User login
American Heart Association (AHA): Scientific Sessions 2016
Nutrition expert to heart patients: ‘Eat some cheese’
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
FROM THE AHA SCIENTIFIC SESSIONS
Cardiologist care for newly diagnosed A-fib improves outcomes
NEW ORLEANS – Patients who see a cardiologist for their newly diagnosed atrial fibrillation are at significantly lower subsequent risk for ischemic stroke and death than those who receive their care exclusively from primary care physicians, according to a huge Veterans Affairs study.
These superior outcomes were mediated in part by a significantly higher rate of oral anticoagulation prescription within 90 days after diagnosis of atrial fibrillation (AF) among patients who saw a cardiologist, Alexander C. Perino, MD, reported at the American Heart Association scientific sessions.
He presented results from TREAT-AF (The Retrospective Evaluation and Assessment of Therapies in AF) study, a nationwide Veterans Affairs retrospective observational cohort study including 181,161 patients with newly diagnosed AF during 2003-2012. Forty percent of them saw a cardiologist and often a primary care physician as well, while 60% received all their AF-related care in primary care clinics.
The rate of oral anticoagulant prescription within 90 days of diagnosis of AF was 70.3% in patients seen by cardiologists, compared with 58.8% in those seen by primary care physicians only.
“One way to look at this is to say, ‘Wow, look how great cardiologists are doing.’ Another way to look at it is to say, ‘What is going on here that 30% of atrial fibrillation patients seen by cardiologists are not being given an oral anticoagulant prescription? How do we increase that number?’ That needs to be looked into,” Dr. Perino said.
The incidence of ischemic stroke was 7.6 per 1,000 person-years in the cardiology group, significantly lower than the 8.8 per 1,000 in the primary care–only group.
While the two groups shared similar CHA2DS2-VASc scores, the cohort seen by cardiologists had significantly higher rates of comorbid diabetes, hypertension, coronary artery disease, prior MI, and stroke.
In a multivariate analysis adjusted for these comorbidities as well as patient demographics, distance to VA medical care, and use of medications other than oral anticoagulants, cardiology care was associated with a 9% relative risk reduction in ischemic stroke, an 11% reduction in all-cause mortality, and a 3% increase in MI, all statistically significant.
In a more sophisticated analysis featuring propensity score matching, the cardiology group had a 12% relative risk reduction in stroke and a 10% reduction in the risk of death.
The investigators determined that 17% of the improvement in outcomes seen in the cardiology group was attributable to their higher rate of early anticoagulant therapy. Another potential contributor to the outcome differences might be cardiologists’ greater use of rate and rhythm control: Rate control medication was prescribed for 90.1% of the cardiology patients, compared with 80.5% of the primary care patients. And rhythm control therapy was prescribed for 20.8% of the cardiology group versus just 11% of the primary care–only patients.
It’s also possible that cardiology care had a differential impact on non-AF conditions, although this is speculation, Dr. Perino noted.
He said it’s unrealistic to propose that all patients with newly diagnosed AF be seen by a cardiologist because the cardiology workforce isn’t big enough. But more widespread use of specialized AF clinics staffed by expert nurse practitioners and physician assistants could be a practical way for health care plans to achieve cardiologylike outcomes in patients with newly diagnosed AF, in his view.
Dr. Perino reported having no financial conflicts of interest regarding his study.
NEW ORLEANS – Patients who see a cardiologist for their newly diagnosed atrial fibrillation are at significantly lower subsequent risk for ischemic stroke and death than those who receive their care exclusively from primary care physicians, according to a huge Veterans Affairs study.
These superior outcomes were mediated in part by a significantly higher rate of oral anticoagulation prescription within 90 days after diagnosis of atrial fibrillation (AF) among patients who saw a cardiologist, Alexander C. Perino, MD, reported at the American Heart Association scientific sessions.
He presented results from TREAT-AF (The Retrospective Evaluation and Assessment of Therapies in AF) study, a nationwide Veterans Affairs retrospective observational cohort study including 181,161 patients with newly diagnosed AF during 2003-2012. Forty percent of them saw a cardiologist and often a primary care physician as well, while 60% received all their AF-related care in primary care clinics.
The rate of oral anticoagulant prescription within 90 days of diagnosis of AF was 70.3% in patients seen by cardiologists, compared with 58.8% in those seen by primary care physicians only.
“One way to look at this is to say, ‘Wow, look how great cardiologists are doing.’ Another way to look at it is to say, ‘What is going on here that 30% of atrial fibrillation patients seen by cardiologists are not being given an oral anticoagulant prescription? How do we increase that number?’ That needs to be looked into,” Dr. Perino said.
The incidence of ischemic stroke was 7.6 per 1,000 person-years in the cardiology group, significantly lower than the 8.8 per 1,000 in the primary care–only group.
While the two groups shared similar CHA2DS2-VASc scores, the cohort seen by cardiologists had significantly higher rates of comorbid diabetes, hypertension, coronary artery disease, prior MI, and stroke.
In a multivariate analysis adjusted for these comorbidities as well as patient demographics, distance to VA medical care, and use of medications other than oral anticoagulants, cardiology care was associated with a 9% relative risk reduction in ischemic stroke, an 11% reduction in all-cause mortality, and a 3% increase in MI, all statistically significant.
In a more sophisticated analysis featuring propensity score matching, the cardiology group had a 12% relative risk reduction in stroke and a 10% reduction in the risk of death.
The investigators determined that 17% of the improvement in outcomes seen in the cardiology group was attributable to their higher rate of early anticoagulant therapy. Another potential contributor to the outcome differences might be cardiologists’ greater use of rate and rhythm control: Rate control medication was prescribed for 90.1% of the cardiology patients, compared with 80.5% of the primary care patients. And rhythm control therapy was prescribed for 20.8% of the cardiology group versus just 11% of the primary care–only patients.
It’s also possible that cardiology care had a differential impact on non-AF conditions, although this is speculation, Dr. Perino noted.
He said it’s unrealistic to propose that all patients with newly diagnosed AF be seen by a cardiologist because the cardiology workforce isn’t big enough. But more widespread use of specialized AF clinics staffed by expert nurse practitioners and physician assistants could be a practical way for health care plans to achieve cardiologylike outcomes in patients with newly diagnosed AF, in his view.
Dr. Perino reported having no financial conflicts of interest regarding his study.
NEW ORLEANS – Patients who see a cardiologist for their newly diagnosed atrial fibrillation are at significantly lower subsequent risk for ischemic stroke and death than those who receive their care exclusively from primary care physicians, according to a huge Veterans Affairs study.
These superior outcomes were mediated in part by a significantly higher rate of oral anticoagulation prescription within 90 days after diagnosis of atrial fibrillation (AF) among patients who saw a cardiologist, Alexander C. Perino, MD, reported at the American Heart Association scientific sessions.
He presented results from TREAT-AF (The Retrospective Evaluation and Assessment of Therapies in AF) study, a nationwide Veterans Affairs retrospective observational cohort study including 181,161 patients with newly diagnosed AF during 2003-2012. Forty percent of them saw a cardiologist and often a primary care physician as well, while 60% received all their AF-related care in primary care clinics.
The rate of oral anticoagulant prescription within 90 days of diagnosis of AF was 70.3% in patients seen by cardiologists, compared with 58.8% in those seen by primary care physicians only.
“One way to look at this is to say, ‘Wow, look how great cardiologists are doing.’ Another way to look at it is to say, ‘What is going on here that 30% of atrial fibrillation patients seen by cardiologists are not being given an oral anticoagulant prescription? How do we increase that number?’ That needs to be looked into,” Dr. Perino said.
The incidence of ischemic stroke was 7.6 per 1,000 person-years in the cardiology group, significantly lower than the 8.8 per 1,000 in the primary care–only group.
While the two groups shared similar CHA2DS2-VASc scores, the cohort seen by cardiologists had significantly higher rates of comorbid diabetes, hypertension, coronary artery disease, prior MI, and stroke.
In a multivariate analysis adjusted for these comorbidities as well as patient demographics, distance to VA medical care, and use of medications other than oral anticoagulants, cardiology care was associated with a 9% relative risk reduction in ischemic stroke, an 11% reduction in all-cause mortality, and a 3% increase in MI, all statistically significant.
In a more sophisticated analysis featuring propensity score matching, the cardiology group had a 12% relative risk reduction in stroke and a 10% reduction in the risk of death.
The investigators determined that 17% of the improvement in outcomes seen in the cardiology group was attributable to their higher rate of early anticoagulant therapy. Another potential contributor to the outcome differences might be cardiologists’ greater use of rate and rhythm control: Rate control medication was prescribed for 90.1% of the cardiology patients, compared with 80.5% of the primary care patients. And rhythm control therapy was prescribed for 20.8% of the cardiology group versus just 11% of the primary care–only patients.
It’s also possible that cardiology care had a differential impact on non-AF conditions, although this is speculation, Dr. Perino noted.
He said it’s unrealistic to propose that all patients with newly diagnosed AF be seen by a cardiologist because the cardiology workforce isn’t big enough. But more widespread use of specialized AF clinics staffed by expert nurse practitioners and physician assistants could be a practical way for health care plans to achieve cardiologylike outcomes in patients with newly diagnosed AF, in his view.
Dr. Perino reported having no financial conflicts of interest regarding his study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The incidence of ischemic stroke in patients who saw a cardiologist for their newly diagnosed atrial fibrillation was 7.6 per 1,000 person-years, significantly lower than the 8.8 per 1,000 in those cared for exclusively by primary care physicians.
Data source: The TREAT-AF study was a national retrospective cohort study of 181,161 Veterans Affairs patients with newly diagnosed AF during 2003-2012.
Disclosures: The study presenter reported having no financial conflicts of interest.
The Barbershop Study: Hypertension causes nocturia
NEW ORLEANS – Uncontrolled systolic hypertension is a strong independent determinant of nocturia in middle-aged African American men, O’Neil Mason, MD, reported at the American Heart Association scientific sessions.
