User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Chronic pain and depression: Treatment of 2 culprits in common
Patients who have chronic pain and those with a major depressive disorder (MDD) share clinical features, including fatigue, cognitive complaints, and functional limitation. Sleep disturbance and anxiety are common with both disorders. Because pain and depression share common neurobiological pathways (see Part 1 of this article in the February 2016 issue and at CurrentPsychiatry.com) and clinical manifestations, you can use similar strategies and, often, the same agents to treat both conditions when they occur together (Table 1).
What are the medical options?
Antidepressants. Using an antidepressant to treat chronic pain is a common practice in primary care and specialty practice. Antidepressants that modulate multiple neurotransmitters appear to be more efficacious than those with a single mechanism of action.1 Convergent evidence from preclinical and clinical studies supports the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) as more effective analgesic agents, compared with the mostly noradrenergic antidepressants, which, in turn, are more effective than selective serotonin reuptake inhibitors (SSRIs).2 The mechanism of the analgesic action of antidepressants appears to rely on their inhibitory effects of norepinephrine and serotonin reuptake, thereby elevating the performance of endogenous descending pain regulatory pathways.3
Tricyclic antidepressants (TCAs), primarily amitriptyline, nortriptyline, and desipramine, have the advantage of years of clinical experience and low cost. Their side effect burden, however, is higher, especially in geriatric patients. Dose-dependent side effects include sedation, constipation, dry mouth, urinary retention, and orthostatic hypotension.
TCAs must be used with caution in patients with suicidal ideation because of the risk of a potentially lethal intentional overdose.
The key to using a TCA is to start with a low dosage, followed by slow titration. Typically, the dosages of TCAs used in clinical trials that focused on pain have been lower (25 to 100 mg/d of amitriptyline or equivalent) than the dosage typically necessary for treating depression; however, some experts have found that titrating TCAs to higher dosages with an option of monitoring serum levels may benefit some patients.4
SNRIs are considered first-line agents for both neuropathic pain and fibromyalgia. Duloxetine has been shown to be effective in both conditions5; venlafaxine also has shown efficacy in neuropathic pain.6 Milnacipran, another SNRI that blocks 5-HT, and norepinephrine equally and exerts a mild N-methyl-D-aspartate inhibition, has proven efficacy in fibromyalgia.7,8
SSRIs for alleviating central pain or neuropathic pain are supported by minimal evidence only.9 A review of the effectiveness of various antidepressants on pain in diabetic neuropathy concluded that fluoxetine was no more effective than placebo.10,11 Schreiber and Pick11 evaluated the antinociceptive properties of several SSRIs and offered the opinion that fluoxetine, fluvoxamine, and citalopram were, at best, weak antinociceptors.
Opioids. Data on the long-term benefits of opioids are limited, except for use in carefully selected patients; in any case, risk of abuse, diversion, and even death with these agents is quite high.12 Also, there is evidence that opioid-induced hyperalgesia might limit the usefulness of opioids for controlling chronic pain.13
Gabapentin and pregabalin, both anticonvulsants, act by binding to the α-2-σ subunit of voltage-gated calcium channels within the CNS.14 By reducing calcium influx at nerve terminals, the drugs diminish the release of several neurotransmitters, including glutamate, noradrenaline, and substance P. This mechanism is thought to be the basis for the analgesic, anticonvulsant, and anxiolytic effects of these drugs.15
Gabapentin and pregabalin have been shown to decrease pain intensity and improve quality of life and function in patients with neuropathic pain conditions. Pregabalin also has shown efficacy in treating central neuropathic pain and fibromyalgia.16
Added benefits of these drugs is that they have (1) a better side effect profile than TCAs and (2) fewer drug interactions when they are used as a component of combination therapy. Pregabalin has the additional advantage of less-frequent dosing, linear pharmacokinetics, and a predictable dose-response relationship.17
Addressing other comorbid psychiatric conditions
Sleep disturbance is common among patients with chronic pain. Sleep deprivation causes a hyperexcitable state that amplifies the pain response.18
When a patient presents with chronic pain, depression, and disturbed sleep, consider using a sedating antidepressant, such as a TCA. Alternatively, gabapentin or pregabalin can be added to an SNRI; anticonvulsants have been shown to improve quality of sleep.19 Cognitive-behavioral interventions targeting sleep disturbance may be a helpful adjunct in these patients.20
When anxiety is comorbid with chronic pain, antidepressants with proven efficacy in treating anxiety disorders, such as duloxetine or venlafaxine, can be used. When chronic pain coexists with a specific anxiety disorder (social anxiety disorder, obsessive-compulsive disorder, panic disorder), an SSRI might be more advantageous than an SNRI,21 especially if it is combined with a more efficacious analgesic.
Benzodiazepines should be avoided as a routine treatment for comorbid anxiety and pain, because these agents can produce sedation and cognitive interference, and carry the potential for dependence.
Fatigue. In patients who, in addition to pain and depression, complain of fatigue, an activating agent such as milnacipran or adjunct bupropion might be preferable to other agents. Modafinil has been shown to be a well-tolerated and potentially effective augmenting agent for antidepressants when fatigue and sleepiness are present as residual symptoms22; consider them as adjuncts when managing patients who have chronic pain and depression that manifests as excessive sleepiness and/or fatigue.
Cognitive complaints. We have noted that disturbances of cognition are common in patients with depression and chronic pain, and that cognitive dysfunction might improve after antidepressant treatment.
Studies suggest that SSRIs, duloxetine, and other antidepressants, such as bupropion, might exert a positive effect on learning, memory, and executive function in depressed patients.23 Beneficial effects of antidepressants may be “pseudo-specific,” however—that is, predominantly a reflection of overall improvement in mood, not on specific amelioration of the cognitive disturbance.
Vortioxetine has shown promise in improving cognitive function in adults with MDD; its cognitive benefits are largely independent of its antidepressant effect.24 The utility of vortioxetine in chronic pain patients has not been studied, but its positive impact on mood, anxiety, sleep, and cognition might make it a consideration for patients with comorbid depression—although it is uncertain at this time whether putative noradrenergic activity makes it suitable for use in chronic pain disorders.
Last, avoid TCAs in patients who have cognitive complaints. These agents have anticholinergic effects that can have an adverse impact on cognitive function.
Cautions: Drug−drug interactions, suicide risk, disrupted sleep
Avoiding drug−drug interactions is an important consideration when treating comorbid disorders. Many chronic pain patients take over-the-counter or prescribed nonsteroidal anti-inflammatory drugs for analgesia; these agents can increase the risk of gastrointestinal bleeding when they are combined with an SSRI or an SNRI.
The use of the opioid tramadol with an SNRI or a TCA is discouraged because of the risk of serotonin syndrome.
Combining a sedating antidepressant, such as a TCA, with gabapentin or pregabalin can increase the risk of CNS depression and psychomotor impairment, especially in geriatric patients. An opioid analgesic is likely to amplify these effects.
Suicidal ideation is not uncommon in patients with chronic pain and depression. To minimize the risk of suicide in patients with a chronic pain disorder, you should ensure optimal pain control by combining the most efficacious analgesic agent with psychotherapeutic interventions and optimal antidepressant treatment. Furthermore, cognitive-behavioral therapy (CBT) (see the discussion below) might not only improve pain coping skills, but also ameliorate catastrophizing, anxiety, and concomitant sleep disturbance.
Complaints of sleep disturbance and anxiety can compound the risk of suicide in a chronic pain patient. When possible, these complex patients should be treated by a multidisciplinary team that includes a pain management specialist, psychotherapist, and primary care clinician. It is important to strengthen the clinician−patient relationship to facilitate close monitoring of symptoms and to provide a trusting environment in which patients feel free to discuss thoughts of suicide or self-harm. For such patients, prescribing opiates and TCAs in small quantities is a prudent action.
When a patient struggles with suicidal thoughts, his (her) family might need to dispense these medications. Most important, if a patient is actively suicidal, consider referral to an inpatient facility or intensive outpatient program, where aggressive treatment of depressive symptoms and intensive monitoring and support can be provided.25
Usefulness of non-drug interventions
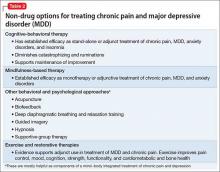
There is, of course, a diversity of non-drug treatments for MDD and for chronic pain; discussion here focuses primarily on modalities with established efficacy in both disease states (Table 2). On rare occasions, non-drug treatments can constitute a stand-alone approach; most often, they are incorporated into a multimodal treatment plan or applied as an adjunct intervention.26
Psychotherapy. The most robust evidence supports the use of CBT in addressing MDD and chronic pain—occurring individually and comorbidly.26-28 Efficacy is well established in MDD, as monotherapy and adjunct treatment, spanning acute and maintenance phases.
Furthermore, CBT also has support from randomized trials, meta-analyses, and treatment guidelines, either as monotherapy or co-therapy for both short-term relief and long-term pain reduction. Additionally, CBT has demonstrated value for relieving pain-related disability.26,28
Combination of a special form of CBT, rumination-focused CBT with ongoing pharmacological therapy over a 26-week period in a group of medication-refractory MDD patients produced a remission rate of 62%, compared with 21% in a treatment-as-usual group.29 This is of particular interest in chronic pain patients, because rumination-related phenomena of pain catastrophizing and avoidance facilitate a transition from acute to chronic pain, while augmenting pain severity and associated disability.30
Catastrophizing also has been implicated in mediating the relationship between pain and sleep disturbance. Not surprisingly, a randomized controlled study demonstrated the benefit of 8-week, Internet-delivered CBT in patients suffering from comorbid chronic pain, depression, and anxiety. Treatment significantly diminished pain catastrophizing, depression, and anxiety; maintenance of improvement was demonstrated after 1 year of follow-up.31
Other behavioral and psychological approaches. Biofeedback, mindfulness-based stress reduction, relaxation training and diaphragmatic breathing, guided imagery, hypnosis, and supportive groups might play an important role as components of an integrated mind−body approach to chronic pain,28,32,33 while also providing mood benefits.
Exercise. The role of exercise as a primary treatment of MDD continues to be controversial, but its benefits as an add-on intervention are indisputable. Exercise not only complements pharmacotherapy to produce greater reduction in depressive scores and improvement in quality of life, it might aid in reestablishing social contacts when conducted in a group setting—an effect that can be of great value in both MDD and chronic pain.34
Exercise and restorative therapies provide several benefits for chronic pain patients, including:
- improved pain control, cognition, and mood
- greater strength and endurance
- cardiovascular and metabolic benefits
- improved bone health and functionality.26,28,32,33,35
To achieve optimal benefit, an exercise program must be customized to fit the patient’s physical condition, level of fitness, and specific type of pain.35 Preliminary evidence suggests that, beyond improvement in pain and functionality, exercise might reduce depressive symptoms in chronic pain patients.36
1. Sharp J, Keefe B. Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep. 2005;7(3):213-219.
2. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
3. Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263-275.
4. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
5. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3.
6. Rowbotham MC, Goli V, Kunz NR, et al. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697-706.
7. Kranzler JD, Gendreau JF, Rao SG. The psychopharmacology of fibromyalgia: a drug development perspective. Psychopharmacol Bull. 2002;36(1):165-213.
8. Pae CU, Marks DM, Shah M, et al. Milnacipran: beyond a role of antidepressant. Clin Neuropharmacol. 2009;32(6):355-363.
9. Depression and pain. J Clin Psychiatry. 2008;69(12):1970-1978.
10. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
11. Schreiber S, Pick CG. From selective to highly selective SSRIs: a comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. Eur Neuropsychopharmacol. 2006;16(6):464-468.
12. Freynhagen R, Geisslinger G, Schug SA. Opioids for chronic non-cancer pain. BMJ. 2013;346:f2937. doi: 10.1136/bmj.f2937.
13. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679-684.
14. Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit α2σ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2σ ligand pregabalin. J Neurosci. 2009;29(13):4076-4088.
15. Dooley DJ, Taylor CP, Donevan S, et al. Ca2+ channel α2σ ligands: novel modulators of neurotransmission [Erratum in: Trends Pharmacol Sci. 2007;28(4):151]. Trends Pharmacol Sci. 2007;28(2):75-82.
16. Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. doi: 10.1002/14651858.CD010567.pub2.
17. Finnerup NB, Otto M, Jensen TS, et al. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9(2):36.
18. Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(suppl 1):S9-S27.
19. Sammaritano M, Sherwin A. Effect of anticonvulsants on sleep. Neurology. 2000;54(5 suppl 1):S16-S24.
20. Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005-2015.
21. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
22. Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85-93.
23. Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219(1):25-50.
24. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
25. Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(suppl 2):S43-S48.
26. Chang KL, Fillingim R, Hurley RW, et al. Chronic pain management: nonpharmacological therapies for chronic pain. FP Essent. 2015;432:21-26.
27. Cuijpers P, Smit F, Bohlmeijer E, et al. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196(3):173-178.
28. Lambert M. ICSI releases guideline on chronic pain assessment and management. Am Fam Physician. 2010;82(4):434-439.
29. Watkins ER, Mullan E, Wingrove J, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry. 2011;199(4):317-322.
30. Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2):88-95.
31. Buhrman M, Syk M, Burvall O, et al. Individualized guided Internet-delivered cognitive-behavior therapy for chronic pain patients with comorbid depression and anxiety: a randomized controlled trial. Clin J Pain. 2015;31(6):504-516.
32. American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810-833.
33. Theadom A, Cropley M, Smith HE, et al. Mind and body therapy for fibromyalgia. Cochrane Database Syst Rev. 2015;4:CD001980. doi: 10.1002/14651858.CD001980.pub3.
34. Mura G, Moro MF, Patten SB, et al. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr. 2014;19(6):496-508.
35. Kroll HR. Exercise therapy for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):263-281.
36. Liang H, Zhang H, Ji H, et al. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol. 2015;34(10):1737-1744.
Patients who have chronic pain and those with a major depressive disorder (MDD) share clinical features, including fatigue, cognitive complaints, and functional limitation. Sleep disturbance and anxiety are common with both disorders. Because pain and depression share common neurobiological pathways (see Part 1 of this article in the February 2016 issue and at CurrentPsychiatry.com) and clinical manifestations, you can use similar strategies and, often, the same agents to treat both conditions when they occur together (Table 1).
What are the medical options?
Antidepressants. Using an antidepressant to treat chronic pain is a common practice in primary care and specialty practice. Antidepressants that modulate multiple neurotransmitters appear to be more efficacious than those with a single mechanism of action.1 Convergent evidence from preclinical and clinical studies supports the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) as more effective analgesic agents, compared with the mostly noradrenergic antidepressants, which, in turn, are more effective than selective serotonin reuptake inhibitors (SSRIs).2 The mechanism of the analgesic action of antidepressants appears to rely on their inhibitory effects of norepinephrine and serotonin reuptake, thereby elevating the performance of endogenous descending pain regulatory pathways.3
Tricyclic antidepressants (TCAs), primarily amitriptyline, nortriptyline, and desipramine, have the advantage of years of clinical experience and low cost. Their side effect burden, however, is higher, especially in geriatric patients. Dose-dependent side effects include sedation, constipation, dry mouth, urinary retention, and orthostatic hypotension.
TCAs must be used with caution in patients with suicidal ideation because of the risk of a potentially lethal intentional overdose.
The key to using a TCA is to start with a low dosage, followed by slow titration. Typically, the dosages of TCAs used in clinical trials that focused on pain have been lower (25 to 100 mg/d of amitriptyline or equivalent) than the dosage typically necessary for treating depression; however, some experts have found that titrating TCAs to higher dosages with an option of monitoring serum levels may benefit some patients.4
SNRIs are considered first-line agents for both neuropathic pain and fibromyalgia. Duloxetine has been shown to be effective in both conditions5; venlafaxine also has shown efficacy in neuropathic pain.6 Milnacipran, another SNRI that blocks 5-HT, and norepinephrine equally and exerts a mild N-methyl-D-aspartate inhibition, has proven efficacy in fibromyalgia.7,8
SSRIs for alleviating central pain or neuropathic pain are supported by minimal evidence only.9 A review of the effectiveness of various antidepressants on pain in diabetic neuropathy concluded that fluoxetine was no more effective than placebo.10,11 Schreiber and Pick11 evaluated the antinociceptive properties of several SSRIs and offered the opinion that fluoxetine, fluvoxamine, and citalopram were, at best, weak antinociceptors.
Opioids. Data on the long-term benefits of opioids are limited, except for use in carefully selected patients; in any case, risk of abuse, diversion, and even death with these agents is quite high.12 Also, there is evidence that opioid-induced hyperalgesia might limit the usefulness of opioids for controlling chronic pain.13
Gabapentin and pregabalin, both anticonvulsants, act by binding to the α-2-σ subunit of voltage-gated calcium channels within the CNS.14 By reducing calcium influx at nerve terminals, the drugs diminish the release of several neurotransmitters, including glutamate, noradrenaline, and substance P. This mechanism is thought to be the basis for the analgesic, anticonvulsant, and anxiolytic effects of these drugs.15
Gabapentin and pregabalin have been shown to decrease pain intensity and improve quality of life and function in patients with neuropathic pain conditions. Pregabalin also has shown efficacy in treating central neuropathic pain and fibromyalgia.16
Added benefits of these drugs is that they have (1) a better side effect profile than TCAs and (2) fewer drug interactions when they are used as a component of combination therapy. Pregabalin has the additional advantage of less-frequent dosing, linear pharmacokinetics, and a predictable dose-response relationship.17
Addressing other comorbid psychiatric conditions
Sleep disturbance is common among patients with chronic pain. Sleep deprivation causes a hyperexcitable state that amplifies the pain response.18
When a patient presents with chronic pain, depression, and disturbed sleep, consider using a sedating antidepressant, such as a TCA. Alternatively, gabapentin or pregabalin can be added to an SNRI; anticonvulsants have been shown to improve quality of sleep.19 Cognitive-behavioral interventions targeting sleep disturbance may be a helpful adjunct in these patients.20
When anxiety is comorbid with chronic pain, antidepressants with proven efficacy in treating anxiety disorders, such as duloxetine or venlafaxine, can be used. When chronic pain coexists with a specific anxiety disorder (social anxiety disorder, obsessive-compulsive disorder, panic disorder), an SSRI might be more advantageous than an SNRI,21 especially if it is combined with a more efficacious analgesic.
Benzodiazepines should be avoided as a routine treatment for comorbid anxiety and pain, because these agents can produce sedation and cognitive interference, and carry the potential for dependence.
Fatigue. In patients who, in addition to pain and depression, complain of fatigue, an activating agent such as milnacipran or adjunct bupropion might be preferable to other agents. Modafinil has been shown to be a well-tolerated and potentially effective augmenting agent for antidepressants when fatigue and sleepiness are present as residual symptoms22; consider them as adjuncts when managing patients who have chronic pain and depression that manifests as excessive sleepiness and/or fatigue.
Cognitive complaints. We have noted that disturbances of cognition are common in patients with depression and chronic pain, and that cognitive dysfunction might improve after antidepressant treatment.
Studies suggest that SSRIs, duloxetine, and other antidepressants, such as bupropion, might exert a positive effect on learning, memory, and executive function in depressed patients.23 Beneficial effects of antidepressants may be “pseudo-specific,” however—that is, predominantly a reflection of overall improvement in mood, not on specific amelioration of the cognitive disturbance.
Vortioxetine has shown promise in improving cognitive function in adults with MDD; its cognitive benefits are largely independent of its antidepressant effect.24 The utility of vortioxetine in chronic pain patients has not been studied, but its positive impact on mood, anxiety, sleep, and cognition might make it a consideration for patients with comorbid depression—although it is uncertain at this time whether putative noradrenergic activity makes it suitable for use in chronic pain disorders.
Last, avoid TCAs in patients who have cognitive complaints. These agents have anticholinergic effects that can have an adverse impact on cognitive function.
Cautions: Drug−drug interactions, suicide risk, disrupted sleep
Avoiding drug−drug interactions is an important consideration when treating comorbid disorders. Many chronic pain patients take over-the-counter or prescribed nonsteroidal anti-inflammatory drugs for analgesia; these agents can increase the risk of gastrointestinal bleeding when they are combined with an SSRI or an SNRI.
The use of the opioid tramadol with an SNRI or a TCA is discouraged because of the risk of serotonin syndrome.
Combining a sedating antidepressant, such as a TCA, with gabapentin or pregabalin can increase the risk of CNS depression and psychomotor impairment, especially in geriatric patients. An opioid analgesic is likely to amplify these effects.
Suicidal ideation is not uncommon in patients with chronic pain and depression. To minimize the risk of suicide in patients with a chronic pain disorder, you should ensure optimal pain control by combining the most efficacious analgesic agent with psychotherapeutic interventions and optimal antidepressant treatment. Furthermore, cognitive-behavioral therapy (CBT) (see the discussion below) might not only improve pain coping skills, but also ameliorate catastrophizing, anxiety, and concomitant sleep disturbance.
Complaints of sleep disturbance and anxiety can compound the risk of suicide in a chronic pain patient. When possible, these complex patients should be treated by a multidisciplinary team that includes a pain management specialist, psychotherapist, and primary care clinician. It is important to strengthen the clinician−patient relationship to facilitate close monitoring of symptoms and to provide a trusting environment in which patients feel free to discuss thoughts of suicide or self-harm. For such patients, prescribing opiates and TCAs in small quantities is a prudent action.
