User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
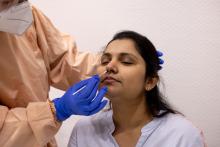
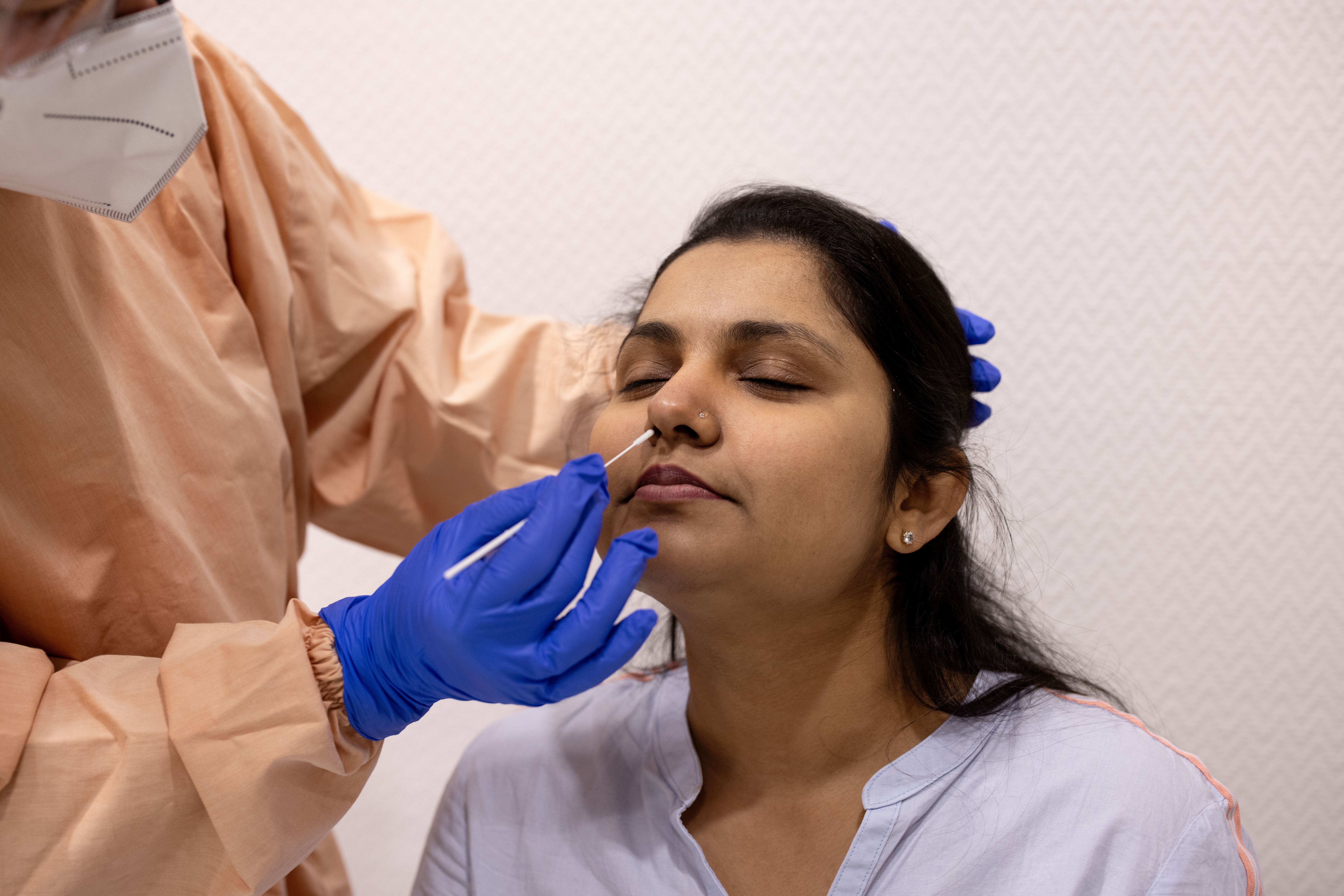
2021 AGA Rapid Review and Guideline Update: Pre-endoscopy SARS-CoV-2 testing post vaccination
The American Gastroenterological Association recently updated their guideline for preendoscopy SARS-CoV-2 testing in light of population-wide vaccination programs, now recommending against routine viral screening regardless of patient vaccination status and local disease prevalence.
Centers electing to maintain a preprocedure testing strategy should use standard nucleic acid testing, preferably rapid reverse transcription polymerase chain reaction (RT-PCR) because this can be performed on the day of the procedure, thereby limiting patient testing burden, reported authors led by co–first authors Shahnaz Sultan, MD, of the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Healthcare System, and Shazia M. Siddique, MD, of the University of Pennsylvania, Philadelphia.
These new recommendations, both of which are conditional and based on very-low-certainty evidence, were drawn from a rapid evidence review of benefits and risks in the postvaccination period.
“Since the start of the pandemic, our increased understanding of transmission has facilitated the implementation of practices to promote patient and health care worker (HCW) safety,” the guideline authors wrote in Gastroenterology. “Simultaneously, there has been increasing recognition of the potential harm associated with delays in patient care, as well as inefficiency of endoscopy units. With widespread vaccination of HCWs and the general population, a reevaluation of AGA’s prior recommendations was warranted.”
The 2020 AGA guideline, also led by Dr. Sultan, issued viral screening recommendations based on local prevalence rates of asymptomatic COVID-19, with pretesting reserved for moderately affected locations. Mildly affected areas were advised against pretesting, whereas centers in pandemic hot spots were cautioned against performing all but “emergency or time-sensitive procedures.”
Those recommendations have now been replaced by the present guideline, which no longer distinguishes between local prevalence rates. This decision was based on a variety of factors, the panelists noted, including endoscopy volumes, vaccine efficacy, HCW and patient anxiety, endoscopy-related risk of infection to both patients and HCWs, prevalence of asymptomatic COVID-19 among patients undergoing endoscopy, and the impact of delaying care on cancer burden.
“The panel placed a high value on minimizing additional delays in patient care, acknowledging the reduced endoscopy volumes, downstream impact on delayed cancer diagnoses, and burden of testing on patients,” Dr. Sultan and colleagues wrote.
The guideline includes a summary of evidence related to the two new recommendations, including several studies reporting prevalence of asymptomatic SARS-CoV-2 infection among patients tested prior to endoscopy procedures.
“Across 13 studies, asymptomatic prevalence ranged from 0% to 1.5%, but most studies reported a range from 0% to 0.5%,” the panelists wrote, “regardless of local surges of COVID-19 cases.”
Although Dr. Sultan and colleagues acknowledged that pretesting may be reassuring, they noted that, based on available evidence, “there were few to no cases of infections reported among HCWs (performing endoscopy) and patients. Among the few reported cases, the authors could not clearly distinguish between community-acquired infections or health care–acquired infections.”
They went on to quantify the relationship between delays in care and cancer burden, reviewing data from 14 studies that demonstrated an overall reduction in endoscopic-detected colorectal cancers by 31%-71%, esophageal cancers by 27%-37%, and gastric cancer by 27%-52% since the start of the pandemic. A recent study by Ahmad Khan, MD, and colleagues, which focused on the United States from July to November 2020, demonstrated an 11.74% decrease in diagnoses of malignant colorectal cancer, and a 19.78% decline in diagnoses of esophageal and gastric cancer.
The second recommendation – calling for standard nucleic acid testing among centers electing to maintain a pretesting strategy – was also presented with a summary of supporting evidence, largely pertaining to test accuracy.
“Rapid RT-PCR tests that can be easily performed on the day of endoscopy (results within 1 hour) are preferable as they pose less burden to patients,” the panelists wrote. “In the preprocedure setting, the utility of rapid isothermal tests or antigen tests is limited due to concerns of assay sensitivity. There is no role of antibody tests for preprocedure testing.”
For both new recommendations, it is assumed that “all centers have access to PPE, including face shield, eye protection, and surgical mask or N95 (or N99, powered air-purifying respirators)” and that “all centers have implemented universal screening of patients for COVID-19 symptoms, using a screening checklist, and have implemented universal precautions, including physical distancing, masks, and hand hygiene in the endoscopy unit.”
As COVID-19 cases rise in the United States because of the Delta variant, there is renewed concern about infection and transmission of SARS-CoV2 during endoscopy. Stay tuned for updates and visit https://gastro.org/practice-guidance/practice-updates/covid-19/.
Guideline development was funded by the AGA. No panel members received any payments.
The American Gastroenterological Association recently updated their guideline for preendoscopy SARS-CoV-2 testing in light of population-wide vaccination programs, now recommending against routine viral screening regardless of patient vaccination status and local disease prevalence.
Centers electing to maintain a preprocedure testing strategy should use standard nucleic acid testing, preferably rapid reverse transcription polymerase chain reaction (RT-PCR) because this can be performed on the day of the procedure, thereby limiting patient testing burden, reported authors led by co–first authors Shahnaz Sultan, MD, of the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Healthcare System, and Shazia M. Siddique, MD, of the University of Pennsylvania, Philadelphia.
These new recommendations, both of which are conditional and based on very-low-certainty evidence, were drawn from a rapid evidence review of benefits and risks in the postvaccination period.
“Since the start of the pandemic, our increased understanding of transmission has facilitated the implementation of practices to promote patient and health care worker (HCW) safety,” the guideline authors wrote in Gastroenterology. “Simultaneously, there has been increasing recognition of the potential harm associated with delays in patient care, as well as inefficiency of endoscopy units. With widespread vaccination of HCWs and the general population, a reevaluation of AGA’s prior recommendations was warranted.”
The 2020 AGA guideline, also led by Dr. Sultan, issued viral screening recommendations based on local prevalence rates of asymptomatic COVID-19, with pretesting reserved for moderately affected locations. Mildly affected areas were advised against pretesting, whereas centers in pandemic hot spots were cautioned against performing all but “emergency or time-sensitive procedures.”
Those recommendations have now been replaced by the present guideline, which no longer distinguishes between local prevalence rates. This decision was based on a variety of factors, the panelists noted, including endoscopy volumes, vaccine efficacy, HCW and patient anxiety, endoscopy-related risk of infection to both patients and HCWs, prevalence of asymptomatic COVID-19 among patients undergoing endoscopy, and the impact of delaying care on cancer burden.
“The panel placed a high value on minimizing additional delays in patient care, acknowledging the reduced endoscopy volumes, downstream impact on delayed cancer diagnoses, and burden of testing on patients,” Dr. Sultan and colleagues wrote.
The guideline includes a summary of evidence related to the two new recommendations, including several studies reporting prevalence of asymptomatic SARS-CoV-2 infection among patients tested prior to endoscopy procedures.
“Across 13 studies, asymptomatic prevalence ranged from 0% to 1.5%, but most studies reported a range from 0% to 0.5%,” the panelists wrote, “regardless of local surges of COVID-19 cases.”
