User login
Mental health legislative proposals proliferate in Congress
Mental health reform is hot in Congress right now, with several bills having been introduced in the House and Senate.
The most comprehensive House bill – and the one that has received the most consideration to date – is H.R. 2646, the Helping Families in Mental Health Crisis Act of 2015, introduced by Rep. Tim Murphy (R-Penn.), a clinical psychologist.
If enacted, the bill would combine mental and physical health electronic medical records and adjust HIPAA (the Health Insurance Portability and Accountability Act of 1996) to allow caregivers to access a patient’s mental health records without his or her consent, although psychotherapy notes would be redacted.
In the Senate, S. 1945, the Mental Health Reform Act of 2015, introduced by Sen. Chris Murphy (D-Conn.) and Sen. Bill Cassidy (R-La.), would integrate physical and mental health records and would lift the cap on inpatient psychiatric services covered under Medicare.
Also on the Senate side is S. 1893, the Mental Health Awareness and Improvement Act of 2015 introduced by Lamar Alexander (R-Tenn.). This bill covers much of the same ground as both Murphys’ bills, however not much would directly impact daily clinical practice.
A bill introduced by Senate Majority Whip John Cornyn (R-Tex.) would clarify which mental health records could be included in the National Instant Criminal Background Check System and would provide funding to identify and treat prisoners with serious mental illness. To date, however, the bill has no cosponsors.
On Twitter @whitneymcknight
Mental health reform is hot in Congress right now, with several bills having been introduced in the House and Senate.
The most comprehensive House bill – and the one that has received the most consideration to date – is H.R. 2646, the Helping Families in Mental Health Crisis Act of 2015, introduced by Rep. Tim Murphy (R-Penn.), a clinical psychologist.
If enacted, the bill would combine mental and physical health electronic medical records and adjust HIPAA (the Health Insurance Portability and Accountability Act of 1996) to allow caregivers to access a patient’s mental health records without his or her consent, although psychotherapy notes would be redacted.
In the Senate, S. 1945, the Mental Health Reform Act of 2015, introduced by Sen. Chris Murphy (D-Conn.) and Sen. Bill Cassidy (R-La.), would integrate physical and mental health records and would lift the cap on inpatient psychiatric services covered under Medicare.
Also on the Senate side is S. 1893, the Mental Health Awareness and Improvement Act of 2015 introduced by Lamar Alexander (R-Tenn.). This bill covers much of the same ground as both Murphys’ bills, however not much would directly impact daily clinical practice.
A bill introduced by Senate Majority Whip John Cornyn (R-Tex.) would clarify which mental health records could be included in the National Instant Criminal Background Check System and would provide funding to identify and treat prisoners with serious mental illness. To date, however, the bill has no cosponsors.
On Twitter @whitneymcknight
Mental health reform is hot in Congress right now, with several bills having been introduced in the House and Senate.
The most comprehensive House bill – and the one that has received the most consideration to date – is H.R. 2646, the Helping Families in Mental Health Crisis Act of 2015, introduced by Rep. Tim Murphy (R-Penn.), a clinical psychologist.
If enacted, the bill would combine mental and physical health electronic medical records and adjust HIPAA (the Health Insurance Portability and Accountability Act of 1996) to allow caregivers to access a patient’s mental health records without his or her consent, although psychotherapy notes would be redacted.
In the Senate, S. 1945, the Mental Health Reform Act of 2015, introduced by Sen. Chris Murphy (D-Conn.) and Sen. Bill Cassidy (R-La.), would integrate physical and mental health records and would lift the cap on inpatient psychiatric services covered under Medicare.
Also on the Senate side is S. 1893, the Mental Health Awareness and Improvement Act of 2015 introduced by Lamar Alexander (R-Tenn.). This bill covers much of the same ground as both Murphys’ bills, however not much would directly impact daily clinical practice.
A bill introduced by Senate Majority Whip John Cornyn (R-Tex.) would clarify which mental health records could be included in the National Instant Criminal Background Check System and would provide funding to identify and treat prisoners with serious mental illness. To date, however, the bill has no cosponsors.
On Twitter @whitneymcknight
FDA okays prophylactic Pradaxa for VTE in hip replacement
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight
The Food and Drug Administration has approved dabigatran for the prevention of deep venous thrombosis and pulmonary embolism for patients after hip replacement surgery.
The FDA’s approval was based on the results of two randomized, double-blind, phase III trials in patients undergoing total hip replacement, Boehringer Ingelheim, the manufacturer of the direct thrombin inhibitor, announced.
In RE-NOVATE I, the first trial, 3,494 patients were randomly assigned to three groups receiving prophylactic treatment with one of two doses of dabigatran (220 mg or 150 mg) once daily, or to the low-molecular-weight heparin enoxaparin at 40 mg once daily for 28-35 days. The first study drug arm was given 110 mg on the day of surgery and 220 mg daily thereafter; the second study drug arm received a dose of 75 mg on the day of surgery and 150 mg daily thereafter. Patients taking the dabigatran (Pradaxa) at 220 mg had a lower composite total of venous thromboembolism (VTE) and all-cause mortality (6.0%) than did those on enoxaparin 40 mg (6.7%), meeting the noninferiority mark (Lancet. 2007 Sep 15;370[9591]:949-56).
In RE-NOVATE II, 2,055 patients were randomly assigned prophylactic treatment for 28-35 days with the study drug dosed at 220 mg once daily, or enoxaparin 40 mg once daily. Patients receiving the study drug were treated with a dose of 110 mg on the day of surgery and 220 mg daily thereafter. The composite total of VTE and all-cause death occurred in 7.7% of patients in the study group vs. 8.8% of patients in the enoxaparin group, which was within the margin for noninferiority (Thromb Haemost. 2011 Apr;105[4]:721-9).
However, there were higher rates of major bleeding in RE-NOVATE I (2.0%, 1.6%) and II (1.4%, 0.9%) with 220 mg vs. enoxaparin. In both studies, the rate of major gastrointestinal bleeds in patients was the same (0.1%) for both the study and control drugs. The rate of any GI bleeds was 1.4% for the study drug and 0.9% for enoxaparin. The most common adverse events in both studies were GI disorders. The incidence rate was the same across all treatment groups (39.5%). Dyspepsia occurred more frequently in patients receiving the study drug (4.1%), compared with those taking enoxaparin (3.8%). Gastritislike symptoms were less common in patients receiving the study drug (0.6%), compared with enoxaparin (1.0%). Clinical myocardial infarction was reported in two (0.1%) study patients and six (0.3%) enoxaparin patients.
Pradaxa was initially indicated by the FDA in 2010 to reduce stroke and systemic embolism risk in patients with nonvalvular atrial fibrillation. In 2014, the FDA approved two additional indications for the drug for the treatment of VTE in patients treated with a parenteral anticoagulant for 5-10 day and to reduce the risk of recurrent VTE in patients who have been previously treated.
On Twitter @whitneymcknight
AUDIO: Do you know the most important question to ask a patient who presents with depressive symptoms?
WASHINGTON – When patients present with what you suspect is depression, how confident are you in your ability to differentiate your diagnosis from distress or demoralization? In this interview, Dr. James L. Griffith, the Leon M. Yochelson Professor and chair of the department of psychiatry and behavioral Sciences at George Washington University, Washington, discusses the clinical signs of all three, and offers his thoughts on how to confidently diagnose and treat them.
“Humans are built to heal through many redundant pathways,” Dr. Griffith says. “We don’t have to hit all of them.”
In addition to using antidepressants and forms of talk therapy, Dr. Griffith talks about how leveraging hope, a patient’s relationships, and the insights they have into their lives and ailments can lead to better outcomes. Dr. Griffith also shares the single most important question to ask patients when addressing their behavioral health needs.
On Twitter @whitneymcknight
WASHINGTON – When patients present with what you suspect is depression, how confident are you in your ability to differentiate your diagnosis from distress or demoralization? In this interview, Dr. James L. Griffith, the Leon M. Yochelson Professor and chair of the department of psychiatry and behavioral Sciences at George Washington University, Washington, discusses the clinical signs of all three, and offers his thoughts on how to confidently diagnose and treat them.
“Humans are built to heal through many redundant pathways,” Dr. Griffith says. “We don’t have to hit all of them.”
In addition to using antidepressants and forms of talk therapy, Dr. Griffith talks about how leveraging hope, a patient’s relationships, and the insights they have into their lives and ailments can lead to better outcomes. Dr. Griffith also shares the single most important question to ask patients when addressing their behavioral health needs.
