User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Deaf and self-signing
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
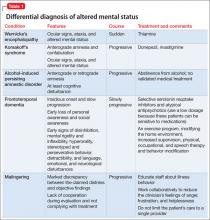
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Self Signing
Mrs. H, a 47-year-old, deaf, African American woman, is brought into the emergency room because she is becoming increasingly withdrawn and is signing to herself. She was hospitalized more than 10 years ago after developing psychotic symptoms and received a diagnosis of psychotic disorder, not otherwise specified. She was treated with olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d, but she has not seen a psychiatrist or taken any psychotropics in 8 years. Upon admission to the inpatient psychiatric unit, Mrs. H reports, through an American Sign Language (ASL) interpreter, that she has had “problems with her parents” and with “being fair” and that she is 18 months pregnant. Urine pregnancy test is negative. Mrs. H also reports that her mother is pregnant. She indicates that it is difficult for her to describe what she is trying to say and that it is difficult to be deaf.
She endorses “very strong” racing thoughts, which she first states have been present for 15 years, then reports it has been 20 months. She endorses high-energy levels, feeling like there is “work to do,” and poor sleep. However, when asked, she indicates that she sleeps for 15 hours a day.
Which is critical when conducting a psychiatric assessment for a deaf patient?
a) rely only on the ASL interpreter
b) inquire about the patient’s communication preferences
c) use written language to communicate instead of speech
d) use a family member as interpreter
The authors’ observations
Mental health assessment of a deaf a patient involves a unique set of challenges and requires a specialized skill set for mental health practitioners—a skill set that is not routinely covered in psychiatric training programs.
a We use the term “deaf” to describe patients who have severe hearing loss. Other terms, such as “hearing impaired,” might be considered pejorative in the Deaf community. The term “Deaf” (capitalized) refers to Deaf culture and community, which deaf patients may or may not identify with.
Deafness history
It is important to assess the cause of deafness,1,2 if known, and its age of onset (Table 1). A person is considered to be prelingually deaf if hearing loss was diagnosed before age 3.2 Clinicians should establish the patient’s communication preferences (use of assistive devices or interpreters or preference for lip reading), home communication dynamic,2 and language fluency level.1-3 Ask the patient if she attended a specialized school for the deaf and, if so, if there was an emphasis on oral communication or signing.2
HISTORY Conflicting reports
Mrs. H reports that she has been deaf since age 9, and that she learned sign language in India, where she became the “star king.” Mrs. H states that she then moved to the United States where she went to a school for the deaf. When asked if her family is able to communicate with her in sign language, she nods and indicates that they speak to her in “African and Indian.”
Mrs. H’s husband, who is hearing, says that Mrs. H is congenitally deaf, and was raised in the Midwestern United States where she attended a specialized school for the deaf. Mr. H and his 2 adult sons are hearing but communicate with Mrs. H in basic ASL. He states that Mrs. H sometimes uses signs that he and his sons cannot interpret. In addition to increased self-preoccupation and self-signing, Mrs. H has become more impulsive.
What are limitations of the mental status examination when evaluating a deaf patient?
a) facial expressions have a specific linguistic function in ASL
b) there is no differentiation in the mental status exam of deaf patients from that of hearing patients
c) the Mini-Mental State Examination (MMSE) is a validated tool to assess cognition in deaf patients
d) the clinician should not rely on the interpreter to assist with the mental status examination
The authors’ observation
Performing a mental status examination of a deaf patient without recognizing some of the challenges inherent to this task can lead to misleading findings. For example, signing and gesturing can give the clinician an impression of psychomotor agitation.2 What appears to be socially withdrawn behavior might be a reaction to the patient’s inability to communicate with others.2,3 Social skills may be affected by language deprivation, if present.3 In ASL, facial expressions have specific linguistic functions in addition to representing emotions,2 and can affect the meaning of the sign used. An exaggerated or intense facial expression with the sign “quiet,” for example, usually means “very quiet.”4 In assessing cognition, the MMSE is not available in ASL and has not been validated in deaf patients.5 Also, deaf people have reduced access to information, and a lack of knowledge does not necessarily correlate with low IQ.2
The interpreter’s role
An ASL interpreter can aid in assessing a deaf patient’s communication skills. The interpreter can help with a thorough language evaluation1,6 and provide information about socio-cultural norms in the Deaf community.7 Using an ASL interpreter with special training in mental health1,3,6,7 is important to accurately diagnose thought disorders in deaf patients.1
EVALUATION Mental status exam
Mrs. H is poorly groomed and is wearing a pink housecoat, with her hair in disarray. She seems to be distracted by something next to the interpreter, because her eyes keep roving in this direction. She has moderate psychomotor agitation, based on the rapidity of her signing and gesturing. Mrs. H makes indecipherable vocalizations while signing, often loud and with an urgent quality. Her affect is elevated and expansive. She is not oriented to place or time and when asked where she is, signs, “many times, every day, 6-9-9, 2-5, more trouble…”
The ASL interpreter notes that Mrs. H signs so quickly that only about one-half of her signs are interpretable. Mrs. H’s grammar is not always correct and that her syntax is, at times, inappropriate. Mrs. H’s letters are difficult to interpret because she often starts and concludes a word with a clear sign, but the intervening letters are rapid and uninterpretable. She also uses several non-alphabet signs that cannot be interpreted (approximately 10% to 15% of signs) and repeats signs without clear context, such as “nothing off.” Mrs. H can pause to clarify for the interpreter at the beginning of the interview but is not able to do so by the end of the interview.
How does assessment of psychosis differ when evaluating deaf patients?
a) language dysfluency must be carefully differentiated from a thought disorder
b) signing to oneself does not necessarily indicate a response to internal stimuli
c) norms in Deaf culture might be misconstrued as delusions
d) all of the above
The authors’ observations
The prevalence of psychotic disorders among deaf patients is unknown.8 Although older studies have reported an increased prevalence of psychotic disorders among deaf patients, these studies suffer from methodological problems.1 Other studies are at odds with each other, variably reporting a greater,9 equivalent,10 and lesser incidence of psychotic disorders in deaf psychiatric inpatients.11 Deaf patients with psychotic disorders experience delusions, hallucinations, and thought disorders,1,3 and assessing for these symptoms in deaf patients can present a diagnostic challenge (Table 2).
Delusions are thought to present similarly in deaf patients with psychotic disorders compared with hearing patients.1,3 Paranoia may be increased in patients who are postlingually deaf, but has not been associated with prelingual deafness. Deficits in theory of mind related to hearing impairment have been thought to contribute to delusions in deaf patients.1,12
Many deaf patients distrust health care systems and providers,2,3,13 which may be misinterpreted as paranoia. Poor communication between deaf patients and clinicians and poor health literacy among deaf patients contribute to feelings of mistrust. Deaf patients often report experiencing prejudice within the health care system, and think that providers lack sufficient knowledge of deafness.13 Care must be taken to ensure that Deaf cultural norms are not misinterpreted as delusions.
Hallucinations. How deaf patients experience hallucinations, especially in prelingual deafness, likely is different from hallucinatory experiences of hearing patients.1,14 Deaf people with psychosis have described ”ideas coming into one’s head” and an almost “telepathic” process of “knowing.”14 Deaf patients with schizophrenia are more likely to report visual elements to their hallucinations; however, these may be subvisual precepts rather than true visual hallucinations.1,15 For example, hallucination might include the perception of being signed to.1
Deaf patients’ experience of auditory hallucinations is thought to be closely related to past auditory experiences. It is unlikely that prelingually deaf patients experience true auditory hallucinations.1,14 An endorsement of hearing a “voice” in ASL does not necessarily translate to an audiological experience.15 If profoundly prelingually deaf patients endorse hearing voices, generally they cannot assign acoustic properties (pitch, tone, volume, accent, etc.).1,14,15 It may not be necessary to fully comprehend the precise modality of how hallucinations are experienced by deaf patients to provide therapy.14
Self-signing, or signing to oneself, does not necessarily indicate that a deaf person is responding to a hallucinatory experience. Non-verbal patients may gesture to themselves without clear evidence of psychosis. When considering whether a patient is experiencing hallucinations, it is important to look for other evidence of psychosis.3
Possible approaches to evaluating hallucinations in deaf patients include asking,, “is someone signing in your head?” or “Is someone who is not in the room trying to communicate with you?”
Thought disorders in deaf psychiatric inpatients are difficult to diagnose, in part because of a high rate of language dysfluency in deaf patients; in samples of psychiatric inpatients, 75% are not fluent in ASL, 66% are not fluent in any language).1,3,11 Commonly, language dysfluency is related to language deprivation because of late or inadequate exposure to ASL, although it may be related to neurologic damage or aphasia.1,3,6,16 Deaf patients can have additional disabilities, including learning disabilities, that might contribute to language dysfluency.2 Language dysfluency can be misattributed to a psychotic process1-3,7 (Table 3).1
Language dysfluency and thought disorders can be difficult to differentiate and may be comorbid. Loose associations and flight of ideas can be hard to assess in patients with language dysfluency. In general, increasing looseness of association between concepts corresponds to an increasing likelihood that a patient has true loose associations rather than language dysfluency alone.3 Deaf patients with schizophrenia can be identified by the presence of associated symptoms of psychosis, especially if delusions are present.1,3
EVALUATION Psychotic symptoms
Mrs. H’s thought process appears disorganized and illogical, with flight of ideas. She might have an underlying language dysfluency. It is likely that Mrs. H is using neologisms to communicate because of her family’s lack of familiarity with some of her signs. She also demonstrates perseveration, with use of certain signs repeatedly without clear context (ie, “nothing off”).
Her thought content includes racial themes—she mentions Russia, Germany, and Vietnam without clear context—and delusions of being the “star king” and of being pregnant. She endorses paranoid feelings that people on the inpatient unit are trying to hurt her, although it isn’t clear whether this represents a true paranoid delusion because of the hectic climate of the unit, and she did not show unnecessarily defensive or guarded behaviors.
She is seen signing to herself in the dayroom and endorses feeling as though someone who is not in the room—described as an Indian teacher (and sometimes as a boss or principal) known as “Mr. Smith” or “Mr. Donald”—is trying to communicate with her. She describes this person as being male and female. She mentions that sometimes she sees an Indian man and another man fighting. It is likely that Mrs. H is experiencing hallucinations from decompensated psychosis, because of the constellation and trajectory of her symptoms. Her nonverbal behavior—her eyes rove around the room during interviews—also supports this conclusion.
Because of evidence of mood and psychotic symptoms, and with a collateral history that suggests significant baseline disorganization, Mrs. H receives a diagnosis of schizoaffective disorder, bipolar type. She is restarted on olanzapine, 10 mg/d, and valproic acid, 1,000 mg/d.
Mrs. H’s psychomotor acceleration and affective elevation gradually improve with pharmacotherapy. After a 2-week hospitalization, despite ongoing disorganization and self-signing, Mrs. H’s husband says that he feels she is improved enough to return home, with plans to continue to take her medications and to reestablish outpatient follow-up.
Bottom Line
Psychiatric assessment of deaf patients presents distinctive challenges related to cultural and language barriers—making it important to engage an ASL interpreter with training in mental health during assessment of a deaf patient. Clinicians must become familiar with these challenges to provide effective care for mentally ill deaf patients.
Related Resources
• Landsberger SA, Diaz DR. Communicating with deaf patients: 10 tips to deliver appropriate care. Current Psychiatry. 2010;9(6):36-37.
• Deaf Wellness Center. University of Rochester School of Medicine. www.urmc.rochester.edu/deaf-wellness-center.
• Gallaudet University Mental Health Center. www.gallaudet.edu/
mental_health_center.html.
Drug Brand Names
Olanzapine • Zyprexa
Valproic acid • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
1. Landsberger SA, Diaz DR. Identifying and assessing psychosis in deaf psychiatric patients. Curr Psychiatry Rep. 2011;13(3):198-202.
2. Fellinger J, Holzinger D, Pollard R. Mental health of deaf people. Lancet. 2012;379(9820):1037-1044.
3. Glickman N. Do you hear voices? Problems in assessment of mental status in deaf persons with severe language deprivation. J Deaf Stud Deaf Educ. 2007;12(2):127-147.
4. Vicars W. ASL University. Facial expressions. http://www.lifeprint.com/asl101/pages-layout/facialexpressions.htm. Accessed April 2, 2013.
5. Dean PM, Feldman DM, Morere D, et al. Clinical evaluation of the mini-mental state exam with culturally deaf senior citizens. Arch Clin Neuropsychol. 2009;24(8):753-760.
6. Crump C, Glickman N. Mental health interpreting with language dysfluent deaf clients. Journal of Interpretation. 2011;21(1):21-36.
7. Leigh IW, Pollard RQ Jr. Mental health and deaf adults. In: Marschark M, Spencer PE, eds. Oxford handbook of deaf studies, language, and education. Vol 1. New York, NY: Oxford University Press. 2011:214-226.
8. Øhre B, von Tezchner S, Falkum E. Deaf adults and mental health: A review of recent research on the prevalence and distribution of psychiatric symptoms and disorders in the prelingually deaf adult population. International Journal on Mental Health and Deafness. 2011;1(1):3-22.
9. Appleford J. Clinical activity within a specialist mental health service for deaf people: comparison with a general psychiatric service. Psychiatric Bulletin. 2003;27(10): 375-377.
10. Landsberger SA, Diaz DR. Inpatient psychiatric treatment of deaf adults: demographic and diagnostic comparisons with hearing inpatients. Psychiatr Serv. 2010;61(2):196-199.
11. Black PA, Glickman NS. Demographics, psychiatric diagnoses, and other characteristics of North American deaf and hard-of-hearing inpatients. J Deaf Stud Deaf Educ. 2006; 11(3):303-321.
12. Thewissen V, Myin-Germeys I, Bentall R, et al. Hearing impairment and psychosis revisited. Schizophr Res. 2005; 76(1):99-103.
13. Steinberg AG, Barnett S, Meador HE, et al. Health care system accessibility. Experiences and perceptions of deaf people. J Gen Inter Med. 2006;21(3):260-266.
14. Paijmans R, Cromwell J, Austen S. Do profoundly prelingually deaf patients with psychosis really hear voices? Am Ann Deaf. 2006;151(1):42-48.
15. Atkinson JR. The perceptual characteristics of voice-hallucinations in deaf people: insights into the nature of subvocal thought and sensory feedback loops. Schizophr Bull. 2006;32(4):701-708.
16. Trumbetta SL, Bonvillian JD, Siedlecki T, et al. Language-related symptoms in persons with schizophrenia and how deaf persons may manifest these symptoms. Sign Language Studies. 2001;1(3):228-253.
Should you use an anticonvulsant to treat impulsivity and aggression?
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
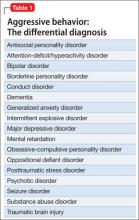
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. V, age 29, is a US Army veteran who presents to the psychiatric emergency department because of increasing aggression. He recently returned from deployment overseas and lives with his parents. Mr. V’s mother reports that he has been increasingly “unstable” and describes an incident during which he punched a hole in his bedroom window after a temporary slow-down in the home’s Internet connection.
The workup and review of the history rules out substance abuse, posttraumatic stress disorder, bipolar disorder, seizure disorder, and personality disorders. He is currently taking only omeprazole, 40 mg/d, for acid reflux. The psychiatrist considers prescribing an antiepileptic medication to treat the agitation. Why this choice of agent?
According to DSM-5, patients who have repeated episodes of aggression can be given a diagnosis of intermittent explosive disorder, but such behavior can occur secondary to other psychiatric diagnoses (Table 1). No medications are FDA approved for aggression.1
Aggression and associated verbal and physical acts fall into 2 subtypes: impulsive type and premeditated (predatory) type. Impulsive aggression generally is described as an emotionally charged aggressive response characterized by a loss of behavioral control.
Premeditated aggression
Pharmacotherapy is directed primarily at treating impulsive aggression because this subtype is thought to be caused by neurologic deficits that can affect a person’s ability to process, and react appropriately to, external stimuli. Agitation can result from neuronal hyperactivity.2 Agents such as antiepileptic drugs (AEDs) have the potential to reduce the intensity and frequency of such behaviors.2
In this article, we focus on the use of AEDs for treating impulsive aggression in adults.
Reviewing the evidence for AEDs
The neurobiology of aggression involves multiple neurotransmitters, intracellular pathways, and ion channels.3 AEDs have several mechanisms of action, however; primary mechanisms include action on sodium and calcium channels and modulation of γ-aminobutyric acid (GABA), glutamate, and carbonic anhydrase.2,3 Agent-specific mechanisms of actions are listed in Table 2.
Phenytoin. Several double-blind, placebo-controlled trials have found a statistically significant difference between phenytoin and placebo for treating impulsive aggression, as measured by the Overt Aggression Scale (OAS)a or a modified version (MOAS/ OAS-M).1,2,4 Researchers found that phenytoin, 300 mg/d, but not 100 mg/d, decreased impulsive aggression.4
a Studies generally used the OAS, or one of its modifications, to evaluate aggressive behavior.2,4
Valproate. Trials of valproate for decreasing aggressive behaviors have produced mixed results with regard to primary outcome when used at standard dosages and within the therapeutic range measured by serum concentration.2,3 In a pooled analysis of studies that met stringent criteria (randomized, controlled trial, aggressive behavior as primary outcome, patients free of organic illness or neurologic illness), Jones and colleagues1 reported that valproate/divalproex did not produce statistically significant results compared with placebo for treating impulsive aggression.
Carbamazepine and oxcarbazepine. Double-blind, placebo-controlled trials and case studies of carbamazepine have shown mixed results. In contrast, oxcarbazepine has been found to significantly decrease aggressive behavior, measured by OAS/MOAS/ OAS-M scores.2,3 Total daily dosages of oxcarbazepine ranged from 1,500 to 2,400 mg.2-4 It has been speculated that oxcarbazepine might be a useful option for treating impulsive aggression because of its therapeutic value in temporal lobe seizures—a subtype of seizure disorder that involves the limbic system, which also modulates aggressiveness.5
Additionally, when compared with carbamazepine, oxcarbazepine has a lower risk of cardiotoxicity, neurotoxicity, and blood dyscrasia. Oxcarbazepine has fewer drug-drug interactions because of a lower degree of hepatic enzyme induction.
Topiramate. Several studies have confirmed the efficacy of topiramate for aggressive behavior.2,3 However, there have been reports that topiramate can induce or exacerbate aggression in some patients, an effect that might be dose-related. Aggression might respond better to a higher, short-term dosage (eg, 400 mg/d) than to lower (100 to 300 mg/d) dosages, which might exacerbate aggression.3
Gabapentin. Research on using gabapentin for aggression is limited. Speculation is that the combined activity of gabapentin on GABA and glutamate give the drug its antiaggressive effect.3 No randomized, double-blind, placebo-controlled trials are underway comparing gabapentin and placebo or other active medication for impulsive aggression.
Some case reports and small-scale, open-label studies report a decrease in aggression with gabapentin. As is the case with topiramate, a lower dosage (200 mg to 400 mg) has been reported to result in increased aggression—whereas a higher dosages (800 mg) decreases aggressive behavior.2,3
Lamotrigine. The results of several studies, including double-blind, placebo-controlled trials, support the use of lamotrigine for aggressive behavior. A number of these studies, however, used scales other than OAS (or its modifications) to determine this outcome. One trial showed increased aggression in several patients on lower-dosage lamotrigine (100 mg/d) that resolved when the dosage was increased.2,3
Treatment recommendations
Although all AEDs have some documented efficacy against aggression, choosing the appropriate agent depends on patient-specific variables. Avoiding divalproex in patients with liver dysfunction, for example, or carbamazepine in those with a preexisting cardiac conduction abnormality will improve outcomes by avoiding complications.
It is important to rule out all other causes of aggression before selecting a treatment. The presence of one or more of the diagnoses listed in Table 1 could lead to selection of an alternate class of medication. Nondrug therapies, such as cognitive-behavioral therapy, also should be considered.
Related Resources
• Coccaro EF. Aggression. Psychiatric assessment and treatment. Chicago, IL: Marcel Dekker, Inc.; 2003.
• Citrome LL. Aggression. http://emedicine.medscape.com/article/288689-overview. Updated June 18, 2012. Accessed February 28, 2014.
Drug Brand Names
Carbamazepine • Tegretol Phenytoin • Dilantin
Gabapentin • Neurontin Topiramate • Topamax
Lamotrigine • Lamictal Valproate/Divalproex
Omeprazole • Prilosec • Depakote
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.
1. Jones RM, Arlidge J, Gilham R, et al. Efficacy of mood stabilizers in the treatment of impulsive or repetitive aggression: systemic review and meta-analysis. Br J Psychiatry. 2011;198(2):93-98.
2. Stanford MS, Anderson NE, Lake SL, et al. Pharmacologic treatment of impulsive aggression with antiepileptic drugs. Curr Treat Options Neurol. 2009;11(5):383-390.
3. Comai S, Tau M, Pavlovic Z, et al. The psychopharmacology of aggressive behavior: a translational approach: part 2: clinical studies using atypical antipsychotics, anticonvulsants, and lithium. J Clin Psychopharmacol. 2012;32(2):237-260.
4. Huband N, Ferriter M, Nathan R, et al. Antiepileptics for aggression and associated impulsivity. Cochrane Database Sys Rev. 2010;2:CD003499.
5. Mattes JA. Medications for aggressiveness in prison: focus on oxcarbazepine. J Am Acad Psychiatry Law. 2012;40(2):234-238.
The first of 2 parts: A practical approach to subtyping depression among your patients
Depression carries a wide differential diagnosis. Practitioners sometimes think polarity is the fundamental distinction when they conceptualize depression as a clinical entity; in fact, many nosologic frameworks have been described for defining and subtyping clinically meaningful forms of depression, and each waxed and waned in popularity.
Kraepelin, writing in the early 20th century, linked manic-depressive illness with “the greater part of the morbid states termed melancholia,”1 but many features other than polarity remain important components of depression, and those features often carry implications for how individual patients respond to treatment.
In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
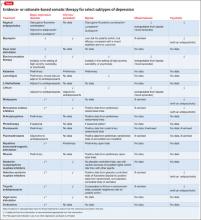
The first part of this article examines major depressive disorder (MDD), minor depression, chronic depression, depression in bipolar disorder, depression that is severe or mild, and psychotic depression. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
The subtypes of depression that I’ll discuss in the second part of the article are listed on page 47.
Major and minor depression
MDD has been the focus of most drug trials seeking FDA approval. As a syndrome, MDD is defined by a constellation of features that are related not only to mood but also to sleep, energy, cognition, motivation, and motor behavior, persisting for ≥2 weeks.
DSM-5 has imposed few changes to the basic definition of MDD:
• bereavement (the aftermath of death of a loved one), formerly an exclusion criterion, no longer precludes making a diagnosis of MDD when syndromal criteria are otherwise fulfilled
• “with anxious distress” is a new course specifier that designates prominent anxiety features (feeling worried, restless, tense, or keyed up; fearful of losing control or something terrible happening)
• “with mixed features” is a new course specifier pertinent when ≥3 mania or hypomania symptoms coexist (that is, might be a subsyndromal mania or hypomania) with a depressive syndrome; the mixed features specifier can be applied to depressed patients whether or not they have ever had a manic or hypomanic episode, but MDD—rather than bipolar disorder— remains the overarching diagnosis, unless criteria have ever been met for a full mania or hypomania.
More than 2 dozen medications are FDA-approved to treat MDD. Evidence-based psychotherapies (eg, cognitive-behavioral therapy [CBT] and interpersonal therapy), as adjuncts to pharmacotherapy, further improve outcomes, but with only modest additional effect.2
Minor depression. Depressive states that involve 2 to 4 associated symptoms lasting ≥2 weeks but <2 years are sometimes described as minor depression, captured within DSM-5 as “depression not elsewhere defined.” The terminology of so-called “minor depression” generally is shunned, in part because it might wrongly connote low severity and therefore discourage treatment—even though it confers more than a 5-fold increase in risk of MDD.3
Chronicity
DSM-IV-TR identified long-standing depression by 2 constructs:
• chronic major depression (an episode of MDD lasting ≥2 years in adults, ≥1 year in children and adolescents)
• dysthymic disorder (2 to 4 depressive symptoms for ≥2 years in adults and ≥1 year in children and adolescents), affecting 3% to 6% of adults and carrying a 2-fold increased risk of MDD, eventually.4
Depression that begins as dysthymic disorder and blossoms into syndromal MDD is described as “double depression”—although it is not recognized as a unique condition in any edition of the DSM. Subsequent incomplete recovery may revert to dysthymic disorder. DSM-5 has subsumed chronic major depression and dysthymia under the unified heading of persistent depressive disorder.
There are no FDA-approved drugs for treating dysthymia. A meta-analysis of 9 controlled trials of off-label use of antidepressants to treat dysthymia revealed an overall response rate of 52.4%, compared with 29.9% for placebo.5 Notably, although the active drug response rate in these studies is comparable to what seen in MDD, the placebo response rate was approximately 10% lower than what was seen in major depression.
Positive therapeutic findings (typically, treatment for 6 to 12 weeks) have been reported in so-called “pure” dysthymic disorder with sertraline, fluoxetine, imipramine, ritanserin, moclobemide (not approved for use in the United States), and phenelzine; the results of additional, positive placebo-controlled studies support the utility of duloxetine6 and paroxetine.7 Randomized trials have reported negative findings for desipramine,8 fluoxetine,9 and escitalopram10 escitalopram10—although the sample size in these latter studies might have been too small to detect a drug-placebo difference.
In dysthymic and minor-depressive middle-age and older adult men who have a low serum level of testosterone, hormone replacement was shown to be superior to placebo in several randomized trials.11 Studies of adjunctive atypical antipsychotics for dysthymic disorder are scarce; a Cochrane review identified controlled data only with amisulpride (not approved for use in the United States), which yielded a modest therapeutic effect.12
Polarity
In recent years, depression in bipolar disorder (BD) has been contrasted with unipolar MDD based on a difference in:
• duration (briefer in BD)
• severity (worse in BD)
• risk of suicide (higher)
• comorbidity (more extensive)
• family history (often present for BD and highly recurrent depression)
• treatment outcome (generally less favorable).
DSM-5 has at least somewhat blurred the distinctions in polarity by way of the new construct of “major depression with mixed features” (see the discussion of MDD above), identifiable even when a person has never had a full manic or hypomanic episode.
No randomized trials have been conducted to identify the best treatments for such presentations, which has invited extrapolation from the literature in regard to bipolar mixed episodes, and suggesting that 1) some mood stabilizers (eg, divalproex) might have value and 2) antidepressants might exacerbate manic symptoms.
Perhaps most noteworthy in regard to treating bipolar depression is the unresolved, but hotly debated, controversy over whether and, if so, when, an antidepressant is inappropriate (based on concerns about possible induction or exacerbation of manic symptoms). In addition, nearly all of the large, randomized controlled trials of antidepressants for bipolar depression have shown that they offer no advantage over placebo.
Some authors argue that a lack of response to antidepressants might, itself, be a “soft” indicator of “bipolarity.” However, nonresponse to antidepressants should prompt a wider assessment of features other than polarity—including psychosis, anxiety, substance abuse, a personality disorder, psychiatric adverse effects from concomitant medications, medical comor bidity, adequacy of trials of medical therapy, and potential non-adherence to such trials—to account for poor antidepressant outcomes.
Severity
Severity of depression warrants consideration when formulating impressions about the nature and treatment of all presentations of depression.
High-severity forms prompt decisions about treatment setting (inpatient or outpatient); suicide assessment; and therapeutic modalities (eg, electroconvulsive therapy is more appropriate than psychotherapy for catatonic depression).
Mild forms. A recent meta-analysis of 6 randomized trials (each of >6 weeks’ duration) of antidepressants for mild depression demonstrated that these agents exert only a modest effect compared with placebo, owing largely to higher placebo-responsivity in mild depressive episodes than in moderate and severe episodes.13 In contrast, another meta-analysis of subjects who had “mild” baseline depression severity scores found that antidepressant medication had greater efficacy than placebo in 4 of 6 randomized trials.14 Higher depression severity levels typically diminish the placebo response rate but also reduce the magnitude of drug efficacy.
Psychosis
Before DSM-III, psychotic (as opposed to neurotic) depression was perhaps the key nosologic distinction when characterizing forms of depression. The presence of psychosis and related components (eg, mood-congruence) is closely linked with the severity of depression (high) and prognosis and longitudinal outcome (poorer), and has implications for treatment (Table).
Bottom Line
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including polarity, chronicity, recurrence, psychosis, treatment resistance, comorbidity, and atypicality, among other classifications. Clinical characteristics vary across subtypes—and so do corresponding preferred treatments, which should be tailored to the needs of each of your patients.
Related Resources
• Goldberg JF, Thase ME. Monoamine oxidase inhibitors revisited: what you should know. J Clin Psychiatry. 2013;74(2):189-191.
• Goldberg JF. Antidepressants in bipolar disorder: 7 myths and realities. Current Psychiatry. 2010;9(5):41-49.
• Ketamine cousin rapidly lifts depression without side effects. National Institute of Mental Health. http://www. nimh.nih.gov/news/science-news/2013/ketamine-cousin-rapidly-lifts-depression-without-side-effects.shtml. Published May 23, 2013. Accessed March 20, 2014.
• Research Domain Criteria (RDoC). National Institute of Mental Health. http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml?u tm_ source = govdelivery&utm_medium=email&utm_campaign= govdelivery. Accessed March 20, 2014.
Drug Brand Names
Amisulpride • Amazeo, Lurasidone • Latuda
Amival, Amipride, Sulpitax Mirtazapine • Remeron
Aripiprazole • Abilify Moclobemide • Amira,
Armodafinil • Nuvigil Aurorix, Clobemix,
Bupropion • Wellbutrin Depnil, Manerix
Desipramine • Norpramin Modafinil • Provigil
Divalproex • Depakote, Olanzapine/fluoxetine
Depakene • Symbyax
Duloxetine • Cymbalta Paroxetine • Paxil
Escitalopram • Lexapro Phenelzine • Nardil
Fluoxetine • Prozac Pramipexole • Mirapex
Imipramine • Tofranil Quetiapine • Seroquel
Ketamine • Ketalar Riluzole • Rilutek
Lamotrigine • Lamictal Sertraline • Zoloft
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion
Pharmaceuticals, and Takeda and Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
Editor’s note: The second part of Dr. Goldberg’s review of depression subtypes—focusing on “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder—will appear in the May 2014 issue of Current Psychiatry.
1. Kraepelin E. Manic-depressive insanity and paranoia. Barclay RM, trans. Robertson GM, ed. Edinburgh, Scotland: E&S Livingstone; 1921:1.
2. Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70(9):1219-1229.
3. Fogel J, Eaton WW, Ford DE. Minor depression as a predictor of the first onset of major depressive disorder over a 15-year follow-up. Acta Psychiatr Scand. 2006; 113(1):36-43.
4. Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79(1-3):71-79.
5. Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(4):509-514.
6. Hellerstein DJ, Stewart JW, McGrath PJ, et al. A randomized controlled trial of duloxetine versus placebo in the treatment of nonmajor chronic depression. J Clin Psychiatry. 2012;73(7):984-991.
7. Ravindran AV, Cameron C, Bhatla R, et al. Paroxetine in the treatment of dysthymic disorder without co-morbidities: a double-blind, placebo-controlled, flexible-dose study. Asian J Psychiatry. 2013;6(2):157-161.
8. Stewart JW, McGrath PJ, Liebowitz MR, et al. Treatment outcome validation of DSM-III depressive subtypes. Clinical usefulness in outpatients with mild to moderate depression. Arch Gen Psychiatry. 1985;42(12):1148-1153.
9. Serrano-Blanco A, Gabarron E, Garcia-Bayo I, et al. Effectiveness and cost-effectiveness of antidepressant treatment in primary health care: a six-month randomised study comparing fluoxetine to imipramine. J Affect Disord. 2006;91(2-3):153-163.
10. Hellerstein DJ, Batchelder ST, Hyler S, et al. Escitalopram versus placebo in the treatment of dysthymic disorders. Int Clin Psychopharmacol. 2010;25(3):143-148.
11. Seidman SN, Orr G, Raviv G, et al. Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol. 2009;29(3):216-221.
12. Komossa K, Depping AM, Gaudchau A, et al. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010; 8:(12):CD008121.
13. Fournier JC, DeRubeis RJ, Hollom SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
14. Stewart JA, Deliyannides DA, Hellerstein DJ, et al. Can people with nonsevere major depression benefit from antidepressant medication? J Clin Psychiatry. 2012;73(4):518-525.
Depression carries a wide differential diagnosis. Practitioners sometimes think polarity is the fundamental distinction when they conceptualize depression as a clinical entity; in fact, many nosologic frameworks have been described for defining and subtyping clinically meaningful forms of depression, and each waxed and waned in popularity.
