User login
In Case You Missed It: COVID
63% of long COVID patients are women, study says
according to a new study published in JAMA.
The global study also found that about 6% of people with symptomatic infections had long COVID in 2020 and 2021. The risk for long COVID seemed to be greater among those who needed hospitalization, especially those who needed intensive care.
“Quantifying the number of individuals with long COVID may help policy makers ensure adequate access to services to guide people toward recovery, return to the workplace or school, and restore their mental health and social life,” the researchers wrote.
The study team, which included dozens of researchers across nearly every continent, analyzed data from 54 studies and two databases for more than 1 million patients in 22 countries who had symptomatic COVID infections in 2020 and 2021. They looked at three long COVID symptom types: persistent fatigue with bodily pain or mood swings, ongoing respiratory problems, and cognitive issues. The study included people aged 4-66.
Overall, 6.2% of people reported one of the long COVID symptom types, including 3.7% with ongoing respiratory problems, 3.2% with persistent fatigue and bodily pain or mood swings, and 2.2% with cognitive problems. Among those with long COVID, 38% of people reported more than one symptom cluster.
At 3 months after infection, long COVID symptoms were nearly twice as common in women who were at least 20 years old at 10.6%, compared with men who were at least 20 years old at 5.4%.
Children and teens appeared to have lower risks of long COVID. About 2.8% of patients under age 20 with symptomatic infection developed long-term issues.
The estimated average duration of long COVID symptoms was 9 months among hospitalized patients and 4 months among those who weren’t hospitalized. About 15% of people with long COVID symptoms 3 months after the initial infection continued to have symptoms at 12 months.
The study was largely based on detailed data from ongoing COVID-19 studies in the United States, Austria, the Faroe Islands, Germany, Iran, Italy, the Netherlands, Russia, Sweden, and Switzerland, according to UPI. It was supplemented by published data and research conducted as part of the Global Burden of Diseases, Injuries and Risk Factors Study. The dozens of researchers are referred to as “Global Burden of Disease Long COVID Collaborators.”
The study had limitations, the researchers said, including the assumption that long COVID follows a similar course in all countries. Additional studies may show how long COVID symptoms and severity may vary in different countries and continents.
Ultimately, ongoing studies of large numbers of people with long COVID could help scientists and public health officials understand risk factors and ways to treat the debilitating condition, the study authors wrote, noting that “postinfection fatigue syndrome” has been reported before, namely during the 1918 flu pandemic, after the SARS outbreak in 2003, and after the Ebola epidemic in West Africa in 2014.
“Similar symptoms have been reported after other viral infections, including the Epstein-Barr virus, mononucleosis, and dengue, as well as after nonviral infections such as Q fever, Lyme disease and giardiasis,” they wrote.
Several study investigators reported receiving grants and personal fees from a variety of sources.
A version of this article first appeared on Medscape.com.
according to a new study published in JAMA.
The global study also found that about 6% of people with symptomatic infections had long COVID in 2020 and 2021. The risk for long COVID seemed to be greater among those who needed hospitalization, especially those who needed intensive care.
“Quantifying the number of individuals with long COVID may help policy makers ensure adequate access to services to guide people toward recovery, return to the workplace or school, and restore their mental health and social life,” the researchers wrote.
The study team, which included dozens of researchers across nearly every continent, analyzed data from 54 studies and two databases for more than 1 million patients in 22 countries who had symptomatic COVID infections in 2020 and 2021. They looked at three long COVID symptom types: persistent fatigue with bodily pain or mood swings, ongoing respiratory problems, and cognitive issues. The study included people aged 4-66.
Overall, 6.2% of people reported one of the long COVID symptom types, including 3.7% with ongoing respiratory problems, 3.2% with persistent fatigue and bodily pain or mood swings, and 2.2% with cognitive problems. Among those with long COVID, 38% of people reported more than one symptom cluster.
At 3 months after infection, long COVID symptoms were nearly twice as common in women who were at least 20 years old at 10.6%, compared with men who were at least 20 years old at 5.4%.
Children and teens appeared to have lower risks of long COVID. About 2.8% of patients under age 20 with symptomatic infection developed long-term issues.
The estimated average duration of long COVID symptoms was 9 months among hospitalized patients and 4 months among those who weren’t hospitalized. About 15% of people with long COVID symptoms 3 months after the initial infection continued to have symptoms at 12 months.
The study was largely based on detailed data from ongoing COVID-19 studies in the United States, Austria, the Faroe Islands, Germany, Iran, Italy, the Netherlands, Russia, Sweden, and Switzerland, according to UPI. It was supplemented by published data and research conducted as part of the Global Burden of Diseases, Injuries and Risk Factors Study. The dozens of researchers are referred to as “Global Burden of Disease Long COVID Collaborators.”
The study had limitations, the researchers said, including the assumption that long COVID follows a similar course in all countries. Additional studies may show how long COVID symptoms and severity may vary in different countries and continents.
Ultimately, ongoing studies of large numbers of people with long COVID could help scientists and public health officials understand risk factors and ways to treat the debilitating condition, the study authors wrote, noting that “postinfection fatigue syndrome” has been reported before, namely during the 1918 flu pandemic, after the SARS outbreak in 2003, and after the Ebola epidemic in West Africa in 2014.
“Similar symptoms have been reported after other viral infections, including the Epstein-Barr virus, mononucleosis, and dengue, as well as after nonviral infections such as Q fever, Lyme disease and giardiasis,” they wrote.
Several study investigators reported receiving grants and personal fees from a variety of sources.
A version of this article first appeared on Medscape.com.
according to a new study published in JAMA.
The global study also found that about 6% of people with symptomatic infections had long COVID in 2020 and 2021. The risk for long COVID seemed to be greater among those who needed hospitalization, especially those who needed intensive care.
“Quantifying the number of individuals with long COVID may help policy makers ensure adequate access to services to guide people toward recovery, return to the workplace or school, and restore their mental health and social life,” the researchers wrote.
The study team, which included dozens of researchers across nearly every continent, analyzed data from 54 studies and two databases for more than 1 million patients in 22 countries who had symptomatic COVID infections in 2020 and 2021. They looked at three long COVID symptom types: persistent fatigue with bodily pain or mood swings, ongoing respiratory problems, and cognitive issues. The study included people aged 4-66.
Overall, 6.2% of people reported one of the long COVID symptom types, including 3.7% with ongoing respiratory problems, 3.2% with persistent fatigue and bodily pain or mood swings, and 2.2% with cognitive problems. Among those with long COVID, 38% of people reported more than one symptom cluster.
At 3 months after infection, long COVID symptoms were nearly twice as common in women who were at least 20 years old at 10.6%, compared with men who were at least 20 years old at 5.4%.
Children and teens appeared to have lower risks of long COVID. About 2.8% of patients under age 20 with symptomatic infection developed long-term issues.
The estimated average duration of long COVID symptoms was 9 months among hospitalized patients and 4 months among those who weren’t hospitalized. About 15% of people with long COVID symptoms 3 months after the initial infection continued to have symptoms at 12 months.
The study was largely based on detailed data from ongoing COVID-19 studies in the United States, Austria, the Faroe Islands, Germany, Iran, Italy, the Netherlands, Russia, Sweden, and Switzerland, according to UPI. It was supplemented by published data and research conducted as part of the Global Burden of Diseases, Injuries and Risk Factors Study. The dozens of researchers are referred to as “Global Burden of Disease Long COVID Collaborators.”
The study had limitations, the researchers said, including the assumption that long COVID follows a similar course in all countries. Additional studies may show how long COVID symptoms and severity may vary in different countries and continents.
Ultimately, ongoing studies of large numbers of people with long COVID could help scientists and public health officials understand risk factors and ways to treat the debilitating condition, the study authors wrote, noting that “postinfection fatigue syndrome” has been reported before, namely during the 1918 flu pandemic, after the SARS outbreak in 2003, and after the Ebola epidemic in West Africa in 2014.
“Similar symptoms have been reported after other viral infections, including the Epstein-Barr virus, mononucleosis, and dengue, as well as after nonviral infections such as Q fever, Lyme disease and giardiasis,” they wrote.
Several study investigators reported receiving grants and personal fees from a variety of sources.
A version of this article first appeared on Medscape.com.
FROM JAMA
Why people lie about COVID
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Have you ever lied about COVID-19?
Before you get upset, before the “how dare you,” I want you to think carefully.
Did you have COVID-19 (or think you did) and not mention it to someone you were going to be with? Did you tell someone you were taking more COVID precautions than you really were? Did you tell someone you were vaccinated when you weren’t? Have you avoided getting a COVID test even though you knew you should have?
Researchers appreciated the fact that public health interventions in COVID are important but are only as good as the percentage of people who actually abide by them. So, they designed a survey to ask the questions that many people don’t want to hear the answer to.
A total of 1,733 participants – 80% of those invited – responded to the survey. By design, approximately one-third of respondents (477) had already had COVID, one-third (499) were vaccinated and not yet infected, and one-third (509) were unvaccinated and not yet infected.
Of those surveyed, 41.6% admitted that they lied about COVID or didn’t adhere to COVID guidelines - a conservative estimate, if you ask me.
Breaking down some of the results, about 20% of people who previously were infected with COVID said they didn’t mention it when meeting with someone. A similar number said they didn’t tell anyone when they were entering a public place. A bit more concerning to me, roughly 20% reported not disclosing their COVID-positive status when going to a health care provider’s office.
About 10% of those who had not been vaccinated reported lying about their vaccination status. That’s actually less than the 15% of vaccinated people who lied and told someone they weren’t vaccinated.
About 17% of people lied about the need to quarantine, and many more broke quarantine rules.
The authors tried to see if certain personal characteristics predicted people who were more likely to lie about COVID-19–related issues. Turns out there was only one thing that predicted honesty: age.
Older people were more honest about their COVID status and COVID habits. Other factors – gender, education, race, political affiliation, COVID-19 conspiracy beliefs, and where you got your COVID information – did not seem to make much of a difference. Why are older people more honest? Because older people take COVID more seriously. And they should; COVID is more severe in older people.
The problem arises, of course, because people who are at lower risk for COVID complications interact with people at higher risk – and in those situations, honesty matters more.
On the other hand, isn’t lying about COVID stuff inevitable? If you know that a positive test means you can’t go to work, and not going to work means you won’t get paid, might you not be more likely to lie about the test? Or not get the test at all?
The authors explored the reasons for dishonesty and they are fairly broad, ranging from the desire for life to feel normal (more than half of people who lied) to not believing that COVID was real (a whopping 30%). Some of the reasons for lying included:
- Wanted life to feel normal (50%).
- Freedom (45%).
- It’s no one’s business (40%).
- COVID isn’t real (30%).
In the end, though, we need to realize that public health recommendations are not going to be universally followed, and people may tell us they are following them when, in fact, they are not.
What this adds is another data point to a trend we’ve seen across the course of the pandemic, a shift from collective to individual responsibility. If you can’t be sure what others are doing in regard to COVID, you need to focus on protecting yourself. Perhaps that shift was inevitable. Doesn’t mean we have to like it.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Have you ever lied about COVID-19?
Before you get upset, before the “how dare you,” I want you to think carefully.
Did you have COVID-19 (or think you did) and not mention it to someone you were going to be with? Did you tell someone you were taking more COVID precautions than you really were? Did you tell someone you were vaccinated when you weren’t? Have you avoided getting a COVID test even though you knew you should have?
Researchers appreciated the fact that public health interventions in COVID are important but are only as good as the percentage of people who actually abide by them. So, they designed a survey to ask the questions that many people don’t want to hear the answer to.
A total of 1,733 participants – 80% of those invited – responded to the survey. By design, approximately one-third of respondents (477) had already had COVID, one-third (499) were vaccinated and not yet infected, and one-third (509) were unvaccinated and not yet infected.
Of those surveyed, 41.6% admitted that they lied about COVID or didn’t adhere to COVID guidelines - a conservative estimate, if you ask me.
Breaking down some of the results, about 20% of people who previously were infected with COVID said they didn’t mention it when meeting with someone. A similar number said they didn’t tell anyone when they were entering a public place. A bit more concerning to me, roughly 20% reported not disclosing their COVID-positive status when going to a health care provider’s office.
About 10% of those who had not been vaccinated reported lying about their vaccination status. That’s actually less than the 15% of vaccinated people who lied and told someone they weren’t vaccinated.
About 17% of people lied about the need to quarantine, and many more broke quarantine rules.
The authors tried to see if certain personal characteristics predicted people who were more likely to lie about COVID-19–related issues. Turns out there was only one thing that predicted honesty: age.
Older people were more honest about their COVID status and COVID habits. Other factors – gender, education, race, political affiliation, COVID-19 conspiracy beliefs, and where you got your COVID information – did not seem to make much of a difference. Why are older people more honest? Because older people take COVID more seriously. And they should; COVID is more severe in older people.
The problem arises, of course, because people who are at lower risk for COVID complications interact with people at higher risk – and in those situations, honesty matters more.
On the other hand, isn’t lying about COVID stuff inevitable? If you know that a positive test means you can’t go to work, and not going to work means you won’t get paid, might you not be more likely to lie about the test? Or not get the test at all?
The authors explored the reasons for dishonesty and they are fairly broad, ranging from the desire for life to feel normal (more than half of people who lied) to not believing that COVID was real (a whopping 30%). Some of the reasons for lying included:
- Wanted life to feel normal (50%).
- Freedom (45%).
- It’s no one’s business (40%).
- COVID isn’t real (30%).
In the end, though, we need to realize that public health recommendations are not going to be universally followed, and people may tell us they are following them when, in fact, they are not.
What this adds is another data point to a trend we’ve seen across the course of the pandemic, a shift from collective to individual responsibility. If you can’t be sure what others are doing in regard to COVID, you need to focus on protecting yourself. Perhaps that shift was inevitable. Doesn’t mean we have to like it.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Have you ever lied about COVID-19?
Before you get upset, before the “how dare you,” I want you to think carefully.
Did you have COVID-19 (or think you did) and not mention it to someone you were going to be with? Did you tell someone you were taking more COVID precautions than you really were? Did you tell someone you were vaccinated when you weren’t? Have you avoided getting a COVID test even though you knew you should have?
Researchers appreciated the fact that public health interventions in COVID are important but are only as good as the percentage of people who actually abide by them. So, they designed a survey to ask the questions that many people don’t want to hear the answer to.
A total of 1,733 participants – 80% of those invited – responded to the survey. By design, approximately one-third of respondents (477) had already had COVID, one-third (499) were vaccinated and not yet infected, and one-third (509) were unvaccinated and not yet infected.
Of those surveyed, 41.6% admitted that they lied about COVID or didn’t adhere to COVID guidelines - a conservative estimate, if you ask me.
Breaking down some of the results, about 20% of people who previously were infected with COVID said they didn’t mention it when meeting with someone. A similar number said they didn’t tell anyone when they were entering a public place. A bit more concerning to me, roughly 20% reported not disclosing their COVID-positive status when going to a health care provider’s office.
About 10% of those who had not been vaccinated reported lying about their vaccination status. That’s actually less than the 15% of vaccinated people who lied and told someone they weren’t vaccinated.
About 17% of people lied about the need to quarantine, and many more broke quarantine rules.
The authors tried to see if certain personal characteristics predicted people who were more likely to lie about COVID-19–related issues. Turns out there was only one thing that predicted honesty: age.
Older people were more honest about their COVID status and COVID habits. Other factors – gender, education, race, political affiliation, COVID-19 conspiracy beliefs, and where you got your COVID information – did not seem to make much of a difference. Why are older people more honest? Because older people take COVID more seriously. And they should; COVID is more severe in older people.
The problem arises, of course, because people who are at lower risk for COVID complications interact with people at higher risk – and in those situations, honesty matters more.
