User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
‘Long haul’ COVID recovery worse than cancer rehab for some: CDC
People experiencing ongoing or “long-haul” symptoms after COVID-19 illness were more likely to report pain, challenges with physical activities, and “substantially worse health,” compared with people needing rehabilitation because of cancer, lead author Jessica Rogers-Brown, PhD, and colleagues report.
The study was published online July 9 in Morbidity and Mortality Weekly Report (MMWR).
The CDC investigators compared the self-reported physical and mental health symptoms, physical endurance, and use of health services of 1,295 outpatients recovering from COVID-19 and a control group of another 2,395 outpatients rehabilitating from a previous or current cancer diagnosis who had not experienced COVID-19.
Researchers used electronic health record data from January 2020 to March 2021 in the Select Medical network of outpatient clinics. The study included patients from 36 states and the District of Columbia.
Compared with people referred for cancer rehabilitation, those with COVID-19 symptoms lasting beyond 4 weeks were 2.3 times more likely to report pain, 1.8 times more likely to report worse physical health, and 1.6 times more likely to report difficulty with physical activities, an adjusted odds ratio analysis reveals.
The COVID-19 rehabilitation group also performed significantly worse on a 6-minute walk test, suggesting less physical endurance than people recovering from cancer (P < .001). They also used more rehabilitation services overall than the control group.
The researchers suggest services tailored to the unique physical and mental health rehabilitation needs of the post–COVID-19 patient population could be warranted.
The study does not suggest all people recovering with COVID-19 will fare worse than people recovering from cancer, the authors caution. They note that “these results should not be interpreted to mean that post–COVID-19 patients overall had poorer physical and mental health than patients with cancer.”
“Instead, results indicate that post–COVID-19 patients specifically referred to a large physical rehabilitation network had poorer health measures than those referred for cancer, which indicates that some patients recovering from COVID-19 had substantial rehabilitation needs.”
A version of this article first appeared on Medscape.com.
People experiencing ongoing or “long-haul” symptoms after COVID-19 illness were more likely to report pain, challenges with physical activities, and “substantially worse health,” compared with people needing rehabilitation because of cancer, lead author Jessica Rogers-Brown, PhD, and colleagues report.
The study was published online July 9 in Morbidity and Mortality Weekly Report (MMWR).
The CDC investigators compared the self-reported physical and mental health symptoms, physical endurance, and use of health services of 1,295 outpatients recovering from COVID-19 and a control group of another 2,395 outpatients rehabilitating from a previous or current cancer diagnosis who had not experienced COVID-19.
Researchers used electronic health record data from January 2020 to March 2021 in the Select Medical network of outpatient clinics. The study included patients from 36 states and the District of Columbia.
Compared with people referred for cancer rehabilitation, those with COVID-19 symptoms lasting beyond 4 weeks were 2.3 times more likely to report pain, 1.8 times more likely to report worse physical health, and 1.6 times more likely to report difficulty with physical activities, an adjusted odds ratio analysis reveals.
The COVID-19 rehabilitation group also performed significantly worse on a 6-minute walk test, suggesting less physical endurance than people recovering from cancer (P < .001). They also used more rehabilitation services overall than the control group.
The researchers suggest services tailored to the unique physical and mental health rehabilitation needs of the post–COVID-19 patient population could be warranted.
The study does not suggest all people recovering with COVID-19 will fare worse than people recovering from cancer, the authors caution. They note that “these results should not be interpreted to mean that post–COVID-19 patients overall had poorer physical and mental health than patients with cancer.”
“Instead, results indicate that post–COVID-19 patients specifically referred to a large physical rehabilitation network had poorer health measures than those referred for cancer, which indicates that some patients recovering from COVID-19 had substantial rehabilitation needs.”
A version of this article first appeared on Medscape.com.
People experiencing ongoing or “long-haul” symptoms after COVID-19 illness were more likely to report pain, challenges with physical activities, and “substantially worse health,” compared with people needing rehabilitation because of cancer, lead author Jessica Rogers-Brown, PhD, and colleagues report.
The study was published online July 9 in Morbidity and Mortality Weekly Report (MMWR).
The CDC investigators compared the self-reported physical and mental health symptoms, physical endurance, and use of health services of 1,295 outpatients recovering from COVID-19 and a control group of another 2,395 outpatients rehabilitating from a previous or current cancer diagnosis who had not experienced COVID-19.
Researchers used electronic health record data from January 2020 to March 2021 in the Select Medical network of outpatient clinics. The study included patients from 36 states and the District of Columbia.
Compared with people referred for cancer rehabilitation, those with COVID-19 symptoms lasting beyond 4 weeks were 2.3 times more likely to report pain, 1.8 times more likely to report worse physical health, and 1.6 times more likely to report difficulty with physical activities, an adjusted odds ratio analysis reveals.
The COVID-19 rehabilitation group also performed significantly worse on a 6-minute walk test, suggesting less physical endurance than people recovering from cancer (P < .001). They also used more rehabilitation services overall than the control group.
The researchers suggest services tailored to the unique physical and mental health rehabilitation needs of the post–COVID-19 patient population could be warranted.
The study does not suggest all people recovering with COVID-19 will fare worse than people recovering from cancer, the authors caution. They note that “these results should not be interpreted to mean that post–COVID-19 patients overall had poorer physical and mental health than patients with cancer.”
“Instead, results indicate that post–COVID-19 patients specifically referred to a large physical rehabilitation network had poorer health measures than those referred for cancer, which indicates that some patients recovering from COVID-19 had substantial rehabilitation needs.”
A version of this article first appeared on Medscape.com.
Are oncologists liable for pandemic-related treatment delays?
Albuquerque oncologist Barbara McAneny, MD, has a patient in his 30s who experienced rectal bleeding for 6 months in 2020 but didn’t see a physician because he was afraid of catching COVID-19. He hoped it was just hemorrhoids.
When he finally came in to see her recently, Dr. McAneny diagnosed a large colon cancer. She fears the delay could prove fatal. “We’ll do our best to cure him, but I don’t know if he’ll be treatable,” she said. “Six months absolutely can make a difference.”
She and other oncologists around the country are seeing many patients in the past few months with advanced breast, colon, lung, and other cancers who were not diagnosed and treated during the COVID-19 pandemic because the patients didn’t want to come in, or because medical facilities weren’t taking nonemergency or non-COVID patients.
Given that failure to diagnose cancer is among the most common medical malpractice allegations, should oncologists be worried that they are at legal risk?
Pandemic provides ‘safe harbor’
In a March survey done by medical malpractice insurer The Doctors Company, one-third of physicians said they were very concerned or somewhat concerned that malpractice claims related to care during the pandemic will rise.
But in most of these cases, physicians and hospitals have little to worry about in terms of medical malpractice liability, according to veteran plaintiff and defense attorneys and the head of a large medical liability insurer.
“You had people with diseases like cancer not getting care because health care systems were overwhelmed,” said Sean Domnick, JD, a malpractice plaintiff attorney in Boca Raton, Fla. “Will those lead to successful malpractice lawsuits? Most likely not.”
“The risks will be low because it’s hard to pin it on the doctor if the patient didn’t want to come in or facilities weren’t scheduling appointments because of the public health emergency,” said Richard Roberts, MD, JD, a professor of family medicine at the University of Wisconsin–Madison who is also a malpractice defense attorney.
In addition, liability protections enacted in more than 30 states because of the COVID-19 pandemic will help shield clinicians from lawsuits. Those laws generally require allegations of gross negligence or reckless conduct far beyond ordinary negligence, which are hard to prove. But the immunity provisions remain largely untested in the courts, and it’s unclear how they will affect cases involving care for conditions other than COVID-19.
Another helpful factor is the widespread public appreciation of the valiant work by health care professionals throughout the pandemic, though that halo effect could fade over the next several years as malpractice claims from the pandemic period are filed and tried.
“In many circumstances, the pandemic will prove to be a safe harbor for providers,” said Steven Wigrizer, JD, a malpractice plaintiff attorney in Philadelphia. “Jurors will be reluctant to impose liability on providers who were doing their best in a global pandemic the world hadn’t seen in 100 years.”
Risky situations
These predictions from liability experts should reassure physicians who are anxious over reports that many cancer diagnoses were missed as a result of the COVID-19 pandemic.
Still, there are situations where physicians and hospitals could be vulnerable to malpractice claims despite the pandemic conditions. The highest-risk cases are those where patients recognized a potential cancer symptom like a breast lump or rectal bleeding, and tried to visit a doctor’s office or hospital, but were told they couldn’t be seen.
“Those kinds of cases lend themselves to delayed diagnosis claims,” said Richard Anderson, MD, an oncologist who is chairman and CEO of The Doctors Company. “My guess is we will see claims,” though he expects a reduced number arising from 2020 care scenarios, compared with previous years.
So far, his company has seen 20% fewer claims in 2020, which he said isn’t surprising given that the volume of physician and hospital visits plummeted.
Another risky situation is where physicians – particularly primary care physicians but also specialists like gynecologists and urologists – did not inform patients about concerning test results and order a follow-up test or visit. That is dangerous even if the physician did try to schedule a visit but the patient canceled the appointment.
“The jury will ask, ‘What did you do to get the patient back?’ ” said Sean Byrne, JD, a malpractice defense attorney in Richmond, Va. “The provider will say: ‘I’m sure we called.’ But it’s a difficult defense to say the patient didn’t return the call. I need written proof.”
Mr. Domnick said failures to follow up on suspicious test results could produce viable malpractice claims, pandemic or not. “The question becomes to what extent doctors will try to hide behind COVID to explain otherwise run-of-the-mill negligence,” he said. “We’ll have to see how that plays out.”
There are also worries about missed cancer diagnoses during telemedicine visits. “On telemedicine, I can’t feel a lymph node, I can’t palpate a breast mass, and I can’t see if someone’s liver is enlarged,” Dr. McAneny fretted. “I think you’ll get suits because you’ll miss stuff.”
One other area of exposure cited by the experts: Radiologists and pathologists could be sued for missing tumors in reading imaging tests. “The COVID-19 demand on resources has been immense,” Mr. Byrne said. “If that production pressure resulted in any quality loss in testing services, we could see claims.”
Patient protocols provide protection
There’s no question that cancer screenings dropped sharply during the pandemic. In June 2020, the National Cancer Institute estimated there was a 75% decrease in mammograms and colonoscopies during the first few months of the pandemic. It projected that delays in screenings, diagnoses, and treatment likely would result in 10,000 more breast and colorectal cancer deaths than otherwise expected over the next decade.
Delays of even 1 month in treatment for seven common forms of cancer can increase mortality risk by 6%-13%, according to a BMJ study.
While many medical facilities stopped doing preventive screening tests during the height of the pandemic last year, health care professionals still found ways to bring in patients with diagnosed cancers or who were at heightened cancer risk for tests and treatment.
Most facilities convened multidisciplinary tumor boards to decide which patients could wait for treatment, which patients could be maintained on drug therapy, and which ones needed immediate surgery, said Carla Fisher, MD, director of breast surgery at Indiana University, Indianapolis. For breast cancer, they used guidelines from her professional group, the American Society of Breast Surgeons.
Following such protocols for prioritizing patients for treatment during the pandemic should help protect against liability, experts said.
Even if it can be shown that a clinician’s negligence led to delayed diagnosis or treatment of a patient’s cancer, plaintiff attorneys will be wary about filing such claims. That is because it is difficult in most cases to prove that the delay significantly worsened the course of the patient’s disease or the odds of survival. Showing harm may be more possible with certain cancers known to be particularly aggressive.
“The plaintiff attorney will have to get an expert to say that the 3-month delay in getting the patient a mammogram caused her great harm,” said Dr. Roberts. “But it’s hard to calculate that scientifically, and it’s really hard to lay that all on the doctor or health system, because they were supposed to lock down during the pandemic.”
Playing catch-up
With patients now feeling more comfortable about coming in for physician visits, Mr. Byrne urges clinicians to make a special effort to mitigate potential liability arising from the past year. Physicians should carefully review patients’ charts and make sure to catch them up on preventive screenings. Some health systems, like Kaiser Permanente, have been doing proactive patient outreach for cancer screening throughout the pandemic.
“Providers may need to be extra diligent, and consider expanding the exam into a wellness visit and remind patients about cancer surveillance,” he said.
Overall, however, the expert consensus is that physicians should focus on providing the best quality care going forward, and not worry excessively about the care they wish they could have delivered over the past year during the extraordinary pandemic conditions.
“Liability risks will be decreased, because state laws have changed and doctors will be cut some slack, not just by judges and juries but by patients themselves,” Dr. Roberts said. “As you are running down the hall to take care of the next person, don’t be looking over your shoulder or you’ll run into the wall.”
A version of this article first appeared on Medscape.com.
Albuquerque oncologist Barbara McAneny, MD, has a patient in his 30s who experienced rectal bleeding for 6 months in 2020 but didn’t see a physician because he was afraid of catching COVID-19. He hoped it was just hemorrhoids.
When he finally came in to see her recently, Dr. McAneny diagnosed a large colon cancer. She fears the delay could prove fatal. “We’ll do our best to cure him, but I don’t know if he’ll be treatable,” she said. “Six months absolutely can make a difference.”
She and other oncologists around the country are seeing many patients in the past few months with advanced breast, colon, lung, and other cancers who were not diagnosed and treated during the COVID-19 pandemic because the patients didn’t want to come in, or because medical facilities weren’t taking nonemergency or non-COVID patients.
Given that failure to diagnose cancer is among the most common medical malpractice allegations, should oncologists be worried that they are at legal risk?
Pandemic provides ‘safe harbor’
In a March survey done by medical malpractice insurer The Doctors Company, one-third of physicians said they were very concerned or somewhat concerned that malpractice claims related to care during the pandemic will rise.
But in most of these cases, physicians and hospitals have little to worry about in terms of medical malpractice liability, according to veteran plaintiff and defense attorneys and the head of a large medical liability insurer.
“You had people with diseases like cancer not getting care because health care systems were overwhelmed,” said Sean Domnick, JD, a malpractice plaintiff attorney in Boca Raton, Fla. “Will those lead to successful malpractice lawsuits? Most likely not.”
“The risks will be low because it’s hard to pin it on the doctor if the patient didn’t want to come in or facilities weren’t scheduling appointments because of the public health emergency,” said Richard Roberts, MD, JD, a professor of family medicine at the University of Wisconsin–Madison who is also a malpractice defense attorney.
In addition, liability protections enacted in more than 30 states because of the COVID-19 pandemic will help shield clinicians from lawsuits. Those laws generally require allegations of gross negligence or reckless conduct far beyond ordinary negligence, which are hard to prove. But the immunity provisions remain largely untested in the courts, and it’s unclear how they will affect cases involving care for conditions other than COVID-19.
Another helpful factor is the widespread public appreciation of the valiant work by health care professionals throughout the pandemic, though that halo effect could fade over the next several years as malpractice claims from the pandemic period are filed and tried.
“In many circumstances, the pandemic will prove to be a safe harbor for providers,” said Steven Wigrizer, JD, a malpractice plaintiff attorney in Philadelphia. “Jurors will be reluctant to impose liability on providers who were doing their best in a global pandemic the world hadn’t seen in 100 years.”
Risky situations
These predictions from liability experts should reassure physicians who are anxious over reports that many cancer diagnoses were missed as a result of the COVID-19 pandemic.
Still, there are situations where physicians and hospitals could be vulnerable to malpractice claims despite the pandemic conditions. The highest-risk cases are those where patients recognized a potential cancer symptom like a breast lump or rectal bleeding, and tried to visit a doctor’s office or hospital, but were told they couldn’t be seen.
“Those kinds of cases lend themselves to delayed diagnosis claims,” said Richard Anderson, MD, an oncologist who is chairman and CEO of The Doctors Company. “My guess is we will see claims,” though he expects a reduced number arising from 2020 care scenarios, compared with previous years.