On Twitter @whitneymcknight
WASHINGTON – When patients present with what you suspect is depression, how confident are you in your ability to differentiate your diagnosis from distress or demoralization? In this interview, Dr. James L. Griffith, the Leon M. Yochelson Professor and chair of the department of psychiatry and behavioral Sciences at George Washington University, Washington, discusses the clinical signs of all three, and offers his thoughts on how to confidently diagnose and treat them.
“Humans are built to heal through many redundant pathways,” Dr. Griffith says. “We don’t have to hit all of them.”
In addition to using antidepressants and forms of talk therapy, Dr. Griffith talks about how leveraging hope, a patient’s relationships, and the insights they have into their lives and ailments can lead to better outcomes. Dr. Griffith also shares the single most important question to ask patients when addressing their behavioral health needs.
On Twitter @whitneymcknight
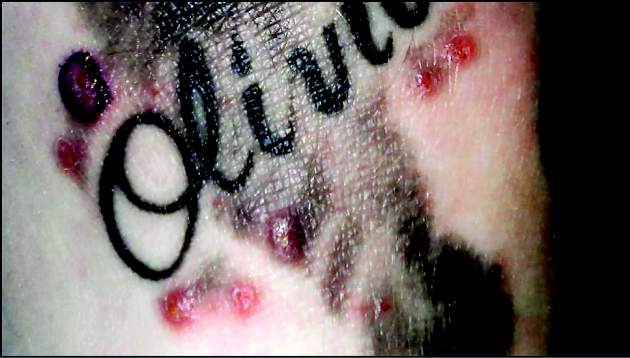
Survey illustrates opportunities for dermatologists to help manage tattoo risks, regrets
About a fifth of adults reported a history of an itchy tattoo, and most said they believed that dermatologists are knowledgeable about managing tattoo complications, according to a survey of 501 adults with one or more tattoos.
The survey, conducted by researchers at Tulane University, New Orleans, found that almost 23% had had pruritus at the tattoo site more than a month after being tattooed. In addition, 3.2% said they had a tattoo that had become infected; these people were significantly more likely to have more tattoos and to have undergone more tattooing sessions, compared with those who had not had an infected tattoo.
In addition, 3.8% said they had pain at the site of at least one tattoo after the 1-month mark, reported Dr. Walter Liszewski of Tulane University and his coauthors (Dermatol Surg. 2015 Nov;41[11]:1283-9).
Their 18-question survey was administered to 501 people aged 18 years and older with at least one tattoo in the French Quarter over a 3-day period in January 2015. They were from 38 states, Puerto Rico, and Washington, D.C.; almost 55% were women.
When it came to where to seek help managing these tattoo-related complications and misgivings, the dermatologist’s office ran second to the tattoo parlor. Of those surveyed, 93.2% of all participants believed tattoo artists were “very knowledgeable” or “knowledgeable” about the allergic and infectious tattoo complications, while 78.8% reported thinking the same about dermatologists – which suggests “that there is considerable trust in the ability of dermatologists to manage tattoo complications,” the authors pointed out.
Almost 70% thought primary care physicians and almost 40% thought pharmacists were similarly competent.
Almost a fifth of those surveyed said they had received at least one tattoo in a setting other than a tattoo parlor, such as in prison or at a party. And about a fifth said they had received one or more tattoos while inebriated.
The rates of tattoo regret were higher among those who were younger when they had received their first tattoo, ranging from 11.4% of those who were aged 21 years and older when they got their first tattoo to 35.1% of those who had their first tattoo before aged 18 years. Moreover, almost 43% of those who had their first tattoo before age 18 said they were would be interested in having at least one tattoo removed for free, compared with 19.1% and 14.4% of those who received their first tattoo between ages 18 and 20 years and at ages 21 and older, respectively.
“Given the incidence of pruritic tattoos, tattoo regret, unsafe tattooing practices, and desire to have tattoos removed among the study participants, dermatologists have an opportunity to provide medical and cosmetic care, as well as anticipatory guidance, for these patients,” the authors concluded.
On Twitter @whitneymcknight
About a fifth of adults reported a history of an itchy tattoo, and most said they believed that dermatologists are knowledgeable about managing tattoo complications, according to a survey of 501 adults with one or more tattoos.
The survey, conducted by researchers at Tulane University, New Orleans, found that almost 23% had had pruritus at the tattoo site more than a month after being tattooed. In addition, 3.2% said they had a tattoo that had become infected; these people were significantly more likely to have more tattoos and to have undergone more tattooing sessions, compared with those who had not had an infected tattoo.
In addition, 3.8% said they had pain at the site of at least one tattoo after the 1-month mark, reported Dr. Walter Liszewski of Tulane University and his coauthors (Dermatol Surg. 2015 Nov;41[11]:1283-9).
Their 18-question survey was administered to 501 people aged 18 years and older with at least one tattoo in the French Quarter over a 3-day period in January 2015. They were from 38 states, Puerto Rico, and Washington, D.C.; almost 55% were women.
When it came to where to seek help managing these tattoo-related complications and misgivings, the dermatologist’s office ran second to the tattoo parlor. Of those surveyed, 93.2% of all participants believed tattoo artists were “very knowledgeable” or “knowledgeable” about the allergic and infectious tattoo complications, while 78.8% reported thinking the same about dermatologists – which suggests “that there is considerable trust in the ability of dermatologists to manage tattoo complications,” the authors pointed out.
Almost 70% thought primary care physicians and almost 40% thought pharmacists were similarly competent.
Almost a fifth of those surveyed said they had received at least one tattoo in a setting other than a tattoo parlor, such as in prison or at a party. And about a fifth said they had received one or more tattoos while inebriated.
The rates of tattoo regret were higher among those who were younger when they had received their first tattoo, ranging from 11.4% of those who were aged 21 years and older when they got their first tattoo to 35.1% of those who had their first tattoo before aged 18 years. Moreover, almost 43% of those who had their first tattoo before age 18 said they were would be interested in having at least one tattoo removed for free, compared with 19.1% and 14.4% of those who received their first tattoo between ages 18 and 20 years and at ages 21 and older, respectively.
“Given the incidence of pruritic tattoos, tattoo regret, unsafe tattooing practices, and desire to have tattoos removed among the study participants, dermatologists have an opportunity to provide medical and cosmetic care, as well as anticipatory guidance, for these patients,” the authors concluded.
On Twitter @whitneymcknight
About a fifth of adults reported a history of an itchy tattoo, and most said they believed that dermatologists are knowledgeable about managing tattoo complications, according to a survey of 501 adults with one or more tattoos.
The survey, conducted by researchers at Tulane University, New Orleans, found that almost 23% had had pruritus at the tattoo site more than a month after being tattooed. In addition, 3.2% said they had a tattoo that had become infected; these people were significantly more likely to have more tattoos and to have undergone more tattooing sessions, compared with those who had not had an infected tattoo.
In addition, 3.8% said they had pain at the site of at least one tattoo after the 1-month mark, reported Dr. Walter Liszewski of Tulane University and his coauthors (Dermatol Surg. 2015 Nov;41[11]:1283-9).
Their 18-question survey was administered to 501 people aged 18 years and older with at least one tattoo in the French Quarter over a 3-day period in January 2015. They were from 38 states, Puerto Rico, and Washington, D.C.; almost 55% were women.
When it came to where to seek help managing these tattoo-related complications and misgivings, the dermatologist’s office ran second to the tattoo parlor. Of those surveyed, 93.2% of all participants believed tattoo artists were “very knowledgeable” or “knowledgeable” about the allergic and infectious tattoo complications, while 78.8% reported thinking the same about dermatologists – which suggests “that there is considerable trust in the ability of dermatologists to manage tattoo complications,” the authors pointed out.
Almost 70% thought primary care physicians and almost 40% thought pharmacists were similarly competent.
Almost a fifth of those surveyed said they had received at least one tattoo in a setting other than a tattoo parlor, such as in prison or at a party. And about a fifth said they had received one or more tattoos while inebriated.
The rates of tattoo regret were higher among those who were younger when they had received their first tattoo, ranging from 11.4% of those who were aged 21 years and older when they got their first tattoo to 35.1% of those who had their first tattoo before aged 18 years. Moreover, almost 43% of those who had their first tattoo before age 18 said they were would be interested in having at least one tattoo removed for free, compared with 19.1% and 14.4% of those who received their first tattoo between ages 18 and 20 years and at ages 21 and older, respectively.
“Given the incidence of pruritic tattoos, tattoo regret, unsafe tattooing practices, and desire to have tattoos removed among the study participants, dermatologists have an opportunity to provide medical and cosmetic care, as well as anticipatory guidance, for these patients,” the authors concluded.
