User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
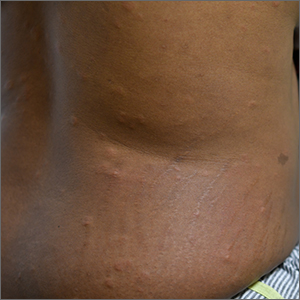
Colorectal polyps often recur after incomplete resection
When gastroenterologists resect polyps during colonoscopy, they may inadvertently leave some neoplastic tissue behind. Biopsies from resection margins sometimes contain polyp remnants, even after a lesion has been visibly removed, research has shown. But the significance of incomplete resection has been unclear.
A new study indicates that incomplete resection, compared with complete resection, substantially increases the likelihood that a polyp will be found during follow-up colonoscopy.
The evidence “strongly supports the hypothesis” that residual polyp tissue likely contributes to neoplastic polyp recurrence and, by extension, interval colorectal cancer, said study author Heiko Pohl, MD, and colleagues. Dr. Pohl is affiliated with the White River Junction Veterans Affairs Medical Center in Vermont and Dartmouth-Hitchcock Medical Center and Dartmouth Geisel School of Medicine in Hanover, N.H.
Among 166 participants in the Complete Adenoma Resection (CARE) study who went on to have a surveillance colonoscopy in the following years, metachronous neoplasia was detected more often in colon segments where polyps had been incompletely resected, compared with colon segments with complete resections (52% vs. 23%), according to a study in Annals of Internal Medicine.
In addition, colon segments that had had incomplete resections in the CARE study were more likely to have polyps that were 10 mm or greater (18% vs. 3%). The 32 participants who had at least one incomplete resection during the original CARE study were three times more likely to have a polyp at follow-up, Dr. Pohl and coauthors found.
There were no instances of cancer or high-grade dysplasia during follow-up, however.
Key questions remain, including “Why do people get cancer after colonoscopy?” said Shai Friedland, MD, MS, who was not involved in the study. “Maybe it is because of these polyps that were not removed completely.”
On the other hand, lesions that are more difficult to see in the first place and more dangerous might be responsible when colorectal cancer does occur after colonoscopy, said Dr. Friedland, professor of gastroenterology and hepatology at Stanford (Calif.) University and a gastroenterologist at Veterans Affairs Palo Alto (Calif.) Health Care System.
Although it is not surprising that polyp remnants may appear as polyps during follow-up examinations, the difference in polyp recurrence between groups “must be quite striking” for it to clearly be seen in this small study, Dr. Friedland said. The results suggest that there is a need to “redouble our efforts to make sure that we remove polyps completely and not just hope that they disappear after an incomplete job,” he added.
In the CARE study, which was conducted between 2009 and 2012 at two academic medical centers, researchers found that about 10% of neoplastic polyps with visibly complete removal may nonetheless be incompletely resected, based on the presence of neoplastic tissue in biopsies taken from resection margins.
The investigators focused on 5- to 20-mm nonpedunculated colorectal polyps that were removed during routine polypectomy using electrocautery snare resection.
Participants with incomplete polyp resections were encouraged to have a surveillance examination within 1 year, and those with complete resections received guideline-based surveillance recommendations. In the follow-up analysis, those with an incomplete resection had a median time to first examination of 17 months, and those with complete resections had a median time to first examination of 45 months.
“These results highlight the importance of achieving complete resection because the risk for subsequent advanced neoplasia detection statistically significantly increases without it,” Dr. Pohl said. “The large polyp size after incomplete resection within a generally short follow-up time may imply fast growth, even if none of the presumed recurrent polyps contained advanced histologic characteristics.”
Between-group differences in the participants and polyps could confound the observations, the authors noted. It is possible that endoscopists who did the follow-up colonoscopies looked harder for polyps in colon segments that had prior incomplete resections, on account of the initial study findings, they said. “It is also plausible that endoscopists with a greater incomplete resection rate would be more likely to miss polyps, which could then be detected at follow-up,” though detection and resection skills do not necessarily correlate, the investigators said.
With resection, the goal is to remove the polyp in one piece with clear margins, and researchers have known that the polyp removal techniques used in the study “are far from perfect,” Dr. Friedland said. One question is whether the field should focus on improving standard techniques, or incorporating newer, more advanced techniques such as endoscopic submucosal dissection, which is effective but may entail significant perforation risk when performed by someone who is not proficient, he said.
“One of the major focuses we had over the years is finding the polyps to prevent future cancer,” Dr. Pohl said in an interview. But the CARE study showed that “we do not do a good job of taking polyps off completely,” he added. The present analysis underscores that clinicians should do their best to remove polyps completely, even if they lack a way to confirm complete resection in routine practice, as is currently the case, Dr. Pohl said. To that end, clinicians should aim to refine their skills and techniques. Video evaluations with guidance from experts may prove helpful, he suggested. “We need to think hard about quality assessment,” Dr. Pohl said.
Dr. Pohl disclosed grants from Boston Scientific, Cosmo Pharmaceuticals, and Steris outside of the study. Dr. Friedland had no disclosures.
When gastroenterologists resect polyps during colonoscopy, they may inadvertently leave some neoplastic tissue behind. Biopsies from resection margins sometimes contain polyp remnants, even after a lesion has been visibly removed, research has shown. But the significance of incomplete resection has been unclear.
A new study indicates that incomplete resection, compared with complete resection, substantially increases the likelihood that a polyp will be found during follow-up colonoscopy.
The evidence “strongly supports the hypothesis” that residual polyp tissue likely contributes to neoplastic polyp recurrence and, by extension, interval colorectal cancer, said study author Heiko Pohl, MD, and colleagues. Dr. Pohl is affiliated with the White River Junction Veterans Affairs Medical Center in Vermont and Dartmouth-Hitchcock Medical Center and Dartmouth Geisel School of Medicine in Hanover, N.H.
Among 166 participants in the Complete Adenoma Resection (CARE) study who went on to have a surveillance colonoscopy in the following years, metachronous neoplasia was detected more often in colon segments where polyps had been incompletely resected, compared with colon segments with complete resections (52% vs. 23%), according to a study in Annals of Internal Medicine.
In addition, colon segments that had had incomplete resections in the CARE study were more likely to have polyps that were 10 mm or greater (18% vs. 3%). The 32 participants who had at least one incomplete resection during the original CARE study were three times more likely to have a polyp at follow-up, Dr. Pohl and coauthors found.
There were no instances of cancer or high-grade dysplasia during follow-up, however.
Key questions remain, including “Why do people get cancer after colonoscopy?” said Shai Friedland, MD, MS, who was not involved in the study. “Maybe it is because of these polyps that were not removed completely.”
On the other hand, lesions that are more difficult to see in the first place and more dangerous might be responsible when colorectal cancer does occur after colonoscopy, said Dr. Friedland, professor of gastroenterology and hepatology at Stanford (Calif.) University and a gastroenterologist at Veterans Affairs Palo Alto (Calif.) Health Care System.
Although it is not surprising that polyp remnants may appear as polyps during follow-up examinations, the difference in polyp recurrence between groups “must be quite striking” for it to clearly be seen in this small study, Dr. Friedland said. The results suggest that there is a need to “redouble our efforts to make sure that we remove polyps completely and not just hope that they disappear after an incomplete job,” he added.
In the CARE study, which was conducted between 2009 and 2012 at two academic medical centers, researchers found that about 10% of neoplastic polyps with visibly complete removal may nonetheless be incompletely resected, based on the presence of neoplastic tissue in biopsies taken from resection margins.
The investigators focused on 5- to 20-mm nonpedunculated colorectal polyps that were removed during routine polypectomy using electrocautery snare resection.
Participants with incomplete polyp resections were encouraged to have a surveillance examination within 1 year, and those with complete resections received guideline-based surveillance recommendations. In the follow-up analysis, those with an incomplete resection had a median time to first examination of 17 months, and those with complete resections had a median time to first examination of 45 months.
“These results highlight the importance of achieving complete resection because the risk for subsequent advanced neoplasia detection statistically significantly increases without it,” Dr. Pohl said. “The large polyp size after incomplete resection within a generally short follow-up time may imply fast growth, even if none of the presumed recurrent polyps contained advanced histologic characteristics.”
Between-group differences in the participants and polyps could confound the observations, the authors noted. It is possible that endoscopists who did the follow-up colonoscopies looked harder for polyps in colon segments that had prior incomplete resections, on account of the initial study findings, they said. “It is also plausible that endoscopists with a greater incomplete resection rate would be more likely to miss polyps, which could then be detected at follow-up,” though detection and resection skills do not necessarily correlate, the investigators said.
With resection, the goal is to remove the polyp in one piece with clear margins, and researchers have known that the polyp removal techniques used in the study “are far from perfect,” Dr. Friedland said. One question is whether the field should focus on improving standard techniques, or incorporating newer, more advanced techniques such as endoscopic submucosal dissection, which is effective but may entail significant perforation risk when performed by someone who is not proficient, he said.
“One of the major focuses we had over the years is finding the polyps to prevent future cancer,” Dr. Pohl said in an interview. But the CARE study showed that “we do not do a good job of taking polyps off completely,” he added. The present analysis underscores that clinicians should do their best to remove polyps completely, even if they lack a way to confirm complete resection in routine practice, as is currently the case, Dr. Pohl said. To that end, clinicians should aim to refine their skills and techniques. Video evaluations with guidance from experts may prove helpful, he suggested. “We need to think hard about quality assessment,” Dr. Pohl said.
Dr. Pohl disclosed grants from Boston Scientific, Cosmo Pharmaceuticals, and Steris outside of the study. Dr. Friedland had no disclosures.
When gastroenterologists resect polyps during colonoscopy, they may inadvertently leave some neoplastic tissue behind. Biopsies from resection margins sometimes contain polyp remnants, even after a lesion has been visibly removed, research has shown. But the significance of incomplete resection has been unclear.
A new study indicates that incomplete resection, compared with complete resection, substantially increases the likelihood that a polyp will be found during follow-up colonoscopy.
The evidence “strongly supports the hypothesis” that residual polyp tissue likely contributes to neoplastic polyp recurrence and, by extension, interval colorectal cancer, said study author Heiko Pohl, MD, and colleagues. Dr. Pohl is affiliated with the White River Junction Veterans Affairs Medical Center in Vermont and Dartmouth-Hitchcock Medical Center and Dartmouth Geisel School of Medicine in Hanover, N.H.
Among 166 participants in the Complete Adenoma Resection (CARE) study who went on to have a surveillance colonoscopy in the following years, metachronous neoplasia was detected more often in colon segments where polyps had been incompletely resected, compared with colon segments with complete resections (52% vs. 23%), according to a study in Annals of Internal Medicine.
In addition, colon segments that had had incomplete resections in the CARE study were more likely to have polyps that were 10 mm or greater (18% vs. 3%). The 32 participants who had at least one incomplete resection during the original CARE study were three times more likely to have a polyp at follow-up, Dr. Pohl and coauthors found.
There were no instances of cancer or high-grade dysplasia during follow-up, however.
Key questions remain, including “Why do people get cancer after colonoscopy?” said Shai Friedland, MD, MS, who was not involved in the study. “Maybe it is because of these polyps that were not removed completely.”
On the other hand, lesions that are more difficult to see in the first place and more dangerous might be responsible when colorectal cancer does occur after colonoscopy, said Dr. Friedland, professor of gastroenterology and hepatology at Stanford (Calif.) University and a gastroenterologist at Veterans Affairs Palo Alto (Calif.) Health Care System.
Although it is not surprising that polyp remnants may appear as polyps during follow-up examinations, the difference in polyp recurrence between groups “must be quite striking” for it to clearly be seen in this small study, Dr. Friedland said. The results suggest that there is a need to “redouble our efforts to make sure that we remove polyps completely and not just hope that they disappear after an incomplete job,” he added.
In the CARE study, which was conducted between 2009 and 2012 at two academic medical centers, researchers found that about 10% of neoplastic polyps with visibly complete removal may nonetheless be incompletely resected, based on the presence of neoplastic tissue in biopsies taken from resection margins.
The investigators focused on 5- to 20-mm nonpedunculated colorectal polyps that were removed during routine polypectomy using electrocautery snare resection.
Participants with incomplete polyp resections were encouraged to have a surveillance examination within 1 year, and those with complete resections received guideline-based surveillance recommendations. In the follow-up analysis, those with an incomplete resection had a median time to first examination of 17 months, and those with complete resections had a median time to first examination of 45 months.
“These results highlight the importance of achieving complete resection because the risk for subsequent advanced neoplasia detection statistically significantly increases without it,” Dr. Pohl said. “The large polyp size after incomplete resection within a generally short follow-up time may imply fast growth, even if none of the presumed recurrent polyps contained advanced histologic characteristics.”
Between-group differences in the participants and polyps could confound the observations, the authors noted. It is possible that endoscopists who did the follow-up colonoscopies looked harder for polyps in colon segments that had prior incomplete resections, on account of the initial study findings, they said. “It is also plausible that endoscopists with a greater incomplete resection rate would be more likely to miss polyps, which could then be detected at follow-up,” though detection and resection skills do not necessarily correlate, the investigators said.
With resection, the goal is to remove the polyp in one piece with clear margins, and researchers have known that the polyp removal techniques used in the study “are far from perfect,” Dr. Friedland said. One question is whether the field should focus on improving standard techniques, or incorporating newer, more advanced techniques such as endoscopic submucosal dissection, which is effective but may entail significant perforation risk when performed by someone who is not proficient, he said.
“One of the major focuses we had over the years is finding the polyps to prevent future cancer,” Dr. Pohl said in an interview. But the CARE study showed that “we do not do a good job of taking polyps off completely,” he added. The present analysis underscores that clinicians should do their best to remove polyps completely, even if they lack a way to confirm complete resection in routine practice, as is currently the case, Dr. Pohl said. To that end, clinicians should aim to refine their skills and techniques. Video evaluations with guidance from experts may prove helpful, he suggested. “We need to think hard about quality assessment,” Dr. Pohl said.
Dr. Pohl disclosed grants from Boston Scientific, Cosmo Pharmaceuticals, and Steris outside of the study. Dr. Friedland had no disclosures.
New-AFib risk may not rise with light drinking, may fall with wine
Alcoholic drinks are in the news again, served with a twist. A large cohort study saw a familiar J-shaped curve detailing risk for new atrial fibrillation (AFib) in which the risk rose steadily with greater number of drinks per week, except at the lowest levels of alcohol intake.
There, the curve turned the other way. Light drinkers overall showed no higher AFib risk than nondrinkers, and the risk was lowest at any degree of alcohol intake up to 56 g per week.
On closer analysis of risk patterns, the type of alcoholic beverage mattered.
Alcohol content per drink was defined by standards in the United Kingdom, where the cohort was based.
The risk of AFib also didn’t climb at low intake levels of white wine or with “very low” use of liquor or spirits. But it went up consistently at any level of beer or cider consumption, and to be sure, “high intake of any beverage was associated with greater AF[ib] risk,” notes a report on the study published July 27, 2021, in JACC: Clinical Electrophysiology.
The results, based on more than 400,000 adults in the community, “raise the possibility that, for current consumers, drinking red or white wine could potentially be a safer alternative to other types of alcoholic beverages with respect to AF[ib] risk,” the report proposes.
The J-shaped risk curve for new AFib by degree of alcohol consumption follows the pattern sometimes seen for cardiovascular risk in general. But the intake level at which AFib risk is flat or reduced “is at a far lower dose of alcohol than what we’ve seen for cardiovascular disease,” lead author Samuel J. Tu, BHlthMedSc, said in an interview.
“That being said, even with the threshold sitting quite low, it still tells us that cutting down on alcohol is a good thing and perhaps one of the best things for our heart,” said Mr. Tu, University of Adelaide and Royal Adelaide Hospital, who also presented the findings at the Heart Rhythm Society 2021 Scientific Sessions, held in Boston and virtually.
How much alcohol is in a drink?
In a caution for anyone looking to beer, wine, or liquor to protect against AFib, or at least not cause it, the weekly number of drinks associated with the lowest AFib risk may be fewer than expected. That bottom of 56 g per week works out to one drink a day or less for British and only four or fewer per week for Americans, according to the study’s internationally varying definitions for the alcohol content of one drink.
For example, a drink was considered to have 8 g of alcohol in the United Kingdom, 14 g in the United States and some other countries, and up to 20 g in Austria. Those numbers came from definitions used by the respective national health agencies, such as the National Health Service in the United Kingdom and Centers for Disease Control and Prevention in the United States, Mr. Tu explained.
“They all defined standard drinks slightly differently. But wherever we looked, the threshold we found was far lower than what our governments recommend” based on what is known about alcohol and overall cardiovascular risk, he said.
First to show a hint of protection
The current study “is especially noteworthy because it’s the really the first to demonstrate any hint that there could be a protective effect from any particular amount of alcohol in regard to atrial fibrillation,” Gregory M. Marcus, MD, MAS, University of California, San Francisco, said in an interview. “The J-shaped association fits with what’s been observed with myocardial infarction and overall mortality, and hasn’t previously been seen in the setting of atrial fibrillation.”
Quite interestingly, “it appeared to be the wine drinkers, rather than those who consumed other types of alcohol, that enjoyed this benefit,” said Dr. Marcus, who was not involved in the research but co-authored an accompanying editorial with UCSF colleague Thomas A. Dewland, MD.
“It’s important to recognize the overwhelming evidence that alcohol in general increases the risk for atrial fibrillation,” he said. But “perhaps there’s something in wine that is anti-inflammatory that has some beneficial effect that maybe overwhelms the proarrhythmic aspect.”
The current study “opens the door to the question as to whether there is a small amount of alcohol, perhaps in the form of wine, where there are some benefits that outweigh the risks of atrial fibrillation.”
