User login
Patient Handout: Safe practices during the COVID-19 pandemic
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
Oncology dominates clinical trial landscape
Oncology will account for a substantial majority of clinical trials to be launched in 2020, as well as accounting for most of those to be completed this year, according to a new analysis.
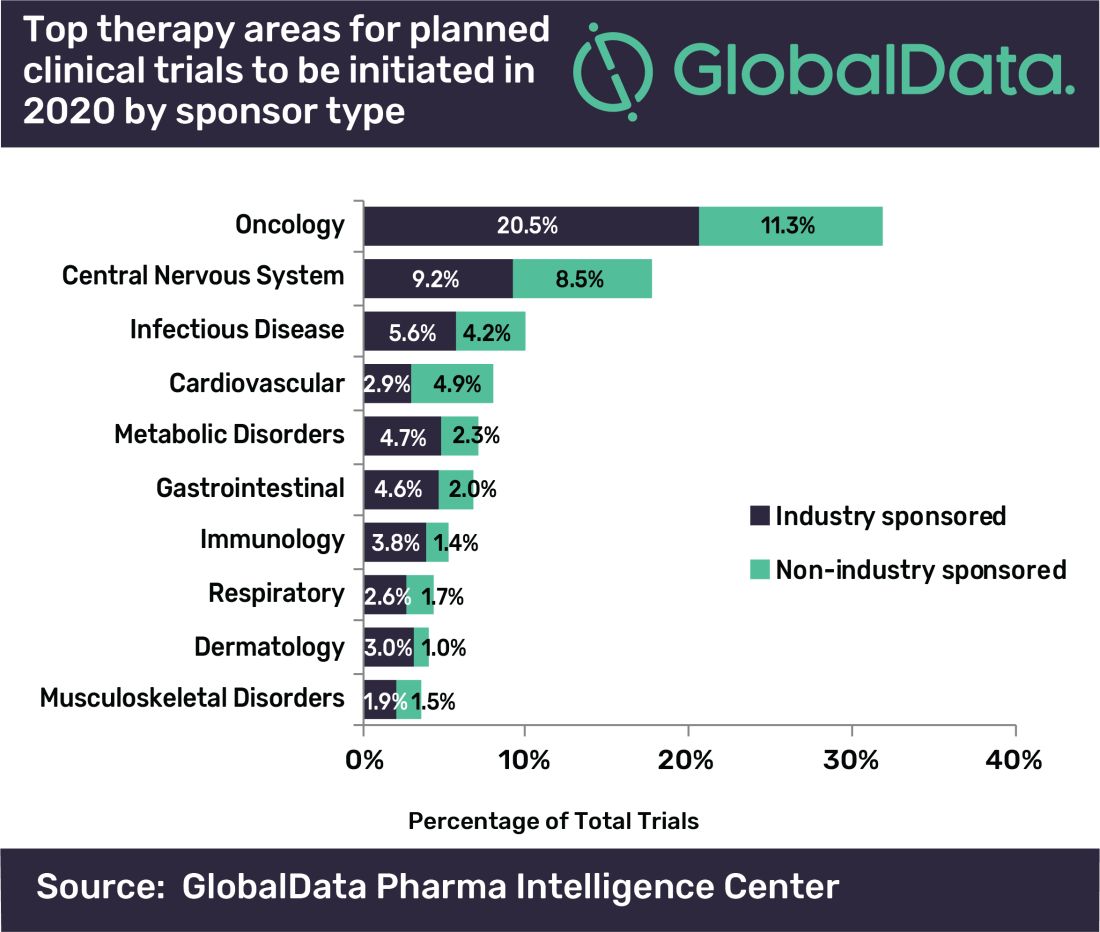
“A large number of early stage clinical trials within this field are likely to be due to the demand for novel therapeutic approaches addressing unmet medical need,” commented Mohamed Abukar, pharma analyst at GlobalData.
Most oncology studies planned to start in 2020 are phase 1 and 2, and 61.9% are industry sponsored. Eli Lilly and Novartis have announced the most upcoming studies.
