User login
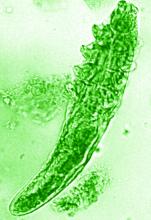
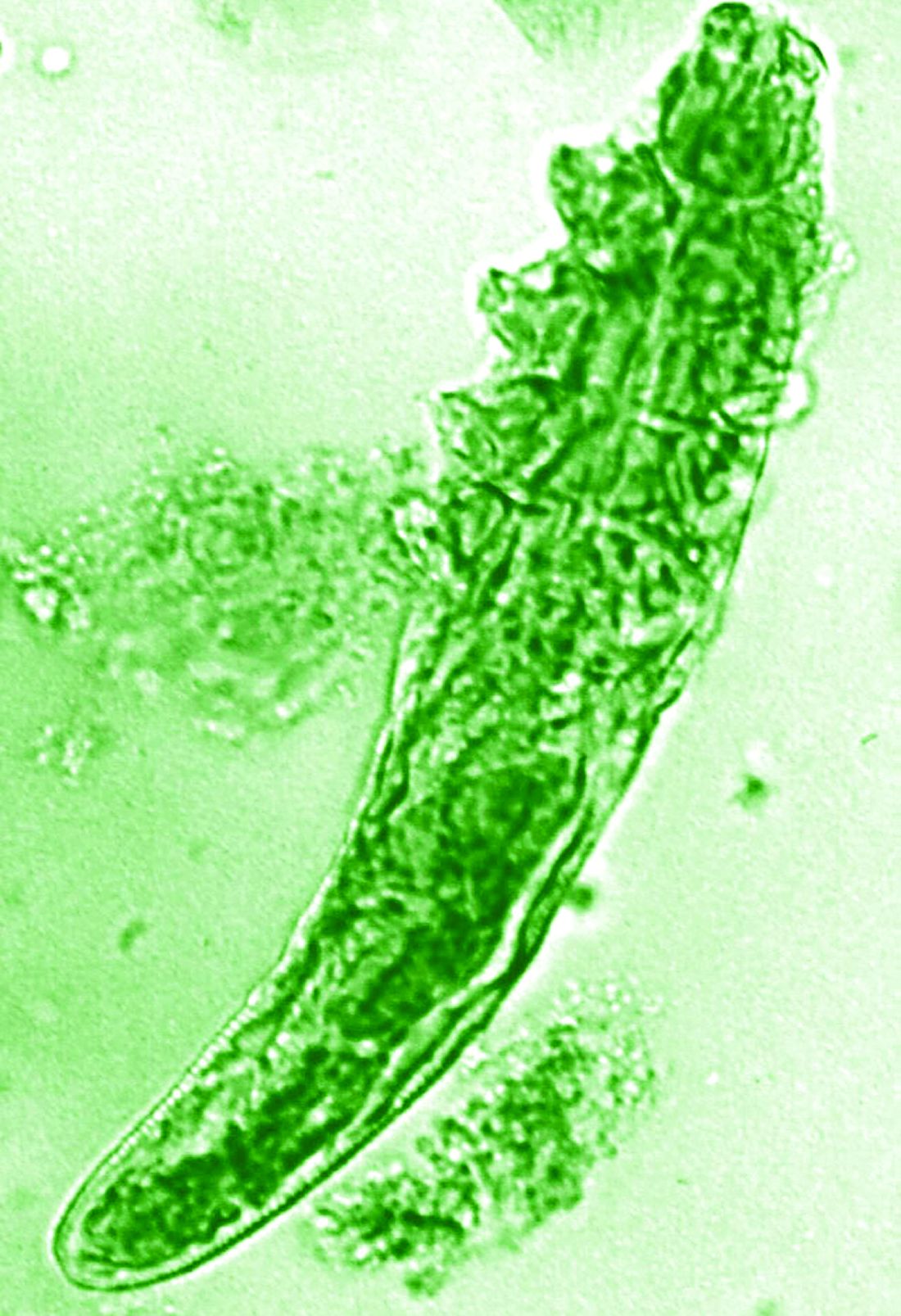
Topical ivermectin study sheds light on dysbiosis in rosacea
, according to a report presented at the recent European Academy of Dermatology and Venereology (EADV) 2023 Congress.
“This is the first hint that the host’s cutaneous microbiome plays a secondary role in the immunopathogenesis of rosacea,” said Bernard Homey, MD, director of the department of dermatology at University Hospital Düsseldorf in Germany.
“In rosacea, we are well aware of trigger factors such as stress, UV light, heat, cold, food, and alcohol,” he said. “We are also well aware that there is an increase in Demodex mites in the pilosebaceous unit.”
Research over the past decade has also started to look at the potential role of the skin microbiome in the disease process, but answers have remained “largely elusive,” Dr. Homey said.
Ivermectin helps, but how?
Ivermectin 1% cream (Soolantra) has been approved by the U.S. Food and Drug Administration since 2014 for the treatment of the inflammatory lesions that are characteristic of rosacea, but its mechanism of action is not clear.
Dr. Homey presented the results of a study of 61 patients designed to look at how ivermectin might be working in the treatment of people with rosacea and investigate if there was any relation to the skin microbiome and transcriptome of patients.
The trial included 41 individuals with papulopustular rosacea and 20 individuals who did not have rosacea. For all patients, surface skin biopsies were performed twice 30 days apart using cyanoacrylate glue; patients with rosacea were treated with topical ivermectin 1% between biopsies. Skin samples obtained at day 0 and day 30 were examined under the microscope, and Demodex counts (mites/cm2) of skin and RNA sequencing of the cutaneous microbiome were undertaken.
The mean age of the patients with rosacea was 54.9 years, and the mean Demodex counts before and after treatment were a respective 7.2 cm2 and 0.9 cm2.
Using the Investigator’s General Assessment to assess the severity of rosacea, Homey reported that 43.9% of patients with rosacea had a decrease in scores at day 30, indicating improvement.
In addition, topical ivermectin resulted in a marked or total decrease in Demodex mite density for 87.5% of patients (n = 24) who were identified as having the mites.
Skin microbiome changes seen
As a form of quality control, skin microbiome changes among the patients were compared with control patients using 16S rRNA sequencing.
“The taxa we find within the cutaneous niche of inflammatory lesions of rosacea patients are significantly different from healthy volunteers,” Dr. Homey said.
Cutibacterium species are predominant in healthy control persons but are not present when there is inflammation in patients with rosacea. Instead, staphylococcus species “take over the niche, similar to atopic dermatitis,” he noted.
Looking at how treatment with ivermectin influences the organisms, the decrease in C. acnes seen in patients with rosacea persisted despite treatment, and the abundance of Staphylococcus epidermidis, S. hominis, and S. capitis increased further. This suggests a possible protective or homeostatic role of C. acnes but a pathogenic role for staphylococci, explained Dr. Homey.
“Surprisingly, although inflammatory lesions decrease, patients get better, the cutaneous microbiome does not revert to homeostatic conditions during topical ivermectin treatment,” he observed.
There is, of course, variability among individuals.
Dr. Homey also reported that Snodgrassella alvi – a microorganism believed to reside in the gut of Demodex folliculorum mites – was found in the skin microbiome of patients with rosacea before but not after ivermectin treatment. This may mean that this microorganism could be partially triggering inflammation in rosacea patients.
Looking at the transcriptome of patients, Dr. Homey said that there was downregulation of distinct genes that might make for more favorable conditions for Demodex mites.
Moreover, insufficient upregulation of interleukin-17 pathways might be working together with barrier defects in the skin and metabolic changes to “pave the way” for colonization by S. epidermidis.
Pulling it together
Dr. Homey and associates conclude in their abstract that the findings “support that rosacea lesions are associated with dysbiosis.”
Although treatment with ivermectin did not normalize the skin’s microbiome, it was associated with a decrease in Demodex mite density and the reduction of microbes associated with Demodex.
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra in Portugal, who cochaired the late-breaking news session where the data were presented, asked whether healthy and affected skin in patients with rosacea had been compared, rather than comparing the skin of rosacea lesions with healthy control samples.
“No, we did not this, as this is methodologically a little bit more difficult,” Dr. Homey responded.
Also cochairing the session was Michel Gilliet, MD, chair of the department of dermatology at the University Hospital CHUV in Lausanne, Switzerland. He commented that these “data suggest that there’s an intimate link between Demodex and the skin microbiota and dysbiosis in in rosacea.”
Dr. Gilliet added: “You have a whole dysbiosis going on in rosacea, which is probably only dependent on these bacteria.”
It would be “very interesting,” as a “proof-of-concept” study, to look at whether depleting Demodex would also delete S. alvi, he suggested.
The study was funded by Galderma. Dr. Homey has acted as a consultant, speaker or investigator for many pharmaceutical companies including Galderma.
A version of this article first appeared on Medscape.com.
, according to a report presented at the recent European Academy of Dermatology and Venereology (EADV) 2023 Congress.
“This is the first hint that the host’s cutaneous microbiome plays a secondary role in the immunopathogenesis of rosacea,” said Bernard Homey, MD, director of the department of dermatology at University Hospital Düsseldorf in Germany.
“In rosacea, we are well aware of trigger factors such as stress, UV light, heat, cold, food, and alcohol,” he said. “We are also well aware that there is an increase in Demodex mites in the pilosebaceous unit.”
Research over the past decade has also started to look at the potential role of the skin microbiome in the disease process, but answers have remained “largely elusive,” Dr. Homey said.
Ivermectin helps, but how?
Ivermectin 1% cream (Soolantra) has been approved by the U.S. Food and Drug Administration since 2014 for the treatment of the inflammatory lesions that are characteristic of rosacea, but its mechanism of action is not clear.
Dr. Homey presented the results of a study of 61 patients designed to look at how ivermectin might be working in the treatment of people with rosacea and investigate if there was any relation to the skin microbiome and transcriptome of patients.
The trial included 41 individuals with papulopustular rosacea and 20 individuals who did not have rosacea. For all patients, surface skin biopsies were performed twice 30 days apart using cyanoacrylate glue; patients with rosacea were treated with topical ivermectin 1% between biopsies. Skin samples obtained at day 0 and day 30 were examined under the microscope, and Demodex counts (mites/cm2) of skin and RNA sequencing of the cutaneous microbiome were undertaken.
The mean age of the patients with rosacea was 54.9 years, and the mean Demodex counts before and after treatment were a respective 7.2 cm2 and 0.9 cm2.
Using the Investigator’s General Assessment to assess the severity of rosacea, Homey reported that 43.9% of patients with rosacea had a decrease in scores at day 30, indicating improvement.
In addition, topical ivermectin resulted in a marked or total decrease in Demodex mite density for 87.5% of patients (n = 24) who were identified as having the mites.
Skin microbiome changes seen
As a form of quality control, skin microbiome changes among the patients were compared with control patients using 16S rRNA sequencing.
“The taxa we find within the cutaneous niche of inflammatory lesions of rosacea patients are significantly different from healthy volunteers,” Dr. Homey said.
Cutibacterium species are predominant in healthy control persons but are not present when there is inflammation in patients with rosacea. Instead, staphylococcus species “take over the niche, similar to atopic dermatitis,” he noted.
Looking at how treatment with ivermectin influences the organisms, the decrease in C. acnes seen in patients with rosacea persisted despite treatment, and the abundance of Staphylococcus epidermidis, S. hominis, and S. capitis increased further. This suggests a possible protective or homeostatic role of C. acnes but a pathogenic role for staphylococci, explained Dr. Homey.
“Surprisingly, although inflammatory lesions decrease, patients get better, the cutaneous microbiome does not revert to homeostatic conditions during topical ivermectin treatment,” he observed.
There is, of course, variability among individuals.
Dr. Homey also reported that Snodgrassella alvi – a microorganism believed to reside in the gut of Demodex folliculorum mites – was found in the skin microbiome of patients with rosacea before but not after ivermectin treatment. This may mean that this microorganism could be partially triggering inflammation in rosacea patients.
