User login
Standardize Communication to Reduce Medical Errors
DENVER – Do you speak in SBAR?
If not, communication in your department probably could be better, two speakers said at the annual meeting of the American College of Emergency Physicians, in a session on improving teamwork.
SBAR is the acronym for a standardized communication format that organizes the transfer of information under four themes – situation, background; assessment, and recommendation. Developed by anesthesiologist Dr. Michael Leonard and colleagues at Kaiser Permanente of Colorado, Evergreen, the SBAR tool has been endorsed by many national organizations and medical accreditation bodies, said Dr. Jennifer L. Wiler.
It originally was used for acute-care communications from nurses to physicians but now is being used more widely for communication from nurse to nurse and from physician to physician in a variety of care settings. One study found that it was particularly useful for reporting changes in a patient’s status or a patient’s deterioration between health care services or shifts (Healthcare Quarterly 2008;11:72-9).
Using SBAR won’t necessarily save you time – at least one study suggests it increases time for communications. The limited data from seven published studies so far suggest that SBAR use improves the transfer of important information, the perception of a culture of safety, and patient satisfaction, though SBAR has yet to be validated in an emergency department setting, said Dr. Wiler of the University of Colorado, Aurora.
It has worked in hospital medicine. Hospitalists with Kaiser Permanente found SBAR to be helpful, according to a study conducted from May 2008 to August 2009. SBAR was used for end-of-rotation patient handoffs, the researchers wrote. "The Hospitalist Chief finds SBAR useful when communicating with consultants, especially during the initial telephone conversation when it is important for hospitalists to clearly state information needs." Hospitalists communicate primarily with ED physicians, and "communications with KP ED physicians is superb; [we] get a complete diagnosis" (Perm. J. 2011 Summer;15:51-60).
The Joint Commission and the Agency for Healthcare Research and Quality (AHRQ) list communication problems as the No. 1 cause of medical error, she noted. "Addressing errors related to communication is really critical," Dr. Wiler said.
To communicate in SBAR, use the following structure:
– Situation. Identify yourself, your occupation and where you are calling from, if speaking by phone. Identify the patient – the name, date of birth, age, and sex, and the reason for your report. Describe the current status of the patient or your reason for calling. If it’s urgent, say so.
– Background. Give the patient’s presenting complaint, any relevant past medical history, and a brief summary of background.
– Assessment. Describe abnormal vital signs (heart rate, respiratory rate, blood pressure, temperature, oxygen saturation, pain scale, or level of consciousness). Provide your clinical impression, the severity of the patient’s situation, and any additional concerns.
– Recommendation. Explain what you require, how urgently, and when action needs to be taken. Suggest what action might be taken. Clarify what action you expect to be taken.
One of the challenges to good communication is that physicians and nurses practice in parallel environments and too seldom prioritize teamwork, said Eric Christensen, R.N.
Beyond SBAR, a variety of strategies can improve communication between team members, he said. For example, the AHRQ suggests a structured handoff sign-out protocol dubbed ANTICipate (for Administrative data, New clinical information, Tasks to be performed, Illness severity, and Contingency plans).
Develop structured communication events specific to your hospital or department, suggested Mr. Christensen, a staff nurse at Denver Health Medical Center.
These might include a patient’s physiologic parameters that trigger an alert to the nurse and doctor, or required communication to reconcile abnormal vital signs. A "time out" at discharge will lead to safer discharge if all providers are aware of the treatment plan and pending issues are resolved. Face-to-face debriefings at shift changes are a good idea. He also recommended greater use of physician-nurse huddles to discuss significant patient issues, therapies, oxygen status and last vital signs, and any pending issues in clinical management.
"One of the things we’ve done at our institution is to institute huddles throughout the shift," he said in an interview. Every 2-3 hours the nurses and physicians round together to make sure throughout the shift that everyone is "on the same page."
At Dr. Wiler’s institution, team-based huddles are standard practice at presentation of more acute, sicker patient or trauma patients. "We’re moving to that methodology with all our patients," she said in an interview.
If you have concerns to raise with nurses, resist the temptation to direct them only to the most senior nursing staff, Mr. Christensen said. "It makes a world of difference to address a nurse by name," he added. If you don’t know a nurse’s name, ask directly, so the nurse feels included.
Taking 2 minutes to discuss the plan of care for complicated patients directly with the nurse and seek the nurse’s input will pay off in the long run, he said. With any patient, providing brief targeted bedside education for nurses by not just saying what will be done, but why, gets nurses more engaged in the clinical care team and better prepared to anticipate treatment of similar patients in the future.
Being approachable and complimenting nurses who are doing a good job also pays off, Mr. Christensen said.
"Be a leader, not a commander," he said. "Managers manage process and leaders lead people. If you want to know which you are, just look behind you. If there is no one there, you are just going for a walk."
Dr. Wiler and Mr. Christensen reported having no financial disclosures.
DENVER – Do you speak in SBAR?
If not, communication in your department probably could be better, two speakers said at the annual meeting of the American College of Emergency Physicians, in a session on improving teamwork.
SBAR is the acronym for a standardized communication format that organizes the transfer of information under four themes – situation, background; assessment, and recommendation. Developed by anesthesiologist Dr. Michael Leonard and colleagues at Kaiser Permanente of Colorado, Evergreen, the SBAR tool has been endorsed by many national organizations and medical accreditation bodies, said Dr. Jennifer L. Wiler.
It originally was used for acute-care communications from nurses to physicians but now is being used more widely for communication from nurse to nurse and from physician to physician in a variety of care settings. One study found that it was particularly useful for reporting changes in a patient’s status or a patient’s deterioration between health care services or shifts (Healthcare Quarterly 2008;11:72-9).
Using SBAR won’t necessarily save you time – at least one study suggests it increases time for communications. The limited data from seven published studies so far suggest that SBAR use improves the transfer of important information, the perception of a culture of safety, and patient satisfaction, though SBAR has yet to be validated in an emergency department setting, said Dr. Wiler of the University of Colorado, Aurora.
It has worked in hospital medicine. Hospitalists with Kaiser Permanente found SBAR to be helpful, according to a study conducted from May 2008 to August 2009. SBAR was used for end-of-rotation patient handoffs, the researchers wrote. "The Hospitalist Chief finds SBAR useful when communicating with consultants, especially during the initial telephone conversation when it is important for hospitalists to clearly state information needs." Hospitalists communicate primarily with ED physicians, and "communications with KP ED physicians is superb; [we] get a complete diagnosis" (Perm. J. 2011 Summer;15:51-60).
The Joint Commission and the Agency for Healthcare Research and Quality (AHRQ) list communication problems as the No. 1 cause of medical error, she noted. "Addressing errors related to communication is really critical," Dr. Wiler said.
To communicate in SBAR, use the following structure:
– Situation. Identify yourself, your occupation and where you are calling from, if speaking by phone. Identify the patient – the name, date of birth, age, and sex, and the reason for your report. Describe the current status of the patient or your reason for calling. If it’s urgent, say so.
– Background. Give the patient’s presenting complaint, any relevant past medical history, and a brief summary of background.
– Assessment. Describe abnormal vital signs (heart rate, respiratory rate, blood pressure, temperature, oxygen saturation, pain scale, or level of consciousness). Provide your clinical impression, the severity of the patient’s situation, and any additional concerns.
– Recommendation. Explain what you require, how urgently, and when action needs to be taken. Suggest what action might be taken. Clarify what action you expect to be taken.
One of the challenges to good communication is that physicians and nurses practice in parallel environments and too seldom prioritize teamwork, said Eric Christensen, R.N.
Beyond SBAR, a variety of strategies can improve communication between team members, he said. For example, the AHRQ suggests a structured handoff sign-out protocol dubbed ANTICipate (for Administrative data, New clinical information, Tasks to be performed, Illness severity, and Contingency plans).
Develop structured communication events specific to your hospital or department, suggested Mr. Christensen, a staff nurse at Denver Health Medical Center.
These might include a patient’s physiologic parameters that trigger an alert to the nurse and doctor, or required communication to reconcile abnormal vital signs. A "time out" at discharge will lead to safer discharge if all providers are aware of the treatment plan and pending issues are resolved. Face-to-face debriefings at shift changes are a good idea. He also recommended greater use of physician-nurse huddles to discuss significant patient issues, therapies, oxygen status and last vital signs, and any pending issues in clinical management.
"One of the things we’ve done at our institution is to institute huddles throughout the shift," he said in an interview. Every 2-3 hours the nurses and physicians round together to make sure throughout the shift that everyone is "on the same page."
At Dr. Wiler’s institution, team-based huddles are standard practice at presentation of more acute, sicker patient or trauma patients. "We’re moving to that methodology with all our patients," she said in an interview.
If you have concerns to raise with nurses, resist the temptation to direct them only to the most senior nursing staff, Mr. Christensen said. "It makes a world of difference to address a nurse by name," he added. If you don’t know a nurse’s name, ask directly, so the nurse feels included.
Taking 2 minutes to discuss the plan of care for complicated patients directly with the nurse and seek the nurse’s input will pay off in the long run, he said. With any patient, providing brief targeted bedside education for nurses by not just saying what will be done, but why, gets nurses more engaged in the clinical care team and better prepared to anticipate treatment of similar patients in the future.
Being approachable and complimenting nurses who are doing a good job also pays off, Mr. Christensen said.
"Be a leader, not a commander," he said. "Managers manage process and leaders lead people. If you want to know which you are, just look behind you. If there is no one there, you are just going for a walk."
Dr. Wiler and Mr. Christensen reported having no financial disclosures.
DENVER – Do you speak in SBAR?
If not, communication in your department probably could be better, two speakers said at the annual meeting of the American College of Emergency Physicians, in a session on improving teamwork.
SBAR is the acronym for a standardized communication format that organizes the transfer of information under four themes – situation, background; assessment, and recommendation. Developed by anesthesiologist Dr. Michael Leonard and colleagues at Kaiser Permanente of Colorado, Evergreen, the SBAR tool has been endorsed by many national organizations and medical accreditation bodies, said Dr. Jennifer L. Wiler.
It originally was used for acute-care communications from nurses to physicians but now is being used more widely for communication from nurse to nurse and from physician to physician in a variety of care settings. One study found that it was particularly useful for reporting changes in a patient’s status or a patient’s deterioration between health care services or shifts (Healthcare Quarterly 2008;11:72-9).
Using SBAR won’t necessarily save you time – at least one study suggests it increases time for communications. The limited data from seven published studies so far suggest that SBAR use improves the transfer of important information, the perception of a culture of safety, and patient satisfaction, though SBAR has yet to be validated in an emergency department setting, said Dr. Wiler of the University of Colorado, Aurora.
It has worked in hospital medicine. Hospitalists with Kaiser Permanente found SBAR to be helpful, according to a study conducted from May 2008 to August 2009. SBAR was used for end-of-rotation patient handoffs, the researchers wrote. "The Hospitalist Chief finds SBAR useful when communicating with consultants, especially during the initial telephone conversation when it is important for hospitalists to clearly state information needs." Hospitalists communicate primarily with ED physicians, and "communications with KP ED physicians is superb; [we] get a complete diagnosis" (Perm. J. 2011 Summer;15:51-60).
The Joint Commission and the Agency for Healthcare Research and Quality (AHRQ) list communication problems as the No. 1 cause of medical error, she noted. "Addressing errors related to communication is really critical," Dr. Wiler said.
To communicate in SBAR, use the following structure:
– Situation. Identify yourself, your occupation and where you are calling from, if speaking by phone. Identify the patient – the name, date of birth, age, and sex, and the reason for your report. Describe the current status of the patient or your reason for calling. If it’s urgent, say so.
– Background. Give the patient’s presenting complaint, any relevant past medical history, and a brief summary of background.
– Assessment. Describe abnormal vital signs (heart rate, respiratory rate, blood pressure, temperature, oxygen saturation, pain scale, or level of consciousness). Provide your clinical impression, the severity of the patient’s situation, and any additional concerns.
– Recommendation. Explain what you require, how urgently, and when action needs to be taken. Suggest what action might be taken. Clarify what action you expect to be taken.
One of the challenges to good communication is that physicians and nurses practice in parallel environments and too seldom prioritize teamwork, said Eric Christensen, R.N.
Beyond SBAR, a variety of strategies can improve communication between team members, he said. For example, the AHRQ suggests a structured handoff sign-out protocol dubbed ANTICipate (for Administrative data, New clinical information, Tasks to be performed, Illness severity, and Contingency plans).
Develop structured communication events specific to your hospital or department, suggested Mr. Christensen, a staff nurse at Denver Health Medical Center.
These might include a patient’s physiologic parameters that trigger an alert to the nurse and doctor, or required communication to reconcile abnormal vital signs. A "time out" at discharge will lead to safer discharge if all providers are aware of the treatment plan and pending issues are resolved. Face-to-face debriefings at shift changes are a good idea. He also recommended greater use of physician-nurse huddles to discuss significant patient issues, therapies, oxygen status and last vital signs, and any pending issues in clinical management.
"One of the things we’ve done at our institution is to institute huddles throughout the shift," he said in an interview. Every 2-3 hours the nurses and physicians round together to make sure throughout the shift that everyone is "on the same page."
At Dr. Wiler’s institution, team-based huddles are standard practice at presentation of more acute, sicker patient or trauma patients. "We’re moving to that methodology with all our patients," she said in an interview.
If you have concerns to raise with nurses, resist the temptation to direct them only to the most senior nursing staff, Mr. Christensen said. "It makes a world of difference to address a nurse by name," he added. If you don’t know a nurse’s name, ask directly, so the nurse feels included.
Taking 2 minutes to discuss the plan of care for complicated patients directly with the nurse and seek the nurse’s input will pay off in the long run, he said. With any patient, providing brief targeted bedside education for nurses by not just saying what will be done, but why, gets nurses more engaged in the clinical care team and better prepared to anticipate treatment of similar patients in the future.
Being approachable and complimenting nurses who are doing a good job also pays off, Mr. Christensen said.
"Be a leader, not a commander," he said. "Managers manage process and leaders lead people. If you want to know which you are, just look behind you. If there is no one there, you are just going for a walk."
Dr. Wiler and Mr. Christensen reported having no financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF EMERGENCY PHYSICIANS
Higher MI Mortality in Hospitalized HIV Patients
SAN FRANCISCO – Hospitalized patients who have an acute MI are 53% more likely to die if they are infected with HIV, based on a secondary analysis of data from the Nationwide Inpatient Sample database.
The mortality rates for in-hospital acute MI were 4.3% for HIV-positive patients and 2.4% for HIV-negative ones, a statistically significant difference.
HIV-positive patients had a greater burden of comorbidities, as evidenced by a mean Charlson’s Comorbidity Index score of 1.14, as compared with HIV-negative patients with an average score of 0.94. Comorbidities that were significantly more prevalent in the HIV-positive cases, compared with HIV-negative cases, included renal disease (13% vs. 5%, respectively), mild liver disease (8% vs. 1%), and heart failure (26% vs. 20%), Dr. Daniel Pearce reported at the meeting, sponsored by the American Society for Microbiology.
He and his associates analyzed data from 1997 to 2006 for nearly 1.5 million adults who were hospitalized for more than a day and had an acute MI. The data approximated a stratified 20% sample of all nonfederal, short-term, general, and specialty hospitals serving adults in the United States.
The hazard ratio for death after MI was 53% higher among the 5,984 HIV-positive patients, compared with those without HIV after adjusting for the effects of age, race, gender, comorbidity, and type of insurance (as a marker for socioeconomic status), Dr. Pearce, of the Riverside County (Calif.) Department of Public Health, and his associates reported in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The HIV-positive group was significantly younger compared with the HIV-negative group (mean ages of 54 and 64 years, respectively), more likely to be male (65% vs. 72%), and more likely to be insured primarily by Medicare or Medicaid (62% vs. 25%).
On the other hand, the HIV-positive cases had lower rates of the most common cardiometabolic risk factors, including hypertension, diabetes, and cardiac arrhythmias. Substance abuse was more prevalent in the HIV-positive group than the HIV-negative group.
Dr. Pearce speculated the higher death rate could be related to the pathological effects of HIV viremia on cardiac vasculature and function.
Dr. Pearce reported having no financial disclosures.

|
|
The higher rate of in-hospital MI mortality in HIV-infected patients may be due to risk factors other than HIV infection itself. Many of these patients use illicit drugs and they’re in and out of care, disenfranchised, or don’t have access to medical care. We know that people with very limited resources don’t do as well with any diagnosed illness.
There may or may not be some truth to an underlying role of inflammation related to HIV infection in driving the higher rate of death, but we haven’t really locked that down yet, especially for early infection.
I’m not sure that these results show that HIV infection is the cause of the increased risk for fatal in-hospital acute MI. The risk may be related to HIV, but it may also be related to lifestyles and other risk factors.
Dr. Howard Edelstein is an infectious diseases specialist at Alameda County Medical Center, Oakland, Calif. He reported having no financial disclosures.

|
|
The higher rate of in-hospital MI mortality in HIV-infected patients may be due to risk factors other than HIV infection itself. Many of these patients use illicit drugs and they’re in and out of care, disenfranchised, or don’t have access to medical care. We know that people with very limited resources don’t do as well with any diagnosed illness.
There may or may not be some truth to an underlying role of inflammation related to HIV infection in driving the higher rate of death, but we haven’t really locked that down yet, especially for early infection.
I’m not sure that these results show that HIV infection is the cause of the increased risk for fatal in-hospital acute MI. The risk may be related to HIV, but it may also be related to lifestyles and other risk factors.
Dr. Howard Edelstein is an infectious diseases specialist at Alameda County Medical Center, Oakland, Calif. He reported having no financial disclosures.

|
|
The higher rate of in-hospital MI mortality in HIV-infected patients may be due to risk factors other than HIV infection itself. Many of these patients use illicit drugs and they’re in and out of care, disenfranchised, or don’t have access to medical care. We know that people with very limited resources don’t do as well with any diagnosed illness.
There may or may not be some truth to an underlying role of inflammation related to HIV infection in driving the higher rate of death, but we haven’t really locked that down yet, especially for early infection.
I’m not sure that these results show that HIV infection is the cause of the increased risk for fatal in-hospital acute MI. The risk may be related to HIV, but it may also be related to lifestyles and other risk factors.
Dr. Howard Edelstein is an infectious diseases specialist at Alameda County Medical Center, Oakland, Calif. He reported having no financial disclosures.
SAN FRANCISCO – Hospitalized patients who have an acute MI are 53% more likely to die if they are infected with HIV, based on a secondary analysis of data from the Nationwide Inpatient Sample database.
