User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Ketamine promising for rare condition linked to autism
Also known as Helsmoortel–Van Der Aa syndrome, ADNP syndrome is caused by mutations in the ADNP gene. Studies in animal models suggest that low-dose ketamine increases expression of ADNP and is neuroprotective.
Intrigued by the preclinical evidence, Alexander Kolevzon, MD, clinical director of the Seaver Autism Center at Mount Sinai, New York, and colleagues treated 10 children with ADNP syndrome with a single low dose of ketamine (0.5mg/kg) infused intravenously over 40 minutes. The children ranged in ages 6-12 years.
Using parent-report instruments to assess treatment effects, ketamine was associated with “nominally significant” improvement in a variety of domains, including social behavior, attention-deficit and hyperactivity, restricted and repetitive behaviors, and sensory sensitivities.
Parent reports of improvement in these domains aligned with clinician-rated assessments based on the Clinical Global Impressions–Improvement scale.
The results also highlight the potential utility of electrophysiological measurement of auditory steady-state response and eye-tracking to track change with ketamine treatment, the researchers say.
The study was published online in Human Genetics and Genomic (HGG) Advances.
Hypothesis-generating
Ketamine was generally well tolerated. There were no clinically significant abnormalities in laboratory or cardiac monitoring, and there were no serious adverse events (AEs).
Treatment emergent AEs were all mild to moderate and no child required any interventions.
The most common AEs were elation/silliness in five children (50%), all of whom had a history of similar symptoms. Drowsiness and fatigue occurred in four children (40%) and two of them had a history of drowsiness. Aggression was likewise relatively common, reported in four children (40%), all of whom had aggression at baseline.
Decreased appetite emerged as a new AE in three children (30%), increased anxiety occurred in three children (30%), and irritability, nausea/vomiting, and restlessness each occurred in two children (20%).
The researchers caution that the findings are intended to be “hypothesis generating.”
“We are encouraged by these findings, which provide preliminary support for ketamine to help reduce negative effects of this devastating syndrome,” Dr. Kolevzon said in a news release from Mount Sinai.
Ketamine might help ease symptoms of ADNP syndrome “by increasing expression of the ADNP gene or by promoting synaptic plasticity through glutamatergic pathways,” Dr. Kolevzon told this news organization.
The next step, he said, is to get “a larger, placebo-controlled study approved for funding using repeated dosing over a longer duration of time. We are working with the FDA to get the design approved for an investigational new drug application.”
Support for the study was provided by the ADNP Kids Foundation and the Foundation for Mood Disorders. Support for mediKanren was provided by the National Center for Advancing Translational Sciences, and National Institutes of Health through the Biomedical Data Translator Program. Dr. Kolevzon is on the scientific advisory board of Ovid Therapeutics, Ritrova Therapeutics, and Jaguar Therapeutics and consults to Acadia, Alkermes, GW Pharmaceuticals, Neuren Pharmaceuticals, Clinilabs Drug Development Corporation, and Scioto Biosciences.
A version of this article first appeared on Medscape.com.
Also known as Helsmoortel–Van Der Aa syndrome, ADNP syndrome is caused by mutations in the ADNP gene. Studies in animal models suggest that low-dose ketamine increases expression of ADNP and is neuroprotective.
Intrigued by the preclinical evidence, Alexander Kolevzon, MD, clinical director of the Seaver Autism Center at Mount Sinai, New York, and colleagues treated 10 children with ADNP syndrome with a single low dose of ketamine (0.5mg/kg) infused intravenously over 40 minutes. The children ranged in ages 6-12 years.
Using parent-report instruments to assess treatment effects, ketamine was associated with “nominally significant” improvement in a variety of domains, including social behavior, attention-deficit and hyperactivity, restricted and repetitive behaviors, and sensory sensitivities.
Parent reports of improvement in these domains aligned with clinician-rated assessments based on the Clinical Global Impressions–Improvement scale.
The results also highlight the potential utility of electrophysiological measurement of auditory steady-state response and eye-tracking to track change with ketamine treatment, the researchers say.
The study was published online in Human Genetics and Genomic (HGG) Advances.
Hypothesis-generating
Ketamine was generally well tolerated. There were no clinically significant abnormalities in laboratory or cardiac monitoring, and there were no serious adverse events (AEs).
Treatment emergent AEs were all mild to moderate and no child required any interventions.
The most common AEs were elation/silliness in five children (50%), all of whom had a history of similar symptoms. Drowsiness and fatigue occurred in four children (40%) and two of them had a history of drowsiness. Aggression was likewise relatively common, reported in four children (40%), all of whom had aggression at baseline.
Decreased appetite emerged as a new AE in three children (30%), increased anxiety occurred in three children (30%), and irritability, nausea/vomiting, and restlessness each occurred in two children (20%).
The researchers caution that the findings are intended to be “hypothesis generating.”
“We are encouraged by these findings, which provide preliminary support for ketamine to help reduce negative effects of this devastating syndrome,” Dr. Kolevzon said in a news release from Mount Sinai.
Ketamine might help ease symptoms of ADNP syndrome “by increasing expression of the ADNP gene or by promoting synaptic plasticity through glutamatergic pathways,” Dr. Kolevzon told this news organization.
The next step, he said, is to get “a larger, placebo-controlled study approved for funding using repeated dosing over a longer duration of time. We are working with the FDA to get the design approved for an investigational new drug application.”
Support for the study was provided by the ADNP Kids Foundation and the Foundation for Mood Disorders. Support for mediKanren was provided by the National Center for Advancing Translational Sciences, and National Institutes of Health through the Biomedical Data Translator Program. Dr. Kolevzon is on the scientific advisory board of Ovid Therapeutics, Ritrova Therapeutics, and Jaguar Therapeutics and consults to Acadia, Alkermes, GW Pharmaceuticals, Neuren Pharmaceuticals, Clinilabs Drug Development Corporation, and Scioto Biosciences.
A version of this article first appeared on Medscape.com.
Also known as Helsmoortel–Van Der Aa syndrome, ADNP syndrome is caused by mutations in the ADNP gene. Studies in animal models suggest that low-dose ketamine increases expression of ADNP and is neuroprotective.
Intrigued by the preclinical evidence, Alexander Kolevzon, MD, clinical director of the Seaver Autism Center at Mount Sinai, New York, and colleagues treated 10 children with ADNP syndrome with a single low dose of ketamine (0.5mg/kg) infused intravenously over 40 minutes. The children ranged in ages 6-12 years.
Using parent-report instruments to assess treatment effects, ketamine was associated with “nominally significant” improvement in a variety of domains, including social behavior, attention-deficit and hyperactivity, restricted and repetitive behaviors, and sensory sensitivities.
Parent reports of improvement in these domains aligned with clinician-rated assessments based on the Clinical Global Impressions–Improvement scale.
The results also highlight the potential utility of electrophysiological measurement of auditory steady-state response and eye-tracking to track change with ketamine treatment, the researchers say.
The study was published online in Human Genetics and Genomic (HGG) Advances.
Hypothesis-generating
Ketamine was generally well tolerated. There were no clinically significant abnormalities in laboratory or cardiac monitoring, and there were no serious adverse events (AEs).
Treatment emergent AEs were all mild to moderate and no child required any interventions.
The most common AEs were elation/silliness in five children (50%), all of whom had a history of similar symptoms. Drowsiness and fatigue occurred in four children (40%) and two of them had a history of drowsiness. Aggression was likewise relatively common, reported in four children (40%), all of whom had aggression at baseline.
Decreased appetite emerged as a new AE in three children (30%), increased anxiety occurred in three children (30%), and irritability, nausea/vomiting, and restlessness each occurred in two children (20%).
The researchers caution that the findings are intended to be “hypothesis generating.”
“We are encouraged by these findings, which provide preliminary support for ketamine to help reduce negative effects of this devastating syndrome,” Dr. Kolevzon said in a news release from Mount Sinai.
Ketamine might help ease symptoms of ADNP syndrome “by increasing expression of the ADNP gene or by promoting synaptic plasticity through glutamatergic pathways,” Dr. Kolevzon told this news organization.
The next step, he said, is to get “a larger, placebo-controlled study approved for funding using repeated dosing over a longer duration of time. We are working with the FDA to get the design approved for an investigational new drug application.”
Support for the study was provided by the ADNP Kids Foundation and the Foundation for Mood Disorders. Support for mediKanren was provided by the National Center for Advancing Translational Sciences, and National Institutes of Health through the Biomedical Data Translator Program. Dr. Kolevzon is on the scientific advisory board of Ovid Therapeutics, Ritrova Therapeutics, and Jaguar Therapeutics and consults to Acadia, Alkermes, GW Pharmaceuticals, Neuren Pharmaceuticals, Clinilabs Drug Development Corporation, and Scioto Biosciences.
A version of this article first appeared on Medscape.com.
From Human Genetics and Genomic Advances
Clozapine may be best choice for cutting SUD risk in schizophrenia
results of a real-world study show.
“Our findings are in line with a recent meta-analysis showing superior efficacy of clozapine in schizophrenia and comorbid SUD and other studies pointing toward clozapine’s superiority over other antipsychotics in the treatment of individuals with schizophrenia and comorbid SUD,” the investigators, led by Jari Tiihonen MD, PhD, department of clinical neuroscience, Karolinska Institutet, Stockholm, write.
“The results on polypharmacy are in line with previous results from nationwide cohorts showing a favorable outcome, compared with oral monotherapies among persons with schizophrenia in general,” they add.
The study was published online Aug. 25 in The British Journal of Psychiatry.
Research gap
Research on the effectiveness of pharmacotherapies for schizophrenia and comorbid SUD is “very sparse, and more importantly, non-existent on the prevention of the development of SUDs in patients with schizophrenia,” the researchers note.
To investigate, they analyzed data on more than 45,000 patients with schizophrenia from Finnish and Swedish national registries, with follow-up lasting 22 years in Finland and 11 years in Sweden.
In patients with schizophrenia without SUD, treatment with clozapine was associated with lowest risk for an initial SUD in both Finland (adjusted hazard ratio, 0.20; 95% confidence interval, 0.16-0.24) and Sweden (aHR, 0.35; 95% CI, 0.24-0.50), compared with no use or use of other antipsychotics.
In Finland, aripiprazole was associated with the second lowest risk for an initial SUD (aHR, 0.36; 95% CI, 0.24-0.55) and antipsychotic polytherapy the third lowest risk (aHR, 0.47; 95% CI, 0.42-0.53).
In Sweden, antipsychotic polytherapy was associated with second lowest risk for an initial SUD (aHR, 0.54; 95% CI, 0.44-0.66) and olanzapine the third lowest risk (aHR, 0.67; 95% CI, 0.53-0.84).
In both countries, the risk for relapse as indicated by psychiatric hospital admission and SUD-related hospital admission were lowest for clozapine, antipsychotic polytherapy and long-acting injectables, the investigators report.
Interpret with caution
Reached for comment, Christoph U. Correll, MD, professor of psychiatry and molecular medicine, the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, urged caution in interpreting the results.
“While the authors are experts in national database analyses and the study was conducted with state-of-the-art methodology, the onset of SUD analyses favoring clozapine are subject to survival bias and order effects,” Dr. Correll said.
“Since clozapine is generally used later in the illness and treatment course, after multiple other antipsychotics have been used, and since SUDs generally occur early in the illness course, most SUDs will already have arisen by the time that clozapine is considered and used,” Dr. Correll said.
“A similar potential bias exists for long-acting injectables (LAIs), as these have generally also been used late in the treatment algorithm,” he noted.
In terms of the significant reduction of SUD-related hospitalizations observed with clozapine, the “order effect” could also be relevant, Dr. Correll said, because over time, patients are less likely to be nonadherent and hospitalized and clozapine is systematically used later in life than other antipsychotics.
“Why antipsychotic polytherapy came out as the second-best treatment is much less clear. Clearly head-to-head randomized trials are needed to follow up on these interesting and intriguing naturalistic database study data,” said Dr. Correll.
This study was funded by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital. Dr. Tiihonen and three co-authors have participated in research projects funded by grants from Janssen-Cilag and Eli Lilly to their institution. Dr. Correll reports having been a consultant and/or advisor to or receiving honoraria from many companies. He has also provided expert testimony for Janssen and Otsuka; served on a Data Safety Monitoring Board for Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva; received royalties from UpToDate; and is a stock option holder of Cardio Diagnostics, Mindpax, LB Pharma, and Quantic.
A version of this article first appeared on Medscape.com.
results of a real-world study show.
“Our findings are in line with a recent meta-analysis showing superior efficacy of clozapine in schizophrenia and comorbid SUD and other studies pointing toward clozapine’s superiority over other antipsychotics in the treatment of individuals with schizophrenia and comorbid SUD,” the investigators, led by Jari Tiihonen MD, PhD, department of clinical neuroscience, Karolinska Institutet, Stockholm, write.
“The results on polypharmacy are in line with previous results from nationwide cohorts showing a favorable outcome, compared with oral monotherapies among persons with schizophrenia in general,” they add.
The study was published online Aug. 25 in The British Journal of Psychiatry.
Research gap
Research on the effectiveness of pharmacotherapies for schizophrenia and comorbid SUD is “very sparse, and more importantly, non-existent on the prevention of the development of SUDs in patients with schizophrenia,” the researchers note.
To investigate, they analyzed data on more than 45,000 patients with schizophrenia from Finnish and Swedish national registries, with follow-up lasting 22 years in Finland and 11 years in Sweden.
In patients with schizophrenia without SUD, treatment with clozapine was associated with lowest risk for an initial SUD in both Finland (adjusted hazard ratio, 0.20; 95% confidence interval, 0.16-0.24) and Sweden (aHR, 0.35; 95% CI, 0.24-0.50), compared with no use or use of other antipsychotics.
In Finland, aripiprazole was associated with the second lowest risk for an initial SUD (aHR, 0.36; 95% CI, 0.24-0.55) and antipsychotic polytherapy the third lowest risk (aHR, 0.47; 95% CI, 0.42-0.53).
