User login
American Heart Association (AHA): Scientific Sessions 2016
Nutrition expert to heart patients: ‘Eat some cheese’
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
This article is included so that vascular surgeons can adequately advise patients who request dietary information about ]dairy products. However, as a cheese lover myself, it will also permit cheese aficionados like myself to “cut the cheese” in an appropriate manner! My only concern is the long list of dairy groups that support Dr. Astrup’s research grants. I note that he also serves on the advisory board of the Dutch Beer Knowledge Institute. I look forward to his upcoming research project explaining the benefits of consuming large quantities of beer!
Russell H. Samson, MD, is the Medical Editor of Vascular Specialist.
This article is included so that vascular surgeons can adequately advise patients who request dietary information about ]dairy products. However, as a cheese lover myself, it will also permit cheese aficionados like myself to “cut the cheese” in an appropriate manner! My only concern is the long list of dairy groups that support Dr. Astrup’s research grants. I note that he also serves on the advisory board of the Dutch Beer Knowledge Institute. I look forward to his upcoming research project explaining the benefits of consuming large quantities of beer!
Russell H. Samson, MD, is the Medical Editor of Vascular Specialist.
This article is included so that vascular surgeons can adequately advise patients who request dietary information about ]dairy products. However, as a cheese lover myself, it will also permit cheese aficionados like myself to “cut the cheese” in an appropriate manner! My only concern is the long list of dairy groups that support Dr. Astrup’s research grants. I note that he also serves on the advisory board of the Dutch Beer Knowledge Institute. I look forward to his upcoming research project explaining the benefits of consuming large quantities of beer!
Russell H. Samson, MD, is the Medical Editor of Vascular Specialist.
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
NEW ORLEANS – While many Americans have been dithering over the relative health benefits of high- versus low-carbohydrate diets, various pop-culture weight loss programs, vegetarianism, gluten-free living, and other nutritional matters, a quiet revolution in mainstream scientific thinking has occurred regarding the role of full-fat dairy products.
Saturated fatty acid–rich dairy products, formerly viewed as the enemy of cardiovascular health, have gone from foe to friend, according to Arne Astrup, MD, professor and head of the department of nutrition, exercise and sports at the University of Copenhagen.
“From all I have seen, I think it’s quite safe to recommend that our diabetics and heart patients eat some cheese without being afraid of it. I don’t think there’s any harmful effect, and it could actually be very beneficial,” Dr. Astrup continued.
For example, a recent comprehensive meta-analysis of 31 prospective cohort studies found that a high dairy intake was associated with a 9% reduction in the risk of stroke, compared with low or no dairy consumption. Of note, high cheese intake was associated with an 18% lower risk of coronary heart disease (CHD) and a 13% reduction in risk of stroke (Br J Nutr. 2016;115[4]:737-50).
Dutch investigators reported based upon their meta-analysis of 18 prospective cohort studies with 8-26 years of follow-up that stroke risk fell by 7% for each 200 mL of milk consumed per day. Consumption of 25 g/day or more of cheese was associated with a 13% reduction in stroke risk and an 8% lower risk of CHD (J Am Heart Assoc. 2016 May 20;5[5]. doi: 10.1161/JAHA.115.002787).
“The totality of evidence – meta-analyses of both observational studies and randomized controlled trials – cannot find any harmful effects of cheese on body fat, metabolic syndrome, type 2 diabetes, or cardiovascular disease,” he said. “And cheese has beneficial effects on LDL cholesterol, blood pressure, and postprandial triglycerides as compared with butter containing the same amount of saturated fatty acids.”
The classic lipid hypothesis of cardiovascular disease holds that dietary saturated fat raises blood cholesterol, in turn accelerating atherosclerosis and resultant coronary heart disease. But the published literature of the past few years indicates it’s not that simple. All saturated fats are not equally harmful. They have very different biologic effects, and the food matrix in which they occur seems to be important. The saturated fatty acids found in red meat are clearly damaging. Ditto trans fats.
In contrast, the saturated fats present in milk, hard cheeses, and fermented dairy products such as yogurt have been shown in a variety of study formats to be cardioprotective. They also appear to protect against other chronic diseases as well, according to the researcher.
“If we look at all the different meta-analyses addressing the various cardiovascular risk factors, it really looks like cheese, despite its high content of sodium and saturated fat, seems to exert some beneficial effects. So I think we need to address the food matrix much more. We’ve done controlled feeding trials in humans and found that if we give subjects the same amount of saturated fat from either butter or cheese, you see following the cheese [that] the subjects do not increase their total or LDL-cholesterol as you would expect based upon their intake of saturated fat. So there’s something going on with cheese,” Dr. Astrup said.
What’s going on, he continued, is the saturated fats in cheese benefit from the company they keep. Fermented dairy products contain an arm-long list of potentially beneficial nutrients, including protein, calcium, short-chain fatty acids, bioactive peptides, and phospholipids.
Take, for example, calcium: “We’ve found the calcium content of cheese completely modifies the metabolism of the saturated fat. The calcium seems to bind the bile acids and fatty acids, resulting in increased fecal fat secretion,” according to Dr. Astrup.
Although at the AHA meeting he focused mainly on the effects of cheese and other dairy products on cardiovascular health, in a recent review article he expanded upon the scientific evidence regarding the impact of these foods on the risks of obesity, type 2 diabetes, cancer, and osteoporosis (Food Nutr Res. 2016 Nov 22;60:32527).
There is solid evidence that a diet high in dairy products reduces the risk of childhood obesity and enhances body composition in adults. It aids in weight loss by promoting satiety during periods of energy restriction. A recent meta-analysis of observational studies found an inverse relationship between consumption of fermented dairy products – yogurt and cheese – and risk of type 2 diabetes (Am J Clin Nutr. 2016 Apr;103[4]:1111-24).
Regarding cancer, the World Cancer Research Fund has issued a series of evidence reviews concluding that dairy products probably protect against colorectal, breast, gastric, and bladder cancer. The jury is still out regarding prostate cancer risk.
A wealth of evidence indicates dairy consumption has a beneficial effect on bone health in children and adolescents. However, meta-analyses haven’t shown a protective effect against osteoporosis and fractures in adults. This is consistent with the adage that osteoporosis is a pediatric disease with geriatric consequences, Dr. Astrup noted.
He reported receiving research grants from the Danish Dairy Research Foundation, the Global Dairy Platform, the Danish Agriculture and Food Council, and the European Milk Forum. He serves on advisory boards for the Dutch Beer Knowledge Institute, Suntory, Weight Watchers, and several food companies.
Strokes cut by extended NOAC prophylaxis in hospitalized, medically ill patients
NEW ORLEANS – Thromboprophylaxis for 35-42 days with the new oral anticoagulant betrixaban led to a significant reduction in all-cause and ischemic strokes in medically ill patients who required hospitalization as compared with conventional prophylaxis for 10 days, based on a post-hoc analysis of data from a randomized trial with more than 7,500 patients.
But the trial’s unusual design left it unclear whether the incremental benefit seen from prolonged prophylaxis with a NOAC resulted primarily from a longer period of treatment, the drug used, or both.
The safety analysis showed that prolonged treatment with betrixaban roughly doubled the rate of major or clinically relevant nonmajor bleeding events during the period of treatment and for the first 7 days after treatment stopped. The incidence of these bleeds was 1.6% among control patients on 10 days of enoxaparin treatment and 3.1% among patients who received extended treatment with betrixaban, a statistically significant difference. The rates of fatal bleeds and intracranial hemorrhages in the two study groups did not significantly differ.
The data Dr. Gibson reported came from the Multicenter, Randomized, Active-Controlled Efficacy And Safety Study Comparing Extended Duration Betrixaban With Standard Of Care Enoxaparin For The Prevention Of Venous Thromboembolism In Acute Medically Ill Patients (APEX). The study’s primary aim was testing in 7,513 hospitalized medically ill patients the safety and efficacy of prolonged prophylaxis with the oral, factor Xa inhibitor betrixaban, compared with 10 days of prophylaxis with the low molecular weight heparin enoxaparin. The primary endpoint was the rate of venous thromboembolic events and deaths from venous thromboembolism (VTE) out to 47 days after the start of treatment.
APEX enrolled patients hospitalized for acute decompensated heart failure, chronic respiratory failure, acute infection without septic shock, acute rheumatic disorders or acute ischemic stroke. All enrolled patients had to be expected to be immobilized for at least 24 hours following randomization and to be hospitalized for at least 3 days. Patients also had to have an additional risk marker for high thrombotic risk: They had to be at least 75 years old, or 60-74 years old with a D-dimer level at least twice the upper limit of normal, or 40-59 years old with a D-dimer level at least twice the upper limit of normal and a history of either VTE or cancer.
Results for the primary endpoint, reported in 2016, showed that prolonged betrixaban prophylaxis linked with an absolute 1.6% reduction in the combined endpoint, which resulted in a 19% relative risk reduction that fell just short of the trial’s prespecified definition of statistical significance. The study’s primary safety endpoint was the occurrence of major bleeding events through 7 days after the stop of treatment, which occurred in 0.7% of the betrixaban patients and in 0.6% of those on enoxaparin (N Engl J Med. 2016 Aug 11;375[6]:534-44).
Even thought the primary results from this pivotal trial failed to meet the prespecified threshold for statistical significance, the company developing betrixaban, Portola, submitted an application to the Food and Drug Administration to approve marketing of extended-duration betrixaban for VTE prophylaxis in acute medically-ill patients with VTE risk factors. In December 2016, Portola announced that the FDA had given the application priority status for a decision.
The post-hoc analysis that Dr. Gibson presented at the meeting looked at the impact of betrixaban compared with enoxaparin on the incidence of all-cause and ischemic stroke during 77 days of follow-up after the start of treatment in the 7,432 patients who received at least one dose of their assigned drug, two endpoints that weren’t even secondary outcomes in APEX’s original design.
Among the 3,716 treated with betrixaban, the all-cause stroke incidence was 0.54%; among the 3,716 patients treated with enoxaparin, the all-cause stroke incidence was 0.97%. The 56% relative risk reduction was statistically significant. The incidence of ischemic strokes was 0.48% with betrixaban and 0.91% with enoxaparin, a 53% relative risk reduction that was also statistically significant.
The post-hoc analysis also looked specifically at the comparison between betrixaban and enoxaparin for stroke prevention in a subgroup of patients who had the highest stroke rate, the patients who were hospitalized because of an index stroke or an index heart failure episode. In this high-risk subgroup, prophylaxis with betrixaban cut the all-cause stroke rate compared with enoxaparin by 49% and the ischemic stroke rate by 45%, both statistically significant effects. When the high-risk subgroup also included patients hospitalized for an index episode of atrial fibrillation, betrixaban cut the rate of all-cause strokes by a relative 48% and ischemic strokes by a relative 44%.
Concurrently with Dr. Gibson’s report at the meeting, the results also appeared online (Circulation. 2016 Nov 14. doi: 10.1161/CIRCULATIONAHA.116.025427).
APEX was sponsored by Portola, the company developing betrixaban. Dr. Gibson has been a consultant to Eli Lilly, Gilead, The Medicines Company, Novo Nordisk, Pfizer, and St. Jude. He has received research support from Portola and several other companies.
