User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Survey finds European dermatologists unhappy with pandemic teledermatology experience
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
intensely, according to the findings of a survey presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“The results of our survey clearly show 7 out of 10 participating dermatologists declared that they were not happy with teledermatology, and most of them declared that they were not at all happy,” according to Mariano Suppa, MD, PhD, of the department of dermatology and venereology, Free University of Brussels.
“It was very interesting: it was not just about the lack of a good quality of consultation, but was also related to some extent to a lack of respect from some patients, and also a lack of empathy. The majority of survey respondents felt [attacked] by their own patients because they were proposing teledermatology. So, yes, we were forced to go to teledermatology, and I think we will be again to some extent, but clearly we’re not happy about it,” he elaborated in response to a question from session chair Brigitte Dreno, MD, professor of dermatology and vice dean of the faculty of medicine at the University of Nantes (France).
The survey, conducted by the EADV communication committee, assessed the pandemic’s impact on European dermatologists’ professional practices and personal lives through 30 brief questions, with space at the end for additional open-ended comments. In the comments section, many dermatologists vented about their income loss, the disorganized response to round one of the pandemic, and most of all about teledermatology. Common complaints were that teledermatology required a huge consumption of energy and constituted a major intrusion upon the physicians’ personal lives. And then there was the common theme of unkind treatment by some patients.
The survey was sent twice in June 2020 to more than 4,800 EADV members. It was completed by 490 dermatologists from 39 countries. Dr. Suppa attributed the low response rate to physician weariness of the topic due to saturation news media coverage of the pandemic.
Sixty-nine percent of responding dermatologists were women. Fifty-two percent of participants were over age 50, 81% lived in a city, and 53% worked in a university or public hospital or clinic. Twelve percent lived alone.
Impact on professional practice
Many European dermatologists were on the front lines in dealing with the first wave of COVID-19. Twenty-eight percent worked in a COVID-19 unit. Forty-eight percent of dermatologists performed COVID-19 tests, and those who didn’t either had no patient contact or couldn’t get test kits. Thirty-five percent of dermatologists saw patients who presented with skin signs of COVID-19. Four percent of survey respondents became infected.
Seventy percent rescheduled or canceled all or most patient appointments. Clinical care was prioritized: during the peak of the pandemic, 76% of dermatologists saw only urgent cases – mostly potentially serious rashes – and dermato-oncology patients. Seventy-six percent of dermatologists performed teledermatology, although by June 60% of respondents reported seeing at least three-quarters of their patients face-to-face.
Twenty-three percent of dermatologists reported having lost most or all of their income during March through June, and another 26% lost about half.
Impact on dermatologists’ personal lives
About half of survey respondents reported feeling stressed, and a similar percentage checked the box marked ‘anxiety.’ Nine percent reported depressive symptoms, 15% mentioned feeling anger, 17% uselessness, and 2% admitted suicidal ideation. But 30% of dermatologists reported experiencing no negative psychological effects whatsoever stemming from the pandemic.
Sixteen percent of dermatologists reported drinking more alcohol during sequestration.
But respondents cited positive effects as well: a renewed appreciation of the importance of time, and enjoyment of the additional time spent with family and alone. Many dermatologists relished the opportunity to spend more time cooking, reading literature, doing research, listening to or playing music, and practicing yoga or meditation. And dermatologists took solace and pride in being members of the vital medical community.
Dr. Dreno asked if the survey revealed evidence of underdiagnosis and undertreatment of dermatologic diseases during the pandemic. Dr. Suppa replied that the survey didn’t address that issue, but it’s his personal opinion that this was no doubt the case. Roughly one-quarter of dermatologists canceled all appointments, and when dermatology clinics became filled beginning in June, he and his colleagues saw a number of cases of delayed-diagnosis advanced skin cancer.
“I think that the diseases that were really penalized were the chronic inflammatory diseases, such as psoriasis, hidradenitis suppurativa, and also atopic dermatitis. We were doing a lot of telephone consultations for those patients at that time, and we saw in June that for those particular patients there was an unmet need in the pandemic because some of them really needed to have been seen. I think this is a lesson we should learn for the second wave that we’re unfortunately facing right now: We need to adopt restrictive measures to avoid spreading the pandemic, yes, for sure, but we need to keep in mind that there is not just COVID-19, but also other important diseases,” Dr. Suppa said.
A second EADV survey will be performed during the fall/winter wave of the pandemic.
Dr. Suppa reported having no financial conflicts regarding the EADV-funded survey.
SOURCE: Suppa M. EADV 2020. Presentation D3T03.4D
FROM THE EADV CONGRESS
COVID-19: U.S. sets new weekly high in children
the American Academy of Pediatrics announced Nov. 2.
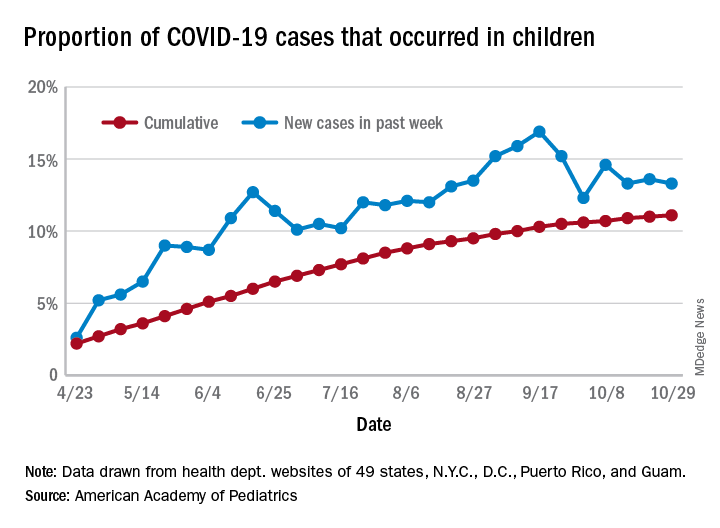
For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
the American Academy of Pediatrics announced Nov. 2.

For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
the American Academy of Pediatrics announced Nov. 2.

For the week, over 61,000 cases were reported in children, bringing the number of COVID-19 cases for the month of October to nearly 200,000 and the total since the start of the pandemic to over 853,000, the AAP and the Children’s Hospital Association said in their weekly report.
“These numbers reflect a disturbing increase in cases throughout most of the United States in all populations, especially among young adults,” Yvonne Maldonado, MD, chair of the AAP Committee on Infectious Diseases, said in a separate statement. “We are entering a heightened wave of infections around the country. We would encourage family holiday gatherings to be avoided if possible, especially if there are high-risk individuals in the household.”
For the week ending Oct. 29, children represented 13.3% of all cases, possibly constituting a minitrend of stability over the past 3 weeks. For the full length of the pandemic, 11.1% of all COVID-19 cases have occurred in children, although severe illness is much less common: 1.7% of all hospitalizations (data from 24 states and New York City) and 0.06% of all deaths (data from 42 states and New York City), the AAP and CHA report said.
Other data show that 1,134 per 100,000 children in the United States have been infected by the coronavirus, up from 1,053 the previous week, with state rates ranging from 221 per 100,000 in Vermont to 3,321 in North Dakota. In Wyoming, 25.5% of all COVID-19 cases have occurred in children, the highest of any state, while New Jersey has the lowest rate at 4.9%, the AAP/CHA report showed.
In the 10 states making testing data available, children represent the lowest percentage of tests in Iowa (5.0%) and the highest in Indiana (16.9%). Iowa, however, has the highest positivity rate for children at 14.6%, along with Nevada, while West Virginia has the lowest at 3.6%, the AAP and CHA said in the report.
These numbers, however, may not be telling the whole story. “The number of reported COVID-19 cases in children is likely an undercount because children’s symptoms are often mild and they may not be tested for every illness,” the AAP said in its statement.
“We urge policy makers to listen to doctors and public health experts rather than level baseless accusations against them. Physicians, nurses and other health care professionals have put their lives on the line to protect our communities. We can all do our part to protect them, and our communities, by wearing masks, practicing physical distancing, and getting our flu immunizations,” AAP President Sally Goza, MD, said in the AAP statement.
HF an added risk in COVID-19, regardless of ejection fraction
People with a history of heart failure – no matter the type – face more complications and death than their peers without HF once hospitalized with COVID-19, a new observational study shows.
A history of HF was associated with a near doubling risk of in-hospital mortality and ICU care and more than a tripling risk of mechanical ventilation despite adjustment for 18 factors including race, obesity, diabetes, previous treatment with renin-angiotensin-aldosterone system (RAAS) inhibitors, and severity of illness.
Adverse outcomes were high regardless of whether patients had HF with a preserved, mid-range, or reduced left ventricular ejection fraction (HFpEF/HFmrEF/HFrEF).
“That for me was the real zinger,” senior author Anuradha Lala, MD, said in an interview . “Because as clinicians, oftentimes, and wrongly so, we think this person has preserved ejection fraction, so they’re not needing my heart failure expertise as much as someone with heart failure with reduced ejection fraction.”
In the peak of the pandemic, that may have meant triaging patients with HFpEF to a regular floor, whereas those with HFrEF were seen by the specialist team.
“What this alerted me to is to take heart failure as a diagnosis very seriously, regardless of ejection fraction, and that is very much in line with all of the emerging data about heart failure with preserved ejection fraction,” said Dr. Lala, from the Icahn School of Medicine at Mount Sinai, New York.
“Now when I see patients in the clinic, I incorporate part of our visit to talking about what they are doing to prevent COVID, which I really wasn’t doing before. It was like ‘Oh yeah, what crazy times we’re dealing with’ and then addressing their heart failure as I normally would,” she said. “But now, interwoven into every visit is: Are you wearing a mask, what’s your social distancing policy, who are you living with at home, has anyone at home or who you’ve interacted with been sick? I’m asking those questions just as a knee-jerk reaction for these patients because I know the repercussions. We have to keep in mind these are observational studies, so I can’t prove causality but these are observations that are, nonetheless, quite robust.”
Although cardiovascular disease, including HF, is recognized as a risk factor for worse outcomes in COVID-19 patients, data are sparse on the clinical course and prognosis of patients with preexisting HF.
“I would have expected that there would have been a gradation of risk from the people with very low ejection fractions up into the normal range, but here it didn’t seem to matter at all. So that’s an important point that bad outcomes were independent of ejection fraction,” commented Lee Goldberg, MD, professor of medicine and chief of advanced heart failure and cardiac transplant at the University of Pennsylvania, Philadelphia.
The study also validated that there is no association between use of RAAS inhibitors and bad outcomes in patients with COVID-19, he said.
Although this has been demonstrated in several studies, concerns were raised early in the pandemic that ACE inhibitors and angiotensin receptor blockers could facilitate infection with SARS-CoV-2 and increase the risk of severe or lethal COVID-19.
“For most clinicians that question has been put to bed, but we’re still getting patients that will ask during office visits ‘Is it safe for me to stay on?’ They still have that doubt [about] ‘Are we doing the right thing?’ ” Dr. Goldberg said.
“We can reassure them now. A lot of us are able to say there’s nothing to that, we’re very clear about this, stay on the meds. If anything, there’s data that suggest actually it may be better to be on an ACE inhibitor; that the hospitalizations were shorter and the outcomes were a little bit better.”
For the current study, published online Oct. 28 in the Journal of the American College of Cardiology, the investigators analyzed 6,439 patients admitted for COVID-19 at one of five Mount Sinai Health System hospitals in New York between Feb. 27 and June 26. Their mean age was 65.3 years, 45% were women, and one-third were treated with RAAS inhibitors before admission.
Using ICD-9/10 codes and individual chart review, HF was identified in 422 patients (6.6%), of which 250 patients had HFpEF (≥50%), 44 had HFmrEF (41%-49%), and 128 had HFrEF (≤40%).
Patients with HFpEF were older, more frequently women with a higher body mass index and history of lung disease than patients with HFrEF, whereas those with HFmrEF fell in between.
The HFpEF group was also treated with hydroxychloroquine or macrolides and noninvasive ventilation more frequently than the other two groups, whereas antiplatelet and neurohormonal therapies were more common in the HFrEF group.
Patients with a history of HF had significantly longer hospital stays than those without HF (8 days vs. 6 days), increased need for intubation (22.8% vs. 11.9%) and ICU care (23.2% vs. 16.6%), and worse in-hospital mortality (40% vs. 24.9%).
After multivariable regression adjustment, HF persisted as an independent risk factor for ICU care (odds ratio, 1.71; 95% CI, 1.25-2.34), intubation and mechanical ventilation (OR, 3.64; 95% CI, 2.56-5.16), and in-hospital mortality (OR, 1.88; 95% CI, 1.27-2.78).
“I knew to expect higher rates of adverse outcomes but I didn’t expect it to be nearly a twofold increase,” Dr. Lala said. “I thought that was pretty powerful.”
No significant differences were seen across LVEF categories in length of stay, need for ICU care, intubation and mechanical ventilation, acute kidney injury, shock, thromboembolic events, arrhythmias, or 30-day readmission rates.
However, cardiogenic shock (7.8% vs. 2.3% vs. 2%) and HF-related causes for 30-day readmissions (47.1% vs. 0% vs. 8.6%) were significantly higher in patients with HFrEF than in those with HFmrEF or HFpEF.
Also, mortality was lower in those with HFmrEF (22.7%) than with HFrEF (38.3%) and HFpEF (44%). The group was small but the “results suggested that patients with HFmrEF could have a better prognosis, because they can represent a distinct and more favorable HF phenotype,” the authors wrote.
The statistical testing didn’t show much difference and the patient numbers were very small, noted Dr. Goldberg. “So they might be overreaching a little bit there.”
“To me, the take-home message is that just having the phenotype of heart failure, regardless of EF, is associated with bad outcomes and we need to be vigilant on two fronts,” he said. “We really need to be doing prevention in the folks with heart failure because if they get COVID their outcomes are not going to be as good. Second, as clinicians, if we see a patient presenting with COVID who has a history of heart failure we may want to be much more vigilant with that individual than we might otherwise be. So I think there’s something to be said for kind of risk-stratifying people in that way.”
Dr. Goldberg pointed out that the study had many “amazing strengths,” including a large, racially diverse population, direct chart review to identify HF patients, and capturing a patient’s specific HF phenotype.
Weaknesses are that it was a single-center study, so the biases of how these patients were treated are not easily controlled for, he said. “We also don’t know when the hospital system was very strained as they were making some decisions: Were the older patients who had advanced heart and lung disease ultimately less aggressively treated because they felt they wouldn’t survive?”
Dr. Lala has received personal fees from Zoll, outside the submitted work. Dr. Goldberg reported research funding with Respicardia and consulting fees from Abbott.
This article first appeared on Medscape.com.
People with a history of heart failure – no matter the type – face more complications and death than their peers without HF once hospitalized with COVID-19, a new observational study shows.
A history of HF was associated with a near doubling risk of in-hospital mortality and ICU care and more than a tripling risk of mechanical ventilation despite adjustment for 18 factors including race, obesity, diabetes, previous treatment with renin-angiotensin-aldosterone system (RAAS) inhibitors, and severity of illness.
Adverse outcomes were high regardless of whether patients had HF with a preserved, mid-range, or reduced left ventricular ejection fraction (HFpEF/HFmrEF/HFrEF).
“That for me was the real zinger,” senior author Anuradha Lala, MD, said in an interview . “Because as clinicians, oftentimes, and wrongly so, we think this person has preserved ejection fraction, so they’re not needing my heart failure expertise as much as someone with heart failure with reduced ejection fraction.”
In the peak of the pandemic, that may have meant triaging patients with HFpEF to a regular floor, whereas those with HFrEF were seen by the specialist team.
“What this alerted me to is to take heart failure as a diagnosis very seriously, regardless of ejection fraction, and that is very much in line with all of the emerging data about heart failure with preserved ejection fraction,” said Dr. Lala, from the Icahn School of Medicine at Mount Sinai, New York.
