User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Obtaining informed consent for research in an acute inpatient psychiatric setting
Conducting clinical research with patients in an acute inpatient psychiatric setting raises possible ethical difficulties, in part because of concern about patients’ ability to give informed consent to participate in research.
We propose the acronym CHECK (for capacity, heredity, ethics, coercion-free, and knowledge) to provide researchers with guidance on the process of addressing informed consent in an acute inpatient setting.
Capacity. Ensure that the patient has the decisional capacity to:
• understand disclosed information about proposed research
• appreciate the impact of participation and nonparticipation
• reason about risks and benefits of participation
• communicate a consistent choice.1
The standards for disclosing information to a potential participant are higher for research than in clinical practice, because patients must understand and accept randomization, placebo control, blinding, and possible exposure to non-approved treatment interventions—yet there is a balance regarding how much information is necessary for consent in a given situation.2
Be mindful that the severity of the patient’s psychiatric illness can impair understanding and insight that might preclude giving informed consent (eg, major depression can produce a slowing of intellectual processes; mania can display distractibility; schizophrenia can compromise decisional capacity because of disorganized thinking or delusions; and neurocognitive disorders can affect the ability to process information).
The MacArthur Competence Assessment Tool for Clinical Research, designed as an aid to assessing capacity, has the most empirical support, although other instruments might be equally or better suited to some situations.1
Heredity. When undertaking human genetic and genomic research, create a precise, robust consent process. Genome sequencing studies can reveal information about the health of patients and their families, provoking discussion about appropriate protections for such data. Informed consent should include:
• how the data will be used now and in the future
• the extent to which patients can control future use of the data
• benefits and risks of participation, including the potential for unknown future risks
• what information, including incidental findings, will be returned to the patient
• what methods will be used to safeguard genetic testing data.3
Ethics. Researchers are bound by a code of ethics:
• Patients have the right to decline participation in research and to withdraw at any stage without prejudice; exclusion recognizes the need to protect those who may be incapable of exercising that right.2 Avoid research with dissenting patients, whether or not they are considered capable.2 Do not routinely invite treatment-refusing patients to participate in research projects, other than in extraordinary circumstances; eg, treatment refusing patients who have been adjudicated as “incompetent,” in which case the court-appointed surrogate decision-maker could be approached for informed consent. You should routinely seek a legal opinion in such a circumstance.
• Unless the research is examining interventions for acute and disabling psychiatric illness, consent should not be sought until patients are well enough to make an informed decision. However, clinical assessment is always needed (despite psychiatric illness category) because it cannot be assumed that psychiatric patients are unable to make such a decision (eg, in some cases, substance abuse should not automatically eliminate a participant, as long as the patient retains adequate cognitive status for informed consent).
• Capacity for consent is not “all-or-nothing,” but is specific to the research paradigm. In cases of impaired decisional capacity, researchers can obtain informed consent by obtaining agreement of family, legal representative, or caregiver; therefore, research with assenting adults, who are nonetheless incapable, is unlikely to be regarded as unethical.2
Coercion-free. Avoid covert pressures:
• Ensure that consent is given freely without coercion or duress. This is important if the participant has a physician-patient relationship with a member of the research team. Exercise caution when research methods involve physical contact. Such contact, in incapable patients—even those who assent— could create a medico-legal conflict (eg, taking a blood sample specific for research purposes without consent could result in a charge of battery).2 When in doubt, seek a legal opinion before enrolling decisionally incapable patients (and/or those adjudicated as incompetent) in research trials.
• Consider that participation be initiated by a third party (eg, an approach from a staff member who is not part of their care team and not involved in the research to ask if the potential participant has made a decision that he wants to have communicated to the researcher4).
• Require that a family member, legal representative, or caregiver be present at the time of consent with decisionally incapacitated patients.
Knowledge. The participant must be given adequate information about the project. Understand consent as an ongoing process occurring within a specific context:
• Give participants a fair explanation of the proposed project, the risks and benefits that might ensue, and, when applicable, what appropriate procedures may be offered if the participant experiences discomfort. If a study is to be blinded, patients must understand and appreciate that they could receive no benefit at all.
• Consider the importance of using appropriate language, repeating information, ensuring adequate time for questions and answers, and providing written material to the patient.2 Avoid leaving the patient alone with an information sheet to avoid coercion, because this risks denying patients the opportunity to participate because they lack the occasion to receive information and ask questions.4 Rather, go over the research consent document item by item with the patient in an iterative process, encouraging questions. Ensure private individual discussion between study team members and the patient to address questions related to the study.4
• Reapproach patients to discuss or revisit consent as needed, because their capacity to provide informed consent may vary over time. This is especially important in CNS illnesses, in which the level of cognitive function is variable. An item such as “consent status” for each encounter can be added to the checklist.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dunn LB, Nowrangi MA, Palmer BW, et al. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2006;163(8): 1323-1334.
2. Fulford KW, Howse K. Ethics of research with psychiatric patients: principles, problems and the primary responsibilities of researchers. J Med Ethics. 1993;19(2):85-91.
3. Kuehn BM. Growing use of genomic data reveals need to improve consent and privacy standards. JAMA. 2013; 309(20):2083-2084.
4. Cameron J, Hart A. Ethical issues in obtaining informed consent for research from those recovering from acute mental health problems: a commentary. Research Ethics Review. 2007;3(4):127-129.
Conducting clinical research with patients in an acute inpatient psychiatric setting raises possible ethical difficulties, in part because of concern about patients’ ability to give informed consent to participate in research.
We propose the acronym CHECK (for capacity, heredity, ethics, coercion-free, and knowledge) to provide researchers with guidance on the process of addressing informed consent in an acute inpatient setting.
Capacity. Ensure that the patient has the decisional capacity to:
• understand disclosed information about proposed research
• appreciate the impact of participation and nonparticipation
• reason about risks and benefits of participation
• communicate a consistent choice.1
The standards for disclosing information to a potential participant are higher for research than in clinical practice, because patients must understand and accept randomization, placebo control, blinding, and possible exposure to non-approved treatment interventions—yet there is a balance regarding how much information is necessary for consent in a given situation.2
Be mindful that the severity of the patient’s psychiatric illness can impair understanding and insight that might preclude giving informed consent (eg, major depression can produce a slowing of intellectual processes; mania can display distractibility; schizophrenia can compromise decisional capacity because of disorganized thinking or delusions; and neurocognitive disorders can affect the ability to process information).
The MacArthur Competence Assessment Tool for Clinical Research, designed as an aid to assessing capacity, has the most empirical support, although other instruments might be equally or better suited to some situations.1
Heredity. When undertaking human genetic and genomic research, create a precise, robust consent process. Genome sequencing studies can reveal information about the health of patients and their families, provoking discussion about appropriate protections for such data. Informed consent should include:
• how the data will be used now and in the future
• the extent to which patients can control future use of the data
• benefits and risks of participation, including the potential for unknown future risks
• what information, including incidental findings, will be returned to the patient
• what methods will be used to safeguard genetic testing data.3
Ethics. Researchers are bound by a code of ethics:
• Patients have the right to decline participation in research and to withdraw at any stage without prejudice; exclusion recognizes the need to protect those who may be incapable of exercising that right.2 Avoid research with dissenting patients, whether or not they are considered capable.2 Do not routinely invite treatment-refusing patients to participate in research projects, other than in extraordinary circumstances; eg, treatment refusing patients who have been adjudicated as “incompetent,” in which case the court-appointed surrogate decision-maker could be approached for informed consent. You should routinely seek a legal opinion in such a circumstance.
• Unless the research is examining interventions for acute and disabling psychiatric illness, consent should not be sought until patients are well enough to make an informed decision. However, clinical assessment is always needed (despite psychiatric illness category) because it cannot be assumed that psychiatric patients are unable to make such a decision (eg, in some cases, substance abuse should not automatically eliminate a participant, as long as the patient retains adequate cognitive status for informed consent).
• Capacity for consent is not “all-or-nothing,” but is specific to the research paradigm. In cases of impaired decisional capacity, researchers can obtain informed consent by obtaining agreement of family, legal representative, or caregiver; therefore, research with assenting adults, who are nonetheless incapable, is unlikely to be regarded as unethical.2
Coercion-free. Avoid covert pressures:
• Ensure that consent is given freely without coercion or duress. This is important if the participant has a physician-patient relationship with a member of the research team. Exercise caution when research methods involve physical contact. Such contact, in incapable patients—even those who assent— could create a medico-legal conflict (eg, taking a blood sample specific for research purposes without consent could result in a charge of battery).2 When in doubt, seek a legal opinion before enrolling decisionally incapable patients (and/or those adjudicated as incompetent) in research trials.
• Consider that participation be initiated by a third party (eg, an approach from a staff member who is not part of their care team and not involved in the research to ask if the potential participant has made a decision that he wants to have communicated to the researcher4).
• Require that a family member, legal representative, or caregiver be present at the time of consent with decisionally incapacitated patients.
Knowledge. The participant must be given adequate information about the project. Understand consent as an ongoing process occurring within a specific context:
• Give participants a fair explanation of the proposed project, the risks and benefits that might ensue, and, when applicable, what appropriate procedures may be offered if the participant experiences discomfort. If a study is to be blinded, patients must understand and appreciate that they could receive no benefit at all.
• Consider the importance of using appropriate language, repeating information, ensuring adequate time for questions and answers, and providing written material to the patient.2 Avoid leaving the patient alone with an information sheet to avoid coercion, because this risks denying patients the opportunity to participate because they lack the occasion to receive information and ask questions.4 Rather, go over the research consent document item by item with the patient in an iterative process, encouraging questions. Ensure private individual discussion between study team members and the patient to address questions related to the study.4
• Reapproach patients to discuss or revisit consent as needed, because their capacity to provide informed consent may vary over time. This is especially important in CNS illnesses, in which the level of cognitive function is variable. An item such as “consent status” for each encounter can be added to the checklist.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Conducting clinical research with patients in an acute inpatient psychiatric setting raises possible ethical difficulties, in part because of concern about patients’ ability to give informed consent to participate in research.
We propose the acronym CHECK (for capacity, heredity, ethics, coercion-free, and knowledge) to provide researchers with guidance on the process of addressing informed consent in an acute inpatient setting.
Capacity. Ensure that the patient has the decisional capacity to:
• understand disclosed information about proposed research
• appreciate the impact of participation and nonparticipation
• reason about risks and benefits of participation
• communicate a consistent choice.1
The standards for disclosing information to a potential participant are higher for research than in clinical practice, because patients must understand and accept randomization, placebo control, blinding, and possible exposure to non-approved treatment interventions—yet there is a balance regarding how much information is necessary for consent in a given situation.2
Be mindful that the severity of the patient’s psychiatric illness can impair understanding and insight that might preclude giving informed consent (eg, major depression can produce a slowing of intellectual processes; mania can display distractibility; schizophrenia can compromise decisional capacity because of disorganized thinking or delusions; and neurocognitive disorders can affect the ability to process information).
The MacArthur Competence Assessment Tool for Clinical Research, designed as an aid to assessing capacity, has the most empirical support, although other instruments might be equally or better suited to some situations.1
Heredity. When undertaking human genetic and genomic research, create a precise, robust consent process. Genome sequencing studies can reveal information about the health of patients and their families, provoking discussion about appropriate protections for such data. Informed consent should include:
• how the data will be used now and in the future
• the extent to which patients can control future use of the data
• benefits and risks of participation, including the potential for unknown future risks
• what information, including incidental findings, will be returned to the patient
• what methods will be used to safeguard genetic testing data.3
Ethics. Researchers are bound by a code of ethics:
• Patients have the right to decline participation in research and to withdraw at any stage without prejudice; exclusion recognizes the need to protect those who may be incapable of exercising that right.2 Avoid research with dissenting patients, whether or not they are considered capable.2 Do not routinely invite treatment-refusing patients to participate in research projects, other than in extraordinary circumstances; eg, treatment refusing patients who have been adjudicated as “incompetent,” in which case the court-appointed surrogate decision-maker could be approached for informed consent. You should routinely seek a legal opinion in such a circumstance.
• Unless the research is examining interventions for acute and disabling psychiatric illness, consent should not be sought until patients are well enough to make an informed decision. However, clinical assessment is always needed (despite psychiatric illness category) because it cannot be assumed that psychiatric patients are unable to make such a decision (eg, in some cases, substance abuse should not automatically eliminate a participant, as long as the patient retains adequate cognitive status for informed consent).
• Capacity for consent is not “all-or-nothing,” but is specific to the research paradigm. In cases of impaired decisional capacity, researchers can obtain informed consent by obtaining agreement of family, legal representative, or caregiver; therefore, research with assenting adults, who are nonetheless incapable, is unlikely to be regarded as unethical.2
Coercion-free. Avoid covert pressures:
• Ensure that consent is given freely without coercion or duress. This is important if the participant has a physician-patient relationship with a member of the research team. Exercise caution when research methods involve physical contact. Such contact, in incapable patients—even those who assent— could create a medico-legal conflict (eg, taking a blood sample specific for research purposes without consent could result in a charge of battery).2 When in doubt, seek a legal opinion before enrolling decisionally incapable patients (and/or those adjudicated as incompetent) in research trials.
• Consider that participation be initiated by a third party (eg, an approach from a staff member who is not part of their care team and not involved in the research to ask if the potential participant has made a decision that he wants to have communicated to the researcher4).
• Require that a family member, legal representative, or caregiver be present at the time of consent with decisionally incapacitated patients.
Knowledge. The participant must be given adequate information about the project. Understand consent as an ongoing process occurring within a specific context:
• Give participants a fair explanation of the proposed project, the risks and benefits that might ensue, and, when applicable, what appropriate procedures may be offered if the participant experiences discomfort. If a study is to be blinded, patients must understand and appreciate that they could receive no benefit at all.
• Consider the importance of using appropriate language, repeating information, ensuring adequate time for questions and answers, and providing written material to the patient.2 Avoid leaving the patient alone with an information sheet to avoid coercion, because this risks denying patients the opportunity to participate because they lack the occasion to receive information and ask questions.4 Rather, go over the research consent document item by item with the patient in an iterative process, encouraging questions. Ensure private individual discussion between study team members and the patient to address questions related to the study.4
• Reapproach patients to discuss or revisit consent as needed, because their capacity to provide informed consent may vary over time. This is especially important in CNS illnesses, in which the level of cognitive function is variable. An item such as “consent status” for each encounter can be added to the checklist.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Dunn LB, Nowrangi MA, Palmer BW, et al. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2006;163(8): 1323-1334.
2. Fulford KW, Howse K. Ethics of research with psychiatric patients: principles, problems and the primary responsibilities of researchers. J Med Ethics. 1993;19(2):85-91.
3. Kuehn BM. Growing use of genomic data reveals need to improve consent and privacy standards. JAMA. 2013; 309(20):2083-2084.
4. Cameron J, Hart A. Ethical issues in obtaining informed consent for research from those recovering from acute mental health problems: a commentary. Research Ethics Review. 2007;3(4):127-129.
1. Dunn LB, Nowrangi MA, Palmer BW, et al. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2006;163(8): 1323-1334.
2. Fulford KW, Howse K. Ethics of research with psychiatric patients: principles, problems and the primary responsibilities of researchers. J Med Ethics. 1993;19(2):85-91.
3. Kuehn BM. Growing use of genomic data reveals need to improve consent and privacy standards. JAMA. 2013; 309(20):2083-2084.
4. Cameron J, Hart A. Ethical issues in obtaining informed consent for research from those recovering from acute mental health problems: a commentary. Research Ethics Review. 2007;3(4):127-129.
Managing psychiatric illness in patients with epilepsy
Patients who have epilepsy have a higher incidence of psychiatric illness than the general population—at a prevalence of 60%.1 Establishing a temporal association and making a psychiatric diagnosis can be vexing, but awareness of potential comorbidities does improve the clinical outcome2 (Box). As this article discusses, psychiatric presentations and ictal disorders can share common pathology and exacerbate one another.3 Their coexistence often results in frequent hospitalization, higher treatment cost, and drug-resistant seizures.4 Risk factors for psychopathology in people who have epilepsy include psychosocial stressors, genetic factors, early age of onset of seizures, and each ictal event.5 Among ictal disorders, temporal-lobe epilepsy confers the highest rate of comorbidity.3
Mood disorders
Mood disorders are the most common psychiatric disorder comorbid with epilepsy (irrespective of age, socioeconomic status, and ethnicity), affecting 43% of patients who have a seizure disorder.5 These disorders present as an ictal aura in 1% of cases; the presence of a comorbid mood disorder implies a more severe form of epilepsy.2 Most mood disorders are underdiagnosed in epilepsy, however, because of the mistaken assumption that depression is a normal reaction to having a seizure disorder.
Interictal depression is the most commonly reported complaint, although dysphoria also can present peri-ictally.6 The severity of depression and the seizure disorder often are directly proportional to each other.1 Decreased levels of serotonin and norepinephrine, or abnormalities in their transport or postsynaptic binding, have been reported in epilepsy and in affective illness.6 MRI studies have documented that patients who have a depressive disorder have more gray-matter loss compared with healthy controls.7 Depression diminishes the quality of seizure remission after medical and surgical interventions for epilepsy.8
Taking a multidisciplinary approach to treating a mood disorder in a patient who has epilepsy might improve ictal and mood outcomes.9 Anhedonia is the most common presenting symptom, but some patients do not meet DSM-5 criteria. Depression exhibits atypically, with fatigue, irritability, poor frustration tolerance, anxiety, and mood lability.6 Self-report screening scales, such as the Neurological Disorders Depression Inventory for Epilepsy, are helpful for making a diagnosis.10
Treatment. Prompt antidepressant treatment is indicated. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are the most common agents in this setting.11 Consider possible cytochrome P450 interactions between antiepileptic drugs (AEDs) and antidepressants; sertraline, citalopram, and escitalopram have the lowest incidence of adverse effects. Because tricyclic antidepressants have proconvulsant properties, they are not commonly prescribed in these patients12 (Table 1).13
Electroconvulsive therapy and vagus nerve stimulation14 are effective interventions in treatment-resistant depression. The efficacy of transcranial magnetic stimulation remains to be clarified.
