User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
List of COVID-19 high-risk comorbidities expanded
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
Risk for erectile dysfunction sixfold higher in men with COVID-19
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 increases the risk of developing erectile dysfunction (ED) by nearly sixfold, according to data from the first study to investigate the association between ED and COVID-19 in young men in a real-life setting.
Men with ED are more than five times more likely to have COVID-19 (odds ratio, 5.27).
For men with a history of COVID-19, the odds ratio of developing ED was 5.66. The strength of the association remained after adjusting for factors considered to affect ED.
The study, which was led by Emmanuele A. Jannini, MD, professor of endocrinology and medical sexology, University of Rome Tor Vergata, was published on March 20 in Andrology.
‘Mask up to keep it up’
ED can be both a short-term and a long-term complication of COVID-19, Dr. Jannini suggests.
“When offered, men should have the COVID vaccination. It also gives a whole new meaning to wearing the mask – mask up to keep it up,” he said. “It could possibly have the added benefit of preventing sexual dysfunction.”
He points out that older age, diabetes, high body mass index, and smoking increase the risk of contracting COVID-19.
“These are the same as risk factors for ED. Results of our study agree with the pathophysiological mechanisms linking ED, endothelial dysfunction, and COVID-19. Basically, endothelial dysfunction is common in both conditions [COVID-10 and ED].
“We would like to find some sort of biomarker of endothelial dysfunction post COVID, because it seems that there are many sequelae that coexist for a long time after infection,” added Dr. Jannini. “Asking a patient if they have ED after COVID might provide a measure of systemic wellness.”
Allan Pacey, MD, professor of andrology at the University of Sheffield (England), welcomed the research, noting, “This seems to be a well-conducted study. However, at the moment, the relationship is just a correlation, and it might be that some of the comorbidities that increased the men’s chances of getting a significant COVID-19 infection may have also independently increased their chances of erectile dysfunction.
“But the authors offer a plausible mechanism by which COVID-19 may impact directly on erectile function,” agrees Dr. Pacey. However, “There’s more work to be done,” he said. “I’d also argue it’s a good reason for men to wear a mask, practice social distancing, and take the vaccine when it’s offered to them.”
Urologist John Mulhall, MD, from Memorial Sloan Kettering Cancer Center, New York, remarked, “It was a highly preliminary study, but the data are suggestive of a potential link between COVID-19 infection and ED.
“However, it raises enough questions such that further large, more long-term analyses are required to define causation. Future studies assessing testosterone levels and erectile hemodynamics will be needed to provide definite evidence of a causative link,» he stressed.
Erectile problems a ‘hallmark’ of systemic endothelial dysfunction
Prior research has suggested that asymptomatic COVID-19 could be associated with subclinical microvascular involvement with long-term cardiovascular effects.
“Indeed, COVID-19 is by all means an endothelial disease, in which systemic manifestations ... can potentially be due to alterations in the endothelial thrombotic/fibrinolytic balance,” emphasized Dr. Jannini. “In addition, endothelial cells express many of the cofactors used by SARS-CoV-2 to invade host cells.
“Erectile dysfunction has often been considered a hallmark of endothelial dysfunction, and as such, a potential association between ED and COVID-19 has also been postulated and underpinned the investigation in this study,” he explained.
The study was predicated on the fact that ED is often considered a clinical marker of impaired overall health status, which often features cardiovascular events at an early age. It aimed to investigate the bidirectional relationship between COVID-19 and ED. It asked whether ED could be a risk factor for contracting COVID-19 and whether having COVID-19 predisposes to developing ED.
“This would possibly suggest that men with ED, due to the underlying conditions which impair erectile response, could also be more susceptible to contracting COVID-19,” said Dr. Jannini.
Data were drawn from the Sex@COVID online survey, which was conducted from April 7 to May 4, 2020, in Italy. The survey included 6,821 participants aged 18 years or older (4,177 women; 2,644 men; mean age, 32.83 ± 11.24 years). Participants were stratified on the basis of marital status and sexual activity during lockdown. From these participants, 985 sexually active men were identified, among whom 25 (2.54%) reported having tested positive for COVID-19. These persons were matched with 75 COVID-19–negative men using propensity score matching in a 1:3 ratio.