Kraepelin, writing in the early 20th century, linked manic-depressive illness with “the greater part of the morbid states termed melancholia,”1 but many features other than polarity remain important components of depression, and those features often carry implications for how individual patients respond to treatment.
In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
The first part of this article examines major depressive disorder (MDD), minor depression, chronic depression, depression in bipolar disorder, depression that is severe or mild, and psychotic depression. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
The subtypes of depression that I’ll discuss in the second part of the article are listed on page 47.
Major and minor depression
MDD has been the focus of most drug trials seeking FDA approval. As a syndrome, MDD is defined by a constellation of features that are related not only to mood but also to sleep, energy, cognition, motivation, and motor behavior, persisting for ≥2 weeks.
DSM-5 has imposed few changes to the basic definition of MDD:
• bereavement (the aftermath of death of a loved one), formerly an exclusion criterion, no longer precludes making a diagnosis of MDD when syndromal criteria are otherwise fulfilled
• “with anxious distress” is a new course specifier that designates prominent anxiety features (feeling worried, restless, tense, or keyed up; fearful of losing control or something terrible happening)
• “with mixed features” is a new course specifier pertinent when ≥3 mania or hypomania symptoms coexist (that is, might be a subsyndromal mania or hypomania) with a depressive syndrome; the mixed features specifier can be applied to depressed patients whether or not they have ever had a manic or hypomanic episode, but MDD—rather than bipolar disorder— remains the overarching diagnosis, unless criteria have ever been met for a full mania or hypomania.
More than 2 dozen medications are FDA-approved to treat MDD. Evidence-based psychotherapies (eg, cognitive-behavioral therapy [CBT] and interpersonal therapy), as adjuncts to pharmacotherapy, further improve outcomes, but with only modest additional effect.2
Minor depression. Depressive states that involve 2 to 4 associated symptoms lasting ≥2 weeks but <2 years are sometimes described as minor depression, captured within DSM-5 as “depression not elsewhere defined.” The terminology of so-called “minor depression” generally is shunned, in part because it might wrongly connote low severity and therefore discourage treatment—even though it confers more than a 5-fold increase in risk of MDD.3
Chronicity
DSM-IV-TR identified long-standing depression by 2 constructs:
• chronic major depression (an episode of MDD lasting ≥2 years in adults, ≥1 year in children and adolescents)
• dysthymic disorder (2 to 4 depressive symptoms for ≥2 years in adults and ≥1 year in children and adolescents), affecting 3% to 6% of adults and carrying a 2-fold increased risk of MDD, eventually.4
Depression that begins as dysthymic disorder and blossoms into syndromal MDD is described as “double depression”—although it is not recognized as a unique condition in any edition of the DSM. Subsequent incomplete recovery may revert to dysthymic disorder. DSM-5 has subsumed chronic major depression and dysthymia under the unified heading of persistent depressive disorder.
There are no FDA-approved drugs for treating dysthymia. A meta-analysis of 9 controlled trials of off-label use of antidepressants to treat dysthymia revealed an overall response rate of 52.4%, compared with 29.9% for placebo.5 Notably, although the active drug response rate in these studies is comparable to what seen in MDD, the placebo response rate was approximately 10% lower than what was seen in major depression.
Positive therapeutic findings (typically, treatment for 6 to 12 weeks) have been reported in so-called “pure” dysthymic disorder with sertraline, fluoxetine, imipramine, ritanserin, moclobemide (not approved for use in the United States), and phenelzine; the results of additional, positive placebo-controlled studies support the utility of duloxetine6 and paroxetine.7 Randomized trials have reported negative findings for desipramine,8 fluoxetine,9 and escitalopram10 escitalopram10—although the sample size in these latter studies might have been too small to detect a drug-placebo difference.
In dysthymic and minor-depressive middle-age and older adult men who have a low serum level of testosterone, hormone replacement was shown to be superior to placebo in several randomized trials.11 Studies of adjunctive atypical antipsychotics for dysthymic disorder are scarce; a Cochrane review identified controlled data only with amisulpride (not approved for use in the United States), which yielded a modest therapeutic effect.12
Polarity
In recent years, depression in bipolar disorder (BD) has been contrasted with unipolar MDD based on a difference in:
• duration (briefer in BD)
• severity (worse in BD)
• risk of suicide (higher)
• comorbidity (more extensive)
• family history (often present for BD and highly recurrent depression)
• treatment outcome (generally less favorable).
DSM-5 has at least somewhat blurred the distinctions in polarity by way of the new construct of “major depression with mixed features” (see the discussion of MDD above), identifiable even when a person has never had a full manic or hypomanic episode.
No randomized trials have been conducted to identify the best treatments for such presentations, which has invited extrapolation from the literature in regard to bipolar mixed episodes, and suggesting that 1) some mood stabilizers (eg, divalproex) might have value and 2) antidepressants might exacerbate manic symptoms.
Perhaps most noteworthy in regard to treating bipolar depression is the unresolved, but hotly debated, controversy over whether and, if so, when, an antidepressant is inappropriate (based on concerns about possible induction or exacerbation of manic symptoms). In addition, nearly all of the large, randomized controlled trials of antidepressants for bipolar depression have shown that they offer no advantage over placebo.
Some authors argue that a lack of response to antidepressants might, itself, be a “soft” indicator of “bipolarity.” However, nonresponse to antidepressants should prompt a wider assessment of features other than polarity—including psychosis, anxiety, substance abuse, a personality disorder, psychiatric adverse effects from concomitant medications, medical comor bidity, adequacy of trials of medical therapy, and potential non-adherence to such trials—to account for poor antidepressant outcomes.
Severity
Severity of depression warrants consideration when formulating impressions about the nature and treatment of all presentations of depression.
High-severity forms prompt decisions about treatment setting (inpatient or outpatient); suicide assessment; and therapeutic modalities (eg, electroconvulsive therapy is more appropriate than psychotherapy for catatonic depression).
Mild forms. A recent meta-analysis of 6 randomized trials (each of >6 weeks’ duration) of antidepressants for mild depression demonstrated that these agents exert only a modest effect compared with placebo, owing largely to higher placebo-responsivity in mild depressive episodes than in moderate and severe episodes.13 In contrast, another meta-analysis of subjects who had “mild” baseline depression severity scores found that antidepressant medication had greater efficacy than placebo in 4 of 6 randomized trials.14 Higher depression severity levels typically diminish the placebo response rate but also reduce the magnitude of drug efficacy.
Psychosis
Before DSM-III, psychotic (as opposed to neurotic) depression was perhaps the key nosologic distinction when characterizing forms of depression. The presence of psychosis and related components (eg, mood-congruence) is closely linked with the severity of depression (high) and prognosis and longitudinal outcome (poorer), and has implications for treatment (Table).
Bottom Line
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including polarity, chronicity, recurrence, psychosis, treatment resistance, comorbidity, and atypicality, among other classifications. Clinical characteristics vary across subtypes—and so do corresponding preferred treatments, which should be tailored to the needs of each of your patients.
Related Resources
• Goldberg JF, Thase ME. Monoamine oxidase inhibitors revisited: what you should know. J Clin Psychiatry. 2013;74(2):189-191.
• Goldberg JF. Antidepressants in bipolar disorder: 7 myths and realities. Current Psychiatry. 2010;9(5):41-49.
• Ketamine cousin rapidly lifts depression without side effects. National Institute of Mental Health. http://www. nimh.nih.gov/news/science-news/2013/ketamine-cousin-rapidly-lifts-depression-without-side-effects.shtml. Published May 23, 2013. Accessed March 20, 2014.
• Research Domain Criteria (RDoC). National Institute of Mental Health. http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml?u tm_ source = govdelivery&utm_medium=email&utm_campaign= govdelivery. Accessed March 20, 2014.
Drug Brand Names
Amisulpride • Amazeo, Lurasidone • Latuda
Amival, Amipride, Sulpitax Mirtazapine • Remeron
Aripiprazole • Abilify Moclobemide • Amira,
Armodafinil • Nuvigil Aurorix, Clobemix,
Bupropion • Wellbutrin Depnil, Manerix
Desipramine • Norpramin Modafinil • Provigil
Divalproex • Depakote, Olanzapine/fluoxetine
Depakene • Symbyax
Duloxetine • Cymbalta Paroxetine • Paxil
Escitalopram • Lexapro Phenelzine • Nardil
Fluoxetine • Prozac Pramipexole • Mirapex
Imipramine • Tofranil Quetiapine • Seroquel
Ketamine • Ketalar Riluzole • Rilutek
Lamotrigine • Lamictal Sertraline • Zoloft
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion
Pharmaceuticals, and Takeda and Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
Editor’s note: The second part of Dr. Goldberg’s review of depression subtypes—focusing on “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder—will appear in the May 2014 issue of Current Psychiatry.
Depression carries a wide differential diagnosis. Practitioners sometimes think polarity is the fundamental distinction when they conceptualize depression as a clinical entity; in fact, many nosologic frameworks have been described for defining and subtyping clinically meaningful forms of depression, and each waxed and waned in popularity.
Kraepelin, writing in the early 20th century, linked manic-depressive illness with “the greater part of the morbid states termed melancholia,”1 but many features other than polarity remain important components of depression, and those features often carry implications for how individual patients respond to treatment.
In this 2-part article [April and May 2014 issues], I summarize information about clinically distinct subtypes of depression, as they are recognized within diagnostic systems or as descriptors of treatment outcomes for particular subgroups of patients. My focus is on practical considerations for assessing and managing depression. Because many forms of the disorder respond inadequately to initial antidepressant treatment, optimal “next-step” pharmacotherapy, after nonresponse or partial response, often hinges on clinical subtyping.
The first part of this article examines major depressive disorder (MDD), minor depression, chronic depression, depression in bipolar disorder, depression that is severe or mild, and psychotic depression. Treatments for these subtypes for which there is evidence, or a clinical rationale, are given in the Table.
The subtypes of depression that I’ll discuss in the second part of the article are listed on page 47.
Major and minor depression
MDD has been the focus of most drug trials seeking FDA approval. As a syndrome, MDD is defined by a constellation of features that are related not only to mood but also to sleep, energy, cognition, motivation, and motor behavior, persisting for ≥2 weeks.
DSM-5 has imposed few changes to the basic definition of MDD:
• bereavement (the aftermath of death of a loved one), formerly an exclusion criterion, no longer precludes making a diagnosis of MDD when syndromal criteria are otherwise fulfilled
• “with anxious distress” is a new course specifier that designates prominent anxiety features (feeling worried, restless, tense, or keyed up; fearful of losing control or something terrible happening)
• “with mixed features” is a new course specifier pertinent when ≥3 mania or hypomania symptoms coexist (that is, might be a subsyndromal mania or hypomania) with a depressive syndrome; the mixed features specifier can be applied to depressed patients whether or not they have ever had a manic or hypomanic episode, but MDD—rather than bipolar disorder— remains the overarching diagnosis, unless criteria have ever been met for a full mania or hypomania.
More than 2 dozen medications are FDA-approved to treat MDD. Evidence-based psychotherapies (eg, cognitive-behavioral therapy [CBT] and interpersonal therapy), as adjuncts to pharmacotherapy, further improve outcomes, but with only modest additional effect.2
Minor depression. Depressive states that involve 2 to 4 associated symptoms lasting ≥2 weeks but <2 years are sometimes described as minor depression, captured within DSM-5 as “depression not elsewhere defined.” The terminology of so-called “minor depression” generally is shunned, in part because it might wrongly connote low severity and therefore discourage treatment—even though it confers more than a 5-fold increase in risk of MDD.3
Chronicity
DSM-IV-TR identified long-standing depression by 2 constructs:
• chronic major depression (an episode of MDD lasting ≥2 years in adults, ≥1 year in children and adolescents)
• dysthymic disorder (2 to 4 depressive symptoms for ≥2 years in adults and ≥1 year in children and adolescents), affecting 3% to 6% of adults and carrying a 2-fold increased risk of MDD, eventually.4
Depression that begins as dysthymic disorder and blossoms into syndromal MDD is described as “double depression”—although it is not recognized as a unique condition in any edition of the DSM. Subsequent incomplete recovery may revert to dysthymic disorder. DSM-5 has subsumed chronic major depression and dysthymia under the unified heading of persistent depressive disorder.
There are no FDA-approved drugs for treating dysthymia. A meta-analysis of 9 controlled trials of off-label use of antidepressants to treat dysthymia revealed an overall response rate of 52.4%, compared with 29.9% for placebo.5 Notably, although the active drug response rate in these studies is comparable to what seen in MDD, the placebo response rate was approximately 10% lower than what was seen in major depression.
Positive therapeutic findings (typically, treatment for 6 to 12 weeks) have been reported in so-called “pure” dysthymic disorder with sertraline, fluoxetine, imipramine, ritanserin, moclobemide (not approved for use in the United States), and phenelzine; the results of additional, positive placebo-controlled studies support the utility of duloxetine6 and paroxetine.7 Randomized trials have reported negative findings for desipramine,8 fluoxetine,9 and escitalopram10 escitalopram10—although the sample size in these latter studies might have been too small to detect a drug-placebo difference.
In dysthymic and minor-depressive middle-age and older adult men who have a low serum level of testosterone, hormone replacement was shown to be superior to placebo in several randomized trials.11 Studies of adjunctive atypical antipsychotics for dysthymic disorder are scarce; a Cochrane review identified controlled data only with amisulpride (not approved for use in the United States), which yielded a modest therapeutic effect.12
Polarity
In recent years, depression in bipolar disorder (BD) has been contrasted with unipolar MDD based on a difference in:
• duration (briefer in BD)
• severity (worse in BD)
• risk of suicide (higher)
• comorbidity (more extensive)
• family history (often present for BD and highly recurrent depression)
• treatment outcome (generally less favorable).
DSM-5 has at least somewhat blurred the distinctions in polarity by way of the new construct of “major depression with mixed features” (see the discussion of MDD above), identifiable even when a person has never had a full manic or hypomanic episode.
No randomized trials have been conducted to identify the best treatments for such presentations, which has invited extrapolation from the literature in regard to bipolar mixed episodes, and suggesting that 1) some mood stabilizers (eg, divalproex) might have value and 2) antidepressants might exacerbate manic symptoms.
Perhaps most noteworthy in regard to treating bipolar depression is the unresolved, but hotly debated, controversy over whether and, if so, when, an antidepressant is inappropriate (based on concerns about possible induction or exacerbation of manic symptoms). In addition, nearly all of the large, randomized controlled trials of antidepressants for bipolar depression have shown that they offer no advantage over placebo.
Some authors argue that a lack of response to antidepressants might, itself, be a “soft” indicator of “bipolarity.” However, nonresponse to antidepressants should prompt a wider assessment of features other than polarity—including psychosis, anxiety, substance abuse, a personality disorder, psychiatric adverse effects from concomitant medications, medical comor bidity, adequacy of trials of medical therapy, and potential non-adherence to such trials—to account for poor antidepressant outcomes.
Severity
Severity of depression warrants consideration when formulating impressions about the nature and treatment of all presentations of depression.
High-severity forms prompt decisions about treatment setting (inpatient or outpatient); suicide assessment; and therapeutic modalities (eg, electroconvulsive therapy is more appropriate than psychotherapy for catatonic depression).
Mild forms. A recent meta-analysis of 6 randomized trials (each of >6 weeks’ duration) of antidepressants for mild depression demonstrated that these agents exert only a modest effect compared with placebo, owing largely to higher placebo-responsivity in mild depressive episodes than in moderate and severe episodes.13 In contrast, another meta-analysis of subjects who had “mild” baseline depression severity scores found that antidepressant medication had greater efficacy than placebo in 4 of 6 randomized trials.14 Higher depression severity levels typically diminish the placebo response rate but also reduce the magnitude of drug efficacy.
Psychosis
Before DSM-III, psychotic (as opposed to neurotic) depression was perhaps the key nosologic distinction when characterizing forms of depression. The presence of psychosis and related components (eg, mood-congruence) is closely linked with the severity of depression (high) and prognosis and longitudinal outcome (poorer), and has implications for treatment (Table).
Bottom Line
Depressive disorders comprise a range of conditions that can be viewed along many dimensions, including polarity, chronicity, recurrence, psychosis, treatment resistance, comorbidity, and atypicality, among other classifications. Clinical characteristics vary across subtypes—and so do corresponding preferred treatments, which should be tailored to the needs of each of your patients.
Related Resources
• Goldberg JF, Thase ME. Monoamine oxidase inhibitors revisited: what you should know. J Clin Psychiatry. 2013;74(2):189-191.
• Goldberg JF. Antidepressants in bipolar disorder: 7 myths and realities. Current Psychiatry. 2010;9(5):41-49.
• Ketamine cousin rapidly lifts depression without side effects. National Institute of Mental Health. http://www. nimh.nih.gov/news/science-news/2013/ketamine-cousin-rapidly-lifts-depression-without-side-effects.shtml. Published May 23, 2013. Accessed March 20, 2014.
• Research Domain Criteria (RDoC). National Institute of Mental Health. http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml?u tm_ source = govdelivery&utm_medium=email&utm_campaign= govdelivery. Accessed March 20, 2014.
Drug Brand Names
Amisulpride • Amazeo, Lurasidone • Latuda
Amival, Amipride, Sulpitax Mirtazapine • Remeron
Aripiprazole • Abilify Moclobemide • Amira,
Armodafinil • Nuvigil Aurorix, Clobemix,
Bupropion • Wellbutrin Depnil, Manerix
Desipramine • Norpramin Modafinil • Provigil
Divalproex • Depakote, Olanzapine/fluoxetine
Depakene • Symbyax
Duloxetine • Cymbalta Paroxetine • Paxil
Escitalopram • Lexapro Phenelzine • Nardil
Fluoxetine • Prozac Pramipexole • Mirapex
Imipramine • Tofranil Quetiapine • Seroquel
Ketamine • Ketalar Riluzole • Rilutek
Lamotrigine • Lamictal Sertraline • Zoloft
Lithium • Eskalith, Lithobid Vortioxetine • Brintellix
Disclosure
Dr. Goldberg has been a consultant to Avanir Pharmaceuticals and Merck; has served on the speakers’ bureau for AstraZeneca, Merck, Novartis, Sunovion
Pharmaceuticals, and Takeda and Lundbeck; and has received royalties from American Psychiatric Publishing and honoraria from Medscape and WebMD.
Editor’s note: The second part of Dr. Goldberg’s review of depression subtypes—focusing on “situational,” treatment-resistant, melancholic, agitated, anxious, and atypical depression; depression occurring with a substance use disorder; premenstrual dysphoric disorder; and seasonal affective disorder—will appear in the May 2014 issue of Current Psychiatry.
1. Kraepelin E. Manic-depressive insanity and paranoia. Barclay RM, trans. Robertson GM, ed. Edinburgh, Scotland: E&S Livingstone; 1921:1.
2. Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70(9):1219-1229.
3. Fogel J, Eaton WW, Ford DE. Minor depression as a predictor of the first onset of major depressive disorder over a 15-year follow-up. Acta Psychiatr Scand. 2006; 113(1):36-43.
4. Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79(1-3):71-79.
5. Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(4):509-514.
6. Hellerstein DJ, Stewart JW, McGrath PJ, et al. A randomized controlled trial of duloxetine versus placebo in the treatment of nonmajor chronic depression. J Clin Psychiatry. 2012;73(7):984-991.
7. Ravindran AV, Cameron C, Bhatla R, et al. Paroxetine in the treatment of dysthymic disorder without co-morbidities: a double-blind, placebo-controlled, flexible-dose study. Asian J Psychiatry. 2013;6(2):157-161.
8. Stewart JW, McGrath PJ, Liebowitz MR, et al. Treatment outcome validation of DSM-III depressive subtypes. Clinical usefulness in outpatients with mild to moderate depression. Arch Gen Psychiatry. 1985;42(12):1148-1153.
9. Serrano-Blanco A, Gabarron E, Garcia-Bayo I, et al. Effectiveness and cost-effectiveness of antidepressant treatment in primary health care: a six-month randomised study comparing fluoxetine to imipramine. J Affect Disord. 2006;91(2-3):153-163.
10. Hellerstein DJ, Batchelder ST, Hyler S, et al. Escitalopram versus placebo in the treatment of dysthymic disorders. Int Clin Psychopharmacol. 2010;25(3):143-148.
11. Seidman SN, Orr G, Raviv G, et al. Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol. 2009;29(3):216-221.
12. Komossa K, Depping AM, Gaudchau A, et al. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010; 8:(12):CD008121.
13. Fournier JC, DeRubeis RJ, Hollom SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
14. Stewart JA, Deliyannides DA, Hellerstein DJ, et al. Can people with nonsevere major depression benefit from antidepressant medication? J Clin Psychiatry. 2012;73(4):518-525.
1. Kraepelin E. Manic-depressive insanity and paranoia. Barclay RM, trans. Robertson GM, ed. Edinburgh, Scotland: E&S Livingstone; 1921:1.
2. Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70(9):1219-1229.
3. Fogel J, Eaton WW, Ford DE. Minor depression as a predictor of the first onset of major depressive disorder over a 15-year follow-up. Acta Psychiatr Scand. 2006; 113(1):36-43.
4. Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79(1-3):71-79.
5. Levkovitz Y, Tedeschini E, Papakostas GI. Efficacy of antidepressants for dysthymia: a meta-analysis of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72(4):509-514.
6. Hellerstein DJ, Stewart JW, McGrath PJ, et al. A randomized controlled trial of duloxetine versus placebo in the treatment of nonmajor chronic depression. J Clin Psychiatry. 2012;73(7):984-991.
7. Ravindran AV, Cameron C, Bhatla R, et al. Paroxetine in the treatment of dysthymic disorder without co-morbidities: a double-blind, placebo-controlled, flexible-dose study. Asian J Psychiatry. 2013;6(2):157-161.
8. Stewart JW, McGrath PJ, Liebowitz MR, et al. Treatment outcome validation of DSM-III depressive subtypes. Clinical usefulness in outpatients with mild to moderate depression. Arch Gen Psychiatry. 1985;42(12):1148-1153.
9. Serrano-Blanco A, Gabarron E, Garcia-Bayo I, et al. Effectiveness and cost-effectiveness of antidepressant treatment in primary health care: a six-month randomised study comparing fluoxetine to imipramine. J Affect Disord. 2006;91(2-3):153-163.
10. Hellerstein DJ, Batchelder ST, Hyler S, et al. Escitalopram versus placebo in the treatment of dysthymic disorders. Int Clin Psychopharmacol. 2010;25(3):143-148.
11. Seidman SN, Orr G, Raviv G, et al. Effects of testosterone replacement in middle-aged men with dysthymia: a randomized, placebo-controlled clinical trial. J Clin Psychopharmacol. 2009;29(3):216-221.
12. Komossa K, Depping AM, Gaudchau A, et al. Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. 2010; 8:(12):CD008121.
13. Fournier JC, DeRubeis RJ, Hollom SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53.
14. Stewart JA, Deliyannides DA, Hellerstein DJ, et al. Can people with nonsevere major depression benefit from antidepressant medication? J Clin Psychiatry. 2012;73(4):518-525.
To prevent depression recurrence, interpersonal psychotherapy is a first-line treatment with long-term benefits
Major depressive disorder (MDD) frequently is recurrent, with new episodes causing substantial social and economic impairment1 and increasing the likelihood of future episodes.2 For this reason, contemporary psychiatric practitioners think of depression treatment as long-term and plan thoughtfully for maintenance therapy.
Recognizing the importance of engaging depressed individuals beyond the initial response,3 American Psychiatric Association practice guidelines conceptualize depression treatment as 3 phases:
• acute treatment, with the aim of remission (symptom removal)
• continuation treatment, with the aim of preventing relapse (symptom return)
• maintenance treatment, with the aim of preventing recurrence (new episodes).4
Interpersonal psychotherapy (IPT) is an evidence-based psychosocial treatment that adheres to this model.5 As a time-limited, manual-driven6,7 approach, IPT focuses on interpersonal distresses as precipitating and perpetuating factors of depression.8
Acute IPT’s efficacy is well-established across >200 empirical studies—making it an evidence-based, first-line treatment for adult depression.4,9,10 Meta-analyses show that acute IPT is superior to placebo and no-treatment controls, and largely comparable to antidepressant medication and other active, first-line psychotherapies, such as cognitive-behavioral therapy (CBT).11,12
Although this review, as well as the literature, focuses largely on adult outpatients with depression, evidence of IPT’s general efficacy exists for adolescents,13 chronically depressed patients,11 and depressed inpatients.14 This article presents a case study to describe the structure of IPT when used to treat depressed adults. We also present evidence of IPT’s acute and long-term efficacy in preventing depression recurrence and data to guide its use in practice.
CASE REPORT
‘Safe’ but depressed
Timothy, age 18, is a first-year college student who presents for outpatient psychotherapy to address recurrent depression. He reports general unhappiness, loss of interest in things, low energy, sleep problems, poor academic and work functioning, and low self-esteem. He experienced at least 3 similar depressive episodes while in high school.
The therapist’s diagnostic and interpersonal assessment suggests that Timothy’s depression is interpersonally driven. Timothy longs for relational intimacy but fears he will fail or burden people with his needs. He has difficulty gauging appropriate levels of enmeshment with others and either becomes overdependent or stays at a distance. This “safe” approach to relationships contributes to boredom, loneliness, and isolation. His recent transition to college away from home and the failure of a romantic relationship have compounded these experiences.
Interpersonal model of IPT
IPT conceptualizes depression as involving predisposing, precipitating, and perpetuating biopsychosocial factors, including:
• underlying biological and social vulnerability, such as insecure attach ment (ie, tenuous and often negative views of self and others)
• current interpersonal life stressors
• inadequate social supports.15,16
For example, poor early attachment to caregivers can give rise to despair, isolation, and low mood. In turn, this can be exacerbated by poor social and communication skills that promote further rejection and withdrawal of social support and thus, intensified despair, isolation, and low mood. As in Timothy’s case, this vicious cycle underscores psychosocial stressors as a causal factor, maintaining factor, and result of depression. Specifically, IPT conceptualizes 4 main biopsychosocial problem domains:
• grief and loss
• interpersonal disputes
• role transitions
• interpersonal/communication deficits (often connected to isolation).
Working within 1 or 2 of the most salient problem domains, IPT centers on strategies for helping patients solve interpersonal problems based on the notion that modified relationships, revised interpersonal expectations, improved communications, and increased social support will lead to symptom reduction.15-17
Many techniques are utilized in IPT (Table 1) to help patients modify their interpersonal relationships as a mechanism for decreasing their distress. IPT is problem-focused, aiming to improve patients’ relationships by drawing on their assets and helping to build skills around shortcomings. Therefore, IPT focuses on observable interpersonal patterns, as opposed to latent personality dynamics.
CASE CONTINUED
Setting goals
When the clinical explains in the non-technical terms the data supporting IPT’s efficacy for depression, including with young adults, Timothy agrees to teeatment with acute IPT. The therapist behins with consciousness-raising techniques to help Timothy adopt the “sick role” by viewing depressing as an illness to be cured. Collaboratively, they establish treatment goals that fit the IPT formulation of depression— ie, revising current relationships and expectations of them, increasing social support, improving communication skills, and solving problems within 1 or 2 of the IPT problem domains.
For Timothy, the most pressing psychosocial problems seem to be interpersonal deficits and role transitions. He appears to be insecurely attached to others, which is a risk factor for poor facilitation of, and boundaries around, good relationships. A transition to a new and intimidating interpersonal context—living on a college campus—compounded his vulnerabilities and increased his depression.
Acute treatment. The acute phase of IPT is time-limited—often, 12 to 16 sessions with gradual tapering toward the end (akin to a continuation phase). The time limit’s purpose is to focus both patient and therapist on the specific goal of removing the acute “illness” of depression. The IPT clinician takes an interpersonal inventory to learn about the patient’s most important relationships and hones in on the IPT domain foci. Working collaboratively, the therapist might help the patient mourn a loss, reconstruct a narrative with a deceased loved one, consider ways to increase social contact, develop assertiveness, label feelings and needs, resolve an impasse with a significant other, and so forth.
The IPT therapist is an advocate for the patient and adopts an active stance laced with empathy and warmth. However, the therapist is more than unconditionally accepting as depression is viewed as a problem to be actively resolved.
CASE CONTINUED
Creating new patterns
The therapist uses various IPT strategies to work collaboratively with Timothy. She attempts to develop a strong working alliance by building interpersonal safety and trust— which take time with an insecurely attached patient. She tries to provide a new model for how close relationships can develop, while also focusing on current relationships. She and Timothy address his romantic desire for a coworker and work on developing realistic expectations and effective methods for conveying his interest.
When Timothy approaches his coworker, she does not reject him—as he expected— but wants to pursue friendship before possibly dating. The therapist then works with Timothy’s emotional reaction and explores ways to effectively convey his emotions to this young woman. Drawing on communication analysis and problem-solving strategies, Timothy is able to sustain this friendship—a shift from his typical retreat when relationships have not gone as hoped or expected.
Timothy develops confidence to take more risks in initiating social encounters and starts to confide in his roommates when he feels upset. After 3 months of treatment, his expanded social network and improved interpersonal skills result in decreased depression. When Timothy suggests termination, he and the therapist agree to end acute IPT but—given his history of depression—to continue maintenance sessions.
Limited data exist on variables that relate to IPT’s acute success or conditions under which it works best. Although process research lags behind acute IPT outcome research, some findings can help guide the IPT practitioner. For example, variables shown to predict outcomes of acute IPT for depression include a positive therapeutic alliance, therapist warmth, and psycho psychotherapist use of exploratory techniques (Table 2).
Similarly, IPT has been shown to be more effective in some patients than others, depending on various moderators of depression. For example:
• For patients with high cognitive dysfunction, IPT outperforms CBT.
• For patients with higher need for medical reassurance, IPT outperforms selective serotonin reuptake inhibitor (SSRI) pharmacotherapy.
• For patients with severe depression, CBT outperforms IPT.
• For patients with low psychomotor activation, response is more rapid with an SSRI than with IPT (Table 3).18
Durability of acute IPT
One way to understand recurrence prevention is to examine the durability of a treatment’s acute effect in the absence of a specific maintenance plan. In theory, patients will continue to apply the skills learned in acute IPT to maintain gains and prevent recurrences, even after they stop seeing the psychotherapist.
Initial findings. Some research speaks to IPT’s acute-phase durability. The inaugural clinical trial of IPT by Weissman et al19 included 4 months of acute treatment and a 1-year uncontrolled naturalistic follow-up assessment. At follow-up, depression and global clinical symptoms were the same, whether patients had been acutely treated with IPT alone, pharmacotherapy alone (amitriptyline), combined IPT and pharmacotherapy, or nonscheduled treatment with a psychiatrist.
Some patients continued to function well, whereas others did not fully maintain acute treatment gains. Patients who received IPT acutely, either singly or with medication, showed better social functioning at follow-up compared with patients who did not receive IPT. This long-term durability of social improvements was an obvious target of IPT.
Support from TDCRP. In the National Institute of Mental Health Treatment of Depression Collaborative Research Project (TDCRP),20 patients in the acute phase of depression were assigned to 16 weeks of IPT, CBT, pharmacotherapy (imipramine) and clinical management (CM), or placebo plus CM. Among those who recovered by acute treatment’s end, MDD relapse rates at 18-month naturalistic follow-up were 33% for IPT, 36% for CBT, 50% for imipramine, and 33% for placebo. Between-group differences were not statistically significant.
Because acute responders to different types of treatment might have different inherent relapse tendencies, these data do not support causal attributions about the enduring effects of acute-phase treatment. The relapse rates do suggest, however, that 16 weeks of acute treatment, irrespective of kind, was insufficient for some patients to achieve full recovery and lasting remission. Consistent with the initial IPT trial,19 IPT (and CBT) outperformed medi cation and placebo in maintaining relationship quality.21
Long-term benefits. A more recent trial by Zobel et al22 examined the durability of benefits from 5 weeks of acute IPT plus pharmacotherapy and pharmacotherapy plus CM for inpatients with MDD. Although caution is required in interpreting naturalistic follow-up studies, patients in both groups showed decreased depression from baseline to 5-year follow-up. Early symptom reduction was more rapid for patients in the IPT plus pharmacotherapy group, but no significant difference existed at 5 years. More IPT patients than CM patients showed sustained remission (28% vs 11%, respectively). These rates demonstrate a need for longer-term potency of acute treatments and more targeted maintenance treatments.
IPT-M for preventing recurrence
A second way to understand recurrence prevention is to examine the efficacy of a treatment’s maintenance protocol added to an acute treatment phase. IPT has been adapted as a maintenance treatment (IPT-M), with emphasis on keeping patients well. With this revised focus, IPT-M differs somewhat from acute IPT. Although treatment continues to center on interpersonal functioning, IPT-M favors:
• vigilance for possible triggers of new depressive episodes
• longer-term contact with a therapist
• reinforcing skills learned
• addressing an expanded number of interpersonal problem areas (given that such problems can be addressed more efficiently relative to acute treatment).
