User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Digital therapeutics could help patients with IBS
, according to a new review of available products.
These tools aren’t widely used by gastroenterologists yet, but the market is expected to grow broadly during the next decade.
“Digital therapeutics make so much sense and solve so many access issues,” coauthor William Chey, MD, chief of gastroenterology at the University of Michigan, Ann Arbor, said in an interview. “Because of this, their promise could easily outstrip their substance. We need to hold digital therapeutics companies accountable for proper evidence of benefit, so patients and doctors don’t end up chasing the latest shiny object.”
The review was published online in The American Journal of Gastroenterology.
Understanding the apps
IBS is most effectively treated with a combination of medications, diet changes, and behavioral interventions that are specific to the patient, the authors write. Cognitive behavioral therapy (CBT) and gut-directed hypnotherapy (GDH) have been effective at modifying behaviors and thought patterns, they add.
However, many gastroenterologists and their patients with IBS don’t have easy access to the mental health services component of integrated gastrointestinal (GI) care. DTx may offer a solution.
The review by Dr. Chey and colleagues is intended to serve as a primer for gastroenterologists about the current generation of DTx that provide virtual behavioral health interventions. For each product, they include a description of its services, evidence supporting its use, and other key information.
Mahana IBS, made by Mahana Therapeutics, is an FDA-approved, prescription-only CBT program for adults with IBS. The maximum out-of-pocket cost is $90. The product includes 10 sessions over 12 weeks.
Available as a mobile app or Web-based platform, Mahana IBS was validated in a randomized comparative effectiveness trial in a group of 558 patients, divided into three groups who received Web-based CBT, phone-based CBT, or treatment as usual. Before treatment, the mean IBS Symptom Severity Score for the entire group was 265.
At 12 weeks, the control group had an average reduction of 52.9 points, while the phone-based therapy group had a reduction of 133.3 points, and the Web-based therapy group had a reduction of 101.2 points. The average Work and Social Adjustment Scale (WSAS) decreased by an additional 3.5 points in the phone-based group and 3 points in the Web-based group, compared with the control group.
Zemedy, made by Bold Health, is a mobile app that provides virtual CBT through a chat bot for patients with IBS. It costs $19.49 per month or $154.99 per year. The app isn’t FDA-approved and doesn’t require a prescription.
The program includes six weekly psychoeducational modules with information about IBS and CBT, followed by CBT training modules. Users can chat with an automated system that provides computer-generated responses for support. A “flare module” supports patients when symptoms worsen.
Zemedy was evaluated in a crossover randomized controlled trial with 62 people in an active treatment group and 59 people in a wait-list control group. The app improved several measures, including self-reported IBS-quality of life, GI symptoms on the IBS rating scale, the Fear of Food Questionnaire, the Visceral Sensitivity Index, and the Depression Anxiety Stress Scale.
A larger clinical trial to validate the results is ongoing.
Regulora, made by metaMe Health, is an FDA-approved, prescription-only GDH program aimed at addressing abdominal pain related to IBS. The maximum out-of-pocket cost is $75. The protocols were developed by GI behavioral health researchers at the University of North Carolina at Chapel Hill. Available on a Web-based platform or as a mobile app, the program includes seven sessions of 30 minutes each over 12 weeks.
Regulora was evaluated in a randomized comparative effectiveness trial of 362 patients who used either this program or an app focused on muscle relaxation. The primary endpoint was the proportion of patients with a 30% or more reduction in abdominal pain intensity, and although the researchers found no significant difference between them, there was some relief. In the GDH group, 31% of participants reported a 30% or greater reduction in abdominal pain intensity, and 45% experienced a 30% or greater improvement in the proportion of stools with normal consistency.
The complete results of the trial still need to receive formal peer review and publication in a scientific journal.
Nerva, made by Mindset Health, is a GDH program delivered by mobile app or Web browser that costs $79.99 for 3 months. It isn’t FDA-approved and doesn’t require a prescription. The protocols were developed in collaboration with researchers from Monash University in Melbourne. The program features 6 weeks of daily sessions, psychoeducation readings, and breathing techniques.
Nerva was evaluated in an observational cohort study of 190 patients who completed all 42 sessions, typically within 2 months. About 64% responded to the program, with a 20 mm or greater symptom reduction on the Visual Analog Scale and median improvement of 33 mm. Participants also reported improvements in abdominal pain, bloating, dissatisfaction with stool consistency, flatulence, and nausea.
Results were reported as an abstract, and full findings from a formal randomized controlled trial aren’t yet available.
Patient and provider benefits
Although DTx tools are still in the early stages of development and validation, they can improve patient care and add value to a gastroenterologist’s practice, the authors write.
The products should undergo the same level of scientific rigor as pharmaceutical therapies, including randomized controlled trials in diverse patient groups, and patient data handling must be secure and transparent, the authors write. Cost analyses will be an important factor in clinical integration and adoption, they add.
“Change is inevitable, and the right change will bring benefits to providers and their patients,” Dr. Chey said. “Don’t be afraid of it, but do your due diligence before you embrace it. Our primer is intended to help providers conduct that due diligence.”
While behavioral health care is essential for many patients with IBS, there aren’t enough therapists with GI knowledge to meet the demand, Melissa Hunt, PhD, associate director of clinical training in psychology at the University of Pennsylvania, Philadelphia, said in an interview. The population prevalence of IBS is 6%, which means about 18 million people in the United States need guidance, she said.
Dr. Hunt, who wasn’t involved with this paper, has evaluated DTx options for patients with IBS, including the randomized controlled trial of Zemedy. Her research suggests that about 50% of IBS patients could benefit from self-help DTx.
“I get two to three new patient referrals a week and have a 6-month wait-list for my private practice,” Dr. Hunt said. “DTx is a cutting edge, evidence-based way to address the gaps in service and meet the needs of this population.”
The study didn’t receive any funding. The authors disclosed research, consultant, and leadership relationships with several companies not related to this report. Dr. Hunt declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, according to a new review of available products.
These tools aren’t widely used by gastroenterologists yet, but the market is expected to grow broadly during the next decade.
“Digital therapeutics make so much sense and solve so many access issues,” coauthor William Chey, MD, chief of gastroenterology at the University of Michigan, Ann Arbor, said in an interview. “Because of this, their promise could easily outstrip their substance. We need to hold digital therapeutics companies accountable for proper evidence of benefit, so patients and doctors don’t end up chasing the latest shiny object.”
The review was published online in The American Journal of Gastroenterology.
Understanding the apps
IBS is most effectively treated with a combination of medications, diet changes, and behavioral interventions that are specific to the patient, the authors write. Cognitive behavioral therapy (CBT) and gut-directed hypnotherapy (GDH) have been effective at modifying behaviors and thought patterns, they add.
However, many gastroenterologists and their patients with IBS don’t have easy access to the mental health services component of integrated gastrointestinal (GI) care. DTx may offer a solution.
The review by Dr. Chey and colleagues is intended to serve as a primer for gastroenterologists about the current generation of DTx that provide virtual behavioral health interventions. For each product, they include a description of its services, evidence supporting its use, and other key information.
Mahana IBS, made by Mahana Therapeutics, is an FDA-approved, prescription-only CBT program for adults with IBS. The maximum out-of-pocket cost is $90. The product includes 10 sessions over 12 weeks.
Available as a mobile app or Web-based platform, Mahana IBS was validated in a randomized comparative effectiveness trial in a group of 558 patients, divided into three groups who received Web-based CBT, phone-based CBT, or treatment as usual. Before treatment, the mean IBS Symptom Severity Score for the entire group was 265.
At 12 weeks, the control group had an average reduction of 52.9 points, while the phone-based therapy group had a reduction of 133.3 points, and the Web-based therapy group had a reduction of 101.2 points. The average Work and Social Adjustment Scale (WSAS) decreased by an additional 3.5 points in the phone-based group and 3 points in the Web-based group, compared with the control group.
Zemedy, made by Bold Health, is a mobile app that provides virtual CBT through a chat bot for patients with IBS. It costs $19.49 per month or $154.99 per year. The app isn’t FDA-approved and doesn’t require a prescription.
The program includes six weekly psychoeducational modules with information about IBS and CBT, followed by CBT training modules. Users can chat with an automated system that provides computer-generated responses for support. A “flare module” supports patients when symptoms worsen.
Zemedy was evaluated in a crossover randomized controlled trial with 62 people in an active treatment group and 59 people in a wait-list control group. The app improved several measures, including self-reported IBS-quality of life, GI symptoms on the IBS rating scale, the Fear of Food Questionnaire, the Visceral Sensitivity Index, and the Depression Anxiety Stress Scale.
A larger clinical trial to validate the results is ongoing.
Regulora, made by metaMe Health, is an FDA-approved, prescription-only GDH program aimed at addressing abdominal pain related to IBS. The maximum out-of-pocket cost is $75. The protocols were developed by GI behavioral health researchers at the University of North Carolina at Chapel Hill. Available on a Web-based platform or as a mobile app, the program includes seven sessions of 30 minutes each over 12 weeks.
Regulora was evaluated in a randomized comparative effectiveness trial of 362 patients who used either this program or an app focused on muscle relaxation. The primary endpoint was the proportion of patients with a 30% or more reduction in abdominal pain intensity, and although the researchers found no significant difference between them, there was some relief. In the GDH group, 31% of participants reported a 30% or greater reduction in abdominal pain intensity, and 45% experienced a 30% or greater improvement in the proportion of stools with normal consistency.
The complete results of the trial still need to receive formal peer review and publication in a scientific journal.
Nerva, made by Mindset Health, is a GDH program delivered by mobile app or Web browser that costs $79.99 for 3 months. It isn’t FDA-approved and doesn’t require a prescription. The protocols were developed in collaboration with researchers from Monash University in Melbourne. The program features 6 weeks of daily sessions, psychoeducation readings, and breathing techniques.
