User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Key questions to ask patients who are veterans
The Mission Act—signed into law in 2018—recognizes that the health care needs of patients who are veterans can no longer be fully served by the Veterans Health Administration.1 This act allows some veterans who are enrolled in the Veterans Affairs (VA) health care system or otherwise entitled to VA care to access treatment outside of VA facilities.1 As a result, psychiatrists may treat veterans more frequently.
During such patients’ initial visit, obtaining a detailed history of their military service can reveal vital clinical information and establish a therapeutic alliance that can help foster positive treatment outcomes. Here we offer an A-to-L list of important questions to ask veterans about their military service, and explanations of why these questions are valuable.
Attained rank. What rank did you attain during your military service? Did you retire from the military? How many years did you serve?
Asking about your patient’s rank, retirement status, and time in service is vital to understanding their military experience. By military law, only individuals who retired from the military can use their rank as an identifier after they leave the military, although some veterans may not wish to be called by their rank in a clinical setting.
Branch. Which branch of the military did you serve? Were you in Active Duty, the Reserves, or the National Guard?
Military members often take great pride in service of their specific branch. Each branch has its own language, culture, values, and exposures. If your patient has served in a combination of Active Duty, Reserves, and/or National Guard, ask how much time they spent in each.
Culture. What part of the military culture was positive or negative for you?
Continue to: There is a clear culture...
There is a clear culture within the military. Some veterans may feel lost without the military structure, and even devalued without the respect of rank. Others may feel jaded and spiteful about the strict military culture, procedures, and expectations.
Discharge. When, why, and under what circumstances were you discharged? What type of discharge did you receive?
There are 6 types of discharge: Honorable, General, Other than Honorable (OTH), Entry Level Separation, Bad Conduct, and Dishonorable. The type of discharge a veteran received may impact what resources are available to them. It also can influence a veteran’s perception of their military career.
Exposures. Were you exposed to combat, death, explosive blasts, or hazardous chemicals?
Do not ask a veteran if they have killed anyone. This question is both disrespectful and highly presumptuous because most veterans have not killed anyone. Be respectful of their experiences. Depending on the veteran’s mission, they may have unique exposures (Agent Orange, burn pits, detainee camps, etc.). Consider asking follow-up questions to learn the details of these exposures.
Continue to: Family impact
Family impact. How has your military service impacted your family?
A veteran’s military service often affects family members. Deployments can cause strain on marital relationships, children’s birthdays and special events may be missed, and extended family may have negative reactions to military service. Understanding the impact on the veteran’s family members can help uncover potential stressful relationships as well as help enhance any positive support systems that are available at home.
Go. Where were you stationed? Were you deployed?
Training location, geography of combat theater, peace-keeping locations, and area of station can all profoundly impact a veteran’s military experience. Ask follow-up questions about their duty stations, deployment locations, and experiences with these locations.
Hot water. Did you ever get into “trouble” while serving the military (eg, lose rank, get arrested, etc.)? How did you respond to the military’s method of discipline?
Continue to: Although it may be difficult...
Although it may be difficult or uncomfortable to ask your patient if they experienced any disciplinary action, this information may prove useful. It can help provide context when you discuss the veteran’s ease of assimilation into civilian life and other important information regarding the type of discharge.
Injuries. Have you experienced any moral, physical, sexual, emotional, or concussive injuries?
Moral injury, guilt, and regret are common for veterans. Not all injuries are from combat. Your patient may have experienced sexual assault, hazing rituals, pranks, etc.
Job. What was your job in the military? What kind of security clearance did you have?
Note that not all veterans’ “jobs” in the military accurately reflect the duties and tasks that they actually performed. Security clearance will often influence the duties and tasks they were required to perform.
Continue to: Keeping it inside
Keeping it inside. Do you have anyone to talk with about your military experiences?
Many veterans feel uncomfortable discussing their experiences with others. Some veterans may be concerned that others will not understand what they went through. Some might perceive that disclosing their experiences could burden other people, or they may be concerned that explaining their experiences may be too shocking. Asking this question may present an opportunity for you to suggest psychotherapy for your patient.
Life as a civilian. How is your life different as a civilian? How have you adjusted to civilian life?
During the process of assimilation into civilian life, veterans may experience symptoms of depression, posttraumatic stress disorder, anxiety, or other disorders. These symptoms may emerge and/or become exacerbated during their transition to civilian life.
1. VA MISSION Act of 2018 (VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act), S 2372, 115th Cong, 2nd Sess, HR Doc No. 115-671 (2018).
The Mission Act—signed into law in 2018—recognizes that the health care needs of patients who are veterans can no longer be fully served by the Veterans Health Administration.1 This act allows some veterans who are enrolled in the Veterans Affairs (VA) health care system or otherwise entitled to VA care to access treatment outside of VA facilities.1 As a result, psychiatrists may treat veterans more frequently.
During such patients’ initial visit, obtaining a detailed history of their military service can reveal vital clinical information and establish a therapeutic alliance that can help foster positive treatment outcomes. Here we offer an A-to-L list of important questions to ask veterans about their military service, and explanations of why these questions are valuable.
Attained rank. What rank did you attain during your military service? Did you retire from the military? How many years did you serve?
Asking about your patient’s rank, retirement status, and time in service is vital to understanding their military experience. By military law, only individuals who retired from the military can use their rank as an identifier after they leave the military, although some veterans may not wish to be called by their rank in a clinical setting.
Branch. Which branch of the military did you serve? Were you in Active Duty, the Reserves, or the National Guard?
Military members often take great pride in service of their specific branch. Each branch has its own language, culture, values, and exposures. If your patient has served in a combination of Active Duty, Reserves, and/or National Guard, ask how much time they spent in each.
Culture. What part of the military culture was positive or negative for you?
Continue to: There is a clear culture...
There is a clear culture within the military. Some veterans may feel lost without the military structure, and even devalued without the respect of rank. Others may feel jaded and spiteful about the strict military culture, procedures, and expectations.
Discharge. When, why, and under what circumstances were you discharged? What type of discharge did you receive?
There are 6 types of discharge: Honorable, General, Other than Honorable (OTH), Entry Level Separation, Bad Conduct, and Dishonorable. The type of discharge a veteran received may impact what resources are available to them. It also can influence a veteran’s perception of their military career.
Exposures. Were you exposed to combat, death, explosive blasts, or hazardous chemicals?
Do not ask a veteran if they have killed anyone. This question is both disrespectful and highly presumptuous because most veterans have not killed anyone. Be respectful of their experiences. Depending on the veteran’s mission, they may have unique exposures (Agent Orange, burn pits, detainee camps, etc.). Consider asking follow-up questions to learn the details of these exposures.
Continue to: Family impact
Family impact. How has your military service impacted your family?
A veteran’s military service often affects family members. Deployments can cause strain on marital relationships, children’s birthdays and special events may be missed, and extended family may have negative reactions to military service. Understanding the impact on the veteran’s family members can help uncover potential stressful relationships as well as help enhance any positive support systems that are available at home.
Go. Where were you stationed? Were you deployed?
Training location, geography of combat theater, peace-keeping locations, and area of station can all profoundly impact a veteran’s military experience. Ask follow-up questions about their duty stations, deployment locations, and experiences with these locations.
Hot water. Did you ever get into “trouble” while serving the military (eg, lose rank, get arrested, etc.)? How did you respond to the military’s method of discipline?
Continue to: Although it may be difficult...
Although it may be difficult or uncomfortable to ask your patient if they experienced any disciplinary action, this information may prove useful. It can help provide context when you discuss the veteran’s ease of assimilation into civilian life and other important information regarding the type of discharge.
Injuries. Have you experienced any moral, physical, sexual, emotional, or concussive injuries?
Moral injury, guilt, and regret are common for veterans. Not all injuries are from combat. Your patient may have experienced sexual assault, hazing rituals, pranks, etc.
Job. What was your job in the military? What kind of security clearance did you have?
Note that not all veterans’ “jobs” in the military accurately reflect the duties and tasks that they actually performed. Security clearance will often influence the duties and tasks they were required to perform.
Continue to: Keeping it inside
Keeping it inside. Do you have anyone to talk with about your military experiences?
Many veterans feel uncomfortable discussing their experiences with others. Some veterans may be concerned that others will not understand what they went through. Some might perceive that disclosing their experiences could burden other people, or they may be concerned that explaining their experiences may be too shocking. Asking this question may present an opportunity for you to suggest psychotherapy for your patient.
Life as a civilian. How is your life different as a civilian? How have you adjusted to civilian life?
During the process of assimilation into civilian life, veterans may experience symptoms of depression, posttraumatic stress disorder, anxiety, or other disorders. These symptoms may emerge and/or become exacerbated during their transition to civilian life.
The Mission Act—signed into law in 2018—recognizes that the health care needs of patients who are veterans can no longer be fully served by the Veterans Health Administration.1 This act allows some veterans who are enrolled in the Veterans Affairs (VA) health care system or otherwise entitled to VA care to access treatment outside of VA facilities.1 As a result, psychiatrists may treat veterans more frequently.
During such patients’ initial visit, obtaining a detailed history of their military service can reveal vital clinical information and establish a therapeutic alliance that can help foster positive treatment outcomes. Here we offer an A-to-L list of important questions to ask veterans about their military service, and explanations of why these questions are valuable.
Attained rank. What rank did you attain during your military service? Did you retire from the military? How many years did you serve?
Asking about your patient’s rank, retirement status, and time in service is vital to understanding their military experience. By military law, only individuals who retired from the military can use their rank as an identifier after they leave the military, although some veterans may not wish to be called by their rank in a clinical setting.
Branch. Which branch of the military did you serve? Were you in Active Duty, the Reserves, or the National Guard?
Military members often take great pride in service of their specific branch. Each branch has its own language, culture, values, and exposures. If your patient has served in a combination of Active Duty, Reserves, and/or National Guard, ask how much time they spent in each.
Culture. What part of the military culture was positive or negative for you?
Continue to: There is a clear culture...
There is a clear culture within the military. Some veterans may feel lost without the military structure, and even devalued without the respect of rank. Others may feel jaded and spiteful about the strict military culture, procedures, and expectations.
Discharge. When, why, and under what circumstances were you discharged? What type of discharge did you receive?
There are 6 types of discharge: Honorable, General, Other than Honorable (OTH), Entry Level Separation, Bad Conduct, and Dishonorable. The type of discharge a veteran received may impact what resources are available to them. It also can influence a veteran’s perception of their military career.
Exposures. Were you exposed to combat, death, explosive blasts, or hazardous chemicals?