So far, his company has seen 20% fewer claims in 2020, which he said isn’t surprising given that the volume of physician and hospital visits plummeted.
Another risky situation is where physicians – particularly primary care physicians but also specialists like gynecologists and urologists – did not inform patients about concerning test results and order a follow-up test or visit. That is dangerous even if the physician did try to schedule a visit but the patient canceled the appointment.
“The jury will ask, ‘What did you do to get the patient back?’ ” said Sean Byrne, JD, a malpractice defense attorney in Richmond, Va. “The provider will say: ‘I’m sure we called.’ But it’s a difficult defense to say the patient didn’t return the call. I need written proof.”
Mr. Domnick said failures to follow up on suspicious test results could produce viable malpractice claims, pandemic or not. “The question becomes to what extent doctors will try to hide behind COVID to explain otherwise run-of-the-mill negligence,” he said. “We’ll have to see how that plays out.”
There are also worries about missed cancer diagnoses during telemedicine visits. “On telemedicine, I can’t feel a lymph node, I can’t palpate a breast mass, and I can’t see if someone’s liver is enlarged,” Dr. McAneny fretted. “I think you’ll get suits because you’ll miss stuff.”
One other area of exposure cited by the experts: Radiologists and pathologists could be sued for missing tumors in reading imaging tests. “The COVID-19 demand on resources has been immense,” Mr. Byrne said. “If that production pressure resulted in any quality loss in testing services, we could see claims.”
Patient protocols provide protection
There’s no question that cancer screenings dropped sharply during the pandemic. In June 2020, the National Cancer Institute estimated there was a 75% decrease in mammograms and colonoscopies during the first few months of the pandemic. It projected that delays in screenings, diagnoses, and treatment likely would result in 10,000 more breast and colorectal cancer deaths than otherwise expected over the next decade.
Delays of even 1 month in treatment for seven common forms of cancer can increase mortality risk by 6%-13%, according to a BMJ study.
While many medical facilities stopped doing preventive screening tests during the height of the pandemic last year, health care professionals still found ways to bring in patients with diagnosed cancers or who were at heightened cancer risk for tests and treatment.
Most facilities convened multidisciplinary tumor boards to decide which patients could wait for treatment, which patients could be maintained on drug therapy, and which ones needed immediate surgery, said Carla Fisher, MD, director of breast surgery at Indiana University, Indianapolis. For breast cancer, they used guidelines from her professional group, the American Society of Breast Surgeons.
Following such protocols for prioritizing patients for treatment during the pandemic should help protect against liability, experts said.
Even if it can be shown that a clinician’s negligence led to delayed diagnosis or treatment of a patient’s cancer, plaintiff attorneys will be wary about filing such claims. That is because it is difficult in most cases to prove that the delay significantly worsened the course of the patient’s disease or the odds of survival. Showing harm may be more possible with certain cancers known to be particularly aggressive.
“The plaintiff attorney will have to get an expert to say that the 3-month delay in getting the patient a mammogram caused her great harm,” said Dr. Roberts. “But it’s hard to calculate that scientifically, and it’s really hard to lay that all on the doctor or health system, because they were supposed to lock down during the pandemic.”
Playing catch-up
With patients now feeling more comfortable about coming in for physician visits, Mr. Byrne urges clinicians to make a special effort to mitigate potential liability arising from the past year. Physicians should carefully review patients’ charts and make sure to catch them up on preventive screenings. Some health systems, like Kaiser Permanente, have been doing proactive patient outreach for cancer screening throughout the pandemic.
“Providers may need to be extra diligent, and consider expanding the exam into a wellness visit and remind patients about cancer surveillance,” he said.
Overall, however, the expert consensus is that physicians should focus on providing the best quality care going forward, and not worry excessively about the care they wish they could have delivered over the past year during the extraordinary pandemic conditions.
“Liability risks will be decreased, because state laws have changed and doctors will be cut some slack, not just by judges and juries but by patients themselves,” Dr. Roberts said. “As you are running down the hall to take care of the next person, don’t be looking over your shoulder or you’ll run into the wall.”
A version of this article first appeared on Medscape.com.
Albuquerque oncologist Barbara McAneny, MD, has a patient in his 30s who experienced rectal bleeding for 6 months in 2020 but didn’t see a physician because he was afraid of catching COVID-19. He hoped it was just hemorrhoids.
When he finally came in to see her recently, Dr. McAneny diagnosed a large colon cancer. She fears the delay could prove fatal. “We’ll do our best to cure him, but I don’t know if he’ll be treatable,” she said. “Six months absolutely can make a difference.”
She and other oncologists around the country are seeing many patients in the past few months with advanced breast, colon, lung, and other cancers who were not diagnosed and treated during the COVID-19 pandemic because the patients didn’t want to come in, or because medical facilities weren’t taking nonemergency or non-COVID patients.
Given that failure to diagnose cancer is among the most common medical malpractice allegations, should oncologists be worried that they are at legal risk?
Pandemic provides ‘safe harbor’
In a March survey done by medical malpractice insurer The Doctors Company, one-third of physicians said they were very concerned or somewhat concerned that malpractice claims related to care during the pandemic will rise.
But in most of these cases, physicians and hospitals have little to worry about in terms of medical malpractice liability, according to veteran plaintiff and defense attorneys and the head of a large medical liability insurer.
“You had people with diseases like cancer not getting care because health care systems were overwhelmed,” said Sean Domnick, JD, a malpractice plaintiff attorney in Boca Raton, Fla. “Will those lead to successful malpractice lawsuits? Most likely not.”
“The risks will be low because it’s hard to pin it on the doctor if the patient didn’t want to come in or facilities weren’t scheduling appointments because of the public health emergency,” said Richard Roberts, MD, JD, a professor of family medicine at the University of Wisconsin–Madison who is also a malpractice defense attorney.
In addition, liability protections enacted in more than 30 states because of the COVID-19 pandemic will help shield clinicians from lawsuits. Those laws generally require allegations of gross negligence or reckless conduct far beyond ordinary negligence, which are hard to prove. But the immunity provisions remain largely untested in the courts, and it’s unclear how they will affect cases involving care for conditions other than COVID-19.
Another helpful factor is the widespread public appreciation of the valiant work by health care professionals throughout the pandemic, though that halo effect could fade over the next several years as malpractice claims from the pandemic period are filed and tried.
“In many circumstances, the pandemic will prove to be a safe harbor for providers,” said Steven Wigrizer, JD, a malpractice plaintiff attorney in Philadelphia. “Jurors will be reluctant to impose liability on providers who were doing their best in a global pandemic the world hadn’t seen in 100 years.”
Risky situations
These predictions from liability experts should reassure physicians who are anxious over reports that many cancer diagnoses were missed as a result of the COVID-19 pandemic.
Still, there are situations where physicians and hospitals could be vulnerable to malpractice claims despite the pandemic conditions. The highest-risk cases are those where patients recognized a potential cancer symptom like a breast lump or rectal bleeding, and tried to visit a doctor’s office or hospital, but were told they couldn’t be seen.
“Those kinds of cases lend themselves to delayed diagnosis claims,” said Richard Anderson, MD, an oncologist who is chairman and CEO of The Doctors Company. “My guess is we will see claims,” though he expects a reduced number arising from 2020 care scenarios, compared with previous years.
So far, his company has seen 20% fewer claims in 2020, which he said isn’t surprising given that the volume of physician and hospital visits plummeted.
Another risky situation is where physicians – particularly primary care physicians but also specialists like gynecologists and urologists – did not inform patients about concerning test results and order a follow-up test or visit. That is dangerous even if the physician did try to schedule a visit but the patient canceled the appointment.
“The jury will ask, ‘What did you do to get the patient back?’ ” said Sean Byrne, JD, a malpractice defense attorney in Richmond, Va. “The provider will say: ‘I’m sure we called.’ But it’s a difficult defense to say the patient didn’t return the call. I need written proof.”
Mr. Domnick said failures to follow up on suspicious test results could produce viable malpractice claims, pandemic or not. “The question becomes to what extent doctors will try to hide behind COVID to explain otherwise run-of-the-mill negligence,” he said. “We’ll have to see how that plays out.”
There are also worries about missed cancer diagnoses during telemedicine visits. “On telemedicine, I can’t feel a lymph node, I can’t palpate a breast mass, and I can’t see if someone’s liver is enlarged,” Dr. McAneny fretted. “I think you’ll get suits because you’ll miss stuff.”
One other area of exposure cited by the experts: Radiologists and pathologists could be sued for missing tumors in reading imaging tests. “The COVID-19 demand on resources has been immense,” Mr. Byrne said. “If that production pressure resulted in any quality loss in testing services, we could see claims.”
Patient protocols provide protection
There’s no question that cancer screenings dropped sharply during the pandemic. In June 2020, the National Cancer Institute estimated there was a 75% decrease in mammograms and colonoscopies during the first few months of the pandemic. It projected that delays in screenings, diagnoses, and treatment likely would result in 10,000 more breast and colorectal cancer deaths than otherwise expected over the next decade.
Delays of even 1 month in treatment for seven common forms of cancer can increase mortality risk by 6%-13%, according to a BMJ study.
While many medical facilities stopped doing preventive screening tests during the height of the pandemic last year, health care professionals still found ways to bring in patients with diagnosed cancers or who were at heightened cancer risk for tests and treatment.
Most facilities convened multidisciplinary tumor boards to decide which patients could wait for treatment, which patients could be maintained on drug therapy, and which ones needed immediate surgery, said Carla Fisher, MD, director of breast surgery at Indiana University, Indianapolis. For breast cancer, they used guidelines from her professional group, the American Society of Breast Surgeons.
Following such protocols for prioritizing patients for treatment during the pandemic should help protect against liability, experts said.
Even if it can be shown that a clinician’s negligence led to delayed diagnosis or treatment of a patient’s cancer, plaintiff attorneys will be wary about filing such claims. That is because it is difficult in most cases to prove that the delay significantly worsened the course of the patient’s disease or the odds of survival. Showing harm may be more possible with certain cancers known to be particularly aggressive.
“The plaintiff attorney will have to get an expert to say that the 3-month delay in getting the patient a mammogram caused her great harm,” said Dr. Roberts. “But it’s hard to calculate that scientifically, and it’s really hard to lay that all on the doctor or health system, because they were supposed to lock down during the pandemic.”
Playing catch-up
With patients now feeling more comfortable about coming in for physician visits, Mr. Byrne urges clinicians to make a special effort to mitigate potential liability arising from the past year. Physicians should carefully review patients’ charts and make sure to catch them up on preventive screenings. Some health systems, like Kaiser Permanente, have been doing proactive patient outreach for cancer screening throughout the pandemic.
“Providers may need to be extra diligent, and consider expanding the exam into a wellness visit and remind patients about cancer surveillance,” he said.
Overall, however, the expert consensus is that physicians should focus on providing the best quality care going forward, and not worry excessively about the care they wish they could have delivered over the past year during the extraordinary pandemic conditions.
“Liability risks will be decreased, because state laws have changed and doctors will be cut some slack, not just by judges and juries but by patients themselves,” Dr. Roberts said. “As you are running down the hall to take care of the next person, don’t be looking over your shoulder or you’ll run into the wall.”
A version of this article first appeared on Medscape.com.
Network meta-analysis ranks first-line H. pylori regimens
A network meta-analysis of current first-line dual, triple, and quadruple therapies for Helicobacter pylori infection found that vonoprazan triple therapy was most effective, while standard triple therapy of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin was least effective. Levofloxacin-containing triple therapy performed best in Western countries and West Asia, while reverse hybrid therapy was most effective in East Asia.
The results “[suggest that] a new approach concerning H. pylori treatment is now needed and that the time for transitioning from trial and error to antimicrobial stewardship [of H. pylori infection] has arrived,” wrote Theodore Rokkas, PhD, MD, of the European University of Cyprus in Engomi, and colleagues. Their study was published in Gastroenterology.
H. pylori infection is the primary cause of gastritis, peptic ulcer disease, gastric mucosa–associated lymphoid tissue lymphoma, and gastric cancer.
Since H. pylori infection was first recognized, physicians have employed a range of drugs in double, triple, and quadruple combinations to combat it.
Despite those efforts, treatment success is lower than with many other infectious diseases. A newcomer is the potassium-competing acid blocker vonoprazan, which increases efficacy of amoxicillin combination therapies and has, thereby, generated renewed interest in all combination therapies, according to the study authors. Vonoprazan is currently available in some Asian countries, but not the United States or Europe.
Current guidelines for H. pylori treatment relied on randomized controlled trials and relevant pair-wise meta-analyses, but no previous pairwise analysis has included all currently available medications, the authors noted. Network meta-analyses can help fill this evidence gap: They incorporate both direct and indirect evidence from a collection of randomized controlled trials to estimate the comparative effectiveness of three or more regimens.
The researchers conducted a network meta-analysis that included 68 randomized, controlled trials totaling 22,975 patients. The following regimens were included in the analysis: Concomitant quadruple bismuth treatment (bismuth quadruple therapy), concomitant quadruple nonbismuth treatment (nonbismuth quadruple therapy), high-dose amoxicillin double treatment (Amox-dual therapy), levofloxacin-containing treatment (Levo-therapy), reverse hybrid therapy (R-hybrid therapy), sequential quadruple treatment (sequential therapy), standard triple treatment (triple therapy), and vonoprazan-containing therapy (Vono-triple therapy).
Statistically significant results were found with Vono-triple therapy versus triple therapy (odds ratio, 3.80; 95% confidence interval, 1.62-8.94), sequential therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53), nonbismuth quadruple therapy versus triple therapy (OR, 2.08; 95% CI, 1.45-2.98), bismuth quadruple therapy versus triple therapy (OR, 1.47; 95% CI, 1.02-2.11), and Levo-therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53).
In the overall data, mean cure rates greater than 90% were seen only in Vono-triple therapy (91.4%; 95% CI, 88.5-93.5%) and R-hybrid therapy (93.6%; 95% CI, 90.4-96.8%). Cure rates were lower for Nonbismuth quadruple therapy (84.3%; 95% CI, 82.7-85.8%), Levo-therapy (83.8%; 95% CI, 82.1-85.4%), Sequential therapy (83.7%; 95% CI, 82.7-84.7%), bismuth quadruple therapy (81.3%; 95% CI, 79.5-83.1%), Amox-dual therapy (80.2%; 75.3%-84.4%), and triple therapy (75.7%; 95% CI, 74.9-76.4%). Levo-therapy performed best in Western countries (88.5%; 95% CI, 86.5-90.5%) and West Asia (88.4%; 95% CI, 84.6-91.1%). R-hybrid therapy performed best in East Asia (93.6%; 95% CI, 90.4-96.8%).
A surface under the cumulative ranking (SUCRA) value, which represents the efficacy of the intervention compared to an ideal intervention, was 92.4% for Vono-triple therapy. The second highest SUCRA value was for 68.8% for nonbismuth quadruple therapy. The SUCRA value of standard triple therapy was 4.7%.
A key limitation to the study is that Vono-triple therapy was tested only in Japan, and requires additional study in other geographic regions.
The study received support from the Department of Veteran Affairs. The authors have consulted for and received research funding from various pharmaceutical companies.
A network meta-analysis of current first-line dual, triple, and quadruple therapies for Helicobacter pylori infection found that vonoprazan triple therapy was most effective, while standard triple therapy of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin was least effective. Levofloxacin-containing triple therapy performed best in Western countries and West Asia, while reverse hybrid therapy was most effective in East Asia.
The results “[suggest that] a new approach concerning H. pylori treatment is now needed and that the time for transitioning from trial and error to antimicrobial stewardship [of H. pylori infection] has arrived,” wrote Theodore Rokkas, PhD, MD, of the European University of Cyprus in Engomi, and colleagues. Their study was published in Gastroenterology.