Looking at the transcriptome of patients, Dr. Homey said that there was downregulation of distinct genes that might make for more favorable conditions for Demodex mites.
Moreover, insufficient upregulation of interleukin-17 pathways might be working together with barrier defects in the skin and metabolic changes to “pave the way” for colonization by S. epidermidis.
Pulling it together
Dr. Homey and associates conclude in their abstract that the findings “support that rosacea lesions are associated with dysbiosis.”
Although treatment with ivermectin did not normalize the skin’s microbiome, it was associated with a decrease in Demodex mite density and the reduction of microbes associated with Demodex.
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra in Portugal, who cochaired the late-breaking news session where the data were presented, asked whether healthy and affected skin in patients with rosacea had been compared, rather than comparing the skin of rosacea lesions with healthy control samples.
“No, we did not this, as this is methodologically a little bit more difficult,” Dr. Homey responded.
Also cochairing the session was Michel Gilliet, MD, chair of the department of dermatology at the University Hospital CHUV in Lausanne, Switzerland. He commented that these “data suggest that there’s an intimate link between Demodex and the skin microbiota and dysbiosis in in rosacea.”
Dr. Gilliet added: “You have a whole dysbiosis going on in rosacea, which is probably only dependent on these bacteria.”
It would be “very interesting,” as a “proof-of-concept” study, to look at whether depleting Demodex would also delete S. alvi, he suggested.
The study was funded by Galderma. Dr. Homey has acted as a consultant, speaker or investigator for many pharmaceutical companies including Galderma.
A version of this article first appeared on Medscape.com.
, according to a report presented at the recent European Academy of Dermatology and Venereology (EADV) 2023 Congress.
“This is the first hint that the host’s cutaneous microbiome plays a secondary role in the immunopathogenesis of rosacea,” said Bernard Homey, MD, director of the department of dermatology at University Hospital Düsseldorf in Germany.
“In rosacea, we are well aware of trigger factors such as stress, UV light, heat, cold, food, and alcohol,” he said. “We are also well aware that there is an increase in Demodex mites in the pilosebaceous unit.”
Research over the past decade has also started to look at the potential role of the skin microbiome in the disease process, but answers have remained “largely elusive,” Dr. Homey said.
Ivermectin helps, but how?
Ivermectin 1% cream (Soolantra) has been approved by the U.S. Food and Drug Administration since 2014 for the treatment of the inflammatory lesions that are characteristic of rosacea, but its mechanism of action is not clear.
Dr. Homey presented the results of a study of 61 patients designed to look at how ivermectin might be working in the treatment of people with rosacea and investigate if there was any relation to the skin microbiome and transcriptome of patients.
The trial included 41 individuals with papulopustular rosacea and 20 individuals who did not have rosacea. For all patients, surface skin biopsies were performed twice 30 days apart using cyanoacrylate glue; patients with rosacea were treated with topical ivermectin 1% between biopsies. Skin samples obtained at day 0 and day 30 were examined under the microscope, and Demodex counts (mites/cm2) of skin and RNA sequencing of the cutaneous microbiome were undertaken.
The mean age of the patients with rosacea was 54.9 years, and the mean Demodex counts before and after treatment were a respective 7.2 cm2 and 0.9 cm2.
Using the Investigator’s General Assessment to assess the severity of rosacea, Homey reported that 43.9% of patients with rosacea had a decrease in scores at day 30, indicating improvement.
In addition, topical ivermectin resulted in a marked or total decrease in Demodex mite density for 87.5% of patients (n = 24) who were identified as having the mites.
Skin microbiome changes seen
As a form of quality control, skin microbiome changes among the patients were compared with control patients using 16S rRNA sequencing.
“The taxa we find within the cutaneous niche of inflammatory lesions of rosacea patients are significantly different from healthy volunteers,” Dr. Homey said.
Cutibacterium species are predominant in healthy control persons but are not present when there is inflammation in patients with rosacea. Instead, staphylococcus species “take over the niche, similar to atopic dermatitis,” he noted.
Looking at how treatment with ivermectin influences the organisms, the decrease in C. acnes seen in patients with rosacea persisted despite treatment, and the abundance of Staphylococcus epidermidis, S. hominis, and S. capitis increased further. This suggests a possible protective or homeostatic role of C. acnes but a pathogenic role for staphylococci, explained Dr. Homey.
“Surprisingly, although inflammatory lesions decrease, patients get better, the cutaneous microbiome does not revert to homeostatic conditions during topical ivermectin treatment,” he observed.
There is, of course, variability among individuals.
Dr. Homey also reported that Snodgrassella alvi – a microorganism believed to reside in the gut of Demodex folliculorum mites – was found in the skin microbiome of patients with rosacea before but not after ivermectin treatment. This may mean that this microorganism could be partially triggering inflammation in rosacea patients.
Looking at the transcriptome of patients, Dr. Homey said that there was downregulation of distinct genes that might make for more favorable conditions for Demodex mites.
Moreover, insufficient upregulation of interleukin-17 pathways might be working together with barrier defects in the skin and metabolic changes to “pave the way” for colonization by S. epidermidis.
Pulling it together
Dr. Homey and associates conclude in their abstract that the findings “support that rosacea lesions are associated with dysbiosis.”
Although treatment with ivermectin did not normalize the skin’s microbiome, it was associated with a decrease in Demodex mite density and the reduction of microbes associated with Demodex.
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra in Portugal, who cochaired the late-breaking news session where the data were presented, asked whether healthy and affected skin in patients with rosacea had been compared, rather than comparing the skin of rosacea lesions with healthy control samples.
“No, we did not this, as this is methodologically a little bit more difficult,” Dr. Homey responded.
Also cochairing the session was Michel Gilliet, MD, chair of the department of dermatology at the University Hospital CHUV in Lausanne, Switzerland. He commented that these “data suggest that there’s an intimate link between Demodex and the skin microbiota and dysbiosis in in rosacea.”
Dr. Gilliet added: “You have a whole dysbiosis going on in rosacea, which is probably only dependent on these bacteria.”
It would be “very interesting,” as a “proof-of-concept” study, to look at whether depleting Demodex would also delete S. alvi, he suggested.
The study was funded by Galderma. Dr. Homey has acted as a consultant, speaker or investigator for many pharmaceutical companies including Galderma.
A version of this article first appeared on Medscape.com.
FROM EADV 2023
More phase 3 data support use of nemolizumab for prurigo nodularis
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
reported at the annual Congress of the European Academy of Dermatology and Venereology.
In the OLYMPIA 1 study, clinically significant improvements in both itch and skin lesions were seen after 16 weeks of treatment with nemolizumab compared with placebo (P < .0001).
Indeed, among the 286 patients who participated in the trial (190 on nemolizumab and 96 on placebo), 58.4% of those treated with nemolizumab and 16.7% of those who received placebo had an improvement of 4 points or more in the weekly average peak pruritus numeric rating scale (PP-NRS) at week 16 (P < .0001).
Skin lesions were assessed using an investigators general assessment (IGA) score, where IGA success was defined as a score of 0/1 indicating clear or almost clear skin or where there had been at least a 2-point change from baseline values. Over a quarter (26.3%) of nemolizumab-treated patients met these criteria versus 7.3% for those on placebo (P = .0001).
“These results confirm the results of the OLYMPIA 2 study, the other phase 3 study, and now I hope you understand why we are so excited,” lead investigator Sonja Ständer, MD, of the Center for Chronic Pruritus at University Hospital Münster, Germany, said at the meeting, where she presented the data.
The OLYMPIA 2 study included 274 patients and the results showed a weekly average PP-NRS score improvement of 56.3% vs. 20.9% for placebo and IGA success in 37.7% and 11% of patients, respectively, at 16 weeks.
First-in-class therapy
“We know how difficult it is to treat patients; they are refractory to treatment, frustrated, and this really impacts them regarding their quality of life,” said Dr. Ständer. New options are needed to help patients, and nemolizumab, a first-in-class interleukin-31 (IL-31) receptor alpha antagonist, is one treatment that may answer this call.
Prurigo nodularis is a chronic neuroimmune skin condition characterized by severe itch and multiple nodular skin lesions, Dr. Ständer explained. She added that there is evidence that IL-31 has a key role to play in the development of itch, and in differentiation of keratinocytes, type 2 and type 17 immune responses, and fibrosis associated with the condition.
The OLYMPIA 1 and 2 trials are part of a large developmental program that includes two ongoing trials. One is assessing the durability of response over 24 weeks in 40 patients and the other is a long-term extension trial involving 450 patients from the OLYMPIA 1 and 2 trials.
Inclusion criteria and additional results
For inclusion in the study, adults with prurigo nodularis for at least 6 months had to have 20 or more nodules on the body with a bilateral distribution, an IGA score of 3 or more, and an average PP-NRS of 7 or higher. The latter “was really a high bar for them to qualify for the trial,” said Dr. Ständer.
After an initial 4-week screening period, patients were randomly assigned to 24 weeks of treatment with nemolizumab or placebo given as a subcutaneous injection every 4 weeks. An 8-week “off-treatment” period followed.
The nemolizumab dose was based on the patient’s body weight, with patients weighing less than 90 kg (198 pounds) getting a loading dose of 60 mg followed by further doses of 30 mg; while patients weighing 90 kg or more receiving 50 mg of nemolizumab.
Dr. Ständer reported that nemolizumab met all of the trials’ secondary endpoints; this included at least a 4-point improvement in sleep disturbance. She noted that changes in itch and subsequent sleep disturbance occurred early, at 4 weeks of treatment – after just one injection of nemolizumab.
The response rates seen in the moderate to severe prurigo nodularis population studies are quite unique when compared with conventional therapies, Dr. Ständer maintained. “We’ve never seen something like this before.”
No safety concerns
No significant difference in tolerability was seen between the nemolizumab and placebo groups, Dr. Ständer observed. Any adverse event occurred in 71.7% and 65.3% of patients, respectively, and serious adverse events in 8.6% and 10.5%.
There was a similar rate of adverse events leading to discontinuation, respectively (4.8% vs. 4.2%).
Headache was seen more frequently among those on nemolizumab than those on placebo (7.0% vs. 2.1%), and there was a higher number of eczema cases among those on nemolizumab (5.3% vs. 1.1%). The latter is somewhat paradoxical because nemolizumab is also being studied as a treatment for atopic dermatitis, with good results seen in phase 3 trials. Asked about this finding after her presentation, Dr. Ständer said “we are following up on that to know exactly what is going on; this is a side effect of nemolizumab that is seen also with other biologics.”
JAK inhibitor trial for PN, CPUO
Nemolizumab is not the only promising new approach to treating prurigo nodularis. During a separate late-breaking news session at the meeting, Shawn Kwatra, MD, director of the Johns Hopkins Itch Center in Baltimore, presented “dramatic” data from a “proof-of-concept” phase 2 study with the Janus kinase (JAK) inhibitor abrocitinib (Cibinqo), which is approved for atopic dermatitis in the United States and Europe.
The investigator-initiated trial took a different approach from most other trials, Dr. Kwatra said. The starting point was to look at studying multiple rather than single dermatologic diseases that were perhaps being left a little by the wayside but may share some common ground. Those two diseases were prurigo nodularis and chronic pruritus of unknown origin (CPUO).
“They’re actually very analogous conditions in the way we treat, so I thought those would be a good pair,” Dr. Kwatra said, noting that there were several studies that made him think that JAK inhibition “would be an interesting concept to try.”
On that basis, 10 women with prurigo nodularis (mean age, 58 years) and two women and eight men with CPUO (mean age, 70 years) were recruited and all were treated with abrocitinib at a once-daily oral dose of 200 mg for 12 weeks.
“They all had really intense itch,” before treatment, Dr. Kwatra said. The mean baseline PP-NRS was 9.2 and 8.2 in the prurigo nodularis and CPUO groups, respectively. By the end of treatment, however, “the improvement in itch was pretty dramatic,” especially for prurigo nodularis, he said.
