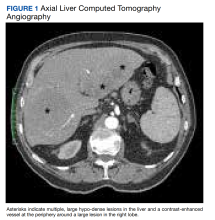
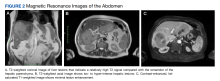
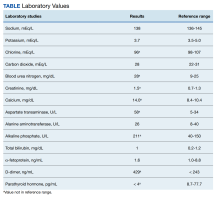
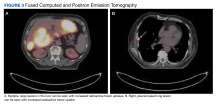
User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Patritumab deruxtecan shows promise for breast cancer patients
according to data presented from Abstract 1240 at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Patritumab deruxtecan (HER3-DXd) has previously demonstrated an acceptable safety profile and antitumor activity in phase I studies involving heavily pretreated patients with metastatic breast cancer and various levels of HER3 protein expression, said Mafalda Oliveira, MD, of the Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology in Barcelona.
Antibody-drug conjugates (ADCs) are a combination of a monoclonal antibody chemically linked to a drug, as defined by the National Cancer Institute. ADCs work by binding to receptors or proteins and selectively delivering cytotoxic drugs to the site of a tumor.
Dr. Oliveira presented results from part B the of SOLTI TOT-HER3 trial, a window-of-opportunity trial that evaluated the effect of a single dose of HER3-Dxd in patients with treatment-naive HR+/HER2– early breast cancer.
In such trials, patients receive one or more new compounds between the time of cancer diagnosis and standard treatment. Biological and clinical activity from part A of the SOLTI TOT-HER3 trial were presented at last year’s ESMO Breast Cancer Congress, Dr. Oliveira said.
In the current study, Dr. Oliveira and colleagues recruited 37 women with HR+/HER2– early breast cancer, including 20 who were hormone receptor–positive and 17 who had triple negative breast cancer (TNBC). The age of the participants ranged from 30 to 81 years, with a median age of 51 years; 54% were premenopausal. The mean tumor size was 21 mm, with a range of 10-81 mm.
Distinct from part A of the SOLTI TOT-HER3 trial, part B included a subset of patients with TNBC to assess preliminary efficacy in this subtype, Dr. Oliveira noted.
All patients in part B received a single dose of 5.6 mg/kg of HER3-DXd. The primary outcome was the variation in the tumor cellularity and tumor-infiltrating lymphocyte (CelTIL) score at baseline and after 21 days via breast ultrasound.
At day 21, the total CelTIL score increased by a significant mean difference of 9.4 points after a single dose; the mean differences for TNBC and HR+/HER2– patients, were 17.9 points and 2.2 points, respectively, Dr. Oliveira said. The overall response rate was 32% (35% in TNBC patients and 30% in HR+/HER2– patients) and was significantly associated with the absolute change in CelTIL (area under the curve = 0.693; P = .049).
In a subtype analysis, a statistically significant change in CelTIL was observed between paired samples overall (P = .046) and in TNBC (P = .016), but not in HR+ (P = .793).
Baseline levels of ERBB3 (also known as human epidermal growth factor receptor type 3, or HER3) were not associated with changes in CelTIL or in overall response rate.
HER3-DXd induced high expression of immune-related genes (such as PD1, CD8, and CD19), and suppressed proliferation-related genes, she said.
A total of 31 patients (84%) reported any adverse events. Of these, the most common were nausea, fatigue, alopecia, diarrhea, constipation, and vomiting, and one patient experienced grade 3 treatment-related nausea. No interstitial lung disease events were reported during the study, and the incidence of hematological and hepatic toxicity was lower with the lower dose in part B, compared with the 6.5 mg/kg dose used in part A, Dr. Oliveira noted.
To further validate the findings of the current study and assess the activity of HER3-DXd in early breast cancer, Dr. Oliveira and colleagues are conducting a neoadjuvant phase II trial known as SOLTI-2103 VALENTINE. In this study, they are testing six cycles of HER3-DXd at a 5.6 mg/kg dose in HR+/HER2– breast cancer patients, she said.
During a question-and-answer session, Dr. Oliveira was asked whether CelTIL is the best endpoint for assessing HER3-DXd. Finding the best endpoint is always a challenge when conducting window-of-opportunity trials, she said. The CelTIL score has been correlated with pathologic complete response (pCR), as well as with disease-free survival and overall survival, she added.
ICARUS-BREAST01
In a presentation of Abstract 1890 during the same session, Barbari Pistilli, MD, of Gustave Roussy Cancer Center, Villejuif, France, shared data from a phase II study known as ICARUS-BREAST.
The study population included women with unresectable locally advanced breast cancer (ABC) who had undergone a median of two previous systemic therapies. In the current study, the patients underwent a median of eight cycles of HER3-DXd. The dosage was 5.6 mg/kg every 3 weeks until disease progression or unacceptable toxicity.
The primary outcome was overall response and disease progression after 3 months. Dr. Pistilli, who is also a coauthor of the research, provided data from 56 evaluable patients.
After 3 months, 16 patients (28.6%) showed a partial response, 30 patients showed stable disease (54%), and 10 (18%) showed disease progression. “No patients had a complete response,” Dr. Pistilli noted.
As for the safety profile, all patients reported at least one treatment-emergent event of any grade, but less than half (48.2%) were grade 3 or higher, and 12.5% led to treatment discontinuation. Fatigue and nausea were the most frequently reported adverse events overall, and occurred in 89.3% and 76.8% of patients, respectively. All grade and grade 3 or higher neutropenia occurred in four patients and six patients, respectively; all grade and grade 3 or higher thrombocytopenia occurred in four patients and two patients, respectively, Dr. Pistilli said.
Data on circulating tumor cells (CTCs) were available for 31 patients, and the researchers reviewed CTC counts after the first HER3-DXd cycle.
“We found that the median number of CTCs decreased by one to two cell cycles of HER3-DXd,” said Dr. Pistilli. She and her coauthors found no substantial impact of the treatment on HER3 negative CTC counts, and “more importantly, no increase of HER3 negative CTC counts at disease progression,” Dr. Pistilli continued.
In addition, patients with higher HER+ CTC counts at baseline or a greater decrease in HER3+ CTC counts after one cycle of HER3-DXd were more likely to have an early treatment response, but this association was not statistically significant.
Looking ahead, further analysis will be performed to evaluate the association between HER3+ CTC counts and dynamics and the main outcomes of overall response rate and progression-free survival to determine the potential of HER3+ CTC counts to identify patients who can benefit from HER3-DXd, said Dr. Pistilli. The ICARUS-BREAST01 study is ongoing, and further efficacy and biomarker analysis will be presented, she added.
In the question-and-answer session, Dr. Pistilli was asked why she chose CTC as a measure.
Dr. Pistilli responded that she and her coauthors wanted to understand whether CTC could serve as a biomarker to help in patient selection.
Also, when asked about which genes might be upregulated and downregulated in responders vs. nonresponders, she noted that some genes related to DNA repair were involved in patients who were responders, but more research is needed.
Early results merit further exploration
Although patritumab deruxtecan is early in development, “there is a clear signal to expand,” based on preliminary research, said Rebecca A. Dent, MD, who served as discussant for the two studies.
“There is no clear role for a specific subtype in both protein and gene expression,” noted Dr. Dent, who is a professor at Duke NUS Medical School, a collaboration between Duke University, Durham, N.C., and the National University of Singapore.
In the SOLTI TOT-HER3 trial, the small numbers make teasing out correlations a challenge, said Dr. Dent. However, changes were observed after just one cycle of the drug, and the upregulation of immune signature genes was reassuring, she said.
“A single dose of HER3-DXd induced an overall response of approximately 30% independently of hormone receptor status,” she emphasized, and the lower incidence of hematological and hepatic toxicity with the lower dose is good news as well. The findings were limited by the small sample size, but the results support moving forward with clinical development of HER3-DXd, she said.
The ICARUS-BREAST01 study researchers tried to show whether they could identify potential markers of early treatment response, and they examined CTCs and gene alterations, said Dr. Dent. “I think it is reassuring that despite these patients being heavily pretreated, HER-DXd seems to be active regardless of most frequent breast cancer genomic alterations,” she noted. Remaining questions include the need for more data on primary resistance.
“We are able to get these patients to respond, but what makes patients resistant to ADCs is just as important,” she said. “We see exciting data across all these subtypes,”
In Dr. Dent’s opinion, future research should focus on triple negative breast cancer, an opinion supported by the stronger response in this subset of patients in the SOLTI TOT-HER3 trial. “I think you need to bring triple negative to the table,” she said.
The SOLTI TOT-HER3 study was funded by Daiichi Sankyo. Dr. Oliveira disclosed relationships with companies including AstraZeneca, Ayala Pharmaceuticals, Boehringer-Ingelheim, Genentech, Gilead, GSK, Novartis, Roche, Seagen, Zenith Epigenetics, Daiichi Sankyo, iTEOS, MSD, Pierre-Fabre, Relay Therapeutics, and Eisai. ICARUS-BREAST01 was sponsored by the Gustave Roussy Cancer Center and supported by Daiichi Sankyo. Dr. Pistilli disclosed relationships with multiple companies including Daiichi-Sankyo, AstraZeneca, Gilead, Seagen, MSD, Novartis, Lilly, and Pierre Fabre. Dr. Dent disclosed financial relationships with companies including AstraZeneca, Roche, Eisai, Lilly, MSD, Novartis, and Pfizer.
according to data presented from Abstract 1240 at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Patritumab deruxtecan (HER3-DXd) has previously demonstrated an acceptable safety profile and antitumor activity in phase I studies involving heavily pretreated patients with metastatic breast cancer and various levels of HER3 protein expression, said Mafalda Oliveira, MD, of the Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology in Barcelona.
Antibody-drug conjugates (ADCs) are a combination of a monoclonal antibody chemically linked to a drug, as defined by the National Cancer Institute. ADCs work by binding to receptors or proteins and selectively delivering cytotoxic drugs to the site of a tumor.
Dr. Oliveira presented results from part B the of SOLTI TOT-HER3 trial, a window-of-opportunity trial that evaluated the effect of a single dose of HER3-Dxd in patients with treatment-naive HR+/HER2– early breast cancer.
In such trials, patients receive one or more new compounds between the time of cancer diagnosis and standard treatment. Biological and clinical activity from part A of the SOLTI TOT-HER3 trial were presented at last year’s ESMO Breast Cancer Congress, Dr. Oliveira said.
In the current study, Dr. Oliveira and colleagues recruited 37 women with HR+/HER2– early breast cancer, including 20 who were hormone receptor–positive and 17 who had triple negative breast cancer (TNBC). The age of the participants ranged from 30 to 81 years, with a median age of 51 years; 54% were premenopausal. The mean tumor size was 21 mm, with a range of 10-81 mm.
Distinct from part A of the SOLTI TOT-HER3 trial, part B included a subset of patients with TNBC to assess preliminary efficacy in this subtype, Dr. Oliveira noted.
All patients in part B received a single dose of 5.6 mg/kg of HER3-DXd. The primary outcome was the variation in the tumor cellularity and tumor-infiltrating lymphocyte (CelTIL) score at baseline and after 21 days via breast ultrasound.
At day 21, the total CelTIL score increased by a significant mean difference of 9.4 points after a single dose; the mean differences for TNBC and HR+/HER2– patients, were 17.9 points and 2.2 points, respectively, Dr. Oliveira said. The overall response rate was 32% (35% in TNBC patients and 30% in HR+/HER2– patients) and was significantly associated with the absolute change in CelTIL (area under the curve = 0.693; P = .049).
In a subtype analysis, a statistically significant change in CelTIL was observed between paired samples overall (P = .046) and in TNBC (P = .016), but not in HR+ (P = .793).
Baseline levels of ERBB3 (also known as human epidermal growth factor receptor type 3, or HER3) were not associated with changes in CelTIL or in overall response rate.
