms of each stage are similar, but congestion is the hallmark of the late phase. While both phases are clinically important because of their contribution to the patient’s symptoms, most patients experience continued exposure to allergens, resulting in constant, overlapping symptoms.
HISTORY AND PHYSICAL EXAMINATION
Patients with AR relate a history of congestion, excessive mucous production, itchy, watery eyes, bouts of sneezing, and more systemic symptoms, such as headache, malaise, and excessive fatigue. It is important to evaluate the degree and duration of the symptoms, noting patterns and triggers, in an effort to confirm the diagnosis and to help the patient evaluate treatment options.
When taking the patient history, always review the family history, which is often notable for allergies and other atopic diseases. Be sure to ask about medications and recreational drug use; a number of substances have been implicated in the development of rhinitis, including anticholinergic medications, oxymetazoline (when overused), and cocaine. Also, question the patient about self-medication and treatment to determine what may or may not have provided relief. Further questioning may also reveal a history of comorbidities, including contact dermatitis, asthma, eczema, and chronic sinusitis.
The physical examination of the patient with rhinitis begins with observation of the patient’s outward appearance, which may reveal allergic shiners (dark to purplish areas under the eyes), conjunctivitis, an allergic salute (a transverse crease of the nose caused by upward rubbing of an itchy nose), mouth breathing, and a generally tired appearance. The nasal turbinates are swollen and often pale. Mucous secretions are usually thin and clear. Enlarged tonsils and posterior nasal drainage may be visualized. The types of AR are listed in Table 1.
On the next page: Diagnosis and treatment >>
DIAGNOSIS
Most of the clues needed to arrive at a diagnosis are discovered by taking a careful history and completing a physical examination. AR frequently underlies and/or coexists with acute upper respiratory infection (URI) and acute and chronic sinusitis. Differentiating acute URI and acute sinusitis from AR is usually relatively straightforward, based on the symptoms of the illness. The diagnosis of chronic sinusitis is made by radiologic imaging with CT scan.
Distinguishing nonallergic rhinitis (NAR) from AR can be far more difficult, because the symptoms of these conditions are similar and chronic in nature (see Table 2). Empiric treatment for AR may be attempted; however, further testing is often needed to differentiate the two. At this point, clinicians may choose to proceed with specific IgE blood tests. Alternatively, many medical practices are prepared to perform or refer for allergy skin testing.
TREATMENT
Avoidance of known triggers is the cornerstone of allergy treatment. Currently, the most effective pharmaceutical treatment for the majority of AR symptoms is inhaled nasal corticosteroids. Although less effective than corticosteroids, antihistamines—both nasal and oral—are a recommended addition to the regimen if the adverse effects and costs to the patient are tolerable. Other treatments include the leukotriene receptor antagonists, intranasal formulations of cromolyn, and the anticholinergic ipratropium bromide nasal spray, which is effective primarily on watery rhinorrhea. If symptoms are not controlled with medication, allergy immunotherapy (AI), the only known disease-modifying therapy for AR, may be indicated.
On the next page: Allergy testing >>
ALLERGY TESTING
In order to distinguish between AR and NAR and to direct treatment toward specific allergen avoidance and immunotherapy, providers have the choice of ordering in vitro blood IgE testing (to measure the antibodies that mediate an allergic response) or in vivo allergy skin testing (to measure the immune response to allergens that induces an allergic atopic reaction). Allergy testing is not a contemporary concept; the first allergy testing was documented in 1656 when Pierre Borel applied egg to a patient’s skin, which exhibited an allergic reaction.2
Allergy skin testing consists of applying multiple allergens to the skin of the patient’s forearms via tiny pinpricks while watching for immediate hypersensitivity reactions. The test begins with the placement of a drop of histamine to serve as a control. If after 10 minutes of watchful waiting the patient develops a reaction to the histamine (a positive test result), it is appropriate to test for antigens by placing drops of suspected allergen extracts on the skin.
After a period of time (usually 20 to 30 minutes), the area is inspected for allergic reaction. An immediate (early phase) wheal and flare (surrounding erythema) reaction may develop. This positive reaction indicates the presence of a mast cell–bound IgE antibody specific to the tested allergen. The size of the reactions is measured in millimeters, allowing for comparison to the histamine control.
A list of the commonly tested antigens in Arizona, as an example, is shown in Table 3. Antigens vary geographically and even from practice to practice. For up-to-date information on pollen counts by region, visit the American Academy of Allergy Asthma & Immunology Web site (see “Pollen Counts”).
On the next page: Patient selection and allergy immunotherapy >>
Patient Selection
After the clinician has determined that there is a high likelihood that the diagnosis is AR, allergy testing, needed to guide AI, is appropriate. Although not true of specific IgE testing, the accuracy of allergy skin testing results can be adversely affected by several medications. For example, some practitioners may choose to stop first-generation antihistamines two to three days before testing. It is generally accepted that the newer, second-generation antihistamines, which can affect skin-testing results longer, be stopped a week prior to testing.
Patients should be reminded that OTC sleep aids frequently contain antihistamines (particularly diphenhydramine) and that they must be discontinued prior to testing as well. Histamine H2-receptor antagonists such as cimetidine and ranitidine may be stopped a day or two before testing. Although β-blockers are only relatively contraindicated in both allergy testing and AI, many health care providers avoid testing and AI in patients taking oral and/or topical (eye drops) β-blocker therapy. Ultimately, the decision is made by individual health care practices.
In vivo allergy skin testing should not be performed on patients taking tricyclic antidepressants and monoamine oxidase inhibitors. Patients with significant cardiovascular disease should not undergo testing or treatment. Pregnancy is a relative contraindication, and allergy skin testing and AI are done only with obstetrician approval. Most allergists avoid allergy skin testing in pregnant women, however, because use of epinephrine, if required, introduces the risk for preterm labor.12,13 Special consideration should also be given to patients with immune deficiencies.
Setting for Allergy Evaluation and Treatment
Recently, many primary care practices have added allergy evaluation and management to the procedures and treatments they offer; however, evaluations and testing traditionally have been performed by allergy and immunology specialists, many of whom include PAs and NPs on their staff. PAs and NPs frequently manage the practices’ allergy programs. Allergists who are listed as American Board of Allergy and Immunology (ABAI)–certified have successfully passed the ABAI’s certifying examination. Other medical specialists, including otolaryngologists and the primary care specialties, are also well placed to evaluate patients with common allergy symptoms and provide appropriate treatment.
