User login
Nonwhite race, lower socioeconomic status predicts persistently active AD
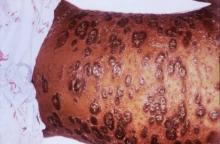
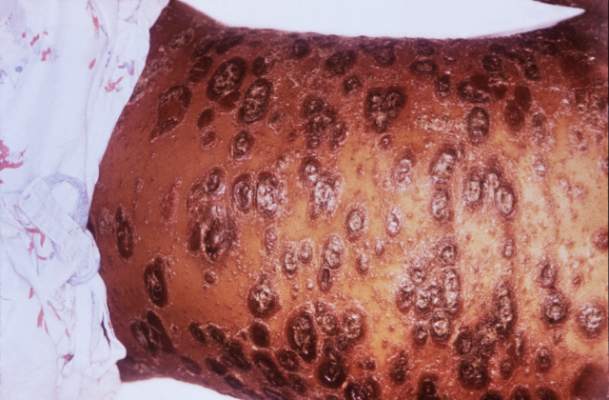
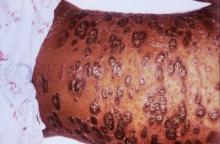
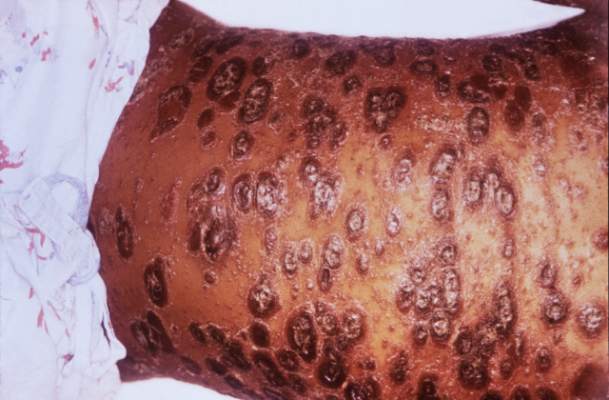
SCOTTSDALE, ARIZ. –Among patients with atopic dermatitis, persistently active disease was significantly more common among females of nonwhite race with a history of atopy than among patients without these characteristics, in an analysis of survey data from the Pediatric Elective Eczema Registry.
Annual household income under $50,000 also was a significant predictor of persistently active eczema, according to Katrina Abuabara, MD, of the department of dermatology, University of California, San Francisco, and her associates, who reported their results in a poster at the annual meeting of the Society for Investigative Dermatology.
Atopic dermatitis often persists into adulthood, but few studies have explored contributors to poor disease control. To help fill that gap, the investigators analyzed 65,237 surveys from the Pediatric Eczema Elective Registry (PEER), which tracks children and young adults aged 2-26 years with physician-diagnosed atopic dermatitis. The average age of the 6,237 patients was 7 years at enrollment (standard deviation, 4 years). They were followed at 6-month intervals for up to 10 years, with an average of about 10 surveys per respondent (standard deviation, 6.3 surveys).
In all, 4,607 patients (74% of the cohort) returned surveys spanning early childhood through their mid-20s. Only 15% of patients had “resolving” disease, meaning that as they aged, they increasingly reported complete disease control for periods of 6 months and longer.
The remaining 85% of patients had persistently active disease. In this group, 54% were female, 77% had a household income under $50,000 per year, 71% were nonwhite, and 75% had a history of atopy. Each of these characteristics significantly increased the odds of persistently active atopic dermatitis in the multivariable model (P less than .05 for each association).
Nonwhite race and history of atopy were the strongest predictors of persistently active disease – each lowered the odds of complete disease control by almost 50% (odds ratio, 0.53). Furthermore, females had 37% lower odds of complete disease control compared with males (OR, 0.63), and individuals with household income under $50,000 had 16% lower odds of complete disease control compared with those with higher annual incomes (OR, 0.84).
The link between lower socioeconomic status and persistently active eczema belies previous findings, the researchers noted. Those studies found that individuals of higher socioeconomic status were at greater risk for developing atopic dermatitis, but “failed to account for the chronic nature of the disease. In contrast, our results suggest that atopic dermatitis persistence may be associated with lower income and nonwhite race, and highlight the importance of longitudinal studies that permit analysis of mechanisms of disease control over time.”
Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
SCOTTSDALE, ARIZ. –Among patients with atopic dermatitis, persistently active disease was significantly more common among females of nonwhite race with a history of atopy than among patients without these characteristics, in an analysis of survey data from the Pediatric Elective Eczema Registry.
Annual household income under $50,000 also was a significant predictor of persistently active eczema, according to Katrina Abuabara, MD, of the department of dermatology, University of California, San Francisco, and her associates, who reported their results in a poster at the annual meeting of the Society for Investigative Dermatology.
Atopic dermatitis often persists into adulthood, but few studies have explored contributors to poor disease control. To help fill that gap, the investigators analyzed 65,237 surveys from the Pediatric Eczema Elective Registry (PEER), which tracks children and young adults aged 2-26 years with physician-diagnosed atopic dermatitis. The average age of the 6,237 patients was 7 years at enrollment (standard deviation, 4 years). They were followed at 6-month intervals for up to 10 years, with an average of about 10 surveys per respondent (standard deviation, 6.3 surveys).
In all, 4,607 patients (74% of the cohort) returned surveys spanning early childhood through their mid-20s. Only 15% of patients had “resolving” disease, meaning that as they aged, they increasingly reported complete disease control for periods of 6 months and longer.
The remaining 85% of patients had persistently active disease. In this group, 54% were female, 77% had a household income under $50,000 per year, 71% were nonwhite, and 75% had a history of atopy. Each of these characteristics significantly increased the odds of persistently active atopic dermatitis in the multivariable model (P less than .05 for each association).
Nonwhite race and history of atopy were the strongest predictors of persistently active disease – each lowered the odds of complete disease control by almost 50% (odds ratio, 0.53). Furthermore, females had 37% lower odds of complete disease control compared with males (OR, 0.63), and individuals with household income under $50,000 had 16% lower odds of complete disease control compared with those with higher annual incomes (OR, 0.84).
The link between lower socioeconomic status and persistently active eczema belies previous findings, the researchers noted. Those studies found that individuals of higher socioeconomic status were at greater risk for developing atopic dermatitis, but “failed to account for the chronic nature of the disease. In contrast, our results suggest that atopic dermatitis persistence may be associated with lower income and nonwhite race, and highlight the importance of longitudinal studies that permit analysis of mechanisms of disease control over time.”
Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
SCOTTSDALE, ARIZ. –Among patients with atopic dermatitis, persistently active disease was significantly more common among females of nonwhite race with a history of atopy than among patients without these characteristics, in an analysis of survey data from the Pediatric Elective Eczema Registry.
