User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Even small changes can make endoscopy more sustainable: Study
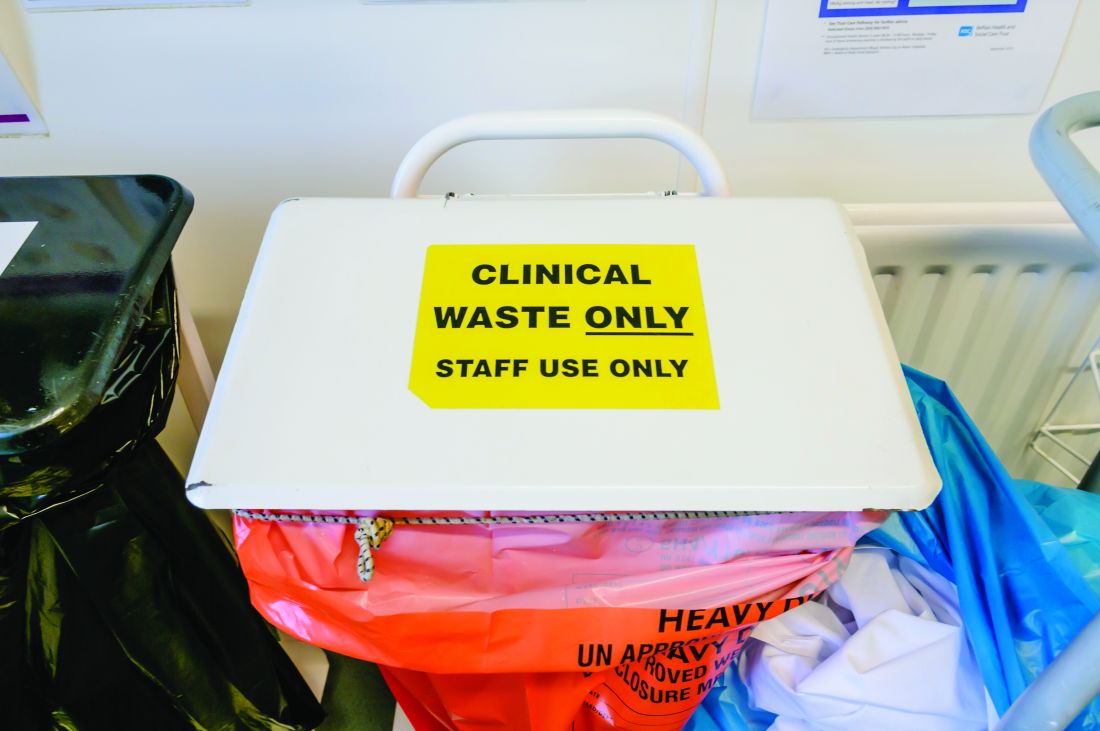
Moving recycling bins and providing education to staff led to a reduction in the carbon footprint at a Portuguese hospital’s endoscopy unit, according to a study published in Gut.
“Sustainable endoscopy regarding waste handling was achievable and sustained over time, did not compromise productivity, and may be cost-effective for stakeholders,” wrote João A Cunha Neves, MD, MMSc, of Algarve University Hospital Centre in Portimão, Portugal, and his colleagues.
Although other work has shown that several of the strategies for reducing endoscopic waste are ‘easy wins,’ the authors noted that “lack of awareness by most endoscopy staff regarding the expenses and correct categorisation of endoscopic waste is the primary barrier to recycling in many endoscopy units.”
The four-stage prospective study took place at the Portimão endoscopy unit of Algarve University Hospital Centre in Portugal. It began with a 4-week audit that involved weighing regulated medical waste (“material fully contaminated with blood or body fluids or containing infectious agents”) and landfill waste (nonrecyclable material that isn’t fully contaminated). The researchers excluded from the analysis all waste from sharps containers, pre- and postprocedure activities, and endoscope reprocessing.
The second stage was one week of medical, nursing, and auxiliary team education about handling waste, in part based on observations collected during the first stage. Each endoscopy generates an estimated 1.5 kg of plastic waste, but recycling bins often are not available for the 0.3 kg of waste that’s recyclable. During the second stage, recycling bins were placed in endoscopy rooms while regulated medical waste and landfill bins were moved elsewhere.
The final two stages involved weighing both types of waste 1 month after the training and then 4 months after the training. For their calculations, the researchers assumed that 1 kg of landfill waste equated to 1 kg of carbon dioxide and 1 kg of regulated medical waste equated to 3 kg of carbon dioxide.
At the third stage, mean total waste fell by 12.9%, albeit not statistically significantly (P = .16), while the 41.4% regulated medical waste reduction was significant (P = .01). Landfill waste had increased 12.3% and both paper (0 to 1.2 kg) and plastic (0 to 2.1 kg) recycling waste increased. Mean endoscopy load had not significantly changed (46.2 vs. 44.5). Overall carbon dioxide was reduced by 31.6%, from 109.7 kg of carbon dioxide to 74.9 kg (P = .018), equating to an annual decrease of 1,665.6 kg. At four months postintervention, these effects remained.
“In both assessment periods, total waste produced by diagnostic standard endoscopic procedures was similar, but both regulated medical waste and overall carbon footprint were reduced,” the authors reported.
In a four-question assessment of the intervention with staff, “the entire team agreed that the study did not interfere with the daily work routine and was helpful in raising awareness about waste sorting within the unit,” the authors reported. The staff “also acknowledged that recycling waste allowed for more sustainable activity within the endoscopy unit, and that the achievements of the study were to be maintained in the future.”
How feasible is change?
The authors noted that health care accounts for 4.4% of the world’s carbon footprint and that endoscopy is the third largest generator of hospital medical waste, primarily because of single-use consumables. However, 71% of that health care carbon footprint comes from supply chain issues, particularly transportation, John I. Allen, MD, MBA, a retired clinical professor of medicine at the University Michigan, Ann Arbor, said in an interview. “Facility emissions add 17% and heating/cooling add 12%,” he said. “So, the actual footprint of endoscopy is quite small.”
Reducing single-use equipment and disposables by a third is a small overall impact considering the “labor-intensive education and monitoring system,” said Dr. Allen, who was not involved in the study. “That said, this paper and numerous others remind us of small steps that we can take – mostly to raise awareness about new technologies and more sustainable processes that are available or should be studied – to help transition us from the current [terrible] state to a more climate-friendly style of practice and life.”
One of the study’s limitations was the lack of a cost-benefit or impact analysis, according to Dr. Allen. Climate policies claiming to have local impact can be problematic when they “ignore both externalities and the actual wider impact on gas or temperature mitigation,” he added.
But he noted that “many health care systems are already implementing new ways of working with temperature- and carbon-mitigation in mind,” such as his own institution’s pledge to become carbon-neutral within a year. One step on that path has included transitioning more than 30% of their clinic visits to virtual visits, “thus saving [literally] millions of miles in travel and altering our parking construction plans,” Dr. Allen said.
“Reduction in climate impact will come from new ways to manufacture endoscopes, less reliance on single-use equipment, and increasing use of materials that encourage recycling,” Dr. Allen said. “In order to achieve meaningful climate impact, we need research into what current regulations and processes are necessary to protect patients and staff from infection transmission while creating an environmentally favorable workflow.”
Where does making a difference begin?
Another limitation was simply the small size of the department itself, which limits how much impact the intervention can have, but the study also showed the feasibility of getting buy-in from a department to make meaningful changes, according to Bishr Omary, MD, PhD, professor of medicine at Rutgers University’s Robert Wood Johnson Medical School, Piscataway, N.J. He was not involved in the study.
“Culture is an important aspect of this, but that’s where education comes in,” Dr. Omary said in an interview. “Leadership has to try to encourage and incentivize different units, not just endoscopy, but other surgery, too. I think the most effective approach is going to be top-down, where hospitals and health systems buy into this.”
One challenge to that buy-in is that climate change has become political, said Linda Anh B. Nguyen, MD, clinical professor of medicine and vice chief of clinical operations in Stanford (Calif.) University’s division of gastroenterology and hepatology.
“There may be resistance to implementation by those who do not see the value of reducing waste,” Dr. Nguyen, who was not involved in this study, said in an interview. “Successful implementation requires a culture change and reducing the physical barriers to make waste reduction easier. Having an advocate for the program embedded within the endoscopy unit will help with implementation.”
One of the advantages of the intervention in this study, Dr. Nguyen said, was its relative ease of implementation.
“The next step would be identifying which of the items that go into landfill can be replaced with reusable products,” Dr. Nguyen added.
