User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Helping older adults overcome the challenges of technology
Technology is pervasive, and for many people, it is central to their daily activities. Younger people who have been exposed to technology for their entire lives take this for granted, but older individuals often have had much less experience with it. Many technological developments that are now a part of most people’s daily life, such as personal computers, cell phones, and automated teller machines (ATMs), have occurred in the past 4 decades, with the pace accelerating in the last 15 to 20 years.
Such changes have had a substantial impact on older adults who were never exposed to these technologies during their working life. For example, an 85-year-old person who retired at age 65 would probably have not been exposed to wireless internet prior to retirement. Therefore, all of the tasks that they are now required to complete online would have been performed in other ways. Banking, accessing instruction manuals for new devices, and even scheduling and confirming health care appointments and accessing medical records all now require individuals to have a level of technological skills that many older individuals find challenging. At times, this can limit their ability to complete routine daily activities, and also can have clinical implications (Table).
Fortunately, there are strategies clinicians can use to help their older patients face these challenges. In this article, we describe the cognitive domains associated with learning technological skills, how aging affects these domains, and what can be done to help older adults improve their technological skills.
Limited training on how to use new technology
Technological skills are similar to any other skills in one critical way: they need to be learned. At the same time, technological skills also differ from many other skills, such as playing a musical instrument, because of the constant updating of devices, programs, and applications. When smartphones or computers update their operating systems, the visual appearance of the screen and the way that tasks are performed also can change. Buttons can move and sequences of commands can be altered. Updates often happen with little or no notice, and users may need to navigate a completely different device landscape in order to perform tasks that they had previously mastered.
In addition, the creators/distributors of technology typically provide little training or documentation. Further, institutions such as banks or health care systems frequently do not provide any specific training for using their systems. For example, when patients are required to use technology to refill prescriptions, typically there is no training available on how the system operates.
Cognitive domains associated with technological skills
Because there are minimal opportunities to receive training in how to use most aspects of technology, users have to be able to learn by exposure and experience. This requires several different cognitive abilities to work together. In a recent review, Harvey1 described cognition and cognitive assessment in the general population, with a focus on cognitive domains. Here we discuss several of these domains in terms of the relationship to real-world functional tasks and discuss their importance for mastering technology.
Reasoning and problem solving. Because most technological devices and applications are designed to be “intuitive,” the user needs to be able to adopt a sequential approach to learning the task. For example, using the internet to refill a prescription requires several steps:
- accessing the internet
- finding the pharmacy web site
- establishing a user ID and password
- navigating the web site to the prescriptions section
- identifying the correct prescription
- requesting the refill
- selecting the pickup date and time.
Continue to: After navigating these steps...
After navigating these steps, an individual still needs other cognitive abilities to refill other prescriptions later. However, executive functioning is also critical for maintaining organization across different technological demands. For example, web sites have different password rules and require frequent changes without re-using old passwords, so it becomes critical to maintain an organized list of web site addresses and their passwords.
Refilling a prescription with a telephone voice menu also requires a series of steps. Typically, this process is simpler than an internet refill, because no log-in information is necessary. However, it still requires a structured series of tasks.
Working memory refers to the ability to hold information in consciousness long enough to operate on it. At each step of the navigation process, the user needs to remember which steps he/she has already completed, because repeating steps can slow down the process or lead to error messages. Thus, remembering which steps have been completed is as critical for performing tasks as is correctly understanding the anticipated sequence of steps. Further, when a password is forgotten, the user needs to remember the newly provided password.
Working memory can be spatial as well. For example, most web sites do not display a password while it is being entered, which eliminates spatial working memory from the equation. Thus, the ability to remember which characters have been entered and which still need to be entered is necessary.
Episodic memory is the process of learning and retaining newly presented verbal or spatial information as well as recalling it later for adaptive use. After successfully using a new technology, it is critical to be able to remember what to do the next time it is used. This includes both recalling how to access the technology (including the web address, user ID, and password), recalling the steps needed to be performed and their sequence, and recognizing the buttons and instructions presented onscreen.
Continue to: Procedural memory
Procedural memory is memory for motor acts and sequences. For instance, remembering how to ride a bicycle is a procedural memory, as is the ability to perform motor acts in sequence, such as peeling, cutting, and cooking vegetables. Interestingly, procedural memory can be spared in individuals with major challenges in episodic memory, such as those with amnestic conditions or cortical dementia. Thus, it may be possible for people to continue to perform technology-based skills despite declines in episodic memory. Many current technological functional tasks have fixed sequences of events that, if remembered, can lead to increased efficiency and higher chances of success in performance of functional tasks.
Prospective memory is the ability to remember to perform tasks in the future. This can include event-related tasks (eg, enter your password before trying to make a hotel reservation on a web site) or time-related tasks (eg, refill your prescriptions next Friday). Technology can actually facilitate prospective memory by providing reminders to individuals, such as alarms for appointments. However, prospective memory is required to initially set up such alarms, and setting up confusing or incorrect alarms can impede task performance.
Processing speed is the ability to perform cognitively demanding tasks under time constraints. Traditional processing speed tasks include coding and sorting tasks, which require processing new information and effort for relatively short periods of time. In our research, we discovered that processing speed measured with traditional tests was strongly correlated with the time required to perform functional tasks such as an ATM banking task.2,3 This correlation makes sense in terms of the fact that many real-world functional tasks with technology often have a series of sequential demands that must be accomplished before progression to the next task.
Manual dexterity is also important for using technology. Many electronic devices have small, touch screen-based keyboards. Being able to touch the correct key requires dexterity and can be made more difficult by age-related vision changes, a tremor, or reduced sensation in extremities.
Cognitive changes and aging
It is normal for certain cognitive abilities to change with aging. There are a set of cognitive skills that are generally stable from early adulthood until the early “senescent” period. Some of these skills decline normatively after age 60 to 65, or earlier in some individuals. These include processing new information, solving new problems, and learning and remembering information. Referred to as “fluid intelligence,” these abilities show age-related decline during healthy aging, and even greater decline in individuals with age-related cognitive conditions.
Continue to: On the other hand...
On the other hand, some cognitive abilities do not decline with aging. These include previously acquired knowledge, such as vocabulary and mathematics skills, as well as factual information, such as academic information and the faces of familiar people. These are referred to as “crystallized intelligence,” and there is limited evidence that they decline with age. In fact, these abilities do not decline until the moderately severe stage of cortical dementias, and are commonly used to index premorbid cognitive functioning and cognitive reserve.
Why is this distinction between fluid intelligence and crystallized intelligence important? As noted above, many older people do not have early-life experience with technology. Thus, their crystallized intelligence, which is not as vulnerable to decline with aging, does not include information about how to perform many technological tasks. In contrast to today’s adolescents and young adults, older adults’ academic history typically does not include using smartphones, doing homework via Google Docs, or having homework and classwork assigned via the internet.
Learning how to use new technology requires fluid intelligence, and these abilities are less efficient in older adults. So for many older people, technological tasks can be complex and unfamiliar, and the skills needed to learn how to perform them are also more limited, even in comparison to older adults’ own ability when younger. Because many technology-based activities require concurrent performance of multiple tasks, older adults are at a disadvantage.4 It is not surprising, therefore, that a subset of older adults rate their technology skills as weak, and technology-based tasks as challenging or anxiety-provoking.
However, studies show most older adults’ attitudes toward technology remain largely positive, and that they are capable of attaining the necessary skills to use information and communication technology.4,5 An individual’s perception of his/her age, age-related beliefs, and self-efficacy are associated not only with attitudes toward technology, but possibly with cognition itself.6
Education level and socioeconomic factors also influence a person’s ability to become proficient in using technology.7-9 In fact, socioeconomic factors are more strongly related to access to the internet than age. Many older adults have internet access, but this access does not always translate into full use of its services.
Continue to: The Box...
The Box10-22 describes some of the effects of aging on the brain, and how these changes are reflected in cognitive abilities.
Box
The global baseline intensity of human brain activity, determined by indirectly measuring blood oxygenation, decreases with age.10 Multiple domains of fluid cognition decline with age; these cognitive abilities include processing speed,11,12 working memory,11 episodic memory,11 and executive function.11 Expected neuroanatomic changes of aging include a decrease in cerebral grey matter volume as well as decreased white matter integrity, which is associated with diminished executive function and impaired working memory.13 Processing speed is associated with increased white matter microstructure during neurodevelopment.14 Diminished processing speed in older adults also may predict increased mortality risk.15 Individuals with advanced age may have augmented difficulty with episodic memory, especially when they are required to integrate information from more than one source.11 Diminished hippocampal volume13 and reduced activity of the middle frontal gyrus are associated with age-related decline in episodic memory retrieval.10 Working memory16 is known to share a neurocircuitry overlap with attention processes.17 Working memory capacity also is closely associated with other cognitive functions, such as shifting and inhibition.10 Enhanced cerebellar activity is related to working memory; increased cerebellar activity is likely due to compensatory recruitment of neurons due to reduced activity in the superior frontal gyrus.10 The superior frontal gyrus contributes to both working memory as well as executive processing.10
Although the cognitive decline associated with aging is inevitable, individuals who experience cognitive decline at an increased rate are predisposed to worse outcomes. One longitudinal cohort study found that adults in their 8th and 9th decades of life with preserved cognitive function had a lower risk of disability and death.18
On the other hand, crystallized cognitive functions such as semantic memory,13 shortterm memory,13 and emotion regulation16 remain largely intact throughout the aging process. Semantic memory, a subtype of episodic memory, is related to associated facts or interpretations of previous occurrences.19 This type of memory is detached from an individual’s personal experience.20 Semantic memory loss classically presents with anomia and detectable lesions in the anterior and temporal lobes.20 Emotion regulation deficits are not a part of normal aging; in fact, emotional well-being is known to either improve or remain consistent with age.21 Emotional experiences in patients of advanced age may be more complex and unique in comparison to other cognitive abilities.22
The role of cognitive training
Existing interventions for helping older adults improve their technology proficiency generally focus on improving cognition, and not necessarily on addressing skills learning. Skills learning and cognition are related; however, the brain depends on neural plasticity for skills learning, whereas cognitive declines are a result of gradual and functional worsening of memory, processing speed, executive functioning, and attention.23 Interventions such as cognitive strategy training are capable of altering brain neurocircuitry to improve attention and memory.10,11 Other interventions known to improve cognition include exercise10 and processing speed training.24 On the other hand, skills learning is more effectively targeted by interventions that focus on stimulating realistic environments to mimic activities of daily living that involve technology.
Studies have consistently demonstrated cognitive improvements associated with computerized cognitive training (CCT). The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study was designed to evaluate the efficacy of cognitive training in 2,832 healthy adults age >65 across 6 recruitment sites in the United States.25 Participants were randomized to a control group (no treatment) or to 1 of 3 treatment groups:
- memory strategy training (instructor-led, not computerized)
- reasoning training (instructor-led, not computerized)
- speed training (no instructor, adaptive computerized training).
Each treatment group received 10 sessions of classroom-based training (1 hour each, twice per week for 5 weeks). Following the intervention, participants who had completed ≥8 sessions were randomized to receive 4 booster sessions at 11 and 35 months after the initial training, or no booster sessions.
Each cognitive training program significantly improved performance on within-domain cognitive tests relative to the control group. Effect sizes were large immediately following training; they declined over time, but were still significant at 10-year follow-up. As hypothesized, training effects did not generalize to neuropsychological tests in other training domains. The booster subgroup of speed training showed improved performance on a separate functional speed measure at 2-year26 and 5-year follow-up.27 Each condition showed slower decline in instrumental activities of daily living relative to the control group.
Continue to: The Figure...
The Figure shows the type of stimuli presented in the speed training, a procedure where individuals are taught high-speed multitasking by having to identify and locate visual information quickly in a divided-attention format. A stimulus appears in the center of the screen—either a car or a truck—and at the same time, a “Route 66” sign appears in the periphery. For every successful response, the next stimulus is presented at a shorter duration after every successful response, and more slowly after errors.

Secondary outcome analyses demonstrated that for older adults, speed training reduced rates of driving cessation,27 improved driving habits, and lowered the incidence of at-fault crashes28 (based on motor vehicle records). Speed training also resulted in improvements in health-related quality of life,29,30 depression,31 locus of control,32 and medical expenditures.33 An analysis of 10-year outcomes34 found that speed training was associated with a 29% reduction in risk of developing of dementia, while the other 2 interventions were not. However, despite these multiple areas of benefit, there was no evidence that new functional skills were acquired as a result of the training.26-34
Functional skills training
While there is a long history of using functional skills training to help patients with schizophrenia, for healthy older people, there are considerably more challenges. First, aging is not a disease. Consequently, functional skills training is typically not covered by health insurance. Second, functional skills training delivered by a human trainer can be expensive and is not readily available. Finally, there are no real curricula for training functional skills, particularly those that are device-based (phone, tablet, or computer).
Recently, researchers have developed a functional skills assessment and training program that was originally piloted as a fixed difficulty simulation as described in 2 studies by Czaja et al.2,3 The original assessment was used to compare healthy control individuals with people with mild cognitive impairment (MCI) or schizophrenia. Most recently, training modules for 6 different technology-based functional tasks have been developed and piloted in samples of healthy controls and patients with MCI in a randomized trial.35 Half of the participants in each of the 2 groups were randomized to receive speed training similar to the ACTIVE study, and the other half received skills training alone. All participants were trained for 24 sessions over 12 weeks or until they mastered all 6 simulations.
Both patients with MCI and healthy controls improved in all 6 simulations. Although patients with MCI were considerably less efficient at baseline, their training gains per session were equivalent to that of healthy controls. Finally, concurrent cognitive training increased the efficiency of skills training. At the end of the study, functional gains were the same for people in both groups randomized to either condition, even though individuals in the combined cognitive and skills training interventions received only half as much skills training time.
Continue to: What to tell patients
What to tell patients
Older patients might ask their clinicians what they can do to “exercise their brain.” Let them know that CCT has been shown to improve cognitive performance in healthy older people, and that there are several evidence-based, commercially available products for this purpose. Two such self-administrable systems with supportive data are BrainHQ (www.brainhq.com) and Happy Neuron (www.happy-neuron.com). Explain that it is likely that the best strategy is a combination of cognitive and functional skills training. One commercially available functional skills training program with supportive data is i-Function (www.i-Function.com). (Editor’s note: One of the authors, PDH, is an employee of i-Function, Inc.)
Bottom Line
Clinicians should ensure older patients that they have the cognitive capacity to learn new technology-related functional skills, and that such patients have the opportunity to learn these skills. Clinicians need to be able to identify people who are at high risk of not being able to adhere to instructions and suggestions that require interactions with technology. Treatment options include computerized cognitive training and functional skills training.
Related Resources
- Hill NT, Mowszowski L, Naismith SL, et al. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and metaanalysis. Am J Psychiatry. 2017;174(4):329-340.
- Harvey PD, McGurk SR, Mahncke H, et al. Controversies in computerized cognitive training. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(11):907-915.
1. Harvey PD. Domains of cognition and their assessment. Dialogues Clin Neuro. 2019;21(3):227-237.
2. Czaja SJ, Loewenstein DA, Sabbag SA, et al. A novel method for direct assessment of everyday competence among older adults. J Alzheimers Dis. 2017;57(4):1229-1238.
3. Czaja SJ, Loewenstein DA, Lee CC, et al. Assessing functional performance using computer-based simulations of everyday activities. Schizophr Res. 2017;183:130-136.
4. Tsai HS, Shillair R, Cotten SR. Social support and “playing around”: an examination of how older adults acquire digital literacy with tablet computers. J Appl Gerontol. 2017;36(1):29-55.
5. Cabrita M, Tabak M, Vollenbroek-Hutten MM. Older adults’ attitudes toward ambulatory technology to support monitoring and coaching of healthy behaviors: qualitative study. JMIR Aging. 2019;2(1):e10476. doi: 10.2196/10476.
6. Lim KY, Chang KJ, Kim HJ, et al. P.5.a.010 association between memory age identity and cognition in the elderly. Eur Neuropsychopharmacol. 2010;20(suppl 3):S555.
7. Moraes C, Pinto JA Jr, Lopes MA, et al. Impact of sociodemographic and health variables on mini-mental state examination in a community-based sample of older people. Eur Arch Psychiatry Clin Neurosci. 2010;260(7):535-542.
8. Freitas S, Simões MR, Alves L, et al. The relevance of sociodemographic and health variables on MMSE normative data. Appl Neuropsychol Adult. 2015;22(4):311-319.
9. Han C, Jo SA, Jo I, et al. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study). Arch Gerontol Geriatr. 2008;47(3):302-310.
10. Yin S, Zhu X, Li R, et al. Intervention-induced enhancement in intrinsic brain activity in healthy older adults. Sci Rep. 2014;4:7309.
11. Bender AR, Prindle JJ, Brandmaier AM, et al. White matter and memory in healthy adults: coupled changes over two years. Neuroimage. 2016;131:193-204.
12. Guye S, von Bastian CC. Working memory training in older adults: Bayesian evidence supporting the absence of transfer. Psychol Aging. 2017;32(8):732-746.
13. Taki Y, Kinomura S, Sato K, et al. Correlation between gray/white matter volume and cognition in healthy elderly people. Brain Cogn. 2011;75(2):170-176.
14. Cassidy AR, White MT, DeMaso DR, et al. Processing speed, executive function, and academic achievement in children with dextro-transposition of the great arteries: Testing a longitudinal developmental cascade model. Neuropsychology. 2016;30(7):874-885.
15. Aichele S, Rabbitt P, Ghisletta P. Life span decrements in fluid intelligence and processing speed predict mortality risk. Psychol Aging. 2015;30(3):598-612.
16. Eich TS, Castel AD. The cognitive control of emotional versus value-based information in younger and older adults. Psychol Aging. 2016;31(5):503-512.
17. Rolle CE, Anguera JA, Skinner SN, et al. Enhancing spatial attention and working memory in younger and older adults. J Cogn Neurosci. 2017;29(9):1483-1497.
18. Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc. 2010;58(5):889-894.
19. Madore KP, Schacter DL. An episodic specificity induction enhances means-end problem solving in young and older adults. Psychol Aging. 2014;29(4):913-924.
20. Matthews BR. Memory dysfunction. Continuum (Minneap Minn). 2015;21(3 Behavioral Neurology and Neuropsychiatry):613-626.
21. Mather M. The emotion paradox in the aging brain. Ann N Y Acad Sci. 2012;1251(1):33-49.
22. Gurera JW, Isaacowitz DM. Emotion regulation and emotion perception in aging: A perspective on age-related differences and similarities. Prog Brain Res. 2019;247:329-351.
23. Cai L, Chan JS, Yan JH, et al. Brain plasticity and motor practice in cognitive aging. Front Aging Neurosci. 2014;6:31.
24. Cassetta BD, Tomfohr-Madsen LM, Goghari VM. A randomized controlled trial of working memory and processing speed training in schizophrenia. Psychol Med. 2019;49(12):2009-2019.
25. Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271-2281.
26. Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16-24.
27. Edwards JD, Delahunt PB, Mahncke HW. Cognitive speed of processing training delays driving cessation. J Gerontol A Biol Sci Med Sci. 2009;64(12):1262-1267.
28. Ball K, Edwards JD, Ross LA, et al. Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2010;58(11):2107-2113.
29. Wolinsky FD, Unverzagt FW, Smith DM, et al. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J Gerontol B Psychol Sci Soc Sci. 2006;61(5):S281-S287.
30. Wolinsky FD, Unverzagt FW, Smith DM, et al. The ACTIVE cognitive training trial and health-related quality of life: protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61(12):1324-1329.
31. Wolinsky FD, Vander Weg MW, Martin R, et al. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci. 2009;64(4):468-472.
32. Wolinsky FD, Vander Weg MW, Martin R, et al. Does cognitive training improve internal locus of control among older adults? J Gerontol B Psychol Sci Soc Sci. 2010;65(5):591-598.
33. Wolinsky FD, Mahncke HW, Kosinski M, et al. The ACTIVE cognitive training trial and predicted medical expenditures. BMC Health Serv Res. 2009;9:109.
34. Edwards JD, Xu H, Clark DO, et al. Speed of processing training results in lower risk of dementia. Alzheimers Dement (N Y). 2017;3(4):603-611.
35. Harvey PD, Tibiriçá L, Kallestrup P, et al. A computerized functional skills assessment and training program targeting technology based everyday functional skills. J Vis Exp. 2020;156:e60330. doi: 10.3791/60330.
Technology is pervasive, and for many people, it is central to their daily activities. Younger people who have been exposed to technology for their entire lives take this for granted, but older individuals often have had much less experience with it. Many technological developments that are now a part of most people’s daily life, such as personal computers, cell phones, and automated teller machines (ATMs), have occurred in the past 4 decades, with the pace accelerating in the last 15 to 20 years.
Such changes have had a substantial impact on older adults who were never exposed to these technologies during their working life. For example, an 85-year-old person who retired at age 65 would probably have not been exposed to wireless internet prior to retirement. Therefore, all of the tasks that they are now required to complete online would have been performed in other ways. Banking, accessing instruction manuals for new devices, and even scheduling and confirming health care appointments and accessing medical records all now require individuals to have a level of technological skills that many older individuals find challenging. At times, this can limit their ability to complete routine daily activities, and also can have clinical implications (Table).
Fortunately, there are strategies clinicians can use to help their older patients face these challenges. In this article, we describe the cognitive domains associated with learning technological skills, how aging affects these domains, and what can be done to help older adults improve their technological skills.
Limited training on how to use new technology
Technological skills are similar to any other skills in one critical way: they need to be learned. At the same time, technological skills also differ from many other skills, such as playing a musical instrument, because of the constant updating of devices, programs, and applications. When smartphones or computers update their operating systems, the visual appearance of the screen and the way that tasks are performed also can change. Buttons can move and sequences of commands can be altered. Updates often happen with little or no notice, and users may need to navigate a completely different device landscape in order to perform tasks that they had previously mastered.
In addition, the creators/distributors of technology typically provide little training or documentation. Further, institutions such as banks or health care systems frequently do not provide any specific training for using their systems. For example, when patients are required to use technology to refill prescriptions, typically there is no training available on how the system operates.
Cognitive domains associated with technological skills
Because there are minimal opportunities to receive training in how to use most aspects of technology, users have to be able to learn by exposure and experience. This requires several different cognitive abilities to work together. In a recent review, Harvey1 described cognition and cognitive assessment in the general population, with a focus on cognitive domains. Here we discuss several of these domains in terms of the relationship to real-world functional tasks and discuss their importance for mastering technology.
Reasoning and problem solving. Because most technological devices and applications are designed to be “intuitive,” the user needs to be able to adopt a sequential approach to learning the task. For example, using the internet to refill a prescription requires several steps:
- accessing the internet
- finding the pharmacy web site
- establishing a user ID and password
- navigating the web site to the prescriptions section
- identifying the correct prescription
- requesting the refill
- selecting the pickup date and time.
Continue to: After navigating these steps...
After navigating these steps, an individual still needs other cognitive abilities to refill other prescriptions later. However, executive functioning is also critical for maintaining organization across different technological demands. For example, web sites have different password rules and require frequent changes without re-using old passwords, so it becomes critical to maintain an organized list of web site addresses and their passwords.
Refilling a prescription with a telephone voice menu also requires a series of steps. Typically, this process is simpler than an internet refill, because no log-in information is necessary. However, it still requires a structured series of tasks.
Working memory refers to the ability to hold information in consciousness long enough to operate on it. At each step of the navigation process, the user needs to remember which steps he/she has already completed, because repeating steps can slow down the process or lead to error messages. Thus, remembering which steps have been completed is as critical for performing tasks as is correctly understanding the anticipated sequence of steps. Further, when a password is forgotten, the user needs to remember the newly provided password.
Working memory can be spatial as well. For example, most web sites do not display a password while it is being entered, which eliminates spatial working memory from the equation. Thus, the ability to remember which characters have been entered and which still need to be entered is necessary.
Episodic memory is the process of learning and retaining newly presented verbal or spatial information as well as recalling it later for adaptive use. After successfully using a new technology, it is critical to be able to remember what to do the next time it is used. This includes both recalling how to access the technology (including the web address, user ID, and password), recalling the steps needed to be performed and their sequence, and recognizing the buttons and instructions presented onscreen.
Continue to: Procedural memory
Procedural memory is memory for motor acts and sequences. For instance, remembering how to ride a bicycle is a procedural memory, as is the ability to perform motor acts in sequence, such as peeling, cutting, and cooking vegetables. Interestingly, procedural memory can be spared in individuals with major challenges in episodic memory, such as those with amnestic conditions or cortical dementia. Thus, it may be possible for people to continue to perform technology-based skills despite declines in episodic memory. Many current technological functional tasks have fixed sequences of events that, if remembered, can lead to increased efficiency and higher chances of success in performance of functional tasks.
Prospective memory is the ability to remember to perform tasks in the future. This can include event-related tasks (eg, enter your password before trying to make a hotel reservation on a web site) or time-related tasks (eg, refill your prescriptions next Friday). Technology can actually facilitate prospective memory by providing reminders to individuals, such as alarms for appointments. However, prospective memory is required to initially set up such alarms, and setting up confusing or incorrect alarms can impede task performance.
Processing speed is the ability to perform cognitively demanding tasks under time constraints. Traditional processing speed tasks include coding and sorting tasks, which require processing new information and effort for relatively short periods of time. In our research, we discovered that processing speed measured with traditional tests was strongly correlated with the time required to perform functional tasks such as an ATM banking task.2,3 This correlation makes sense in terms of the fact that many real-world functional tasks with technology often have a series of sequential demands that must be accomplished before progression to the next task.
Manual dexterity is also important for using technology. Many electronic devices have small, touch screen-based keyboards. Being able to touch the correct key requires dexterity and can be made more difficult by age-related vision changes, a tremor, or reduced sensation in extremities.
Cognitive changes and aging
It is normal for certain cognitive abilities to change with aging. There are a set of cognitive skills that are generally stable from early adulthood until the early “senescent” period. Some of these skills decline normatively after age 60 to 65, or earlier in some individuals. These include processing new information, solving new problems, and learning and remembering information. Referred to as “fluid intelligence,” these abilities show age-related decline during healthy aging, and even greater decline in individuals with age-related cognitive conditions.
Continue to: On the other hand...
On the other hand, some cognitive abilities do not decline with aging. These include previously acquired knowledge, such as vocabulary and mathematics skills, as well as factual information, such as academic information and the faces of familiar people. These are referred to as “crystallized intelligence,” and there is limited evidence that they decline with age. In fact, these abilities do not decline until the moderately severe stage of cortical dementias, and are commonly used to index premorbid cognitive functioning and cognitive reserve.
Why is this distinction between fluid intelligence and crystallized intelligence important? As noted above, many older people do not have early-life experience with technology. Thus, their crystallized intelligence, which is not as vulnerable to decline with aging, does not include information about how to perform many technological tasks. In contrast to today’s adolescents and young adults, older adults’ academic history typically does not include using smartphones, doing homework via Google Docs, or having homework and classwork assigned via the internet.
Learning how to use new technology requires fluid intelligence, and these abilities are less efficient in older adults. So for many older people, technological tasks can be complex and unfamiliar, and the skills needed to learn how to perform them are also more limited, even in comparison to older adults’ own ability when younger. Because many technology-based activities require concurrent performance of multiple tasks, older adults are at a disadvantage.4 It is not surprising, therefore, that a subset of older adults rate their technology skills as weak, and technology-based tasks as challenging or anxiety-provoking.
However, studies show most older adults’ attitudes toward technology remain largely positive, and that they are capable of attaining the necessary skills to use information and communication technology.4,5 An individual’s perception of his/her age, age-related beliefs, and self-efficacy are associated not only with attitudes toward technology, but possibly with cognition itself.6
Education level and socioeconomic factors also influence a person’s ability to become proficient in using technology.7-9 In fact, socioeconomic factors are more strongly related to access to the internet than age. Many older adults have internet access, but this access does not always translate into full use of its services.
Continue to: The Box...
The Box10-22 describes some of the effects of aging on the brain, and how these changes are reflected in cognitive abilities.
Box
The global baseline intensity of human brain activity, determined by indirectly measuring blood oxygenation, decreases with age.10 Multiple domains of fluid cognition decline with age; these cognitive abilities include processing speed,11,12 working memory,11 episodic memory,11 and executive function.11 Expected neuroanatomic changes of aging include a decrease in cerebral grey matter volume as well as decreased white matter integrity, which is associated with diminished executive function and impaired working memory.13 Processing speed is associated with increased white matter microstructure during neurodevelopment.14 Diminished processing speed in older adults also may predict increased mortality risk.15 Individuals with advanced age may have augmented difficulty with episodic memory, especially when they are required to integrate information from more than one source.11 Diminished hippocampal volume13 and reduced activity of the middle frontal gyrus are associated with age-related decline in episodic memory retrieval.10 Working memory16 is known to share a neurocircuitry overlap with attention processes.17 Working memory capacity also is closely associated with other cognitive functions, such as shifting and inhibition.10 Enhanced cerebellar activity is related to working memory; increased cerebellar activity is likely due to compensatory recruitment of neurons due to reduced activity in the superior frontal gyrus.10 The superior frontal gyrus contributes to both working memory as well as executive processing.10
Although the cognitive decline associated with aging is inevitable, individuals who experience cognitive decline at an increased rate are predisposed to worse outcomes. One longitudinal cohort study found that adults in their 8th and 9th decades of life with preserved cognitive function had a lower risk of disability and death.18
On the other hand, crystallized cognitive functions such as semantic memory,13 shortterm memory,13 and emotion regulation16 remain largely intact throughout the aging process. Semantic memory, a subtype of episodic memory, is related to associated facts or interpretations of previous occurrences.19 This type of memory is detached from an individual’s personal experience.20 Semantic memory loss classically presents with anomia and detectable lesions in the anterior and temporal lobes.20 Emotion regulation deficits are not a part of normal aging; in fact, emotional well-being is known to either improve or remain consistent with age.21 Emotional experiences in patients of advanced age may be more complex and unique in comparison to other cognitive abilities.22
The role of cognitive training
Existing interventions for helping older adults improve their technology proficiency generally focus on improving cognition, and not necessarily on addressing skills learning. Skills learning and cognition are related; however, the brain depends on neural plasticity for skills learning, whereas cognitive declines are a result of gradual and functional worsening of memory, processing speed, executive functioning, and attention.23 Interventions such as cognitive strategy training are capable of altering brain neurocircuitry to improve attention and memory.10,11 Other interventions known to improve cognition include exercise10 and processing speed training.24 On the other hand, skills learning is more effectively targeted by interventions that focus on stimulating realistic environments to mimic activities of daily living that involve technology.
Studies have consistently demonstrated cognitive improvements associated with computerized cognitive training (CCT). The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study was designed to evaluate the efficacy of cognitive training in 2,832 healthy adults age >65 across 6 recruitment sites in the United States.25 Participants were randomized to a control group (no treatment) or to 1 of 3 treatment groups:
- memory strategy training (instructor-led, not computerized)
- reasoning training (instructor-led, not computerized)
- speed training (no instructor, adaptive computerized training).
Each treatment group received 10 sessions of classroom-based training (1 hour each, twice per week for 5 weeks). Following the intervention, participants who had completed ≥8 sessions were randomized to receive 4 booster sessions at 11 and 35 months after the initial training, or no booster sessions.
Each cognitive training program significantly improved performance on within-domain cognitive tests relative to the control group. Effect sizes were large immediately following training; they declined over time, but were still significant at 10-year follow-up. As hypothesized, training effects did not generalize to neuropsychological tests in other training domains. The booster subgroup of speed training showed improved performance on a separate functional speed measure at 2-year26 and 5-year follow-up.27 Each condition showed slower decline in instrumental activities of daily living relative to the control group.
Continue to: The Figure...
