User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Continuous glucose monitoring recommended over finger sticks for type 1 diabetes
“Studies have found that people with type 1 diabetes who use CGMs [continuous glucose monitors] are able to maintain better control of their blood sugar without increasing episodes of hypoglycemia when blood sugar drops to dangerous levels, compared to those who self-monitor blood glucose with periodic finger sticks,” the chair of the guideline task force, Anne Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, said in a Sept. 26 statement.
The group recommended CGMs for well-controlled type 1 patients as well as those above hemoglobin A1c (HbA1c) targets, so long as they want and understand how to use the devices. It also suggested short-term, intermittent CGM use to help type 2 patients meet HbA1cgoals. Insulin pumps were recommended over multiple daily injections for type 1 patients above target HbA1clevels, as well as those who meet their target but continue to have severe hypoglycemia or high glucose variability. For patients with type 2 disease, pumps were suggested for cases of poor glycemic control despite intensive insulin therapy, oral agents, and other measures.
The Endocrine Society and others have been pushing Medicare to cover CGMs for a while; the new guideline seems to support the effort. Although the devices are used by type 1 patients and covered by some insurance plans, they are only indicated as finger-stick adjuncts, not replacements. For Medicare coverage, they would “need to serve a primary medical purpose and not be used adjunctively,” according to a recent review by Dexcom, a company seeking a primary monitoring indication for its CGM.
The Endocrine Society noted in its evidence-based guideline that standard capillary blood glucose measurements “offer only a limited perspective on the constant daily changes in blood glucose levels,” and, unlike continuous monitoring, “do not provide alarms that indicate when blood glucose levels are above or below various thresholds, and do not indicate trends in blood glucose levels.”
The society commissioned a pooled analysis of 11 randomized trials that showed a 0.3% reduction in HbA1c with real-time glucose monitoring, mostly in patients 15 years or older. Other studies cited by the group also showed better HbA1c control than with finger sticks, without an increased risk of hypoglycemia.
The guideline also suggested insulin pumps for type 1 patients who want greater flexibility and convenience and that insulin pump therapy should continue during hospitalizations. It also suggested encouraging patients to use the embedded bolus calculators in their pumps so long as they “have appropriate education regarding their use and limitations.”
The Endocrine Society funded the work, with cosponsorship from the American Association for Clinical Chemistry, the American Association of Diabetes Educators, and the European Society of Endocrinology. Several of the authors have industry ties to companies that make CGMs or insulin pumps. Dr. Peters is an advisor for Abbott, Becton Dickinson, AstraZeneca, Biodel, Medtronic, and other companies, as well as a speaker, investigator, and advisor for Janssen.
“Studies have found that people with type 1 diabetes who use CGMs [continuous glucose monitors] are able to maintain better control of their blood sugar without increasing episodes of hypoglycemia when blood sugar drops to dangerous levels, compared to those who self-monitor blood glucose with periodic finger sticks,” the chair of the guideline task force, Anne Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, said in a Sept. 26 statement.
The group recommended CGMs for well-controlled type 1 patients as well as those above hemoglobin A1c (HbA1c) targets, so long as they want and understand how to use the devices. It also suggested short-term, intermittent CGM use to help type 2 patients meet HbA1cgoals. Insulin pumps were recommended over multiple daily injections for type 1 patients above target HbA1clevels, as well as those who meet their target but continue to have severe hypoglycemia or high glucose variability. For patients with type 2 disease, pumps were suggested for cases of poor glycemic control despite intensive insulin therapy, oral agents, and other measures.
The Endocrine Society and others have been pushing Medicare to cover CGMs for a while; the new guideline seems to support the effort. Although the devices are used by type 1 patients and covered by some insurance plans, they are only indicated as finger-stick adjuncts, not replacements. For Medicare coverage, they would “need to serve a primary medical purpose and not be used adjunctively,” according to a recent review by Dexcom, a company seeking a primary monitoring indication for its CGM.
The Endocrine Society noted in its evidence-based guideline that standard capillary blood glucose measurements “offer only a limited perspective on the constant daily changes in blood glucose levels,” and, unlike continuous monitoring, “do not provide alarms that indicate when blood glucose levels are above or below various thresholds, and do not indicate trends in blood glucose levels.”
The society commissioned a pooled analysis of 11 randomized trials that showed a 0.3% reduction in HbA1c with real-time glucose monitoring, mostly in patients 15 years or older. Other studies cited by the group also showed better HbA1c control than with finger sticks, without an increased risk of hypoglycemia.
The guideline also suggested insulin pumps for type 1 patients who want greater flexibility and convenience and that insulin pump therapy should continue during hospitalizations. It also suggested encouraging patients to use the embedded bolus calculators in their pumps so long as they “have appropriate education regarding their use and limitations.”
The Endocrine Society funded the work, with cosponsorship from the American Association for Clinical Chemistry, the American Association of Diabetes Educators, and the European Society of Endocrinology. Several of the authors have industry ties to companies that make CGMs or insulin pumps. Dr. Peters is an advisor for Abbott, Becton Dickinson, AstraZeneca, Biodel, Medtronic, and other companies, as well as a speaker, investigator, and advisor for Janssen.
“Studies have found that people with type 1 diabetes who use CGMs [continuous glucose monitors] are able to maintain better control of their blood sugar without increasing episodes of hypoglycemia when blood sugar drops to dangerous levels, compared to those who self-monitor blood glucose with periodic finger sticks,” the chair of the guideline task force, Anne Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, said in a Sept. 26 statement.
The group recommended CGMs for well-controlled type 1 patients as well as those above hemoglobin A1c (HbA1c) targets, so long as they want and understand how to use the devices. It also suggested short-term, intermittent CGM use to help type 2 patients meet HbA1cgoals. Insulin pumps were recommended over multiple daily injections for type 1 patients above target HbA1clevels, as well as those who meet their target but continue to have severe hypoglycemia or high glucose variability. For patients with type 2 disease, pumps were suggested for cases of poor glycemic control despite intensive insulin therapy, oral agents, and other measures.
The Endocrine Society and others have been pushing Medicare to cover CGMs for a while; the new guideline seems to support the effort. Although the devices are used by type 1 patients and covered by some insurance plans, they are only indicated as finger-stick adjuncts, not replacements. For Medicare coverage, they would “need to serve a primary medical purpose and not be used adjunctively,” according to a recent review by Dexcom, a company seeking a primary monitoring indication for its CGM.
The Endocrine Society noted in its evidence-based guideline that standard capillary blood glucose measurements “offer only a limited perspective on the constant daily changes in blood glucose levels,” and, unlike continuous monitoring, “do not provide alarms that indicate when blood glucose levels are above or below various thresholds, and do not indicate trends in blood glucose levels.”
The society commissioned a pooled analysis of 11 randomized trials that showed a 0.3% reduction in HbA1c with real-time glucose monitoring, mostly in patients 15 years or older. Other studies cited by the group also showed better HbA1c control than with finger sticks, without an increased risk of hypoglycemia.
The guideline also suggested insulin pumps for type 1 patients who want greater flexibility and convenience and that insulin pump therapy should continue during hospitalizations. It also suggested encouraging patients to use the embedded bolus calculators in their pumps so long as they “have appropriate education regarding their use and limitations.”
The Endocrine Society funded the work, with cosponsorship from the American Association for Clinical Chemistry, the American Association of Diabetes Educators, and the European Society of Endocrinology. Several of the authors have industry ties to companies that make CGMs or insulin pumps. Dr. Peters is an advisor for Abbott, Becton Dickinson, AstraZeneca, Biodel, Medtronic, and other companies, as well as a speaker, investigator, and advisor for Janssen.
Analysis yields ‘strong evidence’ for benefit of physical activity in NAFLD
Regular physical exercise significantly improved measures of nonalcoholic fatty liver disease independently of dietary changes, according to a meta-analysis of randomized clinical trials published in the October issue of Clinical Gastroenterology and Hepatology.
“On the basis of the current findings, physical activity should be recommended not only in combination with dietary changes but also independently as an effective approach to manage NAFLD,” wrote Lorenzo Orci, MD, and his associates at the University of Geneva. “We propose that the level of evidence surrounding the specific role of physical activity in the management of NAFLD is now sufficient to be awarded a grade of Ia.”
Nonalcoholic fatty liver disease, “the hepatic manifestation of metabolic syndrome,” affects at least one in four U.S. adults and 15%-35% of individuals in Europe, the Middle East, China, and Japan, the researchers noted. Dietary changes are the cornerstone of NAFLD management, and there is less evidence for how physical exercise affects liver fat content. Therefore, the researchers searched MEDLINE, Embase, and the Cochrane databases from inception through October 2015 to find randomized trials of the impact of physical activity on markers of liver steatosis and liver inflammation in patients diagnosed with NAFLD, obesity, type 2 diabetes, or metabolic syndrome. This approach yielded 28 trials with data from more than 1,600 patients. Only two trials were multicenter, 13 required participants to have an NAFLD diagnosis, four focused on type 2 diabetes, and most of the rest included sedentary obese patients without requiring a diagnosis of NAFLD, the researchers said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.036).
After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001). “Because effect sizes such as standard mean difference [SMD] are difficult to interpret, the translation of such a statistical measure into a clinically relevant notion has been the focus of research for more than a decade,” the investigators added. “A commonly used interpretation was proposed by Cohen, who suggested that SMDs of 0.2, 0.5, and 0.8 correspond to small, moderate, and large effect sizes, respectively. By using this rule of thumb, our results indicate that physical activity exerts a moderate-to-large impact on the reduction of intrahepatic lipid content.”
Exercise reduced liver fat content even more in pediatric patients (SMD, –0.75; 95% CI, –0.1 to –0.5; P less than .0001) and in patients who had been specifically diagnosed with NAFLD (SMD, –0.86; 95% CI, –1.26 to –0.46; P less than .0001). Patients with the highest baseline body mass index also seemed to benefit more than patients with lower baseline BMI (P = .04). Indeed, exercise reduced BMI itself by a weighted mean difference of 0.8 (95% CI, –1.22 to 0.38; P less than .001), the researchers noted. Exercise intensity did not seem to affect the likelihood of benefit. There was a trend toward a greater effect of aerobic over resistance training (P = .06), and few studies examined the effects of combining both types of exercise.
The multivariable analysis also linked physical activity to an average 3.30 IU/L drop in alanine aminotransferase levels (95% CI, –5.57 to –1.04) and to a 4.9 IU/L decrease in aspartate aminotransferase levels (95% CI, –8.68 to –1.02). The investigators were unable to assess the long-term effects of physical exercise, nor its effects on hepatic fibrosis or inflammation, they noted. Nonetheless, the moderate to large effect size “provides strong evidence for the recommendation of physical activity as an effective intervention in the treatment of NAFLD,” they concluded. “Physical activity is also associated with an improvement in blood levels of aminotransferases and is particularly beneficial in patients presenting with severe obesity at baseline.”
