User login
News and Views that Matter to Physicians
Candida auris in Venezuela outbreak is triazole-resistant, opportunistic
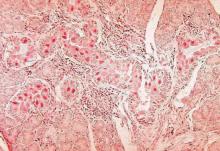
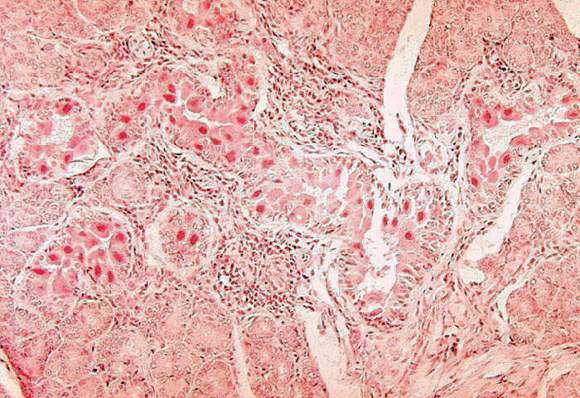
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
AT ASM 2016
Key clinical point: Isolates in an outbreak of nosocomially acquired Candida auris were fluconazole-resistant.
Major finding: All C. auris isolates were resistant to fluconazole, with geometric mean minimum inhibitory concentrations greater than 64 mcg/mL.
Data source: Retrospective, single-center study of 18 pediatric and adult patients with C. auris infections at a tertiary care center in Venezuela.
Disclosures: The study investigators reported no outside sources of funding and no disclosures.
Intensified rifampicin boosts outcomes in TB/HIV coinfection
DURBAN, SOUTH AFRICA – Prescribing high-dose rifampicin in addition to antiretroviral therapy reduces 12-month all-cause mortality in patients who are coinfected with tuberculosis and HIV and who have a low CD4 cell count, Corinne S. Merle, MD, reported at the 21st International AIDS Conference.
“Current strategies to reduce TB/HIV mortality rely largely on optimal management of HIV disease with early ART [antiretroviral therapy]. We wanted to look at whether there is value in focusing on the TB side of the problem. This is the first study to look at more intensive TB therapy for reducing mortality; and we think that, at least in patients who are immunosuppressed, there might be some benefit in a more aggressive TB treatment from the start,” said Dr. Merle of the London School of Hygiene and Tropical Medicine.
She presented the results of the open-label, multicenter trial of 747 ART-naive adults from West Africa. All were coinfected with TB/HIV and had a CD4 count of at least 50 cells/mm3 at enrollment. They were randomized to one of three treatment arms: ART starting at 2 weeks combined with standard TB treatment; ART starting at 8 weeks plus standard TB therapy; or ART initiation at 8 weeks coupled with 2 months of high-dose rifampicin (Rifadin) at 15 mg/kg daily, followed by standard TB therapy. None of the participants had multidrug-resistant TB. More than one-quarter of them were undernourished as evidenced by a baseline body mass index below 16 kg/m2.
The primary outcome was all-cause mortality at 12 months. There was no significant difference between the study arms, with a 10% rate in the intensified TB treatment arm and mortality rates of 11% and 14% with standard TB therapy and ART starting after 2 and 8 weeks, respectively.
However, a prespecified secondary analysis restricted to the 159 subjects with a baseline CD4 count below 100 cells/mm3 struck gold. Overall 12-month mortality was 4% in the intensified TB treatment subgroup, compared with 19% in patients on standard TB therapy with ART starting at 2 weeks and 28% with ART starting at 8 weeks. In a Cox regression analysis, severely immunosuppressed patients in the high-dose rifampicin group were an adjusted 88% less likely to die within 12 months than those on standard TB treatment with ART starting at 8 weeks and 80% less likely to die than those starting ART at 2 weeks.
At 18 months after randomization, roughly three-quarters of patients in each study arm had undetectable HIV viral loads.
There was no evidence of an increased risk of hepatotoxicity with 2 months of high-dose rifampicin. Only 4 of nearly 3,800 aspartate aminotransferase measurements obtained during the trial showed grade 3 or 4 hepatotoxicity, Dr. Merle noted.
In a plenary lecture on TB/HIV coinfection at the AIDS 2016 conference, Anton Pozniak, MD, singled out the trial as a sterling example of how to optimize available clinical management tools while awaiting a desperately needed new TB vaccine and better drugs.
More than 1 million new TB cases occur annually in HIV-infected persons, roughly 80% of them in sub-Saharan Africa. There are now 400,000 deaths per year worldwide in coinfected TB/HIV patients. Indeed, TB has become the No. 1 cause of death among people living with HIV infection, said Dr. Pozniak, director of HIV services at Chelsea and Westminster Hospital in London.
He offered a road map to eliminating TB by the year 2035. At present, the global trend is a 2% per year decline in new cases. Optimizing TB case finding, treatment, and preventive therapy could achieve a 10% per year decrease in new cases. That rate still wouldn’t reach the goal by 2035. But more than a dozen candidate TB vaccines are in the developmental pipeline, including a mycobacterial whole cell extract in phase III testing in China. If a new vaccine can be introduced by 2025, that would be a game changer.
“A new vaccine that could prevent adolescents and adults from developing and transmitting TB would be the single most cost-effective tool in mitigating the epidemic,” he said. “Even if we had only a 60% efficacious vaccine and delivered it to 20% of the target population, it could potentially avert 30-50 million incident cases of TB by 2050.”
A new vaccine plus effective alternatives to the standard 6 months of isoniazid for latency prophylaxis by 2025 are estimated to reduce new cases of TB by an average of 17% per year. That circumstance would mean the end of TB by 2035, Dr. Pozniak declared.
The trial was funded by the European and Developing Countries Clinical Trials Partnership. Dr. Merle reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Prescribing high-dose rifampicin in addition to antiretroviral therapy reduces 12-month all-cause mortality in patients who are coinfected with tuberculosis and HIV and who have a low CD4 cell count, Corinne S. Merle, MD, reported at the 21st International AIDS Conference.
“Current strategies to reduce TB/HIV mortality rely largely on optimal management of HIV disease with early ART [antiretroviral therapy]. We wanted to look at whether there is value in focusing on the TB side of the problem. This is the first study to look at more intensive TB therapy for reducing mortality; and we think that, at least in patients who are immunosuppressed, there might be some benefit in a more aggressive TB treatment from the start,” said Dr. Merle of the London School of Hygiene and Tropical Medicine.
She presented the results of the open-label, multicenter trial of 747 ART-naive adults from West Africa. All were coinfected with TB/HIV and had a CD4 count of at least 50 cells/mm3 at enrollment. They were randomized to one of three treatment arms: ART starting at 2 weeks combined with standard TB treatment; ART starting at 8 weeks plus standard TB therapy; or ART initiation at 8 weeks coupled with 2 months of high-dose rifampicin (Rifadin) at 15 mg/kg daily, followed by standard TB therapy. None of the participants had multidrug-resistant TB. More than one-quarter of them were undernourished as evidenced by a baseline body mass index below 16 kg/m2.
The primary outcome was all-cause mortality at 12 months. There was no significant difference between the study arms, with a 10% rate in the intensified TB treatment arm and mortality rates of 11% and 14% with standard TB therapy and ART starting after 2 and 8 weeks, respectively.
However, a prespecified secondary analysis restricted to the 159 subjects with a baseline CD4 count below 100 cells/mm3 struck gold. Overall 12-month mortality was 4% in the intensified TB treatment subgroup, compared with 19% in patients on standard TB therapy with ART starting at 2 weeks and 28% with ART starting at 8 weeks. In a Cox regression analysis, severely immunosuppressed patients in the high-dose rifampicin group were an adjusted 88% less likely to die within 12 months than those on standard TB treatment with ART starting at 8 weeks and 80% less likely to die than those starting ART at 2 weeks.
At 18 months after randomization, roughly three-quarters of patients in each study arm had undetectable HIV viral loads.
There was no evidence of an increased risk of hepatotoxicity with 2 months of high-dose rifampicin. Only 4 of nearly 3,800 aspartate aminotransferase measurements obtained during the trial showed grade 3 or 4 hepatotoxicity, Dr. Merle noted.
In a plenary lecture on TB/HIV coinfection at the AIDS 2016 conference, Anton Pozniak, MD, singled out the trial as a sterling example of how to optimize available clinical management tools while awaiting a desperately needed new TB vaccine and better drugs.
More than 1 million new TB cases occur annually in HIV-infected persons, roughly 80% of them in sub-Saharan Africa. There are now 400,000 deaths per year worldwide in coinfected TB/HIV patients. Indeed, TB has become the No. 1 cause of death among people living with HIV infection, said Dr. Pozniak, director of HIV services at Chelsea and Westminster Hospital in London.
He offered a road map to eliminating TB by the year 2035. At present, the global trend is a 2% per year decline in new cases. Optimizing TB case finding, treatment, and preventive therapy could achieve a 10% per year decrease in new cases. That rate still wouldn’t reach the goal by 2035. But more than a dozen candidate TB vaccines are in the developmental pipeline, including a mycobacterial whole cell extract in phase III testing in China. If a new vaccine can be introduced by 2025, that would be a game changer.
“A new vaccine that could prevent adolescents and adults from developing and transmitting TB would be the single most cost-effective tool in mitigating the epidemic,” he said. “Even if we had only a 60% efficacious vaccine and delivered it to 20% of the target population, it could potentially avert 30-50 million incident cases of TB by 2050.”
A new vaccine plus effective alternatives to the standard 6 months of isoniazid for latency prophylaxis by 2025 are estimated to reduce new cases of TB by an average of 17% per year. That circumstance would mean the end of TB by 2035, Dr. Pozniak declared.
The trial was funded by the European and Developing Countries Clinical Trials Partnership. Dr. Merle reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Prescribing high-dose rifampicin in addition to antiretroviral therapy reduces 12-month all-cause mortality in patients who are coinfected with tuberculosis and HIV and who have a low CD4 cell count, Corinne S. Merle, MD, reported at the 21st International AIDS Conference.
“Current strategies to reduce TB/HIV mortality rely largely on optimal management of HIV disease with early ART [antiretroviral therapy]. We wanted to look at whether there is value in focusing on the TB side of the problem. This is the first study to look at more intensive TB therapy for reducing mortality; and we think that, at least in patients who are immunosuppressed, there might be some benefit in a more aggressive TB treatment from the start,” said Dr. Merle of the London School of Hygiene and Tropical Medicine.
She presented the results of the open-label, multicenter trial of 747 ART-naive adults from West Africa. All were coinfected with TB/HIV and had a CD4 count of at least 50 cells/mm3 at enrollment. They were randomized to one of three treatment arms: ART starting at 2 weeks combined with standard TB treatment; ART starting at 8 weeks plus standard TB therapy; or ART initiation at 8 weeks coupled with 2 months of high-dose rifampicin (Rifadin) at 15 mg/kg daily, followed by standard TB therapy. None of the participants had multidrug-resistant TB. More than one-quarter of them were undernourished as evidenced by a baseline body mass index below 16 kg/m2.
The primary outcome was all-cause mortality at 12 months. There was no significant difference between the study arms, with a 10% rate in the intensified TB treatment arm and mortality rates of 11% and 14% with standard TB therapy and ART starting after 2 and 8 weeks, respectively.
