User login
In Case You Missed It: COVID
Pandemic pushed death rates to historic highs
Excess mortality is a way of quantifying the impact of a pandemic, based on overall mortality from nonpandemic periods. Mortality data over long periods of time are not available for many countries, but Switzerland, Sweden, and Spain have accumulated death count data for an uninterrupted period of more than 100 years.
In a study published in the Annals of Internal Medicine, Kaspar Staub, PhD, of the University of Zurich led a team of researchers in reviewing data on monthly excess deaths from all causes for Switzerland, Sweden, and Spain for 2020 to 2021. Dr. Staub and colleagues also compared these numbers to other pandemic and nonpandemic periods since the end of the 19th century. The starting years were 1877 for Switzerland, 1851 for Sweden, and 1908 for Spain.
The researchers collected data for monthly all-cause deaths from the statistical offices of each country and determined excess mortality by comparing these numbers to population size and age structure.
They found that 2020 showed the highest number of excess deaths since 1918, with relative excess of deaths of 12.5% in Switzerland, 8.5% in Sweden, and 17.3 % in Spain.
To put it another way, the number of excess deaths per 100,000 people was 100 for Switzerland, 75 for Sweden, and 155 for Spain.
“Our findings suggest that the pandemic led to the second-largest mortality disaster driven by a viral infection in more than 100 years in the three countries we studied, second only to the 1918 influenza pandemic,” the researchers wrote.
They explained that the excess mortality for the year 1918 was six to seven times higher than the 2020 numbers, but that the 2020 numbers might have been higher without the strong public health interventions taken worldwide to mitigate the impact of the COVID-19 pandemic.
“Early estimates suggest that vaccination prevented approximately 470,000 deaths in persons aged 60 years or older across 33 European countries between December 2019 and November 2021,” they wrote. However, because the COVID-19 pandemic is ongoing, “a more conclusive assessment will have to wait,” they added.
The 2020 numbers also were higher than most mortality rates since 1918, including peak years of previous influenza pandemics that occurred in 1957, 1968, 1977, and, most recently, the swine flu pandemic of 2009 which was caused by a novel strain of the H1N1 influenza virus.
The study findings had some limitations. For example, only three countries were included. Also, monthly death numbers according to sex, age, and cause of death were available only for the past 60 years, and data from years before the 20th century may not be reliable, the researchers said.
The new study does not account for the long-term effects of patients suffering from long COVID, they noted.
Study findings support strong public health response
“With the COVID-19 pandemic ongoing, this study reinforces the historic magnitude of the problem in terms of mortality and could add to the justification for ongoing public health measures such as vaccination drives and vaccine mandates to curb deaths,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
“The results are surprising because when we view the rapid advancement in medical science over the last few decades, which have led to a decline in mortality from many previously fatal diseases, the scale of excess mortality from COVID-19 seems to have offset many such gains in the past 2 years.”
Prior studies of United States mortality data have estimated that excess deaths in the United States in 2020 exceeded the deaths attributed to COVID-19, said Dr. Pal. “The findings of this study could help clinicians in their discussion of the need for COVID-19 prevention measures with their patients” and inform discussions between doctors and patients about prevention strategies, he explained.
“Emphasizing that this pandemic is the second-largest cause of death due to a viral infection in a century could help patients understand the need for public health measures that may be viewed as unprecedented, such as government-imposed lockdowns, contact tracing, mask requirements, restrictions on travel, and vaccine mandates,” Dr. Pal noted. Better understanding of the evidence behind such measures may decrease the public’s resistance to following them, he added.As for additional research, “region-specific analysis of excess deaths may help estimate the impact of COVID-19 better, especially in regions where data reporting may be unreliable.”
Dr. F. Perry Wilson's take on study
“All-cause mortality is a key metric to assess the impact of the pandemic, because each death is treated equally,” said F. Perry Wilson, MD, of Yale University, in an interview. “With this type of analysis, there is no vague definition of a death from COVID or with COVID,” he explained. “A death is a death, and more deaths than expected is, of course, a bad thing. These analyses give a high-level view of the true human cost of the pandemic,” he said.
Dr. Wilson said he was not surprised by the findings. “There have been multiple studies, across multiple countries including the United States, which show similar findings—that observed deaths during this pandemic are substantially higher than expected,” he said. The current study findings are unique in that they compare the current pandemic to death rates in a nearly unbroken chain into the last century using data that only a few countries can provide, he noted.
The mortality data are “quite similar to what we see in the United States, with the exception that Spain was particularly hard-hit in the first COVID-19 wave in April 2020, said Dr. Wilson. By contrast, “the U.S. had substantially more excess deaths in the recent Delta wave, presumably due to lower vaccination uptake,” he added.
The current study is important for clinicians and their patients, said Dr. Wilson. “Data like these can help cut through some of the misinformation, such as the idea that only people who would have died anyway die of COVID, or that COVID is not severe,” he emphasized. “Overall death data are quite clear that far more people, millions more people, died over the last 22 months than could possibly be explained except by a global-level mortality event,” he said.
“One thing this study reminds us of is the value of high-quality data,” said Dr. Wilson. “Few countries have near complete vital statistics records on their entire populations and these can be so crucial to understand the true impact of pandemics and other disasters,” he explained. Of course, mortality data also serve as a reminder “that COVID is a serious disease: a once-in-a-century (we hope) pandemic,” he added.
The current study showed that excess death rates were similar, but not the same, from country to country, Dr. Wilson noted. “Moving forward, we need to learn what factors, from vaccination to social distancing strategies,” saved lives around the world,” he said.
The study was supported by the Foundation for Research in Science and the Humanities at the University of Zurich, the Swiss National Science Foundation, and the U.S. National Institute of Allergy and Infectious Diseases. The researchers, Dr. Pal, and Dr. Wilson had no financial conflicts.
*This article was updated on 2/1/2022.
Excess mortality is a way of quantifying the impact of a pandemic, based on overall mortality from nonpandemic periods. Mortality data over long periods of time are not available for many countries, but Switzerland, Sweden, and Spain have accumulated death count data for an uninterrupted period of more than 100 years.
In a study published in the Annals of Internal Medicine, Kaspar Staub, PhD, of the University of Zurich led a team of researchers in reviewing data on monthly excess deaths from all causes for Switzerland, Sweden, and Spain for 2020 to 2021. Dr. Staub and colleagues also compared these numbers to other pandemic and nonpandemic periods since the end of the 19th century. The starting years were 1877 for Switzerland, 1851 for Sweden, and 1908 for Spain.
The researchers collected data for monthly all-cause deaths from the statistical offices of each country and determined excess mortality by comparing these numbers to population size and age structure.
They found that 2020 showed the highest number of excess deaths since 1918, with relative excess of deaths of 12.5% in Switzerland, 8.5% in Sweden, and 17.3 % in Spain.
To put it another way, the number of excess deaths per 100,000 people was 100 for Switzerland, 75 for Sweden, and 155 for Spain.
“Our findings suggest that the pandemic led to the second-largest mortality disaster driven by a viral infection in more than 100 years in the three countries we studied, second only to the 1918 influenza pandemic,” the researchers wrote.
They explained that the excess mortality for the year 1918 was six to seven times higher than the 2020 numbers, but that the 2020 numbers might have been higher without the strong public health interventions taken worldwide to mitigate the impact of the COVID-19 pandemic.
“Early estimates suggest that vaccination prevented approximately 470,000 deaths in persons aged 60 years or older across 33 European countries between December 2019 and November 2021,” they wrote. However, because the COVID-19 pandemic is ongoing, “a more conclusive assessment will have to wait,” they added.
The 2020 numbers also were higher than most mortality rates since 1918, including peak years of previous influenza pandemics that occurred in 1957, 1968, 1977, and, most recently, the swine flu pandemic of 2009 which was caused by a novel strain of the H1N1 influenza virus.
The study findings had some limitations. For example, only three countries were included. Also, monthly death numbers according to sex, age, and cause of death were available only for the past 60 years, and data from years before the 20th century may not be reliable, the researchers said.
The new study does not account for the long-term effects of patients suffering from long COVID, they noted.
Study findings support strong public health response
“With the COVID-19 pandemic ongoing, this study reinforces the historic magnitude of the problem in terms of mortality and could add to the justification for ongoing public health measures such as vaccination drives and vaccine mandates to curb deaths,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
“The results are surprising because when we view the rapid advancement in medical science over the last few decades, which have led to a decline in mortality from many previously fatal diseases, the scale of excess mortality from COVID-19 seems to have offset many such gains in the past 2 years.”
Prior studies of United States mortality data have estimated that excess deaths in the United States in 2020 exceeded the deaths attributed to COVID-19, said Dr. Pal. “The findings of this study could help clinicians in their discussion of the need for COVID-19 prevention measures with their patients” and inform discussions between doctors and patients about prevention strategies, he explained.