Nerva was evaluated in an observational cohort study of 190 patients who completed all 42 sessions, typically within 2 months. About 64% responded to the program, with a 20 mm or greater symptom reduction on the Visual Analog Scale and median improvement of 33 mm. Participants also reported improvements in abdominal pain, bloating, dissatisfaction with stool consistency, flatulence, and nausea.
Results were reported as an abstract, and full findings from a formal randomized controlled trial aren’t yet available.
Patient and provider benefits
Although DTx tools are still in the early stages of development and validation, they can improve patient care and add value to a gastroenterologist’s practice, the authors write.
The products should undergo the same level of scientific rigor as pharmaceutical therapies, including randomized controlled trials in diverse patient groups, and patient data handling must be secure and transparent, the authors write. Cost analyses will be an important factor in clinical integration and adoption, they add.
“Change is inevitable, and the right change will bring benefits to providers and their patients,” Dr. Chey said. “Don’t be afraid of it, but do your due diligence before you embrace it. Our primer is intended to help providers conduct that due diligence.”
While behavioral health care is essential for many patients with IBS, there aren’t enough therapists with GI knowledge to meet the demand, Melissa Hunt, PhD, associate director of clinical training in psychology at the University of Pennsylvania, Philadelphia, said in an interview. The population prevalence of IBS is 6%, which means about 18 million people in the United States need guidance, she said.
Dr. Hunt, who wasn’t involved with this paper, has evaluated DTx options for patients with IBS, including the randomized controlled trial of Zemedy. Her research suggests that about 50% of IBS patients could benefit from self-help DTx.
“I get two to three new patient referrals a week and have a 6-month wait-list for my private practice,” Dr. Hunt said. “DTx is a cutting edge, evidence-based way to address the gaps in service and meet the needs of this population.”
The study didn’t receive any funding. The authors disclosed research, consultant, and leadership relationships with several companies not related to this report. Dr. Hunt declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
, according to a new review of available products.
These tools aren’t widely used by gastroenterologists yet, but the market is expected to grow broadly during the next decade.
“Digital therapeutics make so much sense and solve so many access issues,” coauthor William Chey, MD, chief of gastroenterology at the University of Michigan, Ann Arbor, said in an interview. “Because of this, their promise could easily outstrip their substance. We need to hold digital therapeutics companies accountable for proper evidence of benefit, so patients and doctors don’t end up chasing the latest shiny object.”
The review was published online in The American Journal of Gastroenterology.
Understanding the apps
IBS is most effectively treated with a combination of medications, diet changes, and behavioral interventions that are specific to the patient, the authors write. Cognitive behavioral therapy (CBT) and gut-directed hypnotherapy (GDH) have been effective at modifying behaviors and thought patterns, they add.
However, many gastroenterologists and their patients with IBS don’t have easy access to the mental health services component of integrated gastrointestinal (GI) care. DTx may offer a solution.
The review by Dr. Chey and colleagues is intended to serve as a primer for gastroenterologists about the current generation of DTx that provide virtual behavioral health interventions. For each product, they include a description of its services, evidence supporting its use, and other key information.
Mahana IBS, made by Mahana Therapeutics, is an FDA-approved, prescription-only CBT program for adults with IBS. The maximum out-of-pocket cost is $90. The product includes 10 sessions over 12 weeks.
Available as a mobile app or Web-based platform, Mahana IBS was validated in a randomized comparative effectiveness trial in a group of 558 patients, divided into three groups who received Web-based CBT, phone-based CBT, or treatment as usual. Before treatment, the mean IBS Symptom Severity Score for the entire group was 265.
At 12 weeks, the control group had an average reduction of 52.9 points, while the phone-based therapy group had a reduction of 133.3 points, and the Web-based therapy group had a reduction of 101.2 points. The average Work and Social Adjustment Scale (WSAS) decreased by an additional 3.5 points in the phone-based group and 3 points in the Web-based group, compared with the control group.
Zemedy, made by Bold Health, is a mobile app that provides virtual CBT through a chat bot for patients with IBS. It costs $19.49 per month or $154.99 per year. The app isn’t FDA-approved and doesn’t require a prescription.
The program includes six weekly psychoeducational modules with information about IBS and CBT, followed by CBT training modules. Users can chat with an automated system that provides computer-generated responses for support. A “flare module” supports patients when symptoms worsen.
Zemedy was evaluated in a crossover randomized controlled trial with 62 people in an active treatment group and 59 people in a wait-list control group. The app improved several measures, including self-reported IBS-quality of life, GI symptoms on the IBS rating scale, the Fear of Food Questionnaire, the Visceral Sensitivity Index, and the Depression Anxiety Stress Scale.
A larger clinical trial to validate the results is ongoing.
Regulora, made by metaMe Health, is an FDA-approved, prescription-only GDH program aimed at addressing abdominal pain related to IBS. The maximum out-of-pocket cost is $75. The protocols were developed by GI behavioral health researchers at the University of North Carolina at Chapel Hill. Available on a Web-based platform or as a mobile app, the program includes seven sessions of 30 minutes each over 12 weeks.
Regulora was evaluated in a randomized comparative effectiveness trial of 362 patients who used either this program or an app focused on muscle relaxation. The primary endpoint was the proportion of patients with a 30% or more reduction in abdominal pain intensity, and although the researchers found no significant difference between them, there was some relief. In the GDH group, 31% of participants reported a 30% or greater reduction in abdominal pain intensity, and 45% experienced a 30% or greater improvement in the proportion of stools with normal consistency.
The complete results of the trial still need to receive formal peer review and publication in a scientific journal.
Nerva, made by Mindset Health, is a GDH program delivered by mobile app or Web browser that costs $79.99 for 3 months. It isn’t FDA-approved and doesn’t require a prescription. The protocols were developed in collaboration with researchers from Monash University in Melbourne. The program features 6 weeks of daily sessions, psychoeducation readings, and breathing techniques.
Nerva was evaluated in an observational cohort study of 190 patients who completed all 42 sessions, typically within 2 months. About 64% responded to the program, with a 20 mm or greater symptom reduction on the Visual Analog Scale and median improvement of 33 mm. Participants also reported improvements in abdominal pain, bloating, dissatisfaction with stool consistency, flatulence, and nausea.
Results were reported as an abstract, and full findings from a formal randomized controlled trial aren’t yet available.
Patient and provider benefits
Although DTx tools are still in the early stages of development and validation, they can improve patient care and add value to a gastroenterologist’s practice, the authors write.
The products should undergo the same level of scientific rigor as pharmaceutical therapies, including randomized controlled trials in diverse patient groups, and patient data handling must be secure and transparent, the authors write. Cost analyses will be an important factor in clinical integration and adoption, they add.
“Change is inevitable, and the right change will bring benefits to providers and their patients,” Dr. Chey said. “Don’t be afraid of it, but do your due diligence before you embrace it. Our primer is intended to help providers conduct that due diligence.”
While behavioral health care is essential for many patients with IBS, there aren’t enough therapists with GI knowledge to meet the demand, Melissa Hunt, PhD, associate director of clinical training in psychology at the University of Pennsylvania, Philadelphia, said in an interview. The population prevalence of IBS is 6%, which means about 18 million people in the United States need guidance, she said.
Dr. Hunt, who wasn’t involved with this paper, has evaluated DTx options for patients with IBS, including the randomized controlled trial of Zemedy. Her research suggests that about 50% of IBS patients could benefit from self-help DTx.
“I get two to three new patient referrals a week and have a 6-month wait-list for my private practice,” Dr. Hunt said. “DTx is a cutting edge, evidence-based way to address the gaps in service and meet the needs of this population.”
The study didn’t receive any funding. The authors disclosed research, consultant, and leadership relationships with several companies not related to this report. Dr. Hunt declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Old drug verapamil may have new use in type 1 diabetes
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
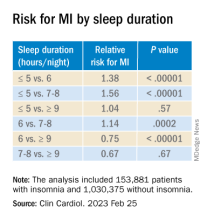
Insomnia, short sleep linked to greater risk for MI
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Fewer than 10% of eligible type 2 diabetes patients get new, pricey drugs
Fewer than 10% of American adults with type 2 diabetes who qualified for treatment with newer agents – such as an SGLT2 inhibitor or GLP-1 agonist – actually received treatment with at least one drug from drug class in 2017-2020, based on a new analysis of just over a thousand adults who participated in a representative, biannual survey and self-reported a diabetes diagnosis.
The cost of these agents, and their uncertain cost-effectiveness at current prices, is likely a key driver of the low usage rate, say the authors of a brief report published in Annals of Internal Medicine.
“Clinical studies have shown that both GLP-1 [glucagonlike peptide–1] receptor agonists and SGLT2 [sodium-glucose cotransporter 2] inhibitors yield additional clinical benefits, compared with older treatments in reducing body weight and progression of cardiovascular disease and chronic kidney disease,” write Shichao Tang, PhD, from the U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
“However, these medications come at a substantially higher cost,” they stress.
Dr. Tang explained in an interview that the new study “points to prior studies about the high cost of these medications as a potential barrier to use, but more research is needed to understand cost-effectiveness and any potential barriers to use, including cost.”
The work “did not include research into cost-effectiveness or why the percentage of people already using these medications was low,” he emphasized.
Dr. Tang and colleagues used data collected by the U.S. National Health and Nutrition Examination Survey during two 2-year cycles between 2017 and 2020 that included 1,417 people who self-identified a diagnosis of diabetes.
Excluding those who likely had type 1 diabetes and those with incomplete data left 1,330 survey participants, including 1,133 (85%) who fit criteria for the treatment of type 2 diabetes with an agent from one of the two studied classes, as recommended in 2022 by a panel representing the American Diabetes Association and the European Association for the Study of Diabetes.