“Now when I see patients in the clinic, I incorporate part of our visit to talking about what they are doing to prevent COVID, which I really wasn’t doing before. It was like ‘Oh yeah, what crazy times we’re dealing with’ and then addressing their heart failure as I normally would,” she said. “But now, interwoven into every visit is: Are you wearing a mask, what’s your social distancing policy, who are you living with at home, has anyone at home or who you’ve interacted with been sick? I’m asking those questions just as a knee-jerk reaction for these patients because I know the repercussions. We have to keep in mind these are observational studies, so I can’t prove causality but these are observations that are, nonetheless, quite robust.”
Although cardiovascular disease, including HF, is recognized as a risk factor for worse outcomes in COVID-19 patients, data are sparse on the clinical course and prognosis of patients with preexisting HF.
“I would have expected that there would have been a gradation of risk from the people with very low ejection fractions up into the normal range, but here it didn’t seem to matter at all. So that’s an important point that bad outcomes were independent of ejection fraction,” commented Lee Goldberg, MD, professor of medicine and chief of advanced heart failure and cardiac transplant at the University of Pennsylvania, Philadelphia.
The study also validated that there is no association between use of RAAS inhibitors and bad outcomes in patients with COVID-19, he said.
Although this has been demonstrated in several studies, concerns were raised early in the pandemic that ACE inhibitors and angiotensin receptor blockers could facilitate infection with SARS-CoV-2 and increase the risk of severe or lethal COVID-19.
“For most clinicians that question has been put to bed, but we’re still getting patients that will ask during office visits ‘Is it safe for me to stay on?’ They still have that doubt [about] ‘Are we doing the right thing?’ ” Dr. Goldberg said.
“We can reassure them now. A lot of us are able to say there’s nothing to that, we’re very clear about this, stay on the meds. If anything, there’s data that suggest actually it may be better to be on an ACE inhibitor; that the hospitalizations were shorter and the outcomes were a little bit better.”
For the current study, published online Oct. 28 in the Journal of the American College of Cardiology, the investigators analyzed 6,439 patients admitted for COVID-19 at one of five Mount Sinai Health System hospitals in New York between Feb. 27 and June 26. Their mean age was 65.3 years, 45% were women, and one-third were treated with RAAS inhibitors before admission.
Using ICD-9/10 codes and individual chart review, HF was identified in 422 patients (6.6%), of which 250 patients had HFpEF (≥50%), 44 had HFmrEF (41%-49%), and 128 had HFrEF (≤40%).
Patients with HFpEF were older, more frequently women with a higher body mass index and history of lung disease than patients with HFrEF, whereas those with HFmrEF fell in between.
The HFpEF group was also treated with hydroxychloroquine or macrolides and noninvasive ventilation more frequently than the other two groups, whereas antiplatelet and neurohormonal therapies were more common in the HFrEF group.
Patients with a history of HF had significantly longer hospital stays than those without HF (8 days vs. 6 days), increased need for intubation (22.8% vs. 11.9%) and ICU care (23.2% vs. 16.6%), and worse in-hospital mortality (40% vs. 24.9%).
After multivariable regression adjustment, HF persisted as an independent risk factor for ICU care (odds ratio, 1.71; 95% CI, 1.25-2.34), intubation and mechanical ventilation (OR, 3.64; 95% CI, 2.56-5.16), and in-hospital mortality (OR, 1.88; 95% CI, 1.27-2.78).
“I knew to expect higher rates of adverse outcomes but I didn’t expect it to be nearly a twofold increase,” Dr. Lala said. “I thought that was pretty powerful.”
No significant differences were seen across LVEF categories in length of stay, need for ICU care, intubation and mechanical ventilation, acute kidney injury, shock, thromboembolic events, arrhythmias, or 30-day readmission rates.
However, cardiogenic shock (7.8% vs. 2.3% vs. 2%) and HF-related causes for 30-day readmissions (47.1% vs. 0% vs. 8.6%) were significantly higher in patients with HFrEF than in those with HFmrEF or HFpEF.
Also, mortality was lower in those with HFmrEF (22.7%) than with HFrEF (38.3%) and HFpEF (44%). The group was small but the “results suggested that patients with HFmrEF could have a better prognosis, because they can represent a distinct and more favorable HF phenotype,” the authors wrote.
The statistical testing didn’t show much difference and the patient numbers were very small, noted Dr. Goldberg. “So they might be overreaching a little bit there.”
“To me, the take-home message is that just having the phenotype of heart failure, regardless of EF, is associated with bad outcomes and we need to be vigilant on two fronts,” he said. “We really need to be doing prevention in the folks with heart failure because if they get COVID their outcomes are not going to be as good. Second, as clinicians, if we see a patient presenting with COVID who has a history of heart failure we may want to be much more vigilant with that individual than we might otherwise be. So I think there’s something to be said for kind of risk-stratifying people in that way.”
Dr. Goldberg pointed out that the study had many “amazing strengths,” including a large, racially diverse population, direct chart review to identify HF patients, and capturing a patient’s specific HF phenotype.
Weaknesses are that it was a single-center study, so the biases of how these patients were treated are not easily controlled for, he said. “We also don’t know when the hospital system was very strained as they were making some decisions: Were the older patients who had advanced heart and lung disease ultimately less aggressively treated because they felt they wouldn’t survive?”
Dr. Lala has received personal fees from Zoll, outside the submitted work. Dr. Goldberg reported research funding with Respicardia and consulting fees from Abbott.
This article first appeared on Medscape.com.
People with a history of heart failure – no matter the type – face more complications and death than their peers without HF once hospitalized with COVID-19, a new observational study shows.
A history of HF was associated with a near doubling risk of in-hospital mortality and ICU care and more than a tripling risk of mechanical ventilation despite adjustment for 18 factors including race, obesity, diabetes, previous treatment with renin-angiotensin-aldosterone system (RAAS) inhibitors, and severity of illness.
Adverse outcomes were high regardless of whether patients had HF with a preserved, mid-range, or reduced left ventricular ejection fraction (HFpEF/HFmrEF/HFrEF).
“That for me was the real zinger,” senior author Anuradha Lala, MD, said in an interview . “Because as clinicians, oftentimes, and wrongly so, we think this person has preserved ejection fraction, so they’re not needing my heart failure expertise as much as someone with heart failure with reduced ejection fraction.”
In the peak of the pandemic, that may have meant triaging patients with HFpEF to a regular floor, whereas those with HFrEF were seen by the specialist team.
“What this alerted me to is to take heart failure as a diagnosis very seriously, regardless of ejection fraction, and that is very much in line with all of the emerging data about heart failure with preserved ejection fraction,” said Dr. Lala, from the Icahn School of Medicine at Mount Sinai, New York.
“Now when I see patients in the clinic, I incorporate part of our visit to talking about what they are doing to prevent COVID, which I really wasn’t doing before. It was like ‘Oh yeah, what crazy times we’re dealing with’ and then addressing their heart failure as I normally would,” she said. “But now, interwoven into every visit is: Are you wearing a mask, what’s your social distancing policy, who are you living with at home, has anyone at home or who you’ve interacted with been sick? I’m asking those questions just as a knee-jerk reaction for these patients because I know the repercussions. We have to keep in mind these are observational studies, so I can’t prove causality but these are observations that are, nonetheless, quite robust.”
Although cardiovascular disease, including HF, is recognized as a risk factor for worse outcomes in COVID-19 patients, data are sparse on the clinical course and prognosis of patients with preexisting HF.
“I would have expected that there would have been a gradation of risk from the people with very low ejection fractions up into the normal range, but here it didn’t seem to matter at all. So that’s an important point that bad outcomes were independent of ejection fraction,” commented Lee Goldberg, MD, professor of medicine and chief of advanced heart failure and cardiac transplant at the University of Pennsylvania, Philadelphia.
The study also validated that there is no association between use of RAAS inhibitors and bad outcomes in patients with COVID-19, he said.
Although this has been demonstrated in several studies, concerns were raised early in the pandemic that ACE inhibitors and angiotensin receptor blockers could facilitate infection with SARS-CoV-2 and increase the risk of severe or lethal COVID-19.
“For most clinicians that question has been put to bed, but we’re still getting patients that will ask during office visits ‘Is it safe for me to stay on?’ They still have that doubt [about] ‘Are we doing the right thing?’ ” Dr. Goldberg said.
“We can reassure them now. A lot of us are able to say there’s nothing to that, we’re very clear about this, stay on the meds. If anything, there’s data that suggest actually it may be better to be on an ACE inhibitor; that the hospitalizations were shorter and the outcomes were a little bit better.”
For the current study, published online Oct. 28 in the Journal of the American College of Cardiology, the investigators analyzed 6,439 patients admitted for COVID-19 at one of five Mount Sinai Health System hospitals in New York between Feb. 27 and June 26. Their mean age was 65.3 years, 45% were women, and one-third were treated with RAAS inhibitors before admission.
Using ICD-9/10 codes and individual chart review, HF was identified in 422 patients (6.6%), of which 250 patients had HFpEF (≥50%), 44 had HFmrEF (41%-49%), and 128 had HFrEF (≤40%).
Patients with HFpEF were older, more frequently women with a higher body mass index and history of lung disease than patients with HFrEF, whereas those with HFmrEF fell in between.
The HFpEF group was also treated with hydroxychloroquine or macrolides and noninvasive ventilation more frequently than the other two groups, whereas antiplatelet and neurohormonal therapies were more common in the HFrEF group.
Patients with a history of HF had significantly longer hospital stays than those without HF (8 days vs. 6 days), increased need for intubation (22.8% vs. 11.9%) and ICU care (23.2% vs. 16.6%), and worse in-hospital mortality (40% vs. 24.9%).
After multivariable regression adjustment, HF persisted as an independent risk factor for ICU care (odds ratio, 1.71; 95% CI, 1.25-2.34), intubation and mechanical ventilation (OR, 3.64; 95% CI, 2.56-5.16), and in-hospital mortality (OR, 1.88; 95% CI, 1.27-2.78).
“I knew to expect higher rates of adverse outcomes but I didn’t expect it to be nearly a twofold increase,” Dr. Lala said. “I thought that was pretty powerful.”
No significant differences were seen across LVEF categories in length of stay, need for ICU care, intubation and mechanical ventilation, acute kidney injury, shock, thromboembolic events, arrhythmias, or 30-day readmission rates.
However, cardiogenic shock (7.8% vs. 2.3% vs. 2%) and HF-related causes for 30-day readmissions (47.1% vs. 0% vs. 8.6%) were significantly higher in patients with HFrEF than in those with HFmrEF or HFpEF.
Also, mortality was lower in those with HFmrEF (22.7%) than with HFrEF (38.3%) and HFpEF (44%). The group was small but the “results suggested that patients with HFmrEF could have a better prognosis, because they can represent a distinct and more favorable HF phenotype,” the authors wrote.
The statistical testing didn’t show much difference and the patient numbers were very small, noted Dr. Goldberg. “So they might be overreaching a little bit there.”
“To me, the take-home message is that just having the phenotype of heart failure, regardless of EF, is associated with bad outcomes and we need to be vigilant on two fronts,” he said. “We really need to be doing prevention in the folks with heart failure because if they get COVID their outcomes are not going to be as good. Second, as clinicians, if we see a patient presenting with COVID who has a history of heart failure we may want to be much more vigilant with that individual than we might otherwise be. So I think there’s something to be said for kind of risk-stratifying people in that way.”
Dr. Goldberg pointed out that the study had many “amazing strengths,” including a large, racially diverse population, direct chart review to identify HF patients, and capturing a patient’s specific HF phenotype.
Weaknesses are that it was a single-center study, so the biases of how these patients were treated are not easily controlled for, he said. “We also don’t know when the hospital system was very strained as they were making some decisions: Were the older patients who had advanced heart and lung disease ultimately less aggressively treated because they felt they wouldn’t survive?”
Dr. Lala has received personal fees from Zoll, outside the submitted work. Dr. Goldberg reported research funding with Respicardia and consulting fees from Abbott.
This article first appeared on Medscape.com.
Preemptive CMV monitoring beats prophylaxis post liver transplant
Preemptive monitoring and treatment of cytomegalovirus infections in CMV-seronegative liver transplant recipients who receive organs from CMV-positive donors appears to be better at preventing infections than a viral prophylaxis strategy, according to infectious disease and organ transplant specialists.
In a study published in JAMA that may have gotten scant notice because of its publication during the early days of the COVID-19 pandemic, investigators at the University of Pittsburgh and other transplant centers reported results of a randomized clinical trial comparing the two CMV management strategies, and found that the incidence of CMV disease was significantly lower for patients who were started on valganciclovir when asymptomatic CMV viremia was detected, compared with patients on antiviral prophylaxis with valganciclovir.
The study “is a significant game changer for the field of transplantation,” commented Michael G. Ison, MD, professor of infectious diseases and organ transplantation at Northwestern University, Chicago.
Dr. Ison discussed the study and its implications during a session on potentially practice-changing clinical trials presented virtually during IDWeek 2020, an annual scientific meeting on infectious diseases.
In the trial, Nina Singh, MD, and colleagues randomly assigned 100 CMV-seronegative liver transplant recipients to receive preemptive therapy, in which patients underwent weekly testing for 100 days with a highly sensitive real-time plasma polymerase chain reaction assay for CMV. If viremia at any level was detected, the patients received oral valganciclovir 900 mg twice daily until two consecutive tests performed 1 week apart came back negative.
The remaining 105 patients were randomly assigned to 100 days of oral prophylaxis with 900 mg valganciclovir twice daily, started within 10 days of transplant.
CMV disease incidence lower
The incidence of CMV disease within 12 months of transplants, the primary outcome, was 9% in the preemptive therapy group, compared with 19% in the prophylaxis group (P = .04)
The difference between the groups was largely accounted for by a reduction in disease onset beyond 100 days in the preemptive therapy group (6% vs. 17%, respectively, P = .01)
There were no significant differences in secondary endpoints of rejection, opportunistic infections, graft loss because of retransplantation, neutropenia, or receipt of one or more doses of granulocyte colony–stimulating factor for the management of neutropenia.
At 1-year follow-up, the incidence of all-cause mortality was 15% in the preemptive therapy group, and 19% in the prophylaxis group; the difference was not statistically significant.
“While most transplant centers utilize universal prophylaxis, I think that this study really suggests that preemptive monitoring, if it can be safely accomplished at your center, may be of the greatest benefit to your patients,” Dr. Ison said.
He noted that Singh et al. also looked in an exploratory analysis at CMV-specific immunity and observed that patients assigned to preemptive therapy “clearly had better CMV-specific immunity, whether CD4 or CD8 cells, and had higher lymphocyte numbers than those patients that had received universal prophylaxis.”
In a comment, Sarah Doernberg, MD, from the division of infectious diseases at the University of California, San Francisco, agreed that “exploratory analysis of CMV-specific immune responses suggested increased CMV-specific immunity in those in the preemptive group, a finding that warrants further study. The feasibility of adopting reliable preemptive monitoring must be considered as individual centers ponder adopting this approach.”
Dr. Doernberg moderated the session where Dr. Ison discussed the data, but was not involved in the research.
The study by Singh et al. was supported by the National Institutes of Health. Dr. Singh reported research grants from NIH. Dr. Ison disclosed research support and paid consultation for several companies. Dr. Doernberg disclosed consulting for Basilea and Genentech.
Preemptive monitoring and treatment of cytomegalovirus infections in CMV-seronegative liver transplant recipients who receive organs from CMV-positive donors appears to be better at preventing infections than a viral prophylaxis strategy, according to infectious disease and organ transplant specialists.
In a study published in JAMA that may have gotten scant notice because of its publication during the early days of the COVID-19 pandemic, investigators at the University of Pittsburgh and other transplant centers reported results of a randomized clinical trial comparing the two CMV management strategies, and found that the incidence of CMV disease was significantly lower for patients who were started on valganciclovir when asymptomatic CMV viremia was detected, compared with patients on antiviral prophylaxis with valganciclovir.
