User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Top research findings of 2018-2019 for clinical practice
In Part 1 of this article, published in
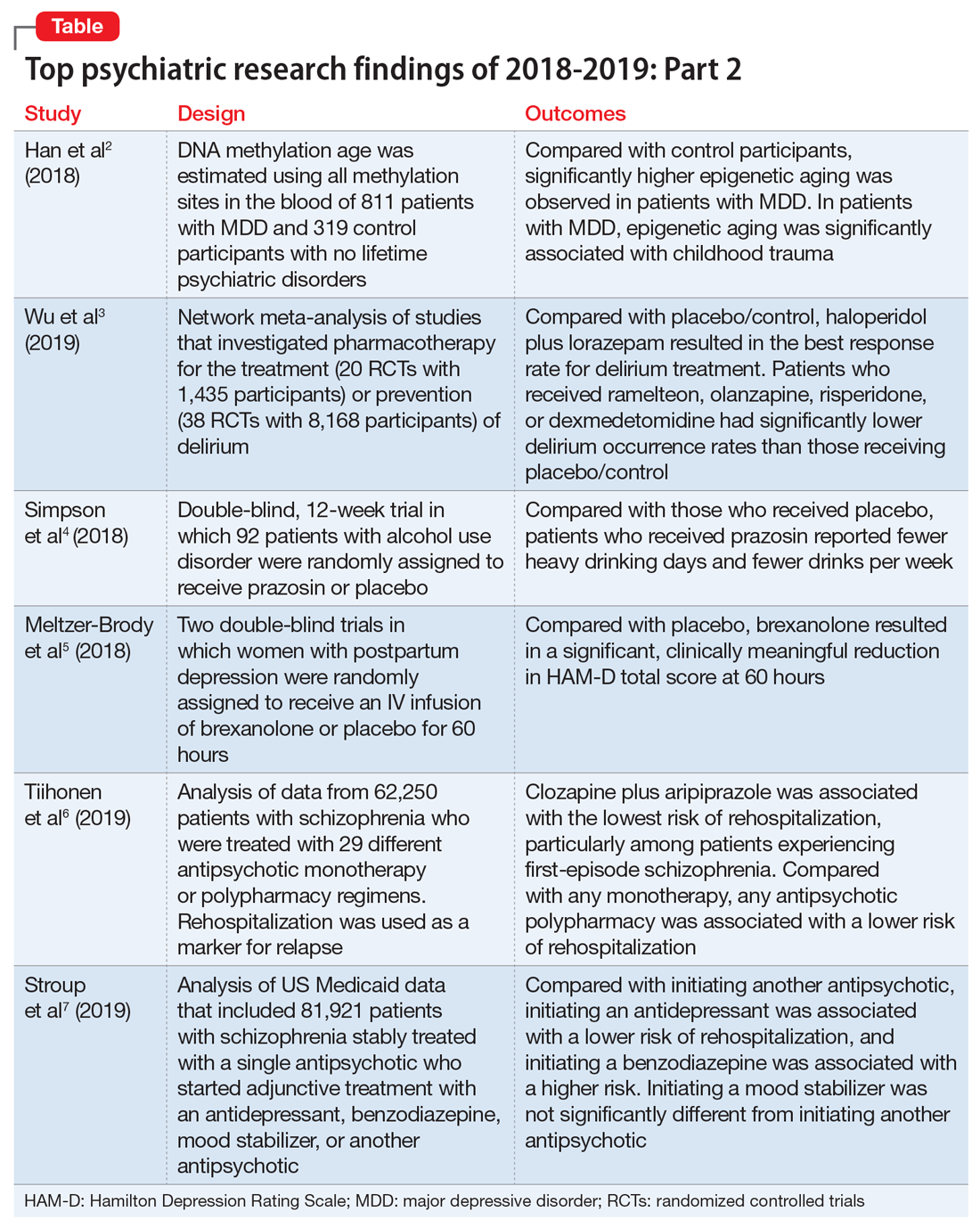
1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
Legalization of marijuana and youths’ attitudes toward its use
The legal status of marijuana has changed a great deal during the last 4 decades. In the United States, several states have legalized the use of marijuana to treat several medical conditions. Some states have decriminalized marijuana possession, and several have legalized marijuana for recreational use by adults. These changes have contributed to a growing misperception among young people that marijuana is harmless or not as risky as other illicit
In this article, I explore the effect the legalization of marijuana has had on young peoples’ attitudes toward its use.
Marijuana use among adolescents
Among adolescents, marijuana is the most commonly used illicit substance, after alcohol.1 According to data from the 2019 Monitoring the Future Survey, while past month, past year, and lifetime marijuana use among 8th and 10th graders remained steady from 2018 to 2019, daily marijuana use among these adolescents increased.2 This survey also reported increases in adolescent marijuana vaping from 2018 to 2019.2 Further, the percentage of adolescents who think that the regular use of marijuana is risky has been trending down since the mid-2000s.2
Youth substance use rates depend on numerous factors, including legal status, availability, ease of access to the substance, and perception of harm.3 Although the legalization of marijuana for recreational use has been for adults only, based on rates of tobacco and alcohol use in adolescents (both of which are legal for adults), the legalization of marijuana is likely to have implications for adolescents.4
Adverse effects among adolescents
During adolescence, the brain is still developing, and marijuana use during this time could cause decreased cognitive functioning, especially executive fun
- an increased risk of mental health disorders, including depression, anxiety, and psychosis, particularly among adolescents at higher risk, such as those with a family history of psychiatric illness
- a decline in school performance
- an increased school dropout rate
- an increased risk of marijuana dependence
- an elevated rate of engaging in risky behaviors.
Factors by which the legalization of marijuana might increase its use among adolescents include4:
- perceived decreased risk of marijuana use
- increased availability
- lower cost
- decreased fear of legal consequences of marijuana use.
Increased parental use is an indirect way in which legalization of marijuana for adult recreational use might increase use in youth.
Continue to: What the evidence says
What the evidence says
Colorado legalized marijuana for medical use in 2000, and for adult recreational use in 2014. A 2012 study of adolescents receiving substance abuse treatment in Colorado found diversion of medical marijuana to these adolescents was common.6 This study also reported that compared with those who did not use medical marijuana, adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana use disorder symptoms, and more symptoms of conduct disorder.6 However, data from the US Substance Abuse and Mental Health Services Administration7 and from the Colorado Department of Public Health & Environment8 suggest that marijuana use among adolescents has not increased since legalization in Colorado.
In 2012, voters in Washington state legalized marijuana for recreational use. In 2013, Moreno et al9 interviewed college students in Washington, where marijuana had just been legalized, and Wisconsin, where it had not. In both states, most participants indicated that legalization would not change their attitude towards use. A small proportion of students felt that legalization would signify an endorsement of marijuana, and they were likely to perceive it as safe to use.
In an analysis of data on more than 250,000 students in 8th, 10th, and 12th grade, Cerdá et al10 found that after legalization in Washington, the perceived harmfulness of marijuana decreased and marijuana use increased among 8th and 10th graders in Washington; however, there were no significant differences noted among adolescents in Colorado.
In 2010, voters in California passed legislation to decriminalize marijuana. In an analysis of data from 8th, 10th, and 12th graders in California, Miech et al11 found a positive correlation between decriminalization and increases in youth future marijuana use. They also found that compared with their peers in other states, 12thgraders in California were more likely to have used marijuana in the last 30 days, less likely to perceive marijuana use as a health risk, and less likely to disapprove of its use.11
Although some studies have suggested that legalization of marijuana might increase use among adolescents, limitations of these studies include that they relied on self-reported use by adolescents, and they did not evaluate adolescent populations outside of school settings.
Continue to: Addressing adolescents' marijuana use
Addressing adolescents’ marijuana use
Strategies for preventing or reducing marijuana use among adolescents might include imposing restrictions and passing stricter laws on the sale of marijuana to individuals age <21, regulating marijuana advertising, increasing adolescent substance use prevention program initiatives, and educating youth about the negative effects of marijuana. Further research is needed to clearly establish if the legalization of marijuana for adult recreational use will increase its use among adolescents.
1. US Department of Health & Human Services. Marijuana use in adolescence. https://www.hhs.gov/ash/oah/adolescent-development/substance-use/marijuana/index.html. Updated April 19, 2019. Accessed January 15, 2020.
2. University of Michigan Institute for Social Research. National adolescent drug trends in 2019: Findings released. http://monitoringthefuture.org//pressreleases/19drugpr.pdf. Updated December 18, 2019. Accessed January 13, 2020.
3. Ammerman S, Ryan S, William P; Committee on Substance Abuse, the Committee on Adolescence. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135(3):584-587.
4. Hopfer C. Implications of marijuana legalization for adolescent substance use. Subst Abus. 2014;35(4):331-335.
5. Silins E, Horwood LJ, Patton GC, et al. Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry. 2014;1(4):286-293.
6. Salomonsen-Sautel S, Sakai JT, Thurstone C, et al. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;51(7):694-702.
7. US Department of Health & Human Services. Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health: Comparison of 2014-2015 and 2015-2016 Population Percentages (50 States and the District of Columbia). https://www.samhsa.gov/data/sites/default/files/NSDUHsaeShortTermCHG2016/NSDUHsaeShortTermCHG2016.htm. Accessed January 15, 2020.
8. Colorado Department of Public Health & Environment. Data Brief: Colorado youth marijuana use 2017. https://drive.google.com/file/d/1AX_2RWWgygGXtGpAGoOMTe84Crzsv62T/view. Accessed January 15, 2020.
9. Moreno MA, Whitehill JM, Quach V, et al. Marijuana experiences, voting behaviors, and early perspectives regarding marijuana legalization among college students from 2 states. J Am Coll Health. 2016;64(1):9-18.
10. Cerdá M, Wall M, Feng T, et al. Association of state recreational marijuana laws with adolescent marijuana use. JAMA Pediatrics. 2017;171(2):142-149.
11. Miech RA, Johnston L, O’Malley PM, et al. Trends in use of marijuana and attitudes toward marijuana among youth before and after decriminalization: the case of California 2007-2013. Int J Drug Policy. 2015;26(4):336-344.
The legal status of marijuana has changed a great deal during the last 4 decades. In the United States, several states have legalized the use of marijuana to treat several medical conditions. Some states have decriminalized marijuana possession, and several have legalized marijuana for recreational use by adults. These changes have contributed to a growing misperception among young people that marijuana is harmless or not as risky as other illicit
In this article, I explore the effect the legalization of marijuana has had on young peoples’ attitudes toward its use.
Marijuana use among adolescents
Among adolescents, marijuana is the most commonly used illicit substance, after alcohol.1 According to data from the 2019 Monitoring the Future Survey, while past month, past year, and lifetime marijuana use among 8th and 10th graders remained steady from 2018 to 2019, daily marijuana use among these adolescents increased.2 This survey also reported increases in adolescent marijuana vaping from 2018 to 2019.2 Further, the percentage of adolescents who think that the regular use of marijuana is risky has been trending down since the mid-2000s.2
Youth substance use rates depend on numerous factors, including legal status, availability, ease of access to the substance, and perception of harm.3 Although the legalization of marijuana for recreational use has been for adults only, based on rates of tobacco and alcohol use in adolescents (both of which are legal for adults), the legalization of marijuana is likely to have implications for adolescents.4
Adverse effects among adolescents
During adolescence, the brain is still developing, and marijuana use during this time could cause decreased cognitive functioning, especially executive fun
- an increased risk of mental health disorders, including depression, anxiety, and psychosis, particularly among adolescents at higher risk, such as those with a family history of psychiatric illness
- a decline in school performance
- an increased school dropout rate
- an increased risk of marijuana dependence
- an elevated rate of engaging in risky behaviors.
Factors by which the legalization of marijuana might increase its use among adolescents include4:
- perceived decreased risk of marijuana use
- increased availability
- lower cost
- decreased fear of legal consequences of marijuana use.
Increased parental use is an indirect way in which legalization of marijuana for adult recreational use might increase use in youth.
Continue to: What the evidence says
What the evidence says
Colorado legalized marijuana for medical use in 2000, and for adult recreational use in 2014. A 2012 study of adolescents receiving substance abuse treatment in Colorado found diversion of medical marijuana to these adolescents was common.6 This study also reported that compared with those who did not use medical marijuana, adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana use disorder symptoms, and more symptoms of conduct disorder.6 However, data from the US Substance Abuse and Mental Health Services Administration7 and from the Colorado Department of Public Health & Environment8 suggest that marijuana use among adolescents has not increased since legalization in Colorado.
In 2012, voters in Washington state legalized marijuana for recreational use. In 2013, Moreno et al9 interviewed college students in Washington, where marijuana had just been legalized, and Wisconsin, where it had not. In both states, most participants indicated that legalization would not change their attitude towards use. A small proportion of students felt that legalization would signify an endorsement of marijuana, and they were likely to perceive it as safe to use.