This finding from the ongoing National Heart, Lung, and Blood Institute–sponsored Barbershop Study challenges the traditional notion of hypertension as an asymptomatic disease. It also provides a novel health promotion message aimed at improving compliance with blood pressure medication.
The Barbershop Study is a hypertension intervention trial that’s being conducted in Los Angeles barbershops frequented by black men. In the initial screening phase for study eligibility, 2,577 African American men aged 35-79 years underwent highly accurate blood pressure measurements using an average of three readings taken via an oscillometric monitor.
The mean age of the men was 53 years. It was an obese group, with a mean body mass index of 30 kg/m2. Fifty percent of the men had hypertension, and among that cohort fully one-third weren’t on antihypertensive medication and another 28% were treated but uncontrolled, with on-treatment blood pressures of 140/90 mm Hg or more. Thus, only 39% of these middle-aged African American men with high blood pressure were treated and controlled at baseline.
Seventy-seven percent of the screened men reported awakening once or more per night to urinate. A progressive increase in nocturia severity was seen with increasing systolic blood pressure. The prevalence of nocturia ranged from 68% among normotensive men to 91% among those with treated but uncontrolled hypertension, Dr. Mason reported.
In a multivariate logistic regression analysis controlling for the standard risk factors for nocturia – including advancing age, an enlarged prostate, and diabetes, which was present in 16% of the men – stage 1 systolic hypertension in the range of 140-159 mm Hg was independently associated with a 1.57-fold increased likelihood of nocturia, compared with normotensive subjects. Stage 2 hypertension, with a systolic blood pressure of 160 mm Hg or more, was associated with a 2.32-fold increased risk; that’s in the same ballpark as having an enlarged prostate, which carried a 2.1-fold increased risk. Prehypertension – that is, a systolic pressure of 120-139 mm Hg – was associated with a nonsignificant 1.18-fold risk.
Diastolic blood pressure wasn’t an independent determinant of nocturia.
In a similar multivariate analysis focused on severe nocturia, defined as three or more episodes per night, stage 1 systolic hypertension was independently associated with a 2.29-fold increased risk, compared with normotension, and stage 2 systolic hypertension carried a 2.77-fold increased risk.
Audience members were clearly intrigued by this novel finding. They were quick to speculate as to potential underlying pathophysiologic mechanisms, including atrial stretch, increased renal blood flow, or perhaps a side effect of diuretic therapy. However, Dr. Mason and his coinvestigators favor another possibility: “African Americans have more salt-sensitive hypertension and they have less nocturnal blood pressure dipping,” he noted. “So if nighttime blood pressure is high it could lead through increased pressure natriuresis to increased urine production. More activity in getting up to go to the bathroom increases the blood pressure and creates a cycle that begets more urine.”
Asked if uncontrolled systolic hypertension is also a determinant of nocturia in African American women, Dr. Mason replied that he would assume so. But that question hasn’t ever been studied. The Barbershop Study is restricted to African American men with hypertension because studies have shown they have a particularly low rate of controlled hypertension. In contrast, the controlled hypertension rate among hypertensive African American women is comparable with their white counterparts.
In the next phase of the Barbershop Study, participants’ use of various classes of antihypertensive medication will be prospectively tracked. Among other things, this will enable investigators to determine whether diuretics contribute to nocturia.
Dr. Mason reported having no conflicts of interest regarding the study.
NEW ORLEANS – Uncontrolled systolic hypertension is a strong independent determinant of nocturia in middle-aged African American men, O’Neil Mason, MD, reported at the American Heart Association scientific sessions.
This finding from the ongoing National Heart, Lung, and Blood Institute–sponsored Barbershop Study challenges the traditional notion of hypertension as an asymptomatic disease. It also provides a novel health promotion message aimed at improving compliance with blood pressure medication.
The Barbershop Study is a hypertension intervention trial that’s being conducted in Los Angeles barbershops frequented by black men. In the initial screening phase for study eligibility, 2,577 African American men aged 35-79 years underwent highly accurate blood pressure measurements using an average of three readings taken via an oscillometric monitor.
The mean age of the men was 53 years. It was an obese group, with a mean body mass index of 30 kg/m2. Fifty percent of the men had hypertension, and among that cohort fully one-third weren’t on antihypertensive medication and another 28% were treated but uncontrolled, with on-treatment blood pressures of 140/90 mm Hg or more. Thus, only 39% of these middle-aged African American men with high blood pressure were treated and controlled at baseline.
Seventy-seven percent of the screened men reported awakening once or more per night to urinate. A progressive increase in nocturia severity was seen with increasing systolic blood pressure. The prevalence of nocturia ranged from 68% among normotensive men to 91% among those with treated but uncontrolled hypertension, Dr. Mason reported.
In a multivariate logistic regression analysis controlling for the standard risk factors for nocturia – including advancing age, an enlarged prostate, and diabetes, which was present in 16% of the men – stage 1 systolic hypertension in the range of 140-159 mm Hg was independently associated with a 1.57-fold increased likelihood of nocturia, compared with normotensive subjects. Stage 2 hypertension, with a systolic blood pressure of 160 mm Hg or more, was associated with a 2.32-fold increased risk; that’s in the same ballpark as having an enlarged prostate, which carried a 2.1-fold increased risk. Prehypertension – that is, a systolic pressure of 120-139 mm Hg – was associated with a nonsignificant 1.18-fold risk.
Diastolic blood pressure wasn’t an independent determinant of nocturia.
In a similar multivariate analysis focused on severe nocturia, defined as three or more episodes per night, stage 1 systolic hypertension was independently associated with a 2.29-fold increased risk, compared with normotension, and stage 2 systolic hypertension carried a 2.77-fold increased risk.
Audience members were clearly intrigued by this novel finding. They were quick to speculate as to potential underlying pathophysiologic mechanisms, including atrial stretch, increased renal blood flow, or perhaps a side effect of diuretic therapy. However, Dr. Mason and his coinvestigators favor another possibility: “African Americans have more salt-sensitive hypertension and they have less nocturnal blood pressure dipping,” he noted. “So if nighttime blood pressure is high it could lead through increased pressure natriuresis to increased urine production. More activity in getting up to go to the bathroom increases the blood pressure and creates a cycle that begets more urine.”
Asked if uncontrolled systolic hypertension is also a determinant of nocturia in African American women, Dr. Mason replied that he would assume so. But that question hasn’t ever been studied. The Barbershop Study is restricted to African American men with hypertension because studies have shown they have a particularly low rate of controlled hypertension. In contrast, the controlled hypertension rate among hypertensive African American women is comparable with their white counterparts.
In the next phase of the Barbershop Study, participants’ use of various classes of antihypertensive medication will be prospectively tracked. Among other things, this will enable investigators to determine whether diuretics contribute to nocturia.
Dr. Mason reported having no conflicts of interest regarding the study.
NEW ORLEANS – Uncontrolled systolic hypertension is a strong independent determinant of nocturia in middle-aged African American men, O’Neil Mason, MD, reported at the American Heart Association scientific sessions.
This finding from the ongoing National Heart, Lung, and Blood Institute–sponsored Barbershop Study challenges the traditional notion of hypertension as an asymptomatic disease. It also provides a novel health promotion message aimed at improving compliance with blood pressure medication.
The Barbershop Study is a hypertension intervention trial that’s being conducted in Los Angeles barbershops frequented by black men. In the initial screening phase for study eligibility, 2,577 African American men aged 35-79 years underwent highly accurate blood pressure measurements using an average of three readings taken via an oscillometric monitor.
The mean age of the men was 53 years. It was an obese group, with a mean body mass index of 30 kg/m2. Fifty percent of the men had hypertension, and among that cohort fully one-third weren’t on antihypertensive medication and another 28% were treated but uncontrolled, with on-treatment blood pressures of 140/90 mm Hg or more. Thus, only 39% of these middle-aged African American men with high blood pressure were treated and controlled at baseline.
Seventy-seven percent of the screened men reported awakening once or more per night to urinate. A progressive increase in nocturia severity was seen with increasing systolic blood pressure. The prevalence of nocturia ranged from 68% among normotensive men to 91% among those with treated but uncontrolled hypertension, Dr. Mason reported.
In a multivariate logistic regression analysis controlling for the standard risk factors for nocturia – including advancing age, an enlarged prostate, and diabetes, which was present in 16% of the men – stage 1 systolic hypertension in the range of 140-159 mm Hg was independently associated with a 1.57-fold increased likelihood of nocturia, compared with normotensive subjects. Stage 2 hypertension, with a systolic blood pressure of 160 mm Hg or more, was associated with a 2.32-fold increased risk; that’s in the same ballpark as having an enlarged prostate, which carried a 2.1-fold increased risk. Prehypertension – that is, a systolic pressure of 120-139 mm Hg – was associated with a nonsignificant 1.18-fold risk.
Diastolic blood pressure wasn’t an independent determinant of nocturia.
In a similar multivariate analysis focused on severe nocturia, defined as three or more episodes per night, stage 1 systolic hypertension was independently associated with a 2.29-fold increased risk, compared with normotension, and stage 2 systolic hypertension carried a 2.77-fold increased risk.
Audience members were clearly intrigued by this novel finding. They were quick to speculate as to potential underlying pathophysiologic mechanisms, including atrial stretch, increased renal blood flow, or perhaps a side effect of diuretic therapy. However, Dr. Mason and his coinvestigators favor another possibility: “African Americans have more salt-sensitive hypertension and they have less nocturnal blood pressure dipping,” he noted. “So if nighttime blood pressure is high it could lead through increased pressure natriuresis to increased urine production. More activity in getting up to go to the bathroom increases the blood pressure and creates a cycle that begets more urine.”
