User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Delta variant infects six vaccinated guests at outdoor wedding
In April, 92 people gathered in Texas for a wedding. To lower the chances of COVID-19 infection, the festivities were held outside under a large, open-air tent. All 92 guests were required to be fully vaccinated.
Despite those precautions, six people tested positive for the coronavirus and one of them died, Forbes magazine reported, citing a preprint published in medRxiv.
Researchers from Baylor College of Medicine said viral sequencing suggests “the strain containing the Delta variant was transmitted to wedding guests from two patients traveling from India. With no history of vaccine failure in these patients, our observations suggest these are true cases of vaccine breakthrough, mediated by the Delta variant.”
Three females and three males aged 53-69 tested positive for COVID-19. Three were overweight, but none had significant comorbidities or a history of failed vaccination.
The first people to get sick were a man and woman who traveled from India, Forbes reported. The man had no health problems, but the woman had diabetes. Both had gotten two doses of the Covaxin BBV152 vaccine before leaving India.
They tested positive for COVID-19 4 days after the wedding, and the man became so ill he was hospitalized. Six days after the wedding, he died, according to Forbes.
Two people who’d gotten the Pfizer/BioNTech vaccine and two people who received the Moderna vaccine interacted with the first two people, and they also tested positive. One of them, a man in his 60s, had to be hospitalized.
Forbes summed it up this way: “While the available COVID-19 vaccines can offer good protection against COVID-19, the protection is not perfect. As long as the pandemic is continuing, it is better to maintain multiple layers of COVID-19 precautions when you can.”
A version of this article first appeared on WebMD.com.
In April, 92 people gathered in Texas for a wedding. To lower the chances of COVID-19 infection, the festivities were held outside under a large, open-air tent. All 92 guests were required to be fully vaccinated.
Despite those precautions, six people tested positive for the coronavirus and one of them died, Forbes magazine reported, citing a preprint published in medRxiv.
Researchers from Baylor College of Medicine said viral sequencing suggests “the strain containing the Delta variant was transmitted to wedding guests from two patients traveling from India. With no history of vaccine failure in these patients, our observations suggest these are true cases of vaccine breakthrough, mediated by the Delta variant.”
Three females and three males aged 53-69 tested positive for COVID-19. Three were overweight, but none had significant comorbidities or a history of failed vaccination.
The first people to get sick were a man and woman who traveled from India, Forbes reported. The man had no health problems, but the woman had diabetes. Both had gotten two doses of the Covaxin BBV152 vaccine before leaving India.
They tested positive for COVID-19 4 days after the wedding, and the man became so ill he was hospitalized. Six days after the wedding, he died, according to Forbes.
Two people who’d gotten the Pfizer/BioNTech vaccine and two people who received the Moderna vaccine interacted with the first two people, and they also tested positive. One of them, a man in his 60s, had to be hospitalized.
Forbes summed it up this way: “While the available COVID-19 vaccines can offer good protection against COVID-19, the protection is not perfect. As long as the pandemic is continuing, it is better to maintain multiple layers of COVID-19 precautions when you can.”
A version of this article first appeared on WebMD.com.
In April, 92 people gathered in Texas for a wedding. To lower the chances of COVID-19 infection, the festivities were held outside under a large, open-air tent. All 92 guests were required to be fully vaccinated.
Despite those precautions, six people tested positive for the coronavirus and one of them died, Forbes magazine reported, citing a preprint published in medRxiv.
Researchers from Baylor College of Medicine said viral sequencing suggests “the strain containing the Delta variant was transmitted to wedding guests from two patients traveling from India. With no history of vaccine failure in these patients, our observations suggest these are true cases of vaccine breakthrough, mediated by the Delta variant.”
Three females and three males aged 53-69 tested positive for COVID-19. Three were overweight, but none had significant comorbidities or a history of failed vaccination.
The first people to get sick were a man and woman who traveled from India, Forbes reported. The man had no health problems, but the woman had diabetes. Both had gotten two doses of the Covaxin BBV152 vaccine before leaving India.
They tested positive for COVID-19 4 days after the wedding, and the man became so ill he was hospitalized. Six days after the wedding, he died, according to Forbes.
Two people who’d gotten the Pfizer/BioNTech vaccine and two people who received the Moderna vaccine interacted with the first two people, and they also tested positive. One of them, a man in his 60s, had to be hospitalized.
Forbes summed it up this way: “While the available COVID-19 vaccines can offer good protection against COVID-19, the protection is not perfect. As long as the pandemic is continuing, it is better to maintain multiple layers of COVID-19 precautions when you can.”
A version of this article first appeared on WebMD.com.
Medicare proposes direct payments to PAs, telehealth expansion
It also intends to change the approach to payments for office visits and for coaching programs for diabetes prevention.
The Centers for Medicare & Medicaid Services recently posted its proposed 2022 physician fee schedule. Running to more than 1,700 pages, the draft rule contains myriad other changes in how the giant federal health program pays for medical care, including revisions to its approach to evaluation and management (E/M) services, which represent many office visits. In addition, Medicare is seeking to increase participation in a program intended to prevent people from developing diabetes.
Physician groups posted quick complaints about a proposed 3.75% reduction to the conversion factor because of budget neutrality requirements. The cut reinstates a reduction Congress prevented in late 2020.
In a statement, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, called the draft rule a “mixed bag for physician practices.” Mr. Gilberg said the MGMA will seek congressional intervention to avert the cut for services in 2022.
In keeping with a provision Congress included in a massive spending bill enacted in December, Medicare will let PAs directly bill, as nurse practitioners already can. In a press release, CMS on July 13 described this as a move likely to expand access to care and reduce administrative burden. In 2020, the American Academy of PAs praised the inclusion in the spending bill of the provision allowing its members to directly bill Medicare.
In the draft rule, CMS also intends to remove certain geographic restrictions regarding use of telehealth services for diagnosis, evaluation, and treatment of mental health disorders. CMS also is proposing to allow payment to eligible clinicians for certain mental health and behavioral health services to patients via audio-only telephone calls. These services would include counseling and therapy services provided through opioid treatment programs.
“These changes would be particularly helpful for those in areas with poor broadband infrastructure and among people with Medicare who are not capable of, or do not consent to the use of, devices that permit a two-way, audio/video interaction for their health care visits,” CMS said in a statement.
Slimmer Medicare enrollees, bigger payments for coaches?
CMS is seeking to draw more participants to the Medicare Diabetes Prevention Program (MDPP). This program includes organizations that provide structured, coach-led sessions in community and health care settings to help people lose weight and exercise more. During the COVID-19 public health emergency, CMS waived an enrollment fee for new suppliers of services in MDPP. CMS now is proposing to waive this fee for all organizations that submit an application to enroll in Medicare as an MDPP supplier on or after Jan. 1, 2022.
Another proposed change in MDPP services is a restructuring of payments so that organizations involved in coaching would receive larger payments when their participants reach milestones for attendance and for becoming slimmer.
“We propose to increase performance payments for MDPP beneficiary achievement of the 5% weight-loss goal, as well as continued attendance during each core maintenance interval,” CMS said in a statement.
Medicare remains engaged in a review of its payments for E/M services. In the draft rule, CMS is proposing a number of refinements to current policies for split, or shared, E/M visits, critical care services, and services furnished by teaching physicians involving residents. The intention of these changes is to “better reflect the current practice of medicine, the evolving role of nonphysician practitioners as members of the medical team, and to clarify conditions of payment that must be met to bill Medicare for these services,” CMS said.
A version of this article first appeared on Medscape.com.
It also intends to change the approach to payments for office visits and for coaching programs for diabetes prevention.
The Centers for Medicare & Medicaid Services recently posted its proposed 2022 physician fee schedule. Running to more than 1,700 pages, the draft rule contains myriad other changes in how the giant federal health program pays for medical care, including revisions to its approach to evaluation and management (E/M) services, which represent many office visits. In addition, Medicare is seeking to increase participation in a program intended to prevent people from developing diabetes.
Physician groups posted quick complaints about a proposed 3.75% reduction to the conversion factor because of budget neutrality requirements. The cut reinstates a reduction Congress prevented in late 2020.
In a statement, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, called the draft rule a “mixed bag for physician practices.” Mr. Gilberg said the MGMA will seek congressional intervention to avert the cut for services in 2022.
In keeping with a provision Congress included in a massive spending bill enacted in December, Medicare will let PAs directly bill, as nurse practitioners already can. In a press release, CMS on July 13 described this as a move likely to expand access to care and reduce administrative burden. In 2020, the American Academy of PAs praised the inclusion in the spending bill of the provision allowing its members to directly bill Medicare.
In the draft rule, CMS also intends to remove certain geographic restrictions regarding use of telehealth services for diagnosis, evaluation, and treatment of mental health disorders. CMS also is proposing to allow payment to eligible clinicians for certain mental health and behavioral health services to patients via audio-only telephone calls. These services would include counseling and therapy services provided through opioid treatment programs.
“These changes would be particularly helpful for those in areas with poor broadband infrastructure and among people with Medicare who are not capable of, or do not consent to the use of, devices that permit a two-way, audio/video interaction for their health care visits,” CMS said in a statement.
Slimmer Medicare enrollees, bigger payments for coaches?
