User login
Psoriasiform Dermatitis Associated With the Moderna COVID-19 Messenger RNA Vaccine
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

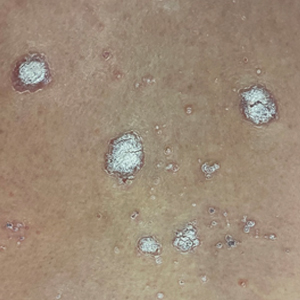
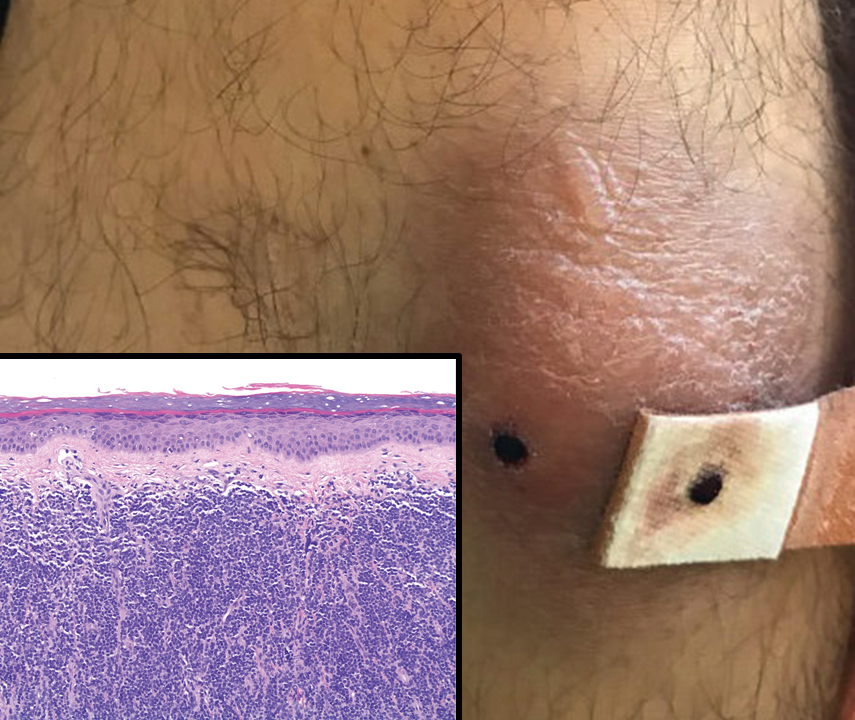
A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
To the Editor:
The Moderna COVID-19 messenger RNA (mRNA) vaccine was authorized for use on December 18, 2020, with the second dose beginning on January 15, 2021.1-3 Some individuals who received the Moderna vaccine experienced an intense rash known as “COVID arm,” a harmless but bothersome adverse effect that typically appears within a week and is a localized and transient immunogenic response.4 COVID arm differs from most vaccine adverse effects. The rash emerges not immediately but 5 to 9 days after the initial dose—on average, 1 week later. Apart from being itchy, the rash does not appear to be harmful and is not a reason to hesitate getting vaccinated.
Dermatologists and allergists have been studying this adverse effect, which has been formally termed delayed cutaneous hypersensitivity. Of potential clinical consequence is that the efficacy of the mRNA COVID-19 vaccine may be harmed if postvaccination dermal reactions necessitate systemic corticosteroid therapy. Because this vaccine stimulates an immune response as viral RNA integrates in cells secondary to production of the spike protein of the virus, the skin may be affected secondarily and manifestations of any underlying disease may be aggravated.5 We report a patient who developed a psoriasiform dermatitis after the first dose of the Moderna vaccine.

A 65-year-old woman presented to her primary care physician because of the severity of psoriasiform dermatitis that developed 5 days after she received the first dose of the Moderna COVID-19 mRNA vaccine. The patient had a medical history of Sjögren syndrome. Her medication history was negative, and her family history was negative for autoimmune disease. Physical examination by primary care revealed an erythematous scaly rash with plaques and papules on the neck and back (Figure 1). The patient presented again to primary care 2 days later with swollen, painful, discolored digits (Figure 2) and a stiff, sore neck.

Laboratory results were positive for anti–Sjögren syndrome–related antigens A and B. A complete blood cell count; comprehensive metabolic panel; erythrocyte sedimentation rate; and assays of rheumatoid factor, C-reactive protein, and anti–cyclic citrullinated peptide were within reference range. A biopsy of a lesion on the back showed psoriasiform dermatitis with confluent parakeratosis and scattered necrotic keratinocytes. There was superficial perivascular inflammation with rare eosinophils (Figure 3).

The patient was treated with a course of systemic corticosteroids. The rash resolved in 1 week. She did not receive the second dose due to the rash.
Two mRNA COVID-19 vaccines—Pfizer BioNTech and Moderna—have been granted emergency use authorization by the US Food and Drug Administration.6 The safety profile of the mRNA-1273 vaccine for the median 2-month follow-up showed no safety concerns.3 Minor localized adverse effects (eg, pain, redness, swelling) have been observed more frequently with the vaccines than with placebo. Systemic symptoms, such as fever, fatigue, headache, and muscle and joint pain, also were seen somewhat more often with the vaccines than with placebo; most such effects occurred 24 to 48 hours after vaccination.3,6,7 The frequency of unsolicited adverse events and serious adverse events reported during the 28-day period after vaccination generally was similar among participants in the vaccine and placebo groups.3
There are 2 types of reactions to COVID-19 vaccination: immediate and delayed. Immediate reactions usually are due to anaphylaxis, requiring prompt recognition and treatment with epinephrine to stop rapid progression of life-threatening symptoms. Delayed reactions include localized reactions, such as urticaria and benign exanthema; serum sickness and serum sickness–like reactions; fever; and rare skin, organ, and neurologic sequelae.1,6-8
Cutaneous manifestations, present in 16% to 50% of patients with Sjögren syndrome, are considered one of the most common extraglandular presentations of the syndrome. They are classified as nonvascular (eg, xerosis, angular cheilitis, eyelid dermatitis, annular erythema) and vascular (eg, Raynaud phenomenon, vasculitis).9-11 Our patient did not have any of those findings. She had not taken any medications before the rash appeared, thereby ruling out a drug reaction.
The differential for our patient included post–urinary tract infection immune-reactive arthritis and rash, which is not typical with Escherichia coli infection but is described with infection with Chlamydia species and Salmonella species. Moreover, post–urinary tract infection immune-reactive arthritis and rash appear mostly on the palms and soles. Systemic lupus erythematosus–like rashes have a different histology and appear on sun-exposed areas; our patient’s rash was found mainly on unexposed areas.12
Because our patient received the Moderna vaccine 5 days before the rash appeared and later developed swelling of the digits with morning stiffness, a delayed serum sickness–like reaction secondary to COVID-19 vaccination was possible.3,6
COVID-19 mRNA vaccines developed by Pfizer-BioNTech and Moderna incorporate a lipid-based nanoparticle carrier system that prevents rapid enzymatic degradation of mRNA and facilitates in vivo delivery of mRNA. This lipid-based nanoparticle carrier system is further stabilized by a polyethylene glycol 2000 lipid conjugate that provides a hydrophilic layer, thus prolonging half-life. The presence of lipid polyethylene glycol 2000 in mRNA vaccines has led to concern that this component could be implicated in anaphylaxis.6
COVID-19 antigens can give rise to varying clinical manifestations that are directly related to viral tissue damage or are indirectly induced by the antiviral immune response.13,14 Hyperactivation of the immune system to eradicate COVID-19 may trigger autoimmunity; several immune-mediated disorders have been described in individuals infected with SARS-CoV-2. Dermal manifestations include cutaneous rash and vasculitis.13-16 Crucial immunologic steps occur during SARS-CoV-2 infection that may link autoimmunity to COVID-19.13,14 In preliminary published data on the efficacy of the Moderna vaccine on 45 trial enrollees, 3 did not receive the second dose of vaccination, including 1 who developed urticaria on both legs 5 days after the first dose.1
Introduction of viral RNA can induce autoimmunity that can be explained by various phenomena, including epitope spreading, molecular mimicry, cryptic antigen, and bystander activation. Remarkably, more than one-third of immunogenic proteins in SARS-CoV-2 have potentially problematic homology to proteins that are key to the human adaptive immune system.5
Moreover, SARS-CoV-2 seems to induce organ injury through alternative mechanisms beyond direct viral infection, including immunologic injury. In some situations, hyperactivation of the immune response to SARS-CoV-2 RNA can result in autoimmune disease. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. It is unclear whether those medical disorders are the result of transitory postinfectious epiphenomena.5
A few studies have shown that patients with rheumatic disease have an incidence and prevalence of COVID-19 that is similar to the general population. A similar pattern has been detected in COVID-19 morbidity and mortality rates, even among patients with an autoimmune disease, such as rheumatoid arthritis and Sjögren syndrome.5,17 Furthermore, exacerbation of preexisting rheumatic symptoms may be due to hyperactivation of antiviral pathways in a person with an autoimmune disease.17-19 The findings in our patient suggested a direct role for the vaccine in skin manifestations, rather than for reactivation or development of new systemic autoimmune processes, such as systemic lupus erythematosus.