HER3-DXd induced high expression of immune-related genes (such as PD1, CD8, and CD19), and suppressed proliferation-related genes, she said.
A total of 31 patients (84%) reported any adverse events. Of these, the most common were nausea, fatigue, alopecia, diarrhea, constipation, and vomiting, and one patient experienced grade 3 treatment-related nausea. No interstitial lung disease events were reported during the study, and the incidence of hematological and hepatic toxicity was lower with the lower dose in part B, compared with the 6.5 mg/kg dose used in part A, Dr. Oliveira noted.
To further validate the findings of the current study and assess the activity of HER3-DXd in early breast cancer, Dr. Oliveira and colleagues are conducting a neoadjuvant phase II trial known as SOLTI-2103 VALENTINE. In this study, they are testing six cycles of HER3-DXd at a 5.6 mg/kg dose in HR+/HER2– breast cancer patients, she said.
During a question-and-answer session, Dr. Oliveira was asked whether CelTIL is the best endpoint for assessing HER3-DXd. Finding the best endpoint is always a challenge when conducting window-of-opportunity trials, she said. The CelTIL score has been correlated with pathologic complete response (pCR), as well as with disease-free survival and overall survival, she added.
ICARUS-BREAST01
In a presentation of Abstract 1890 during the same session, Barbari Pistilli, MD, of Gustave Roussy Cancer Center, Villejuif, France, shared data from a phase II study known as ICARUS-BREAST.
The study population included women with unresectable locally advanced breast cancer (ABC) who had undergone a median of two previous systemic therapies. In the current study, the patients underwent a median of eight cycles of HER3-DXd. The dosage was 5.6 mg/kg every 3 weeks until disease progression or unacceptable toxicity.
The primary outcome was overall response and disease progression after 3 months. Dr. Pistilli, who is also a coauthor of the research, provided data from 56 evaluable patients.
After 3 months, 16 patients (28.6%) showed a partial response, 30 patients showed stable disease (54%), and 10 (18%) showed disease progression. “No patients had a complete response,” Dr. Pistilli noted.
As for the safety profile, all patients reported at least one treatment-emergent event of any grade, but less than half (48.2%) were grade 3 or higher, and 12.5% led to treatment discontinuation. Fatigue and nausea were the most frequently reported adverse events overall, and occurred in 89.3% and 76.8% of patients, respectively. All grade and grade 3 or higher neutropenia occurred in four patients and six patients, respectively; all grade and grade 3 or higher thrombocytopenia occurred in four patients and two patients, respectively, Dr. Pistilli said.
Data on circulating tumor cells (CTCs) were available for 31 patients, and the researchers reviewed CTC counts after the first HER3-DXd cycle.
“We found that the median number of CTCs decreased by one to two cell cycles of HER3-DXd,” said Dr. Pistilli. She and her coauthors found no substantial impact of the treatment on HER3 negative CTC counts, and “more importantly, no increase of HER3 negative CTC counts at disease progression,” Dr. Pistilli continued.
In addition, patients with higher HER+ CTC counts at baseline or a greater decrease in HER3+ CTC counts after one cycle of HER3-DXd were more likely to have an early treatment response, but this association was not statistically significant.
Looking ahead, further analysis will be performed to evaluate the association between HER3+ CTC counts and dynamics and the main outcomes of overall response rate and progression-free survival to determine the potential of HER3+ CTC counts to identify patients who can benefit from HER3-DXd, said Dr. Pistilli. The ICARUS-BREAST01 study is ongoing, and further efficacy and biomarker analysis will be presented, she added.
In the question-and-answer session, Dr. Pistilli was asked why she chose CTC as a measure.
Dr. Pistilli responded that she and her coauthors wanted to understand whether CTC could serve as a biomarker to help in patient selection.
Also, when asked about which genes might be upregulated and downregulated in responders vs. nonresponders, she noted that some genes related to DNA repair were involved in patients who were responders, but more research is needed.
Early results merit further exploration
Although patritumab deruxtecan is early in development, “there is a clear signal to expand,” based on preliminary research, said Rebecca A. Dent, MD, who served as discussant for the two studies.
“There is no clear role for a specific subtype in both protein and gene expression,” noted Dr. Dent, who is a professor at Duke NUS Medical School, a collaboration between Duke University, Durham, N.C., and the National University of Singapore.
In the SOLTI TOT-HER3 trial, the small numbers make teasing out correlations a challenge, said Dr. Dent. However, changes were observed after just one cycle of the drug, and the upregulation of immune signature genes was reassuring, she said.
“A single dose of HER3-DXd induced an overall response of approximately 30% independently of hormone receptor status,” she emphasized, and the lower incidence of hematological and hepatic toxicity with the lower dose is good news as well. The findings were limited by the small sample size, but the results support moving forward with clinical development of HER3-DXd, she said.
The ICARUS-BREAST01 study researchers tried to show whether they could identify potential markers of early treatment response, and they examined CTCs and gene alterations, said Dr. Dent. “I think it is reassuring that despite these patients being heavily pretreated, HER-DXd seems to be active regardless of most frequent breast cancer genomic alterations,” she noted. Remaining questions include the need for more data on primary resistance.
“We are able to get these patients to respond, but what makes patients resistant to ADCs is just as important,” she said. “We see exciting data across all these subtypes,”
In Dr. Dent’s opinion, future research should focus on triple negative breast cancer, an opinion supported by the stronger response in this subset of patients in the SOLTI TOT-HER3 trial. “I think you need to bring triple negative to the table,” she said.
The SOLTI TOT-HER3 study was funded by Daiichi Sankyo. Dr. Oliveira disclosed relationships with companies including AstraZeneca, Ayala Pharmaceuticals, Boehringer-Ingelheim, Genentech, Gilead, GSK, Novartis, Roche, Seagen, Zenith Epigenetics, Daiichi Sankyo, iTEOS, MSD, Pierre-Fabre, Relay Therapeutics, and Eisai. ICARUS-BREAST01 was sponsored by the Gustave Roussy Cancer Center and supported by Daiichi Sankyo. Dr. Pistilli disclosed relationships with multiple companies including Daiichi-Sankyo, AstraZeneca, Gilead, Seagen, MSD, Novartis, Lilly, and Pierre Fabre. Dr. Dent disclosed financial relationships with companies including AstraZeneca, Roche, Eisai, Lilly, MSD, Novartis, and Pfizer.
according to data presented from Abstract 1240 at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Patritumab deruxtecan (HER3-DXd) has previously demonstrated an acceptable safety profile and antitumor activity in phase I studies involving heavily pretreated patients with metastatic breast cancer and various levels of HER3 protein expression, said Mafalda Oliveira, MD, of the Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology in Barcelona.
Antibody-drug conjugates (ADCs) are a combination of a monoclonal antibody chemically linked to a drug, as defined by the National Cancer Institute. ADCs work by binding to receptors or proteins and selectively delivering cytotoxic drugs to the site of a tumor.
Dr. Oliveira presented results from part B the of SOLTI TOT-HER3 trial, a window-of-opportunity trial that evaluated the effect of a single dose of HER3-Dxd in patients with treatment-naive HR+/HER2– early breast cancer.
In such trials, patients receive one or more new compounds between the time of cancer diagnosis and standard treatment. Biological and clinical activity from part A of the SOLTI TOT-HER3 trial were presented at last year’s ESMO Breast Cancer Congress, Dr. Oliveira said.
In the current study, Dr. Oliveira and colleagues recruited 37 women with HR+/HER2– early breast cancer, including 20 who were hormone receptor–positive and 17 who had triple negative breast cancer (TNBC). The age of the participants ranged from 30 to 81 years, with a median age of 51 years; 54% were premenopausal. The mean tumor size was 21 mm, with a range of 10-81 mm.
Distinct from part A of the SOLTI TOT-HER3 trial, part B included a subset of patients with TNBC to assess preliminary efficacy in this subtype, Dr. Oliveira noted.
All patients in part B received a single dose of 5.6 mg/kg of HER3-DXd. The primary outcome was the variation in the tumor cellularity and tumor-infiltrating lymphocyte (CelTIL) score at baseline and after 21 days via breast ultrasound.
At day 21, the total CelTIL score increased by a significant mean difference of 9.4 points after a single dose; the mean differences for TNBC and HR+/HER2– patients, were 17.9 points and 2.2 points, respectively, Dr. Oliveira said. The overall response rate was 32% (35% in TNBC patients and 30% in HR+/HER2– patients) and was significantly associated with the absolute change in CelTIL (area under the curve = 0.693; P = .049).
In a subtype analysis, a statistically significant change in CelTIL was observed between paired samples overall (P = .046) and in TNBC (P = .016), but not in HR+ (P = .793).
Baseline levels of ERBB3 (also known as human epidermal growth factor receptor type 3, or HER3) were not associated with changes in CelTIL or in overall response rate.
HER3-DXd induced high expression of immune-related genes (such as PD1, CD8, and CD19), and suppressed proliferation-related genes, she said.
A total of 31 patients (84%) reported any adverse events. Of these, the most common were nausea, fatigue, alopecia, diarrhea, constipation, and vomiting, and one patient experienced grade 3 treatment-related nausea. No interstitial lung disease events were reported during the study, and the incidence of hematological and hepatic toxicity was lower with the lower dose in part B, compared with the 6.5 mg/kg dose used in part A, Dr. Oliveira noted.
To further validate the findings of the current study and assess the activity of HER3-DXd in early breast cancer, Dr. Oliveira and colleagues are conducting a neoadjuvant phase II trial known as SOLTI-2103 VALENTINE. In this study, they are testing six cycles of HER3-DXd at a 5.6 mg/kg dose in HR+/HER2– breast cancer patients, she said.
During a question-and-answer session, Dr. Oliveira was asked whether CelTIL is the best endpoint for assessing HER3-DXd. Finding the best endpoint is always a challenge when conducting window-of-opportunity trials, she said. The CelTIL score has been correlated with pathologic complete response (pCR), as well as with disease-free survival and overall survival, she added.
ICARUS-BREAST01
In a presentation of Abstract 1890 during the same session, Barbari Pistilli, MD, of Gustave Roussy Cancer Center, Villejuif, France, shared data from a phase II study known as ICARUS-BREAST.
The study population included women with unresectable locally advanced breast cancer (ABC) who had undergone a median of two previous systemic therapies. In the current study, the patients underwent a median of eight cycles of HER3-DXd. The dosage was 5.6 mg/kg every 3 weeks until disease progression or unacceptable toxicity.
The primary outcome was overall response and disease progression after 3 months. Dr. Pistilli, who is also a coauthor of the research, provided data from 56 evaluable patients.
After 3 months, 16 patients (28.6%) showed a partial response, 30 patients showed stable disease (54%), and 10 (18%) showed disease progression. “No patients had a complete response,” Dr. Pistilli noted.
As for the safety profile, all patients reported at least one treatment-emergent event of any grade, but less than half (48.2%) were grade 3 or higher, and 12.5% led to treatment discontinuation. Fatigue and nausea were the most frequently reported adverse events overall, and occurred in 89.3% and 76.8% of patients, respectively. All grade and grade 3 or higher neutropenia occurred in four patients and six patients, respectively; all grade and grade 3 or higher thrombocytopenia occurred in four patients and two patients, respectively, Dr. Pistilli said.
Data on circulating tumor cells (CTCs) were available for 31 patients, and the researchers reviewed CTC counts after the first HER3-DXd cycle.
“We found that the median number of CTCs decreased by one to two cell cycles of HER3-DXd,” said Dr. Pistilli. She and her coauthors found no substantial impact of the treatment on HER3 negative CTC counts, and “more importantly, no increase of HER3 negative CTC counts at disease progression,” Dr. Pistilli continued.
In addition, patients with higher HER+ CTC counts at baseline or a greater decrease in HER3+ CTC counts after one cycle of HER3-DXd were more likely to have an early treatment response, but this association was not statistically significant.