Do not ask a veteran if they have killed anyone. This question is both disrespectful and highly presumptuous because most veterans have not killed anyone. Be respectful of their experiences. Depending on the veteran’s mission, they may have unique exposures (Agent Orange, burn pits, detainee camps, etc.). Consider asking follow-up questions to learn the details of these exposures.
Continue to: Family impact
Family impact. How has your military service impacted your family?
A veteran’s military service often affects family members. Deployments can cause strain on marital relationships, children’s birthdays and special events may be missed, and extended family may have negative reactions to military service. Understanding the impact on the veteran’s family members can help uncover potential stressful relationships as well as help enhance any positive support systems that are available at home.
Go. Where were you stationed? Were you deployed?
Training location, geography of combat theater, peace-keeping locations, and area of station can all profoundly impact a veteran’s military experience. Ask follow-up questions about their duty stations, deployment locations, and experiences with these locations.
Hot water. Did you ever get into “trouble” while serving the military (eg, lose rank, get arrested, etc.)? How did you respond to the military’s method of discipline?
Continue to: Although it may be difficult...
Although it may be difficult or uncomfortable to ask your patient if they experienced any disciplinary action, this information may prove useful. It can help provide context when you discuss the veteran’s ease of assimilation into civilian life and other important information regarding the type of discharge.
Injuries. Have you experienced any moral, physical, sexual, emotional, or concussive injuries?
Moral injury, guilt, and regret are common for veterans. Not all injuries are from combat. Your patient may have experienced sexual assault, hazing rituals, pranks, etc.
Job. What was your job in the military? What kind of security clearance did you have?
Note that not all veterans’ “jobs” in the military accurately reflect the duties and tasks that they actually performed. Security clearance will often influence the duties and tasks they were required to perform.
Continue to: Keeping it inside
Keeping it inside. Do you have anyone to talk with about your military experiences?
Many veterans feel uncomfortable discussing their experiences with others. Some veterans may be concerned that others will not understand what they went through. Some might perceive that disclosing their experiences could burden other people, or they may be concerned that explaining their experiences may be too shocking. Asking this question may present an opportunity for you to suggest psychotherapy for your patient.
Life as a civilian. How is your life different as a civilian? How have you adjusted to civilian life?
During the process of assimilation into civilian life, veterans may experience symptoms of depression, posttraumatic stress disorder, anxiety, or other disorders. These symptoms may emerge and/or become exacerbated during their transition to civilian life.
1. VA MISSION Act of 2018 (VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act), S 2372, 115th Cong, 2nd Sess, HR Doc No. 115-671 (2018).
1. VA MISSION Act of 2018 (VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act), S 2372, 115th Cong, 2nd Sess, HR Doc No. 115-671 (2018).
Helping survivors of human trafficking
Human trafficking (HT) is a secretive, multibillion dollar criminal industry involving the use of coercion, threats, and fraud to force individuals to engage in labor or commercial sex acts. In 2017, the International Labour Organization estimated that 24.9 million people worldwide were victims of forced labor (ie, working under threat or coercion).1 Risk factors for individuals who are vulnerable to HT include recent migration, substance use, housing insecurity, runaway youth, and mental illness. Traffickers continue the cycle of HT through isolation and emotional, physical, financial, and verbal abuse.
Survivors of HT may avoid seeking health care due to cultural reasons or feelings of guilt, isolation, distrust, or fear of criminal sanctions. There can be missed opportunities for victims to obtain help through health care services, law enforcement, child welfare services, or even family or friends. In a study of 173 survivors of HT in the United States, 68% of those who were currently trafficked visited with a health care professional at least once and were not identified as being trafficked.2 Psychiatrists rarely receive education on HT, which can lead to missed opportunities for identifying victims. Table 1 lists screening questions psychiatrists can ask patients they suspect may be trafficked.
The psychiatric sequelae of trafficking
Survivors of HT commonly experience psychiatric illness, substance use, pain, sexually transmitted diseases, and unplanned pregnancies.3 Here we discuss some of the psychiatric conditions that are common among HT survivors, and outline a multidisciplinary approach to their care.
PTSD, mood disorders, and anxiety disorders. Studies suggest survivors of HT who seek care have a high prevalence of depression, anxiety, and posttraumatic stress disorder (PTSD).3 Survivors may have experienced multiple repetitive trauma, such as physical and sexual abuse.3 Compared with survivors of forced labor trafficking, survivors of sex trafficking have higher rates of childhood abuse, violence during trafficking, severe symptoms of PTSD, and comorbid depression and PTSD.4 For survivors with PTSD, consider psychosocial interventions that address social support, coping strategies, and community reintegration.5 Survivors can also benefit from trauma-informed care that focuses on the cognitive aspect of the trauma, such as cognitive processing therapy, which involves cognitive restructuring without a written account of the trauma.6
Substance use disorders. Some individuals who are trafficked may be forced to use drugs of abuse or alcohol, while others may use substances to help cope while they are being trafficked or afterwards.3 For these patients, motivational interviewing may be beneficial. Also, consider referring them to detoxification or rehabilitation programs.
Suicide and self-harm. In a study of 98 HT survivors in England, 33% reported a history of self-harm before receiving care and 25% engaged in self-harm during care.7 After engaging in self-harm, survivors of HT were more likely to be admitted to psychiatric inpatient units than were patients who had not been trafficked.7 It is crucial to conduct a suicide risk assessment as part of the trauma-informed care of these patients.
Other conditions. In addition to psychiatric illness, survivors of HT may experience physical symptoms such as headache, back pain, stomach pain, fatigue, dizziness, memory problems, and weight loss.3 Referral to other specialties may be necessary for addressing any of the patient’s other conditions.
Continue to: Use a multidisciplinary approach
Use a multidisciplinary approach
Treatment for survivors of HT should be tailored to their specific mental health needs by including psychopharmacology; individual, group, or family psychotherapy; and peer advocate support. Rehabilitation, social services, and case management should also be considered. The care of survivors of HT benefits from a multidisciplinary, culturally-sensitive, and trauma-informed approach. Table 28 describes the PEARR Tool (Provide privacy, Educate, Ask, Respect, and Respond), which offers physicians 4 steps for addressing abuse, neglect, or violence with their patients. Also, the National Human Trafficking Hotline (1-888-373-7888) is available 24/7 for trafficked persons, survivors, and health care professionals to provide guidance on reporting laws and finding additional resources such as housing and legal services.

1. International Labour Organization, the Walk Free Foundation. Global Estimates of Modern Slavery: forced labour and forced marriage. Published 2017. Accessed January 14, 2021. www.ilo.org/global/publications/books/WCMS_575479/lang--en/index.htm
2. Chisolm-Straker M, Baldwin S, Gaïgbé-Togbé B, et al. Health care and human trafficking: we are seeing the unseen. J Health Care Poor Underserved. 2016;27(3):1220-1233.
3. Ottisova L, Hemmings S, Howard LM, et al. Prevalence and risk of violence and the mental, physical and sexual health problems associated with human trafficking: an updated systematic review. Epidemiol Psychiatr Sci. 2016;25(4):317-341.
4. Hopper EK, Gonzalez LD. A comparison of psychological symptoms in survivors of sex and labor trafficking. Behav Med. 2018;44(3):177-188.
5. Okech D, Hanseen N, Howard W, et al. Social support, dysfunctional coping, and community reintegration as predictors of PTSD among human trafficking survivors. Behav Med. 2018;44(3):209-218.
6. Salami T, Gordon M, Coverdale J, et al. What therapies are favored in the treatment of the psychological sequelae of trauma in human trafficking victims? J Psychiatr Pract. 2018;24(2):87-96.
7. Borschmann R, Oram S, Kinner SA, et al. Self-harm among adult victims of human trafficking who accessed secondary mental health services in England. Psychiatr Serv. 2017;68(2):207-210.
8. Using the PEARR Tool. Dignity Health. Published 2019. Accessed January 14, 2021. https://www.dignityhealth.org/hello-humankindness/human-trafficking/victimcentered-and-trauma-informed/using-the-pearr-tool
Human trafficking (HT) is a secretive, multibillion dollar criminal industry involving the use of coercion, threats, and fraud to force individuals to engage in labor or commercial sex acts. In 2017, the International Labour Organization estimated that 24.9 million people worldwide were victims of forced labor (ie, working under threat or coercion).1 Risk factors for individuals who are vulnerable to HT include recent migration, substance use, housing insecurity, runaway youth, and mental illness. Traffickers continue the cycle of HT through isolation and emotional, physical, financial, and verbal abuse.
Survivors of HT may avoid seeking health care due to cultural reasons or feelings of guilt, isolation, distrust, or fear of criminal sanctions. There can be missed opportunities for victims to obtain help through health care services, law enforcement, child welfare services, or even family or friends. In a study of 173 survivors of HT in the United States, 68% of those who were currently trafficked visited with a health care professional at least once and were not identified as being trafficked.2 Psychiatrists rarely receive education on HT, which can lead to missed opportunities for identifying victims. Table 1 lists screening questions psychiatrists can ask patients they suspect may be trafficked.
The psychiatric sequelae of trafficking
Survivors of HT commonly experience psychiatric illness, substance use, pain, sexually transmitted diseases, and unplanned pregnancies.3 Here we discuss some of the psychiatric conditions that are common among HT survivors, and outline a multidisciplinary approach to their care.
PTSD, mood disorders, and anxiety disorders. Studies suggest survivors of HT who seek care have a high prevalence of depression, anxiety, and posttraumatic stress disorder (PTSD).3 Survivors may have experienced multiple repetitive trauma, such as physical and sexual abuse.3 Compared with survivors of forced labor trafficking, survivors of sex trafficking have higher rates of childhood abuse, violence during trafficking, severe symptoms of PTSD, and comorbid depression and PTSD.4 For survivors with PTSD, consider psychosocial interventions that address social support, coping strategies, and community reintegration.5 Survivors can also benefit from trauma-informed care that focuses on the cognitive aspect of the trauma, such as cognitive processing therapy, which involves cognitive restructuring without a written account of the trauma.6
Substance use disorders. Some individuals who are trafficked may be forced to use drugs of abuse or alcohol, while others may use substances to help cope while they are being trafficked or afterwards.3 For these patients, motivational interviewing may be beneficial. Also, consider referring them to detoxification or rehabilitation programs.
