User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Psoriasis topical combination maintenance strategy hits mark in phase 3
A proactive long-term strategy of maintenance therapy involving twice-weekly application of combined calcipotriene and betamethasone dipropionate spray foam was safe and effective in patients with moderate plaque psoriasis in the international, randomized PSO-LONG clinical trial, Mark Lebwohl, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The median time to first relapse – the primary study endpoint – was 56 days in patients randomized to the twice-weekly fixed-dose combination calcipotriene 0.005% and betamethasone dipropionate 0.064% foam (Enstilar), a significantly better outcome than the median 30 days for controls assigned to foam vehicle. Moreover, it took 169 days for 75% of patients on the combination foam to experience their first relapse: three times longer than in controls, added Dr. Lebwohl, principal investigator for PSO-LONG and professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
The positive results “could have been predicted,” he said in an interview. “But what really distinguishes this study from others is that no one before has ever done a placebo-controlled, double-blind trial with a topical steroid that lasted a year. This is a first, and we’ve shown that if you limit treatment to twice a week you get dramatic improvements in efficacy at no cost in terms of safety.”
The combination spray foam is approved by the Food and Drug Administration as once-daily therapy in psoriasis patients aged 12 years and older, but only for up to 4 weeks because of safety concerns regarding longer use of the potent topical steroid. However, psoriasis is a chronic disease. The PSO-LONG trial was designed to study the impact of a for-now still-investigational long-term maintenance treatment strategy.
The open-label run-in period of the study included 640 adults with plaque psoriasis, 82% of whom had moderate disease at baseline as rated by Physician Global Assessment (PGA). Participants applied the combination foam once daily for 4 weeks. At that point, 80% of them had achieved a PGA rating of clear or almost clear with at least a two-grade improvement from baseline; these 521 responders were then randomized to 52 weeks of double-blind treatment with the combination foam or vehicle foam. Anyone who relapsed went on 4 weeks of once-daily active treatment with the combination foam, then returned to their original treatment arm.
The risk of a first relapse during the course of 1 year was 43% lower with the combination foam than in controls. The relapse rate over the year was 46% lower. Patients in the active treatment arm spent an average of 256.5 days in remission during the year, compared with 222 days in controls.
“That’s more than 1 month more time in remission during the year with active treatment. And remember, if patients flared, they went on daily therapy for a month,” the dermatologist noted.
The rate of treatment-related adverse events was similar in the two groups at 2.8 events per 100 patient-years in the combination foam arm and 4.5 per 100 patient-years in controls. The twice-weekly active treatment group had no increase in stretch marks, telangiectasias, skin atrophy, serum calcium, or abnormalities of the hypothalamic-pituitary-adrenal axis.
Although the combination foam is approved for daily use for a maximum of 1 month in adolescents and adults, PSO-LONG was restricted to adults.
“I think that what will happen in the marketplace is that the data obtained from this adult study will likely be applied to younger patients,” Dr. Lebwohl predicted.
He reported receiving an institutional research grant to conduct the trial from LEO Pharma, the study sponsor, as well as serving as a consultant to and researcher for the company.
A proactive long-term strategy of maintenance therapy involving twice-weekly application of combined calcipotriene and betamethasone dipropionate spray foam was safe and effective in patients with moderate plaque psoriasis in the international, randomized PSO-LONG clinical trial, Mark Lebwohl, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The median time to first relapse – the primary study endpoint – was 56 days in patients randomized to the twice-weekly fixed-dose combination calcipotriene 0.005% and betamethasone dipropionate 0.064% foam (Enstilar), a significantly better outcome than the median 30 days for controls assigned to foam vehicle. Moreover, it took 169 days for 75% of patients on the combination foam to experience their first relapse: three times longer than in controls, added Dr. Lebwohl, principal investigator for PSO-LONG and professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
The positive results “could have been predicted,” he said in an interview. “But what really distinguishes this study from others is that no one before has ever done a placebo-controlled, double-blind trial with a topical steroid that lasted a year. This is a first, and we’ve shown that if you limit treatment to twice a week you get dramatic improvements in efficacy at no cost in terms of safety.”
The combination spray foam is approved by the Food and Drug Administration as once-daily therapy in psoriasis patients aged 12 years and older, but only for up to 4 weeks because of safety concerns regarding longer use of the potent topical steroid. However, psoriasis is a chronic disease. The PSO-LONG trial was designed to study the impact of a for-now still-investigational long-term maintenance treatment strategy.
The open-label run-in period of the study included 640 adults with plaque psoriasis, 82% of whom had moderate disease at baseline as rated by Physician Global Assessment (PGA). Participants applied the combination foam once daily for 4 weeks. At that point, 80% of them had achieved a PGA rating of clear or almost clear with at least a two-grade improvement from baseline; these 521 responders were then randomized to 52 weeks of double-blind treatment with the combination foam or vehicle foam. Anyone who relapsed went on 4 weeks of once-daily active treatment with the combination foam, then returned to their original treatment arm.
The risk of a first relapse during the course of 1 year was 43% lower with the combination foam than in controls. The relapse rate over the year was 46% lower. Patients in the active treatment arm spent an average of 256.5 days in remission during the year, compared with 222 days in controls.
“That’s more than 1 month more time in remission during the year with active treatment. And remember, if patients flared, they went on daily therapy for a month,” the dermatologist noted.
The rate of treatment-related adverse events was similar in the two groups at 2.8 events per 100 patient-years in the combination foam arm and 4.5 per 100 patient-years in controls. The twice-weekly active treatment group had no increase in stretch marks, telangiectasias, skin atrophy, serum calcium, or abnormalities of the hypothalamic-pituitary-adrenal axis.
Although the combination foam is approved for daily use for a maximum of 1 month in adolescents and adults, PSO-LONG was restricted to adults.
“I think that what will happen in the marketplace is that the data obtained from this adult study will likely be applied to younger patients,” Dr. Lebwohl predicted.
He reported receiving an institutional research grant to conduct the trial from LEO Pharma, the study sponsor, as well as serving as a consultant to and researcher for the company.
A proactive long-term strategy of maintenance therapy involving twice-weekly application of combined calcipotriene and betamethasone dipropionate spray foam was safe and effective in patients with moderate plaque psoriasis in the international, randomized PSO-LONG clinical trial, Mark Lebwohl, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
The median time to first relapse – the primary study endpoint – was 56 days in patients randomized to the twice-weekly fixed-dose combination calcipotriene 0.005% and betamethasone dipropionate 0.064% foam (Enstilar), a significantly better outcome than the median 30 days for controls assigned to foam vehicle. Moreover, it took 169 days for 75% of patients on the combination foam to experience their first relapse: three times longer than in controls, added Dr. Lebwohl, principal investigator for PSO-LONG and professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
The positive results “could have been predicted,” he said in an interview. “But what really distinguishes this study from others is that no one before has ever done a placebo-controlled, double-blind trial with a topical steroid that lasted a year. This is a first, and we’ve shown that if you limit treatment to twice a week you get dramatic improvements in efficacy at no cost in terms of safety.”
The combination spray foam is approved by the Food and Drug Administration as once-daily therapy in psoriasis patients aged 12 years and older, but only for up to 4 weeks because of safety concerns regarding longer use of the potent topical steroid. However, psoriasis is a chronic disease. The PSO-LONG trial was designed to study the impact of a for-now still-investigational long-term maintenance treatment strategy.
The open-label run-in period of the study included 640 adults with plaque psoriasis, 82% of whom had moderate disease at baseline as rated by Physician Global Assessment (PGA). Participants applied the combination foam once daily for 4 weeks. At that point, 80% of them had achieved a PGA rating of clear or almost clear with at least a two-grade improvement from baseline; these 521 responders were then randomized to 52 weeks of double-blind treatment with the combination foam or vehicle foam. Anyone who relapsed went on 4 weeks of once-daily active treatment with the combination foam, then returned to their original treatment arm.
The risk of a first relapse during the course of 1 year was 43% lower with the combination foam than in controls. The relapse rate over the year was 46% lower. Patients in the active treatment arm spent an average of 256.5 days in remission during the year, compared with 222 days in controls.
“That’s more than 1 month more time in remission during the year with active treatment. And remember, if patients flared, they went on daily therapy for a month,” the dermatologist noted.
The rate of treatment-related adverse events was similar in the two groups at 2.8 events per 100 patient-years in the combination foam arm and 4.5 per 100 patient-years in controls. The twice-weekly active treatment group had no increase in stretch marks, telangiectasias, skin atrophy, serum calcium, or abnormalities of the hypothalamic-pituitary-adrenal axis.
Although the combination foam is approved for daily use for a maximum of 1 month in adolescents and adults, PSO-LONG was restricted to adults.
“I think that what will happen in the marketplace is that the data obtained from this adult study will likely be applied to younger patients,” Dr. Lebwohl predicted.
He reported receiving an institutional research grant to conduct the trial from LEO Pharma, the study sponsor, as well as serving as a consultant to and researcher for the company.
FROM AAD 2020
Study spotlights the skin microbiome’s evolving nature
, while the skin microbiome of the mothers of the children whose microbiome was analyzed remained relatively constant over the same time period.
The findings come from what is believed to be the longest longitudinal study specific to the skin microbiome of infants and mothers.
“Even at 10 years, the skin microbiome does not look like an adult skin microbiome, based on composition of the ecosystem,” lead author Kimberly A. Capone, PhD, said in an interview during the virtual annual meeting of the American Academy of Dermatology. “The diversity of the microbiome in children’s skin is distinct to that of an adult’s skin. We all have the same bacteria present, but in children it is distributed differently because the bacteria set themselves up based on the nutrients and topography that they find on the skin. Since infant skin is unique to infants, so too is their microbiome when we compare it to adults. It’s been fascinating to observe these children grow and mature, and follow the skin microbiome along this same period.”
During five time points over a period of 10 years, Dr. Capone and her colleagues at the Skillman, N.J.–based Johnson & Johnson Consumer Experience Center, a research and development site, used 16s rRNA gene sequencing to evaluate the skin microbiome on the forearms and foreheads of 30 mothers and their 31 children. The study participants had Fitzpatrick skin types I-IV and the mean age of mothers was 37 years. “We used 16s rRNA gene sequencing for microbiome analysis at the beginning, as that was what was available 10 years ago,” said Dr. Capone, head of the microbiome platform for Johnson & Johnson Consumer Health. “Since then, we continue to use 16s for continuity, but also collected additional swabs for deeper analysis later.”
She and her colleagues often draw samples from the forearm in baby skin clinical studies, “as the arm is a good site to collect relevant data on the body overall,” she explained. “We chose the forearm and forehead specifically here so we can make same body site comparisons to adult sample data which we took from the mothers on the same areas of their body.” Time point 1 was 3 months to 1 year, time point 2 was 2-3 years, time point 3 was 5-6 years, time point 4 was 7-8 years, and time point 5 was 9-10 years.
