User login
In Case You Missed It: COVID
Independent physicians finally get vaccine for selves, but not patients
Physicians unaffiliated with health care systems continue to have difficulties obtaining COVID-19 vaccinations for themselves and their staffs, but that challenge appears to be fading in some states. Yet, in many places, primary care physicians (PCPs) still aren’t being enlisted in the national vaccination effort, despite their numbers and their relationships with patients.
In the first few weeks after the Pfizer and Moderna vaccines received emergency-use authorizations from the Food and Drug Administration, they were distributed mostly to hospitals, pharmacies, and long-term care facilities. Naturally, the hospitals and health care systems vaccinated their own staffs and employed physicians first.
So, even though the guidelines from the Centers for Disease Control and Prevention specify that all frontline health care workers should be included in the first vaccination group, many non–hospital-affiliated private practices have been left out in the cold. Non–patient-facing hospital staff members in some facilities, as well as first responders such as police officers and firefighters, have taken precedence over independent primary care physicians.
In Florida, residents older than 65 years were invited to get vaccinated before some physicians had received shots, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, said in an interview.
While the Department of Health & Human Services is now telling states to give vaccinations to everyone over 65, that wasn’t the case back then.
Community doctors in some areas are still finding it hard to get vaccinated or even find out how to get shots. Yul Ejnes, MD, an internist and partner in Coastal Medical, an independent medical group based in Cranston, R.I., said in an interview that he and his practice staff haven’t been vaccinated, while the staffs of local hospitals have received their shots.
In response to repeated inquiries from his group, he said, the state health department recently said independent practice staffs will start getting vaccinated the week of Jan. 25.
Dr. Ejnes said he understood why hospital personnel went first: Hospitals have the necessary infrastructure, “and the staff in the emergency department and the ICU are caring for the sickest of the sick.”
For primary care doctors like himself who don’t work for the hospital, he said, “I don’t think an infrastructure to get us the vaccine in a timely manner was developed – or if it was developed, it hasn’t been communicated to us.”
Nevertheless, Dr. Ejnes stressed that primary care physicians in the community are just as vulnerable to the coronavirus as hospital clinicians. “We’re seeing patients who have COVID but don’t know they have it. I’m seeing 15 patients a day, and we screen them – as everyone else does – for symptoms and contact and travel, and check their temp,” he said. “But not a day goes by that one of the clinicians in this office doesn’t get a phone call from a patient who was seen a day or 2 earlier to tell them it turns out they were COVID positive. I’m spending 15 minutes in a 100–sq ft room with a patient for a routine visit. And as much as we’re masking and gloving and wearing eye protection, I wouldn’t consider us to be at low risk, especially with the high prevalence of disease.”
In some other states, the situation seems to be improving. Ada Stewart, MD, president of the American Academy of Family Physicians, said that she and her colleagues in a community health center in Columbia, S.C., are in the process of being vaccinated. She got her own shot Jan. 6 at a local hospital.
Her clinic’s staff hadn’t been vaccinated earlier, she said, because nobody in the practice knew the contact person at the hospital who could help access the vaccine doses. Other independent practices in her state are now getting vaccinated, she said, after Gov. Henry McMaster of South Carolina ordered that all health care providers in the top priority category be inoculated by Jan. 15. “At this point, the issues have been diminished.”
However, Dr. Stewart added, independent doctors in some states are still unable to get their shots. AAFP state chapters, as well as the national organization, are trying to persuade governors to ensure all of these physicians are vaccinated. “We’re trying to make sure that the voices of physicians not affiliated with health systems are being heard,” she said.
Lucky shot for doctor
David Boles, DO, a family doctor in Clarksville, Tenn., was able to get his first dose of vaccine just before Christmas, he said in an interview, because he was medical director of a hospice that had received vaccine doses for first responders. When some firefighters and police officers failed to show up for their appointments, the hospice called him and said he had 45 minutes to get to the site if he wanted to be vaccinated.
In early January, his colleagues and staff were also vaccinated, he said, after they were notified of their eligibility as frontline health care workers.
Dr. Boles agreed with Dr. Ejnes that community physicians and nurses are as much at risk as hospital clinicians, except for those intubating patients in the ICU. They may be even more vulnerable, he added, because they have less personal protective equipment than hospital doctors and nurses.
Jennifer Brull, MD, a family physician in Plainville, Kan., said there have been plenty of COVID-19 cases in her small rural community, and the local critical access hospital nearly ran out of beds at one point. Through a collaborative relationship among her clinic (the lone one in the area), the hospital, and the county health department, nearly every frontline health care worker has been vaccinated, and most clinicians in her group have gotten their second doses.
Both the hospital and the health department received vaccine supplies, she said, and everyone in the high-priority category was offered shots. So far, about 170 health care workers have been vaccinated, and only a few declined. More than 300 other people – most of them essential non–health care workers and people older than 65 – have signed up for the next round of shots.
Expanding vaccination effort
Dr. Brull’s practice is the exception among private medical groups around the country. Mr. Gilberg said the MGMA is “concerned that independent practices are playing second fiddle because they’ve been left behind.” Physicians and patient-facing staff in private groups should be getting vaccinated before hospital information technology workers and other non–patient-facing staffers.
Medical practices also can and should play a much bigger role in the overall vaccination effort. Mr. Gilberg has spoken to leaders of several large primary care groups “that have the freezers [for vaccines] and the capacity but haven’t been folded into the distribution plan, especially if they’re not part of the hospital system.”
While hospitals have the storage, he said, they’re not set up to distribute vaccines throughout their communities. “Most health care in this country is delivered outside of the hospital setting. That’s how you’re going to get people vaccinated.”
Ironically, he added, “the same PCPs that are having trouble getting themselves and their staffs vaccinated would be the physicians who could help with vaccine distribution.”
Dr. Brull’s clinic stands ready to help the hospital and health department vaccinate the local population. When sufficient vaccine supplies arrive, she said, she envisioned the doctors and staff administering 200-400 shots per day on Saturdays or weekends.
Dr. Brull was the exception – the other physicians interviewed hadn’t been invited to participate in vaccination efforts.
Dr. Ejnes said his group is capable of vaccinating its patients if it uses the Moderna vaccine, which doesn’t require a super-cold freezer. There are logistical challenges, including social distancing and finding space to observe vaccinated patients for 15 minutes after their shots, he noted. “We’re ready and willing, but realistic about how much we’ll be able to do in this effort.”
The fact that doctors haven’t been enlisted yet in this campaign speaks volumes about “the neglect of the public health infrastructure,” Dr. Ejnes said. “We’re not mobilizing as quickly as we should.”
Alternative routes
Dr. Boles’ group has a refrigerator for pediatric vaccines, which could be used to store the Moderna vaccine, he noted. Shots could be administered to patients in their cars in the parking lot, and they could wait for a while afterward until a nurse came out to verify they were okay.
Mass vaccination sites might also be deployed, as Los Angeles is doing with Dodger Stadium, and physicians could take shifts there in their spare time, Dr. Boles said. But for right now, he views pharmacies as the primary venues for community vaccination.
Of course, the number of pharmacists and pharmacy-employed advanced practice nurses is tiny, compared with the number of primary care doctors, mid-level practitioners, and nurses in ambulatory care practices. Moreover, Mr. Gilberg said, practices know from their electronic health records which patients are most at risk and should be vaccinated first. “Walgreens and CVS don’t know that.”
Physicians should also take the lead in vaccinations because of their patient relationships, he noted. “They can help educate [vaccine-hesitant] patients on why it’s important and dispel some of the rumors and the misinformation that has been politicized. That’s why we should engage physicians in an outpatient setting. And we have to vaccinate them and their staffs. Otherwise, we’re never going to get this rollout underway.”
Dr. Stewart agreed. “We are really the foundation of how we’re going to accomplish this. Most folks are seen by a primary care physician. We touch millions of lives,” she said. “We’re part of the community. Our patients trust us. We’re out there doing it already. We’re doing prevention, giving flu shots, and we’re trying to encourage people to get the COVID vaccine.”
A version of this article first appeared on Medscape.com.
Physicians unaffiliated with health care systems continue to have difficulties obtaining COVID-19 vaccinations for themselves and their staffs, but that challenge appears to be fading in some states. Yet, in many places, primary care physicians (PCPs) still aren’t being enlisted in the national vaccination effort, despite their numbers and their relationships with patients.
In the first few weeks after the Pfizer and Moderna vaccines received emergency-use authorizations from the Food and Drug Administration, they were distributed mostly to hospitals, pharmacies, and long-term care facilities. Naturally, the hospitals and health care systems vaccinated their own staffs and employed physicians first.
So, even though the guidelines from the Centers for Disease Control and Prevention specify that all frontline health care workers should be included in the first vaccination group, many non–hospital-affiliated private practices have been left out in the cold. Non–patient-facing hospital staff members in some facilities, as well as first responders such as police officers and firefighters, have taken precedence over independent primary care physicians.
In Florida, residents older than 65 years were invited to get vaccinated before some physicians had received shots, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, said in an interview.
While the Department of Health & Human Services is now telling states to give vaccinations to everyone over 65, that wasn’t the case back then.
Community doctors in some areas are still finding it hard to get vaccinated or even find out how to get shots. Yul Ejnes, MD, an internist and partner in Coastal Medical, an independent medical group based in Cranston, R.I., said in an interview that he and his practice staff haven’t been vaccinated, while the staffs of local hospitals have received their shots.
In response to repeated inquiries from his group, he said, the state health department recently said independent practice staffs will start getting vaccinated the week of Jan. 25.
Dr. Ejnes said he understood why hospital personnel went first: Hospitals have the necessary infrastructure, “and the staff in the emergency department and the ICU are caring for the sickest of the sick.”
For primary care doctors like himself who don’t work for the hospital, he said, “I don’t think an infrastructure to get us the vaccine in a timely manner was developed – or if it was developed, it hasn’t been communicated to us.”
Nevertheless, Dr. Ejnes stressed that primary care physicians in the community are just as vulnerable to the coronavirus as hospital clinicians. “We’re seeing patients who have COVID but don’t know they have it. I’m seeing 15 patients a day, and we screen them – as everyone else does – for symptoms and contact and travel, and check their temp,” he said. “But not a day goes by that one of the clinicians in this office doesn’t get a phone call from a patient who was seen a day or 2 earlier to tell them it turns out they were COVID positive. I’m spending 15 minutes in a 100–sq ft room with a patient for a routine visit. And as much as we’re masking and gloving and wearing eye protection, I wouldn’t consider us to be at low risk, especially with the high prevalence of disease.”
In some other states, the situation seems to be improving. Ada Stewart, MD, president of the American Academy of Family Physicians, said that she and her colleagues in a community health center in Columbia, S.C., are in the process of being vaccinated. She got her own shot Jan. 6 at a local hospital.
Her clinic’s staff hadn’t been vaccinated earlier, she said, because nobody in the practice knew the contact person at the hospital who could help access the vaccine doses. Other independent practices in her state are now getting vaccinated, she said, after Gov. Henry McMaster of South Carolina ordered that all health care providers in the top priority category be inoculated by Jan. 15. “At this point, the issues have been diminished.”
However, Dr. Stewart added, independent doctors in some states are still unable to get their shots. AAFP state chapters, as well as the national organization, are trying to persuade governors to ensure all of these physicians are vaccinated. “We’re trying to make sure that the voices of physicians not affiliated with health systems are being heard,” she said.
Lucky shot for doctor
David Boles, DO, a family doctor in Clarksville, Tenn., was able to get his first dose of vaccine just before Christmas, he said in an interview, because he was medical director of a hospice that had received vaccine doses for first responders. When some firefighters and police officers failed to show up for their appointments, the hospice called him and said he had 45 minutes to get to the site if he wanted to be vaccinated.
In early January, his colleagues and staff were also vaccinated, he said, after they were notified of their eligibility as frontline health care workers.
Dr. Boles agreed with Dr. Ejnes that community physicians and nurses are as much at risk as hospital clinicians, except for those intubating patients in the ICU. They may be even more vulnerable, he added, because they have less personal protective equipment than hospital doctors and nurses.
Jennifer Brull, MD, a family physician in Plainville, Kan., said there have been plenty of COVID-19 cases in her small rural community, and the local critical access hospital nearly ran out of beds at one point. Through a collaborative relationship among her clinic (the lone one in the area), the hospital, and the county health department, nearly every frontline health care worker has been vaccinated, and most clinicians in her group have gotten their second doses.
Both the hospital and the health department received vaccine supplies, she said, and everyone in the high-priority category was offered shots. So far, about 170 health care workers have been vaccinated, and only a few declined. More than 300 other people – most of them essential non–health care workers and people older than 65 – have signed up for the next round of shots.
Expanding vaccination effort
Dr. Brull’s practice is the exception among private medical groups around the country. Mr. Gilberg said the MGMA is “concerned that independent practices are playing second fiddle because they’ve been left behind.” Physicians and patient-facing staff in private groups should be getting vaccinated before hospital information technology workers and other non–patient-facing staffers.
Medical practices also can and should play a much bigger role in the overall vaccination effort. Mr. Gilberg has spoken to leaders of several large primary care groups “that have the freezers [for vaccines] and the capacity but haven’t been folded into the distribution plan, especially if they’re not part of the hospital system.”
While hospitals have the storage, he said, they’re not set up to distribute vaccines throughout their communities. “Most health care in this country is delivered outside of the hospital setting. That’s how you’re going to get people vaccinated.”
Ironically, he added, “the same PCPs that are having trouble getting themselves and their staffs vaccinated would be the physicians who could help with vaccine distribution.”
Dr. Brull’s clinic stands ready to help the hospital and health department vaccinate the local population. When sufficient vaccine supplies arrive, she said, she envisioned the doctors and staff administering 200-400 shots per day on Saturdays or weekends.
Dr. Brull was the exception – the other physicians interviewed hadn’t been invited to participate in vaccination efforts.
Dr. Ejnes said his group is capable of vaccinating its patients if it uses the Moderna vaccine, which doesn’t require a super-cold freezer. There are logistical challenges, including social distancing and finding space to observe vaccinated patients for 15 minutes after their shots, he noted. “We’re ready and willing, but realistic about how much we’ll be able to do in this effort.”
The fact that doctors haven’t been enlisted yet in this campaign speaks volumes about “the neglect of the public health infrastructure,” Dr. Ejnes said. “We’re not mobilizing as quickly as we should.”
Alternative routes
Dr. Boles’ group has a refrigerator for pediatric vaccines, which could be used to store the Moderna vaccine, he noted. Shots could be administered to patients in their cars in the parking lot, and they could wait for a while afterward until a nurse came out to verify they were okay.
Mass vaccination sites might also be deployed, as Los Angeles is doing with Dodger Stadium, and physicians could take shifts there in their spare time, Dr. Boles said. But for right now, he views pharmacies as the primary venues for community vaccination.
Of course, the number of pharmacists and pharmacy-employed advanced practice nurses is tiny, compared with the number of primary care doctors, mid-level practitioners, and nurses in ambulatory care practices. Moreover, Mr. Gilberg said, practices know from their electronic health records which patients are most at risk and should be vaccinated first. “Walgreens and CVS don’t know that.”
Physicians should also take the lead in vaccinations because of their patient relationships, he noted. “They can help educate [vaccine-hesitant] patients on why it’s important and dispel some of the rumors and the misinformation that has been politicized. That’s why we should engage physicians in an outpatient setting. And we have to vaccinate them and their staffs. Otherwise, we’re never going to get this rollout underway.”
Dr. Stewart agreed. “We are really the foundation of how we’re going to accomplish this. Most folks are seen by a primary care physician. We touch millions of lives,” she said. “We’re part of the community. Our patients trust us. We’re out there doing it already. We’re doing prevention, giving flu shots, and we’re trying to encourage people to get the COVID vaccine.”
A version of this article first appeared on Medscape.com.
Physicians unaffiliated with health care systems continue to have difficulties obtaining COVID-19 vaccinations for themselves and their staffs, but that challenge appears to be fading in some states. Yet, in many places, primary care physicians (PCPs) still aren’t being enlisted in the national vaccination effort, despite their numbers and their relationships with patients.
In the first few weeks after the Pfizer and Moderna vaccines received emergency-use authorizations from the Food and Drug Administration, they were distributed mostly to hospitals, pharmacies, and long-term care facilities. Naturally, the hospitals and health care systems vaccinated their own staffs and employed physicians first.
So, even though the guidelines from the Centers for Disease Control and Prevention specify that all frontline health care workers should be included in the first vaccination group, many non–hospital-affiliated private practices have been left out in the cold. Non–patient-facing hospital staff members in some facilities, as well as first responders such as police officers and firefighters, have taken precedence over independent primary care physicians.
In Florida, residents older than 65 years were invited to get vaccinated before some physicians had received shots, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, said in an interview.
While the Department of Health & Human Services is now telling states to give vaccinations to everyone over 65, that wasn’t the case back then.
Community doctors in some areas are still finding it hard to get vaccinated or even find out how to get shots. Yul Ejnes, MD, an internist and partner in Coastal Medical, an independent medical group based in Cranston, R.I., said in an interview that he and his practice staff haven’t been vaccinated, while the staffs of local hospitals have received their shots.