The study “is a significant game changer for the field of transplantation,” commented Michael G. Ison, MD, professor of infectious diseases and organ transplantation at Northwestern University, Chicago.
Dr. Ison discussed the study and its implications during a session on potentially practice-changing clinical trials presented virtually during IDWeek 2020, an annual scientific meeting on infectious diseases.
In the trial, Nina Singh, MD, and colleagues randomly assigned 100 CMV-seronegative liver transplant recipients to receive preemptive therapy, in which patients underwent weekly testing for 100 days with a highly sensitive real-time plasma polymerase chain reaction assay for CMV. If viremia at any level was detected, the patients received oral valganciclovir 900 mg twice daily until two consecutive tests performed 1 week apart came back negative.
The remaining 105 patients were randomly assigned to 100 days of oral prophylaxis with 900 mg valganciclovir twice daily, started within 10 days of transplant.
CMV disease incidence lower
The incidence of CMV disease within 12 months of transplants, the primary outcome, was 9% in the preemptive therapy group, compared with 19% in the prophylaxis group (P = .04)
The difference between the groups was largely accounted for by a reduction in disease onset beyond 100 days in the preemptive therapy group (6% vs. 17%, respectively, P = .01)
There were no significant differences in secondary endpoints of rejection, opportunistic infections, graft loss because of retransplantation, neutropenia, or receipt of one or more doses of granulocyte colony–stimulating factor for the management of neutropenia.
At 1-year follow-up, the incidence of all-cause mortality was 15% in the preemptive therapy group, and 19% in the prophylaxis group; the difference was not statistically significant.
“While most transplant centers utilize universal prophylaxis, I think that this study really suggests that preemptive monitoring, if it can be safely accomplished at your center, may be of the greatest benefit to your patients,” Dr. Ison said.
He noted that Singh et al. also looked in an exploratory analysis at CMV-specific immunity and observed that patients assigned to preemptive therapy “clearly had better CMV-specific immunity, whether CD4 or CD8 cells, and had higher lymphocyte numbers than those patients that had received universal prophylaxis.”
In a comment, Sarah Doernberg, MD, from the division of infectious diseases at the University of California, San Francisco, agreed that “exploratory analysis of CMV-specific immune responses suggested increased CMV-specific immunity in those in the preemptive group, a finding that warrants further study. The feasibility of adopting reliable preemptive monitoring must be considered as individual centers ponder adopting this approach.”
Dr. Doernberg moderated the session where Dr. Ison discussed the data, but was not involved in the research.
The study by Singh et al. was supported by the National Institutes of Health. Dr. Singh reported research grants from NIH. Dr. Ison disclosed research support and paid consultation for several companies. Dr. Doernberg disclosed consulting for Basilea and Genentech.
Preemptive monitoring and treatment of cytomegalovirus infections in CMV-seronegative liver transplant recipients who receive organs from CMV-positive donors appears to be better at preventing infections than a viral prophylaxis strategy, according to infectious disease and organ transplant specialists.
In a study published in JAMA that may have gotten scant notice because of its publication during the early days of the COVID-19 pandemic, investigators at the University of Pittsburgh and other transplant centers reported results of a randomized clinical trial comparing the two CMV management strategies, and found that the incidence of CMV disease was significantly lower for patients who were started on valganciclovir when asymptomatic CMV viremia was detected, compared with patients on antiviral prophylaxis with valganciclovir.
The study “is a significant game changer for the field of transplantation,” commented Michael G. Ison, MD, professor of infectious diseases and organ transplantation at Northwestern University, Chicago.
Dr. Ison discussed the study and its implications during a session on potentially practice-changing clinical trials presented virtually during IDWeek 2020, an annual scientific meeting on infectious diseases.
In the trial, Nina Singh, MD, and colleagues randomly assigned 100 CMV-seronegative liver transplant recipients to receive preemptive therapy, in which patients underwent weekly testing for 100 days with a highly sensitive real-time plasma polymerase chain reaction assay for CMV. If viremia at any level was detected, the patients received oral valganciclovir 900 mg twice daily until two consecutive tests performed 1 week apart came back negative.
The remaining 105 patients were randomly assigned to 100 days of oral prophylaxis with 900 mg valganciclovir twice daily, started within 10 days of transplant.
CMV disease incidence lower
The incidence of CMV disease within 12 months of transplants, the primary outcome, was 9% in the preemptive therapy group, compared with 19% in the prophylaxis group (P = .04)
The difference between the groups was largely accounted for by a reduction in disease onset beyond 100 days in the preemptive therapy group (6% vs. 17%, respectively, P = .01)
There were no significant differences in secondary endpoints of rejection, opportunistic infections, graft loss because of retransplantation, neutropenia, or receipt of one or more doses of granulocyte colony–stimulating factor for the management of neutropenia.
At 1-year follow-up, the incidence of all-cause mortality was 15% in the preemptive therapy group, and 19% in the prophylaxis group; the difference was not statistically significant.
“While most transplant centers utilize universal prophylaxis, I think that this study really suggests that preemptive monitoring, if it can be safely accomplished at your center, may be of the greatest benefit to your patients,” Dr. Ison said.
He noted that Singh et al. also looked in an exploratory analysis at CMV-specific immunity and observed that patients assigned to preemptive therapy “clearly had better CMV-specific immunity, whether CD4 or CD8 cells, and had higher lymphocyte numbers than those patients that had received universal prophylaxis.”
In a comment, Sarah Doernberg, MD, from the division of infectious diseases at the University of California, San Francisco, agreed that “exploratory analysis of CMV-specific immune responses suggested increased CMV-specific immunity in those in the preemptive group, a finding that warrants further study. The feasibility of adopting reliable preemptive monitoring must be considered as individual centers ponder adopting this approach.”
Dr. Doernberg moderated the session where Dr. Ison discussed the data, but was not involved in the research.
The study by Singh et al. was supported by the National Institutes of Health. Dr. Singh reported research grants from NIH. Dr. Ison disclosed research support and paid consultation for several companies. Dr. Doernberg disclosed consulting for Basilea and Genentech.
FROM IDWEEK 2020
Biologics may protect psoriasis patients against severe COVID-19
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
FROM THE EADV CONGRESS
Health sector has spent $464 million on lobbying in 2020
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
, according to the Center for Responsive Politics.

PhRMA spent $20.7 million on lobbying through the end of September, good enough for third on the overall list of U.S. companies and organizations. Three other members of the health sector made the top 10: the American Hospital Association ($18.3 million), BlueCross/BlueShield ($16.3 million), and the American Medical Association ($15.2 million), the center reported.
Total spending by the health sector was $464 million from Jan. 1 to Sept. 30, topping the finance/insurance/real estate sector at $403 million, and miscellaneous business at $371 million. Miscellaneous business is the home of the U.S. Chamber of Commerce, the annual leader in such spending for the last 20 years, based on data from the Senate Office of Public Records.
The largest share of health sector spending came from pharmaceuticals/health products, with a total of almost $233 million, just slightly more than the sector’s four other constituents combined: hospitals/nursing homes ($80 million), health services/HMOs ($75 million), health professionals ($67 million), and miscellaneous health ($9.5 million), the center said on OpenSecrets.org.
Taking one step down from the sector level, that $233 million made pharmaceuticals/health products the highest spending of about 100 industries in 2020, nearly doubling the efforts of electronics manufacturing and equipment ($118 million), which came a distant second. Hospitals/nursing homes was eighth on the industry list, the center noted.
Med student’s cardiac crisis a COVID-era medical mystery
Within minutes of her arrival at Community North Hospital in Indianapolis, Ramya Yeleti’s vital signs plummeted; her pulse was at 45 beats per minute and her ejection fraction was hovering near 10%. “I definitely thought there was a chance I would close my eyes and never open them again, but I only had a few seconds to process that,” she recalled. Then everything went black. Ramya fell unconscious as shock pads were positioned and a swarm of clinicians prepared to insert an Impella heart pump through a catheter into her aorta.
The third-year medical student and aspiring psychiatrist had been doing in-person neurology rotations in July when she began to experience fever and uncontrolled vomiting. Her initial thought was that she must have caught the flu from a patient.
After all, Ramya, along with her father Ram Yeleti, MD, mother Indira, and twin sister Divya, had all weathered COVID-19 in previous months and later tested positive for SARS-CoV-2 antibodies. The only family member who had been spared was her younger brother Rohith.
Indira suffered a severe case, requiring ICU care for 2 days but no ventilator; the others experienced mostly mild symptoms. Ramya — who was studying for her third-year board exams after classes at Marian University College of Osteopathic Medicine in Indianapolis went virtual in March — was left with lingering fatigue; however, her cough and muscle aches abated and her sense of taste and smell returned. When she started rotations, she thought her life was getting back to normal.
Ramya’s flu symptoms did not improve. A university-mandated rapid COVID test came back negative, but 2 more days of vomiting started to worry both her and her father, who is a cardiologist and chief physician executive at Community Health Network in Indianapolis. After Ramya felt some chest pain, she asked her father to listen to her heart. All sounded normal, and Ram prescribed ondansetron for her nausea.
But the antiemetic didn’t work, and by the next morning both father and daughter were convinced that they needed to head to the emergency department.
“I wanted to double-check if I was missing something about her being dehydrated,” Ram told Medscape Medical News. “Several things can cause protracted nausea, like hepatitis, appendicitis, or another infection. I feel terribly guilty I didn’t realize she had a heart condition.”
A surprising turn for the worst
Ramya’s subtle symptoms quickly gave way to the dramatic cardiac crisis that unfolded just after her arrival at Community North. “Her EKG looked absolutely horrendous, like a 75-year-old having a heart attack,” Ram said.
As a cardiologist, he knew his daughter’s situation was growing dire when he heard physicians shouting that the Impella wasn’t working and she needed extracorporeal membrane oxygenation (ECMO).
“At that point, I didn’t think she’d survive,” her father recalled. “We had 10 physicians in the room who worked on her for 5 hours to get her stabilized.”
“It was especially traumatic because, obviously, I knew exactly what was happening,” he added. “You can’t sugarcoat anything.”
After being connected to the heart–lung equipment, Ramya was transferred to IU Health Methodist Hospital, also in Indianapolis, where she was tested again for COVID-19. Unlike the rapid test administered just days earlier, the PCR assay came back positive.
“I knew she had acute myocarditis, but coronavirus never crossed my mind,” said Ram.
“As we were dealing with her heart, we were also dealing with this challenge: she was coming back positive for COVID-19 again,” said Roopa Rao, MD, the heart failure transplant cardiologist at IU Health who treated Ramya.
“We weren’t sure whether we were dealing with an active infection or dead virus” from her previous infection, Rao said, “so we started treating her like she had active COVID-19 and gave her remdesivir, convalescent plasma, and steroids, which was the protocol in our hospital.”
A biopsy of Ramya’s heart tissue, along with blood tests, indicated a past parvovirus infection. It’s possible that Ramya’s previous coronavirus infection made her susceptible to heart damage from a newer parvovirus infection, said Rao. Either virus, or both together, could have been responsible for the calamity.
Although it was unheard of during Ramya’s cardiac crisis in early August, evolving evidence now raises the possibility that she is one of a handful of people in the world to be reinfected with SARS-CoV-2. Also emerging are cases of COVID-related myocarditis and other extreme heart complications, particularly in young people.
“At the time, it wasn’t really clear if people could have another infection so quickly,” Rao told Medscape Medical News. “It is possible she is one of these rare individuals to have COVID-19 twice. I’m hoping at some point we will have some clarity.”
“I would favor a coinfection as probably the triggering factor for her sickness,” she said. “It may take some time, but like any other disease — and it doesn’t look like COVID will go away magically — I hope we’ll have some answers down the road.”
Another wrinkle
The next 48 hours brought astonishing news: Ramya’s heart function had rebounded to nearly normal, and her ejection fraction increased to about 45%. Heart transplantation wouldn’t be necessary, although Rao stood poised to follow through if ECMO only sustained, rather than improved, Ramya’s prognosis.
“Ramya was so sick that if she didn’t recover, the only option would be a heart transplant,” said Rao. “But we wanted to do everything to keep that heart.”
After steroid and COVID treatment, Ramya’s heart started to come back. “It didn’t make sense to me,” said Rao. “I don’t know what helped. If we hadn’t done ECMO, her heart probably wouldn’t have recovered, so I would say we have to support these patients and give them time for the heart to recover, even to the point of ECMO.”
Despite the good news, Ramya’s survival still hung in the balance. When she was disconnected from ECMO, clinicians discovered that the Impella device had caused a rare complication, damaging her mitral valve. The valve could be repaired surgically, but both Rao and Ram felt great trepidation at the prospect of cardiopulmonary bypass during the open-heart procedure.
“They would need to stop her heart and restart it, and I was concerned it would not restart,” Ram explained. “I didn’t like the idea of open-heart surgery, but my biggest fear was she was not going to survive it because of a really fresh, sick heart.”
The cardiologists’ fears did, in fact, come to pass: it took an hour to coax Ramya’s heart back at the end of surgery. But, just as the surgeon was preparing to reconnect Ramya to ECMO in desperation, “her heart recovered again,” Rao reported.
“Some things you never forget in life,” she said. “I can’t describe how everyone in the OR felt, all taking care of her. I told Ramya, ‘you are a fighter’.”
New strength
Six days would pass before Ramya woke up and learned of the astounding series of events that saved her. She knew “something was really wrong” because of the incision at the center of her chest, but learning she’d been on ECMO and the heart transplant list drove home how close to death she’d actually come.
“Most people don’t get off ECMO; they die on it,” she said. “And the chances of dying on the heart transplant list are very high. It was very strange to me that this was my story all of a sudden, when a week and a half earlier I was on rotation.”
Ongoing physical therapy over the past 3 months has transformed Ramya from a state of profound physical weakness to a place of relative strength. The now-fourth-year med student is turning 26 in November and is hungry to restart in-person rotations. Her downtime has been filled in part with researching myocarditis and collaborating with Rao on her own case study for journal publication.
But the mental trauma from her experience has girded her in ways she knows will make her stronger personally and professionally in the years ahead.
“It’s still very hard. I’m still recovering,” she acknowledged. “I described it to my therapist as an invisible wound on my brain.”
“When I came out of the hospital, I still had ECMO wounds, deep gashes on my legs that affected how fast and how long I could walk,” she said. “I felt like the same thing was going on my brain — a huge cut no one could see.”
Her intention to specialize in psychiatry has become more pressing now that Ramya has realized the impact of trauma on mental health.
“My body failing me was awful, but I could handle it,” she said. “Losing any part of my mind would have been way worse. I want to take care of that in my patients.”
This article first appeared on Medscape.com.
Within minutes of her arrival at Community North Hospital in Indianapolis, Ramya Yeleti’s vital signs plummeted; her pulse was at 45 beats per minute and her ejection fraction was hovering near 10%. “I definitely thought there was a chance I would close my eyes and never open them again, but I only had a few seconds to process that,” she recalled. Then everything went black. Ramya fell unconscious as shock pads were positioned and a swarm of clinicians prepared to insert an Impella heart pump through a catheter into her aorta.
The third-year medical student and aspiring psychiatrist had been doing in-person neurology rotations in July when she began to experience fever and uncontrolled vomiting. Her initial thought was that she must have caught the flu from a patient.
After all, Ramya, along with her father Ram Yeleti, MD, mother Indira, and twin sister Divya, had all weathered COVID-19 in previous months and later tested positive for SARS-CoV-2 antibodies. The only family member who had been spared was her younger brother Rohith.
Indira suffered a severe case, requiring ICU care for 2 days but no ventilator; the others experienced mostly mild symptoms. Ramya — who was studying for her third-year board exams after classes at Marian University College of Osteopathic Medicine in Indianapolis went virtual in March — was left with lingering fatigue; however, her cough and muscle aches abated and her sense of taste and smell returned. When she started rotations, she thought her life was getting back to normal.