The Figure shows the type of stimuli presented in the speed training, a procedure where individuals are taught high-speed multitasking by having to identify and locate visual information quickly in a divided-attention format. A stimulus appears in the center of the screen—either a car or a truck—and at the same time, a “Route 66” sign appears in the periphery. For every successful response, the next stimulus is presented at a shorter duration after every successful response, and more slowly after errors.

Secondary outcome analyses demonstrated that for older adults, speed training reduced rates of driving cessation,27 improved driving habits, and lowered the incidence of at-fault crashes28 (based on motor vehicle records). Speed training also resulted in improvements in health-related quality of life,29,30 depression,31 locus of control,32 and medical expenditures.33 An analysis of 10-year outcomes34 found that speed training was associated with a 29% reduction in risk of developing of dementia, while the other 2 interventions were not. However, despite these multiple areas of benefit, there was no evidence that new functional skills were acquired as a result of the training.26-34
Functional skills training
While there is a long history of using functional skills training to help patients with schizophrenia, for healthy older people, there are considerably more challenges. First, aging is not a disease. Consequently, functional skills training is typically not covered by health insurance. Second, functional skills training delivered by a human trainer can be expensive and is not readily available. Finally, there are no real curricula for training functional skills, particularly those that are device-based (phone, tablet, or computer).
Recently, researchers have developed a functional skills assessment and training program that was originally piloted as a fixed difficulty simulation as described in 2 studies by Czaja et al.2,3 The original assessment was used to compare healthy control individuals with people with mild cognitive impairment (MCI) or schizophrenia. Most recently, training modules for 6 different technology-based functional tasks have been developed and piloted in samples of healthy controls and patients with MCI in a randomized trial.35 Half of the participants in each of the 2 groups were randomized to receive speed training similar to the ACTIVE study, and the other half received skills training alone. All participants were trained for 24 sessions over 12 weeks or until they mastered all 6 simulations.
Both patients with MCI and healthy controls improved in all 6 simulations. Although patients with MCI were considerably less efficient at baseline, their training gains per session were equivalent to that of healthy controls. Finally, concurrent cognitive training increased the efficiency of skills training. At the end of the study, functional gains were the same for people in both groups randomized to either condition, even though individuals in the combined cognitive and skills training interventions received only half as much skills training time.
Continue to: What to tell patients
What to tell patients
Older patients might ask their clinicians what they can do to “exercise their brain.” Let them know that CCT has been shown to improve cognitive performance in healthy older people, and that there are several evidence-based, commercially available products for this purpose. Two such self-administrable systems with supportive data are BrainHQ (www.brainhq.com) and Happy Neuron (www.happy-neuron.com). Explain that it is likely that the best strategy is a combination of cognitive and functional skills training. One commercially available functional skills training program with supportive data is i-Function (www.i-Function.com). (Editor’s note: One of the authors, PDH, is an employee of i-Function, Inc.)
Bottom Line
Clinicians should ensure older patients that they have the cognitive capacity to learn new technology-related functional skills, and that such patients have the opportunity to learn these skills. Clinicians need to be able to identify people who are at high risk of not being able to adhere to instructions and suggestions that require interactions with technology. Treatment options include computerized cognitive training and functional skills training.
Related Resources
- Hill NT, Mowszowski L, Naismith SL, et al. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and metaanalysis. Am J Psychiatry. 2017;174(4):329-340.
- Harvey PD, McGurk SR, Mahncke H, et al. Controversies in computerized cognitive training. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(11):907-915.
Technology is pervasive, and for many people, it is central to their daily activities. Younger people who have been exposed to technology for their entire lives take this for granted, but older individuals often have had much less experience with it. Many technological developments that are now a part of most people’s daily life, such as personal computers, cell phones, and automated teller machines (ATMs), have occurred in the past 4 decades, with the pace accelerating in the last 15 to 20 years.
Such changes have had a substantial impact on older adults who were never exposed to these technologies during their working life. For example, an 85-year-old person who retired at age 65 would probably have not been exposed to wireless internet prior to retirement. Therefore, all of the tasks that they are now required to complete online would have been performed in other ways. Banking, accessing instruction manuals for new devices, and even scheduling and confirming health care appointments and accessing medical records all now require individuals to have a level of technological skills that many older individuals find challenging. At times, this can limit their ability to complete routine daily activities, and also can have clinical implications (Table).
Fortunately, there are strategies clinicians can use to help their older patients face these challenges. In this article, we describe the cognitive domains associated with learning technological skills, how aging affects these domains, and what can be done to help older adults improve their technological skills.
Limited training on how to use new technology
Technological skills are similar to any other skills in one critical way: they need to be learned. At the same time, technological skills also differ from many other skills, such as playing a musical instrument, because of the constant updating of devices, programs, and applications. When smartphones or computers update their operating systems, the visual appearance of the screen and the way that tasks are performed also can change. Buttons can move and sequences of commands can be altered. Updates often happen with little or no notice, and users may need to navigate a completely different device landscape in order to perform tasks that they had previously mastered.
In addition, the creators/distributors of technology typically provide little training or documentation. Further, institutions such as banks or health care systems frequently do not provide any specific training for using their systems. For example, when patients are required to use technology to refill prescriptions, typically there is no training available on how the system operates.
Cognitive domains associated with technological skills
Because there are minimal opportunities to receive training in how to use most aspects of technology, users have to be able to learn by exposure and experience. This requires several different cognitive abilities to work together. In a recent review, Harvey1 described cognition and cognitive assessment in the general population, with a focus on cognitive domains. Here we discuss several of these domains in terms of the relationship to real-world functional tasks and discuss their importance for mastering technology.
Reasoning and problem solving. Because most technological devices and applications are designed to be “intuitive,” the user needs to be able to adopt a sequential approach to learning the task. For example, using the internet to refill a prescription requires several steps:
- accessing the internet
- finding the pharmacy web site
- establishing a user ID and password
- navigating the web site to the prescriptions section
- identifying the correct prescription
- requesting the refill
- selecting the pickup date and time.
Continue to: After navigating these steps...
After navigating these steps, an individual still needs other cognitive abilities to refill other prescriptions later. However, executive functioning is also critical for maintaining organization across different technological demands. For example, web sites have different password rules and require frequent changes without re-using old passwords, so it becomes critical to maintain an organized list of web site addresses and their passwords.
Refilling a prescription with a telephone voice menu also requires a series of steps. Typically, this process is simpler than an internet refill, because no log-in information is necessary. However, it still requires a structured series of tasks.
Working memory refers to the ability to hold information in consciousness long enough to operate on it. At each step of the navigation process, the user needs to remember which steps he/she has already completed, because repeating steps can slow down the process or lead to error messages. Thus, remembering which steps have been completed is as critical for performing tasks as is correctly understanding the anticipated sequence of steps. Further, when a password is forgotten, the user needs to remember the newly provided password.
Working memory can be spatial as well. For example, most web sites do not display a password while it is being entered, which eliminates spatial working memory from the equation. Thus, the ability to remember which characters have been entered and which still need to be entered is necessary.
Episodic memory is the process of learning and retaining newly presented verbal or spatial information as well as recalling it later for adaptive use. After successfully using a new technology, it is critical to be able to remember what to do the next time it is used. This includes both recalling how to access the technology (including the web address, user ID, and password), recalling the steps needed to be performed and their sequence, and recognizing the buttons and instructions presented onscreen.
Continue to: Procedural memory
Procedural memory is memory for motor acts and sequences. For instance, remembering how to ride a bicycle is a procedural memory, as is the ability to perform motor acts in sequence, such as peeling, cutting, and cooking vegetables. Interestingly, procedural memory can be spared in individuals with major challenges in episodic memory, such as those with amnestic conditions or cortical dementia. Thus, it may be possible for people to continue to perform technology-based skills despite declines in episodic memory. Many current technological functional tasks have fixed sequences of events that, if remembered, can lead to increased efficiency and higher chances of success in performance of functional tasks.
Prospective memory is the ability to remember to perform tasks in the future. This can include event-related tasks (eg, enter your password before trying to make a hotel reservation on a web site) or time-related tasks (eg, refill your prescriptions next Friday). Technology can actually facilitate prospective memory by providing reminders to individuals, such as alarms for appointments. However, prospective memory is required to initially set up such alarms, and setting up confusing or incorrect alarms can impede task performance.
Processing speed is the ability to perform cognitively demanding tasks under time constraints. Traditional processing speed tasks include coding and sorting tasks, which require processing new information and effort for relatively short periods of time. In our research, we discovered that processing speed measured with traditional tests was strongly correlated with the time required to perform functional tasks such as an ATM banking task.2,3 This correlation makes sense in terms of the fact that many real-world functional tasks with technology often have a series of sequential demands that must be accomplished before progression to the next task.
Manual dexterity is also important for using technology. Many electronic devices have small, touch screen-based keyboards. Being able to touch the correct key requires dexterity and can be made more difficult by age-related vision changes, a tremor, or reduced sensation in extremities.
Cognitive changes and aging
It is normal for certain cognitive abilities to change with aging. There are a set of cognitive skills that are generally stable from early adulthood until the early “senescent” period. Some of these skills decline normatively after age 60 to 65, or earlier in some individuals. These include processing new information, solving new problems, and learning and remembering information. Referred to as “fluid intelligence,” these abilities show age-related decline during healthy aging, and even greater decline in individuals with age-related cognitive conditions.
Continue to: On the other hand...
On the other hand, some cognitive abilities do not decline with aging. These include previously acquired knowledge, such as vocabulary and mathematics skills, as well as factual information, such as academic information and the faces of familiar people. These are referred to as “crystallized intelligence,” and there is limited evidence that they decline with age. In fact, these abilities do not decline until the moderately severe stage of cortical dementias, and are commonly used to index premorbid cognitive functioning and cognitive reserve.
Why is this distinction between fluid intelligence and crystallized intelligence important? As noted above, many older people do not have early-life experience with technology. Thus, their crystallized intelligence, which is not as vulnerable to decline with aging, does not include information about how to perform many technological tasks. In contrast to today’s adolescents and young adults, older adults’ academic history typically does not include using smartphones, doing homework via Google Docs, or having homework and classwork assigned via the internet.
Learning how to use new technology requires fluid intelligence, and these abilities are less efficient in older adults. So for many older people, technological tasks can be complex and unfamiliar, and the skills needed to learn how to perform them are also more limited, even in comparison to older adults’ own ability when younger. Because many technology-based activities require concurrent performance of multiple tasks, older adults are at a disadvantage.4 It is not surprising, therefore, that a subset of older adults rate their technology skills as weak, and technology-based tasks as challenging or anxiety-provoking.
However, studies show most older adults’ attitudes toward technology remain largely positive, and that they are capable of attaining the necessary skills to use information and communication technology.4,5 An individual’s perception of his/her age, age-related beliefs, and self-efficacy are associated not only with attitudes toward technology, but possibly with cognition itself.6
Education level and socioeconomic factors also influence a person’s ability to become proficient in using technology.7-9 In fact, socioeconomic factors are more strongly related to access to the internet than age. Many older adults have internet access, but this access does not always translate into full use of its services.
Continue to: The Box...
The Box10-22 describes some of the effects of aging on the brain, and how these changes are reflected in cognitive abilities.
Box
The global baseline intensity of human brain activity, determined by indirectly measuring blood oxygenation, decreases with age.10 Multiple domains of fluid cognition decline with age; these cognitive abilities include processing speed,11,12 working memory,11 episodic memory,11 and executive function.11 Expected neuroanatomic changes of aging include a decrease in cerebral grey matter volume as well as decreased white matter integrity, which is associated with diminished executive function and impaired working memory.13 Processing speed is associated with increased white matter microstructure during neurodevelopment.14 Diminished processing speed in older adults also may predict increased mortality risk.15 Individuals with advanced age may have augmented difficulty with episodic memory, especially when they are required to integrate information from more than one source.11 Diminished hippocampal volume13 and reduced activity of the middle frontal gyrus are associated with age-related decline in episodic memory retrieval.10 Working memory16 is known to share a neurocircuitry overlap with attention processes.17 Working memory capacity also is closely associated with other cognitive functions, such as shifting and inhibition.10 Enhanced cerebellar activity is related to working memory; increased cerebellar activity is likely due to compensatory recruitment of neurons due to reduced activity in the superior frontal gyrus.10 The superior frontal gyrus contributes to both working memory as well as executive processing.10
Although the cognitive decline associated with aging is inevitable, individuals who experience cognitive decline at an increased rate are predisposed to worse outcomes. One longitudinal cohort study found that adults in their 8th and 9th decades of life with preserved cognitive function had a lower risk of disability and death.18
On the other hand, crystallized cognitive functions such as semantic memory,13 shortterm memory,13 and emotion regulation16 remain largely intact throughout the aging process. Semantic memory, a subtype of episodic memory, is related to associated facts or interpretations of previous occurrences.19 This type of memory is detached from an individual’s personal experience.20 Semantic memory loss classically presents with anomia and detectable lesions in the anterior and temporal lobes.20 Emotion regulation deficits are not a part of normal aging; in fact, emotional well-being is known to either improve or remain consistent with age.21 Emotional experiences in patients of advanced age may be more complex and unique in comparison to other cognitive abilities.22
The role of cognitive training
Existing interventions for helping older adults improve their technology proficiency generally focus on improving cognition, and not necessarily on addressing skills learning. Skills learning and cognition are related; however, the brain depends on neural plasticity for skills learning, whereas cognitive declines are a result of gradual and functional worsening of memory, processing speed, executive functioning, and attention.23 Interventions such as cognitive strategy training are capable of altering brain neurocircuitry to improve attention and memory.10,11 Other interventions known to improve cognition include exercise10 and processing speed training.24 On the other hand, skills learning is more effectively targeted by interventions that focus on stimulating realistic environments to mimic activities of daily living that involve technology.
Studies have consistently demonstrated cognitive improvements associated with computerized cognitive training (CCT). The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study was designed to evaluate the efficacy of cognitive training in 2,832 healthy adults age >65 across 6 recruitment sites in the United States.25 Participants were randomized to a control group (no treatment) or to 1 of 3 treatment groups:
- memory strategy training (instructor-led, not computerized)
- reasoning training (instructor-led, not computerized)
- speed training (no instructor, adaptive computerized training).
Each treatment group received 10 sessions of classroom-based training (1 hour each, twice per week for 5 weeks). Following the intervention, participants who had completed ≥8 sessions were randomized to receive 4 booster sessions at 11 and 35 months after the initial training, or no booster sessions.
Each cognitive training program significantly improved performance on within-domain cognitive tests relative to the control group. Effect sizes were large immediately following training; they declined over time, but were still significant at 10-year follow-up. As hypothesized, training effects did not generalize to neuropsychological tests in other training domains. The booster subgroup of speed training showed improved performance on a separate functional speed measure at 2-year26 and 5-year follow-up.27 Each condition showed slower decline in instrumental activities of daily living relative to the control group.
Continue to: The Figure...
The Figure shows the type of stimuli presented in the speed training, a procedure where individuals are taught high-speed multitasking by having to identify and locate visual information quickly in a divided-attention format. A stimulus appears in the center of the screen—either a car or a truck—and at the same time, a “Route 66” sign appears in the periphery. For every successful response, the next stimulus is presented at a shorter duration after every successful response, and more slowly after errors.

Secondary outcome analyses demonstrated that for older adults, speed training reduced rates of driving cessation,27 improved driving habits, and lowered the incidence of at-fault crashes28 (based on motor vehicle records). Speed training also resulted in improvements in health-related quality of life,29,30 depression,31 locus of control,32 and medical expenditures.33 An analysis of 10-year outcomes34 found that speed training was associated with a 29% reduction in risk of developing of dementia, while the other 2 interventions were not. However, despite these multiple areas of benefit, there was no evidence that new functional skills were acquired as a result of the training.26-34
Functional skills training
While there is a long history of using functional skills training to help patients with schizophrenia, for healthy older people, there are considerably more challenges. First, aging is not a disease. Consequently, functional skills training is typically not covered by health insurance. Second, functional skills training delivered by a human trainer can be expensive and is not readily available. Finally, there are no real curricula for training functional skills, particularly those that are device-based (phone, tablet, or computer).
Recently, researchers have developed a functional skills assessment and training program that was originally piloted as a fixed difficulty simulation as described in 2 studies by Czaja et al.2,3 The original assessment was used to compare healthy control individuals with people with mild cognitive impairment (MCI) or schizophrenia. Most recently, training modules for 6 different technology-based functional tasks have been developed and piloted in samples of healthy controls and patients with MCI in a randomized trial.35 Half of the participants in each of the 2 groups were randomized to receive speed training similar to the ACTIVE study, and the other half received skills training alone. All participants were trained for 24 sessions over 12 weeks or until they mastered all 6 simulations.
Both patients with MCI and healthy controls improved in all 6 simulations. Although patients with MCI were considerably less efficient at baseline, their training gains per session were equivalent to that of healthy controls. Finally, concurrent cognitive training increased the efficiency of skills training. At the end of the study, functional gains were the same for people in both groups randomized to either condition, even though individuals in the combined cognitive and skills training interventions received only half as much skills training time.
Continue to: What to tell patients
What to tell patients
Older patients might ask their clinicians what they can do to “exercise their brain.” Let them know that CCT has been shown to improve cognitive performance in healthy older people, and that there are several evidence-based, commercially available products for this purpose. Two such self-administrable systems with supportive data are BrainHQ (www.brainhq.com) and Happy Neuron (www.happy-neuron.com). Explain that it is likely that the best strategy is a combination of cognitive and functional skills training. One commercially available functional skills training program with supportive data is i-Function (www.i-Function.com). (Editor’s note: One of the authors, PDH, is an employee of i-Function, Inc.)
Bottom Line
Clinicians should ensure older patients that they have the cognitive capacity to learn new technology-related functional skills, and that such patients have the opportunity to learn these skills. Clinicians need to be able to identify people who are at high risk of not being able to adhere to instructions and suggestions that require interactions with technology. Treatment options include computerized cognitive training and functional skills training.
Related Resources
- Hill NT, Mowszowski L, Naismith SL, et al. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and metaanalysis. Am J Psychiatry. 2017;174(4):329-340.
- Harvey PD, McGurk SR, Mahncke H, et al. Controversies in computerized cognitive training. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(11):907-915.
1. Harvey PD. Domains of cognition and their assessment. Dialogues Clin Neuro. 2019;21(3):227-237.
2. Czaja SJ, Loewenstein DA, Sabbag SA, et al. A novel method for direct assessment of everyday competence among older adults. J Alzheimers Dis. 2017;57(4):1229-1238.
3. Czaja SJ, Loewenstein DA, Lee CC, et al. Assessing functional performance using computer-based simulations of everyday activities. Schizophr Res. 2017;183:130-136.
4. Tsai HS, Shillair R, Cotten SR. Social support and “playing around”: an examination of how older adults acquire digital literacy with tablet computers. J Appl Gerontol. 2017;36(1):29-55.
5. Cabrita M, Tabak M, Vollenbroek-Hutten MM. Older adults’ attitudes toward ambulatory technology to support monitoring and coaching of healthy behaviors: qualitative study. JMIR Aging. 2019;2(1):e10476. doi: 10.2196/10476.
6. Lim KY, Chang KJ, Kim HJ, et al. P.5.a.010 association between memory age identity and cognition in the elderly. Eur Neuropsychopharmacol. 2010;20(suppl 3):S555.
7. Moraes C, Pinto JA Jr, Lopes MA, et al. Impact of sociodemographic and health variables on mini-mental state examination in a community-based sample of older people. Eur Arch Psychiatry Clin Neurosci. 2010;260(7):535-542.
8. Freitas S, Simões MR, Alves L, et al. The relevance of sociodemographic and health variables on MMSE normative data. Appl Neuropsychol Adult. 2015;22(4):311-319.
9. Han C, Jo SA, Jo I, et al. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study). Arch Gerontol Geriatr. 2008;47(3):302-310.
10. Yin S, Zhu X, Li R, et al. Intervention-induced enhancement in intrinsic brain activity in healthy older adults. Sci Rep. 2014;4:7309.
11. Bender AR, Prindle JJ, Brandmaier AM, et al. White matter and memory in healthy adults: coupled changes over two years. Neuroimage. 2016;131:193-204.
12. Guye S, von Bastian CC. Working memory training in older adults: Bayesian evidence supporting the absence of transfer. Psychol Aging. 2017;32(8):732-746.
13. Taki Y, Kinomura S, Sato K, et al. Correlation between gray/white matter volume and cognition in healthy elderly people. Brain Cogn. 2011;75(2):170-176.
14. Cassidy AR, White MT, DeMaso DR, et al. Processing speed, executive function, and academic achievement in children with dextro-transposition of the great arteries: Testing a longitudinal developmental cascade model. Neuropsychology. 2016;30(7):874-885.
15. Aichele S, Rabbitt P, Ghisletta P. Life span decrements in fluid intelligence and processing speed predict mortality risk. Psychol Aging. 2015;30(3):598-612.
16. Eich TS, Castel AD. The cognitive control of emotional versus value-based information in younger and older adults. Psychol Aging. 2016;31(5):503-512.
17. Rolle CE, Anguera JA, Skinner SN, et al. Enhancing spatial attention and working memory in younger and older adults. J Cogn Neurosci. 2017;29(9):1483-1497.
18. Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc. 2010;58(5):889-894.
19. Madore KP, Schacter DL. An episodic specificity induction enhances means-end problem solving in young and older adults. Psychol Aging. 2014;29(4):913-924.
20. Matthews BR. Memory dysfunction. Continuum (Minneap Minn). 2015;21(3 Behavioral Neurology and Neuropsychiatry):613-626.
21. Mather M. The emotion paradox in the aging brain. Ann N Y Acad Sci. 2012;1251(1):33-49.
22. Gurera JW, Isaacowitz DM. Emotion regulation and emotion perception in aging: A perspective on age-related differences and similarities. Prog Brain Res. 2019;247:329-351.
23. Cai L, Chan JS, Yan JH, et al. Brain plasticity and motor practice in cognitive aging. Front Aging Neurosci. 2014;6:31.
24. Cassetta BD, Tomfohr-Madsen LM, Goghari VM. A randomized controlled trial of working memory and processing speed training in schizophrenia. Psychol Med. 2019;49(12):2009-2019.
25. Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271-2281.
26. Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16-24.
27. Edwards JD, Delahunt PB, Mahncke HW. Cognitive speed of processing training delays driving cessation. J Gerontol A Biol Sci Med Sci. 2009;64(12):1262-1267.
28. Ball K, Edwards JD, Ross LA, et al. Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2010;58(11):2107-2113.
29. Wolinsky FD, Unverzagt FW, Smith DM, et al. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J Gerontol B Psychol Sci Soc Sci. 2006;61(5):S281-S287.
30. Wolinsky FD, Unverzagt FW, Smith DM, et al. The ACTIVE cognitive training trial and health-related quality of life: protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61(12):1324-1329.
31. Wolinsky FD, Vander Weg MW, Martin R, et al. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci. 2009;64(4):468-472.
32. Wolinsky FD, Vander Weg MW, Martin R, et al. Does cognitive training improve internal locus of control among older adults? J Gerontol B Psychol Sci Soc Sci. 2010;65(5):591-598.
33. Wolinsky FD, Mahncke HW, Kosinski M, et al. The ACTIVE cognitive training trial and predicted medical expenditures. BMC Health Serv Res. 2009;9:109.
34. Edwards JD, Xu H, Clark DO, et al. Speed of processing training results in lower risk of dementia. Alzheimers Dement (N Y). 2017;3(4):603-611.
35. Harvey PD, Tibiriçá L, Kallestrup P, et al. A computerized functional skills assessment and training program targeting technology based everyday functional skills. J Vis Exp. 2020;156:e60330. doi: 10.3791/60330.
1. Harvey PD. Domains of cognition and their assessment. Dialogues Clin Neuro. 2019;21(3):227-237.
2. Czaja SJ, Loewenstein DA, Sabbag SA, et al. A novel method for direct assessment of everyday competence among older adults. J Alzheimers Dis. 2017;57(4):1229-1238.
3. Czaja SJ, Loewenstein DA, Lee CC, et al. Assessing functional performance using computer-based simulations of everyday activities. Schizophr Res. 2017;183:130-136.
4. Tsai HS, Shillair R, Cotten SR. Social support and “playing around”: an examination of how older adults acquire digital literacy with tablet computers. J Appl Gerontol. 2017;36(1):29-55.
5. Cabrita M, Tabak M, Vollenbroek-Hutten MM. Older adults’ attitudes toward ambulatory technology to support monitoring and coaching of healthy behaviors: qualitative study. JMIR Aging. 2019;2(1):e10476. doi: 10.2196/10476.
6. Lim KY, Chang KJ, Kim HJ, et al. P.5.a.010 association between memory age identity and cognition in the elderly. Eur Neuropsychopharmacol. 2010;20(suppl 3):S555.
7. Moraes C, Pinto JA Jr, Lopes MA, et al. Impact of sociodemographic and health variables on mini-mental state examination in a community-based sample of older people. Eur Arch Psychiatry Clin Neurosci. 2010;260(7):535-542.
8. Freitas S, Simões MR, Alves L, et al. The relevance of sociodemographic and health variables on MMSE normative data. Appl Neuropsychol Adult. 2015;22(4):311-319.
9. Han C, Jo SA, Jo I, et al. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study). Arch Gerontol Geriatr. 2008;47(3):302-310.
10. Yin S, Zhu X, Li R, et al. Intervention-induced enhancement in intrinsic brain activity in healthy older adults. Sci Rep. 2014;4:7309.
11. Bender AR, Prindle JJ, Brandmaier AM, et al. White matter and memory in healthy adults: coupled changes over two years. Neuroimage. 2016;131:193-204.
12. Guye S, von Bastian CC. Working memory training in older adults: Bayesian evidence supporting the absence of transfer. Psychol Aging. 2017;32(8):732-746.
13. Taki Y, Kinomura S, Sato K, et al. Correlation between gray/white matter volume and cognition in healthy elderly people. Brain Cogn. 2011;75(2):170-176.
14. Cassidy AR, White MT, DeMaso DR, et al. Processing speed, executive function, and academic achievement in children with dextro-transposition of the great arteries: Testing a longitudinal developmental cascade model. Neuropsychology. 2016;30(7):874-885.
15. Aichele S, Rabbitt P, Ghisletta P. Life span decrements in fluid intelligence and processing speed predict mortality risk. Psychol Aging. 2015;30(3):598-612.
16. Eich TS, Castel AD. The cognitive control of emotional versus value-based information in younger and older adults. Psychol Aging. 2016;31(5):503-512.
17. Rolle CE, Anguera JA, Skinner SN, et al. Enhancing spatial attention and working memory in younger and older adults. J Cogn Neurosci. 2017;29(9):1483-1497.
18. Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc. 2010;58(5):889-894.
19. Madore KP, Schacter DL. An episodic specificity induction enhances means-end problem solving in young and older adults. Psychol Aging. 2014;29(4):913-924.
20. Matthews BR. Memory dysfunction. Continuum (Minneap Minn). 2015;21(3 Behavioral Neurology and Neuropsychiatry):613-626.
21. Mather M. The emotion paradox in the aging brain. Ann N Y Acad Sci. 2012;1251(1):33-49.
22. Gurera JW, Isaacowitz DM. Emotion regulation and emotion perception in aging: A perspective on age-related differences and similarities. Prog Brain Res. 2019;247:329-351.
23. Cai L, Chan JS, Yan JH, et al. Brain plasticity and motor practice in cognitive aging. Front Aging Neurosci. 2014;6:31.
24. Cassetta BD, Tomfohr-Madsen LM, Goghari VM. A randomized controlled trial of working memory and processing speed training in schizophrenia. Psychol Med. 2019;49(12):2009-2019.
25. Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271-2281.
26. Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16-24.
27. Edwards JD, Delahunt PB, Mahncke HW. Cognitive speed of processing training delays driving cessation. J Gerontol A Biol Sci Med Sci. 2009;64(12):1262-1267.
28. Ball K, Edwards JD, Ross LA, et al. Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2010;58(11):2107-2113.
29. Wolinsky FD, Unverzagt FW, Smith DM, et al. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J Gerontol B Psychol Sci Soc Sci. 2006;61(5):S281-S287.
30. Wolinsky FD, Unverzagt FW, Smith DM, et al. The ACTIVE cognitive training trial and health-related quality of life: protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61(12):1324-1329.
31. Wolinsky FD, Vander Weg MW, Martin R, et al. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci. 2009;64(4):468-472.
32. Wolinsky FD, Vander Weg MW, Martin R, et al. Does cognitive training improve internal locus of control among older adults? J Gerontol B Psychol Sci Soc Sci. 2010;65(5):591-598.
33. Wolinsky FD, Mahncke HW, Kosinski M, et al. The ACTIVE cognitive training trial and predicted medical expenditures. BMC Health Serv Res. 2009;9:109.
34. Edwards JD, Xu H, Clark DO, et al. Speed of processing training results in lower risk of dementia. Alzheimers Dement (N Y). 2017;3(4):603-611.
35. Harvey PD, Tibiriçá L, Kallestrup P, et al. A computerized functional skills assessment and training program targeting technology based everyday functional skills. J Vis Exp. 2020;156:e60330. doi: 10.3791/60330.
COVID-19 and patients with serious mental illness
“This whole thing is not about heroism. It’s about decency. It may seem a ridiculous idea, but the only way to fight the plague is with decency . ”
– Albert Camus, La Peste (1947)1
Severe acute respiratory syndrome (SARS), H1N1 swine flu, Ebola, Zika, and Middle East respiratory syndrome (MERS): the 21st century has already been witness to several serious infectious outbreaks and pandemics,2 but none has been as deadly and consequential as the current one. The ongoing SARS-coronavirus-2 (SARS-CoV-2) pandemic is shaping not only current psychiatric care but the future of psychiatry. Now that we are beyond the initial stages of the coronavirus disease 2019 (COVID-19) pandemic, when psychiatrists had a crash course in disaster psychiatry, our attention must shift to rebuilding and managing disillusionment and other psychological fallout of the intense early days.3
In this article, we offer guidance to psychiatrists caring for patients with serious mental illness (SMI) during the SARS-CoV-2 pandemic. Patients with SMI are easily forgotten as other issues (eg, preserving ICU capacity) overshadow the already historically neglected needs of this impoverished group.4 From both human and public-health perspectives, this inattention is a mistake. Assuring psychiatric stability is critically important to prevent the spread of COVID-19 in marginalized communities comprised of individuals who are poor, members of racial minorities, and others who already experience health disparities.5 Without controlling transmission in these groups, the pandemic will not be sufficiently contained.
We begin by highlighting general principles of pandemic management because caring for patients with SMI does not occur in a vacuum. Infectious outbreaks require not only helping those who need direct medical care because they are infected, but also managing populations that are at risk of getting infected, including health care and other essential workers.
Principles of pandemic management
Delivery of medical care during a pandemic differs from routine care. An effective disaster response requires collaboration and coordination among public-health, treatment, and emergency systems. Many institutions shift to an incident management system and crisis leadership, with clear lines of authority to coordinate responders and build medical surge capacity. Such a top-down leadership approach must plan and allow for the emergence of other credible leaders and for the restoration of people’s agency.
Unfortunately, adaptive capacity may be limited, especially in the public sector and psychiatric care system, where resources are already poor. Particularly early in a pandemic, services considered non-essential—which includes most psychiatric outpatient care—can become unavailable. A major effort is needed to prevent the psychiatric care system from contracting further, as happened during 9/11.6 Additionally, “essential” cannot be conflated with “emergent,” as can easily occur in extreme circumstances. Early and sustained efforts are required to ensure that patients with SMI who may be teetering on the edge of emergency status do not slip off that edge, especially when the emergency medical system is operating over capacity.
A comprehensive outbreak response must consider that a pandemic is not only a medical crisis but a mental health crisis and a communication emergency.7 Mental health clinicians need to provide accurate information and help patients cope with their fears.
