User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Whooping Cough Rising Fast, Especially Among Teens
Whooping cough is surging in the United States, with four times as many cases reported so far this year, compared to all of 2023.
The CDC said 14,569 cases had been reported as of Sept. 14, compared to 3475 in all of 2023.
Whooping cough, also called pertussis, is a respiratory illness spread through coughing, sneezing, or breathing very close to another person. Babies are given the DTaP vaccine to protect against whooping cough, diphtheria, and tetanus. Because the vaccine effectiveness wanes faster for whooping cough than the two other illnesses, boosters are recommended every decade or so.
Why the Whooping Cough Vaccine Is Important
Whooping cough is a very contagious bacteria, so vaccination is an important step to avoid it.
But many children in their tweens aren’t getting boosters, and that age group is driving the whooping cough outbreak.
“With the increase in vaccine hesitancy that has been going on since the COVID-19 pandemic, we’re seeing outbreaks occurring in kids who are not vaccinated,” Tina Tan, MD, president-elect of the Infectious Diseases Society of America, told NBC News.
Also, people are not social distancing the way they did during the height of the COVID pandemic, when whooping cough numbers went down.
“Levels of pertussis dropped dramatically when we were all masking, and now this huge increase is getting us back to pre-pandemic levels, and probably a little above that,” Thomas Murray, MD, a Yale Medicine pediatric infectious diseases specialist, said in a news release from the school. “It’s a contagious respiratory virus that can spread fairly quickly through the population.”
FDA advisers were scheduled to meet Sept. 20 to discuss developing more effective boosters for whooping cough.
A version of this article appeared on WebMD.com.
Whooping cough is surging in the United States, with four times as many cases reported so far this year, compared to all of 2023.
The CDC said 14,569 cases had been reported as of Sept. 14, compared to 3475 in all of 2023.
Whooping cough, also called pertussis, is a respiratory illness spread through coughing, sneezing, or breathing very close to another person. Babies are given the DTaP vaccine to protect against whooping cough, diphtheria, and tetanus. Because the vaccine effectiveness wanes faster for whooping cough than the two other illnesses, boosters are recommended every decade or so.
Why the Whooping Cough Vaccine Is Important
Whooping cough is a very contagious bacteria, so vaccination is an important step to avoid it.
But many children in their tweens aren’t getting boosters, and that age group is driving the whooping cough outbreak.
“With the increase in vaccine hesitancy that has been going on since the COVID-19 pandemic, we’re seeing outbreaks occurring in kids who are not vaccinated,” Tina Tan, MD, president-elect of the Infectious Diseases Society of America, told NBC News.
Also, people are not social distancing the way they did during the height of the COVID pandemic, when whooping cough numbers went down.
“Levels of pertussis dropped dramatically when we were all masking, and now this huge increase is getting us back to pre-pandemic levels, and probably a little above that,” Thomas Murray, MD, a Yale Medicine pediatric infectious diseases specialist, said in a news release from the school. “It’s a contagious respiratory virus that can spread fairly quickly through the population.”
FDA advisers were scheduled to meet Sept. 20 to discuss developing more effective boosters for whooping cough.
A version of this article appeared on WebMD.com.
Whooping cough is surging in the United States, with four times as many cases reported so far this year, compared to all of 2023.
The CDC said 14,569 cases had been reported as of Sept. 14, compared to 3475 in all of 2023.
Whooping cough, also called pertussis, is a respiratory illness spread through coughing, sneezing, or breathing very close to another person. Babies are given the DTaP vaccine to protect against whooping cough, diphtheria, and tetanus. Because the vaccine effectiveness wanes faster for whooping cough than the two other illnesses, boosters are recommended every decade or so.
Why the Whooping Cough Vaccine Is Important
Whooping cough is a very contagious bacteria, so vaccination is an important step to avoid it.
But many children in their tweens aren’t getting boosters, and that age group is driving the whooping cough outbreak.
“With the increase in vaccine hesitancy that has been going on since the COVID-19 pandemic, we’re seeing outbreaks occurring in kids who are not vaccinated,” Tina Tan, MD, president-elect of the Infectious Diseases Society of America, told NBC News.
Also, people are not social distancing the way they did during the height of the COVID pandemic, when whooping cough numbers went down.
“Levels of pertussis dropped dramatically when we were all masking, and now this huge increase is getting us back to pre-pandemic levels, and probably a little above that,” Thomas Murray, MD, a Yale Medicine pediatric infectious diseases specialist, said in a news release from the school. “It’s a contagious respiratory virus that can spread fairly quickly through the population.”
FDA advisers were scheduled to meet Sept. 20 to discuss developing more effective boosters for whooping cough.