On Twitter @whitneymcknight
FROM DERMATOLOGIC SURGERY
Key clinical point: Opportunities exist for dermatologists to provide tattoo-related medical and cosmetic care.
Major finding: About one-fifth of surveyed adults reported having had a pruritic tattoo at least 1 month after receiving the tattoo; most said they believed dermatologists could help manage tattoo complications.
Data source: A survey of 501 adults with at least one tattoo, conducted in New Orleans, which included questions about tattoo complications, regret, and practices.
Disclosures: The authors reported no relevant financial disclosures.
AK clearance rates maintained 1 year after thermally modulated PDT
Clearance rates for actinic keratoses (AKs) on the extremities were prolonged by adding thermal modulation to photodynamic therapy (PDT), a study has shown.
The single-site study of 17 adults with 10 or more AKs on their arms or legs evaluated thermally modulated PDT: 20% 5-aminolevulinic acid (ALA) applied to skin, occluded with plastic wrap and warmed with an electric heating pad to a median 41.2° C for one hour, then exposed to blue light. Treatment resulted in 90% clearance rates at 3 months and 12 months, reported Dr. Andrea Willey, a dermatologist in Sacramento, Calif., and her associates (Dermatol Surg. 2015 Nov;41[11]:1290-5).
They were inspired to conduct the study as a follow-on to a recently published pilot study of three patients, which found that clearance rates of AKs on the arm treated with thermally modulated 5-ALA blue light PDT persisted up to 14 months after treatment, when compared with the control arm, which was not heated.
In their study, there were 724 grade 1 and 2 AKs at baseline – a median of 15 per extremity; patients were followed up at 3, 6, 9, and 12 months. The total lesion count across the entire cohort was 70 at 3 months, 69 at 6 months, 64 at 9 months, and 72 at 1 year. No new AKs were seen in the treated areas during the 1-year follow-up.
There were 13 grade 3 hypertrophic AK lesions among all study participants. Although these lesions persisted, Dr. Willey and her coauthors noted that they did shrink initially after treatment.
Adverse events associated with the combination therapy were low, and included mild erythema and crusting up to 1 week post treatment, indicating the therapy was well tolerated, and patient satisfaction was high.
The results “suggest that mild skin warming may both improve efficacy and reduce variability of response to PDT in practice,” the authors wrote.
On Twitter @whitneymcknight
Clearance rates for actinic keratoses (AKs) on the extremities were prolonged by adding thermal modulation to photodynamic therapy (PDT), a study has shown.
The single-site study of 17 adults with 10 or more AKs on their arms or legs evaluated thermally modulated PDT: 20% 5-aminolevulinic acid (ALA) applied to skin, occluded with plastic wrap and warmed with an electric heating pad to a median 41.2° C for one hour, then exposed to blue light. Treatment resulted in 90% clearance rates at 3 months and 12 months, reported Dr. Andrea Willey, a dermatologist in Sacramento, Calif., and her associates (Dermatol Surg. 2015 Nov;41[11]:1290-5).
They were inspired to conduct the study as a follow-on to a recently published pilot study of three patients, which found that clearance rates of AKs on the arm treated with thermally modulated 5-ALA blue light PDT persisted up to 14 months after treatment, when compared with the control arm, which was not heated.
In their study, there were 724 grade 1 and 2 AKs at baseline – a median of 15 per extremity; patients were followed up at 3, 6, 9, and 12 months. The total lesion count across the entire cohort was 70 at 3 months, 69 at 6 months, 64 at 9 months, and 72 at 1 year. No new AKs were seen in the treated areas during the 1-year follow-up.
There were 13 grade 3 hypertrophic AK lesions among all study participants. Although these lesions persisted, Dr. Willey and her coauthors noted that they did shrink initially after treatment.
Adverse events associated with the combination therapy were low, and included mild erythema and crusting up to 1 week post treatment, indicating the therapy was well tolerated, and patient satisfaction was high.
The results “suggest that mild skin warming may both improve efficacy and reduce variability of response to PDT in practice,” the authors wrote.
On Twitter @whitneymcknight
Clearance rates for actinic keratoses (AKs) on the extremities were prolonged by adding thermal modulation to photodynamic therapy (PDT), a study has shown.
The single-site study of 17 adults with 10 or more AKs on their arms or legs evaluated thermally modulated PDT: 20% 5-aminolevulinic acid (ALA) applied to skin, occluded with plastic wrap and warmed with an electric heating pad to a median 41.2° C for one hour, then exposed to blue light. Treatment resulted in 90% clearance rates at 3 months and 12 months, reported Dr. Andrea Willey, a dermatologist in Sacramento, Calif., and her associates (Dermatol Surg. 2015 Nov;41[11]:1290-5).
They were inspired to conduct the study as a follow-on to a recently published pilot study of three patients, which found that clearance rates of AKs on the arm treated with thermally modulated 5-ALA blue light PDT persisted up to 14 months after treatment, when compared with the control arm, which was not heated.
In their study, there were 724 grade 1 and 2 AKs at baseline – a median of 15 per extremity; patients were followed up at 3, 6, 9, and 12 months. The total lesion count across the entire cohort was 70 at 3 months, 69 at 6 months, 64 at 9 months, and 72 at 1 year. No new AKs were seen in the treated areas during the 1-year follow-up.
There were 13 grade 3 hypertrophic AK lesions among all study participants. Although these lesions persisted, Dr. Willey and her coauthors noted that they did shrink initially after treatment.
Adverse events associated with the combination therapy were low, and included mild erythema and crusting up to 1 week post treatment, indicating the therapy was well tolerated, and patient satisfaction was high.
The results “suggest that mild skin warming may both improve efficacy and reduce variability of response to PDT in practice,” the authors wrote.
On Twitter @whitneymcknight
FROM DERMATOLOGIC SURGERY
Key clinical point: Mildly warming affected skin sites during photodynamic therapy can prolong the clearance rates for AKs on the extremities.
Major finding: AK clearance rates on the extremities remained at about 90% during the 1 year after treatment with thermally modulated PDT.
Data source: A single site, prospective 1-year study evaluating treatment with thermally modulated PDT for 17 adults with 10 or more AKs on their extremities.
Disclosures: Dr. Willey is a member of the scientific advisory board of DUSA Pharmaceuticals; she received a research grant, equipment loan, and study drug from the company. Her two coauthors had no relevant disclosures.
Physicians contribute to opioid addiction crisis
Underlying the nation’s opioid abuse epidemic is the fact that physicians are undertrained, underresourced, and using incorrect assessment tools to diagnose and treat chronic pain, according to an expert panel.
“I don’t know how a primary care doctor can manage a chronic pain patient,” said Dr. William S. Jacobs, chief of addiction medicine at the Medical College of Georgia, Augusta. “They’re given 15 minutes to treat multiple medical problems, from diabetes, hypertension, heart failure, to asthma, and oh, by the way, the patient has chronic pain. I couldn’t handle one of those problems in 15 minutes. How can they manage to do it?” asked Dr. Jacobs, who also serves as medical director of the Bluff Plantation addiction recovery center, also in Augusta.
He spoke during a Nov. 17 webcast called “Opiate Misuse, Overdose & Addiction – Causes & Solutions” as part of the Fall Lecture Series at the Drug Enforcement Agency Museum in Arlington, Va.
About 2.5 million Americans are addicted to prescription pain medications and increasingly to heroin, according to the National Institute on Drug Abuse. Nearly two-thirds of those people were introduced to opiates in their physician’s office, according to panelist Theodore Cicero, Ph.D., a professor of psychiatry and vice chairman for research at Washington University in St. Louis.
According to NIDA, in 1991, 76 million opioid prescriptions were written, compared with the 207 million written in 2013.
Although that figure is down from the 219 million opioid prescriptions written in 2011, Dr. Cicero presented new data indicating this is not as promising as it might seem, because the less legal opiates are prescribed, the more people addicted to them are crossing over to heroin. “It’s cheaper, easy to get, easy to inject, and provides an intense high,” Dr. Cicero said.
Whereas stigma might have prevented users in the past from crossing over to heroin, once addicted, users care less about their reputation and more about avoiding withdrawal, Dr. Cicero said.
Compounding the situation, said Dr. Jacobs, is the insidious nature of a legal drug being prescribed by a trusted source, who too often has not been specifically educated in pain management, and who does not pay enough attention to patient education and informed consent. Dr. Jacobs said chronic pain patients already treated with opiates such as oxycontin and hydrocodone before their referral to a pain specialist often “have no idea how dangerous the medications that they were being prescribed were.”