H. pylori infection is the primary cause of gastritis, peptic ulcer disease, gastric mucosa–associated lymphoid tissue lymphoma, and gastric cancer.
Since H. pylori infection was first recognized, physicians have employed a range of drugs in double, triple, and quadruple combinations to combat it.
Despite those efforts, treatment success is lower than with many other infectious diseases. A newcomer is the potassium-competing acid blocker vonoprazan, which increases efficacy of amoxicillin combination therapies and has, thereby, generated renewed interest in all combination therapies, according to the study authors. Vonoprazan is currently available in some Asian countries, but not the United States or Europe.
Current guidelines for H. pylori treatment relied on randomized controlled trials and relevant pair-wise meta-analyses, but no previous pairwise analysis has included all currently available medications, the authors noted. Network meta-analyses can help fill this evidence gap: They incorporate both direct and indirect evidence from a collection of randomized controlled trials to estimate the comparative effectiveness of three or more regimens.
The researchers conducted a network meta-analysis that included 68 randomized, controlled trials totaling 22,975 patients. The following regimens were included in the analysis: Concomitant quadruple bismuth treatment (bismuth quadruple therapy), concomitant quadruple nonbismuth treatment (nonbismuth quadruple therapy), high-dose amoxicillin double treatment (Amox-dual therapy), levofloxacin-containing treatment (Levo-therapy), reverse hybrid therapy (R-hybrid therapy), sequential quadruple treatment (sequential therapy), standard triple treatment (triple therapy), and vonoprazan-containing therapy (Vono-triple therapy).
Statistically significant results were found with Vono-triple therapy versus triple therapy (odds ratio, 3.80; 95% confidence interval, 1.62-8.94), sequential therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53), nonbismuth quadruple therapy versus triple therapy (OR, 2.08; 95% CI, 1.45-2.98), bismuth quadruple therapy versus triple therapy (OR, 1.47; 95% CI, 1.02-2.11), and Levo-therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53).
In the overall data, mean cure rates greater than 90% were seen only in Vono-triple therapy (91.4%; 95% CI, 88.5-93.5%) and R-hybrid therapy (93.6%; 95% CI, 90.4-96.8%). Cure rates were lower for Nonbismuth quadruple therapy (84.3%; 95% CI, 82.7-85.8%), Levo-therapy (83.8%; 95% CI, 82.1-85.4%), Sequential therapy (83.7%; 95% CI, 82.7-84.7%), bismuth quadruple therapy (81.3%; 95% CI, 79.5-83.1%), Amox-dual therapy (80.2%; 75.3%-84.4%), and triple therapy (75.7%; 95% CI, 74.9-76.4%). Levo-therapy performed best in Western countries (88.5%; 95% CI, 86.5-90.5%) and West Asia (88.4%; 95% CI, 84.6-91.1%). R-hybrid therapy performed best in East Asia (93.6%; 95% CI, 90.4-96.8%).
A surface under the cumulative ranking (SUCRA) value, which represents the efficacy of the intervention compared to an ideal intervention, was 92.4% for Vono-triple therapy. The second highest SUCRA value was for 68.8% for nonbismuth quadruple therapy. The SUCRA value of standard triple therapy was 4.7%.
A key limitation to the study is that Vono-triple therapy was tested only in Japan, and requires additional study in other geographic regions.
The study received support from the Department of Veteran Affairs. The authors have consulted for and received research funding from various pharmaceutical companies.
A network meta-analysis of current first-line dual, triple, and quadruple therapies for Helicobacter pylori infection found that vonoprazan triple therapy was most effective, while standard triple therapy of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin was least effective. Levofloxacin-containing triple therapy performed best in Western countries and West Asia, while reverse hybrid therapy was most effective in East Asia.
The results “[suggest that] a new approach concerning H. pylori treatment is now needed and that the time for transitioning from trial and error to antimicrobial stewardship [of H. pylori infection] has arrived,” wrote Theodore Rokkas, PhD, MD, of the European University of Cyprus in Engomi, and colleagues. Their study was published in Gastroenterology.
H. pylori infection is the primary cause of gastritis, peptic ulcer disease, gastric mucosa–associated lymphoid tissue lymphoma, and gastric cancer.
Since H. pylori infection was first recognized, physicians have employed a range of drugs in double, triple, and quadruple combinations to combat it.
Despite those efforts, treatment success is lower than with many other infectious diseases. A newcomer is the potassium-competing acid blocker vonoprazan, which increases efficacy of amoxicillin combination therapies and has, thereby, generated renewed interest in all combination therapies, according to the study authors. Vonoprazan is currently available in some Asian countries, but not the United States or Europe.
Current guidelines for H. pylori treatment relied on randomized controlled trials and relevant pair-wise meta-analyses, but no previous pairwise analysis has included all currently available medications, the authors noted. Network meta-analyses can help fill this evidence gap: They incorporate both direct and indirect evidence from a collection of randomized controlled trials to estimate the comparative effectiveness of three or more regimens.
The researchers conducted a network meta-analysis that included 68 randomized, controlled trials totaling 22,975 patients. The following regimens were included in the analysis: Concomitant quadruple bismuth treatment (bismuth quadruple therapy), concomitant quadruple nonbismuth treatment (nonbismuth quadruple therapy), high-dose amoxicillin double treatment (Amox-dual therapy), levofloxacin-containing treatment (Levo-therapy), reverse hybrid therapy (R-hybrid therapy), sequential quadruple treatment (sequential therapy), standard triple treatment (triple therapy), and vonoprazan-containing therapy (Vono-triple therapy).
Statistically significant results were found with Vono-triple therapy versus triple therapy (odds ratio, 3.80; 95% confidence interval, 1.62-8.94), sequential therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53), nonbismuth quadruple therapy versus triple therapy (OR, 2.08; 95% CI, 1.45-2.98), bismuth quadruple therapy versus triple therapy (OR, 1.47; 95% CI, 1.02-2.11), and Levo-therapy versus triple therapy (OR, 1.79; 95% CI, 1.26-2.53).
In the overall data, mean cure rates greater than 90% were seen only in Vono-triple therapy (91.4%; 95% CI, 88.5-93.5%) and R-hybrid therapy (93.6%; 95% CI, 90.4-96.8%). Cure rates were lower for Nonbismuth quadruple therapy (84.3%; 95% CI, 82.7-85.8%), Levo-therapy (83.8%; 95% CI, 82.1-85.4%), Sequential therapy (83.7%; 95% CI, 82.7-84.7%), bismuth quadruple therapy (81.3%; 95% CI, 79.5-83.1%), Amox-dual therapy (80.2%; 75.3%-84.4%), and triple therapy (75.7%; 95% CI, 74.9-76.4%). Levo-therapy performed best in Western countries (88.5%; 95% CI, 86.5-90.5%) and West Asia (88.4%; 95% CI, 84.6-91.1%). R-hybrid therapy performed best in East Asia (93.6%; 95% CI, 90.4-96.8%).
A surface under the cumulative ranking (SUCRA) value, which represents the efficacy of the intervention compared to an ideal intervention, was 92.4% for Vono-triple therapy. The second highest SUCRA value was for 68.8% for nonbismuth quadruple therapy. The SUCRA value of standard triple therapy was 4.7%.
A key limitation to the study is that Vono-triple therapy was tested only in Japan, and requires additional study in other geographic regions.
The study received support from the Department of Veteran Affairs. The authors have consulted for and received research funding from various pharmaceutical companies.
FROM GASTROENTEROLOGY
Fitbit stats show lingering physiologic hit after COVID-19
People infected with SARS-CoV-2 can experience lingering physiologic effects after they recover, according to early data from an ongoing study that is harnessing the power of Fitbits and other wearable trackers to gauge long-term effects of COVID-19.
“To our knowledge, this is the first study to examine longer duration wearable sensor data. We found a prolonged physiological impact of COVID-19 infection, lasting approximately 2-3 months, on average, but with substantial intra-individual variability,” report Jennifer Radin, PhD, MPH, and colleagues with the Scripps Research Translational Institute, San Diego.
The study was published online July 7 in JAMA Network Open.
The DETECT study is enrolling adults from all over the United States and is collecting their health data from different wearable devices to better understand changes associated with viral illness, including COVID-19.
The current analysis focuses on a subset of 875 device wearers who reported symptoms of an acute respiratory illness and underwent testing for SARS-CoV-2. A total of 234 individuals tested positive for SARS-CoV-2; 641 were presumed to have other viral infections (COVID-19-negative symptomatic individuals).
The investigators found that among people with COVID-19, it took longer to return to baseline status with respect to resting heart rate (RHR), sleep, and activity compared with those who had symptoms of viral illness but who did not have COVID-19.
“This difference was most marked for RHR, with COVID-19-positive individuals initially experiencing a transient bradycardia followed by a prolonged relative tachycardia that did not return to baseline, on average, until 79 days after symptom onset,” Dr. Radin and colleagues reported.
Step count and sleep quantity returned to baseline values sooner than RHR, at 32 days and 24 days, respectively.
Among people with COVID-19, during recovery, trajectories differed with respect to return of RHR to normal in comparison with persons who did not have COVID-19.
The RHR of 32 COVID-19–positive participants (13.7%) remained 5 beats/min greater than their baseline RHR for more than 133 days, on average. During the acute phase of COVID-19, these individuals were more apt to report cough, body ache, and shortness of breath compared with other groups.
Limitation
The researchers say a limitation of this analysis is that symptom data were collected only during the acute phase of infection, which limits the ability to compare long-term physiologic and behavioral changes with long-term symptoms.
“In the future, with larger sample sizes and more comprehensive participant-reported outcomes, it will be possible to better understand factors associated with inter-individualized variability in COVID-19 recovery,” they concluded.
Earlier data from the DETECT study showed that pairing wearable tracker data with self-reported symptoms can improve COVID-19 prediction.
As previously reported by this news organization, DETECT investigators found that associating participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and RHR, resulted in an area under the curve of 0.80 for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
Funding for the current study was provided by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People infected with SARS-CoV-2 can experience lingering physiologic effects after they recover, according to early data from an ongoing study that is harnessing the power of Fitbits and other wearable trackers to gauge long-term effects of COVID-19.
“To our knowledge, this is the first study to examine longer duration wearable sensor data. We found a prolonged physiological impact of COVID-19 infection, lasting approximately 2-3 months, on average, but with substantial intra-individual variability,” report Jennifer Radin, PhD, MPH, and colleagues with the Scripps Research Translational Institute, San Diego.
The study was published online July 7 in JAMA Network Open.
The DETECT study is enrolling adults from all over the United States and is collecting their health data from different wearable devices to better understand changes associated with viral illness, including COVID-19.
The current analysis focuses on a subset of 875 device wearers who reported symptoms of an acute respiratory illness and underwent testing for SARS-CoV-2. A total of 234 individuals tested positive for SARS-CoV-2; 641 were presumed to have other viral infections (COVID-19-negative symptomatic individuals).
The investigators found that among people with COVID-19, it took longer to return to baseline status with respect to resting heart rate (RHR), sleep, and activity compared with those who had symptoms of viral illness but who did not have COVID-19.
“This difference was most marked for RHR, with COVID-19-positive individuals initially experiencing a transient bradycardia followed by a prolonged relative tachycardia that did not return to baseline, on average, until 79 days after symptom onset,” Dr. Radin and colleagues reported.
Step count and sleep quantity returned to baseline values sooner than RHR, at 32 days and 24 days, respectively.
Among people with COVID-19, during recovery, trajectories differed with respect to return of RHR to normal in comparison with persons who did not have COVID-19.
The RHR of 32 COVID-19–positive participants (13.7%) remained 5 beats/min greater than their baseline RHR for more than 133 days, on average. During the acute phase of COVID-19, these individuals were more apt to report cough, body ache, and shortness of breath compared with other groups.
Limitation
The researchers say a limitation of this analysis is that symptom data were collected only during the acute phase of infection, which limits the ability to compare long-term physiologic and behavioral changes with long-term symptoms.
“In the future, with larger sample sizes and more comprehensive participant-reported outcomes, it will be possible to better understand factors associated with inter-individualized variability in COVID-19 recovery,” they concluded.
Earlier data from the DETECT study showed that pairing wearable tracker data with self-reported symptoms can improve COVID-19 prediction.
As previously reported by this news organization, DETECT investigators found that associating participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and RHR, resulted in an area under the curve of 0.80 for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
Funding for the current study was provided by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People infected with SARS-CoV-2 can experience lingering physiologic effects after they recover, according to early data from an ongoing study that is harnessing the power of Fitbits and other wearable trackers to gauge long-term effects of COVID-19.
“To our knowledge, this is the first study to examine longer duration wearable sensor data. We found a prolonged physiological impact of COVID-19 infection, lasting approximately 2-3 months, on average, but with substantial intra-individual variability,” report Jennifer Radin, PhD, MPH, and colleagues with the Scripps Research Translational Institute, San Diego.
The study was published online July 7 in JAMA Network Open.
The DETECT study is enrolling adults from all over the United States and is collecting their health data from different wearable devices to better understand changes associated with viral illness, including COVID-19.
The current analysis focuses on a subset of 875 device wearers who reported symptoms of an acute respiratory illness and underwent testing for SARS-CoV-2. A total of 234 individuals tested positive for SARS-CoV-2; 641 were presumed to have other viral infections (COVID-19-negative symptomatic individuals).
The investigators found that among people with COVID-19, it took longer to return to baseline status with respect to resting heart rate (RHR), sleep, and activity compared with those who had symptoms of viral illness but who did not have COVID-19.
“This difference was most marked for RHR, with COVID-19-positive individuals initially experiencing a transient bradycardia followed by a prolonged relative tachycardia that did not return to baseline, on average, until 79 days after symptom onset,” Dr. Radin and colleagues reported.
Step count and sleep quantity returned to baseline values sooner than RHR, at 32 days and 24 days, respectively.
Among people with COVID-19, during recovery, trajectories differed with respect to return of RHR to normal in comparison with persons who did not have COVID-19.
The RHR of 32 COVID-19–positive participants (13.7%) remained 5 beats/min greater than their baseline RHR for more than 133 days, on average. During the acute phase of COVID-19, these individuals were more apt to report cough, body ache, and shortness of breath compared with other groups.
Limitation
The researchers say a limitation of this analysis is that symptom data were collected only during the acute phase of infection, which limits the ability to compare long-term physiologic and behavioral changes with long-term symptoms.
“In the future, with larger sample sizes and more comprehensive participant-reported outcomes, it will be possible to better understand factors associated with inter-individualized variability in COVID-19 recovery,” they concluded.
Earlier data from the DETECT study showed that pairing wearable tracker data with self-reported symptoms can improve COVID-19 prediction.
As previously reported by this news organization, DETECT investigators found that associating participant-reported symptoms with personal sensor data, such as deviation from normal sleep duration and RHR, resulted in an area under the curve of 0.80 for differentiating between symptomatic individuals who were positive and those who were negative for COVID-19.
Funding for the current study was provided by a grant from the National Center for Advancing Translational Sciences at the National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Limited English proficiency linked with less health care in U.S.
Jessica Himmelstein, MD, a Harvard research fellow and primary care physician at Cambridge Health Alliance in Cambridge, Mass., led a study of more than 120,000 adults published July 6, 2021. The study population included 17,776 Hispanic adults with limited English proficiency, 14,936 Hispanic adults proficient in English and 87,834 non-Hispanic, English-proficient adults.
Researchers compared several measures of care usage from information in the Agency for Healthcare Research and Quality’s Medical Expenditure Panel Survey from 1998 to 2018.
They found that, in adjusted analyses, total use of care per capita from 2014-2018, measured by health care expenditures, was $1,463 lower (98% confidence interval, $1,030-$1,897), or 35% lower for primary-Spanish speakers than for Hispanic adults who were English proficient and $2,802 lower (98% CI, $2,356-$3,247), or 42% lower versus non-Hispanic adults who were English proficient.