In response to repeated inquiries from his group, he said, the state health department recently said independent practice staffs will start getting vaccinated the week of Jan. 25.
Dr. Ejnes said he understood why hospital personnel went first: Hospitals have the necessary infrastructure, “and the staff in the emergency department and the ICU are caring for the sickest of the sick.”
For primary care doctors like himself who don’t work for the hospital, he said, “I don’t think an infrastructure to get us the vaccine in a timely manner was developed – or if it was developed, it hasn’t been communicated to us.”
Nevertheless, Dr. Ejnes stressed that primary care physicians in the community are just as vulnerable to the coronavirus as hospital clinicians. “We’re seeing patients who have COVID but don’t know they have it. I’m seeing 15 patients a day, and we screen them – as everyone else does – for symptoms and contact and travel, and check their temp,” he said. “But not a day goes by that one of the clinicians in this office doesn’t get a phone call from a patient who was seen a day or 2 earlier to tell them it turns out they were COVID positive. I’m spending 15 minutes in a 100–sq ft room with a patient for a routine visit. And as much as we’re masking and gloving and wearing eye protection, I wouldn’t consider us to be at low risk, especially with the high prevalence of disease.”
In some other states, the situation seems to be improving. Ada Stewart, MD, president of the American Academy of Family Physicians, said that she and her colleagues in a community health center in Columbia, S.C., are in the process of being vaccinated. She got her own shot Jan. 6 at a local hospital.
Her clinic’s staff hadn’t been vaccinated earlier, she said, because nobody in the practice knew the contact person at the hospital who could help access the vaccine doses. Other independent practices in her state are now getting vaccinated, she said, after Gov. Henry McMaster of South Carolina ordered that all health care providers in the top priority category be inoculated by Jan. 15. “At this point, the issues have been diminished.”
However, Dr. Stewart added, independent doctors in some states are still unable to get their shots. AAFP state chapters, as well as the national organization, are trying to persuade governors to ensure all of these physicians are vaccinated. “We’re trying to make sure that the voices of physicians not affiliated with health systems are being heard,” she said.
Lucky shot for doctor
David Boles, DO, a family doctor in Clarksville, Tenn., was able to get his first dose of vaccine just before Christmas, he said in an interview, because he was medical director of a hospice that had received vaccine doses for first responders. When some firefighters and police officers failed to show up for their appointments, the hospice called him and said he had 45 minutes to get to the site if he wanted to be vaccinated.
In early January, his colleagues and staff were also vaccinated, he said, after they were notified of their eligibility as frontline health care workers.
Dr. Boles agreed with Dr. Ejnes that community physicians and nurses are as much at risk as hospital clinicians, except for those intubating patients in the ICU. They may be even more vulnerable, he added, because they have less personal protective equipment than hospital doctors and nurses.
Jennifer Brull, MD, a family physician in Plainville, Kan., said there have been plenty of COVID-19 cases in her small rural community, and the local critical access hospital nearly ran out of beds at one point. Through a collaborative relationship among her clinic (the lone one in the area), the hospital, and the county health department, nearly every frontline health care worker has been vaccinated, and most clinicians in her group have gotten their second doses.
Both the hospital and the health department received vaccine supplies, she said, and everyone in the high-priority category was offered shots. So far, about 170 health care workers have been vaccinated, and only a few declined. More than 300 other people – most of them essential non–health care workers and people older than 65 – have signed up for the next round of shots.
Expanding vaccination effort
Dr. Brull’s practice is the exception among private medical groups around the country. Mr. Gilberg said the MGMA is “concerned that independent practices are playing second fiddle because they’ve been left behind.” Physicians and patient-facing staff in private groups should be getting vaccinated before hospital information technology workers and other non–patient-facing staffers.
Medical practices also can and should play a much bigger role in the overall vaccination effort. Mr. Gilberg has spoken to leaders of several large primary care groups “that have the freezers [for vaccines] and the capacity but haven’t been folded into the distribution plan, especially if they’re not part of the hospital system.”
While hospitals have the storage, he said, they’re not set up to distribute vaccines throughout their communities. “Most health care in this country is delivered outside of the hospital setting. That’s how you’re going to get people vaccinated.”
Ironically, he added, “the same PCPs that are having trouble getting themselves and their staffs vaccinated would be the physicians who could help with vaccine distribution.”
Dr. Brull’s clinic stands ready to help the hospital and health department vaccinate the local population. When sufficient vaccine supplies arrive, she said, she envisioned the doctors and staff administering 200-400 shots per day on Saturdays or weekends.
Dr. Brull was the exception – the other physicians interviewed hadn’t been invited to participate in vaccination efforts.
Dr. Ejnes said his group is capable of vaccinating its patients if it uses the Moderna vaccine, which doesn’t require a super-cold freezer. There are logistical challenges, including social distancing and finding space to observe vaccinated patients for 15 minutes after their shots, he noted. “We’re ready and willing, but realistic about how much we’ll be able to do in this effort.”
The fact that doctors haven’t been enlisted yet in this campaign speaks volumes about “the neglect of the public health infrastructure,” Dr. Ejnes said. “We’re not mobilizing as quickly as we should.”
Alternative routes
Dr. Boles’ group has a refrigerator for pediatric vaccines, which could be used to store the Moderna vaccine, he noted. Shots could be administered to patients in their cars in the parking lot, and they could wait for a while afterward until a nurse came out to verify they were okay.
Mass vaccination sites might also be deployed, as Los Angeles is doing with Dodger Stadium, and physicians could take shifts there in their spare time, Dr. Boles said. But for right now, he views pharmacies as the primary venues for community vaccination.
Of course, the number of pharmacists and pharmacy-employed advanced practice nurses is tiny, compared with the number of primary care doctors, mid-level practitioners, and nurses in ambulatory care practices. Moreover, Mr. Gilberg said, practices know from their electronic health records which patients are most at risk and should be vaccinated first. “Walgreens and CVS don’t know that.”
Physicians should also take the lead in vaccinations because of their patient relationships, he noted. “They can help educate [vaccine-hesitant] patients on why it’s important and dispel some of the rumors and the misinformation that has been politicized. That’s why we should engage physicians in an outpatient setting. And we have to vaccinate them and their staffs. Otherwise, we’re never going to get this rollout underway.”
Dr. Stewart agreed. “We are really the foundation of how we’re going to accomplish this. Most folks are seen by a primary care physician. We touch millions of lives,” she said. “We’re part of the community. Our patients trust us. We’re out there doing it already. We’re doing prevention, giving flu shots, and we’re trying to encourage people to get the COVID vaccine.”
A version of this article first appeared on Medscape.com.
AMA president: Biden team must create national pandemic strategy
The incoming Biden administration must formulate an effective national strategy for the COVID-19 pandemic, Susan R. Bailey, MD, president of the American Medical Association (AMA), said in a speech delivered Jan. 12 at the National Press Club in Washington.
Dr. Bailey noted that America’s fight against the pandemic is in a critical phase, as evidenced by the escalation in cases, hospitalizations, and deaths in recent weeks. Emergency departments and ICUs are overwhelmed; many frontline clinicians are burned out; and the state- and local-level mechanisms for vaccine distribution have been slow and inconsistent, she said.
“The most important lesson for this moment, and for the year ahead, is that leaving state and local officials to shoulder this burden alone without adequate support from the federal government is not going to work,” Dr. Bailey emphasized.
She called on the Biden administration, which takes over on Jan. 20, to “provide states and local jurisdictions with additional resources, guidance, and support to enable rapid distribution and administration of vaccines.”
In addition, she said, the incoming administration needs to develop a more robust, national strategy for continued COVID-19 testing and PPE production “by tapping into the full powers of the Defense Production Act.”
Biden vaccine distribution policy
In a question-and-answer period following her speech, however, Dr. Bailey said she opposed the president-elect’s decision to release nearly all available vaccine supplies immediately, rather than hold back some doses for the second shots that the Pfizer and Moderna vaccines require. On Jan. 12, the Trump administration announced that it plans to do the same thing.
“We’re a little bit concerned about the announcement that [the Department of Health and Human Services] will not hold back vaccine doses to make sure that everyone who’s gotten their first dose will have a second dose in reserve,” Dr. Bailey said. “We don’t have adequate data to tell us that one dose is sufficient – we don’t think it is – and how long you can wait for the second dose without losing the benefits of the first dose.”
She added that it’s not recommended that people mix the two vaccines in the first and second doses. “Since the Pfizer vaccine has such rigid storage requirements, I want to make sure there’s plenty of vaccine for frontline health care workers who got the Pfizer vaccine because it was the first one to come out in December. I want to make sure they get their second dose on time and [do] not have to wait.”
Dr. Bailey said she hoped there will be plenty of vaccine supply. But she suggested that state and local health authorities be in communication with the federal government about whether there will be enough vaccine to guarantee people can get both doses.
Bolstering public health
In her speech, Dr. Bailey outlined five areas in which steps should be taken to improve the health system so that it isn’t overwhelmed the next time the United States has a public health crisis:
- Restore trust in science and science-based decision making. Make sure that scientific institutions such as the Centers for Disease Control and Prevention and the Food and Drug Administration are “free from political pressure, and that their actions are guided by the best available scientific evidence.”
- Ensure that the health system provides all Americans with affordable access to comprehensive health care. Dr. Bailey wasn’t talking about Medicare for All; she suggested that perhaps there be a second enrollment period for the Affordable Care Act’s individual insurance exchanges.
- Work to remove health care inequities that have hurt communities of color, who have been disproportionately impacted by the pandemic. She referred to a recent AMA that recognized racism as a public health threat.
- Improve public health domestically and globally. Among other things, she noted, the public health infrastructure needs to be revitalized after “decades of disinvestment and neglect,” which has contributed to the slow vaccine rollout.
- Recognize the global health community and restore America’s leadership in global efforts to combat disease, which are critical to preventing future threats. She praised President-Elect Biden for his promise that the United States will rejoin the World Health Organization.
At several points in her presentation, Dr. Bailey rejected political interference with science and health care. Among other things, she said public health could be improved by protecting the doctor-patient relationship from political interference.
Answering a question about how to separate politics from the pandemic, she replied, “The key is in sticking to the science and listening to our public health authorities. They all have to deliver the same message. Also, leaders at all levels, including in our communities, our schools, churches and college campuses, should wear masks and socially distance. This isn’t about anything other than the desire to get out of the pandemic and get our country on the right track again. Masks shouldn’t be political. Going back to school shouldn’t be political. Taking a certain medication or not shouldn’t be political. We need to stick to the science and listen to our public health authorities. That’s the quickest way out.”
Asked when she thought that life might get back to normal again in the United States, Dr. Bailey said a lot depends on the extent of vaccine uptake and how much self-discipline people exhibit in following public health advice. “I think we’re looking at the end of this year. I’m hopeful that by fall, things will have opened up quite a bit as the Venn diagrams of those who’ve gotten vaccines grow larger.”
A version of this article first appeared on Medscape.com.
The incoming Biden administration must formulate an effective national strategy for the COVID-19 pandemic, Susan R. Bailey, MD, president of the American Medical Association (AMA), said in a speech delivered Jan. 12 at the National Press Club in Washington.
Dr. Bailey noted that America’s fight against the pandemic is in a critical phase, as evidenced by the escalation in cases, hospitalizations, and deaths in recent weeks. Emergency departments and ICUs are overwhelmed; many frontline clinicians are burned out; and the state- and local-level mechanisms for vaccine distribution have been slow and inconsistent, she said.
“The most important lesson for this moment, and for the year ahead, is that leaving state and local officials to shoulder this burden alone without adequate support from the federal government is not going to work,” Dr. Bailey emphasized.
She called on the Biden administration, which takes over on Jan. 20, to “provide states and local jurisdictions with additional resources, guidance, and support to enable rapid distribution and administration of vaccines.”
In addition, she said, the incoming administration needs to develop a more robust, national strategy for continued COVID-19 testing and PPE production “by tapping into the full powers of the Defense Production Act.”
Biden vaccine distribution policy
In a question-and-answer period following her speech, however, Dr. Bailey said she opposed the president-elect’s decision to release nearly all available vaccine supplies immediately, rather than hold back some doses for the second shots that the Pfizer and Moderna vaccines require. On Jan. 12, the Trump administration announced that it plans to do the same thing.
“We’re a little bit concerned about the announcement that [the Department of Health and Human Services] will not hold back vaccine doses to make sure that everyone who’s gotten their first dose will have a second dose in reserve,” Dr. Bailey said. “We don’t have adequate data to tell us that one dose is sufficient – we don’t think it is – and how long you can wait for the second dose without losing the benefits of the first dose.”
She added that it’s not recommended that people mix the two vaccines in the first and second doses. “Since the Pfizer vaccine has such rigid storage requirements, I want to make sure there’s plenty of vaccine for frontline health care workers who got the Pfizer vaccine because it was the first one to come out in December. I want to make sure they get their second dose on time and [do] not have to wait.”
Dr. Bailey said she hoped there will be plenty of vaccine supply. But she suggested that state and local health authorities be in communication with the federal government about whether there will be enough vaccine to guarantee people can get both doses.
Bolstering public health
In her speech, Dr. Bailey outlined five areas in which steps should be taken to improve the health system so that it isn’t overwhelmed the next time the United States has a public health crisis:
- Restore trust in science and science-based decision making. Make sure that scientific institutions such as the Centers for Disease Control and Prevention and the Food and Drug Administration are “free from political pressure, and that their actions are guided by the best available scientific evidence.”
- Ensure that the health system provides all Americans with affordable access to comprehensive health care. Dr. Bailey wasn’t talking about Medicare for All; she suggested that perhaps there be a second enrollment period for the Affordable Care Act’s individual insurance exchanges.
- Work to remove health care inequities that have hurt communities of color, who have been disproportionately impacted by the pandemic. She referred to a recent AMA that recognized racism as a public health threat.
- Improve public health domestically and globally. Among other things, she noted, the public health infrastructure needs to be revitalized after “decades of disinvestment and neglect,” which has contributed to the slow vaccine rollout.
- Recognize the global health community and restore America’s leadership in global efforts to combat disease, which are critical to preventing future threats. She praised President-Elect Biden for his promise that the United States will rejoin the World Health Organization.
At several points in her presentation, Dr. Bailey rejected political interference with science and health care. Among other things, she said public health could be improved by protecting the doctor-patient relationship from political interference.
Answering a question about how to separate politics from the pandemic, she replied, “The key is in sticking to the science and listening to our public health authorities. They all have to deliver the same message. Also, leaders at all levels, including in our communities, our schools, churches and college campuses, should wear masks and socially distance. This isn’t about anything other than the desire to get out of the pandemic and get our country on the right track again. Masks shouldn’t be political. Going back to school shouldn’t be political. Taking a certain medication or not shouldn’t be political. We need to stick to the science and listen to our public health authorities. That’s the quickest way out.”
Asked when she thought that life might get back to normal again in the United States, Dr. Bailey said a lot depends on the extent of vaccine uptake and how much self-discipline people exhibit in following public health advice. “I think we’re looking at the end of this year. I’m hopeful that by fall, things will have opened up quite a bit as the Venn diagrams of those who’ve gotten vaccines grow larger.”
A version of this article first appeared on Medscape.com.
The incoming Biden administration must formulate an effective national strategy for the COVID-19 pandemic, Susan R. Bailey, MD, president of the American Medical Association (AMA), said in a speech delivered Jan. 12 at the National Press Club in Washington.
Dr. Bailey noted that America’s fight against the pandemic is in a critical phase, as evidenced by the escalation in cases, hospitalizations, and deaths in recent weeks. Emergency departments and ICUs are overwhelmed; many frontline clinicians are burned out; and the state- and local-level mechanisms for vaccine distribution have been slow and inconsistent, she said.
“The most important lesson for this moment, and for the year ahead, is that leaving state and local officials to shoulder this burden alone without adequate support from the federal government is not going to work,” Dr. Bailey emphasized.
She called on the Biden administration, which takes over on Jan. 20, to “provide states and local jurisdictions with additional resources, guidance, and support to enable rapid distribution and administration of vaccines.”
In addition, she said, the incoming administration needs to develop a more robust, national strategy for continued COVID-19 testing and PPE production “by tapping into the full powers of the Defense Production Act.”
Biden vaccine distribution policy
In a question-and-answer period following her speech, however, Dr. Bailey said she opposed the president-elect’s decision to release nearly all available vaccine supplies immediately, rather than hold back some doses for the second shots that the Pfizer and Moderna vaccines require. On Jan. 12, the Trump administration announced that it plans to do the same thing.
“We’re a little bit concerned about the announcement that [the Department of Health and Human Services] will not hold back vaccine doses to make sure that everyone who’s gotten their first dose will have a second dose in reserve,” Dr. Bailey said. “We don’t have adequate data to tell us that one dose is sufficient – we don’t think it is – and how long you can wait for the second dose without losing the benefits of the first dose.”
She added that it’s not recommended that people mix the two vaccines in the first and second doses. “Since the Pfizer vaccine has such rigid storage requirements, I want to make sure there’s plenty of vaccine for frontline health care workers who got the Pfizer vaccine because it was the first one to come out in December. I want to make sure they get their second dose on time and [do] not have to wait.”
Dr. Bailey said she hoped there will be plenty of vaccine supply. But she suggested that state and local health authorities be in communication with the federal government about whether there will be enough vaccine to guarantee people can get both doses.