In an analysis of data on more than 250,000 students in 8th, 10th, and 12th grade, Cerdá et al10 found that after legalization in Washington, the perceived harmfulness of marijuana decreased and marijuana use increased among 8th and 10th graders in Washington; however, there were no significant differences noted among adolescents in Colorado.
In 2010, voters in California passed legislation to decriminalize marijuana. In an analysis of data from 8th, 10th, and 12th graders in California, Miech et al11 found a positive correlation between decriminalization and increases in youth future marijuana use. They also found that compared with their peers in other states, 12thgraders in California were more likely to have used marijuana in the last 30 days, less likely to perceive marijuana use as a health risk, and less likely to disapprove of its use.11
Although some studies have suggested that legalization of marijuana might increase use among adolescents, limitations of these studies include that they relied on self-reported use by adolescents, and they did not evaluate adolescent populations outside of school settings.
Continue to: Addressing adolescents' marijuana use
Addressing adolescents’ marijuana use
Strategies for preventing or reducing marijuana use among adolescents might include imposing restrictions and passing stricter laws on the sale of marijuana to individuals age <21, regulating marijuana advertising, increasing adolescent substance use prevention program initiatives, and educating youth about the negative effects of marijuana. Further research is needed to clearly establish if the legalization of marijuana for adult recreational use will increase its use among adolescents.
The legal status of marijuana has changed a great deal during the last 4 decades. In the United States, several states have legalized the use of marijuana to treat several medical conditions. Some states have decriminalized marijuana possession, and several have legalized marijuana for recreational use by adults. These changes have contributed to a growing misperception among young people that marijuana is harmless or not as risky as other illicit
In this article, I explore the effect the legalization of marijuana has had on young peoples’ attitudes toward its use.
Marijuana use among adolescents
Among adolescents, marijuana is the most commonly used illicit substance, after alcohol.1 According to data from the 2019 Monitoring the Future Survey, while past month, past year, and lifetime marijuana use among 8th and 10th graders remained steady from 2018 to 2019, daily marijuana use among these adolescents increased.2 This survey also reported increases in adolescent marijuana vaping from 2018 to 2019.2 Further, the percentage of adolescents who think that the regular use of marijuana is risky has been trending down since the mid-2000s.2
Youth substance use rates depend on numerous factors, including legal status, availability, ease of access to the substance, and perception of harm.3 Although the legalization of marijuana for recreational use has been for adults only, based on rates of tobacco and alcohol use in adolescents (both of which are legal for adults), the legalization of marijuana is likely to have implications for adolescents.4
Adverse effects among adolescents
During adolescence, the brain is still developing, and marijuana use during this time could cause decreased cognitive functioning, especially executive fun
- an increased risk of mental health disorders, including depression, anxiety, and psychosis, particularly among adolescents at higher risk, such as those with a family history of psychiatric illness
- a decline in school performance
- an increased school dropout rate
- an increased risk of marijuana dependence
- an elevated rate of engaging in risky behaviors.
Factors by which the legalization of marijuana might increase its use among adolescents include4:
- perceived decreased risk of marijuana use
- increased availability
- lower cost
- decreased fear of legal consequences of marijuana use.
Increased parental use is an indirect way in which legalization of marijuana for adult recreational use might increase use in youth.
Continue to: What the evidence says
What the evidence says
Colorado legalized marijuana for medical use in 2000, and for adult recreational use in 2014. A 2012 study of adolescents receiving substance abuse treatment in Colorado found diversion of medical marijuana to these adolescents was common.6 This study also reported that compared with those who did not use medical marijuana, adolescents who used medical marijuana had an earlier age of regular marijuana use, more marijuana use disorder symptoms, and more symptoms of conduct disorder.6 However, data from the US Substance Abuse and Mental Health Services Administration7 and from the Colorado Department of Public Health & Environment8 suggest that marijuana use among adolescents has not increased since legalization in Colorado.
In 2012, voters in Washington state legalized marijuana for recreational use. In 2013, Moreno et al9 interviewed college students in Washington, where marijuana had just been legalized, and Wisconsin, where it had not. In both states, most participants indicated that legalization would not change their attitude towards use. A small proportion of students felt that legalization would signify an endorsement of marijuana, and they were likely to perceive it as safe to use.
In an analysis of data on more than 250,000 students in 8th, 10th, and 12th grade, Cerdá et al10 found that after legalization in Washington, the perceived harmfulness of marijuana decreased and marijuana use increased among 8th and 10th graders in Washington; however, there were no significant differences noted among adolescents in Colorado.
In 2010, voters in California passed legislation to decriminalize marijuana. In an analysis of data from 8th, 10th, and 12th graders in California, Miech et al11 found a positive correlation between decriminalization and increases in youth future marijuana use. They also found that compared with their peers in other states, 12thgraders in California were more likely to have used marijuana in the last 30 days, less likely to perceive marijuana use as a health risk, and less likely to disapprove of its use.11
Although some studies have suggested that legalization of marijuana might increase use among adolescents, limitations of these studies include that they relied on self-reported use by adolescents, and they did not evaluate adolescent populations outside of school settings.
Continue to: Addressing adolescents' marijuana use
Addressing adolescents’ marijuana use
Strategies for preventing or reducing marijuana use among adolescents might include imposing restrictions and passing stricter laws on the sale of marijuana to individuals age <21, regulating marijuana advertising, increasing adolescent substance use prevention program initiatives, and educating youth about the negative effects of marijuana. Further research is needed to clearly establish if the legalization of marijuana for adult recreational use will increase its use among adolescents.
1. US Department of Health & Human Services. Marijuana use in adolescence. https://www.hhs.gov/ash/oah/adolescent-development/substance-use/marijuana/index.html. Updated April 19, 2019. Accessed January 15, 2020.
2. University of Michigan Institute for Social Research. National adolescent drug trends in 2019: Findings released. http://monitoringthefuture.org//pressreleases/19drugpr.pdf. Updated December 18, 2019. Accessed January 13, 2020.
3. Ammerman S, Ryan S, William P; Committee on Substance Abuse, the Committee on Adolescence. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135(3):584-587.
4. Hopfer C. Implications of marijuana legalization for adolescent substance use. Subst Abus. 2014;35(4):331-335.
5. Silins E, Horwood LJ, Patton GC, et al. Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry. 2014;1(4):286-293.
6. Salomonsen-Sautel S, Sakai JT, Thurstone C, et al. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;51(7):694-702.
7. US Department of Health & Human Services. Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health: Comparison of 2014-2015 and 2015-2016 Population Percentages (50 States and the District of Columbia). https://www.samhsa.gov/data/sites/default/files/NSDUHsaeShortTermCHG2016/NSDUHsaeShortTermCHG2016.htm. Accessed January 15, 2020.
8. Colorado Department of Public Health & Environment. Data Brief: Colorado youth marijuana use 2017. https://drive.google.com/file/d/1AX_2RWWgygGXtGpAGoOMTe84Crzsv62T/view. Accessed January 15, 2020.
9. Moreno MA, Whitehill JM, Quach V, et al. Marijuana experiences, voting behaviors, and early perspectives regarding marijuana legalization among college students from 2 states. J Am Coll Health. 2016;64(1):9-18.
10. Cerdá M, Wall M, Feng T, et al. Association of state recreational marijuana laws with adolescent marijuana use. JAMA Pediatrics. 2017;171(2):142-149.
11. Miech RA, Johnston L, O’Malley PM, et al. Trends in use of marijuana and attitudes toward marijuana among youth before and after decriminalization: the case of California 2007-2013. Int J Drug Policy. 2015;26(4):336-344.
1. US Department of Health & Human Services. Marijuana use in adolescence. https://www.hhs.gov/ash/oah/adolescent-development/substance-use/marijuana/index.html. Updated April 19, 2019. Accessed January 15, 2020.
2. University of Michigan Institute for Social Research. National adolescent drug trends in 2019: Findings released. http://monitoringthefuture.org//pressreleases/19drugpr.pdf. Updated December 18, 2019. Accessed January 13, 2020.
3. Ammerman S, Ryan S, William P; Committee on Substance Abuse, the Committee on Adolescence. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135(3):584-587.
4. Hopfer C. Implications of marijuana legalization for adolescent substance use. Subst Abus. 2014;35(4):331-335.
5. Silins E, Horwood LJ, Patton GC, et al. Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry. 2014;1(4):286-293.
6. Salomonsen-Sautel S, Sakai JT, Thurstone C, et al. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;51(7):694-702.
7. US Department of Health & Human Services. Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health: Comparison of 2014-2015 and 2015-2016 Population Percentages (50 States and the District of Columbia). https://www.samhsa.gov/data/sites/default/files/NSDUHsaeShortTermCHG2016/NSDUHsaeShortTermCHG2016.htm. Accessed January 15, 2020.
8. Colorado Department of Public Health & Environment. Data Brief: Colorado youth marijuana use 2017. https://drive.google.com/file/d/1AX_2RWWgygGXtGpAGoOMTe84Crzsv62T/view. Accessed January 15, 2020.
9. Moreno MA, Whitehill JM, Quach V, et al. Marijuana experiences, voting behaviors, and early perspectives regarding marijuana legalization among college students from 2 states. J Am Coll Health. 2016;64(1):9-18.
10. Cerdá M, Wall M, Feng T, et al. Association of state recreational marijuana laws with adolescent marijuana use. JAMA Pediatrics. 2017;171(2):142-149.
11. Miech RA, Johnston L, O’Malley PM, et al. Trends in use of marijuana and attitudes toward marijuana among youth before and after decriminalization: the case of California 2007-2013. Int J Drug Policy. 2015;26(4):336-344.
Caution on pharmacogenetic testing
The general public may have been led to believe that by decoding genes into their constituent parts, clinicians can prevent or predict serious illnesses and personalize treatment. While this may be true in some areas of medicine, such as oncology, using a pharmacogenetic testing-based “lookup table” to prescribe psychiatric medications is disturbing. This practice could lead to incorrect prescriptions, as well as a lack of follow-up or appropriate dosage titration or medication switching. These problems could put a patient’s life at risk and, consequently, bring the field of psychiatry into disrepute.
In the last few years, using pharmacogenetics to predict or prevent illness and personalize treatment has become very attractive. A 2019 meta-analysis of 5 randomized controlled trials examined the use of pharmacogenetic-guided decision support tools for major depressive disorder (MDD). Researchers randomized 1,737 participants with MDD to either pharmacogenetic-guided decision support tools or treatment as usual.1 Patients were assessed using the Hamilton Depression Rating Scale–17 three times over 8 weeks. Compared with those who received treatment as usual, those who were managed using pharmacogenetic-guided decision support tools were more likely to achieve remission from depressive symptoms (relative risk = 1.71; 95% CI, 1.17 to 2.48; P = .005). However, these results are controversial because the included studies were industry-funded, and proprietary algorithms were used to interpret the results. (Editor's note: For more information about this study and pharmacogenetic testing, see “Pharmacogenomics testing: What the FDA says,” Savvy Psychopharmacology,
In a policy statement on the use of pharmacogenetic testing in psychiatry, the International Society of Psychiatric Genetics (ISPG) explained that such testing should be viewed as a decision support tool to assist in implementing good clinical care, rather than as an alternative to standard protocols.2 Furthermore, the ISPG stated that “common genetic variants are not sufficient to cause psychiatric disorders such as depression, bipolar disorder, substance dependence, or schizophrenia.”2
Some manufacturers have claimed that their pharmacogenetic tests can provide information on how a patient will respond to medications for treating depression and other conditions, and when a clinician can or should change a patient’s medication. However, the relationship between DNA variations and the effectiveness of antidepressant medications has not been established, and basing clinical decisions on the results of these tests may lead to inappropriate medication changes.
Pharmacogenetic tests are being advertised to both clinicians and patients, but the FDA has not approved the use of any test for providing information on a patient’s ability to respond to any specific medication.3 Therefore, psychiatrists should discuss the use of pharmacogenetic testing with their patients, and advise patients to avoid stopping or changing their medications based on the results of any pharmacogenetic test. Clinicians should not change a patient’s medication regimen solely based on the results of pharmacogenetic testing. These tests are not supported by scientific or clinical evidence, and using these tests for clinical decisions may put the patient at risk for potentially serious health consequences.
Aneela Jafri, MD, MS
Research Volunteer
Ocean Medical Center
Nutley, New Jersey
Ramon Solhkhah, MD
Founding Chair and Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Chair
Department of Psychiatry
Jersey Shore University Medical Center
Neptune, New Jersey
Residency Training Director
General Psychiatry
Ocean Medical Center
Brick, New Jersey
Stacy Doumas, MD
Vice Chair
Associate Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Vice Chair for Education & Research
Residency Training Director
General Psychiatry
Jersey Shore University Medical Center Neptune, New Jersey
Saba Afzal, MD
Assistant Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Associate Residency Training Director General Psychiatry
Ocean Medical Center
Brick, New Jersey
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenetics. 2019;20(1):37-47.
