User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Increased risk for IBS among women with endometriosis
Key clinical point: Endometriosis is associated with a 3-fold increase in the risk for irritable bowel syndrome (IBS), with 1 in every 5 women with endometriosis having IBS.
Major finding: The odds of IBS were significantly higher in women with endometriosis compared with healthy controls (odds ratio 2.97; 95% CI 2.17-4.06), and the pooled prevalence of IBS among women with endometriosis was 23.4% (95% CI 9.7%-37.2%).
Study details: This study evaluated the prevalence of IBS in endometriosis (meta-analysis of 6 studies) and association between endometriosis and IBS (meta-analysis of 11 studies involving 18,887 patients with endometriosis and 77,171 healthy controls).
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Nabi MY et al. Endometriosis and irritable bowel syndrome: A systematic review and meta-analyses. Front Med (Lausanne). 2022;9:914356 (Jul 25). Doi: 10.3389/fmed.2022.914356
Key clinical point: Endometriosis is associated with a 3-fold increase in the risk for irritable bowel syndrome (IBS), with 1 in every 5 women with endometriosis having IBS.
Major finding: The odds of IBS were significantly higher in women with endometriosis compared with healthy controls (odds ratio 2.97; 95% CI 2.17-4.06), and the pooled prevalence of IBS among women with endometriosis was 23.4% (95% CI 9.7%-37.2%).
Study details: This study evaluated the prevalence of IBS in endometriosis (meta-analysis of 6 studies) and association between endometriosis and IBS (meta-analysis of 11 studies involving 18,887 patients with endometriosis and 77,171 healthy controls).
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Nabi MY et al. Endometriosis and irritable bowel syndrome: A systematic review and meta-analyses. Front Med (Lausanne). 2022;9:914356 (Jul 25). Doi: 10.3389/fmed.2022.914356
Key clinical point: Endometriosis is associated with a 3-fold increase in the risk for irritable bowel syndrome (IBS), with 1 in every 5 women with endometriosis having IBS.
Major finding: The odds of IBS were significantly higher in women with endometriosis compared with healthy controls (odds ratio 2.97; 95% CI 2.17-4.06), and the pooled prevalence of IBS among women with endometriosis was 23.4% (95% CI 9.7%-37.2%).
Study details: This study evaluated the prevalence of IBS in endometriosis (meta-analysis of 6 studies) and association between endometriosis and IBS (meta-analysis of 11 studies involving 18,887 patients with endometriosis and 77,171 healthy controls).
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Nabi MY et al. Endometriosis and irritable bowel syndrome: A systematic review and meta-analyses. Front Med (Lausanne). 2022;9:914356 (Jul 25). Doi: 10.3389/fmed.2022.914356
IBS symptoms affecting more than two-thirds of patients with IBD
Key clinical point: More than two-thirds of patients with inflammatory bowel disease (IBD) were affected by irritable bowel syndrome (IBS)-type symptoms, which were associated with worse depression, anxiety, somatoform symptoms, and quality-of-life scores.
Major finding: Overall, 67.2% of patients reported IBS-type symptoms at least once during the follow-up, with anxiety, depression, and somatoform symptom scores being worse in patients with vs without IBS-type symptoms (P < .001).
Study details: The data come from a 6-year longitudinal follow-up study including 760 individuals with well-characterized IBD.
Disclosures: This study was funded by The Leeds Teaching Hospitals Charitable Foundation and Tillotts Pharma U.K. Ltd. The authors declared no conflicts of interest.
Source: Fairbrass KM et al. Natural history and impact of irritable bowel syndrome-type symptoms in inflammatory bowel disease during 6 years of longitudinal follow-up. Aliment Pharmacol Ther. 2022 (Aug 22). Doi: 10.1111/apt.17193
Key clinical point: More than two-thirds of patients with inflammatory bowel disease (IBD) were affected by irritable bowel syndrome (IBS)-type symptoms, which were associated with worse depression, anxiety, somatoform symptoms, and quality-of-life scores.
Major finding: Overall, 67.2% of patients reported IBS-type symptoms at least once during the follow-up, with anxiety, depression, and somatoform symptom scores being worse in patients with vs without IBS-type symptoms (P < .001).
Study details: The data come from a 6-year longitudinal follow-up study including 760 individuals with well-characterized IBD.
Disclosures: This study was funded by The Leeds Teaching Hospitals Charitable Foundation and Tillotts Pharma U.K. Ltd. The authors declared no conflicts of interest.
Source: Fairbrass KM et al. Natural history and impact of irritable bowel syndrome-type symptoms in inflammatory bowel disease during 6 years of longitudinal follow-up. Aliment Pharmacol Ther. 2022 (Aug 22). Doi: 10.1111/apt.17193
Key clinical point: More than two-thirds of patients with inflammatory bowel disease (IBD) were affected by irritable bowel syndrome (IBS)-type symptoms, which were associated with worse depression, anxiety, somatoform symptoms, and quality-of-life scores.
Major finding: Overall, 67.2% of patients reported IBS-type symptoms at least once during the follow-up, with anxiety, depression, and somatoform symptom scores being worse in patients with vs without IBS-type symptoms (P < .001).
Study details: The data come from a 6-year longitudinal follow-up study including 760 individuals with well-characterized IBD.
Disclosures: This study was funded by The Leeds Teaching Hospitals Charitable Foundation and Tillotts Pharma U.K. Ltd. The authors declared no conflicts of interest.
Source: Fairbrass KM et al. Natural history and impact of irritable bowel syndrome-type symptoms in inflammatory bowel disease during 6 years of longitudinal follow-up. Aliment Pharmacol Ther. 2022 (Aug 22). Doi: 10.1111/apt.17193
High degree of fatty liver and NAFLD tied to increased incident IBS risk
Key clinical point: The risk for incident irritable bowel syndrome (IBS) was significantly higher in individuals in the highest vs lowest quartile of nonalcoholic fatty liver index and in those with a diagnosis of nonalcoholic fatty liver disease (NAFLD).
Major finding: The risk of developing IBS was 21% higher among individuals in the highest vs lowest quartile of fatty liver index (hazard ratio [HR] 1.21; Ptrend < .001) and 13% higher among patients with vs without NAFLD (HR 1.13; 95% CI 1.05-1.17).
Study details: Findings are from an analysis of 396,838 participants from a large-scale prospective cohort who were free from IBS, any cancer, inflammatory bowel disease, alcoholic liver disease, and celiac disease, of which 38.6% had an NAFLD diagnosis.
Disclosures: This study was funded by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Wu S et al. Non-alcoholic fatty liver is associated with increased risk of irritable bowel syndrome: A prospective cohort study. BMC Med. 2022;20(1):262 (Aug 22). Doi: 10.1186/s12916-022-02460-8
Key clinical point: The risk for incident irritable bowel syndrome (IBS) was significantly higher in individuals in the highest vs lowest quartile of nonalcoholic fatty liver index and in those with a diagnosis of nonalcoholic fatty liver disease (NAFLD).
Major finding: The risk of developing IBS was 21% higher among individuals in the highest vs lowest quartile of fatty liver index (hazard ratio [HR] 1.21; Ptrend < .001) and 13% higher among patients with vs without NAFLD (HR 1.13; 95% CI 1.05-1.17).