Dr. Omary pointed to Practice Greenhealth as an example of an organization working toward the goal of climate change mitigation through a wide range of initiatives, including ones he has written about. The responsibility for reducing carbon footprints should be shared among individuals, institutions, and systems, Dr. Omary said, adding that individuals’ travel, such as to medical meetings, is a major contributor to greenhouse gas emissions.
A forthcoming publication from the four major gastroenterology medical organizations will outline additional ways gastroenterology can address climate change mitigation, Dr. Omary noted.
Dr. Nguyen said that gastroenterologists, as well as the entire health care industry, have a responsibility to combat climate change through waste reduction and that it can be done without sacrificing individual patient safety.
“Climate change must be at the forefront of our priorities for present and future generations,” Dr. Nguyen said. “We need to leave this world better than when we entered.”
But much of that change must especially occur at levels far higher than individual physicians or institutions, Dr. Allen said.
“Major responsibility for altering gastroenterology practices in order to mitigate climate change must originate in regulatory agencies and the manufacturers of our equipment,” Dr. Allen said. “Regulations must be based on demonstrated positive impact that is cost-effective for practices and health care systems.”
The research did not use external funding, and the authors reported no financial disclosures or conflicts of interests. Dr. Allen has consulted for Topography Health, OSHI Health, and Lynxmd. Dr. Nguyen has consulted for Alnylam, Ardelyx, Eli Lilly, Evoke Pharma, Ironwood, Pendulum, Phathom, Neurogastx, Sanofi, and Takeda; has served on the advisory board of Gemelli Biotech; and has received grants from Bold Health and Vanda. Dr. Omary had no disclosures.
Moving recycling bins and providing education to staff led to a reduction in the carbon footprint at a Portuguese hospital’s endoscopy unit, according to a study published in Gut.
“Sustainable endoscopy regarding waste handling was achievable and sustained over time, did not compromise productivity, and may be cost-effective for stakeholders,” wrote João A Cunha Neves, MD, MMSc, of Algarve University Hospital Centre in Portimão, Portugal, and his colleagues.
Although other work has shown that several of the strategies for reducing endoscopic waste are ‘easy wins,’ the authors noted that “lack of awareness by most endoscopy staff regarding the expenses and correct categorisation of endoscopic waste is the primary barrier to recycling in many endoscopy units.”
The four-stage prospective study took place at the Portimão endoscopy unit of Algarve University Hospital Centre in Portugal. It began with a 4-week audit that involved weighing regulated medical waste (“material fully contaminated with blood or body fluids or containing infectious agents”) and landfill waste (nonrecyclable material that isn’t fully contaminated). The researchers excluded from the analysis all waste from sharps containers, pre- and postprocedure activities, and endoscope reprocessing.
The second stage was one week of medical, nursing, and auxiliary team education about handling waste, in part based on observations collected during the first stage. Each endoscopy generates an estimated 1.5 kg of plastic waste, but recycling bins often are not available for the 0.3 kg of waste that’s recyclable. During the second stage, recycling bins were placed in endoscopy rooms while regulated medical waste and landfill bins were moved elsewhere.
The final two stages involved weighing both types of waste 1 month after the training and then 4 months after the training. For their calculations, the researchers assumed that 1 kg of landfill waste equated to 1 kg of carbon dioxide and 1 kg of regulated medical waste equated to 3 kg of carbon dioxide.
At the third stage, mean total waste fell by 12.9%, albeit not statistically significantly (P = .16), while the 41.4% regulated medical waste reduction was significant (P = .01). Landfill waste had increased 12.3% and both paper (0 to 1.2 kg) and plastic (0 to 2.1 kg) recycling waste increased. Mean endoscopy load had not significantly changed (46.2 vs. 44.5). Overall carbon dioxide was reduced by 31.6%, from 109.7 kg of carbon dioxide to 74.9 kg (P = .018), equating to an annual decrease of 1,665.6 kg. At four months postintervention, these effects remained.
“In both assessment periods, total waste produced by diagnostic standard endoscopic procedures was similar, but both regulated medical waste and overall carbon footprint were reduced,” the authors reported.
In a four-question assessment of the intervention with staff, “the entire team agreed that the study did not interfere with the daily work routine and was helpful in raising awareness about waste sorting within the unit,” the authors reported. The staff “also acknowledged that recycling waste allowed for more sustainable activity within the endoscopy unit, and that the achievements of the study were to be maintained in the future.”
How feasible is change?
The authors noted that health care accounts for 4.4% of the world’s carbon footprint and that endoscopy is the third largest generator of hospital medical waste, primarily because of single-use consumables. However, 71% of that health care carbon footprint comes from supply chain issues, particularly transportation, John I. Allen, MD, MBA, a retired clinical professor of medicine at the University Michigan, Ann Arbor, said in an interview. “Facility emissions add 17% and heating/cooling add 12%,” he said. “So, the actual footprint of endoscopy is quite small.”
Reducing single-use equipment and disposables by a third is a small overall impact considering the “labor-intensive education and monitoring system,” said Dr. Allen, who was not involved in the study. “That said, this paper and numerous others remind us of small steps that we can take – mostly to raise awareness about new technologies and more sustainable processes that are available or should be studied – to help transition us from the current [terrible] state to a more climate-friendly style of practice and life.”
One of the study’s limitations was the lack of a cost-benefit or impact analysis, according to Dr. Allen. Climate policies claiming to have local impact can be problematic when they “ignore both externalities and the actual wider impact on gas or temperature mitigation,” he added.
But he noted that “many health care systems are already implementing new ways of working with temperature- and carbon-mitigation in mind,” such as his own institution’s pledge to become carbon-neutral within a year. One step on that path has included transitioning more than 30% of their clinic visits to virtual visits, “thus saving [literally] millions of miles in travel and altering our parking construction plans,” Dr. Allen said.
“Reduction in climate impact will come from new ways to manufacture endoscopes, less reliance on single-use equipment, and increasing use of materials that encourage recycling,” Dr. Allen said. “In order to achieve meaningful climate impact, we need research into what current regulations and processes are necessary to protect patients and staff from infection transmission while creating an environmentally favorable workflow.”
Where does making a difference begin?
Another limitation was simply the small size of the department itself, which limits how much impact the intervention can have, but the study also showed the feasibility of getting buy-in from a department to make meaningful changes, according to Bishr Omary, MD, PhD, professor of medicine at Rutgers University’s Robert Wood Johnson Medical School, Piscataway, N.J. He was not involved in the study.
“Culture is an important aspect of this, but that’s where education comes in,” Dr. Omary said in an interview. “Leadership has to try to encourage and incentivize different units, not just endoscopy, but other surgery, too. I think the most effective approach is going to be top-down, where hospitals and health systems buy into this.”
One challenge to that buy-in is that climate change has become political, said Linda Anh B. Nguyen, MD, clinical professor of medicine and vice chief of clinical operations in Stanford (Calif.) University’s division of gastroenterology and hepatology.
“There may be resistance to implementation by those who do not see the value of reducing waste,” Dr. Nguyen, who was not involved in this study, said in an interview. “Successful implementation requires a culture change and reducing the physical barriers to make waste reduction easier. Having an advocate for the program embedded within the endoscopy unit will help with implementation.”
One of the advantages of the intervention in this study, Dr. Nguyen said, was its relative ease of implementation.
“The next step would be identifying which of the items that go into landfill can be replaced with reusable products,” Dr. Nguyen added.
Dr. Omary pointed to Practice Greenhealth as an example of an organization working toward the goal of climate change mitigation through a wide range of initiatives, including ones he has written about. The responsibility for reducing carbon footprints should be shared among individuals, institutions, and systems, Dr. Omary said, adding that individuals’ travel, such as to medical meetings, is a major contributor to greenhouse gas emissions.
A forthcoming publication from the four major gastroenterology medical organizations will outline additional ways gastroenterology can address climate change mitigation, Dr. Omary noted.
Dr. Nguyen said that gastroenterologists, as well as the entire health care industry, have a responsibility to combat climate change through waste reduction and that it can be done without sacrificing individual patient safety.
“Climate change must be at the forefront of our priorities for present and future generations,” Dr. Nguyen said. “We need to leave this world better than when we entered.”
But much of that change must especially occur at levels far higher than individual physicians or institutions, Dr. Allen said.
“Major responsibility for altering gastroenterology practices in order to mitigate climate change must originate in regulatory agencies and the manufacturers of our equipment,” Dr. Allen said. “Regulations must be based on demonstrated positive impact that is cost-effective for practices and health care systems.”