Spanish speakers also had 36% fewer outpatient visits and 48% fewer prescription medications than non-Hispanic adults, and 35% fewer outpatient visits and 37% fewer prescription medications than English-proficient Hispanic adults.
Even when accounting for differences in health, age, sex, income and insurance, adults with language barriers fared worse.
Gaps span all types of care
The services that those with limited English skills are missing are “the types of care people need to lead a healthy life,” from routine visits and medications to urgent or emergency care, Dr. Himmelstein said in an interview.
She said the gaps were greater in outpatient care and in medication use, compared with emergency department visits and inpatient care, but the inequities were present in all the categories she and her coinvestigators studied.
Underlying causes for having less care may include that people who struggle with English may not feel comfortable accessing the health system or may feel unwelcome or discriminated against.
“An undercurrent of biases, including racism, could also be contributing,” she said.
The data show that, despite several federal policy changes aimed at promoting language services in hospitals and clinics, several language-based disparities have not improved over 2 decades.
Some of the changes have included an executive order in 2000 requiring interpreters to be available in federally funded health facilities. In 2010, the Affordable Care Act enhanced the definition of meaningful access to language services and setting standards for qualified interpreters.
Gap widened over 2 decades
The adjusted gap in annual health care expenditures per capita between adults with limited English skills and non-Hispanic, English-proficient adults widened by $1,596 (98% CI, $837-$2,356) between 1999-2000 and 2017-2018, after accounting for inflation.
Dr. Himmelstein said that though this study period predated COVID-19, its findings may help explain the disproportionate burden the pandemic placed on the Hispanic population.
“This is a community that traditionally wasn’t getting access to care and then suddenly something like COVID-19 comes and they were even more devastated,” she noted.
Telehealth, which proved an important way to access care during the pandemic, also added a degree of communication difficulty for those with fewer English skills, she said.
Many of the telehealth changes are here to stay, and it will be important to ask: “Are we ensuring equity in telehealth use for individuals who face language barriers?” Dr. Himmelstein said.
Olga Garcia-Bedoya, MD, an associate professor at University of Illinois at Chicago’s department of medicine and medical director of UIC’s Institute for Minority Health Research, said having access to interpreters with high accuracy is key to narrowing the gaps.
“The literature is very clear that access to professional medical interpreters is associated with decreased health disparities for patients with limited English proficiency,” she said.
More cultural training for clinicians is needed surrounding beliefs about illness and that some care may be declined not because of a person’s limited English proficiency, but because their beliefs may keep them from getting care, Dr. Garcia-Bedoya added. When it comes to getting a flu shot, for example, sometimes belief systems, rather than English proficiency, keep people from accessing care.
What can be done?
Addressing barriers caused by lack of English proficiency will likely take change in policies, including one related reimbursement for medical interpreters, Dr. Himmelstein said.
Currently, only 15 states’ Medicaid programs or Children’s Health Insurance Programs reimburse providers for language services, the paper notes, and neither Medicare nor private insurers routinely pay for those services.
Recruiting bilingual providers and staff at health care facilities and in medical and nursing schools will also be important to narrow the gaps, Dr. Himmelstein said.
Strengthening standards for interpreters also will help. “Currently such standards vary by state or by institution and are not necessarily enforced,” she explained.
It will also be important to make sure patients know that they are entitled by law to care, free of discriminatory practices and to have certain language services including qualified interpreters, Dr. Himmelstein said.
Dr. Garcia-Bedoya said changes need to come from health systems working in combination with clinicians, providing resources so that quality interpreters can be accessed and making sure that equipment supports clear communication in telehealth. Patients’ language preferences should also be noted as soon as they make the appointment.
The findings of the study may have large significance as one in seven people in the United States speak Spanish at home, and 25 million people in the United States have limited English proficiency, the authors noted.
Dr. Himmelstein receives funding support from an Institutional National Research Service Award. Dr. Garcia-Bedoya reports no relevant financial relationships.
Jessica Himmelstein, MD, a Harvard research fellow and primary care physician at Cambridge Health Alliance in Cambridge, Mass., led a study of more than 120,000 adults published July 6, 2021. The study population included 17,776 Hispanic adults with limited English proficiency, 14,936 Hispanic adults proficient in English and 87,834 non-Hispanic, English-proficient adults.
Researchers compared several measures of care usage from information in the Agency for Healthcare Research and Quality’s Medical Expenditure Panel Survey from 1998 to 2018.
They found that, in adjusted analyses, total use of care per capita from 2014-2018, measured by health care expenditures, was $1,463 lower (98% confidence interval, $1,030-$1,897), or 35% lower for primary-Spanish speakers than for Hispanic adults who were English proficient and $2,802 lower (98% CI, $2,356-$3,247), or 42% lower versus non-Hispanic adults who were English proficient.
Spanish speakers also had 36% fewer outpatient visits and 48% fewer prescription medications than non-Hispanic adults, and 35% fewer outpatient visits and 37% fewer prescription medications than English-proficient Hispanic adults.
Even when accounting for differences in health, age, sex, income and insurance, adults with language barriers fared worse.
Gaps span all types of care
The services that those with limited English skills are missing are “the types of care people need to lead a healthy life,” from routine visits and medications to urgent or emergency care, Dr. Himmelstein said in an interview.
She said the gaps were greater in outpatient care and in medication use, compared with emergency department visits and inpatient care, but the inequities were present in all the categories she and her coinvestigators studied.
Underlying causes for having less care may include that people who struggle with English may not feel comfortable accessing the health system or may feel unwelcome or discriminated against.
“An undercurrent of biases, including racism, could also be contributing,” she said.
The data show that, despite several federal policy changes aimed at promoting language services in hospitals and clinics, several language-based disparities have not improved over 2 decades.
Some of the changes have included an executive order in 2000 requiring interpreters to be available in federally funded health facilities. In 2010, the Affordable Care Act enhanced the definition of meaningful access to language services and setting standards for qualified interpreters.
Gap widened over 2 decades
The adjusted gap in annual health care expenditures per capita between adults with limited English skills and non-Hispanic, English-proficient adults widened by $1,596 (98% CI, $837-$2,356) between 1999-2000 and 2017-2018, after accounting for inflation.
Dr. Himmelstein said that though this study period predated COVID-19, its findings may help explain the disproportionate burden the pandemic placed on the Hispanic population.
“This is a community that traditionally wasn’t getting access to care and then suddenly something like COVID-19 comes and they were even more devastated,” she noted.
Telehealth, which proved an important way to access care during the pandemic, also added a degree of communication difficulty for those with fewer English skills, she said.
Many of the telehealth changes are here to stay, and it will be important to ask: “Are we ensuring equity in telehealth use for individuals who face language barriers?” Dr. Himmelstein said.
Olga Garcia-Bedoya, MD, an associate professor at University of Illinois at Chicago’s department of medicine and medical director of UIC’s Institute for Minority Health Research, said having access to interpreters with high accuracy is key to narrowing the gaps.
“The literature is very clear that access to professional medical interpreters is associated with decreased health disparities for patients with limited English proficiency,” she said.
More cultural training for clinicians is needed surrounding beliefs about illness and that some care may be declined not because of a person’s limited English proficiency, but because their beliefs may keep them from getting care, Dr. Garcia-Bedoya added. When it comes to getting a flu shot, for example, sometimes belief systems, rather than English proficiency, keep people from accessing care.
What can be done?
Addressing barriers caused by lack of English proficiency will likely take change in policies, including one related reimbursement for medical interpreters, Dr. Himmelstein said.
Currently, only 15 states’ Medicaid programs or Children’s Health Insurance Programs reimburse providers for language services, the paper notes, and neither Medicare nor private insurers routinely pay for those services.
Recruiting bilingual providers and staff at health care facilities and in medical and nursing schools will also be important to narrow the gaps, Dr. Himmelstein said.
Strengthening standards for interpreters also will help. “Currently such standards vary by state or by institution and are not necessarily enforced,” she explained.
It will also be important to make sure patients know that they are entitled by law to care, free of discriminatory practices and to have certain language services including qualified interpreters, Dr. Himmelstein said.
Dr. Garcia-Bedoya said changes need to come from health systems working in combination with clinicians, providing resources so that quality interpreters can be accessed and making sure that equipment supports clear communication in telehealth. Patients’ language preferences should also be noted as soon as they make the appointment.
The findings of the study may have large significance as one in seven people in the United States speak Spanish at home, and 25 million people in the United States have limited English proficiency, the authors noted.
Dr. Himmelstein receives funding support from an Institutional National Research Service Award. Dr. Garcia-Bedoya reports no relevant financial relationships.
Jessica Himmelstein, MD, a Harvard research fellow and primary care physician at Cambridge Health Alliance in Cambridge, Mass., led a study of more than 120,000 adults published July 6, 2021. The study population included 17,776 Hispanic adults with limited English proficiency, 14,936 Hispanic adults proficient in English and 87,834 non-Hispanic, English-proficient adults.
Researchers compared several measures of care usage from information in the Agency for Healthcare Research and Quality’s Medical Expenditure Panel Survey from 1998 to 2018.
They found that, in adjusted analyses, total use of care per capita from 2014-2018, measured by health care expenditures, was $1,463 lower (98% confidence interval, $1,030-$1,897), or 35% lower for primary-Spanish speakers than for Hispanic adults who were English proficient and $2,802 lower (98% CI, $2,356-$3,247), or 42% lower versus non-Hispanic adults who were English proficient.
Spanish speakers also had 36% fewer outpatient visits and 48% fewer prescription medications than non-Hispanic adults, and 35% fewer outpatient visits and 37% fewer prescription medications than English-proficient Hispanic adults.
Even when accounting for differences in health, age, sex, income and insurance, adults with language barriers fared worse.
Gaps span all types of care
The services that those with limited English skills are missing are “the types of care people need to lead a healthy life,” from routine visits and medications to urgent or emergency care, Dr. Himmelstein said in an interview.
She said the gaps were greater in outpatient care and in medication use, compared with emergency department visits and inpatient care, but the inequities were present in all the categories she and her coinvestigators studied.
Underlying causes for having less care may include that people who struggle with English may not feel comfortable accessing the health system or may feel unwelcome or discriminated against.
“An undercurrent of biases, including racism, could also be contributing,” she said.
The data show that, despite several federal policy changes aimed at promoting language services in hospitals and clinics, several language-based disparities have not improved over 2 decades.
Some of the changes have included an executive order in 2000 requiring interpreters to be available in federally funded health facilities. In 2010, the Affordable Care Act enhanced the definition of meaningful access to language services and setting standards for qualified interpreters.
Gap widened over 2 decades
The adjusted gap in annual health care expenditures per capita between adults with limited English skills and non-Hispanic, English-proficient adults widened by $1,596 (98% CI, $837-$2,356) between 1999-2000 and 2017-2018, after accounting for inflation.
Dr. Himmelstein said that though this study period predated COVID-19, its findings may help explain the disproportionate burden the pandemic placed on the Hispanic population.
“This is a community that traditionally wasn’t getting access to care and then suddenly something like COVID-19 comes and they were even more devastated,” she noted.
Telehealth, which proved an important way to access care during the pandemic, also added a degree of communication difficulty for those with fewer English skills, she said.
Many of the telehealth changes are here to stay, and it will be important to ask: “Are we ensuring equity in telehealth use for individuals who face language barriers?” Dr. Himmelstein said.
Olga Garcia-Bedoya, MD, an associate professor at University of Illinois at Chicago’s department of medicine and medical director of UIC’s Institute for Minority Health Research, said having access to interpreters with high accuracy is key to narrowing the gaps.
“The literature is very clear that access to professional medical interpreters is associated with decreased health disparities for patients with limited English proficiency,” she said.
More cultural training for clinicians is needed surrounding beliefs about illness and that some care may be declined not because of a person’s limited English proficiency, but because their beliefs may keep them from getting care, Dr. Garcia-Bedoya added. When it comes to getting a flu shot, for example, sometimes belief systems, rather than English proficiency, keep people from accessing care.
What can be done?
Addressing barriers caused by lack of English proficiency will likely take change in policies, including one related reimbursement for medical interpreters, Dr. Himmelstein said.
Currently, only 15 states’ Medicaid programs or Children’s Health Insurance Programs reimburse providers for language services, the paper notes, and neither Medicare nor private insurers routinely pay for those services.
Recruiting bilingual providers and staff at health care facilities and in medical and nursing schools will also be important to narrow the gaps, Dr. Himmelstein said.
Strengthening standards for interpreters also will help. “Currently such standards vary by state or by institution and are not necessarily enforced,” she explained.
It will also be important to make sure patients know that they are entitled by law to care, free of discriminatory practices and to have certain language services including qualified interpreters, Dr. Himmelstein said.
Dr. Garcia-Bedoya said changes need to come from health systems working in combination with clinicians, providing resources so that quality interpreters can be accessed and making sure that equipment supports clear communication in telehealth. Patients’ language preferences should also be noted as soon as they make the appointment.
The findings of the study may have large significance as one in seven people in the United States speak Spanish at home, and 25 million people in the United States have limited English proficiency, the authors noted.
Dr. Himmelstein receives funding support from an Institutional National Research Service Award. Dr. Garcia-Bedoya reports no relevant financial relationships.
FROM HEALTH AFFAIRS
Delta variant key to breakthrough infections in vaccinated Israelis
Israeli officials are reporting a 30% decrease in the effectiveness of the Pfizer/BioNTech vaccine to prevent SARS-CoV-2 infection and mild to moderate cases of COVID-19. At the same time, protection against hospitalization and severe illness remains robust.
The country’s Ministry of Health data cited high levels of circulating Delta variant and a relaxation of public health measures in early June for the drop in the vaccine’s prevention of “breakthrough” cases from 94% to 64% in recent weeks.
However, it is important to consider the findings in context, experts cautioned.
“My overall take on this that the vaccine is highly protective against the endpoints that matter – hospitalization and severe disease,” Anna Durbin, MD, told this news organization.
“I was very pleasantly surprised with the very high efficacy against hospitalization and severe disease – even against the Delta variant,” added Dr. Durbin, professor of medicine at Johns Hopkins University, Baltimore.
Ali Mokdad, PhD, of the Institute for Health Metrics at the University of Washington, Seattle, agreed that the high degree of protection against severe outcomes should be the focus.
“That’s the whole idea. You want to defend against COVID-19. So even if someone is infected, they don’t end up in the hospital or in the morgue,” he said in an interview.
Compared with an earlier report, the efficacy of the Pfizer vaccine against hospitalization fell slightly from 98% to 93%.
“For me, the fact that there is increased infection from the Delta variant after the vaccines such as Pfizer is of course a concern. But the positive news is that there is 93% prevention against severe disease or mortality,” added Dr. Mokdad, who is also professor of global health at University of Washington.
In addition, the absolute numbers remain relatively small. The Ministry of Health data show that, of the 63 Israelis hospitalized with COVID-19 nationwide on July 3, 34 were in critical condition.
Unrealistic expectations?
People may have unrealistic expectations regarding breakthrough infections, Dr. Durbin said. “It seems that people are almost expecting ‘sterilizing immunity’ from these vaccines,” she said, explaining that would mean complete protection from infection.
Expectations may be high “because these vaccines have been so effective,” added Dr. Durbin, who is also affiliated with the Johns Hopkins Center for Global Health.
The higher the number of vaccinated residents, the more breakthrough cases will be reported, epidemiologist Katelyn Jetelina, PhD, MPH, assistant professor of epidemiology, human genetics, and environmental sciences at the University of Texas Science Center at Houston, wrote in her “Your Local Epidemiologist” blog.
This could apply to Israel, with an estimated 60% of adults in Israel fully vaccinated and 65% receiving at least one dose as of July 5, Our World in Data figures show.