Bolstering public health
In her speech, Dr. Bailey outlined five areas in which steps should be taken to improve the health system so that it isn’t overwhelmed the next time the United States has a public health crisis:
- Restore trust in science and science-based decision making. Make sure that scientific institutions such as the Centers for Disease Control and Prevention and the Food and Drug Administration are “free from political pressure, and that their actions are guided by the best available scientific evidence.”
- Ensure that the health system provides all Americans with affordable access to comprehensive health care. Dr. Bailey wasn’t talking about Medicare for All; she suggested that perhaps there be a second enrollment period for the Affordable Care Act’s individual insurance exchanges.
- Work to remove health care inequities that have hurt communities of color, who have been disproportionately impacted by the pandemic. She referred to a recent AMA that recognized racism as a public health threat.
- Improve public health domestically and globally. Among other things, she noted, the public health infrastructure needs to be revitalized after “decades of disinvestment and neglect,” which has contributed to the slow vaccine rollout.
- Recognize the global health community and restore America’s leadership in global efforts to combat disease, which are critical to preventing future threats. She praised President-Elect Biden for his promise that the United States will rejoin the World Health Organization.
At several points in her presentation, Dr. Bailey rejected political interference with science and health care. Among other things, she said public health could be improved by protecting the doctor-patient relationship from political interference.
Answering a question about how to separate politics from the pandemic, she replied, “The key is in sticking to the science and listening to our public health authorities. They all have to deliver the same message. Also, leaders at all levels, including in our communities, our schools, churches and college campuses, should wear masks and socially distance. This isn’t about anything other than the desire to get out of the pandemic and get our country on the right track again. Masks shouldn’t be political. Going back to school shouldn’t be political. Taking a certain medication or not shouldn’t be political. We need to stick to the science and listen to our public health authorities. That’s the quickest way out.”
Asked when she thought that life might get back to normal again in the United States, Dr. Bailey said a lot depends on the extent of vaccine uptake and how much self-discipline people exhibit in following public health advice. “I think we’re looking at the end of this year. I’m hopeful that by fall, things will have opened up quite a bit as the Venn diagrams of those who’ve gotten vaccines grow larger.”
A version of this article first appeared on Medscape.com.
Feds to states: Give COVID-19 vaccine to 65+ and those with comorbidities
Federal health officials are urging states to vaccinate all Americans over age 65 and those aged 16-64 who have a documented underlying health condition that makes them more vulnerable to COVID-19.
U.S. Department of Health and Human Services (HHS) Secretary Alex Azar and Centers for Disease Control and Prevention Director Robert Redfield, MD, made the recommendation in a briefing with reporters on Jan. 12, saying that the current vaccine supply was sufficient to meet demand for the next phase of immunization as recommended by the CDC’s Advisory Committee on Immunization Practices.
“We are ready for a transition that we outlined last September in the playbook we sent to states,” Mr. Azar said. Both he and U.S. Army General Gustave F. Perna, chief operations officer for Operation Warp Speed, said that confidence in the distribution system had led to the decision to urge wider access.
The federal government will also increase the number of sites eligible to receive vaccine – including some 13,000 federally qualified community health centers – and will not keep doses in reserve as insurance against issues that might prevent people from receiving a second dose on a timely basis.
“We don’t need to hold back reserve doses,” Mr. Azar said, noting that if there were any “glitches in production” the federal government would move to fulfill obligations for second doses first and delay initial doses.
Azar: Use it or lose it
In a move that is sure to generate pushback, Mr. Azar said that states that don’t quickly administer vaccines will receive fewer doses in the future. That policy will not go into effect until later in February, which leaves open the possibility that it could be reversed by the incoming Biden administration.
“We have too much vaccine sitting in freezers at hospitals with hospitals not using it,” said Mr. Azar, who also blamed the slow administration process on a reporting lag and states being what he called “overly prescriptive” in who has been eligible to receive a shot.
“I would rather have people working to get appointments to get vaccinated than having vaccine going to waste sitting in freezers,” he told reporters.
Mr. Azar had already been pushing for broader vaccination, telling states to do so in an Operation Warp Speed briefing on Jan. 6. At that briefing, he also said that the federal government would be stepping up vaccination through an “early launch” of a federal partnership with 19 pharmacy chains, which will let states allocate vaccines directly to some 40,000 pharmacy sites.
Gen. Perna said during the Jan. 12 briefing that the aim is to further expand that to some 70,000 locations total.
The CDC reported that as of Jan. 11 some 25.4 million doses have been distributed, with 8.9 million administered. An additional 4.2 million doses were distributed to long-term care facilities, and 937,000 residents and staff have received a dose.
“Pace of administration”
Alaska, Connecticut, North Dakota, South Dakota, the District of Columbia, West Virginia, and the Northern Mariana Islands have administered the most vaccines per capita, according to the CDC. But even these locations have immunized only 4%-5% of their populations, the New York Times reports. At the bottom: Alabama, Arizona, Arkansas, Georgia, Mississippi, and South Carolina.
The federal government can encourage but not require states to move on to new phases of vaccination.
“States ultimately determine how they will proceed with vaccination,” said Marcus Plescia, MD, MPH, chief medical officer for the Association of State and Territorial Health Officials. “Most will be cautious about assuring there are doses for those needing a second dose,” he said in an interview.
Dr. Plescia said that ensuring a second dose is available is especially important for health care workers “who need to be confident that they are protected and not inadvertently transmitting the disease themselves.”
He added that “once we reach a steady state of supply and administration, the rate-limiting factor will be supply of vaccine.”
That supply could now be threatened if states don’t comply with a just-announced federal action that will change how doses are allocated.
Beginning in late February, vaccine allocations to states will be based on “the pace of administration reported by states,” and the size of the 65-and-older population, said Mr. Azar, who has previously criticized New York Governor Andrew Cuomo for fining hospitals that didn’t use up vaccine supply within a week.
“This new system gives states a strong incentive to ensure that all vaccinations are being promptly reported, which they currently are not,” he said.
Currently, allocations are based on a state’s or territory’s population.
Prepandemic, states were required to report vaccinations within 30 days. Since COVID-19 vaccines became available, the CDC has required reporting of shots within 72 hours.
Dr. Redfield said the requirement has caused some difficulty, and that the CDC is investigating why some states have reported using only 15% of doses while others have used 80%.
States have been scrambling to ramp up vaccinations.
Just ahead of the federal briefing, Gov. Cuomo tweeted that New York would be opening up vaccinations to anyone older than 65.
The Associated Press is reporting that some states have started mass vaccination sites.
Arizona has begun operating a 24/7 appointment-only vaccination program at State Farm Stadium outside of Phoenix, with the aim of immunizing 6,000 people each day, according to local radio station KJZZ.
California and Florida have also taken steps to use stadiums, while Michigan, New Jersey, New York, and Texas will use convention centers and fairgrounds, Axios has reported.
In Florida, Palm Beach County Health Director Alina Alonso, MD, told county commissioners on Jan. 12 that there isn’t enough vaccine to meet demand, WPTV reported. “We need to realize that there’s a shortage of vaccine. So it’s not the plan, it’s not our ability to do it. It’s simply supply and demand at this point,” Dr. Alonso said, according to the TV station report.
A version of this article first appeared on Medscape.com.
Federal health officials are urging states to vaccinate all Americans over age 65 and those aged 16-64 who have a documented underlying health condition that makes them more vulnerable to COVID-19.
U.S. Department of Health and Human Services (HHS) Secretary Alex Azar and Centers for Disease Control and Prevention Director Robert Redfield, MD, made the recommendation in a briefing with reporters on Jan. 12, saying that the current vaccine supply was sufficient to meet demand for the next phase of immunization as recommended by the CDC’s Advisory Committee on Immunization Practices.
“We are ready for a transition that we outlined last September in the playbook we sent to states,” Mr. Azar said. Both he and U.S. Army General Gustave F. Perna, chief operations officer for Operation Warp Speed, said that confidence in the distribution system had led to the decision to urge wider access.
The federal government will also increase the number of sites eligible to receive vaccine – including some 13,000 federally qualified community health centers – and will not keep doses in reserve as insurance against issues that might prevent people from receiving a second dose on a timely basis.
“We don’t need to hold back reserve doses,” Mr. Azar said, noting that if there were any “glitches in production” the federal government would move to fulfill obligations for second doses first and delay initial doses.
Azar: Use it or lose it
In a move that is sure to generate pushback, Mr. Azar said that states that don’t quickly administer vaccines will receive fewer doses in the future. That policy will not go into effect until later in February, which leaves open the possibility that it could be reversed by the incoming Biden administration.
“We have too much vaccine sitting in freezers at hospitals with hospitals not using it,” said Mr. Azar, who also blamed the slow administration process on a reporting lag and states being what he called “overly prescriptive” in who has been eligible to receive a shot.
“I would rather have people working to get appointments to get vaccinated than having vaccine going to waste sitting in freezers,” he told reporters.
Mr. Azar had already been pushing for broader vaccination, telling states to do so in an Operation Warp Speed briefing on Jan. 6. At that briefing, he also said that the federal government would be stepping up vaccination through an “early launch” of a federal partnership with 19 pharmacy chains, which will let states allocate vaccines directly to some 40,000 pharmacy sites.
Gen. Perna said during the Jan. 12 briefing that the aim is to further expand that to some 70,000 locations total.
The CDC reported that as of Jan. 11 some 25.4 million doses have been distributed, with 8.9 million administered. An additional 4.2 million doses were distributed to long-term care facilities, and 937,000 residents and staff have received a dose.
“Pace of administration”
Alaska, Connecticut, North Dakota, South Dakota, the District of Columbia, West Virginia, and the Northern Mariana Islands have administered the most vaccines per capita, according to the CDC. But even these locations have immunized only 4%-5% of their populations, the New York Times reports. At the bottom: Alabama, Arizona, Arkansas, Georgia, Mississippi, and South Carolina.
The federal government can encourage but not require states to move on to new phases of vaccination.
“States ultimately determine how they will proceed with vaccination,” said Marcus Plescia, MD, MPH, chief medical officer for the Association of State and Territorial Health Officials. “Most will be cautious about assuring there are doses for those needing a second dose,” he said in an interview.
Dr. Plescia said that ensuring a second dose is available is especially important for health care workers “who need to be confident that they are protected and not inadvertently transmitting the disease themselves.”
He added that “once we reach a steady state of supply and administration, the rate-limiting factor will be supply of vaccine.”
That supply could now be threatened if states don’t comply with a just-announced federal action that will change how doses are allocated.
Beginning in late February, vaccine allocations to states will be based on “the pace of administration reported by states,” and the size of the 65-and-older population, said Mr. Azar, who has previously criticized New York Governor Andrew Cuomo for fining hospitals that didn’t use up vaccine supply within a week.
“This new system gives states a strong incentive to ensure that all vaccinations are being promptly reported, which they currently are not,” he said.
Currently, allocations are based on a state’s or territory’s population.
Prepandemic, states were required to report vaccinations within 30 days. Since COVID-19 vaccines became available, the CDC has required reporting of shots within 72 hours.
Dr. Redfield said the requirement has caused some difficulty, and that the CDC is investigating why some states have reported using only 15% of doses while others have used 80%.
States have been scrambling to ramp up vaccinations.
Just ahead of the federal briefing, Gov. Cuomo tweeted that New York would be opening up vaccinations to anyone older than 65.
The Associated Press is reporting that some states have started mass vaccination sites.
Arizona has begun operating a 24/7 appointment-only vaccination program at State Farm Stadium outside of Phoenix, with the aim of immunizing 6,000 people each day, according to local radio station KJZZ.
California and Florida have also taken steps to use stadiums, while Michigan, New Jersey, New York, and Texas will use convention centers and fairgrounds, Axios has reported.
In Florida, Palm Beach County Health Director Alina Alonso, MD, told county commissioners on Jan. 12 that there isn’t enough vaccine to meet demand, WPTV reported. “We need to realize that there’s a shortage of vaccine. So it’s not the plan, it’s not our ability to do it. It’s simply supply and demand at this point,” Dr. Alonso said, according to the TV station report.
A version of this article first appeared on Medscape.com.
Federal health officials are urging states to vaccinate all Americans over age 65 and those aged 16-64 who have a documented underlying health condition that makes them more vulnerable to COVID-19.
U.S. Department of Health and Human Services (HHS) Secretary Alex Azar and Centers for Disease Control and Prevention Director Robert Redfield, MD, made the recommendation in a briefing with reporters on Jan. 12, saying that the current vaccine supply was sufficient to meet demand for the next phase of immunization as recommended by the CDC’s Advisory Committee on Immunization Practices.
“We are ready for a transition that we outlined last September in the playbook we sent to states,” Mr. Azar said. Both he and U.S. Army General Gustave F. Perna, chief operations officer for Operation Warp Speed, said that confidence in the distribution system had led to the decision to urge wider access.
The federal government will also increase the number of sites eligible to receive vaccine – including some 13,000 federally qualified community health centers – and will not keep doses in reserve as insurance against issues that might prevent people from receiving a second dose on a timely basis.
“We don’t need to hold back reserve doses,” Mr. Azar said, noting that if there were any “glitches in production” the federal government would move to fulfill obligations for second doses first and delay initial doses.
Azar: Use it or lose it
In a move that is sure to generate pushback, Mr. Azar said that states that don’t quickly administer vaccines will receive fewer doses in the future. That policy will not go into effect until later in February, which leaves open the possibility that it could be reversed by the incoming Biden administration.
“We have too much vaccine sitting in freezers at hospitals with hospitals not using it,” said Mr. Azar, who also blamed the slow administration process on a reporting lag and states being what he called “overly prescriptive” in who has been eligible to receive a shot.
“I would rather have people working to get appointments to get vaccinated than having vaccine going to waste sitting in freezers,” he told reporters.
Mr. Azar had already been pushing for broader vaccination, telling states to do so in an Operation Warp Speed briefing on Jan. 6. At that briefing, he also said that the federal government would be stepping up vaccination through an “early launch” of a federal partnership with 19 pharmacy chains, which will let states allocate vaccines directly to some 40,000 pharmacy sites.
Gen. Perna said during the Jan. 12 briefing that the aim is to further expand that to some 70,000 locations total.
The CDC reported that as of Jan. 11 some 25.4 million doses have been distributed, with 8.9 million administered. An additional 4.2 million doses were distributed to long-term care facilities, and 937,000 residents and staff have received a dose.
“Pace of administration”
Alaska, Connecticut, North Dakota, South Dakota, the District of Columbia, West Virginia, and the Northern Mariana Islands have administered the most vaccines per capita, according to the CDC. But even these locations have immunized only 4%-5% of their populations, the New York Times reports. At the bottom: Alabama, Arizona, Arkansas, Georgia, Mississippi, and South Carolina.
The federal government can encourage but not require states to move on to new phases of vaccination.
“States ultimately determine how they will proceed with vaccination,” said Marcus Plescia, MD, MPH, chief medical officer for the Association of State and Territorial Health Officials. “Most will be cautious about assuring there are doses for those needing a second dose,” he said in an interview.
Dr. Plescia said that ensuring a second dose is available is especially important for health care workers “who need to be confident that they are protected and not inadvertently transmitting the disease themselves.”
He added that “once we reach a steady state of supply and administration, the rate-limiting factor will be supply of vaccine.”
That supply could now be threatened if states don’t comply with a just-announced federal action that will change how doses are allocated.
Beginning in late February, vaccine allocations to states will be based on “the pace of administration reported by states,” and the size of the 65-and-older population, said Mr. Azar, who has previously criticized New York Governor Andrew Cuomo for fining hospitals that didn’t use up vaccine supply within a week.
“This new system gives states a strong incentive to ensure that all vaccinations are being promptly reported, which they currently are not,” he said.
Currently, allocations are based on a state’s or territory’s population.
Prepandemic, states were required to report vaccinations within 30 days. Since COVID-19 vaccines became available, the CDC has required reporting of shots within 72 hours.
Dr. Redfield said the requirement has caused some difficulty, and that the CDC is investigating why some states have reported using only 15% of doses while others have used 80%.
States have been scrambling to ramp up vaccinations.
Just ahead of the federal briefing, Gov. Cuomo tweeted that New York would be opening up vaccinations to anyone older than 65.
The Associated Press is reporting that some states have started mass vaccination sites.
Arizona has begun operating a 24/7 appointment-only vaccination program at State Farm Stadium outside of Phoenix, with the aim of immunizing 6,000 people each day, according to local radio station KJZZ.
California and Florida have also taken steps to use stadiums, while Michigan, New Jersey, New York, and Texas will use convention centers and fairgrounds, Axios has reported.
In Florida, Palm Beach County Health Director Alina Alonso, MD, told county commissioners on Jan. 12 that there isn’t enough vaccine to meet demand, WPTV reported. “We need to realize that there’s a shortage of vaccine. So it’s not the plan, it’s not our ability to do it. It’s simply supply and demand at this point,” Dr. Alonso said, according to the TV station report.
A version of this article first appeared on Medscape.com.
Averting COVID hospitalizations with monoclonal antibodies
The United States has allocated more than 641,000 monoclonal antibody treatments for outpatients to ease pressure on strained hospitals, but officials from Operation Warp Speed report that more than half of that reserve sits unused as clinicians grapple with best practices.