The researchers found that the skin of infants during the first few weeks of life was similar at age 3 and 4 years. “From that second time point on, we see significant increases in richness and diversity, richness being presence or absence of various bacterial species, and diversity being the relative abundance of those species,” Dr. Capone said. “What you’re basically seeing is that there are new organisms, i.e., richness, coming into the microbiome. We start to detect new ones. Over time, the ecosystem is expanding. It’s evolving because it’s not yet set up.” The evolving skin microbiome on children was dominated by Streptococcus and Staphylococcus. In addition, children had higher levels of Streptococcus, Moraxella, Granulicatella, Gemella and Veillonella, compared with their adult mothers. Adult skin was colonized predominantly by Propionibacterium/Cutibacterium and Staphylococcus.
“The skin microbiome also increased in diversity over time on the forearm, but not face, of the mothers,” Dr. Capone said. “Previous studies have shown how stable the adult skin microbiome is, so it’s intriguing to see the changes that we saw on the mothers in this study.”
The increase in skin microbiome diversity observed in the children is likely due to a variety of factors, she continued, including inherent growth and development, dietary changes, as well as exposure to various environments and other people. “The fact remains that diversity is increasing over time, as the ecosystem evolves,” she said. “Eventually, the skin microbiome will become ‘adultlike’ in puberty, when lipid production increases. This drives increases in Cutibacterium acnes, particularly on the face.”
She acknowledged certain limitations of the study, including its relatively small size and the fact that some of the original subjects did not return for microbiome analysis at later time points. “We need larger cohort studies, additional deeper sequence data, metabolomics and transcriptomics to better understand the function of the skin microbiome over these various ages,” she said.
The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
, while the skin microbiome of the mothers of the children whose microbiome was analyzed remained relatively constant over the same time period.
The findings come from what is believed to be the longest longitudinal study specific to the skin microbiome of infants and mothers.
“Even at 10 years, the skin microbiome does not look like an adult skin microbiome, based on composition of the ecosystem,” lead author Kimberly A. Capone, PhD, said in an interview during the virtual annual meeting of the American Academy of Dermatology. “The diversity of the microbiome in children’s skin is distinct to that of an adult’s skin. We all have the same bacteria present, but in children it is distributed differently because the bacteria set themselves up based on the nutrients and topography that they find on the skin. Since infant skin is unique to infants, so too is their microbiome when we compare it to adults. It’s been fascinating to observe these children grow and mature, and follow the skin microbiome along this same period.”
During five time points over a period of 10 years, Dr. Capone and her colleagues at the Skillman, N.J.–based Johnson & Johnson Consumer Experience Center, a research and development site, used 16s rRNA gene sequencing to evaluate the skin microbiome on the forearms and foreheads of 30 mothers and their 31 children. The study participants had Fitzpatrick skin types I-IV and the mean age of mothers was 37 years. “We used 16s rRNA gene sequencing for microbiome analysis at the beginning, as that was what was available 10 years ago,” said Dr. Capone, head of the microbiome platform for Johnson & Johnson Consumer Health. “Since then, we continue to use 16s for continuity, but also collected additional swabs for deeper analysis later.”
She and her colleagues often draw samples from the forearm in baby skin clinical studies, “as the arm is a good site to collect relevant data on the body overall,” she explained. “We chose the forearm and forehead specifically here so we can make same body site comparisons to adult sample data which we took from the mothers on the same areas of their body.” Time point 1 was 3 months to 1 year, time point 2 was 2-3 years, time point 3 was 5-6 years, time point 4 was 7-8 years, and time point 5 was 9-10 years.
The researchers found that the skin of infants during the first few weeks of life was similar at age 3 and 4 years. “From that second time point on, we see significant increases in richness and diversity, richness being presence or absence of various bacterial species, and diversity being the relative abundance of those species,” Dr. Capone said. “What you’re basically seeing is that there are new organisms, i.e., richness, coming into the microbiome. We start to detect new ones. Over time, the ecosystem is expanding. It’s evolving because it’s not yet set up.” The evolving skin microbiome on children was dominated by Streptococcus and Staphylococcus. In addition, children had higher levels of Streptococcus, Moraxella, Granulicatella, Gemella and Veillonella, compared with their adult mothers. Adult skin was colonized predominantly by Propionibacterium/Cutibacterium and Staphylococcus.
“The skin microbiome also increased in diversity over time on the forearm, but not face, of the mothers,” Dr. Capone said. “Previous studies have shown how stable the adult skin microbiome is, so it’s intriguing to see the changes that we saw on the mothers in this study.”
The increase in skin microbiome diversity observed in the children is likely due to a variety of factors, she continued, including inherent growth and development, dietary changes, as well as exposure to various environments and other people. “The fact remains that diversity is increasing over time, as the ecosystem evolves,” she said. “Eventually, the skin microbiome will become ‘adultlike’ in puberty, when lipid production increases. This drives increases in Cutibacterium acnes, particularly on the face.”
She acknowledged certain limitations of the study, including its relatively small size and the fact that some of the original subjects did not return for microbiome analysis at later time points. “We need larger cohort studies, additional deeper sequence data, metabolomics and transcriptomics to better understand the function of the skin microbiome over these various ages,” she said.
The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
, while the skin microbiome of the mothers of the children whose microbiome was analyzed remained relatively constant over the same time period.
The findings come from what is believed to be the longest longitudinal study specific to the skin microbiome of infants and mothers.
“Even at 10 years, the skin microbiome does not look like an adult skin microbiome, based on composition of the ecosystem,” lead author Kimberly A. Capone, PhD, said in an interview during the virtual annual meeting of the American Academy of Dermatology. “The diversity of the microbiome in children’s skin is distinct to that of an adult’s skin. We all have the same bacteria present, but in children it is distributed differently because the bacteria set themselves up based on the nutrients and topography that they find on the skin. Since infant skin is unique to infants, so too is their microbiome when we compare it to adults. It’s been fascinating to observe these children grow and mature, and follow the skin microbiome along this same period.”
During five time points over a period of 10 years, Dr. Capone and her colleagues at the Skillman, N.J.–based Johnson & Johnson Consumer Experience Center, a research and development site, used 16s rRNA gene sequencing to evaluate the skin microbiome on the forearms and foreheads of 30 mothers and their 31 children. The study participants had Fitzpatrick skin types I-IV and the mean age of mothers was 37 years. “We used 16s rRNA gene sequencing for microbiome analysis at the beginning, as that was what was available 10 years ago,” said Dr. Capone, head of the microbiome platform for Johnson & Johnson Consumer Health. “Since then, we continue to use 16s for continuity, but also collected additional swabs for deeper analysis later.”
She and her colleagues often draw samples from the forearm in baby skin clinical studies, “as the arm is a good site to collect relevant data on the body overall,” she explained. “We chose the forearm and forehead specifically here so we can make same body site comparisons to adult sample data which we took from the mothers on the same areas of their body.” Time point 1 was 3 months to 1 year, time point 2 was 2-3 years, time point 3 was 5-6 years, time point 4 was 7-8 years, and time point 5 was 9-10 years.
The researchers found that the skin of infants during the first few weeks of life was similar at age 3 and 4 years. “From that second time point on, we see significant increases in richness and diversity, richness being presence or absence of various bacterial species, and diversity being the relative abundance of those species,” Dr. Capone said. “What you’re basically seeing is that there are new organisms, i.e., richness, coming into the microbiome. We start to detect new ones. Over time, the ecosystem is expanding. It’s evolving because it’s not yet set up.” The evolving skin microbiome on children was dominated by Streptococcus and Staphylococcus. In addition, children had higher levels of Streptococcus, Moraxella, Granulicatella, Gemella and Veillonella, compared with their adult mothers. Adult skin was colonized predominantly by Propionibacterium/Cutibacterium and Staphylococcus.
“The skin microbiome also increased in diversity over time on the forearm, but not face, of the mothers,” Dr. Capone said. “Previous studies have shown how stable the adult skin microbiome is, so it’s intriguing to see the changes that we saw on the mothers in this study.”
The increase in skin microbiome diversity observed in the children is likely due to a variety of factors, she continued, including inherent growth and development, dietary changes, as well as exposure to various environments and other people. “The fact remains that diversity is increasing over time, as the ecosystem evolves,” she said. “Eventually, the skin microbiome will become ‘adultlike’ in puberty, when lipid production increases. This drives increases in Cutibacterium acnes, particularly on the face.”
She acknowledged certain limitations of the study, including its relatively small size and the fact that some of the original subjects did not return for microbiome analysis at later time points. “We need larger cohort studies, additional deeper sequence data, metabolomics and transcriptomics to better understand the function of the skin microbiome over these various ages,” she said.
The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
FROM AAD 20
Key clinical point: The skin’s microbial diversity changes with increasing age in children while remaining stable in adult mothers.
Major finding: The skin microbiome in children becomes more diverse between the ages of 3-4 to age 10.
Study details: A longitudinal analysis of 30 mothers and their 31 children.
Disclosures: The study was sponsored by Johnson & Johnson Consumer. Dr. Capone and her two coauthors are employees of the company.
Source: Capone K et al. AAD 20, Abstract F053.
FDA revokes emergency use of hydroxychloroquine
The U.S. Food and Drug Administration revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
“Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19 for the authorized uses in the EUA,” the agency announced in a June 15 statement.
The FDA also warned today that the use of hydroxychloroquine or chloroquine may have a potential drug interaction with the investigational antiviral drug remdesivir that limits its effectiveness against COVID-19.
Remdesivir was granted emergency use authorization by the FDA on May 1.
“Based on a recently completed nonclinical laboratory study, the FDA is revising the fact sheet for healthcare providers that accompanies the drug to state that coadministration of remdesivir and chloroquine phosphate or hydroxychloroquine sulfate is not recommended as it may result in reduced antiviral activity of remdesivir. The agency is not aware of instances of this reduced activity occurring in the clinical setting but is continuing to evaluate all data related to remdesivir,” the FDA said in a news release.
Controversy over hydroxychloroquine
Even with such federal permission, since late March the use of these two agents has been mired in controversy.
President Donald J. Trump promoted the use of hydroxychloroquine and chloroquine to treat Americans with COVID-19, while scientific studies raised questions about their safety and effectiveness. Recent research, for example, pointed to elevated cardiovascular risks, as reported by Medscape Medical News.
The FDA acknowledged this recent evidence. “Additionally, in light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use.”
The full suspension of the EUA follows a warning the agency issued on April 24. The FDA’s Safety Communication cautioned against use of the two agents outside of a hospital setting, citing an increase in outpatient prescriptions and “reports of serious heart rhythm problems.”
“While additional clinical trials continue to evaluate the potential benefit of these drugs in treating or preventing COVID-19, we determined the emergency use authorization was no longer appropriate,” based on a rigorous assessment by scientists in our Center for Drug Evaluation and Research,” Patrizia Cavazzoni, MD, acting director of CDER, noted in the FDA statement.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
“Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19 for the authorized uses in the EUA,” the agency announced in a June 15 statement.
The FDA also warned today that the use of hydroxychloroquine or chloroquine may have a potential drug interaction with the investigational antiviral drug remdesivir that limits its effectiveness against COVID-19.
Remdesivir was granted emergency use authorization by the FDA on May 1.
“Based on a recently completed nonclinical laboratory study, the FDA is revising the fact sheet for healthcare providers that accompanies the drug to state that coadministration of remdesivir and chloroquine phosphate or hydroxychloroquine sulfate is not recommended as it may result in reduced antiviral activity of remdesivir. The agency is not aware of instances of this reduced activity occurring in the clinical setting but is continuing to evaluate all data related to remdesivir,” the FDA said in a news release.