At 12 weeks, the PP-NRS score had fallen to 2.0 in the prurigo nodularis group, equating to a significant 78% change from baseline (P < .001). And, in the CPUO group, the 12-week PP-NRS score was 3.8, nearly a 54% drop from baseline (P = .01).
Sleep disturbance was improved for both conditions, and in the patients with prurigo nodularis, there were improvements in skin lesions. Looking at the patients who responded to treatment, Dr. Kwatra noted that “if you responded, you respond fast, and you respond almost entirely.”
Additional findings from cutaneous transcriptome analysis showed that JAK inhibition with abrocitinib was modulating Th1-, Th2-, Th17-, and Th22-mediated pathways in both groups of patients.
The overall frequency of adverse events was low, and no serious adverse events occurred.
Commenting on the potential use of abrocitinib in managing patients with PN and CPUO, Tiago dos Reis Matos, MD, PhD, MSc, Amsterdam University Medical Centers, told this news organization that JAK1 inhibitors “are showing promising results in treating several diseases.”
Dr. Matos, who was not involved in the study, added that JAK inhibition was “of special interest in prurigo nodularis and chronic pruritus, since these are some of the most difficult diseases to treat with limited therapeutic options.”
Dr. Kwatra observed: “Obviously, we need further development. But we also have clues here about how to design phase 3 trials.”
Galderma funded the OLYMPIA 1 and 2 studies. Dr. Ständer was an investigator for the trial and reported serving as a consultant, speaker, or investigator for multiple pharmaceutical companies, including Galderma.
Johns Hopkins University supported the abrocitinib study with funding from Pfizer. Dr. Kwatra is an advisory board member or consultant to several pharmaceutical companies and is an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM THE EADV CONGRESS
Birch bark–derived treatment reduces daily dressings in patients with epidermolysis bullosa
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
Additional when compared with a control gel.
In a final, post hoc analysis to come from the trial, 15 of 45 (33%) patients treated with Oleogel-S10 versus 5 of 48 (10.4%) treated with the control gel were reported as no longer needing daily dressing changes at 45 days of follow-up.
Moreover, the effect was sustained, with similar percentages of patients no longer requiring daily dressing changes at 60 days (34% vs. 13%, respectively) and 90 days (36% vs. 11%) of follow-up.
The mean reduction in daily dressing changes was 1.36 for Oleogel-S10 and 0.41 for the control gel (P = .005).
“Patients who, in the beginning, had daily dressing changes had almost three fewer dressing changes every 2 weeks if they were treated with Oleogel-S10,” Dimitra Kiritsi, MD, PhD, of the department of dermatology at the University of Freiburg (Germany), reported at the annual congress of the European Academy of Dermatology and Venereology. By comparison, patients in the control group had just one fewer daily dressing change in 2 weeks.
“You might say okay, but what does this mean in terms of time?” added Dr. Kiritsi. Using historical data on the time required for whole body care (Orphanet J Rare Dis. 2020 Jan 3. doi: 10.1186/s13023-019-1279-y), it was estimated that treatment with Oleogel-S10 was associated with an overall time-saving per week of 11 hours (6.6 hours for the patient and 4.4 hours for the caregiver) and use of the control gel was associated with an overall time-saving of 4 hours (2.4 hours for the patient and 1.6 hours for the caregiver).
“This is, for our patients, important,” said Dr. Kiritsi, as “it is time that they can spend doing something nice with the family” instead, avoiding the pain and distress associated with frequent dressing changes.
Approved in Europe, not in the United States
Oleogel-S10, classified as an herbal product, contains triterpenes derived from birch bark extract, which have been formulated with sunflower oil to form a gel.
Despite being approved for use in Europe, Oleogel-S10 has not yet been approved to treat EB in the United States. The FDA did not approve Amryt Pharma’s new drug application in February 2022. The application had included data from the EASE trial.
EASE included 223 patients with dystrophic or junctional EB, including 156 children, at 58 sites in 28 countries. As such, this makes it the largest treatment study in this rare genetic disease to date.
The trial had consisted of an initial 90-day, double-blind treatment period, during which time 109 patients had used Oleogel-S10 and 114 had used a control gel. This was followed by a 24-month open-label phase, during which time all remaining patients (n = 205) had used Oleogel-S10 on top of their standard of care.
Dr. Kiritsi summarized the main results of the EASE trial as follows.
- Complete healing of target wounds (primary endpoint) in 41.3% of patients treated with Oleogel-S10 and 28.9% of patients treated with the control gel (P = .013).
- Improved total body wound burden measured by both Epidermolysis Bullosa Disease Activity and Scarring Index and Body Surface Area Percentage scores.
- Reduced frequency of dressing changes (1 less per 2 weeks for Oleogel-S10 versus 0 less per 2 weeks for control gel).
- Improved pain among participants aged 4 years and older while their dressings were being changed.
- Reduced rates of wound infection (0.9% Oleogel-S10 vs. 4.4% control gel).
- Similar rates of treatment-emergent adverse events (24.8% vs. 22.8%, respectively), which were mostly deemed to be mild or moderate.
The EASE study – an important trial for EB
EASE is an important trial for EB, the study’s principal investigator Dédée Murrell, MD, DSc, University of New South Wales, Sydney, has pointed out previously.
“This was the first EB study to meet its primary endpoint and demonstrated a statistically significant acceleration of target wound healing by day 45,” Dr. Murrell said in a press release issued by Amryt Pharma to coincide with the online publication of the trial results.
“In addition, the favorable trends we see with key secondary endpoints such as reduced wound burden, pain, and frequency of dressing changes are considered as being very meaningful for patients,” Dr. Murrell said.
The EASE study was funded by Amryt Research Limited. Dr. Kiritsi reported receiving honoraria or consultation fees from Amryt, RHEACELL GmbH, and Fibrx Derm. She also acknowledged grant or research support from DEBRA International, EB Research Partnership, Fritz-Thyssen Foundation, German Research Foundation, and RHEACELL. Dr. Murrell has ties to Amryt and Amicus and is a co-owner of the patent for topical sirolimus for EB simplex.
A version of this article appeared on Medscape.com.
FROM THE EADV CONGRESS
Roflumilast side effect benefits patients with psoriasis and overweight/obesity
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
AT THE EADV CONGRESS
Topical botanical drug coacillium curbs childhood alopecia
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
AT THE EADV CONGRESS
Axial spondyloarthritis: Does visibility with x-rays make a difference in management?
Knowing whether a patient has radiographic or nonradiographic axial spondyloarthritis will not change management, experts say.
Out with the old, in with the new
Axial spondyloarthritis is characterized by chronic inflammation of the sacroiliac (SI) joints, and spine. It’s a modern term that includes ankylosing spondylitis (AS) and that refers to opposite ends of a disease spectrum.
Nonradiographic axial spondyloarthritis (nr-axSpA) is so termed because there are no definitive visible changes on plain x-rays, although inflammatory changes may be seen on MRI.
Radiographic axial spondyloarthritis (r-axSpA) is the same as AS to some extent and is associated with clear signs of joint damage (that is, of past inflammation) on x-rays.
“Axial spondyloarthritis is one disease, and whether it is radiographic or nonradiographic makes zero difference in the management of the patient,” says Atul Deodhar, MD, professor of medicine and medical director of rheumatology clinics at Oregon Health and Science University, Portland. The distinction came about in 2009 to facilitate scientific and clinical research, he explains, and to enable the use of tumor necrosis factor inhibitors, which were new at the time, for patients who could not be classified as having AS.
“We have known what ankylosing spondylitis is for a long time because we have been doing plain x-rays of the sacroiliac joints, and if we see classical changes of sacroiliitis, we have the diagnosis. However, MRI changed everything,” Dr. Deodhar says. Now it’s possible to see inflammatory changes in the SI joints and early joint damage, which was not possible to see on x-ray until many years later.
Reassuring for patients?
“Currently, we don’t really have different treatments,” Dr. Deodhar notes. Perhaps the only benefit is that it might be reassuring for patients to know that they have the nonradiographic form. Receiving a diagnosis of axial spondyloarthritis comes as quite a shock. It’s a diagnosis that is potentially going to affect them for the rest of their lives, and some patients worry that they’ll develop the classic “bamboo” spine of AS, he adds. So, being able to tell patients that they have nr-axSpA and that they are going to be treated early and aggressively may be somewhat comforting.
“It’s a continuum of a disease state, but a lot of people will stay at the nonradiographic stage,” points out Portland-based internist Beth Smith, DO, associate professor of medicine at OHSU.
“A good portion of individuals who may have an MRI that’s positive will either go into remission or just stay at that stage of the disease; they won’t necessarily progress to radiographic sacroiliitis,” she adds.
Spotting nr-axSpA in practice
Nr-axSpA can be tricky to spot in clinical practice, and its diagnosis in primary care largely relies on patients’ clinical presentation and identifying IBP. This is the key symptom. When someone younger than 45 years experiences back pain that is characterized by insidious and chronic onset and that improves with anti-inflammatory agents and activity but that worsens with rest and is worse at night, then imaging of the SI joints may be appropriate.
“You have to have that index of suspicion in order to even think about ordering the appropriate imaging test,” Dr. Smith says. IBP may be the big clue, but patients may also return on separate occasions with multiple associated complaints, such as plantar fasciitis, tennis elbow, or other conditions, such as psoriasis, she says.
Ordering HLA-B27 and C-reactive protein tests may be useful prior to conducting any imaging, Dr. Smith says, “and if imaging is ordered, make sure it is an x-ray of the sacroiliac joint, not the lumbar spine.”
Dr. Deodhar cautions: “A single anterior-posterior view of the pelvis is enough to look at the sacroiliac joint.” There is no need to order separate views of the right and left SI joints; doing so will provide no additional useful information and exposes the patient to unnecessary radiation.
Importantly, consider whether an x-ray of the lumbar spine is needed for a patient with chronic back pain, he says. “You should do an investigation that is going to make a difference to your management. If you take 100 patients with back pain, 95% of the time, it is going to be mechanical back pain. Why do an x-ray of the lumbar spine?” Dr. Deodhar asks rhetorically.
It should also be borne in mind that x-rays can be nonspecific, and several conditions may mimic sacroiliitis, such as osteitis condensans ilii in women who have given birth, osteoarthritis of the SI joints, and old infection of the SI joints.
MRIs need specialist interpretation
MRIs of the lumbar spine are overused to diagnose back pain, and while they might be sensitive to early inflammatory changes in SI joints, they require an expert eye for interpretation.
“MRI of the SI joints is to be used wisely in patients when there is enough clinical suspicion,” Dr. Deodhar advises. Even when an MRI is negative for sacroiliitis, patients could still have axial spondyloarthritis.
MRIs of the SI joints are needed, but not of the lumbar spine, he stresses. Views of the lumbar spine may show only signs of disk degeneration and perhaps osteoarthritis.
Moreover, Dr. Deodhar says, “MRI is so sensitive that we used to think that bone marrow edema is good enough for telling us there is sacroiliitis.” However, even people without IBP can have bone marrow edema; “exercise can show bone marrow edema,” he says.
So, “If there’s a suspicion of axial spondyloarthritis, the patient should be referred to a rheumatologist,” who will discuss the interpretation with highly specialized musculoskeletal radiologists.
Take-home messages
Whether it is nr-axSpA or r-axSpA, “the burden of disease for the patient is the same; treatment is the same,” says Dr. Deodhar. Patients should be referred to a rheumatologist as soon as possible if axial spondyloarthritis is suspected. A single x-ray of the pelvis should be performed to see the SI joints, but MRIs should be left to secondary care, he suggests.
Dr. Smith notes: “Having that index of suspicion of an inflammatory etiology for the back pain is essential.” It ensures that “patients can get early and appropriate treatment for a disease that’s very different from the mechanical back pain that we mostly see in primary care.”
Dr. Deodhar has received research grants or has acted as a consultant to multiple pharmaceutical companies, including AbbVie, Bristol-Myers Squibb, Celgene, Janssen, UCB, Novartis, Pfizer, and Eli Lilly. Dr. Smith reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Knowing whether a patient has radiographic or nonradiographic axial spondyloarthritis will not change management, experts say.