Still, the findings are observational and “clearly prone to confounding,” Dr. Marcus said. “We need to be very cautious in inferring causality.”
For example, it’s possible that “there is something about individuals that are able to drink alcohol on a regular basis and in small amounts that is the actual causal factor in reducing atrial fibrillation episodes.”
The analysis was based on 403,281 participants in the UK Biobank registry, a prospective cohort study in the United Kingdom, who were aged 40-69 when recruited from 2006 to 2010; it excluded anyone with a history of AFib or who was a former drinker. About 52% were women, the report noted.
Their median alcohol consumption was eight U.K. drinks per week, with 5.5% reporting they had never consumed alcohol. About 21,300 incident cases of AFib or atrial flutter were documented over almost 4.5 million person-years, or a median follow-up of 11.4 years.
The hazard ratio for incident AFib among those with a weekly alcohol consumption corresponding to 1-7 U.K. drinks, compared with intake of less than 1 U.K. drink per week, was 0.95 (95% confidence interval, 0.91-1.00). Within that range of 1-7 drinks, the absolute lowest AFib risk on the J curve was at 5 per week.
No increased risk of new AFib was seen in association with weekly U.K. drink levels of 10 for red wine, 8 for white wine, and 3 for spirits.
Compared with weekly intake of less than 1 U.K. drink per week, red wine intake at 1-7 per week showed an HR for AFib of 0.94 (95% CI, 0.91-0.97). Indeed, at no observed consumption level was red wine associated with a significant increase in AFib risk. White wine until the highest observed level of intake, above 28 U.K. drinks per week, at which point the HR for AFib was 1.48 (98% CI 1.19-1.86). The curve for spirit intake followed a similar but steeper curve, its HR risk reaching 1.61 (95% CI, 1.34-1.93) at intake levels beyond 28 U.K. drinks per week.
Consumption of beer or cider showed a linear association with AFib risk, which was elevated at all recorded intake levels, including 8-14 U.K. drinks per week (HR, 1.11; 95% CI 1.06-1.17) and up to 28 or more per week (HR, 1.35; 95% CI, 1.26-1.45).
The analysis is hypothesis generating at best, Dr. Marcus emphasized. “Ultimately, a randomized trial would be the only way to be fairly certain if there is indeed a causal protective relationship between red wine, in low amounts, and atrial fib.”
The message for patients, proposed Dr. Dewland and Dr. Marcus, is that alcohol abstinence is best for secondary AFib prevention, “especially if alcohol is a personal trigger for acute AF[ib] episodes,” and that for primary AFib prevention, “continued consumption of some alcohol may be reasonable, but the exact threshold is unclear and is likely a very low amount.”
Mr. Tu has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Marcus disclosed receiving research funding from Baylis Medical; consulting for Johnson & Johnson and InCarda; and holding equity interest in InCarda. Dr. Dewland reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alcoholic drinks are in the news again, served with a twist. A large cohort study saw a familiar J-shaped curve detailing risk for new atrial fibrillation (AFib) in which the risk rose steadily with greater number of drinks per week, except at the lowest levels of alcohol intake.
There, the curve turned the other way. Light drinkers overall showed no higher AFib risk than nondrinkers, and the risk was lowest at any degree of alcohol intake up to 56 g per week.
On closer analysis of risk patterns, the type of alcoholic beverage mattered.
Alcohol content per drink was defined by standards in the United Kingdom, where the cohort was based.
The risk of AFib also didn’t climb at low intake levels of white wine or with “very low” use of liquor or spirits. But it went up consistently at any level of beer or cider consumption, and to be sure, “high intake of any beverage was associated with greater AF[ib] risk,” notes a report on the study published July 27, 2021, in JACC: Clinical Electrophysiology.
The results, based on more than 400,000 adults in the community, “raise the possibility that, for current consumers, drinking red or white wine could potentially be a safer alternative to other types of alcoholic beverages with respect to AF[ib] risk,” the report proposes.
The J-shaped risk curve for new AFib by degree of alcohol consumption follows the pattern sometimes seen for cardiovascular risk in general. But the intake level at which AFib risk is flat or reduced “is at a far lower dose of alcohol than what we’ve seen for cardiovascular disease,” lead author Samuel J. Tu, BHlthMedSc, said in an interview.
“That being said, even with the threshold sitting quite low, it still tells us that cutting down on alcohol is a good thing and perhaps one of the best things for our heart,” said Mr. Tu, University of Adelaide and Royal Adelaide Hospital, who also presented the findings at the Heart Rhythm Society 2021 Scientific Sessions, held in Boston and virtually.
How much alcohol is in a drink?
In a caution for anyone looking to beer, wine, or liquor to protect against AFib, or at least not cause it, the weekly number of drinks associated with the lowest AFib risk may be fewer than expected. That bottom of 56 g per week works out to one drink a day or less for British and only four or fewer per week for Americans, according to the study’s internationally varying definitions for the alcohol content of one drink.
For example, a drink was considered to have 8 g of alcohol in the United Kingdom, 14 g in the United States and some other countries, and up to 20 g in Austria. Those numbers came from definitions used by the respective national health agencies, such as the National Health Service in the United Kingdom and Centers for Disease Control and Prevention in the United States, Mr. Tu explained.
“They all defined standard drinks slightly differently. But wherever we looked, the threshold we found was far lower than what our governments recommend” based on what is known about alcohol and overall cardiovascular risk, he said.
First to show a hint of protection
The current study “is especially noteworthy because it’s the really the first to demonstrate any hint that there could be a protective effect from any particular amount of alcohol in regard to atrial fibrillation,” Gregory M. Marcus, MD, MAS, University of California, San Francisco, said in an interview. “The J-shaped association fits with what’s been observed with myocardial infarction and overall mortality, and hasn’t previously been seen in the setting of atrial fibrillation.”
Quite interestingly, “it appeared to be the wine drinkers, rather than those who consumed other types of alcohol, that enjoyed this benefit,” said Dr. Marcus, who was not involved in the research but co-authored an accompanying editorial with UCSF colleague Thomas A. Dewland, MD.
“It’s important to recognize the overwhelming evidence that alcohol in general increases the risk for atrial fibrillation,” he said. But “perhaps there’s something in wine that is anti-inflammatory that has some beneficial effect that maybe overwhelms the proarrhythmic aspect.”
The current study “opens the door to the question as to whether there is a small amount of alcohol, perhaps in the form of wine, where there are some benefits that outweigh the risks of atrial fibrillation.”
Still, the findings are observational and “clearly prone to confounding,” Dr. Marcus said. “We need to be very cautious in inferring causality.”
For example, it’s possible that “there is something about individuals that are able to drink alcohol on a regular basis and in small amounts that is the actual causal factor in reducing atrial fibrillation episodes.”
The analysis was based on 403,281 participants in the UK Biobank registry, a prospective cohort study in the United Kingdom, who were aged 40-69 when recruited from 2006 to 2010; it excluded anyone with a history of AFib or who was a former drinker. About 52% were women, the report noted.
Their median alcohol consumption was eight U.K. drinks per week, with 5.5% reporting they had never consumed alcohol. About 21,300 incident cases of AFib or atrial flutter were documented over almost 4.5 million person-years, or a median follow-up of 11.4 years.
The hazard ratio for incident AFib among those with a weekly alcohol consumption corresponding to 1-7 U.K. drinks, compared with intake of less than 1 U.K. drink per week, was 0.95 (95% confidence interval, 0.91-1.00). Within that range of 1-7 drinks, the absolute lowest AFib risk on the J curve was at 5 per week.
No increased risk of new AFib was seen in association with weekly U.K. drink levels of 10 for red wine, 8 for white wine, and 3 for spirits.
Compared with weekly intake of less than 1 U.K. drink per week, red wine intake at 1-7 per week showed an HR for AFib of 0.94 (95% CI, 0.91-0.97). Indeed, at no observed consumption level was red wine associated with a significant increase in AFib risk. White wine until the highest observed level of intake, above 28 U.K. drinks per week, at which point the HR for AFib was 1.48 (98% CI 1.19-1.86). The curve for spirit intake followed a similar but steeper curve, its HR risk reaching 1.61 (95% CI, 1.34-1.93) at intake levels beyond 28 U.K. drinks per week.
Consumption of beer or cider showed a linear association with AFib risk, which was elevated at all recorded intake levels, including 8-14 U.K. drinks per week (HR, 1.11; 95% CI 1.06-1.17) and up to 28 or more per week (HR, 1.35; 95% CI, 1.26-1.45).
The analysis is hypothesis generating at best, Dr. Marcus emphasized. “Ultimately, a randomized trial would be the only way to be fairly certain if there is indeed a causal protective relationship between red wine, in low amounts, and atrial fib.”
The message for patients, proposed Dr. Dewland and Dr. Marcus, is that alcohol abstinence is best for secondary AFib prevention, “especially if alcohol is a personal trigger for acute AF[ib] episodes,” and that for primary AFib prevention, “continued consumption of some alcohol may be reasonable, but the exact threshold is unclear and is likely a very low amount.”
Mr. Tu has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Marcus disclosed receiving research funding from Baylis Medical; consulting for Johnson & Johnson and InCarda; and holding equity interest in InCarda. Dr. Dewland reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alcoholic drinks are in the news again, served with a twist. A large cohort study saw a familiar J-shaped curve detailing risk for new atrial fibrillation (AFib) in which the risk rose steadily with greater number of drinks per week, except at the lowest levels of alcohol intake.
There, the curve turned the other way. Light drinkers overall showed no higher AFib risk than nondrinkers, and the risk was lowest at any degree of alcohol intake up to 56 g per week.
On closer analysis of risk patterns, the type of alcoholic beverage mattered.
Alcohol content per drink was defined by standards in the United Kingdom, where the cohort was based.
The risk of AFib also didn’t climb at low intake levels of white wine or with “very low” use of liquor or spirits. But it went up consistently at any level of beer or cider consumption, and to be sure, “high intake of any beverage was associated with greater AF[ib] risk,” notes a report on the study published July 27, 2021, in JACC: Clinical Electrophysiology.
The results, based on more than 400,000 adults in the community, “raise the possibility that, for current consumers, drinking red or white wine could potentially be a safer alternative to other types of alcoholic beverages with respect to AF[ib] risk,” the report proposes.
The J-shaped risk curve for new AFib by degree of alcohol consumption follows the pattern sometimes seen for cardiovascular risk in general. But the intake level at which AFib risk is flat or reduced “is at a far lower dose of alcohol than what we’ve seen for cardiovascular disease,” lead author Samuel J. Tu, BHlthMedSc, said in an interview.
“That being said, even with the threshold sitting quite low, it still tells us that cutting down on alcohol is a good thing and perhaps one of the best things for our heart,” said Mr. Tu, University of Adelaide and Royal Adelaide Hospital, who also presented the findings at the Heart Rhythm Society 2021 Scientific Sessions, held in Boston and virtually.
How much alcohol is in a drink?
In a caution for anyone looking to beer, wine, or liquor to protect against AFib, or at least not cause it, the weekly number of drinks associated with the lowest AFib risk may be fewer than expected. That bottom of 56 g per week works out to one drink a day or less for British and only four or fewer per week for Americans, according to the study’s internationally varying definitions for the alcohol content of one drink.
For example, a drink was considered to have 8 g of alcohol in the United Kingdom, 14 g in the United States and some other countries, and up to 20 g in Austria. Those numbers came from definitions used by the respective national health agencies, such as the National Health Service in the United Kingdom and Centers for Disease Control and Prevention in the United States, Mr. Tu explained.
“They all defined standard drinks slightly differently. But wherever we looked, the threshold we found was far lower than what our governments recommend” based on what is known about alcohol and overall cardiovascular risk, he said.
First to show a hint of protection
The current study “is especially noteworthy because it’s the really the first to demonstrate any hint that there could be a protective effect from any particular amount of alcohol in regard to atrial fibrillation,” Gregory M. Marcus, MD, MAS, University of California, San Francisco, said in an interview. “The J-shaped association fits with what’s been observed with myocardial infarction and overall mortality, and hasn’t previously been seen in the setting of atrial fibrillation.”
Quite interestingly, “it appeared to be the wine drinkers, rather than those who consumed other types of alcohol, that enjoyed this benefit,” said Dr. Marcus, who was not involved in the research but co-authored an accompanying editorial with UCSF colleague Thomas A. Dewland, MD.
“It’s important to recognize the overwhelming evidence that alcohol in general increases the risk for atrial fibrillation,” he said. But “perhaps there’s something in wine that is anti-inflammatory that has some beneficial effect that maybe overwhelms the proarrhythmic aspect.”
The current study “opens the door to the question as to whether there is a small amount of alcohol, perhaps in the form of wine, where there are some benefits that outweigh the risks of atrial fibrillation.”
Still, the findings are observational and “clearly prone to confounding,” Dr. Marcus said. “We need to be very cautious in inferring causality.”
For example, it’s possible that “there is something about individuals that are able to drink alcohol on a regular basis and in small amounts that is the actual causal factor in reducing atrial fibrillation episodes.”
The analysis was based on 403,281 participants in the UK Biobank registry, a prospective cohort study in the United Kingdom, who were aged 40-69 when recruited from 2006 to 2010; it excluded anyone with a history of AFib or who was a former drinker. About 52% were women, the report noted.
Their median alcohol consumption was eight U.K. drinks per week, with 5.5% reporting they had never consumed alcohol. About 21,300 incident cases of AFib or atrial flutter were documented over almost 4.5 million person-years, or a median follow-up of 11.4 years.
The hazard ratio for incident AFib among those with a weekly alcohol consumption corresponding to 1-7 U.K. drinks, compared with intake of less than 1 U.K. drink per week, was 0.95 (95% confidence interval, 0.91-1.00). Within that range of 1-7 drinks, the absolute lowest AFib risk on the J curve was at 5 per week.
No increased risk of new AFib was seen in association with weekly U.K. drink levels of 10 for red wine, 8 for white wine, and 3 for spirits.
Compared with weekly intake of less than 1 U.K. drink per week, red wine intake at 1-7 per week showed an HR for AFib of 0.94 (95% CI, 0.91-0.97). Indeed, at no observed consumption level was red wine associated with a significant increase in AFib risk. White wine until the highest observed level of intake, above 28 U.K. drinks per week, at which point the HR for AFib was 1.48 (98% CI 1.19-1.86). The curve for spirit intake followed a similar but steeper curve, its HR risk reaching 1.61 (95% CI, 1.34-1.93) at intake levels beyond 28 U.K. drinks per week.
Consumption of beer or cider showed a linear association with AFib risk, which was elevated at all recorded intake levels, including 8-14 U.K. drinks per week (HR, 1.11; 95% CI 1.06-1.17) and up to 28 or more per week (HR, 1.35; 95% CI, 1.26-1.45).
The analysis is hypothesis generating at best, Dr. Marcus emphasized. “Ultimately, a randomized trial would be the only way to be fairly certain if there is indeed a causal protective relationship between red wine, in low amounts, and atrial fib.”
The message for patients, proposed Dr. Dewland and Dr. Marcus, is that alcohol abstinence is best for secondary AFib prevention, “especially if alcohol is a personal trigger for acute AF[ib] episodes,” and that for primary AFib prevention, “continued consumption of some alcohol may be reasonable, but the exact threshold is unclear and is likely a very low amount.”
Mr. Tu has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Marcus disclosed receiving research funding from Baylis Medical; consulting for Johnson & Johnson and InCarda; and holding equity interest in InCarda. Dr. Dewland reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Aerobic exercise reduces BP in resistant hypertension
Aerobic exercise may help reduce blood pressure in patients whose hypertension responds poorly to medications, a new study suggests.
A randomized controlled clinical trial showed that patients with resistant hypertension assigned to a moderate-intensity aerobic exercise training program had lower blood pressure compared with patients who received usual care.
“Resistant hypertension persists as a big clinical challenge because the available treatment options to lower blood pressure in this clinical population, namely drugs and renal denervation, show limited success,” Fernando Ribeiro, PhD, University of Aveiro, Portugal, told this news organization. “Aerobic exercise was safe and associated with a significant and clinically relevant reduction in 24-hour, daytime ambulatory, and office blood pressure.”
The findings were published online August 4 in JAMA Cardiology.
The researchers enrolled 53 patients aged 40-75 years with a diagnosis of resistant hypertension in this prospective, single-blinded trial. Nearly half (24) were women.
Resistant hypertension was defined as having a “mean systolic BP of 130 mm Hg or greater on 24-hour ambulatory BP monitoring and/or 135 mm Hg or greater during daytime hours while taking maximally tolerated doses of at least 3 antihypertensive agents, including a diuretic, or to have a controlled BP while taking 4 or more antihypertensive agents.”
From March 2017 to December 2019 at two sites in Portugal, 26 patients were randomly assigned to a 12-week aerobic exercise training program involving three 40-minute supervised sessions per week in addition to usual care. Another 27 patients in the control group were allocated to receive usual care only.
24-hour ambulatory systolic blood pressure was reduced by 7.1 mm Hg (95% confidence interval, -12.8 to -1.4; P = .02) in patients in the exercise group compared with the control group. In the exercise group, there were additional reductions of:
- -5.1 mm Hg of 24-hour ambulatory diastolic blood pressure (95% CI, -7.9 to -2.3; P = .001)
- -8.4 mm Hg of daytime systolic blood pressure (95% CI, -14.3 to -2.5, P = .006)
- -5.7 mm Hg of daytime diastolic blood pressure (95% CI, -9.0 to -2.4; P = .001)
- -10.0 mm Hg of office systolic blood pressure (95% CI, -17.6 to -2.5; P = .01)
Additionally, a significant improvement in cardiorespiratory fitness (5.05 mL/kg per minute of oxygen consumption; 95% CI, 3.5-6.6; P < .001) was observed in the exercise group compared with the control group.