Controversy over hydroxychloroquine
Even with such federal permission, since late March the use of these two agents has been mired in controversy.
President Donald J. Trump promoted the use of hydroxychloroquine and chloroquine to treat Americans with COVID-19, while scientific studies raised questions about their safety and effectiveness. Recent research, for example, pointed to elevated cardiovascular risks, as reported by Medscape Medical News.
The FDA acknowledged this recent evidence. “Additionally, in light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use.”
The full suspension of the EUA follows a warning the agency issued on April 24. The FDA’s Safety Communication cautioned against use of the two agents outside of a hospital setting, citing an increase in outpatient prescriptions and “reports of serious heart rhythm problems.”
“While additional clinical trials continue to evaluate the potential benefit of these drugs in treating or preventing COVID-19, we determined the emergency use authorization was no longer appropriate,” based on a rigorous assessment by scientists in our Center for Drug Evaluation and Research,” Patrizia Cavazzoni, MD, acting director of CDER, noted in the FDA statement.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration revoked its decision from March 28 allowing use of hydroxychloroquine and chloroquine to treat people hospitalized with COVID-19 under an emergency use authorization (EUA).
“Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19 for the authorized uses in the EUA,” the agency announced in a June 15 statement.
The FDA also warned today that the use of hydroxychloroquine or chloroquine may have a potential drug interaction with the investigational antiviral drug remdesivir that limits its effectiveness against COVID-19.
Remdesivir was granted emergency use authorization by the FDA on May 1.
“Based on a recently completed nonclinical laboratory study, the FDA is revising the fact sheet for healthcare providers that accompanies the drug to state that coadministration of remdesivir and chloroquine phosphate or hydroxychloroquine sulfate is not recommended as it may result in reduced antiviral activity of remdesivir. The agency is not aware of instances of this reduced activity occurring in the clinical setting but is continuing to evaluate all data related to remdesivir,” the FDA said in a news release.
Controversy over hydroxychloroquine
Even with such federal permission, since late March the use of these two agents has been mired in controversy.
President Donald J. Trump promoted the use of hydroxychloroquine and chloroquine to treat Americans with COVID-19, while scientific studies raised questions about their safety and effectiveness. Recent research, for example, pointed to elevated cardiovascular risks, as reported by Medscape Medical News.
The FDA acknowledged this recent evidence. “Additionally, in light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use.”
The full suspension of the EUA follows a warning the agency issued on April 24. The FDA’s Safety Communication cautioned against use of the two agents outside of a hospital setting, citing an increase in outpatient prescriptions and “reports of serious heart rhythm problems.”
“While additional clinical trials continue to evaluate the potential benefit of these drugs in treating or preventing COVID-19, we determined the emergency use authorization was no longer appropriate,” based on a rigorous assessment by scientists in our Center for Drug Evaluation and Research,” Patrizia Cavazzoni, MD, acting director of CDER, noted in the FDA statement.
This article first appeared on Medscape.com.
Perfect storm of SARS-CoV-2 during flu season
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at [email protected].
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at [email protected].
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
COVID-19 now. The urban phase of the U.S. pandemic is leveling somewhat, while the rural phase is accelerating – in part because of food processing and handling industries. The pediatric burden has been surprisingly small, with the multisystem inflammatory disease (MIS-c) in children noted in several hundred cases now being seen across the country.

Next wave? Given ongoing COVID-19 disease, controversy rages about when and how to re-open the country. Regardless how more reopening occurs over the next months, we should expect a next or ongoing COVID-19 wave, particularly given loss of social distancing during social justice protests. A sawtooth disease prevalence pattern is predicted by many experts: a drop in prevalence leading to reopening, leading to scattered prevalence increases and regional if not local restriction tightening, followed by another drop in prevalence. Then “rinse and repeat” until 70% of the population is immune either by disease experience or vaccine-induced immunity, likely sometime in 2021.
Influenza too. A COVID-19 up-cycle is likely during influenza season, although influenza season’s onset could be altered because of whatever social distancing rules are in place in November and December. That said, we need to consider the worst. We have seen what happens if we fail to prepare and then react only after a prevalent respiratory infection has surged into the overall population. Best estimates are that at most 20% of the U.S. population is currently immune to SARS-CoV-2. Given that at least some of that 20% of individuals currently immune to SARS-CoV-2 will lose their neutralizing antibody over the next 4-6 months, we can still expect 70%-80% of the U.S. population to be susceptible to SARS-CoV-2 infection in the fall of 2020.
Pediatric preparedness. As pediatric providers, we have struggled with lower patient loads and dramatic income losses/declines. Many clinics/offices’ attendance remain less than 50% of pre–COVID-19 levels, with necessary furloughs of personnel and spotty office hours. But influenza is coming, and SARS-CoV-2 will not be gone yet. How do we prepare for concurrent influenza and COVID-19?
The annual purchase/administration of influenza vaccine in summer/fall is expensive, time consuming, and logistically difficult even in the best times. Given the loss of income, likely reluctance of patients to come to clinics/offices if COVID-19 is still circulating, and likely need for some form of social distancing during late summer and early fall, how will providers, health departments, and hospitals implement influenza vaccine administration this year?
Minimize double whammy infections. It is easy to understand why we should maximize influenza protection in SARS-CoV-2 vulnerables (elderly or persons with existing comorbidities). But is it as critical for otherwise healthy children? My answer is yes.
Children are not currently known as SARS-CoV-2 vectors, but children are excellent influenza vectors, shedding higher titers for longer than other age groups. As with SARS-CoV-2, influenza exposure is cumulative, i.e., the more intense and more frequently a person is exposed, the more likely that infection/disease will result. So, the fewer who get and can transmit influenza during the COVID-19 pandemic, the fewer people are likely to get a double whammy of SARS-CoV-2 concurrent or in tandem with influenza. Double whammy infections likely would further increase the medical care burden and return us to March-April crisis mode.
One alarming new question is whether recent influenza could make children vulnerable to SARS-CoV-2 and trigger hospitalizations. A surge in pediatric plus adult COVID-19 disease plus a surge in all-ages influenza disease would likely break the medical care system, at least in some areas.
Staggering COVID-19 burden. As of June 8, we have had approximately 2 million SARS-CoV-2 cases with 500,000 hospitalizations and 120,000 deaths. Over the past 10 years, total annual U.S. influenza hospitalizations ranged from 180,000 (2011-2012) to 825,000 (2017-2018). The interquartile range for hospitalization length of stay for influenza is 4-6 days1 vs. 15-23 days2 for SARS-CoV-2. One COVID-19 hospitalization uses hospital resources roughly equal to four influenza hospitalizations. To date COVID-19 hospitalizations have used resources equal to an estimated 1.9 million influenza hospitalizations – over twice the worst influenza season in this century – and we are still on the rise. We are likely not even halfway to truly controlling the U.S. pandemic, so expect another 500,000 hospitalizations – equal to another 1.9 million influenza hospitalizations. Further, pneumonia deaths have skyrocketed this year when COVID-19 was superimposed on the last third of influenza season. One hope is that widespread use of antivirals (for example, new antivirals, convalescent plasma, or other interventions) can reduce length of stay by 30% for COVID-19 hospitalizations, yet even with that the numbers remain grim.
Less influenza disease can free up medical resources. Planning ahead could prevent a bad influenza season (for example, up to 850,000 hospitalizations just for influenza). Can we preemptively use vaccine to reduce influenza hospitalizations below 2011-2012 levels – less than 150,000 hospitalizations? Perhaps, if we start by reducing pediatric influenza.
1. Aim to exceed 75% influenza vaccine uptake in your patients.
a. It is ambitious, but if there was ever a year that needed influenza herd immunity, it is 2020-2021.
2. Review practice/group/institution plans for vaccine purchase and ensure adequate personnel to administer vaccine.
3. Plan safe and efficient processes to vaccinate large numbers in August through November.
a. Consider that routine and influenza vaccines can be given concurrently with the annual uptick in school and sports physical examinations.
b. What social distancing and masking rules will be needed?
i. Will patients need to bring their own masks, or will you supply them?
c. What extra supplies and efforts are needed, e.g. hand sanitizer, new signage, 6-foot interval markings on floors or sidewalks, families calling from parking lot to announce their arrivals, etc.?
d. Remember younger patients need two doses before Dec 1, 2020.
e. Be creative, for example, are parking-lot tents for influenza vaccination feasible?
f. Can we partner with other providers to implement influenza vaccine–specific mass clinics?
Ramping up to give seasonal influenza vaccine in 2020 is daunting. But if we do not prepare, it will be even more difficult. Let’s make this the mildest influenza season in memory by vaccinating more than any time in memory – and by doing so, we can hope to blunt medical care burdens despite ongoing COVID-19 disease.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Kansas City (Mo.). Children’s Mercy receives funding from GlaxoSmithKline, Merck, and Pfizer for vaccine research studies on which Dr. Harrison is an investigator. Email him at [email protected].
References
1.. HCUP Statistical Brief #253. 2019 Oct.
2. medrxiv. 2020 Apr 10. doi: 10.1101/2020.04.07.20057299.
Learning the ICU
Although deployment of hospitalists into ICUs during the COVID-19 crisis varies widely, in that sense it reflects the pre-COVID hospital landscape of variable involvement, in which many hospitalists pressed into this role expressed discomfort practicing critical care beyond their scope of training, according to a survey published in the Journal of Hospital Medicine in 2018.1 “Hospitalists frequently deliver critical care services without adequate training or support, most prevalently in rural hospitals,” the authors concluded.
A Critical Care for the Hospitalist Series of resources and lectures developed by Eric Siegal, MD, a pulmonologist in Milwaukee, Wisc., and David Aymond, MD, a hospitalist in Alexandria, La., is available on the SHM website. They recommend that hospitalists trying to get oriented to working in the ICU start with the online courses on fluid resuscitation, mechanical ventilation, and noninvasive ventilation.
“Ninety-five percent of management of COVID-19 patients is nothing other than practicing sound critical care medicine,” Dr. Siegal said. “If you want to take effective care of sick COVID patients, you need to develop good foundational critical care skills and knowledge. Without them, you’re doing stuff without understand it.”
Dr. Aymond also encourages hospitalists to develop a stronger understanding of key physiological concepts by reviewing the critical care clinical topics compiled at SHM’s website.
References
1. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan;13(1):6-12.
Although deployment of hospitalists into ICUs during the COVID-19 crisis varies widely, in that sense it reflects the pre-COVID hospital landscape of variable involvement, in which many hospitalists pressed into this role expressed discomfort practicing critical care beyond their scope of training, according to a survey published in the Journal of Hospital Medicine in 2018.1 “Hospitalists frequently deliver critical care services without adequate training or support, most prevalently in rural hospitals,” the authors concluded.
A Critical Care for the Hospitalist Series of resources and lectures developed by Eric Siegal, MD, a pulmonologist in Milwaukee, Wisc., and David Aymond, MD, a hospitalist in Alexandria, La., is available on the SHM website. They recommend that hospitalists trying to get oriented to working in the ICU start with the online courses on fluid resuscitation, mechanical ventilation, and noninvasive ventilation.
