User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Sneak Peek: Journal of Hospital Medicine – July 2017
BACKGROUND: Medicare patients account for approximately 50% of hospital days. Hospitalization in older adults often results in poor outcomes.
OBJECTIVE: To test the feasibility and impact of using Assessing Care of Vulnerable Elders (ACOVE) quality indicators (QIs) as a therapeutic intervention to improve care of hospitalized older adults.
SETTING: Large tertiary hospital in the greater New York Metropolitan area.
PATIENTS: Hospitalized patients, 75 and over, admitted to medical units.
INTERVENTION: A checklist, comprised of four ACOVE QIs, administered during daily interdisciplinary rounds: venous thrombosis prophylaxis (VTE) (QI 1), indwelling bladder catheters (QI 2), mobilization (QI 3), and delirium evaluation (QI 4).
MEASUREMENTS: Variables were extracted from electronic medical records with QI compliance as the primary outcome, and length of stay (LOS), discharge disposition, and readmissions as secondary outcomes. Generalized linear mixed models for binary clustered data were used to estimate compliance rates for each group (intervention group or control group) in the postintervention period, along with their corresponding 95% confidence intervals.
RESULTS: Of the 2,396 patients, 530 were on an intervention unit. In those patients not already compliant with VTE, the compliance rate was 57% in intervention vs. 39% in control (P less than .0056). For indwelling catheters, mobilization, and delirium evaluation, overall compliance was significantly higher in the intervention group 72.2% vs. 54.4% (P = .1061), 62.9% vs. 48.2% (P less than .0001), and 27.9% vs. 21.7% (P = .0027), respectively.
CONCLUSIONS: The study demonstrates the feasibility and effectiveness of integrating ACOVE QIs to improve the quality of care in hospitalized older adults.
Also in JHM
Use of simulation to assess incoming interns’ recognition of opportunities to choose wisely
AUTHORS: Kathleen M. Wiest, Jeanne M. Farnan, MD, MHPE, Ellen Byrne, Lukas Matern, Melissa Cappaert, MA, Kristen Hirsch, Vineet M. Arora, MD, MAPP
Clinician attitudes regarding ICD deactivation in DNR/DNI patients
AUTHORS: Andrew J. Bradley, MD, Adam D. Marks, MD, MPH
Using standardized patients to assess hospitalist communication skills
AUTHORS: Dennis T. Chang, MD, Micah Mann, MD, Terry Sommer, BFA, Robert Fallar, PhD, Alan Weinberg, MS, Erica Friedman, MD
Techniques and behaviors associated with exemplary inpatient general medicine teaching: An exploratory qualitative study
AUTHORS: Nathan Houchens, MD, Molly Harrod, PhD, Stephanie Moody, PhD, Karen E. Fowler, MPH, Sanjay Saint, MD, MPH
A simple algorithm for predicting bacteremia using food consumption and shaking chills: A prospective observational study
AUTHORS: Takayuki Komatsu, MD, PhD, Erika Takahashi, MD, Kentaro Mishima, MD, Takeo Toyoda, MD, Fumihiro Saitoh, MD, Akari Yasuda, RN, Joe Matsuoka, PhD, Manabu Sugita, MD, PhD, Joel Branch, MD, Makoto Aoki, MD, Lawrence M. Tierney Jr., MD, Kenji Inoue, MD, PhD
For more articles and subscription information, visit www.journalofhospitalmedicine.com.
BACKGROUND: Medicare patients account for approximately 50% of hospital days. Hospitalization in older adults often results in poor outcomes.
OBJECTIVE: To test the feasibility and impact of using Assessing Care of Vulnerable Elders (ACOVE) quality indicators (QIs) as a therapeutic intervention to improve care of hospitalized older adults.
SETTING: Large tertiary hospital in the greater New York Metropolitan area.
PATIENTS: Hospitalized patients, 75 and over, admitted to medical units.
INTERVENTION: A checklist, comprised of four ACOVE QIs, administered during daily interdisciplinary rounds: venous thrombosis prophylaxis (VTE) (QI 1), indwelling bladder catheters (QI 2), mobilization (QI 3), and delirium evaluation (QI 4).
MEASUREMENTS: Variables were extracted from electronic medical records with QI compliance as the primary outcome, and length of stay (LOS), discharge disposition, and readmissions as secondary outcomes. Generalized linear mixed models for binary clustered data were used to estimate compliance rates for each group (intervention group or control group) in the postintervention period, along with their corresponding 95% confidence intervals.
RESULTS: Of the 2,396 patients, 530 were on an intervention unit. In those patients not already compliant with VTE, the compliance rate was 57% in intervention vs. 39% in control (P less than .0056). For indwelling catheters, mobilization, and delirium evaluation, overall compliance was significantly higher in the intervention group 72.2% vs. 54.4% (P = .1061), 62.9% vs. 48.2% (P less than .0001), and 27.9% vs. 21.7% (P = .0027), respectively.
CONCLUSIONS: The study demonstrates the feasibility and effectiveness of integrating ACOVE QIs to improve the quality of care in hospitalized older adults.
Also in JHM
Use of simulation to assess incoming interns’ recognition of opportunities to choose wisely
AUTHORS: Kathleen M. Wiest, Jeanne M. Farnan, MD, MHPE, Ellen Byrne, Lukas Matern, Melissa Cappaert, MA, Kristen Hirsch, Vineet M. Arora, MD, MAPP
Clinician attitudes regarding ICD deactivation in DNR/DNI patients
AUTHORS: Andrew J. Bradley, MD, Adam D. Marks, MD, MPH
Using standardized patients to assess hospitalist communication skills
AUTHORS: Dennis T. Chang, MD, Micah Mann, MD, Terry Sommer, BFA, Robert Fallar, PhD, Alan Weinberg, MS, Erica Friedman, MD
Techniques and behaviors associated with exemplary inpatient general medicine teaching: An exploratory qualitative study
AUTHORS: Nathan Houchens, MD, Molly Harrod, PhD, Stephanie Moody, PhD, Karen E. Fowler, MPH, Sanjay Saint, MD, MPH
A simple algorithm for predicting bacteremia using food consumption and shaking chills: A prospective observational study
AUTHORS: Takayuki Komatsu, MD, PhD, Erika Takahashi, MD, Kentaro Mishima, MD, Takeo Toyoda, MD, Fumihiro Saitoh, MD, Akari Yasuda, RN, Joe Matsuoka, PhD, Manabu Sugita, MD, PhD, Joel Branch, MD, Makoto Aoki, MD, Lawrence M. Tierney Jr., MD, Kenji Inoue, MD, PhD
For more articles and subscription information, visit www.journalofhospitalmedicine.com.
BACKGROUND: Medicare patients account for approximately 50% of hospital days. Hospitalization in older adults often results in poor outcomes.
OBJECTIVE: To test the feasibility and impact of using Assessing Care of Vulnerable Elders (ACOVE) quality indicators (QIs) as a therapeutic intervention to improve care of hospitalized older adults.
SETTING: Large tertiary hospital in the greater New York Metropolitan area.
PATIENTS: Hospitalized patients, 75 and over, admitted to medical units.
INTERVENTION: A checklist, comprised of four ACOVE QIs, administered during daily interdisciplinary rounds: venous thrombosis prophylaxis (VTE) (QI 1), indwelling bladder catheters (QI 2), mobilization (QI 3), and delirium evaluation (QI 4).
MEASUREMENTS: Variables were extracted from electronic medical records with QI compliance as the primary outcome, and length of stay (LOS), discharge disposition, and readmissions as secondary outcomes. Generalized linear mixed models for binary clustered data were used to estimate compliance rates for each group (intervention group or control group) in the postintervention period, along with their corresponding 95% confidence intervals.
RESULTS: Of the 2,396 patients, 530 were on an intervention unit. In those patients not already compliant with VTE, the compliance rate was 57% in intervention vs. 39% in control (P less than .0056). For indwelling catheters, mobilization, and delirium evaluation, overall compliance was significantly higher in the intervention group 72.2% vs. 54.4% (P = .1061), 62.9% vs. 48.2% (P less than .0001), and 27.9% vs. 21.7% (P = .0027), respectively.
CONCLUSIONS: The study demonstrates the feasibility and effectiveness of integrating ACOVE QIs to improve the quality of care in hospitalized older adults.
Also in JHM
Use of simulation to assess incoming interns’ recognition of opportunities to choose wisely
AUTHORS: Kathleen M. Wiest, Jeanne M. Farnan, MD, MHPE, Ellen Byrne, Lukas Matern, Melissa Cappaert, MA, Kristen Hirsch, Vineet M. Arora, MD, MAPP
Clinician attitudes regarding ICD deactivation in DNR/DNI patients
AUTHORS: Andrew J. Bradley, MD, Adam D. Marks, MD, MPH
Using standardized patients to assess hospitalist communication skills
AUTHORS: Dennis T. Chang, MD, Micah Mann, MD, Terry Sommer, BFA, Robert Fallar, PhD, Alan Weinberg, MS, Erica Friedman, MD
Techniques and behaviors associated with exemplary inpatient general medicine teaching: An exploratory qualitative study
AUTHORS: Nathan Houchens, MD, Molly Harrod, PhD, Stephanie Moody, PhD, Karen E. Fowler, MPH, Sanjay Saint, MD, MPH
A simple algorithm for predicting bacteremia using food consumption and shaking chills: A prospective observational study
AUTHORS: Takayuki Komatsu, MD, PhD, Erika Takahashi, MD, Kentaro Mishima, MD, Takeo Toyoda, MD, Fumihiro Saitoh, MD, Akari Yasuda, RN, Joe Matsuoka, PhD, Manabu Sugita, MD, PhD, Joel Branch, MD, Makoto Aoki, MD, Lawrence M. Tierney Jr., MD, Kenji Inoue, MD, PhD
For more articles and subscription information, visit www.journalofhospitalmedicine.com.
Rest dyspnea dims as acute heart failure treatment target
PARIS – During the most recent pharmaceutical generation, drug development for heart failure largely focused on acute heart failure, and specifically on patients with rest dyspnea as the primary manifestation of their acute heart failure decompensation events.
That has now changed, agreed heart failure experts as they debated the upshot of sobering results from two neutral trials that failed to show a midterm mortality benefit in patients hospitalized for acute heart failure who underwent aggressive management of their congestion using 2 days of intravenous treatment with either of two potent vasodilating drugs. Results first reported in November 2016 failed to show a survival benefit from ularitide in the 2,100-patient TRUE-AHF (Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure) trial (N Engl J Med. 2017 May 18;376[20]:1956-64). And results reported at a meeting of the Heart Failure Association of the ESC failed to show a survival benefit from serelaxin in more than 6,500 acute heart failure patients in the RELAX-AHF-2 (Efficacy, Safety and Tolerability of Serelaxin When Added to Standard Therapy in AHF) trial.
The failure of a 2-day infusion of serelaxin to produce a significant reduction in cardiovascular death in RELAX-AHF-2 was especially surprising because the predecessor trial, RELAX-AHF, which randomized only 1,160 patients and used a surrogate endpoint of dyspnea improvement, had shown significant benefit that hinted more clinically meaningful benefits might also result from serelaxin treatment (Lancet. 2013 Jan 5;381[9860]:29-39). The disappointing serelaxin and ularitide results also culminate a series of studies using several different agents or procedures to treat acute decompensated heart failure patients that all failed to produce a reduction in deaths.
“This is a sea change; make no mistake. We will need a more targeted, selective approach. It was always a daunting proposition to believe that short-term infusion could have an effect 6 months later. We were misled by the analogy [of acute heart failure] to acute coronary syndrome,” said Dr. Ruschitzka, professor of medicine at the University of Zürich.
The right time to intervene
Meeting attendees offered several hypotheses to explain why the acute ularitide and serelaxin trials both failed to show a mortality benefit, with timing of treatment the most common denominator.
Acute heart failure “is an event, not a disease,” declared Milton Packer, MD, lead investigator of TRUE-AHF, during a session devoted to vasodilator treatment of acute heart failure. Acute heart failure decompensations “are fluctuations in a chronic disease. It doesn’t matter what you do during the episode – it matters what you do between acute episodes. We focus all our attention on which vasodilator and which dose of Lasix [furosemide], but we send patients home on inadequate chronic therapy. It doesn’t matter what you do to the dyspnea, the shortness of breath will get better. Do we need a new drug that makes dyspnea go away an hour sooner and doesn’t cost a fortune? What really matters is what patients do between acute episodes and how to prevent them, ” said Dr. Packer, distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
Dr. Packer strongly urged clinicians to put heart failure patients on the full regimen of guideline-directed drugs and at full dosages, a step he thinks would go a long way toward preventing a majority of decompensation episodes. “Chronic heart failure treatment has improved dramatically, but implementation is abysmal,” he said.
Of course, at this phase of their disease heart failure patients are usually at home, which more or less demands that the treatments they take are oral or at least delivered by subcutaneous injection.
“We’ve had a mismatch of candidate drugs, which have mostly been IV infusions, with a clinical setting where an IV infusion is challenging to use.”
