User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Psychiatric emergency? What to consider before prescribing
Psychiatric emergencies—such as a patient who is agitated, self-destructive, or suicidal—may arise in a variety of settings, including emergency departments and inpatient units.1 Before emergently prescribing psychotropic medications to address acute psychiatric symptoms, there are numerous factors a clinician needs to consider.1-3 Asking the following questions may help you quickly obtain important clinical information to determine which medication to use during a psychiatric emergency:
Age. Is the patient a child, adolescent, adult, or older adult?
Allergies. Does the patient have any medication allergies or sensitivities?
Behaviors. What are the imminent dangerous behaviors that warrant emergent medication use
Collateral information. If the patient was brought by police or family, how was he/she behaving in the community or at home? If brought from a correctional facility or other institution, how did he/she behave in that setting?
Concurrent diagnoses/interventions. Does the patient have a psychiatric or medical diagnosis? Is the patient receiving any pharmacologic or nonpharmacologic treatments?
First visit. Is this the patient’s first visit to your facility? Or has the patient been to the facility previously and/or repeatedly? Has the patient ever been prescribed psychotropic medications? If the patient has received emergent medications before, which medications were used, and were they helpful?
Continue to: Legal status
Legal status. Is the patient voluntary for treatment or involuntary for treatment? If voluntary, is involuntary treatment needed?
Street. Was this patient evaluated in a medical setting before presenting to your facility? Or did this patient arrive directly from the community/street?
Substance use. Has the patient been using any licit and/or illicit substances?
In my experience with psychiatric emergencies, asking these questions has helped guide my decision-making during these situations. They have helped me to determine the appropriate medication, route of administration, dose, and monitoring requirements. Although other factors can impact clinicians’ decision-making in these situations, I have found these questions to be a good starting point.
1. Mavrogiorgou P, Brüne M, Juckel G. The management of psychiatric emergencies. Dtsch Arztebl Int. 2011;108(13):222-230.
2. Glick RL, Berlin JS, Fishkind AB, et al (eds). Emergency psychiatry: principles and practice. 2nd ed. Philadelphia, PA: Wolter Kluwer; 2020.
3. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatry. 2016;17(2):86-128.
Psychiatric emergencies—such as a patient who is agitated, self-destructive, or suicidal—may arise in a variety of settings, including emergency departments and inpatient units.1 Before emergently prescribing psychotropic medications to address acute psychiatric symptoms, there are numerous factors a clinician needs to consider.1-3 Asking the following questions may help you quickly obtain important clinical information to determine which medication to use during a psychiatric emergency:
Age. Is the patient a child, adolescent, adult, or older adult?
Allergies. Does the patient have any medication allergies or sensitivities?
Behaviors. What are the imminent dangerous behaviors that warrant emergent medication use
Collateral information. If the patient was brought by police or family, how was he/she behaving in the community or at home? If brought from a correctional facility or other institution, how did he/she behave in that setting?
Concurrent diagnoses/interventions. Does the patient have a psychiatric or medical diagnosis? Is the patient receiving any pharmacologic or nonpharmacologic treatments?
First visit. Is this the patient’s first visit to your facility? Or has the patient been to the facility previously and/or repeatedly? Has the patient ever been prescribed psychotropic medications? If the patient has received emergent medications before, which medications were used, and were they helpful?
Continue to: Legal status
Legal status. Is the patient voluntary for treatment or involuntary for treatment? If voluntary, is involuntary treatment needed?
Street. Was this patient evaluated in a medical setting before presenting to your facility? Or did this patient arrive directly from the community/street?
Substance use. Has the patient been using any licit and/or illicit substances?
In my experience with psychiatric emergencies, asking these questions has helped guide my decision-making during these situations. They have helped me to determine the appropriate medication, route of administration, dose, and monitoring requirements. Although other factors can impact clinicians’ decision-making in these situations, I have found these questions to be a good starting point.
Psychiatric emergencies—such as a patient who is agitated, self-destructive, or suicidal—may arise in a variety of settings, including emergency departments and inpatient units.1 Before emergently prescribing psychotropic medications to address acute psychiatric symptoms, there are numerous factors a clinician needs to consider.1-3 Asking the following questions may help you quickly obtain important clinical information to determine which medication to use during a psychiatric emergency:
Age. Is the patient a child, adolescent, adult, or older adult?
Allergies. Does the patient have any medication allergies or sensitivities?
Behaviors. What are the imminent dangerous behaviors that warrant emergent medication use
Collateral information. If the patient was brought by police or family, how was he/she behaving in the community or at home? If brought from a correctional facility or other institution, how did he/she behave in that setting?
Concurrent diagnoses/interventions. Does the patient have a psychiatric or medical diagnosis? Is the patient receiving any pharmacologic or nonpharmacologic treatments?
First visit. Is this the patient’s first visit to your facility? Or has the patient been to the facility previously and/or repeatedly? Has the patient ever been prescribed psychotropic medications? If the patient has received emergent medications before, which medications were used, and were they helpful?
Continue to: Legal status
Legal status. Is the patient voluntary for treatment or involuntary for treatment? If voluntary, is involuntary treatment needed?
Street. Was this patient evaluated in a medical setting before presenting to your facility? Or did this patient arrive directly from the community/street?
Substance use. Has the patient been using any licit and/or illicit substances?
In my experience with psychiatric emergencies, asking these questions has helped guide my decision-making during these situations. They have helped me to determine the appropriate medication, route of administration, dose, and monitoring requirements. Although other factors can impact clinicians’ decision-making in these situations, I have found these questions to be a good starting point.
1. Mavrogiorgou P, Brüne M, Juckel G. The management of psychiatric emergencies. Dtsch Arztebl Int. 2011;108(13):222-230.
2. Glick RL, Berlin JS, Fishkind AB, et al (eds). Emergency psychiatry: principles and practice. 2nd ed. Philadelphia, PA: Wolter Kluwer; 2020.
3. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatry. 2016;17(2):86-128.
1. Mavrogiorgou P, Brüne M, Juckel G. The management of psychiatric emergencies. Dtsch Arztebl Int. 2011;108(13):222-230.
2. Glick RL, Berlin JS, Fishkind AB, et al (eds). Emergency psychiatry: principles and practice. 2nd ed. Philadelphia, PA: Wolter Kluwer; 2020.
3. Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatry. 2016;17(2):86-128.
Schizoaffective disorder: A challenging diagnosis
Mr. C, age 34, presented to the emergency department with his wife because of increasingly bizarre behavior. He reported auditory and visual hallucinations, and believed that the “mob had ordered a hit” against him. He had threatened to shoot his wife and children, which led to his arrest and being briefly jailed. In jail, he was agitated, defecated on the floor, and disrobed. His wife reported that Mr. C had a long history of bipolar disorder and had experienced his first manic episode and hospitalization at age 17. Since then, he had been treated with many different antidepressants, antipsychotics, and mood stabilizers.
Mr. C was admitted to the hospital, where he developed a catatonic syndrome that was treated with a course of electroconvulsive therapy. He was eventually stabilized with
Over the next 8 years, Mr. C was often noncompliant with medication and frequently was hospitalized for mania. His symptoms included poor sleep, grandiosity, pressured speech, racing and disorganized thoughts, increased risk-taking behavior (ie, driving at excessive speeds), and hyperreligiosity (ie, speaking with God). Mr. C also occasionally used methamphetamine, cannabis, and cocaine. Although he had responded well to treatment early in the course of his illness, as he entered his late 30s, his response was less complete, and by his 40s, Mr. C was no longer able to function independently. He eventually was prescribed a long-acting injectable antipsychotic, paliperidone palmitate, 156 mg monthly. Eventually, his family was no longer able to care for him at home, so he was admitted to a residential care facility.
In this facility, based on the long-standing nature of Mr. C’s psychotic disorder and frequency with which he presented with mania, his clinicians changed his diagnosis to schizoaffective disorder, bipolar type. It had become clear that mood symptoms comprised >50% of the total duration of his illness.
Schizoaffective disorder (SAD) often has been used as a diagnosis for patients who have an admixture of mood and psychotic symptoms whose diagnosis is uncertain. Its hallmark is the presence of symptoms of a major mood episode (either a depressive or manic episode) concurrent with symptoms characteristic of schizophrenia, such as delusions, hallucinations, or disorganized speech.1
SAD is a controversial diagnosis. There has been inadequate research regarding the epidemiology, course, etiologic factors, and treatment of this disorder. Debate continues to swirl around its conceptualization; some experts view SAD as an independent disorder, while others see SAD as either a form of schizophrenia or a mood disorder.1 In this review, we describe the classification of SAD and its features, diagnosis, and treatment.
An evolving diagnosis
The term schizoaffective was first used by Jacob Kasanin, MD, in 1933.2 He described 9 patients with “acute schizoaffective psychoses,” each of whom had an abrupt onset. The term was used in the first edition of the DSM as a subtype of schizophrenia.3 In DSM-I, the “schizo-affective type” was defined as a diagnosis for patients with a “significant admixture of schizophrenic and affective reactions.”3 Diagnostic criteria for SAD were developed for DSM-III-R, published in 1987.4 These criteria continued to evolve with subsequent editions of the DSM.
Continue to: DSM-5 provides...
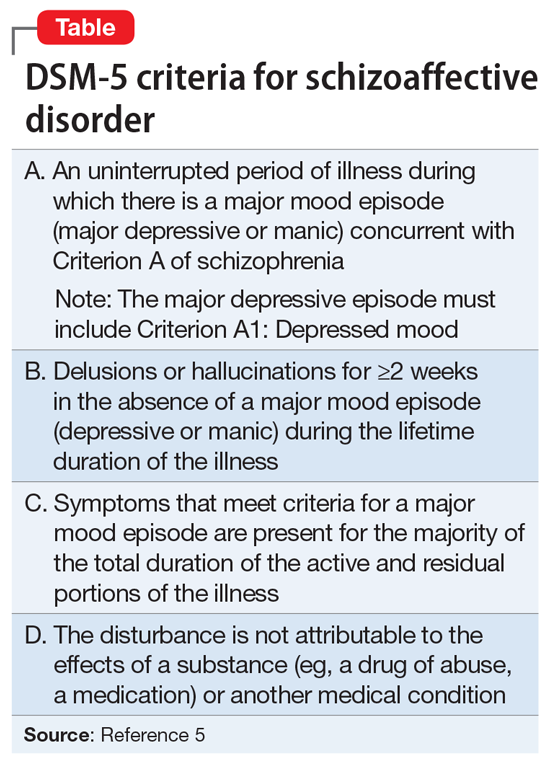
DSM-5 provides a clearer separation between schizophrenia with mood symptoms, bipolar disorder, and SAD (Table5). In addition, DSM-5 shifts away from the DSM-IV diagnosis of SAD as an episode, and instead focuses more on the longitudinal course of the illness. It has been suggested that this change will likely lead to reduced rates of diagnosis of SAD.6 Despite improvements in classification, the diagnosis remains controversial (Box7-11).
Box 1
Despite improvements in classification, controversy continues to swirl around the question of whether schizoaffective disorder (SAD) represents an independent disorder that stands apart from schizophrenia and bipolar disorder, whether it is a form of schizophrenia, or whether it is a form of bipolar disorder or a depressive disorder.7,8 Other possibilities are that SAD is heterogeneous or that it represents a middle point on a spectrum that bridges mood and psychotic disorders. While the merits of each possibility are beyond the scope of this review, it is safe to say that each possibility has its proponents. For these reasons, some argue that the concept itself lacks validity and shows the pitfalls of our classification system.7
Poor diagnostic reliability is one reason for concerns about validity. Most recently, a field trial using DSM-5 criteria produced a kappa of 0.50, which is moderate,9 but earlier definitions produced relatively poor results. Wilson et al10 point out that Criterion C, which concerns duration of mood symptoms, produces a particularly low kappa. Another reason is diagnostic switching, whereby patients initially diagnosed with 1 disorder receive a different diagnosis at followup. Diagnostic switching is especially problematic for SAD. In a large meta-analysis by Santelmann et al,11 36% of patients initially diagnosed with SAD had their diagnosis changed when reassessed. This diagnostic shift tended more toward schizophrenia than bipolar disorder. In addition, more than one-half of all patients initially diagnosed with schizophrenia, bipolar disorder, or major depressive disorder were re-diagnosed with SAD when reassessed.
DSM-5 subtypes and specifiers
In DSM-5,SAD has 2 subtypes5:
- Bipolar type. The bipolar type is marked by the presence of a manic episode (major depressive episodes may also occur)
- Depressive type. The depressive type is marked by the presence of only major depressive episodes.
SAD also includes several specifiers, with the express purpose of giving clinicians greater descriptive ability. The course of SAD can be described as either “first episode,” defined as the first manifestation of the disorder, or as having “multiple episodes,” defined as a minimum of 2 episodes with 1 relapse. In addition, SAD can be described as an acute episode, in partial remission, or in full remission. The course can be described as “continuous” if it is clear that symptoms have been present for the majority of the illness with very brief subthreshold periods. The course is designated as “unspecified” when information is unavailable or lacking. The 5-point Clinician-Rated Dimensions of Psychosis Symptoms was introduced to enable clinicians to make a quantitative assessment of the psychotic symptoms, although its use is not required.
Epidemiology and gender ratio
The epidemiology of SAD has not been well studied. DSM-5 estimates that SAD is approximately one-third as common as schizophrenia, which has a lifetime prevalence of 0.5% to 0.8%.5 This is similar to an estimate by Perälä et al12 of a 0.32% lifetime prevalence based on a nationally representative sample of persons in Finland age ≥30. Scully et al13 calculated a prevalence estimate of 1.1% in a representative sample of adults in rural Ireland. Based on pooled clinical data, Keck et al14 estimated the prevalence in clinical settings at 16%, similar to the figure of 19% reported by Levinson et al15 based on data from New York State psychiatric hospitals. In clinical practice, the diagnosis of SAD is used frequently when there is diagnostic uncertainty, which potentially inflates estimates of lifetime prevalence.
The prevalence of SAD is higher in women than men, with a sex ratio of about 2:1, similar to that seen in mood disorders.13,16-19 There are an equal number of men and women with the bipolar subtype, but a female preponderance with the depressive subtype.5 The bipolar subtype is more common in younger patients, while the depressive subtype is more common in older patients. SAD is a rare diagnosis in children.20
Continue to: Course and outcome
Course and outcome
The onset of SAD typically occurs in early adulthood, but can range from childhood to senescence. Approximately one-third of patients are diagnosed before age 25, one-third between age 25 and 35, and one-third after age 35.21-23 Based on a literature review, Cheniaux et al7 concluded that that age at onset for patients with SAD is between those with schizophrenia and those with mood disorders.
The course of SAD is variable but represents a middle ground between that of schizophrenia and the mood disorders. In a 4- to 5-year follow-up,24 patients with SAD had a better overall course than patients with schizophrenia but had poorer functioning than those with bipolar mania, and much poorer than those with unipolar depression. Mood-incongruent psychotic features predict a particularly worse outcome. These findings were reaffirmed at a 10-year follow-up.25 Mood symptoms portend a better outcome than do symptoms of schizophrenia.
The lifetime suicide risk for patients with SAD is estimated at 5%, with a higher risk associated with the presence of depressive symptoms.26 One study found that women with SAD had a 17.5-year reduced life expectancy (64.1 years) compared with a reduction of 8.0 years for men (69.4 years).27
Comorbidity
Patients with SAD are commonly diagnosed with other psychiatric disorders, including anxiety disorders, obsessive-compulsive disorder, posttraumatic stress disorder, and substance use disorders.21,28,29 When compared with the general population, patients with SAD are at higher risk for coronary heart disease, stroke, obesity, and smoking, likely contributing to their decreased life expectancy.27,30 Because second-generation antipsychotics (SGAs) are often used to treat SAD, patients with SAD are at risk for metabolic syndrome and diabetes mellitus.30
Clinical assessment
Because there are no diagnostic, laboratory, or neuroimaging tests for SAD, the most important basis for making the diagnosis is the patient’s history, supplemented by collateral history from family members or friends, and medical records. Determining the percentage of time spent in a mood episode (DSM-5 Criterion C) is especially important.31 This requires the clinician to pay close attention to the temporal relationship of psychotic and mood symptoms.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis for SAD is broad because it includes all of the possibilities usually considered for major mood disorders and for psychotic disorders5:
- schizophrenia
- bipolar disorder with psychotic features
- major depressive disorder with psychotic features
- depressive or bipolar disorders with catatonic features
- personality disorders (especially the schizotypal, paranoid, and borderline types)
- major neurocognitive disorders in which there are mood and psychotic symptoms
- substance/medication-induced psychotic disorder
- disorders induced by medical conditions.
With schizophrenia, the duration of all episodes of a mood syndrome is brief (<50% of the total duration of the illness) relative to the duration of the psychotic symptoms. Although psychotic symptoms may occur in persons with mood disorders, they are generally not present in the absence of depression or mania, helping to set the boundary between SAD and psychotic mania or depression. As for personality disorders, the individual will not have a true psychosis, although some symptoms, such as feelings of unreality, paranoia, or magical thinking, may cause diagnostic confusion.
Medical conditions also can present with psychotic and mood symptoms and need to be ruled out. These include psychotic disorder due to another medical condition, and delirium. A thorough medical workup should be performed to rule out any possible medical causes for the symptoms.
Substance use should also be ruled out as the cause of the symptoms because many substances are associated with mood and psychotic symptoms. It is usually clear from the history, physical examination, or laboratory tests when a medication/illicit substance has initiated and maintained the disorder.
Neurologic conditions. If a neurologic condition is suspected, a neurologic evaluation may be warranted, including laboratory tests, brain imaging to identify specific anatomical abnormalities, lumbar puncture with cerebrospinal fluid analysis, and an electroencephalogram to rule out a convulsive disorder.
Continue to: Clinical symptoms
Clinical symptoms
The signs and symptoms of SAD include those typically seen in schizophrenia and the mood disorders. Thus, the patient may exhibit elated mood and/or grandiosity, or severe depression, combined with mood-incongruent psychotic features such as paranoid delusions. The symptoms may present together or in an alternating fashion, and psychotic symptoms may be mood-congruent or mood-incongruent. Mr. C’s case illustrates some of the symptoms of the disorder.
Brain imaging
Significant changes have been reported to occur in the brain structure and function in persons with SAD. Neuroimaging studies using voxel-based morphometry have shown significant reductions in gray matter volume in several areas of the brain, including the medial prefrontal cortex, insula, Rolandic operculum, parts of the temporal lobe, and the hippocampus.32-35 Amann et al32 found that patients with SAD and schizophrenia had widespread and overlapping areas of significant volume reduction, but patients with bipolar disorder did not. These studies suggest that at least from a neuroimaging standpoint, SAD is more closely related to schizophrenia than bipolar disorder, and could represent a variant of schizophrenia.
Treatment of SAD
The pharmacotherapy of SAD is mostly empirical because of the lack of randomized controlled trials. Clinicians have traditionally prescribed an antipsychotic agent along with either a mood stabilizer (eg,
Since that exhaustive review,
Patients with SAD will require maintenance treatment for ongoing symptom control. Medication that is effective for treatment of an acute episode should be considered for maintenance treatment. Both the extended-release and long-acting injectable (LAI) formulations of paliperidone have been shown to be efficacious in the maintenance treatment of patients with SAD.40 The LAI form of paliperidone significantly delayed psychotic, depressive, and manic relapses, improved clinical rating scale scores, and increased medication adherence.41,42 In an open-label study, olanzapine LAI was effective in long-term maintenance treatment, although approximately 40% of patients experienced significant weight gain.43 One concern with olanzapine is the possible occurrence of a post-injection delirium/sedation syndrome. For that reason, patients receiving olanzapine must be monitored for at least 3 hours post-injection. The paliperidone LAI does not require monitoring after injection.
Continue to: There is a single clinical trial...
There is a single clinical trial showing that patients with SAD can be successfully switched from other antipsychotics to
Other approaches
Electroconvulsive therapy (ECT) should be considered for patients with SAD who are acutely ill and have failed to respond adequately to medication. ECT is especially relevant in the setting of acute mood symptoms (ie, depressive or manic symptoms co-occurring with psychosis or in the absence of psychosis).45
As currently conceptualized, the diagnosis of SAD is made in persons having an admixture of mood and psychotic symptoms, although by definition mood symptoms must take up the majority (≥50%) of the total duration of the illness. Unfortunately, SAD has been inadequately researched due to the unreliability of its definition and concerns about its validity. The long-term course of SAD is midway between mood and psychotic disorders, and the disorder can cause significant disability.
Bottom Line
Schizoaffective disorder (SAD) is characterized by the presence of symptoms of a major mood episode (a depressive or manic episode) concurrent with symptoms of schizophrenia. The most important basis for establishing the diagnosis is the patient’s history. Determining the percentage of time spent in a mood episode is especially important. Treatment usually consists of an antipsychotic plus a mood stabilizer or antidepressant. Electroconvulsive therapy is an option for patients with SAD who do not respond well to medication.
Related Resources
- Wy TJP, Saadabadi A. Schizoaffective disorder. NCBI Bookshelf: StatPearls Publishing. Published January 2020. https://www.ncbi.nlm.nih.gov/books/NBK541012/. Updated April 15, 2020.
- Parker G. How well does the DSM-5 capture schizoaffective disorder? Can J Psychiatry. 2019;64(9):607-610.
Drug Brand Names
Aripiprazole • Abilify
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Olanzapine long-acting injectable • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate • Invega sustenna
Valproate • Depacon
1. Miller JN, Black DW. Schizoaffective disorder: a review. Ann Clin Psychiatry. 2019;31(1):47-53.
2. Kasanin J. The acute schizoaffective psychoses. Am J Psychiatry. 1933;90:97-126.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington, DC: American Psychiatric Association; 1952.
4. Diagnostic and statistical manual of mental disorders, 3rd ed, revision. Washington, DC: American Psychiatric Association; 1987.
5. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
6. Malaspina D, Owen M, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150:21-25.
7. Cheniaux E, Landeria-Fernandez J, Telles LL, et al. Does schizoaffective disorder really exist? A systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J Affect Disord. 2008;106:209-217.
8. Kantrowitz JT, Citrome L. Schizoaffective disorder: a review of current research themes and pharmacologic management. CNS Drugs. 2011;25:317-331.
9. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170:59-70.
10. Wilson JE, Nian H, Heckers S. The schizoaffective disorder diagnosis: a conundrum in the clinical setting. Eur Arch Psychiatry Clin Neurosci. 2014;264:29-34.
11. Santelmann H, Franklin J, Bußhoff J, Baethge C. Test-retest reliability of schizoaffective disorder compared with schizophrenia, bipolar disorder, and unipolar depression--a systematic review and meta-analysis. Bipolar Disord. 2015;17:753-768.
12. Perälä J, Suvisaari J, Saarni SI, et al. Lifetime Prevalence of psychotic and bipolar I disorders in a general population. JAMA Psychiatry. 2007;64:19-28.
13. Scully PJ, Owens JM, Kinsella A, et al. Schizophrenia, schizoaffective and bipolar disorder within an epidemiologically complete, homogeneous population in rural Ireland: small area variation in rate. Schizophr Res. 2004;67:143-155.
14. Keck PE Jr, McElroy SE, Strakowski SM, et al. Pharmacologic treatment of schizoaffective disorder. Psychopharmacol. 1994;114:529-538.
15. Levinson DF, Umapathy C, Musthaq M. Treatment of schizoaffective disorder and schizophrenia with mood symptoms. Am J Psychiatry. 1999;156:1138-1148.
16. Angst J, Felder W, Lohmeyer B. Course of schizoaffective psychoses: results of a follow-up study. Schizophr Bull. 1980;6:579-585.
17. Lenz G, Simhandl C, Thau K, et al. Temporal stability of diagnostic criteria for functional psychoses. Psychopathol. 1991;24:328-335.
18. Malhi GS, Green M, Fagiolini A, et al. Schizoaffective disorder: diagnostic issues and future recommendations. Bipolar Disord. 2008;10:215-230.
19. Marneros A, Deister A, Rohde A. Psychopathological and social status of patients with affective, schizophrenic and schizoaffective disorders after long‐term course. Acta Psychiatr Scand. 1990;82:352-358.
20. Werry JS, McClellan JM, Chard L. Childhood and adolescent schizophrenic, bipolar, and schizoaffective disorders: a clinical and outcome study. J Am Acad Child Adolesc Psychiatry. 1991;30:457-465.
21. Abrams DJ, Rojas DC, Arciniegas DB. Is schizoaffective disorder a distinct categorical diagnosis? A critical review of the literature. Neuropsychiatr Dis Treat. 2008;4:1089-1109.
22. Bromet EJ, Kotov R, Fochtmann LJ, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168:1186-1194.
23. Salvatore P, Baldessarini RJ, Tohen M, et al. The McLean-Harvard First Episode Project: two-year stability of DSM-IV diagnoses in 500 first-episode psychotic disorder patients. J Clin Psychiatry. 2009;70:458-466.
24. Grossman LS, Harrow M, Goldberg JF, et al. Outcome of schizoaffective disorder at two long term follow-ups: comparisons with outcome of schizophrenia and affective disorders. Am J Psychiatry. 1991;148:1359-1365.
25. Harrow M, Grossman L, Herbener E, et al. Ten-year outcome: patients with schizoaffective disorders, schizophrenia, affective disorders and mood-incongruent psychotic symptoms. Br J Psychiatry. 2000;177:421-426.
26. Hor K, Taylor M. Review: suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24:81-90.
27. Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS ONE. 2011;6:e19590.
28. Byerly M, Goodman W, Acholonu W, et al. Obsessive compulsive symptoms in schizophrenia: frequency and clinical features. Schizophr Res. 2005;76:309-316.
29. Strauss JL, Calhoun PS, Marx CE, et al. Comorbid posttraumatic stress disorder is associated with suicidality in male veterans with schizophrenia or schizoaffective disorder. Schizophr Res. 2006;84:165-169.
30. Fagiolini A, Goracci A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. J Clin Psychiatry. 2009;70:22-29.
31. Black DW, Grant JE. DSM-5 guidebook: the essential companion to the diagnostic and statistical manual of mental disorders, 5th edition. Washington, DC: American Psychiatric Publishing; 2014.
32. Amann BL, Canales-Rodríguez EJ, Madre M, et al. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2016;133:23-33.
33. Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285-1296.
34. Ivleva EI, Bidesi AS, Thomas BP, et al. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res. 2012;204:13-24.
35. Radonic
36. Jäger M, Becker T, Weinmann S, et al. Treatment of schizoaffective disorder - a challenge for evidence-based psychiatry. Acta Psychiatr Scand. 2010;121:22-32.
37. Glick ID, Mankosli R, Eudicone JM, et al. The efficacy, safety, and tolerability of aripiprazole for the treatment of schizoaffective disorder: results from a pooled analysis of a sub-population of subjects from two randomized, double-blind, placebo controlled, pivotal trials. J Affect Disord. 2009;115:18-26.
38. Canuso CM, Lindenmayer JP, Kosik-Gonzalez C, et al. A randomized, double-blind, placebo controlled study of 2 dose ranges of paliperidone extended-release in the treatment of subjects with schizoaffective disorder. J Clin Psychiatry. 2010;71:587-598.
39. Canuso CM, Schooler NR, Carothers J, et al. Paliperidone extended-release in schizoaffective disorder: a randomized controlled trial comparing a flexible-dose with placebo in patients treated with and without antidepressants and/or mood stabilizers. J Clin Psychopharmacol. 2010;30:487-495.
40. Lindenmayer JP, Kaur A. Antipsychotic management of schizoaffective disorder: a review. Drugs. 2016;76:589-604.
41. Alphs L, Fu DJ, Turkoz I. Paliperidone for the treatment of schizoaffective disorder. Expert Opin Pharmacother. 2016;176:871-883.
42. Bossie CA, Turkoz I, Alphs L, et al. Paliperidone palmitate once-monthly treatment in recent onset and chronic illness patients with schizoaffective disorder. J Nerv Ment Dis. 2017;205:324-328.
43. McDonnell DP, Landry J, Detke HC. Long-term safety and efficacy of olanzapine long-acting injection in patients with schizophrenia or schizoaffective disorder: a 6-year, multinational, single-arm, open-label study. Int Clin Psychopharmacol. 2014;29:322-331.
44. McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74:170-179.
45. Mankad MV, Beyer JL, Wiener RD, et al. Manual of electroconvulsive therapy. Washington, DC: American Psychiatric Publishing; 2010.
Mr. C, age 34, presented to the emergency department with his wife because of increasingly bizarre behavior. He reported auditory and visual hallucinations, and believed that the “mob had ordered a hit” against him. He had threatened to shoot his wife and children, which led to his arrest and being briefly jailed. In jail, he was agitated, defecated on the floor, and disrobed. His wife reported that Mr. C had a long history of bipolar disorder and had experienced his first manic episode and hospitalization at age 17. Since then, he had been treated with many different antidepressants, antipsychotics, and mood stabilizers.
Mr. C was admitted to the hospital, where he developed a catatonic syndrome that was treated with a course of electroconvulsive therapy. He was eventually stabilized with
Over the next 8 years, Mr. C was often noncompliant with medication and frequently was hospitalized for mania. His symptoms included poor sleep, grandiosity, pressured speech, racing and disorganized thoughts, increased risk-taking behavior (ie, driving at excessive speeds), and hyperreligiosity (ie, speaking with God). Mr. C also occasionally used methamphetamine, cannabis, and cocaine. Although he had responded well to treatment early in the course of his illness, as he entered his late 30s, his response was less complete, and by his 40s, Mr. C was no longer able to function independently. He eventually was prescribed a long-acting injectable antipsychotic, paliperidone palmitate, 156 mg monthly. Eventually, his family was no longer able to care for him at home, so he was admitted to a residential care facility.
In this facility, based on the long-standing nature of Mr. C’s psychotic disorder and frequency with which he presented with mania, his clinicians changed his diagnosis to schizoaffective disorder, bipolar type. It had become clear that mood symptoms comprised >50% of the total duration of his illness.
Schizoaffective disorder (SAD) often has been used as a diagnosis for patients who have an admixture of mood and psychotic symptoms whose diagnosis is uncertain. Its hallmark is the presence of symptoms of a major mood episode (either a depressive or manic episode) concurrent with symptoms characteristic of schizophrenia, such as delusions, hallucinations, or disorganized speech.1
SAD is a controversial diagnosis. There has been inadequate research regarding the epidemiology, course, etiologic factors, and treatment of this disorder. Debate continues to swirl around its conceptualization; some experts view SAD as an independent disorder, while others see SAD as either a form of schizophrenia or a mood disorder.1 In this review, we describe the classification of SAD and its features, diagnosis, and treatment.
An evolving diagnosis
The term schizoaffective was first used by Jacob Kasanin, MD, in 1933.2 He described 9 patients with “acute schizoaffective psychoses,” each of whom had an abrupt onset. The term was used in the first edition of the DSM as a subtype of schizophrenia.3 In DSM-I, the “schizo-affective type” was defined as a diagnosis for patients with a “significant admixture of schizophrenic and affective reactions.”3 Diagnostic criteria for SAD were developed for DSM-III-R, published in 1987.4 These criteria continued to evolve with subsequent editions of the DSM.
Continue to: DSM-5 provides...