However, a prespecified secondary analysis restricted to the 159 subjects with a baseline CD4 count below 100 cells/mm3 struck gold. Overall 12-month mortality was 4% in the intensified TB treatment subgroup, compared with 19% in patients on standard TB therapy with ART starting at 2 weeks and 28% with ART starting at 8 weeks. In a Cox regression analysis, severely immunosuppressed patients in the high-dose rifampicin group were an adjusted 88% less likely to die within 12 months than those on standard TB treatment with ART starting at 8 weeks and 80% less likely to die than those starting ART at 2 weeks.
At 18 months after randomization, roughly three-quarters of patients in each study arm had undetectable HIV viral loads.
There was no evidence of an increased risk of hepatotoxicity with 2 months of high-dose rifampicin. Only 4 of nearly 3,800 aspartate aminotransferase measurements obtained during the trial showed grade 3 or 4 hepatotoxicity, Dr. Merle noted.
In a plenary lecture on TB/HIV coinfection at the AIDS 2016 conference, Anton Pozniak, MD, singled out the trial as a sterling example of how to optimize available clinical management tools while awaiting a desperately needed new TB vaccine and better drugs.
More than 1 million new TB cases occur annually in HIV-infected persons, roughly 80% of them in sub-Saharan Africa. There are now 400,000 deaths per year worldwide in coinfected TB/HIV patients. Indeed, TB has become the No. 1 cause of death among people living with HIV infection, said Dr. Pozniak, director of HIV services at Chelsea and Westminster Hospital in London.
He offered a road map to eliminating TB by the year 2035. At present, the global trend is a 2% per year decline in new cases. Optimizing TB case finding, treatment, and preventive therapy could achieve a 10% per year decrease in new cases. That rate still wouldn’t reach the goal by 2035. But more than a dozen candidate TB vaccines are in the developmental pipeline, including a mycobacterial whole cell extract in phase III testing in China. If a new vaccine can be introduced by 2025, that would be a game changer.
“A new vaccine that could prevent adolescents and adults from developing and transmitting TB would be the single most cost-effective tool in mitigating the epidemic,” he said. “Even if we had only a 60% efficacious vaccine and delivered it to 20% of the target population, it could potentially avert 30-50 million incident cases of TB by 2050.”
A new vaccine plus effective alternatives to the standard 6 months of isoniazid for latency prophylaxis by 2025 are estimated to reduce new cases of TB by an average of 17% per year. That circumstance would mean the end of TB by 2035, Dr. Pozniak declared.
The trial was funded by the European and Developing Countries Clinical Trials Partnership. Dr. Merle reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: High-dose rifampicin improves survival in patients who are coinfected with tuberculosis and HIV and have low CD4 counts.
Major finding: Overall 12-month mortality was 4% in the intensified TB treatment subgroup, 19% in patients on standard TB therapy with ART starting at 2 weeks, and 28% with standard TB therapy and ART starting at 8 weeks.
Data source: This was a randomized, prospective, three-arm, open-label trial including 747 patients coinfected with tuberculosis and HIV.
Disclosures: The trial was funded by the European and Developing Countries Clinical Trials Partnership. The presenter reported having no financial conflicts of interest.
CMV viremia not culprit in high mortality of TB/HIV coinfection
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Cytomegalovirus viremia is common among patients hospitalized for HIV-associated tuberculosis, but it appears to be a bystander rather than a contributor to the high mortality seen in this population, Amy Ward, MD, said at the 21st International AIDS Conference.
“CMV [cytomegalovirus] viremia is likely a marker of more severe immunodeficiency rather than a direct contributor to mortality,” she concluded based upon the findings of her prospective cohort study. The finding means therapies for CMV viremia will not open up a new avenue of potentially life-saving treatments for these patients.
In other severe immunodeficiency conditions, such as after organ transplant, CMV viremia is directly related to increased mortality, and ganciclovir therapy can prevent progression to clinical disease and death, explained Dr. Ward of the University of Cape Town, South Africa.
She presented a prospective cohort study of 256 HIV-infected South African adults, median age 36 years, who were hospitalized with a new diagnosis of TB. At enrollment, their median CD4 count was 64 cells/mm3. Only 35% were on antiretroviral therapy (ART); 44% had previously been on ART, 21% were ART-naive, and 41% had a positive TB blood culture.
CMV viremia was present in 31%, and CMV viral load was 1,000 copies/mL or more in half of them. None had CMV retinitis, based on panoptic fundoscopy at enrollment. HIV-related retinal pathologies at enrollment included disseminated cryptococcal disease, ocular TB granules, and HIV retinitis.
The primary endpoint of the study was mortality at 12 weeks on anti-TB therapy. The mortality rate was 38% in the CMV viremic group, significantly higher than the 17.8% mortality rate seen in the CMV-negative patients.
In a univariate Cox proportional hazards regression analysis, CMV viremia was associated with a 2.1-fold increased risk for 12-week mortality. But advancing age, a low CD4 count, and decreasing serum albumin were also risk factors.
When these variables were incorporated in a multivariate regression analysis along with HIV viral load, tuberculosis blood culture results, and gender, CMV viremia was no longer a significant risk factor for 12-week mortality. Age was the sole significant predictor of death. Patients who were at least 36 years old had a 32.8% mortality rate, compared with a 14.1% rate in those who were younger. The CD4 count didn’t differ significantly by age; however, the prevalence of CMV viremia was 38% in the older group and 26.3% in patients under age 36.
“Those patients who were 36 years old and above had a higher mortality and were more likely to have CMV viremia, both findings perhaps reflecting premature aging of the immune system,” Dr. Ward said.
Also, no dose-response was seen between CMV viral load and mortality risk. The 12-week mortality rate was 33.3% in patients with a CMV viral load below 1,000 copies/mL and similar at 34.1% in those with a viral load above that cutpoint, she noted.
The study was funded by the Wellcome Trust and the South African Medical Research Council. Dr. Ward reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Cytomegalovirus viremia is common in patients hospitalized for HIV-associated tuberculosis, but treating the CMV infection is unlikely to reduce the coinfected group’s high mortality rate.
Major finding: Cytomegalovirus viremia was present in nearly one-third of a group of hospitalized patients with HIV infection and tuberculosis, but was not an independent risk factor for their 23% mortality rate at 12 weeks.
Data source: This was a prospective cohort study including 256 hospitalized patients coinfected with HIV and newly diagnosed tuberculosis.
Disclosures: The study was funded by the Wellcome Trust and the South African Medical Research Council. The presenter reported having no financial conflicts of interest.
FDA approves generic version of Tamiflu
The Food and Drug Administration has approved the first generic version of Tamiflu (oseltamivir phosphate), a medication for the treatment of influenza A and B.
The announcement was made Aug. 3, 2016, on the Drugs@FDA website and in an email from the FDA’s Division of Drug Information (DDI). Tamiflu was first approved in 1999.
Oseltamivir phosphate is intended for use in patients 2 weeks of age and older who have had flu symptoms for no more than 48 hours, and for prevention of influenza in patients 1 year of age and older. According to the FDA, the drug does not treat or prevent illness caused by viral infections other than the influenza virus, and does not prevent bacterial infections that may happen with the flu.
Products in the FDA generic approval application submitted by Natco Pharma Ltd., an India-based drug company, include the oral capsule form of the drug, in 30-, 45-, and 75-mg strengths.
The FDA acknowledged in its approval that it does not know if oseltamivir phosphate is effective in patients who start treatment after 2 days of developing symptoms, or have weakened immune systems. The most common side effects reported by patients using oseltamivir phosphate in clinical trials included nausea and vomiting.
For more information on oseltamivir phosphate, see the Tamiflu drug label.
On Twitter @richpizzi
The Food and Drug Administration has approved the first generic version of Tamiflu (oseltamivir phosphate), a medication for the treatment of influenza A and B.
The announcement was made Aug. 3, 2016, on the Drugs@FDA website and in an email from the FDA’s Division of Drug Information (DDI). Tamiflu was first approved in 1999.
Oseltamivir phosphate is intended for use in patients 2 weeks of age and older who have had flu symptoms for no more than 48 hours, and for prevention of influenza in patients 1 year of age and older. According to the FDA, the drug does not treat or prevent illness caused by viral infections other than the influenza virus, and does not prevent bacterial infections that may happen with the flu.
Products in the FDA generic approval application submitted by Natco Pharma Ltd., an India-based drug company, include the oral capsule form of the drug, in 30-, 45-, and 75-mg strengths.
The FDA acknowledged in its approval that it does not know if oseltamivir phosphate is effective in patients who start treatment after 2 days of developing symptoms, or have weakened immune systems. The most common side effects reported by patients using oseltamivir phosphate in clinical trials included nausea and vomiting.
For more information on oseltamivir phosphate, see the Tamiflu drug label.
On Twitter @richpizzi
The Food and Drug Administration has approved the first generic version of Tamiflu (oseltamivir phosphate), a medication for the treatment of influenza A and B.
The announcement was made Aug. 3, 2016, on the Drugs@FDA website and in an email from the FDA’s Division of Drug Information (DDI). Tamiflu was first approved in 1999.
Oseltamivir phosphate is intended for use in patients 2 weeks of age and older who have had flu symptoms for no more than 48 hours, and for prevention of influenza in patients 1 year of age and older. According to the FDA, the drug does not treat or prevent illness caused by viral infections other than the influenza virus, and does not prevent bacterial infections that may happen with the flu.
Products in the FDA generic approval application submitted by Natco Pharma Ltd., an India-based drug company, include the oral capsule form of the drug, in 30-, 45-, and 75-mg strengths.
The FDA acknowledged in its approval that it does not know if oseltamivir phosphate is effective in patients who start treatment after 2 days of developing symptoms, or have weakened immune systems. The most common side effects reported by patients using oseltamivir phosphate in clinical trials included nausea and vomiting.
For more information on oseltamivir phosphate, see the Tamiflu drug label.
On Twitter @richpizzi
Post-TORS neck dissections extend hospital stay
SEATTLE – Oncologic transoral robotic surgery patients spend less time in the hospital if they have neck dissections at the same time, instead of later, according to a review of 441 patients by Stony Brook (N.Y.) University.
The average hospital length of stay (LOS) was 6 days for the 349 patients (79.1%) who had lymphadenectomy neck dissections at the same time as transoral robotic surgery (TORS). The 92 patients (20.9%) who had staged procedures - neck dissections and TORS about a month apart, with TORS usually done first - stayed in the hospital an average of 8 days (P less than .0001). After risk adjustment, LOS was 43% shorter for concurrent dissections.
Cardiac arrhythmias were also more common in staged patients, perhaps because they had general anesthesia twice in a short period or maybe because staged patients were more likely to be obese (18.5% vs. 7.5%; P less than .01).
However, there were no statistically significant outcome differences otherwise, and the investigators concluded that “concurrent and staged procedures are equally safe. It is therefore reasonable to allow operator preference and patient factors to determine surgical logistics.”
Neck dissection timing has been controversial since the advent of TORS several years ago, when surgeons and administrators realized they could fit more cases into the schedule by doing neck dissections, which can take a few hours, at a different time.
Proponents of the staged approach argue, among other things, that it reduces the risk of fistulas and tracheotomies, and allows surgeons a second go at positive margins. Advocates of concurrent procedures counter that fistulas, if found, can be repaired right away, and that same-time surgery saves money, allows for earlier adjuvant therapy, and cuts anesthesia risks.