Ramya’s flu symptoms did not improve. A university-mandated rapid COVID test came back negative, but 2 more days of vomiting started to worry both her and her father, who is a cardiologist and chief physician executive at Community Health Network in Indianapolis. After Ramya felt some chest pain, she asked her father to listen to her heart. All sounded normal, and Ram prescribed ondansetron for her nausea.
But the antiemetic didn’t work, and by the next morning both father and daughter were convinced that they needed to head to the emergency department.
“I wanted to double-check if I was missing something about her being dehydrated,” Ram told Medscape Medical News. “Several things can cause protracted nausea, like hepatitis, appendicitis, or another infection. I feel terribly guilty I didn’t realize she had a heart condition.”
A surprising turn for the worst
Ramya’s subtle symptoms quickly gave way to the dramatic cardiac crisis that unfolded just after her arrival at Community North. “Her EKG looked absolutely horrendous, like a 75-year-old having a heart attack,” Ram said.
As a cardiologist, he knew his daughter’s situation was growing dire when he heard physicians shouting that the Impella wasn’t working and she needed extracorporeal membrane oxygenation (ECMO).
“At that point, I didn’t think she’d survive,” her father recalled. “We had 10 physicians in the room who worked on her for 5 hours to get her stabilized.”
“It was especially traumatic because, obviously, I knew exactly what was happening,” he added. “You can’t sugarcoat anything.”
After being connected to the heart–lung equipment, Ramya was transferred to IU Health Methodist Hospital, also in Indianapolis, where she was tested again for COVID-19. Unlike the rapid test administered just days earlier, the PCR assay came back positive.
“I knew she had acute myocarditis, but coronavirus never crossed my mind,” said Ram.
“As we were dealing with her heart, we were also dealing with this challenge: she was coming back positive for COVID-19 again,” said Roopa Rao, MD, the heart failure transplant cardiologist at IU Health who treated Ramya.
“We weren’t sure whether we were dealing with an active infection or dead virus” from her previous infection, Rao said, “so we started treating her like she had active COVID-19 and gave her remdesivir, convalescent plasma, and steroids, which was the protocol in our hospital.”
A biopsy of Ramya’s heart tissue, along with blood tests, indicated a past parvovirus infection. It’s possible that Ramya’s previous coronavirus infection made her susceptible to heart damage from a newer parvovirus infection, said Rao. Either virus, or both together, could have been responsible for the calamity.
Although it was unheard of during Ramya’s cardiac crisis in early August, evolving evidence now raises the possibility that she is one of a handful of people in the world to be reinfected with SARS-CoV-2. Also emerging are cases of COVID-related myocarditis and other extreme heart complications, particularly in young people.
“At the time, it wasn’t really clear if people could have another infection so quickly,” Rao told Medscape Medical News. “It is possible she is one of these rare individuals to have COVID-19 twice. I’m hoping at some point we will have some clarity.”
“I would favor a coinfection as probably the triggering factor for her sickness,” she said. “It may take some time, but like any other disease — and it doesn’t look like COVID will go away magically — I hope we’ll have some answers down the road.”
Another wrinkle
The next 48 hours brought astonishing news: Ramya’s heart function had rebounded to nearly normal, and her ejection fraction increased to about 45%. Heart transplantation wouldn’t be necessary, although Rao stood poised to follow through if ECMO only sustained, rather than improved, Ramya’s prognosis.
“Ramya was so sick that if she didn’t recover, the only option would be a heart transplant,” said Rao. “But we wanted to do everything to keep that heart.”
After steroid and COVID treatment, Ramya’s heart started to come back. “It didn’t make sense to me,” said Rao. “I don’t know what helped. If we hadn’t done ECMO, her heart probably wouldn’t have recovered, so I would say we have to support these patients and give them time for the heart to recover, even to the point of ECMO.”
Despite the good news, Ramya’s survival still hung in the balance. When she was disconnected from ECMO, clinicians discovered that the Impella device had caused a rare complication, damaging her mitral valve. The valve could be repaired surgically, but both Rao and Ram felt great trepidation at the prospect of cardiopulmonary bypass during the open-heart procedure.
“They would need to stop her heart and restart it, and I was concerned it would not restart,” Ram explained. “I didn’t like the idea of open-heart surgery, but my biggest fear was she was not going to survive it because of a really fresh, sick heart.”
The cardiologists’ fears did, in fact, come to pass: it took an hour to coax Ramya’s heart back at the end of surgery. But, just as the surgeon was preparing to reconnect Ramya to ECMO in desperation, “her heart recovered again,” Rao reported.
“Some things you never forget in life,” she said. “I can’t describe how everyone in the OR felt, all taking care of her. I told Ramya, ‘you are a fighter’.”
New strength
Six days would pass before Ramya woke up and learned of the astounding series of events that saved her. She knew “something was really wrong” because of the incision at the center of her chest, but learning she’d been on ECMO and the heart transplant list drove home how close to death she’d actually come.
“Most people don’t get off ECMO; they die on it,” she said. “And the chances of dying on the heart transplant list are very high. It was very strange to me that this was my story all of a sudden, when a week and a half earlier I was on rotation.”
Ongoing physical therapy over the past 3 months has transformed Ramya from a state of profound physical weakness to a place of relative strength. The now-fourth-year med student is turning 26 in November and is hungry to restart in-person rotations. Her downtime has been filled in part with researching myocarditis and collaborating with Rao on her own case study for journal publication.
But the mental trauma from her experience has girded her in ways she knows will make her stronger personally and professionally in the years ahead.
“It’s still very hard. I’m still recovering,” she acknowledged. “I described it to my therapist as an invisible wound on my brain.”
“When I came out of the hospital, I still had ECMO wounds, deep gashes on my legs that affected how fast and how long I could walk,” she said. “I felt like the same thing was going on my brain — a huge cut no one could see.”
Her intention to specialize in psychiatry has become more pressing now that Ramya has realized the impact of trauma on mental health.
“My body failing me was awful, but I could handle it,” she said. “Losing any part of my mind would have been way worse. I want to take care of that in my patients.”
This article first appeared on Medscape.com.
Within minutes of her arrival at Community North Hospital in Indianapolis, Ramya Yeleti’s vital signs plummeted; her pulse was at 45 beats per minute and her ejection fraction was hovering near 10%. “I definitely thought there was a chance I would close my eyes and never open them again, but I only had a few seconds to process that,” she recalled. Then everything went black. Ramya fell unconscious as shock pads were positioned and a swarm of clinicians prepared to insert an Impella heart pump through a catheter into her aorta.
The third-year medical student and aspiring psychiatrist had been doing in-person neurology rotations in July when she began to experience fever and uncontrolled vomiting. Her initial thought was that she must have caught the flu from a patient.
After all, Ramya, along with her father Ram Yeleti, MD, mother Indira, and twin sister Divya, had all weathered COVID-19 in previous months and later tested positive for SARS-CoV-2 antibodies. The only family member who had been spared was her younger brother Rohith.
Indira suffered a severe case, requiring ICU care for 2 days but no ventilator; the others experienced mostly mild symptoms. Ramya — who was studying for her third-year board exams after classes at Marian University College of Osteopathic Medicine in Indianapolis went virtual in March — was left with lingering fatigue; however, her cough and muscle aches abated and her sense of taste and smell returned. When she started rotations, she thought her life was getting back to normal.
Ramya’s flu symptoms did not improve. A university-mandated rapid COVID test came back negative, but 2 more days of vomiting started to worry both her and her father, who is a cardiologist and chief physician executive at Community Health Network in Indianapolis. After Ramya felt some chest pain, she asked her father to listen to her heart. All sounded normal, and Ram prescribed ondansetron for her nausea.
But the antiemetic didn’t work, and by the next morning both father and daughter were convinced that they needed to head to the emergency department.
“I wanted to double-check if I was missing something about her being dehydrated,” Ram told Medscape Medical News. “Several things can cause protracted nausea, like hepatitis, appendicitis, or another infection. I feel terribly guilty I didn’t realize she had a heart condition.”
A surprising turn for the worst
Ramya’s subtle symptoms quickly gave way to the dramatic cardiac crisis that unfolded just after her arrival at Community North. “Her EKG looked absolutely horrendous, like a 75-year-old having a heart attack,” Ram said.
As a cardiologist, he knew his daughter’s situation was growing dire when he heard physicians shouting that the Impella wasn’t working and she needed extracorporeal membrane oxygenation (ECMO).
“At that point, I didn’t think she’d survive,” her father recalled. “We had 10 physicians in the room who worked on her for 5 hours to get her stabilized.”
“It was especially traumatic because, obviously, I knew exactly what was happening,” he added. “You can’t sugarcoat anything.”
After being connected to the heart–lung equipment, Ramya was transferred to IU Health Methodist Hospital, also in Indianapolis, where she was tested again for COVID-19. Unlike the rapid test administered just days earlier, the PCR assay came back positive.
“I knew she had acute myocarditis, but coronavirus never crossed my mind,” said Ram.
“As we were dealing with her heart, we were also dealing with this challenge: she was coming back positive for COVID-19 again,” said Roopa Rao, MD, the heart failure transplant cardiologist at IU Health who treated Ramya.
“We weren’t sure whether we were dealing with an active infection or dead virus” from her previous infection, Rao said, “so we started treating her like she had active COVID-19 and gave her remdesivir, convalescent plasma, and steroids, which was the protocol in our hospital.”
A biopsy of Ramya’s heart tissue, along with blood tests, indicated a past parvovirus infection. It’s possible that Ramya’s previous coronavirus infection made her susceptible to heart damage from a newer parvovirus infection, said Rao. Either virus, or both together, could have been responsible for the calamity.
Although it was unheard of during Ramya’s cardiac crisis in early August, evolving evidence now raises the possibility that she is one of a handful of people in the world to be reinfected with SARS-CoV-2. Also emerging are cases of COVID-related myocarditis and other extreme heart complications, particularly in young people.
“At the time, it wasn’t really clear if people could have another infection so quickly,” Rao told Medscape Medical News. “It is possible she is one of these rare individuals to have COVID-19 twice. I’m hoping at some point we will have some clarity.”
“I would favor a coinfection as probably the triggering factor for her sickness,” she said. “It may take some time, but like any other disease — and it doesn’t look like COVID will go away magically — I hope we’ll have some answers down the road.”
Another wrinkle
The next 48 hours brought astonishing news: Ramya’s heart function had rebounded to nearly normal, and her ejection fraction increased to about 45%. Heart transplantation wouldn’t be necessary, although Rao stood poised to follow through if ECMO only sustained, rather than improved, Ramya’s prognosis.
“Ramya was so sick that if she didn’t recover, the only option would be a heart transplant,” said Rao. “But we wanted to do everything to keep that heart.”
After steroid and COVID treatment, Ramya’s heart started to come back. “It didn’t make sense to me,” said Rao. “I don’t know what helped. If we hadn’t done ECMO, her heart probably wouldn’t have recovered, so I would say we have to support these patients and give them time for the heart to recover, even to the point of ECMO.”
Despite the good news, Ramya’s survival still hung in the balance. When she was disconnected from ECMO, clinicians discovered that the Impella device had caused a rare complication, damaging her mitral valve. The valve could be repaired surgically, but both Rao and Ram felt great trepidation at the prospect of cardiopulmonary bypass during the open-heart procedure.
“They would need to stop her heart and restart it, and I was concerned it would not restart,” Ram explained. “I didn’t like the idea of open-heart surgery, but my biggest fear was she was not going to survive it because of a really fresh, sick heart.”
The cardiologists’ fears did, in fact, come to pass: it took an hour to coax Ramya’s heart back at the end of surgery. But, just as the surgeon was preparing to reconnect Ramya to ECMO in desperation, “her heart recovered again,” Rao reported.
“Some things you never forget in life,” she said. “I can’t describe how everyone in the OR felt, all taking care of her. I told Ramya, ‘you are a fighter’.”
New strength
Six days would pass before Ramya woke up and learned of the astounding series of events that saved her. She knew “something was really wrong” because of the incision at the center of her chest, but learning she’d been on ECMO and the heart transplant list drove home how close to death she’d actually come.
“Most people don’t get off ECMO; they die on it,” she said. “And the chances of dying on the heart transplant list are very high. It was very strange to me that this was my story all of a sudden, when a week and a half earlier I was on rotation.”
Ongoing physical therapy over the past 3 months has transformed Ramya from a state of profound physical weakness to a place of relative strength. The now-fourth-year med student is turning 26 in November and is hungry to restart in-person rotations. Her downtime has been filled in part with researching myocarditis and collaborating with Rao on her own case study for journal publication.
But the mental trauma from her experience has girded her in ways she knows will make her stronger personally and professionally in the years ahead.
“It’s still very hard. I’m still recovering,” she acknowledged. “I described it to my therapist as an invisible wound on my brain.”
“When I came out of the hospital, I still had ECMO wounds, deep gashes on my legs that affected how fast and how long I could walk,” she said. “I felt like the same thing was going on my brain — a huge cut no one could see.”
Her intention to specialize in psychiatry has become more pressing now that Ramya has realized the impact of trauma on mental health.
“My body failing me was awful, but I could handle it,” she said. “Losing any part of my mind would have been way worse. I want to take care of that in my patients.”
This article first appeared on Medscape.com.
About 17% of COVID-19 survivors retest positive in follow-up study
For reasons unknown, about one in six people who recovered from COVID-19 subsequently retested positive at least 2 weeks later, researchers reported in a study in Italy.
Sore throat and rhinitis were the only symptoms associated with a positive result. “Patients who continued to have respiratory symptoms, especially, were more likely to have a new positive test result,” lead author Francesco Landi, MD, PhD, said in an interview.
“This suggests the persistence of respiratory symptoms should not be underestimated and should be adequately assessed in all patients considered recovered from COVID-19,” he said.
“The study results are interesting,” Akiko Iwasaki, PhD, an immunobiologist at Yale University and the Howard Hughes Medical Institute, both in New Haven, Conn.,, said in an interview. “There are other reports of RNA detection postdischarge, but this study ... found that only two symptoms out of many – sore throat and rhinitis – were higher in those with PCR [polymerase chain reaction]-positive status.”
The study was published online Sept. 18 in the American Journal of Preventive Medicine.
The findings could carry important implications for people who continue to be symptomatic. “It is reasonable to be cautious and avoid close contact with others, wear a face mask, and possibly undergo an additional nasopharyngeal swab,” said Dr. Landi, associate professor of internal medicine at Catholic University of the Sacred Heart in Rome.
“One of most interesting findings is that persistent symptoms do not correlate with PCR positivity, suggesting that symptoms are in many cases not due to ongoing viral replication,” Jonathan Karn, PhD, professor and chair of the department of molecular biology and microbiology at Case Western Reserve University, Cleveland, said in an interview.
“The key technical problem, which they have discussed, is that a viral RNA signal in the PCR assay does not necessarily mean that infectious virus is present,” Dr. Karn said. He added that new comprehensive viral RNA analyses would be needed to answer this question.
Official COVID-19 recovery
To identify risk factors and COVID-19 survivors more likely to retest positive, Dr. Landi and members of the Gemelli Against COVID-19 Post-Acute Care Study Group evaluated 131 people after hospital discharge.
All participants met World Health Organization criteria for release from isolation, including two negative test results at least 24 hours apart, and were studied between April 21 and May 21. Mean age was 56 and 39% were women. Only a slightly higher mean body mass index of 27.6 kg/m2 in the positive group versus 25.9 in the negative group, was significant.
Although 51% of survivors reported fatigue, 44% had dyspnea, and 17% were coughing, the rates did not differ significantly between groups. In contrast, 18% of positive survivors and 4% of negative survivors had a sore throat (P = .04), and 27% versus 12%, respectively, reported rhinitis (P = .05).
People returned for follow-up visits a mean 17 days after the second negative swab test.
Asymptomatic COVID-19 carriers
“These findings indicate that a noteworthy rate of recovered patients with COVID-19 could still be asymptomatic carriers of the virus,” the researchers noted in the paper. “Even in the absence of specific guidelines, the 22 patients who tested positive for COVID-19 again were suggested to quarantine for a second time.”
