User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Long-term oxygen for COPD with moderate desaturation
Clinical question: Does using supplemental oxygen in patients with stable chronic obstructive pulmonary disease (COPD) result in a longer time to death or first hospitalization?
Background: Previous trials have shown that use of long-term, supplemental oxygen in COPD and severe resting hypoxia reduced mortality, however, data is inconclusive if its use in mild-moderate COPD has the same effect.
Study design: Parallel-group, randomized, unblinded clinical trial.
Synopsis: Researchers randomized 738 patients from 42 outpatient centers with stable COPD and moderate resting desaturation (SpO2, 89%-93%) or moderate exercise induced desaturation (6-minute walk test, SpO2 greater than 80% for five minutes, and greater than 90% for 10 seconds) to long-term supplemental oxygen or no supplemental oxygen. Time-to-event analysis found no differences in the composite primary outcome of death or first hospitalization (HR, 0.94; 95% confidence interval, 0.79-1.12), or in any other secondary outcomes of COPD exacerbations, or COPD-related or all-cause hospitalizations.
Limitations included lack of blinding, possible exclusion of patients with higher COPD severity, and lack of assessment of immediate effects of oxygen on symptoms or exercise performance.
Bottom line: Long-term, supplemental oxygen provided no benefit in mortality or time to first hospitalization among other outcomes in patients with stable COPD and resting or exercise-induced moderate desaturations.
Citation: The Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617-27.
Dr. Ciarkowski is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: Does using supplemental oxygen in patients with stable chronic obstructive pulmonary disease (COPD) result in a longer time to death or first hospitalization?
Background: Previous trials have shown that use of long-term, supplemental oxygen in COPD and severe resting hypoxia reduced mortality, however, data is inconclusive if its use in mild-moderate COPD has the same effect.
Study design: Parallel-group, randomized, unblinded clinical trial.
Synopsis: Researchers randomized 738 patients from 42 outpatient centers with stable COPD and moderate resting desaturation (SpO2, 89%-93%) or moderate exercise induced desaturation (6-minute walk test, SpO2 greater than 80% for five minutes, and greater than 90% for 10 seconds) to long-term supplemental oxygen or no supplemental oxygen. Time-to-event analysis found no differences in the composite primary outcome of death or first hospitalization (HR, 0.94; 95% confidence interval, 0.79-1.12), or in any other secondary outcomes of COPD exacerbations, or COPD-related or all-cause hospitalizations.
Limitations included lack of blinding, possible exclusion of patients with higher COPD severity, and lack of assessment of immediate effects of oxygen on symptoms or exercise performance.
Bottom line: Long-term, supplemental oxygen provided no benefit in mortality or time to first hospitalization among other outcomes in patients with stable COPD and resting or exercise-induced moderate desaturations.
Citation: The Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617-27.
Dr. Ciarkowski is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: Does using supplemental oxygen in patients with stable chronic obstructive pulmonary disease (COPD) result in a longer time to death or first hospitalization?
Background: Previous trials have shown that use of long-term, supplemental oxygen in COPD and severe resting hypoxia reduced mortality, however, data is inconclusive if its use in mild-moderate COPD has the same effect.
Study design: Parallel-group, randomized, unblinded clinical trial.
Synopsis: Researchers randomized 738 patients from 42 outpatient centers with stable COPD and moderate resting desaturation (SpO2, 89%-93%) or moderate exercise induced desaturation (6-minute walk test, SpO2 greater than 80% for five minutes, and greater than 90% for 10 seconds) to long-term supplemental oxygen or no supplemental oxygen. Time-to-event analysis found no differences in the composite primary outcome of death or first hospitalization (HR, 0.94; 95% confidence interval, 0.79-1.12), or in any other secondary outcomes of COPD exacerbations, or COPD-related or all-cause hospitalizations.
Limitations included lack of blinding, possible exclusion of patients with higher COPD severity, and lack of assessment of immediate effects of oxygen on symptoms or exercise performance.
Bottom line: Long-term, supplemental oxygen provided no benefit in mortality or time to first hospitalization among other outcomes in patients with stable COPD and resting or exercise-induced moderate desaturations.
Citation: The Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617-27.
Dr. Ciarkowski is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Hospital-acquired VTE with high risk of recurrence
Clinical question: Is the risk of recurrence for a venous thromboembolism (VTE) acquired as an inpatient higher than other reversible risk factors?
Background: In patients with acute VTE, transient provoking factors place patients at lower risks for recurrent VTE, while persistent factors (that is, cancer) increase risk for recurrence. Unprovoked VTE places patients at intermediate to high risk, but few data are present for VTE experienced while in the hospital.
Study design: Single-center, population-based, prospective cohort study.
Setting: Tromso, Norway.
Synopsis: Using repeat health surveys from 1994 to 2012, researchers followed 822 patients with a validated, first-lifetime VTE. Hospital-related VTE was defined as a VTE within 8 weeks of hospitalization related to medical illness, surgery, or in patients with active cancer.
This global definition of hospital-related VTE was not associated with an increased risk of recurrent VTE (hazard ratio, 0.99; 0.69-1.41). However, in separate groups, the cumulative risk of recurrence after 5 years in hospital-related VTE due to medical illness was similar to nonhospital-related VTE (20.1% vs. 18.4%), higher than VTE related to surgery (11%), and lower than VTE related to cancer (27.4%).
Risk-adjusted analyses maintained these differences in recurrence risk dependent on reason for hospitalization (cancer, medical illness, surgery). When compared with non-hospital VTE, however, hospital-related VTE was associated with a threefold higher risk of death.
Study limitations included being from a single center, possibly underpowered due to low number of events.
Bottom line: Hospital-related VTE has a high risk of recurrence, but risk level is variable and dependent on the reason for hospitalization.
Citation: Bjøri E, Arshad N, Johnsen HS, Hansen J-B, Brækkan SK. Hospital-related first venous thromboembolism and risk of recurrence [published online ahead of print, Sept. 2, 2016]. J Thromb Haemost. doi: 10.1111/jth.13492
Dr. Ciarkowski is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: Is the risk of recurrence for a venous thromboembolism (VTE) acquired as an inpatient higher than other reversible risk factors?
Background: In patients with acute VTE, transient provoking factors place patients at lower risks for recurrent VTE, while persistent factors (that is, cancer) increase risk for recurrence. Unprovoked VTE places patients at intermediate to high risk, but few data are present for VTE experienced while in the hospital.
Study design: Single-center, population-based, prospective cohort study.
Setting: Tromso, Norway.
Synopsis: Using repeat health surveys from 1994 to 2012, researchers followed 822 patients with a validated, first-lifetime VTE. Hospital-related VTE was defined as a VTE within 8 weeks of hospitalization related to medical illness, surgery, or in patients with active cancer.
This global definition of hospital-related VTE was not associated with an increased risk of recurrent VTE (hazard ratio, 0.99; 0.69-1.41). However, in separate groups, the cumulative risk of recurrence after 5 years in hospital-related VTE due to medical illness was similar to nonhospital-related VTE (20.1% vs. 18.4%), higher than VTE related to surgery (11%), and lower than VTE related to cancer (27.4%).
Risk-adjusted analyses maintained these differences in recurrence risk dependent on reason for hospitalization (cancer, medical illness, surgery). When compared with non-hospital VTE, however, hospital-related VTE was associated with a threefold higher risk of death.
Study limitations included being from a single center, possibly underpowered due to low number of events.
Bottom line: Hospital-related VTE has a high risk of recurrence, but risk level is variable and dependent on the reason for hospitalization.
Citation: Bjøri E, Arshad N, Johnsen HS, Hansen J-B, Brækkan SK. Hospital-related first venous thromboembolism and risk of recurrence [published online ahead of print, Sept. 2, 2016]. J Thromb Haemost. doi: 10.1111/jth.13492
Dr. Ciarkowski is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: Is the risk of recurrence for a venous thromboembolism (VTE) acquired as an inpatient higher than other reversible risk factors?
Background: In patients with acute VTE, transient provoking factors place patients at lower risks for recurrent VTE, while persistent factors (that is, cancer) increase risk for recurrence. Unprovoked VTE places patients at intermediate to high risk, but few data are present for VTE experienced while in the hospital.
Study design: Single-center, population-based, prospective cohort study.
Setting: Tromso, Norway.
Synopsis: Using repeat health surveys from 1994 to 2012, researchers followed 822 patients with a validated, first-lifetime VTE. Hospital-related VTE was defined as a VTE within 8 weeks of hospitalization related to medical illness, surgery, or in patients with active cancer.
This global definition of hospital-related VTE was not associated with an increased risk of recurrent VTE (hazard ratio, 0.99; 0.69-1.41). However, in separate groups, the cumulative risk of recurrence after 5 years in hospital-related VTE due to medical illness was similar to nonhospital-related VTE (20.1% vs. 18.4%), higher than VTE related to surgery (11%), and lower than VTE related to cancer (27.4%).
Risk-adjusted analyses maintained these differences in recurrence risk dependent on reason for hospitalization (cancer, medical illness, surgery). When compared with non-hospital VTE, however, hospital-related VTE was associated with a threefold higher risk of death.
Study limitations included being from a single center, possibly underpowered due to low number of events.
Bottom line: Hospital-related VTE has a high risk of recurrence, but risk level is variable and dependent on the reason for hospitalization.
Citation: Bjøri E, Arshad N, Johnsen HS, Hansen J-B, Brækkan SK. Hospital-related first venous thromboembolism and risk of recurrence [published online ahead of print, Sept. 2, 2016]. J Thromb Haemost. doi: 10.1111/jth.13492
Dr. Ciarkowski is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Do not use steroids in patients with severe sepsis without shock
Clinical question: Does hydrocortisone therapy prevent progression to septic shock in patients with severe sepsis without shock?
Background: Current sepsis management guidelines recommend use of hydrocortisone in patients with septic shock who are unable to restore hemodynamic stability with IV fluids and pressors; current guidelines also recommend against use of corticosteroids without shock. However, these recommendations are based on two RCTs and remain controversial.
Study design: Multicenter, placebo-controlled, double-blind RCT.
Setting: Thirty-four intermediate or intensive care units in German university and community hospitals.
Synopsis: Investigators randomly assigned 380 patients to hydrocortisone or placebo. Patients were included if they had clinical evidence of infection, evidence of SIRS (systemic inflammatory response syndrome), and evidence of organ dysfunction. Patients were excluded if they had any of the following: sepsis-induced hypotension, separate indication for systemic steroid use, or hypersensitivity to steroids. Primary outcome was the occurrence of septic shock within 14 days. Secondary outcomes included time to septic shock or death, death in the ICU or hospital, organ dysfunction, ventilator therapy, renal replacement therapy, and secondary infection.
Study results showed no significant difference in the primary outcome between groups, or in any of the secondary outcomes. In a post-hoc analysis, there was more hyperglycemia and less delirium in the study group.
Study limitations are inclusion of patients only after consent, potentially missing early septic shock, and the fact that many analyses were done post-hoc.
Bottom line: Steroids should be avoided in severe sepsis without shock.
Citation: Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis. JAMA. 2016;316(17):1775-85.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does hydrocortisone therapy prevent progression to septic shock in patients with severe sepsis without shock?
Background: Current sepsis management guidelines recommend use of hydrocortisone in patients with septic shock who are unable to restore hemodynamic stability with IV fluids and pressors; current guidelines also recommend against use of corticosteroids without shock. However, these recommendations are based on two RCTs and remain controversial.
Study design: Multicenter, placebo-controlled, double-blind RCT.
Setting: Thirty-four intermediate or intensive care units in German university and community hospitals.
Synopsis: Investigators randomly assigned 380 patients to hydrocortisone or placebo. Patients were included if they had clinical evidence of infection, evidence of SIRS (systemic inflammatory response syndrome), and evidence of organ dysfunction. Patients were excluded if they had any of the following: sepsis-induced hypotension, separate indication for systemic steroid use, or hypersensitivity to steroids. Primary outcome was the occurrence of septic shock within 14 days. Secondary outcomes included time to septic shock or death, death in the ICU or hospital, organ dysfunction, ventilator therapy, renal replacement therapy, and secondary infection.
Study results showed no significant difference in the primary outcome between groups, or in any of the secondary outcomes. In a post-hoc analysis, there was more hyperglycemia and less delirium in the study group.
Study limitations are inclusion of patients only after consent, potentially missing early septic shock, and the fact that many analyses were done post-hoc.
Bottom line: Steroids should be avoided in severe sepsis without shock.
Citation: Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis. JAMA. 2016;316(17):1775-85.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does hydrocortisone therapy prevent progression to septic shock in patients with severe sepsis without shock?
Background: Current sepsis management guidelines recommend use of hydrocortisone in patients with septic shock who are unable to restore hemodynamic stability with IV fluids and pressors; current guidelines also recommend against use of corticosteroids without shock. However, these recommendations are based on two RCTs and remain controversial.
Study design: Multicenter, placebo-controlled, double-blind RCT.
Setting: Thirty-four intermediate or intensive care units in German university and community hospitals.
Synopsis: Investigators randomly assigned 380 patients to hydrocortisone or placebo. Patients were included if they had clinical evidence of infection, evidence of SIRS (systemic inflammatory response syndrome), and evidence of organ dysfunction. Patients were excluded if they had any of the following: sepsis-induced hypotension, separate indication for systemic steroid use, or hypersensitivity to steroids. Primary outcome was the occurrence of septic shock within 14 days. Secondary outcomes included time to septic shock or death, death in the ICU or hospital, organ dysfunction, ventilator therapy, renal replacement therapy, and secondary infection.
Study results showed no significant difference in the primary outcome between groups, or in any of the secondary outcomes. In a post-hoc analysis, there was more hyperglycemia and less delirium in the study group.
Study limitations are inclusion of patients only after consent, potentially missing early septic shock, and the fact that many analyses were done post-hoc.
Bottom line: Steroids should be avoided in severe sepsis without shock.
Citation: Keh D, Trips E, Marx G, et al. Effect of hydrocortisone on development of shock among patients with severe sepsis. JAMA. 2016;316(17):1775-85.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Dabigatran has less bleeding than rivaroxaban in atrial fibrillation
Clinical question: Does dabigatran or rivaroxaban have more bleeding episodes?