On the other hand, isn’t lying about COVID stuff inevitable? If you know that a positive test means you can’t go to work, and not going to work means you won’t get paid, might you not be more likely to lie about the test? Or not get the test at all?
The authors explored the reasons for dishonesty and they are fairly broad, ranging from the desire for life to feel normal (more than half of people who lied) to not believing that COVID was real (a whopping 30%). Some of the reasons for lying included:
- Wanted life to feel normal (50%).
- Freedom (45%).
- It’s no one’s business (40%).
- COVID isn’t real (30%).
In the end, though, we need to realize that public health recommendations are not going to be universally followed, and people may tell us they are following them when, in fact, they are not.
What this adds is another data point to a trend we’ve seen across the course of the pandemic, a shift from collective to individual responsibility. If you can’t be sure what others are doing in regard to COVID, you need to focus on protecting yourself. Perhaps that shift was inevitable. Doesn’t mean we have to like it.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com.
Epidemic of brain fog? Long COVID’s effects worry experts
Weeks after Jeannie Volpe caught COVID-19 in November 2020, she could no longer do her job running sexual assault support groups in Anniston, Ala., because she kept forgetting the details that survivors had shared with her. “People were telling me they were having to revisit their traumatic memories, which isn’t fair to anybody,” the 47-year-old says.
Ms. Volpe has been diagnosed with long-COVID autonomic dysfunction, which includes severe muscle pain, depression, anxiety, and a loss of thinking skills. Some of her symptoms are more commonly known as brain fog, and they’re among the most frequent problems reported by people who have long-term issues after a bout of COVID-19.
Many experts and medical professionals say they haven’t even begun to scratch the surface of what impact this will have in years to come.
“I’m very worried that we have an epidemic of neurologic dysfunction coming down the pike,” says Pamela Davis, MD, PhD, a research professor at Case Western Reserve University, Cleveland.
In the 2 years Ms. Volpe has been living with long COVID, her executive function – the mental processes that enable people to focus attention, retain information, and multitask – has been so diminished that she had to relearn to drive. One of the various doctors assessing her has suggested speech therapy to help Ms. Volpe relearn how to form words. “I can see the words I want to say in my mind, but I can’t make them come out of my mouth,” she says in a sluggish voice that gives away her condition.
All of those symptoms make it difficult for her to care for herself. Without a job and health insurance, Ms. Volpe says she’s researched assisted suicide in the states that allow it but has ultimately decided she wants to live.
“People tell you things like you should be grateful you survived it, and you should; but you shouldn’t expect somebody to not grieve after losing their autonomy, their career, their finances.”
The findings of researchers studying the brain effects of COVID-19 reinforce what people with long COVID have been dealing with from the start. Their experiences aren’t imaginary; they’re consistent with neurological disorders – including myalgic encephalomyelitis, also known as chronic fatigue syndrome, or ME/CFS – which carry much more weight in the public imagination than the term brain fog, which can often be used dismissively.
Studies have found that COVID-19 is linked to conditions such as strokes; seizures; and mood, memory, and movement disorders.
While there are still a lot of unanswered questions about exactly how COVID-19 affects the brain and what the long-term effects are, there’s enough reason to suggest people should be trying to avoid both infection and reinfection until researchers get more answers.
Worldwide, it’s estimated that COVID-19 has contributed to more than 40 million new cases of neurological disorders, says Ziyad Al-Aly, MD, a clinical epidemiologist and long COVID researcher at Washington University in St. Louis. In his latest study of 14 million medical records of the U.S. Department of Veterans Affairs, the country’s largest integrated health care system, researchers found that regardless of age, gender, race, and lifestyle,
He noted that some of the conditions, such as headaches and mild decline in memory and sharpness, may improve and go away over time. But others that showed up, such as stroke, encephalitis (inflammation of the brain), and Guillain-Barré syndrome (a rare disorder in which the body’s immune system attacks the nerves), often lead to lasting damage. Dr. Al-Aly’s team found that neurological conditions were 7% more likely in those who had COVID-19 than in those who had never been infected.
What’s more, researchers noticed that compared with control groups, the risk of post-COVID thinking problems was more pronounced in people in their 30s, 40s, and 50s – a group that usually would be very unlikely to have these problems. For those over the age of 60, the risks stood out less because at that stage of life, such thinking problems aren’t as rare.
Another study of the veterans system last year showed that COVID-19 survivors were at a 46% higher risk of considering suicide after 1 year.
“We need to be paying attention to this,” says Dr. Al-Aly. “What we’ve seen is really the tip of the iceberg.” He worries that millions of people, including youths, will lose out on employment and education while dealing with long-term disabilities – and the economic and societal implications of such a fallout. “What we will all be left with is the aftermath of sheer devastation in some people’s lives,” he says.
Igor Koralnik, MD, chief of neuro-infectious disease and global neurology at Northwestern University, Chicago, has been running a specialized long COVID clinic. His team published a paper in March 2021 detailing what they saw in their first 100 patients. “About half the population in the study missed at least 10 days of work. This is going to have persistent impact on the workforce,” Dr. Koralnik said in a podcast posted on the Northwestern website. “We have seen that not only [do] patients have symptoms, but they have decreased quality of life.”
For older people and their caregivers, the risk of potential neurodegenerative diseases that the virus has shown to accelerate, such as dementia, is also a big concern. Alzheimer’s is already the fifth leading cause of death for people 65 and older.
In a recent study of more than 6 million people over the age of 65, Dr. Davis and her team at Case Western found the risk of Alzheimer’s in the year after COVID-19 increased by 50%-80%. The chances were especially high for women older than 85.
To date, there are no good treatments for Alzheimer’s, yet total health care costs for long-term care and hospice services for people with dementia topped $300 billion in 2020. That doesn’t even include the related costs to families.
“The downstream effect of having someone with Alzheimer’s being taken care of by a family member can be devastating on everyone,” she says. “Sometimes the caregivers don’t weather that very well.”
When Dr. Davis’s own father got Alzheimer’s at age 86, her mother took care of him until she had a stroke one morning while making breakfast. Dr. Davis attributes the stroke to the stress of caregiving. That left Dr. Davis no choice but to seek housing where both her parents could get care.
Looking at the broader picture, Dr. Davis believes widespread isolation, loneliness, and grief during the pandemic, and the disease of COVID-19 itself, will continue to have a profound impact on psychiatric diagnoses. This in turn could trigger a wave of new substance abuse as a result of unchecked mental health problems.
Still, not all brain experts are jumping to worst-case scenarios, with a lot yet to be understood before sounding the alarm. Joanna Hellmuth, MD, a neurologist and researcher at the University of California, San Francisco, cautions against reading too much into early data, including any assumptions that COVID-19 causes neurodegeneration or irreversible damage in the brain.
Even with before-and-after brain scans by University of Oxford, England, researchers that show structural changes to the brain after infection, she points out that they didn’t actually study the clinical symptoms of the people in the study, so it’s too soon to reach conclusions about associated cognitive problems.
“It’s an important piece of the puzzle, but we don’t know how that fits together with everything else,” says Dr. Hellmuth. “Some of my patients get better. … I haven’t seen a single person get worse since the pandemic started, and so I’m hopeful.”
A version of this article first appeared on WebMD.com.
Weeks after Jeannie Volpe caught COVID-19 in November 2020, she could no longer do her job running sexual assault support groups in Anniston, Ala., because she kept forgetting the details that survivors had shared with her. “People were telling me they were having to revisit their traumatic memories, which isn’t fair to anybody,” the 47-year-old says.
Ms. Volpe has been diagnosed with long-COVID autonomic dysfunction, which includes severe muscle pain, depression, anxiety, and a loss of thinking skills. Some of her symptoms are more commonly known as brain fog, and they’re among the most frequent problems reported by people who have long-term issues after a bout of COVID-19.
Many experts and medical professionals say they haven’t even begun to scratch the surface of what impact this will have in years to come.
“I’m very worried that we have an epidemic of neurologic dysfunction coming down the pike,” says Pamela Davis, MD, PhD, a research professor at Case Western Reserve University, Cleveland.
In the 2 years Ms. Volpe has been living with long COVID, her executive function – the mental processes that enable people to focus attention, retain information, and multitask – has been so diminished that she had to relearn to drive. One of the various doctors assessing her has suggested speech therapy to help Ms. Volpe relearn how to form words. “I can see the words I want to say in my mind, but I can’t make them come out of my mouth,” she says in a sluggish voice that gives away her condition.
All of those symptoms make it difficult for her to care for herself. Without a job and health insurance, Ms. Volpe says she’s researched assisted suicide in the states that allow it but has ultimately decided she wants to live.
“People tell you things like you should be grateful you survived it, and you should; but you shouldn’t expect somebody to not grieve after losing their autonomy, their career, their finances.”
The findings of researchers studying the brain effects of COVID-19 reinforce what people with long COVID have been dealing with from the start. Their experiences aren’t imaginary; they’re consistent with neurological disorders – including myalgic encephalomyelitis, also known as chronic fatigue syndrome, or ME/CFS – which carry much more weight in the public imagination than the term brain fog, which can often be used dismissively.
Studies have found that COVID-19 is linked to conditions such as strokes; seizures; and mood, memory, and movement disorders.
While there are still a lot of unanswered questions about exactly how COVID-19 affects the brain and what the long-term effects are, there’s enough reason to suggest people should be trying to avoid both infection and reinfection until researchers get more answers.
Worldwide, it’s estimated that COVID-19 has contributed to more than 40 million new cases of neurological disorders, says Ziyad Al-Aly, MD, a clinical epidemiologist and long COVID researcher at Washington University in St. Louis. In his latest study of 14 million medical records of the U.S. Department of Veterans Affairs, the country’s largest integrated health care system, researchers found that regardless of age, gender, race, and lifestyle,
He noted that some of the conditions, such as headaches and mild decline in memory and sharpness, may improve and go away over time. But others that showed up, such as stroke, encephalitis (inflammation of the brain), and Guillain-Barré syndrome (a rare disorder in which the body’s immune system attacks the nerves), often lead to lasting damage. Dr. Al-Aly’s team found that neurological conditions were 7% more likely in those who had COVID-19 than in those who had never been infected.
What’s more, researchers noticed that compared with control groups, the risk of post-COVID thinking problems was more pronounced in people in their 30s, 40s, and 50s – a group that usually would be very unlikely to have these problems. For those over the age of 60, the risks stood out less because at that stage of life, such thinking problems aren’t as rare.
Another study of the veterans system last year showed that COVID-19 survivors were at a 46% higher risk of considering suicide after 1 year.
“We need to be paying attention to this,” says Dr. Al-Aly. “What we’ve seen is really the tip of the iceberg.” He worries that millions of people, including youths, will lose out on employment and education while dealing with long-term disabilities – and the economic and societal implications of such a fallout. “What we will all be left with is the aftermath of sheer devastation in some people’s lives,” he says.
Igor Koralnik, MD, chief of neuro-infectious disease and global neurology at Northwestern University, Chicago, has been running a specialized long COVID clinic. His team published a paper in March 2021 detailing what they saw in their first 100 patients. “About half the population in the study missed at least 10 days of work. This is going to have persistent impact on the workforce,” Dr. Koralnik said in a podcast posted on the Northwestern website. “We have seen that not only [do] patients have symptoms, but they have decreased quality of life.”
For older people and their caregivers, the risk of potential neurodegenerative diseases that the virus has shown to accelerate, such as dementia, is also a big concern. Alzheimer’s is already the fifth leading cause of death for people 65 and older.
In a recent study of more than 6 million people over the age of 65, Dr. Davis and her team at Case Western found the risk of Alzheimer’s in the year after COVID-19 increased by 50%-80%. The chances were especially high for women older than 85.
To date, there are no good treatments for Alzheimer’s, yet total health care costs for long-term care and hospice services for people with dementia topped $300 billion in 2020. That doesn’t even include the related costs to families.
“The downstream effect of having someone with Alzheimer’s being taken care of by a family member can be devastating on everyone,” she says. “Sometimes the caregivers don’t weather that very well.”
When Dr. Davis’s own father got Alzheimer’s at age 86, her mother took care of him until she had a stroke one morning while making breakfast. Dr. Davis attributes the stroke to the stress of caregiving. That left Dr. Davis no choice but to seek housing where both her parents could get care.
Looking at the broader picture, Dr. Davis believes widespread isolation, loneliness, and grief during the pandemic, and the disease of COVID-19 itself, will continue to have a profound impact on psychiatric diagnoses. This in turn could trigger a wave of new substance abuse as a result of unchecked mental health problems.
Still, not all brain experts are jumping to worst-case scenarios, with a lot yet to be understood before sounding the alarm. Joanna Hellmuth, MD, a neurologist and researcher at the University of California, San Francisco, cautions against reading too much into early data, including any assumptions that COVID-19 causes neurodegeneration or irreversible damage in the brain.
Even with before-and-after brain scans by University of Oxford, England, researchers that show structural changes to the brain after infection, she points out that they didn’t actually study the clinical symptoms of the people in the study, so it’s too soon to reach conclusions about associated cognitive problems.
“It’s an important piece of the puzzle, but we don’t know how that fits together with everything else,” says Dr. Hellmuth. “Some of my patients get better. … I haven’t seen a single person get worse since the pandemic started, and so I’m hopeful.”
A version of this article first appeared on WebMD.com.
Weeks after Jeannie Volpe caught COVID-19 in November 2020, she could no longer do her job running sexual assault support groups in Anniston, Ala., because she kept forgetting the details that survivors had shared with her. “People were telling me they were having to revisit their traumatic memories, which isn’t fair to anybody,” the 47-year-old says.
Ms. Volpe has been diagnosed with long-COVID autonomic dysfunction, which includes severe muscle pain, depression, anxiety, and a loss of thinking skills. Some of her symptoms are more commonly known as brain fog, and they’re among the most frequent problems reported by people who have long-term issues after a bout of COVID-19.
Many experts and medical professionals say they haven’t even begun to scratch the surface of what impact this will have in years to come.
“I’m very worried that we have an epidemic of neurologic dysfunction coming down the pike,” says Pamela Davis, MD, PhD, a research professor at Case Western Reserve University, Cleveland.
In the 2 years Ms. Volpe has been living with long COVID, her executive function – the mental processes that enable people to focus attention, retain information, and multitask – has been so diminished that she had to relearn to drive. One of the various doctors assessing her has suggested speech therapy to help Ms. Volpe relearn how to form words. “I can see the words I want to say in my mind, but I can’t make them come out of my mouth,” she says in a sluggish voice that gives away her condition.
All of those symptoms make it difficult for her to care for herself. Without a job and health insurance, Ms. Volpe says she’s researched assisted suicide in the states that allow it but has ultimately decided she wants to live.
“People tell you things like you should be grateful you survived it, and you should; but you shouldn’t expect somebody to not grieve after losing their autonomy, their career, their finances.”
The findings of researchers studying the brain effects of COVID-19 reinforce what people with long COVID have been dealing with from the start. Their experiences aren’t imaginary; they’re consistent with neurological disorders – including myalgic encephalomyelitis, also known as chronic fatigue syndrome, or ME/CFS – which carry much more weight in the public imagination than the term brain fog, which can often be used dismissively.
Studies have found that COVID-19 is linked to conditions such as strokes; seizures; and mood, memory, and movement disorders.
While there are still a lot of unanswered questions about exactly how COVID-19 affects the brain and what the long-term effects are, there’s enough reason to suggest people should be trying to avoid both infection and reinfection until researchers get more answers.
