User login
In Case You Missed It: COVID
The next likely COVID-19 vaccine has its advantages
Among the multiple vaccine candidates around the globe, next up in the arsenal against COVID-19 is likely the single-dose Ad26.COV2.S vaccine in development from Johnson & Johnson/Janssen, infectious disease experts predict.
And it got closer with promising interim phase 1/2a trial results, published online Jan. 13 in The New England Journal of Medicine.
A single Ad26.COV2.S dose was associated with S-binding and neutralizing antibodies in more than 90% of the participants. The finding was observed in both adults aged 18-55 years and participants 65 and older, as well as for participants given low-dose or high-dose vaccinations.
The results also suggest a durable vaccine response. “The take-home message [includes] a high neutralizing antibody responder rate to a single dose of our Ad26.COV2.S COVID-19 vaccine candidate. In addition, we see that these responses and antibody titers are stable for at least 71 days,” senior study author Hanneke Schuitemaker, PhD, global head of viral vaccine discovery and translational medicine at Johnson & Johnson in Leiden, the Netherlands, said in an interview.
If the single-dose Johnson & Johnson product gains Food and Drug Administration emergency use authorization (EUA), it could significantly boost the number of overall immunizations available. Less stringent storage requirements – only regular refrigeration vs. a need to freeze the Pfizer/BioNTech and Moderna COVID-19 vaccines – is another potential advantage. The Ad26.COV2.S vaccine can be refrigerated for up to 3 months at 36°-46 °F (2°-8 °C).
“Phase 1-2 trial data on the J&J vaccine: If it works as well as the mRNA options, it will have substantial advantages,” Jeremy Faust, MD, an emergency room physician affiliated with Brigham & Women’s Hospital and Harvard Medical School, Boston, tweeted on Jan. 13.
Unlike the Pfizer/BioNTech and Moderna messenger RNA vaccines, the Johnson & Johnson product is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein.
Phase 3 efficacy/safety results pending
Under normal circumstances, phase 3 trial results would not be anticipated within weeks of phase 1/2a trial findings. However, the urgency of the COVID-19 pandemic accelerated the vaccine development process, so preclinical trials were conducted simultaneously and not sequentially. For this reason, phase 3 interim results for the Johnson & Johnson vaccine are expected within weeks, and a company executive told Reuters that the rollout is on track for March.
“We hope to report data from our first phase 3 study, ENSEMBLE, in which we are testing the protective efficacy of a single dose of Ad26.COV2.S, by the end of this month or early February,” Dr. Schuitemaker said.
In the meantime, the phase 1/2a ongoing, multicenter, randomized, double-blind, and placebo-controlled trial interim results have drawn positive reactions.
“Data is highly encouraging and supports the single inoculation approach that makes this vaccine unique,” Carlos del Rio, executive associate dean for Emory University at Grady in Atlanta, wrote in a tweet on Jan. 13.
“Encouraging COVID vaccine data from J&J published [Jan. 13]. Solid antibody, CD4 T cell, and CD8 T cell responses – a nice trifecta of vaccine immune responses to see! And safe!” tweeted Shane Crotty, PhD, vaccine scientist and professor at the La Jolla (Calif.) Institute for Immunology.
First results in 800+ participants
At baseline for the phase 1/2a trial, 2% of the younger group and 1% of the 65+ group were seropositive for SARS-CoV-2 S-specific antibodies.
A total of 402 people in the younger age cohort and 403 in the 65 and older group received a first dose of the Johnson & Johnson vaccine. Many participants also received a second dose 56 days later for a separate trial, ENSEMBLE2, designed to compare safety and efficacy between single- and double-dose regimens. Results of that trial are still pending.
Safety profile
A single dose was associated with a higher incidence of solicited systemic adverse events in the higher vaccine dose group. They also found that grade 3 adverse events decreased with increasing age.
Injection site pain on the day of immunization or the next day was the most common local reaction. The pain generally resolved within 24 hours. Fever was reported by 15% of the low-dose vaccine group and 39% of the high-dose cohort. Fatigue, headache, and myalgia were the most common grade 1 or 2 solicited systemic adverse events reported.
Five serious adverse events were reported, including four that investigators deemed unrelated to vaccination: hypotension, bilateral nephrolithiasis, legionella pneumonia, and one case of worsening of multiple sclerosis. The vaccine-related serious adverse event was a fever that resulted in hospitalization because of suspicion of COVID-19. The patient recovered within 12 hours.
“These data confirm our previous experience with vaccine candidates based on our Ad26 viral vector platform in the younger age group. The almost similar performance in older adults is promising,” Dr. Schuitemaker said.
A potential limitation of the phase 1/2a trial is “the lack of representation of minority groups,” the researchers noted. Johnson & Johnson is working on improving the diversity of study participants “with respect to groups that seem to be affected most by the COVID-19 pandemic.”
AstraZeneca/Oxford vaccine status
The AstraZeneca/Oxford AZD1222 vaccine in development received approval for use in the United Kingdom on Dec. 30. The approval came after Public Health England said the country was facing “unprecedented” levels of infections, the BBC reported. AstraZeneca applied for European Medical Agency approval earlier in the week of Jan. 10, which could lead to more widespread use across Europe.
The status of the vaccine remains uncertain in the United States. A phase 3 trial that started in August was paused for about 6 weeks in September and October after an adverse event in a British volunteer halted studies worldwide. On Oct. 23, the FDA permitted researchers to continue the trial with approximately 40,000 participants.
There was some suggestion in the clinical trials that a half dose of the AstraZeneca vaccine was more effective than a full dose, 90% vs. 62%, but some irregularities in the research require further investigation.
Although the AstraZeneca vaccine is delivered to cells by an adenovirus – as with the Johnson & Johnson product – it is designed to be delivered in two doses 28 days apart, like the administration schedule of the Moderna mRNA vaccine.
A need for speed, and more doses
Regardless of which vaccine product is next to gain an EUA in the United States, many experts agree the COVID-19 vaccine rollouts so far have been problematic, at a time when cases are climbing to record-breaking levels, and likely more related to logistics over administration of the vaccine than production of the doses.
“Lots of doses being manufactured. In December 20 million, January 40 million, February 80 million and J&J hopefully soon to add to the count. The shortage is the number arms not getting vaccinated. Freezers do not get COVID. They do not need all those vaccines,” Daniel Griffin, MD, PhD, an infectious disease expert in Port Washington, N.Y., tweeted on Jan. 12.
“Unfortunately, the rollout has not gone smoothly, partly due to a lack of resources for this distribution phase we’re in,” Andrew T. Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, said during a media briefing Jan. 14 sponsored by the Infectious Diseases Society of America (IDSA).
“We’re concerned about the mismatch between the number of people who are being told they are eligible and the amount of vaccine that is being distributed,” he said.
Complicating the rollout is a directive from U.S. Health and Human Services Secretary Alex Azar that states should start vaccinating everyone 65 and older as well as those with underlying conditions.
Expanding distribution to the 15% of Americans in just this age group is a big challenge, Dr. Pavia said. “We have enough vaccine maybe to vaccinate 40 million by the end of this month. There is a huge disconnect, and that creates a lot of problems.”
“One of the biggest problems is we are trying to do this mass vaccination program in the middle of the biggest surge we’ve ever seen,” Julie Vaishampayan, MD, MPH, chair of the IDSA Public Health Committee, said during the briefing. Without sufficient time for public health officials to plan for vaccinating a larger population, “people will come and stand in extremely long lines.”
Trying to expand immunization access without a proportionate increase in available doses prompted Dr. Vaishampayan to share an analogy from a colleague: “We are trying to fill a lake with a garden hose. Rather than making the lake bigger, what we really need is more water.”
Dr. Pavia emphasized that infectious disease experts “know the measures that work.” Not using masks, physical distancing, and hand hygiene, he said, “is a bit like knowing that really good shark repellents will be available in summer, so I’m going to jump into the ocean covered in blood while the great whites are swimming around.”
An official at the World Health Organization agreed. “Vaccines are coming online and I do believe vaccines will make a huge difference. But they are not here yet in enough quantities and in enough people to make that difference,” Michael Ryan, MB, WHO executive director of health emergencies, said during an online media briefing Jan. 13, held in conjunction with Emory University.
Dr. Ryan predicted that “we’ve got weeks if not months ahead of us in which our weapon is our knowledge ... what we know about this virus, its transmission, and stopping that transmission.
“And as the vaccines roll in, we can hopefully end this horrific pandemic.”
Dr. Schuitemaker reports grants from BARDA during the conduct of the study; personal fees and other from Janssen Vaccines and Prevention, a J&J company, outside the submitted work. Johnson & Johnson and the Biomedical Advanced Research and Development Authority of the Department of Health and Human Services funded the phase 1/2a study.
A version of this article first appeared on Medscape.com.
Among the multiple vaccine candidates around the globe, next up in the arsenal against COVID-19 is likely the single-dose Ad26.COV2.S vaccine in development from Johnson & Johnson/Janssen, infectious disease experts predict.
And it got closer with promising interim phase 1/2a trial results, published online Jan. 13 in The New England Journal of Medicine.
A single Ad26.COV2.S dose was associated with S-binding and neutralizing antibodies in more than 90% of the participants. The finding was observed in both adults aged 18-55 years and participants 65 and older, as well as for participants given low-dose or high-dose vaccinations.
The results also suggest a durable vaccine response. “The take-home message [includes] a high neutralizing antibody responder rate to a single dose of our Ad26.COV2.S COVID-19 vaccine candidate. In addition, we see that these responses and antibody titers are stable for at least 71 days,” senior study author Hanneke Schuitemaker, PhD, global head of viral vaccine discovery and translational medicine at Johnson & Johnson in Leiden, the Netherlands, said in an interview.
If the single-dose Johnson & Johnson product gains Food and Drug Administration emergency use authorization (EUA), it could significantly boost the number of overall immunizations available. Less stringent storage requirements – only regular refrigeration vs. a need to freeze the Pfizer/BioNTech and Moderna COVID-19 vaccines – is another potential advantage. The Ad26.COV2.S vaccine can be refrigerated for up to 3 months at 36°-46 °F (2°-8 °C).
“Phase 1-2 trial data on the J&J vaccine: If it works as well as the mRNA options, it will have substantial advantages,” Jeremy Faust, MD, an emergency room physician affiliated with Brigham & Women’s Hospital and Harvard Medical School, Boston, tweeted on Jan. 13.
Unlike the Pfizer/BioNTech and Moderna messenger RNA vaccines, the Johnson & Johnson product is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein.