“We are killing good drugs by the way we’re testing them,” commented Javed Butler, MD, who bemoaned the ignominious outcome of serelaxin treatment in RELAX-AHF-2. “The available data show it makes no sense to treat for just 2 days. We should take true worsening heart failure patients, those who are truly failing standard treatment, and look at new chronic oral therapies to try on them.” Oral drugs similar to serelaxin and ularitide could be used chronically, suggested Dr. Butler, professor of medicine and chief of cardiology at Stony Brook (N.Y.) School of Medicine.
Wrong patients with the wrong presentation
Perhaps just as big a flaw of the acute heart failure trials has been their target patient population, patients with rest dyspnea at the time of admission. “Why do we think that dyspnea is a clinically relevant symptom for acute heart failure?” Dr. Packer asked.
Dr. Cleland and his associates analyzed data on 116,752 hospitalizations for acute heart failure in England and Wales during April 2007–March 2013, a database that included more than 90% of hospitals for these regions. “We found that a large proportion of admitted patients did not have breathlessness at rest as their primary reason for seeking hospitalization. For about half the patients, moderate or severe peripheral edema was the main problem,” he reported. Roughly a third of patients had rest dyspnea as their main symptom.
An unadjusted analysis also showed a stronger link between peripheral edema and the rate of mortality during a median follow-up of about a year following hospitalization, compared with rest dyspnea. Compared with the lowest-risk subgroup, the patients with severe peripheral edema (18% of the population) had more than twice the mortality. In contrast, the patients with the most severe rest dyspnea and no evidence at all of peripheral edema, just 6% of the population, had a 50% higher mortality rate than the lowest-risk patients.
”It’s peripheral edema rather than breathlessness that is the important determinant of length of stay and prognosis. The disastrous neutral trials for acute heart failure have all targeted the breathless subset of patients. Maybe a reason for the failures has been that they’ve been treating a problem that does not exist. The trials have looked at the wrong patients,” Dr. Cleland said.
‘We’ve told the wrong story to industry” about the importance of rest dyspnea to acute heart failure patients. “When we say acute heart failure, we mean an ambulance and oxygen and the emergency department and rapid IV treatment. That’s breathlessness. Patients with peripheral edema usually get driven in and walk from the car to a wheelchair and they wait 4 hours to be seen. I think that, following the TRUE-AHF and RELAX-AHF-2 results, we’ll see a radical change.”
But just because the focus should be on peripheral edema rather than dyspnea, that doesn’t mean better drugs aren’t needed, Dr. Cleland added.
“We need better treatments to deal with congestion. Once a patient is congested, we are not very good at getting rid of it. We depend on diuretics, which we don’t use properly. Ultimately I’d like to see agents as adjuncts to diuretics, to produce better kidney function.” But treatments for breathlessness are decent as they now exist: furosemide plus oxygen. When a simple, cheap drug works 80% of the time, it is really hard to improve on that.” The real unmet needs for treating acute decompensated heart failure are patients with rest dyspnea who don’t respond to conventional treatment, and especially patients with gross peripheral edema plus low blood pressure and renal dysfunction for whom no good treatments have been developed, Dr. Cleland said.
Another flaw in the patient selection criteria for the acute heart failure studies has been the focus on patients with elevated blood pressures, the logical target for vasodilator drugs that lower blood pressure, noted Dr. Felker. “But these are the patients at lowest risk. We’ve had a mismatch between the patients with the biggest need and the patients we actually study, which relates to the types of drugs we’ve developed.”
Acute heart failure remains a target
Despite all the talk of refocusing attention on chronic heart failure and peripheral edema, at least one expert remained steadfast in talking up the importance of new acute interventions.
Part of the problem in believing that existing treatments can adequately manage heart failure and prevent acute decompensations is that about 75% of acute heart failure episodes either occur as the first presentation of heart failure, or occur in patients with heart failure with preserved ejection fraction (HFpEF), a type of heart failure that, until recently, had no chronic drug regimens with widely acknowledged efficacy for HFpEF. (The 2017 U.S. heart failure management guidelines update listed aldosterone receptor antagonists as a class IIb recommendation for treating HFpEF, the first time guidelines have sanctioned a drug class for treating HFpEF [Circulation. 2017 Apr 28. doi: 10.1161/CIR.0000000000000509].)
“For patients with heart failure with reduced ejection fraction, you can give optimal treatments and [decompensations] are prevented,” noted Dr. Mebazaa, a professor of anesthesiology and resuscitation at Lariboisière Hospital in Paris. “But for the huge number of HFpEF patients we have nothing. Acute heart failure will remain prevalent, and we still don’t have the right drugs to use on these patients.”
The TRUE-AHF trial was sponsored by Cardiorentis. RELAX-AHF-2 was sponsored by Novartis. Dr. Ruschitzka has been a speaker on behalf of Novartis, and has been a speaker for or consultant to several companies and was a coinvestigator for TRUE-AHF and received fees from Cardiorentis for his participation. Dr. Packer is a consultant to and stockholder in Cardiorentis and has been a consultant to several other companies. Dr. Felker has been a consultant to Novartis and several other companies and was a coinvestigator on RELAX-AHF-2. Dr. Butler has been a consultant to several companies. Dr. Cleland has received research support from Novartis, and he has been a consultant to and received research support from several other companies. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis as well as from several other companies and was a coinvestigator on TRUE-AHF.
[email protected]
On Twitter @mitchelzoler
PARIS – During the most recent pharmaceutical generation, drug development for heart failure largely focused on acute heart failure, and specifically on patients with rest dyspnea as the primary manifestation of their acute heart failure decompensation events.
That has now changed, agreed heart failure experts as they debated the upshot of sobering results from two neutral trials that failed to show a midterm mortality benefit in patients hospitalized for acute heart failure who underwent aggressive management of their congestion using 2 days of intravenous treatment with either of two potent vasodilating drugs. Results first reported in November 2016 failed to show a survival benefit from ularitide in the 2,100-patient TRUE-AHF (Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure) trial (N Engl J Med. 2017 May 18;376[20]:1956-64). And results reported at a meeting of the Heart Failure Association of the ESC failed to show a survival benefit from serelaxin in more than 6,500 acute heart failure patients in the RELAX-AHF-2 (Efficacy, Safety and Tolerability of Serelaxin When Added to Standard Therapy in AHF) trial.
The failure of a 2-day infusion of serelaxin to produce a significant reduction in cardiovascular death in RELAX-AHF-2 was especially surprising because the predecessor trial, RELAX-AHF, which randomized only 1,160 patients and used a surrogate endpoint of dyspnea improvement, had shown significant benefit that hinted more clinically meaningful benefits might also result from serelaxin treatment (Lancet. 2013 Jan 5;381[9860]:29-39). The disappointing serelaxin and ularitide results also culminate a series of studies using several different agents or procedures to treat acute decompensated heart failure patients that all failed to produce a reduction in deaths.
“This is a sea change; make no mistake. We will need a more targeted, selective approach. It was always a daunting proposition to believe that short-term infusion could have an effect 6 months later. We were misled by the analogy [of acute heart failure] to acute coronary syndrome,” said Dr. Ruschitzka, professor of medicine at the University of Zürich.
The right time to intervene
Meeting attendees offered several hypotheses to explain why the acute ularitide and serelaxin trials both failed to show a mortality benefit, with timing of treatment the most common denominator.
Acute heart failure “is an event, not a disease,” declared Milton Packer, MD, lead investigator of TRUE-AHF, during a session devoted to vasodilator treatment of acute heart failure. Acute heart failure decompensations “are fluctuations in a chronic disease. It doesn’t matter what you do during the episode – it matters what you do between acute episodes. We focus all our attention on which vasodilator and which dose of Lasix [furosemide], but we send patients home on inadequate chronic therapy. It doesn’t matter what you do to the dyspnea, the shortness of breath will get better. Do we need a new drug that makes dyspnea go away an hour sooner and doesn’t cost a fortune? What really matters is what patients do between acute episodes and how to prevent them, ” said Dr. Packer, distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
Dr. Packer strongly urged clinicians to put heart failure patients on the full regimen of guideline-directed drugs and at full dosages, a step he thinks would go a long way toward preventing a majority of decompensation episodes. “Chronic heart failure treatment has improved dramatically, but implementation is abysmal,” he said.
Of course, at this phase of their disease heart failure patients are usually at home, which more or less demands that the treatments they take are oral or at least delivered by subcutaneous injection.
“We’ve had a mismatch of candidate drugs, which have mostly been IV infusions, with a clinical setting where an IV infusion is challenging to use.”
“We are killing good drugs by the way we’re testing them,” commented Javed Butler, MD, who bemoaned the ignominious outcome of serelaxin treatment in RELAX-AHF-2. “The available data show it makes no sense to treat for just 2 days. We should take true worsening heart failure patients, those who are truly failing standard treatment, and look at new chronic oral therapies to try on them.” Oral drugs similar to serelaxin and ularitide could be used chronically, suggested Dr. Butler, professor of medicine and chief of cardiology at Stony Brook (N.Y.) School of Medicine.
Wrong patients with the wrong presentation
Perhaps just as big a flaw of the acute heart failure trials has been their target patient population, patients with rest dyspnea at the time of admission. “Why do we think that dyspnea is a clinically relevant symptom for acute heart failure?” Dr. Packer asked.
Dr. Cleland and his associates analyzed data on 116,752 hospitalizations for acute heart failure in England and Wales during April 2007–March 2013, a database that included more than 90% of hospitals for these regions. “We found that a large proportion of admitted patients did not have breathlessness at rest as their primary reason for seeking hospitalization. For about half the patients, moderate or severe peripheral edema was the main problem,” he reported. Roughly a third of patients had rest dyspnea as their main symptom.
An unadjusted analysis also showed a stronger link between peripheral edema and the rate of mortality during a median follow-up of about a year following hospitalization, compared with rest dyspnea. Compared with the lowest-risk subgroup, the patients with severe peripheral edema (18% of the population) had more than twice the mortality. In contrast, the patients with the most severe rest dyspnea and no evidence at all of peripheral edema, just 6% of the population, had a 50% higher mortality rate than the lowest-risk patients.
”It’s peripheral edema rather than breathlessness that is the important determinant of length of stay and prognosis. The disastrous neutral trials for acute heart failure have all targeted the breathless subset of patients. Maybe a reason for the failures has been that they’ve been treating a problem that does not exist. The trials have looked at the wrong patients,” Dr. Cleland said.
‘We’ve told the wrong story to industry” about the importance of rest dyspnea to acute heart failure patients. “When we say acute heart failure, we mean an ambulance and oxygen and the emergency department and rapid IV treatment. That’s breathlessness. Patients with peripheral edema usually get driven in and walk from the car to a wheelchair and they wait 4 hours to be seen. I think that, following the TRUE-AHF and RELAX-AHF-2 results, we’ll see a radical change.”
But just because the focus should be on peripheral edema rather than dyspnea, that doesn’t mean better drugs aren’t needed, Dr. Cleland added.
“We need better treatments to deal with congestion. Once a patient is congested, we are not very good at getting rid of it. We depend on diuretics, which we don’t use properly. Ultimately I’d like to see agents as adjuncts to diuretics, to produce better kidney function.” But treatments for breathlessness are decent as they now exist: furosemide plus oxygen. When a simple, cheap drug works 80% of the time, it is really hard to improve on that.” The real unmet needs for treating acute decompensated heart failure are patients with rest dyspnea who don’t respond to conventional treatment, and especially patients with gross peripheral edema plus low blood pressure and renal dysfunction for whom no good treatments have been developed, Dr. Cleland said.
Another flaw in the patient selection criteria for the acute heart failure studies has been the focus on patients with elevated blood pressures, the logical target for vasodilator drugs that lower blood pressure, noted Dr. Felker. “But these are the patients at lowest risk. We’ve had a mismatch between the patients with the biggest need and the patients we actually study, which relates to the types of drugs we’ve developed.”
Acute heart failure remains a target
Despite all the talk of refocusing attention on chronic heart failure and peripheral edema, at least one expert remained steadfast in talking up the importance of new acute interventions.
Part of the problem in believing that existing treatments can adequately manage heart failure and prevent acute decompensations is that about 75% of acute heart failure episodes either occur as the first presentation of heart failure, or occur in patients with heart failure with preserved ejection fraction (HFpEF), a type of heart failure that, until recently, had no chronic drug regimens with widely acknowledged efficacy for HFpEF. (The 2017 U.S. heart failure management guidelines update listed aldosterone receptor antagonists as a class IIb recommendation for treating HFpEF, the first time guidelines have sanctioned a drug class for treating HFpEF [Circulation. 2017 Apr 28. doi: 10.1161/CIR.0000000000000509].)
“For patients with heart failure with reduced ejection fraction, you can give optimal treatments and [decompensations] are prevented,” noted Dr. Mebazaa, a professor of anesthesiology and resuscitation at Lariboisière Hospital in Paris. “But for the huge number of HFpEF patients we have nothing. Acute heart failure will remain prevalent, and we still don’t have the right drugs to use on these patients.”