The research did not use external funding, and the authors reported no financial disclosures or conflicts of interests. Dr. Allen has consulted for Topography Health, OSHI Health, and Lynxmd. Dr. Nguyen has consulted for Alnylam, Ardelyx, Eli Lilly, Evoke Pharma, Ironwood, Pendulum, Phathom, Neurogastx, Sanofi, and Takeda; has served on the advisory board of Gemelli Biotech; and has received grants from Bold Health and Vanda. Dr. Omary had no disclosures.
Moving recycling bins and providing education to staff led to a reduction in the carbon footprint at a Portuguese hospital’s endoscopy unit, according to a study published in Gut.
“Sustainable endoscopy regarding waste handling was achievable and sustained over time, did not compromise productivity, and may be cost-effective for stakeholders,” wrote João A Cunha Neves, MD, MMSc, of Algarve University Hospital Centre in Portimão, Portugal, and his colleagues.
Although other work has shown that several of the strategies for reducing endoscopic waste are ‘easy wins,’ the authors noted that “lack of awareness by most endoscopy staff regarding the expenses and correct categorisation of endoscopic waste is the primary barrier to recycling in many endoscopy units.”
The four-stage prospective study took place at the Portimão endoscopy unit of Algarve University Hospital Centre in Portugal. It began with a 4-week audit that involved weighing regulated medical waste (“material fully contaminated with blood or body fluids or containing infectious agents”) and landfill waste (nonrecyclable material that isn’t fully contaminated). The researchers excluded from the analysis all waste from sharps containers, pre- and postprocedure activities, and endoscope reprocessing.
The second stage was one week of medical, nursing, and auxiliary team education about handling waste, in part based on observations collected during the first stage. Each endoscopy generates an estimated 1.5 kg of plastic waste, but recycling bins often are not available for the 0.3 kg of waste that’s recyclable. During the second stage, recycling bins were placed in endoscopy rooms while regulated medical waste and landfill bins were moved elsewhere.
The final two stages involved weighing both types of waste 1 month after the training and then 4 months after the training. For their calculations, the researchers assumed that 1 kg of landfill waste equated to 1 kg of carbon dioxide and 1 kg of regulated medical waste equated to 3 kg of carbon dioxide.
At the third stage, mean total waste fell by 12.9%, albeit not statistically significantly (P = .16), while the 41.4% regulated medical waste reduction was significant (P = .01). Landfill waste had increased 12.3% and both paper (0 to 1.2 kg) and plastic (0 to 2.1 kg) recycling waste increased. Mean endoscopy load had not significantly changed (46.2 vs. 44.5). Overall carbon dioxide was reduced by 31.6%, from 109.7 kg of carbon dioxide to 74.9 kg (P = .018), equating to an annual decrease of 1,665.6 kg. At four months postintervention, these effects remained.
“In both assessment periods, total waste produced by diagnostic standard endoscopic procedures was similar, but both regulated medical waste and overall carbon footprint were reduced,” the authors reported.
In a four-question assessment of the intervention with staff, “the entire team agreed that the study did not interfere with the daily work routine and was helpful in raising awareness about waste sorting within the unit,” the authors reported. The staff “also acknowledged that recycling waste allowed for more sustainable activity within the endoscopy unit, and that the achievements of the study were to be maintained in the future.”
How feasible is change?
The authors noted that health care accounts for 4.4% of the world’s carbon footprint and that endoscopy is the third largest generator of hospital medical waste, primarily because of single-use consumables. However, 71% of that health care carbon footprint comes from supply chain issues, particularly transportation, John I. Allen, MD, MBA, a retired clinical professor of medicine at the University Michigan, Ann Arbor, said in an interview. “Facility emissions add 17% and heating/cooling add 12%,” he said. “So, the actual footprint of endoscopy is quite small.”
Reducing single-use equipment and disposables by a third is a small overall impact considering the “labor-intensive education and monitoring system,” said Dr. Allen, who was not involved in the study. “That said, this paper and numerous others remind us of small steps that we can take – mostly to raise awareness about new technologies and more sustainable processes that are available or should be studied – to help transition us from the current [terrible] state to a more climate-friendly style of practice and life.”
One of the study’s limitations was the lack of a cost-benefit or impact analysis, according to Dr. Allen. Climate policies claiming to have local impact can be problematic when they “ignore both externalities and the actual wider impact on gas or temperature mitigation,” he added.
But he noted that “many health care systems are already implementing new ways of working with temperature- and carbon-mitigation in mind,” such as his own institution’s pledge to become carbon-neutral within a year. One step on that path has included transitioning more than 30% of their clinic visits to virtual visits, “thus saving [literally] millions of miles in travel and altering our parking construction plans,” Dr. Allen said.
“Reduction in climate impact will come from new ways to manufacture endoscopes, less reliance on single-use equipment, and increasing use of materials that encourage recycling,” Dr. Allen said. “In order to achieve meaningful climate impact, we need research into what current regulations and processes are necessary to protect patients and staff from infection transmission while creating an environmentally favorable workflow.”
Where does making a difference begin?
Another limitation was simply the small size of the department itself, which limits how much impact the intervention can have, but the study also showed the feasibility of getting buy-in from a department to make meaningful changes, according to Bishr Omary, MD, PhD, professor of medicine at Rutgers University’s Robert Wood Johnson Medical School, Piscataway, N.J. He was not involved in the study.
“Culture is an important aspect of this, but that’s where education comes in,” Dr. Omary said in an interview. “Leadership has to try to encourage and incentivize different units, not just endoscopy, but other surgery, too. I think the most effective approach is going to be top-down, where hospitals and health systems buy into this.”
One challenge to that buy-in is that climate change has become political, said Linda Anh B. Nguyen, MD, clinical professor of medicine and vice chief of clinical operations in Stanford (Calif.) University’s division of gastroenterology and hepatology.
“There may be resistance to implementation by those who do not see the value of reducing waste,” Dr. Nguyen, who was not involved in this study, said in an interview. “Successful implementation requires a culture change and reducing the physical barriers to make waste reduction easier. Having an advocate for the program embedded within the endoscopy unit will help with implementation.”
One of the advantages of the intervention in this study, Dr. Nguyen said, was its relative ease of implementation.
“The next step would be identifying which of the items that go into landfill can be replaced with reusable products,” Dr. Nguyen added.
Dr. Omary pointed to Practice Greenhealth as an example of an organization working toward the goal of climate change mitigation through a wide range of initiatives, including ones he has written about. The responsibility for reducing carbon footprints should be shared among individuals, institutions, and systems, Dr. Omary said, adding that individuals’ travel, such as to medical meetings, is a major contributor to greenhouse gas emissions.
A forthcoming publication from the four major gastroenterology medical organizations will outline additional ways gastroenterology can address climate change mitigation, Dr. Omary noted.
Dr. Nguyen said that gastroenterologists, as well as the entire health care industry, have a responsibility to combat climate change through waste reduction and that it can be done without sacrificing individual patient safety.
“Climate change must be at the forefront of our priorities for present and future generations,” Dr. Nguyen said. “We need to leave this world better than when we entered.”
But much of that change must especially occur at levels far higher than individual physicians or institutions, Dr. Allen said.
“Major responsibility for altering gastroenterology practices in order to mitigate climate change must originate in regulatory agencies and the manufacturers of our equipment,” Dr. Allen said. “Regulations must be based on demonstrated positive impact that is cost-effective for practices and health care systems.”
The research did not use external funding, and the authors reported no financial disclosures or conflicts of interests. Dr. Allen has consulted for Topography Health, OSHI Health, and Lynxmd. Dr. Nguyen has consulted for Alnylam, Ardelyx, Eli Lilly, Evoke Pharma, Ironwood, Pendulum, Phathom, Neurogastx, Sanofi, and Takeda; has served on the advisory board of Gemelli Biotech; and has received grants from Bold Health and Vanda. Dr. Omary had no disclosures.
FROM GUT
COVID-19 linked to increased Alzheimer’s risk
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Polio in 2022: Some concerns but vaccine still works
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Pandemic has helped clinicians to gain better insight on pernio, expert says
PORTLAND, ORE. – while others are not, according to Lindy P. Fox, MD, professor of dermatology and director of the hospital consultation service at the University of California, San Francisco.