“Emphasizing that this pandemic is the second-largest cause of death due to a viral infection in a century could help patients understand the need for public health measures that may be viewed as unprecedented, such as government-imposed lockdowns, contact tracing, mask requirements, restrictions on travel, and vaccine mandates,” Dr. Pal noted. Better understanding of the evidence behind such measures may decrease the public’s resistance to following them, he added.As for additional research, “region-specific analysis of excess deaths may help estimate the impact of COVID-19 better, especially in regions where data reporting may be unreliable.”
Dr. F. Perry Wilson's take on study
“All-cause mortality is a key metric to assess the impact of the pandemic, because each death is treated equally,” said F. Perry Wilson, MD, of Yale University, in an interview. “With this type of analysis, there is no vague definition of a death from COVID or with COVID,” he explained. “A death is a death, and more deaths than expected is, of course, a bad thing. These analyses give a high-level view of the true human cost of the pandemic,” he said.
Dr. Wilson said he was not surprised by the findings. “There have been multiple studies, across multiple countries including the United States, which show similar findings—that observed deaths during this pandemic are substantially higher than expected,” he said. The current study findings are unique in that they compare the current pandemic to death rates in a nearly unbroken chain into the last century using data that only a few countries can provide, he noted.
The mortality data are “quite similar to what we see in the United States, with the exception that Spain was particularly hard-hit in the first COVID-19 wave in April 2020, said Dr. Wilson. By contrast, “the U.S. had substantially more excess deaths in the recent Delta wave, presumably due to lower vaccination uptake,” he added.
The current study is important for clinicians and their patients, said Dr. Wilson. “Data like these can help cut through some of the misinformation, such as the idea that only people who would have died anyway die of COVID, or that COVID is not severe,” he emphasized. “Overall death data are quite clear that far more people, millions more people, died over the last 22 months than could possibly be explained except by a global-level mortality event,” he said.
“One thing this study reminds us of is the value of high-quality data,” said Dr. Wilson. “Few countries have near complete vital statistics records on their entire populations and these can be so crucial to understand the true impact of pandemics and other disasters,” he explained. Of course, mortality data also serve as a reminder “that COVID is a serious disease: a once-in-a-century (we hope) pandemic,” he added.
The current study showed that excess death rates were similar, but not the same, from country to country, Dr. Wilson noted. “Moving forward, we need to learn what factors, from vaccination to social distancing strategies,” saved lives around the world,” he said.
The study was supported by the Foundation for Research in Science and the Humanities at the University of Zurich, the Swiss National Science Foundation, and the U.S. National Institute of Allergy and Infectious Diseases. The researchers, Dr. Pal, and Dr. Wilson had no financial conflicts.
*This article was updated on 2/1/2022.
Excess mortality is a way of quantifying the impact of a pandemic, based on overall mortality from nonpandemic periods. Mortality data over long periods of time are not available for many countries, but Switzerland, Sweden, and Spain have accumulated death count data for an uninterrupted period of more than 100 years.
In a study published in the Annals of Internal Medicine, Kaspar Staub, PhD, of the University of Zurich led a team of researchers in reviewing data on monthly excess deaths from all causes for Switzerland, Sweden, and Spain for 2020 to 2021. Dr. Staub and colleagues also compared these numbers to other pandemic and nonpandemic periods since the end of the 19th century. The starting years were 1877 for Switzerland, 1851 for Sweden, and 1908 for Spain.
The researchers collected data for monthly all-cause deaths from the statistical offices of each country and determined excess mortality by comparing these numbers to population size and age structure.
They found that 2020 showed the highest number of excess deaths since 1918, with relative excess of deaths of 12.5% in Switzerland, 8.5% in Sweden, and 17.3 % in Spain.
To put it another way, the number of excess deaths per 100,000 people was 100 for Switzerland, 75 for Sweden, and 155 for Spain.
“Our findings suggest that the pandemic led to the second-largest mortality disaster driven by a viral infection in more than 100 years in the three countries we studied, second only to the 1918 influenza pandemic,” the researchers wrote.
They explained that the excess mortality for the year 1918 was six to seven times higher than the 2020 numbers, but that the 2020 numbers might have been higher without the strong public health interventions taken worldwide to mitigate the impact of the COVID-19 pandemic.
“Early estimates suggest that vaccination prevented approximately 470,000 deaths in persons aged 60 years or older across 33 European countries between December 2019 and November 2021,” they wrote. However, because the COVID-19 pandemic is ongoing, “a more conclusive assessment will have to wait,” they added.
The 2020 numbers also were higher than most mortality rates since 1918, including peak years of previous influenza pandemics that occurred in 1957, 1968, 1977, and, most recently, the swine flu pandemic of 2009 which was caused by a novel strain of the H1N1 influenza virus.
The study findings had some limitations. For example, only three countries were included. Also, monthly death numbers according to sex, age, and cause of death were available only for the past 60 years, and data from years before the 20th century may not be reliable, the researchers said.
The new study does not account for the long-term effects of patients suffering from long COVID, they noted.
Study findings support strong public health response
“With the COVID-19 pandemic ongoing, this study reinforces the historic magnitude of the problem in terms of mortality and could add to the justification for ongoing public health measures such as vaccination drives and vaccine mandates to curb deaths,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
“The results are surprising because when we view the rapid advancement in medical science over the last few decades, which have led to a decline in mortality from many previously fatal diseases, the scale of excess mortality from COVID-19 seems to have offset many such gains in the past 2 years.”
Prior studies of United States mortality data have estimated that excess deaths in the United States in 2020 exceeded the deaths attributed to COVID-19, said Dr. Pal. “The findings of this study could help clinicians in their discussion of the need for COVID-19 prevention measures with their patients” and inform discussions between doctors and patients about prevention strategies, he explained.
“Emphasizing that this pandemic is the second-largest cause of death due to a viral infection in a century could help patients understand the need for public health measures that may be viewed as unprecedented, such as government-imposed lockdowns, contact tracing, mask requirements, restrictions on travel, and vaccine mandates,” Dr. Pal noted. Better understanding of the evidence behind such measures may decrease the public’s resistance to following them, he added.As for additional research, “region-specific analysis of excess deaths may help estimate the impact of COVID-19 better, especially in regions where data reporting may be unreliable.”
Dr. F. Perry Wilson's take on study
“All-cause mortality is a key metric to assess the impact of the pandemic, because each death is treated equally,” said F. Perry Wilson, MD, of Yale University, in an interview. “With this type of analysis, there is no vague definition of a death from COVID or with COVID,” he explained. “A death is a death, and more deaths than expected is, of course, a bad thing. These analyses give a high-level view of the true human cost of the pandemic,” he said.
Dr. Wilson said he was not surprised by the findings. “There have been multiple studies, across multiple countries including the United States, which show similar findings—that observed deaths during this pandemic are substantially higher than expected,” he said. The current study findings are unique in that they compare the current pandemic to death rates in a nearly unbroken chain into the last century using data that only a few countries can provide, he noted.
The mortality data are “quite similar to what we see in the United States, with the exception that Spain was particularly hard-hit in the first COVID-19 wave in April 2020, said Dr. Wilson. By contrast, “the U.S. had substantially more excess deaths in the recent Delta wave, presumably due to lower vaccination uptake,” he added.
The current study is important for clinicians and their patients, said Dr. Wilson. “Data like these can help cut through some of the misinformation, such as the idea that only people who would have died anyway die of COVID, or that COVID is not severe,” he emphasized. “Overall death data are quite clear that far more people, millions more people, died over the last 22 months than could possibly be explained except by a global-level mortality event,” he said.
“One thing this study reminds us of is the value of high-quality data,” said Dr. Wilson. “Few countries have near complete vital statistics records on their entire populations and these can be so crucial to understand the true impact of pandemics and other disasters,” he explained. Of course, mortality data also serve as a reminder “that COVID is a serious disease: a once-in-a-century (we hope) pandemic,” he added.
The current study showed that excess death rates were similar, but not the same, from country to country, Dr. Wilson noted. “Moving forward, we need to learn what factors, from vaccination to social distancing strategies,” saved lives around the world,” he said.
The study was supported by the Foundation for Research in Science and the Humanities at the University of Zurich, the Swiss National Science Foundation, and the U.S. National Institute of Allergy and Infectious Diseases. The researchers, Dr. Pal, and Dr. Wilson had no financial conflicts.
*This article was updated on 2/1/2022.
FROM ANNALS OF INTERNAL MEDICINE
FDA grants full approval to Moderna COVID-19 vaccine
Moderna announced today that its mRNA COVID-19 vaccine has received full Food and Drug Administration approval for adults 18 years and older.
The move lifts an FDA emergency use authorization for the vaccine, which started Dec. 18, 2020.
The Moderna vaccine also now has a new trade name: Spikevax.
The FDA approval comes a little more than 5 months after the agency granted full approval to the Pfizer/BioNTech COVID-19 vaccine on Aug. 23. At the time, the Pfizer vaccine received the trade name Comirnaty.