In Sweden, antipsychotic polytherapy was associated with second lowest risk for an initial SUD (aHR, 0.54; 95% CI, 0.44-0.66) and olanzapine the third lowest risk (aHR, 0.67; 95% CI, 0.53-0.84).
In both countries, the risk for relapse as indicated by psychiatric hospital admission and SUD-related hospital admission were lowest for clozapine, antipsychotic polytherapy and long-acting injectables, the investigators report.
Interpret with caution
Reached for comment, Christoph U. Correll, MD, professor of psychiatry and molecular medicine, the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, urged caution in interpreting the results.
“While the authors are experts in national database analyses and the study was conducted with state-of-the-art methodology, the onset of SUD analyses favoring clozapine are subject to survival bias and order effects,” Dr. Correll said.
“Since clozapine is generally used later in the illness and treatment course, after multiple other antipsychotics have been used, and since SUDs generally occur early in the illness course, most SUDs will already have arisen by the time that clozapine is considered and used,” Dr. Correll said.
“A similar potential bias exists for long-acting injectables (LAIs), as these have generally also been used late in the treatment algorithm,” he noted.
In terms of the significant reduction of SUD-related hospitalizations observed with clozapine, the “order effect” could also be relevant, Dr. Correll said, because over time, patients are less likely to be nonadherent and hospitalized and clozapine is systematically used later in life than other antipsychotics.
“Why antipsychotic polytherapy came out as the second-best treatment is much less clear. Clearly head-to-head randomized trials are needed to follow up on these interesting and intriguing naturalistic database study data,” said Dr. Correll.
This study was funded by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital. Dr. Tiihonen and three co-authors have participated in research projects funded by grants from Janssen-Cilag and Eli Lilly to their institution. Dr. Correll reports having been a consultant and/or advisor to or receiving honoraria from many companies. He has also provided expert testimony for Janssen and Otsuka; served on a Data Safety Monitoring Board for Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva; received royalties from UpToDate; and is a stock option holder of Cardio Diagnostics, Mindpax, LB Pharma, and Quantic.
A version of this article first appeared on Medscape.com.
results of a real-world study show.
“Our findings are in line with a recent meta-analysis showing superior efficacy of clozapine in schizophrenia and comorbid SUD and other studies pointing toward clozapine’s superiority over other antipsychotics in the treatment of individuals with schizophrenia and comorbid SUD,” the investigators, led by Jari Tiihonen MD, PhD, department of clinical neuroscience, Karolinska Institutet, Stockholm, write.
“The results on polypharmacy are in line with previous results from nationwide cohorts showing a favorable outcome, compared with oral monotherapies among persons with schizophrenia in general,” they add.
The study was published online Aug. 25 in The British Journal of Psychiatry.
Research gap
Research on the effectiveness of pharmacotherapies for schizophrenia and comorbid SUD is “very sparse, and more importantly, non-existent on the prevention of the development of SUDs in patients with schizophrenia,” the researchers note.
To investigate, they analyzed data on more than 45,000 patients with schizophrenia from Finnish and Swedish national registries, with follow-up lasting 22 years in Finland and 11 years in Sweden.
In patients with schizophrenia without SUD, treatment with clozapine was associated with lowest risk for an initial SUD in both Finland (adjusted hazard ratio, 0.20; 95% confidence interval, 0.16-0.24) and Sweden (aHR, 0.35; 95% CI, 0.24-0.50), compared with no use or use of other antipsychotics.
In Finland, aripiprazole was associated with the second lowest risk for an initial SUD (aHR, 0.36; 95% CI, 0.24-0.55) and antipsychotic polytherapy the third lowest risk (aHR, 0.47; 95% CI, 0.42-0.53).
In Sweden, antipsychotic polytherapy was associated with second lowest risk for an initial SUD (aHR, 0.54; 95% CI, 0.44-0.66) and olanzapine the third lowest risk (aHR, 0.67; 95% CI, 0.53-0.84).
In both countries, the risk for relapse as indicated by psychiatric hospital admission and SUD-related hospital admission were lowest for clozapine, antipsychotic polytherapy and long-acting injectables, the investigators report.
Interpret with caution
Reached for comment, Christoph U. Correll, MD, professor of psychiatry and molecular medicine, the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, urged caution in interpreting the results.
“While the authors are experts in national database analyses and the study was conducted with state-of-the-art methodology, the onset of SUD analyses favoring clozapine are subject to survival bias and order effects,” Dr. Correll said.
“Since clozapine is generally used later in the illness and treatment course, after multiple other antipsychotics have been used, and since SUDs generally occur early in the illness course, most SUDs will already have arisen by the time that clozapine is considered and used,” Dr. Correll said.
“A similar potential bias exists for long-acting injectables (LAIs), as these have generally also been used late in the treatment algorithm,” he noted.
In terms of the significant reduction of SUD-related hospitalizations observed with clozapine, the “order effect” could also be relevant, Dr. Correll said, because over time, patients are less likely to be nonadherent and hospitalized and clozapine is systematically used later in life than other antipsychotics.
“Why antipsychotic polytherapy came out as the second-best treatment is much less clear. Clearly head-to-head randomized trials are needed to follow up on these interesting and intriguing naturalistic database study data,” said Dr. Correll.
This study was funded by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital. Dr. Tiihonen and three co-authors have participated in research projects funded by grants from Janssen-Cilag and Eli Lilly to their institution. Dr. Correll reports having been a consultant and/or advisor to or receiving honoraria from many companies. He has also provided expert testimony for Janssen and Otsuka; served on a Data Safety Monitoring Board for Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva; received royalties from UpToDate; and is a stock option holder of Cardio Diagnostics, Mindpax, LB Pharma, and Quantic.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF PSYCHIATRY
Psychedelics may ease fear of death and dying
Psychedelics can produce positive changes in attitudes about death and dying – and may be a way to help ease anxiety and depression toward the end of life, new research suggests.
In a retrospective study of more than 3,000 participants,
“Individuals with existential anxiety and depression at end of life account for substantial suffering and significantly increased health care expenses from desperate and often futile seeking of intensive and expensive medical treatments,” co-investigator Roland Griffiths, PhD, Center for Psychedelics and Consciousness Research at Johns Hopkins Medicine, Baltimore, told this news organization.
“The present findings, which show that both psychedelic and non–drug-occasioned experiences can produce positive and enduring changes in attitudes about death, suggest the importance of future prospective experimental and clinical observational studies to better understand mechanisms of such changes as well as their potential clinical utility in ameliorating suffering related to fear of death,” Dr. Griffiths said.
The results were published online Aug. 24 in PLOS ONE.
Direct comparisons
Both psychedelic drug experiences and near-death experiences can alter perspectives on death and dying, but there have been few direct comparisons of these phenomena, the investigators note.
In the current study, they directly compared psychedelic-occasioned and nondrug experiences, which altered individuals’ beliefs about death.
The researchers surveyed 3,192 mostly White adults from the United States, including 933 who had a natural, nondrug near-death experience and 2,259 who had psychedelic near-death experiences induced with lysergic acid diethylamide, psilocybin, ayahuasca, or N,N-dimethyltryptamine.
The psychedelic group had more men than women and tended to be younger at the time of the experience than was the nondrug group.
Nearly 90% of individuals in both groups said that they were less afraid of death than they were before their experiences.
About half of both groups said they’d encountered something they might call “God” during the experience.
Three-quarters of the psychedelic group and 85% of the nondrug group rated their experiences as among the top five most personally meaningful and spiritually significant events of their life.
Individuals in both groups also reported moderate- to strong-lasting positive changes in personal well-being and life purpose and meaning after their experiences.
However, there were some differences between the groups.
More research needed
Compared with the psychedelic group, the nondrug group was more likely to report being unconscious, clinically dead, or that their life was in imminent danger.
The nonpsychedelic group was also more likely to report that their experience was very brief, lasting 5 minutes or less.
Both the psychedelic and nondrug participants showed robust increases on standardized measures of mystical and near-death experiences, but these measures were significantly greater in the psychedelic group.
The survey findings are in line with several recent clinical trials showing that a single treatment with the psychedelic psilocybin produced sustained decreases in anxiety and depression among patients with a life-threatening cancer diagnosis.
This includes a 2016 study by Dr. Griffiths and colleagues, which included 51 patients with late-stage cancer. As reported at the time, results showed a single, high dose of psilocybin had rapid, clinically significant, and lasting effects on mood and anxiety.
Limitations of the current survey cited by the researchers include the use of retrospective self-report to describe changes in death attitudes and the subjective features of the experiences. Also, respondents were a self-selected study population that may not be representative of all psychedelic or near-death experiences.
In addition, the study did not attempt to document worldview and other belief changes, such as increased belief in afterlife, that might help explain why death attitudes changed.
Looking ahead, the researchers note that future studies are needed to better understand the potential clinical use of psychedelics in ameliorating suffering related to fear of death.
Support through the Johns Hopkins Center for Psychedelic and Consciousness Research was provided by Tim Ferriss, Matt Mullenweg, Blake Mycoskie, Craig Nerenberg, and the Steven and Alexandra Cohen Foundation. Funding was also provided by the Y.C. Ho/Helen and Michael Chiang Foundation. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychedelics can produce positive changes in attitudes about death and dying – and may be a way to help ease anxiety and depression toward the end of life, new research suggests.
In a retrospective study of more than 3,000 participants,
“Individuals with existential anxiety and depression at end of life account for substantial suffering and significantly increased health care expenses from desperate and often futile seeking of intensive and expensive medical treatments,” co-investigator Roland Griffiths, PhD, Center for Psychedelics and Consciousness Research at Johns Hopkins Medicine, Baltimore, told this news organization.
“The present findings, which show that both psychedelic and non–drug-occasioned experiences can produce positive and enduring changes in attitudes about death, suggest the importance of future prospective experimental and clinical observational studies to better understand mechanisms of such changes as well as their potential clinical utility in ameliorating suffering related to fear of death,” Dr. Griffiths said.
The results were published online Aug. 24 in PLOS ONE.
Direct comparisons
Both psychedelic drug experiences and near-death experiences can alter perspectives on death and dying, but there have been few direct comparisons of these phenomena, the investigators note.
In the current study, they directly compared psychedelic-occasioned and nondrug experiences, which altered individuals’ beliefs about death.
The researchers surveyed 3,192 mostly White adults from the United States, including 933 who had a natural, nondrug near-death experience and 2,259 who had psychedelic near-death experiences induced with lysergic acid diethylamide, psilocybin, ayahuasca, or N,N-dimethyltryptamine.
The psychedelic group had more men than women and tended to be younger at the time of the experience than was the nondrug group.
Nearly 90% of individuals in both groups said that they were less afraid of death than they were before their experiences.
About half of both groups said they’d encountered something they might call “God” during the experience.
Three-quarters of the psychedelic group and 85% of the nondrug group rated their experiences as among the top five most personally meaningful and spiritually significant events of their life.
Individuals in both groups also reported moderate- to strong-lasting positive changes in personal well-being and life purpose and meaning after their experiences.
However, there were some differences between the groups.
More research needed
Compared with the psychedelic group, the nondrug group was more likely to report being unconscious, clinically dead, or that their life was in imminent danger.
The nonpsychedelic group was also more likely to report that their experience was very brief, lasting 5 minutes or less.
Both the psychedelic and nondrug participants showed robust increases on standardized measures of mystical and near-death experiences, but these measures were significantly greater in the psychedelic group.
The survey findings are in line with several recent clinical trials showing that a single treatment with the psychedelic psilocybin produced sustained decreases in anxiety and depression among patients with a life-threatening cancer diagnosis.
This includes a 2016 study by Dr. Griffiths and colleagues, which included 51 patients with late-stage cancer. As reported at the time, results showed a single, high dose of psilocybin had rapid, clinically significant, and lasting effects on mood and anxiety.
Limitations of the current survey cited by the researchers include the use of retrospective self-report to describe changes in death attitudes and the subjective features of the experiences. Also, respondents were a self-selected study population that may not be representative of all psychedelic or near-death experiences.
In addition, the study did not attempt to document worldview and other belief changes, such as increased belief in afterlife, that might help explain why death attitudes changed.
Looking ahead, the researchers note that future studies are needed to better understand the potential clinical use of psychedelics in ameliorating suffering related to fear of death.
Support through the Johns Hopkins Center for Psychedelic and Consciousness Research was provided by Tim Ferriss, Matt Mullenweg, Blake Mycoskie, Craig Nerenberg, and the Steven and Alexandra Cohen Foundation. Funding was also provided by the Y.C. Ho/Helen and Michael Chiang Foundation. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychedelics can produce positive changes in attitudes about death and dying – and may be a way to help ease anxiety and depression toward the end of life, new research suggests.
In a retrospective study of more than 3,000 participants,
“Individuals with existential anxiety and depression at end of life account for substantial suffering and significantly increased health care expenses from desperate and often futile seeking of intensive and expensive medical treatments,” co-investigator Roland Griffiths, PhD, Center for Psychedelics and Consciousness Research at Johns Hopkins Medicine, Baltimore, told this news organization.
“The present findings, which show that both psychedelic and non–drug-occasioned experiences can produce positive and enduring changes in attitudes about death, suggest the importance of future prospective experimental and clinical observational studies to better understand mechanisms of such changes as well as their potential clinical utility in ameliorating suffering related to fear of death,” Dr. Griffiths said.
The results were published online Aug. 24 in PLOS ONE.
Direct comparisons
Both psychedelic drug experiences and near-death experiences can alter perspectives on death and dying, but there have been few direct comparisons of these phenomena, the investigators note.
In the current study, they directly compared psychedelic-occasioned and nondrug experiences, which altered individuals’ beliefs about death.
The researchers surveyed 3,192 mostly White adults from the United States, including 933 who had a natural, nondrug near-death experience and 2,259 who had psychedelic near-death experiences induced with lysergic acid diethylamide, psilocybin, ayahuasca, or N,N-dimethyltryptamine.
The psychedelic group had more men than women and tended to be younger at the time of the experience than was the nondrug group.
Nearly 90% of individuals in both groups said that they were less afraid of death than they were before their experiences.
About half of both groups said they’d encountered something they might call “God” during the experience.