Asked if uncontrolled systolic hypertension is also a determinant of nocturia in African American women, Dr. Mason replied that he would assume so. But that question hasn’t ever been studied. The Barbershop Study is restricted to African American men with hypertension because studies have shown they have a particularly low rate of controlled hypertension. In contrast, the controlled hypertension rate among hypertensive African American women is comparable with their white counterparts.
In the next phase of the Barbershop Study, participants’ use of various classes of antihypertensive medication will be prospectively tracked. Among other things, this will enable investigators to determine whether diuretics contribute to nocturia.
Dr. Mason reported having no conflicts of interest regarding the study.
Key clinical point:
Major finding: A systolic blood pressure of 140-159 mm Hg was independently associated with a 2.29-fold increased risk of severe nocturia, compared with normotension in middle-aged African American men, while a pressure of 160 mm Hg or more conferred a 2.77-fold increased risk.
Data source: A report on the initial cross-sectional screening phase of the Barbershop Study, in which 2,577 middle-aged African American men underwent blood pressure measurements in Los Angeles barbershops.
Disclosures: The Barbershop Study is funded by the National Heart, Lung, and Blood Institute. The presenter reported having no financial conflicts.
High-intensity statins cut amputations and mortality in PAD
NEW ORLEANS – High-intensity statin therapy in patients with peripheral artery disease was associated with significant reductions in amputations as well as mortality during up to 5 years of follow-up in the first large study to examine the relationship, Shipra Arya, MD, reported at the American Heart Association scientific sessions.
Low- or moderate-intensity statin therapy also improved survival compared to no statin, albeit to a significantly lesser magnitude than high-intensity therapy. But high- and low/intermediate-intensity statins were similarly effective in reducing amputation risk, according to Dr. Arya, a vascular surgeon at Emory University in Atlanta.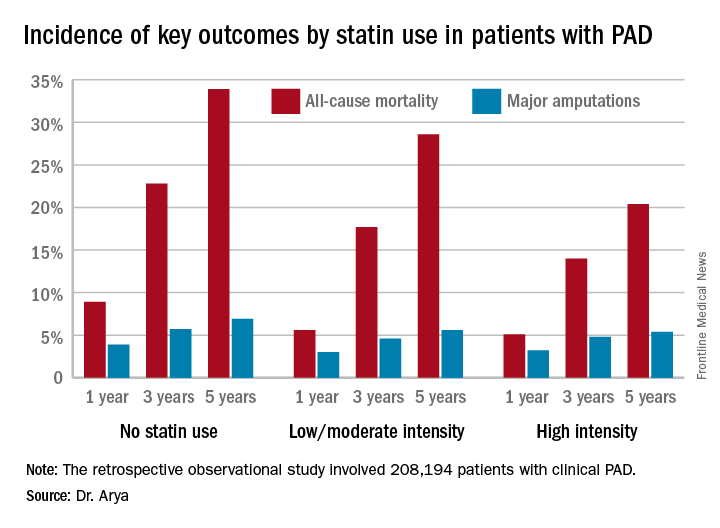
The 2013 AHA/American College of Cardiology treatment guidelines recommend high-intensity statins for all patients with clinical atherosclerotic disease, including those with PAD (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). (Updated PAD guidelines unveiled at the AHA meeting strongly recommend statin medication for all patients with PAD [Circulation. 2016 Nov 13. doi: 10.1161/CIR.0000000000000470]).However, the bulk of patients in Dr. Arya’s study were captured in the database prior to release of the 2013 guidelines. That may account for the sparse use of high-intensity statin therapy in the study cohort. Indeed, only 11.3% of the PAD patients were on a high-intensity statin. Another 36.2% were on moderate-intensity statin therapy, 3.5% were on low-intensity therapy, and 27.6% weren’t on a statin at all.
The relationship between statin therapy and mortality was strongly dose-dependent.
This study was funded by the AHA and the Atlanta Veterans Affairs Medical Center. Dr. Arya reported having no financial conflicts of interest.
NEW ORLEANS – High-intensity statin therapy in patients with peripheral artery disease was associated with significant reductions in amputations as well as mortality during up to 5 years of follow-up in the first large study to examine the relationship, Shipra Arya, MD, reported at the American Heart Association scientific sessions.
Low- or moderate-intensity statin therapy also improved survival compared to no statin, albeit to a significantly lesser magnitude than high-intensity therapy. But high- and low/intermediate-intensity statins were similarly effective in reducing amputation risk, according to Dr. Arya, a vascular surgeon at Emory University in Atlanta.
The 2013 AHA/American College of Cardiology treatment guidelines recommend high-intensity statins for all patients with clinical atherosclerotic disease, including those with PAD (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). (Updated PAD guidelines unveiled at the AHA meeting strongly recommend statin medication for all patients with PAD [Circulation. 2016 Nov 13. doi: 10.1161/CIR.0000000000000470]).However, the bulk of patients in Dr. Arya’s study were captured in the database prior to release of the 2013 guidelines. That may account for the sparse use of high-intensity statin therapy in the study cohort. Indeed, only 11.3% of the PAD patients were on a high-intensity statin. Another 36.2% were on moderate-intensity statin therapy, 3.5% were on low-intensity therapy, and 27.6% weren’t on a statin at all.
The relationship between statin therapy and mortality was strongly dose-dependent.
This study was funded by the AHA and the Atlanta Veterans Affairs Medical Center. Dr. Arya reported having no financial conflicts of interest.
NEW ORLEANS – High-intensity statin therapy in patients with peripheral artery disease was associated with significant reductions in amputations as well as mortality during up to 5 years of follow-up in the first large study to examine the relationship, Shipra Arya, MD, reported at the American Heart Association scientific sessions.
Low- or moderate-intensity statin therapy also improved survival compared to no statin, albeit to a significantly lesser magnitude than high-intensity therapy. But high- and low/intermediate-intensity statins were similarly effective in reducing amputation risk, according to Dr. Arya, a vascular surgeon at Emory University in Atlanta.
The 2013 AHA/American College of Cardiology treatment guidelines recommend high-intensity statins for all patients with clinical atherosclerotic disease, including those with PAD (Circulation. 2014 Jun 24;129[25 Suppl 2]:S1-45). (Updated PAD guidelines unveiled at the AHA meeting strongly recommend statin medication for all patients with PAD [Circulation. 2016 Nov 13. doi: 10.1161/CIR.0000000000000470]).However, the bulk of patients in Dr. Arya’s study were captured in the database prior to release of the 2013 guidelines. That may account for the sparse use of high-intensity statin therapy in the study cohort. Indeed, only 11.3% of the PAD patients were on a high-intensity statin. Another 36.2% were on moderate-intensity statin therapy, 3.5% were on low-intensity therapy, and 27.6% weren’t on a statin at all.
The relationship between statin therapy and mortality was strongly dose-dependent.
This study was funded by the AHA and the Atlanta Veterans Affairs Medical Center. Dr. Arya reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The 5-year all-cause mortality rate after diagnosis of peripheral artery disease was 20.4% in patients on high-intensity statin therapy, 28.6% in those on a low- or moderate-intensity statin, and 33.9% in patients not on a statin.
Data source: A retrospective observational study of 208,194 patients with clinical peripheral artery disease in the national Veterans Affairs database for 2003-2014.
Disclosures: The AHA and the Atlanta Veterans Affairs Medical Center funded the study. The presenter reported having no financial conflicts of interest.
First-trimester blood glucose predicts congenital heart disease risk
NEW ORLEANS – A single, random, first-trimester maternal plasma glucose measurement is superior to an oral glucose tolerance test later in pregnancy as a predictor of congenital heart disease in newborns, Emmi Helle, MD, reported at the American Heart Association scientific sessions.
This finding from a large retrospective study, if confirmed in a prospective data set, is likely to be practice changing. At present, a 1-hour oral glucose tolerance test in the second or third trimester is considered the best means of identifying pregnant women who ought to undergo fetal echocardiography for prenatal diagnosis of congenital heart disease, noted Dr. Helle of Stanford (Calif.) University.
An elevated random plasma glucose value in the first trimester was broadly predictive of increased risk for a variety of congenital heart anomalies, not just, for example, cyanotic conditions.
Fetal heart development is completed during the first trimester, Dr. Helle observed.
Her study received a warm reception. Michael A. Portman, MD, singled it out in his final-day wrap-up of the meeting’s highlights in the field of congenital heart disease.
Several studies have demonstrated that prenatal diagnosis of congenital heart disease results in improved surgical outcomes in newborns. The question is, how to get the right women – those at increased risk – to diagnostic fetal echocardiography. Guidelines suggest but don’t mandate on the basis of weak evidence that an oral glucose tolerance test performed in the second or early third trimester may be a useful means of screening mothers for fetal imaging. Dr. Helle’s study points to a better way.
“Hopefully we can change our guidelines and make them more scientific for identification of mothers who should undergo fetal echocardiography,” said Dr. Portman, professor of pediatrics at the University of Washington, Seattle, and director of pediatric cardiovascular research at Seattle Children’s Hospital.
Dr. Helle and Dr. Portman reported having no relevant financial interests.
NEW ORLEANS – A single, random, first-trimester maternal plasma glucose measurement is superior to an oral glucose tolerance test later in pregnancy as a predictor of congenital heart disease in newborns, Emmi Helle, MD, reported at the American Heart Association scientific sessions.
This finding from a large retrospective study, if confirmed in a prospective data set, is likely to be practice changing. At present, a 1-hour oral glucose tolerance test in the second or third trimester is considered the best means of identifying pregnant women who ought to undergo fetal echocardiography for prenatal diagnosis of congenital heart disease, noted Dr. Helle of Stanford (Calif.) University.
An elevated random plasma glucose value in the first trimester was broadly predictive of increased risk for a variety of congenital heart anomalies, not just, for example, cyanotic conditions.
Fetal heart development is completed during the first trimester, Dr. Helle observed.