The TRUE-AHF trial was sponsored by Cardiorentis. RELAX-AHF-2 was sponsored by Novartis. Dr. Ruschitzka has been a speaker on behalf of Novartis, and has been a speaker for or consultant to several companies and was a coinvestigator for TRUE-AHF and received fees from Cardiorentis for his participation. Dr. Packer is a consultant to and stockholder in Cardiorentis and has been a consultant to several other companies. Dr. Felker has been a consultant to Novartis and several other companies and was a coinvestigator on RELAX-AHF-2. Dr. Butler has been a consultant to several companies. Dr. Cleland has received research support from Novartis, and he has been a consultant to and received research support from several other companies. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis as well as from several other companies and was a coinvestigator on TRUE-AHF.
[email protected]
On Twitter @mitchelzoler
PARIS – During the most recent pharmaceutical generation, drug development for heart failure largely focused on acute heart failure, and specifically on patients with rest dyspnea as the primary manifestation of their acute heart failure decompensation events.
That has now changed, agreed heart failure experts as they debated the upshot of sobering results from two neutral trials that failed to show a midterm mortality benefit in patients hospitalized for acute heart failure who underwent aggressive management of their congestion using 2 days of intravenous treatment with either of two potent vasodilating drugs. Results first reported in November 2016 failed to show a survival benefit from ularitide in the 2,100-patient TRUE-AHF (Efficacy and Safety of Ularitide for the Treatment of Acute Decompensated Heart Failure) trial (N Engl J Med. 2017 May 18;376[20]:1956-64). And results reported at a meeting of the Heart Failure Association of the ESC failed to show a survival benefit from serelaxin in more than 6,500 acute heart failure patients in the RELAX-AHF-2 (Efficacy, Safety and Tolerability of Serelaxin When Added to Standard Therapy in AHF) trial.
The failure of a 2-day infusion of serelaxin to produce a significant reduction in cardiovascular death in RELAX-AHF-2 was especially surprising because the predecessor trial, RELAX-AHF, which randomized only 1,160 patients and used a surrogate endpoint of dyspnea improvement, had shown significant benefit that hinted more clinically meaningful benefits might also result from serelaxin treatment (Lancet. 2013 Jan 5;381[9860]:29-39). The disappointing serelaxin and ularitide results also culminate a series of studies using several different agents or procedures to treat acute decompensated heart failure patients that all failed to produce a reduction in deaths.
“This is a sea change; make no mistake. We will need a more targeted, selective approach. It was always a daunting proposition to believe that short-term infusion could have an effect 6 months later. We were misled by the analogy [of acute heart failure] to acute coronary syndrome,” said Dr. Ruschitzka, professor of medicine at the University of Zürich.
The right time to intervene
Meeting attendees offered several hypotheses to explain why the acute ularitide and serelaxin trials both failed to show a mortality benefit, with timing of treatment the most common denominator.
Acute heart failure “is an event, not a disease,” declared Milton Packer, MD, lead investigator of TRUE-AHF, during a session devoted to vasodilator treatment of acute heart failure. Acute heart failure decompensations “are fluctuations in a chronic disease. It doesn’t matter what you do during the episode – it matters what you do between acute episodes. We focus all our attention on which vasodilator and which dose of Lasix [furosemide], but we send patients home on inadequate chronic therapy. It doesn’t matter what you do to the dyspnea, the shortness of breath will get better. Do we need a new drug that makes dyspnea go away an hour sooner and doesn’t cost a fortune? What really matters is what patients do between acute episodes and how to prevent them, ” said Dr. Packer, distinguished scholar in cardiovascular science at Baylor University Medical Center in Dallas.
Dr. Packer strongly urged clinicians to put heart failure patients on the full regimen of guideline-directed drugs and at full dosages, a step he thinks would go a long way toward preventing a majority of decompensation episodes. “Chronic heart failure treatment has improved dramatically, but implementation is abysmal,” he said.
Of course, at this phase of their disease heart failure patients are usually at home, which more or less demands that the treatments they take are oral or at least delivered by subcutaneous injection.
“We’ve had a mismatch of candidate drugs, which have mostly been IV infusions, with a clinical setting where an IV infusion is challenging to use.”
“We are killing good drugs by the way we’re testing them,” commented Javed Butler, MD, who bemoaned the ignominious outcome of serelaxin treatment in RELAX-AHF-2. “The available data show it makes no sense to treat for just 2 days. We should take true worsening heart failure patients, those who are truly failing standard treatment, and look at new chronic oral therapies to try on them.” Oral drugs similar to serelaxin and ularitide could be used chronically, suggested Dr. Butler, professor of medicine and chief of cardiology at Stony Brook (N.Y.) School of Medicine.
Wrong patients with the wrong presentation
Perhaps just as big a flaw of the acute heart failure trials has been their target patient population, patients with rest dyspnea at the time of admission. “Why do we think that dyspnea is a clinically relevant symptom for acute heart failure?” Dr. Packer asked.
Dr. Cleland and his associates analyzed data on 116,752 hospitalizations for acute heart failure in England and Wales during April 2007–March 2013, a database that included more than 90% of hospitals for these regions. “We found that a large proportion of admitted patients did not have breathlessness at rest as their primary reason for seeking hospitalization. For about half the patients, moderate or severe peripheral edema was the main problem,” he reported. Roughly a third of patients had rest dyspnea as their main symptom.
An unadjusted analysis also showed a stronger link between peripheral edema and the rate of mortality during a median follow-up of about a year following hospitalization, compared with rest dyspnea. Compared with the lowest-risk subgroup, the patients with severe peripheral edema (18% of the population) had more than twice the mortality. In contrast, the patients with the most severe rest dyspnea and no evidence at all of peripheral edema, just 6% of the population, had a 50% higher mortality rate than the lowest-risk patients.
”It’s peripheral edema rather than breathlessness that is the important determinant of length of stay and prognosis. The disastrous neutral trials for acute heart failure have all targeted the breathless subset of patients. Maybe a reason for the failures has been that they’ve been treating a problem that does not exist. The trials have looked at the wrong patients,” Dr. Cleland said.
‘We’ve told the wrong story to industry” about the importance of rest dyspnea to acute heart failure patients. “When we say acute heart failure, we mean an ambulance and oxygen and the emergency department and rapid IV treatment. That’s breathlessness. Patients with peripheral edema usually get driven in and walk from the car to a wheelchair and they wait 4 hours to be seen. I think that, following the TRUE-AHF and RELAX-AHF-2 results, we’ll see a radical change.”
But just because the focus should be on peripheral edema rather than dyspnea, that doesn’t mean better drugs aren’t needed, Dr. Cleland added.
“We need better treatments to deal with congestion. Once a patient is congested, we are not very good at getting rid of it. We depend on diuretics, which we don’t use properly. Ultimately I’d like to see agents as adjuncts to diuretics, to produce better kidney function.” But treatments for breathlessness are decent as they now exist: furosemide plus oxygen. When a simple, cheap drug works 80% of the time, it is really hard to improve on that.” The real unmet needs for treating acute decompensated heart failure are patients with rest dyspnea who don’t respond to conventional treatment, and especially patients with gross peripheral edema plus low blood pressure and renal dysfunction for whom no good treatments have been developed, Dr. Cleland said.
Another flaw in the patient selection criteria for the acute heart failure studies has been the focus on patients with elevated blood pressures, the logical target for vasodilator drugs that lower blood pressure, noted Dr. Felker. “But these are the patients at lowest risk. We’ve had a mismatch between the patients with the biggest need and the patients we actually study, which relates to the types of drugs we’ve developed.”
Acute heart failure remains a target
Despite all the talk of refocusing attention on chronic heart failure and peripheral edema, at least one expert remained steadfast in talking up the importance of new acute interventions.
Part of the problem in believing that existing treatments can adequately manage heart failure and prevent acute decompensations is that about 75% of acute heart failure episodes either occur as the first presentation of heart failure, or occur in patients with heart failure with preserved ejection fraction (HFpEF), a type of heart failure that, until recently, had no chronic drug regimens with widely acknowledged efficacy for HFpEF. (The 2017 U.S. heart failure management guidelines update listed aldosterone receptor antagonists as a class IIb recommendation for treating HFpEF, the first time guidelines have sanctioned a drug class for treating HFpEF [Circulation. 2017 Apr 28. doi: 10.1161/CIR.0000000000000509].)
“For patients with heart failure with reduced ejection fraction, you can give optimal treatments and [decompensations] are prevented,” noted Dr. Mebazaa, a professor of anesthesiology and resuscitation at Lariboisière Hospital in Paris. “But for the huge number of HFpEF patients we have nothing. Acute heart failure will remain prevalent, and we still don’t have the right drugs to use on these patients.”
The TRUE-AHF trial was sponsored by Cardiorentis. RELAX-AHF-2 was sponsored by Novartis. Dr. Ruschitzka has been a speaker on behalf of Novartis, and has been a speaker for or consultant to several companies and was a coinvestigator for TRUE-AHF and received fees from Cardiorentis for his participation. Dr. Packer is a consultant to and stockholder in Cardiorentis and has been a consultant to several other companies. Dr. Felker has been a consultant to Novartis and several other companies and was a coinvestigator on RELAX-AHF-2. Dr. Butler has been a consultant to several companies. Dr. Cleland has received research support from Novartis, and he has been a consultant to and received research support from several other companies. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis as well as from several other companies and was a coinvestigator on TRUE-AHF.
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM HEART FAILURE 2017
HM17 session summary: Rheumatology pearls for the inpatient provider
Presenter
Neal Birnbaum, MD
Session Summary
Dr. Birnbaum began with the differential diagnosis of acute monoarthritis, which is one of the more common reasons for inpatient rheumatology consultation and includes crystalline (e.g., gout), septic, autoimmune (psoriasis), traumatic, and hemorrhagic.
The synovial fluid will give an idea as to whether one is more likely than the other, he said. Normal synovial fluid is transparent, clear, has a low cell count, and is very viscous in nature. Noninflammatory etiologies (osteoarthritis) will have some cells but will largely be similar to normal synovial fluid. Inflammatory causes will have higher cell counts (2-10K WBC) but will have much lower viscosity. Septic joints will look pustular with very high cell counts (sometimes too high to be recorded) and will be positive on fluid culture (unless the patient has already received antimicrobial therapy). Hemorrhagic fluid will look like blood, and the history will give clues as to whether that is the case (recent trauma, history of hemophilia).
Pseudogout (CPPD) is more likely to manifest in different joints (knees more so than wrist more so than shoulders/hips). One should suspect pseudogout in patients with acute arthritis in patients more than 70 years old. Crystals will be positively bifringent and more rectangular or square shaped, compared with gout crystals. Finding chondrocalcinosis on x-ray on a symptomatic patient can make the diagnosis much more likely. However, a patient can have chondrocalcinosis on an x-ray and not have CPPD. A patient can also have no chondrocalcinosis and have CPPD. It is the combination of the x-ray sign and symptoms that creates the high accuracy of the test.
The treatment for both in the acute setting includes colchicine (2 tabs x 1, then 1 more 1 hour later), NSAIDS (although may not be ideal inpatient because of potential toxicities), and corticosteroids (this can be either oral [prednisone 40 mg q24 with rapid taper], intraarticular [triamcinolone 10 mg-40 mg depending on the joint size], or IV [solumedrol or solucortef equivalent to prednisone 40 q24]).
For management of gout chronically, one should strive for a uric acid level of less than 6.0. Contrary to what is commonly believed, one can start urate lowering agents like allopurinol acutely (start with 100 mg for 2 weeks, then titrate up every 2 weeks until one hits the target uric acid level). Clinicians can consider using febuxostat for those patients who have renal insufficiency. While on the urate lowering agent, use low dose colchicine or NSAIDS for the first few months. Unfortunately, there is no long-term chronic strategy to prevent pseudogout flares. If there is an underlying cause for the pseudogout, then try to address it.
Consults for positive antinuclear antibodies (ANA) are common reasons for rheumatology referrals. The patterns of the ANA and the titer are important to the differential diagnosis. Up to 30% of healthy individuals have a positive ANA. ANA can be helpful as a rule out test for systemic lupus erythematosus (SLE), as it has a high sensitivity and a low specificity. However, because SLE is a clinical diagnosis and because of the high ANA positivity in the population, a high ANA alone does not prove a patient has SLE.
Concerning vasculitis, Dr. Birnbaum recommended thinking about it in terms of small versus large vessel disease. For initial evaluation, one should draw a CBC, erythrocyte sedimentation rate/C-reactive protein, urinalysis, chemistry panel, ANA, antineutrophil cytoplasmic antibodies, rheumatoid factor, hepatitis C antibody, and complement levels (C3, C4, CH50). One can also think of drawing cryoglobulins, especially in settings where one is suspicious that hepatitis C may be present. The differential diagnosis for vasculitis includes drug reactions, infections (mostly viral), malignancy, collagen vascular disease, and idiopathic causes (33%-50% of cases). The treatment is to remove offending agents (i.e., drug-induced vasculitis), treat infections (if applicable), and use steroids (the dosing depends on the situation).
Dr. Birnbaum finished with two relatively new illnesses that should be on clinicians’ radars. Chikungunya virus is transmitted by mosquitoes in the same distribution that one may see Zika virus. The symptoms include headaches, fevers, extreme joint pain, and joint swelling (this aspect is different from many other viral illnesses). The illness is usually acute. However, some patients will continue to have symptoms for up to a year. There is no specific treatment other than symptom relief (pain medications, NSAIDs).
