User login
EMERGENCY MEDICINE is a practical, peer-reviewed monthly publication and Web site that meets the educational needs of emergency clinicians and urgent care clinicians for their practice.
New study of diabetes drug for COVID-19 raises eyebrows
A just-launched study of the type 2 diabetes agent dapagliflozin (Farxiga, AstraZeneca) in patients with mild to moderate COVID-19 is raising eyebrows, given that several expert groups have advised that drugs in this class – the sodium-glucose cotransporter 2 (SGLT2) inhibitors – be stopped in all patients hospitalized with COVID-19 because of the increased risk for diabetic ketoacidosis (DKA).
The randomized, double-blind, placebo-controlled, phase 3 Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) study is sponsored by AstraZeneca and Saint Luke’s Mid America Heart Institute.
The trial will assess whether dapagliflozin reduces the risks of disease progression, clinical complications, and death because of COVID-19 in patients with type 2 diabetes, cardiovascular disease, and/or mild to moderate chronic kidney disease (CKD).
“Dapagliflozin has demonstrated cardio- and renal-protective benefits and improved outcomes in high-risk patients with type 2 diabetes, heart failure with reduced ejection fraction, and CKD,” said the principal investigator of DARE-19, Mikhail N. Kosiborod, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo.
And “patients with COVID-19 and underlying cardiometabolic disease appear to be at the highest risk of morbid complications,” he explained in an AstraZeneca statement.
“Through DARE-19, we hope to decrease the severity of illness, and prevent cardiovascular, respiratory, and kidney decompensation, which are common in patients with COVID-19,” Dr. Kosiborod continued.
However, advice to stop SGLT2 inhibitors in patients hospitalized with COVID-19 because of its associated DKA risk has come from several channels.
These include initial guidance from Diabetes UK; experts who spoke during an American Diabetes Association webinar; and most recently, an international panel of diabetes experts.
Some clinicians went so far as to say that they view the trial as potentially dangerous, while others said they could see some logic to it, as long as it is carefully managed.
“A dangerous proposition – a DARE I would not take”
Partha Kar, MD, of Portsmouth Hospitals NHS Trust and national clinical director of diabetes at NHS England, said in an interview: “It’s interesting to see [AstraZeneca] embark on a study with a particular class of drug whereby ... [in] the UK we have said that if you get sent to hospital with COVID-19 you should stop [SGLT2 inhibitors] immediately.”
It “sounds like a risky proposition to go ahead with, [and it] definitely made me raise an eyebrow,” he added.
Nephrologist Bruce R. Leslie, MD, of Seventh Doctor Consulting in Princeton, N.J., agreed with Dr. Kar.
“Giving SGLT2 inhibitors to patients in the DARE-19 study is a dangerous proposition because these drugs can induce ketoacidosis during the stress of acute illness such as COVID-19. ... Moreover, ketoacidosis is associated with hypercoagulability which could be especially dangerous in COVID-19, given that it has been causing thrombophilia with large-vessel occlusive strokes in young patients,” he said in an interview.
“One wonders how these risks were assessed by the authorities that approved the DARE-19 study,” said Dr. Leslie, who formerly worked for Bristol-Myers Squibb.
“How does the sponsor intend to secure informed consent given the risks? This is a DARE I would not take,” he said.
Asked to address these concerns, Dr. Kosiborod said in an interview that “the DARE-19 trial will assess both the efficacy and the safety of dapagliflozin in this patient population in a closely monitored environment of a rigorously designed randomized clinical trial. The trial protocol excludes patients with type 1 diabetes or at high risk for DKA.
“Furthermore, the protocol includes detailed specific instructions to ensure careful monitoring for DKA, including frequent assessments of acid-base status in the hospital setting. The safety data will be closely monitored by an independent data-monitoring committee,” he continued.
Dr. Kosiborod also pointed out that there is “no systematically collected information on the use of dapagliflozin or any other SGLT2 inhibitor in patients being treated for COVID-19, including the associated potential benefits, possible risks such as DKA, and the balance of these potential benefits and risks.”
DARE-19 design: Several outcomes will be examined
The DARE-19 trial is designed to enroll 900 adults with confirmed SARS-CoV-2 infection and oxygen saturation of 94% or greater.
Inclusion criteria include a medical history of hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure, and/or stage 3-4 CKD. Exclusion criteria include current SGLT2 inhibitor treatment, type 1 diabetes, severe CKD, and severe COVID-19.
Dapagliflozin is approved in the EU for use in some patients with type 1 diabetes; this is not the case in the United States, although SGLT2 inhibitors in general are sometimes used off label in these patients.
Patients in DARE-19 will be randomized to 10 mg/day dapagliflozin or placebo for 30 days, in addition to standard care, in participating hospital. Primary outcomes are time to first occurrence of either death or new or worsened organ dysfunction, including respiratory decompensation, new or worsening heart failure, requirement for vasopressor therapy, ventricular tachycardia, and renal failure.
Secondary outcomes include a composite of time to death from any cause, time to new/worsened organ dysfunction, clinical status at day 30, and time to hospital discharge.
Rationale for the study
Irl B. Hirsch, MD, professor and diabetes treatment and teaching chair at the University of Washington, Seattle, said in an interview that he does see some logic to the trial.
Admitting that he doesn’t know much about “COVID-19 cardiomyopathy” – which would be one of the targets of dapagliflozin – other than it is quite common, he said that this, along with the potential renal benefits of dapagliflozin in the setting of COVID-19, make the study “intriguing.”
“Perhaps there is some rationale to it,” he said. However, “my concern is these sick COVID-19 patients are often acidemic, and besides the very complex acid-base challenges we see with intubated patients, these patients likely have combination lactic and ketoacidemia, the latter at least some from starvation.
“Still, if enough dextrose and insulin are provided to prevent ketoacid accumulation, my guess is it would do at least as well as hydroxychloroquine,” he said.
And Simon Heller, MD, professor of clinical diabetes at the University of Sheffield (England), said in an interview: “I think it is quite a brave study, mainly because of the increased risk of DKA.
“However, on the basis that these patients will be carefully monitored, the risk of DKA shouldn’t be great. I think it is important that patients with type 2 diabetes can participate whenever possible in such trials,” he said.
The estimated completion date for DARE-19 is December 2020.
Dr. Kosiborod has reported receiving grant support, honoraria, and/or research support from AstraZeneca, Boehringer Ingelheim, Sanofi, Amgen, Novo Nordisk, Merck, Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia Therapeutics, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. Dr. Leslie has reported owning stock in Bristol-Myers Squibb, Pfizer, and Lilly. Dr. Hirsch has reported consulting for Abbott Diabetes Care, Roche, and Bigfoot Biomedical, conducting research for Medtronic, and is a diabetes editor for UpToDate. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, MSD, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic UK. He is on the advisory board for Medscape. Dr. Kar has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A just-launched study of the type 2 diabetes agent dapagliflozin (Farxiga, AstraZeneca) in patients with mild to moderate COVID-19 is raising eyebrows, given that several expert groups have advised that drugs in this class – the sodium-glucose cotransporter 2 (SGLT2) inhibitors – be stopped in all patients hospitalized with COVID-19 because of the increased risk for diabetic ketoacidosis (DKA).
The randomized, double-blind, placebo-controlled, phase 3 Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) study is sponsored by AstraZeneca and Saint Luke’s Mid America Heart Institute.
The trial will assess whether dapagliflozin reduces the risks of disease progression, clinical complications, and death because of COVID-19 in patients with type 2 diabetes, cardiovascular disease, and/or mild to moderate chronic kidney disease (CKD).
“Dapagliflozin has demonstrated cardio- and renal-protective benefits and improved outcomes in high-risk patients with type 2 diabetes, heart failure with reduced ejection fraction, and CKD,” said the principal investigator of DARE-19, Mikhail N. Kosiborod, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo.
And “patients with COVID-19 and underlying cardiometabolic disease appear to be at the highest risk of morbid complications,” he explained in an AstraZeneca statement.
“Through DARE-19, we hope to decrease the severity of illness, and prevent cardiovascular, respiratory, and kidney decompensation, which are common in patients with COVID-19,” Dr. Kosiborod continued.
However, advice to stop SGLT2 inhibitors in patients hospitalized with COVID-19 because of its associated DKA risk has come from several channels.
These include initial guidance from Diabetes UK; experts who spoke during an American Diabetes Association webinar; and most recently, an international panel of diabetes experts.
Some clinicians went so far as to say that they view the trial as potentially dangerous, while others said they could see some logic to it, as long as it is carefully managed.
“A dangerous proposition – a DARE I would not take”
Partha Kar, MD, of Portsmouth Hospitals NHS Trust and national clinical director of diabetes at NHS England, said in an interview: “It’s interesting to see [AstraZeneca] embark on a study with a particular class of drug whereby ... [in] the UK we have said that if you get sent to hospital with COVID-19 you should stop [SGLT2 inhibitors] immediately.”
It “sounds like a risky proposition to go ahead with, [and it] definitely made me raise an eyebrow,” he added.
Nephrologist Bruce R. Leslie, MD, of Seventh Doctor Consulting in Princeton, N.J., agreed with Dr. Kar.
“Giving SGLT2 inhibitors to patients in the DARE-19 study is a dangerous proposition because these drugs can induce ketoacidosis during the stress of acute illness such as COVID-19. ... Moreover, ketoacidosis is associated with hypercoagulability which could be especially dangerous in COVID-19, given that it has been causing thrombophilia with large-vessel occlusive strokes in young patients,” he said in an interview.
“One wonders how these risks were assessed by the authorities that approved the DARE-19 study,” said Dr. Leslie, who formerly worked for Bristol-Myers Squibb.
“How does the sponsor intend to secure informed consent given the risks? This is a DARE I would not take,” he said.
Asked to address these concerns, Dr. Kosiborod said in an interview that “the DARE-19 trial will assess both the efficacy and the safety of dapagliflozin in this patient population in a closely monitored environment of a rigorously designed randomized clinical trial. The trial protocol excludes patients with type 1 diabetes or at high risk for DKA.
“Furthermore, the protocol includes detailed specific instructions to ensure careful monitoring for DKA, including frequent assessments of acid-base status in the hospital setting. The safety data will be closely monitored by an independent data-monitoring committee,” he continued.
Dr. Kosiborod also pointed out that there is “no systematically collected information on the use of dapagliflozin or any other SGLT2 inhibitor in patients being treated for COVID-19, including the associated potential benefits, possible risks such as DKA, and the balance of these potential benefits and risks.”
DARE-19 design: Several outcomes will be examined
The DARE-19 trial is designed to enroll 900 adults with confirmed SARS-CoV-2 infection and oxygen saturation of 94% or greater.
Inclusion criteria include a medical history of hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure, and/or stage 3-4 CKD. Exclusion criteria include current SGLT2 inhibitor treatment, type 1 diabetes, severe CKD, and severe COVID-19.
Dapagliflozin is approved in the EU for use in some patients with type 1 diabetes; this is not the case in the United States, although SGLT2 inhibitors in general are sometimes used off label in these patients.
Patients in DARE-19 will be randomized to 10 mg/day dapagliflozin or placebo for 30 days, in addition to standard care, in participating hospital. Primary outcomes are time to first occurrence of either death or new or worsened organ dysfunction, including respiratory decompensation, new or worsening heart failure, requirement for vasopressor therapy, ventricular tachycardia, and renal failure.
Secondary outcomes include a composite of time to death from any cause, time to new/worsened organ dysfunction, clinical status at day 30, and time to hospital discharge.
Rationale for the study
Irl B. Hirsch, MD, professor and diabetes treatment and teaching chair at the University of Washington, Seattle, said in an interview that he does see some logic to the trial.
Admitting that he doesn’t know much about “COVID-19 cardiomyopathy” – which would be one of the targets of dapagliflozin – other than it is quite common, he said that this, along with the potential renal benefits of dapagliflozin in the setting of COVID-19, make the study “intriguing.”
“Perhaps there is some rationale to it,” he said. However, “my concern is these sick COVID-19 patients are often acidemic, and besides the very complex acid-base challenges we see with intubated patients, these patients likely have combination lactic and ketoacidemia, the latter at least some from starvation.
“Still, if enough dextrose and insulin are provided to prevent ketoacid accumulation, my guess is it would do at least as well as hydroxychloroquine,” he said.
And Simon Heller, MD, professor of clinical diabetes at the University of Sheffield (England), said in an interview: “I think it is quite a brave study, mainly because of the increased risk of DKA.
“However, on the basis that these patients will be carefully monitored, the risk of DKA shouldn’t be great. I think it is important that patients with type 2 diabetes can participate whenever possible in such trials,” he said.
The estimated completion date for DARE-19 is December 2020.