The FDA approved the Moderna vaccine based on how well it works and its safety for 6 months after a second dose, including follow-up data from a phase 3 study, Moderna announced this morning through a news release. The FDA also announced the news.
Spikevax is the first Moderna product to be fully licensed in the United States.
The United States joins more than 70 other countries where regulators have approved the vaccine. A total of 807 million doses of Moderna’s COVID-19 vaccine were shipped worldwide in 2021, the company reported.
“The full licensure of Spikevax in the U.S. now joins that in Canada, Japan, the European Union, the U.K., Israel, and other countries, where the adolescent indication is also approved,” Stéphane Bancel, Moderna chief executive officer, said in the release.
A version of this article first appeared on WebMD.com.
Moderna announced today that its mRNA COVID-19 vaccine has received full Food and Drug Administration approval for adults 18 years and older.
The move lifts an FDA emergency use authorization for the vaccine, which started Dec. 18, 2020.
The Moderna vaccine also now has a new trade name: Spikevax.
The FDA approval comes a little more than 5 months after the agency granted full approval to the Pfizer/BioNTech COVID-19 vaccine on Aug. 23. At the time, the Pfizer vaccine received the trade name Comirnaty.
The FDA approved the Moderna vaccine based on how well it works and its safety for 6 months after a second dose, including follow-up data from a phase 3 study, Moderna announced this morning through a news release. The FDA also announced the news.
Spikevax is the first Moderna product to be fully licensed in the United States.
The United States joins more than 70 other countries where regulators have approved the vaccine. A total of 807 million doses of Moderna’s COVID-19 vaccine were shipped worldwide in 2021, the company reported.
“The full licensure of Spikevax in the U.S. now joins that in Canada, Japan, the European Union, the U.K., Israel, and other countries, where the adolescent indication is also approved,” Stéphane Bancel, Moderna chief executive officer, said in the release.
A version of this article first appeared on WebMD.com.
Moderna announced today that its mRNA COVID-19 vaccine has received full Food and Drug Administration approval for adults 18 years and older.
The move lifts an FDA emergency use authorization for the vaccine, which started Dec. 18, 2020.
The Moderna vaccine also now has a new trade name: Spikevax.
The FDA approval comes a little more than 5 months after the agency granted full approval to the Pfizer/BioNTech COVID-19 vaccine on Aug. 23. At the time, the Pfizer vaccine received the trade name Comirnaty.
The FDA approved the Moderna vaccine based on how well it works and its safety for 6 months after a second dose, including follow-up data from a phase 3 study, Moderna announced this morning through a news release. The FDA also announced the news.
Spikevax is the first Moderna product to be fully licensed in the United States.
The United States joins more than 70 other countries where regulators have approved the vaccine. A total of 807 million doses of Moderna’s COVID-19 vaccine were shipped worldwide in 2021, the company reported.
“The full licensure of Spikevax in the U.S. now joins that in Canada, Japan, the European Union, the U.K., Israel, and other countries, where the adolescent indication is also approved,” Stéphane Bancel, Moderna chief executive officer, said in the release.
A version of this article first appeared on WebMD.com.
Long COVID is real, and many real questions remain
Long story short, we still have a lot to learn about long COVID-19.
But it is a real phenomenon with real long-term health effects for people recovering from coronavirus infections. And diagnosing and managing it can get tricky, as some symptoms of long COVID-19 overlap with those of other conditions – and what many people have as they recover from any challenging stay in the ICU.
Risk factors remain largely unknown as well: What makes one person more likely to have symptoms like fatigue, “brain fog,” or headaches versus someone else? Researchers are just starting to offer some intriguing answers, but the evidence is preliminary at this point, experts said at a media briefing sponsored by the Infectious Diseases Society of America.
Unanswered questions include: Does an autoimmune reaction drive long COVID? Does the coronavirus linger in reservoirs within the body and reactivate later? What protection against long COVID do vaccines and treatments offer, if any?
To get a handle on these and other questions, nailing down a standard definition of long COVID would be a good start.
“Studies so far have used different definitions of long COVID,” Nahid Bhadelia, MD, founding director of the Boston University Center for Emerging Infectious Diseases Policy and Research, said during the briefing.
Fatigue is the most commonly symptom of long COVID in research so far, said Dr. Bhadelia, who is also an associate professor of medicine at Boston University.
“What’s difficult in this situation is it’s been 2 years in a global pandemic. We’re all fatigued. How do you tease this apart?” she asked.
Other common symptoms are a hard time thinking quickly – also known as “brain fog” – and the feeling that, despite normal oxygen levels, breathing is difficult, said Kathleen Bell, MD.
Headache, joint and muscle pain, and persistent loss of smell and taste are also widely reported, said Dr. Bell, a professor and chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center in Dallas.
Not all the symptoms are physical either.
“Pretty prominent things that we’re seeing are very high levels of anxiety, depression, and insomnia,” Dr. Bell said. These “actually seem to be associated independently with the virus as opposed to just being a completely reactive component.”
More research will be needed to distinguish the causes of these conditions.
A difficult diagnosis
the experts said.
“We are starting to see some interesting features of inaccurate attributions to COVID, both on the part of perhaps the person with long COVID symptoms and health care providers,” Dr. Bell said.“It’s sometimes a little difficult to sort it out.”
Dr. Bell said she was not suggesting misdiagnoses are common, “but it is difficult for physicians that don’t see a lot of people with long COVID.”
The advice is to consider other conditions. “You can have both a long COVID syndrome and other syndromes as well,” she said. “As one of my teachers used to say: ‘You can have both ticks and fleas.’ ”
Predicting long COVID
In a study getting attention, researchers identified four early things linked to greater chances that someone with COVID-19 will have long-term effects: type 2 diabetes at the time of diagnosis, the presence of specific autoantibodies, unusual levels of SARS-CoV-2 RNA in the blood, and signs of the Epstein-Barr virus in the blood.
The study, published in Cell, followed 309 people 2-3 months after COVID-19.
“That’s important work, but it’s early work,” Dr. Bhadelia said. “I think we still have a while to go in terms of understanding the mechanism of long COVID.”
Unexpected patients getting long COVID care
“We are seeing different populations than we all expected to see when this pandemic first started,” Dr. Bell said.
Instead of seeing primarily patients who had severe COVID-19, “the preponderance of people that we’re seeing in long COVID clinics are people who are enabled, were never hospitalized, and have what people might call mild to moderate cases of coronavirus infection,” she said.
Also, instead of just older patients, people of all ages are seeking long COVID care.
One thing that appears more certain is a lack of diversity in people seeking care at long COVID clinics nationwide.
“Many of us who have long COVID specialty clinics will tell you that we are tending to see fairly educated, socioeconomically stable population in these clinics,” Dr. Bell said. “We know that based on the early statistics of who’s getting COVID and having significant COVID that we may not be seeing those populations for follow-up.”
Is an autoinflammatory process to blame?
It remains unclear if a hyperinflammatory response is driving persistent post–COVID-19 symptoms. Children and some adults have developed multisystem inflammatory conditions associated with COVID-19, for example.
There is a signal, and “I think there is enough data now to show something does happen,” Dr. Bhadelia said. “The question is, how often does it happen?”
Spending time in critical care, even without COVID-19, can result in persistent symptoms after a hospital stay, such as acute respiratory distress syndrome. Recovery can take time because being in an ICU is “basically the physiologically equivalent of a car crash,” Dr. Bhadelia said. “So you’re recovering from that, too.”
Dr. Bell agreed. “You’re not only recovering from the virus itself, you’re recovering from intubation, secondary infections, secondary lung conditions, perhaps other organ failure, and prolonged bed rest. There are so many things that go into that, that it’s a little bit hard to sort that out from what long COVID is and what the direct effects of the virus are.”
Also a research opportunity
“I hate to call it this, but we’ve never had an opportunity [where] we have so many people in such a short amount of time with the same viral disorder,” Dr. Bell said. “We also have the technology to investigate it. This has never happened.
“SARS-CoV-2 is not the only virus. This is just the only one we’ve gotten whacked with in such a huge quantity at one time,” she said.
What researchers learn now about COVID-19 and long COVID “is a model that’s going to be able to be applied in the future to infectious diseases in general,” Dr. Bell predicted.
How long will long COVID last?
The vast majority of people with long COVID will get better over time, given enough support and relief of their symptoms, Dr. Bell said.
Type 2 diabetes, preexisting pulmonary disease, and other things could affect how long it takes to recover from long COVID, she said, although more evidence is needed.
“I don’t think at this point that anyone can say how long this long COVID will last because there are a variety of factors,” Dr. Bell said.
A version of this article first appeared on WebMD.com.
Long story short, we still have a lot to learn about long COVID-19.
But it is a real phenomenon with real long-term health effects for people recovering from coronavirus infections. And diagnosing and managing it can get tricky, as some symptoms of long COVID-19 overlap with those of other conditions – and what many people have as they recover from any challenging stay in the ICU.