Looking ahead, further analysis will be performed to evaluate the association between HER3+ CTC counts and dynamics and the main outcomes of overall response rate and progression-free survival to determine the potential of HER3+ CTC counts to identify patients who can benefit from HER3-DXd, said Dr. Pistilli. The ICARUS-BREAST01 study is ongoing, and further efficacy and biomarker analysis will be presented, she added.
In the question-and-answer session, Dr. Pistilli was asked why she chose CTC as a measure.
Dr. Pistilli responded that she and her coauthors wanted to understand whether CTC could serve as a biomarker to help in patient selection.
Also, when asked about which genes might be upregulated and downregulated in responders vs. nonresponders, she noted that some genes related to DNA repair were involved in patients who were responders, but more research is needed.
Early results merit further exploration
Although patritumab deruxtecan is early in development, “there is a clear signal to expand,” based on preliminary research, said Rebecca A. Dent, MD, who served as discussant for the two studies.
“There is no clear role for a specific subtype in both protein and gene expression,” noted Dr. Dent, who is a professor at Duke NUS Medical School, a collaboration between Duke University, Durham, N.C., and the National University of Singapore.
In the SOLTI TOT-HER3 trial, the small numbers make teasing out correlations a challenge, said Dr. Dent. However, changes were observed after just one cycle of the drug, and the upregulation of immune signature genes was reassuring, she said.
“A single dose of HER3-DXd induced an overall response of approximately 30% independently of hormone receptor status,” she emphasized, and the lower incidence of hematological and hepatic toxicity with the lower dose is good news as well. The findings were limited by the small sample size, but the results support moving forward with clinical development of HER3-DXd, she said.
The ICARUS-BREAST01 study researchers tried to show whether they could identify potential markers of early treatment response, and they examined CTCs and gene alterations, said Dr. Dent. “I think it is reassuring that despite these patients being heavily pretreated, HER-DXd seems to be active regardless of most frequent breast cancer genomic alterations,” she noted. Remaining questions include the need for more data on primary resistance.
“We are able to get these patients to respond, but what makes patients resistant to ADCs is just as important,” she said. “We see exciting data across all these subtypes,”
In Dr. Dent’s opinion, future research should focus on triple negative breast cancer, an opinion supported by the stronger response in this subset of patients in the SOLTI TOT-HER3 trial. “I think you need to bring triple negative to the table,” she said.
The SOLTI TOT-HER3 study was funded by Daiichi Sankyo. Dr. Oliveira disclosed relationships with companies including AstraZeneca, Ayala Pharmaceuticals, Boehringer-Ingelheim, Genentech, Gilead, GSK, Novartis, Roche, Seagen, Zenith Epigenetics, Daiichi Sankyo, iTEOS, MSD, Pierre-Fabre, Relay Therapeutics, and Eisai. ICARUS-BREAST01 was sponsored by the Gustave Roussy Cancer Center and supported by Daiichi Sankyo. Dr. Pistilli disclosed relationships with multiple companies including Daiichi-Sankyo, AstraZeneca, Gilead, Seagen, MSD, Novartis, Lilly, and Pierre Fabre. Dr. Dent disclosed financial relationships with companies including AstraZeneca, Roche, Eisai, Lilly, MSD, Novartis, and Pfizer.
FROM ESMO BREAST CANCER 2023
Does the current age cutoff for screening miss too many cases of cervical cancer in older women?
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
Key red flags for early-onset colorectal cancer
As the number of cases of early-onset colorectal cancer (CRC) diagnosed before age 50 continues to rise, early detection has become increasingly important.
The signs and symptoms are abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia.
Two symptoms in particular – rectal bleeding and iron-deficiency anemia – point to the need for timely endoscopy and follow-up, the researchers say.
“Colorectal cancer is not simply a disease affecting older people; we want younger adults to be aware of and act on these potentially very telling signs and symptoms – particularly because people under 50 are considered to be at low risk, and they don’t receive routine colorectal cancer screening,” senior investigator Yin Cao, ScD, with Washington University School of Medicine, St. Louis, said in a news release.
“It’s also crucial to spread awareness among primary care doctors, gastroenterologists, and emergency medicine doctors,” Dr. Cao added. “To date, many early-onset colorectal cancers are detected in emergency rooms, and there often are significant diagnostic delays with this cancer.”
The study was published online in the Journal of the National Cancer Institute.
Although previous research has identified rectal bleeding, iron-deficiency anemia, and rectal/abdominal pain as symptoms of early-onset CRC, most studies “have aggregated symptoms till the time of diagnosis,” which limits their use for early detection, the authors explain.
In the current study, the researchers analyzed data from more than 5,000 cases of early-onset CRC and from more than 22,000 control patients using the IBM MarketScan commercial database.
Dr. Cao and colleagues found that between 3 months and 2 years before diagnosis, abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia each indicated an increased risk for early-onset CRC.
Among patients with early-onset CRC, 19.3% presented with one or more of the four red flags between 3 months and 2 years prior to the index date; 15.6% had one symptom, and 3.7% had two or more.
After multivariable adjustment, having one symptom almost doubled the risk for early-onset CRC (odds ratio, 1.94); having two symptoms increased risk by more than threefold (OR, 3.59); and having three or more boosted the risk by more than 6.5-fold (OR, 6.52).
Abdominal pain was associated with a 34% higher risk of early-onset CRC (11.6% among case patients vs. 7.7% among controls; OR, 1.34).
Although not as common, rectal bleeding was associated with the highest odds for early-onset CRC (7.2% case patients vs. 1.3% controls; OR, 5.13).
The other predictive signs and symptoms included diarrhea (2.8% case patients vs. 1.4% controls; OR, 1.43) and iron-deficiency anemia (2.3% case patients vs. 0.9% controls; OR, 2.07).
No differences were observed by gender for each sign or symptom.
Among patients with a red-flag symptom who presented between 3 months and 2 years before diagnosis, for those with early-onset CRC, the median diagnostic interval was 8.7 months.
The researchers suggest that clinicians prioritize prompt diagnostic workups for patients younger than 50 who present with rectal bleeding and/or iron-deficiency anemia and that they also keep abdominal pain and diarrhea in mind as early symptoms.
Dr. Cao noted that since most early-onset CRC cases “have been and will continue to be diagnosed after symptom presentation, it is crucial to recognize these red-flag signs and symptoms promptly and conduct a diagnostic workup as soon as possible.
“By doing so, we can diagnose the disease earlier, which in turn can reduce the need for more aggressive treatment and improve patients’ quality of life and survival rates,” said Dr. Cao.
The study was supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article originally appeared on Medscape.com.
As the number of cases of early-onset colorectal cancer (CRC) diagnosed before age 50 continues to rise, early detection has become increasingly important.
The signs and symptoms are abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia.
Two symptoms in particular – rectal bleeding and iron-deficiency anemia – point to the need for timely endoscopy and follow-up, the researchers say.
“Colorectal cancer is not simply a disease affecting older people; we want younger adults to be aware of and act on these potentially very telling signs and symptoms – particularly because people under 50 are considered to be at low risk, and they don’t receive routine colorectal cancer screening,” senior investigator Yin Cao, ScD, with Washington University School of Medicine, St. Louis, said in a news release.
“It’s also crucial to spread awareness among primary care doctors, gastroenterologists, and emergency medicine doctors,” Dr. Cao added. “To date, many early-onset colorectal cancers are detected in emergency rooms, and there often are significant diagnostic delays with this cancer.”
The study was published online in the Journal of the National Cancer Institute.
Although previous research has identified rectal bleeding, iron-deficiency anemia, and rectal/abdominal pain as symptoms of early-onset CRC, most studies “have aggregated symptoms till the time of diagnosis,” which limits their use for early detection, the authors explain.
In the current study, the researchers analyzed data from more than 5,000 cases of early-onset CRC and from more than 22,000 control patients using the IBM MarketScan commercial database.
Dr. Cao and colleagues found that between 3 months and 2 years before diagnosis, abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia each indicated an increased risk for early-onset CRC.
Among patients with early-onset CRC, 19.3% presented with one or more of the four red flags between 3 months and 2 years prior to the index date; 15.6% had one symptom, and 3.7% had two or more.
After multivariable adjustment, having one symptom almost doubled the risk for early-onset CRC (odds ratio, 1.94); having two symptoms increased risk by more than threefold (OR, 3.59); and having three or more boosted the risk by more than 6.5-fold (OR, 6.52).
Abdominal pain was associated with a 34% higher risk of early-onset CRC (11.6% among case patients vs. 7.7% among controls; OR, 1.34).
Although not as common, rectal bleeding was associated with the highest odds for early-onset CRC (7.2% case patients vs. 1.3% controls; OR, 5.13).
The other predictive signs and symptoms included diarrhea (2.8% case patients vs. 1.4% controls; OR, 1.43) and iron-deficiency anemia (2.3% case patients vs. 0.9% controls; OR, 2.07).
No differences were observed by gender for each sign or symptom.
Among patients with a red-flag symptom who presented between 3 months and 2 years before diagnosis, for those with early-onset CRC, the median diagnostic interval was 8.7 months.
The researchers suggest that clinicians prioritize prompt diagnostic workups for patients younger than 50 who present with rectal bleeding and/or iron-deficiency anemia and that they also keep abdominal pain and diarrhea in mind as early symptoms.
Dr. Cao noted that since most early-onset CRC cases “have been and will continue to be diagnosed after symptom presentation, it is crucial to recognize these red-flag signs and symptoms promptly and conduct a diagnostic workup as soon as possible.
“By doing so, we can diagnose the disease earlier, which in turn can reduce the need for more aggressive treatment and improve patients’ quality of life and survival rates,” said Dr. Cao.
The study was supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article originally appeared on Medscape.com.
As the number of cases of early-onset colorectal cancer (CRC) diagnosed before age 50 continues to rise, early detection has become increasingly important.
The signs and symptoms are abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia.
Two symptoms in particular – rectal bleeding and iron-deficiency anemia – point to the need for timely endoscopy and follow-up, the researchers say.
“Colorectal cancer is not simply a disease affecting older people; we want younger adults to be aware of and act on these potentially very telling signs and symptoms – particularly because people under 50 are considered to be at low risk, and they don’t receive routine colorectal cancer screening,” senior investigator Yin Cao, ScD, with Washington University School of Medicine, St. Louis, said in a news release.
“It’s also crucial to spread awareness among primary care doctors, gastroenterologists, and emergency medicine doctors,” Dr. Cao added. “To date, many early-onset colorectal cancers are detected in emergency rooms, and there often are significant diagnostic delays with this cancer.”
The study was published online in the Journal of the National Cancer Institute.
Although previous research has identified rectal bleeding, iron-deficiency anemia, and rectal/abdominal pain as symptoms of early-onset CRC, most studies “have aggregated symptoms till the time of diagnosis,” which limits their use for early detection, the authors explain.
In the current study, the researchers analyzed data from more than 5,000 cases of early-onset CRC and from more than 22,000 control patients using the IBM MarketScan commercial database.
Dr. Cao and colleagues found that between 3 months and 2 years before diagnosis, abdominal pain, rectal bleeding, diarrhea, and iron-deficiency anemia each indicated an increased risk for early-onset CRC.
Among patients with early-onset CRC, 19.3% presented with one or more of the four red flags between 3 months and 2 years prior to the index date; 15.6% had one symptom, and 3.7% had two or more.
After multivariable adjustment, having one symptom almost doubled the risk for early-onset CRC (odds ratio, 1.94); having two symptoms increased risk by more than threefold (OR, 3.59); and having three or more boosted the risk by more than 6.5-fold (OR, 6.52).
Abdominal pain was associated with a 34% higher risk of early-onset CRC (11.6% among case patients vs. 7.7% among controls; OR, 1.34).