Efficacy of IPT-M. In the initial trial, Frank et al23 compared the efficacy of IPT-M with that of pharmacotherapy (imipramine) in preventing depressive relapse among patients with recurrent depression who had responded to ≥16 sessions of acute IPT and imipramine and remained well during a 17- week continuation phase. For maintenance, patients were assigned to IPT-M alone, imipramine alone, placebo alone, IPT-M plus imipramine, or IPT-M plus placebo. Maintenance imipramine was continued at the acute dosage (target 200 mg/d; up to 400 mg/d was allowed). Maintenance IPT was monthly sessions. Patients remained in the trial for 3 years or until depression recurred.
On its own, IPT-M showed some efficacy in preventing recurrence, as the mean time to recurrence was 82 weeks for IPT-M alone and 74 weeks for IPT-M plus placebo. The prophylactic effect of imipramine was stronger, however. The mean time to recurrence for imipramine with IPT was 131 weeks, and the mean time to recurrence for imipramine without IPT was 124 weeks. Therefore, whereas monthly IPT-M can certainly help prolong wellness and delay recurrence, IPT maintenance treatment with acute doses of imipramine might be even more effective— if the patient is willing to take medication. These findings must be considered with caution because of the inherent inequity between imipramine and IPT-M in regard to maintenance dosage strength.
Frequency of treatment. In another trial, Frank et al24 examined whether the frequency of maintenance IPT sessions played a role in its prophylactic effect. Adult women who had achieved depression remission with acute IPT (alone or in combination with SSRI pharmacotherapy) were randomized to weekly, bi-weekly, or monthly IPT-M alone for 2 years or until recurrence. Depression recurred during IPT-M in:
• 26% of patients who had received acute IPT alone
• 50% of those who had received acute IPT plus an SSRI.
Frequency of IPT-M sessions did not affect time to recurrence. Thus, for women who can achieve remission with IPT alone, varied frequencies of IPT-M can be good prophylaxis. For women who need an SSRI to augment acute IPT, IPT-M alone at varied dosages is less effective in preventing depression recurrence. Therefore, acute treatment response patterns can inform maintenance plans, with the most prudent maintenance strategy being to maintain the acute treatment strategy over a longer period.
IPT-M for late-life depression. A trial by Reynolds et al25 examined the efficacy of maintenance nortriptyline and IPT-M in preventing depression recurrence in patients age ≥59 who initially recovered after combined acute and continuation IPT plus nortriptyline. The 4 conditions (with their recurrence rates) were:
• monthly IPT-M with nortriptyline (20%)
• monthly IPT-M with placebo (64%)
• nortriptyline plus medication visits (43%)
• placebo plus medication visits (90%).
Clearly, the combined active treatments outperformed placebo and antidepressant alone in terms of delaying or preventing recurrence, which suggests an optimal maintenance strategy with this population.
IPT-M for later life. Another trial by the same group26 enrolled patients age ≥70 with MDD that responded to acute IPT plus paroxetine. The maintenance treatments to which they were randomly assigned (and recurrence rates within 2 years) were:
• paroxetine plus IPT-M (35%)
• placebo plus IPT-M (68%)
• paroxetine plus clinical management (37%)
• placebo plus clinical management (58%).
Recurrence rates were the same for patients receiving medication plus IPT-M and medication plus clinical management, and depression was 2.4 times more likely to recur in patients receiving placebo vs active medication. Therefore, for later life depression, the optimal maintenance strategy was the SSRI.
Secondary analyses of data from these seminal trials of IPT-M point to other predictors of how and for whom maintenance IPT may work (Table 4). For example:
• Greater variability of depression symptoms during all forms of maintenance treatment is related to a greater risk of recurrence.
• Persistent insomnia is related to greater risk of recurrent depression.
• High interpersonal focus in IPT-M sessions is related to longer time to recurrence.
Bottom Line
Interpersonal psychotherapy (IPT) is efficacious for acute depression and for preventing recurrences. Patients treated successfully with acute IPT alone benefit from varied doses of maintenance IPT. Combining IPT-M with antidepressant medication can be more potent than IPT-M alone. For late-life depression, medication appears to be most effective for maintenance treatment.
Related Resources
Media
• Video demonstration, role-play transcripts, lesson plans, and quizzes. In: Appendices in and DVD companion to Ravitz P, Watson P, Grigoriadas S. Interpersonal psychotherapy for depression. New York, NY: Norton; 2013.
• Video demonstration of IPT sessions. In: DVD companion to Dewan, M, Steenbarger, B, Greenberg, R, eds. The art and science of brief psychotherapies: An illustrated guide. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2012.
Text
• Stuart S, Robertson M. Interpersonal psychotherapy: a clinician’s guide. London, United Kingdom: Taylor & Francis; 2012.
• Weissman MM, Markowitz JC, Klerman GL. Comprehensive guide to interpersonal psychotherapy. New York, NY: Basic Books; 2000.
• Weissman M, Markowitz J, Klerman GL. Clinician’s quick guide to interpersonal psychotherapy. New York, NY: Oxford University Press; 2007.
Websites
• Interpersonal Psychotherapy Institute. http://iptinstitute.com.
• International Society for Interpersonal Psychotherapy. http://interpersonalpsychotherapy.org.
Drug Brand Names
Amitriptyline • Elavil Nortriptyline • Pamelor
Imipramine • Tofranil Paroxetine • Paxil
Acknowledgments
The authors are grateful to Samantha L. Bernecker, MS, and Nicholas R. Morrison for their assistance with the research review.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. ten Doesschate MC, Koeter MW, Bockting CL, et al. Health related quality of life in recurrent depression: a comparison with a general population sample. J Affect Disord. 2010; 120(1-3):126-132.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-91.
3. Arnow BA, Constantino MJ. Effectiveness of psychotherapy and combination treatment for chronic depression. J Clin Psychol. 2003;59(8):893-905.
4. American Psychiatric Association. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2010.
5. Klerman GL, Weissman MM, Rounsaville BJ, et al. Interpersonal psychotherapy of depression. New York, NY: Basic Books; 1984.
6. Weissman MM, Markowitz JC, Klerman G. Comprehensive guide to interpersonal psychotherapy. New York, NY: Basic Books; 2000.
7. Weissman M, Markowitz J, Klerman G. Clinician’s quick guide to interpersonal psychotherapy. New York, NY: Oxford University Press; 2007.
8. Brakemeier EL, Frase L. Interpersonal psychotherapy (IPT) in major depressive disorder. Eur Arch Psychiatry Clin Neurosci. 2012;262(suppl 2):S117-1121.
9. Depression in adults (update): NICE guideline CG90). National Institute for Health and Care Excellence. (2009). http://www.nice.org.uk/cg90. Updated October 2009. Accessed March 5, 2014.
10. Depression. National Institutes of Mental Health. http://www.nimh.nih.gov/health/publications/depression/ index.shtml. Revised 2011. Accessed March 5, 2014.
11. Cuijpers P, van Straten A, Andersson G, et al. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008;76(6):909-922.
12. Cuijpers P, Geraedts AS, van Oppen P, et al. Interpersonal psychotherapy for depression: a meta-analysis [Erratum in: Am J Psychiatry. 2011;168(6):652]. Am J Psychiatry. 2011; 168(6):581-592.
13. Mufson L, Dorta K, Wickramaratne P, et al. A randomized effectiveness trial of interpersonal psychotherapy for depressed adolescents. Arch Gen Psychiatry. 2004;61(6): 577-584.
14. Schramm E, Schneider D, Zobel I, et al. Efficacy of interpersonal psychotherapy plus pharmacotherapy in chronically depressed inpatients. J Affect Disord. 2008; 109(1-2):65-73.
15. Bernecker SL. How and for whom does interpersonal psychotherapy work? Psychotherapy Bulletin. 2012;47(2):13-17.
16. Stuart S. Interpersonal psychotherapy. In: Dewan MJ, Steenbarger BN, Greenberg RP, eds. The art and science of brief psychotherapies: an illustrated guide. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2012: 157-193.
17. Grigoriadas S, Watson P, Maunder R, eds. Psychotherapy essentials to go: Interpersonal psychotherapy for depression. New York, NY: W. W. Norton & Company, Inc.; 2013.
18. Bleiberg KL, Markowitz JC. Interpersonal psychotherapy for depression. In: Barlow D, ed. Clinical handbook of psychological disorders: a step-by-step treatment manual. New York, NY: The Guilford Press; 2008:306-327.
19. Weissman MM, Klerman GL, Prusoff BA, et al. Depressed outpatients. Results one year after treatment with drugs and/or interpersonal psychotherapy. Arch Gen Psychiatry. 1981;38(1):51-55.
20. Shea MT, Elkin I, Imber SD, et al. Course of depressive symptoms over follow-up. Findings from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Arch Gen Psychiatry. 1992;49(10):782-787.
21. Blatt S, Zuroff D, Bondi C, et al. Short- and long-term effect of medication and psychotherapy in the brief treatment of depression: further analyses of data from the NIMH TDCRP. Psychother Res. 2000;10(2):215-234.
22. Zobel I, Kech S, van Calker D, et al. Long-term effect of combined interpersonal psychotherapy and pharmacotherapy in a randomized trial of depressed patients. Acta Psychiatr Scand. 2011;123(4):276-282.
23. Frank E, Kupfer DJ, Perel JM, et al. Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry. 1990;47(12):1093-1099.
24. Frank E, Kupfer DJ, Buysse DJ, et al. Randomized trial of weekly, twice-monthly, and monthly interpersonal psychotherapy as maintenance treatment for women with recurrent depression. Am J Psychiatry. 2007;164(5): 761-767.
25. Reynolds CF 3rd, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA. 1999;281(1): 39-45.
26. Reynolds CF 3rd, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354(11):1130-1138.
Major depressive disorder (MDD) frequently is recurrent, with new episodes causing substantial social and economic impairment1 and increasing the likelihood of future episodes.2 For this reason, contemporary psychiatric practitioners think of depression treatment as long-term and plan thoughtfully for maintenance therapy.
Recognizing the importance of engaging depressed individuals beyond the initial response,3 American Psychiatric Association practice guidelines conceptualize depression treatment as 3 phases:
• acute treatment, with the aim of remission (symptom removal)
• continuation treatment, with the aim of preventing relapse (symptom return)
• maintenance treatment, with the aim of preventing recurrence (new episodes).4
Interpersonal psychotherapy (IPT) is an evidence-based psychosocial treatment that adheres to this model.5 As a time-limited, manual-driven6,7 approach, IPT focuses on interpersonal distresses as precipitating and perpetuating factors of depression.8
Acute IPT’s efficacy is well-established across >200 empirical studies—making it an evidence-based, first-line treatment for adult depression.4,9,10 Meta-analyses show that acute IPT is superior to placebo and no-treatment controls, and largely comparable to antidepressant medication and other active, first-line psychotherapies, such as cognitive-behavioral therapy (CBT).11,12
Although this review, as well as the literature, focuses largely on adult outpatients with depression, evidence of IPT’s general efficacy exists for adolescents,13 chronically depressed patients,11 and depressed inpatients.14 This article presents a case study to describe the structure of IPT when used to treat depressed adults. We also present evidence of IPT’s acute and long-term efficacy in preventing depression recurrence and data to guide its use in practice.
CASE REPORT
‘Safe’ but depressed
Timothy, age 18, is a first-year college student who presents for outpatient psychotherapy to address recurrent depression. He reports general unhappiness, loss of interest in things, low energy, sleep problems, poor academic and work functioning, and low self-esteem. He experienced at least 3 similar depressive episodes while in high school.
The therapist’s diagnostic and interpersonal assessment suggests that Timothy’s depression is interpersonally driven. Timothy longs for relational intimacy but fears he will fail or burden people with his needs. He has difficulty gauging appropriate levels of enmeshment with others and either becomes overdependent or stays at a distance. This “safe” approach to relationships contributes to boredom, loneliness, and isolation. His recent transition to college away from home and the failure of a romantic relationship have compounded these experiences.
Interpersonal model of IPT
IPT conceptualizes depression as involving predisposing, precipitating, and perpetuating biopsychosocial factors, including:
• underlying biological and social vulnerability, such as insecure attach ment (ie, tenuous and often negative views of self and others)
• current interpersonal life stressors
• inadequate social supports.15,16
For example, poor early attachment to caregivers can give rise to despair, isolation, and low mood. In turn, this can be exacerbated by poor social and communication skills that promote further rejection and withdrawal of social support and thus, intensified despair, isolation, and low mood. As in Timothy’s case, this vicious cycle underscores psychosocial stressors as a causal factor, maintaining factor, and result of depression. Specifically, IPT conceptualizes 4 main biopsychosocial problem domains:
• grief and loss
• interpersonal disputes
• role transitions
• interpersonal/communication deficits (often connected to isolation).
Working within 1 or 2 of the most salient problem domains, IPT centers on strategies for helping patients solve interpersonal problems based on the notion that modified relationships, revised interpersonal expectations, improved communications, and increased social support will lead to symptom reduction.15-17
Many techniques are utilized in IPT (Table 1) to help patients modify their interpersonal relationships as a mechanism for decreasing their distress. IPT is problem-focused, aiming to improve patients’ relationships by drawing on their assets and helping to build skills around shortcomings. Therefore, IPT focuses on observable interpersonal patterns, as opposed to latent personality dynamics.
CASE CONTINUED
Setting goals
When the clinical explains in the non-technical terms the data supporting IPT’s efficacy for depression, including with young adults, Timothy agrees to teeatment with acute IPT. The therapist behins with consciousness-raising techniques to help Timothy adopt the “sick role” by viewing depressing as an illness to be cured. Collaboratively, they establish treatment goals that fit the IPT formulation of depression— ie, revising current relationships and expectations of them, increasing social support, improving communication skills, and solving problems within 1 or 2 of the IPT problem domains.
For Timothy, the most pressing psychosocial problems seem to be interpersonal deficits and role transitions. He appears to be insecurely attached to others, which is a risk factor for poor facilitation of, and boundaries around, good relationships. A transition to a new and intimidating interpersonal context—living on a college campus—compounded his vulnerabilities and increased his depression.
Acute treatment. The acute phase of IPT is time-limited—often, 12 to 16 sessions with gradual tapering toward the end (akin to a continuation phase). The time limit’s purpose is to focus both patient and therapist on the specific goal of removing the acute “illness” of depression. The IPT clinician takes an interpersonal inventory to learn about the patient’s most important relationships and hones in on the IPT domain foci. Working collaboratively, the therapist might help the patient mourn a loss, reconstruct a narrative with a deceased loved one, consider ways to increase social contact, develop assertiveness, label feelings and needs, resolve an impasse with a significant other, and so forth.
The IPT therapist is an advocate for the patient and adopts an active stance laced with empathy and warmth. However, the therapist is more than unconditionally accepting as depression is viewed as a problem to be actively resolved.
CASE CONTINUED
Creating new patterns
The therapist uses various IPT strategies to work collaboratively with Timothy. She attempts to develop a strong working alliance by building interpersonal safety and trust— which take time with an insecurely attached patient. She tries to provide a new model for how close relationships can develop, while also focusing on current relationships. She and Timothy address his romantic desire for a coworker and work on developing realistic expectations and effective methods for conveying his interest.
When Timothy approaches his coworker, she does not reject him—as he expected— but wants to pursue friendship before possibly dating. The therapist then works with Timothy’s emotional reaction and explores ways to effectively convey his emotions to this young woman. Drawing on communication analysis and problem-solving strategies, Timothy is able to sustain this friendship—a shift from his typical retreat when relationships have not gone as hoped or expected.
Timothy develops confidence to take more risks in initiating social encounters and starts to confide in his roommates when he feels upset. After 3 months of treatment, his expanded social network and improved interpersonal skills result in decreased depression. When Timothy suggests termination, he and the therapist agree to end acute IPT but—given his history of depression—to continue maintenance sessions.
Limited data exist on variables that relate to IPT’s acute success or conditions under which it works best. Although process research lags behind acute IPT outcome research, some findings can help guide the IPT practitioner. For example, variables shown to predict outcomes of acute IPT for depression include a positive therapeutic alliance, therapist warmth, and psycho psychotherapist use of exploratory techniques (Table 2).
Similarly, IPT has been shown to be more effective in some patients than others, depending on various moderators of depression. For example:
• For patients with high cognitive dysfunction, IPT outperforms CBT.
• For patients with higher need for medical reassurance, IPT outperforms selective serotonin reuptake inhibitor (SSRI) pharmacotherapy.
• For patients with severe depression, CBT outperforms IPT.
• For patients with low psychomotor activation, response is more rapid with an SSRI than with IPT (Table 3).18
Durability of acute IPT
One way to understand recurrence prevention is to examine the durability of a treatment’s acute effect in the absence of a specific maintenance plan. In theory, patients will continue to apply the skills learned in acute IPT to maintain gains and prevent recurrences, even after they stop seeing the psychotherapist.
Initial findings. Some research speaks to IPT’s acute-phase durability. The inaugural clinical trial of IPT by Weissman et al19 included 4 months of acute treatment and a 1-year uncontrolled naturalistic follow-up assessment. At follow-up, depression and global clinical symptoms were the same, whether patients had been acutely treated with IPT alone, pharmacotherapy alone (amitriptyline), combined IPT and pharmacotherapy, or nonscheduled treatment with a psychiatrist.
Some patients continued to function well, whereas others did not fully maintain acute treatment gains. Patients who received IPT acutely, either singly or with medication, showed better social functioning at follow-up compared with patients who did not receive IPT. This long-term durability of social improvements was an obvious target of IPT.
Support from TDCRP. In the National Institute of Mental Health Treatment of Depression Collaborative Research Project (TDCRP),20 patients in the acute phase of depression were assigned to 16 weeks of IPT, CBT, pharmacotherapy (imipramine) and clinical management (CM), or placebo plus CM. Among those who recovered by acute treatment’s end, MDD relapse rates at 18-month naturalistic follow-up were 33% for IPT, 36% for CBT, 50% for imipramine, and 33% for placebo. Between-group differences were not statistically significant.
Because acute responders to different types of treatment might have different inherent relapse tendencies, these data do not support causal attributions about the enduring effects of acute-phase treatment. The relapse rates do suggest, however, that 16 weeks of acute treatment, irrespective of kind, was insufficient for some patients to achieve full recovery and lasting remission. Consistent with the initial IPT trial,19 IPT (and CBT) outperformed medi cation and placebo in maintaining relationship quality.21
Long-term benefits. A more recent trial by Zobel et al22 examined the durability of benefits from 5 weeks of acute IPT plus pharmacotherapy and pharmacotherapy plus CM for inpatients with MDD. Although caution is required in interpreting naturalistic follow-up studies, patients in both groups showed decreased depression from baseline to 5-year follow-up. Early symptom reduction was more rapid for patients in the IPT plus pharmacotherapy group, but no significant difference existed at 5 years. More IPT patients than CM patients showed sustained remission (28% vs 11%, respectively). These rates demonstrate a need for longer-term potency of acute treatments and more targeted maintenance treatments.
IPT-M for preventing recurrence
A second way to understand recurrence prevention is to examine the efficacy of a treatment’s maintenance protocol added to an acute treatment phase. IPT has been adapted as a maintenance treatment (IPT-M), with emphasis on keeping patients well. With this revised focus, IPT-M differs somewhat from acute IPT. Although treatment continues to center on interpersonal functioning, IPT-M favors:
• vigilance for possible triggers of new depressive episodes
• longer-term contact with a therapist
• reinforcing skills learned
• addressing an expanded number of interpersonal problem areas (given that such problems can be addressed more efficiently relative to acute treatment).
Efficacy of IPT-M. In the initial trial, Frank et al23 compared the efficacy of IPT-M with that of pharmacotherapy (imipramine) in preventing depressive relapse among patients with recurrent depression who had responded to ≥16 sessions of acute IPT and imipramine and remained well during a 17- week continuation phase. For maintenance, patients were assigned to IPT-M alone, imipramine alone, placebo alone, IPT-M plus imipramine, or IPT-M plus placebo. Maintenance imipramine was continued at the acute dosage (target 200 mg/d; up to 400 mg/d was allowed). Maintenance IPT was monthly sessions. Patients remained in the trial for 3 years or until depression recurred.
On its own, IPT-M showed some efficacy in preventing recurrence, as the mean time to recurrence was 82 weeks for IPT-M alone and 74 weeks for IPT-M plus placebo. The prophylactic effect of imipramine was stronger, however. The mean time to recurrence for imipramine with IPT was 131 weeks, and the mean time to recurrence for imipramine without IPT was 124 weeks. Therefore, whereas monthly IPT-M can certainly help prolong wellness and delay recurrence, IPT maintenance treatment with acute doses of imipramine might be even more effective— if the patient is willing to take medication. These findings must be considered with caution because of the inherent inequity between imipramine and IPT-M in regard to maintenance dosage strength.
Frequency of treatment. In another trial, Frank et al24 examined whether the frequency of maintenance IPT sessions played a role in its prophylactic effect. Adult women who had achieved depression remission with acute IPT (alone or in combination with SSRI pharmacotherapy) were randomized to weekly, bi-weekly, or monthly IPT-M alone for 2 years or until recurrence. Depression recurred during IPT-M in:
• 26% of patients who had received acute IPT alone
• 50% of those who had received acute IPT plus an SSRI.
Frequency of IPT-M sessions did not affect time to recurrence. Thus, for women who can achieve remission with IPT alone, varied frequencies of IPT-M can be good prophylaxis. For women who need an SSRI to augment acute IPT, IPT-M alone at varied dosages is less effective in preventing depression recurrence. Therefore, acute treatment response patterns can inform maintenance plans, with the most prudent maintenance strategy being to maintain the acute treatment strategy over a longer period.
IPT-M for late-life depression. A trial by Reynolds et al25 examined the efficacy of maintenance nortriptyline and IPT-M in preventing depression recurrence in patients age ≥59 who initially recovered after combined acute and continuation IPT plus nortriptyline. The 4 conditions (with their recurrence rates) were:
• monthly IPT-M with nortriptyline (20%)
• monthly IPT-M with placebo (64%)
• nortriptyline plus medication visits (43%)
• placebo plus medication visits (90%).
Clearly, the combined active treatments outperformed placebo and antidepressant alone in terms of delaying or preventing recurrence, which suggests an optimal maintenance strategy with this population.
IPT-M for later life. Another trial by the same group26 enrolled patients age ≥70 with MDD that responded to acute IPT plus paroxetine. The maintenance treatments to which they were randomly assigned (and recurrence rates within 2 years) were:
• paroxetine plus IPT-M (35%)
• placebo plus IPT-M (68%)
• paroxetine plus clinical management (37%)
• placebo plus clinical management (58%).
Recurrence rates were the same for patients receiving medication plus IPT-M and medication plus clinical management, and depression was 2.4 times more likely to recur in patients receiving placebo vs active medication. Therefore, for later life depression, the optimal maintenance strategy was the SSRI.
Secondary analyses of data from these seminal trials of IPT-M point to other predictors of how and for whom maintenance IPT may work (Table 4). For example:
• Greater variability of depression symptoms during all forms of maintenance treatment is related to a greater risk of recurrence.
• Persistent insomnia is related to greater risk of recurrent depression.
• High interpersonal focus in IPT-M sessions is related to longer time to recurrence.
Bottom Line
Interpersonal psychotherapy (IPT) is efficacious for acute depression and for preventing recurrences. Patients treated successfully with acute IPT alone benefit from varied doses of maintenance IPT. Combining IPT-M with antidepressant medication can be more potent than IPT-M alone. For late-life depression, medication appears to be most effective for maintenance treatment.
Related Resources
Media
• Video demonstration, role-play transcripts, lesson plans, and quizzes. In: Appendices in and DVD companion to Ravitz P, Watson P, Grigoriadas S. Interpersonal psychotherapy for depression. New York, NY: Norton; 2013.
• Video demonstration of IPT sessions. In: DVD companion to Dewan, M, Steenbarger, B, Greenberg, R, eds. The art and science of brief psychotherapies: An illustrated guide. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2012.
Text
• Stuart S, Robertson M. Interpersonal psychotherapy: a clinician’s guide. London, United Kingdom: Taylor & Francis; 2012.
• Weissman MM, Markowitz JC, Klerman GL. Comprehensive guide to interpersonal psychotherapy. New York, NY: Basic Books; 2000.
• Weissman M, Markowitz J, Klerman GL. Clinician’s quick guide to interpersonal psychotherapy. New York, NY: Oxford University Press; 2007.
Websites
• Interpersonal Psychotherapy Institute. http://iptinstitute.com.
• International Society for Interpersonal Psychotherapy. http://interpersonalpsychotherapy.org.
Drug Brand Names
Amitriptyline • Elavil Nortriptyline • Pamelor
Imipramine • Tofranil Paroxetine • Paxil
Acknowledgments
The authors are grateful to Samantha L. Bernecker, MS, and Nicholas R. Morrison for their assistance with the research review.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Major depressive disorder (MDD) frequently is recurrent, with new episodes causing substantial social and economic impairment1 and increasing the likelihood of future episodes.2 For this reason, contemporary psychiatric practitioners think of depression treatment as long-term and plan thoughtfully for maintenance therapy.
Recognizing the importance of engaging depressed individuals beyond the initial response,3 American Psychiatric Association practice guidelines conceptualize depression treatment as 3 phases:
• acute treatment, with the aim of remission (symptom removal)
• continuation treatment, with the aim of preventing relapse (symptom return)
• maintenance treatment, with the aim of preventing recurrence (new episodes).4
Interpersonal psychotherapy (IPT) is an evidence-based psychosocial treatment that adheres to this model.5 As a time-limited, manual-driven6,7 approach, IPT focuses on interpersonal distresses as precipitating and perpetuating factors of depression.8
Acute IPT’s efficacy is well-established across >200 empirical studies—making it an evidence-based, first-line treatment for adult depression.4,9,10 Meta-analyses show that acute IPT is superior to placebo and no-treatment controls, and largely comparable to antidepressant medication and other active, first-line psychotherapies, such as cognitive-behavioral therapy (CBT).11,12
Although this review, as well as the literature, focuses largely on adult outpatients with depression, evidence of IPT’s general efficacy exists for adolescents,13 chronically depressed patients,11 and depressed inpatients.14 This article presents a case study to describe the structure of IPT when used to treat depressed adults. We also present evidence of IPT’s acute and long-term efficacy in preventing depression recurrence and data to guide its use in practice.
CASE REPORT
‘Safe’ but depressed
Timothy, age 18, is a first-year college student who presents for outpatient psychotherapy to address recurrent depression. He reports general unhappiness, loss of interest in things, low energy, sleep problems, poor academic and work functioning, and low self-esteem. He experienced at least 3 similar depressive episodes while in high school.
The therapist’s diagnostic and interpersonal assessment suggests that Timothy’s depression is interpersonally driven. Timothy longs for relational intimacy but fears he will fail or burden people with his needs. He has difficulty gauging appropriate levels of enmeshment with others and either becomes overdependent or stays at a distance. This “safe” approach to relationships contributes to boredom, loneliness, and isolation. His recent transition to college away from home and the failure of a romantic relationship have compounded these experiences.
Interpersonal model of IPT
IPT conceptualizes depression as involving predisposing, precipitating, and perpetuating biopsychosocial factors, including:
• underlying biological and social vulnerability, such as insecure attach ment (ie, tenuous and often negative views of self and others)
• current interpersonal life stressors
• inadequate social supports.15,16
For example, poor early attachment to caregivers can give rise to despair, isolation, and low mood. In turn, this can be exacerbated by poor social and communication skills that promote further rejection and withdrawal of social support and thus, intensified despair, isolation, and low mood. As in Timothy’s case, this vicious cycle underscores psychosocial stressors as a causal factor, maintaining factor, and result of depression. Specifically, IPT conceptualizes 4 main biopsychosocial problem domains:
• grief and loss
• interpersonal disputes
• role transitions
• interpersonal/communication deficits (often connected to isolation).
Working within 1 or 2 of the most salient problem domains, IPT centers on strategies for helping patients solve interpersonal problems based on the notion that modified relationships, revised interpersonal expectations, improved communications, and increased social support will lead to symptom reduction.15-17
Many techniques are utilized in IPT (Table 1) to help patients modify their interpersonal relationships as a mechanism for decreasing their distress. IPT is problem-focused, aiming to improve patients’ relationships by drawing on their assets and helping to build skills around shortcomings. Therefore, IPT focuses on observable interpersonal patterns, as opposed to latent personality dynamics.
CASE CONTINUED
Setting goals
When the clinical explains in the non-technical terms the data supporting IPT’s efficacy for depression, including with young adults, Timothy agrees to teeatment with acute IPT. The therapist behins with consciousness-raising techniques to help Timothy adopt the “sick role” by viewing depressing as an illness to be cured. Collaboratively, they establish treatment goals that fit the IPT formulation of depression— ie, revising current relationships and expectations of them, increasing social support, improving communication skills, and solving problems within 1 or 2 of the IPT problem domains.
For Timothy, the most pressing psychosocial problems seem to be interpersonal deficits and role transitions. He appears to be insecurely attached to others, which is a risk factor for poor facilitation of, and boundaries around, good relationships. A transition to a new and intimidating interpersonal context—living on a college campus—compounded his vulnerabilities and increased his depression.
Acute treatment. The acute phase of IPT is time-limited—often, 12 to 16 sessions with gradual tapering toward the end (akin to a continuation phase). The time limit’s purpose is to focus both patient and therapist on the specific goal of removing the acute “illness” of depression. The IPT clinician takes an interpersonal inventory to learn about the patient’s most important relationships and hones in on the IPT domain foci. Working collaboratively, the therapist might help the patient mourn a loss, reconstruct a narrative with a deceased loved one, consider ways to increase social contact, develop assertiveness, label feelings and needs, resolve an impasse with a significant other, and so forth.
The IPT therapist is an advocate for the patient and adopts an active stance laced with empathy and warmth. However, the therapist is more than unconditionally accepting as depression is viewed as a problem to be actively resolved.
CASE CONTINUED
Creating new patterns
The therapist uses various IPT strategies to work collaboratively with Timothy. She attempts to develop a strong working alliance by building interpersonal safety and trust— which take time with an insecurely attached patient. She tries to provide a new model for how close relationships can develop, while also focusing on current relationships. She and Timothy address his romantic desire for a coworker and work on developing realistic expectations and effective methods for conveying his interest.
When Timothy approaches his coworker, she does not reject him—as he expected— but wants to pursue friendship before possibly dating. The therapist then works with Timothy’s emotional reaction and explores ways to effectively convey his emotions to this young woman. Drawing on communication analysis and problem-solving strategies, Timothy is able to sustain this friendship—a shift from his typical retreat when relationships have not gone as hoped or expected.
Timothy develops confidence to take more risks in initiating social encounters and starts to confide in his roommates when he feels upset. After 3 months of treatment, his expanded social network and improved interpersonal skills result in decreased depression. When Timothy suggests termination, he and the therapist agree to end acute IPT but—given his history of depression—to continue maintenance sessions.
Limited data exist on variables that relate to IPT’s acute success or conditions under which it works best. Although process research lags behind acute IPT outcome research, some findings can help guide the IPT practitioner. For example, variables shown to predict outcomes of acute IPT for depression include a positive therapeutic alliance, therapist warmth, and psycho psychotherapist use of exploratory techniques (Table 2).
Similarly, IPT has been shown to be more effective in some patients than others, depending on various moderators of depression. For example:
• For patients with high cognitive dysfunction, IPT outperforms CBT.
• For patients with higher need for medical reassurance, IPT outperforms selective serotonin reuptake inhibitor (SSRI) pharmacotherapy.
• For patients with severe depression, CBT outperforms IPT.
• For patients with low psychomotor activation, response is more rapid with an SSRI than with IPT (Table 3).18
Durability of acute IPT
One way to understand recurrence prevention is to examine the durability of a treatment’s acute effect in the absence of a specific maintenance plan. In theory, patients will continue to apply the skills learned in acute IPT to maintain gains and prevent recurrences, even after they stop seeing the psychotherapist.
Initial findings. Some research speaks to IPT’s acute-phase durability. The inaugural clinical trial of IPT by Weissman et al19 included 4 months of acute treatment and a 1-year uncontrolled naturalistic follow-up assessment. At follow-up, depression and global clinical symptoms were the same, whether patients had been acutely treated with IPT alone, pharmacotherapy alone (amitriptyline), combined IPT and pharmacotherapy, or nonscheduled treatment with a psychiatrist.
Some patients continued to function well, whereas others did not fully maintain acute treatment gains. Patients who received IPT acutely, either singly or with medication, showed better social functioning at follow-up compared with patients who did not receive IPT. This long-term durability of social improvements was an obvious target of IPT.