CMS is seeking to draw more participants to the Medicare Diabetes Prevention Program (MDPP). This program includes organizations that provide structured, coach-led sessions in community and health care settings to help people lose weight and exercise more. During the COVID-19 public health emergency, CMS waived an enrollment fee for new suppliers of services in MDPP. CMS now is proposing to waive this fee for all organizations that submit an application to enroll in Medicare as an MDPP supplier on or after Jan. 1, 2022.
Another proposed change in MDPP services is a restructuring of payments so that organizations involved in coaching would receive larger payments when their participants reach milestones for attendance and for becoming slimmer.
“We propose to increase performance payments for MDPP beneficiary achievement of the 5% weight-loss goal, as well as continued attendance during each core maintenance interval,” CMS said in a statement.
Medicare remains engaged in a review of its payments for E/M services. In the draft rule, CMS is proposing a number of refinements to current policies for split, or shared, E/M visits, critical care services, and services furnished by teaching physicians involving residents. The intention of these changes is to “better reflect the current practice of medicine, the evolving role of nonphysician practitioners as members of the medical team, and to clarify conditions of payment that must be met to bill Medicare for these services,” CMS said.
A version of this article first appeared on Medscape.com.
It also intends to change the approach to payments for office visits and for coaching programs for diabetes prevention.
The Centers for Medicare & Medicaid Services recently posted its proposed 2022 physician fee schedule. Running to more than 1,700 pages, the draft rule contains myriad other changes in how the giant federal health program pays for medical care, including revisions to its approach to evaluation and management (E/M) services, which represent many office visits. In addition, Medicare is seeking to increase participation in a program intended to prevent people from developing diabetes.
Physician groups posted quick complaints about a proposed 3.75% reduction to the conversion factor because of budget neutrality requirements. The cut reinstates a reduction Congress prevented in late 2020.
In a statement, Anders Gilberg, senior vice president of government affairs for the Medical Group Management Association, called the draft rule a “mixed bag for physician practices.” Mr. Gilberg said the MGMA will seek congressional intervention to avert the cut for services in 2022.
In keeping with a provision Congress included in a massive spending bill enacted in December, Medicare will let PAs directly bill, as nurse practitioners already can. In a press release, CMS on July 13 described this as a move likely to expand access to care and reduce administrative burden. In 2020, the American Academy of PAs praised the inclusion in the spending bill of the provision allowing its members to directly bill Medicare.
In the draft rule, CMS also intends to remove certain geographic restrictions regarding use of telehealth services for diagnosis, evaluation, and treatment of mental health disorders. CMS also is proposing to allow payment to eligible clinicians for certain mental health and behavioral health services to patients via audio-only telephone calls. These services would include counseling and therapy services provided through opioid treatment programs.
“These changes would be particularly helpful for those in areas with poor broadband infrastructure and among people with Medicare who are not capable of, or do not consent to the use of, devices that permit a two-way, audio/video interaction for their health care visits,” CMS said in a statement.
Slimmer Medicare enrollees, bigger payments for coaches?
CMS is seeking to draw more participants to the Medicare Diabetes Prevention Program (MDPP). This program includes organizations that provide structured, coach-led sessions in community and health care settings to help people lose weight and exercise more. During the COVID-19 public health emergency, CMS waived an enrollment fee for new suppliers of services in MDPP. CMS now is proposing to waive this fee for all organizations that submit an application to enroll in Medicare as an MDPP supplier on or after Jan. 1, 2022.
Another proposed change in MDPP services is a restructuring of payments so that organizations involved in coaching would receive larger payments when their participants reach milestones for attendance and for becoming slimmer.
“We propose to increase performance payments for MDPP beneficiary achievement of the 5% weight-loss goal, as well as continued attendance during each core maintenance interval,” CMS said in a statement.
Medicare remains engaged in a review of its payments for E/M services. In the draft rule, CMS is proposing a number of refinements to current policies for split, or shared, E/M visits, critical care services, and services furnished by teaching physicians involving residents. The intention of these changes is to “better reflect the current practice of medicine, the evolving role of nonphysician practitioners as members of the medical team, and to clarify conditions of payment that must be met to bill Medicare for these services,” CMS said.
A version of this article first appeared on Medscape.com.
Do patients with cancer need a third shot of COVID vaccine?
Patients with cancer have shown varying responses to COVID-19 vaccination, with good responses in patients with solid tumors (even while on systemic therapy) and poor responses in patients with blood cancers, particularly those on immunosuppressive therapies.
The data are evolving to show factors associated with a poor response but are not strong enough yet to recommend booster shots, say researchers.
The work is defining who will likely need a COVID vaccine booster when they become available. “It’s definitely not all cancer patients,” said Dimpy Shah, MD, PhD, a cancer epidemiologist at the Mays Cancer Center, University of Texas, San Antonio.
Public anxiously awaiting boosters
Boosters aren’t recommended in the United States at the moment, in large part because the Emergency Use Authorization under which the vaccines are being administered allows for only two shots of the Pfizer and Moderna vaccines and one shot of the Johnson & Johnson vaccine.
Even so, regulators and policymakers are “keenly aware that physicians and patients alike are anxious to get going and start doing boosters,” Dr. Shah said. There’s concern that antibody response might wane over time, perhaps even more quickly in patients with cancer.
Pfizer is already in talks with the U.S. Food and Drug Administration to authorize a third dose of its vaccine in the United States. Guidelines could very well change in coming months, said Ghady Haidar, MD, a specialist in infectious diseases and cancer at the University of Pittsburgh.
However, it’s still early in the game, and it’s not clear yet if boosters are necessary in cancer, Dr. Haidar said in an interview.
For one thing, it’s unknown if poor antibody response really means that patients aren’t protected, he explained. The vaccines elicit T-cell responses that could protect patients regardless of antibody levels. It’s also unclear if antibody titer levels are clinically relevant, and there hasn’t been much indication yet that less-than-robust vaccine responses translate to worse COVID outcomes in patients with cancer.
Those and other questions are areas of active investigation by Dr. Shah, Dr. Haidar, and others. Dozens of clinical trials are investigating vaccine response in patients with cancer, including the use of boosters.
Meanwhile, some cancer patients aren’t waiting around for more study results. “I get many, many emails a day” about booster shots, Dr. Haidar said. “We recommend against” them for now but some people bend the rules and get an extra shot anyway. “I get it. People are apprehensive.”
Three COVID deaths despite full vaccination
The vaccine clinical trials had fewer patients with cancer, so researchers are moving fast to backfill the data. Although there is some variation in what’s being reported, an overall picture is slowly emerging.
Dr. Shah and her team reported on responses to the mRNA COVID vaccines from Pfizer and Moderna and found a 94% seroconversion rate in 131 patients with cancer 3-4 weeks after their second dose of vaccine. They also found good responses among patients on cytotoxic chemotherapy within 6 months of their first vaccine dose, although their antibody titer levels were significantly lower than seen in other patients with cancer.
Investigators from Montefiore Medical Center in New York City also recently reported a 94% seroconversion rate among 200 patients with cancer, including 98% seroconversion in patients with solid tumors. Rates were lower in patients with blood cancers but were still 85% overall, with 70% conversion among patients on anti-CD20 therapies and 73% among stem cell transplant patients.
Dr. Haidar’s group reported a seroconversion rate of 82.4% among patients with solid tumors but only 54.7% among those with blood cancer. Risk factors for poor response included treatment with antimetabolites and anti-CD20 therapies, and, in the solid tumor group, radiation therapy, likely because of its overall toxicity and impact on lymphocyte function.
Israeli investigators reported in May a 90% seroconversion rate after two doses of the Pfizer vaccine among 102 patients with solid tumors on active treatment, which compared favorably to the 100% conversion rate in healthy controls, but they noted that antibody titers were considerably lower in patients with cancer.
The only variable associated with lower titer levels was combined use of chemotherapy and immunotherapy, they noted. There were also three women on dose-dense chemotherapy for breast cancer who did not produce any antibodies.
In a study limited to patients with blood cancers, a Lithuanian team recently reported that among 885 patients, those on Bruton tyrosine kinase inhibitors, ruxolitinib (Jakafi), venetoclax (Venclexta), or anti-CD20 therapies mounted almost no antibody response to the Pfizer vaccine.
The Lithuanian group also reported nine breakthrough COVID infections among their fully vaccinated blood cancer patients, including three deaths.
A team from the Icahn School of Medicine at Mount Sinai, New York reported that more than 15% of 260 patients with multiple myeloma also had no response to the Pfizer or Moderna vaccine; they were on BCMA-targeted therapy or anti-CD38 monoclonal antibody therapy at the time of vaccination, but a few had undergone CAR-T cell therapy more than 3 months beforehand.
Heated debate about antibody testing
Despite these reports of some patients with cancer having poorer responses, there’s some uncertainty over the benefit of giving a third (booster) shot.
There’s the question about the clinical relevance of antibody titer levels, and very little work has been done to date on cellular T-cell immunity from the vaccines.
“Right now, we are using titer levels like they actually mean something when they might not,” said Ravi Parikh, MD, a genitourinary and thoracic oncologist at the University of Pennsylvania, Philadelphia, who co-wrote an editorial that accompanies the Israeli report.
That’s one of the reasons why the FDA and others do not currently recommend antibody tests for COVID vaccine decisions outside of a clinical trial, but not everyone agrees with that position.
There’s been “a lot of heated debate in the medical community” over the issue, Dr. Haidar said.
The Icahn team, for instance, said that their results “underscore the need for routine serological monitoring of [multiple myeloma] patients following COVID-19 vaccination” to see if they might still need to mask-up and socially distance.