Among these 1,133 people – who represent more than 22 million American adults with type 2 diabetes who fit the 2022 criteria – a scant 3.7% were actually taking a GLP-1 agonist and 5.3% were taking an SGLT2 inhibitor.
“While it’s important to note that our data predate the 2022 recommendations, these drugs were offered as second-line therapy for patients with certain diabetes-related complications in 2017-2020” and hence provide potentially useful insights, noted Dr. Tang, a health economist with the CDC National Center for Chronic Disease Prevention and Health Promotion.
Based on retail prices listed on a United States–based website, a 30-day supply of an oral SGLT2 inhibitor can cost about $550-$600 per month, while common subcutaneously injected GLP-1 receptor agonists can run from a few hundred dollars for a daily injection or close to $1,000 for a formulation administered weekly.
“Cost-effectiveness was not formally considered in the current guideline, but an assessment of cost-effectiveness may assist better targeting of interventions to achieve the greatest effect at a sustainable cost,” the researchers conclude.
The study received no commercial funding. None of the authors had relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fewer than 10% of American adults with type 2 diabetes who qualified for treatment with newer agents – such as an SGLT2 inhibitor or GLP-1 agonist – actually received treatment with at least one drug from drug class in 2017-2020, based on a new analysis of just over a thousand adults who participated in a representative, biannual survey and self-reported a diabetes diagnosis.
The cost of these agents, and their uncertain cost-effectiveness at current prices, is likely a key driver of the low usage rate, say the authors of a brief report published in Annals of Internal Medicine.
“Clinical studies have shown that both GLP-1 [glucagonlike peptide–1] receptor agonists and SGLT2 [sodium-glucose cotransporter 2] inhibitors yield additional clinical benefits, compared with older treatments in reducing body weight and progression of cardiovascular disease and chronic kidney disease,” write Shichao Tang, PhD, from the U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
“However, these medications come at a substantially higher cost,” they stress.
Dr. Tang explained in an interview that the new study “points to prior studies about the high cost of these medications as a potential barrier to use, but more research is needed to understand cost-effectiveness and any potential barriers to use, including cost.”
The work “did not include research into cost-effectiveness or why the percentage of people already using these medications was low,” he emphasized.
Dr. Tang and colleagues used data collected by the U.S. National Health and Nutrition Examination Survey during two 2-year cycles between 2017 and 2020 that included 1,417 people who self-identified a diagnosis of diabetes.
Excluding those who likely had type 1 diabetes and those with incomplete data left 1,330 survey participants, including 1,133 (85%) who fit criteria for the treatment of type 2 diabetes with an agent from one of the two studied classes, as recommended in 2022 by a panel representing the American Diabetes Association and the European Association for the Study of Diabetes.
Among these 1,133 people – who represent more than 22 million American adults with type 2 diabetes who fit the 2022 criteria – a scant 3.7% were actually taking a GLP-1 agonist and 5.3% were taking an SGLT2 inhibitor.
“While it’s important to note that our data predate the 2022 recommendations, these drugs were offered as second-line therapy for patients with certain diabetes-related complications in 2017-2020” and hence provide potentially useful insights, noted Dr. Tang, a health economist with the CDC National Center for Chronic Disease Prevention and Health Promotion.
Based on retail prices listed on a United States–based website, a 30-day supply of an oral SGLT2 inhibitor can cost about $550-$600 per month, while common subcutaneously injected GLP-1 receptor agonists can run from a few hundred dollars for a daily injection or close to $1,000 for a formulation administered weekly.
“Cost-effectiveness was not formally considered in the current guideline, but an assessment of cost-effectiveness may assist better targeting of interventions to achieve the greatest effect at a sustainable cost,” the researchers conclude.
The study received no commercial funding. None of the authors had relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fewer than 10% of American adults with type 2 diabetes who qualified for treatment with newer agents – such as an SGLT2 inhibitor or GLP-1 agonist – actually received treatment with at least one drug from drug class in 2017-2020, based on a new analysis of just over a thousand adults who participated in a representative, biannual survey and self-reported a diabetes diagnosis.
The cost of these agents, and their uncertain cost-effectiveness at current prices, is likely a key driver of the low usage rate, say the authors of a brief report published in Annals of Internal Medicine.
“Clinical studies have shown that both GLP-1 [glucagonlike peptide–1] receptor agonists and SGLT2 [sodium-glucose cotransporter 2] inhibitors yield additional clinical benefits, compared with older treatments in reducing body weight and progression of cardiovascular disease and chronic kidney disease,” write Shichao Tang, PhD, from the U.S. Centers for Disease Control and Prevention, Atlanta, and colleagues.
“However, these medications come at a substantially higher cost,” they stress.
Dr. Tang explained in an interview that the new study “points to prior studies about the high cost of these medications as a potential barrier to use, but more research is needed to understand cost-effectiveness and any potential barriers to use, including cost.”
The work “did not include research into cost-effectiveness or why the percentage of people already using these medications was low,” he emphasized.
Dr. Tang and colleagues used data collected by the U.S. National Health and Nutrition Examination Survey during two 2-year cycles between 2017 and 2020 that included 1,417 people who self-identified a diagnosis of diabetes.
Excluding those who likely had type 1 diabetes and those with incomplete data left 1,330 survey participants, including 1,133 (85%) who fit criteria for the treatment of type 2 diabetes with an agent from one of the two studied classes, as recommended in 2022 by a panel representing the American Diabetes Association and the European Association for the Study of Diabetes.
Among these 1,133 people – who represent more than 22 million American adults with type 2 diabetes who fit the 2022 criteria – a scant 3.7% were actually taking a GLP-1 agonist and 5.3% were taking an SGLT2 inhibitor.
“While it’s important to note that our data predate the 2022 recommendations, these drugs were offered as second-line therapy for patients with certain diabetes-related complications in 2017-2020” and hence provide potentially useful insights, noted Dr. Tang, a health economist with the CDC National Center for Chronic Disease Prevention and Health Promotion.
Based on retail prices listed on a United States–based website, a 30-day supply of an oral SGLT2 inhibitor can cost about $550-$600 per month, while common subcutaneously injected GLP-1 receptor agonists can run from a few hundred dollars for a daily injection or close to $1,000 for a formulation administered weekly.
“Cost-effectiveness was not formally considered in the current guideline, but an assessment of cost-effectiveness may assist better targeting of interventions to achieve the greatest effect at a sustainable cost,” the researchers conclude.
The study received no commercial funding. None of the authors had relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Isolated nail psoriasis may bring arthritis into play
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.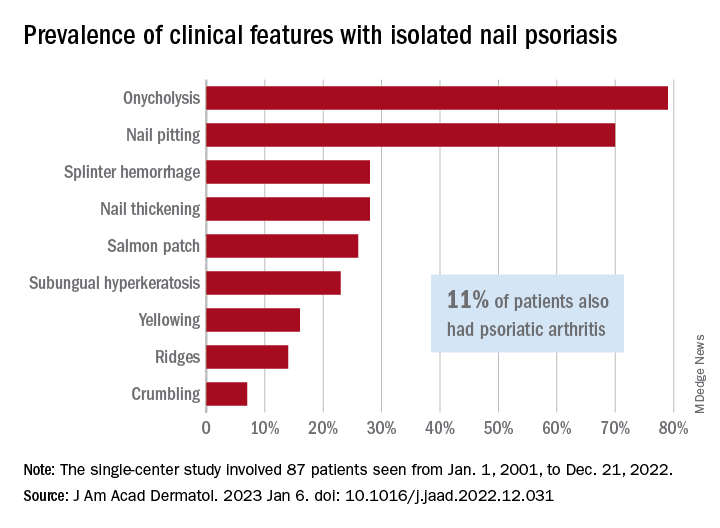
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Chest lesion
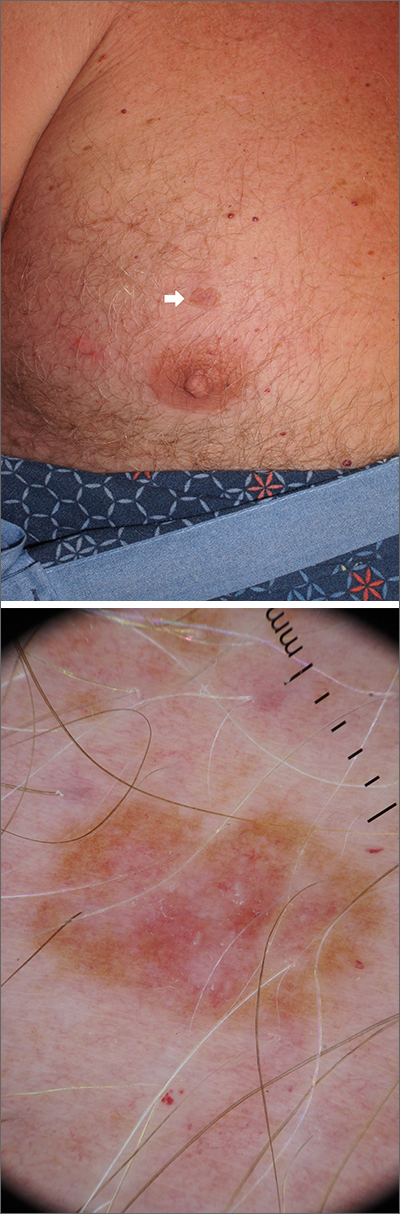
A scoop shave biopsy was performed, including at least a 1-mm margin of normal-looking skin. Pathology was consistent with melanoma in situ.