Three-quarters of the psychedelic group and 85% of the nondrug group rated their experiences as among the top five most personally meaningful and spiritually significant events of their life.
Individuals in both groups also reported moderate- to strong-lasting positive changes in personal well-being and life purpose and meaning after their experiences.
However, there were some differences between the groups.
More research needed
Compared with the psychedelic group, the nondrug group was more likely to report being unconscious, clinically dead, or that their life was in imminent danger.
The nonpsychedelic group was also more likely to report that their experience was very brief, lasting 5 minutes or less.
Both the psychedelic and nondrug participants showed robust increases on standardized measures of mystical and near-death experiences, but these measures were significantly greater in the psychedelic group.
The survey findings are in line with several recent clinical trials showing that a single treatment with the psychedelic psilocybin produced sustained decreases in anxiety and depression among patients with a life-threatening cancer diagnosis.
This includes a 2016 study by Dr. Griffiths and colleagues, which included 51 patients with late-stage cancer. As reported at the time, results showed a single, high dose of psilocybin had rapid, clinically significant, and lasting effects on mood and anxiety.
Limitations of the current survey cited by the researchers include the use of retrospective self-report to describe changes in death attitudes and the subjective features of the experiences. Also, respondents were a self-selected study population that may not be representative of all psychedelic or near-death experiences.
In addition, the study did not attempt to document worldview and other belief changes, such as increased belief in afterlife, that might help explain why death attitudes changed.
Looking ahead, the researchers note that future studies are needed to better understand the potential clinical use of psychedelics in ameliorating suffering related to fear of death.
Support through the Johns Hopkins Center for Psychedelic and Consciousness Research was provided by Tim Ferriss, Matt Mullenweg, Blake Mycoskie, Craig Nerenberg, and the Steven and Alexandra Cohen Foundation. Funding was also provided by the Y.C. Ho/Helen and Michael Chiang Foundation. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
Prior psychological distress tied to ‘long-COVID’ conditions
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.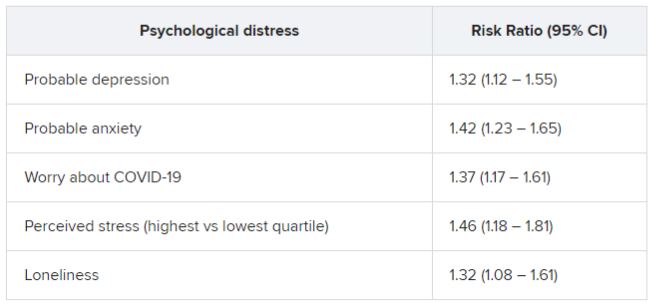
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an analysis of almost 55,000 adult participants in three ongoing studies, having depression, anxiety, worry, perceived stress, or loneliness early in the pandemic, before SARS-CoV-2 infection, was associated with a 50% increased risk for developing long COVID. These types of psychological distress were also associated with a 15% to 51% greater risk for impairment in daily life among individuals with long COVID.
Psychological distress was even more strongly associated with developing long COVID than were physical health risk factors, and the increased risk was not explained by health behaviors such as smoking or physical comorbidities, researchers note.
“Our findings suggest the need to consider psychological health in addition to physical health as risk factors of long COVID-19,” lead author Siwen Wang, MD, postdoctoral fellow, department of nutrition, Harvard T. H. Chan School of Public Health, Boston, said in an interview.
“We need to increase public awareness of the importance of mental health and focus on getting mental health care for people who need it, increasing the supply of mental health clinicians and improving access to care,” she said.
The findings were published online in JAMA Psychiatry.
‘Poorly understood’
Postacute sequelae of SARS-CoV-2 (“long COVID”), which are “signs and symptoms consistent with COVID-19 that extend beyond 4 weeks from onset of infection” constitute “an emerging health issue,” the investigators write.
Dr. Wang noted that it has been estimated that 8-23 million Americans have developed long COVID. However, “despite the high prevalence and daily life impairment associated with long COVID, it is still poorly understood, and few risk factors have been established,” she said.
Although psychological distress may be implicated in long COVID, only three previous studies investigated psychological factors as potential contributors, the researchers note. Also, no study has investigated the potential role of other common manifestations of distress that have increased during the pandemic, such as loneliness and perceived stress, they add.
To investigate these issues, the researchers turned to three large ongoing longitudinal studies: the Nurses’ Health Study II (NSHII), the Nurses’ Health study 3 (NHS3), and the Growing Up Today Study (GUTS).
They analyzed data on 54,960 total participants (96.6% women; mean age, 57.5 years). Of the full group, 38% were active health care workers.
Participants completed an online COVID-19 questionnaire from April 2020 to Sept. 1, 2020 (baseline), and monthly surveys thereafter. Beginning in August 2020, surveys were administered quarterly. The end of follow-up was in November 2021.
The COVID questionnaires included questions about positive SARS-CoV-2 test results, COVID symptoms and hospitalization since March 1, 2020, and the presence of long-term COVID symptoms, such as fatigue, respiratory problems, persistent cough, muscle/joint/chest pain, smell/taste problems, confusion/disorientation/brain fog, depression/anxiety/changes in mood, headache, and memory problems.
Participants who reported these post-COVID conditions were asked about the frequency of symptoms and the degree of impairment in daily life.
Inflammation, immune dysregulation implicated?
The Patient Health Questionnaire–4 (PHQ-4) was used to assess for anxiety and depressive symptoms in the past 2 weeks. It consists of a two-item depression measure (PHQ-2) and a two-item Generalized Anxiety Disorder Scale (GAD-2).
Non–health care providers completed two additional assessments of psychological distress: the four-item Perceived Stress Scale and the three-item UCLA Loneliness Scale.
The researchers included demographic factors, weight, smoking status, marital status, and medical conditions, including diabetes, hypertension, hypercholesterolemia, asthma, and cancer, and socioeconomic factors as covariates.
For each participant, the investigators calculated the number of types of distress experienced at a high level, including probable depression, probable anxiety, worry about COVID-19, being in the top quartile of perceived stress, and loneliness.
During the 19 months of follow-up (1-47 weeks after baseline), 6% of respondents reported a positive result on a SARS-CoV-2 antibody, antigen, or polymerase chain reaction test.
Of these, 43.9% reported long-COVID conditions, with most reporting that symptoms lasted 2 months or longer; 55.8% reported at least occasional daily life impairment.
The most common post-COVID conditions were fatigue (reported by 56%), loss of smell or taste problems (44.6%), shortness of breath (25.5%), confusion/disorientation/ brain fog (24.5%), and memory issues (21.8%).
Among patients who had been infected, there was a considerably higher rate of preinfection psychological distress after adjusting for sociodemographic factors, health behaviors, and comorbidities. Each type of distress was associated with post-COVID conditions.
In addition, participants who had experienced at least two types of distress prior to infection were at nearly 50% increased risk for post–COVID conditions (risk ratio, 1.49; 95% confidence interval, 1.23-1.80).
Among those with post-COVID conditions, all types of distress were associated with increased risk for daily life impairment (RR range, 1.15-1.51).
Senior author Andrea Roberts, PhD, senior research scientist at the Harvard T. H. Chan School of Public Health, Boston, noted that the investigators did not examine biological mechanisms potentially underlying the association they found.
However, “based on prior research, it may be that inflammation and immune dysregulation related to psychological distress play a role in the association of distress with long COVID, but we can’t be sure,” Dr. Roberts said.
Contributes to the field
Commenting for this article, Yapeng Su, PhD, a postdoctoral researcher at the Fred Hutchinson Cancer Research Center in Seattle, called the study “great work contributing to the long-COVID research field and revealing important connections” with psychological stress prior to infection.
Dr. Su, who was not involved with the study, was previously at the Institute for Systems Biology, also in Seattle, and has written about long COVID.
He noted that the “biological mechanism of such intriguing linkage is definitely the important next step, which will likely require deep phenotyping of biological specimens from these patients longitudinally.”
Dr. Wang pointed to past research suggesting that some patients with mental illness “sometimes develop autoantibodies that have also been associated with increased risk of long COVID.” In addition, depression “affects the brain in ways that may explain certain cognitive symptoms in long COVID,” she added.
More studies are now needed to understand how psychological distress increases the risk for long COVID, said Dr. Wang.
The research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institutes of Health, the Dean’s Fund for Scientific Advancement Acceleration Award from the Harvard T. H. Chan School of Public Health, the Massachusetts Consortium on Pathogen Readiness Evergrande COVID-19 Response Fund Award, and the Veterans Affairs Health Services Research and Development Service funds. Dr. Wang and Dr. Roberts have reported no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Su reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Flashy, blingy doc sabotages his own malpractice trial in rural farm town
During a medical malpractice trial in New Jersey, jurors waited nearly 4 hours for the physician defendant to show up. When he did arrive, the body-building surgeon was sporting two thick gold chains and a diamond pinky ring, and had the top buttons of his shirt open enough to reveal his chest hair.
“This trial was in a very rural, farming community,” recalls medical liability defense attorney Catherine Flynn, of Flynn Watts LLC, based in Parsippany, N.J. “Many of the jurors were wearing flannel shirts and jeans. The doctor’s wife walked in wearing a five-carat diamond ring and other jewelry.”
Ms. Flynn took the couple aside and asked them to remove the jewelry. She explained that the opulent accessories could damage the jury’s view of the physician. The surgeon and his wife, however, refused to remove their jewelry, she said. They didn’t think it was a big deal.
The case against the surgeon involved intraoperative damage to a patient when the physician inadvertently removed a portion of nerve in the area of the procedure. After repair of the nerve, the patient had a positive result. However, the patient alleged the surgeon’s negligence resulted in permanent damage despite the successful repair.
Jurors ultimately found the physician negligent in the case and awarded the plaintiff $1.2 million. Ms. Flynn believes that physician’s flamboyant attire and arrogant nature tainted the jury’s decision.
“In certain counties in New Jersey, his attire would not have been a problem,” she said. “In this rural, farming county, it was a huge problem. You have to know your audience. There are a lot of other things that come into play in a medical malpractice case, but when it comes to damages in a case, you don’t want to be sending the message that supports what somebody’s bias may already be telling them about a doctor.”
The surgeon appealed the verdict, and the case ultimately settled for a lesser amount, according to Ms. Flynn.
An over-the-top wardrobe is just one way that physicians can negatively influence jurors during legal trials. From subtle facial expressions to sudden outbursts to downright rudeness, attorneys have witnessed countless examples of physicians sabotaging their own trials.
“The minute you enter the courthouse, jurors or potential jurors are sizing you up,” says health law attorney Michael Clark, of Womble Bond Dickinson (US) LLP, based in Houston. “The same phenomenon occurs in a deposition. Awareness of how you are being assessed at all times, and the image that is needed, is important since a negative impression by jurors can have a detrimental effect on a physician’s case.”
Juror: We didn’t like the doctor’s shoes
In another case, attorneys warned a physician defendant against dressing in his signature wardrobe during his trial. Against their advice, the doctor showed up daily to his trial in bright pastel, monochromatic suits with matching Gucci-brand shoes, said medical liability defense attorney Meredith C. Lander, of Kaufman Borgeest & Ryan LLP, based in Connecticut. On the witness stand, the doctor was long-winded and wasn’t “terribly likable,” Ms. Lander said.
However, the evidence weighed in the physician’s favor, and there was strong testimony by defense experts. The physician won the case, Ms. Lander said, but after the verdict, the jury foreperson approached the trial attorney and made some disparaging remarks about the defendant.
“The foreperson said the jury didn’t like the doctor or his ‘Gucci suits and shoes,’ but they believed the experts,” Ms. Lander said.
Disruptive behavior can also harm jurors’ perception of physicians, Ms. Flynn adds. During one instance, a surgeon insisted on sitting next to Ms. Flynn, although she generally requests clients sit in the first row so that jurors are not so focused on their reactions during testimony. The surgeon loudly peppered Ms. Flynn with questions as witnesses testified, prompting a reprimand from the judge.
“The judge admonished the doctor several times and said, ‘Doctor, you’re raising your voice. You’ll get a chance to speak with your attorney during the break,’ ” Ms. Flynn recalled. “The doctor refused to stop talking, and the judge told him in front of the jury to go sit in the back of the courtroom. His reaction was, ‘Why do I have to move?! I need to sit here!’ ”
The surgeon eventually moved to the back of the courtroom and a sheriff’s deputy stood next to him. Testimony continued until a note in the form of a paper airplane landed on the table in front of Ms. Flynn. She carefully crumpled the note and tossed it in the wastebasket. Luckily, this drew a laugh from jurors, she said.
But things got worse when the surgeon testified. Rather than answer the questions, he interrupted and started telling jurors his own version of events.
“The judge finally said, ‘Doctor, if you don’t listen to your attorney and answer her questions, I’m going to make you get off the stand,’ ” Ms. Flynn said. “That was the most unbelievable, egregious self-sabotage trial moment I’ve ever experienced.”
Fortunately, the physician’s legal case was strong, and the experts who testified drove the defense’s side home, Ms. Flynn said. The surgeon won the case.
Attorney: Watch what you say in the elevator
Other, more subtle behaviors – while often unintentional – can also be damaging.
Physicians often let their guard down while outside the courtroom and can unknowingly wind up next to a juror in an elevator or standing in a hallway, said Laura Postilion, a partner at Quintairos, Prieto, Wood & Boyer, P.A., based in Chicago.
“For instance, a doctor is in an elevator and feels that some witness on the stand was lying,” Ms. Postilion said. “They might be very upset about it and start ranting about a witness lying, not realizing there is a juror is in the elevator with you.”
Physicians should also be cautious when speaking on the phone to their family or friends during a trial break.
“At the Daley Center in downtown Chicago, there are these long corridors and long line of windows; a lot of people will stand there during breaks. A doctor may be talking to his or her spouse and saying, ‘Yeah, this juror is sleeping!’ Jurors are [often] looking for drama. They’re looking for somebody letting their guard down. Hearing a doctor speak badly about them would certainly give them a reason to dislike the physician.”