Continue to: Psychological aspects of pandemics
Psychological aspects of pandemics. Previous infectious outbreaks have reaffirmed that mental health plays an outsized role during epidemics. Chaos, uncertainty, fear of death, and loss of income and housing cause prolonged stress and exact a psychological toll.
Adverse psychological impacts include expectable, normal reactions such as stress-induced anxiety or insomnia. In addition, new-onset psychiatric illnesses or exacerbations of existing ones may emerge.8 As disillusionment and demoralization appear in the wake of the acute phase, with persistently high unemployment, suicide prevention becomes an important goal.9
Pandemics lead to expectable behavioral responses (eg, increases in substance use and interpersonal conflict). Fear-based decisions may result in unhelpful behavior, such as hoarding medications (which may result in shortages) or dangerous, unsupervised use of unproven medications (eg, hydroxychloroquine). Trust is needed to accept public-health measures, and recommendations (eg, wearing masks) must be culturally informed to be credible and effective.
Because people are affected differently, at individual, cultural, and socioeconomic levels, they will view the situation differently. For many people, secondary stressors (eg, job loss) may be more disastrous than the primary medical event (ie, the pandemic). This distinction is critical because concrete financial help, not psychiatric care, is needed. Sometimes, even when a psychiatric disorder such as SMI or major neurocognitive disorder is present, the illusion of an acute decompensation can be created by the loss of social and structural supports that previously scaffolded a person’s life.
Mental illness prevention. Community mental-health surveillance is important to monitor for distress, psychiatric symptoms, health-risk behaviors, risk and safety perception, and preparedness. Clinicians must be ready to normalize expectable and temporary distress, while recognizing when that distress becomes pathological. This may be difficult in patients with SMI who often already have reduced stress tolerance or problem-based coping skills.10
Continue to: Psychological first aid...
Psychological first aid (PFA) is a standard intervention recommended by the World Health Organization for most individuals following a disaster; it is evidence-informed and has face validity.11 Intended to relieve distress by creating an environment that is safe, calm, and connected, PFA fosters self-efficacy and hope. While PFA is a form of universal prevention, it is not designed for patients with SMI, is not a psychiatric intervention, and is not provided by clinicians. Its principles, however, can easily be applied to patients with SMI to prevent distressing symptoms from becoming a relapse.
Communication. Good risk and crisis communication are critical because individual and population behavior will be governed by the perception of risk and fear, and not by facts. Failure to manage the “infodemic”7—with its misinformation, contradictory messages, and rumors—jeopardizes infection control if patients become paralyzed by uncertainty and fear. Scapegoating occurs easily during times of threat, and society must contain the parallel epidemic of xenophobia based on stigma and misinformation.12
Decision-making under uncertainty is not perfect and subject to revision as better information becomes available. Pointing this out to the public is delicate but essential to curtail skepticism and mistrust when policies are adjusted in response to new circumstances and knowledge.
Mistrust of an authority’s legitimacy and fear-based decisions lead to lack of cooperation with public-health measures, which can undermine an effective response to the pandemic. Travel restrictions or quarantine measures will not be followed if individuals question their importance. Like the general public, patients need education and clear communication to address their fear of contagion, dangers posed to family (and pets), and mistrust of authority and government. A lack of appreciation of the seriousness of the pandemic and individual responsibility may need to be addressed. Two important measures to accomplish this are steering patients to reputable sources of information and advising that they limit media exposure.
Resilience-building. Community and workplace resilience are important aspects of making it through a disaster as best as possible. Resilience is not innate and fixed; it must be deliberately built.13 Choosing an attitude of post-traumatic growth over the victim narrative is a helpful stance. Practicing self-care (rest, nutrition, exercise) and self-compassion (self-kindness, common humanity, mindfulness) is good advice for patients and caregivers alike.
Continue to: Workforce protection
Workforce protection. Compared to other disasters, infectious outbreaks disproportionally affect the medical community, and care delivery is at stake. While psychological and psychiatric needs may increase during a pandemic, services often contract, day programs and clinics close, teams are reduced to skeleton crews, and only emergency psychiatric care is available. Workforce protection is critical to avoid illness or simple absenteeism due to mistrust of protective measures.
Only a well-briefed, well-led, well-supported, and adequately resourced workforce is going to be effective in managing this public-health emergency. Burnout and moral injury are feared long-term consequences for health care workers that need to be proactively addressed.14 As opposed to other forms of disasters, managing your own fears about safety is important. Clinicians and their patients sit in the proverbial same boat.
Ethics. The anticipated need to ration life-saving care (eg, ventilators) has been at the forefront of ethical concerns.15 In psychiatry, the question of involuntary public-health interventions for uncooperative psychiatric patients sits uncomfortably between public-health ethics and human rights, and is an opportunity for collaboration with public-health and infectious-disease colleagues.
Redeployed clinicians and those working under substandard conditions may be concerned about civil liability due to a modified standard of care during a crisis. Some clinicians may ask if their duty to care must override their natural instinct to protect themselves. There is a lot of room for resentment in these circumstances. Redeployed or otherwise “conscripted” clinicians may resent administrators, especially those administering from the safety of their homes. Those “left behind” to work in potentially precarious circumstances may resent their absent colleagues. Moreover, these front-line clinicians may have been forced to make ethical decisions for which they were not prepared.16 Maintaining morale is far from trivial, not just during the pandemic, but afterward, when (and if) the entire workforce is reunited. All parties need to be mindful of how their actions and decisions impact and are perceived by others, both in the hospital and at home.
Managing patients with SMI during COVID-19
Patients with SMI are potentially hard hit by COVID-19 due to a “tragic” epidemiologic triad of agent-host-environment: SARS-CoV-2 is a highly infectious agent affecting patients with SMI who are vulnerable hosts in permissive environments (Figure).
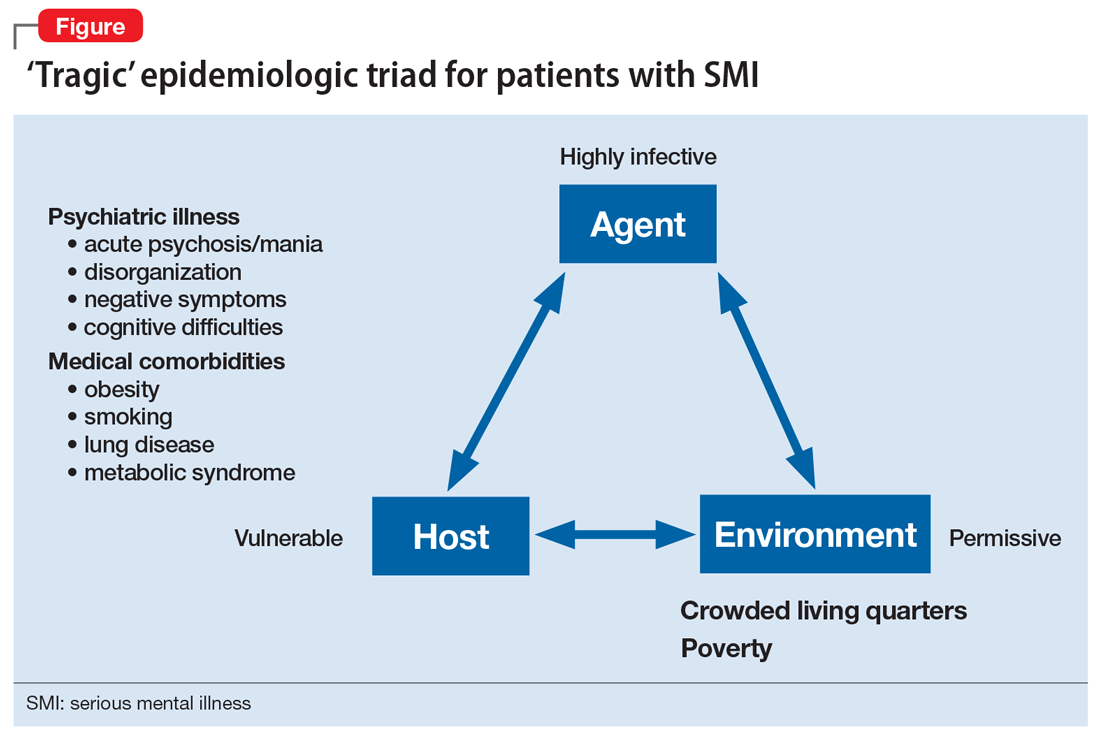
Continue to: While not as infectious as measles...
While not as infectious as measles, COVID-19 is more infectious than the seasonal flu virus.17 It can lead to uncontrolled infection within a short period of time, particularly in enclosed settings. Outbreaks have occurred readily on cruise ships and aircraft carriers as well as in nursing homes, homeless shelters, prisons, and group homes.
Patients with SMI are vulnerable hosts because they have many of the medical risk factors18 that portend a poor prognosis if they become infected, including pre-existing lung conditions and heart disease19 as well as diabetes and obesity.20 Obesity likely creates a hyperinflammatory state and a decrease in vital capacity. Patient-related behavioral factors include poor early-symptom reporting and ineffective infection control.
Unfavorable social determinants of health include not only poverty but crowded housing that is a perfect incubator for COVID-19.
Priority treatment goals. The overarching goal during a pandemic is to keep patients with SMI in psychiatric treatment and prevent them from disengaging from care in the service of infection control. Urgent tasks include infection control, relapse prevention, and preventing treatment disengagement and loneliness.
Infection control. As trusted sources of information, psychiatrists can play an important role in infection control in several important ways:
- educating patients about infection-control measures and public-health recommendations
- helping patients understand what testing can accomplish and when to pursue it
- encouraging protective health behaviors (eg, hand washing, mask wearing, physical distancing)
- assessing patients’ risk appreciation
- assessing for and addressing obstacles to implementing and complying with infection-control measures
- explaining contact tracing
- providing reassurance.
Continue to: Materials and explanations...
Materials and explanations must be adapted for patient understanding.
Patients with disorganization or cognitive disturbances may have difficulties cooperating or problem-solving. Patients with negative symptoms may be inappropriately unconcerned and also inaccurately report symptoms that suggest COVID-19. Acute psychosis or mania can prevent patients from complying with public-health efforts. Some measures may be difficult to implement if the means are simply not there (eg, physical distancing in a crowded apartment). Previously open settings (eg, group homes) have had to develop new mechanisms under the primacy of infection control. Inpatient units—traditionally places where community, shared healing, and group therapy are prized—have had to decrease maximum occupancy, limit the number of patients attending groups, and discourage or outrightly prohibit social interaction (eg, dining together).
Relapse prevention. Patients who take maintenance medications need to be supported. A manic or psychotic relapse during a pandemic puts patients at risk of acquiring and spreading COVID-19. “Treatment as prevention” is a slogan from human immunodeficiency virus (HIV) care that captures the importance of antiretroviral treatment to prevent medical complications from HIV, and also to reduce infecting other people. By analogy, psychiatric treatment for patients with SMI can prevent psychiatric instability and thereby control viral transmission. Avoiding sending psychiatric patients to a potentially stressed acute-care system is important.
Psychosocial support. Clinics need to ensure that patients continue to engage in care beyond medication-taking to proactively prevent psychiatric exacerbations. Healthful, resilience-building behaviors should be encouraged while monitoring and counseling against maladaptive ones (eg, increased substance use). Supporting patients emotionally and helping them solve problems are critical, particularly for those who are subjected to quarantine or isolation. Obviously, in these latter situations, outreach will be necessary and may require creative delivery systems and dedicated clinicians for patients who lack access to the technology necessary for virtual visits. Havens and Ghaemi21 have suggested that a good therapeutic alliance can be viewed as a mood stabilizer. Helping patients grieve losses (loved ones, jobs, sense of safety) may be an important part of support.
Even before COVID-19, loneliness was a major factor for patients with schizophrenia.22 A psychiatric clinic is one aspect of a person with SMI’s social network; during the initial phase of the pandemic, many clinics and treatment programs closed. Patients for whom clinics structure and anchor their activities are at high risk of disconnecting from treatment, staying at home, and becoming lonely.
Continue to: Caregivers are always important...
Caregivers are always important to SMI patients, but they may assume an even bigger role during this pandemic. Some patients may have moved in with a relative, after years of living on their own. In other cases, stable caregiver relationships may be disrupted due to COVID-19–related sickness in the caregiver; if not addressed, this can result in a patient’s clinical decompensation. Clinicians should take the opportunity to understand who a patient’s caregivers are (group home staff, families) and rekindle clinical contact with them. Relationships with caregivers that may have been on “autopilot” during normal times are opportunities for welcome support and guidance, to the benefit of both patients and caregivers.
Table 1 summarizes clinical tasks that need to be kept in mind when conducting clinic visits during COVID-19 in order to achieve the high-priority treatment goals of infection control, relapse prevention, and psychosocial support.
Differential diagnosis. Neuropsychiatric syndromes have long been observed in influenza pandemics,23 due both to direct viral effects and to the effects of critical illness on the brain. Two core symptoms of COVID-19—anosmia and ageusia—suggest that COVID-19 can directly affect the brain. While neurologic manifestations are common,24 it remains unclear to what extent COVID-19 can directly “cause” psychiatric symptoms, or if such symptoms are the result of cytokines25 or other medical processes (eg, thromboembolism).26 Psychosis due to COVID-19 may, in some cases, represent a stress-related brief psychotic disorder.27
Hospitalized patients who have recovered from COVID-19 may have experienced prolonged sedation and severe delirium in an ICU.28 Complications such as posttraumatic stress disorder,29 hypoperfusion-related brain injuries, or other long-term cognitive difficulties may result. In previous flu epidemics, patients developed serious neurologic complications such as post-encephalitic Parkinson’s disease.30
Any person subjected to isolation or quarantine is at risk for psychiatric complications.31 Patients with SMI who live in group homes may be particularly susceptible to new rules, including no-visitor policies.
Continue to: Outpatients whose primary disorder...
Outpatients whose primary disorder is well controlled may, like anyone else, struggle with the effects of the pandemic. It is necessary to carefully differentiate non-specific symptoms associated with stress from the emergence of a new disorder resulting from stress.32 For some patients, grief or adjustment disorders should be considered. Prolonged stress and uncertainty may eventually lead to an exacerbation of a primary disorder, particularly if the situation (eg, financial loss) does not improve or worsens. Demoralization and suicidal thinking need to be monitored. Relapse or increased use of alcohol or other substances as a response to stress may also complicate the clinical picture.33 Last, smoking cessation as a major treatment goal in general should be re-emphasized and not ignored during the ongoing pandemic.34
Table 2 summarizes psychiatric symptoms that need to be considered when managing a patient with SMI during this pandemic.
Treatment tools
Psychopharmacology. Even though crisis-mode prescribing may be necessary, the safe use of psychotropics remains the goal of psychiatric prescribing. Access to medications becomes a larger consideration; for many patients, a 90-day supply may be indicated. Review of polypharmacy, including for pneumonia risk, should be undertaken. Preventing drooling (eg, from sedation, clozapine, extrapyramidal symptoms [EPS]) will decrease aspiration risk.
In general, treatment of psychiatric symptoms in a patient with COVID-19 follows usual guidelines. The best treatment for COVID-19 patients with delirium, however, remains to be established, particularly how to manage severe agitation.28 Pharmacodynamic and pharmacokinetic drug–drug interactions between psychotropics and antiviral treatments for COVID-19 (eg, QTc prolongation) can be expected and need to be reviewed.35 For stress-related anxiety, judicious pharmacotherapy can be helpful. Diazepam given at the earliest signs of a psychotic relapse may stave off a relapse for patients with schizophrenia.36 Even if permitted under relaxed prescribing rules during a public-health emergency, prescribing controlled substances without seeing patients in person requires additional thought. In some cases, adjusting the primary medication to buffer against stress may be preferred (eg, adjusting an antipsychotic in a patient on maintenance treatment for schizophrenia, particularly if a low-dose strategy is pursued).
Clozapine requires registry-based prescribing and bloodwork (“no blood, no drug”). The use of clozapine during this public-health emergency has been made easier because of FDA guidance that allows clozapine to be dispensed without blood work if obtaining blood work is not possible (eg, a patient is quarantined) or can be accomplished only at substantial risk to patients and the population at large. Under certain conditions, clozapine can be dispensed safely and in a way that is consistent with infection prevention. Clozapine-treated patients admitted with COVID-19 should be monitored for clozapine toxicity and the clozapine dose adjusted.37 A consensus statement consistent with the FDA and clinical considerations for using clozapine during COVID-19 is summarized in Table 3.38
Continue to: Long-acting injectable antipsychotics...
Long-acting injectable antipsychotics (LAIs) pose a problem because they require in-person visits. Ideally, during a pandemic, patients should be seen in person as frequently as medically necessary but as infrequently as possible to limit exposure of both patients and staff. Table 4 provides some clinical recommendations on how to use LAIs during the pandemic.39
Supportive psychotherapy may be the most important tool we have in helping patients with loss and uncertainty during these challenging months.40 Simply staying in contact with patients plays a major role in preventing care discontinuity. Even routine interactions have become stressful, with everyone wearing a mask that partially obscures the face. People with impaired hearing may find it even more difficult to understand you.
Education, problem-solving, and a directive, encouraging style are major tools of supportive psychotherapy to reduce symptoms and increase adaptive skills. Clarify that social distancing refers to physical, not emotional, distancing. The judicious and temporary use of anxiolytics is appropriate to reduce anxiety. Concrete help and problem-solving (eg, filling out forms) are examples of proactive crisis intervention.
Telepsychiatry emerged in the pandemic’s early days as the default mode of practice in order to limit in-person contacts.41 Like all new technology, telepsychiatry brings progress and peril.42 While it has gone surprisingly well for most, the “digital divide” does not afford all patients access to the needed technology. The long-term effectiveness and acceptance of telehealth remain to be seen. (Editor’s Note: For more about this topic, see “Telepsychiatry: What you need to know.”
Lessons learned and outlook
Infectious outbreaks have historically inflicted long-term disruptions on societies and altered the course of history. However, each disaster is unique, and lessons from previous disasters may only partially apply.43 We do not yet know how this one will end, including how long it will take for the world’s economies to recover. If nothing else, the current public-health emergency has brought to the forefront what psychiatrists have always known: health disparities are partially responsible for different disease risks (in this case, the risk of getting infected with SARS-CoV-2).5 It may not be a coincidence that the Black Lives Matter movement is becoming a major impetus for social change at a time when the pandemic is exposing health-care inequalities.
Continue to: Some areas of the country...
Some areas of the country succeeded in reducing infections and limiting community spread, which ushered in an uneasy sense of normalcy even while the pandemic continues. At least for now, these locales can focus on rebuilding and preparing for expectable fluctuations in disease activity, including the arrival of the annual flu season on top of COVID-19.44 Recovery is not a return to the status quo ante but building stronger communities—“building back better.”45 Unless there is a continuum of care, shortcomings in one sector will have ripple effects through the entire system, particularly for psychiatric care for patients with SMI, which was inadequate before the pandemic.
Ensuring access to critical care was a priority during the pandemic’s early phase but came at the price of deferring other types of care, such as routine primary care; the coming months will see the downstream consequences of this approach,46 including for patients with SMI.
In the meantime, doing our job as clinicians, as Camus’s fictitious Dr. Bernard Rieux from the epigraph responds when asked how to define decency, may be the best we can do in these times. This includes contributing to and molding our field’s future and fostering a sense of agency in our patients and in ourselves. Major goals will be to preserve lessons learned, maintain flexibility, and avoid a return to unhelpful overregulation and payment models that do not reflect the flexible, person-centered care so important for patients with SMI.47
Bottom Line
During a pandemic, patients with serious mental illness may be easily forgotten as other issues overshadow the needs of this impoverished group. During a pandemic, the priority treatment goals for these patients are infection control, relapse prevention, and preventing treatment disengagement and loneliness. A pandemic requires changes in how patients with serious mental illness will receive psychopharmacology and psychotherapy.
Related Resources
- Huremović D (ed). Psychiatry of pandemics: a mental health response to infection outbreak. Cham, Switzerland: Springer Nature Switzerland AG; 2019.
- Ursano RJ, Fullerton CS, Weisaeth L, et al (eds). Textbook of disaster psychiatry. 2nd ed. Cambridge, UK: Cambridge University Press; 2017.
- Centers for Disease Control and Prevention. Coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- American Psychiatric Association. Coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus.
- SMI Adviser. Make informed decisions related to COVID-19 and mental health. https://smiadviser.org/about/covid.
Drug Brand Names
Clozapine • Clozaril
Diazepam • Valium
Hydroxychloroquine • Plaquenil
1. Camus A. La peste. Paris, France: Éditions Gallimard; 1947.
2. Huremovic
3. Substance Abuse and Mental Health Services Administration. Phases of disaster. https://www.samhsa.gov/dtac/recovering-disasters/phases-disaster. Updated June 17, 2020. Accessed August 7, 2020.
4. Geller J. COVID-19 and advocacy—the good and the unacceptable. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5b13. Published May 7, 2020. Accessed August 7, 2020.
5. Webb Hooper M, Nápoles AM, Perez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466-2467.
6. Sederer LI, Lanzara CB, Essock SM, et al. Lessons learned from the New York State mental health response to the September 11, 2001, attacks. Psychiatr Serv. 2011;62(9):1085-1089.
7. World Health Organization. Infodemic management – infodemiology. https://www.who.int/teams/risk-communication/infodemic-management. Accessed August 7, 2020.
8. Zhou J, Liu L, Xue P, et al. Mental health response to the COVID-19 outbreak in China. Am J Psychiatry. 2020;117(7):574-575.
9. Kawohl W, Nordt C. COVID-19, unemployment, and suicide. Lancet Psychiatry. 2020;7(5):389-390.
10. Yao H, Chen JH, Xu YF. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry. 2020;7(4):e21. doi: 10.1016/S2215-0366(20)30090-0.
11. Minihan E, Gavin B, Kelly BD, et al. Covid-19, mental health and psychological first aid. Ir J Psychol Med. 2020:1-12.
12. Adja KYC, Golinelli D, Lenzi J, et al. Pandemics and social stigma: who’s next? Italy’s experience with COVID-19. Public Health. 2020;185:39-41.
13. Rosenberg AR. Cultivating deliberate resilience during the coronavirus disease 2019 pandemic [published online April 14, 2020]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2020.1436.
14. Dean W, Talbot SG, Caplan A. Clarifying the language of clinician distress [published online January 31, 2020]. JAMA. doi: 10.1001/jama.2019.21576.
15. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055.
16. Rosenbaum L. Facing Covid-19 in Italy - ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875.
17. Viceconte G, Petrosillo N. COVID-19 R0: magic number or conundrum? Infect Dis Rep. 2020;12(1):8516.
18. de Hert M, Schreurs V, Vancampfort D, van Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15-22.
19. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97-105.
20. Finer N, Garnett SP, Bruun JM. COVID-19 and obesity. Clin Obes. 2020;10(3):e12365. doi: 10.1111/cob.12365.
21. Havens LL, Ghaemi SN. Existential despair and bipolar disorder: the therapeutic alliance as a mood stabilizer. Am J Psychother. 2005;59(2):137-147.
22. Trémeau F, Antonius D, Malaspina D, et al. Loneliness in schizophrenia and its possible correlates. An exploratory study. Psychiatry Res. 2016;246:211-217.
23. Menninger KA. Psychoses associated with influenza: I. General data: statistical analysis. JAMA. 1919;72(4):235-241.
24. Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832.
25. Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.012.
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39.
27. Martin Jr. EB. Brief psychotic disorder triggered by fear of coronavirus? Psychiatric Times. https://www.psychiatrictimes.com/view/brief-psychotic-disorder-triggered-fear-coronavirus-small-case-series. Published May 8, 2020. Accessed August 7, 2020.
28. Sher Y, Rabkin B, Maldonado JR, et al. COVID-19-associated hyperactive intensive care unit delirium with proposed pathophysiology and treatment: a case report [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.007.
29. Wolters AE, Peelen LM, Welling MC, et al. Long-term mental health problems after delirium in the ICU. Crit Care Med. 2016;44(10):1808-1813.
30. Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis. 2008;6(3):114-124.
31. Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
32. Maercker A, Brewin CR, Bryant RA, et al. Diagnosis and classification of disorders specifically associated with stress: proposals for ICD-11. World Psychiatry. 2013;12(3):198-206.
33. Ornell F, Moura HF, Scherer JN, et al. The COVID-19 pandemic and its impact on substance use: implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi: 10.1016/j.psychres.2020.113096.
34. Berlin I, Thomas D, Le Faou AL, Cornuz J. COVID-19 and smoking [published online April 3, 2020]. Nicotine Tob Res. https://doi.org/10.1093/ntr/ntaa059.
35. Back D, Marzolini C, Hodge C, et al. COVID-19 treatment in patients with comorbidities: awareness of drug-drug interactions [published online May 8, 2020]. Br J Clin Pharmacol. doi: 10.1111/bcp.14358.
36. Carpenter WT Jr., Buchanan RW, Kirkpatrick B, et al. Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry. 1999;156(2):299-303.
37. Dotson S, Hartvigsen N, Wesner T, et al. Clozapine toxicity in the setting of COVID-19 [published online May 30, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.025.
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223.
39. Schnitzer K, MacLaurin S, Freudenreich O. Long-acting injectable antipsychotics during the COVID-19 pandemic. Current Psychiatry. In press.
40. Winston A, Rosenthal RN, Pinsker H. Learning supportive psychotherapy: an illustrated guide. Washington, DC: American Psychiatric Publishing; 2012.
41. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
42. Jordan A, Dixon LB. Considerations for telepsychiatry service implementation in the era of COVID-19. Psychiatr Serv. 2020;71(6):643-644.
43. DePierro J, Lowe S, Katz C. Lessons learned from 9/11: mental health perspectives on the COVID-19 pandemic. Psychiatry Res. 2020;288:113024.
44. Hussain S. Immunization and vaccination. In: Huremovic
45. Epping-Jordan JE, van Ommeren M, Ashour HN, et al. Beyond the crisis: building back better mental health care in 10 emergency-affected areas using a longer-term perspective. Int J Ment Health Syst. 2015;9:15.
46. Rosenbaum L. The untold toll - the pandemic’s effects on patients without Covid-19. N Engl J Med. 2020;382(24):2368-2371.
47. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness [published online June 3, 2020]. Psychiatr Serv. doi: 10.1176/appi.ps.202000244.
“This whole thing is not about heroism. It’s about decency. It may seem a ridiculous idea, but the only way to fight the plague is with decency . ”
– Albert Camus, La Peste (1947)1
Severe acute respiratory syndrome (SARS), H1N1 swine flu, Ebola, Zika, and Middle East respiratory syndrome (MERS): the 21st century has already been witness to several serious infectious outbreaks and pandemics,2 but none has been as deadly and consequential as the current one. The ongoing SARS-coronavirus-2 (SARS-CoV-2) pandemic is shaping not only current psychiatric care but the future of psychiatry. Now that we are beyond the initial stages of the coronavirus disease 2019 (COVID-19) pandemic, when psychiatrists had a crash course in disaster psychiatry, our attention must shift to rebuilding and managing disillusionment and other psychological fallout of the intense early days.3
In this article, we offer guidance to psychiatrists caring for patients with serious mental illness (SMI) during the SARS-CoV-2 pandemic. Patients with SMI are easily forgotten as other issues (eg, preserving ICU capacity) overshadow the already historically neglected needs of this impoverished group.4 From both human and public-health perspectives, this inattention is a mistake. Assuring psychiatric stability is critically important to prevent the spread of COVID-19 in marginalized communities comprised of individuals who are poor, members of racial minorities, and others who already experience health disparities.5 Without controlling transmission in these groups, the pandemic will not be sufficiently contained.
We begin by highlighting general principles of pandemic management because caring for patients with SMI does not occur in a vacuum. Infectious outbreaks require not only helping those who need direct medical care because they are infected, but also managing populations that are at risk of getting infected, including health care and other essential workers.
Principles of pandemic management
Delivery of medical care during a pandemic differs from routine care. An effective disaster response requires collaboration and coordination among public-health, treatment, and emergency systems. Many institutions shift to an incident management system and crisis leadership, with clear lines of authority to coordinate responders and build medical surge capacity. Such a top-down leadership approach must plan and allow for the emergence of other credible leaders and for the restoration of people’s agency.
Unfortunately, adaptive capacity may be limited, especially in the public sector and psychiatric care system, where resources are already poor. Particularly early in a pandemic, services considered non-essential—which includes most psychiatric outpatient care—can become unavailable. A major effort is needed to prevent the psychiatric care system from contracting further, as happened during 9/11.6 Additionally, “essential” cannot be conflated with “emergent,” as can easily occur in extreme circumstances. Early and sustained efforts are required to ensure that patients with SMI who may be teetering on the edge of emergency status do not slip off that edge, especially when the emergency medical system is operating over capacity.
A comprehensive outbreak response must consider that a pandemic is not only a medical crisis but a mental health crisis and a communication emergency.7 Mental health clinicians need to provide accurate information and help patients cope with their fears.
Continue to: Psychological aspects of pandemics
Psychological aspects of pandemics. Previous infectious outbreaks have reaffirmed that mental health plays an outsized role during epidemics. Chaos, uncertainty, fear of death, and loss of income and housing cause prolonged stress and exact a psychological toll.
Adverse psychological impacts include expectable, normal reactions such as stress-induced anxiety or insomnia. In addition, new-onset psychiatric illnesses or exacerbations of existing ones may emerge.8 As disillusionment and demoralization appear in the wake of the acute phase, with persistently high unemployment, suicide prevention becomes an important goal.9
Pandemics lead to expectable behavioral responses (eg, increases in substance use and interpersonal conflict). Fear-based decisions may result in unhelpful behavior, such as hoarding medications (which may result in shortages) or dangerous, unsupervised use of unproven medications (eg, hydroxychloroquine). Trust is needed to accept public-health measures, and recommendations (eg, wearing masks) must be culturally informed to be credible and effective.
Because people are affected differently, at individual, cultural, and socioeconomic levels, they will view the situation differently. For many people, secondary stressors (eg, job loss) may be more disastrous than the primary medical event (ie, the pandemic). This distinction is critical because concrete financial help, not psychiatric care, is needed. Sometimes, even when a psychiatric disorder such as SMI or major neurocognitive disorder is present, the illusion of an acute decompensation can be created by the loss of social and structural supports that previously scaffolded a person’s life.
Mental illness prevention. Community mental-health surveillance is important to monitor for distress, psychiatric symptoms, health-risk behaviors, risk and safety perception, and preparedness. Clinicians must be ready to normalize expectable and temporary distress, while recognizing when that distress becomes pathological. This may be difficult in patients with SMI who often already have reduced stress tolerance or problem-based coping skills.10
Continue to: Psychological first aid...
Psychological first aid (PFA) is a standard intervention recommended by the World Health Organization for most individuals following a disaster; it is evidence-informed and has face validity.11 Intended to relieve distress by creating an environment that is safe, calm, and connected, PFA fosters self-efficacy and hope. While PFA is a form of universal prevention, it is not designed for patients with SMI, is not a psychiatric intervention, and is not provided by clinicians. Its principles, however, can easily be applied to patients with SMI to prevent distressing symptoms from becoming a relapse.
Communication. Good risk and crisis communication are critical because individual and population behavior will be governed by the perception of risk and fear, and not by facts. Failure to manage the “infodemic”7—with its misinformation, contradictory messages, and rumors—jeopardizes infection control if patients become paralyzed by uncertainty and fear. Scapegoating occurs easily during times of threat, and society must contain the parallel epidemic of xenophobia based on stigma and misinformation.12
Decision-making under uncertainty is not perfect and subject to revision as better information becomes available. Pointing this out to the public is delicate but essential to curtail skepticism and mistrust when policies are adjusted in response to new circumstances and knowledge.