Allergy Immunotherapy
AI has now become more efficacious, safer, and more tolerable for the patient than when it was first introduced in 1911. At that time, Leonard Noon authored a brief article claiming that allergen-specific injections could modify AR. Unfortunately, Noon died at age 36 from tuberculosis, but his work was carried on by his associate, John Freeman. Together, they established the guidelines upon which contemporary AI is based, including the protocol to gradually increase the dose of allergen serum, starting with initial weekly to biweekly injections. They also voiced warnings about the potential for anaphylaxis.14 To this day, immunotherapy is still accomplished by the gradual administration of increasing amounts of the allergen to which the patient is sensitive. This tempers the abnormal immune response to that allergen, easing allergic symptoms.
In patients with IgE-mediated hypersensitivity reactions, confirmed by history, physical examination, and allergy skin testing, immunotherapy can be very effective. The results of immunotherapy may last for years and may even prevent the allergic march, the progression of allergic disease experienced by many patients that frequently begins early in life.15 This includes other allergy-related conditions, such as asthma and eczema (atopic dermatitis), and the acquisition of additional new allergies, including those to foods. AI also has been shown to decrease the frequency of comorbidities such as asthma.5 In addition, AI is used in carefully screened patients who desire to reduce the dosages of medication required to control their symptoms.
In a study published in 1999, Durham and colleagues clarified the questions surrounding the amount of time required for ongoing immunotherapy. They found that the desensitization and tolerance to allergens achieved by AI can last up to three years after a three- to four-year course of therapy. They also found that treatment should not start until an allergic component is identified by allergy skin testing or serum tests for allergen-specific IgE.16
As effective as immunotherapy has been documented to be, it is underused as a therapeutic modality. There are only 2 to 3 million patients receiving subcutaneous immunotherapy out of more than 55 million patients with AR.17
On the next page: Types of allergy immunotherapy >>
Types of AI
There are two categories of allergen desensitization therapy. The most common method is subcutaneous immunotherapy (SCIT), the so-called allergy shots. Less common and still somewhat controversial is sublingual immunotherapy (SLIT). Immunotherapy should be considered for patients who have secondary complications (eg, sinusitis, otitis) or asthma with allergies, or those in whom avoidance measures and medications fail. SCIT or SLIT may be desirable for patients with AR who have difficulty taking regular medications.
Subcutaneous immunotherapy. Currently, SCIT is the most recognized immunotherapy and the only one currently reimbursed by insurance. The procedure involves the subcutaneous injection of increasing doses of therapeutic solutions to which a patient has demonstrated sensitivity. The indications for this treatment are usually inhaled allergens, such as pollens and animal dander. SCIT may also be valuable in patients with asthma and atopic dermatitis.
The most common adverse reaction to allergy injections is a large localized reaction—primarily erythema, pruritus, discomfort, and possibly edema. Severe systemic reactions are extremely rare, with near-fatal to fatal reactions occurring at the rate of only 5.4 per million injections.11 The majority of these rare, albeit serious, complications are caused by higher-than-normal levels of pollen in the environment and dosing errors.11 Because of the uncommon but significant complications, patients undergoing immunotherapy should always receive injections in a medical office equipped with appropriate equipment and staff trained in handling anaphylaxis. It is standard protocol for patients to remain in the office for 30 minutes after administration for observation. Some clinicians prescribe epinephrine injectors (Epi-Pens) for patients to bring to every appointment as a condition for receiving their shot. Because of continuing controversy on this point, others only employ this requirement if the patient has a history of an adverse reaction.
Various protocols exist for the up-dosing of immunotherapy, most of which recommend weekly to twice-weekly injections prior to initiating maintenance therapy. Costs, risks, and benefits must be carefully considered and discussed with the patient prior to initiating immunotherapy.
Many insurance companies reimburse for immunotherapy, with varying copayments. Additionally, the time commitment may be taxing on the patient’s busy schedule. Weekly or biweekly appointments are required initially, and the patient must remain on site for half an hour. Although direct costs of SCIT are relatively easily measured and perhaps compensated, the indirect costs of time spent commuting and at the clinic are less tangible.
Success rates with SCIT may be more than 70% for certain allergens,18 but it is a long-term process with initial improvement often not seen until after six to 12 months of therapy. The benefits of therapy lead not only to reduction and suppression in symptoms (and medication), but also to reduction in comorbidity and lost school or workdays and improvement in quality of life.
Sublingual immunotherapy. Because of the possible safety concerns surrounding SCIT, along with problems relating to patient adherence to weekly office visits, alternative means of achieving allergen desensitization have been implemented. One of these methods, SLIT, has been increasingly supported by clinical evidence, especially in Europe. This therapy consists of applying aqueous allergen extract to the sublingual or oral mucosa, allowing it to be absorbed into the body. Subsequent swallowing of the extract allows the gut to respond with an increase in tolerance. The changes that result from this type of administration appear to be similar to those observed with SCIT.
SLIT has been in use for almost 30 years; the first published controlled study of this therapy was done in 1986 by Scadding and Brostoff.19 The World Allergy Organization recognized the safety and clinical efficacy of SLIT in 2009 after reviewing more than 60 controlled studies.20
A 2010 meta-analysis, reviewing documents from the prior 20 years of research, showed that SLIT decreased medication use and improved symptoms.21 Generally, SLIT was found to be more effective in adults than children. It was not as effective in patients with asthma as in those who were asthma-free. The timing of initiation of SLIT was also an important finding; when SLIT is used for grass pollen allergy, it should be started at least three months before the beginning of grass season.21 For other allergens, SLIT can be started at any time.
Because it is a home-based treatment, SLIT is far more convenient for the patient and therefore has become more popular in the US in the past decade. Its increased safety also contributes to its popularity. Although SLIT is commonly used in many parts of the world, at this writing, medications used in SLIT have not yet been approved by the US FDA. The FDA is currently reviewing two oral tablets for SLIT, and it is expected that its use in the US will increase once an approved product becomes available.22
It should be noted that no studies have directly compared SCIT and SLIT. Careful consultation between clinician and patient can help the patient arrive at the most appropriate modality for his or her condition based on symptomatology and lifestyle needs.
On the next page: Conclusion >>
CONCLUSION
For most patients, AR has little morbidity; however, for some whose rhinitis is moderate to severe, the complications can be a concern. If symptoms are not controlled with avoidance and/or medication, AI may be indicated. It comprises the building up of tolerance to the specific allergens as identified by allergy skin testing or in vitro specific IgE testing. AI, whether SCIT or SLIT, is the only means of altering the abnormal immune system response that underlies AR. Treatment may last as long as three to four years, which provides long-term efficacy of at least three years after cessation of therapy. The long-term prognosis for AR is excellent.
Appreciation is extended to Roxy Irestone, RN, Arizona Asthma & Allergy Institute, for her assistance is gathering factual information for this article.
