User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Called to court? Tips for testifying
As a psychiatrist, you could be called to court to testify as a fact witness in a hearing or trial. Your role as a fact witness would differ from that of an expert witness in that you would likely testify about the information that you have gathered through direct observation of patients or others. Fact witnesses are generally not asked to give expert opinions regarding forensic issues, and treating psychiatrists should not do so about their patients. As a fact witness, depending on the form of litigation, you might be in one of the following 4 roles1:
- Observer. As the term implies, you have observed an event. For example, you are asked to testify about a fight that you witnessed between another clinician’s patient and a nurse while you were making your rounds on an inpatient unit.
- Non-defendant treater. You are the treating psychiatrist for a patient who is involved in litigation to recover damages for injuries sustained from a third party. For example, you are asked to testify about your patient’s premorbid functioning before a claimed injury that spurred the lawsuit.
- Plaintiff. You are suing someone else and may be claiming your own damages. For example, in your attempt to claim damages as a plaintiff, you use your clinical knowledge to testify about your own mental health symptoms and the adverse impact these have had on you.
- Defendant treater. You are being sued by one of your patients. For example, a patient brings a malpractice case against you for allegations of not meeting the standard of care. You testify about your direct observations of the patient, the diagnoses you provided, and your rationale for the implemented treatment plan.
Preparing yourself as a fact witness
For many psychiatrists, testifying can be an intimidating process. Although there are similarities between testifying in a courtroom and giving a deposition, there are also significant differences. For guidelines on providing depositions, see Knoll and Resnick’s “Deposition dos and don’ts: How to answer 8 tricky questions” (
Don’t panic. Although your first reaction may be to panic upon receiving a subpoena or court order, you should “keep your cool” and remember that the observations you made or treatment provided have already taken place.1 Your role as a fact witness is to inform the judge and jury about what you saw and did.1
Continue to: Refresh your memory and practice
Refresh your memory and practice. Gather all required information (eg, medical records, your notes, etc.) and review it before testifying. This will help you to recall the facts more accurately when you are asked a question. Consider practicing your testimony with the attorney who requested you to get feedback on how you present yourself.1 However, do not try to memorize what you are going to say because this could make your testimony sound rehearsed and unconvincing.
Plan ahead, and have a pretrial conference. Because court proceedings are unpredictable, you should clear your schedule to allow enough time to appear in court. Before your court appearance, meet with the attorney who requested you to discuss any new facts or issues as well as learn what the attorney aims to accomplish with your testimony.1
Speak clearly in your own words, and avoid jargon. Courtroom officials are unlikely to understand psychiatric jargon. Therefore, you should explain psychiatric terms in language that laypeople would comprehend. Because the court stenographer will require you to use actual words for the court transcripts, you should answer clearly and verbally or respond with a definitive “yes” or “no” (and not by nodding or shaking your head).
Testimony is also not a time for guessing. If you don’t know the answer, you should say “I don’t know.”
1. Gutheil TG. The psychiatrist in court: a survival guide. Washington, DC: American Psychiatric Press, Inc.; 1998.
2. Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
As a psychiatrist, you could be called to court to testify as a fact witness in a hearing or trial. Your role as a fact witness would differ from that of an expert witness in that you would likely testify about the information that you have gathered through direct observation of patients or others. Fact witnesses are generally not asked to give expert opinions regarding forensic issues, and treating psychiatrists should not do so about their patients. As a fact witness, depending on the form of litigation, you might be in one of the following 4 roles1:
- Observer. As the term implies, you have observed an event. For example, you are asked to testify about a fight that you witnessed between another clinician’s patient and a nurse while you were making your rounds on an inpatient unit.
- Non-defendant treater. You are the treating psychiatrist for a patient who is involved in litigation to recover damages for injuries sustained from a third party. For example, you are asked to testify about your patient’s premorbid functioning before a claimed injury that spurred the lawsuit.
- Plaintiff. You are suing someone else and may be claiming your own damages. For example, in your attempt to claim damages as a plaintiff, you use your clinical knowledge to testify about your own mental health symptoms and the adverse impact these have had on you.
- Defendant treater. You are being sued by one of your patients. For example, a patient brings a malpractice case against you for allegations of not meeting the standard of care. You testify about your direct observations of the patient, the diagnoses you provided, and your rationale for the implemented treatment plan.
Preparing yourself as a fact witness
For many psychiatrists, testifying can be an intimidating process. Although there are similarities between testifying in a courtroom and giving a deposition, there are also significant differences. For guidelines on providing depositions, see Knoll and Resnick’s “Deposition dos and don’ts: How to answer 8 tricky questions” (
Don’t panic. Although your first reaction may be to panic upon receiving a subpoena or court order, you should “keep your cool” and remember that the observations you made or treatment provided have already taken place.1 Your role as a fact witness is to inform the judge and jury about what you saw and did.1
Continue to: Refresh your memory and practice
Refresh your memory and practice. Gather all required information (eg, medical records, your notes, etc.) and review it before testifying. This will help you to recall the facts more accurately when you are asked a question. Consider practicing your testimony with the attorney who requested you to get feedback on how you present yourself.1 However, do not try to memorize what you are going to say because this could make your testimony sound rehearsed and unconvincing.
Plan ahead, and have a pretrial conference. Because court proceedings are unpredictable, you should clear your schedule to allow enough time to appear in court. Before your court appearance, meet with the attorney who requested you to discuss any new facts or issues as well as learn what the attorney aims to accomplish with your testimony.1
Speak clearly in your own words, and avoid jargon. Courtroom officials are unlikely to understand psychiatric jargon. Therefore, you should explain psychiatric terms in language that laypeople would comprehend. Because the court stenographer will require you to use actual words for the court transcripts, you should answer clearly and verbally or respond with a definitive “yes” or “no” (and not by nodding or shaking your head).
Testimony is also not a time for guessing. If you don’t know the answer, you should say “I don’t know.”
As a psychiatrist, you could be called to court to testify as a fact witness in a hearing or trial. Your role as a fact witness would differ from that of an expert witness in that you would likely testify about the information that you have gathered through direct observation of patients or others. Fact witnesses are generally not asked to give expert opinions regarding forensic issues, and treating psychiatrists should not do so about their patients. As a fact witness, depending on the form of litigation, you might be in one of the following 4 roles1:
- Observer. As the term implies, you have observed an event. For example, you are asked to testify about a fight that you witnessed between another clinician’s patient and a nurse while you were making your rounds on an inpatient unit.
- Non-defendant treater. You are the treating psychiatrist for a patient who is involved in litigation to recover damages for injuries sustained from a third party. For example, you are asked to testify about your patient’s premorbid functioning before a claimed injury that spurred the lawsuit.
- Plaintiff. You are suing someone else and may be claiming your own damages. For example, in your attempt to claim damages as a plaintiff, you use your clinical knowledge to testify about your own mental health symptoms and the adverse impact these have had on you.
- Defendant treater. You are being sued by one of your patients. For example, a patient brings a malpractice case against you for allegations of not meeting the standard of care. You testify about your direct observations of the patient, the diagnoses you provided, and your rationale for the implemented treatment plan.
Preparing yourself as a fact witness
For many psychiatrists, testifying can be an intimidating process. Although there are similarities between testifying in a courtroom and giving a deposition, there are also significant differences. For guidelines on providing depositions, see Knoll and Resnick’s “Deposition dos and don’ts: How to answer 8 tricky questions” (
Don’t panic. Although your first reaction may be to panic upon receiving a subpoena or court order, you should “keep your cool” and remember that the observations you made or treatment provided have already taken place.1 Your role as a fact witness is to inform the judge and jury about what you saw and did.1
Continue to: Refresh your memory and practice
Refresh your memory and practice. Gather all required information (eg, medical records, your notes, etc.) and review it before testifying. This will help you to recall the facts more accurately when you are asked a question. Consider practicing your testimony with the attorney who requested you to get feedback on how you present yourself.1 However, do not try to memorize what you are going to say because this could make your testimony sound rehearsed and unconvincing.
Plan ahead, and have a pretrial conference. Because court proceedings are unpredictable, you should clear your schedule to allow enough time to appear in court. Before your court appearance, meet with the attorney who requested you to discuss any new facts or issues as well as learn what the attorney aims to accomplish with your testimony.1
Speak clearly in your own words, and avoid jargon. Courtroom officials are unlikely to understand psychiatric jargon. Therefore, you should explain psychiatric terms in language that laypeople would comprehend. Because the court stenographer will require you to use actual words for the court transcripts, you should answer clearly and verbally or respond with a definitive “yes” or “no” (and not by nodding or shaking your head).
Testimony is also not a time for guessing. If you don’t know the answer, you should say “I don’t know.”
1. Gutheil TG. The psychiatrist in court: a survival guide. Washington, DC: American Psychiatric Press, Inc.; 1998.
2. Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
1. Gutheil TG. The psychiatrist in court: a survival guide. Washington, DC: American Psychiatric Press, Inc.; 1998.
2. Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
The evolution of manic and hypomanic symptoms
Since publication of the first Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1952,1 the diagnosis of manic and hypomanic symptoms has evolved significantly. This evolution has changed my approach to patients who exhibit these symptoms, which include increased goal-directed activity, decreased need for sleep, and racing thoughts. Here I outline these diagnostic changes in each edition of the DSM and discuss their therapeutic importance and the possibility of future changes.
DSM-I (1952) described manic symptoms as having psychotic features.1 The term “manic episode” was not used, but manic symptoms were described as having a “tendency to remission and recurrence.”1
DSM-II (1968) introduced the term “manic episode” as having psychotic features.2 Manic episodes were characterized by symptoms of excessive elation, irritability, talkativeness, flight of ideas, and accelerated speech and motor activity.2
DSM-III (1980) explained that a manic episode could occur without psychotic features.3 The term “hypomanic episode” was introduced. It described manic features that do not meet criteria for a manic episode.3
DSM-IV (1994) reiterated the criteria for a manic episode.4 In addition, it established criteria for a hypomanic episode as lasting at least 4 days and requires ≥3 symptoms.4
DSM-5 (2013) describes hypomanic symptoms that do not meet criteria for a hypomanic episode (Table).5 These symptoms may require treatment with a mood stabilizer or antipsychotic medication.5
Suggested changes for the next DSM
Although DSM-5 does not discuss the duration of different manic or hypomanic symptoms in the same patient, these can vary widely.6 The same patient may have increased activity for 2 days, increased irritability for 2 weeks, and racing thoughts every day. Future versions of the DSM could include the varying durations of different manic or hypomanic symptoms in the same patient.
Continue to: Racing thoughts without...
Racing thoughts without increased energy or activity occur frequently and often go unnoticed.7 They can be mistaken for severe worrying or obsessive ideation. Depending on the severity of the patient’s racing thoughts, treatment might include a mood stabilizer or antipsychotic. All 5 DSM-5 diagnoses listed in the Table5 may include this symptom pattern, but do not specifically mention it. A diagnosis or specifier, such as “racing thoughts without increased energy or activity,” might help clinicians better recognize and treat this symptom pattern.
1. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 1952:24-25.
2. Diagnostic and statistical manual of mental disorders. 2nd ed. Washington, DC: American Psychiatric Association; 1968:35-37.
3. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980:208-210,223.
4. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:332,338.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:139-140,148-149,169,184-185.
6. Wilf TJ. When to treat subthreshold hypomanic episodes. Current Psychiatry. 2012;11(8):55.
7. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59(5):570-575.
Since publication of the first Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1952,1 the diagnosis of manic and hypomanic symptoms has evolved significantly. This evolution has changed my approach to patients who exhibit these symptoms, which include increased goal-directed activity, decreased need for sleep, and racing thoughts. Here I outline these diagnostic changes in each edition of the DSM and discuss their therapeutic importance and the possibility of future changes.
DSM-I (1952) described manic symptoms as having psychotic features.1 The term “manic episode” was not used, but manic symptoms were described as having a “tendency to remission and recurrence.”1
DSM-II (1968) introduced the term “manic episode” as having psychotic features.2 Manic episodes were characterized by symptoms of excessive elation, irritability, talkativeness, flight of ideas, and accelerated speech and motor activity.2
DSM-III (1980) explained that a manic episode could occur without psychotic features.3 The term “hypomanic episode” was introduced. It described manic features that do not meet criteria for a manic episode.3
DSM-IV (1994) reiterated the criteria for a manic episode.4 In addition, it established criteria for a hypomanic episode as lasting at least 4 days and requires ≥3 symptoms.4
DSM-5 (2013) describes hypomanic symptoms that do not meet criteria for a hypomanic episode (Table).5 These symptoms may require treatment with a mood stabilizer or antipsychotic medication.5
Suggested changes for the next DSM
Although DSM-5 does not discuss the duration of different manic or hypomanic symptoms in the same patient, these can vary widely.6 The same patient may have increased activity for 2 days, increased irritability for 2 weeks, and racing thoughts every day. Future versions of the DSM could include the varying durations of different manic or hypomanic symptoms in the same patient.
Continue to: Racing thoughts without...
Racing thoughts without increased energy or activity occur frequently and often go unnoticed.7 They can be mistaken for severe worrying or obsessive ideation. Depending on the severity of the patient’s racing thoughts, treatment might include a mood stabilizer or antipsychotic. All 5 DSM-5 diagnoses listed in the Table5 may include this symptom pattern, but do not specifically mention it. A diagnosis or specifier, such as “racing thoughts without increased energy or activity,” might help clinicians better recognize and treat this symptom pattern.
Since publication of the first Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1952,1 the diagnosis of manic and hypomanic symptoms has evolved significantly. This evolution has changed my approach to patients who exhibit these symptoms, which include increased goal-directed activity, decreased need for sleep, and racing thoughts. Here I outline these diagnostic changes in each edition of the DSM and discuss their therapeutic importance and the possibility of future changes.
DSM-I (1952) described manic symptoms as having psychotic features.1 The term “manic episode” was not used, but manic symptoms were described as having a “tendency to remission and recurrence.”1
DSM-II (1968) introduced the term “manic episode” as having psychotic features.2 Manic episodes were characterized by symptoms of excessive elation, irritability, talkativeness, flight of ideas, and accelerated speech and motor activity.2
DSM-III (1980) explained that a manic episode could occur without psychotic features.3 The term “hypomanic episode” was introduced. It described manic features that do not meet criteria for a manic episode.3
DSM-IV (1994) reiterated the criteria for a manic episode.4 In addition, it established criteria for a hypomanic episode as lasting at least 4 days and requires ≥3 symptoms.4
DSM-5 (2013) describes hypomanic symptoms that do not meet criteria for a hypomanic episode (Table).5 These symptoms may require treatment with a mood stabilizer or antipsychotic medication.5
Suggested changes for the next DSM
Although DSM-5 does not discuss the duration of different manic or hypomanic symptoms in the same patient, these can vary widely.6 The same patient may have increased activity for 2 days, increased irritability for 2 weeks, and racing thoughts every day. Future versions of the DSM could include the varying durations of different manic or hypomanic symptoms in the same patient.
Continue to: Racing thoughts without...
Racing thoughts without increased energy or activity occur frequently and often go unnoticed.7 They can be mistaken for severe worrying or obsessive ideation. Depending on the severity of the patient’s racing thoughts, treatment might include a mood stabilizer or antipsychotic. All 5 DSM-5 diagnoses listed in the Table5 may include this symptom pattern, but do not specifically mention it. A diagnosis or specifier, such as “racing thoughts without increased energy or activity,” might help clinicians better recognize and treat this symptom pattern.
1. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 1952:24-25.
2. Diagnostic and statistical manual of mental disorders. 2nd ed. Washington, DC: American Psychiatric Association; 1968:35-37.
3. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980:208-210,223.
4. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:332,338.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:139-140,148-149,169,184-185.
6. Wilf TJ. When to treat subthreshold hypomanic episodes. Current Psychiatry. 2012;11(8):55.
7. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59(5):570-575.
1. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 1952:24-25.
2. Diagnostic and statistical manual of mental disorders. 2nd ed. Washington, DC: American Psychiatric Association; 1968:35-37.
3. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980:208-210,223.
4. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:332,338.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:139-140,148-149,169,184-185.
6. Wilf TJ. When to treat subthreshold hypomanic episodes. Current Psychiatry. 2012;11(8):55.
7. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59(5):570-575.
Lofexidine: An option for treating opioid withdrawal
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).
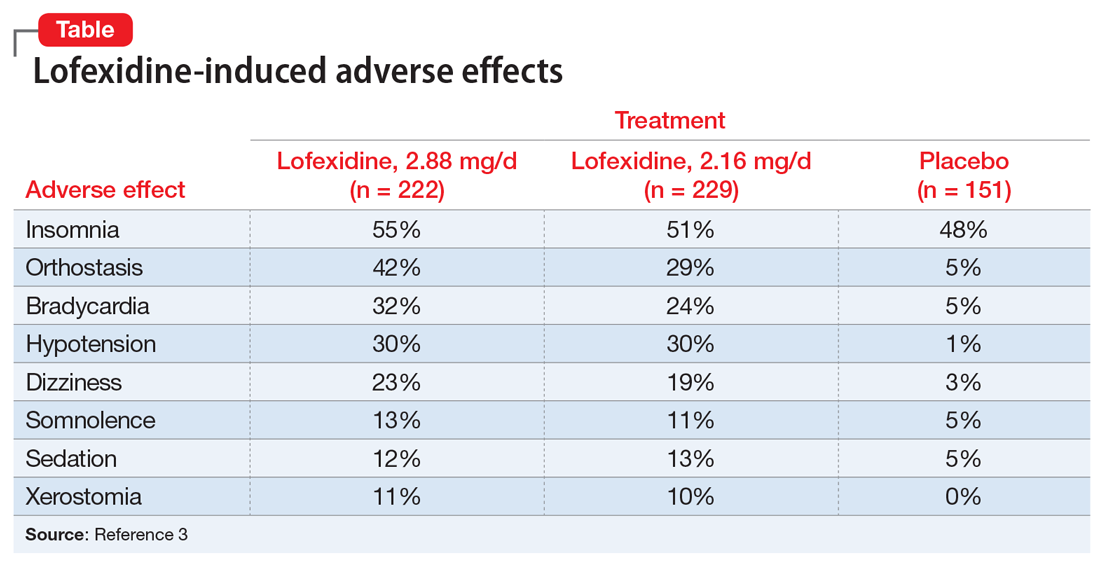
Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).

Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).

Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
The paranoid business executive
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
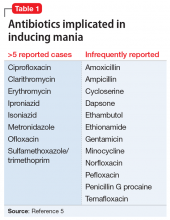
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
Valproic acid-induced hyperammonemic encephalopathy
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1

Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1

Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1

Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.
20 Reasons to celebrate our APA membership in 2020
The American Psychiatric Association (APA) is the largest psychiatric organization in the world, with >38,500 members across 100 countries. At 175 yea
I am truly honored to be nominated as the next APA President-Elect (Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation, click here), which prompted me to delve into the history of this great association that unifies us, empowers us, and gives us a loud voice to advocate for our patients, for our noble medical profession, and for advancing the mental health of society at large.
Our APA was established by 13 superintendents of the “Insane Asylums and Hospitals” in 1844. Its first name was a mouthful—the Association of Medical Superintendents of American Institutions of the Insane, a term now regarded as pejorative and unscientific. Thankfully, the name was changed almost 50 years later (in 1893) to the American Medico-Psychological Association, which was refined 28 years later in 1921 to the American Psychiatric Association, a name that has lasted for the past 99 years. If I am fortunate enough to be elected by my peers this month as President-Elect, and assume the APA Presidency in May 2021, a full century after the name of APA was adopted in 1921 (the era of Kraepelin, Bleuler, and Freud), I will propose and ask the APA members to approve inserting “physicians” in the APA name so it will become the American Psychiatric Physicians Association, or APPA. This will clearly reflect our medical training and identity, and underscore the remarkable progress achieved by the inspiring and diligent work of countless psychiatric physicians over the past century.
By the way, per a Google search, the term “physician” came about in the 13th century, when the Anglo-Normans used the French term “physique” or remedy, to coin the English word “physic” or medicine. Science historian Howard Markel discussed how “physic” became “physician.” As for the term “psychiatrist,” it was coined in 1808 by the German physician Johann Christian Reil, and it essentially means “medical treatment of the soul.”
The APA has an amazing structure that is very democratic, enabling members to elect their leaders as well as their representatives on the Assembly. It has a Board of Trustees (Table 1) comprised of 22 members, 7 of whom comprise the Executive Committee, plus 3 attendees. Eight standing committees (Table 2) report to the Board. There are also 13 councils (Table 3), 11 caucuses (Table 4), and 7 minority and underrepresented caucuses (Table 5). The APA has a national network of 76 District Branches (DBs), each usually representing one state, except for large states that have several DBs (California has 5, and New York has 13). The District of Columbia, Puerto Rico, Western Canada, and Quebec/Eastern Canada each have DBs as well. The DBs have their own bylaws, governance structures, and annual dues, and within them, they may have local “societies” in large cities. Finally, each DB elects representatives to the Assembly, which is comprised of 7 Areas, each of which contains several states.
I am glad to have been a member of the APA for more than 4 decades, since my residency days. Although most psychiatrists in the United States and Canada belong to the APA, some do not, either because they never joined, or they dropped out because they think the dues are high (although dues are less than half of 1% of the average psychiatrist’s annual income, which is a great bargain). So, for my colleagues who do belong, and especially for those who do not, I provide 20 reasons why being an APA member offers so many advantages, professionally and personally, and has a tremendous benefit to us individually and collectively:
1. It makes eminent sense to unify as members of a medical profession to enable us to be strong and influential, to overcome our challenges, and to achieve our goals.
Continue to: #2
2. The APA’s main objectives are to advocate for our patients, for member psychiatrists, and for the growth and success of the discipline of psychiatric medicine.
3. Being an APA member helps fight the hurtful stigma and disparity of parity, which we must all strive for together every day for our psychiatric patients.
4. A strong APA will fight for us to eliminate practice hassles such as outrageous pre-authorizations, complicated maintenance of certification process, cumbersome and time-consuming electronic medical records, and medico-legal constraints.
5. Unity affords our Association moral authority and social gravitas so that we become more credible when we educate the public to dispel the many myths and misconceptions about mental illness.
6. The APA provides us with the necessary political power and influence because medical care can be significantly impacted by good or bad legislation.
Continue to: #7
7. Our economic welfare needs a strong APA to which we all belong.
8. The antipsychiatry movement is a malignant antiscientific ideology that must be countered by all of us through a robust APA to which we all must belong.
9. The APA provides an enormous array of services and resources to all of us, individually or as groups. Many members don’t know that because they never ask.
10. While it is good to have subspecialty societies within the APA, we are all psychiatric physicians who have the same medical and psychiatric training and share the same core values. By joining the APA as our Mother Organization, we avoid Balkanization of our profession, which weakens all of us if we are divided into smaller groups.
11. The APA helps cultivate and recruit more medical students to choose psychiatry as a career. This is vital for the health of our field.
Continue to: #12
12. Mentoring residents about the professional issues of our specialty and involving them in committees is one of the priorities of the APA, which extends into the post-residency phase (early career psychiatrists).
13. The APA provides a “Big Tent” of diverse groups of colleagues across a rich mosaic of racial and ethnic groups, genders, national origins, sexual orientations, and practice settings. Our patients are diverse, and so are we.
14. Education is a top priority for the APA, providing its members with a wide array of opportunities for ongoing and life-long learning. This includes the spectacular annual meeting with its cornucopia of educational offers and newsletters, as well as many initiatives throughout the year.
15. The APA journals, especially its flagship American Journal of Psychiatry (AJP), are among the most cited publications in the world. We get them for free, even though the cost of a personal subscription to the AJP alone for non-APA members is equivalent to the entire annual dues!
16. The APA has many top researchers among its members, spread across more than 150 medical schools. Those members generate new knowledge that continuously advances the field of psychiatry and provides new evidence-based tools for psychiatric practitioners.
Continue to: #17
17. The APA is our community, an ecosystem that sustains us as psychiatrists, and connects us in many gratifying ways that keep us rejuvenated and helps us avoid burnout that may occur in absence of a supportive network of supportive peers.
18. The APA provides us discounts on malpractice insurance and other products.
19. Opportunities for personal and professional growth are available within the APA. This includes leadership skills via participation in the DBs or at the national level via committees, councils, caucuses, and the Assembly.
20. Last but not least, the APA represents all of us in The House of Medicine. It has very productive partnerships and collaborations with many other medical organizations that support us and help us achieve our cherished mission. Besides adding “Physicians” to the APA name, working closely with other physicians across many specialties (especially primary care) will consolidate our medical identity and lead to better outcomes for our patients through collaborative care initiatives.
I thank all my colleagues who are APA members or Fellows, and urge all the readers of
PS. Please VOTE in this month’s APA election! It’s our sacred duty.
The American Psychiatric Association (APA) is the largest psychiatric organization in the world, with >38,500 members across 100 countries. At 175 yea
I am truly honored to be nominated as the next APA President-Elect (Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation, click here), which prompted me to delve into the history of this great association that unifies us, empowers us, and gives us a loud voice to advocate for our patients, for our noble medical profession, and for advancing the mental health of society at large.
Our APA was established by 13 superintendents of the “Insane Asylums and Hospitals” in 1844. Its first name was a mouthful—the Association of Medical Superintendents of American Institutions of the Insane, a term now regarded as pejorative and unscientific. Thankfully, the name was changed almost 50 years later (in 1893) to the American Medico-Psychological Association, which was refined 28 years later in 1921 to the American Psychiatric Association, a name that has lasted for the past 99 years. If I am fortunate enough to be elected by my peers this month as President-Elect, and assume the APA Presidency in May 2021, a full century after the name of APA was adopted in 1921 (the era of Kraepelin, Bleuler, and Freud), I will propose and ask the APA members to approve inserting “physicians” in the APA name so it will become the American Psychiatric Physicians Association, or APPA. This will clearly reflect our medical training and identity, and underscore the remarkable progress achieved by the inspiring and diligent work of countless psychiatric physicians over the past century.
By the way, per a Google search, the term “physician” came about in the 13th century, when the Anglo-Normans used the French term “physique” or remedy, to coin the English word “physic” or medicine. Science historian Howard Markel discussed how “physic” became “physician.” As for the term “psychiatrist,” it was coined in 1808 by the German physician Johann Christian Reil, and it essentially means “medical treatment of the soul.”
The APA has an amazing structure that is very democratic, enabling members to elect their leaders as well as their representatives on the Assembly. It has a Board of Trustees (Table 1) comprised of 22 members, 7 of whom comprise the Executive Committee, plus 3 attendees. Eight standing committees (Table 2) report to the Board. There are also 13 councils (Table 3), 11 caucuses (Table 4), and 7 minority and underrepresented caucuses (Table 5). The APA has a national network of 76 District Branches (DBs), each usually representing one state, except for large states that have several DBs (California has 5, and New York has 13). The District of Columbia, Puerto Rico, Western Canada, and Quebec/Eastern Canada each have DBs as well. The DBs have their own bylaws, governance structures, and annual dues, and within them, they may have local “societies” in large cities. Finally, each DB elects representatives to the Assembly, which is comprised of 7 Areas, each of which contains several states.
I am glad to have been a member of the APA for more than 4 decades, since my residency days. Although most psychiatrists in the United States and Canada belong to the APA, some do not, either because they never joined, or they dropped out because they think the dues are high (although dues are less than half of 1% of the average psychiatrist’s annual income, which is a great bargain). So, for my colleagues who do belong, and especially for those who do not, I provide 20 reasons why being an APA member offers so many advantages, professionally and personally, and has a tremendous benefit to us individually and collectively:
1. It makes eminent sense to unify as members of a medical profession to enable us to be strong and influential, to overcome our challenges, and to achieve our goals.
Continue to: #2
2. The APA’s main objectives are to advocate for our patients, for member psychiatrists, and for the growth and success of the discipline of psychiatric medicine.
3. Being an APA member helps fight the hurtful stigma and disparity of parity, which we must all strive for together every day for our psychiatric patients.
4. A strong APA will fight for us to eliminate practice hassles such as outrageous pre-authorizations, complicated maintenance of certification process, cumbersome and time-consuming electronic medical records, and medico-legal constraints.
5. Unity affords our Association moral authority and social gravitas so that we become more credible when we educate the public to dispel the many myths and misconceptions about mental illness.
6. The APA provides us with the necessary political power and influence because medical care can be significantly impacted by good or bad legislation.
Continue to: #7
7. Our economic welfare needs a strong APA to which we all belong.
8. The antipsychiatry movement is a malignant antiscientific ideology that must be countered by all of us through a robust APA to which we all must belong.
9. The APA provides an enormous array of services and resources to all of us, individually or as groups. Many members don’t know that because they never ask.
10. While it is good to have subspecialty societies within the APA, we are all psychiatric physicians who have the same medical and psychiatric training and share the same core values. By joining the APA as our Mother Organization, we avoid Balkanization of our profession, which weakens all of us if we are divided into smaller groups.
11. The APA helps cultivate and recruit more medical students to choose psychiatry as a career. This is vital for the health of our field.
Continue to: #12
12. Mentoring residents about the professional issues of our specialty and involving them in committees is one of the priorities of the APA, which extends into the post-residency phase (early career psychiatrists).
13. The APA provides a “Big Tent” of diverse groups of colleagues across a rich mosaic of racial and ethnic groups, genders, national origins, sexual orientations, and practice settings. Our patients are diverse, and so are we.
14. Education is a top priority for the APA, providing its members with a wide array of opportunities for ongoing and life-long learning. This includes the spectacular annual meeting with its cornucopia of educational offers and newsletters, as well as many initiatives throughout the year.
15. The APA journals, especially its flagship American Journal of Psychiatry (AJP), are among the most cited publications in the world. We get them for free, even though the cost of a personal subscription to the AJP alone for non-APA members is equivalent to the entire annual dues!
16. The APA has many top researchers among its members, spread across more than 150 medical schools. Those members generate new knowledge that continuously advances the field of psychiatry and provides new evidence-based tools for psychiatric practitioners.
Continue to: #17
17. The APA is our community, an ecosystem that sustains us as psychiatrists, and connects us in many gratifying ways that keep us rejuvenated and helps us avoid burnout that may occur in absence of a supportive network of supportive peers.
18. The APA provides us discounts on malpractice insurance and other products.
19. Opportunities for personal and professional growth are available within the APA. This includes leadership skills via participation in the DBs or at the national level via committees, councils, caucuses, and the Assembly.
20. Last but not least, the APA represents all of us in The House of Medicine. It has very productive partnerships and collaborations with many other medical organizations that support us and help us achieve our cherished mission. Besides adding “Physicians” to the APA name, working closely with other physicians across many specialties (especially primary care) will consolidate our medical identity and lead to better outcomes for our patients through collaborative care initiatives.
I thank all my colleagues who are APA members or Fellows, and urge all the readers of
PS. Please VOTE in this month’s APA election! It’s our sacred duty.
The American Psychiatric Association (APA) is the largest psychiatric organization in the world, with >38,500 members across 100 countries. At 175 yea
I am truly honored to be nominated as the next APA President-Elect (Note: Dr. Nasrallah has withdrawn his candidacy for APA President-Elect. For a statement of explanation, click here), which prompted me to delve into the history of this great association that unifies us, empowers us, and gives us a loud voice to advocate for our patients, for our noble medical profession, and for advancing the mental health of society at large.
Our APA was established by 13 superintendents of the “Insane Asylums and Hospitals” in 1844. Its first name was a mouthful—the Association of Medical Superintendents of American Institutions of the Insane, a term now regarded as pejorative and unscientific. Thankfully, the name was changed almost 50 years later (in 1893) to the American Medico-Psychological Association, which was refined 28 years later in 1921 to the American Psychiatric Association, a name that has lasted for the past 99 years. If I am fortunate enough to be elected by my peers this month as President-Elect, and assume the APA Presidency in May 2021, a full century after the name of APA was adopted in 1921 (the era of Kraepelin, Bleuler, and Freud), I will propose and ask the APA members to approve inserting “physicians” in the APA name so it will become the American Psychiatric Physicians Association, or APPA. This will clearly reflect our medical training and identity, and underscore the remarkable progress achieved by the inspiring and diligent work of countless psychiatric physicians over the past century.