The mortality rates for in-hospital acute MI were 4.3% for HIV-positive patients and 2.4% for HIV-negative ones, a statistically significant difference.
HIV-positive patients had a greater burden of comorbidities, as evidenced by a mean Charlson’s Comorbidity Index score of 1.14, as compared with HIV-negative patients with an average score of 0.94. Comorbidities that were significantly more prevalent in the HIV-positive cases, compared with HIV-negative cases, included renal disease (13% vs. 5%, respectively), mild liver disease (8% vs. 1%), and heart failure (26% vs. 20%), Dr. Daniel Pearce reported at the meeting, sponsored by the American Society for Microbiology.
He and his associates analyzed data from 1997 to 2006 for nearly 1.5 million adults who were hospitalized for more than a day and had an acute MI. The data approximated a stratified 20% sample of all nonfederal, short-term, general, and specialty hospitals serving adults in the United States.
The hazard ratio for death after MI was 53% higher among the 5,984 HIV-positive patients, compared with those without HIV after adjusting for the effects of age, race, gender, comorbidity, and type of insurance (as a marker for socioeconomic status), Dr. Pearce, of the Riverside County (Calif.) Department of Public Health, and his associates reported in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The HIV-positive group was significantly younger compared with the HIV-negative group (mean ages of 54 and 64 years, respectively), more likely to be male (65% vs. 72%), and more likely to be insured primarily by Medicare or Medicaid (62% vs. 25%).
On the other hand, the HIV-positive cases had lower rates of the most common cardiometabolic risk factors, including hypertension, diabetes, and cardiac arrhythmias. Substance abuse was more prevalent in the HIV-positive group than the HIV-negative group.
Dr. Pearce speculated the higher death rate could be related to the pathological effects of HIV viremia on cardiac vasculature and function.
Dr. Pearce reported having no financial disclosures.
SAN FRANCISCO – Hospitalized patients who have an acute MI are 53% more likely to die if they are infected with HIV, based on a secondary analysis of data from the Nationwide Inpatient Sample database.
The mortality rates for in-hospital acute MI were 4.3% for HIV-positive patients and 2.4% for HIV-negative ones, a statistically significant difference.
HIV-positive patients had a greater burden of comorbidities, as evidenced by a mean Charlson’s Comorbidity Index score of 1.14, as compared with HIV-negative patients with an average score of 0.94. Comorbidities that were significantly more prevalent in the HIV-positive cases, compared with HIV-negative cases, included renal disease (13% vs. 5%, respectively), mild liver disease (8% vs. 1%), and heart failure (26% vs. 20%), Dr. Daniel Pearce reported at the meeting, sponsored by the American Society for Microbiology.
He and his associates analyzed data from 1997 to 2006 for nearly 1.5 million adults who were hospitalized for more than a day and had an acute MI. The data approximated a stratified 20% sample of all nonfederal, short-term, general, and specialty hospitals serving adults in the United States.
The hazard ratio for death after MI was 53% higher among the 5,984 HIV-positive patients, compared with those without HIV after adjusting for the effects of age, race, gender, comorbidity, and type of insurance (as a marker for socioeconomic status), Dr. Pearce, of the Riverside County (Calif.) Department of Public Health, and his associates reported in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The HIV-positive group was significantly younger compared with the HIV-negative group (mean ages of 54 and 64 years, respectively), more likely to be male (65% vs. 72%), and more likely to be insured primarily by Medicare or Medicaid (62% vs. 25%).
On the other hand, the HIV-positive cases had lower rates of the most common cardiometabolic risk factors, including hypertension, diabetes, and cardiac arrhythmias. Substance abuse was more prevalent in the HIV-positive group than the HIV-negative group.
Dr. Pearce speculated the higher death rate could be related to the pathological effects of HIV viremia on cardiac vasculature and function.
Dr. Pearce reported having no financial disclosures.
FROM THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: Hospitalized patients who develop acute MI are 53% more likely to die if they are HIV infected.
Data Source: Results were taken from a secondary analysis of national data on nearly 1.5 million hospitalized adults who had an in-hospital MI, 5,984 of whom had HIV.
Disclosures: Dr. Pearce reported having no financial disclosures.
Apps Proliferate Amid Concerns About Medical Use
DENVER – Do you need a stethoscope, a blood pressure monitor, or a tool to assess cardiac rhythms? There are apps for that. In fact, by recent count there are more than 200,000 applications of technology – or "apps" – available for smartphones or tablet devices, and they’re being used more and more for medical purposes.
Need a convenient way to look up drug interactions, pediatric dosing, or clinical decision rules from guidelines? Or how about a translator, a light to examine a finicky infant’s throat, or a "white board" to draw a picture for your patient? Yup – they’re all in apps, and chances are you already may be using some of these.
Dr. Joshua S. Broder expects an exponential increase in the use of apps in medicine as smartphones and tablets continue to proliferate, but their accuracy needs to be verified and potential problems need to be addressed, he said at the annual meeting of the American College of Emergency Physicians.
Apps will be used increasingly for bedside diagnosis and measurement of hemoglobin or other physiologic parameters. "Some of these tests may be taken over by smartphones in the near future," according to Dr. Broder of Duke University, Durham, N.C.
On the other hand, he cautioned, how do you sterilize a smartphone as you move from one hospital room or patient to another, so that you avoid transmitting infection? There are few independent studies so far testing the accuracy and reliability of medical apps, most of which were designed for lay consumers, not physicians.
The Food and Drug Administration is "very interested" in regulating any apps that might substitute for proven technologies such as stethoscopes or that physicians use as accessories to medical devices that already are regulated, he said. The FDA described its approach to deciding which mobile technology to regulate in a draft report in July 2012.
Even the basic functions of smartphones can be convenient in clinical practice, such as taking photos or videos and transmitting information by text or e-mail, but make sure you protect patient privacy and autonomy in ways that maintain trust and comply with HIPAA, Dr. Broder said.
The Duke University Health System has resolved any issues with HIPAA so that it’s safe for physicians to transmit images and video as long as they’re not sent outside the system. Talk to the HIPAA compliance officer at your medical center to establish the ground rules, he said. You can refresh your memory about which parts of data are considered by HIPAA to be protected information via a University of Miami site.
Dr. Broder reviewed some smartphone functions and apps that may be helpful and others that are not yet ready for medical prime time. Many are available for no cost or for as nominal fee. One study of health and fitness apps suggests that apps costing $0.99 or more tend to be higher quality and more trustworthy than less-expensive ones, he noted (J. Med. Internet Res. 2012;14:e72).
• Sleep: One of his residents swears by "smart alarm clock" apps that claim to use a smartphone’s accelerometer to assess where you are in your sleep cycle (based on your movements in bed) to wake you at a time that will leave you feeling less fatigued. You may set for 6 a.m., but the alarm may wake you at 5:45 a.m. Apps like Sleep Cycle ($0.99) and Sleep as Android have some underlying sleep science behind them, but no independent studies have verified their claims.
• CPR: The accelerometer also is used in the free app PocketCPR to give real-time feedback during CPR on the rate and depth of compression. Its has not been cleared by the FDA for use in humans, however, so the app warns that it’s meant for practice only. One prospective, randomized trial in 1,586 cardiac arrests that happened outside of hospitals found that use by emergency services personnel did not significantly change the likelihood of return of spontaneous circulation or other outcomes (BMJ 2011;342:d512).
• Chest: If you’re trying to teach students and residents about heart and lung sounds, or if you still get confused between mitral regurgitation and aortic stenosis, you might want to have a digital stethoscope app handy. These apps interpret heart and lung sounds heard typically through your smartphone’s microphone, which may not be good enough for clinical use. The Thinklabs Stethoscope app at $70 is pricey, compared with others, but it records sounds directly via the smartphone or through an attached electronic stethoscope.
A case that turns an iPhone into an ECG device has been submitted to the FDA for approval. The AliveCor iPhone ECG is expected to sell for between $100 and $200, compared with the usual price tag of thousands of dollars for conventional ECG machines, according to PC Magazine.
One small prospective study of experimental software that programs an iPhone to detect atrial fibrillation by placing a patient’s finger over the camera lens showed it was 98% sensitive and nearly 100% specific in detecting atrial fibrillation (IEEE Trans. Biomed. Eng. 2012 [doi:10.1109/TBME.2012.2208112]).
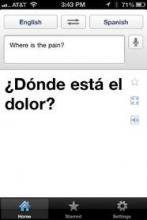
• Translation: When your hospital’s interpreter isn’t available, a free app like Google Translate can help. You can write or speak in one language and your device will write and say the message in a wide selection of language. You’ll need a wireless Internet connection for some translation apps.
Pull up an app like the free EyeChart on your smartphone or tablet.
• Light: You want to inspect a patient’s sore throat, but the light in the exam room is broken. Use the flash on your smartphone camera, or use one of many free "flashlight" apps that turn the smartphone screen into a light source. Be sure to turn it off when you’re done, though, or your battery will run down quickly.
• Ultrasound: The miniaturization of ultrasound devices continues, with systems like the Mobisante MoblUS that attaches a probe to show images on your smartphone screen.
• Skin: For better evaluation of skin lesions, turn your iPhone into a dermatoscope by using the DermScope app ($4.99) and attaching the phone to the DermScope hardware (sold separately).
• Decision support: The PediStat app ($2.99 and up) makes it easy to determine the right pediatric drug dosing, among other features. The free Calculate (Medical Calculator) by QxMD app provides quick intuitive guides to common decision rules and can be customized by medical specialty.
• Drugs: Look up drug dosing, side effects, interactions and other information on free apps from Micromedex and others.
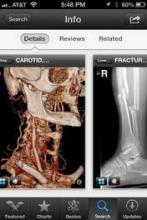
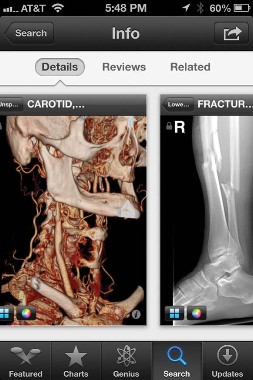
• Photos/videos: These apps are handy for documenting and sharing the appearance of a wound, a patient’s range of motion, or performance on a neurologic exam. Anyone who thinks they see uvula deviation in the throat of a struggling 3-year-old can snap a photo or video for review with other health care providers, medical students, or parents and avoid having to repeat the exam. Images of a wound problem after surgery can be sent to the surgeon when he or she is out of town.
Dr. Broder particularly finds the video useful for children having "pseudoseizures" whose parents demand a neurologic consult, even though the seizure event probably won’t be happening when the neurologist arrives. A video shows the neurologist exactly what Dr. Broder saw. (See the Dos and Don’ts for using photos and videos on the next page.)
Once you’ve got an image or data you want to transmit, avoid texting as first-line means of communication because texts typically are not encrypted. Be careful when e-mailing to make sure it’s going to the correct address and only that address. Use e-mail options such as "confirm delivery" or "request read receipt," and add a sentence to the e-mail saying, "Please delete once no longer necessary for patient care," he advised.
Always document in the patient’s chart that you obtained patient consent and describe what was sent and who received it. Describe any images you send.
Don’t leave images on your portable devices. They’re easily lost, and most have inadequate encryption. Make images part of the medical record by uploading to the patient’s record, printing and scanning, or describing them clearly in the medical record. Then delete them from your device.
Store images and data in "cloud" computing sites with caution, Dr. Broder said. Services such as Google Drive or Dropbox allow sharing of very large files but provide no assurances about the quality of encryption or security. Cloud sites may be best used for giving patients access to instructions, instructional videos, reference papers, anatomic diagrams, etc.
The FDA approved the free Centricity Radiology Mobile Access app, which lets you view CT and MRI images on your iPhone if the images are stored in a GE Centricity PACS (picture archiving and communication system) platform – which may include 20% of U.S. radiology images, according to the company.
The free CloudOn app lets you use MS Office software (including Word, Excel, and Powerpoint) on an iPad.
Various screen replicators that allow you to remotely access your computer desktop from your mobile device (such as ones by Citrix, or Splashtop Remote Desktop) all have the same problem, Dr. Broder said – they’re too clunky and not "touchscreen friendly."
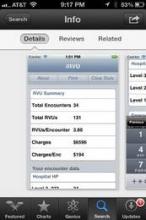
And one final word on an underappreciated perk of medical apps on smartphones: When your medical director stops by, wanting to talk about your productivity, pull out your smartphone to show the data you’ve entered about patient encounters in your free iRVU app, which calculates total RVUs, charges, and average charge per encounter, among other features.
This list only begins to scratch the surface of app use in medicine. Other apps are available for immunization schedules, dictation, infectious disease guides, and teaching aids. Journals provide content to portable devices through apps, and some medical societies offer multifaceted apps such as ACEP Mobile.
Apps on smartphones and tablets will become part of daily medical practice, Dr. Broder predicted, but physicians need to be conscious about their limitations and potential problems as well as their assets.
Dos and Don’ts for Medical Images on Smartphones
Do:
Obtain consent to acquire images or transmit them for the patient’s medical benefit.
Explain to the patient and get consent for any other intended use, such as education or publication.
Tell the patient what you will do with images when their use is completed – delete them or upload them to the medical record.
Confirm receipt if you send to other health care providers.
Specify in your message what that provider should do with the image.
Document in the patient’s chart that consent was obtained, what was sent, who received it, and content of the images.
Don’t:
Obtain images covertly.
Send to any unnecessary recipients.
Show images to anyone for fun.
Post to social media sites.
Blog about "funny" patient encounters.
Dr. Broder owns stock in Apple.
* The photo credits in this story were updated on 10/26/2012.
DENVER – Do you need a stethoscope, a blood pressure monitor, or a tool to assess cardiac rhythms? There are apps for that. In fact, by recent count there are more than 200,000 applications of technology – or "apps" – available for smartphones or tablet devices, and they’re being used more and more for medical purposes.
Need a convenient way to look up drug interactions, pediatric dosing, or clinical decision rules from guidelines? Or how about a translator, a light to examine a finicky infant’s throat, or a "white board" to draw a picture for your patient? Yup – they’re all in apps, and chances are you already may be using some of these.
Dr. Joshua S. Broder expects an exponential increase in the use of apps in medicine as smartphones and tablets continue to proliferate, but their accuracy needs to be verified and potential problems need to be addressed, he said at the annual meeting of the American College of Emergency Physicians.
Apps will be used increasingly for bedside diagnosis and measurement of hemoglobin or other physiologic parameters. "Some of these tests may be taken over by smartphones in the near future," according to Dr. Broder of Duke University, Durham, N.C.
On the other hand, he cautioned, how do you sterilize a smartphone as you move from one hospital room or patient to another, so that you avoid transmitting infection? There are few independent studies so far testing the accuracy and reliability of medical apps, most of which were designed for lay consumers, not physicians.
The Food and Drug Administration is "very interested" in regulating any apps that might substitute for proven technologies such as stethoscopes or that physicians use as accessories to medical devices that already are regulated, he said. The FDA described its approach to deciding which mobile technology to regulate in a draft report in July 2012.
Even the basic functions of smartphones can be convenient in clinical practice, such as taking photos or videos and transmitting information by text or e-mail, but make sure you protect patient privacy and autonomy in ways that maintain trust and comply with HIPAA, Dr. Broder said.
The Duke University Health System has resolved any issues with HIPAA so that it’s safe for physicians to transmit images and video as long as they’re not sent outside the system. Talk to the HIPAA compliance officer at your medical center to establish the ground rules, he said. You can refresh your memory about which parts of data are considered by HIPAA to be protected information via a University of Miami site.
Dr. Broder reviewed some smartphone functions and apps that may be helpful and others that are not yet ready for medical prime time. Many are available for no cost or for as nominal fee. One study of health and fitness apps suggests that apps costing $0.99 or more tend to be higher quality and more trustworthy than less-expensive ones, he noted (J. Med. Internet Res. 2012;14:e72).
• Sleep: One of his residents swears by "smart alarm clock" apps that claim to use a smartphone’s accelerometer to assess where you are in your sleep cycle (based on your movements in bed) to wake you at a time that will leave you feeling less fatigued. You may set for 6 a.m., but the alarm may wake you at 5:45 a.m. Apps like Sleep Cycle ($0.99) and Sleep as Android have some underlying sleep science behind them, but no independent studies have verified their claims.
• CPR: The accelerometer also is used in the free app PocketCPR to give real-time feedback during CPR on the rate and depth of compression. Its has not been cleared by the FDA for use in humans, however, so the app warns that it’s meant for practice only. One prospective, randomized trial in 1,586 cardiac arrests that happened outside of hospitals found that use by emergency services personnel did not significantly change the likelihood of return of spontaneous circulation or other outcomes (BMJ 2011;342:d512).
• Chest: If you’re trying to teach students and residents about heart and lung sounds, or if you still get confused between mitral regurgitation and aortic stenosis, you might want to have a digital stethoscope app handy. These apps interpret heart and lung sounds heard typically through your smartphone’s microphone, which may not be good enough for clinical use. The Thinklabs Stethoscope app at $70 is pricey, compared with others, but it records sounds directly via the smartphone or through an attached electronic stethoscope.
A case that turns an iPhone into an ECG device has been submitted to the FDA for approval. The AliveCor iPhone ECG is expected to sell for between $100 and $200, compared with the usual price tag of thousands of dollars for conventional ECG machines, according to PC Magazine.
One small prospective study of experimental software that programs an iPhone to detect atrial fibrillation by placing a patient’s finger over the camera lens showed it was 98% sensitive and nearly 100% specific in detecting atrial fibrillation (IEEE Trans. Biomed. Eng. 2012 [doi:10.1109/TBME.2012.2208112]).
• Translation: When your hospital’s interpreter isn’t available, a free app like Google Translate can help. You can write or speak in one language and your device will write and say the message in a wide selection of language. You’ll need a wireless Internet connection for some translation apps.
Pull up an app like the free EyeChart on your smartphone or tablet.
• Light: You want to inspect a patient’s sore throat, but the light in the exam room is broken. Use the flash on your smartphone camera, or use one of many free "flashlight" apps that turn the smartphone screen into a light source. Be sure to turn it off when you’re done, though, or your battery will run down quickly.
• Ultrasound: The miniaturization of ultrasound devices continues, with systems like the Mobisante MoblUS that attaches a probe to show images on your smartphone screen.
• Skin: For better evaluation of skin lesions, turn your iPhone into a dermatoscope by using the DermScope app ($4.99) and attaching the phone to the DermScope hardware (sold separately).