Risk factors remain largely unknown as well: What makes one person more likely to have symptoms like fatigue, “brain fog,” or headaches versus someone else? Researchers are just starting to offer some intriguing answers, but the evidence is preliminary at this point, experts said at a media briefing sponsored by the Infectious Diseases Society of America.
Unanswered questions include: Does an autoimmune reaction drive long COVID? Does the coronavirus linger in reservoirs within the body and reactivate later? What protection against long COVID do vaccines and treatments offer, if any?
To get a handle on these and other questions, nailing down a standard definition of long COVID would be a good start.
“Studies so far have used different definitions of long COVID,” Nahid Bhadelia, MD, founding director of the Boston University Center for Emerging Infectious Diseases Policy and Research, said during the briefing.
Fatigue is the most commonly symptom of long COVID in research so far, said Dr. Bhadelia, who is also an associate professor of medicine at Boston University.
“What’s difficult in this situation is it’s been 2 years in a global pandemic. We’re all fatigued. How do you tease this apart?” she asked.
Other common symptoms are a hard time thinking quickly – also known as “brain fog” – and the feeling that, despite normal oxygen levels, breathing is difficult, said Kathleen Bell, MD.
Headache, joint and muscle pain, and persistent loss of smell and taste are also widely reported, said Dr. Bell, a professor and chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center in Dallas.
Not all the symptoms are physical either.
“Pretty prominent things that we’re seeing are very high levels of anxiety, depression, and insomnia,” Dr. Bell said. These “actually seem to be associated independently with the virus as opposed to just being a completely reactive component.”
More research will be needed to distinguish the causes of these conditions.
A difficult diagnosis
the experts said.
“We are starting to see some interesting features of inaccurate attributions to COVID, both on the part of perhaps the person with long COVID symptoms and health care providers,” Dr. Bell said.“It’s sometimes a little difficult to sort it out.”
Dr. Bell said she was not suggesting misdiagnoses are common, “but it is difficult for physicians that don’t see a lot of people with long COVID.”
The advice is to consider other conditions. “You can have both a long COVID syndrome and other syndromes as well,” she said. “As one of my teachers used to say: ‘You can have both ticks and fleas.’ ”
Predicting long COVID
In a study getting attention, researchers identified four early things linked to greater chances that someone with COVID-19 will have long-term effects: type 2 diabetes at the time of diagnosis, the presence of specific autoantibodies, unusual levels of SARS-CoV-2 RNA in the blood, and signs of the Epstein-Barr virus in the blood.
The study, published in Cell, followed 309 people 2-3 months after COVID-19.
“That’s important work, but it’s early work,” Dr. Bhadelia said. “I think we still have a while to go in terms of understanding the mechanism of long COVID.”
Unexpected patients getting long COVID care
“We are seeing different populations than we all expected to see when this pandemic first started,” Dr. Bell said.
Instead of seeing primarily patients who had severe COVID-19, “the preponderance of people that we’re seeing in long COVID clinics are people who are enabled, were never hospitalized, and have what people might call mild to moderate cases of coronavirus infection,” she said.
Also, instead of just older patients, people of all ages are seeking long COVID care.
One thing that appears more certain is a lack of diversity in people seeking care at long COVID clinics nationwide.
“Many of us who have long COVID specialty clinics will tell you that we are tending to see fairly educated, socioeconomically stable population in these clinics,” Dr. Bell said. “We know that based on the early statistics of who’s getting COVID and having significant COVID that we may not be seeing those populations for follow-up.”
Is an autoinflammatory process to blame?
It remains unclear if a hyperinflammatory response is driving persistent post–COVID-19 symptoms. Children and some adults have developed multisystem inflammatory conditions associated with COVID-19, for example.
There is a signal, and “I think there is enough data now to show something does happen,” Dr. Bhadelia said. “The question is, how often does it happen?”
Spending time in critical care, even without COVID-19, can result in persistent symptoms after a hospital stay, such as acute respiratory distress syndrome. Recovery can take time because being in an ICU is “basically the physiologically equivalent of a car crash,” Dr. Bhadelia said. “So you’re recovering from that, too.”
Dr. Bell agreed. “You’re not only recovering from the virus itself, you’re recovering from intubation, secondary infections, secondary lung conditions, perhaps other organ failure, and prolonged bed rest. There are so many things that go into that, that it’s a little bit hard to sort that out from what long COVID is and what the direct effects of the virus are.”
Also a research opportunity
“I hate to call it this, but we’ve never had an opportunity [where] we have so many people in such a short amount of time with the same viral disorder,” Dr. Bell said. “We also have the technology to investigate it. This has never happened.
“SARS-CoV-2 is not the only virus. This is just the only one we’ve gotten whacked with in such a huge quantity at one time,” she said.
What researchers learn now about COVID-19 and long COVID “is a model that’s going to be able to be applied in the future to infectious diseases in general,” Dr. Bell predicted.
How long will long COVID last?
The vast majority of people with long COVID will get better over time, given enough support and relief of their symptoms, Dr. Bell said.
Type 2 diabetes, preexisting pulmonary disease, and other things could affect how long it takes to recover from long COVID, she said, although more evidence is needed.
“I don’t think at this point that anyone can say how long this long COVID will last because there are a variety of factors,” Dr. Bell said.
A version of this article first appeared on WebMD.com.
Long story short, we still have a lot to learn about long COVID-19.
But it is a real phenomenon with real long-term health effects for people recovering from coronavirus infections. And diagnosing and managing it can get tricky, as some symptoms of long COVID-19 overlap with those of other conditions – and what many people have as they recover from any challenging stay in the ICU.
Risk factors remain largely unknown as well: What makes one person more likely to have symptoms like fatigue, “brain fog,” or headaches versus someone else? Researchers are just starting to offer some intriguing answers, but the evidence is preliminary at this point, experts said at a media briefing sponsored by the Infectious Diseases Society of America.
Unanswered questions include: Does an autoimmune reaction drive long COVID? Does the coronavirus linger in reservoirs within the body and reactivate later? What protection against long COVID do vaccines and treatments offer, if any?
To get a handle on these and other questions, nailing down a standard definition of long COVID would be a good start.
“Studies so far have used different definitions of long COVID,” Nahid Bhadelia, MD, founding director of the Boston University Center for Emerging Infectious Diseases Policy and Research, said during the briefing.
Fatigue is the most commonly symptom of long COVID in research so far, said Dr. Bhadelia, who is also an associate professor of medicine at Boston University.
“What’s difficult in this situation is it’s been 2 years in a global pandemic. We’re all fatigued. How do you tease this apart?” she asked.
Other common symptoms are a hard time thinking quickly – also known as “brain fog” – and the feeling that, despite normal oxygen levels, breathing is difficult, said Kathleen Bell, MD.
Headache, joint and muscle pain, and persistent loss of smell and taste are also widely reported, said Dr. Bell, a professor and chair of the department of physical medicine and rehabilitation at the University of Texas Southwestern Medical Center in Dallas.
Not all the symptoms are physical either.
“Pretty prominent things that we’re seeing are very high levels of anxiety, depression, and insomnia,” Dr. Bell said. These “actually seem to be associated independently with the virus as opposed to just being a completely reactive component.”
More research will be needed to distinguish the causes of these conditions.
A difficult diagnosis
the experts said.
“We are starting to see some interesting features of inaccurate attributions to COVID, both on the part of perhaps the person with long COVID symptoms and health care providers,” Dr. Bell said.“It’s sometimes a little difficult to sort it out.”
Dr. Bell said she was not suggesting misdiagnoses are common, “but it is difficult for physicians that don’t see a lot of people with long COVID.”
The advice is to consider other conditions. “You can have both a long COVID syndrome and other syndromes as well,” she said. “As one of my teachers used to say: ‘You can have both ticks and fleas.’ ”
Predicting long COVID
In a study getting attention, researchers identified four early things linked to greater chances that someone with COVID-19 will have long-term effects: type 2 diabetes at the time of diagnosis, the presence of specific autoantibodies, unusual levels of SARS-CoV-2 RNA in the blood, and signs of the Epstein-Barr virus in the blood.
The study, published in Cell, followed 309 people 2-3 months after COVID-19.
“That’s important work, but it’s early work,” Dr. Bhadelia said. “I think we still have a while to go in terms of understanding the mechanism of long COVID.”
Unexpected patients getting long COVID care
“We are seeing different populations than we all expected to see when this pandemic first started,” Dr. Bell said.
Instead of seeing primarily patients who had severe COVID-19, “the preponderance of people that we’re seeing in long COVID clinics are people who are enabled, were never hospitalized, and have what people might call mild to moderate cases of coronavirus infection,” she said.
Also, instead of just older patients, people of all ages are seeking long COVID care.
One thing that appears more certain is a lack of diversity in people seeking care at long COVID clinics nationwide.
