User login
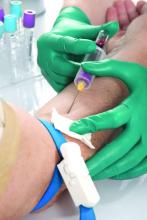
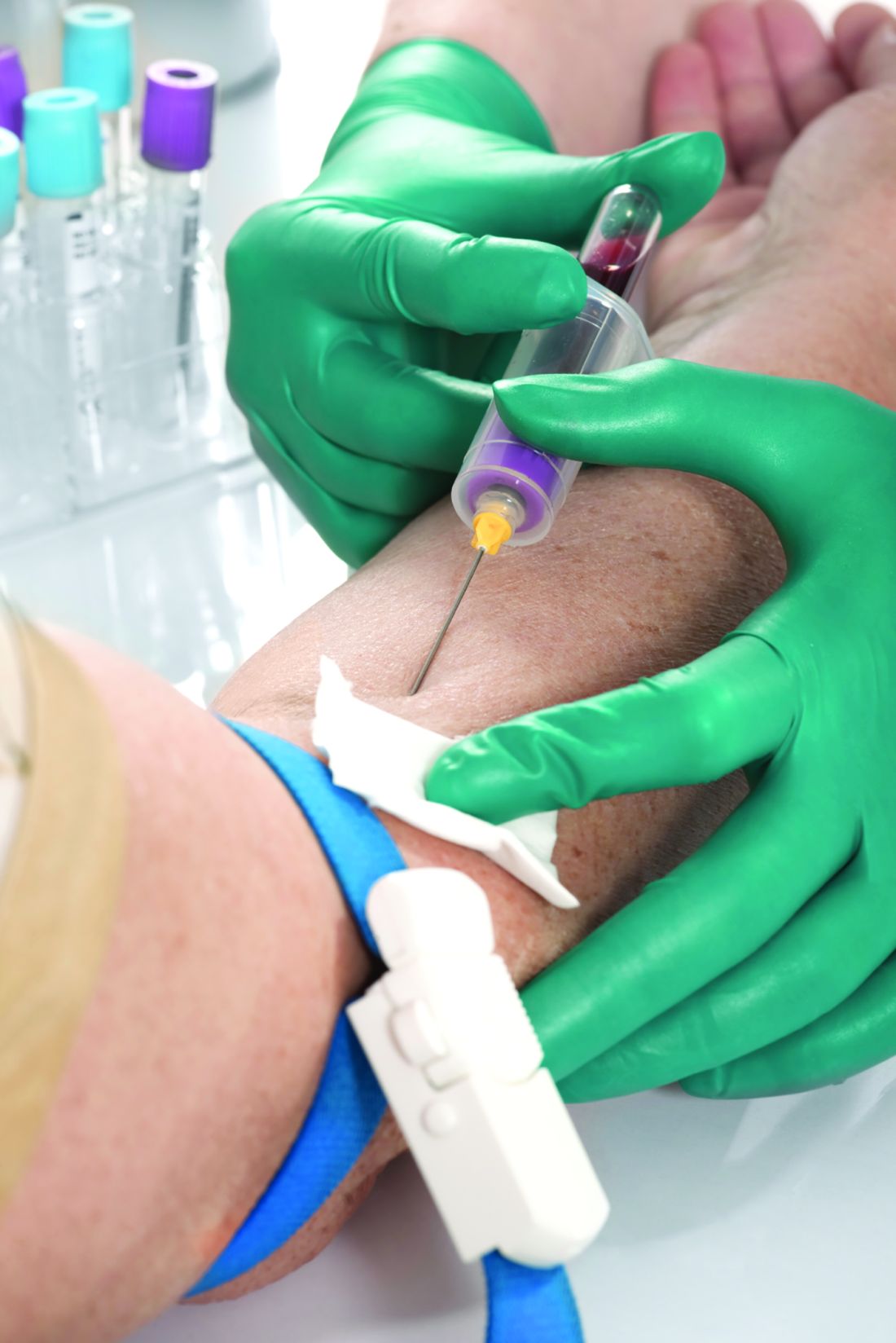
Methotrexate plus leflunomide proves effective for PsA
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new study has found that methotrexate plus leflunomide outperforms methotrexate alone as a treatment option for patients with psoriatic arthritis (PsA).
“We believe that prescribing this combination in routine practice is viable when combined with shared decision-making and strict monitoring of side effects,” write Michelle L.M. Mulder, MD, of the department of rheumatology at Sint Maartenskliniek in Nijmegen, the Netherlands, and her coauthors. Their findings were published in The Lancet Rheumatology.
The latest treatment guidelines from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European Alliance of Associations for Rheumatology recommend conventional synthetic disease-modifying antirheumatic drugs for patients with active PsA, but Dr. Mulder and her colleagues note a distinct lack of information on their effectiveness, especially this particular combination.
To assess the efficacy and safety of methotrexate plus leflunomide, they launched a single-center, double-blind, randomized trial that included 78 Dutch patients with PsA. The majority of the participants in this trial – dubbed COMPLETE-PsA – were men (64%), and the median age of the patients was 55 years. All had active disease at baseline; the median swollen joint count (SJC) and tender joint count were 4.0 in both groups.
Participants were assigned to receive either methotrexate plus leflunomide (n = 39) or methotrexate plus placebo (n = 39). After 16 weeks, mean Psoriatic Arthritis Disease Activity Score (PASDAS) had improved for patients in the combination therapy group in comparison with the monotherapy group (3.1; standard deviation, 1.4 vs. 3.7; SD, 1.3; treatment difference, –0.6; 90% confidence interval, –1.0 to –0.1; P = .025). The combination therapy group also achieved PASDAS low disease activity at a higher rate (59%) than did the monotherapy group (34%; P = .019).
Other notable differences after 16 weeks included improvements in SJC for 66 joints (–3.0 in the combination therapy group vs. –2.0 in the monotherapy group) and significantly better skin and nail measures – such as active psoriasis and change in body surface area – in the methotrexate plus leflunomide group.
When asked who should be prescribed the combination therapy and who should be prescribed methotrexate going forward, Dr. Mulder told this news organization, “At the moment, we have insufficient knowledge on who will benefit most or who will develop clinically relevant side effects. It seems warranted to discuss with every patient which approach they would prefer. This could be a step-down or -up approach.
“We hope to be able to better predict treatment response and side effects in the future via post hoc analysis of our study and via extensive flow-cytometric phenotyping of immune blood cells taken at baseline,” she added.
Three patients in the combination therapy group experienced serious adverse events, two of which were deemed unrelated to leflunomide. The most frequently occurring adverse events were nausea or vomiting, tiredness, and elevated alanine aminotransferase. Mild adverse events were more common in the methotrexate plus leflunomide group. No participants died, and all patients with adverse events recovered completely.
“It appears good practice to do blood draws for laboratory tests on liver enzymes at least monthly for the first 4 months and every 4 months after that once stable dosing is achieved, as well as have a telephone consultation after 6-8 weeks to talk about possible side effects a patient might experience and change or add therapy if necessary,” Dr. Mulder added.
Study turns perception of combination therapy into reality
It had already been perceived by rheumatologists that methotrexate plus leflunomide was an effective combo for PsA, and this study reinforces those beliefs, Clementina López-Medina, MD, PhD, and colleagues from the University of Cordoba (Spain), write in an accompanying editorial.
They highlight this study’s notable strengths, one of which was defining “active disease” as two or more swollen joints, which opened the study up to a larger patient population. The editorialists also underline the confirmation that leflunomide plus methotrexate reduces both joint symptoms and skin involvement in this subset of patients, which had also been found in a previous study.
“Leflunomide is usually considered as a second-line option after methotrexate is unsuccessful,” they note, “despite the fact that methotrexate did not show superiority over placebo in previous trials.”
The editorialists were not surprised that the combination therapy was more toxic than the monotherapy. Rheumatologists could use these data to individualize treatment accordingly, they write, while keeping an eye on “gastrointestinal disturbances.”
Overall, Dr. López-Medina and colleagues say that the study results should “be considered not only in daily clinical practice but also in the development of future recommendations.”
Leflunomide: Forgotten no longer, at least for PsA
“I think we probably underutilize leflunomide,” Arthur Kavanaugh, MD, professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, told this news organization. “Sometimes medicines get ‘old,’ for lack of a better term, and fall a little bit of out of favor, sometimes unnecessarily. Leflunomide falls into that category. Because it’s older, it doesn’t get as much buzz as what’s new and shiny.
“I was not surprised by the results on the joints,” he said, “because we know from previous studies that leflunomide works in that regard. What did surprise me is that the skin got better, especially with the combination.”
Regarding the side effects for the combination therapy, he commended the authors for limiting potential uncertainty by using such a high dose of methotrexate.
“By going with a dose of 25 mg [per week], no one can say, ‘They pulled their punches and methotrexate monotherapy would’ve been just as good if it was given at a higher dose,’ “ he said. “And they also used leflunomide at a high dose. It makes you wonder: Could you use lower doses, and do lower doses mean fewer lab test abnormalities? This positive study does lend itself to some other permutations in terms of study design.
“Even though this was a small study,” he added, “it brings us right back to: We should really consider leflunomide in the treatment of PsA.”
The authors acknowledge their study’s limitations, including the fact that it was conducted in a single country and the absence of a nontreatment placebo group. They also note the higher percentage of women in the methotrexate plus leflunomide group, “which might have lowered the treatment response and increased the adverse event rate, resulting in bias.”
The study was funded by a Regional Junior Researcher Grant from Sint Maartenskliniek. The authors reported numerous potential conflicts of interest, including receiving payment, research grants, and consulting and speaker fees from various pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
EULAR CVD management guidance focuses on gout, lupus, vasculitis
New recommendations from the European Alliance of Associations for Rheumatology provide both broad and detailed advice for cardiovascular risk management in various rheumatic and musculoskeletal diseases (RMDs), many of which can lead to an increased possibility of cardiovascular disease (CVD).
“The panel believes that these recommendations will enable health care providers and patients to mutually engage in a long-term care pathway tailored to patients’ needs and expectations for improving cardiovascular health in RMDs,” write George C. Drosos, National and Kapodistrian University of Athens, and colleagues. The recommendations were published in February in Annals of the Rheumatic Diseases).
EULAR assembled a task force to generate best practices for preventing CVD in patients with gout, vasculitis, systemic sclerosis (SSc), myositis, mixed connective tissue disease (MCTD), Sjögren syndrome (SS), systemic lupus erythematosus (SLE), and antiphospholipid syndrome (APS).
The cardiovascular risk management of patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis was covered in prior EULAR recommendations.
The task force included 20 members from 11 European countries, including 12 rheumatologists, 2 cardiologists, 1 metabolic medicine physician, 1 health care professional, 2 patient representatives, and 2 EMEUNET (Emerging EULAR Network) members. One group of task force members conducted a systematic literature review of 105 articles about gout, vasculitis, SSc, myositis, MCTD, and SS, and another group evaluated 75 articles about SLE and APS. Together, they decided on four overarching principles:
Clinicians need to be aware of increased cardiovascular risk in patients with RMDs, with disease reduction likely decreasing risk.
Rheumatologists – in tandem with other health care providers – are responsible for their patients’ cardiovascular risk assessment and management.
Screening for cardiovascular risk should be performed regularly in all patients with RMDs, with an emphasis on factors like smoking and blood pressure management.
Patient education and counseling on cardiovascular risk, including important lifestyle modifications, is key for RMD patients.
Specific recommendations from the gout, vasculitis, SSc, myositis, MCTD, and SS group include deploying existing cardiovascular prediction tools as they are used in the general population, with the European Vasculitis Society model suggesting to supplement the Framingham Risk Score for patients with antineutrophil cytoplasmic antibody–associated vasculitis. They also recommended avoiding diuretics in patients with gout and beta-blockers in patients with SSc, as well as following the same blood pressure and lipid management strategies that are used among the general population.
Recommendations from the SLE and APS group include thoroughly assessing traditional cardiovascular risk factors in all patients, following typical blood pressure management strategies in patients with APS, and setting a blood pressure target of less than 130/80 mm Hg in patients with SLE. They also recommended administering the lowest possible glucocorticoid dose in patients with SLE, along with treatment with hydroxychloroquine – unless contraindicated – and even common preventive strategies like low-dose aspirin if it suits their cardiovascular risk profile.
As for next steps, the task force noted several areas where additional focus is needed, such as identifying patient subgroups with increased cardiovascular risk. This could include patients with a longer disease duration or more flare-ups, older patients, and those with certain disease characteristics like antiphospholipid positivity in SLE.
Can EULAR’s recommendations be implemented in U.S. rheumatology practices?
“We have been hearing for years that patients with rheumatic diseases have an increased risk of cardiovascular disease,” Ali A. Duarte Garcia, MD, a rheumatologist at the Mayo Clinic in Rochester, Minn., told this news organization. “That has been consistently published for more than a decade now. But any further guidance about it has not been issued. I think there was a void there.”
“Certainly, cardiovascular disease risk in rheumatoid and psoriatic arthritis has been front of mind for the last decade or so,” Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, said when asked to comment on the recommendations. “But in some of these other conditions, it hasn’t been.”
When asked if rheumatologists would be ready and willing to implement these recommendations, Dr. Duarte Garcia acknowledged that it could be challenging for some.
“It’s a different workflow,” he said. “You’ve been trained traditionally to assess inflammation, to keep the disease under control, which is something they recommend, by the way. If you control the disease, patients do better. But I think lipid screening, for example, and testing for cholesterol, smoking cessation, those well-established programs are harder to bring to a rheumatology clinic. It’s doable, but it’s something that needs to be implemented within the current workflows and could take a few years to take hold.”
Dr. Bartels, however, noted that her group has done extensive work over the last 5 years incorporating certain interventions into practice, including sending patients with high blood pressure back to primary care.
“It’s a sustainable intervention in our clinic that basically our medical assistants and nurses do as a routine operation,” she said. “Our primary care providers are grateful to get these patients back. Our patients are grateful because otherwise when they come to the rheumatologist, get their blood pressure measured, and don’t get feedback, they assume they’re OK. So, we’re giving them a false signal.
“We have a similar intervention with smoking,” she added. “Often our patients aren’t even aware that they’re at increased risk of cardiovascular disease or that smoking might make their rheumatic disease and their cardiovascular outcomes worse. No one has had that conversation with them. They really welcome engaging in those discussions.
“Our tobacco intervention takes 90 seconds at point of care. Our blood pressure intervention at point of care, we’ve timed it, takes 3 minutes. There are ways that we can hardwire this into care.”