Exacerbation of psoriasis following COVID-19 vaccination has been described20; however, the case patient did not have a history of psoriasis. The mechanism(s) of such exacerbation remain unclear; COVID-19 vaccine–induced helper T cells (TH17) may play a role.21 Other skin manifestations encountered following COVID-19 vaccination include lichen planus, leukocytoclastic vasculitic rash, erythema multiforme–like rash, and pityriasis rosea–like rash.22-25 The immune mechanisms of these manifestations remain unclear.
The clinical presentation of delayed vaccination reactions can be attributed to the timing of symptoms and, in this case, the immune-mediated background of a psoriasiform reaction. Although adverse reactions to the SARS-CoV-2 mRNA vaccine are rare, more individuals should be studied after vaccination to confirm and better understand this phenomenon.
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
- Jackson LA, Anderson EJ, Rouphael NG, et al; . An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920-1931. doi:10.1056/NEJMoa2022483
- Anderson EJ, Rouphael NG, Widge AT, et al; . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427-2438. doi:10.1056/NEJMoa2028436
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Weise E. ‘COVID arm’ rash seen after Moderna vaccine annoying but harmless, doctors say. USA Today. January 27, 2021. Accessed September 4, 2022. https://www.usatoday.com/story/news/health/2021/01/27/covid-arm-moderna-vaccine-rash-harmless-side-effect-doctors-say/4277725001/
- Talotta R, Robertson E. Autoimmunity as the comet tail of COVID-19 pandemic. World J Clin Cases. 2020;8:3621-3644. doi:10.12998/wjcc.v8.i17.3621
- Castells MC, Phillips EJ. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384:643-649. doi:10.1056/NEJMra2035343
- Polack FP, Thomas SJ, Kitchin N, et al; . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1857-1859. doi:10.15585/mmwr.mm6949e1
- Roguedas AM, Misery L, Sassolas B, et al. Cutaneous manifestations of primary Sjögren’s syndrome are underestimated. Clin Exp Rheumatol. 2004;22:632-636.
- Katayama I. Dry skin manifestations in Sjögren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67:448-454. doi:10.1016/j.alit.2018.07.001
- Generali E, Costanzo A, Mainetti C, et al. Cutaneous and mucosal manifestations of Sjögren’s syndrome. Clin Rev Allergy Immunol. 2017;53:357-370. doi:10.1007/s12016-017-8639-y
- Chanprapaph K, Tankunakorn J, Suchonwanit P, et al. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther (Heidelb). 2021;11:131-147. doi:10.1007/s13555-020-00471-y
- Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, et al. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. 2020;83:e103-e104. doi:10.1016/j.jaad.2020.05.034
- Rahimi H, Tehranchinia Z. A comprehensive review of cutaneous manifestations associated with COVID-19. Biomed Res Int. 2020;2020:1236520. doi:10.1155/2020/1236520
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81. doi:10.1016/j.jdermsci.2020.04.011
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743. doi:10.1111/ijd.14937
- Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305-1308. doi:10.1177/0961203314531346
- Parodi A, Gasparini G, Cozzani E. Could antiphospholipid antibodies contribute to coagulopathy in COVID-19? J Am Acad Dermatol. 2020;83:e249. doi:10.1016/j.jaad.2020.06.003
- Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. doi:10.1111/cts.12805
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010. doi:10.3389/fmed.2021.812010
- Rouai M, Slimane MB, Sassi W, et al. Pustular rash triggered by Pfizer-BioNTech COVID-19 vaccination: a case report. Dermatol Ther. 2022:e15465. doi:10.1111/dth.15465
- Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. 2022;35:e15279. doi:10.1111/dth.15279
- Buckley JE, Landis LN, Rapini RP. Pityriasis rosea-like rash after mRNA COVID-19 vaccination: a case report and review of the literature. JAAD Int. 2022;7:164-168. doi:10.1016/j.jdin.2022.01.009
- Gökçek GE, Öksüm Solak E, Çölgeçen E. Pityriasis rosea like eruption: a dermatological manifestation of Coronavac-COVID-19 vaccine. Dermatol Ther. 2022;35:e15256. doi:10.1111/dth.15256
- Kim MJ, Kim JW, Kim MS, et al. Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech). J Eur Acad Dermatol Venereol. 2022;36:e98-e100. doi:10.1111/jdv.17757
PRACTICE POINTS
- The differential diagnosis for a new-onset psoriasiform rash in an elderly patient should include a vaccine-related rash.
- A rash following vaccination that necessitates systemic corticosteroid therapy can decrease vaccine efficacy.
Isolated Nodule and Generalized Lymphadenopathy
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
A diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN) was rendered. Subsequent needle core biopsy of a left axillary lymph node as well as bone marrow aspiration and biopsy revealed a similar diffuse blastoid infiltrate with an identical immunophenotype to that in the skin biopsy from the pretibial mass and peripheral blood.
Previously known as blastic natural killer cell leukemia/lymphoma or agranular CD4+/CD56+ hematodermic neoplasm/tumor, BPDCN is a rare, clinically aggressive hematologic malignancy derived from the precursors of plasmacytoid dendritic cells. It often is diagnostically challenging, particularly when presenting at noncutaneous sites and in unusual (young) patient populations.1 It was included with other myeloid neoplasms in the 2008 World Health Organization classification; however, in the 2017 classification it was categorized as a separate entity. Blastic plasmacytoid dendritic cell neoplasm typically presents in the skin of elderly patients (age range at diagnosis, 61–67 years) with or without bone marrow involvement and systemic dissemination.1,2 The skin is the most common clinical site of disease in typical cases of BPDCN and often precedes bone marrow involvement. Thus, skin biopsy often is the key to making the diagnosis. Diagnosis of BPDCN may be delayed because of diagnostic pitfalls. Patients usually present with asymptomatic solitary or multiple lesions.3-5 Blastic plasmacytoid dendritic cell neoplasm can present as an isolated purplish nodule or bruiselike papule or more commonly as disseminated purplish nodules, papules, and macules. Isolated nodules are found on the head and lower limbs and can be more than 10 cm in diameter. Peripheral blood and bone marrow may be minimally involved at presentation but invariably become involved with the progression of disease. Cytopenia can occur at diagnosis and in a minority of severe cases indicates bone marrow failure.2-6
Skin involvement of BPDCN is thought to be secondary to the expression of skin migration molecules, such as cutaneous lymphocyte-associated antigen, one of the E-selectin ligands, which binds to E-selectin on high endothelial venules. In addition, the local dermal microenvironment of chemokines binding CXCR3, CXCR4, CCR6, or CCR7 present on neoplastic cells possibly leads to skin involvement. The full mechanism underlying the cutaneous tropism is still to be elucidated.4-7 Infiltration of the oral mucosa is seen in some patients, but it may be underreported. Mucosal disease typically appears similarly to cutaneous disease.