Phase 3 efficacy/safety results pending
Under normal circumstances, phase 3 trial results would not be anticipated within weeks of phase 1/2a trial findings. However, the urgency of the COVID-19 pandemic accelerated the vaccine development process, so preclinical trials were conducted simultaneously and not sequentially. For this reason, phase 3 interim results for the Johnson & Johnson vaccine are expected within weeks, and a company executive told Reuters that the rollout is on track for March.
“We hope to report data from our first phase 3 study, ENSEMBLE, in which we are testing the protective efficacy of a single dose of Ad26.COV2.S, by the end of this month or early February,” Dr. Schuitemaker said.
In the meantime, the phase 1/2a ongoing, multicenter, randomized, double-blind, and placebo-controlled trial interim results have drawn positive reactions.
“Data is highly encouraging and supports the single inoculation approach that makes this vaccine unique,” Carlos del Rio, executive associate dean for Emory University at Grady in Atlanta, wrote in a tweet on Jan. 13.
“Encouraging COVID vaccine data from J&J published [Jan. 13]. Solid antibody, CD4 T cell, and CD8 T cell responses – a nice trifecta of vaccine immune responses to see! And safe!” tweeted Shane Crotty, PhD, vaccine scientist and professor at the La Jolla (Calif.) Institute for Immunology.
First results in 800+ participants
At baseline for the phase 1/2a trial, 2% of the younger group and 1% of the 65+ group were seropositive for SARS-CoV-2 S-specific antibodies.
A total of 402 people in the younger age cohort and 403 in the 65 and older group received a first dose of the Johnson & Johnson vaccine. Many participants also received a second dose 56 days later for a separate trial, ENSEMBLE2, designed to compare safety and efficacy between single- and double-dose regimens. Results of that trial are still pending.
Safety profile
A single dose was associated with a higher incidence of solicited systemic adverse events in the higher vaccine dose group. They also found that grade 3 adverse events decreased with increasing age.
Injection site pain on the day of immunization or the next day was the most common local reaction. The pain generally resolved within 24 hours. Fever was reported by 15% of the low-dose vaccine group and 39% of the high-dose cohort. Fatigue, headache, and myalgia were the most common grade 1 or 2 solicited systemic adverse events reported.
Five serious adverse events were reported, including four that investigators deemed unrelated to vaccination: hypotension, bilateral nephrolithiasis, legionella pneumonia, and one case of worsening of multiple sclerosis. The vaccine-related serious adverse event was a fever that resulted in hospitalization because of suspicion of COVID-19. The patient recovered within 12 hours.
“These data confirm our previous experience with vaccine candidates based on our Ad26 viral vector platform in the younger age group. The almost similar performance in older adults is promising,” Dr. Schuitemaker said.
A potential limitation of the phase 1/2a trial is “the lack of representation of minority groups,” the researchers noted. Johnson & Johnson is working on improving the diversity of study participants “with respect to groups that seem to be affected most by the COVID-19 pandemic.”
AstraZeneca/Oxford vaccine status
The AstraZeneca/Oxford AZD1222 vaccine in development received approval for use in the United Kingdom on Dec. 30. The approval came after Public Health England said the country was facing “unprecedented” levels of infections, the BBC reported. AstraZeneca applied for European Medical Agency approval earlier in the week of Jan. 10, which could lead to more widespread use across Europe.
The status of the vaccine remains uncertain in the United States. A phase 3 trial that started in August was paused for about 6 weeks in September and October after an adverse event in a British volunteer halted studies worldwide. On Oct. 23, the FDA permitted researchers to continue the trial with approximately 40,000 participants.
There was some suggestion in the clinical trials that a half dose of the AstraZeneca vaccine was more effective than a full dose, 90% vs. 62%, but some irregularities in the research require further investigation.
Although the AstraZeneca vaccine is delivered to cells by an adenovirus – as with the Johnson & Johnson product – it is designed to be delivered in two doses 28 days apart, like the administration schedule of the Moderna mRNA vaccine.
A need for speed, and more doses
Regardless of which vaccine product is next to gain an EUA in the United States, many experts agree the COVID-19 vaccine rollouts so far have been problematic, at a time when cases are climbing to record-breaking levels, and likely more related to logistics over administration of the vaccine than production of the doses.
“Lots of doses being manufactured. In December 20 million, January 40 million, February 80 million and J&J hopefully soon to add to the count. The shortage is the number arms not getting vaccinated. Freezers do not get COVID. They do not need all those vaccines,” Daniel Griffin, MD, PhD, an infectious disease expert in Port Washington, N.Y., tweeted on Jan. 12.
“Unfortunately, the rollout has not gone smoothly, partly due to a lack of resources for this distribution phase we’re in,” Andrew T. Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, said during a media briefing Jan. 14 sponsored by the Infectious Diseases Society of America (IDSA).
“We’re concerned about the mismatch between the number of people who are being told they are eligible and the amount of vaccine that is being distributed,” he said.
Complicating the rollout is a directive from U.S. Health and Human Services Secretary Alex Azar that states should start vaccinating everyone 65 and older as well as those with underlying conditions.
Expanding distribution to the 15% of Americans in just this age group is a big challenge, Dr. Pavia said. “We have enough vaccine maybe to vaccinate 40 million by the end of this month. There is a huge disconnect, and that creates a lot of problems.”
“One of the biggest problems is we are trying to do this mass vaccination program in the middle of the biggest surge we’ve ever seen,” Julie Vaishampayan, MD, MPH, chair of the IDSA Public Health Committee, said during the briefing. Without sufficient time for public health officials to plan for vaccinating a larger population, “people will come and stand in extremely long lines.”
Trying to expand immunization access without a proportionate increase in available doses prompted Dr. Vaishampayan to share an analogy from a colleague: “We are trying to fill a lake with a garden hose. Rather than making the lake bigger, what we really need is more water.”
Dr. Pavia emphasized that infectious disease experts “know the measures that work.” Not using masks, physical distancing, and hand hygiene, he said, “is a bit like knowing that really good shark repellents will be available in summer, so I’m going to jump into the ocean covered in blood while the great whites are swimming around.”
An official at the World Health Organization agreed. “Vaccines are coming online and I do believe vaccines will make a huge difference. But they are not here yet in enough quantities and in enough people to make that difference,” Michael Ryan, MB, WHO executive director of health emergencies, said during an online media briefing Jan. 13, held in conjunction with Emory University.
Dr. Ryan predicted that “we’ve got weeks if not months ahead of us in which our weapon is our knowledge ... what we know about this virus, its transmission, and stopping that transmission.
“And as the vaccines roll in, we can hopefully end this horrific pandemic.”
Dr. Schuitemaker reports grants from BARDA during the conduct of the study; personal fees and other from Janssen Vaccines and Prevention, a J&J company, outside the submitted work. Johnson & Johnson and the Biomedical Advanced Research and Development Authority of the Department of Health and Human Services funded the phase 1/2a study.
A version of this article first appeared on Medscape.com.
Among the multiple vaccine candidates around the globe, next up in the arsenal against COVID-19 is likely the single-dose Ad26.COV2.S vaccine in development from Johnson & Johnson/Janssen, infectious disease experts predict.
And it got closer with promising interim phase 1/2a trial results, published online Jan. 13 in The New England Journal of Medicine.
A single Ad26.COV2.S dose was associated with S-binding and neutralizing antibodies in more than 90% of the participants. The finding was observed in both adults aged 18-55 years and participants 65 and older, as well as for participants given low-dose or high-dose vaccinations.
The results also suggest a durable vaccine response. “The take-home message [includes] a high neutralizing antibody responder rate to a single dose of our Ad26.COV2.S COVID-19 vaccine candidate. In addition, we see that these responses and antibody titers are stable for at least 71 days,” senior study author Hanneke Schuitemaker, PhD, global head of viral vaccine discovery and translational medicine at Johnson & Johnson in Leiden, the Netherlands, said in an interview.
If the single-dose Johnson & Johnson product gains Food and Drug Administration emergency use authorization (EUA), it could significantly boost the number of overall immunizations available. Less stringent storage requirements – only regular refrigeration vs. a need to freeze the Pfizer/BioNTech and Moderna COVID-19 vaccines – is another potential advantage. The Ad26.COV2.S vaccine can be refrigerated for up to 3 months at 36°-46 °F (2°-8 °C).
“Phase 1-2 trial data on the J&J vaccine: If it works as well as the mRNA options, it will have substantial advantages,” Jeremy Faust, MD, an emergency room physician affiliated with Brigham & Women’s Hospital and Harvard Medical School, Boston, tweeted on Jan. 13.
Unlike the Pfizer/BioNTech and Moderna messenger RNA vaccines, the Johnson & Johnson product is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein.
Phase 3 efficacy/safety results pending
Under normal circumstances, phase 3 trial results would not be anticipated within weeks of phase 1/2a trial findings. However, the urgency of the COVID-19 pandemic accelerated the vaccine development process, so preclinical trials were conducted simultaneously and not sequentially. For this reason, phase 3 interim results for the Johnson & Johnson vaccine are expected within weeks, and a company executive told Reuters that the rollout is on track for March.
“We hope to report data from our first phase 3 study, ENSEMBLE, in which we are testing the protective efficacy of a single dose of Ad26.COV2.S, by the end of this month or early February,” Dr. Schuitemaker said.
In the meantime, the phase 1/2a ongoing, multicenter, randomized, double-blind, and placebo-controlled trial interim results have drawn positive reactions.
“Data is highly encouraging and supports the single inoculation approach that makes this vaccine unique,” Carlos del Rio, executive associate dean for Emory University at Grady in Atlanta, wrote in a tweet on Jan. 13.
“Encouraging COVID vaccine data from J&J published [Jan. 13]. Solid antibody, CD4 T cell, and CD8 T cell responses – a nice trifecta of vaccine immune responses to see! And safe!” tweeted Shane Crotty, PhD, vaccine scientist and professor at the La Jolla (Calif.) Institute for Immunology.
First results in 800+ participants
At baseline for the phase 1/2a trial, 2% of the younger group and 1% of the 65+ group were seropositive for SARS-CoV-2 S-specific antibodies.
A total of 402 people in the younger age cohort and 403 in the 65 and older group received a first dose of the Johnson & Johnson vaccine. Many participants also received a second dose 56 days later for a separate trial, ENSEMBLE2, designed to compare safety and efficacy between single- and double-dose regimens. Results of that trial are still pending.
Safety profile
A single dose was associated with a higher incidence of solicited systemic adverse events in the higher vaccine dose group. They also found that grade 3 adverse events decreased with increasing age.
Injection site pain on the day of immunization or the next day was the most common local reaction. The pain generally resolved within 24 hours. Fever was reported by 15% of the low-dose vaccine group and 39% of the high-dose cohort. Fatigue, headache, and myalgia were the most common grade 1 or 2 solicited systemic adverse events reported.