Along those lines, Dr. Duarte Garcia stated that the recommendations – although released by EULAR – are largely intuitive and should be very adaptable to an American health care context. He also recognized this moment as an opportunity for rheumatologists to consider patient outcomes beyond what they usually encounter firsthand.
“I don’t think we have many rheumatologists with patients who get a stroke or heart attack because if that happens, it’s in a hospital context or they go see a cardiologist,” he said. “You may see it once it happens if they survive and come and see you – or perhaps if you’re in a more integrated practice – but I don’t think it’s as apparent in our clinics because it is a predominantly outpatient practice and many times those are emergencies or inpatient complications.
“The bottom line,” he added, “is these are practical guidelines. It’s a push in the right direction, but there is still work to be done. And hopefully some of the recommendations, like measuring high blood pressure and addressing it just as in the general population, are something we can start to implement.”
Dr. Duarte Garcia reported receiving grant funding from the Rheumatology Research Foundation and the Centers for Disease Control and Prevention. Dr. Bartels reported that her group’s tobacco cessation work is funded by Pfizer’s Independent Grants for Learning and Change.
A version of this article first appeared on Medscape.com.
New recommendations from the European Alliance of Associations for Rheumatology provide both broad and detailed advice for cardiovascular risk management in various rheumatic and musculoskeletal diseases (RMDs), many of which can lead to an increased possibility of cardiovascular disease (CVD).
“The panel believes that these recommendations will enable health care providers and patients to mutually engage in a long-term care pathway tailored to patients’ needs and expectations for improving cardiovascular health in RMDs,” write George C. Drosos, National and Kapodistrian University of Athens, and colleagues. The recommendations were published in February in Annals of the Rheumatic Diseases).
EULAR assembled a task force to generate best practices for preventing CVD in patients with gout, vasculitis, systemic sclerosis (SSc), myositis, mixed connective tissue disease (MCTD), Sjögren syndrome (SS), systemic lupus erythematosus (SLE), and antiphospholipid syndrome (APS).
The cardiovascular risk management of patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis was covered in prior EULAR recommendations.
The task force included 20 members from 11 European countries, including 12 rheumatologists, 2 cardiologists, 1 metabolic medicine physician, 1 health care professional, 2 patient representatives, and 2 EMEUNET (Emerging EULAR Network) members. One group of task force members conducted a systematic literature review of 105 articles about gout, vasculitis, SSc, myositis, MCTD, and SS, and another group evaluated 75 articles about SLE and APS. Together, they decided on four overarching principles:
Clinicians need to be aware of increased cardiovascular risk in patients with RMDs, with disease reduction likely decreasing risk.
Rheumatologists – in tandem with other health care providers – are responsible for their patients’ cardiovascular risk assessment and management.
Screening for cardiovascular risk should be performed regularly in all patients with RMDs, with an emphasis on factors like smoking and blood pressure management.
Patient education and counseling on cardiovascular risk, including important lifestyle modifications, is key for RMD patients.
Specific recommendations from the gout, vasculitis, SSc, myositis, MCTD, and SS group include deploying existing cardiovascular prediction tools as they are used in the general population, with the European Vasculitis Society model suggesting to supplement the Framingham Risk Score for patients with antineutrophil cytoplasmic antibody–associated vasculitis. They also recommended avoiding diuretics in patients with gout and beta-blockers in patients with SSc, as well as following the same blood pressure and lipid management strategies that are used among the general population.
Recommendations from the SLE and APS group include thoroughly assessing traditional cardiovascular risk factors in all patients, following typical blood pressure management strategies in patients with APS, and setting a blood pressure target of less than 130/80 mm Hg in patients with SLE. They also recommended administering the lowest possible glucocorticoid dose in patients with SLE, along with treatment with hydroxychloroquine – unless contraindicated – and even common preventive strategies like low-dose aspirin if it suits their cardiovascular risk profile.
As for next steps, the task force noted several areas where additional focus is needed, such as identifying patient subgroups with increased cardiovascular risk. This could include patients with a longer disease duration or more flare-ups, older patients, and those with certain disease characteristics like antiphospholipid positivity in SLE.
Can EULAR’s recommendations be implemented in U.S. rheumatology practices?
“We have been hearing for years that patients with rheumatic diseases have an increased risk of cardiovascular disease,” Ali A. Duarte Garcia, MD, a rheumatologist at the Mayo Clinic in Rochester, Minn., told this news organization. “That has been consistently published for more than a decade now. But any further guidance about it has not been issued. I think there was a void there.”
“Certainly, cardiovascular disease risk in rheumatoid and psoriatic arthritis has been front of mind for the last decade or so,” Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, said when asked to comment on the recommendations. “But in some of these other conditions, it hasn’t been.”
When asked if rheumatologists would be ready and willing to implement these recommendations, Dr. Duarte Garcia acknowledged that it could be challenging for some.
“It’s a different workflow,” he said. “You’ve been trained traditionally to assess inflammation, to keep the disease under control, which is something they recommend, by the way. If you control the disease, patients do better. But I think lipid screening, for example, and testing for cholesterol, smoking cessation, those well-established programs are harder to bring to a rheumatology clinic. It’s doable, but it’s something that needs to be implemented within the current workflows and could take a few years to take hold.”
Dr. Bartels, however, noted that her group has done extensive work over the last 5 years incorporating certain interventions into practice, including sending patients with high blood pressure back to primary care.
“It’s a sustainable intervention in our clinic that basically our medical assistants and nurses do as a routine operation,” she said. “Our primary care providers are grateful to get these patients back. Our patients are grateful because otherwise when they come to the rheumatologist, get their blood pressure measured, and don’t get feedback, they assume they’re OK. So, we’re giving them a false signal.
“We have a similar intervention with smoking,” she added. “Often our patients aren’t even aware that they’re at increased risk of cardiovascular disease or that smoking might make their rheumatic disease and their cardiovascular outcomes worse. No one has had that conversation with them. They really welcome engaging in those discussions.
“Our tobacco intervention takes 90 seconds at point of care. Our blood pressure intervention at point of care, we’ve timed it, takes 3 minutes. There are ways that we can hardwire this into care.”
Along those lines, Dr. Duarte Garcia stated that the recommendations – although released by EULAR – are largely intuitive and should be very adaptable to an American health care context. He also recognized this moment as an opportunity for rheumatologists to consider patient outcomes beyond what they usually encounter firsthand.
“I don’t think we have many rheumatologists with patients who get a stroke or heart attack because if that happens, it’s in a hospital context or they go see a cardiologist,” he said. “You may see it once it happens if they survive and come and see you – or perhaps if you’re in a more integrated practice – but I don’t think it’s as apparent in our clinics because it is a predominantly outpatient practice and many times those are emergencies or inpatient complications.
“The bottom line,” he added, “is these are practical guidelines. It’s a push in the right direction, but there is still work to be done. And hopefully some of the recommendations, like measuring high blood pressure and addressing it just as in the general population, are something we can start to implement.”
Dr. Duarte Garcia reported receiving grant funding from the Rheumatology Research Foundation and the Centers for Disease Control and Prevention. Dr. Bartels reported that her group’s tobacco cessation work is funded by Pfizer’s Independent Grants for Learning and Change.
A version of this article first appeared on Medscape.com.
New recommendations from the European Alliance of Associations for Rheumatology provide both broad and detailed advice for cardiovascular risk management in various rheumatic and musculoskeletal diseases (RMDs), many of which can lead to an increased possibility of cardiovascular disease (CVD).
“The panel believes that these recommendations will enable health care providers and patients to mutually engage in a long-term care pathway tailored to patients’ needs and expectations for improving cardiovascular health in RMDs,” write George C. Drosos, National and Kapodistrian University of Athens, and colleagues. The recommendations were published in February in Annals of the Rheumatic Diseases).
EULAR assembled a task force to generate best practices for preventing CVD in patients with gout, vasculitis, systemic sclerosis (SSc), myositis, mixed connective tissue disease (MCTD), Sjögren syndrome (SS), systemic lupus erythematosus (SLE), and antiphospholipid syndrome (APS).
The cardiovascular risk management of patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis was covered in prior EULAR recommendations.
The task force included 20 members from 11 European countries, including 12 rheumatologists, 2 cardiologists, 1 metabolic medicine physician, 1 health care professional, 2 patient representatives, and 2 EMEUNET (Emerging EULAR Network) members. One group of task force members conducted a systematic literature review of 105 articles about gout, vasculitis, SSc, myositis, MCTD, and SS, and another group evaluated 75 articles about SLE and APS. Together, they decided on four overarching principles:
Clinicians need to be aware of increased cardiovascular risk in patients with RMDs, with disease reduction likely decreasing risk.
Rheumatologists – in tandem with other health care providers – are responsible for their patients’ cardiovascular risk assessment and management.
Screening for cardiovascular risk should be performed regularly in all patients with RMDs, with an emphasis on factors like smoking and blood pressure management.
Patient education and counseling on cardiovascular risk, including important lifestyle modifications, is key for RMD patients.
Specific recommendations from the gout, vasculitis, SSc, myositis, MCTD, and SS group include deploying existing cardiovascular prediction tools as they are used in the general population, with the European Vasculitis Society model suggesting to supplement the Framingham Risk Score for patients with antineutrophil cytoplasmic antibody–associated vasculitis. They also recommended avoiding diuretics in patients with gout and beta-blockers in patients with SSc, as well as following the same blood pressure and lipid management strategies that are used among the general population.
Recommendations from the SLE and APS group include thoroughly assessing traditional cardiovascular risk factors in all patients, following typical blood pressure management strategies in patients with APS, and setting a blood pressure target of less than 130/80 mm Hg in patients with SLE. They also recommended administering the lowest possible glucocorticoid dose in patients with SLE, along with treatment with hydroxychloroquine – unless contraindicated – and even common preventive strategies like low-dose aspirin if it suits their cardiovascular risk profile.
As for next steps, the task force noted several areas where additional focus is needed, such as identifying patient subgroups with increased cardiovascular risk. This could include patients with a longer disease duration or more flare-ups, older patients, and those with certain disease characteristics like antiphospholipid positivity in SLE.
Can EULAR’s recommendations be implemented in U.S. rheumatology practices?
“We have been hearing for years that patients with rheumatic diseases have an increased risk of cardiovascular disease,” Ali A. Duarte Garcia, MD, a rheumatologist at the Mayo Clinic in Rochester, Minn., told this news organization. “That has been consistently published for more than a decade now. But any further guidance about it has not been issued. I think there was a void there.”
“Certainly, cardiovascular disease risk in rheumatoid and psoriatic arthritis has been front of mind for the last decade or so,” Christie M. Bartels, MD, chief of the division of rheumatology at the University of Wisconsin, Madison, said when asked to comment on the recommendations. “But in some of these other conditions, it hasn’t been.”
When asked if rheumatologists would be ready and willing to implement these recommendations, Dr. Duarte Garcia acknowledged that it could be challenging for some.
“It’s a different workflow,” he said. “You’ve been trained traditionally to assess inflammation, to keep the disease under control, which is something they recommend, by the way. If you control the disease, patients do better. But I think lipid screening, for example, and testing for cholesterol, smoking cessation, those well-established programs are harder to bring to a rheumatology clinic. It’s doable, but it’s something that needs to be implemented within the current workflows and could take a few years to take hold.”
Dr. Bartels, however, noted that her group has done extensive work over the last 5 years incorporating certain interventions into practice, including sending patients with high blood pressure back to primary care.
“It’s a sustainable intervention in our clinic that basically our medical assistants and nurses do as a routine operation,” she said. “Our primary care providers are grateful to get these patients back. Our patients are grateful because otherwise when they come to the rheumatologist, get their blood pressure measured, and don’t get feedback, they assume they’re OK. So, we’re giving them a false signal.
“We have a similar intervention with smoking,” she added. “Often our patients aren’t even aware that they’re at increased risk of cardiovascular disease or that smoking might make their rheumatic disease and their cardiovascular outcomes worse. No one has had that conversation with them. They really welcome engaging in those discussions.
“Our tobacco intervention takes 90 seconds at point of care. Our blood pressure intervention at point of care, we’ve timed it, takes 3 minutes. There are ways that we can hardwire this into care.”
Along those lines, Dr. Duarte Garcia stated that the recommendations – although released by EULAR – are largely intuitive and should be very adaptable to an American health care context. He also recognized this moment as an opportunity for rheumatologists to consider patient outcomes beyond what they usually encounter firsthand.
“I don’t think we have many rheumatologists with patients who get a stroke or heart attack because if that happens, it’s in a hospital context or they go see a cardiologist,” he said. “You may see it once it happens if they survive and come and see you – or perhaps if you’re in a more integrated practice – but I don’t think it’s as apparent in our clinics because it is a predominantly outpatient practice and many times those are emergencies or inpatient complications.
“The bottom line,” he added, “is these are practical guidelines. It’s a push in the right direction, but there is still work to be done. And hopefully some of the recommendations, like measuring high blood pressure and addressing it just as in the general population, are something we can start to implement.”
Dr. Duarte Garcia reported receiving grant funding from the Rheumatology Research Foundation and the Centers for Disease Control and Prevention. Dr. Bartels reported that her group’s tobacco cessation work is funded by Pfizer’s Independent Grants for Learning and Change.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
PACAP38- and VIP-induced cluster headache attacks do not appear to alter CGRP levels
such as tryptase and histamine, a new study has found.
“Whether cluster headache attacks provoked by CGRP and PACAP38/VIP are mediated by distinct signaling pathways will be worth investigating in forthcoming studies,” wrote Lanfranco Pellesi, MD, of the Danish Headache Center at the University of Copenhagen, and his coauthors. The study was published in Cephalalgia.
To assess how these biochemical variables might contribute to cluster headache attacks, the researchers launched a randomized, double-blind trial of data from 44 Danish participants with cluster headache. The average age of the patients was 38 years; 14 had active episodic cluster headache, 15 had episodic cluster headache in remission, and 15 had chronic cluster headache.
All patients received a continuous infusion of either PACAP38 (10 pmol/kg per minute) or VIP (8 pmol/kg per minute) over a 20-minute period, using a time- and volume-controlled infusion pump. Blood was collected for analysis at fixed time points, including at baseline, at the end of the infusion, 10 minutes after the infusion, and 70 minutes after the infusion. Technical problems led to missing values in 285 out of 1,144 planned plasma samples.
PACAP38 infusion resulted in a cluster headache attack in 13 of the 44 participants and VIP induced an attack in 12 of the 44. No differences in plasma CGRP (P = .7074), tryptase (P = .6673), and histamine (P = .4792) levels were found between patients who developed attacks and those who did not, and the plasma concentrations did not differ among the various blood-drawing time points.