Dr. Kosiborod has reported receiving grant support, honoraria, and/or research support from AstraZeneca, Boehringer Ingelheim, Sanofi, Amgen, Novo Nordisk, Merck, Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia Therapeutics, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. Dr. Leslie has reported owning stock in Bristol-Myers Squibb, Pfizer, and Lilly. Dr. Hirsch has reported consulting for Abbott Diabetes Care, Roche, and Bigfoot Biomedical, conducting research for Medtronic, and is a diabetes editor for UpToDate. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, MSD, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic UK. He is on the advisory board for Medscape. Dr. Kar has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A just-launched study of the type 2 diabetes agent dapagliflozin (Farxiga, AstraZeneca) in patients with mild to moderate COVID-19 is raising eyebrows, given that several expert groups have advised that drugs in this class – the sodium-glucose cotransporter 2 (SGLT2) inhibitors – be stopped in all patients hospitalized with COVID-19 because of the increased risk for diabetic ketoacidosis (DKA).
The randomized, double-blind, placebo-controlled, phase 3 Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) study is sponsored by AstraZeneca and Saint Luke’s Mid America Heart Institute.
The trial will assess whether dapagliflozin reduces the risks of disease progression, clinical complications, and death because of COVID-19 in patients with type 2 diabetes, cardiovascular disease, and/or mild to moderate chronic kidney disease (CKD).
“Dapagliflozin has demonstrated cardio- and renal-protective benefits and improved outcomes in high-risk patients with type 2 diabetes, heart failure with reduced ejection fraction, and CKD,” said the principal investigator of DARE-19, Mikhail N. Kosiborod, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo.
And “patients with COVID-19 and underlying cardiometabolic disease appear to be at the highest risk of morbid complications,” he explained in an AstraZeneca statement.
“Through DARE-19, we hope to decrease the severity of illness, and prevent cardiovascular, respiratory, and kidney decompensation, which are common in patients with COVID-19,” Dr. Kosiborod continued.
However, advice to stop SGLT2 inhibitors in patients hospitalized with COVID-19 because of its associated DKA risk has come from several channels.
These include initial guidance from Diabetes UK; experts who spoke during an American Diabetes Association webinar; and most recently, an international panel of diabetes experts.
Some clinicians went so far as to say that they view the trial as potentially dangerous, while others said they could see some logic to it, as long as it is carefully managed.
“A dangerous proposition – a DARE I would not take”
Partha Kar, MD, of Portsmouth Hospitals NHS Trust and national clinical director of diabetes at NHS England, said in an interview: “It’s interesting to see [AstraZeneca] embark on a study with a particular class of drug whereby ... [in] the UK we have said that if you get sent to hospital with COVID-19 you should stop [SGLT2 inhibitors] immediately.”
It “sounds like a risky proposition to go ahead with, [and it] definitely made me raise an eyebrow,” he added.
Nephrologist Bruce R. Leslie, MD, of Seventh Doctor Consulting in Princeton, N.J., agreed with Dr. Kar.
“Giving SGLT2 inhibitors to patients in the DARE-19 study is a dangerous proposition because these drugs can induce ketoacidosis during the stress of acute illness such as COVID-19. ... Moreover, ketoacidosis is associated with hypercoagulability which could be especially dangerous in COVID-19, given that it has been causing thrombophilia with large-vessel occlusive strokes in young patients,” he said in an interview.
“One wonders how these risks were assessed by the authorities that approved the DARE-19 study,” said Dr. Leslie, who formerly worked for Bristol-Myers Squibb.
“How does the sponsor intend to secure informed consent given the risks? This is a DARE I would not take,” he said.
Asked to address these concerns, Dr. Kosiborod said in an interview that “the DARE-19 trial will assess both the efficacy and the safety of dapagliflozin in this patient population in a closely monitored environment of a rigorously designed randomized clinical trial. The trial protocol excludes patients with type 1 diabetes or at high risk for DKA.
“Furthermore, the protocol includes detailed specific instructions to ensure careful monitoring for DKA, including frequent assessments of acid-base status in the hospital setting. The safety data will be closely monitored by an independent data-monitoring committee,” he continued.
Dr. Kosiborod also pointed out that there is “no systematically collected information on the use of dapagliflozin or any other SGLT2 inhibitor in patients being treated for COVID-19, including the associated potential benefits, possible risks such as DKA, and the balance of these potential benefits and risks.”
DARE-19 design: Several outcomes will be examined
The DARE-19 trial is designed to enroll 900 adults with confirmed SARS-CoV-2 infection and oxygen saturation of 94% or greater.
Inclusion criteria include a medical history of hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure, and/or stage 3-4 CKD. Exclusion criteria include current SGLT2 inhibitor treatment, type 1 diabetes, severe CKD, and severe COVID-19.
Dapagliflozin is approved in the EU for use in some patients with type 1 diabetes; this is not the case in the United States, although SGLT2 inhibitors in general are sometimes used off label in these patients.
Patients in DARE-19 will be randomized to 10 mg/day dapagliflozin or placebo for 30 days, in addition to standard care, in participating hospital. Primary outcomes are time to first occurrence of either death or new or worsened organ dysfunction, including respiratory decompensation, new or worsening heart failure, requirement for vasopressor therapy, ventricular tachycardia, and renal failure.
Secondary outcomes include a composite of time to death from any cause, time to new/worsened organ dysfunction, clinical status at day 30, and time to hospital discharge.
Rationale for the study
Irl B. Hirsch, MD, professor and diabetes treatment and teaching chair at the University of Washington, Seattle, said in an interview that he does see some logic to the trial.
Admitting that he doesn’t know much about “COVID-19 cardiomyopathy” – which would be one of the targets of dapagliflozin – other than it is quite common, he said that this, along with the potential renal benefits of dapagliflozin in the setting of COVID-19, make the study “intriguing.”
“Perhaps there is some rationale to it,” he said. However, “my concern is these sick COVID-19 patients are often acidemic, and besides the very complex acid-base challenges we see with intubated patients, these patients likely have combination lactic and ketoacidemia, the latter at least some from starvation.
“Still, if enough dextrose and insulin are provided to prevent ketoacid accumulation, my guess is it would do at least as well as hydroxychloroquine,” he said.
And Simon Heller, MD, professor of clinical diabetes at the University of Sheffield (England), said in an interview: “I think it is quite a brave study, mainly because of the increased risk of DKA.
“However, on the basis that these patients will be carefully monitored, the risk of DKA shouldn’t be great. I think it is important that patients with type 2 diabetes can participate whenever possible in such trials,” he said.
The estimated completion date for DARE-19 is December 2020.
Dr. Kosiborod has reported receiving grant support, honoraria, and/or research support from AstraZeneca, Boehringer Ingelheim, Sanofi, Amgen, Novo Nordisk, Merck, Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia Therapeutics, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. Dr. Leslie has reported owning stock in Bristol-Myers Squibb, Pfizer, and Lilly. Dr. Hirsch has reported consulting for Abbott Diabetes Care, Roche, and Bigfoot Biomedical, conducting research for Medtronic, and is a diabetes editor for UpToDate. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, MSD, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic UK. He is on the advisory board for Medscape. Dr. Kar has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Survey: Hydroxychloroquine use fairly common in COVID-19
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.
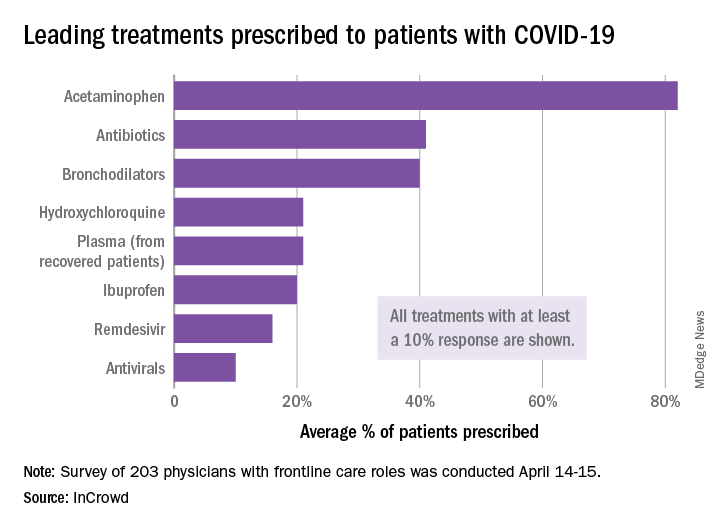
The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.

The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
One of five physicians in front-line treatment roles has prescribed hydroxychloroquine for COVID-19, according to a new survey from health care market research company InCrowd.

The most common treatments were acetaminophen, prescribed to 82% of patients, antibiotics (41%), and bronchodilators (40%), InCrowd said after surveying 203 primary care physicians, pediatricians, and emergency medicine or critical care physicians who are treating at least 20 patients with flulike symptoms.
On April 24, the Food and Drug Administration warned against the use of hydroxychloroquine or chloroquine outside of hospitals and clinical trials.
The InCrowd survey, which took place April 14-15 and is the fourth in a series investigating COVID-19’s impact on physicians, showed that access to testing was up to 82% in mid-April, compared with 67% in March and 20% in late February. The April respondents also were twice as likely (59% vs. 24% in March) to say that their facilities were prepared to treat patients, InCrowd reported.
“U.S. physicians report sluggish optimism around preparedness, safety, and institutional efforts, while many worry about the future, including a second outbreak and job security,” the company said in a separate written statement.
The average estimate for a return to normal was just over 6 months among respondents, and only 28% believed that their facility was prepared for a second outbreak later in the year, InCrowd noted.
On a personal level, 45% of the respondents were concerned about the safety of their job. An emergency/critical care physician from Tennessee said, “We’ve been cutting back on staff due to overall revenue reductions, but have increased acuity and complexity which requires more staffing. This puts even more of a burden on those of us still here.”
Support for institutional responses to slow the pandemic was strongest for state governments, which gained approval from 54% of front-line physicians, up from 33% in March. Actions taken by the federal government were supported by 21% of respondents, compared with 38% for the World Health Organization and 46% for governments outside the United States, InCrowd reported.
Suggestions for further actions by state and local authorities included this comment from an emergency/critical care physician in Florida: “Continued, broad and properly enforced stay at home and social distancing measures MUST remain in place to keep citizens and healthcare workers safe, and the latter alive and in adequate supply.”
Consensus recommendations on AMI management during COVID-19
A consensus statement from the American College of Cardiology (ACC), the American College of Emergency Physicians (ACEP), and the Society for Cardiovascular Angiography & Interventions (SCAI) outlines recommendations for a systematic approach for the care of patients with an acute myocardial infarction (AMI) during the COVID-19 pandemic.
The statement was published in the Journal of the American College of Cardiology.
During the COVID-19 pandemic, percutaneous coronary intervention (PCI) remains the standard of care for patients with ST-segment elevation MI (STEMI) at PCI-capable hospitals when it can be provided in a timely fashion in a dedicated cardiac catheterization laboratory with an expert care team wearing personal protection equipment (PPE), the writing group advised.
“A fibrinolysis-based strategy may be entertained at non-PCI capable referral hospitals or in specific situations where primary PCI cannot be executed or is not deemed the best option,” they said.
SCAI President Ehtisham Mahmud, MD, of the University of California, San Diego, and the writing group also said that clinicians should recognize that cardiovascular manifestations of COVID-19 are “complex” in patients presenting with AMI, myocarditis simulating a STEMI, stress cardiomyopathy, nonischemic cardiomyopathy, coronary spasm, or nonspecific myocardial injury.
A “broad differential diagnosis for ST elevations (including COVID-associated myocarditis) should be considered in the ED prior to choosing a reperfusion strategy,” they advised.
In the absence of hemodynamic instability or ongoing ischemic symptoms, non-STEMI patients with known or suspected COVID-19 are best managed with an initial medical stabilization strategy, the group said.
They also said it is “imperative that health care workers use appropriate PPE for all invasive procedures during this pandemic” and that new rapid COVID-19 testing be “expeditiously” disseminated to all hospitals that manage patients with AMI.
Major challenges are that the prevalence of the COVID-19 in the United States remains unknown and there is the risk for asymptomatic spread.
The writing group said it’s “critical” to “inform the public that we can minimize exposure to the coronavirus so they can continue to call the Emergency Medical System (EMS) for acute ischemic heart disease symptoms and therefore get the appropriate level of cardiac care that their presentation warrants.”
This research had no commercial funding. Dr. Mahmud reported receiving clinical trial research support from Corindus, Abbott Vascular, and CSI; consulting with Medtronic; and consulting and equity with Abiomed. A complete list of author disclosures is included with the original article.
A version of this article originally appeared on Medscape.com.
A consensus statement from the American College of Cardiology (ACC), the American College of Emergency Physicians (ACEP), and the Society for Cardiovascular Angiography & Interventions (SCAI) outlines recommendations for a systematic approach for the care of patients with an acute myocardial infarction (AMI) during the COVID-19 pandemic.
The statement was published in the Journal of the American College of Cardiology.
During the COVID-19 pandemic, percutaneous coronary intervention (PCI) remains the standard of care for patients with ST-segment elevation MI (STEMI) at PCI-capable hospitals when it can be provided in a timely fashion in a dedicated cardiac catheterization laboratory with an expert care team wearing personal protection equipment (PPE), the writing group advised.
“A fibrinolysis-based strategy may be entertained at non-PCI capable referral hospitals or in specific situations where primary PCI cannot be executed or is not deemed the best option,” they said.