The cutaneous differential diagnosis for BPDCN depends on the clinical presentation, extent of disease spread, and thickness of infiltration. It includes common nonneoplastic diseases such as traumatic ecchymoses; purpuric disorders; extramedullary hematopoiesis; and soft-tissue neoplasms such as angiosarcoma, Kaposi sarcoma, neuroblastoma, and vascular metastases, as well as skin involvement by other hematologic neoplasms. An adequate incisional biopsy rather than a punch or shave biopsy is recommended for diagnosis. Dermatologists should alert the pathologist that BPDCN is in the clinical differential diagnosis when possible so that judicious use of appropriate immunophenotypic markers such as CD123, CD4, CD56, and T-cell leukemia/lymphoma protein 1 will avoid misdiagnosis of this aggressive condition, in addition to excluding acute myeloid leukemia, which also may express 3 of the above markers. However, most cases of acute myeloid leukemia lack terminal deoxynucleotidyl transferase (TdT) and express monocytic and other myeloid markers. Terminal deoxynucleotidyl transferase is positive in approximately one-third of cases of BPDCN, with expression in 10% to 80% of cells.1
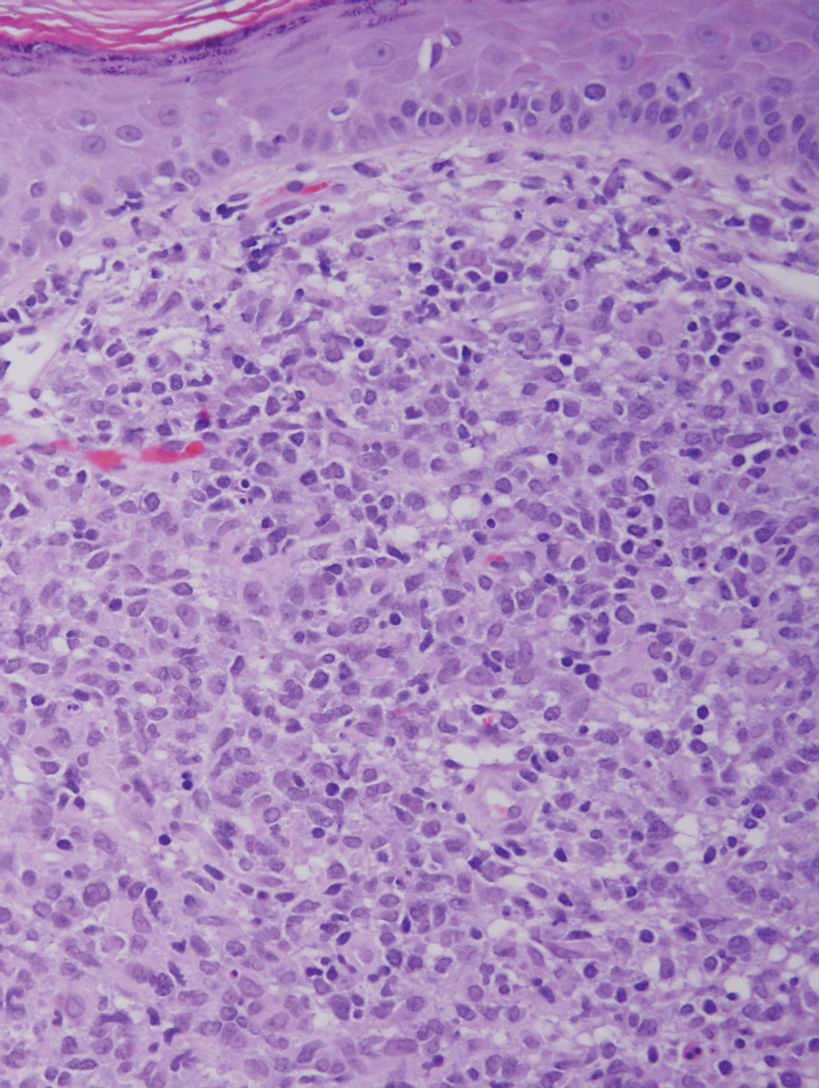
It is important to include BPDCN in the differential diagnosis of immunophenotypically aberrant hematologic tumors. Diffuse large B-cell lymphoma, leg type, accounts for 4% of all primary cutaneous B-cell lymphomas.1 Compared with BPDCN, diffuse large B-cell lymphoma usually occurs in an older age group and is of B-cell lineage. Morphologically, these neoplasms are composed of a monotonous, diffuse, nonepidermotropic infiltrate of confluent sheets of centroblasts and immunoblasts (Figure 1). They may share immunohistochemical markers of CD79a, multiple myeloma 1, Bcl-2, and Bcl-6; however, they lack plasmacytoid dendritic cell (PDC)– associated antigens such as CD4, CD56, CD123, and T-cell leukemia/lymphoma protein 1.1
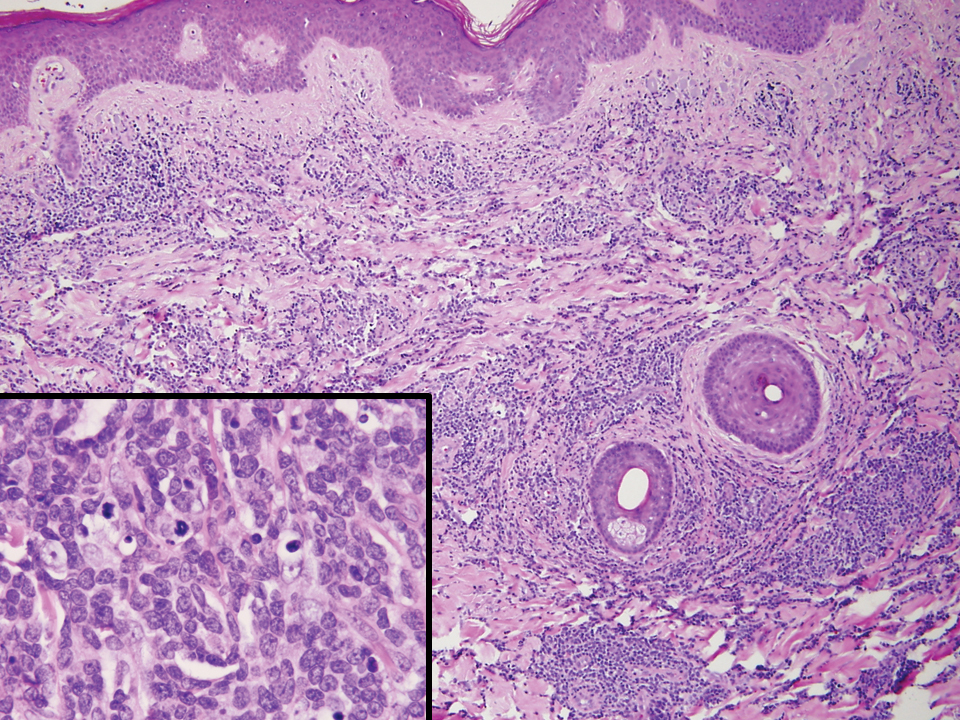
Adult T-cell leukemia/lymphoma is a neoplasm histologically composed of highly pleomorphic medium- to large-sized T cells with an irregular multilobated nuclear contour, so-called flower cells, in the peripheral blood. The nuclear chromatin is coarse and clumped with prominent nucleoli. Blastlike cells with dispersed chromatin are present in variable proportions. Most patients present with widespread lymph node and peripheral blood involvement. Skin is involved in more than half of patients with an epidermal as well as dermal pattern of infiltration (mainly perivascular)(Figure 2). Adult T-cell leukemia/lymphoma is endemic in several regions of the world, and the distribution is closely linked to the prevalence of human T-cell lymphotropic virus type 1 in the population. This neoplasm is of T-cell lineage and may share CD4 but not PDC-associated antigens with BPDCN.1
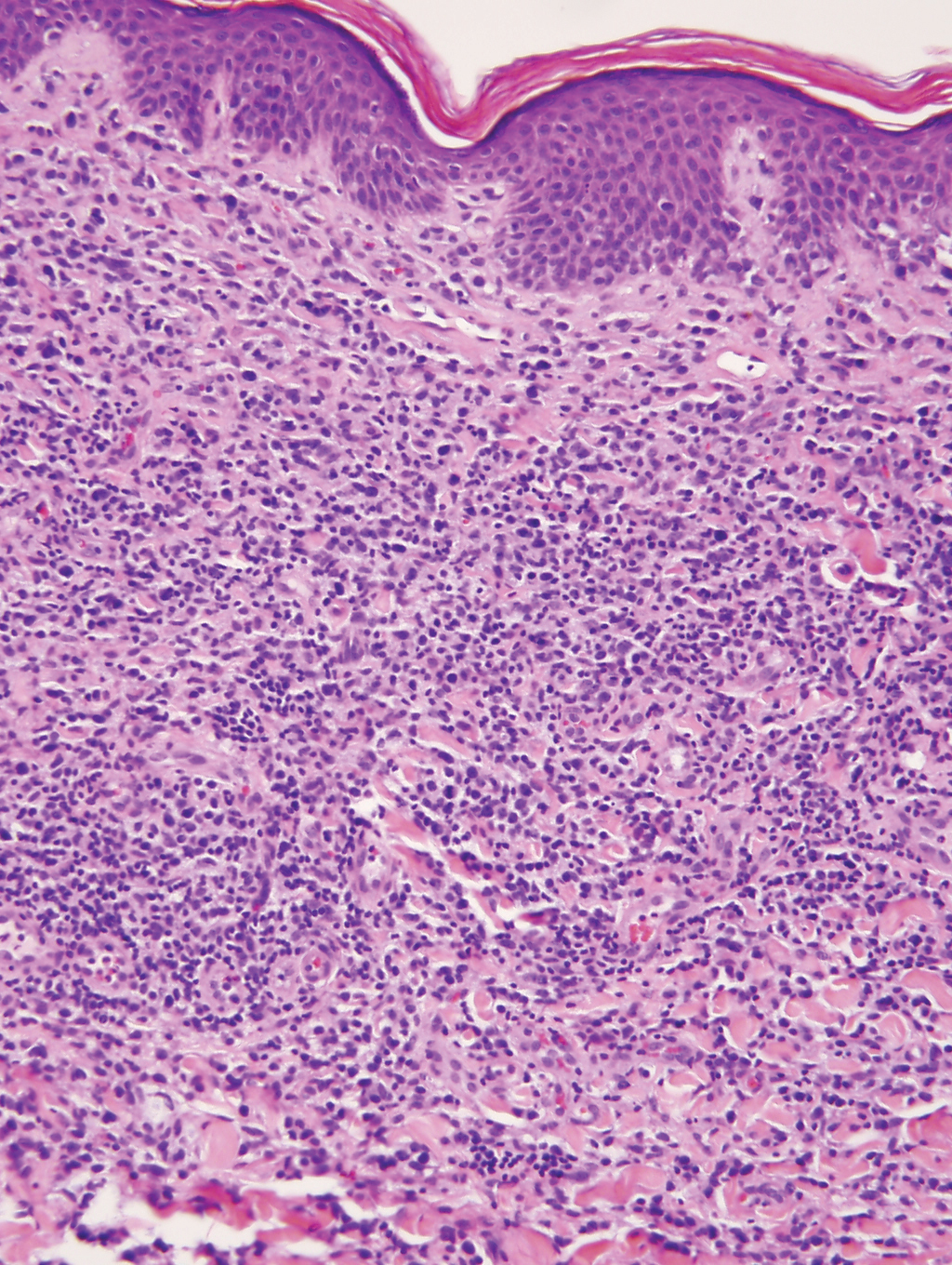
Cutaneous involvement by T-cell lymphoblastic leukemia/lymphoma (T-LBL) is a rare occurrence with a frequency of approximately 4.3%.8 T-cell lymphoblastic leukemia/lymphoma usually presents as multiple skin lesions throughout the body. Almost all cutaneous T-LBL cases are seen in association with bone marrow and/or mediastinal, lymph node, or extranodal involvement. Cutaneous T-LBLs present as a diffuse monomorphous infiltrate located in the entire dermis and subcutis without epidermotropism, composed of medium to large blasts with finely dispersed chromatin and relatively prominent nucleoli (Figure 3). Immunophenotyping studies show an immature T-cell immunophenotype, with expression of TdT (usually uniform), CD7, and cytoplasmic CD3 and an absence of PDC-associated antigens.8
Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a neoplasm primarily involving the skin. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection. Histologically, they show variable epidermotropism as well as dermal and subcutaneous involvement by medium to large cells with coarse clumped chromatin (Figure 4). Large blastic cells with vesicular nuclei and prominent nucleoli are infrequent. In contrast to BPCDN, the neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are positive for T-cell intracellular antigen-1 and granzyme B with loss of CD4.9
At the time of presentation, 27% to 87% of BPDCN patients will have bone marrow involvement, 22% to 28% will have blood involvement, and 6% to 41% will have lymph node involvement.1-4,6,7,10,11 The clinical course is aggressive, with a median survival of 10.0 to 19.8 months, irrespective of the initial pattern of disease.1 Most cases have shown initial response to multiagent chemotherapy, but relapses with subsequent resistance to drugs regularly have been observed. Age has an adverse impact of prognosis. Low TdT expression has been associated with shorter survival.1 Approximately 10% to 20% of cases of BPDCN are associated with or develop into chronic myelogenous leukemia, myelodysplastic syndrome, or acute myeloid leukemia.1,4 Pediatric patients have a greater 5-year overall survival rate than older patients, and overall survival worsens with increasing age. The extent of cutaneous involvement and presence of systemic involvement at initial presentation do not seem to be strong predictors of survival.1,2,5-7,10-12 In a retrospective analysis of 90 patients, Julia et al12 found that the type of skin disease did not predict survival. Specifically, the presence of nodular lesions and disseminated skin involvement were not adverse prognostic factors compared with macular lesions limited to 1 or 2 body areas.12
- Facchetti F, Petrella T, Pileri SA. Blastic plasmacytoid dendritic cells neoplasm. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2017:174-177.
- Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392-404.
- Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med. 2014;138:564-569.
- Khoury JD, Medeiros LJ, Manning JT, et al. CD56(+) TdT(+) blastic natural killer cell tumor of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer. 2002;94:2401-2408.
- Kolerova A, Sergeeva I, Krinitsyna J, et al. Blastic plasmacytoid dendritic cell neoplasm: case report and literature overview. Indian J Dermatol. 2020;65:217-221.
- Hirner JP, O’Malley JT, LeBoeuf NR. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34:501-509.
- Guiducii C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931-2942.
- Khurana S, Beltran M, Jiang L, et al. Primary cutaneous T-cell lymphoblastic lymphoma: case report and literature review. Case Rep Hematol. 2019;2019:3540487. doi:10.1155/2019/3540487
- Gladys TE, Helm MF, Anderson BE, et al. Rapid onset of widespread nodules and lymphadenopathy. Cutis. 2020;106:132, 153-155.
- Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921-2930.