The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The investigators had no disclosures.
Regular physical exercise significantly improved measures of nonalcoholic fatty liver disease independently of dietary changes, according to a meta-analysis of randomized clinical trials published in the October issue of Clinical Gastroenterology and Hepatology.
“On the basis of the current findings, physical activity should be recommended not only in combination with dietary changes but also independently as an effective approach to manage NAFLD,” wrote Lorenzo Orci, MD, and his associates at the University of Geneva. “We propose that the level of evidence surrounding the specific role of physical activity in the management of NAFLD is now sufficient to be awarded a grade of Ia.”
Nonalcoholic fatty liver disease, “the hepatic manifestation of metabolic syndrome,” affects at least one in four U.S. adults and 15%-35% of individuals in Europe, the Middle East, China, and Japan, the researchers noted. Dietary changes are the cornerstone of NAFLD management, and there is less evidence for how physical exercise affects liver fat content. Therefore, the researchers searched MEDLINE, Embase, and the Cochrane databases from inception through October 2015 to find randomized trials of the impact of physical activity on markers of liver steatosis and liver inflammation in patients diagnosed with NAFLD, obesity, type 2 diabetes, or metabolic syndrome. This approach yielded 28 trials with data from more than 1,600 patients. Only two trials were multicenter, 13 required participants to have an NAFLD diagnosis, four focused on type 2 diabetes, and most of the rest included sedentary obese patients without requiring a diagnosis of NAFLD, the researchers said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.036).
After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001). “Because effect sizes such as standard mean difference [SMD] are difficult to interpret, the translation of such a statistical measure into a clinically relevant notion has been the focus of research for more than a decade,” the investigators added. “A commonly used interpretation was proposed by Cohen, who suggested that SMDs of 0.2, 0.5, and 0.8 correspond to small, moderate, and large effect sizes, respectively. By using this rule of thumb, our results indicate that physical activity exerts a moderate-to-large impact on the reduction of intrahepatic lipid content.”
Exercise reduced liver fat content even more in pediatric patients (SMD, –0.75; 95% CI, –0.1 to –0.5; P less than .0001) and in patients who had been specifically diagnosed with NAFLD (SMD, –0.86; 95% CI, –1.26 to –0.46; P less than .0001). Patients with the highest baseline body mass index also seemed to benefit more than patients with lower baseline BMI (P = .04). Indeed, exercise reduced BMI itself by a weighted mean difference of 0.8 (95% CI, –1.22 to 0.38; P less than .001), the researchers noted. Exercise intensity did not seem to affect the likelihood of benefit. There was a trend toward a greater effect of aerobic over resistance training (P = .06), and few studies examined the effects of combining both types of exercise.
The multivariable analysis also linked physical activity to an average 3.30 IU/L drop in alanine aminotransferase levels (95% CI, –5.57 to –1.04) and to a 4.9 IU/L decrease in aspartate aminotransferase levels (95% CI, –8.68 to –1.02). The investigators were unable to assess the long-term effects of physical exercise, nor its effects on hepatic fibrosis or inflammation, they noted. Nonetheless, the moderate to large effect size “provides strong evidence for the recommendation of physical activity as an effective intervention in the treatment of NAFLD,” they concluded. “Physical activity is also associated with an improvement in blood levels of aminotransferases and is particularly beneficial in patients presenting with severe obesity at baseline.”
The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The investigators had no disclosures.
Regular physical exercise significantly improved measures of nonalcoholic fatty liver disease independently of dietary changes, according to a meta-analysis of randomized clinical trials published in the October issue of Clinical Gastroenterology and Hepatology.
“On the basis of the current findings, physical activity should be recommended not only in combination with dietary changes but also independently as an effective approach to manage NAFLD,” wrote Lorenzo Orci, MD, and his associates at the University of Geneva. “We propose that the level of evidence surrounding the specific role of physical activity in the management of NAFLD is now sufficient to be awarded a grade of Ia.”
Nonalcoholic fatty liver disease, “the hepatic manifestation of metabolic syndrome,” affects at least one in four U.S. adults and 15%-35% of individuals in Europe, the Middle East, China, and Japan, the researchers noted. Dietary changes are the cornerstone of NAFLD management, and there is less evidence for how physical exercise affects liver fat content. Therefore, the researchers searched MEDLINE, Embase, and the Cochrane databases from inception through October 2015 to find randomized trials of the impact of physical activity on markers of liver steatosis and liver inflammation in patients diagnosed with NAFLD, obesity, type 2 diabetes, or metabolic syndrome. This approach yielded 28 trials with data from more than 1,600 patients. Only two trials were multicenter, 13 required participants to have an NAFLD diagnosis, four focused on type 2 diabetes, and most of the rest included sedentary obese patients without requiring a diagnosis of NAFLD, the researchers said (Clin Gastroenterol Hepatol. 2016 May 4. doi: 10.1016/j.cgh.2016.04.036).
After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001). “Because effect sizes such as standard mean difference [SMD] are difficult to interpret, the translation of such a statistical measure into a clinically relevant notion has been the focus of research for more than a decade,” the investigators added. “A commonly used interpretation was proposed by Cohen, who suggested that SMDs of 0.2, 0.5, and 0.8 correspond to small, moderate, and large effect sizes, respectively. By using this rule of thumb, our results indicate that physical activity exerts a moderate-to-large impact on the reduction of intrahepatic lipid content.”
Exercise reduced liver fat content even more in pediatric patients (SMD, –0.75; 95% CI, –0.1 to –0.5; P less than .0001) and in patients who had been specifically diagnosed with NAFLD (SMD, –0.86; 95% CI, –1.26 to –0.46; P less than .0001). Patients with the highest baseline body mass index also seemed to benefit more than patients with lower baseline BMI (P = .04). Indeed, exercise reduced BMI itself by a weighted mean difference of 0.8 (95% CI, –1.22 to 0.38; P less than .001), the researchers noted. Exercise intensity did not seem to affect the likelihood of benefit. There was a trend toward a greater effect of aerobic over resistance training (P = .06), and few studies examined the effects of combining both types of exercise.
The multivariable analysis also linked physical activity to an average 3.30 IU/L drop in alanine aminotransferase levels (95% CI, –5.57 to –1.04) and to a 4.9 IU/L decrease in aspartate aminotransferase levels (95% CI, –8.68 to –1.02). The investigators were unable to assess the long-term effects of physical exercise, nor its effects on hepatic fibrosis or inflammation, they noted. Nonetheless, the moderate to large effect size “provides strong evidence for the recommendation of physical activity as an effective intervention in the treatment of NAFLD,” they concluded. “Physical activity is also associated with an improvement in blood levels of aminotransferases and is particularly beneficial in patients presenting with severe obesity at baseline.”
The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The investigators had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Physical activity benefits measures of nonalcoholic fatty liver disease independently of diet.
Major finding: After researchers accounted for dietary changes, physical activity led to a significant drop in intrahepatic lipid content with a standardized mean difference of –0.69 compared with controls (95% confidence interval, –0.90 to –0.48; P less than .0001).
Data source: A systematic review and meta-analysis of 28 randomized controlled trials comprising more than 16,000 patients.
Disclosures: The work was funded by the Ligue Genevoise contre le Cancer and the Dr Henri Dubois-Ferrière/Dinu Lipatti Foundation and by the Swiss National Science Foundation. The researchers had no disclosures.
CABG best for diabetes patients with CKD – or is it?
ROME – The use of coronary artery bypass graft surgery for revascularization in patients with multivessel CAD and comorbid diabetes plus chronic kidney disease was associated with a significantly lower risk of major cardiovascular and cerebrovascular events than was PCI with first-generation drug-eluting stents in a new secondary analysis from the landmark FREEDOM trial.
“The reason for this presentation is that even though chronic kidney disease is common in patients with diabetes, until now there has not been a large study of the efficacy and safety of coronary revascularization with drug-eluting stents versus CABG in this population in a randomized trial cohort,” explained Usman Baber, MD, who reported the results at the annual congress of the European Society of Cardiology.
FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) randomized 1,900 diabetic patients with multivessel CAD to PCI or CABG. As previously reported, CABG proved superior to PCI, with a significantly lower rate of the composite primary endpoint composed of all-cause mortality, MI, or stroke (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
Dr. Baber presented a post hoc analysis of the 451 FREEDOM participants with baseline comorbid chronic kidney disease (CKD). Their mean SYNTAX score was 27, and their mean baseline estimated glomerular filtration rate was 44 mL/min per 1.73 m2, indicative of mild to moderate CKD.
“Only 28 patients in the FREEDOM trial had an estimated GFR below 30, therefore we can’t make any inferences about revascularization in that setting, which I think is a completely different population,” he noted.
The 5-year rate of major adverse cardiovascular and cerebrovascular events in patients with CKD was 26% in the CABG group, an absolute 9.4% less than the 35.6% rate in subjects randomized to PCI.
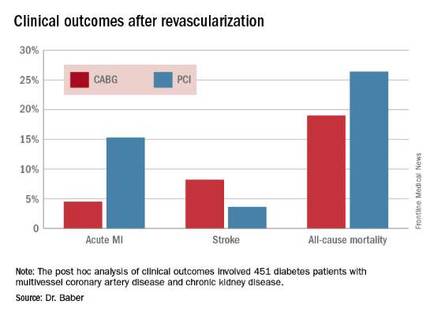
Roughly one-quarter of FREEDOM participants had CKD. They fared significantly worse than did those without CKD. The 5-year incidence of major adverse cardiovascular and cerebrovascular events was 30.8% in patients with CKD and 20.1% in patients without renal impairment. In a multivariate analysis adjusted for age, gender, hypertension, peripheral vascular disease, and other potential confounders, the risk of all-cause mortality was twofold higher in the CKD group. Their risk of cardiac death was increased 1.8-fold, and they were at 1.9-fold increased risk for stroke. Interestingly, however, the acute MI risk did not differ between patients with or without CKD, Dr. Baber observed.
Drilling deeper into the data, the cardiologist reported that CABG was associated with significantly lower rates of MI and a nonsignificant trend for fewer deaths, but with a significantly higher stroke rate than PCI.
One audience member rose to complain that this information won’t be helpful in counseling his diabetic patients with CKD and multivessel CAD because the choices look so grim: a higher risk of MI with percutaneous therapy, and a greater risk of stroke with surgery.
Dr. Baber replied by pointing out that the 10.8% absolute reduction in the risk of MI with CABG compared with PCI was more than twice as large as the absolute 4.6% increase in stroke risk with surgery.
“Most people would say that a heart attack is an inconvenience, and a stroke is a life-changing experience for them and their family,” said session cochair Kim A. Williams, MD, professor of medicine and chairman of cardiology at Rush University Medical Center in Chicago.