A version of this article appeared on WebMD.com.
Treating Family: Ethicist Discusses Whether It’s Appropriate
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There’s a very interesting story in the medical press. A few years ago, a plastic surgeon named Edmond Cabbabe was preparing to do a follow-up cosmetic procedure on his wife at Mercy Hospital South, which is a big hospital in the St. Louis, Missouri, area.
He put her on the operating schedule, and he had done that when he had performed the original operation on her. On the day of the surgery, he got a call from the hospital saying the procedure was canceled. They said that the hospital’s policy, maybe a new one, would not allow doctors to operate on family members.
This physician was a past president of the Missouri State Medical Association. I think he was also on the board or president of the American Medical Association (AMA) Foundation. This was a physician not only in a skilled area where he felt confident he could take care of his wife, but also someone who was prominent in medical politics and medical policy.
The AMA forever has had a policy that says don’t treat relatives. This physician basically said, I think that policy is too restrictive, too cautious, and it doesn’t make much sense to continue to say that you can’t treat family and friends.
By implication, he was saying, I know exactly what I’m doing in my field and I know exactly what I’m doing with her procedure. I should have a right to perform it. I think I do a great job and I’d be best for her.
If you look at medical boards, every once in a while in some state, someone is brought up on a charge of doing different things with family members and saying that they’re going to get censured. They don’t usually lose their license, but they get a reprimand or get told that is just not ethical to do.
I think, in the long run, the policy about not treating your family and friends makes sense. The problem is, as is well known from the social sciences and psychology, people get biased when they deal with those they care about, love, and hold close to them.
It’s hard for the doctor to be objective when dealing with people that they really like or love. It’s also difficult for patients because they may not want to bring up something or they are uncomfortable talking with a doctor who’s a family member or close friend. They may not want to complain. They may be a little bit embarrassed about things. It just adds an emotional edge, I think, that’s difficult.
All that said, do I know doctors who regularly prescribe, say, an ointment for something that’s itchy or some kind of a pill when allergy season breaks out? I do. Do I think they’re acting in a horribly unethical manner? I don’t.
You need some judgment here. There are absolutely minor things where objectivity, fear, and anxiety are not in play. You’re going to be able to prescribe the routine thing for the routine itch without worrying too much about whether it’s a stranger, a friend, or your daughter.
What sorts of things am I really talking about when I say that minor variability ought to be allowed? It’s one thing when someone has poison ivy and they’re going to need some kind of standard medicine to treat it. A very different area that’s much more dangerous, and one I would avoid, is in the mental health field, and for that matter, the pain field.
It’s tempting to say: “Oh, my relative is just having a bad time. I’ll give her a little bit of antidepressant medicine,” or “They seem to be having pain after an operation or something, and I’m going to give them a little bit of pain meds just to get them through.”
Those areas are flying red flags. It’s easy to abuse and easy for someone to become a user and manipulate a friend or a doctor who’s a relative into getting things that another doctor wouldn’t be giving. I think that’s the space where you’ve got to exercise extreme caution.
Time and again, when those people get called up in front of the boards for treating relatives, it’s in those spaces of mental health, anxiety, and pain control. Again, when you know that there’s a likelihood of abuse, I think that’s the place where the line has to hold. Don’t treat the relative. Don’t treat the friend.
At the end of the day, I wouldn’t change the AMA policy. I think we should keep it in place and morally try to discourage doctors from caring for those they’re close to or they have emotional ties to.
At the same time, as with all ethical situations, there has to be a little bit of wiggle room for those super-minor cases where it just makes sense to say: “You don’t have to go find somebody else to do this. I can prescribe this ointment or this minor thing for you. No one’s objectivity is going to be soured, and you’re not going to feel in any way at risk because I’m going to prescribe this for you.”
Common sense ought to prevail. The default position is don’t do it; however, maybe with a tiny bit of space for what’s minor, what’s routine, and what really does just save people some inconvenience, there I might just give a little.
Dr. Caplan, Director, Division of Medical Ethics, New York University Langone Medical Center, New York City, has disclosed relationships with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
Are You Using the Correct Medication or a Look-Alike?
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Cancer Risk: Are Pesticides the New Smoking?
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Should There Be a Mandatory Retirement Age for Physicians?
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
Benralizumab Now FDA Approved to Treat EGPA Vasculitis
The Food and Drug Administration (FDA) has approved benralizumab (Fasenra) for the treatment of adults with eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome.
The drug is the second approved biologic for the treatment of EGPA. The first, mepolizumab (Nucala), was approved in 2017.