“Ninety-five percent of management of COVID-19 patients is nothing other than practicing sound critical care medicine,” Dr. Siegal said. “If you want to take effective care of sick COVID patients, you need to develop good foundational critical care skills and knowledge. Without them, you’re doing stuff without understand it.”
Dr. Aymond also encourages hospitalists to develop a stronger understanding of key physiological concepts by reviewing the critical care clinical topics compiled at SHM’s website.
References
1. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan;13(1):6-12.
Although deployment of hospitalists into ICUs during the COVID-19 crisis varies widely, in that sense it reflects the pre-COVID hospital landscape of variable involvement, in which many hospitalists pressed into this role expressed discomfort practicing critical care beyond their scope of training, according to a survey published in the Journal of Hospital Medicine in 2018.1 “Hospitalists frequently deliver critical care services without adequate training or support, most prevalently in rural hospitals,” the authors concluded.
A Critical Care for the Hospitalist Series of resources and lectures developed by Eric Siegal, MD, a pulmonologist in Milwaukee, Wisc., and David Aymond, MD, a hospitalist in Alexandria, La., is available on the SHM website. They recommend that hospitalists trying to get oriented to working in the ICU start with the online courses on fluid resuscitation, mechanical ventilation, and noninvasive ventilation.
“Ninety-five percent of management of COVID-19 patients is nothing other than practicing sound critical care medicine,” Dr. Siegal said. “If you want to take effective care of sick COVID patients, you need to develop good foundational critical care skills and knowledge. Without them, you’re doing stuff without understand it.”
Dr. Aymond also encourages hospitalists to develop a stronger understanding of key physiological concepts by reviewing the critical care clinical topics compiled at SHM’s website.
References
1. Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan;13(1):6-12.
Antinuclear antibody test interpretation guidance gets updated
New recommendations from the European League Against Rheumatism on interpreting the results of antinuclear antibody (ANA) testing advised taking the test methodology into account because of differences in performance.
ANA results vary not only by the test being used but also by the underlying disease they are being used to assess, warned Pier Luigi Meroni, MD, director of the Immunorheumatology Research Laboratory at the IRCCS Istituto Auxologico Italiano in Milan.
“Antinuclear antibody testing is a known diagnostic tool. But the recent advances in methodologies strongly suggests that we have to update our knowledge for a better interpretation of the results,” Dr. Meroni said in his presentation at the annual European Congress of Rheumatology, held online this year due to COVID-19.
There is “no doubt that ANA testing is useful,” he continued, adding that ANA is used as a primary screening tool in many rheumatic diseases, notably systemic lupus erythematosus (SLE), primary Sjögren’s syndrome, and systemic sclerosis. It’s also recently been suggested as an important entry criterion for the classification of SLE.
In fact, the 2019 SLE classification criteria – developed by EULAR in collaboration with the American College of Rheumatology – state that “testing by immunofluorescence on HEp-2 cells or a solid-phase ANA screening immunoassay with at least equivalent performance is highly recommended,” Dr. Meroni said.
The ideas underpinning that recommendation was that “ANA expression is invariable in SLE, and that ANA-negative lupus is quite rare,” he explained. Also, as SLE expression persists over time, ANA testing could be used for classification at any point in the disease course. These assumptions have been borne out in several studies, with very small percentages of patients (6% or less) having ANA-negative lupus, and more than 80% having a positive HEp-2 test over time, even with immunosuppressive treatment.
Which test methodology to use?
There are several methods that can be used to detect ANA, including the preferred HEp-2 indirect fluorescence assay (IFA), several solid-phase assays (SpA), and line- or dot-blot immunoassays. The issue is which assay should be used in which disease?
The performance of a particular assay can depend on the disease in which they are used. For instance, while the HEp-2 IFA and SpA are equivalent in SLE and in other connective tissue diseases, “this is not the case for other autoimmune diseases in which basically we don’t know exactly all the autoantigens,” Dr. Meroni explained. “Most of the autoantigens are undefined. They cannot be found in solid-phase kits, and we have to use the IFA for detecting all these autoantibodies.”
Importantly, neither the IFA nor the SpA is superior to the other. “We just say that one technique can detect relevant antibodies that are not detectable by the other one, and maybe the combination of the two techniques can be the right strategy to get the highest sensitivity,” Dr. Meroni said.
“Clinicians should be aware of the type of assay used for ANA detection,” he said, “because there are strong differences in the performance, for example between IFA and SpA, and such differences can have important clinical and relevant consequences.”
The test selected will depend on if the aim is to exclude or confirm a disease, and the optimal strategy will depend on pretest probability. For instance, IFA is more sensitive than SpA for SLE and scleroderma, whereas IFA is less sensitive than SpA for Sjögren’s. For SLE, it is suggested to use both the IFA and SpA. A combination of both tests is also considered optimal for scleroderma. SpA testing offers the best sensitivity for Sjögren’s.
“The story is a little bit more complicated for inflammatory myopathies in which we don’t have assays able to detect all the autoantibodies,” Dr. Meroni said. In that situation, several different techniques have to be used to check if the SpA results fit with the IFA pattern.
In 2019, the ACR released its own position statement on ANA testing, highlighting that it supported the use of the HEp-2 IFA assay as the preferred option for ANA testing and that labs should specify the methods being used to test for ANA when reporting their results. The ACR position statement also noted that “ordering health care professionals should select specific ANA subserologies based on a patient’s signs and symptoms and when there is a high pretest suspicion for a specific condition.”
Dr. Meroni disclosed serving as a consultant to Inova Diagnostics, Thermo Fisher Scientific, Pfizer, AbbVie, Merck Sharp & Dohme, and UCB.
New recommendations from the European League Against Rheumatism on interpreting the results of antinuclear antibody (ANA) testing advised taking the test methodology into account because of differences in performance.
ANA results vary not only by the test being used but also by the underlying disease they are being used to assess, warned Pier Luigi Meroni, MD, director of the Immunorheumatology Research Laboratory at the IRCCS Istituto Auxologico Italiano in Milan.
“Antinuclear antibody testing is a known diagnostic tool. But the recent advances in methodologies strongly suggests that we have to update our knowledge for a better interpretation of the results,” Dr. Meroni said in his presentation at the annual European Congress of Rheumatology, held online this year due to COVID-19.
There is “no doubt that ANA testing is useful,” he continued, adding that ANA is used as a primary screening tool in many rheumatic diseases, notably systemic lupus erythematosus (SLE), primary Sjögren’s syndrome, and systemic sclerosis. It’s also recently been suggested as an important entry criterion for the classification of SLE.
In fact, the 2019 SLE classification criteria – developed by EULAR in collaboration with the American College of Rheumatology – state that “testing by immunofluorescence on HEp-2 cells or a solid-phase ANA screening immunoassay with at least equivalent performance is highly recommended,” Dr. Meroni said.
The ideas underpinning that recommendation was that “ANA expression is invariable in SLE, and that ANA-negative lupus is quite rare,” he explained. Also, as SLE expression persists over time, ANA testing could be used for classification at any point in the disease course. These assumptions have been borne out in several studies, with very small percentages of patients (6% or less) having ANA-negative lupus, and more than 80% having a positive HEp-2 test over time, even with immunosuppressive treatment.
Which test methodology to use?
There are several methods that can be used to detect ANA, including the preferred HEp-2 indirect fluorescence assay (IFA), several solid-phase assays (SpA), and line- or dot-blot immunoassays. The issue is which assay should be used in which disease?
The performance of a particular assay can depend on the disease in which they are used. For instance, while the HEp-2 IFA and SpA are equivalent in SLE and in other connective tissue diseases, “this is not the case for other autoimmune diseases in which basically we don’t know exactly all the autoantigens,” Dr. Meroni explained. “Most of the autoantigens are undefined. They cannot be found in solid-phase kits, and we have to use the IFA for detecting all these autoantibodies.”
Importantly, neither the IFA nor the SpA is superior to the other. “We just say that one technique can detect relevant antibodies that are not detectable by the other one, and maybe the combination of the two techniques can be the right strategy to get the highest sensitivity,” Dr. Meroni said.
“Clinicians should be aware of the type of assay used for ANA detection,” he said, “because there are strong differences in the performance, for example between IFA and SpA, and such differences can have important clinical and relevant consequences.”
The test selected will depend on if the aim is to exclude or confirm a disease, and the optimal strategy will depend on pretest probability. For instance, IFA is more sensitive than SpA for SLE and scleroderma, whereas IFA is less sensitive than SpA for Sjögren’s. For SLE, it is suggested to use both the IFA and SpA. A combination of both tests is also considered optimal for scleroderma. SpA testing offers the best sensitivity for Sjögren’s.
“The story is a little bit more complicated for inflammatory myopathies in which we don’t have assays able to detect all the autoantibodies,” Dr. Meroni said. In that situation, several different techniques have to be used to check if the SpA results fit with the IFA pattern.
In 2019, the ACR released its own position statement on ANA testing, highlighting that it supported the use of the HEp-2 IFA assay as the preferred option for ANA testing and that labs should specify the methods being used to test for ANA when reporting their results. The ACR position statement also noted that “ordering health care professionals should select specific ANA subserologies based on a patient’s signs and symptoms and when there is a high pretest suspicion for a specific condition.”
Dr. Meroni disclosed serving as a consultant to Inova Diagnostics, Thermo Fisher Scientific, Pfizer, AbbVie, Merck Sharp & Dohme, and UCB.
New recommendations from the European League Against Rheumatism on interpreting the results of antinuclear antibody (ANA) testing advised taking the test methodology into account because of differences in performance.
ANA results vary not only by the test being used but also by the underlying disease they are being used to assess, warned Pier Luigi Meroni, MD, director of the Immunorheumatology Research Laboratory at the IRCCS Istituto Auxologico Italiano in Milan.
“Antinuclear antibody testing is a known diagnostic tool. But the recent advances in methodologies strongly suggests that we have to update our knowledge for a better interpretation of the results,” Dr. Meroni said in his presentation at the annual European Congress of Rheumatology, held online this year due to COVID-19.
There is “no doubt that ANA testing is useful,” he continued, adding that ANA is used as a primary screening tool in many rheumatic diseases, notably systemic lupus erythematosus (SLE), primary Sjögren’s syndrome, and systemic sclerosis. It’s also recently been suggested as an important entry criterion for the classification of SLE.
In fact, the 2019 SLE classification criteria – developed by EULAR in collaboration with the American College of Rheumatology – state that “testing by immunofluorescence on HEp-2 cells or a solid-phase ANA screening immunoassay with at least equivalent performance is highly recommended,” Dr. Meroni said.
The ideas underpinning that recommendation was that “ANA expression is invariable in SLE, and that ANA-negative lupus is quite rare,” he explained. Also, as SLE expression persists over time, ANA testing could be used for classification at any point in the disease course. These assumptions have been borne out in several studies, with very small percentages of patients (6% or less) having ANA-negative lupus, and more than 80% having a positive HEp-2 test over time, even with immunosuppressive treatment.
Which test methodology to use?
There are several methods that can be used to detect ANA, including the preferred HEp-2 indirect fluorescence assay (IFA), several solid-phase assays (SpA), and line- or dot-blot immunoassays. The issue is which assay should be used in which disease?
