User login
Trial immunosuppressive therapy benefits outweigh long-term lymphoma risks in IBD
HOLLYWOOD, FLA. – The benefits of short-term trial immunosuppressive therapy outweigh the risks in inflammatory bowel disease, but certain patient populations require more vigilance than others, Dr. James D. Lewis said at the 2013 Advances in IBD meeting.
"We can probably all agree that thiopurines increase the risk of lymphoma," said Dr. Lewis, of the University of Pennsylvania, Philadelphia, at the conference on inflammatory bowel diseases. "But I’ve gone from ‘probably’ to ‘possibly’ when it comes to using anti-TNF [anti–tumor necrosis factor] therapy. I believe that a short trial of biologics therapy can really inform the risk-benefit balance, moving the conversation from ‘it might make a patient better’ to either it did or it didn’t."
In young males and the elderly, the benefit of combination therapy might not prove worth the risks of the treatment, said Dr. Lewis, "but in the middle ground, I think we have a sweet spot."
Overall, unless ineffective treatment is justifiable for other reasons such as the prevention of antibody formation, it should be discontinued, he said.
Results from the CESAME study, published in 2009, reinforce previously published data, indicating the risk of lymphoma is up to five times higher in IBD patients exposed to thiopurines than in the general population (Lancet 2009;374:1617-25).
"The most important data from CESAME, however, was that patients who discontinued thiopurines had a lymphoma incidence rate that went back to that of the general population," Dr. Lewis said at the meeting, sponsored by the Crohn’s & Colitis Foundation of America.
The link between lymphoma and anti-TNF treatment is harder to establish in IBD patients, because many in this cohort also have been exposed to thiopurines as part of combination therapies, he said.
Combination therapy is possibly associated with a higher risk of lymphoma than thiopurine monotherapy, and it has been shown to lead to a higher incidence rate than biologics monotherapy, said Dr. Lewis.
If you counsel patients that there is a 1 in 2,000 risk of lymphoma per year, he said, but it takes only a quarter of a year to figure out if the drugs are going to work, then the short-term risk is 1.25 per 10,000 that an additional lymphoma would develop because of that 3-month treatment. "And if you stop the treatment, presumably that risk goes away."
The risk-to-benefit ratio would therefore be favorable for most patients who took thiopurines versus those who did not, he said. "The caveat being, the older you get, the more marginal that risk-benefit balance becomes, probably because as you age, your baseline risk for lymphoma is going up."
Regarding the risk of hepatosplenic T-cell lymphoma in patients exposed to immunosuppressive therapy, Dr. Lewis said that when he pooled the risk in person-years from two studies, he "did some back-of-the-envelope calculations and found that in males, the risk might be on the order of 11 in 100,000 person-years of thiopurine exposure."
That means the number needed to harm is about 9,000 patients, which when combined with the possible risk of developing other lymphomas, the number needed to harm is about 6,000 young males for 1 lymphoma death per year, according to Dr. Lewis.
"But I want to caution you that I am almost sure this is an overestimate of the risk, because if this is true, then it means in young males treated with thiopurines who get lymphoma, a third of them would be hepatosplenic lymphomas, and that seems unlikely," he said.
The ultimate magnitude of risk for adding thiopurine therapy for patients, particularly young males, is on par with that of the risk of death from annual use of automobiles, which Dr. Lewis said was approximately 1 in 9,090.
Since "a substantial proportion" of lymphomas associated with immunosuppression are related to the Epstein-Barr virus, according to Dr. Lewis, aside from minimizing unnecessary treatment, which can be hard to define, clinicians might consider discontinuing just thiopurine therapy, or all therapy in the setting of long-term remission. "I’m not endorsing that," he said. "I am saying that is a question for you to consider."
Other considerations include determining the minimum dose of thiopurine or methotrexate necessary to augment the effectiveness of anti-TNF treatment, as well as whether methotrexate is as effective as thiopurines without having an increased lymphoma risk, although only "indirect evidence" currently exists. "It would be nice to see a head-to-head comparison of those," he said.
As for using Epstein-Barr virus serology to help stratify risk, Dr. Lewis said that currently there are no guidelines for it in IBD, and he does not have a set rule to recommend it.
Dr. Lewis disclosed that he consults for AbbVie, Janssen, Prometheus, and Millennium and has received research funding from Centocor and Takeda.
HOLLYWOOD, FLA. – The benefits of short-term trial immunosuppressive therapy outweigh the risks in inflammatory bowel disease, but certain patient populations require more vigilance than others, Dr. James D. Lewis said at the 2013 Advances in IBD meeting.
"We can probably all agree that thiopurines increase the risk of lymphoma," said Dr. Lewis, of the University of Pennsylvania, Philadelphia, at the conference on inflammatory bowel diseases. "But I’ve gone from ‘probably’ to ‘possibly’ when it comes to using anti-TNF [anti–tumor necrosis factor] therapy. I believe that a short trial of biologics therapy can really inform the risk-benefit balance, moving the conversation from ‘it might make a patient better’ to either it did or it didn’t."
In young males and the elderly, the benefit of combination therapy might not prove worth the risks of the treatment, said Dr. Lewis, "but in the middle ground, I think we have a sweet spot."
Overall, unless ineffective treatment is justifiable for other reasons such as the prevention of antibody formation, it should be discontinued, he said.
Results from the CESAME study, published in 2009, reinforce previously published data, indicating the risk of lymphoma is up to five times higher in IBD patients exposed to thiopurines than in the general population (Lancet 2009;374:1617-25).
"The most important data from CESAME, however, was that patients who discontinued thiopurines had a lymphoma incidence rate that went back to that of the general population," Dr. Lewis said at the meeting, sponsored by the Crohn’s & Colitis Foundation of America.
The link between lymphoma and anti-TNF treatment is harder to establish in IBD patients, because many in this cohort also have been exposed to thiopurines as part of combination therapies, he said.
Combination therapy is possibly associated with a higher risk of lymphoma than thiopurine monotherapy, and it has been shown to lead to a higher incidence rate than biologics monotherapy, said Dr. Lewis.
If you counsel patients that there is a 1 in 2,000 risk of lymphoma per year, he said, but it takes only a quarter of a year to figure out if the drugs are going to work, then the short-term risk is 1.25 per 10,000 that an additional lymphoma would develop because of that 3-month treatment. "And if you stop the treatment, presumably that risk goes away."
The risk-to-benefit ratio would therefore be favorable for most patients who took thiopurines versus those who did not, he said. "The caveat being, the older you get, the more marginal that risk-benefit balance becomes, probably because as you age, your baseline risk for lymphoma is going up."
Regarding the risk of hepatosplenic T-cell lymphoma in patients exposed to immunosuppressive therapy, Dr. Lewis said that when he pooled the risk in person-years from two studies, he "did some back-of-the-envelope calculations and found that in males, the risk might be on the order of 11 in 100,000 person-years of thiopurine exposure."
That means the number needed to harm is about 9,000 patients, which when combined with the possible risk of developing other lymphomas, the number needed to harm is about 6,000 young males for 1 lymphoma death per year, according to Dr. Lewis.
"But I want to caution you that I am almost sure this is an overestimate of the risk, because if this is true, then it means in young males treated with thiopurines who get lymphoma, a third of them would be hepatosplenic lymphomas, and that seems unlikely," he said.
The ultimate magnitude of risk for adding thiopurine therapy for patients, particularly young males, is on par with that of the risk of death from annual use of automobiles, which Dr. Lewis said was approximately 1 in 9,090.
Since "a substantial proportion" of lymphomas associated with immunosuppression are related to the Epstein-Barr virus, according to Dr. Lewis, aside from minimizing unnecessary treatment, which can be hard to define, clinicians might consider discontinuing just thiopurine therapy, or all therapy in the setting of long-term remission. "I’m not endorsing that," he said. "I am saying that is a question for you to consider."
Other considerations include determining the minimum dose of thiopurine or methotrexate necessary to augment the effectiveness of anti-TNF treatment, as well as whether methotrexate is as effective as thiopurines without having an increased lymphoma risk, although only "indirect evidence" currently exists. "It would be nice to see a head-to-head comparison of those," he said.
As for using Epstein-Barr virus serology to help stratify risk, Dr. Lewis said that currently there are no guidelines for it in IBD, and he does not have a set rule to recommend it.
Dr. Lewis disclosed that he consults for AbbVie, Janssen, Prometheus, and Millennium and has received research funding from Centocor and Takeda.
HOLLYWOOD, FLA. – The benefits of short-term trial immunosuppressive therapy outweigh the risks in inflammatory bowel disease, but certain patient populations require more vigilance than others, Dr. James D. Lewis said at the 2013 Advances in IBD meeting.
"We can probably all agree that thiopurines increase the risk of lymphoma," said Dr. Lewis, of the University of Pennsylvania, Philadelphia, at the conference on inflammatory bowel diseases. "But I’ve gone from ‘probably’ to ‘possibly’ when it comes to using anti-TNF [anti–tumor necrosis factor] therapy. I believe that a short trial of biologics therapy can really inform the risk-benefit balance, moving the conversation from ‘it might make a patient better’ to either it did or it didn’t."
In young males and the elderly, the benefit of combination therapy might not prove worth the risks of the treatment, said Dr. Lewis, "but in the middle ground, I think we have a sweet spot."
Overall, unless ineffective treatment is justifiable for other reasons such as the prevention of antibody formation, it should be discontinued, he said.
Results from the CESAME study, published in 2009, reinforce previously published data, indicating the risk of lymphoma is up to five times higher in IBD patients exposed to thiopurines than in the general population (Lancet 2009;374:1617-25).
"The most important data from CESAME, however, was that patients who discontinued thiopurines had a lymphoma incidence rate that went back to that of the general population," Dr. Lewis said at the meeting, sponsored by the Crohn’s & Colitis Foundation of America.
The link between lymphoma and anti-TNF treatment is harder to establish in IBD patients, because many in this cohort also have been exposed to thiopurines as part of combination therapies, he said.
Combination therapy is possibly associated with a higher risk of lymphoma than thiopurine monotherapy, and it has been shown to lead to a higher incidence rate than biologics monotherapy, said Dr. Lewis.
If you counsel patients that there is a 1 in 2,000 risk of lymphoma per year, he said, but it takes only a quarter of a year to figure out if the drugs are going to work, then the short-term risk is 1.25 per 10,000 that an additional lymphoma would develop because of that 3-month treatment. "And if you stop the treatment, presumably that risk goes away."
The risk-to-benefit ratio would therefore be favorable for most patients who took thiopurines versus those who did not, he said. "The caveat being, the older you get, the more marginal that risk-benefit balance becomes, probably because as you age, your baseline risk for lymphoma is going up."
Regarding the risk of hepatosplenic T-cell lymphoma in patients exposed to immunosuppressive therapy, Dr. Lewis said that when he pooled the risk in person-years from two studies, he "did some back-of-the-envelope calculations and found that in males, the risk might be on the order of 11 in 100,000 person-years of thiopurine exposure."
That means the number needed to harm is about 9,000 patients, which when combined with the possible risk of developing other lymphomas, the number needed to harm is about 6,000 young males for 1 lymphoma death per year, according to Dr. Lewis.
"But I want to caution you that I am almost sure this is an overestimate of the risk, because if this is true, then it means in young males treated with thiopurines who get lymphoma, a third of them would be hepatosplenic lymphomas, and that seems unlikely," he said.
The ultimate magnitude of risk for adding thiopurine therapy for patients, particularly young males, is on par with that of the risk of death from annual use of automobiles, which Dr. Lewis said was approximately 1 in 9,090.
Since "a substantial proportion" of lymphomas associated with immunosuppression are related to the Epstein-Barr virus, according to Dr. Lewis, aside from minimizing unnecessary treatment, which can be hard to define, clinicians might consider discontinuing just thiopurine therapy, or all therapy in the setting of long-term remission. "I’m not endorsing that," he said. "I am saying that is a question for you to consider."
Other considerations include determining the minimum dose of thiopurine or methotrexate necessary to augment the effectiveness of anti-TNF treatment, as well as whether methotrexate is as effective as thiopurines without having an increased lymphoma risk, although only "indirect evidence" currently exists. "It would be nice to see a head-to-head comparison of those," he said.
As for using Epstein-Barr virus serology to help stratify risk, Dr. Lewis said that currently there are no guidelines for it in IBD, and he does not have a set rule to recommend it.
Dr. Lewis disclosed that he consults for AbbVie, Janssen, Prometheus, and Millennium and has received research funding from Centocor and Takeda.
EXPERT ANALYSIS AT 2013 ADVANCES IN IBD
DVT risk higher in cardiac and vascular surgery
WASHINGTON - Cardiac and vascular surgery patients are at higher risk for deep vein thrombosis than are general surgery patients, according to data presented at the annual clinical congress of the American College of Surgeons.
In a retrospective analysis of 2,669,772 patients with a median age of 64 years, 43% of whom were males, in the ACS-National Surgical Quality Improvement Program (NSQIP) during 2005-2009, Dr. Faisal Aziz of Penn State Hershey (Pa.) Heart and Vascular Institute and his colleagues sought to determine the actual rate of deep vein thrombosis (DVT) during revascularization procedures, compared with general surgery.
The researchers sorted patients according to DVT risk factors such as age, gender, body mass index over 40 kg/m2, and whether the surgery was acute. They then assessed intraoperative factors such as total time to completion and the American Society of Anesthesiology score. They then considered the postoperative factors associated with DVT, such as blood transfusions, return to the operating room, deep wound infection, cardiac arrest, and mortality.
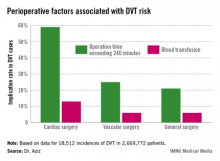
There were 18,512 incidences of DVT, equaling 0.69% of all patients studied. Of those, 0.66% occurred during general surgery, 2.08% occurred during cardiac surgery, and 1% occurred during vascular surgery.
"The implications of our study are that, contrary to popular belief, the incidence of postop DVT is actually higher after cardiac surgery and vascular surgery procedures," he said.
The cardiac surgery procedures associated with the highest DVT incidence rate were tricuspid valve replacement (8%), thoracic endovascular aortic repair (5%), thoracic aortic graft replacement (4%), and pericardial window (4%).
In a comparison of cardiac procedures, tricuspid valve replacement vs. aortic valve replacement had a risk ratio of 3.5 (P < .001). In tricuspid valve replacement vs. coronary artery bypass, the former had a risk ratio of 11.24 (P < .001).
Vascular surgeries with the highest DVT incidence rates were peripheral bypass (1%), amputation (trans-metatarsal, 0.75%; below knee, 1%; above the knee, 1%), and ruptured aortic aneurysms (3.5%).
Comparatively, in ruptured endovascular aneurysm repair (EVAR) vs. elective EVAR, the risk ratio was 3.55 (P < .001). In abdominal aortic aneurysm (AAA) repair, ruptured vs. elective surgeries had a risk ratio of 2.37 (P < .001).
Compared with 80% of general surgery patients, 74% of cardiac surgery patients were 70 years or older (relative risk, 1.12; P = .13); 86% of vascular surgery patients were 70 years or older (RR, 1.1; P < .05).
Male gender was an associated risk factor in 49% of general surgery patients, compared with 70% for cardiac patients (RR, 1.4; P < .001) and 51% for vascular patients (RR, 1.1; P < .001).
Intra- and postoperative factors associated with DVT risk included operation times exceeding 240 minutes and previous DVT. Compared with 21% of general surgery patients, operation time was implicated in 59% of cardiac surgery patients (relative risk, 2.72; P < .001) and 25% of vascular surgery patients (RR, 1.14; P <.001). Blood transfusions affected 13% of cardiac surgery patients (RR, 2.3; P < .001), 6% of vascular surgery patients (RR, 1.3; P < .001), and 6% of general surgery patients.
Compared with 24% for general surgery patients, returning to the operating room was implicated in 27% of cardiac patients (RR, 1.4; P = .27) and 32% of vascular surgery patients (RR, 1.3; P < .001).
"Procedures and perioperative factors associated with high risk of postoperative DVT should be identified, and adequate DVT prophylaxis should be ensured for these patients," Dr. Aziz concluded.
He had no disclosures.
WASHINGTON - Cardiac and vascular surgery patients are at higher risk for deep vein thrombosis than are general surgery patients, according to data presented at the annual clinical congress of the American College of Surgeons.
In a retrospective analysis of 2,669,772 patients with a median age of 64 years, 43% of whom were males, in the ACS-National Surgical Quality Improvement Program (NSQIP) during 2005-2009, Dr. Faisal Aziz of Penn State Hershey (Pa.) Heart and Vascular Institute and his colleagues sought to determine the actual rate of deep vein thrombosis (DVT) during revascularization procedures, compared with general surgery.
The researchers sorted patients according to DVT risk factors such as age, gender, body mass index over 40 kg/m2, and whether the surgery was acute. They then assessed intraoperative factors such as total time to completion and the American Society of Anesthesiology score. They then considered the postoperative factors associated with DVT, such as blood transfusions, return to the operating room, deep wound infection, cardiac arrest, and mortality.
There were 18,512 incidences of DVT, equaling 0.69% of all patients studied. Of those, 0.66% occurred during general surgery, 2.08% occurred during cardiac surgery, and 1% occurred during vascular surgery.
