User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Semaglutide for weight loss? A good first STEP, with caveats
The phase 3a STEP 1 trial that investigated the use of semaglutide (Novo Nordisk), a glucagonlike peptide–1 (GLP-1) agonist, for weight loss is aptly named, some say.
“In sum, we have a long way to go to control the obesity epidemic ... but on the face of it, the STEP 1 trial (like its name) is a good beginning,” wrote coeditorialists Julie R. Ingelfinger, MD, from Harvard Medical School, Boston, and a deputy editor of the New England Journal of Medicine, and Clifford J. Rosen, MD, from Tufts University School of Medicine, also in Boston.
The trial findings by John P.H. Wilding, DM, University of Liverpool (England), and colleagues and an accompanying editorial were published online Feb. 10, 2021, in the New England Journal of Medicine.
“The results are encouraging, with significantly more patients in the semaglutide group having clinically important weight loss,” Dr. Ingelfinger and Dr. Rosen stressed.
However, they also cautioned that “despite the positive results of this trial, the present study has some important limitations” and “there are concerns, including adverse events (mostly gastrointestinal – nausea, sometimes vomiting, and diarrhea) related primarily to the class of the agent.”
Two U.K. experts drew similar takeaways, speaking to the U.K. Science Media Centre.
“This was a well-designed study with unequivocal findings,” which showed that semaglutide “is indeed likely to be a game-changer in the fight against obesity,” according to Baptiste Leurent, PhD, London School of Hygiene and Tropical Medicine.
However, if the drug is approved at this dose for this use, patients would need close monitoring for gastrointestinal disorders, and “we also need to better understand what is happening once the treatment is stopped, and whether it could be taken for a shorter period of time.”
Sir Stephen O’Rahilly, MD, MRC Metabolic Diseases Unit, University of Cambridge (England), pointed out that “GLP-1 is made by cells in the intestine and levels increase in the blood after a meal, providing some of the signal to the brain that tells us we are ‘full,’ ” so GLP-1 agonists have been studied as appetite suppressants, in addition to their approved use to treat type 2 diabetes.
Only about 4.5% of participants in STEP 1 stopped taking semaglutide because of gastrointestinal issues, he noted, although more participants in that group reported problems with gallstones, which can follow rapid weight loss.
And “unlike some previous appetite suppressant drugs which caused significant psychological and psychiatric side effects, there is no evidence that semaglutide has any adverse effects of that nature,” Dr. O’Rahilly noted.
In sum, he said, “this is the start of a new era for obesity drug development with the future direction being to achieve levels of weight loss comparable to semaglutide, while having fewer side effects.”
‘Pressing need’ to address obesity; semaglutide filed for obesity
There is a “pressing need” to address the worldwide increase in obesity and weight-related coexisting conditions, Dr. Ingelfinger and Dr. Rosen noted.
Sustained long-term weight loss with diet and exercise is challenging; behavioral weight-loss strategies “fail more often than not,” bariatric surgery is invasive and often followed by eventual weight regain, they wrote.
In addition, said Dr. Wilding and colleagues, the “use of available [weight-loss] medications remains limited by modest efficacy, safety concerns, and cost.”
Subcutaneous semaglutide, approved for treating type 2 diabetes (as Ozempic) in adults at doses of up to 1 mg/week, induced weight loss at higher doses. The current study is part of the global Semaglutide Treatment Effect in People With Obesity program of four trials (STEP 1, 2, 3, and 4) that aimed to test the safety and efficacy of subcutaneous semaglutide 2.4 mg/week for weight loss.
Topline results from STEP 1 were presented June 4, 2020.
And as reported earlier, results from STEP 3 – a 68-week trial of semaglutide versus placebo in 611 participants who all received very intensive diet and exercise counseling – were presented at the virtual ObesityWeek 2020 meeting.
The four trials of semaglutide for weight loss have been completed and the data were submitted to the Food and Drug Administration on Dec. 4, 2020 (with a decision expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
Most patients had 5% weight loss with semaglutide
The STEP 1 trial enrolled 1,961 adults with a body mass index (BMI) of at least 30 kg/m2 or at least 27 with at least one weight-related coexisting condition, but without type 2 diabetes, at 129 sites in 16 countries in Asia, Europe, North America, and South America.
Participants were a mean age of 47 and three-quarters were women. Most participants were White (76%), followed by Asian (13%), Black or African American (6%), or other (5%).
On average, they had a BMI of 38 and weighed 105 kg. Three-quarters had one or more coexisting conditions.
Participants were randomized to receive semaglutide (1,306 patients) or placebo (655 patients), added to lifestyle intervention.
Everyone received 17 monthly individual counseling sessions during which they learned about adhering to a diet with a 500-calorie/day deficit, were encouraged to build up to walking 150 minutes each week, and recorded their daily diet and exercise (in a diary or using an app).
Semaglutide was administered with a prefilled pen injector at a dose of 0.25 mg/week for the first 4 weeks, escalated to 2.4 mg/week by week 16 (or lower if the patient had unacceptable side effects).
At 68 weeks, participants in the semaglutide versus placebo group had greater mean weight loss (14.9% vs. 2.4%, or 15.3 kg vs. 2.6 kg).
Participants in the semaglutide versus placebo group were much more likely to have lost at least 5% of their initial weight (86% vs. 31.5%) or at least 10% of their initial weight (69.1% vs. 12.0%), or at least 15% of their initial weight (50.5% vs. 4.9%; P < .001 for all three comparisons).
About 80% of participants adhered to the study treatment. A third of participants in the semaglutide group who completed the study lost at least 20% of their initial weight, which approaches the 20%-30% reported weight loss 1-3 years after sleeve gastrectomy, the researchers noted.
Participants in the semaglutide group also had greater improvements in waist circumference and levels of hemoglobin A1c, C-reactive protein (a marker of inflammation), and fasting lipids, as well as in physical function scores on SF-36 and IWQOL-Lite-CT questionnaires.
In their editorial, Dr. Ingelfinger and Dr. Rosen noted that “daily oral semaglutide [already approved in 7-mg and 14-mg doses for the treatment of type 2 diabetes as Rybelsus] might be more appealing to many people,” as a weight-loss medication than a once-weekly subcutaneous dose. Semaglutide is the first GLP-1 agonist available as an oral agent.
The ongoing Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT) trial (with expected completion in 2023) will shed light on cardiovascular outcomes after 2.5-5 years.
GI disorders and ‘important limitations’
More participants in the semaglutide than the placebo group reported gastrointestinal disorders (typically nausea, diarrhea, vomiting, and constipation; 74.2% vs. 47.9%), which were mostly transient and mild to moderate in severity, but also led to more treatment discontinuation (7.0% vs. 3.1%).
More patients in the semaglutide versus placebo group had a gall bladder–related disorder (2.6% vs. 1.2%, mostly cholelithiasis) and mild acute pancreatitis (3 vs. 0 participants), but there were no between-group differences in neoplasms.
Dr. Wilding and colleagues acknowledge the limitations of the study, including the fact that it enrolled mainly women, mainly non-White participants, was relatively short, and excluded patients with type 2 diabetes.
Mean placebo-corrected weight loss with 2.4 mg/weekly subcutaneous semaglutide was greater than with 3.0 mg once-daily subcutaneous liraglutide (Saxenda, Novo Nordisk) – the only GLP-1 agonist approved for weight management – in the 56-week SCALE trial (12.4% vs. 4.5%); however, the two studies had different populations.
The study was supported by Novo Nordisk. Dr. Ingelfinger is a deputy editor and Dr. Rosen is an associate editor of the New England Journal of Medicine. Dr. Ingelfinger, Dr. Rosen, and Dr. Leurent have reported no relevant financial relationships. Dr. O’Rahilly has a current research collaboration with Novo Nordisk scientists in an unrelated area and has been a consultant for the company.
A version of this article first appeared on Medscape.com.
The phase 3a STEP 1 trial that investigated the use of semaglutide (Novo Nordisk), a glucagonlike peptide–1 (GLP-1) agonist, for weight loss is aptly named, some say.
“In sum, we have a long way to go to control the obesity epidemic ... but on the face of it, the STEP 1 trial (like its name) is a good beginning,” wrote coeditorialists Julie R. Ingelfinger, MD, from Harvard Medical School, Boston, and a deputy editor of the New England Journal of Medicine, and Clifford J. Rosen, MD, from Tufts University School of Medicine, also in Boston.
The trial findings by John P.H. Wilding, DM, University of Liverpool (England), and colleagues and an accompanying editorial were published online Feb. 10, 2021, in the New England Journal of Medicine.
“The results are encouraging, with significantly more patients in the semaglutide group having clinically important weight loss,” Dr. Ingelfinger and Dr. Rosen stressed.
However, they also cautioned that “despite the positive results of this trial, the present study has some important limitations” and “there are concerns, including adverse events (mostly gastrointestinal – nausea, sometimes vomiting, and diarrhea) related primarily to the class of the agent.”
Two U.K. experts drew similar takeaways, speaking to the U.K. Science Media Centre.
“This was a well-designed study with unequivocal findings,” which showed that semaglutide “is indeed likely to be a game-changer in the fight against obesity,” according to Baptiste Leurent, PhD, London School of Hygiene and Tropical Medicine.
However, if the drug is approved at this dose for this use, patients would need close monitoring for gastrointestinal disorders, and “we also need to better understand what is happening once the treatment is stopped, and whether it could be taken for a shorter period of time.”
Sir Stephen O’Rahilly, MD, MRC Metabolic Diseases Unit, University of Cambridge (England), pointed out that “GLP-1 is made by cells in the intestine and levels increase in the blood after a meal, providing some of the signal to the brain that tells us we are ‘full,’ ” so GLP-1 agonists have been studied as appetite suppressants, in addition to their approved use to treat type 2 diabetes.
Only about 4.5% of participants in STEP 1 stopped taking semaglutide because of gastrointestinal issues, he noted, although more participants in that group reported problems with gallstones, which can follow rapid weight loss.
And “unlike some previous appetite suppressant drugs which caused significant psychological and psychiatric side effects, there is no evidence that semaglutide has any adverse effects of that nature,” Dr. O’Rahilly noted.
In sum, he said, “this is the start of a new era for obesity drug development with the future direction being to achieve levels of weight loss comparable to semaglutide, while having fewer side effects.”
‘Pressing need’ to address obesity; semaglutide filed for obesity
There is a “pressing need” to address the worldwide increase in obesity and weight-related coexisting conditions, Dr. Ingelfinger and Dr. Rosen noted.
Sustained long-term weight loss with diet and exercise is challenging; behavioral weight-loss strategies “fail more often than not,” bariatric surgery is invasive and often followed by eventual weight regain, they wrote.
In addition, said Dr. Wilding and colleagues, the “use of available [weight-loss] medications remains limited by modest efficacy, safety concerns, and cost.”
Subcutaneous semaglutide, approved for treating type 2 diabetes (as Ozempic) in adults at doses of up to 1 mg/week, induced weight loss at higher doses. The current study is part of the global Semaglutide Treatment Effect in People With Obesity program of four trials (STEP 1, 2, 3, and 4) that aimed to test the safety and efficacy of subcutaneous semaglutide 2.4 mg/week for weight loss.
Topline results from STEP 1 were presented June 4, 2020.
And as reported earlier, results from STEP 3 – a 68-week trial of semaglutide versus placebo in 611 participants who all received very intensive diet and exercise counseling – were presented at the virtual ObesityWeek 2020 meeting.
The four trials of semaglutide for weight loss have been completed and the data were submitted to the Food and Drug Administration on Dec. 4, 2020 (with a decision expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
Most patients had 5% weight loss with semaglutide
The STEP 1 trial enrolled 1,961 adults with a body mass index (BMI) of at least 30 kg/m2 or at least 27 with at least one weight-related coexisting condition, but without type 2 diabetes, at 129 sites in 16 countries in Asia, Europe, North America, and South America.
Participants were a mean age of 47 and three-quarters were women. Most participants were White (76%), followed by Asian (13%), Black or African American (6%), or other (5%).
On average, they had a BMI of 38 and weighed 105 kg. Three-quarters had one or more coexisting conditions.
Participants were randomized to receive semaglutide (1,306 patients) or placebo (655 patients), added to lifestyle intervention.
Everyone received 17 monthly individual counseling sessions during which they learned about adhering to a diet with a 500-calorie/day deficit, were encouraged to build up to walking 150 minutes each week, and recorded their daily diet and exercise (in a diary or using an app).
Semaglutide was administered with a prefilled pen injector at a dose of 0.25 mg/week for the first 4 weeks, escalated to 2.4 mg/week by week 16 (or lower if the patient had unacceptable side effects).
At 68 weeks, participants in the semaglutide versus placebo group had greater mean weight loss (14.9% vs. 2.4%, or 15.3 kg vs. 2.6 kg).
Participants in the semaglutide versus placebo group were much more likely to have lost at least 5% of their initial weight (86% vs. 31.5%) or at least 10% of their initial weight (69.1% vs. 12.0%), or at least 15% of their initial weight (50.5% vs. 4.9%; P < .001 for all three comparisons).
About 80% of participants adhered to the study treatment. A third of participants in the semaglutide group who completed the study lost at least 20% of their initial weight, which approaches the 20%-30% reported weight loss 1-3 years after sleeve gastrectomy, the researchers noted.
