User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Zika vaccine candidate shows promise in phase 1 trial
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
FROM ANNALS OF INTERNAL MEDICINE
The lost year – even for common respiratory viruses
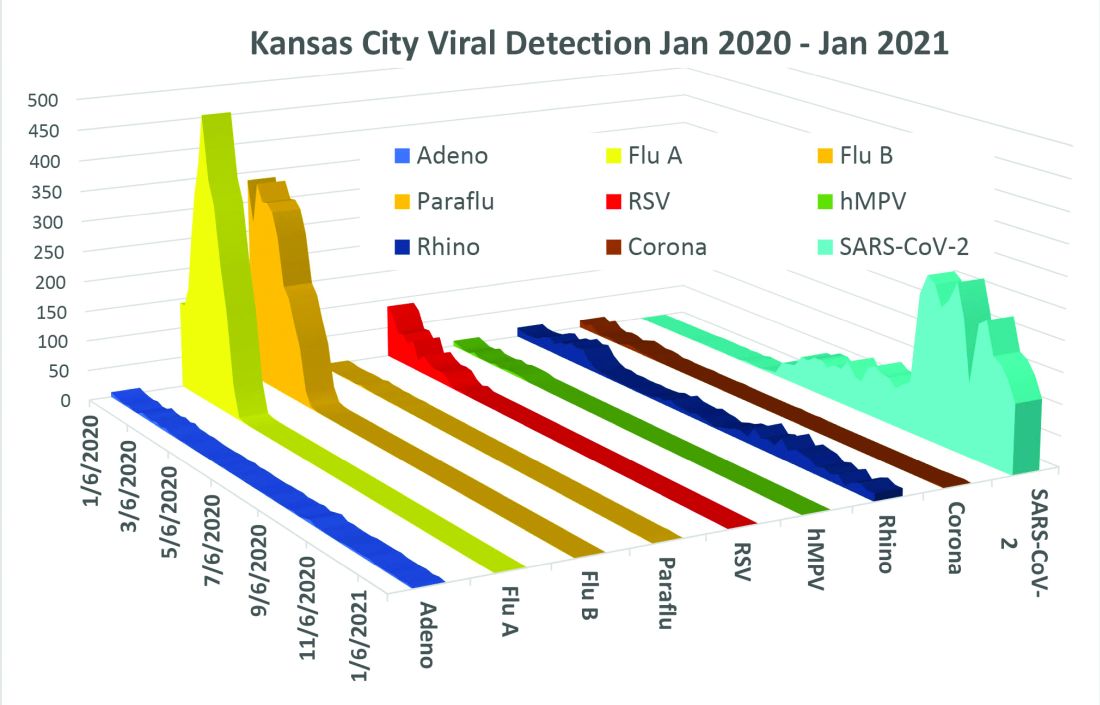
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.
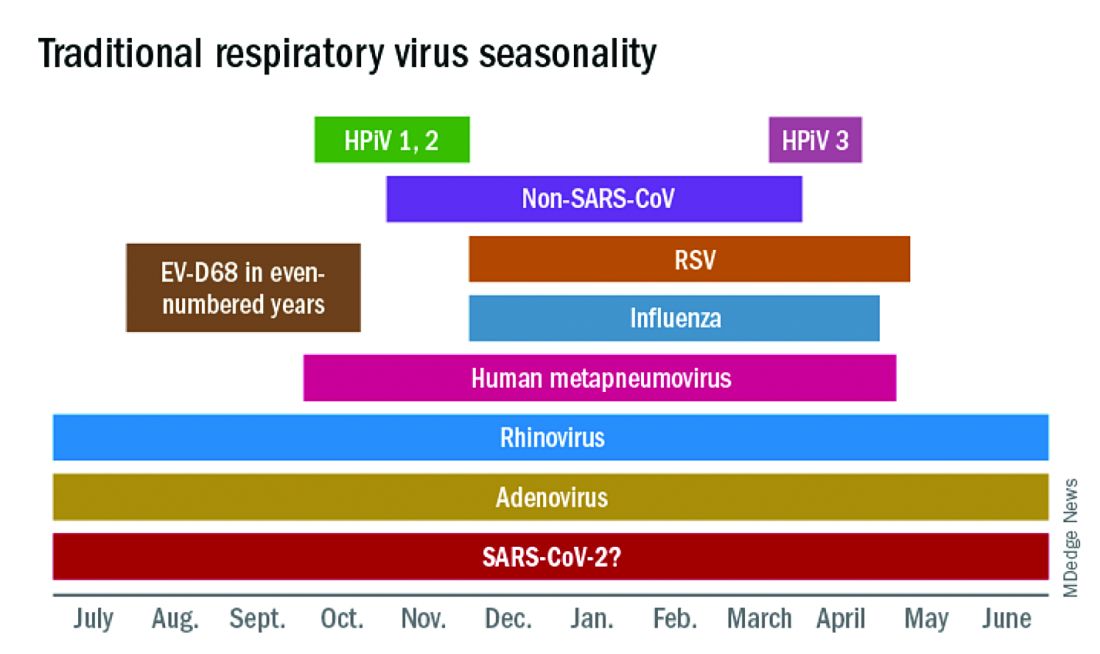
The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).

What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).

What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
Infectious diseases ‘giant’ John Bartlett: His ‘impact will endure’
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.
The cause of death was not immediately disclosed.
Dr. Bartlett is remembered by colleagues for his wide range of infectious disease expertise, an ability to repeatedly predict emerging issues in the field, and for inspiring students and trainees to choose the same specialty.
“What I consistently found so extraordinary about John was his excitement for ID – the whole field. He had a wonderful sixth sense about what was going to be the next ‘big thing,’” Paul Edward Sax, MD, clinical director of the Infectious Disease Clinic at Brigham and Women’s Hospital in Boston, told this news organization.
“He thoroughly absorbed the emerging research on the topic and then provided the most wonderful clinical summaries,” Dr. Sax said. “His range of expert content areas was unbelievably broad.” Dr. Bartlett was “a true ID polymath.”
Dr. Bartlett was “a giant in the field of infectious diseases,” David Lee Thomas, MD, MPH, said in an interview. He agreed that Dr. Bartlett was a visionary who could anticipate the most exciting developments in the specialty.
Dr. Bartlett also “led the efforts to combat the foes, from HIV to antimicrobial resistance,” said Dr. Thomas, director of the division of infectious diseases and professor of medicine at Johns Hopkins University.
A pioneer in HIV research and care
Dr. Bartlett’s early research focused on anaerobic pulmonary and other infections, Bacteroides fragilis pathogenesis, and colitis caused by Clostridioides difficile.
Shortly after joining Johns Hopkins in 1980, he focused on HIV/AIDS research and caring for people with HIV. Dr. Bartlett led clinical trials of new treatments and developed years of HIV clinical treatment guidelines.
“Back when most hospitals, university medical centers, and ID divisions were running away from the AIDS epidemic, John took it on, both as a scientific priority and a moral imperative,” Dr. Sax writes in a blog post for NEJM Journal Watch. “With the help of Frank Polk and the Hopkins president, he established an outpatient AIDS clinic and an inpatient AIDS ward – both of which were way ahead of their time.”
In the same post, Dr. Sax points out that Dr. Bartlett was an expert in multiple areas – any one of which could be a sole career focus. “How many ID doctors are true experts in all of the following distinct topics? HIV, Clostridium difficile, respiratory tract infections, antimicrobial resistance, and anaerobic pulmonary infections.” Dr. Sax writes.