Her study received a warm reception. Michael A. Portman, MD, singled it out in his final-day wrap-up of the meeting’s highlights in the field of congenital heart disease.
Several studies have demonstrated that prenatal diagnosis of congenital heart disease results in improved surgical outcomes in newborns. The question is, how to get the right women – those at increased risk – to diagnostic fetal echocardiography. Guidelines suggest but don’t mandate on the basis of weak evidence that an oral glucose tolerance test performed in the second or early third trimester may be a useful means of screening mothers for fetal imaging. Dr. Helle’s study points to a better way.
“Hopefully we can change our guidelines and make them more scientific for identification of mothers who should undergo fetal echocardiography,” said Dr. Portman, professor of pediatrics at the University of Washington, Seattle, and director of pediatric cardiovascular research at Seattle Children’s Hospital.
Dr. Helle and Dr. Portman reported having no relevant financial interests.
NEW ORLEANS – A single, random, first-trimester maternal plasma glucose measurement is superior to an oral glucose tolerance test later in pregnancy as a predictor of congenital heart disease in newborns, Emmi Helle, MD, reported at the American Heart Association scientific sessions.
This finding from a large retrospective study, if confirmed in a prospective data set, is likely to be practice changing. At present, a 1-hour oral glucose tolerance test in the second or third trimester is considered the best means of identifying pregnant women who ought to undergo fetal echocardiography for prenatal diagnosis of congenital heart disease, noted Dr. Helle of Stanford (Calif.) University.
An elevated random plasma glucose value in the first trimester was broadly predictive of increased risk for a variety of congenital heart anomalies, not just, for example, cyanotic conditions.
Fetal heart development is completed during the first trimester, Dr. Helle observed.
Her study received a warm reception. Michael A. Portman, MD, singled it out in his final-day wrap-up of the meeting’s highlights in the field of congenital heart disease.
Several studies have demonstrated that prenatal diagnosis of congenital heart disease results in improved surgical outcomes in newborns. The question is, how to get the right women – those at increased risk – to diagnostic fetal echocardiography. Guidelines suggest but don’t mandate on the basis of weak evidence that an oral glucose tolerance test performed in the second or early third trimester may be a useful means of screening mothers for fetal imaging. Dr. Helle’s study points to a better way.
“Hopefully we can change our guidelines and make them more scientific for identification of mothers who should undergo fetal echocardiography,” said Dr. Portman, professor of pediatrics at the University of Washington, Seattle, and director of pediatric cardiovascular research at Seattle Children’s Hospital.
Dr. Helle and Dr. Portman reported having no relevant financial interests.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: For every 10-mg/dL increase in maternal plasma glucose on a random first-trimester measurement, the risk of giving birth to a baby with congenital heart disease rose by 8%.
Data source: A retrospective study of 19,197 pregnancies, 811 of which resulted in congenital heart disease in the offspring.
Disclosures: The presenter reported having no financial conflicts of interest regarding the study.
Ischemia-repairing cells fall short for treating intermittent claudication
NEW ORLEANS – Therapy with cells known to repair ischemic damage does not improve intermittent claudication of the legs in unselected patients, according to data from the randomized, phase II PACE trial reported at the American Heart Association scientific sessions. But some patients had evidence of new vessel formation.
“Administration of ALDH [aldehyde dehydrogenase] bright cells was feasible and safe, [but] administration at this dose and in this PAD [peripheral artery disease] cohort did not change peak walking time or MRI-based anatomic and perfusion endpoints,” reported Emerson C. Perin, MD, director of Clinical Research for Cardiovascular Medicine and medical director of the Stem Cell Center, both at the Texas Heart Institute, Houston.
However, “the MRI techniques developed and applied for the first time in a multicenter PAD clinical trial are now available for application in future PAD clinical research to determine if a clinically relevant therapeutic benefit might be achieved from cells or any other promising intervention,” he noted.
“One of the things in peripheral vascular disease that’s always been true is that peak walking time is a good clinical endpoint,” said session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute. “[You] proposed some MRI parameters, but those didn’t correlate with peak walking time. So is the takeaway from this trial these MRI parameters? And if they don’t necessarily correlate, why would you advocate for them?”
Dr. Perin replied: “PAD is kind of the stepchild of cardiovascular medicine, it’s very poorly understood. And I think with the PACE trial, we’ve actually taken a huge step in understanding how we can treat these patients and how to study these patients.”
“Even though intermittent claudication or PAD starts with the flow limitation, what you wind up getting later down the road is not something that just relates to flow,” he elaborated. “We were able to study flow completely in this study – we owned it. What we weren’t able to study, and at the time we couldn’t, but now we can, is the metabolic, endothelial, and mitochondrial function. That is, what’s happening at the level of the muscle that is the missing link, together with the flow, that will give us these answers. So I think PACE [Patients With Intermittent Claudication Injected With ALDH Bright Cells] was very important to give us a greater understanding of where we can go now in PAD research.”
Trial details
Between 1 and 3 million people in the United States live with claudication, Dr. Perin noted when introducing the study. “It’s a very significant problem and a problem for which we really don’t have good solutions. We have one medicine [cilostazol], revascularization surgery, and stents that have recurrence – things that are less than perfect. There are also exercise programs, which not everyone has access to.”
The ALDH bright cells tested in PACE are collected from a patient’s bone marrow and express high levels of that enzyme. They are enriched for hematopoietic, endothelial progenitor, and multipotent mesenchymal colony-forming cells, and have shown ischemic repair capacity in preclinical models, with an increase in capillary density.
The investigators enrolled 82 patients with atherosclerotic peripheral arterial disease and symptom-limiting intermittent claudication of the legs. All had a pre-exercise ankle-brachial index of less than 0.9 or a pre-exercise toe-brachial index of less than 0.7, as well as stenosis greater than 50% or occlusion of infra-inguinal arteries by advanced imaging.
The patients were treated with 10 1-mL injections of ALDH bright cells or placebo into muscles of the posterior lower thigh and calf.
Results showed that after 6 months, peak treadmill walking time had improved by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, but the difference was not significant, Dr. Perin reported. The groups also were statistically indistinguishable overall with respect to changes in ankle-brachial index, walking impairment, and symptoms, and in MRI-assessed collateral count, peak hyperemic flow in the popliteal artery, and capillary perfusion.
However, among the subgroup of patients having a pre-exercise ankle-brachial index of 0.6 or less at baseline, collateral count increased by 2.4 in the cell therapy group, compared with 0.5 in the placebo group (P = .021).
In addition, among patients who had occluded femoral arteries at baseline (having more collateral vessels than peers with patent femoral arteries), the number of collaterals increased by 1.5 in the cell therapy group, compared with 0.3 in the placebo group (P = .047).
“This suggests an arteriogenic effect of cell therapy in patients with an occluded femoral artery substrate,” said Dr. Perin, who disclosed that he received a research grant from the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Therapy with cells known to repair ischemic damage does not improve intermittent claudication of the legs in unselected patients, according to data from the randomized, phase II PACE trial reported at the American Heart Association scientific sessions. But some patients had evidence of new vessel formation.
“Administration of ALDH [aldehyde dehydrogenase] bright cells was feasible and safe, [but] administration at this dose and in this PAD [peripheral artery disease] cohort did not change peak walking time or MRI-based anatomic and perfusion endpoints,” reported Emerson C. Perin, MD, director of Clinical Research for Cardiovascular Medicine and medical director of the Stem Cell Center, both at the Texas Heart Institute, Houston.
However, “the MRI techniques developed and applied for the first time in a multicenter PAD clinical trial are now available for application in future PAD clinical research to determine if a clinically relevant therapeutic benefit might be achieved from cells or any other promising intervention,” he noted.
“One of the things in peripheral vascular disease that’s always been true is that peak walking time is a good clinical endpoint,” said session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute. “[You] proposed some MRI parameters, but those didn’t correlate with peak walking time. So is the takeaway from this trial these MRI parameters? And if they don’t necessarily correlate, why would you advocate for them?”
Dr. Perin replied: “PAD is kind of the stepchild of cardiovascular medicine, it’s very poorly understood. And I think with the PACE trial, we’ve actually taken a huge step in understanding how we can treat these patients and how to study these patients.”
“Even though intermittent claudication or PAD starts with the flow limitation, what you wind up getting later down the road is not something that just relates to flow,” he elaborated. “We were able to study flow completely in this study – we owned it. What we weren’t able to study, and at the time we couldn’t, but now we can, is the metabolic, endothelial, and mitochondrial function. That is, what’s happening at the level of the muscle that is the missing link, together with the flow, that will give us these answers. So I think PACE [Patients With Intermittent Claudication Injected With ALDH Bright Cells] was very important to give us a greater understanding of where we can go now in PAD research.”
Trial details
Between 1 and 3 million people in the United States live with claudication, Dr. Perin noted when introducing the study. “It’s a very significant problem and a problem for which we really don’t have good solutions. We have one medicine [cilostazol], revascularization surgery, and stents that have recurrence – things that are less than perfect. There are also exercise programs, which not everyone has access to.”
The ALDH bright cells tested in PACE are collected from a patient’s bone marrow and express high levels of that enzyme. They are enriched for hematopoietic, endothelial progenitor, and multipotent mesenchymal colony-forming cells, and have shown ischemic repair capacity in preclinical models, with an increase in capillary density.
The investigators enrolled 82 patients with atherosclerotic peripheral arterial disease and symptom-limiting intermittent claudication of the legs. All had a pre-exercise ankle-brachial index of less than 0.9 or a pre-exercise toe-brachial index of less than 0.7, as well as stenosis greater than 50% or occlusion of infra-inguinal arteries by advanced imaging.
The patients were treated with 10 1-mL injections of ALDH bright cells or placebo into muscles of the posterior lower thigh and calf.
Results showed that after 6 months, peak treadmill walking time had improved by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, but the difference was not significant, Dr. Perin reported. The groups also were statistically indistinguishable overall with respect to changes in ankle-brachial index, walking impairment, and symptoms, and in MRI-assessed collateral count, peak hyperemic flow in the popliteal artery, and capillary perfusion.