Study details: Findings are from an analysis of 396,838 participants from a large-scale prospective cohort who were free from IBS, any cancer, inflammatory bowel disease, alcoholic liver disease, and celiac disease, of which 38.6% had an NAFLD diagnosis.
Disclosures: This study was funded by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Wu S et al. Non-alcoholic fatty liver is associated with increased risk of irritable bowel syndrome: A prospective cohort study. BMC Med. 2022;20(1):262 (Aug 22). Doi: 10.1186/s12916-022-02460-8
Key clinical point: The risk for incident irritable bowel syndrome (IBS) was significantly higher in individuals in the highest vs lowest quartile of nonalcoholic fatty liver index and in those with a diagnosis of nonalcoholic fatty liver disease (NAFLD).
Major finding: The risk of developing IBS was 21% higher among individuals in the highest vs lowest quartile of fatty liver index (hazard ratio [HR] 1.21; Ptrend < .001) and 13% higher among patients with vs without NAFLD (HR 1.13; 95% CI 1.05-1.17).
Study details: Findings are from an analysis of 396,838 participants from a large-scale prospective cohort who were free from IBS, any cancer, inflammatory bowel disease, alcoholic liver disease, and celiac disease, of which 38.6% had an NAFLD diagnosis.
Disclosures: This study was funded by the National Natural Science Foundation of China. The authors declared no conflicts of interest.
Source: Wu S et al. Non-alcoholic fatty liver is associated with increased risk of irritable bowel syndrome: A prospective cohort study. BMC Med. 2022;20(1):262 (Aug 22). Doi: 10.1186/s12916-022-02460-8
Home BP monitoring in older adults falls short of recommendations
Just over 51% of older hypertensive adults regularly check their own blood pressure, compared with 48% of those with blood pressure–related health conditions (BPHCs), based on a 2021 survey of individuals aged 50-80 years.
“Guidelines recommend that patients use self-measured blood pressure monitoring (SBPM) outside the clinic to diagnose and manage hypertension,” but just 61% of respondents with a BPHC and 68% of those with hypertension said that they had received such a recommendation from a physician, nurse, or other health care professional, Melanie V. Springer, MD, and associates said in JAMA Network Open.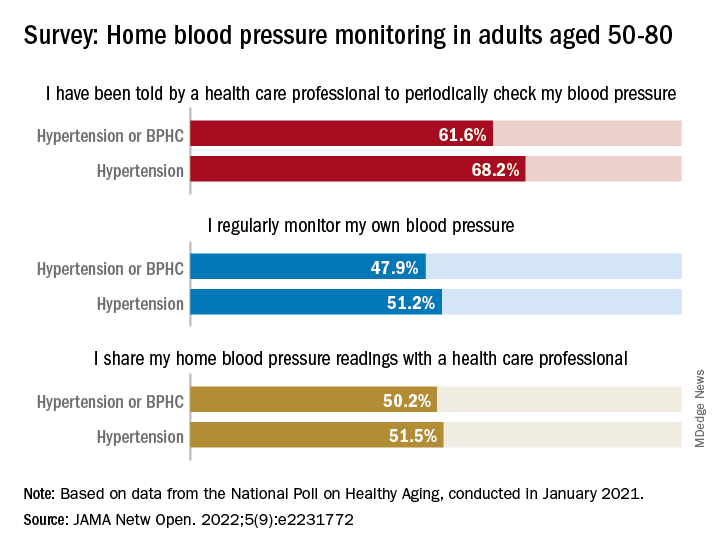
The prevalence of regular monitoring among those with hypertension, 51.2%, does, however, compare favorably with an earlier study showing that 43% of adults aged 18 and older regularly monitored their BP in 2005 and 2008, “which is perhaps associated with our sample’s older age,” said Dr. Springer and associates of the University of Michigan, Ann Arbor.
The current study, they noted, is the first to report “SBPM prevalence in adults ages 50 to 80 years with hypertension or BPHCs, who have a higher risk of adverse outcomes from uncontrolled BP than younger adults.” The analysis is based on data from the National Poll on Healthy Aging, conducted by the University of Michigan in January 2021 and completed by 2,023 individuals.
The frequency of home monitoring varied among adults with BPHCs, as just under 15% reported daily checks and the largest proportion, about 28%, used their device one to three times per month. The results of home monitoring were shared with health care professionals by 50.2% of respondents with a BPHC and by 51.5% of those with hypertension, they said in the research letter.
Home monitoring’s less-than-universal recommendation by providers and use by patients “suggest that protocols should be developed to educate patients about the importance of SBPM and sharing readings with clinicians and the frequency that SBPM should be performed,” Dr. Springer and associates wrote.
The study was funded by AARP, Michigan Medicine, the National Institute of Neurological Disorders and Stroke, and the Department of Veterans Affairs. One investigator has received consulting fees or honoraria from SeeChange Health, HealthMine, the Kaiser Permanente Washington Health Research Institute, the Robert Wood Johnson Foundation, AbilTo, Kansas City Area Life Sciences Institute, American Diabetes Association, Donaghue Foundation, and Luxembourg National Research Fund.
Just over 51% of older hypertensive adults regularly check their own blood pressure, compared with 48% of those with blood pressure–related health conditions (BPHCs), based on a 2021 survey of individuals aged 50-80 years.
“Guidelines recommend that patients use self-measured blood pressure monitoring (SBPM) outside the clinic to diagnose and manage hypertension,” but just 61% of respondents with a BPHC and 68% of those with hypertension said that they had received such a recommendation from a physician, nurse, or other health care professional, Melanie V. Springer, MD, and associates said in JAMA Network Open.
The prevalence of regular monitoring among those with hypertension, 51.2%, does, however, compare favorably with an earlier study showing that 43% of adults aged 18 and older regularly monitored their BP in 2005 and 2008, “which is perhaps associated with our sample’s older age,” said Dr. Springer and associates of the University of Michigan, Ann Arbor.
The current study, they noted, is the first to report “SBPM prevalence in adults ages 50 to 80 years with hypertension or BPHCs, who have a higher risk of adverse outcomes from uncontrolled BP than younger adults.” The analysis is based on data from the National Poll on Healthy Aging, conducted by the University of Michigan in January 2021 and completed by 2,023 individuals.
The frequency of home monitoring varied among adults with BPHCs, as just under 15% reported daily checks and the largest proportion, about 28%, used their device one to three times per month. The results of home monitoring were shared with health care professionals by 50.2% of respondents with a BPHC and by 51.5% of those with hypertension, they said in the research letter.
Home monitoring’s less-than-universal recommendation by providers and use by patients “suggest that protocols should be developed to educate patients about the importance of SBPM and sharing readings with clinicians and the frequency that SBPM should be performed,” Dr. Springer and associates wrote.
The study was funded by AARP, Michigan Medicine, the National Institute of Neurological Disorders and Stroke, and the Department of Veterans Affairs. One investigator has received consulting fees or honoraria from SeeChange Health, HealthMine, the Kaiser Permanente Washington Health Research Institute, the Robert Wood Johnson Foundation, AbilTo, Kansas City Area Life Sciences Institute, American Diabetes Association, Donaghue Foundation, and Luxembourg National Research Fund.