There is precedence, too, for vaccine boosters in cancer. As Dr. Parikh noted in his editorial, guidelines recommend revaccination after stem cell transplant for meningococcus, tetanus, and varicella, and other infections.
In France, COVID booster shots are already standard care for patients on dialysis and those on anti-CD20 agents, as well as for solid organ transplant recipients, for whom the literature supporting the benefit of COVID boosters is much more evolved than in cancer.
Israel has also authorized vaccine boosters for immunocompromised patients, including those with cancer, according to news reports.
It is also almost certain that the FDA will grant a formal approval for the COVID vaccines, at which point doctors will be free to administer boosters as they see fit.
“People are going to have to think really hard about what to do with them” if guidance hasn’t changed by then, Dr. Haidar said.
As the story unfolds, Dr. Haidar and others said in an interview that the take-home message for oncologists remains largely what it has been – namely to get patients vaccinated but also to consider masks and social distancing afterward for those at risk of a poor response.
Dr. Shah, Dr. Haidar, and Dr. Parikh have disclosed no relevant financial relationships. Dr. Parikh is a regular contributor to Medscape Oncology.
A version of this article first appeared on Medscape.com.
Patients with cancer have shown varying responses to COVID-19 vaccination, with good responses in patients with solid tumors (even while on systemic therapy) and poor responses in patients with blood cancers, particularly those on immunosuppressive therapies.
The data are evolving to show factors associated with a poor response but are not strong enough yet to recommend booster shots, say researchers.
The work is defining who will likely need a COVID vaccine booster when they become available. “It’s definitely not all cancer patients,” said Dimpy Shah, MD, PhD, a cancer epidemiologist at the Mays Cancer Center, University of Texas, San Antonio.
Public anxiously awaiting boosters
Boosters aren’t recommended in the United States at the moment, in large part because the Emergency Use Authorization under which the vaccines are being administered allows for only two shots of the Pfizer and Moderna vaccines and one shot of the Johnson & Johnson vaccine.
Even so, regulators and policymakers are “keenly aware that physicians and patients alike are anxious to get going and start doing boosters,” Dr. Shah said. There’s concern that antibody response might wane over time, perhaps even more quickly in patients with cancer.
Pfizer is already in talks with the U.S. Food and Drug Administration to authorize a third dose of its vaccine in the United States. Guidelines could very well change in coming months, said Ghady Haidar, MD, a specialist in infectious diseases and cancer at the University of Pittsburgh.
However, it’s still early in the game, and it’s not clear yet if boosters are necessary in cancer, Dr. Haidar said in an interview.
For one thing, it’s unknown if poor antibody response really means that patients aren’t protected, he explained. The vaccines elicit T-cell responses that could protect patients regardless of antibody levels. It’s also unclear if antibody titer levels are clinically relevant, and there hasn’t been much indication yet that less-than-robust vaccine responses translate to worse COVID outcomes in patients with cancer.
Those and other questions are areas of active investigation by Dr. Shah, Dr. Haidar, and others. Dozens of clinical trials are investigating vaccine response in patients with cancer, including the use of boosters.
Meanwhile, some cancer patients aren’t waiting around for more study results. “I get many, many emails a day” about booster shots, Dr. Haidar said. “We recommend against” them for now but some people bend the rules and get an extra shot anyway. “I get it. People are apprehensive.”
Three COVID deaths despite full vaccination
The vaccine clinical trials had fewer patients with cancer, so researchers are moving fast to backfill the data. Although there is some variation in what’s being reported, an overall picture is slowly emerging.
Dr. Shah and her team reported on responses to the mRNA COVID vaccines from Pfizer and Moderna and found a 94% seroconversion rate in 131 patients with cancer 3-4 weeks after their second dose of vaccine. They also found good responses among patients on cytotoxic chemotherapy within 6 months of their first vaccine dose, although their antibody titer levels were significantly lower than seen in other patients with cancer.
Investigators from Montefiore Medical Center in New York City also recently reported a 94% seroconversion rate among 200 patients with cancer, including 98% seroconversion in patients with solid tumors. Rates were lower in patients with blood cancers but were still 85% overall, with 70% conversion among patients on anti-CD20 therapies and 73% among stem cell transplant patients.
Dr. Haidar’s group reported a seroconversion rate of 82.4% among patients with solid tumors but only 54.7% among those with blood cancer. Risk factors for poor response included treatment with antimetabolites and anti-CD20 therapies, and, in the solid tumor group, radiation therapy, likely because of its overall toxicity and impact on lymphocyte function.
Israeli investigators reported in May a 90% seroconversion rate after two doses of the Pfizer vaccine among 102 patients with solid tumors on active treatment, which compared favorably to the 100% conversion rate in healthy controls, but they noted that antibody titers were considerably lower in patients with cancer.
The only variable associated with lower titer levels was combined use of chemotherapy and immunotherapy, they noted. There were also three women on dose-dense chemotherapy for breast cancer who did not produce any antibodies.
In a study limited to patients with blood cancers, a Lithuanian team recently reported that among 885 patients, those on Bruton tyrosine kinase inhibitors, ruxolitinib (Jakafi), venetoclax (Venclexta), or anti-CD20 therapies mounted almost no antibody response to the Pfizer vaccine.
The Lithuanian group also reported nine breakthrough COVID infections among their fully vaccinated blood cancer patients, including three deaths.
A team from the Icahn School of Medicine at Mount Sinai, New York reported that more than 15% of 260 patients with multiple myeloma also had no response to the Pfizer or Moderna vaccine; they were on BCMA-targeted therapy or anti-CD38 monoclonal antibody therapy at the time of vaccination, but a few had undergone CAR-T cell therapy more than 3 months beforehand.
Heated debate about antibody testing
Despite these reports of some patients with cancer having poorer responses, there’s some uncertainty over the benefit of giving a third (booster) shot.
There’s the question about the clinical relevance of antibody titer levels, and very little work has been done to date on cellular T-cell immunity from the vaccines.
“Right now, we are using titer levels like they actually mean something when they might not,” said Ravi Parikh, MD, a genitourinary and thoracic oncologist at the University of Pennsylvania, Philadelphia, who co-wrote an editorial that accompanies the Israeli report.
That’s one of the reasons why the FDA and others do not currently recommend antibody tests for COVID vaccine decisions outside of a clinical trial, but not everyone agrees with that position.
There’s been “a lot of heated debate in the medical community” over the issue, Dr. Haidar said.
The Icahn team, for instance, said that their results “underscore the need for routine serological monitoring of [multiple myeloma] patients following COVID-19 vaccination” to see if they might still need to mask-up and socially distance.
There is precedence, too, for vaccine boosters in cancer. As Dr. Parikh noted in his editorial, guidelines recommend revaccination after stem cell transplant for meningococcus, tetanus, and varicella, and other infections.
In France, COVID booster shots are already standard care for patients on dialysis and those on anti-CD20 agents, as well as for solid organ transplant recipients, for whom the literature supporting the benefit of COVID boosters is much more evolved than in cancer.
Israel has also authorized vaccine boosters for immunocompromised patients, including those with cancer, according to news reports.
It is also almost certain that the FDA will grant a formal approval for the COVID vaccines, at which point doctors will be free to administer boosters as they see fit.
“People are going to have to think really hard about what to do with them” if guidance hasn’t changed by then, Dr. Haidar said.
As the story unfolds, Dr. Haidar and others said in an interview that the take-home message for oncologists remains largely what it has been – namely to get patients vaccinated but also to consider masks and social distancing afterward for those at risk of a poor response.
Dr. Shah, Dr. Haidar, and Dr. Parikh have disclosed no relevant financial relationships. Dr. Parikh is a regular contributor to Medscape Oncology.
A version of this article first appeared on Medscape.com.
Patients with cancer have shown varying responses to COVID-19 vaccination, with good responses in patients with solid tumors (even while on systemic therapy) and poor responses in patients with blood cancers, particularly those on immunosuppressive therapies.
The data are evolving to show factors associated with a poor response but are not strong enough yet to recommend booster shots, say researchers.
The work is defining who will likely need a COVID vaccine booster when they become available. “It’s definitely not all cancer patients,” said Dimpy Shah, MD, PhD, a cancer epidemiologist at the Mays Cancer Center, University of Texas, San Antonio.
Public anxiously awaiting boosters
Boosters aren’t recommended in the United States at the moment, in large part because the Emergency Use Authorization under which the vaccines are being administered allows for only two shots of the Pfizer and Moderna vaccines and one shot of the Johnson & Johnson vaccine.
Even so, regulators and policymakers are “keenly aware that physicians and patients alike are anxious to get going and start doing boosters,” Dr. Shah said. There’s concern that antibody response might wane over time, perhaps even more quickly in patients with cancer.
Pfizer is already in talks with the U.S. Food and Drug Administration to authorize a third dose of its vaccine in the United States. Guidelines could very well change in coming months, said Ghady Haidar, MD, a specialist in infectious diseases and cancer at the University of Pittsburgh.
However, it’s still early in the game, and it’s not clear yet if boosters are necessary in cancer, Dr. Haidar said in an interview.
For one thing, it’s unknown if poor antibody response really means that patients aren’t protected, he explained. The vaccines elicit T-cell responses that could protect patients regardless of antibody levels. It’s also unclear if antibody titer levels are clinically relevant, and there hasn’t been much indication yet that less-than-robust vaccine responses translate to worse COVID outcomes in patients with cancer.