Worldwide, it’s estimated that COVID-19 has contributed to more than 40 million new cases of neurological disorders, says Ziyad Al-Aly, MD, a clinical epidemiologist and long COVID researcher at Washington University in St. Louis. In his latest study of 14 million medical records of the U.S. Department of Veterans Affairs, the country’s largest integrated health care system, researchers found that regardless of age, gender, race, and lifestyle,
He noted that some of the conditions, such as headaches and mild decline in memory and sharpness, may improve and go away over time. But others that showed up, such as stroke, encephalitis (inflammation of the brain), and Guillain-Barré syndrome (a rare disorder in which the body’s immune system attacks the nerves), often lead to lasting damage. Dr. Al-Aly’s team found that neurological conditions were 7% more likely in those who had COVID-19 than in those who had never been infected.
What’s more, researchers noticed that compared with control groups, the risk of post-COVID thinking problems was more pronounced in people in their 30s, 40s, and 50s – a group that usually would be very unlikely to have these problems. For those over the age of 60, the risks stood out less because at that stage of life, such thinking problems aren’t as rare.
Another study of the veterans system last year showed that COVID-19 survivors were at a 46% higher risk of considering suicide after 1 year.
“We need to be paying attention to this,” says Dr. Al-Aly. “What we’ve seen is really the tip of the iceberg.” He worries that millions of people, including youths, will lose out on employment and education while dealing with long-term disabilities – and the economic and societal implications of such a fallout. “What we will all be left with is the aftermath of sheer devastation in some people’s lives,” he says.
Igor Koralnik, MD, chief of neuro-infectious disease and global neurology at Northwestern University, Chicago, has been running a specialized long COVID clinic. His team published a paper in March 2021 detailing what they saw in their first 100 patients. “About half the population in the study missed at least 10 days of work. This is going to have persistent impact on the workforce,” Dr. Koralnik said in a podcast posted on the Northwestern website. “We have seen that not only [do] patients have symptoms, but they have decreased quality of life.”
For older people and their caregivers, the risk of potential neurodegenerative diseases that the virus has shown to accelerate, such as dementia, is also a big concern. Alzheimer’s is already the fifth leading cause of death for people 65 and older.
In a recent study of more than 6 million people over the age of 65, Dr. Davis and her team at Case Western found the risk of Alzheimer’s in the year after COVID-19 increased by 50%-80%. The chances were especially high for women older than 85.
To date, there are no good treatments for Alzheimer’s, yet total health care costs for long-term care and hospice services for people with dementia topped $300 billion in 2020. That doesn’t even include the related costs to families.
“The downstream effect of having someone with Alzheimer’s being taken care of by a family member can be devastating on everyone,” she says. “Sometimes the caregivers don’t weather that very well.”
When Dr. Davis’s own father got Alzheimer’s at age 86, her mother took care of him until she had a stroke one morning while making breakfast. Dr. Davis attributes the stroke to the stress of caregiving. That left Dr. Davis no choice but to seek housing where both her parents could get care.
Looking at the broader picture, Dr. Davis believes widespread isolation, loneliness, and grief during the pandemic, and the disease of COVID-19 itself, will continue to have a profound impact on psychiatric diagnoses. This in turn could trigger a wave of new substance abuse as a result of unchecked mental health problems.
Still, not all brain experts are jumping to worst-case scenarios, with a lot yet to be understood before sounding the alarm. Joanna Hellmuth, MD, a neurologist and researcher at the University of California, San Francisco, cautions against reading too much into early data, including any assumptions that COVID-19 causes neurodegeneration or irreversible damage in the brain.
Even with before-and-after brain scans by University of Oxford, England, researchers that show structural changes to the brain after infection, she points out that they didn’t actually study the clinical symptoms of the people in the study, so it’s too soon to reach conclusions about associated cognitive problems.
“It’s an important piece of the puzzle, but we don’t know how that fits together with everything else,” says Dr. Hellmuth. “Some of my patients get better. … I haven’t seen a single person get worse since the pandemic started, and so I’m hopeful.”
A version of this article first appeared on WebMD.com.
At the front lines of long COVID, local clinics prove vital
Big-name hospital chains across the United States are opening dedicated centers to help patients dealing with long COVID. But so are the lower-profile clinics and hospitals run by cities, counties and states – including Harborview Medical Center in Seattle.
They serve areas ranging from Campbell County, Wyo., with 47,000 residents, to New York City, with its 8.4 million people. Many providers working there are searching for innovative ways to approach this lingering illness with its variety of symptoms, from brain fog to shortness of breath to depression and more.
Their efforts often fall below the radar, with still-scant serious media attention to long COVID or the public health employees working to treat ailing patients.
Why are state and local health agencies taking on these duties?
They’re leading the way in part because the federal government has made only limited efforts, said Lisa McCorkell, a cofounder of the Patient-Led Research Collaborative. The international group was founded in spring 2020 by researchers who are also long COVID patients.
“It’s a big reason why long COVID isn’t talked about as much,” Ms. McCorkell said. “It’s definitely a national issue. But it trickles down to state and local health departments, and there’s not enough resources.”
The government clinics may be accessible to people without insurance and often are cheaper than clinics at private hospitals.
Harborview has treated more than 1,000 patients with long COVID, and another 200 patients are awaiting treatment, said Jessica Bender, MD, a codirector of the University of Washington Post-COVID Rehabilitation and Recovery Clinic in Seattle’s First Hill neighborhood.
The group Survivor Corps offers lists by states of clinics. While the publicly run clinics may be less expensive or even free for some patients, methods of payment vary from clinic to clinic. Federally qualified health clinics offer treatment on a sliding scale. For instance, the Riverside University Health System in California has federally qualified centers. And other providers who are not federally qualified also offer care paid for on a sliding scale. They include Campbell County Health, where some residents are eligible for discounts of 25%-100%, said spokesperson Norberto Orellana.
At Harborview, Dr. Bender said the public hospital’s post-COVID clinic initially began with a staff of rehabilitation doctors but expanded in 2021 to include family and internal medicine doctors. And it offers mental health programs with rehabilitation psychologists who instruct on how to deal with doctors or loved ones who don’t believe that long COVID exists.
“I have patients who really have been devastated by the lack of support from coworkers [and] family,” Dr. Bender said.
In Campbell County, Wyo., the pandemic surge did not arrive in earnest until late 2021. Physical therapists at Campbell County’s Health Rehabilitation Services organized a rehabilitation program for residents with long COVID after recognizing the need, said Shannon Sorensen, rehabilitation director at Campbell County Health.
“We had patients coming in showing chest pain, or heart palpitations. There were people trying to get back to work. They were frustrated,” Ms. Sorensen said.
Myalgic encephalomyelitis and chronic fatigue syndrome activists have embraced the fight to recognize and help long COVID patients, noting the similarities between the conditions, and hope to help kickstart more organized research, treatment and benefits for long COVID sufferers and myalgic encephalomyelitis/chronic fatigue syndrome patients alike.
In Ft. Collins, Colo., disability activist Alison Sbrana has long had myalgic encephalomyelitis. She and other members of the local chapter of ME Action have met with state officials for several years and are finally seeing the results of those efforts.
Colorado Gov. Jared Polis has created the full-time position of policy adviser for long COVID and post–viral infection planning.
“This is one way forward of how state governments are (finally) paying attention to infection-triggered chronic illnesses and starting to think ahead on them,” Ms. Sbrana said.
New York City’s Health + Hospitals launched what may be the most expansive long COVID treatment program in the nation in April 2021. Called AfterCare, it provides physical and mental health services as well as community support systems and financial assistance.
A persistent issue for patients is that there isn’t yet a test for long COVID, like there is for COVID-19, said Amanda Johnson, MD, assistant vice president for ambulatory care and population health at New York Health + Hospitals. “It’s in many ways a diagnosis of exclusion. You have to make sure their shortness of breath isn’t caused by something else. The same with anemia,” she said.
California’s Department of Public Health has a detailed website devoted to the topic, including videos of “long haulers” describing their experiences.
Vermont is one of several states studying long COVID, said Mark Levine, MD, the state health commissioner. The state, in collaboration with the University of Vermont, has established a surveillance project to determine how many people have long COVID, as well as how severe it is, how long it lasts, and potential predispositions.
The University of Utah, Salt Lake City, established a comprehensive COVID-19 clinic more than a year ago that also handles long COVID patients, said Jeannette Brown, MD, PhD, an associate professor at the school and director of the COVID-19 clinic.
Jennifer Chevinsky, MD, MPH, already had a deep understanding of long COVID when she landed in Riverside County, Calif., in the summer of 2021. She came from Atlanta, where as part of her job as an epidemic intelligence service officer at the CDC, she heard stories of COVID-19 patients who were not getting better.
Now she is a deputy public health officer for Riverside County, in a region known for its deserts, sizzling summer temperatures and diverse populations. She said her department has helped launch programs such as post–COVID-19 follow-up phone calls and long COVID training programs that reach out to the many Latino residents in this county of 2.4 million people. It also includes Black and Native American residents.
“We’re making sure information is circulated with community and faith-based organizations, and community health workers,” she said.
Ms. McCorkell said there is still much work to do to raise public awareness of the risks of long COVID and how to obtain care for patients. She would like to see a national public health campaign about long COVID, possibly spearheaded by the Centers for Disease Control and Prevention in partnership with local health workers and community-based organizations.
“That,” she said, “could make a big difference.”
A version of this article first appeared on WebMD.com.
Big-name hospital chains across the United States are opening dedicated centers to help patients dealing with long COVID. But so are the lower-profile clinics and hospitals run by cities, counties and states – including Harborview Medical Center in Seattle.
They serve areas ranging from Campbell County, Wyo., with 47,000 residents, to New York City, with its 8.4 million people. Many providers working there are searching for innovative ways to approach this lingering illness with its variety of symptoms, from brain fog to shortness of breath to depression and more.
Their efforts often fall below the radar, with still-scant serious media attention to long COVID or the public health employees working to treat ailing patients.
Why are state and local health agencies taking on these duties?
They’re leading the way in part because the federal government has made only limited efforts, said Lisa McCorkell, a cofounder of the Patient-Led Research Collaborative. The international group was founded in spring 2020 by researchers who are also long COVID patients.
“It’s a big reason why long COVID isn’t talked about as much,” Ms. McCorkell said. “It’s definitely a national issue. But it trickles down to state and local health departments, and there’s not enough resources.”
The government clinics may be accessible to people without insurance and often are cheaper than clinics at private hospitals.
Harborview has treated more than 1,000 patients with long COVID, and another 200 patients are awaiting treatment, said Jessica Bender, MD, a codirector of the University of Washington Post-COVID Rehabilitation and Recovery Clinic in Seattle’s First Hill neighborhood.
The group Survivor Corps offers lists by states of clinics. While the publicly run clinics may be less expensive or even free for some patients, methods of payment vary from clinic to clinic. Federally qualified health clinics offer treatment on a sliding scale. For instance, the Riverside University Health System in California has federally qualified centers. And other providers who are not federally qualified also offer care paid for on a sliding scale. They include Campbell County Health, where some residents are eligible for discounts of 25%-100%, said spokesperson Norberto Orellana.
At Harborview, Dr. Bender said the public hospital’s post-COVID clinic initially began with a staff of rehabilitation doctors but expanded in 2021 to include family and internal medicine doctors. And it offers mental health programs with rehabilitation psychologists who instruct on how to deal with doctors or loved ones who don’t believe that long COVID exists.
“I have patients who really have been devastated by the lack of support from coworkers [and] family,” Dr. Bender said.
In Campbell County, Wyo., the pandemic surge did not arrive in earnest until late 2021. Physical therapists at Campbell County’s Health Rehabilitation Services organized a rehabilitation program for residents with long COVID after recognizing the need, said Shannon Sorensen, rehabilitation director at Campbell County Health.
“We had patients coming in showing chest pain, or heart palpitations. There were people trying to get back to work. They were frustrated,” Ms. Sorensen said.
Myalgic encephalomyelitis and chronic fatigue syndrome activists have embraced the fight to recognize and help long COVID patients, noting the similarities between the conditions, and hope to help kickstart more organized research, treatment and benefits for long COVID sufferers and myalgic encephalomyelitis/chronic fatigue syndrome patients alike.
In Ft. Collins, Colo., disability activist Alison Sbrana has long had myalgic encephalomyelitis. She and other members of the local chapter of ME Action have met with state officials for several years and are finally seeing the results of those efforts.
Colorado Gov. Jared Polis has created the full-time position of policy adviser for long COVID and post–viral infection planning.
“This is one way forward of how state governments are (finally) paying attention to infection-triggered chronic illnesses and starting to think ahead on them,” Ms. Sbrana said.
New York City’s Health + Hospitals launched what may be the most expansive long COVID treatment program in the nation in April 2021. Called AfterCare, it provides physical and mental health services as well as community support systems and financial assistance.
A persistent issue for patients is that there isn’t yet a test for long COVID, like there is for COVID-19, said Amanda Johnson, MD, assistant vice president for ambulatory care and population health at New York Health + Hospitals. “It’s in many ways a diagnosis of exclusion. You have to make sure their shortness of breath isn’t caused by something else. The same with anemia,” she said.
California’s Department of Public Health has a detailed website devoted to the topic, including videos of “long haulers” describing their experiences.
Vermont is one of several states studying long COVID, said Mark Levine, MD, the state health commissioner. The state, in collaboration with the University of Vermont, has established a surveillance project to determine how many people have long COVID, as well as how severe it is, how long it lasts, and potential predispositions.
The University of Utah, Salt Lake City, established a comprehensive COVID-19 clinic more than a year ago that also handles long COVID patients, said Jeannette Brown, MD, PhD, an associate professor at the school and director of the COVID-19 clinic.
Jennifer Chevinsky, MD, MPH, already had a deep understanding of long COVID when she landed in Riverside County, Calif., in the summer of 2021. She came from Atlanta, where as part of her job as an epidemic intelligence service officer at the CDC, she heard stories of COVID-19 patients who were not getting better.
Now she is a deputy public health officer for Riverside County, in a region known for its deserts, sizzling summer temperatures and diverse populations. She said her department has helped launch programs such as post–COVID-19 follow-up phone calls and long COVID training programs that reach out to the many Latino residents in this county of 2.4 million people. It also includes Black and Native American residents.
“We’re making sure information is circulated with community and faith-based organizations, and community health workers,” she said.
Ms. McCorkell said there is still much work to do to raise public awareness of the risks of long COVID and how to obtain care for patients. She would like to see a national public health campaign about long COVID, possibly spearheaded by the Centers for Disease Control and Prevention in partnership with local health workers and community-based organizations.
“That,” she said, “could make a big difference.”
A version of this article first appeared on WebMD.com.
Big-name hospital chains across the United States are opening dedicated centers to help patients dealing with long COVID. But so are the lower-profile clinics and hospitals run by cities, counties and states – including Harborview Medical Center in Seattle.
They serve areas ranging from Campbell County, Wyo., with 47,000 residents, to New York City, with its 8.4 million people. Many providers working there are searching for innovative ways to approach this lingering illness with its variety of symptoms, from brain fog to shortness of breath to depression and more.
Their efforts often fall below the radar, with still-scant serious media attention to long COVID or the public health employees working to treat ailing patients.
Why are state and local health agencies taking on these duties?
They’re leading the way in part because the federal government has made only limited efforts, said Lisa McCorkell, a cofounder of the Patient-Led Research Collaborative. The international group was founded in spring 2020 by researchers who are also long COVID patients.
“It’s a big reason why long COVID isn’t talked about as much,” Ms. McCorkell said. “It’s definitely a national issue. But it trickles down to state and local health departments, and there’s not enough resources.”
The government clinics may be accessible to people without insurance and often are cheaper than clinics at private hospitals.