Among the new drugs being evaluated in these clinical trials, four of the top seven drugs in phase 1–3 development are monoclonal antibodies, with the most studies being conducted on the experimental agents ZW25 (Zymeworks) and KSI-301 (Kodiak Sciences), the report notes.
As for clinical trials due for completion this year, many are funded by nonindustry sources, with Memorial Sloan Kettering Cancer Center accounting for the most number of trials.
Top Indications Explored in Clinical Trials
Oncology also accounts for eight of the top ten indications for clinical trials planned to start in 2020, with solid tumors, breast cancer, and non–small cell lung cancer accounting for the second, third, and fourth top spots, respectively, regardless of sponsor type.
However, for industry-sponsored clinical trials, the predominant area is solid tumors for new investigations to start this year, followed by breast cancer, then pain.
“This is attributed to the manner in which the burden of cancer worldwide necessitates industry investment to allow for capitalization on the increasing market size,” Abukar said.
This article first appeared on Medscape.com.
Oncology will account for a substantial majority of clinical trials to be launched in 2020, as well as accounting for most of those to be completed this year, according to a new analysis.

“A large number of early stage clinical trials within this field are likely to be due to the demand for novel therapeutic approaches addressing unmet medical need,” commented Mohamed Abukar, pharma analyst at GlobalData.
Most oncology studies planned to start in 2020 are phase 1 and 2, and 61.9% are industry sponsored. Eli Lilly and Novartis have announced the most upcoming studies.
Among the new drugs being evaluated in these clinical trials, four of the top seven drugs in phase 1–3 development are monoclonal antibodies, with the most studies being conducted on the experimental agents ZW25 (Zymeworks) and KSI-301 (Kodiak Sciences), the report notes.
As for clinical trials due for completion this year, many are funded by nonindustry sources, with Memorial Sloan Kettering Cancer Center accounting for the most number of trials.
Top Indications Explored in Clinical Trials
Oncology also accounts for eight of the top ten indications for clinical trials planned to start in 2020, with solid tumors, breast cancer, and non–small cell lung cancer accounting for the second, third, and fourth top spots, respectively, regardless of sponsor type.
However, for industry-sponsored clinical trials, the predominant area is solid tumors for new investigations to start this year, followed by breast cancer, then pain.
“This is attributed to the manner in which the burden of cancer worldwide necessitates industry investment to allow for capitalization on the increasing market size,” Abukar said.
This article first appeared on Medscape.com.
Oncology will account for a substantial majority of clinical trials to be launched in 2020, as well as accounting for most of those to be completed this year, according to a new analysis.

“A large number of early stage clinical trials within this field are likely to be due to the demand for novel therapeutic approaches addressing unmet medical need,” commented Mohamed Abukar, pharma analyst at GlobalData.
Most oncology studies planned to start in 2020 are phase 1 and 2, and 61.9% are industry sponsored. Eli Lilly and Novartis have announced the most upcoming studies.
Among the new drugs being evaluated in these clinical trials, four of the top seven drugs in phase 1–3 development are monoclonal antibodies, with the most studies being conducted on the experimental agents ZW25 (Zymeworks) and KSI-301 (Kodiak Sciences), the report notes.
As for clinical trials due for completion this year, many are funded by nonindustry sources, with Memorial Sloan Kettering Cancer Center accounting for the most number of trials.
Top Indications Explored in Clinical Trials
Oncology also accounts for eight of the top ten indications for clinical trials planned to start in 2020, with solid tumors, breast cancer, and non–small cell lung cancer accounting for the second, third, and fourth top spots, respectively, regardless of sponsor type.
However, for industry-sponsored clinical trials, the predominant area is solid tumors for new investigations to start this year, followed by breast cancer, then pain.
“This is attributed to the manner in which the burden of cancer worldwide necessitates industry investment to allow for capitalization on the increasing market size,” Abukar said.
This article first appeared on Medscape.com.
CDC creates interactive education module to improve RMSF recognition
The Centers for Disease Control and Prevention has created a first-of-its-kind interactive training module to help physicians both recognize and diagnose Rocky Mountain spotted fever (RMSF).
