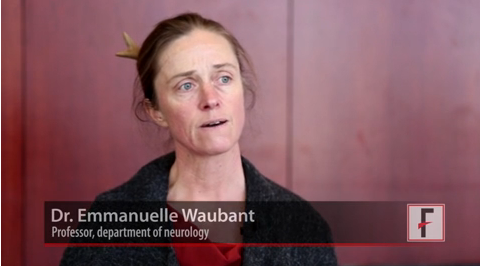User login
American Academy of Neurology (AAN): Annual Meeting
VIDEO: New meds, remyelination therapies move ahead in multiple sclerosis
PHILADELPHIA – From biomarkers for better diagnosis to new biologic agents for more effective treatment, multiple sclerosis patients may soon enjoy a wave of promising advances.
In an interview at the annual meeting of the American Academy of Neurology, Dr. Emmanuelle Waubant, professor of neurology at the University of California, San Francisco, talked about progress being made with remyelination therapies and discussed three investigational drugs that could reach the market soon.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
PHILADELPHIA – From biomarkers for better diagnosis to new biologic agents for more effective treatment, multiple sclerosis patients may soon enjoy a wave of promising advances.
In an interview at the annual meeting of the American Academy of Neurology, Dr. Emmanuelle Waubant, professor of neurology at the University of California, San Francisco, talked about progress being made with remyelination therapies and discussed three investigational drugs that could reach the market soon.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
PHILADELPHIA – From biomarkers for better diagnosis to new biologic agents for more effective treatment, multiple sclerosis patients may soon enjoy a wave of promising advances.
In an interview at the annual meeting of the American Academy of Neurology, Dr. Emmanuelle Waubant, professor of neurology at the University of California, San Francisco, talked about progress being made with remyelination therapies and discussed three investigational drugs that could reach the market soon.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE AAN 2014 ANNUAL MEETING
Eteplirsen showed safety, efficacy over 2 years in Duchenne muscular dystrophy
PHILADELPHIA – Eteplirsen safely maintained its beneficial effect on walking speeds for certain patients with Duchenne muscular dystrophy for more than 2 years and was the first drug to show stabilized diaphragm function over the same period.
The investigational gene therapy drug had a stabilizing effect on patients’ walking speed over the course of 120 weeks regardless of whether they started it earlier or later in the open-label extension of the initial 24-week, randomized trial, but those who started treatment earlier maintained a higher walking speed, Dr. Jerry R. Mendell said at the annual meeting of the American Academy of Neurology.
Coinvestigator Dr. Edward M. Kaye of Sarepta Therapeutics, the study sponsor, presented in-depth safety and pharmacokinetics data that showed no significant treatment-related adverse events with eteplirsen in the small study. No patients have discontinued or disrupted treatment, and no laboratory evidence of toxicity has been seen to date.
The initial 24-week, double-blind, randomized, placebo-controlled study involved four patients who were given the equivalent of 30 mg/kg eteplirsen weekly, four given 50 mg/kg eteplirsen weekly, and four given placebo. At the start of the open-label extension study, two of the placebo-treated patients were transitioned to 30 mg/kg weekly and two to 50 mg/kg weekly.
All patients underwent muscle biopsy at baseline, followed by biopsies at 12 weeks in two of the high-dose patients and in two of the placebo-treated patients, and at 24 weeks in the four patients taking 30 mg/kg weekly and in two placebo-treated patients.
"This design permitted a comparison between high dose over a shorter time interval and low dose over a longer time interval," said Dr. Mendell of the Center for Gene Therapy at Nationwide Children’s Hospital, Columbus, Ohio.
All patients underwent a biopsy at 1 year (48 weeks) in the open-label extension (Ann. Neurol. 2013;74:637-47).
By 48 weeks, the mean percentage of dystrophin-positive muscle fibers was 34% in the placebo/30 mg/kg delayed-treatment group, 43% in the placebo/50 mg/kg delayed-treatment group, 42% in the 50-mg/kg group, and 52% in the 30-mg/kg group.
Distance traveled on the 6-minute walk test began to diverge between active and placebo-treated patients at 12 weeks and stabilized at 24 weeks among those who received active treatment, but continued to decline until 36 weeks for placebo-treated patients who started treatment at 24 weeks.
At 120 weeks, the patients who had received continuous treatment with eteplirsen declined by a mean of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen. In comparison, studies of the natural history of Duchenne muscular dystrophy (DMD) have shown steady declines on the 6-minute walk test, ranging from 30 to 115 m at 1 year and 97 to 125 m at 2 years.
There was "remarkable stability" in diaphragm function over the course of treatment with eteplirsen, "which is quite different from the natural history of untreated patients," Dr. Mendell said. In all 12 patients, maximal expiratory pressure remained stable, going from a mean of 90% of predicted at baseline to 95% at 120 weeks. The same was true for maximal inspiratory pressure, moving from 79% of expected at baseline to 80% at 120 weeks. In contrast, the natural history of untreated DMD shows that pulmonary function declines substantially over time: by 3.9%/year for maximal inspiratory pressure after starting at 90% of predicted at 9 years of age, and by 3.6%/year after starting at 45% of predicted at 9 years of age (Pediatr. Pulmonol. 2014;49:473-81).
Eteplirsen was created to help patients with a deletion of exon 51 in dystrophin, which causes a nonfunctional dystrophin protein. The drug is a charge-neutral phosphorodiamidate morpholino oligomer (PMO) that targets the 13% of DMD patients with this mutation by directing alternative splicing of the dystrophin gene through its ability to bind to exon 51 of dystrophin pre-mRNA. This restores transcription and translation of a truncated, yet functional, dystrophin protein, such as those found in Becker muscular dystrophy.
The neutral charge of eteplirsen helps it to avoid binding to serum proteins, which is one of the problems that have occurred with phosphorothioate antisense oligonucleotide-based drugs. Phosphorothioate antisense drugs have also been linked to immune activation, hepatotoxicity, thrombocytopenia, coagulopathy, renal toxicity, and proteinuria. But Dr. Kaye reported that eteplirsen is mainly excreted by the kidneys, has a half-life of about 3 hours, and showed no evidence of those problems. Only 13 of 453 urine protein assessments tested positive, but all were low, transient, and resolved spontaneously.
"The reason that this is important is there has actually been very little human data until recently, despite the fact that [PMOs have] been around for over 30 years. Most of the work has been done in animals and in research laboratories, and it’s only until recently that people have been exposed to the drug," Dr. Kaye said.
The study was funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
PHILADELPHIA – Eteplirsen safely maintained its beneficial effect on walking speeds for certain patients with Duchenne muscular dystrophy for more than 2 years and was the first drug to show stabilized diaphragm function over the same period.
The investigational gene therapy drug had a stabilizing effect on patients’ walking speed over the course of 120 weeks regardless of whether they started it earlier or later in the open-label extension of the initial 24-week, randomized trial, but those who started treatment earlier maintained a higher walking speed, Dr. Jerry R. Mendell said at the annual meeting of the American Academy of Neurology.
Coinvestigator Dr. Edward M. Kaye of Sarepta Therapeutics, the study sponsor, presented in-depth safety and pharmacokinetics data that showed no significant treatment-related adverse events with eteplirsen in the small study. No patients have discontinued or disrupted treatment, and no laboratory evidence of toxicity has been seen to date.
The initial 24-week, double-blind, randomized, placebo-controlled study involved four patients who were given the equivalent of 30 mg/kg eteplirsen weekly, four given 50 mg/kg eteplirsen weekly, and four given placebo. At the start of the open-label extension study, two of the placebo-treated patients were transitioned to 30 mg/kg weekly and two to 50 mg/kg weekly.
All patients underwent muscle biopsy at baseline, followed by biopsies at 12 weeks in two of the high-dose patients and in two of the placebo-treated patients, and at 24 weeks in the four patients taking 30 mg/kg weekly and in two placebo-treated patients.
"This design permitted a comparison between high dose over a shorter time interval and low dose over a longer time interval," said Dr. Mendell of the Center for Gene Therapy at Nationwide Children’s Hospital, Columbus, Ohio.
All patients underwent a biopsy at 1 year (48 weeks) in the open-label extension (Ann. Neurol. 2013;74:637-47).
By 48 weeks, the mean percentage of dystrophin-positive muscle fibers was 34% in the placebo/30 mg/kg delayed-treatment group, 43% in the placebo/50 mg/kg delayed-treatment group, 42% in the 50-mg/kg group, and 52% in the 30-mg/kg group.
Distance traveled on the 6-minute walk test began to diverge between active and placebo-treated patients at 12 weeks and stabilized at 24 weeks among those who received active treatment, but continued to decline until 36 weeks for placebo-treated patients who started treatment at 24 weeks.
At 120 weeks, the patients who had received continuous treatment with eteplirsen declined by a mean of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen. In comparison, studies of the natural history of Duchenne muscular dystrophy (DMD) have shown steady declines on the 6-minute walk test, ranging from 30 to 115 m at 1 year and 97 to 125 m at 2 years.
There was "remarkable stability" in diaphragm function over the course of treatment with eteplirsen, "which is quite different from the natural history of untreated patients," Dr. Mendell said. In all 12 patients, maximal expiratory pressure remained stable, going from a mean of 90% of predicted at baseline to 95% at 120 weeks. The same was true for maximal inspiratory pressure, moving from 79% of expected at baseline to 80% at 120 weeks. In contrast, the natural history of untreated DMD shows that pulmonary function declines substantially over time: by 3.9%/year for maximal inspiratory pressure after starting at 90% of predicted at 9 years of age, and by 3.6%/year after starting at 45% of predicted at 9 years of age (Pediatr. Pulmonol. 2014;49:473-81).
Eteplirsen was created to help patients with a deletion of exon 51 in dystrophin, which causes a nonfunctional dystrophin protein. The drug is a charge-neutral phosphorodiamidate morpholino oligomer (PMO) that targets the 13% of DMD patients with this mutation by directing alternative splicing of the dystrophin gene through its ability to bind to exon 51 of dystrophin pre-mRNA. This restores transcription and translation of a truncated, yet functional, dystrophin protein, such as those found in Becker muscular dystrophy.
The neutral charge of eteplirsen helps it to avoid binding to serum proteins, which is one of the problems that have occurred with phosphorothioate antisense oligonucleotide-based drugs. Phosphorothioate antisense drugs have also been linked to immune activation, hepatotoxicity, thrombocytopenia, coagulopathy, renal toxicity, and proteinuria. But Dr. Kaye reported that eteplirsen is mainly excreted by the kidneys, has a half-life of about 3 hours, and showed no evidence of those problems. Only 13 of 453 urine protein assessments tested positive, but all were low, transient, and resolved spontaneously.
"The reason that this is important is there has actually been very little human data until recently, despite the fact that [PMOs have] been around for over 30 years. Most of the work has been done in animals and in research laboratories, and it’s only until recently that people have been exposed to the drug," Dr. Kaye said.
The study was funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
PHILADELPHIA – Eteplirsen safely maintained its beneficial effect on walking speeds for certain patients with Duchenne muscular dystrophy for more than 2 years and was the first drug to show stabilized diaphragm function over the same period.