The performance of a particular assay can depend on the disease in which they are used. For instance, while the HEp-2 IFA and SpA are equivalent in SLE and in other connective tissue diseases, “this is not the case for other autoimmune diseases in which basically we don’t know exactly all the autoantigens,” Dr. Meroni explained. “Most of the autoantigens are undefined. They cannot be found in solid-phase kits, and we have to use the IFA for detecting all these autoantibodies.”
Importantly, neither the IFA nor the SpA is superior to the other. “We just say that one technique can detect relevant antibodies that are not detectable by the other one, and maybe the combination of the two techniques can be the right strategy to get the highest sensitivity,” Dr. Meroni said.
“Clinicians should be aware of the type of assay used for ANA detection,” he said, “because there are strong differences in the performance, for example between IFA and SpA, and such differences can have important clinical and relevant consequences.”
The test selected will depend on if the aim is to exclude or confirm a disease, and the optimal strategy will depend on pretest probability. For instance, IFA is more sensitive than SpA for SLE and scleroderma, whereas IFA is less sensitive than SpA for Sjögren’s. For SLE, it is suggested to use both the IFA and SpA. A combination of both tests is also considered optimal for scleroderma. SpA testing offers the best sensitivity for Sjögren’s.
“The story is a little bit more complicated for inflammatory myopathies in which we don’t have assays able to detect all the autoantibodies,” Dr. Meroni said. In that situation, several different techniques have to be used to check if the SpA results fit with the IFA pattern.
In 2019, the ACR released its own position statement on ANA testing, highlighting that it supported the use of the HEp-2 IFA assay as the preferred option for ANA testing and that labs should specify the methods being used to test for ANA when reporting their results. The ACR position statement also noted that “ordering health care professionals should select specific ANA subserologies based on a patient’s signs and symptoms and when there is a high pretest suspicion for a specific condition.”
Dr. Meroni disclosed serving as a consultant to Inova Diagnostics, Thermo Fisher Scientific, Pfizer, AbbVie, Merck Sharp & Dohme, and UCB.
FROM THE EULAR 2020 E-CONGRESS
It’s official: COVID-19 was bad for the health care business
COVID-19 took a huge cut of clinicians’ business in March and April
In the first 2 months of the COVID-19 pandemic, health care professionals experienced sharp drops in both utilization and revenue, according to an analysis of the nation’s largest collection of private health care claims data.
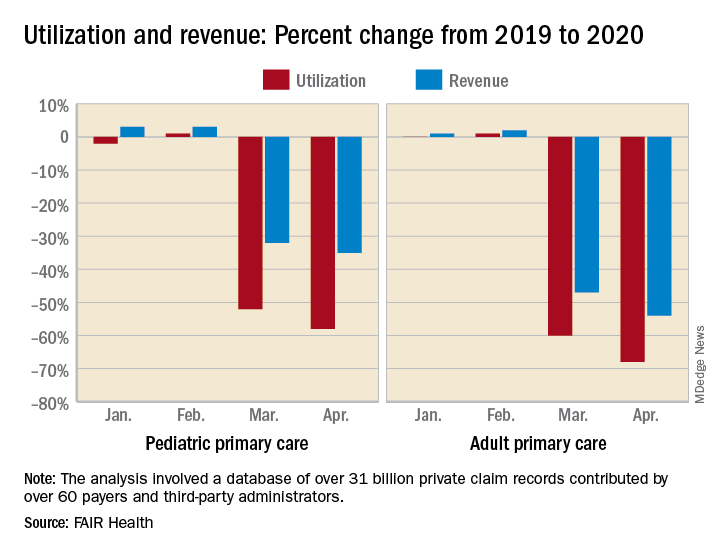
For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
For the Northeast states – the epicenter of the pandemic in March and April – patient volume was down by 60% in March and 80% in April, while revenue fell by 55% in March and 79% in April, the organization said.
For this analysis, “a professional service was defined as any service provided by an individual (e.g., physician, nurse, nurse practitioner, physician assistant) instead of being billed by a facility,” FAIR Health noted. Figures for 2019 were adjusted using the Consumer Price Index.
The size of the pandemic-related decreases in utilization and income varied by specialty. Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
After experiencing a 2% drop in utilization this January and an increase of 4% in February, compared with 2019, gastroenterology saw corresponding drops of 73% in March and 77% in April. Estimated revenue for the specialty was flat in January and rose by 10% in February, but plummeted by 75% in March and 80% in April, the FAIR Health data show.
In cardiology, patient volume from 2019 to 2020 looked like this: Down by 4% in January, up 5% in February, down by 62% in March, and down by 71% in April. The earnings numbers tell a similar story: Down by 2% in January, up by 15% in February, down by 57% in March, and down by 73% in April, the organization reported.
Dermatology did the best among the non–primary care specialties, but that was just a relative success. Utilization still dropped by 62% and 68% in March and April of 2020, compared with last year, and revenue declined by 50% in March and 59% in April, FAIR Health said.
For adult primary care, the utilization numbers were similar, but revenue took a somewhat smaller hit. Patient volume from 2019 to 2020 was fairly steady in January and February, then nosedived in March (down 60%) and April (down 68%). Earnings were up initially, rising 1% in January and 2% in February, but fell 47% in March and 54% in April, FAIR Health said.
Pediatric primary care, it appears, may have been buoyed somewhat by its younger patients. The specialty as a whole saw utilization tumble by 52% in March and 58% in April, but revenue dropped by just 32% and 35%, respectively, according to the report.
A little extra data diving showed that the figures for preventive care visits for patients aged 0-4 years in March and April were –2% and 0% for volume and –2% and 1% for revenue. Meanwhile, the volume of immunizations only dropped by 14% and 10% and vaccine-related revenue slipped by just 7% and 2%, FAIR Health noted.
“Across many specialties from January to April 2020, office or other outpatient [evaluation and management] visits became more common relative to other procedures. ... This may have been due in part to the fact that many of these E&M services could be rendered via telehealth,” FAIR Health said.
Telehealth, however, was no panacea, the report explained: “Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology.”
COVID-19 took a huge cut of clinicians’ business in March and April
COVID-19 took a huge cut of clinicians’ business in March and April
In the first 2 months of the COVID-19 pandemic, health care professionals experienced sharp drops in both utilization and revenue, according to an analysis of the nation’s largest collection of private health care claims data.

For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
For the Northeast states – the epicenter of the pandemic in March and April – patient volume was down by 60% in March and 80% in April, while revenue fell by 55% in March and 79% in April, the organization said.
For this analysis, “a professional service was defined as any service provided by an individual (e.g., physician, nurse, nurse practitioner, physician assistant) instead of being billed by a facility,” FAIR Health noted. Figures for 2019 were adjusted using the Consumer Price Index.
The size of the pandemic-related decreases in utilization and income varied by specialty. Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
After experiencing a 2% drop in utilization this January and an increase of 4% in February, compared with 2019, gastroenterology saw corresponding drops of 73% in March and 77% in April. Estimated revenue for the specialty was flat in January and rose by 10% in February, but plummeted by 75% in March and 80% in April, the FAIR Health data show.
In cardiology, patient volume from 2019 to 2020 looked like this: Down by 4% in January, up 5% in February, down by 62% in March, and down by 71% in April. The earnings numbers tell a similar story: Down by 2% in January, up by 15% in February, down by 57% in March, and down by 73% in April, the organization reported.
Dermatology did the best among the non–primary care specialties, but that was just a relative success. Utilization still dropped by 62% and 68% in March and April of 2020, compared with last year, and revenue declined by 50% in March and 59% in April, FAIR Health said.
For adult primary care, the utilization numbers were similar, but revenue took a somewhat smaller hit. Patient volume from 2019 to 2020 was fairly steady in January and February, then nosedived in March (down 60%) and April (down 68%). Earnings were up initially, rising 1% in January and 2% in February, but fell 47% in March and 54% in April, FAIR Health said.
Pediatric primary care, it appears, may have been buoyed somewhat by its younger patients. The specialty as a whole saw utilization tumble by 52% in March and 58% in April, but revenue dropped by just 32% and 35%, respectively, according to the report.
A little extra data diving showed that the figures for preventive care visits for patients aged 0-4 years in March and April were –2% and 0% for volume and –2% and 1% for revenue. Meanwhile, the volume of immunizations only dropped by 14% and 10% and vaccine-related revenue slipped by just 7% and 2%, FAIR Health noted.
“Across many specialties from January to April 2020, office or other outpatient [evaluation and management] visits became more common relative to other procedures. ... This may have been due in part to the fact that many of these E&M services could be rendered via telehealth,” FAIR Health said.
Telehealth, however, was no panacea, the report explained: “Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology.”
In the first 2 months of the COVID-19 pandemic, health care professionals experienced sharp drops in both utilization and revenue, according to an analysis of the nation’s largest collection of private health care claims data.

For the months of March and April 2020, use of medical professional services dropped by 65% and 68%, respectively, compared with last year, and estimated revenue fell by 45% and 48%, FAIR Health, a nonprofit organization that manages a database of 31 billion claim records, said in a new report.
For the Northeast states – the epicenter of the pandemic in March and April – patient volume was down by 60% in March and 80% in April, while revenue fell by 55% in March and 79% in April, the organization said.
For this analysis, “a professional service was defined as any service provided by an individual (e.g., physician, nurse, nurse practitioner, physician assistant) instead of being billed by a facility,” FAIR Health noted. Figures for 2019 were adjusted using the Consumer Price Index.
The size of the pandemic-related decreases in utilization and income varied by specialty. Of the seven specialties included in the study, oral surgery was hit the hardest, followed by gastroenterology, cardiology, orthopedics, dermatology, adult primary care, and pediatric primary care, FAIR Health said.
After experiencing a 2% drop in utilization this January and an increase of 4% in February, compared with 2019, gastroenterology saw corresponding drops of 73% in March and 77% in April. Estimated revenue for the specialty was flat in January and rose by 10% in February, but plummeted by 75% in March and 80% in April, the FAIR Health data show.
In cardiology, patient volume from 2019 to 2020 looked like this: Down by 4% in January, up 5% in February, down by 62% in March, and down by 71% in April. The earnings numbers tell a similar story: Down by 2% in January, up by 15% in February, down by 57% in March, and down by 73% in April, the organization reported.
Dermatology did the best among the non–primary care specialties, but that was just a relative success. Utilization still dropped by 62% and 68% in March and April of 2020, compared with last year, and revenue declined by 50% in March and 59% in April, FAIR Health said.
For adult primary care, the utilization numbers were similar, but revenue took a somewhat smaller hit. Patient volume from 2019 to 2020 was fairly steady in January and February, then nosedived in March (down 60%) and April (down 68%). Earnings were up initially, rising 1% in January and 2% in February, but fell 47% in March and 54% in April, FAIR Health said.
Pediatric primary care, it appears, may have been buoyed somewhat by its younger patients. The specialty as a whole saw utilization tumble by 52% in March and 58% in April, but revenue dropped by just 32% and 35%, respectively, according to the report.