2. International Society for Psychiatric Genetics. Genetics testing statement: genetic testing and psychiatric disorders. https://ispg.net/genetic-testing-statement. Updated March 11, 2019. Accessed January 9, 2020.
3. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry; a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
The general public may have been led to believe that by decoding genes into their constituent parts, clinicians can prevent or predict serious illnesses and personalize treatment. While this may be true in some areas of medicine, such as oncology, using a pharmacogenetic testing-based “lookup table” to prescribe psychiatric medications is disturbing. This practice could lead to incorrect prescriptions, as well as a lack of follow-up or appropriate dosage titration or medication switching. These problems could put a patient’s life at risk and, consequently, bring the field of psychiatry into disrepute.
In the last few years, using pharmacogenetics to predict or prevent illness and personalize treatment has become very attractive. A 2019 meta-analysis of 5 randomized controlled trials examined the use of pharmacogenetic-guided decision support tools for major depressive disorder (MDD). Researchers randomized 1,737 participants with MDD to either pharmacogenetic-guided decision support tools or treatment as usual.1 Patients were assessed using the Hamilton Depression Rating Scale–17 three times over 8 weeks. Compared with those who received treatment as usual, those who were managed using pharmacogenetic-guided decision support tools were more likely to achieve remission from depressive symptoms (relative risk = 1.71; 95% CI, 1.17 to 2.48; P = .005). However, these results are controversial because the included studies were industry-funded, and proprietary algorithms were used to interpret the results. (Editor's note: For more information about this study and pharmacogenetic testing, see “Pharmacogenomics testing: What the FDA says,” Savvy Psychopharmacology,
In a policy statement on the use of pharmacogenetic testing in psychiatry, the International Society of Psychiatric Genetics (ISPG) explained that such testing should be viewed as a decision support tool to assist in implementing good clinical care, rather than as an alternative to standard protocols.2 Furthermore, the ISPG stated that “common genetic variants are not sufficient to cause psychiatric disorders such as depression, bipolar disorder, substance dependence, or schizophrenia.”2
Some manufacturers have claimed that their pharmacogenetic tests can provide information on how a patient will respond to medications for treating depression and other conditions, and when a clinician can or should change a patient’s medication. However, the relationship between DNA variations and the effectiveness of antidepressant medications has not been established, and basing clinical decisions on the results of these tests may lead to inappropriate medication changes.
Pharmacogenetic tests are being advertised to both clinicians and patients, but the FDA has not approved the use of any test for providing information on a patient’s ability to respond to any specific medication.3 Therefore, psychiatrists should discuss the use of pharmacogenetic testing with their patients, and advise patients to avoid stopping or changing their medications based on the results of any pharmacogenetic test. Clinicians should not change a patient’s medication regimen solely based on the results of pharmacogenetic testing. These tests are not supported by scientific or clinical evidence, and using these tests for clinical decisions may put the patient at risk for potentially serious health consequences.
Aneela Jafri, MD, MS
Research Volunteer
Ocean Medical Center
Nutley, New Jersey
Ramon Solhkhah, MD
Founding Chair and Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Chair
Department of Psychiatry
Jersey Shore University Medical Center
Neptune, New Jersey
Residency Training Director
General Psychiatry
Ocean Medical Center
Brick, New Jersey
Stacy Doumas, MD
Vice Chair
Associate Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Vice Chair for Education & Research
Residency Training Director
General Psychiatry
Jersey Shore University Medical Center Neptune, New Jersey
Saba Afzal, MD
Assistant Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Associate Residency Training Director General Psychiatry
Ocean Medical Center
Brick, New Jersey
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The general public may have been led to believe that by decoding genes into their constituent parts, clinicians can prevent or predict serious illnesses and personalize treatment. While this may be true in some areas of medicine, such as oncology, using a pharmacogenetic testing-based “lookup table” to prescribe psychiatric medications is disturbing. This practice could lead to incorrect prescriptions, as well as a lack of follow-up or appropriate dosage titration or medication switching. These problems could put a patient’s life at risk and, consequently, bring the field of psychiatry into disrepute.
In the last few years, using pharmacogenetics to predict or prevent illness and personalize treatment has become very attractive. A 2019 meta-analysis of 5 randomized controlled trials examined the use of pharmacogenetic-guided decision support tools for major depressive disorder (MDD). Researchers randomized 1,737 participants with MDD to either pharmacogenetic-guided decision support tools or treatment as usual.1 Patients were assessed using the Hamilton Depression Rating Scale–17 three times over 8 weeks. Compared with those who received treatment as usual, those who were managed using pharmacogenetic-guided decision support tools were more likely to achieve remission from depressive symptoms (relative risk = 1.71; 95% CI, 1.17 to 2.48; P = .005). However, these results are controversial because the included studies were industry-funded, and proprietary algorithms were used to interpret the results. (Editor's note: For more information about this study and pharmacogenetic testing, see “Pharmacogenomics testing: What the FDA says,” Savvy Psychopharmacology,
In a policy statement on the use of pharmacogenetic testing in psychiatry, the International Society of Psychiatric Genetics (ISPG) explained that such testing should be viewed as a decision support tool to assist in implementing good clinical care, rather than as an alternative to standard protocols.2 Furthermore, the ISPG stated that “common genetic variants are not sufficient to cause psychiatric disorders such as depression, bipolar disorder, substance dependence, or schizophrenia.”2
Some manufacturers have claimed that their pharmacogenetic tests can provide information on how a patient will respond to medications for treating depression and other conditions, and when a clinician can or should change a patient’s medication. However, the relationship between DNA variations and the effectiveness of antidepressant medications has not been established, and basing clinical decisions on the results of these tests may lead to inappropriate medication changes.
Pharmacogenetic tests are being advertised to both clinicians and patients, but the FDA has not approved the use of any test for providing information on a patient’s ability to respond to any specific medication.3 Therefore, psychiatrists should discuss the use of pharmacogenetic testing with their patients, and advise patients to avoid stopping or changing their medications based on the results of any pharmacogenetic test. Clinicians should not change a patient’s medication regimen solely based on the results of pharmacogenetic testing. These tests are not supported by scientific or clinical evidence, and using these tests for clinical decisions may put the patient at risk for potentially serious health consequences.
Aneela Jafri, MD, MS
Research Volunteer
Ocean Medical Center
Nutley, New Jersey
Ramon Solhkhah, MD
Founding Chair and Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Chair
Department of Psychiatry
Jersey Shore University Medical Center
Neptune, New Jersey
Residency Training Director
General Psychiatry
Ocean Medical Center
Brick, New Jersey
Stacy Doumas, MD
Vice Chair
Associate Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Vice Chair for Education & Research
Residency Training Director
General Psychiatry
Jersey Shore University Medical Center Neptune, New Jersey
Saba Afzal, MD
Assistant Professor
Department of Psychiatry and Behavioral Health
Hackensack Meridian School of Medicine at Seton Hall University
Nutley, New Jersey
Associate Residency Training Director General Psychiatry
Ocean Medical Center
Brick, New Jersey
Disclosures: The authors report no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenetics. 2019;20(1):37-47.
2. International Society for Psychiatric Genetics. Genetics testing statement: genetic testing and psychiatric disorders. https://ispg.net/genetic-testing-statement. Updated March 11, 2019. Accessed January 9, 2020.
3. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry; a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
1. Bousman CA, Arandjelovic K, Mancuso SG, et al. Pharmacogenetic tests and depressive symptom remission: a meta-analysis of randomized controlled trials. Pharmacogenetics. 2019;20(1):37-47.
2. International Society for Psychiatric Genetics. Genetics testing statement: genetic testing and psychiatric disorders. https://ispg.net/genetic-testing-statement. Updated March 11, 2019. Accessed January 9, 2020.
3. Zubenko GS, Sommer BR, Cohen BM. Pharmacogenetics in psychiatry; a companion, rather than competitor, to protocol-based care-reply. JAMA Psychiatry. 2018;75(10):1090-1091.
We are physicians, not providers, and we treat patients, not clients!
One of the most malignant threats that is adversely impacting physicians is the insidious metastasis of the term “provider” within the national health care system over the past 2 to 3 decades.
This demeaning adjective is outrageously inappropriate and beneath the stature of medical doctors (MDs) who sacrificed 12 to 15 years of their lives in college, medical schools, residency programs, and post-residency fellowships to become physicians, specialists, and subspecialists. It is distressing to see hospitals, clinics, pharmacies, insurance corporations, and managed care companies refer to psychiatrists and other physicians as “providers.” It is time to fight back and restore our noble medical identity, which society has always respected and appreciated.
Our unique professional identify is at stake. We do not want to be lumped with nonphysicians as if we are interchangeable parts of a health care system or cogs in a wheel. No other mental health professional has the extensive training, scientific knowledge, clinical expertise, research accomplishments, and teaching/supervisory abilities that physicians have. We strongly uphold the sacred tenet of the physician-patient relationship, and adamantly reject its corruption into a provider-consumer transaction.
Even plumbers and electricians are not referred to as “providers.” Lawyers are not called legal aid providers. Teachers are not called knowledge providers, and administrators and CEOs are not called management providers. So why should physicians in any specialty, including psychiatry, obsequiously accept the denigration of their esteemed medical identify into the vague, amorphous ipseity of a “provider”? Family physicians, internists, and pediatricians used to be called primary care physicians, but have been reduced to primary care providers, which is insulting and degrading to these highly trained MD specialists.
The corruption and debasement of the professional identify of physicians and the propagation of the usage of the belittling term “provider” can be traced back to 3 entities:
1. The Nazi Third Reich. This is the most evil origin of the term “provider,” inflicted on Jewish physicians as part of the despicable persecution of German Jews in the 1930s. The Nazis decided to deprive pediatricians of being called physicians (“Arzt” in German) and forcefully relabeled them as “behandlers” or “providers,” thus erasing their noble medical identity.1 In 1933, all Jewish pediatricians were expelled or forced to resign from the German Society of Pediatrics and were no longer allowed to be called doctors. This deliberate and systematic humiliation of pediatric clinicians and scientists was followed by deporting the lowly “providers” to concentration camps. So why perpetuate this pernicious Nazi terminology?
2. The Federal Government. The term “provider” was introduced and propagated in Public Law 93-641 titled “The National Health Planning and Resource Development Act of 1974.” In that document, patients were referred to as “consumers” and physicians as “providers” (this term was used 19 times in that law). At that time, the civil service employees who drafted the law that marginalized physicians by using generic, nonmedical nomenclature may not have realized the dire consequences of relabeling physicians as “providers.”
Continue to: Insurance companies, managed care companies, and consolidated health systems...
3. Insurance companies, managed care companies, and consolidated health systems have jubilantly adopted the term “provider” because they can equate physicians with less expensive, nonphysician clinicians (physician assistants, nurse practitioners, and certified registered nurse anesthetists), especially when physicians across several specialties (particularly psychiatry) are in short supply. None of these clinicians deserve to be labeled “providers,” either.
To understand why the term “provider” was used instead of “clinicians” or “clinical practitioner,” one must recognize the “businessification” of medicine and the commoditization of clinical care in our country. In some ways, health care has adopted a model similar to a fast-food joint, where workers provide customers with a hamburger. The question here is why did the 1.1 million physicians in the United States not halt this terminology shift before it spread and permeated the national health care system? Physicians who graduate from medical schools (not “provider” schools!) must vigorously and loudly fight back and put this wicked genie back in its bottle. This is feasible only if the American Medical Association (which would never conceive of itself as the “American Provider Association”), along with all 48 specialty organizations (Table), including the American Psychiatric Association (APA), unite and demand that physicians be called medical doctors or physicians, or by a term that reflects their specialty (orthopedists, psychiatrists, oncologists, gastroenterologists, anesthesiologists, cardiologists, etc.). This is an urgent issue to prevent the dissolution of our professional identity and its highly regarded societal image. It is a travesty that we physicians have allowed it to go on unopposed and to become entrenched in the dumbed-down jargon of health care. Physicians tend to avoid confrontation and adversarial stances, but we must unite and demand a return to the traditional nomenclature of medicine.
Much debate has emerged lately about an epidemic of “burnout” among physicians. Proposed causes include the savage increase in the amount of paperwork at the expense of patient care, the sense of helplessness that pre-authorization has inflicted on physicians’ decision-making, and the tyranny of relative value units (RVUs) as a benchmark for physician performance, as if healing patients is like manufacturing widgets. However, the blow to the self-esteem of physicians by being called “providers” daily is certainly another major factor contributing to burnout. It is perfectly legitimate for physicians to expect recognition for their long, rigorous, and uniquely advanced medical training, instead of being lumped together with less qualified professionals as anonymous “providers” in the name of politically correct pseudo-equality of all clinical practitioners. Let the administrators stop and contemplate whether tertiary or quaternary care for the most complex and severely ill patients in medical, surgical, or psychiatric intensive care units can operate without highly specialized physicians.