Although Dr. Sultan and colleagues acknowledged that pretesting may be reassuring, they noted that, based on available evidence, “there were few to no cases of infections reported among HCWs (performing endoscopy) and patients. Among the few reported cases, the authors could not clearly distinguish between community-acquired infections or health care–acquired infections.”
They went on to quantify the relationship between delays in care and cancer burden, reviewing data from 14 studies that demonstrated an overall reduction in endoscopic-detected colorectal cancers by 31%-71%, esophageal cancers by 27%-37%, and gastric cancer by 27%-52% since the start of the pandemic. A recent study by Ahmad Khan, MD, and colleagues, which focused on the United States from July to November 2020, demonstrated an 11.74% decrease in diagnoses of malignant colorectal cancer, and a 19.78% decline in diagnoses of esophageal and gastric cancer.
The second recommendation – calling for standard nucleic acid testing among centers electing to maintain a pretesting strategy – was also presented with a summary of supporting evidence, largely pertaining to test accuracy.
“Rapid RT-PCR tests that can be easily performed on the day of endoscopy (results within 1 hour) are preferable as they pose less burden to patients,” the panelists wrote. “In the preprocedure setting, the utility of rapid isothermal tests or antigen tests is limited due to concerns of assay sensitivity. There is no role of antibody tests for preprocedure testing.”
For both new recommendations, it is assumed that “all centers have access to PPE, including face shield, eye protection, and surgical mask or N95 (or N99, powered air-purifying respirators)” and that “all centers have implemented universal screening of patients for COVID-19 symptoms, using a screening checklist, and have implemented universal precautions, including physical distancing, masks, and hand hygiene in the endoscopy unit.”
As COVID-19 cases rise in the United States because of the Delta variant, there is renewed concern about infection and transmission of SARS-CoV2 during endoscopy. Stay tuned for updates and visit https://gastro.org/practice-guidance/practice-updates/covid-19/.
Guideline development was funded by the AGA. No panel members received any payments.
The American Gastroenterological Association recently updated their guideline for preendoscopy SARS-CoV-2 testing in light of population-wide vaccination programs, now recommending against routine viral screening regardless of patient vaccination status and local disease prevalence.
Centers electing to maintain a preprocedure testing strategy should use standard nucleic acid testing, preferably rapid reverse transcription polymerase chain reaction (RT-PCR) because this can be performed on the day of the procedure, thereby limiting patient testing burden, reported authors led by co–first authors Shahnaz Sultan, MD, of the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Healthcare System, and Shazia M. Siddique, MD, of the University of Pennsylvania, Philadelphia.
These new recommendations, both of which are conditional and based on very-low-certainty evidence, were drawn from a rapid evidence review of benefits and risks in the postvaccination period.
“Since the start of the pandemic, our increased understanding of transmission has facilitated the implementation of practices to promote patient and health care worker (HCW) safety,” the guideline authors wrote in Gastroenterology. “Simultaneously, there has been increasing recognition of the potential harm associated with delays in patient care, as well as inefficiency of endoscopy units. With widespread vaccination of HCWs and the general population, a reevaluation of AGA’s prior recommendations was warranted.”
The 2020 AGA guideline, also led by Dr. Sultan, issued viral screening recommendations based on local prevalence rates of asymptomatic COVID-19, with pretesting reserved for moderately affected locations. Mildly affected areas were advised against pretesting, whereas centers in pandemic hot spots were cautioned against performing all but “emergency or time-sensitive procedures.”
Those recommendations have now been replaced by the present guideline, which no longer distinguishes between local prevalence rates. This decision was based on a variety of factors, the panelists noted, including endoscopy volumes, vaccine efficacy, HCW and patient anxiety, endoscopy-related risk of infection to both patients and HCWs, prevalence of asymptomatic COVID-19 among patients undergoing endoscopy, and the impact of delaying care on cancer burden.
“The panel placed a high value on minimizing additional delays in patient care, acknowledging the reduced endoscopy volumes, downstream impact on delayed cancer diagnoses, and burden of testing on patients,” Dr. Sultan and colleagues wrote.
The guideline includes a summary of evidence related to the two new recommendations, including several studies reporting prevalence of asymptomatic SARS-CoV-2 infection among patients tested prior to endoscopy procedures.
“Across 13 studies, asymptomatic prevalence ranged from 0% to 1.5%, but most studies reported a range from 0% to 0.5%,” the panelists wrote, “regardless of local surges of COVID-19 cases.”
Although Dr. Sultan and colleagues acknowledged that pretesting may be reassuring, they noted that, based on available evidence, “there were few to no cases of infections reported among HCWs (performing endoscopy) and patients. Among the few reported cases, the authors could not clearly distinguish between community-acquired infections or health care–acquired infections.”
They went on to quantify the relationship between delays in care and cancer burden, reviewing data from 14 studies that demonstrated an overall reduction in endoscopic-detected colorectal cancers by 31%-71%, esophageal cancers by 27%-37%, and gastric cancer by 27%-52% since the start of the pandemic. A recent study by Ahmad Khan, MD, and colleagues, which focused on the United States from July to November 2020, demonstrated an 11.74% decrease in diagnoses of malignant colorectal cancer, and a 19.78% decline in diagnoses of esophageal and gastric cancer.
The second recommendation – calling for standard nucleic acid testing among centers electing to maintain a pretesting strategy – was also presented with a summary of supporting evidence, largely pertaining to test accuracy.
“Rapid RT-PCR tests that can be easily performed on the day of endoscopy (results within 1 hour) are preferable as they pose less burden to patients,” the panelists wrote. “In the preprocedure setting, the utility of rapid isothermal tests or antigen tests is limited due to concerns of assay sensitivity. There is no role of antibody tests for preprocedure testing.”
For both new recommendations, it is assumed that “all centers have access to PPE, including face shield, eye protection, and surgical mask or N95 (or N99, powered air-purifying respirators)” and that “all centers have implemented universal screening of patients for COVID-19 symptoms, using a screening checklist, and have implemented universal precautions, including physical distancing, masks, and hand hygiene in the endoscopy unit.”
As COVID-19 cases rise in the United States because of the Delta variant, there is renewed concern about infection and transmission of SARS-CoV2 during endoscopy. Stay tuned for updates and visit https://gastro.org/practice-guidance/practice-updates/covid-19/.
Guideline development was funded by the AGA. No panel members received any payments.
FROM GASTROENTEROLOGY
Even highly allergic adults unlikely to react to COVID-19 vaccine
published Aug. 31, 2021, in JAMA Network Open. Symptoms resolved in a few hours with medication, and no patients required hospitalization.
Risk for allergic reaction has been one of several obstacles in global vaccination efforts, the authors, led by Nancy Agmon-Levin, MD, of the Sheba Medical Center, Ramat Gan, Israel, wrote. Clinical trials for the Moderna and Pfizer-BioNTech COVID-19 vaccines excluded individuals with allergies to any component of the vaccine or with previous allergies to other vaccines. Early reports of anaphylaxis in reaction to the vaccines caused concern among patients and practitioners. Soon after, the Centers for Disease Control and Prevention and other authorities released guidance on preparing for allergic reactions. “Despite these recommendations, uncertainty remains, particularly among patients with a history of anaphylaxis and/or multiple allergies,” the authors added.
In response to early concerns, the Sheba Medical Center opened a COVID-19 referral center to address safety questions and to conduct assessments of allergy risk for the Pfizer-BioNTech vaccine, the first COVID-19 vaccine approved in Israel. From Dec. 27, 2020, to Feb. 22, 2021, the referral center assessed 8,102 patients with allergies. Those who were not clearly at low risk filled out a questionnaire about prior allergic or anaphylactic reactions to drugs or vaccines, other allergies, and other relevant medical history. Patients were considered to be at high risk for allergic reactions if they met at least one of the following criteria: previous anaphylactic reaction to any drug or vaccine, multiple drug allergies, multiple other allergies, and mast cell disorders. Individuals were also classified as high risk if their health care practitioner deferred vaccination because of allergy concerns.
Nearly 95% of the cohort (7,668 individuals) were classified as low risk and received both Pfizer vaccine doses at standard immunization sites and underwent 30 minutes of observation after immunization. Although the study did not follow these lower-risk patients, “no serious allergic reactions were reported back to our referral center by patients or their general practitioner after immunization in the regular settings,” the authors wrote.
Five patients were considered ineligible for immunization because of known sensitivity to polyethylene glycol or multiple anaphylactic reactions to different injectable drugs, following recommendations from the Ministry of Health of Israel at the time. The remaining 429 individuals were deemed high risk and underwent observation for 2 hours from a dedicated allergy team after immunization. For these high-risk patients, both vaccine doses were administered in the same setting. Patients also reported any adverse reactions in the 21 days between the first and second dose.
Women made up most of the high-risk cohort (70.9%). The average age of participants was 52 years. Of the high-risk individuals, 63.2% reported prior anaphylaxis, 32.9% had multiple drug allergies, and 30.3% had multiple other allergies.
During the first 2 hours following immunization, nine individuals (2.1%), all women, experienced allergic reactions. Six individuals (1.4%) experienced minor reactions, including skin flushing, tongue or uvula swelling, or a cough that resolved with antihistamine treatment during the observation period. Three patients (0.7%) had anaphylactic reactions that occurred 10 to 20 minutes after injection. All three patients experienced significant bronchospasm, skin eruption, itching, and shortness of breath. Two patients experienced angioedema, and one patient had gastrointestinal symptoms. They were treated with adrenaline, antihistamines, and an inhaled bronchodilator. All symptoms resolved within 2-6 hours, and no patient required hospitalization.
In the days following vaccination, patients commonly reported pain at the injection site, fatigue, muscle pain, and headache; 14.7% of patients reported skin eruption, itching, or urticaria.
As of Feb. 22, 2021, 218 patients from this highly allergic cohort received their second dose of the vaccine. Four patients (1.8%) had mild allergic reactions. All four developed flushing, and one patient also developed a cough that resolved with antihistamine treatment. Three of these patients had experienced mild allergic reactions to the first dose and were premedicated for the second dose. One patient only reacted to the second dose.
The findings should be “very reassuring” to individuals hesitant to receive the vaccine, Elizabeth Phillips, MD, the director of the Center for Drug Safety and Immunology at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview. She was not involved with the research and wrote an invited commentary on the study. “The rates of anaphylaxis and allergic reactions are truly quite low,” she said. Although about 2% of the high-risk group developed allergic reactions to immunization, the overall percentage for the entire cohort would be much lower.
The study did not investigate specific risk factors for and mechanisms of allergic reactions to COVID-19 vaccines, Dr. Phillips said, which is a study limitation that the authors also acknowledge. The National Institute for Allergy and Infectious Diseases is currently trying to answer some of these questions with a multisite, randomized, double-blinded study. The study is intended to help understand why people have these allergic reactions, Dr. Phillips added. Vanderbilt is one of the sites for the study.