When a patient struggles with suicidal thoughts, his (her) family might need to dispense these medications. Most important, if a patient is actively suicidal, consider referral to an inpatient facility or intensive outpatient program, where aggressive treatment of depressive symptoms and intensive monitoring and support can be provided.25
Usefulness of non-drug interventions
There is, of course, a diversity of non-drug treatments for MDD and for chronic pain; discussion here focuses primarily on modalities with established efficacy in both disease states (Table 2). On rare occasions, non-drug treatments can constitute a stand-alone approach; most often, they are incorporated into a multimodal treatment plan or applied as an adjunct intervention.26
Psychotherapy. The most robust evidence supports the use of CBT in addressing MDD and chronic pain—occurring individually and comorbidly.26-28 Efficacy is well established in MDD, as monotherapy and adjunct treatment, spanning acute and maintenance phases.
Furthermore, CBT also has support from randomized trials, meta-analyses, and treatment guidelines, either as monotherapy or co-therapy for both short-term relief and long-term pain reduction. Additionally, CBT has demonstrated value for relieving pain-related disability.26,28
Combination of a special form of CBT, rumination-focused CBT with ongoing pharmacological therapy over a 26-week period in a group of medication-refractory MDD patients produced a remission rate of 62%, compared with 21% in a treatment-as-usual group.29 This is of particular interest in chronic pain patients, because rumination-related phenomena of pain catastrophizing and avoidance facilitate a transition from acute to chronic pain, while augmenting pain severity and associated disability.30
Catastrophizing also has been implicated in mediating the relationship between pain and sleep disturbance. Not surprisingly, a randomized controlled study demonstrated the benefit of 8-week, Internet-delivered CBT in patients suffering from comorbid chronic pain, depression, and anxiety. Treatment significantly diminished pain catastrophizing, depression, and anxiety; maintenance of improvement was demonstrated after 1 year of follow-up.31
Other behavioral and psychological approaches. Biofeedback, mindfulness-based stress reduction, relaxation training and diaphragmatic breathing, guided imagery, hypnosis, and supportive groups might play an important role as components of an integrated mind−body approach to chronic pain,28,32,33 while also providing mood benefits.
Exercise. The role of exercise as a primary treatment of MDD continues to be controversial, but its benefits as an add-on intervention are indisputable. Exercise not only complements pharmacotherapy to produce greater reduction in depressive scores and improvement in quality of life, it might aid in reestablishing social contacts when conducted in a group setting—an effect that can be of great value in both MDD and chronic pain.34
Exercise and restorative therapies provide several benefits for chronic pain patients, including:
- improved pain control, cognition, and mood
- greater strength and endurance
- cardiovascular and metabolic benefits
- improved bone health and functionality.26,28,32,33,35
To achieve optimal benefit, an exercise program must be customized to fit the patient’s physical condition, level of fitness, and specific type of pain.35 Preliminary evidence suggests that, beyond improvement in pain and functionality, exercise might reduce depressive symptoms in chronic pain patients.36
Patients who have chronic pain and those with a major depressive disorder (MDD) share clinical features, including fatigue, cognitive complaints, and functional limitation. Sleep disturbance and anxiety are common with both disorders. Because pain and depression share common neurobiological pathways (see Part 1 of this article in the February 2016 issue and at CurrentPsychiatry.com) and clinical manifestations, you can use similar strategies and, often, the same agents to treat both conditions when they occur together (Table 1).
What are the medical options?
Antidepressants. Using an antidepressant to treat chronic pain is a common practice in primary care and specialty practice. Antidepressants that modulate multiple neurotransmitters appear to be more efficacious than those with a single mechanism of action.1 Convergent evidence from preclinical and clinical studies supports the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) as more effective analgesic agents, compared with the mostly noradrenergic antidepressants, which, in turn, are more effective than selective serotonin reuptake inhibitors (SSRIs).2 The mechanism of the analgesic action of antidepressants appears to rely on their inhibitory effects of norepinephrine and serotonin reuptake, thereby elevating the performance of endogenous descending pain regulatory pathways.3
Tricyclic antidepressants (TCAs), primarily amitriptyline, nortriptyline, and desipramine, have the advantage of years of clinical experience and low cost. Their side effect burden, however, is higher, especially in geriatric patients. Dose-dependent side effects include sedation, constipation, dry mouth, urinary retention, and orthostatic hypotension.
TCAs must be used with caution in patients with suicidal ideation because of the risk of a potentially lethal intentional overdose.
The key to using a TCA is to start with a low dosage, followed by slow titration. Typically, the dosages of TCAs used in clinical trials that focused on pain have been lower (25 to 100 mg/d of amitriptyline or equivalent) than the dosage typically necessary for treating depression; however, some experts have found that titrating TCAs to higher dosages with an option of monitoring serum levels may benefit some patients.4
SNRIs are considered first-line agents for both neuropathic pain and fibromyalgia. Duloxetine has been shown to be effective in both conditions5; venlafaxine also has shown efficacy in neuropathic pain.6 Milnacipran, another SNRI that blocks 5-HT, and norepinephrine equally and exerts a mild N-methyl-D-aspartate inhibition, has proven efficacy in fibromyalgia.7,8
SSRIs for alleviating central pain or neuropathic pain are supported by minimal evidence only.9 A review of the effectiveness of various antidepressants on pain in diabetic neuropathy concluded that fluoxetine was no more effective than placebo.10,11 Schreiber and Pick11 evaluated the antinociceptive properties of several SSRIs and offered the opinion that fluoxetine, fluvoxamine, and citalopram were, at best, weak antinociceptors.
Opioids. Data on the long-term benefits of opioids are limited, except for use in carefully selected patients; in any case, risk of abuse, diversion, and even death with these agents is quite high.12 Also, there is evidence that opioid-induced hyperalgesia might limit the usefulness of opioids for controlling chronic pain.13
Gabapentin and pregabalin, both anticonvulsants, act by binding to the α-2-σ subunit of voltage-gated calcium channels within the CNS.14 By reducing calcium influx at nerve terminals, the drugs diminish the release of several neurotransmitters, including glutamate, noradrenaline, and substance P. This mechanism is thought to be the basis for the analgesic, anticonvulsant, and anxiolytic effects of these drugs.15
Gabapentin and pregabalin have been shown to decrease pain intensity and improve quality of life and function in patients with neuropathic pain conditions. Pregabalin also has shown efficacy in treating central neuropathic pain and fibromyalgia.16
Added benefits of these drugs is that they have (1) a better side effect profile than TCAs and (2) fewer drug interactions when they are used as a component of combination therapy. Pregabalin has the additional advantage of less-frequent dosing, linear pharmacokinetics, and a predictable dose-response relationship.17
Addressing other comorbid psychiatric conditions
Sleep disturbance is common among patients with chronic pain. Sleep deprivation causes a hyperexcitable state that amplifies the pain response.18
When a patient presents with chronic pain, depression, and disturbed sleep, consider using a sedating antidepressant, such as a TCA. Alternatively, gabapentin or pregabalin can be added to an SNRI; anticonvulsants have been shown to improve quality of sleep.19 Cognitive-behavioral interventions targeting sleep disturbance may be a helpful adjunct in these patients.20
When anxiety is comorbid with chronic pain, antidepressants with proven efficacy in treating anxiety disorders, such as duloxetine or venlafaxine, can be used. When chronic pain coexists with a specific anxiety disorder (social anxiety disorder, obsessive-compulsive disorder, panic disorder), an SSRI might be more advantageous than an SNRI,21 especially if it is combined with a more efficacious analgesic.
Benzodiazepines should be avoided as a routine treatment for comorbid anxiety and pain, because these agents can produce sedation and cognitive interference, and carry the potential for dependence.
Fatigue. In patients who, in addition to pain and depression, complain of fatigue, an activating agent such as milnacipran or adjunct bupropion might be preferable to other agents. Modafinil has been shown to be a well-tolerated and potentially effective augmenting agent for antidepressants when fatigue and sleepiness are present as residual symptoms22; consider them as adjuncts when managing patients who have chronic pain and depression that manifests as excessive sleepiness and/or fatigue.
Cognitive complaints. We have noted that disturbances of cognition are common in patients with depression and chronic pain, and that cognitive dysfunction might improve after antidepressant treatment.
Studies suggest that SSRIs, duloxetine, and other antidepressants, such as bupropion, might exert a positive effect on learning, memory, and executive function in depressed patients.23 Beneficial effects of antidepressants may be “pseudo-specific,” however—that is, predominantly a reflection of overall improvement in mood, not on specific amelioration of the cognitive disturbance.
Vortioxetine has shown promise in improving cognitive function in adults with MDD; its cognitive benefits are largely independent of its antidepressant effect.24 The utility of vortioxetine in chronic pain patients has not been studied, but its positive impact on mood, anxiety, sleep, and cognition might make it a consideration for patients with comorbid depression—although it is uncertain at this time whether putative noradrenergic activity makes it suitable for use in chronic pain disorders.
Last, avoid TCAs in patients who have cognitive complaints. These agents have anticholinergic effects that can have an adverse impact on cognitive function.
Cautions: Drug−drug interactions, suicide risk, disrupted sleep
Avoiding drug−drug interactions is an important consideration when treating comorbid disorders. Many chronic pain patients take over-the-counter or prescribed nonsteroidal anti-inflammatory drugs for analgesia; these agents can increase the risk of gastrointestinal bleeding when they are combined with an SSRI or an SNRI.
The use of the opioid tramadol with an SNRI or a TCA is discouraged because of the risk of serotonin syndrome.
Combining a sedating antidepressant, such as a TCA, with gabapentin or pregabalin can increase the risk of CNS depression and psychomotor impairment, especially in geriatric patients. An opioid analgesic is likely to amplify these effects.
Suicidal ideation is not uncommon in patients with chronic pain and depression. To minimize the risk of suicide in patients with a chronic pain disorder, you should ensure optimal pain control by combining the most efficacious analgesic agent with psychotherapeutic interventions and optimal antidepressant treatment. Furthermore, cognitive-behavioral therapy (CBT) (see the discussion below) might not only improve pain coping skills, but also ameliorate catastrophizing, anxiety, and concomitant sleep disturbance.
Complaints of sleep disturbance and anxiety can compound the risk of suicide in a chronic pain patient. When possible, these complex patients should be treated by a multidisciplinary team that includes a pain management specialist, psychotherapist, and primary care clinician. It is important to strengthen the clinician−patient relationship to facilitate close monitoring of symptoms and to provide a trusting environment in which patients feel free to discuss thoughts of suicide or self-harm. For such patients, prescribing opiates and TCAs in small quantities is a prudent action.
When a patient struggles with suicidal thoughts, his (her) family might need to dispense these medications. Most important, if a patient is actively suicidal, consider referral to an inpatient facility or intensive outpatient program, where aggressive treatment of depressive symptoms and intensive monitoring and support can be provided.25
Usefulness of non-drug interventions
There is, of course, a diversity of non-drug treatments for MDD and for chronic pain; discussion here focuses primarily on modalities with established efficacy in both disease states (Table 2). On rare occasions, non-drug treatments can constitute a stand-alone approach; most often, they are incorporated into a multimodal treatment plan or applied as an adjunct intervention.26
Psychotherapy. The most robust evidence supports the use of CBT in addressing MDD and chronic pain—occurring individually and comorbidly.26-28 Efficacy is well established in MDD, as monotherapy and adjunct treatment, spanning acute and maintenance phases.
Furthermore, CBT also has support from randomized trials, meta-analyses, and treatment guidelines, either as monotherapy or co-therapy for both short-term relief and long-term pain reduction. Additionally, CBT has demonstrated value for relieving pain-related disability.26,28
Combination of a special form of CBT, rumination-focused CBT with ongoing pharmacological therapy over a 26-week period in a group of medication-refractory MDD patients produced a remission rate of 62%, compared with 21% in a treatment-as-usual group.29 This is of particular interest in chronic pain patients, because rumination-related phenomena of pain catastrophizing and avoidance facilitate a transition from acute to chronic pain, while augmenting pain severity and associated disability.30
Catastrophizing also has been implicated in mediating the relationship between pain and sleep disturbance. Not surprisingly, a randomized controlled study demonstrated the benefit of 8-week, Internet-delivered CBT in patients suffering from comorbid chronic pain, depression, and anxiety. Treatment significantly diminished pain catastrophizing, depression, and anxiety; maintenance of improvement was demonstrated after 1 year of follow-up.31
Other behavioral and psychological approaches. Biofeedback, mindfulness-based stress reduction, relaxation training and diaphragmatic breathing, guided imagery, hypnosis, and supportive groups might play an important role as components of an integrated mind−body approach to chronic pain,28,32,33 while also providing mood benefits.
Exercise. The role of exercise as a primary treatment of MDD continues to be controversial, but its benefits as an add-on intervention are indisputable. Exercise not only complements pharmacotherapy to produce greater reduction in depressive scores and improvement in quality of life, it might aid in reestablishing social contacts when conducted in a group setting—an effect that can be of great value in both MDD and chronic pain.34
Exercise and restorative therapies provide several benefits for chronic pain patients, including:
- improved pain control, cognition, and mood
- greater strength and endurance
- cardiovascular and metabolic benefits
- improved bone health and functionality.26,28,32,33,35
To achieve optimal benefit, an exercise program must be customized to fit the patient’s physical condition, level of fitness, and specific type of pain.35 Preliminary evidence suggests that, beyond improvement in pain and functionality, exercise might reduce depressive symptoms in chronic pain patients.36
1. Sharp J, Keefe B. Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep. 2005;7(3):213-219.
2. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
3. Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263-275.
4. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
5. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3.
6. Rowbotham MC, Goli V, Kunz NR, et al. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697-706.
7. Kranzler JD, Gendreau JF, Rao SG. The psychopharmacology of fibromyalgia: a drug development perspective. Psychopharmacol Bull. 2002;36(1):165-213.
8. Pae CU, Marks DM, Shah M, et al. Milnacipran: beyond a role of antidepressant. Clin Neuropharmacol. 2009;32(6):355-363.
9. Depression and pain. J Clin Psychiatry. 2008;69(12):1970-1978.
10. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
11. Schreiber S, Pick CG. From selective to highly selective SSRIs: a comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. Eur Neuropsychopharmacol. 2006;16(6):464-468.
12. Freynhagen R, Geisslinger G, Schug SA. Opioids for chronic non-cancer pain. BMJ. 2013;346:f2937. doi: 10.1136/bmj.f2937.
13. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679-684.
14. Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit α2σ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2σ ligand pregabalin. J Neurosci. 2009;29(13):4076-4088.
15. Dooley DJ, Taylor CP, Donevan S, et al. Ca2+ channel α2σ ligands: novel modulators of neurotransmission [Erratum in: Trends Pharmacol Sci. 2007;28(4):151]. Trends Pharmacol Sci. 2007;28(2):75-82.
16. Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. doi: 10.1002/14651858.CD010567.pub2.
17. Finnerup NB, Otto M, Jensen TS, et al. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9(2):36.
18. Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(suppl 1):S9-S27.
19. Sammaritano M, Sherwin A. Effect of anticonvulsants on sleep. Neurology. 2000;54(5 suppl 1):S16-S24.
20. Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005-2015.
21. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
22. Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85-93.
23. Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219(1):25-50.
24. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
25. Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(suppl 2):S43-S48.
26. Chang KL, Fillingim R, Hurley RW, et al. Chronic pain management: nonpharmacological therapies for chronic pain. FP Essent. 2015;432:21-26.
27. Cuijpers P, Smit F, Bohlmeijer E, et al. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196(3):173-178.
28. Lambert M. ICSI releases guideline on chronic pain assessment and management. Am Fam Physician. 2010;82(4):434-439.
29. Watkins ER, Mullan E, Wingrove J, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry. 2011;199(4):317-322.
30. Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2):88-95.
31. Buhrman M, Syk M, Burvall O, et al. Individualized guided Internet-delivered cognitive-behavior therapy for chronic pain patients with comorbid depression and anxiety: a randomized controlled trial. Clin J Pain. 2015;31(6):504-516.
32. American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810-833.
33. Theadom A, Cropley M, Smith HE, et al. Mind and body therapy for fibromyalgia. Cochrane Database Syst Rev. 2015;4:CD001980. doi: 10.1002/14651858.CD001980.pub3.
34. Mura G, Moro MF, Patten SB, et al. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr. 2014;19(6):496-508.
35. Kroll HR. Exercise therapy for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):263-281.
36. Liang H, Zhang H, Ji H, et al. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol. 2015;34(10):1737-1744.
1. Sharp J, Keefe B. Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep. 2005;7(3):213-219.
2. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
3. Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263-275.
4. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
5. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3.
6. Rowbotham MC, Goli V, Kunz NR, et al. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697-706.
7. Kranzler JD, Gendreau JF, Rao SG. The psychopharmacology of fibromyalgia: a drug development perspective. Psychopharmacol Bull. 2002;36(1):165-213.
8. Pae CU, Marks DM, Shah M, et al. Milnacipran: beyond a role of antidepressant. Clin Neuropharmacol. 2009;32(6):355-363.
9. Depression and pain. J Clin Psychiatry. 2008;69(12):1970-1978.
10. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
11. Schreiber S, Pick CG. From selective to highly selective SSRIs: a comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. Eur Neuropsychopharmacol. 2006;16(6):464-468.
12. Freynhagen R, Geisslinger G, Schug SA. Opioids for chronic non-cancer pain. BMJ. 2013;346:f2937. doi: 10.1136/bmj.f2937.
13. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679-684.
14. Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit α2σ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2σ ligand pregabalin. J Neurosci. 2009;29(13):4076-4088.
15. Dooley DJ, Taylor CP, Donevan S, et al. Ca2+ channel α2σ ligands: novel modulators of neurotransmission [Erratum in: Trends Pharmacol Sci. 2007;28(4):151]. Trends Pharmacol Sci. 2007;28(2):75-82.
16. Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. doi: 10.1002/14651858.CD010567.pub2.
17. Finnerup NB, Otto M, Jensen TS, et al. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9(2):36.
18. Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(suppl 1):S9-S27.
19. Sammaritano M, Sherwin A. Effect of anticonvulsants on sleep. Neurology. 2000;54(5 suppl 1):S16-S24.
20. Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005-2015.
21. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
22. Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85-93.
23. Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219(1):25-50.
24. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
25. Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(suppl 2):S43-S48.
26. Chang KL, Fillingim R, Hurley RW, et al. Chronic pain management: nonpharmacological therapies for chronic pain. FP Essent. 2015;432:21-26.
27. Cuijpers P, Smit F, Bohlmeijer E, et al. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196(3):173-178.
28. Lambert M. ICSI releases guideline on chronic pain assessment and management. Am Fam Physician. 2010;82(4):434-439.
29. Watkins ER, Mullan E, Wingrove J, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry. 2011;199(4):317-322.
30. Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2):88-95.
31. Buhrman M, Syk M, Burvall O, et al. Individualized guided Internet-delivered cognitive-behavior therapy for chronic pain patients with comorbid depression and anxiety: a randomized controlled trial. Clin J Pain. 2015;31(6):504-516.
32. American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810-833.
33. Theadom A, Cropley M, Smith HE, et al. Mind and body therapy for fibromyalgia. Cochrane Database Syst Rev. 2015;4:CD001980. doi: 10.1002/14651858.CD001980.pub3.
34. Mura G, Moro MF, Patten SB, et al. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr. 2014;19(6):496-508.
35. Kroll HR. Exercise therapy for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):263-281.
36. Liang H, Zhang H, Ji H, et al. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol. 2015;34(10):1737-1744.
‘We need to protect the brain’ Addressing the growing problem of chronic traumatic encephalopathy
The National Football League (NFL) had its highest concussion tally last year: 182 such injuries reported1 in the 2014-2015 regular season. The true rate of concussion in the NFL is likely higher, as a result of multiple factors (fear of “letting the team [or the coach] down,” fear of retaliation from team owners,2 etc.).
To simply call a head injury a “concussion” is a disservice to players and their family: Any blow to the head, severe or otherwise, has the potential to cause microvascular disruption in the brain; repeated blows to the head undoubtedly cause further damage.
In reality, a “concussion” is a mild traumatic brain injury (mTBI). With repeated blows, an mTBI can lead to chronic traumatic encephalopathy (CTE). In 2015, eighty-seven of 91 brains from autopsied former NFL players displayed some stage of CTE.3
Pathophysiology and presentation
CTE comprises 4 histological stages; Stage 4 is the most advanced. Alzheimer’s disease (AD) and CTE display similarities, which suggests a separate classification of CTE-AD; the presence of amyloid β plaques correlates with (1) more severe hyperphosphorylated tau (pTau) pathology and (2) advanced stages of the disease and clinical presentations. Death tends to occur 10 years earlier in CTE-AD than in AD, suggesting that repetitive mTBI might change the deposition and accumulation of amyloid β plaques, and even accelerate the aging process in the brain.4
Symptoms. The case series by Omalu et al4 (which inspired the 2015 motion picture Concussion) and the case series presented by McKee et al5 described severe psychiatric symptoms associated with CTE:
- decreased speed of information processing
- increase in religiosity
- lack of insight
- poor judgment
- involvement in illegal activities
- substance abuse
- indiscretion
- verbal and physical abuse
- problems with interpersonal relationships
- isolation
- restlessness and hyperactivity
- somatic complaints.