I urge APA leadership to take a visible and strong stand to rid psychiatrists of this assault on our medical identity. As I mentioned in my January 2020 editorial,2 it is vital that the name of our national psychiatric organization (APA) be modified to the American Psychiatric Physicians Association, to remind all health care systems, as well as patients, the public, and the media, of our medical identity as physicians before we specialized in psychiatry.
Continue to: Patients, not clients
Patients, not clients
We should also emphasize that our suffering and medically ill patients with serious neuropsychiatric disorders such as schizophrenia, bipolar disorder, depression, panic disorder, or obsessive-compulsive disorder are patients, not clients. The terminology used in community mental health centers around the country almost universally includes “providers” and “clients.” This de-medicalization of psychiatrists and our patients must be corrected and reversed so that the public understands that treating mental illness is not a business transaction between a “provider” and a “client.” Using the correct terminology may help generate sympathy and compassion towards patients with serious psychiatric illnesses, just as it does for patients with cancer, heart disease, or stroke. The term “client” will never evoke the public sympathy and support that our patients truly deserve.
Let’s keep this issue alive and translate our demands into actions, both locally and nationally. Psychiatrists and physicians of all other specialties must stand up for their rights and inform their systems of care that they must be called by their legitimate and lawful name: physicians or medical doctors (never “providers”). This is an issue that unites all 1.1 million of us. The US health care system would collapse without us, and asking that we be called exactly what our medical license displays is our right and our professional identity.
1. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
2. Nasrallah HA. 20 Reasons to celebrate our APA membership in 2020. Current Psychiatry. 2020;19(1):6-9.
One of the most malignant threats that is adversely impacting physicians is the insidious metastasis of the term “provider” within the national health care system over the past 2 to 3 decades.
This demeaning adjective is outrageously inappropriate and beneath the stature of medical doctors (MDs) who sacrificed 12 to 15 years of their lives in college, medical schools, residency programs, and post-residency fellowships to become physicians, specialists, and subspecialists. It is distressing to see hospitals, clinics, pharmacies, insurance corporations, and managed care companies refer to psychiatrists and other physicians as “providers.” It is time to fight back and restore our noble medical identity, which society has always respected and appreciated.
Our unique professional identify is at stake. We do not want to be lumped with nonphysicians as if we are interchangeable parts of a health care system or cogs in a wheel. No other mental health professional has the extensive training, scientific knowledge, clinical expertise, research accomplishments, and teaching/supervisory abilities that physicians have. We strongly uphold the sacred tenet of the physician-patient relationship, and adamantly reject its corruption into a provider-consumer transaction.
Even plumbers and electricians are not referred to as “providers.” Lawyers are not called legal aid providers. Teachers are not called knowledge providers, and administrators and CEOs are not called management providers. So why should physicians in any specialty, including psychiatry, obsequiously accept the denigration of their esteemed medical identify into the vague, amorphous ipseity of a “provider”? Family physicians, internists, and pediatricians used to be called primary care physicians, but have been reduced to primary care providers, which is insulting and degrading to these highly trained MD specialists.
The corruption and debasement of the professional identify of physicians and the propagation of the usage of the belittling term “provider” can be traced back to 3 entities:
1. The Nazi Third Reich. This is the most evil origin of the term “provider,” inflicted on Jewish physicians as part of the despicable persecution of German Jews in the 1930s. The Nazis decided to deprive pediatricians of being called physicians (“Arzt” in German) and forcefully relabeled them as “behandlers” or “providers,” thus erasing their noble medical identity.1 In 1933, all Jewish pediatricians were expelled or forced to resign from the German Society of Pediatrics and were no longer allowed to be called doctors. This deliberate and systematic humiliation of pediatric clinicians and scientists was followed by deporting the lowly “providers” to concentration camps. So why perpetuate this pernicious Nazi terminology?
2. The Federal Government. The term “provider” was introduced and propagated in Public Law 93-641 titled “The National Health Planning and Resource Development Act of 1974.” In that document, patients were referred to as “consumers” and physicians as “providers” (this term was used 19 times in that law). At that time, the civil service employees who drafted the law that marginalized physicians by using generic, nonmedical nomenclature may not have realized the dire consequences of relabeling physicians as “providers.”
Continue to: Insurance companies, managed care companies, and consolidated health systems...
3. Insurance companies, managed care companies, and consolidated health systems have jubilantly adopted the term “provider” because they can equate physicians with less expensive, nonphysician clinicians (physician assistants, nurse practitioners, and certified registered nurse anesthetists), especially when physicians across several specialties (particularly psychiatry) are in short supply. None of these clinicians deserve to be labeled “providers,” either.
To understand why the term “provider” was used instead of “clinicians” or “clinical practitioner,” one must recognize the “businessification” of medicine and the commoditization of clinical care in our country. In some ways, health care has adopted a model similar to a fast-food joint, where workers provide customers with a hamburger. The question here is why did the 1.1 million physicians in the United States not halt this terminology shift before it spread and permeated the national health care system? Physicians who graduate from medical schools (not “provider” schools!) must vigorously and loudly fight back and put this wicked genie back in its bottle. This is feasible only if the American Medical Association (which would never conceive of itself as the “American Provider Association”), along with all 48 specialty organizations (Table), including the American Psychiatric Association (APA), unite and demand that physicians be called medical doctors or physicians, or by a term that reflects their specialty (orthopedists, psychiatrists, oncologists, gastroenterologists, anesthesiologists, cardiologists, etc.). This is an urgent issue to prevent the dissolution of our professional identity and its highly regarded societal image. It is a travesty that we physicians have allowed it to go on unopposed and to become entrenched in the dumbed-down jargon of health care. Physicians tend to avoid confrontation and adversarial stances, but we must unite and demand a return to the traditional nomenclature of medicine.

Much debate has emerged lately about an epidemic of “burnout” among physicians. Proposed causes include the savage increase in the amount of paperwork at the expense of patient care, the sense of helplessness that pre-authorization has inflicted on physicians’ decision-making, and the tyranny of relative value units (RVUs) as a benchmark for physician performance, as if healing patients is like manufacturing widgets. However, the blow to the self-esteem of physicians by being called “providers” daily is certainly another major factor contributing to burnout. It is perfectly legitimate for physicians to expect recognition for their long, rigorous, and uniquely advanced medical training, instead of being lumped together with less qualified professionals as anonymous “providers” in the name of politically correct pseudo-equality of all clinical practitioners. Let the administrators stop and contemplate whether tertiary or quaternary care for the most complex and severely ill patients in medical, surgical, or psychiatric intensive care units can operate without highly specialized physicians.
I urge APA leadership to take a visible and strong stand to rid psychiatrists of this assault on our medical identity. As I mentioned in my January 2020 editorial,2 it is vital that the name of our national psychiatric organization (APA) be modified to the American Psychiatric Physicians Association, to remind all health care systems, as well as patients, the public, and the media, of our medical identity as physicians before we specialized in psychiatry.
Continue to: Patients, not clients
Patients, not clients
We should also emphasize that our suffering and medically ill patients with serious neuropsychiatric disorders such as schizophrenia, bipolar disorder, depression, panic disorder, or obsessive-compulsive disorder are patients, not clients. The terminology used in community mental health centers around the country almost universally includes “providers” and “clients.” This de-medicalization of psychiatrists and our patients must be corrected and reversed so that the public understands that treating mental illness is not a business transaction between a “provider” and a “client.” Using the correct terminology may help generate sympathy and compassion towards patients with serious psychiatric illnesses, just as it does for patients with cancer, heart disease, or stroke. The term “client” will never evoke the public sympathy and support that our patients truly deserve.
Let’s keep this issue alive and translate our demands into actions, both locally and nationally. Psychiatrists and physicians of all other specialties must stand up for their rights and inform their systems of care that they must be called by their legitimate and lawful name: physicians or medical doctors (never “providers”). This is an issue that unites all 1.1 million of us. The US health care system would collapse without us, and asking that we be called exactly what our medical license displays is our right and our professional identity.
One of the most malignant threats that is adversely impacting physicians is the insidious metastasis of the term “provider” within the national health care system over the past 2 to 3 decades.
This demeaning adjective is outrageously inappropriate and beneath the stature of medical doctors (MDs) who sacrificed 12 to 15 years of their lives in college, medical schools, residency programs, and post-residency fellowships to become physicians, specialists, and subspecialists. It is distressing to see hospitals, clinics, pharmacies, insurance corporations, and managed care companies refer to psychiatrists and other physicians as “providers.” It is time to fight back and restore our noble medical identity, which society has always respected and appreciated.
Our unique professional identify is at stake. We do not want to be lumped with nonphysicians as if we are interchangeable parts of a health care system or cogs in a wheel. No other mental health professional has the extensive training, scientific knowledge, clinical expertise, research accomplishments, and teaching/supervisory abilities that physicians have. We strongly uphold the sacred tenet of the physician-patient relationship, and adamantly reject its corruption into a provider-consumer transaction.
Even plumbers and electricians are not referred to as “providers.” Lawyers are not called legal aid providers. Teachers are not called knowledge providers, and administrators and CEOs are not called management providers. So why should physicians in any specialty, including psychiatry, obsequiously accept the denigration of their esteemed medical identify into the vague, amorphous ipseity of a “provider”? Family physicians, internists, and pediatricians used to be called primary care physicians, but have been reduced to primary care providers, which is insulting and degrading to these highly trained MD specialists.
The corruption and debasement of the professional identify of physicians and the propagation of the usage of the belittling term “provider” can be traced back to 3 entities:
1. The Nazi Third Reich. This is the most evil origin of the term “provider,” inflicted on Jewish physicians as part of the despicable persecution of German Jews in the 1930s. The Nazis decided to deprive pediatricians of being called physicians (“Arzt” in German) and forcefully relabeled them as “behandlers” or “providers,” thus erasing their noble medical identity.1 In 1933, all Jewish pediatricians were expelled or forced to resign from the German Society of Pediatrics and were no longer allowed to be called doctors. This deliberate and systematic humiliation of pediatric clinicians and scientists was followed by deporting the lowly “providers” to concentration camps. So why perpetuate this pernicious Nazi terminology?
2. The Federal Government. The term “provider” was introduced and propagated in Public Law 93-641 titled “The National Health Planning and Resource Development Act of 1974.” In that document, patients were referred to as “consumers” and physicians as “providers” (this term was used 19 times in that law). At that time, the civil service employees who drafted the law that marginalized physicians by using generic, nonmedical nomenclature may not have realized the dire consequences of relabeling physicians as “providers.”
Continue to: Insurance companies, managed care companies, and consolidated health systems...
3. Insurance companies, managed care companies, and consolidated health systems have jubilantly adopted the term “provider” because they can equate physicians with less expensive, nonphysician clinicians (physician assistants, nurse practitioners, and certified registered nurse anesthetists), especially when physicians across several specialties (particularly psychiatry) are in short supply. None of these clinicians deserve to be labeled “providers,” either.
To understand why the term “provider” was used instead of “clinicians” or “clinical practitioner,” one must recognize the “businessification” of medicine and the commoditization of clinical care in our country. In some ways, health care has adopted a model similar to a fast-food joint, where workers provide customers with a hamburger. The question here is why did the 1.1 million physicians in the United States not halt this terminology shift before it spread and permeated the national health care system? Physicians who graduate from medical schools (not “provider” schools!) must vigorously and loudly fight back and put this wicked genie back in its bottle. This is feasible only if the American Medical Association (which would never conceive of itself as the “American Provider Association”), along with all 48 specialty organizations (Table), including the American Psychiatric Association (APA), unite and demand that physicians be called medical doctors or physicians, or by a term that reflects their specialty (orthopedists, psychiatrists, oncologists, gastroenterologists, anesthesiologists, cardiologists, etc.). This is an urgent issue to prevent the dissolution of our professional identity and its highly regarded societal image. It is a travesty that we physicians have allowed it to go on unopposed and to become entrenched in the dumbed-down jargon of health care. Physicians tend to avoid confrontation and adversarial stances, but we must unite and demand a return to the traditional nomenclature of medicine.

Much debate has emerged lately about an epidemic of “burnout” among physicians. Proposed causes include the savage increase in the amount of paperwork at the expense of patient care, the sense of helplessness that pre-authorization has inflicted on physicians’ decision-making, and the tyranny of relative value units (RVUs) as a benchmark for physician performance, as if healing patients is like manufacturing widgets. However, the blow to the self-esteem of physicians by being called “providers” daily is certainly another major factor contributing to burnout. It is perfectly legitimate for physicians to expect recognition for their long, rigorous, and uniquely advanced medical training, instead of being lumped together with less qualified professionals as anonymous “providers” in the name of politically correct pseudo-equality of all clinical practitioners. Let the administrators stop and contemplate whether tertiary or quaternary care for the most complex and severely ill patients in medical, surgical, or psychiatric intensive care units can operate without highly specialized physicians.
I urge APA leadership to take a visible and strong stand to rid psychiatrists of this assault on our medical identity. As I mentioned in my January 2020 editorial,2 it is vital that the name of our national psychiatric organization (APA) be modified to the American Psychiatric Physicians Association, to remind all health care systems, as well as patients, the public, and the media, of our medical identity as physicians before we specialized in psychiatry.
