User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
COVID-19 hospitalization 80% more likely for smokers
Observational data was analyzed alongside hospital coronavirus test data and UK Biobank genetic information for the first time, and the findings are published in Thorax.
The data cover 421,469 people overall. Of these, 3.2% took a polymerase chain reaction swab test, 0.4% of these tested positive, 0.2% of them required hospitalization for COVID-19, and 0.1% of them died because of COVID-19.
When it came to smoking status, 59% had never smoked, 37% were ex-smokers, and 3% were current smokers.
Current smokers were 80% more likely to be admitted to hospital, and significantly more likely to die from COVID-19, than nonsmokers.
Time to quit
Heavy smokers who smoked more than 20 cigarettes a day were 6.11 times more likely to die from COVID-19 than people who had never smoked.
Analysis also showed those with a genetic predisposition to being smokers had a 45% higher infection risk, and 60% higher hospitalization risk.
The authors wrote: “Overall, the congruence of observational analyses indicating associations with recent smoking behaviors and [Mendelian randomization] analyses indicating associations with lifelong predisposition to smoking and smoking heaviness support a causal effect of smoking on COVID-19 severity.”
In a linked podcast, lead researcher Dr. Ashley Clift, said: “Our results strongly suggest that smoking is related to your risk of getting severe COVID, and just as smoking affects your risk of heart disease, different cancers, and all those other conditions we know smoking is linked to, it appears that it’s the same for COVID. So now might be as good a time as any to quit cigarettes and quit smoking.”
These results contrast with previous studies that have suggested a protective effect of smoking against COVID-19. In a linked editorial, Anthony Laverty, PhD, and Christopher Millet, PhD, Imperial College London, wrote: “The idea that tobacco smoking may protect against COVID-19 was always an improbable one.”
A version of this article first appeared on Medscape.com.
Observational data was analyzed alongside hospital coronavirus test data and UK Biobank genetic information for the first time, and the findings are published in Thorax.
The data cover 421,469 people overall. Of these, 3.2% took a polymerase chain reaction swab test, 0.4% of these tested positive, 0.2% of them required hospitalization for COVID-19, and 0.1% of them died because of COVID-19.
When it came to smoking status, 59% had never smoked, 37% were ex-smokers, and 3% were current smokers.
Current smokers were 80% more likely to be admitted to hospital, and significantly more likely to die from COVID-19, than nonsmokers.
Time to quit
Heavy smokers who smoked more than 20 cigarettes a day were 6.11 times more likely to die from COVID-19 than people who had never smoked.
Analysis also showed those with a genetic predisposition to being smokers had a 45% higher infection risk, and 60% higher hospitalization risk.
The authors wrote: “Overall, the congruence of observational analyses indicating associations with recent smoking behaviors and [Mendelian randomization] analyses indicating associations with lifelong predisposition to smoking and smoking heaviness support a causal effect of smoking on COVID-19 severity.”
In a linked podcast, lead researcher Dr. Ashley Clift, said: “Our results strongly suggest that smoking is related to your risk of getting severe COVID, and just as smoking affects your risk of heart disease, different cancers, and all those other conditions we know smoking is linked to, it appears that it’s the same for COVID. So now might be as good a time as any to quit cigarettes and quit smoking.”
These results contrast with previous studies that have suggested a protective effect of smoking against COVID-19. In a linked editorial, Anthony Laverty, PhD, and Christopher Millet, PhD, Imperial College London, wrote: “The idea that tobacco smoking may protect against COVID-19 was always an improbable one.”
A version of this article first appeared on Medscape.com.
Observational data was analyzed alongside hospital coronavirus test data and UK Biobank genetic information for the first time, and the findings are published in Thorax.
The data cover 421,469 people overall. Of these, 3.2% took a polymerase chain reaction swab test, 0.4% of these tested positive, 0.2% of them required hospitalization for COVID-19, and 0.1% of them died because of COVID-19.
When it came to smoking status, 59% had never smoked, 37% were ex-smokers, and 3% were current smokers.
Current smokers were 80% more likely to be admitted to hospital, and significantly more likely to die from COVID-19, than nonsmokers.
Time to quit
Heavy smokers who smoked more than 20 cigarettes a day were 6.11 times more likely to die from COVID-19 than people who had never smoked.
Analysis also showed those with a genetic predisposition to being smokers had a 45% higher infection risk, and 60% higher hospitalization risk.
The authors wrote: “Overall, the congruence of observational analyses indicating associations with recent smoking behaviors and [Mendelian randomization] analyses indicating associations with lifelong predisposition to smoking and smoking heaviness support a causal effect of smoking on COVID-19 severity.”
In a linked podcast, lead researcher Dr. Ashley Clift, said: “Our results strongly suggest that smoking is related to your risk of getting severe COVID, and just as smoking affects your risk of heart disease, different cancers, and all those other conditions we know smoking is linked to, it appears that it’s the same for COVID. So now might be as good a time as any to quit cigarettes and quit smoking.”
These results contrast with previous studies that have suggested a protective effect of smoking against COVID-19. In a linked editorial, Anthony Laverty, PhD, and Christopher Millet, PhD, Imperial College London, wrote: “The idea that tobacco smoking may protect against COVID-19 was always an improbable one.”
A version of this article first appeared on Medscape.com.
Children and COVID: New cases topped 200,000 after 3 weeks of declines
Weekly COVID-19 cases in children dropped again, but the count remained above 200,000 for the fifth consecutive week, according to the American Academy of Pediatrics and the Children’s Hospital Association.
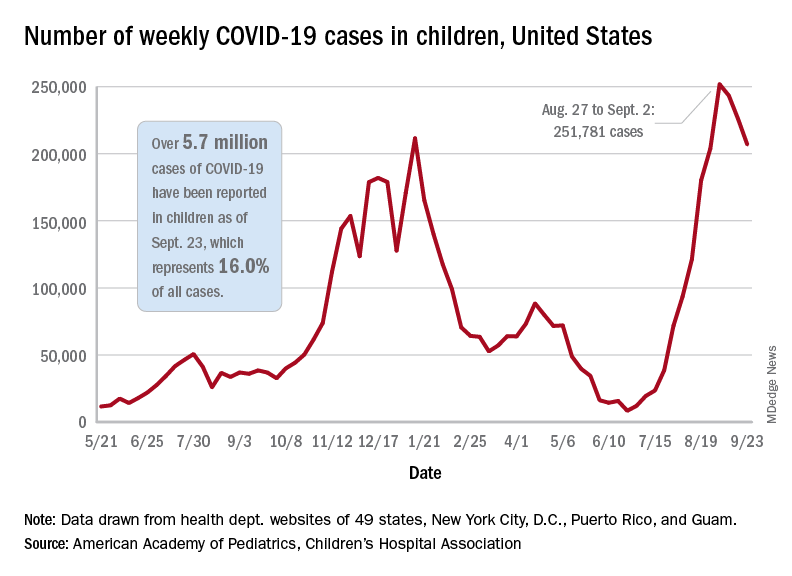
based on the data in the AAP/CHA joint weekly report on COVID in children.
In the most recent week, Sept. 17-23, there were almost 207,000 new cases of COVID-19 in children, which represented 26.7% of all cases reported in the 46 states that are currently posting data by age on their COVID dashboards, the AAP and CHA said. (New York has never reported such data by age, and Alabama, Nebraska, and Texas have not updated their websites since July 29, June 24, and Aug. 26, respectively.)
The decline in new vaccinations among children, however, began before the summer surge in new cases hit its peak – 251,781 during the week of Aug. 27 to Sept. 2 – and has continued for 7 straight weeks in children aged 12-17 years, based on data from the Centers for Disease Control and Prevention.
There were about 172,000 COVID vaccine initiations in children aged 12-17 for the week of Sept. 21-27, the lowest number since April, before it was approved for use in 12- to 15-year-olds. That figure is down by almost a third from the previous week and by more than two-thirds since early August, just before the decline in vaccinations began, according to the CDC’s COVID Data Tracker.
The cumulative vaccine situation looks like this: Just over 13 million children under age 18 years have received at least one dose as of Sept. 27, and almost 10.6 million are fully vaccinated. By age group, 53.9% of 12- to 15-year-olds and 61.6% of 16- to 17-year-olds have received at least one dose, with corresponding figures of 43.3% and 51.3% for full vaccination, the CDC said.
COVID-related hospital admissions also continue to fall after peaking at 0.51 children aged 0-17 per 100,000 population on Sept. 4. The admission rate was down to 0.45 per 100,000 as of Sept. 17, and the latest 7-day average (Sept. 19-25) was 258 admissions, compared with a peak of 371 for the week of Aug. 29 to Sept. 4, the CDC reported.
“Although we have seen slight improvements in COVID-19 volumes in the past week, we are at the beginning of an anticipated increase in” multi-inflammatory syndrome in children, Margaret Rush, MD, president of Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said at a recent hearing of the House Committee on Energy and Commerce’s Oversight subcommittee. That increase would be expected to produce “a secondary wave of seriously ill children 3-6 weeks after acute infection peaks in the community,” the American Hospital Association said.
Meanwhile, Dr. Rush noted, there are signs that seasonal viruses are coming into play. “With the emergence of the Delta variant, we’ve experienced a steep increase in COVID-19 hospitalizations among children on top of an early surge of [respiratory syncytial virus], a serious respiratory illness we usually see in the winter months,” she said in a prepared statement before her testimony.
Weekly COVID-19 cases in children dropped again, but the count remained above 200,000 for the fifth consecutive week, according to the American Academy of Pediatrics and the Children’s Hospital Association.

based on the data in the AAP/CHA joint weekly report on COVID in children.
In the most recent week, Sept. 17-23, there were almost 207,000 new cases of COVID-19 in children, which represented 26.7% of all cases reported in the 46 states that are currently posting data by age on their COVID dashboards, the AAP and CHA said. (New York has never reported such data by age, and Alabama, Nebraska, and Texas have not updated their websites since July 29, June 24, and Aug. 26, respectively.)
The decline in new vaccinations among children, however, began before the summer surge in new cases hit its peak – 251,781 during the week of Aug. 27 to Sept. 2 – and has continued for 7 straight weeks in children aged 12-17 years, based on data from the Centers for Disease Control and Prevention.
There were about 172,000 COVID vaccine initiations in children aged 12-17 for the week of Sept. 21-27, the lowest number since April, before it was approved for use in 12- to 15-year-olds. That figure is down by almost a third from the previous week and by more than two-thirds since early August, just before the decline in vaccinations began, according to the CDC’s COVID Data Tracker.
The cumulative vaccine situation looks like this: Just over 13 million children under age 18 years have received at least one dose as of Sept. 27, and almost 10.6 million are fully vaccinated. By age group, 53.9% of 12- to 15-year-olds and 61.6% of 16- to 17-year-olds have received at least one dose, with corresponding figures of 43.3% and 51.3% for full vaccination, the CDC said.
COVID-related hospital admissions also continue to fall after peaking at 0.51 children aged 0-17 per 100,000 population on Sept. 4. The admission rate was down to 0.45 per 100,000 as of Sept. 17, and the latest 7-day average (Sept. 19-25) was 258 admissions, compared with a peak of 371 for the week of Aug. 29 to Sept. 4, the CDC reported.
“Although we have seen slight improvements in COVID-19 volumes in the past week, we are at the beginning of an anticipated increase in” multi-inflammatory syndrome in children, Margaret Rush, MD, president of Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said at a recent hearing of the House Committee on Energy and Commerce’s Oversight subcommittee. That increase would be expected to produce “a secondary wave of seriously ill children 3-6 weeks after acute infection peaks in the community,” the American Hospital Association said.
Meanwhile, Dr. Rush noted, there are signs that seasonal viruses are coming into play. “With the emergence of the Delta variant, we’ve experienced a steep increase in COVID-19 hospitalizations among children on top of an early surge of [respiratory syncytial virus], a serious respiratory illness we usually see in the winter months,” she said in a prepared statement before her testimony.
Weekly COVID-19 cases in children dropped again, but the count remained above 200,000 for the fifth consecutive week, according to the American Academy of Pediatrics and the Children’s Hospital Association.

based on the data in the AAP/CHA joint weekly report on COVID in children.
In the most recent week, Sept. 17-23, there were almost 207,000 new cases of COVID-19 in children, which represented 26.7% of all cases reported in the 46 states that are currently posting data by age on their COVID dashboards, the AAP and CHA said. (New York has never reported such data by age, and Alabama, Nebraska, and Texas have not updated their websites since July 29, June 24, and Aug. 26, respectively.)
The decline in new vaccinations among children, however, began before the summer surge in new cases hit its peak – 251,781 during the week of Aug. 27 to Sept. 2 – and has continued for 7 straight weeks in children aged 12-17 years, based on data from the Centers for Disease Control and Prevention.
There were about 172,000 COVID vaccine initiations in children aged 12-17 for the week of Sept. 21-27, the lowest number since April, before it was approved for use in 12- to 15-year-olds. That figure is down by almost a third from the previous week and by more than two-thirds since early August, just before the decline in vaccinations began, according to the CDC’s COVID Data Tracker.
The cumulative vaccine situation looks like this: Just over 13 million children under age 18 years have received at least one dose as of Sept. 27, and almost 10.6 million are fully vaccinated. By age group, 53.9% of 12- to 15-year-olds and 61.6% of 16- to 17-year-olds have received at least one dose, with corresponding figures of 43.3% and 51.3% for full vaccination, the CDC said.
COVID-related hospital admissions also continue to fall after peaking at 0.51 children aged 0-17 per 100,000 population on Sept. 4. The admission rate was down to 0.45 per 100,000 as of Sept. 17, and the latest 7-day average (Sept. 19-25) was 258 admissions, compared with a peak of 371 for the week of Aug. 29 to Sept. 4, the CDC reported.
“Although we have seen slight improvements in COVID-19 volumes in the past week, we are at the beginning of an anticipated increase in” multi-inflammatory syndrome in children, Margaret Rush, MD, president of Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said at a recent hearing of the House Committee on Energy and Commerce’s Oversight subcommittee. That increase would be expected to produce “a secondary wave of seriously ill children 3-6 weeks after acute infection peaks in the community,” the American Hospital Association said.
Meanwhile, Dr. Rush noted, there are signs that seasonal viruses are coming into play. “With the emergence of the Delta variant, we’ve experienced a steep increase in COVID-19 hospitalizations among children on top of an early surge of [respiratory syncytial virus], a serious respiratory illness we usually see in the winter months,” she said in a prepared statement before her testimony.
Polyethylene glycol linked to rare allergic reactions seen with mRNA COVID-19 vaccines
A common inert ingredient may be the culprit behind the rare allergic reactions reported among individuals who have received mRNA COVID-19 vaccines, according to investigators at a large regional health center that was among the first to administer the shots.
Blood samples from 10 of 11 individuals with suspected allergic reactions reacted to polyethylene glycol (PEG), a component of both the Pfizer and Moderna mRNA vaccines, according to a report in JAMA Network Open.