There hasn’t been much data to settle the debate, and no one has compared LOS before, so it was “important” to look into the matter, lead investigator Catherine Frenkel, MD, a Stony Brook general surgery resident, said at the American Head and Neck Society International Conference on Head and Neck Cancer.
German investigators also recently concluded that it’s pretty much a draw between concurrent and staged dissections. In a study of 41 TORS cases, “the timing of neck dissection did not make a significant difference in the outcomes. We suggest, therefore, that aspiring and established TORS teams do not restrict their appropriate indications due to robotic slot and theatre time constraints, but perform each indicated TORS case as soon as possible within their given systems, even if the neck dissections cannot be done on the same day,” they said (Eur J Surg Oncol. 2015 Jun;41[6]:773-8).
In addition to obese patients, those who had tongue or tonsil lesions were more likely to be staged in the Stony Brook analysis. About half of the surgeons in the study stuck solely to concurrent procedures, while a handful opted for the staged approach, and the rest did both. Perhaps not surprisingly, high-volume surgeons – those who did five or more TORS cases per year – were more likely to stage.
Almost two-thirds of patients had at least one complication, most commonly renal failure, heart problems, extended ventilation, and surgical errors, which included accidental punctures, postop fistulas, hemorrhages, and wound complications. A total of 13% of patients had at least one postop readmission. Apart from arrhythmias, there were no statistically significant differences in complication or 30-day readmission rates between concurrent and staged patients. High-volume surgeons were less likely to have complications.
Postop bleeding was another common problem, and more likely with staged surgeries (12% vs. 7%). Concurrent procedures had a slightly higher rate of new tracheotomies and gastrostomies, but again the differences were not statistically significant, even with pedicle and free-flap reconstruction. There was no outside funding for the work, and the investigators had no relevant conflicts of interest.
SEATTLE – Oncologic transoral robotic surgery patients spend less time in the hospital if they have neck dissections at the same time, instead of later, according to a review of 441 patients by Stony Brook (N.Y.) University.
The average hospital length of stay (LOS) was 6 days for the 349 patients (79.1%) who had lymphadenectomy neck dissections at the same time as transoral robotic surgery (TORS). The 92 patients (20.9%) who had staged procedures - neck dissections and TORS about a month apart, with TORS usually done first - stayed in the hospital an average of 8 days (P less than .0001). After risk adjustment, LOS was 43% shorter for concurrent dissections.
Cardiac arrhythmias were also more common in staged patients, perhaps because they had general anesthesia twice in a short period or maybe because staged patients were more likely to be obese (18.5% vs. 7.5%; P less than .01).
However, there were no statistically significant outcome differences otherwise, and the investigators concluded that “concurrent and staged procedures are equally safe. It is therefore reasonable to allow operator preference and patient factors to determine surgical logistics.”
Neck dissection timing has been controversial since the advent of TORS several years ago, when surgeons and administrators realized they could fit more cases into the schedule by doing neck dissections, which can take a few hours, at a different time.
Proponents of the staged approach argue, among other things, that it reduces the risk of fistulas and tracheotomies, and allows surgeons a second go at positive margins. Advocates of concurrent procedures counter that fistulas, if found, can be repaired right away, and that same-time surgery saves money, allows for earlier adjuvant therapy, and cuts anesthesia risks.
There hasn’t been much data to settle the debate, and no one has compared LOS before, so it was “important” to look into the matter, lead investigator Catherine Frenkel, MD, a Stony Brook general surgery resident, said at the American Head and Neck Society International Conference on Head and Neck Cancer.
German investigators also recently concluded that it’s pretty much a draw between concurrent and staged dissections. In a study of 41 TORS cases, “the timing of neck dissection did not make a significant difference in the outcomes. We suggest, therefore, that aspiring and established TORS teams do not restrict their appropriate indications due to robotic slot and theatre time constraints, but perform each indicated TORS case as soon as possible within their given systems, even if the neck dissections cannot be done on the same day,” they said (Eur J Surg Oncol. 2015 Jun;41[6]:773-8).
In addition to obese patients, those who had tongue or tonsil lesions were more likely to be staged in the Stony Brook analysis. About half of the surgeons in the study stuck solely to concurrent procedures, while a handful opted for the staged approach, and the rest did both. Perhaps not surprisingly, high-volume surgeons – those who did five or more TORS cases per year – were more likely to stage.
Almost two-thirds of patients had at least one complication, most commonly renal failure, heart problems, extended ventilation, and surgical errors, which included accidental punctures, postop fistulas, hemorrhages, and wound complications. A total of 13% of patients had at least one postop readmission. Apart from arrhythmias, there were no statistically significant differences in complication or 30-day readmission rates between concurrent and staged patients. High-volume surgeons were less likely to have complications.
Postop bleeding was another common problem, and more likely with staged surgeries (12% vs. 7%). Concurrent procedures had a slightly higher rate of new tracheotomies and gastrostomies, but again the differences were not statistically significant, even with pedicle and free-flap reconstruction. There was no outside funding for the work, and the investigators had no relevant conflicts of interest.
SEATTLE – Oncologic transoral robotic surgery patients spend less time in the hospital if they have neck dissections at the same time, instead of later, according to a review of 441 patients by Stony Brook (N.Y.) University.
The average hospital length of stay (LOS) was 6 days for the 349 patients (79.1%) who had lymphadenectomy neck dissections at the same time as transoral robotic surgery (TORS). The 92 patients (20.9%) who had staged procedures - neck dissections and TORS about a month apart, with TORS usually done first - stayed in the hospital an average of 8 days (P less than .0001). After risk adjustment, LOS was 43% shorter for concurrent dissections.
Cardiac arrhythmias were also more common in staged patients, perhaps because they had general anesthesia twice in a short period or maybe because staged patients were more likely to be obese (18.5% vs. 7.5%; P less than .01).
However, there were no statistically significant outcome differences otherwise, and the investigators concluded that “concurrent and staged procedures are equally safe. It is therefore reasonable to allow operator preference and patient factors to determine surgical logistics.”
Neck dissection timing has been controversial since the advent of TORS several years ago, when surgeons and administrators realized they could fit more cases into the schedule by doing neck dissections, which can take a few hours, at a different time.
Proponents of the staged approach argue, among other things, that it reduces the risk of fistulas and tracheotomies, and allows surgeons a second go at positive margins. Advocates of concurrent procedures counter that fistulas, if found, can be repaired right away, and that same-time surgery saves money, allows for earlier adjuvant therapy, and cuts anesthesia risks.
There hasn’t been much data to settle the debate, and no one has compared LOS before, so it was “important” to look into the matter, lead investigator Catherine Frenkel, MD, a Stony Brook general surgery resident, said at the American Head and Neck Society International Conference on Head and Neck Cancer.
German investigators also recently concluded that it’s pretty much a draw between concurrent and staged dissections. In a study of 41 TORS cases, “the timing of neck dissection did not make a significant difference in the outcomes. We suggest, therefore, that aspiring and established TORS teams do not restrict their appropriate indications due to robotic slot and theatre time constraints, but perform each indicated TORS case as soon as possible within their given systems, even if the neck dissections cannot be done on the same day,” they said (Eur J Surg Oncol. 2015 Jun;41[6]:773-8).
In addition to obese patients, those who had tongue or tonsil lesions were more likely to be staged in the Stony Brook analysis. About half of the surgeons in the study stuck solely to concurrent procedures, while a handful opted for the staged approach, and the rest did both. Perhaps not surprisingly, high-volume surgeons – those who did five or more TORS cases per year – were more likely to stage.
Almost two-thirds of patients had at least one complication, most commonly renal failure, heart problems, extended ventilation, and surgical errors, which included accidental punctures, postop fistulas, hemorrhages, and wound complications. A total of 13% of patients had at least one postop readmission. Apart from arrhythmias, there were no statistically significant differences in complication or 30-day readmission rates between concurrent and staged patients. High-volume surgeons were less likely to have complications.
Postop bleeding was another common problem, and more likely with staged surgeries (12% vs. 7%). Concurrent procedures had a slightly higher rate of new tracheotomies and gastrostomies, but again the differences were not statistically significant, even with pedicle and free-flap reconstruction. There was no outside funding for the work, and the investigators had no relevant conflicts of interest.
AT THE INTERNATIONAL CONFERENCE ON HEAD AND NECK CANCER
Key clinical point: Oncologic transoral robotic surgery patients spend less time in the hospital if they have neck dissections at the same time, instead of later.
Major finding: The average hospital length of stay was 6 days for the 349 patients (79.1%) who had lymphadenectomy neck dissections at the same time as TORS. The 92 patients (20.9%) who had staged procedures – neck dissections and TORS about a month apart, with TORS usually done first – stayed in the hospital an average of 8 days (P less than .0001).
Data source: Review of 441 TORS patients.
Disclosures: There was no outside funding for the work, and the investigators had no relevant conflicts of interest.
Viruses on mobile phones
Mobile phones became commonplace in just a few years and are now used everywhere, included remote areas of the world. These communication tools are used for personal and professional purposes, frequently by health care workers (HCWs) during care.
We and others believe that mobile phones improve the quality, rapidity, and efficiency of communication in health care settings and, therefore, improve the management of patients. In fact, professional mobile phones allow communication between HCWs anywhere in the hospital. In addition, personal mobile phones, frequently smartphones, allow the use of medical apps for evidence-based management of patients.
Mobile phones, both professional and personal, are used in close proximity to patients, as reported in behavioral studies. In a recent study we performed in a hospital setting (Clin Microbiol Infect. 2016 May;22[5]:456.e1-e6. doi: 10.1016/j.cmi.2015.12.008), more than 60% of HCWs who participated declared using phones during care, and also declared that they had halted care to patients while answering a call.
Several studies have shown that mobile phones used at hospitals are contaminated by bacteria, including highly pathogenic ones, such as methicillin-resistant Staphylococcus aureus (MRSA), Acinetobacter species, vancomycin-resistant enterococci, Pseudomonas species, and coliforms. Research suggests that these devices may serve as a reservoir of bacteria known to cause nosocomial infections and may play a role in transmission of them to patients through the hands of HCWs.
For the first time, we demonstrated the presence of RNA of epidemic viruses such as rotavirus, influenza virus, syncytial respiratory virus, and metapneumovirus on mobile phones (professional and personal) held by HCWs. In our study, 38.5% of sampled mobile phones were contaminated with RNA from viruses. RNA of rotavirus was the most frequently-detected virus, mainly on phones sampled in the pediatric emergency ward. Interestingly, we found that HCWs in pediatric wards admitted disinfecting their mobile phones less frequently than did other HCWs we interviewed.
Epidemic viruses have already been discovered on other electronic device surfaces, such as keyboards, computers, and telephone handsets. However, in contrast to these other devices, mobile phones are mobile and could be shared and transported anywhere, including in close proximity to patients. Rotaviruses are frequently found on hospital surfaces several months after an epidemic period, after surfaces were cleaned. The high prevalence of rotavirus in pediatric ward patients during our study, and its capacity to persist in the environment, are probably the main factors that explain the high frequency of rotavirus RNA detection on mobile phones in our study.