Five serious adverse events were reported, including four that investigators deemed unrelated to vaccination: hypotension, bilateral nephrolithiasis, legionella pneumonia, and one case of worsening of multiple sclerosis. The vaccine-related serious adverse event was a fever that resulted in hospitalization because of suspicion of COVID-19. The patient recovered within 12 hours.
“These data confirm our previous experience with vaccine candidates based on our Ad26 viral vector platform in the younger age group. The almost similar performance in older adults is promising,” Dr. Schuitemaker said.
A potential limitation of the phase 1/2a trial is “the lack of representation of minority groups,” the researchers noted. Johnson & Johnson is working on improving the diversity of study participants “with respect to groups that seem to be affected most by the COVID-19 pandemic.”
AstraZeneca/Oxford vaccine status
The AstraZeneca/Oxford AZD1222 vaccine in development received approval for use in the United Kingdom on Dec. 30. The approval came after Public Health England said the country was facing “unprecedented” levels of infections, the BBC reported. AstraZeneca applied for European Medical Agency approval earlier in the week of Jan. 10, which could lead to more widespread use across Europe.
The status of the vaccine remains uncertain in the United States. A phase 3 trial that started in August was paused for about 6 weeks in September and October after an adverse event in a British volunteer halted studies worldwide. On Oct. 23, the FDA permitted researchers to continue the trial with approximately 40,000 participants.
There was some suggestion in the clinical trials that a half dose of the AstraZeneca vaccine was more effective than a full dose, 90% vs. 62%, but some irregularities in the research require further investigation.
Although the AstraZeneca vaccine is delivered to cells by an adenovirus – as with the Johnson & Johnson product – it is designed to be delivered in two doses 28 days apart, like the administration schedule of the Moderna mRNA vaccine.
A need for speed, and more doses
Regardless of which vaccine product is next to gain an EUA in the United States, many experts agree the COVID-19 vaccine rollouts so far have been problematic, at a time when cases are climbing to record-breaking levels, and likely more related to logistics over administration of the vaccine than production of the doses.
“Lots of doses being manufactured. In December 20 million, January 40 million, February 80 million and J&J hopefully soon to add to the count. The shortage is the number arms not getting vaccinated. Freezers do not get COVID. They do not need all those vaccines,” Daniel Griffin, MD, PhD, an infectious disease expert in Port Washington, N.Y., tweeted on Jan. 12.
“Unfortunately, the rollout has not gone smoothly, partly due to a lack of resources for this distribution phase we’re in,” Andrew T. Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, said during a media briefing Jan. 14 sponsored by the Infectious Diseases Society of America (IDSA).
“We’re concerned about the mismatch between the number of people who are being told they are eligible and the amount of vaccine that is being distributed,” he said.
Complicating the rollout is a directive from U.S. Health and Human Services Secretary Alex Azar that states should start vaccinating everyone 65 and older as well as those with underlying conditions.
Expanding distribution to the 15% of Americans in just this age group is a big challenge, Dr. Pavia said. “We have enough vaccine maybe to vaccinate 40 million by the end of this month. There is a huge disconnect, and that creates a lot of problems.”
“One of the biggest problems is we are trying to do this mass vaccination program in the middle of the biggest surge we’ve ever seen,” Julie Vaishampayan, MD, MPH, chair of the IDSA Public Health Committee, said during the briefing. Without sufficient time for public health officials to plan for vaccinating a larger population, “people will come and stand in extremely long lines.”
Trying to expand immunization access without a proportionate increase in available doses prompted Dr. Vaishampayan to share an analogy from a colleague: “We are trying to fill a lake with a garden hose. Rather than making the lake bigger, what we really need is more water.”
Dr. Pavia emphasized that infectious disease experts “know the measures that work.” Not using masks, physical distancing, and hand hygiene, he said, “is a bit like knowing that really good shark repellents will be available in summer, so I’m going to jump into the ocean covered in blood while the great whites are swimming around.”
An official at the World Health Organization agreed. “Vaccines are coming online and I do believe vaccines will make a huge difference. But they are not here yet in enough quantities and in enough people to make that difference,” Michael Ryan, MB, WHO executive director of health emergencies, said during an online media briefing Jan. 13, held in conjunction with Emory University.
Dr. Ryan predicted that “we’ve got weeks if not months ahead of us in which our weapon is our knowledge ... what we know about this virus, its transmission, and stopping that transmission.
“And as the vaccines roll in, we can hopefully end this horrific pandemic.”
Dr. Schuitemaker reports grants from BARDA during the conduct of the study; personal fees and other from Janssen Vaccines and Prevention, a J&J company, outside the submitted work. Johnson & Johnson and the Biomedical Advanced Research and Development Authority of the Department of Health and Human Services funded the phase 1/2a study.
A version of this article first appeared on Medscape.com.
Pressure builds on CDC to prioritize both diabetes types for vaccine
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
The American Diabetes Association, along with 18 other organizations, has sent a letter to the U.S. Centers for Disease Control and Prevention urging them to rank people with type 1 diabetes as equally high risk for COVID-19 severity, and therefore vaccination, as those with type 2 diabetes.
On Jan. 12, the CDC recommended states vaccinate all Americans over age 65 and those with underlying health conditions that make them more vulnerable to COVID-19.
Currently, type 2 diabetes is listed among 12 conditions that place adults “at increased risk of severe illness from the virus that causes COVID-19,” with the latter defined as “hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
On the other hand, the autoimmune condition type 1 diabetes is among 11 conditions the CDC says “might be at increased risk” for COVID-19, but limited data were available at the time of the last update on Dec. 23, 2020.
“States are utilizing the CDC risk classification when designing their vaccine distribution plans. This raises an obvious concern as it could result in the approximately 1.6 million with type 1 diabetes receiving the vaccination later than others with the same risk,” states the ADA letter, sent to the CDC on Jan. 13.
Representatives from the Endocrine Society, American Association of Clinical Endocrinology, Pediatric Endocrine Society, Association of Diabetes Care & Education Specialists, and JDRF, among others, cosigned the letter.
Newer data show those with type 1 diabetes at equally high risk
While acknowledging that “early data did not provide as much clarity about the extent to which those with type 1 diabetes are at high risk,” the ADA says newer evidence has emerged, as previously reported by this news organization, that “convincingly demonstrates that COVID-19 severity is more than tripled in individuals with type 1 diabetes.”
The letter also cites another study showing that people with type 1 diabetes “have a 3.3-fold greater risk of severe illness, are 3.9 times more likely to be hospitalized with COVID-19, and have a 3-fold increase in mortality compared to those without type 1 diabetes.”
Those risks, they note, are comparable to the increased risk established for those with type 2 diabetes, as shown in a third study from Scotland, published last month.
Asked for comment, CDC representative Kirsten Nordlund said in an interview, “This list is a living document that will be periodically updated by CDC, and it could rapidly change as the science evolves.”
In addition, Ms. Nordlund said, “Decisions about transitioning to subsequent phases should depend on supply; demand; equitable vaccine distribution; and local, state, or territorial context.”
“Phased vaccine recommendations are meant to be fluid and not restrictive for jurisdictions. It is not necessary to vaccinate all individuals in one phase before initiating the next phase; phases may overlap,” she noted. More information is available here.
Tennessee gives type 1 and type 2 diabetes equal priority for vaccination
Meanwhile, at least one state, Tennessee, has updated its guidance to include both types of diabetes as being priority for COVID-19 vaccination.
Vanderbilt University pediatric endocrinologist Justin M. Gregory, MD, said in an interview: “I was thrilled when our state modified its guidance on December 30th to include both type 1 and type 2 diabetes in the ‘high-risk category.’ Other states have not modified that guidance though.”
It’s unclear how this might play out on the ground, noted Dr. Gregory, who led one of the three studies demonstrating increased COVID-19 risk for people with type 1 diabetes.
“To tell you the truth, I don’t really know how individual organizations dispensing the vaccination [will handle] people who come to their facility saying they have ‘diabetes.’ Individual states set the vaccine-dispensing guidance and individual county health departments and health care systems mirror that guidance,” he said.
Thus, he added, “Although it’s possible an individual nurse may take the ‘I’ll ask you no questions, and you’ll tell me no lies’ approach if someone with type 1 diabetes says they have ‘diabetes’, websites and health department–recorded telephone messages are going to tell people with type 1 diabetes they have to wait further back in line if that is what their state’s guidance directs.”
A version of this article first appeared on Medscape.com.
COVID-19 symptoms persist months after acute infection
, according to a follow-up study involving 1,733 patients.
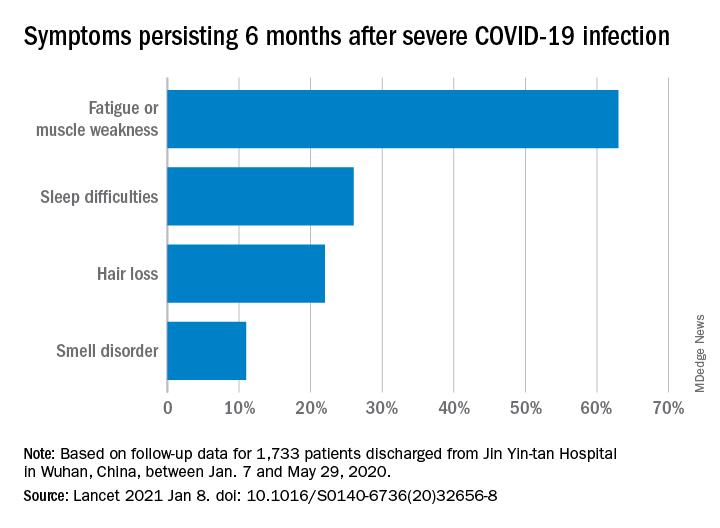
“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
, according to a follow-up study involving 1,733 patients.

“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
, according to a follow-up study involving 1,733 patients.

“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
FROM THE LANCET
Asthma-COPD overlap: Patients have high disease burden
Patients with asthma–chronic obstructive pulmonary disease overlap (ACO) experienced a higher burden of disease than patients with either asthma or COPD alone, a recent study has found.
Approximately 20% of chronic obstructive airway disease cases are ACO, but data on these patients are limited, as they are often excluded from clinical trials, wrote Sarah A. Hiles, MD, of the University of Newcastle (Australia) and colleagues.
“Comparing the burden of eosinophilic ACO, eosinophilic severe asthma, and eosinophilic COPD may also help contextualize findings from phenotype-targeted treatments in different diagnostic groups, such as the limited success of anti-IL [interleukin]–5 monoclonal antibodies as therapy in eosinophilic COPD,” they said.