Although not as common, rectal bleeding was associated with the highest odds for early-onset CRC (7.2% case patients vs. 1.3% controls; OR, 5.13).
The other predictive signs and symptoms included diarrhea (2.8% case patients vs. 1.4% controls; OR, 1.43) and iron-deficiency anemia (2.3% case patients vs. 0.9% controls; OR, 2.07).
No differences were observed by gender for each sign or symptom.
Among patients with a red-flag symptom who presented between 3 months and 2 years before diagnosis, for those with early-onset CRC, the median diagnostic interval was 8.7 months.
The researchers suggest that clinicians prioritize prompt diagnostic workups for patients younger than 50 who present with rectal bleeding and/or iron-deficiency anemia and that they also keep abdominal pain and diarrhea in mind as early symptoms.
Dr. Cao noted that since most early-onset CRC cases “have been and will continue to be diagnosed after symptom presentation, it is crucial to recognize these red-flag signs and symptoms promptly and conduct a diagnostic workup as soon as possible.
“By doing so, we can diagnose the disease earlier, which in turn can reduce the need for more aggressive treatment and improve patients’ quality of life and survival rates,” said Dr. Cao.
The study was supported by grants from the National Institutes of Health. The authors declared no conflicts of interest.
A version of this article originally appeared on Medscape.com.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
T-DXd for HER2-low BC: Analysis confirms adverse effects
Interstitial lung disease (ILD) also remains a concern, and it’s not clear if retreatment after resolution is warranted.
In general, however, “T-DXd demonstrates a manageable safety profile consistent with prior reports. Results from this safety analysis continued to support its use as a new standard of care in patients with HER2-low metastatic breast cancer,” said report lead author Hope Rugo, MD, of the University of California, San Francisco, during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
T-DXd, an antibody-drug conjugate, received FDA approval in August 2022 for patients with HER2-low disease. The drug has been touted as “practice changing” and a “new standard of care.”
However, physicians have noted that the benefits of the drug come at the cost of significant adverse effects, including some that can cause hospitalization. There’s special concern about high-grade interstitial lung disease/pneumonitis, and an FDA boxed warning cautions clinicians about this possible side effect.
For the new analysis, researchers presented additional safety data from the industry-funded DESTINY-Breast04 trial, whose results was published in July 2022 in the New England Journal of Medicine. That study randomized 373 patients to T-DXd and 184 to physician’s choice of treatment. It found that, “among all patients, the median progression-free survival was 9.9 months in the trastuzumab deruxtecan group and 5.1 months in the physician’s choice group (hazard ratio for disease progression or death, 0.50; P < .001), and overall survival was 23.4 months and 16.8 months, respectively (hazard ratio for death, 0.64; P = .001).”
Exposure-adjusted incidence rates for any-grade treatment-emergent adverse events were lower for T-DXd versus physician’s choice of treatment (1.30 vs. 2.66). However, nausea and vomiting events were more than twice as common in patients who took T-DXd versus the physician’s choice (79.5% vs. 35.5%).
A total of 50.9% of patients in the T-DXd arm received antiemetic prophylaxis versus 37.2% in the physician’s choice arm. A single patient discontinued T-DXd treatment because of vomiting, and a single patient discontinued treatment because of nausea. No patients in the physician choice group discontinued treatment because of nausea or vomiting.
Neutropenia and febrile neutropenia were less frequent in the T-DXd arm versus physician’s choice (12.9% vs. 18.0% and 0.3% vs. 2.9%, respectively.)
ILD occurred in 45 patients (12.1%) of those in the T-DXd arm versus 1 (0.6%) in the physician choice arm. Ten patients of the patients in the T-DXd arm had not recovered by the data cutoff point.
Six patients with ILD were retreated following resolution; one discontinued because of an adverse event, two discontinued because of progressive disease, and three remained on the drug. “Given that there was only a small number of patients who were retreated with T-DXd, it’s difficult to make clinically meaningful conclusions on the effect of retreatment following grade IDL events that have resolved,” Dr. Rugo said.
In the big picture, “ILD pneumonitis remains an important identified risk and an adverse event of interest associated with T-DXd,” Dr. Rugo said. “It’s important that we adhere to management guidelines and updated toxicity management guidelines.”
In a discussion, Dr. Rugo said she prescribes three antiemetic drugs to help patients tolerate T-DXd therapy: “It makes a big difference. Anecdotally, it really has improved management of nausea. Start more and back down [as symptoms fade].”
Gustavo Werutsky, MD, PhD, of Moinhos de Vento Hospital, Porto Alegre, Brazil, the discussant for the presentation, also emphasized the importance of prevention and said he prescribes two or three prophylactic drugs. “In the beginning, we didn’t know these events were so important. A big part of the message is that patients from the beginning need to have a good prophylaxis for the nausea and vomiting.”
The researchers also presented a related report at the conference, an analysis of patient-reported outcomes from DESTINY-Breast02, a randomized phase 3 study of T-DXd (n = 406) versus physician’s choice of treatment (n = 202) in patients with HER2-positive metastatic breast cancer who were resistant/refractory to trastuzumab emtansine.
The analysis, led by Tanja Fehm, MD, of University Hospital Düsseldorf (Germany), found that the median time to definitive deterioration was longer with T-DXd versus the other arm per the EORTC QLQ-C30 global health status/quality of life score (14.1 vs. 5.9 months; HR, 0.56; 95% confidence interval, 0.44-0.71).
The studies were funded by Daiichi Sankyo and AstraZeneca, which make T-DXd. Dr. Hugo discloses relationships with Puma, NAPO, Blueprint, Scorpion Therapeutics, Merck, AstraZeneca, Gilead, Astellas, Daiichi Sankyo, F. Hoffmann–La Roche/Genentech, GlaxoSmithKline, Lilly, Novartis, OBI, Pfizer, Pionyr, Sermonix, Taiho Oncology, and Veru. Multiple other authors of both studies have various disclosures.
Interstitial lung disease (ILD) also remains a concern, and it’s not clear if retreatment after resolution is warranted.
In general, however, “T-DXd demonstrates a manageable safety profile consistent with prior reports. Results from this safety analysis continued to support its use as a new standard of care in patients with HER2-low metastatic breast cancer,” said report lead author Hope Rugo, MD, of the University of California, San Francisco, during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
T-DXd, an antibody-drug conjugate, received FDA approval in August 2022 for patients with HER2-low disease. The drug has been touted as “practice changing” and a “new standard of care.”
However, physicians have noted that the benefits of the drug come at the cost of significant adverse effects, including some that can cause hospitalization. There’s special concern about high-grade interstitial lung disease/pneumonitis, and an FDA boxed warning cautions clinicians about this possible side effect.
For the new analysis, researchers presented additional safety data from the industry-funded DESTINY-Breast04 trial, whose results was published in July 2022 in the New England Journal of Medicine. That study randomized 373 patients to T-DXd and 184 to physician’s choice of treatment. It found that, “among all patients, the median progression-free survival was 9.9 months in the trastuzumab deruxtecan group and 5.1 months in the physician’s choice group (hazard ratio for disease progression or death, 0.50; P < .001), and overall survival was 23.4 months and 16.8 months, respectively (hazard ratio for death, 0.64; P = .001).”
Exposure-adjusted incidence rates for any-grade treatment-emergent adverse events were lower for T-DXd versus physician’s choice of treatment (1.30 vs. 2.66). However, nausea and vomiting events were more than twice as common in patients who took T-DXd versus the physician’s choice (79.5% vs. 35.5%).
A total of 50.9% of patients in the T-DXd arm received antiemetic prophylaxis versus 37.2% in the physician’s choice arm. A single patient discontinued T-DXd treatment because of vomiting, and a single patient discontinued treatment because of nausea. No patients in the physician choice group discontinued treatment because of nausea or vomiting.
Neutropenia and febrile neutropenia were less frequent in the T-DXd arm versus physician’s choice (12.9% vs. 18.0% and 0.3% vs. 2.9%, respectively.)
ILD occurred in 45 patients (12.1%) of those in the T-DXd arm versus 1 (0.6%) in the physician choice arm. Ten patients of the patients in the T-DXd arm had not recovered by the data cutoff point.
Six patients with ILD were retreated following resolution; one discontinued because of an adverse event, two discontinued because of progressive disease, and three remained on the drug. “Given that there was only a small number of patients who were retreated with T-DXd, it’s difficult to make clinically meaningful conclusions on the effect of retreatment following grade IDL events that have resolved,” Dr. Rugo said.
In the big picture, “ILD pneumonitis remains an important identified risk and an adverse event of interest associated with T-DXd,” Dr. Rugo said. “It’s important that we adhere to management guidelines and updated toxicity management guidelines.”
In a discussion, Dr. Rugo said she prescribes three antiemetic drugs to help patients tolerate T-DXd therapy: “It makes a big difference. Anecdotally, it really has improved management of nausea. Start more and back down [as symptoms fade].”
Gustavo Werutsky, MD, PhD, of Moinhos de Vento Hospital, Porto Alegre, Brazil, the discussant for the presentation, also emphasized the importance of prevention and said he prescribes two or three prophylactic drugs. “In the beginning, we didn’t know these events were so important. A big part of the message is that patients from the beginning need to have a good prophylaxis for the nausea and vomiting.”
The researchers also presented a related report at the conference, an analysis of patient-reported outcomes from DESTINY-Breast02, a randomized phase 3 study of T-DXd (n = 406) versus physician’s choice of treatment (n = 202) in patients with HER2-positive metastatic breast cancer who were resistant/refractory to trastuzumab emtansine.
The analysis, led by Tanja Fehm, MD, of University Hospital Düsseldorf (Germany), found that the median time to definitive deterioration was longer with T-DXd versus the other arm per the EORTC QLQ-C30 global health status/quality of life score (14.1 vs. 5.9 months; HR, 0.56; 95% confidence interval, 0.44-0.71).
The studies were funded by Daiichi Sankyo and AstraZeneca, which make T-DXd. Dr. Hugo discloses relationships with Puma, NAPO, Blueprint, Scorpion Therapeutics, Merck, AstraZeneca, Gilead, Astellas, Daiichi Sankyo, F. Hoffmann–La Roche/Genentech, GlaxoSmithKline, Lilly, Novartis, OBI, Pfizer, Pionyr, Sermonix, Taiho Oncology, and Veru. Multiple other authors of both studies have various disclosures.
Interstitial lung disease (ILD) also remains a concern, and it’s not clear if retreatment after resolution is warranted.
In general, however, “T-DXd demonstrates a manageable safety profile consistent with prior reports. Results from this safety analysis continued to support its use as a new standard of care in patients with HER2-low metastatic breast cancer,” said report lead author Hope Rugo, MD, of the University of California, San Francisco, during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
T-DXd, an antibody-drug conjugate, received FDA approval in August 2022 for patients with HER2-low disease. The drug has been touted as “practice changing” and a “new standard of care.”
However, physicians have noted that the benefits of the drug come at the cost of significant adverse effects, including some that can cause hospitalization. There’s special concern about high-grade interstitial lung disease/pneumonitis, and an FDA boxed warning cautions clinicians about this possible side effect.
For the new analysis, researchers presented additional safety data from the industry-funded DESTINY-Breast04 trial, whose results was published in July 2022 in the New England Journal of Medicine. That study randomized 373 patients to T-DXd and 184 to physician’s choice of treatment. It found that, “among all patients, the median progression-free survival was 9.9 months in the trastuzumab deruxtecan group and 5.1 months in the physician’s choice group (hazard ratio for disease progression or death, 0.50; P < .001), and overall survival was 23.4 months and 16.8 months, respectively (hazard ratio for death, 0.64; P = .001).”
Exposure-adjusted incidence rates for any-grade treatment-emergent adverse events were lower for T-DXd versus physician’s choice of treatment (1.30 vs. 2.66). However, nausea and vomiting events were more than twice as common in patients who took T-DXd versus the physician’s choice (79.5% vs. 35.5%).