Although prior research has suggested that exercise may lower blood pressure, this study is particularly useful because it “outlines very specifically what types of exercise you can recommend,” said Daniel Lackland, DrPH, Medical University of South Carolina, Charleston.
Although important, exercise is “one part of the overall management of high blood pressure. If people are being prescribed medication, they should continue taking it and work on lifestyle changes like reducing salt intake and drinking in moderation,” added Dr. Lackland, who was not involved in the research.
Also commenting on the findings, Wanpen Vongpatanasin, MD, UT Southwestern Medical Center, Dallas, pointed out that there are many potential benefits from exercise training. “It might improve endothelial function, decrease vascular stiffness and nervous system reactivity to stress, and improve quality of life for patients,” she said.
The study has several limitations, including a small sample size and a patient population that mostly has “relatively mild hypertension,” Dr. Vongpatanasin said, adding, “We don’t know whether these findings will apply to patients with more severe hypertension.”
It would also have been helpful if investigators monitored patient adherence to prescribed medications through urine or blood samples rather than a questionnaire, and to measure nighttime blood pressure, which is a more important predictor of cardiovascular outcomes, said Dr. Vongpatanasin, who was not associated with the research.
Moving forward, it will be important to “investigate why some patients are nonresponders to the exercise intervention and why some are super-responders,” study author Dr. Ribeiro said.
Dr. Ribeiro, Dr. Lackland, and Dr. Vongpatanasin have disclosed no relevant financial relationships. This study was funded by the European Union through the European Regional Development Fund Operational Competitiveness Factors Program (COMPETE) and by the Portuguese government through the Foundation for Science and Technology. The funders had no role in the study.
A version of this article first appeared on Medscape.com.
Aerobic exercise may help reduce blood pressure in patients whose hypertension responds poorly to medications, a new study suggests.
A randomized controlled clinical trial showed that patients with resistant hypertension assigned to a moderate-intensity aerobic exercise training program had lower blood pressure compared with patients who received usual care.
“Resistant hypertension persists as a big clinical challenge because the available treatment options to lower blood pressure in this clinical population, namely drugs and renal denervation, show limited success,” Fernando Ribeiro, PhD, University of Aveiro, Portugal, told this news organization. “Aerobic exercise was safe and associated with a significant and clinically relevant reduction in 24-hour, daytime ambulatory, and office blood pressure.”
The findings were published online August 4 in JAMA Cardiology.
The researchers enrolled 53 patients aged 40-75 years with a diagnosis of resistant hypertension in this prospective, single-blinded trial. Nearly half (24) were women.
Resistant hypertension was defined as having a “mean systolic BP of 130 mm Hg or greater on 24-hour ambulatory BP monitoring and/or 135 mm Hg or greater during daytime hours while taking maximally tolerated doses of at least 3 antihypertensive agents, including a diuretic, or to have a controlled BP while taking 4 or more antihypertensive agents.”
From March 2017 to December 2019 at two sites in Portugal, 26 patients were randomly assigned to a 12-week aerobic exercise training program involving three 40-minute supervised sessions per week in addition to usual care. Another 27 patients in the control group were allocated to receive usual care only.
24-hour ambulatory systolic blood pressure was reduced by 7.1 mm Hg (95% confidence interval, -12.8 to -1.4; P = .02) in patients in the exercise group compared with the control group. In the exercise group, there were additional reductions of:
- -5.1 mm Hg of 24-hour ambulatory diastolic blood pressure (95% CI, -7.9 to -2.3; P = .001)
- -8.4 mm Hg of daytime systolic blood pressure (95% CI, -14.3 to -2.5, P = .006)
- -5.7 mm Hg of daytime diastolic blood pressure (95% CI, -9.0 to -2.4; P = .001)
- -10.0 mm Hg of office systolic blood pressure (95% CI, -17.6 to -2.5; P = .01)
Additionally, a significant improvement in cardiorespiratory fitness (5.05 mL/kg per minute of oxygen consumption; 95% CI, 3.5-6.6; P < .001) was observed in the exercise group compared with the control group.
Although prior research has suggested that exercise may lower blood pressure, this study is particularly useful because it “outlines very specifically what types of exercise you can recommend,” said Daniel Lackland, DrPH, Medical University of South Carolina, Charleston.
Although important, exercise is “one part of the overall management of high blood pressure. If people are being prescribed medication, they should continue taking it and work on lifestyle changes like reducing salt intake and drinking in moderation,” added Dr. Lackland, who was not involved in the research.
Also commenting on the findings, Wanpen Vongpatanasin, MD, UT Southwestern Medical Center, Dallas, pointed out that there are many potential benefits from exercise training. “It might improve endothelial function, decrease vascular stiffness and nervous system reactivity to stress, and improve quality of life for patients,” she said.
The study has several limitations, including a small sample size and a patient population that mostly has “relatively mild hypertension,” Dr. Vongpatanasin said, adding, “We don’t know whether these findings will apply to patients with more severe hypertension.”
It would also have been helpful if investigators monitored patient adherence to prescribed medications through urine or blood samples rather than a questionnaire, and to measure nighttime blood pressure, which is a more important predictor of cardiovascular outcomes, said Dr. Vongpatanasin, who was not associated with the research.
Moving forward, it will be important to “investigate why some patients are nonresponders to the exercise intervention and why some are super-responders,” study author Dr. Ribeiro said.
Dr. Ribeiro, Dr. Lackland, and Dr. Vongpatanasin have disclosed no relevant financial relationships. This study was funded by the European Union through the European Regional Development Fund Operational Competitiveness Factors Program (COMPETE) and by the Portuguese government through the Foundation for Science and Technology. The funders had no role in the study.
A version of this article first appeared on Medscape.com.
Aerobic exercise may help reduce blood pressure in patients whose hypertension responds poorly to medications, a new study suggests.
A randomized controlled clinical trial showed that patients with resistant hypertension assigned to a moderate-intensity aerobic exercise training program had lower blood pressure compared with patients who received usual care.
“Resistant hypertension persists as a big clinical challenge because the available treatment options to lower blood pressure in this clinical population, namely drugs and renal denervation, show limited success,” Fernando Ribeiro, PhD, University of Aveiro, Portugal, told this news organization. “Aerobic exercise was safe and associated with a significant and clinically relevant reduction in 24-hour, daytime ambulatory, and office blood pressure.”
The findings were published online August 4 in JAMA Cardiology.
The researchers enrolled 53 patients aged 40-75 years with a diagnosis of resistant hypertension in this prospective, single-blinded trial. Nearly half (24) were women.
Resistant hypertension was defined as having a “mean systolic BP of 130 mm Hg or greater on 24-hour ambulatory BP monitoring and/or 135 mm Hg or greater during daytime hours while taking maximally tolerated doses of at least 3 antihypertensive agents, including a diuretic, or to have a controlled BP while taking 4 or more antihypertensive agents.”
From March 2017 to December 2019 at two sites in Portugal, 26 patients were randomly assigned to a 12-week aerobic exercise training program involving three 40-minute supervised sessions per week in addition to usual care. Another 27 patients in the control group were allocated to receive usual care only.
24-hour ambulatory systolic blood pressure was reduced by 7.1 mm Hg (95% confidence interval, -12.8 to -1.4; P = .02) in patients in the exercise group compared with the control group. In the exercise group, there were additional reductions of:
- -5.1 mm Hg of 24-hour ambulatory diastolic blood pressure (95% CI, -7.9 to -2.3; P = .001)
- -8.4 mm Hg of daytime systolic blood pressure (95% CI, -14.3 to -2.5, P = .006)
- -5.7 mm Hg of daytime diastolic blood pressure (95% CI, -9.0 to -2.4; P = .001)
- -10.0 mm Hg of office systolic blood pressure (95% CI, -17.6 to -2.5; P = .01)
Additionally, a significant improvement in cardiorespiratory fitness (5.05 mL/kg per minute of oxygen consumption; 95% CI, 3.5-6.6; P < .001) was observed in the exercise group compared with the control group.
Although prior research has suggested that exercise may lower blood pressure, this study is particularly useful because it “outlines very specifically what types of exercise you can recommend,” said Daniel Lackland, DrPH, Medical University of South Carolina, Charleston.
Although important, exercise is “one part of the overall management of high blood pressure. If people are being prescribed medication, they should continue taking it and work on lifestyle changes like reducing salt intake and drinking in moderation,” added Dr. Lackland, who was not involved in the research.
Also commenting on the findings, Wanpen Vongpatanasin, MD, UT Southwestern Medical Center, Dallas, pointed out that there are many potential benefits from exercise training. “It might improve endothelial function, decrease vascular stiffness and nervous system reactivity to stress, and improve quality of life for patients,” she said.
The study has several limitations, including a small sample size and a patient population that mostly has “relatively mild hypertension,” Dr. Vongpatanasin said, adding, “We don’t know whether these findings will apply to patients with more severe hypertension.”
It would also have been helpful if investigators monitored patient adherence to prescribed medications through urine or blood samples rather than a questionnaire, and to measure nighttime blood pressure, which is a more important predictor of cardiovascular outcomes, said Dr. Vongpatanasin, who was not associated with the research.
Moving forward, it will be important to “investigate why some patients are nonresponders to the exercise intervention and why some are super-responders,” study author Dr. Ribeiro said.
Dr. Ribeiro, Dr. Lackland, and Dr. Vongpatanasin have disclosed no relevant financial relationships. This study was funded by the European Union through the European Regional Development Fund Operational Competitiveness Factors Program (COMPETE) and by the Portuguese government through the Foundation for Science and Technology. The funders had no role in the study.
A version of this article first appeared on Medscape.com.
Physicians question the future of TNF inhibitors for psoriasis, PsA
Tumor necrosis factor inhibitors have long been the go-to treatment of choice for patients with psoriasis and psoriatic arthritis (PsA). They’ve served patients well since etanercept was first approved for PsA in 2002, but today, with the availability of more attractive interleukin-17 and IL-23 inhibitors, dermatologists and rheumatologists are asking whether it’s time to reconsider the use of TNF inhibitors as first-line therapy in psoriasis and PsA.
“TNF inhibitors have served psoriasis patients well for many years. The question is, ‘Is it time to move on from them as first-line agents for psoriasis?’ ” said April W. Armstrong, MD, MPH, a dermatologist and associate dean for clinical research at the University of Southern California, Los Angeles. Dr. Armstrong participated in a point/counterpoint debate about the merits of IL-17 and IL-23 inhibitors over TNF inhibitors at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. “For the majority of our patients, IL-17 and IL-23 inhibitors are probably rationally better than TNF inhibitors as first-line agents for moderate to severe plaque psoriasis,” she said.
In this debate, dermatologists and rheumatologists cited studies showing the safety and efficacy of IL-17 and IL-23 inhibitors over TNF inhibitors. TNF inhibitors include etanercept (Enbrel and biosimilars), infliximab (Remicade and biosimilars), adalimumab (Humira and biosimilars), certolizumab pegol (Cimzia), and golimumab (Simponi). IL-12/23 inhibitors are limited to ustekinumab (Stelara). IL-17 inhibitors include secukinumab (Cosentyx), ixekizumab (Taltz), and brodalumab (Siliq). IL-23 inhibitors include guselkumab (Tremfya), tildrakizumab (Ilumya), and risankizumab (Skyrizi).
TNF inhibitors are recommended by the American College of Rheumatology as first-line therapy for treatment-naive patients with active PsA, and they, along with IL-12/23, IL-17, and IL-23 inhibitors are all recommended by the American Academy of Dermatology as monotherapy treatment options in adult patients with moderate to severe plaque psoriasis. However, some studies have shown that non–TNF-inhibitor biologics have a higher efficacy than TNF inhibitors in some cases for some patients, such as those with moderate to severe psoriasis alone or for musculoskeletal efficacy in patients with PsA who have peripheral arthritis, enthesitis, dactylitis, or axial manifestations.
Favorable characteristics of non–TNF-inhibitor biologics
Dr. Armstrong cited a number of head-to-head trials to support her view that IL-17 and IL-23 inhibitors are better than TNF inhibitors as first-line agents for patients with moderate to severe plaque psoriasis. In the first head-to-head study of its kind in patients with moderate to severe psoriasis, ustekinumab proved superior to etanercept. Guselkumab was shown to be superior to adalimumab for patients with moderate to severe psoriasis. Tildrakizumab also proved superior to etanercept for patients with psoriasis. Risankizumab bested adalimumab in patients with moderate to severe psoriasis. Ixekizumab proved superior to etanercept in two pivotal studies of patients with widespread moderate-to-severe psoriasis.
IL-23 and IL-17 inhibitors tend to have less frequent maintenance dosing, with IL-17 inhibitors being once every 2 or 4 weeks and IL-23 inhibitors once every 8 or 12 weeks, compared with frequencies ranging from every week to every 8 weeks with TNF inhibitors, Dr. Armstrong said.
IL-17 and IL-23 inhibitors also appear to have fewer safety concerns than TNF inhibitors, although there is less long-term data for them overall and there are some notable exceptions in certain patient populations. TNF inhibitors should be avoided in patients with a history of demyelinating disease or hepatitis B virus infection, and they are not preferred in patients who have a history of latent tuberculosis or advanced heart failure. IL-17 inhibitors should not be used in patients with a history of inflammatory bowel disease, and their use is associated with a higher rate of oral candidiasis. IL-23 inhibitors have a good safety profile overall, she said.
“The IL-17/23 axis is very important to psoriatic arthritis and should be the focus of our treatments” for PsA, said Deepak Jadon, MBBCh, MRCP, PhD, a rheumatologist and director of the rheumatology research unit at Addenbrooke’s Hospital, Cambridge (England) University Hospitals NHS Foundation Trust. In his presentation, he proposed that IL-17 inhibitors and IL-23 inhibitors be used as first-line therapies in PsA ahead of TNF inhibitors.
One reason to go with IL-17 and IL-23 inhibitors may be to ”get it right immunologically the first time,” Dr. Jadon said. He cited evidence showing substantially better response to guselkumab when given to biologic-naive patients with PsA versus those who had a inadequate response to TNF inhibitors, as well as data indicating better response with secukinumab regardless of previous TNF inhibitor use.
IL-17 inhibitors target more domains of psoriatic disease than do TNF inhibitors, he said, noting that “they have excellent musculoskeletal efficacy in patients with moderate skin psoriasis, not just those with severe psoriasis.” Ixekizumab proved superior to adalimumab in biologic-naive patients with PsA. The results of this study also indicated that IL-17 inhibitors should not be reserved only for patients with severe psoriasis since a higher percentage of patients with moderate psoriasis who were taking ixekizumab achieved very low PsA activity. Secukinumab also beat adalimumab in a head-to-head comparison and showed a greater impact on some measures of health-related quality of life.
IL-17 inhibitors also do not require concomitant methotrexate, he said, “which is a major bonus for our patients. All of my patients wish to stop methotrexate even if tolerated. Not having to cope with prescribed methotrexate improves risk of adverse events and frequency of blood test monitoring.”
IL-17 and IL-23 inhibitors appear to have good efficacy against axial disease in patients with PsA. Randomized trial results for secukinumab versus placebo show high percentages of patients improving either 20% or 40% in Assessment in Spondyloarthritis International Society response criteria and reduced inflammatory MRI lesions in the spine and sacroiliac joints. Analyses of trial results in guselkumab-treated patients with axial manifestations of PsA have shown the IL-23 inhibitor’s efficacy versus placebo across different measures of disease activity.
Dr. Jadon also cited real-world data showing that patients stay longer on IL-17 and IL-12/23 inhibitors versus TNF inhibitors. A 2016 study of patients with psoriasis in the PSOLAR registry showed that patients persisted on treatment longer with ustekinumab than with adalimumab, etanercept, or infliximab. Similarly, a 2020 study of patients with psoriasis from the British Association of Dermatologists Biologics and Immunomodulators Register found that both ustekinumab and secukinumab had better sustained drug survival than did adalimumab.
Accessibility weighs heavily in using TNF inhibitor first
Clinical trials data show that IL-17 inhibitors outperform TNF inhibitors for psoriasis, but in clinical practice, TNF inhibitors still perform very well in individual patients and are well tolerated, said Amit Garg, MD, founding chair of the department of dermatology at Hofstra University, Hempstead, N.Y.
He argued in favor of TNF inhibitors as first-line therapy over IL-17 inhibitors for psoriasis. In this case, treatment decisions often come down to accessibility, Dr. Garg said. Not all insurance companies cover the cost of the newer IL-23 inhibitors. Plus, access to TNF inhibitors is widespread and costs are generally lower.
“As a physician, I don’t have complete autonomy in prescribing what I want. The reality is whether it be because of cross indication or discount pricing, [TNF inhibitors] – in particular adalimumab – is widely available on all plans and is usually the preferred treatment plan, at least in our area,” he said. “I’m not a big fan of plans that allow drugs at low or no cost for a year or 2, and then abandon the patients at that point thereafter. I like to use something that insurance will cover sustainably, and, quite frankly, TNFs have served well in that regard.”
However, TNF inhibitors are associated with more safety signals, plus they carry a greater risk of infection, leading to tolerability and persistence issues with patients.
“Psoriasis is a lifelong disease. I wish I could tell you that every drug is going to work well forever for individual patients, but I don’t think we know that yet. From my perspective, for efficacy, general well tolerance, convenience, and access, TNFs are still an important part of our ability to treat psoriasis effectively. I have no problem starting there and transitioning as needed for individual patients.
“In my experience, I think patients on TNFs generally do well. We don’t always get the patients clear and certainly there’s drop off of efficacy over time, but I’m not sure that’s a rationale for [changing treatment],” Dr. Garg said.