There was also no difference in plasma CGRP levels between patients with active episodic cluster headache, those with episodic cluster headache in remission, and those with chronic cluster headache. After post hoc analysis, plasma tryptase and plasma histamine levels were similar among the three cluster headache patient groups.
The final link to the cluster headache puzzle has not yet been found
“We know a lot about cluster headache: how it presents, how we can stop it acutely, and how we can stop it preventively. But we don’t know everything about all the neurotransmitters involved, the triggers that start an attack, or the causes of pain,” Alan Rapoport, MD, professor of neurology at the University of California, Los Angeles (UCLA), and past president of the International Headache Society, said in an interview. “This study was performed to find the answer to a small piece of the puzzle. Is CGRP the missing link for patients who begin a cluster attack, or should we be looking elsewhere?
“I would be cautious and say it appears that it doesn’t seem to be related, but further studies may show something different,” he added. “The reason for my qualification: There is a monoclonal antibody [galcanezumab], which grabs CGRP and prevents it from docking on its receptor, that has been approved for preventive treatment of episodic cluster headache. When you have episodic cluster, go into a cluster period, and take galcanezumab, it could and should decrease the number of attacks that you would ordinarily have had. That means it is related somewhat. But it certainly doesn’t work for everyone, so more investigations like this are needed.”
“What’s important about this study is that it opens up the possibility that there is another way into the cluster attack that could be operationalized for therapeutic purposes,” Peter Goadsby, MD, PhD, professor of neurology at UCLA and president of the American Headache Society, said in an interview.
When asked about the authors’ stated interest in investigating “if monoclonal antibodies targeting the CGRP pathway prevent PACAP38- or VIP-induced cluster headache attacks” as a follow-up, Dr. Goadsby strongly backed the idea. “If I sound excited about actually exploring whether that was a useful treatment or not, it’s because cluster headache is a dreadful condition. And the sooner you could work out whether it was useful or put the money into something else, well, that’s where I’d go.
“I think the principle here of doing experimental medicine, getting into human work with targets like this at the earliest possible time, is something that is not done as often as would be appropriate,” he added. “There is not enough investment, in my view, in early phase experimental work, which really just gets to that next step. Broadly speaking, the encouragement and support of experimental medicine is crucial to developing new therapies.”
The authors recognized their study’s potential limitations, including it’s being an exploratory study with results that should be interpreted cautiously. They acknowledged discrepancies with previous studies of plasma CGRP during cluster headache attacks, offering “different methodologies, including intra-assay differences and the location of blood sampling” as a possible reason. They also explained that some of the data are missing “completely at random” due to their policy of discarding all observations with incomplete laboratory measurements, adding that the impact on their sample size was “only modest.”
“In spite of these limitations,” Dr. Rapoport said, “this is an excellent study that shows us that PACAP38- and VIP-induced cluster headache attacks are not associated with alterations in plasma CGRP or in histamine and tryptase.”
Regarding potential conflicts of interest, one author reported being employed at the testing lab where the histamine measurements were conducted, as did another author who serves as the lab’s scientific adviser. A third author reported receiving personal fees from various pharmaceutical companies.
such as tryptase and histamine, a new study has found.
“Whether cluster headache attacks provoked by CGRP and PACAP38/VIP are mediated by distinct signaling pathways will be worth investigating in forthcoming studies,” wrote Lanfranco Pellesi, MD, of the Danish Headache Center at the University of Copenhagen, and his coauthors. The study was published in Cephalalgia.
To assess how these biochemical variables might contribute to cluster headache attacks, the researchers launched a randomized, double-blind trial of data from 44 Danish participants with cluster headache. The average age of the patients was 38 years; 14 had active episodic cluster headache, 15 had episodic cluster headache in remission, and 15 had chronic cluster headache.
All patients received a continuous infusion of either PACAP38 (10 pmol/kg per minute) or VIP (8 pmol/kg per minute) over a 20-minute period, using a time- and volume-controlled infusion pump. Blood was collected for analysis at fixed time points, including at baseline, at the end of the infusion, 10 minutes after the infusion, and 70 minutes after the infusion. Technical problems led to missing values in 285 out of 1,144 planned plasma samples.
PACAP38 infusion resulted in a cluster headache attack in 13 of the 44 participants and VIP induced an attack in 12 of the 44. No differences in plasma CGRP (P = .7074), tryptase (P = .6673), and histamine (P = .4792) levels were found between patients who developed attacks and those who did not, and the plasma concentrations did not differ among the various blood-drawing time points.
There was also no difference in plasma CGRP levels between patients with active episodic cluster headache, those with episodic cluster headache in remission, and those with chronic cluster headache. After post hoc analysis, plasma tryptase and plasma histamine levels were similar among the three cluster headache patient groups.
The final link to the cluster headache puzzle has not yet been found
“We know a lot about cluster headache: how it presents, how we can stop it acutely, and how we can stop it preventively. But we don’t know everything about all the neurotransmitters involved, the triggers that start an attack, or the causes of pain,” Alan Rapoport, MD, professor of neurology at the University of California, Los Angeles (UCLA), and past president of the International Headache Society, said in an interview. “This study was performed to find the answer to a small piece of the puzzle. Is CGRP the missing link for patients who begin a cluster attack, or should we be looking elsewhere?
“I would be cautious and say it appears that it doesn’t seem to be related, but further studies may show something different,” he added. “The reason for my qualification: There is a monoclonal antibody [galcanezumab], which grabs CGRP and prevents it from docking on its receptor, that has been approved for preventive treatment of episodic cluster headache. When you have episodic cluster, go into a cluster period, and take galcanezumab, it could and should decrease the number of attacks that you would ordinarily have had. That means it is related somewhat. But it certainly doesn’t work for everyone, so more investigations like this are needed.”
“What’s important about this study is that it opens up the possibility that there is another way into the cluster attack that could be operationalized for therapeutic purposes,” Peter Goadsby, MD, PhD, professor of neurology at UCLA and president of the American Headache Society, said in an interview.
When asked about the authors’ stated interest in investigating “if monoclonal antibodies targeting the CGRP pathway prevent PACAP38- or VIP-induced cluster headache attacks” as a follow-up, Dr. Goadsby strongly backed the idea. “If I sound excited about actually exploring whether that was a useful treatment or not, it’s because cluster headache is a dreadful condition. And the sooner you could work out whether it was useful or put the money into something else, well, that’s where I’d go.
“I think the principle here of doing experimental medicine, getting into human work with targets like this at the earliest possible time, is something that is not done as often as would be appropriate,” he added. “There is not enough investment, in my view, in early phase experimental work, which really just gets to that next step. Broadly speaking, the encouragement and support of experimental medicine is crucial to developing new therapies.”
The authors recognized their study’s potential limitations, including it’s being an exploratory study with results that should be interpreted cautiously. They acknowledged discrepancies with previous studies of plasma CGRP during cluster headache attacks, offering “different methodologies, including intra-assay differences and the location of blood sampling” as a possible reason. They also explained that some of the data are missing “completely at random” due to their policy of discarding all observations with incomplete laboratory measurements, adding that the impact on their sample size was “only modest.”
“In spite of these limitations,” Dr. Rapoport said, “this is an excellent study that shows us that PACAP38- and VIP-induced cluster headache attacks are not associated with alterations in plasma CGRP or in histamine and tryptase.”
Regarding potential conflicts of interest, one author reported being employed at the testing lab where the histamine measurements were conducted, as did another author who serves as the lab’s scientific adviser. A third author reported receiving personal fees from various pharmaceutical companies.
such as tryptase and histamine, a new study has found.
“Whether cluster headache attacks provoked by CGRP and PACAP38/VIP are mediated by distinct signaling pathways will be worth investigating in forthcoming studies,” wrote Lanfranco Pellesi, MD, of the Danish Headache Center at the University of Copenhagen, and his coauthors. The study was published in Cephalalgia.
To assess how these biochemical variables might contribute to cluster headache attacks, the researchers launched a randomized, double-blind trial of data from 44 Danish participants with cluster headache. The average age of the patients was 38 years; 14 had active episodic cluster headache, 15 had episodic cluster headache in remission, and 15 had chronic cluster headache.
All patients received a continuous infusion of either PACAP38 (10 pmol/kg per minute) or VIP (8 pmol/kg per minute) over a 20-minute period, using a time- and volume-controlled infusion pump. Blood was collected for analysis at fixed time points, including at baseline, at the end of the infusion, 10 minutes after the infusion, and 70 minutes after the infusion. Technical problems led to missing values in 285 out of 1,144 planned plasma samples.
PACAP38 infusion resulted in a cluster headache attack in 13 of the 44 participants and VIP induced an attack in 12 of the 44. No differences in plasma CGRP (P = .7074), tryptase (P = .6673), and histamine (P = .4792) levels were found between patients who developed attacks and those who did not, and the plasma concentrations did not differ among the various blood-drawing time points.
There was also no difference in plasma CGRP levels between patients with active episodic cluster headache, those with episodic cluster headache in remission, and those with chronic cluster headache. After post hoc analysis, plasma tryptase and plasma histamine levels were similar among the three cluster headache patient groups.
The final link to the cluster headache puzzle has not yet been found
“We know a lot about cluster headache: how it presents, how we can stop it acutely, and how we can stop it preventively. But we don’t know everything about all the neurotransmitters involved, the triggers that start an attack, or the causes of pain,” Alan Rapoport, MD, professor of neurology at the University of California, Los Angeles (UCLA), and past president of the International Headache Society, said in an interview. “This study was performed to find the answer to a small piece of the puzzle. Is CGRP the missing link for patients who begin a cluster attack, or should we be looking elsewhere?
“I would be cautious and say it appears that it doesn’t seem to be related, but further studies may show something different,” he added. “The reason for my qualification: There is a monoclonal antibody [galcanezumab], which grabs CGRP and prevents it from docking on its receptor, that has been approved for preventive treatment of episodic cluster headache. When you have episodic cluster, go into a cluster period, and take galcanezumab, it could and should decrease the number of attacks that you would ordinarily have had. That means it is related somewhat. But it certainly doesn’t work for everyone, so more investigations like this are needed.”
“What’s important about this study is that it opens up the possibility that there is another way into the cluster attack that could be operationalized for therapeutic purposes,” Peter Goadsby, MD, PhD, professor of neurology at UCLA and president of the American Headache Society, said in an interview.
When asked about the authors’ stated interest in investigating “if monoclonal antibodies targeting the CGRP pathway prevent PACAP38- or VIP-induced cluster headache attacks” as a follow-up, Dr. Goadsby strongly backed the idea. “If I sound excited about actually exploring whether that was a useful treatment or not, it’s because cluster headache is a dreadful condition. And the sooner you could work out whether it was useful or put the money into something else, well, that’s where I’d go.
“I think the principle here of doing experimental medicine, getting into human work with targets like this at the earliest possible time, is something that is not done as often as would be appropriate,” he added. “There is not enough investment, in my view, in early phase experimental work, which really just gets to that next step. Broadly speaking, the encouragement and support of experimental medicine is crucial to developing new therapies.”
The authors recognized their study’s potential limitations, including it’s being an exploratory study with results that should be interpreted cautiously. They acknowledged discrepancies with previous studies of plasma CGRP during cluster headache attacks, offering “different methodologies, including intra-assay differences and the location of blood sampling” as a possible reason. They also explained that some of the data are missing “completely at random” due to their policy of discarding all observations with incomplete laboratory measurements, adding that the impact on their sample size was “only modest.”
“In spite of these limitations,” Dr. Rapoport said, “this is an excellent study that shows us that PACAP38- and VIP-induced cluster headache attacks are not associated with alterations in plasma CGRP or in histamine and tryptase.”
Regarding potential conflicts of interest, one author reported being employed at the testing lab where the histamine measurements were conducted, as did another author who serves as the lab’s scientific adviser. A third author reported receiving personal fees from various pharmaceutical companies.
FROM CEPHALALGIA
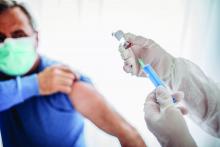
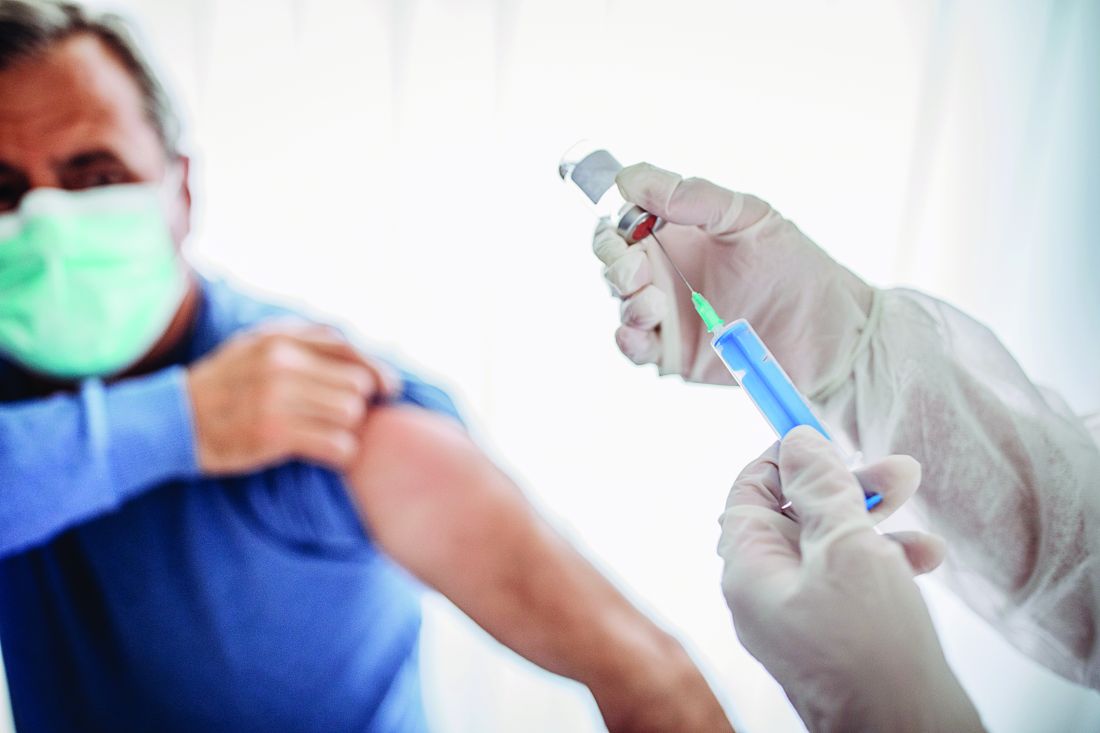
Updated guidance for COVID vaccination in rheumatology patients arrives amid continued hesitancy
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
FROM ARTHRITIS & RHEUMATOLOGY
A range of healthy dietary patterns can reduce risk of gout in women
A new study of thousands of women has found that sticking to recommended healthy dietary patterns can lessen the risk of new-onset gout.