Annual household income under $50,000 also was a significant predictor of persistently active eczema, according to Katrina Abuabara, MD, of the department of dermatology, University of California, San Francisco, and her associates, who reported their results in a poster at the annual meeting of the Society for Investigative Dermatology.
Atopic dermatitis often persists into adulthood, but few studies have explored contributors to poor disease control. To help fill that gap, the investigators analyzed 65,237 surveys from the Pediatric Eczema Elective Registry (PEER), which tracks children and young adults aged 2-26 years with physician-diagnosed atopic dermatitis. The average age of the 6,237 patients was 7 years at enrollment (standard deviation, 4 years). They were followed at 6-month intervals for up to 10 years, with an average of about 10 surveys per respondent (standard deviation, 6.3 surveys).
In all, 4,607 patients (74% of the cohort) returned surveys spanning early childhood through their mid-20s. Only 15% of patients had “resolving” disease, meaning that as they aged, they increasingly reported complete disease control for periods of 6 months and longer.
The remaining 85% of patients had persistently active disease. In this group, 54% were female, 77% had a household income under $50,000 per year, 71% were nonwhite, and 75% had a history of atopy. Each of these characteristics significantly increased the odds of persistently active atopic dermatitis in the multivariable model (P less than .05 for each association).
Nonwhite race and history of atopy were the strongest predictors of persistently active disease – each lowered the odds of complete disease control by almost 50% (odds ratio, 0.53). Furthermore, females had 37% lower odds of complete disease control compared with males (OR, 0.63), and individuals with household income under $50,000 had 16% lower odds of complete disease control compared with those with higher annual incomes (OR, 0.84).
The link between lower socioeconomic status and persistently active eczema belies previous findings, the researchers noted. Those studies found that individuals of higher socioeconomic status were at greater risk for developing atopic dermatitis, but “failed to account for the chronic nature of the disease. In contrast, our results suggest that atopic dermatitis persistence may be associated with lower income and nonwhite race, and highlight the importance of longitudinal studies that permit analysis of mechanisms of disease control over time.”
Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Persistently active atopic dermatitis is associated with nonwhite race, annual household income under $50,000, female sex, and history of atopy.
Major finding: Nonwhite race and history of atopy each lowered the odds of complete disease control by about 43% (odds ratios, 0.53; P less than .05).
Data source: A longitudinal cohort study of 6,237 patients aged 2-26 years from the Pediatric Eczema Elective Registry (PEER).
Disclosures: Dr. Abuabara received a grant from the Clinical & Translational Science Institute of UCSF. She had no disclosures.
Severe Psoriasis Upped Lymphoma Risk in Large Cohort Study
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
AT THE 2016 SID ANNUAL MEETING
Severe psoriasis upped lymphoma risk in large cohort study
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Psoriasis was identified as an independent risk factor for lymphoma, with the risk of lymphoma increasing with disease severity.
Major finding: The strongest association was between severe psoriasis and cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4).
Data source: A population-based longitudinal cohort study of 12,198 patients with severe psoriasis, 184,870 patients with mild psoriasis, and 965,730 nonpsoriatic controls.
Disclosures: The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research support from Pfizer outside the submitted work.
Analysis Supports Daily Folate for Children With Psoriasis on Methotrexate
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
AT THE 2016 SID ANNUAL MEETING
Analysis supports daily folate for children with psoriasis on methotrexate
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
SCOTTSDALE, ARIZ. – Children and adolescents receiving methotrexate for psoriasis were significantly less likely to experience gastrointestinal side effects when they took a folate supplement every day instead of once weekly or 6 days a week, in a retrospective study of more than 400 pediatric psoriasis patients.
Laboratory abnormalities were significantly more common among children who received a folate supplement 6 days per week rather than daily, noted Inge Bronckers of the department of dermatology, Radboud University, Nijmegen, the Netherlands. “These results support the use of daily folate” in this group of patients,” she said in a poster presentation at the annual meeting of the Society for Investigative Dermatology.
Few studies have examined patterns of use or adverse effects of pediatric psoriasis therapies. Although methotrexate is a folate antagonist with related toxicities, whether folate supplementation counteracts the efficacy of methotrexate is also unclear. Because of these uncertainties, some clinicians recommend a supplement 6 days per week, avoiding the day methotrexate is given, while others recommend it daily or once weekly.
To better understand the effects of these regimens, Ms. Inge and her coinvestigators studied 446 children and adolescents who received phototherapy or systemic treatments for moderate to severe psoriasis at 20 centers in the United States, Canada, and Europe between 1990 and 2014. The patients’ average age was 8 years (standard deviation, 4 years); 238 were female and 208 were male.
Among the 390 patients receiving systemic medications, almost 70% were receiving methotrexate, while 27% were being treated with etanercept or another biologic, 15% were using retinoids, 8% were using cyclosporine, and 5% were using fumaric acid. About 19% of patients were receiving more than one of these medications. Methotrexate most often led to nausea (affecting 18% of patients), elevated hepatic transaminases (13%), dyspepsia (7%), and infections (4%), usually of the skin and upper airways. In contrast, biologics most often caused injection-site reactions (19%) and upper airways infections (10%).
Most (253) of the 270 patients on methotrexate had been prescribed folic acid, typically at a dose of about 8 mg/wk, and nearly always in the form of pure folic acid, rather than a multivitamin. Of the patients taking folic acid, about 34% took it 6 days per week, 34% received it daily and 30% – including most patients in Europe – received it once weekly.
Notably, the odds of gastrointestinal side effects were 75% lower for patients who received folic acid daily or 6 days per week, compared with those who received folic acid once a week (odds ratio, 0.25, in both cases; P less than .001), the investigators found. However, laboratory abnormalities were significantly more likely when folic acid was given 6 days a week, compared with daily (OR, 2.31; P = .03) or weekly (OR, 3.9; P = .002). Patients in Europe, who usually received folic acid weekly, were significantly more likely to have methotrexate-related gastrointestinal side effects than were patients in North America (OR, 3.4; P less than .001), and were less likely to have laboratory abnormalities (OR, 0.32; P = .004).
Patients on biologic therapy were less likely to develop laboratory abnormalities or stop treatment because of side effects than were those on other systemic therapies, Ms. Inge and her associates found. Because methotrexate was associated with elevated liver enzymes, it also was dose adjusted more often than other therapies. No patient on any therapy was diagnosed with tuberculosis or malignancy, but three patients on methotrexate had severe adverse effects, including liver disease, methotrexate hypersensitivity pneumonitis, and severe personality changes. In contrast, fumarate was associated with one case each of pericarditis and bone marrow suppression, while one patient on the biologic adalimumab developed appendicitis.