Background: Alternatives to warfarin exist for stroke prevention in nonvalvular atrial fibrillation (AF). The RE-LY and ROCKET-AF trials demonstrated noninferiority to warfarin for both dabigatran (a direct thrombin inhibitor) and rivaroxaban (a factor Xa inhibitor), respectively. Although indirect comparisons have been done using data from these trials, direct, head-to-head comparisons are not available.
Study design: New-user cohort study.
Setting: Medicare beneficiaries 65 years or older with AF and a prescription for either dabigatran or rivaroxaban.
Intracranial hemorrhage and extracranial major bleeding events were significantly greater in the rivaroxaban group than the dabigatran group. There was no significant difference in thromboembolic stroke events.
Limitations include short treatment and follow-up times. Additionally, the study is not generalizable to younger populations.
Bottom line: In elderly patients with non-valvular AF, rivaroxaban was associated with more adverse bleeding events than dabigatran, with no difference in stroke prevention.
Citation: Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662-71.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does dabigatran or rivaroxaban have more bleeding episodes?
Background: Alternatives to warfarin exist for stroke prevention in nonvalvular atrial fibrillation (AF). The RE-LY and ROCKET-AF trials demonstrated noninferiority to warfarin for both dabigatran (a direct thrombin inhibitor) and rivaroxaban (a factor Xa inhibitor), respectively. Although indirect comparisons have been done using data from these trials, direct, head-to-head comparisons are not available.
Study design: New-user cohort study.
Setting: Medicare beneficiaries 65 years or older with AF and a prescription for either dabigatran or rivaroxaban.
Intracranial hemorrhage and extracranial major bleeding events were significantly greater in the rivaroxaban group than the dabigatran group. There was no significant difference in thromboembolic stroke events.
Limitations include short treatment and follow-up times. Additionally, the study is not generalizable to younger populations.
Bottom line: In elderly patients with non-valvular AF, rivaroxaban was associated with more adverse bleeding events than dabigatran, with no difference in stroke prevention.
Citation: Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662-71.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
Clinical question: Does dabigatran or rivaroxaban have more bleeding episodes?
Background: Alternatives to warfarin exist for stroke prevention in nonvalvular atrial fibrillation (AF). The RE-LY and ROCKET-AF trials demonstrated noninferiority to warfarin for both dabigatran (a direct thrombin inhibitor) and rivaroxaban (a factor Xa inhibitor), respectively. Although indirect comparisons have been done using data from these trials, direct, head-to-head comparisons are not available.
Study design: New-user cohort study.
Setting: Medicare beneficiaries 65 years or older with AF and a prescription for either dabigatran or rivaroxaban.
Intracranial hemorrhage and extracranial major bleeding events were significantly greater in the rivaroxaban group than the dabigatran group. There was no significant difference in thromboembolic stroke events.
Limitations include short treatment and follow-up times. Additionally, the study is not generalizable to younger populations.
Bottom line: In elderly patients with non-valvular AF, rivaroxaban was associated with more adverse bleeding events than dabigatran, with no difference in stroke prevention.
Citation: Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662-71.
Dr. Graves is an assistant professor at the University of Utah School of Medicine and associate program director of quality and patient safety for the University of Utah Internal Medicine residency training program.
High-flow oxygen noninferior to noninvasive ventilation postextubation
Clinical Question: Is high-flow oxygen noninferior to noninvasive ventilation (NIV) in preventing postextubation respiratory failure and reintubation?
Background: Studies that suggest NIV usage following extubation reduces the risk of postextubation respiratory failure have led to an increase in use of this practice. Compared with NIV, high-flow, conditioned oxygen therapy has many advantages and fewer adverse effects, suggesting it might be a useful alternative.
Study design: Randomized clinical trial.
Setting: Three ICUs in Spain.
Rates of most secondary outcomes, including infection, mortality, and hospital length of stay (LOS) were similar between the two groups. ICU LOS was significantly less in the high-flow oxygen group (3d vs. 4d; 95% CI, –6.8 to –0.8).
Additionally, every patient tolerated high-flow oxygen therapy, while 40% of patients in the NIV arm required withdrawal of therapy for at least 6 hours due to adverse effects (P less than .001).
Bottom line: High-flow oxygen immediately following extubation may be a useful alternative to NIV in preventing postextubation respiratory failure.
Citation: Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354-61.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical Question: Is high-flow oxygen noninferior to noninvasive ventilation (NIV) in preventing postextubation respiratory failure and reintubation?
Background: Studies that suggest NIV usage following extubation reduces the risk of postextubation respiratory failure have led to an increase in use of this practice. Compared with NIV, high-flow, conditioned oxygen therapy has many advantages and fewer adverse effects, suggesting it might be a useful alternative.
Study design: Randomized clinical trial.
Setting: Three ICUs in Spain.
Rates of most secondary outcomes, including infection, mortality, and hospital length of stay (LOS) were similar between the two groups. ICU LOS was significantly less in the high-flow oxygen group (3d vs. 4d; 95% CI, –6.8 to –0.8).
Additionally, every patient tolerated high-flow oxygen therapy, while 40% of patients in the NIV arm required withdrawal of therapy for at least 6 hours due to adverse effects (P less than .001).
Bottom line: High-flow oxygen immediately following extubation may be a useful alternative to NIV in preventing postextubation respiratory failure.
Citation: Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354-61.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical Question: Is high-flow oxygen noninferior to noninvasive ventilation (NIV) in preventing postextubation respiratory failure and reintubation?
Background: Studies that suggest NIV usage following extubation reduces the risk of postextubation respiratory failure have led to an increase in use of this practice. Compared with NIV, high-flow, conditioned oxygen therapy has many advantages and fewer adverse effects, suggesting it might be a useful alternative.
Study design: Randomized clinical trial.
Setting: Three ICUs in Spain.
Rates of most secondary outcomes, including infection, mortality, and hospital length of stay (LOS) were similar between the two groups. ICU LOS was significantly less in the high-flow oxygen group (3d vs. 4d; 95% CI, –6.8 to –0.8).
Additionally, every patient tolerated high-flow oxygen therapy, while 40% of patients in the NIV arm required withdrawal of therapy for at least 6 hours due to adverse effects (P less than .001).
Bottom line: High-flow oxygen immediately following extubation may be a useful alternative to NIV in preventing postextubation respiratory failure.
Citation: Hernández G, Vaquero C, González P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354-61.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
New guidelines for patients requiring red blood cell transfusion
Clinical question: What is a safe target hemoglobin level for patients requiring red blood cell transfusion, and how long can red blood cells be stored prior to transfusion?
Background: The AABB, formerly the American Association of Blood Banks, notes several new, large, rigorous studies on transfusion thresholds were published since their last guideline in 2012. Additionally, there are concerns from initial studies of increased morbidity and mortality with transfusions of red blood cells stored for longer periods of time.
Study design: Systematic review and meta-analysis.
Setting: Summary findings from the AABB clinical transfusion medicine committee.
Synopsis: Thirty-one randomized clinical trials (RCTs) evaluating blood transfusion thresholds were reviewed and analyzed, including 12,587 patients across various clinical scenarios. The authors recommend a restrictive threshold of 7 g/dL for most hospitalized adult patients in the appropriate clinical context. For patients undergoing orthopedic or cardiac surgery, or with cardiovascular disease, a threshold of 8 g/dL is recommended, as it was the threshold used in studies of these patients (though such patients may actually tolerate a lower value).
No recommendations were made for patients with acute coronary syndrome, hematological or oncological disorders, severe thrombocytopenia, or chronic transfusion-dependent anemia given limited data.
To determine a safe period of time for blood storage prior to transfusion, 13 RCTs were reviewed and analyzed. The authors recommend that patients requiring transfusion receive red blood cell units at any period within the standard issue period (less than 42 days), rather than limit transfusion to fresh units (less than 10 days).
Bottom line: A restrictive red blood cell transfusion threshold of 7-8 g/dL is safe in most clinical settings, and there is no advantage to using fresh units as opposed to those stored for the standard period.
Citation: Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB. JAMA. 2016;316(19):2025-35.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: What is a safe target hemoglobin level for patients requiring red blood cell transfusion, and how long can red blood cells be stored prior to transfusion?
Background: The AABB, formerly the American Association of Blood Banks, notes several new, large, rigorous studies on transfusion thresholds were published since their last guideline in 2012. Additionally, there are concerns from initial studies of increased morbidity and mortality with transfusions of red blood cells stored for longer periods of time.
Study design: Systematic review and meta-analysis.
Setting: Summary findings from the AABB clinical transfusion medicine committee.
Synopsis: Thirty-one randomized clinical trials (RCTs) evaluating blood transfusion thresholds were reviewed and analyzed, including 12,587 patients across various clinical scenarios. The authors recommend a restrictive threshold of 7 g/dL for most hospitalized adult patients in the appropriate clinical context. For patients undergoing orthopedic or cardiac surgery, or with cardiovascular disease, a threshold of 8 g/dL is recommended, as it was the threshold used in studies of these patients (though such patients may actually tolerate a lower value).
No recommendations were made for patients with acute coronary syndrome, hematological or oncological disorders, severe thrombocytopenia, or chronic transfusion-dependent anemia given limited data.
To determine a safe period of time for blood storage prior to transfusion, 13 RCTs were reviewed and analyzed. The authors recommend that patients requiring transfusion receive red blood cell units at any period within the standard issue period (less than 42 days), rather than limit transfusion to fresh units (less than 10 days).
Bottom line: A restrictive red blood cell transfusion threshold of 7-8 g/dL is safe in most clinical settings, and there is no advantage to using fresh units as opposed to those stored for the standard period.
Citation: Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB. JAMA. 2016;316(19):2025-35.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: What is a safe target hemoglobin level for patients requiring red blood cell transfusion, and how long can red blood cells be stored prior to transfusion?
Background: The AABB, formerly the American Association of Blood Banks, notes several new, large, rigorous studies on transfusion thresholds were published since their last guideline in 2012. Additionally, there are concerns from initial studies of increased morbidity and mortality with transfusions of red blood cells stored for longer periods of time.
Study design: Systematic review and meta-analysis.
Setting: Summary findings from the AABB clinical transfusion medicine committee.
Synopsis: Thirty-one randomized clinical trials (RCTs) evaluating blood transfusion thresholds were reviewed and analyzed, including 12,587 patients across various clinical scenarios. The authors recommend a restrictive threshold of 7 g/dL for most hospitalized adult patients in the appropriate clinical context. For patients undergoing orthopedic or cardiac surgery, or with cardiovascular disease, a threshold of 8 g/dL is recommended, as it was the threshold used in studies of these patients (though such patients may actually tolerate a lower value).
No recommendations were made for patients with acute coronary syndrome, hematological or oncological disorders, severe thrombocytopenia, or chronic transfusion-dependent anemia given limited data.
To determine a safe period of time for blood storage prior to transfusion, 13 RCTs were reviewed and analyzed. The authors recommend that patients requiring transfusion receive red blood cell units at any period within the standard issue period (less than 42 days), rather than limit transfusion to fresh units (less than 10 days).
Bottom line: A restrictive red blood cell transfusion threshold of 7-8 g/dL is safe in most clinical settings, and there is no advantage to using fresh units as opposed to those stored for the standard period.
Citation: Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB. JAMA. 2016;316(19):2025-35.
Dr. Murphy is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
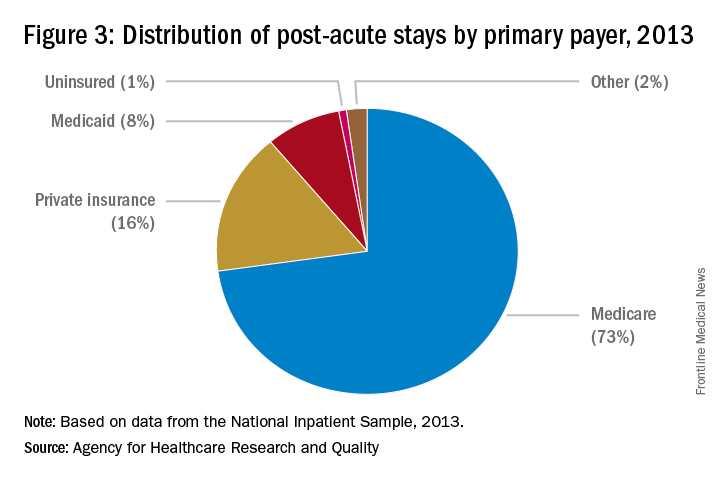
Eight things hospitalists need to know about post-acute care
Whether you’re a hospitalist who works only in a hospital, a hospitalist who works only in a post-acute care (PAC) setting, or a hospitalist who works in both types of facilities, knowing about current trends at PAC facilities and what the future may hold can help you excel in your current capacity and, ultimately, improve patient care.
The Hospitalist tapped experts in the post-acute space to tell us what they thought HM should know about working in PAC – which, in many ways, is quite different from the hospital setting. Here’s a compilation of their top eight must-knows.
1. PAC settings rely more on mid-level medical staff than hospitals do.
PAC facilities employ more mid-level providers, such as nurse practitioners and physician assistants, because they can support the level of medical complexity and decision making 95% of the time, says James D. Tollman, MD, FHM, president of Essex Inpatient Physicians in Boxford, Mass. Further, they are more heavily staffed by licensed practical nurses than are acute-care settings.
Usually, there is no physician or nurse practitioner presence at night. Clinicians rely on nursing staff’s assessment to make decisions regarding changes in patient status during off-hours, says Virginia Cummings, MD, director of long-term care, gerontology division, at Boston-based Beth Israel Deaconess Medical Center.
2. Testing takes longer, and options are limited.
Access to some acute urgent resources such as laboratory testing, imaging tests, and pharmacy products is more challenging at PAC facilities because most of these resources are not on-site. Consequently, there is a time lag between ordering tests and new medications and implementing these orders.