Participants in the semaglutide group also had greater improvements in waist circumference and levels of hemoglobin A1c, C-reactive protein (a marker of inflammation), and fasting lipids, as well as in physical function scores on SF-36 and IWQOL-Lite-CT questionnaires.
In their editorial, Dr. Ingelfinger and Dr. Rosen noted that “daily oral semaglutide [already approved in 7-mg and 14-mg doses for the treatment of type 2 diabetes as Rybelsus] might be more appealing to many people,” as a weight-loss medication than a once-weekly subcutaneous dose. Semaglutide is the first GLP-1 agonist available as an oral agent.
The ongoing Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT) trial (with expected completion in 2023) will shed light on cardiovascular outcomes after 2.5-5 years.
GI disorders and ‘important limitations’
More participants in the semaglutide than the placebo group reported gastrointestinal disorders (typically nausea, diarrhea, vomiting, and constipation; 74.2% vs. 47.9%), which were mostly transient and mild to moderate in severity, but also led to more treatment discontinuation (7.0% vs. 3.1%).
More patients in the semaglutide versus placebo group had a gall bladder–related disorder (2.6% vs. 1.2%, mostly cholelithiasis) and mild acute pancreatitis (3 vs. 0 participants), but there were no between-group differences in neoplasms.
Dr. Wilding and colleagues acknowledge the limitations of the study, including the fact that it enrolled mainly women, mainly non-White participants, was relatively short, and excluded patients with type 2 diabetes.
Mean placebo-corrected weight loss with 2.4 mg/weekly subcutaneous semaglutide was greater than with 3.0 mg once-daily subcutaneous liraglutide (Saxenda, Novo Nordisk) – the only GLP-1 agonist approved for weight management – in the 56-week SCALE trial (12.4% vs. 4.5%); however, the two studies had different populations.
The study was supported by Novo Nordisk. Dr. Ingelfinger is a deputy editor and Dr. Rosen is an associate editor of the New England Journal of Medicine. Dr. Ingelfinger, Dr. Rosen, and Dr. Leurent have reported no relevant financial relationships. Dr. O’Rahilly has a current research collaboration with Novo Nordisk scientists in an unrelated area and has been a consultant for the company.
A version of this article first appeared on Medscape.com.
The phase 3a STEP 1 trial that investigated the use of semaglutide (Novo Nordisk), a glucagonlike peptide–1 (GLP-1) agonist, for weight loss is aptly named, some say.
“In sum, we have a long way to go to control the obesity epidemic ... but on the face of it, the STEP 1 trial (like its name) is a good beginning,” wrote coeditorialists Julie R. Ingelfinger, MD, from Harvard Medical School, Boston, and a deputy editor of the New England Journal of Medicine, and Clifford J. Rosen, MD, from Tufts University School of Medicine, also in Boston.
The trial findings by John P.H. Wilding, DM, University of Liverpool (England), and colleagues and an accompanying editorial were published online Feb. 10, 2021, in the New England Journal of Medicine.
“The results are encouraging, with significantly more patients in the semaglutide group having clinically important weight loss,” Dr. Ingelfinger and Dr. Rosen stressed.
However, they also cautioned that “despite the positive results of this trial, the present study has some important limitations” and “there are concerns, including adverse events (mostly gastrointestinal – nausea, sometimes vomiting, and diarrhea) related primarily to the class of the agent.”
Two U.K. experts drew similar takeaways, speaking to the U.K. Science Media Centre.
“This was a well-designed study with unequivocal findings,” which showed that semaglutide “is indeed likely to be a game-changer in the fight against obesity,” according to Baptiste Leurent, PhD, London School of Hygiene and Tropical Medicine.
However, if the drug is approved at this dose for this use, patients would need close monitoring for gastrointestinal disorders, and “we also need to better understand what is happening once the treatment is stopped, and whether it could be taken for a shorter period of time.”
Sir Stephen O’Rahilly, MD, MRC Metabolic Diseases Unit, University of Cambridge (England), pointed out that “GLP-1 is made by cells in the intestine and levels increase in the blood after a meal, providing some of the signal to the brain that tells us we are ‘full,’ ” so GLP-1 agonists have been studied as appetite suppressants, in addition to their approved use to treat type 2 diabetes.
Only about 4.5% of participants in STEP 1 stopped taking semaglutide because of gastrointestinal issues, he noted, although more participants in that group reported problems with gallstones, which can follow rapid weight loss.
And “unlike some previous appetite suppressant drugs which caused significant psychological and psychiatric side effects, there is no evidence that semaglutide has any adverse effects of that nature,” Dr. O’Rahilly noted.
In sum, he said, “this is the start of a new era for obesity drug development with the future direction being to achieve levels of weight loss comparable to semaglutide, while having fewer side effects.”
‘Pressing need’ to address obesity; semaglutide filed for obesity
There is a “pressing need” to address the worldwide increase in obesity and weight-related coexisting conditions, Dr. Ingelfinger and Dr. Rosen noted.
Sustained long-term weight loss with diet and exercise is challenging; behavioral weight-loss strategies “fail more often than not,” bariatric surgery is invasive and often followed by eventual weight regain, they wrote.
In addition, said Dr. Wilding and colleagues, the “use of available [weight-loss] medications remains limited by modest efficacy, safety concerns, and cost.”
Subcutaneous semaglutide, approved for treating type 2 diabetes (as Ozempic) in adults at doses of up to 1 mg/week, induced weight loss at higher doses. The current study is part of the global Semaglutide Treatment Effect in People With Obesity program of four trials (STEP 1, 2, 3, and 4) that aimed to test the safety and efficacy of subcutaneous semaglutide 2.4 mg/week for weight loss.
Topline results from STEP 1 were presented June 4, 2020.
And as reported earlier, results from STEP 3 – a 68-week trial of semaglutide versus placebo in 611 participants who all received very intensive diet and exercise counseling – were presented at the virtual ObesityWeek 2020 meeting.
The four trials of semaglutide for weight loss have been completed and the data were submitted to the Food and Drug Administration on Dec. 4, 2020 (with a decision expected within 6 months) and to the European Medicines Agency on Dec. 18, 2020.
Most patients had 5% weight loss with semaglutide
The STEP 1 trial enrolled 1,961 adults with a body mass index (BMI) of at least 30 kg/m2 or at least 27 with at least one weight-related coexisting condition, but without type 2 diabetes, at 129 sites in 16 countries in Asia, Europe, North America, and South America.
Participants were a mean age of 47 and three-quarters were women. Most participants were White (76%), followed by Asian (13%), Black or African American (6%), or other (5%).
On average, they had a BMI of 38 and weighed 105 kg. Three-quarters had one or more coexisting conditions.
Participants were randomized to receive semaglutide (1,306 patients) or placebo (655 patients), added to lifestyle intervention.
Everyone received 17 monthly individual counseling sessions during which they learned about adhering to a diet with a 500-calorie/day deficit, were encouraged to build up to walking 150 minutes each week, and recorded their daily diet and exercise (in a diary or using an app).
Semaglutide was administered with a prefilled pen injector at a dose of 0.25 mg/week for the first 4 weeks, escalated to 2.4 mg/week by week 16 (or lower if the patient had unacceptable side effects).
At 68 weeks, participants in the semaglutide versus placebo group had greater mean weight loss (14.9% vs. 2.4%, or 15.3 kg vs. 2.6 kg).
Participants in the semaglutide versus placebo group were much more likely to have lost at least 5% of their initial weight (86% vs. 31.5%) or at least 10% of their initial weight (69.1% vs. 12.0%), or at least 15% of their initial weight (50.5% vs. 4.9%; P < .001 for all three comparisons).
About 80% of participants adhered to the study treatment. A third of participants in the semaglutide group who completed the study lost at least 20% of their initial weight, which approaches the 20%-30% reported weight loss 1-3 years after sleeve gastrectomy, the researchers noted.
Participants in the semaglutide group also had greater improvements in waist circumference and levels of hemoglobin A1c, C-reactive protein (a marker of inflammation), and fasting lipids, as well as in physical function scores on SF-36 and IWQOL-Lite-CT questionnaires.
In their editorial, Dr. Ingelfinger and Dr. Rosen noted that “daily oral semaglutide [already approved in 7-mg and 14-mg doses for the treatment of type 2 diabetes as Rybelsus] might be more appealing to many people,” as a weight-loss medication than a once-weekly subcutaneous dose. Semaglutide is the first GLP-1 agonist available as an oral agent.
The ongoing Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity (SELECT) trial (with expected completion in 2023) will shed light on cardiovascular outcomes after 2.5-5 years.
GI disorders and ‘important limitations’
More participants in the semaglutide than the placebo group reported gastrointestinal disorders (typically nausea, diarrhea, vomiting, and constipation; 74.2% vs. 47.9%), which were mostly transient and mild to moderate in severity, but also led to more treatment discontinuation (7.0% vs. 3.1%).
More patients in the semaglutide versus placebo group had a gall bladder–related disorder (2.6% vs. 1.2%, mostly cholelithiasis) and mild acute pancreatitis (3 vs. 0 participants), but there were no between-group differences in neoplasms.
Dr. Wilding and colleagues acknowledge the limitations of the study, including the fact that it enrolled mainly women, mainly non-White participants, was relatively short, and excluded patients with type 2 diabetes.
Mean placebo-corrected weight loss with 2.4 mg/weekly subcutaneous semaglutide was greater than with 3.0 mg once-daily subcutaneous liraglutide (Saxenda, Novo Nordisk) – the only GLP-1 agonist approved for weight management – in the 56-week SCALE trial (12.4% vs. 4.5%); however, the two studies had different populations.
The study was supported by Novo Nordisk. Dr. Ingelfinger is a deputy editor and Dr. Rosen is an associate editor of the New England Journal of Medicine. Dr. Ingelfinger, Dr. Rosen, and Dr. Leurent have reported no relevant financial relationships. Dr. O’Rahilly has a current research collaboration with Novo Nordisk scientists in an unrelated area and has been a consultant for the company.
A version of this article first appeared on Medscape.com.
FDA approves orphan drug evinacumab-dgnb for homozygous FH
The Food and Drug Administration has approved the fully human monoclonal antibody evinacumab-dgnb (Evkeeza, Regeneron Pharmaceuticals) for use on top of other cholesterol-modifying medication in patients aged 12 years and older with homozygous familial hypercholesterolemia (HoFH), the agency and Regeneron have announced.
Evinacumab had received orphan drug designation and underwent priority regulatory review based primarily on the phase 3 ELIPSE trial, presented at a meeting in March 2020 and published in August 2020 in the New England Journal of Medicine (doi: 10.1056/NEJMoa2004215).
In the trial with 65 patients with HoFH on guideline-based lipid-modifying therapy, those who also received evinacumab 15 mg/kg intravenously every 4 weeks showed a nearly 50% drop in LDL cholesterol levels after 24 weeks, compared with patients given a placebo. Only 2% of patients in both groups discontinued therapy because of adverse reactions.
The drug blocks angiopoietin-like 3, itself an inhibitor of lipoprotein lipase and endothelial lipase. It therefore lowers LDL cholesterol levels by mechanisms that don’t directly involve the LDL receptor.
Regeneron estimates that about 1300 people in the United States have the homozygous genetic disorder, which can lead to LDL cholesterol levels of a 1,000 mg/dL or higher, advanced premature atherosclerosis, and extreme risk for cardiovascular events.
The drug’s average wholesale acquisition cost per patient in the United States is expected to be about $450,000 per year, the company said, adding that it has a financial support program to help qualified patients with out-of-pocket costs.
Regeneron’s announcement included a comment from dyslipidemia-therapy expert Daniel J. Rader, MD, University of Pennsylvania, Philadelphia, who called evinacumab “a potentially transformational new treatment for people with HoFH.”
The drug is currently under regulatory review for the same indication in Europe, the company said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the fully human monoclonal antibody evinacumab-dgnb (Evkeeza, Regeneron Pharmaceuticals) for use on top of other cholesterol-modifying medication in patients aged 12 years and older with homozygous familial hypercholesterolemia (HoFH), the agency and Regeneron have announced.
Evinacumab had received orphan drug designation and underwent priority regulatory review based primarily on the phase 3 ELIPSE trial, presented at a meeting in March 2020 and published in August 2020 in the New England Journal of Medicine (doi: 10.1056/NEJMoa2004215).
In the trial with 65 patients with HoFH on guideline-based lipid-modifying therapy, those who also received evinacumab 15 mg/kg intravenously every 4 weeks showed a nearly 50% drop in LDL cholesterol levels after 24 weeks, compared with patients given a placebo. Only 2% of patients in both groups discontinued therapy because of adverse reactions.
The drug blocks angiopoietin-like 3, itself an inhibitor of lipoprotein lipase and endothelial lipase. It therefore lowers LDL cholesterol levels by mechanisms that don’t directly involve the LDL receptor.
Regeneron estimates that about 1300 people in the United States have the homozygous genetic disorder, which can lead to LDL cholesterol levels of a 1,000 mg/dL or higher, advanced premature atherosclerosis, and extreme risk for cardiovascular events.
