User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Improving nonverbal communication during telepsychiatry sessions
Telepsychiatry appointments (eg, video conferencing) initially replaced face-to-face outpatient encounters during the first phase of the COVID-19 pandemic. However, as offices reopened for in-person appointments, many patients still prefer “virtual” appointments. Telepsychiatry allows for easier delivery of mental health services, including psychotherapy, and may become the new normal.
Although therapy conducted via video conferencing allows you to connect with patients at a safe distance, it alters the basic conditions under which therapy occurs, such as being in the same room.1 While focusing on preserving the verbal elements of communication, you might inadvertently forget the nonverbal elements, which at times might render your words ineffective.1 The main elements of nonverbal communication are facial expression, gaze, posture, gesture, and proxemics (ie, how much space you take up, and your distance from others).2 The following tips can help you preserve the nonverbal elements of communication when conducting telepsychiatry sessions.
Reduce gaze error. Gaze error is the deviation from direct eye contact that occurs during video conferencing. It results from the distance between the image of the person on your screen and the camera above it.1 Gaze error can muddy intended cues and communicate unintended cues.2 Examples of gaze errors include downcast eyes (the most common gaze error), sideways gaze, or gazing over the person’s head.2 These errors can communicate social deference, evasion, insincerity, or even boredom.2 To lessen gaze error, move the patient’s image as close as possible to your camera.1 In addition, avoid looking at yourself on the screen; some video conferencing platforms allow users to hide their self-view.
Create distance and incorporate upper body language. In the office, sitting very close to your patient and staring directly at their face for an hour would be awkward and intrusive.1 Doing so online is no different. While you may be tempted to move close to the screen to compensate for feeling distant or having difficulty hearing or seeing your patient, you should back away from the camera. Doing so will help both parties feel less self-conscious, more at ease, and more focused on the session.1 Backing up from the camera will allow patients to see your upper body language (eg, hand gestures, posture) as well as your facial expressions.1 Empathy improves when patients can see your upper-body cues.2 Keep in mind that the angle of your camera is just as important as the distance. For example, if your camera is positioned so that it is looking up toward your eyes, patients may perceive that you are looking down at them.1 This problem can be remedied by stacking books under the monitor to raise the camera.
Be aware of your facial expressions, posture, gestures, and proxemics. Ensure that your face does not go slack when you are listening to patients talk.3 Just as you would do in person, a slight head tilt and occasional head nod lets patients know that you are engaged and actively listening.3 Maintain an open body posture by keeping your feet firmly on the ground and putting your hands on the table in front of you.3 Lean in when patients share intimate information, just as you would in person. Avoid hunching over the laptop/keyboard because this could make you seem tired or tense.3 Pay attention to your arm and hand movements so that you do not exaggerate them.
Maintain office professionalism. The office setting conveys a therapeutic formality that can get lost online.1 As tempting as it may be to conduct online sessions in pajamas or sweatpants, continue to dress as if you were in the office. Be mindful of your backdrop, set all cell phones to silent, turn off your email alerts, and lock the room.1,3 Stick to the clock as you would in the office, and encourage patients to do the same.
Minor technological improvements—such as headphones with a built-in microphone, a high-definition camera, a larger monitor, or a faster internet connection—might be needed to improve your nonverbal communication during telepsychiatry sessions.1 Although this is not an exhaustive list, these tips can serve as a starting point to ensure effective communication while you are physically distanced from your patients.
1. Arnold C, Franklin T. Seven tips for maintaining the frame in online therapy. Psychiatric News. Published June 25, 2020. Accessed May 26, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.7a21
2. Nguyen DT, Canny J. More than face-to-face: empathy effects of video framing. CHI 2009: Proceedings of the SGCHI Conference on Human Factors in Computing Systems. Published April 6, 2009. Accessed July 31, 2020. https://dl.acm.org/doi/10.1145/1518701.1518770
3. Cossar R, Navarro J. Tips for improving communication during video conferencing: do’s and don’ts for a more professional video-conference. Published March 31, 2020. Accessed July 31, 2020. https://www.psychologytoday.com/us/blog/spycatcher/202003/tips-improving-communication-during-video-conferencing
Telepsychiatry appointments (eg, video conferencing) initially replaced face-to-face outpatient encounters during the first phase of the COVID-19 pandemic. However, as offices reopened for in-person appointments, many patients still prefer “virtual” appointments. Telepsychiatry allows for easier delivery of mental health services, including psychotherapy, and may become the new normal.
Although therapy conducted via video conferencing allows you to connect with patients at a safe distance, it alters the basic conditions under which therapy occurs, such as being in the same room.1 While focusing on preserving the verbal elements of communication, you might inadvertently forget the nonverbal elements, which at times might render your words ineffective.1 The main elements of nonverbal communication are facial expression, gaze, posture, gesture, and proxemics (ie, how much space you take up, and your distance from others).2 The following tips can help you preserve the nonverbal elements of communication when conducting telepsychiatry sessions.
Reduce gaze error. Gaze error is the deviation from direct eye contact that occurs during video conferencing. It results from the distance between the image of the person on your screen and the camera above it.1 Gaze error can muddy intended cues and communicate unintended cues.2 Examples of gaze errors include downcast eyes (the most common gaze error), sideways gaze, or gazing over the person’s head.2 These errors can communicate social deference, evasion, insincerity, or even boredom.2 To lessen gaze error, move the patient’s image as close as possible to your camera.1 In addition, avoid looking at yourself on the screen; some video conferencing platforms allow users to hide their self-view.
Create distance and incorporate upper body language. In the office, sitting very close to your patient and staring directly at their face for an hour would be awkward and intrusive.1 Doing so online is no different. While you may be tempted to move close to the screen to compensate for feeling distant or having difficulty hearing or seeing your patient, you should back away from the camera. Doing so will help both parties feel less self-conscious, more at ease, and more focused on the session.1 Backing up from the camera will allow patients to see your upper body language (eg, hand gestures, posture) as well as your facial expressions.1 Empathy improves when patients can see your upper-body cues.2 Keep in mind that the angle of your camera is just as important as the distance. For example, if your camera is positioned so that it is looking up toward your eyes, patients may perceive that you are looking down at them.1 This problem can be remedied by stacking books under the monitor to raise the camera.
Be aware of your facial expressions, posture, gestures, and proxemics. Ensure that your face does not go slack when you are listening to patients talk.3 Just as you would do in person, a slight head tilt and occasional head nod lets patients know that you are engaged and actively listening.3 Maintain an open body posture by keeping your feet firmly on the ground and putting your hands on the table in front of you.3 Lean in when patients share intimate information, just as you would in person. Avoid hunching over the laptop/keyboard because this could make you seem tired or tense.3 Pay attention to your arm and hand movements so that you do not exaggerate them.
Maintain office professionalism. The office setting conveys a therapeutic formality that can get lost online.1 As tempting as it may be to conduct online sessions in pajamas or sweatpants, continue to dress as if you were in the office. Be mindful of your backdrop, set all cell phones to silent, turn off your email alerts, and lock the room.1,3 Stick to the clock as you would in the office, and encourage patients to do the same.
Minor technological improvements—such as headphones with a built-in microphone, a high-definition camera, a larger monitor, or a faster internet connection—might be needed to improve your nonverbal communication during telepsychiatry sessions.1 Although this is not an exhaustive list, these tips can serve as a starting point to ensure effective communication while you are physically distanced from your patients.
Telepsychiatry appointments (eg, video conferencing) initially replaced face-to-face outpatient encounters during the first phase of the COVID-19 pandemic. However, as offices reopened for in-person appointments, many patients still prefer “virtual” appointments. Telepsychiatry allows for easier delivery of mental health services, including psychotherapy, and may become the new normal.
Although therapy conducted via video conferencing allows you to connect with patients at a safe distance, it alters the basic conditions under which therapy occurs, such as being in the same room.1 While focusing on preserving the verbal elements of communication, you might inadvertently forget the nonverbal elements, which at times might render your words ineffective.1 The main elements of nonverbal communication are facial expression, gaze, posture, gesture, and proxemics (ie, how much space you take up, and your distance from others).2 The following tips can help you preserve the nonverbal elements of communication when conducting telepsychiatry sessions.
Reduce gaze error. Gaze error is the deviation from direct eye contact that occurs during video conferencing. It results from the distance between the image of the person on your screen and the camera above it.1 Gaze error can muddy intended cues and communicate unintended cues.2 Examples of gaze errors include downcast eyes (the most common gaze error), sideways gaze, or gazing over the person’s head.2 These errors can communicate social deference, evasion, insincerity, or even boredom.2 To lessen gaze error, move the patient’s image as close as possible to your camera.1 In addition, avoid looking at yourself on the screen; some video conferencing platforms allow users to hide their self-view.
Create distance and incorporate upper body language. In the office, sitting very close to your patient and staring directly at their face for an hour would be awkward and intrusive.1 Doing so online is no different. While you may be tempted to move close to the screen to compensate for feeling distant or having difficulty hearing or seeing your patient, you should back away from the camera. Doing so will help both parties feel less self-conscious, more at ease, and more focused on the session.1 Backing up from the camera will allow patients to see your upper body language (eg, hand gestures, posture) as well as your facial expressions.1 Empathy improves when patients can see your upper-body cues.2 Keep in mind that the angle of your camera is just as important as the distance. For example, if your camera is positioned so that it is looking up toward your eyes, patients may perceive that you are looking down at them.1 This problem can be remedied by stacking books under the monitor to raise the camera.
Be aware of your facial expressions, posture, gestures, and proxemics. Ensure that your face does not go slack when you are listening to patients talk.3 Just as you would do in person, a slight head tilt and occasional head nod lets patients know that you are engaged and actively listening.3 Maintain an open body posture by keeping your feet firmly on the ground and putting your hands on the table in front of you.3 Lean in when patients share intimate information, just as you would in person. Avoid hunching over the laptop/keyboard because this could make you seem tired or tense.3 Pay attention to your arm and hand movements so that you do not exaggerate them.
Maintain office professionalism. The office setting conveys a therapeutic formality that can get lost online.1 As tempting as it may be to conduct online sessions in pajamas or sweatpants, continue to dress as if you were in the office. Be mindful of your backdrop, set all cell phones to silent, turn off your email alerts, and lock the room.1,3 Stick to the clock as you would in the office, and encourage patients to do the same.
Minor technological improvements—such as headphones with a built-in microphone, a high-definition camera, a larger monitor, or a faster internet connection—might be needed to improve your nonverbal communication during telepsychiatry sessions.1 Although this is not an exhaustive list, these tips can serve as a starting point to ensure effective communication while you are physically distanced from your patients.
1. Arnold C, Franklin T. Seven tips for maintaining the frame in online therapy. Psychiatric News. Published June 25, 2020. Accessed May 26, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.7a21
2. Nguyen DT, Canny J. More than face-to-face: empathy effects of video framing. CHI 2009: Proceedings of the SGCHI Conference on Human Factors in Computing Systems. Published April 6, 2009. Accessed July 31, 2020. https://dl.acm.org/doi/10.1145/1518701.1518770
3. Cossar R, Navarro J. Tips for improving communication during video conferencing: do’s and don’ts for a more professional video-conference. Published March 31, 2020. Accessed July 31, 2020. https://www.psychologytoday.com/us/blog/spycatcher/202003/tips-improving-communication-during-video-conferencing
1. Arnold C, Franklin T. Seven tips for maintaining the frame in online therapy. Psychiatric News. Published June 25, 2020. Accessed May 26, 2021. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.7a21
2. Nguyen DT, Canny J. More than face-to-face: empathy effects of video framing. CHI 2009: Proceedings of the SGCHI Conference on Human Factors in Computing Systems. Published April 6, 2009. Accessed July 31, 2020. https://dl.acm.org/doi/10.1145/1518701.1518770
3. Cossar R, Navarro J. Tips for improving communication during video conferencing: do’s and don’ts for a more professional video-conference. Published March 31, 2020. Accessed July 31, 2020. https://www.psychologytoday.com/us/blog/spycatcher/202003/tips-improving-communication-during-video-conferencing
Treating psychosis in pregnant women: A measured approach
The peak age of onset of schizophrenia coincides with the peak childbearing age of 25 to 35 years.1 So it would not be unusual for your patient with schizophrenia to tell you she is trying to get pregnant, or thinks she might be pregnant. In these situations, you must carefully weigh the risks to the mother (eg, relapse, complications) and to the fetus (eg, possible miscarriage, teratogenesis) when deciding whether to continue or change her treatment regimen. When faced with making these decisions, keep the following factors in mind.
1. Most importantly: Do not make knee-jerk changes. Do not suddenly stop medications. Proceed in a thoughtful and measured way.
2. Discuss the risks with your patient. There is no such thing as a risk-free decision. There are potential risks from untreated psychosis as well as from medications. Mothers with untreated psychosis have an increased risk of suicide and violence, as well as poor self-care. Schizophrenia may be associated with an increased risk of poor birth outcomes, including preterm delivery, low birthweight, and neonatal complications.2 Avoid making absolute statements about specific medications during pregnancy; there needs to be an individualized risk-benefit discussion for each patient, and for each medication.
3. Involve the patient’s partner and family in treatment planning if possible. The patient’s family can be important in promoting mental health during pregnancy and the postpartum. Educating the family as well as the patient regarding medications and the risks of untreated mental illness can go a long way toward compliance.
4. Do not rely on what pregnancy category a medication was. There are multiple dimensions to evaluate when considering the use of an antipsychotic agent during pregnancy. Does it increase the risk of miscarriage? Malformations? Preterm birth? Perinatal toxicity? Behavioral teratogenesis (neurodevelopmental sequelae)? Looking for a simple summary or single letter grade minimizes the understanding of the specific outcome of concern in the specific mother. Instead, look at the Pregnancy section under Use in Specific Populations on the medication’s package insert (prescribing information), consult a web site such as MotherToBaby (mothertobaby.org/healthcare-professionals/), and/or search for the latest research on PubMed.
5. Collaborate with the patient’s obstetrician or family medicine physician. Make sure that you are on the same page regarding treating the patient’s psychosis. Other clinicians often will agree with your treatment plan because they understand the risks of untreated psychosis compared with other risks the patient is facing. However, if you don’t communicate with your patient’s other health care professionals, she might receive mixed messages.
6. As for medication choice, pregnancy is the most important time to conduct a careful medication history to inform your choice of medication. Was Medication X ineffective, or did the patient not pick it up from the pharmacy? Did she really have a trial of 3 months, or did she only take it for a week before she decided to stop?
Continue to: Determine which medication has worked for the patient in the past
7. Determine which medication has worked for the patient in the past. If Medication Y worked before she was pregnant, it is likely to still work during pregnancy. If it is a relatively safe option, it may be the best choice.
8. Avoid multiple medication exposures wherever possible. If a patient is taking Medication Z, it is working, and she tells you she is 3 months pregnant, it is often better to continue it (assuming it is a relatively safe medication) than to switch to Medication A, which has slightly better “safety data.” By switching to a different antipsychotic, you would be exposing the fetus to a second agent that may not even work for the mother.
9. Focus on treating the patient’s present symptoms. Medication doses may need to change due to pregnancy-related changes in symptoms, drug distribution, and/or metabolism.
10. Remain vigilant for other risks. Keep in mind that pregnant women with psychosis often face risks other than psychiatric medications and psychosis. Comorbidities such as substance use disorders, obesity, and poor prenatal care must also be addressed.3
11. Follow your patient more closely during pregnancy. Pregnancy is an uncertain time for any new mother. Be sure to have an open line of communication with the patient, and be responsive to her concerns.
Continue to: Provide psychoeducation about the postpartum period
12. Provide psychoeducation about the postpartum period. Pregnancy is the time to educate your patient about the importance of sleep, warning signs of exacerbation of psychosis, and breastfeeding safety.
13. Be proactive with future female patients of childbearing age, regardless of whether they tell you they are sexually active. Women with psychosis have higher rates of unplanned pregnancy.3,4 When initiating treatment of psychosis in a woman of childbearing age, rather than treating her with the newest available medication that does not yet have safety data in pregnancy, it is best to start with a medication already known to be relatively safe in pregnancy. This way, if she were to become pregnant, your treatment plan would already be safe and appropriate.
14. Consult a reproductive psychiatrist if needed.
1. Einarson A, Boskovic R. Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract. 2009;15(3):183-192.
2. Galbally M, Crabb C. Schizophrenia and psychotic disorders. O&G. 2018;20(3). https://www.ogmagazine.org.au/20/3-20/schizophrenia-and-psychotic-disorders/
3. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635.
4. Friedman SH, Hall RCW, Sorrentino RM. Involuntary treatment of psychosis in pregnancy. J Am Acad Psychiatry Law. 2018;46(2):217-223.
The peak age of onset of schizophrenia coincides with the peak childbearing age of 25 to 35 years.1 So it would not be unusual for your patient with schizophrenia to tell you she is trying to get pregnant, or thinks she might be pregnant. In these situations, you must carefully weigh the risks to the mother (eg, relapse, complications) and to the fetus (eg, possible miscarriage, teratogenesis) when deciding whether to continue or change her treatment regimen. When faced with making these decisions, keep the following factors in mind.
1. Most importantly: Do not make knee-jerk changes. Do not suddenly stop medications. Proceed in a thoughtful and measured way.
2. Discuss the risks with your patient. There is no such thing as a risk-free decision. There are potential risks from untreated psychosis as well as from medications. Mothers with untreated psychosis have an increased risk of suicide and violence, as well as poor self-care. Schizophrenia may be associated with an increased risk of poor birth outcomes, including preterm delivery, low birthweight, and neonatal complications.2 Avoid making absolute statements about specific medications during pregnancy; there needs to be an individualized risk-benefit discussion for each patient, and for each medication.
3. Involve the patient’s partner and family in treatment planning if possible. The patient’s family can be important in promoting mental health during pregnancy and the postpartum. Educating the family as well as the patient regarding medications and the risks of untreated mental illness can go a long way toward compliance.
4. Do not rely on what pregnancy category a medication was. There are multiple dimensions to evaluate when considering the use of an antipsychotic agent during pregnancy. Does it increase the risk of miscarriage? Malformations? Preterm birth? Perinatal toxicity? Behavioral teratogenesis (neurodevelopmental sequelae)? Looking for a simple summary or single letter grade minimizes the understanding of the specific outcome of concern in the specific mother. Instead, look at the Pregnancy section under Use in Specific Populations on the medication’s package insert (prescribing information), consult a web site such as MotherToBaby (mothertobaby.org/healthcare-professionals/), and/or search for the latest research on PubMed.
5. Collaborate with the patient’s obstetrician or family medicine physician. Make sure that you are on the same page regarding treating the patient’s psychosis. Other clinicians often will agree with your treatment plan because they understand the risks of untreated psychosis compared with other risks the patient is facing. However, if you don’t communicate with your patient’s other health care professionals, she might receive mixed messages.
6. As for medication choice, pregnancy is the most important time to conduct a careful medication history to inform your choice of medication. Was Medication X ineffective, or did the patient not pick it up from the pharmacy? Did she really have a trial of 3 months, or did she only take it for a week before she decided to stop?
Continue to: Determine which medication has worked for the patient in the past
7. Determine which medication has worked for the patient in the past. If Medication Y worked before she was pregnant, it is likely to still work during pregnancy. If it is a relatively safe option, it may be the best choice.
8. Avoid multiple medication exposures wherever possible. If a patient is taking Medication Z, it is working, and she tells you she is 3 months pregnant, it is often better to continue it (assuming it is a relatively safe medication) than to switch to Medication A, which has slightly better “safety data.” By switching to a different antipsychotic, you would be exposing the fetus to a second agent that may not even work for the mother.
9. Focus on treating the patient’s present symptoms. Medication doses may need to change due to pregnancy-related changes in symptoms, drug distribution, and/or metabolism.
10. Remain vigilant for other risks. Keep in mind that pregnant women with psychosis often face risks other than psychiatric medications and psychosis. Comorbidities such as substance use disorders, obesity, and poor prenatal care must also be addressed.3
11. Follow your patient more closely during pregnancy. Pregnancy is an uncertain time for any new mother. Be sure to have an open line of communication with the patient, and be responsive to her concerns.
Continue to: Provide psychoeducation about the postpartum period
12. Provide psychoeducation about the postpartum period. Pregnancy is the time to educate your patient about the importance of sleep, warning signs of exacerbation of psychosis, and breastfeeding safety.
13. Be proactive with future female patients of childbearing age, regardless of whether they tell you they are sexually active. Women with psychosis have higher rates of unplanned pregnancy.3,4 When initiating treatment of psychosis in a woman of childbearing age, rather than treating her with the newest available medication that does not yet have safety data in pregnancy, it is best to start with a medication already known to be relatively safe in pregnancy. This way, if she were to become pregnant, your treatment plan would already be safe and appropriate.
14. Consult a reproductive psychiatrist if needed.
The peak age of onset of schizophrenia coincides with the peak childbearing age of 25 to 35 years.1 So it would not be unusual for your patient with schizophrenia to tell you she is trying to get pregnant, or thinks she might be pregnant. In these situations, you must carefully weigh the risks to the mother (eg, relapse, complications) and to the fetus (eg, possible miscarriage, teratogenesis) when deciding whether to continue or change her treatment regimen. When faced with making these decisions, keep the following factors in mind.
1. Most importantly: Do not make knee-jerk changes. Do not suddenly stop medications. Proceed in a thoughtful and measured way.
2. Discuss the risks with your patient. There is no such thing as a risk-free decision. There are potential risks from untreated psychosis as well as from medications. Mothers with untreated psychosis have an increased risk of suicide and violence, as well as poor self-care. Schizophrenia may be associated with an increased risk of poor birth outcomes, including preterm delivery, low birthweight, and neonatal complications.2 Avoid making absolute statements about specific medications during pregnancy; there needs to be an individualized risk-benefit discussion for each patient, and for each medication.
3. Involve the patient’s partner and family in treatment planning if possible. The patient’s family can be important in promoting mental health during pregnancy and the postpartum. Educating the family as well as the patient regarding medications and the risks of untreated mental illness can go a long way toward compliance.
4. Do not rely on what pregnancy category a medication was. There are multiple dimensions to evaluate when considering the use of an antipsychotic agent during pregnancy. Does it increase the risk of miscarriage? Malformations? Preterm birth? Perinatal toxicity? Behavioral teratogenesis (neurodevelopmental sequelae)? Looking for a simple summary or single letter grade minimizes the understanding of the specific outcome of concern in the specific mother. Instead, look at the Pregnancy section under Use in Specific Populations on the medication’s package insert (prescribing information), consult a web site such as MotherToBaby (mothertobaby.org/healthcare-professionals/), and/or search for the latest research on PubMed.
5. Collaborate with the patient’s obstetrician or family medicine physician. Make sure that you are on the same page regarding treating the patient’s psychosis. Other clinicians often will agree with your treatment plan because they understand the risks of untreated psychosis compared with other risks the patient is facing. However, if you don’t communicate with your patient’s other health care professionals, she might receive mixed messages.
6. As for medication choice, pregnancy is the most important time to conduct a careful medication history to inform your choice of medication. Was Medication X ineffective, or did the patient not pick it up from the pharmacy? Did she really have a trial of 3 months, or did she only take it for a week before she decided to stop?
Continue to: Determine which medication has worked for the patient in the past
7. Determine which medication has worked for the patient in the past. If Medication Y worked before she was pregnant, it is likely to still work during pregnancy. If it is a relatively safe option, it may be the best choice.
8. Avoid multiple medication exposures wherever possible. If a patient is taking Medication Z, it is working, and she tells you she is 3 months pregnant, it is often better to continue it (assuming it is a relatively safe medication) than to switch to Medication A, which has slightly better “safety data.” By switching to a different antipsychotic, you would be exposing the fetus to a second agent that may not even work for the mother.