By the way, per a Google search, the term “physician” came about in the 13th century, when the Anglo-Normans used the French term “physique” or remedy, to coin the English word “physic” or medicine. Science historian Howard Markel discussed how “physic” became “physician.” As for the term “psychiatrist,” it was coined in 1808 by the German physician Johann Christian Reil, and it essentially means “medical treatment of the soul.”
The APA has an amazing structure that is very democratic, enabling members to elect their leaders as well as their representatives on the Assembly. It has a Board of Trustees (Table 1) comprised of 22 members, 7 of whom comprise the Executive Committee, plus 3 attendees. Eight standing committees (Table 2) report to the Board. There are also 13 councils (Table 3), 11 caucuses (Table 4), and 7 minority and underrepresented caucuses (Table 5). The APA has a national network of 76 District Branches (DBs), each usually representing one state, except for large states that have several DBs (California has 5, and New York has 13). The District of Columbia, Puerto Rico, Western Canada, and Quebec/Eastern Canada each have DBs as well. The DBs have their own bylaws, governance structures, and annual dues, and within them, they may have local “societies” in large cities. Finally, each DB elects representatives to the Assembly, which is comprised of 7 Areas, each of which contains several states.
I am glad to have been a member of the APA for more than 4 decades, since my residency days. Although most psychiatrists in the United States and Canada belong to the APA, some do not, either because they never joined, or they dropped out because they think the dues are high (although dues are less than half of 1% of the average psychiatrist’s annual income, which is a great bargain). So, for my colleagues who do belong, and especially for those who do not, I provide 20 reasons why being an APA member offers so many advantages, professionally and personally, and has a tremendous benefit to us individually and collectively:
1. It makes eminent sense to unify as members of a medical profession to enable us to be strong and influential, to overcome our challenges, and to achieve our goals.
Continue to: #2
2. The APA’s main objectives are to advocate for our patients, for member psychiatrists, and for the growth and success of the discipline of psychiatric medicine.
3. Being an APA member helps fight the hurtful stigma and disparity of parity, which we must all strive for together every day for our psychiatric patients.
4. A strong APA will fight for us to eliminate practice hassles such as outrageous pre-authorizations, complicated maintenance of certification process, cumbersome and time-consuming electronic medical records, and medico-legal constraints.
5. Unity affords our Association moral authority and social gravitas so that we become more credible when we educate the public to dispel the many myths and misconceptions about mental illness.
6. The APA provides us with the necessary political power and influence because medical care can be significantly impacted by good or bad legislation.
Continue to: #7
7. Our economic welfare needs a strong APA to which we all belong.
8. The antipsychiatry movement is a malignant antiscientific ideology that must be countered by all of us through a robust APA to which we all must belong.
9. The APA provides an enormous array of services and resources to all of us, individually or as groups. Many members don’t know that because they never ask.
10. While it is good to have subspecialty societies within the APA, we are all psychiatric physicians who have the same medical and psychiatric training and share the same core values. By joining the APA as our Mother Organization, we avoid Balkanization of our profession, which weakens all of us if we are divided into smaller groups.
11. The APA helps cultivate and recruit more medical students to choose psychiatry as a career. This is vital for the health of our field.
Continue to: #12
12. Mentoring residents about the professional issues of our specialty and involving them in committees is one of the priorities of the APA, which extends into the post-residency phase (early career psychiatrists).
13. The APA provides a “Big Tent” of diverse groups of colleagues across a rich mosaic of racial and ethnic groups, genders, national origins, sexual orientations, and practice settings. Our patients are diverse, and so are we.
14. Education is a top priority for the APA, providing its members with a wide array of opportunities for ongoing and life-long learning. This includes the spectacular annual meeting with its cornucopia of educational offers and newsletters, as well as many initiatives throughout the year.
15. The APA journals, especially its flagship American Journal of Psychiatry (AJP), are among the most cited publications in the world. We get them for free, even though the cost of a personal subscription to the AJP alone for non-APA members is equivalent to the entire annual dues!
16. The APA has many top researchers among its members, spread across more than 150 medical schools. Those members generate new knowledge that continuously advances the field of psychiatry and provides new evidence-based tools for psychiatric practitioners.
Continue to: #17
17. The APA is our community, an ecosystem that sustains us as psychiatrists, and connects us in many gratifying ways that keep us rejuvenated and helps us avoid burnout that may occur in absence of a supportive network of supportive peers.
18. The APA provides us discounts on malpractice insurance and other products.
19. Opportunities for personal and professional growth are available within the APA. This includes leadership skills via participation in the DBs or at the national level via committees, councils, caucuses, and the Assembly.
20. Last but not least, the APA represents all of us in The House of Medicine. It has very productive partnerships and collaborations with many other medical organizations that support us and help us achieve our cherished mission. Besides adding “Physicians” to the APA name, working closely with other physicians across many specialties (especially primary care) will consolidate our medical identity and lead to better outcomes for our patients through collaborative care initiatives.
I thank all my colleagues who are APA members or Fellows, and urge all the readers of
PS. Please VOTE in this month’s APA election! It’s our sacred duty.
Ketamine/esketamine: Putative mechanisms of action
Since the FDA approved intranasal esketamine, there has understandably been significant dialogue, debate, and discussion about the possible mechanisms of action of its antidepressant effects. Ketamine, the racemate of esketamine and arketamine, has been used off-label since the late 1990s. The first study of IV ketamine’s rapid antidepressant activity was published in 2000.1 In that study, 7 patients with major depressive disorder (MDD) were treated in a double-blind/placebo-controlled manner with IV ketamine or placebo. Researchers found a significant antidepressant effect within 72 hours with the administration of IV ketamine.
There is a tremendous number of publications related to ketamine, which creates a large reservoir of information to review in an attempt to piece together what we currently know about the mechanisms of action of ketamine/esketamine (K/ESK). A search of PubMed using the search word “ketamine” (October 8, 2019; www.ncbi.nlm.nih.gov/pubmed) produced a list of 4,869 articles just in the last 5 years; and the search words “ketamine and depression” produced a list of 1,221 publications over the same time period.
The FDA approval of intranasal esketamine in March 2019 was based on 5 phase III clinical studies (albeit not all were positive studies) and >9 years of intensive preclinical and clinical research on the efficacy and safety of intranasal esketamine in treatment-resistant depression (TRD). At the time the FDA approved it, esketamine had been studied in 1,700 patients with TRD, with 1-year safety data on approximately 800 patients. Despite this established data portfolio, critics of K/ESK continue to opine that we do not have enough long-term experience with these drugs, and some key opinion leaders continue to voice caution about the clinical use of K/ESK until we obtain more information and experience.
An article in the September 2019 issue
Of greater concern to me is the authors’ simplistic and flawed description of the mechanism of action of ketamine. They state “based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms… activation of endogenous opioid receptors… [and] blockade of N-methyl-
Ketamine: A plethora of studies
An impressive body of literature is attempting to piece together the complex and multidimensional neurophysiological mechanisms that result in ketamine’s rapid-acting antidepressant (RAAD) effect, which occurs as soon as 4 hours post-dose. A plethora of pre-clinical and clinical studies, including functional connectivity MRI scans in individuals with MDD, have provided a rough outline, albeit incomplete, of ketamine’s mechanisms of action. Ketamine was discovered in 1962 by chemist Calvin L. Stevens, who was experimenting with novel molecular structures to find a replacement for phencyclidine as a safer dissociative anesthetic. After successful experiments in human prisoners in 1964, ketamine was further studied and became FDA-approved in 1970 as a dissociative anesthetic. Lacking respiratory depression and hypotension, which were common adverse effects of other anesthetics, ketamine became commonly used on the battlefield in the Vietnam War, and continues to be used as a dissociative anesthetic.
Following the publication of the Berman article1 in 2000 that demonstrated apparent RAAD activity of IV ketamine, interest in ketamine’s use for TRD—a huge unmet need in psychiatry—skyrocketed. Since the FDA approval of iproniazid (a monoamine oxidase inhibitor) as the first medication approved to treat major depression in 1958, and the FDA approval of imipramine in 1959, all subsequent FDA-approved antidepressants have shared iproniazid/imipramine’s properties of modulating the monoamines serotonin, dopamine, and norepinephrine. The infamous Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial concluded that only 37% of patients with a major depressive episode achieve remission with their first antidepressant trial, and only 49% respond (50% improvement in symptoms).3 Ketamine/esketamine offered a novel mechanism of action, presumed to be related to the glutamate system, that demonstrated a clinical improvement in depressive symptoms in as few as 4 hours, with benefits that lasted up to 1 week after a single dose.
Continue to: A model of how ketamine works
A model of how ketamine works
Numerous publications from preclinical and clinical research collectively have woven a putative model of how K/ESK may rapidly improve depression by ultimately increasing synaptogenesis in the human prefrontal cortex—a part of the brain known to atrophy in states of chronic stress and depression.4 What is well established is the noncompetitive antagonism of K/ESK at the N-methyl-
A significant body of evidence supports agonism of the glutamate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor as an important step in the cascade of events that ultimately increases levels of the mammalian target of rapamycin (mTOR), which unleashes protein synthesis in synapses facilitating synaptogenesis. Pretreatment with AMPA receptor antagonists blocks the downstream effect of synaptogenesis.6,7 In support of this putative mechanism, hydroxynorketamine, a metabolite of racemic ketamine that has also demonstrated RAAD activity in a ketamine-like manner, is dependent upon AMPA glutamate receptor upregulation and activation, while not requiring activity at the NMDA-glutamate receptor.8,9
A comprehensive model on the putative molecular cascade of events contributing to the antidepressant effect of ketamine has recently been published10 and mirrors the excellent previous review by Abdallah et al.11 Hirota and Lambert10 propose that antagonism of interneuronal NMDA-glutamate receptors on GABAergic interneurons may result in a prefrontal cortex surge of glutamate, which increases agonism of the AMPA-glutamate receptor. This AMPA-glutamate receptor agonism has been shown to increase expression of brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF),12 both of which converge on increasing levels of mTOR, and the subsequent activation of mTOR, which putatively plays a role in increased production of scaffolding proteins and increased synaptogenesis, especially in the prefrontal cortex. In support of this model, during infusion and at 24 hours after a single ketamine infusion in individuals with MDD, functional connectivity MRI demonstrated an increase in global brain connectivity in the prefrontal cortex.13,14 The demonstration of increased global connectivity in the prefrontal cortex of patients with MDD, both during ketamine infusion and at 24 hours post-infusion, supports the clinical observations in clinics treating patients with K/ESK.
Opioid receptors and ketamine
During the past year, there has been significant discussion in psychiatry about the possible role of the mu opioid receptor and opioid system activation in ketamine’s RAAD effect. Remarkably, the literature supporting this hypothesis in humans is based on a single study by Williams et al.15 The authors’ claim: “We now present the first evidence in humans that opioid receptors are necessary for ketamine’s acute antidepressant effect.” In fact, in my opinion, this single study, which has not been replicated, is highly flawed. It included 30 adults with TRD, but only 12 of the 14 participants who qualified for the planned interim analysis completed the double-blind crossover. The population studied was quite treatment-refractory; the average duration of MDD was 24.1 years, the average age at onset was 17.3 years, and the duration of the current depressive episode at the time of the study was 8.6 years. Most significant to me was the reason the study was terminated: “At the interim analysis, given the finding that the combination of ketamine and naltrexone was not only ineffective but also noxious for many participants, we decided to stop enrolling patients in the study.” A distinct possibility is that the noxious adverse effects from the naltrexone impacted the participants’ experience in a negative manner, dampening down any antidepressant effect from ketamine.
In the August 2019 issue of Molecular Psychiatry, these same authors published a second article16 with conclusions based solely on “a secondary analysis of” the data from the same 12 participants in their first publication. Williams et al16 concluded that naltrexone also decreases the anti-suicidality effects of ketamine. Without any additional data or clinical research, these same authors extrapolated their hypothesized opioid receptor activity of ketamine to include it being responsible for ketamine’s established anti-suicidal effects.
Continue to: Mathew and Rivas-Grajales...
Mathew and Rivas-Grajales17 recently published a thoughtful critique and analysis of the study design and conclusions of the original Williams paper.15 They concluded that insufficient evidence exists to answer the question of how ketamine may interface with the opioid system, and they encourage further research into this important topic.
Two additional recent publications18,19 reported that naltrexone pretreatment did not attenuate the antidepressant effects of ketamine in their participants. Additionally, a recent publication in the anesthesiology literature20 concluded that esketamine reversed respiratory depression that was induced by remifentanil. From a clinical perspective, the most compelling argument against a direct mu opioid receptor mechanism for K/ESK is the lack of any craving, tolerance, or withdrawal in patients with TRD treated with K/ESK in numerous clinical publications comparing K/ESK with placebo. In the case of esketamine, during the 5 phase III clinical trials—including both short- and long-term studies—there was no signal for an opioid-like pharmacology. Significantly, both K/ESK are rapidly metabolized by the human body, and the typical dosing is 2 doses/week for the first month, then 1 dose/week for the next month, then 1 dose every week or less for the remainder of treatment.
Curiously, in the May 2019 issue of the American Journal of Psychiatry, Schatzberg21 (one of the co-authors of the prior 2 studies opining that ketamine has direct opioid system activation) wrote a “Reviews and Overviews” article in which he misrepresents the conclusions of an elegant study by Abdallah et al22 published in December 2018.
Abdallah et al22 added rapamycin, an immunosuppressant and a known inhibitor of mTOR, as a pretreatment to patients in a major depressive episode prior to infusion with IV ketamine. Their hypothesis was to see if the rapamycin decreased ketamine’s rapid antidepressant response—putatively by inhibiting the effect of mTOR. Rather than decreasing ketamine’s antidepressant effect, and in contrast to the placebo pretreatment group, at 2 weeks post IV ketamine infusion, patients treated with rapamycin-ketamine had a longer duration/greater improvement in their depressive symptoms compared with the patients receiving placebo-ketamine (improvement of 41% vs 13%, respectively, P = .04). Abdallah et al22 hypothesized that the pretreatment with rapamycin provides anti-inflammatory benefits to the synaptogenesis resulting from ketamine, which protects the newly formed synapses and prolongs ketamine’s antidepressant effect. Schatzberg21 came to a different conclusion than Abdallah et al,22 opining that because the rapamycin “failed to decrease ketamine response,” this result debunks the role of mTOR as a mediator in the antidepressant effect of ketamine through synaptogenesis.
Much more to learn
We still have a great deal to learn about the mechanism of action of K/ESK. However, clinics that are augmenting antidepressants with K/ESK in patients with TRD report significant and rapid symptom improvement in some patients (personal communications). We still do not understand the actual mechanisms of action of antidepressants and antipsychotics, but this does not curtail their use and clinical benefits to our patients. Ketamine has been extensively studied. In the current appropriate climate of concern about the pervasive and lethal opioid epidemic in the United States, we must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our most depressed and refractory patients.
Continue to: Looking at the extensive...
Looking at the extensive published data over the past 20 years, a consistent model has emerged whereby glutamate agonism of the AMPA-glutamate receptor, both with and without antagonism of the NMDA-glutamate receptor, appears to set in motion a molecular cascade involving BDNF and VEGF, and ultimately increasing the activity of mTOR, with resulting synaptogenicity that increases global brain connectivity in the human prefrontal cortex. As we continue to understand the complexities and additional circuitries that are involved in the RAAD effect of K/ESK, the hope is that novel molecular targets for future drug development will emerge.
Bottom Line
Extensive published data over the past 20 years has produced a consistent model to explain the putative mechanisms of action for the rapid antidepressant effects of ketamine and esketamine. We must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our patients with treatment-resistant depression.