No family member or close contact of the positive survivors reported SARS-CoV-2 infection. All patients continued to wear masks and observe social distancing recommendations, which makes it “very difficult to affirm whether these patients were really contagious,” the researchers noted.
Next steps
Evaluating all COVID-19 survivors to identify any who retest positive “will be a crucial contribution to a better understanding of both the natural history of COVID-19 as well as the public health implications of viral shedding,” the authors wrote.
One study limitation is that the reverse transcriptase–PCR test reveals genetic sequences specific to COVID-19. “It is important to underline that this is not a viral culture and cannot determine whether the virus is viable and transmissible,” the researchers noted.
“In this respect, we are trying to better understand if the persistence of long-time positive [reverse transcriptase]–PCR test for COVID-19 is really correlated to a potential contagiousness,” they added.
Dr. Landi and colleagues said their findings should be considered preliminary, and larger data samples are warranted to validate the results.
Dr. Landi and Dr. Karn disclosed no relevant financial relationships. Dr. Iwasaki disclosed a research grant from Condair, a 5% or greater equity interest in RIGImmune, and income of $250 or more from PureTec.
A version of this article originally appeared on Medscape.com.
For reasons unknown, about one in six people who recovered from COVID-19 subsequently retested positive at least 2 weeks later, researchers reported in a study in Italy.
Sore throat and rhinitis were the only symptoms associated with a positive result. “Patients who continued to have respiratory symptoms, especially, were more likely to have a new positive test result,” lead author Francesco Landi, MD, PhD, said in an interview.
“This suggests the persistence of respiratory symptoms should not be underestimated and should be adequately assessed in all patients considered recovered from COVID-19,” he said.
“The study results are interesting,” Akiko Iwasaki, PhD, an immunobiologist at Yale University and the Howard Hughes Medical Institute, both in New Haven, Conn.,, said in an interview. “There are other reports of RNA detection postdischarge, but this study ... found that only two symptoms out of many – sore throat and rhinitis – were higher in those with PCR [polymerase chain reaction]-positive status.”
The study was published online Sept. 18 in the American Journal of Preventive Medicine.
The findings could carry important implications for people who continue to be symptomatic. “It is reasonable to be cautious and avoid close contact with others, wear a face mask, and possibly undergo an additional nasopharyngeal swab,” said Dr. Landi, associate professor of internal medicine at Catholic University of the Sacred Heart in Rome.
“One of most interesting findings is that persistent symptoms do not correlate with PCR positivity, suggesting that symptoms are in many cases not due to ongoing viral replication,” Jonathan Karn, PhD, professor and chair of the department of molecular biology and microbiology at Case Western Reserve University, Cleveland, said in an interview.
“The key technical problem, which they have discussed, is that a viral RNA signal in the PCR assay does not necessarily mean that infectious virus is present,” Dr. Karn said. He added that new comprehensive viral RNA analyses would be needed to answer this question.
Official COVID-19 recovery
To identify risk factors and COVID-19 survivors more likely to retest positive, Dr. Landi and members of the Gemelli Against COVID-19 Post-Acute Care Study Group evaluated 131 people after hospital discharge.
All participants met World Health Organization criteria for release from isolation, including two negative test results at least 24 hours apart, and were studied between April 21 and May 21. Mean age was 56 and 39% were women. Only a slightly higher mean body mass index of 27.6 kg/m2 in the positive group versus 25.9 in the negative group, was significant.
Although 51% of survivors reported fatigue, 44% had dyspnea, and 17% were coughing, the rates did not differ significantly between groups. In contrast, 18% of positive survivors and 4% of negative survivors had a sore throat (P = .04), and 27% versus 12%, respectively, reported rhinitis (P = .05).
People returned for follow-up visits a mean 17 days after the second negative swab test.
Asymptomatic COVID-19 carriers
“These findings indicate that a noteworthy rate of recovered patients with COVID-19 could still be asymptomatic carriers of the virus,” the researchers noted in the paper. “Even in the absence of specific guidelines, the 22 patients who tested positive for COVID-19 again were suggested to quarantine for a second time.”
No family member or close contact of the positive survivors reported SARS-CoV-2 infection. All patients continued to wear masks and observe social distancing recommendations, which makes it “very difficult to affirm whether these patients were really contagious,” the researchers noted.
Next steps
Evaluating all COVID-19 survivors to identify any who retest positive “will be a crucial contribution to a better understanding of both the natural history of COVID-19 as well as the public health implications of viral shedding,” the authors wrote.
One study limitation is that the reverse transcriptase–PCR test reveals genetic sequences specific to COVID-19. “It is important to underline that this is not a viral culture and cannot determine whether the virus is viable and transmissible,” the researchers noted.
“In this respect, we are trying to better understand if the persistence of long-time positive [reverse transcriptase]–PCR test for COVID-19 is really correlated to a potential contagiousness,” they added.
Dr. Landi and colleagues said their findings should be considered preliminary, and larger data samples are warranted to validate the results.
Dr. Landi and Dr. Karn disclosed no relevant financial relationships. Dr. Iwasaki disclosed a research grant from Condair, a 5% or greater equity interest in RIGImmune, and income of $250 or more from PureTec.
A version of this article originally appeared on Medscape.com.
For reasons unknown, about one in six people who recovered from COVID-19 subsequently retested positive at least 2 weeks later, researchers reported in a study in Italy.
Sore throat and rhinitis were the only symptoms associated with a positive result. “Patients who continued to have respiratory symptoms, especially, were more likely to have a new positive test result,” lead author Francesco Landi, MD, PhD, said in an interview.
“This suggests the persistence of respiratory symptoms should not be underestimated and should be adequately assessed in all patients considered recovered from COVID-19,” he said.
“The study results are interesting,” Akiko Iwasaki, PhD, an immunobiologist at Yale University and the Howard Hughes Medical Institute, both in New Haven, Conn.,, said in an interview. “There are other reports of RNA detection postdischarge, but this study ... found that only two symptoms out of many – sore throat and rhinitis – were higher in those with PCR [polymerase chain reaction]-positive status.”
The study was published online Sept. 18 in the American Journal of Preventive Medicine.
The findings could carry important implications for people who continue to be symptomatic. “It is reasonable to be cautious and avoid close contact with others, wear a face mask, and possibly undergo an additional nasopharyngeal swab,” said Dr. Landi, associate professor of internal medicine at Catholic University of the Sacred Heart in Rome.
“One of most interesting findings is that persistent symptoms do not correlate with PCR positivity, suggesting that symptoms are in many cases not due to ongoing viral replication,” Jonathan Karn, PhD, professor and chair of the department of molecular biology and microbiology at Case Western Reserve University, Cleveland, said in an interview.
“The key technical problem, which they have discussed, is that a viral RNA signal in the PCR assay does not necessarily mean that infectious virus is present,” Dr. Karn said. He added that new comprehensive viral RNA analyses would be needed to answer this question.
Official COVID-19 recovery
To identify risk factors and COVID-19 survivors more likely to retest positive, Dr. Landi and members of the Gemelli Against COVID-19 Post-Acute Care Study Group evaluated 131 people after hospital discharge.
All participants met World Health Organization criteria for release from isolation, including two negative test results at least 24 hours apart, and were studied between April 21 and May 21. Mean age was 56 and 39% were women. Only a slightly higher mean body mass index of 27.6 kg/m2 in the positive group versus 25.9 in the negative group, was significant.
Although 51% of survivors reported fatigue, 44% had dyspnea, and 17% were coughing, the rates did not differ significantly between groups. In contrast, 18% of positive survivors and 4% of negative survivors had a sore throat (P = .04), and 27% versus 12%, respectively, reported rhinitis (P = .05).
People returned for follow-up visits a mean 17 days after the second negative swab test.
Asymptomatic COVID-19 carriers
“These findings indicate that a noteworthy rate of recovered patients with COVID-19 could still be asymptomatic carriers of the virus,” the researchers noted in the paper. “Even in the absence of specific guidelines, the 22 patients who tested positive for COVID-19 again were suggested to quarantine for a second time.”
No family member or close contact of the positive survivors reported SARS-CoV-2 infection. All patients continued to wear masks and observe social distancing recommendations, which makes it “very difficult to affirm whether these patients were really contagious,” the researchers noted.
Next steps
Evaluating all COVID-19 survivors to identify any who retest positive “will be a crucial contribution to a better understanding of both the natural history of COVID-19 as well as the public health implications of viral shedding,” the authors wrote.
One study limitation is that the reverse transcriptase–PCR test reveals genetic sequences specific to COVID-19. “It is important to underline that this is not a viral culture and cannot determine whether the virus is viable and transmissible,” the researchers noted.
“In this respect, we are trying to better understand if the persistence of long-time positive [reverse transcriptase]–PCR test for COVID-19 is really correlated to a potential contagiousness,” they added.
Dr. Landi and colleagues said their findings should be considered preliminary, and larger data samples are warranted to validate the results.
Dr. Landi and Dr. Karn disclosed no relevant financial relationships. Dr. Iwasaki disclosed a research grant from Condair, a 5% or greater equity interest in RIGImmune, and income of $250 or more from PureTec.
A version of this article originally appeared on Medscape.com.
CDC panel takes on COVID vaccine rollout, risks, and side effects
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
Federal advisers who will help determine which Americans get the first COVID vaccines took an in-depth look Oct. 30 at the challenges they face in selecting priority groups.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) will face two key decisions once a COVID vaccine wins clearance from the US Food and Drug Administration (FDA).
ACIP will need to decide whether to recommend its use in adults (the age group in which vaccines are currently being tested). The group will also need to offer direction on which groups should get priority in vaccine allocation, inasmuch as early supplies will not be sufficient to vaccinate everyone.
At the Oct. 30 meeting, CDC’s Kathleen Dooling, MD, MPH, suggested that ACIP plan on tackling these issues as two separate questions when it comes time to weigh in on an approved vaccine. Although there was no formal vote among ACIP members at the meeting, Dooling’s proposal for tackling a future recommendation in a two-part fashion drew positive feedback.
ACIP member Katherine A. Poehling, MD, MPH, suggested that the panel and CDC be ready to reexamine the situation frequently regarding COVID vaccination. “Perhaps we could think about reviewing data on a monthly basis and updating the recommendation, so that we can account for the concerns and balance both the benefits and the [potential] harm,” Poehling said.
Dooling agreed. “Both the vaccine recommendation and allocation will be revisited in what is a very dynamic situation,” Dooling replied to Poehling. “So all new evidence will be brought to ACIP, and certainly the allocation as vaccine distribution proceeds will need to be adjusted accordingly.”
Ethics and limited evidence
During the meeting, ACIP members repeatedly expressed discomfort with the prospect of having to weigh in on widespread use of COVID vaccines on the basis of limited evidence.
Within months, FDA may opt for a special clearance, known as an emergency use authorization (EUA), for one or more of the experimental COVID vaccines now in advanced testing. Many of FDA’s past EUA clearances were granted for test kits. For those EUA approvals, the agency considered risks of false results but not longer-term, direct harm to patients from these products.
With a COVID vaccine, there will be strong pressure to distribute doses as quickly as possible with the hope of curbing the pandemic, which has already led to more than 229,000 deaths in the United States alone and has disrupted lives and economies around the world. But questions will persist about the possibility of serious complications from these vaccines, ACIP members noted.
“My personal struggle is the ethical side and how to balance these two,” said ACIP member Robert L. Atmar, MD, of Baylor College of Medicine, Houston, Texas, who noted that he expects his fellow panelists to share this concern.
Currently, four experimental COVID vaccines likely to be used in the United States have advanced to phase 3 testing. Pfizer Inc and BioNtech have enrolled more than 42,000 participants in a test of their candidate, BNT162b2 vaccine, and rival Moderna has enrolled about 30,000 participants in a test of its mRNA-1273 vaccine, CDC staff said.
The other two advanced COVID vaccine candidates have overcome recent hurdles. AstraZeneca Plc on Oct. 23 announced that FDA had removed a hold on the testing of its AZD1222 vaccine candidate; the trial will enroll approximately 30,000 people. Johnson & Johnson’s Janssen unit also announced that day the lifting of a safety pause for its Ad26.COV2.S vaccine; the phase 3 trial for that vaccine will enroll approximately 60,000 volunteers. Federal agencies, states, and territories have developed plans for future distribution of COVID vaccines, CDC staff said in briefing materials for today’s ACIP meeting.
Several ACIP members raised many of the same concerns that members of an FDA advisory committee raised at a meeting earlier in October. ACIP and FDA advisers honed in on the FDA’s decision to set a median follow-up duration of 2 months in phase 3 trials in connection with expected EUA applications for COVID-19 vaccines.
“I struggle with following people for 2 months after their second vaccination as a time point to start making final decisions about safety,” said ACIP member Sharon E. Frey, MD, a professor at St. Louis University School of Medicine, St. Louis, Missouri. “I just want to put that out there.”
Medical front line, then who?
There is consensus that healthcare workers be in the first stage ― Phase 1 ― of distribution. That recommendation was made in a report from the National Academies of Sciences, Engineering, and Medicine (NASEM). Phase 1A would include first responders; Phase 1B might include people of all ages who have two or more comorbidities that put them at significantly higher risk for COVID-19 or death, as well as older adults living in congregate or overcrowded settings, the NASEM report said.
A presentation from the CDC’s Matthew Biggerstaff, ScD, MPH, underscored challenges in distributing what are expected to be limited initial supplies of COVID vaccines.
Biggerstaff showed several scenarios the CDC’s Data, Analytics, and Modeling Task Force had studied. The initial allocation of vaccines would be for healthcare workers, followed by what the CDC called Phase 1B.
Choices for a rollout may include next giving COVID vaccines to people at high risk, such as persons who have one or more chronic medical conditions, including heart disease, diabetes, kidney disease, or obesity. Other options for the rollout could be to vaccinate people aged 65 years and older or essential workers whose employment puts them in contact with the public, thus raising the risk of contracting the virus.
The CDC’s research found that the greatest impact in preventing death was to initially vaccinate adults aged 65 and older in Phase 1B. The agency staff described this approach as likely to result in an about “1 to 11% increase in averted deaths across the scenarios.”
Initially vaccinating essential workers or high-risk adults in Phase 1B would avert the most infections. The agency staff described this approach as yielding about “1 to 5% increase in averted infections across the scenarios,” Biggerstaff said during his presentation.
The following are other findings of the CDC staff:
The earlier the vaccine rollout relative to increasing transmission, the greater the averted percentage and differences between the strategies.
Differences were not substantial in some scenarios.
The need to continue efforts to slow the spread of COVID-19 should be emphasized.
Adverse effects
ACIP members also heard about strategies for tracking potential side effects of future vaccines. A presentation by Tom Shimabukuro, MD, MPH, MBA, from the CDC’s COVID-19 Vaccine Task Force/Vaccine Safety Team, included details about a new smartphone-based active surveillance program for COVID-19 vaccine safety.
Known as v-safe, this system would use Web-based survey monitoring and incorporate text messaging. It would conduct electronic health checks on vaccine recipients, which would occur daily during the first week post vaccination and weekly thereafter for 6 weeks from the time of vaccination.
Clinicians “can play an important role in helping CDC enroll patients in v-safe at the time of vaccination,” Shimabukuro noted in his presentation. This would add another task, though, for clinicians, the CDC staff noted.
Pregnancy and breastfeeding are special concerns
Of special concern with the rollout of a COVID vaccine are recommendations regarding pregnancy and breastfeeding. Women constitute about 75% of the healthcare workforce, CDC staff noted.
At the time the initial ACIP COVID vaccination recommendations are made, there could be approximately 330,000 healthcare personnel who are pregnant or who have recently given birth. Available data indicate potentially increased risks for severe maternal illness and preterm birth associated with SARS-CoV-2 infection, said CDC’s Megan Wallace, DrPH, MPH, in a presentation for the Friday meeting.