A record number of cases of RMSF were reported to the CDC in 2017 (6,248, up from 4,269 in 2016), but less than 1% of those cases had sufficient laboratory evidence to be confirmed. The CDC education module includes scenarios based on real cases to aid providers in recognizing RMSF and differentiating it from similar diseases. CME is available for physicians, nurse practitioners, physician assistants, veterinarians, nurses, epidemiologists, public health professionals, educators, and health communicators.
The disease initially presents with nonspecific symptoms such as fever, headache, or rash, but if left untreated, patients may require the amputation of fingers, toes, or limbs because of low blood flow; heart and lung specialty care; and ICU management. About 20% of untreated cases are fatal; half of these deaths occur within 8 days of initial presentation.
“Rocky Mountain spotted fever can be deadly if not treated early – yet cases often go unrecognized because the signs and symptoms are similar to those of many other diseases. With tickborne diseases on the rise in the U.S., this training will better equip health care providers to identify, diagnose, and treat this potentially fatal disease,” said CDC director Robert R. Redfield, MD.
Find the full press release on the CDC website.
The Centers for Disease Control and Prevention has created a first-of-its-kind interactive training module to help physicians both recognize and diagnose Rocky Mountain spotted fever (RMSF).
A record number of cases of RMSF were reported to the CDC in 2017 (6,248, up from 4,269 in 2016), but less than 1% of those cases had sufficient laboratory evidence to be confirmed. The CDC education module includes scenarios based on real cases to aid providers in recognizing RMSF and differentiating it from similar diseases. CME is available for physicians, nurse practitioners, physician assistants, veterinarians, nurses, epidemiologists, public health professionals, educators, and health communicators.
The disease initially presents with nonspecific symptoms such as fever, headache, or rash, but if left untreated, patients may require the amputation of fingers, toes, or limbs because of low blood flow; heart and lung specialty care; and ICU management. About 20% of untreated cases are fatal; half of these deaths occur within 8 days of initial presentation.
“Rocky Mountain spotted fever can be deadly if not treated early – yet cases often go unrecognized because the signs and symptoms are similar to those of many other diseases. With tickborne diseases on the rise in the U.S., this training will better equip health care providers to identify, diagnose, and treat this potentially fatal disease,” said CDC director Robert R. Redfield, MD.
Find the full press release on the CDC website.
The Centers for Disease Control and Prevention has created a first-of-its-kind interactive training module to help physicians both recognize and diagnose Rocky Mountain spotted fever (RMSF).
A record number of cases of RMSF were reported to the CDC in 2017 (6,248, up from 4,269 in 2016), but less than 1% of those cases had sufficient laboratory evidence to be confirmed. The CDC education module includes scenarios based on real cases to aid providers in recognizing RMSF and differentiating it from similar diseases. CME is available for physicians, nurse practitioners, physician assistants, veterinarians, nurses, epidemiologists, public health professionals, educators, and health communicators.
The disease initially presents with nonspecific symptoms such as fever, headache, or rash, but if left untreated, patients may require the amputation of fingers, toes, or limbs because of low blood flow; heart and lung specialty care; and ICU management. About 20% of untreated cases are fatal; half of these deaths occur within 8 days of initial presentation.
“Rocky Mountain spotted fever can be deadly if not treated early – yet cases often go unrecognized because the signs and symptoms are similar to those of many other diseases. With tickborne diseases on the rise in the U.S., this training will better equip health care providers to identify, diagnose, and treat this potentially fatal disease,” said CDC director Robert R. Redfield, MD.
Find the full press release on the CDC website.
Quick tips: How to get your study published
SAN DIEGO – Looking to get your study published in a top medical journal? Bob Löwenberg, MD, PhD, editor-in-chief of Blood, says to start thinking about what appeals to readers.
“What do readers want? They want important information with impact in a clinical or biological sense,” Dr. Löwenberg of Erasmus University Rotterdam (the Netherlands) said at the annual meeting of the American Society of Hematology. “Usually they want to get novel information – new and cutting-edge insights, if possible. And readers want to receive access to information that is right. This is about quality.”
Dr. Löwenberg offered several tips for getting published:
- Make sure your paper has a “clear message” that comes across in both its title and a concisely written abstract. “When your colleagues are going to scan the journal, they should say ‘Hey, this is an interesting title’ or ‘This is an interesting abstract,’ ” Dr. Löwenberg said.