Harborview has treated more than 1,000 patients with long COVID, and another 200 patients are awaiting treatment, said Jessica Bender, MD, a codirector of the University of Washington Post-COVID Rehabilitation and Recovery Clinic in Seattle’s First Hill neighborhood.
The group Survivor Corps offers lists by states of clinics. While the publicly run clinics may be less expensive or even free for some patients, methods of payment vary from clinic to clinic. Federally qualified health clinics offer treatment on a sliding scale. For instance, the Riverside University Health System in California has federally qualified centers. And other providers who are not federally qualified also offer care paid for on a sliding scale. They include Campbell County Health, where some residents are eligible for discounts of 25%-100%, said spokesperson Norberto Orellana.
At Harborview, Dr. Bender said the public hospital’s post-COVID clinic initially began with a staff of rehabilitation doctors but expanded in 2021 to include family and internal medicine doctors. And it offers mental health programs with rehabilitation psychologists who instruct on how to deal with doctors or loved ones who don’t believe that long COVID exists.
“I have patients who really have been devastated by the lack of support from coworkers [and] family,” Dr. Bender said.
In Campbell County, Wyo., the pandemic surge did not arrive in earnest until late 2021. Physical therapists at Campbell County’s Health Rehabilitation Services organized a rehabilitation program for residents with long COVID after recognizing the need, said Shannon Sorensen, rehabilitation director at Campbell County Health.
“We had patients coming in showing chest pain, or heart palpitations. There were people trying to get back to work. They were frustrated,” Ms. Sorensen said.
Myalgic encephalomyelitis and chronic fatigue syndrome activists have embraced the fight to recognize and help long COVID patients, noting the similarities between the conditions, and hope to help kickstart more organized research, treatment and benefits for long COVID sufferers and myalgic encephalomyelitis/chronic fatigue syndrome patients alike.
In Ft. Collins, Colo., disability activist Alison Sbrana has long had myalgic encephalomyelitis. She and other members of the local chapter of ME Action have met with state officials for several years and are finally seeing the results of those efforts.
Colorado Gov. Jared Polis has created the full-time position of policy adviser for long COVID and post–viral infection planning.
“This is one way forward of how state governments are (finally) paying attention to infection-triggered chronic illnesses and starting to think ahead on them,” Ms. Sbrana said.
New York City’s Health + Hospitals launched what may be the most expansive long COVID treatment program in the nation in April 2021. Called AfterCare, it provides physical and mental health services as well as community support systems and financial assistance.
A persistent issue for patients is that there isn’t yet a test for long COVID, like there is for COVID-19, said Amanda Johnson, MD, assistant vice president for ambulatory care and population health at New York Health + Hospitals. “It’s in many ways a diagnosis of exclusion. You have to make sure their shortness of breath isn’t caused by something else. The same with anemia,” she said.
California’s Department of Public Health has a detailed website devoted to the topic, including videos of “long haulers” describing their experiences.
Vermont is one of several states studying long COVID, said Mark Levine, MD, the state health commissioner. The state, in collaboration with the University of Vermont, has established a surveillance project to determine how many people have long COVID, as well as how severe it is, how long it lasts, and potential predispositions.
The University of Utah, Salt Lake City, established a comprehensive COVID-19 clinic more than a year ago that also handles long COVID patients, said Jeannette Brown, MD, PhD, an associate professor at the school and director of the COVID-19 clinic.
Jennifer Chevinsky, MD, MPH, already had a deep understanding of long COVID when she landed in Riverside County, Calif., in the summer of 2021. She came from Atlanta, where as part of her job as an epidemic intelligence service officer at the CDC, she heard stories of COVID-19 patients who were not getting better.
Now she is a deputy public health officer for Riverside County, in a region known for its deserts, sizzling summer temperatures and diverse populations. She said her department has helped launch programs such as post–COVID-19 follow-up phone calls and long COVID training programs that reach out to the many Latino residents in this county of 2.4 million people. It also includes Black and Native American residents.
“We’re making sure information is circulated with community and faith-based organizations, and community health workers,” she said.
Ms. McCorkell said there is still much work to do to raise public awareness of the risks of long COVID and how to obtain care for patients. She would like to see a national public health campaign about long COVID, possibly spearheaded by the Centers for Disease Control and Prevention in partnership with local health workers and community-based organizations.
“That,” she said, “could make a big difference.”
A version of this article first appeared on WebMD.com.
Children and COVID: Downward trend reverses with small increase in new cases
A small increase in new cases brought COVID-19’s latest losing streak to an end at 4 weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
The 40,656 new cases reported bring the U.S. cumulative count of child COVID-19 cases to over 14.8 million since the pandemic began, which represents 18.4% of all cases, the AAP and CHA said in their weekly report based on state-level data.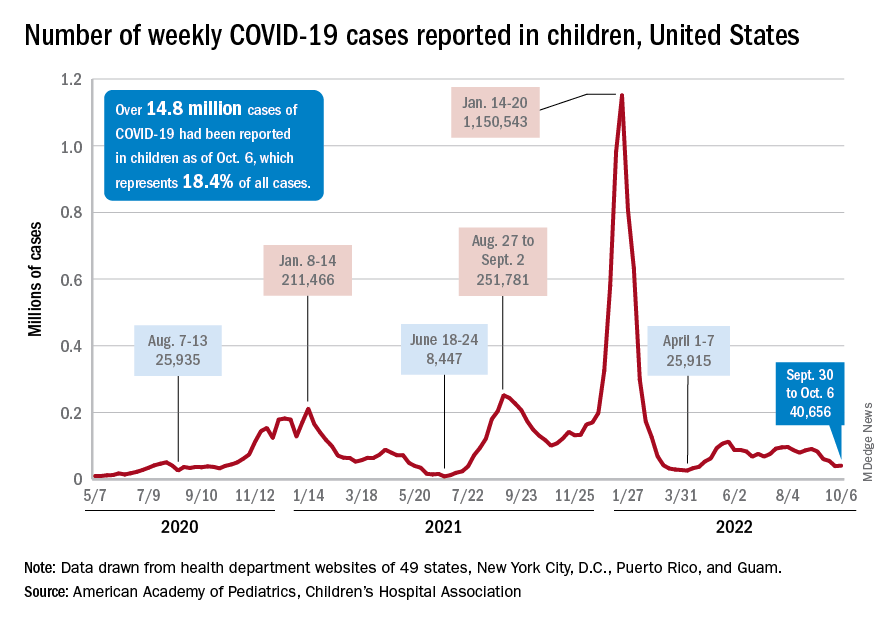
The increase in new cases was not reflected in emergency department visits or hospital admissions, which both continued sustained declines that started in August. In the week from Sept. 27 to Oct. 4, the 7-day averages for ED visits with diagnosed COVID were down by 21.5% (age 0-11), 27.3% (12-15), and 18.2% (16-17), the Centers for Disease Control and Prevention said, while the most recent 7-day average for new admissions – 127 per day for Oct. 2-8 – among children aged 0-17 years with confirmed COVID was down from 161 per day the previous week, a drop of over 21%.
The state-level data that are currently available (several states are no longer reporting) show Alaska (25.5%) and Vermont (25.4%) have the highest proportions of cumulative cases in children, and Florida (12.3%) and Utah (13.5%) have the lowest. Rhode Island has the highest rate of COVID-19 per 100,000 children at 40,427, while Missouri has the lowest at 14,252. The national average is 19,687 per 100,000, the AAP and CHA reported.
Taking a look at vaccination
Vaccinations were up slightly in children aged 12-17 years, as 20,000 initial doses were given during the week of Sept. 29 to Oct. 5, compared with 17,000 and 18,000 the previous 2 weeks. Initial vaccinations in younger children, however, continued declines dating back to August, the AAP said in its weekly vaccination trends report.
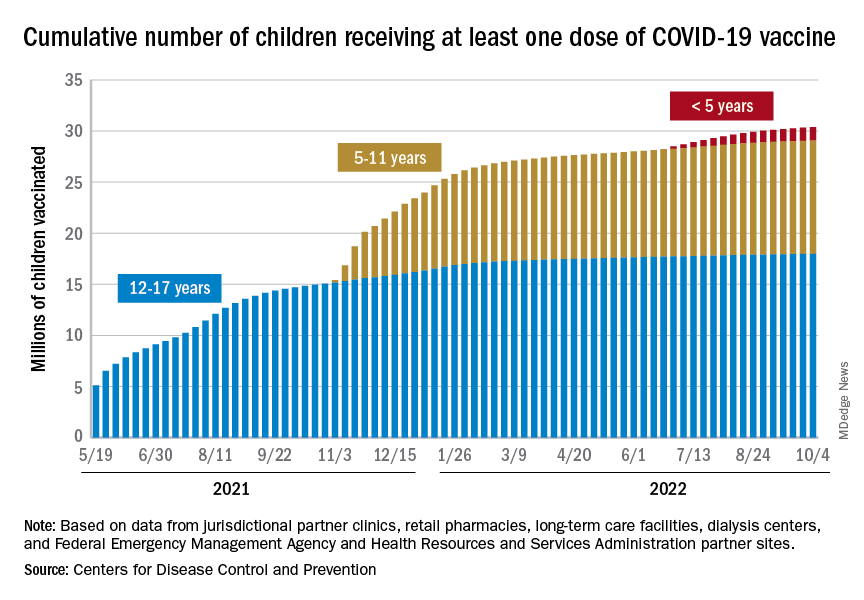
The District of Columbia and Massachusetts have the most highly vaccinated groups of 12- to 17-year-olds, as 100% and 95%, respectively, have received initial doses, while Wyoming (39%) and Idaho (42%) have the lowest. D.C. (73%) and Vermont (68%) have the highest proportions of vaccinated 5- to 11-year-olds, and Alabama (17%) and Mississippi (18%) have the lowest. For children under age 5 years, those in D.C. (33%) and Vermont (26%) are the most likely to have received an initial COVID vaccination, while Alabama, Louisiana, and Mississippi share national-low rates of 2%, the AAP said its report, which is based on CDC data.
When all states and territories are combined, 71% of children aged 12-17 have received at least one dose of vaccine, as have 38.6% of all children 5-11 years old and 6.7% of those under age 5. Almost 61% of the nation’s 16- to 17-year-olds have been fully vaccinated, along with 31.5% of those aged 5-11 and 2.4% of children younger than 5 years, the CDC said on its COVID Data Tracker.
About 42 million children – 58% of the population under the age of 18 years – have not received any vaccine yet, the AAP noted. Meanwhile, CDC data indicate that 36 children died of COVID in the last week, with pediatric deaths now totaling 1,781 over the course of the pandemic.
A small increase in new cases brought COVID-19’s latest losing streak to an end at 4 weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
The 40,656 new cases reported bring the U.S. cumulative count of child COVID-19 cases to over 14.8 million since the pandemic began, which represents 18.4% of all cases, the AAP and CHA said in their weekly report based on state-level data.
The increase in new cases was not reflected in emergency department visits or hospital admissions, which both continued sustained declines that started in August. In the week from Sept. 27 to Oct. 4, the 7-day averages for ED visits with diagnosed COVID were down by 21.5% (age 0-11), 27.3% (12-15), and 18.2% (16-17), the Centers for Disease Control and Prevention said, while the most recent 7-day average for new admissions – 127 per day for Oct. 2-8 – among children aged 0-17 years with confirmed COVID was down from 161 per day the previous week, a drop of over 21%.
The state-level data that are currently available (several states are no longer reporting) show Alaska (25.5%) and Vermont (25.4%) have the highest proportions of cumulative cases in children, and Florida (12.3%) and Utah (13.5%) have the lowest. Rhode Island has the highest rate of COVID-19 per 100,000 children at 40,427, while Missouri has the lowest at 14,252. The national average is 19,687 per 100,000, the AAP and CHA reported.
Taking a look at vaccination
Vaccinations were up slightly in children aged 12-17 years, as 20,000 initial doses were given during the week of Sept. 29 to Oct. 5, compared with 17,000 and 18,000 the previous 2 weeks. Initial vaccinations in younger children, however, continued declines dating back to August, the AAP said in its weekly vaccination trends report.

The District of Columbia and Massachusetts have the most highly vaccinated groups of 12- to 17-year-olds, as 100% and 95%, respectively, have received initial doses, while Wyoming (39%) and Idaho (42%) have the lowest. D.C. (73%) and Vermont (68%) have the highest proportions of vaccinated 5- to 11-year-olds, and Alabama (17%) and Mississippi (18%) have the lowest. For children under age 5 years, those in D.C. (33%) and Vermont (26%) are the most likely to have received an initial COVID vaccination, while Alabama, Louisiana, and Mississippi share national-low rates of 2%, the AAP said its report, which is based on CDC data.
When all states and territories are combined, 71% of children aged 12-17 have received at least one dose of vaccine, as have 38.6% of all children 5-11 years old and 6.7% of those under age 5. Almost 61% of the nation’s 16- to 17-year-olds have been fully vaccinated, along with 31.5% of those aged 5-11 and 2.4% of children younger than 5 years, the CDC said on its COVID Data Tracker.
About 42 million children – 58% of the population under the age of 18 years – have not received any vaccine yet, the AAP noted. Meanwhile, CDC data indicate that 36 children died of COVID in the last week, with pediatric deaths now totaling 1,781 over the course of the pandemic.
A small increase in new cases brought COVID-19’s latest losing streak to an end at 4 weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
The 40,656 new cases reported bring the U.S. cumulative count of child COVID-19 cases to over 14.8 million since the pandemic began, which represents 18.4% of all cases, the AAP and CHA said in their weekly report based on state-level data.
The increase in new cases was not reflected in emergency department visits or hospital admissions, which both continued sustained declines that started in August. In the week from Sept. 27 to Oct. 4, the 7-day averages for ED visits with diagnosed COVID were down by 21.5% (age 0-11), 27.3% (12-15), and 18.2% (16-17), the Centers for Disease Control and Prevention said, while the most recent 7-day average for new admissions – 127 per day for Oct. 2-8 – among children aged 0-17 years with confirmed COVID was down from 161 per day the previous week, a drop of over 21%.
The state-level data that are currently available (several states are no longer reporting) show Alaska (25.5%) and Vermont (25.4%) have the highest proportions of cumulative cases in children, and Florida (12.3%) and Utah (13.5%) have the lowest. Rhode Island has the highest rate of COVID-19 per 100,000 children at 40,427, while Missouri has the lowest at 14,252. The national average is 19,687 per 100,000, the AAP and CHA reported.
Taking a look at vaccination
Vaccinations were up slightly in children aged 12-17 years, as 20,000 initial doses were given during the week of Sept. 29 to Oct. 5, compared with 17,000 and 18,000 the previous 2 weeks. Initial vaccinations in younger children, however, continued declines dating back to August, the AAP said in its weekly vaccination trends report.

The District of Columbia and Massachusetts have the most highly vaccinated groups of 12- to 17-year-olds, as 100% and 95%, respectively, have received initial doses, while Wyoming (39%) and Idaho (42%) have the lowest. D.C. (73%) and Vermont (68%) have the highest proportions of vaccinated 5- to 11-year-olds, and Alabama (17%) and Mississippi (18%) have the lowest. For children under age 5 years, those in D.C. (33%) and Vermont (26%) are the most likely to have received an initial COVID vaccination, while Alabama, Louisiana, and Mississippi share national-low rates of 2%, the AAP said its report, which is based on CDC data.
When all states and territories are combined, 71% of children aged 12-17 have received at least one dose of vaccine, as have 38.6% of all children 5-11 years old and 6.7% of those under age 5. Almost 61% of the nation’s 16- to 17-year-olds have been fully vaccinated, along with 31.5% of those aged 5-11 and 2.4% of children younger than 5 years, the CDC said on its COVID Data Tracker.