SCAI President Ehtisham Mahmud, MD, of the University of California, San Diego, and the writing group also said that clinicians should recognize that cardiovascular manifestations of COVID-19 are “complex” in patients presenting with AMI, myocarditis simulating a STEMI, stress cardiomyopathy, nonischemic cardiomyopathy, coronary spasm, or nonspecific myocardial injury.
A “broad differential diagnosis for ST elevations (including COVID-associated myocarditis) should be considered in the ED prior to choosing a reperfusion strategy,” they advised.
In the absence of hemodynamic instability or ongoing ischemic symptoms, non-STEMI patients with known or suspected COVID-19 are best managed with an initial medical stabilization strategy, the group said.
They also said it is “imperative that health care workers use appropriate PPE for all invasive procedures during this pandemic” and that new rapid COVID-19 testing be “expeditiously” disseminated to all hospitals that manage patients with AMI.
Major challenges are that the prevalence of the COVID-19 in the United States remains unknown and there is the risk for asymptomatic spread.
The writing group said it’s “critical” to “inform the public that we can minimize exposure to the coronavirus so they can continue to call the Emergency Medical System (EMS) for acute ischemic heart disease symptoms and therefore get the appropriate level of cardiac care that their presentation warrants.”
This research had no commercial funding. Dr. Mahmud reported receiving clinical trial research support from Corindus, Abbott Vascular, and CSI; consulting with Medtronic; and consulting and equity with Abiomed. A complete list of author disclosures is included with the original article.
A version of this article originally appeared on Medscape.com.
A consensus statement from the American College of Cardiology (ACC), the American College of Emergency Physicians (ACEP), and the Society for Cardiovascular Angiography & Interventions (SCAI) outlines recommendations for a systematic approach for the care of patients with an acute myocardial infarction (AMI) during the COVID-19 pandemic.
The statement was published in the Journal of the American College of Cardiology.
During the COVID-19 pandemic, percutaneous coronary intervention (PCI) remains the standard of care for patients with ST-segment elevation MI (STEMI) at PCI-capable hospitals when it can be provided in a timely fashion in a dedicated cardiac catheterization laboratory with an expert care team wearing personal protection equipment (PPE), the writing group advised.
“A fibrinolysis-based strategy may be entertained at non-PCI capable referral hospitals or in specific situations where primary PCI cannot be executed or is not deemed the best option,” they said.
SCAI President Ehtisham Mahmud, MD, of the University of California, San Diego, and the writing group also said that clinicians should recognize that cardiovascular manifestations of COVID-19 are “complex” in patients presenting with AMI, myocarditis simulating a STEMI, stress cardiomyopathy, nonischemic cardiomyopathy, coronary spasm, or nonspecific myocardial injury.
A “broad differential diagnosis for ST elevations (including COVID-associated myocarditis) should be considered in the ED prior to choosing a reperfusion strategy,” they advised.
In the absence of hemodynamic instability or ongoing ischemic symptoms, non-STEMI patients with known or suspected COVID-19 are best managed with an initial medical stabilization strategy, the group said.
They also said it is “imperative that health care workers use appropriate PPE for all invasive procedures during this pandemic” and that new rapid COVID-19 testing be “expeditiously” disseminated to all hospitals that manage patients with AMI.
Major challenges are that the prevalence of the COVID-19 in the United States remains unknown and there is the risk for asymptomatic spread.
The writing group said it’s “critical” to “inform the public that we can minimize exposure to the coronavirus so they can continue to call the Emergency Medical System (EMS) for acute ischemic heart disease symptoms and therefore get the appropriate level of cardiac care that their presentation warrants.”
This research had no commercial funding. Dr. Mahmud reported receiving clinical trial research support from Corindus, Abbott Vascular, and CSI; consulting with Medtronic; and consulting and equity with Abiomed. A complete list of author disclosures is included with the original article.
A version of this article originally appeared on Medscape.com.
COVID-19 decimates outpatient visits
There has been a massive decline in outpatient office visits as patients have stayed home – likely deferring needed care – because of COVID-19, new research shows.
The number of visits to ambulatory practices dropped by a whopping 60% in mid-March, and continues to be down by at least 50% since early February, according to new data compiled and analyzed by Harvard University and Phreesia, a health care technology company.
Phreesia – which helps medical practices with patient registration, insurance verification, and payments – has data on 50,000 providers in all 50 states; in a typical year, Phreesia tracks 50 million outpatient visits.
The report was published online April 23 by the Commonwealth Fund.
The company captured data on visits from February 1 through April 16. The decline was greatest in New England and the Mid-Atlantic states, where, at the steepest end of the decline in late March, visits were down 66%.
They have rebounded slightly since then but are still down 64%. Practices in the mountain states had the smallest decline, but visits were down by 45% as of April 16.
Many practices have attempted to reach out to patients through telemedicine. As of April 16, about 30% of all visits tracked by Phreesia were provided via telemedicine – by phone or through video. That’s a monumental increase from mid-February, when zero visits were conducted virtually.
However, the Harvard researchers found that telemedicine visits barely made up for the huge decline in office visits.
Decline by specialty
Not surprisingly, declining visits have been steeper in procedure-oriented specialties.
Overall visits – including telemedicine – to ophthalmologists and otolaryngologists had declined by 79% and 75%, respectively, as of the week of April 5. Dermatology saw a 73% decline. Surgery, pulmonology, urology, orthopedics, cardiology, and gastroenterology all experienced declines ranging from 61% to 66%.
Primary care offices, oncology, endocrinology, and obstetrics/gynecology all fared slightly better, with visits down by half. Behavioral health experienced the lowest rate of decline (30%).
School-aged children were skipping care most often. The study showed a 71% drop in visits in 7- to 17-year-olds, and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Nearly two-thirds of Americans over age 65 also stayed away from their doctors. Only half of those aged 18 to 64 reduced their physician visits.
This article first appeared on Medscape.com.
There has been a massive decline in outpatient office visits as patients have stayed home – likely deferring needed care – because of COVID-19, new research shows.
The number of visits to ambulatory practices dropped by a whopping 60% in mid-March, and continues to be down by at least 50% since early February, according to new data compiled and analyzed by Harvard University and Phreesia, a health care technology company.
Phreesia – which helps medical practices with patient registration, insurance verification, and payments – has data on 50,000 providers in all 50 states; in a typical year, Phreesia tracks 50 million outpatient visits.
The report was published online April 23 by the Commonwealth Fund.
The company captured data on visits from February 1 through April 16. The decline was greatest in New England and the Mid-Atlantic states, where, at the steepest end of the decline in late March, visits were down 66%.
They have rebounded slightly since then but are still down 64%. Practices in the mountain states had the smallest decline, but visits were down by 45% as of April 16.
Many practices have attempted to reach out to patients through telemedicine. As of April 16, about 30% of all visits tracked by Phreesia were provided via telemedicine – by phone or through video. That’s a monumental increase from mid-February, when zero visits were conducted virtually.
However, the Harvard researchers found that telemedicine visits barely made up for the huge decline in office visits.
Decline by specialty
Not surprisingly, declining visits have been steeper in procedure-oriented specialties.
Overall visits – including telemedicine – to ophthalmologists and otolaryngologists had declined by 79% and 75%, respectively, as of the week of April 5. Dermatology saw a 73% decline. Surgery, pulmonology, urology, orthopedics, cardiology, and gastroenterology all experienced declines ranging from 61% to 66%.
Primary care offices, oncology, endocrinology, and obstetrics/gynecology all fared slightly better, with visits down by half. Behavioral health experienced the lowest rate of decline (30%).
School-aged children were skipping care most often. The study showed a 71% drop in visits in 7- to 17-year-olds, and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Nearly two-thirds of Americans over age 65 also stayed away from their doctors. Only half of those aged 18 to 64 reduced their physician visits.
This article first appeared on Medscape.com.
There has been a massive decline in outpatient office visits as patients have stayed home – likely deferring needed care – because of COVID-19, new research shows.
The number of visits to ambulatory practices dropped by a whopping 60% in mid-March, and continues to be down by at least 50% since early February, according to new data compiled and analyzed by Harvard University and Phreesia, a health care technology company.
Phreesia – which helps medical practices with patient registration, insurance verification, and payments – has data on 50,000 providers in all 50 states; in a typical year, Phreesia tracks 50 million outpatient visits.
The report was published online April 23 by the Commonwealth Fund.
The company captured data on visits from February 1 through April 16. The decline was greatest in New England and the Mid-Atlantic states, where, at the steepest end of the decline in late March, visits were down 66%.
They have rebounded slightly since then but are still down 64%. Practices in the mountain states had the smallest decline, but visits were down by 45% as of April 16.
Many practices have attempted to reach out to patients through telemedicine. As of April 16, about 30% of all visits tracked by Phreesia were provided via telemedicine – by phone or through video. That’s a monumental increase from mid-February, when zero visits were conducted virtually.
However, the Harvard researchers found that telemedicine visits barely made up for the huge decline in office visits.
Decline by specialty
Not surprisingly, declining visits have been steeper in procedure-oriented specialties.
Overall visits – including telemedicine – to ophthalmologists and otolaryngologists had declined by 79% and 75%, respectively, as of the week of April 5. Dermatology saw a 73% decline. Surgery, pulmonology, urology, orthopedics, cardiology, and gastroenterology all experienced declines ranging from 61% to 66%.
Primary care offices, oncology, endocrinology, and obstetrics/gynecology all fared slightly better, with visits down by half. Behavioral health experienced the lowest rate of decline (30%).
School-aged children were skipping care most often. The study showed a 71% drop in visits in 7- to 17-year-olds, and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Nearly two-thirds of Americans over age 65 also stayed away from their doctors. Only half of those aged 18 to 64 reduced their physician visits.
This article first appeared on Medscape.com.
FDA reiterates hydroxychloroquine limitations for COVID-19
The U.S. Food and Drug Administration reinforced its March guidance on when it’s permissible to use hydroxychloroquine and chloroquine to treat COVID-19 patients and on the multiple risks these drugs pose in a Safety Communication on April 24.
The new communication reiterated the agency’s position from the Emergency Use Authorization (EUA) it granted on March 28 to allow hydroxychloroquine and chloroquine treatment of COVID-19 patients only when they are hospitalized and participation in a clinical trial is “not available,” or “not feasible.” The April 24 update to the EUA noted that “the FDA is aware of reports of serious heart rhythm problems in patients with COVID-19 treated with hydroxychloroquine or chloroquine, often in combination with azithromycin and other QT-prolonging medicines. We are also aware of increased use of these medicines through outpatient prescriptions.”
In addition to reiterating the prior limitations on permissible patients for these treatment the agency also said in the new communication that “close supervision is strongly recommended, “ specifying that “we recommend initial evaluation and monitoring when using hydroxychloroquine or chloroquine under the EUA or in clinical trials that investigate these medicines for the treatment or prevention of COVID-19. Monitoring may include baseline ECG, electrolytes, renal function, and hepatic tests.” The communication also highlighted several potential serious adverse effects from hydroxychloroquine or chloroquine that include QT prolongation with increased risk in patients with renal insufficiency or failure, increased insulin levels and insulin action causing increased risk of severe hypoglycemia, hemolysis in selected patients, and interaction with other medicines that cause QT prolongation.
“If a healthcare professional is considering use of hydroxychloroquine or chloroquine to treat or prevent COVID-19, FDA recommends checking www.clinicaltrials.gov for a suitable clinical trial and consider enrolling the patient,” the statement added.
The FDA’s Safety Communication came a day after the European Medicines Agency issued a similar reminder about the risk for serious adverse effects from treatment with hydroxychloroquine and chloroquine, the need for adverse effect monitoring, and the unproven status of purported benefits from these agents.
The statement came after ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite a lack of evidence.
The FDA’s communication cited recent case reports sent to the FDA, as well as published findings, and reports to the National Poison Data System that have described serious, heart-related adverse events and death in COVID-19 patients who received hydroxychloroquine and chloroquine, alone or in combination with azithromycin or another QT-prolonging drug. One recent, notable but not peer-reviewed report on 368 patients treated at any of several U.S. VA medical centers showed no apparent benefit to hospitalized COVID-19 patients treated with hydroxychloroquine and a signal for increased mortality among certain patients on this drug (medRxiv. 2020 Apr 23; doi: 10.1101/2020.04.16.20065920). Several cardiology societies have also highlighted the cardiac considerations for using these drugs in patients with COVID-19, including a summary coauthored by the presidents of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society (Circulation. 2020 Apr 8. doi: 10.1161/CIRCULATIONAHA.120.047521), and in guidance from the European Society of Cardiology.
The U.S. Food and Drug Administration reinforced its March guidance on when it’s permissible to use hydroxychloroquine and chloroquine to treat COVID-19 patients and on the multiple risks these drugs pose in a Safety Communication on April 24.
The new communication reiterated the agency’s position from the Emergency Use Authorization (EUA) it granted on March 28 to allow hydroxychloroquine and chloroquine treatment of COVID-19 patients only when they are hospitalized and participation in a clinical trial is “not available,” or “not feasible.” The April 24 update to the EUA noted that “the FDA is aware of reports of serious heart rhythm problems in patients with COVID-19 treated with hydroxychloroquine or chloroquine, often in combination with azithromycin and other QT-prolonging medicines. We are also aware of increased use of these medicines through outpatient prescriptions.”