[email protected]
On Twitter @mitchelzoler
The APEX study identified a group of patients hospitalized for medical reasons who were at high risk for both venous thromboembolism and for stroke. We are comfortable with the concept of thromboprophylaxis for hospitalized patients who are at high risk for venous thromboembolism, but we have generally not paid attention to prophylaxis against stroke during and immediately after hospitalization.
The results suggest that extending thromboprophylaxis beyond the standard period of 10 days may be a good idea. Because patients in the two treatment arms of the study differed in both the drugs they received and in the duration of prophylaxis, the results cannot distinguish which of these two variables was more important. Treating patients with enoxaparin for 35-42 days may provide a similar benefit to what was seen with extended-duration betrixaban.
The findings are a wake-up call to the high thromboembolic risk faced by the types of patients enrolled in APEX, and they point to a new way to manage these patients. Guidelines already call for putting high-risk patients, such as those with heart failure, on anticoagulant prophylaxis if they have no contraindications. These new data suggest that thromboprophylaxis in appropriate patients should extend beyond 10 days and beyond acute hospitalization.
Steven R. Lentz, MD, is a professor of medicine and a hematologist oncologist at the University of Iowa in Iowa City. He has been a consultant to Novo Nordisk and Opko, has an ownership interest in Celgene, and has received research grants from Novo Nordisk. He made these comments in an interview.
The APEX study identified a group of patients hospitalized for medical reasons who were at high risk for both venous thromboembolism and for stroke. We are comfortable with the concept of thromboprophylaxis for hospitalized patients who are at high risk for venous thromboembolism, but we have generally not paid attention to prophylaxis against stroke during and immediately after hospitalization.
The results suggest that extending thromboprophylaxis beyond the standard period of 10 days may be a good idea. Because patients in the two treatment arms of the study differed in both the drugs they received and in the duration of prophylaxis, the results cannot distinguish which of these two variables was more important. Treating patients with enoxaparin for 35-42 days may provide a similar benefit to what was seen with extended-duration betrixaban.
The findings are a wake-up call to the high thromboembolic risk faced by the types of patients enrolled in APEX, and they point to a new way to manage these patients. Guidelines already call for putting high-risk patients, such as those with heart failure, on anticoagulant prophylaxis if they have no contraindications. These new data suggest that thromboprophylaxis in appropriate patients should extend beyond 10 days and beyond acute hospitalization.
Steven R. Lentz, MD, is a professor of medicine and a hematologist oncologist at the University of Iowa in Iowa City. He has been a consultant to Novo Nordisk and Opko, has an ownership interest in Celgene, and has received research grants from Novo Nordisk. He made these comments in an interview.
The APEX study identified a group of patients hospitalized for medical reasons who were at high risk for both venous thromboembolism and for stroke. We are comfortable with the concept of thromboprophylaxis for hospitalized patients who are at high risk for venous thromboembolism, but we have generally not paid attention to prophylaxis against stroke during and immediately after hospitalization.
The results suggest that extending thromboprophylaxis beyond the standard period of 10 days may be a good idea. Because patients in the two treatment arms of the study differed in both the drugs they received and in the duration of prophylaxis, the results cannot distinguish which of these two variables was more important. Treating patients with enoxaparin for 35-42 days may provide a similar benefit to what was seen with extended-duration betrixaban.
The findings are a wake-up call to the high thromboembolic risk faced by the types of patients enrolled in APEX, and they point to a new way to manage these patients. Guidelines already call for putting high-risk patients, such as those with heart failure, on anticoagulant prophylaxis if they have no contraindications. These new data suggest that thromboprophylaxis in appropriate patients should extend beyond 10 days and beyond acute hospitalization.
Steven R. Lentz, MD, is a professor of medicine and a hematologist oncologist at the University of Iowa in Iowa City. He has been a consultant to Novo Nordisk and Opko, has an ownership interest in Celgene, and has received research grants from Novo Nordisk. He made these comments in an interview.
NEW ORLEANS – Thromboprophylaxis for 35-42 days with the new oral anticoagulant betrixaban led to a significant reduction in all-cause and ischemic strokes in medically ill patients who required hospitalization as compared with conventional prophylaxis for 10 days, based on a post-hoc analysis of data from a randomized trial with more than 7,500 patients.
But the trial’s unusual design left it unclear whether the incremental benefit seen from prolonged prophylaxis with a NOAC resulted primarily from a longer period of treatment, the drug used, or both.
The safety analysis showed that prolonged treatment with betrixaban roughly doubled the rate of major or clinically relevant nonmajor bleeding events during the period of treatment and for the first 7 days after treatment stopped. The incidence of these bleeds was 1.6% among control patients on 10 days of enoxaparin treatment and 3.1% among patients who received extended treatment with betrixaban, a statistically significant difference. The rates of fatal bleeds and intracranial hemorrhages in the two study groups did not significantly differ.
The data Dr. Gibson reported came from the Multicenter, Randomized, Active-Controlled Efficacy And Safety Study Comparing Extended Duration Betrixaban With Standard Of Care Enoxaparin For The Prevention Of Venous Thromboembolism In Acute Medically Ill Patients (APEX). The study’s primary aim was testing in 7,513 hospitalized medically ill patients the safety and efficacy of prolonged prophylaxis with the oral, factor Xa inhibitor betrixaban, compared with 10 days of prophylaxis with the low molecular weight heparin enoxaparin. The primary endpoint was the rate of venous thromboembolic events and deaths from venous thromboembolism (VTE) out to 47 days after the start of treatment.
APEX enrolled patients hospitalized for acute decompensated heart failure, chronic respiratory failure, acute infection without septic shock, acute rheumatic disorders or acute ischemic stroke. All enrolled patients had to be expected to be immobilized for at least 24 hours following randomization and to be hospitalized for at least 3 days. Patients also had to have an additional risk marker for high thrombotic risk: They had to be at least 75 years old, or 60-74 years old with a D-dimer level at least twice the upper limit of normal, or 40-59 years old with a D-dimer level at least twice the upper limit of normal and a history of either VTE or cancer.
Results for the primary endpoint, reported in 2016, showed that prolonged betrixaban prophylaxis linked with an absolute 1.6% reduction in the combined endpoint, which resulted in a 19% relative risk reduction that fell just short of the trial’s prespecified definition of statistical significance. The study’s primary safety endpoint was the occurrence of major bleeding events through 7 days after the stop of treatment, which occurred in 0.7% of the betrixaban patients and in 0.6% of those on enoxaparin (N Engl J Med. 2016 Aug 11;375[6]:534-44).
Even thought the primary results from this pivotal trial failed to meet the prespecified threshold for statistical significance, the company developing betrixaban, Portola, submitted an application to the Food and Drug Administration to approve marketing of extended-duration betrixaban for VTE prophylaxis in acute medically-ill patients with VTE risk factors. In December 2016, Portola announced that the FDA had given the application priority status for a decision.
The post-hoc analysis that Dr. Gibson presented at the meeting looked at the impact of betrixaban compared with enoxaparin on the incidence of all-cause and ischemic stroke during 77 days of follow-up after the start of treatment in the 7,432 patients who received at least one dose of their assigned drug, two endpoints that weren’t even secondary outcomes in APEX’s original design.
Among the 3,716 treated with betrixaban, the all-cause stroke incidence was 0.54%; among the 3,716 patients treated with enoxaparin, the all-cause stroke incidence was 0.97%. The 56% relative risk reduction was statistically significant. The incidence of ischemic strokes was 0.48% with betrixaban and 0.91% with enoxaparin, a 53% relative risk reduction that was also statistically significant.
The post-hoc analysis also looked specifically at the comparison between betrixaban and enoxaparin for stroke prevention in a subgroup of patients who had the highest stroke rate, the patients who were hospitalized because of an index stroke or an index heart failure episode. In this high-risk subgroup, prophylaxis with betrixaban cut the all-cause stroke rate compared with enoxaparin by 49% and the ischemic stroke rate by 45%, both statistically significant effects. When the high-risk subgroup also included patients hospitalized for an index episode of atrial fibrillation, betrixaban cut the rate of all-cause strokes by a relative 48% and ischemic strokes by a relative 44%.
Concurrently with Dr. Gibson’s report at the meeting, the results also appeared online (Circulation. 2016 Nov 14. doi: 10.1161/CIRCULATIONAHA.116.025427).
APEX was sponsored by Portola, the company developing betrixaban. Dr. Gibson has been a consultant to Eli Lilly, Gilead, The Medicines Company, Novo Nordisk, Pfizer, and St. Jude. He has received research support from Portola and several other companies.
[email protected]
On Twitter @mitchelzoler
NEW ORLEANS – Thromboprophylaxis for 35-42 days with the new oral anticoagulant betrixaban led to a significant reduction in all-cause and ischemic strokes in medically ill patients who required hospitalization as compared with conventional prophylaxis for 10 days, based on a post-hoc analysis of data from a randomized trial with more than 7,500 patients.
But the trial’s unusual design left it unclear whether the incremental benefit seen from prolonged prophylaxis with a NOAC resulted primarily from a longer period of treatment, the drug used, or both.
The safety analysis showed that prolonged treatment with betrixaban roughly doubled the rate of major or clinically relevant nonmajor bleeding events during the period of treatment and for the first 7 days after treatment stopped. The incidence of these bleeds was 1.6% among control patients on 10 days of enoxaparin treatment and 3.1% among patients who received extended treatment with betrixaban, a statistically significant difference. The rates of fatal bleeds and intracranial hemorrhages in the two study groups did not significantly differ.
The data Dr. Gibson reported came from the Multicenter, Randomized, Active-Controlled Efficacy And Safety Study Comparing Extended Duration Betrixaban With Standard Of Care Enoxaparin For The Prevention Of Venous Thromboembolism In Acute Medically Ill Patients (APEX). The study’s primary aim was testing in 7,513 hospitalized medically ill patients the safety and efficacy of prolonged prophylaxis with the oral, factor Xa inhibitor betrixaban, compared with 10 days of prophylaxis with the low molecular weight heparin enoxaparin. The primary endpoint was the rate of venous thromboembolic events and deaths from venous thromboembolism (VTE) out to 47 days after the start of treatment.
APEX enrolled patients hospitalized for acute decompensated heart failure, chronic respiratory failure, acute infection without septic shock, acute rheumatic disorders or acute ischemic stroke. All enrolled patients had to be expected to be immobilized for at least 24 hours following randomization and to be hospitalized for at least 3 days. Patients also had to have an additional risk marker for high thrombotic risk: They had to be at least 75 years old, or 60-74 years old with a D-dimer level at least twice the upper limit of normal, or 40-59 years old with a D-dimer level at least twice the upper limit of normal and a history of either VTE or cancer.
Results for the primary endpoint, reported in 2016, showed that prolonged betrixaban prophylaxis linked with an absolute 1.6% reduction in the combined endpoint, which resulted in a 19% relative risk reduction that fell just short of the trial’s prespecified definition of statistical significance. The study’s primary safety endpoint was the occurrence of major bleeding events through 7 days after the stop of treatment, which occurred in 0.7% of the betrixaban patients and in 0.6% of those on enoxaparin (N Engl J Med. 2016 Aug 11;375[6]:534-44).