A little extra data diving showed that the figures for preventive care visits for patients aged 0-4 years in March and April were –2% and 0% for volume and –2% and 1% for revenue. Meanwhile, the volume of immunizations only dropped by 14% and 10% and vaccine-related revenue slipped by just 7% and 2%, FAIR Health noted.
“Across many specialties from January to April 2020, office or other outpatient [evaluation and management] visits became more common relative to other procedures. ... This may have been due in part to the fact that many of these E&M services could be rendered via telehealth,” FAIR Health said.
Telehealth, however, was no panacea, the report explained: “Even when medical practices have continued to function via telehealth, many have experienced lower reimbursements for telehealth visits than for in-person visits and more time educating patients on how to use the technology.”
Results from two phase 3 trials of bimekizumab unveiled
Results from two late-breaking
“The rapid and lasting skin clearance observed in the majority of patients in both clinical studies demonstrate bimekizumab’s strong potential to deliver across three key areas: speed, depth and durability,” Kristian Reich, MD, said in an interview during the virtual annual meeting of the American Academy of Dermatology.
Bimekizumab selectively inhibits IL-17A and IL-17F, two key cytokines that drive inflammation and tissue damage across multiple diseases. In BE VIVID, Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 567 patients with moderate to severe psoriasis to bimekizumab 320 mg every 4 weeks (Q4W), ustekinumab (45/90 mg weight-based dosing at baseline and week 4, then every 12 weeks), or placebo (Q4W through week 16 then bimekizumab 320 mg Q4W). Coprimary endpoints were a Psoriasis Area and Severity Index (PASI) of at least 90 and an Investigator Global Assessment (IGA) response of 0 or 1. Secondary/other outcomes included PASI 100 at week 16; PASI 90, IGA 0/1, and PASI 100 at week 52; and safety. (Ustekinumab is an IL-12 and IL-23 antagonist.)
The mean age of patients was 46 years and 72% were male. The researchers found that the proportion of patients who achieved PASI 90 and an IGA of 0/1 was higher in the bimekizumab arm at week 16 (85.0% and 84.1%, respectively), compared with those in the ustekinumab arm (49.7% and 53.4%) and those on placebo (4.8% and 4.8%; P < .001 for all associations). In addition, 58.6% of patients in the bimekizumab arm achieved PASI 100, compared with 20.9% of those in the ustekinumab arm and none of those on placebo.
At week 52, patients in the bimekizumab arm achieved PASI 90, IGA 0/1, and PASI 100 response rates of 81.6%, 77.9%, and 64.2%, respectively, compared with 55.8%, 60.7%, and 38.0% of those in the ustekinumab arm. Over 52 weeks, incidence of serious treatment-emergent adverse events was 6.1% with bimekizumab arm, compared with 7.4% in the ustekinumab arm. Four deaths occurred (two in the bimekizumab arm, and one each in the ustekinumab and placebo arms), all considered unrelated to treatment. The most common reported adverse events in the bimekizumab arm through week 52 were nasopharyngitis (21.8%), oral candidiasis (15.2%), and upper respiratory tract infections (9.1%).
“The rapid and lasting skin clearance observed in the majority of patients treated with bimekizumab provide support for inhibiting IL-17F, in addition to IL-17A, to inhibit the IL-17 pathway,” Dr. Reich said. “This can make a meaningful difference for people living with psoriasis.” He added that the results of the head-to-head study of bimekizumab with secukinumab (an IL-17A antagonist) are expected later this year. “It will be very interesting to see if the marked differences in treatment effect seen in the BE VIVID study remain when comparing to an IL-17.”
In BE READY, a pivotal phase 3, randomized, withdrawal study, investigators led by Kenneth Gordon, MD, randomized 435 patients with moderate to severe psoriasis 4:1 to receive 320 mg Q4W or placebo, and followed them for 16 weeks. In a second part of the study, patients who had achieved at least a PASI 90 response at week 16 were rerandomized to receive continuous bimekizumab at two different dosing regimens: 320 mg Q4W or 320 mg every 8 weeks (Q8W), or to be withdrawn from treatment (placebo Q4W), and followed through week 56. Relapse was defined as a PASI score of less than 75 from week 20.
The mean age of patients was 44 years and 72% were male. At week 16, the proportion of patients who achieved a PASI 90 and an IGA of 0/1 was greatest in the bimekizumab arm (90.8% and 92.6%, respectively), compared with those on placebo. In addition, 68.2% of patients in the bimekizumab arm achieved PASI 100 at week 16, compared with only 1.2% of those on placebo (P < .001 for all associations). In the second part of the study, the researchers found that 86.8% of patients who received continuous bimekizumab 320 mg Q4W maintained PASI 90 at week 56, compared with 91% who were switched to bimekizumab 320 mg Q8W, and 16.2% of patients who were withdrawn from the trial.
“The speed of response and the number of patients who achieved clearance are extremely high, especially in a phase 3 trial,” Dr. Gordon, professor and Thomas R. Russell Family Chair of Dermatology at the Medical College of Wisconsin, Milwaukee, said in an interview. “However, the most surprising aspect may be the impressive maintenance of response in patients, even those who were treated with every-8-week dosing in the maintenance phase. While it is possible that there are some patients who may benefit from more frequent dosing in the long term, the possibility of every-8-week dosing would be a tremendous benefit for patients.”
As in the BE VIVID trial, the most frequently reported adverse events with bimekizumab between week 16 and week 56 in BE READY were nasopharyngitis (10.4% in the Q4W arm vs. 23% in the Q8W arm), oral candidiasis (11.3% Q4W vs. 9% Q8W), and upper respiratory tract infections (11.3% Q4W vs. 8% Q8W). The incidence of serious treatment-emergent adverse events with bimekizumab was 4.7% in the Q4W arm and 3% in the Q8W arm versus 3.8% in the placebo arm at week 56.
“The results from BE READY demonstrate that bimekizumab has the potential to deliver rapid and lasting skin improvement for psoriasis patients,” Dr. Gordon said. “The findings also support the hypothesis that inhibiting IL-17F, in addition to IL-17A, may be more effective in suppressing inflammation in suppressing inflammation in psoriasis than IL-17A inhibition alone.”
Both studies were funded by UCB Pharma. Dr. Reich disclosed that he has served as adviser and/or paid speaker for and/or participated in clinical trials sponsored by companies that include UCB. Dr. Gordon disclosed that he has received honoraria and/or research support from companies that include UCB..
Results from two late-breaking
“The rapid and lasting skin clearance observed in the majority of patients in both clinical studies demonstrate bimekizumab’s strong potential to deliver across three key areas: speed, depth and durability,” Kristian Reich, MD, said in an interview during the virtual annual meeting of the American Academy of Dermatology.
Bimekizumab selectively inhibits IL-17A and IL-17F, two key cytokines that drive inflammation and tissue damage across multiple diseases. In BE VIVID, Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 567 patients with moderate to severe psoriasis to bimekizumab 320 mg every 4 weeks (Q4W), ustekinumab (45/90 mg weight-based dosing at baseline and week 4, then every 12 weeks), or placebo (Q4W through week 16 then bimekizumab 320 mg Q4W). Coprimary endpoints were a Psoriasis Area and Severity Index (PASI) of at least 90 and an Investigator Global Assessment (IGA) response of 0 or 1. Secondary/other outcomes included PASI 100 at week 16; PASI 90, IGA 0/1, and PASI 100 at week 52; and safety. (Ustekinumab is an IL-12 and IL-23 antagonist.)
The mean age of patients was 46 years and 72% were male. The researchers found that the proportion of patients who achieved PASI 90 and an IGA of 0/1 was higher in the bimekizumab arm at week 16 (85.0% and 84.1%, respectively), compared with those in the ustekinumab arm (49.7% and 53.4%) and those on placebo (4.8% and 4.8%; P < .001 for all associations). In addition, 58.6% of patients in the bimekizumab arm achieved PASI 100, compared with 20.9% of those in the ustekinumab arm and none of those on placebo.
At week 52, patients in the bimekizumab arm achieved PASI 90, IGA 0/1, and PASI 100 response rates of 81.6%, 77.9%, and 64.2%, respectively, compared with 55.8%, 60.7%, and 38.0% of those in the ustekinumab arm. Over 52 weeks, incidence of serious treatment-emergent adverse events was 6.1% with bimekizumab arm, compared with 7.4% in the ustekinumab arm. Four deaths occurred (two in the bimekizumab arm, and one each in the ustekinumab and placebo arms), all considered unrelated to treatment. The most common reported adverse events in the bimekizumab arm through week 52 were nasopharyngitis (21.8%), oral candidiasis (15.2%), and upper respiratory tract infections (9.1%).
“The rapid and lasting skin clearance observed in the majority of patients treated with bimekizumab provide support for inhibiting IL-17F, in addition to IL-17A, to inhibit the IL-17 pathway,” Dr. Reich said. “This can make a meaningful difference for people living with psoriasis.” He added that the results of the head-to-head study of bimekizumab with secukinumab (an IL-17A antagonist) are expected later this year. “It will be very interesting to see if the marked differences in treatment effect seen in the BE VIVID study remain when comparing to an IL-17.”
In BE READY, a pivotal phase 3, randomized, withdrawal study, investigators led by Kenneth Gordon, MD, randomized 435 patients with moderate to severe psoriasis 4:1 to receive 320 mg Q4W or placebo, and followed them for 16 weeks. In a second part of the study, patients who had achieved at least a PASI 90 response at week 16 were rerandomized to receive continuous bimekizumab at two different dosing regimens: 320 mg Q4W or 320 mg every 8 weeks (Q8W), or to be withdrawn from treatment (placebo Q4W), and followed through week 56. Relapse was defined as a PASI score of less than 75 from week 20.
The mean age of patients was 44 years and 72% were male. At week 16, the proportion of patients who achieved a PASI 90 and an IGA of 0/1 was greatest in the bimekizumab arm (90.8% and 92.6%, respectively), compared with those on placebo. In addition, 68.2% of patients in the bimekizumab arm achieved PASI 100 at week 16, compared with only 1.2% of those on placebo (P < .001 for all associations). In the second part of the study, the researchers found that 86.8% of patients who received continuous bimekizumab 320 mg Q4W maintained PASI 90 at week 56, compared with 91% who were switched to bimekizumab 320 mg Q8W, and 16.2% of patients who were withdrawn from the trial.
“The speed of response and the number of patients who achieved clearance are extremely high, especially in a phase 3 trial,” Dr. Gordon, professor and Thomas R. Russell Family Chair of Dermatology at the Medical College of Wisconsin, Milwaukee, said in an interview. “However, the most surprising aspect may be the impressive maintenance of response in patients, even those who were treated with every-8-week dosing in the maintenance phase. While it is possible that there are some patients who may benefit from more frequent dosing in the long term, the possibility of every-8-week dosing would be a tremendous benefit for patients.”
As in the BE VIVID trial, the most frequently reported adverse events with bimekizumab between week 16 and week 56 in BE READY were nasopharyngitis (10.4% in the Q4W arm vs. 23% in the Q8W arm), oral candidiasis (11.3% Q4W vs. 9% Q8W), and upper respiratory tract infections (11.3% Q4W vs. 8% Q8W). The incidence of serious treatment-emergent adverse events with bimekizumab was 4.7% in the Q4W arm and 3% in the Q8W arm versus 3.8% in the placebo arm at week 56.