Related Resources
- Mattingly GW, Anderson RH. Intranasal esketamine. Current Psychiatry. 2019;18(5):31-38.
- Thase M. Ketamine and esketamine for treating unipolar depression in adults: Administration, efficacy, and adverse effects. UpToDate. www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects.
Drug Brand Names
Esketamine nasal spray • Spravato
Imipramine • Tofranil
Ketamine • Ketalar
Naltrexone • Vivitrol, ReVia
Rapamycin • Rapamune
Remifentanil • Ultiva
1. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351-354.
2. Epstein K, Farrell HM. ‘Miracle cures’ in psychiatry? Current Psychiatry. 2019;18(9):13-16.
3. Valenstein M. Keeping our eyes on STAR*D. Am J Psychiatry. 2006;163:1484-1486.
4. Abdallah CG, Sanacora G, Duman RS, et al. The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharmacol Ther. 2018;190:148-158.
5. Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921-2927.
6. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959-964.
7. Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory, and disease. Trends Neurosci. 2010;33(2):67-75.
8. Zanos P, Moaddel R, Morris PJ, et al. NMDA inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481-486.
9. Collo G, Cavalleri L, Chiamulera C, et al. (2R,6R)-Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell-derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport. 2018;29(16):1425-1430.
10. Hirota K, Lambert DG. Ketamine and depression. Br J Anaesth. 2018;121(6):1198-1202.
11. Abdallah CG, Adams TG, Kelmendi B, et al. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety. 2016;33(8):689-697.
12. Deyama S, Bang E, Wohleb ES, et al. Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry. 2019;176(5):388-400.
13. Abdallah CG, Dutta A, Averill CL, et al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress (Thousand Oaks). 2018;2. doi: 10.1177/2470547018796102.
14. Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42(6):1210-1219.
15. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205-1215.
16. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786.
17. Mathew SJ, Rivas-Grajales AM. “Does the opioid system block or enhance the antidepressant effects of ketamine?” Chronic Stress. (Thousand Oaks). 2019;3. doi: 10.1177/2470547019852073.
18. Yoon G, Petrakis IL, Krystal JH. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry. 2019;76:337-338.
19. Marton T, Barnes DE, Wallace A, et al. Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol Psychiatry. 2019;85(12):e75-e76.
20. Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117-1127.
21. Schatzberg AF. Scientific issues relevant to improving the diagnosis, risk assessment, and treatment of major depression. Am J Psychiatry. 2019;176(5):342-347.
22. Abdallah C, Averill LA, Gueorgueiva R, et al. Rapamycin, an immunosuppressant and mTORC1 inhibitor, triples the antidepressant response rate of ketamine at 2 weeks following treatment: a double blind, placebo-controlled, cross-over, randomized clinical trial. bioRxiv. December 19, 2018. https://www.biorxiv.org/content/10.1101/500959v1. Accessed December 5, 2019.
Since the FDA approved intranasal esketamine, there has understandably been significant dialogue, debate, and discussion about the possible mechanisms of action of its antidepressant effects. Ketamine, the racemate of esketamine and arketamine, has been used off-label since the late 1990s. The first study of IV ketamine’s rapid antidepressant activity was published in 2000.1 In that study, 7 patients with major depressive disorder (MDD) were treated in a double-blind/placebo-controlled manner with IV ketamine or placebo. Researchers found a significant antidepressant effect within 72 hours with the administration of IV ketamine.
There is a tremendous number of publications related to ketamine, which creates a large reservoir of information to review in an attempt to piece together what we currently know about the mechanisms of action of ketamine/esketamine (K/ESK). A search of PubMed using the search word “ketamine” (October 8, 2019; www.ncbi.nlm.nih.gov/pubmed) produced a list of 4,869 articles just in the last 5 years; and the search words “ketamine and depression” produced a list of 1,221 publications over the same time period.
The FDA approval of intranasal esketamine in March 2019 was based on 5 phase III clinical studies (albeit not all were positive studies) and >9 years of intensive preclinical and clinical research on the efficacy and safety of intranasal esketamine in treatment-resistant depression (TRD). At the time the FDA approved it, esketamine had been studied in 1,700 patients with TRD, with 1-year safety data on approximately 800 patients. Despite this established data portfolio, critics of K/ESK continue to opine that we do not have enough long-term experience with these drugs, and some key opinion leaders continue to voice caution about the clinical use of K/ESK until we obtain more information and experience.
An article in the September 2019 issue
Of greater concern to me is the authors’ simplistic and flawed description of the mechanism of action of ketamine. They state “based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms… activation of endogenous opioid receptors… [and] blockade of N-methyl-
Ketamine: A plethora of studies
An impressive body of literature is attempting to piece together the complex and multidimensional neurophysiological mechanisms that result in ketamine’s rapid-acting antidepressant (RAAD) effect, which occurs as soon as 4 hours post-dose. A plethora of pre-clinical and clinical studies, including functional connectivity MRI scans in individuals with MDD, have provided a rough outline, albeit incomplete, of ketamine’s mechanisms of action. Ketamine was discovered in 1962 by chemist Calvin L. Stevens, who was experimenting with novel molecular structures to find a replacement for phencyclidine as a safer dissociative anesthetic. After successful experiments in human prisoners in 1964, ketamine was further studied and became FDA-approved in 1970 as a dissociative anesthetic. Lacking respiratory depression and hypotension, which were common adverse effects of other anesthetics, ketamine became commonly used on the battlefield in the Vietnam War, and continues to be used as a dissociative anesthetic.
Following the publication of the Berman article1 in 2000 that demonstrated apparent RAAD activity of IV ketamine, interest in ketamine’s use for TRD—a huge unmet need in psychiatry—skyrocketed. Since the FDA approval of iproniazid (a monoamine oxidase inhibitor) as the first medication approved to treat major depression in 1958, and the FDA approval of imipramine in 1959, all subsequent FDA-approved antidepressants have shared iproniazid/imipramine’s properties of modulating the monoamines serotonin, dopamine, and norepinephrine. The infamous Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial concluded that only 37% of patients with a major depressive episode achieve remission with their first antidepressant trial, and only 49% respond (50% improvement in symptoms).3 Ketamine/esketamine offered a novel mechanism of action, presumed to be related to the glutamate system, that demonstrated a clinical improvement in depressive symptoms in as few as 4 hours, with benefits that lasted up to 1 week after a single dose.
Continue to: A model of how ketamine works
A model of how ketamine works
Numerous publications from preclinical and clinical research collectively have woven a putative model of how K/ESK may rapidly improve depression by ultimately increasing synaptogenesis in the human prefrontal cortex—a part of the brain known to atrophy in states of chronic stress and depression.4 What is well established is the noncompetitive antagonism of K/ESK at the N-methyl-
A significant body of evidence supports agonism of the glutamate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor as an important step in the cascade of events that ultimately increases levels of the mammalian target of rapamycin (mTOR), which unleashes protein synthesis in synapses facilitating synaptogenesis. Pretreatment with AMPA receptor antagonists blocks the downstream effect of synaptogenesis.6,7 In support of this putative mechanism, hydroxynorketamine, a metabolite of racemic ketamine that has also demonstrated RAAD activity in a ketamine-like manner, is dependent upon AMPA glutamate receptor upregulation and activation, while not requiring activity at the NMDA-glutamate receptor.8,9
A comprehensive model on the putative molecular cascade of events contributing to the antidepressant effect of ketamine has recently been published10 and mirrors the excellent previous review by Abdallah et al.11 Hirota and Lambert10 propose that antagonism of interneuronal NMDA-glutamate receptors on GABAergic interneurons may result in a prefrontal cortex surge of glutamate, which increases agonism of the AMPA-glutamate receptor. This AMPA-glutamate receptor agonism has been shown to increase expression of brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF),12 both of which converge on increasing levels of mTOR, and the subsequent activation of mTOR, which putatively plays a role in increased production of scaffolding proteins and increased synaptogenesis, especially in the prefrontal cortex. In support of this model, during infusion and at 24 hours after a single ketamine infusion in individuals with MDD, functional connectivity MRI demonstrated an increase in global brain connectivity in the prefrontal cortex.13,14 The demonstration of increased global connectivity in the prefrontal cortex of patients with MDD, both during ketamine infusion and at 24 hours post-infusion, supports the clinical observations in clinics treating patients with K/ESK.
Opioid receptors and ketamine
During the past year, there has been significant discussion in psychiatry about the possible role of the mu opioid receptor and opioid system activation in ketamine’s RAAD effect. Remarkably, the literature supporting this hypothesis in humans is based on a single study by Williams et al.15 The authors’ claim: “We now present the first evidence in humans that opioid receptors are necessary for ketamine’s acute antidepressant effect.” In fact, in my opinion, this single study, which has not been replicated, is highly flawed. It included 30 adults with TRD, but only 12 of the 14 participants who qualified for the planned interim analysis completed the double-blind crossover. The population studied was quite treatment-refractory; the average duration of MDD was 24.1 years, the average age at onset was 17.3 years, and the duration of the current depressive episode at the time of the study was 8.6 years. Most significant to me was the reason the study was terminated: “At the interim analysis, given the finding that the combination of ketamine and naltrexone was not only ineffective but also noxious for many participants, we decided to stop enrolling patients in the study.” A distinct possibility is that the noxious adverse effects from the naltrexone impacted the participants’ experience in a negative manner, dampening down any antidepressant effect from ketamine.
In the August 2019 issue of Molecular Psychiatry, these same authors published a second article16 with conclusions based solely on “a secondary analysis of” the data from the same 12 participants in their first publication. Williams et al16 concluded that naltrexone also decreases the anti-suicidality effects of ketamine. Without any additional data or clinical research, these same authors extrapolated their hypothesized opioid receptor activity of ketamine to include it being responsible for ketamine’s established anti-suicidal effects.
Continue to: Mathew and Rivas-Grajales...
Mathew and Rivas-Grajales17 recently published a thoughtful critique and analysis of the study design and conclusions of the original Williams paper.15 They concluded that insufficient evidence exists to answer the question of how ketamine may interface with the opioid system, and they encourage further research into this important topic.
Two additional recent publications18,19 reported that naltrexone pretreatment did not attenuate the antidepressant effects of ketamine in their participants. Additionally, a recent publication in the anesthesiology literature20 concluded that esketamine reversed respiratory depression that was induced by remifentanil. From a clinical perspective, the most compelling argument against a direct mu opioid receptor mechanism for K/ESK is the lack of any craving, tolerance, or withdrawal in patients with TRD treated with K/ESK in numerous clinical publications comparing K/ESK with placebo. In the case of esketamine, during the 5 phase III clinical trials—including both short- and long-term studies—there was no signal for an opioid-like pharmacology. Significantly, both K/ESK are rapidly metabolized by the human body, and the typical dosing is 2 doses/week for the first month, then 1 dose/week for the next month, then 1 dose every week or less for the remainder of treatment.
Curiously, in the May 2019 issue of the American Journal of Psychiatry, Schatzberg21 (one of the co-authors of the prior 2 studies opining that ketamine has direct opioid system activation) wrote a “Reviews and Overviews” article in which he misrepresents the conclusions of an elegant study by Abdallah et al22 published in December 2018.
Abdallah et al22 added rapamycin, an immunosuppressant and a known inhibitor of mTOR, as a pretreatment to patients in a major depressive episode prior to infusion with IV ketamine. Their hypothesis was to see if the rapamycin decreased ketamine’s rapid antidepressant response—putatively by inhibiting the effect of mTOR. Rather than decreasing ketamine’s antidepressant effect, and in contrast to the placebo pretreatment group, at 2 weeks post IV ketamine infusion, patients treated with rapamycin-ketamine had a longer duration/greater improvement in their depressive symptoms compared with the patients receiving placebo-ketamine (improvement of 41% vs 13%, respectively, P = .04). Abdallah et al22 hypothesized that the pretreatment with rapamycin provides anti-inflammatory benefits to the synaptogenesis resulting from ketamine, which protects the newly formed synapses and prolongs ketamine’s antidepressant effect. Schatzberg21 came to a different conclusion than Abdallah et al,22 opining that because the rapamycin “failed to decrease ketamine response,” this result debunks the role of mTOR as a mediator in the antidepressant effect of ketamine through synaptogenesis.
Much more to learn
We still have a great deal to learn about the mechanism of action of K/ESK. However, clinics that are augmenting antidepressants with K/ESK in patients with TRD report significant and rapid symptom improvement in some patients (personal communications). We still do not understand the actual mechanisms of action of antidepressants and antipsychotics, but this does not curtail their use and clinical benefits to our patients. Ketamine has been extensively studied. In the current appropriate climate of concern about the pervasive and lethal opioid epidemic in the United States, we must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our most depressed and refractory patients.
Continue to: Looking at the extensive...
Looking at the extensive published data over the past 20 years, a consistent model has emerged whereby glutamate agonism of the AMPA-glutamate receptor, both with and without antagonism of the NMDA-glutamate receptor, appears to set in motion a molecular cascade involving BDNF and VEGF, and ultimately increasing the activity of mTOR, with resulting synaptogenicity that increases global brain connectivity in the human prefrontal cortex. As we continue to understand the complexities and additional circuitries that are involved in the RAAD effect of K/ESK, the hope is that novel molecular targets for future drug development will emerge.
Bottom Line
Extensive published data over the past 20 years has produced a consistent model to explain the putative mechanisms of action for the rapid antidepressant effects of ketamine and esketamine. We must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our patients with treatment-resistant depression.
Related Resources
- Mattingly GW, Anderson RH. Intranasal esketamine. Current Psychiatry. 2019;18(5):31-38.
- Thase M. Ketamine and esketamine for treating unipolar depression in adults: Administration, efficacy, and adverse effects. UpToDate. www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects.
Drug Brand Names
Esketamine nasal spray • Spravato
Imipramine • Tofranil
Ketamine • Ketalar
Naltrexone • Vivitrol, ReVia
Rapamycin • Rapamune
Remifentanil • Ultiva
Since the FDA approved intranasal esketamine, there has understandably been significant dialogue, debate, and discussion about the possible mechanisms of action of its antidepressant effects. Ketamine, the racemate of esketamine and arketamine, has been used off-label since the late 1990s. The first study of IV ketamine’s rapid antidepressant activity was published in 2000.1 In that study, 7 patients with major depressive disorder (MDD) were treated in a double-blind/placebo-controlled manner with IV ketamine or placebo. Researchers found a significant antidepressant effect within 72 hours with the administration of IV ketamine.
There is a tremendous number of publications related to ketamine, which creates a large reservoir of information to review in an attempt to piece together what we currently know about the mechanisms of action of ketamine/esketamine (K/ESK). A search of PubMed using the search word “ketamine” (October 8, 2019; www.ncbi.nlm.nih.gov/pubmed) produced a list of 4,869 articles just in the last 5 years; and the search words “ketamine and depression” produced a list of 1,221 publications over the same time period.
The FDA approval of intranasal esketamine in March 2019 was based on 5 phase III clinical studies (albeit not all were positive studies) and >9 years of intensive preclinical and clinical research on the efficacy and safety of intranasal esketamine in treatment-resistant depression (TRD). At the time the FDA approved it, esketamine had been studied in 1,700 patients with TRD, with 1-year safety data on approximately 800 patients. Despite this established data portfolio, critics of K/ESK continue to opine that we do not have enough long-term experience with these drugs, and some key opinion leaders continue to voice caution about the clinical use of K/ESK until we obtain more information and experience.