“The identification of multiple patterns of eating that can similarly reduce a woman’s risk of incident gout in our study allows more choice for potential personalization of dietary recommendations according to culinary traditions and personal preferences to enhance adherence,” Chio Yokose, MD, of Harvard Medical School, Boston, and coauthors wrote. The study was published Jan. 31, 2022, in JAMA Internal Medicine.
To determine whether consistent healthy eating plays a role in preventing gout in women, the authors launched a prospective cohort study tied to the Nurses’ Health Study, an ongoing endeavor that has been questioning its participants’ food and beverage intake since 1984. Based on the 2020 to 2025 Dietary Guidelines for Americans, four healthy eating patterns were identified for assessment: the Dietary Approaches to Stop Hypertension (DASH), the Mediterranean diet, the Alternative Healthy Eating Index, and the Prudent diet, as well as the unhealthy Western dietary pattern for comparison.
Over 34 years of follow-up, the researchers identified 3,890 cases of gout among 80,039 women with an average age of 50.5 and an average body mass index (BMI) of 25.0 kg/m2. Women who strongly adhered to either of the four healthy dietary patterns had a significantly lower risk of gout, especially those who stuck to DASH (multivariable hazard ratio, 0.68; 95% confidence interval, 0.61-0.76) and Prudent (HR, 0.75; 95% CI, 0.73-0.90). In contrast, women with high Western diet scores had a 49% increased risk of gout (HR, 1.49; 95% CI, 1.33-1.68), compared with those who had low scores.
After additional analysis that factored in variables like diuretic use, alcohol use, and obesity, the associations between each diet and their risk of gout persisted in almost every instance. In particular, the most DASH-adherent women with normal BMI had a 68% lower risk of gout (HR, 0.32; 95% CI, 0.26-0.38), compared with the least-adherent women who were overweight or obese. Strong DASH adherence and no diuretic use also led to a 65% gout risk reduction (HR, 0.35; 95% CI, 0.30-0.41).
Healthy eating offers broad benefits for gout patients
“These results are consistent with a lot of the conversations we have on a day-to-day basis with patients,” Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha, said in an interview. “But I will say, I don’t get a lot of patients coming in saying: ‘Hey, what can I do to prevent gout?’ You’re usually seeing them after the fact.”
“These results shouldn’t be confused with that,” he said. “In other words, I wouldn’t want people interpreting this study to mean diet is always a satisfactory treatment for someone with established gout. The fact of the matter is, often it’s not. We need medication to effectively treat gout. I think this and other studies like it call for future research that can look at these dietary interventions as either standalone or probably adjuvant therapies in gout treatment.”
But, he added, that doesn’t mean conversations about diet aren’t of the utmost importance for gout patients.
“That shouldn’t stop clinicians from talking to patients about dietary changes that holistically are going to have positive benefits,” he said. “By the time you meet them, gout patients often already have other health conditions: high blood pressure, diabetes, obesity. The dietary changes that these authors studied are going to have a holistic benefit that goes well beyond gout risk, and that’s important. That’s a conversation that physicians and health care providers can and should be having right now with their patients.”
The authors acknowledged their study’s limitations, including the unmeasured or residual confounding that could come with any observational study as well as these rates of gout and these dietary patterns not necessarily being representative of a random sample of American women. “Future research could examine the population contributions of diets and other risk factors for incident female gout, as done in men.”
The study was funded by the National Institutes of Health. The authors reported several potential conflicts of interest, including receiving grants from the NIH and grants and personal fees from other organizations and pharmaceutical companies. Dr. Mikuls reported receiving past funding from Horizon Therapeutics and serving for them in a consulting capacity.
A new study of thousands of women has found that sticking to recommended healthy dietary patterns can lessen the risk of new-onset gout.
“The identification of multiple patterns of eating that can similarly reduce a woman’s risk of incident gout in our study allows more choice for potential personalization of dietary recommendations according to culinary traditions and personal preferences to enhance adherence,” Chio Yokose, MD, of Harvard Medical School, Boston, and coauthors wrote. The study was published Jan. 31, 2022, in JAMA Internal Medicine.
To determine whether consistent healthy eating plays a role in preventing gout in women, the authors launched a prospective cohort study tied to the Nurses’ Health Study, an ongoing endeavor that has been questioning its participants’ food and beverage intake since 1984. Based on the 2020 to 2025 Dietary Guidelines for Americans, four healthy eating patterns were identified for assessment: the Dietary Approaches to Stop Hypertension (DASH), the Mediterranean diet, the Alternative Healthy Eating Index, and the Prudent diet, as well as the unhealthy Western dietary pattern for comparison.
Over 34 years of follow-up, the researchers identified 3,890 cases of gout among 80,039 women with an average age of 50.5 and an average body mass index (BMI) of 25.0 kg/m2. Women who strongly adhered to either of the four healthy dietary patterns had a significantly lower risk of gout, especially those who stuck to DASH (multivariable hazard ratio, 0.68; 95% confidence interval, 0.61-0.76) and Prudent (HR, 0.75; 95% CI, 0.73-0.90). In contrast, women with high Western diet scores had a 49% increased risk of gout (HR, 1.49; 95% CI, 1.33-1.68), compared with those who had low scores.
After additional analysis that factored in variables like diuretic use, alcohol use, and obesity, the associations between each diet and their risk of gout persisted in almost every instance. In particular, the most DASH-adherent women with normal BMI had a 68% lower risk of gout (HR, 0.32; 95% CI, 0.26-0.38), compared with the least-adherent women who were overweight or obese. Strong DASH adherence and no diuretic use also led to a 65% gout risk reduction (HR, 0.35; 95% CI, 0.30-0.41).
Healthy eating offers broad benefits for gout patients
“These results are consistent with a lot of the conversations we have on a day-to-day basis with patients,” Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha, said in an interview. “But I will say, I don’t get a lot of patients coming in saying: ‘Hey, what can I do to prevent gout?’ You’re usually seeing them after the fact.”
“These results shouldn’t be confused with that,” he said. “In other words, I wouldn’t want people interpreting this study to mean diet is always a satisfactory treatment for someone with established gout. The fact of the matter is, often it’s not. We need medication to effectively treat gout. I think this and other studies like it call for future research that can look at these dietary interventions as either standalone or probably adjuvant therapies in gout treatment.”
But, he added, that doesn’t mean conversations about diet aren’t of the utmost importance for gout patients.
“That shouldn’t stop clinicians from talking to patients about dietary changes that holistically are going to have positive benefits,” he said. “By the time you meet them, gout patients often already have other health conditions: high blood pressure, diabetes, obesity. The dietary changes that these authors studied are going to have a holistic benefit that goes well beyond gout risk, and that’s important. That’s a conversation that physicians and health care providers can and should be having right now with their patients.”
The authors acknowledged their study’s limitations, including the unmeasured or residual confounding that could come with any observational study as well as these rates of gout and these dietary patterns not necessarily being representative of a random sample of American women. “Future research could examine the population contributions of diets and other risk factors for incident female gout, as done in men.”
The study was funded by the National Institutes of Health. The authors reported several potential conflicts of interest, including receiving grants from the NIH and grants and personal fees from other organizations and pharmaceutical companies. Dr. Mikuls reported receiving past funding from Horizon Therapeutics and serving for them in a consulting capacity.
A new study of thousands of women has found that sticking to recommended healthy dietary patterns can lessen the risk of new-onset gout.
“The identification of multiple patterns of eating that can similarly reduce a woman’s risk of incident gout in our study allows more choice for potential personalization of dietary recommendations according to culinary traditions and personal preferences to enhance adherence,” Chio Yokose, MD, of Harvard Medical School, Boston, and coauthors wrote. The study was published Jan. 31, 2022, in JAMA Internal Medicine.
To determine whether consistent healthy eating plays a role in preventing gout in women, the authors launched a prospective cohort study tied to the Nurses’ Health Study, an ongoing endeavor that has been questioning its participants’ food and beverage intake since 1984. Based on the 2020 to 2025 Dietary Guidelines for Americans, four healthy eating patterns were identified for assessment: the Dietary Approaches to Stop Hypertension (DASH), the Mediterranean diet, the Alternative Healthy Eating Index, and the Prudent diet, as well as the unhealthy Western dietary pattern for comparison.
Over 34 years of follow-up, the researchers identified 3,890 cases of gout among 80,039 women with an average age of 50.5 and an average body mass index (BMI) of 25.0 kg/m2. Women who strongly adhered to either of the four healthy dietary patterns had a significantly lower risk of gout, especially those who stuck to DASH (multivariable hazard ratio, 0.68; 95% confidence interval, 0.61-0.76) and Prudent (HR, 0.75; 95% CI, 0.73-0.90). In contrast, women with high Western diet scores had a 49% increased risk of gout (HR, 1.49; 95% CI, 1.33-1.68), compared with those who had low scores.
After additional analysis that factored in variables like diuretic use, alcohol use, and obesity, the associations between each diet and their risk of gout persisted in almost every instance. In particular, the most DASH-adherent women with normal BMI had a 68% lower risk of gout (HR, 0.32; 95% CI, 0.26-0.38), compared with the least-adherent women who were overweight or obese. Strong DASH adherence and no diuretic use also led to a 65% gout risk reduction (HR, 0.35; 95% CI, 0.30-0.41).
Healthy eating offers broad benefits for gout patients
“These results are consistent with a lot of the conversations we have on a day-to-day basis with patients,” Ted Mikuls, MD, of the University of Nebraska Medical Center, Omaha, said in an interview. “But I will say, I don’t get a lot of patients coming in saying: ‘Hey, what can I do to prevent gout?’ You’re usually seeing them after the fact.”
“These results shouldn’t be confused with that,” he said. “In other words, I wouldn’t want people interpreting this study to mean diet is always a satisfactory treatment for someone with established gout. The fact of the matter is, often it’s not. We need medication to effectively treat gout. I think this and other studies like it call for future research that can look at these dietary interventions as either standalone or probably adjuvant therapies in gout treatment.”
But, he added, that doesn’t mean conversations about diet aren’t of the utmost importance for gout patients.
“That shouldn’t stop clinicians from talking to patients about dietary changes that holistically are going to have positive benefits,” he said. “By the time you meet them, gout patients often already have other health conditions: high blood pressure, diabetes, obesity. The dietary changes that these authors studied are going to have a holistic benefit that goes well beyond gout risk, and that’s important. That’s a conversation that physicians and health care providers can and should be having right now with their patients.”
The authors acknowledged their study’s limitations, including the unmeasured or residual confounding that could come with any observational study as well as these rates of gout and these dietary patterns not necessarily being representative of a random sample of American women. “Future research could examine the population contributions of diets and other risk factors for incident female gout, as done in men.”
The study was funded by the National Institutes of Health. The authors reported several potential conflicts of interest, including receiving grants from the NIH and grants and personal fees from other organizations and pharmaceutical companies. Dr. Mikuls reported receiving past funding from Horizon Therapeutics and serving for them in a consulting capacity.
FROM JAMA INTERNAL MEDICINE
Ketamine nasal spray provides slow-acting relief for cluster headache attacks
, a pilot study has found, though it did not reduce pain intensity as quickly as initially anticipated.
“In clinical practice, intranasal ketamine might be a valuable tool for severely affected patients with insufficient response or intolerance to current first-line treatment,” wrote Anja S. Petersen, MD, of the Danish Headache Center at Rigshospitalet-Glostrup (Denmark) and her coauthors. The study was published online ahead of print in Headache.
To assess ketamine’s safety and efficacy in treating cluster headache attacks, the researchers launched a single-center, open-label, proof-of-concept study of 23 Danish patients with chronic cluster headache. Their average age was 51, 70% were males, and their mean disease duration was 18 years. Twenty of the participants suffered a spontaneous attack while under in-hospital observation and were treated with 15 mg of intranasal ketamine every 6 minutes to a maximum of five times.
Fifteen minutes after ketamine was administered, mean pain intensity (±SD) was reduced from 7.2 (±1.3) to 6.1 (±3.1) on an 11-point numeric rating scale, equivalent to a 15% reduction and well below the primary endpoint of a 50% or greater reduction. Only 4 of the 20 participants had a reduction of 50% or more, and 4 patients chose rescue medication at 15 minutes. However, at 30 minutes pain intensity was reduced by 59% (mean difference 4.3, 95% confidence interval, 2.4-6.2, P > 0.001), with 11 out of 16 participants scoring a 4 or below.
Eight of the 20 participants reported feeling complete relief from the ketamine nasal spray, while 6 participants reported feeling no effects. Half of the patients said they preferred ketamine to oxygen and/or sumatriptan injection. Seventeen patients (83%) reported side effects, but 12 of them classified their side effects as “few.” No serious adverse events were identified, with the most common adverse events being dizziness, lightheadedness, nausea/vomiting, and paresthesia.
Debating ketamine’s potential for cluster headache patients
“I’m not crazy about the prospects,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., in an interview. “It was an admirable proof-of-concept trial, and well worth doing. These are desperate patients. But if the aim was to decrease pain intensity within 15 minutes for cluster patients without side effects, this clearly did not do that,” Dr. Tepper said.
“In a sense, this study was to evaluate whether glutamate might be a target for chronic cluster headache, to determine if blocking NMDA glutamate receptors by ketamine would be effective,” Dr. Tepper said. “And I must say, I’m not very impressed.”
He noted his concerns about the study – including 30 minutes being an “unacceptable” wait for patients undergoing a cluster attack, the 20% of patients who required a rescue at 15 minutes, and the various side effects that come with ketamine in nasal form – and said the results did not sway him to consider ketamine a practical option for cluster headache patients.
“You add all of that up, and I would say this was an equivocal study,” he said. “There might be enough there to be worth studying in episodic cluster rather than chronic cluster; there might be enough to consider a randomized, placebo-controlled trial. But it’s not something that I would ring the bell at Wall Street about.”
“The acute treatment of a patient with chronic cluster headache is a real problem for us headache specialists,” added Alan Rapoport, MD, professor of neurology at University of California, Los Angeles, and past president of the International Headache Society, in an interview. “Cluster headache is probably the worst pain we deal with; women who’ve gone through childbirth say that cluster headache is worse. So it’s very reasonable to have tried.”