About 42 million children – 58% of the population under the age of 18 years – have not received any vaccine yet, the AAP noted. Meanwhile, CDC data indicate that 36 children died of COVID in the last week, with pediatric deaths now totaling 1,781 over the course of the pandemic.
Mother-to-child transmission of SARS-CoV-2 may be underestimated
ANAHEIM, CALIF. – The rate of mother-to-child transmission of SARS-CoV-2 infection is likely higher than the current estimate of 2%-8%, suggests a recent study using cord blood serology to determine incidence. The study was presented at the American Academy of Pediatrics National Conference.
“Cord blood screening is a potential tool to identify SARS-CoV-2 infected and/or exposed neonates who should then be followed for long-term consequences of mother-to-child transmission,” Amy Yeh, MD, an assistant professor of clinical pediatrics at the University of Southern California, Los Angeles, told attendees at the meeting.
Dr. Yeh and her colleagues collected cord blood from more than 500 mothers at LAC+USC Medical Center from October 2021 to April 2022 and tested them for IgG antibodies against three SARS-CoV-2 antigens: nucleoprotein (N), receptor-binding domain (RBD), and spike protein (S1). Results with an IgG mean fluorescence intensity (MFI) above 700 were considered positive for IgG antibodies. A positive result for N as well as RBD or S1 indicated a natural infection while a positive result for only RBD or S1 indicated a vaccine response or past infection.
The researchers also tested a subset of the IgG positive samples for IgM and IgA antibodies against N, S1, and RBD, with an IgM MFI greater than 24 and an IgA MFI greater than 102 used as the thresholds for positive results.
Among 384 cord blood samples analyzed, 85.4% were positive for IgG against RBD, indicating that the mother had SARS-CoV-2 immunity from either a past infection or vaccination. Of these anti-RBD positive samples, 60.7% were anti-N IgG negative, suggesting that N had waned since vaccination or the past infection.
Since the other 39.3% that were anti-N IgG positive suggest a past maternal infection, the researchers assessed these 129 samples for IgM and IgA antibodies against RBD. They found that 16 of them had high levels of anti-RBD IgA and/or IgM antibodies, pointing to a rate of mother-to-child-transmission of up to 12.4%.
Sallie Permar, MD, PhD, a professor and the chair of pediatrics at Weill Cornell Medicine in New York, who was not involved in the research, said most studies of placental transmission have focused on virologic testing, such as PCR. “Serologic tests for congenital infections are inherently challenged by the transfer of maternal IgG across the placenta and therefore must rely on non-IgG isotype response detection, which have inherently been more susceptible to false-positive results than IgG-based tests,” Dr. Permar said.
Also, “it is unclear if virologic testing was performed in the infants, which, if positive in the same infants for which cord blood IgM/IgA responses were identified, could further validate positive serologic findings,” added Dr. Permar, who is also pediatrician-in-chief at New York-Presbyterian Komansky Children’s Hospital.
Given these limitations, Dr. Permar reiterated that diagnostics for congenital SARS-CoV-2 continue to evolve, even if congenital SARS-CoV-2 infection currently appears rare. Dr. Permar said she agreed with Dr. Yeh that following those who do develop this infection is important.
“There have been initial reports of neurodevelopmental and other outcomes from long-term follow-up cohorts of infants exposed to SARS-CoV-2 infection in utero with variable results and it should continue to be pursued using cohorts both enrolled early in the pandemic and those enrolled more recently after population-level immunity to SARS-CoV-2 was achieved,” said Dr. Permar.
Dr. Permar serves as a consultant to Moderna, Pfizer, Merck, Dynavax, and Hoopika on their CMV vaccine programs and has led sponsored research programs with Moderna and Merck. Information on study funding and on disclosures for Dr. Yeh was unavailable.
ANAHEIM, CALIF. – The rate of mother-to-child transmission of SARS-CoV-2 infection is likely higher than the current estimate of 2%-8%, suggests a recent study using cord blood serology to determine incidence. The study was presented at the American Academy of Pediatrics National Conference.
“Cord blood screening is a potential tool to identify SARS-CoV-2 infected and/or exposed neonates who should then be followed for long-term consequences of mother-to-child transmission,” Amy Yeh, MD, an assistant professor of clinical pediatrics at the University of Southern California, Los Angeles, told attendees at the meeting.
Dr. Yeh and her colleagues collected cord blood from more than 500 mothers at LAC+USC Medical Center from October 2021 to April 2022 and tested them for IgG antibodies against three SARS-CoV-2 antigens: nucleoprotein (N), receptor-binding domain (RBD), and spike protein (S1). Results with an IgG mean fluorescence intensity (MFI) above 700 were considered positive for IgG antibodies. A positive result for N as well as RBD or S1 indicated a natural infection while a positive result for only RBD or S1 indicated a vaccine response or past infection.
The researchers also tested a subset of the IgG positive samples for IgM and IgA antibodies against N, S1, and RBD, with an IgM MFI greater than 24 and an IgA MFI greater than 102 used as the thresholds for positive results.
Among 384 cord blood samples analyzed, 85.4% were positive for IgG against RBD, indicating that the mother had SARS-CoV-2 immunity from either a past infection or vaccination. Of these anti-RBD positive samples, 60.7% were anti-N IgG negative, suggesting that N had waned since vaccination or the past infection.
Since the other 39.3% that were anti-N IgG positive suggest a past maternal infection, the researchers assessed these 129 samples for IgM and IgA antibodies against RBD. They found that 16 of them had high levels of anti-RBD IgA and/or IgM antibodies, pointing to a rate of mother-to-child-transmission of up to 12.4%.
Sallie Permar, MD, PhD, a professor and the chair of pediatrics at Weill Cornell Medicine in New York, who was not involved in the research, said most studies of placental transmission have focused on virologic testing, such as PCR. “Serologic tests for congenital infections are inherently challenged by the transfer of maternal IgG across the placenta and therefore must rely on non-IgG isotype response detection, which have inherently been more susceptible to false-positive results than IgG-based tests,” Dr. Permar said.
Also, “it is unclear if virologic testing was performed in the infants, which, if positive in the same infants for which cord blood IgM/IgA responses were identified, could further validate positive serologic findings,” added Dr. Permar, who is also pediatrician-in-chief at New York-Presbyterian Komansky Children’s Hospital.
Given these limitations, Dr. Permar reiterated that diagnostics for congenital SARS-CoV-2 continue to evolve, even if congenital SARS-CoV-2 infection currently appears rare. Dr. Permar said she agreed with Dr. Yeh that following those who do develop this infection is important.
“There have been initial reports of neurodevelopmental and other outcomes from long-term follow-up cohorts of infants exposed to SARS-CoV-2 infection in utero with variable results and it should continue to be pursued using cohorts both enrolled early in the pandemic and those enrolled more recently after population-level immunity to SARS-CoV-2 was achieved,” said Dr. Permar.
Dr. Permar serves as a consultant to Moderna, Pfizer, Merck, Dynavax, and Hoopika on their CMV vaccine programs and has led sponsored research programs with Moderna and Merck. Information on study funding and on disclosures for Dr. Yeh was unavailable.
ANAHEIM, CALIF. – The rate of mother-to-child transmission of SARS-CoV-2 infection is likely higher than the current estimate of 2%-8%, suggests a recent study using cord blood serology to determine incidence. The study was presented at the American Academy of Pediatrics National Conference.
“Cord blood screening is a potential tool to identify SARS-CoV-2 infected and/or exposed neonates who should then be followed for long-term consequences of mother-to-child transmission,” Amy Yeh, MD, an assistant professor of clinical pediatrics at the University of Southern California, Los Angeles, told attendees at the meeting.
Dr. Yeh and her colleagues collected cord blood from more than 500 mothers at LAC+USC Medical Center from October 2021 to April 2022 and tested them for IgG antibodies against three SARS-CoV-2 antigens: nucleoprotein (N), receptor-binding domain (RBD), and spike protein (S1). Results with an IgG mean fluorescence intensity (MFI) above 700 were considered positive for IgG antibodies. A positive result for N as well as RBD or S1 indicated a natural infection while a positive result for only RBD or S1 indicated a vaccine response or past infection.
The researchers also tested a subset of the IgG positive samples for IgM and IgA antibodies against N, S1, and RBD, with an IgM MFI greater than 24 and an IgA MFI greater than 102 used as the thresholds for positive results.
Among 384 cord blood samples analyzed, 85.4% were positive for IgG against RBD, indicating that the mother had SARS-CoV-2 immunity from either a past infection or vaccination. Of these anti-RBD positive samples, 60.7% were anti-N IgG negative, suggesting that N had waned since vaccination or the past infection.
Since the other 39.3% that were anti-N IgG positive suggest a past maternal infection, the researchers assessed these 129 samples for IgM and IgA antibodies against RBD. They found that 16 of them had high levels of anti-RBD IgA and/or IgM antibodies, pointing to a rate of mother-to-child-transmission of up to 12.4%.
Sallie Permar, MD, PhD, a professor and the chair of pediatrics at Weill Cornell Medicine in New York, who was not involved in the research, said most studies of placental transmission have focused on virologic testing, such as PCR. “Serologic tests for congenital infections are inherently challenged by the transfer of maternal IgG across the placenta and therefore must rely on non-IgG isotype response detection, which have inherently been more susceptible to false-positive results than IgG-based tests,” Dr. Permar said.
Also, “it is unclear if virologic testing was performed in the infants, which, if positive in the same infants for which cord blood IgM/IgA responses were identified, could further validate positive serologic findings,” added Dr. Permar, who is also pediatrician-in-chief at New York-Presbyterian Komansky Children’s Hospital.
Given these limitations, Dr. Permar reiterated that diagnostics for congenital SARS-CoV-2 continue to evolve, even if congenital SARS-CoV-2 infection currently appears rare. Dr. Permar said she agreed with Dr. Yeh that following those who do develop this infection is important.
“There have been initial reports of neurodevelopmental and other outcomes from long-term follow-up cohorts of infants exposed to SARS-CoV-2 infection in utero with variable results and it should continue to be pursued using cohorts both enrolled early in the pandemic and those enrolled more recently after population-level immunity to SARS-CoV-2 was achieved,” said Dr. Permar.
Dr. Permar serves as a consultant to Moderna, Pfizer, Merck, Dynavax, and Hoopika on their CMV vaccine programs and has led sponsored research programs with Moderna and Merck. Information on study funding and on disclosures for Dr. Yeh was unavailable.
AT AAP 2022
Three COVID scenarios that could spell trouble for the fall
As the United States enters a third fall with COVID-19, the virus for many is seemingly gone – or at least out of mind. But for those keeping watch, it is far from forgotten as deaths and infections continue to mount at a lower but steady pace.
What does that mean for the upcoming months? Experts predict different scenarios, some more dire than others – with one more encouraging.
In the United States, more than 300 people still die every day from COVID and more than 44,000 new daily cases are reported, according to the Centers for Disease Control and Prevention.
But progress is undeniable. The stark daily death tolls of 2020 have plummeted. Vaccines and treatments have dramatically reduced severe illness, and mask requirements have mostly turned to personal preference.
among them more-resistant variants coupled with waning immunity, the potential for a “twindemic” with a flu/COVID onslaught, and underuse of lifesaving vaccines and treatments.
Variants loom/waning immunity
Omicron variant BA.5 still makes up about 80% of infections in the United States, followed by BA4.6, according to the CDC, but other subvariants are emerging and showing signs of resistance to current antiviral treatments.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego, said about COVID this fall: “There will be another wave, magnitude unknown.”
He said subvariants XBB and BQ.1.1 “have extreme levels of immune evasion and both could pose a challenge,” explaining that XBB is more likely to cause trouble than BQ.1.1 because it is even more resistant to natural or vaccine-induced immunity.
Dr. Topol pointed to new research on those variants in a preprint posted on bioRxiv. The authors’ conclusion: “These results suggest that current herd immunity and BA.5 vaccine boosters may not provide sufficiently broad protection against infection.”
Another variant to watch, some experts say, is Omicron subvariant BA.2.75.2, which has shown resistance to antiviral treatments. It is also growing at a rather alarming rate, says Michael Sweat, PhD, director of the Medical University of South Carolina Center for Global Health in Charleston. That subvariant currently makes up under 2% of U.S. cases but has spread to at least 55 countries and 43 U.S. states after first appearing at the end of last year globally and in mid-June in the United States.
A non–peer-reviewed preprint study from Sweden found that the variant in blood samples was neutralized on average “at titers approximately 6.5 times lower than BA.5, making BA.2.75.2 the most [neutralization-resistant] variant evaluated to date.”
Katelyn Jetelina, PhD, assistant professor in the department of epidemiology at University of Texas Health Science Center, Houston, said in an interview the U.S. waves often follow Europe’s, and Europe has seen a recent spike in cases and hospitalizations not related to Omicron subvariants, but to weather changes, waning immunity, and changes in behavior.
The World Health Organization reported on Oct. 5 that, while cases were down in every other region of the world, Europe’s numbers stand out, with an 8% increase in cases from the week before.
Dr. Jetelina cited events such as Oktoberfest in Germany, which ended in the first week of October after drawing nearly 6 million people over 2 weeks, as a potential contributor, and people heading indoors as weather patterns change in Europe.
Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said in an interview he is less worried about the documented variants we know about than he is about the potential for a new immune-escape variety yet to emerge.
“Right now we know the Chinese are gearing up to open up the country, and because they have low immunity and little infection, we expect in China there will be a lot of spread of Omicron,” he said. “It’s possible because of the number of infections we could see a new variant.”
Dr. Mokdad said waning immunity could also leave populations vulnerable to variants.
“Even if you get infected, after about 5 months, you’re susceptible again. Remember, most of the infections from Omicron happened in January or February 2022, and we had two waves after that,” he said.
The new bivalent vaccines tweaked to target some Omicron variants will help, Dr. Mokdad said, but he noted, “people are very reluctant to take it.”
Jennifer Nuzzo, DrPH, professor of epidemiology and director of the Pandemic Center at Brown University, Providence, R.I., worries that in the United States we have less ability this year to track variants as funding has receded for testing kits and testing sites. Most people are testing at home – which doesn’t show up in the numbers – and the United States is relying more on other countries’ data to spot trends.
“I think we’re just going to have less visibility into the circulation of this virus,” she said in an interview.
‘Twindemic’: COVID and flu
Dr. Jetelina noted Australia and New Zealand just wrapped up a flu season that saw flu numbers returning to normal after a sharp drop in the last 2 years, and North America typically follows suit.
“We do expect flu will be here in the United States and probably at levels that we saw prepandemic. We’re all holding our breath to see how our health systems hold up with COVID-19 and flu. We haven’t really experienced that yet,” she said.
There is some disagreement, however, about the possibility of a so-called “twindemic” of influenza and COVID.
Richard Webby, PhD, an infectious disease specialist at St. Jude Children’s Research Hospital in Memphis, said in an interview he thinks the possibility of both viruses spiking at the same time is unlikely.
“That’s not to say we won’t get flu and COVID activity in the same winter,” he explained, “but I think both roaring at the same time is unlikely.”
As an indicator, he said, at the beginning of the flu season last year in the Northern Hemisphere, flu activity started to pick up, but when the Omicron variant came along, “flu just wasn’t able to compete in that same environment and flu numbers dropped right off.” Previous literature suggests that when one virus is spiking it’s hard for another respiratory virus to take hold.
Vaccine, treatment underuse
Another threat is vaccines, boosters, and treatments sitting on shelves.
Dr. Sweat referred to frustration with vaccine uptake that seems to be “frozen in amber.”
As of Oct. 4, only 5.3% of people in the United States who were eligible had received the updated booster launched in early September.
Dr. Nuzzo said boosters for people at least 65 years old will be key to severity of COVID this season.