“The results from BE READY demonstrate that bimekizumab has the potential to deliver rapid and lasting skin improvement for psoriasis patients,” Dr. Gordon said. “The findings also support the hypothesis that inhibiting IL-17F, in addition to IL-17A, may be more effective in suppressing inflammation in suppressing inflammation in psoriasis than IL-17A inhibition alone.”
Both studies were funded by UCB Pharma. Dr. Reich disclosed that he has served as adviser and/or paid speaker for and/or participated in clinical trials sponsored by companies that include UCB. Dr. Gordon disclosed that he has received honoraria and/or research support from companies that include UCB..
Results from two late-breaking
“The rapid and lasting skin clearance observed in the majority of patients in both clinical studies demonstrate bimekizumab’s strong potential to deliver across three key areas: speed, depth and durability,” Kristian Reich, MD, said in an interview during the virtual annual meeting of the American Academy of Dermatology.
Bimekizumab selectively inhibits IL-17A and IL-17F, two key cytokines that drive inflammation and tissue damage across multiple diseases. In BE VIVID, Dr. Reich, of the Center for Translational Research in Inflammatory Skin Diseases at the University Medical Center Hamburg-Eppendorf (Germany), and colleagues randomized 567 patients with moderate to severe psoriasis to bimekizumab 320 mg every 4 weeks (Q4W), ustekinumab (45/90 mg weight-based dosing at baseline and week 4, then every 12 weeks), or placebo (Q4W through week 16 then bimekizumab 320 mg Q4W). Coprimary endpoints were a Psoriasis Area and Severity Index (PASI) of at least 90 and an Investigator Global Assessment (IGA) response of 0 or 1. Secondary/other outcomes included PASI 100 at week 16; PASI 90, IGA 0/1, and PASI 100 at week 52; and safety. (Ustekinumab is an IL-12 and IL-23 antagonist.)
The mean age of patients was 46 years and 72% were male. The researchers found that the proportion of patients who achieved PASI 90 and an IGA of 0/1 was higher in the bimekizumab arm at week 16 (85.0% and 84.1%, respectively), compared with those in the ustekinumab arm (49.7% and 53.4%) and those on placebo (4.8% and 4.8%; P < .001 for all associations). In addition, 58.6% of patients in the bimekizumab arm achieved PASI 100, compared with 20.9% of those in the ustekinumab arm and none of those on placebo.
At week 52, patients in the bimekizumab arm achieved PASI 90, IGA 0/1, and PASI 100 response rates of 81.6%, 77.9%, and 64.2%, respectively, compared with 55.8%, 60.7%, and 38.0% of those in the ustekinumab arm. Over 52 weeks, incidence of serious treatment-emergent adverse events was 6.1% with bimekizumab arm, compared with 7.4% in the ustekinumab arm. Four deaths occurred (two in the bimekizumab arm, and one each in the ustekinumab and placebo arms), all considered unrelated to treatment. The most common reported adverse events in the bimekizumab arm through week 52 were nasopharyngitis (21.8%), oral candidiasis (15.2%), and upper respiratory tract infections (9.1%).
“The rapid and lasting skin clearance observed in the majority of patients treated with bimekizumab provide support for inhibiting IL-17F, in addition to IL-17A, to inhibit the IL-17 pathway,” Dr. Reich said. “This can make a meaningful difference for people living with psoriasis.” He added that the results of the head-to-head study of bimekizumab with secukinumab (an IL-17A antagonist) are expected later this year. “It will be very interesting to see if the marked differences in treatment effect seen in the BE VIVID study remain when comparing to an IL-17.”
In BE READY, a pivotal phase 3, randomized, withdrawal study, investigators led by Kenneth Gordon, MD, randomized 435 patients with moderate to severe psoriasis 4:1 to receive 320 mg Q4W or placebo, and followed them for 16 weeks. In a second part of the study, patients who had achieved at least a PASI 90 response at week 16 were rerandomized to receive continuous bimekizumab at two different dosing regimens: 320 mg Q4W or 320 mg every 8 weeks (Q8W), or to be withdrawn from treatment (placebo Q4W), and followed through week 56. Relapse was defined as a PASI score of less than 75 from week 20.
The mean age of patients was 44 years and 72% were male. At week 16, the proportion of patients who achieved a PASI 90 and an IGA of 0/1 was greatest in the bimekizumab arm (90.8% and 92.6%, respectively), compared with those on placebo. In addition, 68.2% of patients in the bimekizumab arm achieved PASI 100 at week 16, compared with only 1.2% of those on placebo (P < .001 for all associations). In the second part of the study, the researchers found that 86.8% of patients who received continuous bimekizumab 320 mg Q4W maintained PASI 90 at week 56, compared with 91% who were switched to bimekizumab 320 mg Q8W, and 16.2% of patients who were withdrawn from the trial.
“The speed of response and the number of patients who achieved clearance are extremely high, especially in a phase 3 trial,” Dr. Gordon, professor and Thomas R. Russell Family Chair of Dermatology at the Medical College of Wisconsin, Milwaukee, said in an interview. “However, the most surprising aspect may be the impressive maintenance of response in patients, even those who were treated with every-8-week dosing in the maintenance phase. While it is possible that there are some patients who may benefit from more frequent dosing in the long term, the possibility of every-8-week dosing would be a tremendous benefit for patients.”
As in the BE VIVID trial, the most frequently reported adverse events with bimekizumab between week 16 and week 56 in BE READY were nasopharyngitis (10.4% in the Q4W arm vs. 23% in the Q8W arm), oral candidiasis (11.3% Q4W vs. 9% Q8W), and upper respiratory tract infections (11.3% Q4W vs. 8% Q8W). The incidence of serious treatment-emergent adverse events with bimekizumab was 4.7% in the Q4W arm and 3% in the Q8W arm versus 3.8% in the placebo arm at week 56.
“The results from BE READY demonstrate that bimekizumab has the potential to deliver rapid and lasting skin improvement for psoriasis patients,” Dr. Gordon said. “The findings also support the hypothesis that inhibiting IL-17F, in addition to IL-17A, may be more effective in suppressing inflammation in suppressing inflammation in psoriasis than IL-17A inhibition alone.”
Both studies were funded by UCB Pharma. Dr. Reich disclosed that he has served as adviser and/or paid speaker for and/or participated in clinical trials sponsored by companies that include UCB. Dr. Gordon disclosed that he has received honoraria and/or research support from companies that include UCB..
FROM AAD 2020
Be vigilant for scleroderma renal crisis
Scleroderma renal crisis is often the most challenging type of scleroderma emergency to identify promptly, according to Francesco Boin, MD, professor of medicine and director of the scleroderma center at the University of California, San Francisco.
“Fortunately, it’s not a frequent event. But it’s severe enough that all rheumatologists should be aware of it,” he said at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Atypical presentations occur in 30%
Scleroderma renal crisis (SRC) occurs in 5%-10% of scleroderma patients. A vexing feature of this emergency is that not uncommonly it actually precedes the diagnosis of scleroderma. Indeed, 20% of patients with SRC present with sine scleroderma – that is, they have no skin disease and their renal crisis is their first symptom of scleroderma. In contrast, critical digital ischemia – the most common scleroderma emergency – is invariably preceded by worsening episodes of Raynaud’s, and impending intestinal pseudo-obstruction – also among the most common scleroderma emergencies – is heralded by an established history of dysmotility, loss of appetite, abdominal bloating, small intestinal bacterial overgrowth, and bowel distension.
While sine SRC often poses a formidable diagnostic challenge, SRC occurs most often in patients with early, rapidly progressing diffuse scleroderma skin disease. Indeed, the median duration of scleroderma when SRC strikes is just 8 months. The use of glucocorticoids at 15 mg or more per day, or at lower doses for a lengthy period, is an independent risk factor for SRC. Detection of anti–RNA polymerase III antibodies warrants increased vigilance, since 60% of patients with SRC are anti–RNA polymerase III antibody positive. Other autoantibodies are not a risk factor. Neither is preexisting hypertension nor a high baseline serum creatinine.
The classic textbook presentation of SRC is abrupt onset of blood pressures greater than 20 mm Hg above normal for that individual, along with sudden renal failure; a climbing creatinine; proteinuria; and expressions of malignant hypertension such as pulmonary edema, new-onset heart failure, encephalopathy, and/or development of a thrombotic microangiopathy.
Notably, however, 30% of individuals with SRC don’t fit this picture at all. They may present with abrupt-onset severe hypertension but no evidence of renal failure, at least early on. Or they may have sudden renal failure without a hypertensive crisis. Alternatively, they may have no signs of malignant hypertension, just an asymptomatic pericardial effusion or mild arrhythmias.
“Also, the thrombotic microangiopathy can be present without the other features of scleroderma renal crisis, so no renal failure or hypertensive emergency. Be aware of the possibility of atypical presentations, and always suspect this unfolding problem in the right individuals,” the rheumatologist urged.
Anyone with scleroderma who presents with new-onset hypertension needs to begin keeping a careful home blood pressure diary. If the blood pressure shoots up, or symptoms of malignant hypertension develop, or laboratory monitoring reveals evidence of thrombotic microangiopathy, the patient should immediately go to the ED because these events are often followed by accelerated progression to renal crisis.
Inpatient management of SRC is critical. “In the hospital we can monitor renal function in a more refined way, we can manage the malignant hypertension, and early on, hospitalization provides the opportunity to do a renal biopsy. I always consider doing this early. The pathologist often pushes back, but I think it’s relevant. It confirms the diagnosis. We’ve had patients where we were surprised: We thought it was scleroderma renal crisis, but instead they had interstitial nephritis or glomerulonephritis. Most important, biopsy has major prognostic implications: You can measure the extent of damage and therefore have a sense of whether the patient will be able to recover renal function,” Dr. Boin explained.
Prognosis and predictors
Outcome of SRC is often poor: the 1-year mortality is 20%-30%, with a 5-year mortality of 30%-50%. Normotensive SRC with renal crisis, which accounts for about 10% of all cases of SRC, is particularly serious in its implication, with a 1-year mortality of 60%. Half of patients with SRC require hemodialysis, and only one-quarter of them recover spontaneous renal function.
Predictors of worse outcome include older age at onset of SRC, male gender, a serum creatinine level above 3 mg/dL at presentation, incomplete blood pressure control within the first 3 days of the crisis, and normotensive SRC. Use of an ACE inhibitor prior to SRC is also an independent predictor of poor outcome, possibly because by keeping the blood pressure under control the medication blunts recognition of the unfolding renal crisis.
“This is why experts don’t recommend prophylactic ACE inhibitors in patients who are at risk for SRC,” according to Dr. Boin.
He reported having no financial conflicts regarding his presentation.
Scleroderma renal crisis is often the most challenging type of scleroderma emergency to identify promptly, according to Francesco Boin, MD, professor of medicine and director of the scleroderma center at the University of California, San Francisco.