In an Oct. 27 letter to ACIP, Chair Jose Romero, the American College of Obstetricians and Gynecologists (ACOG), urged the panel to ensure that pregnant women and new mothers in the healthcare workforce have priority access to a COVID vaccine. Pregnant and lactating women were “noticeably and alarmingly absent from the NASEM vaccine allocation plan for COVID-19,” wrote Christopher M. Zahn, MD, vice president for practice activities at ACOG, in the letter to Romero.
“ACOG urges ACIP to incorporate pregnant and lactating women clearly and explicitly into its COVID-19 vaccine allocation and prioritization framework,” Zahn wrote. “Should an Emergency Use Authorization be executed for one or more COVID-19 vaccines and provide a permissive recommendation for pregnant and lactating women, pregnant health care workers, pregnant first responders, and pregnant individuals with underlying conditions should be prioritized for vaccination alongside their non-pregnant peers.”
This article first appeared on Medscape.com.
Frivolous lawsuits: Still a big threat to doctors?
Dr. G, a New York surgeon, was only a couple years into practice when he faced his first lawsuit.
After undergoing liposuction surgery on the area of her calf and ankle, a patient claimed she had developed a severe allergic reaction, characterized by small areas of necrosis on the lower extremities, said Dr. G, who asked to remain anonymous. However, the alleged injury seemed suspicious, said Dr. G, considering that 3 weeks after the surgery, the area had shown a successful result with minimal swelling.
Six months into the suit, Dr. G received a shocking phone call. It was the patient’s estranged husband, who revealed that his wife was having an affair with another man, a physician. In recorded phone calls, the patient and her paramour had discussed causing an injury near the patient’s calf in an attempt to sue and get rich, the husband relayed. Dr. G immediately contacted his insurance carrier with the news, but his attorney said the information would not be admissible in court. Instead, the insurer settled with the patient, who received about $125,000.
At the time, Dr. G did not have a consent-to-settle clause in his contract, so the insurer was able to settle without his approval.
In legal practice, a frivolous claim is defined as one that lacks a supporting legal argument or any factual basis. A claim issued with the intent of disturbing, annoying, or harassing the opposing party can also be described as legally frivolous, said Michael Stinson, vice president of government relations and public policy for the Medical Professional Liability Association (MPL Association), a trade association for medical liability insurers.
However, when most physicians refer to “frivolous claims,” they often mean a claim in which there is no attributable negligence. Such suits represent a second category of claims – nonmeritorious lawsuits.
“I think people intermix nonmeritorious and frivolous all the time,” Mr. Stinson said. “In the vast majority of nonmeritorious claims, the patient has suffered an adverse outcome, it’s just that it wasn’t the result of negligence, whereas with a frivolous lawsuit, they really haven’t suffered any damage, so they’ve got no business filing a lawsuit on any level.”
A third type of so-called frivolous suit is that of a fraudulent or fake claim, in which, as Dr. G experienced, a patient causes a self-injury or lies about a condition to craft a false claim against a physician.
If a patient files a claim that the patient knows is false, the patient commits fraud and may be subject to counterclaims for malicious prosecution or abuse of process, said Jeffrey Segal, MD, JD, a neurosurgeon and health law attorney. Further, the patient would be testifying under oath, and such testimony can be considered perjury, a criminal offense with criminal penalties.
Sadly, Dr. G was the target of another frivolous lawsuit years later. In that suit, a patient claimed the surgeon had left a piece of sponge in her breast cavity during surgery. The case was dismissed when medical records proved the patient knew that the foreign body resulted from an unrelated procedure she had undergone years earlier.
“There is so much abuse in the court system,” Dr. G said. “You really don’t think stuff like that will happen to you, especially if you honor the profession. It’s unfortunate. It’s left a very bitter taste in my mouth.”
Frivolous claims have long been a subject of debate. Tort reform advocates often contend that such claims are pervasive. They cite them as key reasons for high health care costs and say that they have led to the rise of defensive medicine. Plaintiffs’ attorneys counter that the rate of frivolous claims is widely exaggerated and argue that the pursuit of frivolous claims would be “bad business” for legal firms.
“I have never seen a frivolous malpractice claim,” says Malcolm P. McConnell III, JD, a Richmond, Va., medical malpractice attorney and chair of the Medical Malpractice Legislative Subcommittee for the Virginia Trial Lawyers Association. “I cannot say that such things never happen, but any lawyer bringing such a thing is foolish, because there is no reward for it.”
Are shotgun lawsuits frivolous?
To many physicians, being dragged into a lawsuit over a complaint or medical outcome in which they were not involved is frivolous, said Stanislaw Stawicki, MD, a trauma surgeon and researcher based in Bethlehem, Pa. Dr. Stawicki was named in a lawsuit along with a long list of medical staff who interacted in some way with the plaintiff. Dr. Stawicki himself saw the patient once and made a note in the chart but had nothing to do with the patient’s surgery or with any critical decisions regarding his care, he said.
“Nothing really prepares you for seeing your name on a legal complaint,” Dr. Stawicki said. “It’s traumatic. I had to block out entire days to give depositions, which were really kind of pointless. Questions like, ‘Is this really your name? Where did you train? Were you there that morning?’ Stuff that was really not consequential to the fact that someone had surgery a month earlier and had some sort of complication.”
Dr. Stawicki was eventually dropped from the claim, but not before a nearly year-long ordeal of legal proceedings, meetings, and paperwork.
It is common practice for plaintiffs’ attorneys to add codefendants in the early stages of a claim, said David M. Studdert, ScD, a leading health law researcher and a professor of law at Stanford (Calif.) Law School. Defendants are gradually dismissed as the case moves forward and details of the incident become clearer, he said.
“Plaintiffs’ attorneys have strong incentives to try and choose claims that will be successful,” Dr. Studdert said. “However, in the early point in the process, neither the patient nor the attorney may have a good idea what has actually happened with care. So sometimes, filing a lawsuit may be the only way to begin the process of opening up that information.”
A study by Dr. Studdert in which medical malpractice claims, errors, and compensation payments were analyzed found that, out of 1,452 claims, about one-third (37%) did not involve errors.
“Many physicians might call those frivolous lawsuits, but in fact, most of those don’t go on to receive compensation,” he said. “We suspect that in many instances, those claims are simply dropped once it becomes apparent that there wasn’t error involved.
“They can still be burdensome, anxiety provoking, and time consuming for physicians who are named in those suits, so I don’t want to suggest that claims that don’t involve errors are not a problem,” said Dr. Studdert. “However, I think it’s wrong to assume, as many people do when they use the term ‘frivolous lawsuit,’ that this is really an extortionary effort by a plaintiffs’ attorney to try to get money out of a hospital or a physician for care that was really unproblematic.”
Certain ‘frivolous’ cases more common than others
Nonmeritorious claims still occur relatively frequently today, according to data from the Medical Professional Liability Association’s Data Sharing Project. Of about 18,000 liability claims reported from 2016 to 2018, 65% were dropped, withdrawn, or dismissed. Of the 6% of claims that went before a jury, more than 85% resulted in a verdict for the defendant, the researchers found.
“Basically, any claim that does not result in a payment because the underlying claim of negligence on the part of a health professional had been demonstrated, proven, or adjudicated false is one we would describe as nonmeritorious,” Mr. Stinson said.
The MPL Association does not track cases that meet the legal definition of frivolous, said Mr. Stinson, and they “don’t see truly frivolous lawsuits very often.”
Malpractice claims are risky, expensive, and aggressively defended, says Mr. McConnell, the plaintiffs’ attorney. Mr. McConnell, who has been practicing for 30 years, said his own claim selection process is very rigorous and that he cannot afford to pursue claims that aren’t well supported by science and medicine.
“Pursuing frivolous cases would bankrupt me and ruin my reputation,” he said. “A lawyer I know once said he would write a check for $10,000 to anyone who could show him a lawyer who makes a living pursuing frivolous medical malpractice cases. It’s a fair challenge. The economics and the practices of liability carriers and defense lawyers make frivolous cases a dead end for plaintiff lawyers.”
Most medical malpractice cases are taken on a contingency fee basis, Mr. McConnell noted, meaning that the plaintiff’s lawyer is not paid unless the claim is successful.
“This means that the plaintiff’s lawyer is risking 2 years of intensive labor on a case which may yield no fee at all,” he said. “Obviously, any reasonable lawyer is going to want to minimize that risk. The only way to minimize that risk is for the case to be solid, not weak, and certainly not frivolous.”
But Dr. Segal, the health law attorney, says that plenty of frivolous liability claims are levied each year, with attorneys willing to pursue them.
It’s true that seasoned plaintiffs’ attorneys generally screen for merit and damages, Dr. Segal said, but in some instances, attorneys who are not trained in malpractice law accept frivolous claims and take them forward. In some cases, they are slip-and-fall accident attorneys accustomed to receiving modest amounts from insurance companies quickly, said Dr. Segal, founder of Medical Justice, a company that helps deter frivolous lawsuits against physicians.
“If we lived in a perfectly rational universe where plaintiffs’ attorneys screened cases well and only took the meritorious cases forward, we would see less frivolous cases filed, but that’s not the universe I live in,” Dr. Segal said. “There are well over a million attorneys in this country, and some are hungrier than others. The attorneys may frequently get burned in the end, and maybe that attorney won’t move another malpractice case forward, but there’s always someone else willing to take their place.”
Medical Justice has twice run a Most Frivolous Lawsuit Contest on its website, one in 2008 and one in late 2018. The first contest drew 30 entries, and the second garnered nearly 40 submissions, primarily from physicians who were defendants in the cases, according to Dr. Segal. (Dr. G’s lawsuit was highlighted in the most recent contest.)
In one case, an emergency physician was drawn into litigation by the family of a deceased patient. The patient experienced sudden cardiac arrythmia at home, and paramedics were unable to intubate her or establish IV access. She was transferred to the hospital, where resuscitation efforts continued, but she remained in asystole and was pronounced dead after 15 minutes.
At the hospital, blood tests were conducted. They showed that her serum potassium concentration was elevated to about 12 mEq/L, Dr. Segal said. The family initiated a claim in which they accused the emergency physician of failure to diagnose hyperkalemia. They alleged that had the hyperkalemia been discovered sooner, the patient’s death could have been prevented.
“If you had no other facts about this, you would wonder how a person with potassium that high would even be alive,” Dr. Segal said. “But what they were looking at was the body decomposing and all the potassium in the cells being released into the bloodstream. It wasn’t the cause of the problem, it was an effect of the problem. She really was dead on arrival, and she was probably dead at home.”
The case was eventually dropped.
Although the outcome for the patient was tragic, says Dr. Segal, the case is one of many types of frivolous claims that exist today.
“Yes, frivolous cases are out there,” he said.
Fraudulent claims uncommon
As for fraudulent medical liability claims, legal experts say they’re rare. J. Richard Moore, JD, an Indianapolis-based medical liability defense attorney, said he’s never personally encountered a medical malpractice claim in which he believed a plaintiff caused an injury or an illness and attempted to blame it on a physician.
However, Mr. Moore has defended many claims in which the illness or condition the plaintiff claimed was caused or was made worse through medical negligence was actually a preexisting condition or a preexisting condition that worsened and was not related to any medical negligence, Mr. Moore said.
“Although I have often felt in such cases that the plaintiff really knew that the condition was not affected by any alleged medical negligence, I would not put that in the ‘fraudulent claim’ category because it can be very difficult to establish a person’s subjective state of mind,” he said. “Usually in those cases, the plaintiff just denies memory of previous medical records or claims that the previous doctor who treated him or her for the same condition ‘got it wrong.’ In those cases, it is generally left to the jury whether to believe the plaintiff or not.”
Mr. Stinson also says he has not come across a truly fraudulent medical liability case. He noted that such a claim might be similar to a person falsely claiming a soft-tissue injury following an alleged slip-and-fall accident.
“Clearly, a fraudulent claim could be viewed as riskier from the plaintiff’s perspective because they could face criminal prosecution for insurance fraud, whereas if a claim is merely frivolous, they probably only run the risk of court-issued fine, if even that. That may be why we don’t often see fraudulent MPL claims.”
Ways to prevent or fight frivolous lawsuits
Since Dr. Stawicki’s legal nightmare as a resident, rules have tightened in Pennsylvania, and it is now more difficult to file frivolous claims, he said.
Pennsylvania is one of at least 28 states that require a certificate of merit in order for a medical liability claim to move forward. The provisions generally state that an appropriately licensed professional must supply a written statement attesting that the care the patient received failed to meet acceptable professional standards and that such conduct was a cause in the alleged harm.
“There is now a much greater burden of proof regarding what can proceed,” Dr. Stawicki said. “I’ve been involved in a couple cases that did not proceed because there was no certificate of merit.”
Although these reforms may help, not all merit rules are created equal. Some states require that the expert who signs the affidavit be knowledgeable in the relevant issues involved in the action. Other states have looser requirements. In one of the cases featured in Medical Justice’s Most Frivolous Lawsuit Contest, a podiatrist signed a supporting declaration for a claim related to obstetric care.
For physicians facing a frivolous claim, fighting it out in court depends on a number of factors. Without a consent-to-settle clause in the contract, an insurer can make the final decision on whether to defend or settle a case.
Resolving a malpractice claim is generally a business decision for the insurer, Dr. Studdert said.
“When the claim is for a relatively low amount of money, the costs of moving forward to defend that claim may be much more than the costs of simply settling it would be,” he said. “On the other hand, liability insurers and their lawyers are repeat players here, as are the plaintiffs’ attorneys. They don’t want to incentivize plaintiffs’ attorneys to bring questionable claims, and if they settle quickly, that may do so.”
Mr. Stinson, of the MPL Association, said a truly frivolous claim – one with no legal basis – is highly unlikely to be settled, “especially by MPL Association members who go beyond having a purely financial interest in their insureds to also focus on their professional reputation/integrity.” MPL Association members insure nearly 2 million health care professionals globally, including 2,500 hospitals and more than two-thirds of America’s physicians who are in private practice.
Physicians should make sure they know what is and what is not included in their policy, Dr. Segal said.
“The broker should sit down with the doctor, ideally before initial purchase or renewal, and explain in clear terms what the carrier’s obligations are and what the physician’s obligations are,” he said. “Know what type of protection is being purchased and what conditions might trigger a surprising and unhappy outcome.”
Should I countersue?
For truly frivolous claims, physicians have the legal right to sue for damages caused by the unfounded complaint.
Perhaps the most well-known case of a successful malpractice countersuit is that of Louisville neurosurgeon John Guarnaschelli, MD, who in 2000 won $72,000 in damages against a plaintiffs’ attorney for malicious prosecution.
The physician’s countersuit followed the dismissal of a negligence claim against Dr. Guarnaschelli by a patient who contracted meningitis. The plaintiffs’ attorney had made little effort to gather evidence to connect Dr. Guarnaschelli to the patient’s injuries and had consulted only one other physician, a client of his, before filing the lawsuit, according to a summary of the case in the American Bar Association Journal.
Malicious prosecution is the most common legal theory of recovery for physicians in countersuits, according to a review of successful countersuits by doctors. Dr. Stawicki is a coauthor of that review. Other legal theories that physicians can raise include abuse of process, negligence, defamation, invasion of privacy, and infliction of emotional distress. Of the 13 cases evaluated in the article by Dr. Stawicki and colleagues, damages awarded to physicians ranged from about $13,000 to $125,000.
Although some doctors have success, pursuing a counterclaim can be a difficult feat, said Benjamin Braslow, MD, a trauma surgeon and professor of clinical surgery at the University of Pennsylvania in Philadelphia.
“The main takeaways were it’s an uphill battle often met with not only resistance but diminishing returns to countersue,” said Dr. Braslow, a coauthor of the countersuits analysis. “You have to meet very specific criteria regarding leveling the suit, and it may end up being a costly, time-consuming battle.”
To prove malicious prosecution, for example, a physician must show that a claim was instituted without probable cause, that the suing party acted maliciously in instituting the action, and that the doctor was damaged by the action, among other essential elements.