"The implications of our study are that, contrary to popular belief, the incidence of postop DVT is actually higher after cardiac surgery and vascular surgery procedures," he said.
The cardiac surgery procedures associated with the highest DVT incidence rate were tricuspid valve replacement (8%), thoracic endovascular aortic repair (5%), thoracic aortic graft replacement (4%), and pericardial window (4%).
In a comparison of cardiac procedures, tricuspid valve replacement vs. aortic valve replacement had a risk ratio of 3.5 (P < .001). In tricuspid valve replacement vs. coronary artery bypass, the former had a risk ratio of 11.24 (P < .001).
Vascular surgeries with the highest DVT incidence rates were peripheral bypass (1%), amputation (trans-metatarsal, 0.75%; below knee, 1%; above the knee, 1%), and ruptured aortic aneurysms (3.5%).
Comparatively, in ruptured endovascular aneurysm repair (EVAR) vs. elective EVAR, the risk ratio was 3.55 (P < .001). In abdominal aortic aneurysm (AAA) repair, ruptured vs. elective surgeries had a risk ratio of 2.37 (P < .001).
Compared with 80% of general surgery patients, 74% of cardiac surgery patients were 70 years or older (relative risk, 1.12; P = .13); 86% of vascular surgery patients were 70 years or older (RR, 1.1; P < .05).
Male gender was an associated risk factor in 49% of general surgery patients, compared with 70% for cardiac patients (RR, 1.4; P < .001) and 51% for vascular patients (RR, 1.1; P < .001).
Intra- and postoperative factors associated with DVT risk included operation times exceeding 240 minutes and previous DVT. Compared with 21% of general surgery patients, operation time was implicated in 59% of cardiac surgery patients (relative risk, 2.72; P < .001) and 25% of vascular surgery patients (RR, 1.14; P <.001). Blood transfusions affected 13% of cardiac surgery patients (RR, 2.3; P < .001), 6% of vascular surgery patients (RR, 1.3; P < .001), and 6% of general surgery patients.
Compared with 24% for general surgery patients, returning to the operating room was implicated in 27% of cardiac patients (RR, 1.4; P = .27) and 32% of vascular surgery patients (RR, 1.3; P < .001).
"Procedures and perioperative factors associated with high risk of postoperative DVT should be identified, and adequate DVT prophylaxis should be ensured for these patients," Dr. Aziz concluded.
He had no disclosures.
WASHINGTON - Cardiac and vascular surgery patients are at higher risk for deep vein thrombosis than are general surgery patients, according to data presented at the annual clinical congress of the American College of Surgeons.
In a retrospective analysis of 2,669,772 patients with a median age of 64 years, 43% of whom were males, in the ACS-National Surgical Quality Improvement Program (NSQIP) during 2005-2009, Dr. Faisal Aziz of Penn State Hershey (Pa.) Heart and Vascular Institute and his colleagues sought to determine the actual rate of deep vein thrombosis (DVT) during revascularization procedures, compared with general surgery.
The researchers sorted patients according to DVT risk factors such as age, gender, body mass index over 40 kg/m2, and whether the surgery was acute. They then assessed intraoperative factors such as total time to completion and the American Society of Anesthesiology score. They then considered the postoperative factors associated with DVT, such as blood transfusions, return to the operating room, deep wound infection, cardiac arrest, and mortality.
There were 18,512 incidences of DVT, equaling 0.69% of all patients studied. Of those, 0.66% occurred during general surgery, 2.08% occurred during cardiac surgery, and 1% occurred during vascular surgery.
"The implications of our study are that, contrary to popular belief, the incidence of postop DVT is actually higher after cardiac surgery and vascular surgery procedures," he said.
The cardiac surgery procedures associated with the highest DVT incidence rate were tricuspid valve replacement (8%), thoracic endovascular aortic repair (5%), thoracic aortic graft replacement (4%), and pericardial window (4%).
In a comparison of cardiac procedures, tricuspid valve replacement vs. aortic valve replacement had a risk ratio of 3.5 (P < .001). In tricuspid valve replacement vs. coronary artery bypass, the former had a risk ratio of 11.24 (P < .001).
Vascular surgeries with the highest DVT incidence rates were peripheral bypass (1%), amputation (trans-metatarsal, 0.75%; below knee, 1%; above the knee, 1%), and ruptured aortic aneurysms (3.5%).
Comparatively, in ruptured endovascular aneurysm repair (EVAR) vs. elective EVAR, the risk ratio was 3.55 (P < .001). In abdominal aortic aneurysm (AAA) repair, ruptured vs. elective surgeries had a risk ratio of 2.37 (P < .001).
Compared with 80% of general surgery patients, 74% of cardiac surgery patients were 70 years or older (relative risk, 1.12; P = .13); 86% of vascular surgery patients were 70 years or older (RR, 1.1; P < .05).
Male gender was an associated risk factor in 49% of general surgery patients, compared with 70% for cardiac patients (RR, 1.4; P < .001) and 51% for vascular patients (RR, 1.1; P < .001).
Intra- and postoperative factors associated with DVT risk included operation times exceeding 240 minutes and previous DVT. Compared with 21% of general surgery patients, operation time was implicated in 59% of cardiac surgery patients (relative risk, 2.72; P < .001) and 25% of vascular surgery patients (RR, 1.14; P <.001). Blood transfusions affected 13% of cardiac surgery patients (RR, 2.3; P < .001), 6% of vascular surgery patients (RR, 1.3; P < .001), and 6% of general surgery patients.
Compared with 24% for general surgery patients, returning to the operating room was implicated in 27% of cardiac patients (RR, 1.4; P = .27) and 32% of vascular surgery patients (RR, 1.3; P < .001).
"Procedures and perioperative factors associated with high risk of postoperative DVT should be identified, and adequate DVT prophylaxis should be ensured for these patients," Dr. Aziz concluded.
He had no disclosures.
OSHA launches hospital worker safety initiative
Improving hospital worker safety will result in improved patient safety, according to the Occupational Safety and Health Administration, which has unveiled a hospital worker safety online resource center.
Worker Safety in Hospitals: Caring for our Caregivers, OSHA's new website aimed at curbing hospital worker injury rates, was introduced during a media teleconference Jan. 16. According to OSHA, injuries occur at a rate of 158 times per 10,000 workers, making hospitals more hazardous work environments than either construction or manufacturing sites.
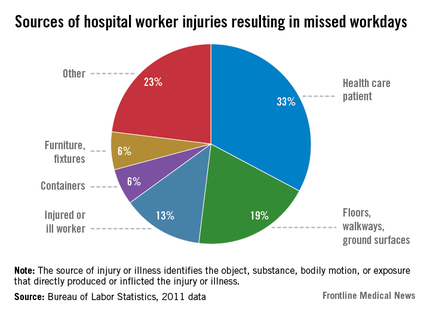
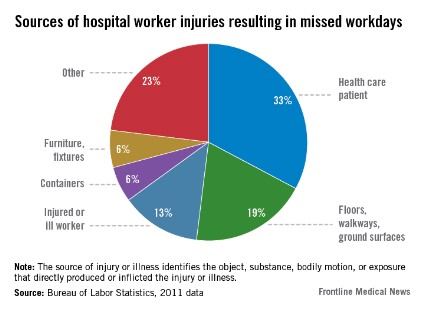
National workers’ compensation losses from hospital workers total $2 billion annually, said OSHA assistant secretary Dr. David Michaels.
Musculoskeletal injuries, primarily from lifting and shifting patients, are the single biggest worker injury in hospitals, he said. According to OSHA, 16,680 hospital workers missed work because of a musculoskeletal injury sustained while handling a patient.
"Other hazards facing hospital workers include workplace violence, slips and falls, exposure to chemicals including hazardous drugs, exposure to infectious diseases, and needle sticks," said Dr. Michaels.
As part of a joint effort to promote the website, also on the conference call were Dr. John Howard, director of the National Institute for Occupational Safety and Health, and Dr. Erin DuPree, chief medical officer and vice president of the Joint Commission Center for Transforming Healthcare, and Dr. Lucian Leape, chairman of the Lucian Leape Institute at the National Patient Safety Foundation.
The OSHA website offers educational materials on avoiding injury when handling patients, as well as on workplace safety needs and the implementation of safety and health management systems.
The materials were culled from best practices employed at hospitals around the country deemed by OSHA to be "high-reliability organizations," and include tips such as having a daily "safety huddle" that includes a discussion of "great catches" and "near misses" that occurred during daily operations.
"One of the major barriers in making progress in patient safety is that when people report that they’ve made a mistake, they often get punished for it," said Dr. Leape. "What we’ve been trying to get people to understand is that mistakes happen because of bad systems, not bad people. You have to create an environment where you really believe that, and make it safe for people to talk about their errors so you can understand what you need to fix."
Dr. DuPree said the OSHA resources align with Joint Commission requirements such as those for hospitals to have a written plan for managing environmental safety for patients and all persons in hospitals. The Joint Commission expects that all individuals who work in the hospital should not fear retribution for speaking up about their safety concerns, Dr. DuPree said. "Leaders need to value and empower their workers. They need to understand that fear of reprisal and failure to share knowledge can compromise an organization’s ability to improve safety for all," he said.
As part of its efforts to advise hospital administrators on how to create safer work environments for their employees, the U.S. Department of Labor Occupational Safety and Health Administration has created a list of attributes of what it calls "high-reliability organizations."
Self-assessment tools, tip sheets, and other materials intended to help hospitals become high-reliability organizations (HROs) are available at OSHA's new website, Worker Safety in Hospitals: Caring for our Caregivers.
"The heart of these new materials are real life lessons from high-performing hospitals [that] have implemented best practices to reduce workplace injuries while also improving patient safety," said OSHA assistant secretary Dr. David Michaels in a press briefing.
The website emphasizes principles endorsed by the Joint Commission, particularly the promotion of a "no-blame" culture to destigmatize safety interventions. Operational strategies derived from the distillation of these best practices and endorsed by OSHA include the following:
• Sensitivity to operations. That means hospital workers should know what the standard procedures are, and follow them.
• Reluctance to simplify. This tenet calls upon hospital administrators to explore the larger context in which safety failures occur.
• Preoccupation with failure. OSHA warns administrators to "never let success breed complacency" and impels hospital leaders to "focus unceasingly on ways the system can fail" and to encourage staff to "listen to their ‘inner voice’ of concern."
• Deference to expertise. Pulling rank rather than going to the one who has the most direct experience in a situation is discouraged by OSHA.
• Resilience. Basically, know there will be failures, but hopefully only small ones; learn from them and rebound.
Dr. Michaels, Dr. Howard, Dr. DuPree, and Dr. Leape did not report any relevant financial disclosures.
*This article has been updated 1/16/2014.
Improving hospital worker safety will result in improved patient safety, according to the Occupational Safety and Health Administration, which has unveiled a hospital worker safety online resource center.
Worker Safety in Hospitals: Caring for our Caregivers, OSHA's new website aimed at curbing hospital worker injury rates, was introduced during a media teleconference Jan. 16. According to OSHA, injuries occur at a rate of 158 times per 10,000 workers, making hospitals more hazardous work environments than either construction or manufacturing sites.

National workers’ compensation losses from hospital workers total $2 billion annually, said OSHA assistant secretary Dr. David Michaels.
Musculoskeletal injuries, primarily from lifting and shifting patients, are the single biggest worker injury in hospitals, he said. According to OSHA, 16,680 hospital workers missed work because of a musculoskeletal injury sustained while handling a patient.
"Other hazards facing hospital workers include workplace violence, slips and falls, exposure to chemicals including hazardous drugs, exposure to infectious diseases, and needle sticks," said Dr. Michaels.
As part of a joint effort to promote the website, also on the conference call were Dr. John Howard, director of the National Institute for Occupational Safety and Health, and Dr. Erin DuPree, chief medical officer and vice president of the Joint Commission Center for Transforming Healthcare, and Dr. Lucian Leape, chairman of the Lucian Leape Institute at the National Patient Safety Foundation.
The OSHA website offers educational materials on avoiding injury when handling patients, as well as on workplace safety needs and the implementation of safety and health management systems.
The materials were culled from best practices employed at hospitals around the country deemed by OSHA to be "high-reliability organizations," and include tips such as having a daily "safety huddle" that includes a discussion of "great catches" and "near misses" that occurred during daily operations.
"One of the major barriers in making progress in patient safety is that when people report that they’ve made a mistake, they often get punished for it," said Dr. Leape. "What we’ve been trying to get people to understand is that mistakes happen because of bad systems, not bad people. You have to create an environment where you really believe that, and make it safe for people to talk about their errors so you can understand what you need to fix."
Dr. DuPree said the OSHA resources align with Joint Commission requirements such as those for hospitals to have a written plan for managing environmental safety for patients and all persons in hospitals. The Joint Commission expects that all individuals who work in the hospital should not fear retribution for speaking up about their safety concerns, Dr. DuPree said. "Leaders need to value and empower their workers. They need to understand that fear of reprisal and failure to share knowledge can compromise an organization’s ability to improve safety for all," he said.
As part of its efforts to advise hospital administrators on how to create safer work environments for their employees, the U.S. Department of Labor Occupational Safety and Health Administration has created a list of attributes of what it calls "high-reliability organizations."
Self-assessment tools, tip sheets, and other materials intended to help hospitals become high-reliability organizations (HROs) are available at OSHA's new website, Worker Safety in Hospitals: Caring for our Caregivers.
"The heart of these new materials are real life lessons from high-performing hospitals [that] have implemented best practices to reduce workplace injuries while also improving patient safety," said OSHA assistant secretary Dr. David Michaels in a press briefing.
The website emphasizes principles endorsed by the Joint Commission, particularly the promotion of a "no-blame" culture to destigmatize safety interventions. Operational strategies derived from the distillation of these best practices and endorsed by OSHA include the following:
• Sensitivity to operations. That means hospital workers should know what the standard procedures are, and follow them.
• Reluctance to simplify. This tenet calls upon hospital administrators to explore the larger context in which safety failures occur.
• Preoccupation with failure. OSHA warns administrators to "never let success breed complacency" and impels hospital leaders to "focus unceasingly on ways the system can fail" and to encourage staff to "listen to their ‘inner voice’ of concern."
• Deference to expertise. Pulling rank rather than going to the one who has the most direct experience in a situation is discouraged by OSHA.
• Resilience. Basically, know there will be failures, but hopefully only small ones; learn from them and rebound.
Dr. Michaels, Dr. Howard, Dr. DuPree, and Dr. Leape did not report any relevant financial disclosures.
*This article has been updated 1/16/2014.
Improving hospital worker safety will result in improved patient safety, according to the Occupational Safety and Health Administration, which has unveiled a hospital worker safety online resource center.
Worker Safety in Hospitals: Caring for our Caregivers, OSHA's new website aimed at curbing hospital worker injury rates, was introduced during a media teleconference Jan. 16. According to OSHA, injuries occur at a rate of 158 times per 10,000 workers, making hospitals more hazardous work environments than either construction or manufacturing sites.

National workers’ compensation losses from hospital workers total $2 billion annually, said OSHA assistant secretary Dr. David Michaels.
Musculoskeletal injuries, primarily from lifting and shifting patients, are the single biggest worker injury in hospitals, he said. According to OSHA, 16,680 hospital workers missed work because of a musculoskeletal injury sustained while handling a patient.
"Other hazards facing hospital workers include workplace violence, slips and falls, exposure to chemicals including hazardous drugs, exposure to infectious diseases, and needle sticks," said Dr. Michaels.
As part of a joint effort to promote the website, also on the conference call were Dr. John Howard, director of the National Institute for Occupational Safety and Health, and Dr. Erin DuPree, chief medical officer and vice president of the Joint Commission Center for Transforming Healthcare, and Dr. Lucian Leape, chairman of the Lucian Leape Institute at the National Patient Safety Foundation.
The OSHA website offers educational materials on avoiding injury when handling patients, as well as on workplace safety needs and the implementation of safety and health management systems.
The materials were culled from best practices employed at hospitals around the country deemed by OSHA to be "high-reliability organizations," and include tips such as having a daily "safety huddle" that includes a discussion of "great catches" and "near misses" that occurred during daily operations.
"One of the major barriers in making progress in patient safety is that when people report that they’ve made a mistake, they often get punished for it," said Dr. Leape. "What we’ve been trying to get people to understand is that mistakes happen because of bad systems, not bad people. You have to create an environment where you really believe that, and make it safe for people to talk about their errors so you can understand what you need to fix."
Dr. DuPree said the OSHA resources align with Joint Commission requirements such as those for hospitals to have a written plan for managing environmental safety for patients and all persons in hospitals. The Joint Commission expects that all individuals who work in the hospital should not fear retribution for speaking up about their safety concerns, Dr. DuPree said. "Leaders need to value and empower their workers. They need to understand that fear of reprisal and failure to share knowledge can compromise an organization’s ability to improve safety for all," he said.
As part of its efforts to advise hospital administrators on how to create safer work environments for their employees, the U.S. Department of Labor Occupational Safety and Health Administration has created a list of attributes of what it calls "high-reliability organizations."
Self-assessment tools, tip sheets, and other materials intended to help hospitals become high-reliability organizations (HROs) are available at OSHA's new website, Worker Safety in Hospitals: Caring for our Caregivers.