The study underscores the need to monitor the long-term risks of pediatric psoriasis treatments, the researchers concluded. Data and lessons from the study are being used to develop a prospective pediatric psoriasis registry. “If industry joins forces to use this prospective international registry to capture prospective pediatric data, we will ensure early detection of safety signals and facilitate comparative analyses of efficacy and safety,” Ms. Inge said in the poster.
The International Psoriasis Council funded the study. The investigators did not list disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Consider daily folate to reduce the likelihood of gastrointestinal side effects of methotrexate in children with psoriasis.
Major finding: The odds of gastrointestinal adverse effects were about 75% lower with daily folate, compared with weekly dosing or 6 days per week dosing that spared the methotrexate day (odds ratio, 0.25; P less than .001).
Data source: An international retrospective study of 446 children receiving phototherapy or systemic therapy for psoriasis.
Disclosures: The International Psoriasis Council funded the study. The investigators did not list disclosures.
Ustekinumab misses primary endpoint in atopic dermatitis
SCOTTSDALE, ARIZ. – Treatment with ustekinumab did not result in significant improvements in atopic dermatitis after 16 weeks, compared with placebo in a phase II study of adults with moderate to severe atopic dermatitis, Dr. Patrick Brunner reported at the annual meeting of the Society for Investigative Dermatology.
However, molecular studies revealed robust modulation of relevant transcriptomic genes after 1 month of ustekinumab, compared with placebo, said Dr. Brunner, who is with the Laboratory for Investigative Dermatology, Rockefeller University, New York. Since the crossover design of this trial and the use of topical corticosteroids by patients probably diluted the differences in clinical outcomes between the intervention and placebo groups, “another study with a different design and dosing is mandated,” he said in an oral presentation at the meeting.
Ustekinumab (Stelara) is a long-acting injectable human interleukin-12 and interleukin-23 antagonist that suppresses Th1, Th17, and Th22 activation. It was approved in 2009 in the United States for moderate to severe plaque psoriasis, and in 2013 for active psoriatic arthritis.
To investigate ustekinumab as therapy for atopic dermatitis, Dr. Brunner and his associates randomly assigned 33 adults aged 18-75 years with refractory atopic dermatitis and baseline scores on the Scoring Atopic Dermatitis (SCORAD) severity scale above 15 to subcutaneous injections of either placebo (17 patients) or ustekinumab (16) at weeks 0, 4, and 16. Ustekinumab was dosed the same way as in psoriasis: 45 mg per injection for patients at or under 100 kg, and 90 mg per injection for heavier patients. At week 16, all patients crossed over to the other treatment for another 16 weeks.
The groups were similar in terms of baseline demographics, intrinsic versus extrinsic IgE status, mean SCORAD, and average levels of IgE and eosinophils. To increase enrollment and retention, both groups were given triamcinolone acetonide 0.025%.
The proportion of SCORAD50 responders (patients with at least a 50% drop from the baseline SCORAD) was greater for ustekinumab than placebo at weeks 12, 16, and 20, but the differences never reached statistical significance, Dr. Brunner reported. At week 16, five (31%) ustekinumab-treated patients had achieved SCORAD50 (the primary endpoint), compared with three (18%) of those on placebo. The ustekinumab SCORAD50 response reached 50% by week 20, but by then patients had crossed over and the two groups began to resemble each other, he said.
“The lessons learned for designing atopic dermatitis trials are to avoid a crossover design with a long-lasting drug, and to keep in mind that even mild background topical steroids can tremendously confound detection of drug effects,” Dr. Brunner commented.
Studies of the atopic dermatitis molecular profile, or transcriptome, revealed similar gene expression levels for both trial arms at baseline, but substantially more gene modulation after 4 weeks of ustekinumab, compared with placebo, he reported. This molecular response involved the Th1, Th17, Th22, but also Th2-related atopic dermatitis genes, and in all cases the differences from placebo were statistically significant (P less than .05). Furthermore, gene modulation became more pronounced through the end of the trial and correlated with clinical response, he added.
The most common adverse effect associated with ustekinumab was respiratory infection, which affected two patients. There were no serious adverse effects reported and none of the patients stopped treatment because of adverse effects.
“Ustekinumab had clear clinical and molecular effects, but clinical outcomes might have been obscured by a profound placebo effect, most likely due to background topical glucocorticosteroids and possibly insufficient dosing for atopic dermatitis,” Dr. Brunner and his associates concluded in a report of their findings, which was published online after the meeting in Experimental Dermatology (Exp Dermatol. 2016 Jun 15. doi: 10.1111/exd.13112).
The study was supported by Janssen, the manufacturer of ustekinumab, and by the National Institutes of Health. Dr. Brunner had no disclosures.
SCOTTSDALE, ARIZ. – Treatment with ustekinumab did not result in significant improvements in atopic dermatitis after 16 weeks, compared with placebo in a phase II study of adults with moderate to severe atopic dermatitis, Dr. Patrick Brunner reported at the annual meeting of the Society for Investigative Dermatology.
However, molecular studies revealed robust modulation of relevant transcriptomic genes after 1 month of ustekinumab, compared with placebo, said Dr. Brunner, who is with the Laboratory for Investigative Dermatology, Rockefeller University, New York. Since the crossover design of this trial and the use of topical corticosteroids by patients probably diluted the differences in clinical outcomes between the intervention and placebo groups, “another study with a different design and dosing is mandated,” he said in an oral presentation at the meeting.
Ustekinumab (Stelara) is a long-acting injectable human interleukin-12 and interleukin-23 antagonist that suppresses Th1, Th17, and Th22 activation. It was approved in 2009 in the United States for moderate to severe plaque psoriasis, and in 2013 for active psoriatic arthritis.
To investigate ustekinumab as therapy for atopic dermatitis, Dr. Brunner and his associates randomly assigned 33 adults aged 18-75 years with refractory atopic dermatitis and baseline scores on the Scoring Atopic Dermatitis (SCORAD) severity scale above 15 to subcutaneous injections of either placebo (17 patients) or ustekinumab (16) at weeks 0, 4, and 16. Ustekinumab was dosed the same way as in psoriasis: 45 mg per injection for patients at or under 100 kg, and 90 mg per injection for heavier patients. At week 16, all patients crossed over to the other treatment for another 16 weeks.
The groups were similar in terms of baseline demographics, intrinsic versus extrinsic IgE status, mean SCORAD, and average levels of IgE and eosinophils. To increase enrollment and retention, both groups were given triamcinolone acetonide 0.025%.
The proportion of SCORAD50 responders (patients with at least a 50% drop from the baseline SCORAD) was greater for ustekinumab than placebo at weeks 12, 16, and 20, but the differences never reached statistical significance, Dr. Brunner reported. At week 16, five (31%) ustekinumab-treated patients had achieved SCORAD50 (the primary endpoint), compared with three (18%) of those on placebo. The ustekinumab SCORAD50 response reached 50% by week 20, but by then patients had crossed over and the two groups began to resemble each other, he said.