“If a patient needs something performed diagnostically immediately, they usually have to be transported to the emergency room or a facility with the necessary testing equipment,” Dr. Tollman says.
However, Paul T. Liistro, managing partner, Arbors of Hop Brook Limited Partnership in Manchester, Conn., and Vernon Manor Health Care Center in Vernon, Conn., and administrator, Manchester Manor Health Care Center, notes that it’s possible for a laboratory service or mobile diagnostic unit to provide laboratory testing or certain imaging at a PAC facility. More-involved diagnostics, such as an MRI or a PET scan, typically require testing at a remote location.
3. Patient populations mainly include rehab and terminally ill patients.
Patients are typically sent to a PAC facility either to recover from an illness or injury or because they are chronically ill and have exhausted treatment options. Regarding the latter, “They are mostly there for palliation; we don’t perform daily tests or prescribe aggressive medications on these patients,” Dr. Nazir says.
Dr. Cummings explains that PAC clinicians go through “the dying process with the patient.”
“They may or may not have assistance from hospice organizations,” she says, “and when they don’t, [hospitalists] take on the role of palliative-care providers.”
Dr. Cummings has seen an increase in psychiatric patients entering PAC facilities.
“Many patients with chronic psychological problems are aging, and there are fewer inpatient psychiatric beds available to those with concurrent medical and psychiatric problems,” she says. Much of this work is now being done in PAC settings. 
4. You can build a relationship with your patient.
Because the pace of a PAC facility is slower and a patient typically stays in a PAC facility longer than at a hospital, there’s time for a hospitalist to have more in-depth conversations with patients and their families.
“Building a deeper relationship with a patient may give the hospitalist an opportunity to discover the cause of an acute problem,” Dr. Nazir says. “They can go in-depth into the psychosocial aspect of medicine and may be able to find out what led to the initial problem and the real root cause, which can help prevent future recurrences, such as repeat falls or forgetting to take a medication.”
5. Using EHRs can improve transitions.
Care transitions between a hospital and PAC facility can be compromised by a lack of information sharing, and they can affect the quality and safety of patient care, says Dori Cross, a doctoral candidate in health services organization and policy at the University of Michigan School of Public Health in Ann Arbor. Handoffs between providers require information continuity – information that is complete, timely, and in a usable format – to ensure appropriate medical decisions and to provide high-quality care during and after transition.
Electronic health records (EHRs) as well as health information exchanges (HIEs) allow providers to communicate and share patient information. For example, hospitals can send information electronically to PAC facilities (“push” exchange) or make information available online securely for PAC providers to log in and access (“pull” exchange). According to a 2014 survey data by the American Hospital Association, more than 50% of hospitals report sending structured summary-of-care records electronically to long-term care settings; a little less than half of those hospitals (23% of the total sample of hospitals) were also receiving information electronically from long-term care sites.1
“This bidirectional exchange, in particular, can make it easier to share information across provider organizations electronically and, in turn, improve care delivery,” says Ms. Cross, who authored an accepted paper on the subject in the Journal of Post-Acute and Long-Term Medicine.
6. Hospitalists can work with providers in PAC settings to improve transitions.
Despite improvements in the electronic transfer of medical information, gaps still exist and can cause problems. One chasm when discharging patients to a PAC facility, is when a hospital IT system is incapable of communicating with the PAC facility system. In this instance, Dr. Nazir says, the hospitalist “can help bridge the gap.”
“[We] can verbally relay relevant information to physicians at PAC facilities so they understand the patient’s status, needs, and expectations,” he says. “Furthermore, hospitalists and a PAC facility’s administration can brainstorm methods to improve the systems of care so the patient receives more effective and timely care.”
7. Hospitalists switching to the PAC setting should have formal training.
The two main obstacles for hospitalists who change from working in a hospital to a PAC facility are the lack of exposure to PAC work in training and the assumption that it requires the same skills sets of a typical hospitalist, according to Manoj K. Mathew, MD, SFHM, national medical director of Los Angeles–based Agilon Health. The PAC setting has quite a number of differences compared with a hospital setting. For example, some regulations apply specifically to PAC facilities. In addition to formal training, hospitalists can benefit from using SHM’s Post-Acute Care Transitions Toolkit, having a mentor, or using resources from other organizations that function in this space such as The Society for Post-Acute and Long-Term Care Medicine, Dr. Nazir says.
8. A variety of payors and payment models are in play.
Commercial insurers continue to be major payors for PAC, especially for individuals younger than 65 years. Medicare and Medicaid, administered by the Centers for Medicare & Medicaid Services, are the primary payors for patients aged 65 years and older.
“These scorecards are using a variety of criteria to rank providers, such as length of stay, cost, readmissions to hospitals, and quality.”
Because Medicare Part A covers many patients discharged to a PAC setting, any changes in payment incentives or benefit structures by the Medicare program will drive changes in PAC.
“For example, as Medicare implements payment adjustments for hospitals that have high rates of readmissions, hospitals have a new incentive to work closely with SNFs and other providers of PAC to ensure patients can avoid unnecessary readmissions,” says Tiffany A. Radcliff, PhD, a health economist and associate professor in the department of health policy and management at Texas A&M University School of Public Health in College Station.
Providers must follow the billing rules for each payor. The rules for Medicare payments are outlined on CMS’ website. Bundled payments for PAC under the Medicare Part A program are scheduled to be implemented by 2018.
Reference
Cross DA, Adler-Milstein J. Investing in post-acute care transitions: electronic information exchange between hospitals and long-term care facilities [published online ahead of print Sept. 14, 2016]. JAMDA. doi: http://dx.doi.org/10.1016/j.jamda.2016.07.024.
Whether you’re a hospitalist who works only in a hospital, a hospitalist who works only in a post-acute care (PAC) setting, or a hospitalist who works in both types of facilities, knowing about current trends at PAC facilities and what the future may hold can help you excel in your current capacity and, ultimately, improve patient care.
The Hospitalist tapped experts in the post-acute space to tell us what they thought HM should know about working in PAC – which, in many ways, is quite different from the hospital setting. Here’s a compilation of their top eight must-knows.
1. PAC settings rely more on mid-level medical staff than hospitals do.
PAC facilities employ more mid-level providers, such as nurse practitioners and physician assistants, because they can support the level of medical complexity and decision making 95% of the time, says James D. Tollman, MD, FHM, president of Essex Inpatient Physicians in Boxford, Mass. Further, they are more heavily staffed by licensed practical nurses than are acute-care settings.
Usually, there is no physician or nurse practitioner presence at night. Clinicians rely on nursing staff’s assessment to make decisions regarding changes in patient status during off-hours, says Virginia Cummings, MD, director of long-term care, gerontology division, at Boston-based Beth Israel Deaconess Medical Center.
2. Testing takes longer, and options are limited.
Access to some acute urgent resources such as laboratory testing, imaging tests, and pharmacy products is more challenging at PAC facilities because most of these resources are not on-site. Consequently, there is a time lag between ordering tests and new medications and implementing these orders.
“If a patient needs something performed diagnostically immediately, they usually have to be transported to the emergency room or a facility with the necessary testing equipment,” Dr. Tollman says.
However, Paul T. Liistro, managing partner, Arbors of Hop Brook Limited Partnership in Manchester, Conn., and Vernon Manor Health Care Center in Vernon, Conn., and administrator, Manchester Manor Health Care Center, notes that it’s possible for a laboratory service or mobile diagnostic unit to provide laboratory testing or certain imaging at a PAC facility. More-involved diagnostics, such as an MRI or a PET scan, typically require testing at a remote location.
3. Patient populations mainly include rehab and terminally ill patients.
Patients are typically sent to a PAC facility either to recover from an illness or injury or because they are chronically ill and have exhausted treatment options. Regarding the latter, “They are mostly there for palliation; we don’t perform daily tests or prescribe aggressive medications on these patients,” Dr. Nazir says.
Dr. Cummings explains that PAC clinicians go through “the dying process with the patient.”
“They may or may not have assistance from hospice organizations,” she says, “and when they don’t, [hospitalists] take on the role of palliative-care providers.”
Dr. Cummings has seen an increase in psychiatric patients entering PAC facilities.
“Many patients with chronic psychological problems are aging, and there are fewer inpatient psychiatric beds available to those with concurrent medical and psychiatric problems,” she says. Much of this work is now being done in PAC settings. 
4. You can build a relationship with your patient.
Because the pace of a PAC facility is slower and a patient typically stays in a PAC facility longer than at a hospital, there’s time for a hospitalist to have more in-depth conversations with patients and their families.
“Building a deeper relationship with a patient may give the hospitalist an opportunity to discover the cause of an acute problem,” Dr. Nazir says. “They can go in-depth into the psychosocial aspect of medicine and may be able to find out what led to the initial problem and the real root cause, which can help prevent future recurrences, such as repeat falls or forgetting to take a medication.”
5. Using EHRs can improve transitions.
Care transitions between a hospital and PAC facility can be compromised by a lack of information sharing, and they can affect the quality and safety of patient care, says Dori Cross, a doctoral candidate in health services organization and policy at the University of Michigan School of Public Health in Ann Arbor. Handoffs between providers require information continuity – information that is complete, timely, and in a usable format – to ensure appropriate medical decisions and to provide high-quality care during and after transition.
Electronic health records (EHRs) as well as health information exchanges (HIEs) allow providers to communicate and share patient information. For example, hospitals can send information electronically to PAC facilities (“push” exchange) or make information available online securely for PAC providers to log in and access (“pull” exchange). According to a 2014 survey data by the American Hospital Association, more than 50% of hospitals report sending structured summary-of-care records electronically to long-term care settings; a little less than half of those hospitals (23% of the total sample of hospitals) were also receiving information electronically from long-term care sites.1
“This bidirectional exchange, in particular, can make it easier to share information across provider organizations electronically and, in turn, improve care delivery,” says Ms. Cross, who authored an accepted paper on the subject in the Journal of Post-Acute and Long-Term Medicine.
6. Hospitalists can work with providers in PAC settings to improve transitions.
Despite improvements in the electronic transfer of medical information, gaps still exist and can cause problems. One chasm when discharging patients to a PAC facility, is when a hospital IT system is incapable of communicating with the PAC facility system. In this instance, Dr. Nazir says, the hospitalist “can help bridge the gap.”
“[We] can verbally relay relevant information to physicians at PAC facilities so they understand the patient’s status, needs, and expectations,” he says. “Furthermore, hospitalists and a PAC facility’s administration can brainstorm methods to improve the systems of care so the patient receives more effective and timely care.”
7. Hospitalists switching to the PAC setting should have formal training.
The two main obstacles for hospitalists who change from working in a hospital to a PAC facility are the lack of exposure to PAC work in training and the assumption that it requires the same skills sets of a typical hospitalist, according to Manoj K. Mathew, MD, SFHM, national medical director of Los Angeles–based Agilon Health. The PAC setting has quite a number of differences compared with a hospital setting. For example, some regulations apply specifically to PAC facilities. In addition to formal training, hospitalists can benefit from using SHM’s Post-Acute Care Transitions Toolkit, having a mentor, or using resources from other organizations that function in this space such as The Society for Post-Acute and Long-Term Care Medicine, Dr. Nazir says.
8. A variety of payors and payment models are in play.
Commercial insurers continue to be major payors for PAC, especially for individuals younger than 65 years. Medicare and Medicaid, administered by the Centers for Medicare & Medicaid Services, are the primary payors for patients aged 65 years and older.
“These scorecards are using a variety of criteria to rank providers, such as length of stay, cost, readmissions to hospitals, and quality.”
Because Medicare Part A covers many patients discharged to a PAC setting, any changes in payment incentives or benefit structures by the Medicare program will drive changes in PAC.

“For example, as Medicare implements payment adjustments for hospitals that have high rates of readmissions, hospitals have a new incentive to work closely with SNFs and other providers of PAC to ensure patients can avoid unnecessary readmissions,” says Tiffany A. Radcliff, PhD, a health economist and associate professor in the department of health policy and management at Texas A&M University School of Public Health in College Station.
Providers must follow the billing rules for each payor. The rules for Medicare payments are outlined on CMS’ website. Bundled payments for PAC under the Medicare Part A program are scheduled to be implemented by 2018.
Reference
Cross DA, Adler-Milstein J. Investing in post-acute care transitions: electronic information exchange between hospitals and long-term care facilities [published online ahead of print Sept. 14, 2016]. JAMDA. doi: http://dx.doi.org/10.1016/j.jamda.2016.07.024.
Whether you’re a hospitalist who works only in a hospital, a hospitalist who works only in a post-acute care (PAC) setting, or a hospitalist who works in both types of facilities, knowing about current trends at PAC facilities and what the future may hold can help you excel in your current capacity and, ultimately, improve patient care.
The Hospitalist tapped experts in the post-acute space to tell us what they thought HM should know about working in PAC – which, in many ways, is quite different from the hospital setting. Here’s a compilation of their top eight must-knows.
1. PAC settings rely more on mid-level medical staff than hospitals do.
PAC facilities employ more mid-level providers, such as nurse practitioners and physician assistants, because they can support the level of medical complexity and decision making 95% of the time, says James D. Tollman, MD, FHM, president of Essex Inpatient Physicians in Boxford, Mass. Further, they are more heavily staffed by licensed practical nurses than are acute-care settings.
Usually, there is no physician or nurse practitioner presence at night. Clinicians rely on nursing staff’s assessment to make decisions regarding changes in patient status during off-hours, says Virginia Cummings, MD, director of long-term care, gerontology division, at Boston-based Beth Israel Deaconess Medical Center.
2. Testing takes longer, and options are limited.
Access to some acute urgent resources such as laboratory testing, imaging tests, and pharmacy products is more challenging at PAC facilities because most of these resources are not on-site. Consequently, there is a time lag between ordering tests and new medications and implementing these orders.
“If a patient needs something performed diagnostically immediately, they usually have to be transported to the emergency room or a facility with the necessary testing equipment,” Dr. Tollman says.