Suicide and self-harm. In a study of 98 HT survivors in England, 33% reported a history of self-harm before receiving care and 25% engaged in self-harm during care.7 After engaging in self-harm, survivors of HT were more likely to be admitted to psychiatric inpatient units than were patients who had not been trafficked.7 It is crucial to conduct a suicide risk assessment as part of the trauma-informed care of these patients.
Other conditions. In addition to psychiatric illness, survivors of HT may experience physical symptoms such as headache, back pain, stomach pain, fatigue, dizziness, memory problems, and weight loss.3 Referral to other specialties may be necessary for addressing any of the patient’s other conditions.
Continue to: Use a multidisciplinary approach
Use a multidisciplinary approach
Treatment for survivors of HT should be tailored to their specific mental health needs by including psychopharmacology; individual, group, or family psychotherapy; and peer advocate support. Rehabilitation, social services, and case management should also be considered. The care of survivors of HT benefits from a multidisciplinary, culturally-sensitive, and trauma-informed approach. Table 28 describes the PEARR Tool (Provide privacy, Educate, Ask, Respect, and Respond), which offers physicians 4 steps for addressing abuse, neglect, or violence with their patients. Also, the National Human Trafficking Hotline (1-888-373-7888) is available 24/7 for trafficked persons, survivors, and health care professionals to provide guidance on reporting laws and finding additional resources such as housing and legal services.

Human trafficking (HT) is a secretive, multibillion dollar criminal industry involving the use of coercion, threats, and fraud to force individuals to engage in labor or commercial sex acts. In 2017, the International Labour Organization estimated that 24.9 million people worldwide were victims of forced labor (ie, working under threat or coercion).1 Risk factors for individuals who are vulnerable to HT include recent migration, substance use, housing insecurity, runaway youth, and mental illness. Traffickers continue the cycle of HT through isolation and emotional, physical, financial, and verbal abuse.
Survivors of HT may avoid seeking health care due to cultural reasons or feelings of guilt, isolation, distrust, or fear of criminal sanctions. There can be missed opportunities for victims to obtain help through health care services, law enforcement, child welfare services, or even family or friends. In a study of 173 survivors of HT in the United States, 68% of those who were currently trafficked visited with a health care professional at least once and were not identified as being trafficked.2 Psychiatrists rarely receive education on HT, which can lead to missed opportunities for identifying victims. Table 1 lists screening questions psychiatrists can ask patients they suspect may be trafficked.
The psychiatric sequelae of trafficking
Survivors of HT commonly experience psychiatric illness, substance use, pain, sexually transmitted diseases, and unplanned pregnancies.3 Here we discuss some of the psychiatric conditions that are common among HT survivors, and outline a multidisciplinary approach to their care.
PTSD, mood disorders, and anxiety disorders. Studies suggest survivors of HT who seek care have a high prevalence of depression, anxiety, and posttraumatic stress disorder (PTSD).3 Survivors may have experienced multiple repetitive trauma, such as physical and sexual abuse.3 Compared with survivors of forced labor trafficking, survivors of sex trafficking have higher rates of childhood abuse, violence during trafficking, severe symptoms of PTSD, and comorbid depression and PTSD.4 For survivors with PTSD, consider psychosocial interventions that address social support, coping strategies, and community reintegration.5 Survivors can also benefit from trauma-informed care that focuses on the cognitive aspect of the trauma, such as cognitive processing therapy, which involves cognitive restructuring without a written account of the trauma.6
Substance use disorders. Some individuals who are trafficked may be forced to use drugs of abuse or alcohol, while others may use substances to help cope while they are being trafficked or afterwards.3 For these patients, motivational interviewing may be beneficial. Also, consider referring them to detoxification or rehabilitation programs.
Suicide and self-harm. In a study of 98 HT survivors in England, 33% reported a history of self-harm before receiving care and 25% engaged in self-harm during care.7 After engaging in self-harm, survivors of HT were more likely to be admitted to psychiatric inpatient units than were patients who had not been trafficked.7 It is crucial to conduct a suicide risk assessment as part of the trauma-informed care of these patients.
Other conditions. In addition to psychiatric illness, survivors of HT may experience physical symptoms such as headache, back pain, stomach pain, fatigue, dizziness, memory problems, and weight loss.3 Referral to other specialties may be necessary for addressing any of the patient’s other conditions.
Continue to: Use a multidisciplinary approach
Use a multidisciplinary approach
Treatment for survivors of HT should be tailored to their specific mental health needs by including psychopharmacology; individual, group, or family psychotherapy; and peer advocate support. Rehabilitation, social services, and case management should also be considered. The care of survivors of HT benefits from a multidisciplinary, culturally-sensitive, and trauma-informed approach. Table 28 describes the PEARR Tool (Provide privacy, Educate, Ask, Respect, and Respond), which offers physicians 4 steps for addressing abuse, neglect, or violence with their patients. Also, the National Human Trafficking Hotline (1-888-373-7888) is available 24/7 for trafficked persons, survivors, and health care professionals to provide guidance on reporting laws and finding additional resources such as housing and legal services.

1. International Labour Organization, the Walk Free Foundation. Global Estimates of Modern Slavery: forced labour and forced marriage. Published 2017. Accessed January 14, 2021. www.ilo.org/global/publications/books/WCMS_575479/lang--en/index.htm
2. Chisolm-Straker M, Baldwin S, Gaïgbé-Togbé B, et al. Health care and human trafficking: we are seeing the unseen. J Health Care Poor Underserved. 2016;27(3):1220-1233.
3. Ottisova L, Hemmings S, Howard LM, et al. Prevalence and risk of violence and the mental, physical and sexual health problems associated with human trafficking: an updated systematic review. Epidemiol Psychiatr Sci. 2016;25(4):317-341.
4. Hopper EK, Gonzalez LD. A comparison of psychological symptoms in survivors of sex and labor trafficking. Behav Med. 2018;44(3):177-188.
5. Okech D, Hanseen N, Howard W, et al. Social support, dysfunctional coping, and community reintegration as predictors of PTSD among human trafficking survivors. Behav Med. 2018;44(3):209-218.
6. Salami T, Gordon M, Coverdale J, et al. What therapies are favored in the treatment of the psychological sequelae of trauma in human trafficking victims? J Psychiatr Pract. 2018;24(2):87-96.
7. Borschmann R, Oram S, Kinner SA, et al. Self-harm among adult victims of human trafficking who accessed secondary mental health services in England. Psychiatr Serv. 2017;68(2):207-210.
8. Using the PEARR Tool. Dignity Health. Published 2019. Accessed January 14, 2021. https://www.dignityhealth.org/hello-humankindness/human-trafficking/victimcentered-and-trauma-informed/using-the-pearr-tool
1. International Labour Organization, the Walk Free Foundation. Global Estimates of Modern Slavery: forced labour and forced marriage. Published 2017. Accessed January 14, 2021. www.ilo.org/global/publications/books/WCMS_575479/lang--en/index.htm
2. Chisolm-Straker M, Baldwin S, Gaïgbé-Togbé B, et al. Health care and human trafficking: we are seeing the unseen. J Health Care Poor Underserved. 2016;27(3):1220-1233.
3. Ottisova L, Hemmings S, Howard LM, et al. Prevalence and risk of violence and the mental, physical and sexual health problems associated with human trafficking: an updated systematic review. Epidemiol Psychiatr Sci. 2016;25(4):317-341.
4. Hopper EK, Gonzalez LD. A comparison of psychological symptoms in survivors of sex and labor trafficking. Behav Med. 2018;44(3):177-188.
5. Okech D, Hanseen N, Howard W, et al. Social support, dysfunctional coping, and community reintegration as predictors of PTSD among human trafficking survivors. Behav Med. 2018;44(3):209-218.
6. Salami T, Gordon M, Coverdale J, et al. What therapies are favored in the treatment of the psychological sequelae of trauma in human trafficking victims? J Psychiatr Pract. 2018;24(2):87-96.
7. Borschmann R, Oram S, Kinner SA, et al. Self-harm among adult victims of human trafficking who accessed secondary mental health services in England. Psychiatr Serv. 2017;68(2):207-210.