DSM-5 provides a clearer separation between schizophrenia with mood symptoms, bipolar disorder, and SAD (Table5). In addition, DSM-5 shifts away from the DSM-IV diagnosis of SAD as an episode, and instead focuses more on the longitudinal course of the illness. It has been suggested that this change will likely lead to reduced rates of diagnosis of SAD.6 Despite improvements in classification, the diagnosis remains controversial (Box7-11).
Box 1
Despite improvements in classification, controversy continues to swirl around the question of whether schizoaffective disorder (SAD) represents an independent disorder that stands apart from schizophrenia and bipolar disorder, whether it is a form of schizophrenia, or whether it is a form of bipolar disorder or a depressive disorder.7,8 Other possibilities are that SAD is heterogeneous or that it represents a middle point on a spectrum that bridges mood and psychotic disorders. While the merits of each possibility are beyond the scope of this review, it is safe to say that each possibility has its proponents. For these reasons, some argue that the concept itself lacks validity and shows the pitfalls of our classification system.7
Poor diagnostic reliability is one reason for concerns about validity. Most recently, a field trial using DSM-5 criteria produced a kappa of 0.50, which is moderate,9 but earlier definitions produced relatively poor results. Wilson et al10 point out that Criterion C, which concerns duration of mood symptoms, produces a particularly low kappa. Another reason is diagnostic switching, whereby patients initially diagnosed with 1 disorder receive a different diagnosis at followup. Diagnostic switching is especially problematic for SAD. In a large meta-analysis by Santelmann et al,11 36% of patients initially diagnosed with SAD had their diagnosis changed when reassessed. This diagnostic shift tended more toward schizophrenia than bipolar disorder. In addition, more than one-half of all patients initially diagnosed with schizophrenia, bipolar disorder, or major depressive disorder were re-diagnosed with SAD when reassessed.
DSM-5 subtypes and specifiers
In DSM-5,SAD has 2 subtypes5:
- Bipolar type. The bipolar type is marked by the presence of a manic episode (major depressive episodes may also occur)
- Depressive type. The depressive type is marked by the presence of only major depressive episodes.
SAD also includes several specifiers, with the express purpose of giving clinicians greater descriptive ability. The course of SAD can be described as either “first episode,” defined as the first manifestation of the disorder, or as having “multiple episodes,” defined as a minimum of 2 episodes with 1 relapse. In addition, SAD can be described as an acute episode, in partial remission, or in full remission. The course can be described as “continuous” if it is clear that symptoms have been present for the majority of the illness with very brief subthreshold periods. The course is designated as “unspecified” when information is unavailable or lacking. The 5-point Clinician-Rated Dimensions of Psychosis Symptoms was introduced to enable clinicians to make a quantitative assessment of the psychotic symptoms, although its use is not required.
Epidemiology and gender ratio
The epidemiology of SAD has not been well studied. DSM-5 estimates that SAD is approximately one-third as common as schizophrenia, which has a lifetime prevalence of 0.5% to 0.8%.5 This is similar to an estimate by Perälä et al12 of a 0.32% lifetime prevalence based on a nationally representative sample of persons in Finland age ≥30. Scully et al13 calculated a prevalence estimate of 1.1% in a representative sample of adults in rural Ireland. Based on pooled clinical data, Keck et al14 estimated the prevalence in clinical settings at 16%, similar to the figure of 19% reported by Levinson et al15 based on data from New York State psychiatric hospitals. In clinical practice, the diagnosis of SAD is used frequently when there is diagnostic uncertainty, which potentially inflates estimates of lifetime prevalence.
The prevalence of SAD is higher in women than men, with a sex ratio of about 2:1, similar to that seen in mood disorders.13,16-19 There are an equal number of men and women with the bipolar subtype, but a female preponderance with the depressive subtype.5 The bipolar subtype is more common in younger patients, while the depressive subtype is more common in older patients. SAD is a rare diagnosis in children.20
Continue to: Course and outcome
Course and outcome
The onset of SAD typically occurs in early adulthood, but can range from childhood to senescence. Approximately one-third of patients are diagnosed before age 25, one-third between age 25 and 35, and one-third after age 35.21-23 Based on a literature review, Cheniaux et al7 concluded that that age at onset for patients with SAD is between those with schizophrenia and those with mood disorders.
The course of SAD is variable but represents a middle ground between that of schizophrenia and the mood disorders. In a 4- to 5-year follow-up,24 patients with SAD had a better overall course than patients with schizophrenia but had poorer functioning than those with bipolar mania, and much poorer than those with unipolar depression. Mood-incongruent psychotic features predict a particularly worse outcome. These findings were reaffirmed at a 10-year follow-up.25 Mood symptoms portend a better outcome than do symptoms of schizophrenia.
The lifetime suicide risk for patients with SAD is estimated at 5%, with a higher risk associated with the presence of depressive symptoms.26 One study found that women with SAD had a 17.5-year reduced life expectancy (64.1 years) compared with a reduction of 8.0 years for men (69.4 years).27
Comorbidity
Patients with SAD are commonly diagnosed with other psychiatric disorders, including anxiety disorders, obsessive-compulsive disorder, posttraumatic stress disorder, and substance use disorders.21,28,29 When compared with the general population, patients with SAD are at higher risk for coronary heart disease, stroke, obesity, and smoking, likely contributing to their decreased life expectancy.27,30 Because second-generation antipsychotics (SGAs) are often used to treat SAD, patients with SAD are at risk for metabolic syndrome and diabetes mellitus.30
Clinical assessment
Because there are no diagnostic, laboratory, or neuroimaging tests for SAD, the most important basis for making the diagnosis is the patient’s history, supplemented by collateral history from family members or friends, and medical records. Determining the percentage of time spent in a mood episode (DSM-5 Criterion C) is especially important.31 This requires the clinician to pay close attention to the temporal relationship of psychotic and mood symptoms.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis for SAD is broad because it includes all of the possibilities usually considered for major mood disorders and for psychotic disorders5:
- schizophrenia
- bipolar disorder with psychotic features
- major depressive disorder with psychotic features
- depressive or bipolar disorders with catatonic features
- personality disorders (especially the schizotypal, paranoid, and borderline types)
- major neurocognitive disorders in which there are mood and psychotic symptoms
- substance/medication-induced psychotic disorder
- disorders induced by medical conditions.
With schizophrenia, the duration of all episodes of a mood syndrome is brief (<50% of the total duration of the illness) relative to the duration of the psychotic symptoms. Although psychotic symptoms may occur in persons with mood disorders, they are generally not present in the absence of depression or mania, helping to set the boundary between SAD and psychotic mania or depression. As for personality disorders, the individual will not have a true psychosis, although some symptoms, such as feelings of unreality, paranoia, or magical thinking, may cause diagnostic confusion.
Medical conditions also can present with psychotic and mood symptoms and need to be ruled out. These include psychotic disorder due to another medical condition, and delirium. A thorough medical workup should be performed to rule out any possible medical causes for the symptoms.
Substance use should also be ruled out as the cause of the symptoms because many substances are associated with mood and psychotic symptoms. It is usually clear from the history, physical examination, or laboratory tests when a medication/illicit substance has initiated and maintained the disorder.
Neurologic conditions. If a neurologic condition is suspected, a neurologic evaluation may be warranted, including laboratory tests, brain imaging to identify specific anatomical abnormalities, lumbar puncture with cerebrospinal fluid analysis, and an electroencephalogram to rule out a convulsive disorder.
Continue to: Clinical symptoms
Clinical symptoms
The signs and symptoms of SAD include those typically seen in schizophrenia and the mood disorders. Thus, the patient may exhibit elated mood and/or grandiosity, or severe depression, combined with mood-incongruent psychotic features such as paranoid delusions. The symptoms may present together or in an alternating fashion, and psychotic symptoms may be mood-congruent or mood-incongruent. Mr. C’s case illustrates some of the symptoms of the disorder.
Brain imaging
Significant changes have been reported to occur in the brain structure and function in persons with SAD. Neuroimaging studies using voxel-based morphometry have shown significant reductions in gray matter volume in several areas of the brain, including the medial prefrontal cortex, insula, Rolandic operculum, parts of the temporal lobe, and the hippocampus.32-35 Amann et al32 found that patients with SAD and schizophrenia had widespread and overlapping areas of significant volume reduction, but patients with bipolar disorder did not. These studies suggest that at least from a neuroimaging standpoint, SAD is more closely related to schizophrenia than bipolar disorder, and could represent a variant of schizophrenia.
Treatment of SAD
The pharmacotherapy of SAD is mostly empirical because of the lack of randomized controlled trials. Clinicians have traditionally prescribed an antipsychotic agent along with either a mood stabilizer (eg,
Since that exhaustive review,
Patients with SAD will require maintenance treatment for ongoing symptom control. Medication that is effective for treatment of an acute episode should be considered for maintenance treatment. Both the extended-release and long-acting injectable (LAI) formulations of paliperidone have been shown to be efficacious in the maintenance treatment of patients with SAD.40 The LAI form of paliperidone significantly delayed psychotic, depressive, and manic relapses, improved clinical rating scale scores, and increased medication adherence.41,42 In an open-label study, olanzapine LAI was effective in long-term maintenance treatment, although approximately 40% of patients experienced significant weight gain.43 One concern with olanzapine is the possible occurrence of a post-injection delirium/sedation syndrome. For that reason, patients receiving olanzapine must be monitored for at least 3 hours post-injection. The paliperidone LAI does not require monitoring after injection.
Continue to: There is a single clinical trial...
There is a single clinical trial showing that patients with SAD can be successfully switched from other antipsychotics to
Other approaches
Electroconvulsive therapy (ECT) should be considered for patients with SAD who are acutely ill and have failed to respond adequately to medication. ECT is especially relevant in the setting of acute mood symptoms (ie, depressive or manic symptoms co-occurring with psychosis or in the absence of psychosis).45
As currently conceptualized, the diagnosis of SAD is made in persons having an admixture of mood and psychotic symptoms, although by definition mood symptoms must take up the majority (≥50%) of the total duration of the illness. Unfortunately, SAD has been inadequately researched due to the unreliability of its definition and concerns about its validity. The long-term course of SAD is midway between mood and psychotic disorders, and the disorder can cause significant disability.
Bottom Line
Schizoaffective disorder (SAD) is characterized by the presence of symptoms of a major mood episode (a depressive or manic episode) concurrent with symptoms of schizophrenia. The most important basis for establishing the diagnosis is the patient’s history. Determining the percentage of time spent in a mood episode is especially important. Treatment usually consists of an antipsychotic plus a mood stabilizer or antidepressant. Electroconvulsive therapy is an option for patients with SAD who do not respond well to medication.
Related Resources
- Wy TJP, Saadabadi A. Schizoaffective disorder. NCBI Bookshelf: StatPearls Publishing. Published January 2020. https://www.ncbi.nlm.nih.gov/books/NBK541012/. Updated April 15, 2020.
- Parker G. How well does the DSM-5 capture schizoaffective disorder? Can J Psychiatry. 2019;64(9):607-610.
Drug Brand Names
Aripiprazole • Abilify
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Olanzapine long-acting injectable • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate • Invega sustenna
Valproate • Depacon
Mr. C, age 34, presented to the emergency department with his wife because of increasingly bizarre behavior. He reported auditory and visual hallucinations, and believed that the “mob had ordered a hit” against him. He had threatened to shoot his wife and children, which led to his arrest and being briefly jailed. In jail, he was agitated, defecated on the floor, and disrobed. His wife reported that Mr. C had a long history of bipolar disorder and had experienced his first manic episode and hospitalization at age 17. Since then, he had been treated with many different antidepressants, antipsychotics, and mood stabilizers.
Mr. C was admitted to the hospital, where he developed a catatonic syndrome that was treated with a course of electroconvulsive therapy. He was eventually stabilized with
Over the next 8 years, Mr. C was often noncompliant with medication and frequently was hospitalized for mania. His symptoms included poor sleep, grandiosity, pressured speech, racing and disorganized thoughts, increased risk-taking behavior (ie, driving at excessive speeds), and hyperreligiosity (ie, speaking with God). Mr. C also occasionally used methamphetamine, cannabis, and cocaine. Although he had responded well to treatment early in the course of his illness, as he entered his late 30s, his response was less complete, and by his 40s, Mr. C was no longer able to function independently. He eventually was prescribed a long-acting injectable antipsychotic, paliperidone palmitate, 156 mg monthly. Eventually, his family was no longer able to care for him at home, so he was admitted to a residential care facility.
In this facility, based on the long-standing nature of Mr. C’s psychotic disorder and frequency with which he presented with mania, his clinicians changed his diagnosis to schizoaffective disorder, bipolar type. It had become clear that mood symptoms comprised >50% of the total duration of his illness.
Schizoaffective disorder (SAD) often has been used as a diagnosis for patients who have an admixture of mood and psychotic symptoms whose diagnosis is uncertain. Its hallmark is the presence of symptoms of a major mood episode (either a depressive or manic episode) concurrent with symptoms characteristic of schizophrenia, such as delusions, hallucinations, or disorganized speech.1
SAD is a controversial diagnosis. There has been inadequate research regarding the epidemiology, course, etiologic factors, and treatment of this disorder. Debate continues to swirl around its conceptualization; some experts view SAD as an independent disorder, while others see SAD as either a form of schizophrenia or a mood disorder.1 In this review, we describe the classification of SAD and its features, diagnosis, and treatment.
An evolving diagnosis
The term schizoaffective was first used by Jacob Kasanin, MD, in 1933.2 He described 9 patients with “acute schizoaffective psychoses,” each of whom had an abrupt onset. The term was used in the first edition of the DSM as a subtype of schizophrenia.3 In DSM-I, the “schizo-affective type” was defined as a diagnosis for patients with a “significant admixture of schizophrenic and affective reactions.”3 Diagnostic criteria for SAD were developed for DSM-III-R, published in 1987.4 These criteria continued to evolve with subsequent editions of the DSM.
Continue to: DSM-5 provides...