Just over 51% of older hypertensive adults regularly check their own blood pressure, compared with 48% of those with blood pressure–related health conditions (BPHCs), based on a 2021 survey of individuals aged 50-80 years.
“Guidelines recommend that patients use self-measured blood pressure monitoring (SBPM) outside the clinic to diagnose and manage hypertension,” but just 61% of respondents with a BPHC and 68% of those with hypertension said that they had received such a recommendation from a physician, nurse, or other health care professional, Melanie V. Springer, MD, and associates said in JAMA Network Open.
The prevalence of regular monitoring among those with hypertension, 51.2%, does, however, compare favorably with an earlier study showing that 43% of adults aged 18 and older regularly monitored their BP in 2005 and 2008, “which is perhaps associated with our sample’s older age,” said Dr. Springer and associates of the University of Michigan, Ann Arbor.
The current study, they noted, is the first to report “SBPM prevalence in adults ages 50 to 80 years with hypertension or BPHCs, who have a higher risk of adverse outcomes from uncontrolled BP than younger adults.” The analysis is based on data from the National Poll on Healthy Aging, conducted by the University of Michigan in January 2021 and completed by 2,023 individuals.
The frequency of home monitoring varied among adults with BPHCs, as just under 15% reported daily checks and the largest proportion, about 28%, used their device one to three times per month. The results of home monitoring were shared with health care professionals by 50.2% of respondents with a BPHC and by 51.5% of those with hypertension, they said in the research letter.
Home monitoring’s less-than-universal recommendation by providers and use by patients “suggest that protocols should be developed to educate patients about the importance of SBPM and sharing readings with clinicians and the frequency that SBPM should be performed,” Dr. Springer and associates wrote.
The study was funded by AARP, Michigan Medicine, the National Institute of Neurological Disorders and Stroke, and the Department of Veterans Affairs. One investigator has received consulting fees or honoraria from SeeChange Health, HealthMine, the Kaiser Permanente Washington Health Research Institute, the Robert Wood Johnson Foundation, AbilTo, Kansas City Area Life Sciences Institute, American Diabetes Association, Donaghue Foundation, and Luxembourg National Research Fund.
FROM JAMA NETWORK OPEN
Vibegron fails to improve IBS-symptoms in phase 2 trial
Key clinical point: Once-daily 75 mg vibegron was not associated with a clinically significant improvement in irritable bowel syndrome (IBS)-associated abdominal pain in women with diarrhea-predominant IBS (IBS-D) or mixed diarrhea/constipation IBS (IBS-M).
Major finding: At week 12, the percentage of women with IBS-D (40.9% vs 42.9%; P = .8222) or IBS-M (28.9% vs 24.4%; P = .6151) experiencing ≥30% improvement in IBS-associated abdominal pain was not significantly different with vibegron vs placebo. The incidence of treatment-emergent adverse events was comparable between the treatment groups.
Study details: The data come from a phase 2 randomized controlled trial including 222 adult women with IBS-D or IBS-M who were randomly assigned to receive 75 mg vibegron (n = 111) or placebo (n = 111).
Disclosures: This study was funded by Urovant Sciences. J King, D Shortino, C Schaumburg, and C Haag-Molkenteller declared being former employees of Urovant Sciences. Some authors declared receiving research grants or serving as consultants or on scientific advisory boards for various sources, including Urovant Sciences.
Source: Lacy BE et al. Efficacy and safety of vibegron for the treatment of irritable bowel syndrome in women: Results of a randomized, double-blind, placebo-controlled phase 2 trial. Neurogastroenterol Motil. 2022;e14448 (Aug 16). Doi: 10.1111/nmo.14448
Key clinical point: Once-daily 75 mg vibegron was not associated with a clinically significant improvement in irritable bowel syndrome (IBS)-associated abdominal pain in women with diarrhea-predominant IBS (IBS-D) or mixed diarrhea/constipation IBS (IBS-M).
Major finding: At week 12, the percentage of women with IBS-D (40.9% vs 42.9%; P = .8222) or IBS-M (28.9% vs 24.4%; P = .6151) experiencing ≥30% improvement in IBS-associated abdominal pain was not significantly different with vibegron vs placebo. The incidence of treatment-emergent adverse events was comparable between the treatment groups.
Study details: The data come from a phase 2 randomized controlled trial including 222 adult women with IBS-D or IBS-M who were randomly assigned to receive 75 mg vibegron (n = 111) or placebo (n = 111).
Disclosures: This study was funded by Urovant Sciences. J King, D Shortino, C Schaumburg, and C Haag-Molkenteller declared being former employees of Urovant Sciences. Some authors declared receiving research grants or serving as consultants or on scientific advisory boards for various sources, including Urovant Sciences.
Source: Lacy BE et al. Efficacy and safety of vibegron for the treatment of irritable bowel syndrome in women: Results of a randomized, double-blind, placebo-controlled phase 2 trial. Neurogastroenterol Motil. 2022;e14448 (Aug 16). Doi: 10.1111/nmo.14448
Key clinical point: Once-daily 75 mg vibegron was not associated with a clinically significant improvement in irritable bowel syndrome (IBS)-associated abdominal pain in women with diarrhea-predominant IBS (IBS-D) or mixed diarrhea/constipation IBS (IBS-M).
Major finding: At week 12, the percentage of women with IBS-D (40.9% vs 42.9%; P = .8222) or IBS-M (28.9% vs 24.4%; P = .6151) experiencing ≥30% improvement in IBS-associated abdominal pain was not significantly different with vibegron vs placebo. The incidence of treatment-emergent adverse events was comparable between the treatment groups.
Study details: The data come from a phase 2 randomized controlled trial including 222 adult women with IBS-D or IBS-M who were randomly assigned to receive 75 mg vibegron (n = 111) or placebo (n = 111).
Disclosures: This study was funded by Urovant Sciences. J King, D Shortino, C Schaumburg, and C Haag-Molkenteller declared being former employees of Urovant Sciences. Some authors declared receiving research grants or serving as consultants or on scientific advisory boards for various sources, including Urovant Sciences.
Source: Lacy BE et al. Efficacy and safety of vibegron for the treatment of irritable bowel syndrome in women: Results of a randomized, double-blind, placebo-controlled phase 2 trial. Neurogastroenterol Motil. 2022;e14448 (Aug 16). Doi: 10.1111/nmo.14448
Mesalazine not superior to placebo for global improvement of IBS symptoms
Key clinical point: An 8-week treatment with mesalazine offered no clear benefits over placebo for global improvement of irritable bowel syndrome (IBS) symptoms.
Major finding: A similar proportion of patients receiving mesalazine vs placebo reported satisfactory relief of IBS symptoms during ≥50% of the treatment weeks (36% vs 30%; P = .40); however, the improvement in abdominal bloating was significantly greater in the mesalazine vs placebo group (P = .02).
Study details: The data come from a randomized controlled trial including 181 patients with IBS who were assigned to an 8-week treatment with 2400 mg mesalazine orally or placebo once daily.
Disclosures: This study was funded by Eurostars project grant from Tillotts Pharma AB and by the Swedish state. Some authors declared receiving research grants or serving as consultants or on advisory boards for various sources, including Tillotts Pharma.