As for Dr. G, the surgeon, he now has a contract with a consent-to-settle clause and has taken other legal precautions since his lawsuits. He requires that his patients sign an agreement that any negligence claims they levy go to arbitration. If an arbitrator finds in the patient’s favor, the case may proceed to court, he said. However, he requires another agreement such that if patients lose in court, they are responsible for his legal fees.
“I’m just more careful,” he said. “I ask all my staff in the office to use their judgment, however superficial, if they feel something is wrong with an individual to tell me so. I’d rather send them away than operate on them and have it result in a lawsuit.”
A version of this article originally appeared on Medscape.com.
Dr. G, a New York surgeon, was only a couple years into practice when he faced his first lawsuit.
After undergoing liposuction surgery on the area of her calf and ankle, a patient claimed she had developed a severe allergic reaction, characterized by small areas of necrosis on the lower extremities, said Dr. G, who asked to remain anonymous. However, the alleged injury seemed suspicious, said Dr. G, considering that 3 weeks after the surgery, the area had shown a successful result with minimal swelling.
Six months into the suit, Dr. G received a shocking phone call. It was the patient’s estranged husband, who revealed that his wife was having an affair with another man, a physician. In recorded phone calls, the patient and her paramour had discussed causing an injury near the patient’s calf in an attempt to sue and get rich, the husband relayed. Dr. G immediately contacted his insurance carrier with the news, but his attorney said the information would not be admissible in court. Instead, the insurer settled with the patient, who received about $125,000.
At the time, Dr. G did not have a consent-to-settle clause in his contract, so the insurer was able to settle without his approval.
In legal practice, a frivolous claim is defined as one that lacks a supporting legal argument or any factual basis. A claim issued with the intent of disturbing, annoying, or harassing the opposing party can also be described as legally frivolous, said Michael Stinson, vice president of government relations and public policy for the Medical Professional Liability Association (MPL Association), a trade association for medical liability insurers.
However, when most physicians refer to “frivolous claims,” they often mean a claim in which there is no attributable negligence. Such suits represent a second category of claims – nonmeritorious lawsuits.
“I think people intermix nonmeritorious and frivolous all the time,” Mr. Stinson said. “In the vast majority of nonmeritorious claims, the patient has suffered an adverse outcome, it’s just that it wasn’t the result of negligence, whereas with a frivolous lawsuit, they really haven’t suffered any damage, so they’ve got no business filing a lawsuit on any level.”
A third type of so-called frivolous suit is that of a fraudulent or fake claim, in which, as Dr. G experienced, a patient causes a self-injury or lies about a condition to craft a false claim against a physician.
If a patient files a claim that the patient knows is false, the patient commits fraud and may be subject to counterclaims for malicious prosecution or abuse of process, said Jeffrey Segal, MD, JD, a neurosurgeon and health law attorney. Further, the patient would be testifying under oath, and such testimony can be considered perjury, a criminal offense with criminal penalties.
Sadly, Dr. G was the target of another frivolous lawsuit years later. In that suit, a patient claimed the surgeon had left a piece of sponge in her breast cavity during surgery. The case was dismissed when medical records proved the patient knew that the foreign body resulted from an unrelated procedure she had undergone years earlier.
“There is so much abuse in the court system,” Dr. G said. “You really don’t think stuff like that will happen to you, especially if you honor the profession. It’s unfortunate. It’s left a very bitter taste in my mouth.”
Frivolous claims have long been a subject of debate. Tort reform advocates often contend that such claims are pervasive. They cite them as key reasons for high health care costs and say that they have led to the rise of defensive medicine. Plaintiffs’ attorneys counter that the rate of frivolous claims is widely exaggerated and argue that the pursuit of frivolous claims would be “bad business” for legal firms.
“I have never seen a frivolous malpractice claim,” says Malcolm P. McConnell III, JD, a Richmond, Va., medical malpractice attorney and chair of the Medical Malpractice Legislative Subcommittee for the Virginia Trial Lawyers Association. “I cannot say that such things never happen, but any lawyer bringing such a thing is foolish, because there is no reward for it.”
Are shotgun lawsuits frivolous?
To many physicians, being dragged into a lawsuit over a complaint or medical outcome in which they were not involved is frivolous, said Stanislaw Stawicki, MD, a trauma surgeon and researcher based in Bethlehem, Pa. Dr. Stawicki was named in a lawsuit along with a long list of medical staff who interacted in some way with the plaintiff. Dr. Stawicki himself saw the patient once and made a note in the chart but had nothing to do with the patient’s surgery or with any critical decisions regarding his care, he said.
“Nothing really prepares you for seeing your name on a legal complaint,” Dr. Stawicki said. “It’s traumatic. I had to block out entire days to give depositions, which were really kind of pointless. Questions like, ‘Is this really your name? Where did you train? Were you there that morning?’ Stuff that was really not consequential to the fact that someone had surgery a month earlier and had some sort of complication.”
Dr. Stawicki was eventually dropped from the claim, but not before a nearly year-long ordeal of legal proceedings, meetings, and paperwork.
It is common practice for plaintiffs’ attorneys to add codefendants in the early stages of a claim, said David M. Studdert, ScD, a leading health law researcher and a professor of law at Stanford (Calif.) Law School. Defendants are gradually dismissed as the case moves forward and details of the incident become clearer, he said.
“Plaintiffs’ attorneys have strong incentives to try and choose claims that will be successful,” Dr. Studdert said. “However, in the early point in the process, neither the patient nor the attorney may have a good idea what has actually happened with care. So sometimes, filing a lawsuit may be the only way to begin the process of opening up that information.”
A study by Dr. Studdert in which medical malpractice claims, errors, and compensation payments were analyzed found that, out of 1,452 claims, about one-third (37%) did not involve errors.
“Many physicians might call those frivolous lawsuits, but in fact, most of those don’t go on to receive compensation,” he said. “We suspect that in many instances, those claims are simply dropped once it becomes apparent that there wasn’t error involved.
“They can still be burdensome, anxiety provoking, and time consuming for physicians who are named in those suits, so I don’t want to suggest that claims that don’t involve errors are not a problem,” said Dr. Studdert. “However, I think it’s wrong to assume, as many people do when they use the term ‘frivolous lawsuit,’ that this is really an extortionary effort by a plaintiffs’ attorney to try to get money out of a hospital or a physician for care that was really unproblematic.”
Certain ‘frivolous’ cases more common than others
Nonmeritorious claims still occur relatively frequently today, according to data from the Medical Professional Liability Association’s Data Sharing Project. Of about 18,000 liability claims reported from 2016 to 2018, 65% were dropped, withdrawn, or dismissed. Of the 6% of claims that went before a jury, more than 85% resulted in a verdict for the defendant, the researchers found.
“Basically, any claim that does not result in a payment because the underlying claim of negligence on the part of a health professional had been demonstrated, proven, or adjudicated false is one we would describe as nonmeritorious,” Mr. Stinson said.
The MPL Association does not track cases that meet the legal definition of frivolous, said Mr. Stinson, and they “don’t see truly frivolous lawsuits very often.”
Malpractice claims are risky, expensive, and aggressively defended, says Mr. McConnell, the plaintiffs’ attorney. Mr. McConnell, who has been practicing for 30 years, said his own claim selection process is very rigorous and that he cannot afford to pursue claims that aren’t well supported by science and medicine.
“Pursuing frivolous cases would bankrupt me and ruin my reputation,” he said. “A lawyer I know once said he would write a check for $10,000 to anyone who could show him a lawyer who makes a living pursuing frivolous medical malpractice cases. It’s a fair challenge. The economics and the practices of liability carriers and defense lawyers make frivolous cases a dead end for plaintiff lawyers.”
Most medical malpractice cases are taken on a contingency fee basis, Mr. McConnell noted, meaning that the plaintiff’s lawyer is not paid unless the claim is successful.
“This means that the plaintiff’s lawyer is risking 2 years of intensive labor on a case which may yield no fee at all,” he said. “Obviously, any reasonable lawyer is going to want to minimize that risk. The only way to minimize that risk is for the case to be solid, not weak, and certainly not frivolous.”
But Dr. Segal, the health law attorney, says that plenty of frivolous liability claims are levied each year, with attorneys willing to pursue them.
It’s true that seasoned plaintiffs’ attorneys generally screen for merit and damages, Dr. Segal said, but in some instances, attorneys who are not trained in malpractice law accept frivolous claims and take them forward. In some cases, they are slip-and-fall accident attorneys accustomed to receiving modest amounts from insurance companies quickly, said Dr. Segal, founder of Medical Justice, a company that helps deter frivolous lawsuits against physicians.
“If we lived in a perfectly rational universe where plaintiffs’ attorneys screened cases well and only took the meritorious cases forward, we would see less frivolous cases filed, but that’s not the universe I live in,” Dr. Segal said. “There are well over a million attorneys in this country, and some are hungrier than others. The attorneys may frequently get burned in the end, and maybe that attorney won’t move another malpractice case forward, but there’s always someone else willing to take their place.”
Medical Justice has twice run a Most Frivolous Lawsuit Contest on its website, one in 2008 and one in late 2018. The first contest drew 30 entries, and the second garnered nearly 40 submissions, primarily from physicians who were defendants in the cases, according to Dr. Segal. (Dr. G’s lawsuit was highlighted in the most recent contest.)
In one case, an emergency physician was drawn into litigation by the family of a deceased patient. The patient experienced sudden cardiac arrythmia at home, and paramedics were unable to intubate her or establish IV access. She was transferred to the hospital, where resuscitation efforts continued, but she remained in asystole and was pronounced dead after 15 minutes.
At the hospital, blood tests were conducted. They showed that her serum potassium concentration was elevated to about 12 mEq/L, Dr. Segal said. The family initiated a claim in which they accused the emergency physician of failure to diagnose hyperkalemia. They alleged that had the hyperkalemia been discovered sooner, the patient’s death could have been prevented.
“If you had no other facts about this, you would wonder how a person with potassium that high would even be alive,” Dr. Segal said. “But what they were looking at was the body decomposing and all the potassium in the cells being released into the bloodstream. It wasn’t the cause of the problem, it was an effect of the problem. She really was dead on arrival, and she was probably dead at home.”
The case was eventually dropped.
Although the outcome for the patient was tragic, says Dr. Segal, the case is one of many types of frivolous claims that exist today.
“Yes, frivolous cases are out there,” he said.
Fraudulent claims uncommon
As for fraudulent medical liability claims, legal experts say they’re rare. J. Richard Moore, JD, an Indianapolis-based medical liability defense attorney, said he’s never personally encountered a medical malpractice claim in which he believed a plaintiff caused an injury or an illness and attempted to blame it on a physician.
However, Mr. Moore has defended many claims in which the illness or condition the plaintiff claimed was caused or was made worse through medical negligence was actually a preexisting condition or a preexisting condition that worsened and was not related to any medical negligence, Mr. Moore said.
“Although I have often felt in such cases that the plaintiff really knew that the condition was not affected by any alleged medical negligence, I would not put that in the ‘fraudulent claim’ category because it can be very difficult to establish a person’s subjective state of mind,” he said. “Usually in those cases, the plaintiff just denies memory of previous medical records or claims that the previous doctor who treated him or her for the same condition ‘got it wrong.’ In those cases, it is generally left to the jury whether to believe the plaintiff or not.”
Mr. Stinson also says he has not come across a truly fraudulent medical liability case. He noted that such a claim might be similar to a person falsely claiming a soft-tissue injury following an alleged slip-and-fall accident.
“Clearly, a fraudulent claim could be viewed as riskier from the plaintiff’s perspective because they could face criminal prosecution for insurance fraud, whereas if a claim is merely frivolous, they probably only run the risk of court-issued fine, if even that. That may be why we don’t often see fraudulent MPL claims.”
Ways to prevent or fight frivolous lawsuits
Since Dr. Stawicki’s legal nightmare as a resident, rules have tightened in Pennsylvania, and it is now more difficult to file frivolous claims, he said.
Pennsylvania is one of at least 28 states that require a certificate of merit in order for a medical liability claim to move forward. The provisions generally state that an appropriately licensed professional must supply a written statement attesting that the care the patient received failed to meet acceptable professional standards and that such conduct was a cause in the alleged harm.
“There is now a much greater burden of proof regarding what can proceed,” Dr. Stawicki said. “I’ve been involved in a couple cases that did not proceed because there was no certificate of merit.”
Although these reforms may help, not all merit rules are created equal. Some states require that the expert who signs the affidavit be knowledgeable in the relevant issues involved in the action. Other states have looser requirements. In one of the cases featured in Medical Justice’s Most Frivolous Lawsuit Contest, a podiatrist signed a supporting declaration for a claim related to obstetric care.
For physicians facing a frivolous claim, fighting it out in court depends on a number of factors. Without a consent-to-settle clause in the contract, an insurer can make the final decision on whether to defend or settle a case.
Resolving a malpractice claim is generally a business decision for the insurer, Dr. Studdert said.
“When the claim is for a relatively low amount of money, the costs of moving forward to defend that claim may be much more than the costs of simply settling it would be,” he said. “On the other hand, liability insurers and their lawyers are repeat players here, as are the plaintiffs’ attorneys. They don’t want to incentivize plaintiffs’ attorneys to bring questionable claims, and if they settle quickly, that may do so.”
Mr. Stinson, of the MPL Association, said a truly frivolous claim – one with no legal basis – is highly unlikely to be settled, “especially by MPL Association members who go beyond having a purely financial interest in their insureds to also focus on their professional reputation/integrity.” MPL Association members insure nearly 2 million health care professionals globally, including 2,500 hospitals and more than two-thirds of America’s physicians who are in private practice.
Physicians should make sure they know what is and what is not included in their policy, Dr. Segal said.
“The broker should sit down with the doctor, ideally before initial purchase or renewal, and explain in clear terms what the carrier’s obligations are and what the physician’s obligations are,” he said. “Know what type of protection is being purchased and what conditions might trigger a surprising and unhappy outcome.”
Should I countersue?
For truly frivolous claims, physicians have the legal right to sue for damages caused by the unfounded complaint.
Perhaps the most well-known case of a successful malpractice countersuit is that of Louisville neurosurgeon John Guarnaschelli, MD, who in 2000 won $72,000 in damages against a plaintiffs’ attorney for malicious prosecution.
The physician’s countersuit followed the dismissal of a negligence claim against Dr. Guarnaschelli by a patient who contracted meningitis. The plaintiffs’ attorney had made little effort to gather evidence to connect Dr. Guarnaschelli to the patient’s injuries and had consulted only one other physician, a client of his, before filing the lawsuit, according to a summary of the case in the American Bar Association Journal.
Malicious prosecution is the most common legal theory of recovery for physicians in countersuits, according to a review of successful countersuits by doctors. Dr. Stawicki is a coauthor of that review. Other legal theories that physicians can raise include abuse of process, negligence, defamation, invasion of privacy, and infliction of emotional distress. Of the 13 cases evaluated in the article by Dr. Stawicki and colleagues, damages awarded to physicians ranged from about $13,000 to $125,000.
Although some doctors have success, pursuing a counterclaim can be a difficult feat, said Benjamin Braslow, MD, a trauma surgeon and professor of clinical surgery at the University of Pennsylvania in Philadelphia.
“The main takeaways were it’s an uphill battle often met with not only resistance but diminishing returns to countersue,” said Dr. Braslow, a coauthor of the countersuits analysis. “You have to meet very specific criteria regarding leveling the suit, and it may end up being a costly, time-consuming battle.”
To prove malicious prosecution, for example, a physician must show that a claim was instituted without probable cause, that the suing party acted maliciously in instituting the action, and that the doctor was damaged by the action, among other essential elements.
As for Dr. G, the surgeon, he now has a contract with a consent-to-settle clause and has taken other legal precautions since his lawsuits. He requires that his patients sign an agreement that any negligence claims they levy go to arbitration. If an arbitrator finds in the patient’s favor, the case may proceed to court, he said. However, he requires another agreement such that if patients lose in court, they are responsible for his legal fees.