“Many of us who have long COVID specialty clinics will tell you that we are tending to see fairly educated, socioeconomically stable population in these clinics,” Dr. Bell said. “We know that based on the early statistics of who’s getting COVID and having significant COVID that we may not be seeing those populations for follow-up.”
Is an autoinflammatory process to blame?
It remains unclear if a hyperinflammatory response is driving persistent post–COVID-19 symptoms. Children and some adults have developed multisystem inflammatory conditions associated with COVID-19, for example.
There is a signal, and “I think there is enough data now to show something does happen,” Dr. Bhadelia said. “The question is, how often does it happen?”
Spending time in critical care, even without COVID-19, can result in persistent symptoms after a hospital stay, such as acute respiratory distress syndrome. Recovery can take time because being in an ICU is “basically the physiologically equivalent of a car crash,” Dr. Bhadelia said. “So you’re recovering from that, too.”
Dr. Bell agreed. “You’re not only recovering from the virus itself, you’re recovering from intubation, secondary infections, secondary lung conditions, perhaps other organ failure, and prolonged bed rest. There are so many things that go into that, that it’s a little bit hard to sort that out from what long COVID is and what the direct effects of the virus are.”
Also a research opportunity
“I hate to call it this, but we’ve never had an opportunity [where] we have so many people in such a short amount of time with the same viral disorder,” Dr. Bell said. “We also have the technology to investigate it. This has never happened.
“SARS-CoV-2 is not the only virus. This is just the only one we’ve gotten whacked with in such a huge quantity at one time,” she said.
What researchers learn now about COVID-19 and long COVID “is a model that’s going to be able to be applied in the future to infectious diseases in general,” Dr. Bell predicted.
How long will long COVID last?
The vast majority of people with long COVID will get better over time, given enough support and relief of their symptoms, Dr. Bell said.
Type 2 diabetes, preexisting pulmonary disease, and other things could affect how long it takes to recover from long COVID, she said, although more evidence is needed.
“I don’t think at this point that anyone can say how long this long COVID will last because there are a variety of factors,” Dr. Bell said.
A version of this article first appeared on WebMD.com.
Immunocompromised patients should receive fourth COVID shot: CDC
The Centers for Disease Control and Prevention contacted pharmacies on Jan. 26 to reinforce the message that people with moderate to severe immune suppression should receive a fourth COVID-19 vaccine, according to Kaiser Health News.
The conference call came a day after the news outlet reported that immunocompromised people were being turned away by pharmacies. White House officials also emphasized on Jan. 26 that immunocompromised people should receive an additional shot.
During the call, the CDC “reiterated the recommendations, running through case examples,” Mitchel Rothholz, RPh, MBA, chief of governance and state affiliates for the American Pharmacists Association, told KHN.
While on the call, Mr. Rothholz asked for a “prepared document” with the CDC’s recommendations “so we can clearly and consistently communicate the message.” The CDC officials on the call said they would create a document but “don’t know how long that will take,” Mr. Rothholz told KHN.
The CDC recommends an additional shot -– or a fourth shot – for those who have weak immune systems, which makes them more at risk for severe COVID-19 and death. About 7 million American adults are considered immunocompromised, KHN reported, which includes people who have certain medical conditions that impair their immune response or who take immune-suppressing drugs because of organ transplants, cancer, or autoimmune diseases.
The CDC first recommended fourth shots for immunocompromised people in October. This month, the CDC shortened the time for booster shots from 6 months to 5 months, and some immunocompromised people who are due for another shot have begun to seek them. The agency has been educating pharmacists and other health providers since then, a CDC spokesperson told KHN.
While patients don’t need to provide proof that they are immunocompromised, according to the CDC, some have been turned away, KHN reported.
To improve communication with the public, large pharmacies could issue news releases and update their websites “explicitly stating that they are offering fourth doses” to immunocompromised people, Ameet Kini, MD, a professor of pathology and laboratory medicine at Loyola University Medical Center in Chicago, told KHN.
Pharmacies should also update their patient portals and provide “clear guidance for their pharmacists,” he said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention contacted pharmacies on Jan. 26 to reinforce the message that people with moderate to severe immune suppression should receive a fourth COVID-19 vaccine, according to Kaiser Health News.
The conference call came a day after the news outlet reported that immunocompromised people were being turned away by pharmacies. White House officials also emphasized on Jan. 26 that immunocompromised people should receive an additional shot.
During the call, the CDC “reiterated the recommendations, running through case examples,” Mitchel Rothholz, RPh, MBA, chief of governance and state affiliates for the American Pharmacists Association, told KHN.
While on the call, Mr. Rothholz asked for a “prepared document” with the CDC’s recommendations “so we can clearly and consistently communicate the message.” The CDC officials on the call said they would create a document but “don’t know how long that will take,” Mr. Rothholz told KHN.
The CDC recommends an additional shot -– or a fourth shot – for those who have weak immune systems, which makes them more at risk for severe COVID-19 and death. About 7 million American adults are considered immunocompromised, KHN reported, which includes people who have certain medical conditions that impair their immune response or who take immune-suppressing drugs because of organ transplants, cancer, or autoimmune diseases.
The CDC first recommended fourth shots for immunocompromised people in October. This month, the CDC shortened the time for booster shots from 6 months to 5 months, and some immunocompromised people who are due for another shot have begun to seek them. The agency has been educating pharmacists and other health providers since then, a CDC spokesperson told KHN.
While patients don’t need to provide proof that they are immunocompromised, according to the CDC, some have been turned away, KHN reported.
To improve communication with the public, large pharmacies could issue news releases and update their websites “explicitly stating that they are offering fourth doses” to immunocompromised people, Ameet Kini, MD, a professor of pathology and laboratory medicine at Loyola University Medical Center in Chicago, told KHN.
Pharmacies should also update their patient portals and provide “clear guidance for their pharmacists,” he said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention contacted pharmacies on Jan. 26 to reinforce the message that people with moderate to severe immune suppression should receive a fourth COVID-19 vaccine, according to Kaiser Health News.
The conference call came a day after the news outlet reported that immunocompromised people were being turned away by pharmacies. White House officials also emphasized on Jan. 26 that immunocompromised people should receive an additional shot.
During the call, the CDC “reiterated the recommendations, running through case examples,” Mitchel Rothholz, RPh, MBA, chief of governance and state affiliates for the American Pharmacists Association, told KHN.
While on the call, Mr. Rothholz asked for a “prepared document” with the CDC’s recommendations “so we can clearly and consistently communicate the message.” The CDC officials on the call said they would create a document but “don’t know how long that will take,” Mr. Rothholz told KHN.
The CDC recommends an additional shot -– or a fourth shot – for those who have weak immune systems, which makes them more at risk for severe COVID-19 and death. About 7 million American adults are considered immunocompromised, KHN reported, which includes people who have certain medical conditions that impair their immune response or who take immune-suppressing drugs because of organ transplants, cancer, or autoimmune diseases.
The CDC first recommended fourth shots for immunocompromised people in October. This month, the CDC shortened the time for booster shots from 6 months to 5 months, and some immunocompromised people who are due for another shot have begun to seek them. The agency has been educating pharmacists and other health providers since then, a CDC spokesperson told KHN.
While patients don’t need to provide proof that they are immunocompromised, according to the CDC, some have been turned away, KHN reported.
To improve communication with the public, large pharmacies could issue news releases and update their websites “explicitly stating that they are offering fourth doses” to immunocompromised people, Ameet Kini, MD, a professor of pathology and laboratory medicine at Loyola University Medical Center in Chicago, told KHN.
Pharmacies should also update their patient portals and provide “clear guidance for their pharmacists,” he said.
A version of this article first appeared on WebMD.com.
Hong Kong, U.S., Israeli data illuminate COVID vaccine myocarditis
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.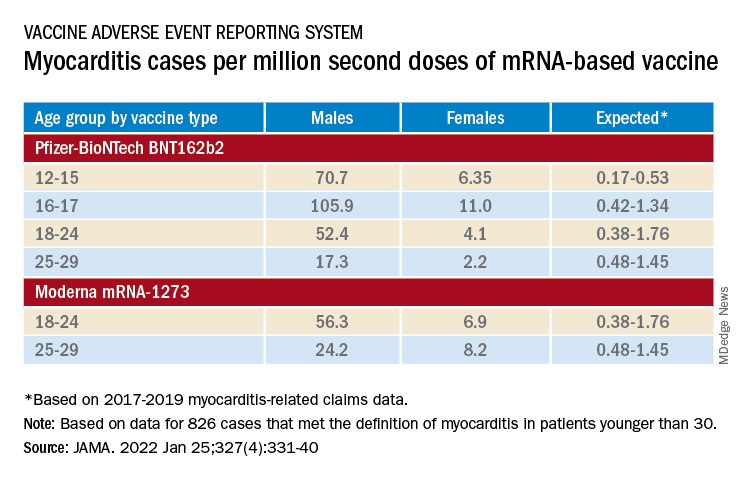
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Get free masks at grocery stores and pharmacies starting Jan. 28
The first batches are expected to arrive in some stores on Jan. 27, and many locations will begin offering them to customers on Jan. 28, according to NPR.
