User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
What to know about COVID-19 vaccines and skin reactions
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
FROM AAD VMX 2021
States ready plans to get Pfizer COVID vaccine to younger teens
after the Food and Drug Administration authorized its use in this age group May 10.
Some states hope to start the vaccinations as early as May 13, officials said at an Association of State and Territorial Health Officials news conference.
There are, however, two more steps before shots can reach younger arms. On May 12, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is expected to recommend use of the vaccine in this age group. Then CDC Director Rochelle Walensky, MD, must make a final decision to begin vaccinating 12- to 15-year-olds.
Some hoping to start this week
Both the CDC panel and Dr. Walensky are expected to sign off on the vaccine’s use. States have been making plans on how to tailor the vaccination message not just to the patient this time, but to parents and guardians as well, some of whom are hesitant to consent.
Some schools, assuming approval May 12, are ready to start vaccinating in cafeterias and gyms.
Anne Zink, MD, president-elect of the Association of State and Territorial Health Officials and Alaska chief medical officer, told reporters that many of her state’s boroughs and districts have booked in-person vaccines for their schools May 12 as the state has dismissal for summer as early as this week.
Maine is readying four types of distribution sites for the vaccines: primary care offices, Walgreen’s and CVS pharmacies, mass vaccination sites, and schools, said Nirav Shah, MD, current ASTHO president and director of the Maine Center for Disease Control and Prevention.
Starting later this week, he said, the state hopes to host large vaccination clinics for people age 12 and over.
Eliminating barriers
States are working to break down barriers through education and improving access.
In Alaska, many of the drive-through evening vaccination sites are being changed to Pfizer sites so parents just getting off work can take their kids.
It’s also important to get young people to speak to their peers about the importance of vaccines, she said. Some teen groups in Alaska are hosting Zoom calls where they share with children and families why they chose to get vaccinated.
In Maine, Dr. Shah said, “the notion of informed consent applies with equal force to adults as it does with adolescents.” But at least in Maine, it is not required that a parent be on site and present during the vaccination itself.
A parent could sign a form allowing the child to be vaccinated in a school-based clinic. Maine also allows verbal consent so a parent can give consent over the phone, Dr. Shah said.
Dividing vaccine trays
Vaccines going to pediatrician and family medicine offices presents a challenge in that smaller numbers of doses are needed for those venues than at large vaccination sites that get trays of 1,170 Pfizer doses each.
Dr. Shah says states have been talking with federal authorities on the need for smaller packaging.
“Breaking the trays up into smaller lot sizes takes a fair amount of effort,” Dr. Shah said. “We understand that later this month the lot size will be going down to 450.”
But even that will be too much for small offices, he said.
Similarly, an effort is being made in Maine to make sure doctors’ offices are not limited by their refrigeration capabilities. The Pfizer vaccine must be kept at ultra-cold temperatures that many primary care doctors’ offices may not have.
“If they need a cool cube with dry ice, we can furnish that to them,” Dr. Shah said.
Should they be mandated?
Dr. Zink said Alaska generally has high acceptance for recommendations around COVID-19 and has no plans to mandate the COVID-19 vaccines for children.
Umair A. Shah, MD, secretary of health at the Washington State Department of Health, said, “Our number one ability to get people vaccinated is for them to be encouraged to do so, to be incentivized to do so, to do everything we can to make the vaccine choice the easy choice,” including eliminating language, cultural and access barriers.
However, he said, “in higher education, University of Washington and Washington State University have indicated they are going to require COVID vaccines for kids to come back to school. I do think that is something that is increasingly being looked at.”
Though the messages will be tailored differently across the states the bottom line will be the same, Dr. Shah said: The vaccines work and they are safe.
But most critically, “Vaccines are our pathway to moving forward and once and for all ending this pandemic,” he said.
A version of this article first appeared on Medscape.com.
after the Food and Drug Administration authorized its use in this age group May 10.
Some states hope to start the vaccinations as early as May 13, officials said at an Association of State and Territorial Health Officials news conference.
There are, however, two more steps before shots can reach younger arms. On May 12, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is expected to recommend use of the vaccine in this age group. Then CDC Director Rochelle Walensky, MD, must make a final decision to begin vaccinating 12- to 15-year-olds.
Some hoping to start this week
Both the CDC panel and Dr. Walensky are expected to sign off on the vaccine’s use. States have been making plans on how to tailor the vaccination message not just to the patient this time, but to parents and guardians as well, some of whom are hesitant to consent.
Some schools, assuming approval May 12, are ready to start vaccinating in cafeterias and gyms.
Anne Zink, MD, president-elect of the Association of State and Territorial Health Officials and Alaska chief medical officer, told reporters that many of her state’s boroughs and districts have booked in-person vaccines for their schools May 12 as the state has dismissal for summer as early as this week.
Maine is readying four types of distribution sites for the vaccines: primary care offices, Walgreen’s and CVS pharmacies, mass vaccination sites, and schools, said Nirav Shah, MD, current ASTHO president and director of the Maine Center for Disease Control and Prevention.
Starting later this week, he said, the state hopes to host large vaccination clinics for people age 12 and over.
Eliminating barriers
States are working to break down barriers through education and improving access.
In Alaska, many of the drive-through evening vaccination sites are being changed to Pfizer sites so parents just getting off work can take their kids.
It’s also important to get young people to speak to their peers about the importance of vaccines, she said. Some teen groups in Alaska are hosting Zoom calls where they share with children and families why they chose to get vaccinated.
In Maine, Dr. Shah said, “the notion of informed consent applies with equal force to adults as it does with adolescents.” But at least in Maine, it is not required that a parent be on site and present during the vaccination itself.
A parent could sign a form allowing the child to be vaccinated in a school-based clinic. Maine also allows verbal consent so a parent can give consent over the phone, Dr. Shah said.
Dividing vaccine trays
Vaccines going to pediatrician and family medicine offices presents a challenge in that smaller numbers of doses are needed for those venues than at large vaccination sites that get trays of 1,170 Pfizer doses each.
Dr. Shah says states have been talking with federal authorities on the need for smaller packaging.
“Breaking the trays up into smaller lot sizes takes a fair amount of effort,” Dr. Shah said. “We understand that later this month the lot size will be going down to 450.”
But even that will be too much for small offices, he said.
Similarly, an effort is being made in Maine to make sure doctors’ offices are not limited by their refrigeration capabilities. The Pfizer vaccine must be kept at ultra-cold temperatures that many primary care doctors’ offices may not have.
“If they need a cool cube with dry ice, we can furnish that to them,” Dr. Shah said.
Should they be mandated?
Dr. Zink said Alaska generally has high acceptance for recommendations around COVID-19 and has no plans to mandate the COVID-19 vaccines for children.
Umair A. Shah, MD, secretary of health at the Washington State Department of Health, said, “Our number one ability to get people vaccinated is for them to be encouraged to do so, to be incentivized to do so, to do everything we can to make the vaccine choice the easy choice,” including eliminating language, cultural and access barriers.
However, he said, “in higher education, University of Washington and Washington State University have indicated they are going to require COVID vaccines for kids to come back to school. I do think that is something that is increasingly being looked at.”
Though the messages will be tailored differently across the states the bottom line will be the same, Dr. Shah said: The vaccines work and they are safe.
But most critically, “Vaccines are our pathway to moving forward and once and for all ending this pandemic,” he said.
A version of this article first appeared on Medscape.com.
after the Food and Drug Administration authorized its use in this age group May 10.
Some states hope to start the vaccinations as early as May 13, officials said at an Association of State and Territorial Health Officials news conference.
There are, however, two more steps before shots can reach younger arms. On May 12, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is expected to recommend use of the vaccine in this age group. Then CDC Director Rochelle Walensky, MD, must make a final decision to begin vaccinating 12- to 15-year-olds.
Some hoping to start this week
Both the CDC panel and Dr. Walensky are expected to sign off on the vaccine’s use. States have been making plans on how to tailor the vaccination message not just to the patient this time, but to parents and guardians as well, some of whom are hesitant to consent.