8. Using the PEARR Tool. Dignity Health. Published 2019. Accessed January 14, 2021. https://www.dignityhealth.org/hello-humankindness/human-trafficking/victimcentered-and-trauma-informed/using-the-pearr-tool
Patient Handout: Safe practices during the COVID-19 pandemic
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
Psychcast: Nursing home consultations supporting documents
Body Text
Body Text
Body Text
Reducing COVID-19 opioid deaths
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.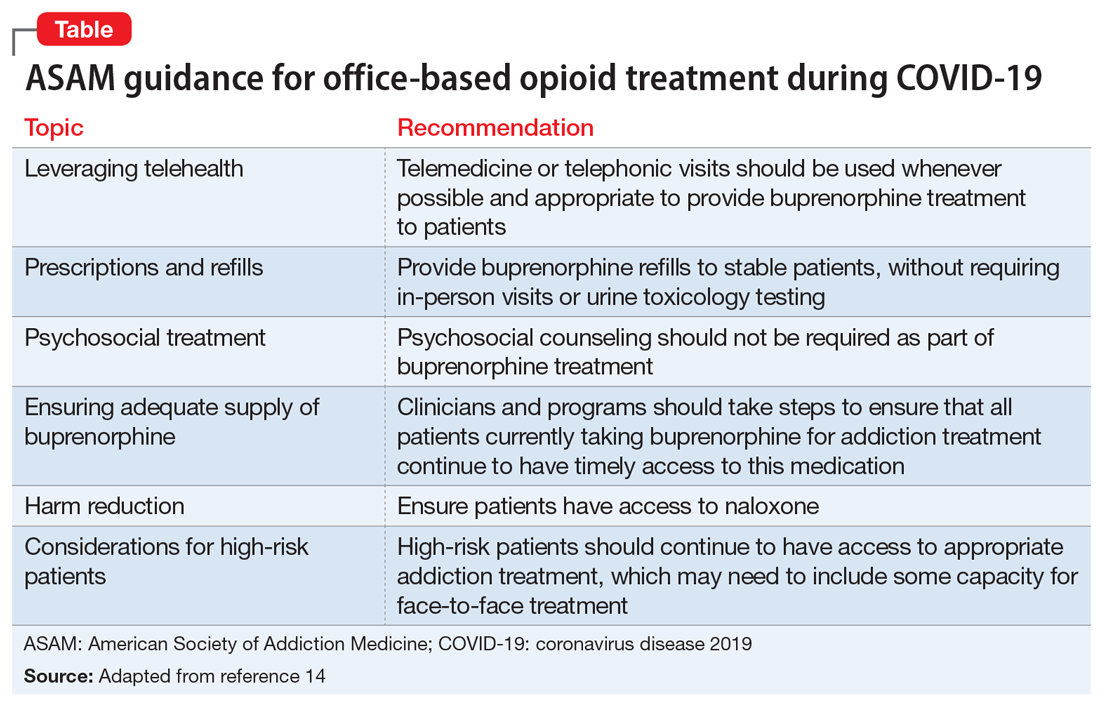
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
Pharmacotherapy for alcohol use disorder in patients with hepatic impairment
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
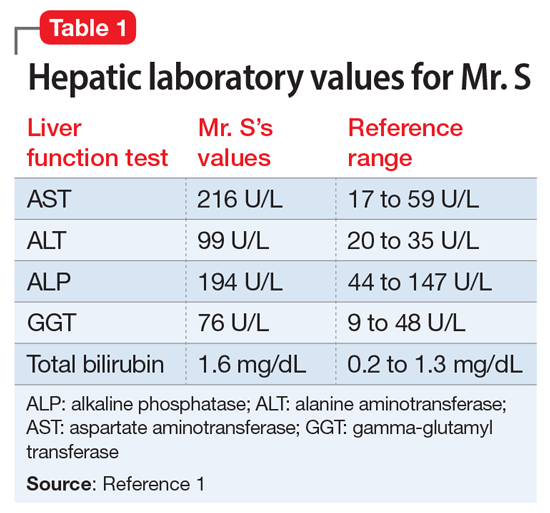
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
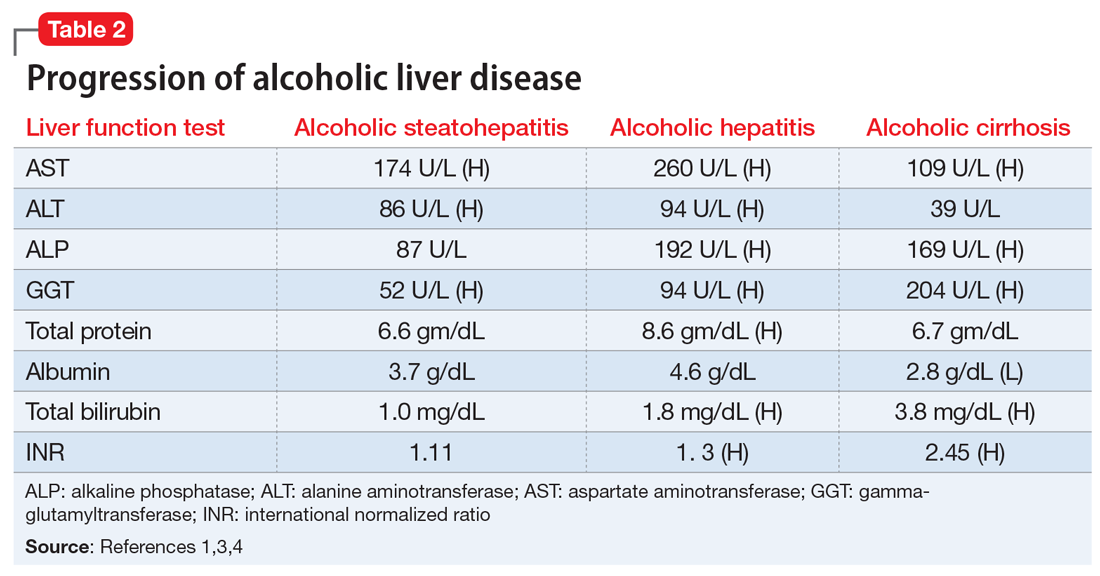
Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
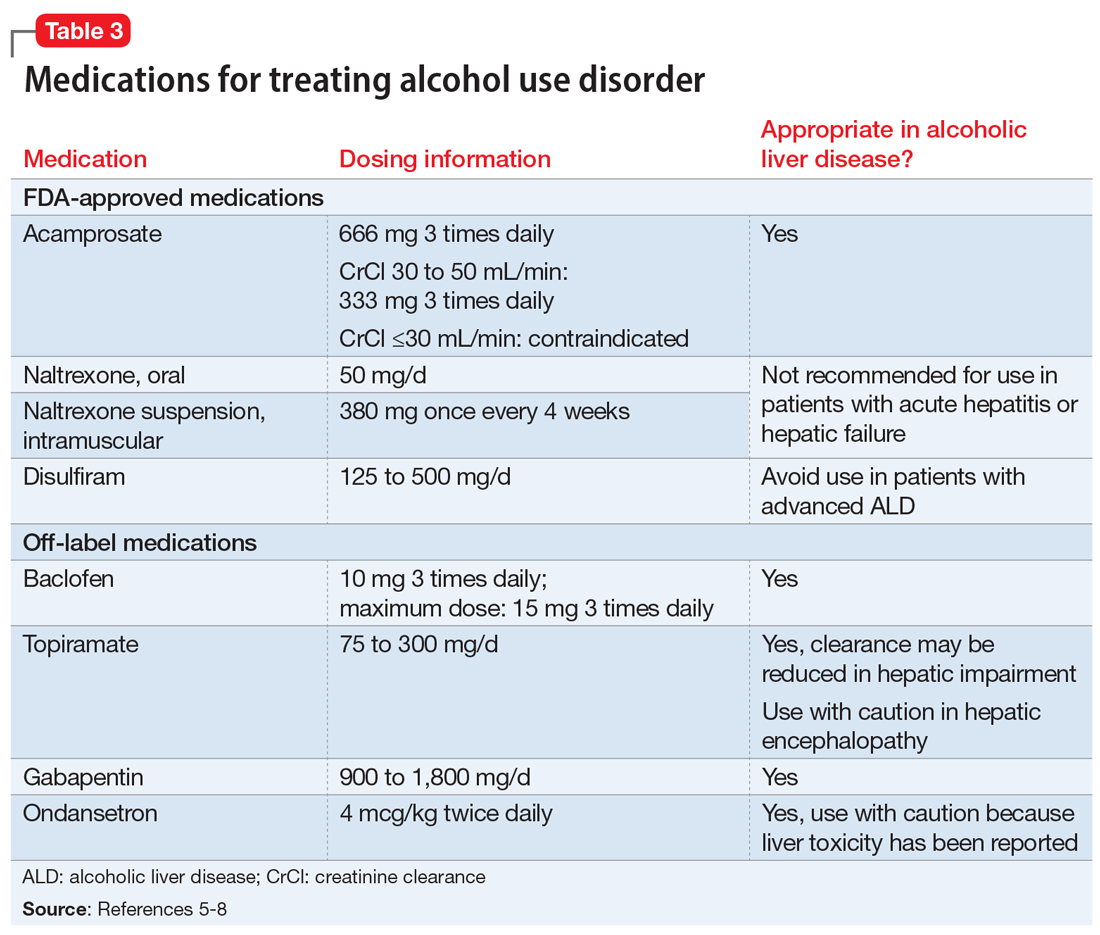
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.

A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4

Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8

Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.

A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4

Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8

Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Threatening to burn the house down
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.
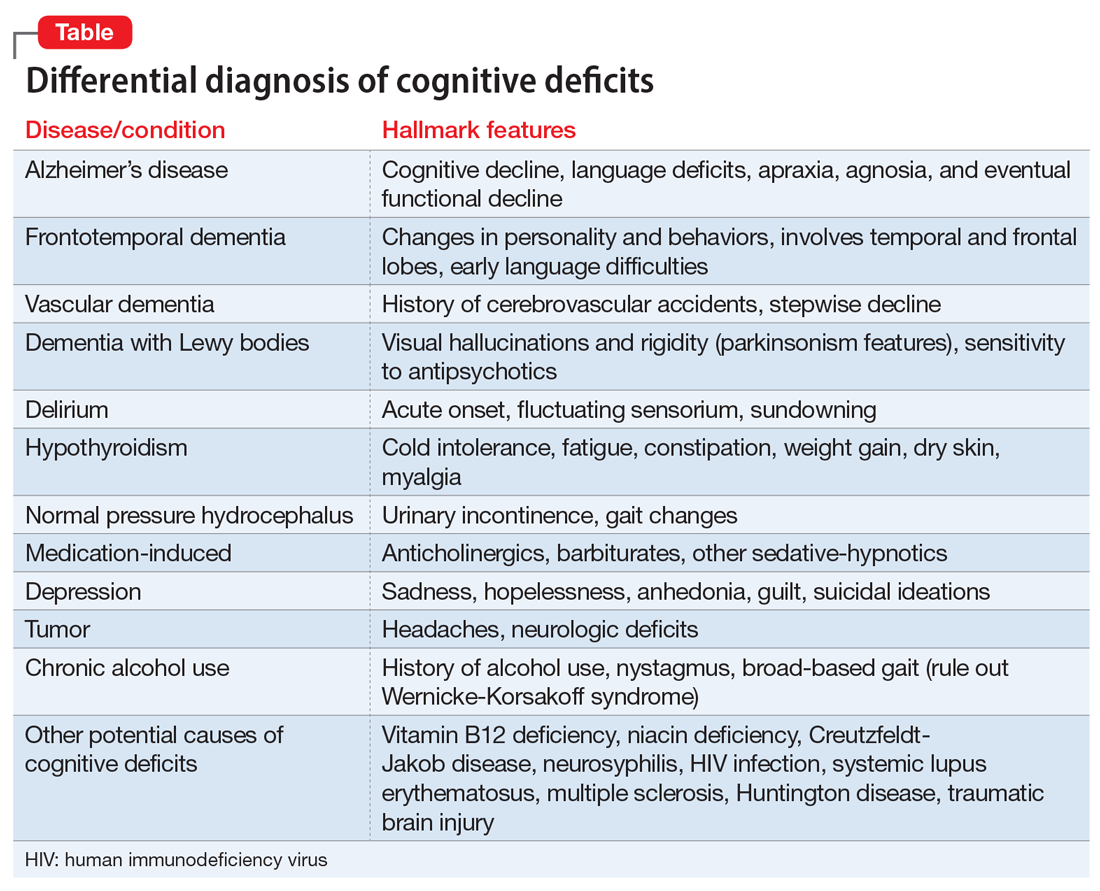
Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.

Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
CASE Agitated and aggressive
Mr. X, age 61, who has Alzheimer’s disease, is brought to the emergency department (ED) by his family after he is found to be confused, becomes physically aggressive with family members, and threatens to burn the house down. His family reports that earlier that day, he was paranoid that somebody was trying to kill him and he tried to leave the house. Mr. X has been experiencing visual hallucinations and delusional thoughts that made him aggressive towards his son. After an initial laboratory workup in the ED, Mr. X’s bloodwork comes back positive for mild leukocytosis, indicating the possibility of an infectious etiology. Mr. X is admitted to the hospital for further evaluation of his altered mental status.