This finding highlights the possible role of mobile phones in cross-transmission of epidemic viruses, with the transfer from nonporous fomites to fingers, and from fingers to fomites – including mobile phones. Due to the difficulty and fastidiousness of viral culture, the viruses were detected only by molecular biology; the viability of the viruses could not be demonstrated. However, we believe that cross-transmission of viruses may occur, notably in health care settings. The recently reported case of a 40-year-old Ugandan man who stole a phone from a patient with Ebola and contracted the disease, also supports this hypothesis.
We also demonstrated in our study that hand hygiene after the use of mobile phones does not seem to be systematic, even for HCWs continuing care that was in process before picking up their phones. Around 30% of HCWs declared that they never perform hand hygiene before or after handling mobile phones. In addition, more than 30% of HCWs admitted that they never disinfect their phones, even their professional ones; this lack of hygiene could contribute to the persistence of RNA of epidemic viruses.
Our study does not support banning the use of mobile phones in hospitals. We just want to make HCWs aware that mobile phones, which are part of our daily practice, can be contaminated by pathogens, notably viruses. The use of disinfection wipes to clean phones, together with adherence to hand hygiene, is crucial to prevent cross-transmission.
Frequent disinfection of personal and professional mobile phones needs to be promoted to reduce contamination of phones by viruses, especially during epidemics.
In practice, each clinician needs to remember that hand hygiene should be the last thing done before patient contact, as recommended by the World Health Organization. Touching a mobile phone could transfer bacteria or viruses onto hands, and we hypothesize that it could be a factor in cross-transmission of pathogens.
Elisabeth Botelho-Nevers, MD, PhD, is an infectious diseases specialist at the University Hospital of Saint-Étienne (France) and Sylvie Pillet, PharmD, PhD, is a virologist in the Laboratory of Infectious Agents and Hygiene, University Hospital of Saint-Étienne.
Mobile phones became commonplace in just a few years and are now used everywhere, included remote areas of the world. These communication tools are used for personal and professional purposes, frequently by health care workers (HCWs) during care.
We and others believe that mobile phones improve the quality, rapidity, and efficiency of communication in health care settings and, therefore, improve the management of patients. In fact, professional mobile phones allow communication between HCWs anywhere in the hospital. In addition, personal mobile phones, frequently smartphones, allow the use of medical apps for evidence-based management of patients.
Mobile phones, both professional and personal, are used in close proximity to patients, as reported in behavioral studies. In a recent study we performed in a hospital setting (Clin Microbiol Infect. 2016 May;22[5]:456.e1-e6. doi: 10.1016/j.cmi.2015.12.008), more than 60% of HCWs who participated declared using phones during care, and also declared that they had halted care to patients while answering a call.
Several studies have shown that mobile phones used at hospitals are contaminated by bacteria, including highly pathogenic ones, such as methicillin-resistant Staphylococcus aureus (MRSA), Acinetobacter species, vancomycin-resistant enterococci, Pseudomonas species, and coliforms. Research suggests that these devices may serve as a reservoir of bacteria known to cause nosocomial infections and may play a role in transmission of them to patients through the hands of HCWs.
For the first time, we demonstrated the presence of RNA of epidemic viruses such as rotavirus, influenza virus, syncytial respiratory virus, and metapneumovirus on mobile phones (professional and personal) held by HCWs. In our study, 38.5% of sampled mobile phones were contaminated with RNA from viruses. RNA of rotavirus was the most frequently-detected virus, mainly on phones sampled in the pediatric emergency ward. Interestingly, we found that HCWs in pediatric wards admitted disinfecting their mobile phones less frequently than did other HCWs we interviewed.
Epidemic viruses have already been discovered on other electronic device surfaces, such as keyboards, computers, and telephone handsets. However, in contrast to these other devices, mobile phones are mobile and could be shared and transported anywhere, including in close proximity to patients. Rotaviruses are frequently found on hospital surfaces several months after an epidemic period, after surfaces were cleaned. The high prevalence of rotavirus in pediatric ward patients during our study, and its capacity to persist in the environment, are probably the main factors that explain the high frequency of rotavirus RNA detection on mobile phones in our study.
This finding highlights the possible role of mobile phones in cross-transmission of epidemic viruses, with the transfer from nonporous fomites to fingers, and from fingers to fomites – including mobile phones. Due to the difficulty and fastidiousness of viral culture, the viruses were detected only by molecular biology; the viability of the viruses could not be demonstrated. However, we believe that cross-transmission of viruses may occur, notably in health care settings. The recently reported case of a 40-year-old Ugandan man who stole a phone from a patient with Ebola and contracted the disease, also supports this hypothesis.
We also demonstrated in our study that hand hygiene after the use of mobile phones does not seem to be systematic, even for HCWs continuing care that was in process before picking up their phones. Around 30% of HCWs declared that they never perform hand hygiene before or after handling mobile phones. In addition, more than 30% of HCWs admitted that they never disinfect their phones, even their professional ones; this lack of hygiene could contribute to the persistence of RNA of epidemic viruses.
Our study does not support banning the use of mobile phones in hospitals. We just want to make HCWs aware that mobile phones, which are part of our daily practice, can be contaminated by pathogens, notably viruses. The use of disinfection wipes to clean phones, together with adherence to hand hygiene, is crucial to prevent cross-transmission.
Frequent disinfection of personal and professional mobile phones needs to be promoted to reduce contamination of phones by viruses, especially during epidemics.
In practice, each clinician needs to remember that hand hygiene should be the last thing done before patient contact, as recommended by the World Health Organization. Touching a mobile phone could transfer bacteria or viruses onto hands, and we hypothesize that it could be a factor in cross-transmission of pathogens.
Elisabeth Botelho-Nevers, MD, PhD, is an infectious diseases specialist at the University Hospital of Saint-Étienne (France) and Sylvie Pillet, PharmD, PhD, is a virologist in the Laboratory of Infectious Agents and Hygiene, University Hospital of Saint-Étienne.
Mobile phones became commonplace in just a few years and are now used everywhere, included remote areas of the world. These communication tools are used for personal and professional purposes, frequently by health care workers (HCWs) during care.
We and others believe that mobile phones improve the quality, rapidity, and efficiency of communication in health care settings and, therefore, improve the management of patients. In fact, professional mobile phones allow communication between HCWs anywhere in the hospital. In addition, personal mobile phones, frequently smartphones, allow the use of medical apps for evidence-based management of patients.
Mobile phones, both professional and personal, are used in close proximity to patients, as reported in behavioral studies. In a recent study we performed in a hospital setting (Clin Microbiol Infect. 2016 May;22[5]:456.e1-e6. doi: 10.1016/j.cmi.2015.12.008), more than 60% of HCWs who participated declared using phones during care, and also declared that they had halted care to patients while answering a call.
Several studies have shown that mobile phones used at hospitals are contaminated by bacteria, including highly pathogenic ones, such as methicillin-resistant Staphylococcus aureus (MRSA), Acinetobacter species, vancomycin-resistant enterococci, Pseudomonas species, and coliforms. Research suggests that these devices may serve as a reservoir of bacteria known to cause nosocomial infections and may play a role in transmission of them to patients through the hands of HCWs.
For the first time, we demonstrated the presence of RNA of epidemic viruses such as rotavirus, influenza virus, syncytial respiratory virus, and metapneumovirus on mobile phones (professional and personal) held by HCWs. In our study, 38.5% of sampled mobile phones were contaminated with RNA from viruses. RNA of rotavirus was the most frequently-detected virus, mainly on phones sampled in the pediatric emergency ward. Interestingly, we found that HCWs in pediatric wards admitted disinfecting their mobile phones less frequently than did other HCWs we interviewed.
Epidemic viruses have already been discovered on other electronic device surfaces, such as keyboards, computers, and telephone handsets. However, in contrast to these other devices, mobile phones are mobile and could be shared and transported anywhere, including in close proximity to patients. Rotaviruses are frequently found on hospital surfaces several months after an epidemic period, after surfaces were cleaned. The high prevalence of rotavirus in pediatric ward patients during our study, and its capacity to persist in the environment, are probably the main factors that explain the high frequency of rotavirus RNA detection on mobile phones in our study.
This finding highlights the possible role of mobile phones in cross-transmission of epidemic viruses, with the transfer from nonporous fomites to fingers, and from fingers to fomites – including mobile phones. Due to the difficulty and fastidiousness of viral culture, the viruses were detected only by molecular biology; the viability of the viruses could not be demonstrated. However, we believe that cross-transmission of viruses may occur, notably in health care settings. The recently reported case of a 40-year-old Ugandan man who stole a phone from a patient with Ebola and contracted the disease, also supports this hypothesis.
We also demonstrated in our study that hand hygiene after the use of mobile phones does not seem to be systematic, even for HCWs continuing care that was in process before picking up their phones. Around 30% of HCWs declared that they never perform hand hygiene before or after handling mobile phones. In addition, more than 30% of HCWs admitted that they never disinfect their phones, even their professional ones; this lack of hygiene could contribute to the persistence of RNA of epidemic viruses.
Our study does not support banning the use of mobile phones in hospitals. We just want to make HCWs aware that mobile phones, which are part of our daily practice, can be contaminated by pathogens, notably viruses. The use of disinfection wipes to clean phones, together with adherence to hand hygiene, is crucial to prevent cross-transmission.
Frequent disinfection of personal and professional mobile phones needs to be promoted to reduce contamination of phones by viruses, especially during epidemics.
In practice, each clinician needs to remember that hand hygiene should be the last thing done before patient contact, as recommended by the World Health Organization. Touching a mobile phone could transfer bacteria or viruses onto hands, and we hypothesize that it could be a factor in cross-transmission of pathogens.
Elisabeth Botelho-Nevers, MD, PhD, is an infectious diseases specialist at the University Hospital of Saint-Étienne (France) and Sylvie Pillet, PharmD, PhD, is a virologist in the Laboratory of Infectious Agents and Hygiene, University Hospital of Saint-Étienne.
Thymectomy improves clinical outcomes for myasthenia gravis
Thymectomy improved 3-year clinical outcomes and proved superior to medical therapy for mild to severe nonthymomatous myasthenia gravis, according to a report published online Aug. 11 in the New England Journal of Medicine.
Compared with standard prednisone therapy, thymectomy plus prednisone decreased the number and severity of symptoms, allowed the lowering of steroid doses, decreased the number and length of hospitalizations for disease exacerbations, reduced the need for immunosuppressive agents, and improved health-related quality of life in an international, randomized clinical trial, said Gil I. Wolfe, MD, of the department of neurology, State University of New York at Buffalo and his associates.
Until now, thymectomy was known to be beneficial in some cases of myasthenia gravis “but with widely varying rates of clinical improvement or remission.” And the success of immunotherapy has raised the question of whether an invasive surgery is necessary. Data from randomized, controlled studies have been sparse.
Moreover, thymectomy rarely causes adverse effects, but “the procedure can cost up to $80,000 and can be associated with operative complications that need to be weighed against benefits.” In comparison, medical therapy with glucocorticoids and other immunosuppressive agents is less invasive but is definitely associated with adverse events, including some that are life threatening, and negatively impacts quality of life, the investigators said.
To address the lack of randomized controlled trial data, they assessed 3-year outcomes in 126 patients treated at 67 medical centers in 18 countries during a 6-year period. The study participants were aged 18-65 years, had a disease duration of less than 5 years at enrollment (median duration, 1 year), and had class II (mild generalized disease) to class IV (severe generalized disease) myasthenia gravis. These patients were randomly assigned to undergo thymectomy and receive standard prednisone therapy (66 participants) or to receive standard prednisone alone (60 participants).