In total, only 22 individuals had suspected allergic reactions out of nearly 39,000 mRNA COVID-19 vaccine doses administered, the investigators reported, noting that the reactions were generally mild and all fully resolved.
Those findings should be reassuring to individuals who are reticent to sign up for a COVID-19 vaccine because of fear of an allergic reaction, said study senior author Kari Nadeau, MD, PhD, director of the Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
“We’re hoping that this word will get out and then that the companies could also think about making vaccines that have other products in them that don’t include polyethylene glycol,” Dr. Nadeau said in an interview.
PEG is a compound used in many products, including pharmaceuticals, cosmetics, and food. In the mRNA COVID-19 vaccines, PEG serves to stabilize the lipid nanoparticles that help protect and transport mRNA. However, its use in this setting has been linked to allergic reactions in this and previous studies.
No immunoglobulin E (IgE) antibodies to PEG were detected among the 22 individuals with suspected allergic reactions to mRNA COVID-19 vaccine, but PEG immunoglobulin G (IgG) was present. That suggests non-IgE mediated allergic reactions to PEG may be implicated for the majority of cases, Dr. Nadeau said.
This case series provides interesting new evidence to confirm previous reports that a mechanism other than the classic IgE-mediated allergic response is behind the suspected allergic reactions that are occurring after mRNA COVID-19 vaccine, said Aleena Banerji, MD, associate professor at Harvard Medical School, Boston, and clinical director of the Drug Allergy Program at Massachusetts General Hospital.
“We need to further understand the mechanism of these reactions, but what we know is that IGE mediated allergy to excipients like PEG is probably not the main cause,” Dr. Banerji, who was not involved in the study, said in an interview.
In a recent research letter published in JAMA Internal Medicine, Dr. Banerji and coauthors reported that all individuals with immediate suspected allergic reactions to mRNA COVID-19 vaccine went on to tolerate the second dose, with mild symptoms reported in the minority of patients (32 out of 159, or about 20%).
“Again, that is very consistent with not having an IgE-mediated allergy, so it seems to all be fitting with that picture,” Dr. Banerji said.
The case series by Dr. Nadeau and coauthors was based on review of nearly 39,000 mRNA COVID-19 vaccine doses administered between December 18, 2020 and January 26, 2021. Most mRNA vaccine recipients were Stanford-affiliated health care workers, according to the report.
Among recipients of those doses, they identified 148 individuals who had anaphylaxis-related ICD-10 codes recorded over the same time period. In a review of medical records, investigators pinpointed 22 individuals as having suspected allergy and invited them to participate in follow-up allergy testing.
A total of 11 individuals underwent skin prick testing, but none of them tested positive to PEG or to polysorbate 80, another excipient that has been linked to vaccine-related allergic reactions. One of the patients tested positive to the same mRNA vaccine they had previously received, according to the report.
Those same 11 individuals also underwent basophil activation testing (BAT). In contrast to the skin testing results, BAT results were positive for PEG in 10 of 11 cases (or 91%) and positive for their administered vaccine in all 11 cases, the report shows.
High levels of IgG to PEG were identified in blood samples of individuals with an allergy to the vaccine. Investigators said it’s possible that the BAT results were activated due to IgG via complement activation–related pseudoallergy, or CARPA, as has been hypothesized by some other investigators.
The negative skin prick testing results for PEG, which contrast with the positive BAT results to PEG, suggest that the former may not be appropriate for use as a predictive marker of potential vaccine allergy, according to Dr. Nadeau.
“The take-home message for doctors is to be careful,” she said. “Don’t assume that just because the person skin-tests negative to PEG or to the vaccine itself that you’re out of the woods, because the skin test would be often negative in those scenarios.”
The study was supported by a grants from the Asthma and Allergic Diseases Cooperative Research Centers, a grant from the National Institutes of Health, the National Institute of Allergy and Infectious Disease SARS Vaccine study, the Parker Foundation, the Crown Foundation, and the Sunshine Foundation. Dr. Nadeau reports numerous conflicts with various sources in the industry. Dr. Banerji has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A common inert ingredient may be the culprit behind the rare allergic reactions reported among individuals who have received mRNA COVID-19 vaccines, according to investigators at a large regional health center that was among the first to administer the shots.
Blood samples from 10 of 11 individuals with suspected allergic reactions reacted to polyethylene glycol (PEG), a component of both the Pfizer and Moderna mRNA vaccines, according to a report in JAMA Network Open.
In total, only 22 individuals had suspected allergic reactions out of nearly 39,000 mRNA COVID-19 vaccine doses administered, the investigators reported, noting that the reactions were generally mild and all fully resolved.
Those findings should be reassuring to individuals who are reticent to sign up for a COVID-19 vaccine because of fear of an allergic reaction, said study senior author Kari Nadeau, MD, PhD, director of the Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
“We’re hoping that this word will get out and then that the companies could also think about making vaccines that have other products in them that don’t include polyethylene glycol,” Dr. Nadeau said in an interview.
PEG is a compound used in many products, including pharmaceuticals, cosmetics, and food. In the mRNA COVID-19 vaccines, PEG serves to stabilize the lipid nanoparticles that help protect and transport mRNA. However, its use in this setting has been linked to allergic reactions in this and previous studies.
No immunoglobulin E (IgE) antibodies to PEG were detected among the 22 individuals with suspected allergic reactions to mRNA COVID-19 vaccine, but PEG immunoglobulin G (IgG) was present. That suggests non-IgE mediated allergic reactions to PEG may be implicated for the majority of cases, Dr. Nadeau said.
This case series provides interesting new evidence to confirm previous reports that a mechanism other than the classic IgE-mediated allergic response is behind the suspected allergic reactions that are occurring after mRNA COVID-19 vaccine, said Aleena Banerji, MD, associate professor at Harvard Medical School, Boston, and clinical director of the Drug Allergy Program at Massachusetts General Hospital.
“We need to further understand the mechanism of these reactions, but what we know is that IGE mediated allergy to excipients like PEG is probably not the main cause,” Dr. Banerji, who was not involved in the study, said in an interview.
In a recent research letter published in JAMA Internal Medicine, Dr. Banerji and coauthors reported that all individuals with immediate suspected allergic reactions to mRNA COVID-19 vaccine went on to tolerate the second dose, with mild symptoms reported in the minority of patients (32 out of 159, or about 20%).
“Again, that is very consistent with not having an IgE-mediated allergy, so it seems to all be fitting with that picture,” Dr. Banerji said.
The case series by Dr. Nadeau and coauthors was based on review of nearly 39,000 mRNA COVID-19 vaccine doses administered between December 18, 2020 and January 26, 2021. Most mRNA vaccine recipients were Stanford-affiliated health care workers, according to the report.
Among recipients of those doses, they identified 148 individuals who had anaphylaxis-related ICD-10 codes recorded over the same time period. In a review of medical records, investigators pinpointed 22 individuals as having suspected allergy and invited them to participate in follow-up allergy testing.
A total of 11 individuals underwent skin prick testing, but none of them tested positive to PEG or to polysorbate 80, another excipient that has been linked to vaccine-related allergic reactions. One of the patients tested positive to the same mRNA vaccine they had previously received, according to the report.
Those same 11 individuals also underwent basophil activation testing (BAT). In contrast to the skin testing results, BAT results were positive for PEG in 10 of 11 cases (or 91%) and positive for their administered vaccine in all 11 cases, the report shows.
High levels of IgG to PEG were identified in blood samples of individuals with an allergy to the vaccine. Investigators said it’s possible that the BAT results were activated due to IgG via complement activation–related pseudoallergy, or CARPA, as has been hypothesized by some other investigators.
The negative skin prick testing results for PEG, which contrast with the positive BAT results to PEG, suggest that the former may not be appropriate for use as a predictive marker of potential vaccine allergy, according to Dr. Nadeau.
“The take-home message for doctors is to be careful,” she said. “Don’t assume that just because the person skin-tests negative to PEG or to the vaccine itself that you’re out of the woods, because the skin test would be often negative in those scenarios.”
The study was supported by a grants from the Asthma and Allergic Diseases Cooperative Research Centers, a grant from the National Institutes of Health, the National Institute of Allergy and Infectious Disease SARS Vaccine study, the Parker Foundation, the Crown Foundation, and the Sunshine Foundation. Dr. Nadeau reports numerous conflicts with various sources in the industry. Dr. Banerji has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A common inert ingredient may be the culprit behind the rare allergic reactions reported among individuals who have received mRNA COVID-19 vaccines, according to investigators at a large regional health center that was among the first to administer the shots.
Blood samples from 10 of 11 individuals with suspected allergic reactions reacted to polyethylene glycol (PEG), a component of both the Pfizer and Moderna mRNA vaccines, according to a report in JAMA Network Open.
In total, only 22 individuals had suspected allergic reactions out of nearly 39,000 mRNA COVID-19 vaccine doses administered, the investigators reported, noting that the reactions were generally mild and all fully resolved.
Those findings should be reassuring to individuals who are reticent to sign up for a COVID-19 vaccine because of fear of an allergic reaction, said study senior author Kari Nadeau, MD, PhD, director of the Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
“We’re hoping that this word will get out and then that the companies could also think about making vaccines that have other products in them that don’t include polyethylene glycol,” Dr. Nadeau said in an interview.
PEG is a compound used in many products, including pharmaceuticals, cosmetics, and food. In the mRNA COVID-19 vaccines, PEG serves to stabilize the lipid nanoparticles that help protect and transport mRNA. However, its use in this setting has been linked to allergic reactions in this and previous studies.
No immunoglobulin E (IgE) antibodies to PEG were detected among the 22 individuals with suspected allergic reactions to mRNA COVID-19 vaccine, but PEG immunoglobulin G (IgG) was present. That suggests non-IgE mediated allergic reactions to PEG may be implicated for the majority of cases, Dr. Nadeau said.
This case series provides interesting new evidence to confirm previous reports that a mechanism other than the classic IgE-mediated allergic response is behind the suspected allergic reactions that are occurring after mRNA COVID-19 vaccine, said Aleena Banerji, MD, associate professor at Harvard Medical School, Boston, and clinical director of the Drug Allergy Program at Massachusetts General Hospital.
“We need to further understand the mechanism of these reactions, but what we know is that IGE mediated allergy to excipients like PEG is probably not the main cause,” Dr. Banerji, who was not involved in the study, said in an interview.
In a recent research letter published in JAMA Internal Medicine, Dr. Banerji and coauthors reported that all individuals with immediate suspected allergic reactions to mRNA COVID-19 vaccine went on to tolerate the second dose, with mild symptoms reported in the minority of patients (32 out of 159, or about 20%).
“Again, that is very consistent with not having an IgE-mediated allergy, so it seems to all be fitting with that picture,” Dr. Banerji said.
The case series by Dr. Nadeau and coauthors was based on review of nearly 39,000 mRNA COVID-19 vaccine doses administered between December 18, 2020 and January 26, 2021. Most mRNA vaccine recipients were Stanford-affiliated health care workers, according to the report.
Among recipients of those doses, they identified 148 individuals who had anaphylaxis-related ICD-10 codes recorded over the same time period. In a review of medical records, investigators pinpointed 22 individuals as having suspected allergy and invited them to participate in follow-up allergy testing.
A total of 11 individuals underwent skin prick testing, but none of them tested positive to PEG or to polysorbate 80, another excipient that has been linked to vaccine-related allergic reactions. One of the patients tested positive to the same mRNA vaccine they had previously received, according to the report.
Those same 11 individuals also underwent basophil activation testing (BAT). In contrast to the skin testing results, BAT results were positive for PEG in 10 of 11 cases (or 91%) and positive for their administered vaccine in all 11 cases, the report shows.
High levels of IgG to PEG were identified in blood samples of individuals with an allergy to the vaccine. Investigators said it’s possible that the BAT results were activated due to IgG via complement activation–related pseudoallergy, or CARPA, as has been hypothesized by some other investigators.
The negative skin prick testing results for PEG, which contrast with the positive BAT results to PEG, suggest that the former may not be appropriate for use as a predictive marker of potential vaccine allergy, according to Dr. Nadeau.
“The take-home message for doctors is to be careful,” she said. “Don’t assume that just because the person skin-tests negative to PEG or to the vaccine itself that you’re out of the woods, because the skin test would be often negative in those scenarios.”
The study was supported by a grants from the Asthma and Allergic Diseases Cooperative Research Centers, a grant from the National Institutes of Health, the National Institute of Allergy and Infectious Disease SARS Vaccine study, the Parker Foundation, the Crown Foundation, and the Sunshine Foundation. Dr. Nadeau reports numerous conflicts with various sources in the industry. Dr. Banerji has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
These schools use weekly testing to keep kids in class – and COVID out
On a recent Monday morning, a group of preschoolers filed into the gymnasium at Hillside School in the west Chicago suburbs. These 4- and 5-year-olds were the first of more than 200 students to get tested for the coronavirus that day – and every Monday – for the foreseeable future.
At the front of the line, a girl in a unicorn headband and sparkly pink skirt clutched a zip-close bag with her name on it. She pulled out a plastic tube with a small funnel attached. Next, Hillside superintendent Kevin Suchinski led the student to a spot marked off with red tape. Mr. Suchinski coached her how to carefully release – but not “spit” – about a half-teaspoon’s worth of saliva into the tube.
“You wait a second, you build up your saliva,” he told her. “You don’t talk, you think about pizza, hamburgers, French fries, ice cream. And you drop it right in there, OK?”
The results will come back within 24 hours. Any students who test positive are instructed to isolate, and the school nurse and administrative staff carry out contact tracing.
Hillside was among the first in Illinois to start regular testing. Now, almost half of Illinois’ 2 million students in grades K-12 attend schools rolling out similar programs. The initiative is supported by federal funding channeled through the state health department.
Schools in other states – such as Massachusetts, Maryland, New York and Colorado – also offer regular testing; Los Angeles public schools have gone further by making it mandatory.
These measures stand in sharp contrast to the confusion in states where people are still fighting about wearing masks in the classroom and other anti-COVID strategies, places where some schools have experienced outbreaks and even teacher deaths.
Within a few weeks of schools reopening, tens of thousands of students across the United States were sent home to quarantine. It’s a concern because options for K-12 students in quarantine are all over the map – with some schools offering virtual instruction and others providing little or no at-home options.
Mr. Suchinski hopes this investment in testing prevents virus detected at Hillside School from spreading into the wider community – and keeps kids learning.
“What we say to ourselves is: If we don’t do this program, we could be losing instruction because we’ve had to close down the school,” he said.
So far, the parents and guardians of two-thirds of all Hillside students have consented to testing. Mr. Suchinski said the school is working hard to get the remaining families on board by educating them about the importance – and benefit – of regular testing.
Every school that can manage it should consider testing students weekly – even twice a week, if possible, said Becky Smith, PhD. She’s an epidemiologist at the University of Illinois in Urbana-Champaign, which developed the saliva test Hillside and other Illinois schools are using. Smith pointed to several studies – including both peer-reviewed and preliminary research – that suggest rigorous testing and contact tracing are key to keeping the virus at bay in K-12 schools.