Out with the old, in with the new
Axial spondyloarthritis is characterized by chronic inflammation of the sacroiliac (SI) joints, and spine. It’s a modern term that includes ankylosing spondylitis (AS) and that refers to opposite ends of a disease spectrum.
Nonradiographic axial spondyloarthritis (nr-axSpA) is so termed because there are no definitive visible changes on plain x-rays, although inflammatory changes may be seen on MRI.
Radiographic axial spondyloarthritis (r-axSpA) is the same as AS to some extent and is associated with clear signs of joint damage (that is, of past inflammation) on x-rays.
“Axial spondyloarthritis is one disease, and whether it is radiographic or nonradiographic makes zero difference in the management of the patient,” says Atul Deodhar, MD, professor of medicine and medical director of rheumatology clinics at Oregon Health and Science University, Portland. The distinction came about in 2009 to facilitate scientific and clinical research, he explains, and to enable the use of tumor necrosis factor inhibitors, which were new at the time, for patients who could not be classified as having AS.
“We have known what ankylosing spondylitis is for a long time because we have been doing plain x-rays of the sacroiliac joints, and if we see classical changes of sacroiliitis, we have the diagnosis. However, MRI changed everything,” Dr. Deodhar says. Now it’s possible to see inflammatory changes in the SI joints and early joint damage, which was not possible to see on x-ray until many years later.
Reassuring for patients?
“Currently, we don’t really have different treatments,” Dr. Deodhar notes. Perhaps the only benefit is that it might be reassuring for patients to know that they have the nonradiographic form. Receiving a diagnosis of axial spondyloarthritis comes as quite a shock. It’s a diagnosis that is potentially going to affect them for the rest of their lives, and some patients worry that they’ll develop the classic “bamboo” spine of AS, he adds. So, being able to tell patients that they have nr-axSpA and that they are going to be treated early and aggressively may be somewhat comforting.
“It’s a continuum of a disease state, but a lot of people will stay at the nonradiographic stage,” points out Portland-based internist Beth Smith, DO, associate professor of medicine at OHSU.
“A good portion of individuals who may have an MRI that’s positive will either go into remission or just stay at that stage of the disease; they won’t necessarily progress to radiographic sacroiliitis,” she adds.
Spotting nr-axSpA in practice
Nr-axSpA can be tricky to spot in clinical practice, and its diagnosis in primary care largely relies on patients’ clinical presentation and identifying IBP. This is the key symptom. When someone younger than 45 years experiences back pain that is characterized by insidious and chronic onset and that improves with anti-inflammatory agents and activity but that worsens with rest and is worse at night, then imaging of the SI joints may be appropriate.
“You have to have that index of suspicion in order to even think about ordering the appropriate imaging test,” Dr. Smith says. IBP may be the big clue, but patients may also return on separate occasions with multiple associated complaints, such as plantar fasciitis, tennis elbow, or other conditions, such as psoriasis, she says.
Ordering HLA-B27 and C-reactive protein tests may be useful prior to conducting any imaging, Dr. Smith says, “and if imaging is ordered, make sure it is an x-ray of the sacroiliac joint, not the lumbar spine.”
Dr. Deodhar cautions: “A single anterior-posterior view of the pelvis is enough to look at the sacroiliac joint.” There is no need to order separate views of the right and left SI joints; doing so will provide no additional useful information and exposes the patient to unnecessary radiation.
Importantly, consider whether an x-ray of the lumbar spine is needed for a patient with chronic back pain, he says. “You should do an investigation that is going to make a difference to your management. If you take 100 patients with back pain, 95% of the time, it is going to be mechanical back pain. Why do an x-ray of the lumbar spine?” Dr. Deodhar asks rhetorically.
It should also be borne in mind that x-rays can be nonspecific, and several conditions may mimic sacroiliitis, such as osteitis condensans ilii in women who have given birth, osteoarthritis of the SI joints, and old infection of the SI joints.
MRIs need specialist interpretation
MRIs of the lumbar spine are overused to diagnose back pain, and while they might be sensitive to early inflammatory changes in SI joints, they require an expert eye for interpretation.
“MRI of the SI joints is to be used wisely in patients when there is enough clinical suspicion,” Dr. Deodhar advises. Even when an MRI is negative for sacroiliitis, patients could still have axial spondyloarthritis.
MRIs of the SI joints are needed, but not of the lumbar spine, he stresses. Views of the lumbar spine may show only signs of disk degeneration and perhaps osteoarthritis.
Moreover, Dr. Deodhar says, “MRI is so sensitive that we used to think that bone marrow edema is good enough for telling us there is sacroiliitis.” However, even people without IBP can have bone marrow edema; “exercise can show bone marrow edema,” he says.
So, “If there’s a suspicion of axial spondyloarthritis, the patient should be referred to a rheumatologist,” who will discuss the interpretation with highly specialized musculoskeletal radiologists.
Take-home messages
Whether it is nr-axSpA or r-axSpA, “the burden of disease for the patient is the same; treatment is the same,” says Dr. Deodhar. Patients should be referred to a rheumatologist as soon as possible if axial spondyloarthritis is suspected. A single x-ray of the pelvis should be performed to see the SI joints, but MRIs should be left to secondary care, he suggests.
Dr. Smith notes: “Having that index of suspicion of an inflammatory etiology for the back pain is essential.” It ensures that “patients can get early and appropriate treatment for a disease that’s very different from the mechanical back pain that we mostly see in primary care.”
Dr. Deodhar has received research grants or has acted as a consultant to multiple pharmaceutical companies, including AbbVie, Bristol-Myers Squibb, Celgene, Janssen, UCB, Novartis, Pfizer, and Eli Lilly. Dr. Smith reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Knowing whether a patient has radiographic or nonradiographic axial spondyloarthritis will not change management, experts say.
Out with the old, in with the new
Axial spondyloarthritis is characterized by chronic inflammation of the sacroiliac (SI) joints, and spine. It’s a modern term that includes ankylosing spondylitis (AS) and that refers to opposite ends of a disease spectrum.
Nonradiographic axial spondyloarthritis (nr-axSpA) is so termed because there are no definitive visible changes on plain x-rays, although inflammatory changes may be seen on MRI.
Radiographic axial spondyloarthritis (r-axSpA) is the same as AS to some extent and is associated with clear signs of joint damage (that is, of past inflammation) on x-rays.
“Axial spondyloarthritis is one disease, and whether it is radiographic or nonradiographic makes zero difference in the management of the patient,” says Atul Deodhar, MD, professor of medicine and medical director of rheumatology clinics at Oregon Health and Science University, Portland. The distinction came about in 2009 to facilitate scientific and clinical research, he explains, and to enable the use of tumor necrosis factor inhibitors, which were new at the time, for patients who could not be classified as having AS.
“We have known what ankylosing spondylitis is for a long time because we have been doing plain x-rays of the sacroiliac joints, and if we see classical changes of sacroiliitis, we have the diagnosis. However, MRI changed everything,” Dr. Deodhar says. Now it’s possible to see inflammatory changes in the SI joints and early joint damage, which was not possible to see on x-ray until many years later.
Reassuring for patients?
“Currently, we don’t really have different treatments,” Dr. Deodhar notes. Perhaps the only benefit is that it might be reassuring for patients to know that they have the nonradiographic form. Receiving a diagnosis of axial spondyloarthritis comes as quite a shock. It’s a diagnosis that is potentially going to affect them for the rest of their lives, and some patients worry that they’ll develop the classic “bamboo” spine of AS, he adds. So, being able to tell patients that they have nr-axSpA and that they are going to be treated early and aggressively may be somewhat comforting.
“It’s a continuum of a disease state, but a lot of people will stay at the nonradiographic stage,” points out Portland-based internist Beth Smith, DO, associate professor of medicine at OHSU.
“A good portion of individuals who may have an MRI that’s positive will either go into remission or just stay at that stage of the disease; they won’t necessarily progress to radiographic sacroiliitis,” she adds.
Spotting nr-axSpA in practice
Nr-axSpA can be tricky to spot in clinical practice, and its diagnosis in primary care largely relies on patients’ clinical presentation and identifying IBP. This is the key symptom. When someone younger than 45 years experiences back pain that is characterized by insidious and chronic onset and that improves with anti-inflammatory agents and activity but that worsens with rest and is worse at night, then imaging of the SI joints may be appropriate.
“You have to have that index of suspicion in order to even think about ordering the appropriate imaging test,” Dr. Smith says. IBP may be the big clue, but patients may also return on separate occasions with multiple associated complaints, such as plantar fasciitis, tennis elbow, or other conditions, such as psoriasis, she says.
Ordering HLA-B27 and C-reactive protein tests may be useful prior to conducting any imaging, Dr. Smith says, “and if imaging is ordered, make sure it is an x-ray of the sacroiliac joint, not the lumbar spine.”
Dr. Deodhar cautions: “A single anterior-posterior view of the pelvis is enough to look at the sacroiliac joint.” There is no need to order separate views of the right and left SI joints; doing so will provide no additional useful information and exposes the patient to unnecessary radiation.
Importantly, consider whether an x-ray of the lumbar spine is needed for a patient with chronic back pain, he says. “You should do an investigation that is going to make a difference to your management. If you take 100 patients with back pain, 95% of the time, it is going to be mechanical back pain. Why do an x-ray of the lumbar spine?” Dr. Deodhar asks rhetorically.
It should also be borne in mind that x-rays can be nonspecific, and several conditions may mimic sacroiliitis, such as osteitis condensans ilii in women who have given birth, osteoarthritis of the SI joints, and old infection of the SI joints.
MRIs need specialist interpretation
MRIs of the lumbar spine are overused to diagnose back pain, and while they might be sensitive to early inflammatory changes in SI joints, they require an expert eye for interpretation.
“MRI of the SI joints is to be used wisely in patients when there is enough clinical suspicion,” Dr. Deodhar advises. Even when an MRI is negative for sacroiliitis, patients could still have axial spondyloarthritis.
MRIs of the SI joints are needed, but not of the lumbar spine, he stresses. Views of the lumbar spine may show only signs of disk degeneration and perhaps osteoarthritis.
Moreover, Dr. Deodhar says, “MRI is so sensitive that we used to think that bone marrow edema is good enough for telling us there is sacroiliitis.” However, even people without IBP can have bone marrow edema; “exercise can show bone marrow edema,” he says.
So, “If there’s a suspicion of axial spondyloarthritis, the patient should be referred to a rheumatologist,” who will discuss the interpretation with highly specialized musculoskeletal radiologists.
Take-home messages
Whether it is nr-axSpA or r-axSpA, “the burden of disease for the patient is the same; treatment is the same,” says Dr. Deodhar. Patients should be referred to a rheumatologist as soon as possible if axial spondyloarthritis is suspected. A single x-ray of the pelvis should be performed to see the SI joints, but MRIs should be left to secondary care, he suggests.
Dr. Smith notes: “Having that index of suspicion of an inflammatory etiology for the back pain is essential.” It ensures that “patients can get early and appropriate treatment for a disease that’s very different from the mechanical back pain that we mostly see in primary care.”
Dr. Deodhar has received research grants or has acted as a consultant to multiple pharmaceutical companies, including AbbVie, Bristol-Myers Squibb, Celgene, Janssen, UCB, Novartis, Pfizer, and Eli Lilly. Dr. Smith reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Diagnosing chronic back pain: When to suspect axial spondyloarthritis
, according to several experts who are championing the need for the earlier diagnosis of the condition.
AxSpA is an inflammatory condition of the spine and joints that often goes undiagnosed for many years. Worldwide, the average time to diagnosis was found to be up to 6 years in a recent systematic review. But patient advocacy groups in both the United Kingdom and United States say that the delay can be much longer, possibly up to 10 years or more.
