User login
Efficacy, Safety, and Tolerability of Halobetasol Propionate 0.01%–Tazarotene 0.045% Lotion for Moderate to Severe Plaque Psoriasis in the Hispanic Population: Post Hoc Analysis
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).
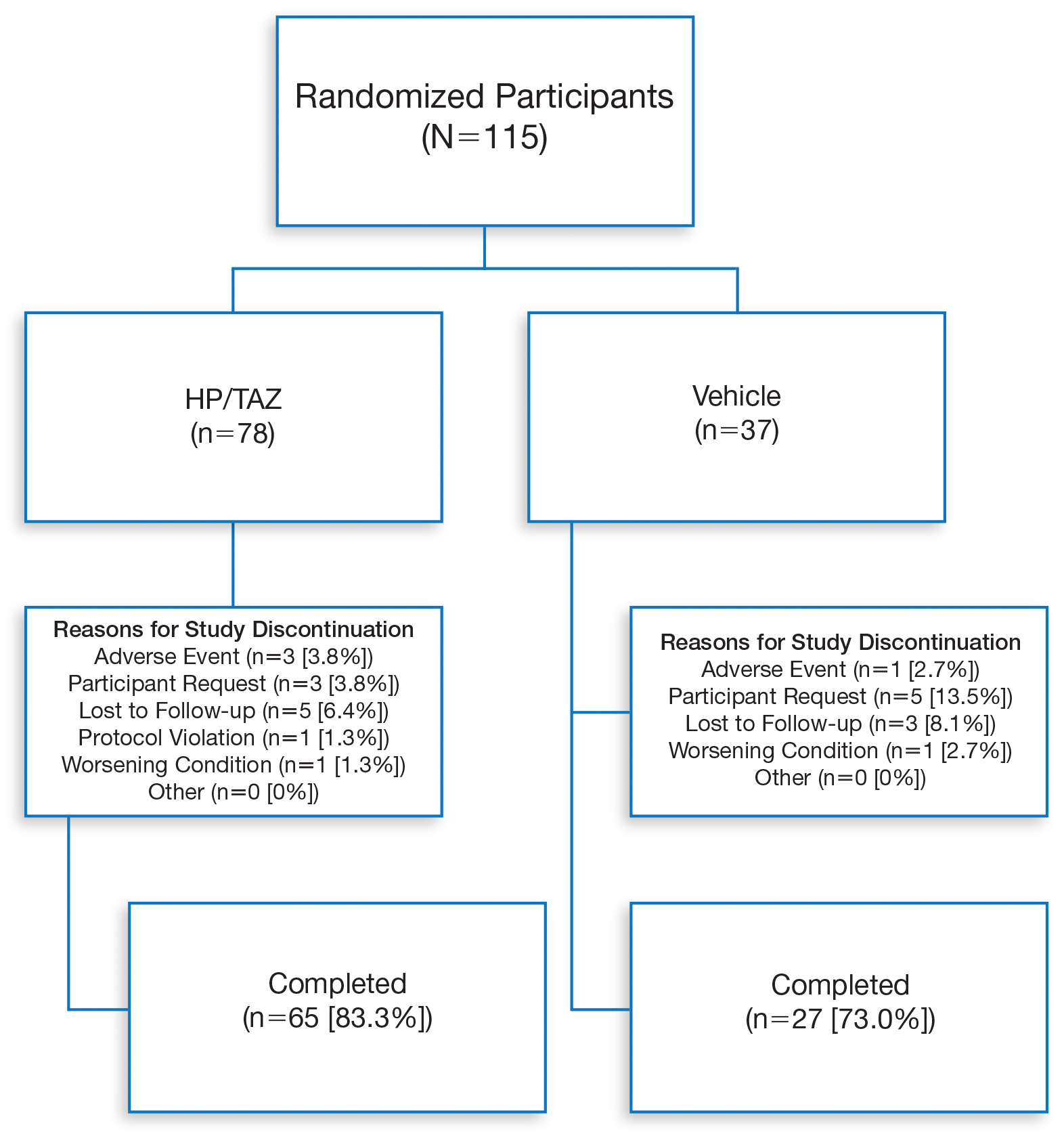
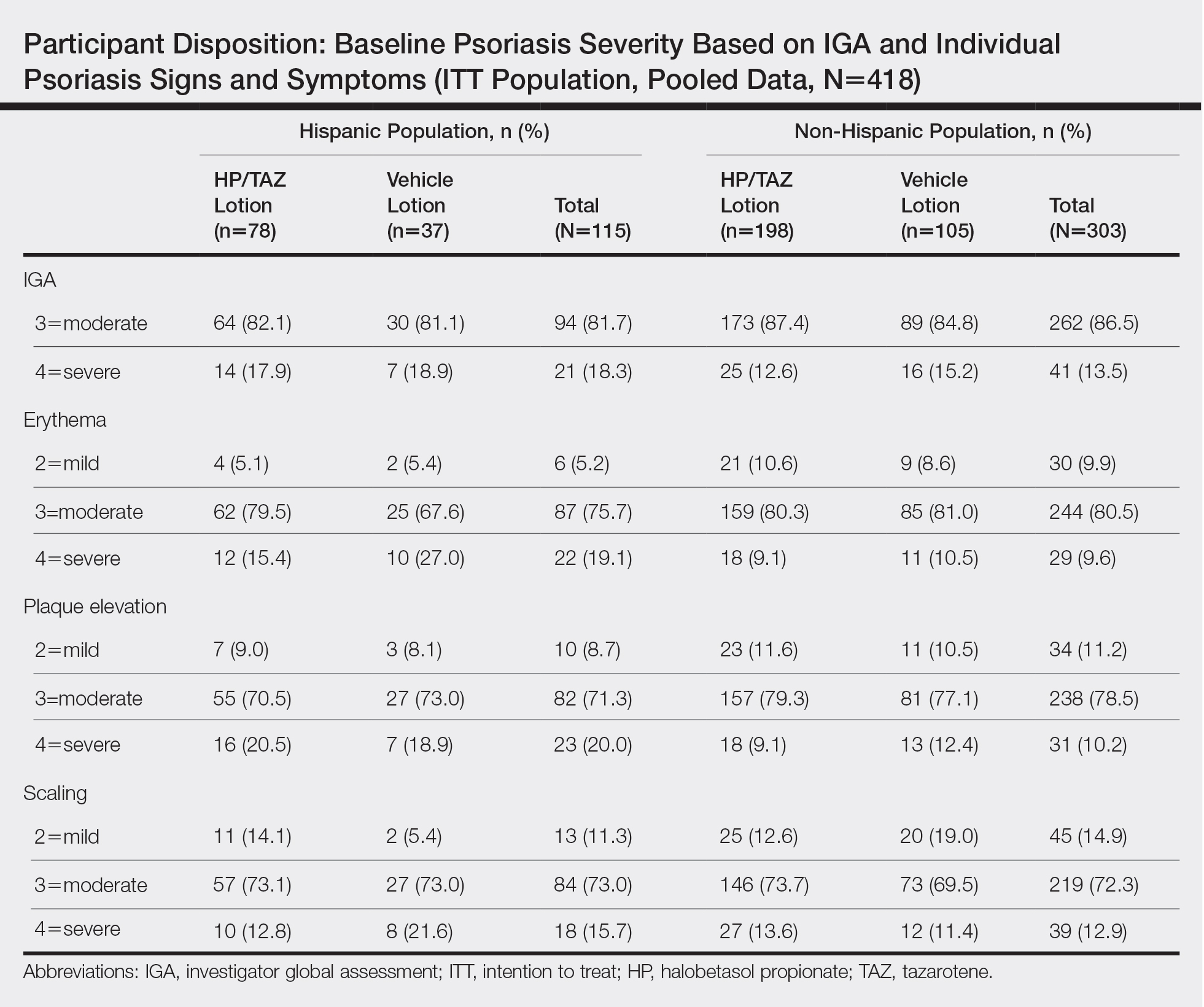
Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).
Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