Ying Ying (Katy) Leung, MD, a rheumatologist with Singapore General Hospital, and a member of the GRAPPA peripheral arthritis working group, argued against the use of IL-17 and IL-23 inhibitors as first-line treatment for PsA over TNF inhibitors. She reasoned that TNF blockers are more accessible, have more long-term safety data (including data indicating safety during pregnancy), and have better cardiovascular protection. She also noted that GRAPPA treatment recommendations strongly advise using TNF blockers (or IL-17 inhibitors) for treatment-naive patients with PsA.
“Accessibility is very important as I learned along the way of leading the peripheral arthritis [GRAPPA] working group. Accessibility [issues] can be coming from a lot of sources, but if you don’t take good care of accessibility, you might be developing a guideline that is way out of reality and nobody is going to use it,” she said.
In her native Singapore, Dr. Leung said that patients pay for biologics out of pocket, so cost is a key factor for her patients. She stated that adalimumab is available as a biosimilar at about $200 monthly for patients with PsA in Singapore, while the average monthly costs are $1,400 for originator infliximab and $1,500 for originator etanercept. By comparison, secukinumab sells for about $750 monthly, ixekizumab $540 monthly, and guselkumab $2,000 monthly.
Treatment choices should be aligned with the disease manifestations of PsA, Dr. Leung said, keeping in mind that accessibility and individual patient needs and preferences should be considered as well. She conducted an informal comparison that found TNF inhibitors are most effective for patients with uveitis or inflammatory bowel disease. Evidence from head-to-head studies indicates that TNF inhibitors and IL-17 inhibitors have similar efficacy for peripheral arthritis, enthesitis, and dactylitis. But caution is warranted, she suggested, for determining the best biologics for axial disease because no head-to-head comparison trials have been conducted for IL-17 or IL-23 inhibitors versus TNF inhibitors.
Dr. Armstrong has been a consultant to AbbVie, Bristol-Myers Squibb, Dermira, Genzyme, Incyte, Janssen, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB. Dr. Jadon has been a consultant to, has been on speakers bureaus for, and has received grant/research support from AbbVie, Amgen, Celgene, Celltrion, Gilead, Janssen, Eli Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, and UCB. Dr. Garg has consulted for AbbVie, Boehringer Ingelheim, Janssen, and UCB. Dr. Leung has been a consultant to AbbVie, Boehringer Ingelheim, Janssen, Eli Lilly, Novartis, and Pfizer. She has been on speakers bureaus for AbbVie, Janssen Eli Lilly, and Novartis. She has received grant/research support from Pfizer and conference support from AbbVie,
Tumor necrosis factor inhibitors have long been the go-to treatment of choice for patients with psoriasis and psoriatic arthritis (PsA). They’ve served patients well since etanercept was first approved for PsA in 2002, but today, with the availability of more attractive interleukin-17 and IL-23 inhibitors, dermatologists and rheumatologists are asking whether it’s time to reconsider the use of TNF inhibitors as first-line therapy in psoriasis and PsA.
“TNF inhibitors have served psoriasis patients well for many years. The question is, ‘Is it time to move on from them as first-line agents for psoriasis?’ ” said April W. Armstrong, MD, MPH, a dermatologist and associate dean for clinical research at the University of Southern California, Los Angeles. Dr. Armstrong participated in a point/counterpoint debate about the merits of IL-17 and IL-23 inhibitors over TNF inhibitors at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. “For the majority of our patients, IL-17 and IL-23 inhibitors are probably rationally better than TNF inhibitors as first-line agents for moderate to severe plaque psoriasis,” she said.
In this debate, dermatologists and rheumatologists cited studies showing the safety and efficacy of IL-17 and IL-23 inhibitors over TNF inhibitors. TNF inhibitors include etanercept (Enbrel and biosimilars), infliximab (Remicade and biosimilars), adalimumab (Humira and biosimilars), certolizumab pegol (Cimzia), and golimumab (Simponi). IL-12/23 inhibitors are limited to ustekinumab (Stelara). IL-17 inhibitors include secukinumab (Cosentyx), ixekizumab (Taltz), and brodalumab (Siliq). IL-23 inhibitors include guselkumab (Tremfya), tildrakizumab (Ilumya), and risankizumab (Skyrizi).
TNF inhibitors are recommended by the American College of Rheumatology as first-line therapy for treatment-naive patients with active PsA, and they, along with IL-12/23, IL-17, and IL-23 inhibitors are all recommended by the American Academy of Dermatology as monotherapy treatment options in adult patients with moderate to severe plaque psoriasis. However, some studies have shown that non–TNF-inhibitor biologics have a higher efficacy than TNF inhibitors in some cases for some patients, such as those with moderate to severe psoriasis alone or for musculoskeletal efficacy in patients with PsA who have peripheral arthritis, enthesitis, dactylitis, or axial manifestations.
Favorable characteristics of non–TNF-inhibitor biologics
Dr. Armstrong cited a number of head-to-head trials to support her view that IL-17 and IL-23 inhibitors are better than TNF inhibitors as first-line agents for patients with moderate to severe plaque psoriasis. In the first head-to-head study of its kind in patients with moderate to severe psoriasis, ustekinumab proved superior to etanercept. Guselkumab was shown to be superior to adalimumab for patients with moderate to severe psoriasis. Tildrakizumab also proved superior to etanercept for patients with psoriasis. Risankizumab bested adalimumab in patients with moderate to severe psoriasis. Ixekizumab proved superior to etanercept in two pivotal studies of patients with widespread moderate-to-severe psoriasis.
IL-23 and IL-17 inhibitors tend to have less frequent maintenance dosing, with IL-17 inhibitors being once every 2 or 4 weeks and IL-23 inhibitors once every 8 or 12 weeks, compared with frequencies ranging from every week to every 8 weeks with TNF inhibitors, Dr. Armstrong said.
IL-17 and IL-23 inhibitors also appear to have fewer safety concerns than TNF inhibitors, although there is less long-term data for them overall and there are some notable exceptions in certain patient populations. TNF inhibitors should be avoided in patients with a history of demyelinating disease or hepatitis B virus infection, and they are not preferred in patients who have a history of latent tuberculosis or advanced heart failure. IL-17 inhibitors should not be used in patients with a history of inflammatory bowel disease, and their use is associated with a higher rate of oral candidiasis. IL-23 inhibitors have a good safety profile overall, she said.
“The IL-17/23 axis is very important to psoriatic arthritis and should be the focus of our treatments” for PsA, said Deepak Jadon, MBBCh, MRCP, PhD, a rheumatologist and director of the rheumatology research unit at Addenbrooke’s Hospital, Cambridge (England) University Hospitals NHS Foundation Trust. In his presentation, he proposed that IL-17 inhibitors and IL-23 inhibitors be used as first-line therapies in PsA ahead of TNF inhibitors.
One reason to go with IL-17 and IL-23 inhibitors may be to ”get it right immunologically the first time,” Dr. Jadon said. He cited evidence showing substantially better response to guselkumab when given to biologic-naive patients with PsA versus those who had a inadequate response to TNF inhibitors, as well as data indicating better response with secukinumab regardless of previous TNF inhibitor use.
IL-17 inhibitors target more domains of psoriatic disease than do TNF inhibitors, he said, noting that “they have excellent musculoskeletal efficacy in patients with moderate skin psoriasis, not just those with severe psoriasis.” Ixekizumab proved superior to adalimumab in biologic-naive patients with PsA. The results of this study also indicated that IL-17 inhibitors should not be reserved only for patients with severe psoriasis since a higher percentage of patients with moderate psoriasis who were taking ixekizumab achieved very low PsA activity. Secukinumab also beat adalimumab in a head-to-head comparison and showed a greater impact on some measures of health-related quality of life.
IL-17 inhibitors also do not require concomitant methotrexate, he said, “which is a major bonus for our patients. All of my patients wish to stop methotrexate even if tolerated. Not having to cope with prescribed methotrexate improves risk of adverse events and frequency of blood test monitoring.”
IL-17 and IL-23 inhibitors appear to have good efficacy against axial disease in patients with PsA. Randomized trial results for secukinumab versus placebo show high percentages of patients improving either 20% or 40% in Assessment in Spondyloarthritis International Society response criteria and reduced inflammatory MRI lesions in the spine and sacroiliac joints. Analyses of trial results in guselkumab-treated patients with axial manifestations of PsA have shown the IL-23 inhibitor’s efficacy versus placebo across different measures of disease activity.
Dr. Jadon also cited real-world data showing that patients stay longer on IL-17 and IL-12/23 inhibitors versus TNF inhibitors. A 2016 study of patients with psoriasis in the PSOLAR registry showed that patients persisted on treatment longer with ustekinumab than with adalimumab, etanercept, or infliximab. Similarly, a 2020 study of patients with psoriasis from the British Association of Dermatologists Biologics and Immunomodulators Register found that both ustekinumab and secukinumab had better sustained drug survival than did adalimumab.
Accessibility weighs heavily in using TNF inhibitor first
Clinical trials data show that IL-17 inhibitors outperform TNF inhibitors for psoriasis, but in clinical practice, TNF inhibitors still perform very well in individual patients and are well tolerated, said Amit Garg, MD, founding chair of the department of dermatology at Hofstra University, Hempstead, N.Y.
He argued in favor of TNF inhibitors as first-line therapy over IL-17 inhibitors for psoriasis. In this case, treatment decisions often come down to accessibility, Dr. Garg said. Not all insurance companies cover the cost of the newer IL-23 inhibitors. Plus, access to TNF inhibitors is widespread and costs are generally lower.
“As a physician, I don’t have complete autonomy in prescribing what I want. The reality is whether it be because of cross indication or discount pricing, [TNF inhibitors] – in particular adalimumab – is widely available on all plans and is usually the preferred treatment plan, at least in our area,” he said. “I’m not a big fan of plans that allow drugs at low or no cost for a year or 2, and then abandon the patients at that point thereafter. I like to use something that insurance will cover sustainably, and, quite frankly, TNFs have served well in that regard.”
However, TNF inhibitors are associated with more safety signals, plus they carry a greater risk of infection, leading to tolerability and persistence issues with patients.
“Psoriasis is a lifelong disease. I wish I could tell you that every drug is going to work well forever for individual patients, but I don’t think we know that yet. From my perspective, for efficacy, general well tolerance, convenience, and access, TNFs are still an important part of our ability to treat psoriasis effectively. I have no problem starting there and transitioning as needed for individual patients.
“In my experience, I think patients on TNFs generally do well. We don’t always get the patients clear and certainly there’s drop off of efficacy over time, but I’m not sure that’s a rationale for [changing treatment],” Dr. Garg said.
Ying Ying (Katy) Leung, MD, a rheumatologist with Singapore General Hospital, and a member of the GRAPPA peripheral arthritis working group, argued against the use of IL-17 and IL-23 inhibitors as first-line treatment for PsA over TNF inhibitors. She reasoned that TNF blockers are more accessible, have more long-term safety data (including data indicating safety during pregnancy), and have better cardiovascular protection. She also noted that GRAPPA treatment recommendations strongly advise using TNF blockers (or IL-17 inhibitors) for treatment-naive patients with PsA.
“Accessibility is very important as I learned along the way of leading the peripheral arthritis [GRAPPA] working group. Accessibility [issues] can be coming from a lot of sources, but if you don’t take good care of accessibility, you might be developing a guideline that is way out of reality and nobody is going to use it,” she said.
In her native Singapore, Dr. Leung said that patients pay for biologics out of pocket, so cost is a key factor for her patients. She stated that adalimumab is available as a biosimilar at about $200 monthly for patients with PsA in Singapore, while the average monthly costs are $1,400 for originator infliximab and $1,500 for originator etanercept. By comparison, secukinumab sells for about $750 monthly, ixekizumab $540 monthly, and guselkumab $2,000 monthly.
Treatment choices should be aligned with the disease manifestations of PsA, Dr. Leung said, keeping in mind that accessibility and individual patient needs and preferences should be considered as well. She conducted an informal comparison that found TNF inhibitors are most effective for patients with uveitis or inflammatory bowel disease. Evidence from head-to-head studies indicates that TNF inhibitors and IL-17 inhibitors have similar efficacy for peripheral arthritis, enthesitis, and dactylitis. But caution is warranted, she suggested, for determining the best biologics for axial disease because no head-to-head comparison trials have been conducted for IL-17 or IL-23 inhibitors versus TNF inhibitors.
Dr. Armstrong has been a consultant to AbbVie, Bristol-Myers Squibb, Dermira, Genzyme, Incyte, Janssen, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB. Dr. Jadon has been a consultant to, has been on speakers bureaus for, and has received grant/research support from AbbVie, Amgen, Celgene, Celltrion, Gilead, Janssen, Eli Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, and UCB. Dr. Garg has consulted for AbbVie, Boehringer Ingelheim, Janssen, and UCB. Dr. Leung has been a consultant to AbbVie, Boehringer Ingelheim, Janssen, Eli Lilly, Novartis, and Pfizer. She has been on speakers bureaus for AbbVie, Janssen Eli Lilly, and Novartis. She has received grant/research support from Pfizer and conference support from AbbVie,
Tumor necrosis factor inhibitors have long been the go-to treatment of choice for patients with psoriasis and psoriatic arthritis (PsA). They’ve served patients well since etanercept was first approved for PsA in 2002, but today, with the availability of more attractive interleukin-17 and IL-23 inhibitors, dermatologists and rheumatologists are asking whether it’s time to reconsider the use of TNF inhibitors as first-line therapy in psoriasis and PsA.
“TNF inhibitors have served psoriasis patients well for many years. The question is, ‘Is it time to move on from them as first-line agents for psoriasis?’ ” said April W. Armstrong, MD, MPH, a dermatologist and associate dean for clinical research at the University of Southern California, Los Angeles. Dr. Armstrong participated in a point/counterpoint debate about the merits of IL-17 and IL-23 inhibitors over TNF inhibitors at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. “For the majority of our patients, IL-17 and IL-23 inhibitors are probably rationally better than TNF inhibitors as first-line agents for moderate to severe plaque psoriasis,” she said.
In this debate, dermatologists and rheumatologists cited studies showing the safety and efficacy of IL-17 and IL-23 inhibitors over TNF inhibitors. TNF inhibitors include etanercept (Enbrel and biosimilars), infliximab (Remicade and biosimilars), adalimumab (Humira and biosimilars), certolizumab pegol (Cimzia), and golimumab (Simponi). IL-12/23 inhibitors are limited to ustekinumab (Stelara). IL-17 inhibitors include secukinumab (Cosentyx), ixekizumab (Taltz), and brodalumab (Siliq). IL-23 inhibitors include guselkumab (Tremfya), tildrakizumab (Ilumya), and risankizumab (Skyrizi).
TNF inhibitors are recommended by the American College of Rheumatology as first-line therapy for treatment-naive patients with active PsA, and they, along with IL-12/23, IL-17, and IL-23 inhibitors are all recommended by the American Academy of Dermatology as monotherapy treatment options in adult patients with moderate to severe plaque psoriasis. However, some studies have shown that non–TNF-inhibitor biologics have a higher efficacy than TNF inhibitors in some cases for some patients, such as those with moderate to severe psoriasis alone or for musculoskeletal efficacy in patients with PsA who have peripheral arthritis, enthesitis, dactylitis, or axial manifestations.
Favorable characteristics of non–TNF-inhibitor biologics
Dr. Armstrong cited a number of head-to-head trials to support her view that IL-17 and IL-23 inhibitors are better than TNF inhibitors as first-line agents for patients with moderate to severe plaque psoriasis. In the first head-to-head study of its kind in patients with moderate to severe psoriasis, ustekinumab proved superior to etanercept. Guselkumab was shown to be superior to adalimumab for patients with moderate to severe psoriasis. Tildrakizumab also proved superior to etanercept for patients with psoriasis. Risankizumab bested adalimumab in patients with moderate to severe psoriasis. Ixekizumab proved superior to etanercept in two pivotal studies of patients with widespread moderate-to-severe psoriasis.
IL-23 and IL-17 inhibitors tend to have less frequent maintenance dosing, with IL-17 inhibitors being once every 2 or 4 weeks and IL-23 inhibitors once every 8 or 12 weeks, compared with frequencies ranging from every week to every 8 weeks with TNF inhibitors, Dr. Armstrong said.
IL-17 and IL-23 inhibitors also appear to have fewer safety concerns than TNF inhibitors, although there is less long-term data for them overall and there are some notable exceptions in certain patient populations. TNF inhibitors should be avoided in patients with a history of demyelinating disease or hepatitis B virus infection, and they are not preferred in patients who have a history of latent tuberculosis or advanced heart failure. IL-17 inhibitors should not be used in patients with a history of inflammatory bowel disease, and their use is associated with a higher rate of oral candidiasis. IL-23 inhibitors have a good safety profile overall, she said.
“The IL-17/23 axis is very important to psoriatic arthritis and should be the focus of our treatments” for PsA, said Deepak Jadon, MBBCh, MRCP, PhD, a rheumatologist and director of the rheumatology research unit at Addenbrooke’s Hospital, Cambridge (England) University Hospitals NHS Foundation Trust. In his presentation, he proposed that IL-17 inhibitors and IL-23 inhibitors be used as first-line therapies in PsA ahead of TNF inhibitors.
One reason to go with IL-17 and IL-23 inhibitors may be to ”get it right immunologically the first time,” Dr. Jadon said. He cited evidence showing substantially better response to guselkumab when given to biologic-naive patients with PsA versus those who had a inadequate response to TNF inhibitors, as well as data indicating better response with secukinumab regardless of previous TNF inhibitor use.