“Fortunately, it’s not a frequent event. But it’s severe enough that all rheumatologists should be aware of it,” he said at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Atypical presentations occur in 30%
Scleroderma renal crisis (SRC) occurs in 5%-10% of scleroderma patients. A vexing feature of this emergency is that not uncommonly it actually precedes the diagnosis of scleroderma. Indeed, 20% of patients with SRC present with sine scleroderma – that is, they have no skin disease and their renal crisis is their first symptom of scleroderma. In contrast, critical digital ischemia – the most common scleroderma emergency – is invariably preceded by worsening episodes of Raynaud’s, and impending intestinal pseudo-obstruction – also among the most common scleroderma emergencies – is heralded by an established history of dysmotility, loss of appetite, abdominal bloating, small intestinal bacterial overgrowth, and bowel distension.
While sine SRC often poses a formidable diagnostic challenge, SRC occurs most often in patients with early, rapidly progressing diffuse scleroderma skin disease. Indeed, the median duration of scleroderma when SRC strikes is just 8 months. The use of glucocorticoids at 15 mg or more per day, or at lower doses for a lengthy period, is an independent risk factor for SRC. Detection of anti–RNA polymerase III antibodies warrants increased vigilance, since 60% of patients with SRC are anti–RNA polymerase III antibody positive. Other autoantibodies are not a risk factor. Neither is preexisting hypertension nor a high baseline serum creatinine.
The classic textbook presentation of SRC is abrupt onset of blood pressures greater than 20 mm Hg above normal for that individual, along with sudden renal failure; a climbing creatinine; proteinuria; and expressions of malignant hypertension such as pulmonary edema, new-onset heart failure, encephalopathy, and/or development of a thrombotic microangiopathy.
Notably, however, 30% of individuals with SRC don’t fit this picture at all. They may present with abrupt-onset severe hypertension but no evidence of renal failure, at least early on. Or they may have sudden renal failure without a hypertensive crisis. Alternatively, they may have no signs of malignant hypertension, just an asymptomatic pericardial effusion or mild arrhythmias.
“Also, the thrombotic microangiopathy can be present without the other features of scleroderma renal crisis, so no renal failure or hypertensive emergency. Be aware of the possibility of atypical presentations, and always suspect this unfolding problem in the right individuals,” the rheumatologist urged.
Anyone with scleroderma who presents with new-onset hypertension needs to begin keeping a careful home blood pressure diary. If the blood pressure shoots up, or symptoms of malignant hypertension develop, or laboratory monitoring reveals evidence of thrombotic microangiopathy, the patient should immediately go to the ED because these events are often followed by accelerated progression to renal crisis.
Inpatient management of SRC is critical. “In the hospital we can monitor renal function in a more refined way, we can manage the malignant hypertension, and early on, hospitalization provides the opportunity to do a renal biopsy. I always consider doing this early. The pathologist often pushes back, but I think it’s relevant. It confirms the diagnosis. We’ve had patients where we were surprised: We thought it was scleroderma renal crisis, but instead they had interstitial nephritis or glomerulonephritis. Most important, biopsy has major prognostic implications: You can measure the extent of damage and therefore have a sense of whether the patient will be able to recover renal function,” Dr. Boin explained.
Prognosis and predictors
Outcome of SRC is often poor: the 1-year mortality is 20%-30%, with a 5-year mortality of 30%-50%. Normotensive SRC with renal crisis, which accounts for about 10% of all cases of SRC, is particularly serious in its implication, with a 1-year mortality of 60%. Half of patients with SRC require hemodialysis, and only one-quarter of them recover spontaneous renal function.
Predictors of worse outcome include older age at onset of SRC, male gender, a serum creatinine level above 3 mg/dL at presentation, incomplete blood pressure control within the first 3 days of the crisis, and normotensive SRC. Use of an ACE inhibitor prior to SRC is also an independent predictor of poor outcome, possibly because by keeping the blood pressure under control the medication blunts recognition of the unfolding renal crisis.
“This is why experts don’t recommend prophylactic ACE inhibitors in patients who are at risk for SRC,” according to Dr. Boin.
He reported having no financial conflicts regarding his presentation.
Scleroderma renal crisis is often the most challenging type of scleroderma emergency to identify promptly, according to Francesco Boin, MD, professor of medicine and director of the scleroderma center at the University of California, San Francisco.
“Fortunately, it’s not a frequent event. But it’s severe enough that all rheumatologists should be aware of it,” he said at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
Atypical presentations occur in 30%
Scleroderma renal crisis (SRC) occurs in 5%-10% of scleroderma patients. A vexing feature of this emergency is that not uncommonly it actually precedes the diagnosis of scleroderma. Indeed, 20% of patients with SRC present with sine scleroderma – that is, they have no skin disease and their renal crisis is their first symptom of scleroderma. In contrast, critical digital ischemia – the most common scleroderma emergency – is invariably preceded by worsening episodes of Raynaud’s, and impending intestinal pseudo-obstruction – also among the most common scleroderma emergencies – is heralded by an established history of dysmotility, loss of appetite, abdominal bloating, small intestinal bacterial overgrowth, and bowel distension.
While sine SRC often poses a formidable diagnostic challenge, SRC occurs most often in patients with early, rapidly progressing diffuse scleroderma skin disease. Indeed, the median duration of scleroderma when SRC strikes is just 8 months. The use of glucocorticoids at 15 mg or more per day, or at lower doses for a lengthy period, is an independent risk factor for SRC. Detection of anti–RNA polymerase III antibodies warrants increased vigilance, since 60% of patients with SRC are anti–RNA polymerase III antibody positive. Other autoantibodies are not a risk factor. Neither is preexisting hypertension nor a high baseline serum creatinine.
The classic textbook presentation of SRC is abrupt onset of blood pressures greater than 20 mm Hg above normal for that individual, along with sudden renal failure; a climbing creatinine; proteinuria; and expressions of malignant hypertension such as pulmonary edema, new-onset heart failure, encephalopathy, and/or development of a thrombotic microangiopathy.
Notably, however, 30% of individuals with SRC don’t fit this picture at all. They may present with abrupt-onset severe hypertension but no evidence of renal failure, at least early on. Or they may have sudden renal failure without a hypertensive crisis. Alternatively, they may have no signs of malignant hypertension, just an asymptomatic pericardial effusion or mild arrhythmias.
“Also, the thrombotic microangiopathy can be present without the other features of scleroderma renal crisis, so no renal failure or hypertensive emergency. Be aware of the possibility of atypical presentations, and always suspect this unfolding problem in the right individuals,” the rheumatologist urged.
Anyone with scleroderma who presents with new-onset hypertension needs to begin keeping a careful home blood pressure diary. If the blood pressure shoots up, or symptoms of malignant hypertension develop, or laboratory monitoring reveals evidence of thrombotic microangiopathy, the patient should immediately go to the ED because these events are often followed by accelerated progression to renal crisis.
Inpatient management of SRC is critical. “In the hospital we can monitor renal function in a more refined way, we can manage the malignant hypertension, and early on, hospitalization provides the opportunity to do a renal biopsy. I always consider doing this early. The pathologist often pushes back, but I think it’s relevant. It confirms the diagnosis. We’ve had patients where we were surprised: We thought it was scleroderma renal crisis, but instead they had interstitial nephritis or glomerulonephritis. Most important, biopsy has major prognostic implications: You can measure the extent of damage and therefore have a sense of whether the patient will be able to recover renal function,” Dr. Boin explained.
Prognosis and predictors
Outcome of SRC is often poor: the 1-year mortality is 20%-30%, with a 5-year mortality of 30%-50%. Normotensive SRC with renal crisis, which accounts for about 10% of all cases of SRC, is particularly serious in its implication, with a 1-year mortality of 60%. Half of patients with SRC require hemodialysis, and only one-quarter of them recover spontaneous renal function.
Predictors of worse outcome include older age at onset of SRC, male gender, a serum creatinine level above 3 mg/dL at presentation, incomplete blood pressure control within the first 3 days of the crisis, and normotensive SRC. Use of an ACE inhibitor prior to SRC is also an independent predictor of poor outcome, possibly because by keeping the blood pressure under control the medication blunts recognition of the unfolding renal crisis.
“This is why experts don’t recommend prophylactic ACE inhibitors in patients who are at risk for SRC,” according to Dr. Boin.
He reported having no financial conflicts regarding his presentation.
FROM SOTA 2020
Combo exhibits activity in metastatic mucosal melanoma
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
according to a presentation made as part of the American Society of Clinical Oncology virtual scientific program.
The combination was well tolerated and “the preliminary efficacy seems to be promising,” which warrants a phase 3 trial, said investigator Jun Guo, MD, of the Peking University Cancer Hospital and Institute in Beijing, who presented the findings.
Mucosal melanoma does not respond as well as cutaneous melanoma to standard programmed death-1 (PD-1) blockade, so investigators are looking for additional options, Dr. Guo noted. Earlier studies have shown that vascular endothelial growth factor expression correlates negatively with clinical outcome, so the combination of VEGF inhibition with PD-1 blockade might provide therapeutic opportunities.
To find out, Dr. Guo and colleagues tested the anti-PD-1 antibody toripalimab in combination with the VEGF inhibitor axitinib in a phase 1 trial. The trial was conducted in China, where mucosal melanoma accounts for up to a quarter of all melanoma cases and where toripalimab is approved to treat mucosal melanoma.
The trial enrolled 33 patients with pathologically confirmed metastatic mucosal melanoma. The esophagus and genital tract were the most common primary lesion sites (both 21.2%). The patients’ average age was 53.4 years, and 60.6% were women. Two patients (6.1%) had previously received systemic chemotherapy. Most (64.6%) were PD–ligand 1 (PD-L1) negative, and most (60.6%) were BRAF/RAS/NF1 wild type.
The patients received axitinib at 5 mg twice daily plus toripalimab at 3 mg/kg every 2 weeks until confirmed disease progression, unacceptable toxicity, or voluntary withdrawal.
As of May 2, 2020, the overall response rate was 48.5%. There were 15 partial responses and 1 complete response. The median duration of response was 13.7 months. The median progression-free survival was 7.5 months, and the median overall survival was 20.7 months.
Progression-free and overall survival were numerically higher in PD-L1-positive subjects and those with higher tumor mutation burdens. An expression profile of 12 genes related to inflammation and angiogenesis showed a significant correlation with response. This might help identify patients most likely to respond to the combination, but further validation is needed, Dr. Guo said.
A total of 32 subjects (97%) have had a treatment-related adverse event, including 13 (39.4%) with grade 3-5 events. The most common of these were proteinuria, hypertension, and neutropenia (all 9.1%).
“So does this study address the unmet need? In many ways, yes,” said Ryan Sullivan, MD, an assistant professor of hematology/oncology at Massachusetts General Hospital in Boston, and the discussant on Dr. Guo’s presentation.
“However, the data to date [don’t] mean we should be treating all of our mucosal melanoma patients with axitinib plus an anti-PD-1 antibody. There needs to be randomized data, but I would describe this data as very encouraging,” he said.
The study was funded by the maker of toripalimab, Shanghai Junshi Bioscience. Dr. Guo disclosed relationships with Shanghai Junshi Bioscience and Pfizer, maker of axitinib. Other investigators are employed by Shanghai Junshi Bioscience. Dr. Sullivan reported institutional research funding from Pfizer.
SOURCE: Guo J et al. ASCO 2020, Abstract 10007.
FROM ASCO 2020