“We’re learning a lot about pernio because of COVID,” Dr. Fox, a member of the American Academy of Dermatology’s Ad Hoc Task Force on COVID-19, said at the annual meeting of the Pacific Dermatologic Association. “Patients with pernio tend to either have bright red or purple individual lesions or an erythromelalgia-like presentation, often waking up in the middle of the night saying ‘my feet hurt. I can’t put sheets over my feet.’ In my experience, the patients with an erythromelalgia-like presentation tend to be a lot harder to treat.”
Establishing terminology to describe pernio-like lesions was a challenge in the early stages of the COVID-19 pandemic, Dr. Fox added, with clinicians using terms like erythema multiforme-like, coxsackie-like, or even necrotic to describe the lesions. “I don’t think pernio is truly necrotic; I think it’s really inflammatory and purpuric,” she said.
Early in the pandemic, studies suggesting a link with these cases and COVID-19 infection include a case series of 318 patients with pernio-like skin lesions who had confirmed or suspected COVID-19. Most of these patients were generally young and healthy and most had relatively mild COVID-19; 7% were laboratory-confirmed COVID-19 positive, and 6% were close contacts of patients with confirmed COVID-19. Pernio-like lesions were the only symptoms in 55% of the patients.
In another study, researchers in France evaluated the clinical, laboratory, and pathologic characteristics of 40 patients who developed chilblain-like lesions (mostly involving the toes) during the COVID-19 pandemic and were seen as outpatients in April 2020 . All were polymerase chain reaction (PCR) negative, 30% were SARS-CoV-2 serology positive, and 60% had elevated D-dimers. Histology obtained from 19 of the patients revealed lymphocytic inflammation and vascular damage, and 8 had IgA positivity.
In a retrospective analysis of seven pediatric chilblains cases during the pandemic, researchers examined the skin biopsies to evaluate histopathological features and explored the presence of SARS-CoV-2 in the tissue. All patients were PCR negative. The authors observed cytoplasmic granular positivity for SARS-CoV-2 spike protein in endothelial cells, a feature that they said showed coronavirus-like particles, consistent with SARS-CoV-2.
Not all studies in the medical literature have demonstrated an association between pernio-like/chilblains-like lesions and COVID-19, though. An analysis of 23 patients, with skin eruptions considered associated with SARS-CoV-2 infections (including 21 cases of chilblains) during the first wave of the pandemic found that the antibody and T-cell response in patients with pandemic chilblains was the same as in negative controls.
“What’s remarkably interesting about this study is that they did autopsies of samples from patients who had died prepandemic, so there was no such thing as COVID-19,” said Dr. Fox, who was not involved with the study. “They stained for viral particles in those patients, and they were positive in a subset of patients. This makes me wonder about what the significance of that staining positivity is.”
Yet another group of investigators looked at what was happening with pernio during the waves of COVID in a study of chilblains cases in children in Spain, and found a stronger association between lockdown and cold temperature, which argues against a direct association between pernio and COVID infection.
In Dr. Fox’s experience, COVID toes can recur, especially upon exposure to cold. “What taught me this in real life is a patient who I saw remotely by video,” she recalled. “It was early on in the pandemic. I could not prove he had COVID no matter how hard I tried, but I do think he had COVID toes at that time.” When he later was confirmed to have COVID, “he got pernio in the same exact location as his original suspected COVID toes.”
According to an analysis of long COVID in the skin, based on cases reported to the American Academy of Dermatology–International League of Dermatological Societies registry from April 8 to Oct. 8, 2020, pernio-like lesions lasted a median of 12 days in patients with lab-confirmed COVID-19 and a median of 15 days in those with suspected COVID-19. But almost 7% of the 103 pernio cases were long-haulers, defined as those with dermatologic signs of COVID that lasted beyond 60 days.
“There are some patients who are resistant to treatment,” Dr. Fox said. “In addition, recurrent lesions make me think that maybe all pernio is triggered by some viral cause. This causes an immunologic phenomenon that’s responding to a viral trigger you’re trying to deal with. That may be the better way to think about COVID toes.”
Different variants of COVID also appear to be changing the characteristics of dermatologic manifestations associated with infection. Results from a large retrospective analysis of nearly 350,000 users of a COVID study App in the United Kingdom found that skin lesions were more predictive of a positive test in the Delta wave, compared with the Omicron wave, while pernio-like lesions were predictive of infection in the Delta wave but not in the Omicron wave.
“And, whether you were vaccinated or unvaccinated really did not influence whether or not you were going to have a skin rash as a presenting sign of COVID, except for the burning rash, which was less in vaccinated patients,” said Dr. Fox, who was not involved with the study.
Dr. Fox reported having no relevant disclosures.
PORTLAND, ORE. – while others are not, according to Lindy P. Fox, MD, professor of dermatology and director of the hospital consultation service at the University of California, San Francisco.
“We’re learning a lot about pernio because of COVID,” Dr. Fox, a member of the American Academy of Dermatology’s Ad Hoc Task Force on COVID-19, said at the annual meeting of the Pacific Dermatologic Association. “Patients with pernio tend to either have bright red or purple individual lesions or an erythromelalgia-like presentation, often waking up in the middle of the night saying ‘my feet hurt. I can’t put sheets over my feet.’ In my experience, the patients with an erythromelalgia-like presentation tend to be a lot harder to treat.”
Establishing terminology to describe pernio-like lesions was a challenge in the early stages of the COVID-19 pandemic, Dr. Fox added, with clinicians using terms like erythema multiforme-like, coxsackie-like, or even necrotic to describe the lesions. “I don’t think pernio is truly necrotic; I think it’s really inflammatory and purpuric,” she said.
Early in the pandemic, studies suggesting a link with these cases and COVID-19 infection include a case series of 318 patients with pernio-like skin lesions who had confirmed or suspected COVID-19. Most of these patients were generally young and healthy and most had relatively mild COVID-19; 7% were laboratory-confirmed COVID-19 positive, and 6% were close contacts of patients with confirmed COVID-19. Pernio-like lesions were the only symptoms in 55% of the patients.
In another study, researchers in France evaluated the clinical, laboratory, and pathologic characteristics of 40 patients who developed chilblain-like lesions (mostly involving the toes) during the COVID-19 pandemic and were seen as outpatients in April 2020 . All were polymerase chain reaction (PCR) negative, 30% were SARS-CoV-2 serology positive, and 60% had elevated D-dimers. Histology obtained from 19 of the patients revealed lymphocytic inflammation and vascular damage, and 8 had IgA positivity.
In a retrospective analysis of seven pediatric chilblains cases during the pandemic, researchers examined the skin biopsies to evaluate histopathological features and explored the presence of SARS-CoV-2 in the tissue. All patients were PCR negative. The authors observed cytoplasmic granular positivity for SARS-CoV-2 spike protein in endothelial cells, a feature that they said showed coronavirus-like particles, consistent with SARS-CoV-2.
Not all studies in the medical literature have demonstrated an association between pernio-like/chilblains-like lesions and COVID-19, though. An analysis of 23 patients, with skin eruptions considered associated with SARS-CoV-2 infections (including 21 cases of chilblains) during the first wave of the pandemic found that the antibody and T-cell response in patients with pandemic chilblains was the same as in negative controls.
“What’s remarkably interesting about this study is that they did autopsies of samples from patients who had died prepandemic, so there was no such thing as COVID-19,” said Dr. Fox, who was not involved with the study. “They stained for viral particles in those patients, and they were positive in a subset of patients. This makes me wonder about what the significance of that staining positivity is.”
Yet another group of investigators looked at what was happening with pernio during the waves of COVID in a study of chilblains cases in children in Spain, and found a stronger association between lockdown and cold temperature, which argues against a direct association between pernio and COVID infection.
In Dr. Fox’s experience, COVID toes can recur, especially upon exposure to cold. “What taught me this in real life is a patient who I saw remotely by video,” she recalled. “It was early on in the pandemic. I could not prove he had COVID no matter how hard I tried, but I do think he had COVID toes at that time.” When he later was confirmed to have COVID, “he got pernio in the same exact location as his original suspected COVID toes.”
According to an analysis of long COVID in the skin, based on cases reported to the American Academy of Dermatology–International League of Dermatological Societies registry from April 8 to Oct. 8, 2020, pernio-like lesions lasted a median of 12 days in patients with lab-confirmed COVID-19 and a median of 15 days in those with suspected COVID-19. But almost 7% of the 103 pernio cases were long-haulers, defined as those with dermatologic signs of COVID that lasted beyond 60 days.
“There are some patients who are resistant to treatment,” Dr. Fox said. “In addition, recurrent lesions make me think that maybe all pernio is triggered by some viral cause. This causes an immunologic phenomenon that’s responding to a viral trigger you’re trying to deal with. That may be the better way to think about COVID toes.”