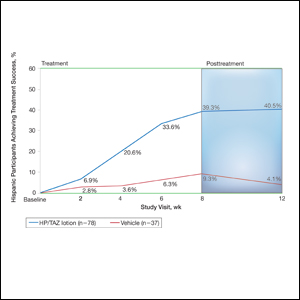
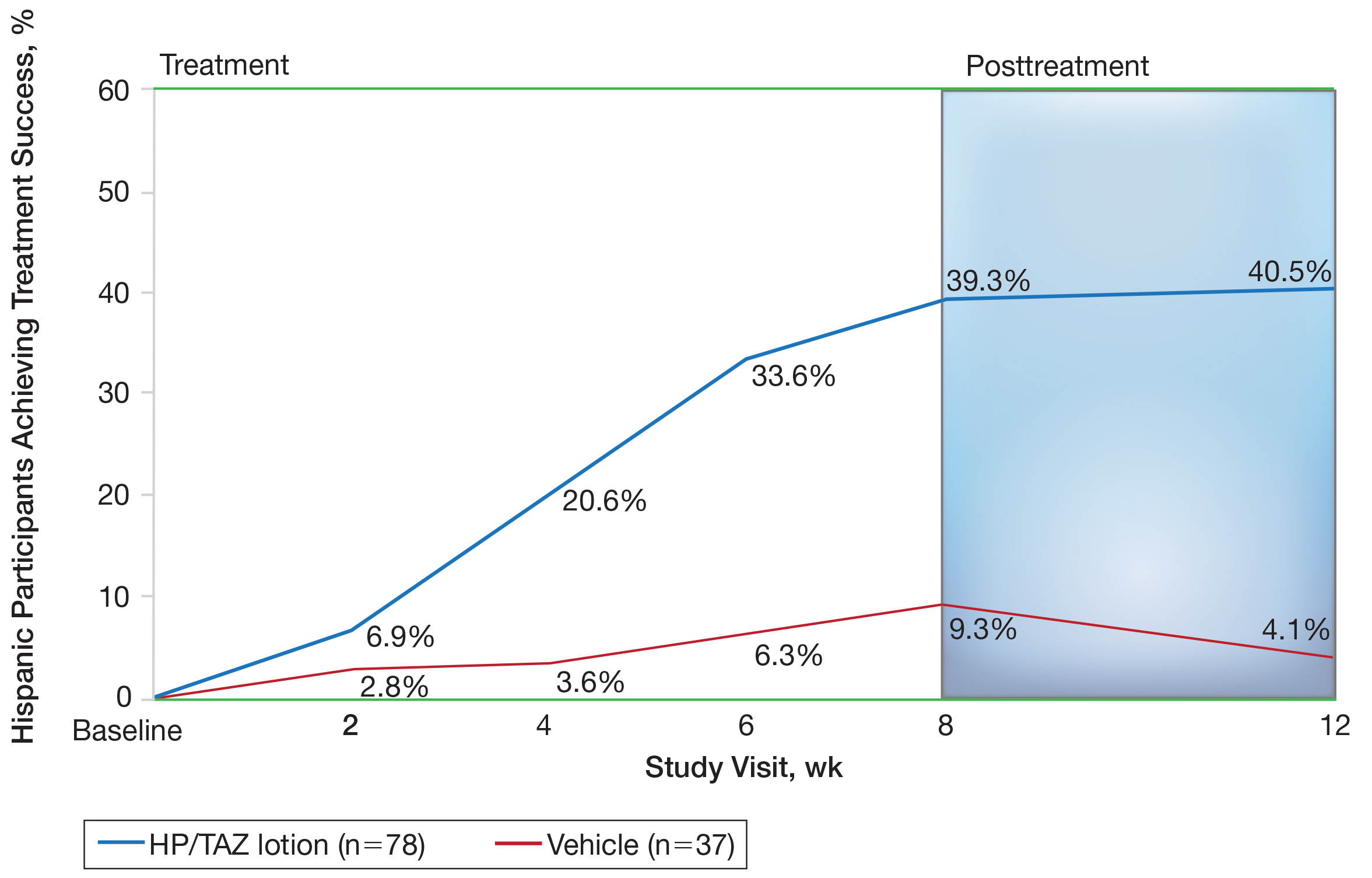
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).
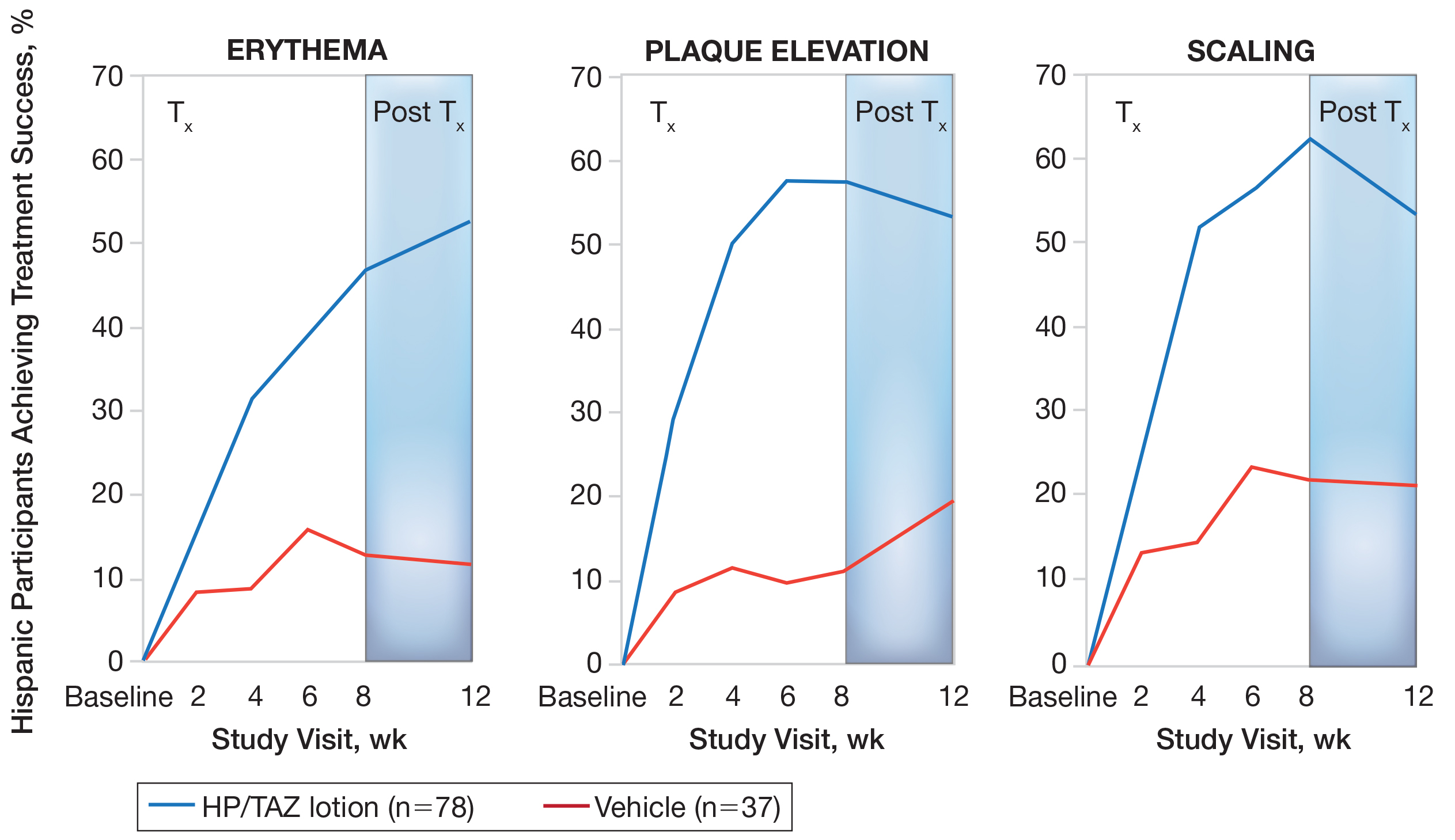
Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.