Finally, immunoglobulin G4–related disease can affect virtually any organ system, but seems to manifest frequently as pancreatitis in the hospital setting. Think about this in patients with pancreatitis not secondary to the usual alcoholic or gallstone variety. The gold standard for diagnosis is biopsy with histologic findings of IgG4 in plasma cells. Most patients will be noted to have elevated IgG4 levels. The treatment is prednisone 40mg q24 with a taper over 2 months. For those who cannot be weaned or for those with recurrent disease, rituximab (1000mg IV x 1 then approximately 2 weeks later) can be used.
Key takeaways for HM
- Know the differential diagnosis of acute monoarticular arthritis and how the synovial fluid will vary depending on the diagnosis.
- Gout can manifest in other joints besides the first toe. One can use allopurinol even in the acute setting. The goal is to attain a uric acid level of less than 6.0.
- Pseudogout should be considered in patients older than 70 years with acute arthritis. There is no allopurinol equivalent for chronic management.
- Positive ANAs are common, but they do not make the diagnosis of SLE (although a negative ANA generally does rule out SLE).
- SLE is a clinical diagnosis that requires multiple symptoms and findings to make the diagnosis. Please refer to the ACR classification criteria.
- Think of vasculitis in terms of small versus large vessel disease and think of the differential diagnosis as to the etiology (realizing that 33%-50% will end up being idiopathic).
- Chikungunya is mosquito-borne and associated with severe joint pains, headaches, and fevers but can also have joint swelling. While often acute, the symptoms can last for up to a year. Treatment is symptomatic management.
- Think of IgG4-related disease in patients with pancreatitis without the usual causes (alcohol, gallstones). Diagnosis is based on pathology and IgG4 levels. Treatment is with steroids and/or rituximab.
Dr. Kim is a hospitalist who works at Emory University Hospital in Atlanta and is an editorial board member of The Hospitalist.
Presenter
Neal Birnbaum, MD
Session Summary
Dr. Birnbaum began with the differential diagnosis of acute monoarthritis, which is one of the more common reasons for inpatient rheumatology consultation and includes crystalline (e.g., gout), septic, autoimmune (psoriasis), traumatic, and hemorrhagic.
The synovial fluid will give an idea as to whether one is more likely than the other, he said. Normal synovial fluid is transparent, clear, has a low cell count, and is very viscous in nature. Noninflammatory etiologies (osteoarthritis) will have some cells but will largely be similar to normal synovial fluid. Inflammatory causes will have higher cell counts (2-10K WBC) but will have much lower viscosity. Septic joints will look pustular with very high cell counts (sometimes too high to be recorded) and will be positive on fluid culture (unless the patient has already received antimicrobial therapy). Hemorrhagic fluid will look like blood, and the history will give clues as to whether that is the case (recent trauma, history of hemophilia).
Pseudogout (CPPD) is more likely to manifest in different joints (knees more so than wrist more so than shoulders/hips). One should suspect pseudogout in patients with acute arthritis in patients more than 70 years old. Crystals will be positively bifringent and more rectangular or square shaped, compared with gout crystals. Finding chondrocalcinosis on x-ray on a symptomatic patient can make the diagnosis much more likely. However, a patient can have chondrocalcinosis on an x-ray and not have CPPD. A patient can also have no chondrocalcinosis and have CPPD. It is the combination of the x-ray sign and symptoms that creates the high accuracy of the test.
The treatment for both in the acute setting includes colchicine (2 tabs x 1, then 1 more 1 hour later), NSAIDS (although may not be ideal inpatient because of potential toxicities), and corticosteroids (this can be either oral [prednisone 40 mg q24 with rapid taper], intraarticular [triamcinolone 10 mg-40 mg depending on the joint size], or IV [solumedrol or solucortef equivalent to prednisone 40 q24]).
For management of gout chronically, one should strive for a uric acid level of less than 6.0. Contrary to what is commonly believed, one can start urate lowering agents like allopurinol acutely (start with 100 mg for 2 weeks, then titrate up every 2 weeks until one hits the target uric acid level). Clinicians can consider using febuxostat for those patients who have renal insufficiency. While on the urate lowering agent, use low dose colchicine or NSAIDS for the first few months. Unfortunately, there is no long-term chronic strategy to prevent pseudogout flares. If there is an underlying cause for the pseudogout, then try to address it.
Consults for positive antinuclear antibodies (ANA) are common reasons for rheumatology referrals. The patterns of the ANA and the titer are important to the differential diagnosis. Up to 30% of healthy individuals have a positive ANA. ANA can be helpful as a rule out test for systemic lupus erythematosus (SLE), as it has a high sensitivity and a low specificity. However, because SLE is a clinical diagnosis and because of the high ANA positivity in the population, a high ANA alone does not prove a patient has SLE.
Concerning vasculitis, Dr. Birnbaum recommended thinking about it in terms of small versus large vessel disease. For initial evaluation, one should draw a CBC, erythrocyte sedimentation rate/C-reactive protein, urinalysis, chemistry panel, ANA, antineutrophil cytoplasmic antibodies, rheumatoid factor, hepatitis C antibody, and complement levels (C3, C4, CH50). One can also think of drawing cryoglobulins, especially in settings where one is suspicious that hepatitis C may be present. The differential diagnosis for vasculitis includes drug reactions, infections (mostly viral), malignancy, collagen vascular disease, and idiopathic causes (33%-50% of cases). The treatment is to remove offending agents (i.e., drug-induced vasculitis), treat infections (if applicable), and use steroids (the dosing depends on the situation).
Dr. Birnbaum finished with two relatively new illnesses that should be on clinicians’ radars. Chikungunya virus is transmitted by mosquitoes in the same distribution that one may see Zika virus. The symptoms include headaches, fevers, extreme joint pain, and joint swelling (this aspect is different from many other viral illnesses). The illness is usually acute. However, some patients will continue to have symptoms for up to a year. There is no specific treatment other than symptom relief (pain medications, NSAIDs).
Finally, immunoglobulin G4–related disease can affect virtually any organ system, but seems to manifest frequently as pancreatitis in the hospital setting. Think about this in patients with pancreatitis not secondary to the usual alcoholic or gallstone variety. The gold standard for diagnosis is biopsy with histologic findings of IgG4 in plasma cells. Most patients will be noted to have elevated IgG4 levels. The treatment is prednisone 40mg q24 with a taper over 2 months. For those who cannot be weaned or for those with recurrent disease, rituximab (1000mg IV x 1 then approximately 2 weeks later) can be used.
Key takeaways for HM
- Know the differential diagnosis of acute monoarticular arthritis and how the synovial fluid will vary depending on the diagnosis.
- Gout can manifest in other joints besides the first toe. One can use allopurinol even in the acute setting. The goal is to attain a uric acid level of less than 6.0.
- Pseudogout should be considered in patients older than 70 years with acute arthritis. There is no allopurinol equivalent for chronic management.
- Positive ANAs are common, but they do not make the diagnosis of SLE (although a negative ANA generally does rule out SLE).
- SLE is a clinical diagnosis that requires multiple symptoms and findings to make the diagnosis. Please refer to the ACR classification criteria.
- Think of vasculitis in terms of small versus large vessel disease and think of the differential diagnosis as to the etiology (realizing that 33%-50% will end up being idiopathic).
- Chikungunya is mosquito-borne and associated with severe joint pains, headaches, and fevers but can also have joint swelling. While often acute, the symptoms can last for up to a year. Treatment is symptomatic management.
- Think of IgG4-related disease in patients with pancreatitis without the usual causes (alcohol, gallstones). Diagnosis is based on pathology and IgG4 levels. Treatment is with steroids and/or rituximab.
Dr. Kim is a hospitalist who works at Emory University Hospital in Atlanta and is an editorial board member of The Hospitalist.
Presenter
Neal Birnbaum, MD
Session Summary
Dr. Birnbaum began with the differential diagnosis of acute monoarthritis, which is one of the more common reasons for inpatient rheumatology consultation and includes crystalline (e.g., gout), septic, autoimmune (psoriasis), traumatic, and hemorrhagic.
The synovial fluid will give an idea as to whether one is more likely than the other, he said. Normal synovial fluid is transparent, clear, has a low cell count, and is very viscous in nature. Noninflammatory etiologies (osteoarthritis) will have some cells but will largely be similar to normal synovial fluid. Inflammatory causes will have higher cell counts (2-10K WBC) but will have much lower viscosity. Septic joints will look pustular with very high cell counts (sometimes too high to be recorded) and will be positive on fluid culture (unless the patient has already received antimicrobial therapy). Hemorrhagic fluid will look like blood, and the history will give clues as to whether that is the case (recent trauma, history of hemophilia).
Pseudogout (CPPD) is more likely to manifest in different joints (knees more so than wrist more so than shoulders/hips). One should suspect pseudogout in patients with acute arthritis in patients more than 70 years old. Crystals will be positively bifringent and more rectangular or square shaped, compared with gout crystals. Finding chondrocalcinosis on x-ray on a symptomatic patient can make the diagnosis much more likely. However, a patient can have chondrocalcinosis on an x-ray and not have CPPD. A patient can also have no chondrocalcinosis and have CPPD. It is the combination of the x-ray sign and symptoms that creates the high accuracy of the test.
The treatment for both in the acute setting includes colchicine (2 tabs x 1, then 1 more 1 hour later), NSAIDS (although may not be ideal inpatient because of potential toxicities), and corticosteroids (this can be either oral [prednisone 40 mg q24 with rapid taper], intraarticular [triamcinolone 10 mg-40 mg depending on the joint size], or IV [solumedrol or solucortef equivalent to prednisone 40 q24]).
For management of gout chronically, one should strive for a uric acid level of less than 6.0. Contrary to what is commonly believed, one can start urate lowering agents like allopurinol acutely (start with 100 mg for 2 weeks, then titrate up every 2 weeks until one hits the target uric acid level). Clinicians can consider using febuxostat for those patients who have renal insufficiency. While on the urate lowering agent, use low dose colchicine or NSAIDS for the first few months. Unfortunately, there is no long-term chronic strategy to prevent pseudogout flares. If there is an underlying cause for the pseudogout, then try to address it.
Consults for positive antinuclear antibodies (ANA) are common reasons for rheumatology referrals. The patterns of the ANA and the titer are important to the differential diagnosis. Up to 30% of healthy individuals have a positive ANA. ANA can be helpful as a rule out test for systemic lupus erythematosus (SLE), as it has a high sensitivity and a low specificity. However, because SLE is a clinical diagnosis and because of the high ANA positivity in the population, a high ANA alone does not prove a patient has SLE.
Concerning vasculitis, Dr. Birnbaum recommended thinking about it in terms of small versus large vessel disease. For initial evaluation, one should draw a CBC, erythrocyte sedimentation rate/C-reactive protein, urinalysis, chemistry panel, ANA, antineutrophil cytoplasmic antibodies, rheumatoid factor, hepatitis C antibody, and complement levels (C3, C4, CH50). One can also think of drawing cryoglobulins, especially in settings where one is suspicious that hepatitis C may be present. The differential diagnosis for vasculitis includes drug reactions, infections (mostly viral), malignancy, collagen vascular disease, and idiopathic causes (33%-50% of cases). The treatment is to remove offending agents (i.e., drug-induced vasculitis), treat infections (if applicable), and use steroids (the dosing depends on the situation).
Dr. Birnbaum finished with two relatively new illnesses that should be on clinicians’ radars. Chikungunya virus is transmitted by mosquitoes in the same distribution that one may see Zika virus. The symptoms include headaches, fevers, extreme joint pain, and joint swelling (this aspect is different from many other viral illnesses). The illness is usually acute. However, some patients will continue to have symptoms for up to a year. There is no specific treatment other than symptom relief (pain medications, NSAIDs).
Finally, immunoglobulin G4–related disease can affect virtually any organ system, but seems to manifest frequently as pancreatitis in the hospital setting. Think about this in patients with pancreatitis not secondary to the usual alcoholic or gallstone variety. The gold standard for diagnosis is biopsy with histologic findings of IgG4 in plasma cells. Most patients will be noted to have elevated IgG4 levels. The treatment is prednisone 40mg q24 with a taper over 2 months. For those who cannot be weaned or for those with recurrent disease, rituximab (1000mg IV x 1 then approximately 2 weeks later) can be used.
Key takeaways for HM
- Know the differential diagnosis of acute monoarticular arthritis and how the synovial fluid will vary depending on the diagnosis.
- Gout can manifest in other joints besides the first toe. One can use allopurinol even in the acute setting. The goal is to attain a uric acid level of less than 6.0.
- Pseudogout should be considered in patients older than 70 years with acute arthritis. There is no allopurinol equivalent for chronic management.
- Positive ANAs are common, but they do not make the diagnosis of SLE (although a negative ANA generally does rule out SLE).
- SLE is a clinical diagnosis that requires multiple symptoms and findings to make the diagnosis. Please refer to the ACR classification criteria.
- Think of vasculitis in terms of small versus large vessel disease and think of the differential diagnosis as to the etiology (realizing that 33%-50% will end up being idiopathic).