However, among the subgroup of patients having a pre-exercise ankle-brachial index of 0.6 or less at baseline, collateral count increased by 2.4 in the cell therapy group, compared with 0.5 in the placebo group (P = .021).
In addition, among patients who had occluded femoral arteries at baseline (having more collateral vessels than peers with patent femoral arteries), the number of collaterals increased by 1.5 in the cell therapy group, compared with 0.3 in the placebo group (P = .047).
“This suggests an arteriogenic effect of cell therapy in patients with an occluded femoral artery substrate,” said Dr. Perin, who disclosed that he received a research grant from the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Therapy with cells known to repair ischemic damage does not improve intermittent claudication of the legs in unselected patients, according to data from the randomized, phase II PACE trial reported at the American Heart Association scientific sessions. But some patients had evidence of new vessel formation.
“Administration of ALDH [aldehyde dehydrogenase] bright cells was feasible and safe, [but] administration at this dose and in this PAD [peripheral artery disease] cohort did not change peak walking time or MRI-based anatomic and perfusion endpoints,” reported Emerson C. Perin, MD, director of Clinical Research for Cardiovascular Medicine and medical director of the Stem Cell Center, both at the Texas Heart Institute, Houston.
However, “the MRI techniques developed and applied for the first time in a multicenter PAD clinical trial are now available for application in future PAD clinical research to determine if a clinically relevant therapeutic benefit might be achieved from cells or any other promising intervention,” he noted.
“One of the things in peripheral vascular disease that’s always been true is that peak walking time is a good clinical endpoint,” said session panelist Doris A. Taylor, PhD, director of Regenerative Medicine Research at the Texas Heart Institute. “[You] proposed some MRI parameters, but those didn’t correlate with peak walking time. So is the takeaway from this trial these MRI parameters? And if they don’t necessarily correlate, why would you advocate for them?”
Dr. Perin replied: “PAD is kind of the stepchild of cardiovascular medicine, it’s very poorly understood. And I think with the PACE trial, we’ve actually taken a huge step in understanding how we can treat these patients and how to study these patients.”
“Even though intermittent claudication or PAD starts with the flow limitation, what you wind up getting later down the road is not something that just relates to flow,” he elaborated. “We were able to study flow completely in this study – we owned it. What we weren’t able to study, and at the time we couldn’t, but now we can, is the metabolic, endothelial, and mitochondrial function. That is, what’s happening at the level of the muscle that is the missing link, together with the flow, that will give us these answers. So I think PACE [Patients With Intermittent Claudication Injected With ALDH Bright Cells] was very important to give us a greater understanding of where we can go now in PAD research.”
Trial details
Between 1 and 3 million people in the United States live with claudication, Dr. Perin noted when introducing the study. “It’s a very significant problem and a problem for which we really don’t have good solutions. We have one medicine [cilostazol], revascularization surgery, and stents that have recurrence – things that are less than perfect. There are also exercise programs, which not everyone has access to.”
The ALDH bright cells tested in PACE are collected from a patient’s bone marrow and express high levels of that enzyme. They are enriched for hematopoietic, endothelial progenitor, and multipotent mesenchymal colony-forming cells, and have shown ischemic repair capacity in preclinical models, with an increase in capillary density.
The investigators enrolled 82 patients with atherosclerotic peripheral arterial disease and symptom-limiting intermittent claudication of the legs. All had a pre-exercise ankle-brachial index of less than 0.9 or a pre-exercise toe-brachial index of less than 0.7, as well as stenosis greater than 50% or occlusion of infra-inguinal arteries by advanced imaging.
The patients were treated with 10 1-mL injections of ALDH bright cells or placebo into muscles of the posterior lower thigh and calf.
Results showed that after 6 months, peak treadmill walking time had improved by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, but the difference was not significant, Dr. Perin reported. The groups also were statistically indistinguishable overall with respect to changes in ankle-brachial index, walking impairment, and symptoms, and in MRI-assessed collateral count, peak hyperemic flow in the popliteal artery, and capillary perfusion.
However, among the subgroup of patients having a pre-exercise ankle-brachial index of 0.6 or less at baseline, collateral count increased by 2.4 in the cell therapy group, compared with 0.5 in the placebo group (P = .021).
In addition, among patients who had occluded femoral arteries at baseline (having more collateral vessels than peers with patent femoral arteries), the number of collaterals increased by 1.5 in the cell therapy group, compared with 0.3 in the placebo group (P = .047).
“This suggests an arteriogenic effect of cell therapy in patients with an occluded femoral artery substrate,” said Dr. Perin, who disclosed that he received a research grant from the National Heart, Lung, and Blood Institute.
Key clinical point:
Major finding: At 6 months, peak walking time had increased by 2.2 minutes in the cell therapy group and 1.2 minutes in the placebo group, a nonsignificant difference (P = .238).
Data source: PACE, a randomized phase II trial of 82 patients with PAD and symptom-limiting intermittent claudication of the legs.
Disclosures: Dr. Perin received a research grant from the National Heart, Lung, and Blood Institute.
VIDEO: New antisense inhibitor nets impressive reductions in lipids
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
The trial shows a very clear proof of concept in regards to the effects on lipids.
In contrast to agents that increase LDL receptor levels (statins, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors, bile acid–binding resins, and ezetimibe), IONIS-ANGPTL3-LRx produces greater reductions in non-HDL cholesterol than in LDL cholesterol, suggesting different mechanisms are at work.
But the key issue at this point is to ascertain the longer-term safety of IONIS-ANGPTL3-LRx in the liver and other organs.
One of the most impressive aspects of the combination of biotechnology and genetics is how rapidly you can come to a proof of concept in humans, and this is unprecedented, compared with what we have seen over the last 20 or 30 years. Unfortunately, the determination of safety is not quite as rapid, and it takes large numbers and long duration of follow-up. Obviously, we need that in terms of understanding benefits and risks.
Christie M. Ballantyne, MD, director of the Center for Cardiovascular Disease Prevention at the Methodist DeBakey Heart Center, Baylor College of Medicine in Houston, made these comments as the invited discussant. He has been a consultant to and received research support from trial sponsor Ionis Pharmaceuticals.
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A new antisense inhibitor to angiopoietinlike protein 3 (ANGPTL3) reduces lipids in healthy adults with elevated triglyceride levels, according to results of a phase I/IIa ascending-dose trial reported at the American Heart Association scientific sessions.
ANGPTL3 regulates lipid and possibly general metabolism through actions in the liver, gut, muscle, and adipose tissue, explained presenting author Sotirios Tsimikas, MD, an investigator with Ionis Pharmaceuticals, Carlsbad, Calif., and a professor of medicine and director of vascular medicine at the University of California, San Diego. Individuals having loss-of-function mutations in the gene encoding this protein have very low plasma levels of cholesterol and triglycerides.
In the trial, sponsored by Ionis Pharmaceuticals, 32 healthy volunteers with elevated triglyceride levels were treated with various doses of the antisense inhibitor, called IONIS-ANGPTL3-LRx, or a placebo, given by weekly subcutaneous injections for 6 weeks.
Results showed that participants treated at the higher-dose levels had a reduction from baseline of 66% in triglycerides, 68% in apoliprotein C-III (ApoC-III), 35% in LDL cholesterol, 36% in total cholesterol, and 40% in non-HDL cholesterol, as well as 25% in HDL cholesterol, Dr. Tsimikas reported. They also had reductions in apolipoprotein B (ApoB).
“All these lipid parameters are really going in the right direction in terms of postulating potential clinical benefit,” he commented.
Safety and tolerability results showed that only a single participant experienced a local injection site adverse event related to the inhibitor (erythema and pruritus). None experienced flulike symptoms, platelet reductions, or serious adverse events, and none left the study because of adverse events.
“Among all known therapies that lower triglycerides, this [IONIS-ANGPTL3-LRx] is also associated with a reduction not only in LDL cholesterol levels, but also in ApoB as well, which portends well for future outcomes trials,” noted Dr. Tsimikas.
“We think this is going to be a promising candidate for patients who have uncontrolled LDL cholesterol, elevated triglycerides, and possibly patients who have hepatic steatosis and NASH [nonalcoholic steatohepatitis],” he concluded.
Dr. Tsimikas, who is an employee of and shareholder in the trial’s sponsor, Ionis Pharmaceuticals, discussed his findings in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Treatment was associated with reductions in levels of triglycerides (–66%), ApoC-III (–68%), LDL cholesterol (–35%), total cholesterol (–36%), and non-HDL cholesterol (–40%), as well as levels of HDL cholesterol (–25%).
Data source: A phase I/IIa ascending-dose trial among 32 healthy adults with hypertriglyceridemia.
Disclosures: Dr. Tsimikas is an employee of and shareholder in Ionis Pharmaceuticals, which sponsored the trial.
Depression further boosts stroke risk in A-fib
NEW ORLEANS – Depression increases the risk of stroke in patients with atrial fibrillation, Bruno B. Lima, MD, reported at the American Heart Association scientific sessions.
This novel finding from what’s believed to be the first prospective observational study to examine the association between depression and stroke risk in patients with atrial fibrillation (AF) raises the exciting possibility that systematic screening for depression and provision of effective antidepressant therapy in affected AF patients could reduce their risk of stroke. But clinical trials will be necessary to answer that question, noted Dr. Lima of the University of Pittsburgh.
He reported on 5,976 consecutive patients diagnosed with paroxysmal or persistent AF and placed on rhythm control medication at the University of Pittsburgh Medical Center and its affiliated clinics. Physician-diagnosed depression was present in 11.4% of the patients’ charts.
During a median follow-up of 39 months, 200 strokes occurred, including 35 in patients with depression. The incidence was 7.5% in AF patients with depression and 4.7% in those without diagnosed depression.