• Decision support: The PediStat app ($2.99 and up) makes it easy to determine the right pediatric drug dosing, among other features. The free Calculate (Medical Calculator) by QxMD app provides quick intuitive guides to common decision rules and can be customized by medical specialty.
• Drugs: Look up drug dosing, side effects, interactions and other information on free apps from Micromedex and others.
• Photos/videos: These apps are handy for documenting and sharing the appearance of a wound, a patient’s range of motion, or performance on a neurologic exam. Anyone who thinks they see uvula deviation in the throat of a struggling 3-year-old can snap a photo or video for review with other health care providers, medical students, or parents and avoid having to repeat the exam. Images of a wound problem after surgery can be sent to the surgeon when he or she is out of town.
Dr. Broder particularly finds the video useful for children having "pseudoseizures" whose parents demand a neurologic consult, even though the seizure event probably won’t be happening when the neurologist arrives. A video shows the neurologist exactly what Dr. Broder saw. (See the Dos and Don’ts for using photos and videos on the next page.)
Once you’ve got an image or data you want to transmit, avoid texting as first-line means of communication because texts typically are not encrypted. Be careful when e-mailing to make sure it’s going to the correct address and only that address. Use e-mail options such as "confirm delivery" or "request read receipt," and add a sentence to the e-mail saying, "Please delete once no longer necessary for patient care," he advised.
Always document in the patient’s chart that you obtained patient consent and describe what was sent and who received it. Describe any images you send.
Don’t leave images on your portable devices. They’re easily lost, and most have inadequate encryption. Make images part of the medical record by uploading to the patient’s record, printing and scanning, or describing them clearly in the medical record. Then delete them from your device.
Store images and data in "cloud" computing sites with caution, Dr. Broder said. Services such as Google Drive or Dropbox allow sharing of very large files but provide no assurances about the quality of encryption or security. Cloud sites may be best used for giving patients access to instructions, instructional videos, reference papers, anatomic diagrams, etc.
The FDA approved the free Centricity Radiology Mobile Access app, which lets you view CT and MRI images on your iPhone if the images are stored in a GE Centricity PACS (picture archiving and communication system) platform – which may include 20% of U.S. radiology images, according to the company.
The free CloudOn app lets you use MS Office software (including Word, Excel, and Powerpoint) on an iPad.
Various screen replicators that allow you to remotely access your computer desktop from your mobile device (such as ones by Citrix, or Splashtop Remote Desktop) all have the same problem, Dr. Broder said – they’re too clunky and not "touchscreen friendly."
And one final word on an underappreciated perk of medical apps on smartphones: When your medical director stops by, wanting to talk about your productivity, pull out your smartphone to show the data you’ve entered about patient encounters in your free iRVU app, which calculates total RVUs, charges, and average charge per encounter, among other features.
This list only begins to scratch the surface of app use in medicine. Other apps are available for immunization schedules, dictation, infectious disease guides, and teaching aids. Journals provide content to portable devices through apps, and some medical societies offer multifaceted apps such as ACEP Mobile.
Apps on smartphones and tablets will become part of daily medical practice, Dr. Broder predicted, but physicians need to be conscious about their limitations and potential problems as well as their assets.
Dos and Don’ts for Medical Images on Smartphones
Do:
Obtain consent to acquire images or transmit them for the patient’s medical benefit.
Explain to the patient and get consent for any other intended use, such as education or publication.
Tell the patient what you will do with images when their use is completed – delete them or upload them to the medical record.
Confirm receipt if you send to other health care providers.
Specify in your message what that provider should do with the image.
Document in the patient’s chart that consent was obtained, what was sent, who received it, and content of the images.
Don’t:
Obtain images covertly.
Send to any unnecessary recipients.
Show images to anyone for fun.
Post to social media sites.
Blog about "funny" patient encounters.
Dr. Broder owns stock in Apple.
* The photo credits in this story were updated on 10/26/2012.
DENVER – Do you need a stethoscope, a blood pressure monitor, or a tool to assess cardiac rhythms? There are apps for that. In fact, by recent count there are more than 200,000 applications of technology – or "apps" – available for smartphones or tablet devices, and they’re being used more and more for medical purposes.
Need a convenient way to look up drug interactions, pediatric dosing, or clinical decision rules from guidelines? Or how about a translator, a light to examine a finicky infant’s throat, or a "white board" to draw a picture for your patient? Yup – they’re all in apps, and chances are you already may be using some of these.
Dr. Joshua S. Broder expects an exponential increase in the use of apps in medicine as smartphones and tablets continue to proliferate, but their accuracy needs to be verified and potential problems need to be addressed, he said at the annual meeting of the American College of Emergency Physicians.
Apps will be used increasingly for bedside diagnosis and measurement of hemoglobin or other physiologic parameters. "Some of these tests may be taken over by smartphones in the near future," according to Dr. Broder of Duke University, Durham, N.C.
On the other hand, he cautioned, how do you sterilize a smartphone as you move from one hospital room or patient to another, so that you avoid transmitting infection? There are few independent studies so far testing the accuracy and reliability of medical apps, most of which were designed for lay consumers, not physicians.
The Food and Drug Administration is "very interested" in regulating any apps that might substitute for proven technologies such as stethoscopes or that physicians use as accessories to medical devices that already are regulated, he said. The FDA described its approach to deciding which mobile technology to regulate in a draft report in July 2012.
Even the basic functions of smartphones can be convenient in clinical practice, such as taking photos or videos and transmitting information by text or e-mail, but make sure you protect patient privacy and autonomy in ways that maintain trust and comply with HIPAA, Dr. Broder said.
The Duke University Health System has resolved any issues with HIPAA so that it’s safe for physicians to transmit images and video as long as they’re not sent outside the system. Talk to the HIPAA compliance officer at your medical center to establish the ground rules, he said. You can refresh your memory about which parts of data are considered by HIPAA to be protected information via a University of Miami site.
Dr. Broder reviewed some smartphone functions and apps that may be helpful and others that are not yet ready for medical prime time. Many are available for no cost or for as nominal fee. One study of health and fitness apps suggests that apps costing $0.99 or more tend to be higher quality and more trustworthy than less-expensive ones, he noted (J. Med. Internet Res. 2012;14:e72).
• Sleep: One of his residents swears by "smart alarm clock" apps that claim to use a smartphone’s accelerometer to assess where you are in your sleep cycle (based on your movements in bed) to wake you at a time that will leave you feeling less fatigued. You may set for 6 a.m., but the alarm may wake you at 5:45 a.m. Apps like Sleep Cycle ($0.99) and Sleep as Android have some underlying sleep science behind them, but no independent studies have verified their claims.
• CPR: The accelerometer also is used in the free app PocketCPR to give real-time feedback during CPR on the rate and depth of compression. Its has not been cleared by the FDA for use in humans, however, so the app warns that it’s meant for practice only. One prospective, randomized trial in 1,586 cardiac arrests that happened outside of hospitals found that use by emergency services personnel did not significantly change the likelihood of return of spontaneous circulation or other outcomes (BMJ 2011;342:d512).
• Chest: If you’re trying to teach students and residents about heart and lung sounds, or if you still get confused between mitral regurgitation and aortic stenosis, you might want to have a digital stethoscope app handy. These apps interpret heart and lung sounds heard typically through your smartphone’s microphone, which may not be good enough for clinical use. The Thinklabs Stethoscope app at $70 is pricey, compared with others, but it records sounds directly via the smartphone or through an attached electronic stethoscope.
A case that turns an iPhone into an ECG device has been submitted to the FDA for approval. The AliveCor iPhone ECG is expected to sell for between $100 and $200, compared with the usual price tag of thousands of dollars for conventional ECG machines, according to PC Magazine.
One small prospective study of experimental software that programs an iPhone to detect atrial fibrillation by placing a patient’s finger over the camera lens showed it was 98% sensitive and nearly 100% specific in detecting atrial fibrillation (IEEE Trans. Biomed. Eng. 2012 [doi:10.1109/TBME.2012.2208112]).
• Translation: When your hospital’s interpreter isn’t available, a free app like Google Translate can help. You can write or speak in one language and your device will write and say the message in a wide selection of language. You’ll need a wireless Internet connection for some translation apps.
Pull up an app like the free EyeChart on your smartphone or tablet.
• Light: You want to inspect a patient’s sore throat, but the light in the exam room is broken. Use the flash on your smartphone camera, or use one of many free "flashlight" apps that turn the smartphone screen into a light source. Be sure to turn it off when you’re done, though, or your battery will run down quickly.
• Ultrasound: The miniaturization of ultrasound devices continues, with systems like the Mobisante MoblUS that attaches a probe to show images on your smartphone screen.
• Skin: For better evaluation of skin lesions, turn your iPhone into a dermatoscope by using the DermScope app ($4.99) and attaching the phone to the DermScope hardware (sold separately).
• Decision support: The PediStat app ($2.99 and up) makes it easy to determine the right pediatric drug dosing, among other features. The free Calculate (Medical Calculator) by QxMD app provides quick intuitive guides to common decision rules and can be customized by medical specialty.
• Drugs: Look up drug dosing, side effects, interactions and other information on free apps from Micromedex and others.
• Photos/videos: These apps are handy for documenting and sharing the appearance of a wound, a patient’s range of motion, or performance on a neurologic exam. Anyone who thinks they see uvula deviation in the throat of a struggling 3-year-old can snap a photo or video for review with other health care providers, medical students, or parents and avoid having to repeat the exam. Images of a wound problem after surgery can be sent to the surgeon when he or she is out of town.
Dr. Broder particularly finds the video useful for children having "pseudoseizures" whose parents demand a neurologic consult, even though the seizure event probably won’t be happening when the neurologist arrives. A video shows the neurologist exactly what Dr. Broder saw. (See the Dos and Don’ts for using photos and videos on the next page.)
Once you’ve got an image or data you want to transmit, avoid texting as first-line means of communication because texts typically are not encrypted. Be careful when e-mailing to make sure it’s going to the correct address and only that address. Use e-mail options such as "confirm delivery" or "request read receipt," and add a sentence to the e-mail saying, "Please delete once no longer necessary for patient care," he advised.
Always document in the patient’s chart that you obtained patient consent and describe what was sent and who received it. Describe any images you send.
Don’t leave images on your portable devices. They’re easily lost, and most have inadequate encryption. Make images part of the medical record by uploading to the patient’s record, printing and scanning, or describing them clearly in the medical record. Then delete them from your device.
Store images and data in "cloud" computing sites with caution, Dr. Broder said. Services such as Google Drive or Dropbox allow sharing of very large files but provide no assurances about the quality of encryption or security. Cloud sites may be best used for giving patients access to instructions, instructional videos, reference papers, anatomic diagrams, etc.
The FDA approved the free Centricity Radiology Mobile Access app, which lets you view CT and MRI images on your iPhone if the images are stored in a GE Centricity PACS (picture archiving and communication system) platform – which may include 20% of U.S. radiology images, according to the company.
The free CloudOn app lets you use MS Office software (including Word, Excel, and Powerpoint) on an iPad.
Various screen replicators that allow you to remotely access your computer desktop from your mobile device (such as ones by Citrix, or Splashtop Remote Desktop) all have the same problem, Dr. Broder said – they’re too clunky and not "touchscreen friendly."
And one final word on an underappreciated perk of medical apps on smartphones: When your medical director stops by, wanting to talk about your productivity, pull out your smartphone to show the data you’ve entered about patient encounters in your free iRVU app, which calculates total RVUs, charges, and average charge per encounter, among other features.
This list only begins to scratch the surface of app use in medicine. Other apps are available for immunization schedules, dictation, infectious disease guides, and teaching aids. Journals provide content to portable devices through apps, and some medical societies offer multifaceted apps such as ACEP Mobile.
Apps on smartphones and tablets will become part of daily medical practice, Dr. Broder predicted, but physicians need to be conscious about their limitations and potential problems as well as their assets.
Dos and Don’ts for Medical Images on Smartphones
Do:
Obtain consent to acquire images or transmit them for the patient’s medical benefit.
Explain to the patient and get consent for any other intended use, such as education or publication.
Tell the patient what you will do with images when their use is completed – delete them or upload them to the medical record.
Confirm receipt if you send to other health care providers.
Specify in your message what that provider should do with the image.
Document in the patient’s chart that consent was obtained, what was sent, who received it, and content of the images.
Don’t:
Obtain images covertly.
Send to any unnecessary recipients.
Show images to anyone for fun.
Post to social media sites.
Blog about "funny" patient encounters.
Dr. Broder owns stock in Apple.
* The photo credits in this story were updated on 10/26/2012.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF EMERGENCY PHYSICIANS
Bedside Tools to ID Severe C. difficile Fall Short
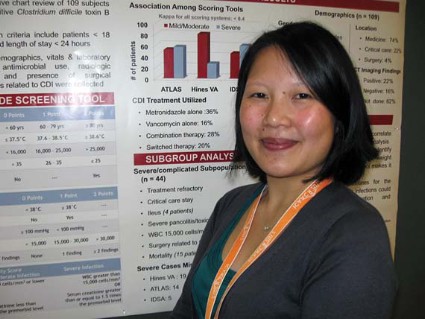
SAN FRANCISCO – A side-by-side comparison of three bedside tools used to identify severe cases of Clostridium difficile infection yielded no clear winner, a reminder that judgment at diagnosis is still the clinician’s best bet.
Criteria from the Infectious Diseases Society of America were more sensitive but the least specific than both the Hines Veterans Affairs (VA) and the ATLAS severity scoring systems, Thien-Ly Doan, Pharm.D. explained in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The Hines VA system for stratifying patients missed 19 of 44 severe/complicated cases of C. difficile infection. The ATLAS scoring system (which incorporates five parameters: age, temperature, leukocytosis, albumin, and systemic concomitant antibiotic use) missed 14 of the 44 cases in a retrospective chart review of 109 patients hospitalized for more than a day with confirmed C. difficile infection.
The IDSA guidelines missed only 5 of the 44 severe/complicated infections, but they cast such a wide net that anyone with a white count above 15,000 cells/mm3 or an elevated creatinine (1.5 times or greater than the premorbid level) is considered to have severe C. difficile infection, she said.
Use of the IDSA guidelines could increase unnecessary use of vancomycin instead of metronidazole, said Dr. Doan, a clinical coordinator at Long Island Jewish Medical Center, New Hyde Park, N.Y.
The IDSA criteria suggested that nearly 60% of the 109 patients had severe infection. However, the 44 severe/complicated C. difficile patients comprised just 40% of the study population. They were defined in the study as patients who were in critical care or whose infections were refractory to treatment and who had ileus, severe pancolitis/toxic megacolon, a WBC of 15,000 cells/mL with hypotension, surgery related to C. difficile infection, or who had died from infection.
Dr. Doan and her associates compared the three stratification systems in evaluating the charts of adults with C. difficile infection at the medical center, who had a mean age of 71 years. A total of 74% of patients were on the medicine service, 22% were in critical care, and 4% were on the surgical service; 34% were female.
The Centers for Disease Control and Prevention also offer severity criteria, but these require the observation of clinical end points and thus are ineffective for assessing patients at initial presentation, she said in a poster presentation at the meeting, sponsored by the American Society for Microbiology.
The Hines VA scoring system, in addition to missing the most severe cases, also gives a great deal of weight to diagnostic imaging, which "makes it impractical at our institution," she said. The Hines VA tool incorporates temperature, the presence of ileus, systolic blood pressure, leukocytosis, and abnormal CT findings to stratify patients by severity.
"We’re going to continue relying on the clinician’s assessment at the bedside at the time of diagnosis to evaluate whether cases are severe or not severe, and not use any of these tools that are available," Dr. Doan said.
A good bedside tool sure would be nice, though, to have a good, objective way of identifying severe C. difficile infection, she added. In a large health system, order sets could be developed based on the tool’s findings "so that everybody would be on the same page in terms of treatment," she said. None of the current tools are good enough for that.
Severe cases of C. difficile are on the rise because of increasing prevalence of the hypervirulent NAP1/BI/027 strain, she noted.
A number of clinicians at the meeting approached her with their own versions of bedside tools for identifying severe C. difficile infection, which Dr. Doan and her associates may evaluate next. They also may compare the tools on different subpopulations of patients with severe infection, such as only patients whose death or surgery was related to C. difficile infection.
Dr. Doan reported having no financial disclosures.
Reported mortality from Clostridium difficile infection (CDI) in the United States has increased dramatically in recent years (Emerg. Infect. Dis. 2007;13: 1417-9). Current guidelines call for the use of oral vancomy-cin as first-line therapy in severe CDI while metronidazole may be used in milder disease (Infect. Control Hosp. Epidemiol. 2010;31:431-55). Thus, it becomes important for therapy to identify those with potentially severe CDI early in their clinical course. However, a systematic review published in 2012 that specifically looked at clinical prediction rules (CPRs) for poor outcomes in CDI concluded that the available tools are inadequate for the task (PLoS One 2012;7:e30258).
The study by Dr. Doan and colleagues assessed the utility of bedside severity-of-illness tools in the treatment of patients with CDI. This was a retrospective chart review of 109 patients hospitalized for more than a day with confirmed CDI. Three CPRs were assessed: The Hines VA system , ; the ATLAS scoring system; and the Infectious Diseases Society of America (IDSA) guidelines. . Sensitivity in detecting severe outcomes of CDI were 57%, 68%, and 89%, respectively. However, the most sensitive CPR, the IDSA guideline, showed poor specificity because it categorized 60% of all subjects as severe. Thus, the IDSA guideline will encourage more widespread use of oral vancomycin in CDI.
Therefore, we lack a risk-scoring system for severe CDI that is easy to use, sensitive, specific, and validated. Such a prediction tool is essential to allow us to follow the current CDI treatment guidelines.
CIARAN P. KELLY, M.D., is director of gastroenterology training and is medical director of the Celiac Center at Beth Israel Deaconess Medical Center, Boston. SAURABH SETHI, M.D., is a fellow in gastroenterology and hepatology at Beth Israe Deaconess. Dr. Kelly reported serving as a consultant or scientific advisor for, being a member of an advisory board for, or receiving research support from many companies developing drugs for C. difficile. Dr. Sethi had no relevant financial disclosures.
Reported mortality from Clostridium difficile infection (CDI) in the United States has increased dramatically in recent years (Emerg. Infect. Dis. 2007;13: 1417-9). Current guidelines call for the use of oral vancomy-cin as first-line therapy in severe CDI while metronidazole may be used in milder disease (Infect. Control Hosp. Epidemiol. 2010;31:431-55). Thus, it becomes important for therapy to identify those with potentially severe CDI early in their clinical course. However, a systematic review published in 2012 that specifically looked at clinical prediction rules (CPRs) for poor outcomes in CDI concluded that the available tools are inadequate for the task (PLoS One 2012;7:e30258).