“This disease has a devastating impact on patients and the quality of their life, and they need more treatment options. The approval of another treatment in EGPA is welcome news to the approximately 15,000 patients living in the US with this difficult-to-treat rare disease,” said Joyce Kullman, executive director of the Vasculitis Foundation, in a press release on September 18.
Benralizumab, developed by AstraZeneca, is a monoclonal antibody against the interleukin-5 alpha receptor expressed on eosinophils. The drug was first approved in 2017 as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and is now approved for use in children aged 6 years and older.
The new indication was based on positive results from a noninferiority trial comparing benralizumab and mepolizumab. For the trial, published in the New England Journal of Medicine earlier in 2024, 140 adults with relapsing or refractory EGPA were randomized to a 30-mg subcutaneous injection of benralizumab or three separate 100-mg mepolizumab injections every 4 weeks for 1 year. At weeks 36 and 48, 59% of patients in the benralizumab group and 56% of patients in the mepolizumab group achieved remission (95% CI, –13 to 18; P = .73 for superiority). From week 42 to 52, 41% of patients who received benralizumab completely stopped taking oral glucocorticoids, compared with 26% of those who received mepolizumab.
“Patients often rely on long-term oral corticosteroids, which can cause serious and lasting side effects. Benralizumab is a much-needed treatment option, with data showing that not only is remission an achievable goal for EGPA patients, but benralizumab can also help patients taper off steroid therapy,” Michael Wechsler, MD, director of The Asthma Institute at National Jewish Health in Denver, Colorado, and the international coordinating investigator for the clinical trial, said in the press release.
Benralizumab is administered via subcutaneous injection. In adults with EGPA, the recommended dosage is 30 mg every 4 weeks for the first three doses, then once every 8 weeks.
The most common adverse reactions include headache and pharyngitis, according to the prescribing information.
Benralizumab is also in development for the treatment of chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps, and hypereosinophilic syndrome.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved benralizumab (Fasenra) for the treatment of adults with eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome.
The drug is the second approved biologic for the treatment of EGPA. The first, mepolizumab (Nucala), was approved in 2017.
“This disease has a devastating impact on patients and the quality of their life, and they need more treatment options. The approval of another treatment in EGPA is welcome news to the approximately 15,000 patients living in the US with this difficult-to-treat rare disease,” said Joyce Kullman, executive director of the Vasculitis Foundation, in a press release on September 18.
Benralizumab, developed by AstraZeneca, is a monoclonal antibody against the interleukin-5 alpha receptor expressed on eosinophils. The drug was first approved in 2017 as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and is now approved for use in children aged 6 years and older.
The new indication was based on positive results from a noninferiority trial comparing benralizumab and mepolizumab. For the trial, published in the New England Journal of Medicine earlier in 2024, 140 adults with relapsing or refractory EGPA were randomized to a 30-mg subcutaneous injection of benralizumab or three separate 100-mg mepolizumab injections every 4 weeks for 1 year. At weeks 36 and 48, 59% of patients in the benralizumab group and 56% of patients in the mepolizumab group achieved remission (95% CI, –13 to 18; P = .73 for superiority). From week 42 to 52, 41% of patients who received benralizumab completely stopped taking oral glucocorticoids, compared with 26% of those who received mepolizumab.
“Patients often rely on long-term oral corticosteroids, which can cause serious and lasting side effects. Benralizumab is a much-needed treatment option, with data showing that not only is remission an achievable goal for EGPA patients, but benralizumab can also help patients taper off steroid therapy,” Michael Wechsler, MD, director of The Asthma Institute at National Jewish Health in Denver, Colorado, and the international coordinating investigator for the clinical trial, said in the press release.
Benralizumab is administered via subcutaneous injection. In adults with EGPA, the recommended dosage is 30 mg every 4 weeks for the first three doses, then once every 8 weeks.
The most common adverse reactions include headache and pharyngitis, according to the prescribing information.
Benralizumab is also in development for the treatment of chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps, and hypereosinophilic syndrome.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) has approved benralizumab (Fasenra) for the treatment of adults with eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome.
The drug is the second approved biologic for the treatment of EGPA. The first, mepolizumab (Nucala), was approved in 2017.
“This disease has a devastating impact on patients and the quality of their life, and they need more treatment options. The approval of another treatment in EGPA is welcome news to the approximately 15,000 patients living in the US with this difficult-to-treat rare disease,” said Joyce Kullman, executive director of the Vasculitis Foundation, in a press release on September 18.
Benralizumab, developed by AstraZeneca, is a monoclonal antibody against the interleukin-5 alpha receptor expressed on eosinophils. The drug was first approved in 2017 as an add-on treatment for patients 12 years and older with severe eosinophilic asthma, and is now approved for use in children aged 6 years and older.