IL-17 inhibitors target more domains of psoriatic disease than do TNF inhibitors, he said, noting that “they have excellent musculoskeletal efficacy in patients with moderate skin psoriasis, not just those with severe psoriasis.” Ixekizumab proved superior to adalimumab in biologic-naive patients with PsA. The results of this study also indicated that IL-17 inhibitors should not be reserved only for patients with severe psoriasis since a higher percentage of patients with moderate psoriasis who were taking ixekizumab achieved very low PsA activity. Secukinumab also beat adalimumab in a head-to-head comparison and showed a greater impact on some measures of health-related quality of life.
IL-17 inhibitors also do not require concomitant methotrexate, he said, “which is a major bonus for our patients. All of my patients wish to stop methotrexate even if tolerated. Not having to cope with prescribed methotrexate improves risk of adverse events and frequency of blood test monitoring.”
IL-17 and IL-23 inhibitors appear to have good efficacy against axial disease in patients with PsA. Randomized trial results for secukinumab versus placebo show high percentages of patients improving either 20% or 40% in Assessment in Spondyloarthritis International Society response criteria and reduced inflammatory MRI lesions in the spine and sacroiliac joints. Analyses of trial results in guselkumab-treated patients with axial manifestations of PsA have shown the IL-23 inhibitor’s efficacy versus placebo across different measures of disease activity.
Dr. Jadon also cited real-world data showing that patients stay longer on IL-17 and IL-12/23 inhibitors versus TNF inhibitors. A 2016 study of patients with psoriasis in the PSOLAR registry showed that patients persisted on treatment longer with ustekinumab than with adalimumab, etanercept, or infliximab. Similarly, a 2020 study of patients with psoriasis from the British Association of Dermatologists Biologics and Immunomodulators Register found that both ustekinumab and secukinumab had better sustained drug survival than did adalimumab.
Accessibility weighs heavily in using TNF inhibitor first
Clinical trials data show that IL-17 inhibitors outperform TNF inhibitors for psoriasis, but in clinical practice, TNF inhibitors still perform very well in individual patients and are well tolerated, said Amit Garg, MD, founding chair of the department of dermatology at Hofstra University, Hempstead, N.Y.
He argued in favor of TNF inhibitors as first-line therapy over IL-17 inhibitors for psoriasis. In this case, treatment decisions often come down to accessibility, Dr. Garg said. Not all insurance companies cover the cost of the newer IL-23 inhibitors. Plus, access to TNF inhibitors is widespread and costs are generally lower.
“As a physician, I don’t have complete autonomy in prescribing what I want. The reality is whether it be because of cross indication or discount pricing, [TNF inhibitors] – in particular adalimumab – is widely available on all plans and is usually the preferred treatment plan, at least in our area,” he said. “I’m not a big fan of plans that allow drugs at low or no cost for a year or 2, and then abandon the patients at that point thereafter. I like to use something that insurance will cover sustainably, and, quite frankly, TNFs have served well in that regard.”
However, TNF inhibitors are associated with more safety signals, plus they carry a greater risk of infection, leading to tolerability and persistence issues with patients.
“Psoriasis is a lifelong disease. I wish I could tell you that every drug is going to work well forever for individual patients, but I don’t think we know that yet. From my perspective, for efficacy, general well tolerance, convenience, and access, TNFs are still an important part of our ability to treat psoriasis effectively. I have no problem starting there and transitioning as needed for individual patients.
“In my experience, I think patients on TNFs generally do well. We don’t always get the patients clear and certainly there’s drop off of efficacy over time, but I’m not sure that’s a rationale for [changing treatment],” Dr. Garg said.
Ying Ying (Katy) Leung, MD, a rheumatologist with Singapore General Hospital, and a member of the GRAPPA peripheral arthritis working group, argued against the use of IL-17 and IL-23 inhibitors as first-line treatment for PsA over TNF inhibitors. She reasoned that TNF blockers are more accessible, have more long-term safety data (including data indicating safety during pregnancy), and have better cardiovascular protection. She also noted that GRAPPA treatment recommendations strongly advise using TNF blockers (or IL-17 inhibitors) for treatment-naive patients with PsA.
“Accessibility is very important as I learned along the way of leading the peripheral arthritis [GRAPPA] working group. Accessibility [issues] can be coming from a lot of sources, but if you don’t take good care of accessibility, you might be developing a guideline that is way out of reality and nobody is going to use it,” she said.
In her native Singapore, Dr. Leung said that patients pay for biologics out of pocket, so cost is a key factor for her patients. She stated that adalimumab is available as a biosimilar at about $200 monthly for patients with PsA in Singapore, while the average monthly costs are $1,400 for originator infliximab and $1,500 for originator etanercept. By comparison, secukinumab sells for about $750 monthly, ixekizumab $540 monthly, and guselkumab $2,000 monthly.
Treatment choices should be aligned with the disease manifestations of PsA, Dr. Leung said, keeping in mind that accessibility and individual patient needs and preferences should be considered as well. She conducted an informal comparison that found TNF inhibitors are most effective for patients with uveitis or inflammatory bowel disease. Evidence from head-to-head studies indicates that TNF inhibitors and IL-17 inhibitors have similar efficacy for peripheral arthritis, enthesitis, and dactylitis. But caution is warranted, she suggested, for determining the best biologics for axial disease because no head-to-head comparison trials have been conducted for IL-17 or IL-23 inhibitors versus TNF inhibitors.
Dr. Armstrong has been a consultant to AbbVie, Bristol-Myers Squibb, Dermira, Genzyme, Incyte, Janssen, Leo Pharma, Eli Lilly, Novartis, Pfizer, and UCB. Dr. Jadon has been a consultant to, has been on speakers bureaus for, and has received grant/research support from AbbVie, Amgen, Celgene, Celltrion, Gilead, Janssen, Eli Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, and UCB. Dr. Garg has consulted for AbbVie, Boehringer Ingelheim, Janssen, and UCB. Dr. Leung has been a consultant to AbbVie, Boehringer Ingelheim, Janssen, Eli Lilly, Novartis, and Pfizer. She has been on speakers bureaus for AbbVie, Janssen Eli Lilly, and Novartis. She has received grant/research support from Pfizer and conference support from AbbVie,
FROM THE GRAPPA 2021 ANNUAL MEETING
Half abandon metformin within a year of diabetes diagnosis
Nearly half of adults prescribed metformin after a new diagnosis of type 2 diabetes have stopped taking it by 1 year, new data show.
The findings, from a retrospective analysis of administrative data from Alberta, Canada, during 2012-2017, also show that the fall-off in metformin adherence was most dramatic during the first 30 days, and in most cases, there was no concomitant substitution of another glucose-lowering drug.
While the majority with newly diagnosed type 2 diabetes were prescribed metformin as first-line therapy, patients started on other agents incurred far higher medication and health care costs.
The data were recently published online in Diabetic Medicine by David J. T. Campbell, MD, PhD, of the University of Calgary (Alta.), and colleagues.
“We realized that even if someone is prescribed metformin that doesn’t mean they’re staying on metformin even for a year ... the drop-off rate is really quite abrupt,” Dr. Campbell said in an interview. Most who discontinued had A1c levels above 7.5%, so it wasn’t that they no longer needed glucose-lowering medication, he noted.
People don’t understand chronic use; meds don’t make you feel better
One reason for the discontinuations, he said, is that patients might not realize they need to keep taking the medication.
“When a physician is seeing a person with newly diagnosed diabetes, I think it’s important to remember that they might not know the implications of having a chronic condition. A lot of times we’re quick to prescribe metformin and forget about it. ... Physicians might write a script for 3 months and three refills and not see the patient again for a year ... We may need to keep a closer eye on these folks and have more regular follow-up, and make sure they’re getting early diabetes education.”
Side effects are an issue, but not for most. “Any clinician who prescribes metformin knows there are side effects, such as upset stomach, diarrhea, and nausea. But certainly, it’s not half [who experience these]. ... A lot of people just aren’t accepting of having to take it lifelong, especially since they probably don’t feel any better on it,” Dr. Campbell said.
James Flory, MD, an endocrinologist at Memorial Sloan Kettering Cancer Center, New York, said in an interview that about 25% of patients taking metformin experience gastrointestinal side effects.
Moreover, he noted that the drop-off in adherence is also seen with antihypertensive and lipid-lowering drugs that have fewer side effects than those of metformin. He pointed to a “striking example” of this, a 2011 randomized trial published in the New England Journal of Medicine, and as reported by this news organization, showing overall rates of adherence to these medications was around 50%, even among people who had already had a myocardial infarction.
“People really don’t want to be on these medications. ... They have an aversion to being medicalized and taking pills. If they’re not being pretty consistently prompted and reminded and urged to take them, I think people will find rationalizations, reasons for stopping. ... I think people want to handle things through lifestyle and not be on a drug,” noted Dr. Flory, who has published on the subject of metformin adherence.
“These drugs don’t make people feel better. None of them do. At best they don’t make you feel worse. You have to really believe in the chronic condition and believe that it’s hurting you and that you can’t handle it without the drugs to motivate you to keep taking them,” Dr. Flory explained.
Communication with the patient is key, he added.
“I don’t have empirical data to support this, but I feel it’s helpful to acknowledge the downsides to patients. I tell them to let me know [if they’re having side effects] and we’ll work on it. Don’t just stop taking the drug and never circle back.” At the same time, he added, “I think it’s important to emphasize metformin’s safety and effectiveness.”
For patients experiencing gastrointestinal side effects, options including switching to extended-release metformin or lowering the dose.
Also, while patients are typically advised to take metformin with food, some experience diarrhea when they do that and prefer to take it at bedtime than with dinner. “If that’s what works for people, that’s what they should do,” Dr. Flory advised.
“It doesn’t take a lot of time to emphasize to patients the safety and this level of flexibility and control they should be able to exercise over how much they take and when. These things should really help.”
Metformin usually prescribed, but not always taken
Dr. Campbell and colleagues analyzed 17,932 individuals with incident type 2 diabetes diagnosed between April 1, 2012, and March 31, 2017. Overall, 89% received metformin monotherapy as their initial diabetes prescription, 7.6% started metformin in combination with another glucose-lowering drug, and 3.3% were prescribed a nonmetformin diabetes medication. (Those prescribed insulin as their first diabetes medication were excluded.)
The most commonly coprescribed drugs with metformin were sulfonylureas (in 47%) and DPP-4 inhibitors (28%). Of those initiated with only nonmetformin medications, sulfonylureas were also the most common (53%) and dipeptidyl peptidase-4 (DPP-4) inhibitors second (21%).
The metformin prescribing rate of 89% reflects current guidelines, Dr. Campbell noted.
“In hypertension, clinicians weren’t really following the guidelines ... they were prescribing more expensive drugs than the guidelines say. ... We showed that in diabetes, contrary to hypertension, clinicians really are generally following the clinical practice guidelines. ... The vast majority who are started on metformin are started on monotherapy. That was reassuring to us. We’re not paying for a bunch of expensive drugs when metformin would do just as well,” he said.
However, the proportion who had been dispensed metformin to cover the prescribed number of days dropped by about 10% after 30 days, by a further 10% after 90 days, and yet again after 100 days, resulting in just 54% remaining on the drug by 1 year.
Factors associated with higher adherence included older age, presence of comorbidities, and highest versus lowest neighborhood income quintile.
Those who had been prescribed nonmetformin monotherapy had about twice the total health care costs of those initially prescribed metformin monotherapy. Higher health care costs were seen among patients who were younger, had lower incomes, had higher baseline A1c, had more comorbidities, and were men.
How will the newer type 2 diabetes drugs change prescribing?
Dr. Campbell noted that “a lot has changed since 2017. ... At least in Canada, the sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists were supposed to be reserved as second-line agents in patients with cardiovascular disease, but more and more they’re being thought of as first-line agents in high-risk patients.”
“I suspect as those guidelines are transmitted to primary care colleagues who are doing the bulk of the prescribing we’ll see more and more uptake of these agents.”
Indeed, Dr. Flory said, “The metformin data at this point are very dated and the body of trials showing health benefits for it is actually very weak compared to the big trials that have been done for the newer agents, to the point where you can imagine a consensus gradually forming where people start to recommend something other than metformin for nearly everybody with type 2 diabetes. The cost implications are just huge, and I think the safety implications as well.”
According to Dr. Flory, the SGLT2 inhibitors “are fundamentally not as safe as metformin. I think they’re very safe drugs – large good studies have established that – but if you’re going to give drugs to a large number of people who are pretty healthy at baseline the safety standards have to be pretty high.”
Just the elevated risk of euglycemic diabetic ketoacidosis alone is reason for pause, Dr. Flory said. “Even though it’s manageable ... metformin just doesn’t have a safety problem like that. I’m very comfortable prescribing SGLT2 inhibitors, but If I’m going to give a drug to a million people and have nothing go wrong with any of them, that would be metformin, not an SGLT2 [inhibitor].”
Dr. Campbell and colleagues will be conducting a follow-up of prescribing data through 2019, which will of course include the newer agents. They’ll also investigate reasons for drug discontinuation and outcomes of those who discontinue versus continue metformin.
Dr. Campbell has reported no relevant financial relationships. Dr. Flory consults for a legal firm on litigation related to insulin analog pricing issues, not for or pertaining to a specific company.
A version of this article first appeared on Medscape.com.
Nearly half of adults prescribed metformin after a new diagnosis of type 2 diabetes have stopped taking it by 1 year, new data show.
The findings, from a retrospective analysis of administrative data from Alberta, Canada, during 2012-2017, also show that the fall-off in metformin adherence was most dramatic during the first 30 days, and in most cases, there was no concomitant substitution of another glucose-lowering drug.
While the majority with newly diagnosed type 2 diabetes were prescribed metformin as first-line therapy, patients started on other agents incurred far higher medication and health care costs.
The data were recently published online in Diabetic Medicine by David J. T. Campbell, MD, PhD, of the University of Calgary (Alta.), and colleagues.
“We realized that even if someone is prescribed metformin that doesn’t mean they’re staying on metformin even for a year ... the drop-off rate is really quite abrupt,” Dr. Campbell said in an interview. Most who discontinued had A1c levels above 7.5%, so it wasn’t that they no longer needed glucose-lowering medication, he noted.
People don’t understand chronic use; meds don’t make you feel better
One reason for the discontinuations, he said, is that patients might not realize they need to keep taking the medication.
“When a physician is seeing a person with newly diagnosed diabetes, I think it’s important to remember that they might not know the implications of having a chronic condition. A lot of times we’re quick to prescribe metformin and forget about it. ... Physicians might write a script for 3 months and three refills and not see the patient again for a year ... We may need to keep a closer eye on these folks and have more regular follow-up, and make sure they’re getting early diabetes education.”
Side effects are an issue, but not for most. “Any clinician who prescribes metformin knows there are side effects, such as upset stomach, diarrhea, and nausea. But certainly, it’s not half [who experience these]. ... A lot of people just aren’t accepting of having to take it lifelong, especially since they probably don’t feel any better on it,” Dr. Campbell said.
James Flory, MD, an endocrinologist at Memorial Sloan Kettering Cancer Center, New York, said in an interview that about 25% of patients taking metformin experience gastrointestinal side effects.
Moreover, he noted that the drop-off in adherence is also seen with antihypertensive and lipid-lowering drugs that have fewer side effects than those of metformin. He pointed to a “striking example” of this, a 2011 randomized trial published in the New England Journal of Medicine, and as reported by this news organization, showing overall rates of adherence to these medications was around 50%, even among people who had already had a myocardial infarction.
“People really don’t want to be on these medications. ... They have an aversion to being medicalized and taking pills. If they’re not being pretty consistently prompted and reminded and urged to take them, I think people will find rationalizations, reasons for stopping. ... I think people want to handle things through lifestyle and not be on a drug,” noted Dr. Flory, who has published on the subject of metformin adherence.
“These drugs don’t make people feel better. None of them do. At best they don’t make you feel worse. You have to really believe in the chronic condition and believe that it’s hurting you and that you can’t handle it without the drugs to motivate you to keep taking them,” Dr. Flory explained.
Communication with the patient is key, he added.
“I don’t have empirical data to support this, but I feel it’s helpful to acknowledge the downsides to patients. I tell them to let me know [if they’re having side effects] and we’ll work on it. Don’t just stop taking the drug and never circle back.” At the same time, he added, “I think it’s important to emphasize metformin’s safety and effectiveness.”
For patients experiencing gastrointestinal side effects, options including switching to extended-release metformin or lowering the dose.
Also, while patients are typically advised to take metformin with food, some experience diarrhea when they do that and prefer to take it at bedtime than with dinner. “If that’s what works for people, that’s what they should do,” Dr. Flory advised.
“It doesn’t take a lot of time to emphasize to patients the safety and this level of flexibility and control they should be able to exercise over how much they take and when. These things should really help.”
Metformin usually prescribed, but not always taken
Dr. Campbell and colleagues analyzed 17,932 individuals with incident type 2 diabetes diagnosed between April 1, 2012, and March 31, 2017. Overall, 89% received metformin monotherapy as their initial diabetes prescription, 7.6% started metformin in combination with another glucose-lowering drug, and 3.3% were prescribed a nonmetformin diabetes medication. (Those prescribed insulin as their first diabetes medication were excluded.)
The most commonly coprescribed drugs with metformin were sulfonylureas (in 47%) and DPP-4 inhibitors (28%). Of those initiated with only nonmetformin medications, sulfonylureas were also the most common (53%) and dipeptidyl peptidase-4 (DPP-4) inhibitors second (21%).
The metformin prescribing rate of 89% reflects current guidelines, Dr. Campbell noted.
“In hypertension, clinicians weren’t really following the guidelines ... they were prescribing more expensive drugs than the guidelines say. ... We showed that in diabetes, contrary to hypertension, clinicians really are generally following the clinical practice guidelines. ... The vast majority who are started on metformin are started on monotherapy. That was reassuring to us. We’re not paying for a bunch of expensive drugs when metformin would do just as well,” he said.