The study by Dr. Doan and colleagues assessed the utility of bedside severity-of-illness tools in the treatment of patients with CDI. This was a retrospective chart review of 109 patients hospitalized for more than a day with confirmed CDI. Three CPRs were assessed: The Hines VA system , ; the ATLAS scoring system; and the Infectious Diseases Society of America (IDSA) guidelines. . Sensitivity in detecting severe outcomes of CDI were 57%, 68%, and 89%, respectively. However, the most sensitive CPR, the IDSA guideline, showed poor specificity because it categorized 60% of all subjects as severe. Thus, the IDSA guideline will encourage more widespread use of oral vancomycin in CDI.
Therefore, we lack a risk-scoring system for severe CDI that is easy to use, sensitive, specific, and validated. Such a prediction tool is essential to allow us to follow the current CDI treatment guidelines.
CIARAN P. KELLY, M.D., is director of gastroenterology training and is medical director of the Celiac Center at Beth Israel Deaconess Medical Center, Boston. SAURABH SETHI, M.D., is a fellow in gastroenterology and hepatology at Beth Israe Deaconess. Dr. Kelly reported serving as a consultant or scientific advisor for, being a member of an advisory board for, or receiving research support from many companies developing drugs for C. difficile. Dr. Sethi had no relevant financial disclosures.
Reported mortality from Clostridium difficile infection (CDI) in the United States has increased dramatically in recent years (Emerg. Infect. Dis. 2007;13: 1417-9). Current guidelines call for the use of oral vancomy-cin as first-line therapy in severe CDI while metronidazole may be used in milder disease (Infect. Control Hosp. Epidemiol. 2010;31:431-55). Thus, it becomes important for therapy to identify those with potentially severe CDI early in their clinical course. However, a systematic review published in 2012 that specifically looked at clinical prediction rules (CPRs) for poor outcomes in CDI concluded that the available tools are inadequate for the task (PLoS One 2012;7:e30258).
The study by Dr. Doan and colleagues assessed the utility of bedside severity-of-illness tools in the treatment of patients with CDI. This was a retrospective chart review of 109 patients hospitalized for more than a day with confirmed CDI. Three CPRs were assessed: The Hines VA system , ; the ATLAS scoring system; and the Infectious Diseases Society of America (IDSA) guidelines. . Sensitivity in detecting severe outcomes of CDI were 57%, 68%, and 89%, respectively. However, the most sensitive CPR, the IDSA guideline, showed poor specificity because it categorized 60% of all subjects as severe. Thus, the IDSA guideline will encourage more widespread use of oral vancomycin in CDI.
Therefore, we lack a risk-scoring system for severe CDI that is easy to use, sensitive, specific, and validated. Such a prediction tool is essential to allow us to follow the current CDI treatment guidelines.
CIARAN P. KELLY, M.D., is director of gastroenterology training and is medical director of the Celiac Center at Beth Israel Deaconess Medical Center, Boston. SAURABH SETHI, M.D., is a fellow in gastroenterology and hepatology at Beth Israe Deaconess. Dr. Kelly reported serving as a consultant or scientific advisor for, being a member of an advisory board for, or receiving research support from many companies developing drugs for C. difficile. Dr. Sethi had no relevant financial disclosures.
SAN FRANCISCO – A side-by-side comparison of three bedside tools used to identify severe cases of Clostridium difficile infection yielded no clear winner, a reminder that judgment at diagnosis is still the clinician’s best bet.
Criteria from the Infectious Diseases Society of America were more sensitive but the least specific than both the Hines Veterans Affairs (VA) and the ATLAS severity scoring systems, Thien-Ly Doan, Pharm.D. explained in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The Hines VA system for stratifying patients missed 19 of 44 severe/complicated cases of C. difficile infection. The ATLAS scoring system (which incorporates five parameters: age, temperature, leukocytosis, albumin, and systemic concomitant antibiotic use) missed 14 of the 44 cases in a retrospective chart review of 109 patients hospitalized for more than a day with confirmed C. difficile infection.
The IDSA guidelines missed only 5 of the 44 severe/complicated infections, but they cast such a wide net that anyone with a white count above 15,000 cells/mm3 or an elevated creatinine (1.5 times or greater than the premorbid level) is considered to have severe C. difficile infection, she said.
Use of the IDSA guidelines could increase unnecessary use of vancomycin instead of metronidazole, said Dr. Doan, a clinical coordinator at Long Island Jewish Medical Center, New Hyde Park, N.Y.
The IDSA criteria suggested that nearly 60% of the 109 patients had severe infection. However, the 44 severe/complicated C. difficile patients comprised just 40% of the study population. They were defined in the study as patients who were in critical care or whose infections were refractory to treatment and who had ileus, severe pancolitis/toxic megacolon, a WBC of 15,000 cells/mL with hypotension, surgery related to C. difficile infection, or who had died from infection.
Dr. Doan and her associates compared the three stratification systems in evaluating the charts of adults with C. difficile infection at the medical center, who had a mean age of 71 years. A total of 74% of patients were on the medicine service, 22% were in critical care, and 4% were on the surgical service; 34% were female.
The Centers for Disease Control and Prevention also offer severity criteria, but these require the observation of clinical end points and thus are ineffective for assessing patients at initial presentation, she said in a poster presentation at the meeting, sponsored by the American Society for Microbiology.
The Hines VA scoring system, in addition to missing the most severe cases, also gives a great deal of weight to diagnostic imaging, which "makes it impractical at our institution," she said. The Hines VA tool incorporates temperature, the presence of ileus, systolic blood pressure, leukocytosis, and abnormal CT findings to stratify patients by severity.
"We’re going to continue relying on the clinician’s assessment at the bedside at the time of diagnosis to evaluate whether cases are severe or not severe, and not use any of these tools that are available," Dr. Doan said.
A good bedside tool sure would be nice, though, to have a good, objective way of identifying severe C. difficile infection, she added. In a large health system, order sets could be developed based on the tool’s findings "so that everybody would be on the same page in terms of treatment," she said. None of the current tools are good enough for that.
Severe cases of C. difficile are on the rise because of increasing prevalence of the hypervirulent NAP1/BI/027 strain, she noted.
A number of clinicians at the meeting approached her with their own versions of bedside tools for identifying severe C. difficile infection, which Dr. Doan and her associates may evaluate next. They also may compare the tools on different subpopulations of patients with severe infection, such as only patients whose death or surgery was related to C. difficile infection.
Dr. Doan reported having no financial disclosures.
SAN FRANCISCO – A side-by-side comparison of three bedside tools used to identify severe cases of Clostridium difficile infection yielded no clear winner, a reminder that judgment at diagnosis is still the clinician’s best bet.
Criteria from the Infectious Diseases Society of America were more sensitive but the least specific than both the Hines Veterans Affairs (VA) and the ATLAS severity scoring systems, Thien-Ly Doan, Pharm.D. explained in an interview at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The Hines VA system for stratifying patients missed 19 of 44 severe/complicated cases of C. difficile infection. The ATLAS scoring system (which incorporates five parameters: age, temperature, leukocytosis, albumin, and systemic concomitant antibiotic use) missed 14 of the 44 cases in a retrospective chart review of 109 patients hospitalized for more than a day with confirmed C. difficile infection.
The IDSA guidelines missed only 5 of the 44 severe/complicated infections, but they cast such a wide net that anyone with a white count above 15,000 cells/mm3 or an elevated creatinine (1.5 times or greater than the premorbid level) is considered to have severe C. difficile infection, she said.
Use of the IDSA guidelines could increase unnecessary use of vancomycin instead of metronidazole, said Dr. Doan, a clinical coordinator at Long Island Jewish Medical Center, New Hyde Park, N.Y.
The IDSA criteria suggested that nearly 60% of the 109 patients had severe infection. However, the 44 severe/complicated C. difficile patients comprised just 40% of the study population. They were defined in the study as patients who were in critical care or whose infections were refractory to treatment and who had ileus, severe pancolitis/toxic megacolon, a WBC of 15,000 cells/mL with hypotension, surgery related to C. difficile infection, or who had died from infection.
Dr. Doan and her associates compared the three stratification systems in evaluating the charts of adults with C. difficile infection at the medical center, who had a mean age of 71 years. A total of 74% of patients were on the medicine service, 22% were in critical care, and 4% were on the surgical service; 34% were female.
The Centers for Disease Control and Prevention also offer severity criteria, but these require the observation of clinical end points and thus are ineffective for assessing patients at initial presentation, she said in a poster presentation at the meeting, sponsored by the American Society for Microbiology.
The Hines VA scoring system, in addition to missing the most severe cases, also gives a great deal of weight to diagnostic imaging, which "makes it impractical at our institution," she said. The Hines VA tool incorporates temperature, the presence of ileus, systolic blood pressure, leukocytosis, and abnormal CT findings to stratify patients by severity.
"We’re going to continue relying on the clinician’s assessment at the bedside at the time of diagnosis to evaluate whether cases are severe or not severe, and not use any of these tools that are available," Dr. Doan said.
A good bedside tool sure would be nice, though, to have a good, objective way of identifying severe C. difficile infection, she added. In a large health system, order sets could be developed based on the tool’s findings "so that everybody would be on the same page in terms of treatment," she said. None of the current tools are good enough for that.
Severe cases of C. difficile are on the rise because of increasing prevalence of the hypervirulent NAP1/BI/027 strain, she noted.
A number of clinicians at the meeting approached her with their own versions of bedside tools for identifying severe C. difficile infection, which Dr. Doan and her associates may evaluate next. They also may compare the tools on different subpopulations of patients with severe infection, such as only patients whose death or surgery was related to C. difficile infection.
Dr. Doan reported having no financial disclosures.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
HPV Vaccination Held Cost Effective for Boys
SAN FRANCISCO – So few girls are receiving the human papillomavirus vaccine that vaccinating boys is becoming cost effective, according to Dr. Janet A. Englund.
That’s an important message from recent data on human papillomavirus (HPV) vaccination rates and cost-effectiveness studies, Dr. Englund said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Federal data show "quite low uptake in girls" in each of the past 3 years, "which makes it important to push for increased vaccination of boys," not only to protect males against genital warts and HPV-associated cancers but to increase protection for girls, she said.
The percentage of U.S. adolescents who received at least one dose of HPV vaccine increased from 69% in 2010 to 78% in 2011, but the proportion of U.S. girls who got all three recommended doses was only 32% in 2010 and 35% in 2011 (MMWR 2012;61:671-7).
That’s "disappointing" compared with larger increases in the use of other vaccines in U.S. girls in the same time period and in comparison with HPV vaccination coverage in other countries, said Dr. Englund, a specialist in infectious diseases at Seattle Children’s Hospital and professor of pediatrics at the University of Washington, Seattle. She was a member of the Advisory Committee on Immunization Practices (ACIP) during much of the time HPV vaccine issues were discussed.
School-based vaccination programs have produced HPV vaccination series coverage rates of 56%-85% in Canadian provinces, 80%-90% in the United Kingdom, and greater than 90% in Australia. The United States does not have a school-based vaccination program. Four years after the HPV vaccination program was started in Australia, genital warts have nearly disappeared in young women and men, one study found (Sex Transm. Infect. 2011;87:544-7).
In late 2011, ACIP recommended routine use of HPV vaccine in boys aged 11-12 years. Studies have shown that immunogenicity after the quadrivalent vaccine series is more than doubled if males get vaccinated at ages 9-15 years compared with 16-26 years, she said at the meeting, sponsored by the American Society for Microbiology. For both boys and girls, the vaccine is most effective if given prior to becoming sexually active.
In males, pivotal trials found efficacy rates for the quadrivalent HPV vaccine to be 89% in previously uninfected males and 67% regardless of infection status at baseline.
"These vaccines remain quite expensive," but they are cost effective in the United States, she said.
Analyses presented to ACIP in 2009 suggest that the HPV vaccine’s cost per quality-adjusted life year (QALY) gained for 12-year-old girls ranges from $3,000 to $45,000, depending on the model used in the analysis, Dr. Englund said.
If just the prevention of cervical disease is considered, the cost of adding HPV vaccinations for males seems too costly, adding $115,400-$182,400 in cost per QALY. But if the vaccine’s benefits in reducing vulvar and vaginal cancers, genital warts, anal cancer, recurrent respiratory papillomatosis, oropharyngeal cancer, and penile cancer are also considered, the cost per QALY when vaccinating males drops to $24,400-$42,700. In the United States, costs below $50,000 per QALY are generally considered to be cost effective, Dr. Englund said.
Other analyses suggest that vaccinating boys against HPV is cost effective when the immunization rate for girls is low, "which it is in the United States," she said. "Although coverage in females is increasing, we believe that male vaccination would remain cost effective into the foreseeable future." When female coverage reaches high levels, male vaccination likely would not be cost effective.
Between 2004 and 2007, invasive cancers associated with HPV types 16 and 18 were diagnosed in more than 11,000 men and more than 20,000 women in the United States, data presented to ACIP in 2011 showed.
One study that enrolled freshmen at the University of Washington found approximately a 50% cumulative incidence of HPV in males during the next 24 months, she added.
The most common adverse events in males after vaccination are injection-site reactions. Dr. Englund said that serious adverse events have been minimized in her office by having the adolescents sit down for half an hour, or at least 15 minutes, before leaving the office.
Dr. Englund has been a consultant for GlaxoSmithKline (which markets an HPV vaccine) and Novavax and has received research support from Novartis and Chimerix.
Advisory Committee on Vaccination Practices, ACIP,
SAN FRANCISCO – So few girls are receiving the human papillomavirus vaccine that vaccinating boys is becoming cost effective, according to Dr. Janet A. Englund.
That’s an important message from recent data on human papillomavirus (HPV) vaccination rates and cost-effectiveness studies, Dr. Englund said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Federal data show "quite low uptake in girls" in each of the past 3 years, "which makes it important to push for increased vaccination of boys," not only to protect males against genital warts and HPV-associated cancers but to increase protection for girls, she said.
The percentage of U.S. adolescents who received at least one dose of HPV vaccine increased from 69% in 2010 to 78% in 2011, but the proportion of U.S. girls who got all three recommended doses was only 32% in 2010 and 35% in 2011 (MMWR 2012;61:671-7).
That’s "disappointing" compared with larger increases in the use of other vaccines in U.S. girls in the same time period and in comparison with HPV vaccination coverage in other countries, said Dr. Englund, a specialist in infectious diseases at Seattle Children’s Hospital and professor of pediatrics at the University of Washington, Seattle. She was a member of the Advisory Committee on Immunization Practices (ACIP) during much of the time HPV vaccine issues were discussed.
School-based vaccination programs have produced HPV vaccination series coverage rates of 56%-85% in Canadian provinces, 80%-90% in the United Kingdom, and greater than 90% in Australia. The United States does not have a school-based vaccination program. Four years after the HPV vaccination program was started in Australia, genital warts have nearly disappeared in young women and men, one study found (Sex Transm. Infect. 2011;87:544-7).
In late 2011, ACIP recommended routine use of HPV vaccine in boys aged 11-12 years. Studies have shown that immunogenicity after the quadrivalent vaccine series is more than doubled if males get vaccinated at ages 9-15 years compared with 16-26 years, she said at the meeting, sponsored by the American Society for Microbiology. For both boys and girls, the vaccine is most effective if given prior to becoming sexually active.
In males, pivotal trials found efficacy rates for the quadrivalent HPV vaccine to be 89% in previously uninfected males and 67% regardless of infection status at baseline.
"These vaccines remain quite expensive," but they are cost effective in the United States, she said.
Analyses presented to ACIP in 2009 suggest that the HPV vaccine’s cost per quality-adjusted life year (QALY) gained for 12-year-old girls ranges from $3,000 to $45,000, depending on the model used in the analysis, Dr. Englund said.
If just the prevention of cervical disease is considered, the cost of adding HPV vaccinations for males seems too costly, adding $115,400-$182,400 in cost per QALY. But if the vaccine’s benefits in reducing vulvar and vaginal cancers, genital warts, anal cancer, recurrent respiratory papillomatosis, oropharyngeal cancer, and penile cancer are also considered, the cost per QALY when vaccinating males drops to $24,400-$42,700. In the United States, costs below $50,000 per QALY are generally considered to be cost effective, Dr. Englund said.
Other analyses suggest that vaccinating boys against HPV is cost effective when the immunization rate for girls is low, "which it is in the United States," she said. "Although coverage in females is increasing, we believe that male vaccination would remain cost effective into the foreseeable future." When female coverage reaches high levels, male vaccination likely would not be cost effective.
Between 2004 and 2007, invasive cancers associated with HPV types 16 and 18 were diagnosed in more than 11,000 men and more than 20,000 women in the United States, data presented to ACIP in 2011 showed.
One study that enrolled freshmen at the University of Washington found approximately a 50% cumulative incidence of HPV in males during the next 24 months, she added.
The most common adverse events in males after vaccination are injection-site reactions. Dr. Englund said that serious adverse events have been minimized in her office by having the adolescents sit down for half an hour, or at least 15 minutes, before leaving the office.
Dr. Englund has been a consultant for GlaxoSmithKline (which markets an HPV vaccine) and Novavax and has received research support from Novartis and Chimerix.
SAN FRANCISCO – So few girls are receiving the human papillomavirus vaccine that vaccinating boys is becoming cost effective, according to Dr. Janet A. Englund.
That’s an important message from recent data on human papillomavirus (HPV) vaccination rates and cost-effectiveness studies, Dr. Englund said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Federal data show "quite low uptake in girls" in each of the past 3 years, "which makes it important to push for increased vaccination of boys," not only to protect males against genital warts and HPV-associated cancers but to increase protection for girls, she said.
The percentage of U.S. adolescents who received at least one dose of HPV vaccine increased from 69% in 2010 to 78% in 2011, but the proportion of U.S. girls who got all three recommended doses was only 32% in 2010 and 35% in 2011 (MMWR 2012;61:671-7).
That’s "disappointing" compared with larger increases in the use of other vaccines in U.S. girls in the same time period and in comparison with HPV vaccination coverage in other countries, said Dr. Englund, a specialist in infectious diseases at Seattle Children’s Hospital and professor of pediatrics at the University of Washington, Seattle. She was a member of the Advisory Committee on Immunization Practices (ACIP) during much of the time HPV vaccine issues were discussed.