There are space and personnel limitations in hospitals right now, Janet Woodcock, MD, therapeutics lead on Operation Warp Speed, acknowledges in an interview with this news organization. “Special areas and procedures must be set up.” And the operation is in the process of broadening availability beyond hospitals, she points out.
But for frontline clinicians, questions about treatment efficacy and the logistics of administering intravenous drugs to infectious outpatients loom large.
More than 50 monoclonal antibody products that target SARS-CoV-2 are now in development. The U.S. Food and Drug Administration has already issued Emergency Use Authorization (EUA) for two such drugs on the basis of phase 2 trial data – bamlanivimab, made by Eli Lilly, and a cocktail of casirivimab plus imdevimab, made by Regeneron – and another two-antibody cocktail from AstraZeneca, AZD7442, has started phase 3 clinical trials. The Regeneron combination was used to treat President Donald Trump when he contracted COVID-19 in October.
Monoclonal antibody drugs are based on the natural antibodies that the body uses to fight infections. They work by binding to a specific target and then blocking its action or flagging it for destruction by other parts of the immune system. Both bamlanivimab and the casirivimab plus imdevimab combination target the spike protein of the virus and stop it from attaching to and entering human cells.
Targeting the spike protein out of the hospital
The antibody drugs covered by EUAs do not cure COVID-19, but they have been shown to reduce hospitalizations and visits to the emergency department for patients at high risk for disease progression. They are approved to treat patients older than 12 years with mild to moderate COVID-19 who are at high risk of progressing to severe disease or hospitalization. They are not authorized for use in patients who have been hospitalized or who are on ventilators. The hope is that antibody drugs will reduce the number of severe cases of COVID-19 and ease pressure on overstretched hospitals.
Most COVID-19 patients are outpatients, so we need something to keep them from getting worse.
This is important because it targets the greatest need in COVID-19 therapeutics, says Rajesh Gandhi, MD, an infectious disease physician at Harvard Medical School in Boston, who is a member of two panels evaluating COVID-19 treatments: one for the Infectious Disease Society of America and the other for the National Institutes of Health. “Up to now, most of the focus has been on hospitalized patients,” he says, but “most COVID-19 patients are outpatients, so we need something to keep them from getting worse.”
Both panels have said that, despite the EUAs, more evidence is needed to be sure of the efficacy of the drugs and to determine which patients will benefit the most from them.
These aren’t the mature data from drug development that guideline groups are accustomed to working with, Dr. Woodcock points out. “But this is an emergency and the data taken as a whole are pretty convincing,” she says. “As I look at the totality of the evidence, monoclonal antibodies will have a big effect in keeping people out of the hospital and helping them recover faster.”
High-risk patients are eligible for treatment, especially those older than 65 years and those with comorbidities who are younger. Access to the drugs is increasing for clinicians who are able to infuse safely or work with a site that will.
In the Boston area, several hospitals, including Massachusetts General where Dr. Gandhi works, have set up infusion centers where newly diagnosed patients can get the antibody treatment if their doctor thinks it will benefit them. And Coram, a provider of at-home infusion therapy owned by the CVS pharmacy chain, is running a pilot program offering the Eli Lilly drug to people in seven cities – including Boston, Chicago, Los Angeles, and Tampa – and their surrounding communities with a physician referral.
Getting that referral could be tricky, however, for patients without a primary care physician or for those whose doctor isn’t already connected to one of the institutions providing the infusions. The hospitals are sending out communications on how patients and physicians can get the therapy, but Dr. Gandhi says that making information about access available should be a priority. The window for the effective treatment is small – the drugs appear to work best before patients begin to make their own antibodies, says Dr. Gandhi – so it’s vital that doctors act quickly if they have a patient who is eligible.
And rolling out the new therapies to patients around the world will be a major logistical undertaking.
The first hurdle will be making enough of them to go around. Case numbers are skyrocketing around the globe, and producing the drugs is a complex time- and labor-intensive process that requires specialized facilities. Antibodies are produced by cell lines in bioreactors, so a plant that churns out generic aspirin tablets can’t simply be converted into an antibody factory.
“These types of drugs are manufactured in a sterile injectables plant, which is different from a plant where oral solids are made,” says Kim Crabtree, senior director of pharma portfolio management for Henry Schein Medical, a medical supplies distributor. “Those are not as plentiful as a standard pill factory.”
The doses required are also relatively high – 1.2 g of each antibody in Regeneron’s cocktail – which will further strain production capacity. Leah Lipsich, PhD, vice president of strategic program direction at Regeneron, says the company is prepared for high demand and has been able to respond, thanks to its rapid development and manufacturing technology, known as VelociSuite, which allows it to rapidly scale-up from discovery to productions in weeks instead of months.
“We knew supply would be a huge problem for COVID-19, but because we had such confidence in our technology, we went immediately from research-scale to our largest-scale manufacturing,” she says. “We’ve been manufacturing our cocktail for months now.”
The company has also partnered with Roche, the biggest manufacturer and vendor of monoclonal antibodies in the world, to manufacture and supply the drugs. Once full manufacturing capacity is reached in 2021, the companies expect to produce at least 2 million doses a year.
Then there is the issue of getting the drugs from the factories to the places they will be used.
Antibodies are temperature sensitive and need to be refrigerated during transport and storage, so a cold-chain-compliant supply chain is required. Fortunately, they can be kept at standard refrigerator temperatures, ranging from 2° C to 8° C, rather than the ultra-low temperatures required by some COVID-19 vaccines.
Two million doses a year
Medical logistics companies have a lot of experience dealing with products like these and are well prepared to handle the new antibody drugs. “There are quite a few products like these on the market, and the supply chain is used to shipping them,” Ms. Crabtree says.
They will be shipped to distribution centers in refrigerated trucks, repacked into smaller lots that can sustain the correct temperature for 24 hours, and then sent to their final destination, often in something as simple as a Styrofoam cooler filled with dry ice.
The expected rise in demand shouldn’t be too much of an issue for distributors either, says Ms. Crabtree; they have built systems that can deal with short-term surges in volume. The annual flu vaccine, for example, involves shipping a lot of product in a very short time, usually from August to November. “The distribution system is used to seasonal variations and peaks in demand,” she says.
The next question is how the treatments will be administered. Although most patients who will receive monoclonal antibodies will be ambulatory and not hospitalized, the administration requires intravenous infusion. Hospitals, of course, have a lot of experience with intravenous drugs, but typically give them only to inpatients. Most other monoclonal antibody drugs – such as those for cancer and autoimmune disorders – are given in specialized suites in doctor’s offices or in stand-alone infusion clinics.
That means that the places best suited to treat COVID-19 patients with antibodies are those that regularly deal with people who are immunocompromised, and such patients should not be interacting with people who have an infectious disease. “How do we protect the staff and other patients?” Dr. Gandhi asks.
Protecting staff and other patients
This is not an insurmountable obstacle, he points out, but it is one that requires careful thought and planning to accommodate COVID-19 patients without unduly disrupting life-saving treatments for other patients. It might involve, for example, treating COVID-19 patients in sequestered parts of the clinic or at different times of day, with even greater attention paid to cleaning, he explains. “We now have many months of experience with infection control, so we know how to do this; it’s just a question of logistics.”
But even once all the details around manufacturing, transporting, and administering the drugs are sorted out, there is still the issue of how they will be distributed fairly and equitably.
Despite multiple companies working to produce an array of different antibody drugs, demand is still expected to exceed supply for many months. “With more than 200,000 new cases a day in the United States, there won’t be enough antibodies to treat all of the high-risk patients,” says Dr. Gandhi. “Most of us are worried that demand will far outstrip supply. People are talking about lotteries to determine who gets them.”
The Department of Health and Human Services will continue to distribute the drugs to states on the basis of their COVID-19 burdens, and the states will then decide how much to provide to each health care facility.
Although the HHS goal is to ensure that the drugs reach as many patients as possible, no matter where they live and regardless of their income, there are still concerns that larger facilities serving more affluent areas will end up being favored, if only because they are the ones best equipped to deal with the drugs right now.
“We are all aware that this has affected certain communities more, so we need to make sure that the drugs are used equitably and made available to the communities that were hardest hit,” says Dr. Gandhi. The ability to monitor drug distribution should be built into the rollout, so that institutions and governments will have some sense of whether they are being doled out evenly, he adds.
Equity in distribution will be an issue for the rest of the world as well. Currently, 80% of monoclonal antibodies are sold in Canada, Europe, and the United States; few, if any, are available in low- and middle-income countries. The treatments are expensive: the cost of producing one g of marketed monoclonal antibodies is between $95 and $200, which does not include the cost of R&D, packaging, shipping, or administration. The median price for antibody treatment not related to COVID-19 runs from $15,000 to $200,000 per year in the United States.
Regeneron’s Dr. Lipsich says that the company has not yet set a price for its antibody cocktail. The government paid $450 million for its 300,000 doses, but that price includes the costs of research, manufacturing, and distribution, so is not a useful indicator of the eventual per-dose price. “We’re not in a position to talk about how it will be priced yet, but we will do our best to make it affordable and accessible to all,” she says.
There are some projects underway to ensure that the drugs are made available in poorer countries. In April, the COVID-19 Therapeutics Accelerator – an initiative launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard to speed-up the response to the global pandemic – reserved manufacturing capacity with Fujifilm Diosynth Biotechnologies in Denmark for future monoclonal antibody therapies that will supply low- and middle-income countries. In October, the initiative announced that Eli Lilly would use that reserved capacity to produce its antibody drug starting in April 2021.
In the meantime, Lilly will make some of its product manufactured in other facilities available to lower-income countries. To help keep costs down, the company’s collaborators have agreed to waive their royalties on antibodies distributed in low- and middle-income countries.
“Everyone is looking carefully at how the drugs are distributed to ensure all will get access,” said Dr. Lipsich.
A version of this article first appeared on Medscape.com.
The United States has allocated more than 641,000 monoclonal antibody treatments for outpatients to ease pressure on strained hospitals, but officials from Operation Warp Speed report that more than half of that reserve sits unused as clinicians grapple with best practices.
There are space and personnel limitations in hospitals right now, Janet Woodcock, MD, therapeutics lead on Operation Warp Speed, acknowledges in an interview with this news organization. “Special areas and procedures must be set up.” And the operation is in the process of broadening availability beyond hospitals, she points out.
But for frontline clinicians, questions about treatment efficacy and the logistics of administering intravenous drugs to infectious outpatients loom large.
More than 50 monoclonal antibody products that target SARS-CoV-2 are now in development. The U.S. Food and Drug Administration has already issued Emergency Use Authorization (EUA) for two such drugs on the basis of phase 2 trial data – bamlanivimab, made by Eli Lilly, and a cocktail of casirivimab plus imdevimab, made by Regeneron – and another two-antibody cocktail from AstraZeneca, AZD7442, has started phase 3 clinical trials. The Regeneron combination was used to treat President Donald Trump when he contracted COVID-19 in October.
Monoclonal antibody drugs are based on the natural antibodies that the body uses to fight infections. They work by binding to a specific target and then blocking its action or flagging it for destruction by other parts of the immune system. Both bamlanivimab and the casirivimab plus imdevimab combination target the spike protein of the virus and stop it from attaching to and entering human cells.
Targeting the spike protein out of the hospital
The antibody drugs covered by EUAs do not cure COVID-19, but they have been shown to reduce hospitalizations and visits to the emergency department for patients at high risk for disease progression. They are approved to treat patients older than 12 years with mild to moderate COVID-19 who are at high risk of progressing to severe disease or hospitalization. They are not authorized for use in patients who have been hospitalized or who are on ventilators. The hope is that antibody drugs will reduce the number of severe cases of COVID-19 and ease pressure on overstretched hospitals.
Most COVID-19 patients are outpatients, so we need something to keep them from getting worse.
This is important because it targets the greatest need in COVID-19 therapeutics, says Rajesh Gandhi, MD, an infectious disease physician at Harvard Medical School in Boston, who is a member of two panels evaluating COVID-19 treatments: one for the Infectious Disease Society of America and the other for the National Institutes of Health. “Up to now, most of the focus has been on hospitalized patients,” he says, but “most COVID-19 patients are outpatients, so we need something to keep them from getting worse.”
Both panels have said that, despite the EUAs, more evidence is needed to be sure of the efficacy of the drugs and to determine which patients will benefit the most from them.
These aren’t the mature data from drug development that guideline groups are accustomed to working with, Dr. Woodcock points out. “But this is an emergency and the data taken as a whole are pretty convincing,” she says. “As I look at the totality of the evidence, monoclonal antibodies will have a big effect in keeping people out of the hospital and helping them recover faster.”
High-risk patients are eligible for treatment, especially those older than 65 years and those with comorbidities who are younger. Access to the drugs is increasing for clinicians who are able to infuse safely or work with a site that will.
In the Boston area, several hospitals, including Massachusetts General where Dr. Gandhi works, have set up infusion centers where newly diagnosed patients can get the antibody treatment if their doctor thinks it will benefit them. And Coram, a provider of at-home infusion therapy owned by the CVS pharmacy chain, is running a pilot program offering the Eli Lilly drug to people in seven cities – including Boston, Chicago, Los Angeles, and Tampa – and their surrounding communities with a physician referral.
Getting that referral could be tricky, however, for patients without a primary care physician or for those whose doctor isn’t already connected to one of the institutions providing the infusions. The hospitals are sending out communications on how patients and physicians can get the therapy, but Dr. Gandhi says that making information about access available should be a priority. The window for the effective treatment is small – the drugs appear to work best before patients begin to make their own antibodies, says Dr. Gandhi – so it’s vital that doctors act quickly if they have a patient who is eligible.
And rolling out the new therapies to patients around the world will be a major logistical undertaking.
The first hurdle will be making enough of them to go around. Case numbers are skyrocketing around the globe, and producing the drugs is a complex time- and labor-intensive process that requires specialized facilities. Antibodies are produced by cell lines in bioreactors, so a plant that churns out generic aspirin tablets can’t simply be converted into an antibody factory.
“These types of drugs are manufactured in a sterile injectables plant, which is different from a plant where oral solids are made,” says Kim Crabtree, senior director of pharma portfolio management for Henry Schein Medical, a medical supplies distributor. “Those are not as plentiful as a standard pill factory.”
The doses required are also relatively high – 1.2 g of each antibody in Regeneron’s cocktail – which will further strain production capacity. Leah Lipsich, PhD, vice president of strategic program direction at Regeneron, says the company is prepared for high demand and has been able to respond, thanks to its rapid development and manufacturing technology, known as VelociSuite, which allows it to rapidly scale-up from discovery to productions in weeks instead of months.
“We knew supply would be a huge problem for COVID-19, but because we had such confidence in our technology, we went immediately from research-scale to our largest-scale manufacturing,” she says. “We’ve been manufacturing our cocktail for months now.”
The company has also partnered with Roche, the biggest manufacturer and vendor of monoclonal antibodies in the world, to manufacture and supply the drugs. Once full manufacturing capacity is reached in 2021, the companies expect to produce at least 2 million doses a year.
Then there is the issue of getting the drugs from the factories to the places they will be used.
Antibodies are temperature sensitive and need to be refrigerated during transport and storage, so a cold-chain-compliant supply chain is required. Fortunately, they can be kept at standard refrigerator temperatures, ranging from 2° C to 8° C, rather than the ultra-low temperatures required by some COVID-19 vaccines.
Two million doses a year
Medical logistics companies have a lot of experience dealing with products like these and are well prepared to handle the new antibody drugs. “There are quite a few products like these on the market, and the supply chain is used to shipping them,” Ms. Crabtree says.
They will be shipped to distribution centers in refrigerated trucks, repacked into smaller lots that can sustain the correct temperature for 24 hours, and then sent to their final destination, often in something as simple as a Styrofoam cooler filled with dry ice.
The expected rise in demand shouldn’t be too much of an issue for distributors either, says Ms. Crabtree; they have built systems that can deal with short-term surges in volume. The annual flu vaccine, for example, involves shipping a lot of product in a very short time, usually from August to November. “The distribution system is used to seasonal variations and peaks in demand,” she says.
The next question is how the treatments will be administered. Although most patients who will receive monoclonal antibodies will be ambulatory and not hospitalized, the administration requires intravenous infusion. Hospitals, of course, have a lot of experience with intravenous drugs, but typically give them only to inpatients. Most other monoclonal antibody drugs – such as those for cancer and autoimmune disorders – are given in specialized suites in doctor’s offices or in stand-alone infusion clinics.
That means that the places best suited to treat COVID-19 patients with antibodies are those that regularly deal with people who are immunocompromised, and such patients should not be interacting with people who have an infectious disease. “How do we protect the staff and other patients?” Dr. Gandhi asks.
Protecting staff and other patients
This is not an insurmountable obstacle, he points out, but it is one that requires careful thought and planning to accommodate COVID-19 patients without unduly disrupting life-saving treatments for other patients. It might involve, for example, treating COVID-19 patients in sequestered parts of the clinic or at different times of day, with even greater attention paid to cleaning, he explains. “We now have many months of experience with infection control, so we know how to do this; it’s just a question of logistics.”