“It’s not an impressive finding,” he said, “but it does indicate that there’s some value here. Maybe they need to change the dose; maybe they need to get it in faster by doing something tricky like combining the drug with another substance that will make it attach to the nasal mucosa better. I urge them to study it again, and I hope that they come up with better results the next time, because what they attempted to study is absolutely vital.”
The authors acknowledged their study’s limitations, including a homogeneous patient population and the lack of placebo-controlled verification of effect after 30 minutes. They added, however, that a pilot study like this provides “critical information and paves the way for subsequent placebo-controlled studies.” They also admitted that “daily usage [of ketamine] seems suboptimal” because of the potential of patients becoming addicted.
The study was funded by CCH Pharmaceuticals. Several authors reported receiving speaker’s fees and being subinvestigators in trials run by various pharmaceutical companies, including CCH Pharmaceuticals.
, a pilot study has found, though it did not reduce pain intensity as quickly as initially anticipated.
“In clinical practice, intranasal ketamine might be a valuable tool for severely affected patients with insufficient response or intolerance to current first-line treatment,” wrote Anja S. Petersen, MD, of the Danish Headache Center at Rigshospitalet-Glostrup (Denmark) and her coauthors. The study was published online ahead of print in Headache.
To assess ketamine’s safety and efficacy in treating cluster headache attacks, the researchers launched a single-center, open-label, proof-of-concept study of 23 Danish patients with chronic cluster headache. Their average age was 51, 70% were males, and their mean disease duration was 18 years. Twenty of the participants suffered a spontaneous attack while under in-hospital observation and were treated with 15 mg of intranasal ketamine every 6 minutes to a maximum of five times.
Fifteen minutes after ketamine was administered, mean pain intensity (±SD) was reduced from 7.2 (±1.3) to 6.1 (±3.1) on an 11-point numeric rating scale, equivalent to a 15% reduction and well below the primary endpoint of a 50% or greater reduction. Only 4 of the 20 participants had a reduction of 50% or more, and 4 patients chose rescue medication at 15 minutes. However, at 30 minutes pain intensity was reduced by 59% (mean difference 4.3, 95% confidence interval, 2.4-6.2, P > 0.001), with 11 out of 16 participants scoring a 4 or below.
Eight of the 20 participants reported feeling complete relief from the ketamine nasal spray, while 6 participants reported feeling no effects. Half of the patients said they preferred ketamine to oxygen and/or sumatriptan injection. Seventeen patients (83%) reported side effects, but 12 of them classified their side effects as “few.” No serious adverse events were identified, with the most common adverse events being dizziness, lightheadedness, nausea/vomiting, and paresthesia.
Debating ketamine’s potential for cluster headache patients
“I’m not crazy about the prospects,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., in an interview. “It was an admirable proof-of-concept trial, and well worth doing. These are desperate patients. But if the aim was to decrease pain intensity within 15 minutes for cluster patients without side effects, this clearly did not do that,” Dr. Tepper said.
“In a sense, this study was to evaluate whether glutamate might be a target for chronic cluster headache, to determine if blocking NMDA glutamate receptors by ketamine would be effective,” Dr. Tepper said. “And I must say, I’m not very impressed.”
He noted his concerns about the study – including 30 minutes being an “unacceptable” wait for patients undergoing a cluster attack, the 20% of patients who required a rescue at 15 minutes, and the various side effects that come with ketamine in nasal form – and said the results did not sway him to consider ketamine a practical option for cluster headache patients.
“You add all of that up, and I would say this was an equivocal study,” he said. “There might be enough there to be worth studying in episodic cluster rather than chronic cluster; there might be enough to consider a randomized, placebo-controlled trial. But it’s not something that I would ring the bell at Wall Street about.”
“The acute treatment of a patient with chronic cluster headache is a real problem for us headache specialists,” added Alan Rapoport, MD, professor of neurology at University of California, Los Angeles, and past president of the International Headache Society, in an interview. “Cluster headache is probably the worst pain we deal with; women who’ve gone through childbirth say that cluster headache is worse. So it’s very reasonable to have tried.”
“It’s not an impressive finding,” he said, “but it does indicate that there’s some value here. Maybe they need to change the dose; maybe they need to get it in faster by doing something tricky like combining the drug with another substance that will make it attach to the nasal mucosa better. I urge them to study it again, and I hope that they come up with better results the next time, because what they attempted to study is absolutely vital.”
The authors acknowledged their study’s limitations, including a homogeneous patient population and the lack of placebo-controlled verification of effect after 30 minutes. They added, however, that a pilot study like this provides “critical information and paves the way for subsequent placebo-controlled studies.” They also admitted that “daily usage [of ketamine] seems suboptimal” because of the potential of patients becoming addicted.
The study was funded by CCH Pharmaceuticals. Several authors reported receiving speaker’s fees and being subinvestigators in trials run by various pharmaceutical companies, including CCH Pharmaceuticals.
, a pilot study has found, though it did not reduce pain intensity as quickly as initially anticipated.
“In clinical practice, intranasal ketamine might be a valuable tool for severely affected patients with insufficient response or intolerance to current first-line treatment,” wrote Anja S. Petersen, MD, of the Danish Headache Center at Rigshospitalet-Glostrup (Denmark) and her coauthors. The study was published online ahead of print in Headache.
To assess ketamine’s safety and efficacy in treating cluster headache attacks, the researchers launched a single-center, open-label, proof-of-concept study of 23 Danish patients with chronic cluster headache. Their average age was 51, 70% were males, and their mean disease duration was 18 years. Twenty of the participants suffered a spontaneous attack while under in-hospital observation and were treated with 15 mg of intranasal ketamine every 6 minutes to a maximum of five times.
Fifteen minutes after ketamine was administered, mean pain intensity (±SD) was reduced from 7.2 (±1.3) to 6.1 (±3.1) on an 11-point numeric rating scale, equivalent to a 15% reduction and well below the primary endpoint of a 50% or greater reduction. Only 4 of the 20 participants had a reduction of 50% or more, and 4 patients chose rescue medication at 15 minutes. However, at 30 minutes pain intensity was reduced by 59% (mean difference 4.3, 95% confidence interval, 2.4-6.2, P > 0.001), with 11 out of 16 participants scoring a 4 or below.
Eight of the 20 participants reported feeling complete relief from the ketamine nasal spray, while 6 participants reported feeling no effects. Half of the patients said they preferred ketamine to oxygen and/or sumatriptan injection. Seventeen patients (83%) reported side effects, but 12 of them classified their side effects as “few.” No serious adverse events were identified, with the most common adverse events being dizziness, lightheadedness, nausea/vomiting, and paresthesia.
Debating ketamine’s potential for cluster headache patients
“I’m not crazy about the prospects,” said Stewart J. Tepper, MD, professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H., in an interview. “It was an admirable proof-of-concept trial, and well worth doing. These are desperate patients. But if the aim was to decrease pain intensity within 15 minutes for cluster patients without side effects, this clearly did not do that,” Dr. Tepper said.
“In a sense, this study was to evaluate whether glutamate might be a target for chronic cluster headache, to determine if blocking NMDA glutamate receptors by ketamine would be effective,” Dr. Tepper said. “And I must say, I’m not very impressed.”
He noted his concerns about the study – including 30 minutes being an “unacceptable” wait for patients undergoing a cluster attack, the 20% of patients who required a rescue at 15 minutes, and the various side effects that come with ketamine in nasal form – and said the results did not sway him to consider ketamine a practical option for cluster headache patients.
“You add all of that up, and I would say this was an equivocal study,” he said. “There might be enough there to be worth studying in episodic cluster rather than chronic cluster; there might be enough to consider a randomized, placebo-controlled trial. But it’s not something that I would ring the bell at Wall Street about.”
“The acute treatment of a patient with chronic cluster headache is a real problem for us headache specialists,” added Alan Rapoport, MD, professor of neurology at University of California, Los Angeles, and past president of the International Headache Society, in an interview. “Cluster headache is probably the worst pain we deal with; women who’ve gone through childbirth say that cluster headache is worse. So it’s very reasonable to have tried.”
“It’s not an impressive finding,” he said, “but it does indicate that there’s some value here. Maybe they need to change the dose; maybe they need to get it in faster by doing something tricky like combining the drug with another substance that will make it attach to the nasal mucosa better. I urge them to study it again, and I hope that they come up with better results the next time, because what they attempted to study is absolutely vital.”
The authors acknowledged their study’s limitations, including a homogeneous patient population and the lack of placebo-controlled verification of effect after 30 minutes. They added, however, that a pilot study like this provides “critical information and paves the way for subsequent placebo-controlled studies.” They also admitted that “daily usage [of ketamine] seems suboptimal” because of the potential of patients becoming addicted.
The study was funded by CCH Pharmaceuticals. Several authors reported receiving speaker’s fees and being subinvestigators in trials run by various pharmaceutical companies, including CCH Pharmaceuticals.
FROM HEADACHE
Proactive infliximab monitoring found best for sustaining control of inflammatory diseases
A new study has found that proactive therapeutic drug monitoring (TDM) with maintenance infliximab is more effective than standard therapy in sustaining control of immune-mediated inflammatory diseases.
The findings from the Norwegian Drug Monitoring B (NOR-DRUM B) trial, published Dec. 21, 2021, in JAMA, provide greater support to the usefulness of TDM in proactively monitoring serum drug levels and antidrug antibodies to infliximab, which has been previously shown to have benefit in patients with inflammatory bowel disease, but leave the benefits of proactive versus reactive monitoring and the cost-effectiveness of the approach in individual immune-mediated inflammatory diseases still open to questioning.
TDM is ‘not the holy grail,’ and that’s OK
“This is an important milestone in the field of TDM with biologics for immunoinflammatory diseases,” Niels Vande Casteele, PharmD, PhD, of the University of California, San Diego, told this news organization. He was not involved in the study.
“When you read through the study, you can see the authors used the TAXIT trial results to inform their study design and the sample size,” he added, referencing his 2015 study on infliximab guide dosing for patients with inflammatory bowel disease, “the first-ever randomized, controlled trial of proactive TDM with any biologic.”
For the TAXIT study’s primary outcome of clinical and biochemical remission at 1 year, “continued concentration-based dosing was not superior to clinically based dosing for achieving remission.” But in regard to their secondary outcome of sustained remission, their results were quite similar to the results of NOR-DRUM B.
“If anything, we already showed a benefit of proactive TDM in 2015,” he said, “but I’m very glad that the authors looked at the trial design and teased out where TDM could be the most important and have the biggest impact, which is to maintain that sustained disease remission over a prolonged period.”
As for next steps, Dr. Vande Casteele noted that TDM isn’t a one-size-fits-all upgrade for drug treatments. But that doesn’t mean it won’t be very useful in many patients.
“What the paper is saying, and what we’ve been finding all along, is that TDM is not the holy grail,” he said. “But it is a tool in the physicians’ toolbox to optimize treatments and maximize efficacy, and there are some patients who truly benefit from it.”
Study details
To determine if proactive TDM with infliximab led to more sustained disease control than standard therapy, first author Silje Watterdal Syversen, MD, PhD, of Diakonhjemmet Hospital in Oslo, and coauthors conducted a 52-week, randomized, parallel-group, open-label trial. From 20 Norwegian hospitals, they recruited 458 patients with rheumatoid arthritis (n = 80), spondyloarthritis (n = 138), psoriatic arthritis (n = 54), ulcerative colitis (n = 81), Crohn’s disease (n = 68), or psoriasis (n = 37) who were undergoing maintenance therapy with the biologic.
The 454 patients who received at least one randomly allocated dose of infliximab were treated with one of two strategies: TDM (n = 227) or standard therapy (n = 227). The TDM group received dose and interval adjustments based on an algorithm that factored in serum drug levels and antidrug antibodies. The standard therapy group was treated on the basis of clinical judgment and physician discretion. The average age across groups was roughly 45 years, and just under 50% were women.
Overall, sustained disease control without worsening was achieved in 167 patients (73.6%) in the TDM group and 127 patients (55.9%) in the standard therapy group, with an estimated adjusted difference of 17.6% (95% confidence interval, 9.0%-26.2%; P < .001). The estimated hazard ratio of disease worsening was 2.1 (95% CI, 1.5-2.9) for standard therapy, compared with TDM. A total of 27 patients (15%) in the standard therapy group and 21 patients (9.2%) in the TDM group developed significant levels of antidrug antibodies, defined here as 50 mcg/L or more.
A total of 34 patients discontinued infliximab in each group; in the TDM group, most discontinued because of antidrug antibody formation, while the main reason for discontinuing in the standard therapy group was disease worsening. Adverse events were reported in 137 patients (60%) in the TDM group and 142 patients (63%) in the standard therapy group.
Removing barriers to TDM
It’s not clear that proactive TDM will benefit treatment with all biologic disease-modifying antirheumatic drugs (bDMARDs), but the findings from Dr. Syversen and colleagues state the clear value of using drug monitoring to guide maintenance therapy with infliximab, Zachary S. Wallace, MD, and Jeffrey A. Sparks, MD, wrote in an accompanying editorial.
“The relatively large sample size and rigorous study design ... helped to overcome some limitations of previous observational studies and small clinical trials that yielded conflicting results regarding TDM,” they added, noting that these findings contrasted somewhat with the NOR-DRUM A trial in which TDM did not improve remission induction in patients initiating infliximab therapy.
Along those lines, they recognized that TDM appears to have a greater effect in patients on maintenance infliximab, compared with those just starting the drug, surmising – among several explanations – that achieving remission in someone beginning treatment is a more difficult outcome to achieve than controlling disease in a patient already in remission.
For now, more clinical trials assessing specific diseases and involving other bDMARDs are needed; Dr. Wallace and Dr. Sparks stated that it’s time to remove barriers to implementing TDM – including the need for medical insurance preauthorization before increasing drug doses – and potentially “introduce a new era in treatment approach to maintenance therapy for patients with immune-mediated inflammatory diseases.”
The authors acknowledged their study’s limitations, including disease worsening being measured in part by patient-physician consensus and thus potentially subject to bias. In addition, they did not have the statistical ability to test TDM effectiveness in each of the six disease groups, noting that “these diseases have inherent differences, and findings may not be completely generalizable across groups.”