The 2 groups of researchers also noted hopelessness, social phobia, anxiety, agitation, mania, labile mood, insomnia, explosivity, and suicidal ideation, attempt, and completion.4,5
By Stage 4, all affected patients are symptomatic. Cognitive impairment is severe; many are described as having “severe memory loss with dementia,”5 “profound” inattention and loss of concentration,5 and dysarthria. Paranoia may develop. Mood symptoms can be severe: Approximately 31% of subjects studied have contemplated suicide; of those, 26% had “suicidal tendencies” and 14% completed suicide.5
Two distinct types of CTE progression are apparent:
- patients who display cognitive deficits first; they progress to dementia but live longer
- patients who display mood and behavioral symptoms first; they tend to be younger, more violent, depressed, and explosive.6
CTE cannot be diagnosed with imaging. There are, however, a few positron emission tomography (PET) ligands for pTau that show promise:
- [F-18]FDDNP, which consistently identifies pTau deposits in brains in which CTE is clinically suspected, in the same distribution of pTau neurofibrillary tangles on autopsy.
- [11C]DPA-713, which detected TBI-related inflammation of neurons in 9 former NFL players in whom CTE was suspected based on the clinical presentation.
- PiB amyloid ligand, under investigation for use in PET neuroimaging.7
Casualties
In January 2016 alone, at least 3 former NFL players were found to have CTE posthumously.
Earl Morrall. Former quarterback who had a 21-year NFL career. Official cause of death in 2014 at age 79 was recorded as “complications of Parkinson’s disease.” In 2016, Stage-4 CTE was discovered on autopsy.8
Ken Stabler. Former quarterback for several NFL teams over 15 seasons. Died of colon cancer at age 69 in 2015. On autopsy, was found to have Stage-3 CTE.9
Tyler Sash. Former University of Iowa and New York Giants football player. Died in September 2015 at age 27 of an apparent drug overdose; posthumously, determined to have Stage-2 CTE. His family reported memory loss, minor fits of rage, confusion, inattention, lack of focus, and chronic pain.
Sash’s mother said, “My son knew something was wrong, but he couldn’t express it. He was such a good person, and it’s sad that he struggled so with this—not knowing where to go with it. Now it makes sense.”10 Sash played 16 years of football in all, sustaining at least 5 concussions. (“If you’ve played football, you know there are often other incidents [of head trauma],” Sash’s father said.10)
Cultural and medical mindsets about contact sports
In the United States, children as young as age 5, with a low weight limit of 35 pounds, routinely are introduced to football.11 Reports of 5 high school players dying from football-related injury in the 2014 season, and 3 deaths in the 2015 season, led a St. Louis, Missouri, area school district to defund their football program entirely. The district’s 2015 homecoming game was a soccer match; students and parents seemed to embrace the change.12
On its face, soccer seems a good alternative to football. When children are instructed to “head” the ball, however, concern arises about CTE: Mild CTE changes have been reported in 2 young soccer players, and late-stage CTE changes were seen in a retired soccer player with dementia.13
Perhaps most disturbing is that players who develop symptoms of CTE, or are at risk, are unlikely to seek psychiatric help. We, as psychiatric clinicians, must be diligent about questioning young patients about their extracurricular activities. It is not enough to simply ask about a history of head trauma: Ask patients about any blow to the head, and don’t limit your questioning to whether they sustained a “concussion” during practice or play.
When speaking with adult and geriatric patients, ask about a history of playing interscholastic or collegiate contact sports, such as football, hockey, and soccer.
Is the solution to better shield the head?
That is not a solution: Helmets and other protective headgear appear to be insufficient to protect the brain from traumatic injury. Perhaps keeping children from engaging in violent sports that put them at high risk of CTE later is the preventive approach that merits the most attention.
1. Blackstone J. NFL tackles alarming increase in concussions. CBS News. http://www.cbsnews.com/news/nfl-studying-how-to-tackle-alarming-increase-in-concussions. Published February 2, 2016. Accessed February 3, 2016.
2. McNamee M, Partridge B, Anderson L. Concussion ethics and sports medicine. Clin Sports Med. 2015;35(2):257-267.
3. Abreu MA, Cromartie FJ, Spradley BD; United States Sports Academy. Chronic traumatic encephalopathy (CTE) and former National Football League player suicides. The Sport Journal. http://thesportjournal.org/article/chronic-traumatic-encephalopathy-cte-and-former-national-football-league-player-suicides. Published January 29, 2016. Accessed January 29, 2016.
4. Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in american athletes. Neurosurgery. 2011;69(1):173-183; discussion 183.
5. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):43-64.
6. Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129.
7. Eisenmenger LB, Huo EJ, Hoffman JM, et al. Advances in PET imaging of degenerative, cerebrovascular, and traumatic causes of dementia. Semin Nucl Med. 2016;46(1):57-87.
8. Jackson B. Report: former Miami Dolphins QB Earl Morrall had brain disease CTE. Miami Herald. http://www.miamiherald.com/sports/nfl/miami-dolphins/article58794523.html. Published February 5, 2016. Accessed February 6, 2016.
9. Fantz A. Ex-NFL player Ken Stabler had concussion disease CTE, doctor says. CNN. http://www.cnn.com/2016/02/03/health/ken-stabler-cte. Updated February 4, 2016. Accessed February 9, 2016.
10. Pennington B. C.T.E. is found in an Ex-Giant Tyler Sash, who died at 27. The New York Times. http://www.nytimes.com/2016/01/27/sports/football/former-giants-safety-tyler-sash-found-to-have-cte.html?_r=0. Published January 26, 2016. Accessed January 27, 2016.
11. Pop Warner Little Scholars, Inc. Ages and weights for tackle football programs. http://www.popwarner.com/football/footballstructure.htm. Accessed February 5, 2016.
12. Fowler L. No football for homecoming? No problem at Maplewood-Richmond Heights High. St. Louis Post Dispatch. http://www.stltoday.com/news/local/education/no-football-for-homecoming-no-problem-at-maplewood-richmond-heights/article_cc8dc31b-5097-5114-ba9b-9b3584f478b9.html. Published October 9, 2015. Accessed February 3, 2016.
13. Hales C, Neill S, Gearing M, et al. Late-stage CTE pathology in a retired soccer player with dementia. Neurology. 2014;83(24):2307-2309. doi: 10.1212/WNL.0000000000001081.
The National Football League (NFL) had its highest concussion tally last year: 182 such injuries reported1 in the 2014-2015 regular season. The true rate of concussion in the NFL is likely higher, as a result of multiple factors (fear of “letting the team [or the coach] down,” fear of retaliation from team owners,2 etc.).
To simply call a head injury a “concussion” is a disservice to players and their family: Any blow to the head, severe or otherwise, has the potential to cause microvascular disruption in the brain; repeated blows to the head undoubtedly cause further damage.
In reality, a “concussion” is a mild traumatic brain injury (mTBI). With repeated blows, an mTBI can lead to chronic traumatic encephalopathy (CTE). In 2015, eighty-seven of 91 brains from autopsied former NFL players displayed some stage of CTE.3
Pathophysiology and presentation
CTE comprises 4 histological stages; Stage 4 is the most advanced. Alzheimer’s disease (AD) and CTE display similarities, which suggests a separate classification of CTE-AD; the presence of amyloid β plaques correlates with (1) more severe hyperphosphorylated tau (pTau) pathology and (2) advanced stages of the disease and clinical presentations. Death tends to occur 10 years earlier in CTE-AD than in AD, suggesting that repetitive mTBI might change the deposition and accumulation of amyloid β plaques, and even accelerate the aging process in the brain.4
Symptoms. The case series by Omalu et al4 (which inspired the 2015 motion picture Concussion) and the case series presented by McKee et al5 described severe psychiatric symptoms associated with CTE:
- decreased speed of information processing
- increase in religiosity
- lack of insight
- poor judgment
- involvement in illegal activities
- substance abuse
- indiscretion
- verbal and physical abuse
- problems with interpersonal relationships
- isolation
- restlessness and hyperactivity
- somatic complaints.
The 2 groups of researchers also noted hopelessness, social phobia, anxiety, agitation, mania, labile mood, insomnia, explosivity, and suicidal ideation, attempt, and completion.4,5
By Stage 4, all affected patients are symptomatic. Cognitive impairment is severe; many are described as having “severe memory loss with dementia,”5 “profound” inattention and loss of concentration,5 and dysarthria. Paranoia may develop. Mood symptoms can be severe: Approximately 31% of subjects studied have contemplated suicide; of those, 26% had “suicidal tendencies” and 14% completed suicide.5
Two distinct types of CTE progression are apparent:
- patients who display cognitive deficits first; they progress to dementia but live longer
- patients who display mood and behavioral symptoms first; they tend to be younger, more violent, depressed, and explosive.6
CTE cannot be diagnosed with imaging. There are, however, a few positron emission tomography (PET) ligands for pTau that show promise:
- [F-18]FDDNP, which consistently identifies pTau deposits in brains in which CTE is clinically suspected, in the same distribution of pTau neurofibrillary tangles on autopsy.
- [11C]DPA-713, which detected TBI-related inflammation of neurons in 9 former NFL players in whom CTE was suspected based on the clinical presentation.
- PiB amyloid ligand, under investigation for use in PET neuroimaging.7
Casualties
In January 2016 alone, at least 3 former NFL players were found to have CTE posthumously.
Earl Morrall. Former quarterback who had a 21-year NFL career. Official cause of death in 2014 at age 79 was recorded as “complications of Parkinson’s disease.” In 2016, Stage-4 CTE was discovered on autopsy.8
Ken Stabler. Former quarterback for several NFL teams over 15 seasons. Died of colon cancer at age 69 in 2015. On autopsy, was found to have Stage-3 CTE.9
Tyler Sash. Former University of Iowa and New York Giants football player. Died in September 2015 at age 27 of an apparent drug overdose; posthumously, determined to have Stage-2 CTE. His family reported memory loss, minor fits of rage, confusion, inattention, lack of focus, and chronic pain.
Sash’s mother said, “My son knew something was wrong, but he couldn’t express it. He was such a good person, and it’s sad that he struggled so with this—not knowing where to go with it. Now it makes sense.”10 Sash played 16 years of football in all, sustaining at least 5 concussions. (“If you’ve played football, you know there are often other incidents [of head trauma],” Sash’s father said.10)
Cultural and medical mindsets about contact sports
In the United States, children as young as age 5, with a low weight limit of 35 pounds, routinely are introduced to football.11 Reports of 5 high school players dying from football-related injury in the 2014 season, and 3 deaths in the 2015 season, led a St. Louis, Missouri, area school district to defund their football program entirely. The district’s 2015 homecoming game was a soccer match; students and parents seemed to embrace the change.12
On its face, soccer seems a good alternative to football. When children are instructed to “head” the ball, however, concern arises about CTE: Mild CTE changes have been reported in 2 young soccer players, and late-stage CTE changes were seen in a retired soccer player with dementia.13
Perhaps most disturbing is that players who develop symptoms of CTE, or are at risk, are unlikely to seek psychiatric help. We, as psychiatric clinicians, must be diligent about questioning young patients about their extracurricular activities. It is not enough to simply ask about a history of head trauma: Ask patients about any blow to the head, and don’t limit your questioning to whether they sustained a “concussion” during practice or play.
When speaking with adult and geriatric patients, ask about a history of playing interscholastic or collegiate contact sports, such as football, hockey, and soccer.
Is the solution to better shield the head?
That is not a solution: Helmets and other protective headgear appear to be insufficient to protect the brain from traumatic injury. Perhaps keeping children from engaging in violent sports that put them at high risk of CTE later is the preventive approach that merits the most attention.
The National Football League (NFL) had its highest concussion tally last year: 182 such injuries reported1 in the 2014-2015 regular season. The true rate of concussion in the NFL is likely higher, as a result of multiple factors (fear of “letting the team [or the coach] down,” fear of retaliation from team owners,2 etc.).
To simply call a head injury a “concussion” is a disservice to players and their family: Any blow to the head, severe or otherwise, has the potential to cause microvascular disruption in the brain; repeated blows to the head undoubtedly cause further damage.
In reality, a “concussion” is a mild traumatic brain injury (mTBI). With repeated blows, an mTBI can lead to chronic traumatic encephalopathy (CTE). In 2015, eighty-seven of 91 brains from autopsied former NFL players displayed some stage of CTE.3
Pathophysiology and presentation
CTE comprises 4 histological stages; Stage 4 is the most advanced. Alzheimer’s disease (AD) and CTE display similarities, which suggests a separate classification of CTE-AD; the presence of amyloid β plaques correlates with (1) more severe hyperphosphorylated tau (pTau) pathology and (2) advanced stages of the disease and clinical presentations. Death tends to occur 10 years earlier in CTE-AD than in AD, suggesting that repetitive mTBI might change the deposition and accumulation of amyloid β plaques, and even accelerate the aging process in the brain.4
Symptoms. The case series by Omalu et al4 (which inspired the 2015 motion picture Concussion) and the case series presented by McKee et al5 described severe psychiatric symptoms associated with CTE:
- decreased speed of information processing
- increase in religiosity
- lack of insight
- poor judgment
- involvement in illegal activities
- substance abuse
- indiscretion
- verbal and physical abuse
- problems with interpersonal relationships
- isolation
- restlessness and hyperactivity
- somatic complaints.
The 2 groups of researchers also noted hopelessness, social phobia, anxiety, agitation, mania, labile mood, insomnia, explosivity, and suicidal ideation, attempt, and completion.4,5
By Stage 4, all affected patients are symptomatic. Cognitive impairment is severe; many are described as having “severe memory loss with dementia,”5 “profound” inattention and loss of concentration,5 and dysarthria. Paranoia may develop. Mood symptoms can be severe: Approximately 31% of subjects studied have contemplated suicide; of those, 26% had “suicidal tendencies” and 14% completed suicide.5
Two distinct types of CTE progression are apparent:
- patients who display cognitive deficits first; they progress to dementia but live longer
- patients who display mood and behavioral symptoms first; they tend to be younger, more violent, depressed, and explosive.6
CTE cannot be diagnosed with imaging. There are, however, a few positron emission tomography (PET) ligands for pTau that show promise:
- [F-18]FDDNP, which consistently identifies pTau deposits in brains in which CTE is clinically suspected, in the same distribution of pTau neurofibrillary tangles on autopsy.
- [11C]DPA-713, which detected TBI-related inflammation of neurons in 9 former NFL players in whom CTE was suspected based on the clinical presentation.
- PiB amyloid ligand, under investigation for use in PET neuroimaging.7
Casualties
In January 2016 alone, at least 3 former NFL players were found to have CTE posthumously.
Earl Morrall. Former quarterback who had a 21-year NFL career. Official cause of death in 2014 at age 79 was recorded as “complications of Parkinson’s disease.” In 2016, Stage-4 CTE was discovered on autopsy.8
Ken Stabler. Former quarterback for several NFL teams over 15 seasons. Died of colon cancer at age 69 in 2015. On autopsy, was found to have Stage-3 CTE.9
Tyler Sash. Former University of Iowa and New York Giants football player. Died in September 2015 at age 27 of an apparent drug overdose; posthumously, determined to have Stage-2 CTE. His family reported memory loss, minor fits of rage, confusion, inattention, lack of focus, and chronic pain.
Sash’s mother said, “My son knew something was wrong, but he couldn’t express it. He was such a good person, and it’s sad that he struggled so with this—not knowing where to go with it. Now it makes sense.”10 Sash played 16 years of football in all, sustaining at least 5 concussions. (“If you’ve played football, you know there are often other incidents [of head trauma],” Sash’s father said.10)
Cultural and medical mindsets about contact sports
In the United States, children as young as age 5, with a low weight limit of 35 pounds, routinely are introduced to football.11 Reports of 5 high school players dying from football-related injury in the 2014 season, and 3 deaths in the 2015 season, led a St. Louis, Missouri, area school district to defund their football program entirely. The district’s 2015 homecoming game was a soccer match; students and parents seemed to embrace the change.12
On its face, soccer seems a good alternative to football. When children are instructed to “head” the ball, however, concern arises about CTE: Mild CTE changes have been reported in 2 young soccer players, and late-stage CTE changes were seen in a retired soccer player with dementia.13
Perhaps most disturbing is that players who develop symptoms of CTE, or are at risk, are unlikely to seek psychiatric help. We, as psychiatric clinicians, must be diligent about questioning young patients about their extracurricular activities. It is not enough to simply ask about a history of head trauma: Ask patients about any blow to the head, and don’t limit your questioning to whether they sustained a “concussion” during practice or play.
When speaking with adult and geriatric patients, ask about a history of playing interscholastic or collegiate contact sports, such as football, hockey, and soccer.
Is the solution to better shield the head?
That is not a solution: Helmets and other protective headgear appear to be insufficient to protect the brain from traumatic injury. Perhaps keeping children from engaging in violent sports that put them at high risk of CTE later is the preventive approach that merits the most attention.
1. Blackstone J. NFL tackles alarming increase in concussions. CBS News. http://www.cbsnews.com/news/nfl-studying-how-to-tackle-alarming-increase-in-concussions. Published February 2, 2016. Accessed February 3, 2016.
2. McNamee M, Partridge B, Anderson L. Concussion ethics and sports medicine. Clin Sports Med. 2015;35(2):257-267.
3. Abreu MA, Cromartie FJ, Spradley BD; United States Sports Academy. Chronic traumatic encephalopathy (CTE) and former National Football League player suicides. The Sport Journal. http://thesportjournal.org/article/chronic-traumatic-encephalopathy-cte-and-former-national-football-league-player-suicides. Published January 29, 2016. Accessed January 29, 2016.
4. Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in american athletes. Neurosurgery. 2011;69(1):173-183; discussion 183.
5. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):43-64.
6. Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129.
7. Eisenmenger LB, Huo EJ, Hoffman JM, et al. Advances in PET imaging of degenerative, cerebrovascular, and traumatic causes of dementia. Semin Nucl Med. 2016;46(1):57-87.
8. Jackson B. Report: former Miami Dolphins QB Earl Morrall had brain disease CTE. Miami Herald. http://www.miamiherald.com/sports/nfl/miami-dolphins/article58794523.html. Published February 5, 2016. Accessed February 6, 2016.
9. Fantz A. Ex-NFL player Ken Stabler had concussion disease CTE, doctor says. CNN. http://www.cnn.com/2016/02/03/health/ken-stabler-cte. Updated February 4, 2016. Accessed February 9, 2016.
10. Pennington B. C.T.E. is found in an Ex-Giant Tyler Sash, who died at 27. The New York Times. http://www.nytimes.com/2016/01/27/sports/football/former-giants-safety-tyler-sash-found-to-have-cte.html?_r=0. Published January 26, 2016. Accessed January 27, 2016.
11. Pop Warner Little Scholars, Inc. Ages and weights for tackle football programs. http://www.popwarner.com/football/footballstructure.htm. Accessed February 5, 2016.
12. Fowler L. No football for homecoming? No problem at Maplewood-Richmond Heights High. St. Louis Post Dispatch. http://www.stltoday.com/news/local/education/no-football-for-homecoming-no-problem-at-maplewood-richmond-heights/article_cc8dc31b-5097-5114-ba9b-9b3584f478b9.html. Published October 9, 2015. Accessed February 3, 2016.
13. Hales C, Neill S, Gearing M, et al. Late-stage CTE pathology in a retired soccer player with dementia. Neurology. 2014;83(24):2307-2309. doi: 10.1212/WNL.0000000000001081.
1. Blackstone J. NFL tackles alarming increase in concussions. CBS News. http://www.cbsnews.com/news/nfl-studying-how-to-tackle-alarming-increase-in-concussions. Published February 2, 2016. Accessed February 3, 2016.
2. McNamee M, Partridge B, Anderson L. Concussion ethics and sports medicine. Clin Sports Med. 2015;35(2):257-267.
3. Abreu MA, Cromartie FJ, Spradley BD; United States Sports Academy. Chronic traumatic encephalopathy (CTE) and former National Football League player suicides. The Sport Journal. http://thesportjournal.org/article/chronic-traumatic-encephalopathy-cte-and-former-national-football-league-player-suicides. Published January 29, 2016. Accessed January 29, 2016.
4. Omalu B, Bailes J, Hamilton RL, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in american athletes. Neurosurgery. 2011;69(1):173-183; discussion 183.
5. McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):43-64.
6. Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129.
7. Eisenmenger LB, Huo EJ, Hoffman JM, et al. Advances in PET imaging of degenerative, cerebrovascular, and traumatic causes of dementia. Semin Nucl Med. 2016;46(1):57-87.
8. Jackson B. Report: former Miami Dolphins QB Earl Morrall had brain disease CTE. Miami Herald. http://www.miamiherald.com/sports/nfl/miami-dolphins/article58794523.html. Published February 5, 2016. Accessed February 6, 2016.
9. Fantz A. Ex-NFL player Ken Stabler had concussion disease CTE, doctor says. CNN. http://www.cnn.com/2016/02/03/health/ken-stabler-cte. Updated February 4, 2016. Accessed February 9, 2016.