HISTORY Decline over 2 years
This is Mr. X’s third inpatient admission for agitation and psychosis. His current medications—twice daily divalproex sodium extended release (ER), 250 mg every morning and 500 mg at every bedtime, and prazosin, 2 mg/d at bedtime—have been only partially effective. His medical history includes osteoarthritis, back pain, and heterozygous factor V Leiden (not on anticoagulation). He quit smoking tobacco several years ago and has no history of substance use. He has no family history of dementia. Previous trials of cholinesterase inhibitors, antipsychotics, and antidepressants resulted in only minimal improvement in his agitation and psychosis.
A chart review shows that 2 years before his current hospital admission, Mr. X had presented to his primary care physician with slurred speech, forgetfulness, missing words, and transient reading difficulties. His initial laboratory workup and MRI came back normal. He was placed on short-term disability due to work-related errors. He was referred to the hospital’s Memory Clinic 2 years ago, where his Mini-Mental State Exam score was 20/30, indicating mild cognitive impairment. Stroke workup was negative. Due to significant language deficits, a differential diagnosis for Alzheimer’s disease vs primary progressive aphasia vs frontotemporal dementia was made. He screened positive for amyloid PET scan, which confirmed the diagnosis of Alzheimer’s disease.
Neuropsychological testing showed similarities with logopenic variant of primary progressive aphasia, which in many cases is present in Alzheimer’s disease. Mr. X was prescribed anticholinesterase inhibitors, including donepezil, 10 mg/d, and rivastigmine patch, 9.5 mg/d; and memantine, 10 mg/d, which he could not tolerate because of adverse effects. During the next year, Mr. X deteriorated and presented to the ED a few times with significant psychotic symptoms and aggression. He had a poor response to various pharmacologic and nonpharmacologic interventions during this time.
EVALUATION Continued problematic behaviors
During his hospitalization, Mr. X continues to be agitated and paranoid and is placed in restraints. He is unable to respond to his name and cannot follow simple verbal commands. Results of his laboratory workup are within normal limits. His mild leukocytosis resolves with no active signs of infection. Psychiatry is consulted for management of his behavioral and psychological symptoms of dementia (BPSD).
Continue to: Mr. X is started on olanzapine...
Mr. X is started on olanzapine and lorazepam as needed for agitation, and his twice daily divalproex sodium ER is increased to 250 every morning and 750 mg at every bedtime. However, Mr. X remains agitated and requires restraints. Olanzapine is switched from an as-needed dose to scheduled doses of 10 mg every morning and 15 mg at every bedtime, to address his psychosis and agitation.
On Day 24 of hospitalization, Mr. X’s ammonia levels are checked and are found to be 69 µ/dL, which is high (normal range: 15 to 45 µ/dL). Divalproex sodium ER is eventually tapered and discontinued. Mr. X is started on carbamazepine, which is titrated to 400 mg twice daily and results in some improvement in his behavior. He continues to receive carbamazepine and is started on dextromethorphan-quinidine, 10 mg/d, and increased to 10 mg twice daily; however, Mr. X continues to be verbally aggressive with staff, throws food, wanders around, and tries to leave the hospital unit, so he is placed in restraints and continues to require a sitter.
[polldaddy:10698428]
The authors' observations
Dementia typically affects older adults, but its onset can occur before age 60. It is a syndrome rather than a specific illness; the most common types are Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia. Diagnostic clarity and an evidence-based treatment plan are crucial for improving the quality of life for both the patient and their caregivers. The Table outlines the differential diagnosis of cognitive deficits. New-onset cognitive deficits warrant neuroimaging, and other testing may also be needed.

Behavioral and psychological symptoms of dementia
Noncognitive symptoms occur in 98% of individuals with dementia at some point in their disease and are often the most distressing to both caregivers and patients.1 Behavioral and psychological symptoms of dementia, including apathy, depression, sleep disorders, hallucinations, delusions, psychosis, agitation, and aggression, are exceedingly prevalent.2 Although these symptoms pose a significant burden, there are no clear published treatment guidelines; however, the American Psychiatric Association and the American Geriatric Society recommend using nonpharmacologic approaches as the first-line of treatment for patients with BPSD.3,4
Nonpharmacologic treatments
Due to the unfavorable adverse effects profiles of medications commonly used to treat dementia, nonpharmacologic treatment approaches have always played a crucial role for managing BPSD. Interventions such as music therapy, aromatherapy, art therapy, behavioral therapy, reality orientation, tailored activities, and physical exercises, have shown promising results for alleviating BPSD.5-7
Continue to: Pharmacologic therapies should be used...
Pharmacologic treatments
Pharmacologic therapies should be used when nonpharmacologic approaches are unsuccessful, or when a patient is at imminent risk to harm themselves or others.
Antipsychotics. Although there is conflicting data regarding the use of antipsychotics in older adults, these agents are the most common pharmacologic treatment for patients with BPSD. Several studies examining the efficacy of antipsychotics for treating BPSD have demonstrated an increased risk of cerebrovascular events, including stroke and death due to any cause.8 While the use of antipsychotics increases the risk of mortality in older adults, the absolute risk is still low.9
Antipsychotics used to treat BPSD include:
- Risperidone is well studied in older adults and has shown benefit for treating aggression, agitation, and psychosis.10
- Quetiapine has a favorable adverse effects profile and may help improve sleep and reduce anxiety.10
- Olanzapine. Low-dose olanzapine has been modestly effective in decreasing agitation and aggression in patients with Alzheimer’s and vascular dementias.11
- Aripiprazole has shown modest benefit in treating psychosis and agitation in patients with dementia but may be associated with insomnia or activation symptoms at lower doses.10
- Ziprasidone. Case reports have found benefit with oral and injectable forms.12
Antidepressants. In the CitAD study, which was a placebo-controlled randomized trial, citalopram titrated to a target of 30 mg/d was found to be effective in reducing BPSD.13 However, QTc prolongation limits the use of citalopram. Sertraline was studied in 1 small, randomized trial against haloperidol but showed no additional benefit.14
Mood stabilizers. In a small, randomized trial, carbamazepine was helpful for patients with BPSD who were resistant to treatment with antipsychotics, with efficacy demonstrated over 6 weeks.15 No other mood stabilizers have had significant positive results in treating BPSD.16
Anxiolytic medications. Some research suggests that the occasional use of lorazepam, as necessary, is acceptable for patients with extreme agitation or aggression when behavioral interventions or sleep aids are ineffective.17 Various case reports and case series have suggested gabapentin may be effective for BPSD.18
Prazosin. In a small randomized placebo-controlled trial, the commonly used antihypertensive agent prazosin reduced agitation and aggression in patients with Alzheimer’s dementia, at doses from 1 to 6 mg/d.19 Postural hypotension, the main adverse effect associated with prazosin, can limit its use.
Trazodone. Some research suggests trazodone can reduce irritability and aggression in patients with Alzheimer’s disease.20
Dextromethorphan/quinidine. In a 10-week phase 2 randomized clinical trial of patients with probable Alzheimer’s disease dementia, combination dextromethorphan/quinidine reduced agitation and was generally well tolerated.21
For patients such as Mr. X who do not respond to multiple pharmacologic treatments, electroconvulsive therapy (ECT) may be an option.
Continue to: Because Mr. X does not respond...
TREATMENT A trial of ECT
Because Mr. X does not respond to the standard treatment protocols, the treatment team and Mr. X’s family discuss the use of ECT to control his agitation. Consent is obtained from his legal guardian and Mr. X is medically cleared to receive ECT. Mr. X receives 3 ECT treatments per week. During the first week, Mr. X experiences post-treatment agitation and confusion. The frequency of ECT treatments is reduced to 2 treatments per week, and then 1 session per week. Mr. X starts to show improvement in his agitation and ECT is continued at 1 session per week for 7 weeks.
The authors’ observations
Electroconvulsive therapy has been an effective treatment for patients with treatment-resistant depression and has shown benefit in treating other psychiatric conditions such as acute mania, catatonia, psychotic disorders, and Parkinson’s disease.22 Its use as an off-label treatment for chronic neuropathic pain has also been well documented.23 Although ECT is not indicated for treating agitation and aggression in patients with dementia, its effectiveness for these symptoms has been discussed extensively in the literature.22,24-26
Electroconvulsive therapy treatment can be divided into 2 phases: an acute phase during which ECT is administered 2 to 3 times a week for 4 to 5 weeks, and a maintenance phase of weekly treatments for 4 weeks and then biweekly treatments for 8 weeks.26 Although extensive research supports the safe use of ECT in older adults, concerns for worsening cognitive impairment can deter patients and families from agreeing to this treatment.
Adverse effects of ECT such as headaches and postictal confusion are generally mild and transient. Severe adverse effects such as seizures, severe confusion, and delirium are uncommon.25 The number of ECT treatments required for a good effect ranges from 2 to 18, and the most common position for electrodes placement is bilateral. Outcomes can be measured by using rating scales such as the Cohen-Mansfield Agitation Inventory, Neuropsychiatric Inventory, Social Dysfunction and Aggression Scale, Clinical Global Impression scale, and Pittsford Agitation Scale.25 Obtaining consent from patients with dementia is generally not possible because these patients generally lack the capacity to make medical decisions. Clinicians should refer to their state laws regarding medical-decision making in such cases. The patient’s next of kin or medical power of attorney should be contacted, and the risks and benefits should be discussed before starting ECT.
OUTCOME Lasting improvement
Due to Mr. X’s improvement after ECT, on hospital Day 124, the restraints are removed and he no longer requires a sitter. He starts responding to his name and following simple verbal commands. Electroconvulsive therapy is tapered to every other week, and eventually stopped as his status improves. Mr. X continues to do well and is maintained on the same dosages of olanzapine, carbamazepine, and dextromethorphan-quinidine he had been receiving prior to discharge.
Related Resources
• Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
• Kales HC, Mulsant BH, Sajatovic M. Prescribing antipsychotics in geriatric patients: Focus on dementia. Current Psychiatry. 2017;16(12):24-30.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Citalopram • Celexa
Dextromethorphan- quinidine • Nuedexta
Divalproex sodium ER • Depakote
Donepezil • Aricept
Gabapentin • Neurontin
Haloperidol • Haldol
Lorazepam • Ativan
Memantine • Namenda
Olanzapine • Zyprexa
Prazosin • Minipress
Quetiapine • Seroquel
Risperidone • Risperdal
Rivastigmine • Exelon
Sertraline • Zoloft
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
1. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
2. Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62(10):1601-1608.
3. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
4. AGS Executive Committee. A guide to the management of psychotic disorders and neuropsychiatric symptoms of dementia in older adults. The American Geriatrics Society. Published April 2011. Accessed September 24, 2020. https://qioprogram.org/sites/default/files/AGS_Guidelines_for_Telligen.pdf
5. Yang MH, Lin LC, Wu SC, et al. Comparison of the efficacy of aroma-acupressure and aromatherapy for the treatment of dementia-associated agitation. BMC Complement Altern Med. 2015;15:93.
6. Cerga-Pashoja A, Lowery D, Bhattacharya R, et al. Evaluation of exercise on individuals with dementia and their carers: a randomised controlled trial. Trials. 2010;11:53.
7. Chen RC, Liu CL, Lin MH, et al. Non-pharmacological treatment reducing not only behavioral symptoms, but also psychotic symptoms of older adults with dementia: a prospective cohort study in Taiwan. Geriatr Gerontol Int. 2014;14(2):440-446.
8. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943.
9. Lenzer J. FDA warns about using antipsychotic drugs for dementia. BMJ. 2005;330(7497):922.
10. Burke AD, Tariot PN. Atypical antipsychotics in the elderly: a review of therapeutic trends and clinical outcomes. Expert Opin Pharmacother. 2009;10(15):2407-2414.
11. Moretti R, Torre R, Antonello T, et al. Olanzapine as a possible treatment of behavioral symptoms in vascular dementia: risks of cerebrovascular events. J Neurol. 2005;252(10):1186-1193.
12. Cole SA, Saleem R, Shea WP, et al. Ziprasidone for agitation or psychosis in dementia: four cases. Int J Psychiatry Med. 2005;35(1):91-98.
13. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA. 2014;311(7):682-691.
14. Gaber S, Ronzoli S, Bruno A, et al. Sertraline versus small doses of haloperidol in the treatment of agitated behavior in patients with dementia. Arch Gerontol Geriatr Suppl. 2001; 7:159-162.
15. Olin JT, Fox LS, Pawluczyk S, et al. A pilot randomized trial of carbamazepine for behavioral symptoms in treatment-resistant outpatients with Alzheimer disease. Am J Geriatr Psychiatry. 2001;9(4):400-405.
16. Konovalov S, Muralee S, Tampi RR. Anticonvulsants for the treatment of behavioral and psychological symptoms of dementia: a literature review. Int Psychogeriatr. 2008;20(2):293-308.
17. Davies SJC, Burhan AM, Kim D. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J Psychopharmacol. 2018;32(5):509-523.
18. Kim Y, Wilkins KM, Tampi RR. Use of gabapentin in the treatment of behavioural and psychological symptoms of dementia: a review of the evidence. Drugs Aging. 2008;25(3):187-196.
19. Wang LY, Shofer JB, Rohde K, et al. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry. 2009; 17(9):744-751.
20. López-Pousa S, Garre-Olmo J, Vilalta-Franch J, et al. Trazodone for Alzheimer’s disease: a naturalistic follow-up study. Arch Gerontol Geriatr. 2008;47(2):207-215.
21. Cummings JL, Lyketsos CG, Peskind ER. Effect of dextromethorphan-quinidine on agitation in patients with Alzheimer disease dementia: a randomized clinical trial. JAMA. 2015;314(12):1242-1254.
22. Ujkaj M, Davidoff DA, Seiner SJ, et al. Safety and efficacy of electroconvulsive therapy for the treatment of agitation and aggression in patients with dementia. Am J Geriatr Psychiatry. 2012;20(1):61-72.
23. McDaniel WW. Electroconvulsive therapy in complex regional pain syndromes. J ECT. 2003;19(4):226-229.
24. Glass OM, Forester BP, Hermida AP. Electroconvulsive therapy (ECT) for treating agitation in dementia (major neurocognitive disorder)–a promising option. Int Psychogeriatr. 2017;29(5):717-726.
25. Van den Berg JF, Kruithof HC, Kok RM, et al. Electroconvulsive therapy for agitation and aggression in dementia: a systematic review. Am J Geriatr Psychiatry. 2018;26(4):419-434.
26. Isserles M, Daskalakis ZJ, Kumar S, et al. Clinical effectiveness and tolerability of electroconvulsive therapy in patients with neuropsychiatric symptoms of dementia. J Alzheimers Dis. 2017;57(1):45-51.
Let’s ‘cancel’ these obsolete terms in DSM
Psychiatry has made significant scientific advances over the past century. However, it is still saddled with archaic terms, with pejorative connotations, disguised as official medical diagnoses. It is time to “cancel” those terms and replace them with ones that are neutral and have not accumulated baggage.
This process of “creative destruction” of psychiatric terminology is long overdue. It is frankly disturbing that the psychiatric jargon used around the time that the American Psychiatric Association was established 175 years ago (1844) is now considered insults and epithets. We no longer work in “lunatic asylums for the insane,” and our patients with intellectual disabilities are no longer classified as “morons,” “idiots,” or “imbeciles.” Such “diagnoses” have certainly contributed to the stigma of psychiatric brain disorders. Even the noble word “asylum” has acquired a negative valence because in the past it referred to hospitals that housed persons with serious mental illness.
Thankfully, some of the outrageous terms fabricated during the condemnable and dark era of slavery 2 centuries ago were never adopted by organized psychiatry. The absurd diagnosis of “negritude,” whose tenet was that black skin is a disease curable by whitening the skin, was “invented” by none other than Benjamin Rush, the Father of Psychiatry, whose conflicted soul was depicted by concomitantly owning a slave and positioning himself as an ardent abolitionist!
Terms that need to be replaced
Fast-forward to the modern era and consider the following:
Borderline personality disorder. It is truly tragic how this confusing and non-scientific term is used as an official diagnosis for a set of seriously ill persons. It is loaded with obloquy, indignity, and derision that completely ignore the tumult, self-harm, and disability with which patients who carry this label are burdened throughout their lives, despite being intelligent. This is a serious brain disorder that has been shown to be highly genetic and is characterized by many well-established structural brain abnormalities that have been documented in neuroimaging studies.1,2 Borderline personality should not be classified as a personality disorder but as an illness with multiple signs and symptoms, including mood lability, anger, impulsivity, self-cutting, suicidal urges, feelings of abandonment, and micro-psychotic episodes. A more clinically accurate term should be coined very soon to replace borderline personality, which should be discarded to the trash heap of obsolete psychiatric terms, and no longer inflicted on patients.
Neurosis. What is the justification for continuing to use the term “neurotic” for a person who has an anxiety disorder? Is it used because Jung and Freud propagated the term “neurosis” (after it was coined by William Cullen in 1769)? Neurosis has degenerated from a psychiatric diagnosis to a scornful snub that must never be used for any patient.
Schizophrenia. This diagnosis, coined by Eugen Bleuler to replace the narrow and pessimistic “dementia praecox” proposed by Emil Kraepelin in the 1920s, initially seemed to be a neutral description of a thought disorder (split associations, not split personality). Bleuler was perceptive enough to call his book Dementia Praecox or the Group of Schizophrenias, which is consistent with the modern scientific research that confirms schizophrenia is a very heterogeneous syndrome with hundreds of genetic and environmental biotypes with a similar phenotype but a wide range of severity, treatment response, and functional outcomes. However, in subsequent decades, schizophrenia became one of the most demeaning labels in psychiatry, casting a shadow of hopelessness and disability on the people who have this serious neurologic condition with many psychiatric symptoms. The term that should replace schizophrenia should be no more degrading than stroke, multiple sclerosis, or myocardial infarction.
Continue to: Over the past 15 years...
Over the past 15 years, an expanding group of schizophrenia experts have agreed that this term must be changed to one that reflects the core features of this syndrome, and have proposed terms such as “salience syndrome,” “psychosis-spectrum,” and “reality distortion and cognitive impairment disorder.”3 In fact, several countries have already adopted a new official diagnosis for schizophrenia.4 Japan now uses the term “integration disorder,” which has significantly reduced the stigma of this brain disorder.5 South Korea changed the name to “attunement disorder.” Hong Kong and Taiwan now use “dysfunction of thought and perception.” Some researchers recommend calling schizophrenia “Bleuler’s syndrome,” a neutral eponymous designation.
One of the most irritating things about the term schizophrenia is the widespread misconception that it means “split personality.” This prompts some sports announcers to call a football team “schizophrenic” if they play well in the first half and badly in the second. The stock market is labeled “schizophrenic” if it goes up one day and way down on the next. No other medical term is misused by the media as often as the term schizophrenia.
Narcissistic personality disorder. The origin of this diagnostic category is the concept of “malignant narcissism” coined by Erich Fromm in 1964, which he designated as “the quintessence of evil.” I strongly object to implying that evil is part of any psychiatric diagnosis. Numerous studies have found structural brain abnormalities (in both gray and white matter) in patients diagnosed with psychopathic traits.6 Later, malignant narcissism was reframed as narcissistic personality disorder in 1971 by Herbert Rosenfeld. Although malignant narcissism was never accepted by either the DSM or the International Classification of Diseases, narcissistic personality disorder has been included in the DSM for the past few decades. This diagnosis reeks of disparagement and negativity. Persons with narcissistic personality disorder have been shown to have pathological brain changes in resting-state functional connectivity,7 weakened frontostriatal white matter connectivity,8,9 and a reduced frontal thickness and cortical volume.10 A distorted sense of self and others is a socially disabling disorder that should generate empathy, not disdain. Narcissistic personality disorder should be replaced by a term that accurately describes its behavioral pathology, and should not incorporate Greek mythology.
Mania. This is another unfortunate diagnosis that immediately evokes a negative image of patients who suffer from a potentially lethal brain disorder. It was fortunate that Robert Kendall coined the term “bipolar disorder” to replace “manic-depressive illness,” but mania is still being used within bipolar disorder as a prominent clinical phase. While depression accurately describes the mood in the other phase of this disorder, the term mania evokes wild, irrational behavior. Because the actual mood symptom cluster in mania is either elation/grandiosity or irritability/anger, why not replace mania with “elation/irritability phase of bipolar disorder”? It is more descriptive of the patient’s mood and is less pejorative.