Thymectomy was performed using a median sternotomy “with the goal of an en bloc resection of all mediastinal tissue that could anatomically contain gross or microscopic thymus.”
At follow-up, time-weighted average scores on the Quantitative Myasthenia Gravis scale were significantly lower by 2.85 points, indicating improved clinical status, in the thymectomy group than in the control group. Time-weighted average prednisone dose also was significantly lower, at an average alternate-day dose of 44 mg in the thymectomy group and 60 mg in the control group, Dr. Wolfe and his associates said (N Engl J Med. 2016 Aug 11. doi: 10.1056/NEJMoa1602489).
On a measure of treatment-related complications, scores favored thymectomy with regard to the number of patients with symptoms, the total number of symptoms, and the distress level related to symptoms throughout the study period. Fewer patients in the thymectomy group required hospitalization for exacerbations of myasthenia gravis (9% vs. 37%), and the mean cumulative number of hospital days was lower with thymectomy (8.4 vs. 19.2).
In addition, scores on the Myasthenia Gravis Activities of Daily Living scale favored thymectomy (2.24 vs. 3.41). Fewer patients in the thymectomy group required azathioprine (17% vs. 0.48%). And the percentage of patients who reported having minimal manifestations of the disease at 3 years was significantly higher with thymectomy (67%) than with prednisone alone (47%).
This study was supported by the National Institute of Neurological Disorders and Stroke, the Muscular Dystrophy Association, and the Myasthenia Gravis Foundation of America and received no commercial support. Dr. Wolfe reported ties to Alexion Pharmaceuticals, Alpha Cancer Technologies, Argenx, Baxalta, CSL Behring, Grifols, and UCB, and his associates reported ties to numerous industry sources.
Landmark trial establishes effectiveness of thymectomy in myasthenia gravis
One of the many challenges of treating patients with myasthenia gravis (MG) is the fluctuating nature of symptoms and deficits. The neurologist or neuromuscular specialist must decide whether the disease is truly worsening, whether the patient is experiencing more pronounced symptoms from intercurrent illness or the effects of a medication known to affect the neuromuscular junction adversely, or whether the patient is concerned that there might be worsening disease when all objective measures indicate stability. These factors make treatment decisions more difficult in MG than for many other neuromuscular disorders.
Similarly, researchers considering a trial investigating treatment efficacy in MG face the complex issues of disease fluctuation in cohorts of individuals with the disease, varying levels of corticosteroid and immunosuppressant doses in different MG patients, and thorny ethical dilemmas in providing accepted therapies but not withholding effective treatments from those in need.
Dr. Wolfe and his colleagues demonstrate that they have navigated these treacherous waters. They have succeeded in completing a landmark controlled clinical trial which establishes the effectiveness of transsternal thymectomy with adjuvant corticosteroid therapy in nonthymomatous MG vs. oral prednisone without surgery. While this international 36-center trial managed to recruit 126 subjects over a 6-year period, using sound inclusion and exclusion criteria and a meticulous trial design, the number of patients is not sufficient to allow for as robust a subgroup analysis for age, gender, and a variety of clinical variables reflecting severity of disease as would have been hoped for by the MG community.
Nonetheless, this paper sets the use of thymectomy in nonthymomatous MG on firmer ground going forward. The investigators will doubtless be presenting further data from the trial, including clinical-pathologic correlates and other relevant novel observations. In addition, Wolfe et al. have opened the door for future trials of thymectomy in MG to address such issues as the benefits vs. risks of performing the operation via the traditional transsternal vs. alternative non–sternal splitting approaches.
Benn E. Smith, MD, is an associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz. and is the director of the sensory laboratory there. Dr. Smith is on the Editorial Advisory Board of Clinical Neurology News.
End to an 80-year controversy
These findings from Wolfe et al. end an 80-year controversy over the effectiveness of thymectomy for patients with myasthenia gravis.
Perhaps the most important benefit for patients is that even when they require prednisone following the surgery, they can take lower doses, endure fewer glucocorticoid-related symptoms, and experience less distress from those symptoms than patients who don’t undergo thymectomy.
Unfortunately, the study results cannot offer further clarity regarding patient selection for thymectomy. The patient population in this trial was so small that subgroup analyses couldn’t allow conclusions regarding the relative effectiveness of thymectomy in men vs. women or younger vs. older patients.
Allan H. Ropper, MD, is in the department of neurology at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. His financial disclosures are available at NEJM.org. Dr. Ropper made these remarks in an editorial accompanying Dr. Wolfe’s report (N Engl J Med. 2016 Aug 11. doi: 10.1056/NEJMe1607953).
Landmark trial establishes effectiveness of thymectomy in myasthenia gravis
One of the many challenges of treating patients with myasthenia gravis (MG) is the fluctuating nature of symptoms and deficits. The neurologist or neuromuscular specialist must decide whether the disease is truly worsening, whether the patient is experiencing more pronounced symptoms from intercurrent illness or the effects of a medication known to affect the neuromuscular junction adversely, or whether the patient is concerned that there might be worsening disease when all objective measures indicate stability. These factors make treatment decisions more difficult in MG than for many other neuromuscular disorders.
Similarly, researchers considering a trial investigating treatment efficacy in MG face the complex issues of disease fluctuation in cohorts of individuals with the disease, varying levels of corticosteroid and immunosuppressant doses in different MG patients, and thorny ethical dilemmas in providing accepted therapies but not withholding effective treatments from those in need.
Dr. Wolfe and his colleagues demonstrate that they have navigated these treacherous waters. They have succeeded in completing a landmark controlled clinical trial which establishes the effectiveness of transsternal thymectomy with adjuvant corticosteroid therapy in nonthymomatous MG vs. oral prednisone without surgery. While this international 36-center trial managed to recruit 126 subjects over a 6-year period, using sound inclusion and exclusion criteria and a meticulous trial design, the number of patients is not sufficient to allow for as robust a subgroup analysis for age, gender, and a variety of clinical variables reflecting severity of disease as would have been hoped for by the MG community.
Nonetheless, this paper sets the use of thymectomy in nonthymomatous MG on firmer ground going forward. The investigators will doubtless be presenting further data from the trial, including clinical-pathologic correlates and other relevant novel observations. In addition, Wolfe et al. have opened the door for future trials of thymectomy in MG to address such issues as the benefits vs. risks of performing the operation via the traditional transsternal vs. alternative non–sternal splitting approaches.
Benn E. Smith, MD, is an associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz. and is the director of the sensory laboratory there. Dr. Smith is on the Editorial Advisory Board of Clinical Neurology News.
End to an 80-year controversy
These findings from Wolfe et al. end an 80-year controversy over the effectiveness of thymectomy for patients with myasthenia gravis.
Perhaps the most important benefit for patients is that even when they require prednisone following the surgery, they can take lower doses, endure fewer glucocorticoid-related symptoms, and experience less distress from those symptoms than patients who don’t undergo thymectomy.
Unfortunately, the study results cannot offer further clarity regarding patient selection for thymectomy. The patient population in this trial was so small that subgroup analyses couldn’t allow conclusions regarding the relative effectiveness of thymectomy in men vs. women or younger vs. older patients.
Allan H. Ropper, MD, is in the department of neurology at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. His financial disclosures are available at NEJM.org. Dr. Ropper made these remarks in an editorial accompanying Dr. Wolfe’s report (N Engl J Med. 2016 Aug 11. doi: 10.1056/NEJMe1607953).
Landmark trial establishes effectiveness of thymectomy in myasthenia gravis
One of the many challenges of treating patients with myasthenia gravis (MG) is the fluctuating nature of symptoms and deficits. The neurologist or neuromuscular specialist must decide whether the disease is truly worsening, whether the patient is experiencing more pronounced symptoms from intercurrent illness or the effects of a medication known to affect the neuromuscular junction adversely, or whether the patient is concerned that there might be worsening disease when all objective measures indicate stability. These factors make treatment decisions more difficult in MG than for many other neuromuscular disorders.
Similarly, researchers considering a trial investigating treatment efficacy in MG face the complex issues of disease fluctuation in cohorts of individuals with the disease, varying levels of corticosteroid and immunosuppressant doses in different MG patients, and thorny ethical dilemmas in providing accepted therapies but not withholding effective treatments from those in need.
Dr. Wolfe and his colleagues demonstrate that they have navigated these treacherous waters. They have succeeded in completing a landmark controlled clinical trial which establishes the effectiveness of transsternal thymectomy with adjuvant corticosteroid therapy in nonthymomatous MG vs. oral prednisone without surgery. While this international 36-center trial managed to recruit 126 subjects over a 6-year period, using sound inclusion and exclusion criteria and a meticulous trial design, the number of patients is not sufficient to allow for as robust a subgroup analysis for age, gender, and a variety of clinical variables reflecting severity of disease as would have been hoped for by the MG community.
Nonetheless, this paper sets the use of thymectomy in nonthymomatous MG on firmer ground going forward. The investigators will doubtless be presenting further data from the trial, including clinical-pathologic correlates and other relevant novel observations. In addition, Wolfe et al. have opened the door for future trials of thymectomy in MG to address such issues as the benefits vs. risks of performing the operation via the traditional transsternal vs. alternative non–sternal splitting approaches.
Benn E. Smith, MD, is an associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz. and is the director of the sensory laboratory there. Dr. Smith is on the Editorial Advisory Board of Clinical Neurology News.
End to an 80-year controversy
These findings from Wolfe et al. end an 80-year controversy over the effectiveness of thymectomy for patients with myasthenia gravis.
Perhaps the most important benefit for patients is that even when they require prednisone following the surgery, they can take lower doses, endure fewer glucocorticoid-related symptoms, and experience less distress from those symptoms than patients who don’t undergo thymectomy.
Unfortunately, the study results cannot offer further clarity regarding patient selection for thymectomy. The patient population in this trial was so small that subgroup analyses couldn’t allow conclusions regarding the relative effectiveness of thymectomy in men vs. women or younger vs. older patients.
Allan H. Ropper, MD, is in the department of neurology at Brigham and Women’s Hospital and Harvard Medical School, both in Boston. His financial disclosures are available at NEJM.org. Dr. Ropper made these remarks in an editorial accompanying Dr. Wolfe’s report (N Engl J Med. 2016 Aug 11. doi: 10.1056/NEJMe1607953).
Thymectomy improved 3-year clinical outcomes and proved superior to medical therapy for mild to severe nonthymomatous myasthenia gravis, according to a report published online Aug. 11 in the New England Journal of Medicine.
Compared with standard prednisone therapy, thymectomy plus prednisone decreased the number and severity of symptoms, allowed the lowering of steroid doses, decreased the number and length of hospitalizations for disease exacerbations, reduced the need for immunosuppressive agents, and improved health-related quality of life in an international, randomized clinical trial, said Gil I. Wolfe, MD, of the department of neurology, State University of New York at Buffalo and his associates.
Until now, thymectomy was known to be beneficial in some cases of myasthenia gravis “but with widely varying rates of clinical improvement or remission.” And the success of immunotherapy has raised the question of whether an invasive surgery is necessary. Data from randomized, controlled studies have been sparse.