School-based vaccination programs have produced HPV vaccination series coverage rates of 56%-85% in Canadian provinces, 80%-90% in the United Kingdom, and greater than 90% in Australia. The United States does not have a school-based vaccination program. Four years after the HPV vaccination program was started in Australia, genital warts have nearly disappeared in young women and men, one study found (Sex Transm. Infect. 2011;87:544-7).
In late 2011, ACIP recommended routine use of HPV vaccine in boys aged 11-12 years. Studies have shown that immunogenicity after the quadrivalent vaccine series is more than doubled if males get vaccinated at ages 9-15 years compared with 16-26 years, she said at the meeting, sponsored by the American Society for Microbiology. For both boys and girls, the vaccine is most effective if given prior to becoming sexually active.
In males, pivotal trials found efficacy rates for the quadrivalent HPV vaccine to be 89% in previously uninfected males and 67% regardless of infection status at baseline.
"These vaccines remain quite expensive," but they are cost effective in the United States, she said.
Analyses presented to ACIP in 2009 suggest that the HPV vaccine’s cost per quality-adjusted life year (QALY) gained for 12-year-old girls ranges from $3,000 to $45,000, depending on the model used in the analysis, Dr. Englund said.
If just the prevention of cervical disease is considered, the cost of adding HPV vaccinations for males seems too costly, adding $115,400-$182,400 in cost per QALY. But if the vaccine’s benefits in reducing vulvar and vaginal cancers, genital warts, anal cancer, recurrent respiratory papillomatosis, oropharyngeal cancer, and penile cancer are also considered, the cost per QALY when vaccinating males drops to $24,400-$42,700. In the United States, costs below $50,000 per QALY are generally considered to be cost effective, Dr. Englund said.
Other analyses suggest that vaccinating boys against HPV is cost effective when the immunization rate for girls is low, "which it is in the United States," she said. "Although coverage in females is increasing, we believe that male vaccination would remain cost effective into the foreseeable future." When female coverage reaches high levels, male vaccination likely would not be cost effective.
Between 2004 and 2007, invasive cancers associated with HPV types 16 and 18 were diagnosed in more than 11,000 men and more than 20,000 women in the United States, data presented to ACIP in 2011 showed.
One study that enrolled freshmen at the University of Washington found approximately a 50% cumulative incidence of HPV in males during the next 24 months, she added.
The most common adverse events in males after vaccination are injection-site reactions. Dr. Englund said that serious adverse events have been minimized in her office by having the adolescents sit down for half an hour, or at least 15 minutes, before leaving the office.
Dr. Englund has been a consultant for GlaxoSmithKline (which markets an HPV vaccine) and Novavax and has received research support from Novartis and Chimerix.
Advisory Committee on Vaccination Practices, ACIP,
Advisory Committee on Vaccination Practices, ACIP,
EXPERT ANALYSIS FROM THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Higher Dose for Severe C. diff Speeds Response
SAN FRANCISCO – Two small studies suggest that treating severe Clostridium difficile infection with a higher initial dose of vancomycin may work better than the recommended dose of 125 mg every six hours.
The most recent study, presented in a poster at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, surprised the investigators.
"Pharmacodynamically, the concentrations in stool of the standard dose of 125 mg are about 500-1,000 times greater than the MIC [minimum inhibitory concentration]," Yleana T. Garcia, Pharm.D., said in an interview. "So, we have enough concentration in the stool. We wanted to see that we have similar outcomes with patients who are treated with standard doses as with higher doses."
Instead, the retrospective review of 62 patients with severe diarrhea who received oral vancomycin for at least 3 days found that symptoms resolved significantly quicker in 19 patients who got 250 mg every 6 hours compared with 43 patients who got 125 mg every 6 hours, she and her associates reported.
Symptoms resolved by day 3 in nine patients (47%) on the high dose and six patients (15%) on the conventional dose, said Dr. Garcia, a palliative care fellow at the James J. Peters Veterans Affairs Medical Center, Bronx, N.Y.
There also were statistically nonsignificant trends toward a higher likelihood of clinical cure, shorter length of stay, and reduced risk of recurrence in patients with the higher dose. An increased death rate in the higher-dose group also was not statistically significant, and might be due to greater severity of illness at baseline in patients who got the higher dose of vancomycin, she said at the meeting, sponsored by the American Society for Microbiology.
The findings support those of a small prospective study that analyzed levels of vancomycin in feces collected from 15 patients with presumed or confirmed C. difficile infection. Drug concentrations were high in patients who got 250 or 500 mg q.i.d. but were inadequate in at least one patient on the first day of treatment with 125 mg q.i.d. (BMC Infect. Dis. 2010;10:363).
"Higher doses like 250-500 mg may be warranted to reach adequate concentrations in the stool in the first 24-48 hours," Dr. Garcia said. "I’m not saying to use 250 for the whole treatment course, but there may be a role for a loading dose of 250 mg q6 for the first 24-48 hours, and then switching to 125 mg q6 for the remainder of the treatment course. We know that 125 does have adequate fecal concentration; it just may not be adequate on day 1."
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends treating severe C. difficile infection with oral vancomycin 125 mg every 6 hours or using 500 mg every 6 hours for patients with severe disease complicated by ileus, megacolon, or hypotension (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
Severe C. difficile infection generally is defined as the presence of the organism plus leukocytosis with a white blood cell count of 15,000 cells/microL or greater, or a serum creatinine level at least 1.5 times baseline. The study reviewed records of patients who received vancomycin for these indications or hypotension, shock, ileus, megacolon, or evidence of colitis. The study excluded patients who were treated with any other medication besides metronidazole.
The study is continuing in order to increase the number of patients reviewed and the power of the findings.
Dr. Garcia reported having no financial disclosures.
SAN FRANCISCO – Two small studies suggest that treating severe Clostridium difficile infection with a higher initial dose of vancomycin may work better than the recommended dose of 125 mg every six hours.
The most recent study, presented in a poster at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, surprised the investigators.
"Pharmacodynamically, the concentrations in stool of the standard dose of 125 mg are about 500-1,000 times greater than the MIC [minimum inhibitory concentration]," Yleana T. Garcia, Pharm.D., said in an interview. "So, we have enough concentration in the stool. We wanted to see that we have similar outcomes with patients who are treated with standard doses as with higher doses."
Instead, the retrospective review of 62 patients with severe diarrhea who received oral vancomycin for at least 3 days found that symptoms resolved significantly quicker in 19 patients who got 250 mg every 6 hours compared with 43 patients who got 125 mg every 6 hours, she and her associates reported.
Symptoms resolved by day 3 in nine patients (47%) on the high dose and six patients (15%) on the conventional dose, said Dr. Garcia, a palliative care fellow at the James J. Peters Veterans Affairs Medical Center, Bronx, N.Y.
There also were statistically nonsignificant trends toward a higher likelihood of clinical cure, shorter length of stay, and reduced risk of recurrence in patients with the higher dose. An increased death rate in the higher-dose group also was not statistically significant, and might be due to greater severity of illness at baseline in patients who got the higher dose of vancomycin, she said at the meeting, sponsored by the American Society for Microbiology.
The findings support those of a small prospective study that analyzed levels of vancomycin in feces collected from 15 patients with presumed or confirmed C. difficile infection. Drug concentrations were high in patients who got 250 or 500 mg q.i.d. but were inadequate in at least one patient on the first day of treatment with 125 mg q.i.d. (BMC Infect. Dis. 2010;10:363).
"Higher doses like 250-500 mg may be warranted to reach adequate concentrations in the stool in the first 24-48 hours," Dr. Garcia said. "I’m not saying to use 250 for the whole treatment course, but there may be a role for a loading dose of 250 mg q6 for the first 24-48 hours, and then switching to 125 mg q6 for the remainder of the treatment course. We know that 125 does have adequate fecal concentration; it just may not be adequate on day 1."
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends treating severe C. difficile infection with oral vancomycin 125 mg every 6 hours or using 500 mg every 6 hours for patients with severe disease complicated by ileus, megacolon, or hypotension (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
Severe C. difficile infection generally is defined as the presence of the organism plus leukocytosis with a white blood cell count of 15,000 cells/microL or greater, or a serum creatinine level at least 1.5 times baseline. The study reviewed records of patients who received vancomycin for these indications or hypotension, shock, ileus, megacolon, or evidence of colitis. The study excluded patients who were treated with any other medication besides metronidazole.
The study is continuing in order to increase the number of patients reviewed and the power of the findings.
Dr. Garcia reported having no financial disclosures.
SAN FRANCISCO – Two small studies suggest that treating severe Clostridium difficile infection with a higher initial dose of vancomycin may work better than the recommended dose of 125 mg every six hours.
The most recent study, presented in a poster at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy, surprised the investigators.
"Pharmacodynamically, the concentrations in stool of the standard dose of 125 mg are about 500-1,000 times greater than the MIC [minimum inhibitory concentration]," Yleana T. Garcia, Pharm.D., said in an interview. "So, we have enough concentration in the stool. We wanted to see that we have similar outcomes with patients who are treated with standard doses as with higher doses."
Instead, the retrospective review of 62 patients with severe diarrhea who received oral vancomycin for at least 3 days found that symptoms resolved significantly quicker in 19 patients who got 250 mg every 6 hours compared with 43 patients who got 125 mg every 6 hours, she and her associates reported.
Symptoms resolved by day 3 in nine patients (47%) on the high dose and six patients (15%) on the conventional dose, said Dr. Garcia, a palliative care fellow at the James J. Peters Veterans Affairs Medical Center, Bronx, N.Y.
There also were statistically nonsignificant trends toward a higher likelihood of clinical cure, shorter length of stay, and reduced risk of recurrence in patients with the higher dose. An increased death rate in the higher-dose group also was not statistically significant, and might be due to greater severity of illness at baseline in patients who got the higher dose of vancomycin, she said at the meeting, sponsored by the American Society for Microbiology.
The findings support those of a small prospective study that analyzed levels of vancomycin in feces collected from 15 patients with presumed or confirmed C. difficile infection. Drug concentrations were high in patients who got 250 or 500 mg q.i.d. but were inadequate in at least one patient on the first day of treatment with 125 mg q.i.d. (BMC Infect. Dis. 2010;10:363).
"Higher doses like 250-500 mg may be warranted to reach adequate concentrations in the stool in the first 24-48 hours," Dr. Garcia said. "I’m not saying to use 250 for the whole treatment course, but there may be a role for a loading dose of 250 mg q6 for the first 24-48 hours, and then switching to 125 mg q6 for the remainder of the treatment course. We know that 125 does have adequate fecal concentration; it just may not be adequate on day 1."
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends treating severe C. difficile infection with oral vancomycin 125 mg every 6 hours or using 500 mg every 6 hours for patients with severe disease complicated by ileus, megacolon, or hypotension (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
Severe C. difficile infection generally is defined as the presence of the organism plus leukocytosis with a white blood cell count of 15,000 cells/microL or greater, or a serum creatinine level at least 1.5 times baseline. The study reviewed records of patients who received vancomycin for these indications or hypotension, shock, ileus, megacolon, or evidence of colitis. The study excluded patients who were treated with any other medication besides metronidazole.
The study is continuing in order to increase the number of patients reviewed and the power of the findings.
Dr. Garcia reported having no financial disclosures.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
New Gout Guidelines Inspired by Recent Data
The first guidelines on the management of gout from the American College of Rheumatology recommend new ways of using old drugs and changes in prophylaxis strategies, among other things.
The two-part guidelines, published online Sept. 28, should help speed up effective treatment of gout and get physicians to treat patients to a target urate level of less than 6 mg/dL in order to improve symptoms, Dr. John D. FitzGerald said in an interview.
"There has been a fair amount of recent movement on gout medications" including new alternatives to allopurinol and colchicine and new data on how to use those traditional drugs in safer ways, said Dr. FitzGerald, acting chief of the rheumatology division at the University of California, Los Angeles. "It’s a fair number of changes for medications that people had been using for decades."
The documents update previous guidelines from medical organizations in Europe, the Netherlands, and Japan. The new guidelines will be published in October 2012 by the journal Arthritis Care & Research.
Part 1 of the American College of Rheumatology (ACR) guidelines covers nonpharmacologic and pharmacologic approaches to managing hyperuricemia (Arthritis Care Res. 2012;64:1431-46 [doi:10.1002/acr.21772]).
Part 2 addresses prophylaxis and treatment for acute gouty arthritis (Arthritis Care Res. 2012;64:1447-61 [doi.wiley.com/10.1002/acr.21773]).
Dr. FitzGerald and two other co-leaders of the project, Dr. Dinesh Khanna of the University of Michigan, Ann Arbor and Dr. Robert Terkeltaub of the University of California, San Diego, reviewed the medical literature on gout from the 1950s to the present and drew up nine clinical case scenarios commonly seen in practice. A task force panel comprising seven rheumatologists, two primary care physicians, a nephrologist, and a patient representative used the scenarios to create consensus recommendations.
Among the recommendations, for example, on the use of allopurinol is to start at a low dose of 100 mg/day (instead of the common practice of starting with 300 mg/day), or even lower for patients with chronic kidney disease, and then gradually titrate upward every 2-5 weeks. That recommendation supports previous statements from the Food and Drug Administration and the European League Against Rheumatism.
Also, allopurinol therapy should be actively managed and patients followed to make sure the uric acid target is achieved. "You can’t just give a prescription and say your job is done," though some recent studies suggest that many physicians do just that, Dr. FitzGerald said. "The corollary would be if someone gave blood pressure medication and then didn’t follow the patient’s blood pressure. That wouldn’t be seen as good medicine."
Maintenance doses of allopurinol to prevent acute gout attacks can exceed 300 mg even in patients with chronic kidney disease provided there is adequate patient education and monitoring.
A new recommendation drops the starting dose of oral colchicine for acute gout attacks to a loading dose of 1.2 mg, followed by 0.6 mg an hour later, and then starting prophylaxis 12 hours later at dosing of 0.6 mg once or twice daily.
"We used to give up to eight tablets a day," Dr. FitzGerald said. "That is dropped down to three to four tablets at the start of an attacks, because of findings that more colchicine didn’t really help outcomes" and that smaller doses are safer. The authors called this recommendation from ACR "a paradigm shift" that’s in accordance with Food and Drug Administration-approved label language.
Other highlights of the new ACR recommendations include sections on screening for HLA-B*5801 in patients at high risk of severe adverse reaction to allopurinol, combination therapy when target urate levels are not achieved, medication options including new drugs, and more.
Although the reports are titled "Guidelines," the text makes clear that they are expert recommendations and that clinicians are expected to take active roles in choosing the best management strategies for their particular patients. The authors were "very concerned" that the guidelines not be used by third-party payers to restrict access to medications or to promote one drug over another if there isn’t clear evidence to support it, Dr. FitzGerald said.
The methodology of the project precluded evaluations of costs and cost effectiveness, instead focusing on efficacy. So, for example, the guidelines say that allopurinol and febuxostat can be used equivalently in some circumstances, but clinicians need to consider all other aspects of these options including cost, patient preference, and more.
The ACR plans to update the guidelines as new data become available. The task force panel did create specific indications for use of imaging studies because results should be available in the next few years from studies on the use of high-resolution ultrasound and dual-energy CT for patients with gout.
In the United States, gout affects an estimated 4% of adults – more than 8 million people.
"I’m most excited and hopeful about trying to get this out to internal medicine and family practice doctors," Dr. FitzGerald said. "They see more gout than rheumatologists."
Dr. FitzGerald reported having no financial disclosures. Some members of the task force reported financial associations with multiple pharmaceutical companies but, by design, a majority of task force members had no perceived potential conflicts of interest.
Writing guidelines on gout is a difficult task. I think they made a very good effort to cover as many treatment issues as they could.
Most patients with gout in the United States are cared for by primary care physicians. The guidelines will be helpful to both primary practitioners and rheumatologists, but the subtleties may be lost on the general practitioner, whereas the rheumatologist would pick these up right away. The devil is often in the details when it comes to treating gout. If physicians use the guidelines employing a cookbook approach, they might run into some problems.
For instance, the guidelines cover the use of colchicine as a first-line agent for an acute attack: It’s a good choice, but even the randomized controlled trials that have been published on this, especially using the low-dose approach, show that a significant proportion of patients will not respond to this regimen. The guidelines recommend a dosage higher than what has been advised previously for the low-dose colchicine approach. This may actually be a better method, so I hope this will allow primary practitioners to be able identify more people using this approach. But there are definitely going to be people who do not respond to the colchicine.
Another example of where the guidelines may mislead primary care physicians is the recommendation on when to start urate-lowering therapy (ULT). Their indications for starting pharmacologic ULT include an established diagnosis of gouty arthritis and at least two attacks per year. My colleagues and I think that may exclude too many people. Theoretically, you could have a patient with one attack per year who is having gout-related joint damage and, with this criteria, wouldn’t qualify for ULT. A rheumatologist would pick that up right away, but general practitioners who adhere to these guidelines might end up undertreating some patients.
Also, they recommend using adrenocorticotropic hormone (ACTH) for people who cannot take oral medications. Not only is ACTH is extremely expensive, but the Food and Drug Administration has taken gout off the list of indications for ACTH, so I doubt it would be readily available in a real clinical situation.
When the recommendations discuss using prednisone as a prophylactic against gout attacks, they suggest using 10 mg or less. I think that the authors are trying for the best of both worlds and ending up not having either. We generally try to avoid using steroids long term, so the authors suggest using low-dose prednisone; the problem is that 10 mg would probably be ineffective. There are data suggesting that gout prophylaxis requires higher doses, maybe as much as 20 mg/day. You could try 10 mg but I anticipate that it is not going to work very well.
In their defense, were the authors to go into the subtleties and side effects, what to do with a patient with liver or coronary disease, or issues of cost effectiveness, the guidelines would have become an unmanageable length. But the devil is in the details.
That said, it’s a major effort here. It’s good work. They tried to answer a lot of questions.
Dr. Christopher M. Burns is a rheumatologist at the Geisel School of Medicine at Dartmouth, Hanover, N.H. He reported having no financial disclosures.
Writing guidelines on gout is a difficult task. I think they made a very good effort to cover as many treatment issues as they could.
Most patients with gout in the United States are cared for by primary care physicians. The guidelines will be helpful to both primary practitioners and rheumatologists, but the subtleties may be lost on the general practitioner, whereas the rheumatologist would pick these up right away. The devil is often in the details when it comes to treating gout. If physicians use the guidelines employing a cookbook approach, they might run into some problems.