The depressed AF patients had more comorbid conditions, including significantly higher rates of hypertension, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, obesity, cancer, and heart failure. They were also more likely to be smokers. But in a multivariate analysis controlling for comorbidities, age, gender, previous stroke, and use of anticoagulant therapy, the depressed AF patients remained at 45% greater risk of stroke compared with AF patients without diagnosed depression.
Strengths of this study include its large size and reasonable duration of follow-up. Limitations include the fact that investigators had to rely upon physician documentation of depressive episodes in the medical record, with no standardized measure of depression severity. Clinical trials addressing the issue of whether effective antidepressant therapy prevents strokes in depressed AF patients will need to use systematic screening for depression and serial measurements of depression severity, according to Dr. Lima.
He reported having no financial conflicts of interest regarding this study, which was conducted free of commercial support.
NEW ORLEANS – Depression increases the risk of stroke in patients with atrial fibrillation, Bruno B. Lima, MD, reported at the American Heart Association scientific sessions.
This novel finding from what’s believed to be the first prospective observational study to examine the association between depression and stroke risk in patients with atrial fibrillation (AF) raises the exciting possibility that systematic screening for depression and provision of effective antidepressant therapy in affected AF patients could reduce their risk of stroke. But clinical trials will be necessary to answer that question, noted Dr. Lima of the University of Pittsburgh.
He reported on 5,976 consecutive patients diagnosed with paroxysmal or persistent AF and placed on rhythm control medication at the University of Pittsburgh Medical Center and its affiliated clinics. Physician-diagnosed depression was present in 11.4% of the patients’ charts.
During a median follow-up of 39 months, 200 strokes occurred, including 35 in patients with depression. The incidence was 7.5% in AF patients with depression and 4.7% in those without diagnosed depression.
The depressed AF patients had more comorbid conditions, including significantly higher rates of hypertension, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, obesity, cancer, and heart failure. They were also more likely to be smokers. But in a multivariate analysis controlling for comorbidities, age, gender, previous stroke, and use of anticoagulant therapy, the depressed AF patients remained at 45% greater risk of stroke compared with AF patients without diagnosed depression.
Strengths of this study include its large size and reasonable duration of follow-up. Limitations include the fact that investigators had to rely upon physician documentation of depressive episodes in the medical record, with no standardized measure of depression severity. Clinical trials addressing the issue of whether effective antidepressant therapy prevents strokes in depressed AF patients will need to use systematic screening for depression and serial measurements of depression severity, according to Dr. Lima.
He reported having no financial conflicts of interest regarding this study, which was conducted free of commercial support.
NEW ORLEANS – Depression increases the risk of stroke in patients with atrial fibrillation, Bruno B. Lima, MD, reported at the American Heart Association scientific sessions.
This novel finding from what’s believed to be the first prospective observational study to examine the association between depression and stroke risk in patients with atrial fibrillation (AF) raises the exciting possibility that systematic screening for depression and provision of effective antidepressant therapy in affected AF patients could reduce their risk of stroke. But clinical trials will be necessary to answer that question, noted Dr. Lima of the University of Pittsburgh.
He reported on 5,976 consecutive patients diagnosed with paroxysmal or persistent AF and placed on rhythm control medication at the University of Pittsburgh Medical Center and its affiliated clinics. Physician-diagnosed depression was present in 11.4% of the patients’ charts.
During a median follow-up of 39 months, 200 strokes occurred, including 35 in patients with depression. The incidence was 7.5% in AF patients with depression and 4.7% in those without diagnosed depression.
The depressed AF patients had more comorbid conditions, including significantly higher rates of hypertension, diabetes, chronic kidney disease, chronic obstructive pulmonary disease, obesity, cancer, and heart failure. They were also more likely to be smokers. But in a multivariate analysis controlling for comorbidities, age, gender, previous stroke, and use of anticoagulant therapy, the depressed AF patients remained at 45% greater risk of stroke compared with AF patients without diagnosed depression.
Strengths of this study include its large size and reasonable duration of follow-up. Limitations include the fact that investigators had to rely upon physician documentation of depressive episodes in the medical record, with no standardized measure of depression severity. Clinical trials addressing the issue of whether effective antidepressant therapy prevents strokes in depressed AF patients will need to use systematic screening for depression and serial measurements of depression severity, according to Dr. Lima.
He reported having no financial conflicts of interest regarding this study, which was conducted free of commercial support.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: After adjustment for numerous potential confounders, depressed patients with atrial fibrillation were 45% more likely to have a stroke during follow-up than were nondepressed patients with the arrhythmia.
Data source: A single-center prospective observational study of nearly 6,000 consecutive AF patients followed for a median of 39 months, 11.4% of whom carried a diagnosis of depression.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study, which was conducted free of commercial support.
VIDEO: ECG screen for cardiac disease in all youths is cost effective
NEW ORLEANS – Results of a first-of-its-kind nationwide U.K. cardiac disease screening program in the general population of teens and young adults indicate that incorporating a 12-lead ECG alongside history and physical examination not only increases the diagnostic yield for conditions predisposing to sudden cardiac death, but it’s actually cost saving, compared with screening by history and physical exam alone, Harshil Dhutia, MD, said at the American Heart Association scientific sessions.
Current practice in the United Kingdom and most other western countries, including the United States, is to screen for cardiac disease in the general population of young people by history and physical exam alone. Only those with symptoms or a positive family history on the initial screen go on to a more comprehensive evaluation including an ECG. The ECG isn’t part of the initial screen primarily because of cost concerns. However, those concerns are based upon conjecture. The diagnostic and financial implications of routine screening by 12-lead ECG in the general population of young people hadn’t been examined prior to the nationwide U.K. screening program – and the results of the U.K. project indicate it’s time for a change in policy, according to Dr. Dhutia, a cardiology research fellow at St. George’s University in London.
The screening project, known as the Cardiac Risk in the Young Program, included 26,900 subjects aged 14-35 years without known cardiovascular disease who responded to public service announcements and voluntarily presented for screening at schools, community centers, and health centers across the U.K. during 2011-2013. The mean age of the subjects was 19.4 years, roughly two-thirds were male, and 90% were white. All screening was performed by cardiologists who followed the AHA protocol for history-taking, performed the physical exam, and followed the European Society of Cardiology 2010 recommendations for ECG interpretation. Individuals with a positive screen were referred to their local hospital for further investigation. Participants were prospectively followed for 2 years.
“This was the first study of comprehensive cardiovascular screening of young individuals outside competitive sport,” Dr. Dhutia noted.
Among the key findings: 3.5% of subjects were deemed by their screening cardiologist to have an abnormal history and/or physical exam warranting further investigation, 8.1% had an abnormal 12-lead ECG, and 0.5% had both. Overall, 11.7% of subjects underwent echocardiography to confirm or refute a diagnosis of cardiac disease, 1.7% underwent Holter monitoring, 1.7% had an exercise stress test, and 0.9% underwent cardiac MRI.
At 2 years of follow-up, 87 individuals, or 0.3% of the overall study cohort, had been diagnosed with a serious cardiac disease predisposing to sudden cardiac death.
“The vast majority of these individuals were asymptomatic and diagnosed on the basis of an ECG abnormality,” Dr. Dhutia observed.
Indeed, 72 of the 87 patients with serious cardiac disease were diagnosed on the basis on their abnormal ECG. This tool proved particularly helpful in identifying individuals with cardiomyopathies or congenital accessory pathways, such as Wolff-Parkinson-White syndrome or long QT syndrome. At 2 years of follow-up, 42 of these 72 patients were on disease-modifying therapies beyond lifestyle interventions, most commonly ablation procedures or antiarrhythmic medication.
The history and physical exam was useful in identifying young people with channelopathies or Marfan syndrome. Roughly half of individuals identified by history and physical exam as having serious cardiac disease were on antiarrhythmic medication – or, less commonly, pacemaker therapy – at 2 years follow-up.
The overall cost per individual screened using history, physical exam, and ECG amounted to $110 on the basis of U.K. National Health Service rates. The cost per serious cardiac condition identified was $33,927. In contrast, the average cost per serious cardiac diagnosis under the current U.K. protocol of screening youth by history and physical exam only is 67% greater, at $56,597.
“Inclusion of ECG to history and physical examination is associated with a significantly improved diagnostic yield and superior economic profile. These findings have the potential to influence health care policy and certainly suggest that the National Health Service framework in the United Kingdom is counterintuitive and may warrant revision,” he concluded.
Session moderator Nisha Parikh, MD, of the University of California, San Francisco, asked if Dr. Dhutia and his coinvestigators had identified any characteristics associated with serious occult cardiac disease in adolescents and young adults that would permit a more refined, selective approach to screening.
Not in the general population of nearly 27,000 subjects in the U.K. study, he replied. Competitive athletes are a different story, though.
“In competitive athletes, certainly individuals who play stop/start sports such as basketball or football [soccer], appear to be at higher risk, especially those of Afro-Caribbean origin. The incidence of sudden cardiac death in Afro-Caribbean basketball players is 1 in 3,000,” according to Dr. Dhutia, who discussed his findings in a video interview.
He reported receiving a small research grant for his study from the Cardiac Risk in the Young Program, a U.K. charitable organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Results of a first-of-its-kind nationwide U.K. cardiac disease screening program in the general population of teens and young adults indicate that incorporating a 12-lead ECG alongside history and physical examination not only increases the diagnostic yield for conditions predisposing to sudden cardiac death, but it’s actually cost saving, compared with screening by history and physical exam alone, Harshil Dhutia, MD, said at the American Heart Association scientific sessions.