The study was funded by grants from the Norwegian Regional Health Authorities and the South-Eastern Norway Regional Health Authorities. The authors reported numerous potential conflicts of interest, including receiving personal fees and grants from various pharmaceutical companies. Dr. Wallace and Dr. Sparks also reported receiving research support and fees from pharmaceutical companies. Dr. Vande Casteele reported receiving research grants and personal fees from multiple pharmaceutical companies, all outside of the reviewed work.
A version of this article first appeared on Medscape.com.
A new study has found that proactive therapeutic drug monitoring (TDM) with maintenance infliximab is more effective than standard therapy in sustaining control of immune-mediated inflammatory diseases.
The findings from the Norwegian Drug Monitoring B (NOR-DRUM B) trial, published Dec. 21, 2021, in JAMA, provide greater support to the usefulness of TDM in proactively monitoring serum drug levels and antidrug antibodies to infliximab, which has been previously shown to have benefit in patients with inflammatory bowel disease, but leave the benefits of proactive versus reactive monitoring and the cost-effectiveness of the approach in individual immune-mediated inflammatory diseases still open to questioning.
TDM is ‘not the holy grail,’ and that’s OK
“This is an important milestone in the field of TDM with biologics for immunoinflammatory diseases,” Niels Vande Casteele, PharmD, PhD, of the University of California, San Diego, told this news organization. He was not involved in the study.
“When you read through the study, you can see the authors used the TAXIT trial results to inform their study design and the sample size,” he added, referencing his 2015 study on infliximab guide dosing for patients with inflammatory bowel disease, “the first-ever randomized, controlled trial of proactive TDM with any biologic.”
For the TAXIT study’s primary outcome of clinical and biochemical remission at 1 year, “continued concentration-based dosing was not superior to clinically based dosing for achieving remission.” But in regard to their secondary outcome of sustained remission, their results were quite similar to the results of NOR-DRUM B.
“If anything, we already showed a benefit of proactive TDM in 2015,” he said, “but I’m very glad that the authors looked at the trial design and teased out where TDM could be the most important and have the biggest impact, which is to maintain that sustained disease remission over a prolonged period.”
As for next steps, Dr. Vande Casteele noted that TDM isn’t a one-size-fits-all upgrade for drug treatments. But that doesn’t mean it won’t be very useful in many patients.
“What the paper is saying, and what we’ve been finding all along, is that TDM is not the holy grail,” he said. “But it is a tool in the physicians’ toolbox to optimize treatments and maximize efficacy, and there are some patients who truly benefit from it.”
Study details
To determine if proactive TDM with infliximab led to more sustained disease control than standard therapy, first author Silje Watterdal Syversen, MD, PhD, of Diakonhjemmet Hospital in Oslo, and coauthors conducted a 52-week, randomized, parallel-group, open-label trial. From 20 Norwegian hospitals, they recruited 458 patients with rheumatoid arthritis (n = 80), spondyloarthritis (n = 138), psoriatic arthritis (n = 54), ulcerative colitis (n = 81), Crohn’s disease (n = 68), or psoriasis (n = 37) who were undergoing maintenance therapy with the biologic.
The 454 patients who received at least one randomly allocated dose of infliximab were treated with one of two strategies: TDM (n = 227) or standard therapy (n = 227). The TDM group received dose and interval adjustments based on an algorithm that factored in serum drug levels and antidrug antibodies. The standard therapy group was treated on the basis of clinical judgment and physician discretion. The average age across groups was roughly 45 years, and just under 50% were women.
Overall, sustained disease control without worsening was achieved in 167 patients (73.6%) in the TDM group and 127 patients (55.9%) in the standard therapy group, with an estimated adjusted difference of 17.6% (95% confidence interval, 9.0%-26.2%; P < .001). The estimated hazard ratio of disease worsening was 2.1 (95% CI, 1.5-2.9) for standard therapy, compared with TDM. A total of 27 patients (15%) in the standard therapy group and 21 patients (9.2%) in the TDM group developed significant levels of antidrug antibodies, defined here as 50 mcg/L or more.
A total of 34 patients discontinued infliximab in each group; in the TDM group, most discontinued because of antidrug antibody formation, while the main reason for discontinuing in the standard therapy group was disease worsening. Adverse events were reported in 137 patients (60%) in the TDM group and 142 patients (63%) in the standard therapy group.
Removing barriers to TDM
It’s not clear that proactive TDM will benefit treatment with all biologic disease-modifying antirheumatic drugs (bDMARDs), but the findings from Dr. Syversen and colleagues state the clear value of using drug monitoring to guide maintenance therapy with infliximab, Zachary S. Wallace, MD, and Jeffrey A. Sparks, MD, wrote in an accompanying editorial.
“The relatively large sample size and rigorous study design ... helped to overcome some limitations of previous observational studies and small clinical trials that yielded conflicting results regarding TDM,” they added, noting that these findings contrasted somewhat with the NOR-DRUM A trial in which TDM did not improve remission induction in patients initiating infliximab therapy.
Along those lines, they recognized that TDM appears to have a greater effect in patients on maintenance infliximab, compared with those just starting the drug, surmising – among several explanations – that achieving remission in someone beginning treatment is a more difficult outcome to achieve than controlling disease in a patient already in remission.
For now, more clinical trials assessing specific diseases and involving other bDMARDs are needed; Dr. Wallace and Dr. Sparks stated that it’s time to remove barriers to implementing TDM – including the need for medical insurance preauthorization before increasing drug doses – and potentially “introduce a new era in treatment approach to maintenance therapy for patients with immune-mediated inflammatory diseases.”
The authors acknowledged their study’s limitations, including disease worsening being measured in part by patient-physician consensus and thus potentially subject to bias. In addition, they did not have the statistical ability to test TDM effectiveness in each of the six disease groups, noting that “these diseases have inherent differences, and findings may not be completely generalizable across groups.”
The study was funded by grants from the Norwegian Regional Health Authorities and the South-Eastern Norway Regional Health Authorities. The authors reported numerous potential conflicts of interest, including receiving personal fees and grants from various pharmaceutical companies. Dr. Wallace and Dr. Sparks also reported receiving research support and fees from pharmaceutical companies. Dr. Vande Casteele reported receiving research grants and personal fees from multiple pharmaceutical companies, all outside of the reviewed work.
A version of this article first appeared on Medscape.com.
A new study has found that proactive therapeutic drug monitoring (TDM) with maintenance infliximab is more effective than standard therapy in sustaining control of immune-mediated inflammatory diseases.
The findings from the Norwegian Drug Monitoring B (NOR-DRUM B) trial, published Dec. 21, 2021, in JAMA, provide greater support to the usefulness of TDM in proactively monitoring serum drug levels and antidrug antibodies to infliximab, which has been previously shown to have benefit in patients with inflammatory bowel disease, but leave the benefits of proactive versus reactive monitoring and the cost-effectiveness of the approach in individual immune-mediated inflammatory diseases still open to questioning.
TDM is ‘not the holy grail,’ and that’s OK
“This is an important milestone in the field of TDM with biologics for immunoinflammatory diseases,” Niels Vande Casteele, PharmD, PhD, of the University of California, San Diego, told this news organization. He was not involved in the study.
“When you read through the study, you can see the authors used the TAXIT trial results to inform their study design and the sample size,” he added, referencing his 2015 study on infliximab guide dosing for patients with inflammatory bowel disease, “the first-ever randomized, controlled trial of proactive TDM with any biologic.”
For the TAXIT study’s primary outcome of clinical and biochemical remission at 1 year, “continued concentration-based dosing was not superior to clinically based dosing for achieving remission.” But in regard to their secondary outcome of sustained remission, their results were quite similar to the results of NOR-DRUM B.
“If anything, we already showed a benefit of proactive TDM in 2015,” he said, “but I’m very glad that the authors looked at the trial design and teased out where TDM could be the most important and have the biggest impact, which is to maintain that sustained disease remission over a prolonged period.”
As for next steps, Dr. Vande Casteele noted that TDM isn’t a one-size-fits-all upgrade for drug treatments. But that doesn’t mean it won’t be very useful in many patients.
“What the paper is saying, and what we’ve been finding all along, is that TDM is not the holy grail,” he said. “But it is a tool in the physicians’ toolbox to optimize treatments and maximize efficacy, and there are some patients who truly benefit from it.”
Study details
To determine if proactive TDM with infliximab led to more sustained disease control than standard therapy, first author Silje Watterdal Syversen, MD, PhD, of Diakonhjemmet Hospital in Oslo, and coauthors conducted a 52-week, randomized, parallel-group, open-label trial. From 20 Norwegian hospitals, they recruited 458 patients with rheumatoid arthritis (n = 80), spondyloarthritis (n = 138), psoriatic arthritis (n = 54), ulcerative colitis (n = 81), Crohn’s disease (n = 68), or psoriasis (n = 37) who were undergoing maintenance therapy with the biologic.
The 454 patients who received at least one randomly allocated dose of infliximab were treated with one of two strategies: TDM (n = 227) or standard therapy (n = 227). The TDM group received dose and interval adjustments based on an algorithm that factored in serum drug levels and antidrug antibodies. The standard therapy group was treated on the basis of clinical judgment and physician discretion. The average age across groups was roughly 45 years, and just under 50% were women.
Overall, sustained disease control without worsening was achieved in 167 patients (73.6%) in the TDM group and 127 patients (55.9%) in the standard therapy group, with an estimated adjusted difference of 17.6% (95% confidence interval, 9.0%-26.2%; P < .001). The estimated hazard ratio of disease worsening was 2.1 (95% CI, 1.5-2.9) for standard therapy, compared with TDM. A total of 27 patients (15%) in the standard therapy group and 21 patients (9.2%) in the TDM group developed significant levels of antidrug antibodies, defined here as 50 mcg/L or more.
A total of 34 patients discontinued infliximab in each group; in the TDM group, most discontinued because of antidrug antibody formation, while the main reason for discontinuing in the standard therapy group was disease worsening. Adverse events were reported in 137 patients (60%) in the TDM group and 142 patients (63%) in the standard therapy group.
Removing barriers to TDM
It’s not clear that proactive TDM will benefit treatment with all biologic disease-modifying antirheumatic drugs (bDMARDs), but the findings from Dr. Syversen and colleagues state the clear value of using drug monitoring to guide maintenance therapy with infliximab, Zachary S. Wallace, MD, and Jeffrey A. Sparks, MD, wrote in an accompanying editorial.
“The relatively large sample size and rigorous study design ... helped to overcome some limitations of previous observational studies and small clinical trials that yielded conflicting results regarding TDM,” they added, noting that these findings contrasted somewhat with the NOR-DRUM A trial in which TDM did not improve remission induction in patients initiating infliximab therapy.
Along those lines, they recognized that TDM appears to have a greater effect in patients on maintenance infliximab, compared with those just starting the drug, surmising – among several explanations – that achieving remission in someone beginning treatment is a more difficult outcome to achieve than controlling disease in a patient already in remission.
For now, more clinical trials assessing specific diseases and involving other bDMARDs are needed; Dr. Wallace and Dr. Sparks stated that it’s time to remove barriers to implementing TDM – including the need for medical insurance preauthorization before increasing drug doses – and potentially “introduce a new era in treatment approach to maintenance therapy for patients with immune-mediated inflammatory diseases.”
The authors acknowledged their study’s limitations, including disease worsening being measured in part by patient-physician consensus and thus potentially subject to bias. In addition, they did not have the statistical ability to test TDM effectiveness in each of the six disease groups, noting that “these diseases have inherent differences, and findings may not be completely generalizable across groups.”
The study was funded by grants from the Norwegian Regional Health Authorities and the South-Eastern Norway Regional Health Authorities. The authors reported numerous potential conflicts of interest, including receiving personal fees and grants from various pharmaceutical companies. Dr. Wallace and Dr. Sparks also reported receiving research support and fees from pharmaceutical companies. Dr. Vande Casteele reported receiving research grants and personal fees from multiple pharmaceutical companies, all outside of the reviewed work.
A version of this article first appeared on Medscape.com.
FROM JAMA
Guselkumab’s efficacy, safety confirmed in patients with psoriatic arthritis and prior TNFi exposure
A new study has established guselkumab (Tremfya) as both a safe and effective treatment option for psoriatic arthritis (PsA) in patients who had previously responded poorly to tumor necrosis factor inhibitors (TNFis).
“While the positive guselkumab benefit-risk profile observed through week 24 was maintained through 1 year, real-world evidence will further inform long-term guselkumab persistence in TNFi-inadequate response patients,” writes Laura C. Coates, MBChB, PhD, of the University of Oxford (England), and her coauthors. The study was published in the Annals of the Rheumatic Diseases.
Previous studies indicated that the anti–interleukin-23p19 monoclonal antibody improved outcomes in patients with PsA, even after 1 year, but some uncertainty remained regarding the surprisingly similar level of effectiveness in biologic-naive and TNFi-treated patients. Guselkumab is approved for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy and adults with active psoriatic arthritis.
Clarity on guselkumab’s effectiveness in certain patients
“In previous studies that cemented guselkumab as a treatment option for PsA, what was odd was that the results were pretty comparable,” Eric M. Ruderman, MD, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago, Illinois, said in an interview. “We didn’t really have a sense of how well it worked in patients who had failed other biologics, which is where you might expect a drug with a new mechanism to be used when it comes into a particular disease category.
“Not surprisingly, in this study, the overall response rate was a little less than the response rate in the other two trials,” said Dr. Ruderman, who was not involved in the study. “You can’t really compare across studies, but it does fit with what we might expect: People who’ve previously failed a TNF inhibitor might be a little less likely to respond to guselkumab, compared to someone who hasn’t seen a TNF inhibitor.”
When asked about potential follow-up studies, Dr. Ruderman noted that “the missing piece of the puzzle is that we still really have no way to compare this to other biologics. The next step would be to ask, in a single trial, what happens if you give some people TNF inhibitors and some people guselkumab? Just to try to give us context. Is this equivalent? Is it less effective? More effective? Where does it fit? Without that information, rheumatologists may struggle to figure out who is the right person for this drug and how often should they use it.”
Study details
To assess the efficacy and safety of guselkumab in patients who had previously taken TNFis but stopped because of inefficacy or intolerance, the researchers launched a randomized, double-blind study called COSMOS at 84 European sites from March 2019 to November 2020. The study’s 285 patients – 52% of whom were women, with an average overall age of 49 – were assigned to two groups: guselkumab (n = 189) or placebo (n = 96). A total of 88% of all patients had used one TNFi prior; 12% had used two.