At that, Dr. Baber backtracked a bit, observing that since this was a post hoc analysis, the FREEDOM findings in patients with CKD must be viewed as hypothesis-generating rather than definitive. And, of course, contemporary second-generation drug-eluting stents have a better risk/benefit profile than do those used in FREEDOM.
“The number needed to treat/number needed to harm ratio for CABG and PCI probably ends up being roughly equal. The pertinence of an analysis like this is if you look at real-world registry-based data, you find a therapeutic nihilism that’s highly prevalent in CKD patients, where many patients who might benefit are not provided with revascularization therapy. It’s clear that we as clinicians – either because we don’t know there is a benefit or we are too concerned about potential harm – deprive patients of a treatment that might be beneficial. This analysis makes clinicians who might be concerned feel somewhat comforted that there is not unacceptable harm and that there is benefit,” Dr. Baber said.
Follow-up of FREEDOM participants continues and will be the subject of future reports, he added.
The FREEDOM trial was sponsored by the National Heart, Lung and Blood Institute. Dr. Baber reported having no financial conflicts of interest.
ROME – The use of coronary artery bypass graft surgery for revascularization in patients with multivessel CAD and comorbid diabetes plus chronic kidney disease was associated with a significantly lower risk of major cardiovascular and cerebrovascular events than was PCI with first-generation drug-eluting stents in a new secondary analysis from the landmark FREEDOM trial.
“The reason for this presentation is that even though chronic kidney disease is common in patients with diabetes, until now there has not been a large study of the efficacy and safety of coronary revascularization with drug-eluting stents versus CABG in this population in a randomized trial cohort,” explained Usman Baber, MD, who reported the results at the annual congress of the European Society of Cardiology.
FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) randomized 1,900 diabetic patients with multivessel CAD to PCI or CABG. As previously reported, CABG proved superior to PCI, with a significantly lower rate of the composite primary endpoint composed of all-cause mortality, MI, or stroke (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
Dr. Baber presented a post hoc analysis of the 451 FREEDOM participants with baseline comorbid chronic kidney disease (CKD). Their mean SYNTAX score was 27, and their mean baseline estimated glomerular filtration rate was 44 mL/min per 1.73 m2, indicative of mild to moderate CKD.
“Only 28 patients in the FREEDOM trial had an estimated GFR below 30, therefore we can’t make any inferences about revascularization in that setting, which I think is a completely different population,” he noted.
The 5-year rate of major adverse cardiovascular and cerebrovascular events in patients with CKD was 26% in the CABG group, an absolute 9.4% less than the 35.6% rate in subjects randomized to PCI.

Roughly one-quarter of FREEDOM participants had CKD. They fared significantly worse than did those without CKD. The 5-year incidence of major adverse cardiovascular and cerebrovascular events was 30.8% in patients with CKD and 20.1% in patients without renal impairment. In a multivariate analysis adjusted for age, gender, hypertension, peripheral vascular disease, and other potential confounders, the risk of all-cause mortality was twofold higher in the CKD group. Their risk of cardiac death was increased 1.8-fold, and they were at 1.9-fold increased risk for stroke. Interestingly, however, the acute MI risk did not differ between patients with or without CKD, Dr. Baber observed.
Drilling deeper into the data, the cardiologist reported that CABG was associated with significantly lower rates of MI and a nonsignificant trend for fewer deaths, but with a significantly higher stroke rate than PCI.
One audience member rose to complain that this information won’t be helpful in counseling his diabetic patients with CKD and multivessel CAD because the choices look so grim: a higher risk of MI with percutaneous therapy, and a greater risk of stroke with surgery.
Dr. Baber replied by pointing out that the 10.8% absolute reduction in the risk of MI with CABG compared with PCI was more than twice as large as the absolute 4.6% increase in stroke risk with surgery.
“Most people would say that a heart attack is an inconvenience, and a stroke is a life-changing experience for them and their family,” said session cochair Kim A. Williams, MD, professor of medicine and chairman of cardiology at Rush University Medical Center in Chicago.
At that, Dr. Baber backtracked a bit, observing that since this was a post hoc analysis, the FREEDOM findings in patients with CKD must be viewed as hypothesis-generating rather than definitive. And, of course, contemporary second-generation drug-eluting stents have a better risk/benefit profile than do those used in FREEDOM.
“The number needed to treat/number needed to harm ratio for CABG and PCI probably ends up being roughly equal. The pertinence of an analysis like this is if you look at real-world registry-based data, you find a therapeutic nihilism that’s highly prevalent in CKD patients, where many patients who might benefit are not provided with revascularization therapy. It’s clear that we as clinicians – either because we don’t know there is a benefit or we are too concerned about potential harm – deprive patients of a treatment that might be beneficial. This analysis makes clinicians who might be concerned feel somewhat comforted that there is not unacceptable harm and that there is benefit,” Dr. Baber said.
Follow-up of FREEDOM participants continues and will be the subject of future reports, he added.
The FREEDOM trial was sponsored by the National Heart, Lung and Blood Institute. Dr. Baber reported having no financial conflicts of interest.
ROME – The use of coronary artery bypass graft surgery for revascularization in patients with multivessel CAD and comorbid diabetes plus chronic kidney disease was associated with a significantly lower risk of major cardiovascular and cerebrovascular events than was PCI with first-generation drug-eluting stents in a new secondary analysis from the landmark FREEDOM trial.
“The reason for this presentation is that even though chronic kidney disease is common in patients with diabetes, until now there has not been a large study of the efficacy and safety of coronary revascularization with drug-eluting stents versus CABG in this population in a randomized trial cohort,” explained Usman Baber, MD, who reported the results at the annual congress of the European Society of Cardiology.
FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) randomized 1,900 diabetic patients with multivessel CAD to PCI or CABG. As previously reported, CABG proved superior to PCI, with a significantly lower rate of the composite primary endpoint composed of all-cause mortality, MI, or stroke (N Engl J Med. 2012 Dec 20;367[25]:2375-84).
Dr. Baber presented a post hoc analysis of the 451 FREEDOM participants with baseline comorbid chronic kidney disease (CKD). Their mean SYNTAX score was 27, and their mean baseline estimated glomerular filtration rate was 44 mL/min per 1.73 m2, indicative of mild to moderate CKD.
“Only 28 patients in the FREEDOM trial had an estimated GFR below 30, therefore we can’t make any inferences about revascularization in that setting, which I think is a completely different population,” he noted.
The 5-year rate of major adverse cardiovascular and cerebrovascular events in patients with CKD was 26% in the CABG group, an absolute 9.4% less than the 35.6% rate in subjects randomized to PCI.

Roughly one-quarter of FREEDOM participants had CKD. They fared significantly worse than did those without CKD. The 5-year incidence of major adverse cardiovascular and cerebrovascular events was 30.8% in patients with CKD and 20.1% in patients without renal impairment. In a multivariate analysis adjusted for age, gender, hypertension, peripheral vascular disease, and other potential confounders, the risk of all-cause mortality was twofold higher in the CKD group. Their risk of cardiac death was increased 1.8-fold, and they were at 1.9-fold increased risk for stroke. Interestingly, however, the acute MI risk did not differ between patients with or without CKD, Dr. Baber observed.
Drilling deeper into the data, the cardiologist reported that CABG was associated with significantly lower rates of MI and a nonsignificant trend for fewer deaths, but with a significantly higher stroke rate than PCI.
One audience member rose to complain that this information won’t be helpful in counseling his diabetic patients with CKD and multivessel CAD because the choices look so grim: a higher risk of MI with percutaneous therapy, and a greater risk of stroke with surgery.
Dr. Baber replied by pointing out that the 10.8% absolute reduction in the risk of MI with CABG compared with PCI was more than twice as large as the absolute 4.6% increase in stroke risk with surgery.
“Most people would say that a heart attack is an inconvenience, and a stroke is a life-changing experience for them and their family,” said session cochair Kim A. Williams, MD, professor of medicine and chairman of cardiology at Rush University Medical Center in Chicago.
At that, Dr. Baber backtracked a bit, observing that since this was a post hoc analysis, the FREEDOM findings in patients with CKD must be viewed as hypothesis-generating rather than definitive. And, of course, contemporary second-generation drug-eluting stents have a better risk/benefit profile than do those used in FREEDOM.
“The number needed to treat/number needed to harm ratio for CABG and PCI probably ends up being roughly equal. The pertinence of an analysis like this is if you look at real-world registry-based data, you find a therapeutic nihilism that’s highly prevalent in CKD patients, where many patients who might benefit are not provided with revascularization therapy. It’s clear that we as clinicians – either because we don’t know there is a benefit or we are too concerned about potential harm – deprive patients of a treatment that might be beneficial. This analysis makes clinicians who might be concerned feel somewhat comforted that there is not unacceptable harm and that there is benefit,” Dr. Baber said.
Follow-up of FREEDOM participants continues and will be the subject of future reports, he added.
The FREEDOM trial was sponsored by the National Heart, Lung and Blood Institute. Dr. Baber reported having no financial conflicts of interest.
AT THE ESC CONGRESS 2016
Key clinical point: Coronary artery bypass graft surgery resulted in fewer myocardial infarctions but more strokes than did percutaneous coronary intervention at 5 years of follow-up in diabetic patients with multivessel coronary artery disease and chronic kidney disease.
Major finding: The cumulative MI rates in patients randomized to CABG versus PCI were 4.5% and 15.3%, respectively, while the stroke rates were 8.2% versus 3.6%.
Data source: A post hoc analysis of clinical outcomes in 451 diabetic patients with multivessel CAD and chronic kidney disease who were randomized to CABG or PCI in the prospective multicenter FREEDOM trial.
Disclosures: The FREEDOM trial was sponsored by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts of interest.
Aspirin not prescribed appropriately to cut cardiovascular risk in diabetes
MUNICH – Many patients with diabetes who could benefit from low-dose aspirin therapy may not be getting it – and many who are getting aspirin should not be, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
A large, randomized trial concluded that 21% of diabetes patients who qualified for aspirin therapy for cardiovascular risk reduction were not getting it, and that it was contraindicated in almost 60% of those who were taking it, Lauren Crain, PhD, reported at the meeting.
Balancing the risks and benefits of aspirin therapy is not an easy challenge, said Dr. Crain, a health behavior researcher at HealthPartners Institute, Minneapolis. The clinical information necessary for the assessment is “rather lengthy, and not always readily available in primary care settings,” she said, and it’s clear from this study that clinicians could use some help in this area. Unfortunately, the electronic algorithm tested, which was meant to improve appropriate aspirin prescribing, didn’t improve the situation very much.
“At the final visit in the diabetes group [after the algorithm was employed], the total proportion of patients using aspirin was higher than at the first visit,” Dr. Crain noted. “However, that was the case regardless of whether patients were over- or underusing aspirin at the first visit.”