Those and other questions are areas of active investigation by Dr. Shah, Dr. Haidar, and others. Dozens of clinical trials are investigating vaccine response in patients with cancer, including the use of boosters.
Meanwhile, some cancer patients aren’t waiting around for more study results. “I get many, many emails a day” about booster shots, Dr. Haidar said. “We recommend against” them for now but some people bend the rules and get an extra shot anyway. “I get it. People are apprehensive.”
Three COVID deaths despite full vaccination
The vaccine clinical trials had fewer patients with cancer, so researchers are moving fast to backfill the data. Although there is some variation in what’s being reported, an overall picture is slowly emerging.
Dr. Shah and her team reported on responses to the mRNA COVID vaccines from Pfizer and Moderna and found a 94% seroconversion rate in 131 patients with cancer 3-4 weeks after their second dose of vaccine. They also found good responses among patients on cytotoxic chemotherapy within 6 months of their first vaccine dose, although their antibody titer levels were significantly lower than seen in other patients with cancer.
Investigators from Montefiore Medical Center in New York City also recently reported a 94% seroconversion rate among 200 patients with cancer, including 98% seroconversion in patients with solid tumors. Rates were lower in patients with blood cancers but were still 85% overall, with 70% conversion among patients on anti-CD20 therapies and 73% among stem cell transplant patients.
Dr. Haidar’s group reported a seroconversion rate of 82.4% among patients with solid tumors but only 54.7% among those with blood cancer. Risk factors for poor response included treatment with antimetabolites and anti-CD20 therapies, and, in the solid tumor group, radiation therapy, likely because of its overall toxicity and impact on lymphocyte function.
Israeli investigators reported in May a 90% seroconversion rate after two doses of the Pfizer vaccine among 102 patients with solid tumors on active treatment, which compared favorably to the 100% conversion rate in healthy controls, but they noted that antibody titers were considerably lower in patients with cancer.
The only variable associated with lower titer levels was combined use of chemotherapy and immunotherapy, they noted. There were also three women on dose-dense chemotherapy for breast cancer who did not produce any antibodies.
In a study limited to patients with blood cancers, a Lithuanian team recently reported that among 885 patients, those on Bruton tyrosine kinase inhibitors, ruxolitinib (Jakafi), venetoclax (Venclexta), or anti-CD20 therapies mounted almost no antibody response to the Pfizer vaccine.
The Lithuanian group also reported nine breakthrough COVID infections among their fully vaccinated blood cancer patients, including three deaths.
A team from the Icahn School of Medicine at Mount Sinai, New York reported that more than 15% of 260 patients with multiple myeloma also had no response to the Pfizer or Moderna vaccine; they were on BCMA-targeted therapy or anti-CD38 monoclonal antibody therapy at the time of vaccination, but a few had undergone CAR-T cell therapy more than 3 months beforehand.
Heated debate about antibody testing
Despite these reports of some patients with cancer having poorer responses, there’s some uncertainty over the benefit of giving a third (booster) shot.
There’s the question about the clinical relevance of antibody titer levels, and very little work has been done to date on cellular T-cell immunity from the vaccines.
“Right now, we are using titer levels like they actually mean something when they might not,” said Ravi Parikh, MD, a genitourinary and thoracic oncologist at the University of Pennsylvania, Philadelphia, who co-wrote an editorial that accompanies the Israeli report.
That’s one of the reasons why the FDA and others do not currently recommend antibody tests for COVID vaccine decisions outside of a clinical trial, but not everyone agrees with that position.
There’s been “a lot of heated debate in the medical community” over the issue, Dr. Haidar said.
The Icahn team, for instance, said that their results “underscore the need for routine serological monitoring of [multiple myeloma] patients following COVID-19 vaccination” to see if they might still need to mask-up and socially distance.
There is precedence, too, for vaccine boosters in cancer. As Dr. Parikh noted in his editorial, guidelines recommend revaccination after stem cell transplant for meningococcus, tetanus, and varicella, and other infections.
In France, COVID booster shots are already standard care for patients on dialysis and those on anti-CD20 agents, as well as for solid organ transplant recipients, for whom the literature supporting the benefit of COVID boosters is much more evolved than in cancer.
Israel has also authorized vaccine boosters for immunocompromised patients, including those with cancer, according to news reports.
It is also almost certain that the FDA will grant a formal approval for the COVID vaccines, at which point doctors will be free to administer boosters as they see fit.
“People are going to have to think really hard about what to do with them” if guidance hasn’t changed by then, Dr. Haidar said.
As the story unfolds, Dr. Haidar and others said in an interview that the take-home message for oncologists remains largely what it has been – namely to get patients vaccinated but also to consider masks and social distancing afterward for those at risk of a poor response.
Dr. Shah, Dr. Haidar, and Dr. Parikh have disclosed no relevant financial relationships. Dr. Parikh is a regular contributor to Medscape Oncology.
A version of this article first appeared on Medscape.com.
Long COVID symptoms reported by 6% of pediatric patients
The prevalence of long COVID in children has been unclear, and is complicated by the lack of a consistent definition, said Anna Funk, PhD, an epidemiologist at the University of Calgary (Alba.), during her online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
In the several small studies conducted to date, rates range from 0% to 67% 2-4 months after infection, Dr. Funk reported.
To examine prevalence, she and her colleagues, as part of the Pediatric Emergency Research Network (PERN) global research consortium, assessed more than 10,500 children who were screened for SARS-CoV-2 when they presented to the ED at 1 of 41 study sites in 10 countries – Australia, Canada, Indonesia, the United States, plus three countries in Latin America and three in Western Europe – from March 2020 to June 15, 2021.
PERN researchers are following up with the more than 3,100 children who tested positive 14, 30, and 90 days after testing, tracking respiratory, neurologic, and psychobehavioral sequelae.
Dr. Funk presented data on the 1,884 children who tested positive for SARS-CoV-2 before Jan. 20, 2021, and who had completed 90-day follow-up; 447 of those children were hospitalized and 1,437 were not.
Symptoms were reported more often by children admitted to the hospital than not admitted (9.8% vs. 4.6%). Common persistent symptoms were respiratory in 2% of cases, systemic (such as fatigue and fever) in 2%, neurologic (such as headache, seizures, and continued loss of taste or smell) in 1%, and psychological (such as new-onset depression and anxiety) in 1%.
“This study provides the first good epidemiological data on persistent symptoms among SARS-CoV-2–infected children, regardless of severity,” said Kevin Messacar, MD, a pediatric infectious disease clinician and researcher at Children’s Hospital Colorado in Aurora, who was not involved in the study.
And the findings show that, although severe COVID and chronic symptoms are less common in children than in adults, they are “not nonexistent and need to be taken seriously,” he said in an interview.
After adjustment for country of enrollment, children aged 10-17 years were more likely to experience persistent symptoms than children younger than 1 year (odds ratio, 2.4; P = .002).
Hospitalized children were more than twice as likely to experience persistent symptoms as nonhospitalized children (OR, 2.5; P < .001). And children who presented to the ED with at least seven symptoms were four times more likely to have long-term symptoms than those who presented with fewer symptoms (OR, 4.02; P = .01).
‘Some reassurance’
“Given that COVID is new and is known to have acute cardiac and neurologic effects, particularly in children with [multisystem inflammatory syndrome], there were initially concerns about persistent cardiovascular and neurologic effects in any infected child,” Dr. Messacar explained. “These data provide some reassurance that this is uncommon among children with mild or moderate infections who are not hospitalized.”
But “the risk is not zero,” he added. “Getting children vaccinated when it is available to them and taking precautions to prevent unvaccinated children getting COVID is the best way to reduce the risk of severe disease or persistent symptoms.”
The study was limited by its lack of data on variants, reliance on self-reported symptoms, and a population drawn solely from EDs, Dr. Funk acknowledged.
No external funding source was noted. Dr. Messacar and Dr. Funk disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The prevalence of long COVID in children has been unclear, and is complicated by the lack of a consistent definition, said Anna Funk, PhD, an epidemiologist at the University of Calgary (Alba.), during her online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
In the several small studies conducted to date, rates range from 0% to 67% 2-4 months after infection, Dr. Funk reported.
To examine prevalence, she and her colleagues, as part of the Pediatric Emergency Research Network (PERN) global research consortium, assessed more than 10,500 children who were screened for SARS-CoV-2 when they presented to the ED at 1 of 41 study sites in 10 countries – Australia, Canada, Indonesia, the United States, plus three countries in Latin America and three in Western Europe – from March 2020 to June 15, 2021.
PERN researchers are following up with the more than 3,100 children who tested positive 14, 30, and 90 days after testing, tracking respiratory, neurologic, and psychobehavioral sequelae.
Dr. Funk presented data on the 1,884 children who tested positive for SARS-CoV-2 before Jan. 20, 2021, and who had completed 90-day follow-up; 447 of those children were hospitalized and 1,437 were not.
Symptoms were reported more often by children admitted to the hospital than not admitted (9.8% vs. 4.6%). Common persistent symptoms were respiratory in 2% of cases, systemic (such as fatigue and fever) in 2%, neurologic (such as headache, seizures, and continued loss of taste or smell) in 1%, and psychological (such as new-onset depression and anxiety) in 1%.
“This study provides the first good epidemiological data on persistent symptoms among SARS-CoV-2–infected children, regardless of severity,” said Kevin Messacar, MD, a pediatric infectious disease clinician and researcher at Children’s Hospital Colorado in Aurora, who was not involved in the study.