Support from TDCRP. In the National Institute of Mental Health Treatment of Depression Collaborative Research Project (TDCRP),20 patients in the acute phase of depression were assigned to 16 weeks of IPT, CBT, pharmacotherapy (imipramine) and clinical management (CM), or placebo plus CM. Among those who recovered by acute treatment’s end, MDD relapse rates at 18-month naturalistic follow-up were 33% for IPT, 36% for CBT, 50% for imipramine, and 33% for placebo. Between-group differences were not statistically significant.
Because acute responders to different types of treatment might have different inherent relapse tendencies, these data do not support causal attributions about the enduring effects of acute-phase treatment. The relapse rates do suggest, however, that 16 weeks of acute treatment, irrespective of kind, was insufficient for some patients to achieve full recovery and lasting remission. Consistent with the initial IPT trial,19 IPT (and CBT) outperformed medi cation and placebo in maintaining relationship quality.21
Long-term benefits. A more recent trial by Zobel et al22 examined the durability of benefits from 5 weeks of acute IPT plus pharmacotherapy and pharmacotherapy plus CM for inpatients with MDD. Although caution is required in interpreting naturalistic follow-up studies, patients in both groups showed decreased depression from baseline to 5-year follow-up. Early symptom reduction was more rapid for patients in the IPT plus pharmacotherapy group, but no significant difference existed at 5 years. More IPT patients than CM patients showed sustained remission (28% vs 11%, respectively). These rates demonstrate a need for longer-term potency of acute treatments and more targeted maintenance treatments.
IPT-M for preventing recurrence
A second way to understand recurrence prevention is to examine the efficacy of a treatment’s maintenance protocol added to an acute treatment phase. IPT has been adapted as a maintenance treatment (IPT-M), with emphasis on keeping patients well. With this revised focus, IPT-M differs somewhat from acute IPT. Although treatment continues to center on interpersonal functioning, IPT-M favors:
• vigilance for possible triggers of new depressive episodes
• longer-term contact with a therapist
• reinforcing skills learned
• addressing an expanded number of interpersonal problem areas (given that such problems can be addressed more efficiently relative to acute treatment).
Efficacy of IPT-M. In the initial trial, Frank et al23 compared the efficacy of IPT-M with that of pharmacotherapy (imipramine) in preventing depressive relapse among patients with recurrent depression who had responded to ≥16 sessions of acute IPT and imipramine and remained well during a 17- week continuation phase. For maintenance, patients were assigned to IPT-M alone, imipramine alone, placebo alone, IPT-M plus imipramine, or IPT-M plus placebo. Maintenance imipramine was continued at the acute dosage (target 200 mg/d; up to 400 mg/d was allowed). Maintenance IPT was monthly sessions. Patients remained in the trial for 3 years or until depression recurred.
On its own, IPT-M showed some efficacy in preventing recurrence, as the mean time to recurrence was 82 weeks for IPT-M alone and 74 weeks for IPT-M plus placebo. The prophylactic effect of imipramine was stronger, however. The mean time to recurrence for imipramine with IPT was 131 weeks, and the mean time to recurrence for imipramine without IPT was 124 weeks. Therefore, whereas monthly IPT-M can certainly help prolong wellness and delay recurrence, IPT maintenance treatment with acute doses of imipramine might be even more effective— if the patient is willing to take medication. These findings must be considered with caution because of the inherent inequity between imipramine and IPT-M in regard to maintenance dosage strength.
Frequency of treatment. In another trial, Frank et al24 examined whether the frequency of maintenance IPT sessions played a role in its prophylactic effect. Adult women who had achieved depression remission with acute IPT (alone or in combination with SSRI pharmacotherapy) were randomized to weekly, bi-weekly, or monthly IPT-M alone for 2 years or until recurrence. Depression recurred during IPT-M in:
• 26% of patients who had received acute IPT alone
• 50% of those who had received acute IPT plus an SSRI.
Frequency of IPT-M sessions did not affect time to recurrence. Thus, for women who can achieve remission with IPT alone, varied frequencies of IPT-M can be good prophylaxis. For women who need an SSRI to augment acute IPT, IPT-M alone at varied dosages is less effective in preventing depression recurrence. Therefore, acute treatment response patterns can inform maintenance plans, with the most prudent maintenance strategy being to maintain the acute treatment strategy over a longer period.
IPT-M for late-life depression. A trial by Reynolds et al25 examined the efficacy of maintenance nortriptyline and IPT-M in preventing depression recurrence in patients age ≥59 who initially recovered after combined acute and continuation IPT plus nortriptyline. The 4 conditions (with their recurrence rates) were:
• monthly IPT-M with nortriptyline (20%)
• monthly IPT-M with placebo (64%)
• nortriptyline plus medication visits (43%)
• placebo plus medication visits (90%).
Clearly, the combined active treatments outperformed placebo and antidepressant alone in terms of delaying or preventing recurrence, which suggests an optimal maintenance strategy with this population.
IPT-M for later life. Another trial by the same group26 enrolled patients age ≥70 with MDD that responded to acute IPT plus paroxetine. The maintenance treatments to which they were randomly assigned (and recurrence rates within 2 years) were:
• paroxetine plus IPT-M (35%)
• placebo plus IPT-M (68%)
• paroxetine plus clinical management (37%)
• placebo plus clinical management (58%).
Recurrence rates were the same for patients receiving medication plus IPT-M and medication plus clinical management, and depression was 2.4 times more likely to recur in patients receiving placebo vs active medication. Therefore, for later life depression, the optimal maintenance strategy was the SSRI.
Secondary analyses of data from these seminal trials of IPT-M point to other predictors of how and for whom maintenance IPT may work (Table 4). For example:
• Greater variability of depression symptoms during all forms of maintenance treatment is related to a greater risk of recurrence.
• Persistent insomnia is related to greater risk of recurrent depression.
• High interpersonal focus in IPT-M sessions is related to longer time to recurrence.
Bottom Line
Interpersonal psychotherapy (IPT) is efficacious for acute depression and for preventing recurrences. Patients treated successfully with acute IPT alone benefit from varied doses of maintenance IPT. Combining IPT-M with antidepressant medication can be more potent than IPT-M alone. For late-life depression, medication appears to be most effective for maintenance treatment.
Related Resources
Media
• Video demonstration, role-play transcripts, lesson plans, and quizzes. In: Appendices in and DVD companion to Ravitz P, Watson P, Grigoriadas S. Interpersonal psychotherapy for depression. New York, NY: Norton; 2013.
• Video demonstration of IPT sessions. In: DVD companion to Dewan, M, Steenbarger, B, Greenberg, R, eds. The art and science of brief psychotherapies: An illustrated guide. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2012.
Text
• Stuart S, Robertson M. Interpersonal psychotherapy: a clinician’s guide. London, United Kingdom: Taylor & Francis; 2012.
• Weissman MM, Markowitz JC, Klerman GL. Comprehensive guide to interpersonal psychotherapy. New York, NY: Basic Books; 2000.
• Weissman M, Markowitz J, Klerman GL. Clinician’s quick guide to interpersonal psychotherapy. New York, NY: Oxford University Press; 2007.
Websites
• Interpersonal Psychotherapy Institute. http://iptinstitute.com.
• International Society for Interpersonal Psychotherapy. http://interpersonalpsychotherapy.org.
Drug Brand Names
Amitriptyline • Elavil Nortriptyline • Pamelor
Imipramine • Tofranil Paroxetine • Paxil
Acknowledgments
The authors are grateful to Samantha L. Bernecker, MS, and Nicholas R. Morrison for their assistance with the research review.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. ten Doesschate MC, Koeter MW, Bockting CL, et al. Health related quality of life in recurrent depression: a comparison with a general population sample. J Affect Disord. 2010; 120(1-3):126-132.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-91.
3. Arnow BA, Constantino MJ. Effectiveness of psychotherapy and combination treatment for chronic depression. J Clin Psychol. 2003;59(8):893-905.
4. American Psychiatric Association. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2010.
5. Klerman GL, Weissman MM, Rounsaville BJ, et al. Interpersonal psychotherapy of depression. New York, NY: Basic Books; 1984.
6. Weissman MM, Markowitz JC, Klerman G. Comprehensive guide to interpersonal psychotherapy. New York, NY: Basic Books; 2000.
7. Weissman M, Markowitz J, Klerman G. Clinician’s quick guide to interpersonal psychotherapy. New York, NY: Oxford University Press; 2007.
8. Brakemeier EL, Frase L. Interpersonal psychotherapy (IPT) in major depressive disorder. Eur Arch Psychiatry Clin Neurosci. 2012;262(suppl 2):S117-1121.
9. Depression in adults (update): NICE guideline CG90). National Institute for Health and Care Excellence. (2009). http://www.nice.org.uk/cg90. Updated October 2009. Accessed March 5, 2014.
10. Depression. National Institutes of Mental Health. http://www.nimh.nih.gov/health/publications/depression/ index.shtml. Revised 2011. Accessed March 5, 2014.
11. Cuijpers P, van Straten A, Andersson G, et al. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008;76(6):909-922.
12. Cuijpers P, Geraedts AS, van Oppen P, et al. Interpersonal psychotherapy for depression: a meta-analysis [Erratum in: Am J Psychiatry. 2011;168(6):652]. Am J Psychiatry. 2011; 168(6):581-592.
13. Mufson L, Dorta K, Wickramaratne P, et al. A randomized effectiveness trial of interpersonal psychotherapy for depressed adolescents. Arch Gen Psychiatry. 2004;61(6): 577-584.
14. Schramm E, Schneider D, Zobel I, et al. Efficacy of interpersonal psychotherapy plus pharmacotherapy in chronically depressed inpatients. J Affect Disord. 2008; 109(1-2):65-73.
15. Bernecker SL. How and for whom does interpersonal psychotherapy work? Psychotherapy Bulletin. 2012;47(2):13-17.
16. Stuart S. Interpersonal psychotherapy. In: Dewan MJ, Steenbarger BN, Greenberg RP, eds. The art and science of brief psychotherapies: an illustrated guide. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2012: 157-193.
17. Grigoriadas S, Watson P, Maunder R, eds. Psychotherapy essentials to go: Interpersonal psychotherapy for depression. New York, NY: W. W. Norton & Company, Inc.; 2013.
18. Bleiberg KL, Markowitz JC. Interpersonal psychotherapy for depression. In: Barlow D, ed. Clinical handbook of psychological disorders: a step-by-step treatment manual. New York, NY: The Guilford Press; 2008:306-327.
19. Weissman MM, Klerman GL, Prusoff BA, et al. Depressed outpatients. Results one year after treatment with drugs and/or interpersonal psychotherapy. Arch Gen Psychiatry. 1981;38(1):51-55.
20. Shea MT, Elkin I, Imber SD, et al. Course of depressive symptoms over follow-up. Findings from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Arch Gen Psychiatry. 1992;49(10):782-787.
21. Blatt S, Zuroff D, Bondi C, et al. Short- and long-term effect of medication and psychotherapy in the brief treatment of depression: further analyses of data from the NIMH TDCRP. Psychother Res. 2000;10(2):215-234.
22. Zobel I, Kech S, van Calker D, et al. Long-term effect of combined interpersonal psychotherapy and pharmacotherapy in a randomized trial of depressed patients. Acta Psychiatr Scand. 2011;123(4):276-282.
23. Frank E, Kupfer DJ, Perel JM, et al. Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry. 1990;47(12):1093-1099.
24. Frank E, Kupfer DJ, Buysse DJ, et al. Randomized trial of weekly, twice-monthly, and monthly interpersonal psychotherapy as maintenance treatment for women with recurrent depression. Am J Psychiatry. 2007;164(5): 761-767.
25. Reynolds CF 3rd, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA. 1999;281(1): 39-45.
26. Reynolds CF 3rd, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354(11):1130-1138.
1. ten Doesschate MC, Koeter MW, Bockting CL, et al. Health related quality of life in recurrent depression: a comparison with a general population sample. J Affect Disord. 2010; 120(1-3):126-132.
2. Hardeveld F, Spijker J, De Graaf R, et al. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122(3):184-91.
3. Arnow BA, Constantino MJ. Effectiveness of psychotherapy and combination treatment for chronic depression. J Clin Psychol. 2003;59(8):893-905.
4. American Psychiatric Association. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2010.
5. Klerman GL, Weissman MM, Rounsaville BJ, et al. Interpersonal psychotherapy of depression. New York, NY: Basic Books; 1984.
6. Weissman MM, Markowitz JC, Klerman G. Comprehensive guide to interpersonal psychotherapy. New York, NY: Basic Books; 2000.
7. Weissman M, Markowitz J, Klerman G. Clinician’s quick guide to interpersonal psychotherapy. New York, NY: Oxford University Press; 2007.
8. Brakemeier EL, Frase L. Interpersonal psychotherapy (IPT) in major depressive disorder. Eur Arch Psychiatry Clin Neurosci. 2012;262(suppl 2):S117-1121.
9. Depression in adults (update): NICE guideline CG90). National Institute for Health and Care Excellence. (2009). http://www.nice.org.uk/cg90. Updated October 2009. Accessed March 5, 2014.
10. Depression. National Institutes of Mental Health. http://www.nimh.nih.gov/health/publications/depression/ index.shtml. Revised 2011. Accessed March 5, 2014.
11. Cuijpers P, van Straten A, Andersson G, et al. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008;76(6):909-922.
12. Cuijpers P, Geraedts AS, van Oppen P, et al. Interpersonal psychotherapy for depression: a meta-analysis [Erratum in: Am J Psychiatry. 2011;168(6):652]. Am J Psychiatry. 2011; 168(6):581-592.
13. Mufson L, Dorta K, Wickramaratne P, et al. A randomized effectiveness trial of interpersonal psychotherapy for depressed adolescents. Arch Gen Psychiatry. 2004;61(6): 577-584.
14. Schramm E, Schneider D, Zobel I, et al. Efficacy of interpersonal psychotherapy plus pharmacotherapy in chronically depressed inpatients. J Affect Disord. 2008; 109(1-2):65-73.
15. Bernecker SL. How and for whom does interpersonal psychotherapy work? Psychotherapy Bulletin. 2012;47(2):13-17.
16. Stuart S. Interpersonal psychotherapy. In: Dewan MJ, Steenbarger BN, Greenberg RP, eds. The art and science of brief psychotherapies: an illustrated guide. 2nd ed. Arlington, VA: American Psychiatric Publishing; 2012: 157-193.
17. Grigoriadas S, Watson P, Maunder R, eds. Psychotherapy essentials to go: Interpersonal psychotherapy for depression. New York, NY: W. W. Norton & Company, Inc.; 2013.
18. Bleiberg KL, Markowitz JC. Interpersonal psychotherapy for depression. In: Barlow D, ed. Clinical handbook of psychological disorders: a step-by-step treatment manual. New York, NY: The Guilford Press; 2008:306-327.
19. Weissman MM, Klerman GL, Prusoff BA, et al. Depressed outpatients. Results one year after treatment with drugs and/or interpersonal psychotherapy. Arch Gen Psychiatry. 1981;38(1):51-55.
20. Shea MT, Elkin I, Imber SD, et al. Course of depressive symptoms over follow-up. Findings from the National Institute of Mental Health Treatment of Depression Collaborative Research Program. Arch Gen Psychiatry. 1992;49(10):782-787.
21. Blatt S, Zuroff D, Bondi C, et al. Short- and long-term effect of medication and psychotherapy in the brief treatment of depression: further analyses of data from the NIMH TDCRP. Psychother Res. 2000;10(2):215-234.
22. Zobel I, Kech S, van Calker D, et al. Long-term effect of combined interpersonal psychotherapy and pharmacotherapy in a randomized trial of depressed patients. Acta Psychiatr Scand. 2011;123(4):276-282.
23. Frank E, Kupfer DJ, Perel JM, et al. Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry. 1990;47(12):1093-1099.
24. Frank E, Kupfer DJ, Buysse DJ, et al. Randomized trial of weekly, twice-monthly, and monthly interpersonal psychotherapy as maintenance treatment for women with recurrent depression. Am J Psychiatry. 2007;164(5): 761-767.
25. Reynolds CF 3rd, Frank E, Perel JM, et al. Nortriptyline and interpersonal psychotherapy as maintenance therapies for recurrent major depression: a randomized controlled trial in patients older than 59 years. JAMA. 1999;281(1): 39-45.
26. Reynolds CF 3rd, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354(11):1130-1138.
Taking the spice route: Psychoactive properties of culinary spices
Many substances that are not typically thought of as “substances of abuse” possess—when adequately dosed—clinically meaningful psychoactive properties. In addition to the more familiar effects of alcohol, psychostimulants, opioids, Cannabis, and hallucinogens, you may encounter psychiatric phenomena resulting from abuse of more obscure substances, including culinary spices.
The clinician treating a patient in an apparent intoxicated state who has a negative drug screen might ask that patient if he (she) abuses spices. This might be particularly relevant when treating patients thought to have limited access to illicit substances or those with ready access to large amounts of spices, such as prisoners, young patients, and those working in the food service industry.
Abuse of spices can be a problematic diagnosis
Patients may misuse culinary spices to achieve euphoria, or a “natural high.” They may present with medical or psychiatric symptoms, including acute altered mental status, but the psychoactive substances are not identified on routine toxicology studies. In addition, patients may not attribute their use of spices for psychoactive effect to “drugs,” because these materials are legal and readily available. This may lead to misdiagnosis of a systemic medical disorder or a primary psychiatric illness to explain the patient’s symptoms and initiating a psychotropic agent and other psychiatric services when a substance abuse program might be a more appropriate clinical intervention.
Some spices contain psychoactive compounds that can alter CNS function (Table1-7), might be abused for recreational purposes, and can be toxic in an excessive amount. Internet resources, including anonymous web-based communications, and anecdotal materials about non-traditional recreational drugs, are available to anyone with Internet access.8 However, little research has been conducted into the prevalence of abuse (Box)9 and spices’ psychoactive properties. The lack of toxicology detection of spices in the medical setting presents a diagnostic challenge.
The psychoactive plants used in “natural high” products mainly are psychoactively inactive in their natural form, but extracts or alkaloids obtained from them might induce 1 or more of 3 classifications of psychoactivity:
• stimulant
• sedative
• hallucinogenic.
Many of these substances are considered to be aphrodisiac, and some may be abused to increase sexual function.
The following is a review of common spices that have been reported to possess potential psychoactive properties.
Nutmeg
Nutmeg (Myristica fragrans) is a common and easily accessible means of reaching euphoria in adults.10 The aromatic oil of nutmeg contains myristicin, a psychoactive substance that is chemically similar to hallucinogenic compounds such as mescaline. Its psychoactive effects could be attributed to metabolic formation of amphetamine derivatives from its core ingredients, elemicin, myristicin, and safrole.11,12
Nutmeg and its active component, myristicin, produce central monoamine oxidase (MAO) inhibition as evidenced by the ability to lower the convulsive dose of IV tryptamine in mice and to increase brain 5-hydroxytryptamine concentrations.13,14 Although myristicin’s potency is not comparable to that of the more potent MAO inhibitors such as tranylcypromine and iproniazid (which is not available in the United States), it seems adequate when compared with its low toxicity.14 Nutmeg extract is associated with a significant antidepressant effect in mice, which seemed to be mediated by interaction with the adrenergic, dopaminergic, and serotonergic systems.13 Nutmeg is associated with sustained increase in sexual activity in animal studies, with no evidence of adverse effects and toxicity, suggesting that nutmeg possesses clinically significant aphrodisiac activity.15
Psychoactive effects can be achieved by ingesting 5 to 15 g of nutmeg.11 Acute nutmeg intoxication produces palpitations, dizziness, anxiety, and hallucinations, mostly resolving within 24 hours, while effects of chronic abuse are reported to be similar to Cannabis use, including euphoria, giddiness, anxiety, fear, sense of impending doom, detachment, confabulation, and hallucinations.11,16 Urine drug screens are negative unless other psychoactive substances have been ingested.17
Suspected nutmeg intoxication or poisoning should be treated with supportive treatment. Use sedatives with caution because of alternating periods of delirium and obtundation during nutmeg intoxication.17
In case reports, myristicin poisoning induced CNS neuromodulatory signs that mimicked an anticholinergic hyperstimulation state.12,18 Fatal myristicin poisoning is rare; 2 cases have been reported, 1 in combination with flunitrazepam (not available in the United States).19,20 Nutmeg also has sedative properties and can cause GI symptoms when ingesting excessive amounts.1,20,21 Grover et al21 described no harmful effects on blood pressure and electrocardiogram; however, Shah et al22 reported palpitations and dry mouth.
Vanilla
Vanilla (species of the genus Vanilla) contains piperonal, also known as heliotropin.1 Piperonal has aromatherapeutic qualities that might elevate mood and well-being. In the early 1990s, the Memorial Sloan- Kettering Cancer Center in New York City described heliotropin as a powerful aromatherapy tool. Patients who were undergoing an MRI in an environment scented with heliotropin demonstrated a 63% reduction in anxiety compared with those who were not exposed to fragrance.23 The Smell and Taste Treatment and Research Foundation in Chicago found that vanilla can promote sexual arousal.24
Short-term effects of vanillin—a major component of vanilla—include a feeling of relaxation and reduced stress; long-term use can produce an antidepressant effect.1 There are no reports of vanilla abuse to achieve these effects; however, patients might abuse vanilla extract because of its alcohol content (up to 35% ethanol).25
Fennel
The essential oil of fennel (Foeniculum vulgare) can be neurotoxic and epileptogenic. Skalli and colleagues recently reported a case of seizure induction in a young woman after ingesting cakes containing fennel oil.26 Fennel oil also has been reported to have significant interaction with the fluoroquinolone-type antibiotics. Be aware of adverse effects associated with fennel ingestion; question patients if atypical seizures or reactions to antibiotics occur.27
Spices such as fennel, dill, cinnamon, saffron, and anise also contain psychoactive substances that are chemically similar to myristicin, which can induce sedation, stimulation, or hallucinations.7
Black pepper
Piperine, which gives black pepper (Piper nigrum) its spiciness, enhances thermogenesis of lipid metabolism, accelerates energy metabolism, and increases serotonin and endorphin production in the brain.28 Black pepper is reported to potentiate γ-aminobutyric acid A receptor subtypes,29 and could present possible applications for treating insomnia, epilepsy, and anxiety disorders.
Cloves
Non-culinary uses of clove (Syzygium aromaticum, a tree in the myrtle family) include flavored cigarettes. However, in 2009 clove cigarettes were banned in the United States as part of a public policy to reduce the number of children who start smoking.30 Eugenol, which constitutes as much as 90% of the essential oil extracted from cloves (and is responsible for the aroma), can cause hepatotoxicity31 and palpitations32; it can be toxic in quantities as low as 5 mL.33 Eugenol is present in other spices, such as nutmeg and cinnamon, and has been reported to have sedative properties.1
Mace
Mace is made from the covering of nutmeg (Myristica fragrans) seeds. It has a strong aroma resembling that of nutmeg. Whole mace contains 4% to 14% of a volatile oil similar to that found in nutmeg. Because mace contains the same oils that make nutmeg psychoactive1 in excessive amounts—although nutmeg seeds are more potent—be aware of the psychoactive potential of mace.
CinnamonCassia cinnamon (Cinnamomum aromaticum) is spicier and tarter than Ceylon cinnamon (Cinnamomum zeylanicum), which has a more flowery aroma. The 2 types of cinnamon can be distinguished by their different chemical composition. Ceylon cinnamon contains eugenol and benzyl benzoate; cassia cinnamon contains coumarin.3 Eugenol is reported to have sedative effects.1 Coumarin is a precursor molecule in the synthesis of a number of synthetic anticoagulant pharmaceuticals, including coumadin. Because of the toxic component of coumarin, European health agencies have warned against consuming high amounts of cassia.34 There are no reports of side effects arising from the occasional use of cinnamon as a spice.
In a study by Frydman-Marom et al,35 cinnamon extract (CEppt) was found to act on the CNS by inhibiting development of Alzheimer’s disease in animal models.
Asarone
Asarone is found in the Asarum family of spices that includes Acorus calamus. Asarone is chemically similar to mescaline. Although anecdotal reports indicate that A. calamus is a hallucinogen, research shows no evidence that it contains hallucinogenic substances.36 Han et al37 reported an antidepressant effect with the essential oil and asarones for the rhizomes of Acorus tatarinowii. In animal studies, asarone was found to reduce spontaneous motor activity, and even in low doses, reduced anxiety without decreasing acuity of perception.38
Ginger
Ginger (Zingiber officinale) is regarded as a sedative, general stimulant, and aphrodisiac.1,4,5 Its main constituents are phenolic compounds such as gingerols and shogaols, and sesquiterpenes such as zingiberene.4 Ginger is an inhibitor of thromboxane synthetase, a property shared by tricyclic antidepressants.39
Research indicates that 9 compounds found in ginger may interact with the serotonin 5-HT1A receptor, suggesting a possible mechanism for reducing anxiety.40 A study by Nievergelt et al41 indicates that by binding to human serotonin receptors, ginger might influence GI function. Ginger extract contains a cholinergic and spasmogenic component, which provides a mechanistic insight for the prokinetic action of ginger.40
Turmeric
Turmeric (Curcuma longa) has been investigated for possible benefit in Alzheimer’s disease42; research into curcumin, the active substance of turmeric, is increasing. Although the original report was retracted after publication, curcumin was reported to selectively bind to human cannabinoid receptors type 1 (CB1) with nanomolar affinities and to function as an antagonist/inverse agonist.43 However, Gertsch et al44 found that curcumin did not interact functionally with the CB1 receptor, although this compound appears to share ability of the CB1 receptor inverse agonist.
Galangal
Major constituents identified in the galangal (or galanga) rhizome and leaf oil were 1,8-cineole, and β-pinene and camphor.6 Galangal, a member of the ginger (Zingiberaceae) family, interacts with MAO inhibitors, H2 receptor antagonists, and proton-pump inhibitors.1 Anxiolytic, hallucinogenic, and stimulant properties have been reported.1 An excessive amount can induce diarrhea, dizziness, nausea, and vomiting.1
Saffron
Stigma of saffron (a member of the family Iridaceae) was found to be significantly more effective than placebo and equally as efficacious as fluoxetine and imipramine in treating depression. Saffron petal was found to be significantly more effective than placebo and as effective as fluoxetine and saffron stigma in a recent systematic review.45-48
Asafetida
Asafetida (Ferula assa-foetida), when combined with valerian root, is used as a sedative to treat hyperactivity.2 The active ingredients of asafetida are the resin, endogenous gum, essential oil, propenyl-isobutylsulfide, umbelliferone, and vanillin. Several of the volatile constituents produce a sedative effect.2 Additive effects can occur between the hypotensive property of asafetida and dopamine receptor agonists such as bromocriptine mesylate. Use caution when combining asafetida in conjunction with a CNS depressant or a stimulant.2
Recommendations for treating spice-abusers
Patients may present to psychiatry services with psychological and physiological evidence of intoxication with culinary spices that may mimic 1) abuse of other substances, 2) primary psychiatric illness, and 3) primary medical illness. When you encounter a patient with a new psychiatric symptom, consider inquiring about the abuse of spices.
Patients might abuse more than 1 spice; a comprehensive screening approach might therefore be useful. Caution patients that ingesting these substance to excess can have harmful effects. Consider appropriate psychopharmacotherapy for underlying psychiatric symptoms to help patients who use spices maladaptively to self-medicate psychiatric symptoms.
Consider abuse of culinary spices in clinical presentations of psychiatric symptoms that do not seem adequate for a diagnosis of a primary anxiety, mood, or psychotic disorder, or in cases atypical psychiatric presentations that are—perhaps to your surprise—associated with negative toxicology studies for common, more familiar substances of abuse.
Physicians practicing in an environment where street drugs are difficult to obtain (eg, prisons) should consider monitoring for possible abuse of spices. Based on the available, albeit limited, literature, it appears that most culinary spice–associated intoxication can be managed:
• with an elevated level of clinical suspicion
• by ruling out other causes of intoxication
• using targeted, empirical psychopharmacotherapy to manage symptoms
• with supportive care that includes close psychiatric follow-up.
Consider comorbid abuse of other, more familiar substances of abuse in patients who misuse spices. As with inhalant abuse, the concept of “substance abuse” in clinical practice may need to be further expanded to include patients who abuse culinary spices. Patients could be screened for psychiatric illnesses known to increase the risk of substance abuse. These might include—but are not limited to:
• comorbid psychotic disorders
• mood disorders, particularly bipolar disorders
• trauma- and stressor-related disorders, particularly posttraumatic stress disorder
• personality disorders, particularly antisocial, borderline, and narcissistic personality disorders.
Pending the availability of population-based studies on abuse of culinary spices, the usual cautions regarding substance abuse seem to be appropriate when caring for these patients. Assessment for and management of comorbid psychiatric conditions is essential in the comprehensive psychiatric care of patients who abuse substances.
Last, general consideration of a 12-step recovery program appears warranted for these patients; the self-reflection and group support of such programs can be useful in helping patients control their use of these substances.
Bottom Line
Presentation of culinary spice intoxication can parallel that of other medical or psychiatric illnesses, or other drugs of abuse. Consideration and questioning for abuse of spices is necessary to ascertain the psychoactive effects of these substances when used surreptitiously. Management should follow substance abuse treatment protocols: inquiry into patterns of problematic use and readiness to change, assessment and management of psychiatric comorbidity, and referral to a recovery program.
Related Resources
• Srinivasan K. Role of spices beyond food flavoring: nutraceuticals with multiple health effects. Food Reviews International. 2005;21(2):167-188.
• Parthasarathi U, Hategan A, Bourgeois JA. Out of the cupboard and into the clinic: Nutmeg-induced mood disorder. Current Psychiatry. 2013;12(12):E1-E2.
Drug Brand Names
Bromocriptine mesylate • Parlodel Imipramine • Tofrani
Flunitrazepam • Rohypnol Iproniazid • Marsilid
Fluoxetine • Prozac Tranylcypromine • Parnate
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. O’Mahony Carey S. Psychoactive substances. A guide to ethnobotanical plants and herbs, synthetic chemicals, compounds and products. http://www.drugs.ie/ resourcesfiles/guides/Psychoactive_substances_low_res. pdf. Accessed March 4, 2014.
2. Asafetida. Applied Health. http://www.appliedhealth.com/index.php?option=com _content&view=article&id= 108207. Accessed March 4, 2014.
3. Jayatilaka A, Poole SK, Poole CF, et al. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupled-column gas chromatography. Analytica Chimica Acta. 1995;302(2-3):147-162.
4. Spices. History & Special Collections UCLA Louise M. Darling Biomedical Library. http://unitproj.library.ucla. edu/biomed/spice/index.cfm?displayID=15. Accessed March 4, 2014.
5. Ginger action and uses. Ginger extract. Gingerols. MDidea Web site. http://www.mdidea.com/products/new/ new02108.html. Accessed March 4, 2014.
6. Raina VK, Srivastava SK, Syamasunder KV. The essential oil of ‘greater galangal’ [Alpinia galanga (L.) Willd.] from the lower Himalayan region of India. Flavour and Fragrance Journal. 2002;17(5):358-360.
7. Wenk G. Psychoactive spices - Bon appetite! http://www.psychologytoday.com/blog/your-brain-food/201008/ psychoactive-spices-bon-appetite. Published August 4, 2010. Accessed March 4, 2014.
8. Wax PM. Just a click away: recreational drug Web sites on the Internet. Pediatrics.2002;109(6):e96.
9. Forrester MB. Nutmeg intoxication in Texas, 1998-2004. Hum Exp Toxicol. 2005;24(11):563-566.
10. Abernethy MK, Becker LB. Acute nutmeg intoxication. Am J Emerg Med. 1992;10(5):429-430.
11. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
12. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
13. Dhingra D, Sharma A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9(1):84-89.
14. Truitt EB Jr, Duritz G, Ebersberger EM. Evidence of monoamine oxidase inhibition by myristicin and nutmeg. Proc Soc Exp Biol Med. 1963;112:647-650.
15. Tajuddin, Ahmad S, Latif A, et al. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med. 2005;5:16.
16. Quin GI, Fanning NF, Plunkett PK. Nutmeg intoxication. J Accid Emerg Med. 1998;15(4):287-288.
17. Barceloux DG. Nutmeg (Myristica fragrans Houtt.) Dis Mon. 2009;55(6):373-379.
18. Demetriades AK, Wallman PD, McGuiness A, et al. Low cost, high risk: accidental nutmeg intoxication. Emerg Med J. 2005;22(3):223-225.
19. Weil A. The use of nutmeg as a psychotropic agent. Bull Narc. 1966;18(4):15-23. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1966-01-01_4_ page003.html. Accessed March 5, 2013.