The new indication was based on positive results from a noninferiority trial comparing benralizumab and mepolizumab. For the trial, published in the New England Journal of Medicine earlier in 2024, 140 adults with relapsing or refractory EGPA were randomized to a 30-mg subcutaneous injection of benralizumab or three separate 100-mg mepolizumab injections every 4 weeks for 1 year. At weeks 36 and 48, 59% of patients in the benralizumab group and 56% of patients in the mepolizumab group achieved remission (95% CI, –13 to 18; P = .73 for superiority). From week 42 to 52, 41% of patients who received benralizumab completely stopped taking oral glucocorticoids, compared with 26% of those who received mepolizumab.
“Patients often rely on long-term oral corticosteroids, which can cause serious and lasting side effects. Benralizumab is a much-needed treatment option, with data showing that not only is remission an achievable goal for EGPA patients, but benralizumab can also help patients taper off steroid therapy,” Michael Wechsler, MD, director of The Asthma Institute at National Jewish Health in Denver, Colorado, and the international coordinating investigator for the clinical trial, said in the press release.
Benralizumab is administered via subcutaneous injection. In adults with EGPA, the recommended dosage is 30 mg every 4 weeks for the first three doses, then once every 8 weeks.
The most common adverse reactions include headache and pharyngitis, according to the prescribing information.
Benralizumab is also in development for the treatment of chronic obstructive pulmonary disease, chronic rhinosinusitis with nasal polyps, and hypereosinophilic syndrome.
A version of this article first appeared on Medscape.com.
When You and Your Malpractice Insurer Disagree on Your Case
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
Coffee’s ‘Sweet Spot’: Daily Consumption and Cardiometabolic Risk
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.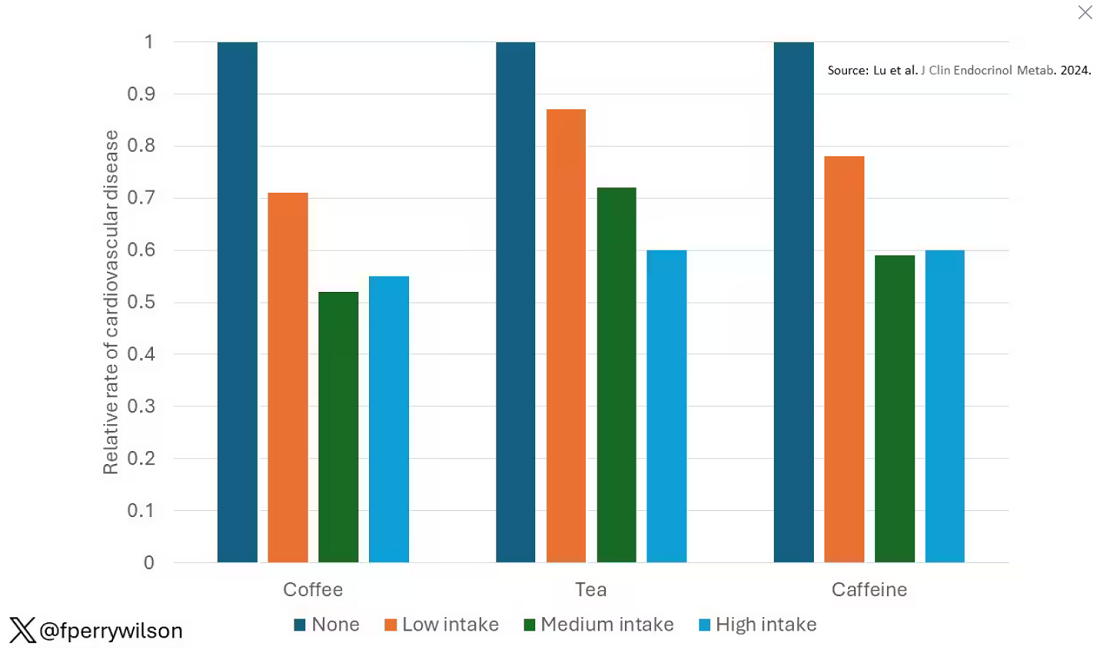
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine. 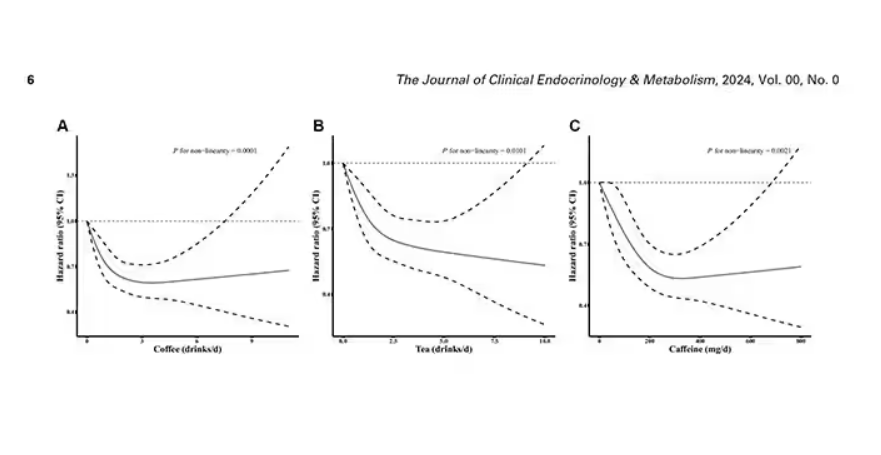
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
This figure summarizes the findings, and yes, this is way too complicated. 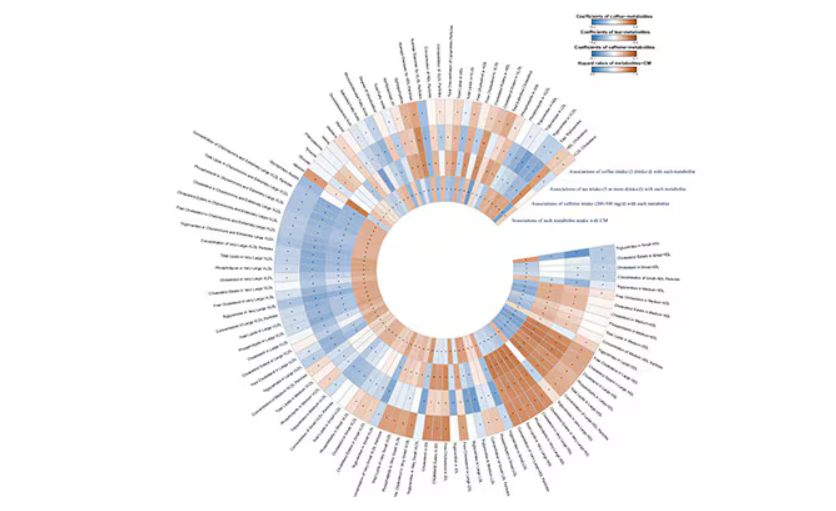
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.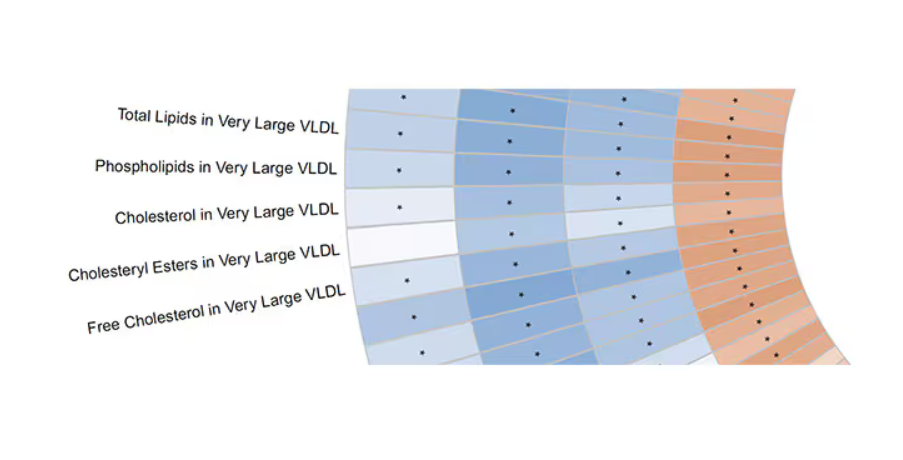
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.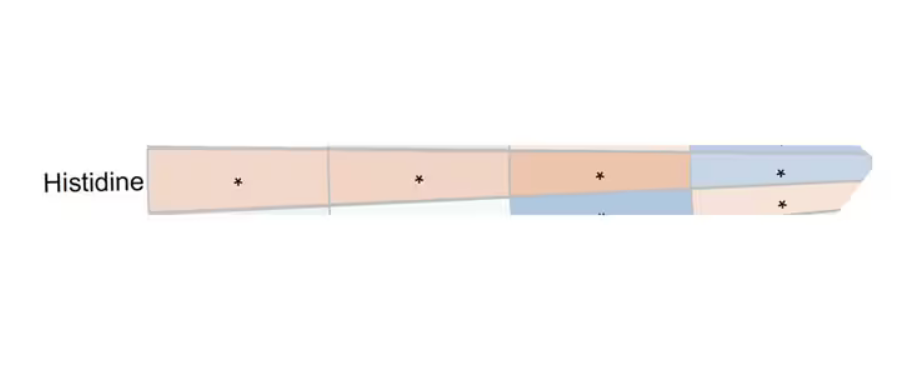
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine. 