“The lessons learned for designing atopic dermatitis trials are to avoid a crossover design with a long-lasting drug, and to keep in mind that even mild background topical steroids can tremendously confound detection of drug effects,” Dr. Brunner commented.
Studies of the atopic dermatitis molecular profile, or transcriptome, revealed similar gene expression levels for both trial arms at baseline, but substantially more gene modulation after 4 weeks of ustekinumab, compared with placebo, he reported. This molecular response involved the Th1, Th17, Th22, but also Th2-related atopic dermatitis genes, and in all cases the differences from placebo were statistically significant (P less than .05). Furthermore, gene modulation became more pronounced through the end of the trial and correlated with clinical response, he added.
The most common adverse effect associated with ustekinumab was respiratory infection, which affected two patients. There were no serious adverse effects reported and none of the patients stopped treatment because of adverse effects.
“Ustekinumab had clear clinical and molecular effects, but clinical outcomes might have been obscured by a profound placebo effect, most likely due to background topical glucocorticosteroids and possibly insufficient dosing for atopic dermatitis,” Dr. Brunner and his associates concluded in a report of their findings, which was published online after the meeting in Experimental Dermatology (Exp Dermatol. 2016 Jun 15. doi: 10.1111/exd.13112).
The study was supported by Janssen, the manufacturer of ustekinumab, and by the National Institutes of Health. Dr. Brunner had no disclosures.
SCOTTSDALE, ARIZ. – Treatment with ustekinumab did not result in significant improvements in atopic dermatitis after 16 weeks, compared with placebo in a phase II study of adults with moderate to severe atopic dermatitis, Dr. Patrick Brunner reported at the annual meeting of the Society for Investigative Dermatology.
However, molecular studies revealed robust modulation of relevant transcriptomic genes after 1 month of ustekinumab, compared with placebo, said Dr. Brunner, who is with the Laboratory for Investigative Dermatology, Rockefeller University, New York. Since the crossover design of this trial and the use of topical corticosteroids by patients probably diluted the differences in clinical outcomes between the intervention and placebo groups, “another study with a different design and dosing is mandated,” he said in an oral presentation at the meeting.
Ustekinumab (Stelara) is a long-acting injectable human interleukin-12 and interleukin-23 antagonist that suppresses Th1, Th17, and Th22 activation. It was approved in 2009 in the United States for moderate to severe plaque psoriasis, and in 2013 for active psoriatic arthritis.
To investigate ustekinumab as therapy for atopic dermatitis, Dr. Brunner and his associates randomly assigned 33 adults aged 18-75 years with refractory atopic dermatitis and baseline scores on the Scoring Atopic Dermatitis (SCORAD) severity scale above 15 to subcutaneous injections of either placebo (17 patients) or ustekinumab (16) at weeks 0, 4, and 16. Ustekinumab was dosed the same way as in psoriasis: 45 mg per injection for patients at or under 100 kg, and 90 mg per injection for heavier patients. At week 16, all patients crossed over to the other treatment for another 16 weeks.
The groups were similar in terms of baseline demographics, intrinsic versus extrinsic IgE status, mean SCORAD, and average levels of IgE and eosinophils. To increase enrollment and retention, both groups were given triamcinolone acetonide 0.025%.
The proportion of SCORAD50 responders (patients with at least a 50% drop from the baseline SCORAD) was greater for ustekinumab than placebo at weeks 12, 16, and 20, but the differences never reached statistical significance, Dr. Brunner reported. At week 16, five (31%) ustekinumab-treated patients had achieved SCORAD50 (the primary endpoint), compared with three (18%) of those on placebo. The ustekinumab SCORAD50 response reached 50% by week 20, but by then patients had crossed over and the two groups began to resemble each other, he said.
“The lessons learned for designing atopic dermatitis trials are to avoid a crossover design with a long-lasting drug, and to keep in mind that even mild background topical steroids can tremendously confound detection of drug effects,” Dr. Brunner commented.
Studies of the atopic dermatitis molecular profile, or transcriptome, revealed similar gene expression levels for both trial arms at baseline, but substantially more gene modulation after 4 weeks of ustekinumab, compared with placebo, he reported. This molecular response involved the Th1, Th17, Th22, but also Th2-related atopic dermatitis genes, and in all cases the differences from placebo were statistically significant (P less than .05). Furthermore, gene modulation became more pronounced through the end of the trial and correlated with clinical response, he added.
The most common adverse effect associated with ustekinumab was respiratory infection, which affected two patients. There were no serious adverse effects reported and none of the patients stopped treatment because of adverse effects.
“Ustekinumab had clear clinical and molecular effects, but clinical outcomes might have been obscured by a profound placebo effect, most likely due to background topical glucocorticosteroids and possibly insufficient dosing for atopic dermatitis,” Dr. Brunner and his associates concluded in a report of their findings, which was published online after the meeting in Experimental Dermatology (Exp Dermatol. 2016 Jun 15. doi: 10.1111/exd.13112).
The study was supported by Janssen, the manufacturer of ustekinumab, and by the National Institutes of Health. Dr. Brunner had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Ustekinumab missed its primary endpoint in a phase II trial of adults with moderate to severe atopic dermatitis.
Major finding: At 16 weeks, five (31%) ustekinumab patients had achieved SCORAD50, compared with three (19%) placebo patients, a difference that was not statistically significant.
Data source: A randomized, double-blind study evaluated ustekinumab in 33 patients with moderate to severe atopic dermatitis.
Disclosures: The study was supported by Janssen, the maker of ustekinumab (Stelara), and by the National Institutes of Health. Dr. Brunner had no disclosures.
SEER data underscore mortality associated with thin melanomas
SCOTTSDALE, ARIZ. – Thin melanomas account for most melanoma deaths, even though T4 lesions have the worst prognosis, according to an analysis of melanoma data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
In 2015, a study from Queensland, Australia, reported that more patients died from T1 melanomas than did from T4 melanomas (J Invest Dermatol. 2015 Apr;135:1190-3). But a similar analysis in the United States was not available, so Dr. Shoshana Landow, a dermatologist at the Providence (R.I.) Veterans Affairs Medical Center,and her associates extracted SEER 13 data for invasive melanomas between 1992 through 2003. These registries covered Atlanta, Connecticut, Detroit, rural Georgia, Hawaii, Iowa, Los Angeles, New Mexico, San Francisco-Oakland, San Jose-Monterey, Seattle-Puget Sound, Utah, and the Alaska Native Tumor Registry, according to the SEER website.
Among 105,264 recorded melanomas during this period, 37,210 tumors were in situ, the investigators reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. They excluded those cases, as well as 4,789 cases that involved more than one primary melanoma, 8,637 that extended beyond the skin at diagnosis, 5,308 with no record of thickness, and one that was not known to be primary.