Mistrust of an authority’s legitimacy and fear-based decisions lead to lack of cooperation with public-health measures, which can undermine an effective response to the pandemic. Travel restrictions or quarantine measures will not be followed if individuals question their importance. Like the general public, patients need education and clear communication to address their fear of contagion, dangers posed to family (and pets), and mistrust of authority and government. A lack of appreciation of the seriousness of the pandemic and individual responsibility may need to be addressed. Two important measures to accomplish this are steering patients to reputable sources of information and advising that they limit media exposure.
Resilience-building. Community and workplace resilience are important aspects of making it through a disaster as best as possible. Resilience is not innate and fixed; it must be deliberately built.13 Choosing an attitude of post-traumatic growth over the victim narrative is a helpful stance. Practicing self-care (rest, nutrition, exercise) and self-compassion (self-kindness, common humanity, mindfulness) is good advice for patients and caregivers alike.
Continue to: Workforce protection
Workforce protection. Compared to other disasters, infectious outbreaks disproportionally affect the medical community, and care delivery is at stake. While psychological and psychiatric needs may increase during a pandemic, services often contract, day programs and clinics close, teams are reduced to skeleton crews, and only emergency psychiatric care is available. Workforce protection is critical to avoid illness or simple absenteeism due to mistrust of protective measures.
Only a well-briefed, well-led, well-supported, and adequately resourced workforce is going to be effective in managing this public-health emergency. Burnout and moral injury are feared long-term consequences for health care workers that need to be proactively addressed.14 As opposed to other forms of disasters, managing your own fears about safety is important. Clinicians and their patients sit in the proverbial same boat.
Ethics. The anticipated need to ration life-saving care (eg, ventilators) has been at the forefront of ethical concerns.15 In psychiatry, the question of involuntary public-health interventions for uncooperative psychiatric patients sits uncomfortably between public-health ethics and human rights, and is an opportunity for collaboration with public-health and infectious-disease colleagues.
Redeployed clinicians and those working under substandard conditions may be concerned about civil liability due to a modified standard of care during a crisis. Some clinicians may ask if their duty to care must override their natural instinct to protect themselves. There is a lot of room for resentment in these circumstances. Redeployed or otherwise “conscripted” clinicians may resent administrators, especially those administering from the safety of their homes. Those “left behind” to work in potentially precarious circumstances may resent their absent colleagues. Moreover, these front-line clinicians may have been forced to make ethical decisions for which they were not prepared.16 Maintaining morale is far from trivial, not just during the pandemic, but afterward, when (and if) the entire workforce is reunited. All parties need to be mindful of how their actions and decisions impact and are perceived by others, both in the hospital and at home.
Managing patients with SMI during COVID-19
Patients with SMI are potentially hard hit by COVID-19 due to a “tragic” epidemiologic triad of agent-host-environment: SARS-CoV-2 is a highly infectious agent affecting patients with SMI who are vulnerable hosts in permissive environments (Figure).

Continue to: While not as infectious as measles...
While not as infectious as measles, COVID-19 is more infectious than the seasonal flu virus.17 It can lead to uncontrolled infection within a short period of time, particularly in enclosed settings. Outbreaks have occurred readily on cruise ships and aircraft carriers as well as in nursing homes, homeless shelters, prisons, and group homes.
Patients with SMI are vulnerable hosts because they have many of the medical risk factors18 that portend a poor prognosis if they become infected, including pre-existing lung conditions and heart disease19 as well as diabetes and obesity.20 Obesity likely creates a hyperinflammatory state and a decrease in vital capacity. Patient-related behavioral factors include poor early-symptom reporting and ineffective infection control.
Unfavorable social determinants of health include not only poverty but crowded housing that is a perfect incubator for COVID-19.
Priority treatment goals. The overarching goal during a pandemic is to keep patients with SMI in psychiatric treatment and prevent them from disengaging from care in the service of infection control. Urgent tasks include infection control, relapse prevention, and preventing treatment disengagement and loneliness.
Infection control. As trusted sources of information, psychiatrists can play an important role in infection control in several important ways:
- educating patients about infection-control measures and public-health recommendations
- helping patients understand what testing can accomplish and when to pursue it
- encouraging protective health behaviors (eg, hand washing, mask wearing, physical distancing)
- assessing patients’ risk appreciation
- assessing for and addressing obstacles to implementing and complying with infection-control measures
- explaining contact tracing
- providing reassurance.
Continue to: Materials and explanations...
Materials and explanations must be adapted for patient understanding.
Patients with disorganization or cognitive disturbances may have difficulties cooperating or problem-solving. Patients with negative symptoms may be inappropriately unconcerned and also inaccurately report symptoms that suggest COVID-19. Acute psychosis or mania can prevent patients from complying with public-health efforts. Some measures may be difficult to implement if the means are simply not there (eg, physical distancing in a crowded apartment). Previously open settings (eg, group homes) have had to develop new mechanisms under the primacy of infection control. Inpatient units—traditionally places where community, shared healing, and group therapy are prized—have had to decrease maximum occupancy, limit the number of patients attending groups, and discourage or outrightly prohibit social interaction (eg, dining together).
Relapse prevention. Patients who take maintenance medications need to be supported. A manic or psychotic relapse during a pandemic puts patients at risk of acquiring and spreading COVID-19. “Treatment as prevention” is a slogan from human immunodeficiency virus (HIV) care that captures the importance of antiretroviral treatment to prevent medical complications from HIV, and also to reduce infecting other people. By analogy, psychiatric treatment for patients with SMI can prevent psychiatric instability and thereby control viral transmission. Avoiding sending psychiatric patients to a potentially stressed acute-care system is important.
Psychosocial support. Clinics need to ensure that patients continue to engage in care beyond medication-taking to proactively prevent psychiatric exacerbations. Healthful, resilience-building behaviors should be encouraged while monitoring and counseling against maladaptive ones (eg, increased substance use). Supporting patients emotionally and helping them solve problems are critical, particularly for those who are subjected to quarantine or isolation. Obviously, in these latter situations, outreach will be necessary and may require creative delivery systems and dedicated clinicians for patients who lack access to the technology necessary for virtual visits. Havens and Ghaemi21 have suggested that a good therapeutic alliance can be viewed as a mood stabilizer. Helping patients grieve losses (loved ones, jobs, sense of safety) may be an important part of support.
Even before COVID-19, loneliness was a major factor for patients with schizophrenia.22 A psychiatric clinic is one aspect of a person with SMI’s social network; during the initial phase of the pandemic, many clinics and treatment programs closed. Patients for whom clinics structure and anchor their activities are at high risk of disconnecting from treatment, staying at home, and becoming lonely.
Continue to: Caregivers are always important...
Caregivers are always important to SMI patients, but they may assume an even bigger role during this pandemic. Some patients may have moved in with a relative, after years of living on their own. In other cases, stable caregiver relationships may be disrupted due to COVID-19–related sickness in the caregiver; if not addressed, this can result in a patient’s clinical decompensation. Clinicians should take the opportunity to understand who a patient’s caregivers are (group home staff, families) and rekindle clinical contact with them. Relationships with caregivers that may have been on “autopilot” during normal times are opportunities for welcome support and guidance, to the benefit of both patients and caregivers.
Table 1 summarizes clinical tasks that need to be kept in mind when conducting clinic visits during COVID-19 in order to achieve the high-priority treatment goals of infection control, relapse prevention, and psychosocial support.
Differential diagnosis. Neuropsychiatric syndromes have long been observed in influenza pandemics,23 due both to direct viral effects and to the effects of critical illness on the brain. Two core symptoms of COVID-19—anosmia and ageusia—suggest that COVID-19 can directly affect the brain. While neurologic manifestations are common,24 it remains unclear to what extent COVID-19 can directly “cause” psychiatric symptoms, or if such symptoms are the result of cytokines25 or other medical processes (eg, thromboembolism).26 Psychosis due to COVID-19 may, in some cases, represent a stress-related brief psychotic disorder.27
Hospitalized patients who have recovered from COVID-19 may have experienced prolonged sedation and severe delirium in an ICU.28 Complications such as posttraumatic stress disorder,29 hypoperfusion-related brain injuries, or other long-term cognitive difficulties may result. In previous flu epidemics, patients developed serious neurologic complications such as post-encephalitic Parkinson’s disease.30
Any person subjected to isolation or quarantine is at risk for psychiatric complications.31 Patients with SMI who live in group homes may be particularly susceptible to new rules, including no-visitor policies.
Continue to: Outpatients whose primary disorder...
Outpatients whose primary disorder is well controlled may, like anyone else, struggle with the effects of the pandemic. It is necessary to carefully differentiate non-specific symptoms associated with stress from the emergence of a new disorder resulting from stress.32 For some patients, grief or adjustment disorders should be considered. Prolonged stress and uncertainty may eventually lead to an exacerbation of a primary disorder, particularly if the situation (eg, financial loss) does not improve or worsens. Demoralization and suicidal thinking need to be monitored. Relapse or increased use of alcohol or other substances as a response to stress may also complicate the clinical picture.33 Last, smoking cessation as a major treatment goal in general should be re-emphasized and not ignored during the ongoing pandemic.34
Table 2 summarizes psychiatric symptoms that need to be considered when managing a patient with SMI during this pandemic.
Treatment tools
Psychopharmacology. Even though crisis-mode prescribing may be necessary, the safe use of psychotropics remains the goal of psychiatric prescribing. Access to medications becomes a larger consideration; for many patients, a 90-day supply may be indicated. Review of polypharmacy, including for pneumonia risk, should be undertaken. Preventing drooling (eg, from sedation, clozapine, extrapyramidal symptoms [EPS]) will decrease aspiration risk.
In general, treatment of psychiatric symptoms in a patient with COVID-19 follows usual guidelines. The best treatment for COVID-19 patients with delirium, however, remains to be established, particularly how to manage severe agitation.28 Pharmacodynamic and pharmacokinetic drug–drug interactions between psychotropics and antiviral treatments for COVID-19 (eg, QTc prolongation) can be expected and need to be reviewed.35 For stress-related anxiety, judicious pharmacotherapy can be helpful. Diazepam given at the earliest signs of a psychotic relapse may stave off a relapse for patients with schizophrenia.36 Even if permitted under relaxed prescribing rules during a public-health emergency, prescribing controlled substances without seeing patients in person requires additional thought. In some cases, adjusting the primary medication to buffer against stress may be preferred (eg, adjusting an antipsychotic in a patient on maintenance treatment for schizophrenia, particularly if a low-dose strategy is pursued).
Clozapine requires registry-based prescribing and bloodwork (“no blood, no drug”). The use of clozapine during this public-health emergency has been made easier because of FDA guidance that allows clozapine to be dispensed without blood work if obtaining blood work is not possible (eg, a patient is quarantined) or can be accomplished only at substantial risk to patients and the population at large. Under certain conditions, clozapine can be dispensed safely and in a way that is consistent with infection prevention. Clozapine-treated patients admitted with COVID-19 should be monitored for clozapine toxicity and the clozapine dose adjusted.37 A consensus statement consistent with the FDA and clinical considerations for using clozapine during COVID-19 is summarized in Table 3.38
Continue to: Long-acting injectable antipsychotics...
Long-acting injectable antipsychotics (LAIs) pose a problem because they require in-person visits. Ideally, during a pandemic, patients should be seen in person as frequently as medically necessary but as infrequently as possible to limit exposure of both patients and staff. Table 4 provides some clinical recommendations on how to use LAIs during the pandemic.39
Supportive psychotherapy may be the most important tool we have in helping patients with loss and uncertainty during these challenging months.40 Simply staying in contact with patients plays a major role in preventing care discontinuity. Even routine interactions have become stressful, with everyone wearing a mask that partially obscures the face. People with impaired hearing may find it even more difficult to understand you.
Education, problem-solving, and a directive, encouraging style are major tools of supportive psychotherapy to reduce symptoms and increase adaptive skills. Clarify that social distancing refers to physical, not emotional, distancing. The judicious and temporary use of anxiolytics is appropriate to reduce anxiety. Concrete help and problem-solving (eg, filling out forms) are examples of proactive crisis intervention.
Telepsychiatry emerged in the pandemic’s early days as the default mode of practice in order to limit in-person contacts.41 Like all new technology, telepsychiatry brings progress and peril.42 While it has gone surprisingly well for most, the “digital divide” does not afford all patients access to the needed technology. The long-term effectiveness and acceptance of telehealth remain to be seen. (Editor’s Note: For more about this topic, see “Telepsychiatry: What you need to know.”
Lessons learned and outlook
Infectious outbreaks have historically inflicted long-term disruptions on societies and altered the course of history. However, each disaster is unique, and lessons from previous disasters may only partially apply.43 We do not yet know how this one will end, including how long it will take for the world’s economies to recover. If nothing else, the current public-health emergency has brought to the forefront what psychiatrists have always known: health disparities are partially responsible for different disease risks (in this case, the risk of getting infected with SARS-CoV-2).5 It may not be a coincidence that the Black Lives Matter movement is becoming a major impetus for social change at a time when the pandemic is exposing health-care inequalities.
Continue to: Some areas of the country...
Some areas of the country succeeded in reducing infections and limiting community spread, which ushered in an uneasy sense of normalcy even while the pandemic continues. At least for now, these locales can focus on rebuilding and preparing for expectable fluctuations in disease activity, including the arrival of the annual flu season on top of COVID-19.44 Recovery is not a return to the status quo ante but building stronger communities—“building back better.”45 Unless there is a continuum of care, shortcomings in one sector will have ripple effects through the entire system, particularly for psychiatric care for patients with SMI, which was inadequate before the pandemic.
Ensuring access to critical care was a priority during the pandemic’s early phase but came at the price of deferring other types of care, such as routine primary care; the coming months will see the downstream consequences of this approach,46 including for patients with SMI.
In the meantime, doing our job as clinicians, as Camus’s fictitious Dr. Bernard Rieux from the epigraph responds when asked how to define decency, may be the best we can do in these times. This includes contributing to and molding our field’s future and fostering a sense of agency in our patients and in ourselves. Major goals will be to preserve lessons learned, maintain flexibility, and avoid a return to unhelpful overregulation and payment models that do not reflect the flexible, person-centered care so important for patients with SMI.47
Bottom Line
During a pandemic, patients with serious mental illness may be easily forgotten as other issues overshadow the needs of this impoverished group. During a pandemic, the priority treatment goals for these patients are infection control, relapse prevention, and preventing treatment disengagement and loneliness. A pandemic requires changes in how patients with serious mental illness will receive psychopharmacology and psychotherapy.
Related Resources
- Huremović D (ed). Psychiatry of pandemics: a mental health response to infection outbreak. Cham, Switzerland: Springer Nature Switzerland AG; 2019.
- Ursano RJ, Fullerton CS, Weisaeth L, et al (eds). Textbook of disaster psychiatry. 2nd ed. Cambridge, UK: Cambridge University Press; 2017.
- Centers for Disease Control and Prevention. Coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- American Psychiatric Association. Coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus.
- SMI Adviser. Make informed decisions related to COVID-19 and mental health. https://smiadviser.org/about/covid.
Drug Brand Names
Clozapine • Clozaril
Diazepam • Valium
Hydroxychloroquine • Plaquenil
“This whole thing is not about heroism. It’s about decency. It may seem a ridiculous idea, but the only way to fight the plague is with decency . ”
– Albert Camus, La Peste (1947)1
Severe acute respiratory syndrome (SARS), H1N1 swine flu, Ebola, Zika, and Middle East respiratory syndrome (MERS): the 21st century has already been witness to several serious infectious outbreaks and pandemics,2 but none has been as deadly and consequential as the current one. The ongoing SARS-coronavirus-2 (SARS-CoV-2) pandemic is shaping not only current psychiatric care but the future of psychiatry. Now that we are beyond the initial stages of the coronavirus disease 2019 (COVID-19) pandemic, when psychiatrists had a crash course in disaster psychiatry, our attention must shift to rebuilding and managing disillusionment and other psychological fallout of the intense early days.3
In this article, we offer guidance to psychiatrists caring for patients with serious mental illness (SMI) during the SARS-CoV-2 pandemic. Patients with SMI are easily forgotten as other issues (eg, preserving ICU capacity) overshadow the already historically neglected needs of this impoverished group.4 From both human and public-health perspectives, this inattention is a mistake. Assuring psychiatric stability is critically important to prevent the spread of COVID-19 in marginalized communities comprised of individuals who are poor, members of racial minorities, and others who already experience health disparities.5 Without controlling transmission in these groups, the pandemic will not be sufficiently contained.
We begin by highlighting general principles of pandemic management because caring for patients with SMI does not occur in a vacuum. Infectious outbreaks require not only helping those who need direct medical care because they are infected, but also managing populations that are at risk of getting infected, including health care and other essential workers.
Principles of pandemic management
Delivery of medical care during a pandemic differs from routine care. An effective disaster response requires collaboration and coordination among public-health, treatment, and emergency systems. Many institutions shift to an incident management system and crisis leadership, with clear lines of authority to coordinate responders and build medical surge capacity. Such a top-down leadership approach must plan and allow for the emergence of other credible leaders and for the restoration of people’s agency.
Unfortunately, adaptive capacity may be limited, especially in the public sector and psychiatric care system, where resources are already poor. Particularly early in a pandemic, services considered non-essential—which includes most psychiatric outpatient care—can become unavailable. A major effort is needed to prevent the psychiatric care system from contracting further, as happened during 9/11.6 Additionally, “essential” cannot be conflated with “emergent,” as can easily occur in extreme circumstances. Early and sustained efforts are required to ensure that patients with SMI who may be teetering on the edge of emergency status do not slip off that edge, especially when the emergency medical system is operating over capacity.
A comprehensive outbreak response must consider that a pandemic is not only a medical crisis but a mental health crisis and a communication emergency.7 Mental health clinicians need to provide accurate information and help patients cope with their fears.
Continue to: Psychological aspects of pandemics
Psychological aspects of pandemics. Previous infectious outbreaks have reaffirmed that mental health plays an outsized role during epidemics. Chaos, uncertainty, fear of death, and loss of income and housing cause prolonged stress and exact a psychological toll.
Adverse psychological impacts include expectable, normal reactions such as stress-induced anxiety or insomnia. In addition, new-onset psychiatric illnesses or exacerbations of existing ones may emerge.8 As disillusionment and demoralization appear in the wake of the acute phase, with persistently high unemployment, suicide prevention becomes an important goal.9
Pandemics lead to expectable behavioral responses (eg, increases in substance use and interpersonal conflict). Fear-based decisions may result in unhelpful behavior, such as hoarding medications (which may result in shortages) or dangerous, unsupervised use of unproven medications (eg, hydroxychloroquine). Trust is needed to accept public-health measures, and recommendations (eg, wearing masks) must be culturally informed to be credible and effective.
Because people are affected differently, at individual, cultural, and socioeconomic levels, they will view the situation differently. For many people, secondary stressors (eg, job loss) may be more disastrous than the primary medical event (ie, the pandemic). This distinction is critical because concrete financial help, not psychiatric care, is needed. Sometimes, even when a psychiatric disorder such as SMI or major neurocognitive disorder is present, the illusion of an acute decompensation can be created by the loss of social and structural supports that previously scaffolded a person’s life.
Mental illness prevention. Community mental-health surveillance is important to monitor for distress, psychiatric symptoms, health-risk behaviors, risk and safety perception, and preparedness. Clinicians must be ready to normalize expectable and temporary distress, while recognizing when that distress becomes pathological. This may be difficult in patients with SMI who often already have reduced stress tolerance or problem-based coping skills.10
Continue to: Psychological first aid...
Psychological first aid (PFA) is a standard intervention recommended by the World Health Organization for most individuals following a disaster; it is evidence-informed and has face validity.11 Intended to relieve distress by creating an environment that is safe, calm, and connected, PFA fosters self-efficacy and hope. While PFA is a form of universal prevention, it is not designed for patients with SMI, is not a psychiatric intervention, and is not provided by clinicians. Its principles, however, can easily be applied to patients with SMI to prevent distressing symptoms from becoming a relapse.
Communication. Good risk and crisis communication are critical because individual and population behavior will be governed by the perception of risk and fear, and not by facts. Failure to manage the “infodemic”7—with its misinformation, contradictory messages, and rumors—jeopardizes infection control if patients become paralyzed by uncertainty and fear. Scapegoating occurs easily during times of threat, and society must contain the parallel epidemic of xenophobia based on stigma and misinformation.12
Decision-making under uncertainty is not perfect and subject to revision as better information becomes available. Pointing this out to the public is delicate but essential to curtail skepticism and mistrust when policies are adjusted in response to new circumstances and knowledge.
Mistrust of an authority’s legitimacy and fear-based decisions lead to lack of cooperation with public-health measures, which can undermine an effective response to the pandemic. Travel restrictions or quarantine measures will not be followed if individuals question their importance. Like the general public, patients need education and clear communication to address their fear of contagion, dangers posed to family (and pets), and mistrust of authority and government. A lack of appreciation of the seriousness of the pandemic and individual responsibility may need to be addressed. Two important measures to accomplish this are steering patients to reputable sources of information and advising that they limit media exposure.
Resilience-building. Community and workplace resilience are important aspects of making it through a disaster as best as possible. Resilience is not innate and fixed; it must be deliberately built.13 Choosing an attitude of post-traumatic growth over the victim narrative is a helpful stance. Practicing self-care (rest, nutrition, exercise) and self-compassion (self-kindness, common humanity, mindfulness) is good advice for patients and caregivers alike.
Continue to: Workforce protection
Workforce protection. Compared to other disasters, infectious outbreaks disproportionally affect the medical community, and care delivery is at stake. While psychological and psychiatric needs may increase during a pandemic, services often contract, day programs and clinics close, teams are reduced to skeleton crews, and only emergency psychiatric care is available. Workforce protection is critical to avoid illness or simple absenteeism due to mistrust of protective measures.
Only a well-briefed, well-led, well-supported, and adequately resourced workforce is going to be effective in managing this public-health emergency. Burnout and moral injury are feared long-term consequences for health care workers that need to be proactively addressed.14 As opposed to other forms of disasters, managing your own fears about safety is important. Clinicians and their patients sit in the proverbial same boat.
Ethics. The anticipated need to ration life-saving care (eg, ventilators) has been at the forefront of ethical concerns.15 In psychiatry, the question of involuntary public-health interventions for uncooperative psychiatric patients sits uncomfortably between public-health ethics and human rights, and is an opportunity for collaboration with public-health and infectious-disease colleagues.
Redeployed clinicians and those working under substandard conditions may be concerned about civil liability due to a modified standard of care during a crisis. Some clinicians may ask if their duty to care must override their natural instinct to protect themselves. There is a lot of room for resentment in these circumstances. Redeployed or otherwise “conscripted” clinicians may resent administrators, especially those administering from the safety of their homes. Those “left behind” to work in potentially precarious circumstances may resent their absent colleagues. Moreover, these front-line clinicians may have been forced to make ethical decisions for which they were not prepared.16 Maintaining morale is far from trivial, not just during the pandemic, but afterward, when (and if) the entire workforce is reunited. All parties need to be mindful of how their actions and decisions impact and are perceived by others, both in the hospital and at home.
Managing patients with SMI during COVID-19
Patients with SMI are potentially hard hit by COVID-19 due to a “tragic” epidemiologic triad of agent-host-environment: SARS-CoV-2 is a highly infectious agent affecting patients with SMI who are vulnerable hosts in permissive environments (Figure).

Continue to: While not as infectious as measles...
While not as infectious as measles, COVID-19 is more infectious than the seasonal flu virus.17 It can lead to uncontrolled infection within a short period of time, particularly in enclosed settings. Outbreaks have occurred readily on cruise ships and aircraft carriers as well as in nursing homes, homeless shelters, prisons, and group homes.
Patients with SMI are vulnerable hosts because they have many of the medical risk factors18 that portend a poor prognosis if they become infected, including pre-existing lung conditions and heart disease19 as well as diabetes and obesity.20 Obesity likely creates a hyperinflammatory state and a decrease in vital capacity. Patient-related behavioral factors include poor early-symptom reporting and ineffective infection control.
Unfavorable social determinants of health include not only poverty but crowded housing that is a perfect incubator for COVID-19.
Priority treatment goals. The overarching goal during a pandemic is to keep patients with SMI in psychiatric treatment and prevent them from disengaging from care in the service of infection control. Urgent tasks include infection control, relapse prevention, and preventing treatment disengagement and loneliness.
Infection control. As trusted sources of information, psychiatrists can play an important role in infection control in several important ways:
- educating patients about infection-control measures and public-health recommendations
- helping patients understand what testing can accomplish and when to pursue it
- encouraging protective health behaviors (eg, hand washing, mask wearing, physical distancing)
- assessing patients’ risk appreciation
- assessing for and addressing obstacles to implementing and complying with infection-control measures
- explaining contact tracing
- providing reassurance.
Continue to: Materials and explanations...
Materials and explanations must be adapted for patient understanding.
Patients with disorganization or cognitive disturbances may have difficulties cooperating or problem-solving. Patients with negative symptoms may be inappropriately unconcerned and also inaccurately report symptoms that suggest COVID-19. Acute psychosis or mania can prevent patients from complying with public-health efforts. Some measures may be difficult to implement if the means are simply not there (eg, physical distancing in a crowded apartment). Previously open settings (eg, group homes) have had to develop new mechanisms under the primacy of infection control. Inpatient units—traditionally places where community, shared healing, and group therapy are prized—have had to decrease maximum occupancy, limit the number of patients attending groups, and discourage or outrightly prohibit social interaction (eg, dining together).
Relapse prevention. Patients who take maintenance medications need to be supported. A manic or psychotic relapse during a pandemic puts patients at risk of acquiring and spreading COVID-19. “Treatment as prevention” is a slogan from human immunodeficiency virus (HIV) care that captures the importance of antiretroviral treatment to prevent medical complications from HIV, and also to reduce infecting other people. By analogy, psychiatric treatment for patients with SMI can prevent psychiatric instability and thereby control viral transmission. Avoiding sending psychiatric patients to a potentially stressed acute-care system is important.
Psychosocial support. Clinics need to ensure that patients continue to engage in care beyond medication-taking to proactively prevent psychiatric exacerbations. Healthful, resilience-building behaviors should be encouraged while monitoring and counseling against maladaptive ones (eg, increased substance use). Supporting patients emotionally and helping them solve problems are critical, particularly for those who are subjected to quarantine or isolation. Obviously, in these latter situations, outreach will be necessary and may require creative delivery systems and dedicated clinicians for patients who lack access to the technology necessary for virtual visits. Havens and Ghaemi21 have suggested that a good therapeutic alliance can be viewed as a mood stabilizer. Helping patients grieve losses (loved ones, jobs, sense of safety) may be an important part of support.
Even before COVID-19, loneliness was a major factor for patients with schizophrenia.22 A psychiatric clinic is one aspect of a person with SMI’s social network; during the initial phase of the pandemic, many clinics and treatment programs closed. Patients for whom clinics structure and anchor their activities are at high risk of disconnecting from treatment, staying at home, and becoming lonely.
Continue to: Caregivers are always important...
Caregivers are always important to SMI patients, but they may assume an even bigger role during this pandemic. Some patients may have moved in with a relative, after years of living on their own. In other cases, stable caregiver relationships may be disrupted due to COVID-19–related sickness in the caregiver; if not addressed, this can result in a patient’s clinical decompensation. Clinicians should take the opportunity to understand who a patient’s caregivers are (group home staff, families) and rekindle clinical contact with them. Relationships with caregivers that may have been on “autopilot” during normal times are opportunities for welcome support and guidance, to the benefit of both patients and caregivers.
Table 1 summarizes clinical tasks that need to be kept in mind when conducting clinic visits during COVID-19 in order to achieve the high-priority treatment goals of infection control, relapse prevention, and psychosocial support.
Differential diagnosis. Neuropsychiatric syndromes have long been observed in influenza pandemics,23 due both to direct viral effects and to the effects of critical illness on the brain. Two core symptoms of COVID-19—anosmia and ageusia—suggest that COVID-19 can directly affect the brain. While neurologic manifestations are common,24 it remains unclear to what extent COVID-19 can directly “cause” psychiatric symptoms, or if such symptoms are the result of cytokines25 or other medical processes (eg, thromboembolism).26 Psychosis due to COVID-19 may, in some cases, represent a stress-related brief psychotic disorder.27
Hospitalized patients who have recovered from COVID-19 may have experienced prolonged sedation and severe delirium in an ICU.28 Complications such as posttraumatic stress disorder,29 hypoperfusion-related brain injuries, or other long-term cognitive difficulties may result. In previous flu epidemics, patients developed serious neurologic complications such as post-encephalitic Parkinson’s disease.30
Any person subjected to isolation or quarantine is at risk for psychiatric complications.31 Patients with SMI who live in group homes may be particularly susceptible to new rules, including no-visitor policies.
Continue to: Outpatients whose primary disorder...
Outpatients whose primary disorder is well controlled may, like anyone else, struggle with the effects of the pandemic. It is necessary to carefully differentiate non-specific symptoms associated with stress from the emergence of a new disorder resulting from stress.32 For some patients, grief or adjustment disorders should be considered. Prolonged stress and uncertainty may eventually lead to an exacerbation of a primary disorder, particularly if the situation (eg, financial loss) does not improve or worsens. Demoralization and suicidal thinking need to be monitored. Relapse or increased use of alcohol or other substances as a response to stress may also complicate the clinical picture.33 Last, smoking cessation as a major treatment goal in general should be re-emphasized and not ignored during the ongoing pandemic.34
Table 2 summarizes psychiatric symptoms that need to be considered when managing a patient with SMI during this pandemic.
Treatment tools
Psychopharmacology. Even though crisis-mode prescribing may be necessary, the safe use of psychotropics remains the goal of psychiatric prescribing. Access to medications becomes a larger consideration; for many patients, a 90-day supply may be indicated. Review of polypharmacy, including for pneumonia risk, should be undertaken. Preventing drooling (eg, from sedation, clozapine, extrapyramidal symptoms [EPS]) will decrease aspiration risk.
In general, treatment of psychiatric symptoms in a patient with COVID-19 follows usual guidelines. The best treatment for COVID-19 patients with delirium, however, remains to be established, particularly how to manage severe agitation.28 Pharmacodynamic and pharmacokinetic drug–drug interactions between psychotropics and antiviral treatments for COVID-19 (eg, QTc prolongation) can be expected and need to be reviewed.35 For stress-related anxiety, judicious pharmacotherapy can be helpful. Diazepam given at the earliest signs of a psychotic relapse may stave off a relapse for patients with schizophrenia.36 Even if permitted under relaxed prescribing rules during a public-health emergency, prescribing controlled substances without seeing patients in person requires additional thought. In some cases, adjusting the primary medication to buffer against stress may be preferred (eg, adjusting an antipsychotic in a patient on maintenance treatment for schizophrenia, particularly if a low-dose strategy is pursued).
Clozapine requires registry-based prescribing and bloodwork (“no blood, no drug”). The use of clozapine during this public-health emergency has been made easier because of FDA guidance that allows clozapine to be dispensed without blood work if obtaining blood work is not possible (eg, a patient is quarantined) or can be accomplished only at substantial risk to patients and the population at large. Under certain conditions, clozapine can be dispensed safely and in a way that is consistent with infection prevention. Clozapine-treated patients admitted with COVID-19 should be monitored for clozapine toxicity and the clozapine dose adjusted.37 A consensus statement consistent with the FDA and clinical considerations for using clozapine during COVID-19 is summarized in Table 3.38
Continue to: Long-acting injectable antipsychotics...
Long-acting injectable antipsychotics (LAIs) pose a problem because they require in-person visits. Ideally, during a pandemic, patients should be seen in person as frequently as medically necessary but as infrequently as possible to limit exposure of both patients and staff. Table 4 provides some clinical recommendations on how to use LAIs during the pandemic.39
Supportive psychotherapy may be the most important tool we have in helping patients with loss and uncertainty during these challenging months.40 Simply staying in contact with patients plays a major role in preventing care discontinuity. Even routine interactions have become stressful, with everyone wearing a mask that partially obscures the face. People with impaired hearing may find it even more difficult to understand you.