Expertise that defined an era
In a piece reviewing the long history of infectious disease medicine at Johns Hopkins published in Clinical Infectious Diseases in 2014, Paul Auwaerter, MD, and colleagues describe his tenure at the institution from 1980 to 2006 as “The Bartlett Era,” notable for the many advances he spearheaded.
“It is nearly impossible to find someone trained in infectious diseases in the past 30 years who has not been impacted by John Bartlett,” Dr. Auwaerter and colleagues note. “His tireless devotion to scholarship, teaching, and patient care remains an inspiration to his faculty members at Johns Hopkins, his colleagues, and coworkers around the world.”
Dr. Bartlett was not only a faculty member in the division of infectious diseases, he also helped establish it. When he joined Johns Hopkins, the infectious disease department featured just three faculty members with a research budget of less than $285,000. By the time he left 26 years later, the division had 44 faculty members on tenure track and a research budget exceeding $40 million.
Sharing memories via social media
Reactions to Dr. Bartlett’s passing on Twitter were swift.
“We have lost one of the greatest physicians I have ever met or had the privilege to learn from. Saddened to hear of Dr. John G. Bartlett’s passing. He inspired so many, including me, to choose the field of infectious diseases,” David Fisk, MD, infectious disease specialist in Santa Barbara, Calif., wrote on Twitter.
“John Bartlett just died – a true visionary and the classic ‘Renaissance’ person in clinical ID. Such a nice guy, too! His IDSA/IDWeek literature summaries (among other things) were amazing. We’ll miss him!” Dr. Sax tweeted on Jan. 19.
A colleague at Johns Hopkins, transplant infectious disease specialist Shmuel Shoham, MD, shared an anecdote about Dr. Bartlett on Twitter: “Year ago. My office is across from his. I ask him what he is doing. He tells me he is reviewing a file from the Vatican to adjudicate whether a miracle happened. True story.”
Infectious disease specialist Graeme Forrest, MBBS, also shared a story about Dr. Bartlett via Twitter. “He described to me in 2001 how the U.S. model of health care would not cope with a pandemic or serious bioterror attack as it’s not connected to disseminate information. How prescient from 20 years ago.”
Dr. Bartlett shared his expertise at many national and international infectious disease conferences over the years. He also authored 470 articles, 282 book chapters, and 61 editions of 14 books.
Dr. Bartlett was also a regular contributor to this news organization. For example, he shared his expertise in perspective pieces that addressed priorities in antibiotic stewardship, upcoming infectious disease predictions, and critical infectious disease topics in a three-part series.
Dr. Bartlett’s education includes a bachelor’s degree from Dartmouth College in Hanover, N.H., in 1959 and an MD from Upstate Medical Center in Syracuse, N.Y., in 1963. He did his first 2 years of residency at Brigham and Women’s Hospital.
He also served as an Army captain from 1965 to 1967, treating patients in fever wards in Vietnam. He then returned to the United States to finish his internal medicine training at the University of Alabama in 1968.
Dr. Bartlett completed his fellowship in infectious diseases at the University of California, Los Angeles. In 1975, he joined the faculty at Tufts University, Boston.
Leaving a legacy
Dr. Bartlett’s influence will likely live on in many ways at Johns Hopkins.
“John is a larger-than-life legend whose impact will endure and after whom we are so proud to have named our clinical service, The Bartlett Specialty Practice,” Dr. Thomas said.
The specialty practice clinic named for him has 23 exam rooms and features multidisciplinary care for people with HIV, hepatitis, bone infections, general infectious diseases, and more. Furthermore, friends, family, and colleagues joined forces to create the “Dr. John G. Bartlett HIV/AIDS Fund.”
They note that it is “only appropriate that we honor him by creating an endowment that will provide support for young trainees and junior faculty in the division, helping them transition to their independent careers.”
In addition to all his professional accomplishments, “He was also a genuinely nice person, approachable and humble,” Dr. Sax said. “We really lost a great one!”
A version of this article first appeared on Medscape.com.
The changing brain signature of HIV
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
“This shift in subcortical signatures may be contributing to the increasing range of neuropsychiatric and cognitive outcomes,” write Neda Jahanshad, PhD, University of Southern California, Los Angeles, and colleagues.
The study was published online Jan. 15 in JAMA Network Open.
Brain signature of HIV
The researchers with the HIV Working Group within the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) consortium examined structural brain associations with CD4+ T cell counts and HIV viral load.
These clinical markers are the most consistently available in studies of HIV and generalize across demographically and clinically diverse HIV-infected individuals, they point out. However, the degree to which they capture central nervous system injury is not fully understood.
In this cross-sectional study of 1,203 HIV-infected adults from 13 HIV neuroimaging studies, a lower CD4+ T-cell count was associated with smaller hippocampal and thalamic volume independent of treatment status. However, in a subset of adults not on cART, a lower CD4+ T-cell count was associated with smaller putamen volume.
Across all participants, detectable viral load was associated with smaller hippocampal volume, but in the subset on cART, detectable viral load was also associated with smaller amygdala volume.
The findings indicate that plasma markers universally used to monitor immune function and response to treatment in patients with HIV infection are associated with subcortical brain volume.
“Our findings,” they add, “extend beyond the classically implicated regions of the basal ganglia and may represent a generalizable brain signature of HIV infection in the cART era.”
A limitation of the analysis is that most of the participants were men (n = 880, 73%). “A more extensive international effort assessing the neurologic effects of HIV infection in women is needed,” they conclude.
This analysis, they add, demonstrates the feasibility and utility of a global collaborative initiative to understand the neurologic signatures of HIV infection. They invite other HIV researchers to join the ENIGMA-HIV consortium.
“With a greater collaborative effort, we will be able to assess factors that may modulate neurologic outcomes, including cART treatment regimens, comorbidities, coinfections, substance use, socioeconomic factors, and demographic factors, as well as the functional implications of such structural brain differences, in well-powered analyses,” the researchers say.
“Understanding the neurobiological changes that may contribute to neuropsychiatric and cognitive outcomes in HIV-positive individuals is critical for identifying individuals at risk for neurologic symptoms, driving novel treatments that may protect the CNS, and monitoring treatment response,” they add.
Support for this research was provided by grants from the National Institutes of Health, the SA Medical Research Council, the National Health and Medical Research Council, and the European Research Council. Dr. Jahanshad received partial research support from Biogen for work unrelated to the topic of this article. A complete list of author disclosures is in the original article.
A version of this article first appeared on Medscape.com.
Steroid and immunoglobulin standard of care for MIS-C
The combination of methylprednisolone and intravenous immunoglobulins works better than intravenous immunoglobulins alone for multisystem inflammatory syndrome in children (MIS-C), researchers say.
“I’m not sure it’s the best treatment because we have not studied every possible treatment,” François Angoulvant, MD, PhD, told this news organization, “but right now, it’s the standard of care.”
Dr. Angoulvant, a professor of pediatrics at University of Paris, and colleagues published a comparison of the two treatments in the Journal of the American Medical Association.
A small percentage of children infected with SARS-CoV-2 develop MIS-C about 2 to 4 weeks later. It is considered a separate disease entity from COVID-19 and is associated with persistent fever, digestive symptoms, rash, bilateral nonpurulent conjunctivitis, mucocutaneous inflammation signs, and frequent cardiovascular involvement. In more than 60% of cases, it leads to hemodynamic failure, with acute cardiac dysfunction.