However, Paul T. Liistro, managing partner, Arbors of Hop Brook Limited Partnership in Manchester, Conn., and Vernon Manor Health Care Center in Vernon, Conn., and administrator, Manchester Manor Health Care Center, notes that it’s possible for a laboratory service or mobile diagnostic unit to provide laboratory testing or certain imaging at a PAC facility. More-involved diagnostics, such as an MRI or a PET scan, typically require testing at a remote location.
3. Patient populations mainly include rehab and terminally ill patients.
Patients are typically sent to a PAC facility either to recover from an illness or injury or because they are chronically ill and have exhausted treatment options. Regarding the latter, “They are mostly there for palliation; we don’t perform daily tests or prescribe aggressive medications on these patients,” Dr. Nazir says.
Dr. Cummings explains that PAC clinicians go through “the dying process with the patient.”
“They may or may not have assistance from hospice organizations,” she says, “and when they don’t, [hospitalists] take on the role of palliative-care providers.”
Dr. Cummings has seen an increase in psychiatric patients entering PAC facilities.
“Many patients with chronic psychological problems are aging, and there are fewer inpatient psychiatric beds available to those with concurrent medical and psychiatric problems,” she says. Much of this work is now being done in PAC settings. 
4. You can build a relationship with your patient.
Because the pace of a PAC facility is slower and a patient typically stays in a PAC facility longer than at a hospital, there’s time for a hospitalist to have more in-depth conversations with patients and their families.
“Building a deeper relationship with a patient may give the hospitalist an opportunity to discover the cause of an acute problem,” Dr. Nazir says. “They can go in-depth into the psychosocial aspect of medicine and may be able to find out what led to the initial problem and the real root cause, which can help prevent future recurrences, such as repeat falls or forgetting to take a medication.”
5. Using EHRs can improve transitions.
Care transitions between a hospital and PAC facility can be compromised by a lack of information sharing, and they can affect the quality and safety of patient care, says Dori Cross, a doctoral candidate in health services organization and policy at the University of Michigan School of Public Health in Ann Arbor. Handoffs between providers require information continuity – information that is complete, timely, and in a usable format – to ensure appropriate medical decisions and to provide high-quality care during and after transition.
Electronic health records (EHRs) as well as health information exchanges (HIEs) allow providers to communicate and share patient information. For example, hospitals can send information electronically to PAC facilities (“push” exchange) or make information available online securely for PAC providers to log in and access (“pull” exchange). According to a 2014 survey data by the American Hospital Association, more than 50% of hospitals report sending structured summary-of-care records electronically to long-term care settings; a little less than half of those hospitals (23% of the total sample of hospitals) were also receiving information electronically from long-term care sites.1
“This bidirectional exchange, in particular, can make it easier to share information across provider organizations electronically and, in turn, improve care delivery,” says Ms. Cross, who authored an accepted paper on the subject in the Journal of Post-Acute and Long-Term Medicine.
6. Hospitalists can work with providers in PAC settings to improve transitions.
Despite improvements in the electronic transfer of medical information, gaps still exist and can cause problems. One chasm when discharging patients to a PAC facility, is when a hospital IT system is incapable of communicating with the PAC facility system. In this instance, Dr. Nazir says, the hospitalist “can help bridge the gap.”
“[We] can verbally relay relevant information to physicians at PAC facilities so they understand the patient’s status, needs, and expectations,” he says. “Furthermore, hospitalists and a PAC facility’s administration can brainstorm methods to improve the systems of care so the patient receives more effective and timely care.”
7. Hospitalists switching to the PAC setting should have formal training.
The two main obstacles for hospitalists who change from working in a hospital to a PAC facility are the lack of exposure to PAC work in training and the assumption that it requires the same skills sets of a typical hospitalist, according to Manoj K. Mathew, MD, SFHM, national medical director of Los Angeles–based Agilon Health. The PAC setting has quite a number of differences compared with a hospital setting. For example, some regulations apply specifically to PAC facilities. In addition to formal training, hospitalists can benefit from using SHM’s Post-Acute Care Transitions Toolkit, having a mentor, or using resources from other organizations that function in this space such as The Society for Post-Acute and Long-Term Care Medicine, Dr. Nazir says.
8. A variety of payors and payment models are in play.
Commercial insurers continue to be major payors for PAC, especially for individuals younger than 65 years. Medicare and Medicaid, administered by the Centers for Medicare & Medicaid Services, are the primary payors for patients aged 65 years and older.
“These scorecards are using a variety of criteria to rank providers, such as length of stay, cost, readmissions to hospitals, and quality.”
Because Medicare Part A covers many patients discharged to a PAC setting, any changes in payment incentives or benefit structures by the Medicare program will drive changes in PAC.

“For example, as Medicare implements payment adjustments for hospitals that have high rates of readmissions, hospitals have a new incentive to work closely with SNFs and other providers of PAC to ensure patients can avoid unnecessary readmissions,” says Tiffany A. Radcliff, PhD, a health economist and associate professor in the department of health policy and management at Texas A&M University School of Public Health in College Station.
Providers must follow the billing rules for each payor. The rules for Medicare payments are outlined on CMS’ website. Bundled payments for PAC under the Medicare Part A program are scheduled to be implemented by 2018.
Reference
Cross DA, Adler-Milstein J. Investing in post-acute care transitions: electronic information exchange between hospitals and long-term care facilities [published online ahead of print Sept. 14, 2016]. JAMDA. doi: http://dx.doi.org/10.1016/j.jamda.2016.07.024.
Female physicians, lower mortality, lower readmissions: A case study
Week in, week out for the past 25 years, I have had a front-row seat to the medical practice of a certain female physician: my wife, Heather. We met when we worked together on the wards during residency in 1991; spent a year in rural Montana working together in clinics, ERs, and hospitals; shared the care of one another’s patients as our practices grew in parallel – hers in skilled nursing facilities, mine in the hospital; and reunited in recent years to work together as part of the same practice.
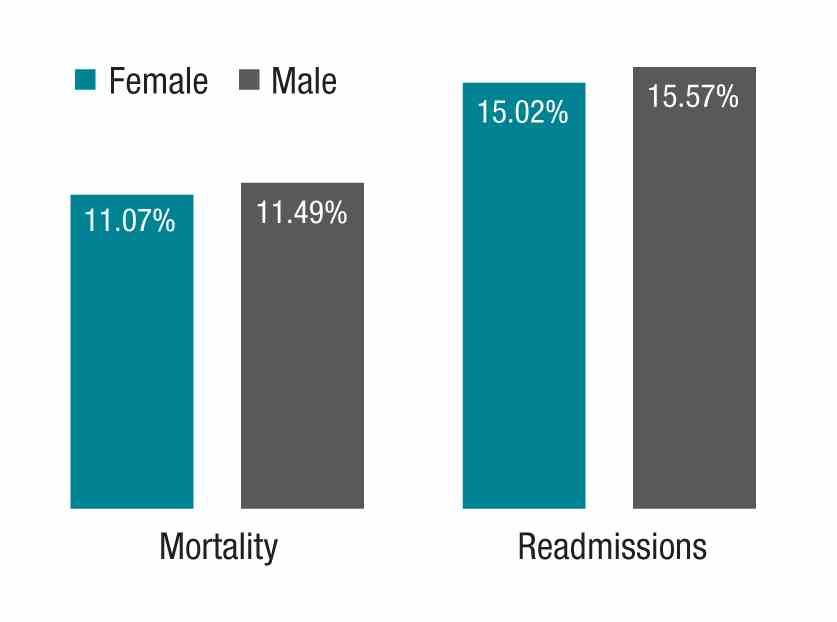
When I saw the paper by Yusuke Tsugawa, MD, MPH, PhD, and his associates showing lower mortality and readmission rates for female physicians versus their male counterparts, I began to wonder if the case of Heather’s practice style, and my observations of it, could help to interpret the findings of the study (JAMA Intern Med. 2016 Dec 19. doi: 10.1001/jamainternmed.2016.7875). The authors suggested that female physicians may produce better outcomes than male physicians.
The study in question, which analyzed more than 1.5 million hospitalizations, looked at Medicare beneficiaries hospitalized with a medical condition treated by general internists between 2011 and 2014. The authors found that patients treated by female physicians had lower 30-day mortality (adjusted rate, 11.07% vs. 11.49%, P<.001) and readmissions (adjusted rate, 15.02% vs. 15.57%, P<.001) than those treated by male physicians within the same hospital. The differences were “modest but important,” coauthor Ashish K. Jha, MD, MPH, wrote in his blog. Numbers needed to treat to prevent one death and one readmission were 233 and 182, respectively.
My observations of Heather’s practice approach, compared with my own, center around three main themes:
She spends more time considering her approach to a challenging case.
She has less urgency in deciding on a definitive course of action and more patience in sorting things out before proceeding with a diagnostic and therapeutic plan. She is more likely to leave open the possibility of changing her mind; she has less of a tendency to anchor on a particular diagnosis and treatment. Put another way, she is more willing to continue with ambiguous findings without lateralizing to one particular approach.
She brings more work-life balance to her professional responsibilities.
Despite being highly productive at work (and at home), she has worked less than full time throughout her career. This means that, during any given patient encounter, she is more likely to be unburdened by overwork and its negative consequences. It is my sense that many full-time physicians would be happier (and more effective) if they simply worked less. Heather has had the self-knowledge to take on a more manageable workload; the result is that she has remained joyous in practice for more than two decades.
She is less dogmatic and more willing to customize care based on the needs of the individual patient.
Although a good fund of knowledge is essential, if such knowledge obscures the physician’s ability to read the patient, then it is best abandoned, at least temporarily. Heather refers to the body of scientific evidence frequently, but she reserves an equal or greater portion of her cognitive bandwidth for the patient she is caring for at a particular moment.
How might the observations of this case study help to derive meaning from the study by Dr. Tsugawa and his associates, so that all patients may benefit from whatever it is that female physicians do to achieve better outcomes?
First, if physicians – regardless of gender – simply have an awareness of anchoring bias or rushing to land on a diagnosis or treatment, they will be less likely to do so in the future.
Next, we can learn that avoiding overwork can make for more joy in work, and if this is so, our patients may fare better. When I say “avoiding overwork,” that might mean rethinking our assumptions underlying the amount of work we take on.
Finally, while amassing a large fund of knowledge is a good thing, balancing medical knowledge with knowledge of the individual patient is crucial to good medical practice.
Dr. Whitcomb is Chief Medical Officer at Remedy Partners in Darien, CT. He is a cofounder and past president of SHM. Email him at [email protected].
Week in, week out for the past 25 years, I have had a front-row seat to the medical practice of a certain female physician: my wife, Heather. We met when we worked together on the wards during residency in 1991; spent a year in rural Montana working together in clinics, ERs, and hospitals; shared the care of one another’s patients as our practices grew in parallel – hers in skilled nursing facilities, mine in the hospital; and reunited in recent years to work together as part of the same practice.
When I saw the paper by Yusuke Tsugawa, MD, MPH, PhD, and his associates showing lower mortality and readmission rates for female physicians versus their male counterparts, I began to wonder if the case of Heather’s practice style, and my observations of it, could help to interpret the findings of the study (JAMA Intern Med. 2016 Dec 19. doi: 10.1001/jamainternmed.2016.7875). The authors suggested that female physicians may produce better outcomes than male physicians.
The study in question, which analyzed more than 1.5 million hospitalizations, looked at Medicare beneficiaries hospitalized with a medical condition treated by general internists between 2011 and 2014. The authors found that patients treated by female physicians had lower 30-day mortality (adjusted rate, 11.07% vs. 11.49%, P<.001) and readmissions (adjusted rate, 15.02% vs. 15.57%, P<.001) than those treated by male physicians within the same hospital. The differences were “modest but important,” coauthor Ashish K. Jha, MD, MPH, wrote in his blog. Numbers needed to treat to prevent one death and one readmission were 233 and 182, respectively.

My observations of Heather’s practice approach, compared with my own, center around three main themes:
She spends more time considering her approach to a challenging case.
She has less urgency in deciding on a definitive course of action and more patience in sorting things out before proceeding with a diagnostic and therapeutic plan. She is more likely to leave open the possibility of changing her mind; she has less of a tendency to anchor on a particular diagnosis and treatment. Put another way, she is more willing to continue with ambiguous findings without lateralizing to one particular approach.
She brings more work-life balance to her professional responsibilities.
Despite being highly productive at work (and at home), she has worked less than full time throughout her career. This means that, during any given patient encounter, she is more likely to be unburdened by overwork and its negative consequences. It is my sense that many full-time physicians would be happier (and more effective) if they simply worked less. Heather has had the self-knowledge to take on a more manageable workload; the result is that she has remained joyous in practice for more than two decades.
She is less dogmatic and more willing to customize care based on the needs of the individual patient.
Although a good fund of knowledge is essential, if such knowledge obscures the physician’s ability to read the patient, then it is best abandoned, at least temporarily. Heather refers to the body of scientific evidence frequently, but she reserves an equal or greater portion of her cognitive bandwidth for the patient she is caring for at a particular moment.
How might the observations of this case study help to derive meaning from the study by Dr. Tsugawa and his associates, so that all patients may benefit from whatever it is that female physicians do to achieve better outcomes?
First, if physicians – regardless of gender – simply have an awareness of anchoring bias or rushing to land on a diagnosis or treatment, they will be less likely to do so in the future.
Next, we can learn that avoiding overwork can make for more joy in work, and if this is so, our patients may fare better. When I say “avoiding overwork,” that might mean rethinking our assumptions underlying the amount of work we take on.
Finally, while amassing a large fund of knowledge is a good thing, balancing medical knowledge with knowledge of the individual patient is crucial to good medical practice.
Dr. Whitcomb is Chief Medical Officer at Remedy Partners in Darien, CT. He is a cofounder and past president of SHM. Email him at [email protected].
Week in, week out for the past 25 years, I have had a front-row seat to the medical practice of a certain female physician: my wife, Heather. We met when we worked together on the wards during residency in 1991; spent a year in rural Montana working together in clinics, ERs, and hospitals; shared the care of one another’s patients as our practices grew in parallel – hers in skilled nursing facilities, mine in the hospital; and reunited in recent years to work together as part of the same practice.