Meijer, which operates more than 250 groceries and pharmacies throughout the Midwest, has received about 3 million masks. Customers can pick up masks from the greeter stand at the store entrance.
More than 2,200 Kroger stores with pharmacies will give out free masks, with the first shipment expected to arrive on Jan. 27, a spokeswoman told NPR.
Walgreens will likely begin offering masks in some stores on Jan. 28, which will continue “on a rolling basis in the days and weeks following,” a spokesman told NPR.
Masks should arrive by Jan. 28 at Southeastern Grocers locations with in-store pharmacies, including Fresco y Mas, Harveys, and Winn-Dixie, according to CNN.
Hy-Vee received and began giving out masks on Jan. 21, and most stores with pharmacies were giving them out Jan. 26, according to Today.
CVS Pharmacy locations will offer free masks as early as Jan. 27, a spokesman told Today. That will include CVS Pharmacy locations inside Target and Schnucks.
Albertsons is “currently working to finalize details regarding inventory and distribution,” the chain told Today.
Rite Aid will have free masks in some stores at the end of the week, with all stores receiving them by early February, Today reported.
Walmart and Sam’s Club will offer free masks late next week at the earliest, according to NBC Chicago.
The Biden administration is sending out 400 million N95 masks from the Strategic National Stockpile. Each person can take up to three free masks, if they’re available, the Department of Health and Human Services has said.
The distribution of masks is meant to align with the CDC’s latest recommendation to wear an N95 or KN95 mask to prevent the spread of the highly transmissible Omicron variant. When worn correctly over the mouth and nose, the high-filtration masks are made to filter out 95% or more of airborne particles.
The Biden administration is also sending masks to community health centers and COVID-19 test kits directly to Americans. The programs are ramping up now and should be fully running by early February, NPR reported.
A version of this article first appeared on WebMD.com.
The first batches are expected to arrive in some stores on Jan. 27, and many locations will begin offering them to customers on Jan. 28, according to NPR.
Meijer, which operates more than 250 groceries and pharmacies throughout the Midwest, has received about 3 million masks. Customers can pick up masks from the greeter stand at the store entrance.
More than 2,200 Kroger stores with pharmacies will give out free masks, with the first shipment expected to arrive on Jan. 27, a spokeswoman told NPR.
Walgreens will likely begin offering masks in some stores on Jan. 28, which will continue “on a rolling basis in the days and weeks following,” a spokesman told NPR.
Masks should arrive by Jan. 28 at Southeastern Grocers locations with in-store pharmacies, including Fresco y Mas, Harveys, and Winn-Dixie, according to CNN.
Hy-Vee received and began giving out masks on Jan. 21, and most stores with pharmacies were giving them out Jan. 26, according to Today.
CVS Pharmacy locations will offer free masks as early as Jan. 27, a spokesman told Today. That will include CVS Pharmacy locations inside Target and Schnucks.
Albertsons is “currently working to finalize details regarding inventory and distribution,” the chain told Today.
Rite Aid will have free masks in some stores at the end of the week, with all stores receiving them by early February, Today reported.
Walmart and Sam’s Club will offer free masks late next week at the earliest, according to NBC Chicago.
The Biden administration is sending out 400 million N95 masks from the Strategic National Stockpile. Each person can take up to three free masks, if they’re available, the Department of Health and Human Services has said.
The distribution of masks is meant to align with the CDC’s latest recommendation to wear an N95 or KN95 mask to prevent the spread of the highly transmissible Omicron variant. When worn correctly over the mouth and nose, the high-filtration masks are made to filter out 95% or more of airborne particles.
The Biden administration is also sending masks to community health centers and COVID-19 test kits directly to Americans. The programs are ramping up now and should be fully running by early February, NPR reported.
A version of this article first appeared on WebMD.com.
The first batches are expected to arrive in some stores on Jan. 27, and many locations will begin offering them to customers on Jan. 28, according to NPR.
Meijer, which operates more than 250 groceries and pharmacies throughout the Midwest, has received about 3 million masks. Customers can pick up masks from the greeter stand at the store entrance.
More than 2,200 Kroger stores with pharmacies will give out free masks, with the first shipment expected to arrive on Jan. 27, a spokeswoman told NPR.
Walgreens will likely begin offering masks in some stores on Jan. 28, which will continue “on a rolling basis in the days and weeks following,” a spokesman told NPR.
Masks should arrive by Jan. 28 at Southeastern Grocers locations with in-store pharmacies, including Fresco y Mas, Harveys, and Winn-Dixie, according to CNN.
Hy-Vee received and began giving out masks on Jan. 21, and most stores with pharmacies were giving them out Jan. 26, according to Today.
CVS Pharmacy locations will offer free masks as early as Jan. 27, a spokesman told Today. That will include CVS Pharmacy locations inside Target and Schnucks.
Albertsons is “currently working to finalize details regarding inventory and distribution,” the chain told Today.
Rite Aid will have free masks in some stores at the end of the week, with all stores receiving them by early February, Today reported.
Walmart and Sam’s Club will offer free masks late next week at the earliest, according to NBC Chicago.
The Biden administration is sending out 400 million N95 masks from the Strategic National Stockpile. Each person can take up to three free masks, if they’re available, the Department of Health and Human Services has said.
The distribution of masks is meant to align with the CDC’s latest recommendation to wear an N95 or KN95 mask to prevent the spread of the highly transmissible Omicron variant. When worn correctly over the mouth and nose, the high-filtration masks are made to filter out 95% or more of airborne particles.
The Biden administration is also sending masks to community health centers and COVID-19 test kits directly to Americans. The programs are ramping up now and should be fully running by early February, NPR reported.
A version of this article first appeared on WebMD.com.
Kids’ mask use linked with fewer childcare closings
Mask-wearing in childcare programs is linked with fewer COVID-19–related program closures, new data released suggest.
Researchers included 6,654 childcare professionals in a prospective, 1-year, longitudinal electronic survey study of home- and center-based childcare programs in all 50 states.
Findings by Thomas S. Murray, MD, PhD, with the department of pediatrics, Yale University, New Haven, Conn., and coauthors, were published in JAMA Network Open on Jan. 28, 2022.
They found that mask-wearing from the May 22, 2020, baseline to June 8, 2020, was associated with a 13% reduction in program closures within the following year (adjusted relative risk, 0.87; 95% confidence interval, 0.77-0.99). Continued mask-wearing throughout the 1-year follow-up was associated with a 14% reduction in program closures (aRR, 0.86; 95% CI, 0.74-1.00).
The authors said the evidence supports current masking recommendation in younger children provided by the Centers for Disease Control and Prevention.
They wrote: “This finding has important public health policy implications for families that rely on childcare to sustain employment.”
The benefits of masking in preventing COVID-19 transmission within kindergarten through 12th-grade classes are well documented. Masks are particularly important in areas where vaccinations are not widespread.
Masks can be worn safely by young children without harming respiratory function, studies have shown.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., pointed out that the American Academy of Pediatrics has said there are no noteworthy effects on breathing function for most children.
“There’s been so much discussion about the contribution of masks to reducing the risk of COVID that it’s nice to have the data,” he said, adding that this is a relationship that has been difficult to study, but this analysis was able to make the connection with hard numbers.
“It’s an important outcome,” he said in an interview.
The authors pointed out there is evidence that school-age children can identify most emotions in masked faces.
They added that “2-year-old children recognize spoken words better through an opaque mask, compared with a clear face shield, suggesting verbal communication to infants is not harmed by face masks.”
Studies have shown that childhood infection with other respiratory viruses also decreased and asthma symptoms were not reported when preschool children wore masks and used other preventative steps.
The authors wrote that a potential reason for that may be that those who wear masks have less face touching, known to increase the spread of COVID-19.
Paloma Beamer, PhD, an engineer and exposure scientist at University of Arizona, Tucson, who also has a 3-year-old son who wears masks at his daycare center, said in an interview that she works closely with his school on training kids how to wear their masks because getting young children to keep them on and finding ones that fit is challenging.
“We need layered controls and protections in place at schools as much as possible,” she said, adding that the authors didn’t mention ventilation, but that’s another important component as well.
“We’re fortunate in Arizona that we are in an old school and the windows are open as much as possible,” she said.
She said this study shows that “masks are a great form of additional control.” Her son is on his third quarantine this month after three kids tested positive, she added.
She said: “I think these newer variants perhaps make the findings of this study more compelling and it will be interesting to see if the researchers do a follow-up study.”
Strengths of the study include that it utilized prospective data from a large national cohort of childcare professionals. Additionally, the retention rate was high at 1 year. And the self-reported information likely gives better information than looking at policies that may or may not be well followed.