While researchers continue to hunt for answers, the algorithm developed by the authors provides “a great strategy to get people that are at higher risk vaccinated in a monitored setting,” she said. The results show that “people should not be avoiding vaccination because of a history of anaphylaxis.”
Dr. Phillips has received institutional grants from the National Institutes of Health and the National Health and Medical Research Council; royalties from UpToDate and Lexicomp; and consulting fees from Janssen, Vertex, Biocryst, and Regeneron.
A version of this article first appeared on Medscape.com.
published Aug. 31, 2021, in JAMA Network Open. Symptoms resolved in a few hours with medication, and no patients required hospitalization.
Risk for allergic reaction has been one of several obstacles in global vaccination efforts, the authors, led by Nancy Agmon-Levin, MD, of the Sheba Medical Center, Ramat Gan, Israel, wrote. Clinical trials for the Moderna and Pfizer-BioNTech COVID-19 vaccines excluded individuals with allergies to any component of the vaccine or with previous allergies to other vaccines. Early reports of anaphylaxis in reaction to the vaccines caused concern among patients and practitioners. Soon after, the Centers for Disease Control and Prevention and other authorities released guidance on preparing for allergic reactions. “Despite these recommendations, uncertainty remains, particularly among patients with a history of anaphylaxis and/or multiple allergies,” the authors added.
In response to early concerns, the Sheba Medical Center opened a COVID-19 referral center to address safety questions and to conduct assessments of allergy risk for the Pfizer-BioNTech vaccine, the first COVID-19 vaccine approved in Israel. From Dec. 27, 2020, to Feb. 22, 2021, the referral center assessed 8,102 patients with allergies. Those who were not clearly at low risk filled out a questionnaire about prior allergic or anaphylactic reactions to drugs or vaccines, other allergies, and other relevant medical history. Patients were considered to be at high risk for allergic reactions if they met at least one of the following criteria: previous anaphylactic reaction to any drug or vaccine, multiple drug allergies, multiple other allergies, and mast cell disorders. Individuals were also classified as high risk if their health care practitioner deferred vaccination because of allergy concerns.
Nearly 95% of the cohort (7,668 individuals) were classified as low risk and received both Pfizer vaccine doses at standard immunization sites and underwent 30 minutes of observation after immunization. Although the study did not follow these lower-risk patients, “no serious allergic reactions were reported back to our referral center by patients or their general practitioner after immunization in the regular settings,” the authors wrote.
Five patients were considered ineligible for immunization because of known sensitivity to polyethylene glycol or multiple anaphylactic reactions to different injectable drugs, following recommendations from the Ministry of Health of Israel at the time. The remaining 429 individuals were deemed high risk and underwent observation for 2 hours from a dedicated allergy team after immunization. For these high-risk patients, both vaccine doses were administered in the same setting. Patients also reported any adverse reactions in the 21 days between the first and second dose.
Women made up most of the high-risk cohort (70.9%). The average age of participants was 52 years. Of the high-risk individuals, 63.2% reported prior anaphylaxis, 32.9% had multiple drug allergies, and 30.3% had multiple other allergies.
During the first 2 hours following immunization, nine individuals (2.1%), all women, experienced allergic reactions. Six individuals (1.4%) experienced minor reactions, including skin flushing, tongue or uvula swelling, or a cough that resolved with antihistamine treatment during the observation period. Three patients (0.7%) had anaphylactic reactions that occurred 10 to 20 minutes after injection. All three patients experienced significant bronchospasm, skin eruption, itching, and shortness of breath. Two patients experienced angioedema, and one patient had gastrointestinal symptoms. They were treated with adrenaline, antihistamines, and an inhaled bronchodilator. All symptoms resolved within 2-6 hours, and no patient required hospitalization.
In the days following vaccination, patients commonly reported pain at the injection site, fatigue, muscle pain, and headache; 14.7% of patients reported skin eruption, itching, or urticaria.
As of Feb. 22, 2021, 218 patients from this highly allergic cohort received their second dose of the vaccine. Four patients (1.8%) had mild allergic reactions. All four developed flushing, and one patient also developed a cough that resolved with antihistamine treatment. Three of these patients had experienced mild allergic reactions to the first dose and were premedicated for the second dose. One patient only reacted to the second dose.
The findings should be “very reassuring” to individuals hesitant to receive the vaccine, Elizabeth Phillips, MD, the director of the Center for Drug Safety and Immunology at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview. She was not involved with the research and wrote an invited commentary on the study. “The rates of anaphylaxis and allergic reactions are truly quite low,” she said. Although about 2% of the high-risk group developed allergic reactions to immunization, the overall percentage for the entire cohort would be much lower.
The study did not investigate specific risk factors for and mechanisms of allergic reactions to COVID-19 vaccines, Dr. Phillips said, which is a study limitation that the authors also acknowledge. The National Institute for Allergy and Infectious Diseases is currently trying to answer some of these questions with a multisite, randomized, double-blinded study. The study is intended to help understand why people have these allergic reactions, Dr. Phillips added. Vanderbilt is one of the sites for the study.
While researchers continue to hunt for answers, the algorithm developed by the authors provides “a great strategy to get people that are at higher risk vaccinated in a monitored setting,” she said. The results show that “people should not be avoiding vaccination because of a history of anaphylaxis.”
Dr. Phillips has received institutional grants from the National Institutes of Health and the National Health and Medical Research Council; royalties from UpToDate and Lexicomp; and consulting fees from Janssen, Vertex, Biocryst, and Regeneron.
A version of this article first appeared on Medscape.com.
published Aug. 31, 2021, in JAMA Network Open. Symptoms resolved in a few hours with medication, and no patients required hospitalization.
Risk for allergic reaction has been one of several obstacles in global vaccination efforts, the authors, led by Nancy Agmon-Levin, MD, of the Sheba Medical Center, Ramat Gan, Israel, wrote. Clinical trials for the Moderna and Pfizer-BioNTech COVID-19 vaccines excluded individuals with allergies to any component of the vaccine or with previous allergies to other vaccines. Early reports of anaphylaxis in reaction to the vaccines caused concern among patients and practitioners. Soon after, the Centers for Disease Control and Prevention and other authorities released guidance on preparing for allergic reactions. “Despite these recommendations, uncertainty remains, particularly among patients with a history of anaphylaxis and/or multiple allergies,” the authors added.
In response to early concerns, the Sheba Medical Center opened a COVID-19 referral center to address safety questions and to conduct assessments of allergy risk for the Pfizer-BioNTech vaccine, the first COVID-19 vaccine approved in Israel. From Dec. 27, 2020, to Feb. 22, 2021, the referral center assessed 8,102 patients with allergies. Those who were not clearly at low risk filled out a questionnaire about prior allergic or anaphylactic reactions to drugs or vaccines, other allergies, and other relevant medical history. Patients were considered to be at high risk for allergic reactions if they met at least one of the following criteria: previous anaphylactic reaction to any drug or vaccine, multiple drug allergies, multiple other allergies, and mast cell disorders. Individuals were also classified as high risk if their health care practitioner deferred vaccination because of allergy concerns.
Nearly 95% of the cohort (7,668 individuals) were classified as low risk and received both Pfizer vaccine doses at standard immunization sites and underwent 30 minutes of observation after immunization. Although the study did not follow these lower-risk patients, “no serious allergic reactions were reported back to our referral center by patients or their general practitioner after immunization in the regular settings,” the authors wrote.
Five patients were considered ineligible for immunization because of known sensitivity to polyethylene glycol or multiple anaphylactic reactions to different injectable drugs, following recommendations from the Ministry of Health of Israel at the time. The remaining 429 individuals were deemed high risk and underwent observation for 2 hours from a dedicated allergy team after immunization. For these high-risk patients, both vaccine doses were administered in the same setting. Patients also reported any adverse reactions in the 21 days between the first and second dose.
Women made up most of the high-risk cohort (70.9%). The average age of participants was 52 years. Of the high-risk individuals, 63.2% reported prior anaphylaxis, 32.9% had multiple drug allergies, and 30.3% had multiple other allergies.
During the first 2 hours following immunization, nine individuals (2.1%), all women, experienced allergic reactions. Six individuals (1.4%) experienced minor reactions, including skin flushing, tongue or uvula swelling, or a cough that resolved with antihistamine treatment during the observation period. Three patients (0.7%) had anaphylactic reactions that occurred 10 to 20 minutes after injection. All three patients experienced significant bronchospasm, skin eruption, itching, and shortness of breath. Two patients experienced angioedema, and one patient had gastrointestinal symptoms. They were treated with adrenaline, antihistamines, and an inhaled bronchodilator. All symptoms resolved within 2-6 hours, and no patient required hospitalization.
In the days following vaccination, patients commonly reported pain at the injection site, fatigue, muscle pain, and headache; 14.7% of patients reported skin eruption, itching, or urticaria.
As of Feb. 22, 2021, 218 patients from this highly allergic cohort received their second dose of the vaccine. Four patients (1.8%) had mild allergic reactions. All four developed flushing, and one patient also developed a cough that resolved with antihistamine treatment. Three of these patients had experienced mild allergic reactions to the first dose and were premedicated for the second dose. One patient only reacted to the second dose.
The findings should be “very reassuring” to individuals hesitant to receive the vaccine, Elizabeth Phillips, MD, the director of the Center for Drug Safety and Immunology at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview. She was not involved with the research and wrote an invited commentary on the study. “The rates of anaphylaxis and allergic reactions are truly quite low,” she said. Although about 2% of the high-risk group developed allergic reactions to immunization, the overall percentage for the entire cohort would be much lower.
The study did not investigate specific risk factors for and mechanisms of allergic reactions to COVID-19 vaccines, Dr. Phillips said, which is a study limitation that the authors also acknowledge. The National Institute for Allergy and Infectious Diseases is currently trying to answer some of these questions with a multisite, randomized, double-blinded study. The study is intended to help understand why people have these allergic reactions, Dr. Phillips added. Vanderbilt is one of the sites for the study.
While researchers continue to hunt for answers, the algorithm developed by the authors provides “a great strategy to get people that are at higher risk vaccinated in a monitored setting,” she said. The results show that “people should not be avoiding vaccination because of a history of anaphylaxis.”
Dr. Phillips has received institutional grants from the National Institutes of Health and the National Health and Medical Research Council; royalties from UpToDate and Lexicomp; and consulting fees from Janssen, Vertex, Biocryst, and Regeneron.
A version of this article first appeared on Medscape.com.
Bystander rescue breathing CPR in kids tied to better survival
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children who receive CPR with both rescue breathing and compressions from a bystander have greater odds of survival without serious brain damage than if they receive CPR with compressions only, according to a study published online in the Journal of the American College of Cardiology.
Specifically, a child has a 61% better chance of surviving with good neurologic outcomes if they receive compression-only CPR versus no bystander resuscitation, but that child is more than twice as likely to survive if he or she receives rescue breathing as well.
The study’s clinical implications are most important for bystander CPR training, lead author Maryam Y. Naim, MD, MSCE, of the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, told this news organization.