"The heart of these new materials are real life lessons from high-performing hospitals [that] have implemented best practices to reduce workplace injuries while also improving patient safety," said OSHA assistant secretary Dr. David Michaels in a press briefing.
The website emphasizes principles endorsed by the Joint Commission, particularly the promotion of a "no-blame" culture to destigmatize safety interventions. Operational strategies derived from the distillation of these best practices and endorsed by OSHA include the following:
• Sensitivity to operations. That means hospital workers should know what the standard procedures are, and follow them.
• Reluctance to simplify. This tenet calls upon hospital administrators to explore the larger context in which safety failures occur.
• Preoccupation with failure. OSHA warns administrators to "never let success breed complacency" and impels hospital leaders to "focus unceasingly on ways the system can fail" and to encourage staff to "listen to their ‘inner voice’ of concern."
• Deference to expertise. Pulling rank rather than going to the one who has the most direct experience in a situation is discouraged by OSHA.
• Resilience. Basically, know there will be failures, but hopefully only small ones; learn from them and rebound.
Dr. Michaels, Dr. Howard, Dr. DuPree, and Dr. Leape did not report any relevant financial disclosures.
*This article has been updated 1/16/2014.
Olmesartan associated with some enteropathies
HOLLYWOOD, FLA. – Patients on angiotensin-receptor blocking therapy who present with severe gastrointestinal symptoms may be experiencing drug-induced enteropathy, Dr. Joseph A. Murray said at the 2013 Advances in IBD meeting.
He discovered the connection in a retrospective analysis of patients after treating two women with collagenous celiac disease whose patient history included olmesartan therapy. "Until a few years ago, I’d never heard of such a thing," he said. "But for a condition like collagenous sprue, which is exceptionally rare, that really got my attention."
Olmesartan was implicated in 14 out of 32 cases of refractory idiopathic sprue treated by Dr. Murray at the Mayo Clinic in Rochester, Minn., between 2008 and 2011. "It’s very much less common for other ARBs [angiotensin-receptor blockers] such as valsartan and irbesartan to have a spruelike effect on patients," Dr. Murray said of his clinical experience.
Since he noted the connection, he has successfully treated 22 additional such cases: When all patients were taken off the ARB, their symptoms – particularly their severe diarrhea – resolved, and the patients required no further therapy. They also were all able to return to a diet that contained gluten.
Autoimmune- vs. drug-induced enteropathy
Drug- and autoimmune-induced enteropathy have similar presentations, including severe chronic diarrhea; villous atrophy and collagen deposition with or without intraepithelial lymphocytes; and negative tissue transglutaminase antibodies and endomysial antibodies.
In drug-induced enteropathy, however, laboratory findings will always be seronegative for celiac disease and there will be no response to a gluten-free diet.
Patients taking olmesartan who previously were diagnosed with celiac disease should be retested, as should patients not currently taking olmesertan but who were on the drug at the time they were diagnosed.
In patients with severe diarrhea, olmesartan should be suspected, Dr. Murray said. Diarrhea in drug-induced cases is severe and protracted, with patients experiencing as many as 42 bowel movements a day. Acute renal failure and the need for parenteral nutrition are also possible with this condition, he said.
Clinical presentation
Of the 22 patients (median age, 70 years) treated for drug-induced enteropathy, 13 were women and 21 were non-Hispanic white. Twelve had one or more multiple electrolyte abnormalities, 14 had normocytic normochromic anemia, and 10 were severely hypoalbuminemic.
The amount of ARB prescribed to patients varied from 10 to 40 mg. "Patients had been on olmesartan for at least a year, and even as long as 3 years, before the symptoms started, which often did so abruptly, without explanation."
Prior treatments that had failed in these patients included a gluten-free diet, systemic steroids or budesonide, antidiarrheal agents, pancreatic enzymes, bile acid sequestrants, metronidazole, azathioprine, and octreotide.
In all of the 17 patients who had follow-up biopsies, the villi recovered. Anecdotal reports of four patients who were rechallenged with olmesartan included a full return of the illness, regardless of the period of time that had elapsed.
Possible mechanisms
"We think it’s a cell-mediated immune response, not the classic, ‘I take medication, I get hypersensistivity’ response," Dr. Murray said.
ARBs inhibit transforming growth factor (TGF)-beta, and TGF-beta "is key for regulating the immune response in the gut," he said. However, because TGF-beta also drives collagen formation, that there would be a high number of collagenous sprue cases casts doubt on this as a clear mechanism, he said.
Another possibility Dr. Murray and his associates tested, but rejected, is that a bioactivating hydrolase enzyme activates olmesartan given as a prodrug, in the epithelial tissue of the intestine and liver, creating polymorphisms.
The role of angiotensin receptors in the gut and on lymphocytes has not yet been explained, although there is some similarity to celiac disease where "lots of regulatory T cells in patients "simply don’t work," Dr. Murray said. "We know that these are CD8-positive T cells, so they are cytotoxic, and there are plenty of FOXP3 cells present, and they don’t change when you take them off the drug."
The conference was sponsored by the Crohn’s & Colitis Foundation of America. Dr. Murray had no relevant disclosures.
HOLLYWOOD, FLA. – Patients on angiotensin-receptor blocking therapy who present with severe gastrointestinal symptoms may be experiencing drug-induced enteropathy, Dr. Joseph A. Murray said at the 2013 Advances in IBD meeting.
He discovered the connection in a retrospective analysis of patients after treating two women with collagenous celiac disease whose patient history included olmesartan therapy. "Until a few years ago, I’d never heard of such a thing," he said. "But for a condition like collagenous sprue, which is exceptionally rare, that really got my attention."
Olmesartan was implicated in 14 out of 32 cases of refractory idiopathic sprue treated by Dr. Murray at the Mayo Clinic in Rochester, Minn., between 2008 and 2011. "It’s very much less common for other ARBs [angiotensin-receptor blockers] such as valsartan and irbesartan to have a spruelike effect on patients," Dr. Murray said of his clinical experience.
Since he noted the connection, he has successfully treated 22 additional such cases: When all patients were taken off the ARB, their symptoms – particularly their severe diarrhea – resolved, and the patients required no further therapy. They also were all able to return to a diet that contained gluten.
Autoimmune- vs. drug-induced enteropathy
Drug- and autoimmune-induced enteropathy have similar presentations, including severe chronic diarrhea; villous atrophy and collagen deposition with or without intraepithelial lymphocytes; and negative tissue transglutaminase antibodies and endomysial antibodies.
In drug-induced enteropathy, however, laboratory findings will always be seronegative for celiac disease and there will be no response to a gluten-free diet.
Patients taking olmesartan who previously were diagnosed with celiac disease should be retested, as should patients not currently taking olmesertan but who were on the drug at the time they were diagnosed.
In patients with severe diarrhea, olmesartan should be suspected, Dr. Murray said. Diarrhea in drug-induced cases is severe and protracted, with patients experiencing as many as 42 bowel movements a day. Acute renal failure and the need for parenteral nutrition are also possible with this condition, he said.
Clinical presentation
Of the 22 patients (median age, 70 years) treated for drug-induced enteropathy, 13 were women and 21 were non-Hispanic white. Twelve had one or more multiple electrolyte abnormalities, 14 had normocytic normochromic anemia, and 10 were severely hypoalbuminemic.
The amount of ARB prescribed to patients varied from 10 to 40 mg. "Patients had been on olmesartan for at least a year, and even as long as 3 years, before the symptoms started, which often did so abruptly, without explanation."
Prior treatments that had failed in these patients included a gluten-free diet, systemic steroids or budesonide, antidiarrheal agents, pancreatic enzymes, bile acid sequestrants, metronidazole, azathioprine, and octreotide.
In all of the 17 patients who had follow-up biopsies, the villi recovered. Anecdotal reports of four patients who were rechallenged with olmesartan included a full return of the illness, regardless of the period of time that had elapsed.
Possible mechanisms
"We think it’s a cell-mediated immune response, not the classic, ‘I take medication, I get hypersensistivity’ response," Dr. Murray said.
ARBs inhibit transforming growth factor (TGF)-beta, and TGF-beta "is key for regulating the immune response in the gut," he said. However, because TGF-beta also drives collagen formation, that there would be a high number of collagenous sprue cases casts doubt on this as a clear mechanism, he said.
Another possibility Dr. Murray and his associates tested, but rejected, is that a bioactivating hydrolase enzyme activates olmesartan given as a prodrug, in the epithelial tissue of the intestine and liver, creating polymorphisms.
The role of angiotensin receptors in the gut and on lymphocytes has not yet been explained, although there is some similarity to celiac disease where "lots of regulatory T cells in patients "simply don’t work," Dr. Murray said. "We know that these are CD8-positive T cells, so they are cytotoxic, and there are plenty of FOXP3 cells present, and they don’t change when you take them off the drug."
The conference was sponsored by the Crohn’s & Colitis Foundation of America. Dr. Murray had no relevant disclosures.
HOLLYWOOD, FLA. – Patients on angiotensin-receptor blocking therapy who present with severe gastrointestinal symptoms may be experiencing drug-induced enteropathy, Dr. Joseph A. Murray said at the 2013 Advances in IBD meeting.
He discovered the connection in a retrospective analysis of patients after treating two women with collagenous celiac disease whose patient history included olmesartan therapy. "Until a few years ago, I’d never heard of such a thing," he said. "But for a condition like collagenous sprue, which is exceptionally rare, that really got my attention."
Olmesartan was implicated in 14 out of 32 cases of refractory idiopathic sprue treated by Dr. Murray at the Mayo Clinic in Rochester, Minn., between 2008 and 2011. "It’s very much less common for other ARBs [angiotensin-receptor blockers] such as valsartan and irbesartan to have a spruelike effect on patients," Dr. Murray said of his clinical experience.
Since he noted the connection, he has successfully treated 22 additional such cases: When all patients were taken off the ARB, their symptoms – particularly their severe diarrhea – resolved, and the patients required no further therapy. They also were all able to return to a diet that contained gluten.
Autoimmune- vs. drug-induced enteropathy
Drug- and autoimmune-induced enteropathy have similar presentations, including severe chronic diarrhea; villous atrophy and collagen deposition with or without intraepithelial lymphocytes; and negative tissue transglutaminase antibodies and endomysial antibodies.
In drug-induced enteropathy, however, laboratory findings will always be seronegative for celiac disease and there will be no response to a gluten-free diet.
Patients taking olmesartan who previously were diagnosed with celiac disease should be retested, as should patients not currently taking olmesertan but who were on the drug at the time they were diagnosed.
In patients with severe diarrhea, olmesartan should be suspected, Dr. Murray said. Diarrhea in drug-induced cases is severe and protracted, with patients experiencing as many as 42 bowel movements a day. Acute renal failure and the need for parenteral nutrition are also possible with this condition, he said.
Clinical presentation
Of the 22 patients (median age, 70 years) treated for drug-induced enteropathy, 13 were women and 21 were non-Hispanic white. Twelve had one or more multiple electrolyte abnormalities, 14 had normocytic normochromic anemia, and 10 were severely hypoalbuminemic.
The amount of ARB prescribed to patients varied from 10 to 40 mg. "Patients had been on olmesartan for at least a year, and even as long as 3 years, before the symptoms started, which often did so abruptly, without explanation."
Prior treatments that had failed in these patients included a gluten-free diet, systemic steroids or budesonide, antidiarrheal agents, pancreatic enzymes, bile acid sequestrants, metronidazole, azathioprine, and octreotide.
In all of the 17 patients who had follow-up biopsies, the villi recovered. Anecdotal reports of four patients who were rechallenged with olmesartan included a full return of the illness, regardless of the period of time that had elapsed.
Possible mechanisms
"We think it’s a cell-mediated immune response, not the classic, ‘I take medication, I get hypersensistivity’ response," Dr. Murray said.
ARBs inhibit transforming growth factor (TGF)-beta, and TGF-beta "is key for regulating the immune response in the gut," he said. However, because TGF-beta also drives collagen formation, that there would be a high number of collagenous sprue cases casts doubt on this as a clear mechanism, he said.
Another possibility Dr. Murray and his associates tested, but rejected, is that a bioactivating hydrolase enzyme activates olmesartan given as a prodrug, in the epithelial tissue of the intestine and liver, creating polymorphisms.
The role of angiotensin receptors in the gut and on lymphocytes has not yet been explained, although there is some similarity to celiac disease where "lots of regulatory T cells in patients "simply don’t work," Dr. Murray said. "We know that these are CD8-positive T cells, so they are cytotoxic, and there are plenty of FOXP3 cells present, and they don’t change when you take them off the drug."
The conference was sponsored by the Crohn’s & Colitis Foundation of America. Dr. Murray had no relevant disclosures.
EXPERT ANALYSIS FROM 2013 ADVANCES IN IBD
Major finding: Olmesartan was implicated in 14 out of 32 cases of refractory idiopathic sprue treated at the Mayo Clinic between 2008 and 2011.
Data source: Retrospective case series analysis.
Disclosures: Dr. Murray had no relevant disclosures.
Metronidazole linked to increased risk for inflammatory bowel disease
HOLLYWOOD, FLA. – Exposure to antibiotics other than penicillins, in particular metronidazole and quinolones, was associated with new-onset Crohn’s disease, based on a meta-analysis of observational and case-control studies presented by Dr. Ryan Ungaro at a conference on inflammatory bowel diseases.
"Exposure to antibiotics may somehow contribute to alterations in the microbiome and result in dysbiosis, which is known to be part of the pathogenesis that leads to IBD," said Dr. Ungaro, of the Icahn School of Medicine at Mount Sinai, New York. Alternatively, antibiotic exposures might just be surrogate markers for an infectious trigger that is actually associated with IBD. The analysis did not detect a link between antibiotic exposure and ulcerative colitis.
Dr. Ungaro and his colleagues performed a meta-analysis of 11 studies that included the records of 7,208 patients who had been newly diagnosed with IBD after antibiotic exposure; 3,937 had Crohn’s disease, 3,207 had ulcerative colitis, and 64 had unclassified IBD. Nine of the studies accounted for the potential confounding of diagnostic delay, with a range of 4 months to 4 years.
All classes of antibiotics except penicillin were implicated in new-onset IBD, with an odds ratio (OR) of 1.55 for the overall risk of new-onset IBD after antibiotic exposure. Three studies provided data on the use of metronidazole, which proved to have the highest associated risk for new cases of IBD with a pooled OR of 5.01 (P = .005). Quinolones were accounted for in three of the studies and carried the next-highest associated risk, an OR of 1.79 (P = .040).
When stratified by age, the OR for new IBD diagnosis in adults was 1.43 and in children was 1.89. The OR for a new diagnosis of Crohn’s disease was 1.56 in adults and 2.7 in children.
The conference was sponsored by the Crohn’s & Colitis Foundation of America.
Dr. Ungaro did not have any relevant disclosures.
HOLLYWOOD, FLA. – Exposure to antibiotics other than penicillins, in particular metronidazole and quinolones, was associated with new-onset Crohn’s disease, based on a meta-analysis of observational and case-control studies presented by Dr. Ryan Ungaro at a conference on inflammatory bowel diseases.
"Exposure to antibiotics may somehow contribute to alterations in the microbiome and result in dysbiosis, which is known to be part of the pathogenesis that leads to IBD," said Dr. Ungaro, of the Icahn School of Medicine at Mount Sinai, New York. Alternatively, antibiotic exposures might just be surrogate markers for an infectious trigger that is actually associated with IBD. The analysis did not detect a link between antibiotic exposure and ulcerative colitis.
Dr. Ungaro and his colleagues performed a meta-analysis of 11 studies that included the records of 7,208 patients who had been newly diagnosed with IBD after antibiotic exposure; 3,937 had Crohn’s disease, 3,207 had ulcerative colitis, and 64 had unclassified IBD. Nine of the studies accounted for the potential confounding of diagnostic delay, with a range of 4 months to 4 years.
All classes of antibiotics except penicillin were implicated in new-onset IBD, with an odds ratio (OR) of 1.55 for the overall risk of new-onset IBD after antibiotic exposure. Three studies provided data on the use of metronidazole, which proved to have the highest associated risk for new cases of IBD with a pooled OR of 5.01 (P = .005). Quinolones were accounted for in three of the studies and carried the next-highest associated risk, an OR of 1.79 (P = .040).
When stratified by age, the OR for new IBD diagnosis in adults was 1.43 and in children was 1.89. The OR for a new diagnosis of Crohn’s disease was 1.56 in adults and 2.7 in children.
The conference was sponsored by the Crohn’s & Colitis Foundation of America.
Dr. Ungaro did not have any relevant disclosures.
HOLLYWOOD, FLA. – Exposure to antibiotics other than penicillins, in particular metronidazole and quinolones, was associated with new-onset Crohn’s disease, based on a meta-analysis of observational and case-control studies presented by Dr. Ryan Ungaro at a conference on inflammatory bowel diseases.
"Exposure to antibiotics may somehow contribute to alterations in the microbiome and result in dysbiosis, which is known to be part of the pathogenesis that leads to IBD," said Dr. Ungaro, of the Icahn School of Medicine at Mount Sinai, New York. Alternatively, antibiotic exposures might just be surrogate markers for an infectious trigger that is actually associated with IBD. The analysis did not detect a link between antibiotic exposure and ulcerative colitis.