Different variants of COVID also appear to be changing the characteristics of dermatologic manifestations associated with infection. Results from a large retrospective analysis of nearly 350,000 users of a COVID study App in the United Kingdom found that skin lesions were more predictive of a positive test in the Delta wave, compared with the Omicron wave, while pernio-like lesions were predictive of infection in the Delta wave but not in the Omicron wave.
“And, whether you were vaccinated or unvaccinated really did not influence whether or not you were going to have a skin rash as a presenting sign of COVID, except for the burning rash, which was less in vaccinated patients,” said Dr. Fox, who was not involved with the study.
Dr. Fox reported having no relevant disclosures.
PORTLAND, ORE. – while others are not, according to Lindy P. Fox, MD, professor of dermatology and director of the hospital consultation service at the University of California, San Francisco.
“We’re learning a lot about pernio because of COVID,” Dr. Fox, a member of the American Academy of Dermatology’s Ad Hoc Task Force on COVID-19, said at the annual meeting of the Pacific Dermatologic Association. “Patients with pernio tend to either have bright red or purple individual lesions or an erythromelalgia-like presentation, often waking up in the middle of the night saying ‘my feet hurt. I can’t put sheets over my feet.’ In my experience, the patients with an erythromelalgia-like presentation tend to be a lot harder to treat.”
Establishing terminology to describe pernio-like lesions was a challenge in the early stages of the COVID-19 pandemic, Dr. Fox added, with clinicians using terms like erythema multiforme-like, coxsackie-like, or even necrotic to describe the lesions. “I don’t think pernio is truly necrotic; I think it’s really inflammatory and purpuric,” she said.
Early in the pandemic, studies suggesting a link with these cases and COVID-19 infection include a case series of 318 patients with pernio-like skin lesions who had confirmed or suspected COVID-19. Most of these patients were generally young and healthy and most had relatively mild COVID-19; 7% were laboratory-confirmed COVID-19 positive, and 6% were close contacts of patients with confirmed COVID-19. Pernio-like lesions were the only symptoms in 55% of the patients.
In another study, researchers in France evaluated the clinical, laboratory, and pathologic characteristics of 40 patients who developed chilblain-like lesions (mostly involving the toes) during the COVID-19 pandemic and were seen as outpatients in April 2020 . All were polymerase chain reaction (PCR) negative, 30% were SARS-CoV-2 serology positive, and 60% had elevated D-dimers. Histology obtained from 19 of the patients revealed lymphocytic inflammation and vascular damage, and 8 had IgA positivity.
In a retrospective analysis of seven pediatric chilblains cases during the pandemic, researchers examined the skin biopsies to evaluate histopathological features and explored the presence of SARS-CoV-2 in the tissue. All patients were PCR negative. The authors observed cytoplasmic granular positivity for SARS-CoV-2 spike protein in endothelial cells, a feature that they said showed coronavirus-like particles, consistent with SARS-CoV-2.
Not all studies in the medical literature have demonstrated an association between pernio-like/chilblains-like lesions and COVID-19, though. An analysis of 23 patients, with skin eruptions considered associated with SARS-CoV-2 infections (including 21 cases of chilblains) during the first wave of the pandemic found that the antibody and T-cell response in patients with pandemic chilblains was the same as in negative controls.
“What’s remarkably interesting about this study is that they did autopsies of samples from patients who had died prepandemic, so there was no such thing as COVID-19,” said Dr. Fox, who was not involved with the study. “They stained for viral particles in those patients, and they were positive in a subset of patients. This makes me wonder about what the significance of that staining positivity is.”
Yet another group of investigators looked at what was happening with pernio during the waves of COVID in a study of chilblains cases in children in Spain, and found a stronger association between lockdown and cold temperature, which argues against a direct association between pernio and COVID infection.
In Dr. Fox’s experience, COVID toes can recur, especially upon exposure to cold. “What taught me this in real life is a patient who I saw remotely by video,” she recalled. “It was early on in the pandemic. I could not prove he had COVID no matter how hard I tried, but I do think he had COVID toes at that time.” When he later was confirmed to have COVID, “he got pernio in the same exact location as his original suspected COVID toes.”
According to an analysis of long COVID in the skin, based on cases reported to the American Academy of Dermatology–International League of Dermatological Societies registry from April 8 to Oct. 8, 2020, pernio-like lesions lasted a median of 12 days in patients with lab-confirmed COVID-19 and a median of 15 days in those with suspected COVID-19. But almost 7% of the 103 pernio cases were long-haulers, defined as those with dermatologic signs of COVID that lasted beyond 60 days.
“There are some patients who are resistant to treatment,” Dr. Fox said. “In addition, recurrent lesions make me think that maybe all pernio is triggered by some viral cause. This causes an immunologic phenomenon that’s responding to a viral trigger you’re trying to deal with. That may be the better way to think about COVID toes.”
Different variants of COVID also appear to be changing the characteristics of dermatologic manifestations associated with infection. Results from a large retrospective analysis of nearly 350,000 users of a COVID study App in the United Kingdom found that skin lesions were more predictive of a positive test in the Delta wave, compared with the Omicron wave, while pernio-like lesions were predictive of infection in the Delta wave but not in the Omicron wave.
“And, whether you were vaccinated or unvaccinated really did not influence whether or not you were going to have a skin rash as a presenting sign of COVID, except for the burning rash, which was less in vaccinated patients,” said Dr. Fox, who was not involved with the study.
Dr. Fox reported having no relevant disclosures.
AT PDA 2022
Walking intensity and step count are linked to health benefits
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
Each additional 2,000 steps per day – up to 10,000 – was associated with 8% to 11% fewer deaths and less heart disease and cancer, the researchers found. Walking quickly had an even stronger link to lower health risks.
The findings were reported in JAMA Internal Medicine. In a separate paper, published in JAMA Neurology, the researchers reported associations between walking and reduced risk of dementia.
Moving faster provides a health ‘bonus’
The findings expand on evidence in smaller studies of middle-aged individuals and older women that suggested health benefits from covering less than the widely promoted target of 10,000 steps a day.
The new study supports the ideas that “every step counts” and moving faster provides a health “bonus,” said one of its co-lead authors, Borja del Pozo Cruz, PhD, an associate professor at the University of Southern Denmark, Odense, and a senior researcher in health at the University of Cadiz, Spain.
Dr. Del Pozo Cruz and his coauthors analyzed median daily step counts for 78,500 adults aged 40-79 years in the U.K. Biobank database who agreed to wear an accelerometer for 1 week. Participants’ average age was 61. Fifty-five percent were women and 97% were White.
Steps were categorized as “incidental,” defined as a pace of less than 40 per minute, and “purposeful,” ones taken at the pace of 40 or more per minute. Researchers also calculated peak 30-minute cadence, the average of an individual’s 30 most active minutes in a day.
Participants’ health records were reviewed after 7 years. Each additional 2,000 steps taken was associated with lower all-cause mortality (mean rate of change [MRC] in the hazard ratio, –0.08; 95% confidence interval, –0.11 to –0.06); cardiovascular mortality (MRC, –0.10; 95% CI, –0.15 to –0.06), and cancer mortality (MRC, –0.11; 95% CI, –0.15 to –0.06).
Similar incremental reductions were observed in the incidence of heart disease, defined as fatal and nonfatal coronary heart disease, stroke, and heart failure; and a composite cancer outcome of 13 sites shown to be associated with low physical activity.
Both incidental and purposeful steps were linked to lower rates of mortality and disease. Particularly encouraging, the researchers said, was the benefit associated with incidental steps, which might be more feasible for some individuals than a planned walk.
The association with better outcomes was especially strong for peak-30 cadence, with individuals in the top fifth of intensity having a 34% lower mortality rate compared with those in the bottom fifth – an observation that researchers wrote “reflects the importance of the natural best effort relative to the individual’s capability.”
The analysis adjusted for a variety of factors including age, sex, race, smoking, alcohol use, fruit and vegetable consumption, medication use, family history of cardiovascular disease or cancer, and sleep quality. It also excluded participants who had deaths and illnesses within 2 years of a step assessment to minimize the problem of reverse causation, in which existing health problems cause participants to move less.
Data contribute evidence toward step count recommendations
The data are observational and do not prove cause and effect, the researchers noted. Still, the authors said the study “contributes critical evidence toward step count–based recommendations” for physical activity.