In a cross-sectional, observational study published in Respirology the researchers recruited patients aged 18 years and older with a confirmed diagnosis of COPD only (153) severe asthma only (64), or ACO (106). Patients were assessed for demographic and clinical factors including health-related quality of life, past-year exacerbation, and other indicators of disease burden. In addition, patients were identified as having eosinophilic airway disease based on a blood eosinophil count of at least 0.3x109/L.
Overall, eosinophilic airway disease was present in 41% of the patients; 55%, 44%, and 29% for those with ACO, severe asthma, and COPD, respectively. Reports of poor health-related quality of life and past-year exacerbations were similar for eosinophilic patients across all three conditions.
However, patients with eosinophilic ACO experienced significantly more past-year exacerbations, notably those requiring oral corticosteroids, compared with patients with asthma alone. In addition, the cumulative number of past-year exacerbations in patient with eosinophilic disease was 164 in those with ACO, compared with severe asthma alone (44) and COPD alone (59).
Patients with ACO also had significantly higher disease burden based on the St. George’s Respiratory Questionnaire (SGRQ), which assessed functional limitation. “For 100 patients, the cumulative SGRQ score attributable to eosinophilic airways disease in ACO was 2,872.8, which was higher than in severe asthma (1,942.5) or COPD (1,638.1),” the researchers said.
The study was limited by several factors including the cross-sectional design and use of a single measurement to classify eosinophilia, the researchers noted. “The non-eosinophilic group likely included a mix of patients with treated eosinophilia and patients without eosinophilia, regardless of treatment, which is a limitation to consider when interpreting the disease burden estimates in this group,” they added.
However, the results add to the understanding of blood eosinophils in airway disease and the study “supports eosinophilia as a phenotype that spans across disease labels of severe asthma and COPD, and their overlap,” they concluded.
The study was supported by AstraZeneca; lead author Dr. Hiles received a salary through a grant from AstraZeneca to the University of Newcastle while conducting the study. Other coauthors disclosed relationships with companies including AstraZeneca, GlaxoSmithKline, Menarini, and Novartis.
Patients with asthma–chronic obstructive pulmonary disease overlap (ACO) experienced a higher burden of disease than patients with either asthma or COPD alone, a recent study has found.
Approximately 20% of chronic obstructive airway disease cases are ACO, but data on these patients are limited, as they are often excluded from clinical trials, wrote Sarah A. Hiles, MD, of the University of Newcastle (Australia) and colleagues.
“Comparing the burden of eosinophilic ACO, eosinophilic severe asthma, and eosinophilic COPD may also help contextualize findings from phenotype-targeted treatments in different diagnostic groups, such as the limited success of anti-IL [interleukin]–5 monoclonal antibodies as therapy in eosinophilic COPD,” they said.
In a cross-sectional, observational study published in Respirology the researchers recruited patients aged 18 years and older with a confirmed diagnosis of COPD only (153) severe asthma only (64), or ACO (106). Patients were assessed for demographic and clinical factors including health-related quality of life, past-year exacerbation, and other indicators of disease burden. In addition, patients were identified as having eosinophilic airway disease based on a blood eosinophil count of at least 0.3x109/L.
Overall, eosinophilic airway disease was present in 41% of the patients; 55%, 44%, and 29% for those with ACO, severe asthma, and COPD, respectively. Reports of poor health-related quality of life and past-year exacerbations were similar for eosinophilic patients across all three conditions.
However, patients with eosinophilic ACO experienced significantly more past-year exacerbations, notably those requiring oral corticosteroids, compared with patients with asthma alone. In addition, the cumulative number of past-year exacerbations in patient with eosinophilic disease was 164 in those with ACO, compared with severe asthma alone (44) and COPD alone (59).
Patients with ACO also had significantly higher disease burden based on the St. George’s Respiratory Questionnaire (SGRQ), which assessed functional limitation. “For 100 patients, the cumulative SGRQ score attributable to eosinophilic airways disease in ACO was 2,872.8, which was higher than in severe asthma (1,942.5) or COPD (1,638.1),” the researchers said.
The study was limited by several factors including the cross-sectional design and use of a single measurement to classify eosinophilia, the researchers noted. “The non-eosinophilic group likely included a mix of patients with treated eosinophilia and patients without eosinophilia, regardless of treatment, which is a limitation to consider when interpreting the disease burden estimates in this group,” they added.
However, the results add to the understanding of blood eosinophils in airway disease and the study “supports eosinophilia as a phenotype that spans across disease labels of severe asthma and COPD, and their overlap,” they concluded.
The study was supported by AstraZeneca; lead author Dr. Hiles received a salary through a grant from AstraZeneca to the University of Newcastle while conducting the study. Other coauthors disclosed relationships with companies including AstraZeneca, GlaxoSmithKline, Menarini, and Novartis.
Patients with asthma–chronic obstructive pulmonary disease overlap (ACO) experienced a higher burden of disease than patients with either asthma or COPD alone, a recent study has found.
Approximately 20% of chronic obstructive airway disease cases are ACO, but data on these patients are limited, as they are often excluded from clinical trials, wrote Sarah A. Hiles, MD, of the University of Newcastle (Australia) and colleagues.
“Comparing the burden of eosinophilic ACO, eosinophilic severe asthma, and eosinophilic COPD may also help contextualize findings from phenotype-targeted treatments in different diagnostic groups, such as the limited success of anti-IL [interleukin]–5 monoclonal antibodies as therapy in eosinophilic COPD,” they said.
In a cross-sectional, observational study published in Respirology the researchers recruited patients aged 18 years and older with a confirmed diagnosis of COPD only (153) severe asthma only (64), or ACO (106). Patients were assessed for demographic and clinical factors including health-related quality of life, past-year exacerbation, and other indicators of disease burden. In addition, patients were identified as having eosinophilic airway disease based on a blood eosinophil count of at least 0.3x109/L.
Overall, eosinophilic airway disease was present in 41% of the patients; 55%, 44%, and 29% for those with ACO, severe asthma, and COPD, respectively. Reports of poor health-related quality of life and past-year exacerbations were similar for eosinophilic patients across all three conditions.
However, patients with eosinophilic ACO experienced significantly more past-year exacerbations, notably those requiring oral corticosteroids, compared with patients with asthma alone. In addition, the cumulative number of past-year exacerbations in patient with eosinophilic disease was 164 in those with ACO, compared with severe asthma alone (44) and COPD alone (59).
Patients with ACO also had significantly higher disease burden based on the St. George’s Respiratory Questionnaire (SGRQ), which assessed functional limitation. “For 100 patients, the cumulative SGRQ score attributable to eosinophilic airways disease in ACO was 2,872.8, which was higher than in severe asthma (1,942.5) or COPD (1,638.1),” the researchers said.
The study was limited by several factors including the cross-sectional design and use of a single measurement to classify eosinophilia, the researchers noted. “The non-eosinophilic group likely included a mix of patients with treated eosinophilia and patients without eosinophilia, regardless of treatment, which is a limitation to consider when interpreting the disease burden estimates in this group,” they added.
However, the results add to the understanding of blood eosinophils in airway disease and the study “supports eosinophilia as a phenotype that spans across disease labels of severe asthma and COPD, and their overlap,” they concluded.
The study was supported by AstraZeneca; lead author Dr. Hiles received a salary through a grant from AstraZeneca to the University of Newcastle while conducting the study. Other coauthors disclosed relationships with companies including AstraZeneca, GlaxoSmithKline, Menarini, and Novartis.
FROM RESPIROLOGY
CVD deaths rose, imaging declined during pandemic
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Natural immunity from COVID-19 ‘may last months’
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
Liver disease associated with worse COVID-19 outcomes
A growing body of evidence suggests that patients with COVID-19 and preexisting liver disease face increased risks of decompensation and mortality, according to a review of recent literature.
The review aimed to bring together the best approaches for caring for patients with preexisting liver conditions based on recommendations from three major hepatology societies. Findings in included studies could guide clinical decision-making, but a reliable framework for patient management has yet to be established, most likely because of limited research, according to lead author Abdul Mohammed, MD, of Case Western Reserve University, Cleveland, and colleagues.
The relationship between chronic liver diseases and “COVID-19 is not well documented in the literature,” Dr. Mohammed and colleagues wrote in the Journal of Clinical Gastroenterology. “The intricate interplay between immune dysfunction in preexisting liver diseases and the immune dysregulation triggered by the SARS-CoV-2 virus needs further evaluation.”
Such knowledge gaps likely explain the inconsistencies in recommendations between major hepatology societies, including clinical guidance from the American Association for the Study of Liver Disease, the European Association for the Study of the Liver, and the Asian Pacific Association for the Study of the Liver.
Both the literature review and the societal guidance address nonalcoholic fatty liver disease (NAFLD), hepatitis B virus (HBV) infection, autoimmune hepatitis, hepatocellular carcinoma (HCC), cirrhosis, and liver transplantation.
Dr. Mohammed and colleagues first offered an update of the relationship between COVID-19 and liver pathology. While it is clear that SARS-CoV-2 gains hepatic access through binding to ACE2 receptors in bile duct epithelial cells, it remains unclear whether this results in direct hepatic injury or indirect damage from virus-mediated cytokine release. Regardless, more than 90% of patients hospitalized for COVID-19 may develop increased levels of ALT and AST, and these elevations “appear to mirror disease severity,” the investigators wrote.
They noted that severity of COVID-19 appears to correlate with type of preexisting liver disease. For example, one study in the review associated NAFLD with a significantly increased risk of progressive COVID-19 (odds ratio, 6.4; 95% confidence interval, 1.5-31.2), and it also found that patients with NAFLD had longer duration of viral shedding than those without (17 vs. 12 days). Although the AASLD and APASL give no specific recommendations, the EASL recommends prioritizing COVID-19 patients with NAFLD.
Cirrhosis has been associated with a fourfold increased risk of mortality (relative risk, 4.6; 95% CI, 2.6-8.3) According to data from two international self-reporting registries, COVIDHep.net and COVIDCirrhosis.org, likelihood of death appears to move in tandem with Child-Turcotte-Pugh scores. Decompensated cirrhosis appears to predispose patients to having pulmonary complications, but more studies exploring this correlation need to be performed, according to the review authors. One study found that acute-on-chronic liver failure or acute decompensation occurred in 20% of patients who had COVID-19 and cirrhosis. It’s little surprise, then, that both the AASLD and the EASL recommend prioritizing in person evaluation for patients with decompensated cirrhosis.
Chronic HBV infection has also been associated with a higher COVID-19 mortality rate, although Dr. Mohammed and colleagues suggested that “larger studies are needed.” The review notes that the three societies recommend initiating HBV treatment only if there is clinical suspicion of hepatitis flare.