20. Stein U, Greyer H, Hentschel H. Nutmeg (myristicin) poisoning - report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci Int. 2001;118(1):87-90.
21. Grover JK, Khandkar S, Vats V, et al. Pharmacological studies on Myristica fragrans—antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24(10):675-680.
22. Shah AM, Calello DP, Quintero-Solivan J, et al. The not-so-nice spice: a teenage girl with palpitations and dry mouth. Pediatr Emerg Care. 2011;27(12):1205-1207.
23. Heliotropin. Polarized light microscopy digital image gallery. http://micro.magnet.fsu.edu/primer/techniques/ polarized/gallery/pages/heliotropinsmall.html. Accessed March 5, 2014.
24. Gage E. Romancing the bean. Budget Travel. http://articles.cnn.com/2007-09-11/travel/vanilla_1_vanilla-orchid-totonaca?_s=PM:TRAVEL. Published September 11, 2007. Updated September 16, 2012. Accessed March 5, 2014.
25. Mazor S, DesLauriers CA, Mycyk MB. Adolescent ethanol intoxication from vanilla extract ingestion: a case report. The Internet Journal of Family Practice. 2005;4(1). doi: 10.5580/bc.
26. Skalli S, Soulaymani Bencheikh R. Epileptic seizure induced by fennel essential oil. Epileptic Disord. 2011;13(3):345-347.
27. Zhu M, Wong PY, Li RC. Effect of oral administration of fennel (Foeniculum vulgare) on ciprofloxacin absorption and disposition in the rat. J Pharm Pharmacol. 1999;51(12):1391-1396.
28. Malini T, Arunakaran J, Aruldhas MM, et al. Effects of piperine on the lipid composition and enzymes of the pyruvate-malate cycle in the testis of the rat in vivo. Biochem Mol Biol Int. 1999;47(3):537-545.
29. Zaugg J, Baburin I, Hering S, et al. Identifying GABAA receptor ligands in black pepper by activity profiling, LC-TOFMS, and offline microprobe NMR. Planta Med. 2009; 75(9):888-889. doi: 10.1055/s-0029-1234276.
30. Flavored tobacco. FDA.gov. http://www.fda.gov/TobaccoProducts/ProtectingKidsfromTobacco/ FlavoredTobacco/default.htm. Published September 22, 2009. Updated March 21, 2013. Accessed March 18, 2014.
31. Fujisawa S, Atsumi T, Kadoma Y, et al. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177(1):39-54.
32. Eugenol oil overdose. New York Times Health Guide. http://health.nytimes.com/health/guides/poison/ eugenol-oil-overdose/overview.html. Accessed March 5, 2014.
33. Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child. 1993;69(3):392-393.
34. Harris E. NPR. German Christmas cookies pose health danger. http://www.npr.org/templates/story/story.php? storyId=6672644. Published December 25, 2006. Accessed March 5, 2014.
35. Frydman-Marom A, Levin A, Farfara D, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011; 6(1):e16564. doi:10.1371/journal.pone.001656453.
36. Björnstad K, Helander A, Hultén P, et al. Bioanalytical investigation of asarone in connection with Acorus calamus oil intoxications. J Anal Toxicol. 2009;33(9):604-609.
37. Han P, Han T, Peng W, et al. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm Biol. 2013;51(5):589-594.
38. Dandiya PC, Menon MK. Actions of asarone on behavior, stress, and hyperpyrexia, and its interaction with central stimulants. J Pharmacol Exp Ther. 1964;145:42-46.
39. Bockon J. Ginger: inhibition of thromboxane synthetase and stimulation of prostacyclin: relevance for medicine and psychiatry. Med Hypotheses. 1986;20(3):271-278.
40. Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50(10):1889-1897.
41. Nievergelt A, Huonker P, Schoop R, et al. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg Med Chem. 2010;18(9):3345-3351.
42. Mishra A, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13-19.
43. Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists [retracted in: J Pharmacol Exp Ther. 2009;331(3):1147]. J Pharmacol Exp Ther. 2009;330(1): 31-39.
44. Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant – do they exist? Br J Pharmacol. 2010;160(3):523-529.
45. Dwyer AV, Whitten DL, Hawrelak JA. Herbal medicines, other than St. John’s Wort, in the treatment of depression: a systematic review. Altern Med Rev. 2011;16(1):40-49.
46. Moshiri E, Basti AA, Noorbala AA, et al. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo controlled trial. Phytomedicine. 2006;13(9-10):607-611.
47. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-284.
48. Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized, and placebo-controlled trial. Phytother Res. 2005;19(2):148-151.
Many substances that are not typically thought of as “substances of abuse” possess—when adequately dosed—clinically meaningful psychoactive properties. In addition to the more familiar effects of alcohol, psychostimulants, opioids, Cannabis, and hallucinogens, you may encounter psychiatric phenomena resulting from abuse of more obscure substances, including culinary spices.
The clinician treating a patient in an apparent intoxicated state who has a negative drug screen might ask that patient if he (she) abuses spices. This might be particularly relevant when treating patients thought to have limited access to illicit substances or those with ready access to large amounts of spices, such as prisoners, young patients, and those working in the food service industry.
Abuse of spices can be a problematic diagnosis
Patients may misuse culinary spices to achieve euphoria, or a “natural high.” They may present with medical or psychiatric symptoms, including acute altered mental status, but the psychoactive substances are not identified on routine toxicology studies. In addition, patients may not attribute their use of spices for psychoactive effect to “drugs,” because these materials are legal and readily available. This may lead to misdiagnosis of a systemic medical disorder or a primary psychiatric illness to explain the patient’s symptoms and initiating a psychotropic agent and other psychiatric services when a substance abuse program might be a more appropriate clinical intervention.
Some spices contain psychoactive compounds that can alter CNS function (Table1-7), might be abused for recreational purposes, and can be toxic in an excessive amount. Internet resources, including anonymous web-based communications, and anecdotal materials about non-traditional recreational drugs, are available to anyone with Internet access.8 However, little research has been conducted into the prevalence of abuse (Box)9 and spices’ psychoactive properties. The lack of toxicology detection of spices in the medical setting presents a diagnostic challenge.
The psychoactive plants used in “natural high” products mainly are psychoactively inactive in their natural form, but extracts or alkaloids obtained from them might induce 1 or more of 3 classifications of psychoactivity:
• stimulant
• sedative
• hallucinogenic.
Many of these substances are considered to be aphrodisiac, and some may be abused to increase sexual function.
The following is a review of common spices that have been reported to possess potential psychoactive properties.
Nutmeg
Nutmeg (Myristica fragrans) is a common and easily accessible means of reaching euphoria in adults.10 The aromatic oil of nutmeg contains myristicin, a psychoactive substance that is chemically similar to hallucinogenic compounds such as mescaline. Its psychoactive effects could be attributed to metabolic formation of amphetamine derivatives from its core ingredients, elemicin, myristicin, and safrole.11,12
Nutmeg and its active component, myristicin, produce central monoamine oxidase (MAO) inhibition as evidenced by the ability to lower the convulsive dose of IV tryptamine in mice and to increase brain 5-hydroxytryptamine concentrations.13,14 Although myristicin’s potency is not comparable to that of the more potent MAO inhibitors such as tranylcypromine and iproniazid (which is not available in the United States), it seems adequate when compared with its low toxicity.14 Nutmeg extract is associated with a significant antidepressant effect in mice, which seemed to be mediated by interaction with the adrenergic, dopaminergic, and serotonergic systems.13 Nutmeg is associated with sustained increase in sexual activity in animal studies, with no evidence of adverse effects and toxicity, suggesting that nutmeg possesses clinically significant aphrodisiac activity.15
Psychoactive effects can be achieved by ingesting 5 to 15 g of nutmeg.11 Acute nutmeg intoxication produces palpitations, dizziness, anxiety, and hallucinations, mostly resolving within 24 hours, while effects of chronic abuse are reported to be similar to Cannabis use, including euphoria, giddiness, anxiety, fear, sense of impending doom, detachment, confabulation, and hallucinations.11,16 Urine drug screens are negative unless other psychoactive substances have been ingested.17
Suspected nutmeg intoxication or poisoning should be treated with supportive treatment. Use sedatives with caution because of alternating periods of delirium and obtundation during nutmeg intoxication.17
In case reports, myristicin poisoning induced CNS neuromodulatory signs that mimicked an anticholinergic hyperstimulation state.12,18 Fatal myristicin poisoning is rare; 2 cases have been reported, 1 in combination with flunitrazepam (not available in the United States).19,20 Nutmeg also has sedative properties and can cause GI symptoms when ingesting excessive amounts.1,20,21 Grover et al21 described no harmful effects on blood pressure and electrocardiogram; however, Shah et al22 reported palpitations and dry mouth.
Vanilla
Vanilla (species of the genus Vanilla) contains piperonal, also known as heliotropin.1 Piperonal has aromatherapeutic qualities that might elevate mood and well-being. In the early 1990s, the Memorial Sloan- Kettering Cancer Center in New York City described heliotropin as a powerful aromatherapy tool. Patients who were undergoing an MRI in an environment scented with heliotropin demonstrated a 63% reduction in anxiety compared with those who were not exposed to fragrance.23 The Smell and Taste Treatment and Research Foundation in Chicago found that vanilla can promote sexual arousal.24
Short-term effects of vanillin—a major component of vanilla—include a feeling of relaxation and reduced stress; long-term use can produce an antidepressant effect.1 There are no reports of vanilla abuse to achieve these effects; however, patients might abuse vanilla extract because of its alcohol content (up to 35% ethanol).25
Fennel
The essential oil of fennel (Foeniculum vulgare) can be neurotoxic and epileptogenic. Skalli and colleagues recently reported a case of seizure induction in a young woman after ingesting cakes containing fennel oil.26 Fennel oil also has been reported to have significant interaction with the fluoroquinolone-type antibiotics. Be aware of adverse effects associated with fennel ingestion; question patients if atypical seizures or reactions to antibiotics occur.27
Spices such as fennel, dill, cinnamon, saffron, and anise also contain psychoactive substances that are chemically similar to myristicin, which can induce sedation, stimulation, or hallucinations.7
Black pepper
Piperine, which gives black pepper (Piper nigrum) its spiciness, enhances thermogenesis of lipid metabolism, accelerates energy metabolism, and increases serotonin and endorphin production in the brain.28 Black pepper is reported to potentiate γ-aminobutyric acid A receptor subtypes,29 and could present possible applications for treating insomnia, epilepsy, and anxiety disorders.
Cloves
Non-culinary uses of clove (Syzygium aromaticum, a tree in the myrtle family) include flavored cigarettes. However, in 2009 clove cigarettes were banned in the United States as part of a public policy to reduce the number of children who start smoking.30 Eugenol, which constitutes as much as 90% of the essential oil extracted from cloves (and is responsible for the aroma), can cause hepatotoxicity31 and palpitations32; it can be toxic in quantities as low as 5 mL.33 Eugenol is present in other spices, such as nutmeg and cinnamon, and has been reported to have sedative properties.1
Mace
Mace is made from the covering of nutmeg (Myristica fragrans) seeds. It has a strong aroma resembling that of nutmeg. Whole mace contains 4% to 14% of a volatile oil similar to that found in nutmeg. Because mace contains the same oils that make nutmeg psychoactive1 in excessive amounts—although nutmeg seeds are more potent—be aware of the psychoactive potential of mace.
CinnamonCassia cinnamon (Cinnamomum aromaticum) is spicier and tarter than Ceylon cinnamon (Cinnamomum zeylanicum), which has a more flowery aroma. The 2 types of cinnamon can be distinguished by their different chemical composition. Ceylon cinnamon contains eugenol and benzyl benzoate; cassia cinnamon contains coumarin.3 Eugenol is reported to have sedative effects.1 Coumarin is a precursor molecule in the synthesis of a number of synthetic anticoagulant pharmaceuticals, including coumadin. Because of the toxic component of coumarin, European health agencies have warned against consuming high amounts of cassia.34 There are no reports of side effects arising from the occasional use of cinnamon as a spice.
In a study by Frydman-Marom et al,35 cinnamon extract (CEppt) was found to act on the CNS by inhibiting development of Alzheimer’s disease in animal models.
Asarone
Asarone is found in the Asarum family of spices that includes Acorus calamus. Asarone is chemically similar to mescaline. Although anecdotal reports indicate that A. calamus is a hallucinogen, research shows no evidence that it contains hallucinogenic substances.36 Han et al37 reported an antidepressant effect with the essential oil and asarones for the rhizomes of Acorus tatarinowii. In animal studies, asarone was found to reduce spontaneous motor activity, and even in low doses, reduced anxiety without decreasing acuity of perception.38
Ginger
Ginger (Zingiber officinale) is regarded as a sedative, general stimulant, and aphrodisiac.1,4,5 Its main constituents are phenolic compounds such as gingerols and shogaols, and sesquiterpenes such as zingiberene.4 Ginger is an inhibitor of thromboxane synthetase, a property shared by tricyclic antidepressants.39
Research indicates that 9 compounds found in ginger may interact with the serotonin 5-HT1A receptor, suggesting a possible mechanism for reducing anxiety.40 A study by Nievergelt et al41 indicates that by binding to human serotonin receptors, ginger might influence GI function. Ginger extract contains a cholinergic and spasmogenic component, which provides a mechanistic insight for the prokinetic action of ginger.40
Turmeric
Turmeric (Curcuma longa) has been investigated for possible benefit in Alzheimer’s disease42; research into curcumin, the active substance of turmeric, is increasing. Although the original report was retracted after publication, curcumin was reported to selectively bind to human cannabinoid receptors type 1 (CB1) with nanomolar affinities and to function as an antagonist/inverse agonist.43 However, Gertsch et al44 found that curcumin did not interact functionally with the CB1 receptor, although this compound appears to share ability of the CB1 receptor inverse agonist.
Galangal
Major constituents identified in the galangal (or galanga) rhizome and leaf oil were 1,8-cineole, and β-pinene and camphor.6 Galangal, a member of the ginger (Zingiberaceae) family, interacts with MAO inhibitors, H2 receptor antagonists, and proton-pump inhibitors.1 Anxiolytic, hallucinogenic, and stimulant properties have been reported.1 An excessive amount can induce diarrhea, dizziness, nausea, and vomiting.1
Saffron
Stigma of saffron (a member of the family Iridaceae) was found to be significantly more effective than placebo and equally as efficacious as fluoxetine and imipramine in treating depression. Saffron petal was found to be significantly more effective than placebo and as effective as fluoxetine and saffron stigma in a recent systematic review.45-48
Asafetida
Asafetida (Ferula assa-foetida), when combined with valerian root, is used as a sedative to treat hyperactivity.2 The active ingredients of asafetida are the resin, endogenous gum, essential oil, propenyl-isobutylsulfide, umbelliferone, and vanillin. Several of the volatile constituents produce a sedative effect.2 Additive effects can occur between the hypotensive property of asafetida and dopamine receptor agonists such as bromocriptine mesylate. Use caution when combining asafetida in conjunction with a CNS depressant or a stimulant.2
Recommendations for treating spice-abusers
Patients may present to psychiatry services with psychological and physiological evidence of intoxication with culinary spices that may mimic 1) abuse of other substances, 2) primary psychiatric illness, and 3) primary medical illness. When you encounter a patient with a new psychiatric symptom, consider inquiring about the abuse of spices.
Patients might abuse more than 1 spice; a comprehensive screening approach might therefore be useful. Caution patients that ingesting these substance to excess can have harmful effects. Consider appropriate psychopharmacotherapy for underlying psychiatric symptoms to help patients who use spices maladaptively to self-medicate psychiatric symptoms.
Consider abuse of culinary spices in clinical presentations of psychiatric symptoms that do not seem adequate for a diagnosis of a primary anxiety, mood, or psychotic disorder, or in cases atypical psychiatric presentations that are—perhaps to your surprise—associated with negative toxicology studies for common, more familiar substances of abuse.
Physicians practicing in an environment where street drugs are difficult to obtain (eg, prisons) should consider monitoring for possible abuse of spices. Based on the available, albeit limited, literature, it appears that most culinary spice–associated intoxication can be managed:
• with an elevated level of clinical suspicion
• by ruling out other causes of intoxication
• using targeted, empirical psychopharmacotherapy to manage symptoms
• with supportive care that includes close psychiatric follow-up.
Consider comorbid abuse of other, more familiar substances of abuse in patients who misuse spices. As with inhalant abuse, the concept of “substance abuse” in clinical practice may need to be further expanded to include patients who abuse culinary spices. Patients could be screened for psychiatric illnesses known to increase the risk of substance abuse. These might include—but are not limited to:
• comorbid psychotic disorders
• mood disorders, particularly bipolar disorders
• trauma- and stressor-related disorders, particularly posttraumatic stress disorder
• personality disorders, particularly antisocial, borderline, and narcissistic personality disorders.
Pending the availability of population-based studies on abuse of culinary spices, the usual cautions regarding substance abuse seem to be appropriate when caring for these patients. Assessment for and management of comorbid psychiatric conditions is essential in the comprehensive psychiatric care of patients who abuse substances.
Last, general consideration of a 12-step recovery program appears warranted for these patients; the self-reflection and group support of such programs can be useful in helping patients control their use of these substances.
Bottom Line
Presentation of culinary spice intoxication can parallel that of other medical or psychiatric illnesses, or other drugs of abuse. Consideration and questioning for abuse of spices is necessary to ascertain the psychoactive effects of these substances when used surreptitiously. Management should follow substance abuse treatment protocols: inquiry into patterns of problematic use and readiness to change, assessment and management of psychiatric comorbidity, and referral to a recovery program.
Related Resources
• Srinivasan K. Role of spices beyond food flavoring: nutraceuticals with multiple health effects. Food Reviews International. 2005;21(2):167-188.
• Parthasarathi U, Hategan A, Bourgeois JA. Out of the cupboard and into the clinic: Nutmeg-induced mood disorder. Current Psychiatry. 2013;12(12):E1-E2.
Drug Brand Names
Bromocriptine mesylate • Parlodel Imipramine • Tofrani
Flunitrazepam • Rohypnol Iproniazid • Marsilid
Fluoxetine • Prozac Tranylcypromine • Parnate
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Many substances that are not typically thought of as “substances of abuse” possess—when adequately dosed—clinically meaningful psychoactive properties. In addition to the more familiar effects of alcohol, psychostimulants, opioids, Cannabis, and hallucinogens, you may encounter psychiatric phenomena resulting from abuse of more obscure substances, including culinary spices.
The clinician treating a patient in an apparent intoxicated state who has a negative drug screen might ask that patient if he (she) abuses spices. This might be particularly relevant when treating patients thought to have limited access to illicit substances or those with ready access to large amounts of spices, such as prisoners, young patients, and those working in the food service industry.
Abuse of spices can be a problematic diagnosis
Patients may misuse culinary spices to achieve euphoria, or a “natural high.” They may present with medical or psychiatric symptoms, including acute altered mental status, but the psychoactive substances are not identified on routine toxicology studies. In addition, patients may not attribute their use of spices for psychoactive effect to “drugs,” because these materials are legal and readily available. This may lead to misdiagnosis of a systemic medical disorder or a primary psychiatric illness to explain the patient’s symptoms and initiating a psychotropic agent and other psychiatric services when a substance abuse program might be a more appropriate clinical intervention.
Some spices contain psychoactive compounds that can alter CNS function (Table1-7), might be abused for recreational purposes, and can be toxic in an excessive amount. Internet resources, including anonymous web-based communications, and anecdotal materials about non-traditional recreational drugs, are available to anyone with Internet access.8 However, little research has been conducted into the prevalence of abuse (Box)9 and spices’ psychoactive properties. The lack of toxicology detection of spices in the medical setting presents a diagnostic challenge.
The psychoactive plants used in “natural high” products mainly are psychoactively inactive in their natural form, but extracts or alkaloids obtained from them might induce 1 or more of 3 classifications of psychoactivity:
• stimulant
• sedative
• hallucinogenic.
Many of these substances are considered to be aphrodisiac, and some may be abused to increase sexual function.
The following is a review of common spices that have been reported to possess potential psychoactive properties.
Nutmeg
Nutmeg (Myristica fragrans) is a common and easily accessible means of reaching euphoria in adults.10 The aromatic oil of nutmeg contains myristicin, a psychoactive substance that is chemically similar to hallucinogenic compounds such as mescaline. Its psychoactive effects could be attributed to metabolic formation of amphetamine derivatives from its core ingredients, elemicin, myristicin, and safrole.11,12
Nutmeg and its active component, myristicin, produce central monoamine oxidase (MAO) inhibition as evidenced by the ability to lower the convulsive dose of IV tryptamine in mice and to increase brain 5-hydroxytryptamine concentrations.13,14 Although myristicin’s potency is not comparable to that of the more potent MAO inhibitors such as tranylcypromine and iproniazid (which is not available in the United States), it seems adequate when compared with its low toxicity.14 Nutmeg extract is associated with a significant antidepressant effect in mice, which seemed to be mediated by interaction with the adrenergic, dopaminergic, and serotonergic systems.13 Nutmeg is associated with sustained increase in sexual activity in animal studies, with no evidence of adverse effects and toxicity, suggesting that nutmeg possesses clinically significant aphrodisiac activity.15
Psychoactive effects can be achieved by ingesting 5 to 15 g of nutmeg.11 Acute nutmeg intoxication produces palpitations, dizziness, anxiety, and hallucinations, mostly resolving within 24 hours, while effects of chronic abuse are reported to be similar to Cannabis use, including euphoria, giddiness, anxiety, fear, sense of impending doom, detachment, confabulation, and hallucinations.11,16 Urine drug screens are negative unless other psychoactive substances have been ingested.17
Suspected nutmeg intoxication or poisoning should be treated with supportive treatment. Use sedatives with caution because of alternating periods of delirium and obtundation during nutmeg intoxication.17
In case reports, myristicin poisoning induced CNS neuromodulatory signs that mimicked an anticholinergic hyperstimulation state.12,18 Fatal myristicin poisoning is rare; 2 cases have been reported, 1 in combination with flunitrazepam (not available in the United States).19,20 Nutmeg also has sedative properties and can cause GI symptoms when ingesting excessive amounts.1,20,21 Grover et al21 described no harmful effects on blood pressure and electrocardiogram; however, Shah et al22 reported palpitations and dry mouth.
Vanilla
Vanilla (species of the genus Vanilla) contains piperonal, also known as heliotropin.1 Piperonal has aromatherapeutic qualities that might elevate mood and well-being. In the early 1990s, the Memorial Sloan- Kettering Cancer Center in New York City described heliotropin as a powerful aromatherapy tool. Patients who were undergoing an MRI in an environment scented with heliotropin demonstrated a 63% reduction in anxiety compared with those who were not exposed to fragrance.23 The Smell and Taste Treatment and Research Foundation in Chicago found that vanilla can promote sexual arousal.24
Short-term effects of vanillin—a major component of vanilla—include a feeling of relaxation and reduced stress; long-term use can produce an antidepressant effect.1 There are no reports of vanilla abuse to achieve these effects; however, patients might abuse vanilla extract because of its alcohol content (up to 35% ethanol).25
Fennel
The essential oil of fennel (Foeniculum vulgare) can be neurotoxic and epileptogenic. Skalli and colleagues recently reported a case of seizure induction in a young woman after ingesting cakes containing fennel oil.26 Fennel oil also has been reported to have significant interaction with the fluoroquinolone-type antibiotics. Be aware of adverse effects associated with fennel ingestion; question patients if atypical seizures or reactions to antibiotics occur.27
Spices such as fennel, dill, cinnamon, saffron, and anise also contain psychoactive substances that are chemically similar to myristicin, which can induce sedation, stimulation, or hallucinations.7
Black pepper
Piperine, which gives black pepper (Piper nigrum) its spiciness, enhances thermogenesis of lipid metabolism, accelerates energy metabolism, and increases serotonin and endorphin production in the brain.28 Black pepper is reported to potentiate γ-aminobutyric acid A receptor subtypes,29 and could present possible applications for treating insomnia, epilepsy, and anxiety disorders.
Cloves
Non-culinary uses of clove (Syzygium aromaticum, a tree in the myrtle family) include flavored cigarettes. However, in 2009 clove cigarettes were banned in the United States as part of a public policy to reduce the number of children who start smoking.30 Eugenol, which constitutes as much as 90% of the essential oil extracted from cloves (and is responsible for the aroma), can cause hepatotoxicity31 and palpitations32; it can be toxic in quantities as low as 5 mL.33 Eugenol is present in other spices, such as nutmeg and cinnamon, and has been reported to have sedative properties.1
Mace
Mace is made from the covering of nutmeg (Myristica fragrans) seeds. It has a strong aroma resembling that of nutmeg. Whole mace contains 4% to 14% of a volatile oil similar to that found in nutmeg. Because mace contains the same oils that make nutmeg psychoactive1 in excessive amounts—although nutmeg seeds are more potent—be aware of the psychoactive potential of mace.
CinnamonCassia cinnamon (Cinnamomum aromaticum) is spicier and tarter than Ceylon cinnamon (Cinnamomum zeylanicum), which has a more flowery aroma. The 2 types of cinnamon can be distinguished by their different chemical composition. Ceylon cinnamon contains eugenol and benzyl benzoate; cassia cinnamon contains coumarin.3 Eugenol is reported to have sedative effects.1 Coumarin is a precursor molecule in the synthesis of a number of synthetic anticoagulant pharmaceuticals, including coumadin. Because of the toxic component of coumarin, European health agencies have warned against consuming high amounts of cassia.34 There are no reports of side effects arising from the occasional use of cinnamon as a spice.
In a study by Frydman-Marom et al,35 cinnamon extract (CEppt) was found to act on the CNS by inhibiting development of Alzheimer’s disease in animal models.
Asarone
Asarone is found in the Asarum family of spices that includes Acorus calamus. Asarone is chemically similar to mescaline. Although anecdotal reports indicate that A. calamus is a hallucinogen, research shows no evidence that it contains hallucinogenic substances.36 Han et al37 reported an antidepressant effect with the essential oil and asarones for the rhizomes of Acorus tatarinowii. In animal studies, asarone was found to reduce spontaneous motor activity, and even in low doses, reduced anxiety without decreasing acuity of perception.38
Ginger
Ginger (Zingiber officinale) is regarded as a sedative, general stimulant, and aphrodisiac.1,4,5 Its main constituents are phenolic compounds such as gingerols and shogaols, and sesquiterpenes such as zingiberene.4 Ginger is an inhibitor of thromboxane synthetase, a property shared by tricyclic antidepressants.39
Research indicates that 9 compounds found in ginger may interact with the serotonin 5-HT1A receptor, suggesting a possible mechanism for reducing anxiety.40 A study by Nievergelt et al41 indicates that by binding to human serotonin receptors, ginger might influence GI function. Ginger extract contains a cholinergic and spasmogenic component, which provides a mechanistic insight for the prokinetic action of ginger.40
Turmeric
Turmeric (Curcuma longa) has been investigated for possible benefit in Alzheimer’s disease42; research into curcumin, the active substance of turmeric, is increasing. Although the original report was retracted after publication, curcumin was reported to selectively bind to human cannabinoid receptors type 1 (CB1) with nanomolar affinities and to function as an antagonist/inverse agonist.43 However, Gertsch et al44 found that curcumin did not interact functionally with the CB1 receptor, although this compound appears to share ability of the CB1 receptor inverse agonist.
Galangal
Major constituents identified in the galangal (or galanga) rhizome and leaf oil were 1,8-cineole, and β-pinene and camphor.6 Galangal, a member of the ginger (Zingiberaceae) family, interacts with MAO inhibitors, H2 receptor antagonists, and proton-pump inhibitors.1 Anxiolytic, hallucinogenic, and stimulant properties have been reported.1 An excessive amount can induce diarrhea, dizziness, nausea, and vomiting.1
Saffron
Stigma of saffron (a member of the family Iridaceae) was found to be significantly more effective than placebo and equally as efficacious as fluoxetine and imipramine in treating depression. Saffron petal was found to be significantly more effective than placebo and as effective as fluoxetine and saffron stigma in a recent systematic review.45-48
Asafetida
Asafetida (Ferula assa-foetida), when combined with valerian root, is used as a sedative to treat hyperactivity.2 The active ingredients of asafetida are the resin, endogenous gum, essential oil, propenyl-isobutylsulfide, umbelliferone, and vanillin. Several of the volatile constituents produce a sedative effect.2 Additive effects can occur between the hypotensive property of asafetida and dopamine receptor agonists such as bromocriptine mesylate. Use caution when combining asafetida in conjunction with a CNS depressant or a stimulant.2
Recommendations for treating spice-abusers
Patients may present to psychiatry services with psychological and physiological evidence of intoxication with culinary spices that may mimic 1) abuse of other substances, 2) primary psychiatric illness, and 3) primary medical illness. When you encounter a patient with a new psychiatric symptom, consider inquiring about the abuse of spices.
Patients might abuse more than 1 spice; a comprehensive screening approach might therefore be useful. Caution patients that ingesting these substance to excess can have harmful effects. Consider appropriate psychopharmacotherapy for underlying psychiatric symptoms to help patients who use spices maladaptively to self-medicate psychiatric symptoms.
Consider abuse of culinary spices in clinical presentations of psychiatric symptoms that do not seem adequate for a diagnosis of a primary anxiety, mood, or psychotic disorder, or in cases atypical psychiatric presentations that are—perhaps to your surprise—associated with negative toxicology studies for common, more familiar substances of abuse.
Physicians practicing in an environment where street drugs are difficult to obtain (eg, prisons) should consider monitoring for possible abuse of spices. Based on the available, albeit limited, literature, it appears that most culinary spice–associated intoxication can be managed:
• with an elevated level of clinical suspicion
• by ruling out other causes of intoxication
• using targeted, empirical psychopharmacotherapy to manage symptoms
• with supportive care that includes close psychiatric follow-up.
Consider comorbid abuse of other, more familiar substances of abuse in patients who misuse spices. As with inhalant abuse, the concept of “substance abuse” in clinical practice may need to be further expanded to include patients who abuse culinary spices. Patients could be screened for psychiatric illnesses known to increase the risk of substance abuse. These might include—but are not limited to:
• comorbid psychotic disorders
• mood disorders, particularly bipolar disorders
• trauma- and stressor-related disorders, particularly posttraumatic stress disorder
• personality disorders, particularly antisocial, borderline, and narcissistic personality disorders.
Pending the availability of population-based studies on abuse of culinary spices, the usual cautions regarding substance abuse seem to be appropriate when caring for these patients. Assessment for and management of comorbid psychiatric conditions is essential in the comprehensive psychiatric care of patients who abuse substances.
Last, general consideration of a 12-step recovery program appears warranted for these patients; the self-reflection and group support of such programs can be useful in helping patients control their use of these substances.
Bottom Line
Presentation of culinary spice intoxication can parallel that of other medical or psychiatric illnesses, or other drugs of abuse. Consideration and questioning for abuse of spices is necessary to ascertain the psychoactive effects of these substances when used surreptitiously. Management should follow substance abuse treatment protocols: inquiry into patterns of problematic use and readiness to change, assessment and management of psychiatric comorbidity, and referral to a recovery program.
Related Resources
• Srinivasan K. Role of spices beyond food flavoring: nutraceuticals with multiple health effects. Food Reviews International. 2005;21(2):167-188.
• Parthasarathi U, Hategan A, Bourgeois JA. Out of the cupboard and into the clinic: Nutmeg-induced mood disorder. Current Psychiatry. 2013;12(12):E1-E2.
Drug Brand Names
Bromocriptine mesylate • Parlodel Imipramine • Tofrani
Flunitrazepam • Rohypnol Iproniazid • Marsilid
Fluoxetine • Prozac Tranylcypromine • Parnate
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. O’Mahony Carey S. Psychoactive substances. A guide to ethnobotanical plants and herbs, synthetic chemicals, compounds and products. http://www.drugs.ie/ resourcesfiles/guides/Psychoactive_substances_low_res. pdf. Accessed March 4, 2014.
2. Asafetida. Applied Health. http://www.appliedhealth.com/index.php?option=com _content&view=article&id= 108207. Accessed March 4, 2014.
3. Jayatilaka A, Poole SK, Poole CF, et al. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupled-column gas chromatography. Analytica Chimica Acta. 1995;302(2-3):147-162.
4. Spices. History & Special Collections UCLA Louise M. Darling Biomedical Library. http://unitproj.library.ucla. edu/biomed/spice/index.cfm?displayID=15. Accessed March 4, 2014.
5. Ginger action and uses. Ginger extract. Gingerols. MDidea Web site. http://www.mdidea.com/products/new/ new02108.html. Accessed March 4, 2014.
6. Raina VK, Srivastava SK, Syamasunder KV. The essential oil of ‘greater galangal’ [Alpinia galanga (L.) Willd.] from the lower Himalayan region of India. Flavour and Fragrance Journal. 2002;17(5):358-360.