An article in the September 2019 issue
Of greater concern to me is the authors’ simplistic and flawed description of the mechanism of action of ketamine. They state “based on available research, ketamine’s long-lasting effects seem to come from 2 mechanisms… activation of endogenous opioid receptors… [and] blockade of N-methyl-
Ketamine: A plethora of studies
An impressive body of literature is attempting to piece together the complex and multidimensional neurophysiological mechanisms that result in ketamine’s rapid-acting antidepressant (RAAD) effect, which occurs as soon as 4 hours post-dose. A plethora of pre-clinical and clinical studies, including functional connectivity MRI scans in individuals with MDD, have provided a rough outline, albeit incomplete, of ketamine’s mechanisms of action. Ketamine was discovered in 1962 by chemist Calvin L. Stevens, who was experimenting with novel molecular structures to find a replacement for phencyclidine as a safer dissociative anesthetic. After successful experiments in human prisoners in 1964, ketamine was further studied and became FDA-approved in 1970 as a dissociative anesthetic. Lacking respiratory depression and hypotension, which were common adverse effects of other anesthetics, ketamine became commonly used on the battlefield in the Vietnam War, and continues to be used as a dissociative anesthetic.
Following the publication of the Berman article1 in 2000 that demonstrated apparent RAAD activity of IV ketamine, interest in ketamine’s use for TRD—a huge unmet need in psychiatry—skyrocketed. Since the FDA approval of iproniazid (a monoamine oxidase inhibitor) as the first medication approved to treat major depression in 1958, and the FDA approval of imipramine in 1959, all subsequent FDA-approved antidepressants have shared iproniazid/imipramine’s properties of modulating the monoamines serotonin, dopamine, and norepinephrine. The infamous Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial concluded that only 37% of patients with a major depressive episode achieve remission with their first antidepressant trial, and only 49% respond (50% improvement in symptoms).3 Ketamine/esketamine offered a novel mechanism of action, presumed to be related to the glutamate system, that demonstrated a clinical improvement in depressive symptoms in as few as 4 hours, with benefits that lasted up to 1 week after a single dose.
Continue to: A model of how ketamine works
A model of how ketamine works
Numerous publications from preclinical and clinical research collectively have woven a putative model of how K/ESK may rapidly improve depression by ultimately increasing synaptogenesis in the human prefrontal cortex—a part of the brain known to atrophy in states of chronic stress and depression.4 What is well established is the noncompetitive antagonism of K/ESK at the N-methyl-
A significant body of evidence supports agonism of the glutamate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor as an important step in the cascade of events that ultimately increases levels of the mammalian target of rapamycin (mTOR), which unleashes protein synthesis in synapses facilitating synaptogenesis. Pretreatment with AMPA receptor antagonists blocks the downstream effect of synaptogenesis.6,7 In support of this putative mechanism, hydroxynorketamine, a metabolite of racemic ketamine that has also demonstrated RAAD activity in a ketamine-like manner, is dependent upon AMPA glutamate receptor upregulation and activation, while not requiring activity at the NMDA-glutamate receptor.8,9
A comprehensive model on the putative molecular cascade of events contributing to the antidepressant effect of ketamine has recently been published10 and mirrors the excellent previous review by Abdallah et al.11 Hirota and Lambert10 propose that antagonism of interneuronal NMDA-glutamate receptors on GABAergic interneurons may result in a prefrontal cortex surge of glutamate, which increases agonism of the AMPA-glutamate receptor. This AMPA-glutamate receptor agonism has been shown to increase expression of brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF),12 both of which converge on increasing levels of mTOR, and the subsequent activation of mTOR, which putatively plays a role in increased production of scaffolding proteins and increased synaptogenesis, especially in the prefrontal cortex. In support of this model, during infusion and at 24 hours after a single ketamine infusion in individuals with MDD, functional connectivity MRI demonstrated an increase in global brain connectivity in the prefrontal cortex.13,14 The demonstration of increased global connectivity in the prefrontal cortex of patients with MDD, both during ketamine infusion and at 24 hours post-infusion, supports the clinical observations in clinics treating patients with K/ESK.
Opioid receptors and ketamine
During the past year, there has been significant discussion in psychiatry about the possible role of the mu opioid receptor and opioid system activation in ketamine’s RAAD effect. Remarkably, the literature supporting this hypothesis in humans is based on a single study by Williams et al.15 The authors’ claim: “We now present the first evidence in humans that opioid receptors are necessary for ketamine’s acute antidepressant effect.” In fact, in my opinion, this single study, which has not been replicated, is highly flawed. It included 30 adults with TRD, but only 12 of the 14 participants who qualified for the planned interim analysis completed the double-blind crossover. The population studied was quite treatment-refractory; the average duration of MDD was 24.1 years, the average age at onset was 17.3 years, and the duration of the current depressive episode at the time of the study was 8.6 years. Most significant to me was the reason the study was terminated: “At the interim analysis, given the finding that the combination of ketamine and naltrexone was not only ineffective but also noxious for many participants, we decided to stop enrolling patients in the study.” A distinct possibility is that the noxious adverse effects from the naltrexone impacted the participants’ experience in a negative manner, dampening down any antidepressant effect from ketamine.
In the August 2019 issue of Molecular Psychiatry, these same authors published a second article16 with conclusions based solely on “a secondary analysis of” the data from the same 12 participants in their first publication. Williams et al16 concluded that naltrexone also decreases the anti-suicidality effects of ketamine. Without any additional data or clinical research, these same authors extrapolated their hypothesized opioid receptor activity of ketamine to include it being responsible for ketamine’s established anti-suicidal effects.
Continue to: Mathew and Rivas-Grajales...
Mathew and Rivas-Grajales17 recently published a thoughtful critique and analysis of the study design and conclusions of the original Williams paper.15 They concluded that insufficient evidence exists to answer the question of how ketamine may interface with the opioid system, and they encourage further research into this important topic.
Two additional recent publications18,19 reported that naltrexone pretreatment did not attenuate the antidepressant effects of ketamine in their participants. Additionally, a recent publication in the anesthesiology literature20 concluded that esketamine reversed respiratory depression that was induced by remifentanil. From a clinical perspective, the most compelling argument against a direct mu opioid receptor mechanism for K/ESK is the lack of any craving, tolerance, or withdrawal in patients with TRD treated with K/ESK in numerous clinical publications comparing K/ESK with placebo. In the case of esketamine, during the 5 phase III clinical trials—including both short- and long-term studies—there was no signal for an opioid-like pharmacology. Significantly, both K/ESK are rapidly metabolized by the human body, and the typical dosing is 2 doses/week for the first month, then 1 dose/week for the next month, then 1 dose every week or less for the remainder of treatment.
Curiously, in the May 2019 issue of the American Journal of Psychiatry, Schatzberg21 (one of the co-authors of the prior 2 studies opining that ketamine has direct opioid system activation) wrote a “Reviews and Overviews” article in which he misrepresents the conclusions of an elegant study by Abdallah et al22 published in December 2018.
Abdallah et al22 added rapamycin, an immunosuppressant and a known inhibitor of mTOR, as a pretreatment to patients in a major depressive episode prior to infusion with IV ketamine. Their hypothesis was to see if the rapamycin decreased ketamine’s rapid antidepressant response—putatively by inhibiting the effect of mTOR. Rather than decreasing ketamine’s antidepressant effect, and in contrast to the placebo pretreatment group, at 2 weeks post IV ketamine infusion, patients treated with rapamycin-ketamine had a longer duration/greater improvement in their depressive symptoms compared with the patients receiving placebo-ketamine (improvement of 41% vs 13%, respectively, P = .04). Abdallah et al22 hypothesized that the pretreatment with rapamycin provides anti-inflammatory benefits to the synaptogenesis resulting from ketamine, which protects the newly formed synapses and prolongs ketamine’s antidepressant effect. Schatzberg21 came to a different conclusion than Abdallah et al,22 opining that because the rapamycin “failed to decrease ketamine response,” this result debunks the role of mTOR as a mediator in the antidepressant effect of ketamine through synaptogenesis.
Much more to learn
We still have a great deal to learn about the mechanism of action of K/ESK. However, clinics that are augmenting antidepressants with K/ESK in patients with TRD report significant and rapid symptom improvement in some patients (personal communications). We still do not understand the actual mechanisms of action of antidepressants and antipsychotics, but this does not curtail their use and clinical benefits to our patients. Ketamine has been extensively studied. In the current appropriate climate of concern about the pervasive and lethal opioid epidemic in the United States, we must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our most depressed and refractory patients.
Continue to: Looking at the extensive...
Looking at the extensive published data over the past 20 years, a consistent model has emerged whereby glutamate agonism of the AMPA-glutamate receptor, both with and without antagonism of the NMDA-glutamate receptor, appears to set in motion a molecular cascade involving BDNF and VEGF, and ultimately increasing the activity of mTOR, with resulting synaptogenicity that increases global brain connectivity in the human prefrontal cortex. As we continue to understand the complexities and additional circuitries that are involved in the RAAD effect of K/ESK, the hope is that novel molecular targets for future drug development will emerge.
Bottom Line
Extensive published data over the past 20 years has produced a consistent model to explain the putative mechanisms of action for the rapid antidepressant effects of ketamine and esketamine. We must remain on solid scientific ground before attributing an opioid mechanism to a novel treatment that has already benefitted many of our patients with treatment-resistant depression.
Related Resources
- Mattingly GW, Anderson RH. Intranasal esketamine. Current Psychiatry. 2019;18(5):31-38.
- Thase M. Ketamine and esketamine for treating unipolar depression in adults: Administration, efficacy, and adverse effects. UpToDate. www.uptodate.com/contents/ketamine-and-esketamine-for-treating-unipolar-depression-in-adults-administration-efficacy-and-adverse-effects.
Drug Brand Names
Esketamine nasal spray • Spravato
Imipramine • Tofranil
Ketamine • Ketalar
Naltrexone • Vivitrol, ReVia
Rapamycin • Rapamune
Remifentanil • Ultiva
1. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351-354.
2. Epstein K, Farrell HM. ‘Miracle cures’ in psychiatry? Current Psychiatry. 2019;18(9):13-16.
3. Valenstein M. Keeping our eyes on STAR*D. Am J Psychiatry. 2006;163:1484-1486.
4. Abdallah CG, Sanacora G, Duman RS, et al. The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharmacol Ther. 2018;190:148-158.
5. Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921-2927.
6. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959-964.
7. Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory, and disease. Trends Neurosci. 2010;33(2):67-75.
8. Zanos P, Moaddel R, Morris PJ, et al. NMDA inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481-486.
9. Collo G, Cavalleri L, Chiamulera C, et al. (2R,6R)-Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell-derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport. 2018;29(16):1425-1430.
10. Hirota K, Lambert DG. Ketamine and depression. Br J Anaesth. 2018;121(6):1198-1202.
11. Abdallah CG, Adams TG, Kelmendi B, et al. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety. 2016;33(8):689-697.
12. Deyama S, Bang E, Wohleb ES, et al. Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry. 2019;176(5):388-400.
13. Abdallah CG, Dutta A, Averill CL, et al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress (Thousand Oaks). 2018;2. doi: 10.1177/2470547018796102.
14. Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42(6):1210-1219.
15. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205-1215.
16. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786.
17. Mathew SJ, Rivas-Grajales AM. “Does the opioid system block or enhance the antidepressant effects of ketamine?” Chronic Stress. (Thousand Oaks). 2019;3. doi: 10.1177/2470547019852073.
18. Yoon G, Petrakis IL, Krystal JH. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry. 2019;76:337-338.
19. Marton T, Barnes DE, Wallace A, et al. Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol Psychiatry. 2019;85(12):e75-e76.
20. Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117-1127.
21. Schatzberg AF. Scientific issues relevant to improving the diagnosis, risk assessment, and treatment of major depression. Am J Psychiatry. 2019;176(5):342-347.
22. Abdallah C, Averill LA, Gueorgueiva R, et al. Rapamycin, an immunosuppressant and mTORC1 inhibitor, triples the antidepressant response rate of ketamine at 2 weeks following treatment: a double blind, placebo-controlled, cross-over, randomized clinical trial. bioRxiv. December 19, 2018. https://www.biorxiv.org/content/10.1101/500959v1. Accessed December 5, 2019.
1. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351-354.
2. Epstein K, Farrell HM. ‘Miracle cures’ in psychiatry? Current Psychiatry. 2019;18(9):13-16.
3. Valenstein M. Keeping our eyes on STAR*D. Am J Psychiatry. 2006;163:1484-1486.
4. Abdallah CG, Sanacora G, Duman RS, et al. The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation? Pharmacol Ther. 2018;190:148-158.
5. Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921-2927.
6. Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959-964.
7. Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory, and disease. Trends Neurosci. 2010;33(2):67-75.
8. Zanos P, Moaddel R, Morris PJ, et al. NMDA inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481-486.
9. Collo G, Cavalleri L, Chiamulera C, et al. (2R,6R)-Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell-derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport. 2018;29(16):1425-1430.
10. Hirota K, Lambert DG. Ketamine and depression. Br J Anaesth. 2018;121(6):1198-1202.
11. Abdallah CG, Adams TG, Kelmendi B, et al. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety. 2016;33(8):689-697.
12. Deyama S, Bang E, Wohleb ES, et al. Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry. 2019;176(5):388-400.
13. Abdallah CG, Dutta A, Averill CL, et al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress (Thousand Oaks). 2018;2. doi: 10.1177/2470547018796102.
14. Abdallah CG, Averill LA, Collins KA, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017;42(6):1210-1219.
15. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205-1215.
16. Williams NR, Heifets BD, Bentzley BS, et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry. 2019;24(12):1779-1786.
17. Mathew SJ, Rivas-Grajales AM. “Does the opioid system block or enhance the antidepressant effects of ketamine?” Chronic Stress. (Thousand Oaks). 2019;3. doi: 10.1177/2470547019852073.
18. Yoon G, Petrakis IL, Krystal JH. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry. 2019;76:337-338.
19. Marton T, Barnes DE, Wallace A, et al. Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol Psychiatry. 2019;85(12):e75-e76.
20. Jonkman K, van Rijnsoever E, Olofsen E, et al. Esketamine counters opioid-induced respiratory depression. Br J Anaesth. 2018;120(5):1117-1127.
21. Schatzberg AF. Scientific issues relevant to improving the diagnosis, risk assessment, and treatment of major depression. Am J Psychiatry. 2019;176(5):342-347.
22. Abdallah C, Averill LA, Gueorgueiva R, et al. Rapamycin, an immunosuppressant and mTORC1 inhibitor, triples the antidepressant response rate of ketamine at 2 weeks following treatment: a double blind, placebo-controlled, cross-over, randomized clinical trial. bioRxiv. December 19, 2018. https://www.biorxiv.org/content/10.1101/500959v1. Accessed December 5, 2019.
Career Choices: Psychiatric oncology
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Addiction Psychiatry Fellow at Boston University, talked with William Pirl, MD, MPH, FACLP, FAPOS. Dr. Pirl is Associate Professor, Psychiatry, Harvard Medical School. He joined Dana-Farber Cancer Institute in 2018 as Vice Chair for Psychosocial Oncology, Department of Psychosocial Oncology and Palliative Care. He is a past president of the American Psychosocial Oncology Society and North American Associate Editor for the journal Psycho-Oncology.
Dr. Ahmed: What made you choose the psychiatric oncology track, and how did your training lead you towards this path?
Dr. Pirl: I went to medical school thinking that I wanted to be a psychiatrist. However, I was really drawn to internal medicine, especially the process of sorting through medical differential diagnoses. I was deciding between applying for residency in medicine or psychiatry when I did an elective rotation in consultation-liaison (CL) psychiatry. Consultation-liaison psychiatry combined both medicine and psychiatry, which is exactly what I wanted to do. After residency, I wanted to do a CL fellowship outside of Boston, which is where I had done all of my medical education and training. One of my residency advisors suggested Memorial Sloan-Kettering Cancer Center, and I ended up going there. On the first day of fellowship, I realized that I’d only be working with cancer over that year, which I had not really thought about beforehand. Luckily, I loved it, and over the year I realized that the work had tremendous impact and meaning.
Dr. Ahmed: What are some of the pros and cons of working in psychiatric oncology?
Dr. Pirl: Things that I think are pros might be cons for some people. Consults in psychiatric oncology tend to be more relationship-based than they might be in other CL subspecialties. Oncology clinicians want to know who they are referring their patients to, and they are used to team-based care. If you like practicing as part of a multidisciplinary team, this is a pro.