Findings are also cloudy among patients with autoimmune hepatitis and liver transplant recipients; however, the investigators noted that COVID-19 causes tissue damage primarily through cytokine release, and suggested that “immunosuppression can potentially curb this response.” Even so, recommendations from leading hepatology societies allude to a safe middle ground of immunosuppression, albeit with indistinct borders. All three caution against withdrawing immunosuppression, but the societies each describe tailoring regimens in different ways and for different patients, emphasizing continued corticosteroid treatments, according to the review.
Guidance also varies for management of HCC. “Since the tumor doubling time is 4-8 months and current guidelines recommend screening every 6 months, in patients at lower risk for developing HCC, a 2-month delay in ultrasound surveillance has been suggested by the AASLD,” the review authors noted. “In patients with a high risk of developing HCC, 6-month interval screening should be continued.” The AASLD recommends proceeding with treatment with newly diagnosed HCC, the EASL suggests that checkpoint inhibitors should be withheld and locoregional therapies should be postponed, and the APASL calls for a less frequent schedule of tyrosine kinase inhibitors and immunotherapy.
“COVID-19 patients with the preexisting liver disease face a higher risk of decompensation and mortality,” the review authors concluded. “We presented the most up-to-date literature on preexisting liver disease and its interaction with COVID-19.”
While such discrepancies may remain unresolved until further data are available, Wajahat Mehal, MD, PhD, director of the fatty liver disease program at Yale University, New Haven, Conn., suggested that clinicians remain vigilant for nonalcoholic steatohepatitis (NASH), which is common among overweight and obese individuals, an overrepresented group among those hospitalized for COVID-19.
“This is of great significance because patients with various forms of liver disease have a worse outcome with COVID-19,” Dr. Mehal said. “When seeing a patient with COVID-19 it is therefore important to ask if they have underlying liver disease, with attention paid to NASH. This can be approached by seeing if they have any evidence of abnormal liver function tests before the onset of COVID and any evidence of abnormal liver imaging. The Fib-4 test is a good screening tool for the presence of advanced liver fibrosis and a positive result should lead to more specific tests of liver fibrosis status such as fibroscan.”
The investigators reported no conflicts of interest. Dr. Mehal reported having nothing to disclose.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
A growing body of evidence suggests that patients with COVID-19 and preexisting liver disease face increased risks of decompensation and mortality, according to a review of recent literature.
The review aimed to bring together the best approaches for caring for patients with preexisting liver conditions based on recommendations from three major hepatology societies. Findings in included studies could guide clinical decision-making, but a reliable framework for patient management has yet to be established, most likely because of limited research, according to lead author Abdul Mohammed, MD, of Case Western Reserve University, Cleveland, and colleagues.
The relationship between chronic liver diseases and “COVID-19 is not well documented in the literature,” Dr. Mohammed and colleagues wrote in the Journal of Clinical Gastroenterology. “The intricate interplay between immune dysfunction in preexisting liver diseases and the immune dysregulation triggered by the SARS-CoV-2 virus needs further evaluation.”
Such knowledge gaps likely explain the inconsistencies in recommendations between major hepatology societies, including clinical guidance from the American Association for the Study of Liver Disease, the European Association for the Study of the Liver, and the Asian Pacific Association for the Study of the Liver.
Both the literature review and the societal guidance address nonalcoholic fatty liver disease (NAFLD), hepatitis B virus (HBV) infection, autoimmune hepatitis, hepatocellular carcinoma (HCC), cirrhosis, and liver transplantation.
Dr. Mohammed and colleagues first offered an update of the relationship between COVID-19 and liver pathology. While it is clear that SARS-CoV-2 gains hepatic access through binding to ACE2 receptors in bile duct epithelial cells, it remains unclear whether this results in direct hepatic injury or indirect damage from virus-mediated cytokine release. Regardless, more than 90% of patients hospitalized for COVID-19 may develop increased levels of ALT and AST, and these elevations “appear to mirror disease severity,” the investigators wrote.
They noted that severity of COVID-19 appears to correlate with type of preexisting liver disease. For example, one study in the review associated NAFLD with a significantly increased risk of progressive COVID-19 (odds ratio, 6.4; 95% confidence interval, 1.5-31.2), and it also found that patients with NAFLD had longer duration of viral shedding than those without (17 vs. 12 days). Although the AASLD and APASL give no specific recommendations, the EASL recommends prioritizing COVID-19 patients with NAFLD.
Cirrhosis has been associated with a fourfold increased risk of mortality (relative risk, 4.6; 95% CI, 2.6-8.3) According to data from two international self-reporting registries, COVIDHep.net and COVIDCirrhosis.org, likelihood of death appears to move in tandem with Child-Turcotte-Pugh scores. Decompensated cirrhosis appears to predispose patients to having pulmonary complications, but more studies exploring this correlation need to be performed, according to the review authors. One study found that acute-on-chronic liver failure or acute decompensation occurred in 20% of patients who had COVID-19 and cirrhosis. It’s little surprise, then, that both the AASLD and the EASL recommend prioritizing in person evaluation for patients with decompensated cirrhosis.
Chronic HBV infection has also been associated with a higher COVID-19 mortality rate, although Dr. Mohammed and colleagues suggested that “larger studies are needed.” The review notes that the three societies recommend initiating HBV treatment only if there is clinical suspicion of hepatitis flare.
Findings are also cloudy among patients with autoimmune hepatitis and liver transplant recipients; however, the investigators noted that COVID-19 causes tissue damage primarily through cytokine release, and suggested that “immunosuppression can potentially curb this response.” Even so, recommendations from leading hepatology societies allude to a safe middle ground of immunosuppression, albeit with indistinct borders. All three caution against withdrawing immunosuppression, but the societies each describe tailoring regimens in different ways and for different patients, emphasizing continued corticosteroid treatments, according to the review.
Guidance also varies for management of HCC. “Since the tumor doubling time is 4-8 months and current guidelines recommend screening every 6 months, in patients at lower risk for developing HCC, a 2-month delay in ultrasound surveillance has been suggested by the AASLD,” the review authors noted. “In patients with a high risk of developing HCC, 6-month interval screening should be continued.” The AASLD recommends proceeding with treatment with newly diagnosed HCC, the EASL suggests that checkpoint inhibitors should be withheld and locoregional therapies should be postponed, and the APASL calls for a less frequent schedule of tyrosine kinase inhibitors and immunotherapy.
“COVID-19 patients with the preexisting liver disease face a higher risk of decompensation and mortality,” the review authors concluded. “We presented the most up-to-date literature on preexisting liver disease and its interaction with COVID-19.”
While such discrepancies may remain unresolved until further data are available, Wajahat Mehal, MD, PhD, director of the fatty liver disease program at Yale University, New Haven, Conn., suggested that clinicians remain vigilant for nonalcoholic steatohepatitis (NASH), which is common among overweight and obese individuals, an overrepresented group among those hospitalized for COVID-19.
“This is of great significance because patients with various forms of liver disease have a worse outcome with COVID-19,” Dr. Mehal said. “When seeing a patient with COVID-19 it is therefore important to ask if they have underlying liver disease, with attention paid to NASH. This can be approached by seeing if they have any evidence of abnormal liver function tests before the onset of COVID and any evidence of abnormal liver imaging. The Fib-4 test is a good screening tool for the presence of advanced liver fibrosis and a positive result should lead to more specific tests of liver fibrosis status such as fibroscan.”
The investigators reported no conflicts of interest. Dr. Mehal reported having nothing to disclose.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
A growing body of evidence suggests that patients with COVID-19 and preexisting liver disease face increased risks of decompensation and mortality, according to a review of recent literature.
The review aimed to bring together the best approaches for caring for patients with preexisting liver conditions based on recommendations from three major hepatology societies. Findings in included studies could guide clinical decision-making, but a reliable framework for patient management has yet to be established, most likely because of limited research, according to lead author Abdul Mohammed, MD, of Case Western Reserve University, Cleveland, and colleagues.
The relationship between chronic liver diseases and “COVID-19 is not well documented in the literature,” Dr. Mohammed and colleagues wrote in the Journal of Clinical Gastroenterology. “The intricate interplay between immune dysfunction in preexisting liver diseases and the immune dysregulation triggered by the SARS-CoV-2 virus needs further evaluation.”
Such knowledge gaps likely explain the inconsistencies in recommendations between major hepatology societies, including clinical guidance from the American Association for the Study of Liver Disease, the European Association for the Study of the Liver, and the Asian Pacific Association for the Study of the Liver.
Both the literature review and the societal guidance address nonalcoholic fatty liver disease (NAFLD), hepatitis B virus (HBV) infection, autoimmune hepatitis, hepatocellular carcinoma (HCC), cirrhosis, and liver transplantation.
Dr. Mohammed and colleagues first offered an update of the relationship between COVID-19 and liver pathology. While it is clear that SARS-CoV-2 gains hepatic access through binding to ACE2 receptors in bile duct epithelial cells, it remains unclear whether this results in direct hepatic injury or indirect damage from virus-mediated cytokine release. Regardless, more than 90% of patients hospitalized for COVID-19 may develop increased levels of ALT and AST, and these elevations “appear to mirror disease severity,” the investigators wrote.
They noted that severity of COVID-19 appears to correlate with type of preexisting liver disease. For example, one study in the review associated NAFLD with a significantly increased risk of progressive COVID-19 (odds ratio, 6.4; 95% confidence interval, 1.5-31.2), and it also found that patients with NAFLD had longer duration of viral shedding than those without (17 vs. 12 days). Although the AASLD and APASL give no specific recommendations, the EASL recommends prioritizing COVID-19 patients with NAFLD.
Cirrhosis has been associated with a fourfold increased risk of mortality (relative risk, 4.6; 95% CI, 2.6-8.3) According to data from two international self-reporting registries, COVIDHep.net and COVIDCirrhosis.org, likelihood of death appears to move in tandem with Child-Turcotte-Pugh scores. Decompensated cirrhosis appears to predispose patients to having pulmonary complications, but more studies exploring this correlation need to be performed, according to the review authors. One study found that acute-on-chronic liver failure or acute decompensation occurred in 20% of patients who had COVID-19 and cirrhosis. It’s little surprise, then, that both the AASLD and the EASL recommend prioritizing in person evaluation for patients with decompensated cirrhosis.
Chronic HBV infection has also been associated with a higher COVID-19 mortality rate, although Dr. Mohammed and colleagues suggested that “larger studies are needed.” The review notes that the three societies recommend initiating HBV treatment only if there is clinical suspicion of hepatitis flare.