7. Wenk G. Psychoactive spices - Bon appetite! http://www.psychologytoday.com/blog/your-brain-food/201008/ psychoactive-spices-bon-appetite. Published August 4, 2010. Accessed March 4, 2014.
8. Wax PM. Just a click away: recreational drug Web sites on the Internet. Pediatrics.2002;109(6):e96.
9. Forrester MB. Nutmeg intoxication in Texas, 1998-2004. Hum Exp Toxicol. 2005;24(11):563-566.
10. Abernethy MK, Becker LB. Acute nutmeg intoxication. Am J Emerg Med. 1992;10(5):429-430.
11. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
12. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
13. Dhingra D, Sharma A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9(1):84-89.
14. Truitt EB Jr, Duritz G, Ebersberger EM. Evidence of monoamine oxidase inhibition by myristicin and nutmeg. Proc Soc Exp Biol Med. 1963;112:647-650.
15. Tajuddin, Ahmad S, Latif A, et al. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med. 2005;5:16.
16. Quin GI, Fanning NF, Plunkett PK. Nutmeg intoxication. J Accid Emerg Med. 1998;15(4):287-288.
17. Barceloux DG. Nutmeg (Myristica fragrans Houtt.) Dis Mon. 2009;55(6):373-379.
18. Demetriades AK, Wallman PD, McGuiness A, et al. Low cost, high risk: accidental nutmeg intoxication. Emerg Med J. 2005;22(3):223-225.
19. Weil A. The use of nutmeg as a psychotropic agent. Bull Narc. 1966;18(4):15-23. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1966-01-01_4_ page003.html. Accessed March 5, 2013.
20. Stein U, Greyer H, Hentschel H. Nutmeg (myristicin) poisoning - report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci Int. 2001;118(1):87-90.
21. Grover JK, Khandkar S, Vats V, et al. Pharmacological studies on Myristica fragrans—antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24(10):675-680.
22. Shah AM, Calello DP, Quintero-Solivan J, et al. The not-so-nice spice: a teenage girl with palpitations and dry mouth. Pediatr Emerg Care. 2011;27(12):1205-1207.
23. Heliotropin. Polarized light microscopy digital image gallery. http://micro.magnet.fsu.edu/primer/techniques/ polarized/gallery/pages/heliotropinsmall.html. Accessed March 5, 2014.
24. Gage E. Romancing the bean. Budget Travel. http://articles.cnn.com/2007-09-11/travel/vanilla_1_vanilla-orchid-totonaca?_s=PM:TRAVEL. Published September 11, 2007. Updated September 16, 2012. Accessed March 5, 2014.
25. Mazor S, DesLauriers CA, Mycyk MB. Adolescent ethanol intoxication from vanilla extract ingestion: a case report. The Internet Journal of Family Practice. 2005;4(1). doi: 10.5580/bc.
26. Skalli S, Soulaymani Bencheikh R. Epileptic seizure induced by fennel essential oil. Epileptic Disord. 2011;13(3):345-347.
27. Zhu M, Wong PY, Li RC. Effect of oral administration of fennel (Foeniculum vulgare) on ciprofloxacin absorption and disposition in the rat. J Pharm Pharmacol. 1999;51(12):1391-1396.
28. Malini T, Arunakaran J, Aruldhas MM, et al. Effects of piperine on the lipid composition and enzymes of the pyruvate-malate cycle in the testis of the rat in vivo. Biochem Mol Biol Int. 1999;47(3):537-545.
29. Zaugg J, Baburin I, Hering S, et al. Identifying GABAA receptor ligands in black pepper by activity profiling, LC-TOFMS, and offline microprobe NMR. Planta Med. 2009; 75(9):888-889. doi: 10.1055/s-0029-1234276.
30. Flavored tobacco. FDA.gov. http://www.fda.gov/TobaccoProducts/ProtectingKidsfromTobacco/ FlavoredTobacco/default.htm. Published September 22, 2009. Updated March 21, 2013. Accessed March 18, 2014.
31. Fujisawa S, Atsumi T, Kadoma Y, et al. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177(1):39-54.
32. Eugenol oil overdose. New York Times Health Guide. http://health.nytimes.com/health/guides/poison/ eugenol-oil-overdose/overview.html. Accessed March 5, 2014.
33. Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child. 1993;69(3):392-393.
34. Harris E. NPR. German Christmas cookies pose health danger. http://www.npr.org/templates/story/story.php? storyId=6672644. Published December 25, 2006. Accessed March 5, 2014.
35. Frydman-Marom A, Levin A, Farfara D, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011; 6(1):e16564. doi:10.1371/journal.pone.001656453.
36. Björnstad K, Helander A, Hultén P, et al. Bioanalytical investigation of asarone in connection with Acorus calamus oil intoxications. J Anal Toxicol. 2009;33(9):604-609.
37. Han P, Han T, Peng W, et al. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm Biol. 2013;51(5):589-594.
38. Dandiya PC, Menon MK. Actions of asarone on behavior, stress, and hyperpyrexia, and its interaction with central stimulants. J Pharmacol Exp Ther. 1964;145:42-46.
39. Bockon J. Ginger: inhibition of thromboxane synthetase and stimulation of prostacyclin: relevance for medicine and psychiatry. Med Hypotheses. 1986;20(3):271-278.
40. Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50(10):1889-1897.
41. Nievergelt A, Huonker P, Schoop R, et al. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg Med Chem. 2010;18(9):3345-3351.
42. Mishra A, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13-19.
43. Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists [retracted in: J Pharmacol Exp Ther. 2009;331(3):1147]. J Pharmacol Exp Ther. 2009;330(1): 31-39.
44. Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant – do they exist? Br J Pharmacol. 2010;160(3):523-529.
45. Dwyer AV, Whitten DL, Hawrelak JA. Herbal medicines, other than St. John’s Wort, in the treatment of depression: a systematic review. Altern Med Rev. 2011;16(1):40-49.
46. Moshiri E, Basti AA, Noorbala AA, et al. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo controlled trial. Phytomedicine. 2006;13(9-10):607-611.
47. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-284.
48. Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized, and placebo-controlled trial. Phytother Res. 2005;19(2):148-151.
1. O’Mahony Carey S. Psychoactive substances. A guide to ethnobotanical plants and herbs, synthetic chemicals, compounds and products. http://www.drugs.ie/ resourcesfiles/guides/Psychoactive_substances_low_res. pdf. Accessed March 4, 2014.
2. Asafetida. Applied Health. http://www.appliedhealth.com/index.php?option=com _content&view=article&id= 108207. Accessed March 4, 2014.
3. Jayatilaka A, Poole SK, Poole CF, et al. Simultaneous micro steam distillation/solvent extraction for the isolation of semivolatile flavor compounds from cinnamon and their separation by series coupled-column gas chromatography. Analytica Chimica Acta. 1995;302(2-3):147-162.
4. Spices. History & Special Collections UCLA Louise M. Darling Biomedical Library. http://unitproj.library.ucla. edu/biomed/spice/index.cfm?displayID=15. Accessed March 4, 2014.
5. Ginger action and uses. Ginger extract. Gingerols. MDidea Web site. http://www.mdidea.com/products/new/ new02108.html. Accessed March 4, 2014.
6. Raina VK, Srivastava SK, Syamasunder KV. The essential oil of ‘greater galangal’ [Alpinia galanga (L.) Willd.] from the lower Himalayan region of India. Flavour and Fragrance Journal. 2002;17(5):358-360.
7. Wenk G. Psychoactive spices - Bon appetite! http://www.psychologytoday.com/blog/your-brain-food/201008/ psychoactive-spices-bon-appetite. Published August 4, 2010. Accessed March 4, 2014.
8. Wax PM. Just a click away: recreational drug Web sites on the Internet. Pediatrics.2002;109(6):e96.
9. Forrester MB. Nutmeg intoxication in Texas, 1998-2004. Hum Exp Toxicol. 2005;24(11):563-566.
10. Abernethy MK, Becker LB. Acute nutmeg intoxication. Am J Emerg Med. 1992;10(5):429-430.
11. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
12. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
13. Dhingra D, Sharma A. Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J Med Food. 2006;9(1):84-89.
14. Truitt EB Jr, Duritz G, Ebersberger EM. Evidence of monoamine oxidase inhibition by myristicin and nutmeg. Proc Soc Exp Biol Med. 1963;112:647-650.
15. Tajuddin, Ahmad S, Latif A, et al. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med. 2005;5:16.
16. Quin GI, Fanning NF, Plunkett PK. Nutmeg intoxication. J Accid Emerg Med. 1998;15(4):287-288.
17. Barceloux DG. Nutmeg (Myristica fragrans Houtt.) Dis Mon. 2009;55(6):373-379.
18. Demetriades AK, Wallman PD, McGuiness A, et al. Low cost, high risk: accidental nutmeg intoxication. Emerg Med J. 2005;22(3):223-225.
19. Weil A. The use of nutmeg as a psychotropic agent. Bull Narc. 1966;18(4):15-23. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1966-01-01_4_ page003.html. Accessed March 5, 2013.
20. Stein U, Greyer H, Hentschel H. Nutmeg (myristicin) poisoning - report on a fatal case and a series of cases recorded by a poison information centre. Forensic Sci Int. 2001;118(1):87-90.
21. Grover JK, Khandkar S, Vats V, et al. Pharmacological studies on Myristica fragrans—antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24(10):675-680.
22. Shah AM, Calello DP, Quintero-Solivan J, et al. The not-so-nice spice: a teenage girl with palpitations and dry mouth. Pediatr Emerg Care. 2011;27(12):1205-1207.
23. Heliotropin. Polarized light microscopy digital image gallery. http://micro.magnet.fsu.edu/primer/techniques/ polarized/gallery/pages/heliotropinsmall.html. Accessed March 5, 2014.
24. Gage E. Romancing the bean. Budget Travel. http://articles.cnn.com/2007-09-11/travel/vanilla_1_vanilla-orchid-totonaca?_s=PM:TRAVEL. Published September 11, 2007. Updated September 16, 2012. Accessed March 5, 2014.
25. Mazor S, DesLauriers CA, Mycyk MB. Adolescent ethanol intoxication from vanilla extract ingestion: a case report. The Internet Journal of Family Practice. 2005;4(1). doi: 10.5580/bc.
26. Skalli S, Soulaymani Bencheikh R. Epileptic seizure induced by fennel essential oil. Epileptic Disord. 2011;13(3):345-347.
27. Zhu M, Wong PY, Li RC. Effect of oral administration of fennel (Foeniculum vulgare) on ciprofloxacin absorption and disposition in the rat. J Pharm Pharmacol. 1999;51(12):1391-1396.
28. Malini T, Arunakaran J, Aruldhas MM, et al. Effects of piperine on the lipid composition and enzymes of the pyruvate-malate cycle in the testis of the rat in vivo. Biochem Mol Biol Int. 1999;47(3):537-545.
29. Zaugg J, Baburin I, Hering S, et al. Identifying GABAA receptor ligands in black pepper by activity profiling, LC-TOFMS, and offline microprobe NMR. Planta Med. 2009; 75(9):888-889. doi: 10.1055/s-0029-1234276.
30. Flavored tobacco. FDA.gov. http://www.fda.gov/TobaccoProducts/ProtectingKidsfromTobacco/ FlavoredTobacco/default.htm. Published September 22, 2009. Updated March 21, 2013. Accessed March 18, 2014.
31. Fujisawa S, Atsumi T, Kadoma Y, et al. Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology. 2002;177(1):39-54.
32. Eugenol oil overdose. New York Times Health Guide. http://health.nytimes.com/health/guides/poison/ eugenol-oil-overdose/overview.html. Accessed March 5, 2014.
33. Hartnoll G, Moore D, Douek D. Near fatal ingestion of oil of cloves. Arch Dis Child. 1993;69(3):392-393.
34. Harris E. NPR. German Christmas cookies pose health danger. http://www.npr.org/templates/story/story.php? storyId=6672644. Published December 25, 2006. Accessed March 5, 2014.
35. Frydman-Marom A, Levin A, Farfara D, et al. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011; 6(1):e16564. doi:10.1371/journal.pone.001656453.
36. Björnstad K, Helander A, Hultén P, et al. Bioanalytical investigation of asarone in connection with Acorus calamus oil intoxications. J Anal Toxicol. 2009;33(9):604-609.
37. Han P, Han T, Peng W, et al. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm Biol. 2013;51(5):589-594.
38. Dandiya PC, Menon MK. Actions of asarone on behavior, stress, and hyperpyrexia, and its interaction with central stimulants. J Pharmacol Exp Ther. 1964;145:42-46.
39. Bockon J. Ginger: inhibition of thromboxane synthetase and stimulation of prostacyclin: relevance for medicine and psychiatry. Med Hypotheses. 1986;20(3):271-278.
40. Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50(10):1889-1897.
41. Nievergelt A, Huonker P, Schoop R, et al. Identification of serotonin 5-HT1A receptor partial agonists in ginger. Bioorg Med Chem. 2010;18(9):3345-3351.
42. Mishra A, Palanivelu K. The effect of curcumin (turmeric) on Alzheimer’s disease: an overview. Ann Indian Acad Neurol. 2008;11(1):13-19.
43. Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists [retracted in: J Pharmacol Exp Ther. 2009;331(3):1147]. J Pharmacol Exp Ther. 2009;330(1): 31-39.
44. Gertsch J, Pertwee RG, Di Marzo V. Phytocannabinoids beyond the Cannabis plant – do they exist? Br J Pharmacol. 2010;160(3):523-529.
45. Dwyer AV, Whitten DL, Hawrelak JA. Herbal medicines, other than St. John’s Wort, in the treatment of depression: a systematic review. Altern Med Rev. 2011;16(1):40-49.
46. Moshiri E, Basti AA, Noorbala AA, et al. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo controlled trial. Phytomedicine. 2006;13(9-10):607-611.
47. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-284.
48. Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized, and placebo-controlled trial. Phytother Res. 2005;19(2):148-151.
Borderline personality disorder is a heritable brain disease
The prevailing view among many psychiatrists and mental health professionals is that borderline personality disorder (BPD) is a “psychological” condition. BPD often is conceptualized as a behavioral consequence of childhood trauma; treatment approaches have emphasized intensive psychotherapeutic modalities, less so biologic interventions. You might not be aware that a large body of research over the past decade provides strong evidence that BPD is a neurobiological illness—a finding that would drastically alter how the disorder should be conceptualized and managed.
Neuropathology underpins the personality disorder
Foremost, BPD must be regarded as a serious, disabling brain disorder, not simply an aberration of personality. In DSM-5, symptoms of BPD are listed as: feelings of abandonment; unstable and intense interpersonal relationships; unstable sense of self; impulsivity; suicidal or self-mutilating behavior; affective instability (dysphoria, irritability, anxiety); chronic feelings of emptiness; intense anger episodes; and transient paranoid or dissociative symptoms. Clearly, these clusters of psychopathological and behavioral symptoms reflect a pervasive brain disorder associated with abnormal neurobiology and neural circuitry that might, at times, stubbornly defy therapeutic intervention.
No wonder that 42 published studies report that, compared with healthy controls, people who have BPD display extensive cortical and subcortical abnormalities in brain structure and function.1 These anomalous patterns have been detected across all 4 available neuroimaging techniques.
Magnetic resonance imaging. MRI studies have revealed the following abnormalities in BPD:
• hypoplasia of the hippocampus, caudate, and dorsolateral prefrontal cortex
• variations in the CA1 region of the hippocampus and subiculum
• smaller-than-normal orbitofrontal cortex (by 24%, compared with healthy controls) and the mid-temporal and left cingulate gyrii (by 26%)
• larger-than-normal volume of the right inferior parietal cortex and the right parahippocampal gyrus
• loss of gray matter in the frontal, temporal, and parietal cortices
• an enlarged third cerebral ventricle
• in women, reduced size of the medial temporal lobe and amygdala
• in men, a decreased concentration of gray matter in the anterior cingulate
• reversal of normal right-greater-than-left asymmetry of the orbitofrontal cortex gray matter, reflecting loss of gray matter on the right side
• a lower concentration of gray matter in the rostral/subgenual anterior cingulate cortex
• a smaller frontal lobe.
In an analysis of MRI studies,2 correlation was found between structural brain abnormalities and specific symptoms of BPD, such as impulsivity, suicidality, and aggression. These findings might someday guide personalized interventions—for example, using neurostimulation techniques such as repetitive transcranial magnetic stimulation and deep brain stimulation—to modulate the activity of a given region of the brain (depending on which symptom is most prominent or disabling).
Magnetic resonance spectroscopy. In BPD, MRS studies reveal:
• compared with controls, a higher glutamate level in the anterior cingulate cortex
• reduced levels of N-acetyl aspartate (NAA; found in neurons) and creatinine in the left amygdala
• a reduction (on average, 19%) in the NAA concentration in the dorsolateral prefrontal cortex.
Functional magnetic resonance imaging. From fMRI studies, there is evidence in BPD of:
• greater activation of the amygdala and prolonged return to baseline
• increased functional connectivity in the left frontopolar cortex and left insula
• decreased connectivity in the left cuneus and left inferior parietal and the right middle temporal lobes
• marked frontal hypometabolism
• hypermetabolism in the motor cortex, medial and anterior cingulate, and occipital and temporal poles
• lower connectivity between the amygdala during a neutral stimulus
• higher connectivity between the amygdala during fear stimulus
• higher connectivity between the amygdala during fear stimulus
• deactivation of the opioid system in the left nucleus accumbens, hypothalamus, and hippocampus
• hyperactivation of the left medial prefrontal cortex during social exclusion
• more mistakes made in differentiating an emotional and a neutral facial expression.
Diffusion tensor imaging. DTI white-matter integrity studies of BPD show:
• a bilateral decrease in fractional anisotropy (FA) in frontal, uncinated, and occipitalfrontal fasciculi
• a decrease in FA in the genu and rostrum of the corpus callosum
• a decrease in inter-hemispheric connectivity between right and left anterior cigulate cortices.
Genetic Studies
There is substantial scientific evidence that BPD is highly heritable—a finding that suggests that brain abnormalities of this disorder are a consequence of genes involved in brain development (similar to what is known about schizophrenia, bipolar disorder, and autism).
A systematic review of the heritability of BPD examined 59 published studies that were categorized into 12 family studies, 18 twin studies, 24 association studies, and 5 gene-environment interaction studies.3 The authors concluded that BPD has a strong genetic component, although there also is evidence of gene-environment (G.E) interactions (ie, how nature and nurture influence each other).
The G.E interaction model appears to be consistent with the theory that expression of plasticity genes is modified by childhood experiences and environment, such as physical or sexual abuse. Some studies have found evidence of hypermethylation in BPD, which can exert epigenetic effects. Childhood abuse might, therefore, disrupt certain neuroplasticity genes, culminating in morphological, neurochemical, metabolic, and white-matter aberrations—leading to pathological behavioral patterns identified as BPD.
The neuropsychiatric basis of BPD must guide treatment
There is no such thing as a purely psychological disorder: Invariably, it is an abnormality of brain circuits that disrupts normal development of emotions, thought, behavior, and social cognition. BPD is an exemplar of such neuropsychiatric illness, and treatment should support psychotherapeutic approaches to mend the mind at the same time it moves aggressively to repair the brain.
1. McKenzie CE, Nasrallah HA. Neuroimaging abnormalities in borderline personality disorder: MRI, MRS, fMRI and DTI findings. Poster presented at: 52nd Annual Meeting of the American College of Neuropsychopharmacology; December 8-12, 2013; Hollywood, FL.
2. McKenzie CE, Nasrallah HA. Clinical symptoms of borderline personality disorder are associated with cortical and subcortical abnormalities on brain magnetic resonance imaging (MRI). Poster presented at: 26th Annual Meeting of the U.S. Psychiatric and Mental Health Congress; September 31-October 3, 2013; Las Vegas, NV.
3. Amad A, Ramoz N, Thomas P, et al. Genetics of borderline personality disorder: systematic review and proposal of an integrative model. Neurosci Biobehav Rev. 2014;40C:6-19.
The prevailing view among many psychiatrists and mental health professionals is that borderline personality disorder (BPD) is a “psychological” condition. BPD often is conceptualized as a behavioral consequence of childhood trauma; treatment approaches have emphasized intensive psychotherapeutic modalities, less so biologic interventions. You might not be aware that a large body of research over the past decade provides strong evidence that BPD is a neurobiological illness—a finding that would drastically alter how the disorder should be conceptualized and managed.
Neuropathology underpins the personality disorder
Foremost, BPD must be regarded as a serious, disabling brain disorder, not simply an aberration of personality. In DSM-5, symptoms of BPD are listed as: feelings of abandonment; unstable and intense interpersonal relationships; unstable sense of self; impulsivity; suicidal or self-mutilating behavior; affective instability (dysphoria, irritability, anxiety); chronic feelings of emptiness; intense anger episodes; and transient paranoid or dissociative symptoms. Clearly, these clusters of psychopathological and behavioral symptoms reflect a pervasive brain disorder associated with abnormal neurobiology and neural circuitry that might, at times, stubbornly defy therapeutic intervention.
No wonder that 42 published studies report that, compared with healthy controls, people who have BPD display extensive cortical and subcortical abnormalities in brain structure and function.1 These anomalous patterns have been detected across all 4 available neuroimaging techniques.
Magnetic resonance imaging. MRI studies have revealed the following abnormalities in BPD:
• hypoplasia of the hippocampus, caudate, and dorsolateral prefrontal cortex
• variations in the CA1 region of the hippocampus and subiculum
• smaller-than-normal orbitofrontal cortex (by 24%, compared with healthy controls) and the mid-temporal and left cingulate gyrii (by 26%)
• larger-than-normal volume of the right inferior parietal cortex and the right parahippocampal gyrus
• loss of gray matter in the frontal, temporal, and parietal cortices
• an enlarged third cerebral ventricle
• in women, reduced size of the medial temporal lobe and amygdala
• in men, a decreased concentration of gray matter in the anterior cingulate
• reversal of normal right-greater-than-left asymmetry of the orbitofrontal cortex gray matter, reflecting loss of gray matter on the right side
• a lower concentration of gray matter in the rostral/subgenual anterior cingulate cortex
• a smaller frontal lobe.
In an analysis of MRI studies,2 correlation was found between structural brain abnormalities and specific symptoms of BPD, such as impulsivity, suicidality, and aggression. These findings might someday guide personalized interventions—for example, using neurostimulation techniques such as repetitive transcranial magnetic stimulation and deep brain stimulation—to modulate the activity of a given region of the brain (depending on which symptom is most prominent or disabling).
Magnetic resonance spectroscopy. In BPD, MRS studies reveal:
• compared with controls, a higher glutamate level in the anterior cingulate cortex
• reduced levels of N-acetyl aspartate (NAA; found in neurons) and creatinine in the left amygdala
• a reduction (on average, 19%) in the NAA concentration in the dorsolateral prefrontal cortex.
Functional magnetic resonance imaging. From fMRI studies, there is evidence in BPD of:
• greater activation of the amygdala and prolonged return to baseline
• increased functional connectivity in the left frontopolar cortex and left insula
• decreased connectivity in the left cuneus and left inferior parietal and the right middle temporal lobes
• marked frontal hypometabolism
• hypermetabolism in the motor cortex, medial and anterior cingulate, and occipital and temporal poles
• lower connectivity between the amygdala during a neutral stimulus
• higher connectivity between the amygdala during fear stimulus
• higher connectivity between the amygdala during fear stimulus
• deactivation of the opioid system in the left nucleus accumbens, hypothalamus, and hippocampus
• hyperactivation of the left medial prefrontal cortex during social exclusion
• more mistakes made in differentiating an emotional and a neutral facial expression.
Diffusion tensor imaging. DTI white-matter integrity studies of BPD show:
• a bilateral decrease in fractional anisotropy (FA) in frontal, uncinated, and occipitalfrontal fasciculi
• a decrease in FA in the genu and rostrum of the corpus callosum
• a decrease in inter-hemispheric connectivity between right and left anterior cigulate cortices.
Genetic Studies
There is substantial scientific evidence that BPD is highly heritable—a finding that suggests that brain abnormalities of this disorder are a consequence of genes involved in brain development (similar to what is known about schizophrenia, bipolar disorder, and autism).
A systematic review of the heritability of BPD examined 59 published studies that were categorized into 12 family studies, 18 twin studies, 24 association studies, and 5 gene-environment interaction studies.3 The authors concluded that BPD has a strong genetic component, although there also is evidence of gene-environment (G.E) interactions (ie, how nature and nurture influence each other).
The G.E interaction model appears to be consistent with the theory that expression of plasticity genes is modified by childhood experiences and environment, such as physical or sexual abuse. Some studies have found evidence of hypermethylation in BPD, which can exert epigenetic effects. Childhood abuse might, therefore, disrupt certain neuroplasticity genes, culminating in morphological, neurochemical, metabolic, and white-matter aberrations—leading to pathological behavioral patterns identified as BPD.
The neuropsychiatric basis of BPD must guide treatment
There is no such thing as a purely psychological disorder: Invariably, it is an abnormality of brain circuits that disrupts normal development of emotions, thought, behavior, and social cognition. BPD is an exemplar of such neuropsychiatric illness, and treatment should support psychotherapeutic approaches to mend the mind at the same time it moves aggressively to repair the brain.
The prevailing view among many psychiatrists and mental health professionals is that borderline personality disorder (BPD) is a “psychological” condition. BPD often is conceptualized as a behavioral consequence of childhood trauma; treatment approaches have emphasized intensive psychotherapeutic modalities, less so biologic interventions. You might not be aware that a large body of research over the past decade provides strong evidence that BPD is a neurobiological illness—a finding that would drastically alter how the disorder should be conceptualized and managed.
Neuropathology underpins the personality disorder
Foremost, BPD must be regarded as a serious, disabling brain disorder, not simply an aberration of personality. In DSM-5, symptoms of BPD are listed as: feelings of abandonment; unstable and intense interpersonal relationships; unstable sense of self; impulsivity; suicidal or self-mutilating behavior; affective instability (dysphoria, irritability, anxiety); chronic feelings of emptiness; intense anger episodes; and transient paranoid or dissociative symptoms. Clearly, these clusters of psychopathological and behavioral symptoms reflect a pervasive brain disorder associated with abnormal neurobiology and neural circuitry that might, at times, stubbornly defy therapeutic intervention.
No wonder that 42 published studies report that, compared with healthy controls, people who have BPD display extensive cortical and subcortical abnormalities in brain structure and function.1 These anomalous patterns have been detected across all 4 available neuroimaging techniques.
Magnetic resonance imaging. MRI studies have revealed the following abnormalities in BPD:
• hypoplasia of the hippocampus, caudate, and dorsolateral prefrontal cortex
• variations in the CA1 region of the hippocampus and subiculum
• smaller-than-normal orbitofrontal cortex (by 24%, compared with healthy controls) and the mid-temporal and left cingulate gyrii (by 26%)
• larger-than-normal volume of the right inferior parietal cortex and the right parahippocampal gyrus
• loss of gray matter in the frontal, temporal, and parietal cortices
• an enlarged third cerebral ventricle
• in women, reduced size of the medial temporal lobe and amygdala
• in men, a decreased concentration of gray matter in the anterior cingulate
• reversal of normal right-greater-than-left asymmetry of the orbitofrontal cortex gray matter, reflecting loss of gray matter on the right side
• a lower concentration of gray matter in the rostral/subgenual anterior cingulate cortex
• a smaller frontal lobe.
In an analysis of MRI studies,2 correlation was found between structural brain abnormalities and specific symptoms of BPD, such as impulsivity, suicidality, and aggression. These findings might someday guide personalized interventions—for example, using neurostimulation techniques such as repetitive transcranial magnetic stimulation and deep brain stimulation—to modulate the activity of a given region of the brain (depending on which symptom is most prominent or disabling).
Magnetic resonance spectroscopy. In BPD, MRS studies reveal:
• compared with controls, a higher glutamate level in the anterior cingulate cortex
• reduced levels of N-acetyl aspartate (NAA; found in neurons) and creatinine in the left amygdala
• a reduction (on average, 19%) in the NAA concentration in the dorsolateral prefrontal cortex.
Functional magnetic resonance imaging. From fMRI studies, there is evidence in BPD of:
• greater activation of the amygdala and prolonged return to baseline
• increased functional connectivity in the left frontopolar cortex and left insula
• decreased connectivity in the left cuneus and left inferior parietal and the right middle temporal lobes
• marked frontal hypometabolism
• hypermetabolism in the motor cortex, medial and anterior cingulate, and occipital and temporal poles
• lower connectivity between the amygdala during a neutral stimulus
• higher connectivity between the amygdala during fear stimulus
• higher connectivity between the amygdala during fear stimulus
• deactivation of the opioid system in the left nucleus accumbens, hypothalamus, and hippocampus
• hyperactivation of the left medial prefrontal cortex during social exclusion
• more mistakes made in differentiating an emotional and a neutral facial expression.
Diffusion tensor imaging. DTI white-matter integrity studies of BPD show:
• a bilateral decrease in fractional anisotropy (FA) in frontal, uncinated, and occipitalfrontal fasciculi
• a decrease in FA in the genu and rostrum of the corpus callosum
• a decrease in inter-hemispheric connectivity between right and left anterior cigulate cortices.
Genetic Studies
There is substantial scientific evidence that BPD is highly heritable—a finding that suggests that brain abnormalities of this disorder are a consequence of genes involved in brain development (similar to what is known about schizophrenia, bipolar disorder, and autism).
A systematic review of the heritability of BPD examined 59 published studies that were categorized into 12 family studies, 18 twin studies, 24 association studies, and 5 gene-environment interaction studies.3 The authors concluded that BPD has a strong genetic component, although there also is evidence of gene-environment (G.E) interactions (ie, how nature and nurture influence each other).
The G.E interaction model appears to be consistent with the theory that expression of plasticity genes is modified by childhood experiences and environment, such as physical or sexual abuse. Some studies have found evidence of hypermethylation in BPD, which can exert epigenetic effects. Childhood abuse might, therefore, disrupt certain neuroplasticity genes, culminating in morphological, neurochemical, metabolic, and white-matter aberrations—leading to pathological behavioral patterns identified as BPD.
The neuropsychiatric basis of BPD must guide treatment
There is no such thing as a purely psychological disorder: Invariably, it is an abnormality of brain circuits that disrupts normal development of emotions, thought, behavior, and social cognition. BPD is an exemplar of such neuropsychiatric illness, and treatment should support psychotherapeutic approaches to mend the mind at the same time it moves aggressively to repair the brain.
1. McKenzie CE, Nasrallah HA. Neuroimaging abnormalities in borderline personality disorder: MRI, MRS, fMRI and DTI findings. Poster presented at: 52nd Annual Meeting of the American College of Neuropsychopharmacology; December 8-12, 2013; Hollywood, FL.
2. McKenzie CE, Nasrallah HA. Clinical symptoms of borderline personality disorder are associated with cortical and subcortical abnormalities on brain magnetic resonance imaging (MRI). Poster presented at: 26th Annual Meeting of the U.S. Psychiatric and Mental Health Congress; September 31-October 3, 2013; Las Vegas, NV.
3. Amad A, Ramoz N, Thomas P, et al. Genetics of borderline personality disorder: systematic review and proposal of an integrative model. Neurosci Biobehav Rev. 2014;40C:6-19.
1. McKenzie CE, Nasrallah HA. Neuroimaging abnormalities in borderline personality disorder: MRI, MRS, fMRI and DTI findings. Poster presented at: 52nd Annual Meeting of the American College of Neuropsychopharmacology; December 8-12, 2013; Hollywood, FL.
2. McKenzie CE, Nasrallah HA. Clinical symptoms of borderline personality disorder are associated with cortical and subcortical abnormalities on brain magnetic resonance imaging (MRI). Poster presented at: 26th Annual Meeting of the U.S. Psychiatric and Mental Health Congress; September 31-October 3, 2013; Las Vegas, NV.
3. Amad A, Ramoz N, Thomas P, et al. Genetics of borderline personality disorder: systematic review and proposal of an integrative model. Neurosci Biobehav Rev. 2014;40C:6-19.
Strategies to enhance patients’ acceptance of voluntary psychiatric admission
Voluntary psychiatric admission has become more problematic because of managed care authorization policies, restrictive inpatient entry criteria, uninsured
patients, and a decline in hospital beds.
In addition, patients often are ambivalent or resistant to hospitalization. It can be challenging to persuade a patient, as well as his (her) family, of the need for psychiatric admission, even when he acknowledges emotional suffering and impaired functioning.
The strategies offered here can enhance the probability that your patient, and his family, will agree to voluntary admission.
Provide a compelling rationale. Stress the need for immediate, specialized, and intensive services. If the patient is receiving outpatient mental health care, advise him that these services have been unsuccessful in achieving safety and clinical stability, and that it is not possible to quickly establish a modified outpatient plan or a day hospital placement that would meet his needs. For a patient who is not receiving outpatient care, explain that it is not feasible to implement a workable plan “from the ground up” in a timely manner.