Source: Tejera VC et al. Randomised clinical trial and meta-analysis: Mesalazine treatment in irritable bowel syndrome—Effects on gastrointestinal symptoms and rectal biomarkers of immune activity. Aliment Pharmacol Ther. 2022;56(6):968-979 (Aug 8). Doi: 10.1111/apt.17182
Key clinical point: An 8-week treatment with mesalazine offered no clear benefits over placebo for global improvement of irritable bowel syndrome (IBS) symptoms.
Major finding: A similar proportion of patients receiving mesalazine vs placebo reported satisfactory relief of IBS symptoms during ≥50% of the treatment weeks (36% vs 30%; P = .40); however, the improvement in abdominal bloating was significantly greater in the mesalazine vs placebo group (P = .02).
Study details: The data come from a randomized controlled trial including 181 patients with IBS who were assigned to an 8-week treatment with 2400 mg mesalazine orally or placebo once daily.
Disclosures: This study was funded by Eurostars project grant from Tillotts Pharma AB and by the Swedish state. Some authors declared receiving research grants or serving as consultants or on advisory boards for various sources, including Tillotts Pharma.
Source: Tejera VC et al. Randomised clinical trial and meta-analysis: Mesalazine treatment in irritable bowel syndrome—Effects on gastrointestinal symptoms and rectal biomarkers of immune activity. Aliment Pharmacol Ther. 2022;56(6):968-979 (Aug 8). Doi: 10.1111/apt.17182
Key clinical point: An 8-week treatment with mesalazine offered no clear benefits over placebo for global improvement of irritable bowel syndrome (IBS) symptoms.
Major finding: A similar proportion of patients receiving mesalazine vs placebo reported satisfactory relief of IBS symptoms during ≥50% of the treatment weeks (36% vs 30%; P = .40); however, the improvement in abdominal bloating was significantly greater in the mesalazine vs placebo group (P = .02).
Study details: The data come from a randomized controlled trial including 181 patients with IBS who were assigned to an 8-week treatment with 2400 mg mesalazine orally or placebo once daily.
Disclosures: This study was funded by Eurostars project grant from Tillotts Pharma AB and by the Swedish state. Some authors declared receiving research grants or serving as consultants or on advisory boards for various sources, including Tillotts Pharma.
Source: Tejera VC et al. Randomised clinical trial and meta-analysis: Mesalazine treatment in irritable bowel syndrome—Effects on gastrointestinal symptoms and rectal biomarkers of immune activity. Aliment Pharmacol Ther. 2022;56(6):968-979 (Aug 8). Doi: 10.1111/apt.17182
Continuous cuffless monitoring may fuel lifestyle change to lower BP
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
Wearing a cuffless device on the wrist to continuously monitor blood pressure was associated with a significantly lower systolic BP at 6 months among hypertensive adults, real-world results from Europe show.
“We don’t know what they did to reduce their blood pressure,” Jay Shah, MD, Division of Cardiology, Mayo Clinic Arizona, Phoenix, told this news organization.
“The idea is that because they were exposed to their data on a continual basis, that may have prompted them to do something that led to an improvement in their blood pressure, whether it be exercise more, go to their doctor, or change their medication,” said Dr. Shah, who is also chief medical officer for Aktiia.
Dr. Shah presented the study at the Hypertension Scientific Sessions, San Diego.
Empowering data
The study used the Aktiia 24/7 BP monitor; Atkiia funded the trial. The monitor passively and continually monitors BP values from photoplethysmography signals collected via optical sensors at the wrist.
After initial individualized calibration using a cuff-based reference, BP measurements are displayed on a smartphone app, allowing users to consistently monitor their own BP for long periods of time.
Aktiia received CE mark in Europe in January 2021 and is currently under review by the U.S. Food and Drug Administration.
Dr. Shah and colleagues analyzed systolic BP (SBP) trends among 838 real-world Aktiia users in Europe (age 57 ± 11 years; 14% women) who consistently used the monitor for 6 months.
Altogether, they had data on 375 (± 287) app interactions, 3,646 (± 1,417) cuffless readings per user, and 9 (± 7) cuff readings per user.
Traditional cuff SBP averages were calculated monthly and compared with the SBP average of the first month. A t-test analysis was used to detect the difference in SBP between the first and successive months.
On the basis of the mean SBP calculated over 6 months, 136 participants were hypertensive (SBP > 140 mm Hg) and the rest had SBP less than 140 mm Hg.
Hypertensive users saw a statistically significant reduction in SBP of –3.2 mm Hg (95% CI, –0.70 to –5.59; P < .02), beginning at 3 months of continual cuffless BP monitoring, which was sustained through 6 months.
Among users with SBP less than 140 mm Hg, the mean SBP remained unchanged.
“The magnitude of improvement might look modest, but even a 5 mm Hg reduction in systolic BP correlates to a 10% decrease in cardiovascular risk,” Dr. Shah told this news organization.
He noted that “one of the major hurdles is that people may not be aware they have high blood pressure because they don’t feel it. And with a regular cuff, they’ll only see that number when they actually check their blood pressure, which is extremely rare, even for people who have hypertension.”
“Having the ability to show someone their continual blood pressure picture really empowers them to do something to make changes and to be aware, [as well as] to be a more active participant in their health,” Dr. Shah said.
He said that a good analogy is diabetes management, which has transitioned from single finger-stick glucose monitoring to continuous glucose monitoring that provides a complete 24/7 picture of glucose levels.
Transforming technology
Offering perspective on the study, Harlan Krumholz, MD, SM, with Yale New Haven Hospital and Yale School of Medicine, New Haven, Conn., said that having an accurate, affordable, unobtrusive cuffless continuous BP monitor would “transform” BP management.
“This could unlock an era of precision BP management with empowerment of patients to view and act on their numbers,” Dr. Krumholz said in an interview.
“We need data to be confident in the devices – and then research to best leverage the streams of information – and strategies to optimize its use in practice,” Dr. Krumholz added.
“Like any new innovation, we need to mitigate risks and monitor for unintended adverse consequences, but I am bullish on the future of cuffless continuous BP monitors,” Dr. Krumholz said.
Dr. Krumholz said that he “applauds Aktiia for doing studies that assess the effect of the information they are producing on BP over time. We need to know that new approaches not only generate valid information but that they can improve health.”
Ready for prime time?
In June, the European Society of Hypertension issued a statement noting that cuffless BP measurement is a fast-growing and promising field with considerable potential for improving hypertension awareness, management, and control, but because the accuracy of these new devices has not yet been validated, they are not yet suitable for clinical use.
Also providing perspective, Stephen Juraschek, MD, PhD, research director, Hypertension Center of Excellence at Healthcare Associates, Beth Israel Deaconess Medical Center, Boston, said that “there is a lot of interest in cuffless BP monitors due to their ease of measurement, comfort, and ability to obtain BP measurements in multiple settings and environments, and this study showed that the monitoring improved BP over time.”
“It is believed that the increased awareness and feedback may promote healthier behaviors aimed at lowering BP. However, this result should not be conflated with the accuracy of these monitors,” Dr. Juraschek cautioned.