Continue to: Patients, not clients
Patients, not clients
We should also emphasize that our suffering and medically ill patients with serious neuropsychiatric disorders such as schizophrenia, bipolar disorder, depression, panic disorder, or obsessive-compulsive disorder are patients, not clients. The terminology used in community mental health centers around the country almost universally includes “providers” and “clients.” This de-medicalization of psychiatrists and our patients must be corrected and reversed so that the public understands that treating mental illness is not a business transaction between a “provider” and a “client.” Using the correct terminology may help generate sympathy and compassion towards patients with serious psychiatric illnesses, just as it does for patients with cancer, heart disease, or stroke. The term “client” will never evoke the public sympathy and support that our patients truly deserve.
Let’s keep this issue alive and translate our demands into actions, both locally and nationally. Psychiatrists and physicians of all other specialties must stand up for their rights and inform their systems of care that they must be called by their legitimate and lawful name: physicians or medical doctors (never “providers”). This is an issue that unites all 1.1 million of us. The US health care system would collapse without us, and asking that we be called exactly what our medical license displays is our right and our professional identity.
1. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
2. Nasrallah HA. 20 Reasons to celebrate our APA membership in 2020. Current Psychiatry. 2020;19(1):6-9.
1. Saenger P. Jewish pediatricians in Nazi Germany: victims of persecution. Isr Med Assoc J. 2006;8(5):324-328.
2. Nasrallah HA. 20 Reasons to celebrate our APA membership in 2020. Current Psychiatry. 2020;19(1):6-9.
Lumateperone for schizophrenia
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9

How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9

How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9

How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
Therapeutic drug monitoring of antipsychotics
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.
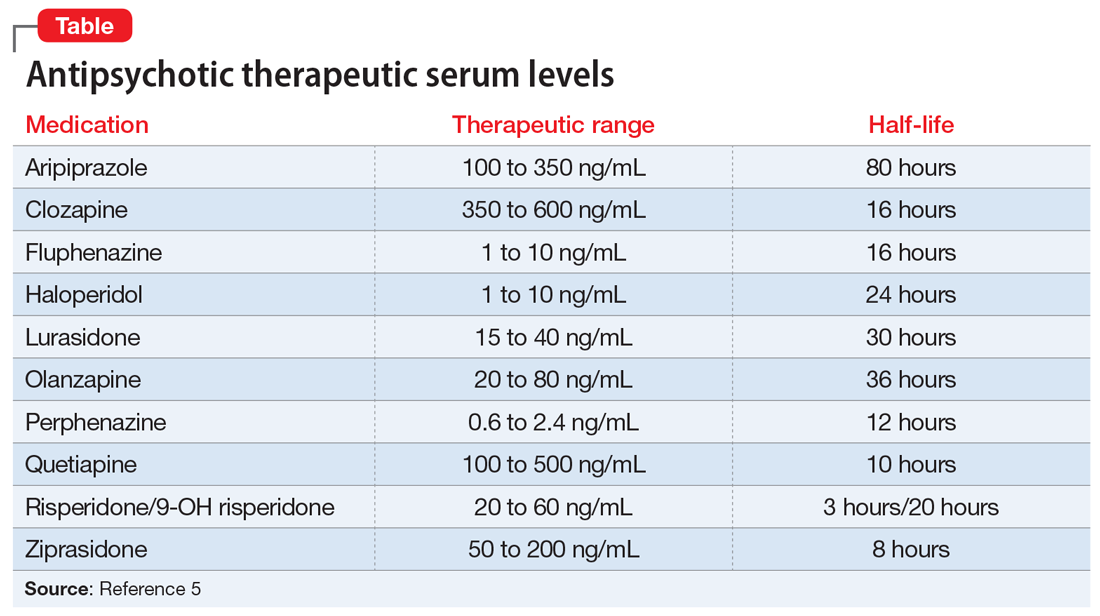
Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.

Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
Mr. Q, age 36, has a history of schizophrenia. He is brought to the hospital due to persistent auditory hallucinations and paranoid delusions. His history documents a trial and failure of risperidone, 4 mg twice daily, and aripiprazole, 20 mg/d. Based on this, the treatment team initiates haloperidol, 5 mg twice daily. Because he experiences persistent auditory hallucinations and paranoid delusions, Mr. Q is titrated to increasing doses of haloperidol over 2 weeks during the course of the hospitalization. Once Mr. Q is receiving a total haloperidol dose of 30 mg/d, the team decides to obtain a serum haloperidol level due to his persistent psychotic symptoms and the development of drug-induced parkinsonism. His serum haloperidol level is 24 ng/mL, which is within the expected range for his dose, but above the therapeutic window for efficacy. The team decides that the severity of Mr. Q’s illness and documented treatment resistance (failing at least 2 adequate trials of antipsychotics) warrant a trial of clozapine.
Despite a long history of therapeutic drug monitoring (TDM) within psychiatry, routine monitoring of antipsychotic serum levels has not been unanimously adopted as standard practice. Clinical practice typically results in mostly a subjective assessment of the safety and efficacy of antipsychotics. This practice is in contrast to agents such as valproic acid and lithium, which are routinely monitored for safety and efficacy using both subjective and laboratory measures. Clinicians may adhere to these monitoring practices for lithium and valproic acid because of these agents’ narrow “therapeutic window” between toxicity and efficacy. However, antipsychotics can be viewed in a similar fashion.
To help conceptualize the therapeutic window for antipsychotics, it is important to understand that in most cases, the pharmacologic target for antipsychotics is dopamine (D2)receptor antagonism between 60% to 80%.1 Total drug exposure would thus determine a patient’s likelihood of minimizing positive symptoms, or exposure to adverse effects related to total dopamine antagonism. Serum drug concentrations are a better metric than total daily dose for determining drug exposure and achieving the pharmacologic target.2 Evaluating serum antipsychotic levels also is a better method of determining true treatment failure than relying on the clinical judgment of the treating psychiatrist.3
Pros and cons of TDM
Benefits of using TDM for patients being treated with antipsychotics include4:
- ensuring adherence
- quantitatively adjusting dosages for medication interactions or genetic variations
- ensuring an adequate trial of a medication before considering it a treatment failure.
Potential drawbacks to TDM include:
- Delayed results. Access to expeditious testing may not be possible in certain laboratories, and this may require send-out testing, which could result in a delay in obtaining results. Continued advocacy and research on the value of TDM in antipsychotics may improve access to these resources in the future. Nonetheless, obtaining antipsychotic serum levels will still give clinicians insight into the antipsychotic exposure at a given dose. Further, obtaining antipsychotic serum levels may strengthen decisions about treatment resistance and the assessment of interactions, adherence, or the likelihood of adverse effects.
- Lack of guidance. Unfortunately, there is no established guidance outlining what to do once antipsychotic serum levels are obtained. The correlation of serum levels of commonly used second-generation antipsychotics with clinical efficacy needs to be more closely investigated; however, certain agents do have more data associated with appropriate ranges for efficacy/toxicity. While researchers should continue to study the precise relationship between antipsychotic serum levels and effect, clinicians still have resources available to help determine what the expected serum value for a given patient may be. Knowing an expected serum level may help clinicians determine whether there is an unknown interaction or genetic variation that is causing lower- or higher-than-expected levels. This may also help determine whether a patient is adhering to their medication regimen.
Growing evidence for TDM
In recent years, evidence supporting the use of TDM in patients receiving antipsychotics has been increasing, and recommendations from consensus groups have been strengthened. One of the most comprehensive assessments of these practices was published by the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie (AGNP), a German-based psychopharmacology group consisting of researchers and clinicians. In 2018, the AGNP published consensus guidelines for TDM in various neuropsychiatric medication classes and recommendations for collecting, interpreting, and decision-making elements of the process.5 The Table5 lists the AGNP-recommended therapeutic serum range for several commonly used second-generation antipsychotics.

Researchers should be encouraged to contribute to the body of knowledge on the correlation of clinical response to serum level. However, there is compelling evidence for the use of TDM across many first- and second-generation antipsychotics. Of the most common, haloperidol and clozapine have evidence of a therapeutic range that is better correlated with serum level than daily dose. Specifically, haloperidol appears to lose benefit with dose increases beyond serum levels of approximately 10 ng/mL.6
Continue to: Clozapine levels may be...
Clozapine levels may be reported by measuring the metabolite norclozapine, which is not correlated with efficacy, or as a total level (combination of clozapine and norclozapine). While norclozapine is not associated with efficacy, the ratio of clozapine to norclozapine may indicate adherence to the medication, or any enzymatic modulation (genetic or drug–drug interaction) that may increase or decrease total exposure. A ratio of 1.5 to 2.0 (clozapine to norclozapine) is optimal; a ratio <0.5 may indicate nonadherence; and a ratio >2.0 may indicate inhibited drug clearance. A 12-hour serum clozapine level of ≥350 ng/mL is more likely to predict treatment response.7
CASE CONTINUED
Mr. Q is carefully tapered from haloperidol while initiating clozapine at 25 mg/d. As he is titrated on clozapine, Mr. Q’s serum levels are periodically checked and compared with expected levels and levels associated with efficacy. Eventually, Mr. Q is titrated to a clozapine dose of 400 mg/d at bedtime.
While receiving clozapine for 4 weeks, Mr. Q’s psychotic symptoms resolve, and he is scheduled for follow-up in the outpatient clozapine clinic.
Related Resources
- De Leon J. A critical commentary on the 2017 AGNP consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology. Pharmacopsychiatry. 2018; 51(1-02):63-68.
- Meyer JM. Is monitoring of plasma antipsychotic levels useful? Current Psychiatry. 2015;14(11):16,19-20.
Drug Brand Names
Aripiprazole • Abilify
Clozapine • Clozaril
Fluphenazine • Prolixin
Haloperidol • Haldol
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Perphenazine • Trilafon
Quetiapine • Seroquel
Risperidone • Risperdal
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.
1. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013:129-236.
2. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
3. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39-46.
4. Horvitz-Lennon M, Mattke S, Predmore Z, et al. The role of antipsychotic plasma levels in the treatment of schizophrenia. Am J Psychiatry. 2017;174(5):421-426.
5. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62.
6. Van Putten T, Marder SR, Mintz J, et al. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149(4):500-505.
7. Couchman L, Morgan PE, Spencer EP, et al. Plasma clozapine, norclozapine, and the clozapine:norclozapine ratio in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 1993-2007. Ther Drug Monit. 2010;32(4):438-447.
Suicidal while receiving treatment for breast cancer
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6
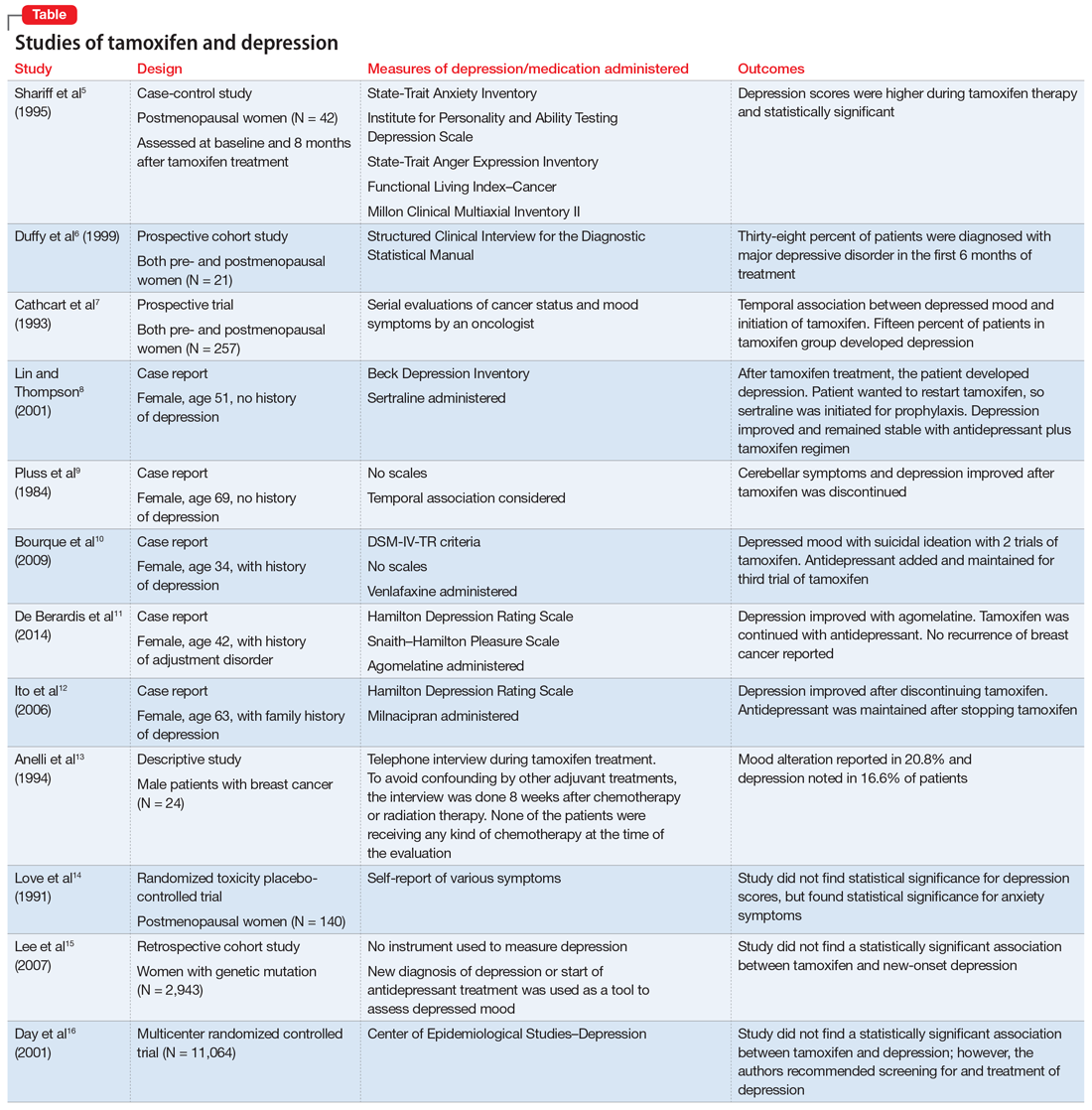
In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6

In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
CASE Worsening mood symptoms and suicidal ideation
On a recent visit to the oncology clinic, where she has been receiving treatment for breast cancer for 11 months, Mrs. L, age 46, reports the abrupt onset of sadness, irritability, difficulty sleeping, and negative self-thoughts.