Reset the clock. Redefine admission as a way to interrupt a downward spiral and offer a new start with a treatment team that has “fresh eyes.”
Use language of the medical model. Explain to the patient that a person who has a dangerously high, poorly controlled body temperature unquestionably needs to be hospitalized and that, by analogy, he—your patient—is running a “high emotional temperature” that warrants inpatient care.
Consider having the patient complete a brief, self-report rating scale, such as the Beck Depression Inventory-II or the Generalized Anxiety Disorder 7-item scale.1 Review findings with him and his family to show the frequency, duration, and severity of symptoms.
Dispel misconceptions and myths. These include catastrophic fears—often based on stereotypes—about coercive treatment and indefinite confinement. Clarifying what a patient can expect with voluntary admission with regard to probable length of stay, participation in the milieu, visitation, and discharge planning is helpful for allaying such fears.
Build bridges with significant others. Ally with parties who support voluntary admission, including the patient’s primary care or mental health provider, if appropriate. Getting family members and significant others on board; having them talk with the patient can go a long way toward reaching an agreement to proceed with hospitalization.
Maintain an empathic stance. For many patients, psychiatric admission evokes considerable distress. Remain sensitive to the situational concerns that typically arise, such as disruption to family and job responsibilities, insurance coverage, and whether there will be an outpatient plan in place at discharge.
A psychiatric admission often triggers long-standing psychological vulnerabilities— such as feelings of humiliation or failure, fear of separation and abandonment, worry about being a burden to family, stigma, and anxiety about having a serious mental illness—all of which might require exploration to allay upset and enhance compliance.
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned with this article or with manufacturers of competing products.
Reference
1. Blais MA. A guide to applying rating scales in clinical psychiatry. Psychiatr Times. 2011;28:58-62.
Voluntary psychiatric admission has become more problematic because of managed care authorization policies, restrictive inpatient entry criteria, uninsured
patients, and a decline in hospital beds.
In addition, patients often are ambivalent or resistant to hospitalization. It can be challenging to persuade a patient, as well as his (her) family, of the need for psychiatric admission, even when he acknowledges emotional suffering and impaired functioning.
The strategies offered here can enhance the probability that your patient, and his family, will agree to voluntary admission.
Provide a compelling rationale. Stress the need for immediate, specialized, and intensive services. If the patient is receiving outpatient mental health care, advise him that these services have been unsuccessful in achieving safety and clinical stability, and that it is not possible to quickly establish a modified outpatient plan or a day hospital placement that would meet his needs. For a patient who is not receiving outpatient care, explain that it is not feasible to implement a workable plan “from the ground up” in a timely manner.
Reset the clock. Redefine admission as a way to interrupt a downward spiral and offer a new start with a treatment team that has “fresh eyes.”
Use language of the medical model. Explain to the patient that a person who has a dangerously high, poorly controlled body temperature unquestionably needs to be hospitalized and that, by analogy, he—your patient—is running a “high emotional temperature” that warrants inpatient care.
Consider having the patient complete a brief, self-report rating scale, such as the Beck Depression Inventory-II or the Generalized Anxiety Disorder 7-item scale.1 Review findings with him and his family to show the frequency, duration, and severity of symptoms.
Dispel misconceptions and myths. These include catastrophic fears—often based on stereotypes—about coercive treatment and indefinite confinement. Clarifying what a patient can expect with voluntary admission with regard to probable length of stay, participation in the milieu, visitation, and discharge planning is helpful for allaying such fears.
Build bridges with significant others. Ally with parties who support voluntary admission, including the patient’s primary care or mental health provider, if appropriate. Getting family members and significant others on board; having them talk with the patient can go a long way toward reaching an agreement to proceed with hospitalization.
Maintain an empathic stance. For many patients, psychiatric admission evokes considerable distress. Remain sensitive to the situational concerns that typically arise, such as disruption to family and job responsibilities, insurance coverage, and whether there will be an outpatient plan in place at discharge.
A psychiatric admission often triggers long-standing psychological vulnerabilities— such as feelings of humiliation or failure, fear of separation and abandonment, worry about being a burden to family, stigma, and anxiety about having a serious mental illness—all of which might require exploration to allay upset and enhance compliance.
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned with this article or with manufacturers of competing products.
Voluntary psychiatric admission has become more problematic because of managed care authorization policies, restrictive inpatient entry criteria, uninsured
patients, and a decline in hospital beds.
In addition, patients often are ambivalent or resistant to hospitalization. It can be challenging to persuade a patient, as well as his (her) family, of the need for psychiatric admission, even when he acknowledges emotional suffering and impaired functioning.
The strategies offered here can enhance the probability that your patient, and his family, will agree to voluntary admission.
Provide a compelling rationale. Stress the need for immediate, specialized, and intensive services. If the patient is receiving outpatient mental health care, advise him that these services have been unsuccessful in achieving safety and clinical stability, and that it is not possible to quickly establish a modified outpatient plan or a day hospital placement that would meet his needs. For a patient who is not receiving outpatient care, explain that it is not feasible to implement a workable plan “from the ground up” in a timely manner.
Reset the clock. Redefine admission as a way to interrupt a downward spiral and offer a new start with a treatment team that has “fresh eyes.”
Use language of the medical model. Explain to the patient that a person who has a dangerously high, poorly controlled body temperature unquestionably needs to be hospitalized and that, by analogy, he—your patient—is running a “high emotional temperature” that warrants inpatient care.
Consider having the patient complete a brief, self-report rating scale, such as the Beck Depression Inventory-II or the Generalized Anxiety Disorder 7-item scale.1 Review findings with him and his family to show the frequency, duration, and severity of symptoms.
Dispel misconceptions and myths. These include catastrophic fears—often based on stereotypes—about coercive treatment and indefinite confinement. Clarifying what a patient can expect with voluntary admission with regard to probable length of stay, participation in the milieu, visitation, and discharge planning is helpful for allaying such fears.
Build bridges with significant others. Ally with parties who support voluntary admission, including the patient’s primary care or mental health provider, if appropriate. Getting family members and significant others on board; having them talk with the patient can go a long way toward reaching an agreement to proceed with hospitalization.
Maintain an empathic stance. For many patients, psychiatric admission evokes considerable distress. Remain sensitive to the situational concerns that typically arise, such as disruption to family and job responsibilities, insurance coverage, and whether there will be an outpatient plan in place at discharge.
A psychiatric admission often triggers long-standing psychological vulnerabilities— such as feelings of humiliation or failure, fear of separation and abandonment, worry about being a burden to family, stigma, and anxiety about having a serious mental illness—all of which might require exploration to allay upset and enhance compliance.
Disclosure
Dr. Pollak reports no financial relationships with any company whose products are mentioned with this article or with manufacturers of competing products.
Reference
1. Blais MA. A guide to applying rating scales in clinical psychiatry. Psychiatr Times. 2011;28:58-62.
Reference
1. Blais MA. A guide to applying rating scales in clinical psychiatry. Psychiatr Times. 2011;28:58-62.
The confused binge drinker
CASE Paranoid and confused
Mr. P, age 46, presents to the emergency department (ED) with a chief complaint of feeling “very weird.” Although he has seen a number of psychiatrists in the past, he does not recall being given a specific diagnosis. He describes his feelings as “1 minute I am fine and the next minute I am confused.” He endorses feeling paranoid for the past 6 to 12 months and reports a history of passive suicidal ideations. On the day he presents to the ED, however, he has a specific plan to shoot himself. He does not report audiovisual hallucinations, but has noticed that he talks to himself often.
Mr. P reports feeling worthless at times. He has a history of manic symptoms, including decreased need for sleep and hypersexuality. He describes verbal and sexual abuse by his foster parents. Mr. P reports using Cannabis and opioids occasionally and to drinking every “now and then” but not every day. He denies using benzodiazepines. When he is evaluated, he is not taking any medication and has no significant medical problems. Mr. P reports a history of several hospitalizations, but he could not describe the reasons or timing of past admissions.
Mr. P has a 10th-grade education. He lives with his fiancée, who reports that he has been behaving oddly for some time. She noticed that he has memory problems and describes violent behavior, such as shaking his fist at her, breaking the television, and attempting to cut his throat once when he was “intoxicated.” She says she does not feel safe around him because of his labile mood and history of
aggression. She confirms that Mr. P does not drink daily but binge-drinks at times.
Initial mental status examination of evaluation reveals hyperverbal, rapid speech. Mr. P is circumstantial and tangential in his thought process. He has poor judgment and insight and exhibits suicidal ideations with a plan. Toxicology screening reveals a blood alcohol level of 50 mg/dL and is positive for Cannabis and opiates.
Which condition most likely accounts for Mr. P’s presentation?
a) bipolar disorder, currently manic
b) substance-induced mood disorder
c) cognitive disorder
d) delirium
TREATMENT Rapid improvement
From the ED, Mr. P was admitted to an inpatient psychiatric unit, where he was found initially to be disoriented to time, place, and person. His thought process remained disorganized and irrational, with significant memory difficulties. He is noted to have an unsteady gait. Nursing staff observes that Mr. P has significant difficulties with activities of daily living and requires assistance. He talks in circles
and uses nonsensical words.
His serum vitamin B12 level, folate level, rapid plasma reagin, magnesium level, and thiamine level are within normal limits; CT scan of the brain is unremarkable. Neuropsychological testing reveals significant and diffuse cognitive deficits suggestive of frontal lobe dysfunction. He is deemed to not have decision-making capacity; because he has no family, his fiancée is appointed as his temporary health care proxy.
Thiamine and lorazepam are prescribed as needed because of Mr. P’s history of alcohol abuse. However, it’s determined that he does not need lorazepam because his vital signs are stable and there is no evidence of alcohol withdrawal symptoms.
During the course of his 10-day hospitalization, Mr. P’s cognitive difficulties resolved. He regains orientation to time, place, and person. He gains skill in all his activities of daily living, to the point of independence, and is discharged with minimal supervision. Vitamin B supplementation is prescribed, with close follow up in an outpatient day program. MRI/SPECT scan is considered to rule out frontotemporal dementia as recommended by the results of his neurocognitive testing profile.
Which condition likely account for Mr. P’s presentation during inpatient hospitalization?
a) Wernicke’s encephalopathy
b) Korsakoff’s syndrome
c) malingering
d) frontotemporal dementia
e) a neurodegenerative disease
The author's observations
Mr. P’s fluctuating mental status, gait instability, and confabulation create high suspicion for Wernicke’s encephalopathy; his dramatic improvement with IV thiamine supports that diagnosis. Mr. P attends the outpatient day program once after his discharge, and is then lost to follow-up.
During inpatient stay, Mr. P eventually admits to binge drinking several times a week, and drinking early in the morning, which would continue throughout the day. His significant cognitive deficits revealed by neuropsychological testing suggests consideration of a differential diagnosis of multifactorial cognitive dysfunction because of:
• long-term substance use
• Korsakoff’s syndrome
• frontotemporal dementia
• a neurodegenerative disease
• malingering (Table 1).
Wernicke’s encephalopathy
Wernicke’s encephalopathy is a life-threatening neurologic disorder caused by thiamine deficiency. The disease is rare, catastrophic in onset, and clinically complex1; as in Mr. P’s case, diagnosis often is delayed. In autopsy studies, the reported prevalence of Wernicke’s encephalopathy is 0.4% to 2.8%.1 Wernicke’s encephalopathy was suspected before death in 33% of alcohol-dependent patientsand 6% of nonalcoholics.1 Other causes of Wernicke’s encephalopathy include cancer, gastrointestinal surgery, hyperemesis gravidarum, a starvation or malnutrition state, GI tract disease, AIDS, parenteral nutrition, repetitive vomiting, and infection.1
Diagnosis. Making the correct diagnosis is challenging because the clinical presentation can be variable. No lab or imaging studies confirm the diagnosis. The triad of signs considered to support the diagnosis include ocular signs such as nystagmus, cerebellar signs, and confusion. These signs occur in only 8% to 10% of patients in whom the diagnosis likely.1,2
Attempts to increase the likelihood of making an accurate lifetime diagnosis of
Wernicke’s encephalopathy include expanding the focus to 8 clinical domains:
• dietary deficiency
• eye signs
• cerebellar signs
• seizures
• frontal lobe dysfunction
• amnesia
• mild memory impairment
• altered mental status.1
The sensitivity of making a correct diagnosis increases to 85% if at least 2 of 4 features—namely dietary deficiency, eye signs, cerebellar signs, memory impairment, and altered mental status—are present. These criteria can be applied to alcoholic and nonalcoholic patients.1Table 23 lists common and uncommon symptoms of Wernicke’s encephalopathy.
Although CT scan of the brain is not a reliable test for the disorder, MRI can be powerful tool that could support a diagnosis of acute Wernicke’s encephalopathy.1 We did not consider MRI in Mr. P’s case because the consulting neurologist thought this was unnecessary because of the quick improvement in his cognitive status with IV thiamine—although MRI might have helped to detect the disease earlier. In some studies, brain MRI revealed lesions in two-thirds of Wernicke’s encephalopathy patients.1 Typically, lesions are symmetrical and seen in the thalamus, mammillary body, and periaqueductal areas.1,4 Atypical lesions commonly are seen in the cerebellum, dentate nuclei, caudate nucleus, and cerebral cortex.1
Treatment. Evidence supports use of IV thiamine, 200 mg 3 times a day, when the disease is suspected or established.1,2 Thiamine has been associated with sporadic anaphylactic reactions, and should be administered when resuscitation facilities are available. Do not delay treatment because resuscitation measures are unavailable because you risk causing irreversible brain damage.1
In Mr. P’s case, prompt recognition of the need for thiamine likely led to a better outcome. Thiamine supplementation can prevent Wernicke’s encephalopathy in some patients. Prophylactic parenteral administration of thiamine before administration of glucose in the ED is recommended, as well as vitamin B supplementation with thiamine included upon discharge.1,2 Studies support several treatment regimens for patients with Wernicke’s encephalopathy and those at risk of it.1,3,5
Neither the optimal dosage of thiamine nor the appropriate duration of treatment have been determined by randomized, double-blind, controlled studies; empirical clinical practice and recommendations by Royal College of Physicians, London, suggest that a more prolonged course of thiamine—administered as long as improvement continues—might be beneficial.6
Left untreated, Wernicke’s encephalopathy can lead to irreversible brain damage.2
Mortality has been reported as 17% to 20%; 82% of patients develop Korsakoff’s syndrome, a chronic condition characterized by short-term memory loss. One-quarter of patients who develop Korsakoff’s syndrome require long-term residential care because of permanent brain damage.2
Making a diagnosis of Wernicke’s encephalopathy is a challenge because no specific symptom or diagnostic test can be relied upon to confirm the diagnosis. Also, patients might deny that they have an alcohol problem or give an inaccurate history of their alcohol use,2 as Mr. P did. The disorder is substantially underdiagnosed; as a consequence, patients are at risk of brain damage.2
Bottom Line
Not all patients who present with aggressive behavior, mania, and psychiatric
symptoms have a primary psychiatric diagnosis. It is important to consider
nutritional deficiencies caused by chronic alcohol abuse in patients presenting
with acute onset of confusion or altered mental status. Wernicke’s encephalopathy
might be the result of alcohol abuse and can be treated with IV thiamine.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Galvin R, Bråthen G, Ivashynka A, et al; EFNS. Guidelines for diagnosis, therapy and prevention of Wernicke’s encephalopathy. Eur J Neurol. 2010;17(12):
1408-1418.
2. Robinson K. Wernicke’s encephalopathy. Emerg Nurse. 2003;11(5):30-33.
3. Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442-455.
4. Celik Y, Kaya M. Brain SPECT findings in Wernicke’s encephalopathy. Neurol Sci. 2004;25(1):23-26.
5. Thomson AD, Guerrini I, Marshall JE. Wernicke’s encephalopathy: role of thiamine. Practical Gastroenterology. 2009;33(6):21-30.
6. Thomson AD, Cook CCH, Guerrini I, et al. Wernicke’s encephalopathy: ‘plus ca change, plus c’est la meme chose’. Alcohol Alcohol. 2008;43:180-186.
CASE Paranoid and confused
Mr. P, age 46, presents to the emergency department (ED) with a chief complaint of feeling “very weird.” Although he has seen a number of psychiatrists in the past, he does not recall being given a specific diagnosis. He describes his feelings as “1 minute I am fine and the next minute I am confused.” He endorses feeling paranoid for the past 6 to 12 months and reports a history of passive suicidal ideations. On the day he presents to the ED, however, he has a specific plan to shoot himself. He does not report audiovisual hallucinations, but has noticed that he talks to himself often.
Mr. P reports feeling worthless at times. He has a history of manic symptoms, including decreased need for sleep and hypersexuality. He describes verbal and sexual abuse by his foster parents. Mr. P reports using Cannabis and opioids occasionally and to drinking every “now and then” but not every day. He denies using benzodiazepines. When he is evaluated, he is not taking any medication and has no significant medical problems. Mr. P reports a history of several hospitalizations, but he could not describe the reasons or timing of past admissions.
Mr. P has a 10th-grade education. He lives with his fiancée, who reports that he has been behaving oddly for some time. She noticed that he has memory problems and describes violent behavior, such as shaking his fist at her, breaking the television, and attempting to cut his throat once when he was “intoxicated.” She says she does not feel safe around him because of his labile mood and history of
aggression. She confirms that Mr. P does not drink daily but binge-drinks at times.
Initial mental status examination of evaluation reveals hyperverbal, rapid speech. Mr. P is circumstantial and tangential in his thought process. He has poor judgment and insight and exhibits suicidal ideations with a plan. Toxicology screening reveals a blood alcohol level of 50 mg/dL and is positive for Cannabis and opiates.
Which condition most likely accounts for Mr. P’s presentation?
a) bipolar disorder, currently manic
b) substance-induced mood disorder
c) cognitive disorder
d) delirium
TREATMENT Rapid improvement
From the ED, Mr. P was admitted to an inpatient psychiatric unit, where he was found initially to be disoriented to time, place, and person. His thought process remained disorganized and irrational, with significant memory difficulties. He is noted to have an unsteady gait. Nursing staff observes that Mr. P has significant difficulties with activities of daily living and requires assistance. He talks in circles
and uses nonsensical words.
His serum vitamin B12 level, folate level, rapid plasma reagin, magnesium level, and thiamine level are within normal limits; CT scan of the brain is unremarkable. Neuropsychological testing reveals significant and diffuse cognitive deficits suggestive of frontal lobe dysfunction. He is deemed to not have decision-making capacity; because he has no family, his fiancée is appointed as his temporary health care proxy.
Thiamine and lorazepam are prescribed as needed because of Mr. P’s history of alcohol abuse. However, it’s determined that he does not need lorazepam because his vital signs are stable and there is no evidence of alcohol withdrawal symptoms.
During the course of his 10-day hospitalization, Mr. P’s cognitive difficulties resolved. He regains orientation to time, place, and person. He gains skill in all his activities of daily living, to the point of independence, and is discharged with minimal supervision. Vitamin B supplementation is prescribed, with close follow up in an outpatient day program. MRI/SPECT scan is considered to rule out frontotemporal dementia as recommended by the results of his neurocognitive testing profile.
Which condition likely account for Mr. P’s presentation during inpatient hospitalization?
a) Wernicke’s encephalopathy
b) Korsakoff’s syndrome
c) malingering
d) frontotemporal dementia
e) a neurodegenerative disease
The author's observations
Mr. P’s fluctuating mental status, gait instability, and confabulation create high suspicion for Wernicke’s encephalopathy; his dramatic improvement with IV thiamine supports that diagnosis. Mr. P attends the outpatient day program once after his discharge, and is then lost to follow-up.
During inpatient stay, Mr. P eventually admits to binge drinking several times a week, and drinking early in the morning, which would continue throughout the day. His significant cognitive deficits revealed by neuropsychological testing suggests consideration of a differential diagnosis of multifactorial cognitive dysfunction because of:
• long-term substance use
• Korsakoff’s syndrome
• frontotemporal dementia
• a neurodegenerative disease
• malingering (Table 1).
Wernicke’s encephalopathy
Wernicke’s encephalopathy is a life-threatening neurologic disorder caused by thiamine deficiency. The disease is rare, catastrophic in onset, and clinically complex1; as in Mr. P’s case, diagnosis often is delayed. In autopsy studies, the reported prevalence of Wernicke’s encephalopathy is 0.4% to 2.8%.1 Wernicke’s encephalopathy was suspected before death in 33% of alcohol-dependent patientsand 6% of nonalcoholics.1 Other causes of Wernicke’s encephalopathy include cancer, gastrointestinal surgery, hyperemesis gravidarum, a starvation or malnutrition state, GI tract disease, AIDS, parenteral nutrition, repetitive vomiting, and infection.1
Diagnosis. Making the correct diagnosis is challenging because the clinical presentation can be variable. No lab or imaging studies confirm the diagnosis. The triad of signs considered to support the diagnosis include ocular signs such as nystagmus, cerebellar signs, and confusion. These signs occur in only 8% to 10% of patients in whom the diagnosis likely.1,2
Attempts to increase the likelihood of making an accurate lifetime diagnosis of
Wernicke’s encephalopathy include expanding the focus to 8 clinical domains:
• dietary deficiency
• eye signs
• cerebellar signs
• seizures
• frontal lobe dysfunction
• amnesia
• mild memory impairment
• altered mental status.1
The sensitivity of making a correct diagnosis increases to 85% if at least 2 of 4 features—namely dietary deficiency, eye signs, cerebellar signs, memory impairment, and altered mental status—are present. These criteria can be applied to alcoholic and nonalcoholic patients.1Table 23 lists common and uncommon symptoms of Wernicke’s encephalopathy.
Although CT scan of the brain is not a reliable test for the disorder, MRI can be powerful tool that could support a diagnosis of acute Wernicke’s encephalopathy.1 We did not consider MRI in Mr. P’s case because the consulting neurologist thought this was unnecessary because of the quick improvement in his cognitive status with IV thiamine—although MRI might have helped to detect the disease earlier. In some studies, brain MRI revealed lesions in two-thirds of Wernicke’s encephalopathy patients.1 Typically, lesions are symmetrical and seen in the thalamus, mammillary body, and periaqueductal areas.1,4 Atypical lesions commonly are seen in the cerebellum, dentate nuclei, caudate nucleus, and cerebral cortex.1
Treatment. Evidence supports use of IV thiamine, 200 mg 3 times a day, when the disease is suspected or established.1,2 Thiamine has been associated with sporadic anaphylactic reactions, and should be administered when resuscitation facilities are available. Do not delay treatment because resuscitation measures are unavailable because you risk causing irreversible brain damage.1
In Mr. P’s case, prompt recognition of the need for thiamine likely led to a better outcome. Thiamine supplementation can prevent Wernicke’s encephalopathy in some patients. Prophylactic parenteral administration of thiamine before administration of glucose in the ED is recommended, as well as vitamin B supplementation with thiamine included upon discharge.1,2 Studies support several treatment regimens for patients with Wernicke’s encephalopathy and those at risk of it.1,3,5
Neither the optimal dosage of thiamine nor the appropriate duration of treatment have been determined by randomized, double-blind, controlled studies; empirical clinical practice and recommendations by Royal College of Physicians, London, suggest that a more prolonged course of thiamine—administered as long as improvement continues—might be beneficial.6
Left untreated, Wernicke’s encephalopathy can lead to irreversible brain damage.2
Mortality has been reported as 17% to 20%; 82% of patients develop Korsakoff’s syndrome, a chronic condition characterized by short-term memory loss. One-quarter of patients who develop Korsakoff’s syndrome require long-term residential care because of permanent brain damage.2
Making a diagnosis of Wernicke’s encephalopathy is a challenge because no specific symptom or diagnostic test can be relied upon to confirm the diagnosis. Also, patients might deny that they have an alcohol problem or give an inaccurate history of their alcohol use,2 as Mr. P did. The disorder is substantially underdiagnosed; as a consequence, patients are at risk of brain damage.2
Bottom Line
Not all patients who present with aggressive behavior, mania, and psychiatric
symptoms have a primary psychiatric diagnosis. It is important to consider
nutritional deficiencies caused by chronic alcohol abuse in patients presenting
with acute onset of confusion or altered mental status. Wernicke’s encephalopathy
might be the result of alcohol abuse and can be treated with IV thiamine.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Paranoid and confused
Mr. P, age 46, presents to the emergency department (ED) with a chief complaint of feeling “very weird.” Although he has seen a number of psychiatrists in the past, he does not recall being given a specific diagnosis. He describes his feelings as “1 minute I am fine and the next minute I am confused.” He endorses feeling paranoid for the past 6 to 12 months and reports a history of passive suicidal ideations. On the day he presents to the ED, however, he has a specific plan to shoot himself. He does not report audiovisual hallucinations, but has noticed that he talks to himself often.
Mr. P reports feeling worthless at times. He has a history of manic symptoms, including decreased need for sleep and hypersexuality. He describes verbal and sexual abuse by his foster parents. Mr. P reports using Cannabis and opioids occasionally and to drinking every “now and then” but not every day. He denies using benzodiazepines. When he is evaluated, he is not taking any medication and has no significant medical problems. Mr. P reports a history of several hospitalizations, but he could not describe the reasons or timing of past admissions.
Mr. P has a 10th-grade education. He lives with his fiancée, who reports that he has been behaving oddly for some time. She noticed that he has memory problems and describes violent behavior, such as shaking his fist at her, breaking the television, and attempting to cut his throat once when he was “intoxicated.” She says she does not feel safe around him because of his labile mood and history of
aggression. She confirms that Mr. P does not drink daily but binge-drinks at times.
Initial mental status examination of evaluation reveals hyperverbal, rapid speech. Mr. P is circumstantial and tangential in his thought process. He has poor judgment and insight and exhibits suicidal ideations with a plan. Toxicology screening reveals a blood alcohol level of 50 mg/dL and is positive for Cannabis and opiates.
Which condition most likely accounts for Mr. P’s presentation?
a) bipolar disorder, currently manic
b) substance-induced mood disorder
c) cognitive disorder
d) delirium
TREATMENT Rapid improvement
From the ED, Mr. P was admitted to an inpatient psychiatric unit, where he was found initially to be disoriented to time, place, and person. His thought process remained disorganized and irrational, with significant memory difficulties. He is noted to have an unsteady gait. Nursing staff observes that Mr. P has significant difficulties with activities of daily living and requires assistance. He talks in circles
and uses nonsensical words.
His serum vitamin B12 level, folate level, rapid plasma reagin, magnesium level, and thiamine level are within normal limits; CT scan of the brain is unremarkable. Neuropsychological testing reveals significant and diffuse cognitive deficits suggestive of frontal lobe dysfunction. He is deemed to not have decision-making capacity; because he has no family, his fiancée is appointed as his temporary health care proxy.
Thiamine and lorazepam are prescribed as needed because of Mr. P’s history of alcohol abuse. However, it’s determined that he does not need lorazepam because his vital signs are stable and there is no evidence of alcohol withdrawal symptoms.
During the course of his 10-day hospitalization, Mr. P’s cognitive difficulties resolved. He regains orientation to time, place, and person. He gains skill in all his activities of daily living, to the point of independence, and is discharged with minimal supervision. Vitamin B supplementation is prescribed, with close follow up in an outpatient day program. MRI/SPECT scan is considered to rule out frontotemporal dementia as recommended by the results of his neurocognitive testing profile.
Which condition likely account for Mr. P’s presentation during inpatient hospitalization?
a) Wernicke’s encephalopathy
b) Korsakoff’s syndrome
c) malingering
d) frontotemporal dementia
e) a neurodegenerative disease
The author's observations
Mr. P’s fluctuating mental status, gait instability, and confabulation create high suspicion for Wernicke’s encephalopathy; his dramatic improvement with IV thiamine supports that diagnosis. Mr. P attends the outpatient day program once after his discharge, and is then lost to follow-up.
During inpatient stay, Mr. P eventually admits to binge drinking several times a week, and drinking early in the morning, which would continue throughout the day. His significant cognitive deficits revealed by neuropsychological testing suggests consideration of a differential diagnosis of multifactorial cognitive dysfunction because of:
• long-term substance use
• Korsakoff’s syndrome
• frontotemporal dementia
• a neurodegenerative disease
• malingering (Table 1).
Wernicke’s encephalopathy
Wernicke’s encephalopathy is a life-threatening neurologic disorder caused by thiamine deficiency. The disease is rare, catastrophic in onset, and clinically complex1; as in Mr. P’s case, diagnosis often is delayed. In autopsy studies, the reported prevalence of Wernicke’s encephalopathy is 0.4% to 2.8%.1 Wernicke’s encephalopathy was suspected before death in 33% of alcohol-dependent patientsand 6% of nonalcoholics.1 Other causes of Wernicke’s encephalopathy include cancer, gastrointestinal surgery, hyperemesis gravidarum, a starvation or malnutrition state, GI tract disease, AIDS, parenteral nutrition, repetitive vomiting, and infection.1
Diagnosis. Making the correct diagnosis is challenging because the clinical presentation can be variable. No lab or imaging studies confirm the diagnosis. The triad of signs considered to support the diagnosis include ocular signs such as nystagmus, cerebellar signs, and confusion. These signs occur in only 8% to 10% of patients in whom the diagnosis likely.1,2
Attempts to increase the likelihood of making an accurate lifetime diagnosis of
Wernicke’s encephalopathy include expanding the focus to 8 clinical domains:
• dietary deficiency
• eye signs
• cerebellar signs
• seizures
• frontal lobe dysfunction
• amnesia
• mild memory impairment
• altered mental status.1
The sensitivity of making a correct diagnosis increases to 85% if at least 2 of 4 features—namely dietary deficiency, eye signs, cerebellar signs, memory impairment, and altered mental status—are present. These criteria can be applied to alcoholic and nonalcoholic patients.1Table 23 lists common and uncommon symptoms of Wernicke’s encephalopathy.
Although CT scan of the brain is not a reliable test for the disorder, MRI can be powerful tool that could support a diagnosis of acute Wernicke’s encephalopathy.1 We did not consider MRI in Mr. P’s case because the consulting neurologist thought this was unnecessary because of the quick improvement in his cognitive status with IV thiamine—although MRI might have helped to detect the disease earlier. In some studies, brain MRI revealed lesions in two-thirds of Wernicke’s encephalopathy patients.1 Typically, lesions are symmetrical and seen in the thalamus, mammillary body, and periaqueductal areas.1,4 Atypical lesions commonly are seen in the cerebellum, dentate nuclei, caudate nucleus, and cerebral cortex.1
Treatment. Evidence supports use of IV thiamine, 200 mg 3 times a day, when the disease is suspected or established.1,2 Thiamine has been associated with sporadic anaphylactic reactions, and should be administered when resuscitation facilities are available. Do not delay treatment because resuscitation measures are unavailable because you risk causing irreversible brain damage.1
In Mr. P’s case, prompt recognition of the need for thiamine likely led to a better outcome. Thiamine supplementation can prevent Wernicke’s encephalopathy in some patients. Prophylactic parenteral administration of thiamine before administration of glucose in the ED is recommended, as well as vitamin B supplementation with thiamine included upon discharge.1,2 Studies support several treatment regimens for patients with Wernicke’s encephalopathy and those at risk of it.1,3,5
Neither the optimal dosage of thiamine nor the appropriate duration of treatment have been determined by randomized, double-blind, controlled studies; empirical clinical practice and recommendations by Royal College of Physicians, London, suggest that a more prolonged course of thiamine—administered as long as improvement continues—might be beneficial.6
Left untreated, Wernicke’s encephalopathy can lead to irreversible brain damage.2
Mortality has been reported as 17% to 20%; 82% of patients develop Korsakoff’s syndrome, a chronic condition characterized by short-term memory loss. One-quarter of patients who develop Korsakoff’s syndrome require long-term residential care because of permanent brain damage.2
Making a diagnosis of Wernicke’s encephalopathy is a challenge because no specific symptom or diagnostic test can be relied upon to confirm the diagnosis. Also, patients might deny that they have an alcohol problem or give an inaccurate history of their alcohol use,2 as Mr. P did. The disorder is substantially underdiagnosed; as a consequence, patients are at risk of brain damage.2
Bottom Line
Not all patients who present with aggressive behavior, mania, and psychiatric
symptoms have a primary psychiatric diagnosis. It is important to consider
nutritional deficiencies caused by chronic alcohol abuse in patients presenting
with acute onset of confusion or altered mental status. Wernicke’s encephalopathy
might be the result of alcohol abuse and can be treated with IV thiamine.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Galvin R, Bråthen G, Ivashynka A, et al; EFNS. Guidelines for diagnosis, therapy and prevention of Wernicke’s encephalopathy. Eur J Neurol. 2010;17(12):
1408-1418.