In addition to reiterating the prior limitations on permissible patients for these treatment the agency also said in the new communication that “close supervision is strongly recommended, “ specifying that “we recommend initial evaluation and monitoring when using hydroxychloroquine or chloroquine under the EUA or in clinical trials that investigate these medicines for the treatment or prevention of COVID-19. Monitoring may include baseline ECG, electrolytes, renal function, and hepatic tests.” The communication also highlighted several potential serious adverse effects from hydroxychloroquine or chloroquine that include QT prolongation with increased risk in patients with renal insufficiency or failure, increased insulin levels and insulin action causing increased risk of severe hypoglycemia, hemolysis in selected patients, and interaction with other medicines that cause QT prolongation.
“If a healthcare professional is considering use of hydroxychloroquine or chloroquine to treat or prevent COVID-19, FDA recommends checking www.clinicaltrials.gov for a suitable clinical trial and consider enrolling the patient,” the statement added.
The FDA’s Safety Communication came a day after the European Medicines Agency issued a similar reminder about the risk for serious adverse effects from treatment with hydroxychloroquine and chloroquine, the need for adverse effect monitoring, and the unproven status of purported benefits from these agents.
The statement came after ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite a lack of evidence.
The FDA’s communication cited recent case reports sent to the FDA, as well as published findings, and reports to the National Poison Data System that have described serious, heart-related adverse events and death in COVID-19 patients who received hydroxychloroquine and chloroquine, alone or in combination with azithromycin or another QT-prolonging drug. One recent, notable but not peer-reviewed report on 368 patients treated at any of several U.S. VA medical centers showed no apparent benefit to hospitalized COVID-19 patients treated with hydroxychloroquine and a signal for increased mortality among certain patients on this drug (medRxiv. 2020 Apr 23; doi: 10.1101/2020.04.16.20065920). Several cardiology societies have also highlighted the cardiac considerations for using these drugs in patients with COVID-19, including a summary coauthored by the presidents of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society (Circulation. 2020 Apr 8. doi: 10.1161/CIRCULATIONAHA.120.047521), and in guidance from the European Society of Cardiology.
The U.S. Food and Drug Administration reinforced its March guidance on when it’s permissible to use hydroxychloroquine and chloroquine to treat COVID-19 patients and on the multiple risks these drugs pose in a Safety Communication on April 24.
The new communication reiterated the agency’s position from the Emergency Use Authorization (EUA) it granted on March 28 to allow hydroxychloroquine and chloroquine treatment of COVID-19 patients only when they are hospitalized and participation in a clinical trial is “not available,” or “not feasible.” The April 24 update to the EUA noted that “the FDA is aware of reports of serious heart rhythm problems in patients with COVID-19 treated with hydroxychloroquine or chloroquine, often in combination with azithromycin and other QT-prolonging medicines. We are also aware of increased use of these medicines through outpatient prescriptions.”
In addition to reiterating the prior limitations on permissible patients for these treatment the agency also said in the new communication that “close supervision is strongly recommended, “ specifying that “we recommend initial evaluation and monitoring when using hydroxychloroquine or chloroquine under the EUA or in clinical trials that investigate these medicines for the treatment or prevention of COVID-19. Monitoring may include baseline ECG, electrolytes, renal function, and hepatic tests.” The communication also highlighted several potential serious adverse effects from hydroxychloroquine or chloroquine that include QT prolongation with increased risk in patients with renal insufficiency or failure, increased insulin levels and insulin action causing increased risk of severe hypoglycemia, hemolysis in selected patients, and interaction with other medicines that cause QT prolongation.
“If a healthcare professional is considering use of hydroxychloroquine or chloroquine to treat or prevent COVID-19, FDA recommends checking www.clinicaltrials.gov for a suitable clinical trial and consider enrolling the patient,” the statement added.
The FDA’s Safety Communication came a day after the European Medicines Agency issued a similar reminder about the risk for serious adverse effects from treatment with hydroxychloroquine and chloroquine, the need for adverse effect monitoring, and the unproven status of purported benefits from these agents.
The statement came after ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite a lack of evidence.
The FDA’s communication cited recent case reports sent to the FDA, as well as published findings, and reports to the National Poison Data System that have described serious, heart-related adverse events and death in COVID-19 patients who received hydroxychloroquine and chloroquine, alone or in combination with azithromycin or another QT-prolonging drug. One recent, notable but not peer-reviewed report on 368 patients treated at any of several U.S. VA medical centers showed no apparent benefit to hospitalized COVID-19 patients treated with hydroxychloroquine and a signal for increased mortality among certain patients on this drug (medRxiv. 2020 Apr 23; doi: 10.1101/2020.04.16.20065920). Several cardiology societies have also highlighted the cardiac considerations for using these drugs in patients with COVID-19, including a summary coauthored by the presidents of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society (Circulation. 2020 Apr 8. doi: 10.1161/CIRCULATIONAHA.120.047521), and in guidance from the European Society of Cardiology.
FROM THE FDA
Angiotensin drugs and COVID-19: More reassuring data
Initial data from one Chinese center on the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in patients hospitalized with COVID-19 appear to give some further reassurance about continued use of these drugs.
The report from one hospital in Wuhan found that among patients with hypertension hospitalized with the COVID-19 virus, there was no difference in disease severity or death rate in patients taking ACE inhibitors or ARBs and those not taking such medications.
The data were published online April 23 in JAMA Cardiology.
The study adds to another recent report in a larger number of COVID-19 patients from nine Chinese hospitals that suggested a beneficial effect of ACE inhibitors or ARBs on mortality.
Additional studies
Two other similar studies have also been recently released. Another study from China, published online March 31 in Emerging Microbes & Infections, included a small sample of 42 hospitalized patients with COVID-19 on antihypertensive therapy. Those on ACE inhibitor/ARB therapy had a lower rate of severe disease and a trend toward a lower level of IL-6 in peripheral blood. In addition, patients on ACE inhibitor/ARB therapy had increased CD3+ and CD8+ T-cell counts in peripheral blood and decreased peak viral load compared with other antihypertensive drugs.
And a preliminary study from the UK, which has not yet been peer reviewed, found that treatment with ACE inhibitors was associated with a reduced risk of rapidly deteriorating severe COVID-19 disease.
The study, available online on MedRxiv, a preprint server for health sciences, reports on 205 acute inpatients with COVID-19 at King’s College Hospital and Princess Royal University Hospital, London.
Of these, 51.2% had hypertension, 30.2% had diabetes, and 14.6% had ischemic heart disease or heart failure. Of the 37 patients on ACE inhibitors, five (14%) died or required critical care support compared with 29% (48/168) of patients not taking an ACE inhibitor.
New Wuhan study
The authors of the new article published in JAMA Cardiology, led by Juyi Li, MD, reported on a case series of 1,178 patients hospitalized with COVID-19 at the Central Hospital of Wuhan, Hubei, China, between Jan. 15 and March 15, 2020.
Patients were a median age of 55 years, and 46% were men. They had an overall in-hospital mortality rate of 11%.
Of the 1,178 patients, 362 (30.7%) had a diagnosis of hypertension. These patients were older (median age, 66 years) and had a greater prevalence of chronic diseases. Patients with hypertension also had more severe manifestations of COVID-19 compared to those without hypertension, including higher rates of acute respiratory distress syndrome and in-hospital mortality (21.3% vs. 6.5%).
Of the 362 patients with hypertension, 31.8% were taking ACE inhibitors or ARBs.
Apart from a greater prevalence of coronary artery disease, patients taking ACE inhibitors or ARBs had similar comorbidities to those not taking these medications, and also similar laboratory profile results including blood counts, inflammatory markers, renal and liver function tests, and cardiac biomarkers, although those taking ACE inhibitors/ARBs had higher levels of alkaline phosphatase.
The most commonly used antihypertensive drugs were calcium blockers. The percentage of patients with hypertension taking any drug or drug combination did not differ between those with severe and nonsevere infections and between those who survived and those who died.
Specifically regarding ACE inhibitors/ARBs, there was no difference between those with severe versus nonsevere illness in the use of ACE inhibitors (9.2% vs. 10.1%; P = .80), ARBs (24.9% vs. 21.2%; P = .40), or the composite of ACE inhibitors or ARBs (32.9% vs. 30.7%; P = .65).
Similarly, there were no differences in nonsurvivors and survivors in the use of ACE inhibitors (9.1% vs. 9.8%; P = .85); ARBs (19.5% vs. 23.9%; P = .42), or the composite of ACE inhibitors or ARBs (27.3% vs. 33.0%; P = .34).
The frequency of severe illness and death also did not differ between those treated with and without ACE inhibitors/ARBs in patients with hypertension and other various chronic conditions including coronary heart disease, cerebrovascular disease, diabetes, neurological disease, and chronic renal disease.
The authors noted that these data confirm previous reports showing that patients with hypertension have more severe illness and higher mortality rates associated with COVID-19 than those without hypertension.
But they added: “Our data provide some reassurance that ACE inhibitors/ARBs are not associated with the progression or outcome of COVID-19 hospitalizations in patients with hypertension.”
They also noted that these results support the recommendations from almost all major cardiovascular societies that patients do not discontinue ACE inhibitors or ARBs because of worries about COVID-19.
However, the authors did point out some limitations of their study, which included a small number of patients with hypertension taking ACE inhibitors or ARBs and the fact that a nonsevere disease course was still severe enough to require hospitalization. In addition, it was not clear whether ACE inhibitor/ARB treatment at baseline was maintained throughout hospitalization for all patients.
This was also an observational comparison and may be biased by differences in patients taking versus not taking ACE inhibitors or ARBs at the time of hospitalization, although the measured baseline characteristics were similar in both groups.
But the authors also highlighted the finding that, in this cohort, patients with hypertension had three times the mortality rate of all other patients hospitalized with COVID-19.
“Hypertension combined with cardiovascular and cerebrovascular disease, diabetes, and chronic kidney disease would predispose patients to an increased risk of severity and mortality of COVID-19. Therefore, patients with these underlying conditions who develop COVID-19 require particularly intensive surveillance and care,” they wrote.
Experts cautiously optimistic
Some cardiovascular experts were cautiously optimistic about these latest results.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, and editor-in-chief of the Journal of Clinical Hypertension, said: “This new report from Wuhan, China, gives modest reassurance that the use of ACE inhibitors or ARBs in hypertensive patients with COVID-19 disease does not increase the risk of clinical deterioration or death.
“Ongoing, more definitive studies should help resolve competing hypotheses regarding the effects of these agents: whether the increased ACE2 enzyme levels they produce can worsen outcomes by increasing access of the COVID virus to lung tissue; or whether there is a benefit linked to a protective effect of increased ACE2 on alveolar cell function,” Dr. Weber noted.
“Though the number of patients included in this new report is small, it is startling that hypertensive patients were three times as likely as nonhypertensives to have a fatal outcome, presumably reflecting vulnerability due to the cardiovascular and metabolic comorbidities associated with hypertension,” he added.
“In any case, for now, clinicians should continue treating hypertensive patients with whichever drugs, including ACE inhibitors and ARBs, best provide protection from adverse outcomes,” Dr. Weber concluded.
John McMurray, MD, professor of medical cardiology, University of Glasgow, Scotland, commented: “This study from Wuhan provides some reassurance about one of the two questions about ACEI/ARBs: Do these drugs increase susceptibility to infection? And if [the patient is] infected, do they increase the severity of infection? This study addresses the latter question and appears to suggest no increased severity.”
However, Dr. McMurray pointed out that the study had many limitations. There were only small patient numbers and the data were unadjusted, “although it looks like the ACE inhibitor/ARB treated patients were higher risk to start with.” It was an observational study, and patients were not randomized and were predominantly treated with ARBs, and not ACE inhibitors, so “we don’t know if the concerns apply equally to these two classes of drug.
“Other data published and unpublished supporting this (even showing better outcomes in patients treated with an ACE inhibitor/ARB), and, to date, any concerns about these drugs remain unsubstantiated and the guidance from medical societies to continue treatment with these agents in patients prescribed them seems wise,” Dr. McMurray added.
Franz H. Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “The study from Wuhan is not a great study. They didn’t even do a multivariable analysis. They could have done a bit more with the data, but it still gives some reassurance.”
Dr. Messerli said it was “interesting” that 30% of the patients hospitalized with COVID-19 in the sample had hypertension. “That corresponds to the general population, so does not suggest that having hypertension increases susceptibility to infection – but it does seem to increase the risk of a bad outcome.”
Dr. Messerli noted that there are two more similar studies due to be published soon, both said to suggest either a beneficial or neutral effect of ACE inhibitors/ARBs on COVID-19 outcomes in hospitalized patients.
“This does help with confidence in prescribing these agents and reinforces the recommendations for patients to stay on these drugs,” he said.
“However, none of these studies address the infectivity issue – whether their use upregulates the ACE2 receptor, which the virus uses to gain entry to cells, thereby increasing susceptibility to the infection,” Dr. Messerli cautioned. “But the similar or better outcomes on these drugs are encouraging,” he added.
The Wuhan study was supported by the Health and Family Planning Commission of Wuhan City, China. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Initial data from one Chinese center on the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in patients hospitalized with COVID-19 appear to give some further reassurance about continued use of these drugs.