- Guru Murthy GS, Pemmaraju N, Attallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21-23.
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2012;169:579-586.
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
A diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN) was rendered. Subsequent needle core biopsy of a left axillary lymph node as well as bone marrow aspiration and biopsy revealed a similar diffuse blastoid infiltrate with an identical immunophenotype to that in the skin biopsy from the pretibial mass and peripheral blood.
Previously known as blastic natural killer cell leukemia/lymphoma or agranular CD4+/CD56+ hematodermic neoplasm/tumor, BPDCN is a rare, clinically aggressive hematologic malignancy derived from the precursors of plasmacytoid dendritic cells. It often is diagnostically challenging, particularly when presenting at noncutaneous sites and in unusual (young) patient populations.1 It was included with other myeloid neoplasms in the 2008 World Health Organization classification; however, in the 2017 classification it was categorized as a separate entity. Blastic plasmacytoid dendritic cell neoplasm typically presents in the skin of elderly patients (age range at diagnosis, 61–67 years) with or without bone marrow involvement and systemic dissemination.1,2 The skin is the most common clinical site of disease in typical cases of BPDCN and often precedes bone marrow involvement. Thus, skin biopsy often is the key to making the diagnosis. Diagnosis of BPDCN may be delayed because of diagnostic pitfalls. Patients usually present with asymptomatic solitary or multiple lesions.3-5 Blastic plasmacytoid dendritic cell neoplasm can present as an isolated purplish nodule or bruiselike papule or more commonly as disseminated purplish nodules, papules, and macules. Isolated nodules are found on the head and lower limbs and can be more than 10 cm in diameter. Peripheral blood and bone marrow may be minimally involved at presentation but invariably become involved with the progression of disease. Cytopenia can occur at diagnosis and in a minority of severe cases indicates bone marrow failure.2-6
Skin involvement of BPDCN is thought to be secondary to the expression of skin migration molecules, such as cutaneous lymphocyte-associated antigen, one of the E-selectin ligands, which binds to E-selectin on high endothelial venules. In addition, the local dermal microenvironment of chemokines binding CXCR3, CXCR4, CCR6, or CCR7 present on neoplastic cells possibly leads to skin involvement. The full mechanism underlying the cutaneous tropism is still to be elucidated.4-7 Infiltration of the oral mucosa is seen in some patients, but it may be underreported. Mucosal disease typically appears similarly to cutaneous disease.
The cutaneous differential diagnosis for BPDCN depends on the clinical presentation, extent of disease spread, and thickness of infiltration. It includes common nonneoplastic diseases such as traumatic ecchymoses; purpuric disorders; extramedullary hematopoiesis; and soft-tissue neoplasms such as angiosarcoma, Kaposi sarcoma, neuroblastoma, and vascular metastases, as well as skin involvement by other hematologic neoplasms. An adequate incisional biopsy rather than a punch or shave biopsy is recommended for diagnosis. Dermatologists should alert the pathologist that BPDCN is in the clinical differential diagnosis when possible so that judicious use of appropriate immunophenotypic markers such as CD123, CD4, CD56, and T-cell leukemia/lymphoma protein 1 will avoid misdiagnosis of this aggressive condition, in addition to excluding acute myeloid leukemia, which also may express 3 of the above markers. However, most cases of acute myeloid leukemia lack terminal deoxynucleotidyl transferase (TdT) and express monocytic and other myeloid markers. Terminal deoxynucleotidyl transferase is positive in approximately one-third of cases of BPDCN, with expression in 10% to 80% of cells.1
It is important to include BPDCN in the differential diagnosis of immunophenotypically aberrant hematologic tumors. Diffuse large B-cell lymphoma, leg type, accounts for 4% of all primary cutaneous B-cell lymphomas.1 Compared with BPDCN, diffuse large B-cell lymphoma usually occurs in an older age group and is of B-cell lineage. Morphologically, these neoplasms are composed of a monotonous, diffuse, nonepidermotropic infiltrate of confluent sheets of centroblasts and immunoblasts (Figure 1). They may share immunohistochemical markers of CD79a, multiple myeloma 1, Bcl-2, and Bcl-6; however, they lack plasmacytoid dendritic cell (PDC)– associated antigens such as CD4, CD56, CD123, and T-cell leukemia/lymphoma protein 1.1

Adult T-cell leukemia/lymphoma is a neoplasm histologically composed of highly pleomorphic medium- to large-sized T cells with an irregular multilobated nuclear contour, so-called flower cells, in the peripheral blood. The nuclear chromatin is coarse and clumped with prominent nucleoli. Blastlike cells with dispersed chromatin are present in variable proportions. Most patients present with widespread lymph node and peripheral blood involvement. Skin is involved in more than half of patients with an epidermal as well as dermal pattern of infiltration (mainly perivascular)(Figure 2). Adult T-cell leukemia/lymphoma is endemic in several regions of the world, and the distribution is closely linked to the prevalence of human T-cell lymphotropic virus type 1 in the population. This neoplasm is of T-cell lineage and may share CD4 but not PDC-associated antigens with BPDCN.1

Cutaneous involvement by T-cell lymphoblastic leukemia/lymphoma (T-LBL) is a rare occurrence with a frequency of approximately 4.3%.8 T-cell lymphoblastic leukemia/lymphoma usually presents as multiple skin lesions throughout the body. Almost all cutaneous T-LBL cases are seen in association with bone marrow and/or mediastinal, lymph node, or extranodal involvement. Cutaneous T-LBLs present as a diffuse monomorphous infiltrate located in the entire dermis and subcutis without epidermotropism, composed of medium to large blasts with finely dispersed chromatin and relatively prominent nucleoli (Figure 3). Immunophenotyping studies show an immature T-cell immunophenotype, with expression of TdT (usually uniform), CD7, and cytoplasmic CD3 and an absence of PDC-associated antigens.8

Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a neoplasm primarily involving the skin. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection. Histologically, they show variable epidermotropism as well as dermal and subcutaneous involvement by medium to large cells with coarse clumped chromatin (Figure 4). Large blastic cells with vesicular nuclei and prominent nucleoli are infrequent. In contrast to BPCDN, the neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are positive for T-cell intracellular antigen-1 and granzyme B with loss of CD4.9

At the time of presentation, 27% to 87% of BPDCN patients will have bone marrow involvement, 22% to 28% will have blood involvement, and 6% to 41% will have lymph node involvement.1-4,6,7,10,11 The clinical course is aggressive, with a median survival of 10.0 to 19.8 months, irrespective of the initial pattern of disease.1 Most cases have shown initial response to multiagent chemotherapy, but relapses with subsequent resistance to drugs regularly have been observed. Age has an adverse impact of prognosis. Low TdT expression has been associated with shorter survival.1 Approximately 10% to 20% of cases of BPDCN are associated with or develop into chronic myelogenous leukemia, myelodysplastic syndrome, or acute myeloid leukemia.1,4 Pediatric patients have a greater 5-year overall survival rate than older patients, and overall survival worsens with increasing age. The extent of cutaneous involvement and presence of systemic involvement at initial presentation do not seem to be strong predictors of survival.1,2,5-7,10-12 In a retrospective analysis of 90 patients, Julia et al12 found that the type of skin disease did not predict survival. Specifically, the presence of nodular lesions and disseminated skin involvement were not adverse prognostic factors compared with macular lesions limited to 1 or 2 body areas.12
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
A diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN) was rendered. Subsequent needle core biopsy of a left axillary lymph node as well as bone marrow aspiration and biopsy revealed a similar diffuse blastoid infiltrate with an identical immunophenotype to that in the skin biopsy from the pretibial mass and peripheral blood.