“Many programs teach compression-only CPR to lay rescuers, and there should be a renewed emphasis on rescue breathing for the possibility a lay rescuer has to perform CPR on a child,” Dr. Naim said.
That said, if a bystander is unfamiliar with how to properly administer rescue breathing or has concerns about hygiene or infection on someone they don’t know, Dr. Naim advises doing compression-only CPR, especially if the child is older than age 1 year. “If a child is younger than a year of age please consider giving rescue breaths with chest compressions,” she added.
Dr. Naim and colleagues analyzed 13,060 pediatric out-of-hospital cardiac arrests from the Cardiac Arrest Registry to Enhance Survival database, which includes data from 911 call centers, emergency medical services (EMS) providers, and receiving hospitals across 28 states. The data sample included all cases age 18 years or younger who experienced nontraumatic out-of-hospital cardiac arrest between January 2013 and December 2019, excluding those with obvious signs of death or a “do not resuscitate” order.
“Because the etiology of cardiac arrest in children is difficult to determine, especially in cases that result in death, all nontraumatic cases were included regardless of presumed etiology, including respiratory, cardiac, drowning, electrocution, or other,” the authors wrote. The researchers defined neurologically favorable survival, the primary endpoint, as “a cerebral performance category score of 1 (no neurologic disability) or 2 (moderate disability)” at discharge. Neurologically unfavorable survival included a score of 3 (severe disability), 4 (coma or vegetative state), or death.
Among the 10,429 cases ultimately analyzed after exclusions and missing data, 46.5% received bystander CPR. Slightly more than half of these (55.6%) received compression-only CPR while the other 45.3% received rescue-breathing CPR.
Dr. Naim was surprised that compression-only CPR was the most common form of CPR given to children with cardiac arrest because the current American Heart Association/International Liaison Committee on Resuscitation recommendations note rescue breathing as the preferred form in children.
That preference exists because respiratory failure occurs more often in children than in adults as a cause of cardiac arrest, explained Sandra Weiss, MD, an interventional cardiologist and the medical director of the cardiac intensive care unit at ChristianaCare’s Christiana Hospital in Newark, Del.
Because of that, “it’s not surprising that if you give respiratory resuscitation to a child who’s arresting from a respiratory cause that they’re going to do better than if you just do chest compressions,” said Dr. Weiss, who was not involved in the study.
The study found the most common presumed cause of arrest to be cardiac, occurring in 44.4% of cases, but it was closely followed by respiratory in nearly one-third of cases (32.8%).
Infants younger than age 1 year were the most common age group to have a cardiac arrest, making up more than all other ages combined. Most out-of-hospital cardiac arrests occurred in a home and were observed by someone when they happened. While rates of bystander CPR did not change during the study’s 6-year period, the incidence of compression-only CPR increased. Lay people without medical training provided the CPR in 93.6% of cases.
Only 8.6% of cardiac arrest cases resulted in neurologically favorable survival, a rate which remained steady throughout the study period. The rate increased with increasing age, at 4.6% of infants, 10.6% of children, and 16.5% of adolescents.
Those who received CPR with rescue breathing had more than double the odds of neurologically favorable survival than if they hadn’t received CPR at all (adjusted odds ratio, 2.16). Survival with a positive neurologic outcome was 1.6 times more likely with compression-only CPR than no CPR (aOR, 1.61). When researchers compared the two forms of CPR, inclusion of rescue breathing increased the child’s likelihood of survival without neurologic sequelae by 36% (aOR, 1.36).
Despite these findings, however, Dr. Weiss agrees with Dr. Naim that offering compression-only CPR is preferable to offering no CPR at all.
“All resuscitation is better than no resuscitation, regardless of whether it’s compression only or respiratory breathing,” Dr. Weiss said in an interview. “The average lay person is probably going to do the easiest thing, and survivability is going to be increased by doing anything rather than nothing.”
Dr. Weiss also noted that it’s easier to instruct people how to do chest compressions, especially, for example, during an emergency phone call with a dispatcher while waiting for EMS to arrive.
“It’s absolutely imperative for people to get the basics, and the basics are compressions,” she said. “That’s really what is the most vital component of all resuscitative efforts, regardless of whether it’s adult or pediatrics.”
Dr. Weiss also acknowledges that laypeople may feel particularly less comfortable administering rescue breaths to a child they don’t know in the midst of the COVID-19 pandemic. Even if the odds are low that the specific child experiencing a cardiac arrest is necessarily infectious, the AHA guidelines include the caveat that, “if there’s a concern for infection transmissibility, that compression only is acceptable,” Dr. Weiss said. “It’s a reality for our current state.”
The superiority of rescue-breathing CPR to compression-only CPR was true across all age groups, but compression-only CPR still resulted in better survival odds than no CPR at all for all age groups except infants, in whom only rescue breathing was associated with a statistically significant increased likelihood of neurologically favorable survival.
Protective factors for positive outcomes included being younger than age 1 year, the arrest being witnessed, and a having shockable rhythm. Risk factors reducing survival included being Black, being in a home, and cardiac arrests linked with automated external defibrillator use before EMS arrived.
The CARES program was previously funded by the Centers for Disease Control and Prevention and is now funded by the American Red Cross, the AHA, Stryker, and Emory University. Dr. Naim was further supported by Children’s Hospital of Philadelphia and the American Red Cross. The authors and Dr. Weiss disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Another COVID-19 patient to get ivermectin after court order
Another case, another state, another judge ordering a hospital to give a patient a controversial horse deworming drug to treat a severe case of COVID-19.
, according to the Ohio Capital Journal. Judge Gregory Howard’s ruling comes after Mr. Smith’s wife sued to force the hospital to provide the controversial drug to her husband, who has been hospitalized since July 15.
Julie Smith has gotten Fred Wagshul, MD, to agree to administer ivermectin to her husband. Dr. Wagshul is known as a member of a group of doctors who say the Centers for Disease Control and Prevention and the Food and Drug Administration are lying about ivermectin’s usefulness in fighting COVID-19. Both agencies have warned against using the drug to treat COVID-19, saying there is no evidence it works and that it can be dangerous in large amounts.
According to the Ohio Capital Journal, Dr. Wagshul accused the CDC and FDA of engaging in a “conspiracy” to prevent ivermectin’s use.
But Arthur L. Caplan, MD, professor of bioethics at New York University’s Langone Medical Center, said, “it is absurd that this order was issued,” according to an interview in Ars Technica. “If I were these doctors, I simply wouldn’t do it.”
It is not the first time a judge has ordered ivermectin’s use against a hospital’s wishes.
A 68-year-old woman with COVID-19 in an Illinois hospital started receiving the controversial drug in May after her family sued the hospital to have someone administer it.
Nurije Fype’s daughter, Desareta, filed suit against Elmhurst Hospital, part of Edward-Elmhurst Health, asking that her mother receive the treatment, which is approved as an antiparasitic drug but not approved for the treatment of COVID-19. Desareta Fype was granted temporary guardianship of her mother.
The FDA has published guidance titled “Why You Should Not Use Ivermectin to Treat or Prevent COVID-19” on its website. The National Institutes of Health said there is not enough data to recommend either for or against its use in treating COVID-19.
But DuPage County Judge James Orel ruled Ms. Fype should be allowed to get the treatment.
Three days later, according to the Daily Herald, the lawyer for the hospital, Joseph Monahan, argued the hospital could not find a hospital-affiliated doctor to administer the ivermectin.
The Herald reported the judge told the hospital to “get out of the way” and allow any board-certified doctor to administer the drug.
When Ms. Fype’s doctor was unable to administer it, the legal team found another doctor, Alan Bain, DO, to do it. Mr. Monahan said Dr. Bain was granted credentials to work at the hospital so he could administer it.
Judge Orel denied a request from Desareta Fype’s lawyer to order the hospital’s nurses to administer further doses. The judge also denied a request to hold the hospital in contempt of court.
A version of this article first appeared on WebMD.com.
Another case, another state, another judge ordering a hospital to give a patient a controversial horse deworming drug to treat a severe case of COVID-19.
, according to the Ohio Capital Journal. Judge Gregory Howard’s ruling comes after Mr. Smith’s wife sued to force the hospital to provide the controversial drug to her husband, who has been hospitalized since July 15.
Julie Smith has gotten Fred Wagshul, MD, to agree to administer ivermectin to her husband. Dr. Wagshul is known as a member of a group of doctors who say the Centers for Disease Control and Prevention and the Food and Drug Administration are lying about ivermectin’s usefulness in fighting COVID-19. Both agencies have warned against using the drug to treat COVID-19, saying there is no evidence it works and that it can be dangerous in large amounts.
According to the Ohio Capital Journal, Dr. Wagshul accused the CDC and FDA of engaging in a “conspiracy” to prevent ivermectin’s use.
But Arthur L. Caplan, MD, professor of bioethics at New York University’s Langone Medical Center, said, “it is absurd that this order was issued,” according to an interview in Ars Technica. “If I were these doctors, I simply wouldn’t do it.”
It is not the first time a judge has ordered ivermectin’s use against a hospital’s wishes.
A 68-year-old woman with COVID-19 in an Illinois hospital started receiving the controversial drug in May after her family sued the hospital to have someone administer it.
Nurije Fype’s daughter, Desareta, filed suit against Elmhurst Hospital, part of Edward-Elmhurst Health, asking that her mother receive the treatment, which is approved as an antiparasitic drug but not approved for the treatment of COVID-19. Desareta Fype was granted temporary guardianship of her mother.
The FDA has published guidance titled “Why You Should Not Use Ivermectin to Treat or Prevent COVID-19” on its website. The National Institutes of Health said there is not enough data to recommend either for or against its use in treating COVID-19.
But DuPage County Judge James Orel ruled Ms. Fype should be allowed to get the treatment.
Three days later, according to the Daily Herald, the lawyer for the hospital, Joseph Monahan, argued the hospital could not find a hospital-affiliated doctor to administer the ivermectin.
The Herald reported the judge told the hospital to “get out of the way” and allow any board-certified doctor to administer the drug.
When Ms. Fype’s doctor was unable to administer it, the legal team found another doctor, Alan Bain, DO, to do it. Mr. Monahan said Dr. Bain was granted credentials to work at the hospital so he could administer it.
Judge Orel denied a request from Desareta Fype’s lawyer to order the hospital’s nurses to administer further doses. The judge also denied a request to hold the hospital in contempt of court.
A version of this article first appeared on WebMD.com.
Another case, another state, another judge ordering a hospital to give a patient a controversial horse deworming drug to treat a severe case of COVID-19.
, according to the Ohio Capital Journal. Judge Gregory Howard’s ruling comes after Mr. Smith’s wife sued to force the hospital to provide the controversial drug to her husband, who has been hospitalized since July 15.