When I saw the paper by Yusuke Tsugawa, MD, MPH, PhD, and his associates showing lower mortality and readmission rates for female physicians versus their male counterparts, I began to wonder if the case of Heather’s practice style, and my observations of it, could help to interpret the findings of the study (JAMA Intern Med. 2016 Dec 19. doi: 10.1001/jamainternmed.2016.7875). The authors suggested that female physicians may produce better outcomes than male physicians.
The study in question, which analyzed more than 1.5 million hospitalizations, looked at Medicare beneficiaries hospitalized with a medical condition treated by general internists between 2011 and 2014. The authors found that patients treated by female physicians had lower 30-day mortality (adjusted rate, 11.07% vs. 11.49%, P<.001) and readmissions (adjusted rate, 15.02% vs. 15.57%, P<.001) than those treated by male physicians within the same hospital. The differences were “modest but important,” coauthor Ashish K. Jha, MD, MPH, wrote in his blog. Numbers needed to treat to prevent one death and one readmission were 233 and 182, respectively.

My observations of Heather’s practice approach, compared with my own, center around three main themes:
She spends more time considering her approach to a challenging case.
She has less urgency in deciding on a definitive course of action and more patience in sorting things out before proceeding with a diagnostic and therapeutic plan. She is more likely to leave open the possibility of changing her mind; she has less of a tendency to anchor on a particular diagnosis and treatment. Put another way, she is more willing to continue with ambiguous findings without lateralizing to one particular approach.
She brings more work-life balance to her professional responsibilities.
Despite being highly productive at work (and at home), she has worked less than full time throughout her career. This means that, during any given patient encounter, she is more likely to be unburdened by overwork and its negative consequences. It is my sense that many full-time physicians would be happier (and more effective) if they simply worked less. Heather has had the self-knowledge to take on a more manageable workload; the result is that she has remained joyous in practice for more than two decades.
She is less dogmatic and more willing to customize care based on the needs of the individual patient.
Although a good fund of knowledge is essential, if such knowledge obscures the physician’s ability to read the patient, then it is best abandoned, at least temporarily. Heather refers to the body of scientific evidence frequently, but she reserves an equal or greater portion of her cognitive bandwidth for the patient she is caring for at a particular moment.
How might the observations of this case study help to derive meaning from the study by Dr. Tsugawa and his associates, so that all patients may benefit from whatever it is that female physicians do to achieve better outcomes?
First, if physicians – regardless of gender – simply have an awareness of anchoring bias or rushing to land on a diagnosis or treatment, they will be less likely to do so in the future.
Next, we can learn that avoiding overwork can make for more joy in work, and if this is so, our patients may fare better. When I say “avoiding overwork,” that might mean rethinking our assumptions underlying the amount of work we take on.
Finally, while amassing a large fund of knowledge is a good thing, balancing medical knowledge with knowledge of the individual patient is crucial to good medical practice.
Dr. Whitcomb is Chief Medical Officer at Remedy Partners in Darien, CT. He is a cofounder and past president of SHM. Email him at [email protected].
How should urine electrolytes be ordered and interpreted in acute kidney injury and electrolyte abnormalities?
The case
A 50-year old woman naive to the health care system presents to the ED with nausea, malaise, and decreased exercise tolerance for several weeks. Physical exam reveals mild bilateral lower extremity edema. Her labs are notable for an elevated creatinine of 7.0. She is admitted for work-up of her renal disease.
Nephrology was consulted and recommended obtaining urine electrolytes. The admitting hospitalist is unsure which urine electrolytes are appropriate to order, and in turn orders all of the urine electrolytes in the order set.
Which urine electrolytes should be ordered in various clinical contexts?
Introduction
Hospitalists have been on the forefront of efforts to tailor testing and resource utilization to eliminate wasteful practices in health care. To order and interpret diagnostic tests appropriately, a hospitalist needs to have a thorough understanding of the diagnostic utility of laboratory tests. There is a lack of clear diagnostic guidelines, so ordering all the urine electrolytes in a “blanket” strategy is a common practice. We will discuss the diagnostic utility of each of the urine electrolytes in a variety of clinical scenarios.
Acute kidney injury
Both the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEUrea) have long been used as part of the standard work-up for determining if acute kidney injury (AKI) is due to prerenal causes. Although these markers prove to be beneficial in the work-up of AKI, both the FENa and FEUrea have several limitations.
FENa measures the ratio of sodium excreted in the urine compared to how much is filtered through the kidney. A FENa of less than 1% in oliguric patients may indicate prerenal azotemia, as an increased reabsorption of sodium is the appropriate response of functioning nephrons to decreased renal perfusion. Values greater than 3% may be consistent with acute tubular necrosis (ATN) due to inappropriate sodium excretion in the setting of tubular damage.
Importantly, a FENa value of less than 1% occurs in a number of conditions other than prerenal azotemia due to dehydration, including hypervolemic prerenal states such as cirrhosis or heart failure; AKI due to radiocontrast or heme pigments; acute glomerulonephritis; transition from prerenal to postischemic ATN or sepsis, and in acute interstitial nephritis (AIN).1,2 Approximately 10% of patients with nonoliguric ATN have a FENa less than 1.0%. Moreover, use of diuretics can falsely elevate the FENa due to inhibition of sodium reabsorption. FENa values above 3% can occur in volume contraction in patients with chronic kidney disease (CKD) or in elderly patients as their sodium reabsorption is impaired.3 Acute volume loss (e.g. blood loss), or more commonly, administration of diuretics or intravenous fluids, can also alter the interpretation of the FENa.2
Many of the limitations of the FENa also apply to the FEUrea, including interpretation in the elderly and use in acute volume changes. However, the FEUrea has unique limitations, particularly in patients with sepsis, as cytokines released in sepsis may interfere with urea transporters in the kidney and colon.2 Its interpretation also relies on intact functioning of the proximal tubule, which can be altered in many conditions including uncontrolled diabetes. Overall, the FENa and FEUrea can be helpful to determine the etiology of AKI, but only in certain clinical scenarios.
Hyponatremia
Hyponatremia is the most common electrolyte abnormality in hospitalized patients, with a prevalence of up to 30% in critically ill patients.4 It often is acquired during the hospitalization itself. A detailed history and physical exam, including careful assessment of volume status, is as important as laboratory values in establishing the cause of hyponatremia.
Urine sodium and urine osmolality are measured to understand whether the renin-aldosterone-angiotensin system (RAAS) and antidiuretic hormone (ADH) are activated. If renal blood flow or renal delivery of sodium is decreased, renin secretion from the juxtaglomerular apparatus will be activated, ultimately leading to increased reabsorption of sodium in the distal tubules and collecting ducts. Thus, low urine sodium signals that the RAAS is activated due to decreased serum sodium concentration or decreased renal blood flow from hypovolemia or low effective arterial circulation from cirrhosis or heart failure.
Most causes of hyponatremia will have low urine sodium values, including hypovolemia, cirrhosis, heart failure, “tea-and-toast” diet, beer potomania, and primary polydipsia. However, the urine sodium may be unreliable in patients who are not oliguric or who have CKD.
Diuretic-induced hyponatremia from thiazide or loop diuretics will likely have elevated urine sodium levels. Similarly, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) will have an elevated urine sodium above 20-40 mEq/L.
Urine osmolality becomes elevated when ADH is secreted in response to reduced plasma volume or increased plasma osmolality. Urine osmolality is low in cases such as primary polydipsia, which creates a maximally dilute urine of 40-100 mEq/L, and in tea-and-toast diets or beer potomania due to low solute intake. Urine osmolality can be elevated in hypovolemic states as well as SIADH, and is variable in hypothyroidism and selective serotonin reuptake inhibitor administration. Thus, urine sodium, and not urine osmolality, is the most useful differentiator between SIADH and hypovolemic states.
In a study of 555 patients with hyponatremia secondary to SIADH, mean urine sodium was found to be 72 (range 30-251) and the median urine osmolality was 379 (range 123-1019).5
In cases of marked hyperglycemia, serum osmolality should be measured to evaluate hyperglycemia as a cause of hyperosmolar hyponatremia. Pseudohyponatremia in the setting of hyperlipidemia, hypertriglyceridemia, or hyperparaproteinemia represents a laboratory artifact due to lower plasma water concentration in the specimen sample and should be excluded.
Hypokalemia
About 20% of patients are hypokalemic during an inpatient hospitalization. There is a broad differential for hypokalemia, including medical, nutritional, and medication-related causes. Exogenous insulin administration or endogenous production in cases of refeeding syndrome drives potassium intracellularly via the N+/K+ ATPase. Increased sympathetic activity from alcohol withdrawal, acute myocardial infarction, head injury, or thyroid imbalance, as well as iatrogenic causes such as albuterol administration, also drive potassium intracellularly. Diarrhea and nasogastric tube suction lead to gastrointestinal (GI) potassium losses, while antibiotics, chemotherapeutic agents, and diuretics can cause hypokalemia through renal potassium wasting. Hyperaldosteronism and renal tubular acidosis are less common causes.6
The history, review of medications, physical exam, and initial basic laboratory testing (electrolytes, BUN, creatinine, magnesium) should assess for pseudohypokalemia, poor oral intake, diuretic use, acid-base disturbances, or GI losses.
Measuring urine potassium is useful in the work-up of the hypokalemic patient when these conditions are not evident. Urine potassium – either 24-hour or spot urine potassium-to-creatinine ratio – can help determine if urinary potassium wasting is a factor. Potassium is excreted at a near constant rate throughout the day. A urine potassium-to-creatinine ratio corrects for variations in urine volume. When this ratio is greater than 13 mEq/g, renal potassium losses should be suspected. If the ratio is less than 13 mEq/g, hypokalemia is likely due to transcellular potassium shifts, GI losses, diuretics, or poor intake.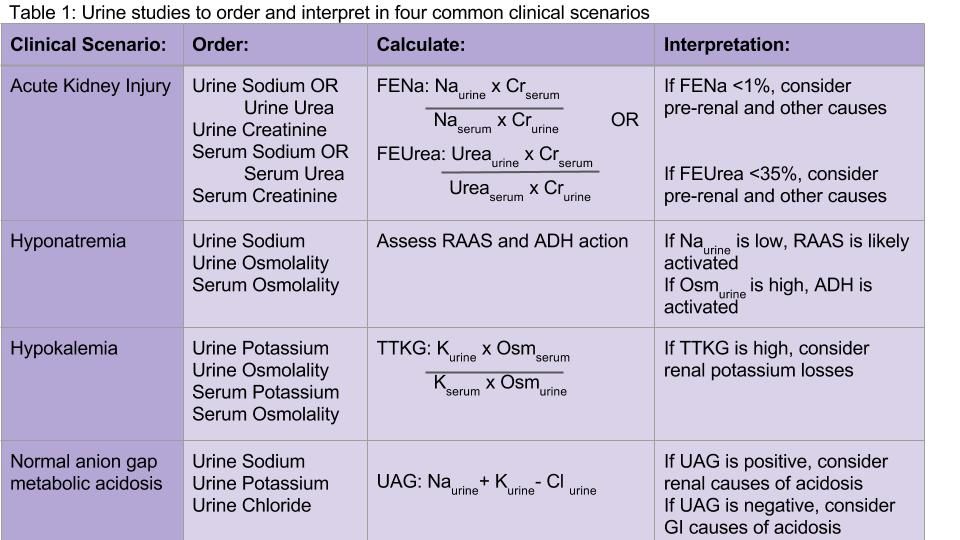
Hyperkalemia
Several concepts in hypokalemia are relevant to hyperkalemia. Redistribution of potassium into the extracellular fluid can cause hyperkalemia when the body tries to counterbalance low extracellular pH by potassium-hydrogen exchange. Medications may cause an extracellular shift of potassium (e.g. digoxin) or induce diminished potassium excretion (e.g. NSAIDs, spironolactone, ACE/ARBs).
CKD and end-stage kidney disease are common causes of hyperkalemia in the hospitalized patient – as functioning nephrons decrease, poor Na-K exchange ensues. Hypoaldosteronism and type 4 renal tubular acidosis are also on the differential diagnosis. Pseudohyperkalemia secondary to thrombocytosis, erythrocytosis, or activated platelets should be considered and evaluated.
Appropriate renal excretion of potassium is mediated by the connecting segment between the distal tubule and the collecting duct, and the cortical collecting duct itself. There are four major causes of hyperkalemia due to reduced urinary potassium secretion: reduced aldosterone secretion, reduced response to aldosterone, reduced distal sodium and water delivery (often related to low effective arterial blood volume), and kidney injury.6
Measurement of 24-hour urinary potassium excretion is of limited utility in patients with persistent stable hyperkalemia because urinary potassium excretion is related to potassium intake. The TTKG was previously used to assess the degree of aldosterone activity by estimating the potassium concentration in the cortical collecting tubule. However, some assumptions upon which this calculation was based have been considered invalid by the original studies’ authors, and the TTKG to evaluate potassium abnormalities is no longer uniformly recommended.7,8 Ultimately, if patients have persistent hyperkalemia, work-up for hypoaldosteronism should be considered.
Normal anion gap metabolic acidosis
The urine anion gap (UAG) is used to determine the cause of normal anion gap hyperchloremic metabolic acidosis by indirectly measuring urinary excretion of ammonium. To maintain a normal acid/base balance, hydrogen ions are excreted in the urine with simultaneous reabsorption of bicarbonate. Hydrogen ions are bound to ammonia (NH3) to form ammonium (NH4+), which is excreted as NH4Cl in the urine.
The UAG is calculated by adding urine sodium and urine potassium and subtracting urine chloride (see Table 1). In a patient without an acid/base disturbance, the UAG is positive because more Na and K is absorbed in the gastrointestinal system compared to Cl, and thus more Na and K is excreted in the urine. In a normal anion gap metabolic acidosis through an acid load or bicarbonate loss, the normal response of the kidney is to excrete more hydrogen ions, resulting in more chloride excretion as NH4Cl. This leads to a negative urine anion gap, as Cl excretion outweighs Na and K excretion. When NH4+ excretion is impaired, such as in distal renal tubular acidosis (RTA), the urine anion gap will remain positive despite the metabolic acidosis. Thus, a positive UAG points to renal causes of the normal anion gap metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.9
Additional considerations
Urine studies can also be useful for assessment of proteinuria and albuminuria in a patient with CKD or diabetes, diagnosis of plasma cell dyscrasias, the diagnosis and prevention of nephrolithiasis, and a wide variety of other conditions.