The report from one hospital in Wuhan found that among patients with hypertension hospitalized with the COVID-19 virus, there was no difference in disease severity or death rate in patients taking ACE inhibitors or ARBs and those not taking such medications.
The data were published online April 23 in JAMA Cardiology.
The study adds to another recent report in a larger number of COVID-19 patients from nine Chinese hospitals that suggested a beneficial effect of ACE inhibitors or ARBs on mortality.
Additional studies
Two other similar studies have also been recently released. Another study from China, published online March 31 in Emerging Microbes & Infections, included a small sample of 42 hospitalized patients with COVID-19 on antihypertensive therapy. Those on ACE inhibitor/ARB therapy had a lower rate of severe disease and a trend toward a lower level of IL-6 in peripheral blood. In addition, patients on ACE inhibitor/ARB therapy had increased CD3+ and CD8+ T-cell counts in peripheral blood and decreased peak viral load compared with other antihypertensive drugs.
And a preliminary study from the UK, which has not yet been peer reviewed, found that treatment with ACE inhibitors was associated with a reduced risk of rapidly deteriorating severe COVID-19 disease.
The study, available online on MedRxiv, a preprint server for health sciences, reports on 205 acute inpatients with COVID-19 at King’s College Hospital and Princess Royal University Hospital, London.
Of these, 51.2% had hypertension, 30.2% had diabetes, and 14.6% had ischemic heart disease or heart failure. Of the 37 patients on ACE inhibitors, five (14%) died or required critical care support compared with 29% (48/168) of patients not taking an ACE inhibitor.
New Wuhan study
The authors of the new article published in JAMA Cardiology, led by Juyi Li, MD, reported on a case series of 1,178 patients hospitalized with COVID-19 at the Central Hospital of Wuhan, Hubei, China, between Jan. 15 and March 15, 2020.
Patients were a median age of 55 years, and 46% were men. They had an overall in-hospital mortality rate of 11%.
Of the 1,178 patients, 362 (30.7%) had a diagnosis of hypertension. These patients were older (median age, 66 years) and had a greater prevalence of chronic diseases. Patients with hypertension also had more severe manifestations of COVID-19 compared to those without hypertension, including higher rates of acute respiratory distress syndrome and in-hospital mortality (21.3% vs. 6.5%).
Of the 362 patients with hypertension, 31.8% were taking ACE inhibitors or ARBs.
Apart from a greater prevalence of coronary artery disease, patients taking ACE inhibitors or ARBs had similar comorbidities to those not taking these medications, and also similar laboratory profile results including blood counts, inflammatory markers, renal and liver function tests, and cardiac biomarkers, although those taking ACE inhibitors/ARBs had higher levels of alkaline phosphatase.
The most commonly used antihypertensive drugs were calcium blockers. The percentage of patients with hypertension taking any drug or drug combination did not differ between those with severe and nonsevere infections and between those who survived and those who died.
Specifically regarding ACE inhibitors/ARBs, there was no difference between those with severe versus nonsevere illness in the use of ACE inhibitors (9.2% vs. 10.1%; P = .80), ARBs (24.9% vs. 21.2%; P = .40), or the composite of ACE inhibitors or ARBs (32.9% vs. 30.7%; P = .65).
Similarly, there were no differences in nonsurvivors and survivors in the use of ACE inhibitors (9.1% vs. 9.8%; P = .85); ARBs (19.5% vs. 23.9%; P = .42), or the composite of ACE inhibitors or ARBs (27.3% vs. 33.0%; P = .34).
The frequency of severe illness and death also did not differ between those treated with and without ACE inhibitors/ARBs in patients with hypertension and other various chronic conditions including coronary heart disease, cerebrovascular disease, diabetes, neurological disease, and chronic renal disease.
The authors noted that these data confirm previous reports showing that patients with hypertension have more severe illness and higher mortality rates associated with COVID-19 than those without hypertension.
But they added: “Our data provide some reassurance that ACE inhibitors/ARBs are not associated with the progression or outcome of COVID-19 hospitalizations in patients with hypertension.”
They also noted that these results support the recommendations from almost all major cardiovascular societies that patients do not discontinue ACE inhibitors or ARBs because of worries about COVID-19.
However, the authors did point out some limitations of their study, which included a small number of patients with hypertension taking ACE inhibitors or ARBs and the fact that a nonsevere disease course was still severe enough to require hospitalization. In addition, it was not clear whether ACE inhibitor/ARB treatment at baseline was maintained throughout hospitalization for all patients.
This was also an observational comparison and may be biased by differences in patients taking versus not taking ACE inhibitors or ARBs at the time of hospitalization, although the measured baseline characteristics were similar in both groups.
But the authors also highlighted the finding that, in this cohort, patients with hypertension had three times the mortality rate of all other patients hospitalized with COVID-19.
“Hypertension combined with cardiovascular and cerebrovascular disease, diabetes, and chronic kidney disease would predispose patients to an increased risk of severity and mortality of COVID-19. Therefore, patients with these underlying conditions who develop COVID-19 require particularly intensive surveillance and care,” they wrote.
Experts cautiously optimistic
Some cardiovascular experts were cautiously optimistic about these latest results.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, and editor-in-chief of the Journal of Clinical Hypertension, said: “This new report from Wuhan, China, gives modest reassurance that the use of ACE inhibitors or ARBs in hypertensive patients with COVID-19 disease does not increase the risk of clinical deterioration or death.
“Ongoing, more definitive studies should help resolve competing hypotheses regarding the effects of these agents: whether the increased ACE2 enzyme levels they produce can worsen outcomes by increasing access of the COVID virus to lung tissue; or whether there is a benefit linked to a protective effect of increased ACE2 on alveolar cell function,” Dr. Weber noted.
“Though the number of patients included in this new report is small, it is startling that hypertensive patients were three times as likely as nonhypertensives to have a fatal outcome, presumably reflecting vulnerability due to the cardiovascular and metabolic comorbidities associated with hypertension,” he added.
“In any case, for now, clinicians should continue treating hypertensive patients with whichever drugs, including ACE inhibitors and ARBs, best provide protection from adverse outcomes,” Dr. Weber concluded.
John McMurray, MD, professor of medical cardiology, University of Glasgow, Scotland, commented: “This study from Wuhan provides some reassurance about one of the two questions about ACEI/ARBs: Do these drugs increase susceptibility to infection? And if [the patient is] infected, do they increase the severity of infection? This study addresses the latter question and appears to suggest no increased severity.”
However, Dr. McMurray pointed out that the study had many limitations. There were only small patient numbers and the data were unadjusted, “although it looks like the ACE inhibitor/ARB treated patients were higher risk to start with.” It was an observational study, and patients were not randomized and were predominantly treated with ARBs, and not ACE inhibitors, so “we don’t know if the concerns apply equally to these two classes of drug.
“Other data published and unpublished supporting this (even showing better outcomes in patients treated with an ACE inhibitor/ARB), and, to date, any concerns about these drugs remain unsubstantiated and the guidance from medical societies to continue treatment with these agents in patients prescribed them seems wise,” Dr. McMurray added.
Franz H. Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “The study from Wuhan is not a great study. They didn’t even do a multivariable analysis. They could have done a bit more with the data, but it still gives some reassurance.”
Dr. Messerli said it was “interesting” that 30% of the patients hospitalized with COVID-19 in the sample had hypertension. “That corresponds to the general population, so does not suggest that having hypertension increases susceptibility to infection – but it does seem to increase the risk of a bad outcome.”
Dr. Messerli noted that there are two more similar studies due to be published soon, both said to suggest either a beneficial or neutral effect of ACE inhibitors/ARBs on COVID-19 outcomes in hospitalized patients.
“This does help with confidence in prescribing these agents and reinforces the recommendations for patients to stay on these drugs,” he said.
“However, none of these studies address the infectivity issue – whether their use upregulates the ACE2 receptor, which the virus uses to gain entry to cells, thereby increasing susceptibility to the infection,” Dr. Messerli cautioned. “But the similar or better outcomes on these drugs are encouraging,” he added.
The Wuhan study was supported by the Health and Family Planning Commission of Wuhan City, China. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Initial data from one Chinese center on the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in patients hospitalized with COVID-19 appear to give some further reassurance about continued use of these drugs.
The report from one hospital in Wuhan found that among patients with hypertension hospitalized with the COVID-19 virus, there was no difference in disease severity or death rate in patients taking ACE inhibitors or ARBs and those not taking such medications.
The data were published online April 23 in JAMA Cardiology.
The study adds to another recent report in a larger number of COVID-19 patients from nine Chinese hospitals that suggested a beneficial effect of ACE inhibitors or ARBs on mortality.
Additional studies
Two other similar studies have also been recently released. Another study from China, published online March 31 in Emerging Microbes & Infections, included a small sample of 42 hospitalized patients with COVID-19 on antihypertensive therapy. Those on ACE inhibitor/ARB therapy had a lower rate of severe disease and a trend toward a lower level of IL-6 in peripheral blood. In addition, patients on ACE inhibitor/ARB therapy had increased CD3+ and CD8+ T-cell counts in peripheral blood and decreased peak viral load compared with other antihypertensive drugs.
And a preliminary study from the UK, which has not yet been peer reviewed, found that treatment with ACE inhibitors was associated with a reduced risk of rapidly deteriorating severe COVID-19 disease.
The study, available online on MedRxiv, a preprint server for health sciences, reports on 205 acute inpatients with COVID-19 at King’s College Hospital and Princess Royal University Hospital, London.
Of these, 51.2% had hypertension, 30.2% had diabetes, and 14.6% had ischemic heart disease or heart failure. Of the 37 patients on ACE inhibitors, five (14%) died or required critical care support compared with 29% (48/168) of patients not taking an ACE inhibitor.
New Wuhan study
The authors of the new article published in JAMA Cardiology, led by Juyi Li, MD, reported on a case series of 1,178 patients hospitalized with COVID-19 at the Central Hospital of Wuhan, Hubei, China, between Jan. 15 and March 15, 2020.
Patients were a median age of 55 years, and 46% were men. They had an overall in-hospital mortality rate of 11%.
Of the 1,178 patients, 362 (30.7%) had a diagnosis of hypertension. These patients were older (median age, 66 years) and had a greater prevalence of chronic diseases. Patients with hypertension also had more severe manifestations of COVID-19 compared to those without hypertension, including higher rates of acute respiratory distress syndrome and in-hospital mortality (21.3% vs. 6.5%).
Of the 362 patients with hypertension, 31.8% were taking ACE inhibitors or ARBs.
Apart from a greater prevalence of coronary artery disease, patients taking ACE inhibitors or ARBs had similar comorbidities to those not taking these medications, and also similar laboratory profile results including blood counts, inflammatory markers, renal and liver function tests, and cardiac biomarkers, although those taking ACE inhibitors/ARBs had higher levels of alkaline phosphatase.
The most commonly used antihypertensive drugs were calcium blockers. The percentage of patients with hypertension taking any drug or drug combination did not differ between those with severe and nonsevere infections and between those who survived and those who died.
Specifically regarding ACE inhibitors/ARBs, there was no difference between those with severe versus nonsevere illness in the use of ACE inhibitors (9.2% vs. 10.1%; P = .80), ARBs (24.9% vs. 21.2%; P = .40), or the composite of ACE inhibitors or ARBs (32.9% vs. 30.7%; P = .65).
Similarly, there were no differences in nonsurvivors and survivors in the use of ACE inhibitors (9.1% vs. 9.8%; P = .85); ARBs (19.5% vs. 23.9%; P = .42), or the composite of ACE inhibitors or ARBs (27.3% vs. 33.0%; P = .34).
The frequency of severe illness and death also did not differ between those treated with and without ACE inhibitors/ARBs in patients with hypertension and other various chronic conditions including coronary heart disease, cerebrovascular disease, diabetes, neurological disease, and chronic renal disease.
The authors noted that these data confirm previous reports showing that patients with hypertension have more severe illness and higher mortality rates associated with COVID-19 than those without hypertension.
But they added: “Our data provide some reassurance that ACE inhibitors/ARBs are not associated with the progression or outcome of COVID-19 hospitalizations in patients with hypertension.”
They also noted that these results support the recommendations from almost all major cardiovascular societies that patients do not discontinue ACE inhibitors or ARBs because of worries about COVID-19.
However, the authors did point out some limitations of their study, which included a small number of patients with hypertension taking ACE inhibitors or ARBs and the fact that a nonsevere disease course was still severe enough to require hospitalization. In addition, it was not clear whether ACE inhibitor/ARB treatment at baseline was maintained throughout hospitalization for all patients.
This was also an observational comparison and may be biased by differences in patients taking versus not taking ACE inhibitors or ARBs at the time of hospitalization, although the measured baseline characteristics were similar in both groups.
But the authors also highlighted the finding that, in this cohort, patients with hypertension had three times the mortality rate of all other patients hospitalized with COVID-19.
“Hypertension combined with cardiovascular and cerebrovascular disease, diabetes, and chronic kidney disease would predispose patients to an increased risk of severity and mortality of COVID-19. Therefore, patients with these underlying conditions who develop COVID-19 require particularly intensive surveillance and care,” they wrote.