Current practice in the United Kingdom and most other western countries, including the United States, is to screen for cardiac disease in the general population of young people by history and physical exam alone. Only those with symptoms or a positive family history on the initial screen go on to a more comprehensive evaluation including an ECG. The ECG isn’t part of the initial screen primarily because of cost concerns. However, those concerns are based upon conjecture. The diagnostic and financial implications of routine screening by 12-lead ECG in the general population of young people hadn’t been examined prior to the nationwide U.K. screening program – and the results of the U.K. project indicate it’s time for a change in policy, according to Dr. Dhutia, a cardiology research fellow at St. George’s University in London.
The screening project, known as the Cardiac Risk in the Young Program, included 26,900 subjects aged 14-35 years without known cardiovascular disease who responded to public service announcements and voluntarily presented for screening at schools, community centers, and health centers across the U.K. during 2011-2013. The mean age of the subjects was 19.4 years, roughly two-thirds were male, and 90% were white. All screening was performed by cardiologists who followed the AHA protocol for history-taking, performed the physical exam, and followed the European Society of Cardiology 2010 recommendations for ECG interpretation. Individuals with a positive screen were referred to their local hospital for further investigation. Participants were prospectively followed for 2 years.
“This was the first study of comprehensive cardiovascular screening of young individuals outside competitive sport,” Dr. Dhutia noted.
Among the key findings: 3.5% of subjects were deemed by their screening cardiologist to have an abnormal history and/or physical exam warranting further investigation, 8.1% had an abnormal 12-lead ECG, and 0.5% had both. Overall, 11.7% of subjects underwent echocardiography to confirm or refute a diagnosis of cardiac disease, 1.7% underwent Holter monitoring, 1.7% had an exercise stress test, and 0.9% underwent cardiac MRI.
At 2 years of follow-up, 87 individuals, or 0.3% of the overall study cohort, had been diagnosed with a serious cardiac disease predisposing to sudden cardiac death.
“The vast majority of these individuals were asymptomatic and diagnosed on the basis of an ECG abnormality,” Dr. Dhutia observed.
Indeed, 72 of the 87 patients with serious cardiac disease were diagnosed on the basis on their abnormal ECG. This tool proved particularly helpful in identifying individuals with cardiomyopathies or congenital accessory pathways, such as Wolff-Parkinson-White syndrome or long QT syndrome. At 2 years of follow-up, 42 of these 72 patients were on disease-modifying therapies beyond lifestyle interventions, most commonly ablation procedures or antiarrhythmic medication.
The history and physical exam was useful in identifying young people with channelopathies or Marfan syndrome. Roughly half of individuals identified by history and physical exam as having serious cardiac disease were on antiarrhythmic medication – or, less commonly, pacemaker therapy – at 2 years follow-up.
The overall cost per individual screened using history, physical exam, and ECG amounted to $110 on the basis of U.K. National Health Service rates. The cost per serious cardiac condition identified was $33,927. In contrast, the average cost per serious cardiac diagnosis under the current U.K. protocol of screening youth by history and physical exam only is 67% greater, at $56,597.
“Inclusion of ECG to history and physical examination is associated with a significantly improved diagnostic yield and superior economic profile. These findings have the potential to influence health care policy and certainly suggest that the National Health Service framework in the United Kingdom is counterintuitive and may warrant revision,” he concluded.
Session moderator Nisha Parikh, MD, of the University of California, San Francisco, asked if Dr. Dhutia and his coinvestigators had identified any characteristics associated with serious occult cardiac disease in adolescents and young adults that would permit a more refined, selective approach to screening.
Not in the general population of nearly 27,000 subjects in the U.K. study, he replied. Competitive athletes are a different story, though.
“In competitive athletes, certainly individuals who play stop/start sports such as basketball or football [soccer], appear to be at higher risk, especially those of Afro-Caribbean origin. The incidence of sudden cardiac death in Afro-Caribbean basketball players is 1 in 3,000,” according to Dr. Dhutia, who discussed his findings in a video interview.
He reported receiving a small research grant for his study from the Cardiac Risk in the Young Program, a U.K. charitable organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Results of a first-of-its-kind nationwide U.K. cardiac disease screening program in the general population of teens and young adults indicate that incorporating a 12-lead ECG alongside history and physical examination not only increases the diagnostic yield for conditions predisposing to sudden cardiac death, but it’s actually cost saving, compared with screening by history and physical exam alone, Harshil Dhutia, MD, said at the American Heart Association scientific sessions.
Current practice in the United Kingdom and most other western countries, including the United States, is to screen for cardiac disease in the general population of young people by history and physical exam alone. Only those with symptoms or a positive family history on the initial screen go on to a more comprehensive evaluation including an ECG. The ECG isn’t part of the initial screen primarily because of cost concerns. However, those concerns are based upon conjecture. The diagnostic and financial implications of routine screening by 12-lead ECG in the general population of young people hadn’t been examined prior to the nationwide U.K. screening program – and the results of the U.K. project indicate it’s time for a change in policy, according to Dr. Dhutia, a cardiology research fellow at St. George’s University in London.
The screening project, known as the Cardiac Risk in the Young Program, included 26,900 subjects aged 14-35 years without known cardiovascular disease who responded to public service announcements and voluntarily presented for screening at schools, community centers, and health centers across the U.K. during 2011-2013. The mean age of the subjects was 19.4 years, roughly two-thirds were male, and 90% were white. All screening was performed by cardiologists who followed the AHA protocol for history-taking, performed the physical exam, and followed the European Society of Cardiology 2010 recommendations for ECG interpretation. Individuals with a positive screen were referred to their local hospital for further investigation. Participants were prospectively followed for 2 years.
“This was the first study of comprehensive cardiovascular screening of young individuals outside competitive sport,” Dr. Dhutia noted.
Among the key findings: 3.5% of subjects were deemed by their screening cardiologist to have an abnormal history and/or physical exam warranting further investigation, 8.1% had an abnormal 12-lead ECG, and 0.5% had both. Overall, 11.7% of subjects underwent echocardiography to confirm or refute a diagnosis of cardiac disease, 1.7% underwent Holter monitoring, 1.7% had an exercise stress test, and 0.9% underwent cardiac MRI.
At 2 years of follow-up, 87 individuals, or 0.3% of the overall study cohort, had been diagnosed with a serious cardiac disease predisposing to sudden cardiac death.
“The vast majority of these individuals were asymptomatic and diagnosed on the basis of an ECG abnormality,” Dr. Dhutia observed.
Indeed, 72 of the 87 patients with serious cardiac disease were diagnosed on the basis on their abnormal ECG. This tool proved particularly helpful in identifying individuals with cardiomyopathies or congenital accessory pathways, such as Wolff-Parkinson-White syndrome or long QT syndrome. At 2 years of follow-up, 42 of these 72 patients were on disease-modifying therapies beyond lifestyle interventions, most commonly ablation procedures or antiarrhythmic medication.
The history and physical exam was useful in identifying young people with channelopathies or Marfan syndrome. Roughly half of individuals identified by history and physical exam as having serious cardiac disease were on antiarrhythmic medication – or, less commonly, pacemaker therapy – at 2 years follow-up.
The overall cost per individual screened using history, physical exam, and ECG amounted to $110 on the basis of U.K. National Health Service rates. The cost per serious cardiac condition identified was $33,927. In contrast, the average cost per serious cardiac diagnosis under the current U.K. protocol of screening youth by history and physical exam only is 67% greater, at $56,597.
“Inclusion of ECG to history and physical examination is associated with a significantly improved diagnostic yield and superior economic profile. These findings have the potential to influence health care policy and certainly suggest that the National Health Service framework in the United Kingdom is counterintuitive and may warrant revision,” he concluded.
Session moderator Nisha Parikh, MD, of the University of California, San Francisco, asked if Dr. Dhutia and his coinvestigators had identified any characteristics associated with serious occult cardiac disease in adolescents and young adults that would permit a more refined, selective approach to screening.
Not in the general population of nearly 27,000 subjects in the U.K. study, he replied. Competitive athletes are a different story, though.
“In competitive athletes, certainly individuals who play stop/start sports such as basketball or football [soccer], appear to be at higher risk, especially those of Afro-Caribbean origin. The incidence of sudden cardiac death in Afro-Caribbean basketball players is 1 in 3,000,” according to Dr. Dhutia, who discussed his findings in a video interview.
He reported receiving a small research grant for his study from the Cardiac Risk in the Young Program, a U.K. charitable organization.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Key clinical point:
Major finding: The cost of identifying one case of serious cardiac disease predisposing to sudden cardiac death in young people was reduced by 40% when initial screening of the general population ages 14-35 years was conducted by history, physical examination, and 12-lead ECG, compared with no ECG.
Data source: A prospective observational study of nearly 27,000 young people ages 14-35 years in the general population across the United Kingdom who presented for cardiac screening conducted by cardiologists using history, physical examination, and a 12-lead ECG.
Disclosures: The study was funded by the Cardiac Risk in the Young Program, a U.K. charitable organization.
Cardiovascular complications in pregnancy quickly boost future risk
NEW ORLEANS – Women who experience peripartum cardiomyopathy or any of a variety of hypertensive disorders in pregnancy are at sharply increased risk of acute MI, stroke, or new-onset heart failure beginning within just a few years, Rima Arnaout, MD, reported at the American Heart Association scientific sessions.
“Our study supports the idea that women who have cardiovascular complications in pregnancy really need to be monitored closely for potential primary prevention of cardiovascular events,” said Dr. Arnaout of the University of California, San Francisco.
At a session devoted to “big data” studies in cardiovascular medicine, she presented one of the biggest: a retrospective cohort study of 1.66 million pregnancies during 2005-2009 in California women without any history of congenital or valvular heart disease or prepregnancy cardiovascular events. The California database was created as part of the U.S. Agency for Healthcare Research and Quality’s comprehensive Healthcare Cost and Utilization Project, which included more than 95% of the state’s hospitals. Women who experienced an MI or a stroke, or who were diagnosed with heart failure, during a median of 2.7 years and a maximum of 6 years of follow-up post pregnancy were identified via ICD-9 codes.