Dr. Ungaro and his colleagues performed a meta-analysis of 11 studies that included the records of 7,208 patients who had been newly diagnosed with IBD after antibiotic exposure; 3,937 had Crohn’s disease, 3,207 had ulcerative colitis, and 64 had unclassified IBD. Nine of the studies accounted for the potential confounding of diagnostic delay, with a range of 4 months to 4 years.
All classes of antibiotics except penicillin were implicated in new-onset IBD, with an odds ratio (OR) of 1.55 for the overall risk of new-onset IBD after antibiotic exposure. Three studies provided data on the use of metronidazole, which proved to have the highest associated risk for new cases of IBD with a pooled OR of 5.01 (P = .005). Quinolones were accounted for in three of the studies and carried the next-highest associated risk, an OR of 1.79 (P = .040).
When stratified by age, the OR for new IBD diagnosis in adults was 1.43 and in children was 1.89. The OR for a new diagnosis of Crohn’s disease was 1.56 in adults and 2.7 in children.
The conference was sponsored by the Crohn’s & Colitis Foundation of America.
Dr. Ungaro did not have any relevant disclosures.
FROM 2013 ADVANCES IN IBD
Major finding: Three studies provided data on use of metronidazole, which proved to have the highest associated risk for new cases of IBD with a pooled odds ratio of 5.01 (P = .005).
Data source: A meta-analysis of 7,208 patients in 11 observational studies with antibiotic exposure prior to IBD diagnosis.
Disclosures: Dr. Ungaro did not have any relevant disclosures.
USPSTF gives final recommendation on lung cancer screening
Low-dose computed tomography screening of those at high risk for lung cancer has received a grade B recommendation from the U.S. Preventive Services Task Force. Initially available for public comment in July 2013, the Task Force’s recommendations are now final and published.
The action allows the Centers for Medicare and Medicaid to mandate this service be provided without charging a copay or deductible. Widespread availability of screening raises concerns about inappropriate use of low-dose computed tomography (LDCT) and the associated costs of the procedure, physician experts noted in editorials and interviews.
More than a third of Americans are current or former smokers. Increasing age and cumulative exposure to tobacco smoke are the leading risk factors for lung cancer.
The USPSTF defines those at high-risk patients as heavy smokers who are aged 55-80 years and have a 30-pack-year or more habit, and former heavy smokers who have quit in the past 15 years. Screening should be discontinued once a person has not smoked for 15 years.
Patients also can be selected for screening based on risk factors other than tobacco use, including occupational exposures, radon exposure, family history, and incidence of pulmonary fibrosis or chronic obstructive lung disease.
Because of the potential for patients to experience "net harm, no net benefit, or at least substantially less benefit" from screening, the USPSTF stated it may be inappropriate to screen patients who have comorbidities that limit life expectancy, or who would be either unwilling or unable to have curative lung surgery.
Other forms of screening, including chest x-rays and sputum cytology, are not recommended because of their "inadequate sensitivity or specificity."
The USPSTF’s recommendations are based largely on a systematic review of several randomized, controlled trials published between 2000 and 2013, including the National Lung Screening Trial. That study of more than 50,000 asymptomatic adults, aged 55-74 years, showed a 16% reduction in lung cancer mortality and a 6.7% reduction in all-cause mortality when patients were screened using LDCT. One cancer death was averted for every 320 patients screened, and one death from all-causes was prevented in every 219 patients screened.
"Lung cancer causes as many deaths in the United States as the next three leading types of cancers combined, all of which already have screening interventions," wrote Dr. Frank C. Detterbeck of Yale University in New Haven, Conn., and Dr. Michael Unger of the Fox Chase Cancer Center in Philadelphia in an editorial accompanying the report.
And while the use of LDCT is part of a structured screening process, not just a scan, the USPSTF report does not address many of the practical aspects of implementing lung cancer screening, they said.
Many patients who are not necessarily high risk will present to their physicians with anxiety about developing lung cancer. "These people have reasons for their concerns; turning them away because they do not meet the criteria does not provide them the reassurance they seek," the editorialists wrote. An educated discussion usually eases the patient’s fear, but "this requires specialized knowledge and time. It is easier to give in and screen an anxious patient who does not meet the criteria."
As noted by the USPSTF, the potential harms of LDCT screening include false-negative and false-positive results, including the potential for incidental findings, overdiagnosis, and radiation exposure. "In a high-quality screening program, further imaging can resolve most false-positive results; however, some patients may require invasive procedures," the recommendations state.
Dr. Detterbeck and Dr. Unger wrote that effective screening hinges on reaching high-risk individuals, yet this is the population least likely to seek screening despite recognizing they are at risk. Further, chest CT is not a simple way to provide reassurance to anxious, lower-risk individuals. It is questionable whether primary care physicians will have the time and skill to advise patients on lung cancer screening and whether the "health care system is willing to support what the USPSTF is recommending."
Dr. Peter B. Bach, director of the Center for Health Policy and Outcomes at Sloan-Kettering Cancer Center in New York, authored a second editorial that accompanied the recommendations.
In an interview, he noted that issues of cost and counseling "matter a lot now that the Affordable Care Act links these recommendations to mandatory insurance benefits, which will then lead to automatic increases in health insurance premiums," according to Dr. Bach.
What is needed, he said, is more granular level of recommendations with more clinical utility.
"The expected degree of net benefit or level of certainty about the evidence is rarely uniform, even for selected populations," he wrote in his editorial. There are subgroups in which we have a lot of insight that screening is quite a bit more likely to help than harm, and the findings from the NLST should drive the approach.
Across the quintiles of lung cancer risk studied in the NLST, those considered to have experienced a probable benefit from screening varied from 5,276 in the lowest-risk group to 161 in the highest-risk group. Similarly, when considering the NLST’s benefit-to-harm ratio across the quintiles from lowest to highest, the number of false-positive results per lung cancer–related death prevented varied from 1,648 false-positive results per prevented death to 65, respectively, he said.
Screening protocols for patients in the low-risk group should receive a grade C from the USPSTF, which means the service should be offered selectively only, according to Dr. Bach.
"Screening should not be mandated for insurance coverage in the low-risk population. Neither should doctors and patients be told that it is definitely a good idea for everyone, nor should it become a quality standard for doctors, hospitals, and insurance plans, which are all things that could happen with this "B" recommendation," Dr. Bach said in an interview.
Dr. Bach was the lead author of practice guidelines issued jointly in 2013 by the American College of Chest Physicians and the American Society of Clinical Oncology. Those guidelines, which are based mostly on the NLST, state that individuals aged 55-74 years who have at least a 30 pack-year smoking history should be screened with LDCT. The American Cancer Society has also endorsed lung cancer screening recommendations based on the same protocols as the ACCP and ASCO (CA Cancer J. Clin. 2013;63:107-17).
"I support the task force’s role in the crafting of essential health benefits absolutely," Dr. Bach said. "But I think their power now to create mandates means they should up their game."
Low-dose computed tomography screening of those at high risk for lung cancer has received a grade B recommendation from the U.S. Preventive Services Task Force. Initially available for public comment in July 2013, the Task Force’s recommendations are now final and published.
The action allows the Centers for Medicare and Medicaid to mandate this service be provided without charging a copay or deductible. Widespread availability of screening raises concerns about inappropriate use of low-dose computed tomography (LDCT) and the associated costs of the procedure, physician experts noted in editorials and interviews.
More than a third of Americans are current or former smokers. Increasing age and cumulative exposure to tobacco smoke are the leading risk factors for lung cancer.
The USPSTF defines those at high-risk patients as heavy smokers who are aged 55-80 years and have a 30-pack-year or more habit, and former heavy smokers who have quit in the past 15 years. Screening should be discontinued once a person has not smoked for 15 years.
Patients also can be selected for screening based on risk factors other than tobacco use, including occupational exposures, radon exposure, family history, and incidence of pulmonary fibrosis or chronic obstructive lung disease.
Because of the potential for patients to experience "net harm, no net benefit, or at least substantially less benefit" from screening, the USPSTF stated it may be inappropriate to screen patients who have comorbidities that limit life expectancy, or who would be either unwilling or unable to have curative lung surgery.
Other forms of screening, including chest x-rays and sputum cytology, are not recommended because of their "inadequate sensitivity or specificity."
The USPSTF’s recommendations are based largely on a systematic review of several randomized, controlled trials published between 2000 and 2013, including the National Lung Screening Trial. That study of more than 50,000 asymptomatic adults, aged 55-74 years, showed a 16% reduction in lung cancer mortality and a 6.7% reduction in all-cause mortality when patients were screened using LDCT. One cancer death was averted for every 320 patients screened, and one death from all-causes was prevented in every 219 patients screened.
"Lung cancer causes as many deaths in the United States as the next three leading types of cancers combined, all of which already have screening interventions," wrote Dr. Frank C. Detterbeck of Yale University in New Haven, Conn., and Dr. Michael Unger of the Fox Chase Cancer Center in Philadelphia in an editorial accompanying the report.
And while the use of LDCT is part of a structured screening process, not just a scan, the USPSTF report does not address many of the practical aspects of implementing lung cancer screening, they said.
Many patients who are not necessarily high risk will present to their physicians with anxiety about developing lung cancer. "These people have reasons for their concerns; turning them away because they do not meet the criteria does not provide them the reassurance they seek," the editorialists wrote. An educated discussion usually eases the patient’s fear, but "this requires specialized knowledge and time. It is easier to give in and screen an anxious patient who does not meet the criteria."
As noted by the USPSTF, the potential harms of LDCT screening include false-negative and false-positive results, including the potential for incidental findings, overdiagnosis, and radiation exposure. "In a high-quality screening program, further imaging can resolve most false-positive results; however, some patients may require invasive procedures," the recommendations state.
Dr. Detterbeck and Dr. Unger wrote that effective screening hinges on reaching high-risk individuals, yet this is the population least likely to seek screening despite recognizing they are at risk. Further, chest CT is not a simple way to provide reassurance to anxious, lower-risk individuals. It is questionable whether primary care physicians will have the time and skill to advise patients on lung cancer screening and whether the "health care system is willing to support what the USPSTF is recommending."
Dr. Peter B. Bach, director of the Center for Health Policy and Outcomes at Sloan-Kettering Cancer Center in New York, authored a second editorial that accompanied the recommendations.
In an interview, he noted that issues of cost and counseling "matter a lot now that the Affordable Care Act links these recommendations to mandatory insurance benefits, which will then lead to automatic increases in health insurance premiums," according to Dr. Bach.
What is needed, he said, is more granular level of recommendations with more clinical utility.
"The expected degree of net benefit or level of certainty about the evidence is rarely uniform, even for selected populations," he wrote in his editorial. There are subgroups in which we have a lot of insight that screening is quite a bit more likely to help than harm, and the findings from the NLST should drive the approach.
Across the quintiles of lung cancer risk studied in the NLST, those considered to have experienced a probable benefit from screening varied from 5,276 in the lowest-risk group to 161 in the highest-risk group. Similarly, when considering the NLST’s benefit-to-harm ratio across the quintiles from lowest to highest, the number of false-positive results per lung cancer–related death prevented varied from 1,648 false-positive results per prevented death to 65, respectively, he said.
Screening protocols for patients in the low-risk group should receive a grade C from the USPSTF, which means the service should be offered selectively only, according to Dr. Bach.
"Screening should not be mandated for insurance coverage in the low-risk population. Neither should doctors and patients be told that it is definitely a good idea for everyone, nor should it become a quality standard for doctors, hospitals, and insurance plans, which are all things that could happen with this "B" recommendation," Dr. Bach said in an interview.
Dr. Bach was the lead author of practice guidelines issued jointly in 2013 by the American College of Chest Physicians and the American Society of Clinical Oncology. Those guidelines, which are based mostly on the NLST, state that individuals aged 55-74 years who have at least a 30 pack-year smoking history should be screened with LDCT. The American Cancer Society has also endorsed lung cancer screening recommendations based on the same protocols as the ACCP and ASCO (CA Cancer J. Clin. 2013;63:107-17).
"I support the task force’s role in the crafting of essential health benefits absolutely," Dr. Bach said. "But I think their power now to create mandates means they should up their game."
Low-dose computed tomography screening of those at high risk for lung cancer has received a grade B recommendation from the U.S. Preventive Services Task Force. Initially available for public comment in July 2013, the Task Force’s recommendations are now final and published.
The action allows the Centers for Medicare and Medicaid to mandate this service be provided without charging a copay or deductible. Widespread availability of screening raises concerns about inappropriate use of low-dose computed tomography (LDCT) and the associated costs of the procedure, physician experts noted in editorials and interviews.
More than a third of Americans are current or former smokers. Increasing age and cumulative exposure to tobacco smoke are the leading risk factors for lung cancer.
The USPSTF defines those at high-risk patients as heavy smokers who are aged 55-80 years and have a 30-pack-year or more habit, and former heavy smokers who have quit in the past 15 years. Screening should be discontinued once a person has not smoked for 15 years.
Patients also can be selected for screening based on risk factors other than tobacco use, including occupational exposures, radon exposure, family history, and incidence of pulmonary fibrosis or chronic obstructive lung disease.
Because of the potential for patients to experience "net harm, no net benefit, or at least substantially less benefit" from screening, the USPSTF stated it may be inappropriate to screen patients who have comorbidities that limit life expectancy, or who would be either unwilling or unable to have curative lung surgery.
Other forms of screening, including chest x-rays and sputum cytology, are not recommended because of their "inadequate sensitivity or specificity."
The USPSTF’s recommendations are based largely on a systematic review of several randomized, controlled trials published between 2000 and 2013, including the National Lung Screening Trial. That study of more than 50,000 asymptomatic adults, aged 55-74 years, showed a 16% reduction in lung cancer mortality and a 6.7% reduction in all-cause mortality when patients were screened using LDCT. One cancer death was averted for every 320 patients screened, and one death from all-causes was prevented in every 219 patients screened.
"Lung cancer causes as many deaths in the United States as the next three leading types of cancers combined, all of which already have screening interventions," wrote Dr. Frank C. Detterbeck of Yale University in New Haven, Conn., and Dr. Michael Unger of the Fox Chase Cancer Center in Philadelphia in an editorial accompanying the report.
And while the use of LDCT is part of a structured screening process, not just a scan, the USPSTF report does not address many of the practical aspects of implementing lung cancer screening, they said.
Many patients who are not necessarily high risk will present to their physicians with anxiety about developing lung cancer. "These people have reasons for their concerns; turning them away because they do not meet the criteria does not provide them the reassurance they seek," the editorialists wrote. An educated discussion usually eases the patient’s fear, but "this requires specialized knowledge and time. It is easier to give in and screen an anxious patient who does not meet the criteria."
As noted by the USPSTF, the potential harms of LDCT screening include false-negative and false-positive results, including the potential for incidental findings, overdiagnosis, and radiation exposure. "In a high-quality screening program, further imaging can resolve most false-positive results; however, some patients may require invasive procedures," the recommendations state.
Dr. Detterbeck and Dr. Unger wrote that effective screening hinges on reaching high-risk individuals, yet this is the population least likely to seek screening despite recognizing they are at risk. Further, chest CT is not a simple way to provide reassurance to anxious, lower-risk individuals. It is questionable whether primary care physicians will have the time and skill to advise patients on lung cancer screening and whether the "health care system is willing to support what the USPSTF is recommending."
Dr. Peter B. Bach, director of the Center for Health Policy and Outcomes at Sloan-Kettering Cancer Center in New York, authored a second editorial that accompanied the recommendations.
In an interview, he noted that issues of cost and counseling "matter a lot now that the Affordable Care Act links these recommendations to mandatory insurance benefits, which will then lead to automatic increases in health insurance premiums," according to Dr. Bach.
What is needed, he said, is more granular level of recommendations with more clinical utility.
"The expected degree of net benefit or level of certainty about the evidence is rarely uniform, even for selected populations," he wrote in his editorial. There are subgroups in which we have a lot of insight that screening is quite a bit more likely to help than harm, and the findings from the NLST should drive the approach.
Across the quintiles of lung cancer risk studied in the NLST, those considered to have experienced a probable benefit from screening varied from 5,276 in the lowest-risk group to 161 in the highest-risk group. Similarly, when considering the NLST’s benefit-to-harm ratio across the quintiles from lowest to highest, the number of false-positive results per lung cancer–related death prevented varied from 1,648 false-positive results per prevented death to 65, respectively, he said.
Screening protocols for patients in the low-risk group should receive a grade C from the USPSTF, which means the service should be offered selectively only, according to Dr. Bach.
"Screening should not be mandated for insurance coverage in the low-risk population. Neither should doctors and patients be told that it is definitely a good idea for everyone, nor should it become a quality standard for doctors, hospitals, and insurance plans, which are all things that could happen with this "B" recommendation," Dr. Bach said in an interview.
Dr. Bach was the lead author of practice guidelines issued jointly in 2013 by the American College of Chest Physicians and the American Society of Clinical Oncology. Those guidelines, which are based mostly on the NLST, state that individuals aged 55-74 years who have at least a 30 pack-year smoking history should be screened with LDCT. The American Cancer Society has also endorsed lung cancer screening recommendations based on the same protocols as the ACCP and ASCO (CA Cancer J. Clin. 2013;63:107-17).