DSM-5 provides a clearer separation between schizophrenia with mood symptoms, bipolar disorder, and SAD (Table5). In addition, DSM-5 shifts away from the DSM-IV diagnosis of SAD as an episode, and instead focuses more on the longitudinal course of the illness. It has been suggested that this change will likely lead to reduced rates of diagnosis of SAD.6 Despite improvements in classification, the diagnosis remains controversial (Box7-11).
Box 1
Despite improvements in classification, controversy continues to swirl around the question of whether schizoaffective disorder (SAD) represents an independent disorder that stands apart from schizophrenia and bipolar disorder, whether it is a form of schizophrenia, or whether it is a form of bipolar disorder or a depressive disorder.7,8 Other possibilities are that SAD is heterogeneous or that it represents a middle point on a spectrum that bridges mood and psychotic disorders. While the merits of each possibility are beyond the scope of this review, it is safe to say that each possibility has its proponents. For these reasons, some argue that the concept itself lacks validity and shows the pitfalls of our classification system.7
Poor diagnostic reliability is one reason for concerns about validity. Most recently, a field trial using DSM-5 criteria produced a kappa of 0.50, which is moderate,9 but earlier definitions produced relatively poor results. Wilson et al10 point out that Criterion C, which concerns duration of mood symptoms, produces a particularly low kappa. Another reason is diagnostic switching, whereby patients initially diagnosed with 1 disorder receive a different diagnosis at followup. Diagnostic switching is especially problematic for SAD. In a large meta-analysis by Santelmann et al,11 36% of patients initially diagnosed with SAD had their diagnosis changed when reassessed. This diagnostic shift tended more toward schizophrenia than bipolar disorder. In addition, more than one-half of all patients initially diagnosed with schizophrenia, bipolar disorder, or major depressive disorder were re-diagnosed with SAD when reassessed.
DSM-5 subtypes and specifiers
In DSM-5,SAD has 2 subtypes5:
- Bipolar type. The bipolar type is marked by the presence of a manic episode (major depressive episodes may also occur)
- Depressive type. The depressive type is marked by the presence of only major depressive episodes.
SAD also includes several specifiers, with the express purpose of giving clinicians greater descriptive ability. The course of SAD can be described as either “first episode,” defined as the first manifestation of the disorder, or as having “multiple episodes,” defined as a minimum of 2 episodes with 1 relapse. In addition, SAD can be described as an acute episode, in partial remission, or in full remission. The course can be described as “continuous” if it is clear that symptoms have been present for the majority of the illness with very brief subthreshold periods. The course is designated as “unspecified” when information is unavailable or lacking. The 5-point Clinician-Rated Dimensions of Psychosis Symptoms was introduced to enable clinicians to make a quantitative assessment of the psychotic symptoms, although its use is not required.
Epidemiology and gender ratio
The epidemiology of SAD has not been well studied. DSM-5 estimates that SAD is approximately one-third as common as schizophrenia, which has a lifetime prevalence of 0.5% to 0.8%.5 This is similar to an estimate by Perälä et al12 of a 0.32% lifetime prevalence based on a nationally representative sample of persons in Finland age ≥30. Scully et al13 calculated a prevalence estimate of 1.1% in a representative sample of adults in rural Ireland. Based on pooled clinical data, Keck et al14 estimated the prevalence in clinical settings at 16%, similar to the figure of 19% reported by Levinson et al15 based on data from New York State psychiatric hospitals. In clinical practice, the diagnosis of SAD is used frequently when there is diagnostic uncertainty, which potentially inflates estimates of lifetime prevalence.
The prevalence of SAD is higher in women than men, with a sex ratio of about 2:1, similar to that seen in mood disorders.13,16-19 There are an equal number of men and women with the bipolar subtype, but a female preponderance with the depressive subtype.5 The bipolar subtype is more common in younger patients, while the depressive subtype is more common in older patients. SAD is a rare diagnosis in children.20
Continue to: Course and outcome
Course and outcome
The onset of SAD typically occurs in early adulthood, but can range from childhood to senescence. Approximately one-third of patients are diagnosed before age 25, one-third between age 25 and 35, and one-third after age 35.21-23 Based on a literature review, Cheniaux et al7 concluded that that age at onset for patients with SAD is between those with schizophrenia and those with mood disorders.
The course of SAD is variable but represents a middle ground between that of schizophrenia and the mood disorders. In a 4- to 5-year follow-up,24 patients with SAD had a better overall course than patients with schizophrenia but had poorer functioning than those with bipolar mania, and much poorer than those with unipolar depression. Mood-incongruent psychotic features predict a particularly worse outcome. These findings were reaffirmed at a 10-year follow-up.25 Mood symptoms portend a better outcome than do symptoms of schizophrenia.
The lifetime suicide risk for patients with SAD is estimated at 5%, with a higher risk associated with the presence of depressive symptoms.26 One study found that women with SAD had a 17.5-year reduced life expectancy (64.1 years) compared with a reduction of 8.0 years for men (69.4 years).27
Comorbidity
Patients with SAD are commonly diagnosed with other psychiatric disorders, including anxiety disorders, obsessive-compulsive disorder, posttraumatic stress disorder, and substance use disorders.21,28,29 When compared with the general population, patients with SAD are at higher risk for coronary heart disease, stroke, obesity, and smoking, likely contributing to their decreased life expectancy.27,30 Because second-generation antipsychotics (SGAs) are often used to treat SAD, patients with SAD are at risk for metabolic syndrome and diabetes mellitus.30
Clinical assessment
Because there are no diagnostic, laboratory, or neuroimaging tests for SAD, the most important basis for making the diagnosis is the patient’s history, supplemented by collateral history from family members or friends, and medical records. Determining the percentage of time spent in a mood episode (DSM-5 Criterion C) is especially important.31 This requires the clinician to pay close attention to the temporal relationship of psychotic and mood symptoms.
Continue to: Differential diagnosis
Differential diagnosis
The differential diagnosis for SAD is broad because it includes all of the possibilities usually considered for major mood disorders and for psychotic disorders5:
- schizophrenia
- bipolar disorder with psychotic features
- major depressive disorder with psychotic features
- depressive or bipolar disorders with catatonic features
- personality disorders (especially the schizotypal, paranoid, and borderline types)
- major neurocognitive disorders in which there are mood and psychotic symptoms
- substance/medication-induced psychotic disorder
- disorders induced by medical conditions.
With schizophrenia, the duration of all episodes of a mood syndrome is brief (<50% of the total duration of the illness) relative to the duration of the psychotic symptoms. Although psychotic symptoms may occur in persons with mood disorders, they are generally not present in the absence of depression or mania, helping to set the boundary between SAD and psychotic mania or depression. As for personality disorders, the individual will not have a true psychosis, although some symptoms, such as feelings of unreality, paranoia, or magical thinking, may cause diagnostic confusion.
Medical conditions also can present with psychotic and mood symptoms and need to be ruled out. These include psychotic disorder due to another medical condition, and delirium. A thorough medical workup should be performed to rule out any possible medical causes for the symptoms.
Substance use should also be ruled out as the cause of the symptoms because many substances are associated with mood and psychotic symptoms. It is usually clear from the history, physical examination, or laboratory tests when a medication/illicit substance has initiated and maintained the disorder.
Neurologic conditions. If a neurologic condition is suspected, a neurologic evaluation may be warranted, including laboratory tests, brain imaging to identify specific anatomical abnormalities, lumbar puncture with cerebrospinal fluid analysis, and an electroencephalogram to rule out a convulsive disorder.
Continue to: Clinical symptoms
Clinical symptoms
The signs and symptoms of SAD include those typically seen in schizophrenia and the mood disorders. Thus, the patient may exhibit elated mood and/or grandiosity, or severe depression, combined with mood-incongruent psychotic features such as paranoid delusions. The symptoms may present together or in an alternating fashion, and psychotic symptoms may be mood-congruent or mood-incongruent. Mr. C’s case illustrates some of the symptoms of the disorder.
Brain imaging
Significant changes have been reported to occur in the brain structure and function in persons with SAD. Neuroimaging studies using voxel-based morphometry have shown significant reductions in gray matter volume in several areas of the brain, including the medial prefrontal cortex, insula, Rolandic operculum, parts of the temporal lobe, and the hippocampus.32-35 Amann et al32 found that patients with SAD and schizophrenia had widespread and overlapping areas of significant volume reduction, but patients with bipolar disorder did not. These studies suggest that at least from a neuroimaging standpoint, SAD is more closely related to schizophrenia than bipolar disorder, and could represent a variant of schizophrenia.
Treatment of SAD
The pharmacotherapy of SAD is mostly empirical because of the lack of randomized controlled trials. Clinicians have traditionally prescribed an antipsychotic agent along with either a mood stabilizer (eg,
Since that exhaustive review,
Patients with SAD will require maintenance treatment for ongoing symptom control. Medication that is effective for treatment of an acute episode should be considered for maintenance treatment. Both the extended-release and long-acting injectable (LAI) formulations of paliperidone have been shown to be efficacious in the maintenance treatment of patients with SAD.40 The LAI form of paliperidone significantly delayed psychotic, depressive, and manic relapses, improved clinical rating scale scores, and increased medication adherence.41,42 In an open-label study, olanzapine LAI was effective in long-term maintenance treatment, although approximately 40% of patients experienced significant weight gain.43 One concern with olanzapine is the possible occurrence of a post-injection delirium/sedation syndrome. For that reason, patients receiving olanzapine must be monitored for at least 3 hours post-injection. The paliperidone LAI does not require monitoring after injection.
Continue to: There is a single clinical trial...
There is a single clinical trial showing that patients with SAD can be successfully switched from other antipsychotics to
Other approaches
Electroconvulsive therapy (ECT) should be considered for patients with SAD who are acutely ill and have failed to respond adequately to medication. ECT is especially relevant in the setting of acute mood symptoms (ie, depressive or manic symptoms co-occurring with psychosis or in the absence of psychosis).45
As currently conceptualized, the diagnosis of SAD is made in persons having an admixture of mood and psychotic symptoms, although by definition mood symptoms must take up the majority (≥50%) of the total duration of the illness. Unfortunately, SAD has been inadequately researched due to the unreliability of its definition and concerns about its validity. The long-term course of SAD is midway between mood and psychotic disorders, and the disorder can cause significant disability.
Bottom Line
Schizoaffective disorder (SAD) is characterized by the presence of symptoms of a major mood episode (a depressive or manic episode) concurrent with symptoms of schizophrenia. The most important basis for establishing the diagnosis is the patient’s history. Determining the percentage of time spent in a mood episode is especially important. Treatment usually consists of an antipsychotic plus a mood stabilizer or antidepressant. Electroconvulsive therapy is an option for patients with SAD who do not respond well to medication.
Related Resources
- Wy TJP, Saadabadi A. Schizoaffective disorder. NCBI Bookshelf: StatPearls Publishing. Published January 2020. https://www.ncbi.nlm.nih.gov/books/NBK541012/. Updated April 15, 2020.
- Parker G. How well does the DSM-5 capture schizoaffective disorder? Can J Psychiatry. 2019;64(9):607-610.
Drug Brand Names
Aripiprazole • Abilify
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Olanzapine long-acting injectable • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate • Invega sustenna
Valproate • Depacon
1. Miller JN, Black DW. Schizoaffective disorder: a review. Ann Clin Psychiatry. 2019;31(1):47-53.
2. Kasanin J. The acute schizoaffective psychoses. Am J Psychiatry. 1933;90:97-126.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington, DC: American Psychiatric Association; 1952.
4. Diagnostic and statistical manual of mental disorders, 3rd ed, revision. Washington, DC: American Psychiatric Association; 1987.
5. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
6. Malaspina D, Owen M, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150:21-25.
7. Cheniaux E, Landeria-Fernandez J, Telles LL, et al. Does schizoaffective disorder really exist? A systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J Affect Disord. 2008;106:209-217.
8. Kantrowitz JT, Citrome L. Schizoaffective disorder: a review of current research themes and pharmacologic management. CNS Drugs. 2011;25:317-331.
9. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170:59-70.
10. Wilson JE, Nian H, Heckers S. The schizoaffective disorder diagnosis: a conundrum in the clinical setting. Eur Arch Psychiatry Clin Neurosci. 2014;264:29-34.
11. Santelmann H, Franklin J, Bußhoff J, Baethge C. Test-retest reliability of schizoaffective disorder compared with schizophrenia, bipolar disorder, and unipolar depression--a systematic review and meta-analysis. Bipolar Disord. 2015;17:753-768.
12. Perälä J, Suvisaari J, Saarni SI, et al. Lifetime Prevalence of psychotic and bipolar I disorders in a general population. JAMA Psychiatry. 2007;64:19-28.
13. Scully PJ, Owens JM, Kinsella A, et al. Schizophrenia, schizoaffective and bipolar disorder within an epidemiologically complete, homogeneous population in rural Ireland: small area variation in rate. Schizophr Res. 2004;67:143-155.
14. Keck PE Jr, McElroy SE, Strakowski SM, et al. Pharmacologic treatment of schizoaffective disorder. Psychopharmacol. 1994;114:529-538.
15. Levinson DF, Umapathy C, Musthaq M. Treatment of schizoaffective disorder and schizophrenia with mood symptoms. Am J Psychiatry. 1999;156:1138-1148.
16. Angst J, Felder W, Lohmeyer B. Course of schizoaffective psychoses: results of a follow-up study. Schizophr Bull. 1980;6:579-585.
17. Lenz G, Simhandl C, Thau K, et al. Temporal stability of diagnostic criteria for functional psychoses. Psychopathol. 1991;24:328-335.
18. Malhi GS, Green M, Fagiolini A, et al. Schizoaffective disorder: diagnostic issues and future recommendations. Bipolar Disord. 2008;10:215-230.
19. Marneros A, Deister A, Rohde A. Psychopathological and social status of patients with affective, schizophrenic and schizoaffective disorders after long‐term course. Acta Psychiatr Scand. 1990;82:352-358.
20. Werry JS, McClellan JM, Chard L. Childhood and adolescent schizophrenic, bipolar, and schizoaffective disorders: a clinical and outcome study. J Am Acad Child Adolesc Psychiatry. 1991;30:457-465.
21. Abrams DJ, Rojas DC, Arciniegas DB. Is schizoaffective disorder a distinct categorical diagnosis? A critical review of the literature. Neuropsychiatr Dis Treat. 2008;4:1089-1109.
22. Bromet EJ, Kotov R, Fochtmann LJ, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168:1186-1194.
23. Salvatore P, Baldessarini RJ, Tohen M, et al. The McLean-Harvard First Episode Project: two-year stability of DSM-IV diagnoses in 500 first-episode psychotic disorder patients. J Clin Psychiatry. 2009;70:458-466.
24. Grossman LS, Harrow M, Goldberg JF, et al. Outcome of schizoaffective disorder at two long term follow-ups: comparisons with outcome of schizophrenia and affective disorders. Am J Psychiatry. 1991;148:1359-1365.
25. Harrow M, Grossman L, Herbener E, et al. Ten-year outcome: patients with schizoaffective disorders, schizophrenia, affective disorders and mood-incongruent psychotic symptoms. Br J Psychiatry. 2000;177:421-426.
26. Hor K, Taylor M. Review: suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24:81-90.
27. Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS ONE. 2011;6:e19590.
28. Byerly M, Goodman W, Acholonu W, et al. Obsessive compulsive symptoms in schizophrenia: frequency and clinical features. Schizophr Res. 2005;76:309-316.
29. Strauss JL, Calhoun PS, Marx CE, et al. Comorbid posttraumatic stress disorder is associated with suicidality in male veterans with schizophrenia or schizoaffective disorder. Schizophr Res. 2006;84:165-169.
30. Fagiolini A, Goracci A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. J Clin Psychiatry. 2009;70:22-29.
31. Black DW, Grant JE. DSM-5 guidebook: the essential companion to the diagnostic and statistical manual of mental disorders, 5th edition. Washington, DC: American Psychiatric Publishing; 2014.
32. Amann BL, Canales-Rodríguez EJ, Madre M, et al. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2016;133:23-33.
33. Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285-1296.
34. Ivleva EI, Bidesi AS, Thomas BP, et al. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res. 2012;204:13-24.
35. Radonic
36. Jäger M, Becker T, Weinmann S, et al. Treatment of schizoaffective disorder - a challenge for evidence-based psychiatry. Acta Psychiatr Scand. 2010;121:22-32.
37. Glick ID, Mankosli R, Eudicone JM, et al. The efficacy, safety, and tolerability of aripiprazole for the treatment of schizoaffective disorder: results from a pooled analysis of a sub-population of subjects from two randomized, double-blind, placebo controlled, pivotal trials. J Affect Disord. 2009;115:18-26.
38. Canuso CM, Lindenmayer JP, Kosik-Gonzalez C, et al. A randomized, double-blind, placebo controlled study of 2 dose ranges of paliperidone extended-release in the treatment of subjects with schizoaffective disorder. J Clin Psychiatry. 2010;71:587-598.
39. Canuso CM, Schooler NR, Carothers J, et al. Paliperidone extended-release in schizoaffective disorder: a randomized controlled trial comparing a flexible-dose with placebo in patients treated with and without antidepressants and/or mood stabilizers. J Clin Psychopharmacol. 2010;30:487-495.
40. Lindenmayer JP, Kaur A. Antipsychotic management of schizoaffective disorder: a review. Drugs. 2016;76:589-604.
41. Alphs L, Fu DJ, Turkoz I. Paliperidone for the treatment of schizoaffective disorder. Expert Opin Pharmacother. 2016;176:871-883.
42. Bossie CA, Turkoz I, Alphs L, et al. Paliperidone palmitate once-monthly treatment in recent onset and chronic illness patients with schizoaffective disorder. J Nerv Ment Dis. 2017;205:324-328.
43. McDonnell DP, Landry J, Detke HC. Long-term safety and efficacy of olanzapine long-acting injection in patients with schizophrenia or schizoaffective disorder: a 6-year, multinational, single-arm, open-label study. Int Clin Psychopharmacol. 2014;29:322-331.
44. McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74:170-179.
45. Mankad MV, Beyer JL, Wiener RD, et al. Manual of electroconvulsive therapy. Washington, DC: American Psychiatric Publishing; 2010.
1. Miller JN, Black DW. Schizoaffective disorder: a review. Ann Clin Psychiatry. 2019;31(1):47-53.
2. Kasanin J. The acute schizoaffective psychoses. Am J Psychiatry. 1933;90:97-126.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington, DC: American Psychiatric Association; 1952.
4. Diagnostic and statistical manual of mental disorders, 3rd ed, revision. Washington, DC: American Psychiatric Association; 1987.
5. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
6. Malaspina D, Owen M, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150:21-25.
7. Cheniaux E, Landeria-Fernandez J, Telles LL, et al. Does schizoaffective disorder really exist? A systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J Affect Disord. 2008;106:209-217.
8. Kantrowitz JT, Citrome L. Schizoaffective disorder: a review of current research themes and pharmacologic management. CNS Drugs. 2011;25:317-331.
9. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170:59-70.
10. Wilson JE, Nian H, Heckers S. The schizoaffective disorder diagnosis: a conundrum in the clinical setting. Eur Arch Psychiatry Clin Neurosci. 2014;264:29-34.
11. Santelmann H, Franklin J, Bußhoff J, Baethge C. Test-retest reliability of schizoaffective disorder compared with schizophrenia, bipolar disorder, and unipolar depression--a systematic review and meta-analysis. Bipolar Disord. 2015;17:753-768.
12. Perälä J, Suvisaari J, Saarni SI, et al. Lifetime Prevalence of psychotic and bipolar I disorders in a general population. JAMA Psychiatry. 2007;64:19-28.
13. Scully PJ, Owens JM, Kinsella A, et al. Schizophrenia, schizoaffective and bipolar disorder within an epidemiologically complete, homogeneous population in rural Ireland: small area variation in rate. Schizophr Res. 2004;67:143-155.
14. Keck PE Jr, McElroy SE, Strakowski SM, et al. Pharmacologic treatment of schizoaffective disorder. Psychopharmacol. 1994;114:529-538.
15. Levinson DF, Umapathy C, Musthaq M. Treatment of schizoaffective disorder and schizophrenia with mood symptoms. Am J Psychiatry. 1999;156:1138-1148.
16. Angst J, Felder W, Lohmeyer B. Course of schizoaffective psychoses: results of a follow-up study. Schizophr Bull. 1980;6:579-585.
17. Lenz G, Simhandl C, Thau K, et al. Temporal stability of diagnostic criteria for functional psychoses. Psychopathol. 1991;24:328-335.
18. Malhi GS, Green M, Fagiolini A, et al. Schizoaffective disorder: diagnostic issues and future recommendations. Bipolar Disord. 2008;10:215-230.
19. Marneros A, Deister A, Rohde A. Psychopathological and social status of patients with affective, schizophrenic and schizoaffective disorders after long‐term course. Acta Psychiatr Scand. 1990;82:352-358.
20. Werry JS, McClellan JM, Chard L. Childhood and adolescent schizophrenic, bipolar, and schizoaffective disorders: a clinical and outcome study. J Am Acad Child Adolesc Psychiatry. 1991;30:457-465.
21. Abrams DJ, Rojas DC, Arciniegas DB. Is schizoaffective disorder a distinct categorical diagnosis? A critical review of the literature. Neuropsychiatr Dis Treat. 2008;4:1089-1109.
22. Bromet EJ, Kotov R, Fochtmann LJ, et al. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168:1186-1194.
23. Salvatore P, Baldessarini RJ, Tohen M, et al. The McLean-Harvard First Episode Project: two-year stability of DSM-IV diagnoses in 500 first-episode psychotic disorder patients. J Clin Psychiatry. 2009;70:458-466.
24. Grossman LS, Harrow M, Goldberg JF, et al. Outcome of schizoaffective disorder at two long term follow-ups: comparisons with outcome of schizophrenia and affective disorders. Am J Psychiatry. 1991;148:1359-1365.
25. Harrow M, Grossman L, Herbener E, et al. Ten-year outcome: patients with schizoaffective disorders, schizophrenia, affective disorders and mood-incongruent psychotic symptoms. Br J Psychiatry. 2000;177:421-426.
26. Hor K, Taylor M. Review: suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24:81-90.
27. Chang CK, Hayes RD, Perera G, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS ONE. 2011;6:e19590.
28. Byerly M, Goodman W, Acholonu W, et al. Obsessive compulsive symptoms in schizophrenia: frequency and clinical features. Schizophr Res. 2005;76:309-316.
29. Strauss JL, Calhoun PS, Marx CE, et al. Comorbid posttraumatic stress disorder is associated with suicidality in male veterans with schizophrenia or schizoaffective disorder. Schizophr Res. 2006;84:165-169.
30. Fagiolini A, Goracci A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. J Clin Psychiatry. 2009;70:22-29.
31. Black DW, Grant JE. DSM-5 guidebook: the essential companion to the diagnostic and statistical manual of mental disorders, 5th edition. Washington, DC: American Psychiatric Publishing; 2014.
32. Amann BL, Canales-Rodríguez EJ, Madre M, et al. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2016;133:23-33.
33. Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285-1296.
34. Ivleva EI, Bidesi AS, Thomas BP, et al. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res. 2012;204:13-24.
35. Radonic
36. Jäger M, Becker T, Weinmann S, et al. Treatment of schizoaffective disorder - a challenge for evidence-based psychiatry. Acta Psychiatr Scand. 2010;121:22-32.
37. Glick ID, Mankosli R, Eudicone JM, et al. The efficacy, safety, and tolerability of aripiprazole for the treatment of schizoaffective disorder: results from a pooled analysis of a sub-population of subjects from two randomized, double-blind, placebo controlled, pivotal trials. J Affect Disord. 2009;115:18-26.
38. Canuso CM, Lindenmayer JP, Kosik-Gonzalez C, et al. A randomized, double-blind, placebo controlled study of 2 dose ranges of paliperidone extended-release in the treatment of subjects with schizoaffective disorder. J Clin Psychiatry. 2010;71:587-598.
39. Canuso CM, Schooler NR, Carothers J, et al. Paliperidone extended-release in schizoaffective disorder: a randomized controlled trial comparing a flexible-dose with placebo in patients treated with and without antidepressants and/or mood stabilizers. J Clin Psychopharmacol. 2010;30:487-495.
40. Lindenmayer JP, Kaur A. Antipsychotic management of schizoaffective disorder: a review. Drugs. 2016;76:589-604.
41. Alphs L, Fu DJ, Turkoz I. Paliperidone for the treatment of schizoaffective disorder. Expert Opin Pharmacother. 2016;176:871-883.
42. Bossie CA, Turkoz I, Alphs L, et al. Paliperidone palmitate once-monthly treatment in recent onset and chronic illness patients with schizoaffective disorder. J Nerv Ment Dis. 2017;205:324-328.
43. McDonnell DP, Landry J, Detke HC. Long-term safety and efficacy of olanzapine long-acting injection in patients with schizophrenia or schizoaffective disorder: a 6-year, multinational, single-arm, open-label study. Int Clin Psychopharmacol. 2014;29:322-331.
44. McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74:170-179.
45. Mankad MV, Beyer JL, Wiener RD, et al. Manual of electroconvulsive therapy. Washington, DC: American Psychiatric Publishing; 2010.
Assessing decision-making capacity
Medical and surgical inpatient teams often consult psychiatrists to help assess a patient’s decision-making capacity (DMC). Completing a DMC assessment can be a source of stress and frustration for psychiatry residents; however, the process can be significantly improved by following a consistent and focused psychiatric interview. In our institution, the most frequently used tool for assessing DMC is the Aid to Capacity Evaluation (ACE).1 The American Academy of Family Physicians offers a printer-friendly adaptation2 of the ACE assessment.
Reasons for a DMC consultation
When accepting a DMC consultation, make sure to specify for which medical decision the primary team would like the patient to be evaluated so that the consultation can be most helpful and specific to primary team’s concerns.3 The 4 most common reasons for a DMC consultation are:
Acute changes in mental status. These changes may be due to hypoxia, infection, medication, metabolic disturbances, or other medical conditions. Often, the diagnosis is delirium due to a medical condition, and assessing for DMC is deferred until the delirium resolves.
Refusal of a recommended treatment. One of the most frequent reasons for a DMC consultation is when a patient declines the primary team’s treatment recommendations. These recommendations may include medications, interventions (procedural or surgical), or discharge planning (such as transfer to a rehabilitation facility or skilled nursing care facility).
Consenting to a risky or invasive treatment too hastily. This occurs less often than the other 3 scenarios, most likely because capacity is rarely questioned when a patient’s decision aligns with the physicians’ recommendations.
Having risk factors for impaired decision-making. One of the most common reasons for involving psychiatry in a DMC consultation is when the patient has a risk factor that may impair his/her decision-making.
These risk factors include:
- a chronic psychiatric or neurologic condition
- a significant cultural or language barrier
- a low or unknown education level
- anxiety or discomfort with institutional health care settings
- age <18 or >85.
Continue to: When should you put off a DMC consultation?
When should you put off a DMC consultation?
There are situations in which a DMC consultation should be declined or postponed. A DMC consultation is not appropriate for patients who have a court-appointed legal guardian because these patients are considered legally incapacitated. Although such patients may still choose to participate in shared decision-making with the treatment team and communicate those decisions to their legal guardian, all treatment decisions are made by their legal guardian.
Consider delaying DMC consultations for patients with acute changes in mental status, including delirium, or those who are sedated or intubated. On the other hand, patients not excluded from a DMC assessment include those with mild or major neurocognitive disorders (such as Alzheimer’s disease or other types of dementia), intellectual disabilities, a durable power of attorney (DPOA), or legal charges. It is important to note that a diagnosis of a neurocognitive disorder does not preclude capacity, and capacity may therefore vary based on the complexity of medical decision.
Prepare for the interview
Prior to meeting the patient, familiarize yourself with his/her reason for admission, diagnosis, medical workup, treatment provided thus far, and proposed future treatment. If possible, attempt to gather the patient’s medical and psychiatric history from chart review, which may be obtained from outside medical records or inferred from the patient’s current medication list. Review the chart for any signs that the patient is in a delirious state, including the use of psychotropic medications for agitation, restraints, or a sitter at bedside. Finally, speak with the patient’s nurse before entering the patient’s room. This may provide valuable information regarding the patient’s willingness to participate (including any hostility or aggression toward clinicians), any barriers to the interview (such as hearing or language difficulties), and if any family or friends are present at the bedside.
During the interview
The structure of a decision-making capacity evaluation has been well documented elsewhere1,2 and, if completed, is sufficient for determining DMC. Asking the patient about additional psychiatric histories, including hospitalizations, suicide attempts, or a family history of psychiatric illness, may be counterproductive and frustrating for the patient, especially if the interview has already been lengthy and emotionally exhausting. Therefore, it is often appropriate to shorten the psychiatric interview to assess for symptoms of anxiety and depression, perform a brief suicide risk assessment, and assess for the presence of auditory or visual hallucinations, or delusions. This allows for a complete mental status exam while still focusing the interview on determining DMC. If time allows and the patient is willing to participate, it can be helpful to perform a Montreal Cognitive Assessment (MoCA) or Mini-Mental State Exam, although a score that indicates some level of cognitive impairment (<26 on MoCA4) does not necessarily preclude patient from having capacity for certain medical decisions. Additionally, if the patient does not understand a question, explain it and return to it later to check for comprehension.
Making a recommendation
Patients are presumed to be capable; therefore, if the determination is not clear, err on the side of capacity. Additional information may be helpful from the patient’s family, friends, DPOA, or guardian, especially as it pertains to the patient’s past wishes and medical decisions made in similar situations. If available and pertinent, review the patient’s advance health care directive.
Communicate your recommendations to the primary team clearly and concisely, including if the evaluation is incomplete, and if the patient will be seen again by the consult team, because this may determine disposition planning. Finally, if the patient is deemed to not have DMC, the primary team must then establish a surrogate decision maker on the patient’s behalf. Because the protocol and hierarchy of next-of-kin varies by state law and institution policy, it is essential to involve social work and case management
1. Joint Centre for Bioethics. Aid to capacity evaluation (ACE). http://www.jcb.utoronto.ca/tools/documents/ace.pdf. Published 1996. Accessed July 20, 2020.
2. American Academy of Family Physicians. Aid to capacity evaluation. https://www.aafp.org/afp/2001/0715/afp20010715p299-f2.pdf. Published 2000. Accessed July 20, 2020.
3. Tunzi M. Can the patient decide? Evaluating patient capacity in practice. Am Fam Physician. 2001;64(2):299-306.
4. Montreal Cognitive Assessment. FAQ. https://www.mocatest.org/faq/. Accessed July 20, 2020.
Medical and surgical inpatient teams often consult psychiatrists to help assess a patient’s decision-making capacity (DMC). Completing a DMC assessment can be a source of stress and frustration for psychiatry residents; however, the process can be significantly improved by following a consistent and focused psychiatric interview. In our institution, the most frequently used tool for assessing DMC is the Aid to Capacity Evaluation (ACE).1 The American Academy of Family Physicians offers a printer-friendly adaptation2 of the ACE assessment.
Reasons for a DMC consultation
When accepting a DMC consultation, make sure to specify for which medical decision the primary team would like the patient to be evaluated so that the consultation can be most helpful and specific to primary team’s concerns.3 The 4 most common reasons for a DMC consultation are:
Acute changes in mental status. These changes may be due to hypoxia, infection, medication, metabolic disturbances, or other medical conditions. Often, the diagnosis is delirium due to a medical condition, and assessing for DMC is deferred until the delirium resolves.
Refusal of a recommended treatment. One of the most frequent reasons for a DMC consultation is when a patient declines the primary team’s treatment recommendations. These recommendations may include medications, interventions (procedural or surgical), or discharge planning (such as transfer to a rehabilitation facility or skilled nursing care facility).
Consenting to a risky or invasive treatment too hastily. This occurs less often than the other 3 scenarios, most likely because capacity is rarely questioned when a patient’s decision aligns with the physicians’ recommendations.
Having risk factors for impaired decision-making. One of the most common reasons for involving psychiatry in a DMC consultation is when the patient has a risk factor that may impair his/her decision-making.
These risk factors include:
- a chronic psychiatric or neurologic condition
- a significant cultural or language barrier
- a low or unknown education level
- anxiety or discomfort with institutional health care settings
- age <18 or >85.
Continue to: When should you put off a DMC consultation?
When should you put off a DMC consultation?
There are situations in which a DMC consultation should be declined or postponed. A DMC consultation is not appropriate for patients who have a court-appointed legal guardian because these patients are considered legally incapacitated. Although such patients may still choose to participate in shared decision-making with the treatment team and communicate those decisions to their legal guardian, all treatment decisions are made by their legal guardian.
Consider delaying DMC consultations for patients with acute changes in mental status, including delirium, or those who are sedated or intubated. On the other hand, patients not excluded from a DMC assessment include those with mild or major neurocognitive disorders (such as Alzheimer’s disease or other types of dementia), intellectual disabilities, a durable power of attorney (DPOA), or legal charges. It is important to note that a diagnosis of a neurocognitive disorder does not preclude capacity, and capacity may therefore vary based on the complexity of medical decision.
Prepare for the interview
Prior to meeting the patient, familiarize yourself with his/her reason for admission, diagnosis, medical workup, treatment provided thus far, and proposed future treatment. If possible, attempt to gather the patient’s medical and psychiatric history from chart review, which may be obtained from outside medical records or inferred from the patient’s current medication list. Review the chart for any signs that the patient is in a delirious state, including the use of psychotropic medications for agitation, restraints, or a sitter at bedside. Finally, speak with the patient’s nurse before entering the patient’s room. This may provide valuable information regarding the patient’s willingness to participate (including any hostility or aggression toward clinicians), any barriers to the interview (such as hearing or language difficulties), and if any family or friends are present at the bedside.
During the interview
The structure of a decision-making capacity evaluation has been well documented elsewhere1,2 and, if completed, is sufficient for determining DMC. Asking the patient about additional psychiatric histories, including hospitalizations, suicide attempts, or a family history of psychiatric illness, may be counterproductive and frustrating for the patient, especially if the interview has already been lengthy and emotionally exhausting. Therefore, it is often appropriate to shorten the psychiatric interview to assess for symptoms of anxiety and depression, perform a brief suicide risk assessment, and assess for the presence of auditory or visual hallucinations, or delusions. This allows for a complete mental status exam while still focusing the interview on determining DMC. If time allows and the patient is willing to participate, it can be helpful to perform a Montreal Cognitive Assessment (MoCA) or Mini-Mental State Exam, although a score that indicates some level of cognitive impairment (<26 on MoCA4) does not necessarily preclude patient from having capacity for certain medical decisions. Additionally, if the patient does not understand a question, explain it and return to it later to check for comprehension.
Making a recommendation
Patients are presumed to be capable; therefore, if the determination is not clear, err on the side of capacity. Additional information may be helpful from the patient’s family, friends, DPOA, or guardian, especially as it pertains to the patient’s past wishes and medical decisions made in similar situations. If available and pertinent, review the patient’s advance health care directive.
Communicate your recommendations to the primary team clearly and concisely, including if the evaluation is incomplete, and if the patient will be seen again by the consult team, because this may determine disposition planning. Finally, if the patient is deemed to not have DMC, the primary team must then establish a surrogate decision maker on the patient’s behalf. Because the protocol and hierarchy of next-of-kin varies by state law and institution policy, it is essential to involve social work and case management
Medical and surgical inpatient teams often consult psychiatrists to help assess a patient’s decision-making capacity (DMC). Completing a DMC assessment can be a source of stress and frustration for psychiatry residents; however, the process can be significantly improved by following a consistent and focused psychiatric interview. In our institution, the most frequently used tool for assessing DMC is the Aid to Capacity Evaluation (ACE).1 The American Academy of Family Physicians offers a printer-friendly adaptation2 of the ACE assessment.
Reasons for a DMC consultation
When accepting a DMC consultation, make sure to specify for which medical decision the primary team would like the patient to be evaluated so that the consultation can be most helpful and specific to primary team’s concerns.3 The 4 most common reasons for a DMC consultation are:
Acute changes in mental status. These changes may be due to hypoxia, infection, medication, metabolic disturbances, or other medical conditions. Often, the diagnosis is delirium due to a medical condition, and assessing for DMC is deferred until the delirium resolves.
Refusal of a recommended treatment. One of the most frequent reasons for a DMC consultation is when a patient declines the primary team’s treatment recommendations. These recommendations may include medications, interventions (procedural or surgical), or discharge planning (such as transfer to a rehabilitation facility or skilled nursing care facility).
Consenting to a risky or invasive treatment too hastily. This occurs less often than the other 3 scenarios, most likely because capacity is rarely questioned when a patient’s decision aligns with the physicians’ recommendations.
Having risk factors for impaired decision-making. One of the most common reasons for involving psychiatry in a DMC consultation is when the patient has a risk factor that may impair his/her decision-making.
These risk factors include:
- a chronic psychiatric or neurologic condition
- a significant cultural or language barrier
- a low or unknown education level
- anxiety or discomfort with institutional health care settings
- age <18 or >85.
Continue to: When should you put off a DMC consultation?
When should you put off a DMC consultation?
There are situations in which a DMC consultation should be declined or postponed. A DMC consultation is not appropriate for patients who have a court-appointed legal guardian because these patients are considered legally incapacitated. Although such patients may still choose to participate in shared decision-making with the treatment team and communicate those decisions to their legal guardian, all treatment decisions are made by their legal guardian.
Consider delaying DMC consultations for patients with acute changes in mental status, including delirium, or those who are sedated or intubated. On the other hand, patients not excluded from a DMC assessment include those with mild or major neurocognitive disorders (such as Alzheimer’s disease or other types of dementia), intellectual disabilities, a durable power of attorney (DPOA), or legal charges. It is important to note that a diagnosis of a neurocognitive disorder does not preclude capacity, and capacity may therefore vary based on the complexity of medical decision.
Prepare for the interview
Prior to meeting the patient, familiarize yourself with his/her reason for admission, diagnosis, medical workup, treatment provided thus far, and proposed future treatment. If possible, attempt to gather the patient’s medical and psychiatric history from chart review, which may be obtained from outside medical records or inferred from the patient’s current medication list. Review the chart for any signs that the patient is in a delirious state, including the use of psychotropic medications for agitation, restraints, or a sitter at bedside. Finally, speak with the patient’s nurse before entering the patient’s room. This may provide valuable information regarding the patient’s willingness to participate (including any hostility or aggression toward clinicians), any barriers to the interview (such as hearing or language difficulties), and if any family or friends are present at the bedside.
During the interview
The structure of a decision-making capacity evaluation has been well documented elsewhere1,2 and, if completed, is sufficient for determining DMC. Asking the patient about additional psychiatric histories, including hospitalizations, suicide attempts, or a family history of psychiatric illness, may be counterproductive and frustrating for the patient, especially if the interview has already been lengthy and emotionally exhausting. Therefore, it is often appropriate to shorten the psychiatric interview to assess for symptoms of anxiety and depression, perform a brief suicide risk assessment, and assess for the presence of auditory or visual hallucinations, or delusions. This allows for a complete mental status exam while still focusing the interview on determining DMC. If time allows and the patient is willing to participate, it can be helpful to perform a Montreal Cognitive Assessment (MoCA) or Mini-Mental State Exam, although a score that indicates some level of cognitive impairment (<26 on MoCA4) does not necessarily preclude patient from having capacity for certain medical decisions. Additionally, if the patient does not understand a question, explain it and return to it later to check for comprehension.
Making a recommendation
Patients are presumed to be capable; therefore, if the determination is not clear, err on the side of capacity. Additional information may be helpful from the patient’s family, friends, DPOA, or guardian, especially as it pertains to the patient’s past wishes and medical decisions made in similar situations. If available and pertinent, review the patient’s advance health care directive.
Communicate your recommendations to the primary team clearly and concisely, including if the evaluation is incomplete, and if the patient will be seen again by the consult team, because this may determine disposition planning. Finally, if the patient is deemed to not have DMC, the primary team must then establish a surrogate decision maker on the patient’s behalf. Because the protocol and hierarchy of next-of-kin varies by state law and institution policy, it is essential to involve social work and case management
1. Joint Centre for Bioethics. Aid to capacity evaluation (ACE). http://www.jcb.utoronto.ca/tools/documents/ace.pdf. Published 1996. Accessed July 20, 2020.
2. American Academy of Family Physicians. Aid to capacity evaluation. https://www.aafp.org/afp/2001/0715/afp20010715p299-f2.pdf. Published 2000. Accessed July 20, 2020.
3. Tunzi M. Can the patient decide? Evaluating patient capacity in practice. Am Fam Physician. 2001;64(2):299-306.
4. Montreal Cognitive Assessment. FAQ. https://www.mocatest.org/faq/. Accessed July 20, 2020.
1. Joint Centre for Bioethics. Aid to capacity evaluation (ACE). http://www.jcb.utoronto.ca/tools/documents/ace.pdf. Published 1996. Accessed July 20, 2020.
2. American Academy of Family Physicians. Aid to capacity evaluation. https://www.aafp.org/afp/2001/0715/afp20010715p299-f2.pdf. Published 2000. Accessed July 20, 2020.
3. Tunzi M. Can the patient decide? Evaluating patient capacity in practice. Am Fam Physician. 2001;64(2):299-306.
4. Montreal Cognitive Assessment. FAQ. https://www.mocatest.org/faq/. Accessed July 20, 2020.
When the professional becomes personal
I arrived at the inpatient psychiatry unit to begin my last overnight call shift as an intern. When I received sign-out from the day-shift resident, Dr. A (all names have been changed to protect anonymity), I was surprised to learn that the medical student assigned for call that day, G, did not present for his shift. In my residency program, a third-year medical student was assigned to accompany an intern during call shifts. While this assignment was for the medical student’s “learning purposes,” the presence of a medical student was vital for the intern. The high volume of patients who needed to be seen while on call was difficult to manage, and the help of a medical student certainly lessened that burden.
During her shift, Dr. A had texted G regarding his missed shift, but he replied that he was busy with a research project and did not intend to attend his call shift. Dr. A and I agreed that G’s behavior was unusual, especially because we had both worked with him before and had found him to be highly motivated and competent. Dr. A said that she was going to follow up with the clerkship director about G’s missed shift. While I was initially angry about having to work the call shift alone, I was quickly overcome with patient care. I labored through the night, and then spent the next day sleeping and recovering.
The following morning, I was back on the inpatient unit to start the work week. I was performing my usual morning pre-rounding when I received shocking news: a medical student had completed suicide on the school’s campus. After processing this news, I asked for the name of the student. It was G.
I sat in disbelief. How could a medical student who was supposed to work with me just 2 days ago have completed suicide? How could such a bright and well-presenting student decide to end his life? I voiced these thoughts to my colleague, who responded, “He must have been preparing to do it instead of coming to your call shift.” This statement hit me like a ton of bricks. Should I have done more to reach out to him? If I had spoken with him, could I have intervened and arrested his planning?
Later, I found out that G had been diagnosed with bipolar disorder. He had shared his diagnosis with some of his classmates and his psychiatry attending. G had wanted to share his success story as someone with mental illness who could have a career in medicine. However, because mental illness carries stigma, the attending had warned G about possible negative consequences in professional settings if he chose to share his diagnosis openly. G expressed disappointment with this advice. Subsequently, he had appeared more withdrawn during his clerkship engagements.
As psychiatrists, we expect to have conversations with our patients about thoughts of suicide, but such discussions with our physician colleagues are far from the norm. We know that the rate of suicide in physicians is higher than in the general population1—particularly in women2—but stigma often prevents those who work in medicine from seeking treatment.3 Unfortunately, professional requirements and attitudes perpetuate barriers to accessing mental health care.4 As long as licensure concerns or other negative professional consequences persist, physicians will avoid seeking potentially life-saving treatment.
I felt guilty for having been angry at G for missing my call shift. I knew that the sequence of events could not be changed, but that did not stop me from wondering, “What if?” What if I had reached out? What if Dr. A had corresponded differently? If we want to prevent tragic outcomes like G’s, then we need to fix our flawed system. We need to allow physicians to seek treatment and share their experience without punitive professional consequences, because suicide is permanent, and there is no undoing.
1. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
2. Duarte D, El-Hagrassy MM, Couto TCE, et al. Male and female physician suicidality: a systematic review and meta-analysis [published online March 4, 2020]. JAMA Psychiatry. 2020;77(6):1-11.
3. Worley LLM. Our fallen peers: a mandate for change. Acad Psychiatry. 2008;32(1):8-12.
4. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166.
I arrived at the inpatient psychiatry unit to begin my last overnight call shift as an intern. When I received sign-out from the day-shift resident, Dr. A (all names have been changed to protect anonymity), I was surprised to learn that the medical student assigned for call that day, G, did not present for his shift. In my residency program, a third-year medical student was assigned to accompany an intern during call shifts. While this assignment was for the medical student’s “learning purposes,” the presence of a medical student was vital for the intern. The high volume of patients who needed to be seen while on call was difficult to manage, and the help of a medical student certainly lessened that burden.
During her shift, Dr. A had texted G regarding his missed shift, but he replied that he was busy with a research project and did not intend to attend his call shift. Dr. A and I agreed that G’s behavior was unusual, especially because we had both worked with him before and had found him to be highly motivated and competent. Dr. A said that she was going to follow up with the clerkship director about G’s missed shift. While I was initially angry about having to work the call shift alone, I was quickly overcome with patient care. I labored through the night, and then spent the next day sleeping and recovering.
The following morning, I was back on the inpatient unit to start the work week. I was performing my usual morning pre-rounding when I received shocking news: a medical student had completed suicide on the school’s campus. After processing this news, I asked for the name of the student. It was G.
I sat in disbelief. How could a medical student who was supposed to work with me just 2 days ago have completed suicide? How could such a bright and well-presenting student decide to end his life? I voiced these thoughts to my colleague, who responded, “He must have been preparing to do it instead of coming to your call shift.” This statement hit me like a ton of bricks. Should I have done more to reach out to him? If I had spoken with him, could I have intervened and arrested his planning?
Later, I found out that G had been diagnosed with bipolar disorder. He had shared his diagnosis with some of his classmates and his psychiatry attending. G had wanted to share his success story as someone with mental illness who could have a career in medicine. However, because mental illness carries stigma, the attending had warned G about possible negative consequences in professional settings if he chose to share his diagnosis openly. G expressed disappointment with this advice. Subsequently, he had appeared more withdrawn during his clerkship engagements.
As psychiatrists, we expect to have conversations with our patients about thoughts of suicide, but such discussions with our physician colleagues are far from the norm. We know that the rate of suicide in physicians is higher than in the general population1—particularly in women2—but stigma often prevents those who work in medicine from seeking treatment.3 Unfortunately, professional requirements and attitudes perpetuate barriers to accessing mental health care.4 As long as licensure concerns or other negative professional consequences persist, physicians will avoid seeking potentially life-saving treatment.
I felt guilty for having been angry at G for missing my call shift. I knew that the sequence of events could not be changed, but that did not stop me from wondering, “What if?” What if I had reached out? What if Dr. A had corresponded differently? If we want to prevent tragic outcomes like G’s, then we need to fix our flawed system. We need to allow physicians to seek treatment and share their experience without punitive professional consequences, because suicide is permanent, and there is no undoing.
I arrived at the inpatient psychiatry unit to begin my last overnight call shift as an intern. When I received sign-out from the day-shift resident, Dr. A (all names have been changed to protect anonymity), I was surprised to learn that the medical student assigned for call that day, G, did not present for his shift. In my residency program, a third-year medical student was assigned to accompany an intern during call shifts. While this assignment was for the medical student’s “learning purposes,” the presence of a medical student was vital for the intern. The high volume of patients who needed to be seen while on call was difficult to manage, and the help of a medical student certainly lessened that burden.
During her shift, Dr. A had texted G regarding his missed shift, but he replied that he was busy with a research project and did not intend to attend his call shift. Dr. A and I agreed that G’s behavior was unusual, especially because we had both worked with him before and had found him to be highly motivated and competent. Dr. A said that she was going to follow up with the clerkship director about G’s missed shift. While I was initially angry about having to work the call shift alone, I was quickly overcome with patient care. I labored through the night, and then spent the next day sleeping and recovering.
The following morning, I was back on the inpatient unit to start the work week. I was performing my usual morning pre-rounding when I received shocking news: a medical student had completed suicide on the school’s campus. After processing this news, I asked for the name of the student. It was G.
I sat in disbelief. How could a medical student who was supposed to work with me just 2 days ago have completed suicide? How could such a bright and well-presenting student decide to end his life? I voiced these thoughts to my colleague, who responded, “He must have been preparing to do it instead of coming to your call shift.” This statement hit me like a ton of bricks. Should I have done more to reach out to him? If I had spoken with him, could I have intervened and arrested his planning?
Later, I found out that G had been diagnosed with bipolar disorder. He had shared his diagnosis with some of his classmates and his psychiatry attending. G had wanted to share his success story as someone with mental illness who could have a career in medicine. However, because mental illness carries stigma, the attending had warned G about possible negative consequences in professional settings if he chose to share his diagnosis openly. G expressed disappointment with this advice. Subsequently, he had appeared more withdrawn during his clerkship engagements.
As psychiatrists, we expect to have conversations with our patients about thoughts of suicide, but such discussions with our physician colleagues are far from the norm. We know that the rate of suicide in physicians is higher than in the general population1—particularly in women2—but stigma often prevents those who work in medicine from seeking treatment.3 Unfortunately, professional requirements and attitudes perpetuate barriers to accessing mental health care.4 As long as licensure concerns or other negative professional consequences persist, physicians will avoid seeking potentially life-saving treatment.
I felt guilty for having been angry at G for missing my call shift. I knew that the sequence of events could not be changed, but that did not stop me from wondering, “What if?” What if I had reached out? What if Dr. A had corresponded differently? If we want to prevent tragic outcomes like G’s, then we need to fix our flawed system. We need to allow physicians to seek treatment and share their experience without punitive professional consequences, because suicide is permanent, and there is no undoing.
1. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
2. Duarte D, El-Hagrassy MM, Couto TCE, et al. Male and female physician suicidality: a systematic review and meta-analysis [published online March 4, 2020]. JAMA Psychiatry. 2020;77(6):1-11.
3. Worley LLM. Our fallen peers: a mandate for change. Acad Psychiatry. 2008;32(1):8-12.
4. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166.
1. Schernhammer ES, Colditz GA. Suicide rates among physicians: a quantitative and gender assessment (meta-analysis). Am J Psychiatry. 2004;161(12):2295-2302.
2. Duarte D, El-Hagrassy MM, Couto TCE, et al. Male and female physician suicidality: a systematic review and meta-analysis [published online March 4, 2020]. JAMA Psychiatry. 2020;77(6):1-11.
3. Worley LLM. Our fallen peers: a mandate for change. Acad Psychiatry. 2008;32(1):8-12.
4. Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289(23):3161-3166.
Enduring the ordeal of a quadruple threat is especially arduous for psychiatric patients
These are unusually stressful days for everyone, especially our patients. We are all experiencing a turbulent mix of emotions as we try to cope with a confluence of threats to both our lives and to life as we know it. Peace of mind has become so elusive due to the relentless overlapping waves of fear, sadness, anger, and uncertainty. We are all grieving in a different way, but our psychiatric patients are suffering the most.
Fear. It only takes 1 traumatic event to trigger posttraumatic stress disorder (PTSD). Yet over the past few months, we have been afflicted by 4 jarring traumatic events, individually and as a society. Just a few months ago, it would have been impossible to imagine the conflux of 4 concurrent seismic threats to our well-being. A toxic political zeitgeist was the backdrop, which we bemoaned and tried to compartmentalize, despite the corrosive political environment shrouding the country. Then the deadly coronavirus disease 2019 (COVID-19) pandemic suddenly arrived, imposing draconian health-preserving measures that impacted every individual’s daily life in countless detrimental ways. Fear prevailed as we all sheltered at home, stopped commuting to work, canceled all trips, distanced ourselves from our friends and relatives, and watched depressing and anxiety-provoking television and read online news throughout our waking hours. Hoarding food and household supplies became endemic due to fear about survival.
Sadness. The agonizing prospect of a national financial necrosis followed the threat of serious illness or death. The economy came to a screeching halt, hemorrhaging millions of jobs. Unemployed parents stayed home with their morose children whose schools were shuttered, leaving them deprived of socializing with their friends. The government hurried with financial chemotherapy, printing trillions of dollars to prevent economic collapse, to avert potential poverty and hunger for many. The fear of the pandemic became coupled with sadness over the loss of livelihoods and grief for the loss of liberty and the ability to pursue happiness, or even small pleasures.
Anger. Then a tsunami of anger was generated by the brutal and sadistic death of a black man in police custody. This was a spark that ignited a massive amount of previously dormant racial tension dating back to the dark days of slavery. Peaceful protests were marred by destructive riots. The explosive fury was perhaps intensified by the protestors having been being locked up for weeks and having to wear masks, both of which were symbolic of being held down and “unable to breathe,” like the murdered Mr. George Floyd.
An epidemic of destroying statues followed. Heavy statues that appeared invincible for decades were dismantled from their plinth in a matter of minutes, signifying extreme frustration with the social injustice that remains despite the transformational laws of the Civil Rights Acts of 1960 and 1964. Suddenly—like falling dominoes—statues, flags, names of military bases, and previously venerated monuments were removed, changed, vandalized, or threatened with destruction. The founders of the republic were also maligned because they were slave owners 2 centuries ago. The paradigm shift spawned by the rage over racial inequality was disconcerting and dramatic. The anger and rampage spawned a sense that a tipping point in our society has been reached.
Uncertainty. The confluence of political instability, a deadly pandemic, economic collapse, and racial tensions were like the 4 horsemen of mass PTSD. The result was an agonizing uncertainty about the impact of these changes, and whether a sense of normalcy will ever return. It became apparent to all of us that our social structure has changed forever across multiple fundamental domains: public health, social, political, and financial. The wait for a vaccine for COVID-19 seems interminable, and racial healing and harmony seems elusive. Economic recovery may be possible, but political detoxification appears unlikely. The fate of police departments, condemned because of the deplorable and illegal acts of a few, and the safety of citizens, usually guaranteed by law and order, seem uncertain. Like COVID-19, angst has rapidly spread across the population.
The price our patients pay