The aspirin findings were part of a large, randomized trial testing the algorithm as a way to reduce cardiovascular risk factors. The study was conducted in 19 primary care practices.
The decision-making algorithm, Cardiovascular Wizard, uses electronic health records to identify and advise patients with uncontrolled cardiovascular risk factors. Priorities and clinical recommendations are displayed for the provider and patient in the hope of facilitating shared decision making, Dr. Crain said.
One of the Wizard’s algorithms concerns aspirin prescribing. It is programmed with data from the United States Preventive Services Task Force, and recommends aspirin if cardiovascular risk scores are high and if consistent with providing a benefit greater than the risk of gastrointestinal bleeding. Aspirin is not recommended if the benefit is determined to be low or if major contraindications are present, including anticoagulant use or history of intracerebral hemorrhage.
The tool also alerts providers to the presence of other potential risks including aspirin allergy or intolerance, history of GI bleeds or risk conditions, and the concomitant use of nonsteroidal anti-inflammatory drugs.
The study comprised 11,000 adults, 4,000 of whom had diabetes. The remainder had high-risk, reversible cardiovascular risk factors (hypertension, dyslipidemia, or tobacco use). Each group was randomized to either cardiovascular risk assessment by usual care or with the Cardiovascular Wizard program.
The aspirin substudy looked at aspirin use at the baseline visit and the patient’s final, 1-year follow-up visit. At both visits, aspirin use was documented, and the clinician used the Wizard to assess whether or not it was indicated.
At the baseline visit, 71% of the diabetes group was using aspirin. However, according to the Wizard tool, more than one-third of them should not have been taking it – and among these, 57% were doing so. Among the remaining two-thirds of patients, all of whom should have been using aspirin, 21% were not taking it, Dr. Crain said.
Among the patients with reversible high-risk factors, 27% were using aspirin. However, according to the Wizard tool, the drug was contraindicated in 34% of those patients. “Most importantly, however, among those for whom aspirin was indicated, only 25% were using it – so, we’re talking about a 75% underusage,” Dr. Crain said.
By the 1-year follow-up visit, the situation was not much changed, despite the tool’s recommendations. Among those with diabetes, 56% in the usual care group and 60% in Wizard group were still overusing aspirin. Underuse was occurring in 21% of the usual care group and 17% of the Wizard group.
Patients with reversible high-risk factors fared a little better at 1 year, especially those who, at baseline, should have been taking aspirin but were not. Among these, 10% in the usual care group and 13% in the Wizard group had started taking aspirin.
The results were a bit of a disappointment, Dr. Crain said, but they don’t invalidate the investigators’ faith in an algorithmic advising system.
“We do think that electronic health record tools like this can help providers follow guidelines and improve the quality of their aspirin recommendations and prescribing, and hopefully reduce cardiovascular events and aspirin-related hazards,” she said. “Unfortunately, that didn’t happen here in the diabetes patients,” and the results in the second group were not stellar.
She added that the Wizard development team will be tweaking the tool to clarify some of the choices available as it guides patients and providers through the algorithm, in hopes of improving its efficacy.
Dr. Crain made no financial disclosures.
On Twitter @alz_gal
MUNICH – Many patients with diabetes who could benefit from low-dose aspirin therapy may not be getting it – and many who are getting aspirin should not be, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
A large, randomized trial concluded that 21% of diabetes patients who qualified for aspirin therapy for cardiovascular risk reduction were not getting it, and that it was contraindicated in almost 60% of those who were taking it, Lauren Crain, PhD, reported at the meeting.
Balancing the risks and benefits of aspirin therapy is not an easy challenge, said Dr. Crain, a health behavior researcher at HealthPartners Institute, Minneapolis. The clinical information necessary for the assessment is “rather lengthy, and not always readily available in primary care settings,” she said, and it’s clear from this study that clinicians could use some help in this area. Unfortunately, the electronic algorithm tested, which was meant to improve appropriate aspirin prescribing, didn’t improve the situation very much.
“At the final visit in the diabetes group [after the algorithm was employed], the total proportion of patients using aspirin was higher than at the first visit,” Dr. Crain noted. “However, that was the case regardless of whether patients were over- or underusing aspirin at the first visit.”
The aspirin findings were part of a large, randomized trial testing the algorithm as a way to reduce cardiovascular risk factors. The study was conducted in 19 primary care practices.
The decision-making algorithm, Cardiovascular Wizard, uses electronic health records to identify and advise patients with uncontrolled cardiovascular risk factors. Priorities and clinical recommendations are displayed for the provider and patient in the hope of facilitating shared decision making, Dr. Crain said.
One of the Wizard’s algorithms concerns aspirin prescribing. It is programmed with data from the United States Preventive Services Task Force, and recommends aspirin if cardiovascular risk scores are high and if consistent with providing a benefit greater than the risk of gastrointestinal bleeding. Aspirin is not recommended if the benefit is determined to be low or if major contraindications are present, including anticoagulant use or history of intracerebral hemorrhage.
The tool also alerts providers to the presence of other potential risks including aspirin allergy or intolerance, history of GI bleeds or risk conditions, and the concomitant use of nonsteroidal anti-inflammatory drugs.
The study comprised 11,000 adults, 4,000 of whom had diabetes. The remainder had high-risk, reversible cardiovascular risk factors (hypertension, dyslipidemia, or tobacco use). Each group was randomized to either cardiovascular risk assessment by usual care or with the Cardiovascular Wizard program.
The aspirin substudy looked at aspirin use at the baseline visit and the patient’s final, 1-year follow-up visit. At both visits, aspirin use was documented, and the clinician used the Wizard to assess whether or not it was indicated.
At the baseline visit, 71% of the diabetes group was using aspirin. However, according to the Wizard tool, more than one-third of them should not have been taking it – and among these, 57% were doing so. Among the remaining two-thirds of patients, all of whom should have been using aspirin, 21% were not taking it, Dr. Crain said.
Among the patients with reversible high-risk factors, 27% were using aspirin. However, according to the Wizard tool, the drug was contraindicated in 34% of those patients. “Most importantly, however, among those for whom aspirin was indicated, only 25% were using it – so, we’re talking about a 75% underusage,” Dr. Crain said.
By the 1-year follow-up visit, the situation was not much changed, despite the tool’s recommendations. Among those with diabetes, 56% in the usual care group and 60% in Wizard group were still overusing aspirin. Underuse was occurring in 21% of the usual care group and 17% of the Wizard group.
Patients with reversible high-risk factors fared a little better at 1 year, especially those who, at baseline, should have been taking aspirin but were not. Among these, 10% in the usual care group and 13% in the Wizard group had started taking aspirin.
The results were a bit of a disappointment, Dr. Crain said, but they don’t invalidate the investigators’ faith in an algorithmic advising system.
“We do think that electronic health record tools like this can help providers follow guidelines and improve the quality of their aspirin recommendations and prescribing, and hopefully reduce cardiovascular events and aspirin-related hazards,” she said. “Unfortunately, that didn’t happen here in the diabetes patients,” and the results in the second group were not stellar.
She added that the Wizard development team will be tweaking the tool to clarify some of the choices available as it guides patients and providers through the algorithm, in hopes of improving its efficacy.
Dr. Crain made no financial disclosures.
On Twitter @alz_gal
MUNICH – Many patients with diabetes who could benefit from low-dose aspirin therapy may not be getting it – and many who are getting aspirin should not be, according to data presented at the annual meeting of the European Association for the Study of Diabetes.
A large, randomized trial concluded that 21% of diabetes patients who qualified for aspirin therapy for cardiovascular risk reduction were not getting it, and that it was contraindicated in almost 60% of those who were taking it, Lauren Crain, PhD, reported at the meeting.
Balancing the risks and benefits of aspirin therapy is not an easy challenge, said Dr. Crain, a health behavior researcher at HealthPartners Institute, Minneapolis. The clinical information necessary for the assessment is “rather lengthy, and not always readily available in primary care settings,” she said, and it’s clear from this study that clinicians could use some help in this area. Unfortunately, the electronic algorithm tested, which was meant to improve appropriate aspirin prescribing, didn’t improve the situation very much.
“At the final visit in the diabetes group [after the algorithm was employed], the total proportion of patients using aspirin was higher than at the first visit,” Dr. Crain noted. “However, that was the case regardless of whether patients were over- or underusing aspirin at the first visit.”
The aspirin findings were part of a large, randomized trial testing the algorithm as a way to reduce cardiovascular risk factors. The study was conducted in 19 primary care practices.
The decision-making algorithm, Cardiovascular Wizard, uses electronic health records to identify and advise patients with uncontrolled cardiovascular risk factors. Priorities and clinical recommendations are displayed for the provider and patient in the hope of facilitating shared decision making, Dr. Crain said.
One of the Wizard’s algorithms concerns aspirin prescribing. It is programmed with data from the United States Preventive Services Task Force, and recommends aspirin if cardiovascular risk scores are high and if consistent with providing a benefit greater than the risk of gastrointestinal bleeding. Aspirin is not recommended if the benefit is determined to be low or if major contraindications are present, including anticoagulant use or history of intracerebral hemorrhage.
The tool also alerts providers to the presence of other potential risks including aspirin allergy or intolerance, history of GI bleeds or risk conditions, and the concomitant use of nonsteroidal anti-inflammatory drugs.
The study comprised 11,000 adults, 4,000 of whom had diabetes. The remainder had high-risk, reversible cardiovascular risk factors (hypertension, dyslipidemia, or tobacco use). Each group was randomized to either cardiovascular risk assessment by usual care or with the Cardiovascular Wizard program.
The aspirin substudy looked at aspirin use at the baseline visit and the patient’s final, 1-year follow-up visit. At both visits, aspirin use was documented, and the clinician used the Wizard to assess whether or not it was indicated.
At the baseline visit, 71% of the diabetes group was using aspirin. However, according to the Wizard tool, more than one-third of them should not have been taking it – and among these, 57% were doing so. Among the remaining two-thirds of patients, all of whom should have been using aspirin, 21% were not taking it, Dr. Crain said.
Among the patients with reversible high-risk factors, 27% were using aspirin. However, according to the Wizard tool, the drug was contraindicated in 34% of those patients. “Most importantly, however, among those for whom aspirin was indicated, only 25% were using it – so, we’re talking about a 75% underusage,” Dr. Crain said.
By the 1-year follow-up visit, the situation was not much changed, despite the tool’s recommendations. Among those with diabetes, 56% in the usual care group and 60% in Wizard group were still overusing aspirin. Underuse was occurring in 21% of the usual care group and 17% of the Wizard group.
Patients with reversible high-risk factors fared a little better at 1 year, especially those who, at baseline, should have been taking aspirin but were not. Among these, 10% in the usual care group and 13% in the Wizard group had started taking aspirin.