How the updated figures were reported could be confusing, Dr. Jetelina said. Israel’s Health Minister Chezy Levy noted that “55% of the newly infected had been vaccinated” in a radio interview announcing the results.
“This language is important because it’s very different than ‘half of vaccinated people were infected,’ ” Dr. Jetelina noted.
Israel had a 7-day rolling average of 324 new confirmed COVID-19 cases as of July 5. Assuming 55% of these cases were among vaccinated people, that would mean 178 people experienced breakthrough infections.
In contrast, almost 6 million people in Israel are fully vaccinated. If 55% of them experienced breakthrough infections, the number would be much higher – more than 3 million.
Dr. Jetelina added that more details about the new Israel figures would be helpful, including the severity of COVID-19 among the vaccinated cases and breakdown of infections between adults and children.
Next steps
Israeli health officials are weighing the necessity of a third or booster dose of the vaccine. Whether they will reinstate public health measures to prevent spread of COVID-19 also remains unknown.
Going forward, Israel intends to study whether factors such as age, comorbidities, or time since immunization affect risk for breakthrough infections among people vaccinated against COVID-19.
“We want to prevent people from getting hospitalized, seriously ill, and of course, dying. It’s encouraging these vaccines will be able to have a high impact on those outcomes,” Dr. Durbin said. “We just need to get people vaccinated.”
A call for better global surveillance
A global surveillance system is a potential solution to track and respond to the growing threat of the Delta variant and other variants of concern, Scott P. Layne, MD, and Jeffery K. Taubenberger, MD, PhD, wrote in a July 7, 2021, editorial in Science Translational Medicine.
One goal, Dr. Layne said in an interview, is to highlight “the compelling need for a new global COVID-19 program of surveillance and offer a blueprint for building it.” A second aim is to promote global cooperation among key advisers and leaders in the G7, G20, and Asia-Pacific Economic Cooperation nations.
“It’s an uphill struggle with superpower discords, global warming, cybersecurity, and pandemics all competing for finite attention,” Dr. Layne said. “However, what other options do we have for taming the so-called forever virus?”
Dr. Mokdad and Dr. Jetelina had no relevant disclosures. Dr. Durban disclosed she was the site primary investigator for the phase 3 AstraZeneca vaccine trial and an investigator on the Pfizer COVID-19 vaccine trial.
A version of this article first appeared on Medscape.com.
Israeli officials are reporting a 30% decrease in the effectiveness of the Pfizer/BioNTech vaccine to prevent SARS-CoV-2 infection and mild to moderate cases of COVID-19. At the same time, protection against hospitalization and severe illness remains robust.
The country’s Ministry of Health data cited high levels of circulating Delta variant and a relaxation of public health measures in early June for the drop in the vaccine’s prevention of “breakthrough” cases from 94% to 64% in recent weeks.
However, it is important to consider the findings in context, experts cautioned.
“My overall take on this that the vaccine is highly protective against the endpoints that matter – hospitalization and severe disease,” Anna Durbin, MD, told this news organization.
“I was very pleasantly surprised with the very high efficacy against hospitalization and severe disease – even against the Delta variant,” added Dr. Durbin, professor of medicine at Johns Hopkins University, Baltimore.
Ali Mokdad, PhD, of the Institute for Health Metrics at the University of Washington, Seattle, agreed that the high degree of protection against severe outcomes should be the focus.
“That’s the whole idea. You want to defend against COVID-19. So even if someone is infected, they don’t end up in the hospital or in the morgue,” he said in an interview.
Compared with an earlier report, the efficacy of the Pfizer vaccine against hospitalization fell slightly from 98% to 93%.
“For me, the fact that there is increased infection from the Delta variant after the vaccines such as Pfizer is of course a concern. But the positive news is that there is 93% prevention against severe disease or mortality,” added Dr. Mokdad, who is also professor of global health at University of Washington.
In addition, the absolute numbers remain relatively small. The Ministry of Health data show that, of the 63 Israelis hospitalized with COVID-19 nationwide on July 3, 34 were in critical condition.
Unrealistic expectations?
People may have unrealistic expectations regarding breakthrough infections, Dr. Durbin said. “It seems that people are almost expecting ‘sterilizing immunity’ from these vaccines,” she said, explaining that would mean complete protection from infection.
Expectations may be high “because these vaccines have been so effective,” added Dr. Durbin, who is also affiliated with the Johns Hopkins Center for Global Health.
The higher the number of vaccinated residents, the more breakthrough cases will be reported, epidemiologist Katelyn Jetelina, PhD, MPH, assistant professor of epidemiology, human genetics, and environmental sciences at the University of Texas Science Center at Houston, wrote in her “Your Local Epidemiologist” blog.
This could apply to Israel, with an estimated 60% of adults in Israel fully vaccinated and 65% receiving at least one dose as of July 5, Our World in Data figures show.
How the updated figures were reported could be confusing, Dr. Jetelina said. Israel’s Health Minister Chezy Levy noted that “55% of the newly infected had been vaccinated” in a radio interview announcing the results.
“This language is important because it’s very different than ‘half of vaccinated people were infected,’ ” Dr. Jetelina noted.
Israel had a 7-day rolling average of 324 new confirmed COVID-19 cases as of July 5. Assuming 55% of these cases were among vaccinated people, that would mean 178 people experienced breakthrough infections.
In contrast, almost 6 million people in Israel are fully vaccinated. If 55% of them experienced breakthrough infections, the number would be much higher – more than 3 million.
Dr. Jetelina added that more details about the new Israel figures would be helpful, including the severity of COVID-19 among the vaccinated cases and breakdown of infections between adults and children.
Next steps
Israeli health officials are weighing the necessity of a third or booster dose of the vaccine. Whether they will reinstate public health measures to prevent spread of COVID-19 also remains unknown.
Going forward, Israel intends to study whether factors such as age, comorbidities, or time since immunization affect risk for breakthrough infections among people vaccinated against COVID-19.
“We want to prevent people from getting hospitalized, seriously ill, and of course, dying. It’s encouraging these vaccines will be able to have a high impact on those outcomes,” Dr. Durbin said. “We just need to get people vaccinated.”
A call for better global surveillance
A global surveillance system is a potential solution to track and respond to the growing threat of the Delta variant and other variants of concern, Scott P. Layne, MD, and Jeffery K. Taubenberger, MD, PhD, wrote in a July 7, 2021, editorial in Science Translational Medicine.
One goal, Dr. Layne said in an interview, is to highlight “the compelling need for a new global COVID-19 program of surveillance and offer a blueprint for building it.” A second aim is to promote global cooperation among key advisers and leaders in the G7, G20, and Asia-Pacific Economic Cooperation nations.
“It’s an uphill struggle with superpower discords, global warming, cybersecurity, and pandemics all competing for finite attention,” Dr. Layne said. “However, what other options do we have for taming the so-called forever virus?”
Dr. Mokdad and Dr. Jetelina had no relevant disclosures. Dr. Durban disclosed she was the site primary investigator for the phase 3 AstraZeneca vaccine trial and an investigator on the Pfizer COVID-19 vaccine trial.
A version of this article first appeared on Medscape.com.
Israeli officials are reporting a 30% decrease in the effectiveness of the Pfizer/BioNTech vaccine to prevent SARS-CoV-2 infection and mild to moderate cases of COVID-19. At the same time, protection against hospitalization and severe illness remains robust.
The country’s Ministry of Health data cited high levels of circulating Delta variant and a relaxation of public health measures in early June for the drop in the vaccine’s prevention of “breakthrough” cases from 94% to 64% in recent weeks.
However, it is important to consider the findings in context, experts cautioned.
“My overall take on this that the vaccine is highly protective against the endpoints that matter – hospitalization and severe disease,” Anna Durbin, MD, told this news organization.
“I was very pleasantly surprised with the very high efficacy against hospitalization and severe disease – even against the Delta variant,” added Dr. Durbin, professor of medicine at Johns Hopkins University, Baltimore.
Ali Mokdad, PhD, of the Institute for Health Metrics at the University of Washington, Seattle, agreed that the high degree of protection against severe outcomes should be the focus.
“That’s the whole idea. You want to defend against COVID-19. So even if someone is infected, they don’t end up in the hospital or in the morgue,” he said in an interview.
Compared with an earlier report, the efficacy of the Pfizer vaccine against hospitalization fell slightly from 98% to 93%.
“For me, the fact that there is increased infection from the Delta variant after the vaccines such as Pfizer is of course a concern. But the positive news is that there is 93% prevention against severe disease or mortality,” added Dr. Mokdad, who is also professor of global health at University of Washington.
In addition, the absolute numbers remain relatively small. The Ministry of Health data show that, of the 63 Israelis hospitalized with COVID-19 nationwide on July 3, 34 were in critical condition.
Unrealistic expectations?
People may have unrealistic expectations regarding breakthrough infections, Dr. Durbin said. “It seems that people are almost expecting ‘sterilizing immunity’ from these vaccines,” she said, explaining that would mean complete protection from infection.
Expectations may be high “because these vaccines have been so effective,” added Dr. Durbin, who is also affiliated with the Johns Hopkins Center for Global Health.
The higher the number of vaccinated residents, the more breakthrough cases will be reported, epidemiologist Katelyn Jetelina, PhD, MPH, assistant professor of epidemiology, human genetics, and environmental sciences at the University of Texas Science Center at Houston, wrote in her “Your Local Epidemiologist” blog.
This could apply to Israel, with an estimated 60% of adults in Israel fully vaccinated and 65% receiving at least one dose as of July 5, Our World in Data figures show.
How the updated figures were reported could be confusing, Dr. Jetelina said. Israel’s Health Minister Chezy Levy noted that “55% of the newly infected had been vaccinated” in a radio interview announcing the results.
“This language is important because it’s very different than ‘half of vaccinated people were infected,’ ” Dr. Jetelina noted.
Israel had a 7-day rolling average of 324 new confirmed COVID-19 cases as of July 5. Assuming 55% of these cases were among vaccinated people, that would mean 178 people experienced breakthrough infections.
In contrast, almost 6 million people in Israel are fully vaccinated. If 55% of them experienced breakthrough infections, the number would be much higher – more than 3 million.
Dr. Jetelina added that more details about the new Israel figures would be helpful, including the severity of COVID-19 among the vaccinated cases and breakdown of infections between adults and children.
Next steps
Israeli health officials are weighing the necessity of a third or booster dose of the vaccine. Whether they will reinstate public health measures to prevent spread of COVID-19 also remains unknown.
Going forward, Israel intends to study whether factors such as age, comorbidities, or time since immunization affect risk for breakthrough infections among people vaccinated against COVID-19.
“We want to prevent people from getting hospitalized, seriously ill, and of course, dying. It’s encouraging these vaccines will be able to have a high impact on those outcomes,” Dr. Durbin said. “We just need to get people vaccinated.”
A call for better global surveillance
A global surveillance system is a potential solution to track and respond to the growing threat of the Delta variant and other variants of concern, Scott P. Layne, MD, and Jeffery K. Taubenberger, MD, PhD, wrote in a July 7, 2021, editorial in Science Translational Medicine.
One goal, Dr. Layne said in an interview, is to highlight “the compelling need for a new global COVID-19 program of surveillance and offer a blueprint for building it.” A second aim is to promote global cooperation among key advisers and leaders in the G7, G20, and Asia-Pacific Economic Cooperation nations.
“It’s an uphill struggle with superpower discords, global warming, cybersecurity, and pandemics all competing for finite attention,” Dr. Layne said. “However, what other options do we have for taming the so-called forever virus?”
Dr. Mokdad and Dr. Jetelina had no relevant disclosures. Dr. Durban disclosed she was the site primary investigator for the phase 3 AstraZeneca vaccine trial and an investigator on the Pfizer COVID-19 vaccine trial.
A version of this article first appeared on Medscape.com.
Clostridioides difficile: Two sets of guidelines disagree
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
With two sets of Clostridioides difficile recommendations being published within a month of each other, clinicians may find themselves trying to reconcile some of the conflicts between the two guidelines.
The first set, published June 1 by the American College of Gastroenterology, focuses on fecal microbiota transplantation (FMT) and the antibiotic vancomycin. The second, published June 24 by the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America, drives a shift in treatment for initial episodes and short-term recurrence from vancomycin to fidaxomicin and, in some cases, adding on the monoclonal antibody bezlotoxumab, both made by Merck.
The updates are timely because researchers are now recognizing that C. difficile can colonize people without causing symptoms, David Johnson, MD, professor of medicine and chief of gastroenterology at the Eastern Virginia School of Medicine, Norfolk, said in an interview. He was not involved in writing either set of guidelines. “C. diff infection was a hospital-type infection, but we’re now seeing it in up to approximately 35%-50% of patients coming from the community, so it’s a big concern.”
Although the guidelines agree on which treatments are effective, the recommendations give the options a different emphasis.
Infectious disease specialist Stuart Johnson, MD, professor of medicine at Loyola University Medical Center in Maywood, Ill., and a physician researcher at Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., is the first author in the IDSA/SHEA guidelines. He told this news organization that one reason the two sets of recommendations may diverge in emphasis for initial and recurrent C. difficile is that “everyone has a different way of looking at things.” Compared with infectious disease specialists like him, he said, gastroenterologists “for the most part see the world a little different and have their own bent on things.”
The differences between the two guidelines relate to the first-line therapy for people with an initial or recurrent C. difficile episode. For an initial episode, the IDSA/SHEA authors conditionally recommend fidaxomicin as first preferred choice over vancomycin, with a moderate certainty of evidence. They noted that implementing this recommendation depends on “available resources,” a reference to the higher cost and difficulty of access associated with fidaxomicin.
Gastroenterologist Monika Fischer, MD, an associate professor of medicine at Indiana University, Indianapolis, is one of the authors of the ACG guidelines. She told this news organization that the cost difference between fidaxomicin and vancomycin is considerable and finds the choice to foreground fidaxomicin puzzling. “They did not reference any new data compared to those we have published.” Their recommendation may make sense in terms of efficacy, but real-world demands require attention to cost and reimbursement. “They themselves state this in their recommendations,” she noted.
Dr. Fischer cited a ballpark of about $100 for a course of vancomycin, compared with about $3,000 for a course of fidaxomicin. The IDSA/SHEA guidelines do cite vancomycin as an acceptable alternative. According to Dr. Fischer, the ACG guidelines authors discussed fidaxomicin and concluded that there just wasn’t enough evidence to justify favoring this antibiotic over vancomycin, given the cost-benefit imbalance. The ACG guidelines call for a standard course of oral vancomycin for a first, nonsevere C. difficile episode, listing oral fidaxomicin or oral metronidazole as alternatives.
For a recurrence, the IDSA/SHEA authors also favor fidaxomicin in a conditional recommendation over a standard course of vancomycin. For multiple recurrences, a tapered and pulsed vancomycin regimen, vancomycin followed by rifaximin, or FMT are also options.
Dr. David Johnson said that these recommendations favoring fidaxomicin are “surprising,” and that lower costs of vancomycin outweigh the benefit of fidaxomicin, given more-or-less comparable data on cure rates.
In contrast, the ACG guidelines recommend that an initial recurrence be treated with a tapering dose of vancomycin, and call for FMT for patients who are eligible and who experience a second or more C. difficile recurrences after a round of pulsed vancomycin.
Dr. Stuart Johnson said that FMT carries its own special set of issues. “If you don’t have a donor program set up, you have to rely on a stool bank,” noting that one widely used stool bank “basically had to stop making the product because of the coronavirus.” Costs for FMT products have doubled in recent years, and because Food and Drug Administration approval of the therapy is lacking, insurance does not cover it.
Dr. David Johnson also said that he is not “terribly happy” about the ACG recommendation for vancomycin prophylaxis. “It may help, but it also can have off-target effects against colonic bacterial flora, so we would not agree with that recommendation.”