But even once all the details around manufacturing, transporting, and administering the drugs are sorted out, there is still the issue of how they will be distributed fairly and equitably.
Despite multiple companies working to produce an array of different antibody drugs, demand is still expected to exceed supply for many months. “With more than 200,000 new cases a day in the United States, there won’t be enough antibodies to treat all of the high-risk patients,” says Dr. Gandhi. “Most of us are worried that demand will far outstrip supply. People are talking about lotteries to determine who gets them.”
The Department of Health and Human Services will continue to distribute the drugs to states on the basis of their COVID-19 burdens, and the states will then decide how much to provide to each health care facility.
Although the HHS goal is to ensure that the drugs reach as many patients as possible, no matter where they live and regardless of their income, there are still concerns that larger facilities serving more affluent areas will end up being favored, if only because they are the ones best equipped to deal with the drugs right now.
“We are all aware that this has affected certain communities more, so we need to make sure that the drugs are used equitably and made available to the communities that were hardest hit,” says Dr. Gandhi. The ability to monitor drug distribution should be built into the rollout, so that institutions and governments will have some sense of whether they are being doled out evenly, he adds.
Equity in distribution will be an issue for the rest of the world as well. Currently, 80% of monoclonal antibodies are sold in Canada, Europe, and the United States; few, if any, are available in low- and middle-income countries. The treatments are expensive: the cost of producing one g of marketed monoclonal antibodies is between $95 and $200, which does not include the cost of R&D, packaging, shipping, or administration. The median price for antibody treatment not related to COVID-19 runs from $15,000 to $200,000 per year in the United States.
Regeneron’s Dr. Lipsich says that the company has not yet set a price for its antibody cocktail. The government paid $450 million for its 300,000 doses, but that price includes the costs of research, manufacturing, and distribution, so is not a useful indicator of the eventual per-dose price. “We’re not in a position to talk about how it will be priced yet, but we will do our best to make it affordable and accessible to all,” she says.
There are some projects underway to ensure that the drugs are made available in poorer countries. In April, the COVID-19 Therapeutics Accelerator – an initiative launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard to speed-up the response to the global pandemic – reserved manufacturing capacity with Fujifilm Diosynth Biotechnologies in Denmark for future monoclonal antibody therapies that will supply low- and middle-income countries. In October, the initiative announced that Eli Lilly would use that reserved capacity to produce its antibody drug starting in April 2021.
In the meantime, Lilly will make some of its product manufactured in other facilities available to lower-income countries. To help keep costs down, the company’s collaborators have agreed to waive their royalties on antibodies distributed in low- and middle-income countries.
“Everyone is looking carefully at how the drugs are distributed to ensure all will get access,” said Dr. Lipsich.
A version of this article first appeared on Medscape.com.
The United States has allocated more than 641,000 monoclonal antibody treatments for outpatients to ease pressure on strained hospitals, but officials from Operation Warp Speed report that more than half of that reserve sits unused as clinicians grapple with best practices.
There are space and personnel limitations in hospitals right now, Janet Woodcock, MD, therapeutics lead on Operation Warp Speed, acknowledges in an interview with this news organization. “Special areas and procedures must be set up.” And the operation is in the process of broadening availability beyond hospitals, she points out.
But for frontline clinicians, questions about treatment efficacy and the logistics of administering intravenous drugs to infectious outpatients loom large.
More than 50 monoclonal antibody products that target SARS-CoV-2 are now in development. The U.S. Food and Drug Administration has already issued Emergency Use Authorization (EUA) for two such drugs on the basis of phase 2 trial data – bamlanivimab, made by Eli Lilly, and a cocktail of casirivimab plus imdevimab, made by Regeneron – and another two-antibody cocktail from AstraZeneca, AZD7442, has started phase 3 clinical trials. The Regeneron combination was used to treat President Donald Trump when he contracted COVID-19 in October.
Monoclonal antibody drugs are based on the natural antibodies that the body uses to fight infections. They work by binding to a specific target and then blocking its action or flagging it for destruction by other parts of the immune system. Both bamlanivimab and the casirivimab plus imdevimab combination target the spike protein of the virus and stop it from attaching to and entering human cells.
Targeting the spike protein out of the hospital
The antibody drugs covered by EUAs do not cure COVID-19, but they have been shown to reduce hospitalizations and visits to the emergency department for patients at high risk for disease progression. They are approved to treat patients older than 12 years with mild to moderate COVID-19 who are at high risk of progressing to severe disease or hospitalization. They are not authorized for use in patients who have been hospitalized or who are on ventilators. The hope is that antibody drugs will reduce the number of severe cases of COVID-19 and ease pressure on overstretched hospitals.
Most COVID-19 patients are outpatients, so we need something to keep them from getting worse.
This is important because it targets the greatest need in COVID-19 therapeutics, says Rajesh Gandhi, MD, an infectious disease physician at Harvard Medical School in Boston, who is a member of two panels evaluating COVID-19 treatments: one for the Infectious Disease Society of America and the other for the National Institutes of Health. “Up to now, most of the focus has been on hospitalized patients,” he says, but “most COVID-19 patients are outpatients, so we need something to keep them from getting worse.”
Both panels have said that, despite the EUAs, more evidence is needed to be sure of the efficacy of the drugs and to determine which patients will benefit the most from them.
These aren’t the mature data from drug development that guideline groups are accustomed to working with, Dr. Woodcock points out. “But this is an emergency and the data taken as a whole are pretty convincing,” she says. “As I look at the totality of the evidence, monoclonal antibodies will have a big effect in keeping people out of the hospital and helping them recover faster.”
High-risk patients are eligible for treatment, especially those older than 65 years and those with comorbidities who are younger. Access to the drugs is increasing for clinicians who are able to infuse safely or work with a site that will.
In the Boston area, several hospitals, including Massachusetts General where Dr. Gandhi works, have set up infusion centers where newly diagnosed patients can get the antibody treatment if their doctor thinks it will benefit them. And Coram, a provider of at-home infusion therapy owned by the CVS pharmacy chain, is running a pilot program offering the Eli Lilly drug to people in seven cities – including Boston, Chicago, Los Angeles, and Tampa – and their surrounding communities with a physician referral.
Getting that referral could be tricky, however, for patients without a primary care physician or for those whose doctor isn’t already connected to one of the institutions providing the infusions. The hospitals are sending out communications on how patients and physicians can get the therapy, but Dr. Gandhi says that making information about access available should be a priority. The window for the effective treatment is small – the drugs appear to work best before patients begin to make their own antibodies, says Dr. Gandhi – so it’s vital that doctors act quickly if they have a patient who is eligible.
And rolling out the new therapies to patients around the world will be a major logistical undertaking.
The first hurdle will be making enough of them to go around. Case numbers are skyrocketing around the globe, and producing the drugs is a complex time- and labor-intensive process that requires specialized facilities. Antibodies are produced by cell lines in bioreactors, so a plant that churns out generic aspirin tablets can’t simply be converted into an antibody factory.
“These types of drugs are manufactured in a sterile injectables plant, which is different from a plant where oral solids are made,” says Kim Crabtree, senior director of pharma portfolio management for Henry Schein Medical, a medical supplies distributor. “Those are not as plentiful as a standard pill factory.”
The doses required are also relatively high – 1.2 g of each antibody in Regeneron’s cocktail – which will further strain production capacity. Leah Lipsich, PhD, vice president of strategic program direction at Regeneron, says the company is prepared for high demand and has been able to respond, thanks to its rapid development and manufacturing technology, known as VelociSuite, which allows it to rapidly scale-up from discovery to productions in weeks instead of months.
“We knew supply would be a huge problem for COVID-19, but because we had such confidence in our technology, we went immediately from research-scale to our largest-scale manufacturing,” she says. “We’ve been manufacturing our cocktail for months now.”
The company has also partnered with Roche, the biggest manufacturer and vendor of monoclonal antibodies in the world, to manufacture and supply the drugs. Once full manufacturing capacity is reached in 2021, the companies expect to produce at least 2 million doses a year.
Then there is the issue of getting the drugs from the factories to the places they will be used.
Antibodies are temperature sensitive and need to be refrigerated during transport and storage, so a cold-chain-compliant supply chain is required. Fortunately, they can be kept at standard refrigerator temperatures, ranging from 2° C to 8° C, rather than the ultra-low temperatures required by some COVID-19 vaccines.
Two million doses a year
Medical logistics companies have a lot of experience dealing with products like these and are well prepared to handle the new antibody drugs. “There are quite a few products like these on the market, and the supply chain is used to shipping them,” Ms. Crabtree says.
They will be shipped to distribution centers in refrigerated trucks, repacked into smaller lots that can sustain the correct temperature for 24 hours, and then sent to their final destination, often in something as simple as a Styrofoam cooler filled with dry ice.
The expected rise in demand shouldn’t be too much of an issue for distributors either, says Ms. Crabtree; they have built systems that can deal with short-term surges in volume. The annual flu vaccine, for example, involves shipping a lot of product in a very short time, usually from August to November. “The distribution system is used to seasonal variations and peaks in demand,” she says.
The next question is how the treatments will be administered. Although most patients who will receive monoclonal antibodies will be ambulatory and not hospitalized, the administration requires intravenous infusion. Hospitals, of course, have a lot of experience with intravenous drugs, but typically give them only to inpatients. Most other monoclonal antibody drugs – such as those for cancer and autoimmune disorders – are given in specialized suites in doctor’s offices or in stand-alone infusion clinics.
That means that the places best suited to treat COVID-19 patients with antibodies are those that regularly deal with people who are immunocompromised, and such patients should not be interacting with people who have an infectious disease. “How do we protect the staff and other patients?” Dr. Gandhi asks.
Protecting staff and other patients
This is not an insurmountable obstacle, he points out, but it is one that requires careful thought and planning to accommodate COVID-19 patients without unduly disrupting life-saving treatments for other patients. It might involve, for example, treating COVID-19 patients in sequestered parts of the clinic or at different times of day, with even greater attention paid to cleaning, he explains. “We now have many months of experience with infection control, so we know how to do this; it’s just a question of logistics.”
But even once all the details around manufacturing, transporting, and administering the drugs are sorted out, there is still the issue of how they will be distributed fairly and equitably.
Despite multiple companies working to produce an array of different antibody drugs, demand is still expected to exceed supply for many months. “With more than 200,000 new cases a day in the United States, there won’t be enough antibodies to treat all of the high-risk patients,” says Dr. Gandhi. “Most of us are worried that demand will far outstrip supply. People are talking about lotteries to determine who gets them.”
The Department of Health and Human Services will continue to distribute the drugs to states on the basis of their COVID-19 burdens, and the states will then decide how much to provide to each health care facility.
Although the HHS goal is to ensure that the drugs reach as many patients as possible, no matter where they live and regardless of their income, there are still concerns that larger facilities serving more affluent areas will end up being favored, if only because they are the ones best equipped to deal with the drugs right now.
“We are all aware that this has affected certain communities more, so we need to make sure that the drugs are used equitably and made available to the communities that were hardest hit,” says Dr. Gandhi. The ability to monitor drug distribution should be built into the rollout, so that institutions and governments will have some sense of whether they are being doled out evenly, he adds.
Equity in distribution will be an issue for the rest of the world as well. Currently, 80% of monoclonal antibodies are sold in Canada, Europe, and the United States; few, if any, are available in low- and middle-income countries. The treatments are expensive: the cost of producing one g of marketed monoclonal antibodies is between $95 and $200, which does not include the cost of R&D, packaging, shipping, or administration. The median price for antibody treatment not related to COVID-19 runs from $15,000 to $200,000 per year in the United States.
Regeneron’s Dr. Lipsich says that the company has not yet set a price for its antibody cocktail. The government paid $450 million for its 300,000 doses, but that price includes the costs of research, manufacturing, and distribution, so is not a useful indicator of the eventual per-dose price. “We’re not in a position to talk about how it will be priced yet, but we will do our best to make it affordable and accessible to all,” she says.
There are some projects underway to ensure that the drugs are made available in poorer countries. In April, the COVID-19 Therapeutics Accelerator – an initiative launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard to speed-up the response to the global pandemic – reserved manufacturing capacity with Fujifilm Diosynth Biotechnologies in Denmark for future monoclonal antibody therapies that will supply low- and middle-income countries. In October, the initiative announced that Eli Lilly would use that reserved capacity to produce its antibody drug starting in April 2021.
In the meantime, Lilly will make some of its product manufactured in other facilities available to lower-income countries. To help keep costs down, the company’s collaborators have agreed to waive their royalties on antibodies distributed in low- and middle-income countries.
“Everyone is looking carefully at how the drugs are distributed to ensure all will get access,” said Dr. Lipsich.
A version of this article first appeared on Medscape.com.
COVID-19 in children: Weekly cases trending downward
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
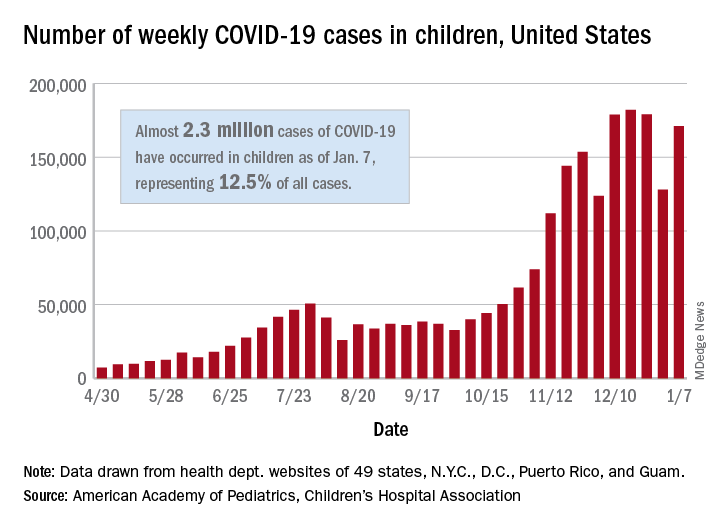
Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.
Concern over response to COVID-19 in patients with blood cancers
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with cancer, particularly those with solid tumors, mounted an immune response to COVID-19 similar to that seen in people without cancer, but among patients with hematologic cancers, immune responses were less pronounced and were highly variable, typically taking longer to clear the virus.
The findings come from a small U.K. study published online Jan. 4 in Cancer Cell as a fast-track preprint article.
The findings may have implications for vaccinating against COVID-19, said the researchers, led by Sheeba Irshad, MD, PhD, a Cancer Research UK clinician scientist based at King’s College London.
“Our study provides some confidence and reassurance to care providers that many of our patients with solid cancers will mount a good immune response against the virus, develop antibodies that last, and hopefully resume their cancer treatment as soon as possible,” Dr. Irshad said in a statement.
“These conclusions imply that many patients, despite being on immunosuppressive therapies, will respond satisfactorily to COVID-19 vaccines,” she added.
Although “the data would suggest that solid cancer patients are likely to mount an efficient immune response to the vaccine ... the same cannot be said for hematological cancers, especially those with B-cell malignancies,” Dr. Irshad said in an interview.
“They may be susceptible to persistent infection despite developing antibodies, so the next stage of our study will focus on monitoring their response to the vaccines.
“At present, the best way to protect them alongside vaccinating them may be to vaccinate all their health care providers and carers to achieve herd immunity and continue to respect the public health measures put in place,” such as wearing a mask, practicing social distancing, and testing asymptomatic persons, she commented.
Study details
This study, known as the SARS-CoV-2 for Cancer Patients study, involved 76 patients with cancer; 41 of these patients had COVID-19, and 35 served as non-COVID cancer control patients.
Peripheral blood was collected from all patients; multiple samples were taken every 2-4 days where possible.
The COVID-19 and control groups were matched for age, body mass index, and tumor type, and both groups included patients with solid and hematologic cancers.
The groups were also comparable in terms of the proportion of patients with stage IV disease, those who received palliative as opposed to radical treatment, and patients who were treated within 4 weeks of recruitment to the study.
The results showed that 24.4% of cancer patients who were exposed to COVID-19 remained asymptomatic, 21.9% had mild disease, 31.7% had moderate disease, and 21.9% had severe disease.
Patients with hematologic cancers were more likely to experience dyspnea than those with solid tumors, and 39% received corticosteroid/antiviral therapies that specifically targeted COVID-19 infection.
The median duration of virus shedding was 39 days across the whole cohort. It was notably longer among patients with hematologic cancers, at a median of 55 days versus 29 days for patients with solid tumors.
Of 46 patients who survived beyond 30 days and for whom complete data were available, the team found that those with moderate or severe COVID-19 were more likely to be diagnosed with progressive cancer at their next assessment in comparison with those who were asymptomatic with COVID-19 or with control patients.
Solid-cancer patients with moderate to severe COVID-19 had sustained lymphopenia and increased neutrophil-to-lymphocyte ratios up to days 40-49 of the infection, whereas among those with mild infection, clinical blood parameters were typically in the normal range.
Although overall blood profiles of patients with hematologic cancers were similar to those of patients with solid cancers, the trajectories between mild and moderate/severe COVID-19 overlapped, and there was a large degree of heterogeneity between patients.
The team also reports that among patients with solid tumors, all parameters returned to values that were close to baseline 4-6 weeks after the patients tested negative for COVID-19 on nasopharyngeal swabbing; by contrast, many of the patients with hematologic cancers experienced ongoing immune dysregulation.