The researchers used standardized psychometric tools to measure the effects of lockdown and social distancing on the intrapsychic, relational, and sexual health of the participants.
Erectile function was measured with the International Index of Erectile Function or the Sexual Health Inventory for Men, which are often used in clinical settings. In light of the two-way interaction between sexual activity and psychological well-being, results were adjusted for any influence of anxiety and depression, which were measured with recognized scales for use in patients with a history of COVID-19.
Results showed that the prevalence of ED was significantly higher among men who self-reported a history of COVID-19, compared with a matching COVID-negative population (28% vs. 9.33%; P = .027).
After adjusting for variables that are considered to have a bearing on the development of ED, such as psychological status, age, and BMI, the odds ratio for developing ED after having had COVID-19 was 5.66 (95% confidence interval, 1.50-24.01).
Similarly, after adjusting for age and BMI, men with ED were more likely to have COVID‐19 (OR, 5.27; 95% CI, 1.49-20.09).
The authors note that persons who experience “a sudden onset or worsening of ED might also consider precautionary quarantine or nasopharyngeal swab, as COVID‐19 might act as a potential initiating trigger for the onset of erectile impairment, or an aggravating factor for its progression to more severe forms.”
Similarly, patients who have ED “should consider their erectile impairment as a sign of possible underlying conditions that could increase the likelihood of suffering from COVID‐19,” they write.
Dr. Mulhall highlighted several limitations of the study, including its retrospective nature, recall bias associated with the use of online questionnaires, and the inclusion of COVID‐19 diagnoses that were based on the response to the survey rather than on testing with nasopharyngeal swabs. In addition, comorbidity data were incomplete, and there was no indication of duration after COVID-19 infection, the severity of COVID-19, or the severity of ED.
The authors have disclosed no relevant financial relationships. Dr. Pacey is chairman of the advisory committee of the U.K. National External Quality Assurance Schemes in Andrology, editor-in-chief of Human Fertility, trustee of the Progress Educational Trust, and trustee of the British Fertility Society (all unpaid). Dr. Mulhall has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular risks elevated in transgender youth
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study suggests no added risk of blood clots in COVID-19 outpatients
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
Cardiovascular disease remains leading cause of type 2 diabetes mortality
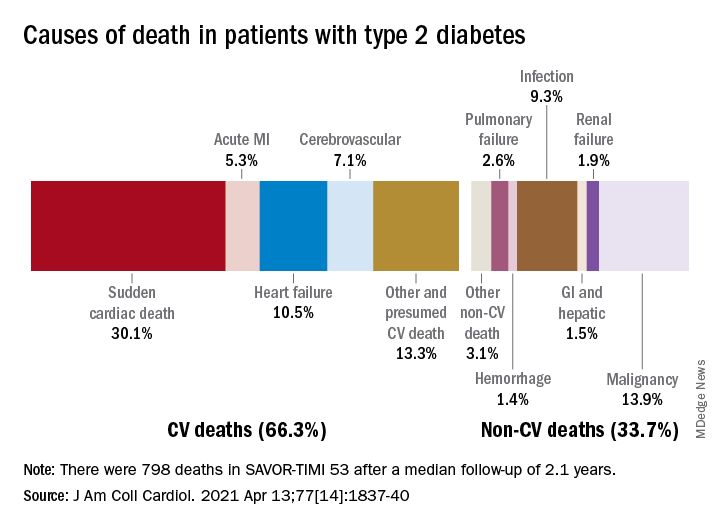
Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Excess deaths jump 23% in U.S. in 2020, mostly because of COVID-19
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
Is there a need for tPA before thrombectomy in patients with stroke?
In a new randomized trial that investigated the question of whether thrombolysis can be omitted for patients with stroke who are undergoing endovascular thrombectomy for a large-vessel occlusion, results were similar for both approaches.
and functional outcomes were not significantly different. In addition, hemorrhage rates with or without intravenous alteplase administration before endovascular treatment were similar.