Some schools, assuming approval May 12, are ready to start vaccinating in cafeterias and gyms.
Anne Zink, MD, president-elect of the Association of State and Territorial Health Officials and Alaska chief medical officer, told reporters that many of her state’s boroughs and districts have booked in-person vaccines for their schools May 12 as the state has dismissal for summer as early as this week.
Maine is readying four types of distribution sites for the vaccines: primary care offices, Walgreen’s and CVS pharmacies, mass vaccination sites, and schools, said Nirav Shah, MD, current ASTHO president and director of the Maine Center for Disease Control and Prevention.
Starting later this week, he said, the state hopes to host large vaccination clinics for people age 12 and over.
Eliminating barriers
States are working to break down barriers through education and improving access.
In Alaska, many of the drive-through evening vaccination sites are being changed to Pfizer sites so parents just getting off work can take their kids.
It’s also important to get young people to speak to their peers about the importance of vaccines, she said. Some teen groups in Alaska are hosting Zoom calls where they share with children and families why they chose to get vaccinated.
In Maine, Dr. Shah said, “the notion of informed consent applies with equal force to adults as it does with adolescents.” But at least in Maine, it is not required that a parent be on site and present during the vaccination itself.
A parent could sign a form allowing the child to be vaccinated in a school-based clinic. Maine also allows verbal consent so a parent can give consent over the phone, Dr. Shah said.
Dividing vaccine trays
Vaccines going to pediatrician and family medicine offices presents a challenge in that smaller numbers of doses are needed for those venues than at large vaccination sites that get trays of 1,170 Pfizer doses each.
Dr. Shah says states have been talking with federal authorities on the need for smaller packaging.
“Breaking the trays up into smaller lot sizes takes a fair amount of effort,” Dr. Shah said. “We understand that later this month the lot size will be going down to 450.”
But even that will be too much for small offices, he said.
Similarly, an effort is being made in Maine to make sure doctors’ offices are not limited by their refrigeration capabilities. The Pfizer vaccine must be kept at ultra-cold temperatures that many primary care doctors’ offices may not have.
“If they need a cool cube with dry ice, we can furnish that to them,” Dr. Shah said.
Should they be mandated?
Dr. Zink said Alaska generally has high acceptance for recommendations around COVID-19 and has no plans to mandate the COVID-19 vaccines for children.
Umair A. Shah, MD, secretary of health at the Washington State Department of Health, said, “Our number one ability to get people vaccinated is for them to be encouraged to do so, to be incentivized to do so, to do everything we can to make the vaccine choice the easy choice,” including eliminating language, cultural and access barriers.
However, he said, “in higher education, University of Washington and Washington State University have indicated they are going to require COVID vaccines for kids to come back to school. I do think that is something that is increasingly being looked at.”
Though the messages will be tailored differently across the states the bottom line will be the same, Dr. Shah said: The vaccines work and they are safe.
But most critically, “Vaccines are our pathway to moving forward and once and for all ending this pandemic,” he said.
A version of this article first appeared on Medscape.com.
Keep antibiotics unchanged in breakthrough UTIs
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
Changing the continuous antibiotic prophylactic agent had no significant effect on the risk of a second infection in children with breakthrough urinary tract infections (UTIs), based on data from 62 children treated at a single center.
Continuous antibiotic prophylaxis (CAP) is often used for UTI prevention in children with febrile UTIs or anomalies that predispose them to UTIs, such as vesicoureteral reflux (VUR) or bladder and bowel dysfunction, said Lane M. Shish, MPH, of the University of Washington, Bothell, and colleagues in a poster (#1245) presented at the Pediatric Academic Societies annual meeting.
CAP, once initiated, is used until a planned endpoint or a breakthrough UTI, at which point alternative treatments usually include surgical intervention or a CAP agent change, the researchers said. However, changing the CAP agent is based on consensus without evidence of benefit, they noted.
To evaluate the potential effect of switching or maintaining CAP in cases of breakthrough UTIs, the researchers conducted a retrospective cohort study of all patients younger than 18 years on CAP for UTI prevention enrolled in a pediatric urology registry between January 2013 and August 2020.
All patients experienced a breakthrough UTI while on CAP; CAP was changed for 24 patients and left unchanged for 38 patients.
The primary outcome of second-breakthrough infections occurred in 12 of the changed CAP group and 22 of the unchanged group, with a relative risk of 0.86. The percentage of second breakthrough UTIs resistant to the current CAP was not significantly different between the changed and unchanged CAP groups (75% vs. 77%; P = 0.88).
The researchers also identified a rate ratio of 0.67 for a second breakthrough UTI in the changed CAP group, and found that approximately one-third of these patients (33.3%) developed antibiotic resistance to their initial antibiotic agent and the changed antibiotic agent.
The study findings were limited by several factors, including the retrospective design and small sample size, the researchers noted.
However, the results suggest that changing the CAP after an initial breakthrough UTI in children did not increase the risk of a second breakthrough UTI, and that CAP changing did introduce a risk of developing a second UTI with increased CAP resistance, the researchers noted. The results support leaving a child’s CAP unchanged after an initial breakthrough UTI, although additional research is needed to verify the findings, including studies involving a larger cohort with a multi-institutional prospective evaluation, they concluded.
Manage UTIs to reduce recurrence and resistance
“As we know, avoiding recurrent UTIs is important in preserving renal function in pediatric patients,” said Tim Joos, MD, a Seattle-based clinician with a combination internal medicine/pediatrics practice, in an interview.
“Avoiding recurrent UTIs is also important to avoid the development and spread of multidrug-resistant organisms,” he said.
Dr. Joos said he was surprised by some of the study findings. “I was surprised that, over the course of this 7-year retrospective review, overall only approximately 50% of patients with a first breakthrough UTI on CAP developed a second breakthrough UTI,” he noted. “Also, the relative risk of a second UTI was not significantly affected by whether the CAP antibiotic was changed after the first infection,” he said. “It would be interesting to see whether these results hold up in a randomized, prospective study,” he added.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Joos had no financial conflicts to disclose, but serves as a member of the Pediatric News Editorial Advisory Board.
FROM PAS 2021
Small increase seen in new COVID-19 cases among children
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
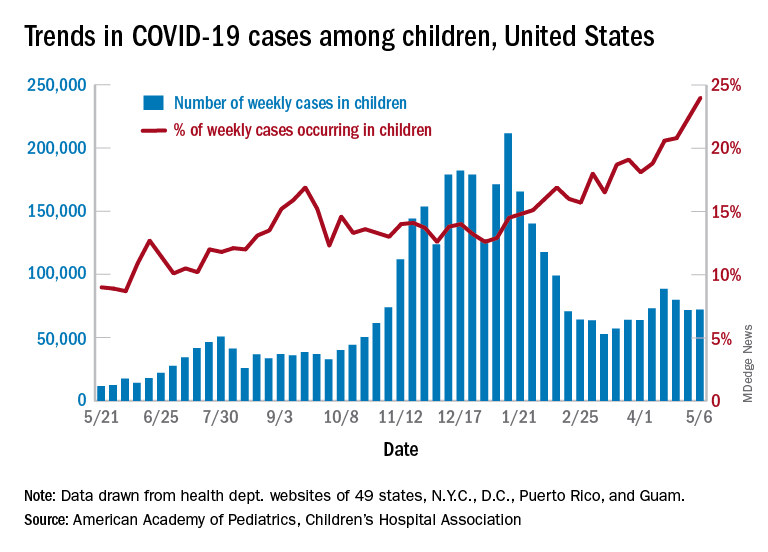
higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
Dr. Fauci: Feds may ease indoor mask mandates soon
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
FDA authorizes Pfizer COVID vaccine for teens 12-15
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
NSAIDs don’t make COVID-19 worse in hospitalized patients
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
FROM THE LANCET RHEUMATOLOGY
Quinolones and tendon health: Third-generation drugs may be safer
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
the findings of a new study suggest.