Ms. Postilion warns against talking about jurors in or outside of the courtroom. This includes parking structures, she said.
Physicians can take additional steps to save themselves from negative judgment from jurors, attorneys say. Even before the trial starts, Ms. Postilion advises clients to make their social media accounts private. Some curious jurors may look up a physician’s social media accounts to learn more about their personal life, political leanings, or social beliefs, which could prejudice them against the doctor, she said.
Once on the stand, the words and tone used are key. The last thing a physician defendant wants is to come across as arrogant or condescending to jurors, said medical liability defense attorney Michael Moroney, of Flynn Watts LLC.
“For instance, a defendant might say, ‘Well, let me make this simple for you,’ as if they’re talking to a bunch of schoolchildren,” he said. “You don’t know who’s on the jury. That type of language can be offensive.”
Ms. Lander counsels her clients to refrain from using the common phrase, “honestly,” before answering questions on the stand.
“Everything you’re saying on the stand is presumed to be honest,” she said. “When you start an answer with, ‘Honestly…’ out of habit, it really does undercut everything that follows and everything else that’s already been said. It suggests that you were not being honest in your other answers.”
Attitude, body language speak volumes
Keep in mind that plaintiffs’ attorneys will try their best to rattle physicians on the stand and get them to appear unlikeable, says Mr. Clark, the Houston-based health law attorney. Physicians who lose their cool and begin arguing with attorneys play into their strategy.
“Plaintiffs’ attorneys have been trained in ways to get under their skin,” he said. “Righteous indignation and annoyance are best left for a rare occasion. Think about how you feel in a social setting when people are bickering in front of you. It’s uncomfortable at best. That’s how a jury feels too.”
Body language is also important, Mr. Clark notes. Physicians should avoid crossed arms, leaning back and rocking, or putting a hand on their mouth while testifying, he said. Many attorneys have practice sessions with their clients and record the interaction so that doctors can watch it and see how they look.
“Know your strengths and weaknesses,” he said. “Get help from your lawyer and perhaps consultants about how to improve these skills. Practice and preparation are important.”
Ms. Postilion goes over courtroom clothing with physician clients before trial. Anything “too flashy, too high-end, or too dumpy” should be avoided, she said. Getting accustomed to the courtroom and practicing in an empty courtroom are good ways to ensure that a physician’s voice is loud enough and projecting far enough in the courtroom, she adds.
“The doctor should try to be the best version of him- or herself to jurors,” she said. “A jury can pick up someone who’s trying to be something they’re not. A good attorney can help the doctor find the best version of themselves and capitalize on it. What is it that you want the jury to know about your care of the patient? Take that overall feeling and make sure it’s clearly expressed to the jury.”
A version of this article first appeared on Medscape.com.
During a medical malpractice trial in New Jersey, jurors waited nearly 4 hours for the physician defendant to show up. When he did arrive, the body-building surgeon was sporting two thick gold chains and a diamond pinky ring, and had the top buttons of his shirt open enough to reveal his chest hair.
“This trial was in a very rural, farming community,” recalls medical liability defense attorney Catherine Flynn, of Flynn Watts LLC, based in Parsippany, N.J. “Many of the jurors were wearing flannel shirts and jeans. The doctor’s wife walked in wearing a five-carat diamond ring and other jewelry.”
Ms. Flynn took the couple aside and asked them to remove the jewelry. She explained that the opulent accessories could damage the jury’s view of the physician. The surgeon and his wife, however, refused to remove their jewelry, she said. They didn’t think it was a big deal.
The case against the surgeon involved intraoperative damage to a patient when the physician inadvertently removed a portion of nerve in the area of the procedure. After repair of the nerve, the patient had a positive result. However, the patient alleged the surgeon’s negligence resulted in permanent damage despite the successful repair.
Jurors ultimately found the physician negligent in the case and awarded the plaintiff $1.2 million. Ms. Flynn believes that physician’s flamboyant attire and arrogant nature tainted the jury’s decision.
“In certain counties in New Jersey, his attire would not have been a problem,” she said. “In this rural, farming county, it was a huge problem. You have to know your audience. There are a lot of other things that come into play in a medical malpractice case, but when it comes to damages in a case, you don’t want to be sending the message that supports what somebody’s bias may already be telling them about a doctor.”
The surgeon appealed the verdict, and the case ultimately settled for a lesser amount, according to Ms. Flynn.
An over-the-top wardrobe is just one way that physicians can negatively influence jurors during legal trials. From subtle facial expressions to sudden outbursts to downright rudeness, attorneys have witnessed countless examples of physicians sabotaging their own trials.
“The minute you enter the courthouse, jurors or potential jurors are sizing you up,” says health law attorney Michael Clark, of Womble Bond Dickinson (US) LLP, based in Houston. “The same phenomenon occurs in a deposition. Awareness of how you are being assessed at all times, and the image that is needed, is important since a negative impression by jurors can have a detrimental effect on a physician’s case.”
Juror: We didn’t like the doctor’s shoes
In another case, attorneys warned a physician defendant against dressing in his signature wardrobe during his trial. Against their advice, the doctor showed up daily to his trial in bright pastel, monochromatic suits with matching Gucci-brand shoes, said medical liability defense attorney Meredith C. Lander, of Kaufman Borgeest & Ryan LLP, based in Connecticut. On the witness stand, the doctor was long-winded and wasn’t “terribly likable,” Ms. Lander said.
However, the evidence weighed in the physician’s favor, and there was strong testimony by defense experts. The physician won the case, Ms. Lander said, but after the verdict, the jury foreperson approached the trial attorney and made some disparaging remarks about the defendant.
“The foreperson said the jury didn’t like the doctor or his ‘Gucci suits and shoes,’ but they believed the experts,” Ms. Lander said.
Disruptive behavior can also harm jurors’ perception of physicians, Ms. Flynn adds. During one instance, a surgeon insisted on sitting next to Ms. Flynn, although she generally requests clients sit in the first row so that jurors are not so focused on their reactions during testimony. The surgeon loudly peppered Ms. Flynn with questions as witnesses testified, prompting a reprimand from the judge.
“The judge admonished the doctor several times and said, ‘Doctor, you’re raising your voice. You’ll get a chance to speak with your attorney during the break,’ ” Ms. Flynn recalled. “The doctor refused to stop talking, and the judge told him in front of the jury to go sit in the back of the courtroom. His reaction was, ‘Why do I have to move?! I need to sit here!’ ”
The surgeon eventually moved to the back of the courtroom and a sheriff’s deputy stood next to him. Testimony continued until a note in the form of a paper airplane landed on the table in front of Ms. Flynn. She carefully crumpled the note and tossed it in the wastebasket. Luckily, this drew a laugh from jurors, she said.
But things got worse when the surgeon testified. Rather than answer the questions, he interrupted and started telling jurors his own version of events.
“The judge finally said, ‘Doctor, if you don’t listen to your attorney and answer her questions, I’m going to make you get off the stand,’ ” Ms. Flynn said. “That was the most unbelievable, egregious self-sabotage trial moment I’ve ever experienced.”
Fortunately, the physician’s legal case was strong, and the experts who testified drove the defense’s side home, Ms. Flynn said. The surgeon won the case.
Attorney: Watch what you say in the elevator
Other, more subtle behaviors – while often unintentional – can also be damaging.
Physicians often let their guard down while outside the courtroom and can unknowingly wind up next to a juror in an elevator or standing in a hallway, said Laura Postilion, a partner at Quintairos, Prieto, Wood & Boyer, P.A., based in Chicago.
“For instance, a doctor is in an elevator and feels that some witness on the stand was lying,” Ms. Postilion said. “They might be very upset about it and start ranting about a witness lying, not realizing there is a juror is in the elevator with you.”
Physicians should also be cautious when speaking on the phone to their family or friends during a trial break.
“At the Daley Center in downtown Chicago, there are these long corridors and long line of windows; a lot of people will stand there during breaks. A doctor may be talking to his or her spouse and saying, ‘Yeah, this juror is sleeping!’ Jurors are [often] looking for drama. They’re looking for somebody letting their guard down. Hearing a doctor speak badly about them would certainly give them a reason to dislike the physician.”
Ms. Postilion warns against talking about jurors in or outside of the courtroom. This includes parking structures, she said.
Physicians can take additional steps to save themselves from negative judgment from jurors, attorneys say. Even before the trial starts, Ms. Postilion advises clients to make their social media accounts private. Some curious jurors may look up a physician’s social media accounts to learn more about their personal life, political leanings, or social beliefs, which could prejudice them against the doctor, she said.
Once on the stand, the words and tone used are key. The last thing a physician defendant wants is to come across as arrogant or condescending to jurors, said medical liability defense attorney Michael Moroney, of Flynn Watts LLC.
“For instance, a defendant might say, ‘Well, let me make this simple for you,’ as if they’re talking to a bunch of schoolchildren,” he said. “You don’t know who’s on the jury. That type of language can be offensive.”
Ms. Lander counsels her clients to refrain from using the common phrase, “honestly,” before answering questions on the stand.
“Everything you’re saying on the stand is presumed to be honest,” she said. “When you start an answer with, ‘Honestly…’ out of habit, it really does undercut everything that follows and everything else that’s already been said. It suggests that you were not being honest in your other answers.”
Attitude, body language speak volumes
Keep in mind that plaintiffs’ attorneys will try their best to rattle physicians on the stand and get them to appear unlikeable, says Mr. Clark, the Houston-based health law attorney. Physicians who lose their cool and begin arguing with attorneys play into their strategy.
“Plaintiffs’ attorneys have been trained in ways to get under their skin,” he said. “Righteous indignation and annoyance are best left for a rare occasion. Think about how you feel in a social setting when people are bickering in front of you. It’s uncomfortable at best. That’s how a jury feels too.”
Body language is also important, Mr. Clark notes. Physicians should avoid crossed arms, leaning back and rocking, or putting a hand on their mouth while testifying, he said. Many attorneys have practice sessions with their clients and record the interaction so that doctors can watch it and see how they look.
“Know your strengths and weaknesses,” he said. “Get help from your lawyer and perhaps consultants about how to improve these skills. Practice and preparation are important.”
Ms. Postilion goes over courtroom clothing with physician clients before trial. Anything “too flashy, too high-end, or too dumpy” should be avoided, she said. Getting accustomed to the courtroom and practicing in an empty courtroom are good ways to ensure that a physician’s voice is loud enough and projecting far enough in the courtroom, she adds.
“The doctor should try to be the best version of him- or herself to jurors,” she said. “A jury can pick up someone who’s trying to be something they’re not. A good attorney can help the doctor find the best version of themselves and capitalize on it. What is it that you want the jury to know about your care of the patient? Take that overall feeling and make sure it’s clearly expressed to the jury.”
A version of this article first appeared on Medscape.com.
During a medical malpractice trial in New Jersey, jurors waited nearly 4 hours for the physician defendant to show up. When he did arrive, the body-building surgeon was sporting two thick gold chains and a diamond pinky ring, and had the top buttons of his shirt open enough to reveal his chest hair.
“This trial was in a very rural, farming community,” recalls medical liability defense attorney Catherine Flynn, of Flynn Watts LLC, based in Parsippany, N.J. “Many of the jurors were wearing flannel shirts and jeans. The doctor’s wife walked in wearing a five-carat diamond ring and other jewelry.”
Ms. Flynn took the couple aside and asked them to remove the jewelry. She explained that the opulent accessories could damage the jury’s view of the physician. The surgeon and his wife, however, refused to remove their jewelry, she said. They didn’t think it was a big deal.
The case against the surgeon involved intraoperative damage to a patient when the physician inadvertently removed a portion of nerve in the area of the procedure. After repair of the nerve, the patient had a positive result. However, the patient alleged the surgeon’s negligence resulted in permanent damage despite the successful repair.
Jurors ultimately found the physician negligent in the case and awarded the plaintiff $1.2 million. Ms. Flynn believes that physician’s flamboyant attire and arrogant nature tainted the jury’s decision.
“In certain counties in New Jersey, his attire would not have been a problem,” she said. “In this rural, farming county, it was a huge problem. You have to know your audience. There are a lot of other things that come into play in a medical malpractice case, but when it comes to damages in a case, you don’t want to be sending the message that supports what somebody’s bias may already be telling them about a doctor.”
The surgeon appealed the verdict, and the case ultimately settled for a lesser amount, according to Ms. Flynn.
An over-the-top wardrobe is just one way that physicians can negatively influence jurors during legal trials. From subtle facial expressions to sudden outbursts to downright rudeness, attorneys have witnessed countless examples of physicians sabotaging their own trials.
“The minute you enter the courthouse, jurors or potential jurors are sizing you up,” says health law attorney Michael Clark, of Womble Bond Dickinson (US) LLP, based in Houston. “The same phenomenon occurs in a deposition. Awareness of how you are being assessed at all times, and the image that is needed, is important since a negative impression by jurors can have a detrimental effect on a physician’s case.”
Juror: We didn’t like the doctor’s shoes
In another case, attorneys warned a physician defendant against dressing in his signature wardrobe during his trial. Against their advice, the doctor showed up daily to his trial in bright pastel, monochromatic suits with matching Gucci-brand shoes, said medical liability defense attorney Meredith C. Lander, of Kaufman Borgeest & Ryan LLP, based in Connecticut. On the witness stand, the doctor was long-winded and wasn’t “terribly likable,” Ms. Lander said.
However, the evidence weighed in the physician’s favor, and there was strong testimony by defense experts. The physician won the case, Ms. Lander said, but after the verdict, the jury foreperson approached the trial attorney and made some disparaging remarks about the defendant.
“The foreperson said the jury didn’t like the doctor or his ‘Gucci suits and shoes,’ but they believed the experts,” Ms. Lander said.
Disruptive behavior can also harm jurors’ perception of physicians, Ms. Flynn adds. During one instance, a surgeon insisted on sitting next to Ms. Flynn, although she generally requests clients sit in the first row so that jurors are not so focused on their reactions during testimony. The surgeon loudly peppered Ms. Flynn with questions as witnesses testified, prompting a reprimand from the judge.