The drug’s average wholesale acquisition cost per patient in the United States is expected to be about $450,000 per year, the company said, adding that it has a financial support program to help qualified patients with out-of-pocket costs.
Regeneron’s announcement included a comment from dyslipidemia-therapy expert Daniel J. Rader, MD, University of Pennsylvania, Philadelphia, who called evinacumab “a potentially transformational new treatment for people with HoFH.”
The drug is currently under regulatory review for the same indication in Europe, the company said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the fully human monoclonal antibody evinacumab-dgnb (Evkeeza, Regeneron Pharmaceuticals) for use on top of other cholesterol-modifying medication in patients aged 12 years and older with homozygous familial hypercholesterolemia (HoFH), the agency and Regeneron have announced.
Evinacumab had received orphan drug designation and underwent priority regulatory review based primarily on the phase 3 ELIPSE trial, presented at a meeting in March 2020 and published in August 2020 in the New England Journal of Medicine (doi: 10.1056/NEJMoa2004215).
In the trial with 65 patients with HoFH on guideline-based lipid-modifying therapy, those who also received evinacumab 15 mg/kg intravenously every 4 weeks showed a nearly 50% drop in LDL cholesterol levels after 24 weeks, compared with patients given a placebo. Only 2% of patients in both groups discontinued therapy because of adverse reactions.
The drug blocks angiopoietin-like 3, itself an inhibitor of lipoprotein lipase and endothelial lipase. It therefore lowers LDL cholesterol levels by mechanisms that don’t directly involve the LDL receptor.
Regeneron estimates that about 1300 people in the United States have the homozygous genetic disorder, which can lead to LDL cholesterol levels of a 1,000 mg/dL or higher, advanced premature atherosclerosis, and extreme risk for cardiovascular events.
The drug’s average wholesale acquisition cost per patient in the United States is expected to be about $450,000 per year, the company said, adding that it has a financial support program to help qualified patients with out-of-pocket costs.
Regeneron’s announcement included a comment from dyslipidemia-therapy expert Daniel J. Rader, MD, University of Pennsylvania, Philadelphia, who called evinacumab “a potentially transformational new treatment for people with HoFH.”
The drug is currently under regulatory review for the same indication in Europe, the company said.
A version of this article first appeared on Medscape.com.
Steroid and immunoglobulin standard of care for MIS-C
The combination of methylprednisolone and intravenous immunoglobulins works better than intravenous immunoglobulins alone for multisystem inflammatory syndrome in children (MIS-C), researchers say.
“I’m not sure it’s the best treatment because we have not studied every possible treatment,” François Angoulvant, MD, PhD, told this news organization, “but right now, it’s the standard of care.”
Dr. Angoulvant, a professor of pediatrics at University of Paris, and colleagues published a comparison of the two treatments in the Journal of the American Medical Association.
A small percentage of children infected with SARS-CoV-2 develop MIS-C about 2 to 4 weeks later. It is considered a separate disease entity from COVID-19 and is associated with persistent fever, digestive symptoms, rash, bilateral nonpurulent conjunctivitis, mucocutaneous inflammation signs, and frequent cardiovascular involvement. In more than 60% of cases, it leads to hemodynamic failure, with acute cardiac dysfunction.
Because MIS-C resembles Kawasaki disease, clinicians modeled their treatment on that condition and started with immunoglobulins alone, Dr. Angoulvant said.
Based on expert opinion, the National Health Service in the United Kingdom published a consensus statement in Sept. listing immunoglobulins alone as the first-line treatment.
But anecdotal reports have emerged that combining the immunoglobulins with a corticosteroid worked better. To investigate this possibility, Dr. Angoulvant and colleagues analyzed records of MIS-C cases in France, where physicians are required to report all suspected cases of MIS-C to the French National Public Health Agency.
Among the 181 cases they scrutinized, 111 fulfilled the World Health Organization criteria for MIS-C. Of these, the researchers were able to match 64 patients who had received immunoglobulins alone with 32 who had received the combined therapy and could be matched using propensity scores.
The researchers defined treatment failure as persistence of fever for 2 days after the start of therapy or recurrence of fever within a week. By this measure, the combination treatment failed in only 9% of cases while immunoglobulins alone failed in 38% of cases. The difference was statistically significant (P = .008). Most of those for whom these treatments failed received second-line treatments such as steroids or biological agents.
Patients treated with the combination therapy also had a lower risk of secondary acute left ventricular dysfunction (odds ratio, 0.20; 95% confidence interval, 0.06-0.66) and a lower risk of needing hemodynamic support (OR, 0.21; 95% CI, 0.06-0.76).
Those receiving the combination therapy spent a mean of 4 days in the pediatric intensive care unit compared with 6 days for those receiving immunoglobulins alone. (Difference in days, −2.4; 95% CI, −4.0 to −0.7; P = .005).
There are few drawbacks to the combination approach, Dr. Angoulvant said, as the side effects of corticosteroids are generally not severe and they can be anticipated because this class of medications has been used for many years.
The study raises the question of whether corticosteroids might work as well by themselves, but it could not be answered with this database as no one is using that approach in France, Dr. Angoulvant said. “I hope other teams around the world could bring us the answer.”
In the United States, most physicians appear to already be using the combination therapy, said David Teachey, MD, an associate professor of pediatrics at the Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia.
The reduction in time in pediatric intensive care and the reduced risk of cardiac dysfunction are important findings, he said.
This retrospective study falls short of the evidence provided by a randomized clinical trial, Dr. Teachey noted. But he acknowledged that few families would agree to participate in such a trial as they would have to take a chance that the sick children would receive a less effective therapy than what they would otherwise get. “It’s hard to [talk] about a therapy reduction,” he told this news organization.
Given that impediment, he agreed with Dr. Angoulvant that the current study and others like it may provide the best data available pointing to a treatment approach for MIS-C.
The study received an unrestricted grant from Pfizer. The French COVID-19 Paediatric Inflammation Consortium received an unrestricted grant from the Square Foundation (Grandir–Fonds de Solidarité pour L’Enfance). Dr. Angoulvant and Dr. Teachey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of methylprednisolone and intravenous immunoglobulins works better than intravenous immunoglobulins alone for multisystem inflammatory syndrome in children (MIS-C), researchers say.
“I’m not sure it’s the best treatment because we have not studied every possible treatment,” François Angoulvant, MD, PhD, told this news organization, “but right now, it’s the standard of care.”
Dr. Angoulvant, a professor of pediatrics at University of Paris, and colleagues published a comparison of the two treatments in the Journal of the American Medical Association.
A small percentage of children infected with SARS-CoV-2 develop MIS-C about 2 to 4 weeks later. It is considered a separate disease entity from COVID-19 and is associated with persistent fever, digestive symptoms, rash, bilateral nonpurulent conjunctivitis, mucocutaneous inflammation signs, and frequent cardiovascular involvement. In more than 60% of cases, it leads to hemodynamic failure, with acute cardiac dysfunction.
Because MIS-C resembles Kawasaki disease, clinicians modeled their treatment on that condition and started with immunoglobulins alone, Dr. Angoulvant said.
Based on expert opinion, the National Health Service in the United Kingdom published a consensus statement in Sept. listing immunoglobulins alone as the first-line treatment.
But anecdotal reports have emerged that combining the immunoglobulins with a corticosteroid worked better. To investigate this possibility, Dr. Angoulvant and colleagues analyzed records of MIS-C cases in France, where physicians are required to report all suspected cases of MIS-C to the French National Public Health Agency.
Among the 181 cases they scrutinized, 111 fulfilled the World Health Organization criteria for MIS-C. Of these, the researchers were able to match 64 patients who had received immunoglobulins alone with 32 who had received the combined therapy and could be matched using propensity scores.
The researchers defined treatment failure as persistence of fever for 2 days after the start of therapy or recurrence of fever within a week. By this measure, the combination treatment failed in only 9% of cases while immunoglobulins alone failed in 38% of cases. The difference was statistically significant (P = .008). Most of those for whom these treatments failed received second-line treatments such as steroids or biological agents.
Patients treated with the combination therapy also had a lower risk of secondary acute left ventricular dysfunction (odds ratio, 0.20; 95% confidence interval, 0.06-0.66) and a lower risk of needing hemodynamic support (OR, 0.21; 95% CI, 0.06-0.76).
Those receiving the combination therapy spent a mean of 4 days in the pediatric intensive care unit compared with 6 days for those receiving immunoglobulins alone. (Difference in days, −2.4; 95% CI, −4.0 to −0.7; P = .005).
There are few drawbacks to the combination approach, Dr. Angoulvant said, as the side effects of corticosteroids are generally not severe and they can be anticipated because this class of medications has been used for many years.
The study raises the question of whether corticosteroids might work as well by themselves, but it could not be answered with this database as no one is using that approach in France, Dr. Angoulvant said. “I hope other teams around the world could bring us the answer.”
In the United States, most physicians appear to already be using the combination therapy, said David Teachey, MD, an associate professor of pediatrics at the Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia.
The reduction in time in pediatric intensive care and the reduced risk of cardiac dysfunction are important findings, he said.
This retrospective study falls short of the evidence provided by a randomized clinical trial, Dr. Teachey noted. But he acknowledged that few families would agree to participate in such a trial as they would have to take a chance that the sick children would receive a less effective therapy than what they would otherwise get. “It’s hard to [talk] about a therapy reduction,” he told this news organization.
Given that impediment, he agreed with Dr. Angoulvant that the current study and others like it may provide the best data available pointing to a treatment approach for MIS-C.
The study received an unrestricted grant from Pfizer. The French COVID-19 Paediatric Inflammation Consortium received an unrestricted grant from the Square Foundation (Grandir–Fonds de Solidarité pour L’Enfance). Dr. Angoulvant and Dr. Teachey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of methylprednisolone and intravenous immunoglobulins works better than intravenous immunoglobulins alone for multisystem inflammatory syndrome in children (MIS-C), researchers say.
“I’m not sure it’s the best treatment because we have not studied every possible treatment,” François Angoulvant, MD, PhD, told this news organization, “but right now, it’s the standard of care.”
Dr. Angoulvant, a professor of pediatrics at University of Paris, and colleagues published a comparison of the two treatments in the Journal of the American Medical Association.
A small percentage of children infected with SARS-CoV-2 develop MIS-C about 2 to 4 weeks later. It is considered a separate disease entity from COVID-19 and is associated with persistent fever, digestive symptoms, rash, bilateral nonpurulent conjunctivitis, mucocutaneous inflammation signs, and frequent cardiovascular involvement. In more than 60% of cases, it leads to hemodynamic failure, with acute cardiac dysfunction.
Because MIS-C resembles Kawasaki disease, clinicians modeled their treatment on that condition and started with immunoglobulins alone, Dr. Angoulvant said.
Based on expert opinion, the National Health Service in the United Kingdom published a consensus statement in Sept. listing immunoglobulins alone as the first-line treatment.
But anecdotal reports have emerged that combining the immunoglobulins with a corticosteroid worked better. To investigate this possibility, Dr. Angoulvant and colleagues analyzed records of MIS-C cases in France, where physicians are required to report all suspected cases of MIS-C to the French National Public Health Agency.
Among the 181 cases they scrutinized, 111 fulfilled the World Health Organization criteria for MIS-C. Of these, the researchers were able to match 64 patients who had received immunoglobulins alone with 32 who had received the combined therapy and could be matched using propensity scores.
The researchers defined treatment failure as persistence of fever for 2 days after the start of therapy or recurrence of fever within a week. By this measure, the combination treatment failed in only 9% of cases while immunoglobulins alone failed in 38% of cases. The difference was statistically significant (P = .008). Most of those for whom these treatments failed received second-line treatments such as steroids or biological agents.
Patients treated with the combination therapy also had a lower risk of secondary acute left ventricular dysfunction (odds ratio, 0.20; 95% confidence interval, 0.06-0.66) and a lower risk of needing hemodynamic support (OR, 0.21; 95% CI, 0.06-0.76).
Those receiving the combination therapy spent a mean of 4 days in the pediatric intensive care unit compared with 6 days for those receiving immunoglobulins alone. (Difference in days, −2.4; 95% CI, −4.0 to −0.7; P = .005).
There are few drawbacks to the combination approach, Dr. Angoulvant said, as the side effects of corticosteroids are generally not severe and they can be anticipated because this class of medications has been used for many years.
The study raises the question of whether corticosteroids might work as well by themselves, but it could not be answered with this database as no one is using that approach in France, Dr. Angoulvant said. “I hope other teams around the world could bring us the answer.”
In the United States, most physicians appear to already be using the combination therapy, said David Teachey, MD, an associate professor of pediatrics at the Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia.
The reduction in time in pediatric intensive care and the reduced risk of cardiac dysfunction are important findings, he said.
This retrospective study falls short of the evidence provided by a randomized clinical trial, Dr. Teachey noted. But he acknowledged that few families would agree to participate in such a trial as they would have to take a chance that the sick children would receive a less effective therapy than what they would otherwise get. “It’s hard to [talk] about a therapy reduction,” he told this news organization.
Given that impediment, he agreed with Dr. Angoulvant that the current study and others like it may provide the best data available pointing to a treatment approach for MIS-C.