CE/CME No: CR-1403
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the pathophysiology and etiology of allergic rhinitis (AR).
• Describe the prevalence and types of AR.
• List the differential diagnoses for AR.
• Describe the historical and physical examination findings that are typical
of AR.
• Explain the indications for and types of allergy testing.
• Discuss the types of allergy desensitization therapies/immunotherapies.
FACULTY
Randy D. Danielsen is a Professor and Dean of the Arizona School of Health Sciences, A.T. Still University in Mesa, Arizona, and a long-time PA with the Arizona Asthma & Allergy Institute. Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies at the Arizona School of Health Sciences, A.T. Still University, and a formally trained otolaryngology PA.
The authors have no financial disclosures to report.
ACCREDITATION STATEMENT
Article begins on next page >>
Allergic rhinitis (AR), one of the most familiar complaints seen in primary care, is a common immunologic condition that occurs in genetically predisposed patients. AR is routinely treated through allergen avoidance and pharmacologic therapy. When these measures fail, however, immunologic treatment may be indicated. This review of AR and its treatment focuses on injection and oral immunotherapy.
Congestion; sneezing (particularly paroxysms); itchy nose, palate, and eyes; and runny nose are symptoms characteristic of allergic rhinitis (AR) seen every day in virtually all primary care offices. Patients are plagued not only by their symptoms, but also by AR-related sleep disturbances, resulting in fatigue and daytime sleepiness, irritability, and memory deficits. In children, these sleep disruptions may even cause behavioral disturbances. Depending on the cause, some patients may experience allergic symptoms only with outdoor environmental exposure and subsequent immunoglobulin E (IgE)–mediated responses to otherwise innocuous allergens, while others find that their symptoms are constant, occurring both indoors and out.
Although the prevalence of AR is increasing,1 allergies are certainly nothing new to humankind. In fact, hieroglyphics and Egyptian wall paintings have been discovered depicting a pharaoh dying from anaphylactic shock after receiving a wasp sting.2 In 1565, Leonardo Botallo described AR, calling it “rose catarrh” (mucous or phlegm) or “rose fever,” based on the mistaken idea that the symptoms were caused by rose pollen.2 John Babcock, an English physician, first diagnosed an upper respiratory disease that he called “hay fever” in 1819.
Seventy years later, Charles Blackley identified pollen as a cause of hay fever, documenting his findings in his 1873 book Experimental Researches on the Causes and Nature of Catarrhus Aestivus.2 Dr. Blackley performed the initial documented attempts at allergy desensitization treatments on himself—a willing patient, as he suffered from AR. He placed rye grass pollen onto his nasal mucosa, finding that after 30 minutes the nostril was completely occluded. He continued his experimentation by repeatedly exposing himself to pollen grains via abraded skin. Alas, he never noted any decrease in his symptoms.3
Presently, AR affects more than 55 million people in the United States4—approximately 10% to 30% of the adult population5 and more than 40% of children.6 The rising prevalence of AR is of concern in older adults, who tend to have related comorbidities (eg, chronic sinusitis, asthma, and otitis media). In fact, AR is the fifth most common chronic disease in the US.7
AR and its treatment impose a great economic burden on the health care system, critical in these days of affordable health care. In fact, in 2005 in the US, the overall (direct medical and indirect) cost of AR was $11.2 billion.8 Direct costs derive from office visits, diagnostic testing, and therapeutics. Costs are considerably higher when indirect expenses, including decreased productivity, missed school and missed workdays to care for children, and costs of travel to medical appointments, are included. In the US, approximately 3.5 million workdays and 2 million school days are lost each year due to AR.9 Decreases in productivity cost an estimated $600 per affected employee per year, all of which results in AR being the fifth costliest chronic disease.10,11
On the next page: Pathophysiology and examination >>
PATHOPHYSIOLOGY
AR is an immunologic disorder that occurs in genetically susceptible individuals who produce allergen-specific IgE antibody responses after environmental exposures. The IgE-mediated response causes inflammation of the nasal mucosa. Compared to controls, individuals with AR demonstrate increased amounts of IgE antibodies in the nasal mucosa.11 IgE binds to basophils in the bloodstream and mast cells in tissue. Allergens then attach to IgE on basophils and mast cells, which release histamines, prostaglandins, cytokines, and leukotrienes, with histamine being the most significant mediator in the inflammatory response.
In response to allergy-provoking substances, patients experience immediate- and late-phase symptoms. Symptoms of each stage are similar, but congestion is the hallmark of the late phase. While both phases are clinically important because of their contribution to the patient’s symptoms, most patients experience continued exposure to allergens, resulting in constant, overlapping symptoms.
HISTORY AND PHYSICAL EXAMINATION
Patients with AR relate a history of congestion, excessive mucous production, itchy, watery eyes, bouts of sneezing, and more systemic symptoms, such as headache, malaise, and excessive fatigue. It is important to evaluate the degree and duration of the symptoms, noting patterns and triggers, in an effort to confirm the diagnosis and to help the patient evaluate treatment options.
When taking the patient history, always review the family history, which is often notable for allergies and other atopic diseases. Be sure to ask about medications and recreational drug use; a number of substances have been implicated in the development of rhinitis, including anticholinergic medications, oxymetazoline (when overused), and cocaine. Also, question the patient about self-medication and treatment to determine what may or may not have provided relief. Further questioning may also reveal a history of comorbidities, including contact dermatitis, asthma, eczema, and chronic sinusitis.
The physical examination of the patient with rhinitis begins with observation of the patient’s outward appearance, which may reveal allergic shiners (dark to purplish areas under the eyes), conjunctivitis, an allergic salute (a transverse crease of the nose caused by upward rubbing of an itchy nose), mouth breathing, and a generally tired appearance. The nasal turbinates are swollen and often pale. Mucous secretions are usually thin and clear. Enlarged tonsils and posterior nasal drainage may be visualized. The types of AR are listed in Table 1.
On the next page: Diagnosis and treatment >>
DIAGNOSIS
Most of the clues needed to arrive at a diagnosis are discovered by taking a careful history and completing a physical examination. AR frequently underlies and/or coexists with acute upper respiratory infection (URI) and acute and chronic sinusitis. Differentiating acute URI and acute sinusitis from AR is usually relatively straightforward, based on the symptoms of the illness. The diagnosis of chronic sinusitis is made by radiologic imaging with CT scan.
Distinguishing nonallergic rhinitis (NAR) from AR can be far more difficult, because the symptoms of these conditions are similar and chronic in nature (see Table 2). Empiric treatment for AR may be attempted; however, further testing is often needed to differentiate the two. At this point, clinicians may choose to proceed with specific IgE blood tests. Alternatively, many medical practices are prepared to perform or refer for allergy skin testing.