Back to the case
Our patient was admitted with an elevated creatinine of unclear chronicity, and subacute symptoms of uremia. Because she was oliguric, urine and serum sodium and creatinine were measured before intravenous fluids were administered. Her FENa was 2%, which was not consistent with prerenal azotemia or ATN. She was found to have CKD secondary to previously undiagnosed diabetes. Upon further questioning, she had been taking high-dose NSAIDs for her chronic knee pain. Her renal function improved mildly by withholding NSAIDs, and she was discharged with appropriate nephrology follow-up.
Bottom line
Urine electrolytes have specific indications and utilities for different clinical scenarios, and should be ordered in a targeted manner that can aide in diagnosing AKI, hyponatremia, hypokalemia, and normal anion gap metabolic acidosis.
Dr. Tummalapalli, Dr. Krouss, and Dr. Goetz are hospitalists in the department of medicine at the Icahn School of Medicine at Mount Sinai in New York City.
References
1. Brosius FC, Lau K. Low fractional excretion of sodium in acute renal failure: role of timing of the test and ischemia. Am J Nephrol. 1986;6(6):450-7.
2. Gottfried J, Weisen J, Raina R, Nally J. Finding the cause of acute kidney injury: Which index of fractional excretion is better? Cleve Clin J Med. 2012;79(2):121-6.
3. Steiner, RW. Interpreting the fractional excretion of sodium. Am J Med. 1984;77(4):699-702.
4. DeVita MV, Gardenswartz MH, Konecky A, Zabetakis PM. Incidence and etiology of hyponatremia in an intensive care unit. Clin Nephrol. 1990;34(4):163-6.
5. Shepshelovich D, Leibovitch C, Klein A, et al. The syndrome of inappropriate antidiuretic hormone secretion: distribution and characterization according to etiologies. Eur J Int Med. 2015;26(10):819-24.
6. Mount DB. Fluid and Electrolyte Disturbances. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 19e. New York, NY: McGraw-Hill; 2015.
7. Kamel KS. Intrarenal urea recycling leads to a higher rate of renal excretion of potassium: an hypothesis with clinical implications. Curr Opin Nephrol Hypertens. 2011 Sep;20(5):547-54.
8. Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-45.e2. Philadelphia, PA: Elsevier; 2016.
9. Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: a clinically useful index of ammonium excretion. Am J Med Sci. 1986;198-202.
Key Points:
• In acute kidney injury, the FENa and FEUrea may be calculated to distinguish prerenal azotemia from ATN; however, FENa and FEUrea may be low in a wide variety of conditions other than prerenal azotemia.
• Urine sodium and osmolality values are helpful in diagnosing the cause of hyponatremia, but have a number of limitations in nonoliguric patients and those with CKD.
• An elevated transtubular potassium gradient (TTKG) may indicate renal loss of potassium in patients with hypokalemia.
• A positive urine anion gap (UAG) in the setting of a normal anion gap metabolic acidosis points to renal causes of the metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.Ad
Additional Reading:
Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: A Clinically Useful Index of Ammonium Excretion. Am J Med Sci. 1986;198-202.
Gotfried J, Wiesen J, Raina R, Nally Jr JV. Finding the cause of acute kidney injury: which index of fractional excretion is better. Cleve Clin J Med. 2012;79(2):121-126.
Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-845.e2. Philadelphia, PA: Elsevier; 2016.
The case
A 50-year old woman naive to the health care system presents to the ED with nausea, malaise, and decreased exercise tolerance for several weeks. Physical exam reveals mild bilateral lower extremity edema. Her labs are notable for an elevated creatinine of 7.0. She is admitted for work-up of her renal disease.
Nephrology was consulted and recommended obtaining urine electrolytes. The admitting hospitalist is unsure which urine electrolytes are appropriate to order, and in turn orders all of the urine electrolytes in the order set.
Which urine electrolytes should be ordered in various clinical contexts?
Introduction
Hospitalists have been on the forefront of efforts to tailor testing and resource utilization to eliminate wasteful practices in health care. To order and interpret diagnostic tests appropriately, a hospitalist needs to have a thorough understanding of the diagnostic utility of laboratory tests. There is a lack of clear diagnostic guidelines, so ordering all the urine electrolytes in a “blanket” strategy is a common practice. We will discuss the diagnostic utility of each of the urine electrolytes in a variety of clinical scenarios.
Acute kidney injury
Both the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEUrea) have long been used as part of the standard work-up for determining if acute kidney injury (AKI) is due to prerenal causes. Although these markers prove to be beneficial in the work-up of AKI, both the FENa and FEUrea have several limitations.
FENa measures the ratio of sodium excreted in the urine compared to how much is filtered through the kidney. A FENa of less than 1% in oliguric patients may indicate prerenal azotemia, as an increased reabsorption of sodium is the appropriate response of functioning nephrons to decreased renal perfusion. Values greater than 3% may be consistent with acute tubular necrosis (ATN) due to inappropriate sodium excretion in the setting of tubular damage.
Importantly, a FENa value of less than 1% occurs in a number of conditions other than prerenal azotemia due to dehydration, including hypervolemic prerenal states such as cirrhosis or heart failure; AKI due to radiocontrast or heme pigments; acute glomerulonephritis; transition from prerenal to postischemic ATN or sepsis, and in acute interstitial nephritis (AIN).1,2 Approximately 10% of patients with nonoliguric ATN have a FENa less than 1.0%. Moreover, use of diuretics can falsely elevate the FENa due to inhibition of sodium reabsorption. FENa values above 3% can occur in volume contraction in patients with chronic kidney disease (CKD) or in elderly patients as their sodium reabsorption is impaired.3 Acute volume loss (e.g. blood loss), or more commonly, administration of diuretics or intravenous fluids, can also alter the interpretation of the FENa.2
Many of the limitations of the FENa also apply to the FEUrea, including interpretation in the elderly and use in acute volume changes. However, the FEUrea has unique limitations, particularly in patients with sepsis, as cytokines released in sepsis may interfere with urea transporters in the kidney and colon.2 Its interpretation also relies on intact functioning of the proximal tubule, which can be altered in many conditions including uncontrolled diabetes. Overall, the FENa and FEUrea can be helpful to determine the etiology of AKI, but only in certain clinical scenarios.
Hyponatremia
Hyponatremia is the most common electrolyte abnormality in hospitalized patients, with a prevalence of up to 30% in critically ill patients.4 It often is acquired during the hospitalization itself. A detailed history and physical exam, including careful assessment of volume status, is as important as laboratory values in establishing the cause of hyponatremia.
Urine sodium and urine osmolality are measured to understand whether the renin-aldosterone-angiotensin system (RAAS) and antidiuretic hormone (ADH) are activated. If renal blood flow or renal delivery of sodium is decreased, renin secretion from the juxtaglomerular apparatus will be activated, ultimately leading to increased reabsorption of sodium in the distal tubules and collecting ducts. Thus, low urine sodium signals that the RAAS is activated due to decreased serum sodium concentration or decreased renal blood flow from hypovolemia or low effective arterial circulation from cirrhosis or heart failure.
Most causes of hyponatremia will have low urine sodium values, including hypovolemia, cirrhosis, heart failure, “tea-and-toast” diet, beer potomania, and primary polydipsia. However, the urine sodium may be unreliable in patients who are not oliguric or who have CKD.
Diuretic-induced hyponatremia from thiazide or loop diuretics will likely have elevated urine sodium levels. Similarly, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) will have an elevated urine sodium above 20-40 mEq/L.
Urine osmolality becomes elevated when ADH is secreted in response to reduced plasma volume or increased plasma osmolality. Urine osmolality is low in cases such as primary polydipsia, which creates a maximally dilute urine of 40-100 mEq/L, and in tea-and-toast diets or beer potomania due to low solute intake. Urine osmolality can be elevated in hypovolemic states as well as SIADH, and is variable in hypothyroidism and selective serotonin reuptake inhibitor administration. Thus, urine sodium, and not urine osmolality, is the most useful differentiator between SIADH and hypovolemic states.
In a study of 555 patients with hyponatremia secondary to SIADH, mean urine sodium was found to be 72 (range 30-251) and the median urine osmolality was 379 (range 123-1019).5
In cases of marked hyperglycemia, serum osmolality should be measured to evaluate hyperglycemia as a cause of hyperosmolar hyponatremia. Pseudohyponatremia in the setting of hyperlipidemia, hypertriglyceridemia, or hyperparaproteinemia represents a laboratory artifact due to lower plasma water concentration in the specimen sample and should be excluded.
Hypokalemia
About 20% of patients are hypokalemic during an inpatient hospitalization. There is a broad differential for hypokalemia, including medical, nutritional, and medication-related causes. Exogenous insulin administration or endogenous production in cases of refeeding syndrome drives potassium intracellularly via the N+/K+ ATPase. Increased sympathetic activity from alcohol withdrawal, acute myocardial infarction, head injury, or thyroid imbalance, as well as iatrogenic causes such as albuterol administration, also drive potassium intracellularly. Diarrhea and nasogastric tube suction lead to gastrointestinal (GI) potassium losses, while antibiotics, chemotherapeutic agents, and diuretics can cause hypokalemia through renal potassium wasting. Hyperaldosteronism and renal tubular acidosis are less common causes.6
The history, review of medications, physical exam, and initial basic laboratory testing (electrolytes, BUN, creatinine, magnesium) should assess for pseudohypokalemia, poor oral intake, diuretic use, acid-base disturbances, or GI losses.
Measuring urine potassium is useful in the work-up of the hypokalemic patient when these conditions are not evident. Urine potassium – either 24-hour or spot urine potassium-to-creatinine ratio – can help determine if urinary potassium wasting is a factor. Potassium is excreted at a near constant rate throughout the day. A urine potassium-to-creatinine ratio corrects for variations in urine volume. When this ratio is greater than 13 mEq/g, renal potassium losses should be suspected. If the ratio is less than 13 mEq/g, hypokalemia is likely due to transcellular potassium shifts, GI losses, diuretics, or poor intake.
Hyperkalemia
Several concepts in hypokalemia are relevant to hyperkalemia. Redistribution of potassium into the extracellular fluid can cause hyperkalemia when the body tries to counterbalance low extracellular pH by potassium-hydrogen exchange. Medications may cause an extracellular shift of potassium (e.g. digoxin) or induce diminished potassium excretion (e.g. NSAIDs, spironolactone, ACE/ARBs).
CKD and end-stage kidney disease are common causes of hyperkalemia in the hospitalized patient – as functioning nephrons decrease, poor Na-K exchange ensues. Hypoaldosteronism and type 4 renal tubular acidosis are also on the differential diagnosis. Pseudohyperkalemia secondary to thrombocytosis, erythrocytosis, or activated platelets should be considered and evaluated.
Appropriate renal excretion of potassium is mediated by the connecting segment between the distal tubule and the collecting duct, and the cortical collecting duct itself. There are four major causes of hyperkalemia due to reduced urinary potassium secretion: reduced aldosterone secretion, reduced response to aldosterone, reduced distal sodium and water delivery (often related to low effective arterial blood volume), and kidney injury.6
Measurement of 24-hour urinary potassium excretion is of limited utility in patients with persistent stable hyperkalemia because urinary potassium excretion is related to potassium intake. The TTKG was previously used to assess the degree of aldosterone activity by estimating the potassium concentration in the cortical collecting tubule. However, some assumptions upon which this calculation was based have been considered invalid by the original studies’ authors, and the TTKG to evaluate potassium abnormalities is no longer uniformly recommended.7,8 Ultimately, if patients have persistent hyperkalemia, work-up for hypoaldosteronism should be considered.
Normal anion gap metabolic acidosis
The urine anion gap (UAG) is used to determine the cause of normal anion gap hyperchloremic metabolic acidosis by indirectly measuring urinary excretion of ammonium. To maintain a normal acid/base balance, hydrogen ions are excreted in the urine with simultaneous reabsorption of bicarbonate. Hydrogen ions are bound to ammonia (NH3) to form ammonium (NH4+), which is excreted as NH4Cl in the urine.
The UAG is calculated by adding urine sodium and urine potassium and subtracting urine chloride (see Table 1). In a patient without an acid/base disturbance, the UAG is positive because more Na and K is absorbed in the gastrointestinal system compared to Cl, and thus more Na and K is excreted in the urine. In a normal anion gap metabolic acidosis through an acid load or bicarbonate loss, the normal response of the kidney is to excrete more hydrogen ions, resulting in more chloride excretion as NH4Cl. This leads to a negative urine anion gap, as Cl excretion outweighs Na and K excretion. When NH4+ excretion is impaired, such as in distal renal tubular acidosis (RTA), the urine anion gap will remain positive despite the metabolic acidosis. Thus, a positive UAG points to renal causes of the normal anion gap metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.9
Additional considerations
Urine studies can also be useful for assessment of proteinuria and albuminuria in a patient with CKD or diabetes, diagnosis of plasma cell dyscrasias, the diagnosis and prevention of nephrolithiasis, and a wide variety of other conditions.
Back to the case
Our patient was admitted with an elevated creatinine of unclear chronicity, and subacute symptoms of uremia. Because she was oliguric, urine and serum sodium and creatinine were measured before intravenous fluids were administered. Her FENa was 2%, which was not consistent with prerenal azotemia or ATN. She was found to have CKD secondary to previously undiagnosed diabetes. Upon further questioning, she had been taking high-dose NSAIDs for her chronic knee pain. Her renal function improved mildly by withholding NSAIDs, and she was discharged with appropriate nephrology follow-up.
Bottom line
Urine electrolytes have specific indications and utilities for different clinical scenarios, and should be ordered in a targeted manner that can aide in diagnosing AKI, hyponatremia, hypokalemia, and normal anion gap metabolic acidosis.