Because MIS-C resembles Kawasaki disease, clinicians modeled their treatment on that condition and started with immunoglobulins alone, Dr. Angoulvant said.
Based on expert opinion, the National Health Service in the United Kingdom published a consensus statement in Sept. listing immunoglobulins alone as the first-line treatment.
But anecdotal reports have emerged that combining the immunoglobulins with a corticosteroid worked better. To investigate this possibility, Dr. Angoulvant and colleagues analyzed records of MIS-C cases in France, where physicians are required to report all suspected cases of MIS-C to the French National Public Health Agency.
Among the 181 cases they scrutinized, 111 fulfilled the World Health Organization criteria for MIS-C. Of these, the researchers were able to match 64 patients who had received immunoglobulins alone with 32 who had received the combined therapy and could be matched using propensity scores.
The researchers defined treatment failure as persistence of fever for 2 days after the start of therapy or recurrence of fever within a week. By this measure, the combination treatment failed in only 9% of cases while immunoglobulins alone failed in 38% of cases. The difference was statistically significant (P = .008). Most of those for whom these treatments failed received second-line treatments such as steroids or biological agents.
Patients treated with the combination therapy also had a lower risk of secondary acute left ventricular dysfunction (odds ratio, 0.20; 95% confidence interval, 0.06-0.66) and a lower risk of needing hemodynamic support (OR, 0.21; 95% CI, 0.06-0.76).
Those receiving the combination therapy spent a mean of 4 days in the pediatric intensive care unit compared with 6 days for those receiving immunoglobulins alone. (Difference in days, −2.4; 95% CI, −4.0 to −0.7; P = .005).
There are few drawbacks to the combination approach, Dr. Angoulvant said, as the side effects of corticosteroids are generally not severe and they can be anticipated because this class of medications has been used for many years.
The study raises the question of whether corticosteroids might work as well by themselves, but it could not be answered with this database as no one is using that approach in France, Dr. Angoulvant said. “I hope other teams around the world could bring us the answer.”
In the United States, most physicians appear to already be using the combination therapy, said David Teachey, MD, an associate professor of pediatrics at the Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia.
The reduction in time in pediatric intensive care and the reduced risk of cardiac dysfunction are important findings, he said.
This retrospective study falls short of the evidence provided by a randomized clinical trial, Dr. Teachey noted. But he acknowledged that few families would agree to participate in such a trial as they would have to take a chance that the sick children would receive a less effective therapy than what they would otherwise get. “It’s hard to [talk] about a therapy reduction,” he told this news organization.
Given that impediment, he agreed with Dr. Angoulvant that the current study and others like it may provide the best data available pointing to a treatment approach for MIS-C.
The study received an unrestricted grant from Pfizer. The French COVID-19 Paediatric Inflammation Consortium received an unrestricted grant from the Square Foundation (Grandir–Fonds de Solidarité pour L’Enfance). Dr. Angoulvant and Dr. Teachey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of methylprednisolone and intravenous immunoglobulins works better than intravenous immunoglobulins alone for multisystem inflammatory syndrome in children (MIS-C), researchers say.
“I’m not sure it’s the best treatment because we have not studied every possible treatment,” François Angoulvant, MD, PhD, told this news organization, “but right now, it’s the standard of care.”
Dr. Angoulvant, a professor of pediatrics at University of Paris, and colleagues published a comparison of the two treatments in the Journal of the American Medical Association.
A small percentage of children infected with SARS-CoV-2 develop MIS-C about 2 to 4 weeks later. It is considered a separate disease entity from COVID-19 and is associated with persistent fever, digestive symptoms, rash, bilateral nonpurulent conjunctivitis, mucocutaneous inflammation signs, and frequent cardiovascular involvement. In more than 60% of cases, it leads to hemodynamic failure, with acute cardiac dysfunction.
Because MIS-C resembles Kawasaki disease, clinicians modeled their treatment on that condition and started with immunoglobulins alone, Dr. Angoulvant said.
Based on expert opinion, the National Health Service in the United Kingdom published a consensus statement in Sept. listing immunoglobulins alone as the first-line treatment.
But anecdotal reports have emerged that combining the immunoglobulins with a corticosteroid worked better. To investigate this possibility, Dr. Angoulvant and colleagues analyzed records of MIS-C cases in France, where physicians are required to report all suspected cases of MIS-C to the French National Public Health Agency.
Among the 181 cases they scrutinized, 111 fulfilled the World Health Organization criteria for MIS-C. Of these, the researchers were able to match 64 patients who had received immunoglobulins alone with 32 who had received the combined therapy and could be matched using propensity scores.
The researchers defined treatment failure as persistence of fever for 2 days after the start of therapy or recurrence of fever within a week. By this measure, the combination treatment failed in only 9% of cases while immunoglobulins alone failed in 38% of cases. The difference was statistically significant (P = .008). Most of those for whom these treatments failed received second-line treatments such as steroids or biological agents.
Patients treated with the combination therapy also had a lower risk of secondary acute left ventricular dysfunction (odds ratio, 0.20; 95% confidence interval, 0.06-0.66) and a lower risk of needing hemodynamic support (OR, 0.21; 95% CI, 0.06-0.76).
Those receiving the combination therapy spent a mean of 4 days in the pediatric intensive care unit compared with 6 days for those receiving immunoglobulins alone. (Difference in days, −2.4; 95% CI, −4.0 to −0.7; P = .005).
There are few drawbacks to the combination approach, Dr. Angoulvant said, as the side effects of corticosteroids are generally not severe and they can be anticipated because this class of medications has been used for many years.
The study raises the question of whether corticosteroids might work as well by themselves, but it could not be answered with this database as no one is using that approach in France, Dr. Angoulvant said. “I hope other teams around the world could bring us the answer.”
In the United States, most physicians appear to already be using the combination therapy, said David Teachey, MD, an associate professor of pediatrics at the Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia.
The reduction in time in pediatric intensive care and the reduced risk of cardiac dysfunction are important findings, he said.
This retrospective study falls short of the evidence provided by a randomized clinical trial, Dr. Teachey noted. But he acknowledged that few families would agree to participate in such a trial as they would have to take a chance that the sick children would receive a less effective therapy than what they would otherwise get. “It’s hard to [talk] about a therapy reduction,” he told this news organization.
Given that impediment, he agreed with Dr. Angoulvant that the current study and others like it may provide the best data available pointing to a treatment approach for MIS-C.
The study received an unrestricted grant from Pfizer. The French COVID-19 Paediatric Inflammation Consortium received an unrestricted grant from the Square Foundation (Grandir–Fonds de Solidarité pour L’Enfance). Dr. Angoulvant and Dr. Teachey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of methylprednisolone and intravenous immunoglobulins works better than intravenous immunoglobulins alone for multisystem inflammatory syndrome in children (MIS-C), researchers say.
“I’m not sure it’s the best treatment because we have not studied every possible treatment,” François Angoulvant, MD, PhD, told this news organization, “but right now, it’s the standard of care.”
Dr. Angoulvant, a professor of pediatrics at University of Paris, and colleagues published a comparison of the two treatments in the Journal of the American Medical Association.
A small percentage of children infected with SARS-CoV-2 develop MIS-C about 2 to 4 weeks later. It is considered a separate disease entity from COVID-19 and is associated with persistent fever, digestive symptoms, rash, bilateral nonpurulent conjunctivitis, mucocutaneous inflammation signs, and frequent cardiovascular involvement. In more than 60% of cases, it leads to hemodynamic failure, with acute cardiac dysfunction.