AEDs can produce psychiatric effects, even in nonconvulsive epilepsies. Twenty-eight percent of cases of depression that are comorbid with epilepsy have an iatrogenic basis, and can be induced by barbiturates, topiramate, vigabatrin, tiagabine, and levetiracetam.13 These adverse effects are a common reason that patients discontinue drug treatment and obtain psychiatric consultation.15
Neurosurgical management of epilepsy carries a low risk of depression compared with pharmacotherapy because the surgery offers better ictal control.16 Because some AEDs have mood-stabilizing properties, discontinuing one might unmask an underlying mood disorder.17
The incidence of adjustment disorder with depressed mood in persons who have epilepsy is 10%; with dysthymia, the incidence is 4%. Adjustment problems with an adverse psychosocial outcome are documented more often in patients who have a long-standing, chronic disorder than in those with a more recent diagnosis.18
Postictal suicidal ideation is more common in persons who have a preexisting mood disorder.6 The rate of suicide among epilepsy patients is 5%, compared with 1.4% in the general population—which is the same rate seen among patients with other psychiatric conditions, but higher than what is observed in many chronic medical conditions.19 Attempted suicide is not a direct result of epilepsy, but is significantly related to underlying psychopathology20; anxiety comorbid with a mood disorder increases the risk of suicide.21
The incidence of bipolar disorder among epilepsy patients is 1.4%.22 Although some AEDs can induce mania and hypomania, valproate and lamotrigine each have mood-stabilizing properties that might prevent such episodes.23
Anxiety disorders
Anxiety. Approximately one-third of epilepsy patients report anxiety. In contrast to what is seen with depression, AEDs do no alleviate anxiety.16,19 Anxiety or fear is the most common ictal-related psychiatric symptom2 making it difficult to differentiate anxiety and a seizure.24
Antidepressants, especially an SSRI, often are the treatment of choice; patients must be warned about the risk of an exacerbation of anxiety precipitated by an antidepressant. Such an adverse reaction might prompt cognitive-behavioral therapy (CBT) or limited use of a benzodiazepine.25
Obsessive-compulsive disorder. The incidence of OCD in epilepsy is 14% to 22%.26 Damage to the orbitofrontal cortex or temporal lobe epilepsy surgery can induce OCD; neurotransmitters involved are serotonin, glutamate, dopamine, and γ-aminobutyric acid (GABA).27 Patients may report obsessive thoughts in the peri-ictal period as well; some AEDs, such as topiramate, have been reported to induce such behaviors.28 Treatment options include CBT, an antidepressant, and, in refractory cases, neurosurgery.29
Psychosis
The prevalence of psychosis is approximately 10% among persons who have epilepsy, and is observed most often in patients who have complex partial seizures.30 Risk factors include a family history of epilepsy or psychosis, temporal lobe epilepsy, a long seizure history, and significant neuropathology.31 Structural abnormalities in the limbic system, especially the hippocampus, predispose patients to psychosis. Abnormal activity of GABA and dopamine are implicated in psychotic symptoms in these patients.32
Depending on the type and focus of the seizure, ictal psychoses present with cognitive and affective symptoms or hallucinations. Delusions can be associated with comorbid traumatic brain injury.32 Postictal psychosis is differentiated from other peri-ictal confusional states by:
• absence of confusion or autonomic dysfunction
• presence of more organized thinking
• absence of EEG changes.33
Alteration of an AED regimen can induce post-ictal psychosis. Iatrogenic psychosis sometimes is observed after right-sided temporal lobe surgery.34
Interictal psychoses probably occur as a result of aberrant nerve regeneration, with an increased concentration of dopamine in the brain after long-term seizure control. Epileptic psychosis is distinguished from schizophrenia by the predominance of visual hallucinations, no alteration of personality or affect, and glial proliferation.35 Some patients exhibit “forced normalization,” in which psychotic features appear after epilepsy has been treated successfully and EEG findings are normalized.36
Management of psychosis in epilepsy includes ensuring the patient’s safety, ruling out medical causes of psychosis, and preventing relapse. Prescribe antipsychotics with caution because many of these agents have epileptogenic potential or can interfere with the hepatic metabolism of AEDs. Quetiapine, risperidone, and haloperidol have low potential for seizure induction; chlorpromazine and clozapine are more likely to precipitate an ictal event.37 Ziprasidone, quetiapine, and aripiprazole often are prescribed for post-ictal and inter-ictal psychoses.38
Sleep disorders
Epilepsy patients often complain about difficulty sleeping, namely:
• 10% to 33% exhibit restless leg syndrome or periodic limb movement disorder
• 10% to 65% have obstructive sleep apnea
• 11% to 28% report excessive daytime sleepiness.3
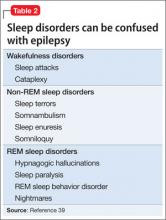
Convulsive activity and the rate of generalization of partial seizures are increased by sleep, especially non-rapid eye movement sleep. Rapid eye movement (REM) sleep suppresses ictal activity, but the pattern of REM sleep is disrupted in epilepsy. Seizures and some sleep disorders present with similar symptoms, such as confusion and amnesia (Table 2).39
Management of comorbid sleep problems includes:
• effective control of seizures
• avoidance of polypharmacy
• assuring sleep hygiene.
Disordered sleep resulting from an AED might be relieved by switching to another medication.39
Substance abuse
Abuse of substances is a significant risk factor for recurrence of seizures.
Alcohol, at a low dose, has antiepileptic properties; intoxication rarely induces a seizure, although seizures often accompany alcohol withdrawal.40
Acute alcohol abuse increases the free level of AEDs by inhibiting 1) microsomal enzyme systems and 2) binding of albumin by metabolites, such as acetaldehyde. These effects can lead to the dangerous outcome of respiratory depression, especially with drugs like phenobarbital.
Chronic alcohol use induces hepatic enzymes, which augments clearance of AEDs, except benzodiazepines. Metabolism of AEDs is decreased because of reduced hepatic blood flow.
Moderate drinking does not increase the incidence of seizures in medication-adherent patients. People who have recurrent alcohol-withdrawal seizures do not have a heightened risk of epilepsy.41
Cannabis. Animal studies have documented the anticonvulsant effect of Cannabis in partial and generalized epilepsy and a proconvulsant effect in absence (petit mal) seizures.42
Tramadol, caffeine. Patients who abuse tramadol or who have an excessive intake of caffeine have a decreased seizure threshold.43
Opiates can exert a proconvulsant or anticonvulsant action, depending on the type of endorphin receptors involved.44
Cocaine decreases the seizure threshold by 1) blocking cerebral GABA receptors and 2) inhibiting dopamine reuptake, thus elevating excitatory neurotransmitters. Cocaine can cause a generalized or focal seizure; the latter is caused by intracerebral stroke or hemorrhage.45
The AEDs topiramate and lamotrigine tend to decrease the desire to abuse alcohol by enhancing inhibitory control by way of decreasing dopamine activity in the mesocorticolimbic system.46
Memory deficits
The relative risk of dementia among epilepsy patients is greater compared with the general population. Recurrent seizures can result in cognitive deficits; epilepsy has been documented in 2% to 64% of Alzheimer’s disease patients.47
Progressive amnesia, with an associated decline in cognition in epilepsy patients despite AED therapy, warrants a dementia workup.48 Patients with an ictal disorder often have difficulty with memory, especially if the hippocampus is affected, such as in temporal lobe epilepsy. Seizures are a common manifestation of several neurodegenerative conditions, and may be associated with a treatable dementia or psychosis in patients with cyanocobalamin deficiency.49
Several memory deficits are associated with seizure disorders:
• Transient epileptic amnesia can be ictal or post-ictal, or can be a manifestation of an underlying seizure disorder. The condition is associated with isolated memory deficits; other cognitive functions usually are intact.
• Accelerated long-term memory deficit occurs when patients forget skills acquired over the past few days or weeks. The problem can be reduced with sleep.50
• Remote memory impairment is characterized by inability to recall personal information from the past.51
When considering a diagnosis of a memory deficit as a manifestation of dementia, keep in mind that cognitive impairment also can develop after epilepsy treatment—although most newer medications cause relatively few such problems.52,53
2-pronged management. It is difficult to establish a temporal association between epilepsy and dementia. When the conditions coexist, appropriate treatment of both is important, because inadequate control of seizures can heighten release of amyloid toxins in the hippocampus. This results in rapidly progressive cognitive decline.54
Neurodevelopmental disorders
The incidence of epilepsy in children who have an autism spectrum disorder is 5% to 38%; the disorder is more common in the presence of mental retardation or cerebral palsy.55
A significant percentage of youth who are referred for evaluation of attention-deficit/ hyperactivity disorder (ADHD) eventually are given a diagnosis of absence seizures. The incidence of ADHD in children with epilepsy is 20%; these patients display epileptiform EEG changes, and require meticulous screening, which includes ictal induction by hyperventilation to differentiate ADHD from a seizure disorder.56 Many AEDs, especially GABAergic drugs, can cause symptoms of ADHD. Methylphenidate is safe in children whose seizures are well-controlled, and has no significant interactions with AEDs.57
Management. Adequate seizure control is the only effective means to slow regression in cases of epilepsy comorbid with autism spectrum disorder, mental retardation, and cerebral palsy.58
BOTTOM LINE
Patients who have epilepsy have a lifetime susceptibility to psychopathology, especially depression and anxiety. Psychiatric practitioners should work collaboratively with patients' primary care provider to evaluate, diagnose, and treat both conditions. Quick action is the key to the best possible outcomes, including reducing the risk of recurrent seizures.
Related Resources
• Clancy MJ, Clarke MC, Connor DJ, et al. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014;14:75.
• Centers for Disease Control and Prevention. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
• Kui C, Yingfu P, Chenling X, et al. What are the predictors of major depression in adult patients with epilepsy? Epileptic Disord. 2014;16(1):74-79.
• Lunde ME, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
Drug Brand Names
Aripiprazole • Abilify Risperidone • Risperdal
Chlorpromazine • Thorazine Quetlapine • Seroquel
Citalopram • Celexa Sertraline • Zoloft
Clozapine • Clozaril, FazaClo Tiagabine • Gabitril
Escitalopram • Lexapro Topiramate • Topamax
Haloperidol • Haldol Tramadol • Ryzolt, Ultram, ConZip
Lamotrigine • Lamictal Valproate • Depokate
Levetiracetam • Keppra Vigabatrin • Sabril
Methylphenidate • Methylin, Ritalin Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Paradiso S, Hermann BP, Blumer D, et al. Impact of depressed mood on neuropsychological status in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(2):180-185.
2. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. 2004;110(4):207-220.
3. Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613-1622.
4. Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs associated with epileptic partial onset seizures among the privately insured in the United States. Epilepsia. 2010;51(5):838-844.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 2005;40(suppl 10): S2-S20.
6. Kanner AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Curr. 2006;6(5):141-146.
7. Salgado PCB, Yasuda CL, Cendes F. Neuroimaging changes in mesial temporal lobe epilepsy are magnified in the presence of depression. Epilepsy Behav. 2010;19(3):422-427.
8. Kanner AM. Psychiatric issues in epilepsy: the complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 2009;15(1):83-87.9. Hedrick SC, Chaney EF, Felker B, et al. Effectiveness of collaborative care depression treatment in Veterans’ Affairs primary care. J Gen Intern Med. 2003;18(1):9-16.
10. Kanner AM. Depression and epilepsy: a bidirectional relation? Epilepsia. 2011;52(suppl 1):21-27.
11. Karouni M, Arulthas S, Larsson PG, et al. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010;66(11):1151-1160.
12. Prueter C, Norra C. Mood disorders and their treatment in patients with epilepsy. J Neuropsychiatry Clin Neurosci. 2005;17(1):20-28.
13. Schmitz B. Antidepressant drugs: indications and guidelines for use in epilepsy. Epilepsia. 2002;43(suppl 2):14-18.
14. Shafique S, Dalsing MC. Vagus nerve stimulation therapy for treatment of drug-resistant epilepsy and depression. Perspect Vasc Surg Endovasc Ther. 2006;18(4):323-327.
15. Schmitz B. Depression and mania in patients with epilepsy. Epilepsia. 2005;46(suppl 4):45-49.
16. Reuber M, Andersen B, Elger CE, et al. Depression and anxiety before and after temporal lobe epilepsy surgery. Seizure. 2004;13(2):129-135.
17. Johannessen Landmark CJ. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27-47.
18. Wiglusz MS, Cubała WJ, Gałuszko-We¸gielnik WG, et al. Mood disorders in epilepsy - diagnostic and methodical considerations. Psychiatr Danub. 2012;24(suppl 1):S44-S50.
19. Jones JE, Hermann BP, Barry JJ, et al. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2003;4(suppl 3):S31-S38.
20. Hara E, Akanuma N, Adachi N, et al. Suicide attempts in adult patients with idiopathic generalized epilepsy. Psychiatry Clin Neurosci. 2009;63(2):225-229.
21. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
22. Mula M, Schmitz B, Jauch R, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13(4): 658-661.
23. Mula M, Monaco F. Antiepileptic drug-induced mania in patients with epilepsy: what do we know? Epilepsy Behav. 2006;9(2):265-267.
24. Kimiskidis VK, Triantafyllou NI, Kararizou E, et al. Depression and anxiety in epilepsy: the association with demographic and seizure-related variables. Ann Gen Psychiatry. 2007;6:28.
25. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
26. Mula M, Cavanna AE, Critchley H, et al. Phenomenology of obsessive compulsive disorder in patients with temporal lobe epilepsy or tourette syndrome. J Neuropsychiatry Clin Neurosci. 2008;20(2):223-226.
27. Fornaro M, Gabrielli F, Albano C, et al. Obsessive-compulsive disorder and related disorders: a comprehensive survey. Ann Gen Psychiatry. 2009;8:13.
28. Thuile J, Even C, Guelfi JD. Topiramate may induce obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2006;60(3):394.
29. Kaplan PW. Epilepsy and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12(2):241-248.
30. Henning OJ, Nakken KO. Psychiatric comorbidity and use of psychotropic drugs in epilepsy patients. Acta Neurol Scand Suppl. 2010;122(suppl 190):18-22.
31. Qin P, Xu H, Laursen TM, et al. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005; 331(7507):23.
32. Kandratavicius L, Lopes-Aguiar C, Bueno-Júnior LS, et al. Psychiatric comorbidities in temporal lobe epilepsy: possible relationships between psychotic disorders and involvement of limbic circuits [Erratum in Rev Bars Psiquiatr. 2013;35(1):107]. Rev Bras Psiquiatr. 2012;34(4):454-466.
33. Lancman ME, Craven WJ, Asconapé JJ, et al. Clinical management of recurrent postictal psychosis. Journal of Epilepsy. 1994;7(1):47-51.
34. Shaw P, Mellers J, Henderson M, et al. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75(7):1003-1008.
35. Perez MM, Trimble MR. Epileptic psychosis–diagnostic comparison with process schizophrenia. Br J Psychiatry. 1980;137:245-249.
36. Krishnamoorthy ES, Trimble MR, Sander JW, et al. Forced normalization at the interface between epilepsy and psychiatry. Epilepsy Behav. 2002;3(4):303-308.
37. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
38. Nadkarni S, Arnedo V, Devinsky O. Psychosis in epilepsy patients. Epilepsia. 2007;48(suppl 9):17-19.
39. Bazil CW. Parasomnias, sleep disorders, and narcolepsy— sleep-time imitators of epilepsy. In: Kaplan PW, Fisher RS, eds. Imitators of epilepsy. 2nd edition. New York, New York: Demos Medical Publishing; 2005:217-230.
40. Chang HJ, Liao CC, Hu CJ, et al. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PloS One. 2013;8(4):e59999.
41. Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42(10):1266-1272.
42. Consroe P. Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol Dis. 1998;5(6, pt B):534-551.
43. Maiga DD, Seyni H, Sidikou A, et al. Convulsive crisis in Tramadol and caffeine abusers: about 8 cases and review of the literature [in French]. Pan Afr Med J. 2012;13:24.
44. Ye JH, Liu PL, Wu WH, et al. Cocaine depresses GABA current of hippocampal neurons. Brain Res. 1997;770(1-2):169-175.
45. Przewłocka B, Stala L, Laso´n W, et al. The effect of various opiate receptor agonists on the seizure threshold in the rat. Is dynorphin an endogenous anticonvulsant? Life Sci. 1983;33(suppl 1):595-598.
46. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370): 1677-1685.
47. Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18(4): 285-294.
48. Cretin B, Blanc F, Gaultier C, et al. Epileptic Amnesic Syndrome revealing Alzheimer’s disease. Epilepsy Res. 2012;102(3):206-209.
49. Vilibié M, Jukié V, Vidovié A, et al. Cobalamin deficiency manifested with seizures, mood oscillations, psychotic features and reversible dementia in the absence of typical neurologic and hematologic signs and symptoms: a case report. Coll Antropol. 2013;37(1):317-319.
50. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008;131(pt 9):2243-2263.
51. Manes F, Hodges JR, Graham KS, et al. Focal autobiographical amnesia in association with transient epileptic amnesia. Brain. 2001;124(pt 3):499-509.
52. Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. 2004;5(4):435-439.
53. Thompson PJ, Baxendale SA, Duncan JS, et al. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69(5):636-641.
54. Noebels JL. A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(suppl 1):39-46.
55. Lewis P, Kopelman MD. Forgetting rates in neuropsychiatric disorders. J Neurol Neurosurg Psychiatry. 1998;65(6):890-898.
56. Tan M, Appleton R. Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy. Arch Dis Child. 2005;90(1):57-59.
57. McBride MC, Wang DD, Torres CF. Methylphenidate in therapeutic doses does not lower seizure threshold [abstract 130]. Ann Neurol. 1986;20(3):428.
58. Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48(suppl 9):33-35.
Patients who have epilepsy have a higher incidence of psychiatric illness than the general population—at a prevalence of 60%.1 Establishing a temporal association and making a psychiatric diagnosis can be vexing, but awareness of potential comorbidities does improve the clinical outcome2 (Box). As this article discusses, psychiatric presentations and ictal disorders can share common pathology and exacerbate one another.3 Their coexistence often results in frequent hospitalization, higher treatment cost, and drug-resistant seizures.4 Risk factors for psychopathology in people who have epilepsy include psychosocial stressors, genetic factors, early age of onset of seizures, and each ictal event.5 Among ictal disorders, temporal-lobe epilepsy confers the highest rate of comorbidity.3
Mood disorders
Mood disorders are the most common psychiatric disorder comorbid with epilepsy (irrespective of age, socioeconomic status, and ethnicity), affecting 43% of patients who have a seizure disorder.5 These disorders present as an ictal aura in 1% of cases; the presence of a comorbid mood disorder implies a more severe form of epilepsy.2 Most mood disorders are underdiagnosed in epilepsy, however, because of the mistaken assumption that depression is a normal reaction to having a seizure disorder.
Interictal depression is the most commonly reported complaint, although dysphoria also can present peri-ictally.6 The severity of depression and the seizure disorder often are directly proportional to each other.1 Decreased levels of serotonin and norepinephrine, or abnormalities in their transport or postsynaptic binding, have been reported in epilepsy and in affective illness.6 MRI studies have documented that patients who have a depressive disorder have more gray-matter loss compared with healthy controls.7 Depression diminishes the quality of seizure remission after medical and surgical interventions for epilepsy.8
Taking a multidisciplinary approach to treating a mood disorder in a patient who has epilepsy might improve ictal and mood outcomes.9 Anhedonia is the most common presenting symptom, but some patients do not meet DSM-5 criteria. Depression exhibits atypically, with fatigue, irritability, poor frustration tolerance, anxiety, and mood lability.6 Self-report screening scales, such as the Neurological Disorders Depression Inventory for Epilepsy, are helpful for making a diagnosis.10
Treatment. Prompt antidepressant treatment is indicated. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are the most common agents in this setting.11 Consider possible cytochrome P450 interactions between antiepileptic drugs (AEDs) and antidepressants; sertraline, citalopram, and escitalopram have the lowest incidence of adverse effects. Because tricyclic antidepressants have proconvulsant properties, they are not commonly prescribed in these patients12 (Table 1).13
Electroconvulsive therapy and vagus nerve stimulation14 are effective interventions in treatment-resistant depression. The efficacy of transcranial magnetic stimulation remains to be clarified.