Psychiatric oncology has more focus on existential issues, which interests me more than some other things in psychiatry. Bearing witness to so much tragedy can be a con at times, but psychiatrists who do this work learn ways to manage this within themselves. Psychiatric oncology also offers many experiences where you can see how much impact you make. It’s rewarding to see results and get positive feedback from patients and their families.
Continue to: Lastly, this is...
Lastly, this is a historic time in oncology. Over the last 15 years, things are happening that I never thought I would live to see. Some patients who 10 or 15 years ago would have had an expected survival of 6 to 9 months are now living years. We are now at a point where we might not actually know a patient’s prognosis, which introduces a whole other layer of uncertainty in cancer. Working as a psychiatrist during this time of rapidly evolving care is amazing. Cancer care will look very different over the next decade.
Dr. Ahmed: Based on your personal experience, what should one consider when choosing a psychiatric oncology program?
Dr. Pirl: I trained in a time before CL was a certified subspecialty of psychiatry. At that time, programs could focus solely on cancer, which cannot be done now. Trainees need to have broader training in certified fellowships. If someone knows that they are interested in psychiatric oncology, there are 2 programs that they should consider: the Dana-Farber Cancer Institute track of the Brigham and Women’s Hospital CL fellowship, and the Memorial Sloan-Kettering Cancer Center/New York Hospital CL fellowship. However, completing a CL fellowship will give someone the skills to do this work, even though they may not know all of the cancer content yet.
Dr. Ahmed: What are some of the career options and work settings in psychiatric oncology?
Dr. Pirl: There are many factors that make it difficult for psychiatrist to have a psychiatric oncology private practice. The amount of late cancellations and no-shows because of illness makes it hard to do this work without some institutional subsidy. Also, being able to communicate and work as a team with oncology providers is much easier if you are in the same place. Most psychiatrists who do psychiatric oncology work in a cancer center or hospital. Practice settings at those places include both inpatient and outpatient work. There is also a shortage of psychiatrists doing this work, which makes it easier to get a job and to advance into leadership roles.
Continue to: Dr. Ahmed...
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Pirl: One challenge is figuring out how to make sure you have income doing something that is not financially viable on its own. This is why most people work for cancer centers or hospitals and have some institutional subsidy for their work. Another challenge is access to care. There are not enough psychiatric resources for all the people with cancer who need them. Traditional referral-based models are getting harder and harder to manage. I think the emotional aspects of the work can also be challenging at times.
Dr. Ahmed: Where do you see the field going?
Dr. Pirl: Psychosocial care is now considered part of quality cancer care, and regulations require cancer centers to do certain aspects of it. This is leading to clinical growth and more integration into oncology. However, I am worried that we are not having enough psychiatry residents choose to do CL and/or psychiatric oncology. Some trainees are choosing to do a palliative care fellowship instead. When those trainees tell me why they want to do palliative care, I say that I do all of that and actually have much more time to do it because I am not managing constipation and vent settings. We need to do a better job of making trainees more aware of psychiatric oncology.
Dr. Ahmed: What advice do you have for those contemplating a career in psychiatric oncology?
Dr. Pirl: Please join the field. There is a shortage of psychiatrists who do this work, which is ironically one of the best and most meaningful jobs in psychiatry.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Addiction Psychiatry Fellow at Boston University, talked with William Pirl, MD, MPH, FACLP, FAPOS. Dr. Pirl is Associate Professor, Psychiatry, Harvard Medical School. He joined Dana-Farber Cancer Institute in 2018 as Vice Chair for Psychosocial Oncology, Department of Psychosocial Oncology and Palliative Care. He is a past president of the American Psychosocial Oncology Society and North American Associate Editor for the journal Psycho-Oncology.
Dr. Ahmed: What made you choose the psychiatric oncology track, and how did your training lead you towards this path?
Dr. Pirl: I went to medical school thinking that I wanted to be a psychiatrist. However, I was really drawn to internal medicine, especially the process of sorting through medical differential diagnoses. I was deciding between applying for residency in medicine or psychiatry when I did an elective rotation in consultation-liaison (CL) psychiatry. Consultation-liaison psychiatry combined both medicine and psychiatry, which is exactly what I wanted to do. After residency, I wanted to do a CL fellowship outside of Boston, which is where I had done all of my medical education and training. One of my residency advisors suggested Memorial Sloan-Kettering Cancer Center, and I ended up going there. On the first day of fellowship, I realized that I’d only be working with cancer over that year, which I had not really thought about beforehand. Luckily, I loved it, and over the year I realized that the work had tremendous impact and meaning.
Dr. Ahmed: What are some of the pros and cons of working in psychiatric oncology?
Dr. Pirl: Things that I think are pros might be cons for some people. Consults in psychiatric oncology tend to be more relationship-based than they might be in other CL subspecialties. Oncology clinicians want to know who they are referring their patients to, and they are used to team-based care. If you like practicing as part of a multidisciplinary team, this is a pro.
Psychiatric oncology has more focus on existential issues, which interests me more than some other things in psychiatry. Bearing witness to so much tragedy can be a con at times, but psychiatrists who do this work learn ways to manage this within themselves. Psychiatric oncology also offers many experiences where you can see how much impact you make. It’s rewarding to see results and get positive feedback from patients and their families.
Continue to: Lastly, this is...
Lastly, this is a historic time in oncology. Over the last 15 years, things are happening that I never thought I would live to see. Some patients who 10 or 15 years ago would have had an expected survival of 6 to 9 months are now living years. We are now at a point where we might not actually know a patient’s prognosis, which introduces a whole other layer of uncertainty in cancer. Working as a psychiatrist during this time of rapidly evolving care is amazing. Cancer care will look very different over the next decade.
Dr. Ahmed: Based on your personal experience, what should one consider when choosing a psychiatric oncology program?
Dr. Pirl: I trained in a time before CL was a certified subspecialty of psychiatry. At that time, programs could focus solely on cancer, which cannot be done now. Trainees need to have broader training in certified fellowships. If someone knows that they are interested in psychiatric oncology, there are 2 programs that they should consider: the Dana-Farber Cancer Institute track of the Brigham and Women’s Hospital CL fellowship, and the Memorial Sloan-Kettering Cancer Center/New York Hospital CL fellowship. However, completing a CL fellowship will give someone the skills to do this work, even though they may not know all of the cancer content yet.
Dr. Ahmed: What are some of the career options and work settings in psychiatric oncology?
Dr. Pirl: There are many factors that make it difficult for psychiatrist to have a psychiatric oncology private practice. The amount of late cancellations and no-shows because of illness makes it hard to do this work without some institutional subsidy. Also, being able to communicate and work as a team with oncology providers is much easier if you are in the same place. Most psychiatrists who do psychiatric oncology work in a cancer center or hospital. Practice settings at those places include both inpatient and outpatient work. There is also a shortage of psychiatrists doing this work, which makes it easier to get a job and to advance into leadership roles.
Continue to: Dr. Ahmed...
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Pirl: One challenge is figuring out how to make sure you have income doing something that is not financially viable on its own. This is why most people work for cancer centers or hospitals and have some institutional subsidy for their work. Another challenge is access to care. There are not enough psychiatric resources for all the people with cancer who need them. Traditional referral-based models are getting harder and harder to manage. I think the emotional aspects of the work can also be challenging at times.
Dr. Ahmed: Where do you see the field going?
Dr. Pirl: Psychosocial care is now considered part of quality cancer care, and regulations require cancer centers to do certain aspects of it. This is leading to clinical growth and more integration into oncology. However, I am worried that we are not having enough psychiatry residents choose to do CL and/or psychiatric oncology. Some trainees are choosing to do a palliative care fellowship instead. When those trainees tell me why they want to do palliative care, I say that I do all of that and actually have much more time to do it because I am not managing constipation and vent settings. We need to do a better job of making trainees more aware of psychiatric oncology.
Dr. Ahmed: What advice do you have for those contemplating a career in psychiatric oncology?
Dr. Pirl: Please join the field. There is a shortage of psychiatrists who do this work, which is ironically one of the best and most meaningful jobs in psychiatry.
Editor’s note: Career Choices features a psychiatry resident/fellow interviewing a psychiatrist about why he or she has chosen a specific career path. The goal is to inform trainees about the various psychiatric career options, and to give them a feel for the pros and cons of the various paths.
In this Career Choices, Saeed Ahmed, MD, Addiction Psychiatry Fellow at Boston University, talked with William Pirl, MD, MPH, FACLP, FAPOS. Dr. Pirl is Associate Professor, Psychiatry, Harvard Medical School. He joined Dana-Farber Cancer Institute in 2018 as Vice Chair for Psychosocial Oncology, Department of Psychosocial Oncology and Palliative Care. He is a past president of the American Psychosocial Oncology Society and North American Associate Editor for the journal Psycho-Oncology.
Dr. Ahmed: What made you choose the psychiatric oncology track, and how did your training lead you towards this path?
Dr. Pirl: I went to medical school thinking that I wanted to be a psychiatrist. However, I was really drawn to internal medicine, especially the process of sorting through medical differential diagnoses. I was deciding between applying for residency in medicine or psychiatry when I did an elective rotation in consultation-liaison (CL) psychiatry. Consultation-liaison psychiatry combined both medicine and psychiatry, which is exactly what I wanted to do. After residency, I wanted to do a CL fellowship outside of Boston, which is where I had done all of my medical education and training. One of my residency advisors suggested Memorial Sloan-Kettering Cancer Center, and I ended up going there. On the first day of fellowship, I realized that I’d only be working with cancer over that year, which I had not really thought about beforehand. Luckily, I loved it, and over the year I realized that the work had tremendous impact and meaning.
Dr. Ahmed: What are some of the pros and cons of working in psychiatric oncology?
Dr. Pirl: Things that I think are pros might be cons for some people. Consults in psychiatric oncology tend to be more relationship-based than they might be in other CL subspecialties. Oncology clinicians want to know who they are referring their patients to, and they are used to team-based care. If you like practicing as part of a multidisciplinary team, this is a pro.
Psychiatric oncology has more focus on existential issues, which interests me more than some other things in psychiatry. Bearing witness to so much tragedy can be a con at times, but psychiatrists who do this work learn ways to manage this within themselves. Psychiatric oncology also offers many experiences where you can see how much impact you make. It’s rewarding to see results and get positive feedback from patients and their families.
Continue to: Lastly, this is...
Lastly, this is a historic time in oncology. Over the last 15 years, things are happening that I never thought I would live to see. Some patients who 10 or 15 years ago would have had an expected survival of 6 to 9 months are now living years. We are now at a point where we might not actually know a patient’s prognosis, which introduces a whole other layer of uncertainty in cancer. Working as a psychiatrist during this time of rapidly evolving care is amazing. Cancer care will look very different over the next decade.
Dr. Ahmed: Based on your personal experience, what should one consider when choosing a psychiatric oncology program?
Dr. Pirl: I trained in a time before CL was a certified subspecialty of psychiatry. At that time, programs could focus solely on cancer, which cannot be done now. Trainees need to have broader training in certified fellowships. If someone knows that they are interested in psychiatric oncology, there are 2 programs that they should consider: the Dana-Farber Cancer Institute track of the Brigham and Women’s Hospital CL fellowship, and the Memorial Sloan-Kettering Cancer Center/New York Hospital CL fellowship. However, completing a CL fellowship will give someone the skills to do this work, even though they may not know all of the cancer content yet.
Dr. Ahmed: What are some of the career options and work settings in psychiatric oncology?
Dr. Pirl: There are many factors that make it difficult for psychiatrist to have a psychiatric oncology private practice. The amount of late cancellations and no-shows because of illness makes it hard to do this work without some institutional subsidy. Also, being able to communicate and work as a team with oncology providers is much easier if you are in the same place. Most psychiatrists who do psychiatric oncology work in a cancer center or hospital. Practice settings at those places include both inpatient and outpatient work. There is also a shortage of psychiatrists doing this work, which makes it easier to get a job and to advance into leadership roles.
Continue to: Dr. Ahmed...
Dr. Ahmed: What are some of the challenges in working in this field?
Dr. Pirl: One challenge is figuring out how to make sure you have income doing something that is not financially viable on its own. This is why most people work for cancer centers or hospitals and have some institutional subsidy for their work. Another challenge is access to care. There are not enough psychiatric resources for all the people with cancer who need them. Traditional referral-based models are getting harder and harder to manage. I think the emotional aspects of the work can also be challenging at times.
Dr. Ahmed: Where do you see the field going?
Dr. Pirl: Psychosocial care is now considered part of quality cancer care, and regulations require cancer centers to do certain aspects of it. This is leading to clinical growth and more integration into oncology. However, I am worried that we are not having enough psychiatry residents choose to do CL and/or psychiatric oncology. Some trainees are choosing to do a palliative care fellowship instead. When those trainees tell me why they want to do palliative care, I say that I do all of that and actually have much more time to do it because I am not managing constipation and vent settings. We need to do a better job of making trainees more aware of psychiatric oncology.
Dr. Ahmed: What advice do you have for those contemplating a career in psychiatric oncology?
Dr. Pirl: Please join the field. There is a shortage of psychiatrists who do this work, which is ironically one of the best and most meaningful jobs in psychiatry.
Antipsychotics, dopamine, and pain
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.

In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.

In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
Buspirone: A forgotten friend
In general, when a medication goes off patent, marketing for it significantly slows down or comes to a halt. Studies have shown that physicians’ prescribing habits are influenced by pharmaceutical representatives and companies.1 This phenomenon may have an unforeseen adverse effect: once an effective and inexpensive medication “goes generic,” its use may fall out of favor. Additionally, physicians may have concerns about prescribing generic medications, such as perceiving them as less effective and conferring more adverse effects compared with brand-name formulations.2 One such generic medication is buspirone, which originally was branded as BuSpar.
Anxiety disorders are the most common psychiatric diagnoses, and at times are the most challenging to treat.3 Anecdotally, we often see benzodiazepines prescribed as first-line monotherapy for acute and chronic anxiety, but because these agents can cause physical dependence and a withdrawal reaction, alternative anxiolytic medications should be strongly considered. Despite its age, buspirone still plays a role in the treatment of anxiety, and its off-label use can also be useful in certain populations and scenarios. In this article, we delve into buspirone’s mechanism of action, discuss its advantages and challenges, and what you need to know when prescribing it.
How buspirone works
Buspirone was originally described as an anxiolytic agent that was pharmacologically unrelated to traditional anxiety-reducing medications (ie, benzodiazepines and barbiturates).4
The antidepressants vortioxetine and vilazodone exhibit dual-action at both serotonin reuptake transporters and 5HT1A receptors; thus, they work like an SSRI and buspirone combined.6 Although some patients may find it more convenient to take a dual-action pill over 2 separate ones, some insurance companies do not cover these newer agents. Additionally, prescribing buspirone separately allows for more precise dosing, which may lower the risk of adverse effects.
Buspirone is a major substrate for cytochrome P450 (CYP) 3A4 and a minor for CYP2D6, so caution must be advised if considering buspirone for a patient receiving any CYP3A4 inducers and/or inhibitors,7 including grapefruit juice.8
Dose adjustments are not necessary for age and sex, which allows for highly consistent dosing.4 However, as with prescribing medications in any geriatric population, lower starting doses and slower titration of buspirone may be necessary to avoid potential adverse effects due to the alterations of pharmacodynamic and pharmacokinetic processes that occur as patients age.9
Advantages of buspirone
Works well as an add-on to other medications. While buspirone in adequate doses may be helpful as monotherapy in GAD, it can also be helpful in other, more complex psychiatric scenarios. Sumiyoshi et al10 observed improvement in scores on the Digit Symbol Substitution Test when buspirone was added to a second-generation antipsychotic (SGA), which suggests buspirone may help improve attention in patients with schizophrenia. It has been postulated that buspirone may also be helpful for cognitive dysfunction in patients with Alzheimer’s disease.11 Buspirone has been used to treat comorbid anxiety and alcohol use disorder, resulting in reduced anxiety, longer latency to relapse, and fewer drinking days during a 12-week treatment program.12 Buspirone has been more effective than placebo for treating post-stroke anxiety.13
Continue to: Patients who receive...