10. Pennington B. C.T.E. is found in an Ex-Giant Tyler Sash, who died at 27. The New York Times. http://www.nytimes.com/2016/01/27/sports/football/former-giants-safety-tyler-sash-found-to-have-cte.html?_r=0. Published January 26, 2016. Accessed January 27, 2016.
11. Pop Warner Little Scholars, Inc. Ages and weights for tackle football programs. http://www.popwarner.com/football/footballstructure.htm. Accessed February 5, 2016.
12. Fowler L. No football for homecoming? No problem at Maplewood-Richmond Heights High. St. Louis Post Dispatch. http://www.stltoday.com/news/local/education/no-football-for-homecoming-no-problem-at-maplewood-richmond-heights/article_cc8dc31b-5097-5114-ba9b-9b3584f478b9.html. Published October 9, 2015. Accessed February 3, 2016.
13. Hales C, Neill S, Gearing M, et al. Late-stage CTE pathology in a retired soccer player with dementia. Neurology. 2014;83(24):2307-2309. doi: 10.1212/WNL.0000000000001081.
Imposing treatment on patients with eating disorders: What are the legal risks?
Dear Dr. Mossman,
At the general hospital where I perform consultations, the medical service asked me to fill out psychiatric “hold” documents to keep a severely malnourished young woman with anorexia nervosa from leaving the hospital. Ms. Q, whose body mass index (BMI) was 12 (yes, 12), came to the hospital to have her “electrolytes fixed.” She was willing to stay the night for electrolyte repletion, but insisted she could gain weight on her own at home.
I’m worried that she might die without prompt inpatient treatment; she needs to stay on the medical service. Should I fill out a psychiatric hold to keep her there? What legal risks could I face if Ms. Q is detained and force-fed against her will? What are the legal risks of letting her leave the hospital before she is medically stable?
Submitted by “Dr. F”
When a severely malnourished patient with an eating disorder arrives on a medical floor, treatment teams often ask psychiatric consultants to help them impose care the patient desperately needs but doesn’t want. This reaction is understandable. After all, an eating disorder is a psychiatric illness, and hospital-based psychiatrists have experience with treating involuntary patients. A psychiatric hold may seem like a sensible way to save the life of a hospitalized patient with a mental illness.
But filling out a psychiatric hold only scratches the surface of what a psychiatric consultant’s contribution should include; in Ms. Q’s case, initiating a psychiatric hold is probably the wrong thing to do.
Why would filling out a psychiatric hold be inappropriate for Ms. Q? What clinical factors and legal issues should a psychiatrist consider when helping medical colleagues provide unwanted treatment to a severely malnourished patient with an eating disorder? We’ll explore these matters as we consider the case of Ms. Q (Figure) and the following questions:
- What type of care is most appropriate for her now?
- Can she refuse medical treatment?
- What are the medicolegal risks of letting her leave the hospital?
- What are the medicolegal risks of detaining and force-feeding her against her will?
- When is a psychiatric “hold” appropriate?
What care is appropriate?
Given her state of self-starvation, Ms. Q’s treatment plan could require close monitoring of her electrolytes and cardiac status, as well as watching her for signs of “refeeding syndrome”—rapid, potentially fatal fluid shifts and metabolic derangements that malnourished patients could experience when they receive artificial refeeding.1
First, the physicians who are caring for Ms. Q should determine whether she needs more intensive medical supervision than is usually available on a psychiatric unit. If she does, but she won’t agree to stay on a medical unit for care, a psychiatric hold is the wrong step, for 2 reasons:
- Once a psychiatric hold has been executed, state statutes require the patient to be placed in a psychiatric facility—a state-approved psychiatric treatment setting, such as a psychiatric unit or free-standing psychiatric hospital—within a specified period.2,3 Most nonpsychiatric medical units would not meet state’s statutory definition for such a facility.
- A psychiatric hold only permits short-term detention. It does not provide legal authority to impose unwanted medical treatment.
Does Ms. Q have capacity?
In the United States, Ms. Q has a legal right to refuse medical care—even if she needs it urgently—provided that her refusal is made competently.4 As Appelbaum and Grisso5 explained in a now-classic 1988 article:
The legal standards for competence include the four related skills of communicating a choice, understanding relevant information, appreciating the current situation and its consequences, and manipulating information rationally.
The Table5 describes these abilities in more detail.
Only courts can make legal determinations of competence, so physicians refer to an evaluation of a patient’s competence-related abilities as a “capacity assessment.” The decision as to whether a patient has capacity ultimately rests with the primary treatment team; however, physicians in other specialties often enlist psychiatrists’ help with this matter because of their interviewing skills and knowledge of how mental illness can impair capacity.
No easy-to-use instrument for evaluating capacity is available. However, Appelbaum6 provides examples of questions that often prove useful in such assessments, and a review by Sessums et al7 on several capacity evaluation tools suggests that the Aid to Capacity Evaluation8 may be the best instrument for performing capacity assessments.
Patients with anorexia nervosa often differ substantially from healthy people in how they assign values to life and death,9 which can make it difficult to evaluate their capacity to refuse life-saving treatment. Malnutrition can alter patients’ ability to think clearly, a phenomenon that some patients with anorexia mention as a reason they are grateful (in retrospect) for the compulsory treatment they received.10 Yet, if an evaluation shows that the patient has the decision-making capacity to refuse care, then her (his) caregivers should carefully document this conclusion and the basis for it. Although caregivers might encourage her to accept the treatment they believe she needs, they should not provide treatment that conflicts with their patient’s wishes.
If evaluation shows that the patient lacks capacity, however, the findings that support this conclusion should be documented clearly. The team then should consult the hospital attorney to determine how to best proceed. The attorney might recommend that a physician on the primary treatment team initiate a “medical hold”—an order that the patient may not leave against medical advice (AMA)—and then seek an emergency guardianship to permit medical treatment, such as refeeding.
To treat or not to treat?
What are the legal risks of allowing Ms. Q to leave AMA before she reaches medical stability?
Powers and Cloak11 describe a case of a 26-year-old woman with anorexia nervosa who came to the hospital with dizziness, weakness, and a very low blood glucose level. She was discharged after 6 days without having received any feeding, only to return to the emergency department 2 days later. This time, she had a letter from her physician stating that she needed medical supervision to start refeeding, yet she was discharged from the emergency department within a few hours. She was re-admitted to the hospital the next day.
Powers and Cloak11 do not report this woman’s medical outcome. But what if she had suffered a fatal cardiac arrhythmia before her third presentation to the emergency department or suffered another injury attributable to her nutritional state: Could her physicians be found at fault?
On Cohen & Associates’ Web site, they essentially answer, “Yes.” They describe a case of “Miss McIntosh,” who had anorexia nervosa and was discharged home from a hospital despite “chronic metabolic problems and not eating properly.” She went into a “hypoglycemic encephalopathic coma” and “suffered irreversible brain damage.” A subsequent lawsuit against the hospital resulted in a 7-figure settlement,12 illustrating the potential for adverse medicolegal consequences if failure to treat a patient with anorexia nervosa could be linked to subsequent physical harm. On the other hand, could a patient with anorexia who is being force-fed take legal action against her providers? At least 3 recent British cases suggest that this is possible.13-15 A British medical student with anorexia, E, made an emergency application to the Court of Protection in London, claiming that being fed against her will was akin to reliving her past experience of sexual abuse. In E’s case, the judge ruled “that the balance tips slowly but unmistakably in the direction of life preserving treatment” and authorized feeding over her objection.6 In 2 other cases, however, British courts have ruled that force-feeding anorexic patients would be futile and disallowed the practice.14,15
Faced with possible legal action, no matter what course you take, how should you respond? Getting legal and ethical consultation is prudent if time allows. In many cases, hospital attorneys might prefer that physicians err on the side of preserving life(D. Vanderpool, MBA, JD, personal communication, February 3, 2016)—even if that means detaining a patient without clear legal authorization to do so—because attorneys would prefer to defend a doctor who acted to save someone’s life than to defend a doctor who knowingly allowed a patient to die.
When might persons with an eating disorder be civilly committed?
Suppose that Ms. Q does not need urgent nonpsychiatric medical care, or that her life-threatening physical problems now have been addressed. Her physicians strongly recommend that she undergo inpatient psychiatric treatment for her eating disorder, but she wants to leave. Would it now be appropriate to fill out paperwork to initiate a psychiatric hold?
All U.S. jurisdictions authorize “civil commitment” proceedings that can lead to involuntary psychiatric hospitalization of people who have a mental disorder and pose a risk to themselves or others because of the disorder.16
In general, to be subject to civil commitment, a person must have a substantial disorder of thought, mood, perception, orientation, or memory. In addition, that disorder must grossly impair her (his) judgment, behavior, reality testing, or ability to meet the demands of everyday life.17
People with psychosis, a severe mood disorder, or dementia often meet these criteria. However, psychiatrists do not usually consider anorexia nervosa to be a thought disorder, mood disorder, or memory disorder. Does this mean that people with anorexia nervosa cannot meet the “substantial” mental disorder criterion?
It does not. Courts interpret the words in statutes based on their “ordinary and natural meaning.”18 If Ms. Q perceived herself as fat, despite having a BMI that was far below the healthy range, most people would regard her thinking to be disordered. If, in addition, her mental disorder impaired her “judgment, behavior, and capacity to meet the ordinary demands of sustaining existence,” then her anorexia nervosa “would qualify as a mental disorder for commitment purposes.”19
To be subject to civil commitment, a person with a substantial mental disorder also must pose a risk of harm to herself or others because of the disorder. That risk can be evidenced via an action, attempt, or threat to do direct physical harm, or it might inhere in the potential for developing grave disability through neglect of one’s basic needs, such as failing to eat adequately. In Ms. Q’s case, if the evidence shows her eating-disordered behavior has placed her at imminent risk of permanent injury or death, she has satisfied the legal criteria that justify court-ordered psychiatric hospitalization.
Bottom Line
When a severely malnourished patient with anorexia nervosa does not agree to allow recommended care, an appropriate clinical response should include judgment about the urgency of the proposed treatment, what treatment setting is best suited to the patient’s condition, and whether the patient has the mental capacity to refuse potentially life-saving care.
1. Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336(7659):1495-1498.
2. Ohio Revised Code §5122.01(F).
3. Oregon Revised Statutes §426.005(c).
4. Schloendorff v Society of New York Hospital, 211 N.Y. 125, 105 N.E. 92 (N1914).
5. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
6. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
7. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
8. Community tools: Aid to Capacity Evaluation (ACE). University of Toronto Joint Centre for Bioethics. http://www.jcb.utoronto.ca/tools/ace_download.shtml. Updated May 8, 2008. Accessed December 21, 2015.
9. Tan J, Hope T, Stewart A. Competence to refuse treatment in anorexia nervosa. Int J Law Psychiatry. 2003;26(6):697-707.
10. Elzakkers IF, Danner UN, Hoek HW, et al. Compulsory treatment in anorexia nervosa: a review. Int J Eat Disord. 2014;47(8):845-852.
11. Powers PS, Cloak NL. Failure to feed patients with anorexia nervosa and other perils and perplexities in the medical care of eating disorder patients. Eat Disord. 2013;21(1):81-89.
12. “Failure to properly treat anorexia nervosa.” Harry S. Cohen & Associates. http://medmal1.com/article/failure-to-properly-treat-anorexia-nervosa. Accessed February 1, 2016.
13. A Local Authority v E. and Others [2012] EWHC 1639 (COP).
14. A NHS Foundation Trust v Ms. X [2014] EWCOP 35.
15. NHS Trust v L [2012] EWHC 2741 (COP).
16. Pinals DA, Mossman D. Evaluation for civil commitment. New York, NY: Oxford University Press; 2011.
17. Castellano-Hoyt DW. Enhancing police response to persons in mental health crisis: providing strategies, communication techniques, and crisis intervention preparation in overcoming institutional challenges. Springfield, IL: Charles C. Thomas Publisher, Ltd; 2003.
18. FDIC v Meyer, 510 U.S. 471 (1994).
19. Appelbaum PS, Rumpf T. Civil commitment of the anorexic patient. Gen Hosp Psychiatry. 1998;20(4):225-230.
Dear Dr. Mossman,
At the general hospital where I perform consultations, the medical service asked me to fill out psychiatric “hold” documents to keep a severely malnourished young woman with anorexia nervosa from leaving the hospital. Ms. Q, whose body mass index (BMI) was 12 (yes, 12), came to the hospital to have her “electrolytes fixed.” She was willing to stay the night for electrolyte repletion, but insisted she could gain weight on her own at home.
I’m worried that she might die without prompt inpatient treatment; she needs to stay on the medical service. Should I fill out a psychiatric hold to keep her there? What legal risks could I face if Ms. Q is detained and force-fed against her will? What are the legal risks of letting her leave the hospital before she is medically stable?
Submitted by “Dr. F”
When a severely malnourished patient with an eating disorder arrives on a medical floor, treatment teams often ask psychiatric consultants to help them impose care the patient desperately needs but doesn’t want. This reaction is understandable. After all, an eating disorder is a psychiatric illness, and hospital-based psychiatrists have experience with treating involuntary patients. A psychiatric hold may seem like a sensible way to save the life of a hospitalized patient with a mental illness.
But filling out a psychiatric hold only scratches the surface of what a psychiatric consultant’s contribution should include; in Ms. Q’s case, initiating a psychiatric hold is probably the wrong thing to do.
Why would filling out a psychiatric hold be inappropriate for Ms. Q? What clinical factors and legal issues should a psychiatrist consider when helping medical colleagues provide unwanted treatment to a severely malnourished patient with an eating disorder? We’ll explore these matters as we consider the case of Ms. Q (Figure) and the following questions:
- What type of care is most appropriate for her now?
- Can she refuse medical treatment?
- What are the medicolegal risks of letting her leave the hospital?
- What are the medicolegal risks of detaining and force-feeding her against her will?
- When is a psychiatric “hold” appropriate?
What care is appropriate?
Given her state of self-starvation, Ms. Q’s treatment plan could require close monitoring of her electrolytes and cardiac status, as well as watching her for signs of “refeeding syndrome”—rapid, potentially fatal fluid shifts and metabolic derangements that malnourished patients could experience when they receive artificial refeeding.1
First, the physicians who are caring for Ms. Q should determine whether she needs more intensive medical supervision than is usually available on a psychiatric unit. If she does, but she won’t agree to stay on a medical unit for care, a psychiatric hold is the wrong step, for 2 reasons:
- Once a psychiatric hold has been executed, state statutes require the patient to be placed in a psychiatric facility—a state-approved psychiatric treatment setting, such as a psychiatric unit or free-standing psychiatric hospital—within a specified period.2,3 Most nonpsychiatric medical units would not meet state’s statutory definition for such a facility.
- A psychiatric hold only permits short-term detention. It does not provide legal authority to impose unwanted medical treatment.
Does Ms. Q have capacity?
In the United States, Ms. Q has a legal right to refuse medical care—even if she needs it urgently—provided that her refusal is made competently.4 As Appelbaum and Grisso5 explained in a now-classic 1988 article:
The legal standards for competence include the four related skills of communicating a choice, understanding relevant information, appreciating the current situation and its consequences, and manipulating information rationally.
The Table5 describes these abilities in more detail.
Only courts can make legal determinations of competence, so physicians refer to an evaluation of a patient’s competence-related abilities as a “capacity assessment.” The decision as to whether a patient has capacity ultimately rests with the primary treatment team; however, physicians in other specialties often enlist psychiatrists’ help with this matter because of their interviewing skills and knowledge of how mental illness can impair capacity.
No easy-to-use instrument for evaluating capacity is available. However, Appelbaum6 provides examples of questions that often prove useful in such assessments, and a review by Sessums et al7 on several capacity evaluation tools suggests that the Aid to Capacity Evaluation8 may be the best instrument for performing capacity assessments.
Patients with anorexia nervosa often differ substantially from healthy people in how they assign values to life and death,9 which can make it difficult to evaluate their capacity to refuse life-saving treatment. Malnutrition can alter patients’ ability to think clearly, a phenomenon that some patients with anorexia mention as a reason they are grateful (in retrospect) for the compulsory treatment they received.10 Yet, if an evaluation shows that the patient has the decision-making capacity to refuse care, then her (his) caregivers should carefully document this conclusion and the basis for it. Although caregivers might encourage her to accept the treatment they believe she needs, they should not provide treatment that conflicts with their patient’s wishes.
If evaluation shows that the patient lacks capacity, however, the findings that support this conclusion should be documented clearly. The team then should consult the hospital attorney to determine how to best proceed. The attorney might recommend that a physician on the primary treatment team initiate a “medical hold”—an order that the patient may not leave against medical advice (AMA)—and then seek an emergency guardianship to permit medical treatment, such as refeeding.
To treat or not to treat?
What are the legal risks of allowing Ms. Q to leave AMA before she reaches medical stability?
Powers and Cloak11 describe a case of a 26-year-old woman with anorexia nervosa who came to the hospital with dizziness, weakness, and a very low blood glucose level. She was discharged after 6 days without having received any feeding, only to return to the emergency department 2 days later. This time, she had a letter from her physician stating that she needed medical supervision to start refeeding, yet she was discharged from the emergency department within a few hours. She was re-admitted to the hospital the next day.
Powers and Cloak11 do not report this woman’s medical outcome. But what if she had suffered a fatal cardiac arrhythmia before her third presentation to the emergency department or suffered another injury attributable to her nutritional state: Could her physicians be found at fault?
On Cohen & Associates’ Web site, they essentially answer, “Yes.” They describe a case of “Miss McIntosh,” who had anorexia nervosa and was discharged home from a hospital despite “chronic metabolic problems and not eating properly.” She went into a “hypoglycemic encephalopathic coma” and “suffered irreversible brain damage.” A subsequent lawsuit against the hospital resulted in a 7-figure settlement,12 illustrating the potential for adverse medicolegal consequences if failure to treat a patient with anorexia nervosa could be linked to subsequent physical harm. On the other hand, could a patient with anorexia who is being force-fed take legal action against her providers? At least 3 recent British cases suggest that this is possible.13-15 A British medical student with anorexia, E, made an emergency application to the Court of Protection in London, claiming that being fed against her will was akin to reliving her past experience of sexual abuse. In E’s case, the judge ruled “that the balance tips slowly but unmistakably in the direction of life preserving treatment” and authorized feeding over her objection.6 In 2 other cases, however, British courts have ruled that force-feeding anorexic patients would be futile and disallowed the practice.14,15
Faced with possible legal action, no matter what course you take, how should you respond? Getting legal and ethical consultation is prudent if time allows. In many cases, hospital attorneys might prefer that physicians err on the side of preserving life(D. Vanderpool, MBA, JD, personal communication, February 3, 2016)—even if that means detaining a patient without clear legal authorization to do so—because attorneys would prefer to defend a doctor who acted to save someone’s life than to defend a doctor who knowingly allowed a patient to die.
When might persons with an eating disorder be civilly committed?
Suppose that Ms. Q does not need urgent nonpsychiatric medical care, or that her life-threatening physical problems now have been addressed. Her physicians strongly recommend that she undergo inpatient psychiatric treatment for her eating disorder, but she wants to leave. Would it now be appropriate to fill out paperwork to initiate a psychiatric hold?
All U.S. jurisdictions authorize “civil commitment” proceedings that can lead to involuntary psychiatric hospitalization of people who have a mental disorder and pose a risk to themselves or others because of the disorder.16
In general, to be subject to civil commitment, a person must have a substantial disorder of thought, mood, perception, orientation, or memory. In addition, that disorder must grossly impair her (his) judgment, behavior, reality testing, or ability to meet the demands of everyday life.17
People with psychosis, a severe mood disorder, or dementia often meet these criteria. However, psychiatrists do not usually consider anorexia nervosa to be a thought disorder, mood disorder, or memory disorder. Does this mean that people with anorexia nervosa cannot meet the “substantial” mental disorder criterion?
It does not. Courts interpret the words in statutes based on their “ordinary and natural meaning.”18 If Ms. Q perceived herself as fat, despite having a BMI that was far below the healthy range, most people would regard her thinking to be disordered. If, in addition, her mental disorder impaired her “judgment, behavior, and capacity to meet the ordinary demands of sustaining existence,” then her anorexia nervosa “would qualify as a mental disorder for commitment purposes.”19
To be subject to civil commitment, a person with a substantial mental disorder also must pose a risk of harm to herself or others because of the disorder. That risk can be evidenced via an action, attempt, or threat to do direct physical harm, or it might inhere in the potential for developing grave disability through neglect of one’s basic needs, such as failing to eat adequately. In Ms. Q’s case, if the evidence shows her eating-disordered behavior has placed her at imminent risk of permanent injury or death, she has satisfied the legal criteria that justify court-ordered psychiatric hospitalization.
Bottom Line
When a severely malnourished patient with anorexia nervosa does not agree to allow recommended care, an appropriate clinical response should include judgment about the urgency of the proposed treatment, what treatment setting is best suited to the patient’s condition, and whether the patient has the mental capacity to refuse potentially life-saving care.