Limitations include potential reporting bias because the self-reports were not independently confirmed. Also, family behavior outside childcare, such as social gatherings where masking is not enforced, also influence COVID-19 cases when children gather and may affect the numbers of closures.
Having the option of childcare centers benefits kids with in-person early education and social interactions with staff, the authors noted. The centers also help parents return to work without interruptions at home.
“Our findings support current national recommendations endorsed by many local and state governments for masking children 2 years and older in childcare programs when community COVID-19 transmission levels are elevated,” the authors wrote.
Dr. Schaffner said the results have implications outside of childcare centers and should be included in discussions of masking in schools and in the general public.
All phases of this study were supported by and coauthors report grants from the Andrew & Julie Klingenstein Family Fund, Esther A. & Joseph Klingenstein Fund, Heising-Simons Foundation, W.K. Kellogg Foundation, Foundation for Child Development, Early Educator Investment Collaborative, and Scholastic. The study was partially funded by the Yale Institute for Global Health. Dr. Schaffner and Dr. Beamer reported no relevant financial relationships.
Mask-wearing in childcare programs is linked with fewer COVID-19–related program closures, new data released suggest.
Researchers included 6,654 childcare professionals in a prospective, 1-year, longitudinal electronic survey study of home- and center-based childcare programs in all 50 states.
Findings by Thomas S. Murray, MD, PhD, with the department of pediatrics, Yale University, New Haven, Conn., and coauthors, were published in JAMA Network Open on Jan. 28, 2022.
They found that mask-wearing from the May 22, 2020, baseline to June 8, 2020, was associated with a 13% reduction in program closures within the following year (adjusted relative risk, 0.87; 95% confidence interval, 0.77-0.99). Continued mask-wearing throughout the 1-year follow-up was associated with a 14% reduction in program closures (aRR, 0.86; 95% CI, 0.74-1.00).
The authors said the evidence supports current masking recommendation in younger children provided by the Centers for Disease Control and Prevention.
They wrote: “This finding has important public health policy implications for families that rely on childcare to sustain employment.”
The benefits of masking in preventing COVID-19 transmission within kindergarten through 12th-grade classes are well documented. Masks are particularly important in areas where vaccinations are not widespread.
Masks can be worn safely by young children without harming respiratory function, studies have shown.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., pointed out that the American Academy of Pediatrics has said there are no noteworthy effects on breathing function for most children.
“There’s been so much discussion about the contribution of masks to reducing the risk of COVID that it’s nice to have the data,” he said, adding that this is a relationship that has been difficult to study, but this analysis was able to make the connection with hard numbers.
“It’s an important outcome,” he said in an interview.
The authors pointed out there is evidence that school-age children can identify most emotions in masked faces.
They added that “2-year-old children recognize spoken words better through an opaque mask, compared with a clear face shield, suggesting verbal communication to infants is not harmed by face masks.”
Studies have shown that childhood infection with other respiratory viruses also decreased and asthma symptoms were not reported when preschool children wore masks and used other preventative steps.
The authors wrote that a potential reason for that may be that those who wear masks have less face touching, known to increase the spread of COVID-19.
Paloma Beamer, PhD, an engineer and exposure scientist at University of Arizona, Tucson, who also has a 3-year-old son who wears masks at his daycare center, said in an interview that she works closely with his school on training kids how to wear their masks because getting young children to keep them on and finding ones that fit is challenging.
“We need layered controls and protections in place at schools as much as possible,” she said, adding that the authors didn’t mention ventilation, but that’s another important component as well.
“We’re fortunate in Arizona that we are in an old school and the windows are open as much as possible,” she said.
She said this study shows that “masks are a great form of additional control.” Her son is on his third quarantine this month after three kids tested positive, she added.
She said: “I think these newer variants perhaps make the findings of this study more compelling and it will be interesting to see if the researchers do a follow-up study.”
Strengths of the study include that it utilized prospective data from a large national cohort of childcare professionals. Additionally, the retention rate was high at 1 year. And the self-reported information likely gives better information than looking at policies that may or may not be well followed.
Limitations include potential reporting bias because the self-reports were not independently confirmed. Also, family behavior outside childcare, such as social gatherings where masking is not enforced, also influence COVID-19 cases when children gather and may affect the numbers of closures.
Having the option of childcare centers benefits kids with in-person early education and social interactions with staff, the authors noted. The centers also help parents return to work without interruptions at home.
“Our findings support current national recommendations endorsed by many local and state governments for masking children 2 years and older in childcare programs when community COVID-19 transmission levels are elevated,” the authors wrote.
Dr. Schaffner said the results have implications outside of childcare centers and should be included in discussions of masking in schools and in the general public.
All phases of this study were supported by and coauthors report grants from the Andrew & Julie Klingenstein Family Fund, Esther A. & Joseph Klingenstein Fund, Heising-Simons Foundation, W.K. Kellogg Foundation, Foundation for Child Development, Early Educator Investment Collaborative, and Scholastic. The study was partially funded by the Yale Institute for Global Health. Dr. Schaffner and Dr. Beamer reported no relevant financial relationships.
Mask-wearing in childcare programs is linked with fewer COVID-19–related program closures, new data released suggest.
Researchers included 6,654 childcare professionals in a prospective, 1-year, longitudinal electronic survey study of home- and center-based childcare programs in all 50 states.
Findings by Thomas S. Murray, MD, PhD, with the department of pediatrics, Yale University, New Haven, Conn., and coauthors, were published in JAMA Network Open on Jan. 28, 2022.
They found that mask-wearing from the May 22, 2020, baseline to June 8, 2020, was associated with a 13% reduction in program closures within the following year (adjusted relative risk, 0.87; 95% confidence interval, 0.77-0.99). Continued mask-wearing throughout the 1-year follow-up was associated with a 14% reduction in program closures (aRR, 0.86; 95% CI, 0.74-1.00).
The authors said the evidence supports current masking recommendation in younger children provided by the Centers for Disease Control and Prevention.
They wrote: “This finding has important public health policy implications for families that rely on childcare to sustain employment.”
The benefits of masking in preventing COVID-19 transmission within kindergarten through 12th-grade classes are well documented. Masks are particularly important in areas where vaccinations are not widespread.
Masks can be worn safely by young children without harming respiratory function, studies have shown.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., pointed out that the American Academy of Pediatrics has said there are no noteworthy effects on breathing function for most children.
“There’s been so much discussion about the contribution of masks to reducing the risk of COVID that it’s nice to have the data,” he said, adding that this is a relationship that has been difficult to study, but this analysis was able to make the connection with hard numbers.
“It’s an important outcome,” he said in an interview.
The authors pointed out there is evidence that school-age children can identify most emotions in masked faces.
They added that “2-year-old children recognize spoken words better through an opaque mask, compared with a clear face shield, suggesting verbal communication to infants is not harmed by face masks.”
Studies have shown that childhood infection with other respiratory viruses also decreased and asthma symptoms were not reported when preschool children wore masks and used other preventative steps.
The authors wrote that a potential reason for that may be that those who wear masks have less face touching, known to increase the spread of COVID-19.
Paloma Beamer, PhD, an engineer and exposure scientist at University of Arizona, Tucson, who also has a 3-year-old son who wears masks at his daycare center, said in an interview that she works closely with his school on training kids how to wear their masks because getting young children to keep them on and finding ones that fit is challenging.
“We need layered controls and protections in place at schools as much as possible,” she said, adding that the authors didn’t mention ventilation, but that’s another important component as well.
“We’re fortunate in Arizona that we are in an old school and the windows are open as much as possible,” she said.
She said this study shows that “masks are a great form of additional control.” Her son is on his third quarantine this month after three kids tested positive, she added.
She said: “I think these newer variants perhaps make the findings of this study more compelling and it will be interesting to see if the researchers do a follow-up study.”
Strengths of the study include that it utilized prospective data from a large national cohort of childcare professionals. Additionally, the retention rate was high at 1 year. And the self-reported information likely gives better information than looking at policies that may or may not be well followed.
Limitations include potential reporting bias because the self-reports were not independently confirmed. Also, family behavior outside childcare, such as social gatherings where masking is not enforced, also influence COVID-19 cases when children gather and may affect the numbers of closures.
Having the option of childcare centers benefits kids with in-person early education and social interactions with staff, the authors noted. The centers also help parents return to work without interruptions at home.
“Our findings support current national recommendations endorsed by many local and state governments for masking children 2 years and older in childcare programs when community COVID-19 transmission levels are elevated,” the authors wrote.
Dr. Schaffner said the results have implications outside of childcare centers and should be included in discussions of masking in schools and in the general public.
All phases of this study were supported by and coauthors report grants from the Andrew & Julie Klingenstein Family Fund, Esther A. & Joseph Klingenstein Fund, Heising-Simons Foundation, W.K. Kellogg Foundation, Foundation for Child Development, Early Educator Investment Collaborative, and Scholastic. The study was partially funded by the Yale Institute for Global Health. Dr. Schaffner and Dr. Beamer reported no relevant financial relationships.