Further analysis revealed differences in immune signatures between patients with solid cancers who had active SARS-CoV-2 infection and noninfected control patients. The former showed, for example, interleukin-8, IL-6, and IL-10, IP-10 enrichment.
In contrast, there were few differences between infected and noninfected hematologic cancer patients.
Across both cohorts, approximately 75% of patients had detectable antibodies against COVID-19. Antibodies were sustained for up to 78 days after exposure to the virus.
However, patients with solid tumors showed earlier seroconversion than those with hematologic cancers. The latter had more varied responses to infection, displaying three distinct phenotypes: failure to mount an antibody response, with prolonged viral shedding, even beyond day 50 after the first positive swab; an antibody response but failure to clear the virus; and an antibody response and successful clearing of the virus.
The team noted that overall patients with hematologic cancers showed a mild response to COVID-19 in the active/early phases of the disease and that the response grew stronger over time, similar to the immune changes typically seen with chronic infections.
This was particularly the case for patients with cancers that affect B cells.
The team acknowledged that there are several limitations to the study, including its small sample size and lack of statistical power to detect differences between, for example, different treatment modalities.
“An important question which remains unanswered is if a ‘reinforced’ immune system following immunotherapy results in an under-/overactivation of the immune response” to COVID-19, the investigators commented. They note that one such patient had a good response.
The SOAP study is sponsored by King’s College London and Guy’s and St. Thomas’ Foundation NHS Trust. It is funded from grants from the KCL Charity funds, MRC, Cancer Research UK, program grants from Breast Cancer Now at King’s College London and by grants to the Breast Cancer Now Toby Robin’s Research Center at the Institute of Cancer Research, London, and the Wellcome Trust Investigator Award, and is supported by the Cancer Research UK Cancer Immunotherapy Accelerator and the UK COVID-Immunology-Consortium. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Overcoming the challenges of COVID-19 for Alzheimer’s patients in long-term care, research
An alarming number of additional Alzheimer’s disease (AD) deaths have been reported across various states within the past several months, according to the Alzheimer’s Association. Centers for Disease Control and Prevention data indicate that no less than 31,000 additional people with the neurodegenerative condition had died from the beginning of the pandemic through the end of September 2020. We know that long-term care facilities have been hit hardest, and access to adequate and/or prompt testing has been cited as the most pressing issue during the onset of the pandemic.1
When ADLs become a matter of survival
For individuals affected with Alzheimer’s disease and other types of dementia, performing routine tasks may seem cumbersome and overwhelming. Many of these patients are dependent upon caregivers and family support to facilitate their activities of daily living (ADLs).
Transitioning into the “new normal” set by the pandemic milieu is not an easy task for the average AD individual, because they are now expected to comply with numerous safety instructions (for example, maintaining hygiene, social distancing, etc.). They are also expected to monitor and communicate information about the onset of any suspected symptom to their caregiver or health care clinician.
The additional tasks added to their list of ADLs are particularly distressing given their already compromised short-term memory and overall cognitive decline. Individuals with AD may also be dealing with a host of psychobehavioral challenges, such as the presence of depression, anxiety, and/or agitation amid self-isolation. Enforced social isolation tied to COVID-19 may compound those issues.
Resource diversion and mitigation strategies
Unfortunately, any disruption in services within a long-term care setting may lead to a suboptimal therapeutic environment for patients. The Washington State LTC, for example, reported experiencing a case fatality rate (CFR) exceeding more than a third of its residents; essential staff and health care clinicians were duly affected from exposure to the virus (the risk of transmission increases considerably during transport between facilities). Access to personal protective equipment (PPE) might have been hindered by availability.
Another issue with far-reaching consequences is diversion of resources for urgent care. Health care professionals may simply not be readily available for those with chronic care needs because of the enormous scale of the impact of COVID-19 upon health care systems.
Continuity of therapy might include evaluations or follow-up services via teleconferencing modalities, but an exhaustive initial onsite physical examination is often necessary for accurate diagnostics and care. Medication management for the newly diagnosed AD or dementia patient necessitates a thorough screening process involving appropriate in-person blood or laboratory work. It is for this reason that clinicians will need to plan ahead by preparing a contingency plan with the corresponding mitigation strategies (for example, telemedicine, proposed solutions to anticipated disruption of services, extended support, and feedback from family members, etc.).2
Resilience and recovery in a retrospective study
A research team from Wuhan Red Cross Hospital in China performed a retrospective cohort study on a sample of patients (n = 42) to determine the severity and prognostic features of COVID-19 pneumonia; 19 AD patients (as per National Institute on Aging/Alzheimer’s Association diagnostic guidelines) were directly compared with 23 age-matched non-AD COVID-19 patients in a similar treatment context.
The study yielded some rather unexpected findings, namely, AD patients experienced remarkably shorter hospital stays and better appetites, especially with respect to their non-AD counterparts. This is even more puzzling when considering that previous studies indicated that dementia patients with concomitant COVID-19 pneumonia are twice as likely to die as those without neurodegenerative compromise.
Aside from a seemingly inexplicable interest in food, the observable positive changes may be attributable to such factors that are particular to the nursing home – residents have immediate access to health care services, which generally allows for timely diagnosis and care. However, the authors of the study speculate that the pathophysiological response of angiotensin-converting enzyme 2 (ACE2) confers to AD patients a therapeutic advantage as they have reduced expression.3 Despite the notoriously high mortality rates of COVID-19 pneumonia among the elderly population, , which underscores the importance of early diagnosis and intervention.
Genetic and environmental susceptibility
One of the more devastating observations about the ongoing pandemic environment is that a whopping 80% of dying patients committed to a long-term facility also include those with AD; it has been reported that almost half of all patients in nursing homes and related services have the neurodegenerative condition. The grim scenario is brought about by several factors, chief of which is the proximity of shared living arrangements within the context of a residential care setting. It should be noted that patients with AD exhibit comorbid conditions (for example, diabetes, cardiovascular disease, and/or respiratory issues) that immediately put them at high risk for COVID exposure. Interestingly enough, the ApoE4 genotype, which is associated with an increased susceptibility for AD, is also correlated with COVID-19 prognosis and severity. Although exact numbers are difficult to come by, it is of utmost importance for clinicians to evaluate the overall scope of the situation, identify at-risk patients such as individuals with AD and related dementias, and work with caregivers to afford care to patients who need it the most.4
Transcending research design
The elderly population, unsurprisingly, experiences the highest COVID-19 mortality rate because of the presence of multiple risk factors, namely, compromised immunity and difficulties maintaining ADLs, and thereby adhering to safety protocols. As far as Alzheimer’s patients are concerned, numerous hurdles affect the domain of neurodegenerative research.
To safeguard the health and well-being of the participants and caregivers, site sponsors and investigators must explore various communication avenues with the goal of facilitating health education (for example, mitigation strategies, adverse effects monitoring, etc.), as well as implementing contingency plans in the event that a site becomes inaccessible (for example, site closure, traveling regulations, lockdowns, etc.).
Alternatives such as telemedicine present viable solutions for ensuring completion of studies. Given the nature of the pandemic, there is a possibility that a research participant may contract the virus, necessitating a break from the established protocol. It is for this reason that site sponsors and corresponding regulatory bodies are advised to proactively engage in dialogue and transparent communications with respect to ensuing protocol deviations. Institutional Review Boards can expedite the review process by making the necessary changes in a timely manner.5
References
1. Ritchie K. KJZZ. 2020 Nov 16.
2. Brown EE et al. Am J Geriatr Psychiatry. 2020 Jul;28(7):712-21.
3. Li J et al. J Alzheimers Dis. 2020;77(1):67-73.
4. Perry G. J Alzheimers Dis. 2020 Jan 1;76(1):1.
5. Alzheimers Dement. 2020 Apr;16(4):587-8.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston. Dr. Dhillon is currently on the speaker bureau/advisory board for Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He has no disclosures.
An alarming number of additional Alzheimer’s disease (AD) deaths have been reported across various states within the past several months, according to the Alzheimer’s Association. Centers for Disease Control and Prevention data indicate that no less than 31,000 additional people with the neurodegenerative condition had died from the beginning of the pandemic through the end of September 2020. We know that long-term care facilities have been hit hardest, and access to adequate and/or prompt testing has been cited as the most pressing issue during the onset of the pandemic.1
When ADLs become a matter of survival
For individuals affected with Alzheimer’s disease and other types of dementia, performing routine tasks may seem cumbersome and overwhelming. Many of these patients are dependent upon caregivers and family support to facilitate their activities of daily living (ADLs).
Transitioning into the “new normal” set by the pandemic milieu is not an easy task for the average AD individual, because they are now expected to comply with numerous safety instructions (for example, maintaining hygiene, social distancing, etc.). They are also expected to monitor and communicate information about the onset of any suspected symptom to their caregiver or health care clinician.
The additional tasks added to their list of ADLs are particularly distressing given their already compromised short-term memory and overall cognitive decline. Individuals with AD may also be dealing with a host of psychobehavioral challenges, such as the presence of depression, anxiety, and/or agitation amid self-isolation. Enforced social isolation tied to COVID-19 may compound those issues.
Resource diversion and mitigation strategies
Unfortunately, any disruption in services within a long-term care setting may lead to a suboptimal therapeutic environment for patients. The Washington State LTC, for example, reported experiencing a case fatality rate (CFR) exceeding more than a third of its residents; essential staff and health care clinicians were duly affected from exposure to the virus (the risk of transmission increases considerably during transport between facilities). Access to personal protective equipment (PPE) might have been hindered by availability.
Another issue with far-reaching consequences is diversion of resources for urgent care. Health care professionals may simply not be readily available for those with chronic care needs because of the enormous scale of the impact of COVID-19 upon health care systems.
Continuity of therapy might include evaluations or follow-up services via teleconferencing modalities, but an exhaustive initial onsite physical examination is often necessary for accurate diagnostics and care. Medication management for the newly diagnosed AD or dementia patient necessitates a thorough screening process involving appropriate in-person blood or laboratory work. It is for this reason that clinicians will need to plan ahead by preparing a contingency plan with the corresponding mitigation strategies (for example, telemedicine, proposed solutions to anticipated disruption of services, extended support, and feedback from family members, etc.).2
Resilience and recovery in a retrospective study
A research team from Wuhan Red Cross Hospital in China performed a retrospective cohort study on a sample of patients (n = 42) to determine the severity and prognostic features of COVID-19 pneumonia; 19 AD patients (as per National Institute on Aging/Alzheimer’s Association diagnostic guidelines) were directly compared with 23 age-matched non-AD COVID-19 patients in a similar treatment context.
The study yielded some rather unexpected findings, namely, AD patients experienced remarkably shorter hospital stays and better appetites, especially with respect to their non-AD counterparts. This is even more puzzling when considering that previous studies indicated that dementia patients with concomitant COVID-19 pneumonia are twice as likely to die as those without neurodegenerative compromise.
Aside from a seemingly inexplicable interest in food, the observable positive changes may be attributable to such factors that are particular to the nursing home – residents have immediate access to health care services, which generally allows for timely diagnosis and care. However, the authors of the study speculate that the pathophysiological response of angiotensin-converting enzyme 2 (ACE2) confers to AD patients a therapeutic advantage as they have reduced expression.3 Despite the notoriously high mortality rates of COVID-19 pneumonia among the elderly population, , which underscores the importance of early diagnosis and intervention.
Genetic and environmental susceptibility
One of the more devastating observations about the ongoing pandemic environment is that a whopping 80% of dying patients committed to a long-term facility also include those with AD; it has been reported that almost half of all patients in nursing homes and related services have the neurodegenerative condition. The grim scenario is brought about by several factors, chief of which is the proximity of shared living arrangements within the context of a residential care setting. It should be noted that patients with AD exhibit comorbid conditions (for example, diabetes, cardiovascular disease, and/or respiratory issues) that immediately put them at high risk for COVID exposure. Interestingly enough, the ApoE4 genotype, which is associated with an increased susceptibility for AD, is also correlated with COVID-19 prognosis and severity. Although exact numbers are difficult to come by, it is of utmost importance for clinicians to evaluate the overall scope of the situation, identify at-risk patients such as individuals with AD and related dementias, and work with caregivers to afford care to patients who need it the most.4
Transcending research design
The elderly population, unsurprisingly, experiences the highest COVID-19 mortality rate because of the presence of multiple risk factors, namely, compromised immunity and difficulties maintaining ADLs, and thereby adhering to safety protocols. As far as Alzheimer’s patients are concerned, numerous hurdles affect the domain of neurodegenerative research.
To safeguard the health and well-being of the participants and caregivers, site sponsors and investigators must explore various communication avenues with the goal of facilitating health education (for example, mitigation strategies, adverse effects monitoring, etc.), as well as implementing contingency plans in the event that a site becomes inaccessible (for example, site closure, traveling regulations, lockdowns, etc.).
Alternatives such as telemedicine present viable solutions for ensuring completion of studies. Given the nature of the pandemic, there is a possibility that a research participant may contract the virus, necessitating a break from the established protocol. It is for this reason that site sponsors and corresponding regulatory bodies are advised to proactively engage in dialogue and transparent communications with respect to ensuing protocol deviations. Institutional Review Boards can expedite the review process by making the necessary changes in a timely manner.5
References
1. Ritchie K. KJZZ. 2020 Nov 16.
2. Brown EE et al. Am J Geriatr Psychiatry. 2020 Jul;28(7):712-21.
3. Li J et al. J Alzheimers Dis. 2020;77(1):67-73.
4. Perry G. J Alzheimers Dis. 2020 Jan 1;76(1):1.
5. Alzheimers Dement. 2020 Apr;16(4):587-8.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston. Dr. Dhillon is currently on the speaker bureau/advisory board for Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He has no disclosures.
An alarming number of additional Alzheimer’s disease (AD) deaths have been reported across various states within the past several months, according to the Alzheimer’s Association. Centers for Disease Control and Prevention data indicate that no less than 31,000 additional people with the neurodegenerative condition had died from the beginning of the pandemic through the end of September 2020. We know that long-term care facilities have been hit hardest, and access to adequate and/or prompt testing has been cited as the most pressing issue during the onset of the pandemic.1
When ADLs become a matter of survival
For individuals affected with Alzheimer’s disease and other types of dementia, performing routine tasks may seem cumbersome and overwhelming. Many of these patients are dependent upon caregivers and family support to facilitate their activities of daily living (ADLs).
Transitioning into the “new normal” set by the pandemic milieu is not an easy task for the average AD individual, because they are now expected to comply with numerous safety instructions (for example, maintaining hygiene, social distancing, etc.). They are also expected to monitor and communicate information about the onset of any suspected symptom to their caregiver or health care clinician.
The additional tasks added to their list of ADLs are particularly distressing given their already compromised short-term memory and overall cognitive decline. Individuals with AD may also be dealing with a host of psychobehavioral challenges, such as the presence of depression, anxiety, and/or agitation amid self-isolation. Enforced social isolation tied to COVID-19 may compound those issues.
Resource diversion and mitigation strategies
Unfortunately, any disruption in services within a long-term care setting may lead to a suboptimal therapeutic environment for patients. The Washington State LTC, for example, reported experiencing a case fatality rate (CFR) exceeding more than a third of its residents; essential staff and health care clinicians were duly affected from exposure to the virus (the risk of transmission increases considerably during transport between facilities). Access to personal protective equipment (PPE) might have been hindered by availability.
Another issue with far-reaching consequences is diversion of resources for urgent care. Health care professionals may simply not be readily available for those with chronic care needs because of the enormous scale of the impact of COVID-19 upon health care systems.
Continuity of therapy might include evaluations or follow-up services via teleconferencing modalities, but an exhaustive initial onsite physical examination is often necessary for accurate diagnostics and care. Medication management for the newly diagnosed AD or dementia patient necessitates a thorough screening process involving appropriate in-person blood or laboratory work. It is for this reason that clinicians will need to plan ahead by preparing a contingency plan with the corresponding mitigation strategies (for example, telemedicine, proposed solutions to anticipated disruption of services, extended support, and feedback from family members, etc.).2
Resilience and recovery in a retrospective study
A research team from Wuhan Red Cross Hospital in China performed a retrospective cohort study on a sample of patients (n = 42) to determine the severity and prognostic features of COVID-19 pneumonia; 19 AD patients (as per National Institute on Aging/Alzheimer’s Association diagnostic guidelines) were directly compared with 23 age-matched non-AD COVID-19 patients in a similar treatment context.
The study yielded some rather unexpected findings, namely, AD patients experienced remarkably shorter hospital stays and better appetites, especially with respect to their non-AD counterparts. This is even more puzzling when considering that previous studies indicated that dementia patients with concomitant COVID-19 pneumonia are twice as likely to die as those without neurodegenerative compromise.
Aside from a seemingly inexplicable interest in food, the observable positive changes may be attributable to such factors that are particular to the nursing home – residents have immediate access to health care services, which generally allows for timely diagnosis and care. However, the authors of the study speculate that the pathophysiological response of angiotensin-converting enzyme 2 (ACE2) confers to AD patients a therapeutic advantage as they have reduced expression.3 Despite the notoriously high mortality rates of COVID-19 pneumonia among the elderly population, , which underscores the importance of early diagnosis and intervention.