Julie Smith has gotten Fred Wagshul, MD, to agree to administer ivermectin to her husband. Dr. Wagshul is known as a member of a group of doctors who say the Centers for Disease Control and Prevention and the Food and Drug Administration are lying about ivermectin’s usefulness in fighting COVID-19. Both agencies have warned against using the drug to treat COVID-19, saying there is no evidence it works and that it can be dangerous in large amounts.
According to the Ohio Capital Journal, Dr. Wagshul accused the CDC and FDA of engaging in a “conspiracy” to prevent ivermectin’s use.
But Arthur L. Caplan, MD, professor of bioethics at New York University’s Langone Medical Center, said, “it is absurd that this order was issued,” according to an interview in Ars Technica. “If I were these doctors, I simply wouldn’t do it.”
It is not the first time a judge has ordered ivermectin’s use against a hospital’s wishes.
A 68-year-old woman with COVID-19 in an Illinois hospital started receiving the controversial drug in May after her family sued the hospital to have someone administer it.
Nurije Fype’s daughter, Desareta, filed suit against Elmhurst Hospital, part of Edward-Elmhurst Health, asking that her mother receive the treatment, which is approved as an antiparasitic drug but not approved for the treatment of COVID-19. Desareta Fype was granted temporary guardianship of her mother.
The FDA has published guidance titled “Why You Should Not Use Ivermectin to Treat or Prevent COVID-19” on its website. The National Institutes of Health said there is not enough data to recommend either for or against its use in treating COVID-19.
But DuPage County Judge James Orel ruled Ms. Fype should be allowed to get the treatment.
Three days later, according to the Daily Herald, the lawyer for the hospital, Joseph Monahan, argued the hospital could not find a hospital-affiliated doctor to administer the ivermectin.
The Herald reported the judge told the hospital to “get out of the way” and allow any board-certified doctor to administer the drug.
When Ms. Fype’s doctor was unable to administer it, the legal team found another doctor, Alan Bain, DO, to do it. Mr. Monahan said Dr. Bain was granted credentials to work at the hospital so he could administer it.
Judge Orel denied a request from Desareta Fype’s lawyer to order the hospital’s nurses to administer further doses. The judge also denied a request to hold the hospital in contempt of court.
A version of this article first appeared on WebMD.com.
COVID-clogged ICUs ‘terrify’ those with chronic or emergency illness
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Type 2 diabetes ‘remission’ is a reality, say major organizations
A new joint consensus statement by four major diabetes organizations aims to standardize the terminology, definition, and assessment to the phenomenon of diabetes “remission.”
The statement was jointly issued by the American Diabetes Association, the Endocrine Society, the European Association for the Study of Diabetes, and Diabetes UK.
The 12-member international writing panel proposed use of the term “remission,” as opposed to others such as “reversal,” “resolution,” or “cure,” to describe the phenomenon of prolonged normoglycemia without the use of glucose-lowering medication in a person previously diagnosed with type 2 diabetes.
“Diabetes remission may be occurring more often due to advances in treatment,” writing group member Amy Rothberg, MD, of the University of Michigan, Ann Arbor, said in a statement.
The group defined “remission” – whether attained via lifestyle, bariatric surgery, or other means – as an A1c < 6.5% (< 48 mmol/mol) at least 3 months after cessation of glucose-lowering pharmacotherapy. The panel also suggested monitoring individuals experiencing diabetes remission and raised questions that need further attention and study.
But it’s not a guideline, panel chair Matthew C. Riddle, MD, said in an interview. Rather, the “main purpose of the statement was to provide definitions, terminology, cut-points, and timing recommendations to allow data collection that will eventually lead to clinical guidelines,” he said.
A great deal of epidemiological research is conducted by analyzing data from medical records, he noted. “If clinicians are more consistent in entering data into the records and in doing measurements, it will be a better database.”
Remission reality: Advice needed for deprescribing, talking to patients
“Increasingly our treatments are getting glucose levels into the normal range, and in many cases, even after withdrawal of drug therapy. That’s not an anomaly or a fiction, it’s reality. Clinicians need to know how to talk to their patients about it,” noted Dr. Riddle, of the division of endocrinology, diabetes, and clinical nutrition at Oregon Health & Science University, Portland.
There is a need for data on the effects of deprescribing once normoglycemia is achieved, he said. “It really goes a long way to have strong epidemiological and interventional evidence. That’s what we need here, and that’s what the group is really hoping for.”
The statement recommends the following:
- The term “remission” should be used to describe a sustained metabolic improvement in type 2 diabetes to near normal levels. The panel agreed the word strikes the best balance, given that insulin resistance and beta-cell dysfunction may still be present despite normoglycemia. “Diabetes doesn’t get cured. The underlying abnormalities are still there. Remission is defined by glucose,” Dr. Riddle said. The panel also decided to do away with ADA’s former terms “partial,” “complete,” and “prolonged” remission because they are ambiguous and unhelpful.
- Remission should be defined as a return to an A1c of < 6.5% (< 48 mmol/mol) – the threshold used to diagnose diabetes – spontaneously or following an intervention and that persists for at least 3 months in the absence of usual glucose-lowering medication.
- When A1c may be unreliable, such as conditions involving variant hemoglobin or erythrocyte survival alterations, acceptable alternatives are a fasting blood glucose < 126 mg/dL (< 7.0 mmol/L) or an estimated A1c < 6.5% calculated from continuous glucose monitoring data.
- A1c testing to document a remission should be performed just prior to an intervention and no sooner than 3 months after initiation of the intervention and withdrawal of any glucose-lowering medication.
- Subsequent ongoing A1c testing should be done at least yearly thereafter, along with routine monitoring for diabetes-related complications, including retinal screening, renal function assessment, foot exams, and cardiovascular risk factor testing. “At present, there is no long-term evidence indicating that any of the usually recommended assessments for complications can safely be discontinued,” the authors wrote.
- Research based on the terminology and definitions in the present statement is needed to determine the frequency, duration, and effects on short- and long-term medical outcomes of type 2 diabetes remissions using available interventions.
Dr. Riddle said in an interview: “We thought that the clinical community needed to understand where this issue stands right now. The feasibility of a remission is greater than it used to be.
“We’re going to see more patients who have what we can now call a remission according to a standardized definition. In the future, there are likely to be guidelines regarding the kind of patients and the kind of tactics appropriate for seeking a remission,” he said.
The statement was simultaneously published online in each of the organizations’ respective journals: Diabetes Care, Journal of Clinical Endocrinology & Metabolism, Diabetologia, and Diabetic Medicine.
Dr. Riddle has reported receiving research grant support through Oregon Health & Science University from Eli Lilly, Novo Nordisk, and AstraZeneca and honoraria for consulting from Adocia, Intercept, and Theracos.
A version of this article first appeared on Medscape.com.
A new joint consensus statement by four major diabetes organizations aims to standardize the terminology, definition, and assessment to the phenomenon of diabetes “remission.”
The statement was jointly issued by the American Diabetes Association, the Endocrine Society, the European Association for the Study of Diabetes, and Diabetes UK.
The 12-member international writing panel proposed use of the term “remission,” as opposed to others such as “reversal,” “resolution,” or “cure,” to describe the phenomenon of prolonged normoglycemia without the use of glucose-lowering medication in a person previously diagnosed with type 2 diabetes.
“Diabetes remission may be occurring more often due to advances in treatment,” writing group member Amy Rothberg, MD, of the University of Michigan, Ann Arbor, said in a statement.
The group defined “remission” – whether attained via lifestyle, bariatric surgery, or other means – as an A1c < 6.5% (< 48 mmol/mol) at least 3 months after cessation of glucose-lowering pharmacotherapy. The panel also suggested monitoring individuals experiencing diabetes remission and raised questions that need further attention and study.
But it’s not a guideline, panel chair Matthew C. Riddle, MD, said in an interview. Rather, the “main purpose of the statement was to provide definitions, terminology, cut-points, and timing recommendations to allow data collection that will eventually lead to clinical guidelines,” he said.
A great deal of epidemiological research is conducted by analyzing data from medical records, he noted. “If clinicians are more consistent in entering data into the records and in doing measurements, it will be a better database.”
Remission reality: Advice needed for deprescribing, talking to patients
“Increasingly our treatments are getting glucose levels into the normal range, and in many cases, even after withdrawal of drug therapy. That’s not an anomaly or a fiction, it’s reality. Clinicians need to know how to talk to their patients about it,” noted Dr. Riddle, of the division of endocrinology, diabetes, and clinical nutrition at Oregon Health & Science University, Portland.
There is a need for data on the effects of deprescribing once normoglycemia is achieved, he said. “It really goes a long way to have strong epidemiological and interventional evidence. That’s what we need here, and that’s what the group is really hoping for.”
The statement recommends the following:
- The term “remission” should be used to describe a sustained metabolic improvement in type 2 diabetes to near normal levels. The panel agreed the word strikes the best balance, given that insulin resistance and beta-cell dysfunction may still be present despite normoglycemia. “Diabetes doesn’t get cured. The underlying abnormalities are still there. Remission is defined by glucose,” Dr. Riddle said. The panel also decided to do away with ADA’s former terms “partial,” “complete,” and “prolonged” remission because they are ambiguous and unhelpful.
- Remission should be defined as a return to an A1c of < 6.5% (< 48 mmol/mol) – the threshold used to diagnose diabetes – spontaneously or following an intervention and that persists for at least 3 months in the absence of usual glucose-lowering medication.
- When A1c may be unreliable, such as conditions involving variant hemoglobin or erythrocyte survival alterations, acceptable alternatives are a fasting blood glucose < 126 mg/dL (< 7.0 mmol/L) or an estimated A1c < 6.5% calculated from continuous glucose monitoring data.
- A1c testing to document a remission should be performed just prior to an intervention and no sooner than 3 months after initiation of the intervention and withdrawal of any glucose-lowering medication.
- Subsequent ongoing A1c testing should be done at least yearly thereafter, along with routine monitoring for diabetes-related complications, including retinal screening, renal function assessment, foot exams, and cardiovascular risk factor testing. “At present, there is no long-term evidence indicating that any of the usually recommended assessments for complications can safely be discontinued,” the authors wrote.
- Research based on the terminology and definitions in the present statement is needed to determine the frequency, duration, and effects on short- and long-term medical outcomes of type 2 diabetes remissions using available interventions.
Dr. Riddle said in an interview: “We thought that the clinical community needed to understand where this issue stands right now. The feasibility of a remission is greater than it used to be.
“We’re going to see more patients who have what we can now call a remission according to a standardized definition. In the future, there are likely to be guidelines regarding the kind of patients and the kind of tactics appropriate for seeking a remission,” he said.
The statement was simultaneously published online in each of the organizations’ respective journals: Diabetes Care, Journal of Clinical Endocrinology & Metabolism, Diabetologia, and Diabetic Medicine.
Dr. Riddle has reported receiving research grant support through Oregon Health & Science University from Eli Lilly, Novo Nordisk, and AstraZeneca and honoraria for consulting from Adocia, Intercept, and Theracos.
A version of this article first appeared on Medscape.com.