AEDs can produce psychiatric effects, even in nonconvulsive epilepsies. Twenty-eight percent of cases of depression that are comorbid with epilepsy have an iatrogenic basis, and can be induced by barbiturates, topiramate, vigabatrin, tiagabine, and levetiracetam.13 These adverse effects are a common reason that patients discontinue drug treatment and obtain psychiatric consultation.15
Neurosurgical management of epilepsy carries a low risk of depression compared with pharmacotherapy because the surgery offers better ictal control.16 Because some AEDs have mood-stabilizing properties, discontinuing one might unmask an underlying mood disorder.17
The incidence of adjustment disorder with depressed mood in persons who have epilepsy is 10%; with dysthymia, the incidence is 4%. Adjustment problems with an adverse psychosocial outcome are documented more often in patients who have a long-standing, chronic disorder than in those with a more recent diagnosis.18
Postictal suicidal ideation is more common in persons who have a preexisting mood disorder.6 The rate of suicide among epilepsy patients is 5%, compared with 1.4% in the general population—which is the same rate seen among patients with other psychiatric conditions, but higher than what is observed in many chronic medical conditions.19 Attempted suicide is not a direct result of epilepsy, but is significantly related to underlying psychopathology20; anxiety comorbid with a mood disorder increases the risk of suicide.21
The incidence of bipolar disorder among epilepsy patients is 1.4%.22 Although some AEDs can induce mania and hypomania, valproate and lamotrigine each have mood-stabilizing properties that might prevent such episodes.23
Anxiety disorders
Anxiety. Approximately one-third of epilepsy patients report anxiety. In contrast to what is seen with depression, AEDs do no alleviate anxiety.16,19 Anxiety or fear is the most common ictal-related psychiatric symptom2 making it difficult to differentiate anxiety and a seizure.24
Antidepressants, especially an SSRI, often are the treatment of choice; patients must be warned about the risk of an exacerbation of anxiety precipitated by an antidepressant. Such an adverse reaction might prompt cognitive-behavioral therapy (CBT) or limited use of a benzodiazepine.25
Obsessive-compulsive disorder. The incidence of OCD in epilepsy is 14% to 22%.26 Damage to the orbitofrontal cortex or temporal lobe epilepsy surgery can induce OCD; neurotransmitters involved are serotonin, glutamate, dopamine, and γ-aminobutyric acid (GABA).27 Patients may report obsessive thoughts in the peri-ictal period as well; some AEDs, such as topiramate, have been reported to induce such behaviors.28 Treatment options include CBT, an antidepressant, and, in refractory cases, neurosurgery.29
Psychosis
The prevalence of psychosis is approximately 10% among persons who have epilepsy, and is observed most often in patients who have complex partial seizures.30 Risk factors include a family history of epilepsy or psychosis, temporal lobe epilepsy, a long seizure history, and significant neuropathology.31 Structural abnormalities in the limbic system, especially the hippocampus, predispose patients to psychosis. Abnormal activity of GABA and dopamine are implicated in psychotic symptoms in these patients.32
Depending on the type and focus of the seizure, ictal psychoses present with cognitive and affective symptoms or hallucinations. Delusions can be associated with comorbid traumatic brain injury.32 Postictal psychosis is differentiated from other peri-ictal confusional states by:
• absence of confusion or autonomic dysfunction
• presence of more organized thinking
• absence of EEG changes.33
Alteration of an AED regimen can induce post-ictal psychosis. Iatrogenic psychosis sometimes is observed after right-sided temporal lobe surgery.34
Interictal psychoses probably occur as a result of aberrant nerve regeneration, with an increased concentration of dopamine in the brain after long-term seizure control. Epileptic psychosis is distinguished from schizophrenia by the predominance of visual hallucinations, no alteration of personality or affect, and glial proliferation.35 Some patients exhibit “forced normalization,” in which psychotic features appear after epilepsy has been treated successfully and EEG findings are normalized.36
Management of psychosis in epilepsy includes ensuring the patient’s safety, ruling out medical causes of psychosis, and preventing relapse. Prescribe antipsychotics with caution because many of these agents have epileptogenic potential or can interfere with the hepatic metabolism of AEDs. Quetiapine, risperidone, and haloperidol have low potential for seizure induction; chlorpromazine and clozapine are more likely to precipitate an ictal event.37 Ziprasidone, quetiapine, and aripiprazole often are prescribed for post-ictal and inter-ictal psychoses.38
Sleep disorders
Epilepsy patients often complain about difficulty sleeping, namely:
• 10% to 33% exhibit restless leg syndrome or periodic limb movement disorder
• 10% to 65% have obstructive sleep apnea
• 11% to 28% report excessive daytime sleepiness.3
Convulsive activity and the rate of generalization of partial seizures are increased by sleep, especially non-rapid eye movement sleep. Rapid eye movement (REM) sleep suppresses ictal activity, but the pattern of REM sleep is disrupted in epilepsy. Seizures and some sleep disorders present with similar symptoms, such as confusion and amnesia (Table 2).39
Management of comorbid sleep problems includes:
• effective control of seizures
• avoidance of polypharmacy
• assuring sleep hygiene.
Disordered sleep resulting from an AED might be relieved by switching to another medication.39
Substance abuse
Abuse of substances is a significant risk factor for recurrence of seizures.
Alcohol, at a low dose, has antiepileptic properties; intoxication rarely induces a seizure, although seizures often accompany alcohol withdrawal.40
Acute alcohol abuse increases the free level of AEDs by inhibiting 1) microsomal enzyme systems and 2) binding of albumin by metabolites, such as acetaldehyde. These effects can lead to the dangerous outcome of respiratory depression, especially with drugs like phenobarbital.
Chronic alcohol use induces hepatic enzymes, which augments clearance of AEDs, except benzodiazepines. Metabolism of AEDs is decreased because of reduced hepatic blood flow.
Moderate drinking does not increase the incidence of seizures in medication-adherent patients. People who have recurrent alcohol-withdrawal seizures do not have a heightened risk of epilepsy.41
Cannabis. Animal studies have documented the anticonvulsant effect of Cannabis in partial and generalized epilepsy and a proconvulsant effect in absence (petit mal) seizures.42
Tramadol, caffeine. Patients who abuse tramadol or who have an excessive intake of caffeine have a decreased seizure threshold.43
Opiates can exert a proconvulsant or anticonvulsant action, depending on the type of endorphin receptors involved.44
Cocaine decreases the seizure threshold by 1) blocking cerebral GABA receptors and 2) inhibiting dopamine reuptake, thus elevating excitatory neurotransmitters. Cocaine can cause a generalized or focal seizure; the latter is caused by intracerebral stroke or hemorrhage.45
The AEDs topiramate and lamotrigine tend to decrease the desire to abuse alcohol by enhancing inhibitory control by way of decreasing dopamine activity in the mesocorticolimbic system.46
Memory deficits
The relative risk of dementia among epilepsy patients is greater compared with the general population. Recurrent seizures can result in cognitive deficits; epilepsy has been documented in 2% to 64% of Alzheimer’s disease patients.47
Progressive amnesia, with an associated decline in cognition in epilepsy patients despite AED therapy, warrants a dementia workup.48 Patients with an ictal disorder often have difficulty with memory, especially if the hippocampus is affected, such as in temporal lobe epilepsy. Seizures are a common manifestation of several neurodegenerative conditions, and may be associated with a treatable dementia or psychosis in patients with cyanocobalamin deficiency.49
Several memory deficits are associated with seizure disorders:
• Transient epileptic amnesia can be ictal or post-ictal, or can be a manifestation of an underlying seizure disorder. The condition is associated with isolated memory deficits; other cognitive functions usually are intact.
• Accelerated long-term memory deficit occurs when patients forget skills acquired over the past few days or weeks. The problem can be reduced with sleep.50
• Remote memory impairment is characterized by inability to recall personal information from the past.51
When considering a diagnosis of a memory deficit as a manifestation of dementia, keep in mind that cognitive impairment also can develop after epilepsy treatment—although most newer medications cause relatively few such problems.52,53
2-pronged management. It is difficult to establish a temporal association between epilepsy and dementia. When the conditions coexist, appropriate treatment of both is important, because inadequate control of seizures can heighten release of amyloid toxins in the hippocampus. This results in rapidly progressive cognitive decline.54
Neurodevelopmental disorders
The incidence of epilepsy in children who have an autism spectrum disorder is 5% to 38%; the disorder is more common in the presence of mental retardation or cerebral palsy.55
A significant percentage of youth who are referred for evaluation of attention-deficit/ hyperactivity disorder (ADHD) eventually are given a diagnosis of absence seizures. The incidence of ADHD in children with epilepsy is 20%; these patients display epileptiform EEG changes, and require meticulous screening, which includes ictal induction by hyperventilation to differentiate ADHD from a seizure disorder.56 Many AEDs, especially GABAergic drugs, can cause symptoms of ADHD. Methylphenidate is safe in children whose seizures are well-controlled, and has no significant interactions with AEDs.57
Management. Adequate seizure control is the only effective means to slow regression in cases of epilepsy comorbid with autism spectrum disorder, mental retardation, and cerebral palsy.58
BOTTOM LINE
Patients who have epilepsy have a lifetime susceptibility to psychopathology, especially depression and anxiety. Psychiatric practitioners should work collaboratively with patients' primary care provider to evaluate, diagnose, and treat both conditions. Quick action is the key to the best possible outcomes, including reducing the risk of recurrent seizures.
Related Resources
• Clancy MJ, Clarke MC, Connor DJ, et al. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014;14:75.
• Centers for Disease Control and Prevention. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
• Kui C, Yingfu P, Chenling X, et al. What are the predictors of major depression in adult patients with epilepsy? Epileptic Disord. 2014;16(1):74-79.
• Lunde ME, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
Drug Brand Names
Aripiprazole • Abilify Risperidone • Risperdal
Chlorpromazine • Thorazine Quetlapine • Seroquel
Citalopram • Celexa Sertraline • Zoloft
Clozapine • Clozaril, FazaClo Tiagabine • Gabitril
Escitalopram • Lexapro Topiramate • Topamax
Haloperidol • Haldol Tramadol • Ryzolt, Ultram, ConZip
Lamotrigine • Lamictal Valproate • Depokate
Levetiracetam • Keppra Vigabatrin • Sabril
Methylphenidate • Methylin, Ritalin Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Patients who have epilepsy have a higher incidence of psychiatric illness than the general population—at a prevalence of 60%.1 Establishing a temporal association and making a psychiatric diagnosis can be vexing, but awareness of potential comorbidities does improve the clinical outcome2 (Box). As this article discusses, psychiatric presentations and ictal disorders can share common pathology and exacerbate one another.3 Their coexistence often results in frequent hospitalization, higher treatment cost, and drug-resistant seizures.4 Risk factors for psychopathology in people who have epilepsy include psychosocial stressors, genetic factors, early age of onset of seizures, and each ictal event.5 Among ictal disorders, temporal-lobe epilepsy confers the highest rate of comorbidity.3
Mood disorders
Mood disorders are the most common psychiatric disorder comorbid with epilepsy (irrespective of age, socioeconomic status, and ethnicity), affecting 43% of patients who have a seizure disorder.5 These disorders present as an ictal aura in 1% of cases; the presence of a comorbid mood disorder implies a more severe form of epilepsy.2 Most mood disorders are underdiagnosed in epilepsy, however, because of the mistaken assumption that depression is a normal reaction to having a seizure disorder.
Interictal depression is the most commonly reported complaint, although dysphoria also can present peri-ictally.6 The severity of depression and the seizure disorder often are directly proportional to each other.1 Decreased levels of serotonin and norepinephrine, or abnormalities in their transport or postsynaptic binding, have been reported in epilepsy and in affective illness.6 MRI studies have documented that patients who have a depressive disorder have more gray-matter loss compared with healthy controls.7 Depression diminishes the quality of seizure remission after medical and surgical interventions for epilepsy.8
Taking a multidisciplinary approach to treating a mood disorder in a patient who has epilepsy might improve ictal and mood outcomes.9 Anhedonia is the most common presenting symptom, but some patients do not meet DSM-5 criteria. Depression exhibits atypically, with fatigue, irritability, poor frustration tolerance, anxiety, and mood lability.6 Self-report screening scales, such as the Neurological Disorders Depression Inventory for Epilepsy, are helpful for making a diagnosis.10
Treatment. Prompt antidepressant treatment is indicated. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors are the most common agents in this setting.11 Consider possible cytochrome P450 interactions between antiepileptic drugs (AEDs) and antidepressants; sertraline, citalopram, and escitalopram have the lowest incidence of adverse effects. Because tricyclic antidepressants have proconvulsant properties, they are not commonly prescribed in these patients12 (Table 1).13
Electroconvulsive therapy and vagus nerve stimulation14 are effective interventions in treatment-resistant depression. The efficacy of transcranial magnetic stimulation remains to be clarified.
AEDs can produce psychiatric effects, even in nonconvulsive epilepsies. Twenty-eight percent of cases of depression that are comorbid with epilepsy have an iatrogenic basis, and can be induced by barbiturates, topiramate, vigabatrin, tiagabine, and levetiracetam.13 These adverse effects are a common reason that patients discontinue drug treatment and obtain psychiatric consultation.15
Neurosurgical management of epilepsy carries a low risk of depression compared with pharmacotherapy because the surgery offers better ictal control.16 Because some AEDs have mood-stabilizing properties, discontinuing one might unmask an underlying mood disorder.17
The incidence of adjustment disorder with depressed mood in persons who have epilepsy is 10%; with dysthymia, the incidence is 4%. Adjustment problems with an adverse psychosocial outcome are documented more often in patients who have a long-standing, chronic disorder than in those with a more recent diagnosis.18
Postictal suicidal ideation is more common in persons who have a preexisting mood disorder.6 The rate of suicide among epilepsy patients is 5%, compared with 1.4% in the general population—which is the same rate seen among patients with other psychiatric conditions, but higher than what is observed in many chronic medical conditions.19 Attempted suicide is not a direct result of epilepsy, but is significantly related to underlying psychopathology20; anxiety comorbid with a mood disorder increases the risk of suicide.21
The incidence of bipolar disorder among epilepsy patients is 1.4%.22 Although some AEDs can induce mania and hypomania, valproate and lamotrigine each have mood-stabilizing properties that might prevent such episodes.23
Anxiety disorders
Anxiety. Approximately one-third of epilepsy patients report anxiety. In contrast to what is seen with depression, AEDs do no alleviate anxiety.16,19 Anxiety or fear is the most common ictal-related psychiatric symptom2 making it difficult to differentiate anxiety and a seizure.24
Antidepressants, especially an SSRI, often are the treatment of choice; patients must be warned about the risk of an exacerbation of anxiety precipitated by an antidepressant. Such an adverse reaction might prompt cognitive-behavioral therapy (CBT) or limited use of a benzodiazepine.25
Obsessive-compulsive disorder. The incidence of OCD in epilepsy is 14% to 22%.26 Damage to the orbitofrontal cortex or temporal lobe epilepsy surgery can induce OCD; neurotransmitters involved are serotonin, glutamate, dopamine, and γ-aminobutyric acid (GABA).27 Patients may report obsessive thoughts in the peri-ictal period as well; some AEDs, such as topiramate, have been reported to induce such behaviors.28 Treatment options include CBT, an antidepressant, and, in refractory cases, neurosurgery.29
Psychosis
The prevalence of psychosis is approximately 10% among persons who have epilepsy, and is observed most often in patients who have complex partial seizures.30 Risk factors include a family history of epilepsy or psychosis, temporal lobe epilepsy, a long seizure history, and significant neuropathology.31 Structural abnormalities in the limbic system, especially the hippocampus, predispose patients to psychosis. Abnormal activity of GABA and dopamine are implicated in psychotic symptoms in these patients.32
Depending on the type and focus of the seizure, ictal psychoses present with cognitive and affective symptoms or hallucinations. Delusions can be associated with comorbid traumatic brain injury.32 Postictal psychosis is differentiated from other peri-ictal confusional states by:
• absence of confusion or autonomic dysfunction
• presence of more organized thinking
• absence of EEG changes.33
Alteration of an AED regimen can induce post-ictal psychosis. Iatrogenic psychosis sometimes is observed after right-sided temporal lobe surgery.34
Interictal psychoses probably occur as a result of aberrant nerve regeneration, with an increased concentration of dopamine in the brain after long-term seizure control. Epileptic psychosis is distinguished from schizophrenia by the predominance of visual hallucinations, no alteration of personality or affect, and glial proliferation.35 Some patients exhibit “forced normalization,” in which psychotic features appear after epilepsy has been treated successfully and EEG findings are normalized.36
Management of psychosis in epilepsy includes ensuring the patient’s safety, ruling out medical causes of psychosis, and preventing relapse. Prescribe antipsychotics with caution because many of these agents have epileptogenic potential or can interfere with the hepatic metabolism of AEDs. Quetiapine, risperidone, and haloperidol have low potential for seizure induction; chlorpromazine and clozapine are more likely to precipitate an ictal event.37 Ziprasidone, quetiapine, and aripiprazole often are prescribed for post-ictal and inter-ictal psychoses.38
Sleep disorders
Epilepsy patients often complain about difficulty sleeping, namely:
• 10% to 33% exhibit restless leg syndrome or periodic limb movement disorder
• 10% to 65% have obstructive sleep apnea
• 11% to 28% report excessive daytime sleepiness.3
Convulsive activity and the rate of generalization of partial seizures are increased by sleep, especially non-rapid eye movement sleep. Rapid eye movement (REM) sleep suppresses ictal activity, but the pattern of REM sleep is disrupted in epilepsy. Seizures and some sleep disorders present with similar symptoms, such as confusion and amnesia (Table 2).39
Management of comorbid sleep problems includes:
• effective control of seizures
• avoidance of polypharmacy
• assuring sleep hygiene.
Disordered sleep resulting from an AED might be relieved by switching to another medication.39
Substance abuse
Abuse of substances is a significant risk factor for recurrence of seizures.
Alcohol, at a low dose, has antiepileptic properties; intoxication rarely induces a seizure, although seizures often accompany alcohol withdrawal.40
Acute alcohol abuse increases the free level of AEDs by inhibiting 1) microsomal enzyme systems and 2) binding of albumin by metabolites, such as acetaldehyde. These effects can lead to the dangerous outcome of respiratory depression, especially with drugs like phenobarbital.
Chronic alcohol use induces hepatic enzymes, which augments clearance of AEDs, except benzodiazepines. Metabolism of AEDs is decreased because of reduced hepatic blood flow.
Moderate drinking does not increase the incidence of seizures in medication-adherent patients. People who have recurrent alcohol-withdrawal seizures do not have a heightened risk of epilepsy.41
Cannabis. Animal studies have documented the anticonvulsant effect of Cannabis in partial and generalized epilepsy and a proconvulsant effect in absence (petit mal) seizures.42
Tramadol, caffeine. Patients who abuse tramadol or who have an excessive intake of caffeine have a decreased seizure threshold.43
Opiates can exert a proconvulsant or anticonvulsant action, depending on the type of endorphin receptors involved.44
Cocaine decreases the seizure threshold by 1) blocking cerebral GABA receptors and 2) inhibiting dopamine reuptake, thus elevating excitatory neurotransmitters. Cocaine can cause a generalized or focal seizure; the latter is caused by intracerebral stroke or hemorrhage.45
The AEDs topiramate and lamotrigine tend to decrease the desire to abuse alcohol by enhancing inhibitory control by way of decreasing dopamine activity in the mesocorticolimbic system.46
Memory deficits
The relative risk of dementia among epilepsy patients is greater compared with the general population. Recurrent seizures can result in cognitive deficits; epilepsy has been documented in 2% to 64% of Alzheimer’s disease patients.47
Progressive amnesia, with an associated decline in cognition in epilepsy patients despite AED therapy, warrants a dementia workup.48 Patients with an ictal disorder often have difficulty with memory, especially if the hippocampus is affected, such as in temporal lobe epilepsy. Seizures are a common manifestation of several neurodegenerative conditions, and may be associated with a treatable dementia or psychosis in patients with cyanocobalamin deficiency.49
Several memory deficits are associated with seizure disorders:
• Transient epileptic amnesia can be ictal or post-ictal, or can be a manifestation of an underlying seizure disorder. The condition is associated with isolated memory deficits; other cognitive functions usually are intact.