Both presenters said not enough data exist currently to predict who is most likely to become addicted to prescription medication, but Dr. Cicero said his research (N Engl J Med. 2015;373:1789-90), based on a survey of 267 prescription painkiller medicine users who crossed over to heroin, indicate they tend to have in common “extremely low self-esteem and lots of problems with anxiety and depression, and they find that, at least temporarily, the opiates relieve these.”
This might be in part tied to what Dr. Jacobs said is the brain’s inability to distinguish physical pain from emotional pain. “It’s no wonder that when we give a patient chronic opiates, and they develop emotional stress in their lives, that they turn to the opiates to try and fix that.”
That pain is subjective, and not as easily quantified as hypertension, for example, which also makes it difficult for physicians to know the appropriate timing of intervening with opiates when a patient reports pain. Plus, said Dr. Jacobs, since the 1990s when pain became “the fifth vital sign,” the parameters of chronic pain gradually have shrunk from a condition that lasted a year, to 6 months, to 3 months, until now, patients undergoing any given surgery are sent home with a prescription for pain medication to last for 2 weeks, whether or not it is indicated.
When prescribers do suspect that a pain medication might be indicated, Dr. Jacobs said, analog scales for assessing pain are “worthless,” because they don’t help to identify the cause of a patient’s pain, nor do they indicate any psychiatric comorbidities. Instead, Dr. Jacobs specifically recommended the validated Mankoski Pain Scale, which he said provides a comprehensive and specific initial assessment, not only of pain, but the context in which it occurs.
Dr. Jacobs and Dr. Cicero had no disclosures.
On Twitter @whitneymcknight
Underlying the nation’s opioid abuse epidemic is the fact that physicians are undertrained, underresourced, and using incorrect assessment tools to diagnose and treat chronic pain, according to an expert panel.
“I don’t know how a primary care doctor can manage a chronic pain patient,” said Dr. William S. Jacobs, chief of addiction medicine at the Medical College of Georgia, Augusta. “They’re given 15 minutes to treat multiple medical problems, from diabetes, hypertension, heart failure, to asthma, and oh, by the way, the patient has chronic pain. I couldn’t handle one of those problems in 15 minutes. How can they manage to do it?” asked Dr. Jacobs, who also serves as medical director of the Bluff Plantation addiction recovery center, also in Augusta.
He spoke during a Nov. 17 webcast called “Opiate Misuse, Overdose & Addiction – Causes & Solutions” as part of the Fall Lecture Series at the Drug Enforcement Agency Museum in Arlington, Va.
About 2.5 million Americans are addicted to prescription pain medications and increasingly to heroin, according to the National Institute on Drug Abuse. Nearly two-thirds of those people were introduced to opiates in their physician’s office, according to panelist Theodore Cicero, Ph.D., a professor of psychiatry and vice chairman for research at Washington University in St. Louis.
According to NIDA, in 1991, 76 million opioid prescriptions were written, compared with the 207 million written in 2013.
Although that figure is down from the 219 million opioid prescriptions written in 2011, Dr. Cicero presented new data indicating this is not as promising as it might seem, because the less legal opiates are prescribed, the more people addicted to them are crossing over to heroin. “It’s cheaper, easy to get, easy to inject, and provides an intense high,” Dr. Cicero said.
Whereas stigma might have prevented users in the past from crossing over to heroin, once addicted, users care less about their reputation and more about avoiding withdrawal, Dr. Cicero said.
Compounding the situation, said Dr. Jacobs, is the insidious nature of a legal drug being prescribed by a trusted source, who too often has not been specifically educated in pain management, and who does not pay enough attention to patient education and informed consent. Dr. Jacobs said chronic pain patients already treated with opiates such as oxycontin and hydrocodone before their referral to a pain specialist often “have no idea how dangerous the medications that they were being prescribed were.”

Both presenters said not enough data exist currently to predict who is most likely to become addicted to prescription medication, but Dr. Cicero said his research (N Engl J Med. 2015;373:1789-90), based on a survey of 267 prescription painkiller medicine users who crossed over to heroin, indicate they tend to have in common “extremely low self-esteem and lots of problems with anxiety and depression, and they find that, at least temporarily, the opiates relieve these.”
This might be in part tied to what Dr. Jacobs said is the brain’s inability to distinguish physical pain from emotional pain. “It’s no wonder that when we give a patient chronic opiates, and they develop emotional stress in their lives, that they turn to the opiates to try and fix that.”
That pain is subjective, and not as easily quantified as hypertension, for example, which also makes it difficult for physicians to know the appropriate timing of intervening with opiates when a patient reports pain. Plus, said Dr. Jacobs, since the 1990s when pain became “the fifth vital sign,” the parameters of chronic pain gradually have shrunk from a condition that lasted a year, to 6 months, to 3 months, until now, patients undergoing any given surgery are sent home with a prescription for pain medication to last for 2 weeks, whether or not it is indicated.
When prescribers do suspect that a pain medication might be indicated, Dr. Jacobs said, analog scales for assessing pain are “worthless,” because they don’t help to identify the cause of a patient’s pain, nor do they indicate any psychiatric comorbidities. Instead, Dr. Jacobs specifically recommended the validated Mankoski Pain Scale, which he said provides a comprehensive and specific initial assessment, not only of pain, but the context in which it occurs.
Dr. Jacobs and Dr. Cicero had no disclosures.
On Twitter @whitneymcknight
Underlying the nation’s opioid abuse epidemic is the fact that physicians are undertrained, underresourced, and using incorrect assessment tools to diagnose and treat chronic pain, according to an expert panel.
“I don’t know how a primary care doctor can manage a chronic pain patient,” said Dr. William S. Jacobs, chief of addiction medicine at the Medical College of Georgia, Augusta. “They’re given 15 minutes to treat multiple medical problems, from diabetes, hypertension, heart failure, to asthma, and oh, by the way, the patient has chronic pain. I couldn’t handle one of those problems in 15 minutes. How can they manage to do it?” asked Dr. Jacobs, who also serves as medical director of the Bluff Plantation addiction recovery center, also in Augusta.
He spoke during a Nov. 17 webcast called “Opiate Misuse, Overdose & Addiction – Causes & Solutions” as part of the Fall Lecture Series at the Drug Enforcement Agency Museum in Arlington, Va.
About 2.5 million Americans are addicted to prescription pain medications and increasingly to heroin, according to the National Institute on Drug Abuse. Nearly two-thirds of those people were introduced to opiates in their physician’s office, according to panelist Theodore Cicero, Ph.D., a professor of psychiatry and vice chairman for research at Washington University in St. Louis.
According to NIDA, in 1991, 76 million opioid prescriptions were written, compared with the 207 million written in 2013.
Although that figure is down from the 219 million opioid prescriptions written in 2011, Dr. Cicero presented new data indicating this is not as promising as it might seem, because the less legal opiates are prescribed, the more people addicted to them are crossing over to heroin. “It’s cheaper, easy to get, easy to inject, and provides an intense high,” Dr. Cicero said.
Whereas stigma might have prevented users in the past from crossing over to heroin, once addicted, users care less about their reputation and more about avoiding withdrawal, Dr. Cicero said.
Compounding the situation, said Dr. Jacobs, is the insidious nature of a legal drug being prescribed by a trusted source, who too often has not been specifically educated in pain management, and who does not pay enough attention to patient education and informed consent. Dr. Jacobs said chronic pain patients already treated with opiates such as oxycontin and hydrocodone before their referral to a pain specialist often “have no idea how dangerous the medications that they were being prescribed were.”

Both presenters said not enough data exist currently to predict who is most likely to become addicted to prescription medication, but Dr. Cicero said his research (N Engl J Med. 2015;373:1789-90), based on a survey of 267 prescription painkiller medicine users who crossed over to heroin, indicate they tend to have in common “extremely low self-esteem and lots of problems with anxiety and depression, and they find that, at least temporarily, the opiates relieve these.”
This might be in part tied to what Dr. Jacobs said is the brain’s inability to distinguish physical pain from emotional pain. “It’s no wonder that when we give a patient chronic opiates, and they develop emotional stress in their lives, that they turn to the opiates to try and fix that.”
That pain is subjective, and not as easily quantified as hypertension, for example, which also makes it difficult for physicians to know the appropriate timing of intervening with opiates when a patient reports pain. Plus, said Dr. Jacobs, since the 1990s when pain became “the fifth vital sign,” the parameters of chronic pain gradually have shrunk from a condition that lasted a year, to 6 months, to 3 months, until now, patients undergoing any given surgery are sent home with a prescription for pain medication to last for 2 weeks, whether or not it is indicated.