9. Focus on treating the patient’s present symptoms. Medication doses may need to change due to pregnancy-related changes in symptoms, drug distribution, and/or metabolism.
10. Remain vigilant for other risks. Keep in mind that pregnant women with psychosis often face risks other than psychiatric medications and psychosis. Comorbidities such as substance use disorders, obesity, and poor prenatal care must also be addressed.3
11. Follow your patient more closely during pregnancy. Pregnancy is an uncertain time for any new mother. Be sure to have an open line of communication with the patient, and be responsive to her concerns.
Continue to: Provide psychoeducation about the postpartum period
12. Provide psychoeducation about the postpartum period. Pregnancy is the time to educate your patient about the importance of sleep, warning signs of exacerbation of psychosis, and breastfeeding safety.
13. Be proactive with future female patients of childbearing age, regardless of whether they tell you they are sexually active. Women with psychosis have higher rates of unplanned pregnancy.3,4 When initiating treatment of psychosis in a woman of childbearing age, rather than treating her with the newest available medication that does not yet have safety data in pregnancy, it is best to start with a medication already known to be relatively safe in pregnancy. This way, if she were to become pregnant, your treatment plan would already be safe and appropriate.
14. Consult a reproductive psychiatrist if needed.
1. Einarson A, Boskovic R. Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract. 2009;15(3):183-192.
2. Galbally M, Crabb C. Schizophrenia and psychotic disorders. O&G. 2018;20(3). https://www.ogmagazine.org.au/20/3-20/schizophrenia-and-psychotic-disorders/
3. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635.
4. Friedman SH, Hall RCW, Sorrentino RM. Involuntary treatment of psychosis in pregnancy. J Am Acad Psychiatry Law. 2018;46(2):217-223.
1. Einarson A, Boskovic R. Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract. 2009;15(3):183-192.
2. Galbally M, Crabb C. Schizophrenia and psychotic disorders. O&G. 2018;20(3). https://www.ogmagazine.org.au/20/3-20/schizophrenia-and-psychotic-disorders/
3. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635.
4. Friedman SH, Hall RCW, Sorrentino RM. Involuntary treatment of psychosis in pregnancy. J Am Acad Psychiatry Law. 2018;46(2):217-223.
Recommending esketamine? 4 factors to consider
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
Since receiving FDA approval in March 2019, intranasal esketamine—the S-enantiomer of ketamine—has become a valuable treatment option for adults with treatment-resistant depression (TRD), owing to its limited adverse effects profile, rapid onset, and potential for significant improvement in depressive symptoms. In August 2020, the FDA expanded esketamine’s indication to include treatment of depressive symptoms in adults with acute suicidal ideation or behavior, thus providing psychiatrists with an additional option for improving the care of their most seriously ill patients. In this article, we review 4 factors to consider before recommending esketamine.
1. Confirm that the patient’s depression qualifies as treatment-resistant. A patient is considered to have TRD if they have long-standing depression that meets DSM-5 criteria for major depressive disorder, and have not adequately responded to at least 2 antidepressant trials of adequate dose and duration.
2. Confirm that the patient’s suicidal ideation and/or suicidal behavior does not require acute hospitalization. The time between the onset of suicidal ideation and a suicide attempt typically is short, which highlights the need to intervene quickly in these patients.1 Being able to provide a treatment that works quickly and effectively may be lifesaving. However, to receive esketamine, patients must be enrolled in the Risk Evaluation Mitigation Strategy (REMS) patient registry through a certified treatment center, and prior authorization from insurance generally is required. These steps take time, so patients at high or imminent risk for suicide may initially require psychiatric hospitalization before they are able to begin esketamine treatment. Parsing out whether the suicidal ideation is chronic or acute can help clinicians assess current dangerousness and determine if esketamine treatment might be appropriate. If a patient with chronic suicidal ideation is stable for outpatient treatment with close monitoring, esketamine might provide an effective treatment option for treating both depression and suicidality. Esketamine’s rapid effect may be an integral part of the treatment for a suicidal patient by bridging the gap caused by the delayed onset of action in typical antidepressants.2
3. Identify a local certified treatment center. Use the online database at www.spravato.com/find-a-center to locate a nearby certified esketamine treatment center. Choosing a center that you can collaborate with regularly is important to determine if the treatment is effective, to provide updates on the treatment course, and to consider tailoring of ongoing treatment.
4. Ensure the patient is also treated with an oral antidepressant. Esketamine should be administered in conjunction with an oral antidepressant. As such, patients must be willing and able to tolerate treatment with a medication that can be construed as an antidepressant while undergoing esketamine treatment. A long-term maintenance trial found that patients with TRD who experienced remission or response after esketamine treatment had a delayed relapse of symptoms when they continued esketamine in addition to an oral antidepressant.3
Considering its rapid onset of action and low adverse effects profile with manageable tolerability, esketamine adjunctive to an oral antidepressant is a reasonable option to consider for patients with TRD, including those with suicidality.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
1. Deisenhammer EA, Ing CM, Strauss R, et al. The duration of the suicidal process: how much time is left for intervention between consideration and accomplishment of a suicide attempt? J Clin Psychiatry. 2009;70(1):19-24.
2. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620-630.
3. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression. JAMA Psychiatry. 2019;76(9):893-903.
4 tips for working with caregivers of children with somatic disorders
Somatic symptom and related disorders—physical complaints that may or may not be medically explained that are associated with significant distress and impairment—are common in children and adolescents, and are often accompanied by anxiety and depression.1 Clinicians are likely to see children with these disorders in emergency departments, consultation services, or outpatient clinics. Common presenting symptoms include abdominal pain, headache, nausea, vomiting, dizziness, and seizures.1 Talking to the caregivers of these children can be challenging due to the subjective nature of the illness. In this article, I offer 4 tips for mental health practitioners to consider when working with caregivers of children with somatic disorders.
1. Support. Talk to the child and caregiver individually, and then together. Try to understand the caregiver’s concerns and express empathy to establish rapport. Being dismissive of their concerns is not going to help the child. Acknowledge the caregiver’s complaints and ask how seriously they feel other clinicians regard their concerns. Ask the caregiver about their perception of their child’s health, how frequently they worry about their child’s health, and the impact their worries have on their lives and their child’s life. Often the caregiver and child must miss out on obligations (eg, work, school, extracurricular activities) due to the child’s care and medical appointments.
2. Educate. This may be difficult, particularly when interacting with a caregiver who is convinced that their child is seriously physically sick. The caregiver may feel that involving psychiatry services is discrediting their concerns. Your initial interaction may be to allow the caregiver to express their frustrations toward the primary service. When talking with caregivers, avoid using medical jargon; in some instances, however, it may be necessary to use medical terminology to reassure the caregiver that you know what you are talking about. Be direct, and do not give false hope. These children often undergo extensive medical workup before psychiatry services are involved. To minimize conflicting messages from multiple clinicians who are caring for the same child, review the patient’s chart in advance, and maintain constant communication with other clinicians involved in the patient’s care.
3. Reassure. When the caregiver finally begins to acknowledge the psychological nature of their child’s illness, provide them with reassurance, but avoid emphasizing that the child is medically healthy because any relief caregivers gain from this can quickly fade and worsen their anxiety. Discuss the importance of treating underlying anxiety or depression with medication and psychotherapy where necessary. Assess the child for substance use disorders, personality disorders, and psychosocial stressors, and if present, target treatment accordingly. Discuss the potential long-term outcomes with and without treatment. Share examples of success stories from your past experiences. Emphasize the importance of noticing even slight improvements. Encourage the child to focus on goals such as attending school or passing online tests, etc.
4. Refer. Connecting the child with a therapist can significantly improve long-term outcomes, especially if coordinated well.2 This becomes more crucial in cases where caregivers are opposed to pharmacotherapy for their child. Whenever possible, communicate with the therapist before the child’s initial appointment to formulate a plan of action. The best approach is integrated care characterized by close collaboration of primary care, a somatic specialist, and mental health care professionals operating on a biopsychosocial model of distress and therapeutic factors.3
The ultimate goal is to help the child and caregiver achieve some level of relief by acknowledgment and support. Utilizing some of these tips can make our work even more meaningful for ourselves and our patients.
1. Malas N, Ortiz-Aguayo R, Giles L, et al. Pediatric somatic symptom disorders. Curr Psychiatry Rep. 2017;19(2):11. doi: 10.1007/s11920-017-0760-3
2. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93(1):49-54.
3. Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-31. doi: 10.31887/DCNS.2018.20.1/phenningsen
Somatic symptom and related disorders—physical complaints that may or may not be medically explained that are associated with significant distress and impairment—are common in children and adolescents, and are often accompanied by anxiety and depression.1 Clinicians are likely to see children with these disorders in emergency departments, consultation services, or outpatient clinics. Common presenting symptoms include abdominal pain, headache, nausea, vomiting, dizziness, and seizures.1 Talking to the caregivers of these children can be challenging due to the subjective nature of the illness. In this article, I offer 4 tips for mental health practitioners to consider when working with caregivers of children with somatic disorders.
1. Support. Talk to the child and caregiver individually, and then together. Try to understand the caregiver’s concerns and express empathy to establish rapport. Being dismissive of their concerns is not going to help the child. Acknowledge the caregiver’s complaints and ask how seriously they feel other clinicians regard their concerns. Ask the caregiver about their perception of their child’s health, how frequently they worry about their child’s health, and the impact their worries have on their lives and their child’s life. Often the caregiver and child must miss out on obligations (eg, work, school, extracurricular activities) due to the child’s care and medical appointments.
2. Educate. This may be difficult, particularly when interacting with a caregiver who is convinced that their child is seriously physically sick. The caregiver may feel that involving psychiatry services is discrediting their concerns. Your initial interaction may be to allow the caregiver to express their frustrations toward the primary service. When talking with caregivers, avoid using medical jargon; in some instances, however, it may be necessary to use medical terminology to reassure the caregiver that you know what you are talking about. Be direct, and do not give false hope. These children often undergo extensive medical workup before psychiatry services are involved. To minimize conflicting messages from multiple clinicians who are caring for the same child, review the patient’s chart in advance, and maintain constant communication with other clinicians involved in the patient’s care.
3. Reassure. When the caregiver finally begins to acknowledge the psychological nature of their child’s illness, provide them with reassurance, but avoid emphasizing that the child is medically healthy because any relief caregivers gain from this can quickly fade and worsen their anxiety. Discuss the importance of treating underlying anxiety or depression with medication and psychotherapy where necessary. Assess the child for substance use disorders, personality disorders, and psychosocial stressors, and if present, target treatment accordingly. Discuss the potential long-term outcomes with and without treatment. Share examples of success stories from your past experiences. Emphasize the importance of noticing even slight improvements. Encourage the child to focus on goals such as attending school or passing online tests, etc.
4. Refer. Connecting the child with a therapist can significantly improve long-term outcomes, especially if coordinated well.2 This becomes more crucial in cases where caregivers are opposed to pharmacotherapy for their child. Whenever possible, communicate with the therapist before the child’s initial appointment to formulate a plan of action. The best approach is integrated care characterized by close collaboration of primary care, a somatic specialist, and mental health care professionals operating on a biopsychosocial model of distress and therapeutic factors.3
The ultimate goal is to help the child and caregiver achieve some level of relief by acknowledgment and support. Utilizing some of these tips can make our work even more meaningful for ourselves and our patients.
Somatic symptom and related disorders—physical complaints that may or may not be medically explained that are associated with significant distress and impairment—are common in children and adolescents, and are often accompanied by anxiety and depression.1 Clinicians are likely to see children with these disorders in emergency departments, consultation services, or outpatient clinics. Common presenting symptoms include abdominal pain, headache, nausea, vomiting, dizziness, and seizures.1 Talking to the caregivers of these children can be challenging due to the subjective nature of the illness. In this article, I offer 4 tips for mental health practitioners to consider when working with caregivers of children with somatic disorders.
1. Support. Talk to the child and caregiver individually, and then together. Try to understand the caregiver’s concerns and express empathy to establish rapport. Being dismissive of their concerns is not going to help the child. Acknowledge the caregiver’s complaints and ask how seriously they feel other clinicians regard their concerns. Ask the caregiver about their perception of their child’s health, how frequently they worry about their child’s health, and the impact their worries have on their lives and their child’s life. Often the caregiver and child must miss out on obligations (eg, work, school, extracurricular activities) due to the child’s care and medical appointments.
2. Educate. This may be difficult, particularly when interacting with a caregiver who is convinced that their child is seriously physically sick. The caregiver may feel that involving psychiatry services is discrediting their concerns. Your initial interaction may be to allow the caregiver to express their frustrations toward the primary service. When talking with caregivers, avoid using medical jargon; in some instances, however, it may be necessary to use medical terminology to reassure the caregiver that you know what you are talking about. Be direct, and do not give false hope. These children often undergo extensive medical workup before psychiatry services are involved. To minimize conflicting messages from multiple clinicians who are caring for the same child, review the patient’s chart in advance, and maintain constant communication with other clinicians involved in the patient’s care.
3. Reassure. When the caregiver finally begins to acknowledge the psychological nature of their child’s illness, provide them with reassurance, but avoid emphasizing that the child is medically healthy because any relief caregivers gain from this can quickly fade and worsen their anxiety. Discuss the importance of treating underlying anxiety or depression with medication and psychotherapy where necessary. Assess the child for substance use disorders, personality disorders, and psychosocial stressors, and if present, target treatment accordingly. Discuss the potential long-term outcomes with and without treatment. Share examples of success stories from your past experiences. Emphasize the importance of noticing even slight improvements. Encourage the child to focus on goals such as attending school or passing online tests, etc.
4. Refer. Connecting the child with a therapist can significantly improve long-term outcomes, especially if coordinated well.2 This becomes more crucial in cases where caregivers are opposed to pharmacotherapy for their child. Whenever possible, communicate with the therapist before the child’s initial appointment to formulate a plan of action. The best approach is integrated care characterized by close collaboration of primary care, a somatic specialist, and mental health care professionals operating on a biopsychosocial model of distress and therapeutic factors.3
The ultimate goal is to help the child and caregiver achieve some level of relief by acknowledgment and support. Utilizing some of these tips can make our work even more meaningful for ourselves and our patients.
1. Malas N, Ortiz-Aguayo R, Giles L, et al. Pediatric somatic symptom disorders. Curr Psychiatry Rep. 2017;19(2):11. doi: 10.1007/s11920-017-0760-3
2. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93(1):49-54.
3. Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-31. doi: 10.31887/DCNS.2018.20.1/phenningsen
1. Malas N, Ortiz-Aguayo R, Giles L, et al. Pediatric somatic symptom disorders. Curr Psychiatry Rep. 2017;19(2):11. doi: 10.1007/s11920-017-0760-3
2. Kurlansik SL, Maffei MS. Somatic symptom disorder. Am Fam Physician. 2016;93(1):49-54.
3. Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-31. doi: 10.31887/DCNS.2018.20.1/phenningsen
Pharmacogenetic testing: Navigating through the confusion
Mr. J, age 30, a Black man with major depressive disorder (MDD), has been your patient for the past year. At the time of his diagnosis, Mr. J received sertraline, 100 mg/d, but had little to no improvement. During the past year, he received trials of citalopram and paroxetine, but they were not effective for his recurrent depressive symptoms and/or resulted in significant adverse effects.
During a recent visit, Mr. J asks you about “the genetic tests that help determine which medications will work.” He mentions that his brother had this testing done and that it had “worked for him,” but offers no other details. You research the different testing panels to see which test you might use. After a brief online review, you identify at least 4 different products, and are not sure which test—if any—you should consider.
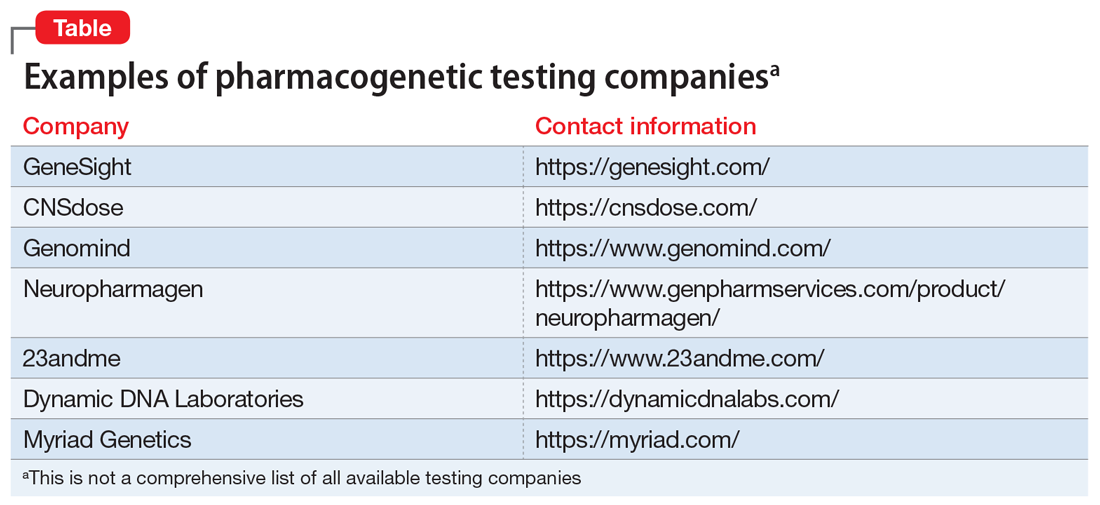
During the last few years, there has been a rise in commercial pharmacogenetic testing options, including tests available to clinicians at academic medical centers as well as direct-to-consumer testing (Table). Clinician and patient interest regarding pharmacogenetic testing in practice is often followed by the question, “Which test is best?” Although this is a logical question, providing an answer is multifactorial.1-3 Because none of the currently available tests have been compared in head-to-head clinical trials, it is nearly impossible to identify the “best” test.
In this article, we focus on the evidence-based principles that clinicians should consider when adopting pharmacogenetic testing in their practice. We discuss which genes are of most interest when prescribing psychotropic medications, the value of decision support tools, cost considerations, and patient education regarding this type of testing.
Which genes and variants should be tested?
The genes relevant to medication treatment outcomes can be broadly classified into those with pharmacokinetic vs pharmacodynamic effects. Pharmacogenes, such as those coding for the drug-metabolizing enzymes cytochrome P450 (CYP) 1A2, CYP2B6, CYP2C19, CYP2C9, CYP2D6, CYP3A4, and UDP-glucuronosyltransferase (UGT)2B1, may alter the rate at which medications are metabolized, thus varying the serum drug concentration across patients. Variants that impact the function of these enzymes are considered pharmacokinetic. Up to 40% of the variance in patients’ response to antidepressants may be due to variations in the pharmacokinetic genes.4 Alternatively, pharmacodynamic pharmacogenes impact drug action and therefore may affect the degree of receptor activation at a given drug concentration, overall drug efficacy, and/or the occurrence of medication sensitivity. These pharmacogenes may include:
- brain-derived neurotrophic factor (BDNF)
- catechol-O-methyltransferase (COMT)
- human leukocyte antigens A (HLA-A)
- serotonin receptor subtype 2 (HTR2)
- serotonin receptor subtype 2C (HTR2C)
- opioid receptor mu 1 (OPRM1)
- solute carrier family 6 member 4 (SLC6A4).
In articles previously published in
Currently, there is no standardization among commercial pharmacogenetic tests on:
- which genes to test
- which variants specific to a gene need to be included
- how the genetic data is translated to phenotype
- how the phenotype is translated to a treatment recommendation.
Continue to: Due to these factors...
Due to these factors, the FDA has advised clinicians to consult the dosing recommendations provided in a medication’s package insert for information regarding how genetic information should be used in making treatment decisions.2
The value of decision support tools
Researchers have assessed how various manufacturers’ decision support tools (DSTs) (ie, the reports the commercial testing companies send to the clinician who orders the test) agree on genotypes, predicted phenotypes, and medication recommendations.4 Overall, this research found varying levels of disagreement in the medication recommendations of the testing panels they studied, which indicates that not all tests are equivalent or interchangeable.4 Of the actionable recommendations for antidepressants, 16% were conflicting; the recommendations for fluoxetine and imipramine were most frequently in disagreement.4 Similarly, 20% of the actionable antipsychotic advice was conflicting, with the recommendations for aripiprazole and clozapine most frequently in disagreement.4 Researchers also reported a situation in which 4 testing panels agreed on the patient’s phenotyping status for CYP2C19, but the dosing recommendations provided for the CYP2C19 substrate, amitriptyline, differed.4 Thus, it is understandable why DSTs can result in confusion, and why clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Additionally, while the genes included on these panels vary, these testing panels also may not evaluate the same variants within a specific gene. These differences may impact the patient’s reported phenotypes and medication recommendations across DSTs. For example, the FDA has recommended HLA gene testing prior to prescribing carbamazepine. However, few of the available tests may include the HLA-B*15:02 variant, which has been associated with carbamazepine-induced severe cutaneous reactions in patients of Asian descent, and fewer may include the HLA-A*31:01 variant, for which testing is recommended prior to prescribing carbamazepine in patients of Caucasian descent.4 Additionally, some of the CYP enzymes—such as CYP2D6*17 and CYP2C19*3 variants, which may be more common in certain populations of patients who are members of ethnic or racial minority groups—may not be consistently included in the various panels. Thus, before deciding on a specific test, clinicians should understand which gene variants are relevant to their patients with regard to race and ethnicity, and key variants for specific medications. Clinicians should refer to FDA guidance and the Clinical Pharmacogenomics Implementation Consortium (CPIC) guidelines to determine the appropriate interpretations of genetic test results.1,2
Despite the disagreement in recommendations from the various testing companies, DSTs are useful and have been shown to facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. A recently published meta-analysis of randomized controlled trials (RCTs) of pharmacogenetic testing found that DSTs improved symptom remission among individuals with MDD by 70%.5 This suggests that pharmacogenetic-guided DSTs may provide superior treatment compared with treatment for DSTs were not used. However, the RCTs in this meta-analysis only included patients who had previously failed an antidepressant trial.5 Therefore, it is currently unknown at what point in care DSTs should be used, and whether they would be more beneficial if they are used when starting a new therapy, or after several trials have failed.
Consider the cost
The cost and availability of pharmacogenetic testing can be an issue when making treatment decisions, and such testing may not be covered by a patient’s insurance plan. Recently, the Centers for Medicare & Medicaid Services announced that Medicare would cover FDA-approved genomic tests that encompass broad gene panels if the evidence supports their use. Similarly, commercial insurers such as UnitedHealthcare have begun to cover some pharmacogenetic tests.6 Medicare or Medicaid plans cover some testing panels’ costs and patients do not incur any out-of-pocket costs; however, some private insurance companies require patients to pay at least a portion of the cost, and many companies offer financial assistance for patients based on income and other factors. Although financial coverage for testing has improved, patients may still face out-of-pocket costs; therefore, clinicians may need to weigh the benefits of pharmacogenetic testing vs its cost.7 Clinicians should also determine what timeline best suits their patient’s financial and clinical needs, and test accordingly.