The IDSA/SHEA authors also conditionally recommend bezlotoxumab, on very low certainty of evidence, as a cotherapy with standard of care antibiotics for recurrence prevention in patients with an episode in the last 6 months, particularly for patients at high recurrence risk “where logistics is not an issue.” The FDA has warned that this monoclonal antibody should be used with great care in patients with heart failure and only when benefits outweigh risks.
The ACG guidelines conditionally recommend considering bezlotoxumab to prevent recurrence in patients with specific risk factors, including age over 65 years and severe presentation. The IDSA/SHEA guidelines expand this population to anyone with a recurrence within 6 months, Dr. Fischer pointed out.
The antibody treatment “does offer another 10% absolute reduction in recurrent C. diff disease,” said Dr. Stuart Johnson, which is a “helpful option and primarily for people who have had recurrent C. diff already.” In general, he said, for both drugs, “access is still something we have to work with.”
In a commentary on the ACG guidelines, Dr. David Johnson wrote that there is good evidence that bezlotoxumab prevents relapse, especially in patients with specific risk factors. The hitch is the $4,500 price tag for a 1,000-mg vial, with a recommended dose of 10 mg/kg.
Dr. Stuart Johnson agreed that the costs of the fidaxomicin and bezlotoxumab are important considerations. In addition, there are logistical issues with using the antibody because most hospitals don’t offer infusions, which pushes patients to infusion centers.
Regardless, he added, “we’re happy that we have new options.”
Dr. Fischer, Dr. Stuart Johnson, and Dr. David Johnson reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Extra COVID-19 vaccine could help immunocompromised people
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People whose immune systems are compromised by therapy or disease may benefit from additional doses of vaccines against SARS-CoV-2, researchers say.
In a study involving 101 people with solid-organ transplants, there was a significant boost in antibodies after the patients received third doses of the Pfizer vaccine, said Nassim Kamar, MD, PhD, professor of nephrology at Toulouse University Hospital, France.
None of the transplant patients had antibodies against the virus before their first dose of the vaccine, and only 4% produced antibodies after the first dose. That proportion rose to 40% after the second dose and to 68% after the third dose.
The effect is so strong that Dr. Kamar and colleagues at Toulouse University Hospital routinely administer three doses of mRNA vaccines to patients with solid-organ transplant without testing them for antibodies.
“When we observed that the second dose was not sufficient to have an immune response, the Francophone Society of Transplantation asked the National Health Authority to allow the third dose,” he told this news organization.
That agency on April 11 approved third doses of mRNA vaccines not only for people with solid-organ transplants but also for those with recent bone marrow transplants, those undergoing dialysis, and those with autoimmune diseases who were receiving strong immunosuppressive treatment, such as anti-CD20 or antimetabolites. Contrary to their procedure for people with solid-organ transplants, clinicians at Toulouse University Hospital test these patients for antibodies and administer third doses of vaccine only to those who test negative or have very low titers.
The researchers’ findings, published on June 23 as a letter to the editor of The New England Journal of Medicine, come as other researchers document more and more categories of patients whose responses to the vaccines typically fall short.
A study at the University of Pittsburgh that was published as a preprint on MedRxiv compared people with various health conditions to healthy health care workers. People with HIV who were taking antivirals against that virus responded almost as well as did the health care workers, said John Mellors, MD, chief of infectious diseases at the university. But people whose immune systems were compromised for other reasons fared less well.
“The areas of concern are hematological malignancy and solid-organ transplants, with the most nonresponsive groups being those who have had lung transplantation,” he said in an interview.
For patients with liver disease, mixed news came from the International Liver Congress (ILC) 2021 annual meeting.
In a study involving patients with liver disease who had received the Pfizer vaccine at Hadassah University Medical Center in Jerusalem, antibody titers were lower in patients who had received liver transplants or who had advanced liver fibrosis, as reported by this news organization.
A multicenter study in China that was presented at the ILC meeting and that was also published in the Journal of Hepatology, provided a more optimistic picture. Among patients with nonalcoholic fatty liver disease who were immunized against SARS-CoV-2 with the Sinopharm vaccine, 95.5% had neutralizing antibodies; the median neutralizing antibody titer was 32.
In the Toulouse University Hospital study, for the 40 patients who were seropositive after the second dose, antibody titers increased from 36 before the third dose to 2,676 a month after the third dose, a statistically significant result (P < .001).
For patients whose immune systems are compromised for reasons other than having received a transplant, clinicians at Toulouse University Hospital use a titer of 14 as the threshold below which they administer a third dose of mRNA vaccines. But Dr. Kamar acknowledged that the threshold is arbitrary and that the assays for antibodies with different vaccines in different populations can’t be compared head to head.
“We can’t tell you simply on the basis of the amount of antibody in your laboratory report how protected you are,” agreed William Schaffner, MD, professor of infectious diseases at Vanderbilt University, Nashville, Tenn., who is a spokesperson for the Infectious Diseases Society of America.
Not enough research has been done to establish that relationship, and results vary from one laboratory to another, he said.
That doesn’t mean that antibody tests don’t help, Dr. Schaffner said. On the basis of views of other experts he has consulted, Dr. Schaffner recommends that people who are immunocompromised undergo an antibody test. If the test is positive – meaning they have some antibodies to SARS-CoV-2, however low the titers – patients can take fewer precautions than before they were vaccinated.
But they should still be more cautious than people with healthy immune systems, he said. “Would I be going to large indoor gatherings without a mask? No. Would I be outside without a mask? Yes. Would I gather with three other people who are vaccinated to play a game of bridge? Yes. You have to titrate things a little and use some common sense,” he added.
If the results are negative, such patients may still be protected. Much research remains to be done on T-cell immunity, a second line of defense against the virus. And the current assays often produce false negative results. But to be on the safe side, people with this result should assume that their vaccine is not protecting them, Dr. Schaffner said.
That suggestion contradicts the Food and Drug Administration, which issued a recommendation on May 19 against using antibody tests to check the effectiveness of SARS-CoV-2 vaccination.
The studies so far suggest that vaccines are safe for people whose immune systems are compromised, Dr. Schaffner and Dr. Kamar agreed. Dr. Kamar is aware of only two case reports of transplant patients rejecting their transplants after vaccination. One of these was his own patient, and the rejection occurred after her second dose. She has not needed dialysis, although her kidney function was impaired.
But the FDA has not approved additional doses of SARS-CoV-2 vaccine to treat patients who are immunocompromised, and Dr. Kamar has not heard of any other national regulatory agencies that have.
In the United States, it may be difficult for anyone to obtain a third dose of vaccine outside of a clinical trial, Dr. Schaffner said, because vaccinators are likely to check databases and deny vaccination to anyone who has already received the recommended number.
Dr. Kamar, Dr. Mellors, and Dr. Schaffner have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
California’s highest COVID infection rates shift to rural counties
Most of us are familiar with the good news: In recent weeks, rates of COVID-19 infection and death have plummeted in California, falling to levels not seen since the early days of the pandemic. The average number of new COVID infections reported each day dropped by an astounding 98% from December to June, according to figures from the California Department of Public Health.
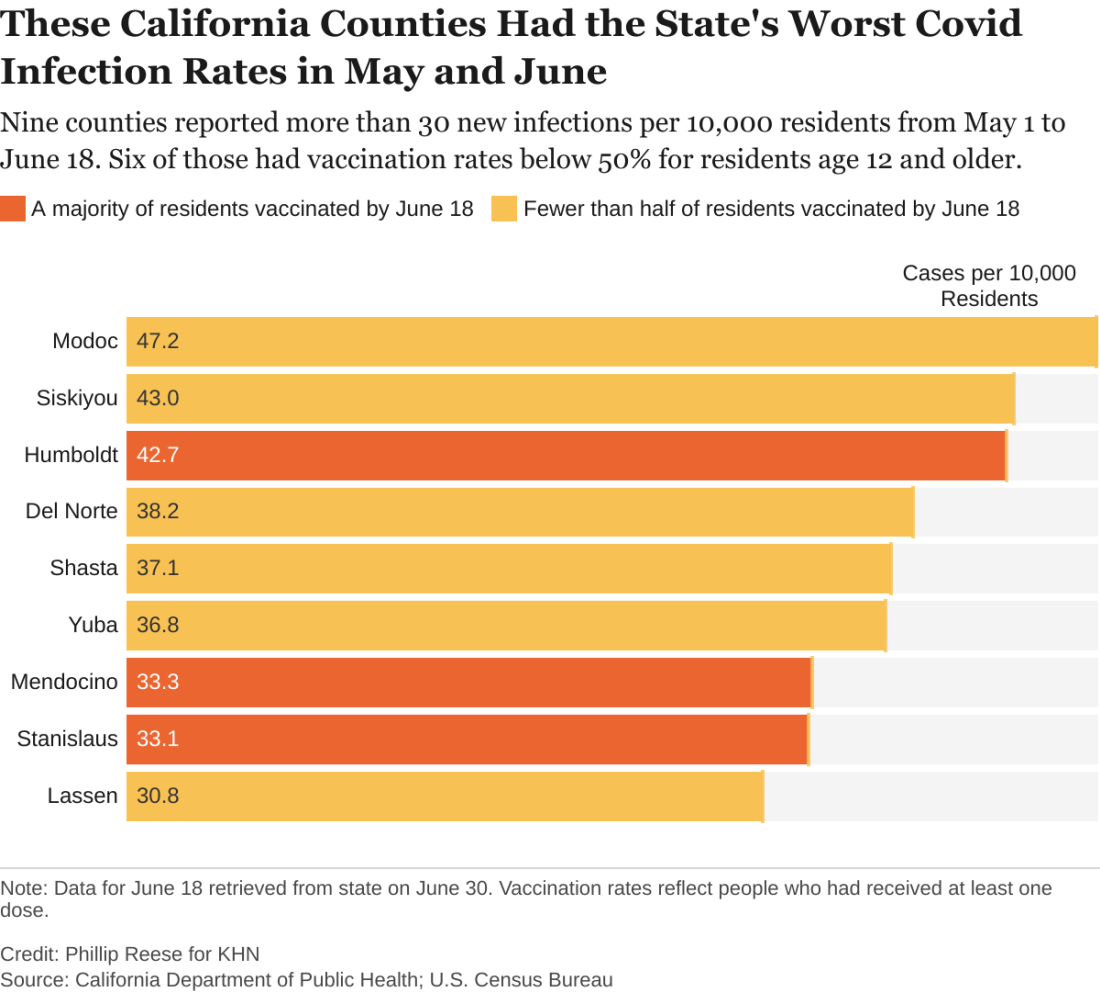
And bolstering that trend, nearly 70% of Californians 12 and older are partially or fully vaccinated.
But state health officials are still reporting nearly 1,000 new COVID cases and more than 2 dozen COVID-related deaths per day. So,
An analysis of state data shows some clear patterns at this stage of the pandemic: As vaccination rates rose across the state, the overall numbers of cases and deaths plunged. But within that broader trend are pronounced regional discrepancies. Counties with relatively low rates of vaccination reported much higher rates of COVID infections and deaths in May and June than counties with high vaccination rates.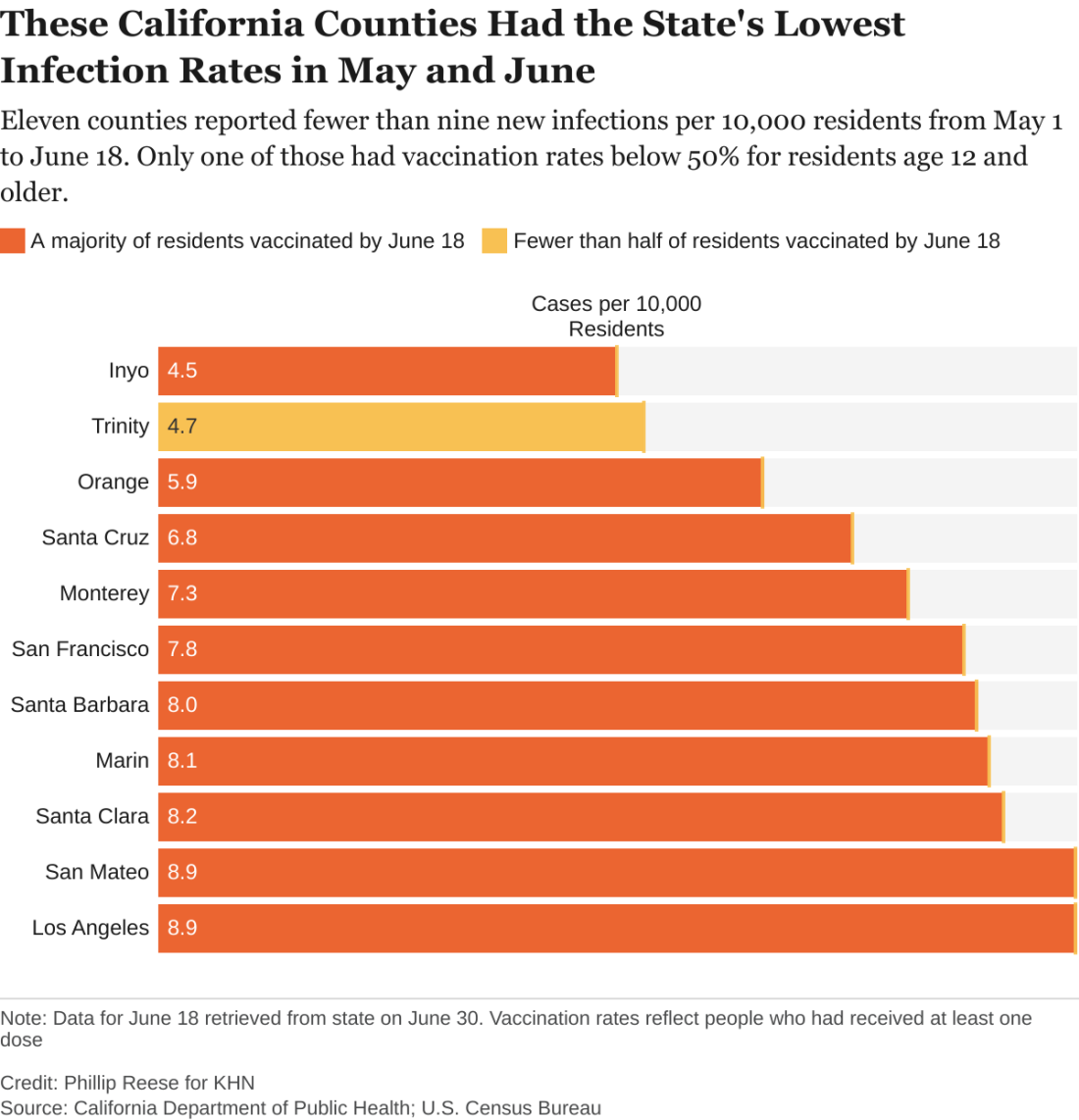
There were about 182 new COVID infections per 100,000 residents from May 1 to June 18 in California counties where fewer than half of residents age 12 and older had received at least one vaccine dose, CDPH data show. By comparison, there were about 102 COVID infections per 100,000 residents in counties where more than two-thirds of residents 12 and up had gotten at least one dose.
“If you live in an area that has low vaccination rates and you have a few people who start to develop a disease, it’s going to spread quickly among those who aren’t vaccinated,” said Rita Burke, PhD, assistant professor of clinical preventive medicine at the University of Southern California, Los Angeles. Dr. Burke noted that the highly contagious Delta variant of the coronavirus now circulating in California amplifies the threat of serious outbreaks in areas with low vaccination rates.