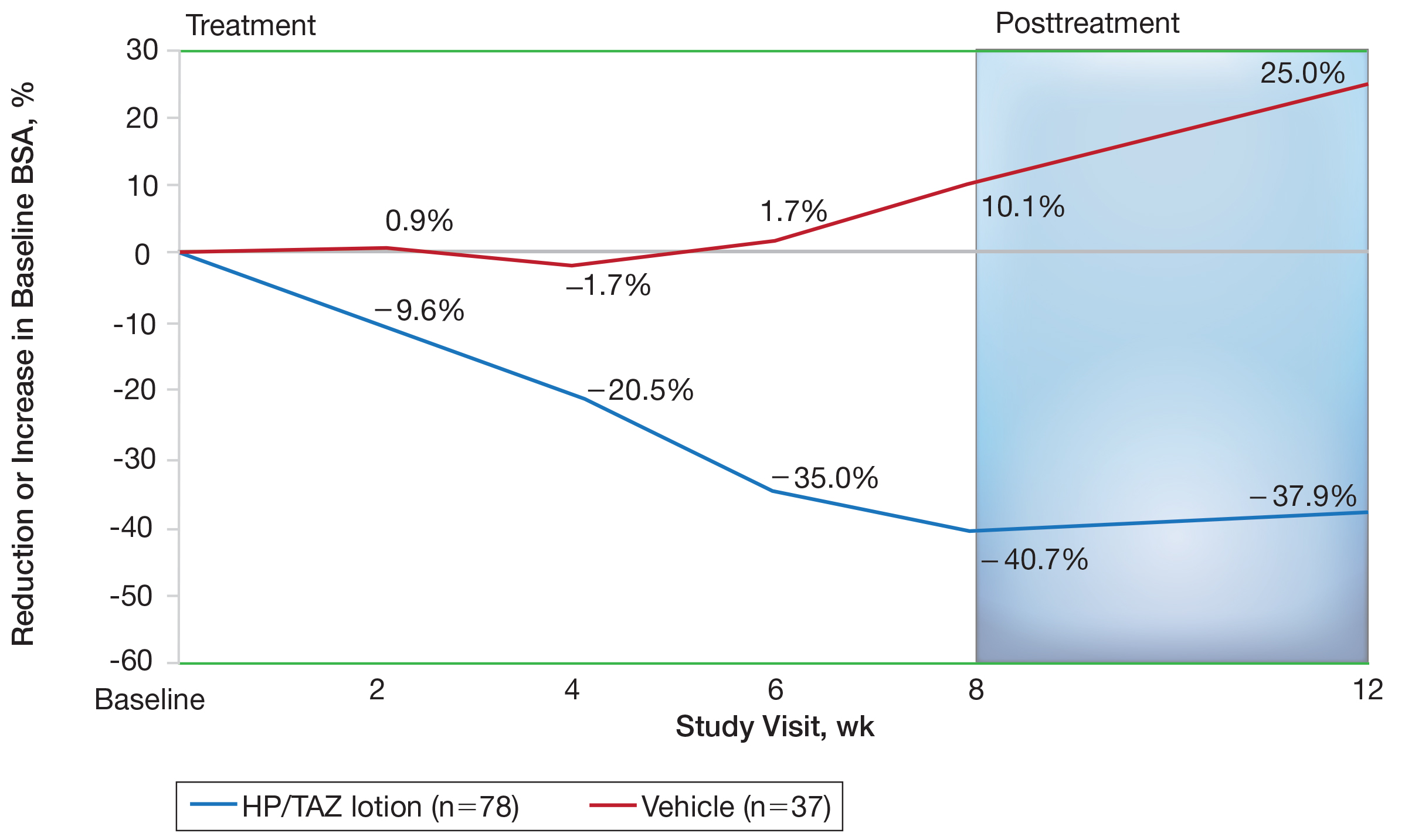
Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).
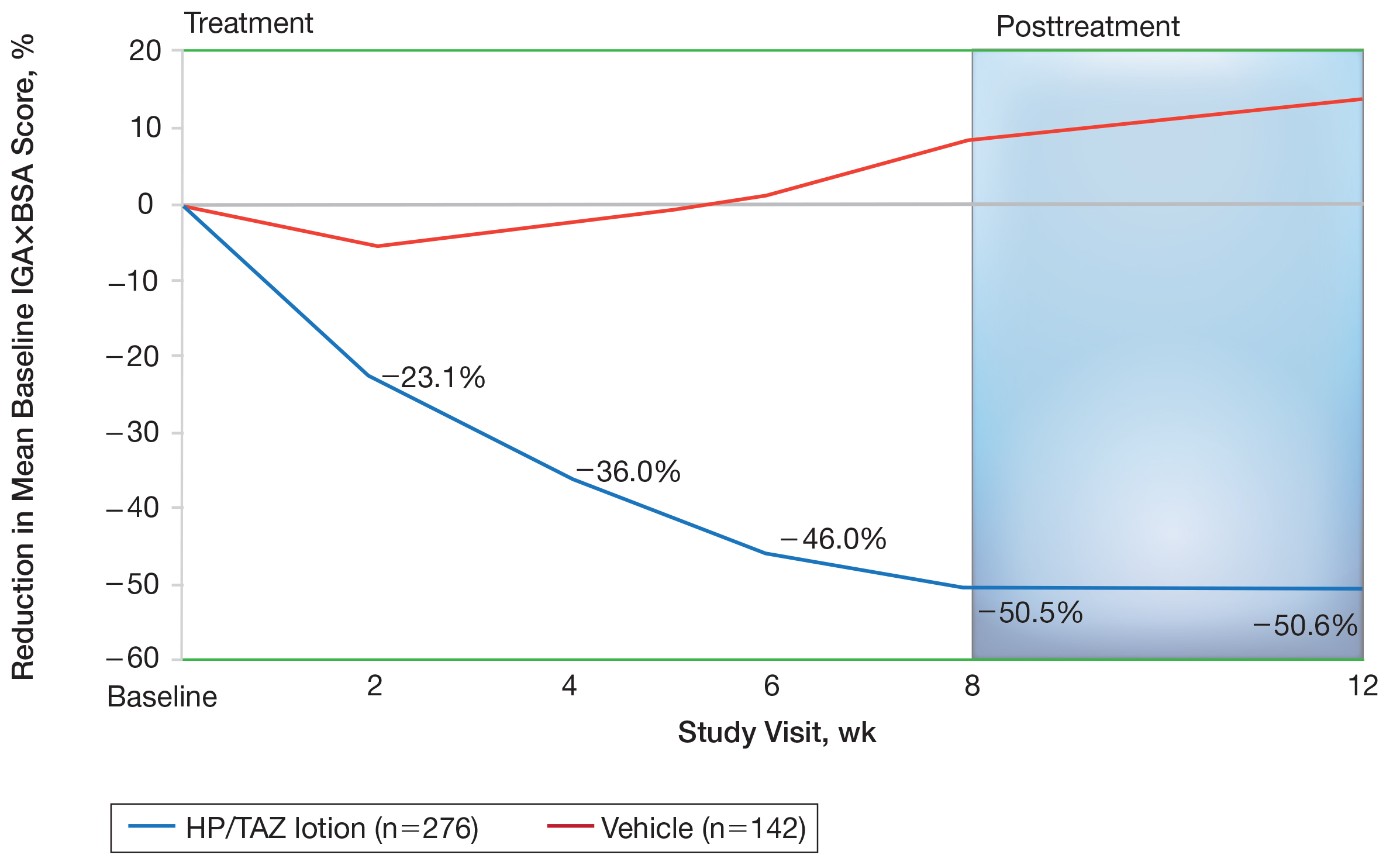
Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10
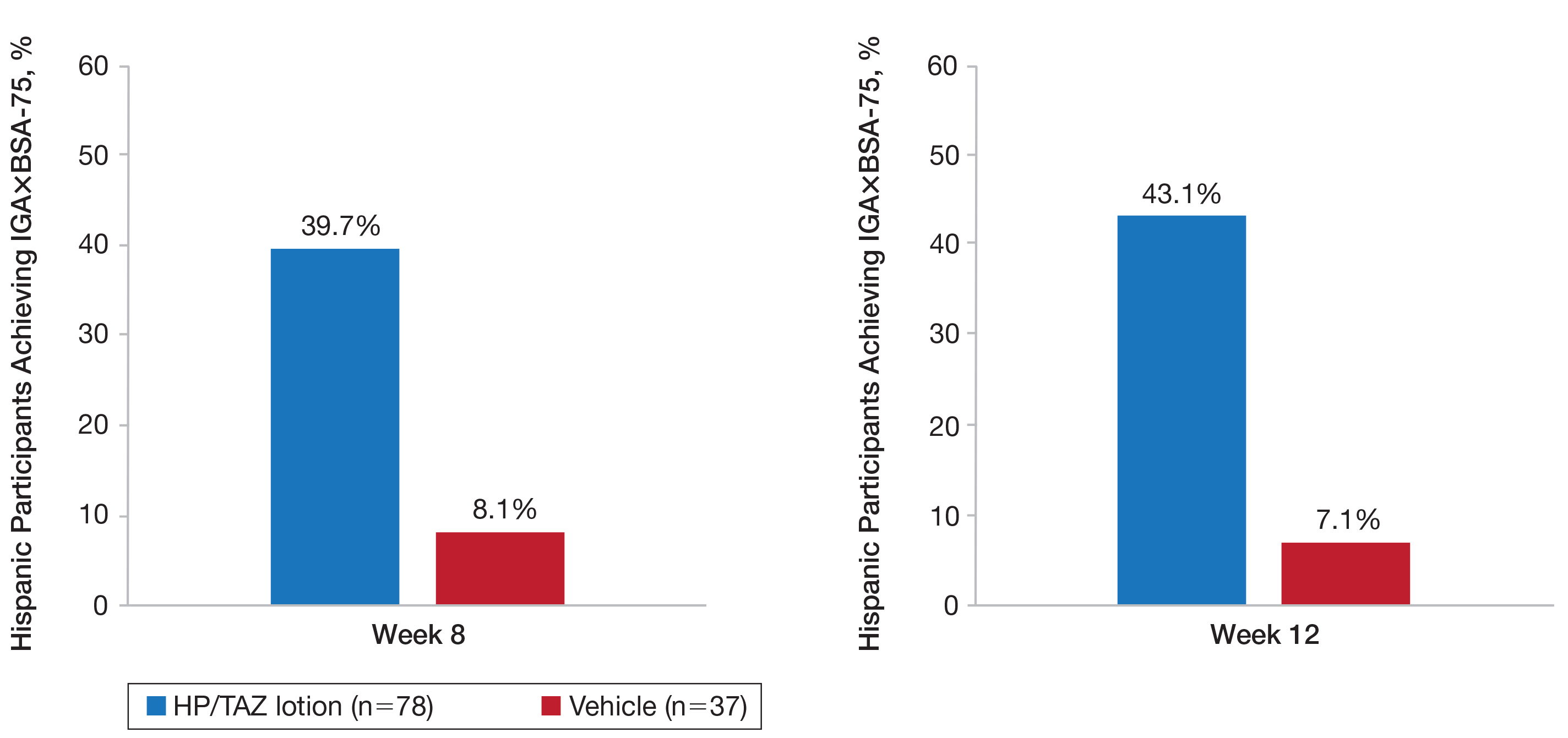