Because MIS-C resembles Kawasaki disease, clinicians modeled their treatment on that condition and started with immunoglobulins alone, Dr. Angoulvant said.
Based on expert opinion, the National Health Service in the United Kingdom published a consensus statement in Sept. listing immunoglobulins alone as the first-line treatment.
But anecdotal reports have emerged that combining the immunoglobulins with a corticosteroid worked better. To investigate this possibility, Dr. Angoulvant and colleagues analyzed records of MIS-C cases in France, where physicians are required to report all suspected cases of MIS-C to the French National Public Health Agency.
Among the 181 cases they scrutinized, 111 fulfilled the World Health Organization criteria for MIS-C. Of these, the researchers were able to match 64 patients who had received immunoglobulins alone with 32 who had received the combined therapy and could be matched using propensity scores.
The researchers defined treatment failure as persistence of fever for 2 days after the start of therapy or recurrence of fever within a week. By this measure, the combination treatment failed in only 9% of cases while immunoglobulins alone failed in 38% of cases. The difference was statistically significant (P = .008). Most of those for whom these treatments failed received second-line treatments such as steroids or biological agents.
Patients treated with the combination therapy also had a lower risk of secondary acute left ventricular dysfunction (odds ratio, 0.20; 95% confidence interval, 0.06-0.66) and a lower risk of needing hemodynamic support (OR, 0.21; 95% CI, 0.06-0.76).
Those receiving the combination therapy spent a mean of 4 days in the pediatric intensive care unit compared with 6 days for those receiving immunoglobulins alone. (Difference in days, −2.4; 95% CI, −4.0 to −0.7; P = .005).
There are few drawbacks to the combination approach, Dr. Angoulvant said, as the side effects of corticosteroids are generally not severe and they can be anticipated because this class of medications has been used for many years.
The study raises the question of whether corticosteroids might work as well by themselves, but it could not be answered with this database as no one is using that approach in France, Dr. Angoulvant said. “I hope other teams around the world could bring us the answer.”
In the United States, most physicians appear to already be using the combination therapy, said David Teachey, MD, an associate professor of pediatrics at the Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia.
The reduction in time in pediatric intensive care and the reduced risk of cardiac dysfunction are important findings, he said.
This retrospective study falls short of the evidence provided by a randomized clinical trial, Dr. Teachey noted. But he acknowledged that few families would agree to participate in such a trial as they would have to take a chance that the sick children would receive a less effective therapy than what they would otherwise get. “It’s hard to [talk] about a therapy reduction,” he told this news organization.
Given that impediment, he agreed with Dr. Angoulvant that the current study and others like it may provide the best data available pointing to a treatment approach for MIS-C.
The study received an unrestricted grant from Pfizer. The French COVID-19 Paediatric Inflammation Consortium received an unrestricted grant from the Square Foundation (Grandir–Fonds de Solidarité pour L’Enfance). Dr. Angoulvant and Dr. Teachey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
2021 ACIP adult schedule released
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
ColCORONA: More questions than answers for colchicine in COVID-19
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Science by press release and preprint has cooled clinician enthusiasm for the use of colchicine in nonhospitalized patients with COVID-19, despite a pressing need for early treatments.
As previously reported by this news organization, a Jan. 22 press release announced that the massive ColCORONA study missed its primary endpoint of hospitalization or death among 4,488 newly diagnosed patients at increased risk for hospitalization.
But it also touted that use of the anti-inflammatory drug significantly reduced the primary endpoint in 4,159 of those patients with polymerase chain reaction–confirmed COVID and led to reductions of 25%, 50%, and 44%, respectively, for hospitalizations, ventilations, and death.
Lead investigator Jean-Claude Tardif, MD, director of the Montreal Heart Institute Research Centre, deemed the findings a “medical breakthrough.”
When the preprint released a few days later, however, newly revealed confidence intervals showed colchicine did not meaningfully reduce the need for mechanical ventilation (odds ratio, 0.50; 95% confidence interval, 0.23-1.07) or death alone (OR, 0.56; 95% CI, 0.19-1.66).
Further, the significant benefit on the primary outcome came at the cost of a fivefold increase in pulmonary embolism (11 vs. 2; P = .01), which was not mentioned in the press release.
“Whether this represents a real phenomenon or simply the play of chance is not known,” Dr. Tardif and colleagues noted later in the preprint.
“I read the preprint on colchicine and I have so many questions,” Aaron E. Glatt, MD, spokesperson for the Infectious Diseases Society of America and chief of infectious diseases, Mount Sinai South Nassau, Hewlett, N.Y., said in an interview. “I’ve been burned too many times with COVID and prefer to see better data.
“People sometimes say if you wait for perfect data, people are going to die,” he said. “Yeah, but we have no idea if people are going to die from getting this drug more than not getting it. That’s what concerns me. How many pulmonary emboli are going to be fatal versus the slight benefit that the study showed?”
The pushback to the non–peer-reviewed data on social media and via emails was so strong that Dr. Tardif posted a nearly 2,000-word letter responding to the many questions at play.
Chief among them was why the trial, originally planned for 6,000 patients, was stopped early by the investigators without consultation with the data safety monitoring board (DSMB).
The explanation in the letter that logistical issues like running the study call center, budget constraints, and a perceived need to quickly communicate the results left some calling foul that the study wasn’t allowed to finish and come to a more definitive conclusion.
“I can be a little bit sympathetic to their cause but at the same time the DSMB should have said no,” said David Boulware, MD, MPH, who led a recent hydroxychloroquine trial in COVID-19. “The problem is we’re sort of left in limbo, where some people kind of believe it and some say it’s not really a thing. So it’s not really moving the needle, as far as guidelines go.”
Indeed, a Twitter poll by cardiologist James Januzzi Jr., MD, captured the uncertainty, with 28% of respondents saying the trial was “neutral,” 58% saying “maybe but meh,” and 14% saying “colchicine for all.”
Another poll cheekily asked whether ColCORONA was the Gamestop/Reddit equivalent of COVID.
“The press release really didn’t help things because it very much oversold the effect. That, I think, poisoned the well,” said Dr. Boulware, professor of medicine in infectious diseases at the University of Minnesota, Minneapolis.
“The question I’m left with is not whether colchicine works, but who does it work in,” he said. “That’s really the fundamental question because it does seem that there are probably high-risk groups in their trial and others where they benefit, whereas other groups don’t benefit. In the subgroup analysis, there was absolutely no beneficial effect in women.”
According to the authors, the number needed to treat to prevent one death or hospitalization was 71 overall, but 29 for patients with diabetes, 31 for those aged 70 years and older, 53 for patients with respiratory disease, and 25 for those with coronary disease or heart failure.
Men are at higher risk overall for poor outcomes. But “the authors didn’t present a multivariable analysis, so it is unclear if another factor, such as a differential prevalence of smoking or cardiovascular risk factors, contributed to the differential benefit,” Rachel Bender Ignacio, MD, MPH, infectious disease specialist, University of Washington, Seattle, said in an interview.
Importantly, in this pragmatic study, duration and severity of symptoms were not reported, observed Dr. Bender Ignacio, who is also a STOP-COVID-2 investigator. “We don’t yet have data as to whether colchicine shortens duration or severity of symptoms or prevents long COVID, so we need more data on that.”