“The judge admonished the doctor several times and said, ‘Doctor, you’re raising your voice. You’ll get a chance to speak with your attorney during the break,’ ” Ms. Flynn recalled. “The doctor refused to stop talking, and the judge told him in front of the jury to go sit in the back of the courtroom. His reaction was, ‘Why do I have to move?! I need to sit here!’ ”
The surgeon eventually moved to the back of the courtroom and a sheriff’s deputy stood next to him. Testimony continued until a note in the form of a paper airplane landed on the table in front of Ms. Flynn. She carefully crumpled the note and tossed it in the wastebasket. Luckily, this drew a laugh from jurors, she said.
But things got worse when the surgeon testified. Rather than answer the questions, he interrupted and started telling jurors his own version of events.
“The judge finally said, ‘Doctor, if you don’t listen to your attorney and answer her questions, I’m going to make you get off the stand,’ ” Ms. Flynn said. “That was the most unbelievable, egregious self-sabotage trial moment I’ve ever experienced.”
Fortunately, the physician’s legal case was strong, and the experts who testified drove the defense’s side home, Ms. Flynn said. The surgeon won the case.
Attorney: Watch what you say in the elevator
Other, more subtle behaviors – while often unintentional – can also be damaging.
Physicians often let their guard down while outside the courtroom and can unknowingly wind up next to a juror in an elevator or standing in a hallway, said Laura Postilion, a partner at Quintairos, Prieto, Wood & Boyer, P.A., based in Chicago.
“For instance, a doctor is in an elevator and feels that some witness on the stand was lying,” Ms. Postilion said. “They might be very upset about it and start ranting about a witness lying, not realizing there is a juror is in the elevator with you.”
Physicians should also be cautious when speaking on the phone to their family or friends during a trial break.
“At the Daley Center in downtown Chicago, there are these long corridors and long line of windows; a lot of people will stand there during breaks. A doctor may be talking to his or her spouse and saying, ‘Yeah, this juror is sleeping!’ Jurors are [often] looking for drama. They’re looking for somebody letting their guard down. Hearing a doctor speak badly about them would certainly give them a reason to dislike the physician.”
Ms. Postilion warns against talking about jurors in or outside of the courtroom. This includes parking structures, she said.
Physicians can take additional steps to save themselves from negative judgment from jurors, attorneys say. Even before the trial starts, Ms. Postilion advises clients to make their social media accounts private. Some curious jurors may look up a physician’s social media accounts to learn more about their personal life, political leanings, or social beliefs, which could prejudice them against the doctor, she said.
Once on the stand, the words and tone used are key. The last thing a physician defendant wants is to come across as arrogant or condescending to jurors, said medical liability defense attorney Michael Moroney, of Flynn Watts LLC.
“For instance, a defendant might say, ‘Well, let me make this simple for you,’ as if they’re talking to a bunch of schoolchildren,” he said. “You don’t know who’s on the jury. That type of language can be offensive.”
Ms. Lander counsels her clients to refrain from using the common phrase, “honestly,” before answering questions on the stand.
“Everything you’re saying on the stand is presumed to be honest,” she said. “When you start an answer with, ‘Honestly…’ out of habit, it really does undercut everything that follows and everything else that’s already been said. It suggests that you were not being honest in your other answers.”
Attitude, body language speak volumes
Keep in mind that plaintiffs’ attorneys will try their best to rattle physicians on the stand and get them to appear unlikeable, says Mr. Clark, the Houston-based health law attorney. Physicians who lose their cool and begin arguing with attorneys play into their strategy.
“Plaintiffs’ attorneys have been trained in ways to get under their skin,” he said. “Righteous indignation and annoyance are best left for a rare occasion. Think about how you feel in a social setting when people are bickering in front of you. It’s uncomfortable at best. That’s how a jury feels too.”
Body language is also important, Mr. Clark notes. Physicians should avoid crossed arms, leaning back and rocking, or putting a hand on their mouth while testifying, he said. Many attorneys have practice sessions with their clients and record the interaction so that doctors can watch it and see how they look.
“Know your strengths and weaknesses,” he said. “Get help from your lawyer and perhaps consultants about how to improve these skills. Practice and preparation are important.”
Ms. Postilion goes over courtroom clothing with physician clients before trial. Anything “too flashy, too high-end, or too dumpy” should be avoided, she said. Getting accustomed to the courtroom and practicing in an empty courtroom are good ways to ensure that a physician’s voice is loud enough and projecting far enough in the courtroom, she adds.
“The doctor should try to be the best version of him- or herself to jurors,” she said. “A jury can pick up someone who’s trying to be something they’re not. A good attorney can help the doctor find the best version of themselves and capitalize on it. What is it that you want the jury to know about your care of the patient? Take that overall feeling and make sure it’s clearly expressed to the jury.”
A version of this article first appeared on Medscape.com.
Parent training pays off for children with autism
“Referrals for parent training should now be considered the expected standard for medical practice,” said a member of the research team, Timothy B. Smith, PhD, a professor of psychology at Brigham Young University, Provo, Utah.
Programs that show parents how to teach functional skills and address maladaptive behaviors, also known as parent-mediated or parent-implemented interventions, offer an alternative to one-on-one professional services, which are in short supply, according to the paper, which was published in the Journal of Autism and Developmental Disorders.
Methods and results
The meta-analysis included 54 papers based on randomized clinical trials involving 2,895 children, which compared the effects of various parent interventions with professional treatment, treatment as usual, or being on a wait-list to receive an intervention.
Overall the research team reported “moderately strong” average benefits from the parent-mediated interventions (Hedges’ g, 0.553), indicating a medium effect size. Parent interventions had the greatest effect on outcomes involving positive behavior and social skills (0.603), followed by language and communication (0.545), maladaptive behavior (0.519), and life skills (0.239).
Similar benefits were observed regardless of a child’s age or sex or which parent or parents implemented an intervention. The effects also appeared to be consistent regardless of intervention characteristics, such as the number of training sessions parents received, although the researchers noted that many studies did not provide data on such details.
Paul Carbone, MD, a professor of pediatrics at the University of Utah, Salt Lake City, who was not involved in the review, said it demonstrates that such parental engagement is “vitally important” and pediatricians “should not hesitate to refer interested families.”
Dr. Carbone, who is the medical director of an assessment program for children with suspected developmental disabilities, said many training programs for parents have adopted telehealth, adding to their convenience. To make appropriate referrals, primary care clinicians should become acquainted with local programs and learn which outcomes they target, he said.
Dr. Smith noted that primary care physicians are “better trained now than ever” to identify autism spectrum disorder and therefore are among the first to identify those conditions and help parents understand “that their actions at home absolutely make a difference in the child’s development.”
Overcoming limitations, future research needs
The research team attempted to overcome limitations with previous reviews by using comprehensive search terms and other methods to identify relevant studies, including some that had not been published. They included only studies that reflect common practice of training multiple parents simultaneously, they wrote.
Dr. Smith noted that long-term outcomes data and further study to compare effects on children with mild, moderate, and severe autism are needed.
Although logic would suggest greater benefits for children with severe disease, there are no data to demonstrate that, he said.
The authors of the study and Dr. Carbone reported no relevant competing interests.
“Referrals for parent training should now be considered the expected standard for medical practice,” said a member of the research team, Timothy B. Smith, PhD, a professor of psychology at Brigham Young University, Provo, Utah.
Programs that show parents how to teach functional skills and address maladaptive behaviors, also known as parent-mediated or parent-implemented interventions, offer an alternative to one-on-one professional services, which are in short supply, according to the paper, which was published in the Journal of Autism and Developmental Disorders.
Methods and results
The meta-analysis included 54 papers based on randomized clinical trials involving 2,895 children, which compared the effects of various parent interventions with professional treatment, treatment as usual, or being on a wait-list to receive an intervention.
Overall the research team reported “moderately strong” average benefits from the parent-mediated interventions (Hedges’ g, 0.553), indicating a medium effect size. Parent interventions had the greatest effect on outcomes involving positive behavior and social skills (0.603), followed by language and communication (0.545), maladaptive behavior (0.519), and life skills (0.239).
Similar benefits were observed regardless of a child’s age or sex or which parent or parents implemented an intervention. The effects also appeared to be consistent regardless of intervention characteristics, such as the number of training sessions parents received, although the researchers noted that many studies did not provide data on such details.
Paul Carbone, MD, a professor of pediatrics at the University of Utah, Salt Lake City, who was not involved in the review, said it demonstrates that such parental engagement is “vitally important” and pediatricians “should not hesitate to refer interested families.”
Dr. Carbone, who is the medical director of an assessment program for children with suspected developmental disabilities, said many training programs for parents have adopted telehealth, adding to their convenience. To make appropriate referrals, primary care clinicians should become acquainted with local programs and learn which outcomes they target, he said.
Dr. Smith noted that primary care physicians are “better trained now than ever” to identify autism spectrum disorder and therefore are among the first to identify those conditions and help parents understand “that their actions at home absolutely make a difference in the child’s development.”
Overcoming limitations, future research needs
The research team attempted to overcome limitations with previous reviews by using comprehensive search terms and other methods to identify relevant studies, including some that had not been published. They included only studies that reflect common practice of training multiple parents simultaneously, they wrote.
Dr. Smith noted that long-term outcomes data and further study to compare effects on children with mild, moderate, and severe autism are needed.
Although logic would suggest greater benefits for children with severe disease, there are no data to demonstrate that, he said.
The authors of the study and Dr. Carbone reported no relevant competing interests.
“Referrals for parent training should now be considered the expected standard for medical practice,” said a member of the research team, Timothy B. Smith, PhD, a professor of psychology at Brigham Young University, Provo, Utah.
Programs that show parents how to teach functional skills and address maladaptive behaviors, also known as parent-mediated or parent-implemented interventions, offer an alternative to one-on-one professional services, which are in short supply, according to the paper, which was published in the Journal of Autism and Developmental Disorders.
Methods and results
The meta-analysis included 54 papers based on randomized clinical trials involving 2,895 children, which compared the effects of various parent interventions with professional treatment, treatment as usual, or being on a wait-list to receive an intervention.
Overall the research team reported “moderately strong” average benefits from the parent-mediated interventions (Hedges’ g, 0.553), indicating a medium effect size. Parent interventions had the greatest effect on outcomes involving positive behavior and social skills (0.603), followed by language and communication (0.545), maladaptive behavior (0.519), and life skills (0.239).
Similar benefits were observed regardless of a child’s age or sex or which parent or parents implemented an intervention. The effects also appeared to be consistent regardless of intervention characteristics, such as the number of training sessions parents received, although the researchers noted that many studies did not provide data on such details.
Paul Carbone, MD, a professor of pediatrics at the University of Utah, Salt Lake City, who was not involved in the review, said it demonstrates that such parental engagement is “vitally important” and pediatricians “should not hesitate to refer interested families.”
Dr. Carbone, who is the medical director of an assessment program for children with suspected developmental disabilities, said many training programs for parents have adopted telehealth, adding to their convenience. To make appropriate referrals, primary care clinicians should become acquainted with local programs and learn which outcomes they target, he said.
Dr. Smith noted that primary care physicians are “better trained now than ever” to identify autism spectrum disorder and therefore are among the first to identify those conditions and help parents understand “that their actions at home absolutely make a difference in the child’s development.”
Overcoming limitations, future research needs
The research team attempted to overcome limitations with previous reviews by using comprehensive search terms and other methods to identify relevant studies, including some that had not been published. They included only studies that reflect common practice of training multiple parents simultaneously, they wrote.
Dr. Smith noted that long-term outcomes data and further study to compare effects on children with mild, moderate, and severe autism are needed.
Although logic would suggest greater benefits for children with severe disease, there are no data to demonstrate that, he said.
The authors of the study and Dr. Carbone reported no relevant competing interests.
FROM JOURNAL OF AUTISM AND DEVELOPMENTAL DISORDERS
Largest-ever study into the effects of cannabis on the brain
The largest-ever independent study into the effects of cannabis on the brain is being carried out in the United Kingdom.
Even though cannabis is the most commonly used illegal drug in the United Kingdom and medicinal cannabis has been legal there since 2018 little is known about why some people react badly to it and others seem to benefit from it.
According to Home Office figures on drug use from 2019, 7.6% of adults aged 16-59 used cannabis in the previous year.
Medicinal cannabis in the United Kingdom can only be prescribed if no other licensed medicine could help the patient. At the moment, GPs can’t prescribe it, only specialist hospital doctors can. The National Health Service says it can only be used in three circumstances: in rare, severe epilepsy; to deal with chemotherapy side effects such as nausea; or to help with multiple sclerosis.
As part of the Cannabis&Me study, KCL needs to get 3,000 current cannabis users and 3,000 non–cannabis users to take part in an online survey, with a third of those survey respondents then taking part in a face-to-face assessment that includes virtual reality (VR) and psychological analysis. The study also aims to determine how the DNA of cannabis users and their endocannabinoid system impacts their experiences, both negative and positive, with the drug.
The study is spearheaded by Marta Di Forti, MD, PhD, and has been allocated over £2.5 million in funding by the Medical Research Council.
This news organization asked Dr. Di Forti about the study.
Question: How do you describe the study?
Answer: “It’s a really unique study. We are aiming to see what’s happening to people using cannabis in the privacy of their homes for medicinal, recreational reasons, or whatever other reason.
“The debate on cannabis has always been quite polarized. There have been people who experience adversities with cannabis use, especially psychosis, whose families may perhaps like cannabis to be abolished if possible. Then there are other people who are saying they get positive benefits from using cannabis.”
Q: So where does the study come in?
A: “The study wants to bring the two sides of the argument together and understand what’s really happening. The group I see as a clinician comes to severe harm when they use cannabis regularly. We want to find out who they are and whether we can identify them. While we need to make sure they never come to harm when using cannabis, we need to consider others who won’t come to harm from using cannabis and give them a chance to use it in a way that’s beneficial.”
Q: How does the study work?