"I support the task force’s role in the crafting of essential health benefits absolutely," Dr. Bach said. "But I think their power now to create mandates means they should up their game."
FROM THE ANNALS OF INTERNAL MEDICINE
Bariatric surgery may have favorable outcomes in patients with HIV
ATLANTA – Bariatric surgery was associated with lowered viral loads and resolution of some antiretroviral-related comorbidities, such as type 2 diabetes, in patients with the human immunodeficiency virus, according to Dr. Raul J. Rosenthal.
Patients with HIV who have extended their life expectancy through highly antiretroviral treatment are now living long enough to develop lipohypertrophy, similar to metabolic syndrome, said Dr. Rosenthal, a bariatric surgeon at the Cleveland Clinic Florida in Weston.
"[Some obese patients with HIV] are developing hypertriglyceridemia, type 2 diabetes, hypercholesterolemia, and coronary artery disease," said Dr. Rosenthal. "We are not surprised that infectious disease and primary care doctors send these patients to us for surgery."
To determine whether bariatric surgery was improving the course of the HIV infection in this patient population, Dr. Rosenthal and his associates reviewed the records of 11 asymptomatic patients with HIV who underwent a bariatric procedure at a single surgery center in the past 10 years.
The researchers found that none of the patients had perioperative complications, and all reduced their preoperative mean body mass index (BMI) from 52 kg/m2 to 36 kg/m2. The patients’ preoperative mean weight dropped, on average, by 50%, Dr. Rosenthal said at the meeting, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
The patients’ total preoperative mean CD4 count went from 825 cells/mm3 to 504 cells/mm3; the viral load went from a preoperative mean of 813 copies/mL plasma to 684 copies/mL plasma. "I think this is the most important part of the whole study," said Dr. Rosenthal, who said that in patients with the HIV infection, it is important to always keep the CD4 (T-cell) level above 200 cells/mm3 and the viral load under 10,000 copies/mL plasma.
Five patients were followed for 2 years postoperatively. None had complications, although one patient did experience a long-term marginal ulcer with malnutrition.
Although the literature about bariatric surgery in this patient population is "scant" said Dr. Rosenthal, the data from his study were comparable to the findings from at least two other small studies that indicated bariatric surgery may be an effective treatment for obesity and its attendant comorbidities in HIV, without negatively impacting virologic suppression (Surg. Obes. Relat. Dis. 2005;1:73-6).
The best bariatric treatment in this patient population may be the sleeve because it was the least likely to preclude future treatments.
"The sleeve has a morbidity rate of 1.6%, and I am not going to fight that," given the unpredictable prognosis in this patient population, Dr. Rosenthal said.
Although larger studies are necessary, "so far, HIV does not appear to increase the rate of perioperative complications, and it does alter the course of HIV infection," he noted.
ATLANTA – Bariatric surgery was associated with lowered viral loads and resolution of some antiretroviral-related comorbidities, such as type 2 diabetes, in patients with the human immunodeficiency virus, according to Dr. Raul J. Rosenthal.
Patients with HIV who have extended their life expectancy through highly antiretroviral treatment are now living long enough to develop lipohypertrophy, similar to metabolic syndrome, said Dr. Rosenthal, a bariatric surgeon at the Cleveland Clinic Florida in Weston.
"[Some obese patients with HIV] are developing hypertriglyceridemia, type 2 diabetes, hypercholesterolemia, and coronary artery disease," said Dr. Rosenthal. "We are not surprised that infectious disease and primary care doctors send these patients to us for surgery."
To determine whether bariatric surgery was improving the course of the HIV infection in this patient population, Dr. Rosenthal and his associates reviewed the records of 11 asymptomatic patients with HIV who underwent a bariatric procedure at a single surgery center in the past 10 years.
The researchers found that none of the patients had perioperative complications, and all reduced their preoperative mean body mass index (BMI) from 52 kg/m2 to 36 kg/m2. The patients’ preoperative mean weight dropped, on average, by 50%, Dr. Rosenthal said at the meeting, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
The patients’ total preoperative mean CD4 count went from 825 cells/mm3 to 504 cells/mm3; the viral load went from a preoperative mean of 813 copies/mL plasma to 684 copies/mL plasma. "I think this is the most important part of the whole study," said Dr. Rosenthal, who said that in patients with the HIV infection, it is important to always keep the CD4 (T-cell) level above 200 cells/mm3 and the viral load under 10,000 copies/mL plasma.
Five patients were followed for 2 years postoperatively. None had complications, although one patient did experience a long-term marginal ulcer with malnutrition.
Although the literature about bariatric surgery in this patient population is "scant" said Dr. Rosenthal, the data from his study were comparable to the findings from at least two other small studies that indicated bariatric surgery may be an effective treatment for obesity and its attendant comorbidities in HIV, without negatively impacting virologic suppression (Surg. Obes. Relat. Dis. 2005;1:73-6).
The best bariatric treatment in this patient population may be the sleeve because it was the least likely to preclude future treatments.
"The sleeve has a morbidity rate of 1.6%, and I am not going to fight that," given the unpredictable prognosis in this patient population, Dr. Rosenthal said.
Although larger studies are necessary, "so far, HIV does not appear to increase the rate of perioperative complications, and it does alter the course of HIV infection," he noted.
ATLANTA – Bariatric surgery was associated with lowered viral loads and resolution of some antiretroviral-related comorbidities, such as type 2 diabetes, in patients with the human immunodeficiency virus, according to Dr. Raul J. Rosenthal.
Patients with HIV who have extended their life expectancy through highly antiretroviral treatment are now living long enough to develop lipohypertrophy, similar to metabolic syndrome, said Dr. Rosenthal, a bariatric surgeon at the Cleveland Clinic Florida in Weston.
"[Some obese patients with HIV] are developing hypertriglyceridemia, type 2 diabetes, hypercholesterolemia, and coronary artery disease," said Dr. Rosenthal. "We are not surprised that infectious disease and primary care doctors send these patients to us for surgery."
To determine whether bariatric surgery was improving the course of the HIV infection in this patient population, Dr. Rosenthal and his associates reviewed the records of 11 asymptomatic patients with HIV who underwent a bariatric procedure at a single surgery center in the past 10 years.
The researchers found that none of the patients had perioperative complications, and all reduced their preoperative mean body mass index (BMI) from 52 kg/m2 to 36 kg/m2. The patients’ preoperative mean weight dropped, on average, by 50%, Dr. Rosenthal said at the meeting, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
The patients’ total preoperative mean CD4 count went from 825 cells/mm3 to 504 cells/mm3; the viral load went from a preoperative mean of 813 copies/mL plasma to 684 copies/mL plasma. "I think this is the most important part of the whole study," said Dr. Rosenthal, who said that in patients with the HIV infection, it is important to always keep the CD4 (T-cell) level above 200 cells/mm3 and the viral load under 10,000 copies/mL plasma.
Five patients were followed for 2 years postoperatively. None had complications, although one patient did experience a long-term marginal ulcer with malnutrition.
Although the literature about bariatric surgery in this patient population is "scant" said Dr. Rosenthal, the data from his study were comparable to the findings from at least two other small studies that indicated bariatric surgery may be an effective treatment for obesity and its attendant comorbidities in HIV, without negatively impacting virologic suppression (Surg. Obes. Relat. Dis. 2005;1:73-6).
The best bariatric treatment in this patient population may be the sleeve because it was the least likely to preclude future treatments.
"The sleeve has a morbidity rate of 1.6%, and I am not going to fight that," given the unpredictable prognosis in this patient population, Dr. Rosenthal said.
Although larger studies are necessary, "so far, HIV does not appear to increase the rate of perioperative complications, and it does alter the course of HIV infection," he noted.
AT OBESITY WEEK
Major finding: Bariatric surgery in HIV was not found to negatively impact the course of infection in HIV patients, and had no significant adverse outcomes.
Data source: A retrospective study of 11 patients at a single site, in addition to an analysis of the literature.
Disclosures: Dr. Rosenthal did not report any financial disclosures.
Misdiagnosis, noncompliance often culprits in refractory celiac disease
HOLLYWOOD, FLA. – Refractory celiac disease is often the result of patients having either received an incorrect diagnosis, or their noncompliance, according to Dr. Joseph Murray of the Mayo Clinic in Rochester, Minn.
"When faced with such a patient, it’s important to reconfirm the original diagnosis," said Dr. Murray, who made his remarks during a clinical track presentation at a conference on inflammatory bowel.
In cases in which the diagnosis can be confirmed and the patient is compliant, discovering if there are other conditions, and whether to intervene and how, are the important next steps, according to Dr. Murray.
When patients present with symptoms of nonresponsive celiac disease, besides taking the patient’s history, which should include whether the patient has any first-degree family members with "true" celiac disease, not just family members who have chosen to stop eating gluten, "I will always ask for the original biopsies," said Dr. Murray.
In addition, original serology tests, if they were done, can confirm whether there are celiac-specific antibodies. "Gliadin antibodies are not celiac specific," said Dr. Murray. "You can get gliadin antibodies in virtually every other disorder that affects the intestines, so they are pretty much worthless." Better specificity comes from tissue transglutaminase or endomysial antibodies, he said at the meeting diseases sponsored by the Crohn’s & Colitis Foundation of America.
Human leukocyte antigen genotyping, and whether the patient had a clinically obvious response to a gluten-free diet also will help the clinician puzzle out if the original diagnosis was correct, according to Dr. Murray. Worth noting is whether the patient has dermatitis herpetiformis, "That’s pathognomonic for celiac disease," said Dr. Murray.
For example, in the case of a 90-year-old woman whose biopsy 10 years before had been interpreted as presumptive celiac disease and who had had an initial response to a gluten-free diet, had symptoms that persisted for a decade because she’d contracted tropical sprue from annual visits to Indonesia that were not noted in her original patient history. Treated properly, her symptoms abated entirely, according to Dr. Murray. "She wasn’t exactly happy about her 10 years of living gluten free," he said.
Dangers of noncompliance
As for patients who claim to follow a gluten-free diet, "That’s not true most of the time," said Dr. Murray. "A positive serology test in a patient who’s been following a gluten-free diet for a year or more means they’re not just getting a little gluten. They’re getting a lot of gluten." It can either be advertent or inadvertently, he said.
However, serology is insensitive for lower levels of gluten contamination, but a gram of gluten, roughly one-half a slice of bread per day, can be detected, according to Dr. Murray.
If noncompliance is the reason for the refractory condition, patients are at greater risk for increased mortality, osteoporosis, lymphomas, and other cancers, and psychological effects such as depression. "Eliminating the gluten may take time. Often we have to use behavioral counselors to help," said Dr. Murray.
Also key is to stay in touch with the patient. "Follow-up in patients with celiac disease is abysmal," Dr. Murray said, "It’s almost like once the disease is diagnosed, it’s forgotten about medically."
"The complicating thing about celiac disease can be that autoimmune disorders and like disorders hang out together," said Dr. Murray. "Complications of celiac disease also can occur in multiples."
Bacterial overgrowth, microscopic colitis, lymphoma, and systemic sclerosis associated–dysmotility are all concurrent conditions Dr. Murray reported seeing in his own practice when treating refractory celiac disease.
Because lactose intolerance is also common in celiac disease, Dr. Murray said he will often advises patients to avoid dairy for a year, and then gradually add that back into the diet with good results. "Often, that will work, so I don’t even test for lactose intolerance initially," he said.
Despite all the possible etiologies for nonresponsive celiac disease, gluten exposure was found in more than a third of cases, while "true refractory celiac disease really makes up only about 10% or 11% of these nonresponsive patients," said Dr. Murray, referring to a study on the topic (Clin. Gastroenterol. Hepatol. 2007;5:445-50).
Patients with celiac disease also can have multifocal strictures in the proximal duodenum that reach the jejunum, "but rarely affect the ileum," according to Dr. Murray.
Possible lymphomas
"The first thing that I think about when I see a really sick patient previously diagnosed with celiac disease several years before is, ‘Does the patient have lymphoma?’" said Dr. Murray. Ulcerative jejunoileitis typically indicates that lymphoma is imminent, although shallower ulcers are often linked to the use of NSAIDs, he said.
Giant cavitating lymphadenopathy, while rare, is also a consideration, according to Dr. Murray. "A premalignant type of disorder, sometimes will respond to immunosuppressives, but often can presage the development of lymphoma," he said.
True refractory celiac disease involves symptomatic malabsorption, severe enteropathy, and a primary or secondary nonresponse to a gluten-free diet. "By definition, there should be no lymphoma," said Dr. Murray.
Refractory celiac disease is either characterized as type 1, which has a normal T-cell population and responds well to immunosuppression, or as type 2 with clonal T cells.
Dr. Murray said he often uses topical budesonide to treat type 1 patients, with good results, since there is about a 90% recovery rate in this patient population. Type 2 is the most pernicious, with nearly half of patients dying within 5 years of diagnosis, either from malignant or infectious complications, according to Dr. Murray. "Type 2 refractory disease is not a trivial disease," he said.
Although most adults with celiac disease don’t heal, many are asymptomatic; however, this does not mean a patient’s risk of mortality from the disease has improved. Patients are also at greater risk for malignant complications. (Am. J. Gastroenterol. 2010;105:1412-20 [doi:10.1038/ajg.2010.10]).
"We really don’t know what we should do about those asymptomatic patients," said Dr. Murray. He noted that, "Failure to heal is not entirely benign, but it’s not refractory celiac disease," said Dr. Murray.
Dr. Murray stated that he had no disclosures.
HOLLYWOOD, FLA. – Refractory celiac disease is often the result of patients having either received an incorrect diagnosis, or their noncompliance, according to Dr. Joseph Murray of the Mayo Clinic in Rochester, Minn.
"When faced with such a patient, it’s important to reconfirm the original diagnosis," said Dr. Murray, who made his remarks during a clinical track presentation at a conference on inflammatory bowel.
In cases in which the diagnosis can be confirmed and the patient is compliant, discovering if there are other conditions, and whether to intervene and how, are the important next steps, according to Dr. Murray.
When patients present with symptoms of nonresponsive celiac disease, besides taking the patient’s history, which should include whether the patient has any first-degree family members with "true" celiac disease, not just family members who have chosen to stop eating gluten, "I will always ask for the original biopsies," said Dr. Murray.
In addition, original serology tests, if they were done, can confirm whether there are celiac-specific antibodies. "Gliadin antibodies are not celiac specific," said Dr. Murray. "You can get gliadin antibodies in virtually every other disorder that affects the intestines, so they are pretty much worthless." Better specificity comes from tissue transglutaminase or endomysial antibodies, he said at the meeting diseases sponsored by the Crohn’s & Colitis Foundation of America.
Human leukocyte antigen genotyping, and whether the patient had a clinically obvious response to a gluten-free diet also will help the clinician puzzle out if the original diagnosis was correct, according to Dr. Murray. Worth noting is whether the patient has dermatitis herpetiformis, "That’s pathognomonic for celiac disease," said Dr. Murray.
For example, in the case of a 90-year-old woman whose biopsy 10 years before had been interpreted as presumptive celiac disease and who had had an initial response to a gluten-free diet, had symptoms that persisted for a decade because she’d contracted tropical sprue from annual visits to Indonesia that were not noted in her original patient history. Treated properly, her symptoms abated entirely, according to Dr. Murray. "She wasn’t exactly happy about her 10 years of living gluten free," he said.
Dangers of noncompliance
As for patients who claim to follow a gluten-free diet, "That’s not true most of the time," said Dr. Murray. "A positive serology test in a patient who’s been following a gluten-free diet for a year or more means they’re not just getting a little gluten. They’re getting a lot of gluten." It can either be advertent or inadvertently, he said.
However, serology is insensitive for lower levels of gluten contamination, but a gram of gluten, roughly one-half a slice of bread per day, can be detected, according to Dr. Murray.
If noncompliance is the reason for the refractory condition, patients are at greater risk for increased mortality, osteoporosis, lymphomas, and other cancers, and psychological effects such as depression. "Eliminating the gluten may take time. Often we have to use behavioral counselors to help," said Dr. Murray.
Also key is to stay in touch with the patient. "Follow-up in patients with celiac disease is abysmal," Dr. Murray said, "It’s almost like once the disease is diagnosed, it’s forgotten about medically."
"The complicating thing about celiac disease can be that autoimmune disorders and like disorders hang out together," said Dr. Murray. "Complications of celiac disease also can occur in multiples."
Bacterial overgrowth, microscopic colitis, lymphoma, and systemic sclerosis associated–dysmotility are all concurrent conditions Dr. Murray reported seeing in his own practice when treating refractory celiac disease.
Because lactose intolerance is also common in celiac disease, Dr. Murray said he will often advises patients to avoid dairy for a year, and then gradually add that back into the diet with good results. "Often, that will work, so I don’t even test for lactose intolerance initially," he said.
Despite all the possible etiologies for nonresponsive celiac disease, gluten exposure was found in more than a third of cases, while "true refractory celiac disease really makes up only about 10% or 11% of these nonresponsive patients," said Dr. Murray, referring to a study on the topic (Clin. Gastroenterol. Hepatol. 2007;5:445-50).
Patients with celiac disease also can have multifocal strictures in the proximal duodenum that reach the jejunum, "but rarely affect the ileum," according to Dr. Murray.