• Accelerated long-term memory deficit occurs when patients forget skills acquired over the past few days or weeks. The problem can be reduced with sleep.50
• Remote memory impairment is characterized by inability to recall personal information from the past.51
When considering a diagnosis of a memory deficit as a manifestation of dementia, keep in mind that cognitive impairment also can develop after epilepsy treatment—although most newer medications cause relatively few such problems.52,53
2-pronged management. It is difficult to establish a temporal association between epilepsy and dementia. When the conditions coexist, appropriate treatment of both is important, because inadequate control of seizures can heighten release of amyloid toxins in the hippocampus. This results in rapidly progressive cognitive decline.54
Neurodevelopmental disorders
The incidence of epilepsy in children who have an autism spectrum disorder is 5% to 38%; the disorder is more common in the presence of mental retardation or cerebral palsy.55
A significant percentage of youth who are referred for evaluation of attention-deficit/ hyperactivity disorder (ADHD) eventually are given a diagnosis of absence seizures. The incidence of ADHD in children with epilepsy is 20%; these patients display epileptiform EEG changes, and require meticulous screening, which includes ictal induction by hyperventilation to differentiate ADHD from a seizure disorder.56 Many AEDs, especially GABAergic drugs, can cause symptoms of ADHD. Methylphenidate is safe in children whose seizures are well-controlled, and has no significant interactions with AEDs.57
Management. Adequate seizure control is the only effective means to slow regression in cases of epilepsy comorbid with autism spectrum disorder, mental retardation, and cerebral palsy.58
BOTTOM LINE
Patients who have epilepsy have a lifetime susceptibility to psychopathology, especially depression and anxiety. Psychiatric practitioners should work collaboratively with patients' primary care provider to evaluate, diagnose, and treat both conditions. Quick action is the key to the best possible outcomes, including reducing the risk of recurrent seizures.
Related Resources
• Clancy MJ, Clarke MC, Connor DJ, et al. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014;14:75.
• Centers for Disease Control and Prevention. Comorbidity in adults with epilepsy—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(43):849-853.
• Kui C, Yingfu P, Chenling X, et al. What are the predictors of major depression in adult patients with epilepsy? Epileptic Disord. 2014;16(1):74-79.
• Lunde ME, Rasmussen KG. Electroconvulsive therapy in patients with epilepsy. Epilepsy Behav. 2006;9(2):355-359.
Drug Brand Names
Aripiprazole • Abilify Risperidone • Risperdal
Chlorpromazine • Thorazine Quetlapine • Seroquel
Citalopram • Celexa Sertraline • Zoloft
Clozapine • Clozaril, FazaClo Tiagabine • Gabitril
Escitalopram • Lexapro Topiramate • Topamax
Haloperidol • Haldol Tramadol • Ryzolt, Ultram, ConZip
Lamotrigine • Lamictal Valproate • Depokate
Levetiracetam • Keppra Vigabatrin • Sabril
Methylphenidate • Methylin, Ritalin Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Paradiso S, Hermann BP, Blumer D, et al. Impact of depressed mood on neuropsychological status in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(2):180-185.
2. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. 2004;110(4):207-220.
3. Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613-1622.
4. Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs associated with epileptic partial onset seizures among the privately insured in the United States. Epilepsia. 2010;51(5):838-844.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 2005;40(suppl 10): S2-S20.
6. Kanner AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Curr. 2006;6(5):141-146.
7. Salgado PCB, Yasuda CL, Cendes F. Neuroimaging changes in mesial temporal lobe epilepsy are magnified in the presence of depression. Epilepsy Behav. 2010;19(3):422-427.
8. Kanner AM. Psychiatric issues in epilepsy: the complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 2009;15(1):83-87.9. Hedrick SC, Chaney EF, Felker B, et al. Effectiveness of collaborative care depression treatment in Veterans’ Affairs primary care. J Gen Intern Med. 2003;18(1):9-16.
10. Kanner AM. Depression and epilepsy: a bidirectional relation? Epilepsia. 2011;52(suppl 1):21-27.
11. Karouni M, Arulthas S, Larsson PG, et al. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010;66(11):1151-1160.
12. Prueter C, Norra C. Mood disorders and their treatment in patients with epilepsy. J Neuropsychiatry Clin Neurosci. 2005;17(1):20-28.
13. Schmitz B. Antidepressant drugs: indications and guidelines for use in epilepsy. Epilepsia. 2002;43(suppl 2):14-18.
14. Shafique S, Dalsing MC. Vagus nerve stimulation therapy for treatment of drug-resistant epilepsy and depression. Perspect Vasc Surg Endovasc Ther. 2006;18(4):323-327.
15. Schmitz B. Depression and mania in patients with epilepsy. Epilepsia. 2005;46(suppl 4):45-49.
16. Reuber M, Andersen B, Elger CE, et al. Depression and anxiety before and after temporal lobe epilepsy surgery. Seizure. 2004;13(2):129-135.
17. Johannessen Landmark CJ. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27-47.
18. Wiglusz MS, Cubała WJ, Gałuszko-We¸gielnik WG, et al. Mood disorders in epilepsy - diagnostic and methodical considerations. Psychiatr Danub. 2012;24(suppl 1):S44-S50.
19. Jones JE, Hermann BP, Barry JJ, et al. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2003;4(suppl 3):S31-S38.
20. Hara E, Akanuma N, Adachi N, et al. Suicide attempts in adult patients with idiopathic generalized epilepsy. Psychiatry Clin Neurosci. 2009;63(2):225-229.
21. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
22. Mula M, Schmitz B, Jauch R, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13(4): 658-661.
23. Mula M, Monaco F. Antiepileptic drug-induced mania in patients with epilepsy: what do we know? Epilepsy Behav. 2006;9(2):265-267.
24. Kimiskidis VK, Triantafyllou NI, Kararizou E, et al. Depression and anxiety in epilepsy: the association with demographic and seizure-related variables. Ann Gen Psychiatry. 2007;6:28.
25. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
26. Mula M, Cavanna AE, Critchley H, et al. Phenomenology of obsessive compulsive disorder in patients with temporal lobe epilepsy or tourette syndrome. J Neuropsychiatry Clin Neurosci. 2008;20(2):223-226.
27. Fornaro M, Gabrielli F, Albano C, et al. Obsessive-compulsive disorder and related disorders: a comprehensive survey. Ann Gen Psychiatry. 2009;8:13.
28. Thuile J, Even C, Guelfi JD. Topiramate may induce obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2006;60(3):394.
29. Kaplan PW. Epilepsy and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12(2):241-248.
30. Henning OJ, Nakken KO. Psychiatric comorbidity and use of psychotropic drugs in epilepsy patients. Acta Neurol Scand Suppl. 2010;122(suppl 190):18-22.
31. Qin P, Xu H, Laursen TM, et al. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005; 331(7507):23.
32. Kandratavicius L, Lopes-Aguiar C, Bueno-Júnior LS, et al. Psychiatric comorbidities in temporal lobe epilepsy: possible relationships between psychotic disorders and involvement of limbic circuits [Erratum in Rev Bars Psiquiatr. 2013;35(1):107]. Rev Bras Psiquiatr. 2012;34(4):454-466.
33. Lancman ME, Craven WJ, Asconapé JJ, et al. Clinical management of recurrent postictal psychosis. Journal of Epilepsy. 1994;7(1):47-51.
34. Shaw P, Mellers J, Henderson M, et al. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75(7):1003-1008.
35. Perez MM, Trimble MR. Epileptic psychosis–diagnostic comparison with process schizophrenia. Br J Psychiatry. 1980;137:245-249.
36. Krishnamoorthy ES, Trimble MR, Sander JW, et al. Forced normalization at the interface between epilepsy and psychiatry. Epilepsy Behav. 2002;3(4):303-308.
37. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
38. Nadkarni S, Arnedo V, Devinsky O. Psychosis in epilepsy patients. Epilepsia. 2007;48(suppl 9):17-19.
39. Bazil CW. Parasomnias, sleep disorders, and narcolepsy— sleep-time imitators of epilepsy. In: Kaplan PW, Fisher RS, eds. Imitators of epilepsy. 2nd edition. New York, New York: Demos Medical Publishing; 2005:217-230.
40. Chang HJ, Liao CC, Hu CJ, et al. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PloS One. 2013;8(4):e59999.
41. Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42(10):1266-1272.
42. Consroe P. Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol Dis. 1998;5(6, pt B):534-551.
43. Maiga DD, Seyni H, Sidikou A, et al. Convulsive crisis in Tramadol and caffeine abusers: about 8 cases and review of the literature [in French]. Pan Afr Med J. 2012;13:24.
44. Ye JH, Liu PL, Wu WH, et al. Cocaine depresses GABA current of hippocampal neurons. Brain Res. 1997;770(1-2):169-175.
45. Przewłocka B, Stala L, Laso´n W, et al. The effect of various opiate receptor agonists on the seizure threshold in the rat. Is dynorphin an endogenous anticonvulsant? Life Sci. 1983;33(suppl 1):595-598.
46. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370): 1677-1685.
47. Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18(4): 285-294.
48. Cretin B, Blanc F, Gaultier C, et al. Epileptic Amnesic Syndrome revealing Alzheimer’s disease. Epilepsy Res. 2012;102(3):206-209.
49. Vilibié M, Jukié V, Vidovié A, et al. Cobalamin deficiency manifested with seizures, mood oscillations, psychotic features and reversible dementia in the absence of typical neurologic and hematologic signs and symptoms: a case report. Coll Antropol. 2013;37(1):317-319.
50. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008;131(pt 9):2243-2263.
51. Manes F, Hodges JR, Graham KS, et al. Focal autobiographical amnesia in association with transient epileptic amnesia. Brain. 2001;124(pt 3):499-509.
52. Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. 2004;5(4):435-439.
53. Thompson PJ, Baxendale SA, Duncan JS, et al. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69(5):636-641.
54. Noebels JL. A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(suppl 1):39-46.
55. Lewis P, Kopelman MD. Forgetting rates in neuropsychiatric disorders. J Neurol Neurosurg Psychiatry. 1998;65(6):890-898.
56. Tan M, Appleton R. Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy. Arch Dis Child. 2005;90(1):57-59.
57. McBride MC, Wang DD, Torres CF. Methylphenidate in therapeutic doses does not lower seizure threshold [abstract 130]. Ann Neurol. 1986;20(3):428.
58. Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48(suppl 9):33-35.
1. Paradiso S, Hermann BP, Blumer D, et al. Impact of depressed mood on neuropsychological status in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2001;70(2):180-185.
2. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. 2004;110(4):207-220.
3. Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia. 2004;45(12):1613-1622.
4. Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs associated with epileptic partial onset seizures among the privately insured in the United States. Epilepsia. 2010;51(5):838-844.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 2005;40(suppl 10): S2-S20.
6. Kanner AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Curr. 2006;6(5):141-146.
7. Salgado PCB, Yasuda CL, Cendes F. Neuroimaging changes in mesial temporal lobe epilepsy are magnified in the presence of depression. Epilepsy Behav. 2010;19(3):422-427.
8. Kanner AM. Psychiatric issues in epilepsy: the complex relation of mood, anxiety disorders, and epilepsy. Epilepsy Behav. 2009;15(1):83-87.9. Hedrick SC, Chaney EF, Felker B, et al. Effectiveness of collaborative care depression treatment in Veterans’ Affairs primary care. J Gen Intern Med. 2003;18(1):9-16.
10. Kanner AM. Depression and epilepsy: a bidirectional relation? Epilepsia. 2011;52(suppl 1):21-27.
11. Karouni M, Arulthas S, Larsson PG, et al. Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol. 2010;66(11):1151-1160.
12. Prueter C, Norra C. Mood disorders and their treatment in patients with epilepsy. J Neuropsychiatry Clin Neurosci. 2005;17(1):20-28.
13. Schmitz B. Antidepressant drugs: indications and guidelines for use in epilepsy. Epilepsia. 2002;43(suppl 2):14-18.
14. Shafique S, Dalsing MC. Vagus nerve stimulation therapy for treatment of drug-resistant epilepsy and depression. Perspect Vasc Surg Endovasc Ther. 2006;18(4):323-327.
15. Schmitz B. Depression and mania in patients with epilepsy. Epilepsia. 2005;46(suppl 4):45-49.
16. Reuber M, Andersen B, Elger CE, et al. Depression and anxiety before and after temporal lobe epilepsy surgery. Seizure. 2004;13(2):129-135.
17. Johannessen Landmark CJ. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27-47.
18. Wiglusz MS, Cubała WJ, Gałuszko-We¸gielnik WG, et al. Mood disorders in epilepsy - diagnostic and methodical considerations. Psychiatr Danub. 2012;24(suppl 1):S44-S50.
19. Jones JE, Hermann BP, Barry JJ, et al. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2003;4(suppl 3):S31-S38.
20. Hara E, Akanuma N, Adachi N, et al. Suicide attempts in adult patients with idiopathic generalized epilepsy. Psychiatry Clin Neurosci. 2009;63(2):225-229.
21. Sareen J, Cox BJ, Afifi TO, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62(11):1249-1257.
22. Mula M, Schmitz B, Jauch R, et al. On the prevalence of bipolar disorder in epilepsy. Epilepsy Behav. 2008;13(4): 658-661.
23. Mula M, Monaco F. Antiepileptic drug-induced mania in patients with epilepsy: what do we know? Epilepsy Behav. 2006;9(2):265-267.
24. Kimiskidis VK, Triantafyllou NI, Kararizou E, et al. Depression and anxiety in epilepsy: the association with demographic and seizure-related variables. Ann Gen Psychiatry. 2007;6:28.
25. Jackson MJ, Turkington D. Depression and anxiety in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i45-i47.
26. Mula M, Cavanna AE, Critchley H, et al. Phenomenology of obsessive compulsive disorder in patients with temporal lobe epilepsy or tourette syndrome. J Neuropsychiatry Clin Neurosci. 2008;20(2):223-226.
27. Fornaro M, Gabrielli F, Albano C, et al. Obsessive-compulsive disorder and related disorders: a comprehensive survey. Ann Gen Psychiatry. 2009;8:13.
28. Thuile J, Even C, Guelfi JD. Topiramate may induce obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2006;60(3):394.
29. Kaplan PW. Epilepsy and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2010;12(2):241-248.
30. Henning OJ, Nakken KO. Psychiatric comorbidity and use of psychotropic drugs in epilepsy patients. Acta Neurol Scand Suppl. 2010;122(suppl 190):18-22.
31. Qin P, Xu H, Laursen TM, et al. Risk for schizophrenia and schizophrenia-like psychosis among patients with epilepsy: population based cohort study. BMJ. 2005; 331(7507):23.
32. Kandratavicius L, Lopes-Aguiar C, Bueno-Júnior LS, et al. Psychiatric comorbidities in temporal lobe epilepsy: possible relationships between psychotic disorders and involvement of limbic circuits [Erratum in Rev Bars Psiquiatr. 2013;35(1):107]. Rev Bras Psiquiatr. 2012;34(4):454-466.
33. Lancman ME, Craven WJ, Asconapé JJ, et al. Clinical management of recurrent postictal psychosis. Journal of Epilepsy. 1994;7(1):47-51.
34. Shaw P, Mellers J, Henderson M, et al. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75(7):1003-1008.
35. Perez MM, Trimble MR. Epileptic psychosis–diagnostic comparison with process schizophrenia. Br J Psychiatry. 1980;137:245-249.
36. Krishnamoorthy ES, Trimble MR, Sander JW, et al. Forced normalization at the interface between epilepsy and psychiatry. Epilepsy Behav. 2002;3(4):303-308.
37. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
38. Nadkarni S, Arnedo V, Devinsky O. Psychosis in epilepsy patients. Epilepsia. 2007;48(suppl 9):17-19.
39. Bazil CW. Parasomnias, sleep disorders, and narcolepsy— sleep-time imitators of epilepsy. In: Kaplan PW, Fisher RS, eds. Imitators of epilepsy. 2nd edition. New York, New York: Demos Medical Publishing; 2005:217-230.
40. Chang HJ, Liao CC, Hu CJ, et al. Psychiatric disorders after epilepsy diagnosis: a population-based retrospective cohort study. PloS One. 2013;8(4):e59999.
41. Gordon E, Devinsky O. Alcohol and marijuana: effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42(10):1266-1272.
42. Consroe P. Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol Dis. 1998;5(6, pt B):534-551.
43. Maiga DD, Seyni H, Sidikou A, et al. Convulsive crisis in Tramadol and caffeine abusers: about 8 cases and review of the literature [in French]. Pan Afr Med J. 2012;13:24.
44. Ye JH, Liu PL, Wu WH, et al. Cocaine depresses GABA current of hippocampal neurons. Brain Res. 1997;770(1-2):169-175.
45. Przewłocka B, Stala L, Laso´n W, et al. The effect of various opiate receptor agonists on the seizure threshold in the rat. Is dynorphin an endogenous anticonvulsant? Life Sci. 1983;33(suppl 1):595-598.
46. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370): 1677-1685.
47. Friedman D, Honig LS, Scarmeas N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci Ther. 2012;18(4): 285-294.
48. Cretin B, Blanc F, Gaultier C, et al. Epileptic Amnesic Syndrome revealing Alzheimer’s disease. Epilepsy Res. 2012;102(3):206-209.
49. Vilibié M, Jukié V, Vidovié A, et al. Cobalamin deficiency manifested with seizures, mood oscillations, psychotic features and reversible dementia in the absence of typical neurologic and hematologic signs and symptoms: a case report. Coll Antropol. 2013;37(1):317-319.
50. Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008;131(pt 9):2243-2263.
51. Manes F, Hodges JR, Graham KS, et al. Focal autobiographical amnesia in association with transient epileptic amnesia. Brain. 2001;124(pt 3):499-509.
52. Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. 2004;5(4):435-439.
53. Thompson PJ, Baxendale SA, Duncan JS, et al. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69(5):636-641.
54. Noebels JL. A perfect storm: converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(suppl 1):39-46.
55. Lewis P, Kopelman MD. Forgetting rates in neuropsychiatric disorders. J Neurol Neurosurg Psychiatry. 1998;65(6):890-898.
56. Tan M, Appleton R. Attention deficit and hyperactivity disorder, methylphenidate, and epilepsy. Arch Dis Child. 2005;90(1):57-59.
57. McBride MC, Wang DD, Torres CF. Methylphenidate in therapeutic doses does not lower seizure threshold [abstract 130]. Ann Neurol. 1986;20(3):428.