Even thought the primary results from this pivotal trial failed to meet the prespecified threshold for statistical significance, the company developing betrixaban, Portola, submitted an application to the Food and Drug Administration to approve marketing of extended-duration betrixaban for VTE prophylaxis in acute medically-ill patients with VTE risk factors. In December 2016, Portola announced that the FDA had given the application priority status for a decision.
The post-hoc analysis that Dr. Gibson presented at the meeting looked at the impact of betrixaban compared with enoxaparin on the incidence of all-cause and ischemic stroke during 77 days of follow-up after the start of treatment in the 7,432 patients who received at least one dose of their assigned drug, two endpoints that weren’t even secondary outcomes in APEX’s original design.
Among the 3,716 treated with betrixaban, the all-cause stroke incidence was 0.54%; among the 3,716 patients treated with enoxaparin, the all-cause stroke incidence was 0.97%. The 56% relative risk reduction was statistically significant. The incidence of ischemic strokes was 0.48% with betrixaban and 0.91% with enoxaparin, a 53% relative risk reduction that was also statistically significant.
The post-hoc analysis also looked specifically at the comparison between betrixaban and enoxaparin for stroke prevention in a subgroup of patients who had the highest stroke rate, the patients who were hospitalized because of an index stroke or an index heart failure episode. In this high-risk subgroup, prophylaxis with betrixaban cut the all-cause stroke rate compared with enoxaparin by 49% and the ischemic stroke rate by 45%, both statistically significant effects. When the high-risk subgroup also included patients hospitalized for an index episode of atrial fibrillation, betrixaban cut the rate of all-cause strokes by a relative 48% and ischemic strokes by a relative 44%.
Concurrently with Dr. Gibson’s report at the meeting, the results also appeared online (Circulation. 2016 Nov 14. doi: 10.1161/CIRCULATIONAHA.116.025427).
APEX was sponsored by Portola, the company developing betrixaban. Dr. Gibson has been a consultant to Eli Lilly, Gilead, The Medicines Company, Novo Nordisk, Pfizer, and St. Jude. He has received research support from Portola and several other companies.
[email protected]
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Strokes occurred in 0.54% of patients on extended-duration betrixaban prophylaxis and in 0.97% of patients on standard-duration enoxaparin.
Data source: APEX, a multicenter randomized trial with 7,513 patients.
Disclosures: APEX was sponsored by Portola, the company developing betrixaban. Dr. Gibson has been a consultant to Eli Lilly, Gilead, The Medicines Company, Novo Nordisk, Pfizer and St. Jude. He has received research support from Portola and several other companies.
Hypertension risk soars in offspring of early-HT parents
NEW ORLEANS – Young adults whose parents develop hypertension before age 55 years are themselves at sharply increased risk of developing the disease, according to a new report from the Framingham (Mass.) Heart Study.
“Our results demonstrate that early-onset but not late-onset hypertension in parents is a strong risk factor for incident hypertension. It may be important for physicians to distinguish between early- and late-onset hypertension as a familial trait when assessing an individual’s risk for hypertension,” Teemu J. Niiranen, MD, said at the American Heart Association scientific sessions.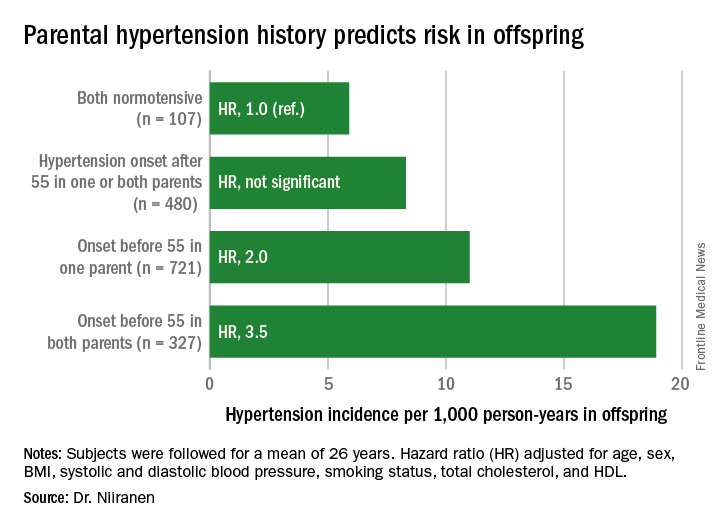
He reported on 1,635 participants in the Offspring cohort of the Framingham Heart Study who were normotensive when they enrolled in the prospective study beginning in 1972. At that time, they averaged 32 years of age. They were followed for a mean of 26 years. Like their parents who enrolled in the Original cohort of the landmark study beginning in 1948, they underwent meticulous blood pressure measurement roughly every 2 years.
Dr. Niiranen and his coinvestigators divided the Offspring cohort into four groups based upon parental hypertension status. There were 107 offspring with normotensive parents, 480 with one or both parents having developed late-onset hypertension after age 55 years, 721 offspring who had one parent with onset of hypertension before age 55 years, and 327 with both parents having early-onset hypertension.
The incidence rate of hypertension in the Offspring cohort climbed in concert with parental early hypertension status. So did the multivariate-adjusted relative risk of the disease, compared with children of normotensive parents.
Moreover, the earlier in life the parents developed hypertension, the earlier their offspring did, too.
Session moderator David J. Maron, MD, of Stanford (Calif.) University, commented, “Everybody’s thinking ‘genetics’ as we look at your findings. But do you have any way to tease out nature versus nurture in understanding the association?”
Dr. Niiranen replied that in a separate study of three generations of Framingham participants, the investigators incorporated two lifestyle factors in their analysis: level of exercise and sodium intake.
“Those didn’t have much effect on the results, so it seems like genetics is driving most of the outcome,” he said.
Dr. Niiranen reported having no financial conflicts of interest regarding his study, sponsored by the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Young adults whose parents develop hypertension before age 55 years are themselves at sharply increased risk of developing the disease, according to a new report from the Framingham (Mass.) Heart Study.
“Our results demonstrate that early-onset but not late-onset hypertension in parents is a strong risk factor for incident hypertension. It may be important for physicians to distinguish between early- and late-onset hypertension as a familial trait when assessing an individual’s risk for hypertension,” Teemu J. Niiranen, MD, said at the American Heart Association scientific sessions.
He reported on 1,635 participants in the Offspring cohort of the Framingham Heart Study who were normotensive when they enrolled in the prospective study beginning in 1972. At that time, they averaged 32 years of age. They were followed for a mean of 26 years. Like their parents who enrolled in the Original cohort of the landmark study beginning in 1948, they underwent meticulous blood pressure measurement roughly every 2 years.
Dr. Niiranen and his coinvestigators divided the Offspring cohort into four groups based upon parental hypertension status. There were 107 offspring with normotensive parents, 480 with one or both parents having developed late-onset hypertension after age 55 years, 721 offspring who had one parent with onset of hypertension before age 55 years, and 327 with both parents having early-onset hypertension.
The incidence rate of hypertension in the Offspring cohort climbed in concert with parental early hypertension status. So did the multivariate-adjusted relative risk of the disease, compared with children of normotensive parents.
Moreover, the earlier in life the parents developed hypertension, the earlier their offspring did, too.
Session moderator David J. Maron, MD, of Stanford (Calif.) University, commented, “Everybody’s thinking ‘genetics’ as we look at your findings. But do you have any way to tease out nature versus nurture in understanding the association?”
Dr. Niiranen replied that in a separate study of three generations of Framingham participants, the investigators incorporated two lifestyle factors in their analysis: level of exercise and sodium intake.
“Those didn’t have much effect on the results, so it seems like genetics is driving most of the outcome,” he said.
Dr. Niiranen reported having no financial conflicts of interest regarding his study, sponsored by the National Heart, Lung, and Blood Institute.
NEW ORLEANS – Young adults whose parents develop hypertension before age 55 years are themselves at sharply increased risk of developing the disease, according to a new report from the Framingham (Mass.) Heart Study.
“Our results demonstrate that early-onset but not late-onset hypertension in parents is a strong risk factor for incident hypertension. It may be important for physicians to distinguish between early- and late-onset hypertension as a familial trait when assessing an individual’s risk for hypertension,” Teemu J. Niiranen, MD, said at the American Heart Association scientific sessions.
He reported on 1,635 participants in the Offspring cohort of the Framingham Heart Study who were normotensive when they enrolled in the prospective study beginning in 1972. At that time, they averaged 32 years of age. They were followed for a mean of 26 years. Like their parents who enrolled in the Original cohort of the landmark study beginning in 1948, they underwent meticulous blood pressure measurement roughly every 2 years.
Dr. Niiranen and his coinvestigators divided the Offspring cohort into four groups based upon parental hypertension status. There were 107 offspring with normotensive parents, 480 with one or both parents having developed late-onset hypertension after age 55 years, 721 offspring who had one parent with onset of hypertension before age 55 years, and 327 with both parents having early-onset hypertension.
The incidence rate of hypertension in the Offspring cohort climbed in concert with parental early hypertension status. So did the multivariate-adjusted relative risk of the disease, compared with children of normotensive parents.
Moreover, the earlier in life the parents developed hypertension, the earlier their offspring did, too.
Session moderator David J. Maron, MD, of Stanford (Calif.) University, commented, “Everybody’s thinking ‘genetics’ as we look at your findings. But do you have any way to tease out nature versus nurture in understanding the association?”
Dr. Niiranen replied that in a separate study of three generations of Framingham participants, the investigators incorporated two lifestyle factors in their analysis: level of exercise and sodium intake.
“Those didn’t have much effect on the results, so it seems like genetics is driving most of the outcome,” he said.
Dr. Niiranen reported having no financial conflicts of interest regarding his study, sponsored by the National Heart, Lung, and Blood Institute.
Key clinical point:
Major finding: Young adults with two parents who developed hypertension before age 55 years are at 3.5-fold greater risk of subsequently developing hypertension than if both parents were normotensive.
Data source: This was an analysis of the incidence of hypertension during a mean prospective follow-up of 26 years in 1,635 members of the Offspring cohort of the Framingham Heart Study who enrolled as young adults.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study sponsored by the National Heart, Lung, and Blood Institute.
Childhood obesity tied to maternal obesity, cesarean birth
NEW ORLEANS – Maternal obesity and cesarean delivery were each independently associated with increased rates of overweight or obesity during childhood in a prospective study of 1,441 mothers and their children.
In addition, these risks for childhood obesity appeared to interact in an additive way, so that women who were both obese and delivered by C-section had a nearly threefold increased rate of having a child who was overweight or obese at about 5 years of age, compared with children born to normal-weight women who delivered vaginally, Noel T. Mueller, PhD, said at the American Heart Association Scientific Sessions.
This finding of a link between maternal overweight and obesity and childhood obesity in the next generation supports results from previously reported studies. The new results “also add to the growing evidence for an association between C-section and obesity [in offspring], as well as C-section and immune-related disorders such as asthma and allergies” in offspring, Dr. Mueller said in an interview.
He hypothesized that delivery mode may contribute to a child’s obesity risk by producing an abnormal gastrointestinal microbiome. For example, vaginal delivery seems to associate with a higher prevalence of Bacteroides species in a child’s gut, bacteria that aid in the digestion of breast milk, Dr. Mueller said.