- Avoid jargon and slang. And don’t fill your paper with abbreviations because that will make it unreadable.
- Don’t just cut and paste the abstract from your meeting submission. Update the information and rewrite it before submitting it. “The abstract is so important because it is the part of your manuscript that’s copied by reference systems,” Dr. Löwenberg said. “It’s more broadly published than your manuscript. Write it in such a way that it tells your entire story in a minimal number of words, without changing the overall message of your paper, and in clear language.”
- Focus on providing important background in the introduction, which usually summarizes existing research.
- “Distill the essentials” in the discussion section. “Don’t repeat the results. Discuss the importance of your findings in relation to the state-of-the-art information that you have presented in the introduction,” he said.
- Beware of plagiarism, which includes “self-plagiarism” – duplicating your own previous research without acknowledgment.
- Understand new rules regarding data-sharing requirements developed by the International Committee of Medical Journal Editors. In order to be considered for publication by the committee’s member journals, clinical trials that begin enrolling participants as of Jan. 1, 2019, must include a data-sharing plan in the trial’s registration.
- Don’t be surprised if your paper is turned down. “We all have experience with rejected papers,” he said. “This is part of the game.”
If you are rejected, you may wish to send a rebuttal – a form of appeal – to the journal. Consider this option if the journal “clearly misunderstood or misrepresented the paper,” he said. “Be polite, try to be unemotional and clear, and never [write] it the same day as when you are still angry about this decision.” Once you send a rebuttal, wait for at least a week for a response. If one doesn’t come, he said, feel free to submit the paper elsewhere.
Dr. Löwenberg reported having no relevant financial disclosures.
SAN DIEGO – Looking to get your study published in a top medical journal? Bob Löwenberg, MD, PhD, editor-in-chief of Blood, says to start thinking about what appeals to readers.
“What do readers want? They want important information with impact in a clinical or biological sense,” Dr. Löwenberg of Erasmus University Rotterdam (the Netherlands) said at the annual meeting of the American Society of Hematology. “Usually they want to get novel information – new and cutting-edge insights, if possible. And readers want to receive access to information that is right. This is about quality.”
Dr. Löwenberg offered several tips for getting published:
- Make sure your paper has a “clear message” that comes across in both its title and a concisely written abstract. “When your colleagues are going to scan the journal, they should say ‘Hey, this is an interesting title’ or ‘This is an interesting abstract,’ ” Dr. Löwenberg said.
- Avoid jargon and slang. And don’t fill your paper with abbreviations because that will make it unreadable.
- Don’t just cut and paste the abstract from your meeting submission. Update the information and rewrite it before submitting it. “The abstract is so important because it is the part of your manuscript that’s copied by reference systems,” Dr. Löwenberg said. “It’s more broadly published than your manuscript. Write it in such a way that it tells your entire story in a minimal number of words, without changing the overall message of your paper, and in clear language.”
- Focus on providing important background in the introduction, which usually summarizes existing research.
- “Distill the essentials” in the discussion section. “Don’t repeat the results. Discuss the importance of your findings in relation to the state-of-the-art information that you have presented in the introduction,” he said.
- Beware of plagiarism, which includes “self-plagiarism” – duplicating your own previous research without acknowledgment.
- Understand new rules regarding data-sharing requirements developed by the International Committee of Medical Journal Editors. In order to be considered for publication by the committee’s member journals, clinical trials that begin enrolling participants as of Jan. 1, 2019, must include a data-sharing plan in the trial’s registration.
- Don’t be surprised if your paper is turned down. “We all have experience with rejected papers,” he said. “This is part of the game.”
If you are rejected, you may wish to send a rebuttal – a form of appeal – to the journal. Consider this option if the journal “clearly misunderstood or misrepresented the paper,” he said. “Be polite, try to be unemotional and clear, and never [write] it the same day as when you are still angry about this decision.” Once you send a rebuttal, wait for at least a week for a response. If one doesn’t come, he said, feel free to submit the paper elsewhere.
Dr. Löwenberg reported having no relevant financial disclosures.