A new joint consensus statement by four major diabetes organizations aims to standardize the terminology, definition, and assessment to the phenomenon of diabetes “remission.”
The statement was jointly issued by the American Diabetes Association, the Endocrine Society, the European Association for the Study of Diabetes, and Diabetes UK.
The 12-member international writing panel proposed use of the term “remission,” as opposed to others such as “reversal,” “resolution,” or “cure,” to describe the phenomenon of prolonged normoglycemia without the use of glucose-lowering medication in a person previously diagnosed with type 2 diabetes.
“Diabetes remission may be occurring more often due to advances in treatment,” writing group member Amy Rothberg, MD, of the University of Michigan, Ann Arbor, said in a statement.
The group defined “remission” – whether attained via lifestyle, bariatric surgery, or other means – as an A1c < 6.5% (< 48 mmol/mol) at least 3 months after cessation of glucose-lowering pharmacotherapy. The panel also suggested monitoring individuals experiencing diabetes remission and raised questions that need further attention and study.
But it’s not a guideline, panel chair Matthew C. Riddle, MD, said in an interview. Rather, the “main purpose of the statement was to provide definitions, terminology, cut-points, and timing recommendations to allow data collection that will eventually lead to clinical guidelines,” he said.
A great deal of epidemiological research is conducted by analyzing data from medical records, he noted. “If clinicians are more consistent in entering data into the records and in doing measurements, it will be a better database.”
Remission reality: Advice needed for deprescribing, talking to patients
“Increasingly our treatments are getting glucose levels into the normal range, and in many cases, even after withdrawal of drug therapy. That’s not an anomaly or a fiction, it’s reality. Clinicians need to know how to talk to their patients about it,” noted Dr. Riddle, of the division of endocrinology, diabetes, and clinical nutrition at Oregon Health & Science University, Portland.
There is a need for data on the effects of deprescribing once normoglycemia is achieved, he said. “It really goes a long way to have strong epidemiological and interventional evidence. That’s what we need here, and that’s what the group is really hoping for.”
The statement recommends the following:
- The term “remission” should be used to describe a sustained metabolic improvement in type 2 diabetes to near normal levels. The panel agreed the word strikes the best balance, given that insulin resistance and beta-cell dysfunction may still be present despite normoglycemia. “Diabetes doesn’t get cured. The underlying abnormalities are still there. Remission is defined by glucose,” Dr. Riddle said. The panel also decided to do away with ADA’s former terms “partial,” “complete,” and “prolonged” remission because they are ambiguous and unhelpful.
- Remission should be defined as a return to an A1c of < 6.5% (< 48 mmol/mol) – the threshold used to diagnose diabetes – spontaneously or following an intervention and that persists for at least 3 months in the absence of usual glucose-lowering medication.
- When A1c may be unreliable, such as conditions involving variant hemoglobin or erythrocyte survival alterations, acceptable alternatives are a fasting blood glucose < 126 mg/dL (< 7.0 mmol/L) or an estimated A1c < 6.5% calculated from continuous glucose monitoring data.
- A1c testing to document a remission should be performed just prior to an intervention and no sooner than 3 months after initiation of the intervention and withdrawal of any glucose-lowering medication.
- Subsequent ongoing A1c testing should be done at least yearly thereafter, along with routine monitoring for diabetes-related complications, including retinal screening, renal function assessment, foot exams, and cardiovascular risk factor testing. “At present, there is no long-term evidence indicating that any of the usually recommended assessments for complications can safely be discontinued,” the authors wrote.
- Research based on the terminology and definitions in the present statement is needed to determine the frequency, duration, and effects on short- and long-term medical outcomes of type 2 diabetes remissions using available interventions.
Dr. Riddle said in an interview: “We thought that the clinical community needed to understand where this issue stands right now. The feasibility of a remission is greater than it used to be.
“We’re going to see more patients who have what we can now call a remission according to a standardized definition. In the future, there are likely to be guidelines regarding the kind of patients and the kind of tactics appropriate for seeking a remission,” he said.
The statement was simultaneously published online in each of the organizations’ respective journals: Diabetes Care, Journal of Clinical Endocrinology & Metabolism, Diabetologia, and Diabetic Medicine.
Dr. Riddle has reported receiving research grant support through Oregon Health & Science University from Eli Lilly, Novo Nordisk, and AstraZeneca and honoraria for consulting from Adocia, Intercept, and Theracos.
A version of this article first appeared on Medscape.com.
Two swings, two misses with colchicine, Vascepa in COVID-19
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agents colchicine and icosapent ethyl (Vascepa; Amarin) failed to provide substantial benefits in separate randomized COVID-19 trials.
Both were reported at the European Society of Cardiology (ESC) Congress 2021.
The open-label ECLA PHRI COLCOVID trial randomized 1,277 hospitalized adults (mean age 62 years) to usual care alone or with colchicine at a loading dose of 1.5 mg for 2 hours followed by 0.5 mg on day 1 and then 0.5 mg twice daily for 14 days or until discharge.
The investigators hypothesized that colchicine, which is widely used to treat gout and other inflammatory conditions, might modulate the hyperinflammatory syndrome, or cytokine storm, associated with COVID-19.
Results showed that the need for mechanical ventilation or death occurred in 25.0% of patients receiving colchicine and 28.8% with usual care (P = .08).
The coprimary endpoint of death at 28 days was also not significantly different between groups (20.5% vs. 22.2%), principal investigator Rafael Diaz, MD, said in a late-breaking COVID-19 trials session at the congress.
Among the secondary outcomes at 28 days, colchicine significantly reduced the incidence of new intubation or death from respiratory failure from 27.0% to 22.3% (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99) but not mortality from respiratory failure (19.5% vs. 16.8%).
The only important adverse effect was severe diarrhea, which was reported in 11.3% of the colchicine group vs. 4.5% in the control group, said Dr. Diaz, director of Estudios Clínicos Latinoamérica (ECLA), Rosario, Argentina.
The results are consistent with those from the massive RECOVERY trial, which earlier this year stopped enrollment in the colchicine arm for lack of efficacy in patients hospitalized with COVID-19, and COLCORONA, which missed its primary endpoint using colchicine among nonhospitalized adults with COVID-19.
Session chair and COLCORONA principal investigator Jean-Claude Tardif, MD, pointed out that, as clinicians, it’s fairly uncommon to combine systemic steroids with colchicine, which was the case in 92% of patients in ECLA PHRI COLCOVID.
“I think it is an inherent limitation of testing colchicine on top of steroids,” said Dr. Tardif, of the Montreal Heart Institute.
Icosapent ethyl in PREPARE-IT
Dr. Diaz returned in the ESC session to present the results of the PREPARE-IT trial, which tested whether icosapent ethyl – at a loading dose of 8 grams (4 capsules) for the first 3 days and 4 g/d on days 4-60 – could reduce the risk for SARS-CoV-2 infection in 2,041 health care and other public workers in Argentina at high risk for infection (mean age 40.5 years).
Vascepa was approved by the Food and Drug Administration in 2012 for the reduction of elevated triglyceride levels, with an added indication in 2019 to reduce cardiovascular (CV) events in people with elevated triglycerides and established CV disease or diabetes with other CV risk factors.
The rationale for using the high-dose prescription eicosapentaenoic acid (EPA) preparation includes its anti-inflammatory and antithrombotic effects, and that unsaturated fatty acids, especially EPA, might inactivate the enveloped virus, he explained.
Among 1,712 participants followed for up to 60 days, however, the SARS-CoV-2 infection rate was 7.9% with icosapent ethyl vs. 7.1% with a mineral oil placebo (P = .58).
There were also no significant changes from baseline in the icosapent ethyl and placebo groups for the secondary outcomes of high-sensitivity C-reactive protein (0 vs. 0), triglycerides (median –2 mg/dL vs. 7 mg/dL), or Influenza Patient-Reported Outcome (FLU-PRO) questionnaire scores (median 0.01 vs. 0.03).
The use of a mineral oil placebo has been the subject of controversy in previous fish oil trials, but, Dr. Diaz noted, it did not have a significant proinflammatory effect or cause any excess adverse events.
Overall, adverse events were similar between the active and placebo groups, including atrial fibrillation (none), major bleeding (none), minor bleeding (7 events vs. 10 events), gastrointestinal symptoms (6.8% vs. 7.0%), and diarrhea (8.6% vs. 7.7%).
Although it missed the primary endpoint, Dr. Diaz said, “this is the first large, randomized blinded trial to demonstrate excellent safety and tolerability of an 8-gram-per-day loading dose of icosapent ethyl, opening up the potential for acute use in randomized trials of myocardial infarction, acute coronary syndromes, strokes, and revascularization.”
During a discussion of the results, Dr. Diaz said the Delta variant was not present at the time of the analysis and that the second half of the trial will report on whether icosapent ethyl can reduce the risk for hospitalization or death in participants diagnosed with COVID-19.
ECLA PHRI COLCOVID was supported by the Estudios Clínicos Latinoamérica Population Health Research Institute. PREPARE-IT was supported by Estudios Clínicos Latinoamérica with collaboration from Amarin. Dr. Diaz reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘This food will kill you, that food will save you’
Not sure if you’ve heard the news, but eating a single hot dog will apparently cost you 36 minutes of healthy life. My first thought when hearing this was of course the same as everyone else’s: Poor Joey Chestnut, multiyear winner of Nathan’s annual hot dog–eating contest.
He won this year’s contest with 76 hot dogs, which puts his total number of competition-consumed hot dogs at 1,089 – which cost him, it would seem, 27.2 days of healthy life. Unless, of course, every hot dog he inhaled came with a bun hosting two portions of sesame seeds, which in turn would buy him 50 extra minutes of life (25 minutes per portion, you see) and would consequently have extended his life by 10.6 days.
Clearly, the obvious solution here is to ensure that all hot dog buns have two portions of sesame seeds on them moving forward; that way, hot dogs can transition from being poisonous killers to antiaging medicine.
The other solution, albeit less exciting, perhaps, is for researchers to stop studying single foods’ impacts on health, and/or for journals to stop publishing them, and/or for the media to stop promoting them – because they are all as ridiculously useless as the example above highlighting findings from a newly published study in Nature Food, entitled “Small targeted dietary changes can yield substantial gains for human health and the environment.”
While no doubt we would all love for diet and health to be so well understood that we could choose specific single foods (knowing that they would prolong our lives) while avoiding single foods that would shorten it, there’s this unfortunate truth that the degree of confounding among food alone is staggering. People eat thousands of different foods in thousands of different dietary combinations. Moreover, most (all?) research conducted on dietary impacts of single foods on health don’t actually track consumption of those specific foods over time, let alone their interactions with all other foods consumed, but rather at moments in time.
In the case of the “hot dogs will kill you unless there are sesame seeds on your bun” article, for example, the researchers utilized one solitary dietary recall session upon which to base their ridiculously specific, ridiculous conclusions.