FROM JAMA NETWORK OPEN
35% of employers to proceed with vaccine mandate, poll shows
despite a recent U.S. Supreme Court ruling that blocked the Biden administration’s vaccine-or-test rule for big businesses.
But the poll by Gartner Inc. showed no consensus among employers. About 4% of polled executives said they’re dropping their vaccine mandate, 29% are in a wait-and-see position, and 12% are less likely to impose a mandate now, Bloomberg reported.
Executives were divided on how a vaccine mandate would affect absenteeism and employee morale. Almost 40% of polled employers said they thought a mandate would attract workers, but about 25% said it would do the opposite, Bloomberg said.
“What is more attractive -- to have a mandate or not?” Brian Kropp, PhD, Gartner’s chief of human resources research, said in an interview with Bloomberg. “Most are not exactly sure what to do.”
Big companies have reacted differently since the court’s ruling.
Starbucks announced it was dropping its vaccine-or-test rule for the company’s approximately 228,000 employees. General Electric dropped its mandate after the ruling, but Honeywell International Inc. announced it was staying with its vaccination policy, Bloomberg said.
The Supreme Court ruled Jan. 13 against the Biden administration’s mandate for businesses. The Occupational Safety and Health Administration had proposed that every company with more than 100 employees would be required to ensure workers were either vaccinated or tested weekly for COVID-19.
State governments and business groups immediately appealed, and the court ruled 6-3 against the mandate. The Biden administration officially dropped its rule on Wednesday.
A version of this article first appeared on WebMD.com.
despite a recent U.S. Supreme Court ruling that blocked the Biden administration’s vaccine-or-test rule for big businesses.
But the poll by Gartner Inc. showed no consensus among employers. About 4% of polled executives said they’re dropping their vaccine mandate, 29% are in a wait-and-see position, and 12% are less likely to impose a mandate now, Bloomberg reported.
Executives were divided on how a vaccine mandate would affect absenteeism and employee morale. Almost 40% of polled employers said they thought a mandate would attract workers, but about 25% said it would do the opposite, Bloomberg said.
“What is more attractive -- to have a mandate or not?” Brian Kropp, PhD, Gartner’s chief of human resources research, said in an interview with Bloomberg. “Most are not exactly sure what to do.”
Big companies have reacted differently since the court’s ruling.
Starbucks announced it was dropping its vaccine-or-test rule for the company’s approximately 228,000 employees. General Electric dropped its mandate after the ruling, but Honeywell International Inc. announced it was staying with its vaccination policy, Bloomberg said.
The Supreme Court ruled Jan. 13 against the Biden administration’s mandate for businesses. The Occupational Safety and Health Administration had proposed that every company with more than 100 employees would be required to ensure workers were either vaccinated or tested weekly for COVID-19.
State governments and business groups immediately appealed, and the court ruled 6-3 against the mandate. The Biden administration officially dropped its rule on Wednesday.
A version of this article first appeared on WebMD.com.
despite a recent U.S. Supreme Court ruling that blocked the Biden administration’s vaccine-or-test rule for big businesses.
But the poll by Gartner Inc. showed no consensus among employers. About 4% of polled executives said they’re dropping their vaccine mandate, 29% are in a wait-and-see position, and 12% are less likely to impose a mandate now, Bloomberg reported.
Executives were divided on how a vaccine mandate would affect absenteeism and employee morale. Almost 40% of polled employers said they thought a mandate would attract workers, but about 25% said it would do the opposite, Bloomberg said.
“What is more attractive -- to have a mandate or not?” Brian Kropp, PhD, Gartner’s chief of human resources research, said in an interview with Bloomberg. “Most are not exactly sure what to do.”
Big companies have reacted differently since the court’s ruling.
Starbucks announced it was dropping its vaccine-or-test rule for the company’s approximately 228,000 employees. General Electric dropped its mandate after the ruling, but Honeywell International Inc. announced it was staying with its vaccination policy, Bloomberg said.
The Supreme Court ruled Jan. 13 against the Biden administration’s mandate for businesses. The Occupational Safety and Health Administration had proposed that every company with more than 100 employees would be required to ensure workers were either vaccinated or tested weekly for COVID-19.
State governments and business groups immediately appealed, and the court ruled 6-3 against the mandate. The Biden administration officially dropped its rule on Wednesday.
A version of this article first appeared on WebMD.com.
Omicron survives longer on plastic, skin than other COVID variants
, one possible explanation for why Omicron has spread so rapidly around the world.
In a lab experiment, samples of different variants were applied to pieces of plastic and human skin collected from autopsies, researchers from Kyoto Prefectural University of Medicine wrote in bioRxiv. A variant “survived” until it could no longer be detected on the surface.
“This study showed that the Omicron variant also has the highest environmental stability among VOCs (variants of concern), which suggests that this high stability might also be one of the factors that have allowed the Omicron variant to replace the Delta variant and spread rapidly,” the researchers wrote.
On plastic, the Omicron variant samples survived an average of 193.5 hours, a little more than 8 days. By comparison, the other survival times on plastic were 56 hours for the original COVID strain, 191.3 hours for Alpha, 156.6 hours for Beta, 59.3 hours for Gamma, and 114 hours for Delta.
On skin samples, the Omicron samples survived an average of 21.1 hours. The other variants had these average survival times on skin: 8.6 hours for the original version, 19.6 hours for Alpha, 19.1 hours for Beta, 11 hours for Gamma, and 16.8 hours for Delta.
The study found that the variants had more resistance to ethanol than the original strain of COVID. That said, all COVID samples were inactivated after being exposed to alcohol-based hand sanitizers for 15 seconds.
“Therefore, it is highly recommended that current infection control (hand hygiene) practices use disinfectants ... as proposed by the World Health Organization,” the researchers said.
The study has not been peer-reviewed.
A version of this article first appeared on WebMD.com.
, one possible explanation for why Omicron has spread so rapidly around the world.
In a lab experiment, samples of different variants were applied to pieces of plastic and human skin collected from autopsies, researchers from Kyoto Prefectural University of Medicine wrote in bioRxiv. A variant “survived” until it could no longer be detected on the surface.
“This study showed that the Omicron variant also has the highest environmental stability among VOCs (variants of concern), which suggests that this high stability might also be one of the factors that have allowed the Omicron variant to replace the Delta variant and spread rapidly,” the researchers wrote.
On plastic, the Omicron variant samples survived an average of 193.5 hours, a little more than 8 days. By comparison, the other survival times on plastic were 56 hours for the original COVID strain, 191.3 hours for Alpha, 156.6 hours for Beta, 59.3 hours for Gamma, and 114 hours for Delta.
On skin samples, the Omicron samples survived an average of 21.1 hours. The other variants had these average survival times on skin: 8.6 hours for the original version, 19.6 hours for Alpha, 19.1 hours for Beta, 11 hours for Gamma, and 16.8 hours for Delta.
The study found that the variants had more resistance to ethanol than the original strain of COVID. That said, all COVID samples were inactivated after being exposed to alcohol-based hand sanitizers for 15 seconds.
“Therefore, it is highly recommended that current infection control (hand hygiene) practices use disinfectants ... as proposed by the World Health Organization,” the researchers said.
The study has not been peer-reviewed.
A version of this article first appeared on WebMD.com.
, one possible explanation for why Omicron has spread so rapidly around the world.
In a lab experiment, samples of different variants were applied to pieces of plastic and human skin collected from autopsies, researchers from Kyoto Prefectural University of Medicine wrote in bioRxiv. A variant “survived” until it could no longer be detected on the surface.
“This study showed that the Omicron variant also has the highest environmental stability among VOCs (variants of concern), which suggests that this high stability might also be one of the factors that have allowed the Omicron variant to replace the Delta variant and spread rapidly,” the researchers wrote.
On plastic, the Omicron variant samples survived an average of 193.5 hours, a little more than 8 days. By comparison, the other survival times on plastic were 56 hours for the original COVID strain, 191.3 hours for Alpha, 156.6 hours for Beta, 59.3 hours for Gamma, and 114 hours for Delta.
On skin samples, the Omicron samples survived an average of 21.1 hours. The other variants had these average survival times on skin: 8.6 hours for the original version, 19.6 hours for Alpha, 19.1 hours for Beta, 11 hours for Gamma, and 16.8 hours for Delta.
The study found that the variants had more resistance to ethanol than the original strain of COVID. That said, all COVID samples were inactivated after being exposed to alcohol-based hand sanitizers for 15 seconds.
“Therefore, it is highly recommended that current infection control (hand hygiene) practices use disinfectants ... as proposed by the World Health Organization,” the researchers said.
The study has not been peer-reviewed.
A version of this article first appeared on WebMD.com.
Presence of autoantibodies most predictive of long COVID in study
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
FROM CELL