“From the MR CLEAN NO IV results, we cannot change standard practice, as we failed to show superiority of the direct endovascular approach, and we also didn’t meet the noninferiority criteria. So, the standard practice of giving tPA to those eligible still holds,” said co–lead investigator Yvo Roos, MD.
“But I think we can say that these results suggest that there may also not be such a need for tPA in patients who can go straight for endovascular therapy,” said Dr. Roos, who is professor of neurology at Amsterdam Medical Center.
“If we are not sure whether a patient is suitable for tPA because they have a higher bleeding risk, I think we can be reassured about missing the tPA out and going straight to endovascular treatment. So, if in doubt, leave it out,” he added.
Results of the MR CLEAN NO IV trial were presented at the International Stroke Conference sponsored by the American Heart Association.
“If in doubt, leave it out”
Dr. Roos noted that three trials have investigated the question regarding dropping thrombolysis for patients who can receive thrombectomy quickly. These are the DIRECT MT, SKIP, and DEVT studies. All of these trials were conducted in Asian countries, and none found differences in functional outcomes between the two approaches.
The largest of these studies – the DIRECT-MT trial, from China, which was a sister study to MR CLEAN NO IV – did show noninferiority of the direct endovascular approach to tPA plus endovascular treatment.
But because of differences in health care logistics and trial populations, the benefits and risks of dropping thrombolysis in Western countries are not known, explained Charles Majoie, MD, who is co–lead investigator of the current trial and is chair of neuroradiology at Amsterdam Medical Center.
The MR CLEAN NO IV trial was designed to show superiority of the direct endovascular approach with noninferiority for hemorrhage. It enrolled 540 European patients who were eligible for both thrombolysis and thrombectomy and who presented to a thrombectomy-capable center. They were randomly assigned to receive thrombolysis plus endovascular therapy or direct endovascular therapy alone.
The mean time from stroke onset to groin puncture (the start of endovascular therapy) was very fast in both groups – 130 minutes in the direct group, and 135 minutes in the tPA group.
The primary outcome was a shift analysis of the Modified Rankin Scale (mRS). On that outcome, the trial failed to show significant superiority of the direct approach (odds ratio, 0.88; 95% confidence interval, 0.65-1.19).
A good functional outcome (mRS, 0-2) was achieved in 49% of the direct thrombectomy group and in 51% of the tPA group (OR, 0.95; 95% CI, 0.65-1.40).
Safety results showed no difference in any of the hemorrhage endpoints between the two groups. The rate of symptomatic intracranial hemorrhage was actually numerically higher in the direct thrombectomy group (5.9% vs. 5.3%).
“One of the most intriguing results of this study is that there was no increase in hemorrhage in the tPA group,” Dr. Roos commented. “This is very surprising, as we have always thought thrombolysis causes an increased bleeding risk. But after these results, we may have to rethink that idea – perhaps it is not the tPA itself that causes bleeding risk but rather the opening up of the vessel.”
On the failure to show noninferiority of the direct approach, Dr. Roos suggested that the trial may have been underpowered in this respect.
“Our sister trial, DIRECT-MT, was a noninferiority study. They had 650 patients, and they just reached noninferiority,” he said. “In MR CLEAN NO IV, we were aiming for superiority, and we had fewer patients – 540. We didn’t show superiority, and we didn’t have quite enough patients to show noninferiority.”
He added that, considering all the four studies together, the results look very similar and suggest no difference between the two approaches.
Individualized approach probable
Dr. Majoie suggested that different patients may be suitable for the different approaches.
“I think we are heading for individualized treatment. If we have a young patient and the angiography suite is ready, we could probably skip tPA, but it would be for the neurologist/neuroradiologist to make individualized decisions on this,” he said. “We need to look at subgroups for more information.”
Another large trial that investigated this issue, SWIFT-DIRECT, is expected to be presented later this year. An Australian trial, DIRECT-SAFE, is ongoing and is at an early stage of recruitment.
Dr. Roos said that the data from all the trials will be combined for a more comprehensive analysis of the benefits and risks of the two approaches in various subgroups.
Commenting on the study was cochair of the ISC session at which it was presented, Tudor Jovin, MD, chair of neurology at Cooper University Hospital, Cherry Hill, N.J.