A: “The first step of the study is to use an online questionnaire that can be filled in by anyone aged 18-45 who lives in the London area or can travel here if selected. The first set of questions are a general idea of their cannabis use: ‘Why do they use it?’ ‘What are its benefits?’ Then, general questions on what their life has been like up to that point: ‘Did they have any adversities in childhood?’ ‘How is their mood and anxiety levels?’ ‘Do they experience any paranoid responses in everyday life?’ It probably takes between 30 and 40 minutes to fill out the questionnaire.”
Q: Can you explain about paranoid responses?
A: “We go through the questionnaires looking at people’s paranoid response to everyday life, not in a clinical disorder term, just in terms of the differences in how we respond to certain circumstances. For example: ‘How do you feel if someone’s staring at you on the Tube?’ Some people are afraid, some feel uncomfortable, some people don’t notice, and others think a person is staring at them as they look good or another such positive feeling. So, we give people a paranoia score and will invite some at the top and some at the bottom of that score for a face-to-face assessment. We want to select those people who are using cannabis daily and they are getting either no paranoia or high paranoia.”
Q: What happens at the face-to-face assessments?
A: “We do two things which are very novel. We ask them to take part in a virtual reality experience. They are in a lovely shop and within this experience they come across challenges, which may or may not induce a benign paranoia response. We will ask them to donate a sample of blood before they go into the VR set. We will test for tetrahydrocannabinol (THC) and cannabidiol (CBD). We will also look at the metabolites of the two. People don’t take into account how differently individuals metabolize cannabis, which could be one of the reasons why some people can tolerate it and others can’t.”
Q: There’s also a genetic aspect of the study?
A: “From the same sample, we will extract DNA to look at the genetics across the genome and compare genetic variations between high and low paranoia in the context of cannabis use. Also, we will look at the epigenetics, as we have learned from neuroscience, and also cancer, that sometimes a substance we ingest has an effect on our health. It’s perhaps an interaction with the way our DNA is written but also with the changes to the way our DNA is read and translated into biology if exposed to that substance. We know that smoking tobacco does have an impact at an epigenetic level on the DNA. We do know that in people who stop smoking, these impacts on the epigenetics are partially reversed. This work hasn’t been done properly for cannabis.
“There have been four published studies that have looked at the effect of cannabis use on epigenetics but they have been quite inconclusive, and they haven’t looked at large numbers of current users taking into account how much they are using. Moreover, we do know that when THC and CBD get into our bodies, they interact with something that is already embedded in our biology which is the endocannabinoid system. Therefore, in the blood samples we also aim to measures the levels of the endocannabinoids we naturally produce.
“All of this data will then be analyzed to see if we can get close to understanding what makes some cannabis users susceptible to paranoia while others who are using cannabis get some benefits, even in the domain of mental health.”
Q: Who are you looking for to take part in your study?
A: “What we don’t want is to get only people who are the classic friends and family of academics to do the study. We want a representative sample of people out there who are using cannabis. My ideal candidate would be someone who hates me and usually sends me abusive emails saying I’m against cannabis, which is wrong. All I want to find out is who is susceptible to harm which will keep everybody else safe. We are not trying to demonize cannabis; it’s exactly the opposite. We would like people from all ethnic and socioeconomic backgrounds to join to give voice to everyone out there using cannabis, the reasons why, and the effects they experience.”
Q: Will this study perhaps give more information of when it’s appropriate to prescribe medicinal cannabis, as it’s still quite unusual for it to be prescribed in the United Kingdom isn’t it?
A: “Absolutely spot on. That’s exactly the point. We want to hear from people who are receiving medicinal cannabis as a prescription, as they are likely to take it on a daily basis and daily use is what epidemiological studies have linked to the highest risk of psychosis. There will be people taking THC everyday for pain, nausea, for Crohn’s disease, and more.
“Normally when you receive a prescription for a medication the physician in charge will tell you the potential side effects which will be monitored to make sure it’s safe, and you may have to swap to a different medication. Now this isn’t really happening with medicinal cannabis, which is one of the reasons clinicians are anxious about prescribing it, and they have been criticized for not prescribing it very much. There’s much less structure and guidance about ‘psychosis-related’ side effects monitoring. If we can really identify those people who are likely to develop psychosis or disabling paranoia when they use cannabis, physicians might be more prepared to prescribe more widely when indicated.
“You could even have a virtual reality scenario available as a screening tool when you get prescribed medicinal cannabis, to see if there are changes in your perception of the world, which is ultimately what psychosis is about. Could this be a way of implementing safe prescribing which will encourage physicians to use safe cannabis compounds and make some people less anxious about it?
“This study is not here to highlight the negativity of cannabis, on the contrary it’s to understand how it can be used recreationally, but even more important, medicinally in a safe way so people that are coming to no harm can continue to do so and people who are at risk can be kept safe, or at least monitored adequately.”
A version of this article first appeared on Medscape UK.
The largest-ever independent study into the effects of cannabis on the brain is being carried out in the United Kingdom.
Even though cannabis is the most commonly used illegal drug in the United Kingdom and medicinal cannabis has been legal there since 2018 little is known about why some people react badly to it and others seem to benefit from it.
According to Home Office figures on drug use from 2019, 7.6% of adults aged 16-59 used cannabis in the previous year.
Medicinal cannabis in the United Kingdom can only be prescribed if no other licensed medicine could help the patient. At the moment, GPs can’t prescribe it, only specialist hospital doctors can. The National Health Service says it can only be used in three circumstances: in rare, severe epilepsy; to deal with chemotherapy side effects such as nausea; or to help with multiple sclerosis.
As part of the Cannabis&Me study, KCL needs to get 3,000 current cannabis users and 3,000 non–cannabis users to take part in an online survey, with a third of those survey respondents then taking part in a face-to-face assessment that includes virtual reality (VR) and psychological analysis. The study also aims to determine how the DNA of cannabis users and their endocannabinoid system impacts their experiences, both negative and positive, with the drug.
The study is spearheaded by Marta Di Forti, MD, PhD, and has been allocated over £2.5 million in funding by the Medical Research Council.
This news organization asked Dr. Di Forti about the study.
Question: How do you describe the study?
Answer: “It’s a really unique study. We are aiming to see what’s happening to people using cannabis in the privacy of their homes for medicinal, recreational reasons, or whatever other reason.
“The debate on cannabis has always been quite polarized. There have been people who experience adversities with cannabis use, especially psychosis, whose families may perhaps like cannabis to be abolished if possible. Then there are other people who are saying they get positive benefits from using cannabis.”
Q: So where does the study come in?
A: “The study wants to bring the two sides of the argument together and understand what’s really happening. The group I see as a clinician comes to severe harm when they use cannabis regularly. We want to find out who they are and whether we can identify them. While we need to make sure they never come to harm when using cannabis, we need to consider others who won’t come to harm from using cannabis and give them a chance to use it in a way that’s beneficial.”
Q: How does the study work?
A: “The first step of the study is to use an online questionnaire that can be filled in by anyone aged 18-45 who lives in the London area or can travel here if selected. The first set of questions are a general idea of their cannabis use: ‘Why do they use it?’ ‘What are its benefits?’ Then, general questions on what their life has been like up to that point: ‘Did they have any adversities in childhood?’ ‘How is their mood and anxiety levels?’ ‘Do they experience any paranoid responses in everyday life?’ It probably takes between 30 and 40 minutes to fill out the questionnaire.”
Q: Can you explain about paranoid responses?
A: “We go through the questionnaires looking at people’s paranoid response to everyday life, not in a clinical disorder term, just in terms of the differences in how we respond to certain circumstances. For example: ‘How do you feel if someone’s staring at you on the Tube?’ Some people are afraid, some feel uncomfortable, some people don’t notice, and others think a person is staring at them as they look good or another such positive feeling. So, we give people a paranoia score and will invite some at the top and some at the bottom of that score for a face-to-face assessment. We want to select those people who are using cannabis daily and they are getting either no paranoia or high paranoia.”
Q: What happens at the face-to-face assessments?
A: “We do two things which are very novel. We ask them to take part in a virtual reality experience. They are in a lovely shop and within this experience they come across challenges, which may or may not induce a benign paranoia response. We will ask them to donate a sample of blood before they go into the VR set. We will test for tetrahydrocannabinol (THC) and cannabidiol (CBD). We will also look at the metabolites of the two. People don’t take into account how differently individuals metabolize cannabis, which could be one of the reasons why some people can tolerate it and others can’t.”
Q: There’s also a genetic aspect of the study?
A: “From the same sample, we will extract DNA to look at the genetics across the genome and compare genetic variations between high and low paranoia in the context of cannabis use. Also, we will look at the epigenetics, as we have learned from neuroscience, and also cancer, that sometimes a substance we ingest has an effect on our health. It’s perhaps an interaction with the way our DNA is written but also with the changes to the way our DNA is read and translated into biology if exposed to that substance. We know that smoking tobacco does have an impact at an epigenetic level on the DNA. We do know that in people who stop smoking, these impacts on the epigenetics are partially reversed. This work hasn’t been done properly for cannabis.
“There have been four published studies that have looked at the effect of cannabis use on epigenetics but they have been quite inconclusive, and they haven’t looked at large numbers of current users taking into account how much they are using. Moreover, we do know that when THC and CBD get into our bodies, they interact with something that is already embedded in our biology which is the endocannabinoid system. Therefore, in the blood samples we also aim to measures the levels of the endocannabinoids we naturally produce.
“All of this data will then be analyzed to see if we can get close to understanding what makes some cannabis users susceptible to paranoia while others who are using cannabis get some benefits, even in the domain of mental health.”
Q: Who are you looking for to take part in your study?
A: “What we don’t want is to get only people who are the classic friends and family of academics to do the study. We want a representative sample of people out there who are using cannabis. My ideal candidate would be someone who hates me and usually sends me abusive emails saying I’m against cannabis, which is wrong. All I want to find out is who is susceptible to harm which will keep everybody else safe. We are not trying to demonize cannabis; it’s exactly the opposite. We would like people from all ethnic and socioeconomic backgrounds to join to give voice to everyone out there using cannabis, the reasons why, and the effects they experience.”
Q: Will this study perhaps give more information of when it’s appropriate to prescribe medicinal cannabis, as it’s still quite unusual for it to be prescribed in the United Kingdom isn’t it?
A: “Absolutely spot on. That’s exactly the point. We want to hear from people who are receiving medicinal cannabis as a prescription, as they are likely to take it on a daily basis and daily use is what epidemiological studies have linked to the highest risk of psychosis. There will be people taking THC everyday for pain, nausea, for Crohn’s disease, and more.
“Normally when you receive a prescription for a medication the physician in charge will tell you the potential side effects which will be monitored to make sure it’s safe, and you may have to swap to a different medication. Now this isn’t really happening with medicinal cannabis, which is one of the reasons clinicians are anxious about prescribing it, and they have been criticized for not prescribing it very much. There’s much less structure and guidance about ‘psychosis-related’ side effects monitoring. If we can really identify those people who are likely to develop psychosis or disabling paranoia when they use cannabis, physicians might be more prepared to prescribe more widely when indicated.
“You could even have a virtual reality scenario available as a screening tool when you get prescribed medicinal cannabis, to see if there are changes in your perception of the world, which is ultimately what psychosis is about. Could this be a way of implementing safe prescribing which will encourage physicians to use safe cannabis compounds and make some people less anxious about it?
“This study is not here to highlight the negativity of cannabis, on the contrary it’s to understand how it can be used recreationally, but even more important, medicinally in a safe way so people that are coming to no harm can continue to do so and people who are at risk can be kept safe, or at least monitored adequately.”
A version of this article first appeared on Medscape UK.
The largest-ever independent study into the effects of cannabis on the brain is being carried out in the United Kingdom.
Even though cannabis is the most commonly used illegal drug in the United Kingdom and medicinal cannabis has been legal there since 2018 little is known about why some people react badly to it and others seem to benefit from it.
According to Home Office figures on drug use from 2019, 7.6% of adults aged 16-59 used cannabis in the previous year.
Medicinal cannabis in the United Kingdom can only be prescribed if no other licensed medicine could help the patient. At the moment, GPs can’t prescribe it, only specialist hospital doctors can. The National Health Service says it can only be used in three circumstances: in rare, severe epilepsy; to deal with chemotherapy side effects such as nausea; or to help with multiple sclerosis.
As part of the Cannabis&Me study, KCL needs to get 3,000 current cannabis users and 3,000 non–cannabis users to take part in an online survey, with a third of those survey respondents then taking part in a face-to-face assessment that includes virtual reality (VR) and psychological analysis. The study also aims to determine how the DNA of cannabis users and their endocannabinoid system impacts their experiences, both negative and positive, with the drug.
The study is spearheaded by Marta Di Forti, MD, PhD, and has been allocated over £2.5 million in funding by the Medical Research Council.
This news organization asked Dr. Di Forti about the study.
Question: How do you describe the study?
Answer: “It’s a really unique study. We are aiming to see what’s happening to people using cannabis in the privacy of their homes for medicinal, recreational reasons, or whatever other reason.
“The debate on cannabis has always been quite polarized. There have been people who experience adversities with cannabis use, especially psychosis, whose families may perhaps like cannabis to be abolished if possible. Then there are other people who are saying they get positive benefits from using cannabis.”
Q: So where does the study come in?
A: “The study wants to bring the two sides of the argument together and understand what’s really happening. The group I see as a clinician comes to severe harm when they use cannabis regularly. We want to find out who they are and whether we can identify them. While we need to make sure they never come to harm when using cannabis, we need to consider others who won’t come to harm from using cannabis and give them a chance to use it in a way that’s beneficial.”
Q: How does the study work?
A: “The first step of the study is to use an online questionnaire that can be filled in by anyone aged 18-45 who lives in the London area or can travel here if selected. The first set of questions are a general idea of their cannabis use: ‘Why do they use it?’ ‘What are its benefits?’ Then, general questions on what their life has been like up to that point: ‘Did they have any adversities in childhood?’ ‘How is their mood and anxiety levels?’ ‘Do they experience any paranoid responses in everyday life?’ It probably takes between 30 and 40 minutes to fill out the questionnaire.”
Q: Can you explain about paranoid responses?