The results were a bit of a disappointment, Dr. Crain said, but they don’t invalidate the investigators’ faith in an algorithmic advising system.
“We do think that electronic health record tools like this can help providers follow guidelines and improve the quality of their aspirin recommendations and prescribing, and hopefully reduce cardiovascular events and aspirin-related hazards,” she said. “Unfortunately, that didn’t happen here in the diabetes patients,” and the results in the second group were not stellar.
She added that the Wizard development team will be tweaking the tool to clarify some of the choices available as it guides patients and providers through the algorithm, in hopes of improving its efficacy.
Dr. Crain made no financial disclosures.
On Twitter @alz_gal
AT EASD 2016
Key clinical point: Many diabetes patients who should be taking aspirin for cardiovascular risk reduction are not doing so, and many who should not be taking it are.
Major finding: Aspirin was underused in 21% of diabetes patients and overused in 57% of patients.
Data source: A randomized study of 11,000 patients.
Disclosures: Dr. Lauren Crain had no financial disclosures.
Cutting routine glucometer readings saves time and money
Eliminating routine glucometer readings makes primary care visits more efficient, according to a 6-month review of activity at a primary care clinic. The results were published online Sept. 26 in JAMA Internal Medicine.
“The routine tasks that are components of rooming the clinic patient are increasing in number,” wrote James L Wofford, MD, of Wake Forest University, Winston-Salem, N.C., and his colleagues (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.5769). Routine glucometer readings are costly in terms of time, money, and mental energy of clinicians, and do not add value, they wrote.
The researchers compared data from a primary care clinic in North Carolina for 3 months before and 3 months after the clinic eliminated routine glucometer readings (Jan. 1, 2015, to March 15, 2015, and March 16, 2015, to June 30, 2015). After a 1-week trial during which no routine clinical glucometry was performed, the option remained available at the request of a nurse or patient.
The number of glucometer readings decreased from approximately 400 per month to 100 per month after the change in policy, yielded a cost savings of at least $2,000 per month, and time saving of 25 hours of nursing time per month.
“Despite the fear of missing an occasional markedly elevated glucose level, clinicians gradually grew comfortable and never reconsidered reinstitution of routine glucometer readings,” Dr. Wofford and his associates wrote. However, some patient education was needed to reassure those who were disappointed by the change.
“As important as the lesson that routine glucometer readings in the clinic is a wasteful practice, the more important lesson is that examining office routines for foolish consistencies should be a regular component of making primary care more efficient,” the researchers added.
They had no financial conflicts to disclose.
Cutting down on unnecessary procedures allows primary care physicians to optimize their time with patients, and curbing routine glucose testing saves time and money, Adam J. Schoenfeld, MD, and Patrick G. O’Malley, MD, wrote in an editorial.
“Changing this policy to leave glucose testing to the discretion of the patient and nurse resulted in a decrease of 300 glucometer tests per month, saving the clinic $2, 000 and 25 hours of nursing time,” they wrote.
“Patients without infectious symptoms likely do not need their temperature taken, and we probably do not need to elicit a pain severity scale in patients without an active complaint of pain,” they noted. “Instead, other issues could take priority, such as medication reconciliation; collection of important psychosocial information, such as screening for commonly undiagnosed illnesses like depression; discussing advanced directives; and preparing the patient to be more active and engaged in addressing their agenda for the visit.”
Dr. Schoenfeld is affiliated with the University of California, San Francisco, and Dr. O’Malley is associated with the Uniformed Services University, Bethesda, Md. They had no financial conflicts to disclose.
Cutting down on unnecessary procedures allows primary care physicians to optimize their time with patients, and curbing routine glucose testing saves time and money, Adam J. Schoenfeld, MD, and Patrick G. O’Malley, MD, wrote in an editorial.
“Changing this policy to leave glucose testing to the discretion of the patient and nurse resulted in a decrease of 300 glucometer tests per month, saving the clinic $2, 000 and 25 hours of nursing time,” they wrote.
“Patients without infectious symptoms likely do not need their temperature taken, and we probably do not need to elicit a pain severity scale in patients without an active complaint of pain,” they noted. “Instead, other issues could take priority, such as medication reconciliation; collection of important psychosocial information, such as screening for commonly undiagnosed illnesses like depression; discussing advanced directives; and preparing the patient to be more active and engaged in addressing their agenda for the visit.”
Dr. Schoenfeld is affiliated with the University of California, San Francisco, and Dr. O’Malley is associated with the Uniformed Services University, Bethesda, Md. They had no financial conflicts to disclose.
Cutting down on unnecessary procedures allows primary care physicians to optimize their time with patients, and curbing routine glucose testing saves time and money, Adam J. Schoenfeld, MD, and Patrick G. O’Malley, MD, wrote in an editorial.
“Changing this policy to leave glucose testing to the discretion of the patient and nurse resulted in a decrease of 300 glucometer tests per month, saving the clinic $2, 000 and 25 hours of nursing time,” they wrote.
“Patients without infectious symptoms likely do not need their temperature taken, and we probably do not need to elicit a pain severity scale in patients without an active complaint of pain,” they noted. “Instead, other issues could take priority, such as medication reconciliation; collection of important psychosocial information, such as screening for commonly undiagnosed illnesses like depression; discussing advanced directives; and preparing the patient to be more active and engaged in addressing their agenda for the visit.”
Dr. Schoenfeld is affiliated with the University of California, San Francisco, and Dr. O’Malley is associated with the Uniformed Services University, Bethesda, Md. They had no financial conflicts to disclose.
Eliminating routine glucometer readings makes primary care visits more efficient, according to a 6-month review of activity at a primary care clinic. The results were published online Sept. 26 in JAMA Internal Medicine.
“The routine tasks that are components of rooming the clinic patient are increasing in number,” wrote James L Wofford, MD, of Wake Forest University, Winston-Salem, N.C., and his colleagues (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.5769). Routine glucometer readings are costly in terms of time, money, and mental energy of clinicians, and do not add value, they wrote.
The researchers compared data from a primary care clinic in North Carolina for 3 months before and 3 months after the clinic eliminated routine glucometer readings (Jan. 1, 2015, to March 15, 2015, and March 16, 2015, to June 30, 2015). After a 1-week trial during which no routine clinical glucometry was performed, the option remained available at the request of a nurse or patient.
The number of glucometer readings decreased from approximately 400 per month to 100 per month after the change in policy, yielded a cost savings of at least $2,000 per month, and time saving of 25 hours of nursing time per month.
“Despite the fear of missing an occasional markedly elevated glucose level, clinicians gradually grew comfortable and never reconsidered reinstitution of routine glucometer readings,” Dr. Wofford and his associates wrote. However, some patient education was needed to reassure those who were disappointed by the change.
“As important as the lesson that routine glucometer readings in the clinic is a wasteful practice, the more important lesson is that examining office routines for foolish consistencies should be a regular component of making primary care more efficient,” the researchers added.
They had no financial conflicts to disclose.
Eliminating routine glucometer readings makes primary care visits more efficient, according to a 6-month review of activity at a primary care clinic. The results were published online Sept. 26 in JAMA Internal Medicine.
“The routine tasks that are components of rooming the clinic patient are increasing in number,” wrote James L Wofford, MD, of Wake Forest University, Winston-Salem, N.C., and his colleagues (JAMA Intern Med. 2016 Sep 26. doi: 10.1001/jamainternmed.2016.5769). Routine glucometer readings are costly in terms of time, money, and mental energy of clinicians, and do not add value, they wrote.
The researchers compared data from a primary care clinic in North Carolina for 3 months before and 3 months after the clinic eliminated routine glucometer readings (Jan. 1, 2015, to March 15, 2015, and March 16, 2015, to June 30, 2015). After a 1-week trial during which no routine clinical glucometry was performed, the option remained available at the request of a nurse or patient.
The number of glucometer readings decreased from approximately 400 per month to 100 per month after the change in policy, yielded a cost savings of at least $2,000 per month, and time saving of 25 hours of nursing time per month.
“Despite the fear of missing an occasional markedly elevated glucose level, clinicians gradually grew comfortable and never reconsidered reinstitution of routine glucometer readings,” Dr. Wofford and his associates wrote. However, some patient education was needed to reassure those who were disappointed by the change.
“As important as the lesson that routine glucometer readings in the clinic is a wasteful practice, the more important lesson is that examining office routines for foolish consistencies should be a regular component of making primary care more efficient,” the researchers added.
They had no financial conflicts to disclose.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Routine glucometer readings are unnecessary as part of a primary care visit.
Major finding: Decreasing glucometer readings from approximately 400 per month to approximately 100 per month saved more than $2,000 and 25 hours of nursing time.
Data source: A comparison of 3 months before and after a policy change eliminating routine glucometer readings in a primary care clinic.
Disclosures: The researchers had no financial conflicts to disclose.
FDA approves first-line combo therapy for type 2 diabetes
The Food and Drug Administration approved an extended-release combination of canagliflozin and metformin for first-line use as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes, according to Janssen Pharmaceuticals.
Once-daily Invokamet XR combines canagliflozin (Invokana) and an extended-release formulation of metformin. Studies in healthy adults have shown that Invokamet XR results in the same levels of canagliflozin and metformin in the body as when corresponding dosages of the two medicines are administered as separate tablets.
Phase III studies showed that using canagliflozin and metformin lowered blood sugar and, in prespecified secondary endpoints, was linked to greater reductions in body weight and systolic blood pressure.
Invokamet XR is available with 50 mg or 150 mg of canagliflozin, and 500 mg or 1,000 mg of extended-release metformin. Invokamet XR contains a boxed warning regarding the risk of lactic acidosis.
Read the full company statement here.
The Food and Drug Administration approved an extended-release combination of canagliflozin and metformin for first-line use as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes, according to Janssen Pharmaceuticals.
Once-daily Invokamet XR combines canagliflozin (Invokana) and an extended-release formulation of metformin. Studies in healthy adults have shown that Invokamet XR results in the same levels of canagliflozin and metformin in the body as when corresponding dosages of the two medicines are administered as separate tablets.
Phase III studies showed that using canagliflozin and metformin lowered blood sugar and, in prespecified secondary endpoints, was linked to greater reductions in body weight and systolic blood pressure.
Invokamet XR is available with 50 mg or 150 mg of canagliflozin, and 500 mg or 1,000 mg of extended-release metformin. Invokamet XR contains a boxed warning regarding the risk of lactic acidosis.
Read the full company statement here.
The Food and Drug Administration approved an extended-release combination of canagliflozin and metformin for first-line use as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes, according to Janssen Pharmaceuticals.
Once-daily Invokamet XR combines canagliflozin (Invokana) and an extended-release formulation of metformin. Studies in healthy adults have shown that Invokamet XR results in the same levels of canagliflozin and metformin in the body as when corresponding dosages of the two medicines are administered as separate tablets.