There were 111,202 cases of various forms of hypertension in pregnancy, for a 6.9% incidence. Peripartum cardiomyopathy was diagnosed in 562 women, for a rate of 3.5 cases per 10,000 pregnancies.
Indeed, in a multivariate Cox proportional hazards analysis adjusted for numerous potential confounders, peripartum cardiomyopathy was associated with a 16-fold increased risk of acute MI during the relatively short follow-up period, as well as 29-fold increased risks of stroke and heart failure, compared with women with no cardiovascular issues during their pregnancy.
Chronic hypertension, regardless of whether it occurred alone or in combination with preeclampsia or gestational hypertension, was associated with roughly a twofold increased risk of each of the three study outcomes, compared with women who didn’t experience a cardiovascular complication during pregnancy. De novo preeclampsia was also associated with roughly a twofold increased risk of later MI, heart failure, or stroke.
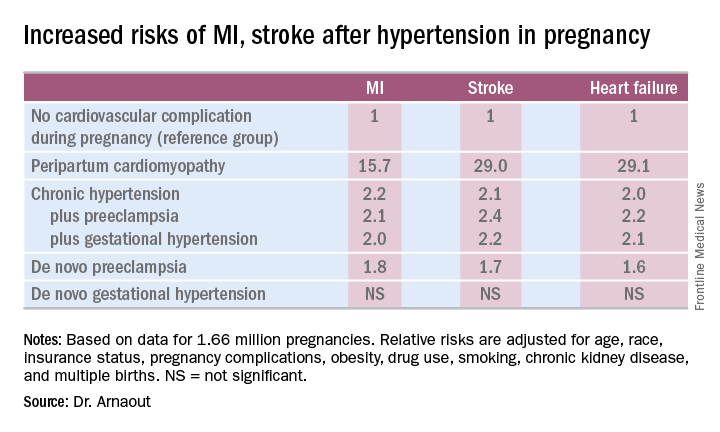
The only form of hypertension in pregnancy that wasn’t associated with a subsequent significantly increased risk of cardiovascular events was de novo gestational hypertension.
Audience member David C. Goff Jr., MD, head of the division of cardiovascular sciences at the National Heart, Lung, and Blood Institute in Bethesda, Md., rose to compliment Dr. Arnaout: “Great work and really important.”
He said that her findings are consistent with the notion that pregnancy constitutes a sort of early-life cardiovascular stress test. He said he wondered, however, just how comfortable Dr. Arnaout is in stating that gestational diabetes isn’t associated with increased subsequent cardiovascular risk, given the relatively short follow-up to date in this population of women who still remain several decades away from the age when cardiovascular event rates really start to climb.
“I completely agree with you,” she replied, noting that other investigators utilizing a different registry have reported an increased longer-term risk for women with gestational diabetes.
Dr. Arnaout said she and her coinvestigators plan to continue to follow the women who experienced peripartum cardiomyopathy or hypertension in pregnancy longer term. They’re also in the process of breaking down the data to look at the risks associated with specific subtypes of MI, stroke, and heart failure.
Dr. Arnaout reported having no financial conflicts regarding her study, which was supported by the American Heart Association and the Sarnoff Cardiovascular Research Foundation.
NEW ORLEANS – Women who experience peripartum cardiomyopathy or any of a variety of hypertensive disorders in pregnancy are at sharply increased risk of acute MI, stroke, or new-onset heart failure beginning within just a few years, Rima Arnaout, MD, reported at the American Heart Association scientific sessions.
“Our study supports the idea that women who have cardiovascular complications in pregnancy really need to be monitored closely for potential primary prevention of cardiovascular events,” said Dr. Arnaout of the University of California, San Francisco.
At a session devoted to “big data” studies in cardiovascular medicine, she presented one of the biggest: a retrospective cohort study of 1.66 million pregnancies during 2005-2009 in California women without any history of congenital or valvular heart disease or prepregnancy cardiovascular events. The California database was created as part of the U.S. Agency for Healthcare Research and Quality’s comprehensive Healthcare Cost and Utilization Project, which included more than 95% of the state’s hospitals. Women who experienced an MI or a stroke, or who were diagnosed with heart failure, during a median of 2.7 years and a maximum of 6 years of follow-up post pregnancy were identified via ICD-9 codes.
There were 111,202 cases of various forms of hypertension in pregnancy, for a 6.9% incidence. Peripartum cardiomyopathy was diagnosed in 562 women, for a rate of 3.5 cases per 10,000 pregnancies.
Indeed, in a multivariate Cox proportional hazards analysis adjusted for numerous potential confounders, peripartum cardiomyopathy was associated with a 16-fold increased risk of acute MI during the relatively short follow-up period, as well as 29-fold increased risks of stroke and heart failure, compared with women with no cardiovascular issues during their pregnancy.
Chronic hypertension, regardless of whether it occurred alone or in combination with preeclampsia or gestational hypertension, was associated with roughly a twofold increased risk of each of the three study outcomes, compared with women who didn’t experience a cardiovascular complication during pregnancy. De novo preeclampsia was also associated with roughly a twofold increased risk of later MI, heart failure, or stroke.

The only form of hypertension in pregnancy that wasn’t associated with a subsequent significantly increased risk of cardiovascular events was de novo gestational hypertension.
Audience member David C. Goff Jr., MD, head of the division of cardiovascular sciences at the National Heart, Lung, and Blood Institute in Bethesda, Md., rose to compliment Dr. Arnaout: “Great work and really important.”
He said that her findings are consistent with the notion that pregnancy constitutes a sort of early-life cardiovascular stress test. He said he wondered, however, just how comfortable Dr. Arnaout is in stating that gestational diabetes isn’t associated with increased subsequent cardiovascular risk, given the relatively short follow-up to date in this population of women who still remain several decades away from the age when cardiovascular event rates really start to climb.
“I completely agree with you,” she replied, noting that other investigators utilizing a different registry have reported an increased longer-term risk for women with gestational diabetes.
Dr. Arnaout said she and her coinvestigators plan to continue to follow the women who experienced peripartum cardiomyopathy or hypertension in pregnancy longer term. They’re also in the process of breaking down the data to look at the risks associated with specific subtypes of MI, stroke, and heart failure.
Dr. Arnaout reported having no financial conflicts regarding her study, which was supported by the American Heart Association and the Sarnoff Cardiovascular Research Foundation.
NEW ORLEANS – Women who experience peripartum cardiomyopathy or any of a variety of hypertensive disorders in pregnancy are at sharply increased risk of acute MI, stroke, or new-onset heart failure beginning within just a few years, Rima Arnaout, MD, reported at the American Heart Association scientific sessions.
“Our study supports the idea that women who have cardiovascular complications in pregnancy really need to be monitored closely for potential primary prevention of cardiovascular events,” said Dr. Arnaout of the University of California, San Francisco.
At a session devoted to “big data” studies in cardiovascular medicine, she presented one of the biggest: a retrospective cohort study of 1.66 million pregnancies during 2005-2009 in California women without any history of congenital or valvular heart disease or prepregnancy cardiovascular events. The California database was created as part of the U.S. Agency for Healthcare Research and Quality’s comprehensive Healthcare Cost and Utilization Project, which included more than 95% of the state’s hospitals. Women who experienced an MI or a stroke, or who were diagnosed with heart failure, during a median of 2.7 years and a maximum of 6 years of follow-up post pregnancy were identified via ICD-9 codes.
There were 111,202 cases of various forms of hypertension in pregnancy, for a 6.9% incidence. Peripartum cardiomyopathy was diagnosed in 562 women, for a rate of 3.5 cases per 10,000 pregnancies.
Indeed, in a multivariate Cox proportional hazards analysis adjusted for numerous potential confounders, peripartum cardiomyopathy was associated with a 16-fold increased risk of acute MI during the relatively short follow-up period, as well as 29-fold increased risks of stroke and heart failure, compared with women with no cardiovascular issues during their pregnancy.
Chronic hypertension, regardless of whether it occurred alone or in combination with preeclampsia or gestational hypertension, was associated with roughly a twofold increased risk of each of the three study outcomes, compared with women who didn’t experience a cardiovascular complication during pregnancy. De novo preeclampsia was also associated with roughly a twofold increased risk of later MI, heart failure, or stroke.

The only form of hypertension in pregnancy that wasn’t associated with a subsequent significantly increased risk of cardiovascular events was de novo gestational hypertension.
Audience member David C. Goff Jr., MD, head of the division of cardiovascular sciences at the National Heart, Lung, and Blood Institute in Bethesda, Md., rose to compliment Dr. Arnaout: “Great work and really important.”
He said that her findings are consistent with the notion that pregnancy constitutes a sort of early-life cardiovascular stress test. He said he wondered, however, just how comfortable Dr. Arnaout is in stating that gestational diabetes isn’t associated with increased subsequent cardiovascular risk, given the relatively short follow-up to date in this population of women who still remain several decades away from the age when cardiovascular event rates really start to climb.
“I completely agree with you,” she replied, noting that other investigators utilizing a different registry have reported an increased longer-term risk for women with gestational diabetes.
Dr. Arnaout said she and her coinvestigators plan to continue to follow the women who experienced peripartum cardiomyopathy or hypertension in pregnancy longer term. They’re also in the process of breaking down the data to look at the risks associated with specific subtypes of MI, stroke, and heart failure.
Dr. Arnaout reported having no financial conflicts regarding her study, which was supported by the American Heart Association and the Sarnoff Cardiovascular Research Foundation.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: California women who developed peripartum cardiomyopathy were at 16- to 29-fold increased risk of experiencing an acute MI, stroke, or heart failure during a median 2.7 years of follow-up.
Data source: A retrospective cohort study of more than 95% of pregnancies in California during 2005-2009.
Disclosures: The presenter reported having no financial conflicts regarding her study, which was supported by the American Heart Association and the Sarnoff Cardiovascular Research Foundation.