“If you’re lucky, you can get away without doing testing, [if] nobody comes to school with a raging infection and takes their mask off at lunchtime and infects everybody sitting at the table with them,” Dr. Smith said. “But relying on luck isn’t what we like to do.”
Julian Hernandez, a Hillside seventh grader, said he feels safer knowing that classmates infected with the virus will be prevented from spreading it to others.
“One of my friends – he got it a couple months ago while we was in school,” Julian recalled. “[He] and his brother had to go back home. ... They were OK. They only had mild symptoms.”
Brandon Muñoz, who’s in the fifth grade, said he’s glad to get tested because he’s too young for the vaccine – and he really doesn’t want to go back to Zoom school.
“Because I wanna really meet more people and friends and just not stay on the computer for too long,” Brandon explained.
Mr. Suchinski said Hillside also improved ventilation throughout the building, installing a new HVAC system and windows with screens in the cafeteria to bring more fresh air in the building.
Regular testing is an added layer of protection, though not the only thing Hillside is relying on: About 90% of Hillside staff are vaccinated, Suchinski said, and students and staffers also wear masks.
Setting up a regular mass-testing program inside a K-12 school takes a good amount of coordination, which Mr. Suchinski can vouch for.
Last school year, Hillside school administrators facilitated the saliva sample collection without outside help. This year, the school tapped funding earmarked for K-12 coronavirus testing to hire COVID testers – who coordinate the collecting, transporting and processing of samples, and reporting results.
A couple of Hillside administrators help oversee the process on Mondays, and also facilitate testing for staff members, plus more frequent testing for a limited group of students: Athletes and children in band and extracurriculars test twice a week because they face greater risks of exposure to the virus from these activities.
Compared with a year ago, COVID testing is now both more affordable and much less invasive, said Mara Aspinall, who studies biomedical testing at Arizona State University. There’s also more help to cover costs.
“The Biden administration has allocated $11 billion to different programs for testing,” Ms. Aspinall said. “There should be no school – public, private or charter – that can’t access that money for testing.”
Creating a mass testing program from scratch is a big lift. But more than half of all states have announced programs to help schools access the money and handle the logistics.
If every school tested every student once a week, the roughly $11 billion earmarked for testing would likely run out in a couple of months. (This assumes $20 to buy and process each test.) Put another way, if a quarter of all U.S. schools tested students weekly, the funds could last the rest of the school year, Ms. Aspinall said.
In its guidance to K-12 schools, updated Aug. 5, the Centers for Disease Control and Prevention does not make a firm recommendation for this surveillance testing.
Instead, the CDC advises schools that choose to offer testing to work with public health officials to determine a suitable approach, given rates of community transmission and other factors.
The agency previously recommended screening at least once a week in all areas experiencing moderate to high levels of community transmission. As of Sept. 21, that included 95% of U.S. counties.
For school leaders looking to explore options, Ms. Aspinall suggests a resource she helped write, which is cited within the CDC guidance to schools: the Rockefeller Foundation’s National Testing Action Plan.
This spring – when Hillside was operating at about half capacity and before the more contagious delta variant took over – the school identified 13 positive cases among students and staffers via its weekly testing program. The overall positivity rate of about half a percent made some wonder if all that testing was necessary.
But Mr. Suchinski said that, by identifying the 13 positive cases, the school perhaps avoided more than a dozen potential outbreaks. Some of the positive cases were among people who weren’t showing symptoms but still could’ve spread the virus.
A couple of weeks into the new school year at Hillside, operating at full capacity, Mr. Suchinski said the excitement is palpable. Nowadays he’s balancing feelings of optimism with caution.
“It is great to hear kids laughing. It’s great to see kids on playgrounds,” Mr. Suchinski said.
“At the same time,” he added, “we know that we’re still fighting against the Delta variant and we have to keep our guard up.”
This story is from a partnership that includes Illinois Public Media, Side Effects Public Media, NPR, and KHN (Kaiser Health News). KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On a recent Monday morning, a group of preschoolers filed into the gymnasium at Hillside School in the west Chicago suburbs. These 4- and 5-year-olds were the first of more than 200 students to get tested for the coronavirus that day – and every Monday – for the foreseeable future.
At the front of the line, a girl in a unicorn headband and sparkly pink skirt clutched a zip-close bag with her name on it. She pulled out a plastic tube with a small funnel attached. Next, Hillside superintendent Kevin Suchinski led the student to a spot marked off with red tape. Mr. Suchinski coached her how to carefully release – but not “spit” – about a half-teaspoon’s worth of saliva into the tube.
“You wait a second, you build up your saliva,” he told her. “You don’t talk, you think about pizza, hamburgers, French fries, ice cream. And you drop it right in there, OK?”
The results will come back within 24 hours. Any students who test positive are instructed to isolate, and the school nurse and administrative staff carry out contact tracing.
Hillside was among the first in Illinois to start regular testing. Now, almost half of Illinois’ 2 million students in grades K-12 attend schools rolling out similar programs. The initiative is supported by federal funding channeled through the state health department.
Schools in other states – such as Massachusetts, Maryland, New York and Colorado – also offer regular testing; Los Angeles public schools have gone further by making it mandatory.
These measures stand in sharp contrast to the confusion in states where people are still fighting about wearing masks in the classroom and other anti-COVID strategies, places where some schools have experienced outbreaks and even teacher deaths.
Within a few weeks of schools reopening, tens of thousands of students across the United States were sent home to quarantine. It’s a concern because options for K-12 students in quarantine are all over the map – with some schools offering virtual instruction and others providing little or no at-home options.
Mr. Suchinski hopes this investment in testing prevents virus detected at Hillside School from spreading into the wider community – and keeps kids learning.
“What we say to ourselves is: If we don’t do this program, we could be losing instruction because we’ve had to close down the school,” he said.
So far, the parents and guardians of two-thirds of all Hillside students have consented to testing. Mr. Suchinski said the school is working hard to get the remaining families on board by educating them about the importance – and benefit – of regular testing.
Every school that can manage it should consider testing students weekly – even twice a week, if possible, said Becky Smith, PhD. She’s an epidemiologist at the University of Illinois in Urbana-Champaign, which developed the saliva test Hillside and other Illinois schools are using. Smith pointed to several studies – including both peer-reviewed and preliminary research – that suggest rigorous testing and contact tracing are key to keeping the virus at bay in K-12 schools.
“If you’re lucky, you can get away without doing testing, [if] nobody comes to school with a raging infection and takes their mask off at lunchtime and infects everybody sitting at the table with them,” Dr. Smith said. “But relying on luck isn’t what we like to do.”
Julian Hernandez, a Hillside seventh grader, said he feels safer knowing that classmates infected with the virus will be prevented from spreading it to others.
“One of my friends – he got it a couple months ago while we was in school,” Julian recalled. “[He] and his brother had to go back home. ... They were OK. They only had mild symptoms.”
Brandon Muñoz, who’s in the fifth grade, said he’s glad to get tested because he’s too young for the vaccine – and he really doesn’t want to go back to Zoom school.
“Because I wanna really meet more people and friends and just not stay on the computer for too long,” Brandon explained.
Mr. Suchinski said Hillside also improved ventilation throughout the building, installing a new HVAC system and windows with screens in the cafeteria to bring more fresh air in the building.
Regular testing is an added layer of protection, though not the only thing Hillside is relying on: About 90% of Hillside staff are vaccinated, Suchinski said, and students and staffers also wear masks.
Setting up a regular mass-testing program inside a K-12 school takes a good amount of coordination, which Mr. Suchinski can vouch for.
Last school year, Hillside school administrators facilitated the saliva sample collection without outside help. This year, the school tapped funding earmarked for K-12 coronavirus testing to hire COVID testers – who coordinate the collecting, transporting and processing of samples, and reporting results.
A couple of Hillside administrators help oversee the process on Mondays, and also facilitate testing for staff members, plus more frequent testing for a limited group of students: Athletes and children in band and extracurriculars test twice a week because they face greater risks of exposure to the virus from these activities.
Compared with a year ago, COVID testing is now both more affordable and much less invasive, said Mara Aspinall, who studies biomedical testing at Arizona State University. There’s also more help to cover costs.
“The Biden administration has allocated $11 billion to different programs for testing,” Ms. Aspinall said. “There should be no school – public, private or charter – that can’t access that money for testing.”
Creating a mass testing program from scratch is a big lift. But more than half of all states have announced programs to help schools access the money and handle the logistics.
If every school tested every student once a week, the roughly $11 billion earmarked for testing would likely run out in a couple of months. (This assumes $20 to buy and process each test.) Put another way, if a quarter of all U.S. schools tested students weekly, the funds could last the rest of the school year, Ms. Aspinall said.
In its guidance to K-12 schools, updated Aug. 5, the Centers for Disease Control and Prevention does not make a firm recommendation for this surveillance testing.
Instead, the CDC advises schools that choose to offer testing to work with public health officials to determine a suitable approach, given rates of community transmission and other factors.
The agency previously recommended screening at least once a week in all areas experiencing moderate to high levels of community transmission. As of Sept. 21, that included 95% of U.S. counties.
For school leaders looking to explore options, Ms. Aspinall suggests a resource she helped write, which is cited within the CDC guidance to schools: the Rockefeller Foundation’s National Testing Action Plan.
This spring – when Hillside was operating at about half capacity and before the more contagious delta variant took over – the school identified 13 positive cases among students and staffers via its weekly testing program. The overall positivity rate of about half a percent made some wonder if all that testing was necessary.
But Mr. Suchinski said that, by identifying the 13 positive cases, the school perhaps avoided more than a dozen potential outbreaks. Some of the positive cases were among people who weren’t showing symptoms but still could’ve spread the virus.
A couple of weeks into the new school year at Hillside, operating at full capacity, Mr. Suchinski said the excitement is palpable. Nowadays he’s balancing feelings of optimism with caution.
“It is great to hear kids laughing. It’s great to see kids on playgrounds,” Mr. Suchinski said.
“At the same time,” he added, “we know that we’re still fighting against the Delta variant and we have to keep our guard up.”
This story is from a partnership that includes Illinois Public Media, Side Effects Public Media, NPR, and KHN (Kaiser Health News). KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On a recent Monday morning, a group of preschoolers filed into the gymnasium at Hillside School in the west Chicago suburbs. These 4- and 5-year-olds were the first of more than 200 students to get tested for the coronavirus that day – and every Monday – for the foreseeable future.
At the front of the line, a girl in a unicorn headband and sparkly pink skirt clutched a zip-close bag with her name on it. She pulled out a plastic tube with a small funnel attached. Next, Hillside superintendent Kevin Suchinski led the student to a spot marked off with red tape. Mr. Suchinski coached her how to carefully release – but not “spit” – about a half-teaspoon’s worth of saliva into the tube.
“You wait a second, you build up your saliva,” he told her. “You don’t talk, you think about pizza, hamburgers, French fries, ice cream. And you drop it right in there, OK?”
The results will come back within 24 hours. Any students who test positive are instructed to isolate, and the school nurse and administrative staff carry out contact tracing.
Hillside was among the first in Illinois to start regular testing. Now, almost half of Illinois’ 2 million students in grades K-12 attend schools rolling out similar programs. The initiative is supported by federal funding channeled through the state health department.
Schools in other states – such as Massachusetts, Maryland, New York and Colorado – also offer regular testing; Los Angeles public schools have gone further by making it mandatory.
These measures stand in sharp contrast to the confusion in states where people are still fighting about wearing masks in the classroom and other anti-COVID strategies, places where some schools have experienced outbreaks and even teacher deaths.
Within a few weeks of schools reopening, tens of thousands of students across the United States were sent home to quarantine. It’s a concern because options for K-12 students in quarantine are all over the map – with some schools offering virtual instruction and others providing little or no at-home options.
Mr. Suchinski hopes this investment in testing prevents virus detected at Hillside School from spreading into the wider community – and keeps kids learning.
“What we say to ourselves is: If we don’t do this program, we could be losing instruction because we’ve had to close down the school,” he said.
So far, the parents and guardians of two-thirds of all Hillside students have consented to testing. Mr. Suchinski said the school is working hard to get the remaining families on board by educating them about the importance – and benefit – of regular testing.
Every school that can manage it should consider testing students weekly – even twice a week, if possible, said Becky Smith, PhD. She’s an epidemiologist at the University of Illinois in Urbana-Champaign, which developed the saliva test Hillside and other Illinois schools are using. Smith pointed to several studies – including both peer-reviewed and preliminary research – that suggest rigorous testing and contact tracing are key to keeping the virus at bay in K-12 schools.
“If you’re lucky, you can get away without doing testing, [if] nobody comes to school with a raging infection and takes their mask off at lunchtime and infects everybody sitting at the table with them,” Dr. Smith said. “But relying on luck isn’t what we like to do.”
Julian Hernandez, a Hillside seventh grader, said he feels safer knowing that classmates infected with the virus will be prevented from spreading it to others.
“One of my friends – he got it a couple months ago while we was in school,” Julian recalled. “[He] and his brother had to go back home. ... They were OK. They only had mild symptoms.”
Brandon Muñoz, who’s in the fifth grade, said he’s glad to get tested because he’s too young for the vaccine – and he really doesn’t want to go back to Zoom school.
“Because I wanna really meet more people and friends and just not stay on the computer for too long,” Brandon explained.
Mr. Suchinski said Hillside also improved ventilation throughout the building, installing a new HVAC system and windows with screens in the cafeteria to bring more fresh air in the building.
Regular testing is an added layer of protection, though not the only thing Hillside is relying on: About 90% of Hillside staff are vaccinated, Suchinski said, and students and staffers also wear masks.
Setting up a regular mass-testing program inside a K-12 school takes a good amount of coordination, which Mr. Suchinski can vouch for.
Last school year, Hillside school administrators facilitated the saliva sample collection without outside help. This year, the school tapped funding earmarked for K-12 coronavirus testing to hire COVID testers – who coordinate the collecting, transporting and processing of samples, and reporting results.
A couple of Hillside administrators help oversee the process on Mondays, and also facilitate testing for staff members, plus more frequent testing for a limited group of students: Athletes and children in band and extracurriculars test twice a week because they face greater risks of exposure to the virus from these activities.
Compared with a year ago, COVID testing is now both more affordable and much less invasive, said Mara Aspinall, who studies biomedical testing at Arizona State University. There’s also more help to cover costs.
“The Biden administration has allocated $11 billion to different programs for testing,” Ms. Aspinall said. “There should be no school – public, private or charter – that can’t access that money for testing.”
Creating a mass testing program from scratch is a big lift. But more than half of all states have announced programs to help schools access the money and handle the logistics.
If every school tested every student once a week, the roughly $11 billion earmarked for testing would likely run out in a couple of months. (This assumes $20 to buy and process each test.) Put another way, if a quarter of all U.S. schools tested students weekly, the funds could last the rest of the school year, Ms. Aspinall said.
In its guidance to K-12 schools, updated Aug. 5, the Centers for Disease Control and Prevention does not make a firm recommendation for this surveillance testing.