For instance, the guidelines cover the use of colchicine as a first-line agent for an acute attack: It’s a good choice, but even the randomized controlled trials that have been published on this, especially using the low-dose approach, show that a significant proportion of patients will not respond to this regimen. The guidelines recommend a dosage higher than what has been advised previously for the low-dose colchicine approach. This may actually be a better method, so I hope this will allow primary practitioners to be able identify more people using this approach. But there are definitely going to be people who do not respond to the colchicine.
Another example of where the guidelines may mislead primary care physicians is the recommendation on when to start urate-lowering therapy (ULT). Their indications for starting pharmacologic ULT include an established diagnosis of gouty arthritis and at least two attacks per year. My colleagues and I think that may exclude too many people. Theoretically, you could have a patient with one attack per year who is having gout-related joint damage and, with this criteria, wouldn’t qualify for ULT. A rheumatologist would pick that up right away, but general practitioners who adhere to these guidelines might end up undertreating some patients.
Also, they recommend using adrenocorticotropic hormone (ACTH) for people who cannot take oral medications. Not only is ACTH is extremely expensive, but the Food and Drug Administration has taken gout off the list of indications for ACTH, so I doubt it would be readily available in a real clinical situation.
When the recommendations discuss using prednisone as a prophylactic against gout attacks, they suggest using 10 mg or less. I think that the authors are trying for the best of both worlds and ending up not having either. We generally try to avoid using steroids long term, so the authors suggest using low-dose prednisone; the problem is that 10 mg would probably be ineffective. There are data suggesting that gout prophylaxis requires higher doses, maybe as much as 20 mg/day. You could try 10 mg but I anticipate that it is not going to work very well.
In their defense, were the authors to go into the subtleties and side effects, what to do with a patient with liver or coronary disease, or issues of cost effectiveness, the guidelines would have become an unmanageable length. But the devil is in the details.
That said, it’s a major effort here. It’s good work. They tried to answer a lot of questions.
Dr. Christopher M. Burns is a rheumatologist at the Geisel School of Medicine at Dartmouth, Hanover, N.H. He reported having no financial disclosures.
Writing guidelines on gout is a difficult task. I think they made a very good effort to cover as many treatment issues as they could.
Most patients with gout in the United States are cared for by primary care physicians. The guidelines will be helpful to both primary practitioners and rheumatologists, but the subtleties may be lost on the general practitioner, whereas the rheumatologist would pick these up right away. The devil is often in the details when it comes to treating gout. If physicians use the guidelines employing a cookbook approach, they might run into some problems.
For instance, the guidelines cover the use of colchicine as a first-line agent for an acute attack: It’s a good choice, but even the randomized controlled trials that have been published on this, especially using the low-dose approach, show that a significant proportion of patients will not respond to this regimen. The guidelines recommend a dosage higher than what has been advised previously for the low-dose colchicine approach. This may actually be a better method, so I hope this will allow primary practitioners to be able identify more people using this approach. But there are definitely going to be people who do not respond to the colchicine.
Another example of where the guidelines may mislead primary care physicians is the recommendation on when to start urate-lowering therapy (ULT). Their indications for starting pharmacologic ULT include an established diagnosis of gouty arthritis and at least two attacks per year. My colleagues and I think that may exclude too many people. Theoretically, you could have a patient with one attack per year who is having gout-related joint damage and, with this criteria, wouldn’t qualify for ULT. A rheumatologist would pick that up right away, but general practitioners who adhere to these guidelines might end up undertreating some patients.
Also, they recommend using adrenocorticotropic hormone (ACTH) for people who cannot take oral medications. Not only is ACTH is extremely expensive, but the Food and Drug Administration has taken gout off the list of indications for ACTH, so I doubt it would be readily available in a real clinical situation.
When the recommendations discuss using prednisone as a prophylactic against gout attacks, they suggest using 10 mg or less. I think that the authors are trying for the best of both worlds and ending up not having either. We generally try to avoid using steroids long term, so the authors suggest using low-dose prednisone; the problem is that 10 mg would probably be ineffective. There are data suggesting that gout prophylaxis requires higher doses, maybe as much as 20 mg/day. You could try 10 mg but I anticipate that it is not going to work very well.
In their defense, were the authors to go into the subtleties and side effects, what to do with a patient with liver or coronary disease, or issues of cost effectiveness, the guidelines would have become an unmanageable length. But the devil is in the details.
That said, it’s a major effort here. It’s good work. They tried to answer a lot of questions.
Dr. Christopher M. Burns is a rheumatologist at the Geisel School of Medicine at Dartmouth, Hanover, N.H. He reported having no financial disclosures.
The first guidelines on the management of gout from the American College of Rheumatology recommend new ways of using old drugs and changes in prophylaxis strategies, among other things.
The two-part guidelines, published online Sept. 28, should help speed up effective treatment of gout and get physicians to treat patients to a target urate level of less than 6 mg/dL in order to improve symptoms, Dr. John D. FitzGerald said in an interview.
"There has been a fair amount of recent movement on gout medications" including new alternatives to allopurinol and colchicine and new data on how to use those traditional drugs in safer ways, said Dr. FitzGerald, acting chief of the rheumatology division at the University of California, Los Angeles. "It’s a fair number of changes for medications that people had been using for decades."
The documents update previous guidelines from medical organizations in Europe, the Netherlands, and Japan. The new guidelines will be published in October 2012 by the journal Arthritis Care & Research.
Part 1 of the American College of Rheumatology (ACR) guidelines covers nonpharmacologic and pharmacologic approaches to managing hyperuricemia (Arthritis Care Res. 2012;64:1431-46 [doi:10.1002/acr.21772]).
Part 2 addresses prophylaxis and treatment for acute gouty arthritis (Arthritis Care Res. 2012;64:1447-61 [doi.wiley.com/10.1002/acr.21773]).
Dr. FitzGerald and two other co-leaders of the project, Dr. Dinesh Khanna of the University of Michigan, Ann Arbor and Dr. Robert Terkeltaub of the University of California, San Diego, reviewed the medical literature on gout from the 1950s to the present and drew up nine clinical case scenarios commonly seen in practice. A task force panel comprising seven rheumatologists, two primary care physicians, a nephrologist, and a patient representative used the scenarios to create consensus recommendations.
Among the recommendations, for example, on the use of allopurinol is to start at a low dose of 100 mg/day (instead of the common practice of starting with 300 mg/day), or even lower for patients with chronic kidney disease, and then gradually titrate upward every 2-5 weeks. That recommendation supports previous statements from the Food and Drug Administration and the European League Against Rheumatism.
Also, allopurinol therapy should be actively managed and patients followed to make sure the uric acid target is achieved. "You can’t just give a prescription and say your job is done," though some recent studies suggest that many physicians do just that, Dr. FitzGerald said. "The corollary would be if someone gave blood pressure medication and then didn’t follow the patient’s blood pressure. That wouldn’t be seen as good medicine."
Maintenance doses of allopurinol to prevent acute gout attacks can exceed 300 mg even in patients with chronic kidney disease provided there is adequate patient education and monitoring.
A new recommendation drops the starting dose of oral colchicine for acute gout attacks to a loading dose of 1.2 mg, followed by 0.6 mg an hour later, and then starting prophylaxis 12 hours later at dosing of 0.6 mg once or twice daily.
"We used to give up to eight tablets a day," Dr. FitzGerald said. "That is dropped down to three to four tablets at the start of an attacks, because of findings that more colchicine didn’t really help outcomes" and that smaller doses are safer. The authors called this recommendation from ACR "a paradigm shift" that’s in accordance with Food and Drug Administration-approved label language.
Other highlights of the new ACR recommendations include sections on screening for HLA-B*5801 in patients at high risk of severe adverse reaction to allopurinol, combination therapy when target urate levels are not achieved, medication options including new drugs, and more.
Although the reports are titled "Guidelines," the text makes clear that they are expert recommendations and that clinicians are expected to take active roles in choosing the best management strategies for their particular patients. The authors were "very concerned" that the guidelines not be used by third-party payers to restrict access to medications or to promote one drug over another if there isn’t clear evidence to support it, Dr. FitzGerald said.
The methodology of the project precluded evaluations of costs and cost effectiveness, instead focusing on efficacy. So, for example, the guidelines say that allopurinol and febuxostat can be used equivalently in some circumstances, but clinicians need to consider all other aspects of these options including cost, patient preference, and more.
The ACR plans to update the guidelines as new data become available. The task force panel did create specific indications for use of imaging studies because results should be available in the next few years from studies on the use of high-resolution ultrasound and dual-energy CT for patients with gout.
In the United States, gout affects an estimated 4% of adults – more than 8 million people.
"I’m most excited and hopeful about trying to get this out to internal medicine and family practice doctors," Dr. FitzGerald said. "They see more gout than rheumatologists."
Dr. FitzGerald reported having no financial disclosures. Some members of the task force reported financial associations with multiple pharmaceutical companies but, by design, a majority of task force members had no perceived potential conflicts of interest.
The first guidelines on the management of gout from the American College of Rheumatology recommend new ways of using old drugs and changes in prophylaxis strategies, among other things.
The two-part guidelines, published online Sept. 28, should help speed up effective treatment of gout and get physicians to treat patients to a target urate level of less than 6 mg/dL in order to improve symptoms, Dr. John D. FitzGerald said in an interview.
"There has been a fair amount of recent movement on gout medications" including new alternatives to allopurinol and colchicine and new data on how to use those traditional drugs in safer ways, said Dr. FitzGerald, acting chief of the rheumatology division at the University of California, Los Angeles. "It’s a fair number of changes for medications that people had been using for decades."
The documents update previous guidelines from medical organizations in Europe, the Netherlands, and Japan. The new guidelines will be published in October 2012 by the journal Arthritis Care & Research.
Part 1 of the American College of Rheumatology (ACR) guidelines covers nonpharmacologic and pharmacologic approaches to managing hyperuricemia (Arthritis Care Res. 2012;64:1431-46 [doi:10.1002/acr.21772]).
Part 2 addresses prophylaxis and treatment for acute gouty arthritis (Arthritis Care Res. 2012;64:1447-61 [doi.wiley.com/10.1002/acr.21773]).
Dr. FitzGerald and two other co-leaders of the project, Dr. Dinesh Khanna of the University of Michigan, Ann Arbor and Dr. Robert Terkeltaub of the University of California, San Diego, reviewed the medical literature on gout from the 1950s to the present and drew up nine clinical case scenarios commonly seen in practice. A task force panel comprising seven rheumatologists, two primary care physicians, a nephrologist, and a patient representative used the scenarios to create consensus recommendations.
Among the recommendations, for example, on the use of allopurinol is to start at a low dose of 100 mg/day (instead of the common practice of starting with 300 mg/day), or even lower for patients with chronic kidney disease, and then gradually titrate upward every 2-5 weeks. That recommendation supports previous statements from the Food and Drug Administration and the European League Against Rheumatism.
Also, allopurinol therapy should be actively managed and patients followed to make sure the uric acid target is achieved. "You can’t just give a prescription and say your job is done," though some recent studies suggest that many physicians do just that, Dr. FitzGerald said. "The corollary would be if someone gave blood pressure medication and then didn’t follow the patient’s blood pressure. That wouldn’t be seen as good medicine."
Maintenance doses of allopurinol to prevent acute gout attacks can exceed 300 mg even in patients with chronic kidney disease provided there is adequate patient education and monitoring.
A new recommendation drops the starting dose of oral colchicine for acute gout attacks to a loading dose of 1.2 mg, followed by 0.6 mg an hour later, and then starting prophylaxis 12 hours later at dosing of 0.6 mg once or twice daily.
"We used to give up to eight tablets a day," Dr. FitzGerald said. "That is dropped down to three to four tablets at the start of an attacks, because of findings that more colchicine didn’t really help outcomes" and that smaller doses are safer. The authors called this recommendation from ACR "a paradigm shift" that’s in accordance with Food and Drug Administration-approved label language.
Other highlights of the new ACR recommendations include sections on screening for HLA-B*5801 in patients at high risk of severe adverse reaction to allopurinol, combination therapy when target urate levels are not achieved, medication options including new drugs, and more.
Although the reports are titled "Guidelines," the text makes clear that they are expert recommendations and that clinicians are expected to take active roles in choosing the best management strategies for their particular patients. The authors were "very concerned" that the guidelines not be used by third-party payers to restrict access to medications or to promote one drug over another if there isn’t clear evidence to support it, Dr. FitzGerald said.
The methodology of the project precluded evaluations of costs and cost effectiveness, instead focusing on efficacy. So, for example, the guidelines say that allopurinol and febuxostat can be used equivalently in some circumstances, but clinicians need to consider all other aspects of these options including cost, patient preference, and more.
The ACR plans to update the guidelines as new data become available. The task force panel did create specific indications for use of imaging studies because results should be available in the next few years from studies on the use of high-resolution ultrasound and dual-energy CT for patients with gout.
In the United States, gout affects an estimated 4% of adults – more than 8 million people.
"I’m most excited and hopeful about trying to get this out to internal medicine and family practice doctors," Dr. FitzGerald said. "They see more gout than rheumatologists."
Dr. FitzGerald reported having no financial disclosures. Some members of the task force reported financial associations with multiple pharmaceutical companies but, by design, a majority of task force members had no perceived potential conflicts of interest.
UV Light Beat Bleach for C. difficile Decontamination
SAN FRANCISCO – The M.D. Anderson Cancer Center is abandoning bleach for cleaning hospital rooms exposed to Clostridium difficile in favor of a new machine that kills the organism using ultraviolet light.
The machine reduced C. difficile counts as much as, or more than, bleach cleaning in a preliminary prospective trial in 30 hospital rooms previously occupied by patients infected with C. difficile. The machine is a bit more expensive than bleach at a cost of approximately $82,000 (or $3,000-$4,000 per month to lease), but it avoids damage to materials and the toxic environment for workers caused by the use of bleach or other corrosive chemicals, Dr. Shashank S. Ghantoji said in an interview at a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Bleach treatment reduced the average number of colony-forming units of C. difficile from 2.39 before cleaning to 0.71, a 70% reduction in the contamination level. Treatment with the Pulsed Xenon UV machine (PX-UV) reduced the average number of colony-forming units from 22.97 to 1.10, a 95% reduction.
The postcleaning contamination levels were not statistically different between the bleach and PX-UV rooms, Dr. Ghantoji and his associates found. However, PX-UV decontamination is faster than using bleach, Dr. Ghantoji said. "It takes at least 45 minutes to clean a room with bleach, and it’s not good for the patients or the health care professionals," plus admissions staff usually are clamoring for the room to be ready as soon as possible, he said. Cleaning a room using the PX-UV method takes perhaps 15 minutes.
The PX-UV machine has been available for some time, but its adoption depends on how proactive hospital infection control teams are, he added. He said he is aware of at least two medical centers beyond M.D. Anderson that are also using the machine.
In the study, 298 samples were taken before and after cleaning from high-touch surfaces – the bathroom handrail, the bed control panel, the bed rail, the top of the bedside table, and the IV pole control panel or other equipment control panel – and analyzed for C. difficile endospores. Fifteen rooms were cleaned by the conventional method using a 1:10 solution of sodium hypochlorite (bleach), and 15 underwent a visual, nonbleach cleaning of surfaces followed by 15 minutes of treatment with the PX-UV.
With the PX-UV method, housekeeping workers clean the bathroom and place the remote-operated PX-UV in the bathroom with the door shut while they finish cleaning the rest of the room. Then the machine is placed on each side of the bed for 4 minutes of operation with workers gone. Sensors stop the machine if any movement is detected.
It works by emitting ultraviolet C light, which kills C. difficile. And here’s a bonus – it also kills vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, Dr. Ghantoji of the M.D. Anderson Cancer Center, Houston, said at the meeting, sponsored by the American Society for Microbiology.
"The PX-UV method may be a promising alternative to the current standard of decontamination, bleach," he said. Future studies should look at whether the PX-UV method decreases not just endospore counts but transmission of C. difficile, he added.
C. difficile causes more than 300,000 health care–associated infections each year in the United States, incurring $2,500-$3,500 in costs per infection aside from any surgical costs, he estimated. Current guidelines recommend that rooms previously occupied by patients infected with C. difficile be cleaned with a disinfectant registered with the Environmental Protection Agency as effective against the organism.
Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
SAN FRANCISCO – The M.D. Anderson Cancer Center is abandoning bleach for cleaning hospital rooms exposed to Clostridium difficile in favor of a new machine that kills the organism using ultraviolet light.
The machine reduced C. difficile counts as much as, or more than, bleach cleaning in a preliminary prospective trial in 30 hospital rooms previously occupied by patients infected with C. difficile. The machine is a bit more expensive than bleach at a cost of approximately $82,000 (or $3,000-$4,000 per month to lease), but it avoids damage to materials and the toxic environment for workers caused by the use of bleach or other corrosive chemicals, Dr. Shashank S. Ghantoji said in an interview at a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Bleach treatment reduced the average number of colony-forming units of C. difficile from 2.39 before cleaning to 0.71, a 70% reduction in the contamination level. Treatment with the Pulsed Xenon UV machine (PX-UV) reduced the average number of colony-forming units from 22.97 to 1.10, a 95% reduction.
The postcleaning contamination levels were not statistically different between the bleach and PX-UV rooms, Dr. Ghantoji and his associates found. However, PX-UV decontamination is faster than using bleach, Dr. Ghantoji said. "It takes at least 45 minutes to clean a room with bleach, and it’s not good for the patients or the health care professionals," plus admissions staff usually are clamoring for the room to be ready as soon as possible, he said. Cleaning a room using the PX-UV method takes perhaps 15 minutes.
The PX-UV machine has been available for some time, but its adoption depends on how proactive hospital infection control teams are, he added. He said he is aware of at least two medical centers beyond M.D. Anderson that are also using the machine.
In the study, 298 samples were taken before and after cleaning from high-touch surfaces – the bathroom handrail, the bed control panel, the bed rail, the top of the bedside table, and the IV pole control panel or other equipment control panel – and analyzed for C. difficile endospores. Fifteen rooms were cleaned by the conventional method using a 1:10 solution of sodium hypochlorite (bleach), and 15 underwent a visual, nonbleach cleaning of surfaces followed by 15 minutes of treatment with the PX-UV.
With the PX-UV method, housekeeping workers clean the bathroom and place the remote-operated PX-UV in the bathroom with the door shut while they finish cleaning the rest of the room. Then the machine is placed on each side of the bed for 4 minutes of operation with workers gone. Sensors stop the machine if any movement is detected.