Experts cautiously optimistic
Some cardiovascular experts were cautiously optimistic about these latest results.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, and editor-in-chief of the Journal of Clinical Hypertension, said: “This new report from Wuhan, China, gives modest reassurance that the use of ACE inhibitors or ARBs in hypertensive patients with COVID-19 disease does not increase the risk of clinical deterioration or death.
“Ongoing, more definitive studies should help resolve competing hypotheses regarding the effects of these agents: whether the increased ACE2 enzyme levels they produce can worsen outcomes by increasing access of the COVID virus to lung tissue; or whether there is a benefit linked to a protective effect of increased ACE2 on alveolar cell function,” Dr. Weber noted.
“Though the number of patients included in this new report is small, it is startling that hypertensive patients were three times as likely as nonhypertensives to have a fatal outcome, presumably reflecting vulnerability due to the cardiovascular and metabolic comorbidities associated with hypertension,” he added.
“In any case, for now, clinicians should continue treating hypertensive patients with whichever drugs, including ACE inhibitors and ARBs, best provide protection from adverse outcomes,” Dr. Weber concluded.
John McMurray, MD, professor of medical cardiology, University of Glasgow, Scotland, commented: “This study from Wuhan provides some reassurance about one of the two questions about ACEI/ARBs: Do these drugs increase susceptibility to infection? And if [the patient is] infected, do they increase the severity of infection? This study addresses the latter question and appears to suggest no increased severity.”
However, Dr. McMurray pointed out that the study had many limitations. There were only small patient numbers and the data were unadjusted, “although it looks like the ACE inhibitor/ARB treated patients were higher risk to start with.” It was an observational study, and patients were not randomized and were predominantly treated with ARBs, and not ACE inhibitors, so “we don’t know if the concerns apply equally to these two classes of drug.
“Other data published and unpublished supporting this (even showing better outcomes in patients treated with an ACE inhibitor/ARB), and, to date, any concerns about these drugs remain unsubstantiated and the guidance from medical societies to continue treatment with these agents in patients prescribed them seems wise,” Dr. McMurray added.
Franz H. Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “The study from Wuhan is not a great study. They didn’t even do a multivariable analysis. They could have done a bit more with the data, but it still gives some reassurance.”
Dr. Messerli said it was “interesting” that 30% of the patients hospitalized with COVID-19 in the sample had hypertension. “That corresponds to the general population, so does not suggest that having hypertension increases susceptibility to infection – but it does seem to increase the risk of a bad outcome.”
Dr. Messerli noted that there are two more similar studies due to be published soon, both said to suggest either a beneficial or neutral effect of ACE inhibitors/ARBs on COVID-19 outcomes in hospitalized patients.
“This does help with confidence in prescribing these agents and reinforces the recommendations for patients to stay on these drugs,” he said.
“However, none of these studies address the infectivity issue – whether their use upregulates the ACE2 receptor, which the virus uses to gain entry to cells, thereby increasing susceptibility to the infection,” Dr. Messerli cautioned. “But the similar or better outcomes on these drugs are encouraging,” he added.
The Wuhan study was supported by the Health and Family Planning Commission of Wuhan City, China. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AUGUSTUS: After ACS or PCI, aspirin gives AFib patients scant benefit
When patients with atrial fibrillation have an acute coronary syndrome event or undergo percutaneous coronary intervention, their window of opportunity for benefiting from a triple antithrombotic regimen was, at best, about 30 days, according to a post hoc analysis of AUGUSTUS, a multicenter, randomized trial with more than 4,600 patients.
Beyond 30 days out to 180 days, the incremental benefit from reduced ischemic events fell to essentially zero, giving it a clear back seat to the ongoing, increased bleeding risk from adding a third antithrombotic drug.
Patients randomized to receive aspirin in addition to an anticoagulant, either apixaban or a vitamin K antagonist such as warfarin, and a P2Y12 inhibitor such as clopidogrel “for up to approximately 30 days” had a roughly similar decrease in severe ischemic events and increase in severe bleeding events, suggesting that even acutely the overall impact of adding aspirin on top of the other two antithrombotics was a wash, John H. Alexander, MD, said in a presentation of research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
Using aspirin as a third antithrombotic in patients with atrial fibrillation (AFib) who have also recently had either an acute coronary syndrome event (ACS) or underwent percutaneous coronary intervention (PCI), “may be reasonable,” for selected patients, but is a decision that requires careful individualization, cautioned Dr. Alexander, professor of medicine and director of Cardiovascular Research at the Duke Clinical Research Institute of Duke University, Durham, N.C.
“This is a superb secondary analysis looking at the time course of potential benefit and harm with aspirin, and they found that aspirin was beneficial only in the first 30 days. After 30 days, it’s startling and remarkable that the ischemic event curves were completely on top of each other,” commented Julia H. Indik, MD, a cardiac electrophysiologist at Banner–University Medical Center Tuscon and designated discussant for the report. “This substudy will be essential for updating the guidelines,” she predicted. “When a treatment’s benefit equals its risks,” which happened when aspirin was part of the regimen during the first 30 days, “then it’s not even a class IIb recommendation; it’s class III,” the classification used by the ACC and collaborating groups to identify treatments where net benefit and net risk are similar and hence the treatment is considered not recommended.
A key element in the analysis Dr. Alexander presented was to define a spectrum of clinical events as representing broad, intermediate, or severe ischemic or bleeding events. The severe category for bleeding events included fatal, intracranial, and any bleed rated as major by the International Society on Thrombosis and Haemostasis (ISTH) criteria, while the broad bleeding definition included all of these plus bleeds that directly resulted in hospitalization and clinically relevant nonmajor bleeds. For ischemic events, the severe group consisted of cardiovascular death, MI, stent thrombosis, and ischemic stroke, while the broad category also tallied urgent revascularizations and cardiovascular hospitalizations.
“I believe the severe bleeds and severe ischemic events we identified are roughly equal in severity,” Dr. Alexander noted. “Where I think we need more analysis is which patients have more bleeding risk and which have more ischemia risk. We need a more tailored approach to identify patient subgroups, perhaps based on angiographic characteristics, or something else,” that modifies the trade-off that, on a population level, seems very evenly balanced.
Applying this approach to scoring the severity of adverse outcomes, Dr. Alexander reported that, during the first 30 days on treatment, patients on aspirin had a net absolute gain of 1.0% in severe bleeding events, compared with placebo, and a 3.4% gain in broad bleeds, while showing a 0.9% drop in severe ischemic events but no between-group difference in the rate of broadly defined ischemic events. During days 31-180, the addition of aspirin resulted in virtually no reductions in ischemic events regardless of whether they were severe, intermediate, or broad, but adding aspirin continued to produce an excess of bleeding episodes in all three categories. The results also appeared in an article published online (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046534).
“We did not see a time window when the ischemia risk was greater than the bleeding risk,” Dr. Alexander noted, and he also highlighted that the one option the analysis could not explore is never giving these patients any aspirin. “Patients received aspirin for some number of days before randomization,” a median of 6 days from the time of their ACS or PCI event until randomization, “so we don’t have great insight into whether no aspirin” is an reasonable option.
The AUGUSTUS trial randomized 4,614 patients with AFib and a recent ACS or PCI event at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the rate of major or clinically relevant nonmajor bleeding by the ISTH criteria during 6 months on treatment, while composites of death or hospitalization, and death plus ischemic events served as secondary outcomes. All patients received an antiplatelet P2Y12 inhibitor, with 93% of patients receiving clopidogrel, and were randomized in a 2 x 2 factorial design to one of four regimens: either apixaban or a vitamin K antagonist (such as warfarin), and to aspirin or placebo. The study’s primary findings showed that using apixaban instead of a vitamin K antagonist significantly reduced bleeding events as well as the rate of death or hospitalization, but the rate of death and ischemic events was similar in the two arms. The primary AUGUSTUS finding for the aspirin versus placebo randomization was that overall throughout the study ischemic events were balanced in the these two treatment arms while aspirin boosted bleeding (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
AUGUSTUS was sponsored by Bristol-Myers Squibb and Pfizer, the companies that market apixaban. Dr. Alexander has been a consultant to and received research funding from Bristol-Myers Squibb and Pfizer; has been a consultant to AbbVie, Bayer, CryoLife, CSL Behring, Novo Nordisk, Portola, Quantum Genomics, XaTek, and Zafgen; and has received research funding from Boehringer Ingelheim, CryoLife, CSL Behring, GlaxoSmithKline, and XaTek. Dr. Indik had no disclosures.
SOURCE: Alexander JH et al. ACC 2020, Abstract 409-08.
When patients with atrial fibrillation have an acute coronary syndrome event or undergo percutaneous coronary intervention, their window of opportunity for benefiting from a triple antithrombotic regimen was, at best, about 30 days, according to a post hoc analysis of AUGUSTUS, a multicenter, randomized trial with more than 4,600 patients.
Beyond 30 days out to 180 days, the incremental benefit from reduced ischemic events fell to essentially zero, giving it a clear back seat to the ongoing, increased bleeding risk from adding a third antithrombotic drug.
Patients randomized to receive aspirin in addition to an anticoagulant, either apixaban or a vitamin K antagonist such as warfarin, and a P2Y12 inhibitor such as clopidogrel “for up to approximately 30 days” had a roughly similar decrease in severe ischemic events and increase in severe bleeding events, suggesting that even acutely the overall impact of adding aspirin on top of the other two antithrombotics was a wash, John H. Alexander, MD, said in a presentation of research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
Using aspirin as a third antithrombotic in patients with atrial fibrillation (AFib) who have also recently had either an acute coronary syndrome event (ACS) or underwent percutaneous coronary intervention (PCI), “may be reasonable,” for selected patients, but is a decision that requires careful individualization, cautioned Dr. Alexander, professor of medicine and director of Cardiovascular Research at the Duke Clinical Research Institute of Duke University, Durham, N.C.
“This is a superb secondary analysis looking at the time course of potential benefit and harm with aspirin, and they found that aspirin was beneficial only in the first 30 days. After 30 days, it’s startling and remarkable that the ischemic event curves were completely on top of each other,” commented Julia H. Indik, MD, a cardiac electrophysiologist at Banner–University Medical Center Tuscon and designated discussant for the report. “This substudy will be essential for updating the guidelines,” she predicted. “When a treatment’s benefit equals its risks,” which happened when aspirin was part of the regimen during the first 30 days, “then it’s not even a class IIb recommendation; it’s class III,” the classification used by the ACC and collaborating groups to identify treatments where net benefit and net risk are similar and hence the treatment is considered not recommended.
A key element in the analysis Dr. Alexander presented was to define a spectrum of clinical events as representing broad, intermediate, or severe ischemic or bleeding events. The severe category for bleeding events included fatal, intracranial, and any bleed rated as major by the International Society on Thrombosis and Haemostasis (ISTH) criteria, while the broad bleeding definition included all of these plus bleeds that directly resulted in hospitalization and clinically relevant nonmajor bleeds. For ischemic events, the severe group consisted of cardiovascular death, MI, stent thrombosis, and ischemic stroke, while the broad category also tallied urgent revascularizations and cardiovascular hospitalizations.
“I believe the severe bleeds and severe ischemic events we identified are roughly equal in severity,” Dr. Alexander noted. “Where I think we need more analysis is which patients have more bleeding risk and which have more ischemia risk. We need a more tailored approach to identify patient subgroups, perhaps based on angiographic characteristics, or something else,” that modifies the trade-off that, on a population level, seems very evenly balanced.
Applying this approach to scoring the severity of adverse outcomes, Dr. Alexander reported that, during the first 30 days on treatment, patients on aspirin had a net absolute gain of 1.0% in severe bleeding events, compared with placebo, and a 3.4% gain in broad bleeds, while showing a 0.9% drop in severe ischemic events but no between-group difference in the rate of broadly defined ischemic events. During days 31-180, the addition of aspirin resulted in virtually no reductions in ischemic events regardless of whether they were severe, intermediate, or broad, but adding aspirin continued to produce an excess of bleeding episodes in all three categories. The results also appeared in an article published online (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046534).
“We did not see a time window when the ischemia risk was greater than the bleeding risk,” Dr. Alexander noted, and he also highlighted that the one option the analysis could not explore is never giving these patients any aspirin. “Patients received aspirin for some number of days before randomization,” a median of 6 days from the time of their ACS or PCI event until randomization, “so we don’t have great insight into whether no aspirin” is an reasonable option.
The AUGUSTUS trial randomized 4,614 patients with AFib and a recent ACS or PCI event at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the rate of major or clinically relevant nonmajor bleeding by the ISTH criteria during 6 months on treatment, while composites of death or hospitalization, and death plus ischemic events served as secondary outcomes. All patients received an antiplatelet P2Y12 inhibitor, with 93% of patients receiving clopidogrel, and were randomized in a 2 x 2 factorial design to one of four regimens: either apixaban or a vitamin K antagonist (such as warfarin), and to aspirin or placebo. The study’s primary findings showed that using apixaban instead of a vitamin K antagonist significantly reduced bleeding events as well as the rate of death or hospitalization, but the rate of death and ischemic events was similar in the two arms. The primary AUGUSTUS finding for the aspirin versus placebo randomization was that overall throughout the study ischemic events were balanced in the these two treatment arms while aspirin boosted bleeding (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
AUGUSTUS was sponsored by Bristol-Myers Squibb and Pfizer, the companies that market apixaban. Dr. Alexander has been a consultant to and received research funding from Bristol-Myers Squibb and Pfizer; has been a consultant to AbbVie, Bayer, CryoLife, CSL Behring, Novo Nordisk, Portola, Quantum Genomics, XaTek, and Zafgen; and has received research funding from Boehringer Ingelheim, CryoLife, CSL Behring, GlaxoSmithKline, and XaTek. Dr. Indik had no disclosures.