People’s diets also change over time for various reasons, and of course people themselves are very different. You might imagine that people whose diets are rich in chicken wings, sugared soda, and hot dogs will have markedly different lifestyles and demographics than those whose diets are rich in walnuts, sashimi, and avocados.
So why do we keep seeing studies like this being published? Is it because they’re basically clickbait catnip for journals and newspapers, and in our publish-or-perish attention-seeking world, that means they not only get a pass but they get a press release? Is it because peer review is broken and everyone knows it? Is it because as a society, we’re frogs who have been steeping for decades in the ever-heated pot of nutritional nonsense, and consequently don’t think to question it?
I don’t know the answer to any of those questions, but one thing I do know: Studies on single foods’ impact on life length are pointless, impossible, and idiotic, and people who share them noncritically should be forever shunned – or at the very least, forever ignored.
Yoni Freedhoff, MD, is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute, a nonsurgical weight-management center.
A version of this article first appeared on Medscape.com.
Not sure if you’ve heard the news, but eating a single hot dog will apparently cost you 36 minutes of healthy life. My first thought when hearing this was of course the same as everyone else’s: Poor Joey Chestnut, multiyear winner of Nathan’s annual hot dog–eating contest.
He won this year’s contest with 76 hot dogs, which puts his total number of competition-consumed hot dogs at 1,089 – which cost him, it would seem, 27.2 days of healthy life. Unless, of course, every hot dog he inhaled came with a bun hosting two portions of sesame seeds, which in turn would buy him 50 extra minutes of life (25 minutes per portion, you see) and would consequently have extended his life by 10.6 days.
Clearly, the obvious solution here is to ensure that all hot dog buns have two portions of sesame seeds on them moving forward; that way, hot dogs can transition from being poisonous killers to antiaging medicine.
The other solution, albeit less exciting, perhaps, is for researchers to stop studying single foods’ impacts on health, and/or for journals to stop publishing them, and/or for the media to stop promoting them – because they are all as ridiculously useless as the example above highlighting findings from a newly published study in Nature Food, entitled “Small targeted dietary changes can yield substantial gains for human health and the environment.”
While no doubt we would all love for diet and health to be so well understood that we could choose specific single foods (knowing that they would prolong our lives) while avoiding single foods that would shorten it, there’s this unfortunate truth that the degree of confounding among food alone is staggering. People eat thousands of different foods in thousands of different dietary combinations. Moreover, most (all?) research conducted on dietary impacts of single foods on health don’t actually track consumption of those specific foods over time, let alone their interactions with all other foods consumed, but rather at moments in time.
In the case of the “hot dogs will kill you unless there are sesame seeds on your bun” article, for example, the researchers utilized one solitary dietary recall session upon which to base their ridiculously specific, ridiculous conclusions.
People’s diets also change over time for various reasons, and of course people themselves are very different. You might imagine that people whose diets are rich in chicken wings, sugared soda, and hot dogs will have markedly different lifestyles and demographics than those whose diets are rich in walnuts, sashimi, and avocados.
So why do we keep seeing studies like this being published? Is it because they’re basically clickbait catnip for journals and newspapers, and in our publish-or-perish attention-seeking world, that means they not only get a pass but they get a press release? Is it because peer review is broken and everyone knows it? Is it because as a society, we’re frogs who have been steeping for decades in the ever-heated pot of nutritional nonsense, and consequently don’t think to question it?
I don’t know the answer to any of those questions, but one thing I do know: Studies on single foods’ impact on life length are pointless, impossible, and idiotic, and people who share them noncritically should be forever shunned – or at the very least, forever ignored.
Yoni Freedhoff, MD, is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute, a nonsurgical weight-management center.
A version of this article first appeared on Medscape.com.
Not sure if you’ve heard the news, but eating a single hot dog will apparently cost you 36 minutes of healthy life. My first thought when hearing this was of course the same as everyone else’s: Poor Joey Chestnut, multiyear winner of Nathan’s annual hot dog–eating contest.
He won this year’s contest with 76 hot dogs, which puts his total number of competition-consumed hot dogs at 1,089 – which cost him, it would seem, 27.2 days of healthy life. Unless, of course, every hot dog he inhaled came with a bun hosting two portions of sesame seeds, which in turn would buy him 50 extra minutes of life (25 minutes per portion, you see) and would consequently have extended his life by 10.6 days.
Clearly, the obvious solution here is to ensure that all hot dog buns have two portions of sesame seeds on them moving forward; that way, hot dogs can transition from being poisonous killers to antiaging medicine.
The other solution, albeit less exciting, perhaps, is for researchers to stop studying single foods’ impacts on health, and/or for journals to stop publishing them, and/or for the media to stop promoting them – because they are all as ridiculously useless as the example above highlighting findings from a newly published study in Nature Food, entitled “Small targeted dietary changes can yield substantial gains for human health and the environment.”
While no doubt we would all love for diet and health to be so well understood that we could choose specific single foods (knowing that they would prolong our lives) while avoiding single foods that would shorten it, there’s this unfortunate truth that the degree of confounding among food alone is staggering. People eat thousands of different foods in thousands of different dietary combinations. Moreover, most (all?) research conducted on dietary impacts of single foods on health don’t actually track consumption of those specific foods over time, let alone their interactions with all other foods consumed, but rather at moments in time.
In the case of the “hot dogs will kill you unless there are sesame seeds on your bun” article, for example, the researchers utilized one solitary dietary recall session upon which to base their ridiculously specific, ridiculous conclusions.
People’s diets also change over time for various reasons, and of course people themselves are very different. You might imagine that people whose diets are rich in chicken wings, sugared soda, and hot dogs will have markedly different lifestyles and demographics than those whose diets are rich in walnuts, sashimi, and avocados.
So why do we keep seeing studies like this being published? Is it because they’re basically clickbait catnip for journals and newspapers, and in our publish-or-perish attention-seeking world, that means they not only get a pass but they get a press release? Is it because peer review is broken and everyone knows it? Is it because as a society, we’re frogs who have been steeping for decades in the ever-heated pot of nutritional nonsense, and consequently don’t think to question it?
I don’t know the answer to any of those questions, but one thing I do know: Studies on single foods’ impact on life length are pointless, impossible, and idiotic, and people who share them noncritically should be forever shunned – or at the very least, forever ignored.
Yoni Freedhoff, MD, is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute, a nonsurgical weight-management center.
A version of this article first appeared on Medscape.com.
‘Deeper dive’ into opioid overdose deaths during COVID pandemic
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Long COVID symptoms can persist for more than 1 year, study shows
Nearly half of people who are hospitalized with COVID-19 suffer at least one lingering symptom 1 year after discharge, according to the largest study yet to assess the dynamic recovery of a group of COVID-19 survivors 12 months after the illness.
The most common lingering symptoms are fatigue and muscle weakness. One-third continue to have shortness of breath.
Overall, at 12 months, COVID-19 survivors had more problems with mobility, pain or discomfort, and anxiety or depression, and had lower self-assessment scores of quality of life than matched COVID-free peers, the investigators report.
The study was published online Aug. 28 in The Lancet.
“While most had made a good recovery, health problems persisted in some patients, especially those who had been critically ill during their hospital stay,” Bin Cao, MD, from the National Center for Respiratory Medicine at the China-Japan Friendship Hospital and Capital Medical University, both in Beijing, said in a Lancet news release.
“Our findings suggest that recovery for some patients will take longer than 1 year, and this should be taken into account when planning delivery of health care services post pandemic,” Dr. Cao said.
“As the COVID-19 pandemic continues, the need to understand and respond to long COVID is increasingly pressing,” says a Lancet editorial.
“Symptoms such as persistent fatigue, breathlessness, brain fog, and depression could debilitate many millions of people globally. Long COVID is a modern medical challenge of the first order,” it reads.
Study details
Dr. Cao and colleagues studied 1,276 COVID-19 patients (median age 59; 53% men) discharged from a hospital in Wuhan, China, between Jan. 7 and May 29, 2020. The patients were assessed at 6 and 12 months from the date they first experienced COVID-19 symptoms.
Many symptoms resolved over time, regardless of the severity of illness. Yet 49% of patients still had at least one symptom 12 months after their acute illness, down from 68% at the 6-month mark, the authors report.
Fatigue and muscle weakness were the most commonly reported symptoms seen in 52% of patients at 6 months and 20% at 12 months. Compared with men, women were 1.4 times more likely to report fatigue or muscle weakness.
Patients treated with corticosteroids during the acute phase of COVID-19 were 1.5 times as likely to experience fatigue or muscle weakness after 12 months, compared with those who had not received corticosteroids.
Thirty percent of patients reported dyspnea at 12 months, slightly more than at 6 months (26%). Dyspnea was more common in the most severely ill patients needing a ventilator during their hospital stay (39%), compared with those who did not need oxygen treatment (25%).
At the 6-month check, 349 study participants underwent pulmonary function tests and 244 of those patients completed the same test at 12 months.
Spirometric and lung volume parameters of most of these patients were within normal limits at 12 months. But lung diffusion impairment was observed in about 20%-30% of patients who had been moderately ill with COVID-19 and as high as 54% in critically ill patients.
Compared with men, women were almost three times as likely to have lung diffusion impairment after 12 months.
Of 186 patients with abnormal lung CT scan at 6 months, 118 patients had a repeat CT scan at 12 months. The lung imaging abnormality gradually recovered during follow-up, yet 76% of the most critically ill patients still had ground glass opacity at 12 months.
Mental health hit
Among those patients who had been employed full- or part-time before catching COVID, the majority had returned to their original job (88%) and most had returned to their pre-COVID-19 level of work (76%) within 12 months.
Among those who did not return to their original work, 32% cited decreased physical function, 25% were unwilling to do their previous job, and 18% were unemployed.
As shown in multiple other studies, COVID-19 can take a toll on mental health. In this cohort, slightly more patients reported anxiety or depression at 12 months than at 6 months (23% vs. 26%), and the proportion was much greater than in matched community-dwelling adults without COVID-19 (5%).
Compared with men, women were twice as likely to report anxiety or depression.
“We do not yet fully understand why psychiatric symptoms are slightly more common at 1 year than at 6 months in COVID-19 survivors,” study author Xiaoying Gu, PhD, from the Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, said in the news release.
“These could be caused by a biological process linked to the virus infection itself, or the body’s immune response to it. Or they could be linked to reduced social contact, loneliness, incomplete recovery of physical health, or loss of employment associated with illness. Large, long-term studies of COVID-19 survivors are needed so that we can better understand the long-term physical and mental health consequences of COVID-19,” Dr. Gu said.
The authors caution that the findings represent a group of patients from a single hospital in China and the cohort included only a small number of patients who had been admitted to intensive care (94 of 1,276; 7.4%).
The Lancet editorial urges the scientific and medical community to “collaborate to explore the mechanism and pathogenesis of long COVID, estimate the global and regional disease burdens, better delineate who is most at risk, understand how vaccines might affect the condition, and find effective treatments via randomized controlled trials.”