However, the proportion who had been dispensed metformin to cover the prescribed number of days dropped by about 10% after 30 days, by a further 10% after 90 days, and yet again after 100 days, resulting in just 54% remaining on the drug by 1 year.
Factors associated with higher adherence included older age, presence of comorbidities, and highest versus lowest neighborhood income quintile.
Those who had been prescribed nonmetformin monotherapy had about twice the total health care costs of those initially prescribed metformin monotherapy. Higher health care costs were seen among patients who were younger, had lower incomes, had higher baseline A1c, had more comorbidities, and were men.
How will the newer type 2 diabetes drugs change prescribing?
Dr. Campbell noted that “a lot has changed since 2017. ... At least in Canada, the sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists were supposed to be reserved as second-line agents in patients with cardiovascular disease, but more and more they’re being thought of as first-line agents in high-risk patients.”
“I suspect as those guidelines are transmitted to primary care colleagues who are doing the bulk of the prescribing we’ll see more and more uptake of these agents.”
Indeed, Dr. Flory said, “The metformin data at this point are very dated and the body of trials showing health benefits for it is actually very weak compared to the big trials that have been done for the newer agents, to the point where you can imagine a consensus gradually forming where people start to recommend something other than metformin for nearly everybody with type 2 diabetes. The cost implications are just huge, and I think the safety implications as well.”
According to Dr. Flory, the SGLT2 inhibitors “are fundamentally not as safe as metformin. I think they’re very safe drugs – large good studies have established that – but if you’re going to give drugs to a large number of people who are pretty healthy at baseline the safety standards have to be pretty high.”
Just the elevated risk of euglycemic diabetic ketoacidosis alone is reason for pause, Dr. Flory said. “Even though it’s manageable ... metformin just doesn’t have a safety problem like that. I’m very comfortable prescribing SGLT2 inhibitors, but If I’m going to give a drug to a million people and have nothing go wrong with any of them, that would be metformin, not an SGLT2 [inhibitor].”
Dr. Campbell and colleagues will be conducting a follow-up of prescribing data through 2019, which will of course include the newer agents. They’ll also investigate reasons for drug discontinuation and outcomes of those who discontinue versus continue metformin.
Dr. Campbell has reported no relevant financial relationships. Dr. Flory consults for a legal firm on litigation related to insulin analog pricing issues, not for or pertaining to a specific company.
A version of this article first appeared on Medscape.com.
Nearly half of adults prescribed metformin after a new diagnosis of type 2 diabetes have stopped taking it by 1 year, new data show.
The findings, from a retrospective analysis of administrative data from Alberta, Canada, during 2012-2017, also show that the fall-off in metformin adherence was most dramatic during the first 30 days, and in most cases, there was no concomitant substitution of another glucose-lowering drug.
While the majority with newly diagnosed type 2 diabetes were prescribed metformin as first-line therapy, patients started on other agents incurred far higher medication and health care costs.
The data were recently published online in Diabetic Medicine by David J. T. Campbell, MD, PhD, of the University of Calgary (Alta.), and colleagues.
“We realized that even if someone is prescribed metformin that doesn’t mean they’re staying on metformin even for a year ... the drop-off rate is really quite abrupt,” Dr. Campbell said in an interview. Most who discontinued had A1c levels above 7.5%, so it wasn’t that they no longer needed glucose-lowering medication, he noted.
People don’t understand chronic use; meds don’t make you feel better
One reason for the discontinuations, he said, is that patients might not realize they need to keep taking the medication.
“When a physician is seeing a person with newly diagnosed diabetes, I think it’s important to remember that they might not know the implications of having a chronic condition. A lot of times we’re quick to prescribe metformin and forget about it. ... Physicians might write a script for 3 months and three refills and not see the patient again for a year ... We may need to keep a closer eye on these folks and have more regular follow-up, and make sure they’re getting early diabetes education.”
Side effects are an issue, but not for most. “Any clinician who prescribes metformin knows there are side effects, such as upset stomach, diarrhea, and nausea. But certainly, it’s not half [who experience these]. ... A lot of people just aren’t accepting of having to take it lifelong, especially since they probably don’t feel any better on it,” Dr. Campbell said.
James Flory, MD, an endocrinologist at Memorial Sloan Kettering Cancer Center, New York, said in an interview that about 25% of patients taking metformin experience gastrointestinal side effects.
Moreover, he noted that the drop-off in adherence is also seen with antihypertensive and lipid-lowering drugs that have fewer side effects than those of metformin. He pointed to a “striking example” of this, a 2011 randomized trial published in the New England Journal of Medicine, and as reported by this news organization, showing overall rates of adherence to these medications was around 50%, even among people who had already had a myocardial infarction.
“People really don’t want to be on these medications. ... They have an aversion to being medicalized and taking pills. If they’re not being pretty consistently prompted and reminded and urged to take them, I think people will find rationalizations, reasons for stopping. ... I think people want to handle things through lifestyle and not be on a drug,” noted Dr. Flory, who has published on the subject of metformin adherence.
“These drugs don’t make people feel better. None of them do. At best they don’t make you feel worse. You have to really believe in the chronic condition and believe that it’s hurting you and that you can’t handle it without the drugs to motivate you to keep taking them,” Dr. Flory explained.
Communication with the patient is key, he added.
“I don’t have empirical data to support this, but I feel it’s helpful to acknowledge the downsides to patients. I tell them to let me know [if they’re having side effects] and we’ll work on it. Don’t just stop taking the drug and never circle back.” At the same time, he added, “I think it’s important to emphasize metformin’s safety and effectiveness.”
For patients experiencing gastrointestinal side effects, options including switching to extended-release metformin or lowering the dose.
Also, while patients are typically advised to take metformin with food, some experience diarrhea when they do that and prefer to take it at bedtime than with dinner. “If that’s what works for people, that’s what they should do,” Dr. Flory advised.
“It doesn’t take a lot of time to emphasize to patients the safety and this level of flexibility and control they should be able to exercise over how much they take and when. These things should really help.”
Metformin usually prescribed, but not always taken
Dr. Campbell and colleagues analyzed 17,932 individuals with incident type 2 diabetes diagnosed between April 1, 2012, and March 31, 2017. Overall, 89% received metformin monotherapy as their initial diabetes prescription, 7.6% started metformin in combination with another glucose-lowering drug, and 3.3% were prescribed a nonmetformin diabetes medication. (Those prescribed insulin as their first diabetes medication were excluded.)
The most commonly coprescribed drugs with metformin were sulfonylureas (in 47%) and DPP-4 inhibitors (28%). Of those initiated with only nonmetformin medications, sulfonylureas were also the most common (53%) and dipeptidyl peptidase-4 (DPP-4) inhibitors second (21%).
The metformin prescribing rate of 89% reflects current guidelines, Dr. Campbell noted.
“In hypertension, clinicians weren’t really following the guidelines ... they were prescribing more expensive drugs than the guidelines say. ... We showed that in diabetes, contrary to hypertension, clinicians really are generally following the clinical practice guidelines. ... The vast majority who are started on metformin are started on monotherapy. That was reassuring to us. We’re not paying for a bunch of expensive drugs when metformin would do just as well,” he said.
However, the proportion who had been dispensed metformin to cover the prescribed number of days dropped by about 10% after 30 days, by a further 10% after 90 days, and yet again after 100 days, resulting in just 54% remaining on the drug by 1 year.
Factors associated with higher adherence included older age, presence of comorbidities, and highest versus lowest neighborhood income quintile.
Those who had been prescribed nonmetformin monotherapy had about twice the total health care costs of those initially prescribed metformin monotherapy. Higher health care costs were seen among patients who were younger, had lower incomes, had higher baseline A1c, had more comorbidities, and were men.
How will the newer type 2 diabetes drugs change prescribing?
Dr. Campbell noted that “a lot has changed since 2017. ... At least in Canada, the sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists were supposed to be reserved as second-line agents in patients with cardiovascular disease, but more and more they’re being thought of as first-line agents in high-risk patients.”
“I suspect as those guidelines are transmitted to primary care colleagues who are doing the bulk of the prescribing we’ll see more and more uptake of these agents.”
Indeed, Dr. Flory said, “The metformin data at this point are very dated and the body of trials showing health benefits for it is actually very weak compared to the big trials that have been done for the newer agents, to the point where you can imagine a consensus gradually forming where people start to recommend something other than metformin for nearly everybody with type 2 diabetes. The cost implications are just huge, and I think the safety implications as well.”
According to Dr. Flory, the SGLT2 inhibitors “are fundamentally not as safe as metformin. I think they’re very safe drugs – large good studies have established that – but if you’re going to give drugs to a large number of people who are pretty healthy at baseline the safety standards have to be pretty high.”
Just the elevated risk of euglycemic diabetic ketoacidosis alone is reason for pause, Dr. Flory said. “Even though it’s manageable ... metformin just doesn’t have a safety problem like that. I’m very comfortable prescribing SGLT2 inhibitors, but If I’m going to give a drug to a million people and have nothing go wrong with any of them, that would be metformin, not an SGLT2 [inhibitor].”
Dr. Campbell and colleagues will be conducting a follow-up of prescribing data through 2019, which will of course include the newer agents. They’ll also investigate reasons for drug discontinuation and outcomes of those who discontinue versus continue metformin.
Dr. Campbell has reported no relevant financial relationships. Dr. Flory consults for a legal firm on litigation related to insulin analog pricing issues, not for or pertaining to a specific company.
A version of this article first appeared on Medscape.com.
Moderna says boosters may be needed after 6 months
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
FDA clears app for FreeStyle Libre 2 glucose monitor
The Food and Drug Administration has cleared the FreeStyle Libre 2 iOS application for use with compatible iPhones.
The new app works with the FreeStyle Libre 2 with optional glucose alarms, which was approved in the United States in June 2020 for people with diabetes aged 4 years and older.
Until now, it was only a reader device with no app compatibility. The older FreeStyle Libre 14-day, available in the United States since July 2018, has both a reader and an app, but not optional alarms.
The new app, which will soon be available for download from the App Store, enables users to view glucose readings on their iPhones and allows for caregivers or other individuals to remotely monitor the patient’s glucose levels and receive real-time alarms via the LibreLinkUp app.
Worn for 14 days before replacement is needed, the FreeStyle Libre 2 is the longest-lasting integrated continuous glucose monitoring (iCGM) sensor currently on the market. The first iCGM, the Dexcom G6, is worn for 10 days.
The Libre 2 is available at pharmacies, typically at a lower cost than other CGM systems based on a list price comparison. The actual cost for patients varies depending on insurance coverage.
Abbott has secured partial or full reimbursement for the FreeStyle Libre system in 38 countries, including Canada, France, Germany, Japan, the United Kingdom, and the United States.
The FreeStyle Libre 3 is approved for use in the European Union.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the FreeStyle Libre 2 iOS application for use with compatible iPhones.
The new app works with the FreeStyle Libre 2 with optional glucose alarms, which was approved in the United States in June 2020 for people with diabetes aged 4 years and older.
Until now, it was only a reader device with no app compatibility. The older FreeStyle Libre 14-day, available in the United States since July 2018, has both a reader and an app, but not optional alarms.
The new app, which will soon be available for download from the App Store, enables users to view glucose readings on their iPhones and allows for caregivers or other individuals to remotely monitor the patient’s glucose levels and receive real-time alarms via the LibreLinkUp app.
Worn for 14 days before replacement is needed, the FreeStyle Libre 2 is the longest-lasting integrated continuous glucose monitoring (iCGM) sensor currently on the market. The first iCGM, the Dexcom G6, is worn for 10 days.
The Libre 2 is available at pharmacies, typically at a lower cost than other CGM systems based on a list price comparison. The actual cost for patients varies depending on insurance coverage.
Abbott has secured partial or full reimbursement for the FreeStyle Libre system in 38 countries, including Canada, France, Germany, Japan, the United Kingdom, and the United States.
The FreeStyle Libre 3 is approved for use in the European Union.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has cleared the FreeStyle Libre 2 iOS application for use with compatible iPhones.
The new app works with the FreeStyle Libre 2 with optional glucose alarms, which was approved in the United States in June 2020 for people with diabetes aged 4 years and older.
Until now, it was only a reader device with no app compatibility. The older FreeStyle Libre 14-day, available in the United States since July 2018, has both a reader and an app, but not optional alarms.
The new app, which will soon be available for download from the App Store, enables users to view glucose readings on their iPhones and allows for caregivers or other individuals to remotely monitor the patient’s glucose levels and receive real-time alarms via the LibreLinkUp app.
Worn for 14 days before replacement is needed, the FreeStyle Libre 2 is the longest-lasting integrated continuous glucose monitoring (iCGM) sensor currently on the market. The first iCGM, the Dexcom G6, is worn for 10 days.
The Libre 2 is available at pharmacies, typically at a lower cost than other CGM systems based on a list price comparison. The actual cost for patients varies depending on insurance coverage.
Abbott has secured partial or full reimbursement for the FreeStyle Libre system in 38 countries, including Canada, France, Germany, Japan, the United Kingdom, and the United States.
The FreeStyle Libre 3 is approved for use in the European Union.
A version of this article first appeared on Medscape.com.
IBD risk rises with higher ultraprocessed food intake
Individuals who consumed more ultraprocessed foods had a significantly increased risk of developing inflammatory bowel disease (IBD) than those who consumed less, according to data from more than 100,000 adults.
“Diet alters the microbiome and modifies the intestinal immune response and so could play a role in the pathogenesis of IBD,” Neeraj Narula, MD, of McMaster University, Hamilton, Ont., and colleagues wrote. Although previous studies have investigated the impact of dietary risk factors on IBD, an association with ultraprocessed foods (defined as foods containing additives and preservatives) in particular has not been examined, they wrote.
In a study published in BMJ, the researchers examined data from 116,087 adults aged 35-70 years from 21 countries between 2003 and 2016 who were part of the large Prospective Urban Rural Epidemiology (PURE) Cohort. Participants completed baseline food frequency questionnaires and were followed at least every 3 years; the median follow-up time was 9.7 years. The primary outcome was the development of Crohn’s disease or ulcerative colitis. In this study, ultraprocessed food included all packaged and formulated foods and beverages that contained food additives, artificial flavors or colors, or other chemical ingredients.
The categories of ultraprocessed foods included processed meat, cold breakfast cereal, various sauces, soft drinks, and fruit drinks, and refined sweetened foods such as candy, chocolate, jam, jelly, and brownies.
Overall, 467 participants developed IBD, including 90 with Crohn’s disease and 377 with ulcerative colitis.
After controlling for confounding factors, the investigators found that increased consumption of ultraprocessed foods was significantly associated with an increased risk of incident IBD. Compared with individuals who consumed less than 1 serving per day of ultraprocessed foods, the hazard ratio was 1.82 for those who consumed 5 or more servings and 1.67 for those who consumed 1-4 servings daily (P = .006).
“The pattern of increased ultraprocessed food intake and higher risk of IBD persisted within each of the regions examined, and effect estimates were generally similar, with overlapping confidence intervals and no significant heterogeneity,” the researchers noted.
The risk of IBD increased among individuals who consumed 1 serving per week or more of processed meat, compared with those who consumed less than 1 serving per week, and the risk increased with the amount consumed (HR, 2.07 for 1 or more servings per day). Similarly, IBD risk was higher among individuals who consumed 100 g/day or more of refined sweetened foods compared with no intake of these foods (HR, 2.58).
Individuals who consumed at least one serving of fried foods per day had the highest risk of IBD (HR, 3.02), the researchers noted. The reason for the association is uncertain, but may occur not only because many fried foods are also processed but also because the action of frying food and the processing of oil, as well as type and quality of oil, might modify the nutrients.
In the subgroup analysis, higher consumption of salty snacks and soft drinks also was associated with higher risk for IBD. However, the researchers found no association between increased risk of IBD and consumption of white meat, unprocessed red meat, dairy, starchy foods, and fruit/vegetables/legumes.
The study findings were limited by several factors including the relatively small number of individuals with Crohn’s disease, potential lack of generalizability to those who develop IBD in childhood or young adulthood, and possible confounding from unmeasured variables. The study also did not account for dietary changes over time, the investigators reported. However, the longitudinal design allowed them “to focus on people with incident IBD and to use medical record review and central adjudication to validate a sample of the diagnoses.”
The results suggest that the way food is processed or ultraprocessed, rather than the food itself, may be what confers the risk for IBD, given the lack of association between IBD and other food categories such as unprocessed red meat and dairy, the researchers concluded.
Next steps: Pin down driving factors
“There is significant interest in the apparent increase in the incidence and prevalence of IBD, particularly in previously low incidence areas,” Edward L. Barnes, MD, MPH, of the University of North Carolina at Chapel Hill said in an interview.
“Many research groups and clinicians suspect that environmental exposures, including dietary exposures, may play a critical role in these trends,” said Dr. Barnes. “This study utilized a large, multinational prospective cohort design to assess the influence of diet on the risk of developing IBD,” which is particularly important considering the potential for processed foods and food additives to impact the gastrointestinal tract.
“The strong associations demonstrated by the authors were impressive, particularly given that the authors performed multiple subanalyses, including evaluations by participant age and evaluations of particular food groups/types [e.g., processed meat, soft drinks, and refined sweet foods],” he noted. Dr. Barnes also found the lack of association with intake of white meat and unprocessed red meat interesting. “In my opinion, these subanalyses strengthen the overall associations demonstrated by the authors given their prospective study design and their attention to evaluating all potential associations that may be driving the relationships present in this cohort.