If confirmed, this will be good news for patients who are allergic to beta-lactam antibiotics and others in whom fluoroquinolones are the antibiotics of choice because of their favorable pharmacokinetic properties and broad-spectrum activity, according to Dr. Takashi Chinen of Jichi Medical University in Tochigi, Japan, lead investigator of the new study, published in Annals of Family Medicine.
“This is especially notable for patients who are at increased risk for tendon disorders, such as athletes,” Dr. Chinen said in an interview.
To investigate the association between third-generation fluoroquinolones and tendinopathy, Dr. Chinen and colleagues conducted a self-controlled case series analysis using administrative claims data for a single prefecture in Japan, focusing specifically on the risk of Achilles tendon rupture.
From a database of 780,000 residents in the Kumamoto Prefecture enrolled in the country’s National Health Insurance and Elderly Health Insurance from April 2012 to March 2017, the investigators identified 504 patients who experienced Achilles tendon rupture during the 5-year period and were prescribed an antibiotic at some time during that period. They divided the observation period into antibiotic exposure (30 days from prescription) and nonexposure periods based on previous research linking this fluoroquinolone exposure window to an elevated risk of tendon injury. They classified antibiotics into fluoroquinolones and nonfluoroquinolones and further classified the fluoroquinolones by first, second, and third generation, including the following agents:
- First generation: Norfloxacin, nalidixic acid, pipemidic acid
- Second generation: Levofloxacin, tosufloxacin, ciprofloxacin, ofloxacin, lomefloxacin
- Third generation: Garenoxacin, sitafloxacin, prulifloxacin, moxifloxacin, pazufloxacin.
Tendon rupture risk varied based on fluoroquinolone class
Comparing the incidence of Achilles tendon rupture in the exposure period relative to the nonexposure period, the risk of rupture was not elevated during exposure to third-generation fluoroquinolones (incidence rate ratio, 1.05; 95% confidence interval, 0.33-3.37) and nonfluoroquinolones (IRR, 1.08; 95% CI, 0.80- 1.47). Contrasting with those findings, the researchers found that the risk of tendon rupture was significantly elevated during exposure to first- and second-generation fluoroquinolones (IRR, 2.94; 95% CI, 1.90-4.54). Similar findings were observed in subgroup analyses by gender and recent corticosteroid use, the authors wrote.
The increased risk associated with exposure to first- and second-generation fluoroquinolones is consistent with the elevated risk observed in previous studies, the majority of which focused on first- and second-generation agents, the authors noted.
“Our study is the first to investigate the risk of Achilles tendon rupture associated with third-generation fluoroquinolones by self-controlled case series analysis and using a large administrative claims database,” they said.
Because the study is based on administrative claims data, it does not support conclusions about differential risks.
“Some preclinical studies suggest that structural differences [in the drugs] may affect the risks,” Dr. Chinen said. In particular, one preclinical study linked methylpiperazinyl substituent with increased risk of tendon injury, and this substituent is more common in first- and second-generation fluoroquinolones.
Outside experts were unable to draw conclusions
The accuracy of the current study is “extremely limited” by its design, according to Dr. Karsten Knobloch, a sports medicine physician in private practice in Hanover, Germany, who has reported on the risk of drug-induced tendon disorders.
“This is a case series only, which is a very strict limitation; therefore, the ability to generalize the data is also very limited,” he said in an interview. “In my view, the study does not add substantial data to support that third-generation [fluoroquinolones] are safer than the prior ones.”
Thomas Lodise, PharmD, PhD, who is a professor at the Albany College of Pharmacy and Health Sciences in New York, pointed out another barrier to determining the value of the new research .
“Without knowing how many received moxifloxacin and descriptors of patients at baseline by each drug, it is hard to draw any definitive results from the paper,” Dr. Lodise noted.
Study design and execution had limitations
The authors acknowledged the limitations in the study design and execution. In particular, reliance on an administrative claims database means that the accuracy of diagnoses cannot be validated. Further, the study sample size may not have been sufficient to estimate the rupture risk for individual fluoroquinolones, they wrote.
Despite these and additional limitations, the findings have merit, according to the authors, who noted that the information may be useful in personalizing antibiotic therapy for individual patients.
“Fluoroquinolone-induced tendon injury is a rare event, and managing risk for even rare adverse events depends on each case,” Dr. Chinen explained. The findings of this study together with previous studies indicate that third-generation fluoroquinolones may be a safer option with respect to risk of Achilles tendon rupture for some patients who can’t be prescribed beta-lactam antibiotics and for some conditions, such as Legionella pneumophila, he said.
To increase internal and external validity of the results, further research including prospective cohort studies in broader populations are necessary, Dr. Chinen stressed.
The authors, Dr. Lodise, and Dr. Knobloch, who is owner of SportPraxis in Hanover, Germany, reported no conflicts.
FROM ANNALS OF FAMILY MEDICINE
‘Malicious peer review’ destroyed doc’s career, he says
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Cardiothoracic surgeon J. Marvin Smith III, MD, had always thrived on a busy practice schedule, often performing 20-30 surgeries a week. A practicing surgeon for more than 40 years, Dr. Smith said he had no plans to slow down anytime soon.
But Dr. Smith said his career was derailed when leaders at Methodist Healthcare System of San Antonio initiated a sudden peer review proceeding against him. The hospital system alleged certain surgeries performed by Dr. Smith had excessive mortality rates. When he proved the data inaccurate, Dr. Smith said administrators next claimed he was cognitively impaired and wasn’t safe to practice.
Dr. Smith has now been embroiled in a peer review dispute with the hospital system for more than 2 years and says the conflict has essentially forced him out of surgical practice. He believes the peer review was “malicious” and was really launched because of complaints he made about nurse staffing and other issues at the hospital.
“I think it is absolutely in bad faith and is disingenuous what they’ve told me along the way,” said Dr. Smith, 73. “It’s because I pointed out deficiencies in nursing care, and they want to get rid of me. It would be a lot easier for them if I had a contract and they could control me better. But the fact that I was independent, meant they had to resort to a malicious peer review to try and push me out.”
Dr. Smith had a peer review hearing with Methodist in March 2021, and in April, a panel found in Dr. Smith’s favor, according to Dr. Smith. The findings were sent to the hospital’s medical board for review, which issued a decision in early May.
Eric A. Pullen, an attorney for Dr. Smith, said he could not go into detail about the board’s decision for legal reasons, but that “the medical board’s decision did not completely resolve the matter, and Dr. Smith intends to exercise his procedural rights, which could include an appeal.”
Methodist Hospital Texsan and its parent company, Methodist Health System of San Antonio, did not respond to messages seeking comment about the case. Without hearing from the hospital system, its side is unknown and it is unclear if there is more to the story from Methodist’s view.
The problem is not new, but some experts, such as Lawrence Huntoon, MD, PhD, say the practice has become more common in recent years, particularly against independent doctors.
Dr. Huntoon believes there is a nationwide trend at many hospitals to get rid of independent physicians and replace them with employed doctors, he said.
However, because most sham peer reviews go on behind closed doors, there are no data to pinpoint its prevalence or measure its growth.
“Independent physicians are basically being purged from medical staffs across the United States,” said Dr. Huntoon, who is chair of the Association of American Physicians and Surgeons’ Committee to Combat Sham Peer Review. “The hospitals want more control over how physicians practice and who they refer to, and they do that by having employees.”
Anthony P. Weiss, MD, MBA, chief medical officer for Beth Israel Deaconess Medical Center said it has not been his experience that independent physicians are being targeted in such a way. Dr. Weiss responded to an inquiry sent to the American Hospital Association for this story.
“As the authority for peer review rests with the organized medical staff (i.e., physicians), and not formally with the hospital per se, the peer review lever is not typically available as a management tool for hospital administration,” said Dr. Weiss, who is a former member of the AHA’s Committee on Clinical Leadership, but who was speaking on behalf of himself.
A spokesman for the AHA said the organization stands behinds Dr. Weiss’ comments.
Peer review remains a foundational aspect of overseeing the safety and appropriateness of healthcare provided by physicians, Dr. Weiss said. Peer review likely varies from hospital to hospital, he added, although the Healthcare Quality Improvement Act provides some level of guidance as does the American Medical Association Code of Medical Ethics (section 9.4.1).