Being aware is key
“We know people get significant pain and functional difficulties if it’s not picked up early, and that impacts on patients financially,” said Toby Wallace, MBChB, a general practitioner based at the Derwent Practice in Malton, North Yorkshire, England, and one of 12 Champions in Primary Care for the National Axial Spondyloarthritis Society in the United Kingdom.
Being aware of the condition is vital to improving the time to patients getting diagnosed and treated, Dr. Wallace said in an interview. The quicker patients can be identified and referred onward on to a specialist rheumatology colleague means the sooner they will receive the appropriate care.
Chronic back pain
One of the key symptoms of axSpA is back pain, said Dr. Wallace. Back pain is an “extremely common” symptom seen in primary care – an estimated 60% or more of adults will have a back problem in their lifetime – but with axSpA, “it’s more about it being a persistent pain that is not going away.”
Fellow NASS Primary Care Champion and advanced practice physiotherapist Sam Bhide, MSc, calls them the “frequent flyers.”
As a first-contact practitioner, much of her practice consists of seeing people presenting with back pain, many of whom may have already been seen by other professionals but diagnosed with mechanical back pain.
“These patients return due to lack of improvement in their ongoing back pain symptoms,” Ms. Bhide noted. But how do you know if it is axSpA causing the pain?
“Normally, we would look for people who have had back pain for more than 3 months, or that gradually progresses on and off over weeks, months, or years, and their symptoms ease but do not resolve completely,” she said.
Eased by exercise and medication
“Essentially we are looking for people with inflammatory back pain,” Ms. Bhide explains.
The pain is often eased with anti-inflammatory medication and with exercise, “which is why these people get missed because they are managing their symptoms with exercises and their anti-inflammatories,” she said.
Sleep disturbance and morning stiffness
Sleep disturbance and feeling stiff in the spine for at least 30 minutes upon waking in the morning are other big indicators that chronic back pain may be due to axSpA, Dr. Wallace said.
“Waking in the early hours of the morning with pain or stiffness and having to get up and move around is fairly usual.”
Signs and symptoms
- Age < 45 years.
- Chronic back pain (3+ months).
- Morning stiffness (> 30 minutes).
- Improvement with exercise, not rest.
- Responds to anti-inflammatory medications.
- Night awakenings due to pain.
- Alternating buttock pain.
- Enthesitis and tendonitis.
- Swollen fingers or toes (dactylitis).
Aged under 45 years
AxSpA typically occurs in younger people, but it can be diagnosed at a later age, said Raj Sengupta, MBBS, a consultant rheumatologist and clinical lead for axSpA at the Royal National Hospital for Rheumatic Diseases in Bath, England.
“In someone who’s under the age of 45, if they’ve had more than 3 months of back pain, then you should be thinking about axial spondyloarthritis already,” he said.
“The proviso is that in someone who’s older, actually asking them when their back pain started is relevant, because that person may have had symptoms that started at age 20, but for whatever reason, they didn’t seek help,” said Dr. Sengupta. “They could still have undiagnosed axial spondyloarthritis.”
Women can be affected as much as men
Importantly, it appears that women can be just as affected as men, particularly in the early stages of the disease, said Dr. Sengupta.
“In the old days, people just thought of it as a ‘men-only’ disease, but what we’ve learned is that the earlier stage of the disease, the prevalence is much more 50:50,” he said.
“The sad part is that over the years women have been really underdiagnosed because of this false message that has gone about, saying women can’t get it. So, sadly, you see greater delays in diagnosis in women because of that.”
Other symptoms and associated conditions
In people with early axSpA, “pain tends to be over the sacroiliac joints, which is over the buttocks, so it’s often confused with sciatica,” explains Dr. Sengupta. Alternating buttock pain is something to take note of, as is tendonitis and enthesitis. The latter is inflammation where the tendons or ligaments are inserted into bone, so it means that people may have problems such as Achilles heel, tennis elbow, or even musculoskeletal chest pain. Dactylitis – swollen fingers or toes – is another sign seen in some people with axSpA.
Associated conditions (including family history)
- Psoriasis.
- Inflammatory bowel disease.
- Eye inflammation (uveitis or iritis).
“Family history is also really important,” although not essential, Dr. Sengupta said. And not only if there is axSpA in the family, but also if there are other conditions such as psoriasis or inflammatory bowel disease. Another commonly associated condition is eye inflammation, which can be uveitis or iritis.
What about tests and tools?
Testing for HLA-B27 – which has a known association with axSpA – and measuring blood levels of C-reactive protein may be helpful, but “even if they are normal, that shouldn’t be reassuring you that this can’t be ankylosing spondylitis [in a patient with a] strong inflammatory back pain story.”
Ordering an MRI scan may be possible within primary care, depending on where you are in the world, but the results do need to be interpreted with expert eyes, Dr. Sengupta advises.
There are online tools available to help with the diagnosis of axSpA, Dr. Sengupta said, such as the Spondyloarthritis Diagnosis Evaluation Tool (SPADE). Efforts are also underway to create online systems that help to flag symptoms in general practice.
Tests and tools
- HLA-B27 association.
- Elevated C-reactive protein.
- Sacroiliitis on MRI.
- SPADE tool.
The bottom line is that many more patients could potentially be identified earlier in primary care by careful assessment of the clinical symptoms and asking about the family history and associated conditions.
At its simplest, if you see “someone under the age of 45, if they’ve had 3 months of back pain, and they keep on coming back to say, ‘My back’s really bad,’ think about axial spondyloarthritis,” said Dr. Sengupta.
A version of this article first appeared on Medscape.com.
, according to several experts who are championing the need for the earlier diagnosis of the condition.
AxSpA is an inflammatory condition of the spine and joints that often goes undiagnosed for many years. Worldwide, the average time to diagnosis was found to be up to 6 years in a recent systematic review. But patient advocacy groups in both the United Kingdom and United States say that the delay can be much longer, possibly up to 10 years or more.
Being aware is key
“We know people get significant pain and functional difficulties if it’s not picked up early, and that impacts on patients financially,” said Toby Wallace, MBChB, a general practitioner based at the Derwent Practice in Malton, North Yorkshire, England, and one of 12 Champions in Primary Care for the National Axial Spondyloarthritis Society in the United Kingdom.
Being aware of the condition is vital to improving the time to patients getting diagnosed and treated, Dr. Wallace said in an interview. The quicker patients can be identified and referred onward on to a specialist rheumatology colleague means the sooner they will receive the appropriate care.
Chronic back pain
One of the key symptoms of axSpA is back pain, said Dr. Wallace. Back pain is an “extremely common” symptom seen in primary care – an estimated 60% or more of adults will have a back problem in their lifetime – but with axSpA, “it’s more about it being a persistent pain that is not going away.”
Fellow NASS Primary Care Champion and advanced practice physiotherapist Sam Bhide, MSc, calls them the “frequent flyers.”
As a first-contact practitioner, much of her practice consists of seeing people presenting with back pain, many of whom may have already been seen by other professionals but diagnosed with mechanical back pain.
“These patients return due to lack of improvement in their ongoing back pain symptoms,” Ms. Bhide noted. But how do you know if it is axSpA causing the pain?
“Normally, we would look for people who have had back pain for more than 3 months, or that gradually progresses on and off over weeks, months, or years, and their symptoms ease but do not resolve completely,” she said.
Eased by exercise and medication
“Essentially we are looking for people with inflammatory back pain,” Ms. Bhide explains.
The pain is often eased with anti-inflammatory medication and with exercise, “which is why these people get missed because they are managing their symptoms with exercises and their anti-inflammatories,” she said.
Sleep disturbance and morning stiffness
Sleep disturbance and feeling stiff in the spine for at least 30 minutes upon waking in the morning are other big indicators that chronic back pain may be due to axSpA, Dr. Wallace said.
“Waking in the early hours of the morning with pain or stiffness and having to get up and move around is fairly usual.”
Signs and symptoms
- Age < 45 years.
- Chronic back pain (3+ months).
- Morning stiffness (> 30 minutes).
- Improvement with exercise, not rest.
- Responds to anti-inflammatory medications.
- Night awakenings due to pain.
- Alternating buttock pain.
- Enthesitis and tendonitis.
- Swollen fingers or toes (dactylitis).
Aged under 45 years
AxSpA typically occurs in younger people, but it can be diagnosed at a later age, said Raj Sengupta, MBBS, a consultant rheumatologist and clinical lead for axSpA at the Royal National Hospital for Rheumatic Diseases in Bath, England.
“In someone who’s under the age of 45, if they’ve had more than 3 months of back pain, then you should be thinking about axial spondyloarthritis already,” he said.
“The proviso is that in someone who’s older, actually asking them when their back pain started is relevant, because that person may have had symptoms that started at age 20, but for whatever reason, they didn’t seek help,” said Dr. Sengupta. “They could still have undiagnosed axial spondyloarthritis.”
Women can be affected as much as men
Importantly, it appears that women can be just as affected as men, particularly in the early stages of the disease, said Dr. Sengupta.
“In the old days, people just thought of it as a ‘men-only’ disease, but what we’ve learned is that the earlier stage of the disease, the prevalence is much more 50:50,” he said.
“The sad part is that over the years women have been really underdiagnosed because of this false message that has gone about, saying women can’t get it. So, sadly, you see greater delays in diagnosis in women because of that.”
Other symptoms and associated conditions
In people with early axSpA, “pain tends to be over the sacroiliac joints, which is over the buttocks, so it’s often confused with sciatica,” explains Dr. Sengupta. Alternating buttock pain is something to take note of, as is tendonitis and enthesitis. The latter is inflammation where the tendons or ligaments are inserted into bone, so it means that people may have problems such as Achilles heel, tennis elbow, or even musculoskeletal chest pain. Dactylitis – swollen fingers or toes – is another sign seen in some people with axSpA.
Associated conditions (including family history)
- Psoriasis.
- Inflammatory bowel disease.
- Eye inflammation (uveitis or iritis).
“Family history is also really important,” although not essential, Dr. Sengupta said. And not only if there is axSpA in the family, but also if there are other conditions such as psoriasis or inflammatory bowel disease. Another commonly associated condition is eye inflammation, which can be uveitis or iritis.
What about tests and tools?
Testing for HLA-B27 – which has a known association with axSpA – and measuring blood levels of C-reactive protein may be helpful, but “even if they are normal, that shouldn’t be reassuring you that this can’t be ankylosing spondylitis [in a patient with a] strong inflammatory back pain story.”
Ordering an MRI scan may be possible within primary care, depending on where you are in the world, but the results do need to be interpreted with expert eyes, Dr. Sengupta advises.
There are online tools available to help with the diagnosis of axSpA, Dr. Sengupta said, such as the Spondyloarthritis Diagnosis Evaluation Tool (SPADE). Efforts are also underway to create online systems that help to flag symptoms in general practice.
Tests and tools
- HLA-B27 association.
- Elevated C-reactive protein.
- Sacroiliitis on MRI.
- SPADE tool.
The bottom line is that many more patients could potentially be identified earlier in primary care by careful assessment of the clinical symptoms and asking about the family history and associated conditions.
At its simplest, if you see “someone under the age of 45, if they’ve had 3 months of back pain, and they keep on coming back to say, ‘My back’s really bad,’ think about axial spondyloarthritis,” said Dr. Sengupta.
A version of this article first appeared on Medscape.com.
, according to several experts who are championing the need for the earlier diagnosis of the condition.
AxSpA is an inflammatory condition of the spine and joints that often goes undiagnosed for many years. Worldwide, the average time to diagnosis was found to be up to 6 years in a recent systematic review. But patient advocacy groups in both the United Kingdom and United States say that the delay can be much longer, possibly up to 10 years or more.
Being aware is key
“We know people get significant pain and functional difficulties if it’s not picked up early, and that impacts on patients financially,” said Toby Wallace, MBChB, a general practitioner based at the Derwent Practice in Malton, North Yorkshire, England, and one of 12 Champions in Primary Care for the National Axial Spondyloarthritis Society in the United Kingdom.
Being aware of the condition is vital to improving the time to patients getting diagnosed and treated, Dr. Wallace said in an interview. The quicker patients can be identified and referred onward on to a specialist rheumatology colleague means the sooner they will receive the appropriate care.