His study used data collected in the Boston Birth Cohort from 1,441 mothers and their children from full-term, singleton pregnancies born to women with a body mass index of at least 18.5 kg/m2 during 1998-2014. The child’s weight was measured at a median age of 4.8 years, with an interquartile range of 3-6 years. Children were deemed overweight if they were at or above the 85th percentile for weight, according to standards from the Centers for Disease Control and Prevention.
Just under half the women were normal weight, slightly more than a quarter were overweight, and a quarter were obese. The incidence of 5-year-old children who were overweight or obese was 70% higher in children of overweight mothers and 80% higher in those with obese mothers, compared with children with normal-weight mothers in an analysis that adjusted for maternal age at delivery, race or ethnicity, and education. Both were statistically significant differences, Dr. Mueller reported.
Two-thirds of the women had vaginal deliveries and a third had C-sections. Overweight or obesity occurred in 40% more of the children delivered by C-section, compared with children born vaginally, a statistically significant difference in an analysis that controlled for the same three covariates as well as prepregnancy body mass index, pregnancy weight gain, and other variables.
When Dr. Mueller and his associates ran a combined analysis they found that the highest risk for childhood overweight or obesity was in children born to obese mothers by C-section, and it was a 2.8-fold higher rate than that in the children born to normal-weight mothers by vaginal delivery, a statistically significant difference.
Dr. Mueller had no disclosures.
[email protected]
On Twitter @mitchelzoler
NEW ORLEANS – Maternal obesity and cesarean delivery were each independently associated with increased rates of overweight or obesity during childhood in a prospective study of 1,441 mothers and their children.
In addition, these risks for childhood obesity appeared to interact in an additive way, so that women who were both obese and delivered by C-section had a nearly threefold increased rate of having a child who was overweight or obese at about 5 years of age, compared with children born to normal-weight women who delivered vaginally, Noel T. Mueller, PhD, said at the American Heart Association Scientific Sessions.
This finding of a link between maternal overweight and obesity and childhood obesity in the next generation supports results from previously reported studies. The new results “also add to the growing evidence for an association between C-section and obesity [in offspring], as well as C-section and immune-related disorders such as asthma and allergies” in offspring, Dr. Mueller said in an interview.
He hypothesized that delivery mode may contribute to a child’s obesity risk by producing an abnormal gastrointestinal microbiome. For example, vaginal delivery seems to associate with a higher prevalence of Bacteroides species in a child’s gut, bacteria that aid in the digestion of breast milk, Dr. Mueller said.
His study used data collected in the Boston Birth Cohort from 1,441 mothers and their children from full-term, singleton pregnancies born to women with a body mass index of at least 18.5 kg/m2 during 1998-2014. The child’s weight was measured at a median age of 4.8 years, with an interquartile range of 3-6 years. Children were deemed overweight if they were at or above the 85th percentile for weight, according to standards from the Centers for Disease Control and Prevention.
Just under half the women were normal weight, slightly more than a quarter were overweight, and a quarter were obese. The incidence of 5-year-old children who were overweight or obese was 70% higher in children of overweight mothers and 80% higher in those with obese mothers, compared with children with normal-weight mothers in an analysis that adjusted for maternal age at delivery, race or ethnicity, and education. Both were statistically significant differences, Dr. Mueller reported.
Two-thirds of the women had vaginal deliveries and a third had C-sections. Overweight or obesity occurred in 40% more of the children delivered by C-section, compared with children born vaginally, a statistically significant difference in an analysis that controlled for the same three covariates as well as prepregnancy body mass index, pregnancy weight gain, and other variables.
When Dr. Mueller and his associates ran a combined analysis they found that the highest risk for childhood overweight or obesity was in children born to obese mothers by C-section, and it was a 2.8-fold higher rate than that in the children born to normal-weight mothers by vaginal delivery, a statistically significant difference.
Dr. Mueller had no disclosures.
[email protected]
On Twitter @mitchelzoler
NEW ORLEANS – Maternal obesity and cesarean delivery were each independently associated with increased rates of overweight or obesity during childhood in a prospective study of 1,441 mothers and their children.
In addition, these risks for childhood obesity appeared to interact in an additive way, so that women who were both obese and delivered by C-section had a nearly threefold increased rate of having a child who was overweight or obese at about 5 years of age, compared with children born to normal-weight women who delivered vaginally, Noel T. Mueller, PhD, said at the American Heart Association Scientific Sessions.
This finding of a link between maternal overweight and obesity and childhood obesity in the next generation supports results from previously reported studies. The new results “also add to the growing evidence for an association between C-section and obesity [in offspring], as well as C-section and immune-related disorders such as asthma and allergies” in offspring, Dr. Mueller said in an interview.
He hypothesized that delivery mode may contribute to a child’s obesity risk by producing an abnormal gastrointestinal microbiome. For example, vaginal delivery seems to associate with a higher prevalence of Bacteroides species in a child’s gut, bacteria that aid in the digestion of breast milk, Dr. Mueller said.
His study used data collected in the Boston Birth Cohort from 1,441 mothers and their children from full-term, singleton pregnancies born to women with a body mass index of at least 18.5 kg/m2 during 1998-2014. The child’s weight was measured at a median age of 4.8 years, with an interquartile range of 3-6 years. Children were deemed overweight if they were at or above the 85th percentile for weight, according to standards from the Centers for Disease Control and Prevention.
Just under half the women were normal weight, slightly more than a quarter were overweight, and a quarter were obese. The incidence of 5-year-old children who were overweight or obese was 70% higher in children of overweight mothers and 80% higher in those with obese mothers, compared with children with normal-weight mothers in an analysis that adjusted for maternal age at delivery, race or ethnicity, and education. Both were statistically significant differences, Dr. Mueller reported.
Two-thirds of the women had vaginal deliveries and a third had C-sections. Overweight or obesity occurred in 40% more of the children delivered by C-section, compared with children born vaginally, a statistically significant difference in an analysis that controlled for the same three covariates as well as prepregnancy body mass index, pregnancy weight gain, and other variables.
When Dr. Mueller and his associates ran a combined analysis they found that the highest risk for childhood overweight or obesity was in children born to obese mothers by C-section, and it was a 2.8-fold higher rate than that in the children born to normal-weight mothers by vaginal delivery, a statistically significant difference.
Dr. Mueller had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Children from obese mothers who had cesarean sections had a 2.8-fold higher obesity rate, compared with children from normal-weight mothers who had vaginal deliveries.
Data source: The Boston Birth Cohort, with prospective data from 1,441 pregnant women and their children.
Disclosures: Dr. Mueller had no disclosures.
Strokes, migraines linked in women with possible CAD
NEW ORLEANS – Women who underwent a clinically indicated coronary angiogram and also reported having migraine headaches had a twofold increased rate of strokes, compared with similar women without a history of migraine, in a prospective, observational study of 888 women followed for a median of 6.5 years.
This finding “underscores that more attention should be placed on evaluating women with a history of migraine headache for cardiovascular disease,” Cecil A. Rambarat, MD, said at the American Heart Association scientific sessions.
Aspirin aside, what’s important for these women is to “modify their risk factors and look at their family history,” reported Dr Rambarat of the University of Florida in Gainesville.
The study used data collected on women enrolled in the Women’s Ischemic Syndrome Evaluation (WISE) study during 1996-1999. WISE entered women at four U.S. centers scheduled for a clinically indicated coronary angiogram as part of their routine care for chest pain symptoms or suspected myocardial ischemia.
Among the 936 women enrolled in WISE, 917 completed a baseline questionnaire about their migraine history that showed 224 women had a migraine history and 693 women did not report having migraine headaches. The average age of women with a migraine history was 54 years, compared with 59 years in those without migraines.
All 917 women were followed for a median of 6.5 years for the incidence of nonfatal myocardial infarction, stroke, or heart failure. A subgroup of 888 of these women were also followed for a median of 9.5 years for mortality, including the incidence of cardiovascular death.
After the investigators adjusted for age, race, body mass index, history of diabetes or hypertension, dyslipidemia, smoking, and other variables, women with migraine were 83% more likely to have a cardiovascular event (cardiovascular death or nonfatal event) during follow-up, compared with women with no migraine history, a statistically significant difference.
Women with migraine were also 2.33-fold more likely to have a nonfatal stroke during follow-up, also a statistically significant difference. The increased stroke rate seems to have largely driven the significant difference in all cardiovascular events.
The mechanisms that might link migraine headaches with stroke are not clear, but Dr. Rambarat suggested several possibilities. Women with migraine may have dysfunction of their vascular endothelium, increased inflammatory markers, increased release of prothrombotic factors, a patent foramen ovale, or certain genetic risk factors that predispose them to migraine and to stroke or other cardiovascular disease, he said.
A report of these findings was recently published online (Am J Med. 2016 Dec 28. doi: 10.1016/j.amjmed.2016.12.028).
Dr. Rambarat had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
NEW ORLEANS – Women who underwent a clinically indicated coronary angiogram and also reported having migraine headaches had a twofold increased rate of strokes, compared with similar women without a history of migraine, in a prospective, observational study of 888 women followed for a median of 6.5 years.
This finding “underscores that more attention should be placed on evaluating women with a history of migraine headache for cardiovascular disease,” Cecil A. Rambarat, MD, said at the American Heart Association scientific sessions.
Aspirin aside, what’s important for these women is to “modify their risk factors and look at their family history,” reported Dr Rambarat of the University of Florida in Gainesville.
The study used data collected on women enrolled in the Women’s Ischemic Syndrome Evaluation (WISE) study during 1996-1999. WISE entered women at four U.S. centers scheduled for a clinically indicated coronary angiogram as part of their routine care for chest pain symptoms or suspected myocardial ischemia.
Among the 936 women enrolled in WISE, 917 completed a baseline questionnaire about their migraine history that showed 224 women had a migraine history and 693 women did not report having migraine headaches. The average age of women with a migraine history was 54 years, compared with 59 years in those without migraines.
All 917 women were followed for a median of 6.5 years for the incidence of nonfatal myocardial infarction, stroke, or heart failure. A subgroup of 888 of these women were also followed for a median of 9.5 years for mortality, including the incidence of cardiovascular death.
After the investigators adjusted for age, race, body mass index, history of diabetes or hypertension, dyslipidemia, smoking, and other variables, women with migraine were 83% more likely to have a cardiovascular event (cardiovascular death or nonfatal event) during follow-up, compared with women with no migraine history, a statistically significant difference.
Women with migraine were also 2.33-fold more likely to have a nonfatal stroke during follow-up, also a statistically significant difference. The increased stroke rate seems to have largely driven the significant difference in all cardiovascular events.
The mechanisms that might link migraine headaches with stroke are not clear, but Dr. Rambarat suggested several possibilities. Women with migraine may have dysfunction of their vascular endothelium, increased inflammatory markers, increased release of prothrombotic factors, a patent foramen ovale, or certain genetic risk factors that predispose them to migraine and to stroke or other cardiovascular disease, he said.
A report of these findings was recently published online (Am J Med. 2016 Dec 28. doi: 10.1016/j.amjmed.2016.12.028).
Dr. Rambarat had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
NEW ORLEANS – Women who underwent a clinically indicated coronary angiogram and also reported having migraine headaches had a twofold increased rate of strokes, compared with similar women without a history of migraine, in a prospective, observational study of 888 women followed for a median of 6.5 years.