A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).
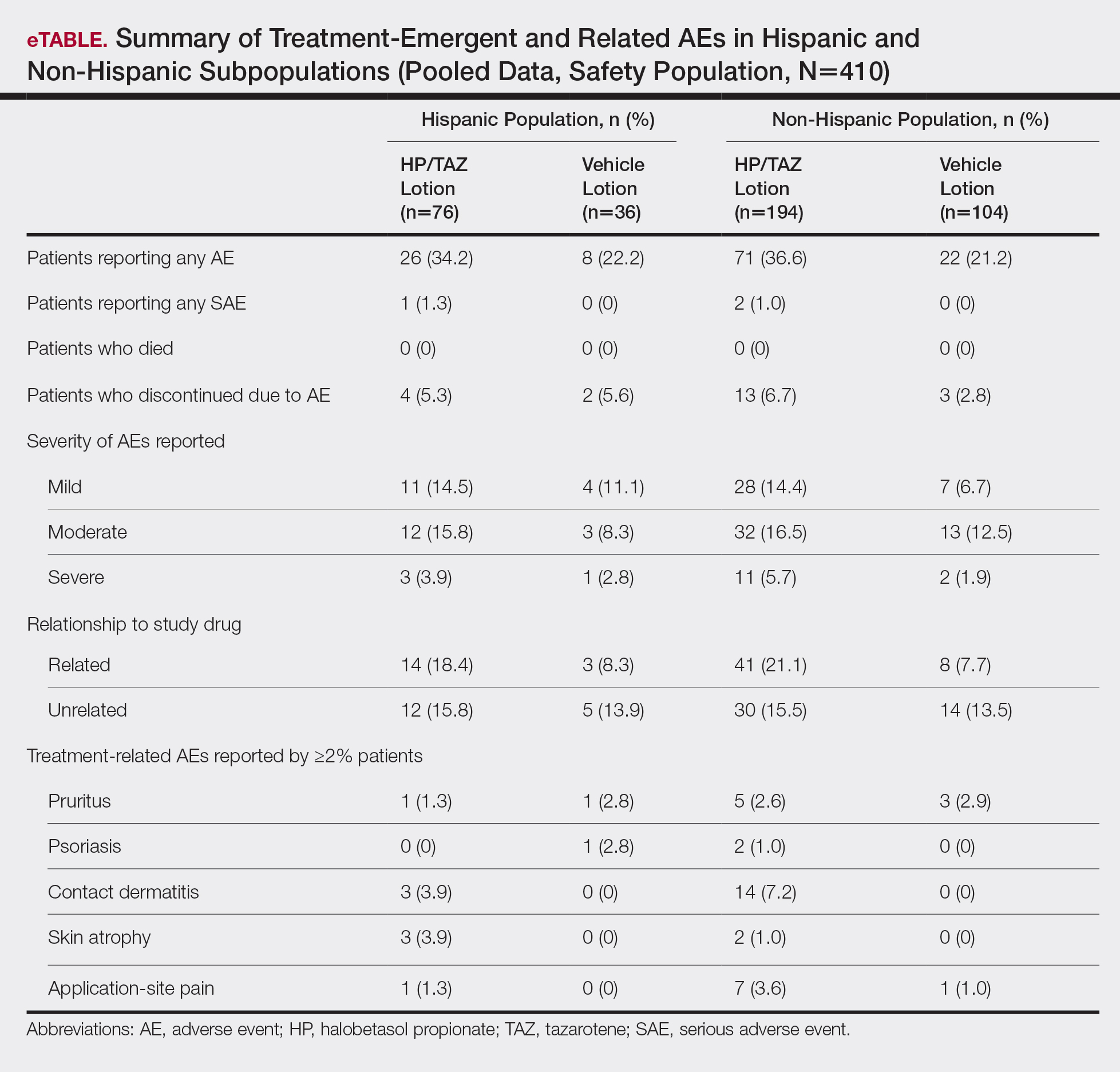
For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.
Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).

Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).

Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).

Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.

Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).

Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10

A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).

For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.

Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
Psoriasis is a common chronic inflammatory disease affecting a diverse patient population, yet epidemiological and clinical data related to psoriasis in patients with skin of color are sparse. The Hispanic ethnic group includes a broad range of skin types and cultures. Prevalence of psoriasis in a Hispanic population has been reported as lower than in a white population1; however, these data may be influenced by the finding that Hispanic patients are less likely to see a dermatologist when they have skin problems.2 In addition, socioeconomic disparities and cultural variations among racial/ethnic groups may contribute to differences in access to care and thresholds for seeking care,3 leading to a tendency for more severe disease in skin of color and Hispanic ethnic groups.4,5 Greater impairments in health-related quality of life have been reported in patients with skin of color and Hispanic racial/ethnic groups compared to white patients, independent of psoriasis severity.4,6 Postinflammatory pigment alteration at the sites of resolving lesions, a common clinical feature in skin of color, may contribute to the impact of psoriasis on quality of life in patients with skin of color. Psoriasis in darker skin types also can present diagnostic challenges due to overlapping features with other papulosquamous disorders and less conspicuous erythema.7
We present a post hoc analysis of the treatment of moderate to severe psoriasis with a novel fixed-combination halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion in a Hispanic patient population. Historically, clinical trials for psoriasis have enrolled low proportions of Hispanic patients and other patients with skin of color; in this analysis, the Hispanic population (115/418) represented 28% of the total study population and provided valuable insights.
Methods
Study Design
Two phase 3 randomized controlled trials were conducted to demonstrate the efficacy and safety of HP/TAZ lotion. Patients with a clinical diagnosis of moderate or severe localized psoriasis (N=418) were randomized to receive HP/TAZ lotion or vehicle (2:1 ratio) once daily for 8 weeks with a 4-week posttreatment follow-up.8,9 A post hoc analysis was conducted on data of the self-identified Hispanic population.
Assessments
Efficacy assessments included treatment success (at least a 2-grade improvement from baseline in the investigator global assessment [IGA] and a score of clear or almost clear) and impact on individual signs of psoriasis (at least a 2-grade improvement in erythema, plaque elevation, and scaling) at the target lesion. In addition, reduction in body surface area (BSA) was recorded, and an IGA×BSA score was calculated by multiplying IGA by BSA at each timepoint for each individual patient. A clinically meaningful improvement in disease severity (percentage of patients achieving a 75% reduction in IGA×BSA [IGA×BSA-75]) also was calculated.
Information on reported and observed adverse events (AEs) was obtained at each visit. The safety population included 112 participants (76 in the HP/TAZ group and 36 in the vehicle group).
Statistical Analysis
The statistical and analytical plan is detailed elsewhere9 and relevant to this post hoc analysis. No statistical analysis was carried out to compare data in the Hispanic population with either the overall study population or the non-Hispanic population.
Results
Overall, 115 Hispanic patients (27.5%) were enrolled (eFigure). Patients had a mean (standard deviation [SD]) age of 46.7 (13.12) years, and more than two-thirds were male (n=80, 69.6%).

Overall completion rates (80.0%) for Hispanic patients were similar to those in the overall study population, though there were more discontinuations in the vehicle group. The main reasons for treatment discontinuation among Hispanic patients were participant request (n=8, 7.0%), lost to follow-up (n=8, 7.0%), and AEs (n=4, 3.5%). Hispanic patients in this study had more severe disease—18.3% (n=21) had an IGA score of 4 compared to 13.5% (n=41) of non-Hispanic patients—and more severe erythema (19.1% vs 9.6%), plaque elevation (20.0% vs 10.2%), and scaling (15.7% vs 12.9%) compared to the non-Hispanic populations (Table).

Efficacy of HP/TAZ lotion in Hispanic patients was similar to the overall study populations,9 though maintenance of effect posttreatment appeared to be better. The incidence of treatment-related AEs also was lower.
Halobetasol propionate 0.01%–TAZ 0.045% lotion demonstrated statistically significant superiority based on treatment success compared to vehicle as early as week 4 (P=.034). By week 8, 39.3% of participants treated with HP/TAZ lotion achieved treatment success compared to 9.3% of participants in the vehicle group (P=.002)(Figure 1). Treatment success was maintained over the 4-week posttreatment period, whereby 40.5% of the HP/TAZ-treated participants were treatment successes at week 12 compared to only 4.1% of participants in the vehicle group (P<.001).

Improvements in psoriasis signs and symptoms at the target lesion were statistically significant compared to vehicle from week 2 (plaque elevation, P=.018) or week 4 (erythema, P=.004; scaling, P<.001)(Figure 2). By week 8, 46.8%, 58.1%, and 63.2% of participants showed at least a 2-grade improvement from baseline and were therefore treatment successes for erythema, plaque elevation, and scaling, respectively (all statistically significant [P<.001] compared to vehicle). The number of participants who achieved at least a 2-grade improvement in erythema with HP/TAZ lotion increased posttreatment from 46.8% to 53.0%.

Mean (SD) baseline BSA was 6.2 (3.07), and the mean (SD) size of the target lesion was 36.3 (21.85) cm2. Overall, BSA also was significantly reduced in participants treated with HP/TAZ lotion compared to vehicle. At week 8, the mean percentage change from baseline was —40.7% compared to an increase (+10.1%) in the vehicle group (P=.002)(Figure 3). Improvements in BSA were maintained posttreatment, whereas in the vehicle group, mean (SD) BSA had increased to 6.1 (4.64).

Halobetasol propionate 0.01%–TAZ 0.045% lotion achieved a 50.5% reduction from baseline IGA×BSA by week 8 compared to an 8.5% increase with vehicle (P<.001)(Figure 4). Differences in treatment groups were significant from week 2 (P=.016). Efficacy was maintained posttreatment, with a 50.6% reduction from baseline IGA×BSA at week 12 compared to an increase of 13.6% in the vehicle group (P<.001). Again, although results were similar to the overall study population at week 8 (50.5% vs 51.9%), maintenance of effect was better posttreatment (50.6% vs 46.6%).10

A clinically meaningful effect (IGA×BSA-75) was achieved in 39.7% of Hispanic participants treated with HP/TAZ lotion compared to 8.1% of participants treated with vehicle (P<.001) at week 8. The benefits were significantly different from week 4 and more participants maintained a clinically meaningful effect posttreatment (43.1% vs 7.1%, P<.001)(Figure 5).

For Hispanic participants overall, 34 participants reported AEs: 26 (34.2%) treated with HP/TAZ lotion and 8 (22.2%) treated with vehicle (eTable). There was 1 (1.3%) serious AE in the HP/TAZ group. Most of the AEs were mild or moderate, with approximately half being related to study treatment. The most common treatment-related AEs in Hispanic participants treated with HP/TAZ lotion were contact dermatitis (n=3, 3.9%) and skin atrophy (n=3, 3.9%) compared to contact dermatitis (n=14, 7.2%) and application-site pain (n=7, 3.6%) in the non-Hispanic population. Pruritus was the most common AE in Hispanic participants treated with vehicle.