“Putting these results together with the previous Asian studies, I think we can say that direct thrombectomy without tPA is clearly not superior to the combined approach of tPA plus thrombectomy,” he said.
Dr. Jovin explained that, in theory, direct thrombectomy could be faster than the combined approach and that the risk for symptomatic intracerebral hemorrhage could be lower. But neither of these two possible benefits were seen in this study.
He agreed with Dr. Roos that MR CLEAN NO IV could have failed to show noninferiority of the direct strategy because the sample was not large enough.
“The results of the two approaches are very similar in this study and in the Asian studies, so it doesn’t appear that tPA adds very much, and it is associated with a significant increase in costs,” he said.
“The answer will probably be that there is not a ‘one-size-fits-all’ strategy, and we may end up using different approaches for different patient groups,” Dr. Jovin added. “Information on this will come from subgroups analyses from these trials.”
MR CLEAN NO-IV trial was part of the CONTRAST consortium, which is supported by the Netherlands Cardiovascular Research Initiative (an initiative of the Dutch Heart Foundation), the Brain Foundation Netherlands, Medtronic, Health-Holland, and Top Sector Life Sciences. The study received additional unrestricted funding from Stryker European Operations. Dr. Roos and Dr. Majoie are shareholders of Nico Lab.
A version of this article first appeared on Medscape.com.
In a new randomized trial that investigated the question of whether thrombolysis can be omitted for patients with stroke who are undergoing endovascular thrombectomy for a large-vessel occlusion, results were similar for both approaches.
and functional outcomes were not significantly different. In addition, hemorrhage rates with or without intravenous alteplase administration before endovascular treatment were similar.
“From the MR CLEAN NO IV results, we cannot change standard practice, as we failed to show superiority of the direct endovascular approach, and we also didn’t meet the noninferiority criteria. So, the standard practice of giving tPA to those eligible still holds,” said co–lead investigator Yvo Roos, MD.
“But I think we can say that these results suggest that there may also not be such a need for tPA in patients who can go straight for endovascular therapy,” said Dr. Roos, who is professor of neurology at Amsterdam Medical Center.
“If we are not sure whether a patient is suitable for tPA because they have a higher bleeding risk, I think we can be reassured about missing the tPA out and going straight to endovascular treatment. So, if in doubt, leave it out,” he added.
Results of the MR CLEAN NO IV trial were presented at the International Stroke Conference sponsored by the American Heart Association.
“If in doubt, leave it out”
Dr. Roos noted that three trials have investigated the question regarding dropping thrombolysis for patients who can receive thrombectomy quickly. These are the DIRECT MT, SKIP, and DEVT studies. All of these trials were conducted in Asian countries, and none found differences in functional outcomes between the two approaches.
The largest of these studies – the DIRECT-MT trial, from China, which was a sister study to MR CLEAN NO IV – did show noninferiority of the direct endovascular approach to tPA plus endovascular treatment.
But because of differences in health care logistics and trial populations, the benefits and risks of dropping thrombolysis in Western countries are not known, explained Charles Majoie, MD, who is co–lead investigator of the current trial and is chair of neuroradiology at Amsterdam Medical Center.
The MR CLEAN NO IV trial was designed to show superiority of the direct endovascular approach with noninferiority for hemorrhage. It enrolled 540 European patients who were eligible for both thrombolysis and thrombectomy and who presented to a thrombectomy-capable center. They were randomly assigned to receive thrombolysis plus endovascular therapy or direct endovascular therapy alone.
The mean time from stroke onset to groin puncture (the start of endovascular therapy) was very fast in both groups – 130 minutes in the direct group, and 135 minutes in the tPA group.
The primary outcome was a shift analysis of the Modified Rankin Scale (mRS). On that outcome, the trial failed to show significant superiority of the direct approach (odds ratio, 0.88; 95% confidence interval, 0.65-1.19).
A good functional outcome (mRS, 0-2) was achieved in 49% of the direct thrombectomy group and in 51% of the tPA group (OR, 0.95; 95% CI, 0.65-1.40).