The overall risk for serious adverse events was lower in the colchicine group, but the difference in pulmonary embolism (PE) was striking, she said. This could be caused by a real biologic effect, or it’s possible that persons with shortness of breath and hypoxia, without evident viral pneumonia on chest x-ray after a positive COVID-19 test, were more likely to receive a CT-PE study.
The press release also failed to include information, later noted in the preprint, that the MHI has submitted two patents related to colchicine: “Methods of treating a coronavirus infection using colchicine” and “Early administration of low-dose colchicine after myocardial infarction.”
Reached for clarification, MHI communications adviser Camille Turbide said in an interview that the first patent “simply refers to the novel concept of preventing complications of COVID-19, such as admission to the hospital, with colchicine as tested in the ColCORONA study.”
The second patent, she said, refers to the “novel concept that administering colchicine early after a major adverse cardiovascular event is better than waiting several days,” as supported by the COLCOT study, which Dr. Tardif also led.
The patents are being reviewed by authorities and “Dr. Tardif has waived his rights in these patents and does not stand to benefit financially at all if colchicine becomes used as a treatment for COVID-19,” Ms. Turbide said.
Dr. Tardif did not respond to interview requests for this story. Dr. Glatt said conflicts of interest must be assessed and are “something that is of great concern in any scientific study.”
Cardiologist Steve Nissen, MD, of the Cleveland Clinic said in an interview that, “despite the negative results, the study does suggest that colchicine might have a benefit and should be studied in future trials. These findings are not sufficient evidence to suggest use of the drug in patients infected with COVID-19.”
He noted that adverse effects like diarrhea were expected but that the excess PE was unexpected and needs greater clarification.
“Stopping the trial for administrative reasons is puzzling and undermined the ability of the trial to give a reliable answer,” Dr. Nissen said. “This is a reasonable pilot study that should be viewed as hypothesis generating but inconclusive.”
Several sources said a new trial is unlikely, particularly given the cost and 28 trials already evaluating colchicine. Among these are RECOVERY and COLCOVID, testing whether colchicine can reduce the duration of hospitalization or death in hospitalized patients with COVID-19.
Because there are so many trials ongoing right now, including for antivirals and other immunomodulators, it’s important that, if colchicine comes to routine clinical use, it provides access to treatment for those not able or willing to access clinical trials, rather than impeding clinical trial enrollment, Dr. Bender Ignacio suggested.
“We have already learned the lesson in the pandemic that early adoption of potentially promising therapies can negatively impact our ability to study and develop other promising treatments,” she said.
The trial was coordinated by the Montreal Heart Institute and funded by the government of Quebec; the National Heart, Lung, and Blood Institute of the National Institutes of Health; Montreal philanthropist Sophie Desmarais, and the COVID-19 Therapeutics Accelerator launched by the Bill & Melinda Gates Foundation, Wellcome, and Mastercard. CGI, Dacima, and Pharmascience of Montreal were also collaborators. Dr. Glatt reported no conflicts of interest. Dr. Boulware reported receiving $18 in food and beverages from Gilead Sciences in 2018.
A version of this article first appeared on Medscape.com.
Study: COVID cases have been ‘severely undercounted’
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.
Some COVID-19 vaccine reactions could be pseudoallergic, experts say
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
On Jan. 13, 2 days after a drive-through vaccination “superstation” opened in San Diego, six people were treated for anaphylaxis after they received the Moderna vaccine, leading the California state epidemiologist to recommend pausing the administration of that particular lot.
A group of allergy and immunology experts and public health officials reviewed the cases, as well as an incident that occurred the day before, and concluded that at least some of the responses were angioedema, or swelling — a serious allergic reaction — but none were actually anaphylaxis. No similar clusters had occurred with the same vaccine lot in other states, and California resumed using the doses.
Yet questions remain about the reactions and the mechanisms for them. Some might have been triggered by an allergy to a vaccine component, most likely the polyethylene glycol (PEG) that stabilizes the lipid surrounding the mRNA, the key vaccine component in both the Moderna and Pfizer vaccines. Another possible explanation is that some could be pseudoallergic reactions to a blood protein known as complement, a little-understood process that resembles an antigen-based reaction but doesn’t leave an immune memory and might not recur.
Cases of complement-activation-related pseudoallergy look like a severe allergic reaction but occur through a different mechanism and don’t require previous exposure to an allergen.
“It has the same signs and symptoms and is treated the same way, but it occurs through a different pathway,” explained Neal Halsey, MD, director emeritus of the Institute for Vaccine Safety and emeritus professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Pseudoallergies are not well understood, but they have been associated with reactions to the contrast media used in imaging, such as with MRI. “If people have had an anaphylaxis-type reaction following the injection of contrast-dye material, that is a strong signal that it might be a complement-activation-related pseudoallergy,” said Dr. Halsey, a member of the Clinical Immunization Safety Assessment Network. “Those are the people who definitely need to consider seeing an allergist before getting the COVID vaccines.”
When Aleena Banerji, MD, clinical director of the allergy and clinical immunology unit at Massachusetts General Hospital in Boston, talks to patients about vaccine reactions, she addresses the risk for COVID-19 infection. All of the people who developed allergies after the Pfizer and Moderna vaccines recovered, but more than 445,000 Americans have died from COVID-19.
Most people with common allergies, such as to food or oral medications, don’t need to worry about reactions, said Dr. Banerji, lead author of a review that assessed the risk for allergic reactions to the Pfizer and Moderna vaccines.
Investigating reactions
As investigators search for the answers to what causes reactions, transparency is crucial to trust, said Kathryn Edwards, MD, principal investigator of the Clinical Immunization Safety Assessment Project, a vaccine safety network funded by the Centers for Disease Control and Prevention.
“Unless the public knows that we’re really investigating and we’re taking this seriously, then I think the vaccine hesitancy is going to increase,” said Dr. Edwards, professor of pediatrics at Vanderbilt University Medical Center and scientific director of the Vanderbilt Vaccine Research Program in Nashville, Tenn.
First reports of anaphylaxis came quickly after COVID-19 vaccinations began. In the 2 weeks before the holidays, almost 2 million health care workers received the Pfizer vaccine, and 21 of them developed anaphylaxis, according to CDC researchers who reviewed case reports from the Vaccine Adverse Event Reporting System (VAERS). That rate of about 1 in 100,000 is 10 times higher than the occurrence with other vaccines. No deaths from anaphylaxis were reported.
As the vaccinations ramped up, the rate declined. As of Jan. 18, 50 cases of anaphylaxis were reported to VAERS after the administration of 9,943,247 Pfizer doses, for a rate of 5.0 per million, according to data presented at the Jan. 27 meeting of the CDC Advisory Committee on Immunization Practices. And 21 cases of anaphylaxis were reported to VAERS after the administration of 7,581,429 Moderna doses, for a rate of 2.8 per million.
The anaphylaxis occurred almost exclusively in women; only three of the VAERS anaphylaxis reports were from men. Only 24% had a history of anaphylaxis.
The earlier CDC report explored the potential link to allergies. One person with anaphylaxis had a history of allergy to iodinated contrast media, and others had allergies to various medications, vaccines, foods, and animals. The researchers reported 86 nonanaphylaxis allergic reactions and 61 nonallergic adverse events among the 175 case reports they reviewed as possible cases of severe allergic reaction.