“In essence, both require that the evaluation be conducted in good faith with the intention to improve care, by physicians with adequate training and knowledge, using a process that is fair and inclusive of the physician under review,” he said. “I believe that most medical staffs abide by these ethical principles, but we have little data to confirm this supposition.”
Did hospital target doc for being vocal?
When members of Methodist’s medical staff first approached Dr. Smith with concerns about his surgery outcomes in November 2018, the physician says he was surprised, but that he was open to an assessment.
“They came to me and said they thought my numbers were bad, and I said: ‘Well my gosh, I certainly don’t want that to be the case. I need to see what numbers you are talking about,’ ” Dr. Smith recalled. “I’ve been president of the Bexar County Medical Society; I’ve been involved with standards and ethics for the Society of Thoracic Surgeons. Quality health care means a whole lot to me.”
The statistical information provided by hospital administrators indicated that Dr. Smith’s mortality rates for coronary artery surgery in 2018 were “excessive” and that his rates for aortic surgery were “unacceptable,” according to a lawsuit Dr. Smith filed against the hospital system. Dr. Smith, who is double boarded with the American Board of Surgery and the American Board of Thoracic Surgery, said his outcomes had never come into question in the past. Dr. Smith said the timing was suspicious to him, however, considering he had recently raised concerns with the hospital through letters about nursing performance, staffing, and compensation.
A peer review investigation was initiated. In the meantime, Dr. Smith agreed to intensivist consults on his postoperative patients and consults with the hospital’s “Heart Team” on all preoperative cardiac, valve, and aortic cases. A vocal critic of the Heart Team, Dr. Smith had long contended the entity provided no meaningful benefit to his patients in most cases and, rather, increased hospital stays and raised medical expenses. Despite his agreement, Dr. Smith was later asked to voluntarily stop performing surgeries at the hospital.
“I agreed, convinced that we’d get this all settled,” he said.
Another report issued by the hospital in 2019 also indicated elevated mortality rates associated with some of Smith’s surgeries, although the document differed from the first report, according to the lawsuit. Dr. Smith says he was ignored when he pointed out problems with the data, including a lack of appropriate risk stratification in the report, departure from Society of Thoracic Surgeons data rules, and improper inclusion of his cases in the denominator of the ratio when a comparison was made of his outcomes with those hospitalwide. A subsequent report from Methodist in March 2019 indicated Dr. Smith’s surgery outcomes were “within the expected parameters of performance,” according to court documents.
The surgery accusations were dropped, but the peer review proceeding against Dr. Smith wasn’t over. The hospital next requested that Dr. Smith undergo a competency evaluation.
“When they realized the data was bad, they then changed their argument in the peer review proceeding and essentially started to argue that Dr. Smith had some sort of cognitive disability that prevented him from continuing to practice,” said Mr. Pullen. “The way I look at it, when the initial basis for the peer review was proven false, the hospital found something else and some other reason to try to keep Dr. Smith from practicing.”
Thus began a lengthy disagreement about which entity would conduct the evaluation, who would pay, and the type of acceptable assessment. An evaluation by the hospital’s preferred organization resulted in a finding of mild cognitive impairment, Dr. Smith said. He hired his own experts who conducted separate evaluations, finding no impairment and no basis for the former evaluation’s conclusion.
“Literally, the determinant as to whether I was normal or below normal on their test was one point, which was associated with a finding that I didn’t draw a clock correctly,” Dr. Smith claimed. “The reviewer said my minute hand was a little too short and docked me a point. It was purely subjective. To me, the gold standard of whether you are learned in thoracic surgery is the American Board of Thoracic Surgery’s test. The board’s test shows my cognitive ability is entirely in keeping with my practice. That contrasts with the one point off I got for drawing a clock wrong in somebody’s estimation.”
Conflict leads to legal case
In September 2020, Dr. Smith filed a lawsuit against Methodist Healthcare System of San Antonio, alleging business disparagement by Methodist for allegedly publishing false and disparaging information about Dr. Smith and tortious interference with business relations. The latter claim stems from Methodist refusing to provide documents to other hospitals about the status of Dr. Smith’s privileges at Methodist, Mr. Pullen said.
Because Methodist refused to confirm his status, the renewal process for Baptist Health System could not be completed and Dr. Smith lost his privileges at Baptist Health System facilities, according to the lawsuit.
Notably, Dr. Smith’s legal challenge also asks the court to take a stance against alleged amendments by Methodist to its Unified Medical Staff Bylaws. The hospital allegedly proposed changes that would prevent physicians from seeking legal action against the hospital for malicious peer review, according to Dr. Smith’s lawsuit.
The amendments would make the peer review process itself the “sole and exclusive remedy with respect to any action or recommendation taken at the hospital affecting medical staff appointment and/or clinical privileges,” according to an excerpt of the proposed amendments included in Dr. Smith’s lawsuit. In addition, the changes would hold practitioners liable for lost revenues if the doctor initiates “any type of legal action challenging credentialing, privileging, or other medical peer review or professional review activity,” according to the lawsuit.
Dr. Smith’s lawsuit seeks a declaration that the proposed amendments to the bylaws are “void as against public policy,” and a declaration that the proposed amendments to the bylaws cannot take away physicians’ statutory right to bring litigation against Methodist for malicious peer review.
“The proposed amendments have a tendency to and will injure the public good,” Dr. Smith argued in the lawsuit. “The proposed amendments allow Methodist to act with malice and in bad faith in conducting peer review proceedings and face no legal repercussions.”
Regardless of the final outcome of the peer review proceeding, Mr. Pullen said the harm Dr. Smith has already endured cannot be reversed.
“Even if comes out in his favor, the damage is already done,” he said. “It will not remedy the damage Dr. Smith has incurred.”
Fighting sham peer review is difficult
Battling a malicious peer review has long been an uphill battle for physicians, according to Dr. Huntoon. That’s because the Health Care Quality Improvement Act (HCQIA), a federal law passed in 1986, provides near absolute immunity to hospitals and peer reviewers in legal disputes.
The HCQIA was created by Congress to extend immunity to good-faith peer review of doctors and to increase overall participation in peer review by removing fear of litigation. However, the act has also enabled abuse of peer review by shielding bad-faith reviewers from accountability, said Dr. Huntoon.
“The Health Care Quality Improvement Act presumes that what the hospital did was warranted and reasonable and shifts the burden to the physician to prove his innocence by a preponderance of evidence,” he said. “That’s an entirely foreign concept to most people who think a person should be considered innocent until proven guilty. Here, it’s the exact opposite.”
The HCQIA has been challenged numerous times over the years and tested at the appellate level, but continues to survive and remain settled law, added Richard B. Willner, DPM, founder and director of the Center for Peer Review Justice, which assists and counsels physicians about sham peer review.
In 2011, former Rep. Joe Heck, DO, (R-Nev.) introduced a bill that would have amended the HCQIA to prohibit a professional review entity from submitting a report to the National Practitioner Data Bank (NPDB) while the doctor was still under investigation and before the doctor was afforded adequate notice and a hearing. Although the measure had 16 cosponsors and plenty of support from the physician community, it failed.
In addition to a heavy legal burden, physicians who experience malicious peer reviews also face ramifications from being reported to the NPDB. Peer review organizations are required to report certain negative actions or findings to the NPDB.
“A databank entry is a scarlet letter on your forehead,” Dr. Willner said. “The rules at a lot of institutions are not to take anyone who has been databanked, rightfully or wrongfully. And what is the evidence necessary to databank you? None. There’s no evidence needed to databank somebody.”
Despite the bleak landscape, experts say progress has been made on a case-by-case basis by physicians who have succeeded in fighting back against questionable peer reviews in recent years.
In January 2020, Indiana ob.gyn. Rebecca Denman, MD, prevailed in her defamation lawsuit against St Vincent Carmel Hospital and St Vincent Carmel Medical Group, winning $4.75 million in damages. Dr. Denman alleged administrators failed to conduct a proper peer review investigation after a false allegation by a nurse that she was under the influence while on the job.