- Chikungunya is mosquito-borne and associated with severe joint pains, headaches, and fevers but can also have joint swelling. While often acute, the symptoms can last for up to a year. Treatment is symptomatic management.
- Think of IgG4-related disease in patients with pancreatitis without the usual causes (alcohol, gallstones). Diagnosis is based on pathology and IgG4 levels. Treatment is with steroids and/or rituximab.
Dr. Kim is a hospitalist who works at Emory University Hospital in Atlanta and is an editorial board member of The Hospitalist.
HFrEF mortality halved when treatment matches guidelines
PARIS – Heart failure patients who received guideline-directed pharmacotherapy, at dosages that approached guideline-directed levels, had roughly half the 6-month mortality as did similar patients who did not receive this level of treatment in a real-world, observational study with more than 6,000 patients.
Adherence to pharmacologic treatment guidelines for patients with heart failure with reduced ejection fraction (HFrEF) “was strongly associated with clinical outcomes during 6-month follow-up,” Michel Komajda, MD, said at a meeting held by the Heart Failure Association of the European Society of Cardiology. The findings highlight the importance of closely following guideline recommendations in routine practice.
[polldaddy:9772629]
The analysis used data collected in the QUALIFY (Quality of Adherence to Guideline Recommendations for Life-Saving Treatment in Heart Failure: an International Survey) registry, which enrolled 7,127 HFrEF patients during September 2013–December 2014 at 547 centers in 36 countries, mostly in Europe, Asia, and Africa but also in Canada, Ecuador, and Australia. All enrolled patients had to have been hospitalized for worsening heart failure at least once during the 1-15 months before they entered QUALIFY.
Dr. Komajda and his associates assessed each enrolled patient at baseline by their treatment with each of four guideline-recommended drug classes: an ACE inhibitor or angiotensin receptor blocker; a beta-blocker; an aldosterone receptor antagonist (ARA) if the patient’s functional status was rated as New York Heart Association class II, III, or IV; and ivabradine (Corlanor) if the patient was in NYHA class II, III, or IV, in sinus rhythm, had a heart rate of at least 70 beats per minute, and if the patient was in a country where ivabradine was available. Because patient enrollment occurred in 2013 and 2014, the study couldn’t include the new formulation of sacubitril plus valsartan (Entresto) in its analysis.
For each eligible drug class a patient received 1 point if their daily prescribed dosage was at least 50% of the recommended dosage (or 100% for an ARA), 0.5 points if the patient received the recommended drug but at a lower dosage, and no points if the drug wasn’t given. A patient also received 1 point if they were appropriately not given a drug because of a documented contraindication or intolerance. The researchers then calculated each patient’s “adherence score” by dividing their point total by the potentially maximum number of points that each patient could have received (a number that ranged from 2 to 4). They defined a score of 1 (which meant the patients received at least half the recommended dosage of all recommended drugs) as good adherence, a score of 0.51-0.99 as moderate adherence, and a score of 0.5 or less as poor adherence.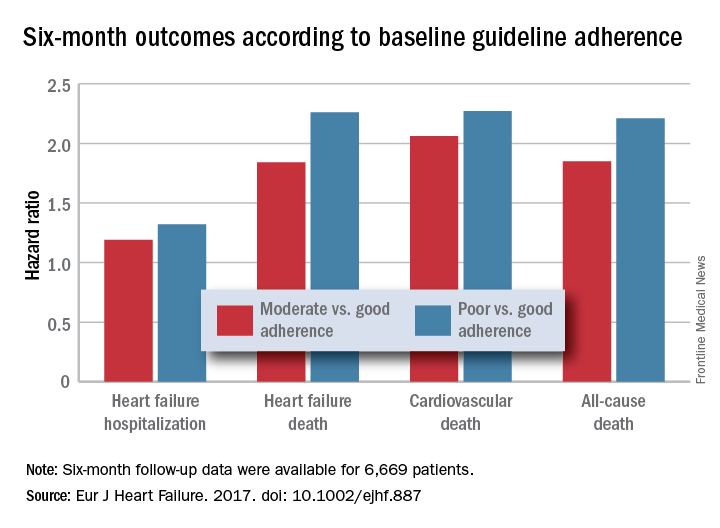
Because patient enrollment occurred during 2013 and 2014, the benchmark heart failure treatment guidelines were those issued by the European Society of Cardiology in 2012 (Eur Heart J. 2012 July;33[14]:1787-1847).
Concurrently with Dr. Komajda’s report at the meeting the results appeared in an article online (Eur J Heart Failure. 2017. doi: 10.1002/ejhf.887).
QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
[email protected]
On Twitter @mitchelzoler
This was a wonderful and useful study. It was large, prospective, covered a wide geographic area, and showed that drug dosage matters when treating heart failure with reduced ejection fraction.
The geographic diversity was a strength, but also a potential weakness because of the resulting differences among the enrolled patients in financial resources, ethnic and genetic makeup, and their tolerance to various drugs. I hope that further research can dissect the role that each of these factors played in the results.
Another limitation is the relatively simplistic approach used for assessing drug dosages to calculate the adherence scores. While it is a very useful step forward to classify patients by the drug dosages they received, there could be some very legitimate variations in dosages based on parameters such as blood pressure. An important question to address in the future is whether it is better to use all the recommended drugs at reduced dosages if necessary, or to use fewer agents at higher dosages.
But these are just quibbles about what is a very important study.
Andrew J.S. Coats, MD , is a cardiologist, professor of medicine, and academic vice-president for the Monash Warwick Alliance of Warwick University in Coventry, England, and Monash University in Melbourne. He has received honoraria from Impulse Dynamics, Menarini, PsiOxus, ResMed, Respicardia, and Servier. He made these comments as designated discussant for Dr. Komajda’s report.
This was a wonderful and useful study. It was large, prospective, covered a wide geographic area, and showed that drug dosage matters when treating heart failure with reduced ejection fraction.
The geographic diversity was a strength, but also a potential weakness because of the resulting differences among the enrolled patients in financial resources, ethnic and genetic makeup, and their tolerance to various drugs. I hope that further research can dissect the role that each of these factors played in the results.
Another limitation is the relatively simplistic approach used for assessing drug dosages to calculate the adherence scores. While it is a very useful step forward to classify patients by the drug dosages they received, there could be some very legitimate variations in dosages based on parameters such as blood pressure. An important question to address in the future is whether it is better to use all the recommended drugs at reduced dosages if necessary, or to use fewer agents at higher dosages.
But these are just quibbles about what is a very important study.
Andrew J.S. Coats, MD , is a cardiologist, professor of medicine, and academic vice-president for the Monash Warwick Alliance of Warwick University in Coventry, England, and Monash University in Melbourne. He has received honoraria from Impulse Dynamics, Menarini, PsiOxus, ResMed, Respicardia, and Servier. He made these comments as designated discussant for Dr. Komajda’s report.
This was a wonderful and useful study. It was large, prospective, covered a wide geographic area, and showed that drug dosage matters when treating heart failure with reduced ejection fraction.
The geographic diversity was a strength, but also a potential weakness because of the resulting differences among the enrolled patients in financial resources, ethnic and genetic makeup, and their tolerance to various drugs. I hope that further research can dissect the role that each of these factors played in the results.
Another limitation is the relatively simplistic approach used for assessing drug dosages to calculate the adherence scores. While it is a very useful step forward to classify patients by the drug dosages they received, there could be some very legitimate variations in dosages based on parameters such as blood pressure. An important question to address in the future is whether it is better to use all the recommended drugs at reduced dosages if necessary, or to use fewer agents at higher dosages.
But these are just quibbles about what is a very important study.
Andrew J.S. Coats, MD , is a cardiologist, professor of medicine, and academic vice-president for the Monash Warwick Alliance of Warwick University in Coventry, England, and Monash University in Melbourne. He has received honoraria from Impulse Dynamics, Menarini, PsiOxus, ResMed, Respicardia, and Servier. He made these comments as designated discussant for Dr. Komajda’s report.
PARIS – Heart failure patients who received guideline-directed pharmacotherapy, at dosages that approached guideline-directed levels, had roughly half the 6-month mortality as did similar patients who did not receive this level of treatment in a real-world, observational study with more than 6,000 patients.
Adherence to pharmacologic treatment guidelines for patients with heart failure with reduced ejection fraction (HFrEF) “was strongly associated with clinical outcomes during 6-month follow-up,” Michel Komajda, MD, said at a meeting held by the Heart Failure Association of the European Society of Cardiology. The findings highlight the importance of closely following guideline recommendations in routine practice.
[polldaddy:9772629]
The analysis used data collected in the QUALIFY (Quality of Adherence to Guideline Recommendations for Life-Saving Treatment in Heart Failure: an International Survey) registry, which enrolled 7,127 HFrEF patients during September 2013–December 2014 at 547 centers in 36 countries, mostly in Europe, Asia, and Africa but also in Canada, Ecuador, and Australia. All enrolled patients had to have been hospitalized for worsening heart failure at least once during the 1-15 months before they entered QUALIFY.
Dr. Komajda and his associates assessed each enrolled patient at baseline by their treatment with each of four guideline-recommended drug classes: an ACE inhibitor or angiotensin receptor blocker; a beta-blocker; an aldosterone receptor antagonist (ARA) if the patient’s functional status was rated as New York Heart Association class II, III, or IV; and ivabradine (Corlanor) if the patient was in NYHA class II, III, or IV, in sinus rhythm, had a heart rate of at least 70 beats per minute, and if the patient was in a country where ivabradine was available. Because patient enrollment occurred in 2013 and 2014, the study couldn’t include the new formulation of sacubitril plus valsartan (Entresto) in its analysis.
For each eligible drug class a patient received 1 point if their daily prescribed dosage was at least 50% of the recommended dosage (or 100% for an ARA), 0.5 points if the patient received the recommended drug but at a lower dosage, and no points if the drug wasn’t given. A patient also received 1 point if they were appropriately not given a drug because of a documented contraindication or intolerance. The researchers then calculated each patient’s “adherence score” by dividing their point total by the potentially maximum number of points that each patient could have received (a number that ranged from 2 to 4). They defined a score of 1 (which meant the patients received at least half the recommended dosage of all recommended drugs) as good adherence, a score of 0.51-0.99 as moderate adherence, and a score of 0.5 or less as poor adherence.
Because patient enrollment occurred during 2013 and 2014, the benchmark heart failure treatment guidelines were those issued by the European Society of Cardiology in 2012 (Eur Heart J. 2012 July;33[14]:1787-1847).
Concurrently with Dr. Komajda’s report at the meeting the results appeared in an article online (Eur J Heart Failure. 2017. doi: 10.1002/ejhf.887).
QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
[email protected]
On Twitter @mitchelzoler
PARIS – Heart failure patients who received guideline-directed pharmacotherapy, at dosages that approached guideline-directed levels, had roughly half the 6-month mortality as did similar patients who did not receive this level of treatment in a real-world, observational study with more than 6,000 patients.
Adherence to pharmacologic treatment guidelines for patients with heart failure with reduced ejection fraction (HFrEF) “was strongly associated with clinical outcomes during 6-month follow-up,” Michel Komajda, MD, said at a meeting held by the Heart Failure Association of the European Society of Cardiology. The findings highlight the importance of closely following guideline recommendations in routine practice.
[polldaddy:9772629]
The analysis used data collected in the QUALIFY (Quality of Adherence to Guideline Recommendations for Life-Saving Treatment in Heart Failure: an International Survey) registry, which enrolled 7,127 HFrEF patients during September 2013–December 2014 at 547 centers in 36 countries, mostly in Europe, Asia, and Africa but also in Canada, Ecuador, and Australia. All enrolled patients had to have been hospitalized for worsening heart failure at least once during the 1-15 months before they entered QUALIFY.
Dr. Komajda and his associates assessed each enrolled patient at baseline by their treatment with each of four guideline-recommended drug classes: an ACE inhibitor or angiotensin receptor blocker; a beta-blocker; an aldosterone receptor antagonist (ARA) if the patient’s functional status was rated as New York Heart Association class II, III, or IV; and ivabradine (Corlanor) if the patient was in NYHA class II, III, or IV, in sinus rhythm, had a heart rate of at least 70 beats per minute, and if the patient was in a country where ivabradine was available. Because patient enrollment occurred in 2013 and 2014, the study couldn’t include the new formulation of sacubitril plus valsartan (Entresto) in its analysis.
For each eligible drug class a patient received 1 point if their daily prescribed dosage was at least 50% of the recommended dosage (or 100% for an ARA), 0.5 points if the patient received the recommended drug but at a lower dosage, and no points if the drug wasn’t given. A patient also received 1 point if they were appropriately not given a drug because of a documented contraindication or intolerance. The researchers then calculated each patient’s “adherence score” by dividing their point total by the potentially maximum number of points that each patient could have received (a number that ranged from 2 to 4). They defined a score of 1 (which meant the patients received at least half the recommended dosage of all recommended drugs) as good adherence, a score of 0.51-0.99 as moderate adherence, and a score of 0.5 or less as poor adherence.
Because patient enrollment occurred during 2013 and 2014, the benchmark heart failure treatment guidelines were those issued by the European Society of Cardiology in 2012 (Eur Heart J. 2012 July;33[14]:1787-1847).