Possible lymphomas
"The first thing that I think about when I see a really sick patient previously diagnosed with celiac disease several years before is, ‘Does the patient have lymphoma?’" said Dr. Murray. Ulcerative jejunoileitis typically indicates that lymphoma is imminent, although shallower ulcers are often linked to the use of NSAIDs, he said.
Giant cavitating lymphadenopathy, while rare, is also a consideration, according to Dr. Murray. "A premalignant type of disorder, sometimes will respond to immunosuppressives, but often can presage the development of lymphoma," he said.
True refractory celiac disease involves symptomatic malabsorption, severe enteropathy, and a primary or secondary nonresponse to a gluten-free diet. "By definition, there should be no lymphoma," said Dr. Murray.
Refractory celiac disease is either characterized as type 1, which has a normal T-cell population and responds well to immunosuppression, or as type 2 with clonal T cells.
Dr. Murray said he often uses topical budesonide to treat type 1 patients, with good results, since there is about a 90% recovery rate in this patient population. Type 2 is the most pernicious, with nearly half of patients dying within 5 years of diagnosis, either from malignant or infectious complications, according to Dr. Murray. "Type 2 refractory disease is not a trivial disease," he said.
Although most adults with celiac disease don’t heal, many are asymptomatic; however, this does not mean a patient’s risk of mortality from the disease has improved. Patients are also at greater risk for malignant complications. (Am. J. Gastroenterol. 2010;105:1412-20 [doi:10.1038/ajg.2010.10]).
"We really don’t know what we should do about those asymptomatic patients," said Dr. Murray. He noted that, "Failure to heal is not entirely benign, but it’s not refractory celiac disease," said Dr. Murray.
Dr. Murray stated that he had no disclosures.
HOLLYWOOD, FLA. – Refractory celiac disease is often the result of patients having either received an incorrect diagnosis, or their noncompliance, according to Dr. Joseph Murray of the Mayo Clinic in Rochester, Minn.
"When faced with such a patient, it’s important to reconfirm the original diagnosis," said Dr. Murray, who made his remarks during a clinical track presentation at a conference on inflammatory bowel.
In cases in which the diagnosis can be confirmed and the patient is compliant, discovering if there are other conditions, and whether to intervene and how, are the important next steps, according to Dr. Murray.
When patients present with symptoms of nonresponsive celiac disease, besides taking the patient’s history, which should include whether the patient has any first-degree family members with "true" celiac disease, not just family members who have chosen to stop eating gluten, "I will always ask for the original biopsies," said Dr. Murray.
In addition, original serology tests, if they were done, can confirm whether there are celiac-specific antibodies. "Gliadin antibodies are not celiac specific," said Dr. Murray. "You can get gliadin antibodies in virtually every other disorder that affects the intestines, so they are pretty much worthless." Better specificity comes from tissue transglutaminase or endomysial antibodies, he said at the meeting diseases sponsored by the Crohn’s & Colitis Foundation of America.
Human leukocyte antigen genotyping, and whether the patient had a clinically obvious response to a gluten-free diet also will help the clinician puzzle out if the original diagnosis was correct, according to Dr. Murray. Worth noting is whether the patient has dermatitis herpetiformis, "That’s pathognomonic for celiac disease," said Dr. Murray.
For example, in the case of a 90-year-old woman whose biopsy 10 years before had been interpreted as presumptive celiac disease and who had had an initial response to a gluten-free diet, had symptoms that persisted for a decade because she’d contracted tropical sprue from annual visits to Indonesia that were not noted in her original patient history. Treated properly, her symptoms abated entirely, according to Dr. Murray. "She wasn’t exactly happy about her 10 years of living gluten free," he said.
Dangers of noncompliance
As for patients who claim to follow a gluten-free diet, "That’s not true most of the time," said Dr. Murray. "A positive serology test in a patient who’s been following a gluten-free diet for a year or more means they’re not just getting a little gluten. They’re getting a lot of gluten." It can either be advertent or inadvertently, he said.
However, serology is insensitive for lower levels of gluten contamination, but a gram of gluten, roughly one-half a slice of bread per day, can be detected, according to Dr. Murray.
If noncompliance is the reason for the refractory condition, patients are at greater risk for increased mortality, osteoporosis, lymphomas, and other cancers, and psychological effects such as depression. "Eliminating the gluten may take time. Often we have to use behavioral counselors to help," said Dr. Murray.
Also key is to stay in touch with the patient. "Follow-up in patients with celiac disease is abysmal," Dr. Murray said, "It’s almost like once the disease is diagnosed, it’s forgotten about medically."
"The complicating thing about celiac disease can be that autoimmune disorders and like disorders hang out together," said Dr. Murray. "Complications of celiac disease also can occur in multiples."
Bacterial overgrowth, microscopic colitis, lymphoma, and systemic sclerosis associated–dysmotility are all concurrent conditions Dr. Murray reported seeing in his own practice when treating refractory celiac disease.
Because lactose intolerance is also common in celiac disease, Dr. Murray said he will often advises patients to avoid dairy for a year, and then gradually add that back into the diet with good results. "Often, that will work, so I don’t even test for lactose intolerance initially," he said.
Despite all the possible etiologies for nonresponsive celiac disease, gluten exposure was found in more than a third of cases, while "true refractory celiac disease really makes up only about 10% or 11% of these nonresponsive patients," said Dr. Murray, referring to a study on the topic (Clin. Gastroenterol. Hepatol. 2007;5:445-50).
Patients with celiac disease also can have multifocal strictures in the proximal duodenum that reach the jejunum, "but rarely affect the ileum," according to Dr. Murray.
Possible lymphomas
"The first thing that I think about when I see a really sick patient previously diagnosed with celiac disease several years before is, ‘Does the patient have lymphoma?’" said Dr. Murray. Ulcerative jejunoileitis typically indicates that lymphoma is imminent, although shallower ulcers are often linked to the use of NSAIDs, he said.
Giant cavitating lymphadenopathy, while rare, is also a consideration, according to Dr. Murray. "A premalignant type of disorder, sometimes will respond to immunosuppressives, but often can presage the development of lymphoma," he said.
True refractory celiac disease involves symptomatic malabsorption, severe enteropathy, and a primary or secondary nonresponse to a gluten-free diet. "By definition, there should be no lymphoma," said Dr. Murray.
Refractory celiac disease is either characterized as type 1, which has a normal T-cell population and responds well to immunosuppression, or as type 2 with clonal T cells.
Dr. Murray said he often uses topical budesonide to treat type 1 patients, with good results, since there is about a 90% recovery rate in this patient population. Type 2 is the most pernicious, with nearly half of patients dying within 5 years of diagnosis, either from malignant or infectious complications, according to Dr. Murray. "Type 2 refractory disease is not a trivial disease," he said.
Although most adults with celiac disease don’t heal, many are asymptomatic; however, this does not mean a patient’s risk of mortality from the disease has improved. Patients are also at greater risk for malignant complications. (Am. J. Gastroenterol. 2010;105:1412-20 [doi:10.1038/ajg.2010.10]).
"We really don’t know what we should do about those asymptomatic patients," said Dr. Murray. He noted that, "Failure to heal is not entirely benign, but it’s not refractory celiac disease," said Dr. Murray.
Dr. Murray stated that he had no disclosures.
EXPERT ANALYSIS FROM 2013 ADVANCES IN IBD
Obesity Treatment Costs
ATLANTA – Obesity and its comorbidities loom, threatening to become an expensive national crisis, given that its treatment costs are nearly double that of other chronic diseases, and third party payers so far have failed to invest in its prevention.
"Obese individuals are about 42% more expensive than their normal weight counterparts," accounting for 9% of all medical expenditures, Eric Finkelstein, Ph.D., said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
With close to half the population expected to have a BMI greater or equal to 40 kg/m2 by 2030, even the slightest reversal of trends can save billions, according to Dr. Finkelstein, a health policy research analyst and professor at the Duke-National University of Singapore Global Health Institute (Am. J. Prev. Med. 2012;42:563-70).
‘Bend the curve’
"By 2020, if you could bend even just the cost percentage point by 1 per year, you could have 2.6 million fewer obese adults, and $3.9 billion less in medical expenditures," said Dr. Finkelstein. "By 2030, the numbers go up to 2.9 million fewer obese adults, and $9.5 billion in savings."
The way to bend the curve is through prevention, said Dr. Finkelstein. But, just what constitutes prevention and who should pay for it are not so straightforward.
Although the Affordable Care Act expanded the Centers for Medicare and Medicaid’s coverage of obesity screening and prevention, "there is some debate as to whether the ACA will help this problem," said Dr. Finkelstein.
A trifecta of politics, prejudice, and inconsistent health insurance policies may undermine the legislation’s ability to meet the challenges posed by obesity, according to the symposium presenters.
‘Ounce of prevention, pound of cure’
"Grandma was right. An ounce of prevention equals a pound of cure," noted Dr. Richard Wild, chief medical officer of the CMS in Atlanta.
To that end, he said that under the ACA, there is "more flexibility to [cover prevention] with no cost sharing to patients." CMS obesity screening, prevention, and treatment are largely tied to the U. S. Preventive Services Task Force advisory committee, said Dr. Wild.
A significant percentage of individuals in their mid-20s with class 1 obesity (BMI between 30 and 35 kg/m2) will have BMIs of 40 kg/m2 or greater before they reach their 40s, according to Dr. Finkelstein. By the time they enter their 40s, 63% of males and 78% of women will have an obesity-related comorbidity. Many of those in the 30 to 35 BMI group are likely to continue to a significant weight gain that will make them eligible for bariatric surgery fairly soon," he said.
Early intervention is key to preventing the cost of comorbidities, Dr. Finkelstein said (Surg. Obes. Relat. Dis. 2013;9:547-53).
However, CMS limits Medicare coverage of bariatric surgery to those with a BMI greater than 35 kg/m2, who have at least one related comorbidity and have proven unsuccessful at past attempts to control their weight.
Beyond that, following the USPTF Recommendation Statement (grade B) for screening and treatment of obesity in adults, behavioral intervention is covered when a person has a BMI of 30 kg/m2. If, after 6 months, the person has demonstrated a 3-kg weight loss, continued "face-to-face" weekly visits with a primary care provider of behavioral intervention can continue up to another 6 months.
Politics over patients
Regardless of the point at which intervention is deemed appropriate, access to all available treatments is still not equal, said Dr. John Morton of Stanford (Calif.) University and president elect of ASBMS.
"Let’s make the playing field level," said Dr. Morton. "Everybody should have the same benefits. One Constitution for all of us, one health care benefit for all of us."
Access to bariatric surgery is limited by a number of factors, including the type of health exchange available in the state where a person lives, or whether their employer-backed health plan offers bariatric surgery and if so, at what cost.
"We believe that a big part of any sort of package should definitely be bariatric surgery," said Dr. Morton, citing data on the "tertiary prevention" provided by bariatric surgery. "We hear about statins and all the good they do. If you look at how much mortality they decrease in 5 years, it’s in the single digits. We’re talking about a 40% decrease in mortality in bariatric surgery." said Dr. Morton (N. Engl. J. Med. 2007; 357:753-61).
Noting that as BMI goes up, costs go up, Dr. Morton said that with bariatric surgery, there is a return on investment in a short amount of time. But politics gets in the way of allowing the cost-saving measures of weight reduction surgery to be applied, he said.
Since the presidential election of 2012, "a lot of states have held their fire about implementing these programs," said Dr. Morton. "But at the end of the day, you need to do what’s right for the patient."
Because bariatric surgery coverage is not mandated at the federal level, millions of American do not have access to obesity care, said Dr. Morton.
Role of prejudice
"The biggest problem we have had with this for 50 years is prejudice," Dr. Henry Buchwald, professor emeritus of the Owen H. and Sarah Davidson Wangensteen Chair in Experimental Surgery at the University of Minnesota, Minneapolis. "People are prejudiced against the obese."
Dr. Morton said that even though obesity is a disease recognized by the AMA, it is often described in exclusionary terms by third-party payers.
"When you look at the ACA, there’s language that says it cannot discriminate on the basis of a health condition, but if you look at some of these [insurance] plans, there is actually language that says ‘we will not cover obesity treatment.’ That’s exclusionary language, and we need to figure out why this is occurring."
However, Dr. Finkelstein suggested that part of the problem with getting coverage for weight loss surgery might be how the field frames their argument in favor of it.
"I think the obesity community has done themselves a disservice by pushing [return on investment] for bariatric procedures," said Dr. Finkelstein. "I don’t think bariatric surgery should be talked about in terms of value for money. The [health] value is there just like any other procedure, and so it should be covered."
Little incentive
Despite the fact that the costs of obesity over a lifetime are high in the aggregate, Dr. Finkelstein said that the costs are highest later in life. That, plus the current trend of employees changing jobs an average of every 3 years, means obesity is often overlooked.
"Even though the net costs from a lifetime perspective are significant, there is not a lot of incentive for any particular payer to do any obesity prevention because the costs are eventually shifted down the line," said Dr. Finkelstein. When the federal government picks up paying for the health care costs of everyone 65 years of age or older, you are unlikely to "see significant investments in prevention," he said.
In cases in which the individual has had no insurance prior to qualifying for Medicare, the costs are even higher, and the cases more complex. "About 25% of patients who have chronic disease with multiple comorbidities make up 85% of our costs," Dr. Wild said, adding that of those 25%, 5% are "superusers" who make up 50% of all CMS costs.
The CMS spends $1.5 billion a day, or $900 billion annually, on health care, according to Dr. Wild.
"When we talk about bending the cost curve and saving money, we need to focus on those patients with multiple comorbidities and chronic diseases," said Dr. Wild.
The agreement on this point brought the panel back to the question of what is the sweet spot for prevention and intervention, and who should pay for it.
Surgery not the only answer
"We’re not going to solve the obesity problem by surgery alone," Dr. Buchwald said, adding that a combination of approaches, including prevention, medical, and other approaches. "We have to look for things that will work together."
Dr. Morton agreed that more research into complimentary medical interventions for obesity was needed. "We have been hamstrung by not having a lot of options," he said.
"Diet and exercise do work, but we don’t diet or exercise as much as we used to and that’s part of the reason we’re in this situation," Dr. Finkelstein said.
The data support bariatric surgery as a viable way to cut costs, said Dr. Morton. "The data are on our side," he said. "We have a lot of patients in need, and I would call for some rational coverage decisions when it comes to health exchanges. I think the government can be our partners in this."
Dr. Finkelstein noted several disclosures including, Jenny Craig, Johnson & Johnson, and Sanofi-Aventis, among several others. Dr. Morton has worked with Covidien. Dr. Buchwald and Dr. Wild did not have any relevant disclosures
ATLANTA – Obesity and its comorbidities loom, threatening to become an expensive national crisis, given that its treatment costs are nearly double that of other chronic diseases, and third party payers so far have failed to invest in its prevention.
"Obese individuals are about 42% more expensive than their normal weight counterparts," accounting for 9% of all medical expenditures, Eric Finkelstein, Ph.D., said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
With close to half the population expected to have a BMI greater or equal to 40 kg/m2 by 2030, even the slightest reversal of trends can save billions, according to Dr. Finkelstein, a health policy research analyst and professor at the Duke-National University of Singapore Global Health Institute (Am. J. Prev. Med. 2012;42:563-70).
‘Bend the curve’
"By 2020, if you could bend even just the cost percentage point by 1 per year, you could have 2.6 million fewer obese adults, and $3.9 billion less in medical expenditures," said Dr. Finkelstein. "By 2030, the numbers go up to 2.9 million fewer obese adults, and $9.5 billion in savings."
The way to bend the curve is through prevention, said Dr. Finkelstein. But, just what constitutes prevention and who should pay for it are not so straightforward.
Although the Affordable Care Act expanded the Centers for Medicare and Medicaid’s coverage of obesity screening and prevention, "there is some debate as to whether the ACA will help this problem," said Dr. Finkelstein.
A trifecta of politics, prejudice, and inconsistent health insurance policies may undermine the legislation’s ability to meet the challenges posed by obesity, according to the symposium presenters.
‘Ounce of prevention, pound of cure’
"Grandma was right. An ounce of prevention equals a pound of cure," noted Dr. Richard Wild, chief medical officer of the CMS in Atlanta.
To that end, he said that under the ACA, there is "more flexibility to [cover prevention] with no cost sharing to patients." CMS obesity screening, prevention, and treatment are largely tied to the U. S. Preventive Services Task Force advisory committee, said Dr. Wild.
A significant percentage of individuals in their mid-20s with class 1 obesity (BMI between 30 and 35 kg/m2) will have BMIs of 40 kg/m2 or greater before they reach their 40s, according to Dr. Finkelstein. By the time they enter their 40s, 63% of males and 78% of women will have an obesity-related comorbidity. Many of those in the 30 to 35 BMI group are likely to continue to a significant weight gain that will make them eligible for bariatric surgery fairly soon," he said.
Early intervention is key to preventing the cost of comorbidities, Dr. Finkelstein said (Surg. Obes. Relat. Dis. 2013;9:547-53).
However, CMS limits Medicare coverage of bariatric surgery to those with a BMI greater than 35 kg/m2, who have at least one related comorbidity and have proven unsuccessful at past attempts to control their weight.