A total of 50.9% of patients in the T-DXd arm received antiemetic prophylaxis versus 37.2% in the physician’s choice arm. A single patient discontinued T-DXd treatment because of vomiting, and a single patient discontinued treatment because of nausea. No patients in the physician choice group discontinued treatment because of nausea or vomiting.
Neutropenia and febrile neutropenia were less frequent in the T-DXd arm versus physician’s choice (12.9% vs. 18.0% and 0.3% vs. 2.9%, respectively.)
ILD occurred in 45 patients (12.1%) of those in the T-DXd arm versus 1 (0.6%) in the physician choice arm. Ten patients of the patients in the T-DXd arm had not recovered by the data cutoff point.
Six patients with ILD were retreated following resolution; one discontinued because of an adverse event, two discontinued because of progressive disease, and three remained on the drug. “Given that there was only a small number of patients who were retreated with T-DXd, it’s difficult to make clinically meaningful conclusions on the effect of retreatment following grade IDL events that have resolved,” Dr. Rugo said.
In the big picture, “ILD pneumonitis remains an important identified risk and an adverse event of interest associated with T-DXd,” Dr. Rugo said. “It’s important that we adhere to management guidelines and updated toxicity management guidelines.”
In a discussion, Dr. Rugo said she prescribes three antiemetic drugs to help patients tolerate T-DXd therapy: “It makes a big difference. Anecdotally, it really has improved management of nausea. Start more and back down [as symptoms fade].”
Gustavo Werutsky, MD, PhD, of Moinhos de Vento Hospital, Porto Alegre, Brazil, the discussant for the presentation, also emphasized the importance of prevention and said he prescribes two or three prophylactic drugs. “In the beginning, we didn’t know these events were so important. A big part of the message is that patients from the beginning need to have a good prophylaxis for the nausea and vomiting.”
The researchers also presented a related report at the conference, an analysis of patient-reported outcomes from DESTINY-Breast02, a randomized phase 3 study of T-DXd (n = 406) versus physician’s choice of treatment (n = 202) in patients with HER2-positive metastatic breast cancer who were resistant/refractory to trastuzumab emtansine.
The analysis, led by Tanja Fehm, MD, of University Hospital Düsseldorf (Germany), found that the median time to definitive deterioration was longer with T-DXd versus the other arm per the EORTC QLQ-C30 global health status/quality of life score (14.1 vs. 5.9 months; HR, 0.56; 95% confidence interval, 0.44-0.71).
The studies were funded by Daiichi Sankyo and AstraZeneca, which make T-DXd. Dr. Hugo discloses relationships with Puma, NAPO, Blueprint, Scorpion Therapeutics, Merck, AstraZeneca, Gilead, Astellas, Daiichi Sankyo, F. Hoffmann–La Roche/Genentech, GlaxoSmithKline, Lilly, Novartis, OBI, Pfizer, Pionyr, Sermonix, Taiho Oncology, and Veru. Multiple other authors of both studies have various disclosures.
FROM ESMO BREAST CANCER 2023
BMI has greater impact on survival in younger breast cancer patients
new data suggest.
Obesity is a well-known risk factor for breast cancer in postmenopausal women and has been associated with adverse prognosis, said Senna W.M. Lammers, MD, of Maastricht (the Netherlands) University during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. In addition, some studies suggest that patients with higher body mass index (BMI) experience reduced benefits from endocrine therapy, she said.
Dr. Lammers and colleagues conducted a study to determine the prognostic and predictive effect of BMI on disease-free survival in postmenopausal women with hormone receptor–positive (HR+) breast cancer who were treated with extended endocrine therapy.
The study population included participants in the randomized, phase III DATA trial, which evaluated the use of 6 years vs. 3 years of anastrozole in postmenopausal women with HR+ breast cancer who were disease-free after 2-3 years of adjuvant tamoxifen therapy.
Patients were categorized based on BMI as having normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), or obese (30 kg/m2 or higher). The primary outcome was disease-free survival (DFS); the median follow-up period was 13.1 years.
DFS for patients with normal weight, overweight, and obesity was 66.2%, 59.5%, and 52.4%, with a P value of less than .001 for the trend, Dr. Lammers said. “These results were confirmed in multivariable analysis,” she said. Overall, patients with overweight and obesity had a worse DFS when compared with patients with normal weight (hazard ratio, 1.16; P = .10, for patients with overweight and HR, 1.26; P = .03 for patients with obesity).
“Next, we aimed to determine whether the prognostic effect of BMI differed by age,” Dr. Lammers said.
In women younger than 60 years, overweight and obesity were significantly associated with worse DFS (HR, 1.29; P = .05 and HR 1.83, P less than .001, respectively). However, this effect was not observed in women aged 60 years and older.
The researchers also examined the treatment effect of extended anastrozole on adapted DFS by weight, and found no significant differences among patients with normal weight, overweight, and obesity (HR, 1.00; HR, 0.74; and HR, 0.97, respectively), said Dr. Lammers.
In the question and answer session, Dr. Lammers was asked about possible explanations for the difference in DFS by age. Potential explanations include possible survival bias “as only the healthier [patients with obesity] survive to old age,” she said. Other potential explanations are biological, such as the potentially higher levels of bone density in older [patients with obesity], she said.
When asked about additional clinical implications, Dr. Lammers emphasized the importance of maintaining a healthy BMI for breast cancer patients of all ages. Other research areas might involve the use of lifestyle interventions, although these are challenging to implement, she noted.
Data draw attention to quality of life and lifestyle factors
The need to “look at drug development with new eyes” is particularly important when reviewing patient-reported outcomes, said Otto Metzger, MD, of the Dana Farber Cancer Institute, Boston, who served as the discussant for the session.
Dr. Metzger brought up the association between age and the effect of BMI on DFS, specifically.
Based on data from multiple studies and meta-analyses, “I do believe that obesity does play a role in prognosis,” he said, but the question is how long will researchers continue to simply record data without acting to add lifestyle interventions while also trying to develop new drugs, he said. Although convincing patients to make lifestyle changes remains a challenge, patients are often more motivated to make such changes after a cancer diagnosis, Dr. Metzger noted.
“I am a firm believer in the use of digital therapeutics in the context of clinical trials,” said Dr. Metzger. Digital technology offers great potential to educate patients on [adverse effects] and also to improve treatment adherence and quality of life, he concluded.
The study was supported by AstraZeneca, and Dr. Lammers disclosed financial relationships with AstraZeneca and Eli Lilly. Dr. Metzger disclosed receiving research funding to his institution from Pfizer, Genentech/Roche, and Sanofi, and serving as an adviser/consultant to AstraZeneca, Merck, Oncoclinicas, Resilience, and Roche.
new data suggest.
Obesity is a well-known risk factor for breast cancer in postmenopausal women and has been associated with adverse prognosis, said Senna W.M. Lammers, MD, of Maastricht (the Netherlands) University during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. In addition, some studies suggest that patients with higher body mass index (BMI) experience reduced benefits from endocrine therapy, she said.
Dr. Lammers and colleagues conducted a study to determine the prognostic and predictive effect of BMI on disease-free survival in postmenopausal women with hormone receptor–positive (HR+) breast cancer who were treated with extended endocrine therapy.
The study population included participants in the randomized, phase III DATA trial, which evaluated the use of 6 years vs. 3 years of anastrozole in postmenopausal women with HR+ breast cancer who were disease-free after 2-3 years of adjuvant tamoxifen therapy.
Patients were categorized based on BMI as having normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), or obese (30 kg/m2 or higher). The primary outcome was disease-free survival (DFS); the median follow-up period was 13.1 years.
DFS for patients with normal weight, overweight, and obesity was 66.2%, 59.5%, and 52.4%, with a P value of less than .001 for the trend, Dr. Lammers said. “These results were confirmed in multivariable analysis,” she said. Overall, patients with overweight and obesity had a worse DFS when compared with patients with normal weight (hazard ratio, 1.16; P = .10, for patients with overweight and HR, 1.26; P = .03 for patients with obesity).
“Next, we aimed to determine whether the prognostic effect of BMI differed by age,” Dr. Lammers said.
In women younger than 60 years, overweight and obesity were significantly associated with worse DFS (HR, 1.29; P = .05 and HR 1.83, P less than .001, respectively). However, this effect was not observed in women aged 60 years and older.
The researchers also examined the treatment effect of extended anastrozole on adapted DFS by weight, and found no significant differences among patients with normal weight, overweight, and obesity (HR, 1.00; HR, 0.74; and HR, 0.97, respectively), said Dr. Lammers.
In the question and answer session, Dr. Lammers was asked about possible explanations for the difference in DFS by age. Potential explanations include possible survival bias “as only the healthier [patients with obesity] survive to old age,” she said. Other potential explanations are biological, such as the potentially higher levels of bone density in older [patients with obesity], she said.
When asked about additional clinical implications, Dr. Lammers emphasized the importance of maintaining a healthy BMI for breast cancer patients of all ages. Other research areas might involve the use of lifestyle interventions, although these are challenging to implement, she noted.
Data draw attention to quality of life and lifestyle factors
The need to “look at drug development with new eyes” is particularly important when reviewing patient-reported outcomes, said Otto Metzger, MD, of the Dana Farber Cancer Institute, Boston, who served as the discussant for the session.
Dr. Metzger brought up the association between age and the effect of BMI on DFS, specifically.
Based on data from multiple studies and meta-analyses, “I do believe that obesity does play a role in prognosis,” he said, but the question is how long will researchers continue to simply record data without acting to add lifestyle interventions while also trying to develop new drugs, he said. Although convincing patients to make lifestyle changes remains a challenge, patients are often more motivated to make such changes after a cancer diagnosis, Dr. Metzger noted.
“I am a firm believer in the use of digital therapeutics in the context of clinical trials,” said Dr. Metzger. Digital technology offers great potential to educate patients on [adverse effects] and also to improve treatment adherence and quality of life, he concluded.
The study was supported by AstraZeneca, and Dr. Lammers disclosed financial relationships with AstraZeneca and Eli Lilly. Dr. Metzger disclosed receiving research funding to his institution from Pfizer, Genentech/Roche, and Sanofi, and serving as an adviser/consultant to AstraZeneca, Merck, Oncoclinicas, Resilience, and Roche.
new data suggest.
Obesity is a well-known risk factor for breast cancer in postmenopausal women and has been associated with adverse prognosis, said Senna W.M. Lammers, MD, of Maastricht (the Netherlands) University during a presentation at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. In addition, some studies suggest that patients with higher body mass index (BMI) experience reduced benefits from endocrine therapy, she said.
Dr. Lammers and colleagues conducted a study to determine the prognostic and predictive effect of BMI on disease-free survival in postmenopausal women with hormone receptor–positive (HR+) breast cancer who were treated with extended endocrine therapy.
The study population included participants in the randomized, phase III DATA trial, which evaluated the use of 6 years vs. 3 years of anastrozole in postmenopausal women with HR+ breast cancer who were disease-free after 2-3 years of adjuvant tamoxifen therapy.
Patients were categorized based on BMI as having normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), or obese (30 kg/m2 or higher). The primary outcome was disease-free survival (DFS); the median follow-up period was 13.1 years.
DFS for patients with normal weight, overweight, and obesity was 66.2%, 59.5%, and 52.4%, with a P value of less than .001 for the trend, Dr. Lammers said. “These results were confirmed in multivariable analysis,” she said. Overall, patients with overweight and obesity had a worse DFS when compared with patients with normal weight (hazard ratio, 1.16; P = .10, for patients with overweight and HR, 1.26; P = .03 for patients with obesity).
“Next, we aimed to determine whether the prognostic effect of BMI differed by age,” Dr. Lammers said.