“At this point, the take-home message for clinicians treating patients with Crohn’s disease and ulcerative colitis should be that this association exists,” said Dr. Barnes. “One question that remains is whether the same risk factors that are present for developing disease also influence the disease course, given that the primary outcome of this study was the development of IBD. Given that much of our data with regard to the interplay between diet and IBD are still emerging, physicians treating patients with IBD can make patients aware of these associations and the potential benefit of limiting ultraprocessed foods in their diet.”
For these important results to become actionable, “further research is likely necessary to identify the factors that are driving this association,” Dr. Barnes explained. “This would likely build on prior animal models that have demonstrated an association between food additives such as emulsifiers and changes in the gastrointestinal tract that could ultimately lead to increased inflammation and the development of IBD.” Such information about specific drivers “would then allow clinicians to determine which population would benefit most from dietary changes/recommendations.”
The overall PURE study was supported by the Population Health Research Institute, Hamilton Health Sciences Research Institute, Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario, CIHR’s Strategy for Patient Oriented Research through the Ontario SPOR Support Unit, and the Ontario Ministry of Health and Long-term Care. PURE also was supported in part by unrestricted grants from several pharmaceutical companies, notably AstraZeneca, Sanofi-Aventis, Boehringer Ingelheim, Servier, and GlaxoSmithKline. The researchers had no relevant financial conflicts to disclose. Dr. Barnes disclosed serving as a consultant for AbbVie, Gilead, Pfizer, Takeda, and Target RWE.
Individuals who consumed more ultraprocessed foods had a significantly increased risk of developing inflammatory bowel disease (IBD) than those who consumed less, according to data from more than 100,000 adults.
“Diet alters the microbiome and modifies the intestinal immune response and so could play a role in the pathogenesis of IBD,” Neeraj Narula, MD, of McMaster University, Hamilton, Ont., and colleagues wrote. Although previous studies have investigated the impact of dietary risk factors on IBD, an association with ultraprocessed foods (defined as foods containing additives and preservatives) in particular has not been examined, they wrote.
In a study published in BMJ, the researchers examined data from 116,087 adults aged 35-70 years from 21 countries between 2003 and 2016 who were part of the large Prospective Urban Rural Epidemiology (PURE) Cohort. Participants completed baseline food frequency questionnaires and were followed at least every 3 years; the median follow-up time was 9.7 years. The primary outcome was the development of Crohn’s disease or ulcerative colitis. In this study, ultraprocessed food included all packaged and formulated foods and beverages that contained food additives, artificial flavors or colors, or other chemical ingredients.
The categories of ultraprocessed foods included processed meat, cold breakfast cereal, various sauces, soft drinks, and fruit drinks, and refined sweetened foods such as candy, chocolate, jam, jelly, and brownies.
Overall, 467 participants developed IBD, including 90 with Crohn’s disease and 377 with ulcerative colitis.
After controlling for confounding factors, the investigators found that increased consumption of ultraprocessed foods was significantly associated with an increased risk of incident IBD. Compared with individuals who consumed less than 1 serving per day of ultraprocessed foods, the hazard ratio was 1.82 for those who consumed 5 or more servings and 1.67 for those who consumed 1-4 servings daily (P = .006).
“The pattern of increased ultraprocessed food intake and higher risk of IBD persisted within each of the regions examined, and effect estimates were generally similar, with overlapping confidence intervals and no significant heterogeneity,” the researchers noted.
The risk of IBD increased among individuals who consumed 1 serving per week or more of processed meat, compared with those who consumed less than 1 serving per week, and the risk increased with the amount consumed (HR, 2.07 for 1 or more servings per day). Similarly, IBD risk was higher among individuals who consumed 100 g/day or more of refined sweetened foods compared with no intake of these foods (HR, 2.58).
Individuals who consumed at least one serving of fried foods per day had the highest risk of IBD (HR, 3.02), the researchers noted. The reason for the association is uncertain, but may occur not only because many fried foods are also processed but also because the action of frying food and the processing of oil, as well as type and quality of oil, might modify the nutrients.
In the subgroup analysis, higher consumption of salty snacks and soft drinks also was associated with higher risk for IBD. However, the researchers found no association between increased risk of IBD and consumption of white meat, unprocessed red meat, dairy, starchy foods, and fruit/vegetables/legumes.
The study findings were limited by several factors including the relatively small number of individuals with Crohn’s disease, potential lack of generalizability to those who develop IBD in childhood or young adulthood, and possible confounding from unmeasured variables. The study also did not account for dietary changes over time, the investigators reported. However, the longitudinal design allowed them “to focus on people with incident IBD and to use medical record review and central adjudication to validate a sample of the diagnoses.”
The results suggest that the way food is processed or ultraprocessed, rather than the food itself, may be what confers the risk for IBD, given the lack of association between IBD and other food categories such as unprocessed red meat and dairy, the researchers concluded.
Next steps: Pin down driving factors
“There is significant interest in the apparent increase in the incidence and prevalence of IBD, particularly in previously low incidence areas,” Edward L. Barnes, MD, MPH, of the University of North Carolina at Chapel Hill said in an interview.
“Many research groups and clinicians suspect that environmental exposures, including dietary exposures, may play a critical role in these trends,” said Dr. Barnes. “This study utilized a large, multinational prospective cohort design to assess the influence of diet on the risk of developing IBD,” which is particularly important considering the potential for processed foods and food additives to impact the gastrointestinal tract.
“The strong associations demonstrated by the authors were impressive, particularly given that the authors performed multiple subanalyses, including evaluations by participant age and evaluations of particular food groups/types [e.g., processed meat, soft drinks, and refined sweet foods],” he noted. Dr. Barnes also found the lack of association with intake of white meat and unprocessed red meat interesting. “In my opinion, these subanalyses strengthen the overall associations demonstrated by the authors given their prospective study design and their attention to evaluating all potential associations that may be driving the relationships present in this cohort.
“At this point, the take-home message for clinicians treating patients with Crohn’s disease and ulcerative colitis should be that this association exists,” said Dr. Barnes. “One question that remains is whether the same risk factors that are present for developing disease also influence the disease course, given that the primary outcome of this study was the development of IBD. Given that much of our data with regard to the interplay between diet and IBD are still emerging, physicians treating patients with IBD can make patients aware of these associations and the potential benefit of limiting ultraprocessed foods in their diet.”
For these important results to become actionable, “further research is likely necessary to identify the factors that are driving this association,” Dr. Barnes explained. “This would likely build on prior animal models that have demonstrated an association between food additives such as emulsifiers and changes in the gastrointestinal tract that could ultimately lead to increased inflammation and the development of IBD.” Such information about specific drivers “would then allow clinicians to determine which population would benefit most from dietary changes/recommendations.”
The overall PURE study was supported by the Population Health Research Institute, Hamilton Health Sciences Research Institute, Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario, CIHR’s Strategy for Patient Oriented Research through the Ontario SPOR Support Unit, and the Ontario Ministry of Health and Long-term Care. PURE also was supported in part by unrestricted grants from several pharmaceutical companies, notably AstraZeneca, Sanofi-Aventis, Boehringer Ingelheim, Servier, and GlaxoSmithKline. The researchers had no relevant financial conflicts to disclose. Dr. Barnes disclosed serving as a consultant for AbbVie, Gilead, Pfizer, Takeda, and Target RWE.
Individuals who consumed more ultraprocessed foods had a significantly increased risk of developing inflammatory bowel disease (IBD) than those who consumed less, according to data from more than 100,000 adults.
“Diet alters the microbiome and modifies the intestinal immune response and so could play a role in the pathogenesis of IBD,” Neeraj Narula, MD, of McMaster University, Hamilton, Ont., and colleagues wrote. Although previous studies have investigated the impact of dietary risk factors on IBD, an association with ultraprocessed foods (defined as foods containing additives and preservatives) in particular has not been examined, they wrote.
In a study published in BMJ, the researchers examined data from 116,087 adults aged 35-70 years from 21 countries between 2003 and 2016 who were part of the large Prospective Urban Rural Epidemiology (PURE) Cohort. Participants completed baseline food frequency questionnaires and were followed at least every 3 years; the median follow-up time was 9.7 years. The primary outcome was the development of Crohn’s disease or ulcerative colitis. In this study, ultraprocessed food included all packaged and formulated foods and beverages that contained food additives, artificial flavors or colors, or other chemical ingredients.
The categories of ultraprocessed foods included processed meat, cold breakfast cereal, various sauces, soft drinks, and fruit drinks, and refined sweetened foods such as candy, chocolate, jam, jelly, and brownies.
Overall, 467 participants developed IBD, including 90 with Crohn’s disease and 377 with ulcerative colitis.
After controlling for confounding factors, the investigators found that increased consumption of ultraprocessed foods was significantly associated with an increased risk of incident IBD. Compared with individuals who consumed less than 1 serving per day of ultraprocessed foods, the hazard ratio was 1.82 for those who consumed 5 or more servings and 1.67 for those who consumed 1-4 servings daily (P = .006).
“The pattern of increased ultraprocessed food intake and higher risk of IBD persisted within each of the regions examined, and effect estimates were generally similar, with overlapping confidence intervals and no significant heterogeneity,” the researchers noted.
The risk of IBD increased among individuals who consumed 1 serving per week or more of processed meat, compared with those who consumed less than 1 serving per week, and the risk increased with the amount consumed (HR, 2.07 for 1 or more servings per day). Similarly, IBD risk was higher among individuals who consumed 100 g/day or more of refined sweetened foods compared with no intake of these foods (HR, 2.58).
Individuals who consumed at least one serving of fried foods per day had the highest risk of IBD (HR, 3.02), the researchers noted. The reason for the association is uncertain, but may occur not only because many fried foods are also processed but also because the action of frying food and the processing of oil, as well as type and quality of oil, might modify the nutrients.
In the subgroup analysis, higher consumption of salty snacks and soft drinks also was associated with higher risk for IBD. However, the researchers found no association between increased risk of IBD and consumption of white meat, unprocessed red meat, dairy, starchy foods, and fruit/vegetables/legumes.
The study findings were limited by several factors including the relatively small number of individuals with Crohn’s disease, potential lack of generalizability to those who develop IBD in childhood or young adulthood, and possible confounding from unmeasured variables. The study also did not account for dietary changes over time, the investigators reported. However, the longitudinal design allowed them “to focus on people with incident IBD and to use medical record review and central adjudication to validate a sample of the diagnoses.”
The results suggest that the way food is processed or ultraprocessed, rather than the food itself, may be what confers the risk for IBD, given the lack of association between IBD and other food categories such as unprocessed red meat and dairy, the researchers concluded.
Next steps: Pin down driving factors
“There is significant interest in the apparent increase in the incidence and prevalence of IBD, particularly in previously low incidence areas,” Edward L. Barnes, MD, MPH, of the University of North Carolina at Chapel Hill said in an interview.
“Many research groups and clinicians suspect that environmental exposures, including dietary exposures, may play a critical role in these trends,” said Dr. Barnes. “This study utilized a large, multinational prospective cohort design to assess the influence of diet on the risk of developing IBD,” which is particularly important considering the potential for processed foods and food additives to impact the gastrointestinal tract.
“The strong associations demonstrated by the authors were impressive, particularly given that the authors performed multiple subanalyses, including evaluations by participant age and evaluations of particular food groups/types [e.g., processed meat, soft drinks, and refined sweet foods],” he noted. Dr. Barnes also found the lack of association with intake of white meat and unprocessed red meat interesting. “In my opinion, these subanalyses strengthen the overall associations demonstrated by the authors given their prospective study design and their attention to evaluating all potential associations that may be driving the relationships present in this cohort.
“At this point, the take-home message for clinicians treating patients with Crohn’s disease and ulcerative colitis should be that this association exists,” said Dr. Barnes. “One question that remains is whether the same risk factors that are present for developing disease also influence the disease course, given that the primary outcome of this study was the development of IBD. Given that much of our data with regard to the interplay between diet and IBD are still emerging, physicians treating patients with IBD can make patients aware of these associations and the potential benefit of limiting ultraprocessed foods in their diet.”
For these important results to become actionable, “further research is likely necessary to identify the factors that are driving this association,” Dr. Barnes explained. “This would likely build on prior animal models that have demonstrated an association between food additives such as emulsifiers and changes in the gastrointestinal tract that could ultimately lead to increased inflammation and the development of IBD.” Such information about specific drivers “would then allow clinicians to determine which population would benefit most from dietary changes/recommendations.”
The overall PURE study was supported by the Population Health Research Institute, Hamilton Health Sciences Research Institute, Canadian Institutes of Health Research, Heart and Stroke Foundation of Ontario, CIHR’s Strategy for Patient Oriented Research through the Ontario SPOR Support Unit, and the Ontario Ministry of Health and Long-term Care. PURE also was supported in part by unrestricted grants from several pharmaceutical companies, notably AstraZeneca, Sanofi-Aventis, Boehringer Ingelheim, Servier, and GlaxoSmithKline. The researchers had no relevant financial conflicts to disclose. Dr. Barnes disclosed serving as a consultant for AbbVie, Gilead, Pfizer, Takeda, and Target RWE.
FROM THE BMJ
Pruritic welts
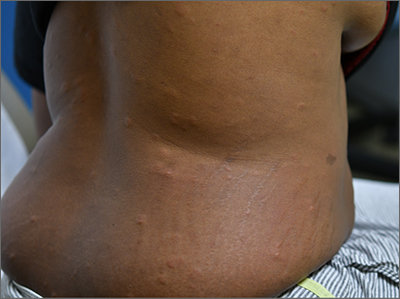
The patient’s history and the classic morphology of raised plaques was consistent with chronic spontaneous urticaria. Erythema may be subtle or not visible in patients with skin of color.
Individual urticarial lesions last less than 24 hours with itching and burning. Angioedema also occurs, and manifests as a deeper edema with itching, burning, and pain. Symptoms typically last for 2 to 3 days and involve the eyelids, lips, and tongue.
Chronic urticaria (CU) is defined as episodes of spontaneous wheals or angioedema lasting for more than 6 weeks and is subdivided into chronic spontaneous urticaria or inducible urticaria (if there is a known cause). Triggers include infection, emotional stressors, or medications (eg, angiotensin-converting enzyme inhibitors, nonsteroidal anti-inflammatory drugs). Autoimmunity is seen in one-third of CU patients.1
Only a limited routine-diagnostic work-up is necessary for CU and it may include a complete blood count with differential and erythrocyte sedimentation rate and C-reactive protein testing. Further diagnostic testing is based on history and may include functional autoantibodies, thyroid stimulating hormone, and thyroid autoantibodies.
The initial step in treatment involves identification and elimination of triggers. If this is not possible, first-line therapy includes second-generation antihistamines. If symptoms persist beyond 2 weeks, the dose of the antihistamine is increased up to 4-fold. Patients should be counseled that the lowest necessary dose needs to be taken habitually to prevent urticaria. If symptoms persist, omalizumab, a recombinant humanized IgG monoclonal antibody against serum IgE, cyclosporine, or montelukast, may be added. Leukotriene-receptor antagonists such as montelukast were previously second-line therapy, but they have had mixed results in randomized controlled trials. With a much lower cost than omalizumab, leukotriene-receptor antagonists are a reasonable alternative for patients who are refractory to antihistamines.1
This patient was given high-dose nonsedating antihistamines; she experienced sedation and inadequate relief. A prior authorization was successfully obtained, and she was prescribed omalizumab.
Image courtesy of Kriti Mishra, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque. Text courtesy of Kriti Mishra, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Antia C, Baquerizo K, Korman A, et al. Urticaria: a comprehensive review: treatment of chronic urticaria, special populations, and disease outcomes. J Am Acad Dermatol. 2018;79:617-633. doi: 10.1016/j.jaad.2018.01.023

The patient’s history and the classic morphology of raised plaques was consistent with chronic spontaneous urticaria. Erythema may be subtle or not visible in patients with skin of color.
Individual urticarial lesions last less than 24 hours with itching and burning. Angioedema also occurs, and manifests as a deeper edema with itching, burning, and pain. Symptoms typically last for 2 to 3 days and involve the eyelids, lips, and tongue.
Chronic urticaria (CU) is defined as episodes of spontaneous wheals or angioedema lasting for more than 6 weeks and is subdivided into chronic spontaneous urticaria or inducible urticaria (if there is a known cause). Triggers include infection, emotional stressors, or medications (eg, angiotensin-converting enzyme inhibitors, nonsteroidal anti-inflammatory drugs). Autoimmunity is seen in one-third of CU patients.1
Only a limited routine-diagnostic work-up is necessary for CU and it may include a complete blood count with differential and erythrocyte sedimentation rate and C-reactive protein testing. Further diagnostic testing is based on history and may include functional autoantibodies, thyroid stimulating hormone, and thyroid autoantibodies.
The initial step in treatment involves identification and elimination of triggers. If this is not possible, first-line therapy includes second-generation antihistamines. If symptoms persist beyond 2 weeks, the dose of the antihistamine is increased up to 4-fold. Patients should be counseled that the lowest necessary dose needs to be taken habitually to prevent urticaria. If symptoms persist, omalizumab, a recombinant humanized IgG monoclonal antibody against serum IgE, cyclosporine, or montelukast, may be added. Leukotriene-receptor antagonists such as montelukast were previously second-line therapy, but they have had mixed results in randomized controlled trials. With a much lower cost than omalizumab, leukotriene-receptor antagonists are a reasonable alternative for patients who are refractory to antihistamines.1
This patient was given high-dose nonsedating antihistamines; she experienced sedation and inadequate relief. A prior authorization was successfully obtained, and she was prescribed omalizumab.
Image courtesy of Kriti Mishra, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque. Text courtesy of Kriti Mishra, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The patient’s history and the classic morphology of raised plaques was consistent with chronic spontaneous urticaria. Erythema may be subtle or not visible in patients with skin of color.