The investigational gene therapy drug had a stabilizing effect on patients’ walking speed over the course of 120 weeks regardless of whether they started it earlier or later in the open-label extension of the initial 24-week, randomized trial, but those who started treatment earlier maintained a higher walking speed, Dr. Jerry R. Mendell said at the annual meeting of the American Academy of Neurology.
Coinvestigator Dr. Edward M. Kaye of Sarepta Therapeutics, the study sponsor, presented in-depth safety and pharmacokinetics data that showed no significant treatment-related adverse events with eteplirsen in the small study. No patients have discontinued or disrupted treatment, and no laboratory evidence of toxicity has been seen to date.
The initial 24-week, double-blind, randomized, placebo-controlled study involved four patients who were given the equivalent of 30 mg/kg eteplirsen weekly, four given 50 mg/kg eteplirsen weekly, and four given placebo. At the start of the open-label extension study, two of the placebo-treated patients were transitioned to 30 mg/kg weekly and two to 50 mg/kg weekly.
All patients underwent muscle biopsy at baseline, followed by biopsies at 12 weeks in two of the high-dose patients and in two of the placebo-treated patients, and at 24 weeks in the four patients taking 30 mg/kg weekly and in two placebo-treated patients.
"This design permitted a comparison between high dose over a shorter time interval and low dose over a longer time interval," said Dr. Mendell of the Center for Gene Therapy at Nationwide Children’s Hospital, Columbus, Ohio.
All patients underwent a biopsy at 1 year (48 weeks) in the open-label extension (Ann. Neurol. 2013;74:637-47).
By 48 weeks, the mean percentage of dystrophin-positive muscle fibers was 34% in the placebo/30 mg/kg delayed-treatment group, 43% in the placebo/50 mg/kg delayed-treatment group, 42% in the 50-mg/kg group, and 52% in the 30-mg/kg group.
Distance traveled on the 6-minute walk test began to diverge between active and placebo-treated patients at 12 weeks and stabilized at 24 weeks among those who received active treatment, but continued to decline until 36 weeks for placebo-treated patients who started treatment at 24 weeks.
At 120 weeks, the patients who had received continuous treatment with eteplirsen declined by a mean of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen. In comparison, studies of the natural history of Duchenne muscular dystrophy (DMD) have shown steady declines on the 6-minute walk test, ranging from 30 to 115 m at 1 year and 97 to 125 m at 2 years.
There was "remarkable stability" in diaphragm function over the course of treatment with eteplirsen, "which is quite different from the natural history of untreated patients," Dr. Mendell said. In all 12 patients, maximal expiratory pressure remained stable, going from a mean of 90% of predicted at baseline to 95% at 120 weeks. The same was true for maximal inspiratory pressure, moving from 79% of expected at baseline to 80% at 120 weeks. In contrast, the natural history of untreated DMD shows that pulmonary function declines substantially over time: by 3.9%/year for maximal inspiratory pressure after starting at 90% of predicted at 9 years of age, and by 3.6%/year after starting at 45% of predicted at 9 years of age (Pediatr. Pulmonol. 2014;49:473-81).
Eteplirsen was created to help patients with a deletion of exon 51 in dystrophin, which causes a nonfunctional dystrophin protein. The drug is a charge-neutral phosphorodiamidate morpholino oligomer (PMO) that targets the 13% of DMD patients with this mutation by directing alternative splicing of the dystrophin gene through its ability to bind to exon 51 of dystrophin pre-mRNA. This restores transcription and translation of a truncated, yet functional, dystrophin protein, such as those found in Becker muscular dystrophy.
The neutral charge of eteplirsen helps it to avoid binding to serum proteins, which is one of the problems that have occurred with phosphorothioate antisense oligonucleotide-based drugs. Phosphorothioate antisense drugs have also been linked to immune activation, hepatotoxicity, thrombocytopenia, coagulopathy, renal toxicity, and proteinuria. But Dr. Kaye reported that eteplirsen is mainly excreted by the kidneys, has a half-life of about 3 hours, and showed no evidence of those problems. Only 13 of 453 urine protein assessments tested positive, but all were low, transient, and resolved spontaneously.
"The reason that this is important is there has actually been very little human data until recently, despite the fact that [PMOs have] been around for over 30 years. Most of the work has been done in animals and in research laboratories, and it’s only until recently that people have been exposed to the drug," Dr. Kaye said.
The study was funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
AT THE AAN 2014 ANNUAL MEETING
Key clinical point: Treatment with eteplirsen stabilized key clinical features of DMD and had no significant adverse events over 2 years.
Major finding: At 120 weeks, patients who received continuous treatment with eteplirsen had a mean decline of 14 m on the 6-minute walk test since baseline, compared with a decline of 79 m in those who underwent delayed treatment with eteplirsen.
Data source: An open-label extension of a 24-week, randomized, double-blind, placebo-controlled trial out to 120 weeks in 12 patients with DMD.
Disclosures: The study is funded by Sarepta Therapeutics. Dr. Kaye and two coauthors are employees of the company. Dr. Mendell had no relevant disclosures.
Rasagiline’s failure as active control halts phase III preladenant trial
PHILADELPHIA – Results of the phase III clinical trial to evaluate preladenant as monotherapy for Parkinson’s disease are difficult to interpret because the lack of observed efficacy of rasagiline as an active control indicates that the study didn’t work.
"There was not a significantly greater benefit in any of the arms, compared with placebo, including all the preladenant arms, and the rasagiline arm, our active-control medication with known efficacy," Dr. Robert A. Hauser revealed to a giggling crowd, some of whom applauded, at the annual meeting of the American Academy of Neurology.
"Interestingly enough, placebo was statistically superior to the lowest dose of preladenant," said Dr. Hauser, director of the Parkinson’s Disease and Movement Disorder Center at the University of South Florida, Tampa.
The randomized, double-blind, placebo- and active-controlled, parallel-group trial was designed to cover two 26-week time periods for a total of 52 weeks, and it was conducted at multiple centers in multiple countries. More than half (58%) of the 1,022 participants were men. The average age was 63 years, and the mean baseline score on the Unified Parkinson’s Disease Rating Scale (UPDRS) parts 2 and 3 was 28.5. The study participants had been diagnosed with Parkinson’s disease within the previous 5 years, and had not yet received any levodopa or dopamine agonists.
The efficacy parameter was based on a change from baseline (CFB) in patient UPDRS scores. There was a dose-ordered response, although the patients in the preladenant 2 mg twice daily arm actually worsened, showing a significant CFB of 0.30 and a 2.60 difference when compared with placebo (P = .0033; 95% confidence interval, 0.86-4.30).
The preladenant 5 mg twice-daily group improved, showing a –1.00 CFB and a 1.30 difference from placebo (P = .1382; 95% CI, –0.41-2.94). The preladenant 10 mg twice-daily arm also improved, showing a CFB of –1.80 with a difference of 0.40 from placebo (P = .6378; 95% CI, –1.29-2.11).
Patients in the 1 mg once-daily rasagiline group improved, but not significantly, with a CFB of -1.90 when compared with placebo (P = .6923; 95% CI, –1.35-2.03), however, the placebo arm had the best results overall with a CFB of –2.20.
"I should point out that rasagiline was our active comparator, and it was included to make sure that the trial worked," said Dr. Hauser. "The fact that [the rasagiline group] did not separate from placebo indicates that this was a failed trial."
Rasagiline is a monoamine oxidase type-B inhibitor, with Food and Drug Administration approval as both monotherapy in early Parkinson’s disease an as an adjunctive treatment in moderate to advanced Parkinson’s disease.
A post hoc analyses revealed that, across the sites, the strongest rasagiline response vs. placebo (when looking at the CFB on the UPDRS parts 2 and 3) occurred in the European Union (–2.4) and North America (–1.5). In Eastern Europe, placebo performed 4 points better than did rasagiline. In Latin America, the rasagiline and placebo arms showed parity. Dr. Hauser speculated that regional differences might reflect experience and expertise of sites in those regions that participated in the trial.
Preladenant previously had been found effective as an adjunct to levodopa when dosed at either 5 mg or 10 mg twice per day.
While it is not possible to draw definitive conclusions about preladenant’s efficacy as a monotherapy for Parkinson’s disease based on this trial, Dr. Hauser stated, "One of things I think this trial demonstrates is the value of having an active-control medication known to have efficacy in a situation when you are testing a new medication."
Dr. Hauser disclosed that he and his associates had many industry relationships, including his own with Abbott Laboratories, Allergan, AstraZeneca, Biotie Therapies, Ceregene, Chelsea Therapeutics, and GE Healthcare. This trial was underwritten by Merck.
On Twitter @whitneymcknight
PHILADELPHIA – Results of the phase III clinical trial to evaluate preladenant as monotherapy for Parkinson’s disease are difficult to interpret because the lack of observed efficacy of rasagiline as an active control indicates that the study didn’t work.
"There was not a significantly greater benefit in any of the arms, compared with placebo, including all the preladenant arms, and the rasagiline arm, our active-control medication with known efficacy," Dr. Robert A. Hauser revealed to a giggling crowd, some of whom applauded, at the annual meeting of the American Academy of Neurology.
"Interestingly enough, placebo was statistically superior to the lowest dose of preladenant," said Dr. Hauser, director of the Parkinson’s Disease and Movement Disorder Center at the University of South Florida, Tampa.
The randomized, double-blind, placebo- and active-controlled, parallel-group trial was designed to cover two 26-week time periods for a total of 52 weeks, and it was conducted at multiple centers in multiple countries. More than half (58%) of the 1,022 participants were men. The average age was 63 years, and the mean baseline score on the Unified Parkinson’s Disease Rating Scale (UPDRS) parts 2 and 3 was 28.5. The study participants had been diagnosed with Parkinson’s disease within the previous 5 years, and had not yet received any levodopa or dopamine agonists.
The efficacy parameter was based on a change from baseline (CFB) in patient UPDRS scores. There was a dose-ordered response, although the patients in the preladenant 2 mg twice daily arm actually worsened, showing a significant CFB of 0.30 and a 2.60 difference when compared with placebo (P = .0033; 95% confidence interval, 0.86-4.30).
The preladenant 5 mg twice-daily group improved, showing a –1.00 CFB and a 1.30 difference from placebo (P = .1382; 95% CI, –0.41-2.94). The preladenant 10 mg twice-daily arm also improved, showing a CFB of –1.80 with a difference of 0.40 from placebo (P = .6378; 95% CI, –1.29-2.11).
Patients in the 1 mg once-daily rasagiline group improved, but not significantly, with a CFB of -1.90 when compared with placebo (P = .6923; 95% CI, –1.35-2.03), however, the placebo arm had the best results overall with a CFB of –2.20.
"I should point out that rasagiline was our active comparator, and it was included to make sure that the trial worked," said Dr. Hauser. "The fact that [the rasagiline group] did not separate from placebo indicates that this was a failed trial."
Rasagiline is a monoamine oxidase type-B inhibitor, with Food and Drug Administration approval as both monotherapy in early Parkinson’s disease an as an adjunctive treatment in moderate to advanced Parkinson’s disease.