Guidelines of the United States and the World Health Organization recommend 150 minutes of moderately intense activity or 75 minutes of vigorous activity weekly plus strength training twice a week.
Given the proliferation of activity trackers in phones and watches, recommendations based on steps could be especially useful for individuals who don’t intentionally record their physical activity, the researchers wrote.
“It’s nice to have a study that puts some science behind steps counts,” cardiologist Nieca Goldberg, MD, a clinical associate professor of medicine at New York University, and a spokesperson for the American Heart Association, said of the findings.
Particularly important, said Dr. Goldberg, who was not involved in the study, is the lack of a minimum threshold for health benefits, since the 10,000-step target may be daunting for some individuals.
Only one in five participants in this latest study achieved 10,000 steps per day, according to the paper.
The authors wrote that promotion of lower step targets “may provide a more realistic and achievable goal for the general adult population,” and longevity gains “may be maximized simply by shifting away from the least-active end of the step-count distribution.”
Dr. Goldberg put it this way: “Take a walk. Try to aspire to 10,000 steps. But if you can only do 6,000 or 8,000, you get benefit there, too.”
Cathy Handy Marshall, MD, MPH, an assistant professor of oncology at Johns Hopkins University, Baltimore, who was not involved in the new study, said the findings can be used to guide “exercise prescriptions,” but more research is needed to tailor recommendations, particularly for individuals who cannot achieve high step counts.
Dr. Del Pozo Cruz said the findings need to be replicated in other populations.
The study authors, Dr. Goldberg, and Dr. Handy Marshall reported no relevant competing interests.
FROM JAMA INTERNAL MEDICINE
Two states aim to curb diet pill sales to minors
California and New York are on the cusp of going further than the Food and Drug Administration in restricting the sale of nonprescription diet pills to minors as pediatricians and public health advocates try to protect kids from extreme weight-loss gimmicks online.
A bill before Gov. Gavin Newsom would bar anyone under 18 in California from buying over-the-counter weight-loss supplements – whether online or in shops – without a prescription. A similar bill passed by New York lawmakers is on Gov. Kathy Hochul’s desk. Neither Democrat has indicated how he or she will act.
If both bills are signed into law, proponents hope the momentum will build to restrict diet pill sales to children in more states. Massachusetts, New Jersey, and Missouri have introduced similar bills and backers plan to continue their push next year.
Nearly 30 million people in the United States will have an eating disorder in their lifetime; 95% of them are aged between 12 and 25, according to Johns Hopkins All Children’s Hospital. The hospital added that eating disorders pose the highest risk of mortality of any mental health disorder. And it has become easier than ever for minors to get pills that are sold online or on drugstore shelves. All dietary supplements, which include those for weight loss, accounted for nearly 35% of the $63 billion over-the-counter health products industry in 2021, according to Vision Research Reports, a market research firm.
Dietary supplements, which encompass a broad range of vitamins, herbs, and minerals, are classified by the FDA as food and don’t undergo scientific and safety testing as prescription drugs and over-the-counter medicines do.
Public health advocates want to keep weight-loss products – with ads that may promise to “Drop 5 pounds a week!” and pill names like Slim Sense – away from young people, particularly girls, since some research has linked some products to eating disorders. A study in the American Journal of Public Health, which followed more than 10,000 women aged 14-36 over 15 years, found that “those who used diet pills had more than 5 times higher adjusted odds of receiving an eating disorder diagnosis from a health care provider within 1-3 years than those who did not.”
Many pills have been found tainted with banned and dangerous ingredients that may cause cancer, heart attacks, strokes, and other ailments. For example, the FDA advised the public to avoid Slim Sense by Dr. Reade because it contains lorcaserin, which has been found to cause psychiatric disturbances and impairments in attention or memory. The FDA ordered it discontinued and the company couldn’t be reached for comment.
“Unscrupulous manufacturers are willing to take risks with consumers’ health – and they are lacing their products with illegal pharmaceuticals, banned pharmaceuticals, steroids, excessive stimulants, even experimental stimulants,” said S. Bryn Austin, ScD, founding director of the Strategic Training Initiative for the Prevention of Eating Disorders, or STRIPED, which supports the restrictions. “Consumers have no idea that this is what’s in these types of products.”
STRIPED is a public health initiative based at the Harvard School of Public Health, Boston, and Boston Children’s Hospital.
An industry trade group, the Natural Products Association, disputes that diet pills cause eating disorders, citing the lack of consumer complaints to the FDA of adverse events from their members’ products. “According to FDA data, there is no association between the two,” said Kyle Turk, the association’s director of government affairs.
The association contends that its members adhere to safe manufacturing processes, random product testing, and appropriate marketing guidelines. Representatives also worry that if minors can’t buy supplements over the counter, they may buy them from “crooks” on the black market and undermine the integrity of the industry. Under the bills, minors purchasing weight-loss products must show identification along with a prescription.
Not all business groups oppose the ban. The American Herbal Products Association, a trade group representing dietary supplement manufacturers and retailers, dropped its opposition to California’s bill once it was amended to remove ingredient categories that are found in non-diet supplements and vitamins, according to Robert Marriott, director of regulatory affairs.
Children’s advocates have found worrisome trends among young people who envision their ideal body type based on what they see on social media. According to a study commissioned by Fairplay, a nonprofit that seeks to stop harmful marketing practices targeting children, kids as young as 9 were found to be following three or more eating disorder accounts on Instagram, while the median age was 19. The authors called it a “pro–eating disorder bubble.”
Meta, which owns Instagram and Facebook, said the report lacks nuance, such as recognizing the human need to share life’s difficult moments. The company argues that blanket censorship isn’t the answer. “Experts and safety organizations have told us it’s important to strike a balance and allow people to share their personal stories while removing any content that encourages or promotes eating disorders,” Liza Crenshaw, a Meta spokesperson, said in an email.
Jason Nagata, MD, a pediatrician who cares for children and young adults with life-threatening eating disorders, believes that easy access to diet pills contributes to his patients’ conditions at UCSF Benioff Children’s Hospital in San Francisco. That was the case for one of his patients, an emaciated 11-year-old girl.
“She had basically entered a starvation state because she was not getting enough nutrition,” said Dr. Nagata, who provided supporting testimony for the California bill. “She was taking these pills and using other kinds of extreme behaviors to lose weight.”
Dr. Nagata said the number of patients he sees with eating disorders has tripled since the pandemic began. They are desperate to get diet pills, some with modest results. “We’ve had patients who have been so dependent on these products that they will be hospitalized and they’re still ordering these products on Amazon,” he said.
Public health advocates turned to state legislatures in response to the federal government’s limited authority to regulate diet pills. Under a 1994 federal law known as the Dietary Supplement Health and Education Act, the FDA “cannot step in until after there is a clear issue of harm to consumers,” said Dr. Austin.
No match for the supplement industry’s heavy lobbying on Capitol Hill, public health advocates shifted to a state-by-state approach.
There is, however, a push for the FDA to improve oversight of what goes into diet pills. Sen. Dick Durbin (D-Ill.) in April introduced a bill that would require dietary supplement manufacturers to register their products – along with the ingredients – with the regulator.
Proponents say the change is needed because manufacturers have been known to include dangerous ingredients. C. Michael White, PharmD, of the University of Connecticut, Storrs, found 35% of tainted health products came from weight-loss supplements in a review of a health fraud database.
A few ingredients have been banned, including sibutramine, a stimulant. “It was a very commonly used weight-loss supplement that ended up being removed from the U.S. market because of its elevated risk of causing things like heart attacks, strokes, and arrhythmias,” Dr. White said.
Another ingredient was phenolphthalein, which was used in laxatives until it was identified as a suspected carcinogen and banned in 1999. “To think,” he said, “that that product would still be on the U.S. market is just unconscionable.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
California and New York are on the cusp of going further than the Food and Drug Administration in restricting the sale of nonprescription diet pills to minors as pediatricians and public health advocates try to protect kids from extreme weight-loss gimmicks online.
A bill before Gov. Gavin Newsom would bar anyone under 18 in California from buying over-the-counter weight-loss supplements – whether online or in shops – without a prescription. A similar bill passed by New York lawmakers is on Gov. Kathy Hochul’s desk. Neither Democrat has indicated how he or she will act.
If both bills are signed into law, proponents hope the momentum will build to restrict diet pill sales to children in more states. Massachusetts, New Jersey, and Missouri have introduced similar bills and backers plan to continue their push next year.