He also noted that there is still no formally approved validation protocol by the Association for the Advancement of Medical Instrumentation.
“While a number of cuffless devices are cleared by the FDA through its 510k mechanism (that is, demonstration of device equivalence), there is no formal stamp of approval or attestation that the measurements are accurate,” Dr. Juraschek said in an interview.
In his view, “more work is needed to understand the validity of these devices. For now, validated, cuff-based home devices are recommended for BP measurement at home, while further work is done to determine the accuracy of these cuffless technologies.”
The study was funded by Aktiia. Dr. Shah is an employee of the company. Dr. Krumholz has no relevant disclosures. Dr. Juraschek is a member of the Validate BP review committee and the AAMI sphygmomanometer committee.
A version of this article first appeared on Medscape.com.
FROM AHA HYPERTENSION 2022
New science reveals the best way to take a pill
I want to tell you a story about forgetfulness and haste, and how the combination of the two can lead to frightening consequences. A few years ago, I was lying in bed about to turn out the light when I realized I’d forgotten to take “my pill.”
Like some 161 million other American adults, I was then a consumer of a prescription medication. Being conscientious, I got up, retrieved said pill, and tossed it back. Being lazy, I didn’t bother to grab a glass of water to help the thing go down. Instead, I promptly returned to bed, threw a pillow over my head, and prepared for sleep.
Within seconds, I began to feel a burning sensation in my chest. After about a minute, that burn became a crippling pain. Not wanting to alarm my wife, I went into the living room, where I spent the next 30 minutes doubled over in agony. Was I having a heart attack? I phoned my sister, a hospitalist in Texas. She advised me to take myself to the ED to get checked out.
If only I’d known then about “Duke.” He could have told me how critical body posture is when people swallow pills.
Who’s Duke?
Duke is a computer representation of a 34-year-old, anatomically normal human male created by computer scientists at the IT’IS Foundation, a nonprofit group based in Switzerland that works on a variety of projects in health care technology. Using Duke, Rajat Mittal, PhD, a professor of medicine at the Johns Hopkins University, Baltimore, created a computer model called “StomachSim” to explore the process of digestion.
Their research, published in the journal Physics of Fluids, turned up several surprising findings about the dynamics of swallowing pills – the most common way medication is used worldwide.
Dr. Mittal said he chose to study the stomach because the functions of most other organ systems, from the heart to the brain, have already attracted plenty of attention from scientists.
“As I was looking to initiate research in some new directions, the implications of stomach biomechanics on important conditions such as diabetes, obesity, and gastroparesis became apparent to me,” he said. “It was clear that bioengineering research in this arena lags other more ‘sexy’ areas such as cardiovascular flows by at least 20 years, and there seemed to be a great opportunity to do impactful work.”
Your posture may help a pill work better
Several well-known things affect a pill’s ability to disperse its contents into the gut and be used by the body, such as the stomach’s contents (a heavy breakfast, a mix of liquids like juice, milk, and coffee) and the motion of the organ’s walls. But Dr. Mittal’s group learned that Duke’s posture also played a major role.
The researchers ran Duke through computer simulations in varying postures: upright, leaning right, leaning left, and leaning back, while keeping all the other parts of their analyses (like the things mentioned above) the same.
They found that posture determined as much as 83% of how quickly a pill disperses into the intestines. The most efficient position was leaning right. The least was leaning left, which prevented the pill from reaching the antrum, or bottom section of the stomach, and thus kept all but traces of the dissolved drug from entering the duodenum, where the stomach joins the small intestine. (Interestingly, Jews who observe Passover are advised to recline to the left during the meal as a symbol of freedom and leisure.)
That makes sense if you think about the stomach’s shape, which looks kind of like a bean, curving from the left to the right side of the body. Because of gravity, your position will change where the pill lands.
a condition in which the stomach loses the ability to empty properly.
How this could help people
Among the groups most likely to benefit from such studies, Dr. Mittal said, are the elderly – who both take a lot of pills and are more prone to trouble swallowing because of age-related changes in their esophagus – and the bedridden, who can’t easily shift their posture. The findings may also lead to improvements in the ability to treat people with gastroparesis, a particular problem for people with diabetes.
Future studies with Duke and similar simulations will look at how the GI system digests proteins, carbohydrates, and fatty meals, Dr. Mittal said.
In the meantime, Dr. Mittal offered the following advice: “Standing or sitting upright after taking a pill is fine. If you have to take a pill lying down, stay on your back or on your right side. Avoid lying on your left side after taking a pill.”
As for what happened to me, any gastroenterologist reading this has figured out that my condition was not heart-related. Instead, I likely was having a bout of pill esophagitis, irritation that can result from medications that aggravate the mucosa of the food tube. Although painful, esophagitis isn’t life-threatening. After about an hour, the pain began to subside, and by the next morning I was fine, with only a faint ache in my chest to remind me of my earlier torment. (Researchers noted an increase in the condition early in the COVID-19 pandemic, linked to the antibiotic doxycycline.)
And, in the interest of accuracy, my pill problem began above the stomach. Nothing in the Hopkins research suggests that the alignment of the esophagus plays a role in how drugs disperse in the gut – unless, of course, it prevents those pills from reaching the stomach in the first place.
A version of this article first appeared on WebMD.com.
I want to tell you a story about forgetfulness and haste, and how the combination of the two can lead to frightening consequences. A few years ago, I was lying in bed about to turn out the light when I realized I’d forgotten to take “my pill.”
Like some 161 million other American adults, I was then a consumer of a prescription medication. Being conscientious, I got up, retrieved said pill, and tossed it back. Being lazy, I didn’t bother to grab a glass of water to help the thing go down. Instead, I promptly returned to bed, threw a pillow over my head, and prepared for sleep.
Within seconds, I began to feel a burning sensation in my chest. After about a minute, that burn became a crippling pain. Not wanting to alarm my wife, I went into the living room, where I spent the next 30 minutes doubled over in agony. Was I having a heart attack? I phoned my sister, a hospitalist in Texas. She advised me to take myself to the ED to get checked out.
If only I’d known then about “Duke.” He could have told me how critical body posture is when people swallow pills.
Who’s Duke?
Duke is a computer representation of a 34-year-old, anatomically normal human male created by computer scientists at the IT’IS Foundation, a nonprofit group based in Switzerland that works on a variety of projects in health care technology. Using Duke, Rajat Mittal, PhD, a professor of medicine at the Johns Hopkins University, Baltimore, created a computer model called “StomachSim” to explore the process of digestion.
Their research, published in the journal Physics of Fluids, turned up several surprising findings about the dynamics of swallowing pills – the most common way medication is used worldwide.
Dr. Mittal said he chose to study the stomach because the functions of most other organ systems, from the heart to the brain, have already attracted plenty of attention from scientists.
“As I was looking to initiate research in some new directions, the implications of stomach biomechanics on important conditions such as diabetes, obesity, and gastroparesis became apparent to me,” he said. “It was clear that bioengineering research in this arena lags other more ‘sexy’ areas such as cardiovascular flows by at least 20 years, and there seemed to be a great opportunity to do impactful work.”