A post hoc analyses revealed that, across the sites, the strongest rasagiline response vs. placebo (when looking at the CFB on the UPDRS parts 2 and 3) occurred in the European Union (–2.4) and North America (–1.5). In Eastern Europe, placebo performed 4 points better than did rasagiline. In Latin America, the rasagiline and placebo arms showed parity. Dr. Hauser speculated that regional differences might reflect experience and expertise of sites in those regions that participated in the trial.
Preladenant previously had been found effective as an adjunct to levodopa when dosed at either 5 mg or 10 mg twice per day.
While it is not possible to draw definitive conclusions about preladenant’s efficacy as a monotherapy for Parkinson’s disease based on this trial, Dr. Hauser stated, "One of things I think this trial demonstrates is the value of having an active-control medication known to have efficacy in a situation when you are testing a new medication."
Dr. Hauser disclosed that he and his associates had many industry relationships, including his own with Abbott Laboratories, Allergan, AstraZeneca, Biotie Therapies, Ceregene, Chelsea Therapeutics, and GE Healthcare. This trial was underwritten by Merck.
On Twitter @whitneymcknight
PHILADELPHIA – Results of the phase III clinical trial to evaluate preladenant as monotherapy for Parkinson’s disease are difficult to interpret because the lack of observed efficacy of rasagiline as an active control indicates that the study didn’t work.
"There was not a significantly greater benefit in any of the arms, compared with placebo, including all the preladenant arms, and the rasagiline arm, our active-control medication with known efficacy," Dr. Robert A. Hauser revealed to a giggling crowd, some of whom applauded, at the annual meeting of the American Academy of Neurology.
"Interestingly enough, placebo was statistically superior to the lowest dose of preladenant," said Dr. Hauser, director of the Parkinson’s Disease and Movement Disorder Center at the University of South Florida, Tampa.
The randomized, double-blind, placebo- and active-controlled, parallel-group trial was designed to cover two 26-week time periods for a total of 52 weeks, and it was conducted at multiple centers in multiple countries. More than half (58%) of the 1,022 participants were men. The average age was 63 years, and the mean baseline score on the Unified Parkinson’s Disease Rating Scale (UPDRS) parts 2 and 3 was 28.5. The study participants had been diagnosed with Parkinson’s disease within the previous 5 years, and had not yet received any levodopa or dopamine agonists.
The efficacy parameter was based on a change from baseline (CFB) in patient UPDRS scores. There was a dose-ordered response, although the patients in the preladenant 2 mg twice daily arm actually worsened, showing a significant CFB of 0.30 and a 2.60 difference when compared with placebo (P = .0033; 95% confidence interval, 0.86-4.30).
The preladenant 5 mg twice-daily group improved, showing a –1.00 CFB and a 1.30 difference from placebo (P = .1382; 95% CI, –0.41-2.94). The preladenant 10 mg twice-daily arm also improved, showing a CFB of –1.80 with a difference of 0.40 from placebo (P = .6378; 95% CI, –1.29-2.11).
Patients in the 1 mg once-daily rasagiline group improved, but not significantly, with a CFB of -1.90 when compared with placebo (P = .6923; 95% CI, –1.35-2.03), however, the placebo arm had the best results overall with a CFB of –2.20.
"I should point out that rasagiline was our active comparator, and it was included to make sure that the trial worked," said Dr. Hauser. "The fact that [the rasagiline group] did not separate from placebo indicates that this was a failed trial."
Rasagiline is a monoamine oxidase type-B inhibitor, with Food and Drug Administration approval as both monotherapy in early Parkinson’s disease an as an adjunctive treatment in moderate to advanced Parkinson’s disease.
A post hoc analyses revealed that, across the sites, the strongest rasagiline response vs. placebo (when looking at the CFB on the UPDRS parts 2 and 3) occurred in the European Union (–2.4) and North America (–1.5). In Eastern Europe, placebo performed 4 points better than did rasagiline. In Latin America, the rasagiline and placebo arms showed parity. Dr. Hauser speculated that regional differences might reflect experience and expertise of sites in those regions that participated in the trial.
Preladenant previously had been found effective as an adjunct to levodopa when dosed at either 5 mg or 10 mg twice per day.
While it is not possible to draw definitive conclusions about preladenant’s efficacy as a monotherapy for Parkinson’s disease based on this trial, Dr. Hauser stated, "One of things I think this trial demonstrates is the value of having an active-control medication known to have efficacy in a situation when you are testing a new medication."
Dr. Hauser disclosed that he and his associates had many industry relationships, including his own with Abbott Laboratories, Allergan, AstraZeneca, Biotie Therapies, Ceregene, Chelsea Therapeutics, and GE Healthcare. This trial was underwritten by Merck.
On Twitter @whitneymcknight
AT THE AAN 2014 ANNUAL MEETING
Key clinical point: An active-control medication with known efficacy is a valuable tool when testing a new medication.
Major finding: Neither preladenant nor rasagiline bested placebo for Parkinson’s disease monotherapy by week 26 of a 1-year, phase III clinical trial.
Data source: Multicenter, double-blind randomized study of 1,022 persons diagnosed with Parkinson’s disease within 5 years, who were not receiving levodopa or dopamine agonists.
Disclosures: Dr. Hauser disclosed that he and his associates had many industry relationships, including his own with Abbott Laboratories, Allergan, AstraZeneca, Biotie Therapies, Ceregene, Chelsea Therapeutics, and GE Healthcare. This trial was underwritten by Merck.
B-cell inhibitor might prevent new brain lesions in multiple sclerosis
A drug that inhibits early-stage B-lymphocyte activation reduced disease activity in patients with relapsing-remitting multiple sclerosis, and showed potential to significantly decrease the annual number of new brain lesions.
The "intriguing" early results of a phase II randomized study suggest that ofatumumab’s targeting of peripheral B cells may be beneficial in treating the disease, according to Daren Austin, Ph.D., head of biopharm clinical pharmacology and biometrics at GlaxoSmithKline, Uxbridge, U.K. He will present these data Wednesday, April 30, at the annual meeting of the American Academy of Neurology.
"These results need to be validated, of course, but the findings are interesting," Dr. Austin said in a press statement. "They provide new insight into the mechanism of B cells in MS [multiple sclerosis] and present a possible new target threshold for exploring the potential benefit of anti-B-cell therapy."
Ofatumumab (Arzerra) is a fully humanized monoclonal antibody against B-lymphocyte antigen CD20, which optimizes B-cell antibody response. On April 17, the U.S. Food and Drug Administration approved the agent for use in combination with chlorambucil in previously untreated patients with chronic lymphocytic leukemia, for whom fludarabine-based therapy is considered inappropriate.
The drug also has shown potential in treating follicular non-Hodgkin’s lymphoma, diffuse large B-cell lymphoma, and rheumatoid arthritis.
The data Dr. Austin will report are part of an ongoing dose-ranging evaluation of 3 to 180 mg of ofatumumab for relapsing-remitting MS. The drug is administered by subcutaneous injection.
The current study included 231 patients who were randomized to placebo or ofatumumab for 24 weeks. The primary endpoint was the correlation of CD19 B-cell count to new gadolinium-enhancing brain lesions (a proxy measure for disease activity). Brain imaging was conducted every 4 weeks throughout the trial.
All of the groups – including placebo – showed lesion activity in the first 4 weeks. However, patients in the active groups showed some lesion suppression after that time. The beneficial effect seemed to appear when patients’ CD19 count fell below 64 cells/mcL, Dr. Austin said.
Researchers correlated B-cell levels to the total number of new brain lesions that appeared on magnetic resonance imaging. If CD19 levels were maintained in the range of 32-64 cells/mcL, patients taking the drug would develop fewer than 1 new lesion per year, compared with 16 new lesions per year in untreated patients, Dr. Austin said in the statement.
The most common side effects, which occurred twice as often in the active group as in the placebo group, were injection-site reaction, dizziness, anxiety, fever, respiratory tract infection, and neuropathy.
Ofatumumab is not approved anywhere in the world for use in the treatment of MS, Dr. Austin added.
GlaxoSmithKline sponsored the study. Dr. Austin is an employee of the company.
A drug that inhibits early-stage B-lymphocyte activation reduced disease activity in patients with relapsing-remitting multiple sclerosis, and showed potential to significantly decrease the annual number of new brain lesions.
The "intriguing" early results of a phase II randomized study suggest that ofatumumab’s targeting of peripheral B cells may be beneficial in treating the disease, according to Daren Austin, Ph.D., head of biopharm clinical pharmacology and biometrics at GlaxoSmithKline, Uxbridge, U.K. He will present these data Wednesday, April 30, at the annual meeting of the American Academy of Neurology.
"These results need to be validated, of course, but the findings are interesting," Dr. Austin said in a press statement. "They provide new insight into the mechanism of B cells in MS [multiple sclerosis] and present a possible new target threshold for exploring the potential benefit of anti-B-cell therapy."
Ofatumumab (Arzerra) is a fully humanized monoclonal antibody against B-lymphocyte antigen CD20, which optimizes B-cell antibody response. On April 17, the U.S. Food and Drug Administration approved the agent for use in combination with chlorambucil in previously untreated patients with chronic lymphocytic leukemia, for whom fludarabine-based therapy is considered inappropriate.
The drug also has shown potential in treating follicular non-Hodgkin’s lymphoma, diffuse large B-cell lymphoma, and rheumatoid arthritis.
The data Dr. Austin will report are part of an ongoing dose-ranging evaluation of 3 to 180 mg of ofatumumab for relapsing-remitting MS. The drug is administered by subcutaneous injection.
The current study included 231 patients who were randomized to placebo or ofatumumab for 24 weeks. The primary endpoint was the correlation of CD19 B-cell count to new gadolinium-enhancing brain lesions (a proxy measure for disease activity). Brain imaging was conducted every 4 weeks throughout the trial.
All of the groups – including placebo – showed lesion activity in the first 4 weeks. However, patients in the active groups showed some lesion suppression after that time. The beneficial effect seemed to appear when patients’ CD19 count fell below 64 cells/mcL, Dr. Austin said.
Researchers correlated B-cell levels to the total number of new brain lesions that appeared on magnetic resonance imaging. If CD19 levels were maintained in the range of 32-64 cells/mcL, patients taking the drug would develop fewer than 1 new lesion per year, compared with 16 new lesions per year in untreated patients, Dr. Austin said in the statement.
The most common side effects, which occurred twice as often in the active group as in the placebo group, were injection-site reaction, dizziness, anxiety, fever, respiratory tract infection, and neuropathy.
Ofatumumab is not approved anywhere in the world for use in the treatment of MS, Dr. Austin added.
GlaxoSmithKline sponsored the study. Dr. Austin is an employee of the company.
A drug that inhibits early-stage B-lymphocyte activation reduced disease activity in patients with relapsing-remitting multiple sclerosis, and showed potential to significantly decrease the annual number of new brain lesions.