SAN DIEGO – Looking to get your study published in a top medical journal? Bob Löwenberg, MD, PhD, editor-in-chief of Blood, says to start thinking about what appeals to readers.
“What do readers want? They want important information with impact in a clinical or biological sense,” Dr. Löwenberg of Erasmus University Rotterdam (the Netherlands) said at the annual meeting of the American Society of Hematology. “Usually they want to get novel information – new and cutting-edge insights, if possible. And readers want to receive access to information that is right. This is about quality.”
Dr. Löwenberg offered several tips for getting published:
- Make sure your paper has a “clear message” that comes across in both its title and a concisely written abstract. “When your colleagues are going to scan the journal, they should say ‘Hey, this is an interesting title’ or ‘This is an interesting abstract,’ ” Dr. Löwenberg said.
- Avoid jargon and slang. And don’t fill your paper with abbreviations because that will make it unreadable.
- Don’t just cut and paste the abstract from your meeting submission. Update the information and rewrite it before submitting it. “The abstract is so important because it is the part of your manuscript that’s copied by reference systems,” Dr. Löwenberg said. “It’s more broadly published than your manuscript. Write it in such a way that it tells your entire story in a minimal number of words, without changing the overall message of your paper, and in clear language.”
- Focus on providing important background in the introduction, which usually summarizes existing research.
- “Distill the essentials” in the discussion section. “Don’t repeat the results. Discuss the importance of your findings in relation to the state-of-the-art information that you have presented in the introduction,” he said.
- Beware of plagiarism, which includes “self-plagiarism” – duplicating your own previous research without acknowledgment.
- Understand new rules regarding data-sharing requirements developed by the International Committee of Medical Journal Editors. In order to be considered for publication by the committee’s member journals, clinical trials that begin enrolling participants as of Jan. 1, 2019, must include a data-sharing plan in the trial’s registration.
- Don’t be surprised if your paper is turned down. “We all have experience with rejected papers,” he said. “This is part of the game.”
If you are rejected, you may wish to send a rebuttal – a form of appeal – to the journal. Consider this option if the journal “clearly misunderstood or misrepresented the paper,” he said. “Be polite, try to be unemotional and clear, and never [write] it the same day as when you are still angry about this decision.” Once you send a rebuttal, wait for at least a week for a response. If one doesn’t come, he said, feel free to submit the paper elsewhere.
Dr. Löwenberg reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM ASH 2018
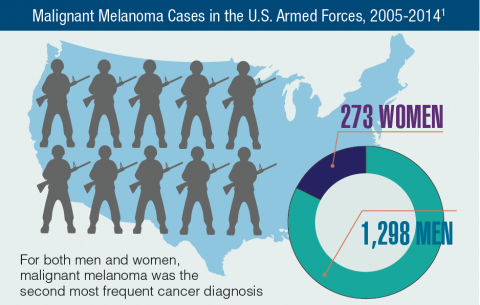
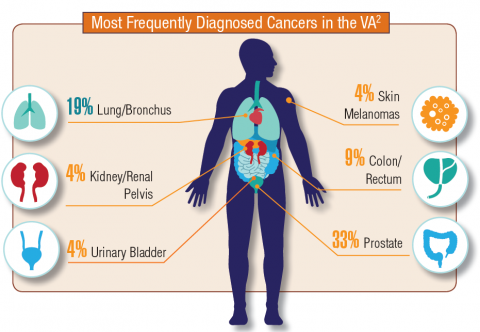
Hematology and Oncology Federal Health Data Trends (FULL)
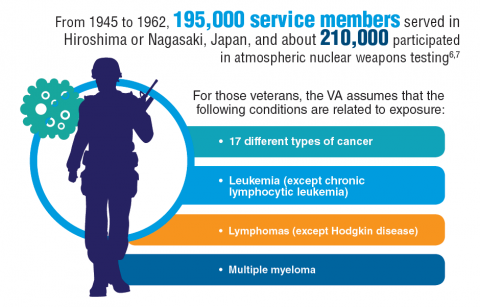
Cancer research is a high priority for the DoD and especially for the VA. Researchers in both agencies played an important role in the early stages of the Cancer Moonshot. As part of this initiative, the VA, DoD, and National Cancer Institute joined forces in the Applied Proteogenomics Organizational Learning and Outcomes (APOLLO) project to develop a system to quickly identify unique targets and pathways of cancer for better interventions.