Your posture may help a pill work better
Several well-known things affect a pill’s ability to disperse its contents into the gut and be used by the body, such as the stomach’s contents (a heavy breakfast, a mix of liquids like juice, milk, and coffee) and the motion of the organ’s walls. But Dr. Mittal’s group learned that Duke’s posture also played a major role.
The researchers ran Duke through computer simulations in varying postures: upright, leaning right, leaning left, and leaning back, while keeping all the other parts of their analyses (like the things mentioned above) the same.
They found that posture determined as much as 83% of how quickly a pill disperses into the intestines. The most efficient position was leaning right. The least was leaning left, which prevented the pill from reaching the antrum, or bottom section of the stomach, and thus kept all but traces of the dissolved drug from entering the duodenum, where the stomach joins the small intestine. (Interestingly, Jews who observe Passover are advised to recline to the left during the meal as a symbol of freedom and leisure.)
That makes sense if you think about the stomach’s shape, which looks kind of like a bean, curving from the left to the right side of the body. Because of gravity, your position will change where the pill lands.
a condition in which the stomach loses the ability to empty properly.
How this could help people
Among the groups most likely to benefit from such studies, Dr. Mittal said, are the elderly – who both take a lot of pills and are more prone to trouble swallowing because of age-related changes in their esophagus – and the bedridden, who can’t easily shift their posture. The findings may also lead to improvements in the ability to treat people with gastroparesis, a particular problem for people with diabetes.
Future studies with Duke and similar simulations will look at how the GI system digests proteins, carbohydrates, and fatty meals, Dr. Mittal said.
In the meantime, Dr. Mittal offered the following advice: “Standing or sitting upright after taking a pill is fine. If you have to take a pill lying down, stay on your back or on your right side. Avoid lying on your left side after taking a pill.”
As for what happened to me, any gastroenterologist reading this has figured out that my condition was not heart-related. Instead, I likely was having a bout of pill esophagitis, irritation that can result from medications that aggravate the mucosa of the food tube. Although painful, esophagitis isn’t life-threatening. After about an hour, the pain began to subside, and by the next morning I was fine, with only a faint ache in my chest to remind me of my earlier torment. (Researchers noted an increase in the condition early in the COVID-19 pandemic, linked to the antibiotic doxycycline.)
And, in the interest of accuracy, my pill problem began above the stomach. Nothing in the Hopkins research suggests that the alignment of the esophagus plays a role in how drugs disperse in the gut – unless, of course, it prevents those pills from reaching the stomach in the first place.
A version of this article first appeared on WebMD.com.
I want to tell you a story about forgetfulness and haste, and how the combination of the two can lead to frightening consequences. A few years ago, I was lying in bed about to turn out the light when I realized I’d forgotten to take “my pill.”
Like some 161 million other American adults, I was then a consumer of a prescription medication. Being conscientious, I got up, retrieved said pill, and tossed it back. Being lazy, I didn’t bother to grab a glass of water to help the thing go down. Instead, I promptly returned to bed, threw a pillow over my head, and prepared for sleep.
Within seconds, I began to feel a burning sensation in my chest. After about a minute, that burn became a crippling pain. Not wanting to alarm my wife, I went into the living room, where I spent the next 30 minutes doubled over in agony. Was I having a heart attack? I phoned my sister, a hospitalist in Texas. She advised me to take myself to the ED to get checked out.
If only I’d known then about “Duke.” He could have told me how critical body posture is when people swallow pills.
Who’s Duke?
Duke is a computer representation of a 34-year-old, anatomically normal human male created by computer scientists at the IT’IS Foundation, a nonprofit group based in Switzerland that works on a variety of projects in health care technology. Using Duke, Rajat Mittal, PhD, a professor of medicine at the Johns Hopkins University, Baltimore, created a computer model called “StomachSim” to explore the process of digestion.
Their research, published in the journal Physics of Fluids, turned up several surprising findings about the dynamics of swallowing pills – the most common way medication is used worldwide.
Dr. Mittal said he chose to study the stomach because the functions of most other organ systems, from the heart to the brain, have already attracted plenty of attention from scientists.
“As I was looking to initiate research in some new directions, the implications of stomach biomechanics on important conditions such as diabetes, obesity, and gastroparesis became apparent to me,” he said. “It was clear that bioengineering research in this arena lags other more ‘sexy’ areas such as cardiovascular flows by at least 20 years, and there seemed to be a great opportunity to do impactful work.”
Your posture may help a pill work better
Several well-known things affect a pill’s ability to disperse its contents into the gut and be used by the body, such as the stomach’s contents (a heavy breakfast, a mix of liquids like juice, milk, and coffee) and the motion of the organ’s walls. But Dr. Mittal’s group learned that Duke’s posture also played a major role.
The researchers ran Duke through computer simulations in varying postures: upright, leaning right, leaning left, and leaning back, while keeping all the other parts of their analyses (like the things mentioned above) the same.
They found that posture determined as much as 83% of how quickly a pill disperses into the intestines. The most efficient position was leaning right. The least was leaning left, which prevented the pill from reaching the antrum, or bottom section of the stomach, and thus kept all but traces of the dissolved drug from entering the duodenum, where the stomach joins the small intestine. (Interestingly, Jews who observe Passover are advised to recline to the left during the meal as a symbol of freedom and leisure.)
That makes sense if you think about the stomach’s shape, which looks kind of like a bean, curving from the left to the right side of the body. Because of gravity, your position will change where the pill lands.
a condition in which the stomach loses the ability to empty properly.
How this could help people
Among the groups most likely to benefit from such studies, Dr. Mittal said, are the elderly – who both take a lot of pills and are more prone to trouble swallowing because of age-related changes in their esophagus – and the bedridden, who can’t easily shift their posture. The findings may also lead to improvements in the ability to treat people with gastroparesis, a particular problem for people with diabetes.
Future studies with Duke and similar simulations will look at how the GI system digests proteins, carbohydrates, and fatty meals, Dr. Mittal said.
In the meantime, Dr. Mittal offered the following advice: “Standing or sitting upright after taking a pill is fine. If you have to take a pill lying down, stay on your back or on your right side. Avoid lying on your left side after taking a pill.”
As for what happened to me, any gastroenterologist reading this has figured out that my condition was not heart-related. Instead, I likely was having a bout of pill esophagitis, irritation that can result from medications that aggravate the mucosa of the food tube. Although painful, esophagitis isn’t life-threatening. After about an hour, the pain began to subside, and by the next morning I was fine, with only a faint ache in my chest to remind me of my earlier torment. (Researchers noted an increase in the condition early in the COVID-19 pandemic, linked to the antibiotic doxycycline.)
And, in the interest of accuracy, my pill problem began above the stomach. Nothing in the Hopkins research suggests that the alignment of the esophagus plays a role in how drugs disperse in the gut – unless, of course, it prevents those pills from reaching the stomach in the first place.
A version of this article first appeared on WebMD.com.
Esophageal motility issues may promote respiratory disease
Individuals with esophageal dysmotility had significantly higher scores on measures of airway reflux symptoms, based on data from 441 patients.
Many patients with chronic respiratory diseases experience persistent symptoms despite optimal treatment, and the reason is often unclear and frustrating for clinicians and patients, Dominic L. Sykes, MD, of Hull (England) University Teaching Hospitals NHS Trust, and colleagues wrote.