A: “We go through the questionnaires looking at people’s paranoid response to everyday life, not in a clinical disorder term, just in terms of the differences in how we respond to certain circumstances. For example: ‘How do you feel if someone’s staring at you on the Tube?’ Some people are afraid, some feel uncomfortable, some people don’t notice, and others think a person is staring at them as they look good or another such positive feeling. So, we give people a paranoia score and will invite some at the top and some at the bottom of that score for a face-to-face assessment. We want to select those people who are using cannabis daily and they are getting either no paranoia or high paranoia.”
Q: What happens at the face-to-face assessments?
A: “We do two things which are very novel. We ask them to take part in a virtual reality experience. They are in a lovely shop and within this experience they come across challenges, which may or may not induce a benign paranoia response. We will ask them to donate a sample of blood before they go into the VR set. We will test for tetrahydrocannabinol (THC) and cannabidiol (CBD). We will also look at the metabolites of the two. People don’t take into account how differently individuals metabolize cannabis, which could be one of the reasons why some people can tolerate it and others can’t.”
Q: There’s also a genetic aspect of the study?
A: “From the same sample, we will extract DNA to look at the genetics across the genome and compare genetic variations between high and low paranoia in the context of cannabis use. Also, we will look at the epigenetics, as we have learned from neuroscience, and also cancer, that sometimes a substance we ingest has an effect on our health. It’s perhaps an interaction with the way our DNA is written but also with the changes to the way our DNA is read and translated into biology if exposed to that substance. We know that smoking tobacco does have an impact at an epigenetic level on the DNA. We do know that in people who stop smoking, these impacts on the epigenetics are partially reversed. This work hasn’t been done properly for cannabis.
“There have been four published studies that have looked at the effect of cannabis use on epigenetics but they have been quite inconclusive, and they haven’t looked at large numbers of current users taking into account how much they are using. Moreover, we do know that when THC and CBD get into our bodies, they interact with something that is already embedded in our biology which is the endocannabinoid system. Therefore, in the blood samples we also aim to measures the levels of the endocannabinoids we naturally produce.
“All of this data will then be analyzed to see if we can get close to understanding what makes some cannabis users susceptible to paranoia while others who are using cannabis get some benefits, even in the domain of mental health.”
Q: Who are you looking for to take part in your study?
A: “What we don’t want is to get only people who are the classic friends and family of academics to do the study. We want a representative sample of people out there who are using cannabis. My ideal candidate would be someone who hates me and usually sends me abusive emails saying I’m against cannabis, which is wrong. All I want to find out is who is susceptible to harm which will keep everybody else safe. We are not trying to demonize cannabis; it’s exactly the opposite. We would like people from all ethnic and socioeconomic backgrounds to join to give voice to everyone out there using cannabis, the reasons why, and the effects they experience.”
Q: Will this study perhaps give more information of when it’s appropriate to prescribe medicinal cannabis, as it’s still quite unusual for it to be prescribed in the United Kingdom isn’t it?
A: “Absolutely spot on. That’s exactly the point. We want to hear from people who are receiving medicinal cannabis as a prescription, as they are likely to take it on a daily basis and daily use is what epidemiological studies have linked to the highest risk of psychosis. There will be people taking THC everyday for pain, nausea, for Crohn’s disease, and more.
“Normally when you receive a prescription for a medication the physician in charge will tell you the potential side effects which will be monitored to make sure it’s safe, and you may have to swap to a different medication. Now this isn’t really happening with medicinal cannabis, which is one of the reasons clinicians are anxious about prescribing it, and they have been criticized for not prescribing it very much. There’s much less structure and guidance about ‘psychosis-related’ side effects monitoring. If we can really identify those people who are likely to develop psychosis or disabling paranoia when they use cannabis, physicians might be more prepared to prescribe more widely when indicated.
“You could even have a virtual reality scenario available as a screening tool when you get prescribed medicinal cannabis, to see if there are changes in your perception of the world, which is ultimately what psychosis is about. Could this be a way of implementing safe prescribing which will encourage physicians to use safe cannabis compounds and make some people less anxious about it?
“This study is not here to highlight the negativity of cannabis, on the contrary it’s to understand how it can be used recreationally, but even more important, medicinally in a safe way so people that are coming to no harm can continue to do so and people who are at risk can be kept safe, or at least monitored adequately.”
A version of this article first appeared on Medscape UK.
Baseline neuromotor abnormalities persist in schizophrenia
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
Neuromotor abnormalities in psychotic disorders have long been ignored as side effects of antipsychotic drugs, but they are gaining new attention as a component of the disease process, with implications for outcomes and management, wrote Victor Peralta, MD, PhD, of Servicio Navarro de Salud, Pamplona, Spain, and colleagues.
Previous research has suggested links between increased levels of parkinsonism, dyskinesia, and NSS and poor symptomatic and functional outcomes, but “the impact of primary neuromotor dysfunction on the long-term course and outcome of psychotic disorders remains largely unknown,” they said.
In a study published in Schizophrenia Research , the investigators identified 243 consecutive schizophrenia patients admitted to a psychiatric ward at a single center.
Patients were assessed at baseline for variables including parkinsonism, dyskinesia, NSS, and catatonia, and were reassessed 21 years later for the same variables, along with psychopathology, functioning, personal recovery, cognitive performance, and comorbidity.
Overall, baseline dyskinesia and NSS measures were stable over time, with Intraclass Correlation Coefficients (ICC) of 0.92 and 0.86, respectively, while rating stability was low for parkinsonism and catatonia (ICC = 0.42 and 0.31, respectively).
Baseline dyskinesia and NSS each were independent predictors of more positive and negative symptoms, poor functioning, and less personal recovery at 21 years. In a multivariate model, neuromotor dysfunction at follow-up was significantly associated with family history of schizophrenia, obstetric complications, neurodevelopmental delay, and premorbid IQ, as well as baseline dyskinesia and NSS; “these variables explained 51% of the variance in the neuromotor outcome, 35% of which corresponded to baseline dyskinesia and NSS,” the researchers said. As for other outcomes, baseline neuromotor ratings predicted a range from 4% for medical comorbidity to 15% for cognitive impairment.
“The distinction between primary and drug-induced neuromotor dysfunction is a very complex issue, mainly because antipsychotic drugs may cause de novo motor dysfunction, such as improve or worsen the disease-based motor dysfunction,” the researchers explained in their discussion.
Baseline parkinsonism, dyskinesia, and NSS were significantly related to increased risk of antipsychotic exposure over the illness course, possibly because primary neuromotor dysfunction was predictive of greater severity of illness in general, which confounds differentiation between primary and drug-induced motor symptoms, they noted.
The study findings were limited by several factors including potential selection bias because of the selection of first-admission psychosis, which may limit generalizability, the researchers noted. Other limitations include the use of standard clinical rating scales rather than instrumental procedures to measuring neuromotor abnormalities.
However, “our findings confirm the significance of baseline and follow-up neuromotor abnormalities as a core dimension of psychosis,” and future studies “should complement clinical rating scales with instrumental assessment to capture neuromotor dysfunction more comprehensively,” they said.
The results highlight the clinical relevance of examining neuromotor abnormalities as a routine part of practice prior to starting antipsychotics because of their potential as predictors of long-term outcomes “and to disentangle the primary versus drug-induced character of neuromotor impairment in treated patients,” they concluded.
The study was supported by the Spanish Ministry of Economy, Industry, and Competitiveness, and the Regional Government of Navarra. The researchers had no financial conflicts to disclose.
FROM SCHIZOPHRENIA RESEARCH
Subtle visual dysfunctions often precede early-stage psychosis
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A multinational group of investigators found that VisDys were reported considerably more often by patients with recent-onset psychosis and CHR than by those with recent-onset depression or a group acting as healthy control participants.
In addition, vision problems of higher severity were associated with less functional remission both for patients at CHR and those with recent-onset psychosis. Among patients with CHR, VisDys was also linked to lower quality of life (QOL), higher depressiveness, and more severe impairment of visuospatial constructability.
The researchers used fMRI imaging to compare resting-state functional brain connectivity in participants with recent-onset psychosis, CHR, and recent-onset depression. They found that the occipital (ON) and frontoparietal (FPN) subnetworks were particularly implicated in VisDys.
“Subtle VisDys should be regarded as a frequent phenomenon across the psychosis spectrum, impinging negatively on patients’ current ability to function in several settings of their daily and social life, their QOL, and visuospatial abilities,” write investigators led by Johanna Schwarzer, Institute for Translational Psychiatry, University of Muenster (Germany).
“These large-sample study findings suggest that VisDys are clinically highly relevant not only in [recent-onset psychosis] but especially in CHR,” they stated.
The findings were published online in Neuropsychopharmacology.
Subtle, underrecognized
Unlike patients with nonpsychotic disorders, approximately 50%-60% of patients diagnosed with schizophrenia report VisDys involving brightness, motion, form, color perception, or distorted perception of their own face, the researchers reported.
These “subtle” VisDys are “often underrecognized during clinical examination, despite their clinical relevance related to suicidal ideation, cognitive impairment, or poorer treatment response,” they wrote.
Most research into these vision problems in patients with schizophrenia has focused on patients in which the illness is in a stable, chronic state – although VisDys often appear years before the diagnosis of a psychotic disorder.
Moreover, there has been little research into the neurobiological underpinnings of VisDys, specifically in early states of psychosis and/or in comparison to other disorders, such as depression.
The Personalised Prognostic Indicators for Early Psychosis Management (PRONIA) Consortium studied the psychophysiological phenomenon of VisDys in a large sample of adolescents and young adults. The sample consisted of three diagnostic groups: those with recent-onset psychosis, those with CHR, and those with recent-onset depression.
VisDys in daily life were measured using the Schizophrenia Proneness Instrument–Adult Scale (SPI-A), which assesses basic symptoms that indicate increased risk for psychosis.
Visual information processing
Resting-state imaging data on intrinsic brain networks were also assessed in the PRONIA sample and were analyzed across 12,720 functional connectivities between 160 regions of interest across the whole brain.
In particular, the researchers were interested in the primary networks involved in visual information processing, especially the dorsal visual stream, with further focus on the ON and FPN intrinsic subnetworks.
The ON was chosen because it comprises “primary visual processing pathways,” while the FPN is “widely suggested to modulate attention related to visual information processing at higher cognitive levels.”
The investigators used a machine-learning multivariate pattern analysis approach that “enables the consideration of multiple interactions within brain systems.”
The current study involved 721 participants from the PRONIA database, including 147 participants with recent-onset psychosis (mean age, 28.45 years; 60.5% men), 143 with CHR (mean age, 26.97 years; about 50% men), 151 with recent-onset depression (mean age, 29.13 years; 47% men), and 280 in the healthy-controls group (mean age, 28.54 years; 39.4% men).
The researchers selected 14 items to assess from the SPI-A that represented different aspects of VisDys. Severity was defined by the maximum frequency within the past 3 months – from 1 (never) to 6 (daily).
The 14 items were as follows: oversensitivity to light and/or certain visual perception objects, photopsia, micropsia/macropsia, near and tele-vision, metamorphopsia, changes in color vision, altered perception of a patient’s own face, pseudomovements of optic stimuli, diplopia or oblique vision, disturbances of the estimation of distances or sizes, disturbances of the perception of straight lines/contours, maintenance of optic stimuli “visual echoes,” partial seeing (including tubular vision), and captivation of attention by details of the visual field.
Participants also completed the Beck Depression Inventory–II scale (BDI-II), the Positive and Negative Syndrome Scale (PANSS), the Functional Remission in General Schizophrenia, and several other scales that measure global and social functioning.
Other assessments included QOL and the Rey-Osterrieth Complex Figure Test, which is a neuropsychological measurement of visuospatial constructability.
Specific to early-stage psychosis?
Results showed that VisDys were reported more frequently in both recent-onset psychosis and CHR groups compared with the recent-onset depression and healthy control groups (50.34% and 55.94% vs. 16.56% and 4.28%, respectively).
The investigators noted that VisDys sum scores “showed high internal consistency” (Cronbachs alpha, 0.78 over all participants).
Among those with recent-onset psychosis, a higher VisDys sum score was correlated with lower scores for functional remission (P = .036) and social functioning (P = .014).
In CHR, higher VisDys sum scores were associated with lower scores for health-related functional remission (P = .024), lower physical and psychological QOL (P = .004 and P = .015, respectively), more severe depression on the BDI-II (P = .021), and more impaired visuospatial constructability (P = .027).
Among those with recent-onset depression and their healthy peers, “no relevant correlations were found between VisDys sum scores and any parameters representing functional remission, QOL, depressiveness, or visuospatial constructability,” the researchers wrote.
A total of 135 participants with recent-onset psychosis, 128 with CHR, and 134 with recent-onset depression also underwent resting-state fMRI.
ON functional connectivity predicted presence of VisDys in patients with recent-onset psychosis and those with CHR, with a balanced accuracy of 60.17% (P = .0001) and 67.38% (P = .029), respectively. In the combined recent-onset psychosis plus CHR sample, VisDys were predicted by FPN functional connectivity (balanced accuracy, 61.1%; P = .006).
“Findings from multivariate pattern analysis support a model of functional integrity within ON and FPN driving the VisDys phenomenon and being implicated in core disease mechanisms of early psychosis states,” the investigators noted.
“The main findings from this large sample study support the idea of VisDys being specific to the psychosis spectrum already at early stages,” while being less frequently reported in recent-onset depression, they wrote. VisDys also “appeared negligible” among those without psychiatric disorders.
Regular assessment needed
Steven Silverstein, PhD, professor of biopsychosocial medicine and professor of psychiatry, neuroscience, and ophthalmology, Center for Visual Science, University of Rochester (N.Y.) Medical Center, called the findings “important” because “they will increase appreciation in the field of mental health for the frequency and disabling nature of visual symptoms and the need for regular assessment in routine clinical practice with people at risk for or with psychotic disorders.”
In addition, “the brain imaging findings are providing needed information that could lead to treatments that target the brain networks generating the visual symptoms,” such as neurofeedback or brain stimulation, said Dr. Silverstein, who was not involved with the research.