Eleven months ago, Mrs. L was diagnosed with invasive lobular carcinoma of the right breast that was classified as T2N0MX, representing relatively early-stage disease. Shortly after her diagnosis, Mrs. L completed 4 cycles of neoadjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by treatment with trastuzumab. Subsequently, she underwent a right segmental mastectomy with bilateral mastopexy and radiation therapy. Recently, Mrs. L’s oncology team prescribed tamoxifen, 20 mg/d, and trastuzumab, 420 mg IV every 3 weeks; however, within 3 weeks after starting tamoxifen, Mrs. L’s mood symptoms worsened to the point where she says she is considering suicide—with a plan to use her husband’s gun to kill herself.
Mrs. L has no other pertinent medical history and no reported history of psychiatric disease.
The primary oncology team discontinues tamoxifen (after 5 weeks of treatment) and refers Mrs. L to psychiatry for further mood evaluation.
[polldaddy:10497042]
The authors’ observations
The prevalence of depression is higher in patients with cancer than in the general population.1 The etiology of depression is often multifactorial.2 In Mrs. L’s case, we hypothesized that the possible cause of her depressive symptoms included concerns about her self-image after mastectomy and the adverse effects of chemotherapy and tamoxifen.
Among these possible causes, estrogen level is particularly important. Estrogen affects the brain in numerous ways, including by modulating different neurotransmitters,3,4 regulating neuroplasticity, providing neuroprotection by preventing formation of oxidative free radicals and of beta amyloid, and possibly avoiding inflammation. From a behavioral standpoint, estrogen acts as an antidepressant while enhancing memory and modulating maternal behavior.4 Therefore, decreased estrogen levels could result in depression and other neuropsychiatric problems. This is illustrated in Mrs. L’s case, where tamoxifen administered after breast cancer treatment coincided with the abrupt onset of depression with suicidal ideation.
Depression in patients receiving tamoxifen might be explained by the fact that tamoxifen is a selective estrogen receptor blocker with dual properties. Specifically, while it has antagonistic action in breast tissue, diminishing the growth-promoting action of estrogen on breast cancer cells, it additionally crosses the blood-brain barrier, so it may block the neuroprotective action of estrogen in the brain.
EXAMINATION Improvement in depression but slightly anxious
During her psychiatric examination, Mrs. L is fairly well-groomed and cooperative. Her speech is normal, thought process is organized, and she has fair insight into her medical situation, with fair judgment. She is alert, attentive, and oriented to time, place, as well as person. She confirms that she has no prior psychiatric history, including no prior suicide attempts. She lives with her husband, who has been supportive. Mrs. L has no children, and she continues to work.
Continue to: Mrs. L reports that per her oncology...
Mrs. L reports that per her oncology team’s instruction, she has not taken tamoxifen for almost 1 week, and notes improvement in her mood. She describes her mood as “fine now,” but appears slightly anxious. She adamantly denies suicidal ideation since stopping tamoxifen; however, she confirms that prior to stopping tamoxifen, she experienced low mood, suicidal thoughts, and a decreased interest in activities. Mrs. L’s Patient Health Questionnaire–9 score is 13, indicating moderate depression. She says she is constantly preoccupied with thoughts about the adverse effects of hormone therapy, and specifically about the oncology team’s suggestion of a retrial of tamoxifen. Due to her constant worry, she has difficulty relaxing; her Generalized Anxiety Disorder–7 item scale score is 12, indicating moderate anxiety. She has a history of cigarette smoking but stopped after her breast cancer diagnosis. She also reports gaining weight since beginning cancer treatment (body mass index: 28.0 kg/m2) and experiencing breast pain.
Mrs. L’s vital signs are normal. Results of her laboratory workup reveal a thyroid-stimulating hormone level of 1.40 µU/mL (reference range: 0.27 to 4.20 µU/mL); a follicle-stimulating hormone (FSH) level of 78.4 mIU/mL (postmenopausal reference range: 25.8 to 134.8 mIU/mL); and an estradiol level of <12.0 pg/mL (postmenopausal range: <55 pg/mL).
The authors’ observations
Studies investigating the effects of tamoxifen on mood have produced varying results (Table5-16). Some researchers have found a significant relationship between depression and tamoxifen in patients with breast cancer. In a case-control study, 42 postmenopausal women with breast cancer who received tamoxifen reported statistically significant elevated depression scores.5 Similarly, in a prospective trial that assessed mood symptoms in 21 pre- and postmenopausal women who developed estrogen deficiency during breast cancer treatment (including treatment with tamoxifen and chemotherapy), 38% of patients met the criteria for major depressive disorder (MDD) in the first 6 months of treatment. Sixty-six percent of these patients were postmenopausal, and 38% were premenopausal. Twenty-five percent of the premenopausal women who experienced MDD symptoms had been treated with tamoxifen and chemotherapy.6

In a larger prospective trial (N = 257), an oncologist assessed mood symptoms in 2 groups of patients with breast cancer: individuals who received tamoxifen, and those who did not receive tamoxifen.7 They found that 15% of patients who received tamoxifen experienced depression, compared with 3% of patients who did not receive tamoxifen; this difference was statistically significant.7 Overall, 31% of the patients had “significant depression” and 27% discontinued tamoxifen because of adverse effects.7 There have been 2 case reports of tamoxifen use and severe depression in patients with no prior psychiatry history8,9 and 3 case reports of tamoxifen use and severe depression in patients who had a psychiatric history.10-12
One study that examined 24 men with breast cancer found that 62.5% of these patients experienced adverse effects related to tamoxifen, and 25% discontinued tamoxifen because of its adverse effects.13 Among the various adverse effects related to tamoxifen, mood alteration was reported in 20.8% of cases, and depressed feelings were reported in 16.6%.13
Continue to: Despite the evidence...
Despite this evidence, other studies have not found an association between tamoxifen and depressed mood in patients with breast cancer. One group of researchers who assessed various symptoms self-reported by postmenopausal women who were breast cancer survivors found that the depression scores were not significant.14 A retrospective cohort study assessed the onset of depression in patients with breast cancer with positive hormone receptor status (who received tamoxifen) vs negative hormone receptor status (who did not receive tamoxifen). These researchers did not find a statistically significant hazard ratio for “new-onset depression.”15 Unfortunately, the criteria for “new-onset depression” used in this study was the diagnosis of depression or use of an antidepressant given or ordered by a clinician, which is not a sensitive assessment of depressed mood.15
A multicenter randomized, placebo-controlled trial (the National Surgical Adjuvant Breast and Bowel Project) assessed the incidence of negative health outcomes, including depression, in a secondary outcome analysis.16 These researchers did not find a statistically significantassociation between tamoxifen and depression. However, in this study, assessment of depression was based on self-report using the Center of Epidemiologic Studies Depression (CES-D) scale, which does not clinically categorize depression. Furthermore, these researchers strongly recommended screening for mood disorders in routine clinical practice. In this study, 3 women completed suicide, 2 of whom were in the tamoxifen arm.16
[polldaddy:10497045]
The authors’ observations
Tamoxifen is a prodrug that converts to the active metabolite, endoxifen, via cytochrome P450 2D6 (CYP2D6) activity. Antidepressants with strong 2D6-inhibiting properties, such as fluoxetine, duloxetine, bupropion, and paroxetine, should be avoided in patients receiving tamoxifen because they interfere with the formation of the active metabolite and could reduce the effectiveness of tamoxifen and its ability to reduce the risk of cancer recurrence.17 Antidepressants can help treat psychological distress, especially depression, which is common in patients with cancer, and vasomotor symptoms, which may impair quality of life and adherence to long-term endocrine therapy. Because tamoxifen can decrease cancer recurrence and associated mortality,18 adherence with treatment is crucial.
TREATMENT Starting an antidepressant
The psychiatry team initiates venlafaxine, 37.5 mg/d, to treat Mrs. L’s anxiety and help prevent the recurrence of severe depression. They prescribe venlafaxine because they anticipate that, based on Mrs. L’s age, the oncology team might reconsider treatment with tamoxifen. Venlafaxine is preferred because it has a favorable pharmacodynamic profile and does not interfere with the metabolism of tamoxifen, as is the case with many selective serotonin reuptake inhibitors.17
Although Mrs. L’s depression had abated once she stopped receiving tamoxifen, she continues to experience anxiety and tearfulness, primarily due to fear of adverse effects of hormone therapy, and due to family as well as work stressors. Therefore, venlafaxine is gradually titrated up to 150 mg/d.
Continue to: The oncology team proposes...
The oncology team proposes a trial of leuprolide, a gonadotropin-releasing hormone agonist that downregulates pituitary receptors, subsequently suppressing female reproductive hormones, which in turn stops the ovaries from producing estrogen so there is a minimal amount of estrogen to promote the growth of estrogen–receptor-positive breast cancer. Mrs. L declines this agent because she is concerned that she will gain weight. Instead, Mrs. L expresses interest in undergoing an oophorectomy to reduce her estrogen level. In the meantime, based on her reproductive hormone levels (FSH and estradiol levels) which are indicative of postmenopausal status, the oncology team prescribes the aromatase inhibitor (AI) exemestane 25 mg/d. The AI helps to decrease the amount of estrogen the body makes peripherally, which is the main source of estrogen in postmenopausal women.
The authors’ observations
Estrogen originates in the ovaries in premenopausal women; it is also produced by peripheral conversion of androgens to estrogen in adipose tissues and muscle in postmenopausal women.19 Aromatase inhibitors block the enzyme aromatase that converts androgen to estrogen, which leads to estrogen deficiency in postmenopausal women and possibly to neuropsychiatric effects.19
The results of studies assessing the adverse psychiatric effects of AIs are mixed. When the results of studies evaluating tamoxifen are compared with those evaluating AIs, overall patients who received AIs had less severe or less frequent mood symptoms. One possible explanation could be that AIs are relatively new compared with tamoxifen. Second, AIs are more commonly used in postmenopausal women with breast cancer, and these patients’ overall estrogen level is significantly lower than that of premenopausal women with breast cancer. Therefore, the degree of hormone fluctuation is less intense in postmenopausal breast cancer survivors.
OUTCOME
After starting exemestane, and while still receiving venlafaxine, Mrs. L no longer experiences severe depressive symptoms. After 8 months, venlafaxine is discontinued. She continues to deny depressive symptoms but has intermittent anxiety, which she is able to manage without psychiatric medication. She continues to remain adherent with ongoing exemestane treatment, with no evidence of disease progression or recurrence.
The authors’ observations
For patients with estrogen-positive breast cancer, the decision to discontinue tamoxifen because of unacceptable adverse effects is an important one because it may increase the risk of cancer recurrence. Psychiatrists have an important role in supporting the patient through this process, helping patients understand alternatives, and working with the oncology team to formulate a plan that is acceptable to everyone.
Continue to: Bottom Line
Bottom Line
For patients with estrogen–positive breast cancer, anti-estrogen treatment can reduce the risk of cancer recurrence. However, it can cause adverse effects, including depression, that might impair quality of life and treatment adherence. For patients with severe depression, stopping estrogen blockers may be warranted. Initiating an antidepressant that does not interfere with the metabolism of tamoxifen may help treat depression and vasomotor symptoms.
Related Resource
- Agarwala P. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11):39-40,45-46,48-49.