In women younger than 60 years, overweight and obesity were significantly associated with worse DFS (HR, 1.29; P = .05 and HR 1.83, P less than .001, respectively). However, this effect was not observed in women aged 60 years and older.
The researchers also examined the treatment effect of extended anastrozole on adapted DFS by weight, and found no significant differences among patients with normal weight, overweight, and obesity (HR, 1.00; HR, 0.74; and HR, 0.97, respectively), said Dr. Lammers.
In the question and answer session, Dr. Lammers was asked about possible explanations for the difference in DFS by age. Potential explanations include possible survival bias “as only the healthier [patients with obesity] survive to old age,” she said. Other potential explanations are biological, such as the potentially higher levels of bone density in older [patients with obesity], she said.
When asked about additional clinical implications, Dr. Lammers emphasized the importance of maintaining a healthy BMI for breast cancer patients of all ages. Other research areas might involve the use of lifestyle interventions, although these are challenging to implement, she noted.
Data draw attention to quality of life and lifestyle factors
The need to “look at drug development with new eyes” is particularly important when reviewing patient-reported outcomes, said Otto Metzger, MD, of the Dana Farber Cancer Institute, Boston, who served as the discussant for the session.
Dr. Metzger brought up the association between age and the effect of BMI on DFS, specifically.
Based on data from multiple studies and meta-analyses, “I do believe that obesity does play a role in prognosis,” he said, but the question is how long will researchers continue to simply record data without acting to add lifestyle interventions while also trying to develop new drugs, he said. Although convincing patients to make lifestyle changes remains a challenge, patients are often more motivated to make such changes after a cancer diagnosis, Dr. Metzger noted.
“I am a firm believer in the use of digital therapeutics in the context of clinical trials,” said Dr. Metzger. Digital technology offers great potential to educate patients on [adverse effects] and also to improve treatment adherence and quality of life, he concluded.
The study was supported by AstraZeneca, and Dr. Lammers disclosed financial relationships with AstraZeneca and Eli Lilly. Dr. Metzger disclosed receiving research funding to his institution from Pfizer, Genentech/Roche, and Sanofi, and serving as an adviser/consultant to AstraZeneca, Merck, Oncoclinicas, Resilience, and Roche.
FROM ESMO BREAST CANCER 2023
CLL: Venetoclax combos top first-line chemoimmunotherapy
according to a phase 3 trial published in the New England Journal of Medicine.
The trial, dubbed GAIA–CLL13, “is a remarkable demonstration of the quality of fixed-duration therapies for younger, fit patients, and it challenges us to continue to work to develop therapeutic strategies that will ultimately cure patients with CLL,” two hematologic cancer specialists said in an accompanying editorial.
In short, “venetoclax-obinutuzumab and venetoclax-obinutuzumab-ibrutinib were superior to chemoimmunotherapy with respect to both the minimal residual disease end point and progression-free survival, but venetoclax-rituximab was not,” Jennifer Woyach, MD, of Ohio State University, Columbus, and John Byrd, MD, University of Cincinnati, said in their commentary.
Noting that randomized trials involving venetoclax combinations in fit CLL patients “have been lacking,” the investigators compared 6 cycles of chemoimmunotherapy (fludarabine-cyclophosphamide-rituximab or bendamustine-rituximab) with 12 cycles of venetoclax plus the anti-CD20 antibody rituximab, venetoclax plus the third generation anti-CD20 antibody obinutuzumab, and venetoclax combined with both obinutuzumab and the Bruton’s tyrosine kinase inhibitor ibrutinib in a novel triple-therapy regimen.
The 926 patients in the study were a mean of 61 years old and split about evenly among the four treatment arms. Ibrutinib was discontinued after two consecutive measurements if patients had undetectable minimal residual disease (uMRD). Subjects did not have TP53 aberrations, a marker of poor prognosis in CLL.
At 15 months, the percentage of patients with uMRD was significantly higher in the triple-therapy arm (92.2%) and the venetoclax-obinutuzumab group (86.5%) than in the chemoimmunotherapy group (52.0%), but there was no statistical difference with venetoclax-rituximab (57%, P = .32).
The three-year progression-free survival (PFS) was 90.5% in the triple-therapy arm versus 87.7% with venetoclax-obinutuzumab. The 3-year PFS with venetoclax-rituximab (80.8%) was again not statistically different than the 75.5% with chemoimmunotherapy (P = .18).
Not ready for prime time
The benefits of triple therapy and venetoclax-obinutuzumab held only in patients with unmutated IgVH. “The high efficacy of the fludarabine, cyclophosphamide, and rituximab regimen in young, fit patients with mutated IgVH may be difficult to improve on,” noted the investigators, led by Barbara Eichhorst, MD, a hematologic malignancy specialist at the University of Cologne (Germany).
Also, although triple-therapy results were impressive, some of the benefits “are neutralized by the need for dose reductions and early treatment discontinuation owing to adverse events,” they said.
For instance, triple therapy had the highest incidence of both grade 3 and 4 infections (21.2%) and atrial fibrillation (7.8%).
The editorialists noted that there has been “a flurry of interest” in trials combining ibrutinib and venetoclax – as was done in the triple-therapy arm – since both emerged as powerful tools against CLL in recent years. However, even with the study results, they said “the use of triplet therapy should be viewed as investigational.”
For one thing, rates of uMRD were not “dramatically different” between triple therapy and venetoclax-obinutuzumab, and longer follow-up is better gauge differences in PFS and long-term toxicities.
Also, ibrutinib is being eclipsed by the second-generation Bruton’s tyrosine kinase inhibitors acalabrutinib and zanubrutinib, because they have better safety profiles, and they are being assessed in CLL combination trials. For now, there are too many unknowns for routine use of triple therapy in fit CLL patients, they said.
The investigators and editorialists both noted that improved uMRD in the study translated into superior PFS, raising the possibility that uMRD might be a valid alternative endpoint to PFS in CLL trials.
With “median remissions in CLL lasting far in excess of 5 years, designing studies that take 8-10 years” to reach a PFS endpoint is simply too slow. Moving to an alternative endpoint such a uMRD would preserve “the momentum that has been generated” with recent advances, Dr. Woyach and Dr. Byrd said.
The work was funded by the companies that market venetoclax, ibrutinib, and obinutuzumab: AbbVie, Janssen, and Roche. Dr. Eichhorst is a consultant and/or speaker for the companies and also reported grants from them. Dr. Byrd is a consultant/adviser for Eilean Therapeutics, Kurome Therapeutics, Newave, and Orbimed. Dr. Woyach disclosed ties with AbbVie, AstraZeneca, Lilly, and other companies.
according to a phase 3 trial published in the New England Journal of Medicine.
The trial, dubbed GAIA–CLL13, “is a remarkable demonstration of the quality of fixed-duration therapies for younger, fit patients, and it challenges us to continue to work to develop therapeutic strategies that will ultimately cure patients with CLL,” two hematologic cancer specialists said in an accompanying editorial.
In short, “venetoclax-obinutuzumab and venetoclax-obinutuzumab-ibrutinib were superior to chemoimmunotherapy with respect to both the minimal residual disease end point and progression-free survival, but venetoclax-rituximab was not,” Jennifer Woyach, MD, of Ohio State University, Columbus, and John Byrd, MD, University of Cincinnati, said in their commentary.
Noting that randomized trials involving venetoclax combinations in fit CLL patients “have been lacking,” the investigators compared 6 cycles of chemoimmunotherapy (fludarabine-cyclophosphamide-rituximab or bendamustine-rituximab) with 12 cycles of venetoclax plus the anti-CD20 antibody rituximab, venetoclax plus the third generation anti-CD20 antibody obinutuzumab, and venetoclax combined with both obinutuzumab and the Bruton’s tyrosine kinase inhibitor ibrutinib in a novel triple-therapy regimen.
The 926 patients in the study were a mean of 61 years old and split about evenly among the four treatment arms. Ibrutinib was discontinued after two consecutive measurements if patients had undetectable minimal residual disease (uMRD). Subjects did not have TP53 aberrations, a marker of poor prognosis in CLL.
At 15 months, the percentage of patients with uMRD was significantly higher in the triple-therapy arm (92.2%) and the venetoclax-obinutuzumab group (86.5%) than in the chemoimmunotherapy group (52.0%), but there was no statistical difference with venetoclax-rituximab (57%, P = .32).
The three-year progression-free survival (PFS) was 90.5% in the triple-therapy arm versus 87.7% with venetoclax-obinutuzumab. The 3-year PFS with venetoclax-rituximab (80.8%) was again not statistically different than the 75.5% with chemoimmunotherapy (P = .18).
Not ready for prime time
The benefits of triple therapy and venetoclax-obinutuzumab held only in patients with unmutated IgVH. “The high efficacy of the fludarabine, cyclophosphamide, and rituximab regimen in young, fit patients with mutated IgVH may be difficult to improve on,” noted the investigators, led by Barbara Eichhorst, MD, a hematologic malignancy specialist at the University of Cologne (Germany).
Also, although triple-therapy results were impressive, some of the benefits “are neutralized by the need for dose reductions and early treatment discontinuation owing to adverse events,” they said.
For instance, triple therapy had the highest incidence of both grade 3 and 4 infections (21.2%) and atrial fibrillation (7.8%).
The editorialists noted that there has been “a flurry of interest” in trials combining ibrutinib and venetoclax – as was done in the triple-therapy arm – since both emerged as powerful tools against CLL in recent years. However, even with the study results, they said “the use of triplet therapy should be viewed as investigational.”
For one thing, rates of uMRD were not “dramatically different” between triple therapy and venetoclax-obinutuzumab, and longer follow-up is better gauge differences in PFS and long-term toxicities.
Also, ibrutinib is being eclipsed by the second-generation Bruton’s tyrosine kinase inhibitors acalabrutinib and zanubrutinib, because they have better safety profiles, and they are being assessed in CLL combination trials. For now, there are too many unknowns for routine use of triple therapy in fit CLL patients, they said.
The investigators and editorialists both noted that improved uMRD in the study translated into superior PFS, raising the possibility that uMRD might be a valid alternative endpoint to PFS in CLL trials.
With “median remissions in CLL lasting far in excess of 5 years, designing studies that take 8-10 years” to reach a PFS endpoint is simply too slow. Moving to an alternative endpoint such a uMRD would preserve “the momentum that has been generated” with recent advances, Dr. Woyach and Dr. Byrd said.
The work was funded by the companies that market venetoclax, ibrutinib, and obinutuzumab: AbbVie, Janssen, and Roche. Dr. Eichhorst is a consultant and/or speaker for the companies and also reported grants from them. Dr. Byrd is a consultant/adviser for Eilean Therapeutics, Kurome Therapeutics, Newave, and Orbimed. Dr. Woyach disclosed ties with AbbVie, AstraZeneca, Lilly, and other companies.
according to a phase 3 trial published in the New England Journal of Medicine.
The trial, dubbed GAIA–CLL13, “is a remarkable demonstration of the quality of fixed-duration therapies for younger, fit patients, and it challenges us to continue to work to develop therapeutic strategies that will ultimately cure patients with CLL,” two hematologic cancer specialists said in an accompanying editorial.
In short, “venetoclax-obinutuzumab and venetoclax-obinutuzumab-ibrutinib were superior to chemoimmunotherapy with respect to both the minimal residual disease end point and progression-free survival, but venetoclax-rituximab was not,” Jennifer Woyach, MD, of Ohio State University, Columbus, and John Byrd, MD, University of Cincinnati, said in their commentary.
Noting that randomized trials involving venetoclax combinations in fit CLL patients “have been lacking,” the investigators compared 6 cycles of chemoimmunotherapy (fludarabine-cyclophosphamide-rituximab or bendamustine-rituximab) with 12 cycles of venetoclax plus the anti-CD20 antibody rituximab, venetoclax plus the third generation anti-CD20 antibody obinutuzumab, and venetoclax combined with both obinutuzumab and the Bruton’s tyrosine kinase inhibitor ibrutinib in a novel triple-therapy regimen.