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
This figure summarizes the findings, and yes, this is way too complicated. 
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine. 
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
This figure summarizes the findings, and yes, this is way too complicated. 
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Locally Acquired Dengue Case Confirmed in California
A case of locally acquired dengue fever has been confirmed in a resident of Baldwin Park, California, according to a press release from the Los Angeles County Department of Public Health.
“Dengue is the most common insect-borne viral infection in the world, with a wide geographic spread; we know that we have mosquitoes capable of carrying and transmitting the virus in the United States already, and Los Angeles county is a major epicenter for international travel and trade,” James Lawler, MD, associate director for International Programs and Innovation at the Global Center for Health Security and professor in the Infectious Diseases Division at the University of Nebraska Medical Center, Omaha, Nebraska, said in an interview.
Although the patient had no known history of travel to a dengue-endemic area, the potential risk for widespread transmission of the virus in the Los Angeles County area remains low, and no additional suspected cases of locally acquired dengue have been identified, according to the release. However, the recent cases highlight the need for vigilance on the part of the public to reduce transmission of mosquito-borne infections, the public health department noted.
Most cases of dengue occur in people who have traveled to areas where the disease is more common, mainly tropical and subtropical areas, according to the press release. However, the types of mosquitoes that spread dengue exist in parts of the United States, so locally acquired infections can occur.
The Centers for Disease Control and Prevention (CDC) issued an official health advisory in June 2024 about an increased risk for dengue infections in the United States. According to the advisory, 745 cases of dengue were identified in US travelers to endemic areas between January 1, 2024, and June 24, 2024.
The CDC advises clinicians to maintain a high level of suspicion for dengue among individuals with fever and recent travel to areas with frequent dengue transmission, but also to consider locally acquired disease in areas of mosquito vectors.
In clinical practice, dengue may be difficult to differentiate from other febrile systemic infections, Dr. Lawler noted. “Joint pain, low back pain, and headache (often retro-orbital) are common and can be severe, and a rash often appears several days into illness,” he noted.
Do not delay treatment in suspected cases while waiting for test results, the CDC emphasized in the advisory. Food and Drug Administration–approved tests for dengue include RT-PCR and IgM antibody tests or NS1 and IgM antibody tests.
“Severe dengue can be life-threatening and progress to a hemorrhagic fever-like syndrome, and patients with severe dengue should be cared for on a high-acuity or intensive care setting, with close monitoring of labs and fluid status,” Dr. Lawler told this news organization.
The World Health Organization has published guidelines for the management of dengue, which Dr. Lawler strongly recommends to clinicians in the rare event that they are facing a severe case. The treatment for dengue is supportive care, according to the CDC; a vaccine that was deemed safe and effective is no longer being manufactured because of low demand.
Most symptoms last for 2-7 days, and most patients recover within a week, but approximately 1 in 20 may develop severe disease, according to the Los Angeles County Department of Public Health.
Approximately one quarter of dengue infections are symptomatic, and clinicians should know the signs of progression to severe disease, which include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and liver enlargement, according to the CDC.
Local Dengue Not Unexpected
“Sadly, I am not surprised at another locally acquired case of dengue fever in the United States,” said Dr. Lawler. “We also have seen a trend of more historically tropical, insect-borne diseases popping up with locally acquired cases in the United States,” he noted.
Dr. Lawler suggested that “the erosion of state and local public health” is a major contributor to the increase in dengue cases. For more than 100 years, activities of state and local public health officials had significantly curtailed mosquito-borne diseases through aggressive control programs, “but we seem to be losing ground over the last several years,” he said.
“Locally acquired dengue cases are still rare in the United States,” he added. “However, people can protect themselves against dengue and more common arthropod-borne infections by taking precautions to cover up and wear insect repellent while outdoors.”
In addition, the Los Angeles County Department of Public Health emphasized in its press release that local residents reduce their risk for contact with mosquitoes by removing areas of standing water on their property and ensuring well-fitted screens on doors and windows.
Dr. Lawler had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
A case of locally acquired dengue fever has been confirmed in a resident of Baldwin Park, California, according to a press release from the Los Angeles County Department of Public Health.
“Dengue is the most common insect-borne viral infection in the world, with a wide geographic spread; we know that we have mosquitoes capable of carrying and transmitting the virus in the United States already, and Los Angeles county is a major epicenter for international travel and trade,” James Lawler, MD, associate director for International Programs and Innovation at the Global Center for Health Security and professor in the Infectious Diseases Division at the University of Nebraska Medical Center, Omaha, Nebraska, said in an interview.