It works by emitting ultraviolet C light, which kills C. difficile. And here’s a bonus – it also kills vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, Dr. Ghantoji of the M.D. Anderson Cancer Center, Houston, said at the meeting, sponsored by the American Society for Microbiology.
"The PX-UV method may be a promising alternative to the current standard of decontamination, bleach," he said. Future studies should look at whether the PX-UV method decreases not just endospore counts but transmission of C. difficile, he added.
C. difficile causes more than 300,000 health care–associated infections each year in the United States, incurring $2,500-$3,500 in costs per infection aside from any surgical costs, he estimated. Current guidelines recommend that rooms previously occupied by patients infected with C. difficile be cleaned with a disinfectant registered with the Environmental Protection Agency as effective against the organism.
Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
SAN FRANCISCO – The M.D. Anderson Cancer Center is abandoning bleach for cleaning hospital rooms exposed to Clostridium difficile in favor of a new machine that kills the organism using ultraviolet light.
The machine reduced C. difficile counts as much as, or more than, bleach cleaning in a preliminary prospective trial in 30 hospital rooms previously occupied by patients infected with C. difficile. The machine is a bit more expensive than bleach at a cost of approximately $82,000 (or $3,000-$4,000 per month to lease), but it avoids damage to materials and the toxic environment for workers caused by the use of bleach or other corrosive chemicals, Dr. Shashank S. Ghantoji said in an interview at a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Bleach treatment reduced the average number of colony-forming units of C. difficile from 2.39 before cleaning to 0.71, a 70% reduction in the contamination level. Treatment with the Pulsed Xenon UV machine (PX-UV) reduced the average number of colony-forming units from 22.97 to 1.10, a 95% reduction.
The postcleaning contamination levels were not statistically different between the bleach and PX-UV rooms, Dr. Ghantoji and his associates found. However, PX-UV decontamination is faster than using bleach, Dr. Ghantoji said. "It takes at least 45 minutes to clean a room with bleach, and it’s not good for the patients or the health care professionals," plus admissions staff usually are clamoring for the room to be ready as soon as possible, he said. Cleaning a room using the PX-UV method takes perhaps 15 minutes.
The PX-UV machine has been available for some time, but its adoption depends on how proactive hospital infection control teams are, he added. He said he is aware of at least two medical centers beyond M.D. Anderson that are also using the machine.
In the study, 298 samples were taken before and after cleaning from high-touch surfaces – the bathroom handrail, the bed control panel, the bed rail, the top of the bedside table, and the IV pole control panel or other equipment control panel – and analyzed for C. difficile endospores. Fifteen rooms were cleaned by the conventional method using a 1:10 solution of sodium hypochlorite (bleach), and 15 underwent a visual, nonbleach cleaning of surfaces followed by 15 minutes of treatment with the PX-UV.
With the PX-UV method, housekeeping workers clean the bathroom and place the remote-operated PX-UV in the bathroom with the door shut while they finish cleaning the rest of the room. Then the machine is placed on each side of the bed for 4 minutes of operation with workers gone. Sensors stop the machine if any movement is detected.
It works by emitting ultraviolet C light, which kills C. difficile. And here’s a bonus – it also kills vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, Dr. Ghantoji of the M.D. Anderson Cancer Center, Houston, said at the meeting, sponsored by the American Society for Microbiology.
"The PX-UV method may be a promising alternative to the current standard of decontamination, bleach," he said. Future studies should look at whether the PX-UV method decreases not just endospore counts but transmission of C. difficile, he added.
C. difficile causes more than 300,000 health care–associated infections each year in the United States, incurring $2,500-$3,500 in costs per infection aside from any surgical costs, he estimated. Current guidelines recommend that rooms previously occupied by patients infected with C. difficile be cleaned with a disinfectant registered with the Environmental Protection Agency as effective against the organism.
Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: Bleach killed 70% of C. difficile spores in hospital rooms compared with 95% decontamination using nonbleach cleaning plus UV light treatment. The difference was not statistically significant.
Data Source: A prospective comparison was performed of the two cleaning methods in 30 rooms after discharge of patients infected with C. difficile.
Disclosures: Xenex Healthcare Services, which markets the PX-UV machine, funded the study, and two of the investigators are employees of the company. Dr. Ghantoji reported having no other relevant financial disclosures.
No Child Too Young for Eye Exam
STANFORD, CALIF. – Myths about pediatric eye abnormalities often mislead parents into thinking an eye evaluation is not necessary, according to Dr. Deborah M. Alcorn, chief of pediatric ophthalmology at Lucile Packard Children’s Hospital, Stanford.
At a pediatric update sponsored by Stanford University, she debunked the top 10 myths about eye problems in children:
1. My child is too young for an eye exam. Dr. Alcorn said she frequently gets called to the neonatal unit to evaluate babies who are only a few months old. Infant eye exams are very objective. The child’s age is irrelevant, except that it’s better to treat a problem earlier rather than later, she said.
2. Tearing must be due to a blocked tear duct. Yes, epiphora is most likely to be secondary to a nasolacrimal duct obstruction, but the differential diagnosis includes corneal abrasion and glaucoma.
Placing yellow fluorescein dye in the eye can easily identify nasolacrimal duct obstruction; within 2-3 minutes, the dye will come out the child’s nose. In infants, treatment with warm compresses and massage will open 95% of lacrimal obstructions by 1 year of age.
Persistent, excessive tearing, especially in the first few weeks of life, may be a sign of an associated dacryocystocele. These blue-domed cysts deserve aggressive treatment with systemic antibiotics, warm compresses, and massage, Dr. Alcorn said. Nasolacrimal duct probing may be needed. If the color of the dacryocystocele changes from blue to very red with erythema, hospitalize the child to treat infection, she said.
Tearing and discharge may be due to congenital nasolacrimal duct obstructions. If you see a child with silicone tubing poking out of the tear duct, don’t pull it out – it’s a stent to enlarge the lacrimal duct. "We like to leave it in for 6 months," she said.
Tearing from corneal abrasion is accompanied by pain, discomfort, and often photophobia. If you use a topical anesthetic to make the eye exam easier, do not dispense it to parents, Dr. Alcorn said. Repeated use will break down the child’s cornea. Treat a corneal abrasion with a topical antibiotic; children usually prefer drops to ointment, which blurs vision, she noted.
You may see tearing from congenital glaucoma, which produces big eyes on the baby that seem beautiful but are abnormal. Unlike adult glaucoma, congenital glaucoma deserves emergency surgery, she said.
3. All red eyes are contagious. Viral conjunctivitis is highly contagious, but the causes of red eye are diverse. Subconjunctival hemorrhage usually is benign and spontaneously resolves. Episcleritis usually resolves with eye drop therapy. Eyes may be red from pinguecula or pterygium – hyperkeratotic reactions to the sun that are slow growing and not malignant. "We’re starting to see more of these because kids are not wearing sunglasses," Dr. Alcorn said.
Treat pinguecula and pterygium with sunglasses, artificial tears, and vasoconstrictors, and consider surgical removal if the lesion is interfering with wearing contact lenses or the pterygium involves the visual axis.
Conjunctivitis can be bacterial, viral, or allergic. The two main clues to differentiate the three kinds of conjunctivitis are the discharge (which is purulent in bacterial conjunctivitis and watery in viral or allergic conjunctivitis) and itching (which is marked in allergic conjunctivitis but minimal with the other two forms).
Viral conjunctivitis is so contagious that Dr. Alcorn said she tries to not let these patients into her examination room, in order to avoid having to wash the room with bleach afterward. These patients usually have bilateral red eyes, preauricular lymphadenopathy, conjunctival inflammation, and watery discharge. Upper respiratory infection, sore throat, and fever are common.
This has been one of the worst years for seasonal allergies causing acute allergic conjunctivitis, in Dr. Alcorn’s experience. Bilateral red eyes, profuse pruritus, chemosis, and ropy mucous discharge are typical.
A variety of topical medications in eye drops can be used to treat allergic conjunctivitis. However, do not prescribe a topical steroid or combination antibiotic-steroid without close follow-up, because steroids can cause glaucoma or cataracts or potentiate infection, she said.
4. Children outgrow crossed eyes. "I don’t care how cute your kids are, they should have straight eyes," she said. Refer any patients with constant strabismus before 12 weeks of age or any strabismus at older ages.
An eye cover test is easy to do to diagnose strabismus. With the patient looking straight ahead, cover one eye, peek behind the cover, and you’ll see the eye turn in or drift outward.
Children with a wide nasal bridge, prominent epicanthal folds, or an abnormally small interpupillary distance may seem to have a crossed eye, but really don’t. Dr. Alcorn usually reevaluates children with pseudostrabismus in 6 months "to be sure we didn’t miss something," she said.
Glasses can fix accommodative esotropia (one eye moving toward the other), but must be worn all the time, except when bathing, swimming, or sleeping. Surgery is not indicated.
If the child is unable to abduct the eye (look outward from the nose), there may be an intracranial process or infection. The eye that can’t turn out will always be turned in if there’s a sixth nerve palsy. In contrast, Duane’s syndrome, a congenital miswiring that prevents an eye from looking outward, allows both eyes to look straight ahead.
The cover test also is very valuable in diagnosing exotropia (outward deviation of an eye), which is much less common than esotropia. These patients may need glasses or another intervention, and should be evaluated.
5. A bump on an eye will go away. Sties often go away with treatment, but chalazia (chronic sties) may need surgical removal. Treat both with warm compresses, topical antibiotic ointment, light massage, and daily oral flaxseed oil (generally 1 tablespoon per 100 pounds of body weight). Eventually, if needed, consider incision and curettage or possibly a steroid injection, she said.
Not all lumps and bumps are sties. Capillary hemangiomas occur in 1%-2% of newborns, usually appear by 6 months of age, and involute spontaneously. Congenital hemangiomas can be treated with topical timolol or oral propranolol, and should be monitored regularly; 50% will resolve by 5 years of age and 70% by 7 years. Orbital dermoids will not go away on their own and usually require surgical excision at age 4 or 5 years. Lymphangiomas typically present in the first decade of life and will grow. Rhabdomyosarcomas deserve emergency care – they can double in size in a day.
6. One eye is bigger, but it’s a family trait. A child with one eye bigger than the other deserves evaluation to determine if this is truly globe asymmetry or if there’s another diagnosis, such as microphthalmia, ptosis, congenital glaucoma, or proptosis from a mass pushing the eye out.
7. Glasses worsen a child’s prescription. No one is too young to wear glasses, which will not worsen vision over time, Dr. Alcorn said. Myopia is becoming a worldwide epidemic, especially in Asian populations, according to a recent report (Lancet 2012;379:1739-48).
Other data suggest that myopia is starting at earlier ages and occurring more frequently, she said. There is some literature to support letting children play outside more often to prevent myopia, so they won’t always be looking at things up close. Laser treatment is not approved in the United States for people younger than 21 years because the eyes are still growing.
Myopia is a "major global health concern" because it increases the risk for blindness, glaucoma, retinal detachment, and other problems. "We’re hoping for a cure," she said.
8. Abnormal light reflexes are just a bad picture. When a child has refractive asymmetry on a vision-screening photograph, be concerned. The child may simply need glasses, or could have leukokoria, a cataract, retinoblastoma, or another problem.
9. Different-colored eyes are cute. Maybe they are, but you wouldn’t want to miss an infection or Horner syndrome, which can affect eye color, Dr. Alcorn said.
10. Parents don’t know best. "Listen to parents," she said. "They know their children!"
Dr. Alcorn reported having no relevant financial disclosures.
STANFORD, CALIF. – Myths about pediatric eye abnormalities often mislead parents into thinking an eye evaluation is not necessary, according to Dr. Deborah M. Alcorn, chief of pediatric ophthalmology at Lucile Packard Children’s Hospital, Stanford.
At a pediatric update sponsored by Stanford University, she debunked the top 10 myths about eye problems in children:
1. My child is too young for an eye exam. Dr. Alcorn said she frequently gets called to the neonatal unit to evaluate babies who are only a few months old. Infant eye exams are very objective. The child’s age is irrelevant, except that it’s better to treat a problem earlier rather than later, she said.
2. Tearing must be due to a blocked tear duct. Yes, epiphora is most likely to be secondary to a nasolacrimal duct obstruction, but the differential diagnosis includes corneal abrasion and glaucoma.
Placing yellow fluorescein dye in the eye can easily identify nasolacrimal duct obstruction; within 2-3 minutes, the dye will come out the child’s nose. In infants, treatment with warm compresses and massage will open 95% of lacrimal obstructions by 1 year of age.
Persistent, excessive tearing, especially in the first few weeks of life, may be a sign of an associated dacryocystocele. These blue-domed cysts deserve aggressive treatment with systemic antibiotics, warm compresses, and massage, Dr. Alcorn said. Nasolacrimal duct probing may be needed. If the color of the dacryocystocele changes from blue to very red with erythema, hospitalize the child to treat infection, she said.
Tearing and discharge may be due to congenital nasolacrimal duct obstructions. If you see a child with silicone tubing poking out of the tear duct, don’t pull it out – it’s a stent to enlarge the lacrimal duct. "We like to leave it in for 6 months," she said.
Tearing from corneal abrasion is accompanied by pain, discomfort, and often photophobia. If you use a topical anesthetic to make the eye exam easier, do not dispense it to parents, Dr. Alcorn said. Repeated use will break down the child’s cornea. Treat a corneal abrasion with a topical antibiotic; children usually prefer drops to ointment, which blurs vision, she noted.
You may see tearing from congenital glaucoma, which produces big eyes on the baby that seem beautiful but are abnormal. Unlike adult glaucoma, congenital glaucoma deserves emergency surgery, she said.
3. All red eyes are contagious. Viral conjunctivitis is highly contagious, but the causes of red eye are diverse. Subconjunctival hemorrhage usually is benign and spontaneously resolves. Episcleritis usually resolves with eye drop therapy. Eyes may be red from pinguecula or pterygium – hyperkeratotic reactions to the sun that are slow growing and not malignant. "We’re starting to see more of these because kids are not wearing sunglasses," Dr. Alcorn said.
Treat pinguecula and pterygium with sunglasses, artificial tears, and vasoconstrictors, and consider surgical removal if the lesion is interfering with wearing contact lenses or the pterygium involves the visual axis.
Conjunctivitis can be bacterial, viral, or allergic. The two main clues to differentiate the three kinds of conjunctivitis are the discharge (which is purulent in bacterial conjunctivitis and watery in viral or allergic conjunctivitis) and itching (which is marked in allergic conjunctivitis but minimal with the other two forms).
Viral conjunctivitis is so contagious that Dr. Alcorn said she tries to not let these patients into her examination room, in order to avoid having to wash the room with bleach afterward. These patients usually have bilateral red eyes, preauricular lymphadenopathy, conjunctival inflammation, and watery discharge. Upper respiratory infection, sore throat, and fever are common.
This has been one of the worst years for seasonal allergies causing acute allergic conjunctivitis, in Dr. Alcorn’s experience. Bilateral red eyes, profuse pruritus, chemosis, and ropy mucous discharge are typical.
A variety of topical medications in eye drops can be used to treat allergic conjunctivitis. However, do not prescribe a topical steroid or combination antibiotic-steroid without close follow-up, because steroids can cause glaucoma or cataracts or potentiate infection, she said.
4. Children outgrow crossed eyes. "I don’t care how cute your kids are, they should have straight eyes," she said. Refer any patients with constant strabismus before 12 weeks of age or any strabismus at older ages.
An eye cover test is easy to do to diagnose strabismus. With the patient looking straight ahead, cover one eye, peek behind the cover, and you’ll see the eye turn in or drift outward.
Children with a wide nasal bridge, prominent epicanthal folds, or an abnormally small interpupillary distance may seem to have a crossed eye, but really don’t. Dr. Alcorn usually reevaluates children with pseudostrabismus in 6 months "to be sure we didn’t miss something," she said.
Glasses can fix accommodative esotropia (one eye moving toward the other), but must be worn all the time, except when bathing, swimming, or sleeping. Surgery is not indicated.
If the child is unable to abduct the eye (look outward from the nose), there may be an intracranial process or infection. The eye that can’t turn out will always be turned in if there’s a sixth nerve palsy. In contrast, Duane’s syndrome, a congenital miswiring that prevents an eye from looking outward, allows both eyes to look straight ahead.
The cover test also is very valuable in diagnosing exotropia (outward deviation of an eye), which is much less common than esotropia. These patients may need glasses or another intervention, and should be evaluated.
5. A bump on an eye will go away. Sties often go away with treatment, but chalazia (chronic sties) may need surgical removal. Treat both with warm compresses, topical antibiotic ointment, light massage, and daily oral flaxseed oil (generally 1 tablespoon per 100 pounds of body weight). Eventually, if needed, consider incision and curettage or possibly a steroid injection, she said.
Not all lumps and bumps are sties. Capillary hemangiomas occur in 1%-2% of newborns, usually appear by 6 months of age, and involute spontaneously. Congenital hemangiomas can be treated with topical timolol or oral propranolol, and should be monitored regularly; 50% will resolve by 5 years of age and 70% by 7 years. Orbital dermoids will not go away on their own and usually require surgical excision at age 4 or 5 years. Lymphangiomas typically present in the first decade of life and will grow. Rhabdomyosarcomas deserve emergency care – they can double in size in a day.
6. One eye is bigger, but it’s a family trait. A child with one eye bigger than the other deserves evaluation to determine if this is truly globe asymmetry or if there’s another diagnosis, such as microphthalmia, ptosis, congenital glaucoma, or proptosis from a mass pushing the eye out.
7. Glasses worsen a child’s prescription. No one is too young to wear glasses, which will not worsen vision over time, Dr. Alcorn said. Myopia is becoming a worldwide epidemic, especially in Asian populations, according to a recent report (Lancet 2012;379:1739-48).
Other data suggest that myopia is starting at earlier ages and occurring more frequently, she said. There is some literature to support letting children play outside more often to prevent myopia, so they won’t always be looking at things up close. Laser treatment is not approved in the United States for people younger than 21 years because the eyes are still growing.
Myopia is a "major global health concern" because it increases the risk for blindness, glaucoma, retinal detachment, and other problems. "We’re hoping for a cure," she said.
8. Abnormal light reflexes are just a bad picture. When a child has refractive asymmetry on a vision-screening photograph, be concerned. The child may simply need glasses, or could have leukokoria, a cataract, retinoblastoma, or another problem.
9. Different-colored eyes are cute. Maybe they are, but you wouldn’t want to miss an infection or Horner syndrome, which can affect eye color, Dr. Alcorn said.