Continue to: Patient education is critical
Patient education is critical
Although the benefits of using pharmacogenetic testing information when making certain treatment decisions is promising, it is important for both patients and clinicians to understand that test results do not always change therapy. A study on the impact of pharmacogenetic testing on clinical outcomes of patients with MDD found that 79% of patients were already prescribed medications that aligned with recommendations.8 Therefore, switching medications based on the test results of a patient who is doing well clinically is not recommended. However, DSTs may help with clinical decisions for ambiguous cases. For example, if a patient has a genotype and/or phenotype that aligns with medication recommendations, the DST might not be able to identify a better medication to use, but may be able to recommend dosing guidance to improve the tolerability of the patient’s current therapy.6 It is also important to understand that the results of such testing may have a broader use beyond the initial reason for obtaining testing, such as when prescribing a common blood thinner such as warfarin or clopidogrel. However, for many of the pharmacodynamic genes that are included in these panels, their use beyond the treatment of depression may be limited because outcome studies for pharmacodynamic pharmacogenes may vary based on psychiatric diagnosis. Regardless, it may be beneficial to securely save and store patient test results in a standardized place within the medical record for future use.
CASE CONTINUED
You work with Mr. J to help him understand the benefits and limitations associated with pharmacogenetic testing. Assuming Mr. J is comfortable with the costs of obtaining testing, you contact the testing companies you identified to determine the specific pharmacogene variants included on each of these panels, and which would be the most appropriate given his race. If the decision is made to order the testing, provide Mr. J with a copy of his testing report so that he can use this information should he need any additional pharmacotherapy in the future, and also maintain a copy in his patient records using a standardized location for easy future access. If Mr. J is not comfortable with the costs associated with the testing, find out which medication his brother is currently receiving for treatment; this information may help identify a treatment plan for Mr. J.
Impact on practice
As psychiatry continues to gain experience in using pharmacogenetic testing and DSTs to help guide treatments for depression and other disorders, clinicians need to learn about these tools and how to use an evidence-based approach to best implement them in their practice. Many academic medical centers have developed continuing education programs or consult services to help with this.9,10 Just as the choice of which medication to use may be based partly on clinician experience, so too may be which pharmacogenetic test to use.
Bottom Line
Pharmacogenetic tests have not been examined in head-to-head clinical trials, which makes it nearly impossible to identify which test is best to use. Although the testing companies’ decision support tools (DSTs) often disagree in their recommendations, research has shown that using DSTs can facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. Clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Related Resources
- PGx Gene-specific information tables. www.pharmgkb.org/page/pgxGeneRef
- Clinical Pharmacogenetics Implementation Consortium. https://cpicpgx.org/guidelines/
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Fluoxetine • Prozac
Imipramine • Tofranil
Paroxetine • Paxil
Sertraline • Zoloft
Warfarin • Coumadin, Jantoven
1. Ellingrod, VL. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
2. Ellingrod VL. Pharmacogenomics testing: what the FDA says. Current Psychiatry. 2019;18(4):29-33.
3. Ramsey LB. Pharmacogenetic testing in children: what to test and how to use it. Current Psychiatry. 2018;17(9):30-36.
4. Bousman CA, Dunlop BW. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. The Pharmacogenomics Journal. 2018;18(5):613-622. doi:10.1038/s41397-018-0027-3
5. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1). doi:10.2217/pgs-2018-0142
6. Nicholson WT, Formea CM, Matey ET, et al. Considerations when applying pharmacogenomics to your practice. Mayo Clin Proc. 2021;96(1);218-230. doi:10.1016/j.mayocp.2020.03.011
7. Krebs K, Milani L. Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good. Human Genomics. 2019;13(1). doi:10.1186/s40246-019-0229-z
8. Greden JF, Parikh S, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111;59-67. doi:10.1016/j.jpsychires.2019.01.003
9. Haga SB. Integrating pharmacogenetic testing into primary care. Expert Review of Precision Medicine and Drug Development. 2017;2(6):327-336. doi:10.1080/23808993.2017.1398046
10. Ward KM, Taubman DS, Pasternak AL, et al. Teaching psychiatric pharmacogenomics effectively: evaluation of a novel interprofessional online course. J Am Coll Clin Pharm. 2021; 4:176-183.
Mr. J, age 30, a Black man with major depressive disorder (MDD), has been your patient for the past year. At the time of his diagnosis, Mr. J received sertraline, 100 mg/d, but had little to no improvement. During the past year, he received trials of citalopram and paroxetine, but they were not effective for his recurrent depressive symptoms and/or resulted in significant adverse effects.
During a recent visit, Mr. J asks you about “the genetic tests that help determine which medications will work.” He mentions that his brother had this testing done and that it had “worked for him,” but offers no other details. You research the different testing panels to see which test you might use. After a brief online review, you identify at least 4 different products, and are not sure which test—if any—you should consider.
During the last few years, there has been a rise in commercial pharmacogenetic testing options, including tests available to clinicians at academic medical centers as well as direct-to-consumer testing (Table). Clinician and patient interest regarding pharmacogenetic testing in practice is often followed by the question, “Which test is best?” Although this is a logical question, providing an answer is multifactorial.1-3 Because none of the currently available tests have been compared in head-to-head clinical trials, it is nearly impossible to identify the “best” test.

In this article, we focus on the evidence-based principles that clinicians should consider when adopting pharmacogenetic testing in their practice. We discuss which genes are of most interest when prescribing psychotropic medications, the value of decision support tools, cost considerations, and patient education regarding this type of testing.
Which genes and variants should be tested?
The genes relevant to medication treatment outcomes can be broadly classified into those with pharmacokinetic vs pharmacodynamic effects. Pharmacogenes, such as those coding for the drug-metabolizing enzymes cytochrome P450 (CYP) 1A2, CYP2B6, CYP2C19, CYP2C9, CYP2D6, CYP3A4, and UDP-glucuronosyltransferase (UGT)2B1, may alter the rate at which medications are metabolized, thus varying the serum drug concentration across patients. Variants that impact the function of these enzymes are considered pharmacokinetic. Up to 40% of the variance in patients’ response to antidepressants may be due to variations in the pharmacokinetic genes.4 Alternatively, pharmacodynamic pharmacogenes impact drug action and therefore may affect the degree of receptor activation at a given drug concentration, overall drug efficacy, and/or the occurrence of medication sensitivity. These pharmacogenes may include:
- brain-derived neurotrophic factor (BDNF)
- catechol-O-methyltransferase (COMT)
- human leukocyte antigens A (HLA-A)
- serotonin receptor subtype 2 (HTR2)
- serotonin receptor subtype 2C (HTR2C)
- opioid receptor mu 1 (OPRM1)
- solute carrier family 6 member 4 (SLC6A4).
In articles previously published in
Currently, there is no standardization among commercial pharmacogenetic tests on:
- which genes to test
- which variants specific to a gene need to be included
- how the genetic data is translated to phenotype
- how the phenotype is translated to a treatment recommendation.
Continue to: Due to these factors...
Due to these factors, the FDA has advised clinicians to consult the dosing recommendations provided in a medication’s package insert for information regarding how genetic information should be used in making treatment decisions.2
The value of decision support tools
Researchers have assessed how various manufacturers’ decision support tools (DSTs) (ie, the reports the commercial testing companies send to the clinician who orders the test) agree on genotypes, predicted phenotypes, and medication recommendations.4 Overall, this research found varying levels of disagreement in the medication recommendations of the testing panels they studied, which indicates that not all tests are equivalent or interchangeable.4 Of the actionable recommendations for antidepressants, 16% were conflicting; the recommendations for fluoxetine and imipramine were most frequently in disagreement.4 Similarly, 20% of the actionable antipsychotic advice was conflicting, with the recommendations for aripiprazole and clozapine most frequently in disagreement.4 Researchers also reported a situation in which 4 testing panels agreed on the patient’s phenotyping status for CYP2C19, but the dosing recommendations provided for the CYP2C19 substrate, amitriptyline, differed.4 Thus, it is understandable why DSTs can result in confusion, and why clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Additionally, while the genes included on these panels vary, these testing panels also may not evaluate the same variants within a specific gene. These differences may impact the patient’s reported phenotypes and medication recommendations across DSTs. For example, the FDA has recommended HLA gene testing prior to prescribing carbamazepine. However, few of the available tests may include the HLA-B*15:02 variant, which has been associated with carbamazepine-induced severe cutaneous reactions in patients of Asian descent, and fewer may include the HLA-A*31:01 variant, for which testing is recommended prior to prescribing carbamazepine in patients of Caucasian descent.4 Additionally, some of the CYP enzymes—such as CYP2D6*17 and CYP2C19*3 variants, which may be more common in certain populations of patients who are members of ethnic or racial minority groups—may not be consistently included in the various panels. Thus, before deciding on a specific test, clinicians should understand which gene variants are relevant to their patients with regard to race and ethnicity, and key variants for specific medications. Clinicians should refer to FDA guidance and the Clinical Pharmacogenomics Implementation Consortium (CPIC) guidelines to determine the appropriate interpretations of genetic test results.1,2
Despite the disagreement in recommendations from the various testing companies, DSTs are useful and have been shown to facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. A recently published meta-analysis of randomized controlled trials (RCTs) of pharmacogenetic testing found that DSTs improved symptom remission among individuals with MDD by 70%.5 This suggests that pharmacogenetic-guided DSTs may provide superior treatment compared with treatment for DSTs were not used. However, the RCTs in this meta-analysis only included patients who had previously failed an antidepressant trial.5 Therefore, it is currently unknown at what point in care DSTs should be used, and whether they would be more beneficial if they are used when starting a new therapy, or after several trials have failed.
Consider the cost
The cost and availability of pharmacogenetic testing can be an issue when making treatment decisions, and such testing may not be covered by a patient’s insurance plan. Recently, the Centers for Medicare & Medicaid Services announced that Medicare would cover FDA-approved genomic tests that encompass broad gene panels if the evidence supports their use. Similarly, commercial insurers such as UnitedHealthcare have begun to cover some pharmacogenetic tests.6 Medicare or Medicaid plans cover some testing panels’ costs and patients do not incur any out-of-pocket costs; however, some private insurance companies require patients to pay at least a portion of the cost, and many companies offer financial assistance for patients based on income and other factors. Although financial coverage for testing has improved, patients may still face out-of-pocket costs; therefore, clinicians may need to weigh the benefits of pharmacogenetic testing vs its cost.7 Clinicians should also determine what timeline best suits their patient’s financial and clinical needs, and test accordingly.
Continue to: Patient education is critical
Patient education is critical
Although the benefits of using pharmacogenetic testing information when making certain treatment decisions is promising, it is important for both patients and clinicians to understand that test results do not always change therapy. A study on the impact of pharmacogenetic testing on clinical outcomes of patients with MDD found that 79% of patients were already prescribed medications that aligned with recommendations.8 Therefore, switching medications based on the test results of a patient who is doing well clinically is not recommended. However, DSTs may help with clinical decisions for ambiguous cases. For example, if a patient has a genotype and/or phenotype that aligns with medication recommendations, the DST might not be able to identify a better medication to use, but may be able to recommend dosing guidance to improve the tolerability of the patient’s current therapy.6 It is also important to understand that the results of such testing may have a broader use beyond the initial reason for obtaining testing, such as when prescribing a common blood thinner such as warfarin or clopidogrel. However, for many of the pharmacodynamic genes that are included in these panels, their use beyond the treatment of depression may be limited because outcome studies for pharmacodynamic pharmacogenes may vary based on psychiatric diagnosis. Regardless, it may be beneficial to securely save and store patient test results in a standardized place within the medical record for future use.
CASE CONTINUED
You work with Mr. J to help him understand the benefits and limitations associated with pharmacogenetic testing. Assuming Mr. J is comfortable with the costs of obtaining testing, you contact the testing companies you identified to determine the specific pharmacogene variants included on each of these panels, and which would be the most appropriate given his race. If the decision is made to order the testing, provide Mr. J with a copy of his testing report so that he can use this information should he need any additional pharmacotherapy in the future, and also maintain a copy in his patient records using a standardized location for easy future access. If Mr. J is not comfortable with the costs associated with the testing, find out which medication his brother is currently receiving for treatment; this information may help identify a treatment plan for Mr. J.
Impact on practice
As psychiatry continues to gain experience in using pharmacogenetic testing and DSTs to help guide treatments for depression and other disorders, clinicians need to learn about these tools and how to use an evidence-based approach to best implement them in their practice. Many academic medical centers have developed continuing education programs or consult services to help with this.9,10 Just as the choice of which medication to use may be based partly on clinician experience, so too may be which pharmacogenetic test to use.
Bottom Line
Pharmacogenetic tests have not been examined in head-to-head clinical trials, which makes it nearly impossible to identify which test is best to use. Although the testing companies’ decision support tools (DSTs) often disagree in their recommendations, research has shown that using DSTs can facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. Clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Related Resources
- PGx Gene-specific information tables. www.pharmgkb.org/page/pgxGeneRef
- Clinical Pharmacogenetics Implementation Consortium. https://cpicpgx.org/guidelines/
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Fluoxetine • Prozac
Imipramine • Tofranil
Paroxetine • Paxil
Sertraline • Zoloft
Warfarin • Coumadin, Jantoven
Mr. J, age 30, a Black man with major depressive disorder (MDD), has been your patient for the past year. At the time of his diagnosis, Mr. J received sertraline, 100 mg/d, but had little to no improvement. During the past year, he received trials of citalopram and paroxetine, but they were not effective for his recurrent depressive symptoms and/or resulted in significant adverse effects.
During a recent visit, Mr. J asks you about “the genetic tests that help determine which medications will work.” He mentions that his brother had this testing done and that it had “worked for him,” but offers no other details. You research the different testing panels to see which test you might use. After a brief online review, you identify at least 4 different products, and are not sure which test—if any—you should consider.
During the last few years, there has been a rise in commercial pharmacogenetic testing options, including tests available to clinicians at academic medical centers as well as direct-to-consumer testing (Table). Clinician and patient interest regarding pharmacogenetic testing in practice is often followed by the question, “Which test is best?” Although this is a logical question, providing an answer is multifactorial.1-3 Because none of the currently available tests have been compared in head-to-head clinical trials, it is nearly impossible to identify the “best” test.

In this article, we focus on the evidence-based principles that clinicians should consider when adopting pharmacogenetic testing in their practice. We discuss which genes are of most interest when prescribing psychotropic medications, the value of decision support tools, cost considerations, and patient education regarding this type of testing.
Which genes and variants should be tested?
The genes relevant to medication treatment outcomes can be broadly classified into those with pharmacokinetic vs pharmacodynamic effects. Pharmacogenes, such as those coding for the drug-metabolizing enzymes cytochrome P450 (CYP) 1A2, CYP2B6, CYP2C19, CYP2C9, CYP2D6, CYP3A4, and UDP-glucuronosyltransferase (UGT)2B1, may alter the rate at which medications are metabolized, thus varying the serum drug concentration across patients. Variants that impact the function of these enzymes are considered pharmacokinetic. Up to 40% of the variance in patients’ response to antidepressants may be due to variations in the pharmacokinetic genes.4 Alternatively, pharmacodynamic pharmacogenes impact drug action and therefore may affect the degree of receptor activation at a given drug concentration, overall drug efficacy, and/or the occurrence of medication sensitivity. These pharmacogenes may include:
- brain-derived neurotrophic factor (BDNF)
- catechol-O-methyltransferase (COMT)
- human leukocyte antigens A (HLA-A)
- serotonin receptor subtype 2 (HTR2)
- serotonin receptor subtype 2C (HTR2C)
- opioid receptor mu 1 (OPRM1)
- solute carrier family 6 member 4 (SLC6A4).
In articles previously published in
Currently, there is no standardization among commercial pharmacogenetic tests on:
- which genes to test
- which variants specific to a gene need to be included
- how the genetic data is translated to phenotype
- how the phenotype is translated to a treatment recommendation.
Continue to: Due to these factors...
Due to these factors, the FDA has advised clinicians to consult the dosing recommendations provided in a medication’s package insert for information regarding how genetic information should be used in making treatment decisions.2
The value of decision support tools
Researchers have assessed how various manufacturers’ decision support tools (DSTs) (ie, the reports the commercial testing companies send to the clinician who orders the test) agree on genotypes, predicted phenotypes, and medication recommendations.4 Overall, this research found varying levels of disagreement in the medication recommendations of the testing panels they studied, which indicates that not all tests are equivalent or interchangeable.4 Of the actionable recommendations for antidepressants, 16% were conflicting; the recommendations for fluoxetine and imipramine were most frequently in disagreement.4 Similarly, 20% of the actionable antipsychotic advice was conflicting, with the recommendations for aripiprazole and clozapine most frequently in disagreement.4 Researchers also reported a situation in which 4 testing panels agreed on the patient’s phenotyping status for CYP2C19, but the dosing recommendations provided for the CYP2C19 substrate, amitriptyline, differed.4 Thus, it is understandable why DSTs can result in confusion, and why clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Additionally, while the genes included on these panels vary, these testing panels also may not evaluate the same variants within a specific gene. These differences may impact the patient’s reported phenotypes and medication recommendations across DSTs. For example, the FDA has recommended HLA gene testing prior to prescribing carbamazepine. However, few of the available tests may include the HLA-B*15:02 variant, which has been associated with carbamazepine-induced severe cutaneous reactions in patients of Asian descent, and fewer may include the HLA-A*31:01 variant, for which testing is recommended prior to prescribing carbamazepine in patients of Caucasian descent.4 Additionally, some of the CYP enzymes—such as CYP2D6*17 and CYP2C19*3 variants, which may be more common in certain populations of patients who are members of ethnic or racial minority groups—may not be consistently included in the various panels. Thus, before deciding on a specific test, clinicians should understand which gene variants are relevant to their patients with regard to race and ethnicity, and key variants for specific medications. Clinicians should refer to FDA guidance and the Clinical Pharmacogenomics Implementation Consortium (CPIC) guidelines to determine the appropriate interpretations of genetic test results.1,2
Despite the disagreement in recommendations from the various testing companies, DSTs are useful and have been shown to facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. A recently published meta-analysis of randomized controlled trials (RCTs) of pharmacogenetic testing found that DSTs improved symptom remission among individuals with MDD by 70%.5 This suggests that pharmacogenetic-guided DSTs may provide superior treatment compared with treatment for DSTs were not used. However, the RCTs in this meta-analysis only included patients who had previously failed an antidepressant trial.5 Therefore, it is currently unknown at what point in care DSTs should be used, and whether they would be more beneficial if they are used when starting a new therapy, or after several trials have failed.
Consider the cost
The cost and availability of pharmacogenetic testing can be an issue when making treatment decisions, and such testing may not be covered by a patient’s insurance plan. Recently, the Centers for Medicare & Medicaid Services announced that Medicare would cover FDA-approved genomic tests that encompass broad gene panels if the evidence supports their use. Similarly, commercial insurers such as UnitedHealthcare have begun to cover some pharmacogenetic tests.6 Medicare or Medicaid plans cover some testing panels’ costs and patients do not incur any out-of-pocket costs; however, some private insurance companies require patients to pay at least a portion of the cost, and many companies offer financial assistance for patients based on income and other factors. Although financial coverage for testing has improved, patients may still face out-of-pocket costs; therefore, clinicians may need to weigh the benefits of pharmacogenetic testing vs its cost.7 Clinicians should also determine what timeline best suits their patient’s financial and clinical needs, and test accordingly.
Continue to: Patient education is critical
Patient education is critical
Although the benefits of using pharmacogenetic testing information when making certain treatment decisions is promising, it is important for both patients and clinicians to understand that test results do not always change therapy. A study on the impact of pharmacogenetic testing on clinical outcomes of patients with MDD found that 79% of patients were already prescribed medications that aligned with recommendations.8 Therefore, switching medications based on the test results of a patient who is doing well clinically is not recommended. However, DSTs may help with clinical decisions for ambiguous cases. For example, if a patient has a genotype and/or phenotype that aligns with medication recommendations, the DST might not be able to identify a better medication to use, but may be able to recommend dosing guidance to improve the tolerability of the patient’s current therapy.6 It is also important to understand that the results of such testing may have a broader use beyond the initial reason for obtaining testing, such as when prescribing a common blood thinner such as warfarin or clopidogrel. However, for many of the pharmacodynamic genes that are included in these panels, their use beyond the treatment of depression may be limited because outcome studies for pharmacodynamic pharmacogenes may vary based on psychiatric diagnosis. Regardless, it may be beneficial to securely save and store patient test results in a standardized place within the medical record for future use.
CASE CONTINUED
You work with Mr. J to help him understand the benefits and limitations associated with pharmacogenetic testing. Assuming Mr. J is comfortable with the costs of obtaining testing, you contact the testing companies you identified to determine the specific pharmacogene variants included on each of these panels, and which would be the most appropriate given his race. If the decision is made to order the testing, provide Mr. J with a copy of his testing report so that he can use this information should he need any additional pharmacotherapy in the future, and also maintain a copy in his patient records using a standardized location for easy future access. If Mr. J is not comfortable with the costs associated with the testing, find out which medication his brother is currently receiving for treatment; this information may help identify a treatment plan for Mr. J.
Impact on practice
As psychiatry continues to gain experience in using pharmacogenetic testing and DSTs to help guide treatments for depression and other disorders, clinicians need to learn about these tools and how to use an evidence-based approach to best implement them in their practice. Many academic medical centers have developed continuing education programs or consult services to help with this.9,10 Just as the choice of which medication to use may be based partly on clinician experience, so too may be which pharmacogenetic test to use.
Bottom Line
Pharmacogenetic tests have not been examined in head-to-head clinical trials, which makes it nearly impossible to identify which test is best to use. Although the testing companies’ decision support tools (DSTs) often disagree in their recommendations, research has shown that using DSTs can facilitate implementation of relevant psychopharmacology dosing guidelines, assist in identifying optimal medication therapy, and improve patient outcomes. Clinicians should use testing panels with recommendations that best align with their individual practices, their patient’s needs, and FDA information.
Related Resources
- PGx Gene-specific information tables. www.pharmgkb.org/page/pgxGeneRef
- Clinical Pharmacogenetics Implementation Consortium. https://cpicpgx.org/guidelines/
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Clopidogrel • Plavix
Clozapine • Clozaril
Fluoxetine • Prozac
Imipramine • Tofranil
Paroxetine • Paxil
Sertraline • Zoloft
Warfarin • Coumadin, Jantoven
1. Ellingrod, VL. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
2. Ellingrod VL. Pharmacogenomics testing: what the FDA says. Current Psychiatry. 2019;18(4):29-33.
3. Ramsey LB. Pharmacogenetic testing in children: what to test and how to use it. Current Psychiatry. 2018;17(9):30-36.
4. Bousman CA, Dunlop BW. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. The Pharmacogenomics Journal. 2018;18(5):613-622. doi:10.1038/s41397-018-0027-3
5. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1). doi:10.2217/pgs-2018-0142
6. Nicholson WT, Formea CM, Matey ET, et al. Considerations when applying pharmacogenomics to your practice. Mayo Clin Proc. 2021;96(1);218-230. doi:10.1016/j.mayocp.2020.03.011
7. Krebs K, Milani L. Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good. Human Genomics. 2019;13(1). doi:10.1186/s40246-019-0229-z
8. Greden JF, Parikh S, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111;59-67. doi:10.1016/j.jpsychires.2019.01.003
9. Haga SB. Integrating pharmacogenetic testing into primary care. Expert Review of Precision Medicine and Drug Development. 2017;2(6):327-336. doi:10.1080/23808993.2017.1398046
10. Ward KM, Taubman DS, Pasternak AL, et al. Teaching psychiatric pharmacogenomics effectively: evaluation of a novel interprofessional online course. J Am Coll Clin Pharm. 2021; 4:176-183.
1. Ellingrod, VL. Using pharmacogenetics guidelines when prescribing: what’s available. Current Psychiatry. 2018;17(1):43-46.