Moreover, thymectomy rarely causes adverse effects, but “the procedure can cost up to $80,000 and can be associated with operative complications that need to be weighed against benefits.” In comparison, medical therapy with glucocorticoids and other immunosuppressive agents is less invasive but is definitely associated with adverse events, including some that are life threatening, and negatively impacts quality of life, the investigators said.
To address the lack of randomized controlled trial data, they assessed 3-year outcomes in 126 patients treated at 67 medical centers in 18 countries during a 6-year period. The study participants were aged 18-65 years, had a disease duration of less than 5 years at enrollment (median duration, 1 year), and had class II (mild generalized disease) to class IV (severe generalized disease) myasthenia gravis. These patients were randomly assigned to undergo thymectomy and receive standard prednisone therapy (66 participants) or to receive standard prednisone alone (60 participants).
Thymectomy was performed using a median sternotomy “with the goal of an en bloc resection of all mediastinal tissue that could anatomically contain gross or microscopic thymus.”
At follow-up, time-weighted average scores on the Quantitative Myasthenia Gravis scale were significantly lower by 2.85 points, indicating improved clinical status, in the thymectomy group than in the control group. Time-weighted average prednisone dose also was significantly lower, at an average alternate-day dose of 44 mg in the thymectomy group and 60 mg in the control group, Dr. Wolfe and his associates said (N Engl J Med. 2016 Aug 11. doi: 10.1056/NEJMoa1602489).
On a measure of treatment-related complications, scores favored thymectomy with regard to the number of patients with symptoms, the total number of symptoms, and the distress level related to symptoms throughout the study period. Fewer patients in the thymectomy group required hospitalization for exacerbations of myasthenia gravis (9% vs. 37%), and the mean cumulative number of hospital days was lower with thymectomy (8.4 vs. 19.2).
In addition, scores on the Myasthenia Gravis Activities of Daily Living scale favored thymectomy (2.24 vs. 3.41). Fewer patients in the thymectomy group required azathioprine (17% vs. 0.48%). And the percentage of patients who reported having minimal manifestations of the disease at 3 years was significantly higher with thymectomy (67%) than with prednisone alone (47%).
This study was supported by the National Institute of Neurological Disorders and Stroke, the Muscular Dystrophy Association, and the Myasthenia Gravis Foundation of America and received no commercial support. Dr. Wolfe reported ties to Alexion Pharmaceuticals, Alpha Cancer Technologies, Argenx, Baxalta, CSL Behring, Grifols, and UCB, and his associates reported ties to numerous industry sources.
Thymectomy improved 3-year clinical outcomes and proved superior to medical therapy for mild to severe nonthymomatous myasthenia gravis, according to a report published online Aug. 11 in the New England Journal of Medicine.
Compared with standard prednisone therapy, thymectomy plus prednisone decreased the number and severity of symptoms, allowed the lowering of steroid doses, decreased the number and length of hospitalizations for disease exacerbations, reduced the need for immunosuppressive agents, and improved health-related quality of life in an international, randomized clinical trial, said Gil I. Wolfe, MD, of the department of neurology, State University of New York at Buffalo and his associates.
Until now, thymectomy was known to be beneficial in some cases of myasthenia gravis “but with widely varying rates of clinical improvement or remission.” And the success of immunotherapy has raised the question of whether an invasive surgery is necessary. Data from randomized, controlled studies have been sparse.
Moreover, thymectomy rarely causes adverse effects, but “the procedure can cost up to $80,000 and can be associated with operative complications that need to be weighed against benefits.” In comparison, medical therapy with glucocorticoids and other immunosuppressive agents is less invasive but is definitely associated with adverse events, including some that are life threatening, and negatively impacts quality of life, the investigators said.
To address the lack of randomized controlled trial data, they assessed 3-year outcomes in 126 patients treated at 67 medical centers in 18 countries during a 6-year period. The study participants were aged 18-65 years, had a disease duration of less than 5 years at enrollment (median duration, 1 year), and had class II (mild generalized disease) to class IV (severe generalized disease) myasthenia gravis. These patients were randomly assigned to undergo thymectomy and receive standard prednisone therapy (66 participants) or to receive standard prednisone alone (60 participants).
Thymectomy was performed using a median sternotomy “with the goal of an en bloc resection of all mediastinal tissue that could anatomically contain gross or microscopic thymus.”
At follow-up, time-weighted average scores on the Quantitative Myasthenia Gravis scale were significantly lower by 2.85 points, indicating improved clinical status, in the thymectomy group than in the control group. Time-weighted average prednisone dose also was significantly lower, at an average alternate-day dose of 44 mg in the thymectomy group and 60 mg in the control group, Dr. Wolfe and his associates said (N Engl J Med. 2016 Aug 11. doi: 10.1056/NEJMoa1602489).
On a measure of treatment-related complications, scores favored thymectomy with regard to the number of patients with symptoms, the total number of symptoms, and the distress level related to symptoms throughout the study period. Fewer patients in the thymectomy group required hospitalization for exacerbations of myasthenia gravis (9% vs. 37%), and the mean cumulative number of hospital days was lower with thymectomy (8.4 vs. 19.2).
In addition, scores on the Myasthenia Gravis Activities of Daily Living scale favored thymectomy (2.24 vs. 3.41). Fewer patients in the thymectomy group required azathioprine (17% vs. 0.48%). And the percentage of patients who reported having minimal manifestations of the disease at 3 years was significantly higher with thymectomy (67%) than with prednisone alone (47%).
This study was supported by the National Institute of Neurological Disorders and Stroke, the Muscular Dystrophy Association, and the Myasthenia Gravis Foundation of America and received no commercial support. Dr. Wolfe reported ties to Alexion Pharmaceuticals, Alpha Cancer Technologies, Argenx, Baxalta, CSL Behring, Grifols, and UCB, and his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Thymectomy improved 3-year clinical outcomes and was superior to medical therapy for mild to severe nonthymomatous myasthenia gravis.
Major finding: Scores on the Quantitative Myasthenia Gravis scale were significantly lower by 2.85 points, indicating improved clinical status, in the thymectomy group than in the control group.
Data source: An international, randomized, medication-controlled trial involving 126 patients at 67 medical centers.
Disclosures: This study was supported by the National Institute of Neurological Disorders and Stroke, the Muscular Dystrophy Association, and the Myasthenia Gravis Foundation of America and received no commercial support. Dr. Wolfe reported ties to Alexion Pharmaceuticals, Alpha Cancer Technologies, Argenx, Baxalta, CSL Behring, Grifols, and UCB, and his associates reported ties to numerous industry sources.
Hospitalist surgical comanagement program reduced complications, costs
The comanagement of surgical patients by a team of surgeons and hospitalists yielded improvements in rates of medical complications, 30-day readmissions, consultant involvement, length of hospital stay, and total cost of care per patient, according to a study published in the Annals of Surgery.
“Traditionally, hospitals have utilized a consultation model of care for surgical patients in which the medicine consultants are involved ‘as needed’ [but] this model, though common, may not be the best approach to care for surgical patients,” wrote the investigators, led by Nidhi Rohatgi, MD, MS, of Stanford (Calif.) University. “To address these limitations in the care of surgical patients, we transitioned from the consultation model to surgical comanagement (SCM) model in Orthopedic and Neurosurgery at our institution in August 2012.”
Dr. Rohatgi and her coinvestigators enrolled 16,930 patients admitted for orthopedic surgery or neurosurgery into the SCM intervention cohort, which was further divided into a preintervention cohort and a postintervention cohort. The former were admitted from January 2009 to July 2012, and the latter from September 2012 to September 2013. A further 3,695 patients were enrolled in a control group, also made up of orthopedic surgery and neurosurgery patients, which was divided into two cohorts of pre- and postcontrol over the same time periods. The two primary outcomes were the proportion of patients that had at least one medical complication – defined as sepsis, pneumonia, urinary tract infections, delirium, acute kidney injury, atrial fibrillation, or ileus – and the proportion of patients with a hospital stay of at least 5 days. Other outcomes included 30-day readmissions, costs, two or more medical consultants, and satisfaction of both patients and providers.
The findings
Overall, the SCM model was associated with a decrease in the proportion of patients with at least one complication (odds ratio, 0.86; 95% confidence interval, 0.74-0.96; P = .008) in the intervention group, compared with the control group. Similarly, there was a reduction in the proportion of patients with a hospital stay of at least 5 days (OR, 0.75; 95% CI, 0.67-0.84; P less than .001). In addition, the SCM model was associated with a reduction in 30-day readmission rates caused by medical complications (OR, 0.67; 95% CI, 0.52-0.81; P less than .001) and a drop in the proportion of patients with two or more medical consultants (OR, 0.55; 95% CI, 0.49-0.63; P less than .001).
The overall rate of provider satisfaction in the SCM cohort was 88.3%, but there was no significant change in patient satisfaction between the pre- and post-intervention cohorts (OR, 1.08; 95% CI, 0.87-1.33; P = .507). Furthermore, Dr. Rohatgi and her coinvestigators estimated that there was at least a $2,642 cost savings per patient, with that number also estimated as being as high as $4,303.
The SCM mode work flow
The SCM model consists of a structured work flow that hospitalists adhere to. On weekdays, an orthopedic and a neurosurgery hospitalist would screen all patients admitted for each respective specialty during the day and would consult with staff after hours to discuss any potential issues they were having with these patients. On weekends, one SCM hospitalist would remain on call to screen patients and consult for both specialities. If a medical intervention was deemed necessary, the SCM on call would formally see the patient to prevent medical complications from occurring.
The SCM would also go on rounds for select patients with the rest of the surgical team, making an effort to keep communication consistent throughout the day. These rounds would be in addition to the daily, multidisciplinary rounds that would include the “surgical team, case managers, social workers, unit nurses, physical and occupational therapists, pharmacist, [and] dietitian.” Additionally, while the surgical team would conduct the discharge summaries, the SCM hospitalists would be the ones response for discharge education, medication reconciliation, and tracking patients every 2 weeks via an electronic tracking system set up by the Stanford Health Care’s Quality and Patient Safety department.
“We postulate the effect of SCM on the quality of patient care has been underestimated due to methodological shortcomings of prior studies,” the authors noted, adding that while “the initial cost of setting up SCM might be perceived as a deterrent, [our] SCM model provided a meaningful return on investment with cost savings.”
No source of funding was provided for this study. Dr. Rohatgi and her coauthors did not report any relevant financial disclosures.
The comanagement of surgical patients by a team of surgeons and hospitalists yielded improvements in rates of medical complications, 30-day readmissions, consultant involvement, length of hospital stay, and total cost of care per patient, according to a study published in the Annals of Surgery.
“Traditionally, hospitals have utilized a consultation model of care for surgical patients in which the medicine consultants are involved ‘as needed’ [but] this model, though common, may not be the best approach to care for surgical patients,” wrote the investigators, led by Nidhi Rohatgi, MD, MS, of Stanford (Calif.) University. “To address these limitations in the care of surgical patients, we transitioned from the consultation model to surgical comanagement (SCM) model in Orthopedic and Neurosurgery at our institution in August 2012.”