That left 49,319 invasive melanomas, of which 35,509 (72%) were T1 (0.01-1.00mm), 7,879 (16%) were T2 (1.01-2.00 mm), 3,948 (8%) were T3 (2.01-4.00 mm), and 1,983 (4%) were T4 (more than 4.00 mm). A total of 17% of the T1 lesions were 0.01-0.25 mm thick at diagnosis, while 42% were 0.26-0.50 mm thick, 25% were 0.51-0.75 mm thick, and 16% were 0.76-1.00 mm thick.
Ten years after diagnosis, 3,660 (7.4%) patients had died of melanoma, according to the study. These deaths included 1,072 T1 patients, 974 T2 patients, 985 T3 patients, and 629 T4 patients. But while T4 lesions accounted for the fewest number of deaths and made up only 4% of all invasive melanomas, the 10-year mortality rate for T4 lesions was nearly 32%, vs. 25% for T3 lesions, 12% for T2 lesions, and 3% for T1 lesions.
Most (42%) T1 lesions were 0.26-0.50 mm thick, 25% of T1 lesions were 0.51-0.75 mm thick, and the remaining T1 lesions were nearly evenly split between the thinnest (0.01-0.25 mm) and the thickest (0.76-1.00mm) categories, the researchers also reported. When considering only the T1 lesions, 10-year mortality rates were 3% for the 0.01-0.25 mm category, 1.9% for the 0.26-0.5 mm category, 3.7% for the 0.51-0.75 mm category, and 5.8% for the 0.76-1.00 mm category.
Thus, the thinnest invasive melanomas had a higher 10-year death rate than did the next-thinnest category, a finding that “demands explanation” and was not caused by ulceration, the researchers noted.
“The greatest total number of deaths was from T1 melanomas and the smallest number from T4, despite the fact that prognosis worsened as melanoma thickened from T1 to T4,” they concluded in their poster. “Our findings highlight the heavy death toll of thin melanomas.”
The authors did not specify funding sources, and had no disclosures.
SCOTTSDALE, ARIZ. – Thin melanomas account for most melanoma deaths, even though T4 lesions have the worst prognosis, according to an analysis of melanoma data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
In 2015, a study from Queensland, Australia, reported that more patients died from T1 melanomas than did from T4 melanomas (J Invest Dermatol. 2015 Apr;135:1190-3). But a similar analysis in the United States was not available, so Dr. Shoshana Landow, a dermatologist at the Providence (R.I.) Veterans Affairs Medical Center,and her associates extracted SEER 13 data for invasive melanomas between 1992 through 2003. These registries covered Atlanta, Connecticut, Detroit, rural Georgia, Hawaii, Iowa, Los Angeles, New Mexico, San Francisco-Oakland, San Jose-Monterey, Seattle-Puget Sound, Utah, and the Alaska Native Tumor Registry, according to the SEER website.
Among 105,264 recorded melanomas during this period, 37,210 tumors were in situ, the investigators reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. They excluded those cases, as well as 4,789 cases that involved more than one primary melanoma, 8,637 that extended beyond the skin at diagnosis, 5,308 with no record of thickness, and one that was not known to be primary.
That left 49,319 invasive melanomas, of which 35,509 (72%) were T1 (0.01-1.00mm), 7,879 (16%) were T2 (1.01-2.00 mm), 3,948 (8%) were T3 (2.01-4.00 mm), and 1,983 (4%) were T4 (more than 4.00 mm). A total of 17% of the T1 lesions were 0.01-0.25 mm thick at diagnosis, while 42% were 0.26-0.50 mm thick, 25% were 0.51-0.75 mm thick, and 16% were 0.76-1.00 mm thick.
Ten years after diagnosis, 3,660 (7.4%) patients had died of melanoma, according to the study. These deaths included 1,072 T1 patients, 974 T2 patients, 985 T3 patients, and 629 T4 patients. But while T4 lesions accounted for the fewest number of deaths and made up only 4% of all invasive melanomas, the 10-year mortality rate for T4 lesions was nearly 32%, vs. 25% for T3 lesions, 12% for T2 lesions, and 3% for T1 lesions.
Most (42%) T1 lesions were 0.26-0.50 mm thick, 25% of T1 lesions were 0.51-0.75 mm thick, and the remaining T1 lesions were nearly evenly split between the thinnest (0.01-0.25 mm) and the thickest (0.76-1.00mm) categories, the researchers also reported. When considering only the T1 lesions, 10-year mortality rates were 3% for the 0.01-0.25 mm category, 1.9% for the 0.26-0.5 mm category, 3.7% for the 0.51-0.75 mm category, and 5.8% for the 0.76-1.00 mm category.
Thus, the thinnest invasive melanomas had a higher 10-year death rate than did the next-thinnest category, a finding that “demands explanation” and was not caused by ulceration, the researchers noted.
“The greatest total number of deaths was from T1 melanomas and the smallest number from T4, despite the fact that prognosis worsened as melanoma thickened from T1 to T4,” they concluded in their poster. “Our findings highlight the heavy death toll of thin melanomas.”
The authors did not specify funding sources, and had no disclosures.
SCOTTSDALE, ARIZ. – Thin melanomas account for most melanoma deaths, even though T4 lesions have the worst prognosis, according to an analysis of melanoma data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program.
In 2015, a study from Queensland, Australia, reported that more patients died from T1 melanomas than did from T4 melanomas (J Invest Dermatol. 2015 Apr;135:1190-3). But a similar analysis in the United States was not available, so Dr. Shoshana Landow, a dermatologist at the Providence (R.I.) Veterans Affairs Medical Center,and her associates extracted SEER 13 data for invasive melanomas between 1992 through 2003. These registries covered Atlanta, Connecticut, Detroit, rural Georgia, Hawaii, Iowa, Los Angeles, New Mexico, San Francisco-Oakland, San Jose-Monterey, Seattle-Puget Sound, Utah, and the Alaska Native Tumor Registry, according to the SEER website.
Among 105,264 recorded melanomas during this period, 37,210 tumors were in situ, the investigators reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. They excluded those cases, as well as 4,789 cases that involved more than one primary melanoma, 8,637 that extended beyond the skin at diagnosis, 5,308 with no record of thickness, and one that was not known to be primary.
That left 49,319 invasive melanomas, of which 35,509 (72%) were T1 (0.01-1.00mm), 7,879 (16%) were T2 (1.01-2.00 mm), 3,948 (8%) were T3 (2.01-4.00 mm), and 1,983 (4%) were T4 (more than 4.00 mm). A total of 17% of the T1 lesions were 0.01-0.25 mm thick at diagnosis, while 42% were 0.26-0.50 mm thick, 25% were 0.51-0.75 mm thick, and 16% were 0.76-1.00 mm thick.