Melanoma in situ, also called Stage 0 melanoma, is defined by atypical melanocytes that have not begun to invade the dermis and, therefore, have a Breslow thickness of 0 mm. While invasive melanoma is responsible for the largest number of skin cancer deaths in the United States (estimated to be 7990 in 2023), melanoma in situ maintains a very high cure rate when treated appropriately.1
Dermoscopy can help differentiate melanoma from benign nevi or other benign skin lesions. In this case, dermoscopy revealed a fine pigment network at the periphery that indicated this lesion was made up of melanocytes. There were also atypical vascular markings (the milky red color) in the center. Taken together, these findings were strongly indicative of melanoma.
Standard of care for melanoma in situ is a wide local excision with a margin of 5 to 10 mm. Melanoma in situ does not require sentinel lymph node biopsy. However, a lymph node biopsy would have been necessary if the melanoma had been ≥ 1 mm in thickness or if it had been ≥ 0.8 mm in thickness with higher-risk features, such as an increased number of mitoses per high-power field on pathology. Mohs micrographic surgery (MMS) is emerging as an alternative method to wide local excision to treat melanoma in situ. However, it can only be done in specialized centers that can do rapid immunohistochemical staining on frozen sections. MMS is especially useful in cosmetically sensitive areas of the body and in areas where the true size of the melanoma in situ is unclear.
This patient subsequently underwent a wide local excision in the office with a margin of 6 mm. A sentinel lymph node biopsy was not performed. The patient will continue with skin surveillance consisting of full skin exams 3 to 4 times in the first year of diagnosis, then twice annually for Years 2 to 5. He will then come in for annual skin exams after that.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. American Cancer Society. Cancer Facts & Figures 2023. Atlanta: American Cancer Society; 2023. Accessed February 20, 2023. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf

A scoop shave biopsy was performed, including at least a 1-mm margin of normal-looking skin. Pathology was consistent with melanoma in situ.
Melanoma in situ, also called Stage 0 melanoma, is defined by atypical melanocytes that have not begun to invade the dermis and, therefore, have a Breslow thickness of 0 mm. While invasive melanoma is responsible for the largest number of skin cancer deaths in the United States (estimated to be 7990 in 2023), melanoma in situ maintains a very high cure rate when treated appropriately.1
Dermoscopy can help differentiate melanoma from benign nevi or other benign skin lesions. In this case, dermoscopy revealed a fine pigment network at the periphery that indicated this lesion was made up of melanocytes. There were also atypical vascular markings (the milky red color) in the center. Taken together, these findings were strongly indicative of melanoma.
Standard of care for melanoma in situ is a wide local excision with a margin of 5 to 10 mm. Melanoma in situ does not require sentinel lymph node biopsy. However, a lymph node biopsy would have been necessary if the melanoma had been ≥ 1 mm in thickness or if it had been ≥ 0.8 mm in thickness with higher-risk features, such as an increased number of mitoses per high-power field on pathology. Mohs micrographic surgery (MMS) is emerging as an alternative method to wide local excision to treat melanoma in situ. However, it can only be done in specialized centers that can do rapid immunohistochemical staining on frozen sections. MMS is especially useful in cosmetically sensitive areas of the body and in areas where the true size of the melanoma in situ is unclear.
This patient subsequently underwent a wide local excision in the office with a margin of 6 mm. A sentinel lymph node biopsy was not performed. The patient will continue with skin surveillance consisting of full skin exams 3 to 4 times in the first year of diagnosis, then twice annually for Years 2 to 5. He will then come in for annual skin exams after that.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A scoop shave biopsy was performed, including at least a 1-mm margin of normal-looking skin. Pathology was consistent with melanoma in situ.
Melanoma in situ, also called Stage 0 melanoma, is defined by atypical melanocytes that have not begun to invade the dermis and, therefore, have a Breslow thickness of 0 mm. While invasive melanoma is responsible for the largest number of skin cancer deaths in the United States (estimated to be 7990 in 2023), melanoma in situ maintains a very high cure rate when treated appropriately.1
Dermoscopy can help differentiate melanoma from benign nevi or other benign skin lesions. In this case, dermoscopy revealed a fine pigment network at the periphery that indicated this lesion was made up of melanocytes. There were also atypical vascular markings (the milky red color) in the center. Taken together, these findings were strongly indicative of melanoma.
Standard of care for melanoma in situ is a wide local excision with a margin of 5 to 10 mm. Melanoma in situ does not require sentinel lymph node biopsy. However, a lymph node biopsy would have been necessary if the melanoma had been ≥ 1 mm in thickness or if it had been ≥ 0.8 mm in thickness with higher-risk features, such as an increased number of mitoses per high-power field on pathology. Mohs micrographic surgery (MMS) is emerging as an alternative method to wide local excision to treat melanoma in situ. However, it can only be done in specialized centers that can do rapid immunohistochemical staining on frozen sections. MMS is especially useful in cosmetically sensitive areas of the body and in areas where the true size of the melanoma in situ is unclear.
This patient subsequently underwent a wide local excision in the office with a margin of 6 mm. A sentinel lymph node biopsy was not performed. The patient will continue with skin surveillance consisting of full skin exams 3 to 4 times in the first year of diagnosis, then twice annually for Years 2 to 5. He will then come in for annual skin exams after that.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. American Cancer Society. Cancer Facts & Figures 2023. Atlanta: American Cancer Society; 2023. Accessed February 20, 2023. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
1. American Cancer Society. Cancer Facts & Figures 2023. Atlanta: American Cancer Society; 2023. Accessed February 20, 2023. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
Artificial sweetener in ‘keto foods’ tied to cardiovascular risk
Erythritol is one of the most widely used artificial sweeteners with rapidly increasing prevalence in processed and “keto-related” foods. Artificial sweeteners are “generally recognized as safe” (GRAS) by the U.S. Food and Drug Administration, so there is no requirement for long-term safety studies, and little is known about the long-term health effects.
The current research, published online in Nature Medicine by Marco Witkowski, MD, of the Lerner Research Institute at Cleveland Clinic and colleagues, had multiple parts.
First, in a group of patients undergoing cardiac risk assessment, the researchers found that high levels of polyols, especially erythritol, were associated with increased 3-year risk of MACE, defined as cardiovascular death or nonfatal myocardial infarction or stroke.
Next, the association of erythritol with this outcome was reproduced in two large U.S. and European groups of stable patients undergoing elective cardiac evaluation.
Next, adding erythritol to whole blood or platelets led to clot activation. And lastly, in eight healthy volunteers, ingesting 30 g of an erythritol-sweetened drink – comparable to a single can of commercially available beverage or a pint of keto ice cream – induced marked and sustained (> 2 day) increases in levels of plasma erythritol.
“Our study shows that when participants consumed an artificially sweetened beverage with an amount of erythritol found in many processed foods, markedly elevated levels in the blood are observed for days – levels well above those observed to enhance clotting risks,” said senior author Stanley L. Hazen, MD, PhD.
“It is important that further safety studies are conducted to examine the long-term effects of artificial sweeteners in general, and erythritol specifically, on risks for heart attack and stroke, particularly in people at higher risk for cardiovascular disease,” Dr. Hazen, co–section head of preventive cardiology at Cleveland Clinic, said in a press release from his institution.
“Sweeteners like erythritol have rapidly increased in popularity in recent years, but there needs to be more in-depth research into their long-term effects. Cardiovascular disease builds over time, and heart disease is the leading cause of death globally. We need to make sure the foods we eat aren’t hidden contributors,” Dr. Hazen urged.
The topic remains controversial.
Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England, told the U.K. Science Media Centre: “This paper effectively shows multiple pieces of a jigsaw exploring the effects of erythritol – although it claims to show an associated risk with the use of erythritol as an artificial sweetener and cardiovascular disease, I believe it fails to do so, as ultimately, erythritol can be made inside our bodies and the intake in most people’s diet is much lower than the amount given in this study.”
Dr. Hazen countered that data from the 2013-2014 National Health and Nutrition Examination Survey (NHANES) in the United States show that, in some individuals, daily intake of erythritol is estimated to reach 30 g/day.
“Many try and reduce sugar intake by taking many teaspoons of erythritol in their tea, coffee, etc., instead of sugar,” Dr. Hazen added. “Or they eat keto processed foods that have significant quantities of erythritol within it.”
“These studies are a warning for how our processed food (keto and zero sugar, especially) may inadvertently be causing risk/harm. … in the very subset of subjects who are most vulnerable,” according to Dr. Hazen.
Erythritol marketed as ‘zero calorie’, ‘non-nutritive’, or ‘natural’
Patients with type 2 diabetes and obesity are often advised to replace sugar with artificial sweeteners for better glucose control and weight loss, but growing epidemiologic evidence links artificial sweetener consumption with weight gain, insulin resistance, type 2 diabetes, and cardiovascular disease, the researchers write.
Erythritol is naturally present in low amounts in fruits and vegetables; the artificial sweetener erythritol that is produced from corn is only 70% as sweet as sugar.
Upon ingestion it is poorly metabolized, and most is excreted in the urine, so it is characterized as a “zero-calorie,” “non-nutritive,” or “natural sweetener.” It is predicted to double in marketshare in the sweetener sector in the next 5 years.
Multipart study
In the first part of their study, in a discovery cohort in 1,157 patients undergoing cardiovascular assessment with 3-year outcomes, the researchers identified polyols that were associated with MACE, and erythritol was among the top MACE-associated molecules.
Next, in a U.S. validation cohort of 2,149 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 1.8-fold higher risk of MACE than patients in the lowest quartile (P = .007), after adjusting for cardiovascular risk factors.
In a European validation cohort of 833 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 2.21-fold higher risk of MACE than patients in the lowest quartile (P = .010, after adjustment).
At physiologic levels, erythritol enhanced platelet reactivity in vitro and thrombosis formation in vivo.
Finally, in a prospective pilot intervention study, erythritol ingestion in healthy volunteers induced marked and sustained increases in plasma erythritol levels well above thresholds associated with heightened platelet reactivity and thrombosis potential in in vitro and in vivo studies.