Concurrently with Dr. Komajda’s report at the meeting the results appeared in an article online (Eur J Heart Failure. 2017. doi: 10.1002/ejhf.887).
QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
[email protected]
On Twitter @mitchelzoler
AT HEART FAILURE 2017
Key clinical point:
Major finding: Six-month all-cause, cardiovascular, and heart failure mortalities were doubled in patients not on guideline-adherent therapy and dosages.
Data source: QUANTIFY, an international registry with 6,669 HFrEF patients followed for 6 months.
Disclosures: QUANTIFY was sponsored by Servier. Dr. Komajda has received honoraria from Servier and from Amgen, AstraZeneca, Bristol-Myers Squibb, Menarini, MSD, Novartis, Novo Nordisk, and Sanofi.
Use of probiotics in hospitalized adults to prevent Clostridium difficile infection
Clinical Question: Does the use and timing of probiotics in hospitalized adult patients with Clostridium difficile infection (CDI) improve clinical outcomes?
Background: The incidence of CDI in hospitalized patients has increased significantly over the past years, resulting in significant morbidity and mortality. Improved prevention of CDI could have substantial public health benefits.
Study design: Systematic review and metaregression analysis.
Setting: 19 studies meeting inclusion criteria.
Synopsis: Computerized bibliography databases were searched for randomized controlled trials (RCTs) evaluating probiotic effects on CDI in hospitalized adults taking antibiotics.
Comprising 6261 subjects, 19 RCTs were analyzed. The incidence of CDI was lower in the probiotic cohort than in the control group (1.6% vs. 3.9%; P less than 0.001). The pooled relative risk of CDI in probiotic users was 0.42 (95% CI, 0.30-0.57). Metaregression analysis demonstrated that probiotics were significantly more effective if given closer to the first antibiotic dose, with a decrease in efficacy for every day of delay in starting probiotics (P = .04). Probiotics given within 2 days of antibiotic initiation produced a greater reduction of risk for CDI (RR, 0.32; 95% CI, 0.22-0.48) than later administration (RR, 0.70; 95% CI, 0.40-1.23; P = .02). There was no increased risk for adverse events among patients receiving probiotics.
Limitations included high risk of bias because of missing data, attrition, restricted patient population, lack of placebo, and conflict of interest.
Bottom Line: Administration of probiotics soon after the first dose of antibiotic reduces the risk of CDI by more than 50% in hospitalized adults without any increased risk of adverse events.
Reference: Shen NT, Maw A, Tmanova LL et al. Timely use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review with Meta-Regression Analysis. Gastroenterology. Published on 9 Feb 2017. doi: 10.1053/j.gastro.2017.02.003.
Dr. Martin is clinical professor in the division of hospital medicine, department of medicine, University of California, San Diego.
Clinical Question: Does the use and timing of probiotics in hospitalized adult patients with Clostridium difficile infection (CDI) improve clinical outcomes?
Background: The incidence of CDI in hospitalized patients has increased significantly over the past years, resulting in significant morbidity and mortality. Improved prevention of CDI could have substantial public health benefits.
Study design: Systematic review and metaregression analysis.
Setting: 19 studies meeting inclusion criteria.
Synopsis: Computerized bibliography databases were searched for randomized controlled trials (RCTs) evaluating probiotic effects on CDI in hospitalized adults taking antibiotics.
Comprising 6261 subjects, 19 RCTs were analyzed. The incidence of CDI was lower in the probiotic cohort than in the control group (1.6% vs. 3.9%; P less than 0.001). The pooled relative risk of CDI in probiotic users was 0.42 (95% CI, 0.30-0.57). Metaregression analysis demonstrated that probiotics were significantly more effective if given closer to the first antibiotic dose, with a decrease in efficacy for every day of delay in starting probiotics (P = .04). Probiotics given within 2 days of antibiotic initiation produced a greater reduction of risk for CDI (RR, 0.32; 95% CI, 0.22-0.48) than later administration (RR, 0.70; 95% CI, 0.40-1.23; P = .02). There was no increased risk for adverse events among patients receiving probiotics.
Limitations included high risk of bias because of missing data, attrition, restricted patient population, lack of placebo, and conflict of interest.
Bottom Line: Administration of probiotics soon after the first dose of antibiotic reduces the risk of CDI by more than 50% in hospitalized adults without any increased risk of adverse events.
Reference: Shen NT, Maw A, Tmanova LL et al. Timely use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review with Meta-Regression Analysis. Gastroenterology. Published on 9 Feb 2017. doi: 10.1053/j.gastro.2017.02.003.
Dr. Martin is clinical professor in the division of hospital medicine, department of medicine, University of California, San Diego.
Clinical Question: Does the use and timing of probiotics in hospitalized adult patients with Clostridium difficile infection (CDI) improve clinical outcomes?
Background: The incidence of CDI in hospitalized patients has increased significantly over the past years, resulting in significant morbidity and mortality. Improved prevention of CDI could have substantial public health benefits.
Study design: Systematic review and metaregression analysis.
Setting: 19 studies meeting inclusion criteria.
Synopsis: Computerized bibliography databases were searched for randomized controlled trials (RCTs) evaluating probiotic effects on CDI in hospitalized adults taking antibiotics.
Comprising 6261 subjects, 19 RCTs were analyzed. The incidence of CDI was lower in the probiotic cohort than in the control group (1.6% vs. 3.9%; P less than 0.001). The pooled relative risk of CDI in probiotic users was 0.42 (95% CI, 0.30-0.57). Metaregression analysis demonstrated that probiotics were significantly more effective if given closer to the first antibiotic dose, with a decrease in efficacy for every day of delay in starting probiotics (P = .04). Probiotics given within 2 days of antibiotic initiation produced a greater reduction of risk for CDI (RR, 0.32; 95% CI, 0.22-0.48) than later administration (RR, 0.70; 95% CI, 0.40-1.23; P = .02). There was no increased risk for adverse events among patients receiving probiotics.
Limitations included high risk of bias because of missing data, attrition, restricted patient population, lack of placebo, and conflict of interest.
Bottom Line: Administration of probiotics soon after the first dose of antibiotic reduces the risk of CDI by more than 50% in hospitalized adults without any increased risk of adverse events.
Reference: Shen NT, Maw A, Tmanova LL et al. Timely use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review with Meta-Regression Analysis. Gastroenterology. Published on 9 Feb 2017. doi: 10.1053/j.gastro.2017.02.003.
Dr. Martin is clinical professor in the division of hospital medicine, department of medicine, University of California, San Diego.
Sooner may not be better: Study shows no benefit of urgent colonoscopy for lower GI bleeding
Clinical Question: In patients hospitalized for a lower gastrointestinal bleeding (LGIB), does an urgent colonoscopy (less than 24 hours after admission) result in any clinical benefits, compared with waiting for an elective colonoscopy?
Background: LGIB is a common cause of morbidity and mortality, often requiring hospitalization. While colonoscopy is necessary for appropriate work-up and treatment, it remains unclear if time to colonoscopy (urgent vs. elective) confers any clinical benefit in hospitalized patients.
Study Design: Systematic review and meta-analysis.
Setting: Twelve studies meeting inclusion criteria.
Synopsis: Computerized bibliography databases were searched for appropriate studies, and 12 met inclusion criteria, resulting in a total sample size of 10,172 patients in the urgent colonoscopy arm and 14,224 patients in the elective colonoscopy.
Outcome measures included bleeding source identified on colonoscopy, therapeutic endoscopic interventions performed, patients requiring blood transfusions, rebleeding, adverse events, and mortality.
Urgent colonoscopy was associated with increased use of endoscopic therapeutic intervention (relative risk, 1.70; 95% CI, 1.08-2.67). There were no significant differences in bleeding source localization (RR, 1.08; 95% CI, 0.92-1.25), adverse event rates (RR, 1.05; 95% CI, 0.65-1.71), rebleeding rates (RR, 1.14; 95% CI, 0.74-1.78), transfusion requirement (RR, 1.02; 95% CI, 0.73-1.41), or mortality (RR, 1.17; 95% CI, 0.45-3.02) between urgent and elective colonoscopy.
Limitations of the study comprise of inclusion of small number of studies, underpowered statistical analysis, and possible variation in quality assessment of articles evaluated.
Bottom Line: Urgent colonoscopy is safe and usually well tolerated in hospitalized patients with LGIB, but, compared with elective colonoscopy, there is no clear evidence it alters important clinical outcomes.
Reference: Kouanda AM, Somsouk M, Sewell JL, Day LW. Urgent colonoscopy in patients with lower GI bleeding: A systematic review and meta-analysis. Gastrointest Endosc. Published online Feb 4, 2017. doi: 10.1016/j.gie.2017.01.035.
Dr. Martin is clinical professor in the division of hospital medicine, department of medicine, University of California, San Diego.
Clinical Question: In patients hospitalized for a lower gastrointestinal bleeding (LGIB), does an urgent colonoscopy (less than 24 hours after admission) result in any clinical benefits, compared with waiting for an elective colonoscopy?
Background: LGIB is a common cause of morbidity and mortality, often requiring hospitalization. While colonoscopy is necessary for appropriate work-up and treatment, it remains unclear if time to colonoscopy (urgent vs. elective) confers any clinical benefit in hospitalized patients.
Study Design: Systematic review and meta-analysis.
Setting: Twelve studies meeting inclusion criteria.
Synopsis: Computerized bibliography databases were searched for appropriate studies, and 12 met inclusion criteria, resulting in a total sample size of 10,172 patients in the urgent colonoscopy arm and 14,224 patients in the elective colonoscopy.
Outcome measures included bleeding source identified on colonoscopy, therapeutic endoscopic interventions performed, patients requiring blood transfusions, rebleeding, adverse events, and mortality.
Urgent colonoscopy was associated with increased use of endoscopic therapeutic intervention (relative risk, 1.70; 95% CI, 1.08-2.67). There were no significant differences in bleeding source localization (RR, 1.08; 95% CI, 0.92-1.25), adverse event rates (RR, 1.05; 95% CI, 0.65-1.71), rebleeding rates (RR, 1.14; 95% CI, 0.74-1.78), transfusion requirement (RR, 1.02; 95% CI, 0.73-1.41), or mortality (RR, 1.17; 95% CI, 0.45-3.02) between urgent and elective colonoscopy.
Limitations of the study comprise of inclusion of small number of studies, underpowered statistical analysis, and possible variation in quality assessment of articles evaluated.
Bottom Line: Urgent colonoscopy is safe and usually well tolerated in hospitalized patients with LGIB, but, compared with elective colonoscopy, there is no clear evidence it alters important clinical outcomes.
Reference: Kouanda AM, Somsouk M, Sewell JL, Day LW. Urgent colonoscopy in patients with lower GI bleeding: A systematic review and meta-analysis. Gastrointest Endosc. Published online Feb 4, 2017. doi: 10.1016/j.gie.2017.01.035.
Dr. Martin is clinical professor in the division of hospital medicine, department of medicine, University of California, San Diego.
Clinical Question: In patients hospitalized for a lower gastrointestinal bleeding (LGIB), does an urgent colonoscopy (less than 24 hours after admission) result in any clinical benefits, compared with waiting for an elective colonoscopy?
Background: LGIB is a common cause of morbidity and mortality, often requiring hospitalization. While colonoscopy is necessary for appropriate work-up and treatment, it remains unclear if time to colonoscopy (urgent vs. elective) confers any clinical benefit in hospitalized patients.
Study Design: Systematic review and meta-analysis.
Setting: Twelve studies meeting inclusion criteria.
Synopsis: Computerized bibliography databases were searched for appropriate studies, and 12 met inclusion criteria, resulting in a total sample size of 10,172 patients in the urgent colonoscopy arm and 14,224 patients in the elective colonoscopy.
Outcome measures included bleeding source identified on colonoscopy, therapeutic endoscopic interventions performed, patients requiring blood transfusions, rebleeding, adverse events, and mortality.
Urgent colonoscopy was associated with increased use of endoscopic therapeutic intervention (relative risk, 1.70; 95% CI, 1.08-2.67). There were no significant differences in bleeding source localization (RR, 1.08; 95% CI, 0.92-1.25), adverse event rates (RR, 1.05; 95% CI, 0.65-1.71), rebleeding rates (RR, 1.14; 95% CI, 0.74-1.78), transfusion requirement (RR, 1.02; 95% CI, 0.73-1.41), or mortality (RR, 1.17; 95% CI, 0.45-3.02) between urgent and elective colonoscopy.
Limitations of the study comprise of inclusion of small number of studies, underpowered statistical analysis, and possible variation in quality assessment of articles evaluated.
Bottom Line: Urgent colonoscopy is safe and usually well tolerated in hospitalized patients with LGIB, but, compared with elective colonoscopy, there is no clear evidence it alters important clinical outcomes.
Reference: Kouanda AM, Somsouk M, Sewell JL, Day LW. Urgent colonoscopy in patients with lower GI bleeding: A systematic review and meta-analysis. Gastrointest Endosc. Published online Feb 4, 2017. doi: 10.1016/j.gie.2017.01.035.