And the findings show that, although severe COVID and chronic symptoms are less common in children than in adults, they are “not nonexistent and need to be taken seriously,” he said in an interview.
After adjustment for country of enrollment, children aged 10-17 years were more likely to experience persistent symptoms than children younger than 1 year (odds ratio, 2.4; P = .002).
Hospitalized children were more than twice as likely to experience persistent symptoms as nonhospitalized children (OR, 2.5; P < .001). And children who presented to the ED with at least seven symptoms were four times more likely to have long-term symptoms than those who presented with fewer symptoms (OR, 4.02; P = .01).
‘Some reassurance’
“Given that COVID is new and is known to have acute cardiac and neurologic effects, particularly in children with [multisystem inflammatory syndrome], there were initially concerns about persistent cardiovascular and neurologic effects in any infected child,” Dr. Messacar explained. “These data provide some reassurance that this is uncommon among children with mild or moderate infections who are not hospitalized.”
But “the risk is not zero,” he added. “Getting children vaccinated when it is available to them and taking precautions to prevent unvaccinated children getting COVID is the best way to reduce the risk of severe disease or persistent symptoms.”
The study was limited by its lack of data on variants, reliance on self-reported symptoms, and a population drawn solely from EDs, Dr. Funk acknowledged.
No external funding source was noted. Dr. Messacar and Dr. Funk disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The prevalence of long COVID in children has been unclear, and is complicated by the lack of a consistent definition, said Anna Funk, PhD, an epidemiologist at the University of Calgary (Alba.), during her online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
In the several small studies conducted to date, rates range from 0% to 67% 2-4 months after infection, Dr. Funk reported.
To examine prevalence, she and her colleagues, as part of the Pediatric Emergency Research Network (PERN) global research consortium, assessed more than 10,500 children who were screened for SARS-CoV-2 when they presented to the ED at 1 of 41 study sites in 10 countries – Australia, Canada, Indonesia, the United States, plus three countries in Latin America and three in Western Europe – from March 2020 to June 15, 2021.
PERN researchers are following up with the more than 3,100 children who tested positive 14, 30, and 90 days after testing, tracking respiratory, neurologic, and psychobehavioral sequelae.
Dr. Funk presented data on the 1,884 children who tested positive for SARS-CoV-2 before Jan. 20, 2021, and who had completed 90-day follow-up; 447 of those children were hospitalized and 1,437 were not.
Symptoms were reported more often by children admitted to the hospital than not admitted (9.8% vs. 4.6%). Common persistent symptoms were respiratory in 2% of cases, systemic (such as fatigue and fever) in 2%, neurologic (such as headache, seizures, and continued loss of taste or smell) in 1%, and psychological (such as new-onset depression and anxiety) in 1%.
“This study provides the first good epidemiological data on persistent symptoms among SARS-CoV-2–infected children, regardless of severity,” said Kevin Messacar, MD, a pediatric infectious disease clinician and researcher at Children’s Hospital Colorado in Aurora, who was not involved in the study.
And the findings show that, although severe COVID and chronic symptoms are less common in children than in adults, they are “not nonexistent and need to be taken seriously,” he said in an interview.
After adjustment for country of enrollment, children aged 10-17 years were more likely to experience persistent symptoms than children younger than 1 year (odds ratio, 2.4; P = .002).
Hospitalized children were more than twice as likely to experience persistent symptoms as nonhospitalized children (OR, 2.5; P < .001). And children who presented to the ED with at least seven symptoms were four times more likely to have long-term symptoms than those who presented with fewer symptoms (OR, 4.02; P = .01).
‘Some reassurance’
“Given that COVID is new and is known to have acute cardiac and neurologic effects, particularly in children with [multisystem inflammatory syndrome], there were initially concerns about persistent cardiovascular and neurologic effects in any infected child,” Dr. Messacar explained. “These data provide some reassurance that this is uncommon among children with mild or moderate infections who are not hospitalized.”
But “the risk is not zero,” he added. “Getting children vaccinated when it is available to them and taking precautions to prevent unvaccinated children getting COVID is the best way to reduce the risk of severe disease or persistent symptoms.”
The study was limited by its lack of data on variants, reliance on self-reported symptoms, and a population drawn solely from EDs, Dr. Funk acknowledged.
No external funding source was noted. Dr. Messacar and Dr. Funk disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: New vaccinations drop as the case count rises
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
With only a quarter of all children aged 12-15 years fully vaccinated against COVID-19, first vaccinations continued to drop and new cases for all children rose for the second consecutive week.

Just under 25% of children aged 12-15 had completed the vaccine regimen as of July 12, and just over one-third (33.5%) had received at least one dose. Meanwhile, that age group represented 11.5% of people who initiated vaccination during the 2 weeks ending July 12, down from 12.1% a week earlier, the Centers for Disease Control and Prevention said. The total number of new vaccinations for the week ending July 12 was just over 201,000, compared with 307,000 for the previous week.
New cases of COVID-19, however, were on the rise in children. The 19,000 new cases reported for the week ending July 8 were up from 12,000 a week earlier and 8,000 the week before that, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
That report also shows that children made up 22.3% of all new cases during the week of July 2-8, compared with 16.8% the previous week, and that there were nine deaths in children that same week, the most since March. COVID-related deaths among children total 344 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting such data by age. “It is not possible to standardize more detailed age ranges for children based on what is publicly available from the states,” the two groups noted.
Such data are available from the CDC’s COVID Data Tracker, however, and they show that children aged 16-17 years, who became eligible for COVID vaccination before the younger age group, are further ahead in the process. Among the older children, almost 46% had gotten at least one dose and 37% were fully vaccinated by July 12.
Patients on methotrexate show T-cell response to Pfizer vaccine
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People taking methotrexate had low antibody responses after the first dose of the Pfizer-BioNTech mRNA COVID-19 vaccine, but did show evidence of T-cell–mediated immune responses, findings from a small study show.
The common immunosuppressant has previously been linked to poor antibody responses to mRNA COVID-19 vaccines, but this appears to be the first study to look at T-cell responses in people taking methotrexate.
The study findings were presented online July 11 at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published in The Lancet Rheumatology.
“These findings indicate that seroconversion alone might not adequately reflect vaccine immunogenicity in individuals with immune-mediated inflammatory diseases receiving therapeutic immunosuppression, and caution against routine use of seroconversion data in isolation in clinical practice,” Satveer K. Mahil, MBBChir, PhD, from St. John’s Institute of Dermatology, Guy’s and St. Thomas’ NHS Foundation Trust, London, and colleagues wrote.
“When taking into account functional humoral immunity and T-cell responses, our data suggest that targeted biologics do not impair vaccine responses and provide some reassurance to this vulnerable population,” they wrote. “Notably, although methotrexate attenuated humoral immunity, cellular responses were preserved.”
Dr. Mahil and colleagues assessed 84 consecutive patients from a psoriasis specialist clinic that serves London and southeast England. Median age of the cohort was 43 years, and 85% were White. All had a confirmed psoriasis diagnosis, received the first dose of the Pfizer-BioNTech COVID-19 vaccine, and were taking either methotrexate (17 patients) or a targeted biologic (27 were taking a tumor necrosis factor inhibitor, 15 an interleukin-17 inhibitor, and 25 an IL-23 inhibitor). In addition, 17 healthy patients not receiving immunosuppression therapy who received the Pfizer-BioNTech vaccine served as the control group.
Four weeks after the study participants received their first dose of the vaccine, 78% of the immunosuppressed patients underwent seroconversion – producing measurable antibodies – as did 100% of the control group. Patients taking methotrexate had the lowest seroconversion rate at 47%, compared with 79% with TNF inhibitors, 83% with IL-23 inhibitors, and 100% with IL-17 inhibitors.
Participants taking methotrexate also had lower neutralizing activity against SARS-CoV-2 than control subjects and those taking a targeted biologic, who had similar levels of neutralizing activity.
All participants had low neutralizing titers against the alpha (B.1.1.7) variant.
The researchers also assessed cellular immunity, “defined as the presence of T cells secreting interferon-gamma, IL-2, or IL-21 in response to stimulation with two peptide pools spanning the entire length of the SARS-CoV-2 spike glycoprotein.”
A T-cell response was seen in 84% of participants taking immunosuppressants, including 93% of those in the methotrexate group and 69% of control subjects.
‘Some protection is better than none’
These findings regarding antibodies match what has been seen in other research, said Ignacio Sanz, MD, director of the Lowance Center for Human Immunology at Emory University, Atlanta.
It would be helpful to see antibody responses after the second doses, he added. Those data will be reported later, according to Dr. Mahil and colleagues.
“The authors make the valid point that T-cell immunity should also be measured. The information is meaningful and supports the idea that there could be protection still provided,” Dr. Sanz said in an interview, adding that it would have been helpful to see CD8 T-cell response as well.
“My message to patients, still, is that some protection is better than none, and that, indeed, protection may be afforded in different ways, including T-cell immunity, which, to the extent tested, seems to be induced,” he said. But discussion of B cells independent of their role in producing antibodies is missing.
“When it comes to B-cell responses, antibodies are the easier and more direct measurement. However, it is perfectly possible that the vaccine may fail to induce high antibody titers and still generate good B-cell immunity,” in the same way virus-specific memory B cells do, he explained. “They would not directly produce antibodies, yet they would be available for a good and quick response in the case of subsequent encounter with the virus and, incidentally, in the case of a booster dose. It is possible that the generation of antibody-producing plasma cells might be uncoupled from the generation of memory B cells.”