Although more studies in recent years have explored the association between gastroesophageal reflux and respiratory diseases such as asthma and chronic obstructive pulmonary disease, data on a potential link between esophageal motility and respiratory disease in adults are limited, they noted.
In a study published in Respiratory Medicine, the researchers reviewed data from 441 adults with refractory respiratory symptoms who were treated at a single center between Jan. 1, 2011, and Dec. 1, 2021. Symptoms included persistent cough and breathlessness despite optimal medication. The participants underwent examination with high-resolution esophageal manometry (HROM). Airway reflux was measured using the Hull Airways Reflux Questionnaire (HARQ). The mean age of the patients was 56.5 years, and 64% were women.
Overall, the most common diagnoses were chronic cough (77%), asthma (10%), and interstitial lung disease (7%). The prevalence of esophageal dysmotility was 66%. Patients with esophageal dysmotility had significantly higher HARQ scores than those with normal motility (40.6 vs. 35.3; P < .001). Approximately one-third of the patients had normal motility (34.5%) on HROM, 54% had ineffective esophageal motility, 7.3% had absent contractility, 3.2% had esophageal-gastric junction outflow obstruction, 0.5% had distal esophageal spasm, 0.5% has achalasia, and one patient had hypercontractile esophagus.
No significant differences in manometric diagnoses appeared between men and women. In addition, HARQ scores showed a significant inverse correlation with esophageal contractility as measured by distal contractile integral (DCI).
“The proportion of patients with esophageal dysmotility is consistently high over a range of respiratory diseases, including interstitial lung disease (72%), airways disease (57%), and chronic cough (68%),” and the findings suggest that esophageal disease may play a role in patients with persistent respiratory symptoms, they noted.
The study authors proposed that “impaired peristaltic activity of the esophagus, leading to aspiration of gaseous nonacidic refluxate into the airways, may be a contributor in the development and progression of respiratory disease.” They added that the HARQ offers clinicians a useful screening tool for assessing the need for esophageal study in patients with persistent respiratory symptoms that should be used before considering antireflux surgery.
The study findings were limited by several factors including the lack of lung function data for patients with airway disease and ILD and the inability to show causality between esophageal dysmotility and refractory respiratory symptoms, the researchers noted. Other limitations include the retrospective design, and the lack of data on symptom severity and the subsequent impact on outcomes.
However, the results support the need for additional research into the relationship between esophageal dysmotility, lung function, and symptom burden in chronic respiratory disease, and may inform investigations of therapeutic targets, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Individuals with esophageal dysmotility had significantly higher scores on measures of airway reflux symptoms, based on data from 441 patients.
Many patients with chronic respiratory diseases experience persistent symptoms despite optimal treatment, and the reason is often unclear and frustrating for clinicians and patients, Dominic L. Sykes, MD, of Hull (England) University Teaching Hospitals NHS Trust, and colleagues wrote.
Although more studies in recent years have explored the association between gastroesophageal reflux and respiratory diseases such as asthma and chronic obstructive pulmonary disease, data on a potential link between esophageal motility and respiratory disease in adults are limited, they noted.
In a study published in Respiratory Medicine, the researchers reviewed data from 441 adults with refractory respiratory symptoms who were treated at a single center between Jan. 1, 2011, and Dec. 1, 2021. Symptoms included persistent cough and breathlessness despite optimal medication. The participants underwent examination with high-resolution esophageal manometry (HROM). Airway reflux was measured using the Hull Airways Reflux Questionnaire (HARQ). The mean age of the patients was 56.5 years, and 64% were women.
Overall, the most common diagnoses were chronic cough (77%), asthma (10%), and interstitial lung disease (7%). The prevalence of esophageal dysmotility was 66%. Patients with esophageal dysmotility had significantly higher HARQ scores than those with normal motility (40.6 vs. 35.3; P < .001). Approximately one-third of the patients had normal motility (34.5%) on HROM, 54% had ineffective esophageal motility, 7.3% had absent contractility, 3.2% had esophageal-gastric junction outflow obstruction, 0.5% had distal esophageal spasm, 0.5% has achalasia, and one patient had hypercontractile esophagus.
No significant differences in manometric diagnoses appeared between men and women. In addition, HARQ scores showed a significant inverse correlation with esophageal contractility as measured by distal contractile integral (DCI).
“The proportion of patients with esophageal dysmotility is consistently high over a range of respiratory diseases, including interstitial lung disease (72%), airways disease (57%), and chronic cough (68%),” and the findings suggest that esophageal disease may play a role in patients with persistent respiratory symptoms, they noted.
The study authors proposed that “impaired peristaltic activity of the esophagus, leading to aspiration of gaseous nonacidic refluxate into the airways, may be a contributor in the development and progression of respiratory disease.” They added that the HARQ offers clinicians a useful screening tool for assessing the need for esophageal study in patients with persistent respiratory symptoms that should be used before considering antireflux surgery.
The study findings were limited by several factors including the lack of lung function data for patients with airway disease and ILD and the inability to show causality between esophageal dysmotility and refractory respiratory symptoms, the researchers noted. Other limitations include the retrospective design, and the lack of data on symptom severity and the subsequent impact on outcomes.
However, the results support the need for additional research into the relationship between esophageal dysmotility, lung function, and symptom burden in chronic respiratory disease, and may inform investigations of therapeutic targets, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Individuals with esophageal dysmotility had significantly higher scores on measures of airway reflux symptoms, based on data from 441 patients.
Many patients with chronic respiratory diseases experience persistent symptoms despite optimal treatment, and the reason is often unclear and frustrating for clinicians and patients, Dominic L. Sykes, MD, of Hull (England) University Teaching Hospitals NHS Trust, and colleagues wrote.
Although more studies in recent years have explored the association between gastroesophageal reflux and respiratory diseases such as asthma and chronic obstructive pulmonary disease, data on a potential link between esophageal motility and respiratory disease in adults are limited, they noted.
In a study published in Respiratory Medicine, the researchers reviewed data from 441 adults with refractory respiratory symptoms who were treated at a single center between Jan. 1, 2011, and Dec. 1, 2021. Symptoms included persistent cough and breathlessness despite optimal medication. The participants underwent examination with high-resolution esophageal manometry (HROM). Airway reflux was measured using the Hull Airways Reflux Questionnaire (HARQ). The mean age of the patients was 56.5 years, and 64% were women.
Overall, the most common diagnoses were chronic cough (77%), asthma (10%), and interstitial lung disease (7%). The prevalence of esophageal dysmotility was 66%. Patients with esophageal dysmotility had significantly higher HARQ scores than those with normal motility (40.6 vs. 35.3; P < .001). Approximately one-third of the patients had normal motility (34.5%) on HROM, 54% had ineffective esophageal motility, 7.3% had absent contractility, 3.2% had esophageal-gastric junction outflow obstruction, 0.5% had distal esophageal spasm, 0.5% has achalasia, and one patient had hypercontractile esophagus.
No significant differences in manometric diagnoses appeared between men and women. In addition, HARQ scores showed a significant inverse correlation with esophageal contractility as measured by distal contractile integral (DCI).