Ten years after diagnosis, 3,660 (7.4%) patients had died of melanoma, according to the study. These deaths included 1,072 T1 patients, 974 T2 patients, 985 T3 patients, and 629 T4 patients. But while T4 lesions accounted for the fewest number of deaths and made up only 4% of all invasive melanomas, the 10-year mortality rate for T4 lesions was nearly 32%, vs. 25% for T3 lesions, 12% for T2 lesions, and 3% for T1 lesions.
Most (42%) T1 lesions were 0.26-0.50 mm thick, 25% of T1 lesions were 0.51-0.75 mm thick, and the remaining T1 lesions were nearly evenly split between the thinnest (0.01-0.25 mm) and the thickest (0.76-1.00mm) categories, the researchers also reported. When considering only the T1 lesions, 10-year mortality rates were 3% for the 0.01-0.25 mm category, 1.9% for the 0.26-0.5 mm category, 3.7% for the 0.51-0.75 mm category, and 5.8% for the 0.76-1.00 mm category.
Thus, the thinnest invasive melanomas had a higher 10-year death rate than did the next-thinnest category, a finding that “demands explanation” and was not caused by ulceration, the researchers noted.
“The greatest total number of deaths was from T1 melanomas and the smallest number from T4, despite the fact that prognosis worsened as melanoma thickened from T1 to T4,” they concluded in their poster. “Our findings highlight the heavy death toll of thin melanomas.”
The authors did not specify funding sources, and had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Thin (T1) melanomas accounted for the greatest number of deaths from melanoma in a large study.
Major finding: Among 3,660 deaths from invasive melanoma, most (1,072) were in people with T1 lesions. While T4 lesions made up only 4% of invasive melanomas, their 10-year mortality rate was nearly 32%.
Data source: A study of 49,139 invasive melanomas recorded in the NCI’s SEER database.
Disclosures: The investigators did not specify funding sources and had no disclosures.
Adding calcipotriene to 5-FU dramatically reduced AKs
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: A combination of 5% 5-fluorouracil cream and 0.005% calcipotriene was significantly more effective at removing actinic keratoses at different anatomic sites as 5-FU monotherapy.
Major finding: At week 8, the combination group had an average 86% reduction in the number of facial AKs, compared with 26% with 5-FU monotherapy.
Data source: A randomized, double-blind, controlled study of 131 patients with at least four AKs on the face, scalp, and/or upper arms.
Disclosures: Dr. Demehri had no disclosures.
Keys to alopecia areata might lie in gut microbiome
SCOTTSDALE, ARIZ. – Wiping out the gut microbiome with antibiotics prevented alopecia areata in a study of mice, providing evidence that the gut microbiome may play a role in alopecia, Dr. James Chen reported at the annual meeting of the Society for Investigative Dermatology.
The finding shows that the bacterial culprits in alopecia “reside in the gut microbiome, and not in the skin,” said Dr. Chen, a postdoctoral research fellow in medical genetics at Columbia University, New York.
Alopecia areata is mediated by autoreactive NKG2D+ CD8+ T cells. Aberrations in the human microbiome underlie several other autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, and type I diabetes, Dr. Chen noted. “The gut microbiome also has been linked to skin conditions, such as acne, psoriasis, and atopic dermatitis,” he added. “So we asked, if we deplete this microbiome with an antibiotic cocktail, do we see an effect on alopecia?”
To find out, he and his coinvestigators grafted skin from C3H/Hej mice, which spontaneously develop alopecia, onto healthy younger mice, causing them to develop alopecia 6-10 weeks later. “Strikingly, we found that treating unaffected mice with an oral antibiotic cocktail prior to grafting completely prevented the development of alopecia areata, and this remained true through 15 weeks,” he said. “This is the first evidence that the gut microbiome could be implicated in alopecia, based on the absence of the phenotype that we see in treated mice.”
The researchers also evaluated whether the skin microbiomes of antibiotic-treated and control mice differed, and determined that the skin samples resembled each other in terms of overall bacterial load and bacterial taxonomic clustering patterns. That suggests that the skin microbiome is not involved in alopecia areata, Dr. Chen said.
Finally, the investigators transferred NKG2D+ CD8+ T cells from the cutaneous lymph nodes of alopecic mice to normal mice that had been pretreated with antibiotics. The treated mice had little infiltration of these T cells into the skin, and lower overall T-cell levels than control mice, Dr. Chen reported.
The investigators are now testing combinations of antibiotics and fecal transplants to pinpoint which gut bacteria make mice susceptible to hair loss. Doing so “will have significant implications on both our understanding of alopecia areata susceptibility, as well as actionable therapeutic targets for treatment” in humans, Dr. Chen said.
The study was funded by the National Institutes of Health, the Medical Research Council, the Dermatology Foundation, Locks of Love Foundation, and NYSTEM (New York State Stem Cell Science). Dr. Chen had no financial disclosures.
SCOTTSDALE, ARIZ. – Wiping out the gut microbiome with antibiotics prevented alopecia areata in a study of mice, providing evidence that the gut microbiome may play a role in alopecia, Dr. James Chen reported at the annual meeting of the Society for Investigative Dermatology.
The finding shows that the bacterial culprits in alopecia “reside in the gut microbiome, and not in the skin,” said Dr. Chen, a postdoctoral research fellow in medical genetics at Columbia University, New York.
Alopecia areata is mediated by autoreactive NKG2D+ CD8+ T cells. Aberrations in the human microbiome underlie several other autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, and type I diabetes, Dr. Chen noted. “The gut microbiome also has been linked to skin conditions, such as acne, psoriasis, and atopic dermatitis,” he added. “So we asked, if we deplete this microbiome with an antibiotic cocktail, do we see an effect on alopecia?”
To find out, he and his coinvestigators grafted skin from C3H/Hej mice, which spontaneously develop alopecia, onto healthy younger mice, causing them to develop alopecia 6-10 weeks later. “Strikingly, we found that treating unaffected mice with an oral antibiotic cocktail prior to grafting completely prevented the development of alopecia areata, and this remained true through 15 weeks,” he said. “This is the first evidence that the gut microbiome could be implicated in alopecia, based on the absence of the phenotype that we see in treated mice.”
The researchers also evaluated whether the skin microbiomes of antibiotic-treated and control mice differed, and determined that the skin samples resembled each other in terms of overall bacterial load and bacterial taxonomic clustering patterns. That suggests that the skin microbiome is not involved in alopecia areata, Dr. Chen said.
Finally, the investigators transferred NKG2D+ CD8+ T cells from the cutaneous lymph nodes of alopecic mice to normal mice that had been pretreated with antibiotics. The treated mice had little infiltration of these T cells into the skin, and lower overall T-cell levels than control mice, Dr. Chen reported.