Others weigh in
“While I think the finding certainly warrants further investigation, don’t throw out your sweeteners just yet,” commented Oliver Jones, PhD, professor of chemistry at the Royal Melbourne Institute of Technology.
“This study only looks at erythritol, and artificial sweeteners are generally considered safe. Any possible (and, as yet unproven) risks of excess erythritol would also need to be balanced against the very real health risks of excess glucose consumption,” he said.
Dr. Hazen responded: “True enough. Erythritol is but one of many artificial sweeteners. That is why it is important to read labels. This study can make patients be informed about how to potentially avoid something that might cause them inadvertent harm.”
“The key findings of this study are that high blood levels of erythritol are strongly associated with cardiovascular outcomes in high-risk patients, which has been replicated in separate validation studies,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London.
“Diabetes UK currently advises diabetes patients not to use polyols,” he added.
Dr. Hazen noted that “About three-quarters of the participants had coronary disease, high blood pressure, and about a fifth had diabetes.”
The researchers acknowledge, however, that the observational studies cannot show cause and effect.
The study was supported by the Office of Dietary Supplements at the National Institutes of Health, the Leducq Foundation, and the German Research Foundation (Deutsche Forschungsgemeinschaft). Dr. Mellor, Dr. Jones, and Dr. Sanders have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Erythritol is one of the most widely used artificial sweeteners with rapidly increasing prevalence in processed and “keto-related” foods. Artificial sweeteners are “generally recognized as safe” (GRAS) by the U.S. Food and Drug Administration, so there is no requirement for long-term safety studies, and little is known about the long-term health effects.
The current research, published online in Nature Medicine by Marco Witkowski, MD, of the Lerner Research Institute at Cleveland Clinic and colleagues, had multiple parts.
First, in a group of patients undergoing cardiac risk assessment, the researchers found that high levels of polyols, especially erythritol, were associated with increased 3-year risk of MACE, defined as cardiovascular death or nonfatal myocardial infarction or stroke.
Next, the association of erythritol with this outcome was reproduced in two large U.S. and European groups of stable patients undergoing elective cardiac evaluation.
Next, adding erythritol to whole blood or platelets led to clot activation. And lastly, in eight healthy volunteers, ingesting 30 g of an erythritol-sweetened drink – comparable to a single can of commercially available beverage or a pint of keto ice cream – induced marked and sustained (> 2 day) increases in levels of plasma erythritol.
“Our study shows that when participants consumed an artificially sweetened beverage with an amount of erythritol found in many processed foods, markedly elevated levels in the blood are observed for days – levels well above those observed to enhance clotting risks,” said senior author Stanley L. Hazen, MD, PhD.
“It is important that further safety studies are conducted to examine the long-term effects of artificial sweeteners in general, and erythritol specifically, on risks for heart attack and stroke, particularly in people at higher risk for cardiovascular disease,” Dr. Hazen, co–section head of preventive cardiology at Cleveland Clinic, said in a press release from his institution.
“Sweeteners like erythritol have rapidly increased in popularity in recent years, but there needs to be more in-depth research into their long-term effects. Cardiovascular disease builds over time, and heart disease is the leading cause of death globally. We need to make sure the foods we eat aren’t hidden contributors,” Dr. Hazen urged.
The topic remains controversial.
Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England, told the U.K. Science Media Centre: “This paper effectively shows multiple pieces of a jigsaw exploring the effects of erythritol – although it claims to show an associated risk with the use of erythritol as an artificial sweetener and cardiovascular disease, I believe it fails to do so, as ultimately, erythritol can be made inside our bodies and the intake in most people’s diet is much lower than the amount given in this study.”
Dr. Hazen countered that data from the 2013-2014 National Health and Nutrition Examination Survey (NHANES) in the United States show that, in some individuals, daily intake of erythritol is estimated to reach 30 g/day.
“Many try and reduce sugar intake by taking many teaspoons of erythritol in their tea, coffee, etc., instead of sugar,” Dr. Hazen added. “Or they eat keto processed foods that have significant quantities of erythritol within it.”
“These studies are a warning for how our processed food (keto and zero sugar, especially) may inadvertently be causing risk/harm. … in the very subset of subjects who are most vulnerable,” according to Dr. Hazen.
Erythritol marketed as ‘zero calorie’, ‘non-nutritive’, or ‘natural’
Patients with type 2 diabetes and obesity are often advised to replace sugar with artificial sweeteners for better glucose control and weight loss, but growing epidemiologic evidence links artificial sweetener consumption with weight gain, insulin resistance, type 2 diabetes, and cardiovascular disease, the researchers write.
Erythritol is naturally present in low amounts in fruits and vegetables; the artificial sweetener erythritol that is produced from corn is only 70% as sweet as sugar.
Upon ingestion it is poorly metabolized, and most is excreted in the urine, so it is characterized as a “zero-calorie,” “non-nutritive,” or “natural sweetener.” It is predicted to double in marketshare in the sweetener sector in the next 5 years.
Multipart study
In the first part of their study, in a discovery cohort in 1,157 patients undergoing cardiovascular assessment with 3-year outcomes, the researchers identified polyols that were associated with MACE, and erythritol was among the top MACE-associated molecules.
Next, in a U.S. validation cohort of 2,149 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 1.8-fold higher risk of MACE than patients in the lowest quartile (P = .007), after adjusting for cardiovascular risk factors.
In a European validation cohort of 833 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 2.21-fold higher risk of MACE than patients in the lowest quartile (P = .010, after adjustment).
At physiologic levels, erythritol enhanced platelet reactivity in vitro and thrombosis formation in vivo.
Finally, in a prospective pilot intervention study, erythritol ingestion in healthy volunteers induced marked and sustained increases in plasma erythritol levels well above thresholds associated with heightened platelet reactivity and thrombosis potential in in vitro and in vivo studies.
Others weigh in
“While I think the finding certainly warrants further investigation, don’t throw out your sweeteners just yet,” commented Oliver Jones, PhD, professor of chemistry at the Royal Melbourne Institute of Technology.
“This study only looks at erythritol, and artificial sweeteners are generally considered safe. Any possible (and, as yet unproven) risks of excess erythritol would also need to be balanced against the very real health risks of excess glucose consumption,” he said.
Dr. Hazen responded: “True enough. Erythritol is but one of many artificial sweeteners. That is why it is important to read labels. This study can make patients be informed about how to potentially avoid something that might cause them inadvertent harm.”
“The key findings of this study are that high blood levels of erythritol are strongly associated with cardiovascular outcomes in high-risk patients, which has been replicated in separate validation studies,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London.
“Diabetes UK currently advises diabetes patients not to use polyols,” he added.
Dr. Hazen noted that “About three-quarters of the participants had coronary disease, high blood pressure, and about a fifth had diabetes.”
The researchers acknowledge, however, that the observational studies cannot show cause and effect.
The study was supported by the Office of Dietary Supplements at the National Institutes of Health, the Leducq Foundation, and the German Research Foundation (Deutsche Forschungsgemeinschaft). Dr. Mellor, Dr. Jones, and Dr. Sanders have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Erythritol is one of the most widely used artificial sweeteners with rapidly increasing prevalence in processed and “keto-related” foods. Artificial sweeteners are “generally recognized as safe” (GRAS) by the U.S. Food and Drug Administration, so there is no requirement for long-term safety studies, and little is known about the long-term health effects.
The current research, published online in Nature Medicine by Marco Witkowski, MD, of the Lerner Research Institute at Cleveland Clinic and colleagues, had multiple parts.
First, in a group of patients undergoing cardiac risk assessment, the researchers found that high levels of polyols, especially erythritol, were associated with increased 3-year risk of MACE, defined as cardiovascular death or nonfatal myocardial infarction or stroke.
Next, the association of erythritol with this outcome was reproduced in two large U.S. and European groups of stable patients undergoing elective cardiac evaluation.
Next, adding erythritol to whole blood or platelets led to clot activation. And lastly, in eight healthy volunteers, ingesting 30 g of an erythritol-sweetened drink – comparable to a single can of commercially available beverage or a pint of keto ice cream – induced marked and sustained (> 2 day) increases in levels of plasma erythritol.
“Our study shows that when participants consumed an artificially sweetened beverage with an amount of erythritol found in many processed foods, markedly elevated levels in the blood are observed for days – levels well above those observed to enhance clotting risks,” said senior author Stanley L. Hazen, MD, PhD.
“It is important that further safety studies are conducted to examine the long-term effects of artificial sweeteners in general, and erythritol specifically, on risks for heart attack and stroke, particularly in people at higher risk for cardiovascular disease,” Dr. Hazen, co–section head of preventive cardiology at Cleveland Clinic, said in a press release from his institution.
“Sweeteners like erythritol have rapidly increased in popularity in recent years, but there needs to be more in-depth research into their long-term effects. Cardiovascular disease builds over time, and heart disease is the leading cause of death globally. We need to make sure the foods we eat aren’t hidden contributors,” Dr. Hazen urged.
The topic remains controversial.
Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England, told the U.K. Science Media Centre: “This paper effectively shows multiple pieces of a jigsaw exploring the effects of erythritol – although it claims to show an associated risk with the use of erythritol as an artificial sweetener and cardiovascular disease, I believe it fails to do so, as ultimately, erythritol can be made inside our bodies and the intake in most people’s diet is much lower than the amount given in this study.”
Dr. Hazen countered that data from the 2013-2014 National Health and Nutrition Examination Survey (NHANES) in the United States show that, in some individuals, daily intake of erythritol is estimated to reach 30 g/day.
“Many try and reduce sugar intake by taking many teaspoons of erythritol in their tea, coffee, etc., instead of sugar,” Dr. Hazen added. “Or they eat keto processed foods that have significant quantities of erythritol within it.”