Indianapolis attorney Kathleen A. DeLaney, who represented Dr. Denman, said hospital leaders misled Dr. Denman into believing a peer review had occurred when no formal peer review hearing or proceeding took place.
“The CMO of the medical group claimed that he performed a peer review ‘screening,’ but he never informed the other members of the peer review executive committee of the matter until after he had placed Dr. Denman on administrative leave,” Ms. DeLaney said. “He also neglected to tell the peer review executive committee that the substance abuse policy had not been followed, or that Dr. Denman had not been tested for alcohol use – due to the 12-hour delay in report.”
Dr. Denman was ultimately required to undergo an alcohol abuse evaluation, enter a treatment program, and sign a 5-year monitoring contract with the Indiana State Medical Association as a condition of her employment, according to the lawsuit. She claimed repercussions from the false allegation resulted in lost compensation, out-of-pocket expenses, emotional distress, and damage to her professional reputation.
She sued the hospital in July 2018, alleging fraud, defamation, tortious interference with an employment relationship, and negligent misrepresentation. After a 4-day trial, jurors found in her favor, awarding Dr. Denman $2 million for her defamation claims, $2 million for her claims of fraud and constructive fraud, $500,000 for her claim of tortious interference with an employment relationship, and $250,000 for her claim of negligent misrepresentation.
A hospital spokesperson said Ascension St Vincent is pursuing an appeal, and that it looks “forward to the opportunity to bring this matter before the Indiana Court of Appeals in June.”
In another case, South Dakota surgeon Linda Miller, MD, was awarded $1.1 million in 2017 after a federal jury found Huron Regional Medical Center breached her contract and violated her due process rights. Dr. Miller became the subject of a peer review at Huron Regional Medical Center when the hospital began analyzing some of her surgery outcomes.
Ken Barker, an attorney for Dr. Miller, said he feels it became evident at trial that the campaign to force Dr. Miller to either resign or lose her privileges was led by the lay board of directors of the hospital and upper-level administration at the hospital.
“They began the process by ordering an unprecedented 90-day review of her medical charts, looking for errors in the medical care she provided patients,” he said. “They could find nothing, so they did a second 90-day review, waiting for a patient’s ‘bad outcome.’ As any general surgeon will say, a ‘bad outcome’ is inevitable. And so it was. Upon that occurrence, they had a medical review committee review the patient’s chart and use it as an excuse to force her to reduce her privileges. Unbeknown to Dr. Miller, an external review had been conducted on another patient’s chart, in which the external review found her care above the standards and, in some measure, ‘exemplary.’ ”
Dr. Miller was eventually pressured to resign, according to her claim. Because of reports made to the NPDB by the medical center, including a patient complication that was allegedly falsified by the hospital, Dr. Miller said she was unable to find work as a general surgeon and went to work as a wound care doctor. At trial, jurors awarded Dr. Miller $586,617 in lost wages, $343,640 for lost future earning capacity, and $250,000 for mental anguish. (The mental anguish award was subsequently struck by a district court.)
Attorneys for Huron Regional Medical Center argued the jury improperly awarded damages and requested a new trial, which was denied by an appeals court.
In the end, the evidence came to light and the jury’s verdict spoke loudly that the hospital had taken unfair advantage of Dr. Miller, Mr. Barker said. But he emphasized that such cases often end differently.
“There are a handful of cases in which physicians like Dr. Miller have challenged the system and won,” he said. “In most cases, however, it is a ‘David vs. Goliath’ scenario where the giant prevails.”
What to do if faced with malicious peer review
An important step when doctors encounter a peer review that they believe is malicious is to consult with an experienced attorney as early as possible, Dr. Huntoon said. “Not all attorneys who set themselves out to be health law attorneys necessarily have knowledge and expertise in sham peer review. And before such a thing happens, I always encourage physicians to read their medical staff bylaws. That’s where everything is set forth, [such as] the corrective action section that tells how peer review is to take place.”
Mr. Barker added that documentation is also key in the event of a potential malicious peer review.
“When a physician senses [the] administration has targeted them, they should start documenting their conversations and actions very carefully, and if possible, recruit another ‘observer’ who can provide a third-party perspective, if necessary,” Mr. Barker said.
Dr. Huntoon recently wrote an article with advice about preparedness and defense of sham peer reviews. The guidance includes that physicians educate themselves about the tactics used by some hospitals to conduct sham peer reviews and the factors that place doctors more at risk. Factors that may raise a doctor’s danger of being targeted include being in solo practice or a small group, being new on staff, or being an older physician approaching retirement as some bad-actor hospitals may view older physicians as being less likely to fight back, said Dr. Huntoon.
Doctors should also keep detailed records and a timeline in the event of a malicious peer review and insist that an independent court reporter record all peer review hearings, even if that means the physician has to pay for the reporter him or herself, according to the guidance. An independent record is invaluable should the physician ultimately issue a future legal challenge against the hospital.
Mr. Willner encourages physicians to call the Center for Peer Review Justice hotline at (504) 621-1670 or visit the website for help with peer review and NPDB issues.
As for Dr. Smith, his days are much quieter and slower today, compared with the active practice he was accustomed to for more than half his life. He misses the fast pace, the patients, and the work that always brought him great joy.
“I hope to get back to doing surgeries eventually,” he said. “I graduated medical school in 1972. Practicing surgery has been my whole life and my career. They have taken my identity and my livelihood away from me based on false numbers and false premises. I want it back.”
A version of this article first appeared on Medscape.com.
Dr. Topol talks: COVID-19 variants are innocent until proven guilty
Editor in Chief of this news organization Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and professor of molecular medicine, has been closely following COVID-19 data since the pandemic began. He spoke with writer Miriam E. Tucker about the latest on SARS-CoV-2 variants and their impact on vaccine efficacy. The conversation serves as a follow-up to his April 13, 2021, New York Times opinion piece, in which he advised readers that “all variants are innocent until proven guilty.”
You have expressed overall confidence in the efficacy of the vaccines thus far despite the emergence of variants, with some caveats. How do you see the current situation?
The Centers for Disease Control and Prevention has designated five “variants of concern,” but only three of them are real concerns – B.1.1.7, first detected in the United Kingdom; P.1, in Brazil and Japan; and B.1.351, in South Africa. Yet, all three are susceptible to our current vaccines.
The U.K. B.1.1.7 is the worst variant of all because it’s hypertransmissible, so I call it a “superspreader strain.” It also causes more severe illness independent of the spread, so it’s a double whammy. It’s clear that it also causes more deaths. The only arguable point is whether it’s 30% or 50% more deaths, but regardless, it’s more lethal and more transmissible.
The B.1.1.7 is going to be the dominant strain worldwide. It could develop new mutations within it that could come back to haunt us. We must keep watch.
But for now, it’s fully responsive to all the vaccines, which is great because if we didn’t have them, we wouldn’t have gotten through this U.S. pandemic like we have, and neither would Israel and the United Kingdom and other countries that have been able to get out of the crisis. We met the enemy and put it in check.
As for the South Africa variant of concern, B.1.351, we just got some encouraging news showing that it›s very responsive to the Pfizer/BioNTech mRNA vaccine in large numbers of people. The study was conducted in Qatar following that country’s mass immunization campaign in which a total of 385,853 people had received at least one vaccine dose and 265,410 had completed the two doses as of March 31, 2021.
At 2 weeks past the second dose, the vaccine was 75% effective at preventing any documented infection with the B.1.351 variant and 89.5% effective against B.1.1.7. The vaccine’s effectiveness against severe, critical, or fatal COVID-19 was greater than 97.4% for all circulating strains in Qatar, where B.1.1.7 and B.1.351 are most prominent.
We also know that B.1.351 is very responsive to the Johnson & Johnson vaccine and the Novavax [vaccine in development] to a lesser degree. It is the most immune-evading variant we’ve seen thus far, with the highest likelihood of providing some vaccine resistance, yet not enough to interfere with vaccination campaigns. So that’s great news.