Dear Dr. Mossman,
At the general hospital where I perform consultations, the medical service asked me to fill out psychiatric “hold” documents to keep a severely malnourished young woman with anorexia nervosa from leaving the hospital. Ms. Q, whose body mass index (BMI) was 12 (yes, 12), came to the hospital to have her “electrolytes fixed.” She was willing to stay the night for electrolyte repletion, but insisted she could gain weight on her own at home.
I’m worried that she might die without prompt inpatient treatment; she needs to stay on the medical service. Should I fill out a psychiatric hold to keep her there? What legal risks could I face if Ms. Q is detained and force-fed against her will? What are the legal risks of letting her leave the hospital before she is medically stable?
Submitted by “Dr. F”
When a severely malnourished patient with an eating disorder arrives on a medical floor, treatment teams often ask psychiatric consultants to help them impose care the patient desperately needs but doesn’t want. This reaction is understandable. After all, an eating disorder is a psychiatric illness, and hospital-based psychiatrists have experience with treating involuntary patients. A psychiatric hold may seem like a sensible way to save the life of a hospitalized patient with a mental illness.
But filling out a psychiatric hold only scratches the surface of what a psychiatric consultant’s contribution should include; in Ms. Q’s case, initiating a psychiatric hold is probably the wrong thing to do.
Why would filling out a psychiatric hold be inappropriate for Ms. Q? What clinical factors and legal issues should a psychiatrist consider when helping medical colleagues provide unwanted treatment to a severely malnourished patient with an eating disorder? We’ll explore these matters as we consider the case of Ms. Q (Figure) and the following questions:
- What type of care is most appropriate for her now?
- Can she refuse medical treatment?
- What are the medicolegal risks of letting her leave the hospital?
- What are the medicolegal risks of detaining and force-feeding her against her will?
- When is a psychiatric “hold” appropriate?
What care is appropriate?
Given her state of self-starvation, Ms. Q’s treatment plan could require close monitoring of her electrolytes and cardiac status, as well as watching her for signs of “refeeding syndrome”—rapid, potentially fatal fluid shifts and metabolic derangements that malnourished patients could experience when they receive artificial refeeding.1
First, the physicians who are caring for Ms. Q should determine whether she needs more intensive medical supervision than is usually available on a psychiatric unit. If she does, but she won’t agree to stay on a medical unit for care, a psychiatric hold is the wrong step, for 2 reasons:
- Once a psychiatric hold has been executed, state statutes require the patient to be placed in a psychiatric facility—a state-approved psychiatric treatment setting, such as a psychiatric unit or free-standing psychiatric hospital—within a specified period.2,3 Most nonpsychiatric medical units would not meet state’s statutory definition for such a facility.
- A psychiatric hold only permits short-term detention. It does not provide legal authority to impose unwanted medical treatment.
Does Ms. Q have capacity?
In the United States, Ms. Q has a legal right to refuse medical care—even if she needs it urgently—provided that her refusal is made competently.4 As Appelbaum and Grisso5 explained in a now-classic 1988 article:
The legal standards for competence include the four related skills of communicating a choice, understanding relevant information, appreciating the current situation and its consequences, and manipulating information rationally.
The Table5 describes these abilities in more detail.
Only courts can make legal determinations of competence, so physicians refer to an evaluation of a patient’s competence-related abilities as a “capacity assessment.” The decision as to whether a patient has capacity ultimately rests with the primary treatment team; however, physicians in other specialties often enlist psychiatrists’ help with this matter because of their interviewing skills and knowledge of how mental illness can impair capacity.
No easy-to-use instrument for evaluating capacity is available. However, Appelbaum6 provides examples of questions that often prove useful in such assessments, and a review by Sessums et al7 on several capacity evaluation tools suggests that the Aid to Capacity Evaluation8 may be the best instrument for performing capacity assessments.
Patients with anorexia nervosa often differ substantially from healthy people in how they assign values to life and death,9 which can make it difficult to evaluate their capacity to refuse life-saving treatment. Malnutrition can alter patients’ ability to think clearly, a phenomenon that some patients with anorexia mention as a reason they are grateful (in retrospect) for the compulsory treatment they received.10 Yet, if an evaluation shows that the patient has the decision-making capacity to refuse care, then her (his) caregivers should carefully document this conclusion and the basis for it. Although caregivers might encourage her to accept the treatment they believe she needs, they should not provide treatment that conflicts with their patient’s wishes.
If evaluation shows that the patient lacks capacity, however, the findings that support this conclusion should be documented clearly. The team then should consult the hospital attorney to determine how to best proceed. The attorney might recommend that a physician on the primary treatment team initiate a “medical hold”—an order that the patient may not leave against medical advice (AMA)—and then seek an emergency guardianship to permit medical treatment, such as refeeding.
To treat or not to treat?
What are the legal risks of allowing Ms. Q to leave AMA before she reaches medical stability?
Powers and Cloak11 describe a case of a 26-year-old woman with anorexia nervosa who came to the hospital with dizziness, weakness, and a very low blood glucose level. She was discharged after 6 days without having received any feeding, only to return to the emergency department 2 days later. This time, she had a letter from her physician stating that she needed medical supervision to start refeeding, yet she was discharged from the emergency department within a few hours. She was re-admitted to the hospital the next day.
Powers and Cloak11 do not report this woman’s medical outcome. But what if she had suffered a fatal cardiac arrhythmia before her third presentation to the emergency department or suffered another injury attributable to her nutritional state: Could her physicians be found at fault?
On Cohen & Associates’ Web site, they essentially answer, “Yes.” They describe a case of “Miss McIntosh,” who had anorexia nervosa and was discharged home from a hospital despite “chronic metabolic problems and not eating properly.” She went into a “hypoglycemic encephalopathic coma” and “suffered irreversible brain damage.” A subsequent lawsuit against the hospital resulted in a 7-figure settlement,12 illustrating the potential for adverse medicolegal consequences if failure to treat a patient with anorexia nervosa could be linked to subsequent physical harm. On the other hand, could a patient with anorexia who is being force-fed take legal action against her providers? At least 3 recent British cases suggest that this is possible.13-15 A British medical student with anorexia, E, made an emergency application to the Court of Protection in London, claiming that being fed against her will was akin to reliving her past experience of sexual abuse. In E’s case, the judge ruled “that the balance tips slowly but unmistakably in the direction of life preserving treatment” and authorized feeding over her objection.6 In 2 other cases, however, British courts have ruled that force-feeding anorexic patients would be futile and disallowed the practice.14,15
Faced with possible legal action, no matter what course you take, how should you respond? Getting legal and ethical consultation is prudent if time allows. In many cases, hospital attorneys might prefer that physicians err on the side of preserving life(D. Vanderpool, MBA, JD, personal communication, February 3, 2016)—even if that means detaining a patient without clear legal authorization to do so—because attorneys would prefer to defend a doctor who acted to save someone’s life than to defend a doctor who knowingly allowed a patient to die.
When might persons with an eating disorder be civilly committed?
Suppose that Ms. Q does not need urgent nonpsychiatric medical care, or that her life-threatening physical problems now have been addressed. Her physicians strongly recommend that she undergo inpatient psychiatric treatment for her eating disorder, but she wants to leave. Would it now be appropriate to fill out paperwork to initiate a psychiatric hold?
All U.S. jurisdictions authorize “civil commitment” proceedings that can lead to involuntary psychiatric hospitalization of people who have a mental disorder and pose a risk to themselves or others because of the disorder.16
In general, to be subject to civil commitment, a person must have a substantial disorder of thought, mood, perception, orientation, or memory. In addition, that disorder must grossly impair her (his) judgment, behavior, reality testing, or ability to meet the demands of everyday life.17
People with psychosis, a severe mood disorder, or dementia often meet these criteria. However, psychiatrists do not usually consider anorexia nervosa to be a thought disorder, mood disorder, or memory disorder. Does this mean that people with anorexia nervosa cannot meet the “substantial” mental disorder criterion?
It does not. Courts interpret the words in statutes based on their “ordinary and natural meaning.”18 If Ms. Q perceived herself as fat, despite having a BMI that was far below the healthy range, most people would regard her thinking to be disordered. If, in addition, her mental disorder impaired her “judgment, behavior, and capacity to meet the ordinary demands of sustaining existence,” then her anorexia nervosa “would qualify as a mental disorder for commitment purposes.”19
To be subject to civil commitment, a person with a substantial mental disorder also must pose a risk of harm to herself or others because of the disorder. That risk can be evidenced via an action, attempt, or threat to do direct physical harm, or it might inhere in the potential for developing grave disability through neglect of one’s basic needs, such as failing to eat adequately. In Ms. Q’s case, if the evidence shows her eating-disordered behavior has placed her at imminent risk of permanent injury or death, she has satisfied the legal criteria that justify court-ordered psychiatric hospitalization.
Bottom Line
When a severely malnourished patient with anorexia nervosa does not agree to allow recommended care, an appropriate clinical response should include judgment about the urgency of the proposed treatment, what treatment setting is best suited to the patient’s condition, and whether the patient has the mental capacity to refuse potentially life-saving care.
1. Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336(7659):1495-1498.
2. Ohio Revised Code §5122.01(F).
3. Oregon Revised Statutes §426.005(c).
4. Schloendorff v Society of New York Hospital, 211 N.Y. 125, 105 N.E. 92 (N1914).
5. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
6. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
7. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
8. Community tools: Aid to Capacity Evaluation (ACE). University of Toronto Joint Centre for Bioethics. http://www.jcb.utoronto.ca/tools/ace_download.shtml. Updated May 8, 2008. Accessed December 21, 2015.
9. Tan J, Hope T, Stewart A. Competence to refuse treatment in anorexia nervosa. Int J Law Psychiatry. 2003;26(6):697-707.
10. Elzakkers IF, Danner UN, Hoek HW, et al. Compulsory treatment in anorexia nervosa: a review. Int J Eat Disord. 2014;47(8):845-852.
11. Powers PS, Cloak NL. Failure to feed patients with anorexia nervosa and other perils and perplexities in the medical care of eating disorder patients. Eat Disord. 2013;21(1):81-89.
12. “Failure to properly treat anorexia nervosa.” Harry S. Cohen & Associates. http://medmal1.com/article/failure-to-properly-treat-anorexia-nervosa. Accessed February 1, 2016.
13. A Local Authority v E. and Others [2012] EWHC 1639 (COP).
14. A NHS Foundation Trust v Ms. X [2014] EWCOP 35.
15. NHS Trust v L [2012] EWHC 2741 (COP).
16. Pinals DA, Mossman D. Evaluation for civil commitment. New York, NY: Oxford University Press; 2011.
17. Castellano-Hoyt DW. Enhancing police response to persons in mental health crisis: providing strategies, communication techniques, and crisis intervention preparation in overcoming institutional challenges. Springfield, IL: Charles C. Thomas Publisher, Ltd; 2003.
18. FDIC v Meyer, 510 U.S. 471 (1994).
19. Appelbaum PS, Rumpf T. Civil commitment of the anorexic patient. Gen Hosp Psychiatry. 1998;20(4):225-230.
1. Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336(7659):1495-1498.
2. Ohio Revised Code §5122.01(F).
3. Oregon Revised Statutes §426.005(c).
4. Schloendorff v Society of New York Hospital, 211 N.Y. 125, 105 N.E. 92 (N1914).
5. Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med. 1988;319(25):1635-1638.
6. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
7. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011;306(4):420-427.
8. Community tools: Aid to Capacity Evaluation (ACE). University of Toronto Joint Centre for Bioethics. http://www.jcb.utoronto.ca/tools/ace_download.shtml. Updated May 8, 2008. Accessed December 21, 2015.
9. Tan J, Hope T, Stewart A. Competence to refuse treatment in anorexia nervosa. Int J Law Psychiatry. 2003;26(6):697-707.
10. Elzakkers IF, Danner UN, Hoek HW, et al. Compulsory treatment in anorexia nervosa: a review. Int J Eat Disord. 2014;47(8):845-852.
11. Powers PS, Cloak NL. Failure to feed patients with anorexia nervosa and other perils and perplexities in the medical care of eating disorder patients. Eat Disord. 2013;21(1):81-89.
12. “Failure to properly treat anorexia nervosa.” Harry S. Cohen & Associates. http://medmal1.com/article/failure-to-properly-treat-anorexia-nervosa. Accessed February 1, 2016.
13. A Local Authority v E. and Others [2012] EWHC 1639 (COP).
14. A NHS Foundation Trust v Ms. X [2014] EWCOP 35.
15. NHS Trust v L [2012] EWHC 2741 (COP).
16. Pinals DA, Mossman D. Evaluation for civil commitment. New York, NY: Oxford University Press; 2011.
17. Castellano-Hoyt DW. Enhancing police response to persons in mental health crisis: providing strategies, communication techniques, and crisis intervention preparation in overcoming institutional challenges. Springfield, IL: Charles C. Thomas Publisher, Ltd; 2003.
18. FDIC v Meyer, 510 U.S. 471 (1994).
19. Appelbaum PS, Rumpf T. Civil commitment of the anorexic patient. Gen Hosp Psychiatry. 1998;20(4):225-230.
A tool to assess behavioral problems in neurocognitive disorder and guide treatment
Non-drug treatment options, such as behavioral techniques and environment adjustment, should be considered before initiating pharmacotherapy in older patients with behavioral deregulation caused by a neurocognitive disorder. Before considering any interventions, including medical therapy, an evaluation and development of a profile of behavioral symptoms is warranted.
The purpose of such a profile is to:
- guide a patient-specific treatment plan
- measure treatment response (whether medication-related or otherwise).
Developing a profile for a patient can lead to a more tailored treatment plan. Such a plan includes identification of mitigating factors for the patient’s behavior and use of specific interventions, with a preference for non-medication interventions.
Profile assessment can guide treatment
The disruptive-behavior profile that I created (Table) can be used as an initial screening device; the score (1 through 4) in each domain indicates the intensity of intervention required. The profile also can be used to evaluate treatment response.
For example, when caring for a person with a neurocognitive disorder with agitation and disruptive behavior, this profile can be used by the caregiver as a reporting tool for the behavioral heath professional providing consultation. Based on this report, the behavioral health professional can evaluate the predisposing, precipitating, and perpetuating factors of the behavioral disturbance, and a treatment plan can be implemented. After interventions are applied, follow-up assessment with the tool can assess the response to the intervention.
The scale can aid in averting overuse of non-specific medication therapy and, if required, can guide pharmacotherapy. This assessment tool can be useful for clinicians providing care for patients with a neurocognitive disorder, not only in choosing treatment, but also to justify clinical rationale.
How does this scale compare with others?
The Neuropsychiatric Inventory (NPI), Behavioral Pathology in Alzheimer’s Disease (Behave-AD), Cohen-Mansfield Agitation Inventory, and Brief Agitation Rating Scale provide valuable information for clinical care. However:
- Use of the NPI in everyday practice is limited; time spent completing the NPI scale remains a significant impediment for the busy clinician.
- Behave-AD requires a higher level of skill for some caregivers to estimate behavioral symptoms and answer questions about severity.
- Cohen-Mansfeld and Brief Agitation Rating Scale provide a limited description of the intensity of behavioral disturbance.
Developing a treatment plan and justifying pharmacotherapy in patients with a neurocognitive disorder is a challenge for clinicians. The scale that I developed aims to (1) assist the busy clinician who must construct a targeted treatment plan and (2) avoid pharmacotherapy when it is unnecessary. If pharmacotherapy is warranted on the basis of any of the domain scores in the profile, it should be documented with a judicious rationale.
Non-drug treatment options, such as behavioral techniques and environment adjustment, should be considered before initiating pharmacotherapy in older patients with behavioral deregulation caused by a neurocognitive disorder. Before considering any interventions, including medical therapy, an evaluation and development of a profile of behavioral symptoms is warranted.
The purpose of such a profile is to:
- guide a patient-specific treatment plan
- measure treatment response (whether medication-related or otherwise).
Developing a profile for a patient can lead to a more tailored treatment plan. Such a plan includes identification of mitigating factors for the patient’s behavior and use of specific interventions, with a preference for non-medication interventions.
Profile assessment can guide treatment
The disruptive-behavior profile that I created (Table) can be used as an initial screening device; the score (1 through 4) in each domain indicates the intensity of intervention required. The profile also can be used to evaluate treatment response.
For example, when caring for a person with a neurocognitive disorder with agitation and disruptive behavior, this profile can be used by the caregiver as a reporting tool for the behavioral heath professional providing consultation. Based on this report, the behavioral health professional can evaluate the predisposing, precipitating, and perpetuating factors of the behavioral disturbance, and a treatment plan can be implemented. After interventions are applied, follow-up assessment with the tool can assess the response to the intervention.
The scale can aid in averting overuse of non-specific medication therapy and, if required, can guide pharmacotherapy. This assessment tool can be useful for clinicians providing care for patients with a neurocognitive disorder, not only in choosing treatment, but also to justify clinical rationale.
How does this scale compare with others?
The Neuropsychiatric Inventory (NPI), Behavioral Pathology in Alzheimer’s Disease (Behave-AD), Cohen-Mansfield Agitation Inventory, and Brief Agitation Rating Scale provide valuable information for clinical care. However:
- Use of the NPI in everyday practice is limited; time spent completing the NPI scale remains a significant impediment for the busy clinician.
- Behave-AD requires a higher level of skill for some caregivers to estimate behavioral symptoms and answer questions about severity.
- Cohen-Mansfeld and Brief Agitation Rating Scale provide a limited description of the intensity of behavioral disturbance.
Developing a treatment plan and justifying pharmacotherapy in patients with a neurocognitive disorder is a challenge for clinicians. The scale that I developed aims to (1) assist the busy clinician who must construct a targeted treatment plan and (2) avoid pharmacotherapy when it is unnecessary. If pharmacotherapy is warranted on the basis of any of the domain scores in the profile, it should be documented with a judicious rationale.
Non-drug treatment options, such as behavioral techniques and environment adjustment, should be considered before initiating pharmacotherapy in older patients with behavioral deregulation caused by a neurocognitive disorder. Before considering any interventions, including medical therapy, an evaluation and development of a profile of behavioral symptoms is warranted.
The purpose of such a profile is to:
- guide a patient-specific treatment plan
- measure treatment response (whether medication-related or otherwise).
Developing a profile for a patient can lead to a more tailored treatment plan. Such a plan includes identification of mitigating factors for the patient’s behavior and use of specific interventions, with a preference for non-medication interventions.
Profile assessment can guide treatment
The disruptive-behavior profile that I created (Table) can be used as an initial screening device; the score (1 through 4) in each domain indicates the intensity of intervention required. The profile also can be used to evaluate treatment response.
For example, when caring for a person with a neurocognitive disorder with agitation and disruptive behavior, this profile can be used by the caregiver as a reporting tool for the behavioral heath professional providing consultation. Based on this report, the behavioral health professional can evaluate the predisposing, precipitating, and perpetuating factors of the behavioral disturbance, and a treatment plan can be implemented. After interventions are applied, follow-up assessment with the tool can assess the response to the intervention.
The scale can aid in averting overuse of non-specific medication therapy and, if required, can guide pharmacotherapy. This assessment tool can be useful for clinicians providing care for patients with a neurocognitive disorder, not only in choosing treatment, but also to justify clinical rationale.
How does this scale compare with others?
The Neuropsychiatric Inventory (NPI), Behavioral Pathology in Alzheimer’s Disease (Behave-AD), Cohen-Mansfield Agitation Inventory, and Brief Agitation Rating Scale provide valuable information for clinical care. However:
- Use of the NPI in everyday practice is limited; time spent completing the NPI scale remains a significant impediment for the busy clinician.
- Behave-AD requires a higher level of skill for some caregivers to estimate behavioral symptoms and answer questions about severity.
- Cohen-Mansfeld and Brief Agitation Rating Scale provide a limited description of the intensity of behavioral disturbance.
Developing a treatment plan and justifying pharmacotherapy in patients with a neurocognitive disorder is a challenge for clinicians. The scale that I developed aims to (1) assist the busy clinician who must construct a targeted treatment plan and (2) avoid pharmacotherapy when it is unnecessary. If pharmacotherapy is warranted on the basis of any of the domain scores in the profile, it should be documented with a judicious rationale.
Extended-release, orally disintegrating mixed amphetamine salts for ADHD: New formulation
An amphetamine-based, extended-release, orally disintegrating tablet for patients age ≥6 diagnosed with attention-deficit/hyperactivity disorder (ADHD) won FDA approval on January 28, 2016 (Table).1
Adzenys XR-ODT is the first extended-release, orally disintegrating tablet for ADHD, Neos Therapeutics, Inc. the drug’s manufacturer, said in a statement.2 The newly approved agent is bioequivalent to Adderall XR (the capsule form of extended-release mixed amphetamine salts), and patients taking Adderall XR can be switched to the new drug. Equivalent dosages of the 2 drugs are outlined on the prescribing information.1
“The novel features of an extended-release orally disintegrating tablet ... make Adzenys XR-ODT attractive for use in both children (6 and older) and adults,” Alice R. Mao, MD, Medical Director, Memorial Park Psychiatry, Houston, Texas, said in the statement.2
As a condition of the approval, Neos must annually report the status of 3 post-marketing studies of children diagnosed with ADHD taking Adzenys XR-ODT, according to the approval letter.2 One is a single-dose, open-label study of children ages 4 and 5; the second is a randomized, double-blind, placebo-controlled titration study of children ages 4 and 5; and the third is a 1-year, open-label safety study of patients ages 4 and 5.