Genetic and environmental susceptibility
One of the more devastating observations about the ongoing pandemic environment is that a whopping 80% of dying patients committed to a long-term facility also include those with AD; it has been reported that almost half of all patients in nursing homes and related services have the neurodegenerative condition. The grim scenario is brought about by several factors, chief of which is the proximity of shared living arrangements within the context of a residential care setting. It should be noted that patients with AD exhibit comorbid conditions (for example, diabetes, cardiovascular disease, and/or respiratory issues) that immediately put them at high risk for COVID exposure. Interestingly enough, the ApoE4 genotype, which is associated with an increased susceptibility for AD, is also correlated with COVID-19 prognosis and severity. Although exact numbers are difficult to come by, it is of utmost importance for clinicians to evaluate the overall scope of the situation, identify at-risk patients such as individuals with AD and related dementias, and work with caregivers to afford care to patients who need it the most.4
Transcending research design
The elderly population, unsurprisingly, experiences the highest COVID-19 mortality rate because of the presence of multiple risk factors, namely, compromised immunity and difficulties maintaining ADLs, and thereby adhering to safety protocols. As far as Alzheimer’s patients are concerned, numerous hurdles affect the domain of neurodegenerative research.
To safeguard the health and well-being of the participants and caregivers, site sponsors and investigators must explore various communication avenues with the goal of facilitating health education (for example, mitigation strategies, adverse effects monitoring, etc.), as well as implementing contingency plans in the event that a site becomes inaccessible (for example, site closure, traveling regulations, lockdowns, etc.).
Alternatives such as telemedicine present viable solutions for ensuring completion of studies. Given the nature of the pandemic, there is a possibility that a research participant may contract the virus, necessitating a break from the established protocol. It is for this reason that site sponsors and corresponding regulatory bodies are advised to proactively engage in dialogue and transparent communications with respect to ensuing protocol deviations. Institutional Review Boards can expedite the review process by making the necessary changes in a timely manner.5
References
1. Ritchie K. KJZZ. 2020 Nov 16.
2. Brown EE et al. Am J Geriatr Psychiatry. 2020 Jul;28(7):712-21.
3. Li J et al. J Alzheimers Dis. 2020;77(1):67-73.
4. Perry G. J Alzheimers Dis. 2020 Jan 1;76(1):1.
5. Alzheimers Dement. 2020 Apr;16(4):587-8.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation, Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Dr. Dhillon is a staff neurologist at Brigham and Women’s Hospital in Boston. Dr. Dhillon is currently on the speaker bureau/advisory board for Biogen, Bristol Myers Squibb, Genzyme, and Teva Neuroscience.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the International Maternal and Child Health Foundation. He has no disclosures.
In COVID-19 patients, risk of bleeding rivals risk of thromboembolism
There is no question that COVID-19 infection increases the risks of serious thromboembolic events, including pulmonary embolism (PE), but it also increases the risk of bleeding, complicating the benefit-to-risk calculations for anticoagulation, according to a review of data at the virtual Going Back to the Heart of Cardiology meeting.
“Bleeding is a significant cause of morbidity in patients with COVID-19, and this is an important concept to appreciate,” reported Rachel P. Rosovsky, MD, director of thrombosis research, Massachusetts General Hospital, Boston.
At least five guidelines, including those issued by the American College of Cardiology, International Society on Thrombosis and Haemostasis (ISTH), and the American College of Chest Physicians, have recently addressed anticoagulation in patients infected with COVID-19, but there are “substantive differences” between them, according to Dr. Rosovsky. The reason is that they are essentially no high quality trials to guide practice. Rather, the recommendations are based primarily on retrospective studies and expert opinion.
The single most common theme from the guidelines is that anticoagulation must be individualized to balance patient-specific risks of venous thromboembolism (VTE) and bleeding, said Dr. Rosovsky, whose group published a recent comparison of these guidelines (Flaczyk A et al. Crit Care 2020;24:559).
Although there is general consensus that all hospitalized patients with COVID-19 should receive anticoagulation unless there are contraindications, there are differences in the recommended intensity of the anticoagulation for different risk groups and there is even less is less consensus on the need to anticoagulate outpatients or patients after discharge, according to Dr. Rosovsky
In her own center, the standard is a prophylactic dose of low molecular weight heparin (LMWH) in an algorithm that calls for dose adjustments for some groups such as those with renal impairment or obesity. Alternative forms of anticoagulation are recommended for patients with a history of thrombocytopenia or are at high risk for hemorrhage. Full dose LMWH is recommended in patients already on an oral anticoagulant at time of hospitalization.
“The biggest question right now is when to consider increasing from a prophylactic dose to intermediate or full dose anticoagulation in high risk patients, especially those in the ICU patients,” Dr. Rosovsky said.
Current practices are diverse, according to a recently published survey led by Dr. Rosovsky (Rosovsky RP et al. Res Pract Thromb Haemost. 2020;4:969-83). According to the survey, which had responses from more than 500 physicians in 41 countries, 30% of centers escalate from a prophylactic dose of anticoagulation to an intermediate dose when patients move to the ICU. Although not all answered this question, 25% reported that they do not escalate at ICU transfer. For 15% of respondents, dose escalation is being offered to patients with a D-dimer exceeding six-times the upper limit of normal.
These practices have developed in the absence of prospective clinical trials, which are urgently needed, according to Dr. Rosovsky. The reason that trials specific to COVID-19 are particularly important is that this infection also engenders a high risk of major bleeding.
For example, in a multicenter retrospective study of 400 hospital-admitted COVID-19 patients the rates of major bleeding was 4.8% or exactly the same as the rate of radiographically confirmed VTE. At 7.6%, the rates of VTE and major bleeding were also exactly the same for ICU patients (Al-Samkari H et al. Blood 2020;136:489-500).
“An elevated D-dimer was a marker for both VTE and major bleeding,” reported Dr. Rosovsky, who was the senior author of this study. On the basis of odds ratio (OR), the risk of VTE was increased more than six-fold (OR, 6.79) and the risk of major bleeding by more than three-fold (OR, 3.56) when the D-dimer exceeded 2,500 ng/mL.
The risk of VTE from COVID-19 infection is well documented. For example, autopsy studies have shown widespread thrombosis, including PE, in patients who have died from COVID-19 infection, according to Dr. Rosovsky.
There is also evidence of benefit from anticoagulation. In an retrospective study from China undertaken early in the pandemic, there was no overall mortality benefit at 28 days among those who did receive LMWH when compared to those who did not, but there was a 20% absolute mortality benefit (52.4% vs. 32.8%; P = .017) in those with a D-dimer six-fold ULN (Tang N et al. J Thromb Haemost 2020;18:1094-9).
These types of data support the use of anticoagulation to manage VTE risk in at least some patients, but the reported rates of VTE across institutions and across inpatient and outpatient settings have varied “dramatically,” according to Dr. Rosovsky. The balance of VTE and major bleeding is delicate. In one retrospective study, the mortality advantage for therapeutic versus prophylactic dose of LMWH did not reach statistical significance, but the rate of major bleeding was nearly doubled (3.0% vs. 1.7%) (Nadkarni GN et al J Am Coll Cardiol 2020;76:1815-26).
Because of the many variables that might affect risk of VTE and risk of major bleeding in any individual patient, the benefit-to-risk calculation of anticoagulation is “complex,” according to Dr. Rosovsky. It is for this reason she urged clinicians to consider entering patients into clinical trials designed to generate evidence-based answers.
There is large and growing body of retrospective data that have helped characterize the risk of VTE and bleeding in patients with COVID-19, but “there is no substitute for a well-controlled clinical trial,” agreed Robert A. Harrington, MD, chairman of the department of medicine, Stanford (Calif.) University.
He and the comoderator of the session in which these data were presented agreed that anticoagulation must be administered within a narrow therapeutic window that will be best defined through controlled trial designs.
“There is a significant risk of doing harm,” said Fatima Rodriguez, MD, assistant professor of cardiology at Stanford University. She seconded the critical role of trial participation when possible and the need for clinical trials to better guide treatment decisions.
The meeting was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
There is no question that COVID-19 infection increases the risks of serious thromboembolic events, including pulmonary embolism (PE), but it also increases the risk of bleeding, complicating the benefit-to-risk calculations for anticoagulation, according to a review of data at the virtual Going Back to the Heart of Cardiology meeting.
“Bleeding is a significant cause of morbidity in patients with COVID-19, and this is an important concept to appreciate,” reported Rachel P. Rosovsky, MD, director of thrombosis research, Massachusetts General Hospital, Boston.
At least five guidelines, including those issued by the American College of Cardiology, International Society on Thrombosis and Haemostasis (ISTH), and the American College of Chest Physicians, have recently addressed anticoagulation in patients infected with COVID-19, but there are “substantive differences” between them, according to Dr. Rosovsky. The reason is that they are essentially no high quality trials to guide practice. Rather, the recommendations are based primarily on retrospective studies and expert opinion.
The single most common theme from the guidelines is that anticoagulation must be individualized to balance patient-specific risks of venous thromboembolism (VTE) and bleeding, said Dr. Rosovsky, whose group published a recent comparison of these guidelines (Flaczyk A et al. Crit Care 2020;24:559).
Although there is general consensus that all hospitalized patients with COVID-19 should receive anticoagulation unless there are contraindications, there are differences in the recommended intensity of the anticoagulation for different risk groups and there is even less is less consensus on the need to anticoagulate outpatients or patients after discharge, according to Dr. Rosovsky
In her own center, the standard is a prophylactic dose of low molecular weight heparin (LMWH) in an algorithm that calls for dose adjustments for some groups such as those with renal impairment or obesity. Alternative forms of anticoagulation are recommended for patients with a history of thrombocytopenia or are at high risk for hemorrhage. Full dose LMWH is recommended in patients already on an oral anticoagulant at time of hospitalization.
“The biggest question right now is when to consider increasing from a prophylactic dose to intermediate or full dose anticoagulation in high risk patients, especially those in the ICU patients,” Dr. Rosovsky said.
Current practices are diverse, according to a recently published survey led by Dr. Rosovsky (Rosovsky RP et al. Res Pract Thromb Haemost. 2020;4:969-83). According to the survey, which had responses from more than 500 physicians in 41 countries, 30% of centers escalate from a prophylactic dose of anticoagulation to an intermediate dose when patients move to the ICU. Although not all answered this question, 25% reported that they do not escalate at ICU transfer. For 15% of respondents, dose escalation is being offered to patients with a D-dimer exceeding six-times the upper limit of normal.
These practices have developed in the absence of prospective clinical trials, which are urgently needed, according to Dr. Rosovsky. The reason that trials specific to COVID-19 are particularly important is that this infection also engenders a high risk of major bleeding.
For example, in a multicenter retrospective study of 400 hospital-admitted COVID-19 patients the rates of major bleeding was 4.8% or exactly the same as the rate of radiographically confirmed VTE. At 7.6%, the rates of VTE and major bleeding were also exactly the same for ICU patients (Al-Samkari H et al. Blood 2020;136:489-500).
“An elevated D-dimer was a marker for both VTE and major bleeding,” reported Dr. Rosovsky, who was the senior author of this study. On the basis of odds ratio (OR), the risk of VTE was increased more than six-fold (OR, 6.79) and the risk of major bleeding by more than three-fold (OR, 3.56) when the D-dimer exceeded 2,500 ng/mL.
The risk of VTE from COVID-19 infection is well documented. For example, autopsy studies have shown widespread thrombosis, including PE, in patients who have died from COVID-19 infection, according to Dr. Rosovsky.
There is also evidence of benefit from anticoagulation. In an retrospective study from China undertaken early in the pandemic, there was no overall mortality benefit at 28 days among those who did receive LMWH when compared to those who did not, but there was a 20% absolute mortality benefit (52.4% vs. 32.8%; P = .017) in those with a D-dimer six-fold ULN (Tang N et al. J Thromb Haemost 2020;18:1094-9).
These types of data support the use of anticoagulation to manage VTE risk in at least some patients, but the reported rates of VTE across institutions and across inpatient and outpatient settings have varied “dramatically,” according to Dr. Rosovsky. The balance of VTE and major bleeding is delicate. In one retrospective study, the mortality advantage for therapeutic versus prophylactic dose of LMWH did not reach statistical significance, but the rate of major bleeding was nearly doubled (3.0% vs. 1.7%) (Nadkarni GN et al J Am Coll Cardiol 2020;76:1815-26).
Because of the many variables that might affect risk of VTE and risk of major bleeding in any individual patient, the benefit-to-risk calculation of anticoagulation is “complex,” according to Dr. Rosovsky. It is for this reason she urged clinicians to consider entering patients into clinical trials designed to generate evidence-based answers.
There is large and growing body of retrospective data that have helped characterize the risk of VTE and bleeding in patients with COVID-19, but “there is no substitute for a well-controlled clinical trial,” agreed Robert A. Harrington, MD, chairman of the department of medicine, Stanford (Calif.) University.
He and the comoderator of the session in which these data were presented agreed that anticoagulation must be administered within a narrow therapeutic window that will be best defined through controlled trial designs.
“There is a significant risk of doing harm,” said Fatima Rodriguez, MD, assistant professor of cardiology at Stanford University. She seconded the critical role of trial participation when possible and the need for clinical trials to better guide treatment decisions.
The meeting was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
There is no question that COVID-19 infection increases the risks of serious thromboembolic events, including pulmonary embolism (PE), but it also increases the risk of bleeding, complicating the benefit-to-risk calculations for anticoagulation, according to a review of data at the virtual Going Back to the Heart of Cardiology meeting.
“Bleeding is a significant cause of morbidity in patients with COVID-19, and this is an important concept to appreciate,” reported Rachel P. Rosovsky, MD, director of thrombosis research, Massachusetts General Hospital, Boston.
At least five guidelines, including those issued by the American College of Cardiology, International Society on Thrombosis and Haemostasis (ISTH), and the American College of Chest Physicians, have recently addressed anticoagulation in patients infected with COVID-19, but there are “substantive differences” between them, according to Dr. Rosovsky. The reason is that they are essentially no high quality trials to guide practice. Rather, the recommendations are based primarily on retrospective studies and expert opinion.
The single most common theme from the guidelines is that anticoagulation must be individualized to balance patient-specific risks of venous thromboembolism (VTE) and bleeding, said Dr. Rosovsky, whose group published a recent comparison of these guidelines (Flaczyk A et al. Crit Care 2020;24:559).
Although there is general consensus that all hospitalized patients with COVID-19 should receive anticoagulation unless there are contraindications, there are differences in the recommended intensity of the anticoagulation for different risk groups and there is even less is less consensus on the need to anticoagulate outpatients or patients after discharge, according to Dr. Rosovsky
In her own center, the standard is a prophylactic dose of low molecular weight heparin (LMWH) in an algorithm that calls for dose adjustments for some groups such as those with renal impairment or obesity. Alternative forms of anticoagulation are recommended for patients with a history of thrombocytopenia or are at high risk for hemorrhage. Full dose LMWH is recommended in patients already on an oral anticoagulant at time of hospitalization.
“The biggest question right now is when to consider increasing from a prophylactic dose to intermediate or full dose anticoagulation in high risk patients, especially those in the ICU patients,” Dr. Rosovsky said.
Current practices are diverse, according to a recently published survey led by Dr. Rosovsky (Rosovsky RP et al. Res Pract Thromb Haemost. 2020;4:969-83). According to the survey, which had responses from more than 500 physicians in 41 countries, 30% of centers escalate from a prophylactic dose of anticoagulation to an intermediate dose when patients move to the ICU. Although not all answered this question, 25% reported that they do not escalate at ICU transfer. For 15% of respondents, dose escalation is being offered to patients with a D-dimer exceeding six-times the upper limit of normal.
These practices have developed in the absence of prospective clinical trials, which are urgently needed, according to Dr. Rosovsky. The reason that trials specific to COVID-19 are particularly important is that this infection also engenders a high risk of major bleeding.
For example, in a multicenter retrospective study of 400 hospital-admitted COVID-19 patients the rates of major bleeding was 4.8% or exactly the same as the rate of radiographically confirmed VTE. At 7.6%, the rates of VTE and major bleeding were also exactly the same for ICU patients (Al-Samkari H et al. Blood 2020;136:489-500).
“An elevated D-dimer was a marker for both VTE and major bleeding,” reported Dr. Rosovsky, who was the senior author of this study. On the basis of odds ratio (OR), the risk of VTE was increased more than six-fold (OR, 6.79) and the risk of major bleeding by more than three-fold (OR, 3.56) when the D-dimer exceeded 2,500 ng/mL.
The risk of VTE from COVID-19 infection is well documented. For example, autopsy studies have shown widespread thrombosis, including PE, in patients who have died from COVID-19 infection, according to Dr. Rosovsky.
There is also evidence of benefit from anticoagulation. In an retrospective study from China undertaken early in the pandemic, there was no overall mortality benefit at 28 days among those who did receive LMWH when compared to those who did not, but there was a 20% absolute mortality benefit (52.4% vs. 32.8%; P = .017) in those with a D-dimer six-fold ULN (Tang N et al. J Thromb Haemost 2020;18:1094-9).