Safety results showed no difference in any of the hemorrhage endpoints between the two groups. The rate of symptomatic intracranial hemorrhage was actually numerically higher in the direct thrombectomy group (5.9% vs. 5.3%).
“One of the most intriguing results of this study is that there was no increase in hemorrhage in the tPA group,” Dr. Roos commented. “This is very surprising, as we have always thought thrombolysis causes an increased bleeding risk. But after these results, we may have to rethink that idea – perhaps it is not the tPA itself that causes bleeding risk but rather the opening up of the vessel.”
On the failure to show noninferiority of the direct approach, Dr. Roos suggested that the trial may have been underpowered in this respect.
“Our sister trial, DIRECT-MT, was a noninferiority study. They had 650 patients, and they just reached noninferiority,” he said. “In MR CLEAN NO IV, we were aiming for superiority, and we had fewer patients – 540. We didn’t show superiority, and we didn’t have quite enough patients to show noninferiority.”
He added that, considering all the four studies together, the results look very similar and suggest no difference between the two approaches.
Individualized approach probable
Dr. Majoie suggested that different patients may be suitable for the different approaches.
“I think we are heading for individualized treatment. If we have a young patient and the angiography suite is ready, we could probably skip tPA, but it would be for the neurologist/neuroradiologist to make individualized decisions on this,” he said. “We need to look at subgroups for more information.”
Another large trial that investigated this issue, SWIFT-DIRECT, is expected to be presented later this year. An Australian trial, DIRECT-SAFE, is ongoing and is at an early stage of recruitment.
Dr. Roos said that the data from all the trials will be combined for a more comprehensive analysis of the benefits and risks of the two approaches in various subgroups.
Commenting on the study was cochair of the ISC session at which it was presented, Tudor Jovin, MD, chair of neurology at Cooper University Hospital, Cherry Hill, N.J.
“Putting these results together with the previous Asian studies, I think we can say that direct thrombectomy without tPA is clearly not superior to the combined approach of tPA plus thrombectomy,” he said.
Dr. Jovin explained that, in theory, direct thrombectomy could be faster than the combined approach and that the risk for symptomatic intracerebral hemorrhage could be lower. But neither of these two possible benefits were seen in this study.
He agreed with Dr. Roos that MR CLEAN NO IV could have failed to show noninferiority of the direct strategy because the sample was not large enough.
“The results of the two approaches are very similar in this study and in the Asian studies, so it doesn’t appear that tPA adds very much, and it is associated with a significant increase in costs,” he said.
“The answer will probably be that there is not a ‘one-size-fits-all’ strategy, and we may end up using different approaches for different patient groups,” Dr. Jovin added. “Information on this will come from subgroups analyses from these trials.”
MR CLEAN NO-IV trial was part of the CONTRAST consortium, which is supported by the Netherlands Cardiovascular Research Initiative (an initiative of the Dutch Heart Foundation), the Brain Foundation Netherlands, Medtronic, Health-Holland, and Top Sector Life Sciences. The study received additional unrestricted funding from Stryker European Operations. Dr. Roos and Dr. Majoie are shareholders of Nico Lab.
A version of this article first appeared on Medscape.com.
In a new randomized trial that investigated the question of whether thrombolysis can be omitted for patients with stroke who are undergoing endovascular thrombectomy for a large-vessel occlusion, results were similar for both approaches.
and functional outcomes were not significantly different. In addition, hemorrhage rates with or without intravenous alteplase administration before endovascular treatment were similar.
“From the MR CLEAN NO IV results, we cannot change standard practice, as we failed to show superiority of the direct endovascular approach, and we also didn’t meet the noninferiority criteria. So, the standard practice of giving tPA to those eligible still holds,” said co–lead investigator Yvo Roos, MD.
“But I think we can say that these results suggest that there may also not be such a need for tPA in patients who can go straight for endovascular therapy,” said Dr. Roos, who is professor of neurology at Amsterdam Medical Center.
“If we are not sure whether a patient is suitable for tPA because they have a higher bleeding risk, I think we can be reassured about missing the tPA out and going straight to endovascular treatment. So, if in doubt, leave it out,” he added.
Results of the MR CLEAN NO IV trial were presented at the International Stroke Conference sponsored by the American Heart Association.