For patients age 6 to 17, the starting dosage is 6.3 mg once daily in the morning; for adults, it is 12.5 mg once daily in the morning, according to the label.1 The medication will be available in 4 other dose strengths: 3.1 mg, 9.4 mg, 15.7 mg, and 18.8 mg.
The most common adverse reactions to the drug among pediatric patients include loss of appetite, insomnia, and abdominal pain. Among adult patients, adverse reactions include dry mouth, loss of appetite, and insomnia.
1. Adzenys XR-ODT [prescription packet]. Grand Prairie, TX: Neos Therapeutics, LP; 2016.
2. Neos Therapeutics announces FDA approval of Adzenys XR-ODT (amphetamine extended-release orally disintegrating tablet) for the treatment of ADHD in patients 6 years and older [news release]. Dallas, TX: Neos Therapeutics, Inc; January 27, 2016. http://investors.neostx.com/phoenix.zhtml?c=254075&p=RssLanding&cat=news&id=2132931. Accessed February 3, 2016.
An amphetamine-based, extended-release, orally disintegrating tablet for patients age ≥6 diagnosed with attention-deficit/hyperactivity disorder (ADHD) won FDA approval on January 28, 2016 (Table).1
Adzenys XR-ODT is the first extended-release, orally disintegrating tablet for ADHD, Neos Therapeutics, Inc. the drug’s manufacturer, said in a statement.2 The newly approved agent is bioequivalent to Adderall XR (the capsule form of extended-release mixed amphetamine salts), and patients taking Adderall XR can be switched to the new drug. Equivalent dosages of the 2 drugs are outlined on the prescribing information.1
“The novel features of an extended-release orally disintegrating tablet ... make Adzenys XR-ODT attractive for use in both children (6 and older) and adults,” Alice R. Mao, MD, Medical Director, Memorial Park Psychiatry, Houston, Texas, said in the statement.2
As a condition of the approval, Neos must annually report the status of 3 post-marketing studies of children diagnosed with ADHD taking Adzenys XR-ODT, according to the approval letter.2 One is a single-dose, open-label study of children ages 4 and 5; the second is a randomized, double-blind, placebo-controlled titration study of children ages 4 and 5; and the third is a 1-year, open-label safety study of patients ages 4 and 5.
For patients age 6 to 17, the starting dosage is 6.3 mg once daily in the morning; for adults, it is 12.5 mg once daily in the morning, according to the label.1 The medication will be available in 4 other dose strengths: 3.1 mg, 9.4 mg, 15.7 mg, and 18.8 mg.
The most common adverse reactions to the drug among pediatric patients include loss of appetite, insomnia, and abdominal pain. Among adult patients, adverse reactions include dry mouth, loss of appetite, and insomnia.
An amphetamine-based, extended-release, orally disintegrating tablet for patients age ≥6 diagnosed with attention-deficit/hyperactivity disorder (ADHD) won FDA approval on January 28, 2016 (Table).1
Adzenys XR-ODT is the first extended-release, orally disintegrating tablet for ADHD, Neos Therapeutics, Inc. the drug’s manufacturer, said in a statement.2 The newly approved agent is bioequivalent to Adderall XR (the capsule form of extended-release mixed amphetamine salts), and patients taking Adderall XR can be switched to the new drug. Equivalent dosages of the 2 drugs are outlined on the prescribing information.1
“The novel features of an extended-release orally disintegrating tablet ... make Adzenys XR-ODT attractive for use in both children (6 and older) and adults,” Alice R. Mao, MD, Medical Director, Memorial Park Psychiatry, Houston, Texas, said in the statement.2
As a condition of the approval, Neos must annually report the status of 3 post-marketing studies of children diagnosed with ADHD taking Adzenys XR-ODT, according to the approval letter.2 One is a single-dose, open-label study of children ages 4 and 5; the second is a randomized, double-blind, placebo-controlled titration study of children ages 4 and 5; and the third is a 1-year, open-label safety study of patients ages 4 and 5.
For patients age 6 to 17, the starting dosage is 6.3 mg once daily in the morning; for adults, it is 12.5 mg once daily in the morning, according to the label.1 The medication will be available in 4 other dose strengths: 3.1 mg, 9.4 mg, 15.7 mg, and 18.8 mg.
The most common adverse reactions to the drug among pediatric patients include loss of appetite, insomnia, and abdominal pain. Among adult patients, adverse reactions include dry mouth, loss of appetite, and insomnia.
1. Adzenys XR-ODT [prescription packet]. Grand Prairie, TX: Neos Therapeutics, LP; 2016.
2. Neos Therapeutics announces FDA approval of Adzenys XR-ODT (amphetamine extended-release orally disintegrating tablet) for the treatment of ADHD in patients 6 years and older [news release]. Dallas, TX: Neos Therapeutics, Inc; January 27, 2016. http://investors.neostx.com/phoenix.zhtml?c=254075&p=RssLanding&cat=news&id=2132931. Accessed February 3, 2016.
1. Adzenys XR-ODT [prescription packet]. Grand Prairie, TX: Neos Therapeutics, LP; 2016.
2. Neos Therapeutics announces FDA approval of Adzenys XR-ODT (amphetamine extended-release orally disintegrating tablet) for the treatment of ADHD in patients 6 years and older [news release]. Dallas, TX: Neos Therapeutics, Inc; January 27, 2016. http://investors.neostx.com/phoenix.zhtml?c=254075&p=RssLanding&cat=news&id=2132931. Accessed February 3, 2016.
We are not ‘psychiatrists’; 'The beauty of the asylum’; Challenges with false-positive urine drug screens
We are not ‘psychiatrists’
I found Dr. Nasrallah’s editorial regarding the future developments in psychiatry interesting (Do you practice sophisticated psychiatry? 10 Proposed foundations of advanced care, From the Editor, Current Psychiatry. August 2015 p. 12-13). As a young psychiatrist in private practice, I understand why the title “psychiatrist” was initially adopted. I am sure that many of my colleagues agree that the word “psyche” is an abstract, confusing concept: How can we claim to treat something that is not part of known human anatomy?
Nevertheless, we need to clarify the specific nature of our work, namely: the diagnosis and treatment of diseases of the brain, considering other medical causes that can present or exacerbate brain nosology, while providing guidance to modify behavior, thus improving the functional, social, and overall lifestyle of our patients.
We need to change our title to what we really are—encephalopathologists, not psychiatrists!
Marios Efstathiou, MD
Psychiatrist, Private Practice
Member, Cyprus Psychiatric Association
Cyprus
'The beauty of the asylum’
I appreciate Dr. Nasrallah’s metaphor of closing asylums to psychosocial abruptio placentae (Needed: A biopsychosocial ‘therapeutic placenta’ for people with schizophrenia, Current Psychiatry. October 2015 pp. 16,19-20). His proposed components of a therapeutic placenta are supported by evidence-based practice and compassion. I wrote a poem about my feelings about this editorial.
Asylum
I inherited an asylum by profession
where past lives listen
when I console a grief stricken heart
watch when medicines are given.
There are names, dates, and why
scribbled on walls begging for closures.
Around me are kindling, plastic
wasting brains waiting for answers.
Where are the lives that belong to them?
Some were sent home alone
others with loved ones, to foster homes.
They had twins, farmed corn, caught
catfish, carved decoys, built roads,
stargazed away from here.
I cried, stumbled when they slept
under bridges, get mugged, homeless
called from morgues, in jail, sent here.
Like a pendulum of serenity, despair
I vacillated from talking to silence
writing then putting away my
prescriptions.
Exhausted I remember past lives
that chattered once with joy and grief.
That is the beauty of the asylum
I inherited this chain of custody
today, I am one among them.
E. Leynes Bautista, MD
Psychiatrist
Lower Shore Clinic
Wicomico Health Department
Salisbury, Maryland
Challenges with false-positive urine drug screens
Drs. Jeffrey Pawlowski’s and Vicki L. Ellingrod’s article, “Urine drug screens: When might a test be false-positive?” (Savvy Psychopharmacology, Current Psychiatry. October 2015 p. 17,22-24), not only was of high clinical relevance, but it also hinted at another issue of crucial importance: namely, not prematurely dismissing a patient’s reports that he (she) has been abstaining from a drug. It is easy for providers to become jaded and assume that patients, particularly those with a history of substance use, are not being truthful when their self-reported abstinence contradicts laboratory results.
I hope that this article encourages us to become intimately familiar with the specifics of the urine drug screens we employ in practice. We owe it to our patients to do so.
Monifa S. Seawell, MD
Assistant Professor of Psychiatry
Morehouse School of Medicine
Atlanta, Georgia
In the article, “Urine drug screens: When might a test be false-positive?”, it was noted that false positives in immunoassays are rare, but that those involving opiates and amphetamines were more common than cocaine-metabolite and cannabinoid false positives. In the Table, the authors noted that dextromethorphan, diphenhydramine, fluoroquinolones, poppy seeds and oil, and rifampin can trigger a false-positive result for opiates.
The importance of false-positive opiate screens cannot be overemphasized, in light of the epidemic of opioid use disorder—especially among clinicians working in a treatment program. Some of the challenging aspects about treating patients with opioid use disorder are:
- high prevalence of the disorder
- diversion of existing medication-assisted treatments (ie, buprenorphine), compliance with treatment
- urine drug monitoring.
The article addressed urine drug screening, particularly cross-reactivity of the different drugs. With buprenorphine treatment, cross-reactivity of the buprenorphine screening assays varies, depending on which assay is being used. In a study comparing the new Lin-Zhi urine buprenorphine enzyme immunoassay (EIA) with the well-known Microgenics cloned enzyme donor immunoassay, investigators concluded that the latter assay generated a higher percentage of opioid cross-reactivity than the former, and that there also was interference from structurally unrelated drugs (ie, chloroquine and hydroxychloroquine).1 The EIA assay demonstrated more highly specific and sensitive detection of buprenorphine, without opioid cross-reactivity.
In a study2 that examined cross-reactivity of naloxone with oxycodone immunoassays, researchers proposed that urine samples with a high naloxone concentration produced higher cross-reactivity with oxycodone. They proposed that such high naloxone concentrations could occur in adulterated or substituted urine when patients have attempted to dissolve buprenorphine in the urine sample to provide the appearance of compliance. The authors mentioned that typical total urine naloxone concentrations are usually quite low for standard buprenorphine formulations, because of their low bioavailability when taken orally. The clinical recommendation in the article states that it is good practice to confirm positive screens with gas chromatography–mass spectrometry tests.
Adegboyega Oyemade, MD, FAPA
Addiction Psychiatrist
Maryland Treatment Centers, Inc.
Baltimore, Maryland
References
1. Melanson SE, Snyder ML, Jarolim P, et al. A new highly specific buprenorphine immunoassay for monitoring buprenorphine compliance and abuse. J Anal Toxicol. 2012;36(3):201-206.
2. Jenkins AJ, Poirier JG 3rd, Juhascik MP. Cross-reactivity of naloxone with oxycodone immunoassays: implications for individuals taking Suboxone. Clin Chem. 2009;55(7):1434-1436.
We are not ‘psychiatrists’
I found Dr. Nasrallah’s editorial regarding the future developments in psychiatry interesting (Do you practice sophisticated psychiatry? 10 Proposed foundations of advanced care, From the Editor, Current Psychiatry. August 2015 p. 12-13). As a young psychiatrist in private practice, I understand why the title “psychiatrist” was initially adopted. I am sure that many of my colleagues agree that the word “psyche” is an abstract, confusing concept: How can we claim to treat something that is not part of known human anatomy?
Nevertheless, we need to clarify the specific nature of our work, namely: the diagnosis and treatment of diseases of the brain, considering other medical causes that can present or exacerbate brain nosology, while providing guidance to modify behavior, thus improving the functional, social, and overall lifestyle of our patients.
We need to change our title to what we really are—encephalopathologists, not psychiatrists!
Marios Efstathiou, MD
Psychiatrist, Private Practice
Member, Cyprus Psychiatric Association
Cyprus
'The beauty of the asylum’
I appreciate Dr. Nasrallah’s metaphor of closing asylums to psychosocial abruptio placentae (Needed: A biopsychosocial ‘therapeutic placenta’ for people with schizophrenia, Current Psychiatry. October 2015 pp. 16,19-20). His proposed components of a therapeutic placenta are supported by evidence-based practice and compassion. I wrote a poem about my feelings about this editorial.
Asylum
I inherited an asylum by profession
where past lives listen
when I console a grief stricken heart
watch when medicines are given.
There are names, dates, and why
scribbled on walls begging for closures.
Around me are kindling, plastic
wasting brains waiting for answers.
Where are the lives that belong to them?
Some were sent home alone
others with loved ones, to foster homes.
They had twins, farmed corn, caught
catfish, carved decoys, built roads,
stargazed away from here.
I cried, stumbled when they slept
under bridges, get mugged, homeless
called from morgues, in jail, sent here.
Like a pendulum of serenity, despair
I vacillated from talking to silence
writing then putting away my
prescriptions.
Exhausted I remember past lives
that chattered once with joy and grief.
That is the beauty of the asylum
I inherited this chain of custody
today, I am one among them.
E. Leynes Bautista, MD
Psychiatrist
Lower Shore Clinic
Wicomico Health Department
Salisbury, Maryland
Challenges with false-positive urine drug screens
Drs. Jeffrey Pawlowski’s and Vicki L. Ellingrod’s article, “Urine drug screens: When might a test be false-positive?” (Savvy Psychopharmacology, Current Psychiatry. October 2015 p. 17,22-24), not only was of high clinical relevance, but it also hinted at another issue of crucial importance: namely, not prematurely dismissing a patient’s reports that he (she) has been abstaining from a drug. It is easy for providers to become jaded and assume that patients, particularly those with a history of substance use, are not being truthful when their self-reported abstinence contradicts laboratory results.
I hope that this article encourages us to become intimately familiar with the specifics of the urine drug screens we employ in practice. We owe it to our patients to do so.
Monifa S. Seawell, MD
Assistant Professor of Psychiatry
Morehouse School of Medicine
Atlanta, Georgia
In the article, “Urine drug screens: When might a test be false-positive?”, it was noted that false positives in immunoassays are rare, but that those involving opiates and amphetamines were more common than cocaine-metabolite and cannabinoid false positives. In the Table, the authors noted that dextromethorphan, diphenhydramine, fluoroquinolones, poppy seeds and oil, and rifampin can trigger a false-positive result for opiates.
The importance of false-positive opiate screens cannot be overemphasized, in light of the epidemic of opioid use disorder—especially among clinicians working in a treatment program. Some of the challenging aspects about treating patients with opioid use disorder are:
- high prevalence of the disorder
- diversion of existing medication-assisted treatments (ie, buprenorphine), compliance with treatment
- urine drug monitoring.
The article addressed urine drug screening, particularly cross-reactivity of the different drugs. With buprenorphine treatment, cross-reactivity of the buprenorphine screening assays varies, depending on which assay is being used. In a study comparing the new Lin-Zhi urine buprenorphine enzyme immunoassay (EIA) with the well-known Microgenics cloned enzyme donor immunoassay, investigators concluded that the latter assay generated a higher percentage of opioid cross-reactivity than the former, and that there also was interference from structurally unrelated drugs (ie, chloroquine and hydroxychloroquine).1 The EIA assay demonstrated more highly specific and sensitive detection of buprenorphine, without opioid cross-reactivity.
In a study2 that examined cross-reactivity of naloxone with oxycodone immunoassays, researchers proposed that urine samples with a high naloxone concentration produced higher cross-reactivity with oxycodone. They proposed that such high naloxone concentrations could occur in adulterated or substituted urine when patients have attempted to dissolve buprenorphine in the urine sample to provide the appearance of compliance. The authors mentioned that typical total urine naloxone concentrations are usually quite low for standard buprenorphine formulations, because of their low bioavailability when taken orally. The clinical recommendation in the article states that it is good practice to confirm positive screens with gas chromatography–mass spectrometry tests.
Adegboyega Oyemade, MD, FAPA
Addiction Psychiatrist
Maryland Treatment Centers, Inc.
Baltimore, Maryland
References
1. Melanson SE, Snyder ML, Jarolim P, et al. A new highly specific buprenorphine immunoassay for monitoring buprenorphine compliance and abuse. J Anal Toxicol. 2012;36(3):201-206.
2. Jenkins AJ, Poirier JG 3rd, Juhascik MP. Cross-reactivity of naloxone with oxycodone immunoassays: implications for individuals taking Suboxone. Clin Chem. 2009;55(7):1434-1436.
We are not ‘psychiatrists’
I found Dr. Nasrallah’s editorial regarding the future developments in psychiatry interesting (Do you practice sophisticated psychiatry? 10 Proposed foundations of advanced care, From the Editor, Current Psychiatry. August 2015 p. 12-13). As a young psychiatrist in private practice, I understand why the title “psychiatrist” was initially adopted. I am sure that many of my colleagues agree that the word “psyche” is an abstract, confusing concept: How can we claim to treat something that is not part of known human anatomy?
Nevertheless, we need to clarify the specific nature of our work, namely: the diagnosis and treatment of diseases of the brain, considering other medical causes that can present or exacerbate brain nosology, while providing guidance to modify behavior, thus improving the functional, social, and overall lifestyle of our patients.
We need to change our title to what we really are—encephalopathologists, not psychiatrists!
Marios Efstathiou, MD
Psychiatrist, Private Practice
Member, Cyprus Psychiatric Association
Cyprus
'The beauty of the asylum’
I appreciate Dr. Nasrallah’s metaphor of closing asylums to psychosocial abruptio placentae (Needed: A biopsychosocial ‘therapeutic placenta’ for people with schizophrenia, Current Psychiatry. October 2015 pp. 16,19-20). His proposed components of a therapeutic placenta are supported by evidence-based practice and compassion. I wrote a poem about my feelings about this editorial.
Asylum
I inherited an asylum by profession
where past lives listen
when I console a grief stricken heart
watch when medicines are given.
There are names, dates, and why
scribbled on walls begging for closures.
Around me are kindling, plastic
wasting brains waiting for answers.
Where are the lives that belong to them?
Some were sent home alone
others with loved ones, to foster homes.
They had twins, farmed corn, caught
catfish, carved decoys, built roads,
stargazed away from here.
I cried, stumbled when they slept
under bridges, get mugged, homeless
called from morgues, in jail, sent here.
Like a pendulum of serenity, despair
I vacillated from talking to silence
writing then putting away my
prescriptions.
Exhausted I remember past lives
that chattered once with joy and grief.
That is the beauty of the asylum
I inherited this chain of custody
today, I am one among them.
E. Leynes Bautista, MD
Psychiatrist
Lower Shore Clinic
Wicomico Health Department
Salisbury, Maryland
Challenges with false-positive urine drug screens
Drs. Jeffrey Pawlowski’s and Vicki L. Ellingrod’s article, “Urine drug screens: When might a test be false-positive?” (Savvy Psychopharmacology, Current Psychiatry. October 2015 p. 17,22-24), not only was of high clinical relevance, but it also hinted at another issue of crucial importance: namely, not prematurely dismissing a patient’s reports that he (she) has been abstaining from a drug. It is easy for providers to become jaded and assume that patients, particularly those with a history of substance use, are not being truthful when their self-reported abstinence contradicts laboratory results.
I hope that this article encourages us to become intimately familiar with the specifics of the urine drug screens we employ in practice. We owe it to our patients to do so.
Monifa S. Seawell, MD
Assistant Professor of Psychiatry
Morehouse School of Medicine
Atlanta, Georgia
In the article, “Urine drug screens: When might a test be false-positive?”, it was noted that false positives in immunoassays are rare, but that those involving opiates and amphetamines were more common than cocaine-metabolite and cannabinoid false positives. In the Table, the authors noted that dextromethorphan, diphenhydramine, fluoroquinolones, poppy seeds and oil, and rifampin can trigger a false-positive result for opiates.
The importance of false-positive opiate screens cannot be overemphasized, in light of the epidemic of opioid use disorder—especially among clinicians working in a treatment program. Some of the challenging aspects about treating patients with opioid use disorder are:
- high prevalence of the disorder
- diversion of existing medication-assisted treatments (ie, buprenorphine), compliance with treatment
- urine drug monitoring.
The article addressed urine drug screening, particularly cross-reactivity of the different drugs. With buprenorphine treatment, cross-reactivity of the buprenorphine screening assays varies, depending on which assay is being used. In a study comparing the new Lin-Zhi urine buprenorphine enzyme immunoassay (EIA) with the well-known Microgenics cloned enzyme donor immunoassay, investigators concluded that the latter assay generated a higher percentage of opioid cross-reactivity than the former, and that there also was interference from structurally unrelated drugs (ie, chloroquine and hydroxychloroquine).1 The EIA assay demonstrated more highly specific and sensitive detection of buprenorphine, without opioid cross-reactivity.