Drug Brand Names
Agomelatine • Valdoxan
Bupropion • Wellbutrin, Zyban
Cyclophosphamide • Cytoxan
Doxorubicin • Adriamycin
Duloxetine • Cymbalta
Exemestane • Aromasin
Fluoxetine • Prozac
Leuprolide • Eligard, Lupron
Milnacipran • Savella
Paroxetine • Paxil
Sertraline • Zoloft
Tamoxifen • Soltamox
Trastuzumab • Herceptin
Venlafaxine • Effexor
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
1. Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
2. Thompson DS, Spanier CA, Vogel VG. The relationship between tamoxifen, estrogen, and depressive symptoms. Breast J. 1999;5(6):375-382.
3. Halbreich U. Role of estrogen in postmenopausal depression. Neurology. 1997;48(5 suppl 7):S16-S19.
4. Schiller CE, Johnson SL, Abate AC, et al. Reproductive steroid regulation of mood and behavior. Compr Physiol. 2016;6(3):1135-1160.
5. Shariff S, Cumming CE, Lees A, et al. Mood disorder in women with early breast cancer taking tamoxifen, an estradiol receptor antagonist. An expected or unexpected effect? Ann N Y Acad Sci. 1995;761:365-368.
6. Duffy LS, Greenberg DB, Younger J, et al. Iatrogenic acute estrogen deficiency and psychiatric syndromes in breast cancer patients. Psychosomatics. 1999;40(4):304-308.
7. Cathcart CK, Jones SE, Pumroy CS, et al. Clinical recognition and management of depression in node negative breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 1993;27(3):277-281.
8. Lin J, Thompson DS. Case report: tamoxifen-induced depression. Primary Care Update for Ob/Gyns. 2001;8(5):207-208.
9. Pluss JL, DiBella NJ. Reversible central nervous system dysfunction due to tamoxifen in a patient with breast cancer. Ann Intern Med. 1984;101(5):652.
10. Bourque F, Karama S, Looper K, et al. Acute tamoxifen-induced depression and its prevention with venlafaxine. Psychosomatics. 2009;50(2):162-165.
11. De Berardis D, Brucchi M, Serroni N, et al. Successful use of agomelatine in the treatment of major depression in a woman taking tamoxifen: a case report. Clin Neuropharmacol. 2014;37(1):31-33.
12. Ito M, Baba H, Kawashima R, et al. A case of prolonged depression with tamoxifen. Japan Med Assoc J. 2006;49(4):167-172.
13. Anelli TF, Anelli A, Tran KN, et al. Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer. 1994;74(1):74-77.
14. Love RR, Cameron L, Connell BL, et al. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151(9):1842-1847.
15. Lee KC, Ray GT, Hunkeler EM, et al. Tamoxifen treatment and new-onset depression in breast cancer patients. Psychosomatics. 2007;48(3):205-210.
16. Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615-1623.
17. Juurlink D. Revisiting the drug interaction between tamoxifen and SSRI antidepressants. BMJ. 2016;354:i5309.
18. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
19. Buijs C, de Vries EGE, Mourits MJE, et al. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev. 2008;34(7):640-655.
MAPS.EDU, GAS POPS, and AEIOU: Acronyms to guide your assessments
Mnemonics and acronyms are part of our daily lives, helping us to memorize and retain clinical information. They play an invaluable role in medical school because they can help students recall vast amounts of information in a moment’s notice, such as psychiatric conditions to consider during a “review of systems.”
Most medical students are trained to conduct a review of systems as a standard approach when a thorough medical history is indicated. Clinicians need to assess all patients for an extremely broad range of syndromes. Because of the extensive comorbidity of many psychiatric disorders, it is important to review the most common conditions before establishing a diagnosis and formulating a treatment plan.1
For example, a patient presenting with a chief complaint consistent with a depressive disorder may have unipolar depression, bipolar depression, or substance-induced depression (after general medical comorbidity has been excluded). In this scenario, it would be equally important to identify co-occurring conditions, such as an anxiety disorder or psychotic symptoms, because these can have a major impact on treatment and prognosis.
In our work as clinical educators, we have noticed that many students struggle with a review of psychiatric systems during their evaluation of a new patient. Acronyms could serve as a map to guide them during assessments. While these may be most valuable to medical students, they are also helpful for clinicians on the frontline of medical practice (primary care, family practice, OB-GYN) as well as for early-career psychiatrists.
MAPS.EDU
The acronym MAPS.EDU covers several common psychiatric conditions seen in routine practice: Mood disorders, Anxiety disorders, Personality disorders, Schizophrenia and related disorders, Eating disorders, Developmental disorders (eg, attention-deficit/hyperactivity disorder), and substance Use disorders. While not comprehensive, MAPS.EDU can be a quick method to help psychiatrists remember these common conditions.
GAS POPS
Anxiety is a core symptom of several psychiatric disorders. The mnemonic GAS POPS can help clinicians recall disorders to consider when screening patients who report anxiety: Generalized anxiety disorder, Agoraphobia, Social anxiety disorder, Panic disorder, Obsessive-compulsive disorder, Posttraumatic stress disorder (PTSD), and Specific phobias.
AEIOU for PTSD
The diagnostic criteria of PTSD can be memorized by using the acronym AEIOU: Avoidance (of triggers), Exposure (to trauma), Intrusions (reliving phenomena), Outbursts (or other manifestations of hyperarousal), and Unhappiness (negative alterations in mood and cognition).
1. Rush J, Zimmerman M, Wisniewski S, et al. Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord. 2005;87(1):43-55.
Mnemonics and acronyms are part of our daily lives, helping us to memorize and retain clinical information. They play an invaluable role in medical school because they can help students recall vast amounts of information in a moment’s notice, such as psychiatric conditions to consider during a “review of systems.”
Most medical students are trained to conduct a review of systems as a standard approach when a thorough medical history is indicated. Clinicians need to assess all patients for an extremely broad range of syndromes. Because of the extensive comorbidity of many psychiatric disorders, it is important to review the most common conditions before establishing a diagnosis and formulating a treatment plan.1
For example, a patient presenting with a chief complaint consistent with a depressive disorder may have unipolar depression, bipolar depression, or substance-induced depression (after general medical comorbidity has been excluded). In this scenario, it would be equally important to identify co-occurring conditions, such as an anxiety disorder or psychotic symptoms, because these can have a major impact on treatment and prognosis.
In our work as clinical educators, we have noticed that many students struggle with a review of psychiatric systems during their evaluation of a new patient. Acronyms could serve as a map to guide them during assessments. While these may be most valuable to medical students, they are also helpful for clinicians on the frontline of medical practice (primary care, family practice, OB-GYN) as well as for early-career psychiatrists.
MAPS.EDU
The acronym MAPS.EDU covers several common psychiatric conditions seen in routine practice: Mood disorders, Anxiety disorders, Personality disorders, Schizophrenia and related disorders, Eating disorders, Developmental disorders (eg, attention-deficit/hyperactivity disorder), and substance Use disorders. While not comprehensive, MAPS.EDU can be a quick method to help psychiatrists remember these common conditions.
GAS POPS
Anxiety is a core symptom of several psychiatric disorders. The mnemonic GAS POPS can help clinicians recall disorders to consider when screening patients who report anxiety: Generalized anxiety disorder, Agoraphobia, Social anxiety disorder, Panic disorder, Obsessive-compulsive disorder, Posttraumatic stress disorder (PTSD), and Specific phobias.
AEIOU for PTSD
The diagnostic criteria of PTSD can be memorized by using the acronym AEIOU: Avoidance (of triggers), Exposure (to trauma), Intrusions (reliving phenomena), Outbursts (or other manifestations of hyperarousal), and Unhappiness (negative alterations in mood and cognition).
Mnemonics and acronyms are part of our daily lives, helping us to memorize and retain clinical information. They play an invaluable role in medical school because they can help students recall vast amounts of information in a moment’s notice, such as psychiatric conditions to consider during a “review of systems.”
Most medical students are trained to conduct a review of systems as a standard approach when a thorough medical history is indicated. Clinicians need to assess all patients for an extremely broad range of syndromes. Because of the extensive comorbidity of many psychiatric disorders, it is important to review the most common conditions before establishing a diagnosis and formulating a treatment plan.1
For example, a patient presenting with a chief complaint consistent with a depressive disorder may have unipolar depression, bipolar depression, or substance-induced depression (after general medical comorbidity has been excluded). In this scenario, it would be equally important to identify co-occurring conditions, such as an anxiety disorder or psychotic symptoms, because these can have a major impact on treatment and prognosis.
In our work as clinical educators, we have noticed that many students struggle with a review of psychiatric systems during their evaluation of a new patient. Acronyms could serve as a map to guide them during assessments. While these may be most valuable to medical students, they are also helpful for clinicians on the frontline of medical practice (primary care, family practice, OB-GYN) as well as for early-career psychiatrists.
MAPS.EDU
The acronym MAPS.EDU covers several common psychiatric conditions seen in routine practice: Mood disorders, Anxiety disorders, Personality disorders, Schizophrenia and related disorders, Eating disorders, Developmental disorders (eg, attention-deficit/hyperactivity disorder), and substance Use disorders. While not comprehensive, MAPS.EDU can be a quick method to help psychiatrists remember these common conditions.
GAS POPS
Anxiety is a core symptom of several psychiatric disorders. The mnemonic GAS POPS can help clinicians recall disorders to consider when screening patients who report anxiety: Generalized anxiety disorder, Agoraphobia, Social anxiety disorder, Panic disorder, Obsessive-compulsive disorder, Posttraumatic stress disorder (PTSD), and Specific phobias.
AEIOU for PTSD
The diagnostic criteria of PTSD can be memorized by using the acronym AEIOU: Avoidance (of triggers), Exposure (to trauma), Intrusions (reliving phenomena), Outbursts (or other manifestations of hyperarousal), and Unhappiness (negative alterations in mood and cognition).
1. Rush J, Zimmerman M, Wisniewski S, et al. Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord. 2005;87(1):43-55.
1. Rush J, Zimmerman M, Wisniewski S, et al. Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord. 2005;87(1):43-55.
Caution about ‘miracle cures’; more
Caution about ‘miracle cures’
I thank Drs. Katherine Epstein and Helen Farrell for the balanced approach in their article “‘Miracle cures’ in psychiatry?” (Psychiatry 2.0,
We need to pay serious attention to the small sample sizes and limited criteria for patient selection in trials of ketamine and MDMA, as well as to what sort of “psychotherapy” follows treatment with these agents. Many of us in psychiatric practice for the past 40 years have been humbled by patients’ idiosyncratic reactions to standard medications, let alone novel ones. Those of us who practiced psychiatry in the heyday of “party drugs” have seen many idiosyncratic reactions. Most early research with cannabinoids and lysergic acid diethylamide (and even Strassman’s trials with N,N-dimethyltryptamine [DMT]1-5) highlighted the significance of response by drug-naïve patients vs drug-savvy individuals. Apart from Veterans Affairs trials for posttraumatic stress disorder, many trials of these drugs for treatment-resistant depression or end-of-life care have attracted non-naïve participants.6-8 Private use of entheogens is quite different from medicalizing their use. This requires our best scrutiny. Our earnest interest in improving outcomes must not be influenced by the promise of a quick fix, let alone a miracle cure.
Sara Hartley, MD
Clinical Faculty
Interim Head of Admissions
UC Berkley/UCSF Joint Medical Program
Berkeley, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73(1-2):121-124.
2. Strassman RJ. DMT: the spirit molecule. A doctor’s revolutionary research into the biology of near-death and mystical experiences. Rochester, VT: Park Street Press; 2001.
3. Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51(2):85-97.
4. Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39(9):784-795.
5. Strassman RJ, Qualls CR, Uhlenhuth EH, et al. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51(2):98-108.
6. Albott CS, et al. Improvement in suicidal ideation after repeated ketamine infusions: Relationship to reductions in symptoms of posttraumatic stress disorder, depression, and pain. Presented at: The Anxiety and Depression Association of America Annual Conference; Mar. 28-31, 2019; Chicago.
7. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509-523.
8. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
Continue to: Physician assistants and the psychiatrist shortage
Physician assistants and the psychiatrist shortage
J. Michael Smith’s article “Physician assistants in psychiatry: Helping to meet America’s mental health needs” (Commentary,
There needs to be a multifocal approach to incentivize medical students to choose psychiatry as a specialty. Several factors have discouraged medical students from going into psychiatry. The low reimbursement rates by insurance companies force psychiatrists to not accept insurances or to work for hospital or clinic organizations, where they become a part of the “medication management industry.” This scenario was created by the pharmaceutical industry and often leaves psychotherapy to other types of clinicians. In the not-too-distant future, advances in both neuroscience and artificial intelligence technologies will further reduce the role of medically trained psychiatrists, and might lead to them being replaced by other emerging professions (eg, psychiatric PAs) that are concentrated in urban settings where they are most profitable.