Dr. Tummalapalli, Dr. Krouss, and Dr. Goetz are hospitalists in the department of medicine at the Icahn School of Medicine at Mount Sinai in New York City.
References
1. Brosius FC, Lau K. Low fractional excretion of sodium in acute renal failure: role of timing of the test and ischemia. Am J Nephrol. 1986;6(6):450-7.
2. Gottfried J, Weisen J, Raina R, Nally J. Finding the cause of acute kidney injury: Which index of fractional excretion is better? Cleve Clin J Med. 2012;79(2):121-6.
3. Steiner, RW. Interpreting the fractional excretion of sodium. Am J Med. 1984;77(4):699-702.
4. DeVita MV, Gardenswartz MH, Konecky A, Zabetakis PM. Incidence and etiology of hyponatremia in an intensive care unit. Clin Nephrol. 1990;34(4):163-6.
5. Shepshelovich D, Leibovitch C, Klein A, et al. The syndrome of inappropriate antidiuretic hormone secretion: distribution and characterization according to etiologies. Eur J Int Med. 2015;26(10):819-24.
6. Mount DB. Fluid and Electrolyte Disturbances. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 19e. New York, NY: McGraw-Hill; 2015.
7. Kamel KS. Intrarenal urea recycling leads to a higher rate of renal excretion of potassium: an hypothesis with clinical implications. Curr Opin Nephrol Hypertens. 2011 Sep;20(5):547-54.
8. Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-45.e2. Philadelphia, PA: Elsevier; 2016.
9. Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: a clinically useful index of ammonium excretion. Am J Med Sci. 1986;198-202.
Key Points:
• In acute kidney injury, the FENa and FEUrea may be calculated to distinguish prerenal azotemia from ATN; however, FENa and FEUrea may be low in a wide variety of conditions other than prerenal azotemia.
• Urine sodium and osmolality values are helpful in diagnosing the cause of hyponatremia, but have a number of limitations in nonoliguric patients and those with CKD.
• An elevated transtubular potassium gradient (TTKG) may indicate renal loss of potassium in patients with hypokalemia.
• A positive urine anion gap (UAG) in the setting of a normal anion gap metabolic acidosis points to renal causes of the metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.Ad
Additional Reading:
Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: A Clinically Useful Index of Ammonium Excretion. Am J Med Sci. 1986;198-202.
Gotfried J, Wiesen J, Raina R, Nally Jr JV. Finding the cause of acute kidney injury: which index of fractional excretion is better. Cleve Clin J Med. 2012;79(2):121-126.
Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-845.e2. Philadelphia, PA: Elsevier; 2016.
The case
A 50-year old woman naive to the health care system presents to the ED with nausea, malaise, and decreased exercise tolerance for several weeks. Physical exam reveals mild bilateral lower extremity edema. Her labs are notable for an elevated creatinine of 7.0. She is admitted for work-up of her renal disease.
Nephrology was consulted and recommended obtaining urine electrolytes. The admitting hospitalist is unsure which urine electrolytes are appropriate to order, and in turn orders all of the urine electrolytes in the order set.
Which urine electrolytes should be ordered in various clinical contexts?
Introduction
Hospitalists have been on the forefront of efforts to tailor testing and resource utilization to eliminate wasteful practices in health care. To order and interpret diagnostic tests appropriately, a hospitalist needs to have a thorough understanding of the diagnostic utility of laboratory tests. There is a lack of clear diagnostic guidelines, so ordering all the urine electrolytes in a “blanket” strategy is a common practice. We will discuss the diagnostic utility of each of the urine electrolytes in a variety of clinical scenarios.
Acute kidney injury
Both the fractional excretion of sodium (FENa) and the fractional excretion of urea (FEUrea) have long been used as part of the standard work-up for determining if acute kidney injury (AKI) is due to prerenal causes. Although these markers prove to be beneficial in the work-up of AKI, both the FENa and FEUrea have several limitations.
FENa measures the ratio of sodium excreted in the urine compared to how much is filtered through the kidney. A FENa of less than 1% in oliguric patients may indicate prerenal azotemia, as an increased reabsorption of sodium is the appropriate response of functioning nephrons to decreased renal perfusion. Values greater than 3% may be consistent with acute tubular necrosis (ATN) due to inappropriate sodium excretion in the setting of tubular damage.
Importantly, a FENa value of less than 1% occurs in a number of conditions other than prerenal azotemia due to dehydration, including hypervolemic prerenal states such as cirrhosis or heart failure; AKI due to radiocontrast or heme pigments; acute glomerulonephritis; transition from prerenal to postischemic ATN or sepsis, and in acute interstitial nephritis (AIN).1,2 Approximately 10% of patients with nonoliguric ATN have a FENa less than 1.0%. Moreover, use of diuretics can falsely elevate the FENa due to inhibition of sodium reabsorption. FENa values above 3% can occur in volume contraction in patients with chronic kidney disease (CKD) or in elderly patients as their sodium reabsorption is impaired.3 Acute volume loss (e.g. blood loss), or more commonly, administration of diuretics or intravenous fluids, can also alter the interpretation of the FENa.2
Many of the limitations of the FENa also apply to the FEUrea, including interpretation in the elderly and use in acute volume changes. However, the FEUrea has unique limitations, particularly in patients with sepsis, as cytokines released in sepsis may interfere with urea transporters in the kidney and colon.2 Its interpretation also relies on intact functioning of the proximal tubule, which can be altered in many conditions including uncontrolled diabetes. Overall, the FENa and FEUrea can be helpful to determine the etiology of AKI, but only in certain clinical scenarios.
Hyponatremia
Hyponatremia is the most common electrolyte abnormality in hospitalized patients, with a prevalence of up to 30% in critically ill patients.4 It often is acquired during the hospitalization itself. A detailed history and physical exam, including careful assessment of volume status, is as important as laboratory values in establishing the cause of hyponatremia.
Urine sodium and urine osmolality are measured to understand whether the renin-aldosterone-angiotensin system (RAAS) and antidiuretic hormone (ADH) are activated. If renal blood flow or renal delivery of sodium is decreased, renin secretion from the juxtaglomerular apparatus will be activated, ultimately leading to increased reabsorption of sodium in the distal tubules and collecting ducts. Thus, low urine sodium signals that the RAAS is activated due to decreased serum sodium concentration or decreased renal blood flow from hypovolemia or low effective arterial circulation from cirrhosis or heart failure.
Most causes of hyponatremia will have low urine sodium values, including hypovolemia, cirrhosis, heart failure, “tea-and-toast” diet, beer potomania, and primary polydipsia. However, the urine sodium may be unreliable in patients who are not oliguric or who have CKD.
Diuretic-induced hyponatremia from thiazide or loop diuretics will likely have elevated urine sodium levels. Similarly, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) will have an elevated urine sodium above 20-40 mEq/L.
Urine osmolality becomes elevated when ADH is secreted in response to reduced plasma volume or increased plasma osmolality. Urine osmolality is low in cases such as primary polydipsia, which creates a maximally dilute urine of 40-100 mEq/L, and in tea-and-toast diets or beer potomania due to low solute intake. Urine osmolality can be elevated in hypovolemic states as well as SIADH, and is variable in hypothyroidism and selective serotonin reuptake inhibitor administration. Thus, urine sodium, and not urine osmolality, is the most useful differentiator between SIADH and hypovolemic states.
In a study of 555 patients with hyponatremia secondary to SIADH, mean urine sodium was found to be 72 (range 30-251) and the median urine osmolality was 379 (range 123-1019).5
In cases of marked hyperglycemia, serum osmolality should be measured to evaluate hyperglycemia as a cause of hyperosmolar hyponatremia. Pseudohyponatremia in the setting of hyperlipidemia, hypertriglyceridemia, or hyperparaproteinemia represents a laboratory artifact due to lower plasma water concentration in the specimen sample and should be excluded.
Hypokalemia
About 20% of patients are hypokalemic during an inpatient hospitalization. There is a broad differential for hypokalemia, including medical, nutritional, and medication-related causes. Exogenous insulin administration or endogenous production in cases of refeeding syndrome drives potassium intracellularly via the N+/K+ ATPase. Increased sympathetic activity from alcohol withdrawal, acute myocardial infarction, head injury, or thyroid imbalance, as well as iatrogenic causes such as albuterol administration, also drive potassium intracellularly. Diarrhea and nasogastric tube suction lead to gastrointestinal (GI) potassium losses, while antibiotics, chemotherapeutic agents, and diuretics can cause hypokalemia through renal potassium wasting. Hyperaldosteronism and renal tubular acidosis are less common causes.6
The history, review of medications, physical exam, and initial basic laboratory testing (electrolytes, BUN, creatinine, magnesium) should assess for pseudohypokalemia, poor oral intake, diuretic use, acid-base disturbances, or GI losses.
Measuring urine potassium is useful in the work-up of the hypokalemic patient when these conditions are not evident. Urine potassium – either 24-hour or spot urine potassium-to-creatinine ratio – can help determine if urinary potassium wasting is a factor. Potassium is excreted at a near constant rate throughout the day. A urine potassium-to-creatinine ratio corrects for variations in urine volume. When this ratio is greater than 13 mEq/g, renal potassium losses should be suspected. If the ratio is less than 13 mEq/g, hypokalemia is likely due to transcellular potassium shifts, GI losses, diuretics, or poor intake.
Hyperkalemia
Several concepts in hypokalemia are relevant to hyperkalemia. Redistribution of potassium into the extracellular fluid can cause hyperkalemia when the body tries to counterbalance low extracellular pH by potassium-hydrogen exchange. Medications may cause an extracellular shift of potassium (e.g. digoxin) or induce diminished potassium excretion (e.g. NSAIDs, spironolactone, ACE/ARBs).
CKD and end-stage kidney disease are common causes of hyperkalemia in the hospitalized patient – as functioning nephrons decrease, poor Na-K exchange ensues. Hypoaldosteronism and type 4 renal tubular acidosis are also on the differential diagnosis. Pseudohyperkalemia secondary to thrombocytosis, erythrocytosis, or activated platelets should be considered and evaluated.
Appropriate renal excretion of potassium is mediated by the connecting segment between the distal tubule and the collecting duct, and the cortical collecting duct itself. There are four major causes of hyperkalemia due to reduced urinary potassium secretion: reduced aldosterone secretion, reduced response to aldosterone, reduced distal sodium and water delivery (often related to low effective arterial blood volume), and kidney injury.6
Measurement of 24-hour urinary potassium excretion is of limited utility in patients with persistent stable hyperkalemia because urinary potassium excretion is related to potassium intake. The TTKG was previously used to assess the degree of aldosterone activity by estimating the potassium concentration in the cortical collecting tubule. However, some assumptions upon which this calculation was based have been considered invalid by the original studies’ authors, and the TTKG to evaluate potassium abnormalities is no longer uniformly recommended.7,8 Ultimately, if patients have persistent hyperkalemia, work-up for hypoaldosteronism should be considered.
Normal anion gap metabolic acidosis
The urine anion gap (UAG) is used to determine the cause of normal anion gap hyperchloremic metabolic acidosis by indirectly measuring urinary excretion of ammonium. To maintain a normal acid/base balance, hydrogen ions are excreted in the urine with simultaneous reabsorption of bicarbonate. Hydrogen ions are bound to ammonia (NH3) to form ammonium (NH4+), which is excreted as NH4Cl in the urine.
The UAG is calculated by adding urine sodium and urine potassium and subtracting urine chloride (see Table 1). In a patient without an acid/base disturbance, the UAG is positive because more Na and K is absorbed in the gastrointestinal system compared to Cl, and thus more Na and K is excreted in the urine. In a normal anion gap metabolic acidosis through an acid load or bicarbonate loss, the normal response of the kidney is to excrete more hydrogen ions, resulting in more chloride excretion as NH4Cl. This leads to a negative urine anion gap, as Cl excretion outweighs Na and K excretion. When NH4+ excretion is impaired, such as in distal renal tubular acidosis (RTA), the urine anion gap will remain positive despite the metabolic acidosis. Thus, a positive UAG points to renal causes of the normal anion gap metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.9
Additional considerations
Urine studies can also be useful for assessment of proteinuria and albuminuria in a patient with CKD or diabetes, diagnosis of plasma cell dyscrasias, the diagnosis and prevention of nephrolithiasis, and a wide variety of other conditions.
Back to the case
Our patient was admitted with an elevated creatinine of unclear chronicity, and subacute symptoms of uremia. Because she was oliguric, urine and serum sodium and creatinine were measured before intravenous fluids were administered. Her FENa was 2%, which was not consistent with prerenal azotemia or ATN. She was found to have CKD secondary to previously undiagnosed diabetes. Upon further questioning, she had been taking high-dose NSAIDs for her chronic knee pain. Her renal function improved mildly by withholding NSAIDs, and she was discharged with appropriate nephrology follow-up.
Bottom line
Urine electrolytes have specific indications and utilities for different clinical scenarios, and should be ordered in a targeted manner that can aide in diagnosing AKI, hyponatremia, hypokalemia, and normal anion gap metabolic acidosis.
Dr. Tummalapalli, Dr. Krouss, and Dr. Goetz are hospitalists in the department of medicine at the Icahn School of Medicine at Mount Sinai in New York City.
References
1. Brosius FC, Lau K. Low fractional excretion of sodium in acute renal failure: role of timing of the test and ischemia. Am J Nephrol. 1986;6(6):450-7.
2. Gottfried J, Weisen J, Raina R, Nally J. Finding the cause of acute kidney injury: Which index of fractional excretion is better? Cleve Clin J Med. 2012;79(2):121-6.
3. Steiner, RW. Interpreting the fractional excretion of sodium. Am J Med. 1984;77(4):699-702.
4. DeVita MV, Gardenswartz MH, Konecky A, Zabetakis PM. Incidence and etiology of hyponatremia in an intensive care unit. Clin Nephrol. 1990;34(4):163-6.
5. Shepshelovich D, Leibovitch C, Klein A, et al. The syndrome of inappropriate antidiuretic hormone secretion: distribution and characterization according to etiologies. Eur J Int Med. 2015;26(10):819-24.