The ingredients of a large-scale societal PTSD, similar to what probably happens during a world war, are now in place. Even resilient individuals may buckle during quadruple ordeals such as this one. So imagine what is happening to our patients, rendered fragile and vulnerable to threats by their pre-existing psychiatric illness. They all pay a heavy price. Patients with anxiety disorders will decompensate, with more panic attacks. Patients burdened by depression will worsen, with more hopelessness, despair, and suicidal ideation due to anxiety and loneliness. Patients with bipolar disorder will become more labile and irritable, and their comorbid anxiety will intensify. Patients with schizophrenia will become more paranoid, depressed, and anxious. Patients with autism will become more agitated and aggressive because their cherished daily routines are disrupted. Patients with obsessive-compulsive disorder will react to their germaphobia by washing their hands and cleaning everything around them even more frequently, and they (along with everyone else) will become hoarders.
Hope and healing
As psychiatrists, we are determined to transcend our own stress, rise above it all, and attend to the pervasive sadness, grief, anger, and uncertainty all around us, but especially among our patients, for whom the anguish of a psychiatric disorder is further compounded by 4 additional ordeals. This is our moment of truth as healers of our patients’ souls, because they look to us to provide them with hope to help navigate these trying times into full health. And we psychiatrists, along with fellow mental health professionals, are up to this unprecedented challenge.
These are unusually stressful days for everyone, especially our patients. We are all experiencing a turbulent mix of emotions as we try to cope with a confluence of threats to both our lives and to life as we know it. Peace of mind has become so elusive due to the relentless overlapping waves of fear, sadness, anger, and uncertainty. We are all grieving in a different way, but our psychiatric patients are suffering the most.
Fear. It only takes 1 traumatic event to trigger posttraumatic stress disorder (PTSD). Yet over the past few months, we have been afflicted by 4 jarring traumatic events, individually and as a society. Just a few months ago, it would have been impossible to imagine the conflux of 4 concurrent seismic threats to our well-being. A toxic political zeitgeist was the backdrop, which we bemoaned and tried to compartmentalize, despite the corrosive political environment shrouding the country. Then the deadly coronavirus disease 2019 (COVID-19) pandemic suddenly arrived, imposing draconian health-preserving measures that impacted every individual’s daily life in countless detrimental ways. Fear prevailed as we all sheltered at home, stopped commuting to work, canceled all trips, distanced ourselves from our friends and relatives, and watched depressing and anxiety-provoking television and read online news throughout our waking hours. Hoarding food and household supplies became endemic due to fear about survival.
Sadness. The agonizing prospect of a national financial necrosis followed the threat of serious illness or death. The economy came to a screeching halt, hemorrhaging millions of jobs. Unemployed parents stayed home with their morose children whose schools were shuttered, leaving them deprived of socializing with their friends. The government hurried with financial chemotherapy, printing trillions of dollars to prevent economic collapse, to avert potential poverty and hunger for many. The fear of the pandemic became coupled with sadness over the loss of livelihoods and grief for the loss of liberty and the ability to pursue happiness, or even small pleasures.
Anger. Then a tsunami of anger was generated by the brutal and sadistic death of a black man in police custody. This was a spark that ignited a massive amount of previously dormant racial tension dating back to the dark days of slavery. Peaceful protests were marred by destructive riots. The explosive fury was perhaps intensified by the protestors having been being locked up for weeks and having to wear masks, both of which were symbolic of being held down and “unable to breathe,” like the murdered Mr. George Floyd.
An epidemic of destroying statues followed. Heavy statues that appeared invincible for decades were dismantled from their plinth in a matter of minutes, signifying extreme frustration with the social injustice that remains despite the transformational laws of the Civil Rights Acts of 1960 and 1964. Suddenly—like falling dominoes—statues, flags, names of military bases, and previously venerated monuments were removed, changed, vandalized, or threatened with destruction. The founders of the republic were also maligned because they were slave owners 2 centuries ago. The paradigm shift spawned by the rage over racial inequality was disconcerting and dramatic. The anger and rampage spawned a sense that a tipping point in our society has been reached.
Uncertainty. The confluence of political instability, a deadly pandemic, economic collapse, and racial tensions were like the 4 horsemen of mass PTSD. The result was an agonizing uncertainty about the impact of these changes, and whether a sense of normalcy will ever return. It became apparent to all of us that our social structure has changed forever across multiple fundamental domains: public health, social, political, and financial. The wait for a vaccine for COVID-19 seems interminable, and racial healing and harmony seems elusive. Economic recovery may be possible, but political detoxification appears unlikely. The fate of police departments, condemned because of the deplorable and illegal acts of a few, and the safety of citizens, usually guaranteed by law and order, seem uncertain. Like COVID-19, angst has rapidly spread across the population.
The price our patients pay
The ingredients of a large-scale societal PTSD, similar to what probably happens during a world war, are now in place. Even resilient individuals may buckle during quadruple ordeals such as this one. So imagine what is happening to our patients, rendered fragile and vulnerable to threats by their pre-existing psychiatric illness. They all pay a heavy price. Patients with anxiety disorders will decompensate, with more panic attacks. Patients burdened by depression will worsen, with more hopelessness, despair, and suicidal ideation due to anxiety and loneliness. Patients with bipolar disorder will become more labile and irritable, and their comorbid anxiety will intensify. Patients with schizophrenia will become more paranoid, depressed, and anxious. Patients with autism will become more agitated and aggressive because their cherished daily routines are disrupted. Patients with obsessive-compulsive disorder will react to their germaphobia by washing their hands and cleaning everything around them even more frequently, and they (along with everyone else) will become hoarders.
Hope and healing
As psychiatrists, we are determined to transcend our own stress, rise above it all, and attend to the pervasive sadness, grief, anger, and uncertainty all around us, but especially among our patients, for whom the anguish of a psychiatric disorder is further compounded by 4 additional ordeals. This is our moment of truth as healers of our patients’ souls, because they look to us to provide them with hope to help navigate these trying times into full health. And we psychiatrists, along with fellow mental health professionals, are up to this unprecedented challenge.
These are unusually stressful days for everyone, especially our patients. We are all experiencing a turbulent mix of emotions as we try to cope with a confluence of threats to both our lives and to life as we know it. Peace of mind has become so elusive due to the relentless overlapping waves of fear, sadness, anger, and uncertainty. We are all grieving in a different way, but our psychiatric patients are suffering the most.
Fear. It only takes 1 traumatic event to trigger posttraumatic stress disorder (PTSD). Yet over the past few months, we have been afflicted by 4 jarring traumatic events, individually and as a society. Just a few months ago, it would have been impossible to imagine the conflux of 4 concurrent seismic threats to our well-being. A toxic political zeitgeist was the backdrop, which we bemoaned and tried to compartmentalize, despite the corrosive political environment shrouding the country. Then the deadly coronavirus disease 2019 (COVID-19) pandemic suddenly arrived, imposing draconian health-preserving measures that impacted every individual’s daily life in countless detrimental ways. Fear prevailed as we all sheltered at home, stopped commuting to work, canceled all trips, distanced ourselves from our friends and relatives, and watched depressing and anxiety-provoking television and read online news throughout our waking hours. Hoarding food and household supplies became endemic due to fear about survival.
Sadness. The agonizing prospect of a national financial necrosis followed the threat of serious illness or death. The economy came to a screeching halt, hemorrhaging millions of jobs. Unemployed parents stayed home with their morose children whose schools were shuttered, leaving them deprived of socializing with their friends. The government hurried with financial chemotherapy, printing trillions of dollars to prevent economic collapse, to avert potential poverty and hunger for many. The fear of the pandemic became coupled with sadness over the loss of livelihoods and grief for the loss of liberty and the ability to pursue happiness, or even small pleasures.
Anger. Then a tsunami of anger was generated by the brutal and sadistic death of a black man in police custody. This was a spark that ignited a massive amount of previously dormant racial tension dating back to the dark days of slavery. Peaceful protests were marred by destructive riots. The explosive fury was perhaps intensified by the protestors having been being locked up for weeks and having to wear masks, both of which were symbolic of being held down and “unable to breathe,” like the murdered Mr. George Floyd.
An epidemic of destroying statues followed. Heavy statues that appeared invincible for decades were dismantled from their plinth in a matter of minutes, signifying extreme frustration with the social injustice that remains despite the transformational laws of the Civil Rights Acts of 1960 and 1964. Suddenly—like falling dominoes—statues, flags, names of military bases, and previously venerated monuments were removed, changed, vandalized, or threatened with destruction. The founders of the republic were also maligned because they were slave owners 2 centuries ago. The paradigm shift spawned by the rage over racial inequality was disconcerting and dramatic. The anger and rampage spawned a sense that a tipping point in our society has been reached.
Uncertainty. The confluence of political instability, a deadly pandemic, economic collapse, and racial tensions were like the 4 horsemen of mass PTSD. The result was an agonizing uncertainty about the impact of these changes, and whether a sense of normalcy will ever return. It became apparent to all of us that our social structure has changed forever across multiple fundamental domains: public health, social, political, and financial. The wait for a vaccine for COVID-19 seems interminable, and racial healing and harmony seems elusive. Economic recovery may be possible, but political detoxification appears unlikely. The fate of police departments, condemned because of the deplorable and illegal acts of a few, and the safety of citizens, usually guaranteed by law and order, seem uncertain. Like COVID-19, angst has rapidly spread across the population.
The price our patients pay
The ingredients of a large-scale societal PTSD, similar to what probably happens during a world war, are now in place. Even resilient individuals may buckle during quadruple ordeals such as this one. So imagine what is happening to our patients, rendered fragile and vulnerable to threats by their pre-existing psychiatric illness. They all pay a heavy price. Patients with anxiety disorders will decompensate, with more panic attacks. Patients burdened by depression will worsen, with more hopelessness, despair, and suicidal ideation due to anxiety and loneliness. Patients with bipolar disorder will become more labile and irritable, and their comorbid anxiety will intensify. Patients with schizophrenia will become more paranoid, depressed, and anxious. Patients with autism will become more agitated and aggressive because their cherished daily routines are disrupted. Patients with obsessive-compulsive disorder will react to their germaphobia by washing their hands and cleaning everything around them even more frequently, and they (along with everyone else) will become hoarders.
Hope and healing
As psychiatrists, we are determined to transcend our own stress, rise above it all, and attend to the pervasive sadness, grief, anger, and uncertainty all around us, but especially among our patients, for whom the anguish of a psychiatric disorder is further compounded by 4 additional ordeals. This is our moment of truth as healers of our patients’ souls, because they look to us to provide them with hope to help navigate these trying times into full health. And we psychiatrists, along with fellow mental health professionals, are up to this unprecedented challenge.
Differing views of ‘behavioral health’
In the wake of Dr. Nasrallah’s recent editorial “Stop calling it ‘behavioral health:’ Psychiatry is much more” (From the Editor,
Naming a field, institute, department, or group of collaborators is crucially important, and must be undertaken with care. We all are familiar with Departments of Psychiatry, Departments of Psychiatry and Psychology, and Institutes for everything from Behavioral Health to Living. Even within the discipline of psychiatry, there have been adjustments over time in subspecialties (as seen with consultation-liaison psychiatry becoming psychosomatic medicine and then back again).
In our hospital system, we have recently adopted the term “Behavioral Health Institute” to denote the work and worth of significant numbers of caregivers (psychiatrists, psychologists, chemical dependency counselors, social workers, child life workers, advanced practice nurses, and others) who strive to improve the health and well-being of patients with both substance abuse and mental illness. We endeavor to remain mindful that a diversity of providers are involved in caring for and about our patients, and that “psychiatry” cannot—and should not—be the extent of how we conceptualize our services.
We submit that the modern view of behavioral health is ahead of other fields of medicine in recognizing that concepts, such as teamwork and diversity, are key to achieving positive patient outcomes. By identifying our providers as part of a Behavioral Health Institute, we acknowledge that not all mental distress is psychiatric illness but may still benefit from intervention and, importantly, that psychiatrists are not the center of the mental health (behavioral health) world. Treatments ranging from medication management to psychiatric procedures to psychotherapeutic modalities show the depth and breadth of our field, and the multiplicity of providers and modalities should be considered laudable. Recognizing the complexities inherent in behavioral health and its varied treatment options does not diminish but, in fact, elevates the field of psychiatry—and psychiatrists themselves.
Further, we note that behavioral health is not the only term that casts a larger net than the physician in a respective field. Does the term “primary care” insult internal medicine, family medicine, and pediatric physicians? Physicians and health care teams join in partnership with patients and families, either to cure or learn how to manage disease. We believe that constructing a health care system centered on physicians and their identities, rather than on patients and treatment outcomes, has been foolish. To that end, the tenor of Dr. Nasrallah’s editorial runs counter to the overall efforts of our field to improve collaboration, and, at its extreme, such articles promote the antiquated notion of physician elitism.
The editorial’s historical context is of course important, and the caution not to water down what “we” do (as psychiatrists) is appropriate. However, instead of comporting ourselves in a psychiatry-centric way, the use of the term behavioral health allows all of us to acknowledge (with appreciation and humility) the many contributors who work in our field. The use of a broad-minded, inclusive term neither minimizes nor trivializes psychiatry as a medical specialty. Rather, accepting this term and this mindset can place psychiatrists in the unique role of being innovators for the rest of medicine, because we embrace multidisciplinary teams and the value that interdisciplinary care can bring to patients and colleagues alike.
Jeanne Lackamp, MD, DFAPA, FACLP
Director, Pain Management Institute
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Patrick Runnels, MD, MBA
Chief Medical Officer, Population Health – Behavioral Health
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Lori Locke, RN, MSN
Director, Psychiatry Service Line and Nursing Practice
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Erum Ahmad, MD
Director, Child and Adolescent Psychiatry Unit
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Robert Ronis, MD, MPH
Douglas Danford Bond Professor and Chairman
Psychiatrist-in-Chief
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
I thank my Cleveland colleagues for their letter, and I welcome their disagreement with the tenets of my editorial. I still insist that the term “behavioral health” has a very narrow meaning that is not equivalent to psychiatry or psychology or social work or psychiatric nursing practice. This term should not be conflated with the widely used “mental health,” which is used as an overarching term for all professionals involved in the care of psychiatric brain disorders that manifest as various mental illnesses and substance use disorders.
While I am an advocate for multidisciplinary collaborations that benefit our patients, I will always uphold psychiatry as a medical specialty whose unique identity should not be sacrificed on the altar of politically correct egalitarianism of the mental health disciplines. Call it elitist if you like, but the fact is that the extensive medical school, residency, and fellowship training of psychiatrists stand out among all the other mental health disciplines. Psychiatrists are the best trained in all components of the biopsychosocial model (which I acquired many years ago from the father of the concept, George Engel, one of my teachers at the University of Rochester Residency Program).
You bring up primary care as an analogy for behavioral health. I assure you, none of the medical specialists included under that umbrella term refer to themselves as primary care physicians (PCPs) (or, God forbid, providers!). They identify themselves as family physicians, internists, pediatricians, and gynecologists. It is for the convenience of the health care systems and insurance companies that clinicians are called PCPs, which homogenizes them into a fuzzy amalgam and disguises their true medical identities as specialists.
So we agree to disagree. Diversity of opinions is a sacred principle. But I still think that a more accurate name for your Behavioral Health Institute would be “Institute of Psychiatric Medicine and Brain Health.” Behavioral health, which actually refers to educating people about implementing principles of evidence-based healthy habits and behaviors that prevent or reduce the risk of mental illness and/or substance use, is a small sliver of your overall mission. As you’ll notice from the other letters we’ve received, the vast majority of our readers agree that psychiatric medicine is far more than behavioral health.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
Continue to: I thoroughly enjoyed...
I thoroughly enjoyed Dr. Nasrallah’s editorial and agree completely. Veterans Affairs, my employer for the last 12 years, has fully bought into the use of “behavioral health” and its implications for its many psychiatrists. I have grown very tired of the constant minimization of psychiatric practice, and it is so good to hear from an affirming voice.
Barbara Day, MD
US Department of Veterans Affairs
Ann Arbor, Michigan
Dr. Nasrallah’s editorial made my heart sing! I have been practicing psychiatry since 1979, and have always bristled when called a “provider” or any of the other terms Dr. Nasrallah described. As a graduate of Johns Hopkins Medical School, I had professors who themselves had been taught by Harry Stack Sullivan and Frida Fromm Reichman, and during my residency at the University of Chicago, I sat in discussions with both Bruno Bettelheim and Heinz Kohut. I felt part of an honorable tradition, and even though biological psychiatry was on the ascendency, these analytical luminaries were part of my learning the “art” of psychiatry. It is not so easy to feel good about a specialty that has had such a history as ours, but my own experiences could never be reduced to being called a behavioral health provider. Dr. Nasrallah’s thoughts are very encouraging, and I thank him!
John Engers, MD
Private psychiatric practice (retired)
Fremont, California
Dr. Nasrallah’s editorial resonated with one of my pet peeves. I’ve been telling my medical students for years that we psychiatrists treat disorders of thinking, emotions, and behavior associated with mental illness, and that the term “behavioral health,” though possibly well intentioned, is a euphemism to reduce stigma.
Irl Extein, MD
Private psychiatric practice
Delray Beach, Florida
Clinical Affiliate Associate Professor, Florida
Atlantic University College of Medicine
Boca Raton, Florida
Continue to: I enjoyed...
I enjoyed Dr. Nasrallah’s editorial regarding “behavioral health.” In New England, we have very clear delineation among psychiatry, mental health, and behavioral health. Only physicians can practice psychiatry because it is a medical specialty. Nurse practitioners and psychologists, on the other hand, are specialists in the field of mental health, as are psychiatrists, so mental health is a more encompassing term. Behavioral health encompasses all of the above plus counselors. Because insurers generally pay counselors, nurse practitioners, and psychiatrists, they use the term behavioral health because it wouldn’t be right for them to pay a counselor for a psychiatric intervention. So as a psychiatrist, I respond when being referred to as a psychiatrist, mental health specialist, or behavioral health specialist. And thankfully, per American Medical Association policy, psychiatrists are not providers.
Stu Gitlow, MD, MPH
Executive Director
Annenberg Physician Training Program in Addictive Disease
Woonsocket, Rhode Island
I was grateful for Dr. Nasrallah’s editorial regarding the misnomer of referring to psychiatry as “behavioral health.” Until this editorial, I had wondered if I was the only one bothered by the term. Many people are under the assumption that behavioral health is a politically correct term that helps to lessen stigmatism. I completely disagree. Without question, it adds to the stigmatism. The term behavioral health is belittling to our patients. For example, calling a psychiatric inpatient unit a “behavioral health unit” implies that if patients would just change their behaviors, they wouldn’t have serious biological psychiatric illness. It insinuates that the patients cause and perpetuate their illnesses, such as schizophrenia or bipolar disorder, by behaving poorly. Granted, we teach behavior modification to help manage psychiatric illness, but so, too, do our colleagues in other medical fields teach behavior modification to manage other organ-related illnesses. Some nearly ubiquitous examples include doctors advising patients to lower stress, modify diet, exercise, and take medications as prescribed. Yet, for example, in the case of a patient with diabetes, we don’t refer to diabetic ketoacidosis treatment as behavioral health treatment, though the patient’s behavior no doubt contributes to this condition. And we certainly would never call the ICU or stepdown unit the “behavioral health unit,” even though adequate holistic treatment in these settings includes counseling the patient with diabetes on changing his/her behaviors that led to the ketoacidosis. Just as in diabetes, the underlying basis of psychiatric illness is biologic processes gone awry. First and foremost, a psychiatric medical illness requires complicated and often precarious medications to treat. As in other medical specialties, modifying behavior does not treat the illness, but merely serves to help transmute the course.
In sum, I wholly agree with Dr. Nasrallah’s eloquent assessment regarding the problems with the title behavioral health in lieu of psychiatry. I also might have taken the discussion a step a further: Because psychiatric illness affects every aspect of a person’s life—such as work, social, and personal—it requires a terminology commensurate with the medical gravity it warrants. So in addition to not referring to the specialty as behavioral health, I have wondered if the name psychiatry could be replaced with a more medical-sounding term such as “cerebrology” or something of the sort. But one step at time.
Stacie Lauro, MD, ABPN
Attending in Psychiatry, Emergency Room, and Consultation Liaison
Mindcare Solutions
Tampa, Florida
The evolution within our field of the use of “behavioral health” has disturbed me to the same extent it has for Dr. Nasrallah. I founded and direct a psychiatric treatment facility in Florida. We are a teaching facility affiliated with 3 psychiatric residencies, 8 medical schools, and 60 physician assistant (PA) schools. In all of the literature (eg, evaluations) from the PA schools, they refer to their rotation with my program as “behavioral health.” I have been attempting to correct them for years! I teach all residents and students to correctly use the terms “psychiatry” and “psychiatric.” I understand there may be stigma associated with the latter terms, but the field reinforces that stigma by avoiding the use of these terms.
Robert A. Moran, MD, FAPA, FASAM
CEO and Medical Director, Family Center for Recovery
Lantana, Florida
Continue to: Pre-authorization and 'hold harmless' clauses
Pre-authorization and ‘hold harmless’ clauses
Regarding Dr. Nasrallah’s editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
Recently to my surprise, while navigating a pre-authorization request for a young patient with bipolar disorder who had accepted the inclusion of lurasidone in his treatment regimen while hospitalized, I found that the CoverMyMeds Business Associate Agreement is required for a user to accomplish pre-authorization online. Having a little extra time for due diligence that day, I read this lengthy agreement carefully. The CoverMyMeds user agreement purports not to offer “medical advice, does not determine medical necessity, insurance coverage or copays and does not otherwise engage in the practice of medicine” (see www.covermymeds.com/main/about/privacy/tos/). Interestingly, the agreement goes on to purport that the whole process is for informational purposes only, not a substitute for clinicians, professional medical judgment, or for individual patient assessments and examinations. Of course, another factor is that the information provided by the process is “solely at the user’s and health care provider’s own risk.” Finally, the agreement requires the user to agree to “indemnify, defend, and hold harmless CoverMyMeds and its affiliates … from any demands, claims, damages, liabilities, expenses, or harms (including attorneys’ fees) arising out of or related to your use of our Services or breach of these Terms of Service.”
Throughout my 25 years of solo private practice, I have refused to sign hold harmless clauses and I refused to sign the CoverMyMeds user agreement. I have made it my practice never to obtain pre-authorization unless the patient is with me in the room during an appointment because the process of navigating pre-authorization does become part of the treatment, however unfortunately. As an alternative, for my patient with bipolar disorder, I was able to use a phone number to talk to a representative of the pharmacy benefit plan that was contracted with CoverMyMeds. Without signing on to be a Business Associate, we accomplished the goal of continuing with the medication as recommended and implemented for 2 preceding months (often pre-authorization actually means continuing authorization, doesn’t it?). I believe if all psychiatrists were to adopt this kind of stance, we could make a change. I know there are anti-trust considerations involved in fee negotiations, but when it comes to the egregious practices of managed care, pre-authorization, and hold harmless clauses, it seems to me that we can mount a counteroffensive to great effect.
Further, I want to stand in strong support of Dr. Nasrallah’s editorial “Stop calling it ‘behavioral health’: Psychiatry is much more.” When I began my first job post-fellowship, I was alarmed to find that our outpatient offices had been named a “counseling center.” Due to such misleading, stigmatizing characterizations, as Dr. Nasrallah pointed out, we have only slid further down the slope into the realm of “providers of behavioral health services.” As an old hand working psychiatric locum tenens told me, we psychiatrists had long since missed the chance to “band together like musk oxen” to defend our profession.
However, I believe it is not too late. With the strength of Dr. Nasrallah’s leadership and a more overt, collective stubbornness coupled with an undying commitment to excellence, we can and must push hard against the insurance and hospital entities, which continue to profiteer from the practice of medicine without a license—using the tools of hold harmless clauses, anti-trust laws in their favor, and misinformation about the scope and efficacy of practicing psychiatry per se. The challenge is to figure out exactly how to proceed.
Although some manage to thrive in independent practice, collectively our struggle seems considerable, but not insurmountable.
David B. Robinson, MD, MPH
Diplomate, American Board of Psychiatry and Neurology in Child, Adolescent and Adult Psychiatry
Fellow, American Psychiatric Association
Private psychiatric practice
Alaska Psychiatric Concepts
Juneau, Alaska
In the wake of Dr. Nasrallah’s recent editorial “Stop calling it ‘behavioral health:’ Psychiatry is much more” (From the Editor,
Naming a field, institute, department, or group of collaborators is crucially important, and must be undertaken with care. We all are familiar with Departments of Psychiatry, Departments of Psychiatry and Psychology, and Institutes for everything from Behavioral Health to Living. Even within the discipline of psychiatry, there have been adjustments over time in subspecialties (as seen with consultation-liaison psychiatry becoming psychosomatic medicine and then back again).
In our hospital system, we have recently adopted the term “Behavioral Health Institute” to denote the work and worth of significant numbers of caregivers (psychiatrists, psychologists, chemical dependency counselors, social workers, child life workers, advanced practice nurses, and others) who strive to improve the health and well-being of patients with both substance abuse and mental illness. We endeavor to remain mindful that a diversity of providers are involved in caring for and about our patients, and that “psychiatry” cannot—and should not—be the extent of how we conceptualize our services.
We submit that the modern view of behavioral health is ahead of other fields of medicine in recognizing that concepts, such as teamwork and diversity, are key to achieving positive patient outcomes. By identifying our providers as part of a Behavioral Health Institute, we acknowledge that not all mental distress is psychiatric illness but may still benefit from intervention and, importantly, that psychiatrists are not the center of the mental health (behavioral health) world. Treatments ranging from medication management to psychiatric procedures to psychotherapeutic modalities show the depth and breadth of our field, and the multiplicity of providers and modalities should be considered laudable. Recognizing the complexities inherent in behavioral health and its varied treatment options does not diminish but, in fact, elevates the field of psychiatry—and psychiatrists themselves.
Further, we note that behavioral health is not the only term that casts a larger net than the physician in a respective field. Does the term “primary care” insult internal medicine, family medicine, and pediatric physicians? Physicians and health care teams join in partnership with patients and families, either to cure or learn how to manage disease. We believe that constructing a health care system centered on physicians and their identities, rather than on patients and treatment outcomes, has been foolish. To that end, the tenor of Dr. Nasrallah’s editorial runs counter to the overall efforts of our field to improve collaboration, and, at its extreme, such articles promote the antiquated notion of physician elitism.
The editorial’s historical context is of course important, and the caution not to water down what “we” do (as psychiatrists) is appropriate. However, instead of comporting ourselves in a psychiatry-centric way, the use of the term behavioral health allows all of us to acknowledge (with appreciation and humility) the many contributors who work in our field. The use of a broad-minded, inclusive term neither minimizes nor trivializes psychiatry as a medical specialty. Rather, accepting this term and this mindset can place psychiatrists in the unique role of being innovators for the rest of medicine, because we embrace multidisciplinary teams and the value that interdisciplinary care can bring to patients and colleagues alike.
Jeanne Lackamp, MD, DFAPA, FACLP
Director, Pain Management Institute
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Patrick Runnels, MD, MBA
Chief Medical Officer, Population Health – Behavioral Health
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Lori Locke, RN, MSN
Director, Psychiatry Service Line and Nursing Practice
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Erum Ahmad, MD
Director, Child and Adolescent Psychiatry Unit
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Robert Ronis, MD, MPH
Douglas Danford Bond Professor and Chairman
Psychiatrist-in-Chief
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
I thank my Cleveland colleagues for their letter, and I welcome their disagreement with the tenets of my editorial. I still insist that the term “behavioral health” has a very narrow meaning that is not equivalent to psychiatry or psychology or social work or psychiatric nursing practice. This term should not be conflated with the widely used “mental health,” which is used as an overarching term for all professionals involved in the care of psychiatric brain disorders that manifest as various mental illnesses and substance use disorders.
While I am an advocate for multidisciplinary collaborations that benefit our patients, I will always uphold psychiatry as a medical specialty whose unique identity should not be sacrificed on the altar of politically correct egalitarianism of the mental health disciplines. Call it elitist if you like, but the fact is that the extensive medical school, residency, and fellowship training of psychiatrists stand out among all the other mental health disciplines. Psychiatrists are the best trained in all components of the biopsychosocial model (which I acquired many years ago from the father of the concept, George Engel, one of my teachers at the University of Rochester Residency Program).
You bring up primary care as an analogy for behavioral health. I assure you, none of the medical specialists included under that umbrella term refer to themselves as primary care physicians (PCPs) (or, God forbid, providers!). They identify themselves as family physicians, internists, pediatricians, and gynecologists. It is for the convenience of the health care systems and insurance companies that clinicians are called PCPs, which homogenizes them into a fuzzy amalgam and disguises their true medical identities as specialists.
So we agree to disagree. Diversity of opinions is a sacred principle. But I still think that a more accurate name for your Behavioral Health Institute would be “Institute of Psychiatric Medicine and Brain Health.” Behavioral health, which actually refers to educating people about implementing principles of evidence-based healthy habits and behaviors that prevent or reduce the risk of mental illness and/or substance use, is a small sliver of your overall mission. As you’ll notice from the other letters we’ve received, the vast majority of our readers agree that psychiatric medicine is far more than behavioral health.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
Continue to: I thoroughly enjoyed...
I thoroughly enjoyed Dr. Nasrallah’s editorial and agree completely. Veterans Affairs, my employer for the last 12 years, has fully bought into the use of “behavioral health” and its implications for its many psychiatrists. I have grown very tired of the constant minimization of psychiatric practice, and it is so good to hear from an affirming voice.
Barbara Day, MD
US Department of Veterans Affairs
Ann Arbor, Michigan
Dr. Nasrallah’s editorial made my heart sing! I have been practicing psychiatry since 1979, and have always bristled when called a “provider” or any of the other terms Dr. Nasrallah described. As a graduate of Johns Hopkins Medical School, I had professors who themselves had been taught by Harry Stack Sullivan and Frida Fromm Reichman, and during my residency at the University of Chicago, I sat in discussions with both Bruno Bettelheim and Heinz Kohut. I felt part of an honorable tradition, and even though biological psychiatry was on the ascendency, these analytical luminaries were part of my learning the “art” of psychiatry. It is not so easy to feel good about a specialty that has had such a history as ours, but my own experiences could never be reduced to being called a behavioral health provider. Dr. Nasrallah’s thoughts are very encouraging, and I thank him!
John Engers, MD
Private psychiatric practice (retired)
Fremont, California
Dr. Nasrallah’s editorial resonated with one of my pet peeves. I’ve been telling my medical students for years that we psychiatrists treat disorders of thinking, emotions, and behavior associated with mental illness, and that the term “behavioral health,” though possibly well intentioned, is a euphemism to reduce stigma.
Irl Extein, MD
Private psychiatric practice
Delray Beach, Florida
Clinical Affiliate Associate Professor, Florida
Atlantic University College of Medicine
Boca Raton, Florida
Continue to: I enjoyed...
I enjoyed Dr. Nasrallah’s editorial regarding “behavioral health.” In New England, we have very clear delineation among psychiatry, mental health, and behavioral health. Only physicians can practice psychiatry because it is a medical specialty. Nurse practitioners and psychologists, on the other hand, are specialists in the field of mental health, as are psychiatrists, so mental health is a more encompassing term. Behavioral health encompasses all of the above plus counselors. Because insurers generally pay counselors, nurse practitioners, and psychiatrists, they use the term behavioral health because it wouldn’t be right for them to pay a counselor for a psychiatric intervention. So as a psychiatrist, I respond when being referred to as a psychiatrist, mental health specialist, or behavioral health specialist. And thankfully, per American Medical Association policy, psychiatrists are not providers.
Stu Gitlow, MD, MPH
Executive Director
Annenberg Physician Training Program in Addictive Disease
Woonsocket, Rhode Island
I was grateful for Dr. Nasrallah’s editorial regarding the misnomer of referring to psychiatry as “behavioral health.” Until this editorial, I had wondered if I was the only one bothered by the term. Many people are under the assumption that behavioral health is a politically correct term that helps to lessen stigmatism. I completely disagree. Without question, it adds to the stigmatism. The term behavioral health is belittling to our patients. For example, calling a psychiatric inpatient unit a “behavioral health unit” implies that if patients would just change their behaviors, they wouldn’t have serious biological psychiatric illness. It insinuates that the patients cause and perpetuate their illnesses, such as schizophrenia or bipolar disorder, by behaving poorly. Granted, we teach behavior modification to help manage psychiatric illness, but so, too, do our colleagues in other medical fields teach behavior modification to manage other organ-related illnesses. Some nearly ubiquitous examples include doctors advising patients to lower stress, modify diet, exercise, and take medications as prescribed. Yet, for example, in the case of a patient with diabetes, we don’t refer to diabetic ketoacidosis treatment as behavioral health treatment, though the patient’s behavior no doubt contributes to this condition. And we certainly would never call the ICU or stepdown unit the “behavioral health unit,” even though adequate holistic treatment in these settings includes counseling the patient with diabetes on changing his/her behaviors that led to the ketoacidosis. Just as in diabetes, the underlying basis of psychiatric illness is biologic processes gone awry. First and foremost, a psychiatric medical illness requires complicated and often precarious medications to treat. As in other medical specialties, modifying behavior does not treat the illness, but merely serves to help transmute the course.
In sum, I wholly agree with Dr. Nasrallah’s eloquent assessment regarding the problems with the title behavioral health in lieu of psychiatry. I also might have taken the discussion a step a further: Because psychiatric illness affects every aspect of a person’s life—such as work, social, and personal—it requires a terminology commensurate with the medical gravity it warrants. So in addition to not referring to the specialty as behavioral health, I have wondered if the name psychiatry could be replaced with a more medical-sounding term such as “cerebrology” or something of the sort. But one step at time.
Stacie Lauro, MD, ABPN
Attending in Psychiatry, Emergency Room, and Consultation Liaison
Mindcare Solutions
Tampa, Florida
The evolution within our field of the use of “behavioral health” has disturbed me to the same extent it has for Dr. Nasrallah. I founded and direct a psychiatric treatment facility in Florida. We are a teaching facility affiliated with 3 psychiatric residencies, 8 medical schools, and 60 physician assistant (PA) schools. In all of the literature (eg, evaluations) from the PA schools, they refer to their rotation with my program as “behavioral health.” I have been attempting to correct them for years! I teach all residents and students to correctly use the terms “psychiatry” and “psychiatric.” I understand there may be stigma associated with the latter terms, but the field reinforces that stigma by avoiding the use of these terms.
Robert A. Moran, MD, FAPA, FASAM
CEO and Medical Director, Family Center for Recovery
Lantana, Florida
Continue to: Pre-authorization and 'hold harmless' clauses
Pre-authorization and ‘hold harmless’ clauses
Regarding Dr. Nasrallah’s editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
Recently to my surprise, while navigating a pre-authorization request for a young patient with bipolar disorder who had accepted the inclusion of lurasidone in his treatment regimen while hospitalized, I found that the CoverMyMeds Business Associate Agreement is required for a user to accomplish pre-authorization online. Having a little extra time for due diligence that day, I read this lengthy agreement carefully. The CoverMyMeds user agreement purports not to offer “medical advice, does not determine medical necessity, insurance coverage or copays and does not otherwise engage in the practice of medicine” (see www.covermymeds.com/main/about/privacy/tos/). Interestingly, the agreement goes on to purport that the whole process is for informational purposes only, not a substitute for clinicians, professional medical judgment, or for individual patient assessments and examinations. Of course, another factor is that the information provided by the process is “solely at the user’s and health care provider’s own risk.” Finally, the agreement requires the user to agree to “indemnify, defend, and hold harmless CoverMyMeds and its affiliates … from any demands, claims, damages, liabilities, expenses, or harms (including attorneys’ fees) arising out of or related to your use of our Services or breach of these Terms of Service.”
Throughout my 25 years of solo private practice, I have refused to sign hold harmless clauses and I refused to sign the CoverMyMeds user agreement. I have made it my practice never to obtain pre-authorization unless the patient is with me in the room during an appointment because the process of navigating pre-authorization does become part of the treatment, however unfortunately. As an alternative, for my patient with bipolar disorder, I was able to use a phone number to talk to a representative of the pharmacy benefit plan that was contracted with CoverMyMeds. Without signing on to be a Business Associate, we accomplished the goal of continuing with the medication as recommended and implemented for 2 preceding months (often pre-authorization actually means continuing authorization, doesn’t it?). I believe if all psychiatrists were to adopt this kind of stance, we could make a change. I know there are anti-trust considerations involved in fee negotiations, but when it comes to the egregious practices of managed care, pre-authorization, and hold harmless clauses, it seems to me that we can mount a counteroffensive to great effect.
Further, I want to stand in strong support of Dr. Nasrallah’s editorial “Stop calling it ‘behavioral health’: Psychiatry is much more.” When I began my first job post-fellowship, I was alarmed to find that our outpatient offices had been named a “counseling center.” Due to such misleading, stigmatizing characterizations, as Dr. Nasrallah pointed out, we have only slid further down the slope into the realm of “providers of behavioral health services.” As an old hand working psychiatric locum tenens told me, we psychiatrists had long since missed the chance to “band together like musk oxen” to defend our profession.
However, I believe it is not too late. With the strength of Dr. Nasrallah’s leadership and a more overt, collective stubbornness coupled with an undying commitment to excellence, we can and must push hard against the insurance and hospital entities, which continue to profiteer from the practice of medicine without a license—using the tools of hold harmless clauses, anti-trust laws in their favor, and misinformation about the scope and efficacy of practicing psychiatry per se. The challenge is to figure out exactly how to proceed.
Although some manage to thrive in independent practice, collectively our struggle seems considerable, but not insurmountable.
David B. Robinson, MD, MPH
Diplomate, American Board of Psychiatry and Neurology in Child, Adolescent and Adult Psychiatry
Fellow, American Psychiatric Association
Private psychiatric practice
Alaska Psychiatric Concepts
Juneau, Alaska
In the wake of Dr. Nasrallah’s recent editorial “Stop calling it ‘behavioral health:’ Psychiatry is much more” (From the Editor,
Naming a field, institute, department, or group of collaborators is crucially important, and must be undertaken with care. We all are familiar with Departments of Psychiatry, Departments of Psychiatry and Psychology, and Institutes for everything from Behavioral Health to Living. Even within the discipline of psychiatry, there have been adjustments over time in subspecialties (as seen with consultation-liaison psychiatry becoming psychosomatic medicine and then back again).
In our hospital system, we have recently adopted the term “Behavioral Health Institute” to denote the work and worth of significant numbers of caregivers (psychiatrists, psychologists, chemical dependency counselors, social workers, child life workers, advanced practice nurses, and others) who strive to improve the health and well-being of patients with both substance abuse and mental illness. We endeavor to remain mindful that a diversity of providers are involved in caring for and about our patients, and that “psychiatry” cannot—and should not—be the extent of how we conceptualize our services.
We submit that the modern view of behavioral health is ahead of other fields of medicine in recognizing that concepts, such as teamwork and diversity, are key to achieving positive patient outcomes. By identifying our providers as part of a Behavioral Health Institute, we acknowledge that not all mental distress is psychiatric illness but may still benefit from intervention and, importantly, that psychiatrists are not the center of the mental health (behavioral health) world. Treatments ranging from medication management to psychiatric procedures to psychotherapeutic modalities show the depth and breadth of our field, and the multiplicity of providers and modalities should be considered laudable. Recognizing the complexities inherent in behavioral health and its varied treatment options does not diminish but, in fact, elevates the field of psychiatry—and psychiatrists themselves.
Further, we note that behavioral health is not the only term that casts a larger net than the physician in a respective field. Does the term “primary care” insult internal medicine, family medicine, and pediatric physicians? Physicians and health care teams join in partnership with patients and families, either to cure or learn how to manage disease. We believe that constructing a health care system centered on physicians and their identities, rather than on patients and treatment outcomes, has been foolish. To that end, the tenor of Dr. Nasrallah’s editorial runs counter to the overall efforts of our field to improve collaboration, and, at its extreme, such articles promote the antiquated notion of physician elitism.
The editorial’s historical context is of course important, and the caution not to water down what “we” do (as psychiatrists) is appropriate. However, instead of comporting ourselves in a psychiatry-centric way, the use of the term behavioral health allows all of us to acknowledge (with appreciation and humility) the many contributors who work in our field. The use of a broad-minded, inclusive term neither minimizes nor trivializes psychiatry as a medical specialty. Rather, accepting this term and this mindset can place psychiatrists in the unique role of being innovators for the rest of medicine, because we embrace multidisciplinary teams and the value that interdisciplinary care can bring to patients and colleagues alike.
Jeanne Lackamp, MD, DFAPA, FACLP
Director, Pain Management Institute
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Patrick Runnels, MD, MBA
Chief Medical Officer, Population Health – Behavioral Health
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Lori Locke, RN, MSN
Director, Psychiatry Service Line and Nursing Practice
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Erum Ahmad, MD
Director, Child and Adolescent Psychiatry Unit
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Robert Ronis, MD, MPH
Douglas Danford Bond Professor and Chairman
Psychiatrist-in-Chief
University Hospitals Health System
Case Western Reserve University School of Medicine
Cleveland, Ohio
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
I thank my Cleveland colleagues for their letter, and I welcome their disagreement with the tenets of my editorial. I still insist that the term “behavioral health” has a very narrow meaning that is not equivalent to psychiatry or psychology or social work or psychiatric nursing practice. This term should not be conflated with the widely used “mental health,” which is used as an overarching term for all professionals involved in the care of psychiatric brain disorders that manifest as various mental illnesses and substance use disorders.
While I am an advocate for multidisciplinary collaborations that benefit our patients, I will always uphold psychiatry as a medical specialty whose unique identity should not be sacrificed on the altar of politically correct egalitarianism of the mental health disciplines. Call it elitist if you like, but the fact is that the extensive medical school, residency, and fellowship training of psychiatrists stand out among all the other mental health disciplines. Psychiatrists are the best trained in all components of the biopsychosocial model (which I acquired many years ago from the father of the concept, George Engel, one of my teachers at the University of Rochester Residency Program).
You bring up primary care as an analogy for behavioral health. I assure you, none of the medical specialists included under that umbrella term refer to themselves as primary care physicians (PCPs) (or, God forbid, providers!). They identify themselves as family physicians, internists, pediatricians, and gynecologists. It is for the convenience of the health care systems and insurance companies that clinicians are called PCPs, which homogenizes them into a fuzzy amalgam and disguises their true medical identities as specialists.
So we agree to disagree. Diversity of opinions is a sacred principle. But I still think that a more accurate name for your Behavioral Health Institute would be “Institute of Psychiatric Medicine and Brain Health.” Behavioral health, which actually refers to educating people about implementing principles of evidence-based healthy habits and behaviors that prevent or reduce the risk of mental illness and/or substance use, is a small sliver of your overall mission. As you’ll notice from the other letters we’ve received, the vast majority of our readers agree that psychiatric medicine is far more than behavioral health.
Henry A. Nasrallah, MD
Professor of Psychiatry, Neurology, and Neuroscience
Medical Director: Neuropsychiatry
Director, Schizophrenia and Neuropsychiatry Programs
University of Cincinnati College of Medicine
Cincinnati, Ohio
Professor Emeritus, Saint Louis University
St. Louis, Missouri
Continue to: I thoroughly enjoyed...
I thoroughly enjoyed Dr. Nasrallah’s editorial and agree completely. Veterans Affairs, my employer for the last 12 years, has fully bought into the use of “behavioral health” and its implications for its many psychiatrists. I have grown very tired of the constant minimization of psychiatric practice, and it is so good to hear from an affirming voice.
Barbara Day, MD
US Department of Veterans Affairs
Ann Arbor, Michigan
Dr. Nasrallah’s editorial made my heart sing! I have been practicing psychiatry since 1979, and have always bristled when called a “provider” or any of the other terms Dr. Nasrallah described. As a graduate of Johns Hopkins Medical School, I had professors who themselves had been taught by Harry Stack Sullivan and Frida Fromm Reichman, and during my residency at the University of Chicago, I sat in discussions with both Bruno Bettelheim and Heinz Kohut. I felt part of an honorable tradition, and even though biological psychiatry was on the ascendency, these analytical luminaries were part of my learning the “art” of psychiatry. It is not so easy to feel good about a specialty that has had such a history as ours, but my own experiences could never be reduced to being called a behavioral health provider. Dr. Nasrallah’s thoughts are very encouraging, and I thank him!
John Engers, MD
Private psychiatric practice (retired)
Fremont, California
Dr. Nasrallah’s editorial resonated with one of my pet peeves. I’ve been telling my medical students for years that we psychiatrists treat disorders of thinking, emotions, and behavior associated with mental illness, and that the term “behavioral health,” though possibly well intentioned, is a euphemism to reduce stigma.
Irl Extein, MD
Private psychiatric practice
Delray Beach, Florida
Clinical Affiliate Associate Professor, Florida
Atlantic University College of Medicine
Boca Raton, Florida
Continue to: I enjoyed...
I enjoyed Dr. Nasrallah’s editorial regarding “behavioral health.” In New England, we have very clear delineation among psychiatry, mental health, and behavioral health. Only physicians can practice psychiatry because it is a medical specialty. Nurse practitioners and psychologists, on the other hand, are specialists in the field of mental health, as are psychiatrists, so mental health is a more encompassing term. Behavioral health encompasses all of the above plus counselors. Because insurers generally pay counselors, nurse practitioners, and psychiatrists, they use the term behavioral health because it wouldn’t be right for them to pay a counselor for a psychiatric intervention. So as a psychiatrist, I respond when being referred to as a psychiatrist, mental health specialist, or behavioral health specialist. And thankfully, per American Medical Association policy, psychiatrists are not providers.
Stu Gitlow, MD, MPH
Executive Director
Annenberg Physician Training Program in Addictive Disease
Woonsocket, Rhode Island
I was grateful for Dr. Nasrallah’s editorial regarding the misnomer of referring to psychiatry as “behavioral health.” Until this editorial, I had wondered if I was the only one bothered by the term. Many people are under the assumption that behavioral health is a politically correct term that helps to lessen stigmatism. I completely disagree. Without question, it adds to the stigmatism. The term behavioral health is belittling to our patients. For example, calling a psychiatric inpatient unit a “behavioral health unit” implies that if patients would just change their behaviors, they wouldn’t have serious biological psychiatric illness. It insinuates that the patients cause and perpetuate their illnesses, such as schizophrenia or bipolar disorder, by behaving poorly. Granted, we teach behavior modification to help manage psychiatric illness, but so, too, do our colleagues in other medical fields teach behavior modification to manage other organ-related illnesses. Some nearly ubiquitous examples include doctors advising patients to lower stress, modify diet, exercise, and take medications as prescribed. Yet, for example, in the case of a patient with diabetes, we don’t refer to diabetic ketoacidosis treatment as behavioral health treatment, though the patient’s behavior no doubt contributes to this condition. And we certainly would never call the ICU or stepdown unit the “behavioral health unit,” even though adequate holistic treatment in these settings includes counseling the patient with diabetes on changing his/her behaviors that led to the ketoacidosis. Just as in diabetes, the underlying basis of psychiatric illness is biologic processes gone awry. First and foremost, a psychiatric medical illness requires complicated and often precarious medications to treat. As in other medical specialties, modifying behavior does not treat the illness, but merely serves to help transmute the course.
In sum, I wholly agree with Dr. Nasrallah’s eloquent assessment regarding the problems with the title behavioral health in lieu of psychiatry. I also might have taken the discussion a step a further: Because psychiatric illness affects every aspect of a person’s life—such as work, social, and personal—it requires a terminology commensurate with the medical gravity it warrants. So in addition to not referring to the specialty as behavioral health, I have wondered if the name psychiatry could be replaced with a more medical-sounding term such as “cerebrology” or something of the sort. But one step at time.
Stacie Lauro, MD, ABPN
Attending in Psychiatry, Emergency Room, and Consultation Liaison
Mindcare Solutions
Tampa, Florida
The evolution within our field of the use of “behavioral health” has disturbed me to the same extent it has for Dr. Nasrallah. I founded and direct a psychiatric treatment facility in Florida. We are a teaching facility affiliated with 3 psychiatric residencies, 8 medical schools, and 60 physician assistant (PA) schools. In all of the literature (eg, evaluations) from the PA schools, they refer to their rotation with my program as “behavioral health.” I have been attempting to correct them for years! I teach all residents and students to correctly use the terms “psychiatry” and “psychiatric.” I understand there may be stigma associated with the latter terms, but the field reinforces that stigma by avoiding the use of these terms.
Robert A. Moran, MD, FAPA, FASAM
CEO and Medical Director, Family Center for Recovery
Lantana, Florida
Continue to: Pre-authorization and 'hold harmless' clauses
Pre-authorization and ‘hold harmless’ clauses
Regarding Dr. Nasrallah’s editorial “Pre-authorization is illegal, unethical, and adversely disrupts patient care” (From the Editor,
Recently to my surprise, while navigating a pre-authorization request for a young patient with bipolar disorder who had accepted the inclusion of lurasidone in his treatment regimen while hospitalized, I found that the CoverMyMeds Business Associate Agreement is required for a user to accomplish pre-authorization online. Having a little extra time for due diligence that day, I read this lengthy agreement carefully. The CoverMyMeds user agreement purports not to offer “medical advice, does not determine medical necessity, insurance coverage or copays and does not otherwise engage in the practice of medicine” (see www.covermymeds.com/main/about/privacy/tos/). Interestingly, the agreement goes on to purport that the whole process is for informational purposes only, not a substitute for clinicians, professional medical judgment, or for individual patient assessments and examinations. Of course, another factor is that the information provided by the process is “solely at the user’s and health care provider’s own risk.” Finally, the agreement requires the user to agree to “indemnify, defend, and hold harmless CoverMyMeds and its affiliates … from any demands, claims, damages, liabilities, expenses, or harms (including attorneys’ fees) arising out of or related to your use of our Services or breach of these Terms of Service.”
Throughout my 25 years of solo private practice, I have refused to sign hold harmless clauses and I refused to sign the CoverMyMeds user agreement. I have made it my practice never to obtain pre-authorization unless the patient is with me in the room during an appointment because the process of navigating pre-authorization does become part of the treatment, however unfortunately. As an alternative, for my patient with bipolar disorder, I was able to use a phone number to talk to a representative of the pharmacy benefit plan that was contracted with CoverMyMeds. Without signing on to be a Business Associate, we accomplished the goal of continuing with the medication as recommended and implemented for 2 preceding months (often pre-authorization actually means continuing authorization, doesn’t it?). I believe if all psychiatrists were to adopt this kind of stance, we could make a change. I know there are anti-trust considerations involved in fee negotiations, but when it comes to the egregious practices of managed care, pre-authorization, and hold harmless clauses, it seems to me that we can mount a counteroffensive to great effect.
Further, I want to stand in strong support of Dr. Nasrallah’s editorial “Stop calling it ‘behavioral health’: Psychiatry is much more.” When I began my first job post-fellowship, I was alarmed to find that our outpatient offices had been named a “counseling center.” Due to such misleading, stigmatizing characterizations, as Dr. Nasrallah pointed out, we have only slid further down the slope into the realm of “providers of behavioral health services.” As an old hand working psychiatric locum tenens told me, we psychiatrists had long since missed the chance to “band together like musk oxen” to defend our profession.
However, I believe it is not too late. With the strength of Dr. Nasrallah’s leadership and a more overt, collective stubbornness coupled with an undying commitment to excellence, we can and must push hard against the insurance and hospital entities, which continue to profiteer from the practice of medicine without a license—using the tools of hold harmless clauses, anti-trust laws in their favor, and misinformation about the scope and efficacy of practicing psychiatry per se. The challenge is to figure out exactly how to proceed.
Although some manage to thrive in independent practice, collectively our struggle seems considerable, but not insurmountable.
David B. Robinson, MD, MPH
Diplomate, American Board of Psychiatry and Neurology in Child, Adolescent and Adult Psychiatry
Fellow, American Psychiatric Association
Private psychiatric practice
Alaska Psychiatric Concepts
Juneau, Alaska
CYP450 interactions between illicit substances and prescription medications
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.

Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.
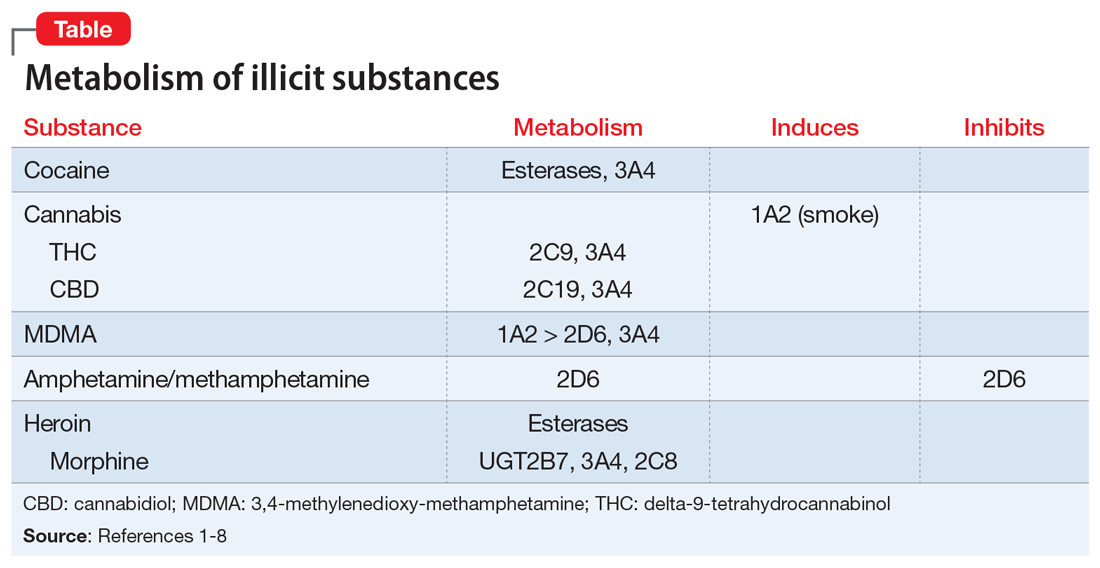
Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.

Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.

Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
Ms. L, age 37, presents to psychiatric emergency services with command auditory hallucinations, ideas of reference, and suicidal ideation.
Ms. L has a 22-year history of schizophrenia. Additionally, she has a history of cocaine use disorder (in remission for 12 years), cannabis use disorder (in remission for 6 months), type 2 diabetes mellitus, and hypertension. Her psychotic symptoms are well controlled on a regimen of
On interview, Ms. L reports smoking cannabis each day for the past month and using $400 worth of cocaine over 2 days. She is experiencing intense guilt over these relapses and is admitted to the inpatient adult psychiatry unit. On admission, Ms. L’s clozapine and norclozapine trough levels (drawn approximately 12 hours after last administration documented by the ACT member) are 300 and 275 ng/mL, respectively. Generally, clozapine levels >350 to 420 ng/mL are considered therapeutic, and a clozapine-to-norclozapine ratio of 2:1 is desirable for maximum efficacy and tolerability. Because Ms. L’s clozapine level is <350 and her ratio is approximately 1:1, her clozapine treatment is subtherapeutic.
Because Ms. L has a history of documented adherence to and benefit from her current medication regimen, no changes are made during her 3-week hospital stay. She notices a gradual reduction in auditory hallucinations, no longer wants to harm herself, and is motivated to regain sobriety.
At the time of discharge, Ms. L’s clozapine and norclozapine trough levels are 550 and 250 ng/mL, respectively, which indicates a more favorable clozapine-to-norclozapine ratio of approximately 2:1 and a clozapine level greater than the recommended minimum threshold of 350 ng/mL. While cocaine ingestion presumably played a role in her acute decompensation, the treatment team determined that Ms. L’s relapse to cannabis use likely contributed to low clozapine levels by induction of cytochrome P450 (CYP) 1A2, and subsequently led to the delayed recovery of symptom control.1
The use of illicit substances is a widespread, growing problem. According to the 2017 National Survey on Drug Use and Health, 11.5% of Americans age ≥12 had used an illicit substance (ie, use of marijuana, cocaine, heroin, hallucinogens, inhalants, or methamphetamine, or misuse of prescription psychotherapeutics) in the past month.2 While illicit substance use is of particular public health interest due to a known increase in mortality and health care spending, there has been little discussion of the impact of illicit drug use on concurrent pharmacologic therapy. Just as prescription medications have pharmacokinetic drug–drug interactions with each other, so do illicit substances, though far less is known about their impact on the treatment of medical conditions.