Nomenclature is vital, and words do matter, especially when used as a diagnostic medical term. Psychiatry must “cancel” its archaic names, which are infused with negative connotations. Reinventing the psychiatric lexicon is a necessary act of renewal in a specialty where a poorly worded diagnostic label can morph into the equivalent of a “scarlet letter.” Think of other contemptuous terms, such as refrigerator mother, male hysteria, moral insanity, toxic parents, inadequate personality disorder, neurasthenia, or catastrophic schizophrenia.
General medicine regularly discards many of its obsolete terms.11 These include terms such as ablepsy, ague, camp fever, bloody flux, chlorosis, catarrh, consumption, dropsy, French pox, phthisis, milk sickness, and scrumpox.
Think also of how society abandoned the antediluvian names of boys and girls. Few parents these days would name their son Ackley, Allard, Arundel, Awarnach, Beldon, Durward, Grower, Kenlm, or Legolan, or name their daughter Afton, Agrona, Arantxa, Corliss, Demelza, Eartha, Maida, Obsession, Radella, or Sacrifice.In summary, a necessary part of psychiatry’s progress is shedding obsolete terminology, even if it means slaughtering some widely used “traditional” vocabulary. It is a necessary act of renewal, and the image of psychiatry will be burnished by it.
1. Nasrallah HA. Borderline personality disorder is a heritable brain disease. Current Psychiatry. 2014;13(4):19-20,32.
2. Sagarwala R, Nasrallah HA. White matter pathology in patients with borderline personality disorder: a review of controlled DTI studies. Ann Clin Psychiatry. 2020;32(4):281-286.
3. Keshavan MS, Tandon R, Nasrallah HA. Renaming schizophrenia: keeping up with the facts. Schizophr Res. 2013;148(1-3):1-2.
4. Lasalvia A, Penta E, Sartorius N, et al. Should the label “schizophrenia” be abandoned? Schizophr Res. 2015;162(1-3):276-284.
5. Takahashi H, Ideno T, Okubo S, et al. Impact of changing the Japanese term for “schizophrenia” for reasons of stereotypical beliefs of schizophrenia in Japanese youth. Schizophr Res. 2009;112(1-3):149-152.
6. Johanson M, Vaurio D, Tiihunen J, et al. A systematic literature review of neuroimaging of psychopathic traits. Front Psychiatry. 2020;10:1027.
7. Yang, W, Cun L, Du X, et al. Gender differences in brain structure and resting-state functional connectivity related to narcissistic personality. Sci Rep. 2015;5:10924.
8. Chester DS, Cynam DR, Powell DK, et al. Narcissismis associated with weakened frontostriatal connectivity: a DTI study. Soc Cogn Affect Neurosci. 2016;11(7):1036-1040.
9. Nenadic I, Gullmar D, Dietzek M, et al. Brain structure in narcissistic personality disorder: a VBM and DTI pilot study. Psychiatry Res. 2015;231(2):184-186.
10. Mao Y, Sang N, Wang Y, et al. Reduced frontal cortex thickness and cortical volume associated with pathological narcissism. Neuroscience. 2016;378:51-57.
11. Nasrallah HA. The transient truths of medical ‘progress.’ Current Psychiatry. 2014;13(6):23-24.
Psychiatry has made significant scientific advances over the past century. However, it is still saddled with archaic terms, with pejorative connotations, disguised as official medical diagnoses. It is time to “cancel” those terms and replace them with ones that are neutral and have not accumulated baggage.
This process of “creative destruction” of psychiatric terminology is long overdue. It is frankly disturbing that the psychiatric jargon used around the time that the American Psychiatric Association was established 175 years ago (1844) is now considered insults and epithets. We no longer work in “lunatic asylums for the insane,” and our patients with intellectual disabilities are no longer classified as “morons,” “idiots,” or “imbeciles.” Such “diagnoses” have certainly contributed to the stigma of psychiatric brain disorders. Even the noble word “asylum” has acquired a negative valence because in the past it referred to hospitals that housed persons with serious mental illness.
Thankfully, some of the outrageous terms fabricated during the condemnable and dark era of slavery 2 centuries ago were never adopted by organized psychiatry. The absurd diagnosis of “negritude,” whose tenet was that black skin is a disease curable by whitening the skin, was “invented” by none other than Benjamin Rush, the Father of Psychiatry, whose conflicted soul was depicted by concomitantly owning a slave and positioning himself as an ardent abolitionist!
Terms that need to be replaced
Fast-forward to the modern era and consider the following:
Borderline personality disorder. It is truly tragic how this confusing and non-scientific term is used as an official diagnosis for a set of seriously ill persons. It is loaded with obloquy, indignity, and derision that completely ignore the tumult, self-harm, and disability with which patients who carry this label are burdened throughout their lives, despite being intelligent. This is a serious brain disorder that has been shown to be highly genetic and is characterized by many well-established structural brain abnormalities that have been documented in neuroimaging studies.1,2 Borderline personality should not be classified as a personality disorder but as an illness with multiple signs and symptoms, including mood lability, anger, impulsivity, self-cutting, suicidal urges, feelings of abandonment, and micro-psychotic episodes. A more clinically accurate term should be coined very soon to replace borderline personality, which should be discarded to the trash heap of obsolete psychiatric terms, and no longer inflicted on patients.
Neurosis. What is the justification for continuing to use the term “neurotic” for a person who has an anxiety disorder? Is it used because Jung and Freud propagated the term “neurosis” (after it was coined by William Cullen in 1769)? Neurosis has degenerated from a psychiatric diagnosis to a scornful snub that must never be used for any patient.
Schizophrenia. This diagnosis, coined by Eugen Bleuler to replace the narrow and pessimistic “dementia praecox” proposed by Emil Kraepelin in the 1920s, initially seemed to be a neutral description of a thought disorder (split associations, not split personality). Bleuler was perceptive enough to call his book Dementia Praecox or the Group of Schizophrenias, which is consistent with the modern scientific research that confirms schizophrenia is a very heterogeneous syndrome with hundreds of genetic and environmental biotypes with a similar phenotype but a wide range of severity, treatment response, and functional outcomes. However, in subsequent decades, schizophrenia became one of the most demeaning labels in psychiatry, casting a shadow of hopelessness and disability on the people who have this serious neurologic condition with many psychiatric symptoms. The term that should replace schizophrenia should be no more degrading than stroke, multiple sclerosis, or myocardial infarction.
Continue to: Over the past 15 years...
Over the past 15 years, an expanding group of schizophrenia experts have agreed that this term must be changed to one that reflects the core features of this syndrome, and have proposed terms such as “salience syndrome,” “psychosis-spectrum,” and “reality distortion and cognitive impairment disorder.”3 In fact, several countries have already adopted a new official diagnosis for schizophrenia.4 Japan now uses the term “integration disorder,” which has significantly reduced the stigma of this brain disorder.5 South Korea changed the name to “attunement disorder.” Hong Kong and Taiwan now use “dysfunction of thought and perception.” Some researchers recommend calling schizophrenia “Bleuler’s syndrome,” a neutral eponymous designation.
One of the most irritating things about the term schizophrenia is the widespread misconception that it means “split personality.” This prompts some sports announcers to call a football team “schizophrenic” if they play well in the first half and badly in the second. The stock market is labeled “schizophrenic” if it goes up one day and way down on the next. No other medical term is misused by the media as often as the term schizophrenia.
Narcissistic personality disorder. The origin of this diagnostic category is the concept of “malignant narcissism” coined by Erich Fromm in 1964, which he designated as “the quintessence of evil.” I strongly object to implying that evil is part of any psychiatric diagnosis. Numerous studies have found structural brain abnormalities (in both gray and white matter) in patients diagnosed with psychopathic traits.6 Later, malignant narcissism was reframed as narcissistic personality disorder in 1971 by Herbert Rosenfeld. Although malignant narcissism was never accepted by either the DSM or the International Classification of Diseases, narcissistic personality disorder has been included in the DSM for the past few decades. This diagnosis reeks of disparagement and negativity. Persons with narcissistic personality disorder have been shown to have pathological brain changes in resting-state functional connectivity,7 weakened frontostriatal white matter connectivity,8,9 and a reduced frontal thickness and cortical volume.10 A distorted sense of self and others is a socially disabling disorder that should generate empathy, not disdain. Narcissistic personality disorder should be replaced by a term that accurately describes its behavioral pathology, and should not incorporate Greek mythology.
Mania. This is another unfortunate diagnosis that immediately evokes a negative image of patients who suffer from a potentially lethal brain disorder. It was fortunate that Robert Kendall coined the term “bipolar disorder” to replace “manic-depressive illness,” but mania is still being used within bipolar disorder as a prominent clinical phase. While depression accurately describes the mood in the other phase of this disorder, the term mania evokes wild, irrational behavior. Because the actual mood symptom cluster in mania is either elation/grandiosity or irritability/anger, why not replace mania with “elation/irritability phase of bipolar disorder”? It is more descriptive of the patient’s mood and is less pejorative.
Nomenclature is vital, and words do matter, especially when used as a diagnostic medical term. Psychiatry must “cancel” its archaic names, which are infused with negative connotations. Reinventing the psychiatric lexicon is a necessary act of renewal in a specialty where a poorly worded diagnostic label can morph into the equivalent of a “scarlet letter.” Think of other contemptuous terms, such as refrigerator mother, male hysteria, moral insanity, toxic parents, inadequate personality disorder, neurasthenia, or catastrophic schizophrenia.
General medicine regularly discards many of its obsolete terms.11 These include terms such as ablepsy, ague, camp fever, bloody flux, chlorosis, catarrh, consumption, dropsy, French pox, phthisis, milk sickness, and scrumpox.
Think also of how society abandoned the antediluvian names of boys and girls. Few parents these days would name their son Ackley, Allard, Arundel, Awarnach, Beldon, Durward, Grower, Kenlm, or Legolan, or name their daughter Afton, Agrona, Arantxa, Corliss, Demelza, Eartha, Maida, Obsession, Radella, or Sacrifice.In summary, a necessary part of psychiatry’s progress is shedding obsolete terminology, even if it means slaughtering some widely used “traditional” vocabulary. It is a necessary act of renewal, and the image of psychiatry will be burnished by it.
Psychiatry has made significant scientific advances over the past century. However, it is still saddled with archaic terms, with pejorative connotations, disguised as official medical diagnoses. It is time to “cancel” those terms and replace them with ones that are neutral and have not accumulated baggage.