This finding “underscores that more attention should be placed on evaluating women with a history of migraine headache for cardiovascular disease,” Cecil A. Rambarat, MD, said at the American Heart Association scientific sessions.
Aspirin aside, what’s important for these women is to “modify their risk factors and look at their family history,” reported Dr Rambarat of the University of Florida in Gainesville.
The study used data collected on women enrolled in the Women’s Ischemic Syndrome Evaluation (WISE) study during 1996-1999. WISE entered women at four U.S. centers scheduled for a clinically indicated coronary angiogram as part of their routine care for chest pain symptoms or suspected myocardial ischemia.
Among the 936 women enrolled in WISE, 917 completed a baseline questionnaire about their migraine history that showed 224 women had a migraine history and 693 women did not report having migraine headaches. The average age of women with a migraine history was 54 years, compared with 59 years in those without migraines.
All 917 women were followed for a median of 6.5 years for the incidence of nonfatal myocardial infarction, stroke, or heart failure. A subgroup of 888 of these women were also followed for a median of 9.5 years for mortality, including the incidence of cardiovascular death.
After the investigators adjusted for age, race, body mass index, history of diabetes or hypertension, dyslipidemia, smoking, and other variables, women with migraine were 83% more likely to have a cardiovascular event (cardiovascular death or nonfatal event) during follow-up, compared with women with no migraine history, a statistically significant difference.
Women with migraine were also 2.33-fold more likely to have a nonfatal stroke during follow-up, also a statistically significant difference. The increased stroke rate seems to have largely driven the significant difference in all cardiovascular events.
The mechanisms that might link migraine headaches with stroke are not clear, but Dr. Rambarat suggested several possibilities. Women with migraine may have dysfunction of their vascular endothelium, increased inflammatory markers, increased release of prothrombotic factors, a patent foramen ovale, or certain genetic risk factors that predispose them to migraine and to stroke or other cardiovascular disease, he said.
A report of these findings was recently published online (Am J Med. 2016 Dec 28. doi: 10.1016/j.amjmed.2016.12.028).
Dr. Rambarat had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The stroke rate during 6.5 years of follow-up was more than twofold greater in women with a migraine headache history.
Data source: WISE, a study of 936 U.S. women enrolled during 1996-1999 and followed prospectively.
Disclosures: Dr. Rambarat had no relevant financial disclosures.
Experts say don’t SPRINT to adopt low blood pressure target
NEW ORLEANS – The key message of the SPRINT trial – that aggressive antihypertensive therapy to a target systolic blood pressure (SBP) of less than 120 mm Hg reduces all-cause mortality, compared with a target SBP under 140 mm Hg – is not broadly applicable as a routine strategy in managing hypertension, experts declared at the American Heart Association scientific sessions.
“My concern is that the patients in the SPRINT trial ended up being highly selected for having a strong ability to achieve and tolerate being at systolic blood pressure levels that we generally don’t see in a lot of treated hypertensives today in this country,” cautioned Peter M. Okin, MD, of Columbia University, New York.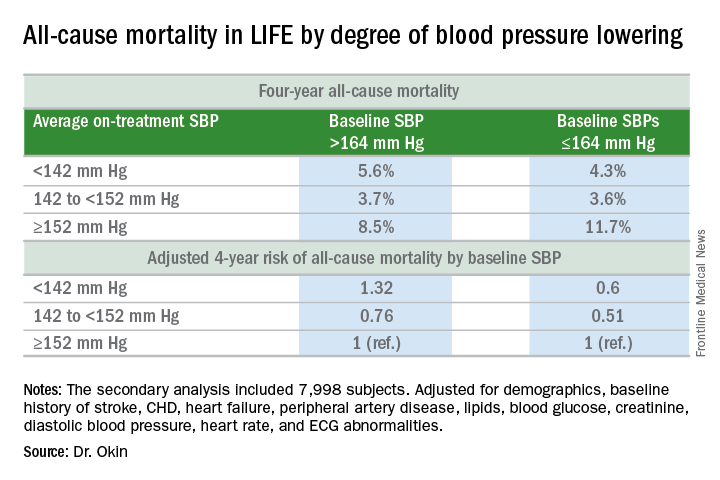
It will be interesting to see how Dr. Okin’s opinion, which is shared by many leading cardiologists, is addressed in new hypertension treatment guidelines from the American College of Cardiology and the American Heart Association. The guidelines are anticipated in March.
Dr. Okin presented a secondary analysis of the earlier landmark LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) trial that’s diametrically at odds with the main finding in SPRINT (the Systolic Blood Pressure Intervention Trial): namely, in LIFE (Lancet. 2002 Mar 23;359[9311]:1004-10), all-cause mortality during follow-up was heavily dependent upon baseline blood pressure.
Among LIFE participants with a baseline SBP below 164 mm Hg, achievement of an average on-treatment SBP below 142 mm Hg was associated with a 40% reduction in all-cause mortality more than 4 years of follow-up, compared with those with an achieved SBP of 152 mm Hg or more. In contrast, LIFE subjects whose baseline SBP was greater than 164 mm Hg actually had a 32% increase in all-cause mortality if their achieved SBP was less than 142 mm Hg, compared with those whose average on-treatment SBP was 152 mm Hg or higher.
How to account for the disparate results of LIFE and SPRINT?
SPRINT (N Engl J Med. 2015 Nov 26; 373:2103-16) enrolled nondiabetic patients aged 50 years or older who had an SBP of 130 mm Hg or more and high cardiovascular risk, with a 10-year Framingham Risk Score greater than 15%. But because the SBP threshold for entry was set so low, at 130 mm Hg, roughly half of SPRINT participants had baseline SBP levels that were already at or below the standard treatment target of 140 mm Hg. For those patients, getting to roughly 120 mm Hg on treatment wasn’t all that big a stretch in terms of the magnitude of blood pressure reduction, Dr. Okin said.
“Our analysis doesn’t invalidate SPRINT in any way, shape, or fashion. It just gives us some pause for thought,” he added.
His post-hoc analysis of LIFE was restricted to the 7,998 participants without diabetes at baseline, since SPRINT excluded diabetics from enrollment.
Audience comments were split between cardiologists who consider SPRINT a game-changer in the treatment of hypertension and those who, like Dr. Okin, have reservations. Among those reservations was the unexpected and difficult-to-explain finding that aggressive SBP lowering didn’t reduce the risk of stroke, compared with less-intensive SBP lowering, unlike the case in other clinical trials and epidemiologic studies in hypertension. Also, audience members took issue with the fact that blood pressure measurements in SPRINT weren’t done in the standard office measurement way employed in other major trials. Instead, SPRINT relied upon automated blood pressure monitoring of a patient alone in a room, which several cardiologists in the audience thought might have skewed the study results, since automated measurements tend to run lower.
Elsewhere at the AHA meeting, former AHA president Clyde W. Yancy, MD, offered a cautionary note regarding SPRINT.
“I think it’s important that we emphasize to this audience that SPRINT is looking at a very select patient population that probably describes only 15% of those with hypertension, specifically those with very high cardiovascular disease risk profiles. So we have to be very careful when we take the blood pressure targets that were identified in SPRINT and try to extrapolate those to other populations,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University, Chicago.
Dr. Okin reported having no financial conflicts of interest.
NEW ORLEANS – The key message of the SPRINT trial – that aggressive antihypertensive therapy to a target systolic blood pressure (SBP) of less than 120 mm Hg reduces all-cause mortality, compared with a target SBP under 140 mm Hg – is not broadly applicable as a routine strategy in managing hypertension, experts declared at the American Heart Association scientific sessions.
“My concern is that the patients in the SPRINT trial ended up being highly selected for having a strong ability to achieve and tolerate being at systolic blood pressure levels that we generally don’t see in a lot of treated hypertensives today in this country,” cautioned Peter M. Okin, MD, of Columbia University, New York.
It will be interesting to see how Dr. Okin’s opinion, which is shared by many leading cardiologists, is addressed in new hypertension treatment guidelines from the American College of Cardiology and the American Heart Association. The guidelines are anticipated in March.
Dr. Okin presented a secondary analysis of the earlier landmark LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) trial that’s diametrically at odds with the main finding in SPRINT (the Systolic Blood Pressure Intervention Trial): namely, in LIFE (Lancet. 2002 Mar 23;359[9311]:1004-10), all-cause mortality during follow-up was heavily dependent upon baseline blood pressure.
Among LIFE participants with a baseline SBP below 164 mm Hg, achievement of an average on-treatment SBP below 142 mm Hg was associated with a 40% reduction in all-cause mortality more than 4 years of follow-up, compared with those with an achieved SBP of 152 mm Hg or more. In contrast, LIFE subjects whose baseline SBP was greater than 164 mm Hg actually had a 32% increase in all-cause mortality if their achieved SBP was less than 142 mm Hg, compared with those whose average on-treatment SBP was 152 mm Hg or higher.
How to account for the disparate results of LIFE and SPRINT?
SPRINT (N Engl J Med. 2015 Nov 26; 373:2103-16) enrolled nondiabetic patients aged 50 years or older who had an SBP of 130 mm Hg or more and high cardiovascular risk, with a 10-year Framingham Risk Score greater than 15%. But because the SBP threshold for entry was set so low, at 130 mm Hg, roughly half of SPRINT participants had baseline SBP levels that were already at or below the standard treatment target of 140 mm Hg. For those patients, getting to roughly 120 mm Hg on treatment wasn’t all that big a stretch in terms of the magnitude of blood pressure reduction, Dr. Okin said.
“Our analysis doesn’t invalidate SPRINT in any way, shape, or fashion. It just gives us some pause for thought,” he added.
His post-hoc analysis of LIFE was restricted to the 7,998 participants without diabetes at baseline, since SPRINT excluded diabetics from enrollment.
Audience comments were split between cardiologists who consider SPRINT a game-changer in the treatment of hypertension and those who, like Dr. Okin, have reservations. Among those reservations was the unexpected and difficult-to-explain finding that aggressive SBP lowering didn’t reduce the risk of stroke, compared with less-intensive SBP lowering, unlike the case in other clinical trials and epidemiologic studies in hypertension. Also, audience members took issue with the fact that blood pressure measurements in SPRINT weren’t done in the standard office measurement way employed in other major trials. Instead, SPRINT relied upon automated blood pressure monitoring of a patient alone in a room, which several cardiologists in the audience thought might have skewed the study results, since automated measurements tend to run lower.
Elsewhere at the AHA meeting, former AHA president Clyde W. Yancy, MD, offered a cautionary note regarding SPRINT.
“I think it’s important that we emphasize to this audience that SPRINT is looking at a very select patient population that probably describes only 15% of those with hypertension, specifically those with very high cardiovascular disease risk profiles. So we have to be very careful when we take the blood pressure targets that were identified in SPRINT and try to extrapolate those to other populations,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University, Chicago.
Dr. Okin reported having no financial conflicts of interest.
NEW ORLEANS – The key message of the SPRINT trial – that aggressive antihypertensive therapy to a target systolic blood pressure (SBP) of less than 120 mm Hg reduces all-cause mortality, compared with a target SBP under 140 mm Hg – is not broadly applicable as a routine strategy in managing hypertension, experts declared at the American Heart Association scientific sessions.