Dr. Martin is clinical professor in the division of hospital medicine, department of medicine, University of California, San Diego.
Angiotensin II may improve vasopressors’ efficacy
WASHINGTON – Adding angiotensin II to available vasopressor therapies correlated with significantly improved arterial pressure in patients with catecholamine-resistant vasodilatory shock and less adverse effects, according to a study presented at the recent international conference of the American Thoracic Society.
In a double blind, controlled, phase III study, 70% of 163 patients given angiotensin II reached arterial pressure of at least 75 mm HG or improved by at least 10 mm Hg three hours later, compared with 23.4% of the 158 patients given a placebo (P less than .001).
Those in the angiotensin II group also saw a mean pressure increase of 12.5 mm Hg in the first 3 hours after initiating treatment, compared with 2.9 mm Hg in the placebo group (P less than .001), according to Ashish Khanna, MD, FCCP, of the Cleveland Clinic, and his fellow researchers (N Engl J Med. 2017 May 21. doi: 10.1056/NEJMoa1704154).
Current vasopressor therapies for vasodilatory patients are associated with dangerous side effects and a 30-day mortality rate of more than 50%, which is a major concern for patients who do not have many options to begin with, the researchers noted.
“Treatment options for patients with catecholamine-resistant vasodilatory shock are limited, and the treatments that are available are often associated with side effects,” said Dr. Khanna and his colleagues.
The researchers added the naturally occurring peptide hormone angiotensin II to vasodilatory patients’ treatment regimen in order to “more closely [mimic] natural physiologic responses to shock, which include increased secretion of catecholamines, vasopressin, and RAAS hormones.”
To test the efficacy of angiotensin II, researchers gathered patients with a median age of 64 years and a mean arterial pressure of 66.3 mm Hg.
Sepsis was the predominant cause of shock for 80.7% of the study’s participants.
Patients were injected with either 20 ng/kg of body weight per minute of angiotensin II or an equivalent dose of a placebo until mean arterial pressure reached 75 mm Hg. After 3 hours and 15 minutes of treatment, the dosages were adjusted to keep pressure between 65 and 75 mm Hg for the next 48 hours.
Among patients in the angiotensin II group, 67% of patients were able to decrease angiotensin II and vasopressor doses within 30 minutes of injection, according to researchers.
When researchers measured improvement using the cardiovascular Sequential Organ Failure Assessment, patients in the angiotensin II group saw an average decrease of 1.75 points, compared with 1.28 points in patients in the placebo group (P = .01) 48 hours after treatment.
The Sequential Organ Failure Assessment is scaled from 0-4, with higher scores indicating more severe organ failure.
When measuring for adverse affects, serious effects occurred in 60.7% of the angiotensin II patients, compared with in 67.1% of those in the placebo group.
At the 28-day mark, 75 angiotensin II patients (46.0%) died, compared with 85 patients (53.8%) of the placebo group.
This study was limited by the small sample size, “so the possibility of clinically important side effects attributable to angiotensin II therapy cannot be exuded,” the researches warned.
Also, the follow-up timeline of 28 days, may not have given researchers enough time to uncover the full extent of positive and negative long-term effects associated with angiotensin II.
This study was supported by La Jolla Pharmaceutical, from which multiple researchers reported receiving financial support in the form of personal fees and grants. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
[email protected]
On Twitter @eaztweets
WASHINGTON – Adding angiotensin II to available vasopressor therapies correlated with significantly improved arterial pressure in patients with catecholamine-resistant vasodilatory shock and less adverse effects, according to a study presented at the recent international conference of the American Thoracic Society.
In a double blind, controlled, phase III study, 70% of 163 patients given angiotensin II reached arterial pressure of at least 75 mm HG or improved by at least 10 mm Hg three hours later, compared with 23.4% of the 158 patients given a placebo (P less than .001).
Those in the angiotensin II group also saw a mean pressure increase of 12.5 mm Hg in the first 3 hours after initiating treatment, compared with 2.9 mm Hg in the placebo group (P less than .001), according to Ashish Khanna, MD, FCCP, of the Cleveland Clinic, and his fellow researchers (N Engl J Med. 2017 May 21. doi: 10.1056/NEJMoa1704154).
Current vasopressor therapies for vasodilatory patients are associated with dangerous side effects and a 30-day mortality rate of more than 50%, which is a major concern for patients who do not have many options to begin with, the researchers noted.
“Treatment options for patients with catecholamine-resistant vasodilatory shock are limited, and the treatments that are available are often associated with side effects,” said Dr. Khanna and his colleagues.
The researchers added the naturally occurring peptide hormone angiotensin II to vasodilatory patients’ treatment regimen in order to “more closely [mimic] natural physiologic responses to shock, which include increased secretion of catecholamines, vasopressin, and RAAS hormones.”
To test the efficacy of angiotensin II, researchers gathered patients with a median age of 64 years and a mean arterial pressure of 66.3 mm Hg.
Sepsis was the predominant cause of shock for 80.7% of the study’s participants.
Patients were injected with either 20 ng/kg of body weight per minute of angiotensin II or an equivalent dose of a placebo until mean arterial pressure reached 75 mm Hg. After 3 hours and 15 minutes of treatment, the dosages were adjusted to keep pressure between 65 and 75 mm Hg for the next 48 hours.
Among patients in the angiotensin II group, 67% of patients were able to decrease angiotensin II and vasopressor doses within 30 minutes of injection, according to researchers.
When researchers measured improvement using the cardiovascular Sequential Organ Failure Assessment, patients in the angiotensin II group saw an average decrease of 1.75 points, compared with 1.28 points in patients in the placebo group (P = .01) 48 hours after treatment.
The Sequential Organ Failure Assessment is scaled from 0-4, with higher scores indicating more severe organ failure.
When measuring for adverse affects, serious effects occurred in 60.7% of the angiotensin II patients, compared with in 67.1% of those in the placebo group.
At the 28-day mark, 75 angiotensin II patients (46.0%) died, compared with 85 patients (53.8%) of the placebo group.
This study was limited by the small sample size, “so the possibility of clinically important side effects attributable to angiotensin II therapy cannot be exuded,” the researches warned.
Also, the follow-up timeline of 28 days, may not have given researchers enough time to uncover the full extent of positive and negative long-term effects associated with angiotensin II.
This study was supported by La Jolla Pharmaceutical, from which multiple researchers reported receiving financial support in the form of personal fees and grants. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
[email protected]
On Twitter @eaztweets
WASHINGTON – Adding angiotensin II to available vasopressor therapies correlated with significantly improved arterial pressure in patients with catecholamine-resistant vasodilatory shock and less adverse effects, according to a study presented at the recent international conference of the American Thoracic Society.
In a double blind, controlled, phase III study, 70% of 163 patients given angiotensin II reached arterial pressure of at least 75 mm HG or improved by at least 10 mm Hg three hours later, compared with 23.4% of the 158 patients given a placebo (P less than .001).
Those in the angiotensin II group also saw a mean pressure increase of 12.5 mm Hg in the first 3 hours after initiating treatment, compared with 2.9 mm Hg in the placebo group (P less than .001), according to Ashish Khanna, MD, FCCP, of the Cleveland Clinic, and his fellow researchers (N Engl J Med. 2017 May 21. doi: 10.1056/NEJMoa1704154).
Current vasopressor therapies for vasodilatory patients are associated with dangerous side effects and a 30-day mortality rate of more than 50%, which is a major concern for patients who do not have many options to begin with, the researchers noted.
“Treatment options for patients with catecholamine-resistant vasodilatory shock are limited, and the treatments that are available are often associated with side effects,” said Dr. Khanna and his colleagues.
The researchers added the naturally occurring peptide hormone angiotensin II to vasodilatory patients’ treatment regimen in order to “more closely [mimic] natural physiologic responses to shock, which include increased secretion of catecholamines, vasopressin, and RAAS hormones.”
To test the efficacy of angiotensin II, researchers gathered patients with a median age of 64 years and a mean arterial pressure of 66.3 mm Hg.
Sepsis was the predominant cause of shock for 80.7% of the study’s participants.
Patients were injected with either 20 ng/kg of body weight per minute of angiotensin II or an equivalent dose of a placebo until mean arterial pressure reached 75 mm Hg. After 3 hours and 15 minutes of treatment, the dosages were adjusted to keep pressure between 65 and 75 mm Hg for the next 48 hours.
Among patients in the angiotensin II group, 67% of patients were able to decrease angiotensin II and vasopressor doses within 30 minutes of injection, according to researchers.
When researchers measured improvement using the cardiovascular Sequential Organ Failure Assessment, patients in the angiotensin II group saw an average decrease of 1.75 points, compared with 1.28 points in patients in the placebo group (P = .01) 48 hours after treatment.
The Sequential Organ Failure Assessment is scaled from 0-4, with higher scores indicating more severe organ failure.
When measuring for adverse affects, serious effects occurred in 60.7% of the angiotensin II patients, compared with in 67.1% of those in the placebo group.
At the 28-day mark, 75 angiotensin II patients (46.0%) died, compared with 85 patients (53.8%) of the placebo group.
This study was limited by the small sample size, “so the possibility of clinically important side effects attributable to angiotensin II therapy cannot be exuded,” the researches warned.
Also, the follow-up timeline of 28 days, may not have given researchers enough time to uncover the full extent of positive and negative long-term effects associated with angiotensin II.
This study was supported by La Jolla Pharmaceutical, from which multiple researchers reported receiving financial support in the form of personal fees and grants. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
[email protected]
On Twitter @eaztweets
At ATS 2017
Key clinical point:
Major finding: In the first 3 hours, patients taking angiotensin II improved arterial pressure by an average of 12.5 mm Hg, compared with 2.9 mm Hg in patients taking the placebo (P less than .001)
Data source: Double blind, randomized, control trial of 321 patients with catecholamine-resistant vasodilatory shock collected from 75 intensive care units globally during May 2015-January 2017.
Disclosures: Multiple investigators reported receiving support from La Jolla Pharmaceutical and similar companies in the form of grants and/or personal fees. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
Assessing the risks associated with MRI patients with a pacemaker or defibrillator
Clinical Question: What are the risks of nonthoracic MRI in patients with pacemakers or implantable cardioverter-defibrillators (ICD) who are not preapproved by the Food and Drug Administration for MRI scanning?
Background: Implantable cardiovascular devices could suffer heating in MRI magnetic fields leading to cardiac thermal injury and changes in pacing properties. The FDA approves “MRI-conditional devices” deemed safe for MRI, but up to six million patients worldwide (and two million in the United States) have non–MRI conditional devices.
Setting: U.S. Centers participating in the MagnaSafe registry.
Synopsis: Adults with non–MRI conditional pacemakers (1000 cases) or ICDs (500 cases) implanted in the thorax after 2001 were scanned with nonthoracic MRI at 1.5 Tesla. Patients with abandoned or inactive leads, other implantable devices, and low batteries and pacing-dependent patients with ICDs were excluded.
Devices were interrogated before each MRI and set to either no pacing or asynchronous pacing with all tachycardia and bradycardia therapies deactivated. Primary endpoints included immediate death, generator or lead failure, loss of capture in paced patients, new arrhythmia, and generator reset.
No patients suffered death or device or lead failure. Six patients developed self-terminating atrial arrhythmias, while an additional six had partial pacemaker electrical reset. Several devices had detectable changes in battery voltage, lead impedance, pacing threshold, and P- or R-wave amplitude without evident clinical significance. Multiple MRIs caused no increase in adverse outcomes. This study suggests that patients with non–MRI conditional devices may be at low risk from nonthoracic imaging if appropriately screened with temporary pacemaker function modification before MRI.
Bottom Line: Appropriately screened and prepared patients with non–MRI conditional thoracic pacemakers or ICDs may be at low risk for complications from nonthoracic MRI at 1.5 Tesla.
Reference: Russo RJ, Costa HS, Silva PD, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med. 2017;376:755-64.
Dr. Frederick is assistant clinical professor in the division of hospital Medicine, department of medicine, University of California, San Diego.
Clinical Question: What are the risks of nonthoracic MRI in patients with pacemakers or implantable cardioverter-defibrillators (ICD) who are not preapproved by the Food and Drug Administration for MRI scanning?
Background: Implantable cardiovascular devices could suffer heating in MRI magnetic fields leading to cardiac thermal injury and changes in pacing properties. The FDA approves “MRI-conditional devices” deemed safe for MRI, but up to six million patients worldwide (and two million in the United States) have non–MRI conditional devices.
Setting: U.S. Centers participating in the MagnaSafe registry.
Synopsis: Adults with non–MRI conditional pacemakers (1000 cases) or ICDs (500 cases) implanted in the thorax after 2001 were scanned with nonthoracic MRI at 1.5 Tesla. Patients with abandoned or inactive leads, other implantable devices, and low batteries and pacing-dependent patients with ICDs were excluded.
Devices were interrogated before each MRI and set to either no pacing or asynchronous pacing with all tachycardia and bradycardia therapies deactivated. Primary endpoints included immediate death, generator or lead failure, loss of capture in paced patients, new arrhythmia, and generator reset.