Beyond that, following the USPTF Recommendation Statement (grade B) for screening and treatment of obesity in adults, behavioral intervention is covered when a person has a BMI of 30 kg/m2. If, after 6 months, the person has demonstrated a 3-kg weight loss, continued "face-to-face" weekly visits with a primary care provider of behavioral intervention can continue up to another 6 months.
Politics over patients
Regardless of the point at which intervention is deemed appropriate, access to all available treatments is still not equal, said Dr. John Morton of Stanford (Calif.) University and president elect of ASBMS.
"Let’s make the playing field level," said Dr. Morton. "Everybody should have the same benefits. One Constitution for all of us, one health care benefit for all of us."
Access to bariatric surgery is limited by a number of factors, including the type of health exchange available in the state where a person lives, or whether their employer-backed health plan offers bariatric surgery and if so, at what cost.
"We believe that a big part of any sort of package should definitely be bariatric surgery," said Dr. Morton, citing data on the "tertiary prevention" provided by bariatric surgery. "We hear about statins and all the good they do. If you look at how much mortality they decrease in 5 years, it’s in the single digits. We’re talking about a 40% decrease in mortality in bariatric surgery." said Dr. Morton (N. Engl. J. Med. 2007; 357:753-61).
Noting that as BMI goes up, costs go up, Dr. Morton said that with bariatric surgery, there is a return on investment in a short amount of time. But politics gets in the way of allowing the cost-saving measures of weight reduction surgery to be applied, he said.
Since the presidential election of 2012, "a lot of states have held their fire about implementing these programs," said Dr. Morton. "But at the end of the day, you need to do what’s right for the patient."
Because bariatric surgery coverage is not mandated at the federal level, millions of American do not have access to obesity care, said Dr. Morton.
Role of prejudice
"The biggest problem we have had with this for 50 years is prejudice," Dr. Henry Buchwald, professor emeritus of the Owen H. and Sarah Davidson Wangensteen Chair in Experimental Surgery at the University of Minnesota, Minneapolis. "People are prejudiced against the obese."
Dr. Morton said that even though obesity is a disease recognized by the AMA, it is often described in exclusionary terms by third-party payers.
"When you look at the ACA, there’s language that says it cannot discriminate on the basis of a health condition, but if you look at some of these [insurance] plans, there is actually language that says ‘we will not cover obesity treatment.’ That’s exclusionary language, and we need to figure out why this is occurring."
However, Dr. Finkelstein suggested that part of the problem with getting coverage for weight loss surgery might be how the field frames their argument in favor of it.
"I think the obesity community has done themselves a disservice by pushing [return on investment] for bariatric procedures," said Dr. Finkelstein. "I don’t think bariatric surgery should be talked about in terms of value for money. The [health] value is there just like any other procedure, and so it should be covered."
Little incentive
Despite the fact that the costs of obesity over a lifetime are high in the aggregate, Dr. Finkelstein said that the costs are highest later in life. That, plus the current trend of employees changing jobs an average of every 3 years, means obesity is often overlooked.
"Even though the net costs from a lifetime perspective are significant, there is not a lot of incentive for any particular payer to do any obesity prevention because the costs are eventually shifted down the line," said Dr. Finkelstein. When the federal government picks up paying for the health care costs of everyone 65 years of age or older, you are unlikely to "see significant investments in prevention," he said.
In cases in which the individual has had no insurance prior to qualifying for Medicare, the costs are even higher, and the cases more complex. "About 25% of patients who have chronic disease with multiple comorbidities make up 85% of our costs," Dr. Wild said, adding that of those 25%, 5% are "superusers" who make up 50% of all CMS costs.
The CMS spends $1.5 billion a day, or $900 billion annually, on health care, according to Dr. Wild.
"When we talk about bending the cost curve and saving money, we need to focus on those patients with multiple comorbidities and chronic diseases," said Dr. Wild.
The agreement on this point brought the panel back to the question of what is the sweet spot for prevention and intervention, and who should pay for it.
Surgery not the only answer
"We’re not going to solve the obesity problem by surgery alone," Dr. Buchwald said, adding that a combination of approaches, including prevention, medical, and other approaches. "We have to look for things that will work together."
Dr. Morton agreed that more research into complimentary medical interventions for obesity was needed. "We have been hamstrung by not having a lot of options," he said.
"Diet and exercise do work, but we don’t diet or exercise as much as we used to and that’s part of the reason we’re in this situation," Dr. Finkelstein said.
The data support bariatric surgery as a viable way to cut costs, said Dr. Morton. "The data are on our side," he said. "We have a lot of patients in need, and I would call for some rational coverage decisions when it comes to health exchanges. I think the government can be our partners in this."
Dr. Finkelstein noted several disclosures including, Jenny Craig, Johnson & Johnson, and Sanofi-Aventis, among several others. Dr. Morton has worked with Covidien. Dr. Buchwald and Dr. Wild did not have any relevant disclosures
ATLANTA – Obesity and its comorbidities loom, threatening to become an expensive national crisis, given that its treatment costs are nearly double that of other chronic diseases, and third party payers so far have failed to invest in its prevention.
"Obese individuals are about 42% more expensive than their normal weight counterparts," accounting for 9% of all medical expenditures, Eric Finkelstein, Ph.D., said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
With close to half the population expected to have a BMI greater or equal to 40 kg/m2 by 2030, even the slightest reversal of trends can save billions, according to Dr. Finkelstein, a health policy research analyst and professor at the Duke-National University of Singapore Global Health Institute (Am. J. Prev. Med. 2012;42:563-70).
‘Bend the curve’
"By 2020, if you could bend even just the cost percentage point by 1 per year, you could have 2.6 million fewer obese adults, and $3.9 billion less in medical expenditures," said Dr. Finkelstein. "By 2030, the numbers go up to 2.9 million fewer obese adults, and $9.5 billion in savings."
The way to bend the curve is through prevention, said Dr. Finkelstein. But, just what constitutes prevention and who should pay for it are not so straightforward.
Although the Affordable Care Act expanded the Centers for Medicare and Medicaid’s coverage of obesity screening and prevention, "there is some debate as to whether the ACA will help this problem," said Dr. Finkelstein.
A trifecta of politics, prejudice, and inconsistent health insurance policies may undermine the legislation’s ability to meet the challenges posed by obesity, according to the symposium presenters.
‘Ounce of prevention, pound of cure’
"Grandma was right. An ounce of prevention equals a pound of cure," noted Dr. Richard Wild, chief medical officer of the CMS in Atlanta.
To that end, he said that under the ACA, there is "more flexibility to [cover prevention] with no cost sharing to patients." CMS obesity screening, prevention, and treatment are largely tied to the U. S. Preventive Services Task Force advisory committee, said Dr. Wild.
A significant percentage of individuals in their mid-20s with class 1 obesity (BMI between 30 and 35 kg/m2) will have BMIs of 40 kg/m2 or greater before they reach their 40s, according to Dr. Finkelstein. By the time they enter their 40s, 63% of males and 78% of women will have an obesity-related comorbidity. Many of those in the 30 to 35 BMI group are likely to continue to a significant weight gain that will make them eligible for bariatric surgery fairly soon," he said.
Early intervention is key to preventing the cost of comorbidities, Dr. Finkelstein said (Surg. Obes. Relat. Dis. 2013;9:547-53).
However, CMS limits Medicare coverage of bariatric surgery to those with a BMI greater than 35 kg/m2, who have at least one related comorbidity and have proven unsuccessful at past attempts to control their weight.
Beyond that, following the USPTF Recommendation Statement (grade B) for screening and treatment of obesity in adults, behavioral intervention is covered when a person has a BMI of 30 kg/m2. If, after 6 months, the person has demonstrated a 3-kg weight loss, continued "face-to-face" weekly visits with a primary care provider of behavioral intervention can continue up to another 6 months.
Politics over patients
Regardless of the point at which intervention is deemed appropriate, access to all available treatments is still not equal, said Dr. John Morton of Stanford (Calif.) University and president elect of ASBMS.
"Let’s make the playing field level," said Dr. Morton. "Everybody should have the same benefits. One Constitution for all of us, one health care benefit for all of us."
Access to bariatric surgery is limited by a number of factors, including the type of health exchange available in the state where a person lives, or whether their employer-backed health plan offers bariatric surgery and if so, at what cost.
"We believe that a big part of any sort of package should definitely be bariatric surgery," said Dr. Morton, citing data on the "tertiary prevention" provided by bariatric surgery. "We hear about statins and all the good they do. If you look at how much mortality they decrease in 5 years, it’s in the single digits. We’re talking about a 40% decrease in mortality in bariatric surgery." said Dr. Morton (N. Engl. J. Med. 2007; 357:753-61).
Noting that as BMI goes up, costs go up, Dr. Morton said that with bariatric surgery, there is a return on investment in a short amount of time. But politics gets in the way of allowing the cost-saving measures of weight reduction surgery to be applied, he said.
Since the presidential election of 2012, "a lot of states have held their fire about implementing these programs," said Dr. Morton. "But at the end of the day, you need to do what’s right for the patient."
Because bariatric surgery coverage is not mandated at the federal level, millions of American do not have access to obesity care, said Dr. Morton.
Role of prejudice
"The biggest problem we have had with this for 50 years is prejudice," Dr. Henry Buchwald, professor emeritus of the Owen H. and Sarah Davidson Wangensteen Chair in Experimental Surgery at the University of Minnesota, Minneapolis. "People are prejudiced against the obese."
Dr. Morton said that even though obesity is a disease recognized by the AMA, it is often described in exclusionary terms by third-party payers.
"When you look at the ACA, there’s language that says it cannot discriminate on the basis of a health condition, but if you look at some of these [insurance] plans, there is actually language that says ‘we will not cover obesity treatment.’ That’s exclusionary language, and we need to figure out why this is occurring."
However, Dr. Finkelstein suggested that part of the problem with getting coverage for weight loss surgery might be how the field frames their argument in favor of it.
"I think the obesity community has done themselves a disservice by pushing [return on investment] for bariatric procedures," said Dr. Finkelstein. "I don’t think bariatric surgery should be talked about in terms of value for money. The [health] value is there just like any other procedure, and so it should be covered."
Little incentive
Despite the fact that the costs of obesity over a lifetime are high in the aggregate, Dr. Finkelstein said that the costs are highest later in life. That, plus the current trend of employees changing jobs an average of every 3 years, means obesity is often overlooked.
"Even though the net costs from a lifetime perspective are significant, there is not a lot of incentive for any particular payer to do any obesity prevention because the costs are eventually shifted down the line," said Dr. Finkelstein. When the federal government picks up paying for the health care costs of everyone 65 years of age or older, you are unlikely to "see significant investments in prevention," he said.
In cases in which the individual has had no insurance prior to qualifying for Medicare, the costs are even higher, and the cases more complex. "About 25% of patients who have chronic disease with multiple comorbidities make up 85% of our costs," Dr. Wild said, adding that of those 25%, 5% are "superusers" who make up 50% of all CMS costs.
The CMS spends $1.5 billion a day, or $900 billion annually, on health care, according to Dr. Wild.
"When we talk about bending the cost curve and saving money, we need to focus on those patients with multiple comorbidities and chronic diseases," said Dr. Wild.
The agreement on this point brought the panel back to the question of what is the sweet spot for prevention and intervention, and who should pay for it.
Surgery not the only answer
"We’re not going to solve the obesity problem by surgery alone," Dr. Buchwald said, adding that a combination of approaches, including prevention, medical, and other approaches. "We have to look for things that will work together."
Dr. Morton agreed that more research into complimentary medical interventions for obesity was needed. "We have been hamstrung by not having a lot of options," he said.
"Diet and exercise do work, but we don’t diet or exercise as much as we used to and that’s part of the reason we’re in this situation," Dr. Finkelstein said.
The data support bariatric surgery as a viable way to cut costs, said Dr. Morton. "The data are on our side," he said. "We have a lot of patients in need, and I would call for some rational coverage decisions when it comes to health exchanges. I think the government can be our partners in this."
Dr. Finkelstein noted several disclosures including, Jenny Craig, Johnson & Johnson, and Sanofi-Aventis, among several others. Dr. Morton has worked with Covidien. Dr. Buchwald and Dr. Wild did not have any relevant disclosures
EXPERT ANALYSIS FROM OBESITY WEEK
Politics, prejudice, inconsistent policies wreak havoc with obesity treatment costs
ATLANTA – Obesity and its comorbidities loom, threatening to become an expensive national crisis, given that its treatment costs are nearly double that of other chronic diseases, and third party payers so far have failed to invest in its prevention.
"Obese individuals are about 42% more expensive than their normal weight counterparts," accounting for 9% of all medical expenditures, Eric Finkelstein, Ph.D., said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
With close to half the population expected to have a BMI greater or equal to 40 kg/m2 by 2030, even the slightest reversal of trends can save billions, according to Dr. Finkelstein, a health policy research analyst and professor at the Duke-National University of Singapore Global Health Institute (Am. J. Prev. Med. 2012;42:563-70).
‘Bend the curve’
"By 2020, if you could bend even just the cost percentage point by 1 per year, you could have 2.6 million fewer obese adults, and $3.9 billion less in medical expenditures," said Dr. Finkelstein. "By 2030, the numbers go up to 2.9 million fewer obese adults, and $9.5 billion in savings."
The way to bend the curve is through prevention, said Dr. Finkelstein. But, just what constitutes prevention and who should pay for it are not so straightforward.
Although the Affordable Care Act expanded the Centers for Medicare and Medicaid’s coverage of obesity screening and prevention, "there is some debate as to whether the ACA will help this problem," said Dr. Finkelstein.
A trifecta of politics, prejudice, and inconsistent health insurance policies may undermine the legislation’s ability to meet the challenges posed by obesity, according to the symposium presenters.
‘Ounce of prevention, pound of cure’
"Grandma was right. An ounce of prevention equals a pound of cure," noted Dr. Richard Wild, chief medical officer of the CMS in Atlanta.
To that end, he said that under the ACA, there is "more flexibility to [cover prevention] with no cost sharing to patients." CMS obesity screening, prevention, and treatment are largely tied to the U. S. Preventive Services Task Force advisory committee, said Dr. Wild.
A significant percentage of individuals in their mid-20s with class 1 obesity (BMI between 30 and 35 kg/m2) will have BMIs of 40 kg/m2 or greater before they reach their 40s, according to Dr. Finkelstein. By the time they enter their 40s, 63% of males and 78% of women will have an obesity-related comorbidity. Many of those in the 30 to 35 BMI group are likely to continue to a significant weight gain that will make them eligible for bariatric surgery fairly soon," he said.
Early intervention is key to preventing the cost of comorbidities, Dr. Finkelstein said (Surg. Obes. Relat. Dis. 2013;9:547-53).
However, CMS limits Medicare coverage of bariatric surgery to those with a BMI greater than 35 kg/m2, who have at least one related comorbidity and have proven unsuccessful at past attempts to control their weight.
Beyond that, following the USPTF Recommendation Statement (grade B) for screening and treatment of obesity in adults, behavioral intervention is covered when a person has a BMI of 30 kg/m2. If, after 6 months, the person has demonstrated a 3-kg weight loss, continued "face-to-face" weekly visits with a primary care provider of behavioral intervention can continue up to another 6 months.
Politics over patients
Regardless of the point at which intervention is deemed appropriate, access to all available treatments is still not equal, said Dr. John Morton of Stanford (Calif.) University and president elect of ASBMS.
"Let’s make the playing field level," said Dr. Morton. "Everybody should have the same benefits. One Constitution for all of us, one health care benefit for all of us."
Access to bariatric surgery is limited by a number of factors, including the type of health exchange available in the state where a person lives, or whether their employer-backed health plan offers bariatric surgery and if so, at what cost.
"We believe that a big part of any sort of package should definitely be bariatric surgery," said Dr. Morton, citing data on the "tertiary prevention" provided by bariatric surgery. "We hear about statins and all the good they do. If you look at how much mortality they decrease in 5 years, it’s in the single digits. We’re talking about a 40% decrease in mortality in bariatric surgery." said Dr. Morton (N. Engl. J. Med. 2007; 357:753-61).
Noting that as BMI goes up, costs go up, Dr. Morton said that with bariatric surgery, there is a return on investment in a short amount of time. But politics gets in the way of allowing the cost-saving measures of weight reduction surgery to be applied, he said.
Since the presidential election of 2012, "a lot of states have held their fire about implementing these programs," said Dr. Morton. "But at the end of the day, you need to do what’s right for the patient."
Because bariatric surgery coverage is not mandated at the federal level, millions of American do not have access to obesity care, said Dr. Morton.
Role of prejudice
"The biggest problem we have had with this for 50 years is prejudice," Dr. Henry Buchwald, professor emeritus of the Owen H. and Sarah Davidson Wangensteen Chair in Experimental Surgery at the University of Minnesota, Minneapolis. "People are prejudiced against the obese."
Dr. Morton said that even though obesity is a disease recognized by the AMA, it is often described in exclusionary terms by third-party payers.
"When you look at the ACA, there’s language that says it cannot discriminate on the basis of a health condition, but if you look at some of these [insurance] plans, there is actually language that says ‘we will not cover obesity treatment.’ That’s exclusionary language, and we need to figure out why this is occurring."