The guselkumab group received 100-mg injections at week 0, week 4, and then every 8 weeks through week 44; the placebo group received injections at weeks 0, 4, 12, and 20, followed by 100 mg of guselkumab at weeks 24, 28, 36, and 44. Patients with less than 5% improvement from baseline in both tender and swollen joint counts at week 16 qualified for early escape to “initiate or increase the dose of one permitted concomitant medication up to the maximum allowed dose at the physician’s discretion.” Ultimately, 88% of patients in the guselkumab arm and 83% of the placebo arm completed the study.
At 24 weeks, more than 44% of the guselkumab group achieved a 20% or greater improvement in American College of Rheumatology criteria (ACR20), compared with just under 20% of the placebo group, a difference of nearly 25% (95% confidence interval, 14.1%-35.2%; multiplicity-adjusted P < .001). At 48 weeks, nearly 58% of the guselkumab group had achieved ACR20; of the 51 patients in the placebo arm who started taking guselkumab at week 24, 55% achieved ACR20 by week 48.
Through 24 weeks, 80 patients in the guselkumab group (42%) and 46 patients in the placebo group (48%) experienced adverse events; only 3.7% and 3.1% developed serious adverse events, respectively. The most common adverse events in the guselkumab group at that point included nasopharyngitis (5%) and upper respiratory tract infection (4%), which occurred at a similar frequency (5% and 3%) in the placebo group.
The authors acknowledge their study’s limitations, including imbalances in baseline characteristics such as gender and weight, as well as the COSMOS study being restricted to European patients and thus potentially limiting diversity. In addition, while the COVID-19 pandemic may have increased major protocol deviations near the end of the study, the authors note that “most were related to timing of study visits and did not impact efficacy.”
The study was funded by Janssen, and six authors reported being employees of the company. The authors also acknowledge numerous potential conflicts of interest, including receiving consulting fees and research grants from various pharmaceutical companies, including Janssen. Dr. Ruderman is a consultant for AbbVie, Bristol-Myers Squibb, Eli Lilly, Novartis, Pfizer, and Janssen and served on the data safety monitoring committee for two other phase 3 guselkumab trials.
A version of this article first appeared on Medscape.com.
A new study has established guselkumab (Tremfya) as both a safe and effective treatment option for psoriatic arthritis (PsA) in patients who had previously responded poorly to tumor necrosis factor inhibitors (TNFis).
“While the positive guselkumab benefit-risk profile observed through week 24 was maintained through 1 year, real-world evidence will further inform long-term guselkumab persistence in TNFi-inadequate response patients,” writes Laura C. Coates, MBChB, PhD, of the University of Oxford (England), and her coauthors. The study was published in the Annals of the Rheumatic Diseases.
Previous studies indicated that the anti–interleukin-23p19 monoclonal antibody improved outcomes in patients with PsA, even after 1 year, but some uncertainty remained regarding the surprisingly similar level of effectiveness in biologic-naive and TNFi-treated patients. Guselkumab is approved for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy and adults with active psoriatic arthritis.
Clarity on guselkumab’s effectiveness in certain patients
“In previous studies that cemented guselkumab as a treatment option for PsA, what was odd was that the results were pretty comparable,” Eric M. Ruderman, MD, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago, Illinois, said in an interview. “We didn’t really have a sense of how well it worked in patients who had failed other biologics, which is where you might expect a drug with a new mechanism to be used when it comes into a particular disease category.
“Not surprisingly, in this study, the overall response rate was a little less than the response rate in the other two trials,” said Dr. Ruderman, who was not involved in the study. “You can’t really compare across studies, but it does fit with what we might expect: People who’ve previously failed a TNF inhibitor might be a little less likely to respond to guselkumab, compared to someone who hasn’t seen a TNF inhibitor.”
When asked about potential follow-up studies, Dr. Ruderman noted that “the missing piece of the puzzle is that we still really have no way to compare this to other biologics. The next step would be to ask, in a single trial, what happens if you give some people TNF inhibitors and some people guselkumab? Just to try to give us context. Is this equivalent? Is it less effective? More effective? Where does it fit? Without that information, rheumatologists may struggle to figure out who is the right person for this drug and how often should they use it.”
Study details
To assess the efficacy and safety of guselkumab in patients who had previously taken TNFis but stopped because of inefficacy or intolerance, the researchers launched a randomized, double-blind study called COSMOS at 84 European sites from March 2019 to November 2020. The study’s 285 patients – 52% of whom were women, with an average overall age of 49 – were assigned to two groups: guselkumab (n = 189) or placebo (n = 96). A total of 88% of all patients had used one TNFi prior; 12% had used two.
The guselkumab group received 100-mg injections at week 0, week 4, and then every 8 weeks through week 44; the placebo group received injections at weeks 0, 4, 12, and 20, followed by 100 mg of guselkumab at weeks 24, 28, 36, and 44. Patients with less than 5% improvement from baseline in both tender and swollen joint counts at week 16 qualified for early escape to “initiate or increase the dose of one permitted concomitant medication up to the maximum allowed dose at the physician’s discretion.” Ultimately, 88% of patients in the guselkumab arm and 83% of the placebo arm completed the study.
At 24 weeks, more than 44% of the guselkumab group achieved a 20% or greater improvement in American College of Rheumatology criteria (ACR20), compared with just under 20% of the placebo group, a difference of nearly 25% (95% confidence interval, 14.1%-35.2%; multiplicity-adjusted P < .001). At 48 weeks, nearly 58% of the guselkumab group had achieved ACR20; of the 51 patients in the placebo arm who started taking guselkumab at week 24, 55% achieved ACR20 by week 48.
Through 24 weeks, 80 patients in the guselkumab group (42%) and 46 patients in the placebo group (48%) experienced adverse events; only 3.7% and 3.1% developed serious adverse events, respectively. The most common adverse events in the guselkumab group at that point included nasopharyngitis (5%) and upper respiratory tract infection (4%), which occurred at a similar frequency (5% and 3%) in the placebo group.
The authors acknowledge their study’s limitations, including imbalances in baseline characteristics such as gender and weight, as well as the COSMOS study being restricted to European patients and thus potentially limiting diversity. In addition, while the COVID-19 pandemic may have increased major protocol deviations near the end of the study, the authors note that “most were related to timing of study visits and did not impact efficacy.”
The study was funded by Janssen, and six authors reported being employees of the company. The authors also acknowledge numerous potential conflicts of interest, including receiving consulting fees and research grants from various pharmaceutical companies, including Janssen. Dr. Ruderman is a consultant for AbbVie, Bristol-Myers Squibb, Eli Lilly, Novartis, Pfizer, and Janssen and served on the data safety monitoring committee for two other phase 3 guselkumab trials.
A version of this article first appeared on Medscape.com.
A new study has established guselkumab (Tremfya) as both a safe and effective treatment option for psoriatic arthritis (PsA) in patients who had previously responded poorly to tumor necrosis factor inhibitors (TNFis).
“While the positive guselkumab benefit-risk profile observed through week 24 was maintained through 1 year, real-world evidence will further inform long-term guselkumab persistence in TNFi-inadequate response patients,” writes Laura C. Coates, MBChB, PhD, of the University of Oxford (England), and her coauthors. The study was published in the Annals of the Rheumatic Diseases.
Previous studies indicated that the anti–interleukin-23p19 monoclonal antibody improved outcomes in patients with PsA, even after 1 year, but some uncertainty remained regarding the surprisingly similar level of effectiveness in biologic-naive and TNFi-treated patients. Guselkumab is approved for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy and adults with active psoriatic arthritis.
Clarity on guselkumab’s effectiveness in certain patients
“In previous studies that cemented guselkumab as a treatment option for PsA, what was odd was that the results were pretty comparable,” Eric M. Ruderman, MD, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago, Illinois, said in an interview. “We didn’t really have a sense of how well it worked in patients who had failed other biologics, which is where you might expect a drug with a new mechanism to be used when it comes into a particular disease category.
“Not surprisingly, in this study, the overall response rate was a little less than the response rate in the other two trials,” said Dr. Ruderman, who was not involved in the study. “You can’t really compare across studies, but it does fit with what we might expect: People who’ve previously failed a TNF inhibitor might be a little less likely to respond to guselkumab, compared to someone who hasn’t seen a TNF inhibitor.”
When asked about potential follow-up studies, Dr. Ruderman noted that “the missing piece of the puzzle is that we still really have no way to compare this to other biologics. The next step would be to ask, in a single trial, what happens if you give some people TNF inhibitors and some people guselkumab? Just to try to give us context. Is this equivalent? Is it less effective? More effective? Where does it fit? Without that information, rheumatologists may struggle to figure out who is the right person for this drug and how often should they use it.”
Study details
To assess the efficacy and safety of guselkumab in patients who had previously taken TNFis but stopped because of inefficacy or intolerance, the researchers launched a randomized, double-blind study called COSMOS at 84 European sites from March 2019 to November 2020. The study’s 285 patients – 52% of whom were women, with an average overall age of 49 – were assigned to two groups: guselkumab (n = 189) or placebo (n = 96). A total of 88% of all patients had used one TNFi prior; 12% had used two.
The guselkumab group received 100-mg injections at week 0, week 4, and then every 8 weeks through week 44; the placebo group received injections at weeks 0, 4, 12, and 20, followed by 100 mg of guselkumab at weeks 24, 28, 36, and 44. Patients with less than 5% improvement from baseline in both tender and swollen joint counts at week 16 qualified for early escape to “initiate or increase the dose of one permitted concomitant medication up to the maximum allowed dose at the physician’s discretion.” Ultimately, 88% of patients in the guselkumab arm and 83% of the placebo arm completed the study.
At 24 weeks, more than 44% of the guselkumab group achieved a 20% or greater improvement in American College of Rheumatology criteria (ACR20), compared with just under 20% of the placebo group, a difference of nearly 25% (95% confidence interval, 14.1%-35.2%; multiplicity-adjusted P < .001). At 48 weeks, nearly 58% of the guselkumab group had achieved ACR20; of the 51 patients in the placebo arm who started taking guselkumab at week 24, 55% achieved ACR20 by week 48.
Through 24 weeks, 80 patients in the guselkumab group (42%) and 46 patients in the placebo group (48%) experienced adverse events; only 3.7% and 3.1% developed serious adverse events, respectively. The most common adverse events in the guselkumab group at that point included nasopharyngitis (5%) and upper respiratory tract infection (4%), which occurred at a similar frequency (5% and 3%) in the placebo group.
The authors acknowledge their study’s limitations, including imbalances in baseline characteristics such as gender and weight, as well as the COSMOS study being restricted to European patients and thus potentially limiting diversity. In addition, while the COVID-19 pandemic may have increased major protocol deviations near the end of the study, the authors note that “most were related to timing of study visits and did not impact efficacy.”
The study was funded by Janssen, and six authors reported being employees of the company. The authors also acknowledge numerous potential conflicts of interest, including receiving consulting fees and research grants from various pharmaceutical companies, including Janssen. Dr. Ruderman is a consultant for AbbVie, Bristol-Myers Squibb, Eli Lilly, Novartis, Pfizer, and Janssen and served on the data safety monitoring committee for two other phase 3 guselkumab trials.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
Largest ever review of new daily persistent headache assesses its clinical features
according to a new retrospective chart review.
“Future prospective studies are needed to better understand this disabling disorder,” wrote Randolph W. Evans, MD, of Baylor College of Medicine of Houston, and Dana P. Turner, PhD, of Massachusetts General Hospital and Harvard Medical School in Boston. Their study was published Oct. 28 in Headache.
To categorize the infrequently reported clinical features of NDPH, the researchers launched a retrospective study of patients who were provisionally diagnosed with NDPH by Dr. Evans at an outpatient clinic in Houston from Sept. 1, 2011, to Feb. 28, 2020. Of the 328 patients whose diagnosis ultimately matched the ICHD-3 criteria, the average age at onset was 40.3 years (range 12-87 years). Approximately 70% were White, and nearly 66% were women. Two hundred and sixty were diagnosed with the migraine phenotype and 68 were diagnosed with the tension-type phenotype.
Key features
The median duration of NDPH at the time of the initial consult with Dr. Evans was 0.7 years, and it was 1.9 years at the time of the last visit. Almost 33% of patients with the migraine phenotype had a history of episodic migraine compared with 16.2% with the tension-type phenotype. Headaches were side-locked unilateral in 8.5% (n = 28) of all patients, and 3.6% (n = 12) had a thunderclap onset.
The most common clinical features across all patients included noise sensitivity (72.1%), light sensitivity (71%), moderate pain at the time of initial consult (57.9%), pressure pain (54.9%), and throbbing pain (50.9%). Nausea was reported in 157 patients and vomiting was reported in 48 patients, all of whom were in the migraine phenotype group. Thunderclap onset was far more prevalent in the migraine phenotype group (11 patients) compared with the tension-type phenotype group (1 patient), as was vertigo (19 patients compared with 1) and visual aura (21 compared with 0).
The top precipitating factors across all patients included stressful life events (20.4%), an antecedent upper respiratory infection or flu-like illness (10.1%), and antecedent extracranial surgery (1.5%). Exacerbating or aggravating factors were far more prevalent in the migraine phenotype group compared with the tension-type phenotype group, with stress (14.6% vs. 5.9%), bright or flashing light (10.4% vs. 1.5%), loud noise (8.5% vs. 0%), and lack of sleep (6.5% vs. 4.4%) leading the way.
The months with the most onsets were June (8.5%), January (7.6%), and February (7.6%); there was no clear seasonal or cyclical variation. The most common prognostic type across all patients was persisting (refractory) at 93%, followed by remitting (self-limiting) at 4.3% and relapsing-remitting at 2.7%.
Unlocking a medical mystery
“This is the largest case review study ever published on NDPH, especially because most people think it’s a fairly rare disorder when it’s actually not,” Herbert G. Markley, MD, of the New England Regional Headache Center in Worcester, Mass., said in an interview.
“The thing people need to understand is that they may have a lot of these patients in their practice and not realize it,” he added. “They keep trying one medication after another, and the patients are giving up, and the doctors are giving up. It’s terrible. We don’t know what causes it, and we don’t know how to treat it. It’s one of the biggest mysteries left in medical science.”
“My idea about this condition, and this is shared by others, is that NDPH is not a diagnosis that describes a cohesive group of patients but rather a group of people who share certain features,” Morris Levin, MD, director of the Headache Center at the University of California, San Francisco, said in an interview. “And they would be better served if this diagnosis was split into different categories.”
While praising Dr. Evans and Dr. Turner for their categorization and classification work, Dr. Levin asked, “Let’s say you diagnose someone with NDPH; does that in any way help you with management of this person? The answer is no. Some might say, ‘If you put the patients in the migraine phenotype group, then you can use migraine treatments.’ My point would be: then call it migraine.