Comment
The large number of Hispanic patients in the 2 phase 3 trials8,9 allowed for this valuable subgroup analysis on the topical treatment of Hispanic patients with plaque psoriasis. Validation of observed differences in maintenance of effect and tolerability warrant further study. Prior clinical studies in psoriasis have tended to enroll a small proportion of Hispanic patients without any post hoc analysis. For example, in a pooled analysis of 4 phase 3 trials with secukinumab, Hispanic patients accounted for only 16% of the overall population.11 In our analysis, the Hispanic cohort represented 28% of the overall study population of 2 phase 3 studies investigating the efficacy, safety, and tolerability of HP/TAZ lotion in patients with moderate to severe psoriasis.8,9 In addition, proportionately more Hispanic patients had severe disease (IGA of 4) or severe signs and symptoms of psoriasis (erythema, plaque elevation, and scaling) than the non-Hispanic population. This finding supports other studies that have suggested Hispanic patients with psoriasis tend to have more severe disease but also may reflect thresholds for seeking care.3-5
Halobetasol propionate 0.01%–TAZ 0.045% lotion was significantly more effective than vehicle for all efficacy assessments. In general, efficacy results with HP/TAZ lotion were similar to those reported in the overall phase 3 study populations over the 8-week treatment period. The only noticeable difference was in the posttreatment period. In the overall study population, efficacy was maintained over the 4-week posttreatment period in the HP/TAZ group. In the Hispanic subpopulation, there appeared to be continued improvement in the number of participants achieving treatment success (IGA and erythema), clinically meaningful success, and further reductions in BSA. Although there is a paucity of studies evaluating psoriasis therapies in Hispanic populations, data on etanercept and secukinumab have been published.6,11
Onset of effect also is an important aspect of treatment. In patients with skin of color, including patients of Hispanic ethnicity and higher Fitzpatrick skin phototypes, early clearance of lesions may help limit the severity and duration of postinflammatory pigment alteration. Improvements in IGA×BSA scores were significant compared to vehicle from week 2 (P=.016), and a clinically meaningful improvement with HP/TAZ lotion (IGA×BSA-75) was seen by week 4 (P=.024).
Halobetasol propionate 0.01%–TAZ 0.045% lotion was well tolerated, both in the 2 phase 3 studies and in the post hoc analysis of the Hispanic subpopulation. The incidence of skin atrophy (n=3, 3.9%) was more common vs the non-Hispanic population (n=2, 1.0%). Other common AEs—contact dermatitis, pruritus, and application-site pain—were more common in the non-Hispanic population.