Instead, the CDC advises schools that choose to offer testing to work with public health officials to determine a suitable approach, given rates of community transmission and other factors.
The agency previously recommended screening at least once a week in all areas experiencing moderate to high levels of community transmission. As of Sept. 21, that included 95% of U.S. counties.
For school leaders looking to explore options, Ms. Aspinall suggests a resource she helped write, which is cited within the CDC guidance to schools: the Rockefeller Foundation’s National Testing Action Plan.
This spring – when Hillside was operating at about half capacity and before the more contagious delta variant took over – the school identified 13 positive cases among students and staffers via its weekly testing program. The overall positivity rate of about half a percent made some wonder if all that testing was necessary.
But Mr. Suchinski said that, by identifying the 13 positive cases, the school perhaps avoided more than a dozen potential outbreaks. Some of the positive cases were among people who weren’t showing symptoms but still could’ve spread the virus.
A couple of weeks into the new school year at Hillside, operating at full capacity, Mr. Suchinski said the excitement is palpable. Nowadays he’s balancing feelings of optimism with caution.
“It is great to hear kids laughing. It’s great to see kids on playgrounds,” Mr. Suchinski said.
“At the same time,” he added, “we know that we’re still fighting against the Delta variant and we have to keep our guard up.”
This story is from a partnership that includes Illinois Public Media, Side Effects Public Media, NPR, and KHN (Kaiser Health News). KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
PCOS linked to menopausal urogenital symptoms but not hot flashes
Women with a history of polycystic ovary syndrome (PCOS) are more likely to experience somatic and urogenital symptoms post menopause, but they were no more likely to experience severe hot flashes than were other women with similar characteristics, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society.
PCOS and vasomotor symptoms are each risk factors for cardiovascular disease, so researchers wanted to find out whether they were linked to one another, which might indicate that they are markers for the same underlying mechanisms that increase heart disease risk. The lack of an association, however, raises questions about how much each of these conditions might independently increase cardiovascular risk.
“Should we take a little more time to truly risk-assess these patients not just with their ASCVD risk score, but take into account that they have PCOS and they’re going through menopause, and how severe their hot flashes are?” asked Angie S. Lobo, MD, an internal medicine specialist at Mayo Clinic in Rochester, Minn., when she discussed her findings in an interview.
The association between PCOS and urogenital symptoms was surprising, Dr. Lobo said, but she said she suspects the reason for the finding may be the self-reported nature of the study.
“If you ask the question, you get the answer,” Dr. Lobo said. ”Are we just not asking the right questions to our patients? And should we be doing this more often? This is an exciting finding because there’s so much room to improve the clinical care of our patients.”
The researchers analyzed data from 3,308 women, ages 45-60, in a cross-sectional study from the Data Registry on the Experiences of Aging, Menopause, and Sexuality (DREAMS). The study occurred at Mayo Clinic locations between May 2015 and December 2019 in Rochester, Minn., in Scottsdale, Ariz., and in Jacksonville, Fla.
The women were an average 53 years old and were primarily White, educated, and postmenopausal. Among the 4.6% of women with a self-reported history of PCOS, 56% of them reported depression symptoms, compared to 42% of women without PCOS. Those with PCOS also had nearly twice the prevalence of obesity – 42% versus 22.5% among women without PCOS – and had a higher average overall score on the Menopause Rating Scale (17.7 vs. 14.7; P < .001).
Although women with PCOS initially had a greater burden of psychological symptoms on the same scale, that association disappeared after adjustment for menopause status, body mass index, depression, anxiety, and current use of hormone therapy. Even after adjustment, however, women with PCOS had higher average scores for somatic symptoms (6.7 vs. 5.6) and urogenital symptoms (5.2 vs. 4.3) than those of women without PCOS (P < .001).
Severe or very severe hot flashes were no more likely in women with a history of PCOS than in the other women in the study.
”The mechanisms underlying the correlation between PCOS and menopause symptoms in the psychological and urogenital symptom domains requires further study, although the well-known association between PCOS and mood disorders may explain the high psychological symptom burden in these women during the menopause transition,” the authors concluded.
Rachael B. Smith, DO, clinical assistant professor of ob.gyn. at the University of Arizona in Phoenix, said she was not surprised to see an association between PCOS and menopause symptoms overall, but she was surprised that PCOS did not correlate with severity of vasomotor symptoms. But Dr. Smith pointed out that the sample size of women with PCOS is fairly small (n = 151).
“Given that PCOS prevalence is about 6%-10%, I feel this association should be further studied to improve our counseling and treatment for this PCOS population,” Dr. Smith, who was not involved in the research, said in an interview. “The take-home message for physicians is improved patient-tailored counseling that takes into account patients’ prior medical history of PCOS.”
Although it will require more research to find out, Dr. Smith said she suspects that PCOS and vasomotor symptoms are additive risk factors for cardiovascular disease. She also noted that the study is limited by the homogeneity of the study population.
The research was funded by the National Institutes of Health. Dr. Lobo and Dr. Smith had no disclosures.
Women with a history of polycystic ovary syndrome (PCOS) are more likely to experience somatic and urogenital symptoms post menopause, but they were no more likely to experience severe hot flashes than were other women with similar characteristics, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society.
PCOS and vasomotor symptoms are each risk factors for cardiovascular disease, so researchers wanted to find out whether they were linked to one another, which might indicate that they are markers for the same underlying mechanisms that increase heart disease risk. The lack of an association, however, raises questions about how much each of these conditions might independently increase cardiovascular risk.
“Should we take a little more time to truly risk-assess these patients not just with their ASCVD risk score, but take into account that they have PCOS and they’re going through menopause, and how severe their hot flashes are?” asked Angie S. Lobo, MD, an internal medicine specialist at Mayo Clinic in Rochester, Minn., when she discussed her findings in an interview.
The association between PCOS and urogenital symptoms was surprising, Dr. Lobo said, but she said she suspects the reason for the finding may be the self-reported nature of the study.
“If you ask the question, you get the answer,” Dr. Lobo said. ”Are we just not asking the right questions to our patients? And should we be doing this more often? This is an exciting finding because there’s so much room to improve the clinical care of our patients.”
The researchers analyzed data from 3,308 women, ages 45-60, in a cross-sectional study from the Data Registry on the Experiences of Aging, Menopause, and Sexuality (DREAMS). The study occurred at Mayo Clinic locations between May 2015 and December 2019 in Rochester, Minn., in Scottsdale, Ariz., and in Jacksonville, Fla.
The women were an average 53 years old and were primarily White, educated, and postmenopausal. Among the 4.6% of women with a self-reported history of PCOS, 56% of them reported depression symptoms, compared to 42% of women without PCOS. Those with PCOS also had nearly twice the prevalence of obesity – 42% versus 22.5% among women without PCOS – and had a higher average overall score on the Menopause Rating Scale (17.7 vs. 14.7; P < .001).
Although women with PCOS initially had a greater burden of psychological symptoms on the same scale, that association disappeared after adjustment for menopause status, body mass index, depression, anxiety, and current use of hormone therapy. Even after adjustment, however, women with PCOS had higher average scores for somatic symptoms (6.7 vs. 5.6) and urogenital symptoms (5.2 vs. 4.3) than those of women without PCOS (P < .001).
Severe or very severe hot flashes were no more likely in women with a history of PCOS than in the other women in the study.
”The mechanisms underlying the correlation between PCOS and menopause symptoms in the psychological and urogenital symptom domains requires further study, although the well-known association between PCOS and mood disorders may explain the high psychological symptom burden in these women during the menopause transition,” the authors concluded.
Rachael B. Smith, DO, clinical assistant professor of ob.gyn. at the University of Arizona in Phoenix, said she was not surprised to see an association between PCOS and menopause symptoms overall, but she was surprised that PCOS did not correlate with severity of vasomotor symptoms. But Dr. Smith pointed out that the sample size of women with PCOS is fairly small (n = 151).
“Given that PCOS prevalence is about 6%-10%, I feel this association should be further studied to improve our counseling and treatment for this PCOS population,” Dr. Smith, who was not involved in the research, said in an interview. “The take-home message for physicians is improved patient-tailored counseling that takes into account patients’ prior medical history of PCOS.”
Although it will require more research to find out, Dr. Smith said she suspects that PCOS and vasomotor symptoms are additive risk factors for cardiovascular disease. She also noted that the study is limited by the homogeneity of the study population.
The research was funded by the National Institutes of Health. Dr. Lobo and Dr. Smith had no disclosures.
Women with a history of polycystic ovary syndrome (PCOS) are more likely to experience somatic and urogenital symptoms post menopause, but they were no more likely to experience severe hot flashes than were other women with similar characteristics, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society.
PCOS and vasomotor symptoms are each risk factors for cardiovascular disease, so researchers wanted to find out whether they were linked to one another, which might indicate that they are markers for the same underlying mechanisms that increase heart disease risk. The lack of an association, however, raises questions about how much each of these conditions might independently increase cardiovascular risk.
“Should we take a little more time to truly risk-assess these patients not just with their ASCVD risk score, but take into account that they have PCOS and they’re going through menopause, and how severe their hot flashes are?” asked Angie S. Lobo, MD, an internal medicine specialist at Mayo Clinic in Rochester, Minn., when she discussed her findings in an interview.
The association between PCOS and urogenital symptoms was surprising, Dr. Lobo said, but she said she suspects the reason for the finding may be the self-reported nature of the study.
“If you ask the question, you get the answer,” Dr. Lobo said. ”Are we just not asking the right questions to our patients? And should we be doing this more often? This is an exciting finding because there’s so much room to improve the clinical care of our patients.”
The researchers analyzed data from 3,308 women, ages 45-60, in a cross-sectional study from the Data Registry on the Experiences of Aging, Menopause, and Sexuality (DREAMS). The study occurred at Mayo Clinic locations between May 2015 and December 2019 in Rochester, Minn., in Scottsdale, Ariz., and in Jacksonville, Fla.
The women were an average 53 years old and were primarily White, educated, and postmenopausal. Among the 4.6% of women with a self-reported history of PCOS, 56% of them reported depression symptoms, compared to 42% of women without PCOS. Those with PCOS also had nearly twice the prevalence of obesity – 42% versus 22.5% among women without PCOS – and had a higher average overall score on the Menopause Rating Scale (17.7 vs. 14.7; P < .001).
Although women with PCOS initially had a greater burden of psychological symptoms on the same scale, that association disappeared after adjustment for menopause status, body mass index, depression, anxiety, and current use of hormone therapy. Even after adjustment, however, women with PCOS had higher average scores for somatic symptoms (6.7 vs. 5.6) and urogenital symptoms (5.2 vs. 4.3) than those of women without PCOS (P < .001).
Severe or very severe hot flashes were no more likely in women with a history of PCOS than in the other women in the study.
”The mechanisms underlying the correlation between PCOS and menopause symptoms in the psychological and urogenital symptom domains requires further study, although the well-known association between PCOS and mood disorders may explain the high psychological symptom burden in these women during the menopause transition,” the authors concluded.
Rachael B. Smith, DO, clinical assistant professor of ob.gyn. at the University of Arizona in Phoenix, said she was not surprised to see an association between PCOS and menopause symptoms overall, but she was surprised that PCOS did not correlate with severity of vasomotor symptoms. But Dr. Smith pointed out that the sample size of women with PCOS is fairly small (n = 151).
“Given that PCOS prevalence is about 6%-10%, I feel this association should be further studied to improve our counseling and treatment for this PCOS population,” Dr. Smith, who was not involved in the research, said in an interview. “The take-home message for physicians is improved patient-tailored counseling that takes into account patients’ prior medical history of PCOS.”
Although it will require more research to find out, Dr. Smith said she suspects that PCOS and vasomotor symptoms are additive risk factors for cardiovascular disease. She also noted that the study is limited by the homogeneity of the study population.
The research was funded by the National Institutes of Health. Dr. Lobo and Dr. Smith had no disclosures.
FROM NAMS 2021
Lifestyle interventions improve resistant hypertension
A 4-month, structured diet and exercise intervention lowered blood pressure in adults with resistant blood pressure, according to results from a randomized, clinical trial. The program also led to improvements in baroreflex sensitivity, heart rate variability, and flow-mediated dilation, compared with individuals who received only a single education session.
The intervention included instruction from a nutritionist on how to follow the Dietary Approaches to Stop Hypertension (DASH) diet, as well as restricting calories and sodium to less than 2,300 mg/day. It included weekly, 45-minute group counseling sessions, run by a clinical psychiatrist, focusing on eating behaviors. The exercise component included 30- to 45-minute sessions at 70%-85% of initial heart rate reserve, carried out three times per week at a cardiac rehabilitation facility.
“While some individuals can make the lifestyle changes on their own, a structured program of supervised exercise and dietary modification conducted by a multidisciplinary team of physicians, psychologists, nutritionists, and physical therapists/exercise physiologists found in cardiac rehabilitation programs throughout the country is likely to be more effective. There are many cardiac rehabilitation programs throughout the country that are accessible to most patients,” said lead author James Blumenthal, PhD, the J.P. Gibbons Professor in Psychiatry and Behavioral Sciences at Duke University, Durham, N.C.
The study, called Treating Resistant Hypertension Using Lifestyle Modification to Promote Health (TRIUMPH), was published in Circulation. It is one of few that have examined lifestyle interventions in resistant hypertension, defined as systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 80 mm Hg after adherence to three or more optimally-dosed antihypertensive medications of three different classes, including one diuretic.
“This is a nice study [that] emphasizes what we often forget: Lifestyle factors, especially salt intake, are important drivers of resistant hypertension. Our own studies have shown that this is predominantly a salt retaining state, and one would expect dietary salt restriction to be particularly effective in this group of patients and that is what this study showed,” said Bryan Williams, MD, who was asked to comment on the study. Dr. Williams is chair of medicine at University College London.
The results should also be reassuring to some who worried that exercise might lead to worsened blood pressure. “This study showed that in patients with resistant hypertension, though not out of control blood pressures, exercise was not only safe, but effective in lowering their blood pressure,” said Deepak L. Bhatt, MD, MPH, who was asked to comment. He is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital, and professor of medicine at Harvard Medical School, both in Boston.
The new research isn’t unique. In August, researchers in Portugal and Brazil showed that a 12-week exercise-only intervention reduced 24-hour and daytime ambulatory systolic and diastolic blood pressure. The two studies communicate the same message. “Lifestyle changes can work for resistant hypertension. So, two independent, modest-sized studies with essentially the same, positive, actionable conclusion,” said Dr. Bhatt, adding that the next step should be a larger, multicenter trial.
In the new study, 90 patients were assigned to the diet and exercise intervention and 50 to the control group. They had a mean age of 63 years, and 48% were women. Participants attended 94% of DASH diet classes and 89% of exercise sessions, and both groups had excellent adherence to medications.