Chronic back pain
One of the key symptoms of axSpA is back pain, said Dr. Wallace. Back pain is an “extremely common” symptom seen in primary care – an estimated 60% or more of adults will have a back problem in their lifetime – but with axSpA, “it’s more about it being a persistent pain that is not going away.”
Fellow NASS Primary Care Champion and advanced practice physiotherapist Sam Bhide, MSc, calls them the “frequent flyers.”
As a first-contact practitioner, much of her practice consists of seeing people presenting with back pain, many of whom may have already been seen by other professionals but diagnosed with mechanical back pain.
“These patients return due to lack of improvement in their ongoing back pain symptoms,” Ms. Bhide noted. But how do you know if it is axSpA causing the pain?
“Normally, we would look for people who have had back pain for more than 3 months, or that gradually progresses on and off over weeks, months, or years, and their symptoms ease but do not resolve completely,” she said.
Eased by exercise and medication
“Essentially we are looking for people with inflammatory back pain,” Ms. Bhide explains.
The pain is often eased with anti-inflammatory medication and with exercise, “which is why these people get missed because they are managing their symptoms with exercises and their anti-inflammatories,” she said.
Sleep disturbance and morning stiffness
Sleep disturbance and feeling stiff in the spine for at least 30 minutes upon waking in the morning are other big indicators that chronic back pain may be due to axSpA, Dr. Wallace said.
“Waking in the early hours of the morning with pain or stiffness and having to get up and move around is fairly usual.”
Signs and symptoms
- Age < 45 years.
- Chronic back pain (3+ months).
- Morning stiffness (> 30 minutes).
- Improvement with exercise, not rest.
- Responds to anti-inflammatory medications.
- Night awakenings due to pain.
- Alternating buttock pain.
- Enthesitis and tendonitis.
- Swollen fingers or toes (dactylitis).
Aged under 45 years
AxSpA typically occurs in younger people, but it can be diagnosed at a later age, said Raj Sengupta, MBBS, a consultant rheumatologist and clinical lead for axSpA at the Royal National Hospital for Rheumatic Diseases in Bath, England.
“In someone who’s under the age of 45, if they’ve had more than 3 months of back pain, then you should be thinking about axial spondyloarthritis already,” he said.
“The proviso is that in someone who’s older, actually asking them when their back pain started is relevant, because that person may have had symptoms that started at age 20, but for whatever reason, they didn’t seek help,” said Dr. Sengupta. “They could still have undiagnosed axial spondyloarthritis.”
Women can be affected as much as men
Importantly, it appears that women can be just as affected as men, particularly in the early stages of the disease, said Dr. Sengupta.
“In the old days, people just thought of it as a ‘men-only’ disease, but what we’ve learned is that the earlier stage of the disease, the prevalence is much more 50:50,” he said.
“The sad part is that over the years women have been really underdiagnosed because of this false message that has gone about, saying women can’t get it. So, sadly, you see greater delays in diagnosis in women because of that.”
Other symptoms and associated conditions
In people with early axSpA, “pain tends to be over the sacroiliac joints, which is over the buttocks, so it’s often confused with sciatica,” explains Dr. Sengupta. Alternating buttock pain is something to take note of, as is tendonitis and enthesitis. The latter is inflammation where the tendons or ligaments are inserted into bone, so it means that people may have problems such as Achilles heel, tennis elbow, or even musculoskeletal chest pain. Dactylitis – swollen fingers or toes – is another sign seen in some people with axSpA.
Associated conditions (including family history)
- Psoriasis.
- Inflammatory bowel disease.
- Eye inflammation (uveitis or iritis).
“Family history is also really important,” although not essential, Dr. Sengupta said. And not only if there is axSpA in the family, but also if there are other conditions such as psoriasis or inflammatory bowel disease. Another commonly associated condition is eye inflammation, which can be uveitis or iritis.
What about tests and tools?
Testing for HLA-B27 – which has a known association with axSpA – and measuring blood levels of C-reactive protein may be helpful, but “even if they are normal, that shouldn’t be reassuring you that this can’t be ankylosing spondylitis [in a patient with a] strong inflammatory back pain story.”
Ordering an MRI scan may be possible within primary care, depending on where you are in the world, but the results do need to be interpreted with expert eyes, Dr. Sengupta advises.
There are online tools available to help with the diagnosis of axSpA, Dr. Sengupta said, such as the Spondyloarthritis Diagnosis Evaluation Tool (SPADE). Efforts are also underway to create online systems that help to flag symptoms in general practice.
Tests and tools
- HLA-B27 association.
- Elevated C-reactive protein.
- Sacroiliitis on MRI.
- SPADE tool.
The bottom line is that many more patients could potentially be identified earlier in primary care by careful assessment of the clinical symptoms and asking about the family history and associated conditions.
At its simplest, if you see “someone under the age of 45, if they’ve had 3 months of back pain, and they keep on coming back to say, ‘My back’s really bad,’ think about axial spondyloarthritis,” said Dr. Sengupta.
A version of this article first appeared on Medscape.com.
Enthesitis, arthritis, tenosynovitis linked to dupilumab use for atopic dermatitis
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
Around 5% of patients treated with dupilumab (Dupixent) for moderate-to-severe atopic dermatitis experience musculoskeletal (MSK) symptoms, according to the results of a descriptive study.
The main MSK symptom seen in the observational cohort was enthesitis, but some patients also experienced arthritis and tenosynovitis a median of 17 weeks after starting dupilumab treatment. Together these symptoms represent a new MSK syndrome, say researchers from the United Kingdom.
“The pattern of MSK symptoms and signs is characteristic of psoriatic arthritis/peripheral spondyloarthritis,” Bruce Kirkham, MD, and collaborators report in Arthritis & Rheumatology.
“We started a few years ago and have been following the patients for quite a long time,” Dr. Kirkham, a consultant rheumatologist at Guy’s and St. Thomas’ NHS Foundation Trust, London, told this news organization.
“We’re still seeing patients with the same type of syndrome presenting occasionally. It’s not a very common adverse event, but we think it continues,” he observed.
“Most of them don’t have very severe problems, and a lot of them can be treated with quite simple drugs or, alternatively, reducing the frequency of the injection,” Dr. Kirkham added.
Characterizing the MSK symptoms
Of 470 patients with atopic dermatitis who started treatment with dupilumab at Guy’s and St. Thomas’ NHS Foundation Trust between October 2018 and February 2021, 36 (7.65%) developed rheumatic symptoms and were referred to the rheumatology department. These individuals had their family history assessed and thorough MSK evaluations, which included antibody and inflammatory markers, ultrasound of the peripheral small joints, and MRI of the large joints and spine.
A total of 26 (5.5%) patients – 14 of whom were male – had inflammatory enthesitis, arthritis, and/or tenosynovitis. Of the others, seven had osteoarthritis and three had degenerative spine disease.
Enthesitis was the most common finding in those with rheumatic symptoms, occurring on its own in 11 patients, with arthritis in three patients, and tenosynovitis in two patients.
These symptoms appeared 2-48 weeks after starting dupilumab treatment and were categorized as mild in 16 (61%) cases, moderate in six cases, and severe in four cases.
No specific predictors of the MSK symptoms seen were noted. Patient age, sex, duration of their atopic dermatitis, or how their skin condition had been previously treated did not help identify those who might develop rheumatic problems.
Conservative management approach
All patients had “outstanding” responses to treatment, Dr. Kirkham noted: The mean Eczema Area and Severity Index score before dupilumab treatment was 21, falling to 4.2 with treatment, indicating a mean 80% improvement.
Co-author Joseph Nathan, MBChB, of London North West Healthcare NHS Trust, who collaborated on the research while working within Dr. Kirkham’s group, said separately: “The concern that patients have is that when they start a medication and develop a side effect is that the medication is going to be stopped.”
Clinicians treating the patients took a conservative approach, prescribing NSAIDs such as cyclooxygenase-2 inhibitors or altering the frequency with which dupilumab was given.
With this approach, MSK symptoms resolved in 15 patients who remained on treatment and in seven who had to stop dupilumab. There were four patients, however, who had unresolved symptoms even once dupilumab treatment had been stopped.
Altering the local cytokine balance
Dupilumab is a monoclonal antibody that binds to the alpha subunit of the interleukin-4 receptor. This results in blocking the function of not only IL-4 but also IL-13.
Dr. Kirkham and colleagues think this might not only alter the balance of cytokines in the skin but also in the joints and entheses with IL-17, IL-23, or even tumor necrosis factor playing a possible role. Another thought is that many circulating T-cells in the skin move to the joints and entheses to trigger symptoms.
IL-13 inhibition does seem to be important, as another British research team, from the Centre for Epidemiology Versus Arthritis at the University of Manchester (England), has found.
At the recent annual meeting of the British Society for Rheumatology, Sizheng Steven Zhao, MBChB, PhD, and colleagues reported that among people who carried a genetic variant predisposing them to having low IL-13 function, there was a higher risk for inflammatory diseases such as psoriatic arthritis and other spondyloarthropathy-related diseases.
Indeed, when the single nucleotide polymorphism rs20541 was present, the odds for having psoriatic arthritis and psoriasis were higher than when it was not.
The findings are consistent with the idea that IL-4 and IL-13 may be acting as a restraint towards MSK diseases in some patients, Dr. Zhao and co-authors suggest.
“The genetic data supports what [Dr. Kirkham and team] have said from a mechanistic point of view,” Dr. Zhao said in an interview. “What you’re observing has a genetic basis.”
Dermatology perspective
Approved by the U.S. Food and Drug Administration in 2017, dupilumab has since been hailed as a “breakthrough” in atopic dermatitis treatment. Given as a subcutaneous injection every 2 weeks, it provides a much-needed option for people who have moderate-to-severe disease and have tried other available treatments, including corticosteroids.
Dupilumab has since also been approved for asthma, chronic sinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis and is used off-label for other skin conditions such as contact dermatitis, chronic spontaneous urticaria, and alopecia areata.
“Dupilumab, like a lot of medications for atopic dermatitis, is a relatively new drug, and we are still learning about its safety,” Joel M. Gelfand, MD, MSCE, of the University of Pennsylvania Perelman School of Medicine, Philadelphia, told this news organization.
“Inflammatory arthritis has been reported in patients treated with dupilumab, and this new study provides some useful estimates,” added Dr. Gelfand, who is a professor of dermatology and epidemiology and directs the Psoriasis and Phototherapy Treatment Center, Philadelphia.
“There was no control group,” Dr. Gelfand said, so “a causal relationship cannot be well established based on these data alone. The mechanism is not known but may result from a shifting of the immune system.”
Dr. Zhao observed: “We don’t know what the natural history of these adverse events is. We don’t know if stopping the drug early will prevent long-term adverse events. So, we don’t know if people will ultimately develop permanent psoriatic arthritis if we don’t intervene quick enough when we observe an adverse event.”
Being aware of the possibility of rheumatic side effects occurring with dupilumab and similar agents is key, Dr. Gelfand and Dr. Kirkham both said independently.
“I have personally seen this entity in my practice,” Dr. Gelfand said. “It is important to clinicians prescribing dupilumab to alert patients about this potential side effect and ask about joint symptoms in follow-up.”
Dr. Kirkham said: “Prescribers need to be aware of it, because up until now it’s been just very vaguely discussed as sort of aches and pains, arthralgias, and it’s a much more specific of a kind of syndrome of enthesitis, arthritis, tenosynovitis – a little like psoriatic arthritis.”
Not everyone has come across these side effects, however, as Steven Daveluy, MD, associate professor and dermatology program director at Wayne State University, Detroit, said in an interview.
“This article and the other case series both noted the musculoskeletal symptoms occurred in about 5% of patients, which surprised me since I haven’t seen it in my practice and have enough patients being treated with dupilumab that I would expect to see a case at that rate,” Dr. Daveluy said.