Previously known as blastic natural killer cell leukemia/lymphoma or agranular CD4+/CD56+ hematodermic neoplasm/tumor, BPDCN is a rare, clinically aggressive hematologic malignancy derived from the precursors of plasmacytoid dendritic cells. It often is diagnostically challenging, particularly when presenting at noncutaneous sites and in unusual (young) patient populations.1 It was included with other myeloid neoplasms in the 2008 World Health Organization classification; however, in the 2017 classification it was categorized as a separate entity. Blastic plasmacytoid dendritic cell neoplasm typically presents in the skin of elderly patients (age range at diagnosis, 61–67 years) with or without bone marrow involvement and systemic dissemination.1,2 The skin is the most common clinical site of disease in typical cases of BPDCN and often precedes bone marrow involvement. Thus, skin biopsy often is the key to making the diagnosis. Diagnosis of BPDCN may be delayed because of diagnostic pitfalls. Patients usually present with asymptomatic solitary or multiple lesions.3-5 Blastic plasmacytoid dendritic cell neoplasm can present as an isolated purplish nodule or bruiselike papule or more commonly as disseminated purplish nodules, papules, and macules. Isolated nodules are found on the head and lower limbs and can be more than 10 cm in diameter. Peripheral blood and bone marrow may be minimally involved at presentation but invariably become involved with the progression of disease. Cytopenia can occur at diagnosis and in a minority of severe cases indicates bone marrow failure.2-6
Skin involvement of BPDCN is thought to be secondary to the expression of skin migration molecules, such as cutaneous lymphocyte-associated antigen, one of the E-selectin ligands, which binds to E-selectin on high endothelial venules. In addition, the local dermal microenvironment of chemokines binding CXCR3, CXCR4, CCR6, or CCR7 present on neoplastic cells possibly leads to skin involvement. The full mechanism underlying the cutaneous tropism is still to be elucidated.4-7 Infiltration of the oral mucosa is seen in some patients, but it may be underreported. Mucosal disease typically appears similarly to cutaneous disease.
The cutaneous differential diagnosis for BPDCN depends on the clinical presentation, extent of disease spread, and thickness of infiltration. It includes common nonneoplastic diseases such as traumatic ecchymoses; purpuric disorders; extramedullary hematopoiesis; and soft-tissue neoplasms such as angiosarcoma, Kaposi sarcoma, neuroblastoma, and vascular metastases, as well as skin involvement by other hematologic neoplasms. An adequate incisional biopsy rather than a punch or shave biopsy is recommended for diagnosis. Dermatologists should alert the pathologist that BPDCN is in the clinical differential diagnosis when possible so that judicious use of appropriate immunophenotypic markers such as CD123, CD4, CD56, and T-cell leukemia/lymphoma protein 1 will avoid misdiagnosis of this aggressive condition, in addition to excluding acute myeloid leukemia, which also may express 3 of the above markers. However, most cases of acute myeloid leukemia lack terminal deoxynucleotidyl transferase (TdT) and express monocytic and other myeloid markers. Terminal deoxynucleotidyl transferase is positive in approximately one-third of cases of BPDCN, with expression in 10% to 80% of cells.1
It is important to include BPDCN in the differential diagnosis of immunophenotypically aberrant hematologic tumors. Diffuse large B-cell lymphoma, leg type, accounts for 4% of all primary cutaneous B-cell lymphomas.1 Compared with BPDCN, diffuse large B-cell lymphoma usually occurs in an older age group and is of B-cell lineage. Morphologically, these neoplasms are composed of a monotonous, diffuse, nonepidermotropic infiltrate of confluent sheets of centroblasts and immunoblasts (Figure 1). They may share immunohistochemical markers of CD79a, multiple myeloma 1, Bcl-2, and Bcl-6; however, they lack plasmacytoid dendritic cell (PDC)– associated antigens such as CD4, CD56, CD123, and T-cell leukemia/lymphoma protein 1.1

Adult T-cell leukemia/lymphoma is a neoplasm histologically composed of highly pleomorphic medium- to large-sized T cells with an irregular multilobated nuclear contour, so-called flower cells, in the peripheral blood. The nuclear chromatin is coarse and clumped with prominent nucleoli. Blastlike cells with dispersed chromatin are present in variable proportions. Most patients present with widespread lymph node and peripheral blood involvement. Skin is involved in more than half of patients with an epidermal as well as dermal pattern of infiltration (mainly perivascular)(Figure 2). Adult T-cell leukemia/lymphoma is endemic in several regions of the world, and the distribution is closely linked to the prevalence of human T-cell lymphotropic virus type 1 in the population. This neoplasm is of T-cell lineage and may share CD4 but not PDC-associated antigens with BPDCN.1

Cutaneous involvement by T-cell lymphoblastic leukemia/lymphoma (T-LBL) is a rare occurrence with a frequency of approximately 4.3%.8 T-cell lymphoblastic leukemia/lymphoma usually presents as multiple skin lesions throughout the body. Almost all cutaneous T-LBL cases are seen in association with bone marrow and/or mediastinal, lymph node, or extranodal involvement. Cutaneous T-LBLs present as a diffuse monomorphous infiltrate located in the entire dermis and subcutis without epidermotropism, composed of medium to large blasts with finely dispersed chromatin and relatively prominent nucleoli (Figure 3). Immunophenotyping studies show an immature T-cell immunophenotype, with expression of TdT (usually uniform), CD7, and cytoplasmic CD3 and an absence of PDC-associated antigens.8

Primary cutaneous γδ T-cell lymphoma (PCGDTL) is a neoplasm primarily involving the skin. Often rapidly fatal, PCGDTL has a broad clinical spectrum that may include indolent variants—subcutaneous, epidermotropic, and dermal. Patients typically present with nodular lesions that progress to ulceration and necrosis. Early lesions can be confused with erythema nodosum, mycosis fungoides, or infection. Histologically, they show variable epidermotropism as well as dermal and subcutaneous involvement by medium to large cells with coarse clumped chromatin (Figure 4). Large blastic cells with vesicular nuclei and prominent nucleoli are infrequent. In contrast to BPCDN, the neoplastic lymphocytes in dermal and subcutaneous PCGDTL typically are positive for T-cell intracellular antigen-1 and granzyme B with loss of CD4.9

At the time of presentation, 27% to 87% of BPDCN patients will have bone marrow involvement, 22% to 28% will have blood involvement, and 6% to 41% will have lymph node involvement.1-4,6,7,10,11 The clinical course is aggressive, with a median survival of 10.0 to 19.8 months, irrespective of the initial pattern of disease.1 Most cases have shown initial response to multiagent chemotherapy, but relapses with subsequent resistance to drugs regularly have been observed. Age has an adverse impact of prognosis. Low TdT expression has been associated with shorter survival.1 Approximately 10% to 20% of cases of BPDCN are associated with or develop into chronic myelogenous leukemia, myelodysplastic syndrome, or acute myeloid leukemia.1,4 Pediatric patients have a greater 5-year overall survival rate than older patients, and overall survival worsens with increasing age. The extent of cutaneous involvement and presence of systemic involvement at initial presentation do not seem to be strong predictors of survival.1,2,5-7,10-12 In a retrospective analysis of 90 patients, Julia et al12 found that the type of skin disease did not predict survival. Specifically, the presence of nodular lesions and disseminated skin involvement were not adverse prognostic factors compared with macular lesions limited to 1 or 2 body areas.12
- Facchetti F, Petrella T, Pileri SA. Blastic plasmacytoid dendritic cells neoplasm. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2017:174-177.
- Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392-404.
- Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med. 2014;138:564-569.
- Khoury JD, Medeiros LJ, Manning JT, et al. CD56(+) TdT(+) blastic natural killer cell tumor of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer. 2002;94:2401-2408.
- Kolerova A, Sergeeva I, Krinitsyna J, et al. Blastic plasmacytoid dendritic cell neoplasm: case report and literature overview. Indian J Dermatol. 2020;65:217-221.