The treatment group had a greater reduction in clinic systolic BP (–12.5 versus –7.1 mm Hg; P = .005) and diastolic BP (–5.9 versus –3.7 mm Hg; P = .034), as well as 24-hour ambulatory systolic BP (–7.0 versus –0.3 mm Hg; P = .001). The treatment group also had more improvement in resting baroreflex sensitivity (2.3 versus –1.1 ms/mm Hg; P = .003), high-frequency heart rate variability (0.4 versus –0.2 ln ms2; P = .025, and flow-mediated dilation (0.3% versus –1.4%, P = .022). The two groups had similar outcomes with respect to pulse wave velocity and left ventricular mass.
“Results of the TRIUMPH study suggest that policymakers should consider resistant hypertension as a new indication for cardiac rehabilitation with appropriate coverage by governmental agencies and private insurers,” Dr. Blumenthal said.
“This is an important new, evidence-based intervention for resistant hypertension. It is safe and relatively inexpensive. It should now be something physicians routinely offer these patients. Hopefully in the future, insurers will cover cardiac rehabilitation for patients with resistant hypertension,” Dr. Bhatt said.
The study also pointed towards an effective approach for patients who may be unable to sustain lifestyle changes. “Of course, not every patient will be able to maintain a healthy diet and an exercise program on their own, thus a cardiac rehabilitation program can be an excellent way to increase the likelihood of successful lifestyle modification,” Dr. Bhatt said.
TRIUMPH was sponsored by grants from the National Heart, Lung, and Blood Institute. None of the authors had disclosures to report. Dr. Bhatt disclosed having financial relationships with more than 40 pharmaceutical companies. Dr. Williams reported having no relevant financial disclosures.
A 4-month, structured diet and exercise intervention lowered blood pressure in adults with resistant blood pressure, according to results from a randomized, clinical trial. The program also led to improvements in baroreflex sensitivity, heart rate variability, and flow-mediated dilation, compared with individuals who received only a single education session.
The intervention included instruction from a nutritionist on how to follow the Dietary Approaches to Stop Hypertension (DASH) diet, as well as restricting calories and sodium to less than 2,300 mg/day. It included weekly, 45-minute group counseling sessions, run by a clinical psychiatrist, focusing on eating behaviors. The exercise component included 30- to 45-minute sessions at 70%-85% of initial heart rate reserve, carried out three times per week at a cardiac rehabilitation facility.
“While some individuals can make the lifestyle changes on their own, a structured program of supervised exercise and dietary modification conducted by a multidisciplinary team of physicians, psychologists, nutritionists, and physical therapists/exercise physiologists found in cardiac rehabilitation programs throughout the country is likely to be more effective. There are many cardiac rehabilitation programs throughout the country that are accessible to most patients,” said lead author James Blumenthal, PhD, the J.P. Gibbons Professor in Psychiatry and Behavioral Sciences at Duke University, Durham, N.C.
The study, called Treating Resistant Hypertension Using Lifestyle Modification to Promote Health (TRIUMPH), was published in Circulation. It is one of few that have examined lifestyle interventions in resistant hypertension, defined as systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 80 mm Hg after adherence to three or more optimally-dosed antihypertensive medications of three different classes, including one diuretic.
“This is a nice study [that] emphasizes what we often forget: Lifestyle factors, especially salt intake, are important drivers of resistant hypertension. Our own studies have shown that this is predominantly a salt retaining state, and one would expect dietary salt restriction to be particularly effective in this group of patients and that is what this study showed,” said Bryan Williams, MD, who was asked to comment on the study. Dr. Williams is chair of medicine at University College London.
The results should also be reassuring to some who worried that exercise might lead to worsened blood pressure. “This study showed that in patients with resistant hypertension, though not out of control blood pressures, exercise was not only safe, but effective in lowering their blood pressure,” said Deepak L. Bhatt, MD, MPH, who was asked to comment. He is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital, and professor of medicine at Harvard Medical School, both in Boston.
The new research isn’t unique. In August, researchers in Portugal and Brazil showed that a 12-week exercise-only intervention reduced 24-hour and daytime ambulatory systolic and diastolic blood pressure. The two studies communicate the same message. “Lifestyle changes can work for resistant hypertension. So, two independent, modest-sized studies with essentially the same, positive, actionable conclusion,” said Dr. Bhatt, adding that the next step should be a larger, multicenter trial.
In the new study, 90 patients were assigned to the diet and exercise intervention and 50 to the control group. They had a mean age of 63 years, and 48% were women. Participants attended 94% of DASH diet classes and 89% of exercise sessions, and both groups had excellent adherence to medications.
The treatment group had a greater reduction in clinic systolic BP (–12.5 versus –7.1 mm Hg; P = .005) and diastolic BP (–5.9 versus –3.7 mm Hg; P = .034), as well as 24-hour ambulatory systolic BP (–7.0 versus –0.3 mm Hg; P = .001). The treatment group also had more improvement in resting baroreflex sensitivity (2.3 versus –1.1 ms/mm Hg; P = .003), high-frequency heart rate variability (0.4 versus –0.2 ln ms2; P = .025, and flow-mediated dilation (0.3% versus –1.4%, P = .022). The two groups had similar outcomes with respect to pulse wave velocity and left ventricular mass.
“Results of the TRIUMPH study suggest that policymakers should consider resistant hypertension as a new indication for cardiac rehabilitation with appropriate coverage by governmental agencies and private insurers,” Dr. Blumenthal said.
“This is an important new, evidence-based intervention for resistant hypertension. It is safe and relatively inexpensive. It should now be something physicians routinely offer these patients. Hopefully in the future, insurers will cover cardiac rehabilitation for patients with resistant hypertension,” Dr. Bhatt said.
The study also pointed towards an effective approach for patients who may be unable to sustain lifestyle changes. “Of course, not every patient will be able to maintain a healthy diet and an exercise program on their own, thus a cardiac rehabilitation program can be an excellent way to increase the likelihood of successful lifestyle modification,” Dr. Bhatt said.
TRIUMPH was sponsored by grants from the National Heart, Lung, and Blood Institute. None of the authors had disclosures to report. Dr. Bhatt disclosed having financial relationships with more than 40 pharmaceutical companies. Dr. Williams reported having no relevant financial disclosures.
A 4-month, structured diet and exercise intervention lowered blood pressure in adults with resistant blood pressure, according to results from a randomized, clinical trial. The program also led to improvements in baroreflex sensitivity, heart rate variability, and flow-mediated dilation, compared with individuals who received only a single education session.
The intervention included instruction from a nutritionist on how to follow the Dietary Approaches to Stop Hypertension (DASH) diet, as well as restricting calories and sodium to less than 2,300 mg/day. It included weekly, 45-minute group counseling sessions, run by a clinical psychiatrist, focusing on eating behaviors. The exercise component included 30- to 45-minute sessions at 70%-85% of initial heart rate reserve, carried out three times per week at a cardiac rehabilitation facility.
“While some individuals can make the lifestyle changes on their own, a structured program of supervised exercise and dietary modification conducted by a multidisciplinary team of physicians, psychologists, nutritionists, and physical therapists/exercise physiologists found in cardiac rehabilitation programs throughout the country is likely to be more effective. There are many cardiac rehabilitation programs throughout the country that are accessible to most patients,” said lead author James Blumenthal, PhD, the J.P. Gibbons Professor in Psychiatry and Behavioral Sciences at Duke University, Durham, N.C.
The study, called Treating Resistant Hypertension Using Lifestyle Modification to Promote Health (TRIUMPH), was published in Circulation. It is one of few that have examined lifestyle interventions in resistant hypertension, defined as systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 80 mm Hg after adherence to three or more optimally-dosed antihypertensive medications of three different classes, including one diuretic.
“This is a nice study [that] emphasizes what we often forget: Lifestyle factors, especially salt intake, are important drivers of resistant hypertension. Our own studies have shown that this is predominantly a salt retaining state, and one would expect dietary salt restriction to be particularly effective in this group of patients and that is what this study showed,” said Bryan Williams, MD, who was asked to comment on the study. Dr. Williams is chair of medicine at University College London.
The results should also be reassuring to some who worried that exercise might lead to worsened blood pressure. “This study showed that in patients with resistant hypertension, though not out of control blood pressures, exercise was not only safe, but effective in lowering their blood pressure,” said Deepak L. Bhatt, MD, MPH, who was asked to comment. He is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital, and professor of medicine at Harvard Medical School, both in Boston.
The new research isn’t unique. In August, researchers in Portugal and Brazil showed that a 12-week exercise-only intervention reduced 24-hour and daytime ambulatory systolic and diastolic blood pressure. The two studies communicate the same message. “Lifestyle changes can work for resistant hypertension. So, two independent, modest-sized studies with essentially the same, positive, actionable conclusion,” said Dr. Bhatt, adding that the next step should be a larger, multicenter trial.
In the new study, 90 patients were assigned to the diet and exercise intervention and 50 to the control group. They had a mean age of 63 years, and 48% were women. Participants attended 94% of DASH diet classes and 89% of exercise sessions, and both groups had excellent adherence to medications.
The treatment group had a greater reduction in clinic systolic BP (–12.5 versus –7.1 mm Hg; P = .005) and diastolic BP (–5.9 versus –3.7 mm Hg; P = .034), as well as 24-hour ambulatory systolic BP (–7.0 versus –0.3 mm Hg; P = .001). The treatment group also had more improvement in resting baroreflex sensitivity (2.3 versus –1.1 ms/mm Hg; P = .003), high-frequency heart rate variability (0.4 versus –0.2 ln ms2; P = .025, and flow-mediated dilation (0.3% versus –1.4%, P = .022). The two groups had similar outcomes with respect to pulse wave velocity and left ventricular mass.
“Results of the TRIUMPH study suggest that policymakers should consider resistant hypertension as a new indication for cardiac rehabilitation with appropriate coverage by governmental agencies and private insurers,” Dr. Blumenthal said.
“This is an important new, evidence-based intervention for resistant hypertension. It is safe and relatively inexpensive. It should now be something physicians routinely offer these patients. Hopefully in the future, insurers will cover cardiac rehabilitation for patients with resistant hypertension,” Dr. Bhatt said.
The study also pointed towards an effective approach for patients who may be unable to sustain lifestyle changes. “Of course, not every patient will be able to maintain a healthy diet and an exercise program on their own, thus a cardiac rehabilitation program can be an excellent way to increase the likelihood of successful lifestyle modification,” Dr. Bhatt said.
TRIUMPH was sponsored by grants from the National Heart, Lung, and Blood Institute. None of the authors had disclosures to report. Dr. Bhatt disclosed having financial relationships with more than 40 pharmaceutical companies. Dr. Williams reported having no relevant financial disclosures.
FROM CIRCULATION
Updates to CDC’s STI guidelines relevant to midlife women too
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
Sexually transmitted infection rates have not increased as dramatically in older women as they have in women in their teens and 20s, but rates of chlamydia and gonorrhea in women over age 35 have seen a steady incline over the past decade, and syphilis rates have climbed steeply, according to data from the Centers for Disease Control and Prevention.
That makes the STI treatment guidelines released by the CDC in July even timelier for practitioners of menopause medicine, according to Michael S. Policar, MD, MPH, a professor emeritus of ob.gyn. and reproductive sciences at the University of California, San Francisco.
Dr. Policar discussed what clinicians need to know about STIs in midlife women at the hybrid annual meeting of the North American Menopause Society. Even the nomenclature change in the guidelines from “sexually transmitted diseases” to “sexually transmitted infections” is important “because they want to acknowledge the fact that a lot of the sexually transmitted infections that we’re treating are asymptomatic, are colonizations, and are not yet diseases,” Dr. Policar said. “We’re trying to be much more expansive in thinking about finding these infections before they actually start causing morbidity in the form of a disease.”
Sexual history
The primary guidelines update for taking sexual history is the recommendation to ask patients about their intentions regarding pregnancy. The “5 Ps” of sexual history are now Partners, Practices, Protection from STIs, Past history of STIs, and Pregnancy intention.
“There should be a sixth P that has to do with pleasure questions,” Policar added. “We ask all the time for patients that we see in the context of perimenopausal and menopausal services, ‘Are you satisfied with your sexual relationship with your partner?’ Hopefully that will make it into the CDC guidelines as the sixth P at some point, but for now, that’s aspirational.”
In asking about partners, instead of asking patients whether they have sex with men, women, or both, clinicians should ask first if the patient is having sex of any kind – oral, vaginal, or anal – with anyone. From there, providers should ask how many sex partners the patient has had, the gender(s) of the partners, and whether they or their partners have other sex partners, using more gender-inclusive language.
When asking about practices, in addition to asking about the type of sexual contact patients have had, additional questions include whether the patient met their partners online or through apps, whether they or any of their partners use drugs, and whether the patient has exchanged sex for any needs, such as money, housing, or drugs. The additional questions can identify those at higher risk for STIs.
After reviewing the CDC’s list of risk factors for gonorrhea and chlamydia screening, Dr. Policar shared the screening list from the California Department of Public Health, which he finds more helpful:
- History of gonorrhea, chlamydia, or pelvic inflammatory disease (PID) in the past 2 years.
- More than 1 sexual partner in the past year.
- New sexual partner within 90 days.
- Reason to believe that a sex partner has had other partners in the past year.
- Exchanging sex for drugs or money within the past year.
- Other factors identified locally, including prevalence of infection in the community.
STI screening guidelines
For those with a positive gonorrhea/chlamydia (GC/CT) screen, a nucleic acid amplification test (NAAT) vaginal swab is the preferred specimen source, and self-collection is fine for women of any age, Dr. Policar said. In addition, cis-women who received anal intercourse in the preceding year should consider undergoing a rectal GC/CT NAAT, and those who performed oral sex should consider a pharyngeal GC/CT NAAT, based on shared clinical decision-making. A rectal swab requires an insertion of 3-4 cm and a 360-degree twirl of the wrist, not the swab, to ensure you get a sample from the entire circumference. Pharyngeal samples require swabbing both tonsillar pillars while taking care for those who may gag.
For contact testing – asymptomatic people who have had a high-risk sexual exposure – providers should test for gonorrhea, chlamydia, HIV, and syphilis but not for herpes, high-risk HPV, hepatitis B, hepatitis C, or bacterial vaginosis. “Maybe we’ll do a screen for trichomoniasis, and maybe we’ll offer herpes type 2 serology or antibody screening,” Dr. Policar said. Providers should also ask patients requesting contact testing if they have been vaccinated for hepatitis B. If not, “the conversation should be how can we get you vaccinated for hepatitis B,” Dr. Policar said.
HIV screening only needs to occur once between the ages of 15 and 65 for low-risk people and then once annually (or more often if necessary) for those who have a sex partner with HIV, use injectable drugs, engage in commercial sex work, have a new sex partner with unknown HIV status, received care at an STD or TB clinic, or were in a correctional facility or homeless shelter.
Those at increased risk for syphilis include men who have sex with men, men under age 29, and anyone living with HIV or who has a history of incarceration or a history of commercial sex work. In addition, African Americans have the greatest risk for syphilis of racial/ethnic groups, followed by Hispanics. Most adults only require hepatitis C screening with anti-hep C antibody testing once in their lifetime. Periodic hepatitis C screening should occur for people who inject drugs. If the screening is positive, providers should conduct an RNA polymerase chain reaction (PCR) test to determine whether a chronic infection is present.