10. Parents don’t know best. "Listen to parents," she said. "They know their children!"
Dr. Alcorn reported having no relevant financial disclosures.
STANFORD, CALIF. – Myths about pediatric eye abnormalities often mislead parents into thinking an eye evaluation is not necessary, according to Dr. Deborah M. Alcorn, chief of pediatric ophthalmology at Lucile Packard Children’s Hospital, Stanford.
At a pediatric update sponsored by Stanford University, she debunked the top 10 myths about eye problems in children:
1. My child is too young for an eye exam. Dr. Alcorn said she frequently gets called to the neonatal unit to evaluate babies who are only a few months old. Infant eye exams are very objective. The child’s age is irrelevant, except that it’s better to treat a problem earlier rather than later, she said.
2. Tearing must be due to a blocked tear duct. Yes, epiphora is most likely to be secondary to a nasolacrimal duct obstruction, but the differential diagnosis includes corneal abrasion and glaucoma.
Placing yellow fluorescein dye in the eye can easily identify nasolacrimal duct obstruction; within 2-3 minutes, the dye will come out the child’s nose. In infants, treatment with warm compresses and massage will open 95% of lacrimal obstructions by 1 year of age.
Persistent, excessive tearing, especially in the first few weeks of life, may be a sign of an associated dacryocystocele. These blue-domed cysts deserve aggressive treatment with systemic antibiotics, warm compresses, and massage, Dr. Alcorn said. Nasolacrimal duct probing may be needed. If the color of the dacryocystocele changes from blue to very red with erythema, hospitalize the child to treat infection, she said.
Tearing and discharge may be due to congenital nasolacrimal duct obstructions. If you see a child with silicone tubing poking out of the tear duct, don’t pull it out – it’s a stent to enlarge the lacrimal duct. "We like to leave it in for 6 months," she said.
Tearing from corneal abrasion is accompanied by pain, discomfort, and often photophobia. If you use a topical anesthetic to make the eye exam easier, do not dispense it to parents, Dr. Alcorn said. Repeated use will break down the child’s cornea. Treat a corneal abrasion with a topical antibiotic; children usually prefer drops to ointment, which blurs vision, she noted.
You may see tearing from congenital glaucoma, which produces big eyes on the baby that seem beautiful but are abnormal. Unlike adult glaucoma, congenital glaucoma deserves emergency surgery, she said.
3. All red eyes are contagious. Viral conjunctivitis is highly contagious, but the causes of red eye are diverse. Subconjunctival hemorrhage usually is benign and spontaneously resolves. Episcleritis usually resolves with eye drop therapy. Eyes may be red from pinguecula or pterygium – hyperkeratotic reactions to the sun that are slow growing and not malignant. "We’re starting to see more of these because kids are not wearing sunglasses," Dr. Alcorn said.
Treat pinguecula and pterygium with sunglasses, artificial tears, and vasoconstrictors, and consider surgical removal if the lesion is interfering with wearing contact lenses or the pterygium involves the visual axis.
Conjunctivitis can be bacterial, viral, or allergic. The two main clues to differentiate the three kinds of conjunctivitis are the discharge (which is purulent in bacterial conjunctivitis and watery in viral or allergic conjunctivitis) and itching (which is marked in allergic conjunctivitis but minimal with the other two forms).
Viral conjunctivitis is so contagious that Dr. Alcorn said she tries to not let these patients into her examination room, in order to avoid having to wash the room with bleach afterward. These patients usually have bilateral red eyes, preauricular lymphadenopathy, conjunctival inflammation, and watery discharge. Upper respiratory infection, sore throat, and fever are common.
This has been one of the worst years for seasonal allergies causing acute allergic conjunctivitis, in Dr. Alcorn’s experience. Bilateral red eyes, profuse pruritus, chemosis, and ropy mucous discharge are typical.
A variety of topical medications in eye drops can be used to treat allergic conjunctivitis. However, do not prescribe a topical steroid or combination antibiotic-steroid without close follow-up, because steroids can cause glaucoma or cataracts or potentiate infection, she said.
4. Children outgrow crossed eyes. "I don’t care how cute your kids are, they should have straight eyes," she said. Refer any patients with constant strabismus before 12 weeks of age or any strabismus at older ages.
An eye cover test is easy to do to diagnose strabismus. With the patient looking straight ahead, cover one eye, peek behind the cover, and you’ll see the eye turn in or drift outward.
Children with a wide nasal bridge, prominent epicanthal folds, or an abnormally small interpupillary distance may seem to have a crossed eye, but really don’t. Dr. Alcorn usually reevaluates children with pseudostrabismus in 6 months "to be sure we didn’t miss something," she said.
Glasses can fix accommodative esotropia (one eye moving toward the other), but must be worn all the time, except when bathing, swimming, or sleeping. Surgery is not indicated.
If the child is unable to abduct the eye (look outward from the nose), there may be an intracranial process or infection. The eye that can’t turn out will always be turned in if there’s a sixth nerve palsy. In contrast, Duane’s syndrome, a congenital miswiring that prevents an eye from looking outward, allows both eyes to look straight ahead.
The cover test also is very valuable in diagnosing exotropia (outward deviation of an eye), which is much less common than esotropia. These patients may need glasses or another intervention, and should be evaluated.
5. A bump on an eye will go away. Sties often go away with treatment, but chalazia (chronic sties) may need surgical removal. Treat both with warm compresses, topical antibiotic ointment, light massage, and daily oral flaxseed oil (generally 1 tablespoon per 100 pounds of body weight). Eventually, if needed, consider incision and curettage or possibly a steroid injection, she said.
Not all lumps and bumps are sties. Capillary hemangiomas occur in 1%-2% of newborns, usually appear by 6 months of age, and involute spontaneously. Congenital hemangiomas can be treated with topical timolol or oral propranolol, and should be monitored regularly; 50% will resolve by 5 years of age and 70% by 7 years. Orbital dermoids will not go away on their own and usually require surgical excision at age 4 or 5 years. Lymphangiomas typically present in the first decade of life and will grow. Rhabdomyosarcomas deserve emergency care – they can double in size in a day.
6. One eye is bigger, but it’s a family trait. A child with one eye bigger than the other deserves evaluation to determine if this is truly globe asymmetry or if there’s another diagnosis, such as microphthalmia, ptosis, congenital glaucoma, or proptosis from a mass pushing the eye out.
7. Glasses worsen a child’s prescription. No one is too young to wear glasses, which will not worsen vision over time, Dr. Alcorn said. Myopia is becoming a worldwide epidemic, especially in Asian populations, according to a recent report (Lancet 2012;379:1739-48).
Other data suggest that myopia is starting at earlier ages and occurring more frequently, she said. There is some literature to support letting children play outside more often to prevent myopia, so they won’t always be looking at things up close. Laser treatment is not approved in the United States for people younger than 21 years because the eyes are still growing.
Myopia is a "major global health concern" because it increases the risk for blindness, glaucoma, retinal detachment, and other problems. "We’re hoping for a cure," she said.
8. Abnormal light reflexes are just a bad picture. When a child has refractive asymmetry on a vision-screening photograph, be concerned. The child may simply need glasses, or could have leukokoria, a cataract, retinoblastoma, or another problem.
9. Different-colored eyes are cute. Maybe they are, but you wouldn’t want to miss an infection or Horner syndrome, which can affect eye color, Dr. Alcorn said.
10. Parents don’t know best. "Listen to parents," she said. "They know their children!"
Dr. Alcorn reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM A PEDIATRIC UPDATE SPONSORED BY STANFORD UNIVERSITY
Two Drugs Not Better Than One for C. Difficile
SAN FRANCISCO – If a patient with Clostridium difficile infection is not responding to single-drug treatment, it’s better to switch drugs than to add a second one, a retrospective study suggests.
The chart review encompassed 248 patients at one institution who received at least 24 hours of monotherapy or combination drug therapy for C. difficile infection from 2008 to 2010. After adjustment for confounding factors, the study showed no significant difference in the time to resolution of diarrhea in patients treated with metronidazole, vancomycin, rifaximin, or nitazoxanide alone, compared with patients who got combinations of these drugs, Jessica C. Njoku, Pharm.D., and her associates reported.
The 39 patients who got combination therapy (16%) also showed no significant differences in clinical cure rate, length of hospital stay, or mortality, compared with patients on monotherapy, Dr. Njoku said in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Diarrhea resolved in the monotherapy group in a mean of 5 days, compared with 10 days in the combination therapy group, a difference that seemed to favor monotherapy but was not statistically significant after adjustment for age, severity of C. difficile infection, immunocompetence, and whether it was a first episode or first recurrence, said Dr. Njoku, currently of Baylor University Medical Center, Dallas. She led the study while she was a fellow at the University of Nebraska Medical Center, Omaha.
The findings suggest that switching drugs instead of adding a drug is the best strategy for a patient who is not responding to initial monotherapy because adding a drug wastes resources, she said in an interview.
The study is one of the first to compare combination therapy to monotherapy for C. difficile infection, Dr. Njoku said. She and her associates conducted the study because clinicians were using drug combinations despite advice from an antimicrobial stewardship team against the practice. "We needed evidence," she said at the meeting, which was sponsored by the American Society for Microbiology.
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends oral metronidazole for mild to moderate C. difficile infections or oral vancomycin for severe infection. For C. difficile infection with severe complications, including ileus or toxic megacolon, expert opinion in the guidelines suggests treating with oral and IV vancomycin with the option of a vancomycin enema (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
"In clinical practice, we don’t follow the guidelines to the letter," she noted. Her records review found that some patients in each category of severity received monotherapy and some were treated with drug combinations.
The C. difficile infection was a first episode in 90% of patients and a first recurrence in 10%. A first episode of infection was significantly more common in the monotherapy group (93%), compared with the combination therapy group (29 [74%] of 39 patients).
The monotherapy group also was significantly more likely to have mild to moderate infection (39%), compared with the combination group (3 patients [ 8%]). Patients with severe infection or severe complications were less common in the monotherapy group (55% vs. 6%, respectively) compared with patients in the combination group, 27 (69%) of whom had severe infection and 9 (23%) of whom had severe complications.
Cure, defined as the resolution of diarrhea (no more than two stools per day) by day 10, was achieved in 74% of patients on monotherapy and in 56% on combination therapy. The mean length of hospitalization was 16 days for monotherapy and 13 days for combination therapy. Eight percent of patients on monotherapy died, compared with 18% of patients on combination therapy. These differences between groups were not statistically significant in multivariate analyses.
Although the findings showed there was no benefit from combination therapy, this was a relatively small retrospective study at a single center, Dr. Njoku acknowledged. A larger, prospective, randomized trial comparing monotherapy with drug combinations is warranted, she said.
Patients in the study were 1 year of age or older, were positive for C. difficile toxin, and had symptoms of the infection. The study excluded patients who were pregnant or had C. difficile infection beyond a first recurrence. Exclusion criteria also cited patients with ileostomy and patients who had other enteric pathogens implicated in infectious diarrhea that were isolated in stool samples.
Dr. Njoku reported having no financial disclosures.
SAN FRANCISCO – If a patient with Clostridium difficile infection is not responding to single-drug treatment, it’s better to switch drugs than to add a second one, a retrospective study suggests.
The chart review encompassed 248 patients at one institution who received at least 24 hours of monotherapy or combination drug therapy for C. difficile infection from 2008 to 2010. After adjustment for confounding factors, the study showed no significant difference in the time to resolution of diarrhea in patients treated with metronidazole, vancomycin, rifaximin, or nitazoxanide alone, compared with patients who got combinations of these drugs, Jessica C. Njoku, Pharm.D., and her associates reported.
The 39 patients who got combination therapy (16%) also showed no significant differences in clinical cure rate, length of hospital stay, or mortality, compared with patients on monotherapy, Dr. Njoku said in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Diarrhea resolved in the monotherapy group in a mean of 5 days, compared with 10 days in the combination therapy group, a difference that seemed to favor monotherapy but was not statistically significant after adjustment for age, severity of C. difficile infection, immunocompetence, and whether it was a first episode or first recurrence, said Dr. Njoku, currently of Baylor University Medical Center, Dallas. She led the study while she was a fellow at the University of Nebraska Medical Center, Omaha.
The findings suggest that switching drugs instead of adding a drug is the best strategy for a patient who is not responding to initial monotherapy because adding a drug wastes resources, she said in an interview.
The study is one of the first to compare combination therapy to monotherapy for C. difficile infection, Dr. Njoku said. She and her associates conducted the study because clinicians were using drug combinations despite advice from an antimicrobial stewardship team against the practice. "We needed evidence," she said at the meeting, which was sponsored by the American Society for Microbiology.
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends oral metronidazole for mild to moderate C. difficile infections or oral vancomycin for severe infection. For C. difficile infection with severe complications, including ileus or toxic megacolon, expert opinion in the guidelines suggests treating with oral and IV vancomycin with the option of a vancomycin enema (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
"In clinical practice, we don’t follow the guidelines to the letter," she noted. Her records review found that some patients in each category of severity received monotherapy and some were treated with drug combinations.
The C. difficile infection was a first episode in 90% of patients and a first recurrence in 10%. A first episode of infection was significantly more common in the monotherapy group (93%), compared with the combination therapy group (29 [74%] of 39 patients).
The monotherapy group also was significantly more likely to have mild to moderate infection (39%), compared with the combination group (3 patients [ 8%]). Patients with severe infection or severe complications were less common in the monotherapy group (55% vs. 6%, respectively) compared with patients in the combination group, 27 (69%) of whom had severe infection and 9 (23%) of whom had severe complications.
Cure, defined as the resolution of diarrhea (no more than two stools per day) by day 10, was achieved in 74% of patients on monotherapy and in 56% on combination therapy. The mean length of hospitalization was 16 days for monotherapy and 13 days for combination therapy. Eight percent of patients on monotherapy died, compared with 18% of patients on combination therapy. These differences between groups were not statistically significant in multivariate analyses.
Although the findings showed there was no benefit from combination therapy, this was a relatively small retrospective study at a single center, Dr. Njoku acknowledged. A larger, prospective, randomized trial comparing monotherapy with drug combinations is warranted, she said.
Patients in the study were 1 year of age or older, were positive for C. difficile toxin, and had symptoms of the infection. The study excluded patients who were pregnant or had C. difficile infection beyond a first recurrence. Exclusion criteria also cited patients with ileostomy and patients who had other enteric pathogens implicated in infectious diarrhea that were isolated in stool samples.
Dr. Njoku reported having no financial disclosures.
SAN FRANCISCO – If a patient with Clostridium difficile infection is not responding to single-drug treatment, it’s better to switch drugs than to add a second one, a retrospective study suggests.
The chart review encompassed 248 patients at one institution who received at least 24 hours of monotherapy or combination drug therapy for C. difficile infection from 2008 to 2010. After adjustment for confounding factors, the study showed no significant difference in the time to resolution of diarrhea in patients treated with metronidazole, vancomycin, rifaximin, or nitazoxanide alone, compared with patients who got combinations of these drugs, Jessica C. Njoku, Pharm.D., and her associates reported.
The 39 patients who got combination therapy (16%) also showed no significant differences in clinical cure rate, length of hospital stay, or mortality, compared with patients on monotherapy, Dr. Njoku said in a poster presentation at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
Diarrhea resolved in the monotherapy group in a mean of 5 days, compared with 10 days in the combination therapy group, a difference that seemed to favor monotherapy but was not statistically significant after adjustment for age, severity of C. difficile infection, immunocompetence, and whether it was a first episode or first recurrence, said Dr. Njoku, currently of Baylor University Medical Center, Dallas. She led the study while she was a fellow at the University of Nebraska Medical Center, Omaha.
The findings suggest that switching drugs instead of adding a drug is the best strategy for a patient who is not responding to initial monotherapy because adding a drug wastes resources, she said in an interview.
The study is one of the first to compare combination therapy to monotherapy for C. difficile infection, Dr. Njoku said. She and her associates conducted the study because clinicians were using drug combinations despite advice from an antimicrobial stewardship team against the practice. "We needed evidence," she said at the meeting, which was sponsored by the American Society for Microbiology.
The 2010 update to clinical practice guidelines for C. difficile infection in adults recommends oral metronidazole for mild to moderate C. difficile infections or oral vancomycin for severe infection. For C. difficile infection with severe complications, including ileus or toxic megacolon, expert opinion in the guidelines suggests treating with oral and IV vancomycin with the option of a vancomycin enema (Infect. Control Hosp. Epidemiol. 2010;31:431-55).
"In clinical practice, we don’t follow the guidelines to the letter," she noted. Her records review found that some patients in each category of severity received monotherapy and some were treated with drug combinations.
The C. difficile infection was a first episode in 90% of patients and a first recurrence in 10%. A first episode of infection was significantly more common in the monotherapy group (93%), compared with the combination therapy group (29 [74%] of 39 patients).
The monotherapy group also was significantly more likely to have mild to moderate infection (39%), compared with the combination group (3 patients [ 8%]). Patients with severe infection or severe complications were less common in the monotherapy group (55% vs. 6%, respectively) compared with patients in the combination group, 27 (69%) of whom had severe infection and 9 (23%) of whom had severe complications.
Cure, defined as the resolution of diarrhea (no more than two stools per day) by day 10, was achieved in 74% of patients on monotherapy and in 56% on combination therapy. The mean length of hospitalization was 16 days for monotherapy and 13 days for combination therapy. Eight percent of patients on monotherapy died, compared with 18% of patients on combination therapy. These differences between groups were not statistically significant in multivariate analyses.
Although the findings showed there was no benefit from combination therapy, this was a relatively small retrospective study at a single center, Dr. Njoku acknowledged. A larger, prospective, randomized trial comparing monotherapy with drug combinations is warranted, she said.
Patients in the study were 1 year of age or older, were positive for C. difficile toxin, and had symptoms of the infection. The study excluded patients who were pregnant or had C. difficile infection beyond a first recurrence. Exclusion criteria also cited patients with ileostomy and patients who had other enteric pathogens implicated in infectious diarrhea that were isolated in stool samples.
Dr. Njoku reported having no financial disclosures.
AT THE ANNUAL INTERSCIENCE CONFERENCE ON ANTIMICROBIAL AGENTS AND CHEMOTHERAPY
Major Finding: C. difficile–associated diarrhea resolved after a mean of 5 days of single-drug treatment or 10 days of combination drug treatment, a difference that was not statistically significant after adjustment for the effects of other factors.
Data Source: Chart review of 248 patients treated at one institution.
Disclosures: Dr. Njoku reported having no financial disclosures.