“My concern is that the patients in the SPRINT trial ended up being highly selected for having a strong ability to achieve and tolerate being at systolic blood pressure levels that we generally don’t see in a lot of treated hypertensives today in this country,” cautioned Peter M. Okin, MD, of Columbia University, New York.
It will be interesting to see how Dr. Okin’s opinion, which is shared by many leading cardiologists, is addressed in new hypertension treatment guidelines from the American College of Cardiology and the American Heart Association. The guidelines are anticipated in March.
Dr. Okin presented a secondary analysis of the earlier landmark LIFE (Losartan Intervention for Endpoint Reduction in Hypertension) trial that’s diametrically at odds with the main finding in SPRINT (the Systolic Blood Pressure Intervention Trial): namely, in LIFE (Lancet. 2002 Mar 23;359[9311]:1004-10), all-cause mortality during follow-up was heavily dependent upon baseline blood pressure.
Among LIFE participants with a baseline SBP below 164 mm Hg, achievement of an average on-treatment SBP below 142 mm Hg was associated with a 40% reduction in all-cause mortality more than 4 years of follow-up, compared with those with an achieved SBP of 152 mm Hg or more. In contrast, LIFE subjects whose baseline SBP was greater than 164 mm Hg actually had a 32% increase in all-cause mortality if their achieved SBP was less than 142 mm Hg, compared with those whose average on-treatment SBP was 152 mm Hg or higher.
How to account for the disparate results of LIFE and SPRINT?
SPRINT (N Engl J Med. 2015 Nov 26; 373:2103-16) enrolled nondiabetic patients aged 50 years or older who had an SBP of 130 mm Hg or more and high cardiovascular risk, with a 10-year Framingham Risk Score greater than 15%. But because the SBP threshold for entry was set so low, at 130 mm Hg, roughly half of SPRINT participants had baseline SBP levels that were already at or below the standard treatment target of 140 mm Hg. For those patients, getting to roughly 120 mm Hg on treatment wasn’t all that big a stretch in terms of the magnitude of blood pressure reduction, Dr. Okin said.
“Our analysis doesn’t invalidate SPRINT in any way, shape, or fashion. It just gives us some pause for thought,” he added.
His post-hoc analysis of LIFE was restricted to the 7,998 participants without diabetes at baseline, since SPRINT excluded diabetics from enrollment.
Audience comments were split between cardiologists who consider SPRINT a game-changer in the treatment of hypertension and those who, like Dr. Okin, have reservations. Among those reservations was the unexpected and difficult-to-explain finding that aggressive SBP lowering didn’t reduce the risk of stroke, compared with less-intensive SBP lowering, unlike the case in other clinical trials and epidemiologic studies in hypertension. Also, audience members took issue with the fact that blood pressure measurements in SPRINT weren’t done in the standard office measurement way employed in other major trials. Instead, SPRINT relied upon automated blood pressure monitoring of a patient alone in a room, which several cardiologists in the audience thought might have skewed the study results, since automated measurements tend to run lower.
Elsewhere at the AHA meeting, former AHA president Clyde W. Yancy, MD, offered a cautionary note regarding SPRINT.
“I think it’s important that we emphasize to this audience that SPRINT is looking at a very select patient population that probably describes only 15% of those with hypertension, specifically those with very high cardiovascular disease risk profiles. So we have to be very careful when we take the blood pressure targets that were identified in SPRINT and try to extrapolate those to other populations,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University, Chicago.
Dr. Okin reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
Subclinical AF found in 1/3 of asymptomatic elderly
NEW ORLEANS – About a third of elderly people at high cardiovascular risk but otherwise healthy and asymptomatic had subclinical atrial fibrillation in a multicenter study of 273 people.
This finding that subclinical atrial fibrillation (AF) is “extremely common” in elderly people with cardiovascular risk factors “weakens the case that detecting subclinical AF in patients following a stroke implies causality” of the stroke “because subclinical AF is so prevalent,” Jeff S. Healey, MD, said at the American Heart Association Scientific Sessions.
“I think that subclinical AF is a distinct subgroup of AF, with a risk for stroke that is quite low, about 1.5%-2% per year,” said Dr. Healey, a cardiologist at McMaster University in Hamilton, Canada. “Given that this was an elderly population [study participants averaged 74 years old] with bleeding risk, it’s reasonable to question” whether many people with subclinical AF need anticoagulation. The question of whether “45 seconds of AF seen 6 months after a stroke is worthy of treatment with an anticoagulant should give people pause,” he said.
The Prevalence of Sub-Clinical Atrial Fibrillation Using an Implantable Cardiac Monitor (ASSERT-II) study initially enrolled 273 people at 26 sites in Canada and The Netherlands. Researchers actually placed a loop recorder in 256, and complete follow-up of at least 9 months occurred for 252. Enrolled patients had to be at 65 years old, and have at least one of these risk factors for AF or stroke: a CHA2DS2-VASc score of 2 or greater; documented obstructive sleep apnea; or a body mass index greater than 30 kg/m2. In addition, enrollees also had to have one of these risk factors for AF: a left atrial volume of at least 58 ml; a left atrial diameter of at least 4.4 cm; or a serum NT-proBNP level of at least 290 pg/mL.
Dr. Healey and his associates prespecified subclinical AF as at least 5 minutes of AF seen in the loop recording during follow-up, which occurred in 34% of the participants during an average 16 months of follow-up, he reported. At least 30 minutes of AF occurred in 22% during follow-up, at least 6 hours in 7%, and at least 24 hours in 3%.
In a prespecified set of subgroup analyses, people with a large left atrium formed the only subgroup with a statistically significant association with outcome. People with a left atrial size at or above the study median of 73.5 ml had an 85% increased rate of subclinical AF compared with those with smaller left atria in the multivariate analysis. But increased left atrial size alone did not fully explain subclinical atrial fibrillation. Even among participants in the lowest quartile for left atrial diameter, less than 4.3 cm, the prevalence of subclinical AF was 27%, Dr. Healey noted.
[email protected]
On Twitter @mitchelzoler
The results reported by Dr. Healey provide robust data that bridges a major gap we have had in our understanding of atrial fibrillation. The new finding of a high prevalence of subclinical atrial fibrillation in elderly people with cardiovascular risk factors, regardless of whether they had a prior stroke, substantially weakens the case that subclinical atrial fibrillation detected following a stroke has a causal relationship to the stroke. This implication is quite important.
Many questions remain about the meaning of subclinical atrial fibrillation. What relationship does it have with stroke, and what thresholds exist for atrial fibrillation to raise stroke risk? Also, what are the risks and benefits of anticoagulation in people with subclinical AF and is intermittent anticoagulation helpful?
N.A. Mark Estes III, MD , is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts Medical Center in Boston. He has been a consultant to Boston Scientific, Medtronic and St. Jude. He made these comments as designated discussant for ASSERT-II.
The results reported by Dr. Healey provide robust data that bridges a major gap we have had in our understanding of atrial fibrillation. The new finding of a high prevalence of subclinical atrial fibrillation in elderly people with cardiovascular risk factors, regardless of whether they had a prior stroke, substantially weakens the case that subclinical atrial fibrillation detected following a stroke has a causal relationship to the stroke. This implication is quite important.
Many questions remain about the meaning of subclinical atrial fibrillation. What relationship does it have with stroke, and what thresholds exist for atrial fibrillation to raise stroke risk? Also, what are the risks and benefits of anticoagulation in people with subclinical AF and is intermittent anticoagulation helpful?
N.A. Mark Estes III, MD , is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts Medical Center in Boston. He has been a consultant to Boston Scientific, Medtronic and St. Jude. He made these comments as designated discussant for ASSERT-II.
The results reported by Dr. Healey provide robust data that bridges a major gap we have had in our understanding of atrial fibrillation. The new finding of a high prevalence of subclinical atrial fibrillation in elderly people with cardiovascular risk factors, regardless of whether they had a prior stroke, substantially weakens the case that subclinical atrial fibrillation detected following a stroke has a causal relationship to the stroke. This implication is quite important.
Many questions remain about the meaning of subclinical atrial fibrillation. What relationship does it have with stroke, and what thresholds exist for atrial fibrillation to raise stroke risk? Also, what are the risks and benefits of anticoagulation in people with subclinical AF and is intermittent anticoagulation helpful?
N.A. Mark Estes III, MD , is professor of medicine and director of the New England Cardiac Arrhythmia Center at Tufts Medical Center in Boston. He has been a consultant to Boston Scientific, Medtronic and St. Jude. He made these comments as designated discussant for ASSERT-II.
NEW ORLEANS – About a third of elderly people at high cardiovascular risk but otherwise healthy and asymptomatic had subclinical atrial fibrillation in a multicenter study of 273 people.
This finding that subclinical atrial fibrillation (AF) is “extremely common” in elderly people with cardiovascular risk factors “weakens the case that detecting subclinical AF in patients following a stroke implies causality” of the stroke “because subclinical AF is so prevalent,” Jeff S. Healey, MD, said at the American Heart Association Scientific Sessions.
“I think that subclinical AF is a distinct subgroup of AF, with a risk for stroke that is quite low, about 1.5%-2% per year,” said Dr. Healey, a cardiologist at McMaster University in Hamilton, Canada. “Given that this was an elderly population [study participants averaged 74 years old] with bleeding risk, it’s reasonable to question” whether many people with subclinical AF need anticoagulation. The question of whether “45 seconds of AF seen 6 months after a stroke is worthy of treatment with an anticoagulant should give people pause,” he said.
The Prevalence of Sub-Clinical Atrial Fibrillation Using an Implantable Cardiac Monitor (ASSERT-II) study initially enrolled 273 people at 26 sites in Canada and The Netherlands. Researchers actually placed a loop recorder in 256, and complete follow-up of at least 9 months occurred for 252. Enrolled patients had to be at 65 years old, and have at least one of these risk factors for AF or stroke: a CHA2DS2-VASc score of 2 or greater; documented obstructive sleep apnea; or a body mass index greater than 30 kg/m2. In addition, enrollees also had to have one of these risk factors for AF: a left atrial volume of at least 58 ml; a left atrial diameter of at least 4.4 cm; or a serum NT-proBNP level of at least 290 pg/mL.
Dr. Healey and his associates prespecified subclinical AF as at least 5 minutes of AF seen in the loop recording during follow-up, which occurred in 34% of the participants during an average 16 months of follow-up, he reported. At least 30 minutes of AF occurred in 22% during follow-up, at least 6 hours in 7%, and at least 24 hours in 3%.
In a prespecified set of subgroup analyses, people with a large left atrium formed the only subgroup with a statistically significant association with outcome. People with a left atrial size at or above the study median of 73.5 ml had an 85% increased rate of subclinical AF compared with those with smaller left atria in the multivariate analysis. But increased left atrial size alone did not fully explain subclinical atrial fibrillation. Even among participants in the lowest quartile for left atrial diameter, less than 4.3 cm, the prevalence of subclinical AF was 27%, Dr. Healey noted.
[email protected]
On Twitter @mitchelzoler
NEW ORLEANS – About a third of elderly people at high cardiovascular risk but otherwise healthy and asymptomatic had subclinical atrial fibrillation in a multicenter study of 273 people.