TREATMENT
Avoidance of known triggers is the cornerstone of allergy treatment. Currently, the most effective pharmaceutical treatment for the majority of AR symptoms is inhaled nasal corticosteroids. Although less effective than corticosteroids, antihistamines—both nasal and oral—are a recommended addition to the regimen if the adverse effects and costs to the patient are tolerable. Other treatments include the leukotriene receptor antagonists, intranasal formulations of cromolyn, and the anticholinergic ipratropium bromide nasal spray, which is effective primarily on watery rhinorrhea. If symptoms are not controlled with medication, allergy immunotherapy (AI), the only known disease-modifying therapy for AR, may be indicated.
On the next page: Allergy testing >>
ALLERGY TESTING
In order to distinguish between AR and NAR and to direct treatment toward specific allergen avoidance and immunotherapy, providers have the choice of ordering in vitro blood IgE testing (to measure the antibodies that mediate an allergic response) or in vivo allergy skin testing (to measure the immune response to allergens that induces an allergic atopic reaction). Allergy testing is not a contemporary concept; the first allergy testing was documented in 1656 when Pierre Borel applied egg to a patient’s skin, which exhibited an allergic reaction.2
Allergy skin testing consists of applying multiple allergens to the skin of the patient’s forearms via tiny pinpricks while watching for immediate hypersensitivity reactions. The test begins with the placement of a drop of histamine to serve as a control. If after 10 minutes of watchful waiting the patient develops a reaction to the histamine (a positive test result), it is appropriate to test for antigens by placing drops of suspected allergen extracts on the skin.
After a period of time (usually 20 to 30 minutes), the area is inspected for allergic reaction. An immediate (early phase) wheal and flare (surrounding erythema) reaction may develop. This positive reaction indicates the presence of a mast cell–bound IgE antibody specific to the tested allergen. The size of the reactions is measured in millimeters, allowing for comparison to the histamine control.
A list of the commonly tested antigens in Arizona, as an example, is shown in Table 3. Antigens vary geographically and even from practice to practice. For up-to-date information on pollen counts by region, visit the American Academy of Allergy Asthma & Immunology Web site (see “Pollen Counts”).
On the next page: Patient selection and allergy immunotherapy >>
Patient Selection
After the clinician has determined that there is a high likelihood that the diagnosis is AR, allergy testing, needed to guide AI, is appropriate. Although not true of specific IgE testing, the accuracy of allergy skin testing results can be adversely affected by several medications. For example, some practitioners may choose to stop first-generation antihistamines two to three days before testing. It is generally accepted that the newer, second-generation antihistamines, which can affect skin-testing results longer, be stopped a week prior to testing.
Patients should be reminded that OTC sleep aids frequently contain antihistamines (particularly diphenhydramine) and that they must be discontinued prior to testing as well. Histamine H2-receptor antagonists such as cimetidine and ranitidine may be stopped a day or two before testing. Although β-blockers are only relatively contraindicated in both allergy testing and AI, many health care providers avoid testing and AI in patients taking oral and/or topical (eye drops) β-blocker therapy. Ultimately, the decision is made by individual health care practices.
In vivo allergy skin testing should not be performed on patients taking tricyclic antidepressants and monoamine oxidase inhibitors. Patients with significant cardiovascular disease should not undergo testing or treatment. Pregnancy is a relative contraindication, and allergy skin testing and AI are done only with obstetrician approval. Most allergists avoid allergy skin testing in pregnant women, however, because use of epinephrine, if required, introduces the risk for preterm labor.12,13 Special consideration should also be given to patients with immune deficiencies.
Setting for Allergy Evaluation and Treatment
Recently, many primary care practices have added allergy evaluation and management to the procedures and treatments they offer; however, evaluations and testing traditionally have been performed by allergy and immunology specialists, many of whom include PAs and NPs on their staff. PAs and NPs frequently manage the practices’ allergy programs. Allergists who are listed as American Board of Allergy and Immunology (ABAI)–certified have successfully passed the ABAI’s certifying examination. Other medical specialists, including otolaryngologists and the primary care specialties, are also well placed to evaluate patients with common allergy symptoms and provide appropriate treatment.
Allergy Immunotherapy
AI has now become more efficacious, safer, and more tolerable for the patient than when it was first introduced in 1911. At that time, Leonard Noon authored a brief article claiming that allergen-specific injections could modify AR. Unfortunately, Noon died at age 36 from tuberculosis, but his work was carried on by his associate, John Freeman. Together, they established the guidelines upon which contemporary AI is based, including the protocol to gradually increase the dose of allergen serum, starting with initial weekly to biweekly injections. They also voiced warnings about the potential for anaphylaxis.14 To this day, immunotherapy is still accomplished by the gradual administration of increasing amounts of the allergen to which the patient is sensitive. This tempers the abnormal immune response to that allergen, easing allergic symptoms.
In patients with IgE-mediated hypersensitivity reactions, confirmed by history, physical examination, and allergy skin testing, immunotherapy can be very effective. The results of immunotherapy may last for years and may even prevent the allergic march, the progression of allergic disease experienced by many patients that frequently begins early in life.15 This includes other allergy-related conditions, such as asthma and eczema (atopic dermatitis), and the acquisition of additional new allergies, including those to foods. AI also has been shown to decrease the frequency of comorbidities such as asthma.5 In addition, AI is used in carefully screened patients who desire to reduce the dosages of medication required to control their symptoms.
In a study published in 1999, Durham and colleagues clarified the questions surrounding the amount of time required for ongoing immunotherapy. They found that the desensitization and tolerance to allergens achieved by AI can last up to three years after a three- to four-year course of therapy. They also found that treatment should not start until an allergic component is identified by allergy skin testing or serum tests for allergen-specific IgE.16
As effective as immunotherapy has been documented to be, it is underused as a therapeutic modality. There are only 2 to 3 million patients receiving subcutaneous immunotherapy out of more than 55 million patients with AR.17
On the next page: Types of allergy immunotherapy >>
Types of AI
There are two categories of allergen desensitization therapy. The most common method is subcutaneous immunotherapy (SCIT), the so-called allergy shots. Less common and still somewhat controversial is sublingual immunotherapy (SLIT). Immunotherapy should be considered for patients who have secondary complications (eg, sinusitis, otitis) or asthma with allergies, or those in whom avoidance measures and medications fail. SCIT or SLIT may be desirable for patients with AR who have difficulty taking regular medications.