The investigators are now testing combinations of antibiotics and fecal transplants to pinpoint which gut bacteria make mice susceptible to hair loss. Doing so “will have significant implications on both our understanding of alopecia areata susceptibility, as well as actionable therapeutic targets for treatment” in humans, Dr. Chen said.
The study was funded by the National Institutes of Health, the Medical Research Council, the Dermatology Foundation, Locks of Love Foundation, and NYSTEM (New York State Stem Cell Science). Dr. Chen had no financial disclosures.
SCOTTSDALE, ARIZ. – Wiping out the gut microbiome with antibiotics prevented alopecia areata in a study of mice, providing evidence that the gut microbiome may play a role in alopecia, Dr. James Chen reported at the annual meeting of the Society for Investigative Dermatology.
The finding shows that the bacterial culprits in alopecia “reside in the gut microbiome, and not in the skin,” said Dr. Chen, a postdoctoral research fellow in medical genetics at Columbia University, New York.
Alopecia areata is mediated by autoreactive NKG2D+ CD8+ T cells. Aberrations in the human microbiome underlie several other autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, and type I diabetes, Dr. Chen noted. “The gut microbiome also has been linked to skin conditions, such as acne, psoriasis, and atopic dermatitis,” he added. “So we asked, if we deplete this microbiome with an antibiotic cocktail, do we see an effect on alopecia?”
To find out, he and his coinvestigators grafted skin from C3H/Hej mice, which spontaneously develop alopecia, onto healthy younger mice, causing them to develop alopecia 6-10 weeks later. “Strikingly, we found that treating unaffected mice with an oral antibiotic cocktail prior to grafting completely prevented the development of alopecia areata, and this remained true through 15 weeks,” he said. “This is the first evidence that the gut microbiome could be implicated in alopecia, based on the absence of the phenotype that we see in treated mice.”
The researchers also evaluated whether the skin microbiomes of antibiotic-treated and control mice differed, and determined that the skin samples resembled each other in terms of overall bacterial load and bacterial taxonomic clustering patterns. That suggests that the skin microbiome is not involved in alopecia areata, Dr. Chen said.
Finally, the investigators transferred NKG2D+ CD8+ T cells from the cutaneous lymph nodes of alopecic mice to normal mice that had been pretreated with antibiotics. The treated mice had little infiltration of these T cells into the skin, and lower overall T-cell levels than control mice, Dr. Chen reported.
The investigators are now testing combinations of antibiotics and fecal transplants to pinpoint which gut bacteria make mice susceptible to hair loss. Doing so “will have significant implications on both our understanding of alopecia areata susceptibility, as well as actionable therapeutic targets for treatment” in humans, Dr. Chen said.
The study was funded by the National Institutes of Health, the Medical Research Council, the Dermatology Foundation, Locks of Love Foundation, and NYSTEM (New York State Stem Cell Science). Dr. Chen had no financial disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Using antibiotics to eliminate the gut microbiome in mice prevented them from developing alopecia.
Major finding: The mice also had lower levels of cytotoxic T-cell infiltration into the skin, compared with alopecic controls.
Data source: A study of C3H/Hej (alopecic) mice and healthy young mice that received skin grafts from the alopecic phenotype.
Disclosures: The study was funded by the National Institutes of Health, the Medical Research Council, the Dermatology Foundation, Locks of Love Foundation, and NYSTEM (New York State Stem Cell Science). Dr. Chen had no financial disclosures.
JAK inhibitor improves alopecia, with caveats
SCOTTSDALE, ARIZ. – The Janus kinase (JAK) inhibitor tofacitinib dramatically improved several cases of alopecia areata (AA), although some patients relapsed to worse than baseline after completing treatment in a small open label pilot trial.
“Regrowth was demonstrated in 11 out of 12 patients on tofacitinib. Seven out of 12 patients achieved more than 50% regrowth,” Dr. Shawn Sidharthan reported at the annual meeting of the Society for Investigative Dermatology.
Worldwide, alopecia areata, which is caused by immune-mediated destruction of hair follicles, has a lifetime incidence of about 2% (Clin Cosmet Investig Dermatol. 2015;8:397-403). But there are no Food and Drug Administration–approved treatments for AA. Tofacitinib (Xeljanz), which is approved by the FDA for moderate to severe rheumatoid arthritis in adults, is a JAK1 and JAK3 inhibitor that curbs the interferon-gamma response inflammatory pathway, said Dr. Sidharthan of the department of dermatology and genetics at Columbia University, New York.
AA shares the same interferon response pathway, and tofacitinib prevented alopecia in mice and led to hair regrowth in a patient with alopecia universalis, he noted.
The single-arm trial included seven patients with moderate to severe patchy AA and five patients with alopecia totalis or alopecia universalis. Patients were treated for 6 months. They initially received 5 mg tofacitinib orally twice daily, which was increased to 10 mg twice daily to improve response. The investigators evaluated patients based on SALT (Severity of Alopecia Tool) scores and the Alopecia Areata Disease Activity Index (ALADIN), which uses three-dimensional bioinformatics to identify groups of genes linked to alopecia.
Seven of 12 patients experienced at least 50% regrowth, including six patients who only improved on 10 mg tofacitinib twice daily, Dr. Sidharthan said. Three additional patients “had good regrowth, but not 50%,” he reported. Among the two remaining patients, one had full regrowth, but dropped out of the study because of uncontrolled hypertension, and one patient with alopecia universalis had little or no regrowth.
Notably, two patients began shedding hair after stopping tofacitinib during the observation period of the study, and their final SALT scores were worse than baseline, Dr. Sidharthan said.
Laboratory monitoring of the cohort revealed no severe adverse events, but one patient paused treatment because of thrombocytopenia. The patient’s platelet count normalized after 2 weeks off tofacitinib, and remained normal when the dose was gradually increased to 10 mg twice daily. Another patient developed leukocytosis that resolved during the off-treatment observation period. One patient who did not comply with instructions to avoid alcohol had elevated liver function tests and was taken off the study. Two patients experienced self-limiting diarrhea, and one patient developed trace hematuria, Dr. Sidharthan noted.
In the study, ALADIN scores correlated with clinical response, he said.
He and his coinvestigators concluded that the overall results “provide a strong rationale for larger clinical trials using JAK inhibitors in alopecia areata,” he said.
Dr. Sidharthan noted that another oral JAK inhibitor, ruxolitinib (Jakafi), led to nearly full hair regrowth in three patients with alopecia in a Columbia University study (Nat Med. 2014 Sep; 20[9]:1043-9).
The Locks of Love Foundation funded the research. Dr. Sidharthan, a clinical research fellow in dermatology at Columbia, had no disclosures.
SCOTTSDALE, ARIZ. – The Janus kinase (JAK) inhibitor tofacitinib dramatically improved several cases of alopecia areata (AA), although some patients relapsed to worse than baseline after completing treatment in a small open label pilot trial.