However, Dr. Finkelstein suggested that part of the problem with getting coverage for weight loss surgery might be how the field frames their argument in favor of it.
"I think the obesity community has done themselves a disservice by pushing [return on investment] for bariatric procedures," said Dr. Finkelstein. "I don’t think bariatric surgery should be talked about in terms of value for money. The [health] value is there just like any other procedure, and so it should be covered."
Little incentive
Despite the fact that the costs of obesity over a lifetime are high in the aggregate, Dr. Finkelstein said that the costs are highest later in life. That, plus the current trend of employees changing jobs an average of every 3 years, means obesity is often overlooked.
"Even though the net costs from a lifetime perspective are significant, there is not a lot of incentive for any particular payer to do any obesity prevention because the costs are eventually shifted down the line," said Dr. Finkelstein. When the federal government picks up paying for the health care costs of everyone 65 years of age or older, you are unlikely to "see significant investments in prevention," he said.
In cases in which the individual has had no insurance prior to qualifying for Medicare, the costs are even higher, and the cases more complex. "About 25% of patients who have chronic disease with multiple comorbidities make up 85% of our costs," Dr. Wild said, adding that of those 25%, 5% are "superusers" who make up 50% of all CMS costs.
The CMS spends $1.5 billion a day, or $900 billion annually, on health care, according to Dr. Wild.
"When we talk about bending the cost curve and saving money, we need to focus on those patients with multiple comorbidities and chronic diseases," said Dr. Wild.
The agreement on this point brought the panel back to the question of what is the sweet spot for prevention and intervention, and who should pay for it.
Surgery not the only answer
"We’re not going to solve the obesity problem by surgery alone," Dr. Buchwald said, adding that a combination of approaches, including prevention, medical, and other approaches. "We have to look for things that will work together."
Dr. Morton agreed that more research into complimentary medical interventions for obesity was needed. "We have been hamstrung by not having a lot of options," he said.
"Diet and exercise do work, but we don’t diet or exercise as much as we used to and that’s part of the reason we’re in this situation," Dr. Finkelstein said.
The data support bariatric surgery as a viable way to cut costs, said Dr. Morton. "The data are on our side," he said. "We have a lot of patients in need, and I would call for some rational coverage decisions when it comes to health exchanges. I think the government can be our partners in this."
Dr. Finkelstein noted several disclosures including, Jenny Craig, Johnson & Johnson, and Sanofi-Aventis, among several others. Dr. Morton has worked with Covidien. Dr. Buchwald and Dr. Wild did not have any relevant disclosures
ATLANTA – Obesity and its comorbidities loom, threatening to become an expensive national crisis, given that its treatment costs are nearly double that of other chronic diseases, and third party payers so far have failed to invest in its prevention.
"Obese individuals are about 42% more expensive than their normal weight counterparts," accounting for 9% of all medical expenditures, Eric Finkelstein, Ph.D., said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
With close to half the population expected to have a BMI greater or equal to 40 kg/m2 by 2030, even the slightest reversal of trends can save billions, according to Dr. Finkelstein, a health policy research analyst and professor at the Duke-National University of Singapore Global Health Institute (Am. J. Prev. Med. 2012;42:563-70).
‘Bend the curve’
"By 2020, if you could bend even just the cost percentage point by 1 per year, you could have 2.6 million fewer obese adults, and $3.9 billion less in medical expenditures," said Dr. Finkelstein. "By 2030, the numbers go up to 2.9 million fewer obese adults, and $9.5 billion in savings."
The way to bend the curve is through prevention, said Dr. Finkelstein. But, just what constitutes prevention and who should pay for it are not so straightforward.
Although the Affordable Care Act expanded the Centers for Medicare and Medicaid’s coverage of obesity screening and prevention, "there is some debate as to whether the ACA will help this problem," said Dr. Finkelstein.
A trifecta of politics, prejudice, and inconsistent health insurance policies may undermine the legislation’s ability to meet the challenges posed by obesity, according to the symposium presenters.
‘Ounce of prevention, pound of cure’
"Grandma was right. An ounce of prevention equals a pound of cure," noted Dr. Richard Wild, chief medical officer of the CMS in Atlanta.
To that end, he said that under the ACA, there is "more flexibility to [cover prevention] with no cost sharing to patients." CMS obesity screening, prevention, and treatment are largely tied to the U. S. Preventive Services Task Force advisory committee, said Dr. Wild.
A significant percentage of individuals in their mid-20s with class 1 obesity (BMI between 30 and 35 kg/m2) will have BMIs of 40 kg/m2 or greater before they reach their 40s, according to Dr. Finkelstein. By the time they enter their 40s, 63% of males and 78% of women will have an obesity-related comorbidity. Many of those in the 30 to 35 BMI group are likely to continue to a significant weight gain that will make them eligible for bariatric surgery fairly soon," he said.
Early intervention is key to preventing the cost of comorbidities, Dr. Finkelstein said (Surg. Obes. Relat. Dis. 2013;9:547-53).
However, CMS limits Medicare coverage of bariatric surgery to those with a BMI greater than 35 kg/m2, who have at least one related comorbidity and have proven unsuccessful at past attempts to control their weight.
Beyond that, following the USPTF Recommendation Statement (grade B) for screening and treatment of obesity in adults, behavioral intervention is covered when a person has a BMI of 30 kg/m2. If, after 6 months, the person has demonstrated a 3-kg weight loss, continued "face-to-face" weekly visits with a primary care provider of behavioral intervention can continue up to another 6 months.
Politics over patients
Regardless of the point at which intervention is deemed appropriate, access to all available treatments is still not equal, said Dr. John Morton of Stanford (Calif.) University and president elect of ASBMS.
"Let’s make the playing field level," said Dr. Morton. "Everybody should have the same benefits. One Constitution for all of us, one health care benefit for all of us."
Access to bariatric surgery is limited by a number of factors, including the type of health exchange available in the state where a person lives, or whether their employer-backed health plan offers bariatric surgery and if so, at what cost.
"We believe that a big part of any sort of package should definitely be bariatric surgery," said Dr. Morton, citing data on the "tertiary prevention" provided by bariatric surgery. "We hear about statins and all the good they do. If you look at how much mortality they decrease in 5 years, it’s in the single digits. We’re talking about a 40% decrease in mortality in bariatric surgery." said Dr. Morton (N. Engl. J. Med. 2007; 357:753-61).
Noting that as BMI goes up, costs go up, Dr. Morton said that with bariatric surgery, there is a return on investment in a short amount of time. But politics gets in the way of allowing the cost-saving measures of weight reduction surgery to be applied, he said.
Since the presidential election of 2012, "a lot of states have held their fire about implementing these programs," said Dr. Morton. "But at the end of the day, you need to do what’s right for the patient."
Because bariatric surgery coverage is not mandated at the federal level, millions of American do not have access to obesity care, said Dr. Morton.
Role of prejudice
"The biggest problem we have had with this for 50 years is prejudice," Dr. Henry Buchwald, professor emeritus of the Owen H. and Sarah Davidson Wangensteen Chair in Experimental Surgery at the University of Minnesota, Minneapolis. "People are prejudiced against the obese."
Dr. Morton said that even though obesity is a disease recognized by the AMA, it is often described in exclusionary terms by third-party payers.
"When you look at the ACA, there’s language that says it cannot discriminate on the basis of a health condition, but if you look at some of these [insurance] plans, there is actually language that says ‘we will not cover obesity treatment.’ That’s exclusionary language, and we need to figure out why this is occurring."
However, Dr. Finkelstein suggested that part of the problem with getting coverage for weight loss surgery might be how the field frames their argument in favor of it.
"I think the obesity community has done themselves a disservice by pushing [return on investment] for bariatric procedures," said Dr. Finkelstein. "I don’t think bariatric surgery should be talked about in terms of value for money. The [health] value is there just like any other procedure, and so it should be covered."
Little incentive
Despite the fact that the costs of obesity over a lifetime are high in the aggregate, Dr. Finkelstein said that the costs are highest later in life. That, plus the current trend of employees changing jobs an average of every 3 years, means obesity is often overlooked.
"Even though the net costs from a lifetime perspective are significant, there is not a lot of incentive for any particular payer to do any obesity prevention because the costs are eventually shifted down the line," said Dr. Finkelstein. When the federal government picks up paying for the health care costs of everyone 65 years of age or older, you are unlikely to "see significant investments in prevention," he said.
In cases in which the individual has had no insurance prior to qualifying for Medicare, the costs are even higher, and the cases more complex. "About 25% of patients who have chronic disease with multiple comorbidities make up 85% of our costs," Dr. Wild said, adding that of those 25%, 5% are "superusers" who make up 50% of all CMS costs.
The CMS spends $1.5 billion a day, or $900 billion annually, on health care, according to Dr. Wild.
"When we talk about bending the cost curve and saving money, we need to focus on those patients with multiple comorbidities and chronic diseases," said Dr. Wild.
The agreement on this point brought the panel back to the question of what is the sweet spot for prevention and intervention, and who should pay for it.
Surgery not the only answer
"We’re not going to solve the obesity problem by surgery alone," Dr. Buchwald said, adding that a combination of approaches, including prevention, medical, and other approaches. "We have to look for things that will work together."
Dr. Morton agreed that more research into complimentary medical interventions for obesity was needed. "We have been hamstrung by not having a lot of options," he said.
"Diet and exercise do work, but we don’t diet or exercise as much as we used to and that’s part of the reason we’re in this situation," Dr. Finkelstein said.
The data support bariatric surgery as a viable way to cut costs, said Dr. Morton. "The data are on our side," he said. "We have a lot of patients in need, and I would call for some rational coverage decisions when it comes to health exchanges. I think the government can be our partners in this."
Dr. Finkelstein noted several disclosures including, Jenny Craig, Johnson & Johnson, and Sanofi-Aventis, among several others. Dr. Morton has worked with Covidien. Dr. Buchwald and Dr. Wild did not have any relevant disclosures
ATLANTA – Obesity and its comorbidities loom, threatening to become an expensive national crisis, given that its treatment costs are nearly double that of other chronic diseases, and third party payers so far have failed to invest in its prevention.
"Obese individuals are about 42% more expensive than their normal weight counterparts," accounting for 9% of all medical expenditures, Eric Finkelstein, Ph.D., said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
With close to half the population expected to have a BMI greater or equal to 40 kg/m2 by 2030, even the slightest reversal of trends can save billions, according to Dr. Finkelstein, a health policy research analyst and professor at the Duke-National University of Singapore Global Health Institute (Am. J. Prev. Med. 2012;42:563-70).
‘Bend the curve’
"By 2020, if you could bend even just the cost percentage point by 1 per year, you could have 2.6 million fewer obese adults, and $3.9 billion less in medical expenditures," said Dr. Finkelstein. "By 2030, the numbers go up to 2.9 million fewer obese adults, and $9.5 billion in savings."
The way to bend the curve is through prevention, said Dr. Finkelstein. But, just what constitutes prevention and who should pay for it are not so straightforward.
Although the Affordable Care Act expanded the Centers for Medicare and Medicaid’s coverage of obesity screening and prevention, "there is some debate as to whether the ACA will help this problem," said Dr. Finkelstein.
A trifecta of politics, prejudice, and inconsistent health insurance policies may undermine the legislation’s ability to meet the challenges posed by obesity, according to the symposium presenters.
‘Ounce of prevention, pound of cure’
"Grandma was right. An ounce of prevention equals a pound of cure," noted Dr. Richard Wild, chief medical officer of the CMS in Atlanta.
To that end, he said that under the ACA, there is "more flexibility to [cover prevention] with no cost sharing to patients." CMS obesity screening, prevention, and treatment are largely tied to the U. S. Preventive Services Task Force advisory committee, said Dr. Wild.
A significant percentage of individuals in their mid-20s with class 1 obesity (BMI between 30 and 35 kg/m2) will have BMIs of 40 kg/m2 or greater before they reach their 40s, according to Dr. Finkelstein. By the time they enter their 40s, 63% of males and 78% of women will have an obesity-related comorbidity. Many of those in the 30 to 35 BMI group are likely to continue to a significant weight gain that will make them eligible for bariatric surgery fairly soon," he said.
Early intervention is key to preventing the cost of comorbidities, Dr. Finkelstein said (Surg. Obes. Relat. Dis. 2013;9:547-53).
However, CMS limits Medicare coverage of bariatric surgery to those with a BMI greater than 35 kg/m2, who have at least one related comorbidity and have proven unsuccessful at past attempts to control their weight.
Beyond that, following the USPTF Recommendation Statement (grade B) for screening and treatment of obesity in adults, behavioral intervention is covered when a person has a BMI of 30 kg/m2. If, after 6 months, the person has demonstrated a 3-kg weight loss, continued "face-to-face" weekly visits with a primary care provider of behavioral intervention can continue up to another 6 months.
Politics over patients
Regardless of the point at which intervention is deemed appropriate, access to all available treatments is still not equal, said Dr. John Morton of Stanford (Calif.) University and president elect of ASBMS.
"Let’s make the playing field level," said Dr. Morton. "Everybody should have the same benefits. One Constitution for all of us, one health care benefit for all of us."
Access to bariatric surgery is limited by a number of factors, including the type of health exchange available in the state where a person lives, or whether their employer-backed health plan offers bariatric surgery and if so, at what cost.
"We believe that a big part of any sort of package should definitely be bariatric surgery," said Dr. Morton, citing data on the "tertiary prevention" provided by bariatric surgery. "We hear about statins and all the good they do. If you look at how much mortality they decrease in 5 years, it’s in the single digits. We’re talking about a 40% decrease in mortality in bariatric surgery." said Dr. Morton (N. Engl. J. Med. 2007; 357:753-61).
Noting that as BMI goes up, costs go up, Dr. Morton said that with bariatric surgery, there is a return on investment in a short amount of time. But politics gets in the way of allowing the cost-saving measures of weight reduction surgery to be applied, he said.
Since the presidential election of 2012, "a lot of states have held their fire about implementing these programs," said Dr. Morton. "But at the end of the day, you need to do what’s right for the patient."
Because bariatric surgery coverage is not mandated at the federal level, millions of American do not have access to obesity care, said Dr. Morton.
Role of prejudice
"The biggest problem we have had with this for 50 years is prejudice," Dr. Henry Buchwald, professor emeritus of the Owen H. and Sarah Davidson Wangensteen Chair in Experimental Surgery at the University of Minnesota, Minneapolis. "People are prejudiced against the obese."
Dr. Morton said that even though obesity is a disease recognized by the AMA, it is often described in exclusionary terms by third-party payers.
"When you look at the ACA, there’s language that says it cannot discriminate on the basis of a health condition, but if you look at some of these [insurance] plans, there is actually language that says ‘we will not cover obesity treatment.’ That’s exclusionary language, and we need to figure out why this is occurring."
However, Dr. Finkelstein suggested that part of the problem with getting coverage for weight loss surgery might be how the field frames their argument in favor of it.
"I think the obesity community has done themselves a disservice by pushing [return on investment] for bariatric procedures," said Dr. Finkelstein. "I don’t think bariatric surgery should be talked about in terms of value for money. The [health] value is there just like any other procedure, and so it should be covered."
Little incentive
Despite the fact that the costs of obesity over a lifetime are high in the aggregate, Dr. Finkelstein said that the costs are highest later in life. That, plus the current trend of employees changing jobs an average of every 3 years, means obesity is often overlooked.
"Even though the net costs from a lifetime perspective are significant, there is not a lot of incentive for any particular payer to do any obesity prevention because the costs are eventually shifted down the line," said Dr. Finkelstein. When the federal government picks up paying for the health care costs of everyone 65 years of age or older, you are unlikely to "see significant investments in prevention," he said.
In cases in which the individual has had no insurance prior to qualifying for Medicare, the costs are even higher, and the cases more complex. "About 25% of patients who have chronic disease with multiple comorbidities make up 85% of our costs," Dr. Wild said, adding that of those 25%, 5% are "superusers" who make up 50% of all CMS costs.
The CMS spends $1.5 billion a day, or $900 billion annually, on health care, according to Dr. Wild.
"When we talk about bending the cost curve and saving money, we need to focus on those patients with multiple comorbidities and chronic diseases," said Dr. Wild.
The agreement on this point brought the panel back to the question of what is the sweet spot for prevention and intervention, and who should pay for it.
Surgery not the only answer
"We’re not going to solve the obesity problem by surgery alone," Dr. Buchwald said, adding that a combination of approaches, including prevention, medical, and other approaches. "We have to look for things that will work together."
Dr. Morton agreed that more research into complimentary medical interventions for obesity was needed. "We have been hamstrung by not having a lot of options," he said.
"Diet and exercise do work, but we don’t diet or exercise as much as we used to and that’s part of the reason we’re in this situation," Dr. Finkelstein said.
The data support bariatric surgery as a viable way to cut costs, said Dr. Morton. "The data are on our side," he said. "We have a lot of patients in need, and I would call for some rational coverage decisions when it comes to health exchanges. I think the government can be our partners in this."
Dr. Finkelstein noted several disclosures including, Jenny Craig, Johnson & Johnson, and Sanofi-Aventis, among several others. Dr. Morton has worked with Covidien. Dr. Buchwald and Dr. Wild did not have any relevant disclosures
EXPERT ANALYSIS FROM OBESITY WEEK