- Hirner JP, O’Malley JT, LeBoeuf NR. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34:501-509.
- Guiducii C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931-2942.
- Khurana S, Beltran M, Jiang L, et al. Primary cutaneous T-cell lymphoblastic lymphoma: case report and literature review. Case Rep Hematol. 2019;2019:3540487. doi:10.1155/2019/3540487
- Gladys TE, Helm MF, Anderson BE, et al. Rapid onset of widespread nodules and lymphadenopathy. Cutis. 2020;106:132, 153-155.
- Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921-2930.
- Guru Murthy GS, Pemmaraju N, Attallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21-23.
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2012;169:579-586.
- Facchetti F, Petrella T, Pileri SA. Blastic plasmacytoid dendritic cells neoplasm. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. World Health Organization; 2017:174-177.
- Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392-404.
- Shi Y, Wang E. Blastic plasmacytoid dendritic cell neoplasm: a clinicopathologic review. Arch Pathol Lab Med. 2014;138:564-569.
- Khoury JD, Medeiros LJ, Manning JT, et al. CD56(+) TdT(+) blastic natural killer cell tumor of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer. 2002;94:2401-2408.
- Kolerova A, Sergeeva I, Krinitsyna J, et al. Blastic plasmacytoid dendritic cell neoplasm: case report and literature overview. Indian J Dermatol. 2020;65:217-221.
- Hirner JP, O’Malley JT, LeBoeuf NR. Blastic plasmacytoid dendritic cell neoplasm: the dermatologist’s perspective. Hematol Oncol Clin North Am. 2020;34:501-509.
- Guiducii C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931-2942.
- Khurana S, Beltran M, Jiang L, et al. Primary cutaneous T-cell lymphoblastic lymphoma: case report and literature review. Case Rep Hematol. 2019;2019:3540487. doi:10.1155/2019/3540487
- Gladys TE, Helm MF, Anderson BE, et al. Rapid onset of widespread nodules and lymphadenopathy. Cutis. 2020;106:132, 153-155.
- Gregorio J, Meller S, Conrad C, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921-2930.
- Guru Murthy GS, Pemmaraju N, Attallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21-23.
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2012;169:579-586.
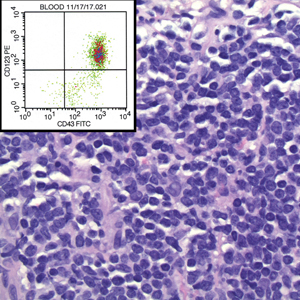
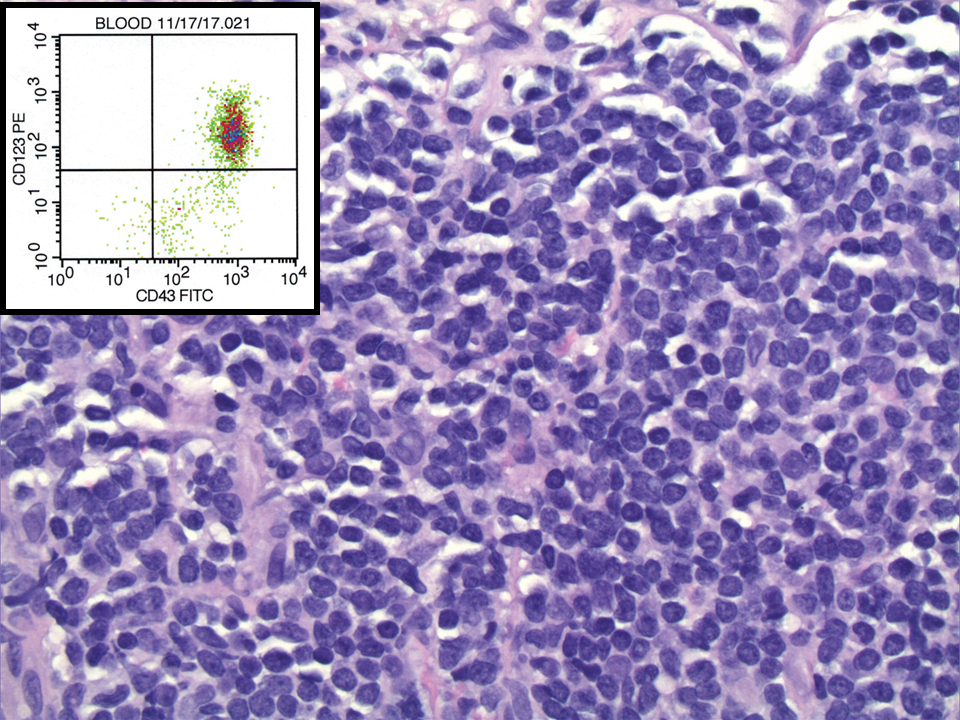
A 23-year-old man presented with skin that bruised easily, pancytopenia, recent fatigue, fever, and loss of appetite, along with a nontender, brown-purple, left anterior pretibial mass of 2 years’ duration (top). Computed tomography showed diffuse lymphadenopathy involving the inguinal, mesenteric, retroperitoneal, mediastinal, and axillary regions. A biopsy of the mass showed a dense monomorphous infiltrate of medium-sized blastoid cells with small or inconspicuous nucleoli (bottom). The lesion diffusely involved the dermis and extended into the subcutaneous tissue but spared the epidermis. Flow cytometry immunophenotyping of peripheral blood neoplastic cells (bottom [inset]) showed high-level expression of CD123 together with expression of CD4, CD56, CD45RA, and CD43 but a lack of expression of any other myelomonocytic or lymphoid lineage–associated markers.
A lump in the umbilicus
A 60-year-old man presented to the emergency department with abdominal pain. The pain was dull and constant, with no radiation and no aggravating or relieving factors. He also reported decreased appetite, weight loss, and constipation over the past 3 months.
He had no history of significant medical problems and was not taking any medications. He had no fever and no evidence of gastrointestinal bleeding.
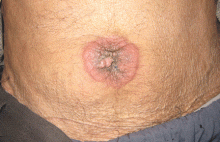
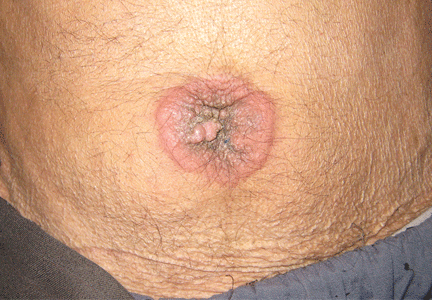
Physical examination showed mild tenderness around the umbilicus and a painless, small nodule (15 mm by 6 mm) protruding through the umbilicus with surrounding erythematous discoloration (Figure 1). A digital rectal examination was normal. Laboratory studies showed only mild normocytic anemia.
The patient underwent abdominal ultrasonography, which showed free fluid in the abdominopelvic cavity. This was followed by computed tomography of the abdominopelvic cavity, which revealed ascites and a small mass in the umbilicus. Punch biopsy of the umbilical lesion was performed, and histologic study indicated a diagnosis of adenocarcinoma.
Based on the biopsy results and the patient’s history of gastrointestinal symptoms, colonoscopy was performed, which showed an exophytic tumor of the transverse colon. The tumor was biopsied, and pathologic evaluation confirmed adenocarcinoma. A diagnosis of metastatic colon cancer was made. The patient received chemotherapy and underwent surgery to relieve the bowel obstruction.