The "intriguing" early results of a phase II randomized study suggest that ofatumumab’s targeting of peripheral B cells may be beneficial in treating the disease, according to Daren Austin, Ph.D., head of biopharm clinical pharmacology and biometrics at GlaxoSmithKline, Uxbridge, U.K. He will present these data Wednesday, April 30, at the annual meeting of the American Academy of Neurology.
"These results need to be validated, of course, but the findings are interesting," Dr. Austin said in a press statement. "They provide new insight into the mechanism of B cells in MS [multiple sclerosis] and present a possible new target threshold for exploring the potential benefit of anti-B-cell therapy."
Ofatumumab (Arzerra) is a fully humanized monoclonal antibody against B-lymphocyte antigen CD20, which optimizes B-cell antibody response. On April 17, the U.S. Food and Drug Administration approved the agent for use in combination with chlorambucil in previously untreated patients with chronic lymphocytic leukemia, for whom fludarabine-based therapy is considered inappropriate.
The drug also has shown potential in treating follicular non-Hodgkin’s lymphoma, diffuse large B-cell lymphoma, and rheumatoid arthritis.
The data Dr. Austin will report are part of an ongoing dose-ranging evaluation of 3 to 180 mg of ofatumumab for relapsing-remitting MS. The drug is administered by subcutaneous injection.
The current study included 231 patients who were randomized to placebo or ofatumumab for 24 weeks. The primary endpoint was the correlation of CD19 B-cell count to new gadolinium-enhancing brain lesions (a proxy measure for disease activity). Brain imaging was conducted every 4 weeks throughout the trial.
All of the groups – including placebo – showed lesion activity in the first 4 weeks. However, patients in the active groups showed some lesion suppression after that time. The beneficial effect seemed to appear when patients’ CD19 count fell below 64 cells/mcL, Dr. Austin said.
Researchers correlated B-cell levels to the total number of new brain lesions that appeared on magnetic resonance imaging. If CD19 levels were maintained in the range of 32-64 cells/mcL, patients taking the drug would develop fewer than 1 new lesion per year, compared with 16 new lesions per year in untreated patients, Dr. Austin said in the statement.
The most common side effects, which occurred twice as often in the active group as in the placebo group, were injection-site reaction, dizziness, anxiety, fever, respiratory tract infection, and neuropathy.
Ofatumumab is not approved anywhere in the world for use in the treatment of MS, Dr. Austin added.
GlaxoSmithKline sponsored the study. Dr. Austin is an employee of the company.
FROM THE AAN 2014 ANNUAL MEETING
Major finding: Patients receiving the biologic agent ofatumumab experienced suppression of multiple sclerosis lesions when their CD19 B-cell counts fell below 64 cells/mcL.
Data source: A phase II, randomized, dose-ranging study of 231 patients.
Disclosures: GlaxoSmithKline sponsored the study. Dr. Austin is an employee of the company.
MCI confers increased risk of death
Patients with mild cognitive impairment were 80% more likely to die over a 6-year period than cognitively normal patients.
The risks were significantly elevated for both patients with amnestic and nonamnestic mild cognitive impairment (MCI), Dr. Maria Vassilaki and her colleagues reported in a study released April 23 in advance of the annual meeting of the American Academy of Neurology.
"Our findings build on the previous awareness of the higher mortality rate associated with MCI [mild cognitive impairment] and could promote greater vigilance in the management of these patients, enhancing timely management of any medical issues that might arise," said Dr. Vassilaki of the Mayo Clinic, Rochester, Minn. "In parallel, this information could serve as a reminder to individuals diagnosed with MCI to consult promptly with their health care team in any instances where health-related issues arise."
The findings were from a substudy of the Mayo Clinic Study on Aging (Neuroepidemiology 2008;30:58-69). The study accrued information on more than 2,000 residents of Olmstead County, Minn., who were aged 70-89 years at the time of enrollment.
Over a median follow-up of about 6 years, 331 of the 862 subjects with MCI died as did 224 of the 1,292 cognitively normal subjects.
Mortality was significantly increased whether the subjects later developed dementia (hazard ratio, 1.47). But it was much higher among those who had amnestic MCI than among the nonamnestic group (HR, 1.68 and 2.26, respectively).
"We still need to continue the research for a longer follow-up period to see whether such differences in death rates persist," Dr. Vassilaki said in an interview. "We also need to understand the factors that lead to greater mortality in individuals with MCI and to determine why individuals with nonamnestic MCI had a higher mortality rate than [did] those with amnestic MCI, compared with cognitively normal individuals."
The causal relationship is still under investigation, she said.
"There is another step to this investigation underway, to look at the causes of death. We just retrieved data on the underlying causes of death and will compare those in the different MCI subgroups and in cognitively normal individuals. This might shed some light," Dr. Vassilaki said.
She noted that previous studies have reported that individuals with MCI (either with amnestic MCI or nonamnestic MCI, depending on what the study investigated) were more likely to have dementia as an underlying cause of death, compared with cognitively normal individuals.
Dr. Vassilaki noted that Dr. Ronald Peterson and Dr. Rosebud O. Roberts are primary investigators on the Mayo Clinic Study on Aging.
She reported having no financial conflicts of interest. The study was supported with a grant from the National Institute on Aging.
On Twitter @alz_gal
Patients with mild cognitive impairment were 80% more likely to die over a 6-year period than cognitively normal patients.
The risks were significantly elevated for both patients with amnestic and nonamnestic mild cognitive impairment (MCI), Dr. Maria Vassilaki and her colleagues reported in a study released April 23 in advance of the annual meeting of the American Academy of Neurology.
"Our findings build on the previous awareness of the higher mortality rate associated with MCI [mild cognitive impairment] and could promote greater vigilance in the management of these patients, enhancing timely management of any medical issues that might arise," said Dr. Vassilaki of the Mayo Clinic, Rochester, Minn. "In parallel, this information could serve as a reminder to individuals diagnosed with MCI to consult promptly with their health care team in any instances where health-related issues arise."
The findings were from a substudy of the Mayo Clinic Study on Aging (Neuroepidemiology 2008;30:58-69). The study accrued information on more than 2,000 residents of Olmstead County, Minn., who were aged 70-89 years at the time of enrollment.
Over a median follow-up of about 6 years, 331 of the 862 subjects with MCI died as did 224 of the 1,292 cognitively normal subjects.
Mortality was significantly increased whether the subjects later developed dementia (hazard ratio, 1.47). But it was much higher among those who had amnestic MCI than among the nonamnestic group (HR, 1.68 and 2.26, respectively).
"We still need to continue the research for a longer follow-up period to see whether such differences in death rates persist," Dr. Vassilaki said in an interview. "We also need to understand the factors that lead to greater mortality in individuals with MCI and to determine why individuals with nonamnestic MCI had a higher mortality rate than [did] those with amnestic MCI, compared with cognitively normal individuals."
The causal relationship is still under investigation, she said.
"There is another step to this investigation underway, to look at the causes of death. We just retrieved data on the underlying causes of death and will compare those in the different MCI subgroups and in cognitively normal individuals. This might shed some light," Dr. Vassilaki said.
She noted that previous studies have reported that individuals with MCI (either with amnestic MCI or nonamnestic MCI, depending on what the study investigated) were more likely to have dementia as an underlying cause of death, compared with cognitively normal individuals.
Dr. Vassilaki noted that Dr. Ronald Peterson and Dr. Rosebud O. Roberts are primary investigators on the Mayo Clinic Study on Aging.
She reported having no financial conflicts of interest. The study was supported with a grant from the National Institute on Aging.
On Twitter @alz_gal
Patients with mild cognitive impairment were 80% more likely to die over a 6-year period than cognitively normal patients.
The risks were significantly elevated for both patients with amnestic and nonamnestic mild cognitive impairment (MCI), Dr. Maria Vassilaki and her colleagues reported in a study released April 23 in advance of the annual meeting of the American Academy of Neurology.
"Our findings build on the previous awareness of the higher mortality rate associated with MCI [mild cognitive impairment] and could promote greater vigilance in the management of these patients, enhancing timely management of any medical issues that might arise," said Dr. Vassilaki of the Mayo Clinic, Rochester, Minn. "In parallel, this information could serve as a reminder to individuals diagnosed with MCI to consult promptly with their health care team in any instances where health-related issues arise."
The findings were from a substudy of the Mayo Clinic Study on Aging (Neuroepidemiology 2008;30:58-69). The study accrued information on more than 2,000 residents of Olmstead County, Minn., who were aged 70-89 years at the time of enrollment.
Over a median follow-up of about 6 years, 331 of the 862 subjects with MCI died as did 224 of the 1,292 cognitively normal subjects.
Mortality was significantly increased whether the subjects later developed dementia (hazard ratio, 1.47). But it was much higher among those who had amnestic MCI than among the nonamnestic group (HR, 1.68 and 2.26, respectively).
"We still need to continue the research for a longer follow-up period to see whether such differences in death rates persist," Dr. Vassilaki said in an interview. "We also need to understand the factors that lead to greater mortality in individuals with MCI and to determine why individuals with nonamnestic MCI had a higher mortality rate than [did] those with amnestic MCI, compared with cognitively normal individuals."
The causal relationship is still under investigation, she said.
"There is another step to this investigation underway, to look at the causes of death. We just retrieved data on the underlying causes of death and will compare those in the different MCI subgroups and in cognitively normal individuals. This might shed some light," Dr. Vassilaki said.
She noted that previous studies have reported that individuals with MCI (either with amnestic MCI or nonamnestic MCI, depending on what the study investigated) were more likely to have dementia as an underlying cause of death, compared with cognitively normal individuals.
Dr. Vassilaki noted that Dr. Ronald Peterson and Dr. Rosebud O. Roberts are primary investigators on the Mayo Clinic Study on Aging.
She reported having no financial conflicts of interest. The study was supported with a grant from the National Institute on Aging.
On Twitter @alz_gal
AT THE AAN 2014 ANNUAL MEETING
Major finding: Over 6 years of follow-up, 331 of 862 patients with MCI died vs. 224 of 1,292 cognitively normal patients.
Data source: A 2,000 patient subset of the Mayo Clinic Study on Aging, which enrolled residents of Olmsted County, Minn., aged 70-89 years.
Disclosures: Dr. Vassilaki reported no conflicts of interest. The study was supported by the National Institute on Aging.
CGRP-targeted migraine prevention drugs succeed in phase II
Two investigational migraine prevention drugs that target a vasodilating peptide significantly decreased the number of migraine days per month, compared with placebo, during a pair of phase II studies.
Both drugs reduced mean migraine headache days per month by more than 4 days overall, and one even eliminated migraine in up to 30% of patients.
Each is a monoclonal antibody that inhibits calcitonin gene–related peptide (CGRP). Activated trigeminal sensory nerves release the peptide, which dilates intra- and extracranial blood vessels and modulates vascular nociception.
The antibodies could be game changers for migraine patients, Dr. Peter Goadsby said in an interview.
"Here are the first mechanism-based preventive treatments for migraine," said Dr. Goadsby of the University of California, San Francisco. "They are easy to use, biweekly or even monthly injections, which are effective and well tolerated. For neurologists it means the first major addition to the preventive armamentarium for a generation or more, and for patients, a tangible advance that they can look forward to enjoying soon."