“These studies are a warning for how our processed food (keto and zero sugar, especially) may inadvertently be causing risk/harm. … in the very subset of subjects who are most vulnerable,” according to Dr. Hazen.
Erythritol marketed as ‘zero calorie’, ‘non-nutritive’, or ‘natural’
Patients with type 2 diabetes and obesity are often advised to replace sugar with artificial sweeteners for better glucose control and weight loss, but growing epidemiologic evidence links artificial sweetener consumption with weight gain, insulin resistance, type 2 diabetes, and cardiovascular disease, the researchers write.
Erythritol is naturally present in low amounts in fruits and vegetables; the artificial sweetener erythritol that is produced from corn is only 70% as sweet as sugar.
Upon ingestion it is poorly metabolized, and most is excreted in the urine, so it is characterized as a “zero-calorie,” “non-nutritive,” or “natural sweetener.” It is predicted to double in marketshare in the sweetener sector in the next 5 years.
Multipart study
In the first part of their study, in a discovery cohort in 1,157 patients undergoing cardiovascular assessment with 3-year outcomes, the researchers identified polyols that were associated with MACE, and erythritol was among the top MACE-associated molecules.
Next, in a U.S. validation cohort of 2,149 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 1.8-fold higher risk of MACE than patients in the lowest quartile (P = .007), after adjusting for cardiovascular risk factors.
In a European validation cohort of 833 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 2.21-fold higher risk of MACE than patients in the lowest quartile (P = .010, after adjustment).
At physiologic levels, erythritol enhanced platelet reactivity in vitro and thrombosis formation in vivo.
Finally, in a prospective pilot intervention study, erythritol ingestion in healthy volunteers induced marked and sustained increases in plasma erythritol levels well above thresholds associated with heightened platelet reactivity and thrombosis potential in in vitro and in vivo studies.
Others weigh in
“While I think the finding certainly warrants further investigation, don’t throw out your sweeteners just yet,” commented Oliver Jones, PhD, professor of chemistry at the Royal Melbourne Institute of Technology.
“This study only looks at erythritol, and artificial sweeteners are generally considered safe. Any possible (and, as yet unproven) risks of excess erythritol would also need to be balanced against the very real health risks of excess glucose consumption,” he said.
Dr. Hazen responded: “True enough. Erythritol is but one of many artificial sweeteners. That is why it is important to read labels. This study can make patients be informed about how to potentially avoid something that might cause them inadvertent harm.”
“The key findings of this study are that high blood levels of erythritol are strongly associated with cardiovascular outcomes in high-risk patients, which has been replicated in separate validation studies,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London.
“Diabetes UK currently advises diabetes patients not to use polyols,” he added.
Dr. Hazen noted that “About three-quarters of the participants had coronary disease, high blood pressure, and about a fifth had diabetes.”
The researchers acknowledge, however, that the observational studies cannot show cause and effect.
The study was supported by the Office of Dietary Supplements at the National Institutes of Health, the Leducq Foundation, and the German Research Foundation (Deutsche Forschungsgemeinschaft). Dr. Mellor, Dr. Jones, and Dr. Sanders have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Red wine’s potential benefits for cardiovascular health
In recent weeks, you may have noticed some familiar headlines about red wine and cardiovascular health. Why the sudden return of these stories? Because of an article recently published in the American Journal of Clinical Nutrition.
Funded in part by a grant from the São Paulo Research Foundation (FAPESP), the “Wine Flora Study” was carried out by prominent researchers from institutions in South America, Europe, and the United States: University of São Paulo; State University of Campinas, São Paulo; University of Brasília; University of Verona (Italy); Austrian Institute of Technology, Tulln; and Harvard Medical School, Boston. The team looked into the effects of red wine on gut flora and plasma levels of trimethylamine-N-oxide (TMAO). And what they found was quite interesting.
The study
Previous results have pointed to the beneficial effect that red wine has on the gut microbiome.
The Wine Flora Study involved 42 men (average age, 60 years) with documented coronary artery disease. The trial encompassed two 3-week interventions. In one, the participants consumed 250 mL of red wine per day; the red wine sample had an alcohol content (% v) of 12.75. The Brazilian Wine Institute produced and supplied the red wine: a 2014 Merlot bottled in August 2016 and customized for the study. The second intervention involved alcohol abstention.
Each intervention was preceded by a 2-week washout period. Because certain foods and drinks could interfere with the results, the participants were instructed not to consume alcoholic beverages, fermented foods (yogurt, kombucha, soy lecithin, kefir, sauerkraut, and other fermented vegetables), synthetic prebiotics (insulin, fructooligosaccharides), fiber, dairy, food polyphenols (grapes, grape juice, cranberries, strawberries), and probiotics.
At each intervention, the gut microbiota was analyzed via 16S ribosomal RNA highthroughput sequencing. This method makes it possible to identify bacterial species. The plasma metabolome of 20 randomly selected participants was evaluated by ultra–high-performance liquid chromatography with tandem mass spectrometry. In this method, liquid chromatography separates the compounds, and a mass spectrometer is used to analyze them.
One of the metabolites of interest was TMAO, which is produced from the trimethylamine released when gut bacteria process protein-rich foods. TMAO has been identified as playing a role in the development of atherosclerosis.
Results
with a difference in beta diversity and predominance of Parasutterella, Ruminococcaceae, several Bacteroides species, and Prevotella.
Plasma metabolomic analysis revealed significant changes in metabolites after red wine consumption, consistent with improved redox homeostasis, which is involved in the oxidative stress that promotes atherosclerosis.
Plasma TMAO, however, did not differ between red wine intervention and alcohol abstention.
Implications
The researchers concluded that modulation of the gut microbiota may contribute to the putative cardiovascular benefits of moderate red wine consumption. But, as they were careful to point out in the very title of the study, a red wine intervention does not modify plasma TMAO. They also mentioned that the 3-week period may have been too short for the findings to serve as the basis for promoting any meaningful modification. In addition, the team emphasized that these data remain hypothesisgenerating and pave the way for future research.
In an interview with FAPESP, the study’s corresponding author, Protásio Lemos da Luz, MD, PhD, warned about the risks associated with drinking too much alcohol (> 8.5 oz., or 250 mL, of wine daily).
It should be kept in mind that, in Brazil, people do not drink nearly as much wine as they do beer or liquor. Furthermore, the evidence that is available does not provide confirmation of the existence or the extent of the protective health effects associated with light or moderate alcohol intake.
This article was translated from the Medscape Portuguese edition. A version appeared on Medscape.com.
In recent weeks, you may have noticed some familiar headlines about red wine and cardiovascular health. Why the sudden return of these stories? Because of an article recently published in the American Journal of Clinical Nutrition.
Funded in part by a grant from the São Paulo Research Foundation (FAPESP), the “Wine Flora Study” was carried out by prominent researchers from institutions in South America, Europe, and the United States: University of São Paulo; State University of Campinas, São Paulo; University of Brasília; University of Verona (Italy); Austrian Institute of Technology, Tulln; and Harvard Medical School, Boston. The team looked into the effects of red wine on gut flora and plasma levels of trimethylamine-N-oxide (TMAO). And what they found was quite interesting.
The study
Previous results have pointed to the beneficial effect that red wine has on the gut microbiome.
The Wine Flora Study involved 42 men (average age, 60 years) with documented coronary artery disease. The trial encompassed two 3-week interventions. In one, the participants consumed 250 mL of red wine per day; the red wine sample had an alcohol content (% v) of 12.75. The Brazilian Wine Institute produced and supplied the red wine: a 2014 Merlot bottled in August 2016 and customized for the study. The second intervention involved alcohol abstention.
Each intervention was preceded by a 2-week washout period. Because certain foods and drinks could interfere with the results, the participants were instructed not to consume alcoholic beverages, fermented foods (yogurt, kombucha, soy lecithin, kefir, sauerkraut, and other fermented vegetables), synthetic prebiotics (insulin, fructooligosaccharides), fiber, dairy, food polyphenols (grapes, grape juice, cranberries, strawberries), and probiotics.
At each intervention, the gut microbiota was analyzed via 16S ribosomal RNA highthroughput sequencing. This method makes it possible to identify bacterial species. The plasma metabolome of 20 randomly selected participants was evaluated by ultra–high-performance liquid chromatography with tandem mass spectrometry. In this method, liquid chromatography separates the compounds, and a mass spectrometer is used to analyze them.
One of the metabolites of interest was TMAO, which is produced from the trimethylamine released when gut bacteria process protein-rich foods. TMAO has been identified as playing a role in the development of atherosclerosis.
Results
with a difference in beta diversity and predominance of Parasutterella, Ruminococcaceae, several Bacteroides species, and Prevotella.
Plasma metabolomic analysis revealed significant changes in metabolites after red wine consumption, consistent with improved redox homeostasis, which is involved in the oxidative stress that promotes atherosclerosis.
Plasma TMAO, however, did not differ between red wine intervention and alcohol abstention.
Implications
The researchers concluded that modulation of the gut microbiota may contribute to the putative cardiovascular benefits of moderate red wine consumption. But, as they were careful to point out in the very title of the study, a red wine intervention does not modify plasma TMAO. They also mentioned that the 3-week period may have been too short for the findings to serve as the basis for promoting any meaningful modification. In addition, the team emphasized that these data remain hypothesisgenerating and pave the way for future research.
In an interview with FAPESP, the study’s corresponding author, Protásio Lemos da Luz, MD, PhD, warned about the risks associated with drinking too much alcohol (> 8.5 oz., or 250 mL, of wine daily).
It should be kept in mind that, in Brazil, people do not drink nearly as much wine as they do beer or liquor. Furthermore, the evidence that is available does not provide confirmation of the existence or the extent of the protective health effects associated with light or moderate alcohol intake.
This article was translated from the Medscape Portuguese edition. A version appeared on Medscape.com.