Education, problem-solving, and a directive, encouraging style are major tools of supportive psychotherapy to reduce symptoms and increase adaptive skills. Clarify that social distancing refers to physical, not emotional, distancing. The judicious and temporary use of anxiolytics is appropriate to reduce anxiety. Concrete help and problem-solving (eg, filling out forms) are examples of proactive crisis intervention.
Telepsychiatry emerged in the pandemic’s early days as the default mode of practice in order to limit in-person contacts.41 Like all new technology, telepsychiatry brings progress and peril.42 While it has gone surprisingly well for most, the “digital divide” does not afford all patients access to the needed technology. The long-term effectiveness and acceptance of telehealth remain to be seen. (Editor’s Note: For more about this topic, see “Telepsychiatry: What you need to know.”
Lessons learned and outlook
Infectious outbreaks have historically inflicted long-term disruptions on societies and altered the course of history. However, each disaster is unique, and lessons from previous disasters may only partially apply.43 We do not yet know how this one will end, including how long it will take for the world’s economies to recover. If nothing else, the current public-health emergency has brought to the forefront what psychiatrists have always known: health disparities are partially responsible for different disease risks (in this case, the risk of getting infected with SARS-CoV-2).5 It may not be a coincidence that the Black Lives Matter movement is becoming a major impetus for social change at a time when the pandemic is exposing health-care inequalities.
Continue to: Some areas of the country...
Some areas of the country succeeded in reducing infections and limiting community spread, which ushered in an uneasy sense of normalcy even while the pandemic continues. At least for now, these locales can focus on rebuilding and preparing for expectable fluctuations in disease activity, including the arrival of the annual flu season on top of COVID-19.44 Recovery is not a return to the status quo ante but building stronger communities—“building back better.”45 Unless there is a continuum of care, shortcomings in one sector will have ripple effects through the entire system, particularly for psychiatric care for patients with SMI, which was inadequate before the pandemic.
Ensuring access to critical care was a priority during the pandemic’s early phase but came at the price of deferring other types of care, such as routine primary care; the coming months will see the downstream consequences of this approach,46 including for patients with SMI.
In the meantime, doing our job as clinicians, as Camus’s fictitious Dr. Bernard Rieux from the epigraph responds when asked how to define decency, may be the best we can do in these times. This includes contributing to and molding our field’s future and fostering a sense of agency in our patients and in ourselves. Major goals will be to preserve lessons learned, maintain flexibility, and avoid a return to unhelpful overregulation and payment models that do not reflect the flexible, person-centered care so important for patients with SMI.47
Bottom Line
During a pandemic, patients with serious mental illness may be easily forgotten as other issues overshadow the needs of this impoverished group. During a pandemic, the priority treatment goals for these patients are infection control, relapse prevention, and preventing treatment disengagement and loneliness. A pandemic requires changes in how patients with serious mental illness will receive psychopharmacology and psychotherapy.
Related Resources
- Huremović D (ed). Psychiatry of pandemics: a mental health response to infection outbreak. Cham, Switzerland: Springer Nature Switzerland AG; 2019.
- Ursano RJ, Fullerton CS, Weisaeth L, et al (eds). Textbook of disaster psychiatry. 2nd ed. Cambridge, UK: Cambridge University Press; 2017.
- Centers for Disease Control and Prevention. Coronavirus (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- American Psychiatric Association. Coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus.
- SMI Adviser. Make informed decisions related to COVID-19 and mental health. https://smiadviser.org/about/covid.
Drug Brand Names
Clozapine • Clozaril
Diazepam • Valium
Hydroxychloroquine • Plaquenil
1. Camus A. La peste. Paris, France: Éditions Gallimard; 1947.
2. Huremovic
3. Substance Abuse and Mental Health Services Administration. Phases of disaster. https://www.samhsa.gov/dtac/recovering-disasters/phases-disaster. Updated June 17, 2020. Accessed August 7, 2020.
4. Geller J. COVID-19 and advocacy—the good and the unacceptable. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5b13. Published May 7, 2020. Accessed August 7, 2020.
5. Webb Hooper M, Nápoles AM, Perez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466-2467.
6. Sederer LI, Lanzara CB, Essock SM, et al. Lessons learned from the New York State mental health response to the September 11, 2001, attacks. Psychiatr Serv. 2011;62(9):1085-1089.
7. World Health Organization. Infodemic management – infodemiology. https://www.who.int/teams/risk-communication/infodemic-management. Accessed August 7, 2020.
8. Zhou J, Liu L, Xue P, et al. Mental health response to the COVID-19 outbreak in China. Am J Psychiatry. 2020;117(7):574-575.
9. Kawohl W, Nordt C. COVID-19, unemployment, and suicide. Lancet Psychiatry. 2020;7(5):389-390.
10. Yao H, Chen JH, Xu YF. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry. 2020;7(4):e21. doi: 10.1016/S2215-0366(20)30090-0.
11. Minihan E, Gavin B, Kelly BD, et al. Covid-19, mental health and psychological first aid. Ir J Psychol Med. 2020:1-12.
12. Adja KYC, Golinelli D, Lenzi J, et al. Pandemics and social stigma: who’s next? Italy’s experience with COVID-19. Public Health. 2020;185:39-41.
13. Rosenberg AR. Cultivating deliberate resilience during the coronavirus disease 2019 pandemic [published online April 14, 2020]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2020.1436.
14. Dean W, Talbot SG, Caplan A. Clarifying the language of clinician distress [published online January 31, 2020]. JAMA. doi: 10.1001/jama.2019.21576.
15. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055.
16. Rosenbaum L. Facing Covid-19 in Italy - ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875.
17. Viceconte G, Petrosillo N. COVID-19 R0: magic number or conundrum? Infect Dis Rep. 2020;12(1):8516.
18. de Hert M, Schreurs V, Vancampfort D, van Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15-22.
19. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97-105.
20. Finer N, Garnett SP, Bruun JM. COVID-19 and obesity. Clin Obes. 2020;10(3):e12365. doi: 10.1111/cob.12365.
21. Havens LL, Ghaemi SN. Existential despair and bipolar disorder: the therapeutic alliance as a mood stabilizer. Am J Psychother. 2005;59(2):137-147.
22. Trémeau F, Antonius D, Malaspina D, et al. Loneliness in schizophrenia and its possible correlates. An exploratory study. Psychiatry Res. 2016;246:211-217.
23. Menninger KA. Psychoses associated with influenza: I. General data: statistical analysis. JAMA. 1919;72(4):235-241.
24. Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832.
25. Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.012.
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39.
27. Martin Jr. EB. Brief psychotic disorder triggered by fear of coronavirus? Psychiatric Times. https://www.psychiatrictimes.com/view/brief-psychotic-disorder-triggered-fear-coronavirus-small-case-series. Published May 8, 2020. Accessed August 7, 2020.
28. Sher Y, Rabkin B, Maldonado JR, et al. COVID-19-associated hyperactive intensive care unit delirium with proposed pathophysiology and treatment: a case report [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.007.
29. Wolters AE, Peelen LM, Welling MC, et al. Long-term mental health problems after delirium in the ICU. Crit Care Med. 2016;44(10):1808-1813.
30. Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis. 2008;6(3):114-124.
31. Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
32. Maercker A, Brewin CR, Bryant RA, et al. Diagnosis and classification of disorders specifically associated with stress: proposals for ICD-11. World Psychiatry. 2013;12(3):198-206.
33. Ornell F, Moura HF, Scherer JN, et al. The COVID-19 pandemic and its impact on substance use: implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi: 10.1016/j.psychres.2020.113096.
34. Berlin I, Thomas D, Le Faou AL, Cornuz J. COVID-19 and smoking [published online April 3, 2020]. Nicotine Tob Res. https://doi.org/10.1093/ntr/ntaa059.
35. Back D, Marzolini C, Hodge C, et al. COVID-19 treatment in patients with comorbidities: awareness of drug-drug interactions [published online May 8, 2020]. Br J Clin Pharmacol. doi: 10.1111/bcp.14358.
36. Carpenter WT Jr., Buchanan RW, Kirkpatrick B, et al. Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry. 1999;156(2):299-303.
37. Dotson S, Hartvigsen N, Wesner T, et al. Clozapine toxicity in the setting of COVID-19 [published online May 30, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.025.
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223.
39. Schnitzer K, MacLaurin S, Freudenreich O. Long-acting injectable antipsychotics during the COVID-19 pandemic. Current Psychiatry. In press.
40. Winston A, Rosenthal RN, Pinsker H. Learning supportive psychotherapy: an illustrated guide. Washington, DC: American Psychiatric Publishing; 2012.
41. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
42. Jordan A, Dixon LB. Considerations for telepsychiatry service implementation in the era of COVID-19. Psychiatr Serv. 2020;71(6):643-644.
43. DePierro J, Lowe S, Katz C. Lessons learned from 9/11: mental health perspectives on the COVID-19 pandemic. Psychiatry Res. 2020;288:113024.
44. Hussain S. Immunization and vaccination. In: Huremovic
45. Epping-Jordan JE, van Ommeren M, Ashour HN, et al. Beyond the crisis: building back better mental health care in 10 emergency-affected areas using a longer-term perspective. Int J Ment Health Syst. 2015;9:15.
46. Rosenbaum L. The untold toll - the pandemic’s effects on patients without Covid-19. N Engl J Med. 2020;382(24):2368-2371.
47. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness [published online June 3, 2020]. Psychiatr Serv. doi: 10.1176/appi.ps.202000244.
1. Camus A. La peste. Paris, France: Éditions Gallimard; 1947.
2. Huremovic
3. Substance Abuse and Mental Health Services Administration. Phases of disaster. https://www.samhsa.gov/dtac/recovering-disasters/phases-disaster. Updated June 17, 2020. Accessed August 7, 2020.
4. Geller J. COVID-19 and advocacy—the good and the unacceptable. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5b13. Published May 7, 2020. Accessed August 7, 2020.
5. Webb Hooper M, Nápoles AM, Perez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323(24):2466-2467.
6. Sederer LI, Lanzara CB, Essock SM, et al. Lessons learned from the New York State mental health response to the September 11, 2001, attacks. Psychiatr Serv. 2011;62(9):1085-1089.
7. World Health Organization. Infodemic management – infodemiology. https://www.who.int/teams/risk-communication/infodemic-management. Accessed August 7, 2020.
8. Zhou J, Liu L, Xue P, et al. Mental health response to the COVID-19 outbreak in China. Am J Psychiatry. 2020;117(7):574-575.
9. Kawohl W, Nordt C. COVID-19, unemployment, and suicide. Lancet Psychiatry. 2020;7(5):389-390.
10. Yao H, Chen JH, Xu YF. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry. 2020;7(4):e21. doi: 10.1016/S2215-0366(20)30090-0.
11. Minihan E, Gavin B, Kelly BD, et al. Covid-19, mental health and psychological first aid. Ir J Psychol Med. 2020:1-12.
12. Adja KYC, Golinelli D, Lenzi J, et al. Pandemics and social stigma: who’s next? Italy’s experience with COVID-19. Public Health. 2020;185:39-41.
13. Rosenberg AR. Cultivating deliberate resilience during the coronavirus disease 2019 pandemic [published online April 14, 2020]. JAMA Pediatr. doi: 10.1001/jamapediatrics.2020.1436.
14. Dean W, Talbot SG, Caplan A. Clarifying the language of clinician distress [published online January 31, 2020]. JAMA. doi: 10.1001/jama.2019.21576.
15. Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382(21):2049-2055.
16. Rosenbaum L. Facing Covid-19 in Italy - ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875.
17. Viceconte G, Petrosillo N. COVID-19 R0: magic number or conundrum? Infect Dis Rep. 2020;12(1):8516.
18. de Hert M, Schreurs V, Vancampfort D, van Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15-22.
19. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97-105.
20. Finer N, Garnett SP, Bruun JM. COVID-19 and obesity. Clin Obes. 2020;10(3):e12365. doi: 10.1111/cob.12365.
21. Havens LL, Ghaemi SN. Existential despair and bipolar disorder: the therapeutic alliance as a mood stabilizer. Am J Psychother. 2005;59(2):137-147.
22. Trémeau F, Antonius D, Malaspina D, et al. Loneliness in schizophrenia and its possible correlates. An exploratory study. Psychiatry Res. 2016;246:211-217.
23. Menninger KA. Psychoses associated with influenza: I. General data: statistical analysis. JAMA. 1919;72(4):235-241.
24. Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832.
25. Ferrando SJ, Klepacz L, Lynch S, et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.012.
26. Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34-39.
27. Martin Jr. EB. Brief psychotic disorder triggered by fear of coronavirus? Psychiatric Times. https://www.psychiatrictimes.com/view/brief-psychotic-disorder-triggered-fear-coronavirus-small-case-series. Published May 8, 2020. Accessed August 7, 2020.
28. Sher Y, Rabkin B, Maldonado JR, et al. COVID-19-associated hyperactive intensive care unit delirium with proposed pathophysiology and treatment: a case report [published online May 19, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.007.
29. Wolters AE, Peelen LM, Welling MC, et al. Long-term mental health problems after delirium in the ICU. Crit Care Med. 2016;44(10):1808-1813.
30. Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis. 2008;6(3):114-124.
31. Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912-920.
32. Maercker A, Brewin CR, Bryant RA, et al. Diagnosis and classification of disorders specifically associated with stress: proposals for ICD-11. World Psychiatry. 2013;12(3):198-206.
33. Ornell F, Moura HF, Scherer JN, et al. The COVID-19 pandemic and its impact on substance use: implications for prevention and treatment. Psychiatry Res. 2020;289:113096. doi: 10.1016/j.psychres.2020.113096.
34. Berlin I, Thomas D, Le Faou AL, Cornuz J. COVID-19 and smoking [published online April 3, 2020]. Nicotine Tob Res. https://doi.org/10.1093/ntr/ntaa059.
35. Back D, Marzolini C, Hodge C, et al. COVID-19 treatment in patients with comorbidities: awareness of drug-drug interactions [published online May 8, 2020]. Br J Clin Pharmacol. doi: 10.1111/bcp.14358.
36. Carpenter WT Jr., Buchanan RW, Kirkpatrick B, et al. Diazepam treatment of early signs of exacerbation in schizophrenia. Am J Psychiatry. 1999;156(2):299-303.
37. Dotson S, Hartvigsen N, Wesner T, et al. Clozapine toxicity in the setting of COVID-19 [published online May 30, 2020]. Psychosomatics. doi: 10.1016/j.psym.2020.05.025.
38. Siskind D, Honer WG, Clark S, et al. Consensus statement on the use of clozapine during the COVID-19 pandemic. J Psychiatry Neurosci. 2020;45(3):222-223.
39. Schnitzer K, MacLaurin S, Freudenreich O. Long-acting injectable antipsychotics during the COVID-19 pandemic. Current Psychiatry. In press.
40. Winston A, Rosenthal RN, Pinsker H. Learning supportive psychotherapy: an illustrated guide. Washington, DC: American Psychiatric Publishing; 2012.
41. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
42. Jordan A, Dixon LB. Considerations for telepsychiatry service implementation in the era of COVID-19. Psychiatr Serv. 2020;71(6):643-644.
43. DePierro J, Lowe S, Katz C. Lessons learned from 9/11: mental health perspectives on the COVID-19 pandemic. Psychiatry Res. 2020;288:113024.
44. Hussain S. Immunization and vaccination. In: Huremovic
45. Epping-Jordan JE, van Ommeren M, Ashour HN, et al. Beyond the crisis: building back better mental health care in 10 emergency-affected areas using a longer-term perspective. Int J Ment Health Syst. 2015;9:15.
46. Rosenbaum L. The untold toll - the pandemic’s effects on patients without Covid-19. N Engl J Med. 2020;382(24):2368-2371.
47. Bartels SJ, Baggett TP, Freudenreich O, et al. COVID-19 emergency reforms in Massachusetts to support behavioral health care and reduce mortality of people with serious mental illness [published online June 3, 2020]. Psychiatr Serv. doi: 10.1176/appi.ps.202000244.
Strengthening faith during coronavirus: An Islamic perspective
"Ramadan will be depressing this year,” a patient told me as I entered the room for an evaluation. This is one of many similar reactions my patients expressed in March, when mosques began to close and social distancing parameters were put in place to limit the spread of coronavirus disease 2019 (COVID-19). Muslims began to adjust to new social norms, such as replacing warm hugs with waving hands from 6 feet away. They were suddenly advised to avoid century-long cultural practices, such as spending time with extended family, visiting the sick and the elderly, and meeting for Jummah (Friday) prayer at mosque. With increasing anxiety and uncertainty in the air, I began thinking about how the pandemic would psychologically affect Islamic spirituality, especially during Ramadan (the Islamic month of fasting) this year.
As a Muslim psychiatry resident working on an inpatient psychiatric unit and in a psychiatry consultation service during the COVID-19 pandemic, I often explore spirituality and faith with my patients as a way of providing supportive therapy for anxiety. Many of my Christian patients endorsed anxiety about how Easter would be “terrible” this year because they could not attend church. Upon hearing this, I realized that I could not picture a Ramadan during which I was not permitted to go to mosque. How was I supposed to provide supportive therapy for my patients when I also felt so uncertain? These concerns led me to take a step back and remind myself of what I frequently tell my patients when they feel hopeless: “With every difficulty, there comes an opportunity to gain a new perspective.”
A time for spirituality
When Ramadan began in April, many people who are Muslim and were working from home told me that it felt strange to have so much time during the day to pray, reflect, and read the Quran. Others mentioned that they enjoyed the peace of Iftar (breaking fast) at home, because they could avoid the hustle and bustle of this at mosque. Halfway through Ramadan, a Muslim patient I was treating reported that her “coronavirus anxiety” had improved as she began focusing her energy on Allah, rather than spending hours watching the news and obsessing over death tolls.
Due to the pandemic, many more opportunities for donating to those in need arose, which led my religious community to perform Zakat (providing charity) and send supplies to food banks in our area. Because of social distancing, Muslim families were able to spend more time preparing meals, learning together, and supporting each other. Although mosques were closed due to the pandemic, it seemed as though each home became its own gathering place for spirituality, gratitude, and self-reflection. By the end of Ramadan, the values of self-discipline, empathy, and patience became self-evident.
Increased attention to mental health among Muslims
Psychologically, I believe resilience has grown stronger among Muslims worldwide during this pandemic. Along with adopting a positive mindset, Muslims have committed to creating their own routines to combat anxiety during this stressful time. The Salat (praying 5 times a day) and Taharat (cleanliness) that Islam emphasizes have been helpful in creating structure to offset the uncertainty and fear that is associated with COVID-19.
The discussion of mental illness, which previously has been regarded as a culturally stigmatized topic, has been gaining significant recognition within Islamic communities. Depression, anxiety, and self-care are now emphasized during virtual sermons, and contact information for mental health hotlines and professionals are being rapidly disseminated. There is now a greater sense of encouragement for people of Islamic faith to seek psychiatric help when needed.
Although COVID-19 has limited some social and physical religious practices, this pandemic has helped to strengthen faith and spirituality not only among Muslims, but also people of other faiths. During periods of stress, change, and uncertainty, it is important to remember that “With every difficulty, there comes an opportunity to gain a new perspective.” Although mosques and churches continue to stay closed and anxiety persists, I can now confidently reassure my patients that through this experience we are becoming resilient and learning to value patience, gratitude, and empathy more than ever.
"Ramadan will be depressing this year,” a patient told me as I entered the room for an evaluation. This is one of many similar reactions my patients expressed in March, when mosques began to close and social distancing parameters were put in place to limit the spread of coronavirus disease 2019 (COVID-19). Muslims began to adjust to new social norms, such as replacing warm hugs with waving hands from 6 feet away. They were suddenly advised to avoid century-long cultural practices, such as spending time with extended family, visiting the sick and the elderly, and meeting for Jummah (Friday) prayer at mosque. With increasing anxiety and uncertainty in the air, I began thinking about how the pandemic would psychologically affect Islamic spirituality, especially during Ramadan (the Islamic month of fasting) this year.
As a Muslim psychiatry resident working on an inpatient psychiatric unit and in a psychiatry consultation service during the COVID-19 pandemic, I often explore spirituality and faith with my patients as a way of providing supportive therapy for anxiety. Many of my Christian patients endorsed anxiety about how Easter would be “terrible” this year because they could not attend church. Upon hearing this, I realized that I could not picture a Ramadan during which I was not permitted to go to mosque. How was I supposed to provide supportive therapy for my patients when I also felt so uncertain? These concerns led me to take a step back and remind myself of what I frequently tell my patients when they feel hopeless: “With every difficulty, there comes an opportunity to gain a new perspective.”
A time for spirituality
When Ramadan began in April, many people who are Muslim and were working from home told me that it felt strange to have so much time during the day to pray, reflect, and read the Quran. Others mentioned that they enjoyed the peace of Iftar (breaking fast) at home, because they could avoid the hustle and bustle of this at mosque. Halfway through Ramadan, a Muslim patient I was treating reported that her “coronavirus anxiety” had improved as she began focusing her energy on Allah, rather than spending hours watching the news and obsessing over death tolls.
Due to the pandemic, many more opportunities for donating to those in need arose, which led my religious community to perform Zakat (providing charity) and send supplies to food banks in our area. Because of social distancing, Muslim families were able to spend more time preparing meals, learning together, and supporting each other. Although mosques were closed due to the pandemic, it seemed as though each home became its own gathering place for spirituality, gratitude, and self-reflection. By the end of Ramadan, the values of self-discipline, empathy, and patience became self-evident.
Increased attention to mental health among Muslims
Psychologically, I believe resilience has grown stronger among Muslims worldwide during this pandemic. Along with adopting a positive mindset, Muslims have committed to creating their own routines to combat anxiety during this stressful time. The Salat (praying 5 times a day) and Taharat (cleanliness) that Islam emphasizes have been helpful in creating structure to offset the uncertainty and fear that is associated with COVID-19.
The discussion of mental illness, which previously has been regarded as a culturally stigmatized topic, has been gaining significant recognition within Islamic communities. Depression, anxiety, and self-care are now emphasized during virtual sermons, and contact information for mental health hotlines and professionals are being rapidly disseminated. There is now a greater sense of encouragement for people of Islamic faith to seek psychiatric help when needed.
Although COVID-19 has limited some social and physical religious practices, this pandemic has helped to strengthen faith and spirituality not only among Muslims, but also people of other faiths. During periods of stress, change, and uncertainty, it is important to remember that “With every difficulty, there comes an opportunity to gain a new perspective.” Although mosques and churches continue to stay closed and anxiety persists, I can now confidently reassure my patients that through this experience we are becoming resilient and learning to value patience, gratitude, and empathy more than ever.
"Ramadan will be depressing this year,” a patient told me as I entered the room for an evaluation. This is one of many similar reactions my patients expressed in March, when mosques began to close and social distancing parameters were put in place to limit the spread of coronavirus disease 2019 (COVID-19). Muslims began to adjust to new social norms, such as replacing warm hugs with waving hands from 6 feet away. They were suddenly advised to avoid century-long cultural practices, such as spending time with extended family, visiting the sick and the elderly, and meeting for Jummah (Friday) prayer at mosque. With increasing anxiety and uncertainty in the air, I began thinking about how the pandemic would psychologically affect Islamic spirituality, especially during Ramadan (the Islamic month of fasting) this year.
As a Muslim psychiatry resident working on an inpatient psychiatric unit and in a psychiatry consultation service during the COVID-19 pandemic, I often explore spirituality and faith with my patients as a way of providing supportive therapy for anxiety. Many of my Christian patients endorsed anxiety about how Easter would be “terrible” this year because they could not attend church. Upon hearing this, I realized that I could not picture a Ramadan during which I was not permitted to go to mosque. How was I supposed to provide supportive therapy for my patients when I also felt so uncertain? These concerns led me to take a step back and remind myself of what I frequently tell my patients when they feel hopeless: “With every difficulty, there comes an opportunity to gain a new perspective.”
A time for spirituality
When Ramadan began in April, many people who are Muslim and were working from home told me that it felt strange to have so much time during the day to pray, reflect, and read the Quran. Others mentioned that they enjoyed the peace of Iftar (breaking fast) at home, because they could avoid the hustle and bustle of this at mosque. Halfway through Ramadan, a Muslim patient I was treating reported that her “coronavirus anxiety” had improved as she began focusing her energy on Allah, rather than spending hours watching the news and obsessing over death tolls.
Due to the pandemic, many more opportunities for donating to those in need arose, which led my religious community to perform Zakat (providing charity) and send supplies to food banks in our area. Because of social distancing, Muslim families were able to spend more time preparing meals, learning together, and supporting each other. Although mosques were closed due to the pandemic, it seemed as though each home became its own gathering place for spirituality, gratitude, and self-reflection. By the end of Ramadan, the values of self-discipline, empathy, and patience became self-evident.
Increased attention to mental health among Muslims
Psychologically, I believe resilience has grown stronger among Muslims worldwide during this pandemic. Along with adopting a positive mindset, Muslims have committed to creating their own routines to combat anxiety during this stressful time. The Salat (praying 5 times a day) and Taharat (cleanliness) that Islam emphasizes have been helpful in creating structure to offset the uncertainty and fear that is associated with COVID-19.
The discussion of mental illness, which previously has been regarded as a culturally stigmatized topic, has been gaining significant recognition within Islamic communities. Depression, anxiety, and self-care are now emphasized during virtual sermons, and contact information for mental health hotlines and professionals are being rapidly disseminated. There is now a greater sense of encouragement for people of Islamic faith to seek psychiatric help when needed.
Although COVID-19 has limited some social and physical religious practices, this pandemic has helped to strengthen faith and spirituality not only among Muslims, but also people of other faiths. During periods of stress, change, and uncertainty, it is important to remember that “With every difficulty, there comes an opportunity to gain a new perspective.” Although mosques and churches continue to stay closed and anxiety persists, I can now confidently reassure my patients that through this experience we are becoming resilient and learning to value patience, gratitude, and empathy more than ever.
Journey from first name to last name: Pursuing my dream
After graduating from medical school in India, where I was born and raised, I came to the United States in 2009 to expand my medical knowledge. At that time, I completed my clinical skills exam and soon after began a volunteer rotation at New York-Presbyterian Queens Hospital. In those early days, as I made my rounds through the emergency department (ED) of the hospital, I would introduce myself as Dr. Siva, which is my first name; this is how the doctors back home in India would introduce themselves to patients. Little did I know that the same name convention was not necessarily used here in the United States. Nonetheless, in those formative days, I learned a great deal from listening to the unique stories of how my patients had ended up in the ED, and I quickly felt right at home getting to know them.
When I first came to the United States, I had limited knowledge of psychiatry because I had only had a few months of psychiatry rotations during medical school. But in 2012, while I served as a volunteer in a research and observership program at Beth Israel Medical Center, one of my colleagues who was a psychiatry resident piqued my interest in the specialty and motivated me to explore and learn more about the various treatment modalities, strategies, and nuances this new modern world of psychiatry had to offer.
So I began by attending training sessions and evening seminars at the New York Psychoanalytic Society & Institute, where I became interested in Sigmund Freud’s work on the development of psychoanalysis. From there, my appetite for knowledge only continued to grow, and I took every opportunity to participate in various learning exercises, present at poster sessions, and give lectures at national conferences. I read and absorbed significant theories and texts and interacted with and learned from colleagues and mentors as I strived to sculpt my mind, with the aim of becoming a well-rounded psychiatrist.
Overcoming challenges
As I worked to further my understanding of psychiatry and understand the different treatment modalities—my goals becoming more clear with each step of my journey—I faced a significant setback. I was unable to secure a residency position to officially enter the specialty. I was devastated in my pursuit to realize the American Dream. At that point, I had been in the United States for 4 years with the financial and emotional support of my parents back home in India. I continued to struggle; another 2 years passed, and I was still coming up empty in my search for a residency position.
In the meantime, I kept moving forward, with my sights firmly on learning more about psychiatry. This time, I sought out several projects, including one where I served as a research assistant (volunteer) for nonpharmacologic clinical trials in patients with bipolar disorder, and another where I served as a research assistant (volunteer) at a substance use disorder clinic at Columbia University. I was also accepted into the “Prelude to Training” program at the Psychoanalytic Association of New York, which is affiliated with the NYU Grossman School of Medicine. Through that program, I was introduced to psychodynamic thinking and practice, which gave me the valuable foundation of thinking beyond oneself.
Grit and determination
To further my education, I studied clinical and translational sciences at Creighton University in Omaha. I was given opportunities to discuss topics related to the historical aspects of and recent advances in psychoanalysis through my involvement with the Professional Reading Alliance on Psychoanalysis at The Circle for the Lacanian Orientation of Omaha. Then came the moment when all my dreams came to fruition—I was accepted into the psychiatry residency program at Creighton University.
Those 4 years of residency passed by in a flash! Recently, I began a neuromodulation fellowship at the University of Florida in Gainesville. Here, my journey continues, as I search for tools to help the disenfranchised and those in need of mental health support. After the neuromodulation fellowship, I plan to pursue a pain medicine fellowship.
Continue to: Through the years...
Through the years, I have grown both professionally and personally. I have also overcome the instinctual urge to introduce myself to patients by my first name and have adapted to the American style of using my last name, and now introduce myself as Dr. Koppolu.
My educational journey in a place far from home has impacted me in ways I never knew possible, and I believe my strength to continue the pursuit is rooted in my passion and ambition to become a psychiatrist. I never gave up working toward that dream—a dream that is slowly becoming a reality.
After graduating from medical school in India, where I was born and raised, I came to the United States in 2009 to expand my medical knowledge. At that time, I completed my clinical skills exam and soon after began a volunteer rotation at New York-Presbyterian Queens Hospital. In those early days, as I made my rounds through the emergency department (ED) of the hospital, I would introduce myself as Dr. Siva, which is my first name; this is how the doctors back home in India would introduce themselves to patients. Little did I know that the same name convention was not necessarily used here in the United States. Nonetheless, in those formative days, I learned a great deal from listening to the unique stories of how my patients had ended up in the ED, and I quickly felt right at home getting to know them.
When I first came to the United States, I had limited knowledge of psychiatry because I had only had a few months of psychiatry rotations during medical school. But in 2012, while I served as a volunteer in a research and observership program at Beth Israel Medical Center, one of my colleagues who was a psychiatry resident piqued my interest in the specialty and motivated me to explore and learn more about the various treatment modalities, strategies, and nuances this new modern world of psychiatry had to offer.
So I began by attending training sessions and evening seminars at the New York Psychoanalytic Society & Institute, where I became interested in Sigmund Freud’s work on the development of psychoanalysis. From there, my appetite for knowledge only continued to grow, and I took every opportunity to participate in various learning exercises, present at poster sessions, and give lectures at national conferences. I read and absorbed significant theories and texts and interacted with and learned from colleagues and mentors as I strived to sculpt my mind, with the aim of becoming a well-rounded psychiatrist.
Overcoming challenges
As I worked to further my understanding of psychiatry and understand the different treatment modalities—my goals becoming more clear with each step of my journey—I faced a significant setback. I was unable to secure a residency position to officially enter the specialty. I was devastated in my pursuit to realize the American Dream. At that point, I had been in the United States for 4 years with the financial and emotional support of my parents back home in India. I continued to struggle; another 2 years passed, and I was still coming up empty in my search for a residency position.
In the meantime, I kept moving forward, with my sights firmly on learning more about psychiatry. This time, I sought out several projects, including one where I served as a research assistant (volunteer) for nonpharmacologic clinical trials in patients with bipolar disorder, and another where I served as a research assistant (volunteer) at a substance use disorder clinic at Columbia University. I was also accepted into the “Prelude to Training” program at the Psychoanalytic Association of New York, which is affiliated with the NYU Grossman School of Medicine. Through that program, I was introduced to psychodynamic thinking and practice, which gave me the valuable foundation of thinking beyond oneself.