Findings are also cloudy among patients with autoimmune hepatitis and liver transplant recipients; however, the investigators noted that COVID-19 causes tissue damage primarily through cytokine release, and suggested that “immunosuppression can potentially curb this response.” Even so, recommendations from leading hepatology societies allude to a safe middle ground of immunosuppression, albeit with indistinct borders. All three caution against withdrawing immunosuppression, but the societies each describe tailoring regimens in different ways and for different patients, emphasizing continued corticosteroid treatments, according to the review.
Guidance also varies for management of HCC. “Since the tumor doubling time is 4-8 months and current guidelines recommend screening every 6 months, in patients at lower risk for developing HCC, a 2-month delay in ultrasound surveillance has been suggested by the AASLD,” the review authors noted. “In patients with a high risk of developing HCC, 6-month interval screening should be continued.” The AASLD recommends proceeding with treatment with newly diagnosed HCC, the EASL suggests that checkpoint inhibitors should be withheld and locoregional therapies should be postponed, and the APASL calls for a less frequent schedule of tyrosine kinase inhibitors and immunotherapy.
“COVID-19 patients with the preexisting liver disease face a higher risk of decompensation and mortality,” the review authors concluded. “We presented the most up-to-date literature on preexisting liver disease and its interaction with COVID-19.”
While such discrepancies may remain unresolved until further data are available, Wajahat Mehal, MD, PhD, director of the fatty liver disease program at Yale University, New Haven, Conn., suggested that clinicians remain vigilant for nonalcoholic steatohepatitis (NASH), which is common among overweight and obese individuals, an overrepresented group among those hospitalized for COVID-19.
“This is of great significance because patients with various forms of liver disease have a worse outcome with COVID-19,” Dr. Mehal said. “When seeing a patient with COVID-19 it is therefore important to ask if they have underlying liver disease, with attention paid to NASH. This can be approached by seeing if they have any evidence of abnormal liver function tests before the onset of COVID and any evidence of abnormal liver imaging. The Fib-4 test is a good screening tool for the presence of advanced liver fibrosis and a positive result should lead to more specific tests of liver fibrosis status such as fibroscan.”
The investigators reported no conflicts of interest. Dr. Mehal reported having nothing to disclose.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
COVID protections suppressed flu season in U.S.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Solutions to the pandemic must include public behavior
Many scientific problems are complex. Finding the solution can require the concerted efforts of a team. Producing a vaccine for COVID-19 involved a multidisciplinary team with a variety of highly specialized expertises, extensive technological resources, and a history of previous scientific discoveries upon whose shoulders today’s scientists can stand.
Many ethical problems are also complex. Finding the ideal, multifaceted answer that addresses all the nuances of a social problem requires brilliant minds, a refined ability for logical analysis and rhetoric, the empowerment of the voices of all stakeholders, and attention to social values such as diversity and justice.
In both endeavors, the typical scientists and ethicists involved tend to presume that if they can determine an ideal solution, it will be rapidly and enthusiastically adopted and implemented for the betterment of society. That is, after all, exactly how those researchers would choose to act. Scientists see moral actions as having two steps. The hard part is deciding what is right. Doing the right thing is the easier task. This delusion is ubiquitous. Many scientists and ethicists recognize the delusion of the existence of a rational society, but proceed anyhow as if one exists.
There is a chorus of voices capable of debunking this delusion. Any priest who hears confessions will testify that the vast majority of harm comes from the failure to do what people already know is right, not from uncertainty, confusion, or ignorance. Psychologists and substance abuse counselors are inundated with people who are stuck doing harmful and self-destructive acts. Internists discuss diet and exercise with their patients, but find the advice is infrequently adopted. Master in business administration programs are devoted to training graduates in methods of motivating people to do what is right.
The response of the scientific establishment to the COVID-19 pandemic was imperfect. There were gaps in knowledge and some early information from China was misleading. The initial CDC test kit production was flawed. The early appeal for the public not to buy masks was strongly driven by a desire to preserve supplies for health care workers. Despite these missteps, the overall advice of scientists was wildly successful and beneficial. The goal was to flatten the curve, and a comparison of the April-June time frame with the November-January period shows markedly fewer COVID-19 cases, hospitalizations, and deaths. Confronted with the pandemic of the century, my assessment is that scientific establishment has performed well.
I am far more negative in my assessment of the institutions that support morality, form the social order, establish justice, and promote the general welfare. For instance, misinformation on social media is rampant, including conspiracy theories and outright denials of the pandemic. Scientific advice has been undercut and impugned. Policy recommendations of esteemed scientific institutions have been ignored. The public’s cooperation has fatigued. Laws on public gatherings, quarantines, and social distancing have been broken. Communitarian ethics and devotion to the common good have been left in a trash heap. The consequences have been hundreds of thousands of lives lost in 2020 and some states are on the brink of much worse.
Medical ethicists have debated in fine detail how to triage ventilators, ration antibody treatments, and prioritize vaccinations. Those policy recommendations have had limited influence. Medical ethics has inadequately addressed the age old problem of morality, which is getting people to behave as they know they ought. Modern medical ethics may have exacerbated the deviancy. Medical ethics for 50 years has emphasized replacing paternalism with autonomy, but it has not adequately promoted communitarian virtues, self-regulation, and personal integrity.
There were many accomplishments and many people to admire in 2020 when compared to historical actions by the health care professionals during crises. Doctors, confronted with the COVID-19 plague, have not abandoned the cities as happened in prior centuries. Patients have not been shunned like lepers, though the total-body protective equipment and the no-visitor policies come very close. Nurses have heroically provided bedside care, though I am haunted by one dissident nurse during a protest carrying a sign saying “Don’t call me a hero. I am being martyred against my will.”
As a scientist, I am prone to the delusion that, if I can build a better mouse trap, people will use it. I’ve lived with that delusion for decades. It carries over into my medical ethics work. Yet I see hospitals in California being overwhelmed by the surge on top of a surge due to unwise and unsafe holiday travel. I can see that optimized solutions aren’t the answer – it is better behavior by the public. I recall when I was a child, my mother would simply command, “Behave yourself.” And never, in any of those recollections, was I in doubt about which correct behavior she meant.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Many scientific problems are complex. Finding the solution can require the concerted efforts of a team. Producing a vaccine for COVID-19 involved a multidisciplinary team with a variety of highly specialized expertises, extensive technological resources, and a history of previous scientific discoveries upon whose shoulders today’s scientists can stand.
Many ethical problems are also complex. Finding the ideal, multifaceted answer that addresses all the nuances of a social problem requires brilliant minds, a refined ability for logical analysis and rhetoric, the empowerment of the voices of all stakeholders, and attention to social values such as diversity and justice.
In both endeavors, the typical scientists and ethicists involved tend to presume that if they can determine an ideal solution, it will be rapidly and enthusiastically adopted and implemented for the betterment of society. That is, after all, exactly how those researchers would choose to act. Scientists see moral actions as having two steps. The hard part is deciding what is right. Doing the right thing is the easier task. This delusion is ubiquitous. Many scientists and ethicists recognize the delusion of the existence of a rational society, but proceed anyhow as if one exists.
There is a chorus of voices capable of debunking this delusion. Any priest who hears confessions will testify that the vast majority of harm comes from the failure to do what people already know is right, not from uncertainty, confusion, or ignorance. Psychologists and substance abuse counselors are inundated with people who are stuck doing harmful and self-destructive acts. Internists discuss diet and exercise with their patients, but find the advice is infrequently adopted. Master in business administration programs are devoted to training graduates in methods of motivating people to do what is right.
The response of the scientific establishment to the COVID-19 pandemic was imperfect. There were gaps in knowledge and some early information from China was misleading. The initial CDC test kit production was flawed. The early appeal for the public not to buy masks was strongly driven by a desire to preserve supplies for health care workers. Despite these missteps, the overall advice of scientists was wildly successful and beneficial. The goal was to flatten the curve, and a comparison of the April-June time frame with the November-January period shows markedly fewer COVID-19 cases, hospitalizations, and deaths. Confronted with the pandemic of the century, my assessment is that scientific establishment has performed well.
I am far more negative in my assessment of the institutions that support morality, form the social order, establish justice, and promote the general welfare. For instance, misinformation on social media is rampant, including conspiracy theories and outright denials of the pandemic. Scientific advice has been undercut and impugned. Policy recommendations of esteemed scientific institutions have been ignored. The public’s cooperation has fatigued. Laws on public gatherings, quarantines, and social distancing have been broken. Communitarian ethics and devotion to the common good have been left in a trash heap. The consequences have been hundreds of thousands of lives lost in 2020 and some states are on the brink of much worse.
Medical ethicists have debated in fine detail how to triage ventilators, ration antibody treatments, and prioritize vaccinations. Those policy recommendations have had limited influence. Medical ethics has inadequately addressed the age old problem of morality, which is getting people to behave as they know they ought. Modern medical ethics may have exacerbated the deviancy. Medical ethics for 50 years has emphasized replacing paternalism with autonomy, but it has not adequately promoted communitarian virtues, self-regulation, and personal integrity.
There were many accomplishments and many people to admire in 2020 when compared to historical actions by the health care professionals during crises. Doctors, confronted with the COVID-19 plague, have not abandoned the cities as happened in prior centuries. Patients have not been shunned like lepers, though the total-body protective equipment and the no-visitor policies come very close. Nurses have heroically provided bedside care, though I am haunted by one dissident nurse during a protest carrying a sign saying “Don’t call me a hero. I am being martyred against my will.”
As a scientist, I am prone to the delusion that, if I can build a better mouse trap, people will use it. I’ve lived with that delusion for decades. It carries over into my medical ethics work. Yet I see hospitals in California being overwhelmed by the surge on top of a surge due to unwise and unsafe holiday travel. I can see that optimized solutions aren’t the answer – it is better behavior by the public. I recall when I was a child, my mother would simply command, “Behave yourself.” And never, in any of those recollections, was I in doubt about which correct behavior she meant.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Many scientific problems are complex. Finding the solution can require the concerted efforts of a team. Producing a vaccine for COVID-19 involved a multidisciplinary team with a variety of highly specialized expertises, extensive technological resources, and a history of previous scientific discoveries upon whose shoulders today’s scientists can stand.
Many ethical problems are also complex. Finding the ideal, multifaceted answer that addresses all the nuances of a social problem requires brilliant minds, a refined ability for logical analysis and rhetoric, the empowerment of the voices of all stakeholders, and attention to social values such as diversity and justice.
In both endeavors, the typical scientists and ethicists involved tend to presume that if they can determine an ideal solution, it will be rapidly and enthusiastically adopted and implemented for the betterment of society. That is, after all, exactly how those researchers would choose to act. Scientists see moral actions as having two steps. The hard part is deciding what is right. Doing the right thing is the easier task. This delusion is ubiquitous. Many scientists and ethicists recognize the delusion of the existence of a rational society, but proceed anyhow as if one exists.