A limitation of our analysis was that it was a post hoc analysis of the Hispanic participants. The phase 3 studies were not designed to specifically study the impact of treatment on ethnicity/race, though the number of Hispanic participants enrolled in the 2 studies was relatively high. The absence of Fitzpatrick skin phototypes in this data set is another limitation of this study.
Conclusion
Halobetasol propionate 0.01%–TAZ 0.045% lotion was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Acknowledgments
We thank Brian Bulley, MSc (Konic Limited, United Kingdom), for assistance with the preparation of the manuscript. Ortho Dermatologics funded Konic’s activities pertaining to this manuscript.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Yan D, Afifi L, Jeon C, et al. A cross-sectional study of the distribution of psoriasis subtypes in different ethno-racial groups. Dermatol Online J. 2018;24. pii:13030/qt5z21q4k2.
- Abrouk M, Lee K, Brodsky M, et al. Ethnicity affects the presenting severity of psoriasis. J Am Acad Dermatol. 2017;77:180-182.
- Shah SK, Arthur A, Yang YC, et al. A retrospective study to investigate racial and ethnic variations in the treatment of psoriasis with etanercept. J Drugs Dermatol. 2011;10:866-872.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18:297-299.
- Adsit S, Zaldivar ER, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327-1339.
Practice Points
- Although psoriasis is a common inflammatory disease, data in the Hispanic population are sparse and disease may be more severe.
- A recent clinical investigation with halobetasol propionate 0.01%–tazarotene 0.045% lotion included a number of Hispanic patients, affording an ideal opportunity to provide important data on this population.
- This fixed-combination therapy was associated with significant, rapid, and sustained reductions in disease severity in a Hispanic population with moderate to severe psoriasis that continued to show improvement posttreatment with good tolerability and safety.
Assessing the Effectiveness of Knowledge-Based Interventions in Increasing Skin Cancer Awareness, Knowledge, and Protective Behaviors in Skin of Color Populations
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
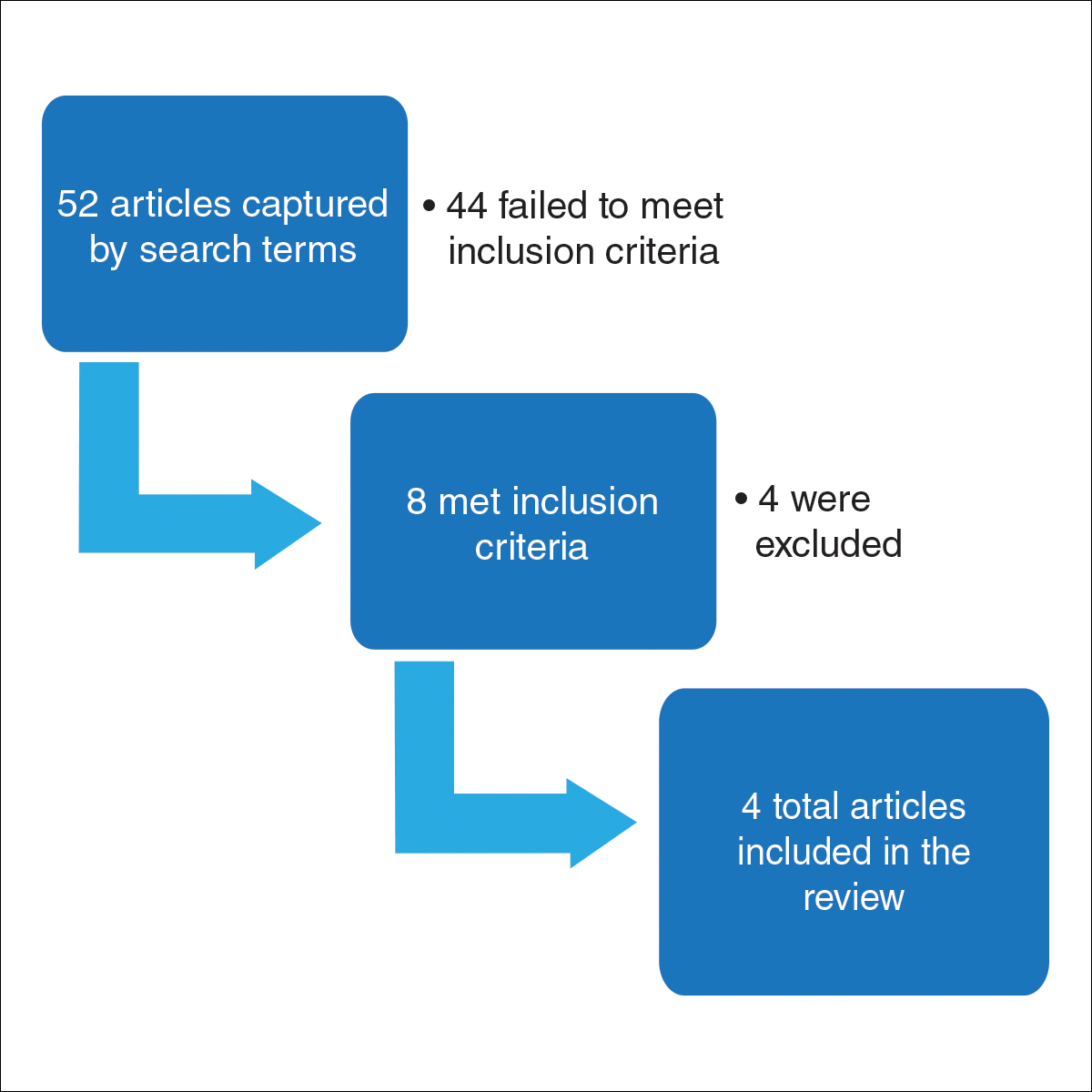
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).
Results
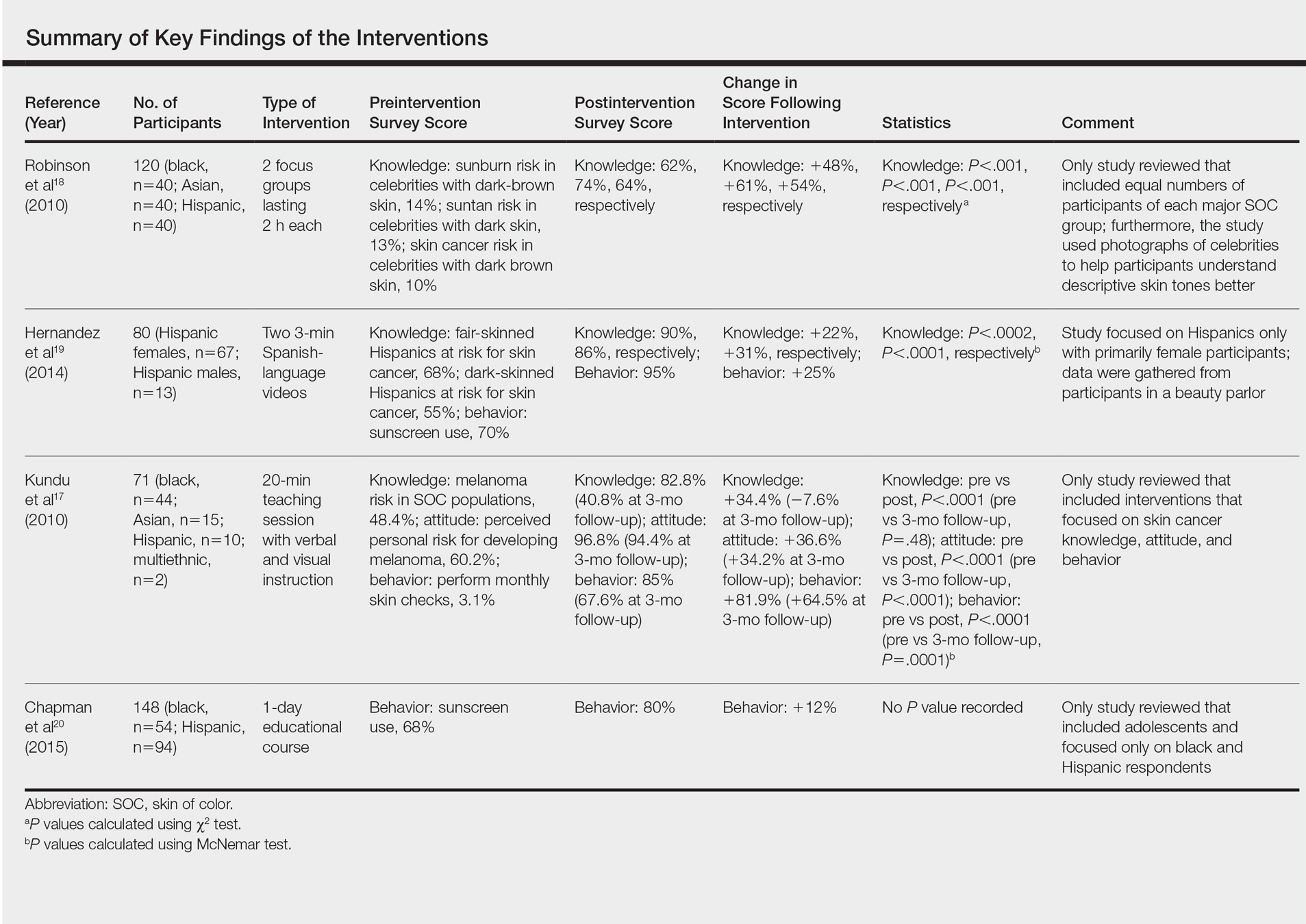
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).


Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).


Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
Practice Points
- Patients of color should be informed that they are at risk for skin cancer including melanoma.
- Patients of color should be taught to identify suspicious skin lesions including the ABCDEs of melanoma.
- Patients of color should be instructed to perform self-body skin examinations, especially of the palms and soles, for any evolving skin lesions. Patients should be instructed on the importance of visiting a physician for an evolving or suspicious mole or lesion.