Patients who receive an SSRI, such as citalopram, but are not able to achieve a substantial improvement in their depressive and/or anxious symptoms may benefit from the addition of buspirone to their treatment regimen.14,15
A favorable adverse-effect profile. There are no absolute contraindications to buspirone except a history of hypersensitivity.4 Buspirone generally is well tolerated and carries a low risk of adverse effects. The most common adverse effects are dizziness and nausea.6 Buspirone is not sedating.
Potentially safe for patients who are pregnant. Unlike many other first-line agents for anxiety, such as SSRIs, buspirone has an FDA Category B classification, meaning animal studies have shown no adverse events during pregnancy.4 The FDA Pregnancy and Lactation Labeling Rule applies only to medications that entered the market on or after June 30, 2001; unfortunately, buspirone is excluded from this updated categorization.16 As with any medication being considered for pregnant or lactating women, the prescriber and patient must weigh the benefits vs the risks to determine if buspirone is appropriate for any individual patient.
No adverse events have been reported from abrupt discontinuation of buspirone.17
Inexpensive. Buspirone is generic and extremely inexpensive. According to GoodRx.com, a 30-day supply of 5-mg tablets for twice-daily dosing can cost $4.18 A maximum daily dose (prescribed as 2 pills, 15 mg twice daily) may cost approximately $18/month.18 Thus, buspirone is a good option for uninsured or underinsured patients, for whom this would be more affordable than other anxiolytic medications.
Continue to: May offset certain adverse effects
May offset certain adverse effects. Sexual dysfunction is a common adverse effect of SSRIs. One strategy to offset this phenomenon is to add bupropion. However, in a randomized controlled trial, Landén et al19 found that sexual adverse effects induced by SSRIs were greatly mitigated by adding buspirone, even within the first week of treatment. This improvement was more marked in women than in men, which is helpful because sexual dysfunction in women is generally resistant to other interventions.20 Unlike
Unlikely to cause extrapyramidal symptoms (EPS). Because of its central D2 antagonism, buspirone has a low potential (<1%) to produce EPS. Buspirone has even been shown to reverse
The Table4 highlights key points to bear in mind when prescribing buspirone.
Challenges with buspirone
Response is not immediate. Unlike benzodiazepines, buspirone does not have an immediate onset of action.22 With buspirone monotherapy, response may be seen in approximately 2 to 4 weeks.23 Therefore, patients transitioning from a quick-onset benzodiazepine to buspirone may not report a good response. However, as noted above, when using buspirone to treat SSRI-induced sexual dysfunction, response may emerge within 1 week.19 Buspirone also lacks the euphoric and sedative qualities of benzodiazepines that patients may prefer.
Not for patients with hepatic and renal impairment. Because plasma levels of buspirone are elevated in patients with hepatic and renal impairment, this medication is not ideal for use in these populations.4
Continue to: Contraindicated in patients receiving MAOIs
Contraindicated in patients receiving MAOIs. Buspirone should not be prescribed to patients with depression who are receiving treatment with a monoamine oxidase inhibitor (MAOI) because the combination may precipitate a hypertensive reaction.4 A minimum washout period of 14 days from the MAOI is necessary before initiating buspirone.9
Idiosyncratic adverse effects. As with all pharmaceuticals, buspirone may produce idiosyncratic adverse effects. Faber and Sansone24 reported a case of a woman who experienced hair loss 3 months into treatment with buspirone. After cessation, her alopecia resolved.
Questionable efficacy for some anxiety subtypes. Buspirone has been studied as a treatment of other common psychiatric conditions, such as social phobia and anxiety in the setting of smoking cessation. However, it has not proven to be effective over placebo in treating these anxiety subtypes.25,26
Short half-life. Because of its relatively short half-life (2 to 3 hours), buspirone requires dosing 2 to 3 times a day, which could increase the risk of noncompliance.4 However, some patients might prefer multiple dosing throughout the day due to perceived better coverage of their anxiety symptoms.
Limited incentive for future research. Because buspirone is available only as a generic formulation, there is little financial incentive for pharmaceutical companies and other interested parties to study what may be valuable uses for buspirone. For example, there is no data available on comparative augmentation of buspirone and SGAs with antidepressants for depression and/or anxiety. There is also little data available about buspirone prescribing trends or why buspirone may be underutilized in clinical practice today.
Continue to: Unfortunately, historical and longitudinal...
Unfortunately, historical and longitudinal data on the prescribing practices of buspirone is limited because the original branded medication, BuSpar, is no longer on the market. However, this medication offers multiple advantages over other agents used to treat anxiety, and it should not be forgotten when formulating a treatment regimen for patients with anxiety and/or depression.
Bottom Line
Buspirone is a safe, low-cost, effective treatment option for patients with anxiety and may be helpful as an augmenting agent for depression. Because of its efficacy and high degree of tolerability, it should be prioritized higher in our treatment algorithms and be a part of our routine pharmacologic armamentarium.
Related Resources
- Howland RH. Buspirone: Back to the future. J Psychosoc Nurs Ment Health Serv. 2015;53(11):21-24.
- Strawn JR, Mills JA, Cornwall GJ, et al. Buspirone in children and adolescents with anxiety: a review and Bayesian analysis of abandoned randomized controlled trials. J Child Adolesc Psychopharmacol. 2018;28(1):2-9.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Citalopram • Celexa
Haloperidol • Haldol
Vilazodone • Viibryd
Vortioxetine • Trintellix
1. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017;7(9):e016408. doi: 10.1136/bmjopen-2017-016408.
2. Haque M. Generic medicine and prescribing: a quick assessment. Adv Hum Biol. 2017;7(3):101-108.
3. National Alliance on Mental Illness. Anxiety disorders. https://www.nami.org/Learn-More/Mental-Health-Conditions/Anxiety-Disorders. Published December 2017. Accessed November 26, 2019.
4. Buspar [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2000.
5. Hjorth S, Carlsson A. Buspirone: effects on central monoaminergic transmission-possible relevance to animal experimental and clinical findings. Eur J Pharmacol. 1982:83;299-303.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
7. Buspirone tablets [package insert]. East Brunswick, NJ: Strides Pharma Inc; 2017.
8. Lilja JJ, Kivistö KT, Backman, JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655-660.
9. Stahl SM. Stahl’s essential psychopharmacology: prescriber’s guide, 6th ed. Cambridge, United Kingdom: Cambridge University Press; 2017.
10. Sumiyoshi T, Park S, Jayathilake K. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2007;95(1-3):158-168.
11. Schechter LE, Dawson LA, Harder JA. The potential utility of 5-HT1A receptor antagonists in the treatment of cognitive dysfunction associated with Alzheimer’s disease. Curr Pharm Des. 2002;8(2):139-145.
12. Kranzler HR, Burleson JA, Del Boca FK. Buspirone treatment of anxious alcoholics: a placebo-controlled trial. Arch Gen Psychiatry. 1994;51(9):720-731.
13. Burton CA, Holmes J, Murray J, et al. Interventions for treating anxiety after stroke. Cochrane Database Syst Rev. 2011;12:1-25.
14. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001; 62(6):448-452.
15. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published May 2010. Accessed November 2019.
16. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. https://www.fda.gov/drugs/labeling/pregnancy-and-lactation-labeling-drugs-final-rule. Published September 11, 2019. Accessed November 26, 2019.
17. Goa KL, Ward A. Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32(2):114-129.
18. GoodRx. Buspar prices, coupons, & savings tips in U.S. area code 08054. https://www.goodrx.com/buspar. Accessed June 6, 2019.
19. Landén M, Eriksson E, Agren H, et al. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999;19(3):268-271.
20. Hensley PL, Nurnberg HG. SSRI sexual dysfunction: a female perspective. J Sex Marital Ther. 2002;28(suppl 1):143-153.
21. Haleem DJ, Samad N, Haleem MA. Reversal of haloperidol-induced extrapyramidal symptoms by buspirone: a time-related study. Behav Pharmacol. 2007;18(2):147-153.
22. Kaplan SS, Saddock BJ, Grebb JA. Synopsis of psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2014.
23. National Alliance on Mental Health. Buspirone (BuSpar). https://www.nami.org/Learn-More/Treatment/Mental-Health-Medications/Types-of-Medication/Buspirone-(BuSpar). Published January 2019. Accessed November 26, 2019.
24. Faber J, Sansone RA. Buspirone: a possible cause of alopecia. Innov Clin Neurosci. 2013;10(1):13.
25. Van Vliet IM, Den Boer JA, Westenberg HGM, et al. Clinical effects of buspirone in social phobia, a double-blind placebo controlled study. J Clin Psychiatry. 1997;58(4):164-168.
26. Schneider NG, Olmstead RE, Steinberg C, et al. Efficacy of buspirone in smoking cessation: a placebo‐controlled trial. Clin Pharmacol Ther. 1996;60(5):568-575.
In general, when a medication goes off patent, marketing for it significantly slows down or comes to a halt. Studies have shown that physicians’ prescribing habits are influenced by pharmaceutical representatives and companies.1 This phenomenon may have an unforeseen adverse effect: once an effective and inexpensive medication “goes generic,” its use may fall out of favor. Additionally, physicians may have concerns about prescribing generic medications, such as perceiving them as less effective and conferring more adverse effects compared with brand-name formulations.2 One such generic medication is buspirone, which originally was branded as BuSpar.
Anxiety disorders are the most common psychiatric diagnoses, and at times are the most challenging to treat.3 Anecdotally, we often see benzodiazepines prescribed as first-line monotherapy for acute and chronic anxiety, but because these agents can cause physical dependence and a withdrawal reaction, alternative anxiolytic medications should be strongly considered. Despite its age, buspirone still plays a role in the treatment of anxiety, and its off-label use can also be useful in certain populations and scenarios. In this article, we delve into buspirone’s mechanism of action, discuss its advantages and challenges, and what you need to know when prescribing it.
How buspirone works
Buspirone was originally described as an anxiolytic agent that was pharmacologically unrelated to traditional anxiety-reducing medications (ie, benzodiazepines and barbiturates).4
The antidepressants vortioxetine and vilazodone exhibit dual-action at both serotonin reuptake transporters and 5HT1A receptors; thus, they work like an SSRI and buspirone combined.6 Although some patients may find it more convenient to take a dual-action pill over 2 separate ones, some insurance companies do not cover these newer agents. Additionally, prescribing buspirone separately allows for more precise dosing, which may lower the risk of adverse effects.
Buspirone is a major substrate for cytochrome P450 (CYP) 3A4 and a minor for CYP2D6, so caution must be advised if considering buspirone for a patient receiving any CYP3A4 inducers and/or inhibitors,7 including grapefruit juice.8
Dose adjustments are not necessary for age and sex, which allows for highly consistent dosing.4 However, as with prescribing medications in any geriatric population, lower starting doses and slower titration of buspirone may be necessary to avoid potential adverse effects due to the alterations of pharmacodynamic and pharmacokinetic processes that occur as patients age.9
Advantages of buspirone
Works well as an add-on to other medications. While buspirone in adequate doses may be helpful as monotherapy in GAD, it can also be helpful in other, more complex psychiatric scenarios. Sumiyoshi et al10 observed improvement in scores on the Digit Symbol Substitution Test when buspirone was added to a second-generation antipsychotic (SGA), which suggests buspirone may help improve attention in patients with schizophrenia. It has been postulated that buspirone may also be helpful for cognitive dysfunction in patients with Alzheimer’s disease.11 Buspirone has been used to treat comorbid anxiety and alcohol use disorder, resulting in reduced anxiety, longer latency to relapse, and fewer drinking days during a 12-week treatment program.12 Buspirone has been more effective than placebo for treating post-stroke anxiety.13
Continue to: Patients who receive...
Patients who receive an SSRI, such as citalopram, but are not able to achieve a substantial improvement in their depressive and/or anxious symptoms may benefit from the addition of buspirone to their treatment regimen.14,15
A favorable adverse-effect profile. There are no absolute contraindications to buspirone except a history of hypersensitivity.4 Buspirone generally is well tolerated and carries a low risk of adverse effects. The most common adverse effects are dizziness and nausea.6 Buspirone is not sedating.
Potentially safe for patients who are pregnant. Unlike many other first-line agents for anxiety, such as SSRIs, buspirone has an FDA Category B classification, meaning animal studies have shown no adverse events during pregnancy.4 The FDA Pregnancy and Lactation Labeling Rule applies only to medications that entered the market on or after June 30, 2001; unfortunately, buspirone is excluded from this updated categorization.16 As with any medication being considered for pregnant or lactating women, the prescriber and patient must weigh the benefits vs the risks to determine if buspirone is appropriate for any individual patient.
No adverse events have been reported from abrupt discontinuation of buspirone.17
Inexpensive. Buspirone is generic and extremely inexpensive. According to GoodRx.com, a 30-day supply of 5-mg tablets for twice-daily dosing can cost $4.18 A maximum daily dose (prescribed as 2 pills, 15 mg twice daily) may cost approximately $18/month.18 Thus, buspirone is a good option for uninsured or underinsured patients, for whom this would be more affordable than other anxiolytic medications.
Continue to: May offset certain adverse effects
May offset certain adverse effects. Sexual dysfunction is a common adverse effect of SSRIs. One strategy to offset this phenomenon is to add bupropion. However, in a randomized controlled trial, Landén et al19 found that sexual adverse effects induced by SSRIs were greatly mitigated by adding buspirone, even within the first week of treatment. This improvement was more marked in women than in men, which is helpful because sexual dysfunction in women is generally resistant to other interventions.20 Unlike
Unlikely to cause extrapyramidal symptoms (EPS). Because of its central D2 antagonism, buspirone has a low potential (<1%) to produce EPS. Buspirone has even been shown to reverse
The Table4 highlights key points to bear in mind when prescribing buspirone.
Challenges with buspirone
Response is not immediate. Unlike benzodiazepines, buspirone does not have an immediate onset of action.22 With buspirone monotherapy, response may be seen in approximately 2 to 4 weeks.23 Therefore, patients transitioning from a quick-onset benzodiazepine to buspirone may not report a good response. However, as noted above, when using buspirone to treat SSRI-induced sexual dysfunction, response may emerge within 1 week.19 Buspirone also lacks the euphoric and sedative qualities of benzodiazepines that patients may prefer.
Not for patients with hepatic and renal impairment. Because plasma levels of buspirone are elevated in patients with hepatic and renal impairment, this medication is not ideal for use in these populations.4
Continue to: Contraindicated in patients receiving MAOIs
Contraindicated in patients receiving MAOIs. Buspirone should not be prescribed to patients with depression who are receiving treatment with a monoamine oxidase inhibitor (MAOI) because the combination may precipitate a hypertensive reaction.4 A minimum washout period of 14 days from the MAOI is necessary before initiating buspirone.9
Idiosyncratic adverse effects. As with all pharmaceuticals, buspirone may produce idiosyncratic adverse effects. Faber and Sansone24 reported a case of a woman who experienced hair loss 3 months into treatment with buspirone. After cessation, her alopecia resolved.
Questionable efficacy for some anxiety subtypes. Buspirone has been studied as a treatment of other common psychiatric conditions, such as social phobia and anxiety in the setting of smoking cessation. However, it has not proven to be effective over placebo in treating these anxiety subtypes.25,26
Short half-life. Because of its relatively short half-life (2 to 3 hours), buspirone requires dosing 2 to 3 times a day, which could increase the risk of noncompliance.4 However, some patients might prefer multiple dosing throughout the day due to perceived better coverage of their anxiety symptoms.
Limited incentive for future research. Because buspirone is available only as a generic formulation, there is little financial incentive for pharmaceutical companies and other interested parties to study what may be valuable uses for buspirone. For example, there is no data available on comparative augmentation of buspirone and SGAs with antidepressants for depression and/or anxiety. There is also little data available about buspirone prescribing trends or why buspirone may be underutilized in clinical practice today.
Continue to: Unfortunately, historical and longitudinal...