In recent weeks, you may have noticed some familiar headlines about red wine and cardiovascular health. Why the sudden return of these stories? Because of an article recently published in the American Journal of Clinical Nutrition.
Funded in part by a grant from the São Paulo Research Foundation (FAPESP), the “Wine Flora Study” was carried out by prominent researchers from institutions in South America, Europe, and the United States: University of São Paulo; State University of Campinas, São Paulo; University of Brasília; University of Verona (Italy); Austrian Institute of Technology, Tulln; and Harvard Medical School, Boston. The team looked into the effects of red wine on gut flora and plasma levels of trimethylamine-N-oxide (TMAO). And what they found was quite interesting.
The study
Previous results have pointed to the beneficial effect that red wine has on the gut microbiome.
The Wine Flora Study involved 42 men (average age, 60 years) with documented coronary artery disease. The trial encompassed two 3-week interventions. In one, the participants consumed 250 mL of red wine per day; the red wine sample had an alcohol content (% v) of 12.75. The Brazilian Wine Institute produced and supplied the red wine: a 2014 Merlot bottled in August 2016 and customized for the study. The second intervention involved alcohol abstention.
Each intervention was preceded by a 2-week washout period. Because certain foods and drinks could interfere with the results, the participants were instructed not to consume alcoholic beverages, fermented foods (yogurt, kombucha, soy lecithin, kefir, sauerkraut, and other fermented vegetables), synthetic prebiotics (insulin, fructooligosaccharides), fiber, dairy, food polyphenols (grapes, grape juice, cranberries, strawberries), and probiotics.
At each intervention, the gut microbiota was analyzed via 16S ribosomal RNA highthroughput sequencing. This method makes it possible to identify bacterial species. The plasma metabolome of 20 randomly selected participants was evaluated by ultra–high-performance liquid chromatography with tandem mass spectrometry. In this method, liquid chromatography separates the compounds, and a mass spectrometer is used to analyze them.
One of the metabolites of interest was TMAO, which is produced from the trimethylamine released when gut bacteria process protein-rich foods. TMAO has been identified as playing a role in the development of atherosclerosis.
Results
with a difference in beta diversity and predominance of Parasutterella, Ruminococcaceae, several Bacteroides species, and Prevotella.
Plasma metabolomic analysis revealed significant changes in metabolites after red wine consumption, consistent with improved redox homeostasis, which is involved in the oxidative stress that promotes atherosclerosis.
Plasma TMAO, however, did not differ between red wine intervention and alcohol abstention.
Implications
The researchers concluded that modulation of the gut microbiota may contribute to the putative cardiovascular benefits of moderate red wine consumption. But, as they were careful to point out in the very title of the study, a red wine intervention does not modify plasma TMAO. They also mentioned that the 3-week period may have been too short for the findings to serve as the basis for promoting any meaningful modification. In addition, the team emphasized that these data remain hypothesisgenerating and pave the way for future research.
In an interview with FAPESP, the study’s corresponding author, Protásio Lemos da Luz, MD, PhD, warned about the risks associated with drinking too much alcohol (> 8.5 oz., or 250 mL, of wine daily).
It should be kept in mind that, in Brazil, people do not drink nearly as much wine as they do beer or liquor. Furthermore, the evidence that is available does not provide confirmation of the existence or the extent of the protective health effects associated with light or moderate alcohol intake.
This article was translated from the Medscape Portuguese edition. A version appeared on Medscape.com.
Topical or intralesional cidofovir an option for recalcitrant warts
HONOLULU – Combining or those located in areas that are challenging to treat, according to John S. Barbieri, MD, MBA.
“There are 5 million office visits per year in the United States for warts and molluscum, and they’re most common in pediatrics,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “In fact, some studies have suggested that one in three children in primary school suffers from warts.”
According to a 2012 Cochrane review of topical therapies for warts, first-line treatments such as salicylic acid, cryotherapy, 5-FU, or Candida antigen injection often have modest efficacy when used alone. For example, the authors found that salicylic acid and cryotherapy cleared warts in about 60%-70% of cases, respectively, but clearance rates were improved by combining the two therapies.
In an earlier literature review and meta-analysis, investigators evaluated the effect of 5-FU plus salicylic acid or salicylic acid alone. The therapeutic effect for common warts across all studies was a 63.4% response rate (complete healing) for 5-FU/SA vversus 23.1% for the 5-FU–free controls, respectively. For plantar warts, the response rate was 63% versus 11%, respectively.
“But what about the person with multiple warts or those in challenging locations where you might worry about destructive treatments hurting the nail fold or causing nail dystrophy?” Dr. Barbieri asked. “Maybe they’ve used salicylic acid or intralesional Candida and they’re still not getting better. What can we do for these patients?”
Emerging research suggests that topical cidofovir can be a valuable option for recalcitrant warts or those in sensitive locations. In a case report of a 10-year-old boy with more than 50 severe verrucous papules on his hands and face that were recalcitrant to multiple conventional therapies, topical 1% cidofovir applied daily for 8 weeks was effective, with no adverse side effects. A young female patient who presented to Dr. Barbieri with multiple warts around the nail matrix of several fingers experienced complete clearance after treatment with topical cidofovir, he said. Other researchers found this approach to be effective for plantar warts as well, in a report of two brothers with severe combined immunodeficiency after hematopoietic stem cell transplantation with persistent warts that did not respond to traditional topical treatments.
“Topical cidofovir is typically a painless treatment, which is nice, especially for our pediatric patients who might be afraid of other therapies like or cryotherapy or intralesional injections,” One limitation is that it is “a bit expensive,” Dr. Barbieri said. “To have topical cidofovir compounded is typically $100-$300, depending on the quantity and strength that you ask for.”
Intralesional cidofovir is another treatment option. In a retrospective study of 58 patients, Dr. Barbieri and colleagues evaluated the outcome of intralesional cidofovir treatment of warts in immunocompromised and nonimmunocompromised patients. Rates of improvement ranged from 98.3% to 100%, while resolution rates ranged from 75.9% to 97.6%.
“Most of the patients had warts for more than 5 years and almost half of them had recalcitrant warts,” Dr. Barbieri said. “These were mostly adult patients, but I think this is a treatment that can work in younger populations as well. About 10%-15% had HIV or cancer or diabetes or were transplant recipients, but despite these challenges and despite these recalcitrant warts, about 100% had improvement.”
He pointed out that cidofovir is available as a 75 mg/mL vial that comes with a 5 mL single-use vial. He dilutes this with normal saline to create a 15 mg/mL solution.
“If you want to be efficient you can try to schedule multiple patients together on the same day as a single vial is sufficient to treat about 25 patients,” assuming about 1 mL is injected per patient, he said. “The challenge with intralesional cidofovir is that it’s painful beyond just the needle part of the injection. Sometimes a nerve block can be helpful. But this can be an effective treatment for patients with recalcitrant warts or those with comorbidities.”
Other intralesional therapies to try for recalcitrant warts, he said, include bleomycin (1 U/mL solution, 1-2 mL per treatment, spaced every 2-4 weeks), and 5-FU (a 4:1 mixture of 5-FU [50 mg/mL] and 2% lidocaine).
Dr. Barbieri disclosed that he receives consulting fees from Dexcel for work unrelated to his presentation. Medscape and this news organization are owned by the same parent company.
HONOLULU – Combining or those located in areas that are challenging to treat, according to John S. Barbieri, MD, MBA.
“There are 5 million office visits per year in the United States for warts and molluscum, and they’re most common in pediatrics,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “In fact, some studies have suggested that one in three children in primary school suffers from warts.”
According to a 2012 Cochrane review of topical therapies for warts, first-line treatments such as salicylic acid, cryotherapy, 5-FU, or Candida antigen injection often have modest efficacy when used alone. For example, the authors found that salicylic acid and cryotherapy cleared warts in about 60%-70% of cases, respectively, but clearance rates were improved by combining the two therapies.
In an earlier literature review and meta-analysis, investigators evaluated the effect of 5-FU plus salicylic acid or salicylic acid alone. The therapeutic effect for common warts across all studies was a 63.4% response rate (complete healing) for 5-FU/SA vversus 23.1% for the 5-FU–free controls, respectively. For plantar warts, the response rate was 63% versus 11%, respectively.
“But what about the person with multiple warts or those in challenging locations where you might worry about destructive treatments hurting the nail fold or causing nail dystrophy?” Dr. Barbieri asked. “Maybe they’ve used salicylic acid or intralesional Candida and they’re still not getting better. What can we do for these patients?”
Emerging research suggests that topical cidofovir can be a valuable option for recalcitrant warts or those in sensitive locations. In a case report of a 10-year-old boy with more than 50 severe verrucous papules on his hands and face that were recalcitrant to multiple conventional therapies, topical 1% cidofovir applied daily for 8 weeks was effective, with no adverse side effects. A young female patient who presented to Dr. Barbieri with multiple warts around the nail matrix of several fingers experienced complete clearance after treatment with topical cidofovir, he said. Other researchers found this approach to be effective for plantar warts as well, in a report of two brothers with severe combined immunodeficiency after hematopoietic stem cell transplantation with persistent warts that did not respond to traditional topical treatments.
“Topical cidofovir is typically a painless treatment, which is nice, especially for our pediatric patients who might be afraid of other therapies like or cryotherapy or intralesional injections,” One limitation is that it is “a bit expensive,” Dr. Barbieri said. “To have topical cidofovir compounded is typically $100-$300, depending on the quantity and strength that you ask for.”
Intralesional cidofovir is another treatment option. In a retrospective study of 58 patients, Dr. Barbieri and colleagues evaluated the outcome of intralesional cidofovir treatment of warts in immunocompromised and nonimmunocompromised patients. Rates of improvement ranged from 98.3% to 100%, while resolution rates ranged from 75.9% to 97.6%.