Temporarily stopping methotrexate
It is well known that methotrexate impairs humoral responses to influenza and pneumococcal vaccines, write Caoilfhionn M. Connolly, MD, and Julie J. Paik, MD, both from the Johns Hopkins University, Baltimore, in an accompanying comment.
Research has also shown that temporarily stopping methotrexate therapy for 2 weeks enhances response to the flu vaccine in patients with rheumatoid arthritis, which prompted the American College of Rheumatology to recommended temporary interruption of methotrexate for 1 week after each dose of the COVID-19 vaccine, the pair notes.
“Although it is encouraging that cellular responses appear to be preserved even in patients with poor humoral responses, these findings are not consistent across study groups,” Dr. Connolly and Dr. Paik explained. “During this period of clinical uncertainty, patients might remain vulnerable, especially after the first dose, and should engage in risk mitigation strategies.”
Mild adverse events after vaccination were reported by 75% of the immunosuppressed patients – most commonly injection-site pain, headache, and fatigue – and by 94% of control subjects. No participants reported moderate or severe adverse effects.
However, 11% of immunosuppressed patients reported a worsening of psoriasis symptoms after vaccination.
This research was funded by the U.K. National Institute for Health Research. Dr. Mahil has received departmental income from AbbVie, Celgene, Eli Lilly, Janssen-Cilag, Novartis, Sano, and UCB unrelated to this study. Seven other authors have relationships with a wide range of pharmaceutical and other companies. Dr. Sanz, Dr. Connolly, and Dr. Paik disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gender pay gap most pronounced in procedural specialties
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
Salary disparities persist in academic internal medicine specialties and are most obvious in procedural specialties, such as cardiology, in which there are fewer women, research suggests.
“Substantial salary inequities persist at the highest faculty levels and specifically in procedural-based specialties,” Teresa Wang, MD, and colleagues reported in a research letter published online July 12, 2021, in JAMA Internal Medicine.
To examine the demographics and salaries of academic internal medicine physician specialists, Dr. Wang, who is with the division of cardiovascular medicine at the University of Pennsylvania, Philadelphia, and coauthors analyzed survey results from faculty at 154 U.S. medical schools.
They used data from the Association of American Medical Colleges Faculty Salary Report of 2018-2019 to assess the median annual salary, faculty rank, and gender for 21,905 faculty in 13 internal medicine specialties.
Overall, women made up less than 40% of full-time faculty across ranks. Female representation was approximately equal at the instructor and assistant ranks – 47% and 46%, respectively – but decreased to 24% at the professor level.
The authors found that women made up the majority in three specialties – general internal medicine, endocrinology, and geriatrics. In contrast, women were least represented in the procedural specialties of pulmonology, critical/intensive care, gastroenterology, and cardiology.
The greatest imbalance was in cardiology, in which only 21% were women, the researchers noted.
Across faculty ranks, the median annual salary was less for women than for men. The median salary for women was within $25,000 of that for men at all ranks except chief and was at least 90% of that for men in 10 of 13 internal medicine specialties.
Cardiology, gastroenterology, and critical/intensive care were the three specialties in which women’s median salary did not reach 90% of men’s. These specialties tended to be better paid overall, “but also demonstrated the largest gender disparities in both representation and salary, particularly within the higher ranks of cardiology and gastroenterology,” the researchers said.
The reasons for gender disparities are unclear, though internal medicine procedural specialties “have long been male dominated in composition and leadership,” the authors noted. The findings indicate that workforce gender parity may be associated with salary equity.
“Despite the growing awareness of workforce disparities in medicine, our findings suggest that women internal medicine specialists remain underpaid and are not promoted to senior level academic ranks when compared with career trajectories of their male counterparts,” study author Nosheen Reza, MD, of the division of cardiovascular medicine at the University of Pennsylvania, told this news organization.
The researchers noted that they were unable to adjust at the individual level for various factors that may influence salary, such as professional service, academic productivity, clinical volume, and supplementary funding sources, and that the results might not apply to all U.S. medical schools, in which departmental structures vary.
Procedures versus evaluation and management
Still, the research “provides an interesting snapshot of current salary disparities in academic internal medicine,” comment Rita F. Redberg, MD, and colleagues in a related editorial. Dr. Redberg, the editor of JAMA Internal Medicine, is affiliated with the department of medicine at the University of California, San Francisco.
Internal medicine has 13 specialties and dozens of subspecialties, and “procedural subspecialties are more male dominated and better paid than nonprocedural subspecialties – both topics deserving of further exploration,” the editorialists wrote.
The field needs to address various issues that drive some women to “shun male-dominated procedural-based fields – including lack of role models, macho ‘cowboy’ culture, unpredictable schedules, longer training periods, or cultural factors,” Dr. Redberg and coauthors suggested. “Concurrently, the medical profession overall, as well as specialties, should thoughtfully and frequently reassess how to distribute pay more equitably and to remove the premium currently paid for procedures over evaluation and management services.”
“Unfortunately, it is not a surprise that there continues to be a gender gap for salary in academic medicine,” Dr. Redberg said in an interview. “It was interesting to see that gender pay disparities were greatest in the procedure-intensive specialties, and we do know that procedures are much more highly reimbursed than evaluation and management time, even in the IM specialties. From a patient perspective, I think what they value most highly is having their doctor talk with them and explain treatment options and risks and benefits. Sadly, our fee-for-service–based reimbursement system values procedures more highly than talking with patients. And part of the gender gap in salary is attributed to less women being proceduralists.”
The Medicare Payment Advisory Commission “has made some excellent recommendations to Congress on helping to correct this imbalance,” Dr. Redberg added.
In a separate viewpoint article, Leah M. Marcotte, MD, of the department of medicine at the University of Washington, Seattle, and colleagues describe reasons why women physicians may have “slower promotional time lines,” compared with men, such as receiving fewer and smaller grants, being underrepresented as speakers at national conferences, and receiving fewer invitations to author editorials.
“To narrow this gap, institutions should proactively nominate women, with a greater focus on those underrepresented in medicine, for internal and external awards and speaking opportunities,” Dr. Marcotte and coauthors wrote. “Institutions should adopt policies to cover child care, breastfeeding/pumping accommodations, and dependent travel. Academic departments should continue to offer virtual speaking opportunities even after COVID-19 pandemic travel restrictions become unnecessary.”
Institutions can also assist women faculty in preparing promotion dossiers.
“Gender disparities in promotion in academic medicine have been described for decades, and yet progress to close the gap has been untenably slow,” they said. “Rather than expecting faculty to adapt to existing systems, we need to change the promotion process to work better for all.”
The authors disclosed no relevant financial relationships. Dr. Redberg has received grants from Arnold Ventures, the Greenwall Foundation, and the National Heart, Lung, and Blood Institute outside the submitted work. One viewpoint coauthor has received honoraria from the American Board of Internal Medicine, and another has received personal fees from F-Prime Capital, both outside the submitted work.
A version of this article first appeared on Medscape.com.
FDA to warn J&J that vaccine can increase Guillain-Barré risk: Media
as early as July 13, according to multiple media reports.
Although the FDA is projected to add the new warning to the labeling for the vaccine, the agency still calculates the benefit of vaccination with the J&J product continues to outweigh the risk. Benefits include protection against the Delta variant and serious COVID-19 outcomes.
More than 100 cases of Guillain-Barré reported to the Vaccine Adverse Event Reporting System, a federal program for reporting vaccine issues, spurred the FDA to act.
Men and people older than 50 appear to be at highest risk, according to reports of a July 12 Centers for Disease Control and Prevention statement. The CDC also revealed that most cases occur about 2 weeks following immunization.
Guillain-Barré syndrome often causes muscle weakness and sometimes temporary paralysis. Most people who develop the rare syndrome recover.
Such was not the case for a 57-year-old man, the New York Times reported July 12. He had a history of both a heart attack and stroke in the previous 4 years and died in April after vaccination with the J&J vaccine and developing Guillain-Barré.
The new warning comes in the wake of a number of setbacks for the company’s COVID-19 vaccine. On April 13, the FDA and CDC both recommended a 10-day pause on administration of the J&J vaccine after reports of rare blood clot events emerged. In mid-June, the FDA requested that Johnson and Johnson discard millions of vaccine doses produced at a manufacturing facility in Baltimore.
The mRNA vaccines from Pfizer/BioNTech and Moderna are not affected by the new FDA warning.
The Biden administration is expected to make a formal announcement of the new warning for the Johnson and Johnson vaccine as early as July 13, the Times reports.
A version of this article first appeared on Medscape.com.
as early as July 13, according to multiple media reports.
Although the FDA is projected to add the new warning to the labeling for the vaccine, the agency still calculates the benefit of vaccination with the J&J product continues to outweigh the risk. Benefits include protection against the Delta variant and serious COVID-19 outcomes.
More than 100 cases of Guillain-Barré reported to the Vaccine Adverse Event Reporting System, a federal program for reporting vaccine issues, spurred the FDA to act.
Men and people older than 50 appear to be at highest risk, according to reports of a July 12 Centers for Disease Control and Prevention statement. The CDC also revealed that most cases occur about 2 weeks following immunization.
Guillain-Barré syndrome often causes muscle weakness and sometimes temporary paralysis. Most people who develop the rare syndrome recover.