2. Robinson K. Wernicke’s encephalopathy. Emerg Nurse. 2003;11(5):30-33.
3. Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442-455.
4. Celik Y, Kaya M. Brain SPECT findings in Wernicke’s encephalopathy. Neurol Sci. 2004;25(1):23-26.
5. Thomson AD, Guerrini I, Marshall JE. Wernicke’s encephalopathy: role of thiamine. Practical Gastroenterology. 2009;33(6):21-30.
6. Thomson AD, Cook CCH, Guerrini I, et al. Wernicke’s encephalopathy: ‘plus ca change, plus c’est la meme chose’. Alcohol Alcohol. 2008;43:180-186.
1. Galvin R, Bråthen G, Ivashynka A, et al; EFNS. Guidelines for diagnosis, therapy and prevention of Wernicke’s encephalopathy. Eur J Neurol. 2010;17(12):
1408-1418.
2. Robinson K. Wernicke’s encephalopathy. Emerg Nurse. 2003;11(5):30-33.
3. Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442-455.
4. Celik Y, Kaya M. Brain SPECT findings in Wernicke’s encephalopathy. Neurol Sci. 2004;25(1):23-26.
5. Thomson AD, Guerrini I, Marshall JE. Wernicke’s encephalopathy: role of thiamine. Practical Gastroenterology. 2009;33(6):21-30.
6. Thomson AD, Cook CCH, Guerrini I, et al. Wernicke’s encephalopathy: ‘plus ca change, plus c’est la meme chose’. Alcohol Alcohol. 2008;43:180-186.
Be aware: Sudden discontinuation of a psychotropic risks a lethal outcome
For mentally ill young men, especially, abruptly stopping a psychotropic medication can be lethal.1 Under such circumstances, excited delirium syndrome (EDS), also known as sudden in-custody death syndrome and Bell’s mania, can occur, warranting your careful observation.
Approximately 10% of EDS cases are fatal2; >95% of fatalities occur in men
(mean age, 36 years).3 Most cases involve stimulant abuse—usually cocaine, although cases associated with methamphetamine, phencyclidine, and LSD have been reported. Patients who present with EDS experience a characteristic loss of
the dopamine transporter in the striatum and excessive dopamine stimulation in
the striatum.
What should you watch for?
Other syndromes and disorders can mimic EDS (Table), but there are certain specific symptoms to look for. Patients who have EDS can present with delirium and an excited or agitated state. Other common symptoms include:
• altered sensorium
• aggressive, agitated behavior
• “superhuman” strength (including a tendency to break glass or unwillingness
to yield to overwhelming force)
• diaphoresis
• hyperthermia
• attraction to light.
Patients who have EDS often exhibit constant physical movement. They are likely to be naked or inadequately clothed; to sweat profusely; and to make unintelligible, animal-like noises. They are insensitive to extreme pain. In a small percentage of cases, EDS progresses to sudden cardiopulmonary arrestand death.3
Medication or restraints?
Many clinicians consider aggressive chemical sedation the first-line intervention for
EDS2,3; choice of medication varies from practice to practice. Restraints often are
necessary to ensure the safety of patient and staff, but use them only in conjunction with aggressive chemical sedation. Physical struggle is a greater contributor to catecholamine surge and metabolic acidosis than other types of exertion; methods of physical control should therefore minimize the time a patient spends struggling while safely achieving physical control.
What is the treatment for EDS?
Begin treatment while you are evaluating the patient for precipitating causes or additional pathology. There are cases of death from EDS even with minimal restraint (such as handcuffs),1,2 without the use of an electronic control device or so-called hog-tie restraint.
When providing pharmacotherapy for EDS, consider a benzodiazepine (midazolam, lorazepam, diazepam), an antipsychotic (haloperidol, droperidol, ziprasidone, olanzapine), or ketamine.4 Because these agents can have depressive respiratory and cardiovascular effects, continuously monitor heart and lungs.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Morrison A, Sadler D. Death of a psychiatric patient during physical restraint. Excited delirium—a case report. Med Sci Law. 2001;41(1):46-50.
2. Vilke GM, Debard ML, Chan TC, et al. Excited delirium syndrome (ExDS): defining based on a review of the literature. J Emerg Med. 2012;43(5):897-905.
4. Vilke GM, Payne-James J, Karch SB. Excited delirium syndrome (ExDS): redefining an old diagnosis. J Forensic Leg Med. 2012;19(1):7-11.
4. Hick JL, Ho JD. Ketamine chemical restraint to facilitate rescue of a combative “jumper.” Prehosp Emerg Care. 2005; 9(1):85-89.
For mentally ill young men, especially, abruptly stopping a psychotropic medication can be lethal.1 Under such circumstances, excited delirium syndrome (EDS), also known as sudden in-custody death syndrome and Bell’s mania, can occur, warranting your careful observation.
Approximately 10% of EDS cases are fatal2; >95% of fatalities occur in men
(mean age, 36 years).3 Most cases involve stimulant abuse—usually cocaine, although cases associated with methamphetamine, phencyclidine, and LSD have been reported. Patients who present with EDS experience a characteristic loss of
the dopamine transporter in the striatum and excessive dopamine stimulation in
the striatum.
What should you watch for?
Other syndromes and disorders can mimic EDS (Table), but there are certain specific symptoms to look for. Patients who have EDS can present with delirium and an excited or agitated state. Other common symptoms include:
• altered sensorium
• aggressive, agitated behavior
• “superhuman” strength (including a tendency to break glass or unwillingness
to yield to overwhelming force)
• diaphoresis
• hyperthermia
• attraction to light.
Patients who have EDS often exhibit constant physical movement. They are likely to be naked or inadequately clothed; to sweat profusely; and to make unintelligible, animal-like noises. They are insensitive to extreme pain. In a small percentage of cases, EDS progresses to sudden cardiopulmonary arrestand death.3
Medication or restraints?
Many clinicians consider aggressive chemical sedation the first-line intervention for
EDS2,3; choice of medication varies from practice to practice. Restraints often are
necessary to ensure the safety of patient and staff, but use them only in conjunction with aggressive chemical sedation. Physical struggle is a greater contributor to catecholamine surge and metabolic acidosis than other types of exertion; methods of physical control should therefore minimize the time a patient spends struggling while safely achieving physical control.
What is the treatment for EDS?
Begin treatment while you are evaluating the patient for precipitating causes or additional pathology. There are cases of death from EDS even with minimal restraint (such as handcuffs),1,2 without the use of an electronic control device or so-called hog-tie restraint.
When providing pharmacotherapy for EDS, consider a benzodiazepine (midazolam, lorazepam, diazepam), an antipsychotic (haloperidol, droperidol, ziprasidone, olanzapine), or ketamine.4 Because these agents can have depressive respiratory and cardiovascular effects, continuously monitor heart and lungs.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
For mentally ill young men, especially, abruptly stopping a psychotropic medication can be lethal.1 Under such circumstances, excited delirium syndrome (EDS), also known as sudden in-custody death syndrome and Bell’s mania, can occur, warranting your careful observation.
Approximately 10% of EDS cases are fatal2; >95% of fatalities occur in men
(mean age, 36 years).3 Most cases involve stimulant abuse—usually cocaine, although cases associated with methamphetamine, phencyclidine, and LSD have been reported. Patients who present with EDS experience a characteristic loss of
the dopamine transporter in the striatum and excessive dopamine stimulation in
the striatum.
What should you watch for?
Other syndromes and disorders can mimic EDS (Table), but there are certain specific symptoms to look for. Patients who have EDS can present with delirium and an excited or agitated state. Other common symptoms include:
• altered sensorium
• aggressive, agitated behavior
• “superhuman” strength (including a tendency to break glass or unwillingness
to yield to overwhelming force)
• diaphoresis
• hyperthermia
• attraction to light.
Patients who have EDS often exhibit constant physical movement. They are likely to be naked or inadequately clothed; to sweat profusely; and to make unintelligible, animal-like noises. They are insensitive to extreme pain. In a small percentage of cases, EDS progresses to sudden cardiopulmonary arrestand death.3
Medication or restraints?
Many clinicians consider aggressive chemical sedation the first-line intervention for
EDS2,3; choice of medication varies from practice to practice. Restraints often are
necessary to ensure the safety of patient and staff, but use them only in conjunction with aggressive chemical sedation. Physical struggle is a greater contributor to catecholamine surge and metabolic acidosis than other types of exertion; methods of physical control should therefore minimize the time a patient spends struggling while safely achieving physical control.
What is the treatment for EDS?
Begin treatment while you are evaluating the patient for precipitating causes or additional pathology. There are cases of death from EDS even with minimal restraint (such as handcuffs),1,2 without the use of an electronic control device or so-called hog-tie restraint.
When providing pharmacotherapy for EDS, consider a benzodiazepine (midazolam, lorazepam, diazepam), an antipsychotic (haloperidol, droperidol, ziprasidone, olanzapine), or ketamine.4 Because these agents can have depressive respiratory and cardiovascular effects, continuously monitor heart and lungs.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Morrison A, Sadler D. Death of a psychiatric patient during physical restraint. Excited delirium—a case report. Med Sci Law. 2001;41(1):46-50.
2. Vilke GM, Debard ML, Chan TC, et al. Excited delirium syndrome (ExDS): defining based on a review of the literature. J Emerg Med. 2012;43(5):897-905.
4. Vilke GM, Payne-James J, Karch SB. Excited delirium syndrome (ExDS): redefining an old diagnosis. J Forensic Leg Med. 2012;19(1):7-11.
4. Hick JL, Ho JD. Ketamine chemical restraint to facilitate rescue of a combative “jumper.” Prehosp Emerg Care. 2005; 9(1):85-89.
1. Morrison A, Sadler D. Death of a psychiatric patient during physical restraint. Excited delirium—a case report. Med Sci Law. 2001;41(1):46-50.
2. Vilke GM, Debard ML, Chan TC, et al. Excited delirium syndrome (ExDS): defining based on a review of the literature. J Emerg Med. 2012;43(5):897-905.
4. Vilke GM, Payne-James J, Karch SB. Excited delirium syndrome (ExDS): redefining an old diagnosis. J Forensic Leg Med. 2012;19(1):7-11.
4. Hick JL, Ho JD. Ketamine chemical restraint to facilitate rescue of a combative “jumper.” Prehosp Emerg Care. 2005; 9(1):85-89.
Strategies for managing drug-induced tardive dyskinesia
Fifteen years ago, Mr. L, age 40, was given a diagnosis of schizophrenia, which has been treated with haloperidol, 10 mg/d. Approximately 1 year ago, he began experiencing consistent lip smacking, a sign of tardive dyskinesia. Vitamin E was added to the treatment regimen, after which the tardive dyskinesia symptoms resolved.
A few months later, however, those symptoms returned and became worse. In addition to lip smacking, Mr. L now also describes involuntary bilateral twitching and muscle spasms in both legs.
Haloperidol and vitamin E are discontinued and Mr. L is switched to olanzapine, 20 mg/d. Although olanzapine is effective for Mr. L's symptoms of schizophrenia, tardive dyskinesia persists, and he gains 60 pounds and develops diabetes. Olanzapine is discontinued and he begins a trial of risperidone, 4 mg/d.
While on risperidone, blood sugar control, measured by hemoglobin A1c, and insulin resistance improve, but Mr. L continues to have symptoms of tardive dyskinesia. Vitamin E is added again, but is ineffective. The treatment team switches Mr. L to clozapine but symptoms of tardive dyskinesia do not improve.
Extrapyramidal side effects are common with first-generation antipsychotics (FGA) such as haloperidol. Types of antipsychotic-induced movement disorders include dystonias, akathisias, pseudoparkinsonism, and tardive dyskinesia. Of these, tardive dyskinesia is the most concerning because it often is difficult to treat and may be irreversible.
Tardive dyskinesia involves abnormal, involuntary movements, usually involving the face and, sometimes, the limbs. Common symptoms include lip smacking, tongue protrusions, and puffing the cheeks1; severe tardive dyskinesia may affect the larynx and diaphragm, which can be life-threatening. The incidence of tardive dyskinesia is approximately 5% after the first year of FGA treatment and 1% with second-generation antipsychotics (SGAs).2 The risk increases with higher doses and longer duration of treatment, with a prevalence of 20% to 25% with long-term FGA use.3
Treatment strategies
There are no FDA-approved drugs for tardive dyskinesia (Table).4-6 The best strategy is to prevent tardive dyskinesia with judicious use of an antipsychotic. If a patient taking a FGA develops tardive dyskinesia, the first-line treatment is to switch to a SGA. Risperidone, olanzapine, quetiapine, and clozapine have a low risk of tardive dyskinesia. Newer agents, such as lurasidone, asenapine, iloperidone, and aripiprazole, might have a lower risk of tardive dyskinesia, possibly because of differences in dopamine blockage between these agents and FGAs. Clozapine is least likely to cause tardive dyskinesia, but it often is used as a last resort because of the risk of agranulocytosis and the need for frequent tests to measurewhite blood cells.1,4
Other treatments include melatonin, donepezil, vitamin B6, and vitamin E.4 These agents can reduce symptoms, but no large clinical trials have proved that the are efficacious. Last-resort treatments include suppressive treatment using FGAs several times a day, because the constant dopamine blockade may stop symptoms for a short time; this approach is not recommended because it can exacerbate symptoms of tardive dyskinesia.4
Other suppressive treatments used in severe or refractory cases include reserpineand tetrabenazine, which are used off-label and work by blocking monoamine transporters. This blockage results in a reduction in neurotransmitters such as dopamine, which have been implicated in the development of tardive dyskinesia. Compared with tetrabenazine, reserpine has a higher affinity for cells in the periphery and therefore causes side effects such as hypotension and diarrhea.7
Tetrabenazine is indicated for chorea associated with Huntington's disease and is used off-label for treating tardive dyskinesia. Tetrabenazine is thought to work by inhibiting human vesicular monoamine transporters. Blocking these transporters prevents monoamines such as dopamine from entering synaptic vesicles.8 Because of its side-effect profile, lack of large clinical trials, and high cost, tetrabenazine is used as a last-line treatment in severe cases of tardive dyskinesia. Adverse effects include somnolence (31%), insomnia (22%), depression (19%), and akathisia (19%). Tetrabenazine carries a black-box warning for depression and suicidalityand is contraindicated in patients with untreated or inadequately treated depression or who are suicidal.8 Assessing the patient's mental state is important when using this medication.
A review by Chen et al7 found that 9 of 11 studies had positive results for tetrabenazine treatment for tardive dyskinesia. Most of the studies were small and retrospective. The biggest prospective blinded study was a videotaped study by Ondo et al of 20 patients with tardive dyskinesia.9 At least 30 days before beginning the study patients discontinued the medication that caused their tardive dyskinesia and any treatments for tardive dyskinesia. Each patient was videotaped before starting tetrabenazine and an average of 20.3 weeks after starting the drug. Investigators' scores showed an average of 54.2% improvement in movement scores and participants' subjective scores reported an average of 60.4% improvement. One patient withdrew because of somnolence. The remaining 19 patients did not experience more than mild side effects and continued treatment with tetrabenazine after study completion.9
Treatment recommendations
Tardive dyskinesia is a difficult condition to treat; it is best, therefore, to prevent its onset by using the minimally effective antipsychotic dose, by preferential use of an SGA rather than a FGA, and by regular screening for tardive dyskinesia using a standardized rating scale such as the Abnormal Involuntary Movement Scale. Symptoms associated with tardive dyskinesia are more likely to resolve if caught early. If a patient develops tardive dyskinesia while taking a FGA, switching to a SGA may alleviate the symptoms.
Several medications can be used off-label to relieve symptoms, including vitamin E and tetrabenazine, which both have the most-although not considerable-literature-based support. Although some studies show benefit with tetrabenazine for tardive dyskinesia, larger clinical trials are needed to more strongly support its use. Tetrabenazine might be a good option for patients with refractory tardive dyskinesia but, given the associated risk of suicidality and depressive symptoms, careful monitoring of suicide risk is essential and may preclude its use for tardive dyskinesia in patients who are experiencing depressive symptoms.
Related Resources
• National Organization for Rare Disorders. Tardive dyskinesia. www.rarediseases.org/rare-disease-information/rare-diseases/byID/493/viewFullReport.
• Caroff SN, Miller DD, Campbell CE. Is there a rational management strategy for tardive dyskinesia? Current Psychiatry. 2011;10(10):22-32.
Drug Brand Names
Aripiprazole • Abilify Lurasidone • Latuda
Asenapine • Saphris Olanzapine • Zyprexa
Clozapine • Clozaril Quetiapine • Seroquel
Donepezil • Aricept Reserpine • Serpasil
Haloperidol • Haldol Risperidone • Risperdal
Iloperidone • Fanapt Tetrabenazine • Xenazine
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Aia PG, Revuelta GJ, Cloud LJ, et al. Tardive dyskinesia. Curr Treat Options Neurol. 2011;13(3):231-241.
2. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
3. Crimson ML, Argo TR, Buckley PF. Schizophrenia. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: a pathophysiologic approach. 8th ed. New York, NY: McGraw- Hill; 2011:1147-1172.
4. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 2: incidence and management strategies in patients with schizophrenia. Can J Psychiatry. 2005;50(11):703-714.
5. Facts & Comparisons. http://online.factsandcomparisons.com/index.aspx. Accessed November 4, 2012.
6. Natural Standard. http://www.naturalstandard.com/index.asp. Accessed November 4, 2012.
7. Chen JJ, Ondo WG, Dashtipour K, et al. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin Ther. 2012;34(7):1487-1504.
8. Xanazine [package insert]. Deerfield, IL: Lundbeck Inc; 2012.
9. Ondo WG, Hanna PA, Jankovic J. Tetrabenazine treatment for tardive dyskinesia: assessment by randomized videotape protocol. Am J Psychiatry. 1999;156(8):1279-1281.
Fifteen years ago, Mr. L, age 40, was given a diagnosis of schizophrenia, which has been treated with haloperidol, 10 mg/d. Approximately 1 year ago, he began experiencing consistent lip smacking, a sign of tardive dyskinesia. Vitamin E was added to the treatment regimen, after which the tardive dyskinesia symptoms resolved.
A few months later, however, those symptoms returned and became worse. In addition to lip smacking, Mr. L now also describes involuntary bilateral twitching and muscle spasms in both legs.
Haloperidol and vitamin E are discontinued and Mr. L is switched to olanzapine, 20 mg/d. Although olanzapine is effective for Mr. L's symptoms of schizophrenia, tardive dyskinesia persists, and he gains 60 pounds and develops diabetes. Olanzapine is discontinued and he begins a trial of risperidone, 4 mg/d.
While on risperidone, blood sugar control, measured by hemoglobin A1c, and insulin resistance improve, but Mr. L continues to have symptoms of tardive dyskinesia. Vitamin E is added again, but is ineffective. The treatment team switches Mr. L to clozapine but symptoms of tardive dyskinesia do not improve.
Extrapyramidal side effects are common with first-generation antipsychotics (FGA) such as haloperidol. Types of antipsychotic-induced movement disorders include dystonias, akathisias, pseudoparkinsonism, and tardive dyskinesia. Of these, tardive dyskinesia is the most concerning because it often is difficult to treat and may be irreversible.
Tardive dyskinesia involves abnormal, involuntary movements, usually involving the face and, sometimes, the limbs. Common symptoms include lip smacking, tongue protrusions, and puffing the cheeks1; severe tardive dyskinesia may affect the larynx and diaphragm, which can be life-threatening. The incidence of tardive dyskinesia is approximately 5% after the first year of FGA treatment and 1% with second-generation antipsychotics (SGAs).2 The risk increases with higher doses and longer duration of treatment, with a prevalence of 20% to 25% with long-term FGA use.3
Treatment strategies
There are no FDA-approved drugs for tardive dyskinesia (Table).4-6 The best strategy is to prevent tardive dyskinesia with judicious use of an antipsychotic. If a patient taking a FGA develops tardive dyskinesia, the first-line treatment is to switch to a SGA. Risperidone, olanzapine, quetiapine, and clozapine have a low risk of tardive dyskinesia. Newer agents, such as lurasidone, asenapine, iloperidone, and aripiprazole, might have a lower risk of tardive dyskinesia, possibly because of differences in dopamine blockage between these agents and FGAs. Clozapine is least likely to cause tardive dyskinesia, but it often is used as a last resort because of the risk of agranulocytosis and the need for frequent tests to measurewhite blood cells.1,4
Other treatments include melatonin, donepezil, vitamin B6, and vitamin E.4 These agents can reduce symptoms, but no large clinical trials have proved that the are efficacious. Last-resort treatments include suppressive treatment using FGAs several times a day, because the constant dopamine blockade may stop symptoms for a short time; this approach is not recommended because it can exacerbate symptoms of tardive dyskinesia.4
Other suppressive treatments used in severe or refractory cases include reserpineand tetrabenazine, which are used off-label and work by blocking monoamine transporters. This blockage results in a reduction in neurotransmitters such as dopamine, which have been implicated in the development of tardive dyskinesia. Compared with tetrabenazine, reserpine has a higher affinity for cells in the periphery and therefore causes side effects such as hypotension and diarrhea.7
Tetrabenazine is indicated for chorea associated with Huntington's disease and is used off-label for treating tardive dyskinesia. Tetrabenazine is thought to work by inhibiting human vesicular monoamine transporters. Blocking these transporters prevents monoamines such as dopamine from entering synaptic vesicles.8 Because of its side-effect profile, lack of large clinical trials, and high cost, tetrabenazine is used as a last-line treatment in severe cases of tardive dyskinesia. Adverse effects include somnolence (31%), insomnia (22%), depression (19%), and akathisia (19%). Tetrabenazine carries a black-box warning for depression and suicidalityand is contraindicated in patients with untreated or inadequately treated depression or who are suicidal.8 Assessing the patient's mental state is important when using this medication.
A review by Chen et al7 found that 9 of 11 studies had positive results for tetrabenazine treatment for tardive dyskinesia. Most of the studies were small and retrospective. The biggest prospective blinded study was a videotaped study by Ondo et al of 20 patients with tardive dyskinesia.9 At least 30 days before beginning the study patients discontinued the medication that caused their tardive dyskinesia and any treatments for tardive dyskinesia. Each patient was videotaped before starting tetrabenazine and an average of 20.3 weeks after starting the drug. Investigators' scores showed an average of 54.2% improvement in movement scores and participants' subjective scores reported an average of 60.4% improvement. One patient withdrew because of somnolence. The remaining 19 patients did not experience more than mild side effects and continued treatment with tetrabenazine after study completion.9
Treatment recommendations
Tardive dyskinesia is a difficult condition to treat; it is best, therefore, to prevent its onset by using the minimally effective antipsychotic dose, by preferential use of an SGA rather than a FGA, and by regular screening for tardive dyskinesia using a standardized rating scale such as the Abnormal Involuntary Movement Scale. Symptoms associated with tardive dyskinesia are more likely to resolve if caught early. If a patient develops tardive dyskinesia while taking a FGA, switching to a SGA may alleviate the symptoms.
Several medications can be used off-label to relieve symptoms, including vitamin E and tetrabenazine, which both have the most-although not considerable-literature-based support. Although some studies show benefit with tetrabenazine for tardive dyskinesia, larger clinical trials are needed to more strongly support its use. Tetrabenazine might be a good option for patients with refractory tardive dyskinesia but, given the associated risk of suicidality and depressive symptoms, careful monitoring of suicide risk is essential and may preclude its use for tardive dyskinesia in patients who are experiencing depressive symptoms.
Related Resources
• National Organization for Rare Disorders. Tardive dyskinesia. www.rarediseases.org/rare-disease-information/rare-diseases/byID/493/viewFullReport.
• Caroff SN, Miller DD, Campbell CE. Is there a rational management strategy for tardive dyskinesia? Current Psychiatry. 2011;10(10):22-32.
Drug Brand Names
Aripiprazole • Abilify Lurasidone • Latuda
Asenapine • Saphris Olanzapine • Zyprexa
Clozapine • Clozaril Quetiapine • Seroquel
Donepezil • Aricept Reserpine • Serpasil
Haloperidol • Haldol Risperidone • Risperdal
Iloperidone • Fanapt Tetrabenazine • Xenazine
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Fifteen years ago, Mr. L, age 40, was given a diagnosis of schizophrenia, which has been treated with haloperidol, 10 mg/d. Approximately 1 year ago, he began experiencing consistent lip smacking, a sign of tardive dyskinesia. Vitamin E was added to the treatment regimen, after which the tardive dyskinesia symptoms resolved.
A few months later, however, those symptoms returned and became worse. In addition to lip smacking, Mr. L now also describes involuntary bilateral twitching and muscle spasms in both legs.
Haloperidol and vitamin E are discontinued and Mr. L is switched to olanzapine, 20 mg/d. Although olanzapine is effective for Mr. L's symptoms of schizophrenia, tardive dyskinesia persists, and he gains 60 pounds and develops diabetes. Olanzapine is discontinued and he begins a trial of risperidone, 4 mg/d.
While on risperidone, blood sugar control, measured by hemoglobin A1c, and insulin resistance improve, but Mr. L continues to have symptoms of tardive dyskinesia. Vitamin E is added again, but is ineffective. The treatment team switches Mr. L to clozapine but symptoms of tardive dyskinesia do not improve.
Extrapyramidal side effects are common with first-generation antipsychotics (FGA) such as haloperidol. Types of antipsychotic-induced movement disorders include dystonias, akathisias, pseudoparkinsonism, and tardive dyskinesia. Of these, tardive dyskinesia is the most concerning because it often is difficult to treat and may be irreversible.
Tardive dyskinesia involves abnormal, involuntary movements, usually involving the face and, sometimes, the limbs. Common symptoms include lip smacking, tongue protrusions, and puffing the cheeks1; severe tardive dyskinesia may affect the larynx and diaphragm, which can be life-threatening. The incidence of tardive dyskinesia is approximately 5% after the first year of FGA treatment and 1% with second-generation antipsychotics (SGAs).2 The risk increases with higher doses and longer duration of treatment, with a prevalence of 20% to 25% with long-term FGA use.3
Treatment strategies
There are no FDA-approved drugs for tardive dyskinesia (Table).4-6 The best strategy is to prevent tardive dyskinesia with judicious use of an antipsychotic. If a patient taking a FGA develops tardive dyskinesia, the first-line treatment is to switch to a SGA. Risperidone, olanzapine, quetiapine, and clozapine have a low risk of tardive dyskinesia. Newer agents, such as lurasidone, asenapine, iloperidone, and aripiprazole, might have a lower risk of tardive dyskinesia, possibly because of differences in dopamine blockage between these agents and FGAs. Clozapine is least likely to cause tardive dyskinesia, but it often is used as a last resort because of the risk of agranulocytosis and the need for frequent tests to measurewhite blood cells.1,4
Other treatments include melatonin, donepezil, vitamin B6, and vitamin E.4 These agents can reduce symptoms, but no large clinical trials have proved that the are efficacious. Last-resort treatments include suppressive treatment using FGAs several times a day, because the constant dopamine blockade may stop symptoms for a short time; this approach is not recommended because it can exacerbate symptoms of tardive dyskinesia.4
Other suppressive treatments used in severe or refractory cases include reserpineand tetrabenazine, which are used off-label and work by blocking monoamine transporters. This blockage results in a reduction in neurotransmitters such as dopamine, which have been implicated in the development of tardive dyskinesia. Compared with tetrabenazine, reserpine has a higher affinity for cells in the periphery and therefore causes side effects such as hypotension and diarrhea.7
Tetrabenazine is indicated for chorea associated with Huntington's disease and is used off-label for treating tardive dyskinesia. Tetrabenazine is thought to work by inhibiting human vesicular monoamine transporters. Blocking these transporters prevents monoamines such as dopamine from entering synaptic vesicles.8 Because of its side-effect profile, lack of large clinical trials, and high cost, tetrabenazine is used as a last-line treatment in severe cases of tardive dyskinesia. Adverse effects include somnolence (31%), insomnia (22%), depression (19%), and akathisia (19%). Tetrabenazine carries a black-box warning for depression and suicidalityand is contraindicated in patients with untreated or inadequately treated depression or who are suicidal.8 Assessing the patient's mental state is important when using this medication.
A review by Chen et al7 found that 9 of 11 studies had positive results for tetrabenazine treatment for tardive dyskinesia. Most of the studies were small and retrospective. The biggest prospective blinded study was a videotaped study by Ondo et al of 20 patients with tardive dyskinesia.9 At least 30 days before beginning the study patients discontinued the medication that caused their tardive dyskinesia and any treatments for tardive dyskinesia. Each patient was videotaped before starting tetrabenazine and an average of 20.3 weeks after starting the drug. Investigators' scores showed an average of 54.2% improvement in movement scores and participants' subjective scores reported an average of 60.4% improvement. One patient withdrew because of somnolence. The remaining 19 patients did not experience more than mild side effects and continued treatment with tetrabenazine after study completion.9
Treatment recommendations
Tardive dyskinesia is a difficult condition to treat; it is best, therefore, to prevent its onset by using the minimally effective antipsychotic dose, by preferential use of an SGA rather than a FGA, and by regular screening for tardive dyskinesia using a standardized rating scale such as the Abnormal Involuntary Movement Scale. Symptoms associated with tardive dyskinesia are more likely to resolve if caught early. If a patient develops tardive dyskinesia while taking a FGA, switching to a SGA may alleviate the symptoms.
Several medications can be used off-label to relieve symptoms, including vitamin E and tetrabenazine, which both have the most-although not considerable-literature-based support. Although some studies show benefit with tetrabenazine for tardive dyskinesia, larger clinical trials are needed to more strongly support its use. Tetrabenazine might be a good option for patients with refractory tardive dyskinesia but, given the associated risk of suicidality and depressive symptoms, careful monitoring of suicide risk is essential and may preclude its use for tardive dyskinesia in patients who are experiencing depressive symptoms.
Related Resources
• National Organization for Rare Disorders. Tardive dyskinesia. www.rarediseases.org/rare-disease-information/rare-diseases/byID/493/viewFullReport.
• Caroff SN, Miller DD, Campbell CE. Is there a rational management strategy for tardive dyskinesia? Current Psychiatry. 2011;10(10):22-32.
Drug Brand Names
Aripiprazole • Abilify Lurasidone • Latuda
Asenapine • Saphris Olanzapine • Zyprexa
Clozapine • Clozaril Quetiapine • Seroquel
Donepezil • Aricept Reserpine • Serpasil
Haloperidol • Haldol Risperidone • Risperdal
Iloperidone • Fanapt Tetrabenazine • Xenazine
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Aia PG, Revuelta GJ, Cloud LJ, et al. Tardive dyskinesia. Curr Treat Options Neurol. 2011;13(3):231-241.
2. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
3. Crimson ML, Argo TR, Buckley PF. Schizophrenia. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: a pathophysiologic approach. 8th ed. New York, NY: McGraw- Hill; 2011:1147-1172.
4. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 2: incidence and management strategies in patients with schizophrenia. Can J Psychiatry. 2005;50(11):703-714.
5. Facts & Comparisons. http://online.factsandcomparisons.com/index.aspx. Accessed November 4, 2012.
6. Natural Standard. http://www.naturalstandard.com/index.asp. Accessed November 4, 2012.
7. Chen JJ, Ondo WG, Dashtipour K, et al. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin Ther. 2012;34(7):1487-1504.
8. Xanazine [package insert]. Deerfield, IL: Lundbeck Inc; 2012.
9. Ondo WG, Hanna PA, Jankovic J. Tetrabenazine treatment for tardive dyskinesia: assessment by randomized videotape protocol. Am J Psychiatry. 1999;156(8):1279-1281.
1. Aia PG, Revuelta GJ, Cloud LJ, et al. Tardive dyskinesia. Curr Treat Options Neurol. 2011;13(3):231-241.
2. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
3. Crimson ML, Argo TR, Buckley PF. Schizophrenia. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: a pathophysiologic approach. 8th ed. New York, NY: McGraw- Hill; 2011:1147-1172.
4. Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 2: incidence and management strategies in patients with schizophrenia. Can J Psychiatry. 2005;50(11):703-714.
5. Facts & Comparisons. http://online.factsandcomparisons.com/index.aspx. Accessed November 4, 2012.
6. Natural Standard. http://www.naturalstandard.com/index.asp. Accessed November 4, 2012.
7. Chen JJ, Ondo WG, Dashtipour K, et al. Tetrabenazine for the treatment of hyperkinetic movement disorders: a review of the literature. Clin Ther. 2012;34(7):1487-1504.
8. Xanazine [package insert]. Deerfield, IL: Lundbeck Inc; 2012.
9. Ondo WG, Hanna PA, Jankovic J. Tetrabenazine treatment for tardive dyskinesia: assessment by randomized videotape protocol. Am J Psychiatry. 1999;156(8):1279-1281.