SOURCE: Alexander JH et al. ACC 2020, Abstract 409-08.
When patients with atrial fibrillation have an acute coronary syndrome event or undergo percutaneous coronary intervention, their window of opportunity for benefiting from a triple antithrombotic regimen was, at best, about 30 days, according to a post hoc analysis of AUGUSTUS, a multicenter, randomized trial with more than 4,600 patients.
Beyond 30 days out to 180 days, the incremental benefit from reduced ischemic events fell to essentially zero, giving it a clear back seat to the ongoing, increased bleeding risk from adding a third antithrombotic drug.
Patients randomized to receive aspirin in addition to an anticoagulant, either apixaban or a vitamin K antagonist such as warfarin, and a P2Y12 inhibitor such as clopidogrel “for up to approximately 30 days” had a roughly similar decrease in severe ischemic events and increase in severe bleeding events, suggesting that even acutely the overall impact of adding aspirin on top of the other two antithrombotics was a wash, John H. Alexander, MD, said in a presentation of research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
Using aspirin as a third antithrombotic in patients with atrial fibrillation (AFib) who have also recently had either an acute coronary syndrome event (ACS) or underwent percutaneous coronary intervention (PCI), “may be reasonable,” for selected patients, but is a decision that requires careful individualization, cautioned Dr. Alexander, professor of medicine and director of Cardiovascular Research at the Duke Clinical Research Institute of Duke University, Durham, N.C.
“This is a superb secondary analysis looking at the time course of potential benefit and harm with aspirin, and they found that aspirin was beneficial only in the first 30 days. After 30 days, it’s startling and remarkable that the ischemic event curves were completely on top of each other,” commented Julia H. Indik, MD, a cardiac electrophysiologist at Banner–University Medical Center Tuscon and designated discussant for the report. “This substudy will be essential for updating the guidelines,” she predicted. “When a treatment’s benefit equals its risks,” which happened when aspirin was part of the regimen during the first 30 days, “then it’s not even a class IIb recommendation; it’s class III,” the classification used by the ACC and collaborating groups to identify treatments where net benefit and net risk are similar and hence the treatment is considered not recommended.
A key element in the analysis Dr. Alexander presented was to define a spectrum of clinical events as representing broad, intermediate, or severe ischemic or bleeding events. The severe category for bleeding events included fatal, intracranial, and any bleed rated as major by the International Society on Thrombosis and Haemostasis (ISTH) criteria, while the broad bleeding definition included all of these plus bleeds that directly resulted in hospitalization and clinically relevant nonmajor bleeds. For ischemic events, the severe group consisted of cardiovascular death, MI, stent thrombosis, and ischemic stroke, while the broad category also tallied urgent revascularizations and cardiovascular hospitalizations.
“I believe the severe bleeds and severe ischemic events we identified are roughly equal in severity,” Dr. Alexander noted. “Where I think we need more analysis is which patients have more bleeding risk and which have more ischemia risk. We need a more tailored approach to identify patient subgroups, perhaps based on angiographic characteristics, or something else,” that modifies the trade-off that, on a population level, seems very evenly balanced.
Applying this approach to scoring the severity of adverse outcomes, Dr. Alexander reported that, during the first 30 days on treatment, patients on aspirin had a net absolute gain of 1.0% in severe bleeding events, compared with placebo, and a 3.4% gain in broad bleeds, while showing a 0.9% drop in severe ischemic events but no between-group difference in the rate of broadly defined ischemic events. During days 31-180, the addition of aspirin resulted in virtually no reductions in ischemic events regardless of whether they were severe, intermediate, or broad, but adding aspirin continued to produce an excess of bleeding episodes in all three categories. The results also appeared in an article published online (Circulation. 2020 Mar 29. doi: 10.1161/CIRCULATIONAHA.120.046534).
“We did not see a time window when the ischemia risk was greater than the bleeding risk,” Dr. Alexander noted, and he also highlighted that the one option the analysis could not explore is never giving these patients any aspirin. “Patients received aspirin for some number of days before randomization,” a median of 6 days from the time of their ACS or PCI event until randomization, “so we don’t have great insight into whether no aspirin” is an reasonable option.
The AUGUSTUS trial randomized 4,614 patients with AFib and a recent ACS or PCI event at any of 492 sites in 33 countries during 2015-2018. The study’s primary endpoint was the rate of major or clinically relevant nonmajor bleeding by the ISTH criteria during 6 months on treatment, while composites of death or hospitalization, and death plus ischemic events served as secondary outcomes. All patients received an antiplatelet P2Y12 inhibitor, with 93% of patients receiving clopidogrel, and were randomized in a 2 x 2 factorial design to one of four regimens: either apixaban or a vitamin K antagonist (such as warfarin), and to aspirin or placebo. The study’s primary findings showed that using apixaban instead of a vitamin K antagonist significantly reduced bleeding events as well as the rate of death or hospitalization, but the rate of death and ischemic events was similar in the two arms. The primary AUGUSTUS finding for the aspirin versus placebo randomization was that overall throughout the study ischemic events were balanced in the these two treatment arms while aspirin boosted bleeding (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
AUGUSTUS was sponsored by Bristol-Myers Squibb and Pfizer, the companies that market apixaban. Dr. Alexander has been a consultant to and received research funding from Bristol-Myers Squibb and Pfizer; has been a consultant to AbbVie, Bayer, CryoLife, CSL Behring, Novo Nordisk, Portola, Quantum Genomics, XaTek, and Zafgen; and has received research funding from Boehringer Ingelheim, CryoLife, CSL Behring, GlaxoSmithKline, and XaTek. Dr. Indik had no disclosures.
SOURCE: Alexander JH et al. ACC 2020, Abstract 409-08.
FROM ACC 2020
Hydroxychloroquine ineffective for COVID-19, VA study suggests
Hydroxychloroquine (HCQ) with or without azithromycin (AZ) is not associated with a lower risk of requiring mechanical ventilation, according to a retrospective study of Veterans Affairs patients hospitalized with COVID-19.
The study, which was posted on a preprint server April 21 and has not been peer reviewed, also showed an increased risk of death associated with COVID-19 patients treated with HCQ alone.
“These findings highlight the importance of awaiting the results of ongoing prospective, randomized controlled studies before widespread adoption of these drugs,” write Joseph Magagnoli with Dorn Research Institute at the Columbia (S.C.) VA Health Care System and the department of clinical pharmacy & outcomes sciences, University of South Carolina, and colleagues.
A spokesperson with the University of Virginia, Charlottesville, where several of coauthors practice, said that the authors declined to comment for this article before peer review is completed.
The new data are not the first to suggest no benefit with HCQ among patients with COVID-19. A randomized trial showed no benefit and more side effects among 75 patients in China treated with HCQ, compared with 75 who received standard of care alone, according to a preprint posted online April 14.
No benefit in ventilation, death rates
The current analysis included data from all 368 male patients hospitalized with confirmed COVID-19 and treated at Veterans Health Administration medical centers in the United States through April 11.
Patients were categorized into three groups: those treated with HCQ in addition to standard of care (n = 97); those treated with HCQ and the antibiotic azithromycin plus standard of care (n = 113); and those who received standard supportive care only (n = 158).
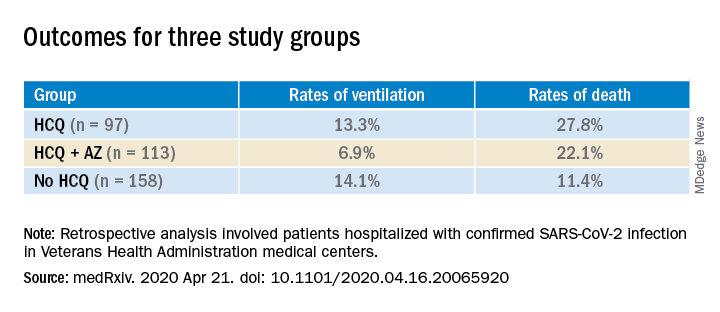
Compared with the no HCQ group, the risk of death from any cause was higher in the HCQ group (adjusted hazard ratio, 2.61; 95% confidence interval, 1.10-6.17; P = .03) but not in the HCQ+AZ group (aHR, 1.14; 95% CI, 0.56-2.32; P = .72).
The risk of ventilation was similar in the HCQ group (aHR, 1.43; 95% CI, 0.53-3.79; P = .48) and in the HCQ+AZ group (aHR, 0.43; 95% CI, 0.16-1.12; P = .09), compared with the no-HCQ group.
This study provides another counterbalance to claims of HCQ efficacy, David R. Wessner, PhD, professor of biology and chair of the department of health and human values at Davidson (N.C.) College, said in an interview.
Interest in HCQ spiked after an open-label, nonrandomized, single-center study of COVID-19 patients in France suggested that hydroxychloroquine helped clear the virus and had a potential enhanced effect when combined with azithromycin.
But the 36-patient trial has since been called into question.
Wait for convincing data
Dr. Wessner, whose research focuses on viral pathogenesis, says that, although the current data don’t definitively answer the question of whether HCQ is effective in treating COVID-19, taking a “let’s try it and see” approach is not reasonable.
“Until we have good, prospective randomized trials, it’s hard to know what to make of this. But this is more evidence that there’s not a good reason to use [HCQ],” Dr. Wessner said. He points out that the small randomized trial from China shows that HCQ comes with potential harms.
Anecdotal evidence is often cited by those who promote HCQ as a potential treatment, but “those are one-off examples,” Wessner continued. “That doesn’t really tell us anything.”
Some HCQ proponents have said that trials finding no benefit are flawed in that the drug is given too late. However, Dr. Wessner says, there’s no way to prove or disprove that claim without randomized controlled trials.
Conflicting messages
Despite lack of clear evidence of benefit for patients with COVID-19, HCQ is recommended off-label by the Chinese National guideline, and the U.S. Food and Drug Administration has issued an emergency-use authorization for the treatment of adult patients with COVID-19.
Conversely, the Infectious Diseases Society of America and a guideline panel convened by the National Institutes of Health each concluded recently that because of insufficient data, they could not recommend any specific treatments for patients with COVID-19.
The VA data for the current study came from the Veterans Affairs Informatics and Computing Infrastructure, which includes inpatient, outpatient and laboratory data and pharmacy claims.
The authors acknowledge some limitations, “including those inherent to all retrospective analyses such as nonrandomization of treatments.”
However, they note that they did adjust for potential confounders, including comorbidities, medications, and clinical and laboratory factors.
A coauthor, Jayakrishna Ambati, MD, is a cofounder of iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics, and has received consultancy fees from Allergan, Biogen, Boehringer Ingelheim, Immunovant, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences, all unrelated to this work. Dr. Ambati is named as an inventor on a patent application filed by the University of Virginia relating to COVID-19 but unrelated to this work. Another coauthor has received research grants from Boehringer Ingelheim, Gilead Sciences, Portola Pharmaceuticals, and United Therapeutics, all unrelated to this work. The other authors and Dr. Wessner have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hydroxychloroquine (HCQ) with or without azithromycin (AZ) is not associated with a lower risk of requiring mechanical ventilation, according to a retrospective study of Veterans Affairs patients hospitalized with COVID-19.
The study, which was posted on a preprint server April 21 and has not been peer reviewed, also showed an increased risk of death associated with COVID-19 patients treated with HCQ alone.
“These findings highlight the importance of awaiting the results of ongoing prospective, randomized controlled studies before widespread adoption of these drugs,” write Joseph Magagnoli with Dorn Research Institute at the Columbia (S.C.) VA Health Care System and the department of clinical pharmacy & outcomes sciences, University of South Carolina, and colleagues.
A spokesperson with the University of Virginia, Charlottesville, where several of coauthors practice, said that the authors declined to comment for this article before peer review is completed.
The new data are not the first to suggest no benefit with HCQ among patients with COVID-19. A randomized trial showed no benefit and more side effects among 75 patients in China treated with HCQ, compared with 75 who received standard of care alone, according to a preprint posted online April 14.
No benefit in ventilation, death rates
The current analysis included data from all 368 male patients hospitalized with confirmed COVID-19 and treated at Veterans Health Administration medical centers in the United States through April 11.
Patients were categorized into three groups: those treated with HCQ in addition to standard of care (n = 97); those treated with HCQ and the antibiotic azithromycin plus standard of care (n = 113); and those who received standard supportive care only (n = 158).

Compared with the no HCQ group, the risk of death from any cause was higher in the HCQ group (adjusted hazard ratio, 2.61; 95% confidence interval, 1.10-6.17; P = .03) but not in the HCQ+AZ group (aHR, 1.14; 95% CI, 0.56-2.32; P = .72).