Dr. Rohatgi and her coinvestigators enrolled 16,930 patients admitted for orthopedic surgery or neurosurgery into the SCM intervention cohort, which was further divided into a preintervention cohort and a postintervention cohort. The former were admitted from January 2009 to July 2012, and the latter from September 2012 to September 2013. A further 3,695 patients were enrolled in a control group, also made up of orthopedic surgery and neurosurgery patients, which was divided into two cohorts of pre- and postcontrol over the same time periods. The two primary outcomes were the proportion of patients that had at least one medical complication – defined as sepsis, pneumonia, urinary tract infections, delirium, acute kidney injury, atrial fibrillation, or ileus – and the proportion of patients with a hospital stay of at least 5 days. Other outcomes included 30-day readmissions, costs, two or more medical consultants, and satisfaction of both patients and providers.
The findings
Overall, the SCM model was associated with a decrease in the proportion of patients with at least one complication (odds ratio, 0.86; 95% confidence interval, 0.74-0.96; P = .008) in the intervention group, compared with the control group. Similarly, there was a reduction in the proportion of patients with a hospital stay of at least 5 days (OR, 0.75; 95% CI, 0.67-0.84; P less than .001). In addition, the SCM model was associated with a reduction in 30-day readmission rates caused by medical complications (OR, 0.67; 95% CI, 0.52-0.81; P less than .001) and a drop in the proportion of patients with two or more medical consultants (OR, 0.55; 95% CI, 0.49-0.63; P less than .001).
The overall rate of provider satisfaction in the SCM cohort was 88.3%, but there was no significant change in patient satisfaction between the pre- and post-intervention cohorts (OR, 1.08; 95% CI, 0.87-1.33; P = .507). Furthermore, Dr. Rohatgi and her coinvestigators estimated that there was at least a $2,642 cost savings per patient, with that number also estimated as being as high as $4,303.
The SCM mode work flow
The SCM model consists of a structured work flow that hospitalists adhere to. On weekdays, an orthopedic and a neurosurgery hospitalist would screen all patients admitted for each respective specialty during the day and would consult with staff after hours to discuss any potential issues they were having with these patients. On weekends, one SCM hospitalist would remain on call to screen patients and consult for both specialities. If a medical intervention was deemed necessary, the SCM on call would formally see the patient to prevent medical complications from occurring.
The SCM would also go on rounds for select patients with the rest of the surgical team, making an effort to keep communication consistent throughout the day. These rounds would be in addition to the daily, multidisciplinary rounds that would include the “surgical team, case managers, social workers, unit nurses, physical and occupational therapists, pharmacist, [and] dietitian.” Additionally, while the surgical team would conduct the discharge summaries, the SCM hospitalists would be the ones response for discharge education, medication reconciliation, and tracking patients every 2 weeks via an electronic tracking system set up by the Stanford Health Care’s Quality and Patient Safety department.
“We postulate the effect of SCM on the quality of patient care has been underestimated due to methodological shortcomings of prior studies,” the authors noted, adding that while “the initial cost of setting up SCM might be perceived as a deterrent, [our] SCM model provided a meaningful return on investment with cost savings.”
No source of funding was provided for this study. Dr. Rohatgi and her coauthors did not report any relevant financial disclosures.
The comanagement of surgical patients by a team of surgeons and hospitalists yielded improvements in rates of medical complications, 30-day readmissions, consultant involvement, length of hospital stay, and total cost of care per patient, according to a study published in the Annals of Surgery.
“Traditionally, hospitals have utilized a consultation model of care for surgical patients in which the medicine consultants are involved ‘as needed’ [but] this model, though common, may not be the best approach to care for surgical patients,” wrote the investigators, led by Nidhi Rohatgi, MD, MS, of Stanford (Calif.) University. “To address these limitations in the care of surgical patients, we transitioned from the consultation model to surgical comanagement (SCM) model in Orthopedic and Neurosurgery at our institution in August 2012.”
Dr. Rohatgi and her coinvestigators enrolled 16,930 patients admitted for orthopedic surgery or neurosurgery into the SCM intervention cohort, which was further divided into a preintervention cohort and a postintervention cohort. The former were admitted from January 2009 to July 2012, and the latter from September 2012 to September 2013. A further 3,695 patients were enrolled in a control group, also made up of orthopedic surgery and neurosurgery patients, which was divided into two cohorts of pre- and postcontrol over the same time periods. The two primary outcomes were the proportion of patients that had at least one medical complication – defined as sepsis, pneumonia, urinary tract infections, delirium, acute kidney injury, atrial fibrillation, or ileus – and the proportion of patients with a hospital stay of at least 5 days. Other outcomes included 30-day readmissions, costs, two or more medical consultants, and satisfaction of both patients and providers.
The findings
Overall, the SCM model was associated with a decrease in the proportion of patients with at least one complication (odds ratio, 0.86; 95% confidence interval, 0.74-0.96; P = .008) in the intervention group, compared with the control group. Similarly, there was a reduction in the proportion of patients with a hospital stay of at least 5 days (OR, 0.75; 95% CI, 0.67-0.84; P less than .001). In addition, the SCM model was associated with a reduction in 30-day readmission rates caused by medical complications (OR, 0.67; 95% CI, 0.52-0.81; P less than .001) and a drop in the proportion of patients with two or more medical consultants (OR, 0.55; 95% CI, 0.49-0.63; P less than .001).
The overall rate of provider satisfaction in the SCM cohort was 88.3%, but there was no significant change in patient satisfaction between the pre- and post-intervention cohorts (OR, 1.08; 95% CI, 0.87-1.33; P = .507). Furthermore, Dr. Rohatgi and her coinvestigators estimated that there was at least a $2,642 cost savings per patient, with that number also estimated as being as high as $4,303.
The SCM mode work flow
The SCM model consists of a structured work flow that hospitalists adhere to. On weekdays, an orthopedic and a neurosurgery hospitalist would screen all patients admitted for each respective specialty during the day and would consult with staff after hours to discuss any potential issues they were having with these patients. On weekends, one SCM hospitalist would remain on call to screen patients and consult for both specialities. If a medical intervention was deemed necessary, the SCM on call would formally see the patient to prevent medical complications from occurring.
The SCM would also go on rounds for select patients with the rest of the surgical team, making an effort to keep communication consistent throughout the day. These rounds would be in addition to the daily, multidisciplinary rounds that would include the “surgical team, case managers, social workers, unit nurses, physical and occupational therapists, pharmacist, [and] dietitian.” Additionally, while the surgical team would conduct the discharge summaries, the SCM hospitalists would be the ones response for discharge education, medication reconciliation, and tracking patients every 2 weeks via an electronic tracking system set up by the Stanford Health Care’s Quality and Patient Safety department.
“We postulate the effect of SCM on the quality of patient care has been underestimated due to methodological shortcomings of prior studies,” the authors noted, adding that while “the initial cost of setting up SCM might be perceived as a deterrent, [our] SCM model provided a meaningful return on investment with cost savings.”
No source of funding was provided for this study. Dr. Rohatgi and her coauthors did not report any relevant financial disclosures.
FROM THE ANNALS OF SURGERY
Key clinical point: Surgical comanagement (SCM) of hospital patients can mitigate medical complications, length of stay, and total cost of care.
Major finding: SCM was associated with a reduction of the proportion of patients with at least one medical complication, LOS of at least 5 days, 30-day readmissions, and those requiring at least two medical consultants.
Data source: A retrospective study of 16,930 patients receiving SCM intervention and 3,695 control patients between January 2009 and September 2013.
Disclosures: No funding source disclosed. Authors did not report any relevant financial disclosures.
West Nile testing underutilized in endemic areas
Testing for West Nile virus (WNV) is underutilized in areas where the disease is endemic, according to Jakapat Vanichanan, MD, and his associates.
In a sample of 751 patients admitted to Houston hospitals for meningitis or encephalitis, 390 patients experienced onset of symptoms during the WNV peak season between June and October, but only 281 of the 751 patients received WNV testing. Of the 281 patients tested for WNV, 32 were diagnosed with acute infection.
Patients tested for WNV were more likely to have acute focal neurologic deficits, be of older age, require an MRI, be prescribed antiviral therapy, have worse clinical outcomes, and experience concomitant testing for mycobacterial, fungal, or other viral infections. Of the 32 patients diagnosed with WNV, 16 were admitted with meningitis and 16 were admitted with encephalitis.
“Although supportive treatment remains the standard of care for patients with WNND [West Nile neuroinvasive diseases], performing appropriate WNV testing may yield several benefits. An accurate diagnosis more precisely defines disease burden and epidemiology, an ongoing surveillance deficiency. Moreover, identifying WNV may lead to early detection of long-term neurologic and neurocognitive sequelae after WNND and thus enable earlier intervention,” the investigators said.
Find the full study in Emerging Infectious Diseases (doi: 10.3201/eid2209.152050).
Testing for West Nile virus (WNV) is underutilized in areas where the disease is endemic, according to Jakapat Vanichanan, MD, and his associates.
In a sample of 751 patients admitted to Houston hospitals for meningitis or encephalitis, 390 patients experienced onset of symptoms during the WNV peak season between June and October, but only 281 of the 751 patients received WNV testing. Of the 281 patients tested for WNV, 32 were diagnosed with acute infection.
Patients tested for WNV were more likely to have acute focal neurologic deficits, be of older age, require an MRI, be prescribed antiviral therapy, have worse clinical outcomes, and experience concomitant testing for mycobacterial, fungal, or other viral infections. Of the 32 patients diagnosed with WNV, 16 were admitted with meningitis and 16 were admitted with encephalitis.
“Although supportive treatment remains the standard of care for patients with WNND [West Nile neuroinvasive diseases], performing appropriate WNV testing may yield several benefits. An accurate diagnosis more precisely defines disease burden and epidemiology, an ongoing surveillance deficiency. Moreover, identifying WNV may lead to early detection of long-term neurologic and neurocognitive sequelae after WNND and thus enable earlier intervention,” the investigators said.
Find the full study in Emerging Infectious Diseases (doi: 10.3201/eid2209.152050).
Testing for West Nile virus (WNV) is underutilized in areas where the disease is endemic, according to Jakapat Vanichanan, MD, and his associates.
In a sample of 751 patients admitted to Houston hospitals for meningitis or encephalitis, 390 patients experienced onset of symptoms during the WNV peak season between June and October, but only 281 of the 751 patients received WNV testing. Of the 281 patients tested for WNV, 32 were diagnosed with acute infection.
Patients tested for WNV were more likely to have acute focal neurologic deficits, be of older age, require an MRI, be prescribed antiviral therapy, have worse clinical outcomes, and experience concomitant testing for mycobacterial, fungal, or other viral infections. Of the 32 patients diagnosed with WNV, 16 were admitted with meningitis and 16 were admitted with encephalitis.
“Although supportive treatment remains the standard of care for patients with WNND [West Nile neuroinvasive diseases], performing appropriate WNV testing may yield several benefits. An accurate diagnosis more precisely defines disease burden and epidemiology, an ongoing surveillance deficiency. Moreover, identifying WNV may lead to early detection of long-term neurologic and neurocognitive sequelae after WNND and thus enable earlier intervention,” the investigators said.
Find the full study in Emerging Infectious Diseases (doi: 10.3201/eid2209.152050).