“I think that’s probably the biggest factor going into the fall and winter,” she said.
Only 38% of people at least 50 years old and 45% of those at least 65 years old had gotten a second booster as of early October.
“If we do nothing else, we have to increase booster uptake in that group,” Dr. Nuzzo said.
She said the treatment nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is greatly underused, often because providers aren’t prescribing it because they don’t think it helps, are worried about drug interactions, or are worried about its “rebound” effect.
Dr. Nuzzo urged greater use of the drug and education on how to manage drug interactions.
“We have very strong data that it does help keep people out of hospital. Sure, there may be a rebound, but that pales in comparison to the risk of being hospitalized,” she said.
Calm COVID season?
Not all predictions are dire. There is another little-talked-about scenario, Dr. Sweat said – that we could be in for a calm COVID season, and those who seem to be only mildly concerned about COVID may find those thoughts justified in the numbers.
Omicron blew through with such strength, he noted, that it may have left wide immunity in its wake. Because variants seem to be staying in the Omicron family, that may signal optimism.
“If the next variant is a descendant of the Omicron lineage, I would suspect that all these people who just got infected will have some protection, not perfect, but quite a bit of protection,” Dr. Sweat said.
Dr. Topol, Dr. Nuzzo, Dr. Sweat, Dr. Webby, Dr. Mokdad, and Dr. Jetelina reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As the United States enters a third fall with COVID-19, the virus for many is seemingly gone – or at least out of mind. But for those keeping watch, it is far from forgotten as deaths and infections continue to mount at a lower but steady pace.
What does that mean for the upcoming months? Experts predict different scenarios, some more dire than others – with one more encouraging.
In the United States, more than 300 people still die every day from COVID and more than 44,000 new daily cases are reported, according to the Centers for Disease Control and Prevention.
But progress is undeniable. The stark daily death tolls of 2020 have plummeted. Vaccines and treatments have dramatically reduced severe illness, and mask requirements have mostly turned to personal preference.
among them more-resistant variants coupled with waning immunity, the potential for a “twindemic” with a flu/COVID onslaught, and underuse of lifesaving vaccines and treatments.
Variants loom/waning immunity
Omicron variant BA.5 still makes up about 80% of infections in the United States, followed by BA4.6, according to the CDC, but other subvariants are emerging and showing signs of resistance to current antiviral treatments.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego, said about COVID this fall: “There will be another wave, magnitude unknown.”
He said subvariants XBB and BQ.1.1 “have extreme levels of immune evasion and both could pose a challenge,” explaining that XBB is more likely to cause trouble than BQ.1.1 because it is even more resistant to natural or vaccine-induced immunity.
Dr. Topol pointed to new research on those variants in a preprint posted on bioRxiv. The authors’ conclusion: “These results suggest that current herd immunity and BA.5 vaccine boosters may not provide sufficiently broad protection against infection.”
Another variant to watch, some experts say, is Omicron subvariant BA.2.75.2, which has shown resistance to antiviral treatments. It is also growing at a rather alarming rate, says Michael Sweat, PhD, director of the Medical University of South Carolina Center for Global Health in Charleston. That subvariant currently makes up under 2% of U.S. cases but has spread to at least 55 countries and 43 U.S. states after first appearing at the end of last year globally and in mid-June in the United States.
A non–peer-reviewed preprint study from Sweden found that the variant in blood samples was neutralized on average “at titers approximately 6.5 times lower than BA.5, making BA.2.75.2 the most [neutralization-resistant] variant evaluated to date.”
Katelyn Jetelina, PhD, assistant professor in the department of epidemiology at University of Texas Health Science Center, Houston, said in an interview the U.S. waves often follow Europe’s, and Europe has seen a recent spike in cases and hospitalizations not related to Omicron subvariants, but to weather changes, waning immunity, and changes in behavior.
The World Health Organization reported on Oct. 5 that, while cases were down in every other region of the world, Europe’s numbers stand out, with an 8% increase in cases from the week before.
Dr. Jetelina cited events such as Oktoberfest in Germany, which ended in the first week of October after drawing nearly 6 million people over 2 weeks, as a potential contributor, and people heading indoors as weather patterns change in Europe.
Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said in an interview he is less worried about the documented variants we know about than he is about the potential for a new immune-escape variety yet to emerge.
“Right now we know the Chinese are gearing up to open up the country, and because they have low immunity and little infection, we expect in China there will be a lot of spread of Omicron,” he said. “It’s possible because of the number of infections we could see a new variant.”
Dr. Mokdad said waning immunity could also leave populations vulnerable to variants.
“Even if you get infected, after about 5 months, you’re susceptible again. Remember, most of the infections from Omicron happened in January or February 2022, and we had two waves after that,” he said.
The new bivalent vaccines tweaked to target some Omicron variants will help, Dr. Mokdad said, but he noted, “people are very reluctant to take it.”
Jennifer Nuzzo, DrPH, professor of epidemiology and director of the Pandemic Center at Brown University, Providence, R.I., worries that in the United States we have less ability this year to track variants as funding has receded for testing kits and testing sites. Most people are testing at home – which doesn’t show up in the numbers – and the United States is relying more on other countries’ data to spot trends.
“I think we’re just going to have less visibility into the circulation of this virus,” she said in an interview.
‘Twindemic’: COVID and flu
Dr. Jetelina noted Australia and New Zealand just wrapped up a flu season that saw flu numbers returning to normal after a sharp drop in the last 2 years, and North America typically follows suit.
“We do expect flu will be here in the United States and probably at levels that we saw prepandemic. We’re all holding our breath to see how our health systems hold up with COVID-19 and flu. We haven’t really experienced that yet,” she said.
There is some disagreement, however, about the possibility of a so-called “twindemic” of influenza and COVID.
Richard Webby, PhD, an infectious disease specialist at St. Jude Children’s Research Hospital in Memphis, said in an interview he thinks the possibility of both viruses spiking at the same time is unlikely.
“That’s not to say we won’t get flu and COVID activity in the same winter,” he explained, “but I think both roaring at the same time is unlikely.”
As an indicator, he said, at the beginning of the flu season last year in the Northern Hemisphere, flu activity started to pick up, but when the Omicron variant came along, “flu just wasn’t able to compete in that same environment and flu numbers dropped right off.” Previous literature suggests that when one virus is spiking it’s hard for another respiratory virus to take hold.
Vaccine, treatment underuse
Another threat is vaccines, boosters, and treatments sitting on shelves.
Dr. Sweat referred to frustration with vaccine uptake that seems to be “frozen in amber.”
As of Oct. 4, only 5.3% of people in the United States who were eligible had received the updated booster launched in early September.
Dr. Nuzzo said boosters for people at least 65 years old will be key to severity of COVID this season.
“I think that’s probably the biggest factor going into the fall and winter,” she said.
Only 38% of people at least 50 years old and 45% of those at least 65 years old had gotten a second booster as of early October.
“If we do nothing else, we have to increase booster uptake in that group,” Dr. Nuzzo said.
She said the treatment nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is greatly underused, often because providers aren’t prescribing it because they don’t think it helps, are worried about drug interactions, or are worried about its “rebound” effect.
Dr. Nuzzo urged greater use of the drug and education on how to manage drug interactions.
“We have very strong data that it does help keep people out of hospital. Sure, there may be a rebound, but that pales in comparison to the risk of being hospitalized,” she said.
Calm COVID season?
Not all predictions are dire. There is another little-talked-about scenario, Dr. Sweat said – that we could be in for a calm COVID season, and those who seem to be only mildly concerned about COVID may find those thoughts justified in the numbers.
Omicron blew through with such strength, he noted, that it may have left wide immunity in its wake. Because variants seem to be staying in the Omicron family, that may signal optimism.
“If the next variant is a descendant of the Omicron lineage, I would suspect that all these people who just got infected will have some protection, not perfect, but quite a bit of protection,” Dr. Sweat said.
Dr. Topol, Dr. Nuzzo, Dr. Sweat, Dr. Webby, Dr. Mokdad, and Dr. Jetelina reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As the United States enters a third fall with COVID-19, the virus for many is seemingly gone – or at least out of mind. But for those keeping watch, it is far from forgotten as deaths and infections continue to mount at a lower but steady pace.
What does that mean for the upcoming months? Experts predict different scenarios, some more dire than others – with one more encouraging.
In the United States, more than 300 people still die every day from COVID and more than 44,000 new daily cases are reported, according to the Centers for Disease Control and Prevention.
But progress is undeniable. The stark daily death tolls of 2020 have plummeted. Vaccines and treatments have dramatically reduced severe illness, and mask requirements have mostly turned to personal preference.
among them more-resistant variants coupled with waning immunity, the potential for a “twindemic” with a flu/COVID onslaught, and underuse of lifesaving vaccines and treatments.
Variants loom/waning immunity
Omicron variant BA.5 still makes up about 80% of infections in the United States, followed by BA4.6, according to the CDC, but other subvariants are emerging and showing signs of resistance to current antiviral treatments.
Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego, said about COVID this fall: “There will be another wave, magnitude unknown.”
He said subvariants XBB and BQ.1.1 “have extreme levels of immune evasion and both could pose a challenge,” explaining that XBB is more likely to cause trouble than BQ.1.1 because it is even more resistant to natural or vaccine-induced immunity.
Dr. Topol pointed to new research on those variants in a preprint posted on bioRxiv. The authors’ conclusion: “These results suggest that current herd immunity and BA.5 vaccine boosters may not provide sufficiently broad protection against infection.”
Another variant to watch, some experts say, is Omicron subvariant BA.2.75.2, which has shown resistance to antiviral treatments. It is also growing at a rather alarming rate, says Michael Sweat, PhD, director of the Medical University of South Carolina Center for Global Health in Charleston. That subvariant currently makes up under 2% of U.S. cases but has spread to at least 55 countries and 43 U.S. states after first appearing at the end of last year globally and in mid-June in the United States.
A non–peer-reviewed preprint study from Sweden found that the variant in blood samples was neutralized on average “at titers approximately 6.5 times lower than BA.5, making BA.2.75.2 the most [neutralization-resistant] variant evaluated to date.”
Katelyn Jetelina, PhD, assistant professor in the department of epidemiology at University of Texas Health Science Center, Houston, said in an interview the U.S. waves often follow Europe’s, and Europe has seen a recent spike in cases and hospitalizations not related to Omicron subvariants, but to weather changes, waning immunity, and changes in behavior.
The World Health Organization reported on Oct. 5 that, while cases were down in every other region of the world, Europe’s numbers stand out, with an 8% increase in cases from the week before.
Dr. Jetelina cited events such as Oktoberfest in Germany, which ended in the first week of October after drawing nearly 6 million people over 2 weeks, as a potential contributor, and people heading indoors as weather patterns change in Europe.
Ali Mokdad, PhD, chief strategy officer for population health at the University of Washington, Seattle, said in an interview he is less worried about the documented variants we know about than he is about the potential for a new immune-escape variety yet to emerge.
“Right now we know the Chinese are gearing up to open up the country, and because they have low immunity and little infection, we expect in China there will be a lot of spread of Omicron,” he said. “It’s possible because of the number of infections we could see a new variant.”
Dr. Mokdad said waning immunity could also leave populations vulnerable to variants.
“Even if you get infected, after about 5 months, you’re susceptible again. Remember, most of the infections from Omicron happened in January or February 2022, and we had two waves after that,” he said.
The new bivalent vaccines tweaked to target some Omicron variants will help, Dr. Mokdad said, but he noted, “people are very reluctant to take it.”
Jennifer Nuzzo, DrPH, professor of epidemiology and director of the Pandemic Center at Brown University, Providence, R.I., worries that in the United States we have less ability this year to track variants as funding has receded for testing kits and testing sites. Most people are testing at home – which doesn’t show up in the numbers – and the United States is relying more on other countries’ data to spot trends.
“I think we’re just going to have less visibility into the circulation of this virus,” she said in an interview.
‘Twindemic’: COVID and flu
Dr. Jetelina noted Australia and New Zealand just wrapped up a flu season that saw flu numbers returning to normal after a sharp drop in the last 2 years, and North America typically follows suit.
“We do expect flu will be here in the United States and probably at levels that we saw prepandemic. We’re all holding our breath to see how our health systems hold up with COVID-19 and flu. We haven’t really experienced that yet,” she said.
There is some disagreement, however, about the possibility of a so-called “twindemic” of influenza and COVID.
Richard Webby, PhD, an infectious disease specialist at St. Jude Children’s Research Hospital in Memphis, said in an interview he thinks the possibility of both viruses spiking at the same time is unlikely.
“That’s not to say we won’t get flu and COVID activity in the same winter,” he explained, “but I think both roaring at the same time is unlikely.”
As an indicator, he said, at the beginning of the flu season last year in the Northern Hemisphere, flu activity started to pick up, but when the Omicron variant came along, “flu just wasn’t able to compete in that same environment and flu numbers dropped right off.” Previous literature suggests that when one virus is spiking it’s hard for another respiratory virus to take hold.
Vaccine, treatment underuse
Another threat is vaccines, boosters, and treatments sitting on shelves.
Dr. Sweat referred to frustration with vaccine uptake that seems to be “frozen in amber.”
As of Oct. 4, only 5.3% of people in the United States who were eligible had received the updated booster launched in early September.
Dr. Nuzzo said boosters for people at least 65 years old will be key to severity of COVID this season.
“I think that’s probably the biggest factor going into the fall and winter,” she said.
Only 38% of people at least 50 years old and 45% of those at least 65 years old had gotten a second booster as of early October.
“If we do nothing else, we have to increase booster uptake in that group,” Dr. Nuzzo said.
She said the treatment nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is greatly underused, often because providers aren’t prescribing it because they don’t think it helps, are worried about drug interactions, or are worried about its “rebound” effect.
Dr. Nuzzo urged greater use of the drug and education on how to manage drug interactions.
“We have very strong data that it does help keep people out of hospital. Sure, there may be a rebound, but that pales in comparison to the risk of being hospitalized,” she said.
Calm COVID season?
Not all predictions are dire. There is another little-talked-about scenario, Dr. Sweat said – that we could be in for a calm COVID season, and those who seem to be only mildly concerned about COVID may find those thoughts justified in the numbers.
Omicron blew through with such strength, he noted, that it may have left wide immunity in its wake. Because variants seem to be staying in the Omicron family, that may signal optimism.
“If the next variant is a descendant of the Omicron lineage, I would suspect that all these people who just got infected will have some protection, not perfect, but quite a bit of protection,” Dr. Sweat said.
Dr. Topol, Dr. Nuzzo, Dr. Sweat, Dr. Webby, Dr. Mokdad, and Dr. Jetelina reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Evusheld PrEP may protect immunocompromised patients from severe COVID-19
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
Tixagevimab copackaged with cilgavimab (Evusheld) is a safe and effective preexposure prophylaxis (PrEP) in patients undergoing B-cell-depleting therapies who have poor immune response to COVID-19 vaccination and are at high risk for serious COVID-19 illness, a small, single-site study suggests.
Evusheld, the only COVID-19 PrEP option available, has Emergency Use Authorization (EUA) from the Food and Drug Administration for treatment of immunocompromised patients who may not respond sufficiently to COVID-19 vaccination and patients who’ve had a severe adverse reaction to COVID-19 vaccination.
“We report the largest real-world experience of Evusheld in this population, and our findings are encouraging,” lead study author Cassandra Calabrese, DO, rheumatologist and infectious disease specialist at Cleveland Clinic, said in an interview.
“Of 412 patients who received Evusheld, 12 [2.9%] developed breakthrough COVID-19, with 11 having mild courses and 1 who required hospitalization but recovered,” she added.
“Our data suggest that Evusheld PrEP, in combination with aggressive outpatient treatment of COVID-19, is likely effective in lowering risk of severe COVID in this vulnerable group.