The regional discrepancies in COVID-related deaths are also striking. There were about 3.2 COVID-related deaths per 100,000 residents from May 1 to June 18 in counties where first-dose vaccination rates were below 50%. That is almost twice as high as the death rate in counties where more than two-thirds of residents had at least one dose.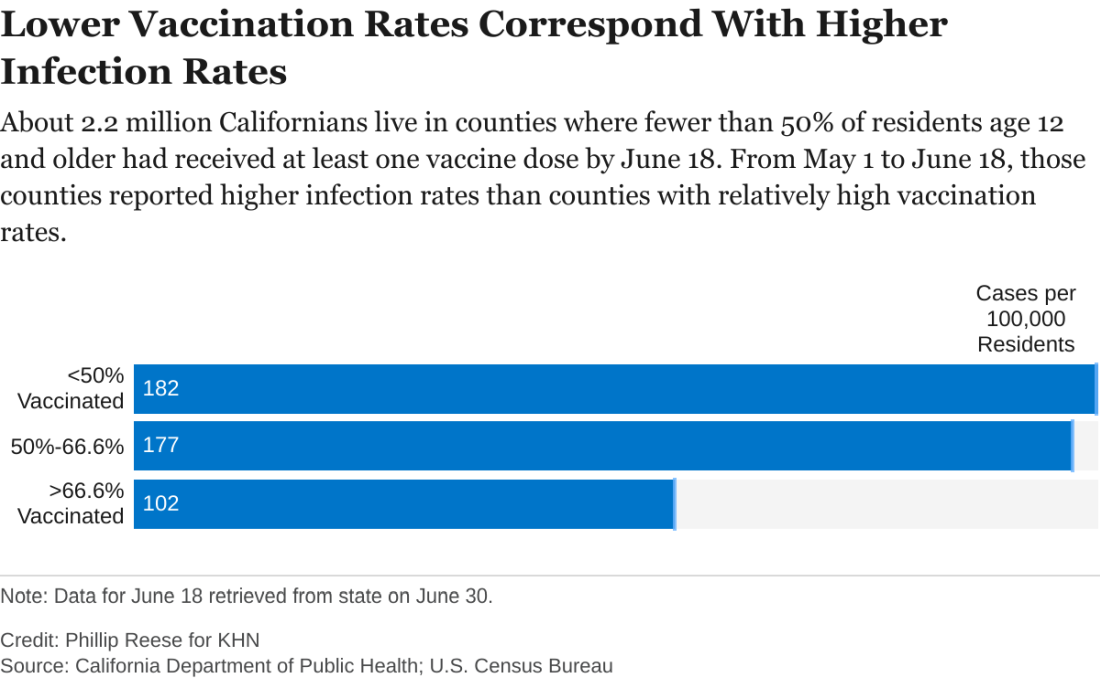
While the pattern is clear, there are exceptions. A couple of sparsely populated mountain counties with low vaccination rates – Trinity and Mariposa – also had relatively low rates of new infections in May and June. Likewise, a few suburban counties with high vaccination rates – among them Sonoma and Contra Costa – had relatively high rates of new infections.
“There are three things that are going on,” said George Rutherford, MD, a professor of epidemiology and biostatistics at the University of California, San Francisco. “One is the vaccine – very important, but not the whole story. One is naturally acquired immunity, which is huge in some places.” A third, he said, is people still managing to evade infection, whether by taking precautions or simply by living in areas with few infections.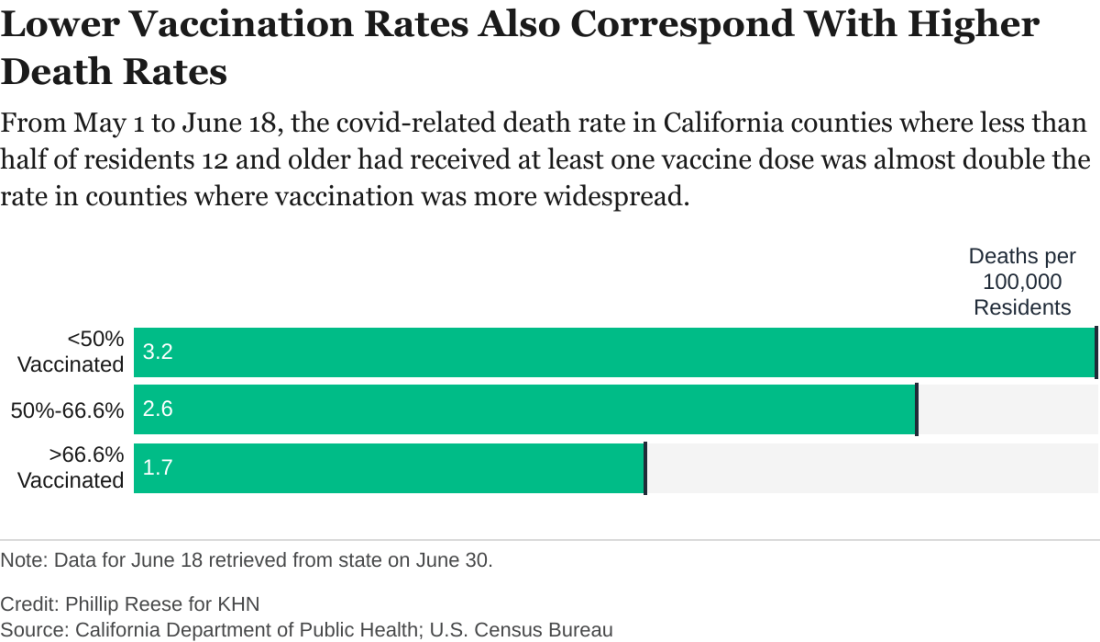
As of June 18, about 67% of Californians age 12 and older had received at least one dose of COVID vaccine, according to the state health department. But that masks a wide variance among the state’s 58 counties. In 14 counties, for example, fewer than half of residents 12 and older had received a shot. In 19 counties, more than two-thirds had.
The counties with low vaccination rates are largely rugged and rural. Nearly all are politically conservative. In January, about 6% of the state’s COVID infections were in the 23 counties where a majority of voters cast ballots for President Donald Trump in November. By May and June, that figure had risen to 11%.
While surveys indicate politics plays a role in vaccine hesitancy in many communities, access also remains an issue in many of California’s rural outposts. It can be hard, or at least inconvenient, for people who live far from the nearest medical facility to get two shots a month apart.
“If you have to drive 30 minutes out to the nearest vaccination site, you may not be as inclined to do that versus if it’s 5 minutes from your house,” Dr. Burke said. “And so we, the public health community, recognize that and have really made a concerted effort in order to eliminate or alleviate that access issue.”
Many of the counties with low vaccination rates had relatively low infection rates in the early months of the pandemic, largely thanks to their remoteness. But, as COVID reaches those communities, that lack of prior exposure and acquired immunity magnifies their vulnerability, Dr. Rutherford said. “We’re going to see cases where people are unvaccinated or where there’s not been a big background level of immunity already.”
As it becomes clearer that new infections will be disproportionately concentrated in areas with low vaccination rates, state officials are working to persuade hesitant Californians to get a vaccine, even introducing a vaccine lottery.
But most persuasive are friends and family members who can help counter the disinformation rampant in some communities, said Lorena Garcia, DrPH, an associate professor of epidemiology at the University of California, Davis. Belittling people for their hesitancy or getting into a political argument likely won’t work.
When talking to her own skeptical relatives, Dr. Garcia avoided politics: “I just explained any questions that they had.”
“Vaccines are a good part of our life,” she said. “It’s something that we’ve done since we were babies. So, it’s just something we’re going to do again.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Most of us are familiar with the good news: In recent weeks, rates of COVID-19 infection and death have plummeted in California, falling to levels not seen since the early days of the pandemic. The average number of new COVID infections reported each day dropped by an astounding 98% from December to June, according to figures from the California Department of Public Health.

And bolstering that trend, nearly 70% of Californians 12 and older are partially or fully vaccinated.
But state health officials are still reporting nearly 1,000 new COVID cases and more than 2 dozen COVID-related deaths per day. So,
An analysis of state data shows some clear patterns at this stage of the pandemic: As vaccination rates rose across the state, the overall numbers of cases and deaths plunged. But within that broader trend are pronounced regional discrepancies. Counties with relatively low rates of vaccination reported much higher rates of COVID infections and deaths in May and June than counties with high vaccination rates.
There were about 182 new COVID infections per 100,000 residents from May 1 to June 18 in California counties where fewer than half of residents age 12 and older had received at least one vaccine dose, CDPH data show. By comparison, there were about 102 COVID infections per 100,000 residents in counties where more than two-thirds of residents 12 and up had gotten at least one dose.
“If you live in an area that has low vaccination rates and you have a few people who start to develop a disease, it’s going to spread quickly among those who aren’t vaccinated,” said Rita Burke, PhD, assistant professor of clinical preventive medicine at the University of Southern California, Los Angeles. Dr. Burke noted that the highly contagious Delta variant of the coronavirus now circulating in California amplifies the threat of serious outbreaks in areas with low vaccination rates.
The regional discrepancies in COVID-related deaths are also striking. There were about 3.2 COVID-related deaths per 100,000 residents from May 1 to June 18 in counties where first-dose vaccination rates were below 50%. That is almost twice as high as the death rate in counties where more than two-thirds of residents had at least one dose.
While the pattern is clear, there are exceptions. A couple of sparsely populated mountain counties with low vaccination rates – Trinity and Mariposa – also had relatively low rates of new infections in May and June. Likewise, a few suburban counties with high vaccination rates – among them Sonoma and Contra Costa – had relatively high rates of new infections.
“There are three things that are going on,” said George Rutherford, MD, a professor of epidemiology and biostatistics at the University of California, San Francisco. “One is the vaccine – very important, but not the whole story. One is naturally acquired immunity, which is huge in some places.” A third, he said, is people still managing to evade infection, whether by taking precautions or simply by living in areas with few infections.
As of June 18, about 67% of Californians age 12 and older had received at least one dose of COVID vaccine, according to the state health department. But that masks a wide variance among the state’s 58 counties. In 14 counties, for example, fewer than half of residents 12 and older had received a shot. In 19 counties, more than two-thirds had.
The counties with low vaccination rates are largely rugged and rural. Nearly all are politically conservative. In January, about 6% of the state’s COVID infections were in the 23 counties where a majority of voters cast ballots for President Donald Trump in November. By May and June, that figure had risen to 11%.
While surveys indicate politics plays a role in vaccine hesitancy in many communities, access also remains an issue in many of California’s rural outposts. It can be hard, or at least inconvenient, for people who live far from the nearest medical facility to get two shots a month apart.
“If you have to drive 30 minutes out to the nearest vaccination site, you may not be as inclined to do that versus if it’s 5 minutes from your house,” Dr. Burke said. “And so we, the public health community, recognize that and have really made a concerted effort in order to eliminate or alleviate that access issue.”
Many of the counties with low vaccination rates had relatively low infection rates in the early months of the pandemic, largely thanks to their remoteness. But, as COVID reaches those communities, that lack of prior exposure and acquired immunity magnifies their vulnerability, Dr. Rutherford said. “We’re going to see cases where people are unvaccinated or where there’s not been a big background level of immunity already.”
As it becomes clearer that new infections will be disproportionately concentrated in areas with low vaccination rates, state officials are working to persuade hesitant Californians to get a vaccine, even introducing a vaccine lottery.
But most persuasive are friends and family members who can help counter the disinformation rampant in some communities, said Lorena Garcia, DrPH, an associate professor of epidemiology at the University of California, Davis. Belittling people for their hesitancy or getting into a political argument likely won’t work.
When talking to her own skeptical relatives, Dr. Garcia avoided politics: “I just explained any questions that they had.”
“Vaccines are a good part of our life,” she said. “It’s something that we’ve done since we were babies. So, it’s just something we’re going to do again.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Most of us are familiar with the good news: In recent weeks, rates of COVID-19 infection and death have plummeted in California, falling to levels not seen since the early days of the pandemic. The average number of new COVID infections reported each day dropped by an astounding 98% from December to June, according to figures from the California Department of Public Health.

And bolstering that trend, nearly 70% of Californians 12 and older are partially or fully vaccinated.
But state health officials are still reporting nearly 1,000 new COVID cases and more than 2 dozen COVID-related deaths per day. So,
An analysis of state data shows some clear patterns at this stage of the pandemic: As vaccination rates rose across the state, the overall numbers of cases and deaths plunged. But within that broader trend are pronounced regional discrepancies. Counties with relatively low rates of vaccination reported much higher rates of COVID infections and deaths in May and June than counties with high vaccination rates.
There were about 182 new COVID infections per 100,000 residents from May 1 to June 18 in California counties where fewer than half of residents age 12 and older had received at least one vaccine dose, CDPH data show. By comparison, there were about 102 COVID infections per 100,000 residents in counties where more than two-thirds of residents 12 and up had gotten at least one dose.
“If you live in an area that has low vaccination rates and you have a few people who start to develop a disease, it’s going to spread quickly among those who aren’t vaccinated,” said Rita Burke, PhD, assistant professor of clinical preventive medicine at the University of Southern California, Los Angeles. Dr. Burke noted that the highly contagious Delta variant of the coronavirus now circulating in California amplifies the threat of serious outbreaks in areas with low vaccination rates.
The regional discrepancies in COVID-related deaths are also striking. There were about 3.2 COVID-related deaths per 100,000 residents from May 1 to June 18 in counties where first-dose vaccination rates were below 50%. That is almost twice as high as the death rate in counties where more than two-thirds of residents had at least one dose.
While the pattern is clear, there are exceptions. A couple of sparsely populated mountain counties with low vaccination rates – Trinity and Mariposa – also had relatively low rates of new infections in May and June. Likewise, a few suburban counties with high vaccination rates – among them Sonoma and Contra Costa – had relatively high rates of new infections.
“There are three things that are going on,” said George Rutherford, MD, a professor of epidemiology and biostatistics at the University of California, San Francisco. “One is the vaccine – very important, but not the whole story. One is naturally acquired immunity, which is huge in some places.” A third, he said, is people still managing to evade infection, whether by taking precautions or simply by living in areas with few infections.
As of June 18, about 67% of Californians age 12 and older had received at least one dose of COVID vaccine, according to the state health department. But that masks a wide variance among the state’s 58 counties. In 14 counties, for example, fewer than half of residents 12 and older had received a shot. In 19 counties, more than two-thirds had.
The counties with low vaccination rates are largely rugged and rural. Nearly all are politically conservative. In January, about 6% of the state’s COVID infections were in the 23 counties where a majority of voters cast ballots for President Donald Trump in November. By May and June, that figure had risen to 11%.
While surveys indicate politics plays a role in vaccine hesitancy in many communities, access also remains an issue in many of California’s rural outposts. It can be hard, or at least inconvenient, for people who live far from the nearest medical facility to get two shots a month apart.
“If you have to drive 30 minutes out to the nearest vaccination site, you may not be as inclined to do that versus if it’s 5 minutes from your house,” Dr. Burke said. “And so we, the public health community, recognize that and have really made a concerted effort in order to eliminate or alleviate that access issue.”
Many of the counties with low vaccination rates had relatively low infection rates in the early months of the pandemic, largely thanks to their remoteness. But, as COVID reaches those communities, that lack of prior exposure and acquired immunity magnifies their vulnerability, Dr. Rutherford said. “We’re going to see cases where people are unvaccinated or where there’s not been a big background level of immunity already.”
As it becomes clearer that new infections will be disproportionately concentrated in areas with low vaccination rates, state officials are working to persuade hesitant Californians to get a vaccine, even introducing a vaccine lottery.
But most persuasive are friends and family members who can help counter the disinformation rampant in some communities, said Lorena Garcia, DrPH, an associate professor of epidemiology at the University of California, Davis. Belittling people for their hesitancy or getting into a political argument likely won’t work.
When talking to her own skeptical relatives, Dr. Garcia avoided politics: “I just explained any questions that they had.”