What can possibly be left for the future of the medically trained psychiatrist if a PA can diagnose and treat psychiatric patients? Why would we need more psychiatrists?
Marco T. Carpio, MD
Psychiatrist, private practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The author responds
I appreciate Dr. Carpio’s comments, and I agree that the shortage of psychiatrists will not be addressed solely by the addition of other types of clinicians, such as PAs and nurse practitioners. However, the use of well-trained health care providers such as PAs will go a long way towards helping patients receive timely and appropriate access to care. Unfortunately, no single plan or method will be adequate to solve the shortage of psychiatrists in the United States, but that does not negate the need for utilizing all available options to improve access to quality mental health care. Physician assistants are well-trained to support this endeavor.
J. Michael Smith, DHSc, MPAS, PA-C, CAQ-Psychiatry
Post-Graduate PA Mental Health Residency Training Director
Physician Assistant, ACCESS Clinic, GMHC
Michael E. DeBakey VA Medical Center
Houston, Texas
Continue to: Additional anathemas in psychiatry
Additional anathemas in psychiatry
While reading Dr. Nasrallah’s “Anathemas of psychiatric practice” (From the Editor,
- Cash-only suboxone clinics. Suboxone was never intended to be used in “suboxone clinics”; it was meant to be part of an integrated treatment provided in an office-based practice. Nevertheless, this treatment has been used as such in this country. As part of this trend, an anathema has grown: cash-only suboxone clinics. Patients with severe substance use disorders can be found in every socioeconomic layer of our society, but many struggle with significant psychosocial adversity and outright poverty. Cash-only suboxone clinics put many patients in a bind. Patients spend their last dollars on a needed treatment or sell these medications to maintain their addiction, or even to purchase food.
- “Medical” marijuana. There is no credible evidence based upon methodologically sound research that cannabis has benefit for treating any mental illness. In fact, there is evidence to the contrary.1 Yet, in many states, physicians—including psychiatrists—are supporting the approval of medical marijuana. I remember taking my Hippocratic Oath when I graduated from medical school, pledging to continue educating myself and my patients about evidenced-based medical science that benefits us all. I have not yet found credible evidence supporting medical marijuana.
Greed in general is a strong anathema in medicine.
Leo Bastiaens, MD
Clinical Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Reference
1. Radhakrishnan R, Ranganathan M, D'Souza DC. Medical marijuana: what physicians need to know. J Clin Psychiatry. 2019;80(5):45-47.
Caution about ‘miracle cures’
I thank Drs. Katherine Epstein and Helen Farrell for the balanced approach in their article “‘Miracle cures’ in psychiatry?” (Psychiatry 2.0,
We need to pay serious attention to the small sample sizes and limited criteria for patient selection in trials of ketamine and MDMA, as well as to what sort of “psychotherapy” follows treatment with these agents. Many of us in psychiatric practice for the past 40 years have been humbled by patients’ idiosyncratic reactions to standard medications, let alone novel ones. Those of us who practiced psychiatry in the heyday of “party drugs” have seen many idiosyncratic reactions. Most early research with cannabinoids and lysergic acid diethylamide (and even Strassman’s trials with N,N-dimethyltryptamine [DMT]1-5) highlighted the significance of response by drug-naïve patients vs drug-savvy individuals. Apart from Veterans Affairs trials for posttraumatic stress disorder, many trials of these drugs for treatment-resistant depression or end-of-life care have attracted non-naïve participants.6-8 Private use of entheogens is quite different from medicalizing their use. This requires our best scrutiny. Our earnest interest in improving outcomes must not be influenced by the promise of a quick fix, let alone a miracle cure.
Sara Hartley, MD
Clinical Faculty
Interim Head of Admissions
UC Berkley/UCSF Joint Medical Program
Berkeley, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73(1-2):121-124.
2. Strassman RJ. DMT: the spirit molecule. A doctor’s revolutionary research into the biology of near-death and mystical experiences. Rochester, VT: Park Street Press; 2001.
3. Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51(2):85-97.
4. Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39(9):784-795.
5. Strassman RJ, Qualls CR, Uhlenhuth EH, et al. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51(2):98-108.
6. Albott CS, et al. Improvement in suicidal ideation after repeated ketamine infusions: Relationship to reductions in symptoms of posttraumatic stress disorder, depression, and pain. Presented at: The Anxiety and Depression Association of America Annual Conference; Mar. 28-31, 2019; Chicago.
7. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509-523.
8. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
Continue to: Physician assistants and the psychiatrist shortage
Physician assistants and the psychiatrist shortage
J. Michael Smith’s article “Physician assistants in psychiatry: Helping to meet America’s mental health needs” (Commentary,
There needs to be a multifocal approach to incentivize medical students to choose psychiatry as a specialty. Several factors have discouraged medical students from going into psychiatry. The low reimbursement rates by insurance companies force psychiatrists to not accept insurances or to work for hospital or clinic organizations, where they become a part of the “medication management industry.” This scenario was created by the pharmaceutical industry and often leaves psychotherapy to other types of clinicians. In the not-too-distant future, advances in both neuroscience and artificial intelligence technologies will further reduce the role of medically trained psychiatrists, and might lead to them being replaced by other emerging professions (eg, psychiatric PAs) that are concentrated in urban settings where they are most profitable.
What can possibly be left for the future of the medically trained psychiatrist if a PA can diagnose and treat psychiatric patients? Why would we need more psychiatrists?
Marco T. Carpio, MD
Psychiatrist, private practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The author responds
I appreciate Dr. Carpio’s comments, and I agree that the shortage of psychiatrists will not be addressed solely by the addition of other types of clinicians, such as PAs and nurse practitioners. However, the use of well-trained health care providers such as PAs will go a long way towards helping patients receive timely and appropriate access to care. Unfortunately, no single plan or method will be adequate to solve the shortage of psychiatrists in the United States, but that does not negate the need for utilizing all available options to improve access to quality mental health care. Physician assistants are well-trained to support this endeavor.
J. Michael Smith, DHSc, MPAS, PA-C, CAQ-Psychiatry
Post-Graduate PA Mental Health Residency Training Director
Physician Assistant, ACCESS Clinic, GMHC
Michael E. DeBakey VA Medical Center
Houston, Texas
Continue to: Additional anathemas in psychiatry
Additional anathemas in psychiatry
While reading Dr. Nasrallah’s “Anathemas of psychiatric practice” (From the Editor,
- Cash-only suboxone clinics. Suboxone was never intended to be used in “suboxone clinics”; it was meant to be part of an integrated treatment provided in an office-based practice. Nevertheless, this treatment has been used as such in this country. As part of this trend, an anathema has grown: cash-only suboxone clinics. Patients with severe substance use disorders can be found in every socioeconomic layer of our society, but many struggle with significant psychosocial adversity and outright poverty. Cash-only suboxone clinics put many patients in a bind. Patients spend their last dollars on a needed treatment or sell these medications to maintain their addiction, or even to purchase food.
- “Medical” marijuana. There is no credible evidence based upon methodologically sound research that cannabis has benefit for treating any mental illness. In fact, there is evidence to the contrary.1 Yet, in many states, physicians—including psychiatrists—are supporting the approval of medical marijuana. I remember taking my Hippocratic Oath when I graduated from medical school, pledging to continue educating myself and my patients about evidenced-based medical science that benefits us all. I have not yet found credible evidence supporting medical marijuana.
Greed in general is a strong anathema in medicine.
Leo Bastiaens, MD
Clinical Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Reference
1. Radhakrishnan R, Ranganathan M, D'Souza DC. Medical marijuana: what physicians need to know. J Clin Psychiatry. 2019;80(5):45-47.
Caution about ‘miracle cures’
I thank Drs. Katherine Epstein and Helen Farrell for the balanced approach in their article “‘Miracle cures’ in psychiatry?” (Psychiatry 2.0,
We need to pay serious attention to the small sample sizes and limited criteria for patient selection in trials of ketamine and MDMA, as well as to what sort of “psychotherapy” follows treatment with these agents. Many of us in psychiatric practice for the past 40 years have been humbled by patients’ idiosyncratic reactions to standard medications, let alone novel ones. Those of us who practiced psychiatry in the heyday of “party drugs” have seen many idiosyncratic reactions. Most early research with cannabinoids and lysergic acid diethylamide (and even Strassman’s trials with N,N-dimethyltryptamine [DMT]1-5) highlighted the significance of response by drug-naïve patients vs drug-savvy individuals. Apart from Veterans Affairs trials for posttraumatic stress disorder, many trials of these drugs for treatment-resistant depression or end-of-life care have attracted non-naïve participants.6-8 Private use of entheogens is quite different from medicalizing their use. This requires our best scrutiny. Our earnest interest in improving outcomes must not be influenced by the promise of a quick fix, let alone a miracle cure.
Sara Hartley, MD
Clinical Faculty
Interim Head of Admissions
UC Berkley/UCSF Joint Medical Program
Berkeley, California
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res. 1996;73(1-2):121-124.
2. Strassman RJ. DMT: the spirit molecule. A doctor’s revolutionary research into the biology of near-death and mystical experiences. Rochester, VT: Park Street Press; 2001.
3. Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51(2):85-97.
4. Strassman RJ, Qualls CR, Berg LM. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry. 1996;39(9):784-795.
5. Strassman RJ, Qualls CR, Uhlenhuth EH, et al. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51(2):98-108.
6. Albott CS, et al. Improvement in suicidal ideation after repeated ketamine infusions: Relationship to reductions in symptoms of posttraumatic stress disorder, depression, and pain. Presented at: The Anxiety and Depression Association of America Annual Conference; Mar. 28-31, 2019; Chicago.
7. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509-523.
8. Mithoefer MC, Mithoefer AT, Feduccia AA, et al. 3,4-Methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. 2018;5(6):486-497.
Continue to: Physician assistants and the psychiatrist shortage
Physician assistants and the psychiatrist shortage
J. Michael Smith’s article “Physician assistants in psychiatry: Helping to meet America’s mental health needs” (Commentary,
There needs to be a multifocal approach to incentivize medical students to choose psychiatry as a specialty. Several factors have discouraged medical students from going into psychiatry. The low reimbursement rates by insurance companies force psychiatrists to not accept insurances or to work for hospital or clinic organizations, where they become a part of the “medication management industry.” This scenario was created by the pharmaceutical industry and often leaves psychotherapy to other types of clinicians. In the not-too-distant future, advances in both neuroscience and artificial intelligence technologies will further reduce the role of medically trained psychiatrists, and might lead to them being replaced by other emerging professions (eg, psychiatric PAs) that are concentrated in urban settings where they are most profitable.
What can possibly be left for the future of the medically trained psychiatrist if a PA can diagnose and treat psychiatric patients? Why would we need more psychiatrists?
Marco T. Carpio, MD
Psychiatrist, private practice
Lynbrook, New York
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
The author responds
I appreciate Dr. Carpio’s comments, and I agree that the shortage of psychiatrists will not be addressed solely by the addition of other types of clinicians, such as PAs and nurse practitioners. However, the use of well-trained health care providers such as PAs will go a long way towards helping patients receive timely and appropriate access to care. Unfortunately, no single plan or method will be adequate to solve the shortage of psychiatrists in the United States, but that does not negate the need for utilizing all available options to improve access to quality mental health care. Physician assistants are well-trained to support this endeavor.
J. Michael Smith, DHSc, MPAS, PA-C, CAQ-Psychiatry
Post-Graduate PA Mental Health Residency Training Director
Physician Assistant, ACCESS Clinic, GMHC
Michael E. DeBakey VA Medical Center
Houston, Texas
Continue to: Additional anathemas in psychiatry
Additional anathemas in psychiatry
While reading Dr. Nasrallah’s “Anathemas of psychiatric practice” (From the Editor,
- Cash-only suboxone clinics. Suboxone was never intended to be used in “suboxone clinics”; it was meant to be part of an integrated treatment provided in an office-based practice. Nevertheless, this treatment has been used as such in this country. As part of this trend, an anathema has grown: cash-only suboxone clinics. Patients with severe substance use disorders can be found in every socioeconomic layer of our society, but many struggle with significant psychosocial adversity and outright poverty. Cash-only suboxone clinics put many patients in a bind. Patients spend their last dollars on a needed treatment or sell these medications to maintain their addiction, or even to purchase food.
- “Medical” marijuana. There is no credible evidence based upon methodologically sound research that cannabis has benefit for treating any mental illness. In fact, there is evidence to the contrary.1 Yet, in many states, physicians—including psychiatrists—are supporting the approval of medical marijuana. I remember taking my Hippocratic Oath when I graduated from medical school, pledging to continue educating myself and my patients about evidenced-based medical science that benefits us all. I have not yet found credible evidence supporting medical marijuana.
Greed in general is a strong anathema in medicine.
Leo Bastiaens, MD
Clinical Associate Professor of Psychiatry
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Reference
1. Radhakrishnan R, Ranganathan M, D'Souza DC. Medical marijuana: what physicians need to know. J Clin Psychiatry. 2019;80(5):45-47.