2. Ellingrod VL. Pharmacogenomics testing: what the FDA says. Current Psychiatry. 2019;18(4):29-33.
3. Ramsey LB. Pharmacogenetic testing in children: what to test and how to use it. Current Psychiatry. 2018;17(9):30-36.
4. Bousman CA, Dunlop BW. Genotype, phenotype, and medication recommendation agreement among commercial pharmacogenetic-based decision support tools. The Pharmacogenomics Journal. 2018;18(5):613-622. doi:10.1038/s41397-018-0027-3
5. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenomics. 2019;20(1). doi:10.2217/pgs-2018-0142
6. Nicholson WT, Formea CM, Matey ET, et al. Considerations when applying pharmacogenomics to your practice. Mayo Clin Proc. 2021;96(1);218-230. doi:10.1016/j.mayocp.2020.03.011
7. Krebs K, Milani L. Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good. Human Genomics. 2019;13(1). doi:10.1186/s40246-019-0229-z
8. Greden JF, Parikh S, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111;59-67. doi:10.1016/j.jpsychires.2019.01.003
9. Haga SB. Integrating pharmacogenetic testing into primary care. Expert Review of Precision Medicine and Drug Development. 2017;2(6):327-336. doi:10.1080/23808993.2017.1398046
10. Ward KM, Taubman DS, Pasternak AL, et al. Teaching psychiatric pharmacogenomics effectively: evaluation of a novel interprofessional online course. J Am Coll Clin Pharm. 2021; 4:176-183.
How COVID-19 affects peripartum women’s mental health
The COVID-19 pandemic has had a negative impact on the mental health of people worldwide, and a disproportionate effect on peripartum women. In this article, we discuss the reasons for this disparity, review the limited literature on this topic, and suggest strategies to safeguard the mental health of peripartum women during the COVID-19 pandemic.
Catastrophic events and women’s mental health
During the peripartum period, women have increased psychosocial and physical health needs.1 In addition, women are disproportionately affected by natural disasters and catastrophic events,2 which are predictors of psychiatric symptoms during the peripartum period.3 Mass tragedies previously associated with maternal stress include wildfires, hurricanes, migrations, earthquakes, and tsunamis.4,5 For example, pregnant women who survived severe exposure during Hurricane Katrina (ie, feeling that one’s life was in danger, experiencing illness or injury to self or a family member, walking through floodwaters) in 2005 had a significantly increased risk of developing posttraumatic stress disorder (PTSD) and depression compared with pregnant women who did not have such exposure.6 After the 2011 Tōhoku earthquake and tsunami in Japan, the prevalence of psychological distress in pregnant women increased, especially among those living in the area directly affected by the tsunami.5
Epidemics and pandemics also can adversely affect peripartum women’s mental health. Studies conducted before the COVID-19 pandemic found that previous infectious disease outbreaks such as severe acute respiratory syndrome (SARS), the 2009 influenza A (H1N1) pandemic, and Zika had negative emotional impacts on pregnant women.7 Our review of the limited literature published to date suggests that COVID-19 is having similar adverse effects.
COVID-19 poses both medical and psychiatric threats
COVID-19 infection is a physical threat to pregnant women who are already vulnerable due to the hormonal and immunological changes inherent to pregnancy. A meta-analysis of 39 studies with a total of 1,316 pregnant women indicated that the most frequently reported symptoms of COVID-19 infection were cough, fever, and myalgias.8 However, COVID-19 infection during pregnancy is also associated with an increase in pregnancy complications and adverse birth outcomes.9 According to the CDC, compared with their nonpregnant counterparts, pregnant women are at greater risk for severe COVID-19 infection and adverse birth outcomes such as preterm birth.10 Pregnant women who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the virus responsible for COVID-19) risk ICU admission, caesarean section, and perinatal death.8 A Swedish study of 2,682 pregnant women found an increase in preeclampsia among women who tested positive for SARS-CoV-2, a finding attributed to COVID-19’s pattern of systemic effects.11 Vertical transmission of the novel coronavirus from mother to fetus appears to be rare but possible.12
In addition to the physical dangers of becoming infected with COVID-19, the perceived threat of infection is an added source of anxiety for some peripartum women. In addition to the concerns involved in any pregnancy, COVID-19–related sources of distress for pregnant women include worrying about harm to the fetus during pregnancy, the possibility of vertical transmission, and exposures during antenatal appointments, during employment, or from a partner.8,13
The death toll from factors associated with COVID-19 adds to the mental health burden. For every person who dies of COVID-19, an estimated 9 others may develop prolonged grief or PTSD due to the loss of someone they loved.14,15 A systematic review found that PTSD in the perinatal period is associated with negative birth and child outcomes, including low birth weight and decreased rates of breastfeeding.16 The COVID-19 pandemic has disrupted human interactions, from social distancing rules and lockdowns of businesses and social activities to panic buying of grocery staples and increased economic insecurity.1 These changes have been accompanied by a rise in mental health challenges. For example, according to an August 2020 CDC survey, 40.9% of US adults reported at least 1 adverse mental or behavioral health condition, including symptoms of anxiety or depression (30.9%), symptoms of a trauma- and stressor-related disorder related to the pandemic (26.3%), and having started or increased substance use to cope with stress or emotions related to COVID-19 (13.3%).17
COVID-19–related traumas and stressors appear to affect women more than men. A study from China found that compared with men, women had significantly higher levels of self-reported pandemic-related anxiety, depression, and posttraumatic stress symptoms (PTSS).18 This trend has been observed in other parts of the world. A study conducted by the UK Office of National Statistics reported anxiety levels were 24% higher in women vs men as reflected by scores on a self-rated anxiety scale.19
Continue to: Many factors influence...
Many factors influence the disproportionate impact of COVID-19 on women in general, and peripartum women in particular (Box20-26).
Box
Factors that predispose women to increased stress during COVID-19 include an increase in child care burdens brought about by school closures and subsequent virtual schooling.20 Intimate partner violence has spiked globally during COVID-19 restrictions.24 Women also represent the majority of the health care workforce (76%) and often take on informal caregiving roles; both of these roles have seen increased burdens during the pandemic.25 Already encumbered by prepandemic gender pay inequalities, women are filing unemployment claims at a significantly increased rate compared to men.26
For women of childbearing age, the disruption of routine clinical care during COVID-19 has decreased access to reproductive health care, resulting in increases in unintended pregnancies, unsafe abortions, and deaths.20 Another source of stress for pregnant women during COVID-19 is feeling unprepared for birth because of the pandemic, a phenomenon described as “preparedness stress.”21 Visitor restriction policies and quarantines have also caused women in labor to experience birth without their support partners, which is associated with increased posttraumatic stress symptoms.22 These restrictions also may be associated with an increase in women choosing out-of-hospital births despite the increased risk of adverse outcomes.23
Psychiatric diagnoses in peripartum women
Multiple studies and meta-analyses have begun to assess the impact of the COVID-19 pandemic on maternal mental health. One meta-analysis of 8 studies conducted in 5 countries determined that COVID-19 significantly increases the risk of anxiety in women during the peripartum period.27 Results of another meta-analysis of 23 studies with >24,000 participants indicated that the prevalence of anxiety, depression, and insomnia in peripartum women was significantly higher during the pandemic than in pre-pandemic times.28
In an online survey of 4,451 pregnant women in the United States, nearly one-third of respondents reported elevated levels of pandemic-related stress as measured by the newly-developed Pandemic-Related Pregnancy Stress Scale.3 The rates were even higher among women who were already at risk for elevated stress levels, such as those who had survived abuse, those giving birth for the first time, or those experiencing high-risk pregnancies.3 Living in a pandemic “hot spot” also appeared to impact peripartum stress levels.
COVID-19 has adverse effects on women’s mental health specifically during the postpartum period. One study from a center in Italy found a high prevalence of depressive symptoms and PTSS in the postpartum period, with COVID-19–related factors playing an “indirect role” compared with prenatal experiences and other individual factors.2 A British study of mothers of infants age ≤12 months found that traveling for work, the impact of lockdown on food affordability, and having an income of less than £30,000 per year (approximately $41,000) predicted poorer mental health during the pandemic.29 Results of a study from China indicated that more than one-quarter of pregnant and postpartum women experienced depression during the pandemic, and women who worried about infection risk or missing pediatric visits were at increased risk.30
How to mitigate these risks
The increase in pandemic-related mental health concerns in the general population and specifically in peripartum women is a global health care challenge. Investing in mitigation strategies is necessary not only to address the current pandemic, but also to help prepare for the possibility of future traumatic events, such as another global pandemic.
Continue to: For pregnant women...
For pregnant women, ensuring access to outdoor space, increasing participation in healthy activities, and minimizing disruptions to prenatal care can protect against pandemic-related stress.3 Physical activity is an effective treatment for mild to moderate depressive symptoms. Because of the significant decrease in exercise among pregnant women during the pandemic, encouraging safe forms of physical activity such as online fitness classes could improve mental health outcomes for these patients.27 When counseling peripartum women, psychiatrists need to be creative in recommending fitness interventions to target mood symptoms, such as by suggesting virtual or at-home programs.
In an online survey, 118 obstetricians called for increased mental health resources for peripartum women, such as easier access to a helpline, educational videos, and mental health professionals.13 Increased screening for psychiatric disorders throughout the peripartum period can help identify women at greater risk, and advancements in telepsychiatry could help meet the increased need for psychiatric care during COVID-19. Psychiatrists and other mental health clinicians should consider reaching out to their colleagues who specialize in women’s health to establish new partnerships and create teams of multidisciplinary professionals.
Similarly, psychiatrists should familiarize themselves with telehealth services available to peripartum patients who could benefit from such services. Telehealth options can increase women’s access to peripartum care for both medical and psychiatric illnesses. Online options such as women’s support groups, parenting classes, and labor coaching seminars also represent valuable virtual tools to strengthen women’s social supports.
Women who need inpatient treatment for severe peripartum depression or anxiety might be particularly reluctant to receive this care during COVID-19 due to fears of becoming infected and of being separated from their infant and family while hospitalized. Clinicians should remain vigilant in screening peripartum women for mood disorders that might represent a danger to mothers and infants, and not allow concerns about COVID-19 to interfere with recommendations for psychiatric hospitalizations, when necessary. The creation of small, women-only inpatient behavioral units can help address this situation, especially given the possibility of frequent visits with infants and other peripartum support. Investment into such units is critical for supporting peripartum mental health, even in nonpandemic times.
What about vaccination? As of mid-May 2021, no large clinical trials of any COVID-19 vaccine that included pregnant women had been completed. However, 2 small preliminary studies suggested that the mRNA vaccines are safe and effective during pregnancy.31,32 When counseling peripartum patients on the risks and benefits, clinicians need to rely on this evidence, animal trials, and limited data from inadvertent exposures during pregnancy. While every woman will weigh the risks and benefits for her own circumstances, the CDC, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine have all stated that the mRNA vaccines should be offered to pregnant and breastfeeding individuals who are eligible for vaccination.33 Rasmussen et al33 have published a useful resource for clinicians regarding COVID-19 vaccination and pregnant women.
Continue to: Bottom Line
Bottom Line
During the COVID-19 pandemic, peripartum women have experienced increased rates of anxiety, depression, and stress. Psychiatric clinicians can help these patients by remaining vigilant in screening for psychiatric disorders, encouraging them to engage in activities to mitigate COVID-19’s adverse psychological effects, and referring them to care via telehealth and other resources as appropriate.
Related Resources
- Hu YJ, Wake M, Saffery R. Clarifying the sweeping consequences of COVID-19 in pregnant women, newborns, and children with existing cohorts. JAMA Pediatr. 2021; 75(2):117-118. doi: 10.1001/jamapediatrics.2020.2395
- Tomfohr-Madsen LM, Racine N, Giesbrecht GF, et al. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021; 300:113912. doi: 10.1016/j.psychres.2021.113912
1. Chivers BR, Garad RM, Boyle JA, et al. Perinatal distress during COVID-19: thematic analysis of an online parenting forum. J Med Internet Res. 2020;22(9):e22002. doi: 10.2196/22002
2. Ostacoli L, Cosma S, Bevilacqua F, et al. Psychosocial factors associated with postpartum psychological distress during the Covid-19 pandemic: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):703. doi: 10.1186/s12884-020-03399-5
3. Preis H, Mahaffey B, Heiselman C, etal. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020;266:113348. doi: 10.1016/j.socscimed.2020.113348
4. Olson DM, Brémault-Phillips S, King S, et al. Recent Canadian efforts to develop population-level pregnancy intervention studies to mitigate effects of natural disasters and other tragedies. J Dev Orig Health Dis. 2019;10(1):108-114. doi: 10.1017/S2040174418001113
5. Watanabe Z, Iwama N, Nishigori H, et al. Japan Environment & Children’s Study Group. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: the Japan Environment and Children’s Study. J Affect Disord. 2016;190:341-348. doi: 10.1016/j.jad.2015.10.024
6. Xiong X, Harville EW, Mattison DR, et al. Hurricane Katrina experience and the risk of post-traumatic stress disorder and depression among pregnant women. Am J Disaster Med. 2010;5(3):181-187. doi: 10.5055/ajdm.2010.0020
7. Brooks SK, Weston D, Greenberg N. Psychological impact of infectious disease outbreaks on pregnant women: rapid evidence review. Public Health. 2020;189:26-36. doi: 10.1016/j.puhe.2020.09.006
8. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):39. doi: 10.1186/s40001-020-00439-w
9. Qi M, Li X, Liu S, et al. Impact of the COVID-19 epidemic on patterns of pregnant women’s perception of threat and its relationship to mental state: a latent class analysis. PLoS One. 2020;15(10):e0239697. doi: 10.1371/journal.pone.0239697
10. Centers for Disease Control and Prevention. Investigating the impact of COVID-19 during pregnancy. Updated February 4, 2021. Accessed April 29, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19/what-cdc-is-doing.html
11. Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782-1785. doi: 10.1001/jama.2020.19124
12. Ashraf MA, Keshavarz P, Hosseinpour P, et al. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157-168.
13. Nanjundaswamy MH, Shiva L, Desai G, et al. COVID-19-related anxiety and concerns expressed by pregnant and postpartum women-a survey among obstetricians. Arch Womens Ment Health. 2020; 23(6):787-790. doi: 10.1007/s00737-020-01060-w
14. Verdery AM, Smith-Greenaway E, Margolis R, et al. Tracking the reach of COVID-19 kin loss with a bereavement multiplier applied to the United States. Proc Natl Acad Sci U S A. 2020;117(30):17695-17701. doi: 10.1073/pnas.2007476117
15. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494. doi: 10.1001/jama.2020.19632
16. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi: 10.1016/j.jad.2017.07.045
17. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. doi:10.15585/mmwr.mm6932a1
18. Almeida M, Shrestha AD, Stojanac D, et al. The impact of the COVID-19 pandemic on women’s mental health. Arch Womens Ment Health. 2020;23(6):741-748. doi:10.1007/s00737-020-01092-2
19. Office for National Statistics. Personal and economic well-being in Great Britain: May 2020. Published May 4, 2020. Accessed April 23, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/bulletins/personalandeconomicwellbeingintheuk/may2020
20. Kuehn BM. COVID-19 halts reproductive care for millions of women. JAMA. 2020;324(15):1489. doi: 10.1001/jama.2020.19025
21. Preis H, Mahaffey B, Lobel M. Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS). J Psychosom Obstet Gynaecol. 2020;41(3):191-197. doi: 10.1080/0167482X.2020.1801625
22. Hermann A, Fitelson EM, Bergink V. Meeting maternal mental health needs during the COVID-19 pandemic. JAMA Psychiatry. 2020;78(2):123-124. doi: 10.1001/jamapsychiatry.2020.1947
23. Arora KS, Mauch JT, Gibson KS. Labor and delivery visitor policies during the COVID-19 pandemic: balancing risks and benefits. JAMA. 2020;323(24):2468-2469. doi: 10.1001/jama.2020.7563
24. Bradbury-Jones C, Isham L. The pandemic paradox: the consequences of COVID-19 on domestic violence. J Clin Nurs. 2020;29(13-14):2047-2049. doi: 10.1111/jocn.15296
25. Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
26. Scharff X, Ryley S. Breaking: some states show alarming spike in women’s share of unemployment claims. The Fuller Project. Accessed April 23, 2021. https://fullerproject.org/story/some-states-shows-alarming-spike-in-womens-share-of-unemployment-claims/
27. Hessami K, Romanelli C, Chiurazzi M, et al. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;1-8. doi: 10.1080/14767058.2020.1843155
28. Yan H, Ding Y, Guo W. Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front Psychol. 2020;11:617001. doi: 10.3389/fpsyg.2020.617001
29. Dib S, Rougeaux E, Vázquez-Vázquez A, et al. Maternal mental health and coping during the COVID-19 lockdown in the UK: data from the COVID-19 New Mum Study. Int J Gynaecol Obstet. 2020;151(3):407-414. doi: 10.1002/ijgo.13397
30. Bo HX, Yang Y, Chen J, et al. Prevalence of depressive symptoms among Chinese pregnant and postpartum women during the COVID-19 pandemic. Psychosom Med. 2020. doi: 10.1097/PSY.0000000000000904
31. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021. doi:10.1001/jama.2021.7563
32. Shanes ED, Otero S, Mithal LB, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol. 2021. doi: 10.1097/AOG.0000000000004457
33. Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408-414. doi: 10.1097/AOG.0000000000004290
The COVID-19 pandemic has had a negative impact on the mental health of people worldwide, and a disproportionate effect on peripartum women. In this article, we discuss the reasons for this disparity, review the limited literature on this topic, and suggest strategies to safeguard the mental health of peripartum women during the COVID-19 pandemic.
Catastrophic events and women’s mental health
During the peripartum period, women have increased psychosocial and physical health needs.1 In addition, women are disproportionately affected by natural disasters and catastrophic events,2 which are predictors of psychiatric symptoms during the peripartum period.3 Mass tragedies previously associated with maternal stress include wildfires, hurricanes, migrations, earthquakes, and tsunamis.4,5 For example, pregnant women who survived severe exposure during Hurricane Katrina (ie, feeling that one’s life was in danger, experiencing illness or injury to self or a family member, walking through floodwaters) in 2005 had a significantly increased risk of developing posttraumatic stress disorder (PTSD) and depression compared with pregnant women who did not have such exposure.6 After the 2011 Tōhoku earthquake and tsunami in Japan, the prevalence of psychological distress in pregnant women increased, especially among those living in the area directly affected by the tsunami.5
Epidemics and pandemics also can adversely affect peripartum women’s mental health. Studies conducted before the COVID-19 pandemic found that previous infectious disease outbreaks such as severe acute respiratory syndrome (SARS), the 2009 influenza A (H1N1) pandemic, and Zika had negative emotional impacts on pregnant women.7 Our review of the limited literature published to date suggests that COVID-19 is having similar adverse effects.
COVID-19 poses both medical and psychiatric threats
COVID-19 infection is a physical threat to pregnant women who are already vulnerable due to the hormonal and immunological changes inherent to pregnancy. A meta-analysis of 39 studies with a total of 1,316 pregnant women indicated that the most frequently reported symptoms of COVID-19 infection were cough, fever, and myalgias.8 However, COVID-19 infection during pregnancy is also associated with an increase in pregnancy complications and adverse birth outcomes.9 According to the CDC, compared with their nonpregnant counterparts, pregnant women are at greater risk for severe COVID-19 infection and adverse birth outcomes such as preterm birth.10 Pregnant women who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the virus responsible for COVID-19) risk ICU admission, caesarean section, and perinatal death.8 A Swedish study of 2,682 pregnant women found an increase in preeclampsia among women who tested positive for SARS-CoV-2, a finding attributed to COVID-19’s pattern of systemic effects.11 Vertical transmission of the novel coronavirus from mother to fetus appears to be rare but possible.12
In addition to the physical dangers of becoming infected with COVID-19, the perceived threat of infection is an added source of anxiety for some peripartum women. In addition to the concerns involved in any pregnancy, COVID-19–related sources of distress for pregnant women include worrying about harm to the fetus during pregnancy, the possibility of vertical transmission, and exposures during antenatal appointments, during employment, or from a partner.8,13
The death toll from factors associated with COVID-19 adds to the mental health burden. For every person who dies of COVID-19, an estimated 9 others may develop prolonged grief or PTSD due to the loss of someone they loved.14,15 A systematic review found that PTSD in the perinatal period is associated with negative birth and child outcomes, including low birth weight and decreased rates of breastfeeding.16 The COVID-19 pandemic has disrupted human interactions, from social distancing rules and lockdowns of businesses and social activities to panic buying of grocery staples and increased economic insecurity.1 These changes have been accompanied by a rise in mental health challenges. For example, according to an August 2020 CDC survey, 40.9% of US adults reported at least 1 adverse mental or behavioral health condition, including symptoms of anxiety or depression (30.9%), symptoms of a trauma- and stressor-related disorder related to the pandemic (26.3%), and having started or increased substance use to cope with stress or emotions related to COVID-19 (13.3%).17
COVID-19–related traumas and stressors appear to affect women more than men. A study from China found that compared with men, women had significantly higher levels of self-reported pandemic-related anxiety, depression, and posttraumatic stress symptoms (PTSS).18 This trend has been observed in other parts of the world. A study conducted by the UK Office of National Statistics reported anxiety levels were 24% higher in women vs men as reflected by scores on a self-rated anxiety scale.19
Continue to: Many factors influence...
Many factors influence the disproportionate impact of COVID-19 on women in general, and peripartum women in particular (Box20-26).
Box
Factors that predispose women to increased stress during COVID-19 include an increase in child care burdens brought about by school closures and subsequent virtual schooling.20 Intimate partner violence has spiked globally during COVID-19 restrictions.24 Women also represent the majority of the health care workforce (76%) and often take on informal caregiving roles; both of these roles have seen increased burdens during the pandemic.25 Already encumbered by prepandemic gender pay inequalities, women are filing unemployment claims at a significantly increased rate compared to men.26
For women of childbearing age, the disruption of routine clinical care during COVID-19 has decreased access to reproductive health care, resulting in increases in unintended pregnancies, unsafe abortions, and deaths.20 Another source of stress for pregnant women during COVID-19 is feeling unprepared for birth because of the pandemic, a phenomenon described as “preparedness stress.”21 Visitor restriction policies and quarantines have also caused women in labor to experience birth without their support partners, which is associated with increased posttraumatic stress symptoms.22 These restrictions also may be associated with an increase in women choosing out-of-hospital births despite the increased risk of adverse outcomes.23
Psychiatric diagnoses in peripartum women
Multiple studies and meta-analyses have begun to assess the impact of the COVID-19 pandemic on maternal mental health. One meta-analysis of 8 studies conducted in 5 countries determined that COVID-19 significantly increases the risk of anxiety in women during the peripartum period.27 Results of another meta-analysis of 23 studies with >24,000 participants indicated that the prevalence of anxiety, depression, and insomnia in peripartum women was significantly higher during the pandemic than in pre-pandemic times.28
In an online survey of 4,451 pregnant women in the United States, nearly one-third of respondents reported elevated levels of pandemic-related stress as measured by the newly-developed Pandemic-Related Pregnancy Stress Scale.3 The rates were even higher among women who were already at risk for elevated stress levels, such as those who had survived abuse, those giving birth for the first time, or those experiencing high-risk pregnancies.3 Living in a pandemic “hot spot” also appeared to impact peripartum stress levels.