Although the patient had no known history of travel to a dengue-endemic area, the potential risk for widespread transmission of the virus in the Los Angeles County area remains low, and no additional suspected cases of locally acquired dengue have been identified, according to the release. However, the recent cases highlight the need for vigilance on the part of the public to reduce transmission of mosquito-borne infections, the public health department noted.
Most cases of dengue occur in people who have traveled to areas where the disease is more common, mainly tropical and subtropical areas, according to the press release. However, the types of mosquitoes that spread dengue exist in parts of the United States, so locally acquired infections can occur.
The Centers for Disease Control and Prevention (CDC) issued an official health advisory in June 2024 about an increased risk for dengue infections in the United States. According to the advisory, 745 cases of dengue were identified in US travelers to endemic areas between January 1, 2024, and June 24, 2024.
The CDC advises clinicians to maintain a high level of suspicion for dengue among individuals with fever and recent travel to areas with frequent dengue transmission, but also to consider locally acquired disease in areas of mosquito vectors.
In clinical practice, dengue may be difficult to differentiate from other febrile systemic infections, Dr. Lawler noted. “Joint pain, low back pain, and headache (often retro-orbital) are common and can be severe, and a rash often appears several days into illness,” he noted.
Do not delay treatment in suspected cases while waiting for test results, the CDC emphasized in the advisory. Food and Drug Administration–approved tests for dengue include RT-PCR and IgM antibody tests or NS1 and IgM antibody tests.
“Severe dengue can be life-threatening and progress to a hemorrhagic fever-like syndrome, and patients with severe dengue should be cared for on a high-acuity or intensive care setting, with close monitoring of labs and fluid status,” Dr. Lawler told this news organization.
The World Health Organization has published guidelines for the management of dengue, which Dr. Lawler strongly recommends to clinicians in the rare event that they are facing a severe case. The treatment for dengue is supportive care, according to the CDC; a vaccine that was deemed safe and effective is no longer being manufactured because of low demand.
Most symptoms last for 2-7 days, and most patients recover within a week, but approximately 1 in 20 may develop severe disease, according to the Los Angeles County Department of Public Health.
Approximately one quarter of dengue infections are symptomatic, and clinicians should know the signs of progression to severe disease, which include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and liver enlargement, according to the CDC.
Local Dengue Not Unexpected
“Sadly, I am not surprised at another locally acquired case of dengue fever in the United States,” said Dr. Lawler. “We also have seen a trend of more historically tropical, insect-borne diseases popping up with locally acquired cases in the United States,” he noted.
Dr. Lawler suggested that “the erosion of state and local public health” is a major contributor to the increase in dengue cases. For more than 100 years, activities of state and local public health officials had significantly curtailed mosquito-borne diseases through aggressive control programs, “but we seem to be losing ground over the last several years,” he said.
“Locally acquired dengue cases are still rare in the United States,” he added. “However, people can protect themselves against dengue and more common arthropod-borne infections by taking precautions to cover up and wear insect repellent while outdoors.”
In addition, the Los Angeles County Department of Public Health emphasized in its press release that local residents reduce their risk for contact with mosquitoes by removing areas of standing water on their property and ensuring well-fitted screens on doors and windows.
Dr. Lawler had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
A case of locally acquired dengue fever has been confirmed in a resident of Baldwin Park, California, according to a press release from the Los Angeles County Department of Public Health.
“Dengue is the most common insect-borne viral infection in the world, with a wide geographic spread; we know that we have mosquitoes capable of carrying and transmitting the virus in the United States already, and Los Angeles county is a major epicenter for international travel and trade,” James Lawler, MD, associate director for International Programs and Innovation at the Global Center for Health Security and professor in the Infectious Diseases Division at the University of Nebraska Medical Center, Omaha, Nebraska, said in an interview.
Although the patient had no known history of travel to a dengue-endemic area, the potential risk for widespread transmission of the virus in the Los Angeles County area remains low, and no additional suspected cases of locally acquired dengue have been identified, according to the release. However, the recent cases highlight the need for vigilance on the part of the public to reduce transmission of mosquito-borne infections, the public health department noted.
Most cases of dengue occur in people who have traveled to areas where the disease is more common, mainly tropical and subtropical areas, according to the press release. However, the types of mosquitoes that spread dengue exist in parts of the United States, so locally acquired infections can occur.
The Centers for Disease Control and Prevention (CDC) issued an official health advisory in June 2024 about an increased risk for dengue infections in the United States. According to the advisory, 745 cases of dengue were identified in US travelers to endemic areas between January 1, 2024, and June 24, 2024.