Nearly 30 million people in the United States will have an eating disorder in their lifetime; 95% of them are aged between 12 and 25, according to Johns Hopkins All Children’s Hospital. The hospital added that eating disorders pose the highest risk of mortality of any mental health disorder. And it has become easier than ever for minors to get pills that are sold online or on drugstore shelves. All dietary supplements, which include those for weight loss, accounted for nearly 35% of the $63 billion over-the-counter health products industry in 2021, according to Vision Research Reports, a market research firm.
Dietary supplements, which encompass a broad range of vitamins, herbs, and minerals, are classified by the FDA as food and don’t undergo scientific and safety testing as prescription drugs and over-the-counter medicines do.
Public health advocates want to keep weight-loss products – with ads that may promise to “Drop 5 pounds a week!” and pill names like Slim Sense – away from young people, particularly girls, since some research has linked some products to eating disorders. A study in the American Journal of Public Health, which followed more than 10,000 women aged 14-36 over 15 years, found that “those who used diet pills had more than 5 times higher adjusted odds of receiving an eating disorder diagnosis from a health care provider within 1-3 years than those who did not.”
Many pills have been found tainted with banned and dangerous ingredients that may cause cancer, heart attacks, strokes, and other ailments. For example, the FDA advised the public to avoid Slim Sense by Dr. Reade because it contains lorcaserin, which has been found to cause psychiatric disturbances and impairments in attention or memory. The FDA ordered it discontinued and the company couldn’t be reached for comment.
“Unscrupulous manufacturers are willing to take risks with consumers’ health – and they are lacing their products with illegal pharmaceuticals, banned pharmaceuticals, steroids, excessive stimulants, even experimental stimulants,” said S. Bryn Austin, ScD, founding director of the Strategic Training Initiative for the Prevention of Eating Disorders, or STRIPED, which supports the restrictions. “Consumers have no idea that this is what’s in these types of products.”
STRIPED is a public health initiative based at the Harvard School of Public Health, Boston, and Boston Children’s Hospital.
An industry trade group, the Natural Products Association, disputes that diet pills cause eating disorders, citing the lack of consumer complaints to the FDA of adverse events from their members’ products. “According to FDA data, there is no association between the two,” said Kyle Turk, the association’s director of government affairs.
The association contends that its members adhere to safe manufacturing processes, random product testing, and appropriate marketing guidelines. Representatives also worry that if minors can’t buy supplements over the counter, they may buy them from “crooks” on the black market and undermine the integrity of the industry. Under the bills, minors purchasing weight-loss products must show identification along with a prescription.
Not all business groups oppose the ban. The American Herbal Products Association, a trade group representing dietary supplement manufacturers and retailers, dropped its opposition to California’s bill once it was amended to remove ingredient categories that are found in non-diet supplements and vitamins, according to Robert Marriott, director of regulatory affairs.
Children’s advocates have found worrisome trends among young people who envision their ideal body type based on what they see on social media. According to a study commissioned by Fairplay, a nonprofit that seeks to stop harmful marketing practices targeting children, kids as young as 9 were found to be following three or more eating disorder accounts on Instagram, while the median age was 19. The authors called it a “pro–eating disorder bubble.”
Meta, which owns Instagram and Facebook, said the report lacks nuance, such as recognizing the human need to share life’s difficult moments. The company argues that blanket censorship isn’t the answer. “Experts and safety organizations have told us it’s important to strike a balance and allow people to share their personal stories while removing any content that encourages or promotes eating disorders,” Liza Crenshaw, a Meta spokesperson, said in an email.
Jason Nagata, MD, a pediatrician who cares for children and young adults with life-threatening eating disorders, believes that easy access to diet pills contributes to his patients’ conditions at UCSF Benioff Children’s Hospital in San Francisco. That was the case for one of his patients, an emaciated 11-year-old girl.
“She had basically entered a starvation state because she was not getting enough nutrition,” said Dr. Nagata, who provided supporting testimony for the California bill. “She was taking these pills and using other kinds of extreme behaviors to lose weight.”
Dr. Nagata said the number of patients he sees with eating disorders has tripled since the pandemic began. They are desperate to get diet pills, some with modest results. “We’ve had patients who have been so dependent on these products that they will be hospitalized and they’re still ordering these products on Amazon,” he said.
Public health advocates turned to state legislatures in response to the federal government’s limited authority to regulate diet pills. Under a 1994 federal law known as the Dietary Supplement Health and Education Act, the FDA “cannot step in until after there is a clear issue of harm to consumers,” said Dr. Austin.
No match for the supplement industry’s heavy lobbying on Capitol Hill, public health advocates shifted to a state-by-state approach.
There is, however, a push for the FDA to improve oversight of what goes into diet pills. Sen. Dick Durbin (D-Ill.) in April introduced a bill that would require dietary supplement manufacturers to register their products – along with the ingredients – with the regulator.
Proponents say the change is needed because manufacturers have been known to include dangerous ingredients. C. Michael White, PharmD, of the University of Connecticut, Storrs, found 35% of tainted health products came from weight-loss supplements in a review of a health fraud database.
A few ingredients have been banned, including sibutramine, a stimulant. “It was a very commonly used weight-loss supplement that ended up being removed from the U.S. market because of its elevated risk of causing things like heart attacks, strokes, and arrhythmias,” Dr. White said.
Another ingredient was phenolphthalein, which was used in laxatives until it was identified as a suspected carcinogen and banned in 1999. “To think,” he said, “that that product would still be on the U.S. market is just unconscionable.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
California and New York are on the cusp of going further than the Food and Drug Administration in restricting the sale of nonprescription diet pills to minors as pediatricians and public health advocates try to protect kids from extreme weight-loss gimmicks online.
A bill before Gov. Gavin Newsom would bar anyone under 18 in California from buying over-the-counter weight-loss supplements – whether online or in shops – without a prescription. A similar bill passed by New York lawmakers is on Gov. Kathy Hochul’s desk. Neither Democrat has indicated how he or she will act.
If both bills are signed into law, proponents hope the momentum will build to restrict diet pill sales to children in more states. Massachusetts, New Jersey, and Missouri have introduced similar bills and backers plan to continue their push next year.
Nearly 30 million people in the United States will have an eating disorder in their lifetime; 95% of them are aged between 12 and 25, according to Johns Hopkins All Children’s Hospital. The hospital added that eating disorders pose the highest risk of mortality of any mental health disorder. And it has become easier than ever for minors to get pills that are sold online or on drugstore shelves. All dietary supplements, which include those for weight loss, accounted for nearly 35% of the $63 billion over-the-counter health products industry in 2021, according to Vision Research Reports, a market research firm.
Dietary supplements, which encompass a broad range of vitamins, herbs, and minerals, are classified by the FDA as food and don’t undergo scientific and safety testing as prescription drugs and over-the-counter medicines do.
Public health advocates want to keep weight-loss products – with ads that may promise to “Drop 5 pounds a week!” and pill names like Slim Sense – away from young people, particularly girls, since some research has linked some products to eating disorders. A study in the American Journal of Public Health, which followed more than 10,000 women aged 14-36 over 15 years, found that “those who used diet pills had more than 5 times higher adjusted odds of receiving an eating disorder diagnosis from a health care provider within 1-3 years than those who did not.”
Many pills have been found tainted with banned and dangerous ingredients that may cause cancer, heart attacks, strokes, and other ailments. For example, the FDA advised the public to avoid Slim Sense by Dr. Reade because it contains lorcaserin, which has been found to cause psychiatric disturbances and impairments in attention or memory. The FDA ordered it discontinued and the company couldn’t be reached for comment.
“Unscrupulous manufacturers are willing to take risks with consumers’ health – and they are lacing their products with illegal pharmaceuticals, banned pharmaceuticals, steroids, excessive stimulants, even experimental stimulants,” said S. Bryn Austin, ScD, founding director of the Strategic Training Initiative for the Prevention of Eating Disorders, or STRIPED, which supports the restrictions. “Consumers have no idea that this is what’s in these types of products.”
STRIPED is a public health initiative based at the Harvard School of Public Health, Boston, and Boston Children’s Hospital.
An industry trade group, the Natural Products Association, disputes that diet pills cause eating disorders, citing the lack of consumer complaints to the FDA of adverse events from their members’ products. “According to FDA data, there is no association between the two,” said Kyle Turk, the association’s director of government affairs.
The association contends that its members adhere to safe manufacturing processes, random product testing, and appropriate marketing guidelines. Representatives also worry that if minors can’t buy supplements over the counter, they may buy them from “crooks” on the black market and undermine the integrity of the industry. Under the bills, minors purchasing weight-loss products must show identification along with a prescription.