Such was not the case for a 57-year-old man, the New York Times reported July 12. He had a history of both a heart attack and stroke in the previous 4 years and died in April after vaccination with the J&J vaccine and developing Guillain-Barré.
The new warning comes in the wake of a number of setbacks for the company’s COVID-19 vaccine. On April 13, the FDA and CDC both recommended a 10-day pause on administration of the J&J vaccine after reports of rare blood clot events emerged. In mid-June, the FDA requested that Johnson and Johnson discard millions of vaccine doses produced at a manufacturing facility in Baltimore.
The mRNA vaccines from Pfizer/BioNTech and Moderna are not affected by the new FDA warning.
The Biden administration is expected to make a formal announcement of the new warning for the Johnson and Johnson vaccine as early as July 13, the Times reports.
A version of this article first appeared on Medscape.com.
as early as July 13, according to multiple media reports.
Although the FDA is projected to add the new warning to the labeling for the vaccine, the agency still calculates the benefit of vaccination with the J&J product continues to outweigh the risk. Benefits include protection against the Delta variant and serious COVID-19 outcomes.
More than 100 cases of Guillain-Barré reported to the Vaccine Adverse Event Reporting System, a federal program for reporting vaccine issues, spurred the FDA to act.
Men and people older than 50 appear to be at highest risk, according to reports of a July 12 Centers for Disease Control and Prevention statement. The CDC also revealed that most cases occur about 2 weeks following immunization.
Guillain-Barré syndrome often causes muscle weakness and sometimes temporary paralysis. Most people who develop the rare syndrome recover.
Such was not the case for a 57-year-old man, the New York Times reported July 12. He had a history of both a heart attack and stroke in the previous 4 years and died in April after vaccination with the J&J vaccine and developing Guillain-Barré.
The new warning comes in the wake of a number of setbacks for the company’s COVID-19 vaccine. On April 13, the FDA and CDC both recommended a 10-day pause on administration of the J&J vaccine after reports of rare blood clot events emerged. In mid-June, the FDA requested that Johnson and Johnson discard millions of vaccine doses produced at a manufacturing facility in Baltimore.
The mRNA vaccines from Pfizer/BioNTech and Moderna are not affected by the new FDA warning.
The Biden administration is expected to make a formal announcement of the new warning for the Johnson and Johnson vaccine as early as July 13, the Times reports.
A version of this article first appeared on Medscape.com.
Standard medical mask can protect wearer from aerosols
A standard medical face mask is more effective at preventing the wearer from inhaling aerosols without causing substantial breathing resistance than various cloth, medical, or respirator masks, new research shows.
“Medical face masks with good filtration efficacies can provide even better protective effects than KN95 respirators,” Christian Sterr, MD, from Philipps University of Marburg (Germany), and colleagues wrote. “FFP2 respirators, on the other hand, could be useful in high-risk situations but require greater breathing effort and therefore physical stress for users.”
Extensive evidence has shown that face masks are an excellent form of source control, preventing infectious people from spreading the SARS-CoV-2 virus into the environment. But evidence has been less clear about how well masks protect the wearer from inhaling particles containing the virus.
The researchers conducted three experiments to test 32 different face masks. The findings were presented at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published online in PLOS One .
First they tested pressure drop, which “relates to how easily air can pass through the material,” said Chris Cappa, PhD, professor of civil and environmental engineering at the University of California, Davis, who was not involved with the study.
“Higher pressure drops mean that there is greater resistance to the air passing through. A higher pressure drop will typically mean breathing through the material will be slightly more challenging, compared to a low pressure drop. There is no relationship between pressure drop and the mask effectiveness,” he said in an interview.
Pressure drop was lowest with type II medical face masks, the typical three-ply surgical masks designed to stop large particles expelled by the wearer from entering the environment, was highest with respirators, including KN95 and FFP2 masks, and varied with the different cloth masks tested.
Next the researchers compared filtration efficacy, which “refers to how well the material removes particles from the air that passes through the mask material,” Dr. Cappa explained. They did this by placing each mask over the opening to an air collector that measured how many particles got through. “A mask that has 100% filtration efficacy will remove all particles from the air that passes through it and 0% means that no particles are removed.”
Cloth masks had the lowest filtration efficacy, at 28%. Certified face masks that met European Standards had a relatively high efficacy, at 70%; for uncertified face masks, filtration efficacy was 63%. As expected, KN95 and FFP2 masks had the highest filtration efficacy, at 94% and 98%, respectively.Finally, the researchers tested as-worn filtration efficacies. They placed each mask on a dummy head with an artificial airway that collected airborne particles. They then pumped a mixture of aerosol particles – ranging in size from 0.3 to 2.0 mcm – and particle-free pressurized air into the air-proof acrylic chamber in which the head was placed.
In this experiment, cloth masks and noncertified face masks were least effective, filtering less than 20% of aerosols. Interestingly, the cloth face mask with the highest filtration on its own (84%) had the lowest filtration efficacy (9%), apparently because of its very high pressure drop (breathing resistance). When more effort is required to breathe through a mask, more air can bypass the filtration system.
Type II medical face masks, however, filtered 47% of aerosols, KN95 masks filtered 41%, and FFP2 masks filtered 65%. Face shields did not prevent the inhalation of any aerosols.
“We know that face shields will only be effective in stopping very large droplets, essentially visible spittle,” Dr. Cappa explained. “Most of the particles that we exhale will travel right around a face shield.”
The “optimal mask effect is a combination of high filter performance and low filter resistance,” which applies to most of the FFP2 and medical type II face masks tested, Dr. Sterr and colleagues wrote. “The type II medical masks in our random sample showed very good as-worn filtration performances with a low additional work of breathing at the same time.”
Although this study showed how well different masks filtered out particles, it could not assess how well different masks prevent actual infection.
“Like any virus, SARS-CoV-2 can only infect people as long as it is viable,” the researchers wrote. “Moreover, a certain number of viable virus particles need to be inhaled to trigger an infection. Thus, the assessed filtration efficacy may differ from the provided protection rate against SARS-CoV-2.”
In addition, particles containing the virus could dry out while going through the mask and become less infectious. “Even a small reduction in inhaled particles might prevent infection or at least lead to a less severe infection,” they noted.
In fact, filtration efficacy does not necessarily indicate how well the mask filters out particles while being worn. “This might be due to the combined effects of mask fit and pressure drop of the mask material and therefore tendency for mask leakage,” the team wrote. “High pressure drop results in higher breathing resistance and therefore supports leakage, especially if combined to a loosely fitting mask.”
These findings are “in line with what we already knew,” Dr. Cappa explained. “Even if the mask material filters out nearly all particles that pass through it, as is the case for high-efficiency masks such as N95 and FFP2, if the mask does not fit well, then it will only provide moderate protection for the wearer.”
Although the findings reaffirm the different levels of filtration provided by various cloth masks, they do not “provide any guidance on which types of cloth masks are better or worse,” he said. But they do show that “medical face masks will generally provide more protection to the wearer.”
It’s not surprising that face shields offer little protection from aerosols, Dr. Cappa said, but they can provide added protection when worn with a mask.
“A face shield could prevent large droplets that might shoot out when a person coughs or sneezes from depositing on a person’s eye,” he pointed out. And it can help “redirect the plume of particles that an infected person exhales, which could be useful in close quarters. However, even then those particles will keep moving around and could be inhaled. A mask can really help to decrease the amount inhaled.”
The study did not use external funding. The authors and Dr. Cappa disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A standard medical face mask is more effective at preventing the wearer from inhaling aerosols without causing substantial breathing resistance than various cloth, medical, or respirator masks, new research shows.
“Medical face masks with good filtration efficacies can provide even better protective effects than KN95 respirators,” Christian Sterr, MD, from Philipps University of Marburg (Germany), and colleagues wrote. “FFP2 respirators, on the other hand, could be useful in high-risk situations but require greater breathing effort and therefore physical stress for users.”
Extensive evidence has shown that face masks are an excellent form of source control, preventing infectious people from spreading the SARS-CoV-2 virus into the environment. But evidence has been less clear about how well masks protect the wearer from inhaling particles containing the virus.
The researchers conducted three experiments to test 32 different face masks. The findings were presented at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published online in PLOS One .
First they tested pressure drop, which “relates to how easily air can pass through the material,” said Chris Cappa, PhD, professor of civil and environmental engineering at the University of California, Davis, who was not involved with the study.
“Higher pressure drops mean that there is greater resistance to the air passing through. A higher pressure drop will typically mean breathing through the material will be slightly more challenging, compared to a low pressure drop. There is no relationship between pressure drop and the mask effectiveness,” he said in an interview.
Pressure drop was lowest with type II medical face masks, the typical three-ply surgical masks designed to stop large particles expelled by the wearer from entering the environment, was highest with respirators, including KN95 and FFP2 masks, and varied with the different cloth masks tested.
Next the researchers compared filtration efficacy, which “refers to how well the material removes particles from the air that passes through the mask material,” Dr. Cappa explained. They did this by placing each mask over the opening to an air collector that measured how many particles got through. “A mask that has 100% filtration efficacy will remove all particles from the air that passes through it and 0% means that no particles are removed.”
Cloth masks had the lowest filtration efficacy, at 28%. Certified face masks that met European Standards had a relatively high efficacy, at 70%; for uncertified face masks, filtration efficacy was 63%. As expected, KN95 and FFP2 masks had the highest filtration efficacy, at 94% and 98%, respectively.Finally, the researchers tested as-worn filtration efficacies. They placed each mask on a dummy head with an artificial airway that collected airborne particles. They then pumped a mixture of aerosol particles – ranging in size from 0.3 to 2.0 mcm – and particle-free pressurized air into the air-proof acrylic chamber in which the head was placed.