The VA also will provide access to the Million Veteran Program database, and > 20 years of electronic health records data for analysis using the U.S. Department of Energy’s advanced computer systems. The enhanced computational infrastructure provided by the departments will facilitate new studies of cancer genomics. The research will begin with prostate cancer, and it is hoped that the project will help researchers distinguish between those prostate cancers that require aggressive management and the more benign cancers that are less likely to progress.
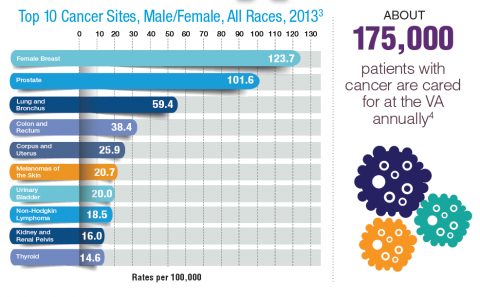
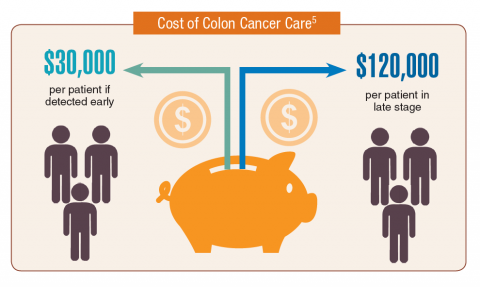
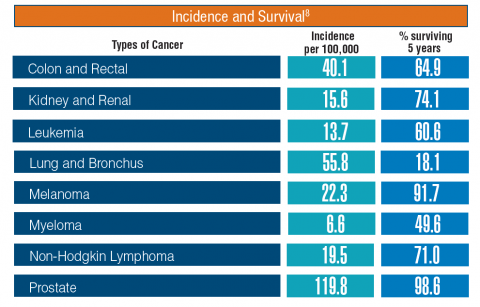
According to the latest VA budget, its researchers are conducting a broad array of research on cancers common in the veteran population, including prostate, lung, colorectal, bladder, kidney, pancreatic, skin, esophageal, and femalespecific cancers (such as breast and cervical cancer), as well as lymphomas and melanomas. For example, one study is focused on improving palliative care for patients with advanced cancer, and another will enroll 50,000 veterans to compare colorectal cancer screening strategies.
Click here to read the digital edition.
Cancer research is a high priority for the DoD and especially for the VA. Researchers in both agencies played an important role in the early stages of the Cancer Moonshot. As part of this initiative, the VA, DoD, and National Cancer Institute joined forces in the Applied Proteogenomics Organizational Learning and Outcomes (APOLLO) project to develop a system to quickly identify unique targets and pathways of cancer for better interventions.
The VA also will provide access to the Million Veteran Program database, and > 20 years of electronic health records data for analysis using the U.S. Department of Energy’s advanced computer systems. The enhanced computational infrastructure provided by the departments will facilitate new studies of cancer genomics. The research will begin with prostate cancer, and it is hoped that the project will help researchers distinguish between those prostate cancers that require aggressive management and the more benign cancers that are less likely to progress.
According to the latest VA budget, its researchers are conducting a broad array of research on cancers common in the veteran population, including prostate, lung, colorectal, bladder, kidney, pancreatic, skin, esophageal, and femalespecific cancers (such as breast and cervical cancer), as well as lymphomas and melanomas. For example, one study is focused on improving palliative care for patients with advanced cancer, and another will enroll 50,000 veterans to compare colorectal cancer screening strategies.
Click here to read the digital edition.
Cancer research is a high priority for the DoD and especially for the VA. Researchers in both agencies played an important role in the early stages of the Cancer Moonshot. As part of this initiative, the VA, DoD, and National Cancer Institute joined forces in the Applied Proteogenomics Organizational Learning and Outcomes (APOLLO) project to develop a system to quickly identify unique targets and pathways of cancer for better interventions.