There is a chorus of voices capable of debunking this delusion. Any priest who hears confessions will testify that the vast majority of harm comes from the failure to do what people already know is right, not from uncertainty, confusion, or ignorance. Psychologists and substance abuse counselors are inundated with people who are stuck doing harmful and self-destructive acts. Internists discuss diet and exercise with their patients, but find the advice is infrequently adopted. Master in business administration programs are devoted to training graduates in methods of motivating people to do what is right.
The response of the scientific establishment to the COVID-19 pandemic was imperfect. There were gaps in knowledge and some early information from China was misleading. The initial CDC test kit production was flawed. The early appeal for the public not to buy masks was strongly driven by a desire to preserve supplies for health care workers. Despite these missteps, the overall advice of scientists was wildly successful and beneficial. The goal was to flatten the curve, and a comparison of the April-June time frame with the November-January period shows markedly fewer COVID-19 cases, hospitalizations, and deaths. Confronted with the pandemic of the century, my assessment is that scientific establishment has performed well.
I am far more negative in my assessment of the institutions that support morality, form the social order, establish justice, and promote the general welfare. For instance, misinformation on social media is rampant, including conspiracy theories and outright denials of the pandemic. Scientific advice has been undercut and impugned. Policy recommendations of esteemed scientific institutions have been ignored. The public’s cooperation has fatigued. Laws on public gatherings, quarantines, and social distancing have been broken. Communitarian ethics and devotion to the common good have been left in a trash heap. The consequences have been hundreds of thousands of lives lost in 2020 and some states are on the brink of much worse.
Medical ethicists have debated in fine detail how to triage ventilators, ration antibody treatments, and prioritize vaccinations. Those policy recommendations have had limited influence. Medical ethics has inadequately addressed the age old problem of morality, which is getting people to behave as they know they ought. Modern medical ethics may have exacerbated the deviancy. Medical ethics for 50 years has emphasized replacing paternalism with autonomy, but it has not adequately promoted communitarian virtues, self-regulation, and personal integrity.
There were many accomplishments and many people to admire in 2020 when compared to historical actions by the health care professionals during crises. Doctors, confronted with the COVID-19 plague, have not abandoned the cities as happened in prior centuries. Patients have not been shunned like lepers, though the total-body protective equipment and the no-visitor policies come very close. Nurses have heroically provided bedside care, though I am haunted by one dissident nurse during a protest carrying a sign saying “Don’t call me a hero. I am being martyred against my will.”
As a scientist, I am prone to the delusion that, if I can build a better mouse trap, people will use it. I’ve lived with that delusion for decades. It carries over into my medical ethics work. Yet I see hospitals in California being overwhelmed by the surge on top of a surge due to unwise and unsafe holiday travel. I can see that optimized solutions aren’t the answer – it is better behavior by the public. I recall when I was a child, my mother would simply command, “Behave yourself.” And never, in any of those recollections, was I in doubt about which correct behavior she meant.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
‘Peer respites’ provide an alternative to psychiatric wards during pandemic
Mia McDermott is no stranger to isolation. Abandoned as an infant in China, she lived in an orphanage until a family in California adopted her as a toddler. She spent her adolescence in boarding schools and early adult years in and out of psychiatric hospitals, where she underwent treatment for bipolar disorder, anxiety, and anorexia.
The pandemic left Ms. McDermott feeling especially lonely. She restricted social interactions because her fatty liver disease put her at greater risk of complications should she contract COVID-19. The 26-year-old Santa Cruz, Calif., resident stopped regularly eating and taking her psychiatric medications, and contemplated suicide.
When Ms. McDermott’s thoughts grew increasingly dark in June, she checked into Second Story, a mental health program based in a home not far from her own, where she finds nonclinical support in a peaceful environment from people who have faced similar challenges.
Second Story is what is known as a “peer respite,” a welcoming place where people can stay when they’re experiencing or nearing a mental health crisis. Betting that a low-key wellness approach, coupled with empathy from people who have “been there,” can help people in distress recover, this unorthodox strategy has gained popularity in recent years as the nation grapples with a severe shortage of psychiatric beds that has been exacerbated by the pandemic.
Peer respites allow guests to avoid psychiatric hospitalization and ED visits. They now operate in at least 14 states. California has five, in the San Francisco Bay Area and Los Angeles County.
“When things are really tough and you need extra support but you don’t need hospitalization, where’s that middle ground?” asked Keris Myrick, founder of Hacienda of Hope, a peer respite in Long Beach, Calif.
People with serious mental illness are more likely to experience emotional distress in the pandemic than the general population, said Benjamin Druss, MD, a psychiatrist and professor at Emory University, Atlanta, elaborating that they tend to have smaller social networks and more medical problems.
That was the case with Ms. McDermott. “I don’t have a full-on relationship with my family. My friends are my family,” she said. She yearned to “give them a hug, see their smile, or stand close and take a selfie.”
The next best thing was Second Story, located in a pewter-gray split-level, five-bedroom house in Aptos, a quaint beach community near Ms. McDermott’s Santa Cruz home.
– people who have experienced mental health conditions and are trained and often certified by states to support others with similar issues – and activities like arts, meditation and support groups.
“You can’t tell who’s the guest and who’s the staff. We don’t wear uniforms or badges,” said Angelica Garcia-Guerrero, associate director of Hacienda of Hope’s parent organization.
Peer respites are free for guests but rarely covered by insurance. States and counties typically pick up the tab. Hacienda of Hope’s $900,000 annual operating costs are covered by Los Angeles County through the Mental Health Services Act, a policy that directs proceeds from a statewide tax on people who earn more than $1 million annually to behavioral health programs.
In September, California Gov. Gavin Newsom signed a bill that would establish a statewide certification process for mental health peer providers by July 2022.
For now, however, peer-respite staff members in California are not licensed or certified. Peer respites typically don’t offer clinical care or dispense psychiatric drugs, though guests can bring theirs. Peers share personal stories with guests but avoid labeling them with diagnoses. Guests must come – and can leave – voluntarily. Some respites have few restrictions on who can stay; others don’t allow guests who express suicidal thoughts or are homeless.
Peer respite is one of several types of programs that divert people facing behavioral health crises from the hospital, but the only one without clinical involvement, said Travis Atkinson, a consultant at TBD Solutions, a behavioral health care company. The first peer respites arose around 2000, said Laysha Ostrow, CEO of Live & Learn, which conducts behavioral health research.
The approach seems to be expanding. Live & Learn currently counts 33 peer respites in the United States, up from 19 6 years ago. All are overseen and staffed by people with histories of psychiatric disorders. About a dozen other programs employ a mix of peers and laypeople who don’t have psychiatric diagnoses, or aren’t peer led, Mr. Atkinson said.
Though she had stayed at Second Story several times over the past 5 years, Ms. McDermott hesitated to return during the pandemic. However, she felt reassured after learning that guests were required to wear a mask in common areas and get a COVID test before their stay. To ensure physical distancing, the respite reduced capacity from six to five guests at a time.
During her 2-week stay, Ms. McDermott played with the respite’s two cats and piano – activities she found therapeutic. But most helpful was talking to peers in a way she couldn’t with her mental health providers. In the past, Ms. McDermott said, she had been involuntarily admitted to a psychiatric hospital after she expressed suicidal thoughts. When she shared similar sentiments with Second Story peers, they offered to talk, or call the hospital if she wanted.
“They were willing to listen,” she said. “But they’re not forceful about helping.”
By the end of the visit, Ms. McDermott said that she felt understood and her loneliness and suicidal feelings had waned. She started eating and taking her medications more consistently.
The small number of studies on respites have found that guests had fewer hospitalizations and accounted for lower Medicaid spending for nearly a year after a respite stay than people with similar conditions who did not stay in a respite. Respite visitors spent less time in the hospital and emergency room the longer they stayed in the respite.
Financial struggles and opposition from neighbors have hindered the growth of respites, however. Live & Learn said that, although five peer respites have been created since 2018, at least two others closed because of budget cuts.
Neighbors have challenged nearby respite placements in a few instances. Santa Cruz–area media outlets reported in 2019 that Second Story neighbors had voiced safety concerns with the respite. Neighbor Tony Crane said in an interview that guests have used drugs and consumed alcohol in the neighborhood, and he worried that peers are not licensed or certified to support people in crisis. He felt it was too risky to let his children ride their bikes near the respite when they were younger.
In a written response, Monica Martinez, whose organization runs Second Story, said neighbors often target community mental health programs because of concerns that “come from misconceptions and stigma surrounding those seeking mental health support.”
Many respites are struggling with increased demand and decreased availability during the pandemic. Sherry Jenkins Tucker, executive director of Georgia Mental Health Consumer Network, said its four respites have had to reduce capacity to enable physical distancing, despite increased demand for services. Other respites have temporarily suspended stays because of the pandemic.
Ms. McDermott said her mental health had improved since staying at Second Story in June, but she still struggles with isolation amid the pandemic. “Holidays are hard for me,” said Ms. McDermott, who returned to Second Story in November. “I really wanted to be able to have Thanksgiving with people.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Mia McDermott is no stranger to isolation. Abandoned as an infant in China, she lived in an orphanage until a family in California adopted her as a toddler. She spent her adolescence in boarding schools and early adult years in and out of psychiatric hospitals, where she underwent treatment for bipolar disorder, anxiety, and anorexia.
The pandemic left Ms. McDermott feeling especially lonely. She restricted social interactions because her fatty liver disease put her at greater risk of complications should she contract COVID-19. The 26-year-old Santa Cruz, Calif., resident stopped regularly eating and taking her psychiatric medications, and contemplated suicide.
When Ms. McDermott’s thoughts grew increasingly dark in June, she checked into Second Story, a mental health program based in a home not far from her own, where she finds nonclinical support in a peaceful environment from people who have faced similar challenges.
Second Story is what is known as a “peer respite,” a welcoming place where people can stay when they’re experiencing or nearing a mental health crisis. Betting that a low-key wellness approach, coupled with empathy from people who have “been there,” can help people in distress recover, this unorthodox strategy has gained popularity in recent years as the nation grapples with a severe shortage of psychiatric beds that has been exacerbated by the pandemic.
Peer respites allow guests to avoid psychiatric hospitalization and ED visits. They now operate in at least 14 states. California has five, in the San Francisco Bay Area and Los Angeles County.
“When things are really tough and you need extra support but you don’t need hospitalization, where’s that middle ground?” asked Keris Myrick, founder of Hacienda of Hope, a peer respite in Long Beach, Calif.