6. Mount DB. Fluid and Electrolyte Disturbances. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. eds. Harrison’s Principles of Internal Medicine, 19e. New York, NY: McGraw-Hill; 2015.
7. Kamel KS. Intrarenal urea recycling leads to a higher rate of renal excretion of potassium: an hypothesis with clinical implications. Curr Opin Nephrol Hypertens. 2011 Sep;20(5):547-54.
8. Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-45.e2. Philadelphia, PA: Elsevier; 2016.
9. Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: a clinically useful index of ammonium excretion. Am J Med Sci. 1986;198-202.
Key Points:
• In acute kidney injury, the FENa and FEUrea may be calculated to distinguish prerenal azotemia from ATN; however, FENa and FEUrea may be low in a wide variety of conditions other than prerenal azotemia.
• Urine sodium and osmolality values are helpful in diagnosing the cause of hyponatremia, but have a number of limitations in nonoliguric patients and those with CKD.
• An elevated transtubular potassium gradient (TTKG) may indicate renal loss of potassium in patients with hypokalemia.
• A positive urine anion gap (UAG) in the setting of a normal anion gap metabolic acidosis points to renal causes of the metabolic acidosis, whereas a negative UAG points to extrarenal causes such as bicarbonate losses in the GI tract.Ad
Additional Reading:
Goldstein MB, Bear R, Richardson RMA, Marsden PA, Halperin ML. The Urine Anion Gap: A Clinically Useful Index of Ammonium Excretion. Am J Med Sci. 1986;198-202.
Gotfried J, Wiesen J, Raina R, Nally Jr JV. Finding the cause of acute kidney injury: which index of fractional excretion is better. Cleve Clin J Med. 2012;79(2):121-126.
Kamel KS, Davids MR, Lin S-H, Halperin ML. Interpretation of Electrolyte and Acid-Base Parameters in Blood and Urine. In: Brenner and Rector’s The Kidney, 27, 804-845.e2. Philadelphia, PA: Elsevier; 2016.
Sneak Peek: Journal of Hospital Medicine
Background: Frailty, history of dementia (HoD), and acute confusional states (ACS) are common in older patients admitted to hospital.
Objective: To study the association of frailty (≥six points in the Clinical Frailty Scale [CFS]), HoD, and ACS with hospital outcomes, controlling for age, gender, acute illness severity (measured by a Modified Early Warning Score in the emergency department), comorbidity (Charlson Comorbidity Index), and discharging specialty (general medicine, geriatric medicine, surgery).
Design: Retrospective, observational study.
Setting: Large university hospital in England.
Patients: We analyzed 8,202 first nonelective inpatient episodes of people ages 75 years and older between October 2014 and October 2015.
Measurements: The outcomes studied were prolonged length of stay (LOS 10 days), inpatient mortality, delayed discharge, institutionalization, and 30-day readmission. Statistical analyses were based on multivariate regression models.
Results: Independently of controlling variables, prolonged LOS was predicted by CFS greater than or equal to 6: odds ratio (OR) = 1.55; 95% confidence interval (CI), 1.36-1.77; P less than .001; HOD: OR = 2.16; 95% CI, 1.79-2.61; P less than .001; and ACS: OR = 3.31; 95% CI, 2.64-4.15; P less than .001. Inpatient mortality was predicted by CFS greater than or equal to 6: OR = 2.29; 95% CI, 1.79-2.94, P less than .001. Delayed discharge was predicted by CFS greater than or equal to 6: OR = 1.46; 95% CI, 1.27-1.67; P less than .001; HOD: OR = 2.17; 95% CI, 1.80-2.62; P less than .001, and ACS: OR = 2.29; 95% CI: 1.83-2.85; P less than .001. Institutionalization was predicted by CFS greater than or equal to 6: OR=2.56; 95% CI, 2.09-3.14; P less than .001; HOD: OR = 2.51; 95% CI, 2.00-3.14; P less than .001; and ACS: OR = 1.93; 95% CI, 1.46-2.56; P less than .001. Readmission was predicted by ACS: OR = 1.36; 95% CI, 1.09-1.71; P = .006.
Conclusion: Routine screening for frailty, HoD, and ACS in hospitals may aid the development of acute care pathways for older adults.
Read the full article at journalofhospitalmedicine.com.
Also in JHM this month…
- Screening for Depression in Hospitalized Medical Patients
AUTHORS: Waguih William IsHak, MD, FAPA, Katherine Collison, Itai Danovitch, MD, MBA, Lili Shek, MD, Payam Kharazi, Tae Kim, DO Candidate, Karim Y. Jaffer, MD Candidate, Lancer Naghdechi, DO Candidate, Enrique Lopez, PsyD, Teryl Nuckols, MD, MSHS, FHM
- Patient-Level Exclusions from mHealth in a Safety-Net Health System
AUTHORS: Keiki Hinami, MD, MS, Bhrandon A. Harris, MD, Ricardo Uriostegui, MD, Wilnise Jasmin, MD, MBA, Mario Lopez, MD, William E. Trick, MD
- Medical and Economic Burden of Heparin-Induced Thrombocytopenia: A Retrospective Nationwide Inpatient Sample (NIS) Study
AUTHORS: Ranjan Pathak, MD, Vijaya Raj Bhatt, MD, Paras Karmacharya, MD, Madan Raj Aryal, MD, Anthony A. Donato, MD, MHPE
- Assessment of the Readability, Understandability and Completeness of Pediatric Hospital Medicine Discharge Instructions
AUTHORS: Ndidi I. Unaka, MD, Med, Angela Statile, MD, Med, Julianne Haney, Andrew F. Beck, MD, MPH, Patrick W. Brady, MD, MSc, Karen E. Jerardi, MD, MEd
- Impact of Patient-Centered Discharge Tools: A Systematic Review
AUTHORS: Karen Okrainec, MD, MSc, Davina Lau, BSc, Howard B Abrams, MD, Shoshanna Hahn-Goldberg, PhD, Ronak Brahmbhatt, MBBS, MPH, Tai Huynh, MBA, Kenneth Lam, MD, Chaim M Bell, MD, PhD
Background: Frailty, history of dementia (HoD), and acute confusional states (ACS) are common in older patients admitted to hospital.
Objective: To study the association of frailty (≥six points in the Clinical Frailty Scale [CFS]), HoD, and ACS with hospital outcomes, controlling for age, gender, acute illness severity (measured by a Modified Early Warning Score in the emergency department), comorbidity (Charlson Comorbidity Index), and discharging specialty (general medicine, geriatric medicine, surgery).
Design: Retrospective, observational study.
Setting: Large university hospital in England.
Patients: We analyzed 8,202 first nonelective inpatient episodes of people ages 75 years and older between October 2014 and October 2015.
Measurements: The outcomes studied were prolonged length of stay (LOS 10 days), inpatient mortality, delayed discharge, institutionalization, and 30-day readmission. Statistical analyses were based on multivariate regression models.
Results: Independently of controlling variables, prolonged LOS was predicted by CFS greater than or equal to 6: odds ratio (OR) = 1.55; 95% confidence interval (CI), 1.36-1.77; P less than .001; HOD: OR = 2.16; 95% CI, 1.79-2.61; P less than .001; and ACS: OR = 3.31; 95% CI, 2.64-4.15; P less than .001. Inpatient mortality was predicted by CFS greater than or equal to 6: OR = 2.29; 95% CI, 1.79-2.94, P less than .001. Delayed discharge was predicted by CFS greater than or equal to 6: OR = 1.46; 95% CI, 1.27-1.67; P less than .001; HOD: OR = 2.17; 95% CI, 1.80-2.62; P less than .001, and ACS: OR = 2.29; 95% CI: 1.83-2.85; P less than .001. Institutionalization was predicted by CFS greater than or equal to 6: OR=2.56; 95% CI, 2.09-3.14; P less than .001; HOD: OR = 2.51; 95% CI, 2.00-3.14; P less than .001; and ACS: OR = 1.93; 95% CI, 1.46-2.56; P less than .001. Readmission was predicted by ACS: OR = 1.36; 95% CI, 1.09-1.71; P = .006.
Conclusion: Routine screening for frailty, HoD, and ACS in hospitals may aid the development of acute care pathways for older adults.
Read the full article at journalofhospitalmedicine.com.
Also in JHM this month…
- Screening for Depression in Hospitalized Medical Patients
AUTHORS: Waguih William IsHak, MD, FAPA, Katherine Collison, Itai Danovitch, MD, MBA, Lili Shek, MD, Payam Kharazi, Tae Kim, DO Candidate, Karim Y. Jaffer, MD Candidate, Lancer Naghdechi, DO Candidate, Enrique Lopez, PsyD, Teryl Nuckols, MD, MSHS, FHM
- Patient-Level Exclusions from mHealth in a Safety-Net Health System
AUTHORS: Keiki Hinami, MD, MS, Bhrandon A. Harris, MD, Ricardo Uriostegui, MD, Wilnise Jasmin, MD, MBA, Mario Lopez, MD, William E. Trick, MD
- Medical and Economic Burden of Heparin-Induced Thrombocytopenia: A Retrospective Nationwide Inpatient Sample (NIS) Study
AUTHORS: Ranjan Pathak, MD, Vijaya Raj Bhatt, MD, Paras Karmacharya, MD, Madan Raj Aryal, MD, Anthony A. Donato, MD, MHPE
- Assessment of the Readability, Understandability and Completeness of Pediatric Hospital Medicine Discharge Instructions
AUTHORS: Ndidi I. Unaka, MD, Med, Angela Statile, MD, Med, Julianne Haney, Andrew F. Beck, MD, MPH, Patrick W. Brady, MD, MSc, Karen E. Jerardi, MD, MEd
- Impact of Patient-Centered Discharge Tools: A Systematic Review
AUTHORS: Karen Okrainec, MD, MSc, Davina Lau, BSc, Howard B Abrams, MD, Shoshanna Hahn-Goldberg, PhD, Ronak Brahmbhatt, MBBS, MPH, Tai Huynh, MBA, Kenneth Lam, MD, Chaim M Bell, MD, PhD
Background: Frailty, history of dementia (HoD), and acute confusional states (ACS) are common in older patients admitted to hospital.
Objective: To study the association of frailty (≥six points in the Clinical Frailty Scale [CFS]), HoD, and ACS with hospital outcomes, controlling for age, gender, acute illness severity (measured by a Modified Early Warning Score in the emergency department), comorbidity (Charlson Comorbidity Index), and discharging specialty (general medicine, geriatric medicine, surgery).
Design: Retrospective, observational study.
Setting: Large university hospital in England.
Patients: We analyzed 8,202 first nonelective inpatient episodes of people ages 75 years and older between October 2014 and October 2015.
Measurements: The outcomes studied were prolonged length of stay (LOS 10 days), inpatient mortality, delayed discharge, institutionalization, and 30-day readmission. Statistical analyses were based on multivariate regression models.
Results: Independently of controlling variables, prolonged LOS was predicted by CFS greater than or equal to 6: odds ratio (OR) = 1.55; 95% confidence interval (CI), 1.36-1.77; P less than .001; HOD: OR = 2.16; 95% CI, 1.79-2.61; P less than .001; and ACS: OR = 3.31; 95% CI, 2.64-4.15; P less than .001. Inpatient mortality was predicted by CFS greater than or equal to 6: OR = 2.29; 95% CI, 1.79-2.94, P less than .001. Delayed discharge was predicted by CFS greater than or equal to 6: OR = 1.46; 95% CI, 1.27-1.67; P less than .001; HOD: OR = 2.17; 95% CI, 1.80-2.62; P less than .001, and ACS: OR = 2.29; 95% CI: 1.83-2.85; P less than .001. Institutionalization was predicted by CFS greater than or equal to 6: OR=2.56; 95% CI, 2.09-3.14; P less than .001; HOD: OR = 2.51; 95% CI, 2.00-3.14; P less than .001; and ACS: OR = 1.93; 95% CI, 1.46-2.56; P less than .001. Readmission was predicted by ACS: OR = 1.36; 95% CI, 1.09-1.71; P = .006.
Conclusion: Routine screening for frailty, HoD, and ACS in hospitals may aid the development of acute care pathways for older adults.
Read the full article at journalofhospitalmedicine.com.
Also in JHM this month…
- Screening for Depression in Hospitalized Medical Patients
AUTHORS: Waguih William IsHak, MD, FAPA, Katherine Collison, Itai Danovitch, MD, MBA, Lili Shek, MD, Payam Kharazi, Tae Kim, DO Candidate, Karim Y. Jaffer, MD Candidate, Lancer Naghdechi, DO Candidate, Enrique Lopez, PsyD, Teryl Nuckols, MD, MSHS, FHM
- Patient-Level Exclusions from mHealth in a Safety-Net Health System
AUTHORS: Keiki Hinami, MD, MS, Bhrandon A. Harris, MD, Ricardo Uriostegui, MD, Wilnise Jasmin, MD, MBA, Mario Lopez, MD, William E. Trick, MD
- Medical and Economic Burden of Heparin-Induced Thrombocytopenia: A Retrospective Nationwide Inpatient Sample (NIS) Study
AUTHORS: Ranjan Pathak, MD, Vijaya Raj Bhatt, MD, Paras Karmacharya, MD, Madan Raj Aryal, MD, Anthony A. Donato, MD, MHPE
- Assessment of the Readability, Understandability and Completeness of Pediatric Hospital Medicine Discharge Instructions
AUTHORS: Ndidi I. Unaka, MD, Med, Angela Statile, MD, Med, Julianne Haney, Andrew F. Beck, MD, MPH, Patrick W. Brady, MD, MSc, Karen E. Jerardi, MD, MEd
- Impact of Patient-Centered Discharge Tools: A Systematic Review
AUTHORS: Karen Okrainec, MD, MSc, Davina Lau, BSc, Howard B Abrams, MD, Shoshanna Hahn-Goldberg, PhD, Ronak Brahmbhatt, MBBS, MPH, Tai Huynh, MBA, Kenneth Lam, MD, Chaim M Bell, MD, PhD