This finding that subclinical atrial fibrillation (AF) is “extremely common” in elderly people with cardiovascular risk factors “weakens the case that detecting subclinical AF in patients following a stroke implies causality” of the stroke “because subclinical AF is so prevalent,” Jeff S. Healey, MD, said at the American Heart Association Scientific Sessions.
“I think that subclinical AF is a distinct subgroup of AF, with a risk for stroke that is quite low, about 1.5%-2% per year,” said Dr. Healey, a cardiologist at McMaster University in Hamilton, Canada. “Given that this was an elderly population [study participants averaged 74 years old] with bleeding risk, it’s reasonable to question” whether many people with subclinical AF need anticoagulation. The question of whether “45 seconds of AF seen 6 months after a stroke is worthy of treatment with an anticoagulant should give people pause,” he said.
The Prevalence of Sub-Clinical Atrial Fibrillation Using an Implantable Cardiac Monitor (ASSERT-II) study initially enrolled 273 people at 26 sites in Canada and The Netherlands. Researchers actually placed a loop recorder in 256, and complete follow-up of at least 9 months occurred for 252. Enrolled patients had to be at 65 years old, and have at least one of these risk factors for AF or stroke: a CHA2DS2-VASc score of 2 or greater; documented obstructive sleep apnea; or a body mass index greater than 30 kg/m2. In addition, enrollees also had to have one of these risk factors for AF: a left atrial volume of at least 58 ml; a left atrial diameter of at least 4.4 cm; or a serum NT-proBNP level of at least 290 pg/mL.
Dr. Healey and his associates prespecified subclinical AF as at least 5 minutes of AF seen in the loop recording during follow-up, which occurred in 34% of the participants during an average 16 months of follow-up, he reported. At least 30 minutes of AF occurred in 22% during follow-up, at least 6 hours in 7%, and at least 24 hours in 3%.
In a prespecified set of subgroup analyses, people with a large left atrium formed the only subgroup with a statistically significant association with outcome. People with a left atrial size at or above the study median of 73.5 ml had an 85% increased rate of subclinical AF compared with those with smaller left atria in the multivariate analysis. But increased left atrial size alone did not fully explain subclinical atrial fibrillation. Even among participants in the lowest quartile for left atrial diameter, less than 4.3 cm, the prevalence of subclinical AF was 27%, Dr. Healey noted.
[email protected]
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: One-third of asymptomatic elderly people with cardiovascular risk factors had subclinical atrial fibrillation.
Data source: A multicenter study with 252 people followed for an average of 16 months.
Disclosures: Dr. Healey has been a consultant to or received honoraria from Bayer, Medtronic, Pfizer and Servier. He has received research support from Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Medtronic and St. Jude.
Cardiac procedures tied to higher cancer risk in adult congenital heart disease
NEW ORLEANS – The greater the lifetime number of cardiac procedures entailing exposure to low-dose ionizing radiation, the higher the associated cancer risk in adults with congenital heart disease, Sarah Cohen, MD, reported at the American Heart Association scientific sessions.
This finding takes on added clinical import because the use of such procedures has increased markedly in patients with adult congenital heart disease, added Dr. Cohen of McGill University in Montreal.
She presented analyses of nearly 25,000 patients in the Quebec congenital heart disease database who were ages 18-64 with no history of cancer in January 1995; 602 of them were diagnosed with cancer during follow-up through 2009. Each was matched to four cancer-free controls in the database on the basis of age, sex, congenital heart disease severity, and calendar year.
Patients with a lifetime total of four or five cardiac procedures involving exposure to low-dose ionizing radiation were 1.7-fold more likely to be diagnosed with cancer during follow-up than were those with a history of no such procedures or just one. With a lifetime history of six or more of the procedures, the relative risk climbed to 2.2-fold.
Nearly 90% of the malignancies were genitourinary, respiratory, digestive, breast, or hematologic cancers.
Dr. Cohen and her coinvestigators also conducted a cohort study comparing the relative risk of developing cancer in 1,781 high-exposure Quebec adults in the database who had a lifetime history of four or more cardiac procedures involving radiation exposure to more than 20,000 others with a history of not more than one such procedure. The high-exposure group had a 3.3-fold greater rate of malignancy during follow-up.
In a separate study, Dr. Cohen’s McGill colleagues analyzed the Quebec database and documented the sharp rise over time in low-dose ionizing radiation cardiac procedures, including catheter-based diagnostic procedures, structural heart interventions, nuclear procedures, coronary interventions, computed tomography scans of the chest, coronary interventions, and insertion or repair of pacemakers and implantable cardioverter-defibrillators. In 1990, the rate was 18.5 such procedures per 1,000 patients per year. By 2005, it had nearly tripled to 51.9 per 1,000 per year. The age at first procedure dropped from 5 years to 9 months (Circulation 2016 Jan 5;133[1]:12-20).
Dr. Cohen reported having no financial disclosures.
NEW ORLEANS – The greater the lifetime number of cardiac procedures entailing exposure to low-dose ionizing radiation, the higher the associated cancer risk in adults with congenital heart disease, Sarah Cohen, MD, reported at the American Heart Association scientific sessions.
This finding takes on added clinical import because the use of such procedures has increased markedly in patients with adult congenital heart disease, added Dr. Cohen of McGill University in Montreal.
She presented analyses of nearly 25,000 patients in the Quebec congenital heart disease database who were ages 18-64 with no history of cancer in January 1995; 602 of them were diagnosed with cancer during follow-up through 2009. Each was matched to four cancer-free controls in the database on the basis of age, sex, congenital heart disease severity, and calendar year.
Patients with a lifetime total of four or five cardiac procedures involving exposure to low-dose ionizing radiation were 1.7-fold more likely to be diagnosed with cancer during follow-up than were those with a history of no such procedures or just one. With a lifetime history of six or more of the procedures, the relative risk climbed to 2.2-fold.
Nearly 90% of the malignancies were genitourinary, respiratory, digestive, breast, or hematologic cancers.
Dr. Cohen and her coinvestigators also conducted a cohort study comparing the relative risk of developing cancer in 1,781 high-exposure Quebec adults in the database who had a lifetime history of four or more cardiac procedures involving radiation exposure to more than 20,000 others with a history of not more than one such procedure. The high-exposure group had a 3.3-fold greater rate of malignancy during follow-up.
In a separate study, Dr. Cohen’s McGill colleagues analyzed the Quebec database and documented the sharp rise over time in low-dose ionizing radiation cardiac procedures, including catheter-based diagnostic procedures, structural heart interventions, nuclear procedures, coronary interventions, computed tomography scans of the chest, coronary interventions, and insertion or repair of pacemakers and implantable cardioverter-defibrillators. In 1990, the rate was 18.5 such procedures per 1,000 patients per year. By 2005, it had nearly tripled to 51.9 per 1,000 per year. The age at first procedure dropped from 5 years to 9 months (Circulation 2016 Jan 5;133[1]:12-20).
Dr. Cohen reported having no financial disclosures.
NEW ORLEANS – The greater the lifetime number of cardiac procedures entailing exposure to low-dose ionizing radiation, the higher the associated cancer risk in adults with congenital heart disease, Sarah Cohen, MD, reported at the American Heart Association scientific sessions.
This finding takes on added clinical import because the use of such procedures has increased markedly in patients with adult congenital heart disease, added Dr. Cohen of McGill University in Montreal.
She presented analyses of nearly 25,000 patients in the Quebec congenital heart disease database who were ages 18-64 with no history of cancer in January 1995; 602 of them were diagnosed with cancer during follow-up through 2009. Each was matched to four cancer-free controls in the database on the basis of age, sex, congenital heart disease severity, and calendar year.
Patients with a lifetime total of four or five cardiac procedures involving exposure to low-dose ionizing radiation were 1.7-fold more likely to be diagnosed with cancer during follow-up than were those with a history of no such procedures or just one. With a lifetime history of six or more of the procedures, the relative risk climbed to 2.2-fold.
Nearly 90% of the malignancies were genitourinary, respiratory, digestive, breast, or hematologic cancers.
Dr. Cohen and her coinvestigators also conducted a cohort study comparing the relative risk of developing cancer in 1,781 high-exposure Quebec adults in the database who had a lifetime history of four or more cardiac procedures involving radiation exposure to more than 20,000 others with a history of not more than one such procedure. The high-exposure group had a 3.3-fold greater rate of malignancy during follow-up.
In a separate study, Dr. Cohen’s McGill colleagues analyzed the Quebec database and documented the sharp rise over time in low-dose ionizing radiation cardiac procedures, including catheter-based diagnostic procedures, structural heart interventions, nuclear procedures, coronary interventions, computed tomography scans of the chest, coronary interventions, and insertion or repair of pacemakers and implantable cardioverter-defibrillators. In 1990, the rate was 18.5 such procedures per 1,000 patients per year. By 2005, it had nearly tripled to 51.9 per 1,000 per year. The age at first procedure dropped from 5 years to 9 months (Circulation 2016 Jan 5;133[1]:12-20).
Dr. Cohen reported having no financial disclosures.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Adults with congenital heart disease who had a lifetime history of six or more cardiac procedures involving exposure to low-dose ionizing radiation were 2.2-fold more likely to develop cancer during follow-up than were those with not more than one such procedure.
Data source: This nested, case-control study included 602 adults with congenital heart disease who developed cancer, each matched to four controls who did not.
Disclosures: The study presenter reported having no financial disclosures.
Cancer risk six times higher in children with congenital heart defects
NEW ORLEANS – Children with congenital heart defects have a five- to sixfold increased risk of developing pediatric cancer, Matthew Oster, MD, reported at the American Heart Association scientific sessions.
“The absolute risk is small, but it’s still a five- to sixfold risk compared to the general pediatric population, and it does warrant monitoring and a high index of suspicion in our children with congenital heart defects,” said Dr. Oster of Children’s Healthcare of Atlanta.
The increased cancer risk in adults with congenital heart defects has recently been the focus of research attention. However, little is known about the risk of cancer during the childhood of patients with congenital heart defects.
This was the impetus for Dr. Oster’s nationwide retrospective study of 6.1 million children and adolescents continuously enrolled in private, employer-sponsored health insurance plans during 2009-2015. The data came from the Truven Health MarketScan administrative database.
Children with Down syndrome were excluded from the study because their condition is known to be associated with increased rates of both congenital heart defects and pediatric cancers.
Among 88,493 individuals under age 18 with a diagnosed congenital heart defect, the incidence of any neoplasm diagnosed at least 30 days after diagnosis of the heart defect was 3.91/1,000, compared with 0.79/1,000 in more than 6 million children and adolescents without congenital heart disease.
Thus, children with a congenital heart defect were at a 4.9-fold increased risk for developing a childhood cancer. The risk for bone tumors was 11.2-fold greater than in the general pediatric population, and their neuroblastoma risk was 9.8-fold greater. Their risks of lymphoma and leukemia were increased 5.2- and 2.8-fold, respectively. Of note, they had no increased risk of brain tumors.
To confirm their results, Dr. Oster and his coinvestigators also conducted a sensitivity analysis limited to the 55,079 children and adolescents with at least two outpatient or one inpatient ICD-9 diagnostic code for congenital heart disease. Here the incidence of childhood malignancies was 5.1/1,000 patients, for an overall 6.4-fold increased relative risk.