Grit and determination
To further my education, I studied clinical and translational sciences at Creighton University in Omaha. I was given opportunities to discuss topics related to the historical aspects of and recent advances in psychoanalysis through my involvement with the Professional Reading Alliance on Psychoanalysis at The Circle for the Lacanian Orientation of Omaha. Then came the moment when all my dreams came to fruition—I was accepted into the psychiatry residency program at Creighton University.
Those 4 years of residency passed by in a flash! Recently, I began a neuromodulation fellowship at the University of Florida in Gainesville. Here, my journey continues, as I search for tools to help the disenfranchised and those in need of mental health support. After the neuromodulation fellowship, I plan to pursue a pain medicine fellowship.
Continue to: Through the years...
Through the years, I have grown both professionally and personally. I have also overcome the instinctual urge to introduce myself to patients by my first name and have adapted to the American style of using my last name, and now introduce myself as Dr. Koppolu.
My educational journey in a place far from home has impacted me in ways I never knew possible, and I believe my strength to continue the pursuit is rooted in my passion and ambition to become a psychiatrist. I never gave up working toward that dream—a dream that is slowly becoming a reality.
After graduating from medical school in India, where I was born and raised, I came to the United States in 2009 to expand my medical knowledge. At that time, I completed my clinical skills exam and soon after began a volunteer rotation at New York-Presbyterian Queens Hospital. In those early days, as I made my rounds through the emergency department (ED) of the hospital, I would introduce myself as Dr. Siva, which is my first name; this is how the doctors back home in India would introduce themselves to patients. Little did I know that the same name convention was not necessarily used here in the United States. Nonetheless, in those formative days, I learned a great deal from listening to the unique stories of how my patients had ended up in the ED, and I quickly felt right at home getting to know them.
When I first came to the United States, I had limited knowledge of psychiatry because I had only had a few months of psychiatry rotations during medical school. But in 2012, while I served as a volunteer in a research and observership program at Beth Israel Medical Center, one of my colleagues who was a psychiatry resident piqued my interest in the specialty and motivated me to explore and learn more about the various treatment modalities, strategies, and nuances this new modern world of psychiatry had to offer.
So I began by attending training sessions and evening seminars at the New York Psychoanalytic Society & Institute, where I became interested in Sigmund Freud’s work on the development of psychoanalysis. From there, my appetite for knowledge only continued to grow, and I took every opportunity to participate in various learning exercises, present at poster sessions, and give lectures at national conferences. I read and absorbed significant theories and texts and interacted with and learned from colleagues and mentors as I strived to sculpt my mind, with the aim of becoming a well-rounded psychiatrist.
Overcoming challenges
As I worked to further my understanding of psychiatry and understand the different treatment modalities—my goals becoming more clear with each step of my journey—I faced a significant setback. I was unable to secure a residency position to officially enter the specialty. I was devastated in my pursuit to realize the American Dream. At that point, I had been in the United States for 4 years with the financial and emotional support of my parents back home in India. I continued to struggle; another 2 years passed, and I was still coming up empty in my search for a residency position.
In the meantime, I kept moving forward, with my sights firmly on learning more about psychiatry. This time, I sought out several projects, including one where I served as a research assistant (volunteer) for nonpharmacologic clinical trials in patients with bipolar disorder, and another where I served as a research assistant (volunteer) at a substance use disorder clinic at Columbia University. I was also accepted into the “Prelude to Training” program at the Psychoanalytic Association of New York, which is affiliated with the NYU Grossman School of Medicine. Through that program, I was introduced to psychodynamic thinking and practice, which gave me the valuable foundation of thinking beyond oneself.
Grit and determination
To further my education, I studied clinical and translational sciences at Creighton University in Omaha. I was given opportunities to discuss topics related to the historical aspects of and recent advances in psychoanalysis through my involvement with the Professional Reading Alliance on Psychoanalysis at The Circle for the Lacanian Orientation of Omaha. Then came the moment when all my dreams came to fruition—I was accepted into the psychiatry residency program at Creighton University.
Those 4 years of residency passed by in a flash! Recently, I began a neuromodulation fellowship at the University of Florida in Gainesville. Here, my journey continues, as I search for tools to help the disenfranchised and those in need of mental health support. After the neuromodulation fellowship, I plan to pursue a pain medicine fellowship.
Continue to: Through the years...
Through the years, I have grown both professionally and personally. I have also overcome the instinctual urge to introduce myself to patients by my first name and have adapted to the American style of using my last name, and now introduce myself as Dr. Koppolu.
My educational journey in a place far from home has impacted me in ways I never knew possible, and I believe my strength to continue the pursuit is rooted in my passion and ambition to become a psychiatrist. I never gave up working toward that dream—a dream that is slowly becoming a reality.
Adolescent e-cigarette use: A public health crisis
The use of electronic cigarettes (e-cigarettes) in teenagers has been increasing rapidly in the United States, leading Surgeon General Jerome Adams, MD, MPH, to label it a public health concern.1 Easy accessibility and extensive marketing for e-cigarettes counteract public education campaigns and policies aimed at decreasing e-cigarette use in teenagers.
E-cigarettes are marketed to teenagers as small, easy-to-use pens or USB flash drive–like devices that can be hidden easily. Some devices can be used to smoke nicotine, delta-9-tetrahydrocannabinol (THC), cannabidiol, and butane hash oil. Some are sold with different nicotine flavors to increase their appeal. E-cigarette ads appear in retail stores, movies, magazines, newspapers, and on the internet.
According to the CDC, the number of middle and high school students using e-cigarettes increased from 3.6 million in 2018 to 5.4 million in 2019.2 Nicotine dependence from e-cigarette use can increase the risk of starting to smoke cigarettes. A 2015-2016 National Institute on Drug Abuse survey found a higher prevalence of e-cigarette use among 9th-, 10th-, and 12th-grade students compared with cigarette smoking (9.5%, 14%, 16.2% vs 3.6%, 6.2%, 11.4%, respectively).3 Due to the growing popularity of vaping among adolescents in the United States, Congress recently raised the legal age to purchase tobacco and vaping products to 21 years.
Evidence of adverse health effects associated with e-cigarette use continues to grow. In 2020, the Department of Health and Services in Wisconsin and the Department of Public Health in Illinois looked at e-cigarette use and pulmonary disease.4 Of 98 participants who reported e-cigarette use, 97% presented with respiratory symptoms, 77% had gastrointestinal symptoms, and 100% had constitutional symptoms. Chest imaging showed bilateral infiltrates in all patients. In addition, 95% were hospitalized, 26% underwent intubation and mechanical ventilation, and 1 patient died. Most participants (89%) reported using THC in their e-cigarette devices.4 Blount et al5 recently found a link between e-cigarette- or vaping-associated lung injury and vitamin E acetate, a toxicant found in bronchoalveolar lavage fluid of some patients who reported using e-cigarettes. Also, nicotine dependency from e-cigarettes may adversely affect brain development in children and adolescents.2
The first step in fighting this crisis is to educate children, parents, teachers, and health care professionals about e-cigarette use, including its prevalence, use compared with cigarette smoking, trends among teenagers, marketing techniques, and adverse effects. Fortunately, the US government and medical professionals and organizations have made ongoing efforts to discourage e-cigarette use. For example, the American Academy of Child and Adolescent Psychiatry supports the FDA’s regulation of e-cigarette use; encourages using evidence-based treatments for tobacco cessation; advocates for vigorous education regarding adolescent e-cigarette use; and endorses restrictions on e-cigarette advertisement.6 We strongly urge clinicians to be vigilant about e-cigarette use in their adolescent patients and to intervene in this public health crisis.
Immad A. Kiani, MD
PGY-3 Psychiatry Resident
Christiana Care Health Services
Department of Psychiatry
Wilmington, Delaware
Narpinder K. Malhi, MD
Child and Adolescent Psychiatrist
Christiana Care Health Services
Wilmington, Delaware
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Adams J. Surgeon General’s advisory on e-cigarette use among youth. US Department of Health & Human Services. https://e-cigarettes.surgeongeneral.gov/documents/surgeon-generals-advisory-on-e-cigarette-use-among-youth-2018.pdf. Published 2018. Accessed August 7, 2020.
2. US Federal Drug and Drug Administration. Results from 2018 National Youth Tobacco Survey show dramatic increase in e-cigarette use among youth over past year. https://www.fda.gov/news-events/press-announcements/results-2018-national-youth-tobacco-survey-show-dramatic-increase-e-cigarette-use-among-youth-over. Published November 15, 2018. Accessed August 7, 2020.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the future national survey results on drug use, 1975-2016: overview, key findings on adolescent drug use. The University of Michigan Institute for Social Research. https://files.eric.ed.gov/fulltext/ED578534.pdf. Published January 2017. Accessed August 7, 2020.
4. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—final report. N Engl J Med. 2020;382(10):903-916.
5. Blount BC, Karwowski MP, Shields PG, et al; Lung Injury Response Laboratory Working Group. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705.
6. Electronic cigarettes. The American Academy of Child and Adolescent Psychiatry. https://www.aacap.org/AACAP/Policy_Statements/2015/Policy_Statement_on_Electronic_Cigarettes.aspx. Published June 2015. Accessed August 7, 2020.
The use of electronic cigarettes (e-cigarettes) in teenagers has been increasing rapidly in the United States, leading Surgeon General Jerome Adams, MD, MPH, to label it a public health concern.1 Easy accessibility and extensive marketing for e-cigarettes counteract public education campaigns and policies aimed at decreasing e-cigarette use in teenagers.
E-cigarettes are marketed to teenagers as small, easy-to-use pens or USB flash drive–like devices that can be hidden easily. Some devices can be used to smoke nicotine, delta-9-tetrahydrocannabinol (THC), cannabidiol, and butane hash oil. Some are sold with different nicotine flavors to increase their appeal. E-cigarette ads appear in retail stores, movies, magazines, newspapers, and on the internet.
According to the CDC, the number of middle and high school students using e-cigarettes increased from 3.6 million in 2018 to 5.4 million in 2019.2 Nicotine dependence from e-cigarette use can increase the risk of starting to smoke cigarettes. A 2015-2016 National Institute on Drug Abuse survey found a higher prevalence of e-cigarette use among 9th-, 10th-, and 12th-grade students compared with cigarette smoking (9.5%, 14%, 16.2% vs 3.6%, 6.2%, 11.4%, respectively).3 Due to the growing popularity of vaping among adolescents in the United States, Congress recently raised the legal age to purchase tobacco and vaping products to 21 years.
Evidence of adverse health effects associated with e-cigarette use continues to grow. In 2020, the Department of Health and Services in Wisconsin and the Department of Public Health in Illinois looked at e-cigarette use and pulmonary disease.4 Of 98 participants who reported e-cigarette use, 97% presented with respiratory symptoms, 77% had gastrointestinal symptoms, and 100% had constitutional symptoms. Chest imaging showed bilateral infiltrates in all patients. In addition, 95% were hospitalized, 26% underwent intubation and mechanical ventilation, and 1 patient died. Most participants (89%) reported using THC in their e-cigarette devices.4 Blount et al5 recently found a link between e-cigarette- or vaping-associated lung injury and vitamin E acetate, a toxicant found in bronchoalveolar lavage fluid of some patients who reported using e-cigarettes. Also, nicotine dependency from e-cigarettes may adversely affect brain development in children and adolescents.2
The first step in fighting this crisis is to educate children, parents, teachers, and health care professionals about e-cigarette use, including its prevalence, use compared with cigarette smoking, trends among teenagers, marketing techniques, and adverse effects. Fortunately, the US government and medical professionals and organizations have made ongoing efforts to discourage e-cigarette use. For example, the American Academy of Child and Adolescent Psychiatry supports the FDA’s regulation of e-cigarette use; encourages using evidence-based treatments for tobacco cessation; advocates for vigorous education regarding adolescent e-cigarette use; and endorses restrictions on e-cigarette advertisement.6 We strongly urge clinicians to be vigilant about e-cigarette use in their adolescent patients and to intervene in this public health crisis.
Immad A. Kiani, MD
PGY-3 Psychiatry Resident
Christiana Care Health Services
Department of Psychiatry
Wilmington, Delaware
Narpinder K. Malhi, MD
Child and Adolescent Psychiatrist
Christiana Care Health Services
Wilmington, Delaware
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The use of electronic cigarettes (e-cigarettes) in teenagers has been increasing rapidly in the United States, leading Surgeon General Jerome Adams, MD, MPH, to label it a public health concern.1 Easy accessibility and extensive marketing for e-cigarettes counteract public education campaigns and policies aimed at decreasing e-cigarette use in teenagers.
E-cigarettes are marketed to teenagers as small, easy-to-use pens or USB flash drive–like devices that can be hidden easily. Some devices can be used to smoke nicotine, delta-9-tetrahydrocannabinol (THC), cannabidiol, and butane hash oil. Some are sold with different nicotine flavors to increase their appeal. E-cigarette ads appear in retail stores, movies, magazines, newspapers, and on the internet.
According to the CDC, the number of middle and high school students using e-cigarettes increased from 3.6 million in 2018 to 5.4 million in 2019.2 Nicotine dependence from e-cigarette use can increase the risk of starting to smoke cigarettes. A 2015-2016 National Institute on Drug Abuse survey found a higher prevalence of e-cigarette use among 9th-, 10th-, and 12th-grade students compared with cigarette smoking (9.5%, 14%, 16.2% vs 3.6%, 6.2%, 11.4%, respectively).3 Due to the growing popularity of vaping among adolescents in the United States, Congress recently raised the legal age to purchase tobacco and vaping products to 21 years.
Evidence of adverse health effects associated with e-cigarette use continues to grow. In 2020, the Department of Health and Services in Wisconsin and the Department of Public Health in Illinois looked at e-cigarette use and pulmonary disease.4 Of 98 participants who reported e-cigarette use, 97% presented with respiratory symptoms, 77% had gastrointestinal symptoms, and 100% had constitutional symptoms. Chest imaging showed bilateral infiltrates in all patients. In addition, 95% were hospitalized, 26% underwent intubation and mechanical ventilation, and 1 patient died. Most participants (89%) reported using THC in their e-cigarette devices.4 Blount et al5 recently found a link between e-cigarette- or vaping-associated lung injury and vitamin E acetate, a toxicant found in bronchoalveolar lavage fluid of some patients who reported using e-cigarettes. Also, nicotine dependency from e-cigarettes may adversely affect brain development in children and adolescents.2
The first step in fighting this crisis is to educate children, parents, teachers, and health care professionals about e-cigarette use, including its prevalence, use compared with cigarette smoking, trends among teenagers, marketing techniques, and adverse effects. Fortunately, the US government and medical professionals and organizations have made ongoing efforts to discourage e-cigarette use. For example, the American Academy of Child and Adolescent Psychiatry supports the FDA’s regulation of e-cigarette use; encourages using evidence-based treatments for tobacco cessation; advocates for vigorous education regarding adolescent e-cigarette use; and endorses restrictions on e-cigarette advertisement.6 We strongly urge clinicians to be vigilant about e-cigarette use in their adolescent patients and to intervene in this public health crisis.
Immad A. Kiani, MD
PGY-3 Psychiatry Resident
Christiana Care Health Services
Department of Psychiatry
Wilmington, Delaware
Narpinder K. Malhi, MD
Child and Adolescent Psychiatrist
Christiana Care Health Services
Wilmington, Delaware
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Adams J. Surgeon General’s advisory on e-cigarette use among youth. US Department of Health & Human Services. https://e-cigarettes.surgeongeneral.gov/documents/surgeon-generals-advisory-on-e-cigarette-use-among-youth-2018.pdf. Published 2018. Accessed August 7, 2020.
2. US Federal Drug and Drug Administration. Results from 2018 National Youth Tobacco Survey show dramatic increase in e-cigarette use among youth over past year. https://www.fda.gov/news-events/press-announcements/results-2018-national-youth-tobacco-survey-show-dramatic-increase-e-cigarette-use-among-youth-over. Published November 15, 2018. Accessed August 7, 2020.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the future national survey results on drug use, 1975-2016: overview, key findings on adolescent drug use. The University of Michigan Institute for Social Research. https://files.eric.ed.gov/fulltext/ED578534.pdf. Published January 2017. Accessed August 7, 2020.
4. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—final report. N Engl J Med. 2020;382(10):903-916.
5. Blount BC, Karwowski MP, Shields PG, et al; Lung Injury Response Laboratory Working Group. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705.
6. Electronic cigarettes. The American Academy of Child and Adolescent Psychiatry. https://www.aacap.org/AACAP/Policy_Statements/2015/Policy_Statement_on_Electronic_Cigarettes.aspx. Published June 2015. Accessed August 7, 2020.
1. Adams J. Surgeon General’s advisory on e-cigarette use among youth. US Department of Health & Human Services. https://e-cigarettes.surgeongeneral.gov/documents/surgeon-generals-advisory-on-e-cigarette-use-among-youth-2018.pdf. Published 2018. Accessed August 7, 2020.
2. US Federal Drug and Drug Administration. Results from 2018 National Youth Tobacco Survey show dramatic increase in e-cigarette use among youth over past year. https://www.fda.gov/news-events/press-announcements/results-2018-national-youth-tobacco-survey-show-dramatic-increase-e-cigarette-use-among-youth-over. Published November 15, 2018. Accessed August 7, 2020.
3. Johnston LD, O’Malley PM, Miech RA, et al. Monitoring the future national survey results on drug use, 1975-2016: overview, key findings on adolescent drug use. The University of Michigan Institute for Social Research. https://files.eric.ed.gov/fulltext/ED578534.pdf. Published January 2017. Accessed August 7, 2020.
4. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—final report. N Engl J Med. 2020;382(10):903-916.
5. Blount BC, Karwowski MP, Shields PG, et al; Lung Injury Response Laboratory Working Group. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705.
6. Electronic cigarettes. The American Academy of Child and Adolescent Psychiatry. https://www.aacap.org/AACAP/Policy_Statements/2015/Policy_Statement_on_Electronic_Cigarettes.aspx. Published June 2015. Accessed August 7, 2020.
Revamp the MOC
There are few things that psychiatrists have come to despise more than the American Board of Psychiatry and Neurology (ABPN) Maintenance of Certification (MOC) program. It has become a professional boondoggle for psychiatric practitioners.
The program needs an overhaul and simplification. There are better, more efficient, cost-effective ways to ensure psychiatric physicians’ ongoing clinical competence after they complete their residency training. Technological advances can also facilitate a more valid assessment of competence without having to jump through more and more hoops between recertifications every 10 years.
I passed the boards long before the MOC was created. For 20 years, I also served as a senior examiner for the oral boards, where clinical competency was rigorously assessed by direct observations of psychiatrists examining and establishing rapport with patients and formulating the data into a differential diagnosis, treatment plan, and prognosis. It is noteworthy that psychiatrists who sat for the oral boards had already passed a written exam that tested their cognitive knowledge. Yet approximately one-third of the candidates failed the live oral exam, which clearly implies that passing a written exam is necessary but not sufficient to establish clinical competence, which is the primary purpose of board certification. It was an unfortunate decision to discontinue the face-to-face oral board exam, which is so vital for psychiatry, and to replace it with a written exam and a barrage of time-consuming activities to document lifelong learning and self-assessment, but not genuine clinical competence. The MOC has been MOCkingly referred to as a major pain in the neck for practically all psychiatrists who were not grandfathered with lifetime certification, as was the case in the first 60 years of the ABPN.
Benefits of the patient-based oral exam
Let’s face it: Passing a patient-based oral exam was the ideal mechanism to establish that a psychiatric physician deserved to be a diplomate of the ABPN. During the oral exam, the candidate’s skills were observed from the minute he/she met the patient. The candidate was then observed as he/she systematically explored a wide range of past and current psychiatric symptoms; reviewed the patient’s developmental, medical, family, and social histories; and conducted a competent mental status exam while demonstrating an empathic stance, responding to the patient’s often subtle verbal and nonverbal cues, establishing rapport, and providing psychoeducation before concluding the interview. All these essential components of a psychiatric exam were observed in a compact 30-minute tour de force of clinical skills, communication, and cognitive acumen. This was followed by another 30 minutes of organizing and presenting the clinical data to 2 or 3 colleagues/examiners, in a coherent fashion, connecting all the dots, formulating the case, presenting a meaningful differential diagnosis, and suggesting a rational array of potential treatment options across the biopsychosocial continuum. To top it off, the candidate had to respond effectively, in an evidence-based manner, to a series of questions related to the disease state, its treatment, adverse effects, and prognosis.
It was a joy to watch many colleagues navigate this clinical examination with skill and competence, without crumbling under the pressure of the examiners’ scrutiny. There were some who passed with flying colors, and others who passed despite having a forgivable minor gap here and there because of their overall strong performance. Finally, there were those who stumbled in several components across data collection, doctor–patient interactions, synthesis of the clinical findings, or treatment recommendations. These candidates inevitably received a failing grade by a consensus of 3 examiners. That they failed to demonstrate clinical competence despite having passed the required written exams a year earlier proved that the true competency of a psychiatrist cannot be judged solely by passing a written test but requires a clinical examination of a live patient.
The oral exams represented an unimpeachable evaluation of clinical competence. The examiners often spoke of how they would feel confident and comfortable with referring a family member to those who successfully passed this rigorous, authentic exam on real patients. It was justifiable to give lifetime certification to those who passed the oral exam. Those permanently certified psychiatrists maintained their lifelong learning by having an unrestricted state medical license, which is contingent on acquiring 50 category 1 continuing medical education (CME) credits annually. Why not restore lifelong certification for those who pass both a written and oral exam, as long as they maintain a valid medical license?
According to the ABPN 2019 Annual Report,1 31,514 psychiatrists have received lifetime certification, of whom an estimated 9,547 were still clinically active in 2019. This is the “grandfathered” cohort of psychiatrists to which I belong. I was tested on neurologic patients, not just psychiatric patients, a tribute to the strong bridge that existed between these sister brain specialties. As of 2019, of the 33,277 psychiatrists who received a time-limited certification, 29,343 were still clinically active, an attrition rate of 12% over the past 25 years. This includes psychiatrists who found the MOC too onerous to complete, or are in private practice where MOC is not a vital requirement. However, these days most psychiatrists are obligated to be recertified because so many entities require it. This includes hiring institutions, government agencies (Medicare/Medicaid), health insurance companies, hospital medical staff for privileging and credentialing, and various regulatory boards, such as The Joint Commission, the Accreditation Council for Graduate Medical Education, and academic medical centers. Because most psychiatrists are involved with at least one of these entities, 29,343 have no choice but to perform all the requirements of the MOC, with its countless hours, numerous documentations, and many fees, to remain certified by the ABPN. Notably absent is an alternative mechanism for a certification process that is widely accepted by all agencies and institutions. Psychiatrists are actively seeking alternatives.
Continue to: The ABPN...
The ABPN, long regarded as an esteemed nonprofit organization, has been accused of being a monopoly. Some angry psychiatrists have filed a class action lawsuit to demand other board certification methods. Some have gone to the media to complain about the American Board of Medical Specialties (of which the ABPN is a member board), accusing both of unfair regulations or of raking in substantial profits to support excessively compensated executives. Perception often trumps reality, so no matter how vigorously the ABPN defends itself, its procedures, or its MOC requirements, its customers—psychiatric physicians—feel oppressed or exploited.
How the MOC can be improved
So what can be done to improve the MOC? The need for recertification is arguably necessary to document clinical competency over an approximately 40-year psychiatric career following residency. I conducted a brief survey of
Significant advances in remote communication technology should be harnessed by the ABPN (or the APA, if it decides to conduct its own board certification) to restore the old model at a fraction of the cost. The oral exams have been replaced by a written exam that is not an accurate reflection or documentation of clinical competence. The traditional oral exam (after passing a written exam) was a magnificent but costly feat of massive logistical complexity, with >1,000 candidates and examiners traveling to a city where the ABPN arranged for several hospitals to shut down their clinics for 2 full days to use their clinical offices for the oral exams. Multiple teams examined the candidates twice on the same day: once with a live patient, and again with a video of a real patient. The examiners filled out scoring cards after observing the candidates conduct the live interview or discussing the video. A consensus grade of pass or fail was documented. At the end of the 2 days, examiners and candidates boarded buses to the airport. It was a highly expensive process (exam fees + airfare + hotel + food). Twice a year, the examiners generously donated their time to the ABPN without compensation, as a token of love for and service to the profession.
That initial certification of a written exam, followed by an oral exam, validated the competence of a psychiatrist both cognitively and clinically. The lifetime certification was truly earned. The same model can now be replicated virtually via videoconferencing at a far lower cost to the ABPN, the candidates, and the examiners. The MOC 10-year recertification can be reduced to a written exam with clinical vignettes and an unrestricted license to practice medicine in any state, which implies that the psychiatrist has received the 50 CME annual credits to renew the license. The rest of the bells and whistles can be strongly recommended but not required. The cost in time and money to both the ABPN and the candidates can be significantly reduced, but more importantly, the clinical competence will be validated at baseline with virtual oral boards after passing the written exam (formerly labeled as part I, preceding the part II oral boards).
The traditional board certification model of the past should be resurrected via videoconferencing and offered as an option to the candidates who prefer it to the current MOC. The MOC can then be simplified to lifetime certification or to only a written exam with clinical vignettes every 10 years to ensure that psychiatrists continue to incorporate relevant clinical and treatment advances in their practice. The KISS principle (keep it simple, stupid) worked very well for many generations of psychiatrists in the past, and will work again going forward if offered as an option. Psychiatrists can then focus on treating patients instead of being burdened by the many time-consuming requirements and hoops of the current MOC.
1. American Board of Psychiatry and Neurology. 2019 Annual Report. https://www.abpn.com/wp-content/uploads/2020/05/ABPN_2019_Annual_Report.pdf. Accessed August 14, 2020.
There are few things that psychiatrists have come to despise more than the American Board of Psychiatry and Neurology (ABPN) Maintenance of Certification (MOC) program. It has become a professional boondoggle for psychiatric practitioners.
The program needs an overhaul and simplification. There are better, more efficient, cost-effective ways to ensure psychiatric physicians’ ongoing clinical competence after they complete their residency training. Technological advances can also facilitate a more valid assessment of competence without having to jump through more and more hoops between recertifications every 10 years.
I passed the boards long before the MOC was created. For 20 years, I also served as a senior examiner for the oral boards, where clinical competency was rigorously assessed by direct observations of psychiatrists examining and establishing rapport with patients and formulating the data into a differential diagnosis, treatment plan, and prognosis. It is noteworthy that psychiatrists who sat for the oral boards had already passed a written exam that tested their cognitive knowledge. Yet approximately one-third of the candidates failed the live oral exam, which clearly implies that passing a written exam is necessary but not sufficient to establish clinical competence, which is the primary purpose of board certification. It was an unfortunate decision to discontinue the face-to-face oral board exam, which is so vital for psychiatry, and to replace it with a written exam and a barrage of time-consuming activities to document lifelong learning and self-assessment, but not genuine clinical competence. The MOC has been MOCkingly referred to as a major pain in the neck for practically all psychiatrists who were not grandfathered with lifetime certification, as was the case in the first 60 years of the ABPN.
Benefits of the patient-based oral exam
Let’s face it: Passing a patient-based oral exam was the ideal mechanism to establish that a psychiatric physician deserved to be a diplomate of the ABPN. During the oral exam, the candidate’s skills were observed from the minute he/she met the patient. The candidate was then observed as he/she systematically explored a wide range of past and current psychiatric symptoms; reviewed the patient’s developmental, medical, family, and social histories; and conducted a competent mental status exam while demonstrating an empathic stance, responding to the patient’s often subtle verbal and nonverbal cues, establishing rapport, and providing psychoeducation before concluding the interview. All these essential components of a psychiatric exam were observed in a compact 30-minute tour de force of clinical skills, communication, and cognitive acumen. This was followed by another 30 minutes of organizing and presenting the clinical data to 2 or 3 colleagues/examiners, in a coherent fashion, connecting all the dots, formulating the case, presenting a meaningful differential diagnosis, and suggesting a rational array of potential treatment options across the biopsychosocial continuum. To top it off, the candidate had to respond effectively, in an evidence-based manner, to a series of questions related to the disease state, its treatment, adverse effects, and prognosis.
It was a joy to watch many colleagues navigate this clinical examination with skill and competence, without crumbling under the pressure of the examiners’ scrutiny. There were some who passed with flying colors, and others who passed despite having a forgivable minor gap here and there because of their overall strong performance. Finally, there were those who stumbled in several components across data collection, doctor–patient interactions, synthesis of the clinical findings, or treatment recommendations. These candidates inevitably received a failing grade by a consensus of 3 examiners. That they failed to demonstrate clinical competence despite having passed the required written exams a year earlier proved that the true competency of a psychiatrist cannot be judged solely by passing a written test but requires a clinical examination of a live patient.
The oral exams represented an unimpeachable evaluation of clinical competence. The examiners often spoke of how they would feel confident and comfortable with referring a family member to those who successfully passed this rigorous, authentic exam on real patients. It was justifiable to give lifetime certification to those who passed the oral exam. Those permanently certified psychiatrists maintained their lifelong learning by having an unrestricted state medical license, which is contingent on acquiring 50 category 1 continuing medical education (CME) credits annually. Why not restore lifelong certification for those who pass both a written and oral exam, as long as they maintain a valid medical license?
According to the ABPN 2019 Annual Report,1 31,514 psychiatrists have received lifetime certification, of whom an estimated 9,547 were still clinically active in 2019. This is the “grandfathered” cohort of psychiatrists to which I belong. I was tested on neurologic patients, not just psychiatric patients, a tribute to the strong bridge that existed between these sister brain specialties. As of 2019, of the 33,277 psychiatrists who received a time-limited certification, 29,343 were still clinically active, an attrition rate of 12% over the past 25 years. This includes psychiatrists who found the MOC too onerous to complete, or are in private practice where MOC is not a vital requirement. However, these days most psychiatrists are obligated to be recertified because so many entities require it. This includes hiring institutions, government agencies (Medicare/Medicaid), health insurance companies, hospital medical staff for privileging and credentialing, and various regulatory boards, such as The Joint Commission, the Accreditation Council for Graduate Medical Education, and academic medical centers. Because most psychiatrists are involved with at least one of these entities, 29,343 have no choice but to perform all the requirements of the MOC, with its countless hours, numerous documentations, and many fees, to remain certified by the ABPN. Notably absent is an alternative mechanism for a certification process that is widely accepted by all agencies and institutions. Psychiatrists are actively seeking alternatives.
Continue to: The ABPN...
The ABPN, long regarded as an esteemed nonprofit organization, has been accused of being a monopoly. Some angry psychiatrists have filed a class action lawsuit to demand other board certification methods. Some have gone to the media to complain about the American Board of Medical Specialties (of which the ABPN is a member board), accusing both of unfair regulations or of raking in substantial profits to support excessively compensated executives. Perception often trumps reality, so no matter how vigorously the ABPN defends itself, its procedures, or its MOC requirements, its customers—psychiatric physicians—feel oppressed or exploited.
How the MOC can be improved
So what can be done to improve the MOC? The need for recertification is arguably necessary to document clinical competency over an approximately 40-year psychiatric career following residency. I conducted a brief survey of
Significant advances in remote communication technology should be harnessed by the ABPN (or the APA, if it decides to conduct its own board certification) to restore the old model at a fraction of the cost. The oral exams have been replaced by a written exam that is not an accurate reflection or documentation of clinical competence. The traditional oral exam (after passing a written exam) was a magnificent but costly feat of massive logistical complexity, with >1,000 candidates and examiners traveling to a city where the ABPN arranged for several hospitals to shut down their clinics for 2 full days to use their clinical offices for the oral exams. Multiple teams examined the candidates twice on the same day: once with a live patient, and again with a video of a real patient. The examiners filled out scoring cards after observing the candidates conduct the live interview or discussing the video. A consensus grade of pass or fail was documented. At the end of the 2 days, examiners and candidates boarded buses to the airport. It was a highly expensive process (exam fees + airfare + hotel + food). Twice a year, the examiners generously donated their time to the ABPN without compensation, as a token of love for and service to the profession.