58. Levisohn PM. The autism-epilepsy connection. Epilepsia. 2007;48(suppl 9):33-35.
Psychosis resolves, but menses stop
CASE Paranoid and hallucinating
Ms. S, age 30, is an unmarried graduate student who has been given a diagnosis of schizophrenia, paranoid type, during inpatient hospitalization that was prompted by impairment in school functioning (difficulty turning in assignments, poor concentration, making careless mistakes on tests), paranoid delusions, and multisensory hallucinations. She says that her roommate and classmates are working together to make her leave school, and recalls seeing them “snare and smirk” as she passes by. Ms. S says that she feels her classmates are calling her names and talking badly about her as soon as she is out of sight.
Ms. S is antipsychotic-naïve and has a baseline body mass index of 17.8 kg/m2, indicating that she is underweight. We believe that olanzapine, 20 mg/d, is a good initial treatment because of its propensity for weight gain; however, she experiences only marginal improvement. Ms. S does not have health insurance, and cannot afford a brand name medication; therefore, she is cross-tapered to perphenazine, 8 mg, and benzatropine, 0.5 mg, both taken twice daily (olanzapine was not available as a generic at the time).
At discharge, Ms. S does not report any hallucinatory experiences, but is guarded, voices suspicions about the treatment team, and asks “What are they doing with all my blood?”—referring to blood draws for laboratory testing during hospitalization.
As an outpatient, Ms. S is continued on the same medications until she has to be switched because she cannot afford the out-of-pocket cost of the antipsychotic, perphenazine ($80 a month). Clozapine is recommended, but Ms. S refuses because of the mandatory weekly blood monitoring. She briefly tries fluphenazine, 2.5 mg/d, but it is discontinued because of malaise and lightheadedness without extrapyramidal symptoms.
Clozapine is again recommended, but Ms. S remains suspicious of the necessary blood draws and refuses. After several trials of antipsychotics, Ms. S starts paliperidone using samples from the clinic, titrated to 6 mg at bedtime. Once tolerance and therapeutic improvement are observed, she is continued on this medication through the manufacturer’s patient assistance program.
Within 3 months, Ms. S and her family find that she has improved significantly. She no longer reports hallucinatory experiences, is less guarded during sessions, and has followed through with paid and volunteer job applications and interviews. She soon finds a job teaching entry-level classes at a community college and is looking forward to a summer trip abroad.
During a follow-up appointment, Ms. S reports that she had missed 2 consecutive menstrual cycles without galactorrhea or fractures. A urine pregnancy test is negative; the prolactin level is 72 μg/L.
Hyperprolactinemia in women is defined as a plasma prolactin level of
a)>2.5 µg/L
b) >5 µg/L
c) >10 µg/L
d) >20 µg/L
e) >25 µg/L
The authors’ observations
A prolactin level >25 μg/L is considered abnormal.1 A level of >250 μg/L may identify a prolactinoma; however, levels >200 μg/L have been observed in patients taking an antipsychotic.1 Given Ms. S’s clinically significant elevation of prolactin, she is referred to her primary care physician. We decide to augment her regimen with aripiprazole, 10 mg/d, because this drug has been noted to help in cases of hyperprolactinemia associated with other antipsychotics.2,3
Prolactin serves several roles in the body, including but not limited to lactation, sexual gratification, proliferation of oligodendrocyte precursor cells, surfactant synthesis of fetal lungs at the end of pregnancy, and neurogenesis in maternal and fetal brains (Figure 1 and Figure 2). A 2004 review reported secondary amenorrhea, galactorrhea, and osteopenia as common symptoms of hyperprolactinemia.5 Hyperprolactinemia has been seen with most antipsychotics, both typical and atypical. Although several studies document prolactin elevation with risperidone, fewer have examined the active metabolite (9-hydroxyrisperidone) paliperidone.5-7
In women, a high prolactin level can cause
a) menstrual disturbance
b) galactorrhea
c) breast engorgement
d) sexual dysfunction
e) all of the above
The authors’ observations
Acutely, hyperprolactinemia can cause menstrual abnormalities, decreased libido, breast engorgement, galactorrhea, and sexual dysfunction in women.8 In men, the most common symptoms of hyperprolactinemia are loss of interest in sex, erectile dysfunction, infertility, and gynecomastia. Osteoporosis has been associated with chronic elevation of the prolactin level8 (Table).
TREATMENT Adjunctive aripiprazole
After 8 weeks of adjunctive aripiprazole, Ms. S’s prolactin level decreases to 42 μg/L, but menses do not return. Because her family and primary care providers are eager to have the prolactin level return to normal, reducing her risk of complications, we decide to decrease paliperidone to 3 mg at bedtime.
Eight weeks later, Ms. S shows functional improvement. A repeat test of prolactin is 24 μg/L; she reports a 4-day period of spotting 1 week ago. One month later, the prolactin level is 21 μg/L, and she reports having a normal menstrual period. She continues treatment with paliperidone, 3 mg/d, and aripiprazole, 10 mg/d, experiences regular menses, and continues teaching.
Pharmacotherapy of hyperprolactinemia includes
a) haloperidol
b) perphenazine
c) bromocriptine
d) olanzapine
e) risperidone
The authors' observations
Our goal in treating Ms. S was to address her schizophrenia symptoms and improve her overall functioning. Often, finding an effective treatment can be challenging, and there is little evidence to support the efficacy of one antipsychotic over another.4 In Ms. S’s case, our care was stymied by the cost of medication, challenges related to delusions intrinsic to the illness (she refused clozapine because of required blood draws), and adverse effects. When Ms. S developed amenorrhea while taking paliperidone— the only medication that showed significant improvement in her psychotic symptoms—our goal was to maintain her functional level without significant long-term adverse effects.
Managing hyperprolactinemia
Management of iatrogenic hyperprolactinemia includes decreasing the dosage of the offending agent, using a prolactin-sparing antipsychotic, or initiating a dopamine agonist, such as bromocriptine or cabergoline, in addition to an antipsychotic.1,4 Aripiprazole is considered to be a prolactin-sparing agent because of its propensity to increase the prolactin level to less of a degree than what is seen with other antipsychotics; in fact, it has been shown to reduce an elevated prolactin level.9-11
Most typical and atypical antipsychotics are dopamine—specifically D2—receptor antagonists. These antipsychotics prevent dopamine from binding to the D2 receptor and from inhibiting prolactin release, therefore causing hyperprolactinemia. Aripiprazole differs from other antipsychotics: It is a partial D2 receptor agonist with high affinity, and therefore suppresses prolactin release.8 In a randomized controlled trial, aripiprazole had a lower rate of prolactin elevation compared with placebo.12
Aripiprazole’s ability to reduce an elevated prolactin level caused by other antipsychotics has been demonstrated in several studies with haloperidol,13 olanzapine,14,15 and risperidone.15-17 There has been 1 case report,18 but no controlled studies, of aripiprazole being used to decrease the prolactin level in patients treated with paliperidone.
In Ms. S’s case, adding aripiprazole, 10 mg/d, reduced her prolactin level by approximately 50%. Because several studies have shown that adjunctive aripiprazole with a D2 antagonist normalizes the prolactin level,19 it is reasonable to conclude that adding aripiprazole facilitated reduction of her prolactin level and might have continued to do so if given more time. Regrettably, because of patient and family concerns, paliperidone was reduced before this could be determined. It is unclear whether normalization of Ms. S’s prolactin level and return of her menstrual cycle was caused by adding aripiprazole or by reducing the dosage of paliperidone.
Although additional randomized controlled trials should be conducted on the utility of this approach, it is reasonable to consider augmentation with aripiprazole when treating a patient who is stable on an antipsychotic, including paliperidone, but has developed hyperprolactinemia secondary to treatment.
BOTTOM LINE
Hyperprolactinemia is a relatively common, underreported side effect of both typical and atypical antipsychotics. Paliperidone and risperidone have been shown to have the highest risk among the atypical antipsychotics; aripiprazole has the lowest risk. Treatment of an elevated prolactin level should include reduction or discontinuation of the offending agent and augmentation with aripiprazole.
Related Resources
• Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review [published online March 28, 2014]. CNS Drugs. doi: 10.1007/s40263-014-0157-3.
• Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179. doi: 10.1371/journal.pone.0070179.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Benzatropine • Cogentin Olanzapine • Zyprexa
Bromocriptine • Parlodel Paliperidone • Invega
Cabergoline • Dostinex Perphenazine • Trilafon
Clozapine • Clozaril Risperidone • Risperdal
Fluphenazine • Prolixin
DisclosureThe authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288.
2. Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25(4):281-297.
3. Hanssens L, L’Italien G, Loze JY, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry. 2008;8:95. doi: 10.1186/1471-244X-8-95.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
5. Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291-2314.
6. Knegtering R, Baselmans P, Castelein S, et al. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162(5): 1010-1012.
7. Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. 2010; 24(7):1011-1018.
8. Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin Endocrinol (Oxf). 2011;74(2):141-147.
9. Friberg LE, Vermeulen AM, Petersson KJ, et al. An agonist-antagonist interaction model for prolactin release following risperidone and paliperidone treatment. Clin Pharmacol Ther. 2009;85(4):409-417.
10. Skopek M, Manoj P. Hyperprolactinaemia during treatment with paliperidone. Australas Psychiatry. 2010; 18(3):261-263.
11. Aihara K, Shimada J, Miwa T, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003(1-2):9-17.
12. Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J Psychopharmacol. 2008;22(2 suppl):46-55.
13. Yasui-Furukori N, Furukori H, Sugawara N, et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596-599.
14. Lorenz RA, Weinstein B. Resolution of haloperidol-induced hyperprolactinemia with aripiprazole. J Clin Psychopharmacol. 2007;27(5):524-525.
15. Aggarwal A, Jain M, Garg A, et al. Aripiprazole for olanzapine-induced symptomatic hyper prolactinemia. Indian J Pharmacol. 2010;42(1):58-59.
16. Byerly MJ, Marcus RN, Tran QV, et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009; 107(2-3):218-222.
17. Chen CK, Huang YS, Ree SC, et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1495-1499.
18. Chen CY, Lin TY, Wang CC, et al. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci. 2011;65(1):95-97.
19. Rocha FL, Hara C, Ramos MG. Using aripiprazole to attenuate paliperidone-induced hyperprolactinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1153-1154.
CASE Paranoid and hallucinating
Ms. S, age 30, is an unmarried graduate student who has been given a diagnosis of schizophrenia, paranoid type, during inpatient hospitalization that was prompted by impairment in school functioning (difficulty turning in assignments, poor concentration, making careless mistakes on tests), paranoid delusions, and multisensory hallucinations. She says that her roommate and classmates are working together to make her leave school, and recalls seeing them “snare and smirk” as she passes by. Ms. S says that she feels her classmates are calling her names and talking badly about her as soon as she is out of sight.
Ms. S is antipsychotic-naïve and has a baseline body mass index of 17.8 kg/m2, indicating that she is underweight. We believe that olanzapine, 20 mg/d, is a good initial treatment because of its propensity for weight gain; however, she experiences only marginal improvement. Ms. S does not have health insurance, and cannot afford a brand name medication; therefore, she is cross-tapered to perphenazine, 8 mg, and benzatropine, 0.5 mg, both taken twice daily (olanzapine was not available as a generic at the time).
At discharge, Ms. S does not report any hallucinatory experiences, but is guarded, voices suspicions about the treatment team, and asks “What are they doing with all my blood?”—referring to blood draws for laboratory testing during hospitalization.
As an outpatient, Ms. S is continued on the same medications until she has to be switched because she cannot afford the out-of-pocket cost of the antipsychotic, perphenazine ($80 a month). Clozapine is recommended, but Ms. S refuses because of the mandatory weekly blood monitoring. She briefly tries fluphenazine, 2.5 mg/d, but it is discontinued because of malaise and lightheadedness without extrapyramidal symptoms.
Clozapine is again recommended, but Ms. S remains suspicious of the necessary blood draws and refuses. After several trials of antipsychotics, Ms. S starts paliperidone using samples from the clinic, titrated to 6 mg at bedtime. Once tolerance and therapeutic improvement are observed, she is continued on this medication through the manufacturer’s patient assistance program.
Within 3 months, Ms. S and her family find that she has improved significantly. She no longer reports hallucinatory experiences, is less guarded during sessions, and has followed through with paid and volunteer job applications and interviews. She soon finds a job teaching entry-level classes at a community college and is looking forward to a summer trip abroad.
During a follow-up appointment, Ms. S reports that she had missed 2 consecutive menstrual cycles without galactorrhea or fractures. A urine pregnancy test is negative; the prolactin level is 72 μg/L.
Hyperprolactinemia in women is defined as a plasma prolactin level of
a)>2.5 µg/L
b) >5 µg/L
c) >10 µg/L
d) >20 µg/L
e) >25 µg/L
The authors’ observations
A prolactin level >25 μg/L is considered abnormal.1 A level of >250 μg/L may identify a prolactinoma; however, levels >200 μg/L have been observed in patients taking an antipsychotic.1 Given Ms. S’s clinically significant elevation of prolactin, she is referred to her primary care physician. We decide to augment her regimen with aripiprazole, 10 mg/d, because this drug has been noted to help in cases of hyperprolactinemia associated with other antipsychotics.2,3
Prolactin serves several roles in the body, including but not limited to lactation, sexual gratification, proliferation of oligodendrocyte precursor cells, surfactant synthesis of fetal lungs at the end of pregnancy, and neurogenesis in maternal and fetal brains (Figure 1 and Figure 2). A 2004 review reported secondary amenorrhea, galactorrhea, and osteopenia as common symptoms of hyperprolactinemia.5 Hyperprolactinemia has been seen with most antipsychotics, both typical and atypical. Although several studies document prolactin elevation with risperidone, fewer have examined the active metabolite (9-hydroxyrisperidone) paliperidone.5-7
In women, a high prolactin level can cause
a) menstrual disturbance
b) galactorrhea
c) breast engorgement
d) sexual dysfunction
e) all of the above
The authors’ observations
Acutely, hyperprolactinemia can cause menstrual abnormalities, decreased libido, breast engorgement, galactorrhea, and sexual dysfunction in women.8 In men, the most common symptoms of hyperprolactinemia are loss of interest in sex, erectile dysfunction, infertility, and gynecomastia. Osteoporosis has been associated with chronic elevation of the prolactin level8 (Table).
TREATMENT Adjunctive aripiprazole
After 8 weeks of adjunctive aripiprazole, Ms. S’s prolactin level decreases to 42 μg/L, but menses do not return. Because her family and primary care providers are eager to have the prolactin level return to normal, reducing her risk of complications, we decide to decrease paliperidone to 3 mg at bedtime.
Eight weeks later, Ms. S shows functional improvement. A repeat test of prolactin is 24 μg/L; she reports a 4-day period of spotting 1 week ago. One month later, the prolactin level is 21 μg/L, and she reports having a normal menstrual period. She continues treatment with paliperidone, 3 mg/d, and aripiprazole, 10 mg/d, experiences regular menses, and continues teaching.
Pharmacotherapy of hyperprolactinemia includes
a) haloperidol
b) perphenazine
c) bromocriptine
d) olanzapine
e) risperidone
The authors' observations
Our goal in treating Ms. S was to address her schizophrenia symptoms and improve her overall functioning. Often, finding an effective treatment can be challenging, and there is little evidence to support the efficacy of one antipsychotic over another.4 In Ms. S’s case, our care was stymied by the cost of medication, challenges related to delusions intrinsic to the illness (she refused clozapine because of required blood draws), and adverse effects. When Ms. S developed amenorrhea while taking paliperidone— the only medication that showed significant improvement in her psychotic symptoms—our goal was to maintain her functional level without significant long-term adverse effects.
Managing hyperprolactinemia
Management of iatrogenic hyperprolactinemia includes decreasing the dosage of the offending agent, using a prolactin-sparing antipsychotic, or initiating a dopamine agonist, such as bromocriptine or cabergoline, in addition to an antipsychotic.1,4 Aripiprazole is considered to be a prolactin-sparing agent because of its propensity to increase the prolactin level to less of a degree than what is seen with other antipsychotics; in fact, it has been shown to reduce an elevated prolactin level.9-11
Most typical and atypical antipsychotics are dopamine—specifically D2—receptor antagonists. These antipsychotics prevent dopamine from binding to the D2 receptor and from inhibiting prolactin release, therefore causing hyperprolactinemia. Aripiprazole differs from other antipsychotics: It is a partial D2 receptor agonist with high affinity, and therefore suppresses prolactin release.8 In a randomized controlled trial, aripiprazole had a lower rate of prolactin elevation compared with placebo.12
Aripiprazole’s ability to reduce an elevated prolactin level caused by other antipsychotics has been demonstrated in several studies with haloperidol,13 olanzapine,14,15 and risperidone.15-17 There has been 1 case report,18 but no controlled studies, of aripiprazole being used to decrease the prolactin level in patients treated with paliperidone.
In Ms. S’s case, adding aripiprazole, 10 mg/d, reduced her prolactin level by approximately 50%. Because several studies have shown that adjunctive aripiprazole with a D2 antagonist normalizes the prolactin level,19 it is reasonable to conclude that adding aripiprazole facilitated reduction of her prolactin level and might have continued to do so if given more time. Regrettably, because of patient and family concerns, paliperidone was reduced before this could be determined. It is unclear whether normalization of Ms. S’s prolactin level and return of her menstrual cycle was caused by adding aripiprazole or by reducing the dosage of paliperidone.
Although additional randomized controlled trials should be conducted on the utility of this approach, it is reasonable to consider augmentation with aripiprazole when treating a patient who is stable on an antipsychotic, including paliperidone, but has developed hyperprolactinemia secondary to treatment.
BOTTOM LINE
Hyperprolactinemia is a relatively common, underreported side effect of both typical and atypical antipsychotics. Paliperidone and risperidone have been shown to have the highest risk among the atypical antipsychotics; aripiprazole has the lowest risk. Treatment of an elevated prolactin level should include reduction or discontinuation of the offending agent and augmentation with aripiprazole.
Related Resources
• Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review [published online March 28, 2014]. CNS Drugs. doi: 10.1007/s40263-014-0157-3.
• Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179. doi: 10.1371/journal.pone.0070179.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Benzatropine • Cogentin Olanzapine • Zyprexa
Bromocriptine • Parlodel Paliperidone • Invega
Cabergoline • Dostinex Perphenazine • Trilafon
Clozapine • Clozaril Risperidone • Risperdal
Fluphenazine • Prolixin
DisclosureThe authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Paranoid and hallucinating
Ms. S, age 30, is an unmarried graduate student who has been given a diagnosis of schizophrenia, paranoid type, during inpatient hospitalization that was prompted by impairment in school functioning (difficulty turning in assignments, poor concentration, making careless mistakes on tests), paranoid delusions, and multisensory hallucinations. She says that her roommate and classmates are working together to make her leave school, and recalls seeing them “snare and smirk” as she passes by. Ms. S says that she feels her classmates are calling her names and talking badly about her as soon as she is out of sight.
Ms. S is antipsychotic-naïve and has a baseline body mass index of 17.8 kg/m2, indicating that she is underweight. We believe that olanzapine, 20 mg/d, is a good initial treatment because of its propensity for weight gain; however, she experiences only marginal improvement. Ms. S does not have health insurance, and cannot afford a brand name medication; therefore, she is cross-tapered to perphenazine, 8 mg, and benzatropine, 0.5 mg, both taken twice daily (olanzapine was not available as a generic at the time).
At discharge, Ms. S does not report any hallucinatory experiences, but is guarded, voices suspicions about the treatment team, and asks “What are they doing with all my blood?”—referring to blood draws for laboratory testing during hospitalization.