This process of “creative destruction” of psychiatric terminology is long overdue. It is frankly disturbing that the psychiatric jargon used around the time that the American Psychiatric Association was established 175 years ago (1844) is now considered insults and epithets. We no longer work in “lunatic asylums for the insane,” and our patients with intellectual disabilities are no longer classified as “morons,” “idiots,” or “imbeciles.” Such “diagnoses” have certainly contributed to the stigma of psychiatric brain disorders. Even the noble word “asylum” has acquired a negative valence because in the past it referred to hospitals that housed persons with serious mental illness.
Thankfully, some of the outrageous terms fabricated during the condemnable and dark era of slavery 2 centuries ago were never adopted by organized psychiatry. The absurd diagnosis of “negritude,” whose tenet was that black skin is a disease curable by whitening the skin, was “invented” by none other than Benjamin Rush, the Father of Psychiatry, whose conflicted soul was depicted by concomitantly owning a slave and positioning himself as an ardent abolitionist!
Terms that need to be replaced
Fast-forward to the modern era and consider the following:
Borderline personality disorder. It is truly tragic how this confusing and non-scientific term is used as an official diagnosis for a set of seriously ill persons. It is loaded with obloquy, indignity, and derision that completely ignore the tumult, self-harm, and disability with which patients who carry this label are burdened throughout their lives, despite being intelligent. This is a serious brain disorder that has been shown to be highly genetic and is characterized by many well-established structural brain abnormalities that have been documented in neuroimaging studies.1,2 Borderline personality should not be classified as a personality disorder but as an illness with multiple signs and symptoms, including mood lability, anger, impulsivity, self-cutting, suicidal urges, feelings of abandonment, and micro-psychotic episodes. A more clinically accurate term should be coined very soon to replace borderline personality, which should be discarded to the trash heap of obsolete psychiatric terms, and no longer inflicted on patients.
Neurosis. What is the justification for continuing to use the term “neurotic” for a person who has an anxiety disorder? Is it used because Jung and Freud propagated the term “neurosis” (after it was coined by William Cullen in 1769)? Neurosis has degenerated from a psychiatric diagnosis to a scornful snub that must never be used for any patient.
Schizophrenia. This diagnosis, coined by Eugen Bleuler to replace the narrow and pessimistic “dementia praecox” proposed by Emil Kraepelin in the 1920s, initially seemed to be a neutral description of a thought disorder (split associations, not split personality). Bleuler was perceptive enough to call his book Dementia Praecox or the Group of Schizophrenias, which is consistent with the modern scientific research that confirms schizophrenia is a very heterogeneous syndrome with hundreds of genetic and environmental biotypes with a similar phenotype but a wide range of severity, treatment response, and functional outcomes. However, in subsequent decades, schizophrenia became one of the most demeaning labels in psychiatry, casting a shadow of hopelessness and disability on the people who have this serious neurologic condition with many psychiatric symptoms. The term that should replace schizophrenia should be no more degrading than stroke, multiple sclerosis, or myocardial infarction.
Continue to: Over the past 15 years...
Over the past 15 years, an expanding group of schizophrenia experts have agreed that this term must be changed to one that reflects the core features of this syndrome, and have proposed terms such as “salience syndrome,” “psychosis-spectrum,” and “reality distortion and cognitive impairment disorder.”3 In fact, several countries have already adopted a new official diagnosis for schizophrenia.4 Japan now uses the term “integration disorder,” which has significantly reduced the stigma of this brain disorder.5 South Korea changed the name to “attunement disorder.” Hong Kong and Taiwan now use “dysfunction of thought and perception.” Some researchers recommend calling schizophrenia “Bleuler’s syndrome,” a neutral eponymous designation.
One of the most irritating things about the term schizophrenia is the widespread misconception that it means “split personality.” This prompts some sports announcers to call a football team “schizophrenic” if they play well in the first half and badly in the second. The stock market is labeled “schizophrenic” if it goes up one day and way down on the next. No other medical term is misused by the media as often as the term schizophrenia.
Narcissistic personality disorder. The origin of this diagnostic category is the concept of “malignant narcissism” coined by Erich Fromm in 1964, which he designated as “the quintessence of evil.” I strongly object to implying that evil is part of any psychiatric diagnosis. Numerous studies have found structural brain abnormalities (in both gray and white matter) in patients diagnosed with psychopathic traits.6 Later, malignant narcissism was reframed as narcissistic personality disorder in 1971 by Herbert Rosenfeld. Although malignant narcissism was never accepted by either the DSM or the International Classification of Diseases, narcissistic personality disorder has been included in the DSM for the past few decades. This diagnosis reeks of disparagement and negativity. Persons with narcissistic personality disorder have been shown to have pathological brain changes in resting-state functional connectivity,7 weakened frontostriatal white matter connectivity,8,9 and a reduced frontal thickness and cortical volume.10 A distorted sense of self and others is a socially disabling disorder that should generate empathy, not disdain. Narcissistic personality disorder should be replaced by a term that accurately describes its behavioral pathology, and should not incorporate Greek mythology.
Mania. This is another unfortunate diagnosis that immediately evokes a negative image of patients who suffer from a potentially lethal brain disorder. It was fortunate that Robert Kendall coined the term “bipolar disorder” to replace “manic-depressive illness,” but mania is still being used within bipolar disorder as a prominent clinical phase. While depression accurately describes the mood in the other phase of this disorder, the term mania evokes wild, irrational behavior. Because the actual mood symptom cluster in mania is either elation/grandiosity or irritability/anger, why not replace mania with “elation/irritability phase of bipolar disorder”? It is more descriptive of the patient’s mood and is less pejorative.
Nomenclature is vital, and words do matter, especially when used as a diagnostic medical term. Psychiatry must “cancel” its archaic names, which are infused with negative connotations. Reinventing the psychiatric lexicon is a necessary act of renewal in a specialty where a poorly worded diagnostic label can morph into the equivalent of a “scarlet letter.” Think of other contemptuous terms, such as refrigerator mother, male hysteria, moral insanity, toxic parents, inadequate personality disorder, neurasthenia, or catastrophic schizophrenia.
General medicine regularly discards many of its obsolete terms.11 These include terms such as ablepsy, ague, camp fever, bloody flux, chlorosis, catarrh, consumption, dropsy, French pox, phthisis, milk sickness, and scrumpox.
Think also of how society abandoned the antediluvian names of boys and girls. Few parents these days would name their son Ackley, Allard, Arundel, Awarnach, Beldon, Durward, Grower, Kenlm, or Legolan, or name their daughter Afton, Agrona, Arantxa, Corliss, Demelza, Eartha, Maida, Obsession, Radella, or Sacrifice.In summary, a necessary part of psychiatry’s progress is shedding obsolete terminology, even if it means slaughtering some widely used “traditional” vocabulary. It is a necessary act of renewal, and the image of psychiatry will be burnished by it.
1. Nasrallah HA. Borderline personality disorder is a heritable brain disease. Current Psychiatry. 2014;13(4):19-20,32.
2. Sagarwala R, Nasrallah HA. White matter pathology in patients with borderline personality disorder: a review of controlled DTI studies. Ann Clin Psychiatry. 2020;32(4):281-286.
3. Keshavan MS, Tandon R, Nasrallah HA. Renaming schizophrenia: keeping up with the facts. Schizophr Res. 2013;148(1-3):1-2.
4. Lasalvia A, Penta E, Sartorius N, et al. Should the label “schizophrenia” be abandoned? Schizophr Res. 2015;162(1-3):276-284.
5. Takahashi H, Ideno T, Okubo S, et al. Impact of changing the Japanese term for “schizophrenia” for reasons of stereotypical beliefs of schizophrenia in Japanese youth. Schizophr Res. 2009;112(1-3):149-152.
6. Johanson M, Vaurio D, Tiihunen J, et al. A systematic literature review of neuroimaging of psychopathic traits. Front Psychiatry. 2020;10:1027.
7. Yang, W, Cun L, Du X, et al. Gender differences in brain structure and resting-state functional connectivity related to narcissistic personality. Sci Rep. 2015;5:10924.
8. Chester DS, Cynam DR, Powell DK, et al. Narcissismis associated with weakened frontostriatal connectivity: a DTI study. Soc Cogn Affect Neurosci. 2016;11(7):1036-1040.
9. Nenadic I, Gullmar D, Dietzek M, et al. Brain structure in narcissistic personality disorder: a VBM and DTI pilot study. Psychiatry Res. 2015;231(2):184-186.
10. Mao Y, Sang N, Wang Y, et al. Reduced frontal cortex thickness and cortical volume associated with pathological narcissism. Neuroscience. 2016;378:51-57.
11. Nasrallah HA. The transient truths of medical ‘progress.’ Current Psychiatry. 2014;13(6):23-24.
1. Nasrallah HA. Borderline personality disorder is a heritable brain disease. Current Psychiatry. 2014;13(4):19-20,32.
2. Sagarwala R, Nasrallah HA. White matter pathology in patients with borderline personality disorder: a review of controlled DTI studies. Ann Clin Psychiatry. 2020;32(4):281-286.
3. Keshavan MS, Tandon R, Nasrallah HA. Renaming schizophrenia: keeping up with the facts. Schizophr Res. 2013;148(1-3):1-2.
4. Lasalvia A, Penta E, Sartorius N, et al. Should the label “schizophrenia” be abandoned? Schizophr Res. 2015;162(1-3):276-284.
5. Takahashi H, Ideno T, Okubo S, et al. Impact of changing the Japanese term for “schizophrenia” for reasons of stereotypical beliefs of schizophrenia in Japanese youth. Schizophr Res. 2009;112(1-3):149-152.
6. Johanson M, Vaurio D, Tiihunen J, et al. A systematic literature review of neuroimaging of psychopathic traits. Front Psychiatry. 2020;10:1027.
7. Yang, W, Cun L, Du X, et al. Gender differences in brain structure and resting-state functional connectivity related to narcissistic personality. Sci Rep. 2015;5:10924.
8. Chester DS, Cynam DR, Powell DK, et al. Narcissismis associated with weakened frontostriatal connectivity: a DTI study. Soc Cogn Affect Neurosci. 2016;11(7):1036-1040.
9. Nenadic I, Gullmar D, Dietzek M, et al. Brain structure in narcissistic personality disorder: a VBM and DTI pilot study. Psychiatry Res. 2015;231(2):184-186.
10. Mao Y, Sang N, Wang Y, et al. Reduced frontal cortex thickness and cortical volume associated with pathological narcissism. Neuroscience. 2016;378:51-57.
11. Nasrallah HA. The transient truths of medical ‘progress.’ Current Psychiatry. 2014;13(6):23-24.