“The proportion of patients with esophageal dysmotility is consistently high over a range of respiratory diseases, including interstitial lung disease (72%), airways disease (57%), and chronic cough (68%),” and the findings suggest that esophageal disease may play a role in patients with persistent respiratory symptoms, they noted.
The study authors proposed that “impaired peristaltic activity of the esophagus, leading to aspiration of gaseous nonacidic refluxate into the airways, may be a contributor in the development and progression of respiratory disease.” They added that the HARQ offers clinicians a useful screening tool for assessing the need for esophageal study in patients with persistent respiratory symptoms that should be used before considering antireflux surgery.
The study findings were limited by several factors including the lack of lung function data for patients with airway disease and ILD and the inability to show causality between esophageal dysmotility and refractory respiratory symptoms, the researchers noted. Other limitations include the retrospective design, and the lack of data on symptom severity and the subsequent impact on outcomes.
However, the results support the need for additional research into the relationship between esophageal dysmotility, lung function, and symptom burden in chronic respiratory disease, and may inform investigations of therapeutic targets, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM RESPIRATORY MEDICINE
Experts express caution over type 2 diabetes/tea-drinking claim
type 2 diabetes/tea-drinking claim
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
A claim that drinking tea might protect people against developing type 2 diabetes has been met with caution from multiple experts ahead of the annual meeting of the European Association for the Study of Diabetes.
The claim is that people who drink four or more cups of tea every day – specifically green, Oolong, or black tea – are 17% less likely to develop type 2 diabetes than those who do not drink tea. Drinking fewer cups of tea per day was not found to confer any benefit.
“Our results are exciting because they suggest that people can do something as simple as drinking four cups of tea a day to potentially lessen their risk of developing type 2 diabetes,” Xiaying Li of Wuhan (China) University of Science and Technology is quoted as saying in an official EASD press release.
“It is possible that particular components in tea, such as polyphenols, may reduce blood glucose levels, but a sufficient amount of these bioactive compounds may be needed to be effective,” Dr. Li added.
“The words ‘suggest’ and ‘potentially’ are crucial here,” said Kevin McConway, PhD, MSc, MBA, emeritus professor of applied statistics at The Open University, said in a separate statement to the press that reeled in Dr. Li’s enthusiasm.
“Tea drinking would only be useful for reducing diabetes risk if the tea drinking causes reductions in risk, that is, if the risk is reduced if you drink the tea and not if you don’t – and this study simply can’t show whether it does this or not,” Dr. Conway stressed.
Naveed Sattar, FMedSci FRCPath FRCPGlas FRSE, professor of metabolic medicine at the University of Glasgow, was also cautiously critical. “There is no good trial evidence whatsoever that the chemicals in tea prevent diabetes,” he observed separately.
“So, I suspect its more about tea being healthier (less calorific) than many alternative drinks or tea drinkers leading healthier lives more generally.”
Dr. Sattar added that it could be that people who drink tea might also be avoiding drinking more harmful sugary drinks and have other health behaviors that might lead them to have a lower risk for type 2 diabetes.
Time for tea?
Dr. Li will present the findings of two analyses on Sept. 21 at the EASD meeting: the first a large observational cohort study and the second an updated systematic review and meta-analysis.
For the cohort study, Dr. Li and her coauthors took data on more than 5,100 adults who had participated in the long-running and ongoing China Health and Nutrition Survey (CHNS). Information on tea drinking behavior was extracted from questionnaires that had been filled out at two time points – 1997 and 2009 – and they determined whether people had developed type 2 diabetes according to American Diabetes Association criteria.
Nearly half, 45.8%, were found to be tea drinkers, and 10% of the population they sampled had developed type 2 diabetes. No association between tea drinking and type 2 diabetes development was found, however, with the hazard ratio comparing tea drinkers and non–tea drinkers sitting firmly at 1.02. Moreover, a sensitivity analysis that excluded participants who had developed type 2 diabetes in the first 3 years of follow-up did not change the result.
Things were slightly different when Dr. Li and associates performed their meta-analysis that involved analyzing data on more than 1 million participants in 19 studies conducted in eight countries that had been published up to September 2021.
Here, they found there was a significant (P < .003) linear association between tea consumption and having type 2 diabetes, with the relative risk of developing type 2 diabetes decreasing by 0.986 for every additional cup of tea that was drunk.
HRs for the development of type 2 diabetes in tea drinkers versus non–tea drinkers were 1.00 for those who drank less than one cup per day, 0.96 for those who had one to two cups, and 0.84 for those who drank four or more cups.
“While more research needs to be done to determine the exact dosage and mechanisms behind these observations, our findings suggest that drinking tea is beneficial in reducing the risk of type 2 diabetes, but only at high doses (at least 4 cups a day)”, said Dr. Li.
Perhaps, “we did not find an association between tea drinking and type 2 diabetes in our cohort study because we did not look at higher tea consumption,” she added.
Tempest in a teacup
“This is large, observational data. It’s not a randomized controlled trial so there’s plenty of room for data to be misunderstood,” warned Matt Sydes, MSc, professor of clinical trials & methodology at the MRC Clinical Trials Unit, University College London.
“Everyone drinks fluids. If there is an effect here (and that’s a big if), it might be not about the tea they drink, but about what they don’t drink. One can’t tell at the moment. It seems unlikely that a large randomized controlled trial could be done to disambiguate” added Dr. Sydes
“Being only a conference abstract, it is difficult to assess the quality of this research,” Baptiste Leurent, PhD, a medical statistician also working at University College London, said. Not only was the cohort study observational, so were all the other studies included in the meta-analysis, he pointed out.
“Therefore, no cause-effect conclusions can be drawn. The association could simply be due to other factors, such as those drinking more tea having a healthier lifestyle. It does not seem that the authors tried to control for confounders, which is usually difficult in meta-analysis,” Dr. Leurent said.
“There is reason to be a bit skeptical at this point; we really need to have the full details to assess it properly,” said Jonathan Cook of the Centre for Statistics in Medicine at the University of Oxford (England). “It’s a fair attempt to look at this, but not cutting edge, [using] fairly standard approaches.”
Similar studies have shown a reduced risk associated with coffee drinking, noted Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University in Birmingham.
“The important take-home message is that lifestyle is important in managing risk of developing type 2 diabetes,” Dr. Mellor said.
“That includes choosing low-calorie drinks including mainly water as well as unsweetened tea and coffee as your drinks of choice as part of a healthy lifestyle.”
The study was funded by the Young Talents Project of Hubei Provincial Health Commission, the Science and Technology Research Key Project of Education Department of Hubei Province, the Sanuo Diabetes Charity Foundation, and the Xiangyang Science and Technology Plan Project, all based in China. Dr. Li had no conflicts of interest to disclose. Dr. McConway is a Trustee and on the advisory committee of The Science Media Centre. Dr. Sattar has consulted for many companies that make diabetes and cardiovascular drugs and has been involved in multiple trials of lifestyle approaches for the prevention and remission of diabetes. Dr. Sydes, Dr. Leurent, Dr. Cook, and Dr. Mellor had no conflicts of interest to report.
type 2 diabetes/tea-drinking claim
type 2 diabetes/tea-drinking claim
FROM EASD 2022