When prescribers do suspect that a pain medication might be indicated, Dr. Jacobs said, analog scales for assessing pain are “worthless,” because they don’t help to identify the cause of a patient’s pain, nor do they indicate any psychiatric comorbidities. Instead, Dr. Jacobs specifically recommended the validated Mankoski Pain Scale, which he said provides a comprehensive and specific initial assessment, not only of pain, but the context in which it occurs.
Dr. Jacobs and Dr. Cicero had no disclosures.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM A DEA LECTURE ON OPIATE MISUSE
Female cardiologists make about $32,000 less than male counterparts
Women in cardiology make considerably less than men do, according to research presented Nov. 8 at the American Heart Association scientific sessions.
“There was a $31,749 gender difference in salary that was not explained by the detailed measures of personal, job, and practice characteristics,” according to study coauthor Dr. Pamela S. Douglas, who presented the findings.
“The lack of gender diversity in the cardiology workforce is striking, with recent workforce estimates suggesting that women constitute only about 12% of general cardiologists and even smaller proportions of specialties like interventional cardiology and clinical cardiac electrophysiology,” according to Dr. Douglas, professor of medicine at Duke University and a member in the Duke Clinical Research Institute, both in Durham, N.C.
Using data from MedAxiom, a membership network and service provider for cardiology practices, hospitals, and academic centers, Dr. Douglas and her colleagues analyzed data on 229 women and 2,450 men from 161 practices.
They found women were less likely to have an interventional subspecialty (11% vs 39%) and were nearly twice as likely to be noninvasive cardiologists (53% vs 28%).
Women worked full time 80% of the time, compared with 91% of men (P less than .001), but worked fewer half-days (387 for women vs. 406 for men; P = .001). The number of mean number of work relative value units generated by women was 7,404, compared with 9,497 for men, (P less than . 001), according to the study, which was published simultaneously in the Journal of the American College of Cardiology.
After researchers adjusted for geographical region; practice size and makeup; measured job and productivity characteristics, such as type of subspecialty, compensation models, and the whether there was a female administrative director, Dr. Douglas said women in the analysis should have earned an average of $31,749 more than they actually did, as confirmed by a further multivariate analysis of the direction and magnitude of the independent association between gender and salary.
Women have made up about half of the enrollment at U.S. medical schools for more than a decade, but only 21% of all first-year cardiology fellows in 2012-2013 were women, according to the American Board of Internal Medicine.
“The findings should also motivate efforts to ensure equity in pay, including the promotion of greater transparency and consistency in salary and reward observed,” according to Dr. Douglas.
On Twitter @whitneymcknight
Women in cardiology make considerably less than men do, according to research presented Nov. 8 at the American Heart Association scientific sessions.
“There was a $31,749 gender difference in salary that was not explained by the detailed measures of personal, job, and practice characteristics,” according to study coauthor Dr. Pamela S. Douglas, who presented the findings.
“The lack of gender diversity in the cardiology workforce is striking, with recent workforce estimates suggesting that women constitute only about 12% of general cardiologists and even smaller proportions of specialties like interventional cardiology and clinical cardiac electrophysiology,” according to Dr. Douglas, professor of medicine at Duke University and a member in the Duke Clinical Research Institute, both in Durham, N.C.
Using data from MedAxiom, a membership network and service provider for cardiology practices, hospitals, and academic centers, Dr. Douglas and her colleagues analyzed data on 229 women and 2,450 men from 161 practices.
They found women were less likely to have an interventional subspecialty (11% vs 39%) and were nearly twice as likely to be noninvasive cardiologists (53% vs 28%).
Women worked full time 80% of the time, compared with 91% of men (P less than .001), but worked fewer half-days (387 for women vs. 406 for men; P = .001). The number of mean number of work relative value units generated by women was 7,404, compared with 9,497 for men, (P less than . 001), according to the study, which was published simultaneously in the Journal of the American College of Cardiology.
After researchers adjusted for geographical region; practice size and makeup; measured job and productivity characteristics, such as type of subspecialty, compensation models, and the whether there was a female administrative director, Dr. Douglas said women in the analysis should have earned an average of $31,749 more than they actually did, as confirmed by a further multivariate analysis of the direction and magnitude of the independent association between gender and salary.
Women have made up about half of the enrollment at U.S. medical schools for more than a decade, but only 21% of all first-year cardiology fellows in 2012-2013 were women, according to the American Board of Internal Medicine.
“The findings should also motivate efforts to ensure equity in pay, including the promotion of greater transparency and consistency in salary and reward observed,” according to Dr. Douglas.
On Twitter @whitneymcknight
Women in cardiology make considerably less than men do, according to research presented Nov. 8 at the American Heart Association scientific sessions.
“There was a $31,749 gender difference in salary that was not explained by the detailed measures of personal, job, and practice characteristics,” according to study coauthor Dr. Pamela S. Douglas, who presented the findings.
“The lack of gender diversity in the cardiology workforce is striking, with recent workforce estimates suggesting that women constitute only about 12% of general cardiologists and even smaller proportions of specialties like interventional cardiology and clinical cardiac electrophysiology,” according to Dr. Douglas, professor of medicine at Duke University and a member in the Duke Clinical Research Institute, both in Durham, N.C.
Using data from MedAxiom, a membership network and service provider for cardiology practices, hospitals, and academic centers, Dr. Douglas and her colleagues analyzed data on 229 women and 2,450 men from 161 practices.
They found women were less likely to have an interventional subspecialty (11% vs 39%) and were nearly twice as likely to be noninvasive cardiologists (53% vs 28%).
Women worked full time 80% of the time, compared with 91% of men (P less than .001), but worked fewer half-days (387 for women vs. 406 for men; P = .001). The number of mean number of work relative value units generated by women was 7,404, compared with 9,497 for men, (P less than . 001), according to the study, which was published simultaneously in the Journal of the American College of Cardiology.
After researchers adjusted for geographical region; practice size and makeup; measured job and productivity characteristics, such as type of subspecialty, compensation models, and the whether there was a female administrative director, Dr. Douglas said women in the analysis should have earned an average of $31,749 more than they actually did, as confirmed by a further multivariate analysis of the direction and magnitude of the independent association between gender and salary.
Women have made up about half of the enrollment at U.S. medical schools for more than a decade, but only 21% of all first-year cardiology fellows in 2012-2013 were women, according to the American Board of Internal Medicine.
“The findings should also motivate efforts to ensure equity in pay, including the promotion of greater transparency and consistency in salary and reward observed,” according to Dr. Douglas.
On Twitter @whitneymcknight
FROM AHA 2015
Key clinical point: Women in cardiology make considerably less than men do.
Major finding: Adjusted for subspecialty tasks, practice characteristics, and productivity, average annual pay for women in cardiology was $31,749 less than expected.
Data source: Data analysis of national sample of 2,679 cardiologists.
Disclosures: Study coauthor Joseph Sasson, Ph.D., is employed by MedAxiom, a sponsor of this study. Coauthor Patrick J. White is a shareholder and president of MedAxiom.
TCT: Lower MI, thrombosis, higher bleeding with extended DAPT in patients with everolimus-eluting stents
SAN FRANCISCO – Continued thienopyridine therapy beyond 1 year in patients treated with an everolimus-eluting stent was associated with significantly lower rates of myocardial infarction and thrombosis, but an increased risk of bleeding, study results have shown.
Dr. James B. Hermiller of St. Vincent´s Medical Group, Indianapolis, presented the data at the Transcatheter Cardiovascular Therapeutics annual meeting. The results were also published online (JACC: Cardiovasc Interv 2015. doi: 10.1016/j.jcin.2015.10.001).
In a post hoc analysis of a subset of patients from the DAPT (Dual Antiplatelet Therapy) study, Dr. James B. Hermiller Jr. and his colleagues evaluated major adverse cardiac and cerebrovascular event (MACCE) and bleeding events in 4,703 patients who’d been treated with an everolimus-eluting stent and 1 year of therapy with thienopyridine and aspirin before being randomly assigned to 18 additional months of the same DAPT, or an additional 18 months of aspirin plus placebo.
The original DAPT Study, a multisite, international, double-blinded, trial of 25,682 coronary stent patients was done to evaluate the efficacy and safety of various combinations of types of drug-eluting stents plus DAPT.