As an outpatient, Ms. S is continued on the same medications until she has to be switched because she cannot afford the out-of-pocket cost of the antipsychotic, perphenazine ($80 a month). Clozapine is recommended, but Ms. S refuses because of the mandatory weekly blood monitoring. She briefly tries fluphenazine, 2.5 mg/d, but it is discontinued because of malaise and lightheadedness without extrapyramidal symptoms.
Clozapine is again recommended, but Ms. S remains suspicious of the necessary blood draws and refuses. After several trials of antipsychotics, Ms. S starts paliperidone using samples from the clinic, titrated to 6 mg at bedtime. Once tolerance and therapeutic improvement are observed, she is continued on this medication through the manufacturer’s patient assistance program.
Within 3 months, Ms. S and her family find that she has improved significantly. She no longer reports hallucinatory experiences, is less guarded during sessions, and has followed through with paid and volunteer job applications and interviews. She soon finds a job teaching entry-level classes at a community college and is looking forward to a summer trip abroad.
During a follow-up appointment, Ms. S reports that she had missed 2 consecutive menstrual cycles without galactorrhea or fractures. A urine pregnancy test is negative; the prolactin level is 72 μg/L.
Hyperprolactinemia in women is defined as a plasma prolactin level of
a)>2.5 µg/L
b) >5 µg/L
c) >10 µg/L
d) >20 µg/L
e) >25 µg/L
The authors’ observations
A prolactin level >25 μg/L is considered abnormal.1 A level of >250 μg/L may identify a prolactinoma; however, levels >200 μg/L have been observed in patients taking an antipsychotic.1 Given Ms. S’s clinically significant elevation of prolactin, she is referred to her primary care physician. We decide to augment her regimen with aripiprazole, 10 mg/d, because this drug has been noted to help in cases of hyperprolactinemia associated with other antipsychotics.2,3
Prolactin serves several roles in the body, including but not limited to lactation, sexual gratification, proliferation of oligodendrocyte precursor cells, surfactant synthesis of fetal lungs at the end of pregnancy, and neurogenesis in maternal and fetal brains (Figure 1 and Figure 2). A 2004 review reported secondary amenorrhea, galactorrhea, and osteopenia as common symptoms of hyperprolactinemia.5 Hyperprolactinemia has been seen with most antipsychotics, both typical and atypical. Although several studies document prolactin elevation with risperidone, fewer have examined the active metabolite (9-hydroxyrisperidone) paliperidone.5-7
In women, a high prolactin level can cause
a) menstrual disturbance
b) galactorrhea
c) breast engorgement
d) sexual dysfunction
e) all of the above
The authors’ observations
Acutely, hyperprolactinemia can cause menstrual abnormalities, decreased libido, breast engorgement, galactorrhea, and sexual dysfunction in women.8 In men, the most common symptoms of hyperprolactinemia are loss of interest in sex, erectile dysfunction, infertility, and gynecomastia. Osteoporosis has been associated with chronic elevation of the prolactin level8 (Table).
TREATMENT Adjunctive aripiprazole
After 8 weeks of adjunctive aripiprazole, Ms. S’s prolactin level decreases to 42 μg/L, but menses do not return. Because her family and primary care providers are eager to have the prolactin level return to normal, reducing her risk of complications, we decide to decrease paliperidone to 3 mg at bedtime.
Eight weeks later, Ms. S shows functional improvement. A repeat test of prolactin is 24 μg/L; she reports a 4-day period of spotting 1 week ago. One month later, the prolactin level is 21 μg/L, and she reports having a normal menstrual period. She continues treatment with paliperidone, 3 mg/d, and aripiprazole, 10 mg/d, experiences regular menses, and continues teaching.
Pharmacotherapy of hyperprolactinemia includes
a) haloperidol
b) perphenazine
c) bromocriptine
d) olanzapine
e) risperidone
The authors' observations
Our goal in treating Ms. S was to address her schizophrenia symptoms and improve her overall functioning. Often, finding an effective treatment can be challenging, and there is little evidence to support the efficacy of one antipsychotic over another.4 In Ms. S’s case, our care was stymied by the cost of medication, challenges related to delusions intrinsic to the illness (she refused clozapine because of required blood draws), and adverse effects. When Ms. S developed amenorrhea while taking paliperidone— the only medication that showed significant improvement in her psychotic symptoms—our goal was to maintain her functional level without significant long-term adverse effects.
Managing hyperprolactinemia
Management of iatrogenic hyperprolactinemia includes decreasing the dosage of the offending agent, using a prolactin-sparing antipsychotic, or initiating a dopamine agonist, such as bromocriptine or cabergoline, in addition to an antipsychotic.1,4 Aripiprazole is considered to be a prolactin-sparing agent because of its propensity to increase the prolactin level to less of a degree than what is seen with other antipsychotics; in fact, it has been shown to reduce an elevated prolactin level.9-11
Most typical and atypical antipsychotics are dopamine—specifically D2—receptor antagonists. These antipsychotics prevent dopamine from binding to the D2 receptor and from inhibiting prolactin release, therefore causing hyperprolactinemia. Aripiprazole differs from other antipsychotics: It is a partial D2 receptor agonist with high affinity, and therefore suppresses prolactin release.8 In a randomized controlled trial, aripiprazole had a lower rate of prolactin elevation compared with placebo.12
Aripiprazole’s ability to reduce an elevated prolactin level caused by other antipsychotics has been demonstrated in several studies with haloperidol,13 olanzapine,14,15 and risperidone.15-17 There has been 1 case report,18 but no controlled studies, of aripiprazole being used to decrease the prolactin level in patients treated with paliperidone.
In Ms. S’s case, adding aripiprazole, 10 mg/d, reduced her prolactin level by approximately 50%. Because several studies have shown that adjunctive aripiprazole with a D2 antagonist normalizes the prolactin level,19 it is reasonable to conclude that adding aripiprazole facilitated reduction of her prolactin level and might have continued to do so if given more time. Regrettably, because of patient and family concerns, paliperidone was reduced before this could be determined. It is unclear whether normalization of Ms. S’s prolactin level and return of her menstrual cycle was caused by adding aripiprazole or by reducing the dosage of paliperidone.
Although additional randomized controlled trials should be conducted on the utility of this approach, it is reasonable to consider augmentation with aripiprazole when treating a patient who is stable on an antipsychotic, including paliperidone, but has developed hyperprolactinemia secondary to treatment.
BOTTOM LINE
Hyperprolactinemia is a relatively common, underreported side effect of both typical and atypical antipsychotics. Paliperidone and risperidone have been shown to have the highest risk among the atypical antipsychotics; aripiprazole has the lowest risk. Treatment of an elevated prolactin level should include reduction or discontinuation of the offending agent and augmentation with aripiprazole.
Related Resources
• Peuskens J, Pani L, Detraux J, et al. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review [published online March 28, 2014]. CNS Drugs. doi: 10.1007/s40263-014-0157-3.
• Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. 2013;8(8):e70179. doi: 10.1371/journal.pone.0070179.
Drug Brand Names
Aripiprazole • Abilify Haloperidol • Haldol
Benzatropine • Cogentin Olanzapine • Zyprexa
Bromocriptine • Parlodel Paliperidone • Invega
Cabergoline • Dostinex Perphenazine • Trilafon
Clozapine • Clozaril Risperidone • Risperdal
Fluphenazine • Prolixin
DisclosureThe authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288.
2. Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25(4):281-297.
3. Hanssens L, L’Italien G, Loze JY, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry. 2008;8:95. doi: 10.1186/1471-244X-8-95.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
5. Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291-2314.
6. Knegtering R, Baselmans P, Castelein S, et al. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162(5): 1010-1012.
7. Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. 2010; 24(7):1011-1018.
8. Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin Endocrinol (Oxf). 2011;74(2):141-147.
9. Friberg LE, Vermeulen AM, Petersson KJ, et al. An agonist-antagonist interaction model for prolactin release following risperidone and paliperidone treatment. Clin Pharmacol Ther. 2009;85(4):409-417.
10. Skopek M, Manoj P. Hyperprolactinaemia during treatment with paliperidone. Australas Psychiatry. 2010; 18(3):261-263.
11. Aihara K, Shimada J, Miwa T, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003(1-2):9-17.
12. Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J Psychopharmacol. 2008;22(2 suppl):46-55.
13. Yasui-Furukori N, Furukori H, Sugawara N, et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596-599.
14. Lorenz RA, Weinstein B. Resolution of haloperidol-induced hyperprolactinemia with aripiprazole. J Clin Psychopharmacol. 2007;27(5):524-525.
15. Aggarwal A, Jain M, Garg A, et al. Aripiprazole for olanzapine-induced symptomatic hyper prolactinemia. Indian J Pharmacol. 2010;42(1):58-59.
16. Byerly MJ, Marcus RN, Tran QV, et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009; 107(2-3):218-222.
17. Chen CK, Huang YS, Ree SC, et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1495-1499.
18. Chen CY, Lin TY, Wang CC, et al. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci. 2011;65(1):95-97.
19. Rocha FL, Hara C, Ramos MG. Using aripiprazole to attenuate paliperidone-induced hyperprolactinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1153-1154.
1. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288.
2. Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25(4):281-297.
3. Hanssens L, L’Italien G, Loze JY, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry. 2008;8:95. doi: 10.1186/1471-244X-8-95.
4. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1223.
5. Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291-2314.
6. Knegtering R, Baselmans P, Castelein S, et al. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162(5): 1010-1012.
7. Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. 2010; 24(7):1011-1018.
8. Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin Endocrinol (Oxf). 2011;74(2):141-147.
9. Friberg LE, Vermeulen AM, Petersson KJ, et al. An agonist-antagonist interaction model for prolactin release following risperidone and paliperidone treatment. Clin Pharmacol Ther. 2009;85(4):409-417.
10. Skopek M, Manoj P. Hyperprolactinaemia during treatment with paliperidone. Australas Psychiatry. 2010; 18(3):261-263.
11. Aihara K, Shimada J, Miwa T, et al. The novel antipsychotic aripiprazole is a partial agonist at short and long isoforms of D2 receptors linked to the regulation of adenylyl cyclase activity and prolactin release. Brain Res. 2004;1003(1-2):9-17.
12. Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J Psychopharmacol. 2008;22(2 suppl):46-55.
13. Yasui-Furukori N, Furukori H, Sugawara N, et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596-599.
14. Lorenz RA, Weinstein B. Resolution of haloperidol-induced hyperprolactinemia with aripiprazole. J Clin Psychopharmacol. 2007;27(5):524-525.
15. Aggarwal A, Jain M, Garg A, et al. Aripiprazole for olanzapine-induced symptomatic hyper prolactinemia. Indian J Pharmacol. 2010;42(1):58-59.
16. Byerly MJ, Marcus RN, Tran QV, et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009; 107(2-3):218-222.
17. Chen CK, Huang YS, Ree SC, et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1495-1499.
18. Chen CY, Lin TY, Wang CC, et al. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci. 2011;65(1):95-97.
19. Rocha FL, Hara C, Ramos MG. Using aripiprazole to attenuate paliperidone-induced hyperprolactinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):1153-1154.
'Neuroscientification' and the mystical are compatible
In the February 2014 issue (Psychiatry’s future shock, Current Psychiatry, February 2014, p. 19-20, 32 [http://bit.ly/1bvrp3t]) Dr. Nasrallah tells us that psychiatrists who are not speaking the new language of the “neuroscientification” of psychiatry will soon be rendered obsolete. He says that we are at the “tipping point” in psychiatry, giving up the “primitive notions that have guided the profession for the past century,” and moving toward explaining our successes and failures in terms of microglial activation, inflammatory markers, apoptosis, S100B, and NOTCH 3. Those who talk about ego strengths, defense mechanisms, resilience, the unconscious mind, and the human spirit are “clinical dinosaurs.”
Sadly, Dr. Nasrallah speaks for many psychiatric academicians today, who believe the best way to understand the complexity of the human condition is to explain it in terms of neurotransmitters, genomics, and MRIs. But the truth is, no matter how much we know about the brain, the mind will always have a mind of its own. The language of science cannot adequately explain the mystery that is an essential part of the human experience.
The brain is hardwired for mystical experiences. We are biologically programmed to experience transcendent states that allow us to see the familiar from a new perspective, and to experience the awesome. It matters less how we explain the mechanics of the mysterious than it does to know it is important. To all my academic colleagues who herald in the “neuroscientification” of psychiatry, and the obsolescence of clinicians who still explore the unconscious mind, I say let’s not take ourselves too seriously. Awe is the mechanism by which we tame the ego.
Carl Hammerschlag, MD
Arizona Health Services Center
University of Arizona
Phoenix, Arizona
In the February 2014 issue (Psychiatry’s future shock, Current Psychiatry, February 2014, p. 19-20, 32 [http://bit.ly/1bvrp3t]) Dr. Nasrallah tells us that psychiatrists who are not speaking the new language of the “neuroscientification” of psychiatry will soon be rendered obsolete. He says that we are at the “tipping point” in psychiatry, giving up the “primitive notions that have guided the profession for the past century,” and moving toward explaining our successes and failures in terms of microglial activation, inflammatory markers, apoptosis, S100B, and NOTCH 3. Those who talk about ego strengths, defense mechanisms, resilience, the unconscious mind, and the human spirit are “clinical dinosaurs.”
Sadly, Dr. Nasrallah speaks for many psychiatric academicians today, who believe the best way to understand the complexity of the human condition is to explain it in terms of neurotransmitters, genomics, and MRIs. But the truth is, no matter how much we know about the brain, the mind will always have a mind of its own. The language of science cannot adequately explain the mystery that is an essential part of the human experience.
The brain is hardwired for mystical experiences. We are biologically programmed to experience transcendent states that allow us to see the familiar from a new perspective, and to experience the awesome. It matters less how we explain the mechanics of the mysterious than it does to know it is important. To all my academic colleagues who herald in the “neuroscientification” of psychiatry, and the obsolescence of clinicians who still explore the unconscious mind, I say let’s not take ourselves too seriously. Awe is the mechanism by which we tame the ego.
Carl Hammerschlag, MD
Arizona Health Services Center
University of Arizona
Phoenix, Arizona
In the February 2014 issue (Psychiatry’s future shock, Current Psychiatry, February 2014, p. 19-20, 32 [http://bit.ly/1bvrp3t]) Dr. Nasrallah tells us that psychiatrists who are not speaking the new language of the “neuroscientification” of psychiatry will soon be rendered obsolete. He says that we are at the “tipping point” in psychiatry, giving up the “primitive notions that have guided the profession for the past century,” and moving toward explaining our successes and failures in terms of microglial activation, inflammatory markers, apoptosis, S100B, and NOTCH 3. Those who talk about ego strengths, defense mechanisms, resilience, the unconscious mind, and the human spirit are “clinical dinosaurs.”
Sadly, Dr. Nasrallah speaks for many psychiatric academicians today, who believe the best way to understand the complexity of the human condition is to explain it in terms of neurotransmitters, genomics, and MRIs. But the truth is, no matter how much we know about the brain, the mind will always have a mind of its own. The language of science cannot adequately explain the mystery that is an essential part of the human experience.
The brain is hardwired for mystical experiences. We are biologically programmed to experience transcendent states that allow us to see the familiar from a new perspective, and to experience the awesome. It matters less how we explain the mechanics of the mysterious than it does to know it is important. To all my academic colleagues who herald in the “neuroscientification” of psychiatry, and the obsolescence of clinicians who still explore the unconscious mind, I say let’s not take ourselves too seriously. Awe is the mechanism by which we tame the ego.
Carl Hammerschlag, MD
Arizona Health Services Center
University of Arizona
Phoenix, Arizona
Consider a 'medical food' for tardive dyskinesia
In the interesting March 2014 Savvy Psychopharmacology article (Strategies for managing drug-induced tardive dyskinesia, Current Psychiatry, March 2014, p. 44-46; [http://bit. ly/1gNALYi]), the authors did not mention a medical food made from the branched-chain amino acids L-Leucine, L-Valine, and L-Isoleucine, which was reviewed by the FDA for the dietary management of tardive dyskinesia (TD) in males.1,2 Although this product, “Tarvil,” is no longer being manufactured, compounding pharmacies can make it using the same ratio of ingredients that was tested in the clinical trial.1 It may be worth considering before using tetrabenazine, a medication approved in the United States under the Orphan Drug Act, and launched at $34.25 for a 12.5 mg tablet and $68.50 for a 25 mg tablet.3
Leslie Citrome, MD, MPH
Clinical Professor of Psychiatry & Behavioral Sciences
New York Medical College
Valhalla, NY
Dr. Ellingrod responds
On behalf of Dr. Kaspar and myself, we thank Dr. Citrome for his interest and clarification. Because of the brevity of Savvy Psychopharmacology in the pages of Current Psychiatry, we decided to include only information on therapies that are readily available for TD. We also agree with Dr. Citrome that other treatment modalities, which often cost less, should be tried before tetrabenazine is utilized. Most important, judicious use of antipsychotics should be considered before any additional medications are added to the patient’s regimen.
Vicki L. Ellingrod, PharmD, FCCP
John Gideon Searle Professor of Clinical and Translational Pharmacy
University of Michigan College of Pharmacy and School of Medicine
Ann Arbor, Michigan
1. Richardson MA, Bevans ML, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry. 2003;160(6):1117-1124.
2. Rosack J. Tardive dyskinesia improves with amino acid cocktail. Psychiatric News. http://www.psychnews.psychiatryonline.org/newsarticle.aspx?articleid=106524. Published August 1, 2003. Accessed March 31, 2014.
3. Specialty TrendsRx Availability Alert Xenazine® (tetrabenazine). CVS Caremark. December 2008. https://www.caremark.com/portal/asset/ SpecialtyTrendsRxAlert_Xenazine2.pdf. Published December 2008. Accessed March 15, 2014.
In the interesting March 2014 Savvy Psychopharmacology article (Strategies for managing drug-induced tardive dyskinesia, Current Psychiatry, March 2014, p. 44-46; [http://bit. ly/1gNALYi]), the authors did not mention a medical food made from the branched-chain amino acids L-Leucine, L-Valine, and L-Isoleucine, which was reviewed by the FDA for the dietary management of tardive dyskinesia (TD) in males.1,2 Although this product, “Tarvil,” is no longer being manufactured, compounding pharmacies can make it using the same ratio of ingredients that was tested in the clinical trial.1 It may be worth considering before using tetrabenazine, a medication approved in the United States under the Orphan Drug Act, and launched at $34.25 for a 12.5 mg tablet and $68.50 for a 25 mg tablet.3
Leslie Citrome, MD, MPH
Clinical Professor of Psychiatry & Behavioral Sciences
New York Medical College
Valhalla, NY
Dr. Ellingrod responds
On behalf of Dr. Kaspar and myself, we thank Dr. Citrome for his interest and clarification. Because of the brevity of Savvy Psychopharmacology in the pages of Current Psychiatry, we decided to include only information on therapies that are readily available for TD. We also agree with Dr. Citrome that other treatment modalities, which often cost less, should be tried before tetrabenazine is utilized. Most important, judicious use of antipsychotics should be considered before any additional medications are added to the patient’s regimen.