The VA also will provide access to the Million Veteran Program database, and > 20 years of electronic health records data for analysis using the U.S. Department of Energy’s advanced computer systems. The enhanced computational infrastructure provided by the departments will facilitate new studies of cancer genomics. The research will begin with prostate cancer, and it is hoped that the project will help researchers distinguish between those prostate cancers that require aggressive management and the more benign cancers that are less likely to progress.
According to the latest VA budget, its researchers are conducting a broad array of research on cancers common in the veteran population, including prostate, lung, colorectal, bladder, kidney, pancreatic, skin, esophageal, and femalespecific cancers (such as breast and cervical cancer), as well as lymphomas and melanomas. For example, one study is focused on improving palliative care for patients with advanced cancer, and another will enroll 50,000 veterans to compare colorectal cancer screening strategies.
Click here to read the digital edition.
Clinical trials summary: AGILE
Study of AG-120 (Ivosidenib) vs. Placebo in Combination With Azacitidine in Patients With Previously Untreated Acute Myeloid Leukemia With an IDH1 Mutation (AGILE)
AG120-C-009 (NCT03173248) will evaluate the efficacy and safety of AG-120 (ivosidenib) plus azacitidine vs. placebo plus azacitidine in adult subjects with previously untreated IDH1m AML who are considered appropriate candidates for non-intensive therapy.
The primary endpoint of this global, phase 3, multicenter, double-blind, randomized, placebo-controlled clinical trial is overall survival. Key secondary efficacy endpoints are event-free survival, rate of complete remission, rate of complete remission with partial hematologic recovery, and overall response rate. Subjects will be randomized 1:1 to receive either oral AG-120 or matched placebo, both administered in combination with subcutaneous or intravenous azacitidine. An estimated 392 subjects will participate in the study, which is sponsored by Agios Pharmaceuticals. Estimated completion date is June 2022.
Contact: Medical Affairs Agios Pharmaceuticals, Inc.; (844) 633-2332, e-mail: [email protected]
Study of AG-120 (Ivosidenib) vs. Placebo in Combination With Azacitidine in Patients With Previously Untreated Acute Myeloid Leukemia With an IDH1 Mutation (AGILE)
AG120-C-009 (NCT03173248) will evaluate the efficacy and safety of AG-120 (ivosidenib) plus azacitidine vs. placebo plus azacitidine in adult subjects with previously untreated IDH1m AML who are considered appropriate candidates for non-intensive therapy.
The primary endpoint of this global, phase 3, multicenter, double-blind, randomized, placebo-controlled clinical trial is overall survival. Key secondary efficacy endpoints are event-free survival, rate of complete remission, rate of complete remission with partial hematologic recovery, and overall response rate. Subjects will be randomized 1:1 to receive either oral AG-120 or matched placebo, both administered in combination with subcutaneous or intravenous azacitidine. An estimated 392 subjects will participate in the study, which is sponsored by Agios Pharmaceuticals. Estimated completion date is June 2022.
Contact: Medical Affairs Agios Pharmaceuticals, Inc.; (844) 633-2332, e-mail: [email protected]
Study of AG-120 (Ivosidenib) vs. Placebo in Combination With Azacitidine in Patients With Previously Untreated Acute Myeloid Leukemia With an IDH1 Mutation (AGILE)
AG120-C-009 (NCT03173248) will evaluate the efficacy and safety of AG-120 (ivosidenib) plus azacitidine vs. placebo plus azacitidine in adult subjects with previously untreated IDH1m AML who are considered appropriate candidates for non-intensive therapy.
The primary endpoint of this global, phase 3, multicenter, double-blind, randomized, placebo-controlled clinical trial is overall survival. Key secondary efficacy endpoints are event-free survival, rate of complete remission, rate of complete remission with partial hematologic recovery, and overall response rate. Subjects will be randomized 1:1 to receive either oral AG-120 or matched placebo, both administered in combination with subcutaneous or intravenous azacitidine. An estimated 392 subjects will participate in the study, which is sponsored by Agios Pharmaceuticals. Estimated completion date is June 2022.