Of 1,266 reports that VAERS received from Dec. 21 to Jan. 10, the CDC identified 108 possible cases of severe allergic reaction after the Moderna vaccine. Only 10 met the case definition of anaphylaxis put forward by the Brighton Collaboration, a vaccine safety organization. All but one case involved a history of allergies or allergic reactions; only five had a previously experienced anaphylaxis.
There were 47 nonanaphylaxis allergic reactions.
The San Diego cluster also met the Brighton case definition for anaphylaxis, Dr. Edwards reported. This discrepancy highlights the difficulties in characterizing vaccine reactions.
Measuring a pseudoallergic reaction is a challenge. It requires that a blood sample be drawn soon after the incident and then frozen to protect heat-sensitive blood markers, Dr. Edwards explained.
And as vaccinations rise, so do adverse-event reports. But unlike in clinical trials, there is no control group for comparison. That is why vaccine safety experts urge caution when evaluating events and, where possible, advise looking at background rates.
“A major way to determine whether the adverse event is causally related is to assess the incidence of the adverse event in vaccines versus nonvaccines,” said Walter Orenstein, MD, who directed the U.S. Immunization Program from 1988 to 2004 and is now associate director of the Emory Vaccine Center and professor of infectious diseases at Emory University in Atlanta. Public health officials could then identify vaccine risk factors, he said.
When a reaction occurs almost immediately after vaccination, vaccine safety investigators look for probable triggers. If allergy to PEG is the culprit in anaphylactic reactions, then the individuals would have had a previous exposure, perhaps from injectable medications, Dr. Edwards said.
It might be feasible to perform a skin test for allergy to PEG. “If the skin testing is negative, that doesn’t completely rule out allergy, but it can be used in the decision-making about giving the first or second vaccine dose,” Dr. Banerji said.
Other vaccines, such as childhood vaccines, contain polysorbate as a stabilizer, which has a similar chemical structure, and it’s not clear why someone would react to PEG but not to polysorbate, Dr. Edwards said.
Meanwhile, other illnesses and even deaths sometimes occur in the days after vaccination, but that doesn’t mean the vaccine caused them, cautioned Steve Black, MD, emeritus professor of pediatrics at Cincinnati Children’s Hospital and cofounder of the Global Vaccine Data Network, an international vaccine safety collaboration.
“Different events and clusters of events will occur by chance alone, as these events can occur without vaccines. We need to not immediately assume that they’re due to the vaccine,” he said. “You don’t want to undermine the whole vaccine program every time something comes up and assume that it’s associated with the vaccine.”
The CDC only has three contraindications for the vaccines:
- Severe allergic reaction (such as anaphylaxis) after a previous dose of an mRNA COVID-19 vaccine or any of its components.
- Immediate allergic reaction of any severity to a previous dose of an mRNA COVID-19 vaccine or any of its components (including PEG).
- Immediate allergic reaction of any severity to polysorbate (due to potential cross-reactive hypersensitivity with PEG).
People who have had an immediate allergic reaction to other vaccines or injectable therapies should consider consulting with an allergist or immunologist before getting the Pfizer or Moderna vaccines, the CDC advises.
The CDC also says that people with a history of anaphylaxis from any cause should be observed for 30 minutes after vaccination. Vaccination protocol calls for everyone else to wait on site for 15 minutes after vaccination.
A version of this article first appeared on Medscape.com.
‘We can do better’: COVID-19 vaccination in patients with cancer
Every day, around 1.5 million doses of the COVID-19 vaccine are being delivered across the United States, but oncologists and patient advocates say that patients with cancer are missing out.
While official bodies recommend that patients with cancer are given priority, only 16 states currently prioritize them in the vaccine rollout. The other 34 states have thus far not singled out patients with cancer for earlier vaccination.
This flies in the face of recommendations from heavy hitters such as the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the National Comprehensive Cancer Network, and the American Association for Cancer Research.
All are in agreement: Patients on active cancer treatment should be prioritized for available vaccine because of their greater risk of death or complications from SARS-CoV-2 infection.
“All municipalities, states, cities, and even individual hospitals have so far been left to their own devices to try to figure out what the best way to do this is and that often conflicts with other recommendations or guidelines,” said E. John Wherry, PhD, chair of the department of systems pharmacology and translational therapeutics at the University of Pennsylvania, Philadelphia.
Dr. Wherry was on a panel at an AACR conference last week that discussed the failings of vaccine delivery to cancer patients.
During the meeting, lung cancer advocate Jill Feldman commented on the situation in Chicago, one of the jurisdictions that has not prioritized patients with cancer: “People don’t know what to do. ‘Do I need to sign up myself somewhere? Is my doctor’s office going to contact me?’ ”
Ms. Feldman said many people have called their cancer centers, “but cancer centers aren’t really providing updates directly to us. And they aren’t because they don’t have the information [either].”
Even in the 16 states that have ushered patients with cancer to the front of the line, the process for flagging these individuals is often unclear or nonexistent.
“Everyone that registers is basically on the same playing field ... because there’s no verification process. That’s very unfortunate,” said patient advocate Grace Cordovano, PhD, describing the vaccine sign-up process in New Jersey.
“It’s an easy fix,” said Dr. Cordovano. “Adding a few more fields [in the form] could really make a difference.”
COVID-19 fatality rates are twice as high in people with cancer than in people without cancer, according to a review published in December 2020 by the AACR’s COVID-19 and Cancer Task Force in the journal Cancer Discovery. Hematologic malignancies conferred an especially high risk.
“Any delay in vaccine access will result in loss of life that could be prevented with earlier access to vaccination,” AACR President Antoni Ribas, MD, told this news organization at the time.
There are also sound epidemiologic reasons to prioritize high-risk cancer patients for the COVID-19 vaccine, Dr. Wherry said in an interview. “What we do in infectious disease is to think about where your transmission and your risks are highest,” he said, citing cancer treatment centers as examples.
People with hematologic malignancies also tend to be long-term viral shedders, he said, putting caregivers and health care staff at increased risk. “There’s a big, big impact [in vaccinating cancer patients] and the numbers are not small.”
The CDC’s Jan. 1 recommendation is that patients with cancer should be assigned to priority group 1c, along with other “persons aged 16-64 with other high-risk medical conditions.”
However, more recent guidance from the NCCN hastened the urgency, advising that “patients with cancer should be assigned to the [CDC] priority group 1b/c.”
Out of 16 states that currently prioritize patients with cancer, 3 states have exceeded official advice, placing patients with cancer in priority group 1a. They opened their first batches of vaccine to everyone “deemed extremely vulnerable to COVID-19 by hospital providers” (Florida), or to “16-64 years old with a chronic health condition” (Mississippi) and to “persons aged 16-64 with high-risk conditions” (Pennsylvania, some jurisdictions).
However, despite these heroic intentions, no jurisdiction appears to have specifically tackled the thorny issue of subgroups of cancer that are more urgent than others, and this worries oncologists.
“Not all cancer patients are the same,” said Marina Garassino, MD, a medical oncologist at the National Tumor Institute of Milan. She shared registry data with the AACR panelists indicating that COVID-19 mortality in thoracic and hematologic malignancies rises to 30%-40%, compared with 13% for cancer overall.
At the AACR meeting, Dr. Ribas summed up his feelings on the issue: “It’s clear to me that patients with cancer should be prioritized. We have to then start defining this population and it should be the patient with an active cancer diagnosis undergoing treatment, in particular patients with lung cancers or hematologic malignancies.”