During the annual meeting of the American Academy of Neurology, Dr. Goadsby will present the findings on ALD403, developed by Alder Biopharmaceuticals. His colleague Dr. David W. Dodick of the Mayo Clinic, Rochester, Minn., will present findings on LY2951742, which is being developed by Arteaus Therapeutics in conjunction with Eli Lily. Both presentations are scheduled for May 1.
The trial of ALD403 comprised 163 patients who experienced 5-14 migraine headache days per month; 81 were randomized to receive a single infusion of 1,000 mg ALD403 and the rest received placebo. Patients were followed for 24 weeks. The primary outcome was the mean change in migraine days between weeks 5-8 and baseline.
Patients in the active treatment group fared significantly better than those in the placebo group, with a mean reduction in migraine headache days of –5.6, compared to –4.6 for the placebo group (66% vs. 52% decrease).
Most patients taking the study drug had at least a 50% reduction in migraine headache days at 12 weeks (60% vs. 30%); the proportions of those with at least a 75% reduction were 32% vs. 9%. A 100% reduction occurred in 16% of the active group and in none of the placebo group. These differences were all statistically significant.
There were no differences in the type or frequency of adverse events, vital signs, or laboratory safety data between the two treatment groups.
The LY2951742 study was similarly successful. It comprised 217 patients who experienced 4-14 migraine headache days per month, said Dr. Dodick. They were randomized to placebo or to biweekly subcutaneous injections of LY2951742 150 mg for 12 weeks. The primary end point was the mean change in number of migraine days per month. Secondary end points were the change in headache days, migraine attacks, and responder rate.
Patients in the active treatment group experienced a significantly greater reduction in migraine days than those taking placebo (–4.2 vs. –3 days; 63% vs. 42% decrease).
LY2951742 was significantly better than placebo in all secondary end points including headache days (–4.9 vs. –3.7), migraine attacks (–3 vs. –2.3), and overall response rate (70% vs. 45%).
In an exploratory analysis, 33% of those in the active treatment group were complete responders, with a 100% reduction in migraine headache days, compared with 17% of the placebo group,
Adverse events seen more frequently with LY2951742 than placebo included injection site pain, upper respiratory tract infections, and abdominal pain.
Both drugs will advance to phase III studies, Dr. Dodick said in an interview. "If they are ultimately confirmed to be effective and safe, this will represent a new era in mechanism-based migraine prevention – in other words, treatment directed at a molecule now known to be very important in generating a migraine attack. This would provide a highly effective treatment option that is delivered intermittently by subcutaneous injection or infusion rather than requiring patients to consume oral medications 1-3 times daily."
And, he said, because the antibodies act on the very specific target of CGRP, they exhibit a very favorable side effect profile.
"This would be a major advantage over currently available medications, which have a high rate of side effects [weight gain, sedation, altered concentration, fatigue, etc]. The lack of efficacy and the side effect profile of currently available preventive migraine medications account in large part for the lack of adherence to these medications. We recently showed that even in patients with chronic migraine who experience headaches every other day, about 86% have discontinued their oral migraine preventive drug within 1 year."
Drugs that hit CGRP may even help other headache types, Dr. Dodick added.
"There is some evidence that CGRP is also important in other serious headache disorders such as chronic migraine, medication overuse headache, and cluster headache – disorders that are currently very difficult to manage."
The ALD403 study was supported by Alder Biopharmaceuticals. The LY2951742 study was supported by Arteaus Therapeutics in conjunction with Eli Lily. Both Dr. Goadsby and Dr. Dodick have financial relationships with numerous pharmaceutical companies, including receiving research monies, and participating as officers or speakers.
Two investigational migraine prevention drugs that target a vasodilating peptide significantly decreased the number of migraine days per month, compared with placebo, during a pair of phase II studies.
Both drugs reduced mean migraine headache days per month by more than 4 days overall, and one even eliminated migraine in up to 30% of patients.
Each is a monoclonal antibody that inhibits calcitonin gene–related peptide (CGRP). Activated trigeminal sensory nerves release the peptide, which dilates intra- and extracranial blood vessels and modulates vascular nociception.
The antibodies could be game changers for migraine patients, Dr. Peter Goadsby said in an interview.
"Here are the first mechanism-based preventive treatments for migraine," said Dr. Goadsby of the University of California, San Francisco. "They are easy to use, biweekly or even monthly injections, which are effective and well tolerated. For neurologists it means the first major addition to the preventive armamentarium for a generation or more, and for patients, a tangible advance that they can look forward to enjoying soon."
During the annual meeting of the American Academy of Neurology, Dr. Goadsby will present the findings on ALD403, developed by Alder Biopharmaceuticals. His colleague Dr. David W. Dodick of the Mayo Clinic, Rochester, Minn., will present findings on LY2951742, which is being developed by Arteaus Therapeutics in conjunction with Eli Lily. Both presentations are scheduled for May 1.
The trial of ALD403 comprised 163 patients who experienced 5-14 migraine headache days per month; 81 were randomized to receive a single infusion of 1,000 mg ALD403 and the rest received placebo. Patients were followed for 24 weeks. The primary outcome was the mean change in migraine days between weeks 5-8 and baseline.
Patients in the active treatment group fared significantly better than those in the placebo group, with a mean reduction in migraine headache days of –5.6, compared to –4.6 for the placebo group (66% vs. 52% decrease).
Most patients taking the study drug had at least a 50% reduction in migraine headache days at 12 weeks (60% vs. 30%); the proportions of those with at least a 75% reduction were 32% vs. 9%. A 100% reduction occurred in 16% of the active group and in none of the placebo group. These differences were all statistically significant.
There were no differences in the type or frequency of adverse events, vital signs, or laboratory safety data between the two treatment groups.
The LY2951742 study was similarly successful. It comprised 217 patients who experienced 4-14 migraine headache days per month, said Dr. Dodick. They were randomized to placebo or to biweekly subcutaneous injections of LY2951742 150 mg for 12 weeks. The primary end point was the mean change in number of migraine days per month. Secondary end points were the change in headache days, migraine attacks, and responder rate.
Patients in the active treatment group experienced a significantly greater reduction in migraine days than those taking placebo (–4.2 vs. –3 days; 63% vs. 42% decrease).
LY2951742 was significantly better than placebo in all secondary end points including headache days (–4.9 vs. –3.7), migraine attacks (–3 vs. –2.3), and overall response rate (70% vs. 45%).
In an exploratory analysis, 33% of those in the active treatment group were complete responders, with a 100% reduction in migraine headache days, compared with 17% of the placebo group,
Adverse events seen more frequently with LY2951742 than placebo included injection site pain, upper respiratory tract infections, and abdominal pain.
Both drugs will advance to phase III studies, Dr. Dodick said in an interview. "If they are ultimately confirmed to be effective and safe, this will represent a new era in mechanism-based migraine prevention – in other words, treatment directed at a molecule now known to be very important in generating a migraine attack. This would provide a highly effective treatment option that is delivered intermittently by subcutaneous injection or infusion rather than requiring patients to consume oral medications 1-3 times daily."
And, he said, because the antibodies act on the very specific target of CGRP, they exhibit a very favorable side effect profile.
"This would be a major advantage over currently available medications, which have a high rate of side effects [weight gain, sedation, altered concentration, fatigue, etc]. The lack of efficacy and the side effect profile of currently available preventive migraine medications account in large part for the lack of adherence to these medications. We recently showed that even in patients with chronic migraine who experience headaches every other day, about 86% have discontinued their oral migraine preventive drug within 1 year."
Drugs that hit CGRP may even help other headache types, Dr. Dodick added.
"There is some evidence that CGRP is also important in other serious headache disorders such as chronic migraine, medication overuse headache, and cluster headache – disorders that are currently very difficult to manage."
The ALD403 study was supported by Alder Biopharmaceuticals. The LY2951742 study was supported by Arteaus Therapeutics in conjunction with Eli Lily. Both Dr. Goadsby and Dr. Dodick have financial relationships with numerous pharmaceutical companies, including receiving research monies, and participating as officers or speakers.
Two investigational migraine prevention drugs that target a vasodilating peptide significantly decreased the number of migraine days per month, compared with placebo, during a pair of phase II studies.
Both drugs reduced mean migraine headache days per month by more than 4 days overall, and one even eliminated migraine in up to 30% of patients.
Each is a monoclonal antibody that inhibits calcitonin gene–related peptide (CGRP). Activated trigeminal sensory nerves release the peptide, which dilates intra- and extracranial blood vessels and modulates vascular nociception.
The antibodies could be game changers for migraine patients, Dr. Peter Goadsby said in an interview.
"Here are the first mechanism-based preventive treatments for migraine," said Dr. Goadsby of the University of California, San Francisco. "They are easy to use, biweekly or even monthly injections, which are effective and well tolerated. For neurologists it means the first major addition to the preventive armamentarium for a generation or more, and for patients, a tangible advance that they can look forward to enjoying soon."
During the annual meeting of the American Academy of Neurology, Dr. Goadsby will present the findings on ALD403, developed by Alder Biopharmaceuticals. His colleague Dr. David W. Dodick of the Mayo Clinic, Rochester, Minn., will present findings on LY2951742, which is being developed by Arteaus Therapeutics in conjunction with Eli Lily. Both presentations are scheduled for May 1.
The trial of ALD403 comprised 163 patients who experienced 5-14 migraine headache days per month; 81 were randomized to receive a single infusion of 1,000 mg ALD403 and the rest received placebo. Patients were followed for 24 weeks. The primary outcome was the mean change in migraine days between weeks 5-8 and baseline.
Patients in the active treatment group fared significantly better than those in the placebo group, with a mean reduction in migraine headache days of –5.6, compared to –4.6 for the placebo group (66% vs. 52% decrease).
Most patients taking the study drug had at least a 50% reduction in migraine headache days at 12 weeks (60% vs. 30%); the proportions of those with at least a 75% reduction were 32% vs. 9%. A 100% reduction occurred in 16% of the active group and in none of the placebo group. These differences were all statistically significant.
There were no differences in the type or frequency of adverse events, vital signs, or laboratory safety data between the two treatment groups.
The LY2951742 study was similarly successful. It comprised 217 patients who experienced 4-14 migraine headache days per month, said Dr. Dodick. They were randomized to placebo or to biweekly subcutaneous injections of LY2951742 150 mg for 12 weeks. The primary end point was the mean change in number of migraine days per month. Secondary end points were the change in headache days, migraine attacks, and responder rate.
Patients in the active treatment group experienced a significantly greater reduction in migraine days than those taking placebo (–4.2 vs. –3 days; 63% vs. 42% decrease).
LY2951742 was significantly better than placebo in all secondary end points including headache days (–4.9 vs. –3.7), migraine attacks (–3 vs. –2.3), and overall response rate (70% vs. 45%).
In an exploratory analysis, 33% of those in the active treatment group were complete responders, with a 100% reduction in migraine headache days, compared with 17% of the placebo group,
Adverse events seen more frequently with LY2951742 than placebo included injection site pain, upper respiratory tract infections, and abdominal pain.