Trichomoniasis screening should occur annually in women living with HIV or in correctional facilities. Others to consider screening include people with new or multiple sex partners, a history of STIs, inconsistent condom use, a history of sex work, and intravenous drug use. Dr. Policar also noted that several new assays, including NAAT, PCR, and a rapid test, are available for trichomoniasis.
STI treatment guidelines
For women with mucoprurulent cervicitis, the cause could be chlamydia, gonorrhea, herpes, trichomonas, mycoplasma, or even progesterone from pregnancy or contraception, Dr. Policar said. The new preferred treatment is 100 mg of doxycycline. The alternative, albeit less preferred, treatment is 1 g azithromycin.
The preferred treatment for chlamydia is now 100 mg oral doxycycline twice daily, or doxycycline 200 mg delayed-release once daily, for 7 days. Alternative regimens include 1 g oral azithromycin in a single dose or 500 mg oral levofloxacin once daily for 7 days. The switch to recommending doxycycline over azithromycin is based on recent evidence showing that doxycycline has a slightly higher efficacy for urogenital chlamydia and a substantially higher efficacy for rectal chlamydia. In addition, an increasing proportion of gonorrheal infections have shown resistance to azithromycin, particularly beginning in 2014.
Preferred treatment of new, uncomplicated gonorrhea infections of the cervix, urethra, rectum, and pharynx is one 500-mg dose of ceftriaxone for those weighing under 150 kg and 1 g for those weighing 150 kg or more. If ceftriaxone is unavailable, the new alternative recommended treatment for gonorrhea is 800 mg cefixime. For pharyngeal gonorrhea only, the CDC recommends a test-of-cure 7-14 days after treatment.
For gonorrheal infections, the CDC also recommends treatment with doxycycline if chlamydia has not been excluded, but the agency no longer recommends dual therapy with azithromycin unless it’s used in place of doxycycline for those who are pregnant, have an allergy, or may not be compliant with a 7-day doxycycline regimen.
The preferred treatment for bacterial vaginosis has not changed. The new recommended regimen for trichomoniasis is 500 mg oral metronidazole for 7 days, with the alternative being a single 2-g dose of tinidazole. Male partners should receive 2 g oral metronidazole. The CDC also notes that patients taking metronidazole no longer need to abstain from alcohol during treatment.
”Another area where the guidelines changed is in their description of expedited partner therapy, which means that, when we find an index case who has gonorrhea or chlamydia, we always have a discussion with her about getting her partners treated,” Dr. Policar said. “The CDC was quite clear that the responsibility for discussing partner treatment rests with us as the diagnosing provider” since city and county health departments don’t have the time or resources for contact tracing these STIs.
The two main ways to treat partners are to have the patient bring their partner(s) to the appointment with them or to do patient-delivered partner therapy. Ideally, clinicians who dispense their own medications can give the patient enough drugs to give her partner(s) a complete dose as well. Otherwise, providers can prescribe extra doses in the index patients’ name or write prescriptions in the partner’s name.
“In every state of the union now, it is legal for you to to prescribe antibiotics for partners sight unseen, Dr. Policar said.
Margaret Sullivan, MD, an ob.gyn. from rural western North Carolina, noted during the Q&A that an obstacle to partner therapy at her practice has been cost, particularly since many of the men don’t have insurance.
“I have not heard before of prescribing the extra doses for partners under the patient’s name,” Dr. Sullivan said. “I’ve thought about doing it, but [was worried about] it potentially being fraudulent if that patient has Medicaid and we’re prescribing extra doses under her name, so how do you work around that?”
Dr. Policar acknowledged that barrier and recommended that patients use the website/app Goodrx.com to find discounts for out-of-pocket generic medications. He also noted the occasional obstacle of pharmacists balking at filling a double or triple dose.
“What we’ve been suggesting in that circumstance is to literally copy that part of the CDC guidelines, which explains expedited partner therapy or patient-delivered partner therapy and send that off to the pharmacist so they can see that it’s a national recommendation of the CDC,” Dr. Policar said.
Claudia Rodriguez, MD, an ob.gyn. who works at Sherman Hospital in Elgin, Ill., asked about the CDC recommendations for HPV vaccination in older women. Although the CDC permits women over age 26 to receive the HPV vaccine, the agency does not “make a solid recommendation to have this done, which oftentimes makes a big difference in whether or not health insurance will actually pay for vaccination in that circumstance,” Dr. Policar said.
Patients are welcome to request the vaccine after shared decision-making, but “we should never present this as something which is routine,” he said. For women in their 50s, for example, “there’s virtually no data about any additional degree of protection that you would get” from HPV vaccination, Dr. Policar said in response to a similar question from Tara Allmen, MD, an ob.gyn. in New York City. “If you ask me for my personal clinical opinion about it, I would say it’s not going to be worth it,” he said.
Dr Policar had no disclosures. Disclosures were unavailable for attendees who spoke.
FROM NAMS 2021
Top questions answered about COVID-19 boosters for your patients
Confusion continues to circulate in the wake of decisions on booster doses of the Pfizer/BioNTech COVID-19 vaccine, all announced within 1 week. Many people – including those now eligible and those who officially have to wait for their shot at a third dose – have questions.
Multiple agencies are involved in the booster decisions, and they have put out multiple – and sometimes conflicting – messages about booster doses, leaving more questions than answers for many people.
On Sept. 22, the Food and Drug Administration granted an emergency use authorization (EUA) for a booster dose of the Pfizer mRNA COVID-19 vaccine for those 65 and older and those at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection – such as frontline health care workers.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, then overruled advice from the agency’s Advisory Committee on Immunization Practices (ACIP) to recommend boosters for essential workers such as those working on the front lines during the pandemic.
As it stands now, the CDC recommends that the following groups should get a third dose of the Pfizer vaccine:
- People aged 65 years and older.
- People aged 18 years and older in long-term care settings.
- People aged 50-64 years with underlying medical conditions.
The CDC also recommends that the following groups may receive a booster shot of the Pfizer vaccine, based on their individual benefits and risks:
- People aged 18-49 years with underlying medical conditions.
- People aged 18-64 years at increased risk for COVID-19 exposure and transmission because of occupational or institutional setting.
The CDC currently considers the following groups at increased risk for COVID-19:
- First responders (health care workers, firefighters, police, congregate care staff).
- Education staff (teachers, support staff, day care workers).
- Food and agriculture workers.
- Manufacturing workers.
- Corrections workers.
- U.S. Postal Service workers.
- Public transit workers.
- Grocery store workers.
Health care professionals, among the most trusted sources of COVID-19 information, are likely to encounter a number of patients wondering how all this will work.
“It’s fantastic that boosters will be available for those who the data supports need [them],” Rachael Piltch-Loeb, PhD, said during a media briefing on Sept. 23, held between the FDA and CDC decisions.
“But we’re really in a place where we have a lot more questions and answers about what the next phase of the vaccine availability and updates are going to be in the United States,” added Dr. Piltch-Loeb, preparedness fellow in the division of policy translation and leadership development and a research associate in the department of biostatistics at the Harvard T. H. Chan School of Public Health in Boston.
1. What is the biggest concern you are hearing from patients about getting a booster?
“The biggest concerns are that everyone wants it and they don’t know where to get it. In health care’s defense, the CDC just figured out what to do,” said Janet Englund, MD, professor of pediatric infectious diseases and an infectious disease and virology expert at Seattle Children’s Hospital in Washington.
“Everyone thinks they should be eligible for a booster ... people in their 50s who are not yet 65+, people with young grandchildren, etc.,” she added. “I’m at Seattle Children’s Hospital, so people are asking about booster shots and about getting their children vaccinated.”
Boosters for all COVID-19 vaccines are completely free.
“All COVID-19 vaccines, including booster doses, will be provided free of charge to the U.S. population,” the CDC has said.
2. Will patients need to prove they meet eligibility criteria for a booster shot or will it be the honor system?
“No, patients will only need to attest that they fall into one of the high-risk groups for whom a booster vaccine is authorized,” said Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston.
Dr. Piltch-Loeb agreed. “It is likely to be an honor system. It is very unlikely that there will be punishments or other ramifications ... if doses are administered, beyond the approved usage.”
3. If a patient who had the Moderna or the Johnson and Johnson vaccination requests a booster, can health care workers give them Pfizer?
The short answer is no. “This only applies to individuals who have received the Pfizer vaccine,” Dr. Piltch-Loeb said.
More data will be needed before other vaccine boosters are authorized, she added.
“My understanding is the Moderna people have just recently submitted their information, all of their data to the FDA and J&J is in line to do that very shortly,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University in Nashville, Tenn. “I would hope that within the next month to 6 weeks, we will get information about both of those vaccines,” Dr. Schaffner said.
4. When are the “mix-and-match” vaccine study results expected to come out?
“We expect that data from the study will be available in the coming weeks,” said Dr. Atmar, who is the national co-principal investigator of a mix-and-match booster trial launched in June 2021.
5. Are side effects of a booster vaccine expected to be about the same as what people experienced during their first or second immunization?
“I’m expecting the side effects will be similar to the second dose,” Dr. Englund said.
“The data presented ... at ACIP suggests that the side effects from the third shot are either the same or actually less than the first two shots,” said Carlos del Rio, MD, distinguished professor of medicine, epidemiology, and global health, and executive associate dean of Emory University School of Medicine at Grady Health System in Atlanta.
”Everyone reacts very differently to vaccines, regardless of vaccine type,” said Eric Ascher, MD, a family medicine physician at Lenox Hill Hospital in New York City. “I have had patients (as well as personal experience) where there were none to minimal symptoms, and others who felt they had a mild flu for 24 hours.”
“I expect no side effects greater than what was felt with you prior doses,” he said. “The vaccine is very safe and the benefit of vaccination outweighs the risks of any mild side effects.”
6. Is it unethical to give a booster to someone outside the approved groups if there are doses remaining at the end of the day in an open vial?
“Offering a booster shot to someone outside of approved groups if remaining doses will go to waste at the end of the day seems like a prudent decision, and relatively harmless action,” said Faith Fletcher, PhD, assistant professor at the Center for Medical Ethics and Health Policy at Baylor College of Medicine.
“However, if doses continue to fall in the laps of unapproved groups, we must evaluate the vaccine systems and structures that advantage some groups and disadvantage others,” she added. “We know that the distribution of COVID-19 vaccines has not been equitable – and some groups have been left behind.”
“I am not an ethicist and there are many competing concerns that this question addresses,” Dr. Atmar said. For example, “there is not a limitation of vaccine supply in the U.S., so that using leftover vaccine to prevent waste is no longer a major concern in the U.S.”
It could be more of a legal than ethical question, Dr. Atmar said. For an individual outside the authorized groups, legally, the FDA’s EUA for boosting does not allow the vaccine to be administered to this person, he said.
“The rationale for the restricted use in the EUA is that at this time the safety and risks associated with such administration are not known, and the benefits also have not been determined,” Dr. Atmar said. “Members of the ACIP raised concerns about other individuals who may potentially benefit from a booster but are not eligible and the importance of making boosters available to them, but from a legal standpoint – I am also not a lawyer, so this is my understanding – administration of the vaccine is limited to those identified in the EUA.”
7. What is the likelihood that one shot will combine COVID and flu protection in the near future?
It is not likely, Dr. Englund said. “The reason is that the flu vaccine changes so much, and it already has four different antigens. This is assuming we keep the same method of making the flu vaccine – the answer could be different if the flu vaccine becomes an mRNA vaccine in the future.”
Companies such as Moderna and Novavax are testing single-dose shots for COVID-19 and influenza, but they are still far from having anything ready for this flu season in the United States.
8. Is there any chance a booster shot distributed now will need to be redesigned for a future variant?
“Absolutely,” Dr. Englund said. “And a booster dose is the time we may want to consider re-engineering a vaccine.”
9. Do you think the FDA/CDC limitations on who is eligible for a booster was in any way influenced by the World Health Organization call for prioritizing shots for the unvaccinated in lower-resource countries?
“This is absolutely still a global problem,” Dr. Piltch-Loeb said. “We need to get more vaccine to more countries and more people as soon as possible, because if there’s anything we’ve seen about the variants it is that ... they can come from all different places.”
“That being said, I think that it is unlikely to change the course of action in the U.S.,” she added, when it comes to comparing the global need with the domestic policy priorities of the administration.
Dr. Atmar was more direct. “No,” he said. “The WHO recommends against boosting of anyone. The U.S. decisions about boosting those in this country who are eligible are aimed toward addressing perceived needs domestically at the same time that vaccines are being provided to other countries.
“The philosophy is to address both ‘needs’ at the same time,” Dr. Atmar said.
10. What does the future hold for booster shots?
“Predicting the future is really hard, especially when it involves COVID,” Dr. del Rio said.
“Having said that, COVID is not the flu, so I doubt there will be need for annual boosters. I think the population eligible for boosters will be expanded ... and the major population not addressed at this point is the people that received either Moderna or J&J [vaccines].”
Kelly Davis contributed to this feature. A version of this article first appeared on Medscape.com.
Confusion continues to circulate in the wake of decisions on booster doses of the Pfizer/BioNTech COVID-19 vaccine, all announced within 1 week. Many people – including those now eligible and those who officially have to wait for their shot at a third dose – have questions.
Multiple agencies are involved in the booster decisions, and they have put out multiple – and sometimes conflicting – messages about booster doses, leaving more questions than answers for many people.
On Sept. 22, the Food and Drug Administration granted an emergency use authorization (EUA) for a booster dose of the Pfizer mRNA COVID-19 vaccine for those 65 and older and those at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection – such as frontline health care workers.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, then overruled advice from the agency’s Advisory Committee on Immunization Practices (ACIP) to recommend boosters for essential workers such as those working on the front lines during the pandemic.
As it stands now, the CDC recommends that the following groups should get a third dose of the Pfizer vaccine:
- People aged 65 years and older.
- People aged 18 years and older in long-term care settings.
- People aged 50-64 years with underlying medical conditions.
The CDC also recommends that the following groups may receive a booster shot of the Pfizer vaccine, based on their individual benefits and risks:
- People aged 18-49 years with underlying medical conditions.
- People aged 18-64 years at increased risk for COVID-19 exposure and transmission because of occupational or institutional setting.
The CDC currently considers the following groups at increased risk for COVID-19:
- First responders (health care workers, firefighters, police, congregate care staff).
- Education staff (teachers, support staff, day care workers).
- Food and agriculture workers.
- Manufacturing workers.
- Corrections workers.
- U.S. Postal Service workers.
- Public transit workers.
- Grocery store workers.
Health care professionals, among the most trusted sources of COVID-19 information, are likely to encounter a number of patients wondering how all this will work.
“It’s fantastic that boosters will be available for those who the data supports need [them],” Rachael Piltch-Loeb, PhD, said during a media briefing on Sept. 23, held between the FDA and CDC decisions.