In a study2 that examined cross-reactivity of naloxone with oxycodone immunoassays, researchers proposed that urine samples with a high naloxone concentration produced higher cross-reactivity with oxycodone. They proposed that such high naloxone concentrations could occur in adulterated or substituted urine when patients have attempted to dissolve buprenorphine in the urine sample to provide the appearance of compliance. The authors mentioned that typical total urine naloxone concentrations are usually quite low for standard buprenorphine formulations, because of their low bioavailability when taken orally. The clinical recommendation in the article states that it is good practice to confirm positive screens with gas chromatography–mass spectrometry tests.
Adegboyega Oyemade, MD, FAPA
Addiction Psychiatrist
Maryland Treatment Centers, Inc.
Baltimore, Maryland
References
1. Melanson SE, Snyder ML, Jarolim P, et al. A new highly specific buprenorphine immunoassay for monitoring buprenorphine compliance and abuse. J Anal Toxicol. 2012;36(3):201-206.
2. Jenkins AJ, Poirier JG 3rd, Juhascik MP. Cross-reactivity of naloxone with oxycodone immunoassays: implications for individuals taking Suboxone. Clin Chem. 2009;55(7):1434-1436.
Flibanserin for hypoactive sexual desire disorder in premenopausal women
Flibanserin, FDA-approved in August 2015, is the first medication approved to treat acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women (Table 1). In clinical trials,1-4 the drug has shown modest efficacy in improving symptoms of low sexual desire (number of satisfying sexual events [SSEs], sexual desire, and overall sexual function). Flibanserin is not indicated to enhance sexual performance, for HSDD in postmenopausal women, or in men.
Clinical implications
Flibanserin could help premenopausal women who have distressing low sexual desire, which must be acquired and generalized:
- “Acquired low sexual desire” means that a patient had an adequate sexual desire that decreased or ceased for an unknown reason.
- “Generalized low sexual desire” means that lack of sexual desire occurs all the time and in all situations, not only with a certain partner or in some situations.
Women taking flibanserin could experience gradually increased sexual desire, increase in SSEs, and decrease of sexual distress. Flibanserin is indicated for long-term use; however, it should be discontinued after 8 weeks if the patient does not report any improvement in symptoms.
The number needed to treat with flibanserin likely would be rather large, but it is not available because of complex outcome measures in clinical trials. Flibanserin was not approved at 2 previous FDA committee hearings—mainly because of safety issues but also because of concerns about efficacy. For example, during the 2013 FDA hearing, the results presented showed statistically significant, but numerically small, treatment differences at 24 weeks compared with placebo. In an FDA responder analysis of the Phase-III trials, after accounting for the placebo effect, approximately 8% to 13% women were at least “much improved” on at least 1 of the primary outcomes.5
Flibanserin is not indicated for women whose sexual desire is due to (1) coexisting medical or psychiatric condition, (2) effects of medication or substance abuse, or (3) a relationship problem. It is unknown whether supplemental treatment would help these patients; however, it seems reasonable that combining flibanserin with psychosocial treatment, such as sex therapy or individual therapy, could be beneficial because it may be difficult to disentangle sexual dysfunction and relationship issues—2 problems that often are interwoven.
How it works
Flibanserin is a serotonin 1A receptor agonist and serotonin 2A receptor antagonist. In vitro, flibanserin demonstrated high affinity for the following 5-HT receptors:
- agonist activity at 5-HT1A
- antagonist activity at 5-HT2A, mostly in the prefrontal cortex.
Flibanserin also has moderate antagonist activities at the 5-HT2B, 5-HT2C, and dopamine D4 receptors. Flibanserin presumably acts centrally in the CNS; it has been suggested that flibanserin could rebalance neural circuitry involved in processing sexual desire by reducing serotonin activity and enhancing dopamine and epinephrine activity. The exact mechanism of how flibanserin improves sexual desire in women is unknown.
Pharmacokinetics
Flibanserin has a mean termination half-life of approximately 11 hours. It is administered once a day (50 to 100 mg) at bedtime. Steady state in healthy women was achieved after 3 days. Based on clinical observations, onset of action seems to be gradual and reaches maximum efficacy in approximately 8 weeks. Patients should discontinue the drug if no improvement is reported after 8 weeks. Flibanserin is readily absorbed from the gastrointestinal tract; however, food slows its absorption. The drug is 98% protein (mostly albumin)-bound.
Flibanserin is primarily metabolized in the liver by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP2C19. Co-administration of moderate (diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil) or strong (eg, ketoconazole, clarithromycin, nefazodone, ritonavir) CYP3A4 inhibitors increases the concentration of flibanserin. This could lead to severe hypotension and syncope; therefore, co-administering flibanserin with a strong CYP3A4 inhibitor is contraindicated. Grapefruit juice is a moderate inhibitor of CYP3A4, and in a study of 26 healthy females, 240 mL of grapefruit juice increased flibanserin concentration 1.4-fold. Flibanserin is excreted though urine and feces. Flibanserin should be taken once a day at bedtime because of sedation, somnolence, and possible syncope.
Efficacy
The efficacy of flibanserin for treating HSDD was established in three 24-week, randomized, double-blind, placebo-controlled studies (Table 2). The target population in these studies was premenopausal women (mean age 36, range 19 to 55) with acquired HSDD lasting at least 6 months (mean duration, approximately 5 years). The 3 studies included 1,187 women who received flibanserin, 100 mg at bedtime, and 1,188 women who received placebo. Participants were mostly white (88.6%), and included black (9.6%) and Asian (1.5%) women. The completion rates were 69% for flibanserin and 78% for placebo. Some of the trials included arms with a lower dosage of flibanserin (25 mg and 50 mg), which are not included in this analysis.
As noted in the package insert, these trials each had 2 co-primary efficacy endpoints, SSEs and sexual desire:
- change from baseline to Week 24 in the number of monthly SSEs (ie, sexual intercourse, oral sex, masturbation, or genital stimulation by the partner)
- change in sexual desire from baseline to 24-week endpoint.
In Study 1 and 2, change in sexual desire from baseline to Week 24 was measured daily by using an electronic diary. Every day, patients rated their sexual desire level by answering the question, “Indicate your most intense level of sexual desire” from 0 (no desire) to 3 (strong desire). These responses were totaled over a 28-day period to yield the monthly sexual desire score, which ranged from 0 to 84. These 2 studies also used the Female Sexual Function Index (FSFI) Desire domain as a secondary endpoint.
Study 3 used the FSFI Desire domain, comprising 2 questions, as the sexual desire co-primary endpoint:
- “Over the past 4 weeks, how often did you feel sexual desire or interest?” Responses ranged from 1 (almost never or never) to 5 (almost always or always).
- “Over the past 4 weeks, how would you rate your level (degree) of sexual desire or interest?” Responses ranged from 1 (very low or none at all) to 5 (very high).
In all 3 trials, flibanserin was associated with a small, yet statistically significant, improvement in change in monthly SSEs from baseline to Week 24 compared with placebo. In Study 1 and 2, there were no statistically significant differences between flibanserin and placebo for the electronic diary sexual desire endpoint. In the third study, there was statistically significant improvement in the change in sexual desire using the FSFI Desire domain with flibanserin compared with placebo. The FSFI Desire domain findings were consistent across all 3 trials. Flibanserin was associated with a decrease in sexual distress compared with placebo in all 3 studies.
Tolerability
Flibanserin was well tolerated in the 3 clinical trials. As the FDA noted, clinical trials are conducted under widely varying conditions and therefore adverse reaction rates observed in trials of flibanserin cannot be directly compared with those reported in clinical trials of another drug and might not reflect rates observed in clinical practice.
The discontinuation rate due to adverse reactions was 13% among patients treated with flibanserin, 100 mg at bedtime, and 6% among those taking placebo. The most common side effects were somnolence, dizziness, fatigue, nausea, insomnia, and dry mouth, which appear dose-dependent. Onset of most of these adverse events was within 14 days after the start of treatment.
Although hypotension and syncope rarely were seen with flibanserin alone in clinical trials, these adverse events occurred more frequently in the morning and when taken with alcohol and with some drugs (moderate or strong CYP3A4 inhibitors), and in patients with hepatic impairment. Therefore, women who drink alcohol or take a moderate or strong inhibitor of CYP3A4—both of which are contraindicated—and those with hepatic impairment should not take flibanserin.
Flibanserin should be taken at bedtime, because the risk of hypotension and syncope is higher when flibanserin is taken in the morning and because of associated sedation and somnolence.
Unique clinical issues
Flibanserin is the first FDA-approved medication for treating HSDD. It is important to note that the drug originally was developed as an antidepressant, but failed to show efficacy. Researchers noted that the drug was more effective than placebo when patients were asked, “How strong is your sexual desire?” The focus of development then shifted to a potential treatment of HSDD.
Flibanserin was not approved at 2 previous FDA hearings, mainly because of safety concerns. For the second hearing, the manufacturer, Boehringer Ingelheim, which sold the rights to the drug to Sprout Pharmaceuticals in 2011,6 did not present any new efficacy data, but provided additional safety data, such as research suggesting the absence of next-day driving impairment and data related to alcohol use (the study confirming hypotension associated with alcohol abuse used a small sample, and only 2 of 25 participants were women).
Contraindications
Flibanserin is contraindicated in patients using alcohol because of an increased risk of hypotension and syncope. A patient’s alcohol use should be evaluated before administering flibanserin, and patients should be counseled about the importance of abstaining from alcohol.
Similarly, concomitant use of flibanserin with a moderate or strong inhibitor of CYP3A4 increases the concentration of flibanserin and raises the risk of hypotension and syncope. Therefore, the use of a moderate or strong inhibitor of CYP3A4 in patients taking flibanserin is contraindicated. Similarly, patients with liver impairment should not take this drug.
Strong CYP2C19 inhibitors (proton-pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, antifungals) could increase flibanserin exposure, which may increase risk of hypotension, syncope, and CNS depression. Discuss these risks with your patients; doing so is particularly important when treating women of Chinese heritage, and some other Asian women, because 20% of these populations are genotypic CYP2C19 poor metabolizers.
Because of the increased risk of hypotension and syncope with alcohol use, flibanserin is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Addyi REMS Program. Flibanserin can be prescribed or dispensed only by physicians and pharmacists who watch this program’s online slide presentation and passed a comprehension test.a
Pregnant women should not take flibanserin because the effect on the fetus is unknown. Also, because the interaction with some oral contraceptives is unknown, patients should be cautioned about unwanted pregnancy. Women who are breastfeeding also should avoid using flibanserin because it is not known whether the drug is excreted in breast milk.
Women taking flibanserin also should avoid grapefruit juice, which increases flibanserin levels, and avoid using herbal products, resveratrol, and some over-the-counter drugs such as cimetidine. Women who have a depressive disorder also should avoid using flibanserin because their low sexual desire is more likely due to depression, which is not a therapeutic target for the drug.
Dosing
Flibanserin is provided in 100-mg film-coated tablets. It should be taken once a day at bedtime; titration is unnecessary. Length of treatment has not been determined, but it is recommended that patients stop flibanserin if they do not experience any benefit after 8 weeks. Although there is no guidance in the prescribing information, the medication probably could be stopped without tapering because withdrawal effects have not been observed.
Bottom Line
Flibanserin is FDA-approved for treating generalized, acquired hypoactive sexual desire disorder in premenopausal women. In clinical trials, the drug increased the number of satisfying sexual events and sexual desire, as measured by a diary and rating scales. Alcohol use and use of any moderate or strong inhibitor of cytochrome P450 3A4 are contraindicated in patients taking flibanserin because of an increased risk of hypotension and syncope.
1. Goldfisher ER, Breaux J, Katz M, et al. Continued efficacy and safety of flibanserin in premenopausal women with Hypoactive Sexual desire Disorder (HSDD): results from a randomized withdrawal trial. J Sex Med. 2011;8(11):3160- 3172.
2. Thorp J, Simon J, Dattani D, et al; DAISY trial investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J Sex Med. 2012;9(3):793-804.
3. Derogatis LR, Komer L, Katz M, et al; VIOLET Trial Investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the VIOLET study. J Sex Med. 2012;9(4):1074-1085.
4. Katz M, DeRogatis LR, Ackerman R, et al; BEGONIA trial investigators. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med. 2013;10(7):1807-1815.
5. Gellad WF, Flynn KE, Alexander GC. Evaluation of flibanserin: science and advocacy at the FDA. JAMA. 2015;314(9):869-870
6. Joffe HV, Chang C, Sewell C, et al. FDA approval of flibanserin—treating hypoactive sexual desire disorder. N Engl J Med. 2016;374(2):101-104.
Flibanserin, FDA-approved in August 2015, is the first medication approved to treat acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women (Table 1). In clinical trials,1-4 the drug has shown modest efficacy in improving symptoms of low sexual desire (number of satisfying sexual events [SSEs], sexual desire, and overall sexual function). Flibanserin is not indicated to enhance sexual performance, for HSDD in postmenopausal women, or in men.
Clinical implications
Flibanserin could help premenopausal women who have distressing low sexual desire, which must be acquired and generalized:
- “Acquired low sexual desire” means that a patient had an adequate sexual desire that decreased or ceased for an unknown reason.
- “Generalized low sexual desire” means that lack of sexual desire occurs all the time and in all situations, not only with a certain partner or in some situations.
Women taking flibanserin could experience gradually increased sexual desire, increase in SSEs, and decrease of sexual distress. Flibanserin is indicated for long-term use; however, it should be discontinued after 8 weeks if the patient does not report any improvement in symptoms.
The number needed to treat with flibanserin likely would be rather large, but it is not available because of complex outcome measures in clinical trials. Flibanserin was not approved at 2 previous FDA committee hearings—mainly because of safety issues but also because of concerns about efficacy. For example, during the 2013 FDA hearing, the results presented showed statistically significant, but numerically small, treatment differences at 24 weeks compared with placebo. In an FDA responder analysis of the Phase-III trials, after accounting for the placebo effect, approximately 8% to 13% women were at least “much improved” on at least 1 of the primary outcomes.5
Flibanserin is not indicated for women whose sexual desire is due to (1) coexisting medical or psychiatric condition, (2) effects of medication or substance abuse, or (3) a relationship problem. It is unknown whether supplemental treatment would help these patients; however, it seems reasonable that combining flibanserin with psychosocial treatment, such as sex therapy or individual therapy, could be beneficial because it may be difficult to disentangle sexual dysfunction and relationship issues—2 problems that often are interwoven.
How it works
Flibanserin is a serotonin 1A receptor agonist and serotonin 2A receptor antagonist. In vitro, flibanserin demonstrated high affinity for the following 5-HT receptors:
- agonist activity at 5-HT1A
- antagonist activity at 5-HT2A, mostly in the prefrontal cortex.
Flibanserin also has moderate antagonist activities at the 5-HT2B, 5-HT2C, and dopamine D4 receptors. Flibanserin presumably acts centrally in the CNS; it has been suggested that flibanserin could rebalance neural circuitry involved in processing sexual desire by reducing serotonin activity and enhancing dopamine and epinephrine activity. The exact mechanism of how flibanserin improves sexual desire in women is unknown.
Pharmacokinetics
Flibanserin has a mean termination half-life of approximately 11 hours. It is administered once a day (50 to 100 mg) at bedtime. Steady state in healthy women was achieved after 3 days. Based on clinical observations, onset of action seems to be gradual and reaches maximum efficacy in approximately 8 weeks. Patients should discontinue the drug if no improvement is reported after 8 weeks. Flibanserin is readily absorbed from the gastrointestinal tract; however, food slows its absorption. The drug is 98% protein (mostly albumin)-bound.
Flibanserin is primarily metabolized in the liver by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP2C19. Co-administration of moderate (diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil) or strong (eg, ketoconazole, clarithromycin, nefazodone, ritonavir) CYP3A4 inhibitors increases the concentration of flibanserin. This could lead to severe hypotension and syncope; therefore, co-administering flibanserin with a strong CYP3A4 inhibitor is contraindicated. Grapefruit juice is a moderate inhibitor of CYP3A4, and in a study of 26 healthy females, 240 mL of grapefruit juice increased flibanserin concentration 1.4-fold. Flibanserin is excreted though urine and feces. Flibanserin should be taken once a day at bedtime because of sedation, somnolence, and possible syncope.
Efficacy
The efficacy of flibanserin for treating HSDD was established in three 24-week, randomized, double-blind, placebo-controlled studies (Table 2). The target population in these studies was premenopausal women (mean age 36, range 19 to 55) with acquired HSDD lasting at least 6 months (mean duration, approximately 5 years). The 3 studies included 1,187 women who received flibanserin, 100 mg at bedtime, and 1,188 women who received placebo. Participants were mostly white (88.6%), and included black (9.6%) and Asian (1.5%) women. The completion rates were 69% for flibanserin and 78% for placebo. Some of the trials included arms with a lower dosage of flibanserin (25 mg and 50 mg), which are not included in this analysis.
As noted in the package insert, these trials each had 2 co-primary efficacy endpoints, SSEs and sexual desire:
- change from baseline to Week 24 in the number of monthly SSEs (ie, sexual intercourse, oral sex, masturbation, or genital stimulation by the partner)
- change in sexual desire from baseline to 24-week endpoint.
In Study 1 and 2, change in sexual desire from baseline to Week 24 was measured daily by using an electronic diary. Every day, patients rated their sexual desire level by answering the question, “Indicate your most intense level of sexual desire” from 0 (no desire) to 3 (strong desire). These responses were totaled over a 28-day period to yield the monthly sexual desire score, which ranged from 0 to 84. These 2 studies also used the Female Sexual Function Index (FSFI) Desire domain as a secondary endpoint.
Study 3 used the FSFI Desire domain, comprising 2 questions, as the sexual desire co-primary endpoint:
- “Over the past 4 weeks, how often did you feel sexual desire or interest?” Responses ranged from 1 (almost never or never) to 5 (almost always or always).
- “Over the past 4 weeks, how would you rate your level (degree) of sexual desire or interest?” Responses ranged from 1 (very low or none at all) to 5 (very high).
In all 3 trials, flibanserin was associated with a small, yet statistically significant, improvement in change in monthly SSEs from baseline to Week 24 compared with placebo. In Study 1 and 2, there were no statistically significant differences between flibanserin and placebo for the electronic diary sexual desire endpoint. In the third study, there was statistically significant improvement in the change in sexual desire using the FSFI Desire domain with flibanserin compared with placebo. The FSFI Desire domain findings were consistent across all 3 trials. Flibanserin was associated with a decrease in sexual distress compared with placebo in all 3 studies.
Tolerability
Flibanserin was well tolerated in the 3 clinical trials. As the FDA noted, clinical trials are conducted under widely varying conditions and therefore adverse reaction rates observed in trials of flibanserin cannot be directly compared with those reported in clinical trials of another drug and might not reflect rates observed in clinical practice.
The discontinuation rate due to adverse reactions was 13% among patients treated with flibanserin, 100 mg at bedtime, and 6% among those taking placebo. The most common side effects were somnolence, dizziness, fatigue, nausea, insomnia, and dry mouth, which appear dose-dependent. Onset of most of these adverse events was within 14 days after the start of treatment.
Although hypotension and syncope rarely were seen with flibanserin alone in clinical trials, these adverse events occurred more frequently in the morning and when taken with alcohol and with some drugs (moderate or strong CYP3A4 inhibitors), and in patients with hepatic impairment. Therefore, women who drink alcohol or take a moderate or strong inhibitor of CYP3A4—both of which are contraindicated—and those with hepatic impairment should not take flibanserin.
Flibanserin should be taken at bedtime, because the risk of hypotension and syncope is higher when flibanserin is taken in the morning and because of associated sedation and somnolence.
Unique clinical issues
Flibanserin is the first FDA-approved medication for treating HSDD. It is important to note that the drug originally was developed as an antidepressant, but failed to show efficacy. Researchers noted that the drug was more effective than placebo when patients were asked, “How strong is your sexual desire?” The focus of development then shifted to a potential treatment of HSDD.
Flibanserin was not approved at 2 previous FDA hearings, mainly because of safety concerns. For the second hearing, the manufacturer, Boehringer Ingelheim, which sold the rights to the drug to Sprout Pharmaceuticals in 2011,6 did not present any new efficacy data, but provided additional safety data, such as research suggesting the absence of next-day driving impairment and data related to alcohol use (the study confirming hypotension associated with alcohol abuse used a small sample, and only 2 of 25 participants were women).
Contraindications
Flibanserin is contraindicated in patients using alcohol because of an increased risk of hypotension and syncope. A patient’s alcohol use should be evaluated before administering flibanserin, and patients should be counseled about the importance of abstaining from alcohol.
Similarly, concomitant use of flibanserin with a moderate or strong inhibitor of CYP3A4 increases the concentration of flibanserin and raises the risk of hypotension and syncope. Therefore, the use of a moderate or strong inhibitor of CYP3A4 in patients taking flibanserin is contraindicated. Similarly, patients with liver impairment should not take this drug.
Strong CYP2C19 inhibitors (proton-pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, antifungals) could increase flibanserin exposure, which may increase risk of hypotension, syncope, and CNS depression. Discuss these risks with your patients; doing so is particularly important when treating women of Chinese heritage, and some other Asian women, because 20% of these populations are genotypic CYP2C19 poor metabolizers.