The study received an unrestricted grant from Pfizer. The French COVID-19 Paediatric Inflammation Consortium received an unrestricted grant from the Square Foundation (Grandir–Fonds de Solidarité pour L’Enfance). Dr. Angoulvant and Dr. Teachey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Menopause transition affects heart health risks
Menopause is a key time to monitor women for the development or increase of cardiovascular risk factors, according to a new consensus statement developed by the Task Force on Gender of the European Society of Cardiology and a multidisciplinary ESC working group on Women’s Health in Menopause.
“After menopause, traditional cardiovascular risk factors are adversely affected – particularly hypertension,” wrote Angela H.E.M. Maas, MD, of Radboud University Medical Center, Nijmegen, Netherlands, and colleagues.
“Since the first ESC consensus paper on the management of cardiovascular risk in perimenopausal women was published in 2007, we have a greater understanding on the role of female-specific risk factors for cardiovascular disease (CVD),” they said.
In a consensus statement published in the European Heart Journal, the authors presented clinical guidance for diagnosis and management of cardiovascular risk factors during the menopause transition. The transition to menopause increases a woman’s risk for developing several CVD risk factors, including central adiposity, increased insulin resistance, a proatherogenic lipid profile, and autonomic dysfunction that can contribute to increased heart rate variability, according to the statement.
Estrogen changes may affect ischemic disease
In general, obstructive coronary artery disease (CAD) strikes women later than men, but coronary vasomotor conditions are a common cause of ischemic heart disease in women with or without CAD, the authors noted.
“Lower estrogen levels after menopause are related to altered vascular function, enhanced inflammation, and up-regulation of other hormonal systems such as the renin–angiotensin–aldosterone system, the sympathetic nervous system, and reduced nitric oxide–dependent vasodilation,” they wrote. They recommended use of the coronary artery calcium score for screening middle-aged women who are symptomatic or at intermediate cardiovascular risk.
The transition to menopause causes changes in lipid profiles, and a rise in blood pressure in particular “may be both a direct effect of hormonal changes on the vasculature and metabolic changes with aging,” but hypertension in early post menopause is “often poorly managed,” the authors noted.
Compared with asymptomatic women, women who suffer from severe menopausal symptoms often have increased cardiovascular disease risk factors. For example, the Women’s Health Initiative (WHI) study showed a 48% increased risk of incident diabetes at follow-up in women with severe symptoms of hot flashes and night sweats, the authors wrote. Clinicians should also be aware of the increased immune reactivity that occurs during and after menopause and the increased CVD risk associated with autoimmune and endocrine disorders, they said.
Multiple strategies to reduce risk
Strategies to address the cardiovascular risk in menopause include assessing glucose, lipid levels, and blood pressure during the transition to menopause, according to the statement.
In addition, they recommended increasing employer awareness of menopause, as changes may interfere with working ability. A healthy lifestyle including healthy diet and regular exercise can help reduce cardiovascular risks and relieve symptoms. Menopausal hormone therapy (MHT) may be indicated to relieve symptoms, including symptoms of depression, and provide cardioprotection for younger women around the time of menopause, according to the statement.
However, “MHT is not recommended in women at high CV risk and after a previous CVD event,” and all women should be assessed for cardiovascular risk factors before starting MHT, they emphasized.
Results raise awareness of cardiovascular health and menopause link
“Over the past 20 years, our knowledge of how menopause might contribute to cardiovascular disease has dramatically evolved,” said Samar El Khoudary, MD, of the University of Pittsburg, in an interview.
“We have accumulated data that consistently point to the menopause transition as a time of change in cardiovascular health. As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” she said. “The goal is to raise awareness for both health care providers and women of the significant adverse cardiovascular health changes accompanying the menopause transition and to point out the importance of adopting prevention strategies early during this stage,” she explained.
The impact of the hormonal changes of menopause on CVD risk “is very complex,” Dr. El Khoudary said. “Until now, we could not prove that using estrogen therapy is cardioprotective,” she emphasized. “Studies point to the need to consider the timing of hormone use, as well as types and route of administration,” she noted. “The truth is that, although the menopause transition is associated with an acceleration in CVD risk, the exact mechanism still is not completely clear. Hormone changes contribute, but they are not the ultimate contributor,” she added.
Research gaps include data on lifestyle and behavioral interventions
“Irrespective of the accumulating findings showing adverse changes in multiple cardiovascular health parameters, as women transition through menopause, we do not have data documenting current status of ideal cardiovascular health components during the menopause transition among women,” said Dr. El Khoudary. “The limited data we have [suggest] that a very small proportion of women transitioning through menopause eat a healthy diet (less than 20%) or practice physical activity (about7.2%) at a level that matches the current recommendations,” she noted.
“Lifestyle and behavioral interventions are critical to maintain a healthy heart and reduce heart disease; we do not have adequate randomized clinical trials testing these interventions specifically during the menopause transition,” she said.
“Similarly, we are in need of randomized clinical trials of therapeutic interventions such as lipid-lowering medications and menopause hormone therapy in women transitioning through menopause,” said Dr. El Khoudary. “This high-risk population has not been the focus of previous clinical trials, leaving us with questions of how the results from these studies might apply to women during the menopause transition,” she said.
Consensus invites collaboration
“I commend the group for putting together a statement that crosses practice and specialty boundaries,” said Lubna Pal, MD, of Yale School of Medicine, New Haven, Conn., in an interview. Although the statement does not present novel information, it “has the power of unifying the various providers by bringing focus on the individual elements spanning a woman’s life that cumulatively determine her lifetime health risk,” she said. Preeclampsia may be a risk factor for cardiovascular disease later in life, and events in reproductive age may determine a woman’s trajectory during the transition to menopause and beyond, Dr. Pal noted.
“The consensus statement will likely be read by internists and family medicine providers as well as ob.gyns.; it encourages all those involved in caring for female patients to take on the responsibility of ‘passing on the baton,’ such that all women who are deemed at an enhanced risk for cardiovascular disease are assured due diligence in care through stringent surveillance and timely interventions,” said Dr. Pal. “It is a call for the various providers who care for women at distinct stages of life to work together toward a shared goal of optimizing every woman’s health across her lifespan,” she said.
“More research is needed for us to better understand the mechanisms at play” in the development of cardiovascular risk and in understanding the continuity of changes across women’s lifespans, Dr. Pal said. “We have associations, but not much information about causation,” she emphasized. However, the statement promotes the dissemination of information about women’s health and sensitizes providers to the potential and the power of preventive care. “We should be much more liberal and loud in holding conversations about risk quantification and risk reduction, and this statement is a resounding effort toward identifying and mitigating long-term cardiovascular risk, even if only through promoting a healthier lifestyle in those deemed at risk,” she added.
The statement received no outside funding. Lead author Dr. Maas had no financial conflicts to disclose. Dr. El Khoudary had no financial conflicts to disclose. Dr. Pal had no relevant financial conflicts to disclose.
Menopause is a key time to monitor women for the development or increase of cardiovascular risk factors, according to a new consensus statement developed by the Task Force on Gender of the European Society of Cardiology and a multidisciplinary ESC working group on Women’s Health in Menopause.
“After menopause, traditional cardiovascular risk factors are adversely affected – particularly hypertension,” wrote Angela H.E.M. Maas, MD, of Radboud University Medical Center, Nijmegen, Netherlands, and colleagues.
“Since the first ESC consensus paper on the management of cardiovascular risk in perimenopausal women was published in 2007, we have a greater understanding on the role of female-specific risk factors for cardiovascular disease (CVD),” they said.
In a consensus statement published in the European Heart Journal, the authors presented clinical guidance for diagnosis and management of cardiovascular risk factors during the menopause transition. The transition to menopause increases a woman’s risk for developing several CVD risk factors, including central adiposity, increased insulin resistance, a proatherogenic lipid profile, and autonomic dysfunction that can contribute to increased heart rate variability, according to the statement.
Estrogen changes may affect ischemic disease
In general, obstructive coronary artery disease (CAD) strikes women later than men, but coronary vasomotor conditions are a common cause of ischemic heart disease in women with or without CAD, the authors noted.
“Lower estrogen levels after menopause are related to altered vascular function, enhanced inflammation, and up-regulation of other hormonal systems such as the renin–angiotensin–aldosterone system, the sympathetic nervous system, and reduced nitric oxide–dependent vasodilation,” they wrote. They recommended use of the coronary artery calcium score for screening middle-aged women who are symptomatic or at intermediate cardiovascular risk.
The transition to menopause causes changes in lipid profiles, and a rise in blood pressure in particular “may be both a direct effect of hormonal changes on the vasculature and metabolic changes with aging,” but hypertension in early post menopause is “often poorly managed,” the authors noted.
Compared with asymptomatic women, women who suffer from severe menopausal symptoms often have increased cardiovascular disease risk factors. For example, the Women’s Health Initiative (WHI) study showed a 48% increased risk of incident diabetes at follow-up in women with severe symptoms of hot flashes and night sweats, the authors wrote. Clinicians should also be aware of the increased immune reactivity that occurs during and after menopause and the increased CVD risk associated with autoimmune and endocrine disorders, they said.
Multiple strategies to reduce risk
Strategies to address the cardiovascular risk in menopause include assessing glucose, lipid levels, and blood pressure during the transition to menopause, according to the statement.
In addition, they recommended increasing employer awareness of menopause, as changes may interfere with working ability. A healthy lifestyle including healthy diet and regular exercise can help reduce cardiovascular risks and relieve symptoms. Menopausal hormone therapy (MHT) may be indicated to relieve symptoms, including symptoms of depression, and provide cardioprotection for younger women around the time of menopause, according to the statement.
However, “MHT is not recommended in women at high CV risk and after a previous CVD event,” and all women should be assessed for cardiovascular risk factors before starting MHT, they emphasized.
Results raise awareness of cardiovascular health and menopause link
“Over the past 20 years, our knowledge of how menopause might contribute to cardiovascular disease has dramatically evolved,” said Samar El Khoudary, MD, of the University of Pittsburg, in an interview.
“We have accumulated data that consistently point to the menopause transition as a time of change in cardiovascular health. As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” she said. “The goal is to raise awareness for both health care providers and women of the significant adverse cardiovascular health changes accompanying the menopause transition and to point out the importance of adopting prevention strategies early during this stage,” she explained.
The impact of the hormonal changes of menopause on CVD risk “is very complex,” Dr. El Khoudary said. “Until now, we could not prove that using estrogen therapy is cardioprotective,” she emphasized. “Studies point to the need to consider the timing of hormone use, as well as types and route of administration,” she noted. “The truth is that, although the menopause transition is associated with an acceleration in CVD risk, the exact mechanism still is not completely clear. Hormone changes contribute, but they are not the ultimate contributor,” she added.
Research gaps include data on lifestyle and behavioral interventions
“Irrespective of the accumulating findings showing adverse changes in multiple cardiovascular health parameters, as women transition through menopause, we do not have data documenting current status of ideal cardiovascular health components during the menopause transition among women,” said Dr. El Khoudary. “The limited data we have [suggest] that a very small proportion of women transitioning through menopause eat a healthy diet (less than 20%) or practice physical activity (about7.2%) at a level that matches the current recommendations,” she noted.
“Lifestyle and behavioral interventions are critical to maintain a healthy heart and reduce heart disease; we do not have adequate randomized clinical trials testing these interventions specifically during the menopause transition,” she said.
“Similarly, we are in need of randomized clinical trials of therapeutic interventions such as lipid-lowering medications and menopause hormone therapy in women transitioning through menopause,” said Dr. El Khoudary. “This high-risk population has not been the focus of previous clinical trials, leaving us with questions of how the results from these studies might apply to women during the menopause transition,” she said.
Consensus invites collaboration
“I commend the group for putting together a statement that crosses practice and specialty boundaries,” said Lubna Pal, MD, of Yale School of Medicine, New Haven, Conn., in an interview. Although the statement does not present novel information, it “has the power of unifying the various providers by bringing focus on the individual elements spanning a woman’s life that cumulatively determine her lifetime health risk,” she said. Preeclampsia may be a risk factor for cardiovascular disease later in life, and events in reproductive age may determine a woman’s trajectory during the transition to menopause and beyond, Dr. Pal noted.
“The consensus statement will likely be read by internists and family medicine providers as well as ob.gyns.; it encourages all those involved in caring for female patients to take on the responsibility of ‘passing on the baton,’ such that all women who are deemed at an enhanced risk for cardiovascular disease are assured due diligence in care through stringent surveillance and timely interventions,” said Dr. Pal. “It is a call for the various providers who care for women at distinct stages of life to work together toward a shared goal of optimizing every woman’s health across her lifespan,” she said.
“More research is needed for us to better understand the mechanisms at play” in the development of cardiovascular risk and in understanding the continuity of changes across women’s lifespans, Dr. Pal said. “We have associations, but not much information about causation,” she emphasized. However, the statement promotes the dissemination of information about women’s health and sensitizes providers to the potential and the power of preventive care. “We should be much more liberal and loud in holding conversations about risk quantification and risk reduction, and this statement is a resounding effort toward identifying and mitigating long-term cardiovascular risk, even if only through promoting a healthier lifestyle in those deemed at risk,” she added.