Both drugs will advance to phase III studies, Dr. Dodick said in an interview. "If they are ultimately confirmed to be effective and safe, this will represent a new era in mechanism-based migraine prevention – in other words, treatment directed at a molecule now known to be very important in generating a migraine attack. This would provide a highly effective treatment option that is delivered intermittently by subcutaneous injection or infusion rather than requiring patients to consume oral medications 1-3 times daily."
And, he said, because the antibodies act on the very specific target of CGRP, they exhibit a very favorable side effect profile.
"This would be a major advantage over currently available medications, which have a high rate of side effects [weight gain, sedation, altered concentration, fatigue, etc]. The lack of efficacy and the side effect profile of currently available preventive migraine medications account in large part for the lack of adherence to these medications. We recently showed that even in patients with chronic migraine who experience headaches every other day, about 86% have discontinued their oral migraine preventive drug within 1 year."
Drugs that hit CGRP may even help other headache types, Dr. Dodick added.
"There is some evidence that CGRP is also important in other serious headache disorders such as chronic migraine, medication overuse headache, and cluster headache – disorders that are currently very difficult to manage."
The ALD403 study was supported by Alder Biopharmaceuticals. The LY2951742 study was supported by Arteaus Therapeutics in conjunction with Eli Lily. Both Dr. Goadsby and Dr. Dodick have financial relationships with numerous pharmaceutical companies, including receiving research monies, and participating as officers or speakers.
FROM THE AAN 2014 ANNUAL MEETING
Key clinical point: These investigational drugs may be an improvement over currently available migraine prevention drugs in terms of side effects and dosing frequency.
Major finding: Both drugs significantly decreased the number of migraine headache days per month, compared with placebo.
Data source: Two randomized, double-blind phase II studies
Disclosures: The ALD403 study was supported by Alder Biopharmaceuticals. The LY2951742 study was supported by Arteaus Therapeutics in conjunction with Eli Lily. Both Dr. Goadsby and Dr. Dodick have financial relationships with numerous pharmaceutical companies
Asymptomatic stenosis could cause cognitive impairment
Asymptomatic carotid stenosis may not be asymptomatic after all.
Patients with a stenosis of 50% or more performed significantly worse on cognitive tests than did those without. Those cognitive issues were very likely clinically significant as well, according to a study to be presented April 30 at the annual meeting of the American Academy of Neurology.
The study is the first to definitively link asymptomatic carotid stenosis with symptomatic cognitive impairment, primary investigator Brajesh K. Lal said in an interview. But since the stenosis itself is not apparent, and the cognitive changes not profound, patients are not being treated for either, said Dr. Lal, chief of vascular surgery at the Baltimore Veterans Affairs Medical Center, and professor of vascular surgery at the University of Maryland.
"If the cognitive decline was severe, it probably would have been enough for the patient to be evaluated for dementia. Instead, this is floating beneath the radar. It’s not as severe as an obvious dementia that would be picked up on cursory evaluation, but it is significant enough to impact activities of daily living, or the patient’s ability to perform a job," he said.
Dr. Lal and his team conducted cognitive testing on 127 patients: 67 with ultrasound-confirmed asymptomatic carotid stenosis of 50% or more, and 60 controls. Although the control patients did not have carotid stenosis, they did have similar vascular comorbidities, including diabetes, hypertension, and coronary artery disease. The investigators used a Cohen’s d test to determine whether any statistically significant cognitive differences were likely to be clinically meaningful. They found that all the detected impairments were at least mild to moderate in severity, Dr. Lal said.
The stenotic group performed worse on the overall neurocognitive composite score, and the motor/processing speed and learning/memory domains. The Cohen’s measure of effect size showed that all of these differences would have a mild to moderate effect on cognitive function.
A trend of poorer performance for executive function and attention/working memory emerged also, although these differences were neither statistically nor clinically significant.
Extrapolating these results out to the number of people who probably have asymptomatic carotid stenosis presents a sobering picture, he added.
"There are probably somewhere between 8 and 13 million people in the U.S. who have an asymptomatic blockage of 50% or more. And while not every one of them is going to have cognitive decline, this does open the door to the idea that there may be a significant population that is affected."
Right now, the pathophysiologic link between stenosis and cognition remains unclear. It might be related to microemboli shedding from the vascular plaque and causing microinfarcts, or to decreased blood flow and oxygenation to the brain, Dr. Lal said.
Dr. Lal reported that he has several NIH and VA Merit grants focused on developing new methods of diagnosing and treating carotid occlusive disease. He reported having no other financial disclosures.
On Twitter @Alz_Gal
Asymptomatic carotid stenosis may not be asymptomatic after all.
Patients with a stenosis of 50% or more performed significantly worse on cognitive tests than did those without. Those cognitive issues were very likely clinically significant as well, according to a study to be presented April 30 at the annual meeting of the American Academy of Neurology.
The study is the first to definitively link asymptomatic carotid stenosis with symptomatic cognitive impairment, primary investigator Brajesh K. Lal said in an interview. But since the stenosis itself is not apparent, and the cognitive changes not profound, patients are not being treated for either, said Dr. Lal, chief of vascular surgery at the Baltimore Veterans Affairs Medical Center, and professor of vascular surgery at the University of Maryland.
"If the cognitive decline was severe, it probably would have been enough for the patient to be evaluated for dementia. Instead, this is floating beneath the radar. It’s not as severe as an obvious dementia that would be picked up on cursory evaluation, but it is significant enough to impact activities of daily living, or the patient’s ability to perform a job," he said.
Dr. Lal and his team conducted cognitive testing on 127 patients: 67 with ultrasound-confirmed asymptomatic carotid stenosis of 50% or more, and 60 controls. Although the control patients did not have carotid stenosis, they did have similar vascular comorbidities, including diabetes, hypertension, and coronary artery disease. The investigators used a Cohen’s d test to determine whether any statistically significant cognitive differences were likely to be clinically meaningful. They found that all the detected impairments were at least mild to moderate in severity, Dr. Lal said.
The stenotic group performed worse on the overall neurocognitive composite score, and the motor/processing speed and learning/memory domains. The Cohen’s measure of effect size showed that all of these differences would have a mild to moderate effect on cognitive function.
A trend of poorer performance for executive function and attention/working memory emerged also, although these differences were neither statistically nor clinically significant.
Extrapolating these results out to the number of people who probably have asymptomatic carotid stenosis presents a sobering picture, he added.
"There are probably somewhere between 8 and 13 million people in the U.S. who have an asymptomatic blockage of 50% or more. And while not every one of them is going to have cognitive decline, this does open the door to the idea that there may be a significant population that is affected."
Right now, the pathophysiologic link between stenosis and cognition remains unclear. It might be related to microemboli shedding from the vascular plaque and causing microinfarcts, or to decreased blood flow and oxygenation to the brain, Dr. Lal said.
Dr. Lal reported that he has several NIH and VA Merit grants focused on developing new methods of diagnosing and treating carotid occlusive disease. He reported having no other financial disclosures.
On Twitter @Alz_Gal
Asymptomatic carotid stenosis may not be asymptomatic after all.
Patients with a stenosis of 50% or more performed significantly worse on cognitive tests than did those without. Those cognitive issues were very likely clinically significant as well, according to a study to be presented April 30 at the annual meeting of the American Academy of Neurology.
The study is the first to definitively link asymptomatic carotid stenosis with symptomatic cognitive impairment, primary investigator Brajesh K. Lal said in an interview. But since the stenosis itself is not apparent, and the cognitive changes not profound, patients are not being treated for either, said Dr. Lal, chief of vascular surgery at the Baltimore Veterans Affairs Medical Center, and professor of vascular surgery at the University of Maryland.
"If the cognitive decline was severe, it probably would have been enough for the patient to be evaluated for dementia. Instead, this is floating beneath the radar. It’s not as severe as an obvious dementia that would be picked up on cursory evaluation, but it is significant enough to impact activities of daily living, or the patient’s ability to perform a job," he said.
Dr. Lal and his team conducted cognitive testing on 127 patients: 67 with ultrasound-confirmed asymptomatic carotid stenosis of 50% or more, and 60 controls. Although the control patients did not have carotid stenosis, they did have similar vascular comorbidities, including diabetes, hypertension, and coronary artery disease. The investigators used a Cohen’s d test to determine whether any statistically significant cognitive differences were likely to be clinically meaningful. They found that all the detected impairments were at least mild to moderate in severity, Dr. Lal said.
The stenotic group performed worse on the overall neurocognitive composite score, and the motor/processing speed and learning/memory domains. The Cohen’s measure of effect size showed that all of these differences would have a mild to moderate effect on cognitive function.
A trend of poorer performance for executive function and attention/working memory emerged also, although these differences were neither statistically nor clinically significant.
Extrapolating these results out to the number of people who probably have asymptomatic carotid stenosis presents a sobering picture, he added.
"There are probably somewhere between 8 and 13 million people in the U.S. who have an asymptomatic blockage of 50% or more. And while not every one of them is going to have cognitive decline, this does open the door to the idea that there may be a significant population that is affected."
Right now, the pathophysiologic link between stenosis and cognition remains unclear. It might be related to microemboli shedding from the vascular plaque and causing microinfarcts, or to decreased blood flow and oxygenation to the brain, Dr. Lal said.
Dr. Lal reported that he has several NIH and VA Merit grants focused on developing new methods of diagnosing and treating carotid occlusive disease. He reported having no other financial disclosures.
On Twitter @Alz_Gal
FROM THE AAN 2014 ANNUAL MEETING
Major finding: Patients with a stenosis of 50% or more performed significantly worse on cognitive tests than did those without stenosis but with similar vascular comorbidities.
Data source: Cognitive testing on 127 patients: 67 with ultrasound-confirmed asymptomatic carotid stenosis of 50% or more, and 60 controls.
Disclosures: Dr. Lal has several NIH and VA Merit grants focused on developing new methods of diagnosing and treating carotid occlusive disease. He reported having no other disclosures.
Vision test has additive effect on concussion detection in athletes
Concussion in athletes was detected with greater accuracy when a simple vision test was added to other established tests in a prospective study of 217 members of the men’s football, women’s soccer, and women’s lacrosse teams at the University of Florida.
All concussive cases were correctly identified when the King-Devick (K-D) test was used in conjunction with the Balance Error Scoring System (BESS), which is part of the Sports Concussion Assessment Tool 3 (SCAT3), Dr. Laura Balcer and her associates reported Feb. 26 in an abstract released in advance of the annual meeting of the American Academy of Neurology in Philadelphia.
Furthermore, differences between baseline assessments and postinjury assessment showed that the K-D test identified a higher percentage of athletes with worsened scores than did the Standardized Assessment of Concussion (SAC) test (79% vs. 52%).
Using the K-D and SAC tests together also was better than using either of the tests alone, with 89% of concussions identified.
In a statement issued by the AAN, Dr. Balcer of the departments of population health and neurology at New York University noted that "the visual pathways are commonly affected in concussion. [And] adding a vision based test to evaluate athletes on the sidelines may allow us to better detect more athletes with concussion more quickly. This is particularly important since not all athletes reliably report their symptoms of concussion."