COVID-19 has adverse effects on women’s mental health specifically during the postpartum period. One study from a center in Italy found a high prevalence of depressive symptoms and PTSS in the postpartum period, with COVID-19–related factors playing an “indirect role” compared with prenatal experiences and other individual factors.2 A British study of mothers of infants age ≤12 months found that traveling for work, the impact of lockdown on food affordability, and having an income of less than £30,000 per year (approximately $41,000) predicted poorer mental health during the pandemic.29 Results of a study from China indicated that more than one-quarter of pregnant and postpartum women experienced depression during the pandemic, and women who worried about infection risk or missing pediatric visits were at increased risk.30
How to mitigate these risks
The increase in pandemic-related mental health concerns in the general population and specifically in peripartum women is a global health care challenge. Investing in mitigation strategies is necessary not only to address the current pandemic, but also to help prepare for the possibility of future traumatic events, such as another global pandemic.
Continue to: For pregnant women...
For pregnant women, ensuring access to outdoor space, increasing participation in healthy activities, and minimizing disruptions to prenatal care can protect against pandemic-related stress.3 Physical activity is an effective treatment for mild to moderate depressive symptoms. Because of the significant decrease in exercise among pregnant women during the pandemic, encouraging safe forms of physical activity such as online fitness classes could improve mental health outcomes for these patients.27 When counseling peripartum women, psychiatrists need to be creative in recommending fitness interventions to target mood symptoms, such as by suggesting virtual or at-home programs.
In an online survey, 118 obstetricians called for increased mental health resources for peripartum women, such as easier access to a helpline, educational videos, and mental health professionals.13 Increased screening for psychiatric disorders throughout the peripartum period can help identify women at greater risk, and advancements in telepsychiatry could help meet the increased need for psychiatric care during COVID-19. Psychiatrists and other mental health clinicians should consider reaching out to their colleagues who specialize in women’s health to establish new partnerships and create teams of multidisciplinary professionals.
Similarly, psychiatrists should familiarize themselves with telehealth services available to peripartum patients who could benefit from such services. Telehealth options can increase women’s access to peripartum care for both medical and psychiatric illnesses. Online options such as women’s support groups, parenting classes, and labor coaching seminars also represent valuable virtual tools to strengthen women’s social supports.
Women who need inpatient treatment for severe peripartum depression or anxiety might be particularly reluctant to receive this care during COVID-19 due to fears of becoming infected and of being separated from their infant and family while hospitalized. Clinicians should remain vigilant in screening peripartum women for mood disorders that might represent a danger to mothers and infants, and not allow concerns about COVID-19 to interfere with recommendations for psychiatric hospitalizations, when necessary. The creation of small, women-only inpatient behavioral units can help address this situation, especially given the possibility of frequent visits with infants and other peripartum support. Investment into such units is critical for supporting peripartum mental health, even in nonpandemic times.
What about vaccination? As of mid-May 2021, no large clinical trials of any COVID-19 vaccine that included pregnant women had been completed. However, 2 small preliminary studies suggested that the mRNA vaccines are safe and effective during pregnancy.31,32 When counseling peripartum patients on the risks and benefits, clinicians need to rely on this evidence, animal trials, and limited data from inadvertent exposures during pregnancy. While every woman will weigh the risks and benefits for her own circumstances, the CDC, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine have all stated that the mRNA vaccines should be offered to pregnant and breastfeeding individuals who are eligible for vaccination.33 Rasmussen et al33 have published a useful resource for clinicians regarding COVID-19 vaccination and pregnant women.
Continue to: Bottom Line
Bottom Line
During the COVID-19 pandemic, peripartum women have experienced increased rates of anxiety, depression, and stress. Psychiatric clinicians can help these patients by remaining vigilant in screening for psychiatric disorders, encouraging them to engage in activities to mitigate COVID-19’s adverse psychological effects, and referring them to care via telehealth and other resources as appropriate.
Related Resources
- Hu YJ, Wake M, Saffery R. Clarifying the sweeping consequences of COVID-19 in pregnant women, newborns, and children with existing cohorts. JAMA Pediatr. 2021; 75(2):117-118. doi: 10.1001/jamapediatrics.2020.2395
- Tomfohr-Madsen LM, Racine N, Giesbrecht GF, et al. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021; 300:113912. doi: 10.1016/j.psychres.2021.113912
The COVID-19 pandemic has had a negative impact on the mental health of people worldwide, and a disproportionate effect on peripartum women. In this article, we discuss the reasons for this disparity, review the limited literature on this topic, and suggest strategies to safeguard the mental health of peripartum women during the COVID-19 pandemic.
Catastrophic events and women’s mental health
During the peripartum period, women have increased psychosocial and physical health needs.1 In addition, women are disproportionately affected by natural disasters and catastrophic events,2 which are predictors of psychiatric symptoms during the peripartum period.3 Mass tragedies previously associated with maternal stress include wildfires, hurricanes, migrations, earthquakes, and tsunamis.4,5 For example, pregnant women who survived severe exposure during Hurricane Katrina (ie, feeling that one’s life was in danger, experiencing illness or injury to self or a family member, walking through floodwaters) in 2005 had a significantly increased risk of developing posttraumatic stress disorder (PTSD) and depression compared with pregnant women who did not have such exposure.6 After the 2011 Tōhoku earthquake and tsunami in Japan, the prevalence of psychological distress in pregnant women increased, especially among those living in the area directly affected by the tsunami.5
Epidemics and pandemics also can adversely affect peripartum women’s mental health. Studies conducted before the COVID-19 pandemic found that previous infectious disease outbreaks such as severe acute respiratory syndrome (SARS), the 2009 influenza A (H1N1) pandemic, and Zika had negative emotional impacts on pregnant women.7 Our review of the limited literature published to date suggests that COVID-19 is having similar adverse effects.
COVID-19 poses both medical and psychiatric threats
COVID-19 infection is a physical threat to pregnant women who are already vulnerable due to the hormonal and immunological changes inherent to pregnancy. A meta-analysis of 39 studies with a total of 1,316 pregnant women indicated that the most frequently reported symptoms of COVID-19 infection were cough, fever, and myalgias.8 However, COVID-19 infection during pregnancy is also associated with an increase in pregnancy complications and adverse birth outcomes.9 According to the CDC, compared with their nonpregnant counterparts, pregnant women are at greater risk for severe COVID-19 infection and adverse birth outcomes such as preterm birth.10 Pregnant women who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; the virus responsible for COVID-19) risk ICU admission, caesarean section, and perinatal death.8 A Swedish study of 2,682 pregnant women found an increase in preeclampsia among women who tested positive for SARS-CoV-2, a finding attributed to COVID-19’s pattern of systemic effects.11 Vertical transmission of the novel coronavirus from mother to fetus appears to be rare but possible.12
In addition to the physical dangers of becoming infected with COVID-19, the perceived threat of infection is an added source of anxiety for some peripartum women. In addition to the concerns involved in any pregnancy, COVID-19–related sources of distress for pregnant women include worrying about harm to the fetus during pregnancy, the possibility of vertical transmission, and exposures during antenatal appointments, during employment, or from a partner.8,13
The death toll from factors associated with COVID-19 adds to the mental health burden. For every person who dies of COVID-19, an estimated 9 others may develop prolonged grief or PTSD due to the loss of someone they loved.14,15 A systematic review found that PTSD in the perinatal period is associated with negative birth and child outcomes, including low birth weight and decreased rates of breastfeeding.16 The COVID-19 pandemic has disrupted human interactions, from social distancing rules and lockdowns of businesses and social activities to panic buying of grocery staples and increased economic insecurity.1 These changes have been accompanied by a rise in mental health challenges. For example, according to an August 2020 CDC survey, 40.9% of US adults reported at least 1 adverse mental or behavioral health condition, including symptoms of anxiety or depression (30.9%), symptoms of a trauma- and stressor-related disorder related to the pandemic (26.3%), and having started or increased substance use to cope with stress or emotions related to COVID-19 (13.3%).17
COVID-19–related traumas and stressors appear to affect women more than men. A study from China found that compared with men, women had significantly higher levels of self-reported pandemic-related anxiety, depression, and posttraumatic stress symptoms (PTSS).18 This trend has been observed in other parts of the world. A study conducted by the UK Office of National Statistics reported anxiety levels were 24% higher in women vs men as reflected by scores on a self-rated anxiety scale.19
Continue to: Many factors influence...
Many factors influence the disproportionate impact of COVID-19 on women in general, and peripartum women in particular (Box20-26).
Box
Factors that predispose women to increased stress during COVID-19 include an increase in child care burdens brought about by school closures and subsequent virtual schooling.20 Intimate partner violence has spiked globally during COVID-19 restrictions.24 Women also represent the majority of the health care workforce (76%) and often take on informal caregiving roles; both of these roles have seen increased burdens during the pandemic.25 Already encumbered by prepandemic gender pay inequalities, women are filing unemployment claims at a significantly increased rate compared to men.26
For women of childbearing age, the disruption of routine clinical care during COVID-19 has decreased access to reproductive health care, resulting in increases in unintended pregnancies, unsafe abortions, and deaths.20 Another source of stress for pregnant women during COVID-19 is feeling unprepared for birth because of the pandemic, a phenomenon described as “preparedness stress.”21 Visitor restriction policies and quarantines have also caused women in labor to experience birth without their support partners, which is associated with increased posttraumatic stress symptoms.22 These restrictions also may be associated with an increase in women choosing out-of-hospital births despite the increased risk of adverse outcomes.23
Psychiatric diagnoses in peripartum women
Multiple studies and meta-analyses have begun to assess the impact of the COVID-19 pandemic on maternal mental health. One meta-analysis of 8 studies conducted in 5 countries determined that COVID-19 significantly increases the risk of anxiety in women during the peripartum period.27 Results of another meta-analysis of 23 studies with >24,000 participants indicated that the prevalence of anxiety, depression, and insomnia in peripartum women was significantly higher during the pandemic than in pre-pandemic times.28
In an online survey of 4,451 pregnant women in the United States, nearly one-third of respondents reported elevated levels of pandemic-related stress as measured by the newly-developed Pandemic-Related Pregnancy Stress Scale.3 The rates were even higher among women who were already at risk for elevated stress levels, such as those who had survived abuse, those giving birth for the first time, or those experiencing high-risk pregnancies.3 Living in a pandemic “hot spot” also appeared to impact peripartum stress levels.
COVID-19 has adverse effects on women’s mental health specifically during the postpartum period. One study from a center in Italy found a high prevalence of depressive symptoms and PTSS in the postpartum period, with COVID-19–related factors playing an “indirect role” compared with prenatal experiences and other individual factors.2 A British study of mothers of infants age ≤12 months found that traveling for work, the impact of lockdown on food affordability, and having an income of less than £30,000 per year (approximately $41,000) predicted poorer mental health during the pandemic.29 Results of a study from China indicated that more than one-quarter of pregnant and postpartum women experienced depression during the pandemic, and women who worried about infection risk or missing pediatric visits were at increased risk.30
How to mitigate these risks
The increase in pandemic-related mental health concerns in the general population and specifically in peripartum women is a global health care challenge. Investing in mitigation strategies is necessary not only to address the current pandemic, but also to help prepare for the possibility of future traumatic events, such as another global pandemic.
Continue to: For pregnant women...
For pregnant women, ensuring access to outdoor space, increasing participation in healthy activities, and minimizing disruptions to prenatal care can protect against pandemic-related stress.3 Physical activity is an effective treatment for mild to moderate depressive symptoms. Because of the significant decrease in exercise among pregnant women during the pandemic, encouraging safe forms of physical activity such as online fitness classes could improve mental health outcomes for these patients.27 When counseling peripartum women, psychiatrists need to be creative in recommending fitness interventions to target mood symptoms, such as by suggesting virtual or at-home programs.
In an online survey, 118 obstetricians called for increased mental health resources for peripartum women, such as easier access to a helpline, educational videos, and mental health professionals.13 Increased screening for psychiatric disorders throughout the peripartum period can help identify women at greater risk, and advancements in telepsychiatry could help meet the increased need for psychiatric care during COVID-19. Psychiatrists and other mental health clinicians should consider reaching out to their colleagues who specialize in women’s health to establish new partnerships and create teams of multidisciplinary professionals.
Similarly, psychiatrists should familiarize themselves with telehealth services available to peripartum patients who could benefit from such services. Telehealth options can increase women’s access to peripartum care for both medical and psychiatric illnesses. Online options such as women’s support groups, parenting classes, and labor coaching seminars also represent valuable virtual tools to strengthen women’s social supports.
Women who need inpatient treatment for severe peripartum depression or anxiety might be particularly reluctant to receive this care during COVID-19 due to fears of becoming infected and of being separated from their infant and family while hospitalized. Clinicians should remain vigilant in screening peripartum women for mood disorders that might represent a danger to mothers and infants, and not allow concerns about COVID-19 to interfere with recommendations for psychiatric hospitalizations, when necessary. The creation of small, women-only inpatient behavioral units can help address this situation, especially given the possibility of frequent visits with infants and other peripartum support. Investment into such units is critical for supporting peripartum mental health, even in nonpandemic times.
What about vaccination? As of mid-May 2021, no large clinical trials of any COVID-19 vaccine that included pregnant women had been completed. However, 2 small preliminary studies suggested that the mRNA vaccines are safe and effective during pregnancy.31,32 When counseling peripartum patients on the risks and benefits, clinicians need to rely on this evidence, animal trials, and limited data from inadvertent exposures during pregnancy. While every woman will weigh the risks and benefits for her own circumstances, the CDC, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine have all stated that the mRNA vaccines should be offered to pregnant and breastfeeding individuals who are eligible for vaccination.33 Rasmussen et al33 have published a useful resource for clinicians regarding COVID-19 vaccination and pregnant women.
Continue to: Bottom Line
Bottom Line
During the COVID-19 pandemic, peripartum women have experienced increased rates of anxiety, depression, and stress. Psychiatric clinicians can help these patients by remaining vigilant in screening for psychiatric disorders, encouraging them to engage in activities to mitigate COVID-19’s adverse psychological effects, and referring them to care via telehealth and other resources as appropriate.
Related Resources
- Hu YJ, Wake M, Saffery R. Clarifying the sweeping consequences of COVID-19 in pregnant women, newborns, and children with existing cohorts. JAMA Pediatr. 2021; 75(2):117-118. doi: 10.1001/jamapediatrics.2020.2395
- Tomfohr-Madsen LM, Racine N, Giesbrecht GF, et al. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021; 300:113912. doi: 10.1016/j.psychres.2021.113912
1. Chivers BR, Garad RM, Boyle JA, et al. Perinatal distress during COVID-19: thematic analysis of an online parenting forum. J Med Internet Res. 2020;22(9):e22002. doi: 10.2196/22002
2. Ostacoli L, Cosma S, Bevilacqua F, et al. Psychosocial factors associated with postpartum psychological distress during the Covid-19 pandemic: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):703. doi: 10.1186/s12884-020-03399-5
3. Preis H, Mahaffey B, Heiselman C, etal. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020;266:113348. doi: 10.1016/j.socscimed.2020.113348
4. Olson DM, Brémault-Phillips S, King S, et al. Recent Canadian efforts to develop population-level pregnancy intervention studies to mitigate effects of natural disasters and other tragedies. J Dev Orig Health Dis. 2019;10(1):108-114. doi: 10.1017/S2040174418001113
5. Watanabe Z, Iwama N, Nishigori H, et al. Japan Environment & Children’s Study Group. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: the Japan Environment and Children’s Study. J Affect Disord. 2016;190:341-348. doi: 10.1016/j.jad.2015.10.024
6. Xiong X, Harville EW, Mattison DR, et al. Hurricane Katrina experience and the risk of post-traumatic stress disorder and depression among pregnant women. Am J Disaster Med. 2010;5(3):181-187. doi: 10.5055/ajdm.2010.0020
7. Brooks SK, Weston D, Greenberg N. Psychological impact of infectious disease outbreaks on pregnant women: rapid evidence review. Public Health. 2020;189:26-36. doi: 10.1016/j.puhe.2020.09.006
8. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):39. doi: 10.1186/s40001-020-00439-w
9. Qi M, Li X, Liu S, et al. Impact of the COVID-19 epidemic on patterns of pregnant women’s perception of threat and its relationship to mental state: a latent class analysis. PLoS One. 2020;15(10):e0239697. doi: 10.1371/journal.pone.0239697
10. Centers for Disease Control and Prevention. Investigating the impact of COVID-19 during pregnancy. Updated February 4, 2021. Accessed April 29, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19/what-cdc-is-doing.html
11. Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782-1785. doi: 10.1001/jama.2020.19124
12. Ashraf MA, Keshavarz P, Hosseinpour P, et al. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157-168.
13. Nanjundaswamy MH, Shiva L, Desai G, et al. COVID-19-related anxiety and concerns expressed by pregnant and postpartum women-a survey among obstetricians. Arch Womens Ment Health. 2020; 23(6):787-790. doi: 10.1007/s00737-020-01060-w
14. Verdery AM, Smith-Greenaway E, Margolis R, et al. Tracking the reach of COVID-19 kin loss with a bereavement multiplier applied to the United States. Proc Natl Acad Sci U S A. 2020;117(30):17695-17701. doi: 10.1073/pnas.2007476117
15. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494. doi: 10.1001/jama.2020.19632
16. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi: 10.1016/j.jad.2017.07.045
17. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. doi:10.15585/mmwr.mm6932a1
18. Almeida M, Shrestha AD, Stojanac D, et al. The impact of the COVID-19 pandemic on women’s mental health. Arch Womens Ment Health. 2020;23(6):741-748. doi:10.1007/s00737-020-01092-2
19. Office for National Statistics. Personal and economic well-being in Great Britain: May 2020. Published May 4, 2020. Accessed April 23, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/bulletins/personalandeconomicwellbeingintheuk/may2020
20. Kuehn BM. COVID-19 halts reproductive care for millions of women. JAMA. 2020;324(15):1489. doi: 10.1001/jama.2020.19025
21. Preis H, Mahaffey B, Lobel M. Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS). J Psychosom Obstet Gynaecol. 2020;41(3):191-197. doi: 10.1080/0167482X.2020.1801625
22. Hermann A, Fitelson EM, Bergink V. Meeting maternal mental health needs during the COVID-19 pandemic. JAMA Psychiatry. 2020;78(2):123-124. doi: 10.1001/jamapsychiatry.2020.1947
23. Arora KS, Mauch JT, Gibson KS. Labor and delivery visitor policies during the COVID-19 pandemic: balancing risks and benefits. JAMA. 2020;323(24):2468-2469. doi: 10.1001/jama.2020.7563
24. Bradbury-Jones C, Isham L. The pandemic paradox: the consequences of COVID-19 on domestic violence. J Clin Nurs. 2020;29(13-14):2047-2049. doi: 10.1111/jocn.15296
25. Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
26. Scharff X, Ryley S. Breaking: some states show alarming spike in women’s share of unemployment claims. The Fuller Project. Accessed April 23, 2021. https://fullerproject.org/story/some-states-shows-alarming-spike-in-womens-share-of-unemployment-claims/
27. Hessami K, Romanelli C, Chiurazzi M, et al. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;1-8. doi: 10.1080/14767058.2020.1843155
28. Yan H, Ding Y, Guo W. Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front Psychol. 2020;11:617001. doi: 10.3389/fpsyg.2020.617001
29. Dib S, Rougeaux E, Vázquez-Vázquez A, et al. Maternal mental health and coping during the COVID-19 lockdown in the UK: data from the COVID-19 New Mum Study. Int J Gynaecol Obstet. 2020;151(3):407-414. doi: 10.1002/ijgo.13397
30. Bo HX, Yang Y, Chen J, et al. Prevalence of depressive symptoms among Chinese pregnant and postpartum women during the COVID-19 pandemic. Psychosom Med. 2020. doi: 10.1097/PSY.0000000000000904
31. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021. doi:10.1001/jama.2021.7563
32. Shanes ED, Otero S, Mithal LB, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol. 2021. doi: 10.1097/AOG.0000000000004457
33. Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408-414. doi: 10.1097/AOG.0000000000004290
1. Chivers BR, Garad RM, Boyle JA, et al. Perinatal distress during COVID-19: thematic analysis of an online parenting forum. J Med Internet Res. 2020;22(9):e22002. doi: 10.2196/22002
2. Ostacoli L, Cosma S, Bevilacqua F, et al. Psychosocial factors associated with postpartum psychological distress during the Covid-19 pandemic: a cross-sectional study. BMC Pregnancy Childbirth. 2020;20(1):703. doi: 10.1186/s12884-020-03399-5
3. Preis H, Mahaffey B, Heiselman C, etal. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020;266:113348. doi: 10.1016/j.socscimed.2020.113348
4. Olson DM, Brémault-Phillips S, King S, et al. Recent Canadian efforts to develop population-level pregnancy intervention studies to mitigate effects of natural disasters and other tragedies. J Dev Orig Health Dis. 2019;10(1):108-114. doi: 10.1017/S2040174418001113
5. Watanabe Z, Iwama N, Nishigori H, et al. Japan Environment & Children’s Study Group. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: the Japan Environment and Children’s Study. J Affect Disord. 2016;190:341-348. doi: 10.1016/j.jad.2015.10.024
6. Xiong X, Harville EW, Mattison DR, et al. Hurricane Katrina experience and the risk of post-traumatic stress disorder and depression among pregnant women. Am J Disaster Med. 2010;5(3):181-187. doi: 10.5055/ajdm.2010.0020
7. Brooks SK, Weston D, Greenberg N. Psychological impact of infectious disease outbreaks on pregnant women: rapid evidence review. Public Health. 2020;189:26-36. doi: 10.1016/j.puhe.2020.09.006
8. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):39. doi: 10.1186/s40001-020-00439-w
9. Qi M, Li X, Liu S, et al. Impact of the COVID-19 epidemic on patterns of pregnant women’s perception of threat and its relationship to mental state: a latent class analysis. PLoS One. 2020;15(10):e0239697. doi: 10.1371/journal.pone.0239697
10. Centers for Disease Control and Prevention. Investigating the impact of COVID-19 during pregnancy. Updated February 4, 2021. Accessed April 29, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19/what-cdc-is-doing.html
11. Ahlberg M, Neovius M, Saltvedt S, et al. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA. 2020;324(17):1782-1785. doi: 10.1001/jama.2020.19124
12. Ashraf MA, Keshavarz P, Hosseinpour P, et al. Coronavirus disease 2019 (COVID-19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21(3):157-168.