The statement received no outside funding. Lead author Dr. Maas had no financial conflicts to disclose. Dr. El Khoudary had no financial conflicts to disclose. Dr. Pal had no relevant financial conflicts to disclose.
Menopause is a key time to monitor women for the development or increase of cardiovascular risk factors, according to a new consensus statement developed by the Task Force on Gender of the European Society of Cardiology and a multidisciplinary ESC working group on Women’s Health in Menopause.
“After menopause, traditional cardiovascular risk factors are adversely affected – particularly hypertension,” wrote Angela H.E.M. Maas, MD, of Radboud University Medical Center, Nijmegen, Netherlands, and colleagues.
“Since the first ESC consensus paper on the management of cardiovascular risk in perimenopausal women was published in 2007, we have a greater understanding on the role of female-specific risk factors for cardiovascular disease (CVD),” they said.
In a consensus statement published in the European Heart Journal, the authors presented clinical guidance for diagnosis and management of cardiovascular risk factors during the menopause transition. The transition to menopause increases a woman’s risk for developing several CVD risk factors, including central adiposity, increased insulin resistance, a proatherogenic lipid profile, and autonomic dysfunction that can contribute to increased heart rate variability, according to the statement.
Estrogen changes may affect ischemic disease
In general, obstructive coronary artery disease (CAD) strikes women later than men, but coronary vasomotor conditions are a common cause of ischemic heart disease in women with or without CAD, the authors noted.
“Lower estrogen levels after menopause are related to altered vascular function, enhanced inflammation, and up-regulation of other hormonal systems such as the renin–angiotensin–aldosterone system, the sympathetic nervous system, and reduced nitric oxide–dependent vasodilation,” they wrote. They recommended use of the coronary artery calcium score for screening middle-aged women who are symptomatic or at intermediate cardiovascular risk.
The transition to menopause causes changes in lipid profiles, and a rise in blood pressure in particular “may be both a direct effect of hormonal changes on the vasculature and metabolic changes with aging,” but hypertension in early post menopause is “often poorly managed,” the authors noted.
Compared with asymptomatic women, women who suffer from severe menopausal symptoms often have increased cardiovascular disease risk factors. For example, the Women’s Health Initiative (WHI) study showed a 48% increased risk of incident diabetes at follow-up in women with severe symptoms of hot flashes and night sweats, the authors wrote. Clinicians should also be aware of the increased immune reactivity that occurs during and after menopause and the increased CVD risk associated with autoimmune and endocrine disorders, they said.
Multiple strategies to reduce risk
Strategies to address the cardiovascular risk in menopause include assessing glucose, lipid levels, and blood pressure during the transition to menopause, according to the statement.
In addition, they recommended increasing employer awareness of menopause, as changes may interfere with working ability. A healthy lifestyle including healthy diet and regular exercise can help reduce cardiovascular risks and relieve symptoms. Menopausal hormone therapy (MHT) may be indicated to relieve symptoms, including symptoms of depression, and provide cardioprotection for younger women around the time of menopause, according to the statement.
However, “MHT is not recommended in women at high CV risk and after a previous CVD event,” and all women should be assessed for cardiovascular risk factors before starting MHT, they emphasized.
Results raise awareness of cardiovascular health and menopause link
“Over the past 20 years, our knowledge of how menopause might contribute to cardiovascular disease has dramatically evolved,” said Samar El Khoudary, MD, of the University of Pittsburg, in an interview.
“We have accumulated data that consistently point to the menopause transition as a time of change in cardiovascular health. As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” she said. “The goal is to raise awareness for both health care providers and women of the significant adverse cardiovascular health changes accompanying the menopause transition and to point out the importance of adopting prevention strategies early during this stage,” she explained.
The impact of the hormonal changes of menopause on CVD risk “is very complex,” Dr. El Khoudary said. “Until now, we could not prove that using estrogen therapy is cardioprotective,” she emphasized. “Studies point to the need to consider the timing of hormone use, as well as types and route of administration,” she noted. “The truth is that, although the menopause transition is associated with an acceleration in CVD risk, the exact mechanism still is not completely clear. Hormone changes contribute, but they are not the ultimate contributor,” she added.
Research gaps include data on lifestyle and behavioral interventions
“Irrespective of the accumulating findings showing adverse changes in multiple cardiovascular health parameters, as women transition through menopause, we do not have data documenting current status of ideal cardiovascular health components during the menopause transition among women,” said Dr. El Khoudary. “The limited data we have [suggest] that a very small proportion of women transitioning through menopause eat a healthy diet (less than 20%) or practice physical activity (about7.2%) at a level that matches the current recommendations,” she noted.
“Lifestyle and behavioral interventions are critical to maintain a healthy heart and reduce heart disease; we do not have adequate randomized clinical trials testing these interventions specifically during the menopause transition,” she said.
“Similarly, we are in need of randomized clinical trials of therapeutic interventions such as lipid-lowering medications and menopause hormone therapy in women transitioning through menopause,” said Dr. El Khoudary. “This high-risk population has not been the focus of previous clinical trials, leaving us with questions of how the results from these studies might apply to women during the menopause transition,” she said.
Consensus invites collaboration
“I commend the group for putting together a statement that crosses practice and specialty boundaries,” said Lubna Pal, MD, of Yale School of Medicine, New Haven, Conn., in an interview. Although the statement does not present novel information, it “has the power of unifying the various providers by bringing focus on the individual elements spanning a woman’s life that cumulatively determine her lifetime health risk,” she said. Preeclampsia may be a risk factor for cardiovascular disease later in life, and events in reproductive age may determine a woman’s trajectory during the transition to menopause and beyond, Dr. Pal noted.
“The consensus statement will likely be read by internists and family medicine providers as well as ob.gyns.; it encourages all those involved in caring for female patients to take on the responsibility of ‘passing on the baton,’ such that all women who are deemed at an enhanced risk for cardiovascular disease are assured due diligence in care through stringent surveillance and timely interventions,” said Dr. Pal. “It is a call for the various providers who care for women at distinct stages of life to work together toward a shared goal of optimizing every woman’s health across her lifespan,” she said.
“More research is needed for us to better understand the mechanisms at play” in the development of cardiovascular risk and in understanding the continuity of changes across women’s lifespans, Dr. Pal said. “We have associations, but not much information about causation,” she emphasized. However, the statement promotes the dissemination of information about women’s health and sensitizes providers to the potential and the power of preventive care. “We should be much more liberal and loud in holding conversations about risk quantification and risk reduction, and this statement is a resounding effort toward identifying and mitigating long-term cardiovascular risk, even if only through promoting a healthier lifestyle in those deemed at risk,” she added.
The statement received no outside funding. Lead author Dr. Maas had no financial conflicts to disclose. Dr. El Khoudary had no financial conflicts to disclose. Dr. Pal had no relevant financial conflicts to disclose.
FROM THE EUROPEAN HEART JOURNAL
2021 ACIP adult schedule released
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
PPE protected critical care staff from COVID-19 transmission
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
FROM CCC50
Women and ACS: Focus on typical symptoms to improve outcomes
There are some differences in how women relative to men report symptoms of an acute coronary syndrome (ACS), but they should not be permitted to get in the way of prompt diagnosis and treatment, according to an expert review at the virtual Going Back to the Heart of Cardiology meeting.
“We need to get away from the idea that symptoms of a myocardial infarction in women are atypical, because women are also having typical symptoms,” said Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix.
Sexes share key symptoms, but not treatment
Although “women are more likely to report additional symptoms,” chest pain “is pretty much equal between men and women” presenting with an ACS, according to Dr. Gulati.
There are several studies that have shown this, including the Variation in Recovery: Role of Gender on Outcomes of Young AMI patients (VIRGO). In VIRGO, which looked at ACS symptom presentation in younger patients (ages 18-55 years), 87.0% of women versus 89.5% of men presented with chest pain defined as pain, pressure, tightness, or discomfort.
Even among those who recognize that more women die of cardiovascular disease (CVD) disease than any other cause, nothing seems to erase the bias that women in an ED are less likely than men to be having a heart attack. About 60 million women in the United States have CVD, so no threat imposes a higher toll in morbidity and mortality.
In comparison, there are only about 3.5 million women with breast cancer. Even though this is a major cause of morbidity and mortality in women, it is dwarfed by CVD, according to statistics cited by Dr. Gulati. Yet, the data show women get inferior care by guideline-based standards.
“After a myocardial infarction, women relative to men are less likely to get aspirin or beta-blockers within 24 hours, they are less likely to undergo any type of invasive procedure, and they are less likely to meet the door-to-balloon time or receive any reperfusion therapy,” Dr. Gulati said. After a CVD event, “the only thing women do better is to die.”
Additional symptoms may muddy the diagnostic waters
In the setting of ACS, the problem is not that women fail to report symptoms that should lead clinicians to consider CVD, but that they report additional symptoms. For the clinician less inclined to consider CVD in women, particularly younger women, there is a greater risk of going down the wrong diagnostic pathway.
In other words, women report symptoms consistent with CVD, “but it is a question of whether we are hearing it,” Dr. Gulati said.
In the VIRGO study, 61.9% of women versus 54.8% of men (P < .001) presented three or more symptoms in addition to chest pain, such as epigastric symptoms, discomfort in the arms or neck, or palpitations. Women were more likely than men to attribute the symptoms to stress or anxiety (20.9% vs. 11.8%; P < .001), while less likely to consider them a result of muscle pain (15.4% vs. 21.2%; P = .029).
There are other gender differences for ACS. For example, women are more likely than men to presented ischemia without obstruction, but Dr. Gulati emphasized that lack of obstruction is not a reason to dismiss the potential for an underlying CV cause.
‘Yentl syndrome’ persists
“Women should not need to present exactly like men to be taken seriously,” she said, describing the “Yentl syndrome,” which now has its own Wikipedia page. A cardiovascular version of this syndrome was first described 30 years ago. Based on a movie of a woman who cross dresses in order to be allowed to undertake Jewish studies, the term captures the societal failure to adapt care for women who do not present disease the same way that men do.
Overall, inadequate urgency to pursue potential symptoms of ACS in women is just another manifestation of the “bikini approach to women’s health,” according to Dr. Gulati. This describes the focus on the breast and reproductive system to the exclusion or other organs and anatomy. Dr. Gulati speculated that this might be the reason that clinicians have failed to apply ACS guidelines to women with the same rigor that they apply to men.
This is hardly a new issue. Calls for improving cardiovascular care in women have been increasing in volume for more than past 20 years, but the issue has proven persistent, according to Dr. Gulati. As an example, she noted that the same types of gaps in care and in outcome reported in a 2008 registry study had not much changed in an article published 8 years later.
The solution is not complex, according to Dr. Gulati. In the ED, guideline-directed diagnostic tests should be offered to any man or woman, including younger women, who present with chest pain, ignoring gender bias that threatens misinterpretation of patient history and symptoms. Once CVD is diagnosed as promptly in women as it is in men, guideline-directed intervention would be expected to reduce the gender gap in outcomes.
“By applying standardized protocols, it will help us to the same for women as we do for men,” Dr. Gulati said.
The meeting was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
There are some differences in how women relative to men report symptoms of an acute coronary syndrome (ACS), but they should not be permitted to get in the way of prompt diagnosis and treatment, according to an expert review at the virtual Going Back to the Heart of Cardiology meeting.
“We need to get away from the idea that symptoms of a myocardial infarction in women are atypical, because women are also having typical symptoms,” said Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix.
Sexes share key symptoms, but not treatment
Although “women are more likely to report additional symptoms,” chest pain “is pretty much equal between men and women” presenting with an ACS, according to Dr. Gulati.
There are several studies that have shown this, including the Variation in Recovery: Role of Gender on Outcomes of Young AMI patients (VIRGO). In VIRGO, which looked at ACS symptom presentation in younger patients (ages 18-55 years), 87.0% of women versus 89.5% of men presented with chest pain defined as pain, pressure, tightness, or discomfort.
Even among those who recognize that more women die of cardiovascular disease (CVD) disease than any other cause, nothing seems to erase the bias that women in an ED are less likely than men to be having a heart attack. About 60 million women in the United States have CVD, so no threat imposes a higher toll in morbidity and mortality.
In comparison, there are only about 3.5 million women with breast cancer. Even though this is a major cause of morbidity and mortality in women, it is dwarfed by CVD, according to statistics cited by Dr. Gulati. Yet, the data show women get inferior care by guideline-based standards.
“After a myocardial infarction, women relative to men are less likely to get aspirin or beta-blockers within 24 hours, they are less likely to undergo any type of invasive procedure, and they are less likely to meet the door-to-balloon time or receive any reperfusion therapy,” Dr. Gulati said. After a CVD event, “the only thing women do better is to die.”
Additional symptoms may muddy the diagnostic waters
In the setting of ACS, the problem is not that women fail to report symptoms that should lead clinicians to consider CVD, but that they report additional symptoms. For the clinician less inclined to consider CVD in women, particularly younger women, there is a greater risk of going down the wrong diagnostic pathway.
In other words, women report symptoms consistent with CVD, “but it is a question of whether we are hearing it,” Dr. Gulati said.