“At the same time, health care providers must acknowledge and validate the toll of the persistent symptoms of long COVID on patients, and health systems need to be prepared to meet individualized, patient-oriented goals, with an appropriately trained workforce involving physical, cognitive, social, and occupational elements,” the editorial states.
“Answering these research questions while providing compassionate and multidisciplinary care will require the full breadth of scientific and medical ingenuity. It is a challenge to which the whole health community must rise,” the editorialists conclude.
The study was funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, the National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, the China Evergrande Group, the Jack Ma Foundation, Sino Biopharmaceutical, the Ping An Insurance (Group), and the New Sunshine Charity Foundation. The full list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Nearly half of people who are hospitalized with COVID-19 suffer at least one lingering symptom 1 year after discharge, according to the largest study yet to assess the dynamic recovery of a group of COVID-19 survivors 12 months after the illness.
The most common lingering symptoms are fatigue and muscle weakness. One-third continue to have shortness of breath.
Overall, at 12 months, COVID-19 survivors had more problems with mobility, pain or discomfort, and anxiety or depression, and had lower self-assessment scores of quality of life than matched COVID-free peers, the investigators report.
The study was published online Aug. 28 in The Lancet.
“While most had made a good recovery, health problems persisted in some patients, especially those who had been critically ill during their hospital stay,” Bin Cao, MD, from the National Center for Respiratory Medicine at the China-Japan Friendship Hospital and Capital Medical University, both in Beijing, said in a Lancet news release.
“Our findings suggest that recovery for some patients will take longer than 1 year, and this should be taken into account when planning delivery of health care services post pandemic,” Dr. Cao said.
“As the COVID-19 pandemic continues, the need to understand and respond to long COVID is increasingly pressing,” says a Lancet editorial.
“Symptoms such as persistent fatigue, breathlessness, brain fog, and depression could debilitate many millions of people globally. Long COVID is a modern medical challenge of the first order,” it reads.
Study details
Dr. Cao and colleagues studied 1,276 COVID-19 patients (median age 59; 53% men) discharged from a hospital in Wuhan, China, between Jan. 7 and May 29, 2020. The patients were assessed at 6 and 12 months from the date they first experienced COVID-19 symptoms.
Many symptoms resolved over time, regardless of the severity of illness. Yet 49% of patients still had at least one symptom 12 months after their acute illness, down from 68% at the 6-month mark, the authors report.
Fatigue and muscle weakness were the most commonly reported symptoms seen in 52% of patients at 6 months and 20% at 12 months. Compared with men, women were 1.4 times more likely to report fatigue or muscle weakness.
Patients treated with corticosteroids during the acute phase of COVID-19 were 1.5 times as likely to experience fatigue or muscle weakness after 12 months, compared with those who had not received corticosteroids.
Thirty percent of patients reported dyspnea at 12 months, slightly more than at 6 months (26%). Dyspnea was more common in the most severely ill patients needing a ventilator during their hospital stay (39%), compared with those who did not need oxygen treatment (25%).
At the 6-month check, 349 study participants underwent pulmonary function tests and 244 of those patients completed the same test at 12 months.
Spirometric and lung volume parameters of most of these patients were within normal limits at 12 months. But lung diffusion impairment was observed in about 20%-30% of patients who had been moderately ill with COVID-19 and as high as 54% in critically ill patients.
Compared with men, women were almost three times as likely to have lung diffusion impairment after 12 months.
Of 186 patients with abnormal lung CT scan at 6 months, 118 patients had a repeat CT scan at 12 months. The lung imaging abnormality gradually recovered during follow-up, yet 76% of the most critically ill patients still had ground glass opacity at 12 months.
Mental health hit
Among those patients who had been employed full- or part-time before catching COVID, the majority had returned to their original job (88%) and most had returned to their pre-COVID-19 level of work (76%) within 12 months.
Among those who did not return to their original work, 32% cited decreased physical function, 25% were unwilling to do their previous job, and 18% were unemployed.
As shown in multiple other studies, COVID-19 can take a toll on mental health. In this cohort, slightly more patients reported anxiety or depression at 12 months than at 6 months (23% vs. 26%), and the proportion was much greater than in matched community-dwelling adults without COVID-19 (5%).
Compared with men, women were twice as likely to report anxiety or depression.
“We do not yet fully understand why psychiatric symptoms are slightly more common at 1 year than at 6 months in COVID-19 survivors,” study author Xiaoying Gu, PhD, from the Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, said in the news release.
“These could be caused by a biological process linked to the virus infection itself, or the body’s immune response to it. Or they could be linked to reduced social contact, loneliness, incomplete recovery of physical health, or loss of employment associated with illness. Large, long-term studies of COVID-19 survivors are needed so that we can better understand the long-term physical and mental health consequences of COVID-19,” Dr. Gu said.
The authors caution that the findings represent a group of patients from a single hospital in China and the cohort included only a small number of patients who had been admitted to intensive care (94 of 1,276; 7.4%).
The Lancet editorial urges the scientific and medical community to “collaborate to explore the mechanism and pathogenesis of long COVID, estimate the global and regional disease burdens, better delineate who is most at risk, understand how vaccines might affect the condition, and find effective treatments via randomized controlled trials.”
“At the same time, health care providers must acknowledge and validate the toll of the persistent symptoms of long COVID on patients, and health systems need to be prepared to meet individualized, patient-oriented goals, with an appropriately trained workforce involving physical, cognitive, social, and occupational elements,” the editorial states.
“Answering these research questions while providing compassionate and multidisciplinary care will require the full breadth of scientific and medical ingenuity. It is a challenge to which the whole health community must rise,” the editorialists conclude.
The study was funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, the National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, the China Evergrande Group, the Jack Ma Foundation, Sino Biopharmaceutical, the Ping An Insurance (Group), and the New Sunshine Charity Foundation. The full list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Nearly half of people who are hospitalized with COVID-19 suffer at least one lingering symptom 1 year after discharge, according to the largest study yet to assess the dynamic recovery of a group of COVID-19 survivors 12 months after the illness.
The most common lingering symptoms are fatigue and muscle weakness. One-third continue to have shortness of breath.
Overall, at 12 months, COVID-19 survivors had more problems with mobility, pain or discomfort, and anxiety or depression, and had lower self-assessment scores of quality of life than matched COVID-free peers, the investigators report.
The study was published online Aug. 28 in The Lancet.
“While most had made a good recovery, health problems persisted in some patients, especially those who had been critically ill during their hospital stay,” Bin Cao, MD, from the National Center for Respiratory Medicine at the China-Japan Friendship Hospital and Capital Medical University, both in Beijing, said in a Lancet news release.
“Our findings suggest that recovery for some patients will take longer than 1 year, and this should be taken into account when planning delivery of health care services post pandemic,” Dr. Cao said.
“As the COVID-19 pandemic continues, the need to understand and respond to long COVID is increasingly pressing,” says a Lancet editorial.
“Symptoms such as persistent fatigue, breathlessness, brain fog, and depression could debilitate many millions of people globally. Long COVID is a modern medical challenge of the first order,” it reads.
Study details
Dr. Cao and colleagues studied 1,276 COVID-19 patients (median age 59; 53% men) discharged from a hospital in Wuhan, China, between Jan. 7 and May 29, 2020. The patients were assessed at 6 and 12 months from the date they first experienced COVID-19 symptoms.
Many symptoms resolved over time, regardless of the severity of illness. Yet 49% of patients still had at least one symptom 12 months after their acute illness, down from 68% at the 6-month mark, the authors report.
Fatigue and muscle weakness were the most commonly reported symptoms seen in 52% of patients at 6 months and 20% at 12 months. Compared with men, women were 1.4 times more likely to report fatigue or muscle weakness.
Patients treated with corticosteroids during the acute phase of COVID-19 were 1.5 times as likely to experience fatigue or muscle weakness after 12 months, compared with those who had not received corticosteroids.
Thirty percent of patients reported dyspnea at 12 months, slightly more than at 6 months (26%). Dyspnea was more common in the most severely ill patients needing a ventilator during their hospital stay (39%), compared with those who did not need oxygen treatment (25%).
At the 6-month check, 349 study participants underwent pulmonary function tests and 244 of those patients completed the same test at 12 months.
Spirometric and lung volume parameters of most of these patients were within normal limits at 12 months. But lung diffusion impairment was observed in about 20%-30% of patients who had been moderately ill with COVID-19 and as high as 54% in critically ill patients.
Compared with men, women were almost three times as likely to have lung diffusion impairment after 12 months.
Of 186 patients with abnormal lung CT scan at 6 months, 118 patients had a repeat CT scan at 12 months. The lung imaging abnormality gradually recovered during follow-up, yet 76% of the most critically ill patients still had ground glass opacity at 12 months.
Mental health hit
Among those patients who had been employed full- or part-time before catching COVID, the majority had returned to their original job (88%) and most had returned to their pre-COVID-19 level of work (76%) within 12 months.
Among those who did not return to their original work, 32% cited decreased physical function, 25% were unwilling to do their previous job, and 18% were unemployed.
As shown in multiple other studies, COVID-19 can take a toll on mental health. In this cohort, slightly more patients reported anxiety or depression at 12 months than at 6 months (23% vs. 26%), and the proportion was much greater than in matched community-dwelling adults without COVID-19 (5%).
Compared with men, women were twice as likely to report anxiety or depression.
“We do not yet fully understand why psychiatric symptoms are slightly more common at 1 year than at 6 months in COVID-19 survivors,” study author Xiaoying Gu, PhD, from the Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, said in the news release.
“These could be caused by a biological process linked to the virus infection itself, or the body’s immune response to it. Or they could be linked to reduced social contact, loneliness, incomplete recovery of physical health, or loss of employment associated with illness. Large, long-term studies of COVID-19 survivors are needed so that we can better understand the long-term physical and mental health consequences of COVID-19,” Dr. Gu said.
The authors caution that the findings represent a group of patients from a single hospital in China and the cohort included only a small number of patients who had been admitted to intensive care (94 of 1,276; 7.4%).
The Lancet editorial urges the scientific and medical community to “collaborate to explore the mechanism and pathogenesis of long COVID, estimate the global and regional disease burdens, better delineate who is most at risk, understand how vaccines might affect the condition, and find effective treatments via randomized controlled trials.”
“At the same time, health care providers must acknowledge and validate the toll of the persistent symptoms of long COVID on patients, and health systems need to be prepared to meet individualized, patient-oriented goals, with an appropriately trained workforce involving physical, cognitive, social, and occupational elements,” the editorial states.
“Answering these research questions while providing compassionate and multidisciplinary care will require the full breadth of scientific and medical ingenuity. It is a challenge to which the whole health community must rise,” the editorialists conclude.
The study was funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, the National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, the China Evergrande Group, the Jack Ma Foundation, Sino Biopharmaceutical, the Ping An Insurance (Group), and the New Sunshine Charity Foundation. The full list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.