“If in doubt, leave it out”
Dr. Roos noted that three trials have investigated the question regarding dropping thrombolysis for patients who can receive thrombectomy quickly. These are the DIRECT MT, SKIP, and DEVT studies. All of these trials were conducted in Asian countries, and none found differences in functional outcomes between the two approaches.
The largest of these studies – the DIRECT-MT trial, from China, which was a sister study to MR CLEAN NO IV – did show noninferiority of the direct endovascular approach to tPA plus endovascular treatment.
But because of differences in health care logistics and trial populations, the benefits and risks of dropping thrombolysis in Western countries are not known, explained Charles Majoie, MD, who is co–lead investigator of the current trial and is chair of neuroradiology at Amsterdam Medical Center.
The MR CLEAN NO IV trial was designed to show superiority of the direct endovascular approach with noninferiority for hemorrhage. It enrolled 540 European patients who were eligible for both thrombolysis and thrombectomy and who presented to a thrombectomy-capable center. They were randomly assigned to receive thrombolysis plus endovascular therapy or direct endovascular therapy alone.
The mean time from stroke onset to groin puncture (the start of endovascular therapy) was very fast in both groups – 130 minutes in the direct group, and 135 minutes in the tPA group.
The primary outcome was a shift analysis of the Modified Rankin Scale (mRS). On that outcome, the trial failed to show significant superiority of the direct approach (odds ratio, 0.88; 95% confidence interval, 0.65-1.19).
A good functional outcome (mRS, 0-2) was achieved in 49% of the direct thrombectomy group and in 51% of the tPA group (OR, 0.95; 95% CI, 0.65-1.40).
Safety results showed no difference in any of the hemorrhage endpoints between the two groups. The rate of symptomatic intracranial hemorrhage was actually numerically higher in the direct thrombectomy group (5.9% vs. 5.3%).
“One of the most intriguing results of this study is that there was no increase in hemorrhage in the tPA group,” Dr. Roos commented. “This is very surprising, as we have always thought thrombolysis causes an increased bleeding risk. But after these results, we may have to rethink that idea – perhaps it is not the tPA itself that causes bleeding risk but rather the opening up of the vessel.”
On the failure to show noninferiority of the direct approach, Dr. Roos suggested that the trial may have been underpowered in this respect.
“Our sister trial, DIRECT-MT, was a noninferiority study. They had 650 patients, and they just reached noninferiority,” he said. “In MR CLEAN NO IV, we were aiming for superiority, and we had fewer patients – 540. We didn’t show superiority, and we didn’t have quite enough patients to show noninferiority.”
He added that, considering all the four studies together, the results look very similar and suggest no difference between the two approaches.
Individualized approach probable
Dr. Majoie suggested that different patients may be suitable for the different approaches.
“I think we are heading for individualized treatment. If we have a young patient and the angiography suite is ready, we could probably skip tPA, but it would be for the neurologist/neuroradiologist to make individualized decisions on this,” he said. “We need to look at subgroups for more information.”
Another large trial that investigated this issue, SWIFT-DIRECT, is expected to be presented later this year. An Australian trial, DIRECT-SAFE, is ongoing and is at an early stage of recruitment.
Dr. Roos said that the data from all the trials will be combined for a more comprehensive analysis of the benefits and risks of the two approaches in various subgroups.
Commenting on the study was cochair of the ISC session at which it was presented, Tudor Jovin, MD, chair of neurology at Cooper University Hospital, Cherry Hill, N.J.
“Putting these results together with the previous Asian studies, I think we can say that direct thrombectomy without tPA is clearly not superior to the combined approach of tPA plus thrombectomy,” he said.
Dr. Jovin explained that, in theory, direct thrombectomy could be faster than the combined approach and that the risk for symptomatic intracerebral hemorrhage could be lower. But neither of these two possible benefits were seen in this study.
He agreed with Dr. Roos that MR CLEAN NO IV could have failed to show noninferiority of the direct strategy because the sample was not large enough.
“The results of the two approaches are very similar in this study and in the Asian studies, so it doesn’t appear that tPA adds very much, and it is associated with a significant increase in costs,” he said.