FROM EMERGING INFECTIOUS DISEASES
Study finds clues to fibrosis progression in chronic HCV infection
Fibrosis progression in hepatitis C virus–infected individuals is not linear, is associated with Clues to fibrosis progression in chronic hepatitis C infectionClues to fibrosis progression in chronic hepatitis C infection–related flares, and varies according to stage, with those who are least fibrotic tending to have the highest progression, according to a study.
Identifying which patients with hepatitis C virus (HCV) infection will likely progress to cirrhosis has historically been a challenge, creating some difficulty adhering to guidelines recommending that patients at greater risk of fibrosis be among those prioritized for treatment. Having an ability to more accurately diagnose those most at risk could help to better guide treatment prioritization and clinical management.
To that end, Marija Zeremski, PhD, and her colleagues at Weill Cornell Medical School, New York, analyzed 936 biopsies taken from 378 patients seen at a single site between 1997 and 2013. At the time of the first biopsy, nearly two-thirds of the patients were white men in their late 40s to mid-50s, with chronic HCV infection. Nearly 88% of all patients were HCV genotype 1 infected (J Infect Dis. 2016. doi: 10.1093/infdis/jiw332).
In the study, investigators found that between the first and a follow-up biopsy, 57.4% of patients progressed by at least one fibrosis stage, 16.1% of patients had more severe fibrosis progressions of at least two stages, and 10.6% developed either stage 3-4 or 4, with nearly 6% of patients progressing to cirrhosis. Fibrosis progression between the first and last biopsies was associated with less fibrosis on the first biopsy (P less than .001).
Increased necroinflammation and the presence of at least one alanine aminotransferase (ALT) flare greater than 200 U/L during follow-up was also significantly associated with fibrosis progression (odds ratio [OR], 2.64, P less than .007). HCV genotype 3–infected patients were significantly more likely to progress to cirrhosis (P less than .001).
Intrahepatic inflammation at the time of the initial biopsy was at grade 1 or lower in 36.4% of patients, while 56.9% of patients had grade 2 (moderate) inflammation. Severe inflammation (grade 3 or higher) was found in 6.7% of patients reviewed. There was no fibrosis in 11.9% of patients, stage 1 level of fibrosis in 32.3%, stage 2 in 39.4%, and stage 3 in 16.4% of patients.
Moderate to severe fibrosis, defined as equal to or greater than stage 2, was significantly associated with elevated inflammation (greater or equal to grade 2) on the initial biopsy (OR, 9.00; P less than .001). Steatosis testing was performed on 222 patients; 59% tested positive for it. This was significantly associated with stage 2 or higher fibrosis (OR, 2.39; P = .002), and grade 2 or higher levels of inflammation (OR, 4.07; P less than .001) on the initial biopsy.
The highest fibrosis progression rate occurred between stages 0 and 1; the lowest, between stages 2 and 3.
Consecutive biopsies were separated by at least 1 year; patients were either HCV treatment naive or were treatment nonresponders. There were a total of 558 consecutive biopsy pairs available to analyze stage progression. The time between the first and last biopsies was 6.5 years (plus or minus 3 years), while the mean duration between adjacent biopsies was 4.4 years (plus or minus 1.9 years). Data regarding HCV treatment between liver biopsies were available for all but 45 patients. Forty-three percent of the remaining patients did not achieve a sustained virologic response after treatment.
Patients who’d had a cirrhosis diagnosis according to the first biopsy, those for whom treatment induced viral eradication, or those who’d had liver transplantation between biopsies were all excluded from the review.
The investigators wrote that their finding about the association between genotype 3 and cirrhosis should be “interpreted cautiously” because of the low number of these patients in their study.
This article was updated August 17, 2016.
On Twitter @whitneymcknight
Fibrosis progression in hepatitis C virus–infected individuals is not linear, is associated with Clues to fibrosis progression in chronic hepatitis C infectionClues to fibrosis progression in chronic hepatitis C infection–related flares, and varies according to stage, with those who are least fibrotic tending to have the highest progression, according to a study.
Identifying which patients with hepatitis C virus (HCV) infection will likely progress to cirrhosis has historically been a challenge, creating some difficulty adhering to guidelines recommending that patients at greater risk of fibrosis be among those prioritized for treatment. Having an ability to more accurately diagnose those most at risk could help to better guide treatment prioritization and clinical management.
To that end, Marija Zeremski, PhD, and her colleagues at Weill Cornell Medical School, New York, analyzed 936 biopsies taken from 378 patients seen at a single site between 1997 and 2013. At the time of the first biopsy, nearly two-thirds of the patients were white men in their late 40s to mid-50s, with chronic HCV infection. Nearly 88% of all patients were HCV genotype 1 infected (J Infect Dis. 2016. doi: 10.1093/infdis/jiw332).
In the study, investigators found that between the first and a follow-up biopsy, 57.4% of patients progressed by at least one fibrosis stage, 16.1% of patients had more severe fibrosis progressions of at least two stages, and 10.6% developed either stage 3-4 or 4, with nearly 6% of patients progressing to cirrhosis. Fibrosis progression between the first and last biopsies was associated with less fibrosis on the first biopsy (P less than .001).
Increased necroinflammation and the presence of at least one alanine aminotransferase (ALT) flare greater than 200 U/L during follow-up was also significantly associated with fibrosis progression (odds ratio [OR], 2.64, P less than .007). HCV genotype 3–infected patients were significantly more likely to progress to cirrhosis (P less than .001).
Intrahepatic inflammation at the time of the initial biopsy was at grade 1 or lower in 36.4% of patients, while 56.9% of patients had grade 2 (moderate) inflammation. Severe inflammation (grade 3 or higher) was found in 6.7% of patients reviewed. There was no fibrosis in 11.9% of patients, stage 1 level of fibrosis in 32.3%, stage 2 in 39.4%, and stage 3 in 16.4% of patients.
Moderate to severe fibrosis, defined as equal to or greater than stage 2, was significantly associated with elevated inflammation (greater or equal to grade 2) on the initial biopsy (OR, 9.00; P less than .001). Steatosis testing was performed on 222 patients; 59% tested positive for it. This was significantly associated with stage 2 or higher fibrosis (OR, 2.39; P = .002), and grade 2 or higher levels of inflammation (OR, 4.07; P less than .001) on the initial biopsy.
The highest fibrosis progression rate occurred between stages 0 and 1; the lowest, between stages 2 and 3.
Consecutive biopsies were separated by at least 1 year; patients were either HCV treatment naive or were treatment nonresponders. There were a total of 558 consecutive biopsy pairs available to analyze stage progression. The time between the first and last biopsies was 6.5 years (plus or minus 3 years), while the mean duration between adjacent biopsies was 4.4 years (plus or minus 1.9 years). Data regarding HCV treatment between liver biopsies were available for all but 45 patients. Forty-three percent of the remaining patients did not achieve a sustained virologic response after treatment.
Patients who’d had a cirrhosis diagnosis according to the first biopsy, those for whom treatment induced viral eradication, or those who’d had liver transplantation between biopsies were all excluded from the review.
The investigators wrote that their finding about the association between genotype 3 and cirrhosis should be “interpreted cautiously” because of the low number of these patients in their study.
This article was updated August 17, 2016.
On Twitter @whitneymcknight
Fibrosis progression in hepatitis C virus–infected individuals is not linear, is associated with Clues to fibrosis progression in chronic hepatitis C infectionClues to fibrosis progression in chronic hepatitis C infection–related flares, and varies according to stage, with those who are least fibrotic tending to have the highest progression, according to a study.
Identifying which patients with hepatitis C virus (HCV) infection will likely progress to cirrhosis has historically been a challenge, creating some difficulty adhering to guidelines recommending that patients at greater risk of fibrosis be among those prioritized for treatment. Having an ability to more accurately diagnose those most at risk could help to better guide treatment prioritization and clinical management.
To that end, Marija Zeremski, PhD, and her colleagues at Weill Cornell Medical School, New York, analyzed 936 biopsies taken from 378 patients seen at a single site between 1997 and 2013. At the time of the first biopsy, nearly two-thirds of the patients were white men in their late 40s to mid-50s, with chronic HCV infection. Nearly 88% of all patients were HCV genotype 1 infected (J Infect Dis. 2016. doi: 10.1093/infdis/jiw332).
In the study, investigators found that between the first and a follow-up biopsy, 57.4% of patients progressed by at least one fibrosis stage, 16.1% of patients had more severe fibrosis progressions of at least two stages, and 10.6% developed either stage 3-4 or 4, with nearly 6% of patients progressing to cirrhosis. Fibrosis progression between the first and last biopsies was associated with less fibrosis on the first biopsy (P less than .001).
Increased necroinflammation and the presence of at least one alanine aminotransferase (ALT) flare greater than 200 U/L during follow-up was also significantly associated with fibrosis progression (odds ratio [OR], 2.64, P less than .007). HCV genotype 3–infected patients were significantly more likely to progress to cirrhosis (P less than .001).
Intrahepatic inflammation at the time of the initial biopsy was at grade 1 or lower in 36.4% of patients, while 56.9% of patients had grade 2 (moderate) inflammation. Severe inflammation (grade 3 or higher) was found in 6.7% of patients reviewed. There was no fibrosis in 11.9% of patients, stage 1 level of fibrosis in 32.3%, stage 2 in 39.4%, and stage 3 in 16.4% of patients.
Moderate to severe fibrosis, defined as equal to or greater than stage 2, was significantly associated with elevated inflammation (greater or equal to grade 2) on the initial biopsy (OR, 9.00; P less than .001). Steatosis testing was performed on 222 patients; 59% tested positive for it. This was significantly associated with stage 2 or higher fibrosis (OR, 2.39; P = .002), and grade 2 or higher levels of inflammation (OR, 4.07; P less than .001) on the initial biopsy.
The highest fibrosis progression rate occurred between stages 0 and 1; the lowest, between stages 2 and 3.
Consecutive biopsies were separated by at least 1 year; patients were either HCV treatment naive or were treatment nonresponders. There were a total of 558 consecutive biopsy pairs available to analyze stage progression. The time between the first and last biopsies was 6.5 years (plus or minus 3 years), while the mean duration between adjacent biopsies was 4.4 years (plus or minus 1.9 years). Data regarding HCV treatment between liver biopsies were available for all but 45 patients. Forty-three percent of the remaining patients did not achieve a sustained virologic response after treatment.
Patients who’d had a cirrhosis diagnosis according to the first biopsy, those for whom treatment induced viral eradication, or those who’d had liver transplantation between biopsies were all excluded from the review.
The investigators wrote that their finding about the association between genotype 3 and cirrhosis should be “interpreted cautiously” because of the low number of these patients in their study.
This article was updated August 17, 2016.
On Twitter @whitneymcknight
FROM THE JOURNAL OF INFECTIOUS DISEASES
Key clinical point: Fibrosis progression is not linear in chronic HCV infection.
Major finding: Between the first and follow-up biopsy, 57.4% of patients progressed by at least one fibrosis stage, 16.1% of patients had more severe fibrosis progressions of at least two stages, and 10.6% developed either stage 3-4 or 4, with nearly 6% of patients progressing to cirrhosis.
Data source: A review of 936 biopsies taken from 378 patients seen at a single site between 1997 and 2013.
Disclosures: Dr. Talal and Dr. Jacobson disclosed numerous industry relationships, including with Abbott, AbbVie, and Gilead. The study was supported by the Troup Fund of the Kaleida Health Foundation and the Greenberg Foundation for Medical Research.