“Practitioners who care for patients with immune-mediated inflammatory diseases should triage high-risk patients for Evusheld as well as rapid diagnosis and aggressive outpatient therapy if infected,” Dr. Calabrese advised.
For the study, Dr. Calabrese and colleagues at Cleveland Clinic searched the health care system pharmacy records for patients with immune‐mediated inflammatory diseases (IMIDs) or inborn errors of humoral immunity (IEI) who met the criteria to receive Evusheld. The researchers included patients on B-cell-depleting therapies or with humoral IEI who had received at least one dose of Evusheld and were later diagnosed with COVID-19, and they excluded those treated with B-cell-depleting therapies for cancer.
EVUSHELD was well tolerated
After extracting data on COVID-19 infection, vaccination status, and outcomes, they found that, between Jan. 18 and May 28, 2022, 412 patients with IMIDs or humoral IEI received Evusheld. No deaths occurred among these patients and, overall, they tolerated the medication well.
All 12 patients who experienced breakthrough COVID-19 infection were treated with B-cell-depleting therapies. Among the 12 patients:
- Six patients developed infection 13-84 (median 19) days after receiving 150 mg/150 mg tixagevimab/cilgavimab.
- Six patients developed infection 19-72 (median of 38.5) days after either a single dose of 300 mg/300 mg or a second dose of 150 mg/150 mg.
- Eleven patients had mild illness and recovered at home; one patient was hospitalized and treated with high-flow oxygen. All cases had been vaccinated against COVID-19 (five received two vaccinations, six received three, and one received four).
- One possible serious adverse event involved a patient with COVID-19 and immune-mediated thrombocytopenia (ITP) who was hospitalized soon after receiving Evusheld with ITP flare that resolved with intravenous immunoglobulin.
Dr. Calabrese acknowledged limitations to the study, including few patients, lack of a comparator group, and the study period falling during the Omicron wave.
“Also, nine of the breakthrough cases received additional COVID-19 therapy (oral antiviral or monoclonal antibody), which falls within standard of care for this high-risk group but prevents ascribing effectiveness to individual components of the regimen,” she added.
“Evusheld is authorized for PrEP against COVID-19 in patients at high risk for severe COVID due to suboptimal vaccine responses. This includes patients receiving B-cell-depleting drugs like rituximab, and patients with inborn errors of humoral immunity,” Dr. Calabrese explained.
“It is well known that this group of patients is at very high risk for severe COVID and death, even when fully vaccinated, and it has become clear that more strategies are needed to protect this vulnerable group, including use of Evusheld as well as aggressive treatment if infected,” she added.
Evusheld not always easy to obtain
Although the medication has been available in the United States since January 2022, Dr. Calabrese said, patients may not receive it because of barriers including lack of both awareness and access.
Davey Smith, MD, professor of medicine and head of infectious diseases and global public health at the University of California San Diego, in La Jolla, said in an interview that he was not surprised by the results, but added that the study was conducted in too few patients to draw any strong conclusions or affect patient care.
“This small study that showed that breakthrough infections occurred but were generally mild, provides a small glimpse of real-world use of tixagevimab/cilgavimab as PrEP for immunocompromised persons,” said Dr. Smith, who was not involved in the study.
“In the setting of Omicron and vaccination, I would expect the same outcomes reported even without the treatment,” he added.
Dr. Smith recommends larger related randomized, controlled trials to provide clinicians with sufficient data to guide them in their patient care.
Graham Snyder, MD, associate professor in the division of infectious diseases at the University of Pittsburgh and medical director of infection prevention and hospital epidemiology at the University of Pittsburgh Medical Center, noted that the study “adds to a quickly growing literature on the real-world benefits of tixagevimab/cilgavimab to protect vulnerable individuals with weakened immune systems from the complications of COVID-19.
“This study provides a modest addition to our understanding of the role and benefit of Evusheld,” Dr. Snyder said in an interview. “By characterizing only patients who have received Evusheld without an untreated comparison group, we can’t draw any inference about the extent of benefit the agent provided to these patients.
“Substantial data already show that this agent is effective in preventing complications of COVID-19 infection in immunocompromised individuals,” added Dr. Snyder, who was not involved in the study.
“ ‘Immunocompromised’ represents a very diverse set of clinical conditions,” he said. “The research agenda should therefore focus on a more refined description of the effect in specific populations and a continued understanding of the effect of Evusheld in the context of updated vaccination strategies and changing virus ecology.”
Dr. Calabrese and her colleagues wrote that larger, controlled trials are underway.
FDA: Evusheld may not neutralize certain SARS-CoV-2 variants
“The biggest unanswered question is how Evusheld will hold up against new variants,” Dr. Calabrese said.
In an Oct. 3, 2022, update, the Food and Drug Administration released a statement about the risk of developing COVID-19 from SARS-CoV-2 variants that are not neutralized by Evusheld. The statement mentions an updated fact sheet that describes reduced protection from Evusheld against the Omicron subvariant BA.4.6, which accounted for nearly 13% of all new COVID-19 cases in the United States in the week ending Oct. 1.
There was no outside funding for the study. Dr. Smith reported no relevant financial conflicts of interest. Dr. Snyder said he is an unpaid adviser to an AstraZeneca observational study that’s assessing the real-world effectiveness of Evusheld.
FROM RMD OPEN
Children and COVID: Weekly cases dropped by 57% in September
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.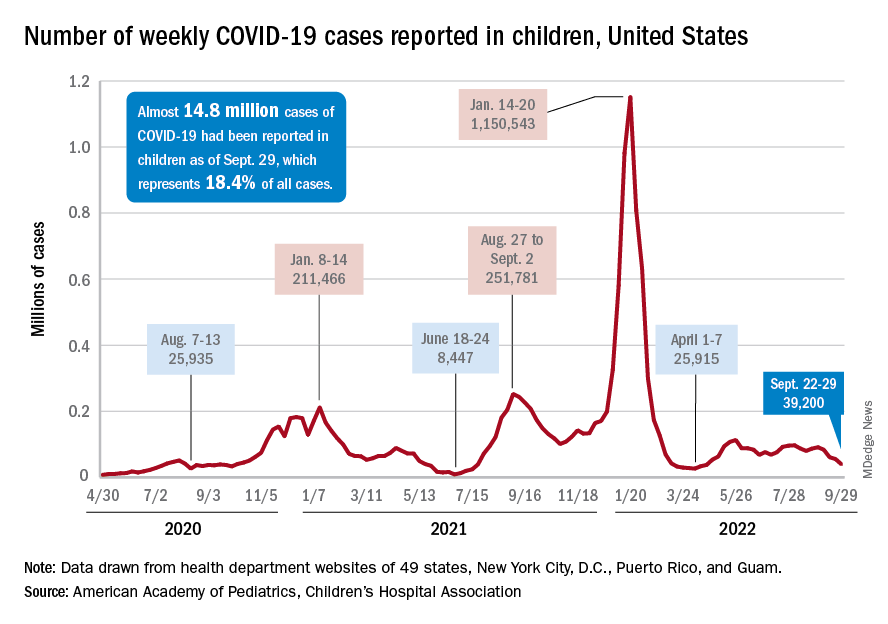
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
Shortage of family physicians in Canada intensified during pandemic
A higher percentage of family physicians quit during the early months of the pandemic than the average yearly percentage that did in the prior decade, according to data from Canada.
The researchers conducted two analyses of billing claims data for family physicians practicing in Ontario. They examined data for a period from 2010 to 2019 – before the onset of the pandemic – and from 2019 through 2020. The findings were published in Annals of Family Medicine.
Overall, the proportion of family physicians who stopped working rose from an average of 1.6% each year for the period between 2010 and 2019 to 3% in the period from 2019 to 2020. The pandemic data set included 12,247 physicians in Ontario. Of these, 385 (3.1%) reported no billings in the first 6 months of the pandemic.
Compared with family physicians billing for work during the pandemic, those reporting no billings were significantly more likely to be 75 years or older (13.0% vs. 3.4%), to have patient panels of less than 500 patients (40.0% vs. 25.8%), and to be eligible for fee-for-service reimbursement (37.7% vs. 24.9%; P less than .001 for all). The family physicians who reported no billing early in the pandemic also had fewer billing days in the previous year (mean of 73 days vs. 101 days, P less than .001).
In a regression analysis, the absolute increase in the percentage of family physicians who stopped working was 0.3% per year from 2010 to 2019, but rose to 1.2% between 2019 and 2020.
Challenges to family physicians in Ontario in the early months of the COVID-19 pandemic included reduced revenue, inability to keep offices fully staffed, and problems obtaining enough personal protective equipment. Such challenges may have prompted some family physicians to stop working prematurely, but more research is needed in other settings, wrote study author Tara Kiran, MD, of the University of Toronto, and colleagues.
“There were a lot of stories and suggestions that more family physicians were choosing to retire due to COVID,” Michael Green, MD, a coauthor of the paper, said in an interview. “Given the preexisting shortages we thought it would be important to see if this was true, and how big of an issue it was,” he said.
Although the absolute number of primary care physicians who stopped working is small, the implications are large given the ongoing shortage of family physicians in Canada, the researchers wrote.
The characteristics of physicians stopping work, such as older age and smaller practice size, were consistent with that of physicians preparing for retirement, the researchers noted. In addition, 56% of the family physicians who stopped working during the pandemic practiced in a patient enrollment model, in which patients are enrolled and between 15% and 70% of payment is based on age and sex. In this study, approximately 80% of physicians worked in this model. The remaining 20% operated in independent, fee-for-service practices.
“Although we cannot directly attribute causation, we hypothesize that some family physicians accelerated their retirement plans because of the pandemic,” the researchers noted. They proposed that possible reasons include health concerns, increased costs of infection prevention and control, reduced revenue from office visits, and burnout. The current study did not examine these issues.
Additional studies are needed to understand the impact on population health, the researchers concluded, but they estimated that the number of family physicians who stopped work during the pandemic would have provided care for approximately 170,000 patients.
The study findings reflect a genuine turnover by family physicians, vs. a departure from family practice to a fellowship and practice in another specialty, Dr. Green said. “We looked at physician billings to determine who stopped practicing, so we report only on those who stopped billing the Ontario Health Insurance Program altogether,” he explained.
The ongoing pandemic accelerated the issue of an upcoming wave of physician retirements and added to an already large number of people without a family physician, Dr. Green noted.
“We know there will be significant shortages of family physicians if we don’t modernize our ways of delivering primary care,” said Dr. Green. More research is needed on how to support family doctors with teams and administrative supports to allow them to provide high quality care to more patients, he said. Better models to estimate health workforce needs in primary care are needed as well, he added.
In the United States, a physician shortage has been growing since before the pandemic, according to a report published in 2021 by the Association of American Medical Colleges. In this report, “The Complexities of Physician Supply and Demand: Projections from 2019 to 2034,” the authors specifically projected a primary care physician shortage of 17,800 to 48,000 by 2034. This projection is in part based on an increase in the percentage of the U.S. population aged 65 years and older, which will increase the demand for care, according to the authors. The report also confirmed that many U.S. physicians are approaching retirement age and that more than two of five active physicians will be 65 years or older within the next 10 years.
However, the authors of this U.S. report acknowledged that the impact of the pandemic on existing primary care shortages remains unclear.
“There are still many unknowns about the direct short-term and long-term impacts of COVID-19 on the physician workforce, and it may be several years before those impacts are clearly understood,” they said in the executive summary of their report.
Alison N. Huffstetler, MD, a coauthor of a recent report that tried to identify the active primary care workforce in Virginia, said, “We know from other research that there are not enough primary care doctors, right now, to do the work that needs to be done – some citations have noted it would take a primary care doc over 20 hours a day just to provide preventive care.
“As our population continues to age, live longer, and need more complex care management, we must ensure we have an accountable, accessible, and knowledgeable primary care network to care for our communities,” she said.
Current state of primary care in Virginia
The study by Dr. Huffstetler, of Virginia Commonwealth University, Richmond, and colleagues was published in Annals of Family Medicine. It used a novel strategy involving the analysis of state all-payer claims data to determine how many physicians were practicing primary care in Virginia.
The researchers used the National Plan and Provider Enumeration System (NPPES) and the Virginia All-Payer Claims Database (VA-APCD) and identified all Virginia physicians and their specialties through the NPPES between 2015 and 2019. Active physicians were defined as those with at least one claim in the VA-APCD during the study period. They identified 20,976 active physicians in Virginia, 28.1% of whom were classified as primary care. Of these, 52% were family medicine physicians, 18.5% were internal medicine physicians, 16.8% were pediatricians, 11.8% were ob.gyns., and 0.5% were other specialists.
Clinician specialties were identified via specialty codes from the NPPES. Physicians were identified as primary care providers in two ways. The first way was by identifying those who had a National Uniform Claim Committee (NUCC) taxonomy of family medicine. The NUCC identifies a provider’s specialty using several levels of classification based on board certification and subspecialty certification data. The second identifier was having been a physician who had billed for at least 10 wellness visit codes from Jan. 1, 2019, through Dec. 31, 2019.
Over the 5-year study period (2015-2019), the counts and percentages of primary care physicians in the workforce remained stable, and the overall number of physicians in the state increased by 3.5%, the researchers noted. A total of 60.45% of all physicians and 60.87% of primary care physicians remained active, and 11.66% of all physicians had a claim in only 1 of the 5 years.
How distribution and access impact patients
In an interview, Dr. Huffstetler said the study she and colleagues authored “offers a transparent and reproducible process for identifying primary care physicians in a state, where they practice, and what changes in staffing occur over time.”
“In Virginia, this is particularly important, as we recently expanded Medicaid, making primary care more affordable for over 500,000 people,” she said. “We also saw the importance of distribution and accessibility to primary care over the past 3 years of COVID. In order to adequately prepare for community needs in the coming years, we must know who is providing primary care, and where they are.”
However, the model used in this study has its limitations, Dr. Huffstetler said, including the lack of a definitive definition of primary care using claims data.
“We used a data-informed wellness visit threshold, but it is likely that primary care is delivered in some locations without claims that are reflected by a wellness visit, and we hope to look at scope in the future to help refine these results,” she said.
Canadian study shows pandemic’s impact on patient care
“The pandemic’s impact on primary care remains palpable, and Dr. Kiran’s team has done an excellent analysis on the practice trends during the past several years,” Dr. Huffstetler said.
“The Canadian analysis uses claims in a similar manner to our study; however, it appears that they already knew who the FPs were in Ontario,” Dr. Huffstetler noted. “Their claims threshold of 50 for active practice was higher than ours, at only 1. Should those FPs have moved to a different specialty, the physicians would still have claims for the patients seen in other subspecialties. As such, I don’t suspect that their analysis miscalculated those that transitioned, rather than stopped practice,” she explained.
The Ontario study was supported by the Initial Credential Evaluation Service, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care, as well as by the Canadian Institutes of Health Research. Additional support came from the INSPIRE Primary Health Care Research Program, which is also funded by the Ontario Ministry of Health and Long-Term Care. The researchers had no financial conflicts to disclose.
The Virginia study was supported by the Department of Medical Assistance Services and the National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
The supply and demand report was conducted for the AAMC by IHS Markit, a global information company.
A higher percentage of family physicians quit during the early months of the pandemic than the average yearly percentage that did in the prior decade, according to data from Canada.
The researchers conducted two analyses of billing claims data for family physicians practicing in Ontario. They examined data for a period from 2010 to 2019 – before the onset of the pandemic – and from 2019 through 2020. The findings were published in Annals of Family Medicine.