The 926 patients in the study were a mean of 61 years old and split about evenly among the four treatment arms. Ibrutinib was discontinued after two consecutive measurements if patients had undetectable minimal residual disease (uMRD). Subjects did not have TP53 aberrations, a marker of poor prognosis in CLL.
At 15 months, the percentage of patients with uMRD was significantly higher in the triple-therapy arm (92.2%) and the venetoclax-obinutuzumab group (86.5%) than in the chemoimmunotherapy group (52.0%), but there was no statistical difference with venetoclax-rituximab (57%, P = .32).
The three-year progression-free survival (PFS) was 90.5% in the triple-therapy arm versus 87.7% with venetoclax-obinutuzumab. The 3-year PFS with venetoclax-rituximab (80.8%) was again not statistically different than the 75.5% with chemoimmunotherapy (P = .18).
Not ready for prime time
The benefits of triple therapy and venetoclax-obinutuzumab held only in patients with unmutated IgVH. “The high efficacy of the fludarabine, cyclophosphamide, and rituximab regimen in young, fit patients with mutated IgVH may be difficult to improve on,” noted the investigators, led by Barbara Eichhorst, MD, a hematologic malignancy specialist at the University of Cologne (Germany).
Also, although triple-therapy results were impressive, some of the benefits “are neutralized by the need for dose reductions and early treatment discontinuation owing to adverse events,” they said.
For instance, triple therapy had the highest incidence of both grade 3 and 4 infections (21.2%) and atrial fibrillation (7.8%).
The editorialists noted that there has been “a flurry of interest” in trials combining ibrutinib and venetoclax – as was done in the triple-therapy arm – since both emerged as powerful tools against CLL in recent years. However, even with the study results, they said “the use of triplet therapy should be viewed as investigational.”
For one thing, rates of uMRD were not “dramatically different” between triple therapy and venetoclax-obinutuzumab, and longer follow-up is better gauge differences in PFS and long-term toxicities.
Also, ibrutinib is being eclipsed by the second-generation Bruton’s tyrosine kinase inhibitors acalabrutinib and zanubrutinib, because they have better safety profiles, and they are being assessed in CLL combination trials. For now, there are too many unknowns for routine use of triple therapy in fit CLL patients, they said.
The investigators and editorialists both noted that improved uMRD in the study translated into superior PFS, raising the possibility that uMRD might be a valid alternative endpoint to PFS in CLL trials.
With “median remissions in CLL lasting far in excess of 5 years, designing studies that take 8-10 years” to reach a PFS endpoint is simply too slow. Moving to an alternative endpoint such a uMRD would preserve “the momentum that has been generated” with recent advances, Dr. Woyach and Dr. Byrd said.
The work was funded by the companies that market venetoclax, ibrutinib, and obinutuzumab: AbbVie, Janssen, and Roche. Dr. Eichhorst is a consultant and/or speaker for the companies and also reported grants from them. Dr. Byrd is a consultant/adviser for Eilean Therapeutics, Kurome Therapeutics, Newave, and Orbimed. Dr. Woyach disclosed ties with AbbVie, AstraZeneca, Lilly, and other companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Number of cancer survivors with functional limitations doubled in 20 years
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
FROM JAMA ONCOLOGY
New AI tool may help predict best treatments for colorectal cancer
according to a new study published in Nature Communications.
Specifically, the tool can aid doctors in identifying a “molecular diagnosis” based on a patient’s tumor and cancer characteristics, Kun-Hsing Yu, MD, PhD, the study’s senior author and an assistant professor of biomedical informatics at Harvard Medical School, Boston, said in an interview.
The Multi-omics Multi-cohort Assessment (MOMA) “successfully identified indicators of how aggressive a tumor was and how likely it was to behave in response to a particular treatment,” as well as patients’ overall and disease-free survival, noted Harvard Medical School in a press release. “Based on an image alone, the model also pinpointed characteristics associated with the presence or absence of specific genetic mutations – something that typically requires genomic sequencing of the tumor.”
The researchers designed the tool to offer “transparent reasoning,” so that if a clinician asks it why it made a certain prediction, it would be able to explain its reasoning and the variables it used, the press release noted.
“We first allow AI to explore any correlation, and then we try to explain those correlations using existing pathology terms that experts will be able to understand,” Dr. Yu said in an interview.
Although the tool is freely available to clinicians and researchers, it’s not yet ready for clinical use. When it is, the tool has the potential to provide timely, accurate decision support based on tumor imaging.
Colorectal cancer is the second most common cause of death from cancer in the United States, with more than 53,000 deaths each year, and the patient population has been gradually skewing younger over the past 2 decades.
Although clinicians already use histopathology and genetic analysis to guide treatment, the process can take several days or weeks in some areas, and these services may not be available in all parts of the world.
“Currently, a clinician has to send a [tissue] sample from the tumor specimen to genomic sequencing labs and wait for a week, sometimes up to 3 or more weeks, to get genomic sequencing results,” Dr. Yu said. That means a patient’s anxiety grows as they wait to find out which treatments might benefit them or how they might respond to a particular treatment.
Additionally, current knowledge for predicting patient survival, beyond considering the patient’s cancer stage, age, and general health status, is limited, Dr. Yu said.
Predictive ability
The MOMA platform was trained on information from 1,888 patients with colorectal cancer from three national cohorts: 628 patients from The Cancer Genome Atlas (TCGA) program, 927 patients from the Nurses’ Health Study with Health Professionals Follow-Up Study (NHS-HPFS), and 333 patients from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
During the training, they fed the model information about the patients’ age, sex, cancer stage, and outcomes, as well as their tumors’ “multi-omic” information: the cancers’ genomic, epigenetic, protein, and metabolic profiles. Researchers showed the AI model digital, whole-slide histopathology images of tumor samples and asked it to look for visual markers related to tumor types, genetic mutations, epigenetic alterations, disease progression, and patient survival with the goal of enabling the platform to detect patterns that are indiscernible to the human eye.
They then tested the MOMA platform’s ability to interpret images by feeding it new tumor sample images from different patients and asking it to predict their survival and progression-free survival.
The researchers found that the tool successfully identified overall survival outcomes in patients with stage I or II cancer in the TCGA cohort, which they further validated with the NHS-HPFS and PLCO cohorts. The platform revealed that “dense clusters of adenocarcinoma cells are highly indicative of worse overall survival outcomes” and that the interaction of cancer cells with smooth muscle cells in cancerous areas predicted poorer overall survival.
MOMA was slightly more effective in predicting progression-free survival for stage I and stage II colorectal cancer across all three cohorts.
“Compared with the overall survival prediction, our progression-free survival model puts more emphasis on infiltrating lymphocytes and regions associated with extracellular mucin in its prediction,” the authors noted.
Prediction of overall survival and progression-free survival for stage III colorectal cancer showed similar levels of accuracy, they noted.
The tool also successfully assessed patients’ likely response to immunotherapy using predictions of microsatellite instability, since high MSI indicates a better response to immune checkpoint inhibitors.
MOMA outperformed a different machine-learning algorithm in predicting the copy number alterations and other features related to cancer development, and it predicted the likelihood of a BRAF mutation, which is linked to poorer prognosis.
Pushing the envelope?
MOMA presents an “intriguing new avenue of adding to how we think about and assess someone who has cancer,” Stacey Cohen, MD, an associate professor in the clinical research division of Fred Hutchinson Cancer Center at the University of Washington Medicine, Seattle, said in an interview.
However, the tool as it’s currently described appears primarily to duplicate what clinicians already are doing, which is considering a wide range of factors – including pathologic features, patient features and demographics, and the patient’s other medical illnesses – to develop a treatment plan within the context of current guidelines, noted Dr. Cohen, who was not involved in the project.
“I’m looking for these types of models to not just prognosticate an outcome but to really predict how someone should be treated, and to do that better than [using] standard clinical features,” Dr. Cohen said. “To some degree, they’re taking this AI model and trying to catch up to what we’re currently doing. Clearly, if they could do that, they can then push the envelope.”
Dr. Cohen acknowledged that a strength of using an AI platform is the speed at which it can provide its predictions in areas with few medical resources and few health care professionals – as long as the necessary imaging is available and physicians have a way to use the platform.
“On the one hand, I do see this as an opportunity to share the wealth of knowledge in a more rapid fashion, but I don’t think anybody is going to let a computer program dictate their treatment without a human medical oncologist being able to interpret that information,” Dr. Cohen said. “It still will require a lot of education by the users and not just by the people who are designing the study.”
Although the MOMA platform looked at multiple pathologic features in multiple cohorts, the results remain limited by the fact that the patients in those cohorts were treated decades ago, before many current treatments may have been available, Dr. Cohen said.
She also added that the cohorts did not have much ethnic diversity. In the NHS-HPFS, the largest cohort, 57% of the patients were White, and researchers lacked data on race for 42% of patients, so only about 1% of participants were of a known non-White race. Similarly, 47% of the TCGA patients were White and 41% had no data on race, leaving only 12% of patients from known, non-White racial backgrounds, including 10% Black or African American.
Additional studies that focus on specific patient populations are needed to evaluate the model’s applicability in clinical settings, the investigators note. More research is required to “identify the optimal prognostic prediction methods and enable personalized treatments and advance care planning,” they added.
These are the early days for this type of technology, Dr. Cohen noted.
“I’m very excited to see how this technology develops and how it could be potentially additive or improve upon our current treatment planning for patients,” she said.
Dr. Yu developed the invention “Quantitative Pathology Analysis and Diagnosis using Neural Networks,” whose patent is held by Harvard University, and has consulted for Curatio. One coauthor is a stakeholder and employee of Vertex Pharmaceuticals. The study’s funding sources included the National Institute of General Medical Sciences, the Google Research Scholar Award, the Blavatnik Center for Computational Biomedicine Award, the National Science and Technology Council Taiwan, and the National Center for High-performance Computing Taiwan. Dr. Cohen has advised or consulted for Natera.
A version of this article first appeared on Medscape.com.
according to a new study published in Nature Communications.
Specifically, the tool can aid doctors in identifying a “molecular diagnosis” based on a patient’s tumor and cancer characteristics, Kun-Hsing Yu, MD, PhD, the study’s senior author and an assistant professor of biomedical informatics at Harvard Medical School, Boston, said in an interview.
The Multi-omics Multi-cohort Assessment (MOMA) “successfully identified indicators of how aggressive a tumor was and how likely it was to behave in response to a particular treatment,” as well as patients’ overall and disease-free survival, noted Harvard Medical School in a press release. “Based on an image alone, the model also pinpointed characteristics associated with the presence or absence of specific genetic mutations – something that typically requires genomic sequencing of the tumor.”
The researchers designed the tool to offer “transparent reasoning,” so that if a clinician asks it why it made a certain prediction, it would be able to explain its reasoning and the variables it used, the press release noted.
“We first allow AI to explore any correlation, and then we try to explain those correlations using existing pathology terms that experts will be able to understand,” Dr. Yu said in an interview.
Although the tool is freely available to clinicians and researchers, it’s not yet ready for clinical use. When it is, the tool has the potential to provide timely, accurate decision support based on tumor imaging.
Colorectal cancer is the second most common cause of death from cancer in the United States, with more than 53,000 deaths each year, and the patient population has been gradually skewing younger over the past 2 decades.
Although clinicians already use histopathology and genetic analysis to guide treatment, the process can take several days or weeks in some areas, and these services may not be available in all parts of the world.
“Currently, a clinician has to send a [tissue] sample from the tumor specimen to genomic sequencing labs and wait for a week, sometimes up to 3 or more weeks, to get genomic sequencing results,” Dr. Yu said. That means a patient’s anxiety grows as they wait to find out which treatments might benefit them or how they might respond to a particular treatment.