People with serious mental illness are more likely to experience emotional distress in the pandemic than the general population, said Benjamin Druss, MD, a psychiatrist and professor at Emory University, Atlanta, elaborating that they tend to have smaller social networks and more medical problems.
That was the case with Ms. McDermott. “I don’t have a full-on relationship with my family. My friends are my family,” she said. She yearned to “give them a hug, see their smile, or stand close and take a selfie.”
The next best thing was Second Story, located in a pewter-gray split-level, five-bedroom house in Aptos, a quaint beach community near Ms. McDermott’s Santa Cruz home.
– people who have experienced mental health conditions and are trained and often certified by states to support others with similar issues – and activities like arts, meditation and support groups.
“You can’t tell who’s the guest and who’s the staff. We don’t wear uniforms or badges,” said Angelica Garcia-Guerrero, associate director of Hacienda of Hope’s parent organization.
Peer respites are free for guests but rarely covered by insurance. States and counties typically pick up the tab. Hacienda of Hope’s $900,000 annual operating costs are covered by Los Angeles County through the Mental Health Services Act, a policy that directs proceeds from a statewide tax on people who earn more than $1 million annually to behavioral health programs.
In September, California Gov. Gavin Newsom signed a bill that would establish a statewide certification process for mental health peer providers by July 2022.
For now, however, peer-respite staff members in California are not licensed or certified. Peer respites typically don’t offer clinical care or dispense psychiatric drugs, though guests can bring theirs. Peers share personal stories with guests but avoid labeling them with diagnoses. Guests must come – and can leave – voluntarily. Some respites have few restrictions on who can stay; others don’t allow guests who express suicidal thoughts or are homeless.
Peer respite is one of several types of programs that divert people facing behavioral health crises from the hospital, but the only one without clinical involvement, said Travis Atkinson, a consultant at TBD Solutions, a behavioral health care company. The first peer respites arose around 2000, said Laysha Ostrow, CEO of Live & Learn, which conducts behavioral health research.
The approach seems to be expanding. Live & Learn currently counts 33 peer respites in the United States, up from 19 6 years ago. All are overseen and staffed by people with histories of psychiatric disorders. About a dozen other programs employ a mix of peers and laypeople who don’t have psychiatric diagnoses, or aren’t peer led, Mr. Atkinson said.
Though she had stayed at Second Story several times over the past 5 years, Ms. McDermott hesitated to return during the pandemic. However, she felt reassured after learning that guests were required to wear a mask in common areas and get a COVID test before their stay. To ensure physical distancing, the respite reduced capacity from six to five guests at a time.
During her 2-week stay, Ms. McDermott played with the respite’s two cats and piano – activities she found therapeutic. But most helpful was talking to peers in a way she couldn’t with her mental health providers. In the past, Ms. McDermott said, she had been involuntarily admitted to a psychiatric hospital after she expressed suicidal thoughts. When she shared similar sentiments with Second Story peers, they offered to talk, or call the hospital if she wanted.
“They were willing to listen,” she said. “But they’re not forceful about helping.”
By the end of the visit, Ms. McDermott said that she felt understood and her loneliness and suicidal feelings had waned. She started eating and taking her medications more consistently.
The small number of studies on respites have found that guests had fewer hospitalizations and accounted for lower Medicaid spending for nearly a year after a respite stay than people with similar conditions who did not stay in a respite. Respite visitors spent less time in the hospital and emergency room the longer they stayed in the respite.
Financial struggles and opposition from neighbors have hindered the growth of respites, however. Live & Learn said that, although five peer respites have been created since 2018, at least two others closed because of budget cuts.
Neighbors have challenged nearby respite placements in a few instances. Santa Cruz–area media outlets reported in 2019 that Second Story neighbors had voiced safety concerns with the respite. Neighbor Tony Crane said in an interview that guests have used drugs and consumed alcohol in the neighborhood, and he worried that peers are not licensed or certified to support people in crisis. He felt it was too risky to let his children ride their bikes near the respite when they were younger.
In a written response, Monica Martinez, whose organization runs Second Story, said neighbors often target community mental health programs because of concerns that “come from misconceptions and stigma surrounding those seeking mental health support.”
Many respites are struggling with increased demand and decreased availability during the pandemic. Sherry Jenkins Tucker, executive director of Georgia Mental Health Consumer Network, said its four respites have had to reduce capacity to enable physical distancing, despite increased demand for services. Other respites have temporarily suspended stays because of the pandemic.
Ms. McDermott said her mental health had improved since staying at Second Story in June, but she still struggles with isolation amid the pandemic. “Holidays are hard for me,” said Ms. McDermott, who returned to Second Story in November. “I really wanted to be able to have Thanksgiving with people.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Mia McDermott is no stranger to isolation. Abandoned as an infant in China, she lived in an orphanage until a family in California adopted her as a toddler. She spent her adolescence in boarding schools and early adult years in and out of psychiatric hospitals, where she underwent treatment for bipolar disorder, anxiety, and anorexia.
The pandemic left Ms. McDermott feeling especially lonely. She restricted social interactions because her fatty liver disease put her at greater risk of complications should she contract COVID-19. The 26-year-old Santa Cruz, Calif., resident stopped regularly eating and taking her psychiatric medications, and contemplated suicide.
When Ms. McDermott’s thoughts grew increasingly dark in June, she checked into Second Story, a mental health program based in a home not far from her own, where she finds nonclinical support in a peaceful environment from people who have faced similar challenges.
Second Story is what is known as a “peer respite,” a welcoming place where people can stay when they’re experiencing or nearing a mental health crisis. Betting that a low-key wellness approach, coupled with empathy from people who have “been there,” can help people in distress recover, this unorthodox strategy has gained popularity in recent years as the nation grapples with a severe shortage of psychiatric beds that has been exacerbated by the pandemic.
Peer respites allow guests to avoid psychiatric hospitalization and ED visits. They now operate in at least 14 states. California has five, in the San Francisco Bay Area and Los Angeles County.
“When things are really tough and you need extra support but you don’t need hospitalization, where’s that middle ground?” asked Keris Myrick, founder of Hacienda of Hope, a peer respite in Long Beach, Calif.
People with serious mental illness are more likely to experience emotional distress in the pandemic than the general population, said Benjamin Druss, MD, a psychiatrist and professor at Emory University, Atlanta, elaborating that they tend to have smaller social networks and more medical problems.
That was the case with Ms. McDermott. “I don’t have a full-on relationship with my family. My friends are my family,” she said. She yearned to “give them a hug, see their smile, or stand close and take a selfie.”
The next best thing was Second Story, located in a pewter-gray split-level, five-bedroom house in Aptos, a quaint beach community near Ms. McDermott’s Santa Cruz home.
– people who have experienced mental health conditions and are trained and often certified by states to support others with similar issues – and activities like arts, meditation and support groups.
“You can’t tell who’s the guest and who’s the staff. We don’t wear uniforms or badges,” said Angelica Garcia-Guerrero, associate director of Hacienda of Hope’s parent organization.
Peer respites are free for guests but rarely covered by insurance. States and counties typically pick up the tab. Hacienda of Hope’s $900,000 annual operating costs are covered by Los Angeles County through the Mental Health Services Act, a policy that directs proceeds from a statewide tax on people who earn more than $1 million annually to behavioral health programs.
In September, California Gov. Gavin Newsom signed a bill that would establish a statewide certification process for mental health peer providers by July 2022.
For now, however, peer-respite staff members in California are not licensed or certified. Peer respites typically don’t offer clinical care or dispense psychiatric drugs, though guests can bring theirs. Peers share personal stories with guests but avoid labeling them with diagnoses. Guests must come – and can leave – voluntarily. Some respites have few restrictions on who can stay; others don’t allow guests who express suicidal thoughts or are homeless.
Peer respite is one of several types of programs that divert people facing behavioral health crises from the hospital, but the only one without clinical involvement, said Travis Atkinson, a consultant at TBD Solutions, a behavioral health care company. The first peer respites arose around 2000, said Laysha Ostrow, CEO of Live & Learn, which conducts behavioral health research.
The approach seems to be expanding. Live & Learn currently counts 33 peer respites in the United States, up from 19 6 years ago. All are overseen and staffed by people with histories of psychiatric disorders. About a dozen other programs employ a mix of peers and laypeople who don’t have psychiatric diagnoses, or aren’t peer led, Mr. Atkinson said.
Though she had stayed at Second Story several times over the past 5 years, Ms. McDermott hesitated to return during the pandemic. However, she felt reassured after learning that guests were required to wear a mask in common areas and get a COVID test before their stay. To ensure physical distancing, the respite reduced capacity from six to five guests at a time.
During her 2-week stay, Ms. McDermott played with the respite’s two cats and piano – activities she found therapeutic. But most helpful was talking to peers in a way she couldn’t with her mental health providers. In the past, Ms. McDermott said, she had been involuntarily admitted to a psychiatric hospital after she expressed suicidal thoughts. When she shared similar sentiments with Second Story peers, they offered to talk, or call the hospital if she wanted.
“They were willing to listen,” she said. “But they’re not forceful about helping.”
By the end of the visit, Ms. McDermott said that she felt understood and her loneliness and suicidal feelings had waned. She started eating and taking her medications more consistently.
The small number of studies on respites have found that guests had fewer hospitalizations and accounted for lower Medicaid spending for nearly a year after a respite stay than people with similar conditions who did not stay in a respite. Respite visitors spent less time in the hospital and emergency room the longer they stayed in the respite.
Financial struggles and opposition from neighbors have hindered the growth of respites, however. Live & Learn said that, although five peer respites have been created since 2018, at least two others closed because of budget cuts.
Neighbors have challenged nearby respite placements in a few instances. Santa Cruz–area media outlets reported in 2019 that Second Story neighbors had voiced safety concerns with the respite. Neighbor Tony Crane said in an interview that guests have used drugs and consumed alcohol in the neighborhood, and he worried that peers are not licensed or certified to support people in crisis. He felt it was too risky to let his children ride their bikes near the respite when they were younger.
In a written response, Monica Martinez, whose organization runs Second Story, said neighbors often target community mental health programs because of concerns that “come from misconceptions and stigma surrounding those seeking mental health support.”
Many respites are struggling with increased demand and decreased availability during the pandemic. Sherry Jenkins Tucker, executive director of Georgia Mental Health Consumer Network, said its four respites have had to reduce capacity to enable physical distancing, despite increased demand for services. Other respites have temporarily suspended stays because of the pandemic.
Ms. McDermott said her mental health had improved since staying at Second Story in June, but she still struggles with isolation amid the pandemic. “Holidays are hard for me,” said Ms. McDermott, who returned to Second Story in November. “I really wanted to be able to have Thanksgiving with people.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.