“I believe another way to approach NDPH might be to create subcategories of migraine and tension-type headaches,” he added. “A migraine that is either intermittent or nonexistent suddenly becomes daily. That could be a subcategory; rather than being called NDPH, call it ‘new persistent chronic migraine.’ Or ‘new persistent chronic tension-type headache.’ Perhaps that would serve us better in terms of grasping the underlying mechanisms and the best treatment for these patients.”
Along the same lines, Dr. Markley echoed Dr. Evans’ call for more prospective studies and more research on possible medication, hoping to fuel further understanding of this debilitating disorder.
“I think this will be a landmark study for people to look back on,” he said, “especially for anyone going into the headache specialty who has never heard of this type of headache and keeps wondering why they can’t help certain patients, no matter how many medications they try.”
The authors acknowledged their study’s limitations, including its single-center nature and the data abstraction process being performed by just one person. They added, however, that Dr. Evans is a “very experienced researcher with more than 30 years of experience in headache medicine who was abstracting his own patients, data that were very familiar to him.”
Dr. Evans and Dr. Turner declared no potential conflicts of interest.
according to a new retrospective chart review.
“Future prospective studies are needed to better understand this disabling disorder,” wrote Randolph W. Evans, MD, of Baylor College of Medicine of Houston, and Dana P. Turner, PhD, of Massachusetts General Hospital and Harvard Medical School in Boston. Their study was published Oct. 28 in Headache.
To categorize the infrequently reported clinical features of NDPH, the researchers launched a retrospective study of patients who were provisionally diagnosed with NDPH by Dr. Evans at an outpatient clinic in Houston from Sept. 1, 2011, to Feb. 28, 2020. Of the 328 patients whose diagnosis ultimately matched the ICHD-3 criteria, the average age at onset was 40.3 years (range 12-87 years). Approximately 70% were White, and nearly 66% were women. Two hundred and sixty were diagnosed with the migraine phenotype and 68 were diagnosed with the tension-type phenotype.
Key features
The median duration of NDPH at the time of the initial consult with Dr. Evans was 0.7 years, and it was 1.9 years at the time of the last visit. Almost 33% of patients with the migraine phenotype had a history of episodic migraine compared with 16.2% with the tension-type phenotype. Headaches were side-locked unilateral in 8.5% (n = 28) of all patients, and 3.6% (n = 12) had a thunderclap onset.
The most common clinical features across all patients included noise sensitivity (72.1%), light sensitivity (71%), moderate pain at the time of initial consult (57.9%), pressure pain (54.9%), and throbbing pain (50.9%). Nausea was reported in 157 patients and vomiting was reported in 48 patients, all of whom were in the migraine phenotype group. Thunderclap onset was far more prevalent in the migraine phenotype group (11 patients) compared with the tension-type phenotype group (1 patient), as was vertigo (19 patients compared with 1) and visual aura (21 compared with 0).
The top precipitating factors across all patients included stressful life events (20.4%), an antecedent upper respiratory infection or flu-like illness (10.1%), and antecedent extracranial surgery (1.5%). Exacerbating or aggravating factors were far more prevalent in the migraine phenotype group compared with the tension-type phenotype group, with stress (14.6% vs. 5.9%), bright or flashing light (10.4% vs. 1.5%), loud noise (8.5% vs. 0%), and lack of sleep (6.5% vs. 4.4%) leading the way.
The months with the most onsets were June (8.5%), January (7.6%), and February (7.6%); there was no clear seasonal or cyclical variation. The most common prognostic type across all patients was persisting (refractory) at 93%, followed by remitting (self-limiting) at 4.3% and relapsing-remitting at 2.7%.
Unlocking a medical mystery
“This is the largest case review study ever published on NDPH, especially because most people think it’s a fairly rare disorder when it’s actually not,” Herbert G. Markley, MD, of the New England Regional Headache Center in Worcester, Mass., said in an interview.
“The thing people need to understand is that they may have a lot of these patients in their practice and not realize it,” he added. “They keep trying one medication after another, and the patients are giving up, and the doctors are giving up. It’s terrible. We don’t know what causes it, and we don’t know how to treat it. It’s one of the biggest mysteries left in medical science.”
“My idea about this condition, and this is shared by others, is that NDPH is not a diagnosis that describes a cohesive group of patients but rather a group of people who share certain features,” Morris Levin, MD, director of the Headache Center at the University of California, San Francisco, said in an interview. “And they would be better served if this diagnosis was split into different categories.”
While praising Dr. Evans and Dr. Turner for their categorization and classification work, Dr. Levin asked, “Let’s say you diagnose someone with NDPH; does that in any way help you with management of this person? The answer is no. Some might say, ‘If you put the patients in the migraine phenotype group, then you can use migraine treatments.’ My point would be: then call it migraine.
“I believe another way to approach NDPH might be to create subcategories of migraine and tension-type headaches,” he added. “A migraine that is either intermittent or nonexistent suddenly becomes daily. That could be a subcategory; rather than being called NDPH, call it ‘new persistent chronic migraine.’ Or ‘new persistent chronic tension-type headache.’ Perhaps that would serve us better in terms of grasping the underlying mechanisms and the best treatment for these patients.”
Along the same lines, Dr. Markley echoed Dr. Evans’ call for more prospective studies and more research on possible medication, hoping to fuel further understanding of this debilitating disorder.
“I think this will be a landmark study for people to look back on,” he said, “especially for anyone going into the headache specialty who has never heard of this type of headache and keeps wondering why they can’t help certain patients, no matter how many medications they try.”
The authors acknowledged their study’s limitations, including its single-center nature and the data abstraction process being performed by just one person. They added, however, that Dr. Evans is a “very experienced researcher with more than 30 years of experience in headache medicine who was abstracting his own patients, data that were very familiar to him.”
Dr. Evans and Dr. Turner declared no potential conflicts of interest.
according to a new retrospective chart review.
“Future prospective studies are needed to better understand this disabling disorder,” wrote Randolph W. Evans, MD, of Baylor College of Medicine of Houston, and Dana P. Turner, PhD, of Massachusetts General Hospital and Harvard Medical School in Boston. Their study was published Oct. 28 in Headache.
To categorize the infrequently reported clinical features of NDPH, the researchers launched a retrospective study of patients who were provisionally diagnosed with NDPH by Dr. Evans at an outpatient clinic in Houston from Sept. 1, 2011, to Feb. 28, 2020. Of the 328 patients whose diagnosis ultimately matched the ICHD-3 criteria, the average age at onset was 40.3 years (range 12-87 years). Approximately 70% were White, and nearly 66% were women. Two hundred and sixty were diagnosed with the migraine phenotype and 68 were diagnosed with the tension-type phenotype.
Key features
The median duration of NDPH at the time of the initial consult with Dr. Evans was 0.7 years, and it was 1.9 years at the time of the last visit. Almost 33% of patients with the migraine phenotype had a history of episodic migraine compared with 16.2% with the tension-type phenotype. Headaches were side-locked unilateral in 8.5% (n = 28) of all patients, and 3.6% (n = 12) had a thunderclap onset.
The most common clinical features across all patients included noise sensitivity (72.1%), light sensitivity (71%), moderate pain at the time of initial consult (57.9%), pressure pain (54.9%), and throbbing pain (50.9%). Nausea was reported in 157 patients and vomiting was reported in 48 patients, all of whom were in the migraine phenotype group. Thunderclap onset was far more prevalent in the migraine phenotype group (11 patients) compared with the tension-type phenotype group (1 patient), as was vertigo (19 patients compared with 1) and visual aura (21 compared with 0).
The top precipitating factors across all patients included stressful life events (20.4%), an antecedent upper respiratory infection or flu-like illness (10.1%), and antecedent extracranial surgery (1.5%). Exacerbating or aggravating factors were far more prevalent in the migraine phenotype group compared with the tension-type phenotype group, with stress (14.6% vs. 5.9%), bright or flashing light (10.4% vs. 1.5%), loud noise (8.5% vs. 0%), and lack of sleep (6.5% vs. 4.4%) leading the way.
The months with the most onsets were June (8.5%), January (7.6%), and February (7.6%); there was no clear seasonal or cyclical variation. The most common prognostic type across all patients was persisting (refractory) at 93%, followed by remitting (self-limiting) at 4.3% and relapsing-remitting at 2.7%.
Unlocking a medical mystery
“This is the largest case review study ever published on NDPH, especially because most people think it’s a fairly rare disorder when it’s actually not,” Herbert G. Markley, MD, of the New England Regional Headache Center in Worcester, Mass., said in an interview.
“The thing people need to understand is that they may have a lot of these patients in their practice and not realize it,” he added. “They keep trying one medication after another, and the patients are giving up, and the doctors are giving up. It’s terrible. We don’t know what causes it, and we don’t know how to treat it. It’s one of the biggest mysteries left in medical science.”
“My idea about this condition, and this is shared by others, is that NDPH is not a diagnosis that describes a cohesive group of patients but rather a group of people who share certain features,” Morris Levin, MD, director of the Headache Center at the University of California, San Francisco, said in an interview. “And they would be better served if this diagnosis was split into different categories.”
While praising Dr. Evans and Dr. Turner for their categorization and classification work, Dr. Levin asked, “Let’s say you diagnose someone with NDPH; does that in any way help you with management of this person? The answer is no. Some might say, ‘If you put the patients in the migraine phenotype group, then you can use migraine treatments.’ My point would be: then call it migraine.
“I believe another way to approach NDPH might be to create subcategories of migraine and tension-type headaches,” he added. “A migraine that is either intermittent or nonexistent suddenly becomes daily. That could be a subcategory; rather than being called NDPH, call it ‘new persistent chronic migraine.’ Or ‘new persistent chronic tension-type headache.’ Perhaps that would serve us better in terms of grasping the underlying mechanisms and the best treatment for these patients.”
Along the same lines, Dr. Markley echoed Dr. Evans’ call for more prospective studies and more research on possible medication, hoping to fuel further understanding of this debilitating disorder.
“I think this will be a landmark study for people to look back on,” he said, “especially for anyone going into the headache specialty who has never heard of this type of headache and keeps wondering why they can’t help certain patients, no matter how many medications they try.”
The authors acknowledged their study’s limitations, including its single-center nature and the data abstraction process being performed by just one person. They added, however, that Dr. Evans is a “very experienced researcher with more than 30 years of experience in headache medicine who was abstracting his own patients, data that were very familiar to him.”
Dr. Evans and Dr. Turner declared no potential conflicts of interest.
FROM HEADACHE
PT may lower risk of long-term opioid use after knee replacement
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.
A new study has found that physical therapy may lead to a reduced risk of long-term opioid use in patients who have undergone total knee replacement (TKR).
“Greater number of PT intervention sessions and earlier initiation of outpatient PT care after TKR were associated with lower odds of long-term opioid use,” authors from Boston University wrote in their report on the study, which was published online Oct. 27 in JAMA Network Open.
“In previous large studies, we’ve seen that physical therapy can reduce pain in people with knee osteoarthritis, which is usually the primary indication for TKR,” study coauthor Deepak Kumar, PT, PhD, said in an interview. “But the association of physical therapy with opioid use in people with knee replacement has not yet been explored.
“The reason we focused on opioid use in these patients is because the number of knee replacement surgeries is going up exponentially,” Dr. Kumar said. “And, depending on which data you look at, from one-third to up to half of people who undergo knee replacement and have used opioids before end up becoming long-term users. Even in people who have not used them before, 5%-8% become long-term users after the surgery.
“Given how many surgeries are happening – and that number is expected to keep going up – the number of people who are becoming long-term opioid users is not trivial,” he said.
Study details
To assess the value of PT in reducing opioid use in this subset of patients, the authors reviewed records from the OptumLabs Data Warehouse insurance claims database to identify 67,322 eligible participants aged 40 or older who underwent TKR from Jan. 1, 2001, to Dec. 31, 2016. Of those patients, 38,408 were opioid naive and 28,914 had taken opioids before. The authors evaluated long-term opioid use – defined as 90 days or more of filled prescriptions – during a 12-month outcome assessment period that varied depending on differences in post-TKR PT start date and duration.
The researchers found a significantly lower likelihood of long-term opioid use associated with receipt of any PT before TKR among patients who had not taken opioids before (adjusted odds ratio [aOR], 0.75; 95% confidence interval, 0.60-0.95) and those who had taken opioids in the past (aOR, 0.75; 95% CI, 0.70-0.80).
Investigators found that 2.2% of participants in the opioid-naive group and 32.5% of those in the opioid-experienced group used opioids long-term after TKR. Approximately 76% of participants overall received outpatient PT within the 90 days after surgery, and the receipt of post-TKR PT at any point was associated with lower odds of long-term opioid use in the opioid-experienced group (aOR, 0.75; 95% CI, 0.70-0.79).
Among the opioid-experienced group, receiving between 6 and 12 PT sessions (aOR, 0.82; 95% CI, 0.75-0.90) or ≥ 13 sessions (aOR, 0.71; 95% CI, 0.65-0.77) were both associated with lower odds of long-term opioid use, compared with those who received 1-5 sessions. Beginning PT 31-60 days or 61-90 days after surgery was associated with greater odds of long-term opioid use across both cohorts, compared with those who initiated therapy within 30 days of TKR.
Physical therapy: Underexplored option for pain in knee replacement
One finding caught the researchers slightly off guard: There was no association between active physical therapy and reduced odds of long-term opioid use. “From prior studies, at least in people with knee osteoarthritis, we know that active interventions were more useful than passive interventions,” Dr. Kumar said.
That said, he added that there is still some professional uncertainty regarding “the right type or the right components of physical therapy for managing pain in this population.” Regardless, he believes their study emphasizes the benefits of PT as a pain alleviator in these patients, especially those who have previously used opioids.
“Pharmaceuticals have side effects. Injections are not super effective,” he said. “The idea behind focusing on physical therapy interventions is that it’s widely available, it does you no harm, and it could potentially be lower cost to both the payers and the providers.”
The authors acknowledged their study’s limitations, including not adjusting for opioid use within the 90 days after surgery as well as the different outcome assessment periods for pre-TKR and post-TKR PT exposures. In addition, they admitted that some of the patients who received PT could have been among those less likely to be treated with opioids, and vice versa. “A randomized clinical trial,” they wrote, “would be required to disentangle these issues.”
The study was supported by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kumar reported receiving grants from the National Institutes of Health during the conduct of the study and grants from Pfizer for unrelated projects outside the submitted work. The full list of author disclosures can be found with the original article.
A version of this article first appeared on Medscape.com.