“The answer will probably be that there is not a ‘one-size-fits-all’ strategy, and we may end up using different approaches for different patient groups,” Dr. Jovin added. “Information on this will come from subgroups analyses from these trials.”
MR CLEAN NO-IV trial was part of the CONTRAST consortium, which is supported by the Netherlands Cardiovascular Research Initiative (an initiative of the Dutch Heart Foundation), the Brain Foundation Netherlands, Medtronic, Health-Holland, and Top Sector Life Sciences. The study received additional unrestricted funding from Stryker European Operations. Dr. Roos and Dr. Majoie are shareholders of Nico Lab.
A version of this article first appeared on Medscape.com.
FROM ISC 2021
Six pregnancy complications flag later heart disease risk
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
AstraZeneca COVID vaccine: Clotting disorder mechanism revealed?
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
FDA okays new indication for alirocumab in homozygous FH
The Food and Drug Administration has approved alirocumab (Praluent, Regeneron Pharmaceuticals) injection as add-on therapy for adults with homozygous familial hypercholesterolemia, the agency announced.
The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor was originally approved in the United States in 2015 as an adjunct to diet, alone or in combination with other lipid-lowering therapies, to reduce LDL cholesterol in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (FH).
Heterozygous FH is one of the most common genetic disorders, affecting 1 in every 200-500 people worldwide, whereas homozygous FH is very rare, affecting about 1 in 1 million people worldwide.
Alirocumab is also approved to reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with cardiovascular disease.
The new indication is based on a 12-week randomized trial in 45 adults who received 150 mg alirocumab every 2 weeks and 24 patients who received placebo, both on top of other therapies to reduce LDL cholesterol. At week 12, patients receiving alirocumab had an average 27% decrease in LDL cholesterol, compared with an average 9% increase among patients on placebo.
Common side effects of alirocumab are nasopharyngitis, injection-site reactions, and influenza. Serious hypersensitivity reactions have occurred among people taking alirocumab.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved alirocumab (Praluent, Regeneron Pharmaceuticals) injection as add-on therapy for adults with homozygous familial hypercholesterolemia, the agency announced.
The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor was originally approved in the United States in 2015 as an adjunct to diet, alone or in combination with other lipid-lowering therapies, to reduce LDL cholesterol in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (FH).
Heterozygous FH is one of the most common genetic disorders, affecting 1 in every 200-500 people worldwide, whereas homozygous FH is very rare, affecting about 1 in 1 million people worldwide.
Alirocumab is also approved to reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with cardiovascular disease.
The new indication is based on a 12-week randomized trial in 45 adults who received 150 mg alirocumab every 2 weeks and 24 patients who received placebo, both on top of other therapies to reduce LDL cholesterol. At week 12, patients receiving alirocumab had an average 27% decrease in LDL cholesterol, compared with an average 9% increase among patients on placebo.
Common side effects of alirocumab are nasopharyngitis, injection-site reactions, and influenza. Serious hypersensitivity reactions have occurred among people taking alirocumab.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved alirocumab (Praluent, Regeneron Pharmaceuticals) injection as add-on therapy for adults with homozygous familial hypercholesterolemia, the agency announced.
The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor was originally approved in the United States in 2015 as an adjunct to diet, alone or in combination with other lipid-lowering therapies, to reduce LDL cholesterol in adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (FH).
Heterozygous FH is one of the most common genetic disorders, affecting 1 in every 200-500 people worldwide, whereas homozygous FH is very rare, affecting about 1 in 1 million people worldwide.
Alirocumab is also approved to reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with cardiovascular disease.
The new indication is based on a 12-week randomized trial in 45 adults who received 150 mg alirocumab every 2 weeks and 24 patients who received placebo, both on top of other therapies to reduce LDL cholesterol. At week 12, patients receiving alirocumab had an average 27% decrease in LDL cholesterol, compared with an average 9% increase among patients on placebo.
Common side effects of alirocumab are nasopharyngitis, injection-site reactions, and influenza. Serious hypersensitivity reactions have occurred among people taking alirocumab.
A version of this article first appeared on Medscape.com.