“Regrowth was demonstrated in 11 out of 12 patients on tofacitinib. Seven out of 12 patients achieved more than 50% regrowth,” Dr. Shawn Sidharthan reported at the annual meeting of the Society for Investigative Dermatology.
Worldwide, alopecia areata, which is caused by immune-mediated destruction of hair follicles, has a lifetime incidence of about 2% (Clin Cosmet Investig Dermatol. 2015;8:397-403). But there are no Food and Drug Administration–approved treatments for AA. Tofacitinib (Xeljanz), which is approved by the FDA for moderate to severe rheumatoid arthritis in adults, is a JAK1 and JAK3 inhibitor that curbs the interferon-gamma response inflammatory pathway, said Dr. Sidharthan of the department of dermatology and genetics at Columbia University, New York.
AA shares the same interferon response pathway, and tofacitinib prevented alopecia in mice and led to hair regrowth in a patient with alopecia universalis, he noted.
The single-arm trial included seven patients with moderate to severe patchy AA and five patients with alopecia totalis or alopecia universalis. Patients were treated for 6 months. They initially received 5 mg tofacitinib orally twice daily, which was increased to 10 mg twice daily to improve response. The investigators evaluated patients based on SALT (Severity of Alopecia Tool) scores and the Alopecia Areata Disease Activity Index (ALADIN), which uses three-dimensional bioinformatics to identify groups of genes linked to alopecia.
Seven of 12 patients experienced at least 50% regrowth, including six patients who only improved on 10 mg tofacitinib twice daily, Dr. Sidharthan said. Three additional patients “had good regrowth, but not 50%,” he reported. Among the two remaining patients, one had full regrowth, but dropped out of the study because of uncontrolled hypertension, and one patient with alopecia universalis had little or no regrowth.
Notably, two patients began shedding hair after stopping tofacitinib during the observation period of the study, and their final SALT scores were worse than baseline, Dr. Sidharthan said.
Laboratory monitoring of the cohort revealed no severe adverse events, but one patient paused treatment because of thrombocytopenia. The patient’s platelet count normalized after 2 weeks off tofacitinib, and remained normal when the dose was gradually increased to 10 mg twice daily. Another patient developed leukocytosis that resolved during the off-treatment observation period. One patient who did not comply with instructions to avoid alcohol had elevated liver function tests and was taken off the study. Two patients experienced self-limiting diarrhea, and one patient developed trace hematuria, Dr. Sidharthan noted.
In the study, ALADIN scores correlated with clinical response, he said.
He and his coinvestigators concluded that the overall results “provide a strong rationale for larger clinical trials using JAK inhibitors in alopecia areata,” he said.
Dr. Sidharthan noted that another oral JAK inhibitor, ruxolitinib (Jakafi), led to nearly full hair regrowth in three patients with alopecia in a Columbia University study (Nat Med. 2014 Sep; 20[9]:1043-9).
The Locks of Love Foundation funded the research. Dr. Sidharthan, a clinical research fellow in dermatology at Columbia, had no disclosures.
SCOTTSDALE, ARIZ. – The Janus kinase (JAK) inhibitor tofacitinib dramatically improved several cases of alopecia areata (AA), although some patients relapsed to worse than baseline after completing treatment in a small open label pilot trial.
“Regrowth was demonstrated in 11 out of 12 patients on tofacitinib. Seven out of 12 patients achieved more than 50% regrowth,” Dr. Shawn Sidharthan reported at the annual meeting of the Society for Investigative Dermatology.
Worldwide, alopecia areata, which is caused by immune-mediated destruction of hair follicles, has a lifetime incidence of about 2% (Clin Cosmet Investig Dermatol. 2015;8:397-403). But there are no Food and Drug Administration–approved treatments for AA. Tofacitinib (Xeljanz), which is approved by the FDA for moderate to severe rheumatoid arthritis in adults, is a JAK1 and JAK3 inhibitor that curbs the interferon-gamma response inflammatory pathway, said Dr. Sidharthan of the department of dermatology and genetics at Columbia University, New York.
AA shares the same interferon response pathway, and tofacitinib prevented alopecia in mice and led to hair regrowth in a patient with alopecia universalis, he noted.
The single-arm trial included seven patients with moderate to severe patchy AA and five patients with alopecia totalis or alopecia universalis. Patients were treated for 6 months. They initially received 5 mg tofacitinib orally twice daily, which was increased to 10 mg twice daily to improve response. The investigators evaluated patients based on SALT (Severity of Alopecia Tool) scores and the Alopecia Areata Disease Activity Index (ALADIN), which uses three-dimensional bioinformatics to identify groups of genes linked to alopecia.
Seven of 12 patients experienced at least 50% regrowth, including six patients who only improved on 10 mg tofacitinib twice daily, Dr. Sidharthan said. Three additional patients “had good regrowth, but not 50%,” he reported. Among the two remaining patients, one had full regrowth, but dropped out of the study because of uncontrolled hypertension, and one patient with alopecia universalis had little or no regrowth.
Notably, two patients began shedding hair after stopping tofacitinib during the observation period of the study, and their final SALT scores were worse than baseline, Dr. Sidharthan said.
Laboratory monitoring of the cohort revealed no severe adverse events, but one patient paused treatment because of thrombocytopenia. The patient’s platelet count normalized after 2 weeks off tofacitinib, and remained normal when the dose was gradually increased to 10 mg twice daily. Another patient developed leukocytosis that resolved during the off-treatment observation period. One patient who did not comply with instructions to avoid alcohol had elevated liver function tests and was taken off the study. Two patients experienced self-limiting diarrhea, and one patient developed trace hematuria, Dr. Sidharthan noted.
In the study, ALADIN scores correlated with clinical response, he said.
He and his coinvestigators concluded that the overall results “provide a strong rationale for larger clinical trials using JAK inhibitors in alopecia areata,” he said.
Dr. Sidharthan noted that another oral JAK inhibitor, ruxolitinib (Jakafi), led to nearly full hair regrowth in three patients with alopecia in a Columbia University study (Nat Med. 2014 Sep; 20[9]:1043-9).
The Locks of Love Foundation funded the research. Dr. Sidharthan, a clinical research fellow in dermatology at Columbia, had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Tofacitinib dramatically improved several cases of alopecia areata, but some patients relapsed after stopping treatment.
Major finding: Eleven of 12 patients experienced regrowth, including seven with at least 50% regrowth, but two patients relapsed to worse than baseline after stopping treatment.
Data source: The single-center open-label pilot trial evaluated tofacitinib in 12 patients with alopecia areata, totalis, or universalis.
Disclosures: The Locks of Love Foundation funded the research. Dr. Shawn Sidharthan had no disclosures.