Individual urticarial lesions last less than 24 hours with itching and burning. Angioedema also occurs, and manifests as a deeper edema with itching, burning, and pain. Symptoms typically last for 2 to 3 days and involve the eyelids, lips, and tongue.
Chronic urticaria (CU) is defined as episodes of spontaneous wheals or angioedema lasting for more than 6 weeks and is subdivided into chronic spontaneous urticaria or inducible urticaria (if there is a known cause). Triggers include infection, emotional stressors, or medications (eg, angiotensin-converting enzyme inhibitors, nonsteroidal anti-inflammatory drugs). Autoimmunity is seen in one-third of CU patients.1
Only a limited routine-diagnostic work-up is necessary for CU and it may include a complete blood count with differential and erythrocyte sedimentation rate and C-reactive protein testing. Further diagnostic testing is based on history and may include functional autoantibodies, thyroid stimulating hormone, and thyroid autoantibodies.
The initial step in treatment involves identification and elimination of triggers. If this is not possible, first-line therapy includes second-generation antihistamines. If symptoms persist beyond 2 weeks, the dose of the antihistamine is increased up to 4-fold. Patients should be counseled that the lowest necessary dose needs to be taken habitually to prevent urticaria. If symptoms persist, omalizumab, a recombinant humanized IgG monoclonal antibody against serum IgE, cyclosporine, or montelukast, may be added. Leukotriene-receptor antagonists such as montelukast were previously second-line therapy, but they have had mixed results in randomized controlled trials. With a much lower cost than omalizumab, leukotriene-receptor antagonists are a reasonable alternative for patients who are refractory to antihistamines.1
This patient was given high-dose nonsedating antihistamines; she experienced sedation and inadequate relief. A prior authorization was successfully obtained, and she was prescribed omalizumab.
Image courtesy of Kriti Mishra, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque. Text courtesy of Kriti Mishra, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Antia C, Baquerizo K, Korman A, et al. Urticaria: a comprehensive review: treatment of chronic urticaria, special populations, and disease outcomes. J Am Acad Dermatol. 2018;79:617-633. doi: 10.1016/j.jaad.2018.01.023
1. Antia C, Baquerizo K, Korman A, et al. Urticaria: a comprehensive review: treatment of chronic urticaria, special populations, and disease outcomes. J Am Acad Dermatol. 2018;79:617-633. doi: 10.1016/j.jaad.2018.01.023
Certain gut bacteria tied to lower risk of diabetes
Having more diverse gut bacteria (greater microbiome richness) and specifically a greater abundance of 12 types of butyrate-producing bacteria were both associated with less insulin resistance and less type 2 diabetes, in a population-based observational study from the Netherlands.
Several studies have reported that there is less microbiome diversity in type 2 diabetes, Zhangling Chen, MD, PhD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues note.
Their study also identified a dozen types of bacteria that ferment dietary fiber (undigested carbohydrates) in the gut to produce butyrate, a short-chain fatty acid, which may play a role in protection against type 2 diabetes.
“The current study is the first, to our knowledge, to comprehensively investigate the associations between gut microbiome composition [and] type 2 diabetes in a large population-based sample … which we adjusted for a series of key confounders,” the researchers write.
“These findings suggest that higher gut microbial diversity, along with specifically more butyrate-producing bacteria, may play a role in the development of type 2 diabetes, which may help guide future prevention and treatment strategies,” they conclude in their study published online July 29 in JAMA Network Open.
Confirmation of previous work, plus some new findings
The study confirms what many smaller ones have repeatedly shown – that low gut microbiome diversity is associated with increased risks of obesity and type 2 diabetes, Nanette I. Steinle, MD, RDN, who was not involved in the research, said in an interview.
A diet rich in fiber and prebiotics promotes gut biome diversity, added Dr. Steinle, chief of the endocrinology and diabetes section at Maryland Veterans Affairs Medical Center in Baltimore.
The findings add to other research, she noted, such as a prospective trial in which a high-fiber diet induced changes in the gut microbe that were linked to better glycemic regulation (Science. 2018;359:1151-6) and a study of a promising probiotic formula to treat diabetes.
“An important next step,” according to Dr. Steinle, “is to provide interventions like healthy diet or specific fiber types to see what can be done to produce lasting shifts in the gut microbiome and if these shifts result in improved metabolic health.”
Natalia Shulzhenko, MD, PhD, said: “Some of associations of taxa [bacteria groupings] with type 2 diabetes reported by this study are new.”
Dr. Shulzhenko and colleagues recently published a review of the role of gut microbiota in type 2 diabetes pathophysiology that summarized evidence from 42 human studies as well as preclinical studies and clinical trials of probiotic treatments (EBioMedicine. 2020;51:102590).
“Besides adding new microbes to the list of potential pathobionts [organisms that can cause harm] and beneficial microbes for type 2 diabetes,” the findings by Dr. Chen and colleagues “support a notion that different members of the gut microbial community may have similar effects on type 2 diabetes in different individuals,” commonly known as “functional redundancy,” Dr. Shulzhenko, associate professor, Carlson College of Veterinary Medicine, Oregon State University, Corvallis, pointed out in an email.
Also “in line with previous studies,” the study shows that butyrate-producing bacteria are associated with type 2 diabetes.
She speculated that “these results will probably contribute to the body of knowledge that is needed to develop microbiota-based therapy and diagnostics.”
Which gut bacteria are linked with diabetes?
It is unclear which gut bacteria are associated with the development of type 2 diabetes, Dr. Chen and colleagues write.
To investigate this, they identified 1,418 participants from the Rotterdam Study and 748 participants from the LifeLines-DEEP study enrolled from January 2018 to December 2020. Of these participants, 193 had type 2 diabetes.
The participants provided stool samples that were used to measure gut microbiome composition using the 16S ribosomal RNA method. They also had blood tests to measure glucose and insulin, and researchers collected other demographic and medical data.
Participants in the Rotterdam study were older than in the LifeLines Deep study (mean age, 62 vs. 45 years). Both cohorts included slightly more men than women (58%).
Dr. Chen and colleagues identified 126 (bacteria) genera in the gut microbiome in the Rotterdam study and 184 genera in the LifeLines Deep study.
After correcting for age, sex, smoking, education, physical activity, alcohol intake, daily calories, body mass index, and use of lipid-lowering medication or proton pump inhibitors, higher microbiome diversity was associated with lower insulin resistance and a lower prevalence of type 2 diabetes.
A higher abundance of each of seven types of butyrate-producing bacteria – Christensenellaceae, Christensenellaceae R7 group, Marvinbryantia, Ruminococcaceae UCG-005, Ruminococcaceae UCG-008, Ruminococcaceae UCG-010, and Ruminococcaceae NK4A214 group – was associated with lower insulin resistance, after adjusting for confounders such as diet and medications (all P < .001).
And a higher abundance of each of five other types of butyrate-producing bacteria – Clostridiaceae 1, Peptostreptococcaceae, Clostridium sensu stricto 1, Intestinibacter, and Romboutsia – was associated with less type 2 diabetes (all P < .001).
Study limitations include that gut microbiome composition was determined from stool (fecal) samples, whereas the actual composition varies in different locations along the intestine, and the study also lacked information about butyrate concentrations in stool or blood, the researchers note.
They call for “future research [to] validate the hypothesis of butyrate-producing bacteria affecting glucose metabolism and diabetes risk via production of butyrate.”
The authors and Dr. Shulzhenko have reported no relevant financial relationships. Dr. Steinle has reported receiving funding from the National Institutes of Health and conducting a study funded by Kowa through the VA.
A version of this article first appeared on Medscape.com.
Having more diverse gut bacteria (greater microbiome richness) and specifically a greater abundance of 12 types of butyrate-producing bacteria were both associated with less insulin resistance and less type 2 diabetes, in a population-based observational study from the Netherlands.
Several studies have reported that there is less microbiome diversity in type 2 diabetes, Zhangling Chen, MD, PhD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues note.
Their study also identified a dozen types of bacteria that ferment dietary fiber (undigested carbohydrates) in the gut to produce butyrate, a short-chain fatty acid, which may play a role in protection against type 2 diabetes.
“The current study is the first, to our knowledge, to comprehensively investigate the associations between gut microbiome composition [and] type 2 diabetes in a large population-based sample … which we adjusted for a series of key confounders,” the researchers write.
“These findings suggest that higher gut microbial diversity, along with specifically more butyrate-producing bacteria, may play a role in the development of type 2 diabetes, which may help guide future prevention and treatment strategies,” they conclude in their study published online July 29 in JAMA Network Open.
Confirmation of previous work, plus some new findings
The study confirms what many smaller ones have repeatedly shown – that low gut microbiome diversity is associated with increased risks of obesity and type 2 diabetes, Nanette I. Steinle, MD, RDN, who was not involved in the research, said in an interview.
A diet rich in fiber and prebiotics promotes gut biome diversity, added Dr. Steinle, chief of the endocrinology and diabetes section at Maryland Veterans Affairs Medical Center in Baltimore.
The findings add to other research, she noted, such as a prospective trial in which a high-fiber diet induced changes in the gut microbe that were linked to better glycemic regulation (Science. 2018;359:1151-6) and a study of a promising probiotic formula to treat diabetes.
“An important next step,” according to Dr. Steinle, “is to provide interventions like healthy diet or specific fiber types to see what can be done to produce lasting shifts in the gut microbiome and if these shifts result in improved metabolic health.”
Natalia Shulzhenko, MD, PhD, said: “Some of associations of taxa [bacteria groupings] with type 2 diabetes reported by this study are new.”
Dr. Shulzhenko and colleagues recently published a review of the role of gut microbiota in type 2 diabetes pathophysiology that summarized evidence from 42 human studies as well as preclinical studies and clinical trials of probiotic treatments (EBioMedicine. 2020;51:102590).
“Besides adding new microbes to the list of potential pathobionts [organisms that can cause harm] and beneficial microbes for type 2 diabetes,” the findings by Dr. Chen and colleagues “support a notion that different members of the gut microbial community may have similar effects on type 2 diabetes in different individuals,” commonly known as “functional redundancy,” Dr. Shulzhenko, associate professor, Carlson College of Veterinary Medicine, Oregon State University, Corvallis, pointed out in an email.
Also “in line with previous studies,” the study shows that butyrate-producing bacteria are associated with type 2 diabetes.
She speculated that “these results will probably contribute to the body of knowledge that is needed to develop microbiota-based therapy and diagnostics.”
Which gut bacteria are linked with diabetes?
It is unclear which gut bacteria are associated with the development of type 2 diabetes, Dr. Chen and colleagues write.
To investigate this, they identified 1,418 participants from the Rotterdam Study and 748 participants from the LifeLines-DEEP study enrolled from January 2018 to December 2020. Of these participants, 193 had type 2 diabetes.
The participants provided stool samples that were used to measure gut microbiome composition using the 16S ribosomal RNA method. They also had blood tests to measure glucose and insulin, and researchers collected other demographic and medical data.
Participants in the Rotterdam study were older than in the LifeLines Deep study (mean age, 62 vs. 45 years). Both cohorts included slightly more men than women (58%).
Dr. Chen and colleagues identified 126 (bacteria) genera in the gut microbiome in the Rotterdam study and 184 genera in the LifeLines Deep study.
After correcting for age, sex, smoking, education, physical activity, alcohol intake, daily calories, body mass index, and use of lipid-lowering medication or proton pump inhibitors, higher microbiome diversity was associated with lower insulin resistance and a lower prevalence of type 2 diabetes.
A higher abundance of each of seven types of butyrate-producing bacteria – Christensenellaceae, Christensenellaceae R7 group, Marvinbryantia, Ruminococcaceae UCG-005, Ruminococcaceae UCG-008, Ruminococcaceae UCG-010, and Ruminococcaceae NK4A214 group – was associated with lower insulin resistance, after adjusting for confounders such as diet and medications (all P < .001).
And a higher abundance of each of five other types of butyrate-producing bacteria – Clostridiaceae 1, Peptostreptococcaceae, Clostridium sensu stricto 1, Intestinibacter, and Romboutsia – was associated with less type 2 diabetes (all P < .001).
Study limitations include that gut microbiome composition was determined from stool (fecal) samples, whereas the actual composition varies in different locations along the intestine, and the study also lacked information about butyrate concentrations in stool or blood, the researchers note.
They call for “future research [to] validate the hypothesis of butyrate-producing bacteria affecting glucose metabolism and diabetes risk via production of butyrate.”
The authors and Dr. Shulzhenko have reported no relevant financial relationships. Dr. Steinle has reported receiving funding from the National Institutes of Health and conducting a study funded by Kowa through the VA.
A version of this article first appeared on Medscape.com.
Having more diverse gut bacteria (greater microbiome richness) and specifically a greater abundance of 12 types of butyrate-producing bacteria were both associated with less insulin resistance and less type 2 diabetes, in a population-based observational study from the Netherlands.
Several studies have reported that there is less microbiome diversity in type 2 diabetes, Zhangling Chen, MD, PhD, of Erasmus Medical Center, Rotterdam, the Netherlands, and colleagues note.
Their study also identified a dozen types of bacteria that ferment dietary fiber (undigested carbohydrates) in the gut to produce butyrate, a short-chain fatty acid, which may play a role in protection against type 2 diabetes.
“The current study is the first, to our knowledge, to comprehensively investigate the associations between gut microbiome composition [and] type 2 diabetes in a large population-based sample … which we adjusted for a series of key confounders,” the researchers write.
“These findings suggest that higher gut microbial diversity, along with specifically more butyrate-producing bacteria, may play a role in the development of type 2 diabetes, which may help guide future prevention and treatment strategies,” they conclude in their study published online July 29 in JAMA Network Open.
Confirmation of previous work, plus some new findings
The study confirms what many smaller ones have repeatedly shown – that low gut microbiome diversity is associated with increased risks of obesity and type 2 diabetes, Nanette I. Steinle, MD, RDN, who was not involved in the research, said in an interview.
A diet rich in fiber and prebiotics promotes gut biome diversity, added Dr. Steinle, chief of the endocrinology and diabetes section at Maryland Veterans Affairs Medical Center in Baltimore.
The findings add to other research, she noted, such as a prospective trial in which a high-fiber diet induced changes in the gut microbe that were linked to better glycemic regulation (Science. 2018;359:1151-6) and a study of a promising probiotic formula to treat diabetes.
“An important next step,” according to Dr. Steinle, “is to provide interventions like healthy diet or specific fiber types to see what can be done to produce lasting shifts in the gut microbiome and if these shifts result in improved metabolic health.”
Natalia Shulzhenko, MD, PhD, said: “Some of associations of taxa [bacteria groupings] with type 2 diabetes reported by this study are new.”
Dr. Shulzhenko and colleagues recently published a review of the role of gut microbiota in type 2 diabetes pathophysiology that summarized evidence from 42 human studies as well as preclinical studies and clinical trials of probiotic treatments (EBioMedicine. 2020;51:102590).
“Besides adding new microbes to the list of potential pathobionts [organisms that can cause harm] and beneficial microbes for type 2 diabetes,” the findings by Dr. Chen and colleagues “support a notion that different members of the gut microbial community may have similar effects on type 2 diabetes in different individuals,” commonly known as “functional redundancy,” Dr. Shulzhenko, associate professor, Carlson College of Veterinary Medicine, Oregon State University, Corvallis, pointed out in an email.
Also “in line with previous studies,” the study shows that butyrate-producing bacteria are associated with type 2 diabetes.
She speculated that “these results will probably contribute to the body of knowledge that is needed to develop microbiota-based therapy and diagnostics.”
Which gut bacteria are linked with diabetes?
It is unclear which gut bacteria are associated with the development of type 2 diabetes, Dr. Chen and colleagues write.
To investigate this, they identified 1,418 participants from the Rotterdam Study and 748 participants from the LifeLines-DEEP study enrolled from January 2018 to December 2020. Of these participants, 193 had type 2 diabetes.
The participants provided stool samples that were used to measure gut microbiome composition using the 16S ribosomal RNA method. They also had blood tests to measure glucose and insulin, and researchers collected other demographic and medical data.
Participants in the Rotterdam study were older than in the LifeLines Deep study (mean age, 62 vs. 45 years). Both cohorts included slightly more men than women (58%).
Dr. Chen and colleagues identified 126 (bacteria) genera in the gut microbiome in the Rotterdam study and 184 genera in the LifeLines Deep study.
After correcting for age, sex, smoking, education, physical activity, alcohol intake, daily calories, body mass index, and use of lipid-lowering medication or proton pump inhibitors, higher microbiome diversity was associated with lower insulin resistance and a lower prevalence of type 2 diabetes.
A higher abundance of each of seven types of butyrate-producing bacteria – Christensenellaceae, Christensenellaceae R7 group, Marvinbryantia, Ruminococcaceae UCG-005, Ruminococcaceae UCG-008, Ruminococcaceae UCG-010, and Ruminococcaceae NK4A214 group – was associated with lower insulin resistance, after adjusting for confounders such as diet and medications (all P < .001).
And a higher abundance of each of five other types of butyrate-producing bacteria – Clostridiaceae 1, Peptostreptococcaceae, Clostridium sensu stricto 1, Intestinibacter, and Romboutsia – was associated with less type 2 diabetes (all P < .001).
Study limitations include that gut microbiome composition was determined from stool (fecal) samples, whereas the actual composition varies in different locations along the intestine, and the study also lacked information about butyrate concentrations in stool or blood, the researchers note.
They call for “future research [to] validate the hypothesis of butyrate-producing bacteria affecting glucose metabolism and diabetes risk via production of butyrate.”
The authors and Dr. Shulzhenko have reported no relevant financial relationships. Dr. Steinle has reported receiving funding from the National Institutes of Health and conducting a study funded by Kowa through the VA.
A version of this article first appeared on Medscape.com.