13. Nanjundaswamy MH, Shiva L, Desai G, et al. COVID-19-related anxiety and concerns expressed by pregnant and postpartum women-a survey among obstetricians. Arch Womens Ment Health. 2020; 23(6):787-790. doi: 10.1007/s00737-020-01060-w
14. Verdery AM, Smith-Greenaway E, Margolis R, et al. Tracking the reach of COVID-19 kin loss with a bereavement multiplier applied to the United States. Proc Natl Acad Sci U S A. 2020;117(30):17695-17701. doi: 10.1073/pnas.2007476117
15. Simon NM, Saxe GN, Marmar CR. Mental health disorders related to COVID-19-related deaths. JAMA. 2020;324(15):1493-1494. doi: 10.1001/jama.2020.19632
16. Cook N, Ayers S, Horsch A. Maternal posttraumatic stress disorder during the perinatal period and child outcomes: a systematic review. J Affect Disord. 2018;225:18-31. doi: 10.1016/j.jad.2017.07.045
17. Czeisler MÉ, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic - United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. doi:10.15585/mmwr.mm6932a1
18. Almeida M, Shrestha AD, Stojanac D, et al. The impact of the COVID-19 pandemic on women’s mental health. Arch Womens Ment Health. 2020;23(6):741-748. doi:10.1007/s00737-020-01092-2
19. Office for National Statistics. Personal and economic well-being in Great Britain: May 2020. Published May 4, 2020. Accessed April 23, 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/bulletins/personalandeconomicwellbeingintheuk/may2020
20. Kuehn BM. COVID-19 halts reproductive care for millions of women. JAMA. 2020;324(15):1489. doi: 10.1001/jama.2020.19025
21. Preis H, Mahaffey B, Lobel M. Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS). J Psychosom Obstet Gynaecol. 2020;41(3):191-197. doi: 10.1080/0167482X.2020.1801625
22. Hermann A, Fitelson EM, Bergink V. Meeting maternal mental health needs during the COVID-19 pandemic. JAMA Psychiatry. 2020;78(2):123-124. doi: 10.1001/jamapsychiatry.2020.1947
23. Arora KS, Mauch JT, Gibson KS. Labor and delivery visitor policies during the COVID-19 pandemic: balancing risks and benefits. JAMA. 2020;323(24):2468-2469. doi: 10.1001/jama.2020.7563
24. Bradbury-Jones C, Isham L. The pandemic paradox: the consequences of COVID-19 on domestic violence. J Clin Nurs. 2020;29(13-14):2047-2049. doi: 10.1111/jocn.15296
25. Connor J, Madhavan S, Mokashi M, et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266:113364. doi: 10.1016/j.socscimed.2020.113364
26. Scharff X, Ryley S. Breaking: some states show alarming spike in women’s share of unemployment claims. The Fuller Project. Accessed April 23, 2021. https://fullerproject.org/story/some-states-shows-alarming-spike-in-womens-share-of-unemployment-claims/
27. Hessami K, Romanelli C, Chiurazzi M, et al. COVID-19 pandemic and maternal mental health: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020;1-8. doi: 10.1080/14767058.2020.1843155
28. Yan H, Ding Y, Guo W. Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front Psychol. 2020;11:617001. doi: 10.3389/fpsyg.2020.617001
29. Dib S, Rougeaux E, Vázquez-Vázquez A, et al. Maternal mental health and coping during the COVID-19 lockdown in the UK: data from the COVID-19 New Mum Study. Int J Gynaecol Obstet. 2020;151(3):407-414. doi: 10.1002/ijgo.13397
30. Bo HX, Yang Y, Chen J, et al. Prevalence of depressive symptoms among Chinese pregnant and postpartum women during the COVID-19 pandemic. Psychosom Med. 2020. doi: 10.1097/PSY.0000000000000904
31. Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021. doi:10.1001/jama.2021.7563
32. Shanes ED, Otero S, Mithal LB, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination in pregnancy: measures of immunity and placental histopathology. Obstet Gynecol. 2021. doi: 10.1097/AOG.0000000000004457
33. Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet Gynecol. 2021;137(3):408-414. doi: 10.1097/AOG.0000000000004290
Ketamine for acute catatonia: A case report
Ms. C, age 44, who has major depressive disorder (MDD), anxiety, obsessive-compulsive disorder (OCD) (religious subtype), and has experienced multiple episodes of treatment-resistant catatonia, is brought to the emergency department (ED) by her parents. She has immobility, mutism, rigidity, and decreased oral intake that she has experienced for 1 day.
The night before, Ms. C had been stressed about an upcoming job interview. She cancelled the interview and went to her bedroom. Later that night her parents found her lying on the floor, immobile.
Before the onset of her psychiatric symptoms, Ms. C had been high functioning. She had been an athlete in college and had a career as a school psychologist. The Sidebar summarizes Ms. C’s psychiatric history, which includes similar complex episodes and multiple hospitalizations. She also has a history of hypothyroidism.
SIDEBAR
In 2013, Ms. C experienced severe social stress from both her work as a psychologist and a divorce. She sold all of her possessions and was living in motels and hotels searching for the “truth of God.” In February 2016, she was hospitalized after refusing to eat and self-discontinuing all medications, including her thyroid medications. She was then placed under the conservatorship of her parents.
In July 2017, Ms. C was hospitalized again for refusing to eat or take her medications; this time she also exhibited selective mutism. Catatonia was suspected and she was started on oral lorazepam, 2 mg 3 times a day. Duloxetine and ziprasidone were also trialed but were stopped due to noncompliance and adverse effects. Ms. C showed little improvement on these regimens. In the hospital, IV lorazepam, 4 mg, was trialed with good effect, and she began to respond to questioning. She was transitioned to oral lorazepam, 4 mg 5 times per day, and mirtazapine, 15 mg/d. With this regimen, Ms. C became progressively more interactive; however, she still refused to eat. Throughout her hospitalization, multiple medications were prescribed, including divalproex sodium, memantine, zolpidem, olanzapine, and dextroamphetamine/levoamphetamine, all of which were not effective in stimulating her appetite. Due to malnutrition, Ms. C was placed on total parenteral nutrition. During this time, the highest dose of IM lorazepam was 20 mg/d in divided doses.
Some improvement with ECT
Four months into her hospitalization, Ms. C’s lorazepam was titrated down to 4 mg 4 times a day, and she underwent a trial of electroconvulsive therapy (ECT). Following the fourth ECT session, she displayed significant improvement. Ms. C engaged with her clinicians, displayed bright mood and affect, began eating again, and was able to recount her depressive symptoms following her divorce. At this time, she received a total of 8 ECT treatments and was started on fluoxetine. At the end of January 2018, after 19 days of hospitalization, she was transitioned to a partial hospitalization program (PHP) on a regimen of lorazepam, 2 mg 3 times daily; fluoxetine, 40 mg/d; midodrine, 10 mg 3 times daily; fludrocortisone; and levothyroxine. Her discharge diagnosis was major depressive disorder with psychotic features and catatonia.
Between her first hospitalization and her current presentation to the emergency department (ED), Ms. C presented several times to the ED with similar symptoms of decreased speech, movement, and oral intake. In February 2018, she was hospitalized and responded after 4 sessions of ECT. She returned to work as a substitute teacher and was stable for >1 year on a regimen of lorazepam, olanzapine, and risperidone. In June 2019, her symptoms returned. She was hospitalized and required a nasogastric tube to address malnutrition. She was eventually stabilized on a regimen of risperidone and lorazepam, which she continued as an outpatient until she was hospitalized again in August 2019. During this hospitalization, Ms. C failed to respond to risperidone or lorazepam, up to 2 mg 3 times a day. After several changes to her regimen, she began to respond to olanzapine, 30 mg/d; mirtazapine, 15 mg/d; and lorazepam, 2 mg 3 times a day.
Throughout her hospitalizations, once she became verbal, Ms. C demonstrated hyper-religiosity. She would ask to read the Bible, and state that her purpose was to find the truth of God. As an outpatient, she would compulsively go to church in the middle of the night and read the Bible for hours. A preliminary diagnosis of obsessive-compulsive disorder was made based on her scrupulosity, and mirtazapine was cross-titrated to fluvoxamine prior to discharge.
Shortly after discharge, she was readmitted to a PHP, and did well on fluvoxamine, 100 mg twice a day; olanzapine, 5 mg every night; levothyroxine, 100 mcg/d; and oral lorazepam, 1 mg 4 times a day. Ms. C displayed full mood, appropriate affect, and began working part-time as a substitute teacher. She had begun to interview for full-time jobs before her most recent ED presentation.
In the ED, the psychiatry team evaluates Ms. C. She displays a similar pattern of mutism, immobility, and rigidity as she did upon her initial presentation. Her father reports that she had been compliant with her medications but had not taken them the previous night. Ms. C screens positive for catatonia on the Bush-Francis Catatonia Rating Scale (BFCRS). Her severity score of 10/69 indicates a mild presentation. She is diagnosed with catatonia and is administered IV
Because Ms. C has been hospitalized many times for similar presentations, the treatment team decides to initiate a trial of IV ketamine.
Catatonia can manifest in many different ways in patients with psychiatric illness. If left untreated, it is associated with a high rate of mortality.1 Catatonia often is described along a continuum from retarded/stuporous to excited, and presentations can vary substantially. The physiologic and psychological mechanisms of catatonia are poorly understood.
Traditionally, most patients respond well to low-dose benzodiazepines, with electroconvulsive therapy as a second-line intervention for refractory and malignant cases. However, these interventions are not always successful or readily available.
Continue to: Research into the anesthetic ketamine...
Research into the anesthetic ketamine is gradually expanding, and the use of this agent for treating various psychiatric illnesses, including both unipolar and bipolar depression, has been increasing.2 Empiric evidence suggests ketamine is effective for certain psychiatric disorders, but the mechanism of action remains unclear. Although the evidence base is small, additional cases demonstrating the effectiveness of ketamine in the treatment of acute catatonia might make it a therapeutic option for use by psychiatrists and emergency medicine clinicians.
In this article, we discuss ketamine’s possible role in the treatment of catatonia, possible adverse effects, dosing strategies, and theories about ketamine’s mechanism of action.
Ketamine’s utility in psychiatry
Ketamine is a rapid-acting anesthetic that acts primarily by antagonizing N-methyl-
Previously, ketamine had been thought to induce a catatonic state, which was supported by a neurophysiologic model of catatonia that suggested the condition was caused in part by glutamate hypoactivity at the NMDA receptor.9 However, recent studies have shown that the NMDA receptor antagonists
Ketamine originally was used for sedation, and much of its safety and risk profile has been developed from decades of administration as an anesthetic. Studies have found that ketamine has a large therapeutic window in children and adults.15,16 Moreover, it does not depress the respiratory system. As an anesthetic, ketamine has a rapid onset and a quick resolution, with its sedative and disorienting effects resolving within 30 to 120 minutes.17 Ketamine’s rapid onset of action extends beyond its sedating effects. Trials with the intranasal spray esketamine for treatment-resistant depression have demonstrated an onset antidepressant effects within 2 days.18 This is much faster than that of traditional antidepressants, such as selective serotonin reuptake inhibitors.18 Based on these features, ketamine has the potential to be a useful medication in the emergency psychiatric setting, particularly for acute presentations such as catatonia.
Continue to: Beware of the potential risks
Beware of the potential risks
Although ketamine may be clinically useful, it also carries some risks. Adverse effects associated with ketamine include sedation, dissociation, hallucinations, elevated blood pressure, nausea, increased heart rate, vomiting, dizziness, fatigue, blurred vision, itching, and emesis. Clinicians also should be aware that some patients may use illicit ketamine, either as self-treatment to control depressive symptoms or for recreational purposes. When misused/abused, long-term use of ketamine can cause neurologic damage.19 Studies also have reported rare occurrences of recurrent hallucinations even after discontinuation of ketamine.20 Animal studies have demonstrated addiction and cognitive deficits with repeated use of ketamine in rodents.21 This research has led to concerns that chronic use of ketamine to treat illnesses such as depression might lead to similar long-term adverse outcomes.
Dosing
As a sedative, IV ketamine dosing is generally 1 to 2 mg/kg, and IM ketamine dosing is 3 to 5 mg/kg.16 As an antidepressant, small clinical trials have suggested that the preferred dose of IV ketamine may be 0.5 to 1 mg/kg, with dose-dependent increases in dissociation and blood pressure.21 Studies have also demonstrated that once-daily IV ketamine, 0.5 mg/kg administered over 40 minutes, led to greater improvements in patients with MDD than placebo, whereas once-daily IV ketamine, 0.2 mg/kg, did not.20
CASE CONTINUED
The team begins to treat Ms. C with IV ketamine. Ketamine, 0.2 mg/kg, is used to calculate the initial dose, and a total of 10 mg is administered over 10 minutes. Fifteen minutes after administration, Ms. C is able to move around in her bed, make eye contact, and nod to questions. She has purposeful movements, such as examining her IV line, scratching her head, and repositioning herself in the bed. After a few more minutes, she makes eye contact with her father, and nods to him during conversation. She is able to make a few noises but does not speak.
Later that day, Ms. C is discharged home (in a wheelchair) with her parents, on a medication regimen of fluvoxamine, 100 mg/d; lorazepam, 1 mg 4 times a day; and olanzapine, 5 mg/d. She is scheduled for an outpatient follow-up appointment 5 days later. Her parents are given instructions and several precautions to ensure that Ms. C receives proper nutrition until her appointment. That evening, Ms. C is able to eat voluntarily.
Five days later, Ms. C visits the outpatient psychiatric clinic and is verbal and ambulatory. Her father reports that she has become more verbal. During her follow-up interview, she is observed to be more subdued and less verbal than her baseline, but is vocal and able to voice her understanding of the treatment plan.
Continue to: After 3 months of being stable...
After 3 months of being stable on her outpatient regimen, Ms. C’s catatonic symptoms return, including refusing to eat and mutism. She is administered IV lorazepam, 4 mg, with no response and is admitted to the hospital for placement of a nasogastric feeding tube to address malnutrition. After several days, Ms. C responds to lorazepam, 4 mg every 6 hours. Six days later, after she begins eating and taking her medications voluntarily and the nasogastric tube is removed, Ms. C is discharged to home.
Findings need to be replicated in larger studies
Although some research has indicated that ketamine may be pro-catatonic, Ms. C’s improvement after receiving ketamine suggests that perhaps the situation is more complex.12,22 The exact mechanisms underlying catatonia remain uncertain. Carroll et al9 described 4 theories, and only 1 of them involved glutamate. Additionally, ketamine’s mechanism of action may extend beyond NMDA antagonism. In our case, Ms. C’s low BFCRS score during her most recent visit to the ED suggests she may have had a milder or less typical form of catatonia compared with her previous presentations (Sidebar). However, Ms. C’s clinical improvement after receiving ketamine is noteworthy.
A review of the literature yielded only 1 other case report that described using ketamine to treat catatonia.23 Iserson et al23 reported that their patient’s catatonic symptoms resolved after a total of 12.5 mg of ketamine was administered in 0.03 mg/kg boluses every 3 minutes. Compared with our own protocol, ketamine was administered at a much slower rate in this case, although both total doses of ketamine were comparable and well below the dose used for sedation. Additionally, in Iserson et al,23 lorazepam was not administered before ketamine because lorazepam was not readily available in the treatment setting. In our case, Ms. C may have had a delayed response to the IV lorazepam she received an hour before the ketamine dose; however, she exhibited a distinct clinical improvement 10 to 15 minutes after IV ketamine was administered. Nevertheless, both cases demonstrated rapid resolution of catatonic symptoms following administration of ketamine.
The marked improvement after the ketamine infusion allowed Ms. C to be discharged from the ED the same day, which was never possible after her previous catatonic episodes. Five days after discharge, she was walking, eating, talking, and able to attend to her activities of daily living without any change to her other medications. Moreover, these effects outlasted the duration of ketamine. Ms. C remained stable for 5 months until she destabilized in June 2020. At that time, she did not respond to lorazepam in the ED, needed to be hospitalized, and required a nasogastric feeding tube. Ketamine was not trialed during this presentation, so it remains to be seen if the patient’s response to ketamine was an isolated incident, or whether it could potentially spare her from future hospitalizations.
Bottom Line
In our case report, a woman with a long history of catatonia responded to a single infusion of IV ketamine, and the beneficial effects lasted for months. More research evaluating the efficacy of ketamine is needed to determine if this agent has a place in the treatment of catatonia.
Continue to: Related Resources
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: How to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Iserson KV, Durga D. Catatonia-like syndrome treated with low-dose ketamine. J Emerg Med. 2020;58(5):771-774.
Drug Brand Names
Amantadine • Gocovri
Dextroamphetamine sulfate/levoamphetamine sulfate • Evekeo
Divalproex sodium • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Fludrocortisone • Florinef
Fluvoxamine • Luvox
Ketamine • Ketalar
Levothyroxine • Synthroid
Lorazepam • Ativan
Memantine • Namenda
Mirtazapine • Remeron
Olanzapine • Zyprexa
Risperidone • Risperdal
Ziprasidone • Geodon
Zolpidem • Ambien
1. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
2. Grady SE, Marsh TA, Tenhouse A, et al. Ketamine for the treatment of major depressive disorder and bipolar depression: a review of the literature. Mental Health Clin. 2017;7(1):16-23.
3. KETALAR (ketamine hydrochloride) injection. (n.d.). Accessed April 29, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/016812s043lbl.pdf
4. Williams NR, Schatzberg AF. NMDA antagonist treatment of depression. Curr Opin Neurobiol. 2016;36:112-117.
5. Parashchanka A, Schelfout S, Coppens M. Role of novel drugs in sedation outside the operating room: dexmedetomidine, ketamine and remifentanil. Curr Opin Anaesthesiol. 2014;27(4):442-447.
6. Radvansky BM, Puri S, Sifonios AN, et al. Ketamine—a narrative review of its uses in medicine. Am J Ther. 2016;23(6):e1414-e1426. doi: 10.1097/MJT.0000000000000257
7. O’Brien SL, Pangarkar S, Prager J. The use of ketamine in neuropathic pain. Current Physical Medicine and Rehabilitation Reports. 2014;2(2):128-145.
8. Swainson J, Thomas RK, Archer S, et al. Esketamine for treatment resistant depression. Expert Rev Neurother. 2019;19(10):899-911.
9. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectr. 2000;5(7):26-33.
10. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
11. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry. 1997;62(4):404-406.
12. Denysenko L, Sica N, Penders TM, et al. Catatonia in the medically ill: etiology, diagnosis, and treatment. The Academy of Consultation-Liaison Psychiatry Evidence-Based Medicine Subcommittee Monograph. Ann Clin Psychiatry. 2018;30(2):140-155.
13. Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A. 2014;111(23):8649-8654.
14. de Bartolomeis A, Sarappa C, Buonaguro EF, et al. Different effects of the NMDA receptor antagonists ketamine, MK-801, and memantine on postsynaptic density transcripts and their topography: role of Homer signaling, and implications for novel antipsychotic and pro-cognitive targets in psychosis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:1-12.
15. Green SM, Johnson NE. Ketamine sedation for pediatric procedures: part 2, review and implications. Ann Emerg Med. 1990;19(9):1033-1046.
16. Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283-290.
17. Majidi S, Parna A, Zamani M, et al. Onset and effect duration of intrabuccal space and intramuscular ketamine in pediatrics. Adv Biomed Res. 2018;7:91.
18. Bahr R, Lopez A, Rey JA. Intranasal esketamine (SpravatoTM) for use in treatment-resistant depression in conjunction with an oral antidepressant. P T. 2019;44(6):340-342,344-346,375.
19. Strong CE, Kabbaj M. On the safety of repeated ketamine infusions for the treatment of depression: effects of sex and developmental periods. Neurobiol Stress. 2018;9:166-175.
20. Su TP, Chen MH, Li CT, et al. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology. 2017;42(13):2482-2492.
21. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
22. Wong DH, Jenkins LC. An experimental study of the mechanism of action of ketamine on the central nervous system. Can Anaesth Soc J. 1974;21(1):57-67.
23. Iserson KV, Durga D. Catatonia-like syndrome treated with low-dose ketamine. J Emerg Med. 2020;58(5):771-774.
Ms. C, age 44, who has major depressive disorder (MDD), anxiety, obsessive-compulsive disorder (OCD) (religious subtype), and has experienced multiple episodes of treatment-resistant catatonia, is brought to the emergency department (ED) by her parents. She has immobility, mutism, rigidity, and decreased oral intake that she has experienced for 1 day.
The night before, Ms. C had been stressed about an upcoming job interview. She cancelled the interview and went to her bedroom. Later that night her parents found her lying on the floor, immobile.
Before the onset of her psychiatric symptoms, Ms. C had been high functioning. She had been an athlete in college and had a career as a school psychologist. The Sidebar summarizes Ms. C’s psychiatric history, which includes similar complex episodes and multiple hospitalizations. She also has a history of hypothyroidism.
SIDEBAR
In 2013, Ms. C experienced severe social stress from both her work as a psychologist and a divorce. She sold all of her possessions and was living in motels and hotels searching for the “truth of God.” In February 2016, she was hospitalized after refusing to eat and self-discontinuing all medications, including her thyroid medications. She was then placed under the conservatorship of her parents.
In July 2017, Ms. C was hospitalized again for refusing to eat or take her medications; this time she also exhibited selective mutism. Catatonia was suspected and she was started on oral lorazepam, 2 mg 3 times a day. Duloxetine and ziprasidone were also trialed but were stopped due to noncompliance and adverse effects. Ms. C showed little improvement on these regimens. In the hospital, IV lorazepam, 4 mg, was trialed with good effect, and she began to respond to questioning. She was transitioned to oral lorazepam, 4 mg 5 times per day, and mirtazapine, 15 mg/d. With this regimen, Ms. C became progressively more interactive; however, she still refused to eat. Throughout her hospitalization, multiple medications were prescribed, including divalproex sodium, memantine, zolpidem, olanzapine, and dextroamphetamine/levoamphetamine, all of which were not effective in stimulating her appetite. Due to malnutrition, Ms. C was placed on total parenteral nutrition. During this time, the highest dose of IM lorazepam was 20 mg/d in divided doses.
Some improvement with ECT
Four months into her hospitalization, Ms. C’s lorazepam was titrated down to 4 mg 4 times a day, and she underwent a trial of electroconvulsive therapy (ECT). Following the fourth ECT session, she displayed significant improvement. Ms. C engaged with her clinicians, displayed bright mood and affect, began eating again, and was able to recount her depressive symptoms following her divorce. At this time, she received a total of 8 ECT treatments and was started on fluoxetine. At the end of January 2018, after 19 days of hospitalization, she was transitioned to a partial hospitalization program (PHP) on a regimen of lorazepam, 2 mg 3 times daily; fluoxetine, 40 mg/d; midodrine, 10 mg 3 times daily; fludrocortisone; and levothyroxine. Her discharge diagnosis was major depressive disorder with psychotic features and catatonia.
Between her first hospitalization and her current presentation to the emergency department (ED), Ms. C presented several times to the ED with similar symptoms of decreased speech, movement, and oral intake. In February 2018, she was hospitalized and responded after 4 sessions of ECT. She returned to work as a substitute teacher and was stable for >1 year on a regimen of lorazepam, olanzapine, and risperidone. In June 2019, her symptoms returned. She was hospitalized and required a nasogastric tube to address malnutrition. She was eventually stabilized on a regimen of risperidone and lorazepam, which she continued as an outpatient until she was hospitalized again in August 2019. During this hospitalization, Ms. C failed to respond to risperidone or lorazepam, up to 2 mg 3 times a day. After several changes to her regimen, she began to respond to olanzapine, 30 mg/d; mirtazapine, 15 mg/d; and lorazepam, 2 mg 3 times a day.
Throughout her hospitalizations, once she became verbal, Ms. C demonstrated hyper-religiosity. She would ask to read the Bible, and state that her purpose was to find the truth of God. As an outpatient, she would compulsively go to church in the middle of the night and read the Bible for hours. A preliminary diagnosis of obsessive-compulsive disorder was made based on her scrupulosity, and mirtazapine was cross-titrated to fluvoxamine prior to discharge.
Shortly after discharge, she was readmitted to a PHP, and did well on fluvoxamine, 100 mg twice a day; olanzapine, 5 mg every night; levothyroxine, 100 mcg/d; and oral lorazepam, 1 mg 4 times a day. Ms. C displayed full mood, appropriate affect, and began working part-time as a substitute teacher. She had begun to interview for full-time jobs before her most recent ED presentation.
In the ED, the psychiatry team evaluates Ms. C. She displays a similar pattern of mutism, immobility, and rigidity as she did upon her initial presentation. Her father reports that she had been compliant with her medications but had not taken them the previous night. Ms. C screens positive for catatonia on the Bush-Francis Catatonia Rating Scale (BFCRS). Her severity score of 10/69 indicates a mild presentation. She is diagnosed with catatonia and is administered IV
Because Ms. C has been hospitalized many times for similar presentations, the treatment team decides to initiate a trial of IV ketamine.