“Most of the patients had warts for more than 5 years and almost half of them had recalcitrant warts,” Dr. Barbieri said. “These were mostly adult patients, but I think this is a treatment that can work in younger populations as well. About 10%-15% had HIV or cancer or diabetes or were transplant recipients, but despite these challenges and despite these recalcitrant warts, about 100% had improvement.”
He pointed out that cidofovir is available as a 75 mg/mL vial that comes with a 5 mL single-use vial. He dilutes this with normal saline to create a 15 mg/mL solution.
“If you want to be efficient you can try to schedule multiple patients together on the same day as a single vial is sufficient to treat about 25 patients,” assuming about 1 mL is injected per patient, he said. “The challenge with intralesional cidofovir is that it’s painful beyond just the needle part of the injection. Sometimes a nerve block can be helpful. But this can be an effective treatment for patients with recalcitrant warts or those with comorbidities.”
Other intralesional therapies to try for recalcitrant warts, he said, include bleomycin (1 U/mL solution, 1-2 mL per treatment, spaced every 2-4 weeks), and 5-FU (a 4:1 mixture of 5-FU [50 mg/mL] and 2% lidocaine).
Dr. Barbieri disclosed that he receives consulting fees from Dexcel for work unrelated to his presentation. Medscape and this news organization are owned by the same parent company.
HONOLULU – Combining or those located in areas that are challenging to treat, according to John S. Barbieri, MD, MBA.
“There are 5 million office visits per year in the United States for warts and molluscum, and they’re most common in pediatrics,” Dr. Barbieri, of the department of dermatology at Brigham and Women’s Hospital, Boston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “In fact, some studies have suggested that one in three children in primary school suffers from warts.”
According to a 2012 Cochrane review of topical therapies for warts, first-line treatments such as salicylic acid, cryotherapy, 5-FU, or Candida antigen injection often have modest efficacy when used alone. For example, the authors found that salicylic acid and cryotherapy cleared warts in about 60%-70% of cases, respectively, but clearance rates were improved by combining the two therapies.
In an earlier literature review and meta-analysis, investigators evaluated the effect of 5-FU plus salicylic acid or salicylic acid alone. The therapeutic effect for common warts across all studies was a 63.4% response rate (complete healing) for 5-FU/SA vversus 23.1% for the 5-FU–free controls, respectively. For plantar warts, the response rate was 63% versus 11%, respectively.
“But what about the person with multiple warts or those in challenging locations where you might worry about destructive treatments hurting the nail fold or causing nail dystrophy?” Dr. Barbieri asked. “Maybe they’ve used salicylic acid or intralesional Candida and they’re still not getting better. What can we do for these patients?”
Emerging research suggests that topical cidofovir can be a valuable option for recalcitrant warts or those in sensitive locations. In a case report of a 10-year-old boy with more than 50 severe verrucous papules on his hands and face that were recalcitrant to multiple conventional therapies, topical 1% cidofovir applied daily for 8 weeks was effective, with no adverse side effects. A young female patient who presented to Dr. Barbieri with multiple warts around the nail matrix of several fingers experienced complete clearance after treatment with topical cidofovir, he said. Other researchers found this approach to be effective for plantar warts as well, in a report of two brothers with severe combined immunodeficiency after hematopoietic stem cell transplantation with persistent warts that did not respond to traditional topical treatments.
“Topical cidofovir is typically a painless treatment, which is nice, especially for our pediatric patients who might be afraid of other therapies like or cryotherapy or intralesional injections,” One limitation is that it is “a bit expensive,” Dr. Barbieri said. “To have topical cidofovir compounded is typically $100-$300, depending on the quantity and strength that you ask for.”
Intralesional cidofovir is another treatment option. In a retrospective study of 58 patients, Dr. Barbieri and colleagues evaluated the outcome of intralesional cidofovir treatment of warts in immunocompromised and nonimmunocompromised patients. Rates of improvement ranged from 98.3% to 100%, while resolution rates ranged from 75.9% to 97.6%.
“Most of the patients had warts for more than 5 years and almost half of them had recalcitrant warts,” Dr. Barbieri said. “These were mostly adult patients, but I think this is a treatment that can work in younger populations as well. About 10%-15% had HIV or cancer or diabetes or were transplant recipients, but despite these challenges and despite these recalcitrant warts, about 100% had improvement.”
He pointed out that cidofovir is available as a 75 mg/mL vial that comes with a 5 mL single-use vial. He dilutes this with normal saline to create a 15 mg/mL solution.
“If you want to be efficient you can try to schedule multiple patients together on the same day as a single vial is sufficient to treat about 25 patients,” assuming about 1 mL is injected per patient, he said. “The challenge with intralesional cidofovir is that it’s painful beyond just the needle part of the injection. Sometimes a nerve block can be helpful. But this can be an effective treatment for patients with recalcitrant warts or those with comorbidities.”
Other intralesional therapies to try for recalcitrant warts, he said, include bleomycin (1 U/mL solution, 1-2 mL per treatment, spaced every 2-4 weeks), and 5-FU (a 4:1 mixture of 5-FU [50 mg/mL] and 2% lidocaine).
Dr. Barbieri disclosed that he receives consulting fees from Dexcel for work unrelated to his presentation. Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
‘Forever chemicals’ disrupt biological processes in children: Study
Exposure to “forever chemicals” widely used in consumer products disrupts important biological processes in children and young adults, a new study says.
That could leave children vulnerable to numerous diseases later in life, including diabetes, cardiovascular disease, and cancer, the study said.
Another important finding was that the disruption appeared to be caused by a mixture of PFAS, rather than a single chemical of that type.
PFAS are known as “forever chemicals” because they don’t break down easily over time and persist in water, soil, and the body. They’re used in numerous consumer products, such as nonstick cookware, stain-resistant carpeting, cosmetics, and water-repellent clothing.
PFAS have previously been linked to a host of health issues, including decreased birth weights and immune system problems. To the study authors’ knowledge, this study is the first to evaluate which biological processes are altered by exposure to multiple PFAS, said a news release from the University of Southern California, Los Angeles.
Researchers studied blood samples from 312 children from the Study of Latino Adolescents at Risk and 137 children from the Southern California Children’s Health Study. All the children had a mixture of common PFAS in their blood, including PFOS, PFHxS, PFHpS, PFOA, and PFNA.
“While current interventions have focused on phasing out the use of individual PFAS, such as PFOS and PFOA, this research shows why the focus should be on reducing exposure to all PFAS chemicals,” said Leda Chatzi, MD, a professor of population and public health sciences at the University of Southern California, Los Angeles. Dr. Chatzi is also one of the study authors.
In October 2021, the Biden administration announced a plan to reduce the amount of PFAS released into the air, drinking and ground water, and food supply chain.
A version of this article first appeared on WebMD.com.
Exposure to “forever chemicals” widely used in consumer products disrupts important biological processes in children and young adults, a new study says.
That could leave children vulnerable to numerous diseases later in life, including diabetes, cardiovascular disease, and cancer, the study said.
Another important finding was that the disruption appeared to be caused by a mixture of PFAS, rather than a single chemical of that type.
PFAS are known as “forever chemicals” because they don’t break down easily over time and persist in water, soil, and the body. They’re used in numerous consumer products, such as nonstick cookware, stain-resistant carpeting, cosmetics, and water-repellent clothing.
PFAS have previously been linked to a host of health issues, including decreased birth weights and immune system problems. To the study authors’ knowledge, this study is the first to evaluate which biological processes are altered by exposure to multiple PFAS, said a news release from the University of Southern California, Los Angeles.
Researchers studied blood samples from 312 children from the Study of Latino Adolescents at Risk and 137 children from the Southern California Children’s Health Study. All the children had a mixture of common PFAS in their blood, including PFOS, PFHxS, PFHpS, PFOA, and PFNA.
“While current interventions have focused on phasing out the use of individual PFAS, such as PFOS and PFOA, this research shows why the focus should be on reducing exposure to all PFAS chemicals,” said Leda Chatzi, MD, a professor of population and public health sciences at the University of Southern California, Los Angeles. Dr. Chatzi is also one of the study authors.
In October 2021, the Biden administration announced a plan to reduce the amount of PFAS released into the air, drinking and ground water, and food supply chain.
A version of this article first appeared on WebMD.com.
Exposure to “forever chemicals” widely used in consumer products disrupts important biological processes in children and young adults, a new study says.
That could leave children vulnerable to numerous diseases later in life, including diabetes, cardiovascular disease, and cancer, the study said.
Another important finding was that the disruption appeared to be caused by a mixture of PFAS, rather than a single chemical of that type.
PFAS are known as “forever chemicals” because they don’t break down easily over time and persist in water, soil, and the body. They’re used in numerous consumer products, such as nonstick cookware, stain-resistant carpeting, cosmetics, and water-repellent clothing.
PFAS have previously been linked to a host of health issues, including decreased birth weights and immune system problems. To the study authors’ knowledge, this study is the first to evaluate which biological processes are altered by exposure to multiple PFAS, said a news release from the University of Southern California, Los Angeles.
Researchers studied blood samples from 312 children from the Study of Latino Adolescents at Risk and 137 children from the Southern California Children’s Health Study. All the children had a mixture of common PFAS in their blood, including PFOS, PFHxS, PFHpS, PFOA, and PFNA.
“While current interventions have focused on phasing out the use of individual PFAS, such as PFOS and PFOA, this research shows why the focus should be on reducing exposure to all PFAS chemicals,” said Leda Chatzi, MD, a professor of population and public health sciences at the University of Southern California, Los Angeles. Dr. Chatzi is also one of the study authors.
In October 2021, the Biden administration announced a plan to reduce the amount of PFAS released into the air, drinking and ground water, and food supply chain.
A version of this article first appeared on WebMD.com.
FROM ENVIRONMENTAL HEALTH PERSPECTIVES