In the VIRGO study, 61.9% of women versus 54.8% of men (P < .001) presented three or more symptoms in addition to chest pain, such as epigastric symptoms, discomfort in the arms or neck, or palpitations. Women were more likely than men to attribute the symptoms to stress or anxiety (20.9% vs. 11.8%; P < .001), while less likely to consider them a result of muscle pain (15.4% vs. 21.2%; P = .029).
There are other gender differences for ACS. For example, women are more likely than men to presented ischemia without obstruction, but Dr. Gulati emphasized that lack of obstruction is not a reason to dismiss the potential for an underlying CV cause.
‘Yentl syndrome’ persists
“Women should not need to present exactly like men to be taken seriously,” she said, describing the “Yentl syndrome,” which now has its own Wikipedia page. A cardiovascular version of this syndrome was first described 30 years ago. Based on a movie of a woman who cross dresses in order to be allowed to undertake Jewish studies, the term captures the societal failure to adapt care for women who do not present disease the same way that men do.
Overall, inadequate urgency to pursue potential symptoms of ACS in women is just another manifestation of the “bikini approach to women’s health,” according to Dr. Gulati. This describes the focus on the breast and reproductive system to the exclusion or other organs and anatomy. Dr. Gulati speculated that this might be the reason that clinicians have failed to apply ACS guidelines to women with the same rigor that they apply to men.
This is hardly a new issue. Calls for improving cardiovascular care in women have been increasing in volume for more than past 20 years, but the issue has proven persistent, according to Dr. Gulati. As an example, she noted that the same types of gaps in care and in outcome reported in a 2008 registry study had not much changed in an article published 8 years later.
The solution is not complex, according to Dr. Gulati. In the ED, guideline-directed diagnostic tests should be offered to any man or woman, including younger women, who present with chest pain, ignoring gender bias that threatens misinterpretation of patient history and symptoms. Once CVD is diagnosed as promptly in women as it is in men, guideline-directed intervention would be expected to reduce the gender gap in outcomes.
“By applying standardized protocols, it will help us to the same for women as we do for men,” Dr. Gulati said.
The meeting was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
There are some differences in how women relative to men report symptoms of an acute coronary syndrome (ACS), but they should not be permitted to get in the way of prompt diagnosis and treatment, according to an expert review at the virtual Going Back to the Heart of Cardiology meeting.
“We need to get away from the idea that symptoms of a myocardial infarction in women are atypical, because women are also having typical symptoms,” said Martha Gulati, MD, chief of cardiology at the University of Arizona, Phoenix.
Sexes share key symptoms, but not treatment
Although “women are more likely to report additional symptoms,” chest pain “is pretty much equal between men and women” presenting with an ACS, according to Dr. Gulati.
There are several studies that have shown this, including the Variation in Recovery: Role of Gender on Outcomes of Young AMI patients (VIRGO). In VIRGO, which looked at ACS symptom presentation in younger patients (ages 18-55 years), 87.0% of women versus 89.5% of men presented with chest pain defined as pain, pressure, tightness, or discomfort.
Even among those who recognize that more women die of cardiovascular disease (CVD) disease than any other cause, nothing seems to erase the bias that women in an ED are less likely than men to be having a heart attack. About 60 million women in the United States have CVD, so no threat imposes a higher toll in morbidity and mortality.
In comparison, there are only about 3.5 million women with breast cancer. Even though this is a major cause of morbidity and mortality in women, it is dwarfed by CVD, according to statistics cited by Dr. Gulati. Yet, the data show women get inferior care by guideline-based standards.
“After a myocardial infarction, women relative to men are less likely to get aspirin or beta-blockers within 24 hours, they are less likely to undergo any type of invasive procedure, and they are less likely to meet the door-to-balloon time or receive any reperfusion therapy,” Dr. Gulati said. After a CVD event, “the only thing women do better is to die.”
Additional symptoms may muddy the diagnostic waters
In the setting of ACS, the problem is not that women fail to report symptoms that should lead clinicians to consider CVD, but that they report additional symptoms. For the clinician less inclined to consider CVD in women, particularly younger women, there is a greater risk of going down the wrong diagnostic pathway.
In other words, women report symptoms consistent with CVD, “but it is a question of whether we are hearing it,” Dr. Gulati said.
In the VIRGO study, 61.9% of women versus 54.8% of men (P < .001) presented three or more symptoms in addition to chest pain, such as epigastric symptoms, discomfort in the arms or neck, or palpitations. Women were more likely than men to attribute the symptoms to stress or anxiety (20.9% vs. 11.8%; P < .001), while less likely to consider them a result of muscle pain (15.4% vs. 21.2%; P = .029).
There are other gender differences for ACS. For example, women are more likely than men to presented ischemia without obstruction, but Dr. Gulati emphasized that lack of obstruction is not a reason to dismiss the potential for an underlying CV cause.
‘Yentl syndrome’ persists
“Women should not need to present exactly like men to be taken seriously,” she said, describing the “Yentl syndrome,” which now has its own Wikipedia page. A cardiovascular version of this syndrome was first described 30 years ago. Based on a movie of a woman who cross dresses in order to be allowed to undertake Jewish studies, the term captures the societal failure to adapt care for women who do not present disease the same way that men do.
Overall, inadequate urgency to pursue potential symptoms of ACS in women is just another manifestation of the “bikini approach to women’s health,” according to Dr. Gulati. This describes the focus on the breast and reproductive system to the exclusion or other organs and anatomy. Dr. Gulati speculated that this might be the reason that clinicians have failed to apply ACS guidelines to women with the same rigor that they apply to men.
This is hardly a new issue. Calls for improving cardiovascular care in women have been increasing in volume for more than past 20 years, but the issue has proven persistent, according to Dr. Gulati. As an example, she noted that the same types of gaps in care and in outcome reported in a 2008 registry study had not much changed in an article published 8 years later.
The solution is not complex, according to Dr. Gulati. In the ED, guideline-directed diagnostic tests should be offered to any man or woman, including younger women, who present with chest pain, ignoring gender bias that threatens misinterpretation of patient history and symptoms. Once CVD is diagnosed as promptly in women as it is in men, guideline-directed intervention would be expected to reduce the gender gap in outcomes.
“By applying standardized protocols, it will help us to the same for women as we do for men,” Dr. Gulati said.
The meeting was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
FROM GOING BACK TO THE HEART OF CARDIOLOGY
Lifestyle coaching for obesity associated with improved cardiometabolic numbers in study
Patients who received intensive lifestyle training by coaches in the primary care setting experienced improvement in several indicators of cardiometabolic health in a 2-year trial.
The 803 trial participants comprised a racially diverse, low-income population with obesity. In this study, primary care clinics were randomly assigned to provide weight-loss coaching or usual care. Patients at the intensive training clinics lost significantly more weight than the other patients, as reported in a paper published in September in the New England Journal of Medicine on the PROmoting Successful Weight Loss in Primary CarE in Louisiana (PROPEL) trial. The patients who received weight loss coaching also had significantly more improvement in HDL cholesterol levels, total to HDL cholesterol ratios, and metabolic syndrome severity score, said researchers in the new paper on the PROPEL trial, which was published in Circulation on February 8 .
“We believe that one reason for success of the program was the use of a health coach [who] was embedded in the primary care office,” said lead author Peter Katzmarzyk, PhD, associate executive director for population and public health sciences at the Pennington Biomedical Research Center, Baton Rouge, La. “This way, the patients could get their counseling in a familiar environment and did not have to go to a different setting. The coaches developed close relationships with the patients over the 2 years, and this helped develop a sense of responsibility in the patients as the coaches were helping the patients to set goals and kept them accountable.”
In the PROPEL study, 67% of patients were Black and had low health literacy scores that corresponded with less than a ninth-grade education level. The intensive lifestyle intervention program included weekly sessions with the trained health coaches over the first 6 months — 16 face-to-face and 6 over the phone — and then at least monthly for the last 18 months. The coaches had higher education degrees in nutrition, physical activity, or behavioral medicine. Before the program started, the coaches also received training in the management of obesity and related health issues, health literacy, and patient communication and education. The goal of the program was 10% weight loss, using personalized action plans on eating, dieting, and physical activity.
Those in the usual-care clinics continued receiving normal care and received newsletters on health topics, such as the importance of sleep and tips for limiting time spent sitting. The primary care physicians at those clinics also were given a presentation with Centers for Medicare & Medicaid Services (CMS) information on intensive lifestyle interventions for obesity.
Cholesterol changes in intervention vs. control group
HDL cholesterol improved significantly among the coached patients, compared with the other patients, with a mean difference of 4.1 mg/dL at 1 year and 4.6 mg/dL at 2 years (P less than .01 for both). The total cholesterol to HDL cholesterol ratio showed a similarly significant difference in decline, with a between-group difference of –0.29 at 1 year and –0.31 at 2 years (P less than .01 for both). Also, the difference in the change in metabolic severity scores were –0.40 at 1 year and –0.21 at 2 years (P less than .01 for both).
Fasting blood glucose had declined after the 1st year by a significantly greater degree in the clinics with coaching, compared with the others, but not after the second year, researchers found.
There were no significant differences seen in total cholesterol, LDL cholesterol, non-HDL cholesterol, or blood pressure. Dr. Katzmarzyk said the likely reason for no change in blood pressure was that it was already relatively well-controlled at baseline for all the patients.
Funding barriers to obesity treatment
The CMS currently cover intensive training for obesity if delivered directly by a primary care physician, according to the authors of the new paper. Dr. Katzmarzyk said he hopes that will change.
“We are hoping that the evidence provided in this study may change the way that CMS funds obesity treatment in the future by allowing an expansion of the care team,” he said.
John Flack, MD, chair of internal medicine at Southern Illinois University, Springfield, said that the main achievement of the study was that it showed that intensive weight-loss training in the primary-care setting could be accomplished in a racially diverse population with low health literacy.
“You can’t just automatically assume just because you’ve seen it in some other populations that you can replicate this in every population, so they’ve done a really good job,” he said.
That programs are eligible for reimbursement only if they’re run by primary-care physicians is an ongoing problem, he said.
“You don’t necessarily need to be a physician to do this,” Dr. Flack said.
For best results, payment for coaching should not be tied to office visits, Dr. Flack noted.
“If they’re de-tethered from the office visits and you’re paid for quality ... you’re going to build out your infrastructure differently to care for people,” he said.
Andrew Freeman, MD, associate professor of medicine at the University of Colorado, Denver, and cochair of the American College of Cardiology’s nutrition and lifestyle work group, said the findings dovetail with his experience.
“I’m a huge believer that when people need to make lifestyle changes, having someone hold their hand and guide them through the effort is incredibly rewarding and incredibly powerful,” said Dr. Freeman, who also oversees the intensive cardiac rehab program at National Jewish Health in Denver.
A program like this needs proper funding in order to work, Dr, Freeman noted. He added that, even with coaches being paid well, “if you are able to prevent just one readmission for, say, heart failure a month . . . you could be saving millions of dollars over just a couple of years.”
Dr. Katzmarzyk, Dr. Flack, and Dr. Freeman reported no relevant disclosures. Louisiana State University, Pennington Biomedical Research Center, and Montclair State University have interest in the intellectual property surrounding a weight graph used in the study. The other researchers reported grants and/or fees from Bayer, Boehringer Ingelheim, Gilead, Takeda, Novo Nordisk, and other companies.
Patients who received intensive lifestyle training by coaches in the primary care setting experienced improvement in several indicators of cardiometabolic health in a 2-year trial.
The 803 trial participants comprised a racially diverse, low-income population with obesity. In this study, primary care clinics were randomly assigned to provide weight-loss coaching or usual care. Patients at the intensive training clinics lost significantly more weight than the other patients, as reported in a paper published in September in the New England Journal of Medicine on the PROmoting Successful Weight Loss in Primary CarE in Louisiana (PROPEL) trial. The patients who received weight loss coaching also had significantly more improvement in HDL cholesterol levels, total to HDL cholesterol ratios, and metabolic syndrome severity score, said researchers in the new paper on the PROPEL trial, which was published in Circulation on February 8 .
“We believe that one reason for success of the program was the use of a health coach [who] was embedded in the primary care office,” said lead author Peter Katzmarzyk, PhD, associate executive director for population and public health sciences at the Pennington Biomedical Research Center, Baton Rouge, La. “This way, the patients could get their counseling in a familiar environment and did not have to go to a different setting. The coaches developed close relationships with the patients over the 2 years, and this helped develop a sense of responsibility in the patients as the coaches were helping the patients to set goals and kept them accountable.”
In the PROPEL study, 67% of patients were Black and had low health literacy scores that corresponded with less than a ninth-grade education level. The intensive lifestyle intervention program included weekly sessions with the trained health coaches over the first 6 months — 16 face-to-face and 6 over the phone — and then at least monthly for the last 18 months. The coaches had higher education degrees in nutrition, physical activity, or behavioral medicine. Before the program started, the coaches also received training in the management of obesity and related health issues, health literacy, and patient communication and education. The goal of the program was 10% weight loss, using personalized action plans on eating, dieting, and physical activity.
Those in the usual-care clinics continued receiving normal care and received newsletters on health topics, such as the importance of sleep and tips for limiting time spent sitting. The primary care physicians at those clinics also were given a presentation with Centers for Medicare & Medicaid Services (CMS) information on intensive lifestyle interventions for obesity.