Phase III studies showed that using canagliflozin and metformin lowered blood sugar and, in prespecified secondary endpoints, was linked to greater reductions in body weight and systolic blood pressure.
Invokamet XR is available with 50 mg or 150 mg of canagliflozin, and 500 mg or 1,000 mg of extended-release metformin. Invokamet XR contains a boxed warning regarding the risk of lactic acidosis.
Read the full company statement here.
Patient-reported outcomes tied to long-term outcomes in bariatric surgery
Clinical outcomes of surgery and patient-reported outcomes of function, disability, and health status are two different measures of surgical success.
A large study of patients who had bariatric surgery showed that patient-reported outcomes were correlated with long-term weight loss but not with short-term complication rates. In addition, obesity-specific patient-reported quality of life scores were associated with a reduction in medications required for the treatment of obesity-related conditions.
“Clinical outcomes, such as perioperative morbidity and mortality, are commonly used to benchmark hospital performance,” reported Jennifer F. Waljee, MD, and her associates at the University of Michigan, Ann Arbor (Ann Surg. 2016. doi: 10.1097/SLA.0000000000001852).
“However, for many surgical procedures, such as bariatric surgery ... complications may be rare, and may not entirely reflect treatment effectiveness. Alternatively, patient-reported measures of function, disability, and health status may offer a unique and more reliable assessment of provider quality and performance,” she explained. Yet despite growing interest in using patient-reported measures, many important questions regarding their accuracy, applicability, and clinical utility remain. The purpose of this study was, therefore, to evaluate how patient-reported quality of life measures compared to short-term and long-term clinical outcomes in patients who underwent bariatric surgery.
The majority of the study’s 11,420 participants were female (79.8%), were white (84.1%), and underwent Roux-en-Y laparoscopic gastric bypass (56.8%). For each study participant, both short-term and long-term clinical outcome measures were obtained from medical board review. Short-term clinical outcomes were defined as the rate of perioperative complications within 30 days of bariatric surgery. Percent excess weight loss at 1 year post surgery was used as a long-term clinical outcome.
In addition, two patient-reported outcomes were collected: an overall health-related quality of life score called the Health and Activities Limitations Index (HALex) and an obesity-specific quality of life score, the Bariatric Quality of Life (BQL) index, which measures well-being, social and physical functioning, and obesity-related symptoms.
Multivariate and linear regression models demonstrated that short-term complication rates were not correlated to the overall patient-reported quality of life score (P = .32) or to the obesity-specific BQL score (P = .74).
However, the long-term measure of excess weight loss at 1 year post surgery was significantly associated with both overall and obesity-specific patient-reported measures of health-related quality of life (P less than .002 and P less than .001 respectively).
Moreover, scores indicating improved quality of life were associated with greater weight loss.
Finally, comorbidity resolution, estimated by the reduction in the use of medications taken to treat conditions related to obesity, was significantly associated with the obesity-specific measure, BQL, but not the overall quality of life measure, HALex.
“In conclusion, [patient-reported outcomes] are distinct from clinical outcomes,” investigators wrote. Patient-reported outcomes “provide an opportunity for improved population-based cost-effectiveness analyses using outcomes germane to procedures performed for symptomatology and improving QOL,” they added.
The Agency for Healthcare Research and Quality supported the research. The investigators reported having no disclosures.
On Twitter @jessnicolecraig
Clinical outcomes of surgery and patient-reported outcomes of function, disability, and health status are two different measures of surgical success.
A large study of patients who had bariatric surgery showed that patient-reported outcomes were correlated with long-term weight loss but not with short-term complication rates. In addition, obesity-specific patient-reported quality of life scores were associated with a reduction in medications required for the treatment of obesity-related conditions.
“Clinical outcomes, such as perioperative morbidity and mortality, are commonly used to benchmark hospital performance,” reported Jennifer F. Waljee, MD, and her associates at the University of Michigan, Ann Arbor (Ann Surg. 2016. doi: 10.1097/SLA.0000000000001852).
“However, for many surgical procedures, such as bariatric surgery ... complications may be rare, and may not entirely reflect treatment effectiveness. Alternatively, patient-reported measures of function, disability, and health status may offer a unique and more reliable assessment of provider quality and performance,” she explained. Yet despite growing interest in using patient-reported measures, many important questions regarding their accuracy, applicability, and clinical utility remain. The purpose of this study was, therefore, to evaluate how patient-reported quality of life measures compared to short-term and long-term clinical outcomes in patients who underwent bariatric surgery.
The majority of the study’s 11,420 participants were female (79.8%), were white (84.1%), and underwent Roux-en-Y laparoscopic gastric bypass (56.8%). For each study participant, both short-term and long-term clinical outcome measures were obtained from medical board review. Short-term clinical outcomes were defined as the rate of perioperative complications within 30 days of bariatric surgery. Percent excess weight loss at 1 year post surgery was used as a long-term clinical outcome.
In addition, two patient-reported outcomes were collected: an overall health-related quality of life score called the Health and Activities Limitations Index (HALex) and an obesity-specific quality of life score, the Bariatric Quality of Life (BQL) index, which measures well-being, social and physical functioning, and obesity-related symptoms.
Multivariate and linear regression models demonstrated that short-term complication rates were not correlated to the overall patient-reported quality of life score (P = .32) or to the obesity-specific BQL score (P = .74).
However, the long-term measure of excess weight loss at 1 year post surgery was significantly associated with both overall and obesity-specific patient-reported measures of health-related quality of life (P less than .002 and P less than .001 respectively).
Moreover, scores indicating improved quality of life were associated with greater weight loss.
Finally, comorbidity resolution, estimated by the reduction in the use of medications taken to treat conditions related to obesity, was significantly associated with the obesity-specific measure, BQL, but not the overall quality of life measure, HALex.
“In conclusion, [patient-reported outcomes] are distinct from clinical outcomes,” investigators wrote. Patient-reported outcomes “provide an opportunity for improved population-based cost-effectiveness analyses using outcomes germane to procedures performed for symptomatology and improving QOL,” they added.
The Agency for Healthcare Research and Quality supported the research. The investigators reported having no disclosures.
On Twitter @jessnicolecraig
Clinical outcomes of surgery and patient-reported outcomes of function, disability, and health status are two different measures of surgical success.
A large study of patients who had bariatric surgery showed that patient-reported outcomes were correlated with long-term weight loss but not with short-term complication rates. In addition, obesity-specific patient-reported quality of life scores were associated with a reduction in medications required for the treatment of obesity-related conditions.
“Clinical outcomes, such as perioperative morbidity and mortality, are commonly used to benchmark hospital performance,” reported Jennifer F. Waljee, MD, and her associates at the University of Michigan, Ann Arbor (Ann Surg. 2016. doi: 10.1097/SLA.0000000000001852).
“However, for many surgical procedures, such as bariatric surgery ... complications may be rare, and may not entirely reflect treatment effectiveness. Alternatively, patient-reported measures of function, disability, and health status may offer a unique and more reliable assessment of provider quality and performance,” she explained. Yet despite growing interest in using patient-reported measures, many important questions regarding their accuracy, applicability, and clinical utility remain. The purpose of this study was, therefore, to evaluate how patient-reported quality of life measures compared to short-term and long-term clinical outcomes in patients who underwent bariatric surgery.
The majority of the study’s 11,420 participants were female (79.8%), were white (84.1%), and underwent Roux-en-Y laparoscopic gastric bypass (56.8%). For each study participant, both short-term and long-term clinical outcome measures were obtained from medical board review. Short-term clinical outcomes were defined as the rate of perioperative complications within 30 days of bariatric surgery. Percent excess weight loss at 1 year post surgery was used as a long-term clinical outcome.
In addition, two patient-reported outcomes were collected: an overall health-related quality of life score called the Health and Activities Limitations Index (HALex) and an obesity-specific quality of life score, the Bariatric Quality of Life (BQL) index, which measures well-being, social and physical functioning, and obesity-related symptoms.
Multivariate and linear regression models demonstrated that short-term complication rates were not correlated to the overall patient-reported quality of life score (P = .32) or to the obesity-specific BQL score (P = .74).
However, the long-term measure of excess weight loss at 1 year post surgery was significantly associated with both overall and obesity-specific patient-reported measures of health-related quality of life (P less than .002 and P less than .001 respectively).
Moreover, scores indicating improved quality of life were associated with greater weight loss.
Finally, comorbidity resolution, estimated by the reduction in the use of medications taken to treat conditions related to obesity, was significantly associated with the obesity-specific measure, BQL, but not the overall quality of life measure, HALex.
“In conclusion, [patient-reported outcomes] are distinct from clinical outcomes,” investigators wrote. Patient-reported outcomes “provide an opportunity for improved population-based cost-effectiveness analyses using outcomes germane to procedures performed for symptomatology and improving QOL,” they added.
The Agency for Healthcare Research and Quality supported the research. The investigators reported having no disclosures.
On Twitter @jessnicolecraig
FROM ANNALS OF SURGERY
Key clinical point: Patient-reported quality of life measures were associated with long-term but not short-term clinical outcomes.
Major finding: Overall and obesity-specific patient-reported quality of life scores were associated with long-term excess weight loss (P less than .002 and P less than .001 respectively).
Data source: A retrospective study of 11,420 patients who underwent bariatric surgery.
Disclosures: The Agency for Healthcare Research and Quality supported the study. The investigators reported having no disclosures.
ESC’s new lipid guidelines keep LDL-cholesterol targets
ROME – LDL cholesterol treatment targets remain alive and well in non-U.S. lipid management guidelines.
New dyslipidemia management guidelines from the European Society of Cardiology, issued in late August, retain the same LDL cholesterol targets as the prior, 2011 guidelines, a sharp and purposeful departure from the “risk-based” U.S. guidelines introduced in 2013 that eliminated treating patients to specific LDL cholesterol targets.
The new ESC guidelines also incorporated the new class of lipid-lowering drugs, PCSK9 inhibitors (evolocumab [Repatha] and alirocumab [Praluent]), into the treatment algorithm, and carved out a role for ezetimibe (Zetia) following its proven success as an add-on agent to statins (Eur Heart J. 2016. doi: 10.1093/eurheartj/ehw272).
But it’s retention of LDL cholesterol targets as a cornerstone of dyslipidemia management in the new ESC report, written jointly with the European Atherosclerosis Society, that especially distinguishes the new guidelines.
“It seemed to us logical that if you have drugs [statins] that lower LDL cholesterol, then you target LDL cholesterol,” explained Ian M. Graham, MD, professor of cardiovascular medicine at Trinity College in Dublin and cochair of the guidelines panel. “If a patient’s risk is high, they still need to lower LDL cholesterol. It’s not really contradictory to the U.S. approach,” Dr. Graham said in an interview.