“But we’re really in a place where we have a lot more questions and answers about what the next phase of the vaccine availability and updates are going to be in the United States,” added Dr. Piltch-Loeb, preparedness fellow in the division of policy translation and leadership development and a research associate in the department of biostatistics at the Harvard T. H. Chan School of Public Health in Boston.
1. What is the biggest concern you are hearing from patients about getting a booster?
“The biggest concerns are that everyone wants it and they don’t know where to get it. In health care’s defense, the CDC just figured out what to do,” said Janet Englund, MD, professor of pediatric infectious diseases and an infectious disease and virology expert at Seattle Children’s Hospital in Washington.
“Everyone thinks they should be eligible for a booster ... people in their 50s who are not yet 65+, people with young grandchildren, etc.,” she added. “I’m at Seattle Children’s Hospital, so people are asking about booster shots and about getting their children vaccinated.”
Boosters for all COVID-19 vaccines are completely free.
“All COVID-19 vaccines, including booster doses, will be provided free of charge to the U.S. population,” the CDC has said.
2. Will patients need to prove they meet eligibility criteria for a booster shot or will it be the honor system?
“No, patients will only need to attest that they fall into one of the high-risk groups for whom a booster vaccine is authorized,” said Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston.
Dr. Piltch-Loeb agreed. “It is likely to be an honor system. It is very unlikely that there will be punishments or other ramifications ... if doses are administered, beyond the approved usage.”
3. If a patient who had the Moderna or the Johnson and Johnson vaccination requests a booster, can health care workers give them Pfizer?
The short answer is no. “This only applies to individuals who have received the Pfizer vaccine,” Dr. Piltch-Loeb said.
More data will be needed before other vaccine boosters are authorized, she added.
“My understanding is the Moderna people have just recently submitted their information, all of their data to the FDA and J&J is in line to do that very shortly,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University in Nashville, Tenn. “I would hope that within the next month to 6 weeks, we will get information about both of those vaccines,” Dr. Schaffner said.
4. When are the “mix-and-match” vaccine study results expected to come out?
“We expect that data from the study will be available in the coming weeks,” said Dr. Atmar, who is the national co-principal investigator of a mix-and-match booster trial launched in June 2021.
5. Are side effects of a booster vaccine expected to be about the same as what people experienced during their first or second immunization?
“I’m expecting the side effects will be similar to the second dose,” Dr. Englund said.
“The data presented ... at ACIP suggests that the side effects from the third shot are either the same or actually less than the first two shots,” said Carlos del Rio, MD, distinguished professor of medicine, epidemiology, and global health, and executive associate dean of Emory University School of Medicine at Grady Health System in Atlanta.
”Everyone reacts very differently to vaccines, regardless of vaccine type,” said Eric Ascher, MD, a family medicine physician at Lenox Hill Hospital in New York City. “I have had patients (as well as personal experience) where there were none to minimal symptoms, and others who felt they had a mild flu for 24 hours.”
“I expect no side effects greater than what was felt with you prior doses,” he said. “The vaccine is very safe and the benefit of vaccination outweighs the risks of any mild side effects.”
6. Is it unethical to give a booster to someone outside the approved groups if there are doses remaining at the end of the day in an open vial?
“Offering a booster shot to someone outside of approved groups if remaining doses will go to waste at the end of the day seems like a prudent decision, and relatively harmless action,” said Faith Fletcher, PhD, assistant professor at the Center for Medical Ethics and Health Policy at Baylor College of Medicine.
“However, if doses continue to fall in the laps of unapproved groups, we must evaluate the vaccine systems and structures that advantage some groups and disadvantage others,” she added. “We know that the distribution of COVID-19 vaccines has not been equitable – and some groups have been left behind.”
“I am not an ethicist and there are many competing concerns that this question addresses,” Dr. Atmar said. For example, “there is not a limitation of vaccine supply in the U.S., so that using leftover vaccine to prevent waste is no longer a major concern in the U.S.”
It could be more of a legal than ethical question, Dr. Atmar said. For an individual outside the authorized groups, legally, the FDA’s EUA for boosting does not allow the vaccine to be administered to this person, he said.
“The rationale for the restricted use in the EUA is that at this time the safety and risks associated with such administration are not known, and the benefits also have not been determined,” Dr. Atmar said. “Members of the ACIP raised concerns about other individuals who may potentially benefit from a booster but are not eligible and the importance of making boosters available to them, but from a legal standpoint – I am also not a lawyer, so this is my understanding – administration of the vaccine is limited to those identified in the EUA.”
7. What is the likelihood that one shot will combine COVID and flu protection in the near future?
It is not likely, Dr. Englund said. “The reason is that the flu vaccine changes so much, and it already has four different antigens. This is assuming we keep the same method of making the flu vaccine – the answer could be different if the flu vaccine becomes an mRNA vaccine in the future.”
Companies such as Moderna and Novavax are testing single-dose shots for COVID-19 and influenza, but they are still far from having anything ready for this flu season in the United States.
8. Is there any chance a booster shot distributed now will need to be redesigned for a future variant?
“Absolutely,” Dr. Englund said. “And a booster dose is the time we may want to consider re-engineering a vaccine.”
9. Do you think the FDA/CDC limitations on who is eligible for a booster was in any way influenced by the World Health Organization call for prioritizing shots for the unvaccinated in lower-resource countries?
“This is absolutely still a global problem,” Dr. Piltch-Loeb said. “We need to get more vaccine to more countries and more people as soon as possible, because if there’s anything we’ve seen about the variants it is that ... they can come from all different places.”
“That being said, I think that it is unlikely to change the course of action in the U.S.,” she added, when it comes to comparing the global need with the domestic policy priorities of the administration.
Dr. Atmar was more direct. “No,” he said. “The WHO recommends against boosting of anyone. The U.S. decisions about boosting those in this country who are eligible are aimed toward addressing perceived needs domestically at the same time that vaccines are being provided to other countries.
“The philosophy is to address both ‘needs’ at the same time,” Dr. Atmar said.
10. What does the future hold for booster shots?
“Predicting the future is really hard, especially when it involves COVID,” Dr. del Rio said.
“Having said that, COVID is not the flu, so I doubt there will be need for annual boosters. I think the population eligible for boosters will be expanded ... and the major population not addressed at this point is the people that received either Moderna or J&J [vaccines].”
Kelly Davis contributed to this feature. A version of this article first appeared on Medscape.com.
Confusion continues to circulate in the wake of decisions on booster doses of the Pfizer/BioNTech COVID-19 vaccine, all announced within 1 week. Many people – including those now eligible and those who officially have to wait for their shot at a third dose – have questions.
Multiple agencies are involved in the booster decisions, and they have put out multiple – and sometimes conflicting – messages about booster doses, leaving more questions than answers for many people.
On Sept. 22, the Food and Drug Administration granted an emergency use authorization (EUA) for a booster dose of the Pfizer mRNA COVID-19 vaccine for those 65 and older and those at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection – such as frontline health care workers.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, then overruled advice from the agency’s Advisory Committee on Immunization Practices (ACIP) to recommend boosters for essential workers such as those working on the front lines during the pandemic.
As it stands now, the CDC recommends that the following groups should get a third dose of the Pfizer vaccine:
- People aged 65 years and older.
- People aged 18 years and older in long-term care settings.
- People aged 50-64 years with underlying medical conditions.
The CDC also recommends that the following groups may receive a booster shot of the Pfizer vaccine, based on their individual benefits and risks:
- People aged 18-49 years with underlying medical conditions.
- People aged 18-64 years at increased risk for COVID-19 exposure and transmission because of occupational or institutional setting.
The CDC currently considers the following groups at increased risk for COVID-19:
- First responders (health care workers, firefighters, police, congregate care staff).
- Education staff (teachers, support staff, day care workers).
- Food and agriculture workers.
- Manufacturing workers.
- Corrections workers.
- U.S. Postal Service workers.
- Public transit workers.
- Grocery store workers.
Health care professionals, among the most trusted sources of COVID-19 information, are likely to encounter a number of patients wondering how all this will work.
“It’s fantastic that boosters will be available for those who the data supports need [them],” Rachael Piltch-Loeb, PhD, said during a media briefing on Sept. 23, held between the FDA and CDC decisions.
“But we’re really in a place where we have a lot more questions and answers about what the next phase of the vaccine availability and updates are going to be in the United States,” added Dr. Piltch-Loeb, preparedness fellow in the division of policy translation and leadership development and a research associate in the department of biostatistics at the Harvard T. H. Chan School of Public Health in Boston.
1. What is the biggest concern you are hearing from patients about getting a booster?
“The biggest concerns are that everyone wants it and they don’t know where to get it. In health care’s defense, the CDC just figured out what to do,” said Janet Englund, MD, professor of pediatric infectious diseases and an infectious disease and virology expert at Seattle Children’s Hospital in Washington.
“Everyone thinks they should be eligible for a booster ... people in their 50s who are not yet 65+, people with young grandchildren, etc.,” she added. “I’m at Seattle Children’s Hospital, so people are asking about booster shots and about getting their children vaccinated.”
Boosters for all COVID-19 vaccines are completely free.
“All COVID-19 vaccines, including booster doses, will be provided free of charge to the U.S. population,” the CDC has said.
2. Will patients need to prove they meet eligibility criteria for a booster shot or will it be the honor system?
“No, patients will only need to attest that they fall into one of the high-risk groups for whom a booster vaccine is authorized,” said Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston.
Dr. Piltch-Loeb agreed. “It is likely to be an honor system. It is very unlikely that there will be punishments or other ramifications ... if doses are administered, beyond the approved usage.”
3. If a patient who had the Moderna or the Johnson and Johnson vaccination requests a booster, can health care workers give them Pfizer?
The short answer is no. “This only applies to individuals who have received the Pfizer vaccine,” Dr. Piltch-Loeb said.
More data will be needed before other vaccine boosters are authorized, she added.
“My understanding is the Moderna people have just recently submitted their information, all of their data to the FDA and J&J is in line to do that very shortly,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University in Nashville, Tenn. “I would hope that within the next month to 6 weeks, we will get information about both of those vaccines,” Dr. Schaffner said.
4. When are the “mix-and-match” vaccine study results expected to come out?
“We expect that data from the study will be available in the coming weeks,” said Dr. Atmar, who is the national co-principal investigator of a mix-and-match booster trial launched in June 2021.
5. Are side effects of a booster vaccine expected to be about the same as what people experienced during their first or second immunization?
“I’m expecting the side effects will be similar to the second dose,” Dr. Englund said.
“The data presented ... at ACIP suggests that the side effects from the third shot are either the same or actually less than the first two shots,” said Carlos del Rio, MD, distinguished professor of medicine, epidemiology, and global health, and executive associate dean of Emory University School of Medicine at Grady Health System in Atlanta.
”Everyone reacts very differently to vaccines, regardless of vaccine type,” said Eric Ascher, MD, a family medicine physician at Lenox Hill Hospital in New York City. “I have had patients (as well as personal experience) where there were none to minimal symptoms, and others who felt they had a mild flu for 24 hours.”
“I expect no side effects greater than what was felt with you prior doses,” he said. “The vaccine is very safe and the benefit of vaccination outweighs the risks of any mild side effects.”
6. Is it unethical to give a booster to someone outside the approved groups if there are doses remaining at the end of the day in an open vial?
“Offering a booster shot to someone outside of approved groups if remaining doses will go to waste at the end of the day seems like a prudent decision, and relatively harmless action,” said Faith Fletcher, PhD, assistant professor at the Center for Medical Ethics and Health Policy at Baylor College of Medicine.
“However, if doses continue to fall in the laps of unapproved groups, we must evaluate the vaccine systems and structures that advantage some groups and disadvantage others,” she added. “We know that the distribution of COVID-19 vaccines has not been equitable – and some groups have been left behind.”
“I am not an ethicist and there are many competing concerns that this question addresses,” Dr. Atmar said. For example, “there is not a limitation of vaccine supply in the U.S., so that using leftover vaccine to prevent waste is no longer a major concern in the U.S.”
It could be more of a legal than ethical question, Dr. Atmar said. For an individual outside the authorized groups, legally, the FDA’s EUA for boosting does not allow the vaccine to be administered to this person, he said.
“The rationale for the restricted use in the EUA is that at this time the safety and risks associated with such administration are not known, and the benefits also have not been determined,” Dr. Atmar said. “Members of the ACIP raised concerns about other individuals who may potentially benefit from a booster but are not eligible and the importance of making boosters available to them, but from a legal standpoint – I am also not a lawyer, so this is my understanding – administration of the vaccine is limited to those identified in the EUA.”
7. What is the likelihood that one shot will combine COVID and flu protection in the near future?
It is not likely, Dr. Englund said. “The reason is that the flu vaccine changes so much, and it already has four different antigens. This is assuming we keep the same method of making the flu vaccine – the answer could be different if the flu vaccine becomes an mRNA vaccine in the future.”
Companies such as Moderna and Novavax are testing single-dose shots for COVID-19 and influenza, but they are still far from having anything ready for this flu season in the United States.
8. Is there any chance a booster shot distributed now will need to be redesigned for a future variant?
“Absolutely,” Dr. Englund said. “And a booster dose is the time we may want to consider re-engineering a vaccine.”
9. Do you think the FDA/CDC limitations on who is eligible for a booster was in any way influenced by the World Health Organization call for prioritizing shots for the unvaccinated in lower-resource countries?
“This is absolutely still a global problem,” Dr. Piltch-Loeb said. “We need to get more vaccine to more countries and more people as soon as possible, because if there’s anything we’ve seen about the variants it is that ... they can come from all different places.”
“That being said, I think that it is unlikely to change the course of action in the U.S.,” she added, when it comes to comparing the global need with the domestic policy priorities of the administration.
Dr. Atmar was more direct. “No,” he said. “The WHO recommends against boosting of anyone. The U.S. decisions about boosting those in this country who are eligible are aimed toward addressing perceived needs domestically at the same time that vaccines are being provided to other countries.
“The philosophy is to address both ‘needs’ at the same time,” Dr. Atmar said.
10. What does the future hold for booster shots?
“Predicting the future is really hard, especially when it involves COVID,” Dr. del Rio said.
“Having said that, COVID is not the flu, so I doubt there will be need for annual boosters. I think the population eligible for boosters will be expanded ... and the major population not addressed at this point is the people that received either Moderna or J&J [vaccines].”
Kelly Davis contributed to this feature. A version of this article first appeared on Medscape.com.
ACOG amicus brief supports case against Mississippi abortion ban
The American College of Obstetricians and Gynecologists (ACOG), took a prominent stand in the battle over abortion legislation by filing an amicus brief to the United States Supreme Court in the case of Dobbs v. Jackson Women’s Health Organization, according to a statement from ACOG issued on Sept. 21.