Vicki L. Ellingrod, PharmD, FCCP
John Gideon Searle Professor of Clinical and Translational Pharmacy
University of Michigan College of Pharmacy and School of Medicine
Ann Arbor, Michigan
In the interesting March 2014 Savvy Psychopharmacology article (Strategies for managing drug-induced tardive dyskinesia, Current Psychiatry, March 2014, p. 44-46; [http://bit. ly/1gNALYi]), the authors did not mention a medical food made from the branched-chain amino acids L-Leucine, L-Valine, and L-Isoleucine, which was reviewed by the FDA for the dietary management of tardive dyskinesia (TD) in males.1,2 Although this product, “Tarvil,” is no longer being manufactured, compounding pharmacies can make it using the same ratio of ingredients that was tested in the clinical trial.1 It may be worth considering before using tetrabenazine, a medication approved in the United States under the Orphan Drug Act, and launched at $34.25 for a 12.5 mg tablet and $68.50 for a 25 mg tablet.3
Leslie Citrome, MD, MPH
Clinical Professor of Psychiatry & Behavioral Sciences
New York Medical College
Valhalla, NY
Dr. Ellingrod responds
On behalf of Dr. Kaspar and myself, we thank Dr. Citrome for his interest and clarification. Because of the brevity of Savvy Psychopharmacology in the pages of Current Psychiatry, we decided to include only information on therapies that are readily available for TD. We also agree with Dr. Citrome that other treatment modalities, which often cost less, should be tried before tetrabenazine is utilized. Most important, judicious use of antipsychotics should be considered before any additional medications are added to the patient’s regimen.
Vicki L. Ellingrod, PharmD, FCCP
John Gideon Searle Professor of Clinical and Translational Pharmacy
University of Michigan College of Pharmacy and School of Medicine
Ann Arbor, Michigan
1. Richardson MA, Bevans ML, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry. 2003;160(6):1117-1124.
2. Rosack J. Tardive dyskinesia improves with amino acid cocktail. Psychiatric News. http://www.psychnews.psychiatryonline.org/newsarticle.aspx?articleid=106524. Published August 1, 2003. Accessed March 31, 2014.
3. Specialty TrendsRx Availability Alert Xenazine® (tetrabenazine). CVS Caremark. December 2008. https://www.caremark.com/portal/asset/ SpecialtyTrendsRxAlert_Xenazine2.pdf. Published December 2008. Accessed March 15, 2014.
1. Richardson MA, Bevans ML, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry. 2003;160(6):1117-1124.
2. Rosack J. Tardive dyskinesia improves with amino acid cocktail. Psychiatric News. http://www.psychnews.psychiatryonline.org/newsarticle.aspx?articleid=106524. Published August 1, 2003. Accessed March 31, 2014.
3. Specialty TrendsRx Availability Alert Xenazine® (tetrabenazine). CVS Caremark. December 2008. https://www.caremark.com/portal/asset/ SpecialtyTrendsRxAlert_Xenazine2.pdf. Published December 2008. Accessed March 15, 2014.
100 years of solicitude: Do global traumatic events have a transgenerational effect?
Yet, important questions about the impact of these events have not been asked: Can there be a transgenerational neurobiological effect on the children and grandchildren of people who have been subjected to life-threatening, traumatic societal events? Could the psychobiology of widespread anxiety and worry (solicitude) be experienced not only by the generation that witnessed and lived through those devastating events, but also by their progeny, who were not yet born during the traumatic events? And could there be epigenetic consequences on a large scale, producing a generation that shares traits induced by the trauma experienced by the previous generation?
Did the rise of delinquency in the 1950s, followed by the anti-war rebellion, unprecedented sexual promiscuity, and substance abuse of the 1960s, be the result of genetic changes in the previous generation induced by living through World War II—after which the generation that grew up in the 1960s was born?
In the late Gabriel García Márquez’s masterpiece novel, One Hundred Years of Solitude, the 1982 Nobel Laureate’s chronicle of the Buendía family across 7 generations is replete with dark and insalubrious events. The fictional family’s story is considered a metaphor for the tumultuous evolution of Márquez’s native Colombia, but that story is consistent with the concept of transgenerational transmission of the biologic effects of stress, as each generation of the Buendía family manifests unusual, even pathological behaviors.
One hundred years of alarm, panic, and anxiety
Psychiatrists are keenly aware of the impact of stressful events on their patients’ mood and behavior, and of the association of life-threatening events with posttraumatic stress disorder (PTSD). For persons who suffer the generalized anxiety of PTSD, further stressful life events can aggravate their condition and result in additional anxiety and solicitude.
It is not surprising that anxiety has been documented as the most common psychiatric condition in the United States.1 Consider the variety of perturbations that have induced alarm, panic, fear, and simmering anxiety on a global scale over the past 100 years— starting with World War I, exactly a century ago.
War. The ruinous 4-year Great War was followed 20 years later by World War II, which caused tens of millions of casualties and the annihilation of Hiroshima and Nagasaki by the atomic bomb— escalating fear of nuclear warfare and radiation poisoning for decades to come. Add to that the Korean War, the Vietnam conflict, the First Gulf War, and the Iraq and Afghanistan wars. The war fatigue and mental exhaustion of the population are palpable.
Economic upheaval. After the Stock Market Crash of 1929 came the Great Depression, the recessions of the 1970s and early 1980s, another stock market crash in 1987, and, most recently, the financial crisis of 2008. Millions saw their wealth wiped out and their livelihoods disrupted, exerting enormous life-changing stresses on countless families.
Disasters. The sinking of the Titanic in 1912, the crash of the Hindenburg, the Three Mile Island nuclear accident, the meltdown of the Chernobyl and Fukushima Daiichi reactors, the space shuttle disasters, and the 9/11 terrorist attacks—all these trigger and perpetuate fear and worry about the one’s own, and one’s loved ones, abrupt and premature mortality.
Epidemics. Millions died in the 1918 influenza pandemic, prompting widespread societal fears that re-intensified during subsequent epidemics: polio in the 1950s, swine flu in the 1970s, SARS (severe acute respiratory syndrome) in the 1990s, West Nile Virus, and avian influenza.
Assassination. The shooting of Archduke Franz Ferdinand of Austria sparked World War I a century ago, but what baby boomers, such as me, vividly remember is our angst over the assassinations of President John F. Kennedy, his brother Robert, and Rev. Dr. Martin Luther King, Jr; the attempted assassination of President Ronald Reagan; and the murder of John Lennon. Each assassination leaves a communal scar on millions, forever reminding them of the ephemeral nature of life at any rung of the social ladder.
Mass murder. The past 100 years began with the Armenian genocide in 1918, followed by the Holocaust of World War II, the Munich Olympics killings, the Jonestown massacre, the Oklahoma City bombing, and, to name a few, the mass murders at Columbine, Virginia Tech, Newtown, and Fort Hood.
Natural disasters can wreak havoc on peoples’ lives. Consider the annual tally of hurricanes (a long list, some—such as Katrina and Sandy—more infamous than others). Add to those storms the earthquakes, tsunamis, erupting volcanoes, floods, and blizzards, and the result is suffering and anxiety on a massive scale, even among those who are not affected directly.
A surprising facet of these disquieting events is the resiliency of people. Life goes on, despite the agony, despair, and solicitude instigated by deadly events. But of those who buckle under the weight of adversity, many end up in a psychiatric clinic or hospital, and are disabled by their symptoms.
Even ‘good’ change can be disquieting
Juxtaposed against these awful events are 100 years of an array of positive, uplifting discoveries, inventions, and medical advances that have completely transformed our lives. Consider: electricity, clean water, women’s right to vote, automobiles, air and space travel, air conditioning, and highway systems; the momentous discoveries of penicillin, antipsychotics, antidepressants, and mood stabilizers; television, the telephone (evolving from dumb to smart), vaccines, oral contraceptives, genetic discoveries, brain imaging technology, and home appliances (refrigerators, microwave ovens, dishwashers); and not at all least, personal computers and the Internet.
But even these advances can generate anxiety and solicitude: Fear of flying, anyone? Embarrassment about a selfie gone viral on the Web? Worry about being a carrier of a breast cancer gene? Claustrophobia inside an MRI scanner?
Hypothesizing about the transfer of anxiety
Could PTSD and solicitude in one generation be transmitted to the next via epigenetic mechanisms (that is, by over-expression or silencing of genes involved in brain development) and could this transmission result in unusual wide-scale stress reactivity? Might this be an example of the infamous Lamarckian “inheritance of acquired characteristics” at the molecular genetic level, in which the anxiety of traumatized parents is transmitted to their offspring? Or could transmission be mediated by being reared in the emotionally oppressive environment of a family still reeling from the effects of war, disaster, and mass murder?
Such questions might sound rhetorical, but they present a reasonable hypothesis that can be answered by research. Findings from animal studies suggest that such a phenomenon might occur in humans.2 If those findings are validated, opportunities for preventing societal solicitude might emerge.
1. Robins LN, Regier DA, eds. Psychiatric disorders in America: The Epidemiologic Catchment Area Study. New York, New York: The Free Press; 1991.
2. Rechavi O, Minevich G, Hobert O. Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell. 2011;147(6):1248-1256.
Yet, important questions about the impact of these events have not been asked: Can there be a transgenerational neurobiological effect on the children and grandchildren of people who have been subjected to life-threatening, traumatic societal events? Could the psychobiology of widespread anxiety and worry (solicitude) be experienced not only by the generation that witnessed and lived through those devastating events, but also by their progeny, who were not yet born during the traumatic events? And could there be epigenetic consequences on a large scale, producing a generation that shares traits induced by the trauma experienced by the previous generation?
Did the rise of delinquency in the 1950s, followed by the anti-war rebellion, unprecedented sexual promiscuity, and substance abuse of the 1960s, be the result of genetic changes in the previous generation induced by living through World War II—after which the generation that grew up in the 1960s was born?
In the late Gabriel García Márquez’s masterpiece novel, One Hundred Years of Solitude, the 1982 Nobel Laureate’s chronicle of the Buendía family across 7 generations is replete with dark and insalubrious events. The fictional family’s story is considered a metaphor for the tumultuous evolution of Márquez’s native Colombia, but that story is consistent with the concept of transgenerational transmission of the biologic effects of stress, as each generation of the Buendía family manifests unusual, even pathological behaviors.
One hundred years of alarm, panic, and anxiety
Psychiatrists are keenly aware of the impact of stressful events on their patients’ mood and behavior, and of the association of life-threatening events with posttraumatic stress disorder (PTSD). For persons who suffer the generalized anxiety of PTSD, further stressful life events can aggravate their condition and result in additional anxiety and solicitude.
It is not surprising that anxiety has been documented as the most common psychiatric condition in the United States.1 Consider the variety of perturbations that have induced alarm, panic, fear, and simmering anxiety on a global scale over the past 100 years— starting with World War I, exactly a century ago.
War. The ruinous 4-year Great War was followed 20 years later by World War II, which caused tens of millions of casualties and the annihilation of Hiroshima and Nagasaki by the atomic bomb— escalating fear of nuclear warfare and radiation poisoning for decades to come. Add to that the Korean War, the Vietnam conflict, the First Gulf War, and the Iraq and Afghanistan wars. The war fatigue and mental exhaustion of the population are palpable.
Economic upheaval. After the Stock Market Crash of 1929 came the Great Depression, the recessions of the 1970s and early 1980s, another stock market crash in 1987, and, most recently, the financial crisis of 2008. Millions saw their wealth wiped out and their livelihoods disrupted, exerting enormous life-changing stresses on countless families.
Disasters. The sinking of the Titanic in 1912, the crash of the Hindenburg, the Three Mile Island nuclear accident, the meltdown of the Chernobyl and Fukushima Daiichi reactors, the space shuttle disasters, and the 9/11 terrorist attacks—all these trigger and perpetuate fear and worry about the one’s own, and one’s loved ones, abrupt and premature mortality.
Epidemics. Millions died in the 1918 influenza pandemic, prompting widespread societal fears that re-intensified during subsequent epidemics: polio in the 1950s, swine flu in the 1970s, SARS (severe acute respiratory syndrome) in the 1990s, West Nile Virus, and avian influenza.
Assassination. The shooting of Archduke Franz Ferdinand of Austria sparked World War I a century ago, but what baby boomers, such as me, vividly remember is our angst over the assassinations of President John F. Kennedy, his brother Robert, and Rev. Dr. Martin Luther King, Jr; the attempted assassination of President Ronald Reagan; and the murder of John Lennon. Each assassination leaves a communal scar on millions, forever reminding them of the ephemeral nature of life at any rung of the social ladder.
Mass murder. The past 100 years began with the Armenian genocide in 1918, followed by the Holocaust of World War II, the Munich Olympics killings, the Jonestown massacre, the Oklahoma City bombing, and, to name a few, the mass murders at Columbine, Virginia Tech, Newtown, and Fort Hood.
Natural disasters can wreak havoc on peoples’ lives. Consider the annual tally of hurricanes (a long list, some—such as Katrina and Sandy—more infamous than others). Add to those storms the earthquakes, tsunamis, erupting volcanoes, floods, and blizzards, and the result is suffering and anxiety on a massive scale, even among those who are not affected directly.
A surprising facet of these disquieting events is the resiliency of people. Life goes on, despite the agony, despair, and solicitude instigated by deadly events. But of those who buckle under the weight of adversity, many end up in a psychiatric clinic or hospital, and are disabled by their symptoms.
Even ‘good’ change can be disquieting
Juxtaposed against these awful events are 100 years of an array of positive, uplifting discoveries, inventions, and medical advances that have completely transformed our lives. Consider: electricity, clean water, women’s right to vote, automobiles, air and space travel, air conditioning, and highway systems; the momentous discoveries of penicillin, antipsychotics, antidepressants, and mood stabilizers; television, the telephone (evolving from dumb to smart), vaccines, oral contraceptives, genetic discoveries, brain imaging technology, and home appliances (refrigerators, microwave ovens, dishwashers); and not at all least, personal computers and the Internet.
But even these advances can generate anxiety and solicitude: Fear of flying, anyone? Embarrassment about a selfie gone viral on the Web? Worry about being a carrier of a breast cancer gene? Claustrophobia inside an MRI scanner?
Hypothesizing about the transfer of anxiety
Could PTSD and solicitude in one generation be transmitted to the next via epigenetic mechanisms (that is, by over-expression or silencing of genes involved in brain development) and could this transmission result in unusual wide-scale stress reactivity? Might this be an example of the infamous Lamarckian “inheritance of acquired characteristics” at the molecular genetic level, in which the anxiety of traumatized parents is transmitted to their offspring? Or could transmission be mediated by being reared in the emotionally oppressive environment of a family still reeling from the effects of war, disaster, and mass murder?
Such questions might sound rhetorical, but they present a reasonable hypothesis that can be answered by research. Findings from animal studies suggest that such a phenomenon might occur in humans.2 If those findings are validated, opportunities for preventing societal solicitude might emerge.
Yet, important questions about the impact of these events have not been asked: Can there be a transgenerational neurobiological effect on the children and grandchildren of people who have been subjected to life-threatening, traumatic societal events? Could the psychobiology of widespread anxiety and worry (solicitude) be experienced not only by the generation that witnessed and lived through those devastating events, but also by their progeny, who were not yet born during the traumatic events? And could there be epigenetic consequences on a large scale, producing a generation that shares traits induced by the trauma experienced by the previous generation?
Did the rise of delinquency in the 1950s, followed by the anti-war rebellion, unprecedented sexual promiscuity, and substance abuse of the 1960s, be the result of genetic changes in the previous generation induced by living through World War II—after which the generation that grew up in the 1960s was born?
In the late Gabriel García Márquez’s masterpiece novel, One Hundred Years of Solitude, the 1982 Nobel Laureate’s chronicle of the Buendía family across 7 generations is replete with dark and insalubrious events. The fictional family’s story is considered a metaphor for the tumultuous evolution of Márquez’s native Colombia, but that story is consistent with the concept of transgenerational transmission of the biologic effects of stress, as each generation of the Buendía family manifests unusual, even pathological behaviors.
One hundred years of alarm, panic, and anxiety
Psychiatrists are keenly aware of the impact of stressful events on their patients’ mood and behavior, and of the association of life-threatening events with posttraumatic stress disorder (PTSD). For persons who suffer the generalized anxiety of PTSD, further stressful life events can aggravate their condition and result in additional anxiety and solicitude.
It is not surprising that anxiety has been documented as the most common psychiatric condition in the United States.1 Consider the variety of perturbations that have induced alarm, panic, fear, and simmering anxiety on a global scale over the past 100 years— starting with World War I, exactly a century ago.
War. The ruinous 4-year Great War was followed 20 years later by World War II, which caused tens of millions of casualties and the annihilation of Hiroshima and Nagasaki by the atomic bomb— escalating fear of nuclear warfare and radiation poisoning for decades to come. Add to that the Korean War, the Vietnam conflict, the First Gulf War, and the Iraq and Afghanistan wars. The war fatigue and mental exhaustion of the population are palpable.
Economic upheaval. After the Stock Market Crash of 1929 came the Great Depression, the recessions of the 1970s and early 1980s, another stock market crash in 1987, and, most recently, the financial crisis of 2008. Millions saw their wealth wiped out and their livelihoods disrupted, exerting enormous life-changing stresses on countless families.
Disasters. The sinking of the Titanic in 1912, the crash of the Hindenburg, the Three Mile Island nuclear accident, the meltdown of the Chernobyl and Fukushima Daiichi reactors, the space shuttle disasters, and the 9/11 terrorist attacks—all these trigger and perpetuate fear and worry about the one’s own, and one’s loved ones, abrupt and premature mortality.
Epidemics. Millions died in the 1918 influenza pandemic, prompting widespread societal fears that re-intensified during subsequent epidemics: polio in the 1950s, swine flu in the 1970s, SARS (severe acute respiratory syndrome) in the 1990s, West Nile Virus, and avian influenza.
Assassination. The shooting of Archduke Franz Ferdinand of Austria sparked World War I a century ago, but what baby boomers, such as me, vividly remember is our angst over the assassinations of President John F. Kennedy, his brother Robert, and Rev. Dr. Martin Luther King, Jr; the attempted assassination of President Ronald Reagan; and the murder of John Lennon. Each assassination leaves a communal scar on millions, forever reminding them of the ephemeral nature of life at any rung of the social ladder.
Mass murder. The past 100 years began with the Armenian genocide in 1918, followed by the Holocaust of World War II, the Munich Olympics killings, the Jonestown massacre, the Oklahoma City bombing, and, to name a few, the mass murders at Columbine, Virginia Tech, Newtown, and Fort Hood.
Natural disasters can wreak havoc on peoples’ lives. Consider the annual tally of hurricanes (a long list, some—such as Katrina and Sandy—more infamous than others). Add to those storms the earthquakes, tsunamis, erupting volcanoes, floods, and blizzards, and the result is suffering and anxiety on a massive scale, even among those who are not affected directly.
A surprising facet of these disquieting events is the resiliency of people. Life goes on, despite the agony, despair, and solicitude instigated by deadly events. But of those who buckle under the weight of adversity, many end up in a psychiatric clinic or hospital, and are disabled by their symptoms.
Even ‘good’ change can be disquieting
Juxtaposed against these awful events are 100 years of an array of positive, uplifting discoveries, inventions, and medical advances that have completely transformed our lives. Consider: electricity, clean water, women’s right to vote, automobiles, air and space travel, air conditioning, and highway systems; the momentous discoveries of penicillin, antipsychotics, antidepressants, and mood stabilizers; television, the telephone (evolving from dumb to smart), vaccines, oral contraceptives, genetic discoveries, brain imaging technology, and home appliances (refrigerators, microwave ovens, dishwashers); and not at all least, personal computers and the Internet.