Results from the subanalysis of the everolimus-eluting stent group were that at 12-30 months post percutaneous intervention, those given additional DAPT had significantly reduced thrombosis (P =.04) and MI (P =.01), compared with aspirin-only controls, but the DAPT test cohort had more moderate to severe bleeding complications (P = .01). Composite death, MI, or stroke rates were also better in the placebo group (P = .42), Dr. Hermiller said at the meeting sponsored by the Cardiovascular Research Foundation.
The continuous-DAPT group had a stent thrombosis rate of 0.3%, compared with 0.7% of the 12-month–only group (hazard ratio, 0.38). The rate of MI in the test group was 2.11% vs. 3.2% in controls (HR, 0.63). The DAPT test group had a 2.5% rate of increased bleeding vs. 1.3% in the placebo group (HR, 1.79).
Patients in the subanalysis were typically older than across all original study groups – typically, men in their early 60s who were more likely to have been treated with clopidogrel (84.4% vs. 48.2%), and to have a slightly lower body mass index, although they were still obese (30.3 vs. 30.8 kg/m2).
The subanalysis group also had a slightly higher rate of history of cancer (P less than .001), a data point that Dr. Hermiller and his coauthors noted in the study accounted for the DAPT continuation group’s significantly increased all-cause mortality, compared with the placebo group (2.2% vs. 1.1%, P =.02).
In an interview, Dr. Spencer B. King, president of the Heart and Vascular Institute of St. Joseph’s Health System in Georgia, and comoderator of the session where the data were presented, also emphasized this finding’s importance, since “cancer increases bleeding risk and is one of the conditions in which prolonged DAPT should usually be avoided.”
The most frequent cause of noncardiovascular death in the DAPT-continuation group was cancer: 18 of the test group vs. 4 in the placebo group (P =.002); 3 of the study group’s cancer deaths involved bleeding. Overall, the second most common cause of death was bleeding, which occurred more in the DAPT test group than in placebo (P = .04).
Dr. Hermiller and his coauthors wrote that the therapeutic window for continued thienopyridine therapy “may be narrow” since the number needed to treat to benefit for stent thrombosis was 235 over 18 months. For MI it was 98. The number needed to treat to harm for moderate or severe bleeding was 84.
“Therefore, meticulous assessment of bleeding risk should always impact decisions regarding thienopyridine therapy duration.”
However, they also noted that current data suggest a net benefit to continuing therapy with absolute reductions in stent thrombosis or MI of about 0.2%, an effect that was exceeded in the everolimus-eluting stent patient subset.
Dr. Hermiller is a consultant for Abbott Vascular, Boston Scientific, Medtronic, and St. Jude Medical. The study also was sponsored by the Harvard Clinical Research Institute Harvard Clinical Research Institute and funded by Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Bristol-Myers Squibb Company/Sanofi Pharmaceuticals Partnership, Eli Lilly and Company, and Daiichi Sankyo Company Limited and the U.S. Department of Health & Human Services.
On Twitter @whitneymcknight
SAN FRANCISCO – Continued thienopyridine therapy beyond 1 year in patients treated with an everolimus-eluting stent was associated with significantly lower rates of myocardial infarction and thrombosis, but an increased risk of bleeding, study results have shown.
Dr. James B. Hermiller of St. Vincent´s Medical Group, Indianapolis, presented the data at the Transcatheter Cardiovascular Therapeutics annual meeting. The results were also published online (JACC: Cardiovasc Interv 2015. doi: 10.1016/j.jcin.2015.10.001).
In a post hoc analysis of a subset of patients from the DAPT (Dual Antiplatelet Therapy) study, Dr. James B. Hermiller Jr. and his colleagues evaluated major adverse cardiac and cerebrovascular event (MACCE) and bleeding events in 4,703 patients who’d been treated with an everolimus-eluting stent and 1 year of therapy with thienopyridine and aspirin before being randomly assigned to 18 additional months of the same DAPT, or an additional 18 months of aspirin plus placebo.
The original DAPT Study, a multisite, international, double-blinded, trial of 25,682 coronary stent patients was done to evaluate the efficacy and safety of various combinations of types of drug-eluting stents plus DAPT.
Results from the subanalysis of the everolimus-eluting stent group were that at 12-30 months post percutaneous intervention, those given additional DAPT had significantly reduced thrombosis (P =.04) and MI (P =.01), compared with aspirin-only controls, but the DAPT test cohort had more moderate to severe bleeding complications (P = .01). Composite death, MI, or stroke rates were also better in the placebo group (P = .42), Dr. Hermiller said at the meeting sponsored by the Cardiovascular Research Foundation.
The continuous-DAPT group had a stent thrombosis rate of 0.3%, compared with 0.7% of the 12-month–only group (hazard ratio, 0.38). The rate of MI in the test group was 2.11% vs. 3.2% in controls (HR, 0.63). The DAPT test group had a 2.5% rate of increased bleeding vs. 1.3% in the placebo group (HR, 1.79).
Patients in the subanalysis were typically older than across all original study groups – typically, men in their early 60s who were more likely to have been treated with clopidogrel (84.4% vs. 48.2%), and to have a slightly lower body mass index, although they were still obese (30.3 vs. 30.8 kg/m2).
The subanalysis group also had a slightly higher rate of history of cancer (P less than .001), a data point that Dr. Hermiller and his coauthors noted in the study accounted for the DAPT continuation group’s significantly increased all-cause mortality, compared with the placebo group (2.2% vs. 1.1%, P =.02).
In an interview, Dr. Spencer B. King, president of the Heart and Vascular Institute of St. Joseph’s Health System in Georgia, and comoderator of the session where the data were presented, also emphasized this finding’s importance, since “cancer increases bleeding risk and is one of the conditions in which prolonged DAPT should usually be avoided.”
The most frequent cause of noncardiovascular death in the DAPT-continuation group was cancer: 18 of the test group vs. 4 in the placebo group (P =.002); 3 of the study group’s cancer deaths involved bleeding. Overall, the second most common cause of death was bleeding, which occurred more in the DAPT test group than in placebo (P = .04).
Dr. Hermiller and his coauthors wrote that the therapeutic window for continued thienopyridine therapy “may be narrow” since the number needed to treat to benefit for stent thrombosis was 235 over 18 months. For MI it was 98. The number needed to treat to harm for moderate or severe bleeding was 84.
“Therefore, meticulous assessment of bleeding risk should always impact decisions regarding thienopyridine therapy duration.”
However, they also noted that current data suggest a net benefit to continuing therapy with absolute reductions in stent thrombosis or MI of about 0.2%, an effect that was exceeded in the everolimus-eluting stent patient subset.
Dr. Hermiller is a consultant for Abbott Vascular, Boston Scientific, Medtronic, and St. Jude Medical. The study also was sponsored by the Harvard Clinical Research Institute Harvard Clinical Research Institute and funded by Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Bristol-Myers Squibb Company/Sanofi Pharmaceuticals Partnership, Eli Lilly and Company, and Daiichi Sankyo Company Limited and the U.S. Department of Health & Human Services.
On Twitter @whitneymcknight
SAN FRANCISCO – Continued thienopyridine therapy beyond 1 year in patients treated with an everolimus-eluting stent was associated with significantly lower rates of myocardial infarction and thrombosis, but an increased risk of bleeding, study results have shown.
Dr. James B. Hermiller of St. Vincent´s Medical Group, Indianapolis, presented the data at the Transcatheter Cardiovascular Therapeutics annual meeting. The results were also published online (JACC: Cardiovasc Interv 2015. doi: 10.1016/j.jcin.2015.10.001).
In a post hoc analysis of a subset of patients from the DAPT (Dual Antiplatelet Therapy) study, Dr. James B. Hermiller Jr. and his colleagues evaluated major adverse cardiac and cerebrovascular event (MACCE) and bleeding events in 4,703 patients who’d been treated with an everolimus-eluting stent and 1 year of therapy with thienopyridine and aspirin before being randomly assigned to 18 additional months of the same DAPT, or an additional 18 months of aspirin plus placebo.
The original DAPT Study, a multisite, international, double-blinded, trial of 25,682 coronary stent patients was done to evaluate the efficacy and safety of various combinations of types of drug-eluting stents plus DAPT.
Results from the subanalysis of the everolimus-eluting stent group were that at 12-30 months post percutaneous intervention, those given additional DAPT had significantly reduced thrombosis (P =.04) and MI (P =.01), compared with aspirin-only controls, but the DAPT test cohort had more moderate to severe bleeding complications (P = .01). Composite death, MI, or stroke rates were also better in the placebo group (P = .42), Dr. Hermiller said at the meeting sponsored by the Cardiovascular Research Foundation.