Subcutaneous immunotherapy. Currently, SCIT is the most recognized immunotherapy and the only one currently reimbursed by insurance. The procedure involves the subcutaneous injection of increasing doses of therapeutic solutions to which a patient has demonstrated sensitivity. The indications for this treatment are usually inhaled allergens, such as pollens and animal dander. SCIT may also be valuable in patients with asthma and atopic dermatitis.
The most common adverse reaction to allergy injections is a large localized reaction—primarily erythema, pruritus, discomfort, and possibly edema. Severe systemic reactions are extremely rare, with near-fatal to fatal reactions occurring at the rate of only 5.4 per million injections.11 The majority of these rare, albeit serious, complications are caused by higher-than-normal levels of pollen in the environment and dosing errors.11 Because of the uncommon but significant complications, patients undergoing immunotherapy should always receive injections in a medical office equipped with appropriate equipment and staff trained in handling anaphylaxis. It is standard protocol for patients to remain in the office for 30 minutes after administration for observation. Some clinicians prescribe epinephrine injectors (Epi-Pens) for patients to bring to every appointment as a condition for receiving their shot. Because of continuing controversy on this point, others only employ this requirement if the patient has a history of an adverse reaction.
Various protocols exist for the up-dosing of immunotherapy, most of which recommend weekly to twice-weekly injections prior to initiating maintenance therapy. Costs, risks, and benefits must be carefully considered and discussed with the patient prior to initiating immunotherapy.
Many insurance companies reimburse for immunotherapy, with varying copayments. Additionally, the time commitment may be taxing on the patient’s busy schedule. Weekly or biweekly appointments are required initially, and the patient must remain on site for half an hour. Although direct costs of SCIT are relatively easily measured and perhaps compensated, the indirect costs of time spent commuting and at the clinic are less tangible.
Success rates with SCIT may be more than 70% for certain allergens,18 but it is a long-term process with initial improvement often not seen until after six to 12 months of therapy. The benefits of therapy lead not only to reduction and suppression in symptoms (and medication), but also to reduction in comorbidity and lost school or workdays and improvement in quality of life.
Sublingual immunotherapy. Because of the possible safety concerns surrounding SCIT, along with problems relating to patient adherence to weekly office visits, alternative means of achieving allergen desensitization have been implemented. One of these methods, SLIT, has been increasingly supported by clinical evidence, especially in Europe. This therapy consists of applying aqueous allergen extract to the sublingual or oral mucosa, allowing it to be absorbed into the body. Subsequent swallowing of the extract allows the gut to respond with an increase in tolerance. The changes that result from this type of administration appear to be similar to those observed with SCIT.
SLIT has been in use for almost 30 years; the first published controlled study of this therapy was done in 1986 by Scadding and Brostoff.19 The World Allergy Organization recognized the safety and clinical efficacy of SLIT in 2009 after reviewing more than 60 controlled studies.20
A 2010 meta-analysis, reviewing documents from the prior 20 years of research, showed that SLIT decreased medication use and improved symptoms.21 Generally, SLIT was found to be more effective in adults than children. It was not as effective in patients with asthma as in those who were asthma-free. The timing of initiation of SLIT was also an important finding; when SLIT is used for grass pollen allergy, it should be started at least three months before the beginning of grass season.21 For other allergens, SLIT can be started at any time.
Because it is a home-based treatment, SLIT is far more convenient for the patient and therefore has become more popular in the US in the past decade. Its increased safety also contributes to its popularity. Although SLIT is commonly used in many parts of the world, at this writing, medications used in SLIT have not yet been approved by the US FDA. The FDA is currently reviewing two oral tablets for SLIT, and it is expected that its use in the US will increase once an approved product becomes available.22
It should be noted that no studies have directly compared SCIT and SLIT. Careful consultation between clinician and patient can help the patient arrive at the most appropriate modality for his or her condition based on symptomatology and lifestyle needs.
On the next page: Conclusion >>
CONCLUSION
For most patients, AR has little morbidity; however, for some whose rhinitis is moderate to severe, the complications can be a concern. If symptoms are not controlled with avoidance and/or medication, AI may be indicated. It comprises the building up of tolerance to the specific allergens as identified by allergy skin testing or in vitro specific IgE testing. AI, whether SCIT or SLIT, is the only means of altering the abnormal immune system response that underlies AR. Treatment may last as long as three to four years, which provides long-term efficacy of at least three years after cessation of therapy. The long-term prognosis for AR is excellent.
Appreciation is extended to Roxy Irestone, RN, Arizona Asthma & Allergy Institute, for her assistance is gathering factual information for this article.
1. Sibbald B, Rink E, D’Souza M. Is the prevalence of atopy increasing? Br J Gen Pract. 1990;40(337):338-340.
2. History of allergy. Allergy and Asthma Specialists Web site. www.allergyasthmaspecialist.com/allergy-and-asthma-clinic-of-kenosha-sc-education.htm. Accessed February 14, 2014.
3. Blackley CH. Hay Fever: Its Causes, Treatment, and Effective Prevention. London, England: Balliere, Tindall, & Cox; 1880.
4. Settipane RA. Rhinitis: a dose of epidemiological reality. Allergy Asthma Proc. 2003;24(3):147-154.
5. Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(2 suppl):S1-S84.
6. Wright AL, Holberg CJ, Halonen M, et al. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94(6):895 -901.
7. Bernstein JA. Allergic and mixed rhinitis: epidemiology and natural history. Allergy Asthma Proc. 2010;31:365-369.
8. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011; 106(suppl 2):S12-S16.
9. American Academy of Allergy, Asthma & Immunology. Task force on allergic disorders: promoting best practice: raising the standard of care for patients with allergic disorders. Executive summary report. 1998.
10. Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007; 28:3-9.
11. Tran NP, Vickery J, Blaiss MS. Management of rhinitis: allergic and non-allergic. Allergy Asthma Immunol Res. 2011;3:148-156.
12. Disease Summaries: Allergic Diseases and Asthma in Pregnancy. World Allergy Organization. www.worldallergy.org/professional/allergic_
diseases_center/allergy_in_pregnancy/. Accessed February 14, 2014.
13. Simons FE, Ardusso LR, Dimov V, et al. World Allergy Organization Anaphylaxis Guidelines: 2013 update of the evidence base. Int Arch Allergy Immunol. 2013;162:193-204.
14. Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;177:1572-1573.
15. Purello-D’Ambrosio F, Gangemi S, Merendino RA, et al. Prevention of new sensitizations in monosensitized subjects submitted to specific immunotherapy or not: a retrospective study. Clin & Exper Allergy. 2001;31:1295-1302.
16. Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468-475.
17. Mohapatra SS, Qazi M, Hellermann G. Immunotherapy for allergies and asthma: present and future. Curr Opin Pharmacol. 2010;10:276-288.
18. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007; Iss 1, Art No: CD001936.
19. Scadding GK, Brostoff J. Low dose sublingual therapy in patients with allergic rhinitis due to house dust mite.Clin Allergy. 1986;16:483-491.
20. Canonica GW, Bousquet J, Casale T, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009;64 suppl 91:1-59.
21. Di Bona D, Plaia A, Scafidi V, et al. Efficacy of sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2010;126:558-566.
22. Sikora JM, Tankersley MS; ACAAI Immunotherapy and Diagnostics Committee. Perception and practice of sublingual immunotherapy among practicing allergists in the United States: a follow-up survey. Ann Allergy Asthma Immunol. 2013;110:194-197.
1. Sibbald B, Rink E, D’Souza M. Is the prevalence of atopy increasing? Br J Gen Pract. 1990;40(337):338-340.
2. History of allergy. Allergy and Asthma Specialists Web site. www.allergyasthmaspecialist.com/allergy-and-asthma-clinic-of-kenosha-sc-education.htm. Accessed February 14, 2014.
3. Blackley CH. Hay Fever: Its Causes, Treatment, and Effective Prevention. London, England: Balliere, Tindall, & Cox; 1880.
4. Settipane RA. Rhinitis: a dose of epidemiological reality. Allergy Asthma Proc. 2003;24(3):147-154.
5. Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(2 suppl):S1-S84.
6. Wright AL, Holberg CJ, Halonen M, et al. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94(6):895 -901.
7. Bernstein JA. Allergic and mixed rhinitis: epidemiology and natural history. Allergy Asthma Proc. 2010;31:365-369.
8. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011; 106(suppl 2):S12-S16.
9. American Academy of Allergy, Asthma & Immunology. Task force on allergic disorders: promoting best practice: raising the standard of care for patients with allergic disorders. Executive summary report. 1998.
10. Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007; 28:3-9.
11. Tran NP, Vickery J, Blaiss MS. Management of rhinitis: allergic and non-allergic. Allergy Asthma Immunol Res. 2011;3:148-156.
12. Disease Summaries: Allergic Diseases and Asthma in Pregnancy. World Allergy Organization. www.worldallergy.org/professional/allergic_
diseases_center/allergy_in_pregnancy/. Accessed February 14, 2014.
13. Simons FE, Ardusso LR, Dimov V, et al. World Allergy Organization Anaphylaxis Guidelines: 2013 update of the evidence base. Int Arch Allergy Immunol. 2013;162:193-204.
14. Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;177:1572-1573.
15. Purello-D’Ambrosio F, Gangemi S, Merendino RA, et al. Prevention of new sensitizations in monosensitized subjects submitted to specific immunotherapy or not: a retrospective study. Clin & Exper Allergy. 2001;31:1295-1302.
16. Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468-475.
17. Mohapatra SS, Qazi M, Hellermann G. Immunotherapy for allergies and asthma: present and future. Curr Opin Pharmacol. 2010;10:276-288.
18. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007; Iss 1, Art No: CD001936.
19. Scadding GK, Brostoff J. Low dose sublingual therapy in patients with allergic rhinitis due to house dust mite.Clin Allergy. 1986;16:483-491.
20. Canonica GW, Bousquet J, Casale T, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009;64 suppl 91:1-59.
21. Di Bona D, Plaia A, Scafidi V, et al. Efficacy of sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a systematic review and meta-analysis. J Allergy Clin Immunol. 2010;126:558-566.
22. Sikora JM, Tankersley MS; ACAAI Immunotherapy and Diagnostics Committee. Perception and practice of sublingual immunotherapy among practicing allergists in the United States: a follow-up survey. Ann Allergy Asthma Immunol. 2013;110:194-197.
The Physician Assistant: An Illustrated History
This timely and well written paperback, authored by icons in the PA world Dr. Tom Piemme, Dr Fred Sadler, Dr Reggie Carter, and Ms. Ruth Ballweg, is presented through the PA History Society and presents a half century of history of the PA profession in a very convenient and easy to read format.
The book is structured around the “Timeline” that is the major feature of the PA History website and is accompanied by abstracted biographies of key leaders through the decades, and illustrations from the history archives. This information by itself is worth the price.
An opening chapter puts the early history of the profession in the context of its time, while a closing discussion anticipates the future of the profession. The authors offers a concise history of the people, places, and events that have propelled the concept of the PA from its inception at Duke University in 1965 to its position as one of the major forces in American medicine today. It is one of those books that once you start reading you can’t put down until the end.
The book is intended, of course, for practicing PAs, but it will also serve PA programs as a useful companion to courses of instruction in the history of the profession for PA students. It will be of interest, as well, to pre-health/medical students considering the PA as a career. My understanding is that the demand for this new book has been so great they are already considering a second printing.
The book is available through the PA History Society for $12 each plus packaging and shipping. The price drops to $10 for orders of 15 or more. It can also be obtained over the Internet from Amazon.
This timely and well written paperback, authored by icons in the PA world Dr. Tom Piemme, Dr Fred Sadler, Dr Reggie Carter, and Ms. Ruth Ballweg, is presented through the PA History Society and presents a half century of history of the PA profession in a very convenient and easy to read format.
The book is structured around the “Timeline” that is the major feature of the PA History website and is accompanied by abstracted biographies of key leaders through the decades, and illustrations from the history archives. This information by itself is worth the price.
An opening chapter puts the early history of the profession in the context of its time, while a closing discussion anticipates the future of the profession. The authors offers a concise history of the people, places, and events that have propelled the concept of the PA from its inception at Duke University in 1965 to its position as one of the major forces in American medicine today. It is one of those books that once you start reading you can’t put down until the end.
The book is intended, of course, for practicing PAs, but it will also serve PA programs as a useful companion to courses of instruction in the history of the profession for PA students. It will be of interest, as well, to pre-health/medical students considering the PA as a career. My understanding is that the demand for this new book has been so great they are already considering a second printing.
The book is available through the PA History Society for $12 each plus packaging and shipping. The price drops to $10 for orders of 15 or more. It can also be obtained over the Internet from Amazon.
This timely and well written paperback, authored by icons in the PA world Dr. Tom Piemme, Dr Fred Sadler, Dr Reggie Carter, and Ms. Ruth Ballweg, is presented through the PA History Society and presents a half century of history of the PA profession in a very convenient and easy to read format.
The book is structured around the “Timeline” that is the major feature of the PA History website and is accompanied by abstracted biographies of key leaders through the decades, and illustrations from the history archives. This information by itself is worth the price.