“I’m just more careful,” he said. “I ask all my staff in the office to use their judgment, however superficial, if they feel something is wrong with an individual to tell me so. I’d rather send them away than operate on them and have it result in a lawsuit.”
A version of this article originally appeared on Medscape.com.
Dr. G, a New York surgeon, was only a couple years into practice when he faced his first lawsuit.
After undergoing liposuction surgery on the area of her calf and ankle, a patient claimed she had developed a severe allergic reaction, characterized by small areas of necrosis on the lower extremities, said Dr. G, who asked to remain anonymous. However, the alleged injury seemed suspicious, said Dr. G, considering that 3 weeks after the surgery, the area had shown a successful result with minimal swelling.
Six months into the suit, Dr. G received a shocking phone call. It was the patient’s estranged husband, who revealed that his wife was having an affair with another man, a physician. In recorded phone calls, the patient and her paramour had discussed causing an injury near the patient’s calf in an attempt to sue and get rich, the husband relayed. Dr. G immediately contacted his insurance carrier with the news, but his attorney said the information would not be admissible in court. Instead, the insurer settled with the patient, who received about $125,000.
At the time, Dr. G did not have a consent-to-settle clause in his contract, so the insurer was able to settle without his approval.
In legal practice, a frivolous claim is defined as one that lacks a supporting legal argument or any factual basis. A claim issued with the intent of disturbing, annoying, or harassing the opposing party can also be described as legally frivolous, said Michael Stinson, vice president of government relations and public policy for the Medical Professional Liability Association (MPL Association), a trade association for medical liability insurers.
However, when most physicians refer to “frivolous claims,” they often mean a claim in which there is no attributable negligence. Such suits represent a second category of claims – nonmeritorious lawsuits.
“I think people intermix nonmeritorious and frivolous all the time,” Mr. Stinson said. “In the vast majority of nonmeritorious claims, the patient has suffered an adverse outcome, it’s just that it wasn’t the result of negligence, whereas with a frivolous lawsuit, they really haven’t suffered any damage, so they’ve got no business filing a lawsuit on any level.”
A third type of so-called frivolous suit is that of a fraudulent or fake claim, in which, as Dr. G experienced, a patient causes a self-injury or lies about a condition to craft a false claim against a physician.
If a patient files a claim that the patient knows is false, the patient commits fraud and may be subject to counterclaims for malicious prosecution or abuse of process, said Jeffrey Segal, MD, JD, a neurosurgeon and health law attorney. Further, the patient would be testifying under oath, and such testimony can be considered perjury, a criminal offense with criminal penalties.
Sadly, Dr. G was the target of another frivolous lawsuit years later. In that suit, a patient claimed the surgeon had left a piece of sponge in her breast cavity during surgery. The case was dismissed when medical records proved the patient knew that the foreign body resulted from an unrelated procedure she had undergone years earlier.
“There is so much abuse in the court system,” Dr. G said. “You really don’t think stuff like that will happen to you, especially if you honor the profession. It’s unfortunate. It’s left a very bitter taste in my mouth.”
Frivolous claims have long been a subject of debate. Tort reform advocates often contend that such claims are pervasive. They cite them as key reasons for high health care costs and say that they have led to the rise of defensive medicine. Plaintiffs’ attorneys counter that the rate of frivolous claims is widely exaggerated and argue that the pursuit of frivolous claims would be “bad business” for legal firms.
“I have never seen a frivolous malpractice claim,” says Malcolm P. McConnell III, JD, a Richmond, Va., medical malpractice attorney and chair of the Medical Malpractice Legislative Subcommittee for the Virginia Trial Lawyers Association. “I cannot say that such things never happen, but any lawyer bringing such a thing is foolish, because there is no reward for it.”
Are shotgun lawsuits frivolous?
To many physicians, being dragged into a lawsuit over a complaint or medical outcome in which they were not involved is frivolous, said Stanislaw Stawicki, MD, a trauma surgeon and researcher based in Bethlehem, Pa. Dr. Stawicki was named in a lawsuit along with a long list of medical staff who interacted in some way with the plaintiff. Dr. Stawicki himself saw the patient once and made a note in the chart but had nothing to do with the patient’s surgery or with any critical decisions regarding his care, he said.
“Nothing really prepares you for seeing your name on a legal complaint,” Dr. Stawicki said. “It’s traumatic. I had to block out entire days to give depositions, which were really kind of pointless. Questions like, ‘Is this really your name? Where did you train? Were you there that morning?’ Stuff that was really not consequential to the fact that someone had surgery a month earlier and had some sort of complication.”
Dr. Stawicki was eventually dropped from the claim, but not before a nearly year-long ordeal of legal proceedings, meetings, and paperwork.
It is common practice for plaintiffs’ attorneys to add codefendants in the early stages of a claim, said David M. Studdert, ScD, a leading health law researcher and a professor of law at Stanford (Calif.) Law School. Defendants are gradually dismissed as the case moves forward and details of the incident become clearer, he said.
“Plaintiffs’ attorneys have strong incentives to try and choose claims that will be successful,” Dr. Studdert said. “However, in the early point in the process, neither the patient nor the attorney may have a good idea what has actually happened with care. So sometimes, filing a lawsuit may be the only way to begin the process of opening up that information.”
A study by Dr. Studdert in which medical malpractice claims, errors, and compensation payments were analyzed found that, out of 1,452 claims, about one-third (37%) did not involve errors.
“Many physicians might call those frivolous lawsuits, but in fact, most of those don’t go on to receive compensation,” he said. “We suspect that in many instances, those claims are simply dropped once it becomes apparent that there wasn’t error involved.
“They can still be burdensome, anxiety provoking, and time consuming for physicians who are named in those suits, so I don’t want to suggest that claims that don’t involve errors are not a problem,” said Dr. Studdert. “However, I think it’s wrong to assume, as many people do when they use the term ‘frivolous lawsuit,’ that this is really an extortionary effort by a plaintiffs’ attorney to try to get money out of a hospital or a physician for care that was really unproblematic.”
Certain ‘frivolous’ cases more common than others
Nonmeritorious claims still occur relatively frequently today, according to data from the Medical Professional Liability Association’s Data Sharing Project. Of about 18,000 liability claims reported from 2016 to 2018, 65% were dropped, withdrawn, or dismissed. Of the 6% of claims that went before a jury, more than 85% resulted in a verdict for the defendant, the researchers found.
“Basically, any claim that does not result in a payment because the underlying claim of negligence on the part of a health professional had been demonstrated, proven, or adjudicated false is one we would describe as nonmeritorious,” Mr. Stinson said.
The MPL Association does not track cases that meet the legal definition of frivolous, said Mr. Stinson, and they “don’t see truly frivolous lawsuits very often.”
Malpractice claims are risky, expensive, and aggressively defended, says Mr. McConnell, the plaintiffs’ attorney. Mr. McConnell, who has been practicing for 30 years, said his own claim selection process is very rigorous and that he cannot afford to pursue claims that aren’t well supported by science and medicine.
“Pursuing frivolous cases would bankrupt me and ruin my reputation,” he said. “A lawyer I know once said he would write a check for $10,000 to anyone who could show him a lawyer who makes a living pursuing frivolous medical malpractice cases. It’s a fair challenge. The economics and the practices of liability carriers and defense lawyers make frivolous cases a dead end for plaintiff lawyers.”
Most medical malpractice cases are taken on a contingency fee basis, Mr. McConnell noted, meaning that the plaintiff’s lawyer is not paid unless the claim is successful.
“This means that the plaintiff’s lawyer is risking 2 years of intensive labor on a case which may yield no fee at all,” he said. “Obviously, any reasonable lawyer is going to want to minimize that risk. The only way to minimize that risk is for the case to be solid, not weak, and certainly not frivolous.”
But Dr. Segal, the health law attorney, says that plenty of frivolous liability claims are levied each year, with attorneys willing to pursue them.
It’s true that seasoned plaintiffs’ attorneys generally screen for merit and damages, Dr. Segal said, but in some instances, attorneys who are not trained in malpractice law accept frivolous claims and take them forward. In some cases, they are slip-and-fall accident attorneys accustomed to receiving modest amounts from insurance companies quickly, said Dr. Segal, founder of Medical Justice, a company that helps deter frivolous lawsuits against physicians.
“If we lived in a perfectly rational universe where plaintiffs’ attorneys screened cases well and only took the meritorious cases forward, we would see less frivolous cases filed, but that’s not the universe I live in,” Dr. Segal said. “There are well over a million attorneys in this country, and some are hungrier than others. The attorneys may frequently get burned in the end, and maybe that attorney won’t move another malpractice case forward, but there’s always someone else willing to take their place.”
Medical Justice has twice run a Most Frivolous Lawsuit Contest on its website, one in 2008 and one in late 2018. The first contest drew 30 entries, and the second garnered nearly 40 submissions, primarily from physicians who were defendants in the cases, according to Dr. Segal. (Dr. G’s lawsuit was highlighted in the most recent contest.)
In one case, an emergency physician was drawn into litigation by the family of a deceased patient. The patient experienced sudden cardiac arrythmia at home, and paramedics were unable to intubate her or establish IV access. She was transferred to the hospital, where resuscitation efforts continued, but she remained in asystole and was pronounced dead after 15 minutes.
At the hospital, blood tests were conducted. They showed that her serum potassium concentration was elevated to about 12 mEq/L, Dr. Segal said. The family initiated a claim in which they accused the emergency physician of failure to diagnose hyperkalemia. They alleged that had the hyperkalemia been discovered sooner, the patient’s death could have been prevented.
“If you had no other facts about this, you would wonder how a person with potassium that high would even be alive,” Dr. Segal said. “But what they were looking at was the body decomposing and all the potassium in the cells being released into the bloodstream. It wasn’t the cause of the problem, it was an effect of the problem. She really was dead on arrival, and she was probably dead at home.”
The case was eventually dropped.
Although the outcome for the patient was tragic, says Dr. Segal, the case is one of many types of frivolous claims that exist today.
“Yes, frivolous cases are out there,” he said.
Fraudulent claims uncommon
As for fraudulent medical liability claims, legal experts say they’re rare. J. Richard Moore, JD, an Indianapolis-based medical liability defense attorney, said he’s never personally encountered a medical malpractice claim in which he believed a plaintiff caused an injury or an illness and attempted to blame it on a physician.
However, Mr. Moore has defended many claims in which the illness or condition the plaintiff claimed was caused or was made worse through medical negligence was actually a preexisting condition or a preexisting condition that worsened and was not related to any medical negligence, Mr. Moore said.
“Although I have often felt in such cases that the plaintiff really knew that the condition was not affected by any alleged medical negligence, I would not put that in the ‘fraudulent claim’ category because it can be very difficult to establish a person’s subjective state of mind,” he said. “Usually in those cases, the plaintiff just denies memory of previous medical records or claims that the previous doctor who treated him or her for the same condition ‘got it wrong.’ In those cases, it is generally left to the jury whether to believe the plaintiff or not.”
Mr. Stinson also says he has not come across a truly fraudulent medical liability case. He noted that such a claim might be similar to a person falsely claiming a soft-tissue injury following an alleged slip-and-fall accident.
“Clearly, a fraudulent claim could be viewed as riskier from the plaintiff’s perspective because they could face criminal prosecution for insurance fraud, whereas if a claim is merely frivolous, they probably only run the risk of court-issued fine, if even that. That may be why we don’t often see fraudulent MPL claims.”
Ways to prevent or fight frivolous lawsuits
Since Dr. Stawicki’s legal nightmare as a resident, rules have tightened in Pennsylvania, and it is now more difficult to file frivolous claims, he said.
Pennsylvania is one of at least 28 states that require a certificate of merit in order for a medical liability claim to move forward. The provisions generally state that an appropriately licensed professional must supply a written statement attesting that the care the patient received failed to meet acceptable professional standards and that such conduct was a cause in the alleged harm.
“There is now a much greater burden of proof regarding what can proceed,” Dr. Stawicki said. “I’ve been involved in a couple cases that did not proceed because there was no certificate of merit.”
Although these reforms may help, not all merit rules are created equal. Some states require that the expert who signs the affidavit be knowledgeable in the relevant issues involved in the action. Other states have looser requirements. In one of the cases featured in Medical Justice’s Most Frivolous Lawsuit Contest, a podiatrist signed a supporting declaration for a claim related to obstetric care.
For physicians facing a frivolous claim, fighting it out in court depends on a number of factors. Without a consent-to-settle clause in the contract, an insurer can make the final decision on whether to defend or settle a case.
Resolving a malpractice claim is generally a business decision for the insurer, Dr. Studdert said.
“When the claim is for a relatively low amount of money, the costs of moving forward to defend that claim may be much more than the costs of simply settling it would be,” he said. “On the other hand, liability insurers and their lawyers are repeat players here, as are the plaintiffs’ attorneys. They don’t want to incentivize plaintiffs’ attorneys to bring questionable claims, and if they settle quickly, that may do so.”
Mr. Stinson, of the MPL Association, said a truly frivolous claim – one with no legal basis – is highly unlikely to be settled, “especially by MPL Association members who go beyond having a purely financial interest in their insureds to also focus on their professional reputation/integrity.” MPL Association members insure nearly 2 million health care professionals globally, including 2,500 hospitals and more than two-thirds of America’s physicians who are in private practice.
Physicians should make sure they know what is and what is not included in their policy, Dr. Segal said.
“The broker should sit down with the doctor, ideally before initial purchase or renewal, and explain in clear terms what the carrier’s obligations are and what the physician’s obligations are,” he said. “Know what type of protection is being purchased and what conditions might trigger a surprising and unhappy outcome.”
Should I countersue?
For truly frivolous claims, physicians have the legal right to sue for damages caused by the unfounded complaint.
Perhaps the most well-known case of a successful malpractice countersuit is that of Louisville neurosurgeon John Guarnaschelli, MD, who in 2000 won $72,000 in damages against a plaintiffs’ attorney for malicious prosecution.
The physician’s countersuit followed the dismissal of a negligence claim against Dr. Guarnaschelli by a patient who contracted meningitis. The plaintiffs’ attorney had made little effort to gather evidence to connect Dr. Guarnaschelli to the patient’s injuries and had consulted only one other physician, a client of his, before filing the lawsuit, according to a summary of the case in the American Bar Association Journal.
Malicious prosecution is the most common legal theory of recovery for physicians in countersuits, according to a review of successful countersuits by doctors. Dr. Stawicki is a coauthor of that review. Other legal theories that physicians can raise include abuse of process, negligence, defamation, invasion of privacy, and infliction of emotional distress. Of the 13 cases evaluated in the article by Dr. Stawicki and colleagues, damages awarded to physicians ranged from about $13,000 to $125,000.
Although some doctors have success, pursuing a counterclaim can be a difficult feat, said Benjamin Braslow, MD, a trauma surgeon and professor of clinical surgery at the University of Pennsylvania in Philadelphia.
“The main takeaways were it’s an uphill battle often met with not only resistance but diminishing returns to countersue,” said Dr. Braslow, a coauthor of the countersuits analysis. “You have to meet very specific criteria regarding leveling the suit, and it may end up being a costly, time-consuming battle.”
To prove malicious prosecution, for example, a physician must show that a claim was instituted without probable cause, that the suing party acted maliciously in instituting the action, and that the doctor was damaged by the action, among other essential elements.
As for Dr. G, the surgeon, he now has a contract with a consent-to-settle clause and has taken other legal precautions since his lawsuits. He requires that his patients sign an agreement that any negligence claims they levy go to arbitration. If an arbitrator finds in the patient’s favor, the case may proceed to court, he said. However, he requires another agreement such that if patients lose in court, they are responsible for his legal fees.
“I’m just more careful,” he said. “I ask all my staff in the office to use their judgment, however superficial, if they feel something is wrong with an individual to tell me so. I’d rather send them away than operate on them and have it result in a lawsuit.”
A version of this article originally appeared on Medscape.com.