The continuous-DAPT group had a stent thrombosis rate of 0.3%, compared with 0.7% of the 12-month–only group (hazard ratio, 0.38). The rate of MI in the test group was 2.11% vs. 3.2% in controls (HR, 0.63). The DAPT test group had a 2.5% rate of increased bleeding vs. 1.3% in the placebo group (HR, 1.79).
Patients in the subanalysis were typically older than across all original study groups – typically, men in their early 60s who were more likely to have been treated with clopidogrel (84.4% vs. 48.2%), and to have a slightly lower body mass index, although they were still obese (30.3 vs. 30.8 kg/m2).
The subanalysis group also had a slightly higher rate of history of cancer (P less than .001), a data point that Dr. Hermiller and his coauthors noted in the study accounted for the DAPT continuation group’s significantly increased all-cause mortality, compared with the placebo group (2.2% vs. 1.1%, P =.02).
In an interview, Dr. Spencer B. King, president of the Heart and Vascular Institute of St. Joseph’s Health System in Georgia, and comoderator of the session where the data were presented, also emphasized this finding’s importance, since “cancer increases bleeding risk and is one of the conditions in which prolonged DAPT should usually be avoided.”
The most frequent cause of noncardiovascular death in the DAPT-continuation group was cancer: 18 of the test group vs. 4 in the placebo group (P =.002); 3 of the study group’s cancer deaths involved bleeding. Overall, the second most common cause of death was bleeding, which occurred more in the DAPT test group than in placebo (P = .04).
Dr. Hermiller and his coauthors wrote that the therapeutic window for continued thienopyridine therapy “may be narrow” since the number needed to treat to benefit for stent thrombosis was 235 over 18 months. For MI it was 98. The number needed to treat to harm for moderate or severe bleeding was 84.
“Therefore, meticulous assessment of bleeding risk should always impact decisions regarding thienopyridine therapy duration.”
However, they also noted that current data suggest a net benefit to continuing therapy with absolute reductions in stent thrombosis or MI of about 0.2%, an effect that was exceeded in the everolimus-eluting stent patient subset.
Dr. Hermiller is a consultant for Abbott Vascular, Boston Scientific, Medtronic, and St. Jude Medical. The study also was sponsored by the Harvard Clinical Research Institute Harvard Clinical Research Institute and funded by Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Bristol-Myers Squibb Company/Sanofi Pharmaceuticals Partnership, Eli Lilly and Company, and Daiichi Sankyo Company Limited and the U.S. Department of Health & Human Services.
On Twitter @whitneymcknight
AT TCT 2015
Key clinical point: The window of extended DAPT therapy may be narrow in patients stented with an everolimus-eluting stent.
Major finding: Patients randomly assigned to 18 months of thienopyridine plus aspirin after 1 year of DAPT had significantly reduced thrombosis (P = .04) and MI (P = .01) vs. placebo plus aspirin but more bleeding (P = .01).
Data source: Subanalysis of 4,703 patients from a double-blind, international, multisite, randomized, controlled trial of 25,682 patients.
Disclosures: Dr. Hermiller is a consultant for Abbott Vascular, Boston Scientific, Medtronic, and St. Jude Medical. The study also was sponsored by the Harvard Clinical Research Institute Harvard Clinical Research Institute and funded by Abbott, Boston Scientific Corporation, Cordis Corporation, Medtronic, Bristol-Myers Squibb Company/Sanofi Pharmaceuticals Partnership, Eli Lilly and Company, and Daiichi Sankyo Company Limited and the U.S. Department of Health & Human Services.
Polymer-free drug-coated stent matches drug-eluting stent at 5 years
A polymer-free, drug-coated stent was as safe and effective as a conventional drug-eluting stent at 5 years in the treatment of de novo coronary lesions, a first-in-man study has shown.
A stent coated with a lower dose of the drug Biolimus A9 did not reach the same efficacy rate as the higher dose.
The results may change practice when treating complex patients at high risk for bleeding, according to lead investigator, Dr. Ricardo A. Costa of the Instituto Dante Pazzanese de Cardiologia, São Paulo. The results were presented the results at this year’s Transcatheter Cardiovascular Therapeutics annual meeting, and simultaneously published online (JACC Cardiovasc Interv. 2015. doi: 10.1016/j.jcin.2015.09.008).
The lipophilic Biolimus A9 is intended for use with a proprietary polymer-free, stainless steel stent (BioFreedom, Biosensors International) that is structured to hold the drug on its abluminal surface. The drug was developed to avoid the chronic inflammation and local toxicity associated with the durable polymers used in first-generation drug-eluting stents. It was also intended to reduce the duration of dual-antiplatelet therapy, which often can surpass 6 months and is contraindicated in patients at high risk for bleeding.
In this first-in-man study conducted at four sites across Germany, 182 patients with de novo lesions were randomly assigned to treatment with a standard-dose drug-coated stent, a low-dose drug-coated stent, or to receive a first-generation paclitaxel-eluting stent.
About two-thirds of all study participants were men in their mid- to late-60s and 70s with stable angina. Prior percutaneous intervention had been performed in a third of the standard-dose drug-coated stent group, 44% of the low-dose group, and 46% of the control group.
Angiographic follow-up at 4-months showed that in-stent late lumen loss, a surrogate for neointimal hyperplasia, in both drug-coated cohorts was significantly lower than in the drug-eluting stent group (0.08 mm and 0.12 mm vs. 0.37 mm, respectively; P less than .0001 for regular dose vs. drug-eluting stent; P = .002 for low-dose vs. drug-eluting stent).
Angiographic follow-up at 1 year showed in-stent late lumen loss was 0.17 mm in the drug-coated stent group vs. 0.35 mm in the drug-eluting stent group (P = .001 for noninferiority; P = .11 for superiority); although at 0.22 mm, the low-dose group did not achieve noninferiority (P = .21).
At 5-year follow-up in 175 of the original 182 patients, there were no significant differences in major adverse cardiac events between the standard-dose (23.8%) and low-dose (26.4%) drug-coated stents and the drug-eluting stent (20.3%), or in clinically-indicated target lesion revascularization, at 10.8%, 13.4%, and 10.2%, respectively. In addition, there were no reported incidences of stent thrombosis across the cohorts.
The results of this trial support those from the LEADERS FREE trial, also presented at this year’s TCT annual meeting, which indicated that the drug-coated stent with only 1 month of dual antiplatelet therapy was safe and effective in complex patients at high risk for post-PCI bleeding.
Dr. Costa and his coinvestigators wrote that although their data were based on patients with simple, discrete lesions, they hope their findings lead to larger studies, and they concluded that the drug-coated stent “may offer less dependence on prolonged dual antiplatelet therapy than polymer-coated drug-eluting stents, while maintaining efficacy; thus, it may be suitable for those at high risk for bleeding.”
This trial was underwritten by Biosensors International. Dr. Costa has received speakers fees from Biosensors, Medtronic, and Daiichi-Sankyo. Three coinvestigators disclosed ties to Bristol-Myers Squibb/Sanofi, the Medicines Company, Lilly/Daiichi-Sankyo, Abbott Vascular, AstraZeneca, Regado Biosciences, and Johnson & Johnson.
On Twitter @whitneymcknight
A polymer-free, drug-coated stent was as safe and effective as a conventional drug-eluting stent at 5 years in the treatment of de novo coronary lesions, a first-in-man study has shown.
A stent coated with a lower dose of the drug Biolimus A9 did not reach the same efficacy rate as the higher dose.
The results may change practice when treating complex patients at high risk for bleeding, according to lead investigator, Dr. Ricardo A. Costa of the Instituto Dante Pazzanese de Cardiologia, São Paulo. The results were presented the results at this year’s Transcatheter Cardiovascular Therapeutics annual meeting, and simultaneously published online (JACC Cardiovasc Interv. 2015. doi: 10.1016/j.jcin.2015.09.008).
The lipophilic Biolimus A9 is intended for use with a proprietary polymer-free, stainless steel stent (BioFreedom, Biosensors International) that is structured to hold the drug on its abluminal surface. The drug was developed to avoid the chronic inflammation and local toxicity associated with the durable polymers used in first-generation drug-eluting stents. It was also intended to reduce the duration of dual-antiplatelet therapy, which often can surpass 6 months and is contraindicated in patients at high risk for bleeding.
In this first-in-man study conducted at four sites across Germany, 182 patients with de novo lesions were randomly assigned to treatment with a standard-dose drug-coated stent, a low-dose drug-coated stent, or to receive a first-generation paclitaxel-eluting stent.