Because of the increased risk of hypotension and syncope with alcohol use, flibanserin is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Addyi REMS Program. Flibanserin can be prescribed or dispensed only by physicians and pharmacists who watch this program’s online slide presentation and passed a comprehension test.a
Pregnant women should not take flibanserin because the effect on the fetus is unknown. Also, because the interaction with some oral contraceptives is unknown, patients should be cautioned about unwanted pregnancy. Women who are breastfeeding also should avoid using flibanserin because it is not known whether the drug is excreted in breast milk.
Women taking flibanserin also should avoid grapefruit juice, which increases flibanserin levels, and avoid using herbal products, resveratrol, and some over-the-counter drugs such as cimetidine. Women who have a depressive disorder also should avoid using flibanserin because their low sexual desire is more likely due to depression, which is not a therapeutic target for the drug.
Dosing
Flibanserin is provided in 100-mg film-coated tablets. It should be taken once a day at bedtime; titration is unnecessary. Length of treatment has not been determined, but it is recommended that patients stop flibanserin if they do not experience any benefit after 8 weeks. Although there is no guidance in the prescribing information, the medication probably could be stopped without tapering because withdrawal effects have not been observed.
Bottom Line
Flibanserin is FDA-approved for treating generalized, acquired hypoactive sexual desire disorder in premenopausal women. In clinical trials, the drug increased the number of satisfying sexual events and sexual desire, as measured by a diary and rating scales. Alcohol use and use of any moderate or strong inhibitor of cytochrome P450 3A4 are contraindicated in patients taking flibanserin because of an increased risk of hypotension and syncope.
Flibanserin, FDA-approved in August 2015, is the first medication approved to treat acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women (Table 1). In clinical trials,1-4 the drug has shown modest efficacy in improving symptoms of low sexual desire (number of satisfying sexual events [SSEs], sexual desire, and overall sexual function). Flibanserin is not indicated to enhance sexual performance, for HSDD in postmenopausal women, or in men.
Clinical implications
Flibanserin could help premenopausal women who have distressing low sexual desire, which must be acquired and generalized:
- “Acquired low sexual desire” means that a patient had an adequate sexual desire that decreased or ceased for an unknown reason.
- “Generalized low sexual desire” means that lack of sexual desire occurs all the time and in all situations, not only with a certain partner or in some situations.
Women taking flibanserin could experience gradually increased sexual desire, increase in SSEs, and decrease of sexual distress. Flibanserin is indicated for long-term use; however, it should be discontinued after 8 weeks if the patient does not report any improvement in symptoms.
The number needed to treat with flibanserin likely would be rather large, but it is not available because of complex outcome measures in clinical trials. Flibanserin was not approved at 2 previous FDA committee hearings—mainly because of safety issues but also because of concerns about efficacy. For example, during the 2013 FDA hearing, the results presented showed statistically significant, but numerically small, treatment differences at 24 weeks compared with placebo. In an FDA responder analysis of the Phase-III trials, after accounting for the placebo effect, approximately 8% to 13% women were at least “much improved” on at least 1 of the primary outcomes.5
Flibanserin is not indicated for women whose sexual desire is due to (1) coexisting medical or psychiatric condition, (2) effects of medication or substance abuse, or (3) a relationship problem. It is unknown whether supplemental treatment would help these patients; however, it seems reasonable that combining flibanserin with psychosocial treatment, such as sex therapy or individual therapy, could be beneficial because it may be difficult to disentangle sexual dysfunction and relationship issues—2 problems that often are interwoven.
How it works
Flibanserin is a serotonin 1A receptor agonist and serotonin 2A receptor antagonist. In vitro, flibanserin demonstrated high affinity for the following 5-HT receptors:
- agonist activity at 5-HT1A
- antagonist activity at 5-HT2A, mostly in the prefrontal cortex.
Flibanserin also has moderate antagonist activities at the 5-HT2B, 5-HT2C, and dopamine D4 receptors. Flibanserin presumably acts centrally in the CNS; it has been suggested that flibanserin could rebalance neural circuitry involved in processing sexual desire by reducing serotonin activity and enhancing dopamine and epinephrine activity. The exact mechanism of how flibanserin improves sexual desire in women is unknown.
Pharmacokinetics
Flibanserin has a mean termination half-life of approximately 11 hours. It is administered once a day (50 to 100 mg) at bedtime. Steady state in healthy women was achieved after 3 days. Based on clinical observations, onset of action seems to be gradual and reaches maximum efficacy in approximately 8 weeks. Patients should discontinue the drug if no improvement is reported after 8 weeks. Flibanserin is readily absorbed from the gastrointestinal tract; however, food slows its absorption. The drug is 98% protein (mostly albumin)-bound.
Flibanserin is primarily metabolized in the liver by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP2C19. Co-administration of moderate (diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil) or strong (eg, ketoconazole, clarithromycin, nefazodone, ritonavir) CYP3A4 inhibitors increases the concentration of flibanserin. This could lead to severe hypotension and syncope; therefore, co-administering flibanserin with a strong CYP3A4 inhibitor is contraindicated. Grapefruit juice is a moderate inhibitor of CYP3A4, and in a study of 26 healthy females, 240 mL of grapefruit juice increased flibanserin concentration 1.4-fold. Flibanserin is excreted though urine and feces. Flibanserin should be taken once a day at bedtime because of sedation, somnolence, and possible syncope.
Efficacy
The efficacy of flibanserin for treating HSDD was established in three 24-week, randomized, double-blind, placebo-controlled studies (Table 2). The target population in these studies was premenopausal women (mean age 36, range 19 to 55) with acquired HSDD lasting at least 6 months (mean duration, approximately 5 years). The 3 studies included 1,187 women who received flibanserin, 100 mg at bedtime, and 1,188 women who received placebo. Participants were mostly white (88.6%), and included black (9.6%) and Asian (1.5%) women. The completion rates were 69% for flibanserin and 78% for placebo. Some of the trials included arms with a lower dosage of flibanserin (25 mg and 50 mg), which are not included in this analysis.
As noted in the package insert, these trials each had 2 co-primary efficacy endpoints, SSEs and sexual desire:
- change from baseline to Week 24 in the number of monthly SSEs (ie, sexual intercourse, oral sex, masturbation, or genital stimulation by the partner)
- change in sexual desire from baseline to 24-week endpoint.
In Study 1 and 2, change in sexual desire from baseline to Week 24 was measured daily by using an electronic diary. Every day, patients rated their sexual desire level by answering the question, “Indicate your most intense level of sexual desire” from 0 (no desire) to 3 (strong desire). These responses were totaled over a 28-day period to yield the monthly sexual desire score, which ranged from 0 to 84. These 2 studies also used the Female Sexual Function Index (FSFI) Desire domain as a secondary endpoint.
Study 3 used the FSFI Desire domain, comprising 2 questions, as the sexual desire co-primary endpoint:
- “Over the past 4 weeks, how often did you feel sexual desire or interest?” Responses ranged from 1 (almost never or never) to 5 (almost always or always).
- “Over the past 4 weeks, how would you rate your level (degree) of sexual desire or interest?” Responses ranged from 1 (very low or none at all) to 5 (very high).
In all 3 trials, flibanserin was associated with a small, yet statistically significant, improvement in change in monthly SSEs from baseline to Week 24 compared with placebo. In Study 1 and 2, there were no statistically significant differences between flibanserin and placebo for the electronic diary sexual desire endpoint. In the third study, there was statistically significant improvement in the change in sexual desire using the FSFI Desire domain with flibanserin compared with placebo. The FSFI Desire domain findings were consistent across all 3 trials. Flibanserin was associated with a decrease in sexual distress compared with placebo in all 3 studies.
Tolerability
Flibanserin was well tolerated in the 3 clinical trials. As the FDA noted, clinical trials are conducted under widely varying conditions and therefore adverse reaction rates observed in trials of flibanserin cannot be directly compared with those reported in clinical trials of another drug and might not reflect rates observed in clinical practice.
The discontinuation rate due to adverse reactions was 13% among patients treated with flibanserin, 100 mg at bedtime, and 6% among those taking placebo. The most common side effects were somnolence, dizziness, fatigue, nausea, insomnia, and dry mouth, which appear dose-dependent. Onset of most of these adverse events was within 14 days after the start of treatment.
Although hypotension and syncope rarely were seen with flibanserin alone in clinical trials, these adverse events occurred more frequently in the morning and when taken with alcohol and with some drugs (moderate or strong CYP3A4 inhibitors), and in patients with hepatic impairment. Therefore, women who drink alcohol or take a moderate or strong inhibitor of CYP3A4—both of which are contraindicated—and those with hepatic impairment should not take flibanserin.
Flibanserin should be taken at bedtime, because the risk of hypotension and syncope is higher when flibanserin is taken in the morning and because of associated sedation and somnolence.
Unique clinical issues
Flibanserin is the first FDA-approved medication for treating HSDD. It is important to note that the drug originally was developed as an antidepressant, but failed to show efficacy. Researchers noted that the drug was more effective than placebo when patients were asked, “How strong is your sexual desire?” The focus of development then shifted to a potential treatment of HSDD.
Flibanserin was not approved at 2 previous FDA hearings, mainly because of safety concerns. For the second hearing, the manufacturer, Boehringer Ingelheim, which sold the rights to the drug to Sprout Pharmaceuticals in 2011,6 did not present any new efficacy data, but provided additional safety data, such as research suggesting the absence of next-day driving impairment and data related to alcohol use (the study confirming hypotension associated with alcohol abuse used a small sample, and only 2 of 25 participants were women).
Contraindications
Flibanserin is contraindicated in patients using alcohol because of an increased risk of hypotension and syncope. A patient’s alcohol use should be evaluated before administering flibanserin, and patients should be counseled about the importance of abstaining from alcohol.
Similarly, concomitant use of flibanserin with a moderate or strong inhibitor of CYP3A4 increases the concentration of flibanserin and raises the risk of hypotension and syncope. Therefore, the use of a moderate or strong inhibitor of CYP3A4 in patients taking flibanserin is contraindicated. Similarly, patients with liver impairment should not take this drug.
Strong CYP2C19 inhibitors (proton-pump inhibitors, selective serotonin reuptake inhibitors, benzodiazepines, antifungals) could increase flibanserin exposure, which may increase risk of hypotension, syncope, and CNS depression. Discuss these risks with your patients; doing so is particularly important when treating women of Chinese heritage, and some other Asian women, because 20% of these populations are genotypic CYP2C19 poor metabolizers.
Because of the increased risk of hypotension and syncope with alcohol use, flibanserin is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the Addyi REMS Program. Flibanserin can be prescribed or dispensed only by physicians and pharmacists who watch this program’s online slide presentation and passed a comprehension test.a
Pregnant women should not take flibanserin because the effect on the fetus is unknown. Also, because the interaction with some oral contraceptives is unknown, patients should be cautioned about unwanted pregnancy. Women who are breastfeeding also should avoid using flibanserin because it is not known whether the drug is excreted in breast milk.
Women taking flibanserin also should avoid grapefruit juice, which increases flibanserin levels, and avoid using herbal products, resveratrol, and some over-the-counter drugs such as cimetidine. Women who have a depressive disorder also should avoid using flibanserin because their low sexual desire is more likely due to depression, which is not a therapeutic target for the drug.
Dosing
Flibanserin is provided in 100-mg film-coated tablets. It should be taken once a day at bedtime; titration is unnecessary. Length of treatment has not been determined, but it is recommended that patients stop flibanserin if they do not experience any benefit after 8 weeks. Although there is no guidance in the prescribing information, the medication probably could be stopped without tapering because withdrawal effects have not been observed.
Bottom Line
Flibanserin is FDA-approved for treating generalized, acquired hypoactive sexual desire disorder in premenopausal women. In clinical trials, the drug increased the number of satisfying sexual events and sexual desire, as measured by a diary and rating scales. Alcohol use and use of any moderate or strong inhibitor of cytochrome P450 3A4 are contraindicated in patients taking flibanserin because of an increased risk of hypotension and syncope.
1. Goldfisher ER, Breaux J, Katz M, et al. Continued efficacy and safety of flibanserin in premenopausal women with Hypoactive Sexual desire Disorder (HSDD): results from a randomized withdrawal trial. J Sex Med. 2011;8(11):3160- 3172.
2. Thorp J, Simon J, Dattani D, et al; DAISY trial investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J Sex Med. 2012;9(3):793-804.
3. Derogatis LR, Komer L, Katz M, et al; VIOLET Trial Investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the VIOLET study. J Sex Med. 2012;9(4):1074-1085.
4. Katz M, DeRogatis LR, Ackerman R, et al; BEGONIA trial investigators. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med. 2013;10(7):1807-1815.
5. Gellad WF, Flynn KE, Alexander GC. Evaluation of flibanserin: science and advocacy at the FDA. JAMA. 2015;314(9):869-870
6. Joffe HV, Chang C, Sewell C, et al. FDA approval of flibanserin—treating hypoactive sexual desire disorder. N Engl J Med. 2016;374(2):101-104.
1. Goldfisher ER, Breaux J, Katz M, et al. Continued efficacy and safety of flibanserin in premenopausal women with Hypoactive Sexual desire Disorder (HSDD): results from a randomized withdrawal trial. J Sex Med. 2011;8(11):3160- 3172.
2. Thorp J, Simon J, Dattani D, et al; DAISY trial investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J Sex Med. 2012;9(3):793-804.
3. Derogatis LR, Komer L, Katz M, et al; VIOLET Trial Investigators. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the VIOLET study. J Sex Med. 2012;9(4):1074-1085.
4. Katz M, DeRogatis LR, Ackerman R, et al; BEGONIA trial investigators. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med. 2013;10(7):1807-1815.
5. Gellad WF, Flynn KE, Alexander GC. Evaluation of flibanserin: science and advocacy at the FDA. JAMA. 2015;314(9):869-870
6. Joffe HV, Chang C, Sewell C, et al. FDA approval of flibanserin—treating hypoactive sexual desire disorder. N Engl J Med. 2016;374(2):101-104.
Tailor chronic pain interventions to the patient’s clinical profile



Existential vs biological depression
RELAPSE: Answers to why a patient is having a new mood episode
A mood disorder is a chronic illness, associated with episodic recurrence over time1,2; when a patient experiences a new mood episode, explore possible underlying causes of that recurrence. The mnemonic RELAPSE can help you take an informed approach to treatment, instead of making reflexive medication changes (Table).
Rhythm disturbances. Seasonal changes, shift work, jet lag, and sleep irregularity can induce a mood episode in a vulnerable patient. Failure of a patient’s circadian clock to resynchronize itself after such disruption in the dark–light cycle can trigger mood symptoms.
Ending treatment. Intentional or unintentional non-adherence to a prescribed medication or psychotherapy can trigger a mood episode. Likewise, switching from a brand-name medication to a generic equivalent can induce a new episode because the generic drug might be as much as 20% less bioavailable than the brand formulation.3
Life change. Some life events, such as divorce or job loss, can be sufficiently overwhelming—despite medical therapy and psychotherapy—to induce a new episode in a vulnerable patient.
Additional drugs. Opiates, interferon, steroids, reserpine, and other drugs can be depressogenic; on the other hand, steroids, anticholinergic agents, and antidepressants can induce mania. If another physician, or the patient, adds a medication or supplement that causes an interaction with the patient’s current psychotropic prescription, the result might be increased metabolism or clearance of the psychotropic—thus decreasing its efficacy and leading to a new mood episode.
Physical health changes. Neurologic conditions (epilepsy, multiple sclerosis, stroke), autoimmune illnesses (eg, lupus), primary sleep disorders (eg, obstructive sleep apnea), and hormone changes (eg, testosterone, estrogen, and thyroid) that can occur over the lifespan of a patient with a mood disorder can trigger a new episode.
Substance use and withdrawal. Chronic use of alcohol and opiates and withdrawal from cocaine and stimulants in a patient with a mood disorder can induce a depressive episode; use of cocaine, stimulants, and caffeine can induce a manic state.
End of drug response. Some patients experience a loss of drug response over time (tachyphylaxis) or a depressive recurrence while taking an antidepressant.4 These phenomena might be caused by brain changes over time. These are a diagnosis of exclusion after other possibilities have been ruled out.
1. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157:229-233.
2. Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry. 2006;163:217-224.
3. Ellingrod VL. How differences among generics might affect your patient’s response. Current Psychiatry. 2010;9(5):31-32,38.
4. Dunlop BW. Depressive recurrence on antidepressant treatment (DRAT): 4 next-step options. Current Psychiatry. 2013;12:54-55.
A mood disorder is a chronic illness, associated with episodic recurrence over time1,2; when a patient experiences a new mood episode, explore possible underlying causes of that recurrence. The mnemonic RELAPSE can help you take an informed approach to treatment, instead of making reflexive medication changes (Table).
Rhythm disturbances. Seasonal changes, shift work, jet lag, and sleep irregularity can induce a mood episode in a vulnerable patient. Failure of a patient’s circadian clock to resynchronize itself after such disruption in the dark–light cycle can trigger mood symptoms.
Ending treatment. Intentional or unintentional non-adherence to a prescribed medication or psychotherapy can trigger a mood episode. Likewise, switching from a brand-name medication to a generic equivalent can induce a new episode because the generic drug might be as much as 20% less bioavailable than the brand formulation.3
Life change. Some life events, such as divorce or job loss, can be sufficiently overwhelming—despite medical therapy and psychotherapy—to induce a new episode in a vulnerable patient.
Additional drugs. Opiates, interferon, steroids, reserpine, and other drugs can be depressogenic; on the other hand, steroids, anticholinergic agents, and antidepressants can induce mania. If another physician, or the patient, adds a medication or supplement that causes an interaction with the patient’s current psychotropic prescription, the result might be increased metabolism or clearance of the psychotropic—thus decreasing its efficacy and leading to a new mood episode.
Physical health changes. Neurologic conditions (epilepsy, multiple sclerosis, stroke), autoimmune illnesses (eg, lupus), primary sleep disorders (eg, obstructive sleep apnea), and hormone changes (eg, testosterone, estrogen, and thyroid) that can occur over the lifespan of a patient with a mood disorder can trigger a new episode.
Substance use and withdrawal. Chronic use of alcohol and opiates and withdrawal from cocaine and stimulants in a patient with a mood disorder can induce a depressive episode; use of cocaine, stimulants, and caffeine can induce a manic state.
End of drug response. Some patients experience a loss of drug response over time (tachyphylaxis) or a depressive recurrence while taking an antidepressant.4 These phenomena might be caused by brain changes over time. These are a diagnosis of exclusion after other possibilities have been ruled out.
A mood disorder is a chronic illness, associated with episodic recurrence over time1,2; when a patient experiences a new mood episode, explore possible underlying causes of that recurrence. The mnemonic RELAPSE can help you take an informed approach to treatment, instead of making reflexive medication changes (Table).
Rhythm disturbances. Seasonal changes, shift work, jet lag, and sleep irregularity can induce a mood episode in a vulnerable patient. Failure of a patient’s circadian clock to resynchronize itself after such disruption in the dark–light cycle can trigger mood symptoms.
Ending treatment. Intentional or unintentional non-adherence to a prescribed medication or psychotherapy can trigger a mood episode. Likewise, switching from a brand-name medication to a generic equivalent can induce a new episode because the generic drug might be as much as 20% less bioavailable than the brand formulation.3
Life change. Some life events, such as divorce or job loss, can be sufficiently overwhelming—despite medical therapy and psychotherapy—to induce a new episode in a vulnerable patient.
Additional drugs. Opiates, interferon, steroids, reserpine, and other drugs can be depressogenic; on the other hand, steroids, anticholinergic agents, and antidepressants can induce mania. If another physician, or the patient, adds a medication or supplement that causes an interaction with the patient’s current psychotropic prescription, the result might be increased metabolism or clearance of the psychotropic—thus decreasing its efficacy and leading to a new mood episode.
Physical health changes. Neurologic conditions (epilepsy, multiple sclerosis, stroke), autoimmune illnesses (eg, lupus), primary sleep disorders (eg, obstructive sleep apnea), and hormone changes (eg, testosterone, estrogen, and thyroid) that can occur over the lifespan of a patient with a mood disorder can trigger a new episode.
Substance use and withdrawal. Chronic use of alcohol and opiates and withdrawal from cocaine and stimulants in a patient with a mood disorder can induce a depressive episode; use of cocaine, stimulants, and caffeine can induce a manic state.
End of drug response. Some patients experience a loss of drug response over time (tachyphylaxis) or a depressive recurrence while taking an antidepressant.4 These phenomena might be caused by brain changes over time. These are a diagnosis of exclusion after other possibilities have been ruled out.
1. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157:229-233.
2. Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry. 2006;163:217-224.
3. Ellingrod VL. How differences among generics might affect your patient’s response. Current Psychiatry. 2010;9(5):31-32,38.
4. Dunlop BW. Depressive recurrence on antidepressant treatment (DRAT): 4 next-step options. Current Psychiatry. 2013;12:54-55.
1. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157:229-233.
2. Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry. 2006;163:217-224.
3. Ellingrod VL. How differences among generics might affect your patient’s response. Current Psychiatry. 2010;9(5):31-32,38.
4. Dunlop BW. Depressive recurrence on antidepressant treatment (DRAT): 4 next-step options. Current Psychiatry. 2013;12:54-55.