The caveats here are that you definitely need two doses of the mRNA vaccines to combat the B.1.351 variant. Also, the AstraZeneca vaccine failed to prevent it in South Africa. However, that study was hard to judge because it was underpowered for number of people with mild infections. So, it didn’t look as if it had any efficacy, but maybe it would if tested in a real trial.
The P.1 (Brazil) variant is the second-highest concern after B.1.1.7 because it’s the only one in the United States that’s still headed up. It seems to be competing a bit with B.1.1.7 here. We know it was associated with the crisis in Brazil, in Chile, and some other South American countries. It has some immune escape, but not as bad as B.1.351. It also appears to have somewhat greater transmissibility but not as much as B.1.1.7.
With P.1, we just don’t know enough yet. It was difficult to assess in Brazil because they were in the midst of a catastrophe – like India is now – and you don’t know how much of it is dragged by the catastrophe vs driving it.
We have to respond to P.1 carefully. There are some good data that it does respond to the Chinese vaccine Sinovac and the AstraZeneca vaccine, and it appears to respond to the others as well, based on serum studies. So it doesn’t look like vaccines will be the worry with this variant. Rather, it could be competing with B.1.1.7 and could lead to breakthrough infections in vaccinated people or reinfections in unvaccinated people who had COVID-19. We need several more weeks to sort it out.
Although the B.1.427 and B.1.429 variants initially seen in California remain on the CDC’s concern list, I’m not worried about them.
You mentioned the current COVID-19 crisis in India, where a new variant has been described as a “double mutant,” but on Twitter you called it a “scariant.” Why?
First of all, the B.1.617 variant isn’t a double mutant. It has 15 mutations. It’s a stupid term, focusing on two mutations which largely have been put aside as to concern. One of them is the L452R, which is the same as one of the California variants, and that hasn’t proved to be particularly serious or concerning. The other is the 484Q, and it’s not clear whether that has any function.
The B.1.617 is not the driver of the catastrophe in India. It may be contributing a small amount, but it has been overhyped as the double mutant that’s causing it all. Adding to that are what I call “scariant” headlines here in the United States when a few cases of that variant have been seen.
I coined the term scariant in early February because it was a pretty clear trend. People don’t know what variants are. They know a little bit about mutations but not variants, and they’re scared. A few variants are concerning, but we keep learning more and more things to decrease the concern. That’s why I wrote the New York Times op-ed, to try to provide some reassurance, since there’s such paranoia.
Do you think booster vaccinations will be necessary? If so, will those be of the original vaccines or new ones that incorporate the variants?
As we go forward, there’s still potential for new variants that we haven’t seen yet that combine the worst of all features – transmissibility and immune evasion – especially since we have a world where COVID-19 is unchecked. So, we’re not out of it yet, but at least for the moment, we have vaccines that are capable of protecting against all variants.
In most people, the immune response against SARS-CoV-2 is very durable and strong and may well last for years. With the most closely related SARS-CoV-1, people still had immune responses up to 18 years later. However, some people will have less robust vaccine responses, including the elderly and the immunocompromised. If they don’t have great responses to the vaccine to start with, over time they’re likely to become more vulnerable, especially if they’re exposed to the variants with some degree of immune evasion.
I think we need to study these individuals post vaccination. A lot of people fit into those categories, including seniors, people being treated for cancer or autoimmune conditions, or post organ transplant. We could set up a prospective study to see whether they develop symptomatic COVID-19 and if so, from what – the original strain, B.1.1.7, or the newer variants.
That’s where I think booster shots may be needed. They may not be necessary across the board, but perhaps just in these special subgroups.
All of the current vaccines can be tweaked to include new variants, but the need for that is uncertain as of now. Moderna is working on a so-called bivalent vaccine that includes the original SARS-CoV-2 strain plus the B.1.351 variant, but it isn’t clear that that’s going to be necessary.
Currently, at least 200 COVID-19 vaccines are in development. There will be vaccines you can inhale, room temperature mRNA vaccines, and potentially even oral vaccines.
In the near future, Novavax is close, and there will likely be a two-dose Johnson & Johnson version that has the same potency as the mRNA vaccines. There are a lot of moving parts here.
There may be a step down in efficacy from mRNA to the others, though, and that shouldn’t be discounted. All of the available vaccines so far protect very well against severe disease and death, but some are less effective against mild to moderate infections, which may then lead to long COVID. We don’t yet know whether those who get mild infection post vaccination can still get long COVID.
What do you think it will take to achieve herd immunity?
I prefer the term “containment.” It’s quantitative. If you get to an infection rate of less than 1 in 100,000 people, as they’ve done in Israel, with 0.8 per 100,000, then you have the virus in check, and there will be very little spread when it’s at that controlled rate, with no outbreaks. The United States is currently at about 15 per 100,000. California is at 4. That still has to get lower.
It will be a challenge to get to President Biden’s goal of having 70% of U.S. adults given at least one dose by July 4. We’re now at about 57%. To get that next 13% of adults is going to take an all-out effort: mobile units, going to homes, making it ultraconvenient, education for people with safety concerns, incentivization, and days off.
We also need to get employers, universities, and health systems to get to the mandatory level. We haven’t done that yet. Some universities have mandated it for students, faculty, and staff. We need it in more health care systems. Right now, we only have a couple. We mandate flu shots, and flu is nothing, compared with COVID-19. And the COVID-19 vaccine is far more efficacious – flu shots are 40% efficacious, while these are 95%. COVID-19 is a tenfold more lethal and serious disease, and much more spreadable.
People are using the lack of full licensure by the Food and Drug Administration – as opposed to emergency use authorization – as an excuse not to get vaccinated. A biologics license application takes time to approve. Meanwhile, we have hundreds of millions of doses that have been well tolerated and incredibly effective.
Another aspect to consider regarding containment is that about 110 million Americans have already had COVID-19, even though only about 30 million cases have been confirmed. Most of these people have immune protection, although it’s not as good as if they have one vaccine dose. But they have enough protection to be part of the story here of the wall against COVID-19 and will help us get through this.
That’s a silver lining of having an unchecked epidemic for the entire year of 2020. The good part is that’s helping to get us to achieve an incredible level of containment when we haven’t even been close. Right now, we’re as good as the country has been in the pandemic, but we still have a long gap to get down to that 1 per 100,000. That’s what we should be working toward, and we can get there.
A version of this article first appeared on Medscape.com.
Editor in Chief of this news organization Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and professor of molecular medicine, has been closely following COVID-19 data since the pandemic began. He spoke with writer Miriam E. Tucker about the latest on SARS-CoV-2 variants and their impact on vaccine efficacy. The conversation serves as a follow-up to his April 13, 2021, New York Times opinion piece, in which he advised readers that “all variants are innocent until proven guilty.”
You have expressed overall confidence in the efficacy of the vaccines thus far despite the emergence of variants, with some caveats. How do you see the current situation?
The Centers for Disease Control and Prevention has designated five “variants of concern,” but only three of them are real concerns – B.1.1.7, first detected in the United Kingdom; P.1, in Brazil and Japan; and B.1.351, in South Africa. Yet, all three are susceptible to our current vaccines.
The U.K. B.1.1.7 is the worst variant of all because it’s hypertransmissible, so I call it a “superspreader strain.” It also causes more severe illness independent of the spread, so it’s a double whammy. It’s clear that it also causes more deaths. The only arguable point is whether it’s 30% or 50% more deaths, but regardless, it’s more lethal and more transmissible.
The B.1.1.7 is going to be the dominant strain worldwide. It could develop new mutations within it that could come back to haunt us. We must keep watch.
But for now, it’s fully responsive to all the vaccines, which is great because if we didn’t have them, we wouldn’t have gotten through this U.S. pandemic like we have, and neither would Israel and the United Kingdom and other countries that have been able to get out of the crisis. We met the enemy and put it in check.
As for the South Africa variant of concern, B.1.351, we just got some encouraging news showing that it›s very responsive to the Pfizer/BioNTech mRNA vaccine in large numbers of people. The study was conducted in Qatar following that country’s mass immunization campaign in which a total of 385,853 people had received at least one vaccine dose and 265,410 had completed the two doses as of March 31, 2021.