The risk of ventilation was similar in the HCQ group (aHR, 1.43; 95% CI, 0.53-3.79; P = .48) and in the HCQ+AZ group (aHR, 0.43; 95% CI, 0.16-1.12; P = .09), compared with the no-HCQ group.
This study provides another counterbalance to claims of HCQ efficacy, David R. Wessner, PhD, professor of biology and chair of the department of health and human values at Davidson (N.C.) College, said in an interview.
Interest in HCQ spiked after an open-label, nonrandomized, single-center study of COVID-19 patients in France suggested that hydroxychloroquine helped clear the virus and had a potential enhanced effect when combined with azithromycin.
But the 36-patient trial has since been called into question.
Wait for convincing data
Dr. Wessner, whose research focuses on viral pathogenesis, says that, although the current data don’t definitively answer the question of whether HCQ is effective in treating COVID-19, taking a “let’s try it and see” approach is not reasonable.
“Until we have good, prospective randomized trials, it’s hard to know what to make of this. But this is more evidence that there’s not a good reason to use [HCQ],” Dr. Wessner said. He points out that the small randomized trial from China shows that HCQ comes with potential harms.
Anecdotal evidence is often cited by those who promote HCQ as a potential treatment, but “those are one-off examples,” Wessner continued. “That doesn’t really tell us anything.”
Some HCQ proponents have said that trials finding no benefit are flawed in that the drug is given too late. However, Dr. Wessner says, there’s no way to prove or disprove that claim without randomized controlled trials.
Conflicting messages
Despite lack of clear evidence of benefit for patients with COVID-19, HCQ is recommended off-label by the Chinese National guideline, and the U.S. Food and Drug Administration has issued an emergency-use authorization for the treatment of adult patients with COVID-19.
Conversely, the Infectious Diseases Society of America and a guideline panel convened by the National Institutes of Health each concluded recently that because of insufficient data, they could not recommend any specific treatments for patients with COVID-19.
The VA data for the current study came from the Veterans Affairs Informatics and Computing Infrastructure, which includes inpatient, outpatient and laboratory data and pharmacy claims.
The authors acknowledge some limitations, “including those inherent to all retrospective analyses such as nonrandomization of treatments.”
However, they note that they did adjust for potential confounders, including comorbidities, medications, and clinical and laboratory factors.
A coauthor, Jayakrishna Ambati, MD, is a cofounder of iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics, and has received consultancy fees from Allergan, Biogen, Boehringer Ingelheim, Immunovant, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences, all unrelated to this work. Dr. Ambati is named as an inventor on a patent application filed by the University of Virginia relating to COVID-19 but unrelated to this work. Another coauthor has received research grants from Boehringer Ingelheim, Gilead Sciences, Portola Pharmaceuticals, and United Therapeutics, all unrelated to this work. The other authors and Dr. Wessner have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hydroxychloroquine (HCQ) with or without azithromycin (AZ) is not associated with a lower risk of requiring mechanical ventilation, according to a retrospective study of Veterans Affairs patients hospitalized with COVID-19.
The study, which was posted on a preprint server April 21 and has not been peer reviewed, also showed an increased risk of death associated with COVID-19 patients treated with HCQ alone.
“These findings highlight the importance of awaiting the results of ongoing prospective, randomized controlled studies before widespread adoption of these drugs,” write Joseph Magagnoli with Dorn Research Institute at the Columbia (S.C.) VA Health Care System and the department of clinical pharmacy & outcomes sciences, University of South Carolina, and colleagues.
A spokesperson with the University of Virginia, Charlottesville, where several of coauthors practice, said that the authors declined to comment for this article before peer review is completed.
The new data are not the first to suggest no benefit with HCQ among patients with COVID-19. A randomized trial showed no benefit and more side effects among 75 patients in China treated with HCQ, compared with 75 who received standard of care alone, according to a preprint posted online April 14.
No benefit in ventilation, death rates
The current analysis included data from all 368 male patients hospitalized with confirmed COVID-19 and treated at Veterans Health Administration medical centers in the United States through April 11.
Patients were categorized into three groups: those treated with HCQ in addition to standard of care (n = 97); those treated with HCQ and the antibiotic azithromycin plus standard of care (n = 113); and those who received standard supportive care only (n = 158).

Compared with the no HCQ group, the risk of death from any cause was higher in the HCQ group (adjusted hazard ratio, 2.61; 95% confidence interval, 1.10-6.17; P = .03) but not in the HCQ+AZ group (aHR, 1.14; 95% CI, 0.56-2.32; P = .72).
The risk of ventilation was similar in the HCQ group (aHR, 1.43; 95% CI, 0.53-3.79; P = .48) and in the HCQ+AZ group (aHR, 0.43; 95% CI, 0.16-1.12; P = .09), compared with the no-HCQ group.
This study provides another counterbalance to claims of HCQ efficacy, David R. Wessner, PhD, professor of biology and chair of the department of health and human values at Davidson (N.C.) College, said in an interview.
Interest in HCQ spiked after an open-label, nonrandomized, single-center study of COVID-19 patients in France suggested that hydroxychloroquine helped clear the virus and had a potential enhanced effect when combined with azithromycin.
But the 36-patient trial has since been called into question.
Wait for convincing data
Dr. Wessner, whose research focuses on viral pathogenesis, says that, although the current data don’t definitively answer the question of whether HCQ is effective in treating COVID-19, taking a “let’s try it and see” approach is not reasonable.
“Until we have good, prospective randomized trials, it’s hard to know what to make of this. But this is more evidence that there’s not a good reason to use [HCQ],” Dr. Wessner said. He points out that the small randomized trial from China shows that HCQ comes with potential harms.
Anecdotal evidence is often cited by those who promote HCQ as a potential treatment, but “those are one-off examples,” Wessner continued. “That doesn’t really tell us anything.”
Some HCQ proponents have said that trials finding no benefit are flawed in that the drug is given too late. However, Dr. Wessner says, there’s no way to prove or disprove that claim without randomized controlled trials.
Conflicting messages
Despite lack of clear evidence of benefit for patients with COVID-19, HCQ is recommended off-label by the Chinese National guideline, and the U.S. Food and Drug Administration has issued an emergency-use authorization for the treatment of adult patients with COVID-19.
Conversely, the Infectious Diseases Society of America and a guideline panel convened by the National Institutes of Health each concluded recently that because of insufficient data, they could not recommend any specific treatments for patients with COVID-19.
The VA data for the current study came from the Veterans Affairs Informatics and Computing Infrastructure, which includes inpatient, outpatient and laboratory data and pharmacy claims.
The authors acknowledge some limitations, “including those inherent to all retrospective analyses such as nonrandomization of treatments.”
However, they note that they did adjust for potential confounders, including comorbidities, medications, and clinical and laboratory factors.
A coauthor, Jayakrishna Ambati, MD, is a cofounder of iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics, and has received consultancy fees from Allergan, Biogen, Boehringer Ingelheim, Immunovant, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences, all unrelated to this work. Dr. Ambati is named as an inventor on a patent application filed by the University of Virginia relating to COVID-19 but unrelated to this work. Another coauthor has received research grants from Boehringer Ingelheim, Gilead Sciences, Portola Pharmaceuticals, and United Therapeutics, all unrelated to this work. The other authors and Dr. Wessner have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Signature STEMI sign may be less diagnostic in the COVID-19 age
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
ACEI/ARBs linked with survival in hypertensive, Chinese COVID-19 patients
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
Hospitalized COVID-19 patients with hypertension and on treatment with an renin-angiotensin system inhibiting drug had significantly better survival, compared with similar hypertensive patients not on these drugs, in observational, propensity score–matched analyses that drew from a pool of more than 3,430 patients hospitalized at any of nine Chinese hospitals during December 2019–February 2020.
“Among patients with hypertension hospitalized with COVID-19, inpatient treatment with ACEI [ACE inhibitor]/ARB [angiotensin receptor blocker] was associated with lower risk of all-cause mortality, compared with ACEI/ARB nonusers, during 28 days of follow-up. While study interpretation needs to consider the potential for residual confounders, it is unlikely that inpatient ACEI/ARB would be associated with an increased risk of mortality,” wrote Peng Zhang, MD, a cardiology researcher at Renmin Hospital of Wuhan University, China, and coauthors in Circulations Research, buttressing recent recommendations from several medical societies to maintain COVID-19 patients on these drugs.
“Our findings in this paper provide evidence supporting continuous use of ACEI/ARB for patients with hypertension infected with SARS-COV-2,” wrote the authors, backing up recent recommendations from cardiology societies that called for not stopping ACEI/ARB prescriptions in patients at risk for contracting or already have COVID-19 infection, including a statement from the American College of Cardiology, American Heart Association, and Heart Failure Society of America, and also guidance from the European Society of Cardiology.
The study included 1,128 patients with a history of hypertension, including 188 (17%) who received an ACEI/ARB drug during hospitalization. During 28-day follow-up, 99 died (9%), including 7 deaths among the 188 patients (4%) on an ACEI/ARB drug and 92 deaths among the 940 other hypertensive patients (10%).
The authors ran several analyses to try to adjust for the influence of possible confounders. A mixed-effect Cox model with four adjusted variables showed that treatment with an ACEI/ARB drug was tied to a statistically significant 58% lower death rate, compared with patients not receiving these drugs.
The researchers also ran several propensity score–adjusted analyses. One matched 174 of the patients who received an ACEI/ARB drug with 522 who did not, and comparing these two matched arms showed that ACEI/ARB use was linked with a statistically significant 63% cut in mortality, compared with patients not getting these drugs. A second propensity score–matched analysis first excluded the 383 patients who were hypertensive but received no antihypertensive medication during hospitalization. From the remaining 745 patients who received at least one antihypertensive medication, the authors identified 181 patients who received an ACEI/ARB and propensity-score matched them with 181 hypertensive patients on a different medication class, finding that ACEI/ARB use linked with a statistically significant 71% lower rate of all-cause mortality.
Additional analyses also showed that patients with hypertension had a statistically significant, 41% increased rate of all-cause death, compared with patients without hypertension, and another propensity score–matched analysis showed that among hypertensives treatment with an ACEI/ARB drug was linked with a statistically significant 68% reduced rate of septic shock.
Although this report was received with caution and some skepticism, it was also acknowledged as a step forward in the creation of an evidence base addressing ACEI/ARB treatment during COVID-19 infection.
“These drugs are lifesaving and should not be discontinued” for patients with hypertension, heart failure, and other cardiovascular disease, commented Gian Paolo Rossi, MD, professor and chair of medicine and director of the high blood pressure unit at the University of Padua (Italy). The analysis by Zhang and associates included the largest number of hospitalized COVID-19 patients with hypertension yet reported to assess the impact of treatment with ACEI/ARB drugs, and adds important evidence in favor of continuing these drugs in patients who develop COVID-19 infection, Dr. Rossi said in an interview. He recently coauthored a review that argued against ACEI/ARB discontinuation in COVID-19 patients based on previously reported evidence (Elife. 2020 Apr 6. doi: 10.7554/eLife.57278).
But other researchers take a wary view of the potential impact of ACEI/ARB agents. “If ACEI/ARB therapy increases ACE2 and the virus down-regulates it, and because ACE2 is the viral entry port into cells, why would ACE2-mediated down-regulation of the renin-angiotensin-aldosterone system lead to amelioration of [COVID-19] disease?” asked Laurence W. Busse, MD, a critical care physician at Emory University, Atlanta. “A number of issues could potentially confound the results, including the definition of COVID-19 and imbalance of antiviral therapy,” added Dr. Busse, who recently coauthored an editorial that posited using angiotensin II (Giapreza), an approved vasopressor drug, as an alternative renin-angiotensin system intervention for COVID-19 patients including both those in shock as well as potentially those not in shock (Crit Care. 2020 Apr 7. doi: 10.1186/s13054-020-02862-1). Despite these caveats, the new Chinese findings reported by Dr. Zhang and associates “are hypothesis generating and worth further exploration.”
The authors of an editorial that accompanied the Zhang study in Circulation Research made similar points. “While the investigators used standard techniques to attempt to reduce bias in this observational study via propensity matching, it is not a randomized study and the residual confounding inherent to this approach renders the conclusions hypothesis generating at best,” wrote Ravi V. Shah, MD, and two coauthors in the editorial (Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317174). They also agreed with the several society statements that have supported continued use of ACEI/ARB drugs in COVID-19 patients. “Withdrawal of these medications in the context of those conditions in which they have proven benefit (e.g., heart failure with reduced left ventricular ejection fraction) may actually inflict more harm than good,” they warned. “In the end we must rely on randomized clinical science,” and while this level of evidence is currently lacking, “the study by Zhang and colleagues is a direct step toward that goal.”
Dr. Zhang and coauthors had no commercial disclosures. Dr. Rossi and Dr. Busse had no disclosures. The authors of the Circulation Research editorial reported several disclosures.
SOURCE: Zhang P et al. Circ Res. 2020 Apr 17. doi: 10.1161/CIRCRESAHA.120.317134.
FROM CIRCULATION RESEARCH