Catatonia can manifest in many different ways in patients with psychiatric illness. If left untreated, it is associated with a high rate of mortality.1 Catatonia often is described along a continuum from retarded/stuporous to excited, and presentations can vary substantially. The physiologic and psychological mechanisms of catatonia are poorly understood.
Traditionally, most patients respond well to low-dose benzodiazepines, with electroconvulsive therapy as a second-line intervention for refractory and malignant cases. However, these interventions are not always successful or readily available.
Continue to: Research into the anesthetic ketamine...
Research into the anesthetic ketamine is gradually expanding, and the use of this agent for treating various psychiatric illnesses, including both unipolar and bipolar depression, has been increasing.2 Empiric evidence suggests ketamine is effective for certain psychiatric disorders, but the mechanism of action remains unclear. Although the evidence base is small, additional cases demonstrating the effectiveness of ketamine in the treatment of acute catatonia might make it a therapeutic option for use by psychiatrists and emergency medicine clinicians.
In this article, we discuss ketamine’s possible role in the treatment of catatonia, possible adverse effects, dosing strategies, and theories about ketamine’s mechanism of action.
Ketamine’s utility in psychiatry
Ketamine is a rapid-acting anesthetic that acts primarily by antagonizing N-methyl-
Previously, ketamine had been thought to induce a catatonic state, which was supported by a neurophysiologic model of catatonia that suggested the condition was caused in part by glutamate hypoactivity at the NMDA receptor.9 However, recent studies have shown that the NMDA receptor antagonists
Ketamine originally was used for sedation, and much of its safety and risk profile has been developed from decades of administration as an anesthetic. Studies have found that ketamine has a large therapeutic window in children and adults.15,16 Moreover, it does not depress the respiratory system. As an anesthetic, ketamine has a rapid onset and a quick resolution, with its sedative and disorienting effects resolving within 30 to 120 minutes.17 Ketamine’s rapid onset of action extends beyond its sedating effects. Trials with the intranasal spray esketamine for treatment-resistant depression have demonstrated an onset antidepressant effects within 2 days.18 This is much faster than that of traditional antidepressants, such as selective serotonin reuptake inhibitors.18 Based on these features, ketamine has the potential to be a useful medication in the emergency psychiatric setting, particularly for acute presentations such as catatonia.
Continue to: Beware of the potential risks
Beware of the potential risks
Although ketamine may be clinically useful, it also carries some risks. Adverse effects associated with ketamine include sedation, dissociation, hallucinations, elevated blood pressure, nausea, increased heart rate, vomiting, dizziness, fatigue, blurred vision, itching, and emesis. Clinicians also should be aware that some patients may use illicit ketamine, either as self-treatment to control depressive symptoms or for recreational purposes. When misused/abused, long-term use of ketamine can cause neurologic damage.19 Studies also have reported rare occurrences of recurrent hallucinations even after discontinuation of ketamine.20 Animal studies have demonstrated addiction and cognitive deficits with repeated use of ketamine in rodents.21 This research has led to concerns that chronic use of ketamine to treat illnesses such as depression might lead to similar long-term adverse outcomes.
Dosing
As a sedative, IV ketamine dosing is generally 1 to 2 mg/kg, and IM ketamine dosing is 3 to 5 mg/kg.16 As an antidepressant, small clinical trials have suggested that the preferred dose of IV ketamine may be 0.5 to 1 mg/kg, with dose-dependent increases in dissociation and blood pressure.21 Studies have also demonstrated that once-daily IV ketamine, 0.5 mg/kg administered over 40 minutes, led to greater improvements in patients with MDD than placebo, whereas once-daily IV ketamine, 0.2 mg/kg, did not.20
CASE CONTINUED
The team begins to treat Ms. C with IV ketamine. Ketamine, 0.2 mg/kg, is used to calculate the initial dose, and a total of 10 mg is administered over 10 minutes. Fifteen minutes after administration, Ms. C is able to move around in her bed, make eye contact, and nod to questions. She has purposeful movements, such as examining her IV line, scratching her head, and repositioning herself in the bed. After a few more minutes, she makes eye contact with her father, and nods to him during conversation. She is able to make a few noises but does not speak.
Later that day, Ms. C is discharged home (in a wheelchair) with her parents, on a medication regimen of fluvoxamine, 100 mg/d; lorazepam, 1 mg 4 times a day; and olanzapine, 5 mg/d. She is scheduled for an outpatient follow-up appointment 5 days later. Her parents are given instructions and several precautions to ensure that Ms. C receives proper nutrition until her appointment. That evening, Ms. C is able to eat voluntarily.
Five days later, Ms. C visits the outpatient psychiatric clinic and is verbal and ambulatory. Her father reports that she has become more verbal. During her follow-up interview, she is observed to be more subdued and less verbal than her baseline, but is vocal and able to voice her understanding of the treatment plan.
Continue to: After 3 months of being stable...
After 3 months of being stable on her outpatient regimen, Ms. C’s catatonic symptoms return, including refusing to eat and mutism. She is administered IV lorazepam, 4 mg, with no response and is admitted to the hospital for placement of a nasogastric feeding tube to address malnutrition. After several days, Ms. C responds to lorazepam, 4 mg every 6 hours. Six days later, after she begins eating and taking her medications voluntarily and the nasogastric tube is removed, Ms. C is discharged to home.
Findings need to be replicated in larger studies
Although some research has indicated that ketamine may be pro-catatonic, Ms. C’s improvement after receiving ketamine suggests that perhaps the situation is more complex.12,22 The exact mechanisms underlying catatonia remain uncertain. Carroll et al9 described 4 theories, and only 1 of them involved glutamate. Additionally, ketamine’s mechanism of action may extend beyond NMDA antagonism. In our case, Ms. C’s low BFCRS score during her most recent visit to the ED suggests she may have had a milder or less typical form of catatonia compared with her previous presentations (Sidebar). However, Ms. C’s clinical improvement after receiving ketamine is noteworthy.
A review of the literature yielded only 1 other case report that described using ketamine to treat catatonia.23 Iserson et al23 reported that their patient’s catatonic symptoms resolved after a total of 12.5 mg of ketamine was administered in 0.03 mg/kg boluses every 3 minutes. Compared with our own protocol, ketamine was administered at a much slower rate in this case, although both total doses of ketamine were comparable and well below the dose used for sedation. Additionally, in Iserson et al,23 lorazepam was not administered before ketamine because lorazepam was not readily available in the treatment setting. In our case, Ms. C may have had a delayed response to the IV lorazepam she received an hour before the ketamine dose; however, she exhibited a distinct clinical improvement 10 to 15 minutes after IV ketamine was administered. Nevertheless, both cases demonstrated rapid resolution of catatonic symptoms following administration of ketamine.
The marked improvement after the ketamine infusion allowed Ms. C to be discharged from the ED the same day, which was never possible after her previous catatonic episodes. Five days after discharge, she was walking, eating, talking, and able to attend to her activities of daily living without any change to her other medications. Moreover, these effects outlasted the duration of ketamine. Ms. C remained stable for 5 months until she destabilized in June 2020. At that time, she did not respond to lorazepam in the ED, needed to be hospitalized, and required a nasogastric feeding tube. Ketamine was not trialed during this presentation, so it remains to be seen if the patient’s response to ketamine was an isolated incident, or whether it could potentially spare her from future hospitalizations.
Bottom Line
In our case report, a woman with a long history of catatonia responded to a single infusion of IV ketamine, and the beneficial effects lasted for months. More research evaluating the efficacy of ketamine is needed to determine if this agent has a place in the treatment of catatonia.
Continue to: Related Resources
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: How to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Iserson KV, Durga D. Catatonia-like syndrome treated with low-dose ketamine. J Emerg Med. 2020;58(5):771-774.
Drug Brand Names
Amantadine • Gocovri
Dextroamphetamine sulfate/levoamphetamine sulfate • Evekeo
Divalproex sodium • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Fludrocortisone • Florinef
Fluvoxamine • Luvox
Ketamine • Ketalar
Levothyroxine • Synthroid
Lorazepam • Ativan
Memantine • Namenda
Mirtazapine • Remeron
Olanzapine • Zyprexa
Risperidone • Risperdal
Ziprasidone • Geodon
Zolpidem • Ambien
Ms. C, age 44, who has major depressive disorder (MDD), anxiety, obsessive-compulsive disorder (OCD) (religious subtype), and has experienced multiple episodes of treatment-resistant catatonia, is brought to the emergency department (ED) by her parents. She has immobility, mutism, rigidity, and decreased oral intake that she has experienced for 1 day.
The night before, Ms. C had been stressed about an upcoming job interview. She cancelled the interview and went to her bedroom. Later that night her parents found her lying on the floor, immobile.
Before the onset of her psychiatric symptoms, Ms. C had been high functioning. She had been an athlete in college and had a career as a school psychologist. The Sidebar summarizes Ms. C’s psychiatric history, which includes similar complex episodes and multiple hospitalizations. She also has a history of hypothyroidism.
SIDEBAR
In 2013, Ms. C experienced severe social stress from both her work as a psychologist and a divorce. She sold all of her possessions and was living in motels and hotels searching for the “truth of God.” In February 2016, she was hospitalized after refusing to eat and self-discontinuing all medications, including her thyroid medications. She was then placed under the conservatorship of her parents.
In July 2017, Ms. C was hospitalized again for refusing to eat or take her medications; this time she also exhibited selective mutism. Catatonia was suspected and she was started on oral lorazepam, 2 mg 3 times a day. Duloxetine and ziprasidone were also trialed but were stopped due to noncompliance and adverse effects. Ms. C showed little improvement on these regimens. In the hospital, IV lorazepam, 4 mg, was trialed with good effect, and she began to respond to questioning. She was transitioned to oral lorazepam, 4 mg 5 times per day, and mirtazapine, 15 mg/d. With this regimen, Ms. C became progressively more interactive; however, she still refused to eat. Throughout her hospitalization, multiple medications were prescribed, including divalproex sodium, memantine, zolpidem, olanzapine, and dextroamphetamine/levoamphetamine, all of which were not effective in stimulating her appetite. Due to malnutrition, Ms. C was placed on total parenteral nutrition. During this time, the highest dose of IM lorazepam was 20 mg/d in divided doses.
Some improvement with ECT
Four months into her hospitalization, Ms. C’s lorazepam was titrated down to 4 mg 4 times a day, and she underwent a trial of electroconvulsive therapy (ECT). Following the fourth ECT session, she displayed significant improvement. Ms. C engaged with her clinicians, displayed bright mood and affect, began eating again, and was able to recount her depressive symptoms following her divorce. At this time, she received a total of 8 ECT treatments and was started on fluoxetine. At the end of January 2018, after 19 days of hospitalization, she was transitioned to a partial hospitalization program (PHP) on a regimen of lorazepam, 2 mg 3 times daily; fluoxetine, 40 mg/d; midodrine, 10 mg 3 times daily; fludrocortisone; and levothyroxine. Her discharge diagnosis was major depressive disorder with psychotic features and catatonia.
Between her first hospitalization and her current presentation to the emergency department (ED), Ms. C presented several times to the ED with similar symptoms of decreased speech, movement, and oral intake. In February 2018, she was hospitalized and responded after 4 sessions of ECT. She returned to work as a substitute teacher and was stable for >1 year on a regimen of lorazepam, olanzapine, and risperidone. In June 2019, her symptoms returned. She was hospitalized and required a nasogastric tube to address malnutrition. She was eventually stabilized on a regimen of risperidone and lorazepam, which she continued as an outpatient until she was hospitalized again in August 2019. During this hospitalization, Ms. C failed to respond to risperidone or lorazepam, up to 2 mg 3 times a day. After several changes to her regimen, she began to respond to olanzapine, 30 mg/d; mirtazapine, 15 mg/d; and lorazepam, 2 mg 3 times a day.
Throughout her hospitalizations, once she became verbal, Ms. C demonstrated hyper-religiosity. She would ask to read the Bible, and state that her purpose was to find the truth of God. As an outpatient, she would compulsively go to church in the middle of the night and read the Bible for hours. A preliminary diagnosis of obsessive-compulsive disorder was made based on her scrupulosity, and mirtazapine was cross-titrated to fluvoxamine prior to discharge.
Shortly after discharge, she was readmitted to a PHP, and did well on fluvoxamine, 100 mg twice a day; olanzapine, 5 mg every night; levothyroxine, 100 mcg/d; and oral lorazepam, 1 mg 4 times a day. Ms. C displayed full mood, appropriate affect, and began working part-time as a substitute teacher. She had begun to interview for full-time jobs before her most recent ED presentation.
In the ED, the psychiatry team evaluates Ms. C. She displays a similar pattern of mutism, immobility, and rigidity as she did upon her initial presentation. Her father reports that she had been compliant with her medications but had not taken them the previous night. Ms. C screens positive for catatonia on the Bush-Francis Catatonia Rating Scale (BFCRS). Her severity score of 10/69 indicates a mild presentation. She is diagnosed with catatonia and is administered IV
Because Ms. C has been hospitalized many times for similar presentations, the treatment team decides to initiate a trial of IV ketamine.
Catatonia can manifest in many different ways in patients with psychiatric illness. If left untreated, it is associated with a high rate of mortality.1 Catatonia often is described along a continuum from retarded/stuporous to excited, and presentations can vary substantially. The physiologic and psychological mechanisms of catatonia are poorly understood.
Traditionally, most patients respond well to low-dose benzodiazepines, with electroconvulsive therapy as a second-line intervention for refractory and malignant cases. However, these interventions are not always successful or readily available.
Continue to: Research into the anesthetic ketamine...
Research into the anesthetic ketamine is gradually expanding, and the use of this agent for treating various psychiatric illnesses, including both unipolar and bipolar depression, has been increasing.2 Empiric evidence suggests ketamine is effective for certain psychiatric disorders, but the mechanism of action remains unclear. Although the evidence base is small, additional cases demonstrating the effectiveness of ketamine in the treatment of acute catatonia might make it a therapeutic option for use by psychiatrists and emergency medicine clinicians.
In this article, we discuss ketamine’s possible role in the treatment of catatonia, possible adverse effects, dosing strategies, and theories about ketamine’s mechanism of action.
Ketamine’s utility in psychiatry
Ketamine is a rapid-acting anesthetic that acts primarily by antagonizing N-methyl-
Previously, ketamine had been thought to induce a catatonic state, which was supported by a neurophysiologic model of catatonia that suggested the condition was caused in part by glutamate hypoactivity at the NMDA receptor.9 However, recent studies have shown that the NMDA receptor antagonists
Ketamine originally was used for sedation, and much of its safety and risk profile has been developed from decades of administration as an anesthetic. Studies have found that ketamine has a large therapeutic window in children and adults.15,16 Moreover, it does not depress the respiratory system. As an anesthetic, ketamine has a rapid onset and a quick resolution, with its sedative and disorienting effects resolving within 30 to 120 minutes.17 Ketamine’s rapid onset of action extends beyond its sedating effects. Trials with the intranasal spray esketamine for treatment-resistant depression have demonstrated an onset antidepressant effects within 2 days.18 This is much faster than that of traditional antidepressants, such as selective serotonin reuptake inhibitors.18 Based on these features, ketamine has the potential to be a useful medication in the emergency psychiatric setting, particularly for acute presentations such as catatonia.
Continue to: Beware of the potential risks
Beware of the potential risks
Although ketamine may be clinically useful, it also carries some risks. Adverse effects associated with ketamine include sedation, dissociation, hallucinations, elevated blood pressure, nausea, increased heart rate, vomiting, dizziness, fatigue, blurred vision, itching, and emesis. Clinicians also should be aware that some patients may use illicit ketamine, either as self-treatment to control depressive symptoms or for recreational purposes. When misused/abused, long-term use of ketamine can cause neurologic damage.19 Studies also have reported rare occurrences of recurrent hallucinations even after discontinuation of ketamine.20 Animal studies have demonstrated addiction and cognitive deficits with repeated use of ketamine in rodents.21 This research has led to concerns that chronic use of ketamine to treat illnesses such as depression might lead to similar long-term adverse outcomes.
Dosing
As a sedative, IV ketamine dosing is generally 1 to 2 mg/kg, and IM ketamine dosing is 3 to 5 mg/kg.16 As an antidepressant, small clinical trials have suggested that the preferred dose of IV ketamine may be 0.5 to 1 mg/kg, with dose-dependent increases in dissociation and blood pressure.21 Studies have also demonstrated that once-daily IV ketamine, 0.5 mg/kg administered over 40 minutes, led to greater improvements in patients with MDD than placebo, whereas once-daily IV ketamine, 0.2 mg/kg, did not.20
CASE CONTINUED
The team begins to treat Ms. C with IV ketamine. Ketamine, 0.2 mg/kg, is used to calculate the initial dose, and a total of 10 mg is administered over 10 minutes. Fifteen minutes after administration, Ms. C is able to move around in her bed, make eye contact, and nod to questions. She has purposeful movements, such as examining her IV line, scratching her head, and repositioning herself in the bed. After a few more minutes, she makes eye contact with her father, and nods to him during conversation. She is able to make a few noises but does not speak.
Later that day, Ms. C is discharged home (in a wheelchair) with her parents, on a medication regimen of fluvoxamine, 100 mg/d; lorazepam, 1 mg 4 times a day; and olanzapine, 5 mg/d. She is scheduled for an outpatient follow-up appointment 5 days later. Her parents are given instructions and several precautions to ensure that Ms. C receives proper nutrition until her appointment. That evening, Ms. C is able to eat voluntarily.
Five days later, Ms. C visits the outpatient psychiatric clinic and is verbal and ambulatory. Her father reports that she has become more verbal. During her follow-up interview, she is observed to be more subdued and less verbal than her baseline, but is vocal and able to voice her understanding of the treatment plan.
Continue to: After 3 months of being stable...
After 3 months of being stable on her outpatient regimen, Ms. C’s catatonic symptoms return, including refusing to eat and mutism. She is administered IV lorazepam, 4 mg, with no response and is admitted to the hospital for placement of a nasogastric feeding tube to address malnutrition. After several days, Ms. C responds to lorazepam, 4 mg every 6 hours. Six days later, after she begins eating and taking her medications voluntarily and the nasogastric tube is removed, Ms. C is discharged to home.
Findings need to be replicated in larger studies
Although some research has indicated that ketamine may be pro-catatonic, Ms. C’s improvement after receiving ketamine suggests that perhaps the situation is more complex.12,22 The exact mechanisms underlying catatonia remain uncertain. Carroll et al9 described 4 theories, and only 1 of them involved glutamate. Additionally, ketamine’s mechanism of action may extend beyond NMDA antagonism. In our case, Ms. C’s low BFCRS score during her most recent visit to the ED suggests she may have had a milder or less typical form of catatonia compared with her previous presentations (Sidebar). However, Ms. C’s clinical improvement after receiving ketamine is noteworthy.
A review of the literature yielded only 1 other case report that described using ketamine to treat catatonia.23 Iserson et al23 reported that their patient’s catatonic symptoms resolved after a total of 12.5 mg of ketamine was administered in 0.03 mg/kg boluses every 3 minutes. Compared with our own protocol, ketamine was administered at a much slower rate in this case, although both total doses of ketamine were comparable and well below the dose used for sedation. Additionally, in Iserson et al,23 lorazepam was not administered before ketamine because lorazepam was not readily available in the treatment setting. In our case, Ms. C may have had a delayed response to the IV lorazepam she received an hour before the ketamine dose; however, she exhibited a distinct clinical improvement 10 to 15 minutes after IV ketamine was administered. Nevertheless, both cases demonstrated rapid resolution of catatonic symptoms following administration of ketamine.
The marked improvement after the ketamine infusion allowed Ms. C to be discharged from the ED the same day, which was never possible after her previous catatonic episodes. Five days after discharge, she was walking, eating, talking, and able to attend to her activities of daily living without any change to her other medications. Moreover, these effects outlasted the duration of ketamine. Ms. C remained stable for 5 months until she destabilized in June 2020. At that time, she did not respond to lorazepam in the ED, needed to be hospitalized, and required a nasogastric feeding tube. Ketamine was not trialed during this presentation, so it remains to be seen if the patient’s response to ketamine was an isolated incident, or whether it could potentially spare her from future hospitalizations.
Bottom Line
In our case report, a woman with a long history of catatonia responded to a single infusion of IV ketamine, and the beneficial effects lasted for months. More research evaluating the efficacy of ketamine is needed to determine if this agent has a place in the treatment of catatonia.
Continue to: Related Resources
Related Resources
- Dubovsky SL, Dubovsky AN. Catatonia: How to identify and treat it. Current Psychiatry. 2018;17(8):16-26.
- Iserson KV, Durga D. Catatonia-like syndrome treated with low-dose ketamine. J Emerg Med. 2020;58(5):771-774.
Drug Brand Names
Amantadine • Gocovri
Dextroamphetamine sulfate/levoamphetamine sulfate • Evekeo
Divalproex sodium • Depakote
Duloxetine • Cymbalta
Esketamine • Spravato
Fluoxetine • Prozac
Fludrocortisone • Florinef
Fluvoxamine • Luvox
Ketamine • Ketalar
Levothyroxine • Synthroid
Lorazepam • Ativan
Memantine • Namenda
Mirtazapine • Remeron
Olanzapine • Zyprexa
Risperidone • Risperdal
Ziprasidone • Geodon
Zolpidem • Ambien
1. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
2. Grady SE, Marsh TA, Tenhouse A, et al. Ketamine for the treatment of major depressive disorder and bipolar depression: a review of the literature. Mental Health Clin. 2017;7(1):16-23.
3. KETALAR (ketamine hydrochloride) injection. (n.d.). Accessed April 29, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/016812s043lbl.pdf
4. Williams NR, Schatzberg AF. NMDA antagonist treatment of depression. Curr Opin Neurobiol. 2016;36:112-117.
5. Parashchanka A, Schelfout S, Coppens M. Role of novel drugs in sedation outside the operating room: dexmedetomidine, ketamine and remifentanil. Curr Opin Anaesthesiol. 2014;27(4):442-447.
6. Radvansky BM, Puri S, Sifonios AN, et al. Ketamine—a narrative review of its uses in medicine. Am J Ther. 2016;23(6):e1414-e1426. doi: 10.1097/MJT.0000000000000257
7. O’Brien SL, Pangarkar S, Prager J. The use of ketamine in neuropathic pain. Current Physical Medicine and Rehabilitation Reports. 2014;2(2):128-145.
8. Swainson J, Thomas RK, Archer S, et al. Esketamine for treatment resistant depression. Expert Rev Neurother. 2019;19(10):899-911.
9. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectr. 2000;5(7):26-33.
10. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
11. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry. 1997;62(4):404-406.
12. Denysenko L, Sica N, Penders TM, et al. Catatonia in the medically ill: etiology, diagnosis, and treatment. The Academy of Consultation-Liaison Psychiatry Evidence-Based Medicine Subcommittee Monograph. Ann Clin Psychiatry. 2018;30(2):140-155.
13. Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A. 2014;111(23):8649-8654.
14. de Bartolomeis A, Sarappa C, Buonaguro EF, et al. Different effects of the NMDA receptor antagonists ketamine, MK-801, and memantine on postsynaptic density transcripts and their topography: role of Homer signaling, and implications for novel antipsychotic and pro-cognitive targets in psychosis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:1-12.
15. Green SM, Johnson NE. Ketamine sedation for pediatric procedures: part 2, review and implications. Ann Emerg Med. 1990;19(9):1033-1046.
16. Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283-290.
17. Majidi S, Parna A, Zamani M, et al. Onset and effect duration of intrabuccal space and intramuscular ketamine in pediatrics. Adv Biomed Res. 2018;7:91.