The ESC panel’s discussions about the LDL cholesterol targets were “difficult and long,” said Guy De Backer, MD, a member of the guidelines committee and professor of cardiology at the University of Ghent, Belgium, in a session devoted to the new guidelines during the annual congress of the ESC.
The ESC’s decision to retain the 2011 LDL cholesterol targets won praise from U.S. cardiologist Eugene Braunwald, MD. “I think that not measuring and following LDL cholesterol is silly. I don’t agree” with current U.S. guidelines, he said in a talk during the congress. “If you don’t follow LDL cholesterol then you don’t know a patient’s compliance.
Regularly measuring LDL cholesterol is important,” said Dr. Braunwald, professor of medicine at Harvard Medical School in Boston. But he questioned the LDL cholesterol targets set by the ESC panel, specifically the LDL cholesterol goal of less than 70 mg/dL for very-high-risk patients.
“I don’t think the ESC went low enough; the goal should be less than 50 mg/dL,” declared Dr. Braunwald, who added “anything above 50 mg/dL is toxic.”
The new guidelines also made the definition of “very-high-risk” patients “stricter and more precise,” said Alberico L. Catapano, MD, cochair of the panel and professor of pharmacology at the University of Milan.
The guideline’s detailed list defining very-high-risk patients includes those with either clinical or “unequivocal” imaging evidence for cardiovascular disease, as well as patients with diabetes and target-organ damage, severe chronic kidney disease, or a 10% or greater 10-year risk for fatal cardiovascular disease calculated by the European Score risk formula.
The potent lipid-lowering PCSK9 inhibitors came onto the U.S. and European markets in 2015, labeled in the United States specifically for patients with familial hypercholesterolemia.
In July 2016, an American College of Cardiology Task Force issued a model clinical-decision pathway for lowering LDL cholesterol levels that for the first time included the PCSK9 inhibitors, specified as a “may be considered” option for selected patients (J Am Coll Cardiol. 2016 Jul;68[1]:92-125). This consensus decision pathway was not a set of actual guidelines, which means the ESC revision is the first guideline to include the PCSK9 inhibitors. The panel took the same stance as the U.S. decision pathway and designated the PCSK9 inhibitors as a “may be considered” option.
Dr. Graham explained that this designation was driven by the current absence of evidence for clinical benefit from the large lipid-lowering effect of the PCSK9 antibodies, although trial results that address this are expected to appear very soon. Experts not on the guidelines panel agreed with this decision.
“We know that it’s crucial to await results from the clinical endpoint studies” before the guidelines committee makes a more forceful recommendation, commented Erik S.G. Stroes, MD, professor of vascular medicine at the Academic Medical Center in Amsterdam. He added, however, that many clinicians, himself included, have been pleased to offer PCSK9-inhibitor treatment to very-high-risk patients with no good alternatives for effectively lowering their LDL cholesterol to target levels, such as statin-intolerant patients or those with LDL cholesterol levels that remain high despite maximal therapy.
The current ESC guideline’s statement on when to use a PCSK9 inhibitor “is vague” said Dr. Braunwald. “Within the next year, we’ll have results from two huge trials that will show clinical outcomes. We know that PCSK9 inhibitors are extremely powerful at lowering LDL cholesterol, but I would like to see the loop closed” with proven effects on clinical outcomes. “I would bet 100 to 1 that they will be effective, but LDL cholesterol is just a surrogate marker, and you need to look in large populations before you make a guideline recommendation to use these drugs, so we’ll wait.”
Once the clinical value of treatment with PCSK9 inhibitors is settled, the next issue will be the cost of these drugs, something that will become a big consideration once they become more widely used, Dr. Braunwald added.
Dr. De Backer and Dr. Graham had no disclosures. Dr. Catapano has been a consultant to Aegerion, Amgen, AstraZeneca, Merck, Pfizer, and Sigma-Tau. Dr. Braunwald has been a consultant to Bayer, Daiichi Sankyo, the Medicines Company, Merck, Novartis, and Sanofi. Dr. Stroes has been a consultant to Amgen, Bristol-Myers Squibb, Merck, and Sanofi.
On Twitter @mitchelzoler
ROME – LDL cholesterol treatment targets remain alive and well in non-U.S. lipid management guidelines.
New dyslipidemia management guidelines from the European Society of Cardiology, issued in late August, retain the same LDL cholesterol targets as the prior, 2011 guidelines, a sharp and purposeful departure from the “risk-based” U.S. guidelines introduced in 2013 that eliminated treating patients to specific LDL cholesterol targets.
The new ESC guidelines also incorporated the new class of lipid-lowering drugs, PCSK9 inhibitors (evolocumab [Repatha] and alirocumab [Praluent]), into the treatment algorithm, and carved out a role for ezetimibe (Zetia) following its proven success as an add-on agent to statins (Eur Heart J. 2016. doi: 10.1093/eurheartj/ehw272).
But it’s retention of LDL cholesterol targets as a cornerstone of dyslipidemia management in the new ESC report, written jointly with the European Atherosclerosis Society, that especially distinguishes the new guidelines.
“It seemed to us logical that if you have drugs [statins] that lower LDL cholesterol, then you target LDL cholesterol,” explained Ian M. Graham, MD, professor of cardiovascular medicine at Trinity College in Dublin and cochair of the guidelines panel. “If a patient’s risk is high, they still need to lower LDL cholesterol. It’s not really contradictory to the U.S. approach,” Dr. Graham said in an interview.
The ESC panel’s discussions about the LDL cholesterol targets were “difficult and long,” said Guy De Backer, MD, a member of the guidelines committee and professor of cardiology at the University of Ghent, Belgium, in a session devoted to the new guidelines during the annual congress of the ESC.
The ESC’s decision to retain the 2011 LDL cholesterol targets won praise from U.S. cardiologist Eugene Braunwald, MD. “I think that not measuring and following LDL cholesterol is silly. I don’t agree” with current U.S. guidelines, he said in a talk during the congress. “If you don’t follow LDL cholesterol then you don’t know a patient’s compliance.
Regularly measuring LDL cholesterol is important,” said Dr. Braunwald, professor of medicine at Harvard Medical School in Boston. But he questioned the LDL cholesterol targets set by the ESC panel, specifically the LDL cholesterol goal of less than 70 mg/dL for very-high-risk patients.
“I don’t think the ESC went low enough; the goal should be less than 50 mg/dL,” declared Dr. Braunwald, who added “anything above 50 mg/dL is toxic.”
The new guidelines also made the definition of “very-high-risk” patients “stricter and more precise,” said Alberico L. Catapano, MD, cochair of the panel and professor of pharmacology at the University of Milan.
The guideline’s detailed list defining very-high-risk patients includes those with either clinical or “unequivocal” imaging evidence for cardiovascular disease, as well as patients with diabetes and target-organ damage, severe chronic kidney disease, or a 10% or greater 10-year risk for fatal cardiovascular disease calculated by the European Score risk formula.
The potent lipid-lowering PCSK9 inhibitors came onto the U.S. and European markets in 2015, labeled in the United States specifically for patients with familial hypercholesterolemia.
In July 2016, an American College of Cardiology Task Force issued a model clinical-decision pathway for lowering LDL cholesterol levels that for the first time included the PCSK9 inhibitors, specified as a “may be considered” option for selected patients (J Am Coll Cardiol. 2016 Jul;68[1]:92-125). This consensus decision pathway was not a set of actual guidelines, which means the ESC revision is the first guideline to include the PCSK9 inhibitors. The panel took the same stance as the U.S. decision pathway and designated the PCSK9 inhibitors as a “may be considered” option.
Dr. Graham explained that this designation was driven by the current absence of evidence for clinical benefit from the large lipid-lowering effect of the PCSK9 antibodies, although trial results that address this are expected to appear very soon. Experts not on the guidelines panel agreed with this decision.
“We know that it’s crucial to await results from the clinical endpoint studies” before the guidelines committee makes a more forceful recommendation, commented Erik S.G. Stroes, MD, professor of vascular medicine at the Academic Medical Center in Amsterdam. He added, however, that many clinicians, himself included, have been pleased to offer PCSK9-inhibitor treatment to very-high-risk patients with no good alternatives for effectively lowering their LDL cholesterol to target levels, such as statin-intolerant patients or those with LDL cholesterol levels that remain high despite maximal therapy.
The current ESC guideline’s statement on when to use a PCSK9 inhibitor “is vague” said Dr. Braunwald. “Within the next year, we’ll have results from two huge trials that will show clinical outcomes. We know that PCSK9 inhibitors are extremely powerful at lowering LDL cholesterol, but I would like to see the loop closed” with proven effects on clinical outcomes. “I would bet 100 to 1 that they will be effective, but LDL cholesterol is just a surrogate marker, and you need to look in large populations before you make a guideline recommendation to use these drugs, so we’ll wait.”
Once the clinical value of treatment with PCSK9 inhibitors is settled, the next issue will be the cost of these drugs, something that will become a big consideration once they become more widely used, Dr. Braunwald added.
Dr. De Backer and Dr. Graham had no disclosures. Dr. Catapano has been a consultant to Aegerion, Amgen, AstraZeneca, Merck, Pfizer, and Sigma-Tau. Dr. Braunwald has been a consultant to Bayer, Daiichi Sankyo, the Medicines Company, Merck, Novartis, and Sanofi. Dr. Stroes has been a consultant to Amgen, Bristol-Myers Squibb, Merck, and Sanofi.
On Twitter @mitchelzoler
ROME – LDL cholesterol treatment targets remain alive and well in non-U.S. lipid management guidelines.
New dyslipidemia management guidelines from the European Society of Cardiology, issued in late August, retain the same LDL cholesterol targets as the prior, 2011 guidelines, a sharp and purposeful departure from the “risk-based” U.S. guidelines introduced in 2013 that eliminated treating patients to specific LDL cholesterol targets.
The new ESC guidelines also incorporated the new class of lipid-lowering drugs, PCSK9 inhibitors (evolocumab [Repatha] and alirocumab [Praluent]), into the treatment algorithm, and carved out a role for ezetimibe (Zetia) following its proven success as an add-on agent to statins (Eur Heart J. 2016. doi: 10.1093/eurheartj/ehw272).
But it’s retention of LDL cholesterol targets as a cornerstone of dyslipidemia management in the new ESC report, written jointly with the European Atherosclerosis Society, that especially distinguishes the new guidelines.
“It seemed to us logical that if you have drugs [statins] that lower LDL cholesterol, then you target LDL cholesterol,” explained Ian M. Graham, MD, professor of cardiovascular medicine at Trinity College in Dublin and cochair of the guidelines panel. “If a patient’s risk is high, they still need to lower LDL cholesterol. It’s not really contradictory to the U.S. approach,” Dr. Graham said in an interview.
The ESC panel’s discussions about the LDL cholesterol targets were “difficult and long,” said Guy De Backer, MD, a member of the guidelines committee and professor of cardiology at the University of Ghent, Belgium, in a session devoted to the new guidelines during the annual congress of the ESC.