Cholesterol changes in intervention vs. control group
HDL cholesterol improved significantly among the coached patients, compared with the other patients, with a mean difference of 4.1 mg/dL at 1 year and 4.6 mg/dL at 2 years (P less than .01 for both). The total cholesterol to HDL cholesterol ratio showed a similarly significant difference in decline, with a between-group difference of –0.29 at 1 year and –0.31 at 2 years (P less than .01 for both). Also, the difference in the change in metabolic severity scores were –0.40 at 1 year and –0.21 at 2 years (P less than .01 for both).
Fasting blood glucose had declined after the 1st year by a significantly greater degree in the clinics with coaching, compared with the others, but not after the second year, researchers found.
There were no significant differences seen in total cholesterol, LDL cholesterol, non-HDL cholesterol, or blood pressure. Dr. Katzmarzyk said the likely reason for no change in blood pressure was that it was already relatively well-controlled at baseline for all the patients.
Funding barriers to obesity treatment
The CMS currently cover intensive training for obesity if delivered directly by a primary care physician, according to the authors of the new paper. Dr. Katzmarzyk said he hopes that will change.
“We are hoping that the evidence provided in this study may change the way that CMS funds obesity treatment in the future by allowing an expansion of the care team,” he said.
John Flack, MD, chair of internal medicine at Southern Illinois University, Springfield, said that the main achievement of the study was that it showed that intensive weight-loss training in the primary-care setting could be accomplished in a racially diverse population with low health literacy.
“You can’t just automatically assume just because you’ve seen it in some other populations that you can replicate this in every population, so they’ve done a really good job,” he said.
That programs are eligible for reimbursement only if they’re run by primary-care physicians is an ongoing problem, he said.
“You don’t necessarily need to be a physician to do this,” Dr. Flack said.
For best results, payment for coaching should not be tied to office visits, Dr. Flack noted.
“If they’re de-tethered from the office visits and you’re paid for quality ... you’re going to build out your infrastructure differently to care for people,” he said.
Andrew Freeman, MD, associate professor of medicine at the University of Colorado, Denver, and cochair of the American College of Cardiology’s nutrition and lifestyle work group, said the findings dovetail with his experience.
“I’m a huge believer that when people need to make lifestyle changes, having someone hold their hand and guide them through the effort is incredibly rewarding and incredibly powerful,” said Dr. Freeman, who also oversees the intensive cardiac rehab program at National Jewish Health in Denver.
A program like this needs proper funding in order to work, Dr, Freeman noted. He added that, even with coaches being paid well, “if you are able to prevent just one readmission for, say, heart failure a month . . . you could be saving millions of dollars over just a couple of years.”
Dr. Katzmarzyk, Dr. Flack, and Dr. Freeman reported no relevant disclosures. Louisiana State University, Pennington Biomedical Research Center, and Montclair State University have interest in the intellectual property surrounding a weight graph used in the study. The other researchers reported grants and/or fees from Bayer, Boehringer Ingelheim, Gilead, Takeda, Novo Nordisk, and other companies.
Patients who received intensive lifestyle training by coaches in the primary care setting experienced improvement in several indicators of cardiometabolic health in a 2-year trial.
The 803 trial participants comprised a racially diverse, low-income population with obesity. In this study, primary care clinics were randomly assigned to provide weight-loss coaching or usual care. Patients at the intensive training clinics lost significantly more weight than the other patients, as reported in a paper published in September in the New England Journal of Medicine on the PROmoting Successful Weight Loss in Primary CarE in Louisiana (PROPEL) trial. The patients who received weight loss coaching also had significantly more improvement in HDL cholesterol levels, total to HDL cholesterol ratios, and metabolic syndrome severity score, said researchers in the new paper on the PROPEL trial, which was published in Circulation on February 8 .
“We believe that one reason for success of the program was the use of a health coach [who] was embedded in the primary care office,” said lead author Peter Katzmarzyk, PhD, associate executive director for population and public health sciences at the Pennington Biomedical Research Center, Baton Rouge, La. “This way, the patients could get their counseling in a familiar environment and did not have to go to a different setting. The coaches developed close relationships with the patients over the 2 years, and this helped develop a sense of responsibility in the patients as the coaches were helping the patients to set goals and kept them accountable.”
In the PROPEL study, 67% of patients were Black and had low health literacy scores that corresponded with less than a ninth-grade education level. The intensive lifestyle intervention program included weekly sessions with the trained health coaches over the first 6 months — 16 face-to-face and 6 over the phone — and then at least monthly for the last 18 months. The coaches had higher education degrees in nutrition, physical activity, or behavioral medicine. Before the program started, the coaches also received training in the management of obesity and related health issues, health literacy, and patient communication and education. The goal of the program was 10% weight loss, using personalized action plans on eating, dieting, and physical activity.
Those in the usual-care clinics continued receiving normal care and received newsletters on health topics, such as the importance of sleep and tips for limiting time spent sitting. The primary care physicians at those clinics also were given a presentation with Centers for Medicare & Medicaid Services (CMS) information on intensive lifestyle interventions for obesity.
Cholesterol changes in intervention vs. control group
HDL cholesterol improved significantly among the coached patients, compared with the other patients, with a mean difference of 4.1 mg/dL at 1 year and 4.6 mg/dL at 2 years (P less than .01 for both). The total cholesterol to HDL cholesterol ratio showed a similarly significant difference in decline, with a between-group difference of –0.29 at 1 year and –0.31 at 2 years (P less than .01 for both). Also, the difference in the change in metabolic severity scores were –0.40 at 1 year and –0.21 at 2 years (P less than .01 for both).
Fasting blood glucose had declined after the 1st year by a significantly greater degree in the clinics with coaching, compared with the others, but not after the second year, researchers found.
There were no significant differences seen in total cholesterol, LDL cholesterol, non-HDL cholesterol, or blood pressure. Dr. Katzmarzyk said the likely reason for no change in blood pressure was that it was already relatively well-controlled at baseline for all the patients.
Funding barriers to obesity treatment
The CMS currently cover intensive training for obesity if delivered directly by a primary care physician, according to the authors of the new paper. Dr. Katzmarzyk said he hopes that will change.
“We are hoping that the evidence provided in this study may change the way that CMS funds obesity treatment in the future by allowing an expansion of the care team,” he said.
John Flack, MD, chair of internal medicine at Southern Illinois University, Springfield, said that the main achievement of the study was that it showed that intensive weight-loss training in the primary-care setting could be accomplished in a racially diverse population with low health literacy.
“You can’t just automatically assume just because you’ve seen it in some other populations that you can replicate this in every population, so they’ve done a really good job,” he said.
That programs are eligible for reimbursement only if they’re run by primary-care physicians is an ongoing problem, he said.
“You don’t necessarily need to be a physician to do this,” Dr. Flack said.
For best results, payment for coaching should not be tied to office visits, Dr. Flack noted.
“If they’re de-tethered from the office visits and you’re paid for quality ... you’re going to build out your infrastructure differently to care for people,” he said.
Andrew Freeman, MD, associate professor of medicine at the University of Colorado, Denver, and cochair of the American College of Cardiology’s nutrition and lifestyle work group, said the findings dovetail with his experience.
“I’m a huge believer that when people need to make lifestyle changes, having someone hold their hand and guide them through the effort is incredibly rewarding and incredibly powerful,” said Dr. Freeman, who also oversees the intensive cardiac rehab program at National Jewish Health in Denver.
A program like this needs proper funding in order to work, Dr, Freeman noted. He added that, even with coaches being paid well, “if you are able to prevent just one readmission for, say, heart failure a month . . . you could be saving millions of dollars over just a couple of years.”
Dr. Katzmarzyk, Dr. Flack, and Dr. Freeman reported no relevant disclosures. Louisiana State University, Pennington Biomedical Research Center, and Montclair State University have interest in the intellectual property surrounding a weight graph used in the study. The other researchers reported grants and/or fees from Bayer, Boehringer Ingelheim, Gilead, Takeda, Novo Nordisk, and other companies.
ColCORONA: More questions than answers for colchicine in COVID-19
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.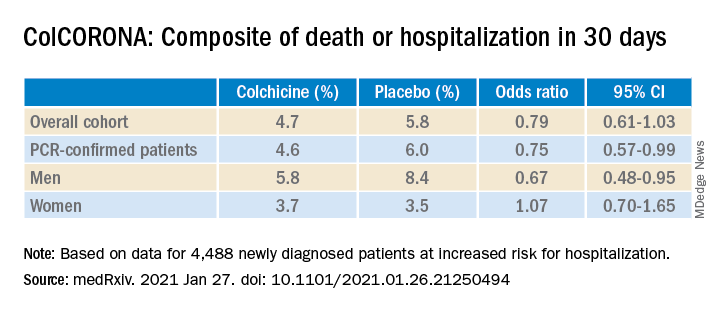
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
PFO closure reduces migraine: New meta-analysis
A meta-analysis of two randomized studies evaluating patent foramen ovale (PFO) closure as a treatment strategy for migraine has shown significant benefits in several key endpoints, prompting the authors to conclude the approach warrants reevaluation.
The pooled analysis of patient-level data from the PRIMA and PREMIUM studies, both of which evaluated the Amplatzer PFO Occluder device (Abbott Vascular), showed that
The study, led by Mohammad K. Mojadidi, MD, Virginia Commonwealth University, Richmond, was published online in the Journal of the American College of Cardiology on Feb. 8, 2021.
Commenting on the article, the coauthor of an accompanying editorial, Zubair Ahmed, MD, of the Cleveland Clinic said the meta-analysis gave some useful new information but is not enough to recommend PFO closure routinely for patients with migraine.
“This meta-analysis looked at different endpoints that are more relevant to current clinical practice than those in the two original studies, and the results show that we shouldn’t rule out PFO closure as a treatment strategy for some migraine patients,” Dr. Ahmed stated. “But we’re still not sure exactly which patients are most likely to benefit from this approach, and we need additional studies to gain more understanding on that.”
The study authors noted that there is an established link between the presence of PFO and migraine, especially migraine with aura. In observational studies of PFO closure for cryptogenic stroke, the vast majority of patients who also had migraine reported a more than 50% reduction in migraine days per month after PFO closure.
However, two recent randomized clinical trials evaluating the Amplatzer PFO Occluder device for reducing the frequency and duration of episodic migraine headaches did not meet their respective primary endpoints, although they did show significant benefit of PFO closure in most of their secondary endpoints.
The current meta-analysis pooled individual participant data from the two trials to increase the power to detect the effect of percutaneous PFO closure for treating patients with episodic migraine compared with medical therapy alone.
In the two studies including a total of 337 patients, 176 were randomized to PFO closure and 161 to medical treatment only. At 12 months, three of the four efficacy endpoints evaluated in the meta-analysis were significantly reduced in the PFO-closure group. These were mean reduction of monthly migraine days (–3.1 days vs. –1.9 days; P = .02), mean reduction of monthly migraine attacks (–2.0 vs. –1.4; P = .01), and number of patients who experienced complete cessation of migraine (9% vs. 0.7%; P < .001).
The responder rate, defined as more than a 50% reduction in migraine attacks, showed a trend towards an increase in the PFO-closure group but did not achieve statistical significance (38% vs. 29%; P = .13).
For the safety analysis, nine procedure-related and four device-related adverse events occurred in 245 patients who eventually received devices. All events were transient and resolved.
Better effect in patients with aura
Patients with migraine with aura, in particular frequent aura, had a significantly greater reduction in migraine days and a higher incidence of complete migraine cessation following PFO closure versus no closure, the authors reported.
In those without aura, PFO closure did not significantly reduce migraine days or improve complete headache cessation. However, some patients without aura did respond to PFO closure, which was statistically significant for reduction of migraine attacks (–2.0 vs. –1.0; P = .03).
“The interaction between the brain that is susceptible to migraine and the plethora of potential triggers is complex. A PFO may be the potential pathway for a variety of chemical triggers, such as serotonin from platelets, and although less frequent, some people with migraine without aura may trigger their migraine through this mechanism,” the researchers suggested. This hypothesis will be tested in the RELIEF trial, which is now being planned.
In the accompanying editorial, Dr. Ahmed and coauthor Robert J. Sommer, MD, Columbia University Medical Center, New York, pointed out that the meta-analysis demonstrates benefit of PFO closure in the migraine population for the first time.
“Moreover, the investigators defined a population of patients who may benefit most from PFO closure, those with migraine with frequent aura, suggesting that these may be different physiologically than other migraine subtypes. The analysis also places the PRIMA and PREMIUM outcomes in the context of endpoints that are more practical and are more commonly assessed in current clinical trials,” the editorialists noted.
Many unanswered questions
But the editorialists highlighted several significant limitations of the analysis, including “pooling of patient cohorts, methods, and outcome measures that might not be entirely comparable,” which they say could have introduced bias.
They also pointed out that the underlying pathophysiological mechanism linking migraine symptoms to PFO remains unknown. They explain that the mechanism is thought to involve the right-to-left passage of systemic venous blood, with some component – which would normally be eliminated or reduced on passage through the pulmonary vasculature – reaching the cerebral circulation via the PFO in supranormal concentrations and acting as a trigger for migraine activity in patients with susceptible brains.