“The majority of cases are mild and respond to treatment with anti-inflammatories like naproxen, which is available over the counter. It’s likely that patients with a mild case could simply treat their pain with naproxen that’s already in their medicine cabinet until it resolves, never bringing it to the doctor’s attention,” he suggested.
“Dupilumab is still a safe and effective medication that can change the lives of patients suffering from atopic dermatitis,” he said.
“Awareness of this potential side effect can help dermatologists recognize it early and work together with patients to determine the best course of action.”
All research mentioned in this article was independently supported. Dr. Kirkham, Mr. Nathan, Dr. Zhao, and Dr. Daveluy report no relevant financial relationships. Dr. Gelfand has served as a consultant for numerous pharmaceutical companies and receives research grants from Amgen, Boehringer Ingelheim, and Pfizer. He is a co-patent holder of resiquimod for treatment of cutaneous T-cell lymphoma.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Low disease state for childhood lupus approaches validation
MANCHESTER, ENGLAND – An age-appropriate version of the Lupus Low Disease Activity State (LLDAS) has been developed by an international task force that will hopefully enable childhood-onset systemic lupus erythematosus (cSLE) to be treated to target in the near future.
The new childhood LLDAS (cLLDAS) has been purposefully developed to align with that already used for adults, Eve Smith, MBChB, PhD, explained at the annual meeting of the British Society for Rheumatology.
“There’s a lot of compelling data that’s accumulating from adult lupus and increasingly from childhood lupus that [treat to target] might be a good idea,” said Dr. Smith, who is a senior clinical fellow and honorary consultant at the University of Liverpool (England) and Alder Hey Children’s NHS Foundation Trust Hospital, also in Liverpool.
Urgent need to improve childhood lupus outcomes
“We urgently need to do something to try and improve outcomes for children,” Dr. Smith said.
“We know that childhood lupus patients have got higher disease activity as compared to adults; they have a greater medication burden, particularly steroids; and they tend to have more severe organ manifestations,” she added.
Moreover, data show that one-fifth of pediatric patients with lupus have already accrued early damage, and there is much higher mortality associated with childhood lupus than there is with adult lupus.
“So, really we want to use treat to target as a way to try and improve on these aspects,” Dr. Smith said.
The treat-to-target (T2T) approach is not a new idea in lupus, with a lot of work already done in adult patients. One large study of more than 3,300 patients conducted in 13 countries has shown that patients who never achieve LLDAS are more likely to have high levels of damage, greater glucocorticoid use, worse quality of life, and higher mortality than are those who do.
Conversely, data have also shown that achieving a LLDAS is associated with a reduction in the risk for new damage, flares, and hospitalization, as well as reducing health care costs and improving patients’ overall health-related quality of life.
T2T is a recognized approach in European adult SLE guidelines, Dr. Smith said, although the approach has not really been fully realized as of yet, even in adult practice.
The cSLE T2T international task force and cLLDAS definition
With evidence accumulating on the benefits of getting children with SLE to a low disease activity state, Dr. Smith and colleague Michael Beresford, MBChB, PhD, Brough Chair, Professor of Child Health at the University of Liverpool, put out a call to develop a task force to look into the feasibility of a T2T approach.
“We had a really enthusiastic response internationally, which we were really encouraged by,” Dr. Smith said, “and we now lead a task force of 20 experts from across all five continents, and we have really strong patient involvement.”
Through a consensus process, an international cSLE T2T Task Force agreed on overarching principles and points to consider that will “lay the foundation for future T2T approaches in cSLE,” according to the recommendations statement, which was endorsed by the Paediatric Rheumatology European Society.
Next, they looked to develop an age-appropriate definition for low disease activity.
“We’re deliberately wanting to maintain sufficient unity with the adult definition, so that we could facilitate life-course studies,” said Dr. Smith, who presented the results of a literature review and series of Delphi surveys at the meeting.
The conceptual definition of cLLDAS is similar to adults in describing it as a sustained state that is associated with a low likelihood of adverse outcome, Dr. Smith said, but with the added wording of “considering disease activity, damage, and medication toxicity.”
The definition is achieved when the SLE Disease Activity Index-2K is ≤ 4 and there is no activity in major organ systems; there are no new features of lupus disease activity since the last assessment; there is a score of ≤ 1 on Physician Global Assessment; steroid doses are ≤ 0.15 mg/kg/day or a maximum of 7.5 mg/day (whichever is lower); and immunosuppressive treatment is stable, with any changes to medication only because of side effects, adherence, changes in weight, or when in the process of reaching a target dose.
“It’s all very well having a definition, but you need to think about how that will work in practice,” Dr. Smith said. This is something that the task force is thinking about very carefully.
The task force next aims to validate the cLLDAS definition, form an extensive research agenda to inform the T2T methods, and develop innovative methods to apply the approach in practice.
The work is supported by the Wellcome Trust, National Institutes for Health Research, Versus Arthritis, and the University of Liverpool, Alder Hey Children’s NHS Foundation Trust and the Alder Hey Charity. Dr. Smith reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – An age-appropriate version of the Lupus Low Disease Activity State (LLDAS) has been developed by an international task force that will hopefully enable childhood-onset systemic lupus erythematosus (cSLE) to be treated to target in the near future.
The new childhood LLDAS (cLLDAS) has been purposefully developed to align with that already used for adults, Eve Smith, MBChB, PhD, explained at the annual meeting of the British Society for Rheumatology.
“There’s a lot of compelling data that’s accumulating from adult lupus and increasingly from childhood lupus that [treat to target] might be a good idea,” said Dr. Smith, who is a senior clinical fellow and honorary consultant at the University of Liverpool (England) and Alder Hey Children’s NHS Foundation Trust Hospital, also in Liverpool.
Urgent need to improve childhood lupus outcomes
“We urgently need to do something to try and improve outcomes for children,” Dr. Smith said.
“We know that childhood lupus patients have got higher disease activity as compared to adults; they have a greater medication burden, particularly steroids; and they tend to have more severe organ manifestations,” she added.
Moreover, data show that one-fifth of pediatric patients with lupus have already accrued early damage, and there is much higher mortality associated with childhood lupus than there is with adult lupus.
“So, really we want to use treat to target as a way to try and improve on these aspects,” Dr. Smith said.
The treat-to-target (T2T) approach is not a new idea in lupus, with a lot of work already done in adult patients. One large study of more than 3,300 patients conducted in 13 countries has shown that patients who never achieve LLDAS are more likely to have high levels of damage, greater glucocorticoid use, worse quality of life, and higher mortality than are those who do.
Conversely, data have also shown that achieving a LLDAS is associated with a reduction in the risk for new damage, flares, and hospitalization, as well as reducing health care costs and improving patients’ overall health-related quality of life.
T2T is a recognized approach in European adult SLE guidelines, Dr. Smith said, although the approach has not really been fully realized as of yet, even in adult practice.
The cSLE T2T international task force and cLLDAS definition
With evidence accumulating on the benefits of getting children with SLE to a low disease activity state, Dr. Smith and colleague Michael Beresford, MBChB, PhD, Brough Chair, Professor of Child Health at the University of Liverpool, put out a call to develop a task force to look into the feasibility of a T2T approach.
“We had a really enthusiastic response internationally, which we were really encouraged by,” Dr. Smith said, “and we now lead a task force of 20 experts from across all five continents, and we have really strong patient involvement.”
Through a consensus process, an international cSLE T2T Task Force agreed on overarching principles and points to consider that will “lay the foundation for future T2T approaches in cSLE,” according to the recommendations statement, which was endorsed by the Paediatric Rheumatology European Society.
Next, they looked to develop an age-appropriate definition for low disease activity.
“We’re deliberately wanting to maintain sufficient unity with the adult definition, so that we could facilitate life-course studies,” said Dr. Smith, who presented the results of a literature review and series of Delphi surveys at the meeting.
The conceptual definition of cLLDAS is similar to adults in describing it as a sustained state that is associated with a low likelihood of adverse outcome, Dr. Smith said, but with the added wording of “considering disease activity, damage, and medication toxicity.”
The definition is achieved when the SLE Disease Activity Index-2K is ≤ 4 and there is no activity in major organ systems; there are no new features of lupus disease activity since the last assessment; there is a score of ≤ 1 on Physician Global Assessment; steroid doses are ≤ 0.15 mg/kg/day or a maximum of 7.5 mg/day (whichever is lower); and immunosuppressive treatment is stable, with any changes to medication only because of side effects, adherence, changes in weight, or when in the process of reaching a target dose.
“It’s all very well having a definition, but you need to think about how that will work in practice,” Dr. Smith said. This is something that the task force is thinking about very carefully.
The task force next aims to validate the cLLDAS definition, form an extensive research agenda to inform the T2T methods, and develop innovative methods to apply the approach in practice.
The work is supported by the Wellcome Trust, National Institutes for Health Research, Versus Arthritis, and the University of Liverpool, Alder Hey Children’s NHS Foundation Trust and the Alder Hey Charity. Dr. Smith reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MANCHESTER, ENGLAND – An age-appropriate version of the Lupus Low Disease Activity State (LLDAS) has been developed by an international task force that will hopefully enable childhood-onset systemic lupus erythematosus (cSLE) to be treated to target in the near future.
The new childhood LLDAS (cLLDAS) has been purposefully developed to align with that already used for adults, Eve Smith, MBChB, PhD, explained at the annual meeting of the British Society for Rheumatology.
“There’s a lot of compelling data that’s accumulating from adult lupus and increasingly from childhood lupus that [treat to target] might be a good idea,” said Dr. Smith, who is a senior clinical fellow and honorary consultant at the University of Liverpool (England) and Alder Hey Children’s NHS Foundation Trust Hospital, also in Liverpool.
Urgent need to improve childhood lupus outcomes
“We urgently need to do something to try and improve outcomes for children,” Dr. Smith said.
“We know that childhood lupus patients have got higher disease activity as compared to adults; they have a greater medication burden, particularly steroids; and they tend to have more severe organ manifestations,” she added.
Moreover, data show that one-fifth of pediatric patients with lupus have already accrued early damage, and there is much higher mortality associated with childhood lupus than there is with adult lupus.
“So, really we want to use treat to target as a way to try and improve on these aspects,” Dr. Smith said.
The treat-to-target (T2T) approach is not a new idea in lupus, with a lot of work already done in adult patients. One large study of more than 3,300 patients conducted in 13 countries has shown that patients who never achieve LLDAS are more likely to have high levels of damage, greater glucocorticoid use, worse quality of life, and higher mortality than are those who do.
Conversely, data have also shown that achieving a LLDAS is associated with a reduction in the risk for new damage, flares, and hospitalization, as well as reducing health care costs and improving patients’ overall health-related quality of life.
T2T is a recognized approach in European adult SLE guidelines, Dr. Smith said, although the approach has not really been fully realized as of yet, even in adult practice.
The cSLE T2T international task force and cLLDAS definition
With evidence accumulating on the benefits of getting children with SLE to a low disease activity state, Dr. Smith and colleague Michael Beresford, MBChB, PhD, Brough Chair, Professor of Child Health at the University of Liverpool, put out a call to develop a task force to look into the feasibility of a T2T approach.
“We had a really enthusiastic response internationally, which we were really encouraged by,” Dr. Smith said, “and we now lead a task force of 20 experts from across all five continents, and we have really strong patient involvement.”
Through a consensus process, an international cSLE T2T Task Force agreed on overarching principles and points to consider that will “lay the foundation for future T2T approaches in cSLE,” according to the recommendations statement, which was endorsed by the Paediatric Rheumatology European Society.
Next, they looked to develop an age-appropriate definition for low disease activity.
“We’re deliberately wanting to maintain sufficient unity with the adult definition, so that we could facilitate life-course studies,” said Dr. Smith, who presented the results of a literature review and series of Delphi surveys at the meeting.
The conceptual definition of cLLDAS is similar to adults in describing it as a sustained state that is associated with a low likelihood of adverse outcome, Dr. Smith said, but with the added wording of “considering disease activity, damage, and medication toxicity.”