The CDC advises clinicians to maintain a high level of suspicion for dengue among individuals with fever and recent travel to areas with frequent dengue transmission, but also to consider locally acquired disease in areas of mosquito vectors.
In clinical practice, dengue may be difficult to differentiate from other febrile systemic infections, Dr. Lawler noted. “Joint pain, low back pain, and headache (often retro-orbital) are common and can be severe, and a rash often appears several days into illness,” he noted.
Do not delay treatment in suspected cases while waiting for test results, the CDC emphasized in the advisory. Food and Drug Administration–approved tests for dengue include RT-PCR and IgM antibody tests or NS1 and IgM antibody tests.
“Severe dengue can be life-threatening and progress to a hemorrhagic fever-like syndrome, and patients with severe dengue should be cared for on a high-acuity or intensive care setting, with close monitoring of labs and fluid status,” Dr. Lawler told this news organization.
The World Health Organization has published guidelines for the management of dengue, which Dr. Lawler strongly recommends to clinicians in the rare event that they are facing a severe case. The treatment for dengue is supportive care, according to the CDC; a vaccine that was deemed safe and effective is no longer being manufactured because of low demand.
Most symptoms last for 2-7 days, and most patients recover within a week, but approximately 1 in 20 may develop severe disease, according to the Los Angeles County Department of Public Health.
Approximately one quarter of dengue infections are symptomatic, and clinicians should know the signs of progression to severe disease, which include abdominal pain or tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy or restlessness, and liver enlargement, according to the CDC.
Local Dengue Not Unexpected
“Sadly, I am not surprised at another locally acquired case of dengue fever in the United States,” said Dr. Lawler. “We also have seen a trend of more historically tropical, insect-borne diseases popping up with locally acquired cases in the United States,” he noted.
Dr. Lawler suggested that “the erosion of state and local public health” is a major contributor to the increase in dengue cases. For more than 100 years, activities of state and local public health officials had significantly curtailed mosquito-borne diseases through aggressive control programs, “but we seem to be losing ground over the last several years,” he said.
“Locally acquired dengue cases are still rare in the United States,” he added. “However, people can protect themselves against dengue and more common arthropod-borne infections by taking precautions to cover up and wear insect repellent while outdoors.”
In addition, the Los Angeles County Department of Public Health emphasized in its press release that local residents reduce their risk for contact with mosquitoes by removing areas of standing water on their property and ensuring well-fitted screens on doors and windows.
Dr. Lawler had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
‘Reform School’ for Pharmacy Benefit Managers: How Might Legislation Help Patients?
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).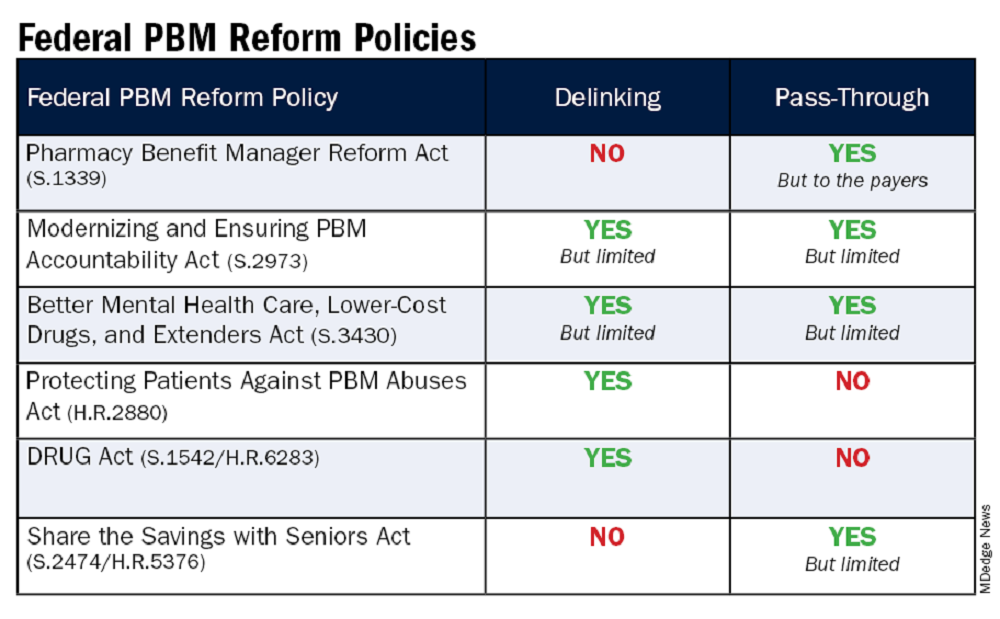
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].