But not all patients with migraine who have PFO benefit from PFO closure, they noted, and therefore presumably have PFO-unrelated migraines. There is no verified way to distinguish between these two groups at present.
“Once we learn to identify the subset of migraine patients in whom PFOs are actually causal of headache symptoms, screening and treatment of PFO for migraine can become a reality,” they wrote.
Although the meta-analysis is a step in the right direction, “it is not a home run,” Dr. Ahmed elaborated. “This was a post hoc analysis of two studies, neither of which showed significant benefits on their primary endpoints. That weakens the findings somewhat.”
He added: “At present, PFO closure is not routinely recommended as a migraine treatment strategy as we haven’t been sure which patients are most likely to benefit. And while this meta-analysis suggests patients with aura may be more likely to benefit, one quarter of patients without aura in the PREMIUM trial responded to PFO closure, so it’s not just about aura.
“There are still many unanswered questions.
“I don’t think the new information from this meta-analysis is enough to persuade me to change my practice, but it is a small building block in the overall picture and suggests this may be a suitable strategy for some patients in future,” he concluded.
The study had no outside funding. Participant-level data were provided by Abbott. Several coauthors were on the steering committee for the PREMIUM or PRIMA trials. Dr. Ahmed reported receiving consulting fees from, Amgen, AbbVie, electroCore, and Eli Lilly; serving on advisory boards for Amgen and Supernus; serving as a speaker for AbbVie; and receiving funding for an investigator-initiated trial from Teva and Eli Lilly.
A version of this article first appeared on Medscape.com.
A meta-analysis of two randomized studies evaluating patent foramen ovale (PFO) closure as a treatment strategy for migraine has shown significant benefits in several key endpoints, prompting the authors to conclude the approach warrants reevaluation.
The pooled analysis of patient-level data from the PRIMA and PREMIUM studies, both of which evaluated the Amplatzer PFO Occluder device (Abbott Vascular), showed that
The study, led by Mohammad K. Mojadidi, MD, Virginia Commonwealth University, Richmond, was published online in the Journal of the American College of Cardiology on Feb. 8, 2021.
Commenting on the article, the coauthor of an accompanying editorial, Zubair Ahmed, MD, of the Cleveland Clinic said the meta-analysis gave some useful new information but is not enough to recommend PFO closure routinely for patients with migraine.
“This meta-analysis looked at different endpoints that are more relevant to current clinical practice than those in the two original studies, and the results show that we shouldn’t rule out PFO closure as a treatment strategy for some migraine patients,” Dr. Ahmed stated. “But we’re still not sure exactly which patients are most likely to benefit from this approach, and we need additional studies to gain more understanding on that.”
The study authors noted that there is an established link between the presence of PFO and migraine, especially migraine with aura. In observational studies of PFO closure for cryptogenic stroke, the vast majority of patients who also had migraine reported a more than 50% reduction in migraine days per month after PFO closure.
However, two recent randomized clinical trials evaluating the Amplatzer PFO Occluder device for reducing the frequency and duration of episodic migraine headaches did not meet their respective primary endpoints, although they did show significant benefit of PFO closure in most of their secondary endpoints.
The current meta-analysis pooled individual participant data from the two trials to increase the power to detect the effect of percutaneous PFO closure for treating patients with episodic migraine compared with medical therapy alone.
In the two studies including a total of 337 patients, 176 were randomized to PFO closure and 161 to medical treatment only. At 12 months, three of the four efficacy endpoints evaluated in the meta-analysis were significantly reduced in the PFO-closure group. These were mean reduction of monthly migraine days (–3.1 days vs. –1.9 days; P = .02), mean reduction of monthly migraine attacks (–2.0 vs. –1.4; P = .01), and number of patients who experienced complete cessation of migraine (9% vs. 0.7%; P < .001).
The responder rate, defined as more than a 50% reduction in migraine attacks, showed a trend towards an increase in the PFO-closure group but did not achieve statistical significance (38% vs. 29%; P = .13).
For the safety analysis, nine procedure-related and four device-related adverse events occurred in 245 patients who eventually received devices. All events were transient and resolved.
Better effect in patients with aura
Patients with migraine with aura, in particular frequent aura, had a significantly greater reduction in migraine days and a higher incidence of complete migraine cessation following PFO closure versus no closure, the authors reported.
In those without aura, PFO closure did not significantly reduce migraine days or improve complete headache cessation. However, some patients without aura did respond to PFO closure, which was statistically significant for reduction of migraine attacks (–2.0 vs. –1.0; P = .03).
“The interaction between the brain that is susceptible to migraine and the plethora of potential triggers is complex. A PFO may be the potential pathway for a variety of chemical triggers, such as serotonin from platelets, and although less frequent, some people with migraine without aura may trigger their migraine through this mechanism,” the researchers suggested. This hypothesis will be tested in the RELIEF trial, which is now being planned.
In the accompanying editorial, Dr. Ahmed and coauthor Robert J. Sommer, MD, Columbia University Medical Center, New York, pointed out that the meta-analysis demonstrates benefit of PFO closure in the migraine population for the first time.
“Moreover, the investigators defined a population of patients who may benefit most from PFO closure, those with migraine with frequent aura, suggesting that these may be different physiologically than other migraine subtypes. The analysis also places the PRIMA and PREMIUM outcomes in the context of endpoints that are more practical and are more commonly assessed in current clinical trials,” the editorialists noted.
Many unanswered questions
But the editorialists highlighted several significant limitations of the analysis, including “pooling of patient cohorts, methods, and outcome measures that might not be entirely comparable,” which they say could have introduced bias.
They also pointed out that the underlying pathophysiological mechanism linking migraine symptoms to PFO remains unknown. They explain that the mechanism is thought to involve the right-to-left passage of systemic venous blood, with some component – which would normally be eliminated or reduced on passage through the pulmonary vasculature – reaching the cerebral circulation via the PFO in supranormal concentrations and acting as a trigger for migraine activity in patients with susceptible brains.
But not all patients with migraine who have PFO benefit from PFO closure, they noted, and therefore presumably have PFO-unrelated migraines. There is no verified way to distinguish between these two groups at present.
“Once we learn to identify the subset of migraine patients in whom PFOs are actually causal of headache symptoms, screening and treatment of PFO for migraine can become a reality,” they wrote.
Although the meta-analysis is a step in the right direction, “it is not a home run,” Dr. Ahmed elaborated. “This was a post hoc analysis of two studies, neither of which showed significant benefits on their primary endpoints. That weakens the findings somewhat.”
He added: “At present, PFO closure is not routinely recommended as a migraine treatment strategy as we haven’t been sure which patients are most likely to benefit. And while this meta-analysis suggests patients with aura may be more likely to benefit, one quarter of patients without aura in the PREMIUM trial responded to PFO closure, so it’s not just about aura.
“There are still many unanswered questions.
“I don’t think the new information from this meta-analysis is enough to persuade me to change my practice, but it is a small building block in the overall picture and suggests this may be a suitable strategy for some patients in future,” he concluded.
The study had no outside funding. Participant-level data were provided by Abbott. Several coauthors were on the steering committee for the PREMIUM or PRIMA trials. Dr. Ahmed reported receiving consulting fees from, Amgen, AbbVie, electroCore, and Eli Lilly; serving on advisory boards for Amgen and Supernus; serving as a speaker for AbbVie; and receiving funding for an investigator-initiated trial from Teva and Eli Lilly.
A version of this article first appeared on Medscape.com.
A meta-analysis of two randomized studies evaluating patent foramen ovale (PFO) closure as a treatment strategy for migraine has shown significant benefits in several key endpoints, prompting the authors to conclude the approach warrants reevaluation.
The pooled analysis of patient-level data from the PRIMA and PREMIUM studies, both of which evaluated the Amplatzer PFO Occluder device (Abbott Vascular), showed that
The study, led by Mohammad K. Mojadidi, MD, Virginia Commonwealth University, Richmond, was published online in the Journal of the American College of Cardiology on Feb. 8, 2021.
Commenting on the article, the coauthor of an accompanying editorial, Zubair Ahmed, MD, of the Cleveland Clinic said the meta-analysis gave some useful new information but is not enough to recommend PFO closure routinely for patients with migraine.
“This meta-analysis looked at different endpoints that are more relevant to current clinical practice than those in the two original studies, and the results show that we shouldn’t rule out PFO closure as a treatment strategy for some migraine patients,” Dr. Ahmed stated. “But we’re still not sure exactly which patients are most likely to benefit from this approach, and we need additional studies to gain more understanding on that.”
The study authors noted that there is an established link between the presence of PFO and migraine, especially migraine with aura. In observational studies of PFO closure for cryptogenic stroke, the vast majority of patients who also had migraine reported a more than 50% reduction in migraine days per month after PFO closure.
However, two recent randomized clinical trials evaluating the Amplatzer PFO Occluder device for reducing the frequency and duration of episodic migraine headaches did not meet their respective primary endpoints, although they did show significant benefit of PFO closure in most of their secondary endpoints.
The current meta-analysis pooled individual participant data from the two trials to increase the power to detect the effect of percutaneous PFO closure for treating patients with episodic migraine compared with medical therapy alone.
In the two studies including a total of 337 patients, 176 were randomized to PFO closure and 161 to medical treatment only. At 12 months, three of the four efficacy endpoints evaluated in the meta-analysis were significantly reduced in the PFO-closure group. These were mean reduction of monthly migraine days (–3.1 days vs. –1.9 days; P = .02), mean reduction of monthly migraine attacks (–2.0 vs. –1.4; P = .01), and number of patients who experienced complete cessation of migraine (9% vs. 0.7%; P < .001).
The responder rate, defined as more than a 50% reduction in migraine attacks, showed a trend towards an increase in the PFO-closure group but did not achieve statistical significance (38% vs. 29%; P = .13).
For the safety analysis, nine procedure-related and four device-related adverse events occurred in 245 patients who eventually received devices. All events were transient and resolved.
Better effect in patients with aura
Patients with migraine with aura, in particular frequent aura, had a significantly greater reduction in migraine days and a higher incidence of complete migraine cessation following PFO closure versus no closure, the authors reported.
In those without aura, PFO closure did not significantly reduce migraine days or improve complete headache cessation. However, some patients without aura did respond to PFO closure, which was statistically significant for reduction of migraine attacks (–2.0 vs. –1.0; P = .03).
“The interaction between the brain that is susceptible to migraine and the plethora of potential triggers is complex. A PFO may be the potential pathway for a variety of chemical triggers, such as serotonin from platelets, and although less frequent, some people with migraine without aura may trigger their migraine through this mechanism,” the researchers suggested. This hypothesis will be tested in the RELIEF trial, which is now being planned.
In the accompanying editorial, Dr. Ahmed and coauthor Robert J. Sommer, MD, Columbia University Medical Center, New York, pointed out that the meta-analysis demonstrates benefit of PFO closure in the migraine population for the first time.
“Moreover, the investigators defined a population of patients who may benefit most from PFO closure, those with migraine with frequent aura, suggesting that these may be different physiologically than other migraine subtypes. The analysis also places the PRIMA and PREMIUM outcomes in the context of endpoints that are more practical and are more commonly assessed in current clinical trials,” the editorialists noted.
Many unanswered questions
But the editorialists highlighted several significant limitations of the analysis, including “pooling of patient cohorts, methods, and outcome measures that might not be entirely comparable,” which they say could have introduced bias.
They also pointed out that the underlying pathophysiological mechanism linking migraine symptoms to PFO remains unknown. They explain that the mechanism is thought to involve the right-to-left passage of systemic venous blood, with some component – which would normally be eliminated or reduced on passage through the pulmonary vasculature – reaching the cerebral circulation via the PFO in supranormal concentrations and acting as a trigger for migraine activity in patients with susceptible brains.
But not all patients with migraine who have PFO benefit from PFO closure, they noted, and therefore presumably have PFO-unrelated migraines. There is no verified way to distinguish between these two groups at present.
“Once we learn to identify the subset of migraine patients in whom PFOs are actually causal of headache symptoms, screening and treatment of PFO for migraine can become a reality,” they wrote.
Although the meta-analysis is a step in the right direction, “it is not a home run,” Dr. Ahmed elaborated. “This was a post hoc analysis of two studies, neither of which showed significant benefits on their primary endpoints. That weakens the findings somewhat.”
He added: “At present, PFO closure is not routinely recommended as a migraine treatment strategy as we haven’t been sure which patients are most likely to benefit. And while this meta-analysis suggests patients with aura may be more likely to benefit, one quarter of patients without aura in the PREMIUM trial responded to PFO closure, so it’s not just about aura.
“There are still many unanswered questions.
“I don’t think the new information from this meta-analysis is enough to persuade me to change my practice, but it is a small building block in the overall picture and suggests this may be a suitable strategy for some patients in future,” he concluded.
The study had no outside funding. Participant-level data were provided by Abbott. Several coauthors were on the steering committee for the PREMIUM or PRIMA trials. Dr. Ahmed reported receiving consulting fees from, Amgen, AbbVie, electroCore, and Eli Lilly; serving on advisory boards for Amgen and Supernus; serving as a speaker for AbbVie; and receiving funding for an investigator-initiated trial from Teva and Eli Lilly.
A version of this article first appeared on Medscape.com.