The ESC’s decision to retain the 2011 LDL cholesterol targets won praise from U.S. cardiologist Eugene Braunwald, MD. “I think that not measuring and following LDL cholesterol is silly. I don’t agree” with current U.S. guidelines, he said in a talk during the congress. “If you don’t follow LDL cholesterol then you don’t know a patient’s compliance.
Regularly measuring LDL cholesterol is important,” said Dr. Braunwald, professor of medicine at Harvard Medical School in Boston. But he questioned the LDL cholesterol targets set by the ESC panel, specifically the LDL cholesterol goal of less than 70 mg/dL for very-high-risk patients.
“I don’t think the ESC went low enough; the goal should be less than 50 mg/dL,” declared Dr. Braunwald, who added “anything above 50 mg/dL is toxic.”
The new guidelines also made the definition of “very-high-risk” patients “stricter and more precise,” said Alberico L. Catapano, MD, cochair of the panel and professor of pharmacology at the University of Milan.
The guideline’s detailed list defining very-high-risk patients includes those with either clinical or “unequivocal” imaging evidence for cardiovascular disease, as well as patients with diabetes and target-organ damage, severe chronic kidney disease, or a 10% or greater 10-year risk for fatal cardiovascular disease calculated by the European Score risk formula.
The potent lipid-lowering PCSK9 inhibitors came onto the U.S. and European markets in 2015, labeled in the United States specifically for patients with familial hypercholesterolemia.
In July 2016, an American College of Cardiology Task Force issued a model clinical-decision pathway for lowering LDL cholesterol levels that for the first time included the PCSK9 inhibitors, specified as a “may be considered” option for selected patients (J Am Coll Cardiol. 2016 Jul;68[1]:92-125). This consensus decision pathway was not a set of actual guidelines, which means the ESC revision is the first guideline to include the PCSK9 inhibitors. The panel took the same stance as the U.S. decision pathway and designated the PCSK9 inhibitors as a “may be considered” option.
Dr. Graham explained that this designation was driven by the current absence of evidence for clinical benefit from the large lipid-lowering effect of the PCSK9 antibodies, although trial results that address this are expected to appear very soon. Experts not on the guidelines panel agreed with this decision.
“We know that it’s crucial to await results from the clinical endpoint studies” before the guidelines committee makes a more forceful recommendation, commented Erik S.G. Stroes, MD, professor of vascular medicine at the Academic Medical Center in Amsterdam. He added, however, that many clinicians, himself included, have been pleased to offer PCSK9-inhibitor treatment to very-high-risk patients with no good alternatives for effectively lowering their LDL cholesterol to target levels, such as statin-intolerant patients or those with LDL cholesterol levels that remain high despite maximal therapy.
The current ESC guideline’s statement on when to use a PCSK9 inhibitor “is vague” said Dr. Braunwald. “Within the next year, we’ll have results from two huge trials that will show clinical outcomes. We know that PCSK9 inhibitors are extremely powerful at lowering LDL cholesterol, but I would like to see the loop closed” with proven effects on clinical outcomes. “I would bet 100 to 1 that they will be effective, but LDL cholesterol is just a surrogate marker, and you need to look in large populations before you make a guideline recommendation to use these drugs, so we’ll wait.”
Once the clinical value of treatment with PCSK9 inhibitors is settled, the next issue will be the cost of these drugs, something that will become a big consideration once they become more widely used, Dr. Braunwald added.
Dr. De Backer and Dr. Graham had no disclosures. Dr. Catapano has been a consultant to Aegerion, Amgen, AstraZeneca, Merck, Pfizer, and Sigma-Tau. Dr. Braunwald has been a consultant to Bayer, Daiichi Sankyo, the Medicines Company, Merck, Novartis, and Sanofi. Dr. Stroes has been a consultant to Amgen, Bristol-Myers Squibb, Merck, and Sanofi.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE ESC CONGRESS 2016
Study suggests link between commonly used antihypertensive drug and skin cancer
Despite its widespread use as a guideline-recommended, first-line agent for the treatment of hypertension, hydrochlorothiazide (HCTZ) may contribute to an increased risk of skin cancer, according to Armand B. Cognetta Jr., MD, and his associates in the division of dermatology, Mohs Micrographic Surgery Unit, Florida State University, Tallahassee.
The common, long-term use of HCTZ for treatment of hypertension, combined with its known photosensitizing effects, makes it a potential candidate for increasing the risk of squamous cell carcinoma (SCC) and other skin cancers.
To further elucidate the association between longterm HCTZ exposure and skin cancer risk, Dr. Cognetta and his associates screened medication lists of 75 patients seen in their Mohs practice over a period of 5 days for lifetime SCCs and HCTZ exposure. For this study, patients with more than 20 lifetime SCCs were considered high risk. They also conducted a literature review of previous studies exploring this relationship, from 1966 to 2015.
Of the 75 patients screened, 4 met the criteria for inclusion in the high risk category. These four patients had a combined lifetime total of 288 SCC and 98 basal cell carcinomas (BCCs), including 10 that were lip SCC. All four patients were non-Hispanic white males and had been taking HCTZ alone or in combination for 3-15 years (Dermatol Surg. 2016 Sep;42[9]:1107-9).
The literature search produced three relevant studies, all of which had large patient populations, published between 2008 and 2012, “demonstrating an increased risk of SCC or lip cancer” associated with HCTZ use, with the highest risk associated with over 5 years of use, the researchers wrote.
“As cutaneous oncologists, it is our duty to look for ‘correctable’ causes of skin cancer,” they noted. “Hydrochlorothiazide, a known photosensitizer, when taken by white non-Hispanic patients with a history of multiple SCC, skin cancers may represent a ‘correctable’ cause.”
In their practice, they screen their high-risk SCC non-Hispanic patients for HCTZ use, “and send a standard letter explaining the association to the primary care provider with a request to change to a different antihypertensive if possible,” they wrote. “Many primary care providers are unaware of the association between HCTZ use and skin cancer,” they observed.
The authors had no disclosures.
Despite its widespread use as a guideline-recommended, first-line agent for the treatment of hypertension, hydrochlorothiazide (HCTZ) may contribute to an increased risk of skin cancer, according to Armand B. Cognetta Jr., MD, and his associates in the division of dermatology, Mohs Micrographic Surgery Unit, Florida State University, Tallahassee.
The common, long-term use of HCTZ for treatment of hypertension, combined with its known photosensitizing effects, makes it a potential candidate for increasing the risk of squamous cell carcinoma (SCC) and other skin cancers.
To further elucidate the association between longterm HCTZ exposure and skin cancer risk, Dr. Cognetta and his associates screened medication lists of 75 patients seen in their Mohs practice over a period of 5 days for lifetime SCCs and HCTZ exposure. For this study, patients with more than 20 lifetime SCCs were considered high risk. They also conducted a literature review of previous studies exploring this relationship, from 1966 to 2015.
Of the 75 patients screened, 4 met the criteria for inclusion in the high risk category. These four patients had a combined lifetime total of 288 SCC and 98 basal cell carcinomas (BCCs), including 10 that were lip SCC. All four patients were non-Hispanic white males and had been taking HCTZ alone or in combination for 3-15 years (Dermatol Surg. 2016 Sep;42[9]:1107-9).
The literature search produced three relevant studies, all of which had large patient populations, published between 2008 and 2012, “demonstrating an increased risk of SCC or lip cancer” associated with HCTZ use, with the highest risk associated with over 5 years of use, the researchers wrote.
“As cutaneous oncologists, it is our duty to look for ‘correctable’ causes of skin cancer,” they noted. “Hydrochlorothiazide, a known photosensitizer, when taken by white non-Hispanic patients with a history of multiple SCC, skin cancers may represent a ‘correctable’ cause.”
In their practice, they screen their high-risk SCC non-Hispanic patients for HCTZ use, “and send a standard letter explaining the association to the primary care provider with a request to change to a different antihypertensive if possible,” they wrote. “Many primary care providers are unaware of the association between HCTZ use and skin cancer,” they observed.
The authors had no disclosures.
Despite its widespread use as a guideline-recommended, first-line agent for the treatment of hypertension, hydrochlorothiazide (HCTZ) may contribute to an increased risk of skin cancer, according to Armand B. Cognetta Jr., MD, and his associates in the division of dermatology, Mohs Micrographic Surgery Unit, Florida State University, Tallahassee.
The common, long-term use of HCTZ for treatment of hypertension, combined with its known photosensitizing effects, makes it a potential candidate for increasing the risk of squamous cell carcinoma (SCC) and other skin cancers.
To further elucidate the association between longterm HCTZ exposure and skin cancer risk, Dr. Cognetta and his associates screened medication lists of 75 patients seen in their Mohs practice over a period of 5 days for lifetime SCCs and HCTZ exposure. For this study, patients with more than 20 lifetime SCCs were considered high risk. They also conducted a literature review of previous studies exploring this relationship, from 1966 to 2015.
Of the 75 patients screened, 4 met the criteria for inclusion in the high risk category. These four patients had a combined lifetime total of 288 SCC and 98 basal cell carcinomas (BCCs), including 10 that were lip SCC. All four patients were non-Hispanic white males and had been taking HCTZ alone or in combination for 3-15 years (Dermatol Surg. 2016 Sep;42[9]:1107-9).
The literature search produced three relevant studies, all of which had large patient populations, published between 2008 and 2012, “demonstrating an increased risk of SCC or lip cancer” associated with HCTZ use, with the highest risk associated with over 5 years of use, the researchers wrote.
“As cutaneous oncologists, it is our duty to look for ‘correctable’ causes of skin cancer,” they noted. “Hydrochlorothiazide, a known photosensitizer, when taken by white non-Hispanic patients with a history of multiple SCC, skin cancers may represent a ‘correctable’ cause.”
In their practice, they screen their high-risk SCC non-Hispanic patients for HCTZ use, “and send a standard letter explaining the association to the primary care provider with a request to change to a different antihypertensive if possible,” they wrote. “Many primary care providers are unaware of the association between HCTZ use and skin cancer,” they observed.
The authors had no disclosures.
Key clinical point: The antihypertensive agent hydrochlorothiazide (HCTZ ) is photosensitizing and may be associated with an increased risk for skin cancers in non-Hispanic white males with a history of squamous cell carcinoma (SCC).
Major finding: All four patients considered high risk based on lifetime SCC history were non-Hispanic white males with a history of HCTZ exposure, an association supported by three previously published studies.
Data sources: Medical records from 75 patients seen in a single dermatology practice over a 5-day period, and a literature search as well as relevant publications detected through a literature search spanning the years 1966-2015.
Disclosures: The authors had no disclosures. A funding source was not identified.
