Unfortunately, historical and longitudinal data on the prescribing practices of buspirone is limited because the original branded medication, BuSpar, is no longer on the market. However, this medication offers multiple advantages over other agents used to treat anxiety, and it should not be forgotten when formulating a treatment regimen for patients with anxiety and/or depression.
Bottom Line
Buspirone is a safe, low-cost, effective treatment option for patients with anxiety and may be helpful as an augmenting agent for depression. Because of its efficacy and high degree of tolerability, it should be prioritized higher in our treatment algorithms and be a part of our routine pharmacologic armamentarium.
Related Resources
- Howland RH. Buspirone: Back to the future. J Psychosoc Nurs Ment Health Serv. 2015;53(11):21-24.
- Strawn JR, Mills JA, Cornwall GJ, et al. Buspirone in children and adolescents with anxiety: a review and Bayesian analysis of abandoned randomized controlled trials. J Child Adolesc Psychopharmacol. 2018;28(1):2-9.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Citalopram • Celexa
Haloperidol • Haldol
Vilazodone • Viibryd
Vortioxetine • Trintellix
In general, when a medication goes off patent, marketing for it significantly slows down or comes to a halt. Studies have shown that physicians’ prescribing habits are influenced by pharmaceutical representatives and companies.1 This phenomenon may have an unforeseen adverse effect: once an effective and inexpensive medication “goes generic,” its use may fall out of favor. Additionally, physicians may have concerns about prescribing generic medications, such as perceiving them as less effective and conferring more adverse effects compared with brand-name formulations.2 One such generic medication is buspirone, which originally was branded as BuSpar.
Anxiety disorders are the most common psychiatric diagnoses, and at times are the most challenging to treat.3 Anecdotally, we often see benzodiazepines prescribed as first-line monotherapy for acute and chronic anxiety, but because these agents can cause physical dependence and a withdrawal reaction, alternative anxiolytic medications should be strongly considered. Despite its age, buspirone still plays a role in the treatment of anxiety, and its off-label use can also be useful in certain populations and scenarios. In this article, we delve into buspirone’s mechanism of action, discuss its advantages and challenges, and what you need to know when prescribing it.
How buspirone works
Buspirone was originally described as an anxiolytic agent that was pharmacologically unrelated to traditional anxiety-reducing medications (ie, benzodiazepines and barbiturates).4
The antidepressants vortioxetine and vilazodone exhibit dual-action at both serotonin reuptake transporters and 5HT1A receptors; thus, they work like an SSRI and buspirone combined.6 Although some patients may find it more convenient to take a dual-action pill over 2 separate ones, some insurance companies do not cover these newer agents. Additionally, prescribing buspirone separately allows for more precise dosing, which may lower the risk of adverse effects.
Buspirone is a major substrate for cytochrome P450 (CYP) 3A4 and a minor for CYP2D6, so caution must be advised if considering buspirone for a patient receiving any CYP3A4 inducers and/or inhibitors,7 including grapefruit juice.8
Dose adjustments are not necessary for age and sex, which allows for highly consistent dosing.4 However, as with prescribing medications in any geriatric population, lower starting doses and slower titration of buspirone may be necessary to avoid potential adverse effects due to the alterations of pharmacodynamic and pharmacokinetic processes that occur as patients age.9
Advantages of buspirone
Works well as an add-on to other medications. While buspirone in adequate doses may be helpful as monotherapy in GAD, it can also be helpful in other, more complex psychiatric scenarios. Sumiyoshi et al10 observed improvement in scores on the Digit Symbol Substitution Test when buspirone was added to a second-generation antipsychotic (SGA), which suggests buspirone may help improve attention in patients with schizophrenia. It has been postulated that buspirone may also be helpful for cognitive dysfunction in patients with Alzheimer’s disease.11 Buspirone has been used to treat comorbid anxiety and alcohol use disorder, resulting in reduced anxiety, longer latency to relapse, and fewer drinking days during a 12-week treatment program.12 Buspirone has been more effective than placebo for treating post-stroke anxiety.13
Continue to: Patients who receive...
Patients who receive an SSRI, such as citalopram, but are not able to achieve a substantial improvement in their depressive and/or anxious symptoms may benefit from the addition of buspirone to their treatment regimen.14,15
A favorable adverse-effect profile. There are no absolute contraindications to buspirone except a history of hypersensitivity.4 Buspirone generally is well tolerated and carries a low risk of adverse effects. The most common adverse effects are dizziness and nausea.6 Buspirone is not sedating.
Potentially safe for patients who are pregnant. Unlike many other first-line agents for anxiety, such as SSRIs, buspirone has an FDA Category B classification, meaning animal studies have shown no adverse events during pregnancy.4 The FDA Pregnancy and Lactation Labeling Rule applies only to medications that entered the market on or after June 30, 2001; unfortunately, buspirone is excluded from this updated categorization.16 As with any medication being considered for pregnant or lactating women, the prescriber and patient must weigh the benefits vs the risks to determine if buspirone is appropriate for any individual patient.
No adverse events have been reported from abrupt discontinuation of buspirone.17
Inexpensive. Buspirone is generic and extremely inexpensive. According to GoodRx.com, a 30-day supply of 5-mg tablets for twice-daily dosing can cost $4.18 A maximum daily dose (prescribed as 2 pills, 15 mg twice daily) may cost approximately $18/month.18 Thus, buspirone is a good option for uninsured or underinsured patients, for whom this would be more affordable than other anxiolytic medications.
Continue to: May offset certain adverse effects
May offset certain adverse effects. Sexual dysfunction is a common adverse effect of SSRIs. One strategy to offset this phenomenon is to add bupropion. However, in a randomized controlled trial, Landén et al19 found that sexual adverse effects induced by SSRIs were greatly mitigated by adding buspirone, even within the first week of treatment. This improvement was more marked in women than in men, which is helpful because sexual dysfunction in women is generally resistant to other interventions.20 Unlike
Unlikely to cause extrapyramidal symptoms (EPS). Because of its central D2 antagonism, buspirone has a low potential (<1%) to produce EPS. Buspirone has even been shown to reverse
The Table4 highlights key points to bear in mind when prescribing buspirone.
Challenges with buspirone
Response is not immediate. Unlike benzodiazepines, buspirone does not have an immediate onset of action.22 With buspirone monotherapy, response may be seen in approximately 2 to 4 weeks.23 Therefore, patients transitioning from a quick-onset benzodiazepine to buspirone may not report a good response. However, as noted above, when using buspirone to treat SSRI-induced sexual dysfunction, response may emerge within 1 week.19 Buspirone also lacks the euphoric and sedative qualities of benzodiazepines that patients may prefer.
Not for patients with hepatic and renal impairment. Because plasma levels of buspirone are elevated in patients with hepatic and renal impairment, this medication is not ideal for use in these populations.4
Continue to: Contraindicated in patients receiving MAOIs
Contraindicated in patients receiving MAOIs. Buspirone should not be prescribed to patients with depression who are receiving treatment with a monoamine oxidase inhibitor (MAOI) because the combination may precipitate a hypertensive reaction.4 A minimum washout period of 14 days from the MAOI is necessary before initiating buspirone.9
Idiosyncratic adverse effects. As with all pharmaceuticals, buspirone may produce idiosyncratic adverse effects. Faber and Sansone24 reported a case of a woman who experienced hair loss 3 months into treatment with buspirone. After cessation, her alopecia resolved.
Questionable efficacy for some anxiety subtypes. Buspirone has been studied as a treatment of other common psychiatric conditions, such as social phobia and anxiety in the setting of smoking cessation. However, it has not proven to be effective over placebo in treating these anxiety subtypes.25,26
Short half-life. Because of its relatively short half-life (2 to 3 hours), buspirone requires dosing 2 to 3 times a day, which could increase the risk of noncompliance.4 However, some patients might prefer multiple dosing throughout the day due to perceived better coverage of their anxiety symptoms.
Limited incentive for future research. Because buspirone is available only as a generic formulation, there is little financial incentive for pharmaceutical companies and other interested parties to study what may be valuable uses for buspirone. For example, there is no data available on comparative augmentation of buspirone and SGAs with antidepressants for depression and/or anxiety. There is also little data available about buspirone prescribing trends or why buspirone may be underutilized in clinical practice today.
Continue to: Unfortunately, historical and longitudinal...
Unfortunately, historical and longitudinal data on the prescribing practices of buspirone is limited because the original branded medication, BuSpar, is no longer on the market. However, this medication offers multiple advantages over other agents used to treat anxiety, and it should not be forgotten when formulating a treatment regimen for patients with anxiety and/or depression.
Bottom Line
Buspirone is a safe, low-cost, effective treatment option for patients with anxiety and may be helpful as an augmenting agent for depression. Because of its efficacy and high degree of tolerability, it should be prioritized higher in our treatment algorithms and be a part of our routine pharmacologic armamentarium.
Related Resources
- Howland RH. Buspirone: Back to the future. J Psychosoc Nurs Ment Health Serv. 2015;53(11):21-24.
- Strawn JR, Mills JA, Cornwall GJ, et al. Buspirone in children and adolescents with anxiety: a review and Bayesian analysis of abandoned randomized controlled trials. J Child Adolesc Psychopharmacol. 2018;28(1):2-9.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Citalopram • Celexa
Haloperidol • Haldol
Vilazodone • Viibryd
Vortioxetine • Trintellix
1. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017;7(9):e016408. doi: 10.1136/bmjopen-2017-016408.
2. Haque M. Generic medicine and prescribing: a quick assessment. Adv Hum Biol. 2017;7(3):101-108.
3. National Alliance on Mental Illness. Anxiety disorders. https://www.nami.org/Learn-More/Mental-Health-Conditions/Anxiety-Disorders. Published December 2017. Accessed November 26, 2019.
4. Buspar [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2000.
5. Hjorth S, Carlsson A. Buspirone: effects on central monoaminergic transmission-possible relevance to animal experimental and clinical findings. Eur J Pharmacol. 1982:83;299-303.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
7. Buspirone tablets [package insert]. East Brunswick, NJ: Strides Pharma Inc; 2017.
8. Lilja JJ, Kivistö KT, Backman, JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655-660.
9. Stahl SM. Stahl’s essential psychopharmacology: prescriber’s guide, 6th ed. Cambridge, United Kingdom: Cambridge University Press; 2017.
10. Sumiyoshi T, Park S, Jayathilake K. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2007;95(1-3):158-168.
11. Schechter LE, Dawson LA, Harder JA. The potential utility of 5-HT1A receptor antagonists in the treatment of cognitive dysfunction associated with Alzheimer’s disease. Curr Pharm Des. 2002;8(2):139-145.
12. Kranzler HR, Burleson JA, Del Boca FK. Buspirone treatment of anxious alcoholics: a placebo-controlled trial. Arch Gen Psychiatry. 1994;51(9):720-731.
13. Burton CA, Holmes J, Murray J, et al. Interventions for treating anxiety after stroke. Cochrane Database Syst Rev. 2011;12:1-25.
14. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001; 62(6):448-452.
15. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published May 2010. Accessed November 2019.
16. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. https://www.fda.gov/drugs/labeling/pregnancy-and-lactation-labeling-drugs-final-rule. Published September 11, 2019. Accessed November 26, 2019.
17. Goa KL, Ward A. Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32(2):114-129.
18. GoodRx. Buspar prices, coupons, & savings tips in U.S. area code 08054. https://www.goodrx.com/buspar. Accessed June 6, 2019.
19. Landén M, Eriksson E, Agren H, et al. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999;19(3):268-271.
20. Hensley PL, Nurnberg HG. SSRI sexual dysfunction: a female perspective. J Sex Marital Ther. 2002;28(suppl 1):143-153.
21. Haleem DJ, Samad N, Haleem MA. Reversal of haloperidol-induced extrapyramidal symptoms by buspirone: a time-related study. Behav Pharmacol. 2007;18(2):147-153.
22. Kaplan SS, Saddock BJ, Grebb JA. Synopsis of psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2014.
23. National Alliance on Mental Health. Buspirone (BuSpar). https://www.nami.org/Learn-More/Treatment/Mental-Health-Medications/Types-of-Medication/Buspirone-(BuSpar). Published January 2019. Accessed November 26, 2019.
24. Faber J, Sansone RA. Buspirone: a possible cause of alopecia. Innov Clin Neurosci. 2013;10(1):13.
25. Van Vliet IM, Den Boer JA, Westenberg HGM, et al. Clinical effects of buspirone in social phobia, a double-blind placebo controlled study. J Clin Psychiatry. 1997;58(4):164-168.
26. Schneider NG, Olmstead RE, Steinberg C, et al. Efficacy of buspirone in smoking cessation: a placebo‐controlled trial. Clin Pharmacol Ther. 1996;60(5):568-575.
1. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017;7(9):e016408. doi: 10.1136/bmjopen-2017-016408.
2. Haque M. Generic medicine and prescribing: a quick assessment. Adv Hum Biol. 2017;7(3):101-108.
3. National Alliance on Mental Illness. Anxiety disorders. https://www.nami.org/Learn-More/Mental-Health-Conditions/Anxiety-Disorders. Published December 2017. Accessed November 26, 2019.
4. Buspar [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2000.
5. Hjorth S, Carlsson A. Buspirone: effects on central monoaminergic transmission-possible relevance to animal experimental and clinical findings. Eur J Pharmacol. 1982:83;299-303.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
7. Buspirone tablets [package insert]. East Brunswick, NJ: Strides Pharma Inc; 2017.
8. Lilja JJ, Kivistö KT, Backman, JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655-660.
9. Stahl SM. Stahl’s essential psychopharmacology: prescriber’s guide, 6th ed. Cambridge, United Kingdom: Cambridge University Press; 2017.
10. Sumiyoshi T, Park S, Jayathilake K. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2007;95(1-3):158-168.
11. Schechter LE, Dawson LA, Harder JA. The potential utility of 5-HT1A receptor antagonists in the treatment of cognitive dysfunction associated with Alzheimer’s disease. Curr Pharm Des. 2002;8(2):139-145.
12. Kranzler HR, Burleson JA, Del Boca FK. Buspirone treatment of anxious alcoholics: a placebo-controlled trial. Arch Gen Psychiatry. 1994;51(9):720-731.
13. Burton CA, Holmes J, Murray J, et al. Interventions for treating anxiety after stroke. Cochrane Database Syst Rev. 2011;12:1-25.
14. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001; 62(6):448-452.
15. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published May 2010. Accessed November 2019.
16. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. https://www.fda.gov/drugs/labeling/pregnancy-and-lactation-labeling-drugs-final-rule. Published September 11, 2019. Accessed November 26, 2019.
17. Goa KL, Ward A. Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32(2):114-129.
18. GoodRx. Buspar prices, coupons, & savings tips in U.S. area code 08054. https://www.goodrx.com/buspar. Accessed June 6, 2019.
19. Landén M, Eriksson E, Agren H, et al. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999;19(3):268-271.
20. Hensley PL, Nurnberg HG. SSRI sexual dysfunction: a female perspective. J Sex Marital Ther. 2002;28(suppl 1):143-153.
21. Haleem DJ, Samad N, Haleem MA. Reversal of haloperidol-induced extrapyramidal symptoms by buspirone: a time-related study. Behav Pharmacol. 2007;18(2):147-153.
22. Kaplan SS, Saddock BJ, Grebb JA. Synopsis of psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2014.
23. National Alliance on Mental Health. Buspirone (BuSpar). https://www.nami.org/Learn-More/Treatment/Mental-Health-Medications/Types-of-Medication/Buspirone-(BuSpar). Published January 2019. Accessed November 26, 2019.
24. Faber J, Sansone RA. Buspirone: a possible cause of alopecia. Innov Clin Neurosci. 2013;10(1):13.
25. Van Vliet IM, Den Boer JA, Westenberg HGM, et al. Clinical effects of buspirone in social phobia, a double-blind placebo controlled study. J Clin Psychiatry. 1997;58(4):164-168.
26. Schneider NG, Olmstead RE, Steinberg C, et al. Efficacy of buspirone in smoking cessation: a placebo‐controlled trial. Clin Pharmacol Ther. 1996;60(5):568-575.