About two-thirds of all study participants were men in their mid- to late-60s and 70s with stable angina. Prior percutaneous intervention had been performed in a third of the standard-dose drug-coated stent group, 44% of the low-dose group, and 46% of the control group.
Angiographic follow-up at 4-months showed that in-stent late lumen loss, a surrogate for neointimal hyperplasia, in both drug-coated cohorts was significantly lower than in the drug-eluting stent group (0.08 mm and 0.12 mm vs. 0.37 mm, respectively; P less than .0001 for regular dose vs. drug-eluting stent; P = .002 for low-dose vs. drug-eluting stent).
Angiographic follow-up at 1 year showed in-stent late lumen loss was 0.17 mm in the drug-coated stent group vs. 0.35 mm in the drug-eluting stent group (P = .001 for noninferiority; P = .11 for superiority); although at 0.22 mm, the low-dose group did not achieve noninferiority (P = .21).
At 5-year follow-up in 175 of the original 182 patients, there were no significant differences in major adverse cardiac events between the standard-dose (23.8%) and low-dose (26.4%) drug-coated stents and the drug-eluting stent (20.3%), or in clinically-indicated target lesion revascularization, at 10.8%, 13.4%, and 10.2%, respectively. In addition, there were no reported incidences of stent thrombosis across the cohorts.
The results of this trial support those from the LEADERS FREE trial, also presented at this year’s TCT annual meeting, which indicated that the drug-coated stent with only 1 month of dual antiplatelet therapy was safe and effective in complex patients at high risk for post-PCI bleeding.
Dr. Costa and his coinvestigators wrote that although their data were based on patients with simple, discrete lesions, they hope their findings lead to larger studies, and they concluded that the drug-coated stent “may offer less dependence on prolonged dual antiplatelet therapy than polymer-coated drug-eluting stents, while maintaining efficacy; thus, it may be suitable for those at high risk for bleeding.”
This trial was underwritten by Biosensors International. Dr. Costa has received speakers fees from Biosensors, Medtronic, and Daiichi-Sankyo. Three coinvestigators disclosed ties to Bristol-Myers Squibb/Sanofi, the Medicines Company, Lilly/Daiichi-Sankyo, Abbott Vascular, AstraZeneca, Regado Biosciences, and Johnson & Johnson.
On Twitter @whitneymcknight
A polymer-free, drug-coated stent was as safe and effective as a conventional drug-eluting stent at 5 years in the treatment of de novo coronary lesions, a first-in-man study has shown.
A stent coated with a lower dose of the drug Biolimus A9 did not reach the same efficacy rate as the higher dose.
The results may change practice when treating complex patients at high risk for bleeding, according to lead investigator, Dr. Ricardo A. Costa of the Instituto Dante Pazzanese de Cardiologia, São Paulo. The results were presented the results at this year’s Transcatheter Cardiovascular Therapeutics annual meeting, and simultaneously published online (JACC Cardiovasc Interv. 2015. doi: 10.1016/j.jcin.2015.09.008).
The lipophilic Biolimus A9 is intended for use with a proprietary polymer-free, stainless steel stent (BioFreedom, Biosensors International) that is structured to hold the drug on its abluminal surface. The drug was developed to avoid the chronic inflammation and local toxicity associated with the durable polymers used in first-generation drug-eluting stents. It was also intended to reduce the duration of dual-antiplatelet therapy, which often can surpass 6 months and is contraindicated in patients at high risk for bleeding.
In this first-in-man study conducted at four sites across Germany, 182 patients with de novo lesions were randomly assigned to treatment with a standard-dose drug-coated stent, a low-dose drug-coated stent, or to receive a first-generation paclitaxel-eluting stent.
About two-thirds of all study participants were men in their mid- to late-60s and 70s with stable angina. Prior percutaneous intervention had been performed in a third of the standard-dose drug-coated stent group, 44% of the low-dose group, and 46% of the control group.
Angiographic follow-up at 4-months showed that in-stent late lumen loss, a surrogate for neointimal hyperplasia, in both drug-coated cohorts was significantly lower than in the drug-eluting stent group (0.08 mm and 0.12 mm vs. 0.37 mm, respectively; P less than .0001 for regular dose vs. drug-eluting stent; P = .002 for low-dose vs. drug-eluting stent).
Angiographic follow-up at 1 year showed in-stent late lumen loss was 0.17 mm in the drug-coated stent group vs. 0.35 mm in the drug-eluting stent group (P = .001 for noninferiority; P = .11 for superiority); although at 0.22 mm, the low-dose group did not achieve noninferiority (P = .21).
At 5-year follow-up in 175 of the original 182 patients, there were no significant differences in major adverse cardiac events between the standard-dose (23.8%) and low-dose (26.4%) drug-coated stents and the drug-eluting stent (20.3%), or in clinically-indicated target lesion revascularization, at 10.8%, 13.4%, and 10.2%, respectively. In addition, there were no reported incidences of stent thrombosis across the cohorts.
The results of this trial support those from the LEADERS FREE trial, also presented at this year’s TCT annual meeting, which indicated that the drug-coated stent with only 1 month of dual antiplatelet therapy was safe and effective in complex patients at high risk for post-PCI bleeding.
Dr. Costa and his coinvestigators wrote that although their data were based on patients with simple, discrete lesions, they hope their findings lead to larger studies, and they concluded that the drug-coated stent “may offer less dependence on prolonged dual antiplatelet therapy than polymer-coated drug-eluting stents, while maintaining efficacy; thus, it may be suitable for those at high risk for bleeding.”
This trial was underwritten by Biosensors International. Dr. Costa has received speakers fees from Biosensors, Medtronic, and Daiichi-Sankyo. Three coinvestigators disclosed ties to Bristol-Myers Squibb/Sanofi, the Medicines Company, Lilly/Daiichi-Sankyo, Abbott Vascular, AstraZeneca, Regado Biosciences, and Johnson & Johnson.
On Twitter @whitneymcknight
AT TCT 2015
Key clinical point: A polymer-free, drug-coated stent demonstrated safety and efficacy rates comparable with a conventional drug-eluting stent at 5 years in the treatment of de novo coronary lesions.
Major finding: Late lumen loss at 1 year was 0.17 mm in the drug-coated stent vs. 0.35 mm in the drug-eluting stent (P = .001 for noninferiority). Major adverse event rates at 5 years were also similar.
Data source: A multisite, prospective, randomly assigned, 182 person, first-in-man study in Germany.
Disclosures: This trial was underwritten by Biosensors International.
VIDEO: Integrated care is key to ‘normal’ life with schizophrenia
WASHINGTON – Integrated, patient-centered care can help young people with serious mental illness stay productive and find meaning in their lives, according to new data from the RAISE (Recovery After an Initial Schizophrenia Episode) studies, sponsored by the National Institute of Mental Health.
With early intervention and the right medication, a combination of goal-oriented psychotherapy, family psychoeducation, and support for helping people with first-episode psychosis stay in school and work is more effective than standard community-based care, explained Robert Heinssen, Ph.D., director of the division of services and intervention research at NIMH.
This video report explores how primary care and psychiatry can work together to help people with schizophrenia, like Maggie Harrigan, aged 20 years, stay in nursing school and function in society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
WASHINGTON – Integrated, patient-centered care can help young people with serious mental illness stay productive and find meaning in their lives, according to new data from the RAISE (Recovery After an Initial Schizophrenia Episode) studies, sponsored by the National Institute of Mental Health.
With early intervention and the right medication, a combination of goal-oriented psychotherapy, family psychoeducation, and support for helping people with first-episode psychosis stay in school and work is more effective than standard community-based care, explained Robert Heinssen, Ph.D., director of the division of services and intervention research at NIMH.
This video report explores how primary care and psychiatry can work together to help people with schizophrenia, like Maggie Harrigan, aged 20 years, stay in nursing school and function in society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
WASHINGTON – Integrated, patient-centered care can help young people with serious mental illness stay productive and find meaning in their lives, according to new data from the RAISE (Recovery After an Initial Schizophrenia Episode) studies, sponsored by the National Institute of Mental Health.
With early intervention and the right medication, a combination of goal-oriented psychotherapy, family psychoeducation, and support for helping people with first-episode psychosis stay in school and work is more effective than standard community-based care, explained Robert Heinssen, Ph.D., director of the division of services and intervention research at NIMH.
This video report explores how primary care and psychiatry can work together to help people with schizophrenia, like Maggie Harrigan, aged 20 years, stay in nursing school and function in society.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM A NATIONAL ALLIANCE ON MENTAL ILLNESS BRIEFING