SISTER MARY JOSEPH NODULE
A periumbilical nodule representing metastatic cancer, also known as Sister Mary Joseph nodule,1 is typically associated with intra-abdominal malignancy. An estimated 1% to 3% of patients with abdominopelvic malignancy present with this nodule,2 most often from gastrointestinal cancer but also from gynecologic malignancies. In about 15% to 30% of cases, no origin is identified.3
How these cancers spread to the umbilicus is not known. Proposed mechanisms include direct transperitoneal, lymphatic, or hematogenous spread, and even iatrogenic spread during laparotomy.4,5
The differential diagnosis includes umbilical hernia, cutaneous endometriosis, lymphangioma, melanoma, pilonidal sinus, and pyogenic granuloma. It is usually described as a painful nodule with irregular margins and a mean diameter of 2 to 3 cm.2 The condition is always a sign of metastatic cancer. Although it can be useful for diagnosing advanced disease, whether this would lead to earlier diagnosis is doubtful. Palliative treatment is generally most appropriate.
- Albano EA, Kanter J. Images in clinical medicine. Sister Mary Joseph’s nodule. N Engl J Med 2005; 352:1913.
- Iavazzo C, Madhuri K, Essapen S, Akrivos N, Tailor A, Butler-Manuel S. Sister Mary Joseph’s nodule as a first manifestation of primary peritoneal cancer. Case Rep Obstet Gynecol 2012; 2012:467240.
- Gabriele R, Borghese M, Conte M, Basso L. Sister Mary Joseph’s nodule as a first sign of cancer of the cecum: report of a case. Dis Colon Rectum 2004; 47:115–117.
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14:385–387.
- Martínez-Palones JM, Gil-Moreno A, Pérez-Benavente MA, Garcia-Giménez A, Xercavins J. Umbilical metastasis after laparoscopic retroperitoneal paraaortic lymphadenectomy for cervical cancer: a true port-site metastasis? Gynecol Oncol 2005; 97:292–295.
A 60-year-old man presented to the emergency department with abdominal pain. The pain was dull and constant, with no radiation and no aggravating or relieving factors. He also reported decreased appetite, weight loss, and constipation over the past 3 months.
He had no history of significant medical problems and was not taking any medications. He had no fever and no evidence of gastrointestinal bleeding.
Physical examination showed mild tenderness around the umbilicus and a painless, small nodule (15 mm by 6 mm) protruding through the umbilicus with surrounding erythematous discoloration (Figure 1). A digital rectal examination was normal. Laboratory studies showed only mild normocytic anemia.
The patient underwent abdominal ultrasonography, which showed free fluid in the abdominopelvic cavity. This was followed by computed tomography of the abdominopelvic cavity, which revealed ascites and a small mass in the umbilicus. Punch biopsy of the umbilical lesion was performed, and histologic study indicated a diagnosis of adenocarcinoma.
Based on the biopsy results and the patient’s history of gastrointestinal symptoms, colonoscopy was performed, which showed an exophytic tumor of the transverse colon. The tumor was biopsied, and pathologic evaluation confirmed adenocarcinoma. A diagnosis of metastatic colon cancer was made. The patient received chemotherapy and underwent surgery to relieve the bowel obstruction.
SISTER MARY JOSEPH NODULE
A periumbilical nodule representing metastatic cancer, also known as Sister Mary Joseph nodule,1 is typically associated with intra-abdominal malignancy. An estimated 1% to 3% of patients with abdominopelvic malignancy present with this nodule,2 most often from gastrointestinal cancer but also from gynecologic malignancies. In about 15% to 30% of cases, no origin is identified.3
How these cancers spread to the umbilicus is not known. Proposed mechanisms include direct transperitoneal, lymphatic, or hematogenous spread, and even iatrogenic spread during laparotomy.4,5
The differential diagnosis includes umbilical hernia, cutaneous endometriosis, lymphangioma, melanoma, pilonidal sinus, and pyogenic granuloma. It is usually described as a painful nodule with irregular margins and a mean diameter of 2 to 3 cm.2 The condition is always a sign of metastatic cancer. Although it can be useful for diagnosing advanced disease, whether this would lead to earlier diagnosis is doubtful. Palliative treatment is generally most appropriate.
A 60-year-old man presented to the emergency department with abdominal pain. The pain was dull and constant, with no radiation and no aggravating or relieving factors. He also reported decreased appetite, weight loss, and constipation over the past 3 months.
He had no history of significant medical problems and was not taking any medications. He had no fever and no evidence of gastrointestinal bleeding.
Physical examination showed mild tenderness around the umbilicus and a painless, small nodule (15 mm by 6 mm) protruding through the umbilicus with surrounding erythematous discoloration (Figure 1). A digital rectal examination was normal. Laboratory studies showed only mild normocytic anemia.
The patient underwent abdominal ultrasonography, which showed free fluid in the abdominopelvic cavity. This was followed by computed tomography of the abdominopelvic cavity, which revealed ascites and a small mass in the umbilicus. Punch biopsy of the umbilical lesion was performed, and histologic study indicated a diagnosis of adenocarcinoma.
Based on the biopsy results and the patient’s history of gastrointestinal symptoms, colonoscopy was performed, which showed an exophytic tumor of the transverse colon. The tumor was biopsied, and pathologic evaluation confirmed adenocarcinoma. A diagnosis of metastatic colon cancer was made. The patient received chemotherapy and underwent surgery to relieve the bowel obstruction.
SISTER MARY JOSEPH NODULE
A periumbilical nodule representing metastatic cancer, also known as Sister Mary Joseph nodule,1 is typically associated with intra-abdominal malignancy. An estimated 1% to 3% of patients with abdominopelvic malignancy present with this nodule,2 most often from gastrointestinal cancer but also from gynecologic malignancies. In about 15% to 30% of cases, no origin is identified.3
How these cancers spread to the umbilicus is not known. Proposed mechanisms include direct transperitoneal, lymphatic, or hematogenous spread, and even iatrogenic spread during laparotomy.4,5
The differential diagnosis includes umbilical hernia, cutaneous endometriosis, lymphangioma, melanoma, pilonidal sinus, and pyogenic granuloma. It is usually described as a painful nodule with irregular margins and a mean diameter of 2 to 3 cm.2 The condition is always a sign of metastatic cancer. Although it can be useful for diagnosing advanced disease, whether this would lead to earlier diagnosis is doubtful. Palliative treatment is generally most appropriate.
- Albano EA, Kanter J. Images in clinical medicine. Sister Mary Joseph’s nodule. N Engl J Med 2005; 352:1913.
- Iavazzo C, Madhuri K, Essapen S, Akrivos N, Tailor A, Butler-Manuel S. Sister Mary Joseph’s nodule as a first manifestation of primary peritoneal cancer. Case Rep Obstet Gynecol 2012; 2012:467240.
- Gabriele R, Borghese M, Conte M, Basso L. Sister Mary Joseph’s nodule as a first sign of cancer of the cecum: report of a case. Dis Colon Rectum 2004; 47:115–117.
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14:385–387.
- Martínez-Palones JM, Gil-Moreno A, Pérez-Benavente MA, Garcia-Giménez A, Xercavins J. Umbilical metastasis after laparoscopic retroperitoneal paraaortic lymphadenectomy for cervical cancer: a true port-site metastasis? Gynecol Oncol 2005; 97:292–295.
- Albano EA, Kanter J. Images in clinical medicine. Sister Mary Joseph’s nodule. N Engl J Med 2005; 352:1913.
- Iavazzo C, Madhuri K, Essapen S, Akrivos N, Tailor A, Butler-Manuel S. Sister Mary Joseph’s nodule as a first manifestation of primary peritoneal cancer. Case Rep Obstet Gynecol 2012; 2012:467240.
- Gabriele R, Borghese M, Conte M, Basso L. Sister Mary Joseph’s nodule as a first sign of cancer of the cecum: report of a case. Dis Colon Rectum 2004; 47:115–117.
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14:385–387.
- Martínez-Palones JM, Gil-Moreno A, Pérez-Benavente MA, Garcia-Giménez A, Xercavins J. Umbilical metastasis after laparoscopic retroperitoneal paraaortic lymphadenectomy for cervical cancer: a true port-site metastasis? Gynecol Oncol 2005; 97:292–295.