In this experiment, cloth masks and noncertified face masks were least effective, filtering less than 20% of aerosols. Interestingly, the cloth face mask with the highest filtration on its own (84%) had the lowest filtration efficacy (9%), apparently because of its very high pressure drop (breathing resistance). When more effort is required to breathe through a mask, more air can bypass the filtration system.
Type II medical face masks, however, filtered 47% of aerosols, KN95 masks filtered 41%, and FFP2 masks filtered 65%. Face shields did not prevent the inhalation of any aerosols.
“We know that face shields will only be effective in stopping very large droplets, essentially visible spittle,” Dr. Cappa explained. “Most of the particles that we exhale will travel right around a face shield.”
The “optimal mask effect is a combination of high filter performance and low filter resistance,” which applies to most of the FFP2 and medical type II face masks tested, Dr. Sterr and colleagues wrote. “The type II medical masks in our random sample showed very good as-worn filtration performances with a low additional work of breathing at the same time.”
Although this study showed how well different masks filtered out particles, it could not assess how well different masks prevent actual infection.
“Like any virus, SARS-CoV-2 can only infect people as long as it is viable,” the researchers wrote. “Moreover, a certain number of viable virus particles need to be inhaled to trigger an infection. Thus, the assessed filtration efficacy may differ from the provided protection rate against SARS-CoV-2.”
In addition, particles containing the virus could dry out while going through the mask and become less infectious. “Even a small reduction in inhaled particles might prevent infection or at least lead to a less severe infection,” they noted.
In fact, filtration efficacy does not necessarily indicate how well the mask filters out particles while being worn. “This might be due to the combined effects of mask fit and pressure drop of the mask material and therefore tendency for mask leakage,” the team wrote. “High pressure drop results in higher breathing resistance and therefore supports leakage, especially if combined to a loosely fitting mask.”
These findings are “in line with what we already knew,” Dr. Cappa explained. “Even if the mask material filters out nearly all particles that pass through it, as is the case for high-efficiency masks such as N95 and FFP2, if the mask does not fit well, then it will only provide moderate protection for the wearer.”
Although the findings reaffirm the different levels of filtration provided by various cloth masks, they do not “provide any guidance on which types of cloth masks are better or worse,” he said. But they do show that “medical face masks will generally provide more protection to the wearer.”
It’s not surprising that face shields offer little protection from aerosols, Dr. Cappa said, but they can provide added protection when worn with a mask.
“A face shield could prevent large droplets that might shoot out when a person coughs or sneezes from depositing on a person’s eye,” he pointed out. And it can help “redirect the plume of particles that an infected person exhales, which could be useful in close quarters. However, even then those particles will keep moving around and could be inhaled. A mask can really help to decrease the amount inhaled.”
The study did not use external funding. The authors and Dr. Cappa disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A standard medical face mask is more effective at preventing the wearer from inhaling aerosols without causing substantial breathing resistance than various cloth, medical, or respirator masks, new research shows.
“Medical face masks with good filtration efficacies can provide even better protective effects than KN95 respirators,” Christian Sterr, MD, from Philipps University of Marburg (Germany), and colleagues wrote. “FFP2 respirators, on the other hand, could be useful in high-risk situations but require greater breathing effort and therefore physical stress for users.”
Extensive evidence has shown that face masks are an excellent form of source control, preventing infectious people from spreading the SARS-CoV-2 virus into the environment. But evidence has been less clear about how well masks protect the wearer from inhaling particles containing the virus.
The researchers conducted three experiments to test 32 different face masks. The findings were presented at the 31st European Congress of Clinical Microbiology & Infectious Diseases and published online in PLOS One .
First they tested pressure drop, which “relates to how easily air can pass through the material,” said Chris Cappa, PhD, professor of civil and environmental engineering at the University of California, Davis, who was not involved with the study.
“Higher pressure drops mean that there is greater resistance to the air passing through. A higher pressure drop will typically mean breathing through the material will be slightly more challenging, compared to a low pressure drop. There is no relationship between pressure drop and the mask effectiveness,” he said in an interview.
Pressure drop was lowest with type II medical face masks, the typical three-ply surgical masks designed to stop large particles expelled by the wearer from entering the environment, was highest with respirators, including KN95 and FFP2 masks, and varied with the different cloth masks tested.
Next the researchers compared filtration efficacy, which “refers to how well the material removes particles from the air that passes through the mask material,” Dr. Cappa explained. They did this by placing each mask over the opening to an air collector that measured how many particles got through. “A mask that has 100% filtration efficacy will remove all particles from the air that passes through it and 0% means that no particles are removed.”
Cloth masks had the lowest filtration efficacy, at 28%. Certified face masks that met European Standards had a relatively high efficacy, at 70%; for uncertified face masks, filtration efficacy was 63%. As expected, KN95 and FFP2 masks had the highest filtration efficacy, at 94% and 98%, respectively.Finally, the researchers tested as-worn filtration efficacies. They placed each mask on a dummy head with an artificial airway that collected airborne particles. They then pumped a mixture of aerosol particles – ranging in size from 0.3 to 2.0 mcm – and particle-free pressurized air into the air-proof acrylic chamber in which the head was placed.
In this experiment, cloth masks and noncertified face masks were least effective, filtering less than 20% of aerosols. Interestingly, the cloth face mask with the highest filtration on its own (84%) had the lowest filtration efficacy (9%), apparently because of its very high pressure drop (breathing resistance). When more effort is required to breathe through a mask, more air can bypass the filtration system.
Type II medical face masks, however, filtered 47% of aerosols, KN95 masks filtered 41%, and FFP2 masks filtered 65%. Face shields did not prevent the inhalation of any aerosols.
“We know that face shields will only be effective in stopping very large droplets, essentially visible spittle,” Dr. Cappa explained. “Most of the particles that we exhale will travel right around a face shield.”
The “optimal mask effect is a combination of high filter performance and low filter resistance,” which applies to most of the FFP2 and medical type II face masks tested, Dr. Sterr and colleagues wrote. “The type II medical masks in our random sample showed very good as-worn filtration performances with a low additional work of breathing at the same time.”
Although this study showed how well different masks filtered out particles, it could not assess how well different masks prevent actual infection.
“Like any virus, SARS-CoV-2 can only infect people as long as it is viable,” the researchers wrote. “Moreover, a certain number of viable virus particles need to be inhaled to trigger an infection. Thus, the assessed filtration efficacy may differ from the provided protection rate against SARS-CoV-2.”
In addition, particles containing the virus could dry out while going through the mask and become less infectious. “Even a small reduction in inhaled particles might prevent infection or at least lead to a less severe infection,” they noted.
In fact, filtration efficacy does not necessarily indicate how well the mask filters out particles while being worn. “This might be due to the combined effects of mask fit and pressure drop of the mask material and therefore tendency for mask leakage,” the team wrote. “High pressure drop results in higher breathing resistance and therefore supports leakage, especially if combined to a loosely fitting mask.”
These findings are “in line with what we already knew,” Dr. Cappa explained. “Even if the mask material filters out nearly all particles that pass through it, as is the case for high-efficiency masks such as N95 and FFP2, if the mask does not fit well, then it will only provide moderate protection for the wearer.”
Although the findings reaffirm the different levels of filtration provided by various cloth masks, they do not “provide any guidance on which types of cloth masks are better or worse,” he said. But they do show that “medical face masks will generally provide more protection to the wearer.”
It’s not surprising that face shields offer little protection from aerosols, Dr. Cappa said, but they can provide added protection when worn with a mask.
“A face shield could prevent large droplets that might shoot out when a person coughs or sneezes from depositing on a person’s eye,” he pointed out. And it can help “redirect the plume of particles that an infected person exhales, which could be useful in close quarters. However, even then those particles will keep moving around and could be inhaled. A mask can really help to decrease the amount inhaled.”
The study did not use external funding. The authors and Dr. Cappa disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Respiratory infection– and asthma-prone children
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).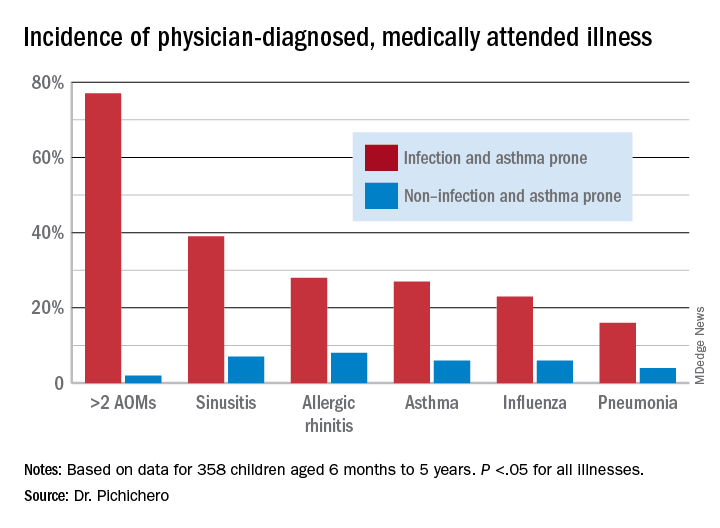
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.