The case, filed by Thomas E. Dobbs, MD, state health officer of the Mississippi Department of Health, and others, appeals the decision by the U.S. Court of Appeals for the Fifth Circuit to throw out Mississippi’s law banning abortion after 15 weeks of pregnancy.*
ACOG’s amicus brief, which was signed by 24 additional medical organizations, including the American Medical Association, “represents an unprecedented level of support from a diverse group of physicians, nurses, and other health care professionals, which demonstrates the concrete medical consensus of opposition to abortion restriction legislation such as the law at the heart of Dobbs v. Jackson,” according to the ACOG statement.
The brief explains how the ban goes against not only the ability of health professionals to provide safe and essential care, but also goes against scientific evidence and medical ethics. “By preventing clinicians from providing patients with necessary medical care, the ban represents gross interference in the patient-clinician relationship,” according to the ACOG brief.
Potential implications if the ban is upheld include health risks to pregnant women at or near 15 weeks’ gestation, who might be forced to travel out of state, attempt self-induced abortion, or carry a pregnancy to term, according to the brief.
“Each of these outcomes increases the likelihood of negative consequences to a woman’s physical and psychological health that could be avoided if care were available,” according to the brief.
The brief also emphasizes that the ban will have a disproportionate effect on women who are already at risk for being medically underserved and who make up a majority of women seeking abortion: women of color, women in rural areas, and women with limited financial resources.
“This law is an example of harmful legislative interference into the practice of medicine,” said ACOG President J. Martin Tucker, MD, FACOG, on behalf of ACOG, in the statement.
“The outcome of this case could overturn decades of legal precedent that safeguards safe, legal abortion before viability, and the consequences of this case have national implications,” said Maureen G. Phipps, MD, MPH, CEO of ACOG, in an interview, as reported by ACOG press person Kate Connors.
“If the court does not strike down this law, clinicians in states across the country may face similar restrictions in their ability to provide necessary, evidence-based medical care,” Dr. Phipps explained. “If states are allowed to create new laws that further restrict abortion access, patients and families across the country will suffer,” she said.
“We hope that the Supreme Court will respond to the arguments of our brief and to the remarkable medical consensus represented by 25 organization signing the brief,” Dr. Phipps said. “We will continue educating and working through the judicial system in support of our patients’ access to evidence-based care and in opposition to legislative interference in the practice of medicine,” she emphasized.
Other medical organizations that signed the brief in support of the case against the Mississippi abortion ban included the American Academy of Pediatrics, the American Association of Family Physicians, the American College of Nurse Midwives, the American College of Physicians, the American Psychological Association, the American Society for Reproductive Medicine, the Association of Women’s Health, Obstetric and Neonatal Nurses, the American Medical Women’s Association, the Council of University Chairs of Obstetrics and Gynecology, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, the Society of OB/GYN Hospitalists, and the Society of Family Planning.
*This story was updated on 10/7/2021.
The American College of Obstetricians and Gynecologists (ACOG), took a prominent stand in the battle over abortion legislation by filing an amicus brief to the United States Supreme Court in the case of Dobbs v. Jackson Women’s Health Organization, according to a statement from ACOG issued on Sept. 21.
The case, filed by Thomas E. Dobbs, MD, state health officer of the Mississippi Department of Health, and others, appeals the decision by the U.S. Court of Appeals for the Fifth Circuit to throw out Mississippi’s law banning abortion after 15 weeks of pregnancy.*
ACOG’s amicus brief, which was signed by 24 additional medical organizations, including the American Medical Association, “represents an unprecedented level of support from a diverse group of physicians, nurses, and other health care professionals, which demonstrates the concrete medical consensus of opposition to abortion restriction legislation such as the law at the heart of Dobbs v. Jackson,” according to the ACOG statement.
The brief explains how the ban goes against not only the ability of health professionals to provide safe and essential care, but also goes against scientific evidence and medical ethics. “By preventing clinicians from providing patients with necessary medical care, the ban represents gross interference in the patient-clinician relationship,” according to the ACOG brief.
Potential implications if the ban is upheld include health risks to pregnant women at or near 15 weeks’ gestation, who might be forced to travel out of state, attempt self-induced abortion, or carry a pregnancy to term, according to the brief.
“Each of these outcomes increases the likelihood of negative consequences to a woman’s physical and psychological health that could be avoided if care were available,” according to the brief.
The brief also emphasizes that the ban will have a disproportionate effect on women who are already at risk for being medically underserved and who make up a majority of women seeking abortion: women of color, women in rural areas, and women with limited financial resources.
“This law is an example of harmful legislative interference into the practice of medicine,” said ACOG President J. Martin Tucker, MD, FACOG, on behalf of ACOG, in the statement.
“The outcome of this case could overturn decades of legal precedent that safeguards safe, legal abortion before viability, and the consequences of this case have national implications,” said Maureen G. Phipps, MD, MPH, CEO of ACOG, in an interview, as reported by ACOG press person Kate Connors.
“If the court does not strike down this law, clinicians in states across the country may face similar restrictions in their ability to provide necessary, evidence-based medical care,” Dr. Phipps explained. “If states are allowed to create new laws that further restrict abortion access, patients and families across the country will suffer,” she said.
“We hope that the Supreme Court will respond to the arguments of our brief and to the remarkable medical consensus represented by 25 organization signing the brief,” Dr. Phipps said. “We will continue educating and working through the judicial system in support of our patients’ access to evidence-based care and in opposition to legislative interference in the practice of medicine,” she emphasized.
Other medical organizations that signed the brief in support of the case against the Mississippi abortion ban included the American Academy of Pediatrics, the American Association of Family Physicians, the American College of Nurse Midwives, the American College of Physicians, the American Psychological Association, the American Society for Reproductive Medicine, the Association of Women’s Health, Obstetric and Neonatal Nurses, the American Medical Women’s Association, the Council of University Chairs of Obstetrics and Gynecology, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, the Society of OB/GYN Hospitalists, and the Society of Family Planning.
*This story was updated on 10/7/2021.
The American College of Obstetricians and Gynecologists (ACOG), took a prominent stand in the battle over abortion legislation by filing an amicus brief to the United States Supreme Court in the case of Dobbs v. Jackson Women’s Health Organization, according to a statement from ACOG issued on Sept. 21.
The case, filed by Thomas E. Dobbs, MD, state health officer of the Mississippi Department of Health, and others, appeals the decision by the U.S. Court of Appeals for the Fifth Circuit to throw out Mississippi’s law banning abortion after 15 weeks of pregnancy.*
ACOG’s amicus brief, which was signed by 24 additional medical organizations, including the American Medical Association, “represents an unprecedented level of support from a diverse group of physicians, nurses, and other health care professionals, which demonstrates the concrete medical consensus of opposition to abortion restriction legislation such as the law at the heart of Dobbs v. Jackson,” according to the ACOG statement.
The brief explains how the ban goes against not only the ability of health professionals to provide safe and essential care, but also goes against scientific evidence and medical ethics. “By preventing clinicians from providing patients with necessary medical care, the ban represents gross interference in the patient-clinician relationship,” according to the ACOG brief.
Potential implications if the ban is upheld include health risks to pregnant women at or near 15 weeks’ gestation, who might be forced to travel out of state, attempt self-induced abortion, or carry a pregnancy to term, according to the brief.
“Each of these outcomes increases the likelihood of negative consequences to a woman’s physical and psychological health that could be avoided if care were available,” according to the brief.
The brief also emphasizes that the ban will have a disproportionate effect on women who are already at risk for being medically underserved and who make up a majority of women seeking abortion: women of color, women in rural areas, and women with limited financial resources.
“This law is an example of harmful legislative interference into the practice of medicine,” said ACOG President J. Martin Tucker, MD, FACOG, on behalf of ACOG, in the statement.
“The outcome of this case could overturn decades of legal precedent that safeguards safe, legal abortion before viability, and the consequences of this case have national implications,” said Maureen G. Phipps, MD, MPH, CEO of ACOG, in an interview, as reported by ACOG press person Kate Connors.
“If the court does not strike down this law, clinicians in states across the country may face similar restrictions in their ability to provide necessary, evidence-based medical care,” Dr. Phipps explained. “If states are allowed to create new laws that further restrict abortion access, patients and families across the country will suffer,” she said.
“We hope that the Supreme Court will respond to the arguments of our brief and to the remarkable medical consensus represented by 25 organization signing the brief,” Dr. Phipps said. “We will continue educating and working through the judicial system in support of our patients’ access to evidence-based care and in opposition to legislative interference in the practice of medicine,” she emphasized.
Other medical organizations that signed the brief in support of the case against the Mississippi abortion ban included the American Academy of Pediatrics, the American Association of Family Physicians, the American College of Nurse Midwives, the American College of Physicians, the American Psychological Association, the American Society for Reproductive Medicine, the Association of Women’s Health, Obstetric and Neonatal Nurses, the American Medical Women’s Association, the Council of University Chairs of Obstetrics and Gynecology, the National Association of Nurse Practitioners in Women’s Health, the North American Society for Pediatric and Adolescent Gynecology, the Society of OB/GYN Hospitalists, and the Society of Family Planning.
*This story was updated on 10/7/2021.
New virus causing ‘Alaskapox’ detected in two more cases
Both people were diagnosed after receiving urgent care in a Fairbanks-area clinic. One was a child with a sore on the left elbow, along with fever and swollen lymph nodes. And the other was an unrelated middle-aged woman with a pox mark on her leg, swollen lymph nodes, and joint pain. In both cases, symptoms improved within 3 weeks.
This isn’t the first time the so-called Alaskapox virus has been detected in the region. In 2015, a woman living near Fairbanks turned up at her doctor’s office with a single reddened pox-like mark on her upper arm and a feeling of fatigue.
Sampling of the pox mark showed that it was caused by a previously unidentified virus of the same family as smallpox and cowpox.
Five years later, another woman showed up with similar signs and symptoms, and her pox also proved to be the result of what public health experts started calling the Alaskapox virus.
In both cases, the women recovered completely.
Smallpox-like illness
Public health sleuths figured out that in three of the four cases, the patients lived in a home with a cat or cats, and one of these cats was known to hunt small animals.
Experts already knew that cats mingling in cow pastures and sickened by cattle virus had helped cowpox make the leap from bovines to humans. And just as in the case of cowpox, they suspected that cats might again be spreading this new virus to people, too.
All four of the infected people lived in sparsely populated areas amid forests. Officials laid animal traps where some of the affected people lived and identified the virus in several species of small wild animals.
The animals that turned up most often with Alaskapox were small mouse-like voles. The rodents with rounded muzzles are known for burrowing in the region. And scientists suspect the Alaskapox virus makes its way from these wild animals to humans through their pet cats or possibly by direct exposure outdoors.
None of the four people identified so far with Alaskapox knew each other or interacted, so officials also suspect that there are more cases going unrecognized, possibly because the symptoms are mild or nonexistent.
There are no documented cases of person-to-person transmission of Alaskapox, according to public health officials monitoring the small number of cases. But other pox viruses can spread by direct contact with skin lesions, so clinicians are recommending that people cover wounds with bandages. Three of the people with Alaskapox mistook their lesions at first for a bite from a spider or insect.
A version of this article first appeared on WebMD.com.
Both people were diagnosed after receiving urgent care in a Fairbanks-area clinic. One was a child with a sore on the left elbow, along with fever and swollen lymph nodes. And the other was an unrelated middle-aged woman with a pox mark on her leg, swollen lymph nodes, and joint pain. In both cases, symptoms improved within 3 weeks.
This isn’t the first time the so-called Alaskapox virus has been detected in the region. In 2015, a woman living near Fairbanks turned up at her doctor’s office with a single reddened pox-like mark on her upper arm and a feeling of fatigue.
Sampling of the pox mark showed that it was caused by a previously unidentified virus of the same family as smallpox and cowpox.
Five years later, another woman showed up with similar signs and symptoms, and her pox also proved to be the result of what public health experts started calling the Alaskapox virus.
In both cases, the women recovered completely.
Smallpox-like illness
Public health sleuths figured out that in three of the four cases, the patients lived in a home with a cat or cats, and one of these cats was known to hunt small animals.
Experts already knew that cats mingling in cow pastures and sickened by cattle virus had helped cowpox make the leap from bovines to humans. And just as in the case of cowpox, they suspected that cats might again be spreading this new virus to people, too.
All four of the infected people lived in sparsely populated areas amid forests. Officials laid animal traps where some of the affected people lived and identified the virus in several species of small wild animals.
The animals that turned up most often with Alaskapox were small mouse-like voles. The rodents with rounded muzzles are known for burrowing in the region. And scientists suspect the Alaskapox virus makes its way from these wild animals to humans through their pet cats or possibly by direct exposure outdoors.
None of the four people identified so far with Alaskapox knew each other or interacted, so officials also suspect that there are more cases going unrecognized, possibly because the symptoms are mild or nonexistent.
There are no documented cases of person-to-person transmission of Alaskapox, according to public health officials monitoring the small number of cases. But other pox viruses can spread by direct contact with skin lesions, so clinicians are recommending that people cover wounds with bandages. Three of the people with Alaskapox mistook their lesions at first for a bite from a spider or insect.
A version of this article first appeared on WebMD.com.
Both people were diagnosed after receiving urgent care in a Fairbanks-area clinic. One was a child with a sore on the left elbow, along with fever and swollen lymph nodes. And the other was an unrelated middle-aged woman with a pox mark on her leg, swollen lymph nodes, and joint pain. In both cases, symptoms improved within 3 weeks.
This isn’t the first time the so-called Alaskapox virus has been detected in the region. In 2015, a woman living near Fairbanks turned up at her doctor’s office with a single reddened pox-like mark on her upper arm and a feeling of fatigue.
Sampling of the pox mark showed that it was caused by a previously unidentified virus of the same family as smallpox and cowpox.
Five years later, another woman showed up with similar signs and symptoms, and her pox also proved to be the result of what public health experts started calling the Alaskapox virus.
In both cases, the women recovered completely.
Smallpox-like illness
Public health sleuths figured out that in three of the four cases, the patients lived in a home with a cat or cats, and one of these cats was known to hunt small animals.
Experts already knew that cats mingling in cow pastures and sickened by cattle virus had helped cowpox make the leap from bovines to humans. And just as in the case of cowpox, they suspected that cats might again be spreading this new virus to people, too.
All four of the infected people lived in sparsely populated areas amid forests. Officials laid animal traps where some of the affected people lived and identified the virus in several species of small wild animals.
The animals that turned up most often with Alaskapox were small mouse-like voles. The rodents with rounded muzzles are known for burrowing in the region. And scientists suspect the Alaskapox virus makes its way from these wild animals to humans through their pet cats or possibly by direct exposure outdoors.
None of the four people identified so far with Alaskapox knew each other or interacted, so officials also suspect that there are more cases going unrecognized, possibly because the symptoms are mild or nonexistent.
There are no documented cases of person-to-person transmission of Alaskapox, according to public health officials monitoring the small number of cases. But other pox viruses can spread by direct contact with skin lesions, so clinicians are recommending that people cover wounds with bandages. Three of the people with Alaskapox mistook their lesions at first for a bite from a spider or insect.
A version of this article first appeared on WebMD.com.