Contact: Medical Affairs Agios Pharmaceuticals, Inc.; (844) 633-2332, e-mail: [email protected]
VIDEO: Social networking offers coping help for endometriosis patients
VANCOUVER – Nearly half of the discussion topics on the social networking site www.myendometriosisteam.com are about pain, but other uncontrolled symptoms are popular topics, including fatigue and depression.
The online social support network includes 30,000 women with endometriosis and offers them a chance to connect with other women with the condition, find a provider, and research treatments. Elise-Marie Menke, director of alliance management at MyHealthTeams, which runs the site, presented data at the World Congress on Endometriosis. Among the findings she presented was how symptoms mapped to a woman’s cycle.
In a video interview, Ms. Menke described how this type of patient-generated data can play a role in the management of disease and serve to highlight unmet needs to physicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VANCOUVER – Nearly half of the discussion topics on the social networking site www.myendometriosisteam.com are about pain, but other uncontrolled symptoms are popular topics, including fatigue and depression.
The online social support network includes 30,000 women with endometriosis and offers them a chance to connect with other women with the condition, find a provider, and research treatments. Elise-Marie Menke, director of alliance management at MyHealthTeams, which runs the site, presented data at the World Congress on Endometriosis. Among the findings she presented was how symptoms mapped to a woman’s cycle.
In a video interview, Ms. Menke described how this type of patient-generated data can play a role in the management of disease and serve to highlight unmet needs to physicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VANCOUVER – Nearly half of the discussion topics on the social networking site www.myendometriosisteam.com are about pain, but other uncontrolled symptoms are popular topics, including fatigue and depression.
The online social support network includes 30,000 women with endometriosis and offers them a chance to connect with other women with the condition, find a provider, and research treatments. Elise-Marie Menke, director of alliance management at MyHealthTeams, which runs the site, presented data at the World Congress on Endometriosis. Among the findings she presented was how symptoms mapped to a woman’s cycle.
In a video interview, Ms. Menke described how this type of patient-generated data can play a role in the management of disease and serve to highlight unmet needs to physicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT WCE 2017
CMS offering educational webinars on MACRA
The Centers for Medicare & Medicaid Services is offering a pair of webinars aimed at helping physicians navigate the new regulation that operationalizes the Medicare Access and CHIP Reauthorization Act (MACRA).
The first webinar, scheduled for Oct. 26, will provide an overview of the two components of the Quality Payment Program – the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs).
The second webinar, scheduled for Nov. 15, is targeted to Medicare Part B fee-for-service clinicians, office managers and administrators, state and national associations that represent health care providers, and other stakeholders and will feature a question-and-answer session.
The webinars are part of the agency’s ongoing efforts to help educate practitioners on the provisions of the final MACRA regulation, which was issued on Oct. 14. CMS also recently launched a website to help in that regard.
The Centers for Medicare & Medicaid Services is offering a pair of webinars aimed at helping physicians navigate the new regulation that operationalizes the Medicare Access and CHIP Reauthorization Act (MACRA).
The first webinar, scheduled for Oct. 26, will provide an overview of the two components of the Quality Payment Program – the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs).
The second webinar, scheduled for Nov. 15, is targeted to Medicare Part B fee-for-service clinicians, office managers and administrators, state and national associations that represent health care providers, and other stakeholders and will feature a question-and-answer session.
The webinars are part of the agency’s ongoing efforts to help educate practitioners on the provisions of the final MACRA regulation, which was issued on Oct. 14. CMS also recently launched a website to help in that regard.
The Centers for Medicare & Medicaid Services is offering a pair of webinars aimed at helping physicians navigate the new regulation that operationalizes the Medicare Access and CHIP Reauthorization Act (MACRA).
The first webinar, scheduled for Oct. 26, will provide an overview of the two components of the Quality Payment Program – the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs).
The second webinar, scheduled for Nov. 15, is targeted to Medicare Part B fee-for-service clinicians, office managers and administrators, state and national associations that represent health care providers, and other stakeholders and will feature a question-and-answer session.
The webinars are part of the agency’s ongoing efforts to help educate practitioners on the provisions of the final MACRA regulation, which was issued on Oct. 14. CMS also recently launched a website to help in that regard.