At 2 weeks past the second dose, the vaccine was 75% effective at preventing any documented infection with the B.1.351 variant and 89.5% effective against B.1.1.7. The vaccine’s effectiveness against severe, critical, or fatal COVID-19 was greater than 97.4% for all circulating strains in Qatar, where B.1.1.7 and B.1.351 are most prominent.
We also know that B.1.351 is very responsive to the Johnson & Johnson vaccine and the Novavax [vaccine in development] to a lesser degree. It is the most immune-evading variant we’ve seen thus far, with the highest likelihood of providing some vaccine resistance, yet not enough to interfere with vaccination campaigns. So that’s great news.
The caveats here are that you definitely need two doses of the mRNA vaccines to combat the B.1.351 variant. Also, the AstraZeneca vaccine failed to prevent it in South Africa. However, that study was hard to judge because it was underpowered for number of people with mild infections. So, it didn’t look as if it had any efficacy, but maybe it would if tested in a real trial.
The P.1 (Brazil) variant is the second-highest concern after B.1.1.7 because it’s the only one in the United States that’s still headed up. It seems to be competing a bit with B.1.1.7 here. We know it was associated with the crisis in Brazil, in Chile, and some other South American countries. It has some immune escape, but not as bad as B.1.351. It also appears to have somewhat greater transmissibility but not as much as B.1.1.7.
With P.1, we just don’t know enough yet. It was difficult to assess in Brazil because they were in the midst of a catastrophe – like India is now – and you don’t know how much of it is dragged by the catastrophe vs driving it.
We have to respond to P.1 carefully. There are some good data that it does respond to the Chinese vaccine Sinovac and the AstraZeneca vaccine, and it appears to respond to the others as well, based on serum studies. So it doesn’t look like vaccines will be the worry with this variant. Rather, it could be competing with B.1.1.7 and could lead to breakthrough infections in vaccinated people or reinfections in unvaccinated people who had COVID-19. We need several more weeks to sort it out.
Although the B.1.427 and B.1.429 variants initially seen in California remain on the CDC’s concern list, I’m not worried about them.
You mentioned the current COVID-19 crisis in India, where a new variant has been described as a “double mutant,” but on Twitter you called it a “scariant.” Why?
First of all, the B.1.617 variant isn’t a double mutant. It has 15 mutations. It’s a stupid term, focusing on two mutations which largely have been put aside as to concern. One of them is the L452R, which is the same as one of the California variants, and that hasn’t proved to be particularly serious or concerning. The other is the 484Q, and it’s not clear whether that has any function.
The B.1.617 is not the driver of the catastrophe in India. It may be contributing a small amount, but it has been overhyped as the double mutant that’s causing it all. Adding to that are what I call “scariant” headlines here in the United States when a few cases of that variant have been seen.
I coined the term scariant in early February because it was a pretty clear trend. People don’t know what variants are. They know a little bit about mutations but not variants, and they’re scared. A few variants are concerning, but we keep learning more and more things to decrease the concern. That’s why I wrote the New York Times op-ed, to try to provide some reassurance, since there’s such paranoia.
Do you think booster vaccinations will be necessary? If so, will those be of the original vaccines or new ones that incorporate the variants?
As we go forward, there’s still potential for new variants that we haven’t seen yet that combine the worst of all features – transmissibility and immune evasion – especially since we have a world where COVID-19 is unchecked. So, we’re not out of it yet, but at least for the moment, we have vaccines that are capable of protecting against all variants.
In most people, the immune response against SARS-CoV-2 is very durable and strong and may well last for years. With the most closely related SARS-CoV-1, people still had immune responses up to 18 years later. However, some people will have less robust vaccine responses, including the elderly and the immunocompromised. If they don’t have great responses to the vaccine to start with, over time they’re likely to become more vulnerable, especially if they’re exposed to the variants with some degree of immune evasion.
I think we need to study these individuals post vaccination. A lot of people fit into those categories, including seniors, people being treated for cancer or autoimmune conditions, or post organ transplant. We could set up a prospective study to see whether they develop symptomatic COVID-19 and if so, from what – the original strain, B.1.1.7, or the newer variants.
That’s where I think booster shots may be needed. They may not be necessary across the board, but perhaps just in these special subgroups.
All of the current vaccines can be tweaked to include new variants, but the need for that is uncertain as of now. Moderna is working on a so-called bivalent vaccine that includes the original SARS-CoV-2 strain plus the B.1.351 variant, but it isn’t clear that that’s going to be necessary.
Currently, at least 200 COVID-19 vaccines are in development. There will be vaccines you can inhale, room temperature mRNA vaccines, and potentially even oral vaccines.
In the near future, Novavax is close, and there will likely be a two-dose Johnson & Johnson version that has the same potency as the mRNA vaccines. There are a lot of moving parts here.
There may be a step down in efficacy from mRNA to the others, though, and that shouldn’t be discounted. All of the available vaccines so far protect very well against severe disease and death, but some are less effective against mild to moderate infections, which may then lead to long COVID. We don’t yet know whether those who get mild infection post vaccination can still get long COVID.
What do you think it will take to achieve herd immunity?
I prefer the term “containment.” It’s quantitative. If you get to an infection rate of less than 1 in 100,000 people, as they’ve done in Israel, with 0.8 per 100,000, then you have the virus in check, and there will be very little spread when it’s at that controlled rate, with no outbreaks. The United States is currently at about 15 per 100,000. California is at 4. That still has to get lower.
It will be a challenge to get to President Biden’s goal of having 70% of U.S. adults given at least one dose by July 4. We’re now at about 57%. To get that next 13% of adults is going to take an all-out effort: mobile units, going to homes, making it ultraconvenient, education for people with safety concerns, incentivization, and days off.
We also need to get employers, universities, and health systems to get to the mandatory level. We haven’t done that yet. Some universities have mandated it for students, faculty, and staff. We need it in more health care systems. Right now, we only have a couple. We mandate flu shots, and flu is nothing, compared with COVID-19. And the COVID-19 vaccine is far more efficacious – flu shots are 40% efficacious, while these are 95%. COVID-19 is a tenfold more lethal and serious disease, and much more spreadable.
People are using the lack of full licensure by the Food and Drug Administration – as opposed to emergency use authorization – as an excuse not to get vaccinated. A biologics license application takes time to approve. Meanwhile, we have hundreds of millions of doses that have been well tolerated and incredibly effective.
Another aspect to consider regarding containment is that about 110 million Americans have already had COVID-19, even though only about 30 million cases have been confirmed. Most of these people have immune protection, although it’s not as good as if they have one vaccine dose. But they have enough protection to be part of the story here of the wall against COVID-19 and will help us get through this.
That’s a silver lining of having an unchecked epidemic for the entire year of 2020. The good part is that’s helping to get us to achieve an incredible level of containment when we haven’t even been close. Right now, we’re as good as the country has been in the pandemic, but we still have a long gap to get down to that 1 per 100,000. That’s what we should be working toward, and we can get there.
A version of this article first appeared on Medscape.com.
Editor in Chief of this news organization Eric Topol, MD, founder and director of the Scripps Research Translational Institute in La Jolla, Calif., and professor of molecular medicine, has been closely following COVID-19 data since the pandemic began. He spoke with writer Miriam E. Tucker about the latest on SARS-CoV-2 variants and their impact on vaccine efficacy. The conversation serves as a follow-up to his April 13, 2021, New York Times opinion piece, in which he advised readers that “all variants are innocent until proven guilty.”
You have expressed overall confidence in the efficacy of the vaccines thus far despite the emergence of variants, with some caveats. How do you see the current situation?
The Centers for Disease Control and Prevention has designated five “variants of concern,” but only three of them are real concerns – B.1.1.7, first detected in the United Kingdom; P.1, in Brazil and Japan; and B.1.351, in South Africa. Yet, all three are susceptible to our current vaccines.