Since patients with cancer as a whole have problems getting timely vaccination – let alone someone with lung cancer or leukemia – the AACR meeting panelists grappled with solutions.
Dr. Cordovano said it was a “no brainer” to start with cancer centers. “Patients there are already registered, they have an account in the electronic health record system, they have insurance information, the care team knows them.”
Vaccination referrals sent directly from oncology centers would eliminate the need for the patient to provide verification or any additional documentation, she pointed out.
However, in New Jersey, cancer centers “have been completely excluded from the process,” she said.
Florida and New Hampshire have somewhat adopted the mechanism suggested by Dr. Cordovano. These states require health care providers to verify that a patient is “especially vulnerable” (Florida) or “medically vulnerable” (New Hampshire) in order for the patient to receive priority vaccine access. In New Hampshire, patients must have at least one other medical condition in addition to cancer to get on the list.
Jia Luo, MD, a medical oncology fellow at Memorial Sloan Kettering Cancer Center, New York, told the meeting that MSKCC has set up a proactive task force that sends “daily emails” to clinic staff highlighting patients eligible for the vaccine. “My sense is, it’s being prioritized to active cancer treatment,” said Dr. Luo. “All of our physicians are currently discussing [it] at each appointment and ... all of our nurses and staff have been talking to our patients on the phone.”
Dr. Cordovano, while advocating hard for cancer patients today, retained optimism about tomorrow: “This isn’t a long-term thing. This is just until things catch up. We knew we were going to have this problem.” Her hope is that, within 6 months, COVID-19 vaccination will become a standard of care in cancer.
Dr. Wherry agreed: “It’s going to take time to catch up with how far behind we are on certain things. ... What we’re seeing is a healthy debate rather than something that we should be concerned about – as long as that debate leads to rapid action.”
“We have to follow the science,” concluded Cordovano. “We can do better than this.”
Dr. Cordovano, Ms. Feldman, and Dr. Wherry have disclosed no relevant financial relationships. Dr. Luo declared a financial relationship with Targeted Oncology. Dr. Ribas declared financial relationships with 4C Biomed, Advaxis, Agilent, Amgen, AstraZeneca, Arcus, Bristol-Myers Squibb, and Kite-Gilead.
A version of this article first appeared on Medscape.com.
Every day, around 1.5 million doses of the COVID-19 vaccine are being delivered across the United States, but oncologists and patient advocates say that patients with cancer are missing out.
While official bodies recommend that patients with cancer are given priority, only 16 states currently prioritize them in the vaccine rollout. The other 34 states have thus far not singled out patients with cancer for earlier vaccination.
This flies in the face of recommendations from heavy hitters such as the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the National Comprehensive Cancer Network, and the American Association for Cancer Research.
All are in agreement: Patients on active cancer treatment should be prioritized for available vaccine because of their greater risk of death or complications from SARS-CoV-2 infection.
“All municipalities, states, cities, and even individual hospitals have so far been left to their own devices to try to figure out what the best way to do this is and that often conflicts with other recommendations or guidelines,” said E. John Wherry, PhD, chair of the department of systems pharmacology and translational therapeutics at the University of Pennsylvania, Philadelphia.
Dr. Wherry was on a panel at an AACR conference last week that discussed the failings of vaccine delivery to cancer patients.
During the meeting, lung cancer advocate Jill Feldman commented on the situation in Chicago, one of the jurisdictions that has not prioritized patients with cancer: “People don’t know what to do. ‘Do I need to sign up myself somewhere? Is my doctor’s office going to contact me?’ ”
Ms. Feldman said many people have called their cancer centers, “but cancer centers aren’t really providing updates directly to us. And they aren’t because they don’t have the information [either].”
Even in the 16 states that have ushered patients with cancer to the front of the line, the process for flagging these individuals is often unclear or nonexistent.
“Everyone that registers is basically on the same playing field ... because there’s no verification process. That’s very unfortunate,” said patient advocate Grace Cordovano, PhD, describing the vaccine sign-up process in New Jersey.
“It’s an easy fix,” said Dr. Cordovano. “Adding a few more fields [in the form] could really make a difference.”
COVID-19 fatality rates are twice as high in people with cancer than in people without cancer, according to a review published in December 2020 by the AACR’s COVID-19 and Cancer Task Force in the journal Cancer Discovery. Hematologic malignancies conferred an especially high risk.
“Any delay in vaccine access will result in loss of life that could be prevented with earlier access to vaccination,” AACR President Antoni Ribas, MD, told this news organization at the time.
There are also sound epidemiologic reasons to prioritize high-risk cancer patients for the COVID-19 vaccine, Dr. Wherry said in an interview. “What we do in infectious disease is to think about where your transmission and your risks are highest,” he said, citing cancer treatment centers as examples.
People with hematologic malignancies also tend to be long-term viral shedders, he said, putting caregivers and health care staff at increased risk. “There’s a big, big impact [in vaccinating cancer patients] and the numbers are not small.”
The CDC’s Jan. 1 recommendation is that patients with cancer should be assigned to priority group 1c, along with other “persons aged 16-64 with other high-risk medical conditions.”
However, more recent guidance from the NCCN hastened the urgency, advising that “patients with cancer should be assigned to the [CDC] priority group 1b/c.”
Out of 16 states that currently prioritize patients with cancer, 3 states have exceeded official advice, placing patients with cancer in priority group 1a. They opened their first batches of vaccine to everyone “deemed extremely vulnerable to COVID-19 by hospital providers” (Florida), or to “16-64 years old with a chronic health condition” (Mississippi) and to “persons aged 16-64 with high-risk conditions” (Pennsylvania, some jurisdictions).
However, despite these heroic intentions, no jurisdiction appears to have specifically tackled the thorny issue of subgroups of cancer that are more urgent than others, and this worries oncologists.
“Not all cancer patients are the same,” said Marina Garassino, MD, a medical oncologist at the National Tumor Institute of Milan. She shared registry data with the AACR panelists indicating that COVID-19 mortality in thoracic and hematologic malignancies rises to 30%-40%, compared with 13% for cancer overall.
At the AACR meeting, Dr. Ribas summed up his feelings on the issue: “It’s clear to me that patients with cancer should be prioritized. We have to then start defining this population and it should be the patient with an active cancer diagnosis undergoing treatment, in particular patients with lung cancers or hematologic malignancies.”
Since patients with cancer as a whole have problems getting timely vaccination – let alone someone with lung cancer or leukemia – the AACR meeting panelists grappled with solutions.
Dr. Cordovano said it was a “no brainer” to start with cancer centers. “Patients there are already registered, they have an account in the electronic health record system, they have insurance information, the care team knows them.”
Vaccination referrals sent directly from oncology centers would eliminate the need for the patient to provide verification or any additional documentation, she pointed out.
However, in New Jersey, cancer centers “have been completely excluded from the process,” she said.
Florida and New Hampshire have somewhat adopted the mechanism suggested by Dr. Cordovano. These states require health care providers to verify that a patient is “especially vulnerable” (Florida) or “medically vulnerable” (New Hampshire) in order for the patient to receive priority vaccine access. In New Hampshire, patients must have at least one other medical condition in addition to cancer to get on the list.
Jia Luo, MD, a medical oncology fellow at Memorial Sloan Kettering Cancer Center, New York, told the meeting that MSKCC has set up a proactive task force that sends “daily emails” to clinic staff highlighting patients eligible for the vaccine. “My sense is, it’s being prioritized to active cancer treatment,” said Dr. Luo. “All of our physicians are currently discussing [it] at each appointment and ... all of our nurses and staff have been talking to our patients on the phone.”