“Vaccines are a good part of our life,” she said. “It’s something that we’ve done since we were babies. So, it’s just something we’re going to do again.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Garlic cloves in the nose and beer dreams and pareidolia faces
Insert clove A into nostril B
Just when you start wondering what crazy and potentially dangerous thing people can do to themselves next, comes a crazy and potentially dangerous new trend. The good folks at TikTok have provided patients a new treatment for stuffed up sinuses.
Dangerous? Well, that’s what doctors say, anyway.
“We typically do not recommend putting anything into the nostril for the obvious fact that it could get dislodged or lodged up into the nasal cavity,” Anthony Del Signore, MD, of Mount Sinai Union Square, New York, told TODAY.
“Not only does it have the potential to rot or cause a nasal obstruction, it can induce an episode of sinusitis,” Omid Mehdizadeh, MD, of Providence Saint John’s Health Center, Santa Monica, Calif., explained to Shape.
But who doesn't want to breathe easier and keep blood-sucking vampires at bay?
TikTokers are posting videos of themselves sticking garlic cloves in their nostrils for several minutes. They, “then, pull the garlic out, followed, typically, by long strands of mucus,” according to The Hill.
That can’t be real, you’re probably saying. Or maybe you think that no one is actually watching this stuff. Well, wake up! This isn’t network television we’re talking about. It’s freakin’ TikTok! One video has been favorited over half a million times. Another is up to 2.2 million.
It’s all true. Really. We couldn’t make this stuff up if we tried.
Seeing faces in random places?
Ever look up at the clouds, at a fast-moving train, or into your morning bowl of cereal and see two eyes, a nose, and a mouth looking back at you? You may shake it off and think you’re imagining something, but it's actually your brain doing what it’s built to do and researchers know why.
The phenomenon is called face pareidolia, and it’s technically an error function of the human brain. Evolution has molded our brains to rapidly identify faces, according to David Alais, PhD, of the University of Sydney, Australia, lead author of the study.
“But the system plays ‘fast and loose’ by applying a crude template of two eyes over a nose and mouth. Lots of things can satisfy that template and thus trigger a face detection response,” he said in a separate statement. But not only are we seeing faces, our brains go one step further and seemingly give those faces feelings.
In the study, Dr. Alais and his team looked for two things about each pareidolia face: Was it analyzed for facial expression or just rejected as a face altogether? The participants were shown a series of faces and then asked to rate the expression on a scale from angry to happy. What the researchers found was that once a face was detected, the brain analyzed the pareidolia face in the same way as a human face. Have you ever seen an angry trash can? Or a smile on an over-easy egg?
The other question faced: Was there a bias on emotion? Yup, and excuse the dad joke.
The researchers showed a mixed series of human faces and pareidolia faces to participants and found that responses were influenced by the previous face seen, no matter if the face was human or not.
So if someone smiled at you on the way to the grocery store and you see a grinning tomato in the produce section, your mind is playing tricks on you, and it’s totally normal.
Corporate dream manipulation
Advertisements are quite literally everywhere. On billboards, in commercials, in videos, in movies; the list goes on and on. Still, at least you can shut your eyes and be mercifully free of corporate interference inside your own head, right? Right?
Early in 2021, Coors launched an ad campaign that seemed to be a b bit of a gimmick, if not a joke. Coors claimed that if people watched an ad before bed, and played an 8-hour soundscape while sleeping, their dreams would be filled with crisp mountains and cold, thirst-quenching beverages. While, the Coors campaign didn’t go viral, someone was paying attention. A group of 35 leading researchers published an open letter on the subject of corporate dream manipulation, in the journal Dream Engineering.
"Multiple marketing studies are openly testing new ways to alter and motivate purchasing behavior through dream and sleep hacking. The commercial, for-profit use of dream incubation is rapidly becoming a reality," wrote the investigators. "As sleep and dream researchers, we are deeply concerned about marketing plans aimed at generating profits at the cost of interfering with our natural nocturnal memory processing."
People have tried to manipulate their dreams for countless years, but only in recent years have scientists attempted to target or manipulate behavior through dreams. In a 2014 study, smokers exposed to tobacco smoke and rotten egg smell while sleeping reduced their cigarette consumption by 30%.
Most research into dream manipulation has been aimed at positive results, but the experts warn that there’s no reason corporations couldn’t use it for their own purposes, especially given the widespread usage of devices such as Alexa. A company could play a certain sound during a commercial, they suggested, and then replay that sound through a device while people are sleeping to trigger a dream about that product.
And just when our COVID-19–driven anxiety dreams were starting to subside.
The experts said that the Federal Trade Commission could intervene to prevent companies from attempting dream manipulation, and have done so in the past to stop subliminal advertising, but as of right now, there’s nothing stopping big business from messing with your dreams. But hey, at least they’re not directly beaming commercials into our heads with gamma radiation. Yet.
Got breast milk?
As we know, breast milk has endless benefits for newbords and babies, but many things can stand in the way of a mother’s ability to breastfeed. Baby formula has served as a good enough substitute. But now, there might be something that’s even better.
A start-up company called BIOMILQ created a product that could be groundbreaking. Using “breakthrough mammary biotechnology,” BIOMILQ created cell-cultured breast milk.
Leila Strickland, a biologist who is the company’s cofounder and chief science officer, said she’s had her own personal experience with breastfeeding and believes the product could benefit many if just given a chance. "Some of the cells we’ve looked at can produce milk for months and months," according to a company statement
Baby formula has done its job feeding and nourishing babies since 1865, but could BIOMILQ do better?
Time – and babies – will tell.
Insert clove A into nostril B
Just when you start wondering what crazy and potentially dangerous thing people can do to themselves next, comes a crazy and potentially dangerous new trend. The good folks at TikTok have provided patients a new treatment for stuffed up sinuses.
Dangerous? Well, that’s what doctors say, anyway.
“We typically do not recommend putting anything into the nostril for the obvious fact that it could get dislodged or lodged up into the nasal cavity,” Anthony Del Signore, MD, of Mount Sinai Union Square, New York, told TODAY.
“Not only does it have the potential to rot or cause a nasal obstruction, it can induce an episode of sinusitis,” Omid Mehdizadeh, MD, of Providence Saint John’s Health Center, Santa Monica, Calif., explained to Shape.
But who doesn't want to breathe easier and keep blood-sucking vampires at bay?
TikTokers are posting videos of themselves sticking garlic cloves in their nostrils for several minutes. They, “then, pull the garlic out, followed, typically, by long strands of mucus,” according to The Hill.
That can’t be real, you’re probably saying. Or maybe you think that no one is actually watching this stuff. Well, wake up! This isn’t network television we’re talking about. It’s freakin’ TikTok! One video has been favorited over half a million times. Another is up to 2.2 million.
It’s all true. Really. We couldn’t make this stuff up if we tried.
Seeing faces in random places?
Ever look up at the clouds, at a fast-moving train, or into your morning bowl of cereal and see two eyes, a nose, and a mouth looking back at you? You may shake it off and think you’re imagining something, but it's actually your brain doing what it’s built to do and researchers know why.
The phenomenon is called face pareidolia, and it’s technically an error function of the human brain. Evolution has molded our brains to rapidly identify faces, according to David Alais, PhD, of the University of Sydney, Australia, lead author of the study.
“But the system plays ‘fast and loose’ by applying a crude template of two eyes over a nose and mouth. Lots of things can satisfy that template and thus trigger a face detection response,” he said in a separate statement. But not only are we seeing faces, our brains go one step further and seemingly give those faces feelings.
In the study, Dr. Alais and his team looked for two things about each pareidolia face: Was it analyzed for facial expression or just rejected as a face altogether? The participants were shown a series of faces and then asked to rate the expression on a scale from angry to happy. What the researchers found was that once a face was detected, the brain analyzed the pareidolia face in the same way as a human face. Have you ever seen an angry trash can? Or a smile on an over-easy egg?
The other question faced: Was there a bias on emotion? Yup, and excuse the dad joke.
The researchers showed a mixed series of human faces and pareidolia faces to participants and found that responses were influenced by the previous face seen, no matter if the face was human or not.
So if someone smiled at you on the way to the grocery store and you see a grinning tomato in the produce section, your mind is playing tricks on you, and it’s totally normal.
Corporate dream manipulation
Advertisements are quite literally everywhere. On billboards, in commercials, in videos, in movies; the list goes on and on. Still, at least you can shut your eyes and be mercifully free of corporate interference inside your own head, right? Right?
Early in 2021, Coors launched an ad campaign that seemed to be a b bit of a gimmick, if not a joke. Coors claimed that if people watched an ad before bed, and played an 8-hour soundscape while sleeping, their dreams would be filled with crisp mountains and cold, thirst-quenching beverages. While, the Coors campaign didn’t go viral, someone was paying attention. A group of 35 leading researchers published an open letter on the subject of corporate dream manipulation, in the journal Dream Engineering.
"Multiple marketing studies are openly testing new ways to alter and motivate purchasing behavior through dream and sleep hacking. The commercial, for-profit use of dream incubation is rapidly becoming a reality," wrote the investigators. "As sleep and dream researchers, we are deeply concerned about marketing plans aimed at generating profits at the cost of interfering with our natural nocturnal memory processing."
People have tried to manipulate their dreams for countless years, but only in recent years have scientists attempted to target or manipulate behavior through dreams. In a 2014 study, smokers exposed to tobacco smoke and rotten egg smell while sleeping reduced their cigarette consumption by 30%.
Most research into dream manipulation has been aimed at positive results, but the experts warn that there’s no reason corporations couldn’t use it for their own purposes, especially given the widespread usage of devices such as Alexa. A company could play a certain sound during a commercial, they suggested, and then replay that sound through a device while people are sleeping to trigger a dream about that product.
And just when our COVID-19–driven anxiety dreams were starting to subside.
The experts said that the Federal Trade Commission could intervene to prevent companies from attempting dream manipulation, and have done so in the past to stop subliminal advertising, but as of right now, there’s nothing stopping big business from messing with your dreams. But hey, at least they’re not directly beaming commercials into our heads with gamma radiation. Yet.
Got breast milk?
As we know, breast milk has endless benefits for newbords and babies, but many things can stand in the way of a mother’s ability to breastfeed. Baby formula has served as a good enough substitute. But now, there might be something that’s even better.
A start-up company called BIOMILQ created a product that could be groundbreaking. Using “breakthrough mammary biotechnology,” BIOMILQ created cell-cultured breast milk.
Leila Strickland, a biologist who is the company’s cofounder and chief science officer, said she’s had her own personal experience with breastfeeding and believes the product could benefit many if just given a chance. "Some of the cells we’ve looked at can produce milk for months and months," according to a company statement
Baby formula has done its job feeding and nourishing babies since 1865, but could BIOMILQ do better?
Time – and babies – will tell.
Insert clove A into nostril B
Just when you start wondering what crazy and potentially dangerous thing people can do to themselves next, comes a crazy and potentially dangerous new trend. The good folks at TikTok have provided patients a new treatment for stuffed up sinuses.
Dangerous? Well, that’s what doctors say, anyway.
“We typically do not recommend putting anything into the nostril for the obvious fact that it could get dislodged or lodged up into the nasal cavity,” Anthony Del Signore, MD, of Mount Sinai Union Square, New York, told TODAY.
“Not only does it have the potential to rot or cause a nasal obstruction, it can induce an episode of sinusitis,” Omid Mehdizadeh, MD, of Providence Saint John’s Health Center, Santa Monica, Calif., explained to Shape.
But who doesn't want to breathe easier and keep blood-sucking vampires at bay?
TikTokers are posting videos of themselves sticking garlic cloves in their nostrils for several minutes. They, “then, pull the garlic out, followed, typically, by long strands of mucus,” according to The Hill.
That can’t be real, you’re probably saying. Or maybe you think that no one is actually watching this stuff. Well, wake up! This isn’t network television we’re talking about. It’s freakin’ TikTok! One video has been favorited over half a million times. Another is up to 2.2 million.
It’s all true. Really. We couldn’t make this stuff up if we tried.
Seeing faces in random places?
Ever look up at the clouds, at a fast-moving train, or into your morning bowl of cereal and see two eyes, a nose, and a mouth looking back at you? You may shake it off and think you’re imagining something, but it's actually your brain doing what it’s built to do and researchers know why.
The phenomenon is called face pareidolia, and it’s technically an error function of the human brain. Evolution has molded our brains to rapidly identify faces, according to David Alais, PhD, of the University of Sydney, Australia, lead author of the study.
“But the system plays ‘fast and loose’ by applying a crude template of two eyes over a nose and mouth. Lots of things can satisfy that template and thus trigger a face detection response,” he said in a separate statement. But not only are we seeing faces, our brains go one step further and seemingly give those faces feelings.
In the study, Dr. Alais and his team looked for two things about each pareidolia face: Was it analyzed for facial expression or just rejected as a face altogether? The participants were shown a series of faces and then asked to rate the expression on a scale from angry to happy. What the researchers found was that once a face was detected, the brain analyzed the pareidolia face in the same way as a human face. Have you ever seen an angry trash can? Or a smile on an over-easy egg?
The other question faced: Was there a bias on emotion? Yup, and excuse the dad joke.
The researchers showed a mixed series of human faces and pareidolia faces to participants and found that responses were influenced by the previous face seen, no matter if the face was human or not.
So if someone smiled at you on the way to the grocery store and you see a grinning tomato in the produce section, your mind is playing tricks on you, and it’s totally normal.
Corporate dream manipulation
Advertisements are quite literally everywhere. On billboards, in commercials, in videos, in movies; the list goes on and on. Still, at least you can shut your eyes and be mercifully free of corporate interference inside your own head, right? Right?
Early in 2021, Coors launched an ad campaign that seemed to be a b bit of a gimmick, if not a joke. Coors claimed that if people watched an ad before bed, and played an 8-hour soundscape while sleeping, their dreams would be filled with crisp mountains and cold, thirst-quenching beverages. While, the Coors campaign didn’t go viral, someone was paying attention. A group of 35 leading researchers published an open letter on the subject of corporate dream manipulation, in the journal Dream Engineering.
"Multiple marketing studies are openly testing new ways to alter and motivate purchasing behavior through dream and sleep hacking. The commercial, for-profit use of dream incubation is rapidly becoming a reality," wrote the investigators. "As sleep and dream researchers, we are deeply concerned about marketing plans aimed at generating profits at the cost of interfering with our natural nocturnal memory processing."
People have tried to manipulate their dreams for countless years, but only in recent years have scientists attempted to target or manipulate behavior through dreams. In a 2014 study, smokers exposed to tobacco smoke and rotten egg smell while sleeping reduced their cigarette consumption by 30%.
Most research into dream manipulation has been aimed at positive results, but the experts warn that there’s no reason corporations couldn’t use it for their own purposes, especially given the widespread usage of devices such as Alexa. A company could play a certain sound during a commercial, they suggested, and then replay that sound through a device while people are sleeping to trigger a dream about that product.
And just when our COVID-19–driven anxiety dreams were starting to subside.
The experts said that the Federal Trade Commission could intervene to prevent companies from attempting dream manipulation, and have done so in the past to stop subliminal advertising, but as of right now, there’s nothing stopping big business from messing with your dreams. But hey, at least they’re not directly beaming commercials into our heads with gamma radiation. Yet.
Got breast milk?
As we know, breast milk has endless benefits for newbords and babies, but many things can stand in the way of a mother’s ability to breastfeed. Baby formula has served as a good enough substitute. But now, there might be something that’s even better.
A start-up company called BIOMILQ created a product that could be groundbreaking. Using “breakthrough mammary biotechnology,” BIOMILQ created cell-cultured breast milk.
Leila Strickland, a biologist who is the company’s cofounder and chief science officer, said she’s had her own personal experience with breastfeeding and believes the product could benefit many if just given a chance. "Some of the cells we’ve looked at can produce milk for months and months," according to a company statement
Baby formula has done its job feeding and nourishing babies since 1865, but could BIOMILQ do better?
Time – and babies – will tell.