Additionally, current knowledge for predicting patient survival, beyond considering the patient’s cancer stage, age, and general health status, is limited, Dr. Yu said.
Predictive ability
The MOMA platform was trained on information from 1,888 patients with colorectal cancer from three national cohorts: 628 patients from The Cancer Genome Atlas (TCGA) program, 927 patients from the Nurses’ Health Study with Health Professionals Follow-Up Study (NHS-HPFS), and 333 patients from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
During the training, they fed the model information about the patients’ age, sex, cancer stage, and outcomes, as well as their tumors’ “multi-omic” information: the cancers’ genomic, epigenetic, protein, and metabolic profiles. Researchers showed the AI model digital, whole-slide histopathology images of tumor samples and asked it to look for visual markers related to tumor types, genetic mutations, epigenetic alterations, disease progression, and patient survival with the goal of enabling the platform to detect patterns that are indiscernible to the human eye.
They then tested the MOMA platform’s ability to interpret images by feeding it new tumor sample images from different patients and asking it to predict their survival and progression-free survival.
The researchers found that the tool successfully identified overall survival outcomes in patients with stage I or II cancer in the TCGA cohort, which they further validated with the NHS-HPFS and PLCO cohorts. The platform revealed that “dense clusters of adenocarcinoma cells are highly indicative of worse overall survival outcomes” and that the interaction of cancer cells with smooth muscle cells in cancerous areas predicted poorer overall survival.
MOMA was slightly more effective in predicting progression-free survival for stage I and stage II colorectal cancer across all three cohorts.
“Compared with the overall survival prediction, our progression-free survival model puts more emphasis on infiltrating lymphocytes and regions associated with extracellular mucin in its prediction,” the authors noted.
Prediction of overall survival and progression-free survival for stage III colorectal cancer showed similar levels of accuracy, they noted.
The tool also successfully assessed patients’ likely response to immunotherapy using predictions of microsatellite instability, since high MSI indicates a better response to immune checkpoint inhibitors.
MOMA outperformed a different machine-learning algorithm in predicting the copy number alterations and other features related to cancer development, and it predicted the likelihood of a BRAF mutation, which is linked to poorer prognosis.
Pushing the envelope?
MOMA presents an “intriguing new avenue of adding to how we think about and assess someone who has cancer,” Stacey Cohen, MD, an associate professor in the clinical research division of Fred Hutchinson Cancer Center at the University of Washington Medicine, Seattle, said in an interview.
However, the tool as it’s currently described appears primarily to duplicate what clinicians already are doing, which is considering a wide range of factors – including pathologic features, patient features and demographics, and the patient’s other medical illnesses – to develop a treatment plan within the context of current guidelines, noted Dr. Cohen, who was not involved in the project.
“I’m looking for these types of models to not just prognosticate an outcome but to really predict how someone should be treated, and to do that better than [using] standard clinical features,” Dr. Cohen said. “To some degree, they’re taking this AI model and trying to catch up to what we’re currently doing. Clearly, if they could do that, they can then push the envelope.”
Dr. Cohen acknowledged that a strength of using an AI platform is the speed at which it can provide its predictions in areas with few medical resources and few health care professionals – as long as the necessary imaging is available and physicians have a way to use the platform.
“On the one hand, I do see this as an opportunity to share the wealth of knowledge in a more rapid fashion, but I don’t think anybody is going to let a computer program dictate their treatment without a human medical oncologist being able to interpret that information,” Dr. Cohen said. “It still will require a lot of education by the users and not just by the people who are designing the study.”
Although the MOMA platform looked at multiple pathologic features in multiple cohorts, the results remain limited by the fact that the patients in those cohorts were treated decades ago, before many current treatments may have been available, Dr. Cohen said.
She also added that the cohorts did not have much ethnic diversity. In the NHS-HPFS, the largest cohort, 57% of the patients were White, and researchers lacked data on race for 42% of patients, so only about 1% of participants were of a known non-White race. Similarly, 47% of the TCGA patients were White and 41% had no data on race, leaving only 12% of patients from known, non-White racial backgrounds, including 10% Black or African American.
Additional studies that focus on specific patient populations are needed to evaluate the model’s applicability in clinical settings, the investigators note. More research is required to “identify the optimal prognostic prediction methods and enable personalized treatments and advance care planning,” they added.
These are the early days for this type of technology, Dr. Cohen noted.
“I’m very excited to see how this technology develops and how it could be potentially additive or improve upon our current treatment planning for patients,” she said.
Dr. Yu developed the invention “Quantitative Pathology Analysis and Diagnosis using Neural Networks,” whose patent is held by Harvard University, and has consulted for Curatio. One coauthor is a stakeholder and employee of Vertex Pharmaceuticals. The study’s funding sources included the National Institute of General Medical Sciences, the Google Research Scholar Award, the Blavatnik Center for Computational Biomedicine Award, the National Science and Technology Council Taiwan, and the National Center for High-performance Computing Taiwan. Dr. Cohen has advised or consulted for Natera.
A version of this article first appeared on Medscape.com.
according to a new study published in Nature Communications.
Specifically, the tool can aid doctors in identifying a “molecular diagnosis” based on a patient’s tumor and cancer characteristics, Kun-Hsing Yu, MD, PhD, the study’s senior author and an assistant professor of biomedical informatics at Harvard Medical School, Boston, said in an interview.
The Multi-omics Multi-cohort Assessment (MOMA) “successfully identified indicators of how aggressive a tumor was and how likely it was to behave in response to a particular treatment,” as well as patients’ overall and disease-free survival, noted Harvard Medical School in a press release. “Based on an image alone, the model also pinpointed characteristics associated with the presence or absence of specific genetic mutations – something that typically requires genomic sequencing of the tumor.”
The researchers designed the tool to offer “transparent reasoning,” so that if a clinician asks it why it made a certain prediction, it would be able to explain its reasoning and the variables it used, the press release noted.
“We first allow AI to explore any correlation, and then we try to explain those correlations using existing pathology terms that experts will be able to understand,” Dr. Yu said in an interview.
Although the tool is freely available to clinicians and researchers, it’s not yet ready for clinical use. When it is, the tool has the potential to provide timely, accurate decision support based on tumor imaging.
Colorectal cancer is the second most common cause of death from cancer in the United States, with more than 53,000 deaths each year, and the patient population has been gradually skewing younger over the past 2 decades.
Although clinicians already use histopathology and genetic analysis to guide treatment, the process can take several days or weeks in some areas, and these services may not be available in all parts of the world.
“Currently, a clinician has to send a [tissue] sample from the tumor specimen to genomic sequencing labs and wait for a week, sometimes up to 3 or more weeks, to get genomic sequencing results,” Dr. Yu said. That means a patient’s anxiety grows as they wait to find out which treatments might benefit them or how they might respond to a particular treatment.
Additionally, current knowledge for predicting patient survival, beyond considering the patient’s cancer stage, age, and general health status, is limited, Dr. Yu said.
Predictive ability
The MOMA platform was trained on information from 1,888 patients with colorectal cancer from three national cohorts: 628 patients from The Cancer Genome Atlas (TCGA) program, 927 patients from the Nurses’ Health Study with Health Professionals Follow-Up Study (NHS-HPFS), and 333 patients from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
During the training, they fed the model information about the patients’ age, sex, cancer stage, and outcomes, as well as their tumors’ “multi-omic” information: the cancers’ genomic, epigenetic, protein, and metabolic profiles. Researchers showed the AI model digital, whole-slide histopathology images of tumor samples and asked it to look for visual markers related to tumor types, genetic mutations, epigenetic alterations, disease progression, and patient survival with the goal of enabling the platform to detect patterns that are indiscernible to the human eye.
They then tested the MOMA platform’s ability to interpret images by feeding it new tumor sample images from different patients and asking it to predict their survival and progression-free survival.
The researchers found that the tool successfully identified overall survival outcomes in patients with stage I or II cancer in the TCGA cohort, which they further validated with the NHS-HPFS and PLCO cohorts. The platform revealed that “dense clusters of adenocarcinoma cells are highly indicative of worse overall survival outcomes” and that the interaction of cancer cells with smooth muscle cells in cancerous areas predicted poorer overall survival.
MOMA was slightly more effective in predicting progression-free survival for stage I and stage II colorectal cancer across all three cohorts.
“Compared with the overall survival prediction, our progression-free survival model puts more emphasis on infiltrating lymphocytes and regions associated with extracellular mucin in its prediction,” the authors noted.
Prediction of overall survival and progression-free survival for stage III colorectal cancer showed similar levels of accuracy, they noted.
The tool also successfully assessed patients’ likely response to immunotherapy using predictions of microsatellite instability, since high MSI indicates a better response to immune checkpoint inhibitors.
MOMA outperformed a different machine-learning algorithm in predicting the copy number alterations and other features related to cancer development, and it predicted the likelihood of a BRAF mutation, which is linked to poorer prognosis.
Pushing the envelope?
MOMA presents an “intriguing new avenue of adding to how we think about and assess someone who has cancer,” Stacey Cohen, MD, an associate professor in the clinical research division of Fred Hutchinson Cancer Center at the University of Washington Medicine, Seattle, said in an interview.
However, the tool as it’s currently described appears primarily to duplicate what clinicians already are doing, which is considering a wide range of factors – including pathologic features, patient features and demographics, and the patient’s other medical illnesses – to develop a treatment plan within the context of current guidelines, noted Dr. Cohen, who was not involved in the project.
“I’m looking for these types of models to not just prognosticate an outcome but to really predict how someone should be treated, and to do that better than [using] standard clinical features,” Dr. Cohen said. “To some degree, they’re taking this AI model and trying to catch up to what we’re currently doing. Clearly, if they could do that, they can then push the envelope.”
Dr. Cohen acknowledged that a strength of using an AI platform is the speed at which it can provide its predictions in areas with few medical resources and few health care professionals – as long as the necessary imaging is available and physicians have a way to use the platform.
“On the one hand, I do see this as an opportunity to share the wealth of knowledge in a more rapid fashion, but I don’t think anybody is going to let a computer program dictate their treatment without a human medical oncologist being able to interpret that information,” Dr. Cohen said. “It still will require a lot of education by the users and not just by the people who are designing the study.”
Although the MOMA platform looked at multiple pathologic features in multiple cohorts, the results remain limited by the fact that the patients in those cohorts were treated decades ago, before many current treatments may have been available, Dr. Cohen said.
She also added that the cohorts did not have much ethnic diversity. In the NHS-HPFS, the largest cohort, 57% of the patients were White, and researchers lacked data on race for 42% of patients, so only about 1% of participants were of a known non-White race. Similarly, 47% of the TCGA patients were White and 41% had no data on race, leaving only 12% of patients from known, non-White racial backgrounds, including 10% Black or African American.
Additional studies that focus on specific patient populations are needed to evaluate the model’s applicability in clinical settings, the investigators note. More research is required to “identify the optimal prognostic prediction methods and enable personalized treatments and advance care planning,” they added.
These are the early days for this type of technology, Dr. Cohen noted.
“I’m very excited to see how this technology develops and how it could be potentially additive or improve upon our current treatment planning for patients,” she said.
Dr. Yu developed the invention “Quantitative Pathology Analysis and Diagnosis using Neural Networks,” whose patent is held by Harvard University, and has consulted for Curatio. One coauthor is a stakeholder and employee of Vertex Pharmaceuticals. The study’s funding sources included the National Institute of General Medical Sciences, the Google Research Scholar Award, the Blavatnik Center for Computational Biomedicine Award, the National Science and Technology Council Taiwan, and the National Center for High-performance Computing Taiwan. Dr. Cohen has advised or consulted for Natera.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Mohs surgery improves survival in early-stage Merkel cell carcinoma
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
AT ACMS 2023
Primary Hepatic Lymphoma: A Rare Form of Diffuse Large B-Cell Lymphoma of the Liver
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187