That initial certification of a written exam, followed by an oral exam, validated the competence of a psychiatrist both cognitively and clinically. The lifetime certification was truly earned. The same model can now be replicated virtually via videoconferencing at a far lower cost to the ABPN, the candidates, and the examiners. The MOC 10-year recertification can be reduced to a written exam with clinical vignettes and an unrestricted license to practice medicine in any state, which implies that the psychiatrist has received the 50 CME annual credits to renew the license. The rest of the bells and whistles can be strongly recommended but not required. The cost in time and money to both the ABPN and the candidates can be significantly reduced, but more importantly, the clinical competence will be validated at baseline with virtual oral boards after passing the written exam (formerly labeled as part I, preceding the part II oral boards).
The traditional board certification model of the past should be resurrected via videoconferencing and offered as an option to the candidates who prefer it to the current MOC. The MOC can then be simplified to lifetime certification or to only a written exam with clinical vignettes every 10 years to ensure that psychiatrists continue to incorporate relevant clinical and treatment advances in their practice. The KISS principle (keep it simple, stupid) worked very well for many generations of psychiatrists in the past, and will work again going forward if offered as an option. Psychiatrists can then focus on treating patients instead of being burdened by the many time-consuming requirements and hoops of the current MOC.
There are few things that psychiatrists have come to despise more than the American Board of Psychiatry and Neurology (ABPN) Maintenance of Certification (MOC) program. It has become a professional boondoggle for psychiatric practitioners.
The program needs an overhaul and simplification. There are better, more efficient, cost-effective ways to ensure psychiatric physicians’ ongoing clinical competence after they complete their residency training. Technological advances can also facilitate a more valid assessment of competence without having to jump through more and more hoops between recertifications every 10 years.
I passed the boards long before the MOC was created. For 20 years, I also served as a senior examiner for the oral boards, where clinical competency was rigorously assessed by direct observations of psychiatrists examining and establishing rapport with patients and formulating the data into a differential diagnosis, treatment plan, and prognosis. It is noteworthy that psychiatrists who sat for the oral boards had already passed a written exam that tested their cognitive knowledge. Yet approximately one-third of the candidates failed the live oral exam, which clearly implies that passing a written exam is necessary but not sufficient to establish clinical competence, which is the primary purpose of board certification. It was an unfortunate decision to discontinue the face-to-face oral board exam, which is so vital for psychiatry, and to replace it with a written exam and a barrage of time-consuming activities to document lifelong learning and self-assessment, but not genuine clinical competence. The MOC has been MOCkingly referred to as a major pain in the neck for practically all psychiatrists who were not grandfathered with lifetime certification, as was the case in the first 60 years of the ABPN.
Benefits of the patient-based oral exam
Let’s face it: Passing a patient-based oral exam was the ideal mechanism to establish that a psychiatric physician deserved to be a diplomate of the ABPN. During the oral exam, the candidate’s skills were observed from the minute he/she met the patient. The candidate was then observed as he/she systematically explored a wide range of past and current psychiatric symptoms; reviewed the patient’s developmental, medical, family, and social histories; and conducted a competent mental status exam while demonstrating an empathic stance, responding to the patient’s often subtle verbal and nonverbal cues, establishing rapport, and providing psychoeducation before concluding the interview. All these essential components of a psychiatric exam were observed in a compact 30-minute tour de force of clinical skills, communication, and cognitive acumen. This was followed by another 30 minutes of organizing and presenting the clinical data to 2 or 3 colleagues/examiners, in a coherent fashion, connecting all the dots, formulating the case, presenting a meaningful differential diagnosis, and suggesting a rational array of potential treatment options across the biopsychosocial continuum. To top it off, the candidate had to respond effectively, in an evidence-based manner, to a series of questions related to the disease state, its treatment, adverse effects, and prognosis.
It was a joy to watch many colleagues navigate this clinical examination with skill and competence, without crumbling under the pressure of the examiners’ scrutiny. There were some who passed with flying colors, and others who passed despite having a forgivable minor gap here and there because of their overall strong performance. Finally, there were those who stumbled in several components across data collection, doctor–patient interactions, synthesis of the clinical findings, or treatment recommendations. These candidates inevitably received a failing grade by a consensus of 3 examiners. That they failed to demonstrate clinical competence despite having passed the required written exams a year earlier proved that the true competency of a psychiatrist cannot be judged solely by passing a written test but requires a clinical examination of a live patient.
The oral exams represented an unimpeachable evaluation of clinical competence. The examiners often spoke of how they would feel confident and comfortable with referring a family member to those who successfully passed this rigorous, authentic exam on real patients. It was justifiable to give lifetime certification to those who passed the oral exam. Those permanently certified psychiatrists maintained their lifelong learning by having an unrestricted state medical license, which is contingent on acquiring 50 category 1 continuing medical education (CME) credits annually. Why not restore lifelong certification for those who pass both a written and oral exam, as long as they maintain a valid medical license?
According to the ABPN 2019 Annual Report,1 31,514 psychiatrists have received lifetime certification, of whom an estimated 9,547 were still clinically active in 2019. This is the “grandfathered” cohort of psychiatrists to which I belong. I was tested on neurologic patients, not just psychiatric patients, a tribute to the strong bridge that existed between these sister brain specialties. As of 2019, of the 33,277 psychiatrists who received a time-limited certification, 29,343 were still clinically active, an attrition rate of 12% over the past 25 years. This includes psychiatrists who found the MOC too onerous to complete, or are in private practice where MOC is not a vital requirement. However, these days most psychiatrists are obligated to be recertified because so many entities require it. This includes hiring institutions, government agencies (Medicare/Medicaid), health insurance companies, hospital medical staff for privileging and credentialing, and various regulatory boards, such as The Joint Commission, the Accreditation Council for Graduate Medical Education, and academic medical centers. Because most psychiatrists are involved with at least one of these entities, 29,343 have no choice but to perform all the requirements of the MOC, with its countless hours, numerous documentations, and many fees, to remain certified by the ABPN. Notably absent is an alternative mechanism for a certification process that is widely accepted by all agencies and institutions. Psychiatrists are actively seeking alternatives.
Continue to: The ABPN...
The ABPN, long regarded as an esteemed nonprofit organization, has been accused of being a monopoly. Some angry psychiatrists have filed a class action lawsuit to demand other board certification methods. Some have gone to the media to complain about the American Board of Medical Specialties (of which the ABPN is a member board), accusing both of unfair regulations or of raking in substantial profits to support excessively compensated executives. Perception often trumps reality, so no matter how vigorously the ABPN defends itself, its procedures, or its MOC requirements, its customers—psychiatric physicians—feel oppressed or exploited.
How the MOC can be improved
So what can be done to improve the MOC? The need for recertification is arguably necessary to document clinical competency over an approximately 40-year psychiatric career following residency. I conducted a brief survey of
Significant advances in remote communication technology should be harnessed by the ABPN (or the APA, if it decides to conduct its own board certification) to restore the old model at a fraction of the cost. The oral exams have been replaced by a written exam that is not an accurate reflection or documentation of clinical competence. The traditional oral exam (after passing a written exam) was a magnificent but costly feat of massive logistical complexity, with >1,000 candidates and examiners traveling to a city where the ABPN arranged for several hospitals to shut down their clinics for 2 full days to use their clinical offices for the oral exams. Multiple teams examined the candidates twice on the same day: once with a live patient, and again with a video of a real patient. The examiners filled out scoring cards after observing the candidates conduct the live interview or discussing the video. A consensus grade of pass or fail was documented. At the end of the 2 days, examiners and candidates boarded buses to the airport. It was a highly expensive process (exam fees + airfare + hotel + food). Twice a year, the examiners generously donated their time to the ABPN without compensation, as a token of love for and service to the profession.
That initial certification of a written exam, followed by an oral exam, validated the competence of a psychiatrist both cognitively and clinically. The lifetime certification was truly earned. The same model can now be replicated virtually via videoconferencing at a far lower cost to the ABPN, the candidates, and the examiners. The MOC 10-year recertification can be reduced to a written exam with clinical vignettes and an unrestricted license to practice medicine in any state, which implies that the psychiatrist has received the 50 CME annual credits to renew the license. The rest of the bells and whistles can be strongly recommended but not required. The cost in time and money to both the ABPN and the candidates can be significantly reduced, but more importantly, the clinical competence will be validated at baseline with virtual oral boards after passing the written exam (formerly labeled as part I, preceding the part II oral boards).
The traditional board certification model of the past should be resurrected via videoconferencing and offered as an option to the candidates who prefer it to the current MOC. The MOC can then be simplified to lifetime certification or to only a written exam with clinical vignettes every 10 years to ensure that psychiatrists continue to incorporate relevant clinical and treatment advances in their practice. The KISS principle (keep it simple, stupid) worked very well for many generations of psychiatrists in the past, and will work again going forward if offered as an option. Psychiatrists can then focus on treating patients instead of being burdened by the many time-consuming requirements and hoops of the current MOC.
1. American Board of Psychiatry and Neurology. 2019 Annual Report. https://www.abpn.com/wp-content/uploads/2020/05/ABPN_2019_Annual_Report.pdf. Accessed August 14, 2020.
1. American Board of Psychiatry and Neurology. 2019 Annual Report. https://www.abpn.com/wp-content/uploads/2020/05/ABPN_2019_Annual_Report.pdf. Accessed August 14, 2020.
Cognitive-behavioral therapy for insomnia: A review of 8 studies
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16
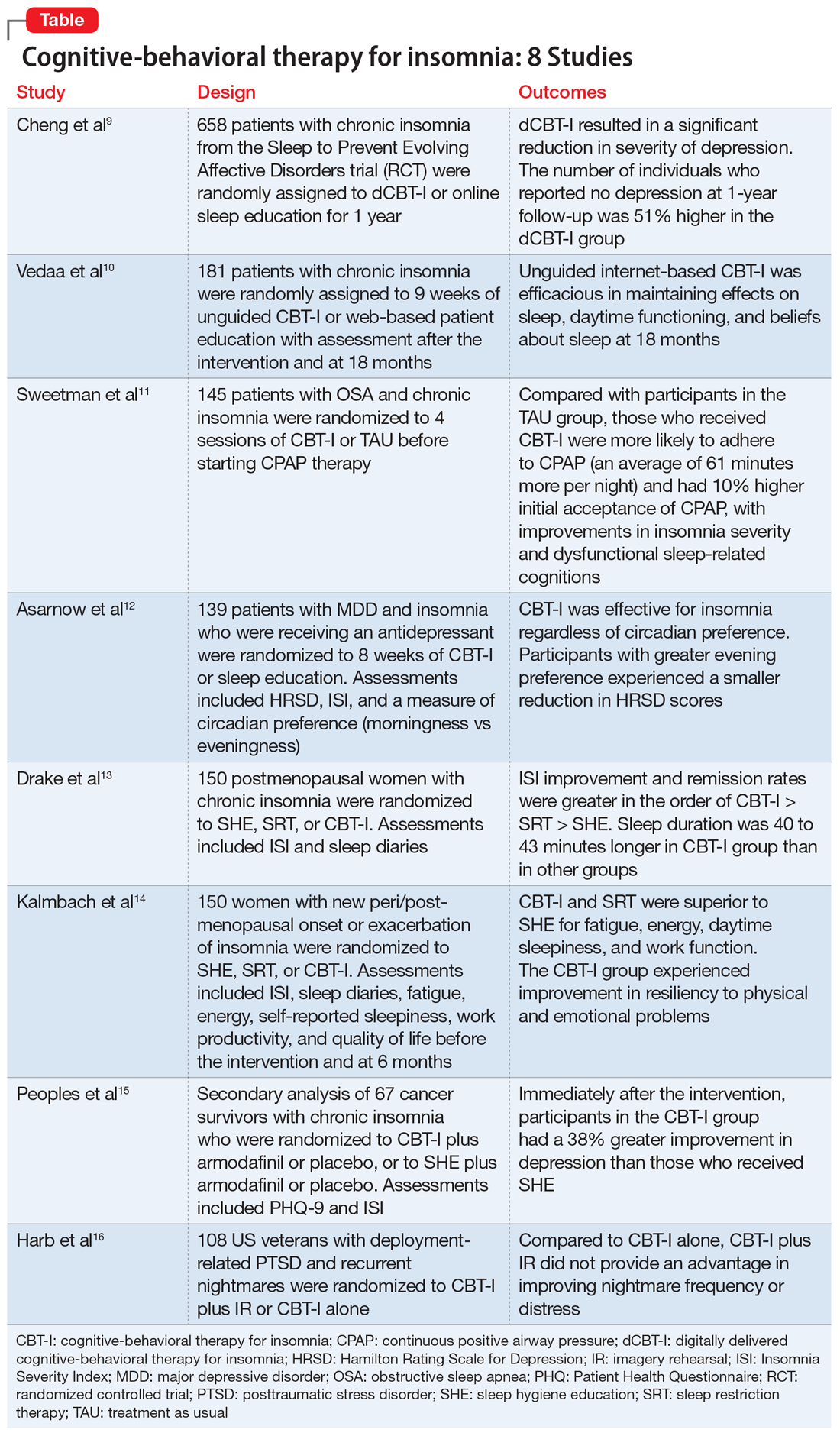
1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16

1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
The prevalence of insomnia in the general population is approximately 6% to 10%.1 In addition, an estimated 30% of the general population may have symptoms of insomnia without meeting the diagnostic criteria.2 As a disorder, insomnia is characterized by a persistent difficulty initiating or maintaining sleep, or early morning awakening with inability to return to sleep, that has been present for at least 3 months. Additionally, the sleep difficulties must occur at least 3 nights a week, result in impaired daytime functioning, and cause significant distress.1
Cognitive-behavioral therapy for insomnia (CBT-I) is an effective treatment, supported by several systematic reviews and meta-analyses.3-5 In the short term, CBT-I is as effective as pharmacotherapy.6 However, CBT-I is the preferred treatment for chronic insomnia, according to recommendations in European and American guidelines.7,8
Here we review 8 recent studies that examined CBT-I. These studies are summarized in the Table.9-16

1. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
Although CBT-I is a first-line treatment for chronic insomnia, it is underutilized in clinical practice primarily due to limited availability. Because few clinicians are certified in CBT-I, it has become necessary to develop alternative modes of delivery for CBT-I, such as fully automated, internet-delivered approaches to reach more patients with insomnia. Digital CBT-I (dCBT-I) is comparable to in-person CBT-I in improving insomnia symptoms and reducing concurrent depressive symptoms with insomnia. It is unclear if unguided, internet-delivered CBT-I is effective for achieving remission from depression or preventing depression in the long term. Chen et al9 examined the efficacy of dCBT-I in reducing and preventing depression over a 1-year follow-up.
Study design
- Participants from various centers in Southeastern Michigan were recruited between 2016 and 2017. Data was obtained from the Sleep to Prevent Evolving Affective Disorders (SPREAD) trial.
- Participants who met DSM-5 criteria for chronic insomnia disorder were randomized to dCBT-I (n = 358) using the Sleepio digital CBT program via the internet or to online sleep education (n = 300).
- The primary outcome was depression, measured using the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR-16) at 1-year follow-up. Depression incidence was also tested against insomnia treatment response.
Outcomes
- The severity of depression was significantly lower at 1-year follow-up in the dCBT-I group compared with the control group.
- The dCBT-I group showed a 51% higher remission rate than the control group at 1-year follow-up.
- The incidence of moderate to severe depression in individuals with minimal to no depression at baseline was halved at 1 year after receiving dCBT-I treatment compared with the control group.
Continue to: Conclusion
Conclusion
- dCBT-I can improve depression and insomnia and has a sustained antidepressant effect.
- dCBT-I is effective for preventing depression. In other words, the risk of developing depression is decreased when dCBT-I is used to treat insomnia in individuals with minimal to no depression at baseline.
2. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
dCBT-I is effective for treating insomnia in the short term; however, little is known about the long-term effectiveness of dCBT-I on sleep and daytime symptoms. Vedaa et al10 evaluated the efficacy of dCBT-I at 18 months after the intervention.
Study design
- In this randomized controlled trial (RCT), the efficacy of unguided, internet-delivered CBT-I (n = 95) was compared with web-based patient education (n = 86) for patients with chronic insomnia.
- Participants were assessed at baseline, after a 9-week intervention period, and at 6-month follow-up. Participants in the internet CBT-I group were reassessed at 18 months after the intervention using online questionnaires, including the Insomnia Severity Index (ISI), Bergen Insomnia Scale (BIS), Brief Dysfunctional Beliefs and Attitudes Scale, Hospital Anxiety and Depression Scale, Chalder Fatigue Questionnaire, and sleep diaries.
Outcomes
- At 18 months, significant improvements were noted from baseline ISI and BIS scores and in levels of daytime fatigue, as well as psychological distress and beliefs about sleep.
- Sleep diary variables—including sleep onset latency, time awake during the night (wake time after sleep onset), early morning awakening, total sleep time, and sleep efficiency—showed significant improvement from baseline to 18-month follow-up (at least moderate effect size).
- Improvements were maintained from the completion of the 9-week intervention to follow-up at 18 months.
Continue to: Conclusion
Conclusion
- Fully-automated, internet-based CBT-I is efficacious in maintaining positive effects on sleep and daytime functioning up to 18 months after completing treatment.
3. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
Comorbid insomnia and sleep apnea (COMISA) can affect a patient’s ability to accept and comply with continuous positive airway pressure (CPAP) therapy. Providing adequate treatment for these patients can be challenging.
Sweetman et al11 evaluated the acceptance and use of CPAP in patients with obstructive sleep apnea and chronic insomnia following initial treatment with CBT-I compared with treatment as usual (TAU).
Study design
- In this RCT, 145 participants with COMISA were randomized to 4 sessions of CBT-I or TAU before starting CPAP therapy until 6 months after randomization.
- Primary outcomes were objective CPAP adherence and objective sleep efficiency at the end of 6 months.
- Secondary outcomes were CPAP acceptance/rejection, changes in sleep parameters, global insomnia severity, and daytime impairments at 6 months.
Continue to: Outcomes
Outcomes
- The CBT-I group had higher initial CPAP acceptance and greater average nightly adherence to CPAP (61 minutes more) than the TAU group.
- Significant improvements were noted in global insomnia severity, nighttime insomnia complaints, and dysfunctional sleep-related cognitions at 6 months in the CBT-I group compared with TAU.
- No differences between the 2 groups were noted in sleep diary parameters or daytime impairments at 6 months.
Conclusions
- Patients with COMISA can benefit from receiving CBT-I before starting CPAP therapy because CBT-I can improve immediate acceptance of CPAP and may help to maintain adherence to CPAP over time.
- Patients with sleep apnea should be evaluated for comorbid insomnia, and CBT-I should be considered before starting CPAP treatment.
4. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
The Treatment of Insomnia and Depression (TRIAD) study reported the effects of combining antidepressants with CBT-I in patients with major depressive disorder (MDD) and insomnia. Asarnow et al12 examined the moderation of circadian preference in the reduction of depression and insomnia symptoms severity during the same trial.
Study design
- In this RCT, 139 participants with MDD and insomnia were treated with an antidepressant (escitalopram, sertraline, or desvenlafaxine) and randomized to 8 weeks of CBT-I or control therapy (sleep education).
- Measurements used were Composite Scale of Morningness for circadian preference (morningness vs eveningness), depression severity with the Hamilton Rating Scale for Depression, and insomnia severity using the ISI.
Continue to: Outcomes
Outcomes
- CBT-I was effective for insomnia regardless of circadian preference.
- A smaller reduction in depression scores was noted in participants with greater evening preference.
- Depression outcomes were better among participants with evening preference if they were assigned to CBT-I vs control therapy.
- The control therapy (sleep education) was particularly ineffective in reducing depression symptoms in participants with evening preference.
Conclusion
- Individuals with MDD and insomnia and an evening preference are at an increased risk for poor response to antidepressants alone.
- Outcomes for both depression and insomnia improve if CBT-I is combined with antidepressants.
- Offering sleep education alone is not sufficient.
5. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
Postmenopausal women with sleep disturbances experience higher medical and psychiatric comorbidities, and have higher alcohol consumption and stress levels than postmenopausal women with good sleep. Nonpharmacologic insomnia treatments with durable effects are imperative for postmenopausal women because they are safer than pharmacologic approaches. Although CBT-I is the recommended first-line treatment for chronic insomnia, its application in menopause-related insomnia is limited. Drake et al13 evaluated the efficacy of CBT-I in menopause-related insomnia compared with sleep restriction therapy (SRT) and sleep hygiene education (SHE).
Study design
- This RCT was conducted at a health system with 6 hospitals in Michigan.
- Postmenopausal women who met DSM-5 criteria for chronic insomnia disorder (n = 150) were randomized into 1 of 3 groups: SHE, SRT, or CBT-I.
- Primary outcome measures were ISI scores and sleep diaries that documented multiple sleep parameters, including sleep onset latency, wake time after sleep onset, number of awakenings in the middle of the night, time in bed, total sleep time, and sleep efficiency. These were measured at baseline, after completion of treatment, and 6 months after treatment.
Continue to: Outcomes
Outcomes
- Both CBT-I and SRT outperformed SHE on the ISI and for most of the sleep parameters on sleep diaries immediately after treatment completion and at 6 months after treatment.
- Total sleep time was 40 to 43 minutes longer in the CBT-I group than in the SRT and SHE groups at 6-month follow-up.
- Remission rates (sleep onset latency ≤30 minutes, wake time after sleep onset ≤30 minutes, sleep efficiency ≥85%) were significantly higher in CBT-I group (CBT-I > SRT > SHE).
Conclusion
- Sleep hygiene education as a standalone treatment is not useful for treating chronic insomnia.
- Both CBT-I and SRT are efficacious for menopause-related insomnia.
- CBT-I may be a better option than SRT because it produces higher remission rates and better long-term outcomes.
6. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
CBT-I has shown efficacy in the treatment of insomnia in postmenopausal women. In this study, Kalmbach et al14 compared 3 nonpharmacologic modalities—CBT-I, SRT, and SHE—for the treatment of menopause-related insomnia and daytime impairment.
Study design
- In this RCT, 150 participants with new peri- and post-menopausal onset or exacerbation of insomnia were randomized to 1 of 3 groups: SHE, SRT, or CBT-I.
- Participants were assessed at baseline, after treatment completion, and at 6-month follow-up using the ISI, sleep diaries, Fatigue Severity Scale, Epworth Sleepiness Scale, Work Productivity and Activity Impairment Questionnaire, and 36-item Medical Outcomes Study Short Form Health Survey.
Continue to: Outcomes
Outcomes
- In both the CBT-I and SRT groups, significant improvements were noted in fatigue, energy, daytime sleepiness, and work function after treatment completion and at 6-month follow-up.
- Improvements were noted in emotional well-being and resiliency to physical and emotional problems in the CBT-I group at 6 months.
- Improvements in overall general health and social functioning, less pain, and fewer hot flashes were reported by postmenopausal women who remitted from insomnia; however, these benefits were not directly related to any specific treatment modality.
Conclusion
- CBT-I and SRT are superior to SHE for improving daytime functioning, and some aspects of life quality and work productivity, in postmenopausal women with insomnia.
- CBT-I may be superior to SRT in producing larger improvements in fatigue, energy level, and daytime sleep propensity.
- CBT-I can improve emotional well-being and resilience to emotional problems in postmenopausal women with insomnia.
7. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
Depression is common in patients with cancer and is usually associated with comorbid insomnia. Depression has significant effect on treatment compliance, coping with illness, and quality of life. Peoples et al15 examined the effects of CBT-I on depression in cancer survivors.
Study design
- This was a secondary analysis of a multicenter, randomized, placebo-controlled trial that evaluated interventions for cancer survivors with chronic insomnia in which the primary outcome measure was insomnia severity.
- Cancer survivors (n = 67) were randomized to CBT-I plus armodafinil or placebo or to SHE plus armodafinil or placebo.
- The Patient Health Questionnaire-9 (PHQ-9) and ISI were used to measure depression and insomnia at baseline, after 7-weeks of intervention, and at 3 months postintervention.
Continue to: Outcomes
Outcomes
- Immediately after completing the intervention, cancer survivors treated with CBT-I had significantly less depression (38% greater improvement in depression) compared with those who received SHE (control group).
- In the CBT-I group, 23% of cancer survivors achieved a clinically important reduction (5-point reduction on PHQ-9 total score) in depression at postintervention compared with 6% of those in the control group.
- At 3 months after the intervention, only 14% of cancer survivors in CBT-I group reported depression (PHQ-9 score >4), whereas 47% of those in the control group (SHE) reported depression.
Conclusion
- CBT-I improves both depression and insomnia in cancer survivors, and the improvements are sustained at 3 months after completing treatment.
- Improvement in insomnia severity appears to mediate the positive effects of CBT-I on depression.
8. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
The American Academy of Sleep Medicine recommends imagery rehearsal (IR) therapy, which incorporates some components of CBT-I, for the treatment of recurrent posttraumatic stress disorder (PTSD)–related nightmares. In this study, Harb et al16 compared CBT-I plus IR to CBT-I alone for the treatment of nightmares in veterans with combat-related PTSD.
Study design
- This RCT included male and female US veterans (n = 108) deployed to Iraq and Afghanistan with current PTSD and recurrent nightmares related to deployment.
- Participants were randomized to 6 sessions of CBT-I plus IR or CBT-I alone.
- Primary outcome measures included frequency of nightmares and distress associated with nightmares.
Continue to: Outcomes
Outcomes
- A significant improvement in nightmares was noted in both groups (29% of participants showed a clinically-significant reduction in nightmare frequency and 22% of participants achieved remission).
- CBT-I plus IR was not superior to CBT-I only at postintervention and at 6-month follow-up.
Conclusion
- Both IR and CBT-I demonstrated efficacy for decreasing nightmare frequency and distress.
- Combining IR and CBT-I may not provide a synergistic advantage over CBT-I alone for treating PTSD-related nightmares in veterans.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123-130.
3. Trauer JM, Qian MY, Doyle JS, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191-204.
4. Wu JQ, Appleman ER, Salazar RD, et al. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. 2015;175(9):1461-1472.
5. van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev. 2018;38:3-16.
6. Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5-11.
7. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
9. Cheng P, Kalmbach DA, Tallent G, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10):zsz150. doi: 10.1093/sleep/zsz150.
10. Vedaa Ø, Hagatun S, Kallestad H, et al. Long-term effects of an unguided online cognitive behavioral therapy for chronic insomnia. J Clin Sleep Med. 2019;15(1):101-110.
11. Sweetman A, Lack L, Catcheside PG, et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with comorbid insomnia: a randomized clinical trial. Sleep. 2019;42(12):zsz178. doi: 10.1093/sleep/zsz178.
12. Asarnow LD, Bei B, Krystal A, et al. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the TRIAD study. J Clin Sleep Med. 2019;15(4):573-580.
13. Drake CL, Kalmbach DA, Arnedt JT, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. doi: 10.1093/sleep/zsy217.
14. Kalmbach DA, Cheng P, Arnedt JT, et al. Improving daytime functioning, work performance, and quality of life in postmenopausal women with insomnia: comparing cognitive behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. J Clin Sleep Med. 2019;15(7):999-1010.
15. Peoples AR, Garland SN, Pigeon WR, et al. Cognitive behavioral therapy for insomnia reduces depression in cancer survivors. J Clin Sleep Med. 2019;15(1):129-137.
16. Harb GC, Cook JM, Phelps AJ, et al. Randomized controlled trial of imagery rehearsal for posttraumatic nightmares in combat veterans. J Clin Sleep Med. 2019;15(5):757-767.
Obsessions or psychosis?
CASE Perseverating on nonexistent sexual assaults
Mr. R, age 17, who has been diagnosed with obsessive-compulsive disorder (OCD), presents to the emergency department (ED) because he thinks that he is being sexually assaulted and is concerned that he is sexually assaulting other people. His family reports that Mr. R has perseverated over these thoughts for months, although there is no evidence to suggest these events have occurred. In order to ameliorate his distress, he performs rituals of looking upwards and repeatedly saying, “It didn’t happen.”
Mr. R is admitted to the inpatient psychiatry unit for further evaluation.
HISTORY Decompensation while attending a PHP
Mr. R had been diagnosed with bipolar disorder and attention-deficit/hyperactivity disorder when he was 13. During that time, he was treated with divalproex sodium and dextroamphetamine. At age 15, Mr. R’s diagnosis was changed to OCD. Seven months before coming to the ED, his symptoms had been getting worse. On one occasion, Mr. R was talking in a nonsensical fashion at school, and the police were called. Mr. R became physically aggressive with the police and was subsequently hospitalized, after which he attended a partial hospitalization program (PHP). At the PHP, Mr. R received exposure and response prevention therapy for OCD, but did not improve, and his symptoms deteriorated until he was unable to brush his teeth or shower regularly. While attending the PHP, Mr. R also developed disorganized speech. The PHP clinicians became concerned that Mr. R’s symptoms may have been prodromal symptoms of schizophrenia because he did not respond to the OCD treatment and his symptoms had worsened over the 3 months he attended the PHP.
EVALUATION Normal laboratory results
Upon admission to the inpatient psychiatric unit, Mr. R is restarted on his home medications, which include
His laboratory workup, including a complete blood count, comprehensive metabolic panel, urine drug screen, and blood ethanol, are all within normal limits. Previous laboratory results, including a thyroid function panel, vitamin D level, and various autoimmune panels, were also within normal limits.
His family reports that Mr. R’s symptoms seem to worsen when he is under increased stress from school and prepping for standardized college admission examinations. The family also says that while he is playing tennis, Mr. R will posture himself in a crouched down position and at times will remain in this position for 30 minutes.
Mr. R says he eventually wants to go to college and have a professional career.
[polldaddy:10600530]
Continue to: The authors' observations
The authors’ observations
When considering Mr. R’s diagnosis, our treatment team considered the possibility of OCD with absent insight/delusional beliefs, OCD with comorbid schizophrenia, bipolar disorder, and psychotic disorder due to another medical condition.