An opening chapter puts the early history of the profession in the context of its time, while a closing discussion anticipates the future of the profession. The authors offers a concise history of the people, places, and events that have propelled the concept of the PA from its inception at Duke University in 1965 to its position as one of the major forces in American medicine today. It is one of those books that once you start reading you can’t put down until the end.
The book is intended, of course, for practicing PAs, but it will also serve PA programs as a useful companion to courses of instruction in the history of the profession for PA students. It will be of interest, as well, to pre-health/medical students considering the PA as a career. My understanding is that the demand for this new book has been so great they are already considering a second printing.
The book is available through the PA History Society for $12 each plus packaging and shipping. The price drops to $10 for orders of 15 or more. It can also be obtained over the Internet from Amazon.
The Mind Knows (But What?)
The other day I was driving across town—a route I have taken many times—when I suddenly found myself not quite sure where I was. This strange and disconcerting feeling lasted only a few seconds. Later, I was talking with a colleague and forgot a common word that I have used millions of times.
Yes, I am not far from becoming an official member of the Medicare generation. I have heard all the jokes about getting old; many of them I have gleefully subjected my older friends to (you know who you are!). But now it is happening to me. My granddaughter called the other day to wish me a happy birthday. She asked how old I was, and I told her: 64. She was quiet for a moment, then asked, “Did you start at 1?”
I don’t know about you, but the animated tales that dominate all discussions about aging—you know, the ones that tell us that age is just a state of mind, that 60 is the new 40 and 80 the new 60—are starting to irritate me. What’s next: 100 as the new middle age? Truth be told, I don’t feel old. In fact, I’m just now getting up to speed.
In my day, I’ve been a messenger with information that the brain cannot grow new cells or older adults cannot learn as well as younger people. I’ve even taught that connections between neurons are relatively fixed throughout life and intelligence is a matter of how many neurons you have and how fast those neurons work.
I now know that those “facts” are entirely wrong! According to James Trefil, a physicist and author, “Your brain never stops developing and changing. It’s been doing it from the time you were an embryo, and will keep doing it all your life. And this ability, perhaps, represents its greatest strength.”1
Research has shown that the brain is more resilient, adaptable, and capable than we long thought. The brain’s emotional circuitry matures and becomes more balanced with age.2 Thank goodness for that! Researchers on the brain are also telling us that older adults more equally use the brain’s two hemispheres—however, I’ve often been accused of using neither!
But before I get too excited about these new findings, I guess I have to admit that the brain is not immune to age-related changes and that our brain cells can indeed wear out with age. Take, for example, the speed with which it can solve math problems. (Hey, I’ve never been that fast with math, anyway.) Researchers are also telling us that much of the decline in mental ability, touted for so long as associated with the aging process, is actually the result of microstrokes, Alzheimer’s disease, or mental illness. Movies like The Notebook and A Beautiful Mind remind us of the sobering symptoms of those diseases.
I recently attended a lecture on aging at our university by an expert on the importance of the “arts” in enhancing brain fitness. The speaker, Dr. Andrea Sherman, enlightened all regarding Creativity: The Critical Link in Person-Centered Care. She described a number of studies that show a connection between leisure activities (a term I am becoming fond of) and decreasing risk for dementia and cognitive decline. Some of those activities include dancing, playing board games, playing a musical instrument, doing crossword puzzles, and even just reading. (Except for the last one, I haven’t been too inclined to participate. I have, however, been an avid viewer of “Dancing with the Stars”—although I’m not sure that activity counts.)
When I was younger, there were two things I looked forward to about getting older. First, I thought by the time I got to this age I’d have it all figured out. Well, I don’t! Second, I expected that when I reached the magic age, I could retire to leisure activities. Well, I’m not seeing it.
Kidding aside, there are reasons to embrace aging. We’ve all heard that “Getting older beats the alternative.” Well, it’s true, and it’s also true that growing “old” today is better than it has ever been before.
Why? For one thing, there will soon be a billion people older than 60, making us 20% of the world’s population. As a result, global corporations—and even Hollywood!—have started gearing their products and services toward baby boomers (who still have more buying power than any other generation). We’re living longer, and many in better health, than our parents, giving us the vitality to continue to contribute to our professions and our communities—and even become entrepreneurs in our “second career”!3
But the #1 reason I’m glad to be in today’s “over-60” crowd? I can prove wrong all those naysayers who said I’d never make it past 30!
So, what is the moral to this editorial? I forget! But I’d love to hear from you: [email protected].
REFERENCES
1. Trefil J. Are We Unique?: A Scientist Explores the Unparalleled Intelligence of the Human Mind. 1998; New York, NY: John Wiley and Sons.
2. Cohen GD. The Mature Mind: The Positive Power of the Aging Brain. 2005; New York, NY: Perseus Books Group.
3. Hodin M. A new middle age [The Blog]. The Huffington Post. www.huffingtonpost.com/michael-hodin/giving-thanks-a-new-middle-age_b_2170083.html. Accessed July 2, 2013.
The other day I was driving across town—a route I have taken many times—when I suddenly found myself not quite sure where I was. This strange and disconcerting feeling lasted only a few seconds. Later, I was talking with a colleague and forgot a common word that I have used millions of times.
Yes, I am not far from becoming an official member of the Medicare generation. I have heard all the jokes about getting old; many of them I have gleefully subjected my older friends to (you know who you are!). But now it is happening to me. My granddaughter called the other day to wish me a happy birthday. She asked how old I was, and I told her: 64. She was quiet for a moment, then asked, “Did you start at 1?”
I don’t know about you, but the animated tales that dominate all discussions about aging—you know, the ones that tell us that age is just a state of mind, that 60 is the new 40 and 80 the new 60—are starting to irritate me. What’s next: 100 as the new middle age? Truth be told, I don’t feel old. In fact, I’m just now getting up to speed.
In my day, I’ve been a messenger with information that the brain cannot grow new cells or older adults cannot learn as well as younger people. I’ve even taught that connections between neurons are relatively fixed throughout life and intelligence is a matter of how many neurons you have and how fast those neurons work.
I now know that those “facts” are entirely wrong! According to James Trefil, a physicist and author, “Your brain never stops developing and changing. It’s been doing it from the time you were an embryo, and will keep doing it all your life. And this ability, perhaps, represents its greatest strength.”1
Research has shown that the brain is more resilient, adaptable, and capable than we long thought. The brain’s emotional circuitry matures and becomes more balanced with age.2 Thank goodness for that! Researchers on the brain are also telling us that older adults more equally use the brain’s two hemispheres—however, I’ve often been accused of using neither!