But even these advances can generate anxiety and solicitude: Fear of flying, anyone? Embarrassment about a selfie gone viral on the Web? Worry about being a carrier of a breast cancer gene? Claustrophobia inside an MRI scanner?
Hypothesizing about the transfer of anxiety
Could PTSD and solicitude in one generation be transmitted to the next via epigenetic mechanisms (that is, by over-expression or silencing of genes involved in brain development) and could this transmission result in unusual wide-scale stress reactivity? Might this be an example of the infamous Lamarckian “inheritance of acquired characteristics” at the molecular genetic level, in which the anxiety of traumatized parents is transmitted to their offspring? Or could transmission be mediated by being reared in the emotionally oppressive environment of a family still reeling from the effects of war, disaster, and mass murder?
Such questions might sound rhetorical, but they present a reasonable hypothesis that can be answered by research. Findings from animal studies suggest that such a phenomenon might occur in humans.2 If those findings are validated, opportunities for preventing societal solicitude might emerge.
1. Robins LN, Regier DA, eds. Psychiatric disorders in America: The Epidemiologic Catchment Area Study. New York, New York: The Free Press; 1991.
2. Rechavi O, Minevich G, Hobert O. Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell. 2011;147(6):1248-1256.
1. Robins LN, Regier DA, eds. Psychiatric disorders in America: The Epidemiologic Catchment Area Study. New York, New York: The Free Press; 1991.
2. Rechavi O, Minevich G, Hobert O. Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell. 2011;147(6):1248-1256.
Binge eating disorder
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Value of subtyping depression
Bicytopenia: Adverse effect of risperidone
Hematologic abnormalities, such as leukopenia, agranulocytosis, and thrombocytopenia, can be life-threatening adverse reactions to atypical antipsychotics. Although clozapine has the highest risk of leukopenia and neutropenia, these side effects also have been associated with other atypical antipsychotics, including risperidone, olanzapine, ziprasidone, paliperdione, and quetiapine. Risperdone-induced leukopenia has been reported,1,2 but risperidone-
induced bicytopenia— that is, leukopenia/ thrombocytopenia—is rare.
Case
Mr. A, age 25, is an African American man admitted to an inpatient psychiatric unit for management of acute psychotic symptoms. He has been taking risperidone, 4 mg/d, for the past 6 months, although his adherence to the regimen is questionable. Baseline blood count shows a white blood cell (WBC) count of 4,400/μL with an absolute neutrophil count (ANC) of 1,900/μL and a platelet count
160×103/μL. A few days after restarting risperidone, repeat blood count shows a drop in the WBC count to 2,900/μL, with an ANC of 900/μL and a platelet count of 130×103/μL.
Mr. A’s physical examination is normal, he does not have any signs or symptoms of infection, and additional lab tests are negative. Risperidone is considered as a possible cause of bicytopenia and is discontinued. Mr. A agrees to start treatment with aripiprazole, 10 mg/d. In next 10 days, the WBC count increases to 6,000/μL. The ANC at 3,100/μL and platelets at 150×103/μL remain stable throughout hospitalization. The slowly increasing WBC count after stopping risperidone is highly suggestive that this agent caused Mr. A’s bicytopenia.
Differential diagnosis
Bone-marrow suppression is associated with first- and second-generation antipsychotics. Blood dyscrasia is a concern in clinical psychiatry because hematologic abnormalities can be life-threatening, requiring close monitoring of the blood count for patients taking an antipsychotic. It is important, therefore, to consider medication side effects in the differential diagnosis of >1 hematologic abnormalities in these patients.
Precise pathophysiologic understanding of the hematologic side effects of antipsychotics is lacking, although different mechanisms of action have been proposed.3 Possible mechanisms when a patient is taking clozapine or olanzapine include:
• direct toxic effect of the drug on bone marrow
• increased peripheral destruction
• oxidative stress induced by unstable metabolites.
There is not enough evidence, however, to identify risperidone’s mechanism of action on blood cells.
Aripiprazole might be a useful alternative when another antipsychotic causes leukopenia and neutropenia. In addition to regularly monitoring the blood cell count during antipsychotic treatment, the neutrophil and platelet counts should be monitored.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Manfredi G, Solfanelli A, Dimitri G, et al. Risperidone-induced leukopenia: a case report and brief review of literature. Gen Hosp Psychiatry. 2013;35(1):102.e3-102.e6.
2. Cosar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18 patients. J Clin Psychopharmacol. 2011;31(2):169-173.
3. Nooijen PM, Carvalho F, Flanagan RJ. Haematological toxicity of clozapine and some other drugs used in psychiatry. Hum Psychopharmacol. 2011;26(2):112-119.
Hematologic abnormalities, such as leukopenia, agranulocytosis, and thrombocytopenia, can be life-threatening adverse reactions to atypical antipsychotics. Although clozapine has the highest risk of leukopenia and neutropenia, these side effects also have been associated with other atypical antipsychotics, including risperidone, olanzapine, ziprasidone, paliperdione, and quetiapine. Risperdone-induced leukopenia has been reported,1,2 but risperidone-
induced bicytopenia— that is, leukopenia/ thrombocytopenia—is rare.
Case
Mr. A, age 25, is an African American man admitted to an inpatient psychiatric unit for management of acute psychotic symptoms. He has been taking risperidone, 4 mg/d, for the past 6 months, although his adherence to the regimen is questionable. Baseline blood count shows a white blood cell (WBC) count of 4,400/μL with an absolute neutrophil count (ANC) of 1,900/μL and a platelet count
160×103/μL. A few days after restarting risperidone, repeat blood count shows a drop in the WBC count to 2,900/μL, with an ANC of 900/μL and a platelet count of 130×103/μL.
Mr. A’s physical examination is normal, he does not have any signs or symptoms of infection, and additional lab tests are negative. Risperidone is considered as a possible cause of bicytopenia and is discontinued. Mr. A agrees to start treatment with aripiprazole, 10 mg/d. In next 10 days, the WBC count increases to 6,000/μL. The ANC at 3,100/μL and platelets at 150×103/μL remain stable throughout hospitalization. The slowly increasing WBC count after stopping risperidone is highly suggestive that this agent caused Mr. A’s bicytopenia.
Differential diagnosis
Bone-marrow suppression is associated with first- and second-generation antipsychotics. Blood dyscrasia is a concern in clinical psychiatry because hematologic abnormalities can be life-threatening, requiring close monitoring of the blood count for patients taking an antipsychotic. It is important, therefore, to consider medication side effects in the differential diagnosis of >1 hematologic abnormalities in these patients.
Precise pathophysiologic understanding of the hematologic side effects of antipsychotics is lacking, although different mechanisms of action have been proposed.3 Possible mechanisms when a patient is taking clozapine or olanzapine include:
• direct toxic effect of the drug on bone marrow
• increased peripheral destruction
• oxidative stress induced by unstable metabolites.
There is not enough evidence, however, to identify risperidone’s mechanism of action on blood cells.
Aripiprazole might be a useful alternative when another antipsychotic causes leukopenia and neutropenia. In addition to regularly monitoring the blood cell count during antipsychotic treatment, the neutrophil and platelet counts should be monitored.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Hematologic abnormalities, such as leukopenia, agranulocytosis, and thrombocytopenia, can be life-threatening adverse reactions to atypical antipsychotics. Although clozapine has the highest risk of leukopenia and neutropenia, these side effects also have been associated with other atypical antipsychotics, including risperidone, olanzapine, ziprasidone, paliperdione, and quetiapine. Risperdone-induced leukopenia has been reported,1,2 but risperidone-
induced bicytopenia— that is, leukopenia/ thrombocytopenia—is rare.
Case
Mr. A, age 25, is an African American man admitted to an inpatient psychiatric unit for management of acute psychotic symptoms. He has been taking risperidone, 4 mg/d, for the past 6 months, although his adherence to the regimen is questionable. Baseline blood count shows a white blood cell (WBC) count of 4,400/μL with an absolute neutrophil count (ANC) of 1,900/μL and a platelet count
160×103/μL. A few days after restarting risperidone, repeat blood count shows a drop in the WBC count to 2,900/μL, with an ANC of 900/μL and a platelet count of 130×103/μL.
Mr. A’s physical examination is normal, he does not have any signs or symptoms of infection, and additional lab tests are negative. Risperidone is considered as a possible cause of bicytopenia and is discontinued. Mr. A agrees to start treatment with aripiprazole, 10 mg/d. In next 10 days, the WBC count increases to 6,000/μL. The ANC at 3,100/μL and platelets at 150×103/μL remain stable throughout hospitalization. The slowly increasing WBC count after stopping risperidone is highly suggestive that this agent caused Mr. A’s bicytopenia.
Differential diagnosis
Bone-marrow suppression is associated with first- and second-generation antipsychotics. Blood dyscrasia is a concern in clinical psychiatry because hematologic abnormalities can be life-threatening, requiring close monitoring of the blood count for patients taking an antipsychotic. It is important, therefore, to consider medication side effects in the differential diagnosis of >1 hematologic abnormalities in these patients.
Precise pathophysiologic understanding of the hematologic side effects of antipsychotics is lacking, although different mechanisms of action have been proposed.3 Possible mechanisms when a patient is taking clozapine or olanzapine include:
• direct toxic effect of the drug on bone marrow
• increased peripheral destruction
• oxidative stress induced by unstable metabolites.
There is not enough evidence, however, to identify risperidone’s mechanism of action on blood cells.
Aripiprazole might be a useful alternative when another antipsychotic causes leukopenia and neutropenia. In addition to regularly monitoring the blood cell count during antipsychotic treatment, the neutrophil and platelet counts should be monitored.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Manfredi G, Solfanelli A, Dimitri G, et al. Risperidone-induced leukopenia: a case report and brief review of literature. Gen Hosp Psychiatry. 2013;35(1):102.e3-102.e6.
2. Cosar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18 patients. J Clin Psychopharmacol. 2011;31(2):169-173.
3. Nooijen PM, Carvalho F, Flanagan RJ. Haematological toxicity of clozapine and some other drugs used in psychiatry. Hum Psychopharmacol. 2011;26(2):112-119.
1. Manfredi G, Solfanelli A, Dimitri G, et al. Risperidone-induced leukopenia: a case report and brief review of literature. Gen Hosp Psychiatry. 2013;35(1):102.e3-102.e6.
2. Cosar B, Taner ME, Eser HY, et al. Does switching to another antipsychotic in patients with clozapine-associated granulocytopenia solve the problem? Case series of 18 patients. J Clin Psychopharmacol. 2011;31(2):169-173.
3. Nooijen PM, Carvalho F, Flanagan RJ. Haematological toxicity of clozapine and some other drugs used in psychiatry. Hum Psychopharmacol. 2011;26(2):112-119.
Pseudobulbar affect: No laughing matter
Pathological laughter and crying— pseudobulbar affect (PBA)—is a disorder of emotional expression characterized by uncontrollable outbursts of laughter or crying without an environmental trigger. Persons with PBA are at an increased risk of depressive and anxiety symptoms associated with an inappropriate outburst of emotion1; such emotional acts might be incongruent with their underlying emotional state.
When should you consider PBA?
Consider PBA in patients with new-onset emotional lability in the presence of certain neurologic conditions. PBA is most common in patients with amyotrophic lateral sclerosis and stroke, in which an incidence of >50% has been estimated.2 Other conditions associated with PBA include Parkinson’s disease, multiple sclerosis, frontotemporal dementia, traumatic brain injury, Alzheimer’s disease, epilepsy, normal pressure hydrocephalus, progressive supranuclear palsy, Wilson disease, and neurosyphilis.3
Avoid PBA misdiagnosis
Depression is the most common PBA misdiagnosis (Table). However, many clinical features distinguish PBA episodes from depressive symptoms; the most prominent difference is duration. Depressive symptoms, including depressed mood, typically last weeks to months, but a PBA episode lasts seconds or minutes. In addition, crying, as a symptom of PBA, might be unrelated or exaggerated relative to the patient’s mood, but crying is congruent with subjective mood in depression. Other symptoms of depression—fatigue, anorexia, insomnia, anhedonia, and feelings of hopelessness and guilt— are not associated with pseudobulbar affect.
PBA also can be differentiated from bipolar disorder (BD) with rapid cycling or mixed mood episodes because of PBA’s relatively brief duration of laughing or crying episodes—with no mood disturbance between episodes—compared with the sustained changes in mood, cognition, and behavior seen in BD.
Options for treating PBA
Serotonergic therapies, such as amitriptyline and fluoxetine, may exert effects by increasing serotonin in the synapse; dextromethorphan may act via antiglutamatergic effects at N-methyl-d-aspartate receptors and sigma-1 receptors.4 Dextromethorphan binding is most prominent in the brainstem and cerebellum, brain areas known to be rich in sigma-1 receptors and key sites implicated in the pathophysiology of PBA. Although the precise mechanisms by which dextromethorphan ameliorates PBA are unknown, modulation of excessive glutamatergic transmission within corticopontine-cerebellar circuits may contribute to its benefits.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tateno A, Jorge RE, Robinson RG. Pathological laughing and crying following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2004;16(4):426-434.
2. Miller A, Pratt H, Schiffer RB. Pseudobulbar affect: the spectrum of clinical presentations, etiologies and treatments. Expert Rev Neurother. 2011;11(7):1077-1088.
3. Haiman G, Pratt H, Miller A. Brain responses to verbal stimuli among multiple sclerosis patients with pseudobulbar affect. J Neurol Sci. 2008;271(1-2):137-147.
4. Werling LL, Keller A, Frank JG, et al. A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder. Exp Neurol. 2007;207(2): 248-257.
Pathological laughter and crying— pseudobulbar affect (PBA)—is a disorder of emotional expression characterized by uncontrollable outbursts of laughter or crying without an environmental trigger. Persons with PBA are at an increased risk of depressive and anxiety symptoms associated with an inappropriate outburst of emotion1; such emotional acts might be incongruent with their underlying emotional state.
When should you consider PBA?
Consider PBA in patients with new-onset emotional lability in the presence of certain neurologic conditions. PBA is most common in patients with amyotrophic lateral sclerosis and stroke, in which an incidence of >50% has been estimated.2 Other conditions associated with PBA include Parkinson’s disease, multiple sclerosis, frontotemporal dementia, traumatic brain injury, Alzheimer’s disease, epilepsy, normal pressure hydrocephalus, progressive supranuclear palsy, Wilson disease, and neurosyphilis.3
Avoid PBA misdiagnosis
Depression is the most common PBA misdiagnosis (Table). However, many clinical features distinguish PBA episodes from depressive symptoms; the most prominent difference is duration. Depressive symptoms, including depressed mood, typically last weeks to months, but a PBA episode lasts seconds or minutes. In addition, crying, as a symptom of PBA, might be unrelated or exaggerated relative to the patient’s mood, but crying is congruent with subjective mood in depression. Other symptoms of depression—fatigue, anorexia, insomnia, anhedonia, and feelings of hopelessness and guilt— are not associated with pseudobulbar affect.
PBA also can be differentiated from bipolar disorder (BD) with rapid cycling or mixed mood episodes because of PBA’s relatively brief duration of laughing or crying episodes—with no mood disturbance between episodes—compared with the sustained changes in mood, cognition, and behavior seen in BD.
Options for treating PBA
Serotonergic therapies, such as amitriptyline and fluoxetine, may exert effects by increasing serotonin in the synapse; dextromethorphan may act via antiglutamatergic effects at N-methyl-d-aspartate receptors and sigma-1 receptors.4 Dextromethorphan binding is most prominent in the brainstem and cerebellum, brain areas known to be rich in sigma-1 receptors and key sites implicated in the pathophysiology of PBA. Although the precise mechanisms by which dextromethorphan ameliorates PBA are unknown, modulation of excessive glutamatergic transmission within corticopontine-cerebellar circuits may contribute to its benefits.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Pathological laughter and crying— pseudobulbar affect (PBA)—is a disorder of emotional expression characterized by uncontrollable outbursts of laughter or crying without an environmental trigger. Persons with PBA are at an increased risk of depressive and anxiety symptoms associated with an inappropriate outburst of emotion1; such emotional acts might be incongruent with their underlying emotional state.
When should you consider PBA?
Consider PBA in patients with new-onset emotional lability in the presence of certain neurologic conditions. PBA is most common in patients with amyotrophic lateral sclerosis and stroke, in which an incidence of >50% has been estimated.2 Other conditions associated with PBA include Parkinson’s disease, multiple sclerosis, frontotemporal dementia, traumatic brain injury, Alzheimer’s disease, epilepsy, normal pressure hydrocephalus, progressive supranuclear palsy, Wilson disease, and neurosyphilis.3
Avoid PBA misdiagnosis
Depression is the most common PBA misdiagnosis (Table). However, many clinical features distinguish PBA episodes from depressive symptoms; the most prominent difference is duration. Depressive symptoms, including depressed mood, typically last weeks to months, but a PBA episode lasts seconds or minutes. In addition, crying, as a symptom of PBA, might be unrelated or exaggerated relative to the patient’s mood, but crying is congruent with subjective mood in depression. Other symptoms of depression—fatigue, anorexia, insomnia, anhedonia, and feelings of hopelessness and guilt— are not associated with pseudobulbar affect.
PBA also can be differentiated from bipolar disorder (BD) with rapid cycling or mixed mood episodes because of PBA’s relatively brief duration of laughing or crying episodes—with no mood disturbance between episodes—compared with the sustained changes in mood, cognition, and behavior seen in BD.
Options for treating PBA
Serotonergic therapies, such as amitriptyline and fluoxetine, may exert effects by increasing serotonin in the synapse; dextromethorphan may act via antiglutamatergic effects at N-methyl-d-aspartate receptors and sigma-1 receptors.4 Dextromethorphan binding is most prominent in the brainstem and cerebellum, brain areas known to be rich in sigma-1 receptors and key sites implicated in the pathophysiology of PBA. Although the precise mechanisms by which dextromethorphan ameliorates PBA are unknown, modulation of excessive glutamatergic transmission within corticopontine-cerebellar circuits may contribute to its benefits.
Disclosure
The author reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tateno A, Jorge RE, Robinson RG. Pathological laughing and crying following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2004;16(4):426-434.
2. Miller A, Pratt H, Schiffer RB. Pseudobulbar affect: the spectrum of clinical presentations, etiologies and treatments. Expert Rev Neurother. 2011;11(7):1077-1088.
3. Haiman G, Pratt H, Miller A. Brain responses to verbal stimuli among multiple sclerosis patients with pseudobulbar affect. J Neurol Sci. 2008;271(1-2):137-147.
4. Werling LL, Keller A, Frank JG, et al. A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder. Exp Neurol. 2007;207(2): 248-257.
1. Tateno A, Jorge RE, Robinson RG. Pathological laughing and crying following traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2004;16(4):426-434.
2. Miller A, Pratt H, Schiffer RB. Pseudobulbar affect: the spectrum of clinical presentations, etiologies and treatments. Expert Rev Neurother. 2011;11(7):1077-1088.
3. Haiman G, Pratt H, Miller A. Brain responses to verbal stimuli among multiple sclerosis patients with pseudobulbar affect. J Neurol Sci. 2008;271(1-2):137-147.
4. Werling LL, Keller A, Frank JG, et al. A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder. Exp Neurol. 2007;207(2): 248-257.