Dr. Cordovano, while advocating hard for cancer patients today, retained optimism about tomorrow: “This isn’t a long-term thing. This is just until things catch up. We knew we were going to have this problem.” Her hope is that, within 6 months, COVID-19 vaccination will become a standard of care in cancer.
Dr. Wherry agreed: “It’s going to take time to catch up with how far behind we are on certain things. ... What we’re seeing is a healthy debate rather than something that we should be concerned about – as long as that debate leads to rapid action.”
“We have to follow the science,” concluded Cordovano. “We can do better than this.”
Dr. Cordovano, Ms. Feldman, and Dr. Wherry have disclosed no relevant financial relationships. Dr. Luo declared a financial relationship with Targeted Oncology. Dr. Ribas declared financial relationships with 4C Biomed, Advaxis, Agilent, Amgen, AstraZeneca, Arcus, Bristol-Myers Squibb, and Kite-Gilead.
A version of this article first appeared on Medscape.com.
Every day, around 1.5 million doses of the COVID-19 vaccine are being delivered across the United States, but oncologists and patient advocates say that patients with cancer are missing out.
While official bodies recommend that patients with cancer are given priority, only 16 states currently prioritize them in the vaccine rollout. The other 34 states have thus far not singled out patients with cancer for earlier vaccination.
This flies in the face of recommendations from heavy hitters such as the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices, the National Comprehensive Cancer Network, and the American Association for Cancer Research.
All are in agreement: Patients on active cancer treatment should be prioritized for available vaccine because of their greater risk of death or complications from SARS-CoV-2 infection.
“All municipalities, states, cities, and even individual hospitals have so far been left to their own devices to try to figure out what the best way to do this is and that often conflicts with other recommendations or guidelines,” said E. John Wherry, PhD, chair of the department of systems pharmacology and translational therapeutics at the University of Pennsylvania, Philadelphia.
Dr. Wherry was on a panel at an AACR conference last week that discussed the failings of vaccine delivery to cancer patients.
During the meeting, lung cancer advocate Jill Feldman commented on the situation in Chicago, one of the jurisdictions that has not prioritized patients with cancer: “People don’t know what to do. ‘Do I need to sign up myself somewhere? Is my doctor’s office going to contact me?’ ”
Ms. Feldman said many people have called their cancer centers, “but cancer centers aren’t really providing updates directly to us. And they aren’t because they don’t have the information [either].”
Even in the 16 states that have ushered patients with cancer to the front of the line, the process for flagging these individuals is often unclear or nonexistent.
“Everyone that registers is basically on the same playing field ... because there’s no verification process. That’s very unfortunate,” said patient advocate Grace Cordovano, PhD, describing the vaccine sign-up process in New Jersey.
“It’s an easy fix,” said Dr. Cordovano. “Adding a few more fields [in the form] could really make a difference.”
COVID-19 fatality rates are twice as high in people with cancer than in people without cancer, according to a review published in December 2020 by the AACR’s COVID-19 and Cancer Task Force in the journal Cancer Discovery. Hematologic malignancies conferred an especially high risk.
“Any delay in vaccine access will result in loss of life that could be prevented with earlier access to vaccination,” AACR President Antoni Ribas, MD, told this news organization at the time.
There are also sound epidemiologic reasons to prioritize high-risk cancer patients for the COVID-19 vaccine, Dr. Wherry said in an interview. “What we do in infectious disease is to think about where your transmission and your risks are highest,” he said, citing cancer treatment centers as examples.
People with hematologic malignancies also tend to be long-term viral shedders, he said, putting caregivers and health care staff at increased risk. “There’s a big, big impact [in vaccinating cancer patients] and the numbers are not small.”
The CDC’s Jan. 1 recommendation is that patients with cancer should be assigned to priority group 1c, along with other “persons aged 16-64 with other high-risk medical conditions.”
However, more recent guidance from the NCCN hastened the urgency, advising that “patients with cancer should be assigned to the [CDC] priority group 1b/c.”
Out of 16 states that currently prioritize patients with cancer, 3 states have exceeded official advice, placing patients with cancer in priority group 1a. They opened their first batches of vaccine to everyone “deemed extremely vulnerable to COVID-19 by hospital providers” (Florida), or to “16-64 years old with a chronic health condition” (Mississippi) and to “persons aged 16-64 with high-risk conditions” (Pennsylvania, some jurisdictions).
However, despite these heroic intentions, no jurisdiction appears to have specifically tackled the thorny issue of subgroups of cancer that are more urgent than others, and this worries oncologists.
“Not all cancer patients are the same,” said Marina Garassino, MD, a medical oncologist at the National Tumor Institute of Milan. She shared registry data with the AACR panelists indicating that COVID-19 mortality in thoracic and hematologic malignancies rises to 30%-40%, compared with 13% for cancer overall.
At the AACR meeting, Dr. Ribas summed up his feelings on the issue: “It’s clear to me that patients with cancer should be prioritized. We have to then start defining this population and it should be the patient with an active cancer diagnosis undergoing treatment, in particular patients with lung cancers or hematologic malignancies.”
Since patients with cancer as a whole have problems getting timely vaccination – let alone someone with lung cancer or leukemia – the AACR meeting panelists grappled with solutions.
Dr. Cordovano said it was a “no brainer” to start with cancer centers. “Patients there are already registered, they have an account in the electronic health record system, they have insurance information, the care team knows them.”
Vaccination referrals sent directly from oncology centers would eliminate the need for the patient to provide verification or any additional documentation, she pointed out.
However, in New Jersey, cancer centers “have been completely excluded from the process,” she said.
Florida and New Hampshire have somewhat adopted the mechanism suggested by Dr. Cordovano. These states require health care providers to verify that a patient is “especially vulnerable” (Florida) or “medically vulnerable” (New Hampshire) in order for the patient to receive priority vaccine access. In New Hampshire, patients must have at least one other medical condition in addition to cancer to get on the list.
Jia Luo, MD, a medical oncology fellow at Memorial Sloan Kettering Cancer Center, New York, told the meeting that MSKCC has set up a proactive task force that sends “daily emails” to clinic staff highlighting patients eligible for the vaccine. “My sense is, it’s being prioritized to active cancer treatment,” said Dr. Luo. “All of our physicians are currently discussing [it] at each appointment and ... all of our nurses and staff have been talking to our patients on the phone.”
Dr. Cordovano, while advocating hard for cancer patients today, retained optimism about tomorrow: “This isn’t a long-term thing. This is just until things catch up. We knew we were going to have this problem.” Her hope is that, within 6 months, COVID-19 vaccination will become a standard of care in cancer.
Dr. Wherry agreed: “It’s going to take time to catch up with how far behind we are on certain things. ... What we’re seeing is a healthy debate rather than something that we should be concerned about – as long as that debate leads to rapid action.”
“We have to follow the science,” concluded Cordovano. “We can do better than this.”
Dr. Cordovano, Ms. Feldman, and Dr. Wherry have disclosed no relevant financial relationships. Dr. Luo declared a financial relationship with Targeted Oncology. Dr. Ribas declared financial relationships with 4C Biomed, Advaxis, Agilent, Amgen, AstraZeneca, Arcus, Bristol-Myers Squibb, and Kite-Gilead.
A version of this article first appeared on Medscape.com.