But before I get too excited about these new findings, I guess I have to admit that the brain is not immune to age-related changes and that our brain cells can indeed wear out with age. Take, for example, the speed with which it can solve math problems. (Hey, I’ve never been that fast with math, anyway.) Researchers are also telling us that much of the decline in mental ability, touted for so long as associated with the aging process, is actually the result of microstrokes, Alzheimer’s disease, or mental illness. Movies like The Notebook and A Beautiful Mind remind us of the sobering symptoms of those diseases.
I recently attended a lecture on aging at our university by an expert on the importance of the “arts” in enhancing brain fitness. The speaker, Dr. Andrea Sherman, enlightened all regarding Creativity: The Critical Link in Person-Centered Care. She described a number of studies that show a connection between leisure activities (a term I am becoming fond of) and decreasing risk for dementia and cognitive decline. Some of those activities include dancing, playing board games, playing a musical instrument, doing crossword puzzles, and even just reading. (Except for the last one, I haven’t been too inclined to participate. I have, however, been an avid viewer of “Dancing with the Stars”—although I’m not sure that activity counts.)
When I was younger, there were two things I looked forward to about getting older. First, I thought by the time I got to this age I’d have it all figured out. Well, I don’t! Second, I expected that when I reached the magic age, I could retire to leisure activities. Well, I’m not seeing it.
Kidding aside, there are reasons to embrace aging. We’ve all heard that “Getting older beats the alternative.” Well, it’s true, and it’s also true that growing “old” today is better than it has ever been before.
Why? For one thing, there will soon be a billion people older than 60, making us 20% of the world’s population. As a result, global corporations—and even Hollywood!—have started gearing their products and services toward baby boomers (who still have more buying power than any other generation). We’re living longer, and many in better health, than our parents, giving us the vitality to continue to contribute to our professions and our communities—and even become entrepreneurs in our “second career”!3
But the #1 reason I’m glad to be in today’s “over-60” crowd? I can prove wrong all those naysayers who said I’d never make it past 30!
So, what is the moral to this editorial? I forget! But I’d love to hear from you: [email protected].
REFERENCES
1. Trefil J. Are We Unique?: A Scientist Explores the Unparalleled Intelligence of the Human Mind. 1998; New York, NY: John Wiley and Sons.
2. Cohen GD. The Mature Mind: The Positive Power of the Aging Brain. 2005; New York, NY: Perseus Books Group.
3. Hodin M. A new middle age [The Blog]. The Huffington Post. www.huffingtonpost.com/michael-hodin/giving-thanks-a-new-middle-age_b_2170083.html. Accessed July 2, 2013.
The other day I was driving across town—a route I have taken many times—when I suddenly found myself not quite sure where I was. This strange and disconcerting feeling lasted only a few seconds. Later, I was talking with a colleague and forgot a common word that I have used millions of times.
Yes, I am not far from becoming an official member of the Medicare generation. I have heard all the jokes about getting old; many of them I have gleefully subjected my older friends to (you know who you are!). But now it is happening to me. My granddaughter called the other day to wish me a happy birthday. She asked how old I was, and I told her: 64. She was quiet for a moment, then asked, “Did you start at 1?”
I don’t know about you, but the animated tales that dominate all discussions about aging—you know, the ones that tell us that age is just a state of mind, that 60 is the new 40 and 80 the new 60—are starting to irritate me. What’s next: 100 as the new middle age? Truth be told, I don’t feel old. In fact, I’m just now getting up to speed.
In my day, I’ve been a messenger with information that the brain cannot grow new cells or older adults cannot learn as well as younger people. I’ve even taught that connections between neurons are relatively fixed throughout life and intelligence is a matter of how many neurons you have and how fast those neurons work.
I now know that those “facts” are entirely wrong! According to James Trefil, a physicist and author, “Your brain never stops developing and changing. It’s been doing it from the time you were an embryo, and will keep doing it all your life. And this ability, perhaps, represents its greatest strength.”1
Research has shown that the brain is more resilient, adaptable, and capable than we long thought. The brain’s emotional circuitry matures and becomes more balanced with age.2 Thank goodness for that! Researchers on the brain are also telling us that older adults more equally use the brain’s two hemispheres—however, I’ve often been accused of using neither!
But before I get too excited about these new findings, I guess I have to admit that the brain is not immune to age-related changes and that our brain cells can indeed wear out with age. Take, for example, the speed with which it can solve math problems. (Hey, I’ve never been that fast with math, anyway.) Researchers are also telling us that much of the decline in mental ability, touted for so long as associated with the aging process, is actually the result of microstrokes, Alzheimer’s disease, or mental illness. Movies like The Notebook and A Beautiful Mind remind us of the sobering symptoms of those diseases.
I recently attended a lecture on aging at our university by an expert on the importance of the “arts” in enhancing brain fitness. The speaker, Dr. Andrea Sherman, enlightened all regarding Creativity: The Critical Link in Person-Centered Care. She described a number of studies that show a connection between leisure activities (a term I am becoming fond of) and decreasing risk for dementia and cognitive decline. Some of those activities include dancing, playing board games, playing a musical instrument, doing crossword puzzles, and even just reading. (Except for the last one, I haven’t been too inclined to participate. I have, however, been an avid viewer of “Dancing with the Stars”—although I’m not sure that activity counts.)
When I was younger, there were two things I looked forward to about getting older. First, I thought by the time I got to this age I’d have it all figured out. Well, I don’t! Second, I expected that when I reached the magic age, I could retire to leisure activities. Well, I’m not seeing it.
Kidding aside, there are reasons to embrace aging. We’ve all heard that “Getting older beats the alternative.” Well, it’s true, and it’s also true that growing “old” today is better than it has ever been before.
Why? For one thing, there will soon be a billion people older than 60, making us 20% of the world’s population. As a result, global corporations—and even Hollywood!—have started gearing their products and services toward baby boomers (who still have more buying power than any other generation). We’re living longer, and many in better health, than our parents, giving us the vitality to continue to contribute to our professions and our communities—and even become entrepreneurs in our “second career”!3
But the #1 reason I’m glad to be in today’s “over-60” crowd? I can prove wrong all those naysayers who said I’d never make it past 30!
So, what is the moral to this editorial? I forget! But I’d love to hear from you: [email protected].
REFERENCES
1. Trefil J. Are We Unique?: A Scientist Explores the Unparalleled Intelligence of the Human Mind. 1998; New York, NY: John Wiley and Sons.
2. Cohen GD. The Mature Mind: The Positive Power of the Aging Brain. 2005; New York, NY: Perseus Books Group.
3. Hodin M. A new middle age [The Blog]. The Huffington Post. www.huffingtonpost.com/michael-hodin/giving-thanks-a-new-middle-age_b_2170083.html. Accessed July 2, 2013.