These types of data support the use of anticoagulation to manage VTE risk in at least some patients, but the reported rates of VTE across institutions and across inpatient and outpatient settings have varied “dramatically,” according to Dr. Rosovsky. The balance of VTE and major bleeding is delicate. In one retrospective study, the mortality advantage for therapeutic versus prophylactic dose of LMWH did not reach statistical significance, but the rate of major bleeding was nearly doubled (3.0% vs. 1.7%) (Nadkarni GN et al J Am Coll Cardiol 2020;76:1815-26).
Because of the many variables that might affect risk of VTE and risk of major bleeding in any individual patient, the benefit-to-risk calculation of anticoagulation is “complex,” according to Dr. Rosovsky. It is for this reason she urged clinicians to consider entering patients into clinical trials designed to generate evidence-based answers.
There is large and growing body of retrospective data that have helped characterize the risk of VTE and bleeding in patients with COVID-19, but “there is no substitute for a well-controlled clinical trial,” agreed Robert A. Harrington, MD, chairman of the department of medicine, Stanford (Calif.) University.
He and the comoderator of the session in which these data were presented agreed that anticoagulation must be administered within a narrow therapeutic window that will be best defined through controlled trial designs.
“There is a significant risk of doing harm,” said Fatima Rodriguez, MD, assistant professor of cardiology at Stanford University. She seconded the critical role of trial participation when possible and the need for clinical trials to better guide treatment decisions.
The meeting was sponsored by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE GOING BACK TO THE HEART OF CARDIOLOGY MEETING
COVID-19 and youth suicide: Do the numbers match the headlines?
There’s little doubt that the COVID-19 pandemic has been hard on many children and adolescents just as it has been difficult for adults. The disruption of routines, reduced contact with friends, concern over getting ill, and financial turmoil suffered by many families is exacting a toll on our mental health, as has been documented by a number of recent surveys and studies.1,2
Quite understandably, concern about rising levels of anxiety and depression in youth prompts additional worries about suicide, the second leading cause of death in adolescents and young adults. In response, many organizations have rallied to provide additional resources to help prevent suicidal thinking and actions. Online mental health tips, support phone and text lines, and the availability of telemedicine have all been mobilized to help people cope and stay safe both physically and psychologically.
But what are the actual numbers when it comes to youth suicide during COVID-19? According to many headlines in the press, the statistics are grim and support many of distressing predictions that have been made. A December story in an Arizona newspaper, “With Teen Suicides on the Rise, Tucson Educators Struggle to Prioritize Mental Health,” described a 67% increase in teen suicides in 2020 compared with 2019 in one county.3 Another post from Psychology Today, “America is Facing a Teen Suicide Pandemic,” raised similar alarms.4 Concern over suicide has even been used politically to argue against restrictions that could reduce the spread of COVID-19 infections.
But despite this common perception shared by both health care professionals and the public, there actually is not evidence at this point that the COVID-19 pandemic has led to a broad spike in youth suicide deaths or attempts. A recent study published in the journal Pediatrics compared suicide screening results on youth presenting to emergency departments for any reason in 2020 to the same month in 2019.5 The authors found no consistent increases in reported suicidal ideation or suicide attempts with scattered elevations found in some months during 2020 compared with the previous year (including February 2020 before the pandemic really began) but not others. Internationally, newly analyzed data from 2020 with regard to suicide deaths have suggested “either no rise in suicide rates ... or a fall in the early months of the pandemic.” In my home and, admittedly small, state of Vermont, data from the Department of Health have shown 93 suicide deaths across all ages as of mid-November 2020 compared with a 5-year average of 96.
Why don’t the data match the headlines? There are a number of possibilities.
1. Suicide rates in youth were going up before the pandemic. As it takes time to verify and analyze data from large populations, many of the reports on suicide that have been published and released in 2020 summarize data from prior years. Without looking closely, a news organization can easily slap on a headline that implies that the data were obtained during the pandemic.
2. Fluctuations tend to occur from year to year. Thankfully, youth suicide remains rare (although not rare enough). With small numbers, regular variations from year to year can look huge in terms of percentages, especially if one doesn’t pull back and look at longer trends over time.
3. People are reaching out for mental health services. The public health message to access support and treatment for COVID 19–related mental health struggles appears to be having an effect, but this increased demand should not necessarily be viewed as a proxy for suicidal ideation and attempts.
While the understanding that we are not actually in the midst of a surge in COVID 19–related youth suicide is reassuring, it is important not to get complacent. Much of the data remains preliminary, and, even if these numbers hold up, there is no guarantee that things will continue this way, especially if the pandemic and it restrictions continue to drag on for many more months. And of course, whether or not the pandemic is making things significantly worse, youth suicide remains an enormous public health imperative with every one being a human tragedy.
It is also quite possible that more detailed analyses will eventually reveal a more complex association between youth suicide and COVID-19, with effects of the pandemic being realized regionally or more for some groups than others. Data from before the pandemic indicated, for example, that suicide rates are increasing more rapidly among African American youth compared with white children and adolescents.6 With the COVID-19 pandemic itself affecting disadvantaged communities more strongly, one could readily expect variable impacts in mental health related to race or socioeconomic status. A recent article voices these concerns for indigenous youth in Montana: a state with one of the highest per capita suicide rates in the country.7 The article notes, however, that the rate of suicide overall in Montana in 2020 is comparable to those of previous years.
Overall, pediatricians should not be needlessly panicked that the COVID-19 pandemic has sparked a surge in youth suicide. The data at this point simply don’t support that assertion despite many headlines to the contrary. At the same time, many children and adolescents are certainly struggling with the stresses the pandemic has created and continue to need our close monitoring and support.
Dr. Rettew is a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont Larner College of Medicine. Follow him on Twitter @PediPsych. His new book, “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood,” launches Feb. 1, 2021.
References
1. Copeland WE et al. Impact of COVID-19 pandemic on college student mental health and wellness. J Am Acad Child Adolesc Psychiatry. 2020;60(1):134-41. doi: 10.1016/j.jaac.2020.08.466.
2. Qiu J et al. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. Gen Psychiatry. 2020;33:e100213. doi: 10.1136/gpsych-2020-100213.
3. Dhmara K. With teen suicides on the rise, Tucson educators struggle to prioritize mental health. Tuscon.com. Dec. 27, 2020.
4. Chafouleas, SM. America is facing a suicide epidemic: New data confirm the urgency of confronting it now. Psychology Today blog. Sept. 4, 2020.
5. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and after COVID-19. Pediatrics. 2020. doi: 10.1542/peds.2020-029280.
6. John A et al. Trends in suicide during the covid-19 pandemic. BMJ 2020;371:m4352. doi: 10.1136/bmj.m4352.
7. Reardon S. Health officials fear COVID-19 pandemic-related suicide spike among indigenous youth. Time Magazine. December 2020.
There’s little doubt that the COVID-19 pandemic has been hard on many children and adolescents just as it has been difficult for adults. The disruption of routines, reduced contact with friends, concern over getting ill, and financial turmoil suffered by many families is exacting a toll on our mental health, as has been documented by a number of recent surveys and studies.1,2
Quite understandably, concern about rising levels of anxiety and depression in youth prompts additional worries about suicide, the second leading cause of death in adolescents and young adults. In response, many organizations have rallied to provide additional resources to help prevent suicidal thinking and actions. Online mental health tips, support phone and text lines, and the availability of telemedicine have all been mobilized to help people cope and stay safe both physically and psychologically.
But what are the actual numbers when it comes to youth suicide during COVID-19? According to many headlines in the press, the statistics are grim and support many of distressing predictions that have been made. A December story in an Arizona newspaper, “With Teen Suicides on the Rise, Tucson Educators Struggle to Prioritize Mental Health,” described a 67% increase in teen suicides in 2020 compared with 2019 in one county.3 Another post from Psychology Today, “America is Facing a Teen Suicide Pandemic,” raised similar alarms.4 Concern over suicide has even been used politically to argue against restrictions that could reduce the spread of COVID-19 infections.
But despite this common perception shared by both health care professionals and the public, there actually is not evidence at this point that the COVID-19 pandemic has led to a broad spike in youth suicide deaths or attempts. A recent study published in the journal Pediatrics compared suicide screening results on youth presenting to emergency departments for any reason in 2020 to the same month in 2019.5 The authors found no consistent increases in reported suicidal ideation or suicide attempts with scattered elevations found in some months during 2020 compared with the previous year (including February 2020 before the pandemic really began) but not others. Internationally, newly analyzed data from 2020 with regard to suicide deaths have suggested “either no rise in suicide rates ... or a fall in the early months of the pandemic.” In my home and, admittedly small, state of Vermont, data from the Department of Health have shown 93 suicide deaths across all ages as of mid-November 2020 compared with a 5-year average of 96.
Why don’t the data match the headlines? There are a number of possibilities.
1. Suicide rates in youth were going up before the pandemic. As it takes time to verify and analyze data from large populations, many of the reports on suicide that have been published and released in 2020 summarize data from prior years. Without looking closely, a news organization can easily slap on a headline that implies that the data were obtained during the pandemic.
2. Fluctuations tend to occur from year to year. Thankfully, youth suicide remains rare (although not rare enough). With small numbers, regular variations from year to year can look huge in terms of percentages, especially if one doesn’t pull back and look at longer trends over time.
3. People are reaching out for mental health services. The public health message to access support and treatment for COVID 19–related mental health struggles appears to be having an effect, but this increased demand should not necessarily be viewed as a proxy for suicidal ideation and attempts.
While the understanding that we are not actually in the midst of a surge in COVID 19–related youth suicide is reassuring, it is important not to get complacent. Much of the data remains preliminary, and, even if these numbers hold up, there is no guarantee that things will continue this way, especially if the pandemic and it restrictions continue to drag on for many more months. And of course, whether or not the pandemic is making things significantly worse, youth suicide remains an enormous public health imperative with every one being a human tragedy.
It is also quite possible that more detailed analyses will eventually reveal a more complex association between youth suicide and COVID-19, with effects of the pandemic being realized regionally or more for some groups than others. Data from before the pandemic indicated, for example, that suicide rates are increasing more rapidly among African American youth compared with white children and adolescents.6 With the COVID-19 pandemic itself affecting disadvantaged communities more strongly, one could readily expect variable impacts in mental health related to race or socioeconomic status. A recent article voices these concerns for indigenous youth in Montana: a state with one of the highest per capita suicide rates in the country.7 The article notes, however, that the rate of suicide overall in Montana in 2020 is comparable to those of previous years.
Overall, pediatricians should not be needlessly panicked that the COVID-19 pandemic has sparked a surge in youth suicide. The data at this point simply don’t support that assertion despite many headlines to the contrary. At the same time, many children and adolescents are certainly struggling with the stresses the pandemic has created and continue to need our close monitoring and support.
Dr. Rettew is a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont Larner College of Medicine. Follow him on Twitter @PediPsych. His new book, “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood,” launches Feb. 1, 2021.
References
1. Copeland WE et al. Impact of COVID-19 pandemic on college student mental health and wellness. J Am Acad Child Adolesc Psychiatry. 2020;60(1):134-41. doi: 10.1016/j.jaac.2020.08.466.
2. Qiu J et al. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. Gen Psychiatry. 2020;33:e100213. doi: 10.1136/gpsych-2020-100213.
3. Dhmara K. With teen suicides on the rise, Tucson educators struggle to prioritize mental health. Tuscon.com. Dec. 27, 2020.
4. Chafouleas, SM. America is facing a suicide epidemic: New data confirm the urgency of confronting it now. Psychology Today blog. Sept. 4, 2020.
5. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and after COVID-19. Pediatrics. 2020. doi: 10.1542/peds.2020-029280.
6. John A et al. Trends in suicide during the covid-19 pandemic. BMJ 2020;371:m4352. doi: 10.1136/bmj.m4352.
7. Reardon S. Health officials fear COVID-19 pandemic-related suicide spike among indigenous youth. Time Magazine. December 2020.
There’s little doubt that the COVID-19 pandemic has been hard on many children and adolescents just as it has been difficult for adults. The disruption of routines, reduced contact with friends, concern over getting ill, and financial turmoil suffered by many families is exacting a toll on our mental health, as has been documented by a number of recent surveys and studies.1,2
Quite understandably, concern about rising levels of anxiety and depression in youth prompts additional worries about suicide, the second leading cause of death in adolescents and young adults. In response, many organizations have rallied to provide additional resources to help prevent suicidal thinking and actions. Online mental health tips, support phone and text lines, and the availability of telemedicine have all been mobilized to help people cope and stay safe both physically and psychologically.
But what are the actual numbers when it comes to youth suicide during COVID-19? According to many headlines in the press, the statistics are grim and support many of distressing predictions that have been made. A December story in an Arizona newspaper, “With Teen Suicides on the Rise, Tucson Educators Struggle to Prioritize Mental Health,” described a 67% increase in teen suicides in 2020 compared with 2019 in one county.3 Another post from Psychology Today, “America is Facing a Teen Suicide Pandemic,” raised similar alarms.4 Concern over suicide has even been used politically to argue against restrictions that could reduce the spread of COVID-19 infections.
But despite this common perception shared by both health care professionals and the public, there actually is not evidence at this point that the COVID-19 pandemic has led to a broad spike in youth suicide deaths or attempts. A recent study published in the journal Pediatrics compared suicide screening results on youth presenting to emergency departments for any reason in 2020 to the same month in 2019.5 The authors found no consistent increases in reported suicidal ideation or suicide attempts with scattered elevations found in some months during 2020 compared with the previous year (including February 2020 before the pandemic really began) but not others. Internationally, newly analyzed data from 2020 with regard to suicide deaths have suggested “either no rise in suicide rates ... or a fall in the early months of the pandemic.” In my home and, admittedly small, state of Vermont, data from the Department of Health have shown 93 suicide deaths across all ages as of mid-November 2020 compared with a 5-year average of 96.
Why don’t the data match the headlines? There are a number of possibilities.
1. Suicide rates in youth were going up before the pandemic. As it takes time to verify and analyze data from large populations, many of the reports on suicide that have been published and released in 2020 summarize data from prior years. Without looking closely, a news organization can easily slap on a headline that implies that the data were obtained during the pandemic.
2. Fluctuations tend to occur from year to year. Thankfully, youth suicide remains rare (although not rare enough). With small numbers, regular variations from year to year can look huge in terms of percentages, especially if one doesn’t pull back and look at longer trends over time.
3. People are reaching out for mental health services. The public health message to access support and treatment for COVID 19–related mental health struggles appears to be having an effect, but this increased demand should not necessarily be viewed as a proxy for suicidal ideation and attempts.
While the understanding that we are not actually in the midst of a surge in COVID 19–related youth suicide is reassuring, it is important not to get complacent. Much of the data remains preliminary, and, even if these numbers hold up, there is no guarantee that things will continue this way, especially if the pandemic and it restrictions continue to drag on for many more months. And of course, whether or not the pandemic is making things significantly worse, youth suicide remains an enormous public health imperative with every one being a human tragedy.
It is also quite possible that more detailed analyses will eventually reveal a more complex association between youth suicide and COVID-19, with effects of the pandemic being realized regionally or more for some groups than others. Data from before the pandemic indicated, for example, that suicide rates are increasing more rapidly among African American youth compared with white children and adolescents.6 With the COVID-19 pandemic itself affecting disadvantaged communities more strongly, one could readily expect variable impacts in mental health related to race or socioeconomic status. A recent article voices these concerns for indigenous youth in Montana: a state with one of the highest per capita suicide rates in the country.7 The article notes, however, that the rate of suicide overall in Montana in 2020 is comparable to those of previous years.
Overall, pediatricians should not be needlessly panicked that the COVID-19 pandemic has sparked a surge in youth suicide. The data at this point simply don’t support that assertion despite many headlines to the contrary. At the same time, many children and adolescents are certainly struggling with the stresses the pandemic has created and continue to need our close monitoring and support.
Dr. Rettew is a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont Larner College of Medicine. Follow him on Twitter @PediPsych. His new book, “Parenting Made Complicated: What Science Really Knows About the Greatest Debates of Early Childhood,” launches Feb. 1, 2021.
References
1. Copeland WE et al. Impact of COVID-19 pandemic on college student mental health and wellness. J Am Acad Child Adolesc Psychiatry. 2020;60(1):134-41. doi: 10.1016/j.jaac.2020.08.466.
2. Qiu J et al. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. Gen Psychiatry. 2020;33:e100213. doi: 10.1136/gpsych-2020-100213.
3. Dhmara K. With teen suicides on the rise, Tucson educators struggle to prioritize mental health. Tuscon.com. Dec. 27, 2020.
4. Chafouleas, SM. America is facing a suicide epidemic: New data confirm the urgency of confronting it now. Psychology Today blog. Sept. 4, 2020.
5. Hill RM et al. Suicide ideation and attempts in a pediatric emergency department before and after COVID-19. Pediatrics. 2020. doi: 10.1542/peds.2020-029280.
6. John A et al. Trends in suicide during the covid-19 pandemic. BMJ 2020;371:m4352. doi: 10.1136/bmj.m4352.
7. Reardon S. Health officials fear COVID-19 pandemic-related suicide spike among indigenous youth. Time Magazine. December 2020.
Cloth masks provide inferior protection vs. medical masks, suggests evidence review
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
FROM ANNALS OF FAMILY MEDICINE