No patients suffered death or device or lead failure. Six patients developed self-terminating atrial arrhythmias, while an additional six had partial pacemaker electrical reset. Several devices had detectable changes in battery voltage, lead impedance, pacing threshold, and P- or R-wave amplitude without evident clinical significance. Multiple MRIs caused no increase in adverse outcomes. This study suggests that patients with non–MRI conditional devices may be at low risk from nonthoracic imaging if appropriately screened with temporary pacemaker function modification before MRI.
Bottom Line: Appropriately screened and prepared patients with non–MRI conditional thoracic pacemakers or ICDs may be at low risk for complications from nonthoracic MRI at 1.5 Tesla.
Reference: Russo RJ, Costa HS, Silva PD, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med. 2017;376:755-64.
Dr. Frederick is assistant clinical professor in the division of hospital Medicine, department of medicine, University of California, San Diego.
Clinical Question: What are the risks of nonthoracic MRI in patients with pacemakers or implantable cardioverter-defibrillators (ICD) who are not preapproved by the Food and Drug Administration for MRI scanning?
Background: Implantable cardiovascular devices could suffer heating in MRI magnetic fields leading to cardiac thermal injury and changes in pacing properties. The FDA approves “MRI-conditional devices” deemed safe for MRI, but up to six million patients worldwide (and two million in the United States) have non–MRI conditional devices.
Setting: U.S. Centers participating in the MagnaSafe registry.
Synopsis: Adults with non–MRI conditional pacemakers (1000 cases) or ICDs (500 cases) implanted in the thorax after 2001 were scanned with nonthoracic MRI at 1.5 Tesla. Patients with abandoned or inactive leads, other implantable devices, and low batteries and pacing-dependent patients with ICDs were excluded.
Devices were interrogated before each MRI and set to either no pacing or asynchronous pacing with all tachycardia and bradycardia therapies deactivated. Primary endpoints included immediate death, generator or lead failure, loss of capture in paced patients, new arrhythmia, and generator reset.
No patients suffered death or device or lead failure. Six patients developed self-terminating atrial arrhythmias, while an additional six had partial pacemaker electrical reset. Several devices had detectable changes in battery voltage, lead impedance, pacing threshold, and P- or R-wave amplitude without evident clinical significance. Multiple MRIs caused no increase in adverse outcomes. This study suggests that patients with non–MRI conditional devices may be at low risk from nonthoracic imaging if appropriately screened with temporary pacemaker function modification before MRI.
Bottom Line: Appropriately screened and prepared patients with non–MRI conditional thoracic pacemakers or ICDs may be at low risk for complications from nonthoracic MRI at 1.5 Tesla.
Reference: Russo RJ, Costa HS, Silva PD, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med. 2017;376:755-64.
Dr. Frederick is assistant clinical professor in the division of hospital Medicine, department of medicine, University of California, San Diego.
Hospitalists and cost control in the U.S. health care system
The rising cost of care has been a major concern in the U.S. health care system. In 1990, about $714 million was spent on health care. In 2010, the cost had risen exponentially to about $2.6 trillion.1 An estimated $750 billion dollars is attributed to health care waste.2
Health care waste includes spending on laboratory testing, diagnostic imaging, procedures or other treatments. Below is a list of the various sources that contribute to health care spending waste:2
1. Unnecessary Services ($210 billion)
2. Excessive Administrative Costs (190 billion)
3. Inefficient Service Delivery ($130 billion)
4. Overpricing ($105 billion)
5. Fraud ($75 billion)
6. Treatment for services that could have been prevented ($55 billion)
Reducing the cost of care
The predominant fee-for-service method of reimbursement does not encourage hospitals or providers to try to control areas of waste. One strategy that puts pressure on the providers of health care to control these areas of waste is the bundled payment system. Bundled payment systems deter unnecessary testing and procedures and encourage care coordination between care providers to promote efficiency.
As hospitalists, we play a key role in the bundled payment arena. Hospitalists are strategically placed to ensure that each episode of care is provided in the most cost-efficient way possible without sacrificing quality.
Training about the evidence supporting bundled payments can be incorporated into medical school and the residency curriculum. Hospitalists can serve as educators for trainees regarding the benefits of bundled payments. This will help drive sustainability by making sure new doctors entering the health field are already equipped with knowledge about bundled payments and their advantages.
Hospitalists can also help spur innovation by engaging with hospital leadership to develop new bundled systems. Payment incentives to organizations that participate will help to drive hospitalist engagement. Hospitalists can also advocate for the development of a risk adjustment system to ensure that each patient’s severity is reflected in the payment. This will allow for more buy-in by hospitals and providers.
Improving the quality of care
The Institute of Medicine published a report that made recommendations for improving the quality of the U.S. health care system by identifying six dimensions that need to be addressed:
1. Safety
2. Effectiveness
3. Patient-centeredness
4. Timeliness
5. Efficiency
6. Equity
The Value Based Purchasing program aims to address these dimensions. The fee-for-service system does not provide an incentive to provide quality care, similar to the way it does not drive cost-conscious care. By linking reimbursement to quality care, hospitals and providers have a significant incentive to ensure that their patients receive high quality care. The passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) is another step in the direction of rewarding providers for quality of care rendered, not just quantity.
Role of hospitalists
Again, hospitalists should serve as educators about the importance of value based purchasing on quality outcomes,\ and its potential for cost savings through rendering appropriate and effective care.
Hospitalists should advocate for expanding value based purchasing across all payers. This will encourage providers to treat all their patients the same, with the expectation of improving quality of care for all patients and not just a limited insurance pool.
Hospitalists can also advocate for the utilization of the same measure for determining quality across all payers. This will allow for more efficient administrative efforts by eliminating the time used to report different measures to different insurance companies.
Unfortunately, the digital era has not made the same advances in the field of medicine as it has in other areas of life. As hospitalists, our clinical perspective puts us in a position of leadership in the area of informatics. We are uniquely qualified to exploit the power of the hospital’s information technology service and push it to its full potential.
Dr. Arole is chief hospitalist, Griffin Faculty Physicians, at Griffin Hospital in Derby, Conn.
References
1. The Healthcare Imperative: Lowering Costs and Improving Outcomes – Workshop Series Summary. 2011 Feb 24. The Institute of Medicine.
2. Health Affairs Policy Brief; Reducing Waste in Health Care. http://www.healthaffairs.org/healthpolicybriefs/brief.
The rising cost of care has been a major concern in the U.S. health care system. In 1990, about $714 million was spent on health care. In 2010, the cost had risen exponentially to about $2.6 trillion.1 An estimated $750 billion dollars is attributed to health care waste.2
Health care waste includes spending on laboratory testing, diagnostic imaging, procedures or other treatments. Below is a list of the various sources that contribute to health care spending waste:2
1. Unnecessary Services ($210 billion)
2. Excessive Administrative Costs (190 billion)
3. Inefficient Service Delivery ($130 billion)
4. Overpricing ($105 billion)
5. Fraud ($75 billion)
6. Treatment for services that could have been prevented ($55 billion)
Reducing the cost of care
The predominant fee-for-service method of reimbursement does not encourage hospitals or providers to try to control areas of waste. One strategy that puts pressure on the providers of health care to control these areas of waste is the bundled payment system. Bundled payment systems deter unnecessary testing and procedures and encourage care coordination between care providers to promote efficiency.
As hospitalists, we play a key role in the bundled payment arena. Hospitalists are strategically placed to ensure that each episode of care is provided in the most cost-efficient way possible without sacrificing quality.
Training about the evidence supporting bundled payments can be incorporated into medical school and the residency curriculum. Hospitalists can serve as educators for trainees regarding the benefits of bundled payments. This will help drive sustainability by making sure new doctors entering the health field are already equipped with knowledge about bundled payments and their advantages.
Hospitalists can also help spur innovation by engaging with hospital leadership to develop new bundled systems. Payment incentives to organizations that participate will help to drive hospitalist engagement. Hospitalists can also advocate for the development of a risk adjustment system to ensure that each patient’s severity is reflected in the payment. This will allow for more buy-in by hospitals and providers.
Improving the quality of care
The Institute of Medicine published a report that made recommendations for improving the quality of the U.S. health care system by identifying six dimensions that need to be addressed:
1. Safety
2. Effectiveness
3. Patient-centeredness
4. Timeliness
5. Efficiency
6. Equity
The Value Based Purchasing program aims to address these dimensions. The fee-for-service system does not provide an incentive to provide quality care, similar to the way it does not drive cost-conscious care. By linking reimbursement to quality care, hospitals and providers have a significant incentive to ensure that their patients receive high quality care. The passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) is another step in the direction of rewarding providers for quality of care rendered, not just quantity.
Role of hospitalists
Again, hospitalists should serve as educators about the importance of value based purchasing on quality outcomes,\ and its potential for cost savings through rendering appropriate and effective care.
Hospitalists should advocate for expanding value based purchasing across all payers. This will encourage providers to treat all their patients the same, with the expectation of improving quality of care for all patients and not just a limited insurance pool.
Hospitalists can also advocate for the utilization of the same measure for determining quality across all payers. This will allow for more efficient administrative efforts by eliminating the time used to report different measures to different insurance companies.
Unfortunately, the digital era has not made the same advances in the field of medicine as it has in other areas of life. As hospitalists, our clinical perspective puts us in a position of leadership in the area of informatics. We are uniquely qualified to exploit the power of the hospital’s information technology service and push it to its full potential.
Dr. Arole is chief hospitalist, Griffin Faculty Physicians, at Griffin Hospital in Derby, Conn.
References
1. The Healthcare Imperative: Lowering Costs and Improving Outcomes – Workshop Series Summary. 2011 Feb 24. The Institute of Medicine.
2. Health Affairs Policy Brief; Reducing Waste in Health Care. http://www.healthaffairs.org/healthpolicybriefs/brief.
The rising cost of care has been a major concern in the U.S. health care system. In 1990, about $714 million was spent on health care. In 2010, the cost had risen exponentially to about $2.6 trillion.1 An estimated $750 billion dollars is attributed to health care waste.2
Health care waste includes spending on laboratory testing, diagnostic imaging, procedures or other treatments. Below is a list of the various sources that contribute to health care spending waste:2
1. Unnecessary Services ($210 billion)
2. Excessive Administrative Costs (190 billion)
3. Inefficient Service Delivery ($130 billion)
4. Overpricing ($105 billion)
5. Fraud ($75 billion)
6. Treatment for services that could have been prevented ($55 billion)
Reducing the cost of care
The predominant fee-for-service method of reimbursement does not encourage hospitals or providers to try to control areas of waste. One strategy that puts pressure on the providers of health care to control these areas of waste is the bundled payment system. Bundled payment systems deter unnecessary testing and procedures and encourage care coordination between care providers to promote efficiency.
As hospitalists, we play a key role in the bundled payment arena. Hospitalists are strategically placed to ensure that each episode of care is provided in the most cost-efficient way possible without sacrificing quality.
Training about the evidence supporting bundled payments can be incorporated into medical school and the residency curriculum. Hospitalists can serve as educators for trainees regarding the benefits of bundled payments. This will help drive sustainability by making sure new doctors entering the health field are already equipped with knowledge about bundled payments and their advantages.
Hospitalists can also help spur innovation by engaging with hospital leadership to develop new bundled systems. Payment incentives to organizations that participate will help to drive hospitalist engagement. Hospitalists can also advocate for the development of a risk adjustment system to ensure that each patient’s severity is reflected in the payment. This will allow for more buy-in by hospitals and providers.
Improving the quality of care
The Institute of Medicine published a report that made recommendations for improving the quality of the U.S. health care system by identifying six dimensions that need to be addressed:
1. Safety
2. Effectiveness
3. Patient-centeredness
4. Timeliness
5. Efficiency
6. Equity
The Value Based Purchasing program aims to address these dimensions. The fee-for-service system does not provide an incentive to provide quality care, similar to the way it does not drive cost-conscious care. By linking reimbursement to quality care, hospitals and providers have a significant incentive to ensure that their patients receive high quality care. The passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) is another step in the direction of rewarding providers for quality of care rendered, not just quantity.
Role of hospitalists
Again, hospitalists should serve as educators about the importance of value based purchasing on quality outcomes,\ and its potential for cost savings through rendering appropriate and effective care.
Hospitalists should advocate for expanding value based purchasing across all payers. This will encourage providers to treat all their patients the same, with the expectation of improving quality of care for all patients and not just a limited insurance pool.
Hospitalists can also advocate for the utilization of the same measure for determining quality across all payers. This will allow for more efficient administrative efforts by eliminating the time used to report different measures to different insurance companies.
Unfortunately, the digital era has not made the same advances in the field of medicine as it has in other areas of life. As hospitalists, our clinical perspective puts us in a position of leadership in the area of informatics. We are uniquely qualified to exploit the power of the hospital’s information technology service and push it to its full potential.
Dr. Arole is chief hospitalist, Griffin Faculty Physicians, at Griffin Hospital in Derby, Conn.
References
1. The Healthcare Imperative: Lowering Costs and Improving Outcomes – Workshop Series Summary. 2011 Feb 24. The Institute of Medicine.
2. Health Affairs Policy Brief; Reducing Waste in Health Care. http://www.healthaffairs.org/healthpolicybriefs/brief.