The findings add to previous work by Dr. Balcer and her colleagues in a similarly sized cohort of athletes from the University of Pennsylvania varsity football, sprint football, and women’s and men’s soccer and basketball teams (J. Neurol. Sci. 2011;309:34-9), and lend further support for the use of the K-D test as a rapid screening tool to assess players’ concussion during sporting events.
Up to 3.8 million sports-related concussive injuries have been estimated to occur in the United States each year, with diagnosis based on a variety of symptoms and signs that may include headache, unsteadiness, confusion, or behavior that is out of character for the individual concerned. It is important to be able to assess head injuries quickly, as concussion may be a result of a more serious neurological injury that needs emergency hospital treatment.
Unlike other tests for concussion that ideally need to be administered by a medical professional, anyone can administer the K-D test. This, together with the fact it takes less than 1 minute to complete, makes it ideal to use on the sidelines as an objective means of whether a concussive injury warrants more urgent attention.
The K-D test was developed in 1976 and assesses saccade, or the quick, simultaneous movement of both eyes in the same direction, as well as subjects’ language and level of concentration. It involves subjects’ quickly reading aloud a series of single-digit numbers shown to them on three test cards of increasing complexity. Subjects read the test cards from left to right and a stopwatch is used to record the time it takes them to complete each one. A time score is then obtained as the sum of all three test card times.
For the present study, the University of Florida sports teams administered several tests for concussion at the beginning of their seasons and again if concussion was suspected during the season. Thirty athletes experienced a first concussion during their athletic season.
The researchers found that worsening symptom severity scores obtained using the Post-Concussion Scale correlated strongly with increasing time to complete the K-D test (P less than .001).
"Among specific symptoms, light and noise sensitivities were particularly well correlated with K-D worsening," Dr. Balcer and her colleagues wrote in their abstract.
Furthermore, athletes who took longer to complete the K-D test at baseline had worse baseline score for visual motor speed assessed using the Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) scale, which was conducted as part of routine clinical practice for concussion management but not diagnoses.
"The implications are that adding a vision-based test to sideline concussion assessment enables us to have a group of tests – a composite – that, when used together, can potentially help identify athletes with concussion," Dr. Balcer commented in an interview.
"A group of tests also helps add to clinical diagnosis of concussion immediately post injury by testing the brain’s many pathways that can be affected, including vision, cognition, and balance," she said.
"While no one test or group of tests can substitute for the fact that concussion is a clinical diagnosis, based on the judgment of the health care provider, trainer, parent, or athlete, having quick sideline tools that can be easily administered by laypeople adds an important element to how we identify concussions among youth athletes," Dr. Balcer observed. Young athletes are a particular group for which athletic trainers and physicians are not usually available on the sidelines.
The K-D test is relevant to any sporting activity in which there is likely to be physical contact between players or there is a chance of head injury or collision. In addition to football, soccer, and lacrosse, its use has been tested in sports such as boxing (Neurology 2011;76:1456-62), basketball (J. Neurol. Sci. 2011;309:34-9), ice hockey (J. Neurol. Sci. 2013;328:28-31), and rugby (J. Neurol. Sci. 2013;326:59-63).
The study was partly supported by the National Institutes of Health. Dr. Balcer declared no relevant conflicts of interest.
Concussion in athletes was detected with greater accuracy when a simple vision test was added to other established tests in a prospective study of 217 members of the men’s football, women’s soccer, and women’s lacrosse teams at the University of Florida.
All concussive cases were correctly identified when the King-Devick (K-D) test was used in conjunction with the Balance Error Scoring System (BESS), which is part of the Sports Concussion Assessment Tool 3 (SCAT3), Dr. Laura Balcer and her associates reported Feb. 26 in an abstract released in advance of the annual meeting of the American Academy of Neurology in Philadelphia.
Furthermore, differences between baseline assessments and postinjury assessment showed that the K-D test identified a higher percentage of athletes with worsened scores than did the Standardized Assessment of Concussion (SAC) test (79% vs. 52%).
Using the K-D and SAC tests together also was better than using either of the tests alone, with 89% of concussions identified.
In a statement issued by the AAN, Dr. Balcer of the departments of population health and neurology at New York University noted that "the visual pathways are commonly affected in concussion. [And] adding a vision based test to evaluate athletes on the sidelines may allow us to better detect more athletes with concussion more quickly. This is particularly important since not all athletes reliably report their symptoms of concussion."
The findings add to previous work by Dr. Balcer and her colleagues in a similarly sized cohort of athletes from the University of Pennsylvania varsity football, sprint football, and women’s and men’s soccer and basketball teams (J. Neurol. Sci. 2011;309:34-9), and lend further support for the use of the K-D test as a rapid screening tool to assess players’ concussion during sporting events.
Up to 3.8 million sports-related concussive injuries have been estimated to occur in the United States each year, with diagnosis based on a variety of symptoms and signs that may include headache, unsteadiness, confusion, or behavior that is out of character for the individual concerned. It is important to be able to assess head injuries quickly, as concussion may be a result of a more serious neurological injury that needs emergency hospital treatment.
Unlike other tests for concussion that ideally need to be administered by a medical professional, anyone can administer the K-D test. This, together with the fact it takes less than 1 minute to complete, makes it ideal to use on the sidelines as an objective means of whether a concussive injury warrants more urgent attention.
The K-D test was developed in 1976 and assesses saccade, or the quick, simultaneous movement of both eyes in the same direction, as well as subjects’ language and level of concentration. It involves subjects’ quickly reading aloud a series of single-digit numbers shown to them on three test cards of increasing complexity. Subjects read the test cards from left to right and a stopwatch is used to record the time it takes them to complete each one. A time score is then obtained as the sum of all three test card times.
For the present study, the University of Florida sports teams administered several tests for concussion at the beginning of their seasons and again if concussion was suspected during the season. Thirty athletes experienced a first concussion during their athletic season.
The researchers found that worsening symptom severity scores obtained using the Post-Concussion Scale correlated strongly with increasing time to complete the K-D test (P less than .001).
"Among specific symptoms, light and noise sensitivities were particularly well correlated with K-D worsening," Dr. Balcer and her colleagues wrote in their abstract.
Furthermore, athletes who took longer to complete the K-D test at baseline had worse baseline score for visual motor speed assessed using the Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) scale, which was conducted as part of routine clinical practice for concussion management but not diagnoses.
"The implications are that adding a vision-based test to sideline concussion assessment enables us to have a group of tests – a composite – that, when used together, can potentially help identify athletes with concussion," Dr. Balcer commented in an interview.
"A group of tests also helps add to clinical diagnosis of concussion immediately post injury by testing the brain’s many pathways that can be affected, including vision, cognition, and balance," she said.
"While no one test or group of tests can substitute for the fact that concussion is a clinical diagnosis, based on the judgment of the health care provider, trainer, parent, or athlete, having quick sideline tools that can be easily administered by laypeople adds an important element to how we identify concussions among youth athletes," Dr. Balcer observed. Young athletes are a particular group for which athletic trainers and physicians are not usually available on the sidelines.
The K-D test is relevant to any sporting activity in which there is likely to be physical contact between players or there is a chance of head injury or collision. In addition to football, soccer, and lacrosse, its use has been tested in sports such as boxing (Neurology 2011;76:1456-62), basketball (J. Neurol. Sci. 2011;309:34-9), ice hockey (J. Neurol. Sci. 2013;328:28-31), and rugby (J. Neurol. Sci. 2013;326:59-63).
The study was partly supported by the National Institutes of Health. Dr. Balcer declared no relevant conflicts of interest.
Concussion in athletes was detected with greater accuracy when a simple vision test was added to other established tests in a prospective study of 217 members of the men’s football, women’s soccer, and women’s lacrosse teams at the University of Florida.
All concussive cases were correctly identified when the King-Devick (K-D) test was used in conjunction with the Balance Error Scoring System (BESS), which is part of the Sports Concussion Assessment Tool 3 (SCAT3), Dr. Laura Balcer and her associates reported Feb. 26 in an abstract released in advance of the annual meeting of the American Academy of Neurology in Philadelphia.
Furthermore, differences between baseline assessments and postinjury assessment showed that the K-D test identified a higher percentage of athletes with worsened scores than did the Standardized Assessment of Concussion (SAC) test (79% vs. 52%).
Using the K-D and SAC tests together also was better than using either of the tests alone, with 89% of concussions identified.
In a statement issued by the AAN, Dr. Balcer of the departments of population health and neurology at New York University noted that "the visual pathways are commonly affected in concussion. [And] adding a vision based test to evaluate athletes on the sidelines may allow us to better detect more athletes with concussion more quickly. This is particularly important since not all athletes reliably report their symptoms of concussion."
The findings add to previous work by Dr. Balcer and her colleagues in a similarly sized cohort of athletes from the University of Pennsylvania varsity football, sprint football, and women’s and men’s soccer and basketball teams (J. Neurol. Sci. 2011;309:34-9), and lend further support for the use of the K-D test as a rapid screening tool to assess players’ concussion during sporting events.
Up to 3.8 million sports-related concussive injuries have been estimated to occur in the United States each year, with diagnosis based on a variety of symptoms and signs that may include headache, unsteadiness, confusion, or behavior that is out of character for the individual concerned. It is important to be able to assess head injuries quickly, as concussion may be a result of a more serious neurological injury that needs emergency hospital treatment.
Unlike other tests for concussion that ideally need to be administered by a medical professional, anyone can administer the K-D test. This, together with the fact it takes less than 1 minute to complete, makes it ideal to use on the sidelines as an objective means of whether a concussive injury warrants more urgent attention.
The K-D test was developed in 1976 and assesses saccade, or the quick, simultaneous movement of both eyes in the same direction, as well as subjects’ language and level of concentration. It involves subjects’ quickly reading aloud a series of single-digit numbers shown to them on three test cards of increasing complexity. Subjects read the test cards from left to right and a stopwatch is used to record the time it takes them to complete each one. A time score is then obtained as the sum of all three test card times.
For the present study, the University of Florida sports teams administered several tests for concussion at the beginning of their seasons and again if concussion was suspected during the season. Thirty athletes experienced a first concussion during their athletic season.
The researchers found that worsening symptom severity scores obtained using the Post-Concussion Scale correlated strongly with increasing time to complete the K-D test (P less than .001).
"Among specific symptoms, light and noise sensitivities were particularly well correlated with K-D worsening," Dr. Balcer and her colleagues wrote in their abstract.
Furthermore, athletes who took longer to complete the K-D test at baseline had worse baseline score for visual motor speed assessed using the Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) scale, which was conducted as part of routine clinical practice for concussion management but not diagnoses.
"The implications are that adding a vision-based test to sideline concussion assessment enables us to have a group of tests – a composite – that, when used together, can potentially help identify athletes with concussion," Dr. Balcer commented in an interview.
"A group of tests also helps add to clinical diagnosis of concussion immediately post injury by testing the brain’s many pathways that can be affected, including vision, cognition, and balance," she said.