18. Bahr R, Lopez A, Rey JA. Intranasal esketamine (SpravatoTM) for use in treatment-resistant depression in conjunction with an oral antidepressant. P T. 2019;44(6):340-342,344-346,375.
19. Strong CE, Kabbaj M. On the safety of repeated ketamine infusions for the treatment of depression: effects of sex and developmental periods. Neurobiol Stress. 2018;9:166-175.
20. Su TP, Chen MH, Li CT, et al. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology. 2017;42(13):2482-2492.
21. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
22. Wong DH, Jenkins LC. An experimental study of the mechanism of action of ketamine on the central nervous system. Can Anaesth Soc J. 1974;21(1):57-67.
23. Iserson KV, Durga D. Catatonia-like syndrome treated with low-dose ketamine. J Emerg Med. 2020;58(5):771-774.
1. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391-398.
2. Grady SE, Marsh TA, Tenhouse A, et al. Ketamine for the treatment of major depressive disorder and bipolar depression: a review of the literature. Mental Health Clin. 2017;7(1):16-23.
3. KETALAR (ketamine hydrochloride) injection. (n.d.). Accessed April 29, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/016812s043lbl.pdf
4. Williams NR, Schatzberg AF. NMDA antagonist treatment of depression. Curr Opin Neurobiol. 2016;36:112-117.
5. Parashchanka A, Schelfout S, Coppens M. Role of novel drugs in sedation outside the operating room: dexmedetomidine, ketamine and remifentanil. Curr Opin Anaesthesiol. 2014;27(4):442-447.
6. Radvansky BM, Puri S, Sifonios AN, et al. Ketamine—a narrative review of its uses in medicine. Am J Ther. 2016;23(6):e1414-e1426. doi: 10.1097/MJT.0000000000000257
7. O’Brien SL, Pangarkar S, Prager J. The use of ketamine in neuropathic pain. Current Physical Medicine and Rehabilitation Reports. 2014;2(2):128-145.
8. Swainson J, Thomas RK, Archer S, et al. Esketamine for treatment resistant depression. Expert Rev Neurother. 2019;19(10):899-911.
9. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectr. 2000;5(7):26-33.
10. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry Clin Neurosci. 2007;19(4):406-412.
11. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry. 1997;62(4):404-406.
12. Denysenko L, Sica N, Penders TM, et al. Catatonia in the medically ill: etiology, diagnosis, and treatment. The Academy of Consultation-Liaison Psychiatry Evidence-Based Medicine Subcommittee Monograph. Ann Clin Psychiatry. 2018;30(2):140-155.
13. Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A. 2014;111(23):8649-8654.
14. de Bartolomeis A, Sarappa C, Buonaguro EF, et al. Different effects of the NMDA receptor antagonists ketamine, MK-801, and memantine on postsynaptic density transcripts and their topography: role of Homer signaling, and implications for novel antipsychotic and pro-cognitive targets in psychosis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:1-12.
15. Green SM, Johnson NE. Ketamine sedation for pediatric procedures: part 2, review and implications. Ann Emerg Med. 1990;19(9):1033-1046.
16. Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283-290.
17. Majidi S, Parna A, Zamani M, et al. Onset and effect duration of intrabuccal space and intramuscular ketamine in pediatrics. Adv Biomed Res. 2018;7:91.
18. Bahr R, Lopez A, Rey JA. Intranasal esketamine (SpravatoTM) for use in treatment-resistant depression in conjunction with an oral antidepressant. P T. 2019;44(6):340-342,344-346,375.
19. Strong CE, Kabbaj M. On the safety of repeated ketamine infusions for the treatment of depression: effects of sex and developmental periods. Neurobiol Stress. 2018;9:166-175.
20. Su TP, Chen MH, Li CT, et al. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology. 2017;42(13):2482-2492.
21. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
22. Wong DH, Jenkins LC. An experimental study of the mechanism of action of ketamine on the central nervous system. Can Anaesth Soc J. 1974;21(1):57-67.
23. Iserson KV, Durga D. Catatonia-like syndrome treated with low-dose ketamine. J Emerg Med. 2020;58(5):771-774.
Inequality in access to technology for telepsychiatry
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The COVID-19 pandemic has brought to the fore inequalities in health care.1 In a letter recently published in the American Journal of Psychiatry, Nadkarni et al2 addressed the reality that there is not equal access to quality care (in this case, telepsychiatry). They reported challenges in converting their psychiatry ambulatory care center to a virtual platform at Brigham and Women’s Hospital, which is affiliated with Harvard Medical School.
Previously, I had reported in
I repeated the survey approximately 3 months later. Preliminary data of these 125 total patients showed a slightly higher percentage (12.6%) had video conferencing sessions.
Factors associated with limited access to technology
Similar to what was reported by Nadkarni et al,2 in our extremely vulnerable population, socioeconomic factors affect access. Our patients are low income, and often do not own computers or smart phones. Nearly all our patients receive medical assistance and/or Medicare. Our patients are more likely to be members of a racial minority group—4 times the national average. Our patients are older.4 Patient age varies from 16 to 83 years; the mean age is 54, and the median age is 56. Educational level is low. Nearly all of our patients who participate in video conferencing sessions are female. Approximately 15 of our patients have comorbid intellectual and developmental disabilities diagnoses, and at least that many have subsyndromal symptoms. Constantino et al5 commented on the multiple negative consequences of the COVID-19 pandemic on individuals with intellectual and developmental disabilities, including “frank disparities in access” to technology as well as gaps left by relying exclusively on telehealth.
Among our patients, being low income, a member of a racial minority group, older, less educated, male, and developmentally and/or intellectually disabled are risk factors for less access to video conferencing.3 Nadkarni et al2 also noted less broadband access for rural residents and less access and lack of digital health literacy in patients with limited English proficiency.
As Nadkarni et al2 suggested, we did contact our legislators, and emergency rules are continuing. For now, we are managing fiscally. Although that certainly is important, it does not address the issue of inequality.
Continue to: With this information...
With this information, we are strongly encouraging our patients to participate in video conferencing sessions. We suspect that for some patients, the possibility of them participating in video conferencing sessions is greater than they have acknowledged. We are stepping up education and support, both informally through the patient’s family and friends, and more formally through case managers who “lend” patients a device during home visits.
In summary, this inequality in access to the technology needed for telepsychiatry will loom even more prominently as we all move forward, both clinically and in policymaking.
1. Geller J. Structural racism in American psychiatry and APA: part 1. Psychiatric News. July 3, 2020. Accessed May 10, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2020.7a18
2. Nadkarni A, Hasler V, AhnAllen CG , et al. Telehealth during COVID-19—does everyone have equal access? Am J Psychiatry. 2020;177(11):1093-1094.
3. Storch, DD. Treating patients during COVID-19: what I observed. Current Psychiatry . 2020;19(10):e5. doi:10.12788/cp.0054
4. Buis L, Singer D, Solway E, et al. Telehealth use among older adults before and during COVID-19. University of Michigan National Poll on Healthy Aging. Published August 2020. Accessed May 10, 2021. https://www.healthyagingpoll.org/report/telehealth-use-among-older-adults-and-during-covid-19
5. Constantino JN, Sahin M, Piven J, et al. The impact of COVID-19 on individuals with intellectual and developmental disabilities: clinical and scientific priorities. Am J Psychiatry. 2020;177(11):1091-1093.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The COVID-19 pandemic has brought to the fore inequalities in health care.1 In a letter recently published in the American Journal of Psychiatry, Nadkarni et al2 addressed the reality that there is not equal access to quality care (in this case, telepsychiatry). They reported challenges in converting their psychiatry ambulatory care center to a virtual platform at Brigham and Women’s Hospital, which is affiliated with Harvard Medical School.
Previously, I had reported in
I repeated the survey approximately 3 months later. Preliminary data of these 125 total patients showed a slightly higher percentage (12.6%) had video conferencing sessions.
Factors associated with limited access to technology
Similar to what was reported by Nadkarni et al,2 in our extremely vulnerable population, socioeconomic factors affect access. Our patients are low income, and often do not own computers or smart phones. Nearly all our patients receive medical assistance and/or Medicare. Our patients are more likely to be members of a racial minority group—4 times the national average. Our patients are older.4 Patient age varies from 16 to 83 years; the mean age is 54, and the median age is 56. Educational level is low. Nearly all of our patients who participate in video conferencing sessions are female. Approximately 15 of our patients have comorbid intellectual and developmental disabilities diagnoses, and at least that many have subsyndromal symptoms. Constantino et al5 commented on the multiple negative consequences of the COVID-19 pandemic on individuals with intellectual and developmental disabilities, including “frank disparities in access” to technology as well as gaps left by relying exclusively on telehealth.
Among our patients, being low income, a member of a racial minority group, older, less educated, male, and developmentally and/or intellectually disabled are risk factors for less access to video conferencing.3 Nadkarni et al2 also noted less broadband access for rural residents and less access and lack of digital health literacy in patients with limited English proficiency.
As Nadkarni et al2 suggested, we did contact our legislators, and emergency rules are continuing. For now, we are managing fiscally. Although that certainly is important, it does not address the issue of inequality.
Continue to: With this information...
With this information, we are strongly encouraging our patients to participate in video conferencing sessions. We suspect that for some patients, the possibility of them participating in video conferencing sessions is greater than they have acknowledged. We are stepping up education and support, both informally through the patient’s family and friends, and more formally through case managers who “lend” patients a device during home visits.
In summary, this inequality in access to the technology needed for telepsychiatry will loom even more prominently as we all move forward, both clinically and in policymaking.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The COVID-19 pandemic has brought to the fore inequalities in health care.1 In a letter recently published in the American Journal of Psychiatry, Nadkarni et al2 addressed the reality that there is not equal access to quality care (in this case, telepsychiatry). They reported challenges in converting their psychiatry ambulatory care center to a virtual platform at Brigham and Women’s Hospital, which is affiliated with Harvard Medical School.
Previously, I had reported in
I repeated the survey approximately 3 months later. Preliminary data of these 125 total patients showed a slightly higher percentage (12.6%) had video conferencing sessions.
Factors associated with limited access to technology
Similar to what was reported by Nadkarni et al,2 in our extremely vulnerable population, socioeconomic factors affect access. Our patients are low income, and often do not own computers or smart phones. Nearly all our patients receive medical assistance and/or Medicare. Our patients are more likely to be members of a racial minority group—4 times the national average. Our patients are older.4 Patient age varies from 16 to 83 years; the mean age is 54, and the median age is 56. Educational level is low. Nearly all of our patients who participate in video conferencing sessions are female. Approximately 15 of our patients have comorbid intellectual and developmental disabilities diagnoses, and at least that many have subsyndromal symptoms. Constantino et al5 commented on the multiple negative consequences of the COVID-19 pandemic on individuals with intellectual and developmental disabilities, including “frank disparities in access” to technology as well as gaps left by relying exclusively on telehealth.
Among our patients, being low income, a member of a racial minority group, older, less educated, male, and developmentally and/or intellectually disabled are risk factors for less access to video conferencing.3 Nadkarni et al2 also noted less broadband access for rural residents and less access and lack of digital health literacy in patients with limited English proficiency.
As Nadkarni et al2 suggested, we did contact our legislators, and emergency rules are continuing. For now, we are managing fiscally. Although that certainly is important, it does not address the issue of inequality.
Continue to: With this information...
With this information, we are strongly encouraging our patients to participate in video conferencing sessions. We suspect that for some patients, the possibility of them participating in video conferencing sessions is greater than they have acknowledged. We are stepping up education and support, both informally through the patient’s family and friends, and more formally through case managers who “lend” patients a device during home visits.
In summary, this inequality in access to the technology needed for telepsychiatry will loom even more prominently as we all move forward, both clinically and in policymaking.
1. Geller J. Structural racism in American psychiatry and APA: part 1. Psychiatric News. July 3, 2020. Accessed May 10, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2020.7a18
2. Nadkarni A, Hasler V, AhnAllen CG , et al. Telehealth during COVID-19—does everyone have equal access? Am J Psychiatry. 2020;177(11):1093-1094.
3. Storch, DD. Treating patients during COVID-19: what I observed. Current Psychiatry . 2020;19(10):e5. doi:10.12788/cp.0054
4. Buis L, Singer D, Solway E, et al. Telehealth use among older adults before and during COVID-19. University of Michigan National Poll on Healthy Aging. Published August 2020. Accessed May 10, 2021. https://www.healthyagingpoll.org/report/telehealth-use-among-older-adults-and-during-covid-19
5. Constantino JN, Sahin M, Piven J, et al. The impact of COVID-19 on individuals with intellectual and developmental disabilities: clinical and scientific priorities. Am J Psychiatry. 2020;177(11):1091-1093.
1. Geller J. Structural racism in American psychiatry and APA: part 1. Psychiatric News. July 3, 2020. Accessed May 10, 2021. https://psychnews.psychiatryonline.org/doi/full/10.1176/appi.pn.2020.7a18
2. Nadkarni A, Hasler V, AhnAllen CG , et al. Telehealth during COVID-19—does everyone have equal access? Am J Psychiatry. 2020;177(11):1093-1094.
3. Storch, DD. Treating patients during COVID-19: what I observed. Current Psychiatry . 2020;19(10):e5. doi:10.12788/cp.0054
4. Buis L, Singer D, Solway E, et al. Telehealth use among older adults before and during COVID-19. University of Michigan National Poll on Healthy Aging. Published August 2020. Accessed May 10, 2021. https://www.healthyagingpoll.org/report/telehealth-use-among-older-adults-and-during-covid-19
5. Constantino JN, Sahin M, Piven J, et al. The impact of COVID-19 on individuals with intellectual and developmental disabilities: clinical and scientific priorities. Am J Psychiatry. 2020;177(11):1091-1093.
More on ‘treatment resistance’
I wanted to thank Dr. Nasrallah for his bold article, “Treatment resistance is a myth!” (From the Editor,
Stanley N. Caroff, MD
Professor of Psychiatry
Perelman School of Medicine
University of Pennsylvania
Philadelphia, Pennsylvania
I thought Dr. Nasrallah’s editorial on treatment resistance was excellent. In my experience, bipolar depression often is not diagnosed in patients with long-standing depression. These patients do worse on antidepressants, which is interpreted by the clinician as treatment-resistant major depressive disorder. The other issue for me is that individuals with bipolar disorder with psychotic features are often diagnosed with schizophrenia or schizoaffective disorder and never receive a trial of lithium, which could alter the course of their illness in a dramatic fashion. For me, the underutilization of lithium is a real quality problem in our field. Keep up the good work!
Bruce J. Schwartz, MD
Deputy Chairman & Professor of PsychiatryMontefiore Medical Center and Albert Einstein College of Medicine
New York, New York
Are psychiatric advances still science fiction?
I read with great enthusiasm Dr. Nasrallah’s editorial “Today’s psychiatric neuroscience advances were science fiction during my residency” (From the Editor,
I have spent all my professional life serving in the public sector, mainly in New York, and can tell you that many of the brain exploration methods, methodologies, and clinical advances mentioned in this article unfortunately are still a dream for us. Still, we remain hopeful that someday those transformative advances will come to us, too, especially as the technology innovates and improves!
Vania Castillo, MD
New York, New York
Dr. Nasrallah responds
Thank you for your comments. Please remember that every single treatment you are currently using in the public mental health system was a research discovery at one point in the past, and it took many years to bring it to clinical practice. Translating basic neuroscience discoveries, such as the ones I mentioned in my editorial, into clinical practice not only takes time to develop and get approved for use, but also requires substantial funding and a cadre of psychiatric physician-scientists, both of which are in short supply.
“Warp speed” COVID-19 vaccine development was possible only because the deadly pandemic became such an urgent national crisis that the government opened its coffers and diverted billions of dollars to pharmaceutical companies, with a massive infrastructure of human talent and biotechnology, making this veritable “moonshot” a reality in 1 year instead of many. Regrettably, even though neuropsychiatric disorders are a serious societal plague that causes disability and early mortality from suicide, homicide, substance use, cardiovascular risk, and accelerated aging, they do not command the urgency of an infectious viral pandemic that rapidly killed millions and shut down societies all over the world.
You probably heard the saying “a journey of a thousand miles begins with a single step.” I believe we are more than one step—maybe more than 100 steps—toward the type of breakthroughs that we all crave for our long-suffering psychiatric patients. I am grateful for the medical advances we have made over the past 10 to 15 years, such as neuromodulation, rapid-acting parenteral antidepressants, nondopaminergic antipsychotics, therapeutic hallucinogens, early recognition and intervention, and many promising neurobiologic leads and novel therapeutic targets for the brain disorders we deal with every day.
The brain is the most complex, challenging, and physically inaccessible organ to explore and treat. In medicine, we can do heart, lung, liver, and kidney biopsies, but it is far too dangerous to do brain biopsies that would help uncover the molecular and cellular underpinnings of neuropsychiatric disorders. Yet thankfully, our knowledge of the brain structure and function in health and disease has grown by >100,000% over the past few decades compared to the preceding millennia of dark ignorance. Someday, we shall overcome.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
I wanted to thank Dr. Nasrallah for his bold article, “Treatment resistance is a myth!” (From the Editor,
Stanley N. Caroff, MD
Professor of Psychiatry
Perelman School of Medicine
University of Pennsylvania
Philadelphia, Pennsylvania
I thought Dr. Nasrallah’s editorial on treatment resistance was excellent. In my experience, bipolar depression often is not diagnosed in patients with long-standing depression. These patients do worse on antidepressants, which is interpreted by the clinician as treatment-resistant major depressive disorder. The other issue for me is that individuals with bipolar disorder with psychotic features are often diagnosed with schizophrenia or schizoaffective disorder and never receive a trial of lithium, which could alter the course of their illness in a dramatic fashion. For me, the underutilization of lithium is a real quality problem in our field. Keep up the good work!
Bruce J. Schwartz, MD
Deputy Chairman & Professor of PsychiatryMontefiore Medical Center and Albert Einstein College of Medicine
New York, New York
Are psychiatric advances still science fiction?
I read with great enthusiasm Dr. Nasrallah’s editorial “Today’s psychiatric neuroscience advances were science fiction during my residency” (From the Editor,
I have spent all my professional life serving in the public sector, mainly in New York, and can tell you that many of the brain exploration methods, methodologies, and clinical advances mentioned in this article unfortunately are still a dream for us. Still, we remain hopeful that someday those transformative advances will come to us, too, especially as the technology innovates and improves!
Vania Castillo, MD
New York, New York
Dr. Nasrallah responds
Thank you for your comments. Please remember that every single treatment you are currently using in the public mental health system was a research discovery at one point in the past, and it took many years to bring it to clinical practice. Translating basic neuroscience discoveries, such as the ones I mentioned in my editorial, into clinical practice not only takes time to develop and get approved for use, but also requires substantial funding and a cadre of psychiatric physician-scientists, both of which are in short supply.
“Warp speed” COVID-19 vaccine development was possible only because the deadly pandemic became such an urgent national crisis that the government opened its coffers and diverted billions of dollars to pharmaceutical companies, with a massive infrastructure of human talent and biotechnology, making this veritable “moonshot” a reality in 1 year instead of many. Regrettably, even though neuropsychiatric disorders are a serious societal plague that causes disability and early mortality from suicide, homicide, substance use, cardiovascular risk, and accelerated aging, they do not command the urgency of an infectious viral pandemic that rapidly killed millions and shut down societies all over the world.
You probably heard the saying “a journey of a thousand miles begins with a single step.” I believe we are more than one step—maybe more than 100 steps—toward the type of breakthroughs that we all crave for our long-suffering psychiatric patients. I am grateful for the medical advances we have made over the past 10 to 15 years, such as neuromodulation, rapid-acting parenteral antidepressants, nondopaminergic antipsychotics, therapeutic hallucinogens, early recognition and intervention, and many promising neurobiologic leads and novel therapeutic targets for the brain disorders we deal with every day.
The brain is the most complex, challenging, and physically inaccessible organ to explore and treat. In medicine, we can do heart, lung, liver, and kidney biopsies, but it is far too dangerous to do brain biopsies that would help uncover the molecular and cellular underpinnings of neuropsychiatric disorders. Yet thankfully, our knowledge of the brain structure and function in health and disease has grown by >100,000% over the past few decades compared to the preceding millennia of dark ignorance. Someday, we shall overcome.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.
I wanted to thank Dr. Nasrallah for his bold article, “Treatment resistance is a myth!” (From the Editor,
Stanley N. Caroff, MD
Professor of Psychiatry
Perelman School of Medicine
University of Pennsylvania
Philadelphia, Pennsylvania
I thought Dr. Nasrallah’s editorial on treatment resistance was excellent. In my experience, bipolar depression often is not diagnosed in patients with long-standing depression. These patients do worse on antidepressants, which is interpreted by the clinician as treatment-resistant major depressive disorder. The other issue for me is that individuals with bipolar disorder with psychotic features are often diagnosed with schizophrenia or schizoaffective disorder and never receive a trial of lithium, which could alter the course of their illness in a dramatic fashion. For me, the underutilization of lithium is a real quality problem in our field. Keep up the good work!
Bruce J. Schwartz, MD
Deputy Chairman & Professor of PsychiatryMontefiore Medical Center and Albert Einstein College of Medicine
New York, New York
Are psychiatric advances still science fiction?
I read with great enthusiasm Dr. Nasrallah’s editorial “Today’s psychiatric neuroscience advances were science fiction during my residency” (From the Editor,
I have spent all my professional life serving in the public sector, mainly in New York, and can tell you that many of the brain exploration methods, methodologies, and clinical advances mentioned in this article unfortunately are still a dream for us. Still, we remain hopeful that someday those transformative advances will come to us, too, especially as the technology innovates and improves!
Vania Castillo, MD
New York, New York
Dr. Nasrallah responds
Thank you for your comments. Please remember that every single treatment you are currently using in the public mental health system was a research discovery at one point in the past, and it took many years to bring it to clinical practice. Translating basic neuroscience discoveries, such as the ones I mentioned in my editorial, into clinical practice not only takes time to develop and get approved for use, but also requires substantial funding and a cadre of psychiatric physician-scientists, both of which are in short supply.
“Warp speed” COVID-19 vaccine development was possible only because the deadly pandemic became such an urgent national crisis that the government opened its coffers and diverted billions of dollars to pharmaceutical companies, with a massive infrastructure of human talent and biotechnology, making this veritable “moonshot” a reality in 1 year instead of many. Regrettably, even though neuropsychiatric disorders are a serious societal plague that causes disability and early mortality from suicide, homicide, substance use, cardiovascular risk, and accelerated aging, they do not command the urgency of an infectious viral pandemic that rapidly killed millions and shut down societies all over the world.
You probably heard the saying “a journey of a thousand miles begins with a single step.” I believe we are more than one step—maybe more than 100 steps—toward the type of breakthroughs that we all crave for our long-suffering psychiatric patients. I am grateful for the medical advances we have made over the past 10 to 15 years, such as neuromodulation, rapid-acting parenteral antidepressants, nondopaminergic antipsychotics, therapeutic hallucinogens, early recognition and intervention, and many promising neurobiologic leads and novel therapeutic targets for the brain disorders we deal with every day.
The brain is the most complex, challenging, and physically inaccessible organ to explore and treat. In medicine, we can do heart, lung, liver, and kidney biopsies, but it is far too dangerous to do brain biopsies that would help uncover the molecular and cellular underpinnings of neuropsychiatric disorders. Yet thankfully, our knowledge of the brain structure and function in health and disease has grown by >100,000% over the past few decades compared to the preceding millennia of dark ignorance. Someday, we shall overcome.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in their letters, or with manufacturers of competing products.