“Bedside-to-bench work is now needed to determine potential mechanisms,” he said. “We believe the increased risk is related more to a common genetic pathway than to an exposure or treatment pathway because it occurs so early. But there may be some impact of exposures or treatment as well. We think that radiation exposure is more of a longer-term risk.”
He and his coinvestigators next plan to look at the impact on pediatric cancer risk of specific types of congenital heart defects.
Dr. Oster reported having no financial conflicts of interest regarding this study, which received Centers for Disease Control and Prevention funding.
NEW ORLEANS – Children with congenital heart defects have a five- to sixfold increased risk of developing pediatric cancer, Matthew Oster, MD, reported at the American Heart Association scientific sessions.
“The absolute risk is small, but it’s still a five- to sixfold risk compared to the general pediatric population, and it does warrant monitoring and a high index of suspicion in our children with congenital heart defects,” said Dr. Oster of Children’s Healthcare of Atlanta.
The increased cancer risk in adults with congenital heart defects has recently been the focus of research attention. However, little is known about the risk of cancer during the childhood of patients with congenital heart defects.
This was the impetus for Dr. Oster’s nationwide retrospective study of 6.1 million children and adolescents continuously enrolled in private, employer-sponsored health insurance plans during 2009-2015. The data came from the Truven Health MarketScan administrative database.
Children with Down syndrome were excluded from the study because their condition is known to be associated with increased rates of both congenital heart defects and pediatric cancers.
Among 88,493 individuals under age 18 with a diagnosed congenital heart defect, the incidence of any neoplasm diagnosed at least 30 days after diagnosis of the heart defect was 3.91/1,000, compared with 0.79/1,000 in more than 6 million children and adolescents without congenital heart disease.
Thus, children with a congenital heart defect were at a 4.9-fold increased risk for developing a childhood cancer. The risk for bone tumors was 11.2-fold greater than in the general pediatric population, and their neuroblastoma risk was 9.8-fold greater. Their risks of lymphoma and leukemia were increased 5.2- and 2.8-fold, respectively. Of note, they had no increased risk of brain tumors.
To confirm their results, Dr. Oster and his coinvestigators also conducted a sensitivity analysis limited to the 55,079 children and adolescents with at least two outpatient or one inpatient ICD-9 diagnostic code for congenital heart disease. Here the incidence of childhood malignancies was 5.1/1,000 patients, for an overall 6.4-fold increased relative risk.
“Bedside-to-bench work is now needed to determine potential mechanisms,” he said. “We believe the increased risk is related more to a common genetic pathway than to an exposure or treatment pathway because it occurs so early. But there may be some impact of exposures or treatment as well. We think that radiation exposure is more of a longer-term risk.”
He and his coinvestigators next plan to look at the impact on pediatric cancer risk of specific types of congenital heart defects.
Dr. Oster reported having no financial conflicts of interest regarding this study, which received Centers for Disease Control and Prevention funding.
NEW ORLEANS – Children with congenital heart defects have a five- to sixfold increased risk of developing pediatric cancer, Matthew Oster, MD, reported at the American Heart Association scientific sessions.
“The absolute risk is small, but it’s still a five- to sixfold risk compared to the general pediatric population, and it does warrant monitoring and a high index of suspicion in our children with congenital heart defects,” said Dr. Oster of Children’s Healthcare of Atlanta.
The increased cancer risk in adults with congenital heart defects has recently been the focus of research attention. However, little is known about the risk of cancer during the childhood of patients with congenital heart defects.
This was the impetus for Dr. Oster’s nationwide retrospective study of 6.1 million children and adolescents continuously enrolled in private, employer-sponsored health insurance plans during 2009-2015. The data came from the Truven Health MarketScan administrative database.
Children with Down syndrome were excluded from the study because their condition is known to be associated with increased rates of both congenital heart defects and pediatric cancers.
Among 88,493 individuals under age 18 with a diagnosed congenital heart defect, the incidence of any neoplasm diagnosed at least 30 days after diagnosis of the heart defect was 3.91/1,000, compared with 0.79/1,000 in more than 6 million children and adolescents without congenital heart disease.
Thus, children with a congenital heart defect were at a 4.9-fold increased risk for developing a childhood cancer. The risk for bone tumors was 11.2-fold greater than in the general pediatric population, and their neuroblastoma risk was 9.8-fold greater. Their risks of lymphoma and leukemia were increased 5.2- and 2.8-fold, respectively. Of note, they had no increased risk of brain tumors.
To confirm their results, Dr. Oster and his coinvestigators also conducted a sensitivity analysis limited to the 55,079 children and adolescents with at least two outpatient or one inpatient ICD-9 diagnostic code for congenital heart disease. Here the incidence of childhood malignancies was 5.1/1,000 patients, for an overall 6.4-fold increased relative risk.
“Bedside-to-bench work is now needed to determine potential mechanisms,” he said. “We believe the increased risk is related more to a common genetic pathway than to an exposure or treatment pathway because it occurs so early. But there may be some impact of exposures or treatment as well. We think that radiation exposure is more of a longer-term risk.”
He and his coinvestigators next plan to look at the impact on pediatric cancer risk of specific types of congenital heart defects.
Dr. Oster reported having no financial conflicts of interest regarding this study, which received Centers for Disease Control and Prevention funding.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Children with congenital heart defects are at a roughly sixfold increased risk of developing a pediatric cancer.
Data source: A retrospective analysis of administrative health insurance plan data on 6.1 million U.S. subjects under age 18 years, more than 88,000 of whom had congenital heart defects.
Disclosures: The presenter reported having no financial conflicts of interest regarding this study, which received Centers for Disease Control and Prevention funding.
LAA closure during cardiac surgery cuts late mortality
NEW ORLEANS – Surgical left atrial appendage closure at the time of open heart surgery in patients with atrial fibrillation doesn’t decrease patients’ early or late risk of stroke, but it does substantially reduce their risk of late mortality, Masahiko Ando, MD, reported at the American Heart Association scientific sessions.
Solid evidence demonstrates that percutaneous left atrial appendage (LAA) closure using the Watchman or other devices in patients with atrial fibrillation offers a potential alternative to lifelong oral anticoagulation.
In contrast, even though surgical LAA closure at the time of cardiac surgery is commonly done, the data as to its long-term impact are scanty. This was the impetus for Dr. Ando and his coinvestigators at Massachusetts General Hospital in Boston to perform a comprehensive systematic review of the medical literature. They also conducted a meta-analysis that involved 7,466 patients who underwent open-heart surgery with or without surgical LAA closure in 12 studies, 3 of which were randomized controlled trials, 2 propensity-matched comparisons, and the rest cohort studies.
At 30-day follow-up, LAA closure was not associated with any significant effect on the risks of stroke, death, reexploration for bleeding, or postoperative atrial fibrillation.
At the latest follow-up in the studies, however, surgical LAA closure was associated with a highly significant 36% reduction in mortality risk compared with the no–LAA-closure control group. This remained the case even after statistical adjustment for demographics, type of cardiac surgery, and the form of preoperative atrial fibrillation.
“Given that we generally add LAA closure to those who have a higher risk of embolization, which could have negatively affected the efficacy of LAA closure, this preventive effect of LAA closure on late mortality cannot be ignored,” said Dr. Ando.
The most likely explanation for the improved survival in surgical LAA closure recipients, he continued, is that the procedure enabled them to avoid aggressive lifelong oral anticoagulation, with its attendant risks.
Dr. Ando reported having no financial conflicts regarding his study.
NEW ORLEANS – Surgical left atrial appendage closure at the time of open heart surgery in patients with atrial fibrillation doesn’t decrease patients’ early or late risk of stroke, but it does substantially reduce their risk of late mortality, Masahiko Ando, MD, reported at the American Heart Association scientific sessions.
Solid evidence demonstrates that percutaneous left atrial appendage (LAA) closure using the Watchman or other devices in patients with atrial fibrillation offers a potential alternative to lifelong oral anticoagulation.
In contrast, even though surgical LAA closure at the time of cardiac surgery is commonly done, the data as to its long-term impact are scanty. This was the impetus for Dr. Ando and his coinvestigators at Massachusetts General Hospital in Boston to perform a comprehensive systematic review of the medical literature. They also conducted a meta-analysis that involved 7,466 patients who underwent open-heart surgery with or without surgical LAA closure in 12 studies, 3 of which were randomized controlled trials, 2 propensity-matched comparisons, and the rest cohort studies.
At 30-day follow-up, LAA closure was not associated with any significant effect on the risks of stroke, death, reexploration for bleeding, or postoperative atrial fibrillation.
At the latest follow-up in the studies, however, surgical LAA closure was associated with a highly significant 36% reduction in mortality risk compared with the no–LAA-closure control group. This remained the case even after statistical adjustment for demographics, type of cardiac surgery, and the form of preoperative atrial fibrillation.
“Given that we generally add LAA closure to those who have a higher risk of embolization, which could have negatively affected the efficacy of LAA closure, this preventive effect of LAA closure on late mortality cannot be ignored,” said Dr. Ando.
The most likely explanation for the improved survival in surgical LAA closure recipients, he continued, is that the procedure enabled them to avoid aggressive lifelong oral anticoagulation, with its attendant risks.
Dr. Ando reported having no financial conflicts regarding his study.
NEW ORLEANS – Surgical left atrial appendage closure at the time of open heart surgery in patients with atrial fibrillation doesn’t decrease patients’ early or late risk of stroke, but it does substantially reduce their risk of late mortality, Masahiko Ando, MD, reported at the American Heart Association scientific sessions.
Solid evidence demonstrates that percutaneous left atrial appendage (LAA) closure using the Watchman or other devices in patients with atrial fibrillation offers a potential alternative to lifelong oral anticoagulation.
In contrast, even though surgical LAA closure at the time of cardiac surgery is commonly done, the data as to its long-term impact are scanty. This was the impetus for Dr. Ando and his coinvestigators at Massachusetts General Hospital in Boston to perform a comprehensive systematic review of the medical literature. They also conducted a meta-analysis that involved 7,466 patients who underwent open-heart surgery with or without surgical LAA closure in 12 studies, 3 of which were randomized controlled trials, 2 propensity-matched comparisons, and the rest cohort studies.
At 30-day follow-up, LAA closure was not associated with any significant effect on the risks of stroke, death, reexploration for bleeding, or postoperative atrial fibrillation.
At the latest follow-up in the studies, however, surgical LAA closure was associated with a highly significant 36% reduction in mortality risk compared with the no–LAA-closure control group. This remained the case even after statistical adjustment for demographics, type of cardiac surgery, and the form of preoperative atrial fibrillation.
“Given that we generally add LAA closure to those who have a higher risk of embolization, which could have negatively affected the efficacy of LAA closure, this preventive effect of LAA closure on late mortality cannot be ignored,” said Dr. Ando.
The most likely explanation for the improved survival in surgical LAA closure recipients, he continued, is that the procedure enabled them to avoid aggressive lifelong oral anticoagulation, with its attendant risks.
Dr. Ando reported having no financial conflicts regarding his study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Late mortality risk was reduced by 36% in patients with atrial fibrillation who underwent surgical LAA closure during open heart surgery, compared with those who did not.
Data source: This meta-analysis included 12 published studies and 7,466 patients who either did or did not undergo surgical LAA closure during open heart surgery.
Disclosures: The study presenter reported having no financial conflicts.