Pharmacokinetic interactions
Key pharmacokinetic interactions have been reported with cocaine, marijuana, amphetamines, and opioids. The Table1-8 summarizes the metabolism of illicit substances.

Continue to: Cocaine
Cocaine is largely metabolized by serum esterases such as pseudocholinesterase, human carboxylesterase-1 (hCE-1), and human carboxylesterase-2 (hCE-2), to inactive metabolites benzoylecgonine (35% to 45%) and ecgonine (32% to 49%).2 However, a smaller portion (2.6% to 6.2%) undergoes hepatic N-demethylation by CYP3A4 to norcocaine.3 Norcocaine is an active metabolite responsible for some of the toxic effects of cocaine (eg, hepatotoxicity).4,5 Several commonly prescribed medications are known inducers of CYP3A4 (eg, phenytoin, carbamazepine) and may lead to increased levels of the toxic metabolite when used concurrently with cocaine. Additionally, the use of cocaine with acetylcholinesterase inhibitors, such as donepezil, may lead to reduction of serum esterases and shunt cocaine metabolism toward the hepatic pathway, thus increasing norcocaine formation.3
Cannabis. The metabolism and drug–drug interactions of cannabis can be separated by its 2 main components: delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A review conducted in 2014 concluded that THC is primarily metabolized by CYP2C9 and 3A4, while CBD is metabolized by CYP2C19 and 3A4.6 Oral administration of ketoconazole, a CYP3A4 inhibitor, along with cannabis extract has been shown to increase the maximum concentration (Cmax) and area under the curve (AUC) of THC by 1.2- and 1.8-fold, respectively, while increasing both Cmaxand AUC of CBD by 2-fold.6 In addition, CYP2C9 poor metabolizers have been shown to experience significant increases in THC exposure and reductions in metabolite formation, further supporting the role of CYP enzymes in cannabis metabolism.6
There is also evidence of enzyme induction by cannabis. Individuals who reported smoking marijuana experienced greater clearance of theophylline, a substrate of CYP1A2, than did those who reported not smoking marijuana.1,6 As with cigarette smoking, this effect appears to be a direct result of the hydrocarbons found in marijuana smoke rather than the cannabis itself, as there is a lack of evidence for enzyme induction when the drug is orally ingested.6
Amphetamine and methamphetamine appear to be both substrates and competitive inhibitors of CYP2D6.7 Rats administered quinidine (a strong 2D6 inhibitor) had 2-fold elevations in AUC and decreased clearance of amphetamine and its metabolites.8 Amphetamine-related recreational drugs, such as 3,4-methylenedioxy-methamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA), are substrates of CYP2D6 and CYP3A4, while MDMA also undergoes substantial metabolism by CYP1A2.3,7,9
Opioids. Heroin is metabolized to 6‑monoacetylmorphine (6-MAM) and morphine by hCE-1, hCE-2, and pseudocholinesterase, and has minimal impact on CYP enzymes. However, while morphine is primarily metabolized to inactive metabolites by UGT2B7, it does undergo minor metabolism through CYP3A4 and 2C8 pathways, creating potential for drug interactions with medications that inhibit or induce CYP3A4.10
Continue to: An underappreciated risk of illicit substance use
An underappreciated risk of illicit substance use
There is a paucity of evidence regarding the metabolism and pharmacokinetic interactions with illicit substances, and further research is needed. Despite the absence of comprehensive data on the subject, the available information indicates the use of illicit substances may have a significant impact on medications used to treat comorbid conditions. Alternatively, those medications may affect the kinetics of recreationally used substances. The risk of adverse consequences of drug–drug interactions is yet another reason patients should be encouraged to avoid use of substances and seek treatment for substance use disorders. When determining the most appropriate therapy for comorbid conditions for patients who are using illicit substances and are likely to continue to do so, clinicians should take into consideration potential interactions among prescription medications and the specific illicit substances the patient uses.
Related Resources
- Lindsey W, Stewart D, Childress D. Drug interactions between common illicit drugs and prescription therapies. Am J Drug Alcohol Abuse. 2012;38(4):334-343.
- Maurer H, Sauer C, Theobald D. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28(3):447-453.
- Dean A. Illicit drugs and drug interactions. Pharmacist. 2006;25(9):684-689.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Ketoconazole • Nizoral
Paliperidone palmitate • Invega sustenna
Phenytoin • Dilantin, Phenytek
Quinidine • Cardioquin, Duraquin
Theophylline • Elixophylline, Theochron
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
1. Jusko WJ, Schentag JJ, Clark JH, et al. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978;24(4):405-410.
2. Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed Tables. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHDetailedTabs2017/NSDUHDetailedTabs2017.htm#tab1-1A. Published 2019. Accessed February 7, 2020.
3. Quinn D, Wodak A, Day R. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997;33(5):344-400.
4. Ndikum-Moffor FM, Schoeb TR, Roberts SM. Liver toxicity from norcocaine nitroxide, an N-oxidative metabolite of cocaine. J Pharmacol Exp Ther. 1998;284(1):413-419.
5. Pellinen P, Honkakoski P, Stenbäck F, et al. Cocaine N-demethylation and the metabolism-related hepatotoxicity can be prevented by cytochrome P450 3A inhibitors. Eur J Pharmacol. 1994;270(1):35-43.
6. Stout S, Cimino N. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2013;46(1):86-95.
7. Kraemer T, Maurer H. Toxicokinetics of amphetamines: metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Ther Drug Monit. 2002;24(2):277-289.
8. Markowitz J, Patrick K. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40(10):753-772.
9. Kreth K, Kovar K, Schwab M, et al. Identification of the human cytochromes P450 involved in the oxidative metabolism of “ecstasy”-related designer drugs. Biochem Pharmacol. 2000;59(12):1563-1571.
10. Meyer M, Maurer H. Absorption, distribution, metabolism and excretion pharmacogenomics of drugs of abuse. Pharmacogenomics. 2011;12(2):215-233.
When the worry is worse than the actual illness
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.

Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1 Table 23 lists clinical indicators that raise suspicion for FDIA.
Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.

Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1 Table 23 lists clinical indicators that raise suspicion for FDIA.

Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
CASE Distraught over a medical illness
Ms. S, age 16, presents to the emergency department (ED) accompanied by her mother with superficial lacerations on her arm. Ms. S states, “I cut my arm because I was afraid I was going to do something serious if I didn’t get to go to the ED.” She says that 6 months earlier, she was diagnosed with superior mesenteric artery syndrome (SMAS), a rare, potentially life-threatening condition that occurs when the duodenum is compressed between the aorta and the superior mesenteric artery, causing a partial or complete blockage of the duodenum. Since receiving this diagnosis, Ms. S reports feeling anxious, depressed, and overwhelmed by both the pain she is experiencing from her illness and uncertainty about her prognosis.
HISTORY In pain and isolated
Since being diagnosed with SMAS, Ms. S has had approximately 30 medical and 7 ED visits for SMAS-related pain. Ms. S was referred to the outpatient clinic for ongoing support and treatment for SMAS.
Because of her pain and anxiety, Ms. S, a junior in high school, no longer attends school but has been working with a tutor. Ms. S says that some of her loneliness and hopelessness are due to the social isolation of being tutored at home. She states that she has been “out of sight and out of mind” from her friends. She also reports feeling different from them due to the pain brought on by SMAS.
Ms. S and her mother live in public housing. Ms. S says that overall, she has a good relationship with her mother, but that in certain situations, her mother’s anxiety causes her significant frustration and anxiety.
EVALUATION Transient suicidal thoughts
A physical examination reveals superficial lacerations to Ms. S’s left arm. Although she appears thin, her current body mass index (BMI) is 20.4 kg/m2, which is within normal range. She says she sees herself as “underweight” and “not fat at all.” Ms. S reports that she likes food and enjoyed eating until it became too painful following her SMAS diagnosis. Ms. S denies a history of binging or purging. Results from her laboratory workup and all values are within normal limits.
During the initial interview, Ms. S’s mother says they came to the ED because Ms. S urgently needs a psychiatric evaluation so she can be cleared for gastrointestinal (GI) surgery and placement of a nasogastric tube. Her mother says a surgeon from a different hospital told them that her insurance company required a psychiatric evaluation to rule out anorexia nervosa before they would authorize the GI surgery. When asked why psychiatry at this hospital was not consulted, Ms. S’s mother does not answer.
When asked about the symptoms she has been experiencing, Ms. S says that her sleep has been poor because of increased pain and excessive worrying about her health. She has limited her food intake. Ms. S reports that after eating, she lays on her left side to alleviate pain and help the food move through her body.
Continue to: Ms. S says...
Ms. S says she feels anxious and depressed due to her SMAS diagnosis, her mother’s online research and oversharing of poor prognoses, and being isolated from her friends. Most of her time outside the home is spent attending medical appointments with specialists. Several months ago, Ms. S had seen a psychotherapist, but her mother was unhappy with the treatment recommendations, which included seeking care from a nutritionist and joining group therapy. Ms. S’s mother says she ended her daughter’s psychotherapy because she was unable to obtain a signature ruling out anorexia nervosa within the first few appointments.
Ms. S also says she has had passive suicidal thoughts during the past month, usually twice a week. She reports that these thoughts lasted as long as several hours and were difficult to control, but she has no specific plan or intent. Ms. S denies current suicidal thoughts or ideation, and works with the treatment team to complete a safety plan, which she signs. Other than her recent visit to the ED, Ms. S denies any other thoughts or behaviors of self-injury or suicide.
[polldaddy:10586905]
The authors’ observations
The treatment team considered the following conditions as part of Ms. S’s differential diagnosis:
Major depressive disorder. The team was able to rule out MDD because Ms. S’s depression was attributed to SMAS. Ms. S reported that all depressive symptoms were manageable or nonexistent before the onset of pain from SMAS. There was no direct pathophysiological consequence of another medical condition. Ms. S was clear that her symptoms of anxiety and depression began after she was isolated from her friends and began having difficulty understanding her diagnosis and prognosis.
Anorexia nervosa also was ruled out. According to the DSM-5, a diagnosis of anorexia nervosa requires the following 3 criteria1:
- restriction of food intake resulting in significantly low body weight (defined as weight that is less than “minimally normal”) relative to age, gender, or development
- intense fear of gaining weight, or persistent behaviors that interfere with weight gain
- disturbance in the way in which one’s body weight or shape is experienced, undue influence of body weight or shape on self-evaluation, or lack of insight with regard to seriousness of current low body weight.
Continue to: Although Ms. S appeared...
Although Ms. S appeared thin, her BMI was within normal range. She added that she likes food and enjoyed eating, but that her medical condition made it too painful. Lastly, Ms. S denied a history of binging or purging.
Somatic symptom disorder.

Factitious disorder imposed on self. An individual with FDIS chronically stimulates, induces, or aggravates illnesses to gain the status of being a patient.
Factitious disorder imposed on another is the deliberate feigning or production of symptoms in another individual who is under the perpetrator’s supervision.1 Table 23 lists clinical indicators that raise suspicion for FDIA.

Before a diagnosis of somatic symptom disorder, FDIS, or FDIA could be established or ruled out, it was imperative to gather collateral information from other clinicians involved in Ms. S’s care. Ms. S and her mother had sought out help from a pediatric surgeon, a pediatric gastroenterologist, a pediatrician, and a psychotherapist.
Continue to: EVALUATION Collateral information
EVALUATION Collateral information
After Ms. S’s mother signs consent forms for exchange of information, the treatment team reaches out to the other clinicians. The therapist confirms that Ms. S’s mother had ended her daughter’s treatment after she was unable to quickly obtain documentation to rule out anorexia nervosa.
Both the pediatric surgeon and gastroenterologist report concerns of FDIA, which is why both clinicians had referred Ms. S and her mother to psychiatry. The pediatric surgeon states that on one occasion when he interviewed Ms. S separately from her mother, she seemed to be going down a checklist of symptoms. The surgeon reports that there was a partial occlusion of the superior mesenteric artery, confirming the diagnosis of SMAS, but he believed it was not severe enough to explain the symptoms Ms. S reported. The surgeon had scheduled another imaging appointment for 1 month later.
The pediatric gastroenterologist reports that Ms. S’s mother had demanded surgery and nasogastric tube placement for her daughter, which raised suspicion of FDIA. The gastroenterologist had convinced Ms. S and her mother to start low-dose doxepin, 20 mg twice a day, for anxiety, sleep, and abdominal pain.
Lastly, the pediatrician reports that she had not seen Ms. S for several months but stated that Ms. S always has been in the low normal BMI range. The pediatrician also reports that 6 months ago, the patient and her mother were frantically visiting EDs and scheduling doctor’s appointments.
[polldaddy:10586906]
The authors’ observations
The treatment team decided that Ms. S was not in imminent danger, and felt it was important to keep her in treatment without raising her mother’s suspicion. The team agreed to raise these concerns to the police, child protective services, and risk management if Ms. S’s health suddenly deteriorated or if her mother decided to remove Ms. S from our care.
Continue to: The treatment team...
The treatment team at the outpatient psychiatry clinic agreed that Ms. S did not currently meet criteria for anorexia nervosa, MDD, FDIS, or FDIA. However, Ms. S reported worries particular to persistent abdominal pain that was exacerbated by either eating or going to bed at night, which indicated that somatic symptom disorder was her likely diagnosis. Further, she endorsed a high level of anxiety and depression with regard to this somatic complaint that interfered with her daily activities and consumed an excessive amount of time, which also pointed to somatic symptom disorder. As a result of this diagnosis, the treatment team helped Ms. S manage her somatic symptoms and monitored for any other changes in her symptoms
Generally, cognitive-behavioral therapy (CBT) and mindfulness-based therapy may help relieve symptoms associated with somatic symptom disorder.4
TREATMENT Therapy sessions and medication management
At the psychiatric clinic, Ms. S is scheduled for biweekly therapy sessions with a social worker and biweekly appointments with a senior psychiatry resident for medication management. At each visit, Ms. S’s vital signs, height, and weight are measured. In the therapy sessions, she is taught mindfulness skills as well as CBT. The senior psychiatry resident maintains regular communication with the other clinicians involved in Ms. S’s care.
After the first month of treatment, Ms. S undergoes repeat imaging at the gastroenterologist’s office that indicates her SMAS is no longer occluded. Ms. S continues to report somatic symptoms, but with mild improvement.
Over the course of approximately 4 months, Ms. S begins to show signs of improvement in her pain, anxiety, and depression. Ms. S begins to feel well enough to get a summer job at a nursing home and expresses enthusiasm when her weight begins to increase. Her mother also became enthused and verbalized her appreciation that her daughter appeared to be improving.
Continue to: In the fall...
In the fall, Ms. S returns to high school for her senior year but has difficulty getting back into the routine and relating to her old friends. Ms. S continues to perseverate on thoughts of getting sick and her physical symptoms become overwhelming once again. She continues to be focused on any new symptoms she experiences, and to limit the types of foods she eats due to fear of the abdominal pain returning.
After several more months of psychiatric treatment, Ms. S reports significant relief from her abdominal pain, and no longer seeks corrective surgery for her SMAS. Although she occasionally struggles with perseverating thoughts and anxiety about her somatic symptoms such as abdominal pain and worrying about the types of foods she eats and becoming ill, she continues to work through symptoms of her somatic symptom disorder.
The authors’ observations
The main challenge of somatic symptom disorder is the patient’s “abnormal illness behavior.”2,5,6 For pediatric patients, there may an association between a parent’s psychological status and the patient’s somatic symptoms. Abdominal symptoms in a pediatric patient have a strong association with a parent who presents with depression, anxiety, or somatization. The effects of the parent’s psychological status could also manifest in the form of modeling catastrophic thinking or through reinforcement. Parents with certain traits, such as disproportionate worry about pain, may pay more attention to their child’s symptoms, and hence, reward the child when he/she reports somatic symptoms.7,8 In the case of Ms. S, her mother did not participate in therapy and the mother’s psychiatric history was never obtained.
OUTCOMES Making personal strides
Ms. S continues to use mindfulness skills as well as CBT to manage her symptoms of somatic symptom disorder. She continues to celebrate her weight gains, denies any thoughts of suicide or self-harm behaviors, and prepares for college by scheduling campus visits and completing admissions applications.
Bottom Line
Patients with somatic symptom disorder tend to have very high levels of worry about illness. Somatic symptoms in such patients may or may not have a medical explanation. Accurate diagnosis and careful management are necessary to reduce patient distress. Cognitive-behavioral therapy and mindfulness-based therapy may help relieve symptoms associated with this disorder.
Related Resources
- Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018;20(1):23-91.
- Rosic T, Kalra S, Samaan Z. Somatic symptom disorder, a new DSM-5 diagnosis of an old clinical challenge. BMJ Case Rep. 2016: bcr2015212553. doi: 10.1136/bcr-2015-212553.
Drug Brand Name
Doxepin • Silenor
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Stern T, Freudenreich O, Smith F, et al. Massachusetts General Hospital Handbook of General Hospital Psychiatry, 7th ed. New York, NY: Elsevier; 2017.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Association; 1997.
4. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry, 11th ed. Philadelphia, PA: Wolters Kluwer; 2014:470.
5. Pilowsky I. The concept of abnormal illness behavior. Psychosomatics. 1990;31(2):207-213.
6. Kirmayer LJ, Looper KJ. Abnormal illness behavior: physiological, psychological and social dimensions of coping with stress. Curr Opin Psychiatry. 2006;19(1):54-60.
7. Walker LS, Garber J, Greene JW. Somatic complaints in pediatric patients: a prospective study of the role of negative life events, child social and academic competence, and parental somatic symptoms. J Consult Clin Psychology. 1994;62(6):1213-1221.
8. Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology. 2016;150(6):1355-1367.
What to tell parents whose child saw them having sex
Many parents find themselves in a difficult situation when their child accidentally sees them having sex. These patients may ask us, as their clinicians, if they should discuss the incident with their child, and if so, what to say. If parents do not address the subject appropriately, their child might be confused and uncomfortable with his/her interpretation of the encounter.1 Some older research suggests that after witnessing their parents in a sexual encounter, children may have difficulty with affectional love, fears of being alone, or feelings of vulnerability.2,3 Clinicians may find themselves at a loss when parents ask them how to handle these situations. Although there are no evidence-based guidelines to consult, consider the following suggestions:
Relax. For patients who have not yet experienced this situation, tell them it is important not to panic if their child witnesses them having sex. They should cover their bodies and calmly respond to their child’s presence. Calm responsiveness is a key to diffusing this awkward situation. Otherwise, children may sense their parents’ embarrassment and conclude that sex is shameful. Parents should gently explain to their child that they are having a private, adult moment. They should ask their child if something is needed immediately, or if it could wait.
Accept that it happened. Parents should not avoid discussing the incident, but should promptly follow up with their child at an appropriate time and place. Waiting for a child to raise the topic puts the responsibility on him/her, instead of on the parent. Although some forthright children may ask questions, others may feel too ashamed or nervous to broach the topic and will prefer their parents to take the lead.
Discuss what happened. Tell parents to explore their child’s impression of what he/she saw. Tailoring the discussion to the child’s age is important. For example, a 3-year-old might wonder if anyone was harmed, and might need reassurance, whereas a 12-year-old is likely to have a better understanding of sex but still feel uncomfortable. Educational conversations about sexuality might be appropriate for children age 8 to 12. The parents’ goal should be to answer questions honestly without oversharing, and to leave the door open—so to speak—for future conversations.
Recommend that parents use plain, factual language to answer any questions their child asks. Statements such as “We were having a private, adult moment” can be helpful. Parents can categorize sex as a universal activity that is not harmful or scary by telling their child something such as, “This is what all parents do.” Parents should avoid providing unnecessary information or answering questions their child is not asking. The Table4 offers guidance on how parents might handle such conversations.

Consider potentially positive outcomes. Although parents may feel guilty or describe this as a terrible situation, remind them that there are some potentially healthy outcomes. For example, such incidents may help reassure the child that their parents love each other, which might give him/her a sense of happiness and security.
Take steps to prevent this from happening again. Advise parents to lock the door when having sex. Remind them to consider the proximity of rooms because their child might hear noises and become curious.
1. Blum HP. On the concept and consequences of the primal scene. Psychoanal Q. 1979;48(1):27-47.
2. Hoyt MF. On the psychology and psychopathology of primal-scene experience. J Am Acad Psychoanal. 1980;8(3):311-335.
3. Ikonen P, Rechardt E. On the universal nature of primal scene fantasies. Int J Psychoanal. 1984;65(pt 1):63-72.
4. Pelly J. Four things my four-year-old already knows about sex. Today’s Parent. https://www.todaysparent.com/family/parenting/4-things-my-4-year-old-already-knows-about-sex/. Published February 1, 2020. Accessed June 20, 2020.
Many parents find themselves in a difficult situation when their child accidentally sees them having sex. These patients may ask us, as their clinicians, if they should discuss the incident with their child, and if so, what to say. If parents do not address the subject appropriately, their child might be confused and uncomfortable with his/her interpretation of the encounter.1 Some older research suggests that after witnessing their parents in a sexual encounter, children may have difficulty with affectional love, fears of being alone, or feelings of vulnerability.2,3 Clinicians may find themselves at a loss when parents ask them how to handle these situations. Although there are no evidence-based guidelines to consult, consider the following suggestions:
Relax. For patients who have not yet experienced this situation, tell them it is important not to panic if their child witnesses them having sex. They should cover their bodies and calmly respond to their child’s presence. Calm responsiveness is a key to diffusing this awkward situation. Otherwise, children may sense their parents’ embarrassment and conclude that sex is shameful. Parents should gently explain to their child that they are having a private, adult moment. They should ask their child if something is needed immediately, or if it could wait.
Accept that it happened. Parents should not avoid discussing the incident, but should promptly follow up with their child at an appropriate time and place. Waiting for a child to raise the topic puts the responsibility on him/her, instead of on the parent. Although some forthright children may ask questions, others may feel too ashamed or nervous to broach the topic and will prefer their parents to take the lead.
Discuss what happened. Tell parents to explore their child’s impression of what he/she saw. Tailoring the discussion to the child’s age is important. For example, a 3-year-old might wonder if anyone was harmed, and might need reassurance, whereas a 12-year-old is likely to have a better understanding of sex but still feel uncomfortable. Educational conversations about sexuality might be appropriate for children age 8 to 12. The parents’ goal should be to answer questions honestly without oversharing, and to leave the door open—so to speak—for future conversations.
Recommend that parents use plain, factual language to answer any questions their child asks. Statements such as “We were having a private, adult moment” can be helpful. Parents can categorize sex as a universal activity that is not harmful or scary by telling their child something such as, “This is what all parents do.” Parents should avoid providing unnecessary information or answering questions their child is not asking. The Table4 offers guidance on how parents might handle such conversations.

Consider potentially positive outcomes. Although parents may feel guilty or describe this as a terrible situation, remind them that there are some potentially healthy outcomes. For example, such incidents may help reassure the child that their parents love each other, which might give him/her a sense of happiness and security.
Take steps to prevent this from happening again. Advise parents to lock the door when having sex. Remind them to consider the proximity of rooms because their child might hear noises and become curious.
Many parents find themselves in a difficult situation when their child accidentally sees them having sex. These patients may ask us, as their clinicians, if they should discuss the incident with their child, and if so, what to say. If parents do not address the subject appropriately, their child might be confused and uncomfortable with his/her interpretation of the encounter.1 Some older research suggests that after witnessing their parents in a sexual encounter, children may have difficulty with affectional love, fears of being alone, or feelings of vulnerability.2,3 Clinicians may find themselves at a loss when parents ask them how to handle these situations. Although there are no evidence-based guidelines to consult, consider the following suggestions:
Relax. For patients who have not yet experienced this situation, tell them it is important not to panic if their child witnesses them having sex. They should cover their bodies and calmly respond to their child’s presence. Calm responsiveness is a key to diffusing this awkward situation. Otherwise, children may sense their parents’ embarrassment and conclude that sex is shameful. Parents should gently explain to their child that they are having a private, adult moment. They should ask their child if something is needed immediately, or if it could wait.
Accept that it happened. Parents should not avoid discussing the incident, but should promptly follow up with their child at an appropriate time and place. Waiting for a child to raise the topic puts the responsibility on him/her, instead of on the parent. Although some forthright children may ask questions, others may feel too ashamed or nervous to broach the topic and will prefer their parents to take the lead.
Discuss what happened. Tell parents to explore their child’s impression of what he/she saw. Tailoring the discussion to the child’s age is important. For example, a 3-year-old might wonder if anyone was harmed, and might need reassurance, whereas a 12-year-old is likely to have a better understanding of sex but still feel uncomfortable. Educational conversations about sexuality might be appropriate for children age 8 to 12. The parents’ goal should be to answer questions honestly without oversharing, and to leave the door open—so to speak—for future conversations.
Recommend that parents use plain, factual language to answer any questions their child asks. Statements such as “We were having a private, adult moment” can be helpful. Parents can categorize sex as a universal activity that is not harmful or scary by telling their child something such as, “This is what all parents do.” Parents should avoid providing unnecessary information or answering questions their child is not asking. The Table4 offers guidance on how parents might handle such conversations.

Consider potentially positive outcomes. Although parents may feel guilty or describe this as a terrible situation, remind them that there are some potentially healthy outcomes. For example, such incidents may help reassure the child that their parents love each other, which might give him/her a sense of happiness and security.
Take steps to prevent this from happening again. Advise parents to lock the door when having sex. Remind them to consider the proximity of rooms because their child might hear noises and become curious.
1. Blum HP. On the concept and consequences of the primal scene. Psychoanal Q. 1979;48(1):27-47.
2. Hoyt MF. On the psychology and psychopathology of primal-scene experience. J Am Acad Psychoanal. 1980;8(3):311-335.
3. Ikonen P, Rechardt E. On the universal nature of primal scene fantasies. Int J Psychoanal. 1984;65(pt 1):63-72.
4. Pelly J. Four things my four-year-old already knows about sex. Today’s Parent. https://www.todaysparent.com/family/parenting/4-things-my-4-year-old-already-knows-about-sex/. Published February 1, 2020. Accessed June 20, 2020.
1. Blum HP. On the concept and consequences of the primal scene. Psychoanal Q. 1979;48(1):27-47.
2. Hoyt MF. On the psychology and psychopathology of primal-scene experience. J Am Acad Psychoanal. 1980;8(3):311-335.
3. Ikonen P, Rechardt E. On the universal nature of primal scene fantasies. Int J Psychoanal. 1984;65(pt 1):63-72.
4. Pelly J. Four things my four-year-old already knows about sex. Today’s Parent. https://www.todaysparent.com/family/parenting/4-things-my-4-year-old-already-knows-about-sex/. Published February 1, 2020. Accessed June 20, 2020.