The definition is achieved when the SLE Disease Activity Index-2K is ≤ 4 and there is no activity in major organ systems; there are no new features of lupus disease activity since the last assessment; there is a score of ≤ 1 on Physician Global Assessment; steroid doses are ≤ 0.15 mg/kg/day or a maximum of 7.5 mg/day (whichever is lower); and immunosuppressive treatment is stable, with any changes to medication only because of side effects, adherence, changes in weight, or when in the process of reaching a target dose.
“It’s all very well having a definition, but you need to think about how that will work in practice,” Dr. Smith said. This is something that the task force is thinking about very carefully.
The task force next aims to validate the cLLDAS definition, form an extensive research agenda to inform the T2T methods, and develop innovative methods to apply the approach in practice.
The work is supported by the Wellcome Trust, National Institutes for Health Research, Versus Arthritis, and the University of Liverpool, Alder Hey Children’s NHS Foundation Trust and the Alder Hey Charity. Dr. Smith reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT BSR 2023
Normal CRP during RA flares: An ‘underappreciated, persistent phenotype’
MANCHESTER, England – Even when C-reactive protein (CRP) levels are normal, patients with seropositive rheumatoid arthritis (RA) could still be experiencing significant disease that persists over time, researchers from University College London have found.
Similar levels of joint erosion and disease activity were observed over a 5-year period; researchers compared patients who had high CRP levels (> 5 mg/L)* with patients whose CRP levels were consistently normal (< 5 mg/L) at the time of an ultrasound-proven disease flare.
“Our data suggests that the phenotype of normal CRP represents at least 5% of our cohort,” Bhavika Sethi, MBChB, reported in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
“They are more likely to require biologic treatment, and this continues on even though they have equivalent DAS28 [disease activity score in 28 joints] and risk of joint damage” to high-CRP patients, she said.
These patients are a significant minority, Dr. Sethi added, and “we need to think about how we provide care for them and allocate resources.”
Diagnostic delay and poor outcomes previously seen
The study is a continuation of a larger project, the corresponding author for the poster, Matthew Hutchinson, MBChB, told this news organization.
A few years ago, Dr. Hutchinson explained, a subset of patients with normal CRP levels during RA flares were identified and were found to be more likely to have experienced diagnostic delay and worse outcomes than did those with high CRP levels.
The aim of the current study was to see whether those findings persisted by longitudinally assessing patient records and seeing what happened 1, 2, and 5 years later. They evaluated 312 patients with seropositive RA, of whom 28 had CRP < 5 mg/L as well as active disease, which was determined on the basis of a DAS28 > 4.5. Of those 28 patients, 16 had persistently low CRP (< 5 mg/L) despite active disease. All patients who were taking tocilizumab were excluded from the study because of its CRP-lowering properties.
“Our project was showing that this group of people exist, trying to characterize them a little better” and that the study serves as a “jumping-off point” for future research, Dr. Hutchinson said.
The study was also conducted to “make people more aware of [patients with normal CRP during flare], because treating clinicians could be falsely reassured by a normal CRP,” he added. “Patients in front of them could actually be undertreated and have worse outcomes if [it is] not picked up,” Dr. Hutchinson suggested.
In comparison with those with high CRP levels, those with normal CRP levels were more likely to be receiving biologic treatment at 5 years (76.6% vs. 44.4%; P = .0323).
At 5 years, DAS28 was similar (P = .9615) among patients with normal CRP levels and those with high CRP levels, at a median of 2.8 and 3.2, respectively. A similar percentage of patients in these two groups also had joint damage (63.3% vs. 71.4%; P = .7384).
Don’t rely only on CRP to diagnose and manage RA flares
“CRP is a generic inflammatory marker in most people,” Dr. Hutchinson said. “In the majority of situations when either there is inflammation or an infection, certainly if it’s systemic infection or inflammation, you will find CRP being elevated on the blood tests.”
For someone presenting with joint pain, high CRP can be a useful indicator that it’s more of an inflammatory process than physical injury, he added. CRP is also frequently used to calculate DAS28 to monitor disease activity.
“This study highlights that CRP may be normal during flares in some people with RA,” Jeffrey A. Sparks, MD, told this news organization.
“These patients may still require advanced therapies and can accrue damage,” the rheumatologist from Brigham and Women’s Hospital and Harvard University, Boston, added.
“Clinicians should not only rely on CRP to diagnose and manage RA flares,” said Dr. Sparks, who was not involved in the study.
The study was independently supported. Dr. Hutchinson and Dr. Sethi report no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care; he has received research support from Bristol-Myers Squibb and has performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
*Correction, 5/9/2023: This article has been updated to correct the units for C-reactive protein from mg/dL to mg/L.
A version of this article first appeared on Medscape.com.
MANCHESTER, England – Even when C-reactive protein (CRP) levels are normal, patients with seropositive rheumatoid arthritis (RA) could still be experiencing significant disease that persists over time, researchers from University College London have found.
Similar levels of joint erosion and disease activity were observed over a 5-year period; researchers compared patients who had high CRP levels (> 5 mg/L)* with patients whose CRP levels were consistently normal (< 5 mg/L) at the time of an ultrasound-proven disease flare.
“Our data suggests that the phenotype of normal CRP represents at least 5% of our cohort,” Bhavika Sethi, MBChB, reported in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
“They are more likely to require biologic treatment, and this continues on even though they have equivalent DAS28 [disease activity score in 28 joints] and risk of joint damage” to high-CRP patients, she said.
These patients are a significant minority, Dr. Sethi added, and “we need to think about how we provide care for them and allocate resources.”
Diagnostic delay and poor outcomes previously seen
The study is a continuation of a larger project, the corresponding author for the poster, Matthew Hutchinson, MBChB, told this news organization.
A few years ago, Dr. Hutchinson explained, a subset of patients with normal CRP levels during RA flares were identified and were found to be more likely to have experienced diagnostic delay and worse outcomes than did those with high CRP levels.
The aim of the current study was to see whether those findings persisted by longitudinally assessing patient records and seeing what happened 1, 2, and 5 years later. They evaluated 312 patients with seropositive RA, of whom 28 had CRP < 5 mg/L as well as active disease, which was determined on the basis of a DAS28 > 4.5. Of those 28 patients, 16 had persistently low CRP (< 5 mg/L) despite active disease. All patients who were taking tocilizumab were excluded from the study because of its CRP-lowering properties.
“Our project was showing that this group of people exist, trying to characterize them a little better” and that the study serves as a “jumping-off point” for future research, Dr. Hutchinson said.
The study was also conducted to “make people more aware of [patients with normal CRP during flare], because treating clinicians could be falsely reassured by a normal CRP,” he added. “Patients in front of them could actually be undertreated and have worse outcomes if [it is] not picked up,” Dr. Hutchinson suggested.
In comparison with those with high CRP levels, those with normal CRP levels were more likely to be receiving biologic treatment at 5 years (76.6% vs. 44.4%; P = .0323).
At 5 years, DAS28 was similar (P = .9615) among patients with normal CRP levels and those with high CRP levels, at a median of 2.8 and 3.2, respectively. A similar percentage of patients in these two groups also had joint damage (63.3% vs. 71.4%; P = .7384).
Don’t rely only on CRP to diagnose and manage RA flares
“CRP is a generic inflammatory marker in most people,” Dr. Hutchinson said. “In the majority of situations when either there is inflammation or an infection, certainly if it’s systemic infection or inflammation, you will find CRP being elevated on the blood tests.”
For someone presenting with joint pain, high CRP can be a useful indicator that it’s more of an inflammatory process than physical injury, he added. CRP is also frequently used to calculate DAS28 to monitor disease activity.
“This study highlights that CRP may be normal during flares in some people with RA,” Jeffrey A. Sparks, MD, told this news organization.
“These patients may still require advanced therapies and can accrue damage,” the rheumatologist from Brigham and Women’s Hospital and Harvard University, Boston, added.
“Clinicians should not only rely on CRP to diagnose and manage RA flares,” said Dr. Sparks, who was not involved in the study.
The study was independently supported. Dr. Hutchinson and Dr. Sethi report no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care; he has received research support from Bristol-Myers Squibb and has performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
*Correction, 5/9/2023: This article has been updated to correct the units for C-reactive protein from mg/dL to mg/L.
A version of this article first appeared on Medscape.com.
MANCHESTER, England – Even when C-reactive protein (CRP) levels are normal, patients with seropositive rheumatoid arthritis (RA) could still be experiencing significant disease that persists over time, researchers from University College London have found.
Similar levels of joint erosion and disease activity were observed over a 5-year period; researchers compared patients who had high CRP levels (> 5 mg/L)* with patients whose CRP levels were consistently normal (< 5 mg/L) at the time of an ultrasound-proven disease flare.
“Our data suggests that the phenotype of normal CRP represents at least 5% of our cohort,” Bhavika Sethi, MBChB, reported in a virtual poster presentation at the annual meeting of the British Society for Rheumatology.
“They are more likely to require biologic treatment, and this continues on even though they have equivalent DAS28 [disease activity score in 28 joints] and risk of joint damage” to high-CRP patients, she said.
These patients are a significant minority, Dr. Sethi added, and “we need to think about how we provide care for them and allocate resources.”
Diagnostic delay and poor outcomes previously seen
The study is a continuation of a larger project, the corresponding author for the poster, Matthew Hutchinson, MBChB, told this news organization.
A few years ago, Dr. Hutchinson explained, a subset of patients with normal CRP levels during RA flares were identified and were found to be more likely to have experienced diagnostic delay and worse outcomes than did those with high CRP levels.
The aim of the current study was to see whether those findings persisted by longitudinally assessing patient records and seeing what happened 1, 2, and 5 years later. They evaluated 312 patients with seropositive RA, of whom 28 had CRP < 5 mg/L as well as active disease, which was determined on the basis of a DAS28 > 4.5. Of those 28 patients, 16 had persistently low CRP (< 5 mg/L) despite active disease. All patients who were taking tocilizumab were excluded from the study because of its CRP-lowering properties.
“Our project was showing that this group of people exist, trying to characterize them a little better” and that the study serves as a “jumping-off point” for future research, Dr. Hutchinson said.
The study was also conducted to “make people more aware of [patients with normal CRP during flare], because treating clinicians could be falsely reassured by a normal CRP,” he added. “Patients in front of them could actually be undertreated and have worse outcomes if [it is] not picked up,” Dr. Hutchinson suggested.
In comparison with those with high CRP levels, those with normal CRP levels were more likely to be receiving biologic treatment at 5 years (76.6% vs. 44.4%; P = .0323).
At 5 years, DAS28 was similar (P = .9615) among patients with normal CRP levels and those with high CRP levels, at a median of 2.8 and 3.2, respectively. A similar percentage of patients in these two groups also had joint damage (63.3% vs. 71.4%; P = .7384).
Don’t rely only on CRP to diagnose and manage RA flares
“CRP is a generic inflammatory marker in most people,” Dr. Hutchinson said. “In the majority of situations when either there is inflammation or an infection, certainly if it’s systemic infection or inflammation, you will find CRP being elevated on the blood tests.”
For someone presenting with joint pain, high CRP can be a useful indicator that it’s more of an inflammatory process than physical injury, he added. CRP is also frequently used to calculate DAS28 to monitor disease activity.
“This study highlights that CRP may be normal during flares in some people with RA,” Jeffrey A. Sparks, MD, told this news organization.
“These patients may still require advanced therapies and can accrue damage,” the rheumatologist from Brigham and Women’s Hospital and Harvard University, Boston, added.
“Clinicians should not only rely on CRP to diagnose and manage RA flares,” said Dr. Sparks, who was not involved in the study.
The study was independently supported. Dr. Hutchinson and Dr. Sethi report no relevant financial relationships. Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care; he has received research support from Bristol-Myers Squibb and has performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer.
*Correction, 5/9/2023: This article has been updated to correct the units for C-reactive protein from mg/dL to mg/L.
A version of this article first appeared on Medscape.com.
AT BSR 2023