"While no one test or group of tests can substitute for the fact that concussion is a clinical diagnosis, based on the judgment of the health care provider, trainer, parent, or athlete, having quick sideline tools that can be easily administered by laypeople adds an important element to how we identify concussions among youth athletes," Dr. Balcer observed. Young athletes are a particular group for which athletic trainers and physicians are not usually available on the sidelines.
The K-D test is relevant to any sporting activity in which there is likely to be physical contact between players or there is a chance of head injury or collision. In addition to football, soccer, and lacrosse, its use has been tested in sports such as boxing (Neurology 2011;76:1456-62), basketball (J. Neurol. Sci. 2011;309:34-9), ice hockey (J. Neurol. Sci. 2013;328:28-31), and rugby (J. Neurol. Sci. 2013;326:59-63).
The study was partly supported by the National Institutes of Health. Dr. Balcer declared no relevant conflicts of interest.
FROM THE 2014 AAN ANNUAL MEETING
Major finding: 89%-100% of concussive injuries were identified when the King-Devick test was combined with standard concussion tests.
Data source: A prospective study of 217 male and female athletes at risk for sporting-related concussion.
Disclosures: The study was partly supported by the National Institutes of Health. Dr. Balcer declared no relevant conflicts of interest.
Small study begins to uncover the role of antibodies to KIR4.1 in MS
Antibodies to the potassium channel KIR4.1 have been discovered in a cohort of people who later developed multiple sclerosis, and not in healthy controls, suggesting that anti-KIR4.1 antibodies may play a role in disease pathogenesis.
In an abstract released Feb. 21 in advance of the annual meeting of the American Academy of Neurology, Dr. Viola Biberacher of Technical University Munich and her colleagues, examined blood samples that had been collected from 16 MS patients between 2 and 9 months before their first clinical attack. The investigators also looked at earlier samples from the same cohort taken up to 6 years before the first attack.
In the cohort, seven patients were positive for anti-KIR4.1 antibodies, two showed borderline activity, and seven were negative. Anti-KIR4.1 antibodies were found in samples taken several years before the first clinical attack, and antibody titers varied at different time points before the first attack; titers before and after disease onset did not differ significantly. None of the 16 age- and sex-matched healthy controls had antibodies to KIR4.1.
Dr. Bernhard Hemmer, also of Technical University Munich and the corresponding author of the study, had been part of the research team that first established KIR4.1 as an immune target in MS. In an observational study of patients with active MS (n = 397), compared with patients with other neurological diseases (n = 329) and healthy blood donors (n = 59), anti-KIR4.1 antibodies were found in almost half (47%) of blood samples from people with MS. No evidence of anti-KIR4 antibodies were found in the healthy controls, and they occurred in less than 1% of people with other neurological diseases (N. Engl. J. Med. 2012;367:115-23).
In the current study as well, about half of the patients who went on to develop MS had antibodies to KIR4.1. The full results of the study will be presented at the meeting in Philadelphia.
In an interview, Dr. Hemmer said that because of the very small sample size of patients in this study, these results must be interpreted with caution. "Replication in independent cohorts is essential," he said.
Dr. Hemmer added that while anti-KIR4.1 antibodies appear to be present in about half of MS patients, "we have not observed a particular phenotype of MS to be associated with these antibodies. However, larger studies are underway to address this issue."
If KIR4.1 is proves to be a relevant immune target in MS, something that will take additional studies to confirm, treatment might involve specific immune therapies that could focus on silencing antibodies to KIR4.1, Dr. Hemmer said. "I could also envision drugs that protect the KIR4.1 channel from damage, or enhance its function if the antibody is disturbed or dysregulated in the MS lesion. But all these therapies require first additional studies to strengthen the hypothesis that the channel plays an important role in MS."
Further studies are looking at expression of KIR4.1 in the brain and MS lesions, and investigating cellular immune responses to KIR4.1.
Currently, no commercial assay is available for KIR4.1, as the protein is difficult to express and purify. "We are working on this too, but it will take time," Dr. Hemmer said.
The KIR4.1 study was funded by the German Ministry for Education and Research and the German Competence Network for Multiple Sclerosis. Dr. Biberacher reported having no conflicts of interest related to the study, but several coauthors hold a patent on KIR4.1 antibody testing in MS. Dr. Hemmer has received past support from Roche, Biogen Idec, Bayer, Novartis, Merck Serono, Metanomics, and Teva.
Antibodies to the potassium channel KIR4.1 have been discovered in a cohort of people who later developed multiple sclerosis, and not in healthy controls, suggesting that anti-KIR4.1 antibodies may play a role in disease pathogenesis.
In an abstract released Feb. 21 in advance of the annual meeting of the American Academy of Neurology, Dr. Viola Biberacher of Technical University Munich and her colleagues, examined blood samples that had been collected from 16 MS patients between 2 and 9 months before their first clinical attack. The investigators also looked at earlier samples from the same cohort taken up to 6 years before the first attack.
In the cohort, seven patients were positive for anti-KIR4.1 antibodies, two showed borderline activity, and seven were negative. Anti-KIR4.1 antibodies were found in samples taken several years before the first clinical attack, and antibody titers varied at different time points before the first attack; titers before and after disease onset did not differ significantly. None of the 16 age- and sex-matched healthy controls had antibodies to KIR4.1.
Dr. Bernhard Hemmer, also of Technical University Munich and the corresponding author of the study, had been part of the research team that first established KIR4.1 as an immune target in MS. In an observational study of patients with active MS (n = 397), compared with patients with other neurological diseases (n = 329) and healthy blood donors (n = 59), anti-KIR4.1 antibodies were found in almost half (47%) of blood samples from people with MS. No evidence of anti-KIR4 antibodies were found in the healthy controls, and they occurred in less than 1% of people with other neurological diseases (N. Engl. J. Med. 2012;367:115-23).
In the current study as well, about half of the patients who went on to develop MS had antibodies to KIR4.1. The full results of the study will be presented at the meeting in Philadelphia.
In an interview, Dr. Hemmer said that because of the very small sample size of patients in this study, these results must be interpreted with caution. "Replication in independent cohorts is essential," he said.
Dr. Hemmer added that while anti-KIR4.1 antibodies appear to be present in about half of MS patients, "we have not observed a particular phenotype of MS to be associated with these antibodies. However, larger studies are underway to address this issue."
If KIR4.1 is proves to be a relevant immune target in MS, something that will take additional studies to confirm, treatment might involve specific immune therapies that could focus on silencing antibodies to KIR4.1, Dr. Hemmer said. "I could also envision drugs that protect the KIR4.1 channel from damage, or enhance its function if the antibody is disturbed or dysregulated in the MS lesion. But all these therapies require first additional studies to strengthen the hypothesis that the channel plays an important role in MS."
Further studies are looking at expression of KIR4.1 in the brain and MS lesions, and investigating cellular immune responses to KIR4.1.
Currently, no commercial assay is available for KIR4.1, as the protein is difficult to express and purify. "We are working on this too, but it will take time," Dr. Hemmer said.
The KIR4.1 study was funded by the German Ministry for Education and Research and the German Competence Network for Multiple Sclerosis. Dr. Biberacher reported having no conflicts of interest related to the study, but several coauthors hold a patent on KIR4.1 antibody testing in MS. Dr. Hemmer has received past support from Roche, Biogen Idec, Bayer, Novartis, Merck Serono, Metanomics, and Teva.
Antibodies to the potassium channel KIR4.1 have been discovered in a cohort of people who later developed multiple sclerosis, and not in healthy controls, suggesting that anti-KIR4.1 antibodies may play a role in disease pathogenesis.
In an abstract released Feb. 21 in advance of the annual meeting of the American Academy of Neurology, Dr. Viola Biberacher of Technical University Munich and her colleagues, examined blood samples that had been collected from 16 MS patients between 2 and 9 months before their first clinical attack. The investigators also looked at earlier samples from the same cohort taken up to 6 years before the first attack.
In the cohort, seven patients were positive for anti-KIR4.1 antibodies, two showed borderline activity, and seven were negative. Anti-KIR4.1 antibodies were found in samples taken several years before the first clinical attack, and antibody titers varied at different time points before the first attack; titers before and after disease onset did not differ significantly. None of the 16 age- and sex-matched healthy controls had antibodies to KIR4.1.
Dr. Bernhard Hemmer, also of Technical University Munich and the corresponding author of the study, had been part of the research team that first established KIR4.1 as an immune target in MS. In an observational study of patients with active MS (n = 397), compared with patients with other neurological diseases (n = 329) and healthy blood donors (n = 59), anti-KIR4.1 antibodies were found in almost half (47%) of blood samples from people with MS. No evidence of anti-KIR4 antibodies were found in the healthy controls, and they occurred in less than 1% of people with other neurological diseases (N. Engl. J. Med. 2012;367:115-23).
In the current study as well, about half of the patients who went on to develop MS had antibodies to KIR4.1. The full results of the study will be presented at the meeting in Philadelphia.
In an interview, Dr. Hemmer said that because of the very small sample size of patients in this study, these results must be interpreted with caution. "Replication in independent cohorts is essential," he said.
Dr. Hemmer added that while anti-KIR4.1 antibodies appear to be present in about half of MS patients, "we have not observed a particular phenotype of MS to be associated with these antibodies. However, larger studies are underway to address this issue."
If KIR4.1 is proves to be a relevant immune target in MS, something that will take additional studies to confirm, treatment might involve specific immune therapies that could focus on silencing antibodies to KIR4.1, Dr. Hemmer said. "I could also envision drugs that protect the KIR4.1 channel from damage, or enhance its function if the antibody is disturbed or dysregulated in the MS lesion. But all these therapies require first additional studies to strengthen the hypothesis that the channel plays an important role in MS."
Further studies are looking at expression of KIR4.1 in the brain and MS lesions, and investigating cellular immune responses to KIR4.1.
Currently, no commercial assay is available for KIR4.1, as the protein is difficult to express and purify. "We are working on this too, but it will take time," Dr. Hemmer said.
The KIR4.1 study was funded by the German Ministry for Education and Research and the German Competence Network for Multiple Sclerosis. Dr. Biberacher reported having no conflicts of interest related to the study, but several coauthors hold a patent on KIR4.1 antibody testing in MS. Dr. Hemmer has received past support from Roche, Biogen Idec, Bayer, Novartis, Merck Serono, Metanomics, and Teva.
FROM THE 2014 AAN ANNUAL MEETING
Major finding: Anti-KIR4.1 antibodies were found in plasma from 7 of 16 patients several years before they developed MS, compared with none of 16 healthy control blood donors.
Data source: A case-control study of 16 patients with MS and 16 healthy control blood donors.
Disclosures: The KIR4.1 study was funded by the German Ministry for Education and Research and the German Competence Network for Multiple Sclerosis. Dr. Biberacher reported having no conflicts related to the study, but several coauthors hold a patent on KIR4.1 antibody testing in MS. Dr. Hemmer has received past support from Roche, Biogen Idec, Bayer, Novartis, Merck Serono, Metanomics, and Teva.