The study was funded by a grant for the PRONIA Consortium. Individual researchers received funding from NARSAD Young Investigator Award of the Brain and Behavior Research Foundation, the Koeln Fortune Program/Faculty of Medicine, the University of Cologne, and the European Union’s Horizon 2020 research and innovation program. Open Access funding was enabled and organized by Projekt DEAL. Ms. Schwarzer and Dr. Silverstein reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROPSYCHOPHARMACOLOGY
Psychiatrists’ views on psychoactive drugs clash with U.S. policy
“The consensus among experts, including psychiatrists, about specific drugs is not consistent or congruent with the schedule of these drugs” in the United States, lead author Adam Levin, MD, third-year psychiatry resident, Ohio State University, Columbus, and affiliate scholar at the Center for Psychedelic Drug Research and Education, Ohio State College of Social Work, told this news organization.
Dr. Levin stressed the importance of appropriate drug scheduling to improve access to treatments such as psilocybin (psychedelic mushrooms) and 4-methylenedioxy methamphetamine (MDMA), which are now being tested for psychiatric disorders.
“We are in the middle of a mental health crisis so having any new tools would be really important,” he said.
The survey findings were published online in the International Journal of Drug Policy.
Five drug schedules
The Controlled Substances Act of 1970 created five “schedules” that organized drugs from most to least dangerous (schedule I-V). However, Dr. Levin said that the schedules do not accurately reflect the harms or therapeutic benefits of the various drugs.
Some drugs in lower, less restrictive schedules have greater potential for harm than do those in higher schedules, he noted. For example, methamphetamine, which has been recalled in multiple formulations because of concerns about abuse and limited medical use, remains a schedule II drug.
In addition, several schedule I drugs, including psilocybin and MDMA that are deemed dangerous and of no medical value, have shown therapeutic potential and low rates of misuse, addiction, or physical harm, the investigators noted.
In fact, the Food and Drug Administration has granted breakthrough therapy status to psilocybin for treatment-resistant depression and major depressive disorder (MDD) and to MDMA for posttraumatic stress disorder. This has positioned these drugs for possible FDA approval within the next few years.
Access to schedule I drugs for research purposes is tightly controlled. “Once psilocybin was placed in schedule I, there was this massive drop-off in the research funding and amount of research; and we’re just now starting to understand the potential therapeutic value of this drug,” said Dr. Levin.
Even with a recent research resurgence, most studies are funded by charitable donations or for-profit companies because of continued hesitancy on the part of grant-making organizations, he added.
Apparent contradictions
Given the pending approval of several schedule I drugs and escalating abuse of drugs in lower schedules, there is a growing need to understand physician attitudes surrounding the apparent contradictions in the drug schedule, the investigators noted.
Their survey included a geographically diverse group of 181 mostly middle-aged psychiatrists (65.2% were men) with an average of 16.2 years of practice after residency.
Participants were randomly assigned to respond to a vignette depicting a clinical scenario where a patient wants one of four drugs to help treat severe depression: psilocybin, a schedule I drug; methamphetamine (Desoxyn), a schedule II drug; ketamine, a Schedule III drug; or alprazolam (Xanax), a schedule IV drug.
Each of these therapies has established antidepressant properties, but none are FDA approved for treatment of MDD. However, an intranasal formulation of the ketamine enantiomer Spravato (esketamine) was recently approved for treatment-resistant depression.
There were significant differences among the groups presented with different vignettes. Participants were more likely to warn against repeated use of and development of a new psychiatric problem with methamphetamine and alprazolam compared with psilocybin or ketamine.
Respondents were most concerned about increased suicide risk after the nonprescribed use of alprazolam compared with psilocybin and ketamine.
Compared with all other drugs, ketamine was more likely to be integrated into treatment plans.
Therapeutic value, abuse potential
Participants were asked to rate the safety, therapeutic value, and abuse potential of the four drugs as well as alcohol, a nonscheduled legal drug, if used properly or as directed.
Respondents viewed psilocybin and ketamine as similarly safe – and safer than methamphetamine and alprazolam. They considered ketamine as having the highest therapeutic potential, followed by psilocybin, and then alprazolam and methamphetamine. “Last was alcohol, which we expected because alcohol is not used therapeutically,” said Dr. Levin.
Survey completers viewed methamphetamine, alprazolam, and alcohol as having similarly high abuse potential, and ketamine as having mid-level abuse potential. Psilocybin was rated as having the lowest abuse potential, “which is exactly the opposite of what is implied by its schedule I status,” noted Dr. Levin.
The results provide evidence these drugs “are incorrectly scheduled,” he said.
“This suggests the schedule does not reflect current evidence, which I think is really important to understand because there are consequences to the drug schedule,” including criminal justice and research consequences, he added.
Dr. Levin pointed out that possession of drugs in more harmful schedules is linked to sometimes lengthy prison sentences.
The psychiatrists’ perceptions of the drugs “overlaps pretty significantly” with recent surveys of other mental health professionals, including psychologists and addiction experts, he noted.
The study was funded by the Drug Enforcement and Policy Center, Moritz College of Law, and The Ohio State University. Dr. Levin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The consensus among experts, including psychiatrists, about specific drugs is not consistent or congruent with the schedule of these drugs” in the United States, lead author Adam Levin, MD, third-year psychiatry resident, Ohio State University, Columbus, and affiliate scholar at the Center for Psychedelic Drug Research and Education, Ohio State College of Social Work, told this news organization.
Dr. Levin stressed the importance of appropriate drug scheduling to improve access to treatments such as psilocybin (psychedelic mushrooms) and 4-methylenedioxy methamphetamine (MDMA), which are now being tested for psychiatric disorders.
“We are in the middle of a mental health crisis so having any new tools would be really important,” he said.
The survey findings were published online in the International Journal of Drug Policy.
Five drug schedules
The Controlled Substances Act of 1970 created five “schedules” that organized drugs from most to least dangerous (schedule I-V). However, Dr. Levin said that the schedules do not accurately reflect the harms or therapeutic benefits of the various drugs.
Some drugs in lower, less restrictive schedules have greater potential for harm than do those in higher schedules, he noted. For example, methamphetamine, which has been recalled in multiple formulations because of concerns about abuse and limited medical use, remains a schedule II drug.
In addition, several schedule I drugs, including psilocybin and MDMA that are deemed dangerous and of no medical value, have shown therapeutic potential and low rates of misuse, addiction, or physical harm, the investigators noted.
In fact, the Food and Drug Administration has granted breakthrough therapy status to psilocybin for treatment-resistant depression and major depressive disorder (MDD) and to MDMA for posttraumatic stress disorder. This has positioned these drugs for possible FDA approval within the next few years.
Access to schedule I drugs for research purposes is tightly controlled. “Once psilocybin was placed in schedule I, there was this massive drop-off in the research funding and amount of research; and we’re just now starting to understand the potential therapeutic value of this drug,” said Dr. Levin.
Even with a recent research resurgence, most studies are funded by charitable donations or for-profit companies because of continued hesitancy on the part of grant-making organizations, he added.
Apparent contradictions
Given the pending approval of several schedule I drugs and escalating abuse of drugs in lower schedules, there is a growing need to understand physician attitudes surrounding the apparent contradictions in the drug schedule, the investigators noted.
Their survey included a geographically diverse group of 181 mostly middle-aged psychiatrists (65.2% were men) with an average of 16.2 years of practice after residency.
Participants were randomly assigned to respond to a vignette depicting a clinical scenario where a patient wants one of four drugs to help treat severe depression: psilocybin, a schedule I drug; methamphetamine (Desoxyn), a schedule II drug; ketamine, a Schedule III drug; or alprazolam (Xanax), a schedule IV drug.
Each of these therapies has established antidepressant properties, but none are FDA approved for treatment of MDD. However, an intranasal formulation of the ketamine enantiomer Spravato (esketamine) was recently approved for treatment-resistant depression.
There were significant differences among the groups presented with different vignettes. Participants were more likely to warn against repeated use of and development of a new psychiatric problem with methamphetamine and alprazolam compared with psilocybin or ketamine.
Respondents were most concerned about increased suicide risk after the nonprescribed use of alprazolam compared with psilocybin and ketamine.
Compared with all other drugs, ketamine was more likely to be integrated into treatment plans.
Therapeutic value, abuse potential
Participants were asked to rate the safety, therapeutic value, and abuse potential of the four drugs as well as alcohol, a nonscheduled legal drug, if used properly or as directed.
Respondents viewed psilocybin and ketamine as similarly safe – and safer than methamphetamine and alprazolam. They considered ketamine as having the highest therapeutic potential, followed by psilocybin, and then alprazolam and methamphetamine. “Last was alcohol, which we expected because alcohol is not used therapeutically,” said Dr. Levin.
Survey completers viewed methamphetamine, alprazolam, and alcohol as having similarly high abuse potential, and ketamine as having mid-level abuse potential. Psilocybin was rated as having the lowest abuse potential, “which is exactly the opposite of what is implied by its schedule I status,” noted Dr. Levin.
The results provide evidence these drugs “are incorrectly scheduled,” he said.
“This suggests the schedule does not reflect current evidence, which I think is really important to understand because there are consequences to the drug schedule,” including criminal justice and research consequences, he added.
Dr. Levin pointed out that possession of drugs in more harmful schedules is linked to sometimes lengthy prison sentences.
The psychiatrists’ perceptions of the drugs “overlaps pretty significantly” with recent surveys of other mental health professionals, including psychologists and addiction experts, he noted.
The study was funded by the Drug Enforcement and Policy Center, Moritz College of Law, and The Ohio State University. Dr. Levin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The consensus among experts, including psychiatrists, about specific drugs is not consistent or congruent with the schedule of these drugs” in the United States, lead author Adam Levin, MD, third-year psychiatry resident, Ohio State University, Columbus, and affiliate scholar at the Center for Psychedelic Drug Research and Education, Ohio State College of Social Work, told this news organization.
Dr. Levin stressed the importance of appropriate drug scheduling to improve access to treatments such as psilocybin (psychedelic mushrooms) and 4-methylenedioxy methamphetamine (MDMA), which are now being tested for psychiatric disorders.
“We are in the middle of a mental health crisis so having any new tools would be really important,” he said.
The survey findings were published online in the International Journal of Drug Policy.
Five drug schedules
The Controlled Substances Act of 1970 created five “schedules” that organized drugs from most to least dangerous (schedule I-V). However, Dr. Levin said that the schedules do not accurately reflect the harms or therapeutic benefits of the various drugs.
Some drugs in lower, less restrictive schedules have greater potential for harm than do those in higher schedules, he noted. For example, methamphetamine, which has been recalled in multiple formulations because of concerns about abuse and limited medical use, remains a schedule II drug.
In addition, several schedule I drugs, including psilocybin and MDMA that are deemed dangerous and of no medical value, have shown therapeutic potential and low rates of misuse, addiction, or physical harm, the investigators noted.
In fact, the Food and Drug Administration has granted breakthrough therapy status to psilocybin for treatment-resistant depression and major depressive disorder (MDD) and to MDMA for posttraumatic stress disorder. This has positioned these drugs for possible FDA approval within the next few years.
Access to schedule I drugs for research purposes is tightly controlled. “Once psilocybin was placed in schedule I, there was this massive drop-off in the research funding and amount of research; and we’re just now starting to understand the potential therapeutic value of this drug,” said Dr. Levin.
Even with a recent research resurgence, most studies are funded by charitable donations or for-profit companies because of continued hesitancy on the part of grant-making organizations, he added.
Apparent contradictions
Given the pending approval of several schedule I drugs and escalating abuse of drugs in lower schedules, there is a growing need to understand physician attitudes surrounding the apparent contradictions in the drug schedule, the investigators noted.
Their survey included a geographically diverse group of 181 mostly middle-aged psychiatrists (65.2% were men) with an average of 16.2 years of practice after residency.
Participants were randomly assigned to respond to a vignette depicting a clinical scenario where a patient wants one of four drugs to help treat severe depression: psilocybin, a schedule I drug; methamphetamine (Desoxyn), a schedule II drug; ketamine, a Schedule III drug; or alprazolam (Xanax), a schedule IV drug.
Each of these therapies has established antidepressant properties, but none are FDA approved for treatment of MDD. However, an intranasal formulation of the ketamine enantiomer Spravato (esketamine) was recently approved for treatment-resistant depression.
There were significant differences among the groups presented with different vignettes. Participants were more likely to warn against repeated use of and development of a new psychiatric problem with methamphetamine and alprazolam compared with psilocybin or ketamine.
Respondents were most concerned about increased suicide risk after the nonprescribed use of alprazolam compared with psilocybin and ketamine.
Compared with all other drugs, ketamine was more likely to be integrated into treatment plans.
Therapeutic value, abuse potential
Participants were asked to rate the safety, therapeutic value, and abuse potential of the four drugs as well as alcohol, a nonscheduled legal drug, if used properly or as directed.
Respondents viewed psilocybin and ketamine as similarly safe – and safer than methamphetamine and alprazolam. They considered ketamine as having the highest therapeutic potential, followed by psilocybin, and then alprazolam and methamphetamine. “Last was alcohol, which we expected because alcohol is not used therapeutically,” said Dr. Levin.
Survey completers viewed methamphetamine, alprazolam, and alcohol as having similarly high abuse potential, and ketamine as having mid-level abuse potential. Psilocybin was rated as having the lowest abuse potential, “which is exactly the opposite of what is implied by its schedule I status,” noted Dr. Levin.
The results provide evidence these drugs “are incorrectly scheduled,” he said.
“This suggests the schedule does not reflect current evidence, which I think is really important to understand because there are consequences to the drug schedule,” including criminal justice and research consequences, he added.
Dr. Levin pointed out that possession of drugs in more harmful schedules is linked to sometimes lengthy prison sentences.
The psychiatrists’ perceptions of the drugs “overlaps pretty significantly” with recent surveys of other mental health professionals, including psychologists and addiction experts, he noted.
The study was funded by the Drug Enforcement and Policy Center, Moritz College of Law, and The Ohio State University. Dr. Levin reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF DRUG POLICY