Overlap between OCD and schizophrenia
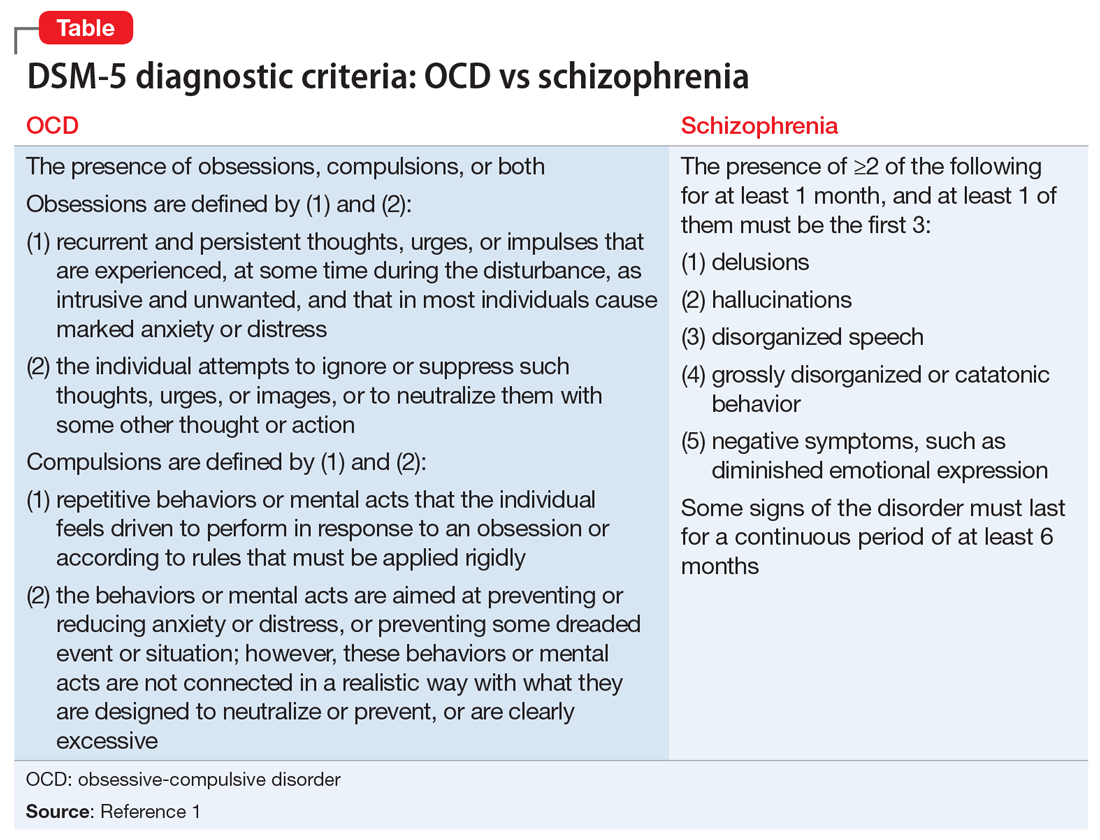
Much of the literature about OCD examines its functional impairment in adults, with findings extrapolated to pediatric patients. Children differ from adults in a variety of meaningful ways. Baytunca et al4 examined adolescents with early-onset schizophrenia, with and without comorbid OCD. Patients with comorbid OCD required higher doses of antipsychotic medication to treat acute psychotic symptoms and maintain a reduction in symptoms. The study controlled for the severity of schizophrenia, and its findings suggest that schizophrenia with comorbid OCD is more treatment-resistant than schizophrenia alone.4
Some researchers have theorized that in adolescents, OCD and psychosis are integrally related such that one disorder could represent a prodrome or a cause of the other disorder. Niendam et al5 studied OCS in the psychosis prodrome. They found that OCS can present as a part of the prodromal picture in youth at high risk for psychosis. However, because none of the patients experiencing OCS converted to full-blown psychosis, these results suggest that OCS may not represent a prodrome to psychosis per se. Instead, these individuals may represent a subset of false positives over the follow-up period.5 Another possible explanation for the increased emergence of pre-psychotic symptoms in adolescents with OCD could be a difference in their threshold of perception. OCS compels adolescents with OCD to self-analyze more critically and frequently. As a result, these patients may more often report depressive symptoms, distress, and exacerbations of pre-psychotic symptoms. These findings highlight that
[polldaddy:10600532]
Continue to: TREATMENT Improvement after switching to haloperidol
TREATMENT Improvement after switching to haloperidol
The treatment team decides to change Mr. R’s medications by cross-titrating risperidone to
The treatment team obtains a consultation on whether electroconvulsive therapy would be appropriate, but this treatment is not recommended. Instead, the team considers
Throughout admission, Mr. R focuses on his lack of improvement and how this episode is negatively impacting his grades and his dream of going to college and having a professional career.
OUTCOME Relief at last
Mr. R improves with the addition of sertraline and tolerates rapid titration well. He continues haloperidol without adverse effects, and is discharged home with close follow-up in a PHP and outpatient psychiatry.
However, after discharge, Mr. R’s symptoms get worse, and he is admitted to a different inpatient facility. At this facility, he continues sertraline, but haloperidol is cross-titrated to
Continue to: Currently...
Currently, Mr. R has greatly improved and is able to function in school. He takes sertraline, 100 mg twice a day, and olanzapine, 7.5 mg twice a day. Mr. R reports his rituals have reduced in frequency to less than 15 minutes each day. His thought processes are organized, and he is confident he will be able to achieve his goals.
The authors’ observations
Given Mr. R’s rapid improvement once an effective pharmacologic regimen was established, we concluded that he had a severe case of OCD with absent insight/delusional beliefs, and that he did not have schizophrenia. Mr. R’s case highlights how a psychiatric diagnosis can produce anxiety as a result of the psychosocial stressors and limitations associated with that diagnosis.
Bottom Line
There is both an epidemiologic and biologic overlap between obsessive-compulsive disorder and schizophrenia. In adolescents, either disorder could represent a prodrome or a cause of the other. It is essential to perform a thorough assessment of individuals with obsessive-compulsive disorder because these patients may exhibit subtle psychotic symptoms.
Related Resources
- Cunill R, Castells X, Simeon D. Relationships between obsessivecompulsive symptomatology and severity of psychosis in schizophrenia: a systematic review and meta-analysis. J Clin Psychiatry. 2009;70(1):70-82.
- Harris E, Delgado SV. Treatment-resistant OCD: there’s more we can do. Current Psychiatry. 2018;17(11):10-12,14-18,51.
Drug Brand Names
Clozapine • Clozaril
Dextroamphetamine • Dexedrine
Divalproex sodium • Depakote
Fluvoxamine • Luvox
Haloperidol • Haldol
Hydroxyzine • Atarax, Vistaril
Lurasidone • Latuda
Olanzapine • Zyprexa
Risperidone • Risperdal
Sertraline • Zoloft
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Schirmbeck F, Swets M, de Haan L. Obsessive-compulsive symptoms in schizophrenia. In: De Haan L, Schirmbeck F, Zink M. Epidemiology: prevalence and clinical characteristics of obsessive-compulsive disorder and obsessive-compulsive symptoms in patients with psychotic disorders. New York, NY: Springer International Publishing; 2015:47-61.
3. de Haan L, Sterk B, Wouters L, et al. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first-episode schizophrenia and related disorders. Schizophr Bull. 2011;39(1):151-160.
4. Baytunca B, Kalyoncu T, Ozel I, et al. Early onset schizophrenia associated with obsessive-compulsive disorder: clinical features and correlates. Clin Neuropharmacol. 2017;40(6):243-245.
5. Niendam TA, Berzak J, Cannon TD, et al. Obsessive compulsive symptoms in the psychosis prodrome: correlates of clinical and functional outcome. Schizophr Res. 2009;108(1-3):170-175.
CASE Perseverating on nonexistent sexual assaults
Mr. R, age 17, who has been diagnosed with obsessive-compulsive disorder (OCD), presents to the emergency department (ED) because he thinks that he is being sexually assaulted and is concerned that he is sexually assaulting other people. His family reports that Mr. R has perseverated over these thoughts for months, although there is no evidence to suggest these events have occurred. In order to ameliorate his distress, he performs rituals of looking upwards and repeatedly saying, “It didn’t happen.”
Mr. R is admitted to the inpatient psychiatry unit for further evaluation.
HISTORY Decompensation while attending a PHP
Mr. R had been diagnosed with bipolar disorder and attention-deficit/hyperactivity disorder when he was 13. During that time, he was treated with divalproex sodium and dextroamphetamine. At age 15, Mr. R’s diagnosis was changed to OCD. Seven months before coming to the ED, his symptoms had been getting worse. On one occasion, Mr. R was talking in a nonsensical fashion at school, and the police were called. Mr. R became physically aggressive with the police and was subsequently hospitalized, after which he attended a partial hospitalization program (PHP). At the PHP, Mr. R received exposure and response prevention therapy for OCD, but did not improve, and his symptoms deteriorated until he was unable to brush his teeth or shower regularly. While attending the PHP, Mr. R also developed disorganized speech. The PHP clinicians became concerned that Mr. R’s symptoms may have been prodromal symptoms of schizophrenia because he did not respond to the OCD treatment and his symptoms had worsened over the 3 months he attended the PHP.
EVALUATION Normal laboratory results
Upon admission to the inpatient psychiatric unit, Mr. R is restarted on his home medications, which include
His laboratory workup, including a complete blood count, comprehensive metabolic panel, urine drug screen, and blood ethanol, are all within normal limits. Previous laboratory results, including a thyroid function panel, vitamin D level, and various autoimmune panels, were also within normal limits.
His family reports that Mr. R’s symptoms seem to worsen when he is under increased stress from school and prepping for standardized college admission examinations. The family also says that while he is playing tennis, Mr. R will posture himself in a crouched down position and at times will remain in this position for 30 minutes.
Mr. R says he eventually wants to go to college and have a professional career.
[polldaddy:10600530]
Continue to: The authors' observations
The authors’ observations
When considering Mr. R’s diagnosis, our treatment team considered the possibility of OCD with absent insight/delusional beliefs, OCD with comorbid schizophrenia, bipolar disorder, and psychotic disorder due to another medical condition.
Overlap between OCD and schizophrenia

Much of the literature about OCD examines its functional impairment in adults, with findings extrapolated to pediatric patients. Children differ from adults in a variety of meaningful ways. Baytunca et al4 examined adolescents with early-onset schizophrenia, with and without comorbid OCD. Patients with comorbid OCD required higher doses of antipsychotic medication to treat acute psychotic symptoms and maintain a reduction in symptoms. The study controlled for the severity of schizophrenia, and its findings suggest that schizophrenia with comorbid OCD is more treatment-resistant than schizophrenia alone.4
Some researchers have theorized that in adolescents, OCD and psychosis are integrally related such that one disorder could represent a prodrome or a cause of the other disorder. Niendam et al5 studied OCS in the psychosis prodrome. They found that OCS can present as a part of the prodromal picture in youth at high risk for psychosis. However, because none of the patients experiencing OCS converted to full-blown psychosis, these results suggest that OCS may not represent a prodrome to psychosis per se. Instead, these individuals may represent a subset of false positives over the follow-up period.5 Another possible explanation for the increased emergence of pre-psychotic symptoms in adolescents with OCD could be a difference in their threshold of perception. OCS compels adolescents with OCD to self-analyze more critically and frequently. As a result, these patients may more often report depressive symptoms, distress, and exacerbations of pre-psychotic symptoms. These findings highlight that
[polldaddy:10600532]
Continue to: TREATMENT Improvement after switching to haloperidol
TREATMENT Improvement after switching to haloperidol
The treatment team decides to change Mr. R’s medications by cross-titrating risperidone to
The treatment team obtains a consultation on whether electroconvulsive therapy would be appropriate, but this treatment is not recommended. Instead, the team considers
Throughout admission, Mr. R focuses on his lack of improvement and how this episode is negatively impacting his grades and his dream of going to college and having a professional career.
OUTCOME Relief at last
Mr. R improves with the addition of sertraline and tolerates rapid titration well. He continues haloperidol without adverse effects, and is discharged home with close follow-up in a PHP and outpatient psychiatry.
However, after discharge, Mr. R’s symptoms get worse, and he is admitted to a different inpatient facility. At this facility, he continues sertraline, but haloperidol is cross-titrated to
Continue to: Currently...
Currently, Mr. R has greatly improved and is able to function in school. He takes sertraline, 100 mg twice a day, and olanzapine, 7.5 mg twice a day. Mr. R reports his rituals have reduced in frequency to less than 15 minutes each day. His thought processes are organized, and he is confident he will be able to achieve his goals.
The authors’ observations
Given Mr. R’s rapid improvement once an effective pharmacologic regimen was established, we concluded that he had a severe case of OCD with absent insight/delusional beliefs, and that he did not have schizophrenia. Mr. R’s case highlights how a psychiatric diagnosis can produce anxiety as a result of the psychosocial stressors and limitations associated with that diagnosis.
Bottom Line
There is both an epidemiologic and biologic overlap between obsessive-compulsive disorder and schizophrenia. In adolescents, either disorder could represent a prodrome or a cause of the other. It is essential to perform a thorough assessment of individuals with obsessive-compulsive disorder because these patients may exhibit subtle psychotic symptoms.
Related Resources
- Cunill R, Castells X, Simeon D. Relationships between obsessivecompulsive symptomatology and severity of psychosis in schizophrenia: a systematic review and meta-analysis. J Clin Psychiatry. 2009;70(1):70-82.
- Harris E, Delgado SV. Treatment-resistant OCD: there’s more we can do. Current Psychiatry. 2018;17(11):10-12,14-18,51.
Drug Brand Names
Clozapine • Clozaril
Dextroamphetamine • Dexedrine
Divalproex sodium • Depakote
Fluvoxamine • Luvox
Haloperidol • Haldol
Hydroxyzine • Atarax, Vistaril
Lurasidone • Latuda
Olanzapine • Zyprexa
Risperidone • Risperdal
Sertraline • Zoloft
CASE Perseverating on nonexistent sexual assaults
Mr. R, age 17, who has been diagnosed with obsessive-compulsive disorder (OCD), presents to the emergency department (ED) because he thinks that he is being sexually assaulted and is concerned that he is sexually assaulting other people. His family reports that Mr. R has perseverated over these thoughts for months, although there is no evidence to suggest these events have occurred. In order to ameliorate his distress, he performs rituals of looking upwards and repeatedly saying, “It didn’t happen.”
Mr. R is admitted to the inpatient psychiatry unit for further evaluation.
HISTORY Decompensation while attending a PHP
Mr. R had been diagnosed with bipolar disorder and attention-deficit/hyperactivity disorder when he was 13. During that time, he was treated with divalproex sodium and dextroamphetamine. At age 15, Mr. R’s diagnosis was changed to OCD. Seven months before coming to the ED, his symptoms had been getting worse. On one occasion, Mr. R was talking in a nonsensical fashion at school, and the police were called. Mr. R became physically aggressive with the police and was subsequently hospitalized, after which he attended a partial hospitalization program (PHP). At the PHP, Mr. R received exposure and response prevention therapy for OCD, but did not improve, and his symptoms deteriorated until he was unable to brush his teeth or shower regularly. While attending the PHP, Mr. R also developed disorganized speech. The PHP clinicians became concerned that Mr. R’s symptoms may have been prodromal symptoms of schizophrenia because he did not respond to the OCD treatment and his symptoms had worsened over the 3 months he attended the PHP.
EVALUATION Normal laboratory results
Upon admission to the inpatient psychiatric unit, Mr. R is restarted on his home medications, which include
His laboratory workup, including a complete blood count, comprehensive metabolic panel, urine drug screen, and blood ethanol, are all within normal limits. Previous laboratory results, including a thyroid function panel, vitamin D level, and various autoimmune panels, were also within normal limits.
His family reports that Mr. R’s symptoms seem to worsen when he is under increased stress from school and prepping for standardized college admission examinations. The family also says that while he is playing tennis, Mr. R will posture himself in a crouched down position and at times will remain in this position for 30 minutes.
Mr. R says he eventually wants to go to college and have a professional career.
[polldaddy:10600530]
Continue to: The authors' observations
The authors’ observations
When considering Mr. R’s diagnosis, our treatment team considered the possibility of OCD with absent insight/delusional beliefs, OCD with comorbid schizophrenia, bipolar disorder, and psychotic disorder due to another medical condition.
Overlap between OCD and schizophrenia

Much of the literature about OCD examines its functional impairment in adults, with findings extrapolated to pediatric patients. Children differ from adults in a variety of meaningful ways. Baytunca et al4 examined adolescents with early-onset schizophrenia, with and without comorbid OCD. Patients with comorbid OCD required higher doses of antipsychotic medication to treat acute psychotic symptoms and maintain a reduction in symptoms. The study controlled for the severity of schizophrenia, and its findings suggest that schizophrenia with comorbid OCD is more treatment-resistant than schizophrenia alone.4
Some researchers have theorized that in adolescents, OCD and psychosis are integrally related such that one disorder could represent a prodrome or a cause of the other disorder. Niendam et al5 studied OCS in the psychosis prodrome. They found that OCS can present as a part of the prodromal picture in youth at high risk for psychosis. However, because none of the patients experiencing OCS converted to full-blown psychosis, these results suggest that OCS may not represent a prodrome to psychosis per se. Instead, these individuals may represent a subset of false positives over the follow-up period.5 Another possible explanation for the increased emergence of pre-psychotic symptoms in adolescents with OCD could be a difference in their threshold of perception. OCS compels adolescents with OCD to self-analyze more critically and frequently. As a result, these patients may more often report depressive symptoms, distress, and exacerbations of pre-psychotic symptoms. These findings highlight that
[polldaddy:10600532]
Continue to: TREATMENT Improvement after switching to haloperidol
TREATMENT Improvement after switching to haloperidol
The treatment team decides to change Mr. R’s medications by cross-titrating risperidone to
The treatment team obtains a consultation on whether electroconvulsive therapy would be appropriate, but this treatment is not recommended. Instead, the team considers
Throughout admission, Mr. R focuses on his lack of improvement and how this episode is negatively impacting his grades and his dream of going to college and having a professional career.
OUTCOME Relief at last
Mr. R improves with the addition of sertraline and tolerates rapid titration well. He continues haloperidol without adverse effects, and is discharged home with close follow-up in a PHP and outpatient psychiatry.
However, after discharge, Mr. R’s symptoms get worse, and he is admitted to a different inpatient facility. At this facility, he continues sertraline, but haloperidol is cross-titrated to
Continue to: Currently...
Currently, Mr. R has greatly improved and is able to function in school. He takes sertraline, 100 mg twice a day, and olanzapine, 7.5 mg twice a day. Mr. R reports his rituals have reduced in frequency to less than 15 minutes each day. His thought processes are organized, and he is confident he will be able to achieve his goals.
The authors’ observations
Given Mr. R’s rapid improvement once an effective pharmacologic regimen was established, we concluded that he had a severe case of OCD with absent insight/delusional beliefs, and that he did not have schizophrenia. Mr. R’s case highlights how a psychiatric diagnosis can produce anxiety as a result of the psychosocial stressors and limitations associated with that diagnosis.
Bottom Line
There is both an epidemiologic and biologic overlap between obsessive-compulsive disorder and schizophrenia. In adolescents, either disorder could represent a prodrome or a cause of the other. It is essential to perform a thorough assessment of individuals with obsessive-compulsive disorder because these patients may exhibit subtle psychotic symptoms.
Related Resources
- Cunill R, Castells X, Simeon D. Relationships between obsessivecompulsive symptomatology and severity of psychosis in schizophrenia: a systematic review and meta-analysis. J Clin Psychiatry. 2009;70(1):70-82.
- Harris E, Delgado SV. Treatment-resistant OCD: there’s more we can do. Current Psychiatry. 2018;17(11):10-12,14-18,51.
Drug Brand Names
Clozapine • Clozaril
Dextroamphetamine • Dexedrine
Divalproex sodium • Depakote
Fluvoxamine • Luvox
Haloperidol • Haldol
Hydroxyzine • Atarax, Vistaril
Lurasidone • Latuda
Olanzapine • Zyprexa
Risperidone • Risperdal
Sertraline • Zoloft
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Schirmbeck F, Swets M, de Haan L. Obsessive-compulsive symptoms in schizophrenia. In: De Haan L, Schirmbeck F, Zink M. Epidemiology: prevalence and clinical characteristics of obsessive-compulsive disorder and obsessive-compulsive symptoms in patients with psychotic disorders. New York, NY: Springer International Publishing; 2015:47-61.
3. de Haan L, Sterk B, Wouters L, et al. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first-episode schizophrenia and related disorders. Schizophr Bull. 2011;39(1):151-160.
4. Baytunca B, Kalyoncu T, Ozel I, et al. Early onset schizophrenia associated with obsessive-compulsive disorder: clinical features and correlates. Clin Neuropharmacol. 2017;40(6):243-245.
5. Niendam TA, Berzak J, Cannon TD, et al. Obsessive compulsive symptoms in the psychosis prodrome: correlates of clinical and functional outcome. Schizophr Res. 2009;108(1-3):170-175.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Schirmbeck F, Swets M, de Haan L. Obsessive-compulsive symptoms in schizophrenia. In: De Haan L, Schirmbeck F, Zink M. Epidemiology: prevalence and clinical characteristics of obsessive-compulsive disorder and obsessive-compulsive symptoms in patients with psychotic disorders. New York, NY: Springer International Publishing; 2015:47-61.
3. de Haan L, Sterk B, Wouters L, et al. The 5-year course of obsessive-compulsive symptoms and obsessive-compulsive disorder in first-episode schizophrenia and related disorders. Schizophr Bull. 2011;39(1):151-160.
4. Baytunca B, Kalyoncu T, Ozel I, et al. Early onset schizophrenia associated with obsessive-compulsive disorder: clinical features and correlates. Clin Neuropharmacol. 2017;40(6):243-245.
5. Niendam TA, Berzak J, Cannon TD, et al. Obsessive compulsive symptoms in the psychosis prodrome: correlates of clinical and functional outcome. Schizophr Res. 2009;108(1-3):170-175.
Off-label prescribing: How to limit your liability
The FDA defines “off-label” prescribing as prescribing an FDA-approved medication for an unapproved use, such as for an unapproved clinical indication, for a higher-than-approved dose, or for a patient who is not part of the FDA-approved population (eg, children or geriatric patients).1 Off-label prescribing is common in psychiatry; approximately 13% of psychiatry patients are prescribed off-label psychotropic medications.2 The American Psychiatric Association strongly supports “the autonomous clinical decision-making authority of a physician” and “a physician’s lawful use of an FDA-approved drug product or medical device for an off-label indication when such use is based upon sound scientific evidence in conjunction with sound medical judgment.”3 Because many psychiatric diagnoses have no FDA-approved medications, off-label prescribing often may be a psychiatrist’s only pharmacologic option.
Unfortunately, off-label prescribing can increase a psychiatrist’s risk for liability when treatment falls short of patients’ expectations, or when patients allege that they were injured by the use of an off-label medication. Off-label prescribing does not automatically lead to losing a malpractice suit because the FDA states that physicians can prescribe approved medications for any scientifically supported use, including off-label.1 Medical malpractice lawsuits alleging negligence in prescribing practices, such as off-label prescribing, typically include allegations against the psychiatrist for failure to4:
- adequately assess the patient
- consult the patient’s medical records
- obtain informed consent from the patient
- appropriately prescribe a medication for the clinical indication, dosage, patient’s age, etc.
- monitor for adverse effects and therapeutic effectiveness.
Steps to minimize your risk
When prescribing a medication off-label, the following approaches can help reduce your liability risk:
Conduct a comprehensive clinical assessment. This should include requesting and reviewing your patient’s medical records.
Explain your motivation. Explain to your patient how prescribing an off-label medication can directly benefit him/her. Make it clear that you are not conducting experimental research by prescribing off-label because some patients might perceive this as a covert form of research.
Know the medications you prescribe. Although this sounds obvious, psychiatrists should thoroughly understand how each medication they prescribe is likely to clinically affect their patient. This information is available from many sources, including the FDA’s medication information sheets and the manufacturer’s medication package inserts. If possible, make sure that your off-label prescribing is supported by reputable, peer-reviewed literature.
Obtain informed consent. Tell your patient that the medication you are recommending is being prescribed off-label. Discuss the medication’s risks, benefits, adverse effects, associated “black-box” warnings, off-label uses, and alternatives to the off-label medication.4 Allow time for the patient to ask questions about these treatments.
Continue to: Document all steps
Document all steps. There is an adage in medicine that “If it’s not written, it wasn’t done.” To help reduce your liability risk when prescribing off-label, be sure to document the following4:
- your clinical assessment
- information you gleaned from the patient’s medical records
- your review of information regarding both therapeutic and adverse effects of the medication you want to prescribe
- your discussion of informed consent, including documentation that the patient is aware that the medication is being prescribed off-label
- your clinical rationale for why the off-label medication is in the patient’s best interest.
Also, document the steps you take to monitor for adverse events and therapeutic effectiveness.4 Overall, the goal of documentation should be to support the adequate continuing care of our patients.
1. US Food and Drug Administration. Understanding unapproved use of approved drugs “off label.” https://www.fda.gov/patients/learn-about-expanded-access-and-other-treatment-options/understanding-unapproved-use-approved-drugs-label. Updated February 5, 2018. Accessed August 6, 2020.
2. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLoS One. 2018;13(7):e0198363. doi: 10.1371/journal.pone.0198363.
3. McLeer S, Mawhinney J; Council on Healthcare Systems and Financing. Position statement on off-label treatments. American Psychiatric Association. https://www.psychiatry.org/File%20Library/About-APA/Organization-Documents-Policies/Policies/Position-2016-Off-Label-Treatment.pdf. Published July 2016. Accessed August 6, 2020.
4. Funicelli A. What to consider when prescribing off-label. Psychiatric News. 2019;54(14):12.
The FDA defines “off-label” prescribing as prescribing an FDA-approved medication for an unapproved use, such as for an unapproved clinical indication, for a higher-than-approved dose, or for a patient who is not part of the FDA-approved population (eg, children or geriatric patients).1 Off-label prescribing is common in psychiatry; approximately 13% of psychiatry patients are prescribed off-label psychotropic medications.2 The American Psychiatric Association strongly supports “the autonomous clinical decision-making authority of a physician” and “a physician’s lawful use of an FDA-approved drug product or medical device for an off-label indication when such use is based upon sound scientific evidence in conjunction with sound medical judgment.”3 Because many psychiatric diagnoses have no FDA-approved medications, off-label prescribing often may be a psychiatrist’s only pharmacologic option.
Unfortunately, off-label prescribing can increase a psychiatrist’s risk for liability when treatment falls short of patients’ expectations, or when patients allege that they were injured by the use of an off-label medication. Off-label prescribing does not automatically lead to losing a malpractice suit because the FDA states that physicians can prescribe approved medications for any scientifically supported use, including off-label.1 Medical malpractice lawsuits alleging negligence in prescribing practices, such as off-label prescribing, typically include allegations against the psychiatrist for failure to4:
- adequately assess the patient
- consult the patient’s medical records
- obtain informed consent from the patient
- appropriately prescribe a medication for the clinical indication, dosage, patient’s age, etc.
- monitor for adverse effects and therapeutic effectiveness.
Steps to minimize your risk
When prescribing a medication off-label, the following approaches can help reduce your liability risk:
Conduct a comprehensive clinical assessment. This should include requesting and reviewing your patient’s medical records.
Explain your motivation. Explain to your patient how prescribing an off-label medication can directly benefit him/her. Make it clear that you are not conducting experimental research by prescribing off-label because some patients might perceive this as a covert form of research.
Know the medications you prescribe. Although this sounds obvious, psychiatrists should thoroughly understand how each medication they prescribe is likely to clinically affect their patient. This information is available from many sources, including the FDA’s medication information sheets and the manufacturer’s medication package inserts. If possible, make sure that your off-label prescribing is supported by reputable, peer-reviewed literature.
Obtain informed consent. Tell your patient that the medication you are recommending is being prescribed off-label. Discuss the medication’s risks, benefits, adverse effects, associated “black-box” warnings, off-label uses, and alternatives to the off-label medication.4 Allow time for the patient to ask questions about these treatments.
Continue to: Document all steps
Document all steps. There is an adage in medicine that “If it’s not written, it wasn’t done.” To help reduce your liability risk when prescribing off-label, be sure to document the following4:
- your clinical assessment
- information you gleaned from the patient’s medical records
- your review of information regarding both therapeutic and adverse effects of the medication you want to prescribe
- your discussion of informed consent, including documentation that the patient is aware that the medication is being prescribed off-label
- your clinical rationale for why the off-label medication is in the patient’s best interest.
Also, document the steps you take to monitor for adverse events and therapeutic effectiveness.4 Overall, the goal of documentation should be to support the adequate continuing care of our patients.
The FDA defines “off-label” prescribing as prescribing an FDA-approved medication for an unapproved use, such as for an unapproved clinical indication, for a higher-than-approved dose, or for a patient who is not part of the FDA-approved population (eg, children or geriatric patients).1 Off-label prescribing is common in psychiatry; approximately 13% of psychiatry patients are prescribed off-label psychotropic medications.2 The American Psychiatric Association strongly supports “the autonomous clinical decision-making authority of a physician” and “a physician’s lawful use of an FDA-approved drug product or medical device for an off-label indication when such use is based upon sound scientific evidence in conjunction with sound medical judgment.”3 Because many psychiatric diagnoses have no FDA-approved medications, off-label prescribing often may be a psychiatrist’s only pharmacologic option.
Unfortunately, off-label prescribing can increase a psychiatrist’s risk for liability when treatment falls short of patients’ expectations, or when patients allege that they were injured by the use of an off-label medication. Off-label prescribing does not automatically lead to losing a malpractice suit because the FDA states that physicians can prescribe approved medications for any scientifically supported use, including off-label.1 Medical malpractice lawsuits alleging negligence in prescribing practices, such as off-label prescribing, typically include allegations against the psychiatrist for failure to4:
- adequately assess the patient
- consult the patient’s medical records
- obtain informed consent from the patient
- appropriately prescribe a medication for the clinical indication, dosage, patient’s age, etc.
- monitor for adverse effects and therapeutic effectiveness.
Steps to minimize your risk
When prescribing a medication off-label, the following approaches can help reduce your liability risk:
Conduct a comprehensive clinical assessment. This should include requesting and reviewing your patient’s medical records.
Explain your motivation. Explain to your patient how prescribing an off-label medication can directly benefit him/her. Make it clear that you are not conducting experimental research by prescribing off-label because some patients might perceive this as a covert form of research.
Know the medications you prescribe. Although this sounds obvious, psychiatrists should thoroughly understand how each medication they prescribe is likely to clinically affect their patient. This information is available from many sources, including the FDA’s medication information sheets and the manufacturer’s medication package inserts. If possible, make sure that your off-label prescribing is supported by reputable, peer-reviewed literature.
Obtain informed consent. Tell your patient that the medication you are recommending is being prescribed off-label. Discuss the medication’s risks, benefits, adverse effects, associated “black-box” warnings, off-label uses, and alternatives to the off-label medication.4 Allow time for the patient to ask questions about these treatments.
Continue to: Document all steps
Document all steps. There is an adage in medicine that “If it’s not written, it wasn’t done.” To help reduce your liability risk when prescribing off-label, be sure to document the following4:
- your clinical assessment
- information you gleaned from the patient’s medical records
- your review of information regarding both therapeutic and adverse effects of the medication you want to prescribe
- your discussion of informed consent, including documentation that the patient is aware that the medication is being prescribed off-label
- your clinical rationale for why the off-label medication is in the patient’s best interest.
Also, document the steps you take to monitor for adverse events and therapeutic effectiveness.4 Overall, the goal of documentation should be to support the adequate continuing care of our patients.
1. US Food and Drug Administration. Understanding unapproved use of approved drugs “off label.” https://www.fda.gov/patients/learn-about-expanded-access-and-other-treatment-options/understanding-unapproved-use-approved-drugs-label. Updated February 5, 2018. Accessed August 6, 2020.
2. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLoS One. 2018;13(7):e0198363. doi: 10.1371/journal.pone.0198363.
3. McLeer S, Mawhinney J; Council on Healthcare Systems and Financing. Position statement on off-label treatments. American Psychiatric Association. https://www.psychiatry.org/File%20Library/About-APA/Organization-Documents-Policies/Policies/Position-2016-Off-Label-Treatment.pdf. Published July 2016. Accessed August 6, 2020.
4. Funicelli A. What to consider when prescribing off-label. Psychiatric News. 2019;54(14):12.
1. US Food and Drug Administration. Understanding unapproved use of approved drugs “off label.” https://www.fda.gov/patients/learn-about-expanded-access-and-other-treatment-options/understanding-unapproved-use-approved-drugs-label. Updated February 5, 2018. Accessed August 6, 2020.
2. Vijay A, Becker JE, Ross JS. Patterns and predictors of off-label prescription of psychiatric drugs. PLoS One. 2018;13(7):e0198363. doi: 10.1371/journal.pone.0198363.
3. McLeer S, Mawhinney J; Council on Healthcare Systems and Financing. Position statement on off-label treatments. American Psychiatric Association. https://www.psychiatry.org/File%20Library/About-APA/Organization-Documents-Policies/Policies/Position-2016-Off-Label-Treatment.pdf. Published July 2016. Accessed August 6, 2020.
4. Funicelli A. What to consider when prescribing off-label. Psychiatric News. 2019;54(14):12.