Not all business groups oppose the ban. The American Herbal Products Association, a trade group representing dietary supplement manufacturers and retailers, dropped its opposition to California’s bill once it was amended to remove ingredient categories that are found in non-diet supplements and vitamins, according to Robert Marriott, director of regulatory affairs.
Children’s advocates have found worrisome trends among young people who envision their ideal body type based on what they see on social media. According to a study commissioned by Fairplay, a nonprofit that seeks to stop harmful marketing practices targeting children, kids as young as 9 were found to be following three or more eating disorder accounts on Instagram, while the median age was 19. The authors called it a “pro–eating disorder bubble.”
Meta, which owns Instagram and Facebook, said the report lacks nuance, such as recognizing the human need to share life’s difficult moments. The company argues that blanket censorship isn’t the answer. “Experts and safety organizations have told us it’s important to strike a balance and allow people to share their personal stories while removing any content that encourages or promotes eating disorders,” Liza Crenshaw, a Meta spokesperson, said in an email.
Jason Nagata, MD, a pediatrician who cares for children and young adults with life-threatening eating disorders, believes that easy access to diet pills contributes to his patients’ conditions at UCSF Benioff Children’s Hospital in San Francisco. That was the case for one of his patients, an emaciated 11-year-old girl.
“She had basically entered a starvation state because she was not getting enough nutrition,” said Dr. Nagata, who provided supporting testimony for the California bill. “She was taking these pills and using other kinds of extreme behaviors to lose weight.”
Dr. Nagata said the number of patients he sees with eating disorders has tripled since the pandemic began. They are desperate to get diet pills, some with modest results. “We’ve had patients who have been so dependent on these products that they will be hospitalized and they’re still ordering these products on Amazon,” he said.
Public health advocates turned to state legislatures in response to the federal government’s limited authority to regulate diet pills. Under a 1994 federal law known as the Dietary Supplement Health and Education Act, the FDA “cannot step in until after there is a clear issue of harm to consumers,” said Dr. Austin.
No match for the supplement industry’s heavy lobbying on Capitol Hill, public health advocates shifted to a state-by-state approach.
There is, however, a push for the FDA to improve oversight of what goes into diet pills. Sen. Dick Durbin (D-Ill.) in April introduced a bill that would require dietary supplement manufacturers to register their products – along with the ingredients – with the regulator.
Proponents say the change is needed because manufacturers have been known to include dangerous ingredients. C. Michael White, PharmD, of the University of Connecticut, Storrs, found 35% of tainted health products came from weight-loss supplements in a review of a health fraud database.
A few ingredients have been banned, including sibutramine, a stimulant. “It was a very commonly used weight-loss supplement that ended up being removed from the U.S. market because of its elevated risk of causing things like heart attacks, strokes, and arrhythmias,” Dr. White said.
Another ingredient was phenolphthalein, which was used in laxatives until it was identified as a suspected carcinogen and banned in 1999. “To think,” he said, “that that product would still be on the U.S. market is just unconscionable.”
This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Cumulative blood pressure load: A better predictor of CV events?
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Should patients stand for office BP readings?
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION 2022
Sustained response at 2 years reported for newly approved oral psoriasis agent
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
AT THE EADV CONGRESS
People of color bearing brunt of long COVID, doctors say
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.
From the earliest days of the COVID-19 pandemic, people of color have been hardest hit by the virus. Now, many doctors and researchers are seeing big disparities come about in who gets care for long COVID.
Long COVID can affect patients from all walks of life. said Alba Miranda Azola, MD, codirector of the post–acute COVID-19 team at Johns Hopkins University, Baltimore.
Non-White patients are more apt to lack access to primary care, face insurance barriers to see specialists, struggle with time off work or transportation for appointments, and have financial barriers to care as copayments for therapy pile up.
“We are getting a very skewed population of Caucasian wealthy people who are coming to our clinic because they have the ability to access care, they have good insurance, and they are looking on the internet and find us,” Dr. Azola said.
This mix of patients at Dr. Azola’s clinic is out of step with the demographics of Baltimore, where the majority of residents are Black, half of them earn less than $52,000 a year, and one in five live in poverty. And this isn’t unique to Hopkins. Many of the dozens of specialized long COVID clinics that have cropped up around the country are also seeing an unequal share of affluent White patients, experts say.
It’s also a patient mix that very likely doesn’t reflect who is most apt to have long COVID.
During the pandemic, people who identified as Black, Hispanic, American Indian, or Alaska Native were more likely to be diagnosed with COVID than people who identified as White, according to the Centers for Disease Control and Prevention. These people of color were also at least twice as likely to be hospitalized with severe infections, and at least 70% more likely to die.
“Data repeatedly show the disproportionate impact of COVID-19 on racial and ethnic minority populations, as well as other population groups such as people living in rural or frontier areas, people experiencing homelessness, essential and frontline workers, people with disabilities, people with substance use disorders, people who are incarcerated, and non–U.S.-born persons,” John Brooks, MD, chief medical officer for COVID-19 response at the CDC, said during testimony before the U.S. House Energy and Commerce Subcommittee on Health in April 2021.
“While we do not yet have clear data on the impact of post-COVID conditions on racial and ethnic minority populations and other disadvantaged communities, we do believe that they are likely to be disproportionately impacted ... and less likely to be able to access health care services,” Dr. Brooks said at the time.
The picture that’s emerging of long COVID suggests that the condition impacts about one in five adults. It’s more common among Hispanic adults than among people who identify as Black, Asian, or White. It’s also more common among those who identify as other races or multiple races, according survey data collected by the CDC.
It’s hard to say how accurate this snapshot is because researchers need to do a better job of identifying and following people with long COVID, said Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the COVID-19 Recovery Clinic at the University of Texas Health Science Center at San Antonio. A major limitation of surveys like the ones done by the CDC to monitor long COVID is that only people who realize they have the condition can get counted.
“Some people from historically marginalized groups may have less health literacy to know about impacts of long COVID,” she said.
Lack of awareness may keep people with persistent symptoms from seeking medical attention, leaving many long COVID cases undiagnosed.
When some patients do seek help, their complaints may not be acknowledged or understood. Often, cultural bias or structural racism can get in the way of diagnosis and treatment, Dr. Azola said.
“I hate to say this, but there is probably bias among providers,” she said. “For example, I am Puerto Rican, and the way we describe symptoms as Latinos may sound exaggerated or may be brushed aside or lost in translation. I think we miss a lot of patients being diagnosed or referred to specialists because the primary care provider they see maybe leans into this cultural bias of thinking this is just a Latino being dramatic.”
There’s some evidence that treatment for long COVID may differ by race even when symptoms are similar. One study of more than 400,000 patients, for example, found no racial differences in the proportion of people who have six common long COVID symptoms: shortness of breath, fatigue, weakness, pain, trouble with thinking skills, and a hard time getting around. Despite this, Black patients were significantly less likely to receive outpatient rehabilitation services to treat these symptoms.
Benjamin Abramoff, MD, who leads the long COVID collaborative for the American Academy of Physical Medicine and Rehabilitation, draws parallels between what happens with long COVID to another common health problem often undertreated among patients of color: pain. With both long COVID and chronic pain, one major barrier to care is “just getting taken seriously by providers,” he said.
“There is significant evidence that racial bias has led to less prescription of pain medications to people of color,” Dr. Abramoff said. “Just as pain can be difficult to get objective measures of, long COVID symptoms can also be difficult to objectively measure and requires trust between the provider and patient.”
Geography can be another barrier to care, said Aaron Friedberg, MD, clinical colead of the post-COVID recovery program at Ohio State University Wexner Medical Center, Columbus. Many communities hardest hit by COVID – particularly in high-poverty urban neighborhoods – have long had limited access to care. The pandemic worsened staffing shortages at many hospitals and clinics in these communities, leaving patients even fewer options close to home.
“I often have patients driving several hours to come to our clinic, and that can create significant challenges both because of the financial burden and time required to coordinate that type of travel, but also because post-COVID symptoms can make it extremely challenging to tolerate that type of travel,” Dr. Friedberg said.
Even though the complete picture of who has long COVID – and who’s getting treated and getting good outcomes – is still emerging, it’s very clear at this point in the pandemic that access isn’t equal among everyone and that many low-income and non-White patients are missing out on needed treatments, Friedberg said.
“One thing that is clear is that there are many people suffering alone from these conditions,” he said.
A version of this article first appeared on WebMD.com.