Overall, the proportion of family physicians who stopped working rose from an average of 1.6% each year for the period between 2010 and 2019 to 3% in the period from 2019 to 2020. The pandemic data set included 12,247 physicians in Ontario. Of these, 385 (3.1%) reported no billings in the first 6 months of the pandemic.
Compared with family physicians billing for work during the pandemic, those reporting no billings were significantly more likely to be 75 years or older (13.0% vs. 3.4%), to have patient panels of less than 500 patients (40.0% vs. 25.8%), and to be eligible for fee-for-service reimbursement (37.7% vs. 24.9%; P less than .001 for all). The family physicians who reported no billing early in the pandemic also had fewer billing days in the previous year (mean of 73 days vs. 101 days, P less than .001).
In a regression analysis, the absolute increase in the percentage of family physicians who stopped working was 0.3% per year from 2010 to 2019, but rose to 1.2% between 2019 and 2020.
Challenges to family physicians in Ontario in the early months of the COVID-19 pandemic included reduced revenue, inability to keep offices fully staffed, and problems obtaining enough personal protective equipment. Such challenges may have prompted some family physicians to stop working prematurely, but more research is needed in other settings, wrote study author Tara Kiran, MD, of the University of Toronto, and colleagues.
“There were a lot of stories and suggestions that more family physicians were choosing to retire due to COVID,” Michael Green, MD, a coauthor of the paper, said in an interview. “Given the preexisting shortages we thought it would be important to see if this was true, and how big of an issue it was,” he said.
Although the absolute number of primary care physicians who stopped working is small, the implications are large given the ongoing shortage of family physicians in Canada, the researchers wrote.
The characteristics of physicians stopping work, such as older age and smaller practice size, were consistent with that of physicians preparing for retirement, the researchers noted. In addition, 56% of the family physicians who stopped working during the pandemic practiced in a patient enrollment model, in which patients are enrolled and between 15% and 70% of payment is based on age and sex. In this study, approximately 80% of physicians worked in this model. The remaining 20% operated in independent, fee-for-service practices.
“Although we cannot directly attribute causation, we hypothesize that some family physicians accelerated their retirement plans because of the pandemic,” the researchers noted. They proposed that possible reasons include health concerns, increased costs of infection prevention and control, reduced revenue from office visits, and burnout. The current study did not examine these issues.
Additional studies are needed to understand the impact on population health, the researchers concluded, but they estimated that the number of family physicians who stopped work during the pandemic would have provided care for approximately 170,000 patients.
The study findings reflect a genuine turnover by family physicians, vs. a departure from family practice to a fellowship and practice in another specialty, Dr. Green said. “We looked at physician billings to determine who stopped practicing, so we report only on those who stopped billing the Ontario Health Insurance Program altogether,” he explained.
The ongoing pandemic accelerated the issue of an upcoming wave of physician retirements and added to an already large number of people without a family physician, Dr. Green noted.
“We know there will be significant shortages of family physicians if we don’t modernize our ways of delivering primary care,” said Dr. Green. More research is needed on how to support family doctors with teams and administrative supports to allow them to provide high quality care to more patients, he said. Better models to estimate health workforce needs in primary care are needed as well, he added.
In the United States, a physician shortage has been growing since before the pandemic, according to a report published in 2021 by the Association of American Medical Colleges. In this report, “The Complexities of Physician Supply and Demand: Projections from 2019 to 2034,” the authors specifically projected a primary care physician shortage of 17,800 to 48,000 by 2034. This projection is in part based on an increase in the percentage of the U.S. population aged 65 years and older, which will increase the demand for care, according to the authors. The report also confirmed that many U.S. physicians are approaching retirement age and that more than two of five active physicians will be 65 years or older within the next 10 years.
However, the authors of this U.S. report acknowledged that the impact of the pandemic on existing primary care shortages remains unclear.
“There are still many unknowns about the direct short-term and long-term impacts of COVID-19 on the physician workforce, and it may be several years before those impacts are clearly understood,” they said in the executive summary of their report.
Alison N. Huffstetler, MD, a coauthor of a recent report that tried to identify the active primary care workforce in Virginia, said, “We know from other research that there are not enough primary care doctors, right now, to do the work that needs to be done – some citations have noted it would take a primary care doc over 20 hours a day just to provide preventive care.
“As our population continues to age, live longer, and need more complex care management, we must ensure we have an accountable, accessible, and knowledgeable primary care network to care for our communities,” she said.
Current state of primary care in Virginia
The study by Dr. Huffstetler, of Virginia Commonwealth University, Richmond, and colleagues was published in Annals of Family Medicine. It used a novel strategy involving the analysis of state all-payer claims data to determine how many physicians were practicing primary care in Virginia.
The researchers used the National Plan and Provider Enumeration System (NPPES) and the Virginia All-Payer Claims Database (VA-APCD) and identified all Virginia physicians and their specialties through the NPPES between 2015 and 2019. Active physicians were defined as those with at least one claim in the VA-APCD during the study period. They identified 20,976 active physicians in Virginia, 28.1% of whom were classified as primary care. Of these, 52% were family medicine physicians, 18.5% were internal medicine physicians, 16.8% were pediatricians, 11.8% were ob.gyns., and 0.5% were other specialists.
Clinician specialties were identified via specialty codes from the NPPES. Physicians were identified as primary care providers in two ways. The first way was by identifying those who had a National Uniform Claim Committee (NUCC) taxonomy of family medicine. The NUCC identifies a provider’s specialty using several levels of classification based on board certification and subspecialty certification data. The second identifier was having been a physician who had billed for at least 10 wellness visit codes from Jan. 1, 2019, through Dec. 31, 2019.
Over the 5-year study period (2015-2019), the counts and percentages of primary care physicians in the workforce remained stable, and the overall number of physicians in the state increased by 3.5%, the researchers noted. A total of 60.45% of all physicians and 60.87% of primary care physicians remained active, and 11.66% of all physicians had a claim in only 1 of the 5 years.
How distribution and access impact patients
In an interview, Dr. Huffstetler said the study she and colleagues authored “offers a transparent and reproducible process for identifying primary care physicians in a state, where they practice, and what changes in staffing occur over time.”
“In Virginia, this is particularly important, as we recently expanded Medicaid, making primary care more affordable for over 500,000 people,” she said. “We also saw the importance of distribution and accessibility to primary care over the past 3 years of COVID. In order to adequately prepare for community needs in the coming years, we must know who is providing primary care, and where they are.”
However, the model used in this study has its limitations, Dr. Huffstetler said, including the lack of a definitive definition of primary care using claims data.
“We used a data-informed wellness visit threshold, but it is likely that primary care is delivered in some locations without claims that are reflected by a wellness visit, and we hope to look at scope in the future to help refine these results,” she said.
Canadian study shows pandemic’s impact on patient care
“The pandemic’s impact on primary care remains palpable, and Dr. Kiran’s team has done an excellent analysis on the practice trends during the past several years,” Dr. Huffstetler said.
“The Canadian analysis uses claims in a similar manner to our study; however, it appears that they already knew who the FPs were in Ontario,” Dr. Huffstetler noted. “Their claims threshold of 50 for active practice was higher than ours, at only 1. Should those FPs have moved to a different specialty, the physicians would still have claims for the patients seen in other subspecialties. As such, I don’t suspect that their analysis miscalculated those that transitioned, rather than stopped practice,” she explained.
The Ontario study was supported by the Initial Credential Evaluation Service, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care, as well as by the Canadian Institutes of Health Research. Additional support came from the INSPIRE Primary Health Care Research Program, which is also funded by the Ontario Ministry of Health and Long-Term Care. The researchers had no financial conflicts to disclose.
The Virginia study was supported by the Department of Medical Assistance Services and the National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
The supply and demand report was conducted for the AAMC by IHS Markit, a global information company.
A higher percentage of family physicians quit during the early months of the pandemic than the average yearly percentage that did in the prior decade, according to data from Canada.
The researchers conducted two analyses of billing claims data for family physicians practicing in Ontario. They examined data for a period from 2010 to 2019 – before the onset of the pandemic – and from 2019 through 2020. The findings were published in Annals of Family Medicine.
Overall, the proportion of family physicians who stopped working rose from an average of 1.6% each year for the period between 2010 and 2019 to 3% in the period from 2019 to 2020. The pandemic data set included 12,247 physicians in Ontario. Of these, 385 (3.1%) reported no billings in the first 6 months of the pandemic.
Compared with family physicians billing for work during the pandemic, those reporting no billings were significantly more likely to be 75 years or older (13.0% vs. 3.4%), to have patient panels of less than 500 patients (40.0% vs. 25.8%), and to be eligible for fee-for-service reimbursement (37.7% vs. 24.9%; P less than .001 for all). The family physicians who reported no billing early in the pandemic also had fewer billing days in the previous year (mean of 73 days vs. 101 days, P less than .001).
In a regression analysis, the absolute increase in the percentage of family physicians who stopped working was 0.3% per year from 2010 to 2019, but rose to 1.2% between 2019 and 2020.
Challenges to family physicians in Ontario in the early months of the COVID-19 pandemic included reduced revenue, inability to keep offices fully staffed, and problems obtaining enough personal protective equipment. Such challenges may have prompted some family physicians to stop working prematurely, but more research is needed in other settings, wrote study author Tara Kiran, MD, of the University of Toronto, and colleagues.
“There were a lot of stories and suggestions that more family physicians were choosing to retire due to COVID,” Michael Green, MD, a coauthor of the paper, said in an interview. “Given the preexisting shortages we thought it would be important to see if this was true, and how big of an issue it was,” he said.
Although the absolute number of primary care physicians who stopped working is small, the implications are large given the ongoing shortage of family physicians in Canada, the researchers wrote.
The characteristics of physicians stopping work, such as older age and smaller practice size, were consistent with that of physicians preparing for retirement, the researchers noted. In addition, 56% of the family physicians who stopped working during the pandemic practiced in a patient enrollment model, in which patients are enrolled and between 15% and 70% of payment is based on age and sex. In this study, approximately 80% of physicians worked in this model. The remaining 20% operated in independent, fee-for-service practices.
“Although we cannot directly attribute causation, we hypothesize that some family physicians accelerated their retirement plans because of the pandemic,” the researchers noted. They proposed that possible reasons include health concerns, increased costs of infection prevention and control, reduced revenue from office visits, and burnout. The current study did not examine these issues.
Additional studies are needed to understand the impact on population health, the researchers concluded, but they estimated that the number of family physicians who stopped work during the pandemic would have provided care for approximately 170,000 patients.
The study findings reflect a genuine turnover by family physicians, vs. a departure from family practice to a fellowship and practice in another specialty, Dr. Green said. “We looked at physician billings to determine who stopped practicing, so we report only on those who stopped billing the Ontario Health Insurance Program altogether,” he explained.
The ongoing pandemic accelerated the issue of an upcoming wave of physician retirements and added to an already large number of people without a family physician, Dr. Green noted.
“We know there will be significant shortages of family physicians if we don’t modernize our ways of delivering primary care,” said Dr. Green. More research is needed on how to support family doctors with teams and administrative supports to allow them to provide high quality care to more patients, he said. Better models to estimate health workforce needs in primary care are needed as well, he added.
In the United States, a physician shortage has been growing since before the pandemic, according to a report published in 2021 by the Association of American Medical Colleges. In this report, “The Complexities of Physician Supply and Demand: Projections from 2019 to 2034,” the authors specifically projected a primary care physician shortage of 17,800 to 48,000 by 2034. This projection is in part based on an increase in the percentage of the U.S. population aged 65 years and older, which will increase the demand for care, according to the authors. The report also confirmed that many U.S. physicians are approaching retirement age and that more than two of five active physicians will be 65 years or older within the next 10 years.
However, the authors of this U.S. report acknowledged that the impact of the pandemic on existing primary care shortages remains unclear.
“There are still many unknowns about the direct short-term and long-term impacts of COVID-19 on the physician workforce, and it may be several years before those impacts are clearly understood,” they said in the executive summary of their report.
Alison N. Huffstetler, MD, a coauthor of a recent report that tried to identify the active primary care workforce in Virginia, said, “We know from other research that there are not enough primary care doctors, right now, to do the work that needs to be done – some citations have noted it would take a primary care doc over 20 hours a day just to provide preventive care.
“As our population continues to age, live longer, and need more complex care management, we must ensure we have an accountable, accessible, and knowledgeable primary care network to care for our communities,” she said.
Current state of primary care in Virginia
The study by Dr. Huffstetler, of Virginia Commonwealth University, Richmond, and colleagues was published in Annals of Family Medicine. It used a novel strategy involving the analysis of state all-payer claims data to determine how many physicians were practicing primary care in Virginia.
The researchers used the National Plan and Provider Enumeration System (NPPES) and the Virginia All-Payer Claims Database (VA-APCD) and identified all Virginia physicians and their specialties through the NPPES between 2015 and 2019. Active physicians were defined as those with at least one claim in the VA-APCD during the study period. They identified 20,976 active physicians in Virginia, 28.1% of whom were classified as primary care. Of these, 52% were family medicine physicians, 18.5% were internal medicine physicians, 16.8% were pediatricians, 11.8% were ob.gyns., and 0.5% were other specialists.
Clinician specialties were identified via specialty codes from the NPPES. Physicians were identified as primary care providers in two ways. The first way was by identifying those who had a National Uniform Claim Committee (NUCC) taxonomy of family medicine. The NUCC identifies a provider’s specialty using several levels of classification based on board certification and subspecialty certification data. The second identifier was having been a physician who had billed for at least 10 wellness visit codes from Jan. 1, 2019, through Dec. 31, 2019.
Over the 5-year study period (2015-2019), the counts and percentages of primary care physicians in the workforce remained stable, and the overall number of physicians in the state increased by 3.5%, the researchers noted. A total of 60.45% of all physicians and 60.87% of primary care physicians remained active, and 11.66% of all physicians had a claim in only 1 of the 5 years.
How distribution and access impact patients
In an interview, Dr. Huffstetler said the study she and colleagues authored “offers a transparent and reproducible process for identifying primary care physicians in a state, where they practice, and what changes in staffing occur over time.”
“In Virginia, this is particularly important, as we recently expanded Medicaid, making primary care more affordable for over 500,000 people,” she said. “We also saw the importance of distribution and accessibility to primary care over the past 3 years of COVID. In order to adequately prepare for community needs in the coming years, we must know who is providing primary care, and where they are.”
However, the model used in this study has its limitations, Dr. Huffstetler said, including the lack of a definitive definition of primary care using claims data.
“We used a data-informed wellness visit threshold, but it is likely that primary care is delivered in some locations without claims that are reflected by a wellness visit, and we hope to look at scope in the future to help refine these results,” she said.
Canadian study shows pandemic’s impact on patient care
“The pandemic’s impact on primary care remains palpable, and Dr. Kiran’s team has done an excellent analysis on the practice trends during the past several years,” Dr. Huffstetler said.
“The Canadian analysis uses claims in a similar manner to our study; however, it appears that they already knew who the FPs were in Ontario,” Dr. Huffstetler noted. “Their claims threshold of 50 for active practice was higher than ours, at only 1. Should those FPs have moved to a different specialty, the physicians would still have claims for the patients seen in other subspecialties. As such, I don’t suspect that their analysis miscalculated those that transitioned, rather than stopped practice,” she explained.
The Ontario study was supported by the Initial Credential Evaluation Service, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care, as well as by the Canadian Institutes of Health Research. Additional support came from the INSPIRE Primary Health Care Research Program, which is also funded by the Ontario Ministry of Health and Long-Term Care. The researchers had no financial conflicts to disclose.
The Virginia study was supported by the Department of Medical Assistance Services and the National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
The supply and demand report was conducted for the AAMC by IHS Markit, a global information company.
FROM ANNALS OF FAMILY MEDICINE