The U.K. B.1.1.7 is the worst variant of all because it’s hypertransmissible, so I call it a “superspreader strain.” It also causes more severe illness independent of the spread, so it’s a double whammy. It’s clear that it also causes more deaths. The only arguable point is whether it’s 30% or 50% more deaths, but regardless, it’s more lethal and more transmissible.
The B.1.1.7 is going to be the dominant strain worldwide. It could develop new mutations within it that could come back to haunt us. We must keep watch.
But for now, it’s fully responsive to all the vaccines, which is great because if we didn’t have them, we wouldn’t have gotten through this U.S. pandemic like we have, and neither would Israel and the United Kingdom and other countries that have been able to get out of the crisis. We met the enemy and put it in check.
As for the South Africa variant of concern, B.1.351, we just got some encouraging news showing that it›s very responsive to the Pfizer/BioNTech mRNA vaccine in large numbers of people. The study was conducted in Qatar following that country’s mass immunization campaign in which a total of 385,853 people had received at least one vaccine dose and 265,410 had completed the two doses as of March 31, 2021.
At 2 weeks past the second dose, the vaccine was 75% effective at preventing any documented infection with the B.1.351 variant and 89.5% effective against B.1.1.7. The vaccine’s effectiveness against severe, critical, or fatal COVID-19 was greater than 97.4% for all circulating strains in Qatar, where B.1.1.7 and B.1.351 are most prominent.
We also know that B.1.351 is very responsive to the Johnson & Johnson vaccine and the Novavax [vaccine in development] to a lesser degree. It is the most immune-evading variant we’ve seen thus far, with the highest likelihood of providing some vaccine resistance, yet not enough to interfere with vaccination campaigns. So that’s great news.
The caveats here are that you definitely need two doses of the mRNA vaccines to combat the B.1.351 variant. Also, the AstraZeneca vaccine failed to prevent it in South Africa. However, that study was hard to judge because it was underpowered for number of people with mild infections. So, it didn’t look as if it had any efficacy, but maybe it would if tested in a real trial.
The P.1 (Brazil) variant is the second-highest concern after B.1.1.7 because it’s the only one in the United States that’s still headed up. It seems to be competing a bit with B.1.1.7 here. We know it was associated with the crisis in Brazil, in Chile, and some other South American countries. It has some immune escape, but not as bad as B.1.351. It also appears to have somewhat greater transmissibility but not as much as B.1.1.7.
With P.1, we just don’t know enough yet. It was difficult to assess in Brazil because they were in the midst of a catastrophe – like India is now – and you don’t know how much of it is dragged by the catastrophe vs driving it.
We have to respond to P.1 carefully. There are some good data that it does respond to the Chinese vaccine Sinovac and the AstraZeneca vaccine, and it appears to respond to the others as well, based on serum studies. So it doesn’t look like vaccines will be the worry with this variant. Rather, it could be competing with B.1.1.7 and could lead to breakthrough infections in vaccinated people or reinfections in unvaccinated people who had COVID-19. We need several more weeks to sort it out.
Although the B.1.427 and B.1.429 variants initially seen in California remain on the CDC’s concern list, I’m not worried about them.
You mentioned the current COVID-19 crisis in India, where a new variant has been described as a “double mutant,” but on Twitter you called it a “scariant.” Why?
First of all, the B.1.617 variant isn’t a double mutant. It has 15 mutations. It’s a stupid term, focusing on two mutations which largely have been put aside as to concern. One of them is the L452R, which is the same as one of the California variants, and that hasn’t proved to be particularly serious or concerning. The other is the 484Q, and it’s not clear whether that has any function.
The B.1.617 is not the driver of the catastrophe in India. It may be contributing a small amount, but it has been overhyped as the double mutant that’s causing it all. Adding to that are what I call “scariant” headlines here in the United States when a few cases of that variant have been seen.
I coined the term scariant in early February because it was a pretty clear trend. People don’t know what variants are. They know a little bit about mutations but not variants, and they’re scared. A few variants are concerning, but we keep learning more and more things to decrease the concern. That’s why I wrote the New York Times op-ed, to try to provide some reassurance, since there’s such paranoia.
Do you think booster vaccinations will be necessary? If so, will those be of the original vaccines or new ones that incorporate the variants?
As we go forward, there’s still potential for new variants that we haven’t seen yet that combine the worst of all features – transmissibility and immune evasion – especially since we have a world where COVID-19 is unchecked. So, we’re not out of it yet, but at least for the moment, we have vaccines that are capable of protecting against all variants.
In most people, the immune response against SARS-CoV-2 is very durable and strong and may well last for years. With the most closely related SARS-CoV-1, people still had immune responses up to 18 years later. However, some people will have less robust vaccine responses, including the elderly and the immunocompromised. If they don’t have great responses to the vaccine to start with, over time they’re likely to become more vulnerable, especially if they’re exposed to the variants with some degree of immune evasion.
I think we need to study these individuals post vaccination. A lot of people fit into those categories, including seniors, people being treated for cancer or autoimmune conditions, or post organ transplant. We could set up a prospective study to see whether they develop symptomatic COVID-19 and if so, from what – the original strain, B.1.1.7, or the newer variants.
That’s where I think booster shots may be needed. They may not be necessary across the board, but perhaps just in these special subgroups.
All of the current vaccines can be tweaked to include new variants, but the need for that is uncertain as of now. Moderna is working on a so-called bivalent vaccine that includes the original SARS-CoV-2 strain plus the B.1.351 variant, but it isn’t clear that that’s going to be necessary.
Currently, at least 200 COVID-19 vaccines are in development. There will be vaccines you can inhale, room temperature mRNA vaccines, and potentially even oral vaccines.
In the near future, Novavax is close, and there will likely be a two-dose Johnson & Johnson version that has the same potency as the mRNA vaccines. There are a lot of moving parts here.
There may be a step down in efficacy from mRNA to the others, though, and that shouldn’t be discounted. All of the available vaccines so far protect very well against severe disease and death, but some are less effective against mild to moderate infections, which may then lead to long COVID. We don’t yet know whether those who get mild infection post vaccination can still get long COVID.
What do you think it will take to achieve herd immunity?
I prefer the term “containment.” It’s quantitative. If you get to an infection rate of less than 1 in 100,000 people, as they’ve done in Israel, with 0.8 per 100,000, then you have the virus in check, and there will be very little spread when it’s at that controlled rate, with no outbreaks. The United States is currently at about 15 per 100,000. California is at 4. That still has to get lower.
It will be a challenge to get to President Biden’s goal of having 70% of U.S. adults given at least one dose by July 4. We’re now at about 57%. To get that next 13% of adults is going to take an all-out effort: mobile units, going to homes, making it ultraconvenient, education for people with safety concerns, incentivization, and days off.
We also need to get employers, universities, and health systems to get to the mandatory level. We haven’t done that yet. Some universities have mandated it for students, faculty, and staff. We need it in more health care systems. Right now, we only have a couple. We mandate flu shots, and flu is nothing, compared with COVID-19. And the COVID-19 vaccine is far more efficacious – flu shots are 40% efficacious, while these are 95%. COVID-19 is a tenfold more lethal and serious disease, and much more spreadable.
People are using the lack of full licensure by the Food and Drug Administration – as opposed to emergency use authorization – as an excuse not to get vaccinated. A biologics license application takes time to approve. Meanwhile, we have hundreds of millions of doses that have been well tolerated and incredibly effective.
Another aspect to consider regarding containment is that about 110 million Americans have already had COVID-19, even though only about 30 million cases have been confirmed. Most of these people have immune protection, although it’s not as good as if they have one vaccine dose. But they have enough protection to be part of the story here of the wall against COVID-19 and will help us get through this.
That’s a silver lining of having an unchecked epidemic for the entire year of 2020. The good part is that’s helping to get us to achieve an incredible level of containment when we haven’t even been close. Right now, we’re as good as the country has been in the pandemic, but we still have a long gap to get down to that 1 per 100,000. That’s what we should be working toward, and we can get there.
A version of this article first appeared on Medscape.com.



